Abstract
Background:
Dietary sodium is a well-known risk factor for cardiovascular and renal disease; however, direct evidence of the longitudinal changes that occur with aging, and the influence of dietary sodium on the age-associated alterations are scarce.
Methods:
C57BL/6 mice were maintained for 13 months on a low (LS, 0.02% Na+), normal (NS, 0.3% Na+) or high (HS, 1.6% Na+) salt diet. We assessed 1) the longitudinal trajectories for two markers of cardiovascular and renal dysfunction (blood pressure (BP) and albuminuria), as well as hormonal changes, and 2) end-of-study cardiac and renal parameters.
Results:
The effect of aging on BP and kidney damage did not reach significance levels in the LS group; however, relative to baseline, there were significant increases in these parameters for animals maintained on NS and HS diets, starting as early as month 7 and month 5, respectively. Furthermore, changes in albuminuria preceded the changes in BP relative to baseline, irrespective of the diet. Circulating aldosterone and plasma renin activity displayed the expected decreasing trends with age and dietary sodium loading. As compared to LS – higher dietary sodium consumption associated with increasing trends in left ventricular mass and volume indices, consistent with an eccentric dilated phenotype. Functional and molecular markers of kidney dysfunction displayed similar trends with increasing long-term sodium levels: higher renovascular resistance, increased glomerular volumes, as well as higher levels of renal angiotensin II type 1 and mineralocorticoid receptors, and lower renal Klotho levels.
Conclusion:
Our study provides a timeline for the development of cardiorenal dysfunction with aging, and documents that increasing dietary salt accelerates the age-induced phenotypes. In addition, we propose albuminuria as a prognostic biomarker for the future development of hypertension. Last, we identified functional and molecular markers of renal dysfunction that associate with long-term dietary salt loading,
Keywords: dietary sodium, blood pressure, aging, albuminuria
1. INTRODUCTION
Hypertension is a leading preventable risk factor for cardiovascular morbidity and mortality1,2. Blood pressure (BP) elevation strongly correlates with aging, and age-dependent development of hypertension has been shown to accelerate most at mid-life3,4. The underlying causes for age-related BP progression are poorly understood; prevailing models to explain the pathogenesis of hypertension include the age-induced alterations in systemic vascular resistance, as well as renal dysfunction leading to volume expansion5,6. However, long-term BP increases have also been described as a leading cause of chronic kidney disease due to the deleterious effects of hypertension on renovascular function7. Further, age-related BP elevations are paralleled by fluid and electrolyte abnormalities, likely the consequence of decreases in plasma renin activity (PRA) and aldosterone (Aldo)8,9. Thus, a major hurdle that needs to be addressed is the unclear timelines in the etiology of age-induced cardiovascular and renal risks.
Studies in humans and animal models have documented a causal relationship between dietary sodium (Na+) intake and a range of chronic diseases, including hypertension, cardiovascular and kidney disease10–14. Consistently, aging-associated BP elevations are not detected in cultures that do not consume salt, supporting the notion that dietary salt is a critical contributor to aging-induced BP elevation and hypertension15–17. However, most data come from cross-sectional observational and short-term dietary intervention studies, in part due to the inherent challenges in performing controlled diet, longitudinal studies, as well as to the slow progression of the phenotypes over the lifetime (particularly in humans). Thus, there is little information regarding the effects of controlled Na+ intake on longitudinal trajectories for cardiovascular and renal risk, from an aging perspective.
The current study aims to address this gap, by assessing, in mice, 1) the effect of long-term exposure to three levels of salt intake (low, moderate and high) on the monthly longitudinal development of cardiovascular and renal risk (BP and albuminuria), and 2) the hormonal, functional and molecular mechanisms that may mediate the salt/aging-induced alterations in cardiorenal homeostasis.
2. MATERIAL AND METHODS
2.1. Animals
Animals were housed in the animal facility in 12:12-h light-dark cycle at 22°C ambient temperature and maintained on ad libitum regular rodent chow and tap water until study initiation. All studies were conducted according to protocols approved by the Institutional Animal Care and Use Committee (IACUC) at Brigham and Women’s Hospital Boston, MA, USA.
Cross-sectional pilot study.
In preliminary studies, we used male and female CL57Bl/6 mice (Charles River, Wilmington, MA, USA) in three age groups (Table S1): young adult (< 20-week-old), middle aged (45–55 week-old) and old (> 65 week-old). Animals were housed in the animal facility in 12:12-h light-dark cycle at 22°C ambient temperature and maintained on ad libitum regular rodent chow and tap water. BP measurements were performed using tail-cuff plethysmography as described below. As age showed a significant effect on BP in male (but not female) mice (Table S1), the subsequent longitudinal studies were performed using male animals only.
Longitudinal study.
Fifteen-week-old male mice CL57Bl/6 (Charles River, Wilmington, MA, USA) were randomized to one of three levels of Na+ intake (Purina, St. Louis, MO) for 13 months: low (LS, 0.03% Na+, #5885), normal (NS, 0.26% Na+, #5755), or high (HS, 1.6% Na+, #26661). Body weight (BW) and BP were measured every month. Blood samples were collected at months 1, 5 and 13 for the assessment of plasma Aldo and PRA. Animals were also placed every month in metabolic cages, for 24h food and water intake measurements; 24h urine was collected bimonthly for the assessment of the albumin (Alb) and creatinine (Cr)), and urine output was measured. Fluid balance was calculated as the difference between water intake and urine output. At the end of the study, animals underwent an echocardiography and Doppler ultrasound assessment (cardiac and renal); the following morning, mice were euthanized under isoflurane anesthesia and heart and kidney tissues were collected.
2.2. Blood pressure measurements
Systolic (SBP) and diastolic BP (DBP), as well as heart rate (HR) were measured in conscious animals every month, for 13 months, using tail-cuff plethysmography and the CODA noninvasive BP system (KENT Scientific Corporation, Torrington, CT), as previously described by us18,19. Mice were warmed at 30°C and allowed to rest quietly. BP measurements were taken in a quiet room in the morning, and the mice were kept calm and handled by the same person. No sedative was used. We have previously demonstrated excellent correlation between SBPs assessed simultaneously by tail cuff and telemetry in mice19,20.
2.3. Blood collection
On months 1, 5 and 13, mice were slightly anesthetized, and blood was collected from the submandibular plexus in purple-top BD Microtainer tubes (EDTA), as previously described by us21. Plasma was separated by centrifugation, and samples stored at −80C until processed for Aldo and PRA assessments.
2.4. Urine and plasma analyte measurements.
Plasma Aldo and PRA were measured in duplicate using a solid-phase radioimmunoassays (IBL International GMBH, Hamburg, Germany; DiaSorin, Stillwater, MN) as previously described22. Urine Alb and Cr were measured using DCA 2000 microalbumin reagent kit (Bayer, Elkhart, IN), as reported by us22, and are shown as Alb/Cr ratios (mg/g). The detectable Alb/Cr range was 1–2000 μg/mg. The inter-assay CVs were < 5%.
2.5. Histological analysis
Following euthanasia, one kidney was dissected, and the cortex portion sectioned and placed in liquid nitrogen immediately after collection, for protein analysis. The other kidney was fixed in 10% formalin and embedded in paraffin blocks for histology analyses. Five-μm-thick sections were cut and stained with Periodic Acid-Schiff reagent for glomerular volume determination. Glomerular volumes measurements were carried out in a blinded fashion as previously described23, using 30 glomeruli per section.
2.6. Binding of conformation specific antibodies in the kidney.
To determine whether renal angiotensin II receptors are activated by long term dietary sodium intake, conformation state-sensitive anti-AT1R and anti-AT2R antibodies24,25 (Proteimax Biotechnology, Cotia, São Paulo, Brazil) were used. These antibodies are sensitive to activity-mediated conformational changes in the receptors being able to specifically recognize the activated state of the receptor, as previously documented26–29. Deparaffinized and rehydrated sections were incubated with blocking solution (1% bovine serum albumin + 5% sucrose in PBS) and then with conformation-specific antibodies against the angiotensin II receptors 1 and 2 (AT1R and AT2R), conjugated with DY-682 (red) and DY-800 (green) fluorophores, respectively. Sections were then washed with PBS, and fluorescence intensity measured using Odyssey equipment (LI-COR Biosciences) as described by us in previous studies24,25. The intensity of each sample was normalized to negative control (i.e. sections treated only with secondary antibody labelled with fluorescent Alexa Fluor dyes DY-682 and DY-800).
2.7. Electrophoresis and Western blotting
Protein expression was measured as previously reported by us22. In brief, kidney cortex homogenates (20 μg total protein) were size-fractionated by electrophoresis on 10% SDS-PAGE gels, then transferred onto nitrocellulose membrane by electroblotting. The membranes were first blocked with 5% non-fat dried milk in Tris-buffered saline (TBS)-Tween (USB Corporation, Cleveland, OH) for 1 h at room temperature, then incubated overnight at 4°C with the following primary antibodies: anti-AT1R, anti-AT2R, anti-MR (Santa Cruz Biotechnology), anti-Klotho (Abcam). Membranes were then washed 3×15 min with TBS-Tween and incubated with peroxidase-conjugated secondary antibody (Dako) for 2 h. The membranes were thoroughly washed, and immunocomplexes detected using enhanced chemiluminescence (Denville Scientific Inc., Holliston, MA). To correct for loading, blots were re-probed with mouse anti-β-actin antibody (Sigma Aldrich). Results were analyzed with optical densitometry; data are presented as fold change relative to measurements in the LS group.
2.8. Imaging protocol for echocardiogram and renal resistive index
At the end of the study, animals were imaged at the Cardiovascular Physiology Rodent Core at Brigham and Women’s Hospital as previously described30,31. In brief, a MS550D probe with center frequency of 40 and 21 MHz was used to capture images of the heart and kidney. Images were acquired using Vevo3100 system and the data were analyzed using the Vevo Lab software (Fujifilm/Visual sonics, Toronto, ON, Canada). Mice were under controlled anesthesia and kept warm on a prewarmed platform to ensure that the body temperature was maintained at physiologic levels. The probe was positioned using the rail system to obtain the left ventricular diastolic and systolic dimensions for the internal diameter (LVID) and posterior wall (LVPW), as well as the calculated ejection fraction (EF), fractional shortening (FS), left ventricle mass index (LVMI), end diastolic (EDVI) and systolic volume indices (ESVI), cardiac output (CO), and left renal artery – resistance index (LRA RI). The LRA RI was calculated by the formula: (peak systolic velocity – end diastolic velocity)/peak systolic velocity. The concentricity was calculated during diastole, using the following formula: 2*LVID/LVPW.
2.9. Statistical analyses
Statistical analyses were performed using GraphPad Prism 9 (GraphPad Software). The longitudinal effects were tested using repeated measures ANOVA. Comparisons between three or more groups were performed using one-way ANOVA followed by post-hoc analyses. Correction for multiple comparisons was performed using the Dunnett test (repeated measures ANOVA) or the Sidak test (one-way ANOVA). Trend analyses were performed via simple linear regression, using the concentration of sodium in the three diets as independent variable. Data were presented as mean ± SEM, and a P value < 0.05 was considered statistically significant.
3. RESULTS
3.1. Aging and dietary sodium effects on body weight and metabolic parameters
As expected, BW progressively increased throughout the study, irrespective of diet (Table S2). Consistent with the increase in BW, 24h food intake and urine output also increased over time in all diet groups; however, 24h water intake remained relatively constant (Table S2). While moderate Na+ loading (NS) did not lead to any changes in food or water intake, or urine output versus LS, HS consumption associated with reduced food intake, yet increased water intake and urine output as compared to the LS diet group. These changes became significant in month 1 (Mo 1) for water intake and Mo 5 for food intake and urine output and maintained significance throughout the study (Table S2). Consistent with previous reports32,33, fluid balance, calculated as the difference between water intake and urine output, progressively declined with aging in the LS and NS groups, but stayed relatively constant in the HS group (data not shown), suggesting potential water retention in this group.
3.2. Aging and dietary sodium effects on blood pressure homeostasis
At the initiation of the study, there were no significant differences between the three diet groups for either SBP (mmHg, LS: 116.2±3.27; NS: 116.3±5.92; HS: 118.9±3.44) or DBP (mmHg, LS: 87.4±5.22; NS: 88.2±7.15; HS: 83.4±4.0). As shown in Fig. 1, long-term LS diet did not induce a significant increase in either SBP or DBP with aging. As compared to Mo 1, animals maintained on a NS diet progressively increased their SBP and DBP, reaching significance starting with Mo 11 and Mo 12 respectively. The HS group displayed a steeper increase in both SBP and DBP, which reached significance (relative to Mo 1) at earlier time points: Mo 6 and Mo 7, respectively. As compared to values on a LS diet, SBP (but not DBP) on a NS diet only reached significant differences in Mo 11 and Mo 12 (Fig. S1–S2); however, SBP and DBP on a HS diet were both significantly higher vs LS in Mo 6–13 (Fig. S1–S2). Thus, at the end of the study (Mo 13), SBP levels were similar in the LS and NS diet groups, but significantly higher in the HS diet group (mm Hg, LS: 123.0±2.52; NS: 130.7±5.25; HS: 138.5±2.40, p =0.0001 vs. LS); the end of study DBP showed a similar trend, with HS (but not NS) values being significantly greater than the LS ones (Fig. S1–S2). Neither aging nor diet had a significant effect on heart rate (HR) or pulse pressure (data not shown).
Figure 1. Longitudinal effect of aging and dietary sodium on blood pressure.
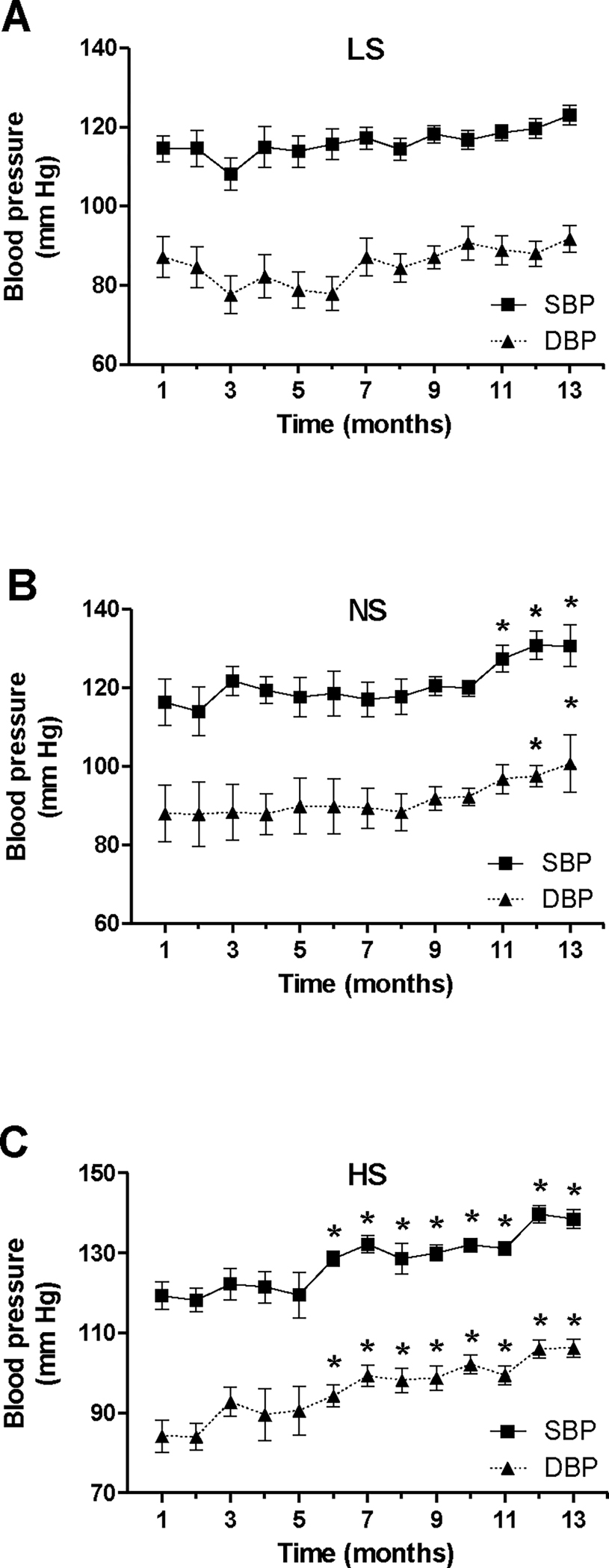
Mice were maintained on a LS (A), NS (B) or HS diet (C) for 13 months; SBP and DBP were assessed monthly in each animal as described under Methods. Data are presented as mean ± SEM. *P<0.05 vs. measurements in month 1.
3.3. Aging and dietary sodium effects on circulating aldosterone and plasma renin activity
To begin to understand the timeline of the hemodynamic changes described above, we then assessed elements of the renin-angiotensin-aldosterone system (RAAS) at the beginning of study (Mo 1), prior to the earliest BP elevation timepoint (Mo 5) and at the end of study (Mo 13) (Fig. 2). As anticipated, dietary Na+ loading was associated with decreased circulating Aldo levels, as compared to the LS group, even after only 1 month of diet. Further, aging induced a progressive decrease in plasma Aldo levels in all groups. This decline was particularly strong on a HS diet, where Aldo suppression at Mo 13 relative to Mo 1 was ~ 18-fold, while only ~4 and ~2 fold in the NS and LS groups. Aging did not significantly affect PRA levels in the LS and NS groups (data not shown) but induced a ~3-fold decrease in the HS group (ng/ml/h, Mo 1: 16.15±0.5; Mo 5: 11.64±0.4; Mo 13: 5.62±2.6, p=0.004 vs Mo 1). Thus, the aging-induced suppression was greater for Aldo than for PRA, particularly on a HS diet.
Figure 2. Longitudinal effect of aging and dietary sodium on plasma aldosterone levels.
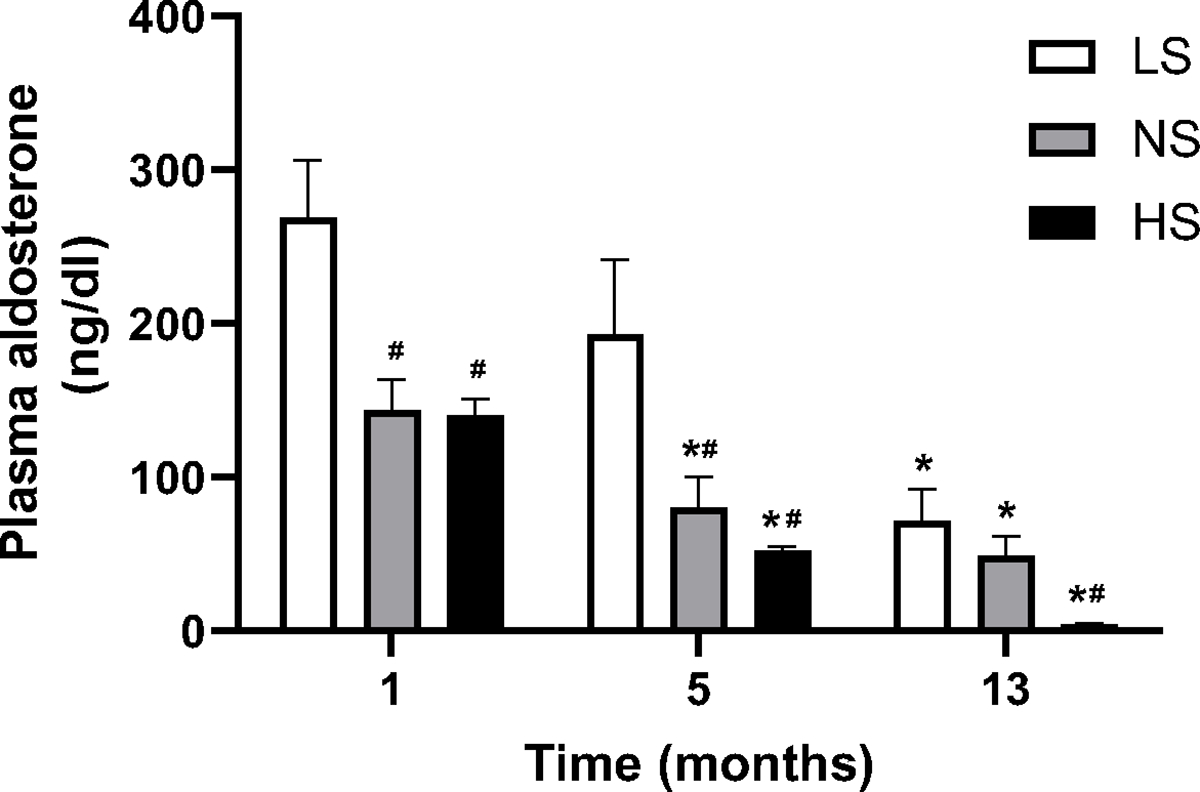
Mice were maintained on three levels of dietary sodium intake (LS, NS, HS) for 13 months; blood samples were collected at indicated times and assessed for Aldo levels as described under Methods. Data are presented as mean ± SEM. *P<0.05 vs. Mo 1 measurements on the same diet. #P<0.05 vs. LS diet in the same month.
3.4. Long-term dietary sodium – effects on cardiac structure and function
As shown in Table S2, BW were not different between the three diet groups in month 13. As compared to LS, long-term NS diet had no significant effect on heart weight (mg, LS: 195.0±6.35; NS: 199.0±6.40) or kidney weight (mg, LS: 249.2±5.36; NS: 247.5±9.21). However, the HS group displayed significant cardiac hypertrophy (228.3±6.13 mg, p=0.001 vs. LS, p=0.01 vs. NS). Consistently, the echocardiographic assessment (Fig. 3A) showed no difference between LVMI in the LS and NS groups; however, there was a positive trend towards higher LVMI levels with increasing dietary Na+ intake, which – relative to LS – only reached significance in the HS group. Further, the left ventricular volume indices (ESVI and EDVI) also displayed significant increases on a HS, but not NS, diet (Fig. 3B–C); however, linear regression analyses revealed a significant trend for higher volume indices with dietary sodium only for EDVI (Fig. 3C). Calculated concentricity values showed no difference between diets (LS: 0.44±0.03; NS: 0.44±0.02; HS: 0.44±0.03), suggesting the hypertrophy seen in the HS group may belong to the “eccentric” dilated phenotype group, as often seen in models of volume expansion34. The cardiac output displayed a significant increase in the HS vs. LS diet groups (Fig. 3 D).
Figure 3. Effect of long-term dietary sodium on echocardiographic measures.
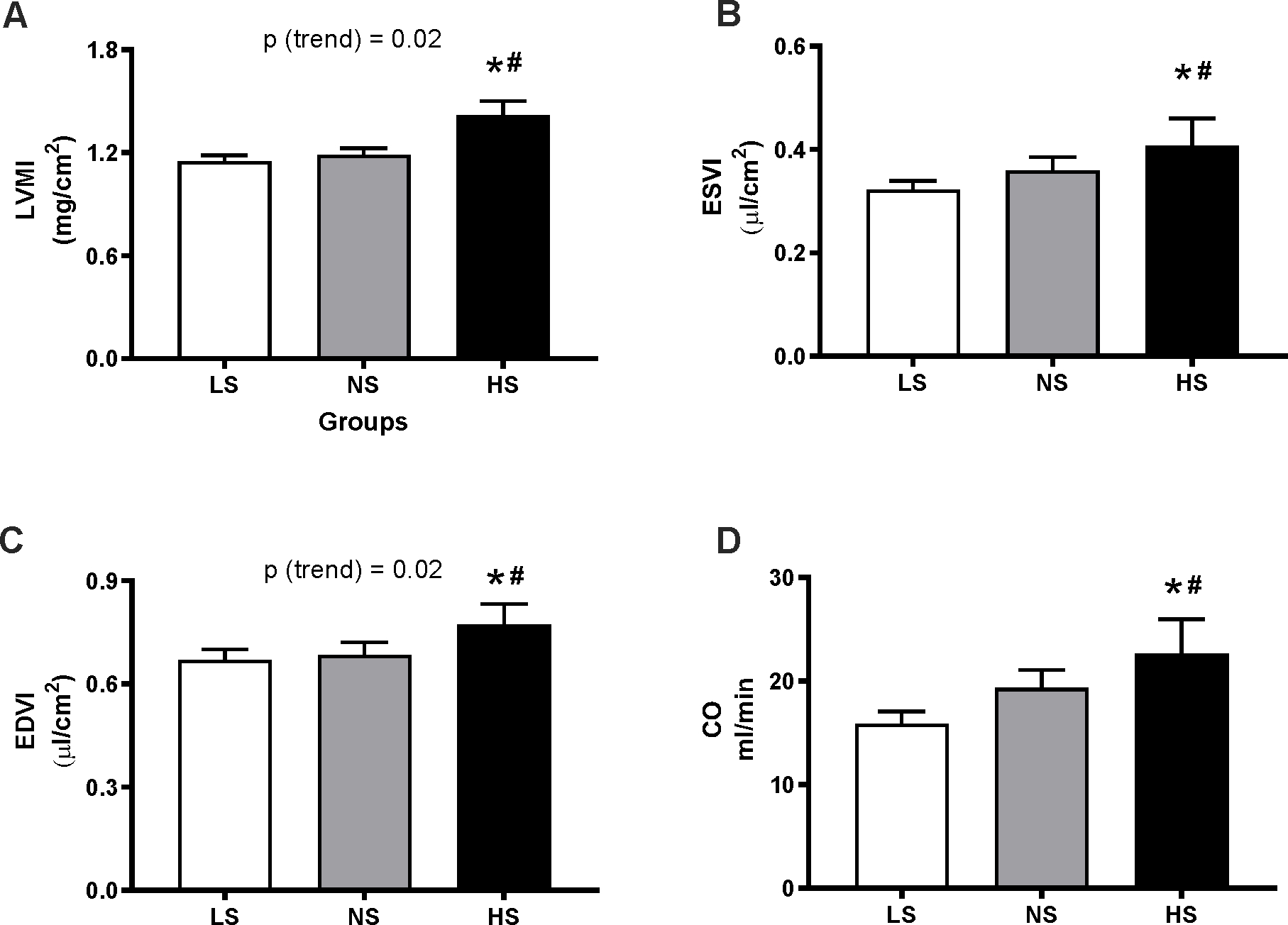
Mice underwent cardiac ultrasonography after 13 months of LS, NS or HS diet. Panels show the left ventricular mass index (LVMI, A), end-systolic volume index (ESVI, B), end-diastolic volume index (EDVI, C) and cardiac output (CO, D). Data are presented as mean ± SEM. *P<0.05 vs LS, #P<0.05 vs NS. P(trend) was obtained from simple linear regression analyses, using the level of sodium in the diets as independent variable, as described in the Methods.
3.5. Aging and dietary sodium effects on albumin/creatinine ratio
As BP increases often lead to kidney damage, we then focused on the longitudinal effects of aging and dietary Na+ on Alb/Cr. At the initiation of the study, there were no significant differences between the three diet groups for Alb/Cr (mg/g, LS: 31.0±1.92; NS: 28.9±1.30; HS: 29.8±1.79). As shown in Fig. 4A, aging on a LS diet was associated with an increasing trend in Alb/Cr but did not reach significance (relative to Mo 1) until Mo 13, where significance was marginal (p=0.051). As compared to baseline, animals maintained on a NS diet progressively increased their Alb/Cr, reaching significance starting with Mo 7 and continuing till the end of study (Fig. 4B). However, there were no significant differences between the LS and NS diets at any time during the study (Fig. S3). The HS group displayed a greater progression for Alb/Cr, which became significant (relative to baseline) starting with Mo 5 (Fig. 4C) and maintained significance until the end of the study. Thus, an all diets, significant upward progression of Alb/Cr relative to baseline occurred earlier than the respective progression in BP: on a LS diet, Alb/Cr progressed in Mo 13 whereas BP did not; on a NS diet, Alb/Cr progressed in Mo 7 while BP – in Mo 11; and on a HS diet, Alb/Cr progressed in Mo 5 whereas BP – in Mo 6. Alb/Cr values on a LS and NS diets were similar throughout the experiment (Fig. S3); however, on a HS diet Alb/Cr reached significantly higher levels in Mo 5, as compared to both other diets. Alb/Cr also displayed positive trends with higher levels of sodium intake in Mo 5–9; however, at the end of the study there was no difference in albuminuria between the three diet groups (mg/g, LS: 42.17±6.27; NS: 46.78±3.78; HS: 48.20±6.51)(Fig. S1–S2).
Figure 4. Longitudinal effect of aging and dietary sodium on albumin/creatinine ratios.
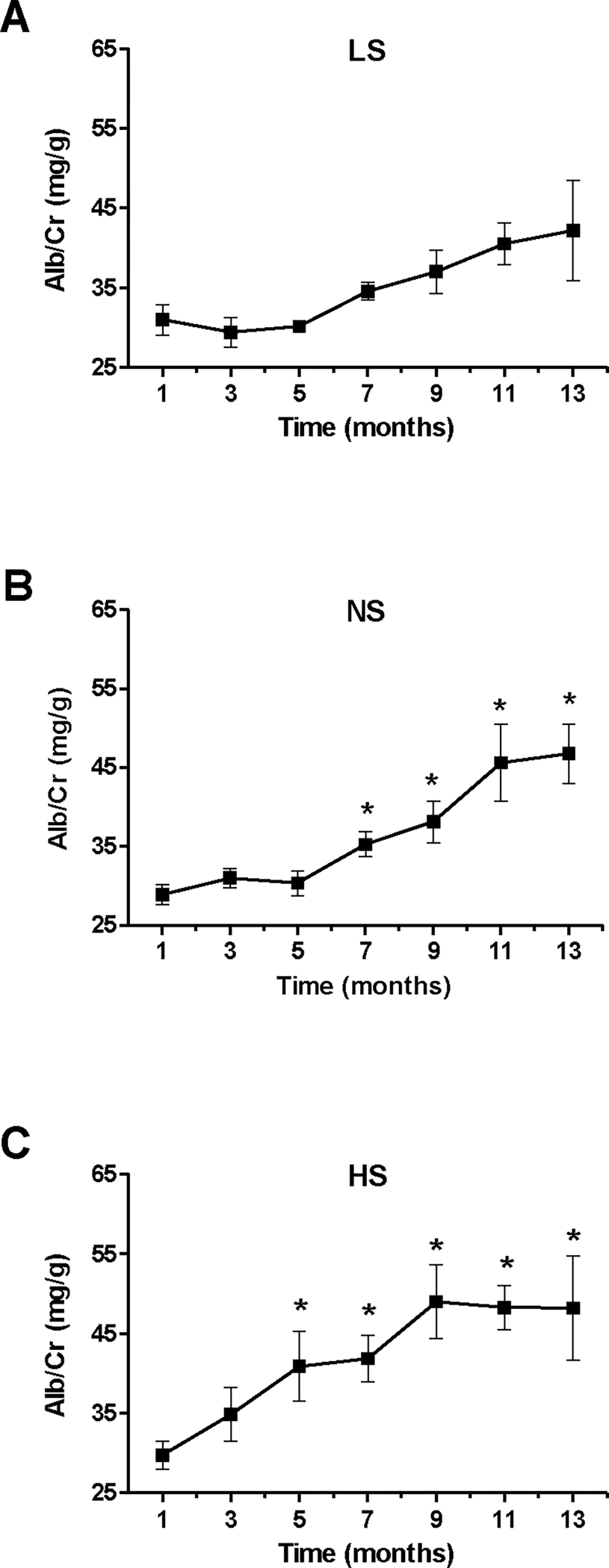
Mice were maintained on a LS (A), NS (B) or HS diet (C) for 13 months; urine Alb/Cr ratios were assessed bimonthly in each animal as described under Methods. Data are presented as mean ± SEM. *P<0.05 vs. measurements in Mo 1.
3.6. Long-term dietary sodium – effects on the kidney
As compared to LS, long-term NS diet had no significant effect on kidney weight (mg, LS: 249.2±5.36; NS: 247.5±9.21). However, the HS group displayed significant renal hypertrophy (273.7±6.51 mg, p=0.009 vs LS). Other groups have described the left renal artery resistive index (LRA RI) as an indicator of renal vascular resistance, with values <0.65 being considered normal, 0.65 – 0.70 high-normal, and values > 0.70 indicating dysfunction35–37. In our current report, end of study LRA RI (Mo 13) was within the high-normal range for animals maintained on a LS diet (0.699±0.01) but trended significantly (p(trend) = 0.03) towards the dysfunctional range for the NS and HS diets (0.710±0.01 and 0.736±0.01, respectively) (Fig. 5A). To further inquire on additional renal dysfunction traits, we then assessed the glomerular volumes in kidney cortex samples from our three diet groups. As shown in Fig. 5B–E, increasing dietary Na+ intake associated with a positive trend for higher glomerular volumes (x106μm3, LS: 0.252±0.01; NS: 0.261±0.01; HS: 0.2868±0.01, p=0.03).
Figure 5. Long-term dietary sodium effect on renal dysfunction.
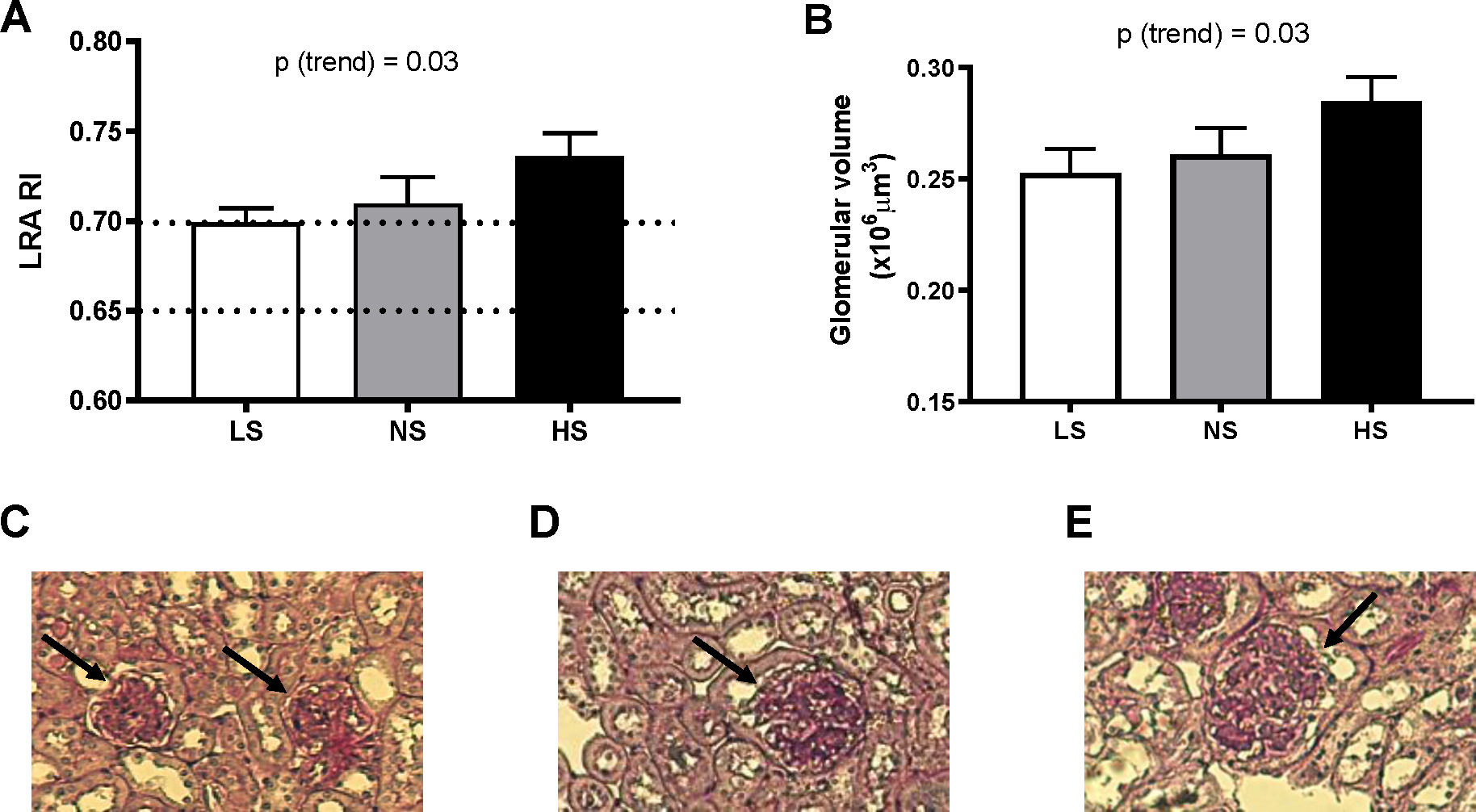
Left renal artery resistive index (LRA RI, A) and glomerular volume (B) was assessed in mice fed a LS, NS, or HS diet for 13 months, as described under Methods. Representative images of glomeruli (PAS stained sections) are shown in C, D and E, for LS, NS and HS respectively. Dotted lines in panel A represent the cutoffs for normal (RI< 0.65), high-normal (0.65 ≤ RI < 0.7) and high resistive index (RI ≥ 0.7). Data are presented as mean ± SEM. *P<0.05 vs LS. P(trend) was obtained from simple linear regression analyses, using the level of sodium in the diets as independent variable, as described in the Methods.
3.7. Long-term dietary sodium – effects on renal protein expression
At the end of the study, the expression of the AT1 receptor in the LS and NS groups was similar. In the HS diet group, AT1 receptor expression was ~ 2-fold higher than in the LS and NS groups; however, this difference only reached significance relative to the NS diet group (p=0.01, Fig 6A). Another RAAS component that was investigated in this study was the mineralocorticoid receptor (MR); as shown in Fig. 6B, dietary Na+ loading led to the progressive increase in MR expression (p (trend) = 0.003), which reached significance only for the HS vs. LS group (p=0.01). On the other hand, the Klotho protein expression displayed an opposite trend: Klotho levels were progressively decreased by dietary sodium, but the difference only reached significance in the HS vs. LS groups (p=0.01) (Fig. 6C), consistent with the reported role of dietary sodium on Klotho levels38–40.
Figure 6. Effect of long-term dietary sodium on renal molecular marker expressions.
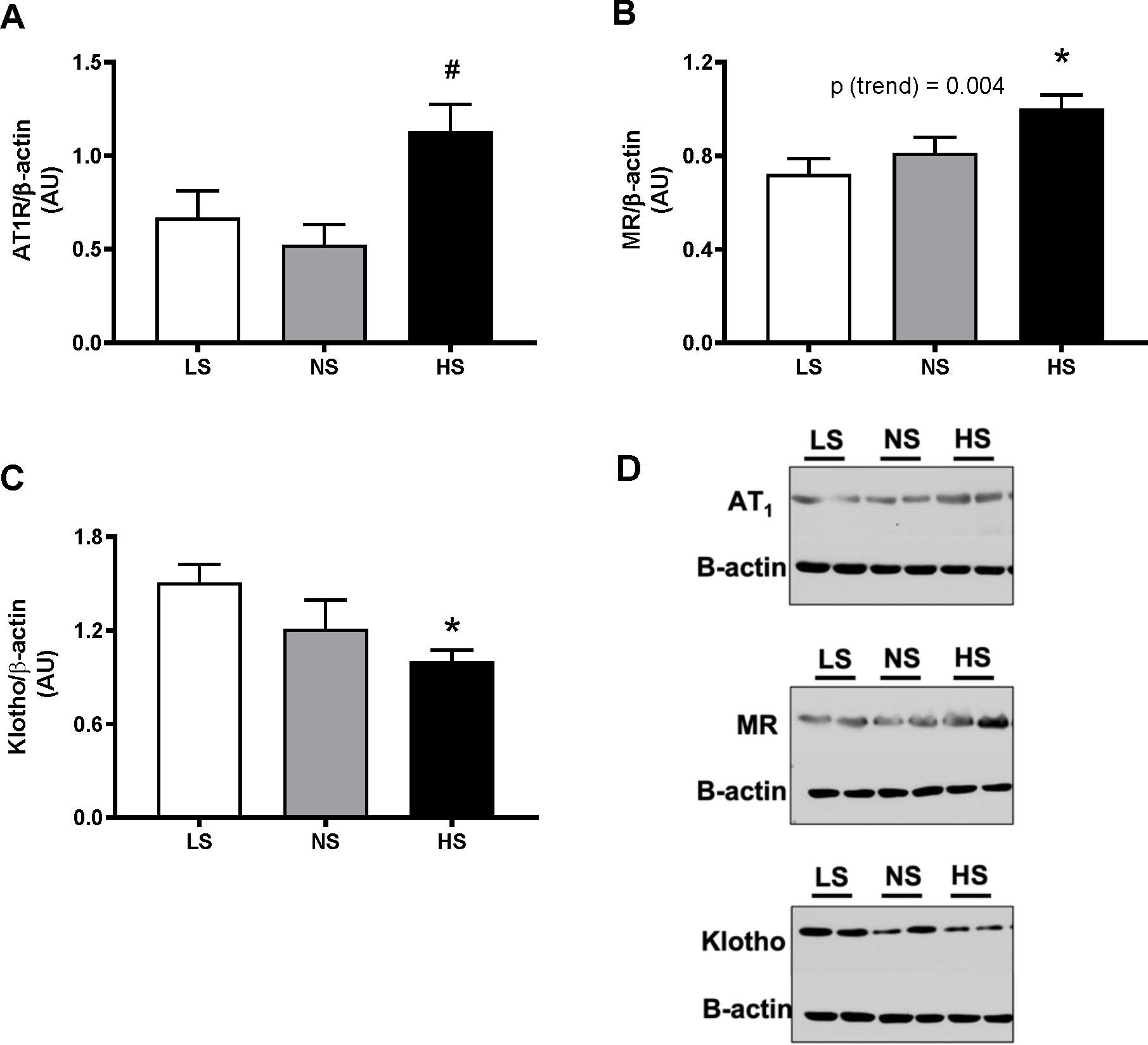
Mouse kidney cortex tissues were collected after 13 months of LS, NS or HS diet, and assessed for levels of AT1R (A), AT2R (B), Klotho (C) and MR (D) by Western blot, as described in Methods. *P<0.05 vs LS, #P<0.05 vs NS. P(trend) was obtained from simple linear regression analyses, using the level of sodium in the diets as independent variable, as described in the Methods.
3.8. Long-term dietary sodium – effects on renal AT1R and AT2R conformation
The conformational change of AT1R and AT2R is induced by the binding of angiotensin II24–29. To assess the effect of long-term dietary Na+ on the activation state of these receptors in the kidney, we used conformation-specific anti-AT1R and anti-AT2R antibodies that recognize the activated form of the receptors. As shown in Fig. 7A–B, increasing Na+ intake levels were associated with a trend for higher AT1R activation levels, and reached significance in the HS vs. LS diet groups. Interestingly, the increase in AT1R activation between the LS and HS diets was only ~ 20%, while the increase in total AT1R protein (Fig. 6A) was much greater (~100%); this discrepancy may be explained by the expected drop in agonist (angiotensin II) levels on a HS diet, or by different rates of dimerization or internalization between the two diets. The AT2R activation state was similar in the three diet groups (Fig. 7C–D).
Figure 7. Effect of long-term dietary sodium on renal AT1R and AT2R conformational status.
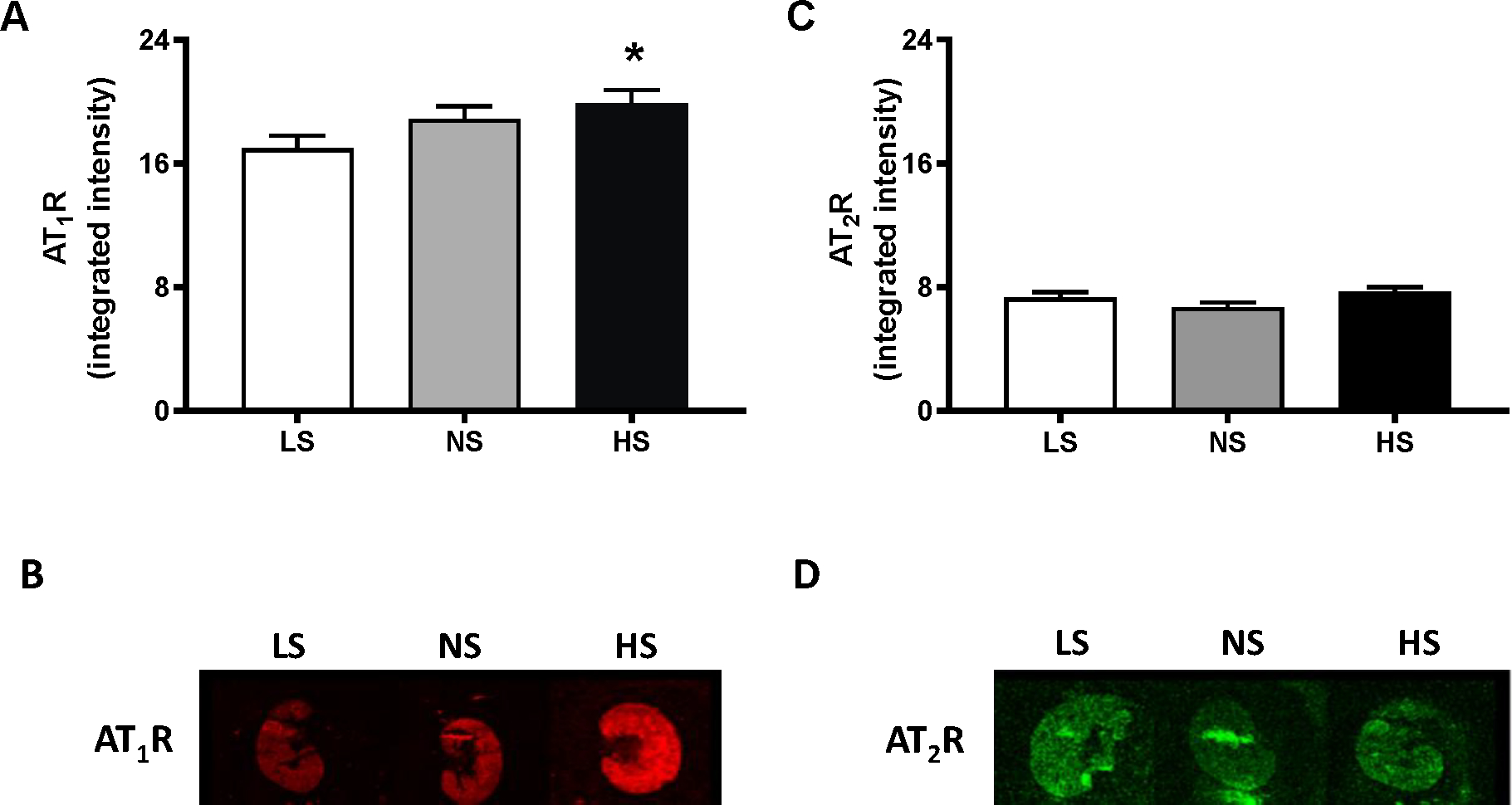
Mouse kidney cortex tissues were collected after 13 months of LS, NS or HS diet. Activation status-related changes in conformation were assessed for the AT1R (A) and AT2R (C), as described in Methods. Representative images are shown in panels B (for AT1R) and D (for AT2R). *P<0.05 vs LS.
4. DISCUSSION
The current study aimed to assess, in normotensive mice, the effect of long-term exposure to three levels of salt intake (low, moderate, and high) on the monthly longitudinal development of cardiovascular and renal risk (BP and albuminuria), as well as the hormonal, functional and molecular mechanisms that may mediate the salt/aging-induced alterations in cardiorenal homeostasis. Our data shows age- and dietary Na+-dependent differences in BP and albuminuria across 13 months of the adult mouse lifespan. BP did not significantly change with aging on a LS diet but displayed an accelerated progression to significantly higher levels (as compared to baseline), after 11 and 6 months of NS and HS diet, respectively. In addition, Alb/Cr showed a similar (but earlier) pattern, with marginal changes during long-term LS, but significant progression after 7 and 5 months of NS and HS diet, respectively – supporting a role for albuminuria as a prognostic biomarker for future BP progression. Furthermore, as compared to LS, long-term exposure to high (but not moderate) salt intake was significantly associated with adverse effects on cardiovascular and renal outcomes – including an eccentric dilated cardiac hypertrophy phenotype, as well as kidney hypertrophy with higher glomerular volumes, increased MR and AT1R (and an activated conformation for the latter), but decreased Klotho expressions. Thus, our findings support the notion that even mild Na+ restriction may extend cardiorenal health span.
Hypertension is a confirmed risk factor for cardiorenal morbidity and mortality1,2; BP elevation strongly correlates with aging, with an accelerated progression in middle adulthood3,4, a timeline suggestive of prolonged exposure to environmental factors (e.g., diet/nutrition). The mechanisms underlying age-related BP progression are yet to be fully understood; the prevalent theory involves a cyclic relationship with imbalances in the RAAS, abnormalities in vascular resistance and renal dysfunction, with hypertension being both a significant contributor to – and the result of any combination of these5–7,41–43. Our findings in animals document that renal dysfunction (as assessed by a rise in Alb/Cr) was an early event and likely driver of the development of BP progression, occurring at least one month (and as long as four months) prior to significant BP elevations – which translates to at least two human year equivalents44. Other groups have found low grade albuminuria as a predictor of hypertension, left ventricular hypertrophy, diastolic dysfunction, as well as cardiovascular and renal morbidity and mortality45–51; however, many of these studies were cross-sectional or focused on at-risk individuals. Our data in mice are consistent with human results that identified microalbuminuria – in short longitudinal studies in normotensives (follow up ~ 3 years) – as a potentially important precursor of essential hypertension and BP progression, even after adjustment for impaired glucose and antecedent blood pressure variables52,53, supporting the translational potential of C57Bl6 mice in aging research. In contrast to these studies, our study herein also assessed the state of the RAAS and highlighted the importance of dietary Na+ on longitudinal progression of albuminuria as a predictor of future development of hypertension.
Studies in humans and animal models have established a clear role for dietary Na+ as a leading modifiable risk factor for several aging-associated chronic diseases, including hypertension, cardiovascular and kidney disease10–14. Indeed, cultures that do not consume salt do not display aging-associated BP increases, supporting the notion that dietary salt is a critical contributor to aging-induced BP elevation and hypertension15–17. However, these findings come from cross-sectional observational and acute dietary intervention studies; there is a paucity of information regarding the effects of Na+ intake on longitudinal trajectories for cardiovascular and renal risk - in part due to inherent challenges in assessing the long-term effects of controlled diets on slowly progressing phenotypes over the lifetime. Because of these limitations, the use of animal models with shorter life span (e.g., mice) offers an attractive alternative for studying environmental effects on aging54. However, Palliyaguru et al.55 recently underlined the lack of a comprehensive analysis of normal aging in mice, thus hindering their use as reliable models of human aging. Our data herein document that long-term dietary salt excess accelerates the age-induced increases in BP and Alb/Cr in C57BL6 mice, suggesting salt-sensitivity of these parameters over the long-term. Interestingly, the C57BL6 strain is used as a salt-resistant control in many studies, as we and others showed that high salt diet alone does not increase BP in this model19,56–58. The reasons for this discrepancy are not fully understood but may relate to the duration of Na+ loading, as most of the above-mentioned studies are short-term. It has also been proposed that salt-sensitivity may be obscured in experiments using BP measurements by tail-cuff59; while our results reported herein did not assess BP responses to changes in dietary salt, tail-cuff measurements were able to detect significant longitudinal changes in BP, as well as differences between three levels of Na+ intake. Our findings on the association of higher levels of Na+ intake with earlier BP and Alb/Cr progression are consistent with the well-documented notion that dietary salt associates – in cross-sectional human studies – with hypertension and microalbuminuria60–63. However, to our knowledge our report is the first to describe the salt-induced acceleration of aging for cardiovascular and renal phenotypes. Consistent with our observations, HS consumption has been recently associated with shorter telomere length and accelerated aging processes at the cellular level64, thus providing a potential mechanism underlying our data in mice.
To gain further understanding of mechanisms underlying the effects of long-term Na+ loading on cardiovascular homeostasis, we performed end-of-study echocardiographic assessments. As compared to LS, 13-month-long exposure to high (but not moderate) salt intake was associated with significant changes in left ventricular mass, lumen, and cardiac output, consistent with an eccentric dilated cardiac hypertrophy phenotype. These data are in agreement with other studies that showed that aging associated with eccentric cardiac hypertrophy in C57BL6 mice65–67; however, in one study these changes occurred only at 24 months of age and were not accompanied by changes in BP66. The reasons for this discrepancy may lie in the Na+ content of the standard chow used in these experiments, likely comparable to our NS diet (where differences in echocardiographic parameters were not significant at the end of the study, in 17 months old animals).
Our current report also provides additional insight into mechanisms underlying the effects of long-term Na+ loading on renal homeostasis. As compared to LS, long-term exposure to high (but not moderate) dietary Na+ was associated with significant changes in renal outcomes – including kidney hypertrophy with higher glomerular volumes, increased renovascular resistance, as well as higher MR and AT1R but decreased Klotho expressions. These results are consistent with previous reports, that support an association between aging and kidney hypertrophy, increased glomerular volumes and activation of the intrarenal RAAS68–71 in animals and humans. Short-term dietary Na+ interventions have also been shown to induce similar changes in the kidney72–76. In our studies, increased renal MR and AT1R levels in response to long-term Na+ loading may mediate both BP and Alb/Cr progression, given their critical roles in vascular and Na+ homeostasis, and despite the expected suppression of circulating RAAS in response to both aging and Na+. Furthermore, 13-month exposure to HS (but not NS) diet significantly suppressed Klotho, a critical anti-aging molecule expressed predominantly in the kidney77,78. In humans, renal Klotho levels were markedly reduced in patients with chronic kidney disease, while decreases in renal Klotho expression in aging mice have been associated with enhanced kidney fibrosis and oxidative stress79,80. Our data is also in agreement with a recent report involving Klotho in dietary salt- and aging-related kidney damage and hypertension81.
Several limitations need to be considered. 1) First, our longitudinal study did not include female mice, based on our pilot data reported herein. However, it is possible that female protection against aging- and dietary Na+-induced dysfunction may cease at later ages, as the anestrus phase has been described to occur in older animals than those included in our pilot study82,83. Thus, future studies are warranted to include similar assessments in female mice. 2) Second, given the duration of our study (13 months) and the limited warranty on the probes, our BP measurements were performed by tail-cuff rather than telemetry. However, we previously demonstrated excellent correlation between SBPs assessed simultaneously by tail-cuff plethysmography and telemetry in mice19,20. 3) Our study does not provide a longitudinal ultrasound assessment of cardiac and renal parameters in response to aging and salt. Recent data suggest that aging does not induce significant variations except for the expected increase in left ventricular mass84; however, future ultrasound studies are needed to investigate the role of extended exposure to dietary Na+ on longitudinal assessments of cardiac and renal parameters. 4) Given the longitudinal study design, molecular assessments are provided only for the end-of-study timepoint (Mo 13). In absence of cross-sectional data at earlier time points (for functional, molecular and histological studies), it is difficult to predict the specific changes that may occur in the aging kidney to result in BP progression. However, based on our end-of-study results, we speculate that AT1R, MR and Klotho are likely to play a role in renal damage initiation, at vascular, tubular and/or glomerular levels. Thus, targeting these mechanisms early on, before overt BP increases, may improve cardiovascular and renal outcomes associated with aging. 5) Although many of our findings appear to match the trajectories of change driving the aging phenotypes in humans, additional studies are needed to establish the C57BL6 mouse as a translatable animal model for aging research55. While the findings in this report support even a moderate reduction of salt intake, as well as the use of angiotensin receptor blockers and MR antagonists as preventive measures of aging- and salt-induced cardiorenal damage, future studies are needed to assess the longitudinal effectiveness of such interventions.
5. CONCLUSIONS
The present study suggests that earlier elevated urinary albumin excretion is associated with the development of hypertension in animals fed by normal and high salt content. Further, our data suggest that renal mechanisms at the vascular, tubular and/or glomerular levels may lead to albuminuria and thus precede the progression to higher blood pressure stages. Additional studies are needed to identify such mechanisms and to determine whether measurement of Alb/Cr alone or in combination with other markers is enough for adequate prognosis of BP progression, thus opening a window of opportunity for early intervention to prevent hypertension and associated conditions.
Supplementary Material
6. ACKNOWLEDGMENTS
We acknowledge the helpful technical assistance of Paul Loutraris and Megan McLean for animal studies.
7. FUNDING
This work was partially supported by: Brigham and Women’s Hospital, Division of Endocrinology, Diabetes and Hypertension, and Harvard Medical School; NIH T32 training grant T32HL007609; NIH research grants R01HL127146 and R01HL104032 from the National Heart, Lung, and Blood Institute; 14GRNT20500000 from the American Heart Association; grants 6752-14-4 from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior and 2016/14696-5 from Fundação de Amparo à Pesquisa do Estado de São Paulo.
Abbreviations.
- Alb/Cr
albumin/creatinine ratio
- Aldo
Aldosterone
- BP
Blood pressure
- BW
Body weight
- HS
High salt
- LS
Low salt
- MR
Mineralocorticoid receptor
- Na+
sodium
- NS
Normal salt
- PRA
Plasma renin activity
- LRA RI
Left renal artery resistive index
REFERENCES
- 1.Mills KT, et al. Global Disparities of Hypertension Prevalence and Control: A Systematic Analysis of Population-Based Studies From 90 Countries. Circulation 2016; 134: 441–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forouzanfar MH, et al. Global Burden of Hypertension and Systolic Blood Pressure of at Least 110 to 115 mm Hg, 1990–2015. JAMA 2017; 317: 165–182. [DOI] [PubMed] [Google Scholar]
- 3.Wills AK, et al. Life course trajectories of systolic blood pressure using longitudinal data from eight UK cohorts. PLoS Med 2011; 8: e1000440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Franklin SS, et al. Hemodynamic patterns of age-related changes in blood pressure. The Framingham Heart Study. Circulation 1997; 96: 308–15. [DOI] [PubMed] [Google Scholar]
- 5.Guyton AC, et al. Arterial pressure regulation. Overriding dominance of the kidneys in long-term regulation and in hypertension. Am J Med 1972; 52: 584–94. [DOI] [PubMed] [Google Scholar]
- 6.Lifton RP, Gharavi AG, Geller DS. Molecular mechanisms of human hypertension. Cell 2001; 104: 545–56. [DOI] [PubMed] [Google Scholar]
- 7.Keane WF, Eknoyan G. Proteinuria, albuminuria, risk, assessment, detection, elimination (PARADE): a position paper of the National Kidney Foundation. Am J Kidney Dis 1999; 33: 1004–10. [DOI] [PubMed] [Google Scholar]
- 8.Bauer JH. Age-related changes in the renin-aldosterone system. Physiological effects and clinical implications. Drugs Aging 1993; 3: 238–45. [DOI] [PubMed] [Google Scholar]
- 9.Jung FF, Kennefick TM, Ingelfinger JR, Vora JP, Anderson S. Down-regulation of the intrarenal renin-angiotensin system in the aging rat. J Am Soc Nephrol 1995; 5: 1573–80. [DOI] [PubMed] [Google Scholar]
- 10.Kang M, et al. Measured sodium excretion is associated with CKD progression: results from the KNOW-CKD study. Nephrol Dial Transplant 2021; 36: 512–519. [DOI] [PubMed] [Google Scholar]
- 11.Abreu D, Sousa P, Matias-Dias C, Pinto FJ. Cardiovascular disease and high blood pressure trend analyses from 2002 to 2016: after the implementation of a salt reduction strategy. BMC Public Health 2018; 18: 722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sacks FM, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med 2001; 344: 3–10. [DOI] [PubMed] [Google Scholar]
- 13.Alderman MH. Dietary Sodium and Cardiovascular Disease Risk. N Engl J Med 2016; 375: 2406. [DOI] [PubMed] [Google Scholar]
- 14.Bibbins-Domingo K The institute of medicine report sodium intake in populations: assessment of evidence: summary of primary findings and implications for clinicians. JAMA Intern Med 2014; 174: 136–7. [DOI] [PubMed] [Google Scholar]
- 15.Hajjar IM, Grim CE, George V, Kotchen TA. Impact of diet on blood pressure and age-related changes in blood pressure in the US population: analysis of NHANES III. Arch Intern Med 2001; 161: 589–93. [DOI] [PubMed] [Google Scholar]
- 16.Oliver WJ, Cohen EL, Neel JV. Blood pressure, sodium intake, and sodium related hormones in the Yanomamo Indians, a “no-salt” culture. Circulation 1975; 52: 146–51. [DOI] [PubMed] [Google Scholar]
- 17.Carvalho JJ, et al. Blood pressure in four remote populations in the INTERSALT Study. Hypertension 1989; 14: 238–46. [DOI] [PubMed] [Google Scholar]
- 18.Pojoga LH, et al. Histone demethylase LSD1 deficiency during high-salt diet is associated with enhanced vascular contraction, altered NO-cGMP relaxation pathway, and hypertension. Am J Physiol Heart Circ Physiol 2011; 301: H1862–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ranjit S, et al. Sex-specific differences in endoplasmic reticulum aminopeptidase 1 modulation influence blood pressure and renin-angiotensin system responses. JCI Insight 2019; 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pojoga LH, et al. Caveolin-1 ablation reduces the adverse cardiovascular effects of N-omega-nitro-L-arginine methyl ester and angiotensin II. Endocrinology 2010; 151: 1236–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pojoga LH, et al. Dissociation of hyperglycemia from altered vascular contraction and relaxation mechanisms in caveolin-1 null mice. J Pharmacol Exp Ther 2014; 348: 260–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang Y, et al. Histone demethylase LSD1 deficiency and biological sex: impact on blood pressure and aldosterone production. J Endocrinol 2019; 240: 111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garza AE, et al. Striatin heterozygous mice are more sensitive to aldosterone-induced injury. J Endocrinol 2020; 245: 439–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferreira DN, et al. Salt-induced cardiac hypertrophy and interstitial fibrosis are due to a blood pressure-independent mechanism in Wistar rats. J Nutr 2010; 140: 1742–51. [DOI] [PubMed] [Google Scholar]
- 25.Katayama IA, et al. High-salt intake induces cardiomyocyte hypertrophy in rats in response to local angiotensin II type 1 receptor activation. J Nutr 2014; 144: 1571–8. [DOI] [PubMed] [Google Scholar]
- 26.Heimann AS, et al. Generation of G protein-coupled receptor antibodies differentially sensitive to conformational states. PLoS One 2017; 12: e0187306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gupta A, et al. Conformation state-sensitive antibodies to G-protein-coupled receptors. J Biol Chem 2007; 282: 5116–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Machado FC, et al. Peripheral interactions between cannabinoid and opioid systems contribute to the antinociceptive effect of crotalphine. Br J Pharmacol 2014; 171: 961–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heimann AS, et al. Hemopressin is an inverse agonist of CB1 cannabinoid receptors. Proc Natl Acad Sci U S A 2007; 104: 20588–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang WT, et al. Ultrasound based assessment of coronary artery flow and coronary flow reserve using the pressure overload model in mice. J Vis Exp 2015: e52598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fisch S, Liao R, Hsiao LL, Lu T. Early Detection of Drug-Induced Renal Hemodynamic Dysfunction Using Sonographic Technology in Rats. J Vis Exp 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schlanger LE, Bailey JL, Sands JM. Electrolytes in the aging. Adv Chronic Kidney Dis 2010; 17: 308–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Begg DP. Disturbances of thirst and fluid balance associated with aging. Physiol Behav 2017; 178: 28–34. [DOI] [PubMed] [Google Scholar]
- 34.de Simone G, Devereux RB, Roman MJ, Alderman MH, Laragh JH. Relation of obesity and gender to left ventricular hypertrophy in normotensive and hypertensive adults. Hypertension 1994; 23: 600–6. [DOI] [PubMed] [Google Scholar]
- 35.Viazzi F, Leoncini G, Derchi LE, Pontremoli R. Ultrasound Doppler renal resistive index: a useful tool for the management of the hypertensive patient. J Hypertens 2014; 32: 149–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tublin ME, Bude RO, Platt JF. Review. The resistive index in renal Doppler sonography: where do we stand? AJR Am J Roentgenol 2003; 180: 885–92. [DOI] [PubMed] [Google Scholar]
- 37.Hanamura K, Tojo A, Kinugasa S, Asaba K, Fujita T. The resistive index is a marker of renal function, pathology, prognosis, and responsiveness to steroid therapy in chronic kidney disease patients. Int J Nephrol 2012; 2012: 139565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Citterio L, et al. Klotho Gene in Human Salt-Sensitive Hypertension. Clin J Am Soc Nephrol 2020; 15: 375–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou X, Chen K, Lei H, Sun Z. Klotho gene deficiency causes salt-sensitive hypertension via monocyte chemotactic protein-1/CC chemokine receptor 2-mediated inflammation. J Am Soc Nephrol 2015; 26: 121–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu JW, Chu C, Shi T, Yan Y, Mu JJ. Effects of salt intervention on serum levels of Klotho influenced by salt sensitivity. J Clin Hypertens (Greenwich) 2020; 22: 2051–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu CH, et al. Renin-Angiotensin System and Cardiovascular Functions. Arterioscler Thromb Vasc Biol 2018; 38: e108–e116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schweda F Salt feedback on the renin-angiotensin-aldosterone system. Pflugers Arch 2015; 467: 565–76. [DOI] [PubMed] [Google Scholar]
- 43.Bidani AK, Griffin KA. Long-term renal consequences of hypertension for normal and diseased kidneys. Curr Opin Nephrol Hypertens 2002; 11: 73–80. [DOI] [PubMed] [Google Scholar]
- 44.The Jackson Laboratory. Life Span as a biomarker. http://www.jax.org/research-and-faculty/research-labs/the-harrison-lab/gerontology/life-span-as-a-biomarker
- 45.Kang M, et al. Albuminuria within the Normal Range Can Predict All-Cause Mortality and Cardiovascular Mortality. Kidney360 2022; 3: 74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang T, et al. Low-Grade Albuminuria Is Associated with Left Ventricular Hypertrophy and Diastolic Dysfunction in Patients with Hypertension. Kidney Blood Press Res 2019; 44: 590–603. [DOI] [PubMed] [Google Scholar]
- 47.Waheed S, et al. Combined association of albuminuria and cystatin C-based estimated GFR with mortality, coronary heart disease, and heart failure outcomes: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Kidney Dis 2012; 60: 207–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pichaiwong W, Homsuwan W, Leelahavanichkul A. The prevalence of normoalbuminuria and renal impairment in type 2 diabetes mellitus. Clin Nephrol 2019; 92: 73–80. [DOI] [PubMed] [Google Scholar]
- 49.Forman JP, Fisher ND, Schopick EL, Curhan GC. Higher levels of albuminuria within the normal range predict incident hypertension. J Am Soc Nephrol 2008; 19: 1983–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mettimano M, Specchia ML, Migneco A, Savi L. Microalbuminuria as a marker of cardiac damage in essential hypertension. Eur Rev Med Pharmacol Sci 2001; 5: 31–6. [PubMed] [Google Scholar]
- 51.Imanishi M, et al. Sodium sensitivity related to albuminuria appearing before hypertension in type 2 diabetic patients. Diabetes Care 2001; 24: 111–6. [DOI] [PubMed] [Google Scholar]
- 52.Wang TJ, et al. Low-grade albuminuria and the risks of hypertension and blood pressure progression. Circulation 2005; 111: 1370–6. [DOI] [PubMed] [Google Scholar]
- 53.Park SK, Moon SY, Oh CM, Ryoo JH, Park MS. High normal urine albumin-to-creatinine ratio predicts development of hypertension in Korean men. Circ J 2014; 78: 656–61. [DOI] [PubMed] [Google Scholar]
- 54.Mitchell SJ, Scheibye-Knudsen M, Longo DL, de Cabo R. Animal models of aging research: implications for human aging and age-related diseases. Annu Rev Anim Biosci 2015; 3: 283–303. [DOI] [PubMed] [Google Scholar]
- 55.Palliyaguru DL, et al. Study of Longitudinal Aging in Mice: Presentation of Experimental Techniques. J Gerontol A Biol Sci Med Sci 2021; 76: 552–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hartner A, Cordasic N, Klanke B, Veelken R, Hilgers KF. Strain differences in the development of hypertension and glomerular lesions induced by deoxycorticosterone acetate salt in mice. Nephrol Dial Transplant 2003; 18: 1999–2004. [DOI] [PubMed] [Google Scholar]
- 57.Garza AE, et al. Variants in striatin gene are associated with salt-sensitive blood pressure in mice and humans. Hypertension 2015; 65: 211–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brooks DL, et al. Aldosterone Modulates the Mechanistic Target of Rapamycin Signaling in Male Mice. Endocrinology 2019; 160: 716–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Combe R, et al. How Does Circadian Rhythm Impact Salt Sensitivity of Blood Pressure in Mice? A Study in Two Close C57Bl/6 Substrains. PLoS One 2016; 11: e0153472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Khaledifar A, Gharipour M, Bahonar A, Sarrafzadegan N, Khosravi A. Association between Salt Intake and Albuminuria in Normotensive and Hypertensive Individuals. Int J Hypertens 2013; 2013: 523682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yilmaz R, et al. Dietary salt intake is related to inflammation and albuminuria in primary hypertensive patients. Eur J Clin Nutr 2012; 66: 1214–8. [DOI] [PubMed] [Google Scholar]
- 62.Swift PA, Markandu ND, Sagnella GA, He FJ, MacGregor GA. Modest salt reduction reduces blood pressure and urine protein excretion in black hypertensives: a randomized control trial. Hypertension 2005; 46: 308–12. [DOI] [PubMed] [Google Scholar]
- 63.He FJ, et al. Effect of modest salt reduction on blood pressure, urinary albumin, and pulse wave velocity in white, black, and Asian mild hypertensives. Hypertension 2009; 54: 482–8. [DOI] [PubMed] [Google Scholar]
- 64.Zhu H, et al. High sodium intake is associated with short leukocyte telomere length in overweight and obese adolescents. Int J Obes (Lond) 2015; 39: 1249–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dai DF, Chen T, Johnson SC, Szeto H, Rabinovitch PS. Cardiac aging: from molecular mechanisms to significance in human health and disease. Antioxid Redox Signal 2012; 16: 1492–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.De Moudt S, et al. Progressive aortic stiffness in aging C57Bl/6 mice displays altered contractile behaviour and extracellular matrix changes. Commun Biol 2022; 5: 605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hoit BD, et al. Naturally occurring variation in cardiovascular traits among inbred mouse strains. Genomics 2002; 79: 679–85. [DOI] [PubMed] [Google Scholar]
- 68.Hodgin JB, et al. Glomerular Aging and Focal Global Glomerulosclerosis: A Podometric Perspective. J Am Soc Nephrol 2015; 26: 3162–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sataranatarajan K, et al. Molecular events in matrix protein metabolism in the aging kidney. Aging Cell 2012; 11: 1065–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yoon HE, Choi BS. The renin-angiotensin system and aging in the kidney. Korean J Intern Med 2014; 29: 291–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Uneda K, et al. Angiotensin II Type 1 Receptor-Associated Protein Regulates Kidney Aging and Lifespan Independent of Angiotensin. J Am Heart Assoc 2017; 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schmid C, Castrop H, Reitbauer J, Della Bruna R, Kurtz A. Dietary salt intake modulates angiotensin II type 1 receptor gene expression. Hypertension 1997; 29: 923–9. [DOI] [PubMed] [Google Scholar]
- 73.Kawarazaki H, et al. Mineralocorticoid receptor activation contributes to salt-induced hypertension and renal injury in prepubertal Dahl salt-sensitive rats. Nephrol Dial Transplant 2010; 25: 2879–89. [DOI] [PubMed] [Google Scholar]
- 74.Domondon M, et al. Renal Glomerular Mitochondria Function in Salt-Sensitive Hypertension. Front Physiol 2019; 10: 1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yu HC, et al. Salt induces myocardial and renal fibrosis in normotensive and hypertensive rats. Circulation 1998; 98: 2621–8. [DOI] [PubMed] [Google Scholar]
- 76.Hosohata K Biomarkers for Chronic Kidney Disease Associated with High Salt Intake. Int J Mol Sci 2017; 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Koh N, et al. Severely reduced production of klotho in human chronic renal failure kidney. Biochem Biophys Res Commun 2001; 280: 1015–20. [DOI] [PubMed] [Google Scholar]
- 78.Kuro-o M, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 1997; 390: 45–51. [DOI] [PubMed] [Google Scholar]
- 79.Lim JH, et al. Age-associated molecular changes in the kidney in aged mice. Oxid Med Cell Longev 2012; 2012: 171383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zuo Z, et al. Aging-related kidney damage is associated with a decrease in klotho expression and an increase in superoxide production. Age (Dordr) 2011; 33: 261–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kawarazaki W, et al. Salt causes aging-associated hypertension via vascular Wnt5a under Klotho deficiency. J Clin Invest 2020; 130: 4152–4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Felicio LS, Nelson JF, Finch CE. Longitudinal studies of estrous cyclicity in aging C57BL/6J mice: II. Cessation of cyclicity and the duration of persistent vaginal cornification. Biol Reprod 1984; 31: 446–53. [DOI] [PubMed] [Google Scholar]
- 83.Finch CE. The menopause and aging, a comparative perspective. J Steroid Biochem Mol Biol 2014; 142: 132–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zuo Z, et al. Assessment of Longitudinal Reproducibility of Mice LV Function Parameters at 11.7 T Derived from Self-Gated CINE MRI. Biomed Res Int 2017; 2017: 8392952. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


