Abstract
Purpose
This study aimed to assess clinical, treatment, and prognostic features in patients with brain metastases (BM) from solid tumors achieving long-term survival (LTS). Further, the accuracy of diagnosis-specific Graded Prognostic Assessment scores (ds-GPA) to predict LTS was evaluated.
Methods
Patients admitted for radiotherapy of BM between 2010 and 2020 at a large tertiary cancer center with survival of at least 3 years from diagnosis of BM were included. Patient, tumor, treatment characteristics and ds-GPA were compiled retrospectively.
Results
From a total of 1248 patients with BM, 61 (4.9%) survived ≥ 3 years. In 40 patients, detailed patient charts were available. Among LTS patients, median survival time from diagnosis of BM was 51.5 months. Most frequent primary tumors were lung cancer (45%), melanoma (20%), and breast cancer (17.5%). At the time of diagnosis of BM, 11/40 patients (27.5%) had oligometastatic disease. Estimated mean survival time based on ds-GPA was 19.7 months (in 8 cases estimated survival < 12 months). Resection followed by focal or whole-brain radiotherapy (WBRT) was often applied (60%), followed by primary stereotactic radiotherapy (SRT) (20%) or WBRT (20%). 80% of patients received systemic treatment, appearing particularly active in specifically altered non-small lung cancer (NSCLC), melanoma, and HER2-positive breast cancer. Karnofsky performance score (KPS) and the presence of oligometastatic disease at BM diagnosis were persisting prognostic factors in LTS patients.
Conclusion
In this monocentric setting reflecting daily pattern of care, LTS with BM is heterogeneous and difficult to predict. Effective local treatment and modern systemic therapies often appear crucial for LTS. The impact of concomitant diseases and frailty is not clear.
Supplementary Information
The online version of this article (10.1007/s00066-023-02123-4) contains supplementary material, which is available to authorized users.
Keywords: Stereotactic radiotherapy, Whole brain radiotherapy, Systemic therapy, ds-GPA, Frailty
Introduction
Brain metastases (BM) are frequent complications in patients with advanced solid tumors [1–3]. The propensity for metastatic spread to the brain varies widely among tumor types, being highest among patients with lung cancer [3–5]. With up to 30% of all cancer patients affected [6–8], BM are the most common brain malignancies in adults [2, 4, 9] and their diagnosis often marks a turning point in the therapeutic approach and prognosis [3].
The frequency of patients diagnosed with BM has increased in recent decades and is expected to rise further [4, 10]. Apart from an improved prognosis of primary tumors, better imaging and the increased availability of magnetic resonance imaging (MRI) facilitates detection of brain lesions [8, 9, 11–13].
Regarding prognosis, median survival for patients with BM is still poor despite improved treatment options for primary tumors over the past years [2–4, 14, 15].
Factors determining survival time after a primary diagnosis of BM are known to a very limited extend. In particular, long-term survival (LTS) after diagnosis of BM is poorly characterized.
Prognostic scores, such as the disease-specific Graded Prognostic Assessment (ds-GPA) score, are used as an aid to predict survival time [16]. These scores mainly utilize parameters like the Karnofsky performance score (KPS), age, number of BM, and tumor subtype. It is unclear whether existing prognostic scores can predict LTS satisfactorily.
Little is known about the impact of other conceivable factors like concurrent diseases and treatment effects in long-term surviving patients. Our aim was to characterize clinical characteristics, treatment patterns, and the value of prognostic factors in detail in long-term surviving patients with BM and to analyze whether LTS is adequately reflected in ds-GPA sores.
Materials and methods
This study was performed according to the principles of the Declaration of Helsinki. Approval was granted by the ethics committee of the Medical Faculty of the University of Leipzig (date 03.08.2021, no. 332/21-ek). All patients consented to anonymized scientific use of their clinical data. Patients who were treated with brain-directed radiotherapy at the Department of Radiotherapy at the University of Leipzig Medical Center between 2010 and 2020 were identified from clinical records. All patients (age ≥ 18 years) diagnosed with BM from a solid primary and with a survival time of ≥ 3 years from diagnosis of BM to date of death or last follow-up were eligible for the study. Survival time was defined as time from diagnosis of BM to death or loss to follow-up and was tabulated according to the Kaplan–Meier method. Differences in survival time were analyzed using a two-sample t-test. Patient data were analyzed from existing patient charts. Various variables including age (at primary cancer and BM diagnosis), sex, primary tumor, and histological subtype, KPS, activity of systemic disease immediately prior to diagnosis of BM, ds-GPA score, time interval between diagnosis of primary and BM, number of BM, treatment modalities, and concurrent diseases were evaluated. Score-based estimated survival time was calculated through ds-GPA score; ds-GPA was determined according to Sperduto et al. [16, 17] for NSCLC adeno- and non-adenocarcinoma, breast cancer, melanoma, renal cell carcinoma, and gastrointestinal (GI) cancer. In other tumor entities, the ds-GPA score was not applicable. Required items used for ds-GPA are displayed in Supplement 1.
Survival was analyzed using the Kaplan–Meier method (α = 0.05). For comparative analysis, univariate and multivariate Cox regression and log-rank tests were used. Data analysis was performed with Microsoft Excel 2016 (Microsoft corporation, Redmond, Washington, USA) and GraphPad Prism version 9.3.1 (350; GraphPad Software Inc., La Jolla, CA, USA).
Results
Patients without LTS
A total of 1248 patients were treated in the described period. 1187 of them did not reach LTS. Among them, 659 (55.5%) were male and 528 (44.5%) were female. Median age at diagnosis of the primary tumor was 62.09 years (mean 60.97, range 7–90). Median age at diagnosis of BM was 63.64 years (mean 62.78, range 18–90).
The distribution of primary tumors was as follows: lung cancer (NSCLC and SCLC): 609 patients (51.3%), breast cancer: 122 patients (10.3%), melanoma: 164 patients (13.8%), renal cancer: 118 (9.9%), colorectal cancer: 40 patients (3.4%), other: 134 (11.3%).
Median survival time from diagnosis of BM was 5 months (mean 7.8, range 12–115). Mean follow-up of the entire cohort was 7.0 years.
Survival and patient characteristics in LTS
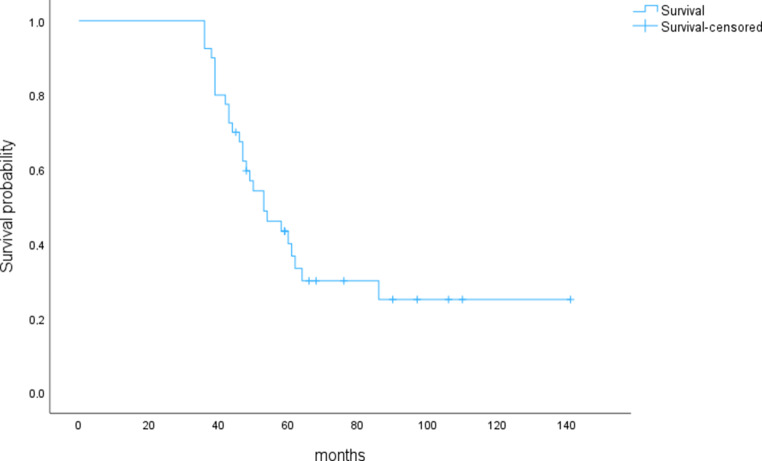
Of 1248 patients, 61 patients (4.9%) survived 3 years or longer from the time of diagnosis of BM. In 40 patients with LTS, detailed charts were available, among whom median (mean, range) survival time from diagnosis of BM was 51.5 months (58.4 months, 36–141 months). 24 patients were male (60%) and 16 female (40%). Data are summarized in Fig. 1 and Table 1.
Fig. 1.
Survival in months after diagnosis of brain metastases
Table 1.
Patient characteristics
| Age at diagnosis of BM (years); median (range) | 59 (39–83) |
| Duration from diagnosis of primary tumor to BM (months); median (range) | 9.5 (0–164) |
| Gender; male (%), female (%) | 24 (60), 16 (40) |
| KPS at diagnosis of BM; median (range) | 90 (60–100) |
| Number of BM at diagnosis of BM; n (% of patients) | |
| 1 | 20 (50) |
| 2–3 | 10 (25) |
| > 4 | 10 (25) |
| Systemic tumor progression at diagnosis of BM; n (% of patients) | |
| Yes (including synchronous BM) | 31 (77.5) |
| No | 9 (22.5) |
| Synchronous BM; n (% of patients) | 17 (42.5) |
| Metachronous BM; n (% of patients) | 23 (57.5) |
| Survival time from diagnosis of BM (months); median (range) | 51.5 (36–141) |
| Concurrent diseases; n (% of patients) | |
| Smoking | 18 (45) |
| Arterial hypertension | 19 (47.5) |
| Hypercholesteremia | 4 (10) |
| Peripheral arterial occlusive disease | 2 (5) |
| Diabetes mellitus | 3 (7.5) |
| Other cardiovascular diseases | 9 (22.5) |
| COPD | 6 (15) |
| History of other tumor disease | 10 (25) |
BM brain metastases, COPD chronic obstructive pulmonary disease
Mean age at initial diagnosis of primary tumor was 57.7 years. Age of patients at diagnosis of BM ranged from 39 to 83 years, with median and mean age of 59 and 59.7 years, respectively.
Compared to non-LTS patients, mean age in LTS patients was significantly lower (p = 0.043, two-sample t-test).
Duration from diagnosis of primary tumor to presentation with BM ranged from 0 to 164 months, with a median time of 9.5 months (mean 24.8 months) and varied according to tumor entity.
KPS at initial diagnosis of BM was 60% in one case, 70% in 5 cases, 80% in 9 cases, 80–90% in one case, 90% in 15 cases, and 100% in 9 cases (median 90%).
Overall, half of the patients were diagnosed with a single BM (20/40; 50%), whereas 2–3 BM were found in 25% (10/40) and ≥ 4 BM in 25% (10/40) of cases.
Immediately prior to diagnosis of BM, 77.5% (31/40) of patients had progressive systemic disease (unstable), as progression of primary or newly diagnosed/progressive metastases outside the brain, whereas 9 patients (22.5%) showed stable disease. Patients with synchronous BM were considered as unstable.
Whereas 42.5% (17/40) of patients were diagnosed with BM synchronous to the primary tumor, 57.5% (23/40) showed metachronous BM.
At the time of diagnosis of BM, 11/40 patients (27.5%) had oligometastatic disease with maximally four metastases.
Concurrent diseases were arterial hypertension (47.5%), hypercholesteremia (10%), peripheral arterial occlusive disease (5%), chronic obstructive lung disease (COPD; 15%), other cardiovascular diseases (22.5%), and diabetes mellitus (7.5%); 45% of patients were smokers (Table 1).
Histologic and molecular features of primary tumors and BM
The most common primary tumor was lung cancer in 18/40 patients (45%), followed by melanoma (8/40; 20%), breast cancer (7/40; 17.5%), renal cell carcinoma (5/40; 12.5%), colorectal cancer (1/40; 2.5%), and bladder cancer (1/40; 2.5%).
Tumor subtypes among patients with lung cancer were non-small cell lung cancer (NSCLC) in 15/18 cases (83.3%) and small cell lung cancer (SCLC), sarcomatoid carcinoma, and adenocarcinoma (of the lung) not further specified in one case each (5.6%). The histologies/subtypes in breast cancer/NSCLC/melanoma are displayed in Table 2.
Table 2.
Tumor subtypes
| Primary tumor | Subtype | Results (%) |
|---|---|---|
| NSCLC (n = 15) | Adenocarcinoma | 13 (86.7) |
| EGFR and/or ALK mutation | 4 (30.8) | |
| EGFR/ALK wildtype | 7 (53.8) | |
| EGFR/ALK status unknown | 2 (15.4) | |
| Squamous cell carcinoma | 2 (13.3) | |
| Melanoma (n = 8) | BRAF-V600E mutation | 4 (50) |
| BRAF-V600E wildtype | 4 (50) | |
| Breast cancer (n = 7) | Luminal A | 1 (14.3) |
| Luminal B (HER2-positive) | 2 (28.6) | |
| HER2-positive | 3 (42.9) | |
| Triple-negative | 1 (14.3) |
NSCLC non-small cell lung cancer, EGFR epidermal growth factor receptor, ALK anaplastic lymphoma kinase, HER2 human epidermal growth factor receptor 2
The distribution of the tumor types lung cancer, melanoma, breast cancer, renal cell carcinoma, and colorectal cancer was not different in LTS patients compared to non-LTS (p = 0.557, chi-square).
Tumor-specific survival and value of initial prognostic parameters among LTS patients
Tumor-specific mean time to BM occurrence was 6.6 months for lung cancer (of those, 12/18 with synchronous BM), 31.5 months for melanoma (0/8 with synchronous BM), 45.1 months for breast cancer (3/7 with synchronous BM), 30.4 months for renal cell carcinoma (2/5 with synchronous BM), 25 months for colon cancer, and 127 months for bladder cancer.
At termination of data acquisition, 13 patients (32.5%) were alive.
Tumor-specific mean survival time from diagnosis of BM was 57.3 months among patients with lung cancer, 66.1 months among patients with melanoma, 62.1 months among patients with breast cancer, and 49.4 months among patients with renal cell carcinoma.
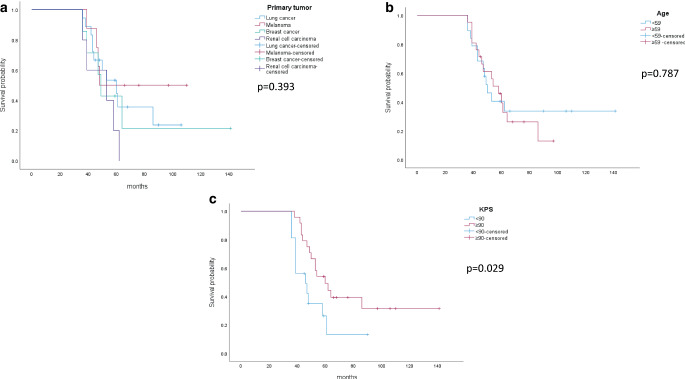
In univariate Cox regression analysis, survival was not significantly different between patients with different tumor entities (Fig. 2a, p = 0.398, log-rank). Age at presentation of BM (above/below median of 59 years) was not associated with survival (Fig. 2b, p = 0.783) while worse KPS (< 90) appeared to be associated with shorter survival (Fig. 2c, p = 0.029).
Fig. 2.
Survival among long-term survival patients depending on primary tumor (a), patient age (b), and KPS (c)
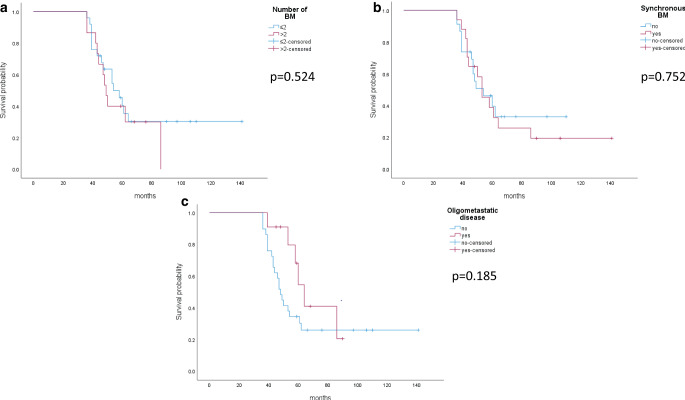
Regarding relevance of number (≤ 2 vs. > 2, p = 0.524) and timing of BM (synchronous vs. metachronous, p = 0.752) and oligometastatic disease (≤ 4 metastases vs. polymetastatic disease, p = 0.185), no significant effects were observed (Fig. 3a, b, and c).
Fig. 3.
Survival among long-term survival patients depending on number (a) and timing of brain metastasis (b) and oligometastasis (c)
In multivariate Cox regression analysis with the mentioned covariates (tumor type, age, KPS, number and timing of BM, presence of oligometastatic disease) only KPS (p = 0.004) and presence of oligometastatic disease (p = 0.036) were significantly associated with survival.
Predicted survival with disease-specific Graded Prognostic Assessment
Expected mean survival time was assessable with ds-GPA for 36/40 patients and was 19.7 months (range 4–46 months). For 8/36 cases (22.2%), the estimated survival time was shorter than 12 months. A survival of 3 or more years was correctly predicted for 2/13 patients (15.4%) with NSCLC adenocarcinoma of the lung and for 1/7 patients (14.3%) with breast cancer. In the majority of patients, the ds-GPA score did not predict survival time correctly. Exemplary, short-term survival of below 1 year was predicted in 1/13 patients (7.7%) with NSCLC adenocarcinoma of the lung, 1/2 patients (50%) with NSCLC squamous cell carcinoma of the lung, 4/8 patients (50%) with melanoma, 1/5 patients (20%) with renal cell carcinoma, and 1/1 patient (100%) with colon cancer. A detailed summary of all ds-GPA scores and predicted survival times is displayed in Table 3.
Table 3.
Distribution of retrospectively assessed disease-specific Graded Prognostic Assessment (ds-GPA) scores among patients per tumor entity
| Primary tumor | Ds-GPA score | Expected survival time (months) | Number of patient (%) |
|---|---|---|---|
| NSCLC adenocarcinoma (n = 13) | 0.5 | 7 | 1 (7.7) |
| 1.5 | 13 | 2 (15.4) | |
| 2.5 | 25 | 3 (23.1) | |
| 3 | 25 | 5 (38.5) | |
| 4 | 46 | 2 (15.4) | |
| NSCLC squamous cell carcinoma (n = 2) | 2 | 10 | 1 (50) |
| 2.5 | 13 | 1 (50) | |
| Melanoma (n = 8) | 0.5 | 5 | 1 (12.5) |
| 2 | 8 | 3 (37.5) | |
| 2.5 | 16 | 2 (25) | |
| 3 | 16 | 1 (12.5) | |
| 4 | 34 | 1 (12.5) | |
| Breast cancer (n = 7) | 2 | 13 | 2 (28.6) |
| 2.5 | 24 | 2 (28.6) | |
| 3 | 24 | 2 (28.6) | |
| 3.5 | 36 | 1 (14.3) | |
| Renal cell carcinoma (n = 5) | 1 | 4 | 1 (20) |
| 2 | 12 | 2 (40) | |
| 3 | 17 | 1 (20) | |
| 3.5 | 35 | 1 (20) | |
| Gastrointestinal cancer (colon cancer; n = 1) | 2.5 | 11 | 1 (100) |
NSCLC non-small cell lung cancer, ds-GPA disease-specific Graded Prognostic Assessment
Local treatment/radiotherapy
All included patients received radiation of the BMs. As initial therapy, resection followed by focal radiotherapy of the metastasis bed (18 patients, 45%) or by WBRT (6 patients, 15%) was the chosen option in the majority of cases (24/40, 60%). Less frequent was sole SRS of metastases (8/40, 20%) and WBRT (8/40, 20%). For details see (Table 4).
Table 4.
Details of initial intracranial treatment and number of brain metastases
| Intracranial treatment | Number of patients (%) | Number of brain metastases (%) | ||
|---|---|---|---|---|
| 1 | 2–3 | ≥ 4 | ||
| Combined treatment | ||||
| Surgery with radiotherapy of metastasis bed (3DCRT) | 11 (27.5) | 9 (81.8) | 2 (18.2) | 0 (0) |
| Surgery with radiotherapy of metastasis bed (IMRT) | 1 (2.5) | 1 (100) | 0 (0) | 0 (0) |
| Surgery with radiotherapy of metastasis bed (SRT) | 4 (10) | 2 (50) | 2 (50) | 0 (0) |
| Surgery with WBRT | 2 (5) | 1 (50) | 0 (0) | 1 (50) |
| Surgery with WBRT and SRT boost | 4 (10) | 0 (0) | 2 (50) | 2 (50) |
| Surgery with radiotherapy of metastasis bed (3DCRT) and SRT of other lesions | 2 (5) | 0 (0) | 1 (50) | 1 (50) |
| Radiotherapy only | ||||
| SRT | 8 (20) | 6 (75) | 1 (12.5) | 1 (12.5) |
| WBRT | 6 (15) | 1 (16.7) | 2 (33.3) | 3 (50) |
| WBRT with SRT boost | 2 (5) | 0 (0) | 0 (0) | 2 (100) |
3DCRT three-dimensional conformal radiotherapy, IMRT intensity-modulated radiotherapy, SRT stereotactic radiotherapy, WBRT whole-brain radiotherapy
Among patients who underwent resection followed by radiotherapy, those with only one lesion comprised 54.2% (13/24). 10/13 patients (77%) had a completely resected brain metastasis according to postoperative MRI. Among patients who underwent WBRT, the majority presented with ≥ 4 BM (5/8, 62.5%).
Among patients with SRS, 6/8 patients had a single BM, one patient had 3 BM, and one patient ≥ 4 BM.
Among patients receiving WBRT only, 5/8 patients had ≥ 4 BM, 2 patients had 2 BM, and one patient had a single BM.
During the later course of disease, 26/40 patients developed intracranial progression and 19/40 patients developed systemic progression.
The mean time to intracranial progression after radiotherapy of the brain was 25.8 months, while the mean time to systemic progression was 25.3 months.
Survival time among long-term survivors not operated for BM was not different between patients treated with SRS or WBRT (51.8 months vs. 49.6 months, p = 0.486).
Systemic treatment
Of the 40 patients, 32 (80%) received systemic treatment, the majority (20/32; 62.5%) started after completion of radiotherapy of the brain. Patient-specific details of treatment (sequence and duration of systemic treatments) are displayed in Supplements 2 and 3.
The treatments of the three largest patient groups (adenocarcinoma of the lung, melanoma, HER2-pos breast cancer) are described here in more detail:
Of the 15 patients with NSCLC, 33.3% (5/15) received EGFR-targeting tyrosine kinase inhibitors (erlotinib, afatinib, osimertinib, lapatinib) at some time after diagnosis of BM. Median duration of this medication was 38 months.
A single patient with an EML4-ALK fusion received crizotinib for an (estimated) period of 31 months.
Of the 15 patients with NSCLC, 9 received mostly short-lasting conventional chemotherapies involving, e.g., cis-/carboplatin or docetaxel.
In melanoma, 5/8 patients (62.5%) received immune checkpoint inhibition (ICI), i.e., pembrolizumab or ipilimumab ± nivolumab as treatment at some time after diagnosis of BM. Median duration of this treatment was 9 months. Only one patient with a BRAF-V600E mutation received a specific inhibitor (vemurafenib) for 10 months.
In breast cancer, 60% (3/5) of patients with HER2-positive disease received anti-HER2 blockage (trastuzumab or lapatinib) after diagnosis of BM, with a median treatment duration of 54 months.
Discussion
Long-term survival with BM was encountered in very few patients in our series. This observation is in agreement with other authors who have described survival of more than 3 years in 2–3.3% of patients [10, 18]. To define LTS as a survival time of more than 3 years is somewhat arbitrary but in line with the prior publications [10, 17]. The most frequent tumor entities among long-term survivors were NSCLC (adenocarcinoma), melanoma, and breast cancer. Independent of survival time, lung cancer, breast cancer, and melanoma are the most common sources of BM [4, 19–21]; this might largely reflect the frequency of patients with BM in the disease and not a proneness to LTS in these entities. In our cohort, the distribution of tumor types among LTS patients resembled that of non-LTS patients. Likewise, in another recent series, NSCLC, breast cancer, and melanoma were the most frequent primary tumors among patients with LTS [22]. In other studies, survival of 3 years after diagnosis of BM occurred in 2.6% (lung), 2% (breast), and 3.3% (melanoma) patients [10, 18].
Although mean age was significantly lower in LTS than in non-LTS patients, there was considerable heterogeneity regarding age, course of disease, number of metastases, and type of local and systemic treatments in LTS patients.
Retrospectively calculated ds-GPA scores appeared not to be able to predict the LTS of our patients with precision. The principal elements of the initial GPA score from 2008 were age, KPS, extracranial metastases, and the number of BM [23]. Later, more specific prognostic factors for the five most frequent primary tumor entities (non-small and small cell lung cancer, melanoma, breast cancer, renal cell carcinoma, and gastrointestinal cancer) were introduced in disease-specific GPA scores (Supplement 1) [24, 25]. However, as a score based on only few clinical items, it appears inherently more accurate for the large majority of patients with survival near to median survival and will lose accuracy at the upper and lower end of survival time. Hence, ds-GPA will likely allow prognostic assessment for the majority but not for all patients. In our series, LTS of ≥ 3 years does not appear to be well covered with ds-GPA. Apart from statistical reasons there might be several contributing factors to this. Firstly, therapeutic aspects like particular sensitivity to local or systemic treatment are not reflected in the score. Secondly, other pretreatment factors, e.g., accurately reflecting frailty [26], are missing in the score. However, relevant positive prognostic factors of ds-GPA like KPS (median 90%), a young age (median 59 years), and non-disseminated BM (below 4 BM: 75% of patients) were frequent among LTS patients [27]. For the clinical setting, cautious use of ds-GPA appears desirable.
Regarding the effect of local treatment, the majority (60%) of patients received resection prior to radiotherapy, which is common practice within the DEGRO Radiosurgery and Stereotactic Radiotherapy Working Group [28]. In clinical trials applying this approach, a median survival of 14 months is achieved [29], proving the efficacy of this treatment.
In our series, 35% of patients remained intracranially stable after radiotherapy and the mean time to intracranial progression was 25.8 months, i.e., particularly long compared to other studies [19]. Most likely this reflects particular sensitivity to local and/or systemic treatment active in the brain. Interestingly, also some patients with multiple BM after WBRT were among the long-term survivors. It is well known that LTS may occur after WBRT for multiple metastases, but this is very rare [18]. Remarkably, 8/40 patients (20%) reached LTS after local treatment without any further systemic treatment.
In recent years, several new treatment modalities in oncology have opened up new prognostic horizons. In our cohort, three larger subgroups (adenocarcinoma of the lung with specific mutations, melanoma, HER2-positive breast cancer) in which new treatments have been introduced were highly represented.
Overall, 40% of NSCLC patients received either a treatment with specific EGFR inhibitors (median treatment duration of 38 months) or crizotinib (median duration of 31 months) for an EML4-ALK fusion. It is well known that these treatments are highly active in BM, and PFS in recent trials with BM ranged from 8.6 to 16.5 months [30–35]. With this background it appears unsurprising that in LTS patients, targetable alterations are much more frequent than among non-selected patients with adenocarcinoma of the lung (EGFR: 41 vs. 11%; ALK: 8 vs. 4.8%) [22, 36]. However, the majority of NSCLC patients still showed a histology of adenocarcinoma without a specific alteration and reached LTS without specific systemic treatment.
In melanoma, 62.5% of patients received ICI as treatment at some time after the diagnosis of BM (median duration of treatment 9 months). Only one patient with a BRAF-V600E mutation received a specific inhibitor (vemurafenib) for 10 months. Several trials indicated high activity of ICI in BM, with around 30% of patients achieving durable responses to ICI [37], intracranial PFS of 59.5% after at 9 months [38], and 5‑year intracranial PFS of 14–52% (Rx naïve) [39]. Our results underline that ICI can contribute to LTS in melanoma with BM, which would otherwise be a rapidly fatal disease in most cases [40, 41].
In breast cancer, 60% (3/5) of patients with HER2-positive disease received trastuzumab after the diagnosis of BM, with a median treatment duration of 32 months. Survival under several cytotoxic treatments in this patient group was shorter, mainly in the range of several months.
HER2 targeting is a successful story in the treatment of breast cancer, with firstly trastuzumab and lapatinib being active in HER2-positive BM patients [42, 43]. More recently, other substances like tucatinib [44] or antibody–drug conjugates like trastuzumab deruxtecan [45–47] proved to be active in BM.
In summary, the effect of systemic treatment on LTS appears considerable and is currently not adequately reflected in prognostic scores.
The role of other concomitant diseases or conditions for prognosis in patients with BM is largely unclear. Among LTS patients in our cohort, arterial hypertension, other cardiovascular diseases, COPD, and smoking were quite frequent, while diabetes mellitus was rather rarely encountered (7.5%). In a recent study, the presence of diabetes mellitus was a significant predictor of poorer overall survival in patients treated for BM with SRS [48]. Similar results were achieved in a recent retrospective analysis of our group (unpublished) showing that diabetes mellitus might be an independent negative prognostic factor in patients with BM. It appears likely that parameters that better reflect multimorbidity or frailty like the Hurria [49] or G8 score [50] may aid in prognostic assessment in BM patients. Further, it would be interesting to validate temporal muscle thickness, which appears to be a relevant independent prognostic parameter in patients with BM, [51, 52] in LTS.
The goal of this work was to generate data in a cohort of patients with characteristics that present to radiotherapy departments on a daily basis and not in the setting of a controlled clinical trial. This approach lacks the accuracy of a controlled clinical trial but can potentially better reflect to what extent the successes of clinical trials and innovations can be translated into a substantial patient benefit in the uncontrolled setting of daily practice involving routine radiotherapy. Our analysis thus has the limitations of a monocentric retrospective series with unfortunate loss of 1/3 of the patient data, but reflects clinical routine and can be an orientation for the treating radiotherapist. We find it somewhat surprising that the results are indeed so heterogenous and that “paths” to LTS can be so different. Larger series and a comprehensive comparison of non-LTS and LTS patients are needed to validated this finding in tumor-specific subgroups. In the absence of high sensitivity of ds-GPA, it is currently very hard to predict LTS. To exaggerate a bit: it is not just the very “fit” patient with very few metastases receiving stereotactic radiotherapy and a particular systemic treatment who can survive for longer than 3 years.
Conclusion
Long-term survival in patients with BM is rare and difficult to predict with current scores. LTS patients are heterogeneous in their clinical features including number of metastases or age. Adequate local treatment appears important in many patients, as well as active systemic treatments. KPS at presentation of BM and oligometastatic disease keep their prognostic value in patients with LTS. The role of other scores encompassing concomitant diseases or frailty in BM remains to be evaluated in the future.
Supplementary Information
Supplement 1 Items assessed for ds-GPA, score calculation, and estimated survival
Supplement 2 Overview of systemic treatments per patient
Supplement 3 Patient-specific course of disease and treatment
Funding
Open Access funding enabled and organized by Projekt DEAL.
Declarations
Conflict of interest
N. H. Nicolay has received research grants from Novocure and speaker honoraria from Merck. C. Seidel has received speaker and/or advisory board honoraria from AbbVie, Bristol-Myers Squibb, HRA Pharma, Medac, Novocure, Roche, Seagen. M. Hügel, J. Stöhr, T. Kuhnt, F. Nägler, K. Papsdorf, S. Klagges, P. Hambsch and E. Güresir declare that they have no competing interests.
Ethical standards
All procedures performed in studies involving human participants or on human tissue were in accordance with the ethical standards of the institutional and/or national research committee and with the 1975 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Footnotes
Availability of data
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
The authors M. Hügel and J. Stöhr contributed equally to the manuscript.
References
- 1.Li AY, Gaebe K, Jerzak KJ, Cheema PK, Sahgal A, Das S (2022) Intracranial Metastatic Disease: Present Challenges, Future Opportunities. Front Oncol 12. 10.3389/fonc.2022.855182. eCollection 2022 [DOI] [PMC free article] [PubMed]
- 2.Habbous S, Forster K, Darling G, Jerzak K, Holloway CMB, Sahgal A, Das S. Incidence and real-world burden of brain metastases from solid tumors and hematologic malignancies in Ontario: a population-based study. Neuro-oncol Adv. 2020;3:vdaa178. doi: 10.1093/noajnl/vdaa178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cagney DN, Martin AM, Catalano PJ, Redig AJ, Lin NU, Lee EQ, Wen PY, Dunn IF, Bi WL, Weiss SE, Haas-Kogan DA, Alexander BM, Aizer AA. Incidence and prognosis of patients with brain metastases at diagnosis of systemic malignancy: a population-based study. Neuro-Oncol. 2017;19:1511–1521. doi: 10.1093/neuonc/nox077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soffietti R, Rudā R, Mutani R. Management of brain metastases. J Neurol. 2002;249:1357–1369. doi: 10.1007/s00415-002-0870-6. [DOI] [PubMed] [Google Scholar]
- 5.Schouten LJ, Rutten J, Huveneers HAM, Twijnstra A. Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer. 2002;94:2698–2705. doi: 10.1002/cncr.10541. [DOI] [PubMed] [Google Scholar]
- 6.Scoccianti S, Ricardi U. Treatment of brain metastases: Review of phase III randomized controlled trials. Radiother Oncol. 2012;102:168–179. doi: 10.1016/j.radonc.2011.08.041. [DOI] [PubMed] [Google Scholar]
- 7.Wen Jay Loeffler PYMDSMD (1999) Oncology. Manag Brain Metastases 13(7):941–954, 957–961; discussion 961–962, 9 [PubMed]
- 8.Arvold ND, Lee EQ, Mehta MP, Margolin K, Alexander BM, Lin NU, Anders CK, Soffietti R, Camidge DR, Vogelbaum MA, Dunn IF, Wen PY. Updates in the management of brain metastases. Neuro-Oncol. 2016;18:1043–1065. doi: 10.1093/neuonc/now127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tabouret E, Chinot O, Metellus P, Tallet A, Viens P, Gonçalves A. Recent Trends in Epidemiology of Brain Metastases: An Overview. Anticancer Res. 2012;32:4655–4662. [PubMed] [Google Scholar]
- 10.Niemiec M, Głogowski M, Tyc-Szczepaniak D, Wierzchowski M, Kępka L. Characteristics of long-term survivors of brain metastases from lung cancer. Rep Pract Oncol Radiother. 2011;16:49–53. doi: 10.1016/j.rpor.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zakaria R, Das K, Bhojak M, Radon M, Walker C, Jenkinson MD. The role of magnetic resonance imaging in the management of brain metastases: diagnosis to prognosis. Cancer Imaging. 2014;14:8. doi: 10.1186/1470-7330-14-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smedby KE, Brandt L, Bäcklund ML, Blomqvist P. Brain metastases admissions in Sweden between 1987 and 2006. Br J Cancer. 2009;101:1919–1924. doi: 10.1038/sj.bjc.6605373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Welzel T, El Shafie RA, v. Nettelbladt B, Bernhardt D, Rieken S, Debus J. Stereotactic radiotherapy of brain metastases: clinical impact of three-dimensional SPACE imaging for 3T-MRI-based treatment planning. Strahlenther Onkol. 2022;198:926–933. doi: 10.1007/s00066-022-01996-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ye C, Handa P, Sahgal A, Lo S, Vellayappan B. Risk-reduction strategies for late complications arising from brain metastases treated with radiotherapy: a narrative review. Chin Clin Oncol. 2022 doi: 10.21037/cco-21-121. [DOI] [PubMed] [Google Scholar]
- 15.Nieder C, Spanne O, Mehta MP, Grosu AL, Geinitz H. Presentation, patterns of care, and survival in patients with brain metastases. Cancer. 2011;117:2505–2512. doi: 10.1002/cncr.25707. [DOI] [PubMed] [Google Scholar]
- 16.Sperduto PW, Mesko S, Li J, Cagney D, Aizer A, Lin NU, Nesbit E, Kruser TJ, Chan J, Braunstein S, Lee J, Kirkpatrick JP, Breen W, Brown PD, Shi D, Shih HA, Soliman H, Sahgal A, Shanley R, Sperduto WA, Lou E, Everett A, Boggs DH, Masucci L, Roberge D, Remick J, Plichta K, Buatti JM, Jain S, Gaspar LE, Wu C-C, Wang TJC, Bryant J, Chuong M, An Y, Chiang V, Nakano T, Aoyama H, Mehta MP. J Clin Oncol Off J Am Soc Clin Oncol. Surviv Patients With Brain Metastases: Summ Rep Updat Diagnosis-specific Graded Progn Assess Defin Eligibility Quotient. 2020;38:3773–3784. doi: 10.1200/JCO.20.01255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sperduto PW, Kased N, Roberge D, Xu Z, Shanley R, Luo X, Sneed PK, Chao ST, Weil RJ, Suh J, Bhatt A, Jensen AW, Brown PD, Shih HA, Kirkpatrick J, Gaspar LE, Fiveash JB, Chiang V, Knisely JPS, Sperduto CM, Lin N, Mehta M. Summary Report on the Graded Prognostic Assessment: An Accurate and Facile Diagnosis-Specific Tool to Estimate Survival for Patients With Brain Metastases. J Clin Oncol. 2012;30:419–425. doi: 10.1200/JCO.2011.38.0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lutterbach J, Bartelt S, Ostertag C. Long-term survival in patients with brain metastases. J Cancer Res Clin Oncol. 2002;128:417–425. doi: 10.1007/s00432-002-0354-1. [DOI] [PubMed] [Google Scholar]
- 19.Berghoff AS, Schur S, Füreder LM, Gatterbauer B, Dieckmann K, Widhalm G, Hainfellner J, Zielinski CC, Birner P, Bartsch R, Preusser M. Descriptive statistical analysis of a real life cohort of 2419 patients with brain metastases of solid cancers. Esmo Open. 2016;1:e000024. doi: 10.1136/esmoopen-2015-000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barnholtz-Sloan JS, Sloan AE, Davis FG, Vigneau FD, Lai P, Sawaya RE. Incidence Proportions of Brain Metastases in Patients Diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol. 2004;22:2865–2872. doi: 10.1200/JCO.2004.12.149. [DOI] [PubMed] [Google Scholar]
- 21.Nayak L, Lee EQ, Wen PY. Epidemiology of Brain Metastases. Curr Oncol Rep. 2012;14:48–54. doi: 10.1007/s11912-011-0203-y. [DOI] [PubMed] [Google Scholar]
- 22.Dasgupta A, Co J, Winter J, Millar B-A, Laperriere N, Tsang DS, van Prooijen M, Damyanovich A, Heaton R, Coolens C, Bernstein M, Kongkham P, Zadeh G, Berlin A, Conrad T, Moraes FY, Shultz DB. Clinicopathologic and Treatment Features of Long-Term Surviving Brain Metastasis Patients. Curr Oncol Tor. 2021. pp. 549–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sperduto PW, Berkey B, Gaspar LE, Mehta M, Curran W. A New Prognostic Index and Comparison to Three Other Indices for Patients With Brain Metastases: An Analysis of 1,960 Patients in the RTOG Database. Int J Radiat Oncol. 2008;70:510–514. doi: 10.1016/j.ijrobp.2007.06.074. [DOI] [PubMed] [Google Scholar]
- 24.Sperduto PW, Chao ST, Sneed PK, Luo X, Suh J, Roberge D, Bhatt A, Jensen AW, Brown PD, Shih H, Kirkpatrick J, Schwer A, Gaspar LE, Fiveash JB, Chiang V, Knisely J, Sperduto CM, Mehta M. Diagnosis-Specific Prognostic Factors, Indexes, and Treatment Outcomes for Patients With Newly Diagnosed Brain Metastases: A Multi-Institutional Analysis of 4,259 Patients. Int J Radiat Oncol. 2010;77:655–661. doi: 10.1016/j.ijrobp.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 25.Sperduto PW, Kased N, Roberge D, Xu Z, Shanley R, Luo X, Sneed PK, Chao ST, Weil RJ, Suh J, Bhatt A, Jensen AW, Brown PD, Shih HA, Kirkpatrick J, Gaspar LE, Fiveash JB, Chiang V, Knisely JPS, Sperduto CM, Lin N, Mehta M. Effect of Tumor Subtype on Survival and the Graded Prognostic Assessment for Patients With Breast Cancer and Brain Metastases. Int J Radiat Oncol. 2012;82:2111–2117. doi: 10.1016/j.ijrobp.2011.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bleckmann A, Kirchner B, Nietert M, Peeck M, Balkenhol M, Egert D, Rohde TV, Beißbarth T, Pukrop T. Impact of pre-OP independence in patients with limited brain metastases on long-term survival. Bmc Cancer. 2020;20:973. doi: 10.1186/s12885-020-07459-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lamba N, Catalano PJ, Bi WL, Wen PY, Haas-Kogan DA, Cagney DN, Aizer AA. Predictors of long-term survival among patients with brain metastases. Neuro-Oncol. 2022;24:494–496. doi: 10.1093/neuonc/noab285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rogers S, Baumert B, Blanck O, Böhmer D, Boström J, Engenhart-Cabillic R, Ermis E, Exner S, Guckenberger M, Habermehl D, Hemmatazad H, Henke G, Lohaus F, Lux S, Mai S, Minasch D, Rezazadeh A, Steffal C, Temming S, Wittig A, Zweifel C, Riesterer O, Combs SE. Stereotactic radiosurgery and radiotherapy for resected brain metastases: current pattern of care in the Radiosurgery and Stereotactic Radiotherapy Working Group of the German Association for Radiation Oncology (DEGRO) Strahlenther Onkol. 2022;198:919–925. doi: 10.1007/s00066-022-01991-6. [DOI] [PubMed] [Google Scholar]
- 29.Gans JH, Raper DMS, Shah AH, Bregy A, Heros D, Lally BE, Morcos JJ, Heros RC, Komotar RJ. The role of radiosurgery to the tumor bed after resection of brain metastases. Neurosurgery. 2013;72:317–325. doi: 10.1227/NEU.0b013e31827fcd60. [DOI] [PubMed] [Google Scholar]
- 30.Rubino S, Oliver DE, Tran ND, Vogelbaum MA, Forsyth PA, H-HM YAK, Etame AB. Improving Brain Metastases Outcomes Through Therapeutic Synergy Between Stereotactic Radiosurgery and Targeted Cancer Therapies. Front Oncol. 2022;12:854402. doi: 10.3389/fonc.2022.854402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang N, Zhang Y, Mi Y, Deng H, Chen G, Tang Z, Mao J, Cui S, Zhang Y, Wang L. Osimertinib for EGFR-mutant lung cancer with central nervous system metastases: a meta-analysis and systematic review. Ann Palliat Med. 2020;9:3038047–3033047. doi: 10.21037/apm-20-605. [DOI] [PubMed] [Google Scholar]
- 32.Solomon BJ, Cappuzzo F, Felip E, Blackhall FH, Costa DB, Kim D-W, Nakagawa K, Wu Y-L, Mekhail T, Paolini J, Tursi J, Usari T, Wilner KD, Selaru P, Mok TSK. J Clin Oncol Off J Am Soc Clin Oncol. Intracranial Efficacy Crizotinib Versus Chemother Patients With Adv Alk-positive Non-small-cell Lung Cancer: Results From Profile 1014. 2016;34:2858–2865. doi: 10.1200/JCO.2015.63.5888. [DOI] [PubMed] [Google Scholar]
- 33.Yang JCH, Kim S-W, Kim D-W, Lee J-S, Cho BC, Ahn J-S, Lee DH, Kim TM, Goldman JW, Natale RB, Brown AP, Collins B, Chmielecki J, Vishwanathan K, Mendoza-Naranjo A, Ahn M-J. Osimertinib in Patients With Epidermal Growth Factor Receptor Mutation–Positive Non–Small-Cell Lung Cancer and Leptomeningeal Metastases: The BLOOM Study. J Clin Oncol. 2020;38:538–547. doi: 10.1200/JCO.19.00457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reungwetwattana T, Nakagawa K, Cho BC, Cobo M, Cho EK, Bertolini A, Bohnet S, Zhou C, Lee KH, Nogami N, Okamoto I, Leighl N, Hodge R, McKeown A, Brown AP, Rukazenkov Y, Ramalingam SS, Vansteenkiste J. CNS Response to Osimertinib Versus Standard Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors in Patients With Untreated EGFR-Mutated Advanced Non–Small-Cell Lung Cancer. J Clin Oncol. 2018;36:3290–3297. doi: 10.1200/JCO.2018.78.3118. [DOI] [PubMed] [Google Scholar]
- 35.Su P-L, Wu Y-L, Chang W-Y, Ho C-L, Tseng Y-L, Lai W-W, Su W-C, Lin C-C, Yang S-C. Preventing and treating brain metastases with three first-line EGFR-tyrosine kinase inhibitors in patients with EGFR mutation-positive advanced non-small cell lung cancer. Ther Adv Med Oncol. 2018;10:1758835918797589. doi: 10.1177/1758835918797589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barlesi F, Mazieres J, Merlio J-P, Debieuvre D, Mosser J, Lena H, Ouafik L, Besse B, Rouquette I, Westeel V, Escande F, Monnet I, Lemoine A, Veillon R, Blons H, Audigier-Valette C, Bringuier P-P, Lamy R, Beau-Faller M, Pujol J-L, Sabourin J-C, Penault-Llorca F, Denis MG, Lantuejoul S, Morin F, Tran Q, Missy P, Langlais A, Milleron B, Cadranel J, Soria J-C, Zalcman G, contributors BF. Routine molecular profiling of patients with advanced non-small-cell lung cancer: results of a 1-year nationwide programme of the French Cooperative Thoracic Intergroup (IFCT) Lancet Lond Engl. 2016;387:1415–1426. doi: 10.1016/S0140-6736(16)00004-0. [DOI] [PubMed] [Google Scholar]
- 37.Le Rhun E, Guckenberger M, Smits M, Dummer R, Bachelot T, Sahm F, Galldiks N, de Azambuja E, Berghoff AS, Metellus P, Peters S, Hong Y-K, Winkler F, Schadendorf D, van den Bent M, Seoane J, Stahel R, Minniti G, Wesseling P, Weller M, Preusser M. EANO—ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up of patients with brain metastasis from solid tumours. Ann Oncol. 2021;32:1332–1347. doi: 10.1016/j.annonc.2021.07.016. [DOI] [PubMed] [Google Scholar]
- 38.Tawbi HA, Forsyth PA, Algazi A, Hamid O, Hodi SF, Moschos SJ, Khushalani NI, Lewis K, Lao CD, Postow MA, Atkins MB, Ernstoff MS, Reardon DA, Puzanov I, Kudchadkar RR, Thomas RP, Tarhini A, Pavlick AC, Jiang J, Avila A, Demelo S, Margolin K. Combined Nivolumab and Ipilimumab in Melanoma Metastatic to the Brain. N Engl J Med. 2018;379:722–730. doi: 10.1056/NEJMoa1805453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Long GV, Atkinson V, Lo S, Guminski AD, Sandhu SK, Brown MP, Gonzalez M, Scolyer RA, Emmett L, McArthur GA, Menzies AM. Five-year overall survival from the anti-PD1 brain collaboration (ABC Study): Randomized phase 2 study of nivolumab (nivo) or nivo+ipilimumab (ipi) in patients (pts) with melanoma brain metastases (mets) J Clin Oncol. 2021;39:9508–9508. doi: 10.1200/JCO.2021.39.15_suppl.9508. [DOI] [Google Scholar]
- 40.Pedersen S, Møller S, Donia M, Persson GF, Svane IM, Ellebaek E. Real-world data on melanoma brain metastases and survival outcome. Melanoma Res. 2022;32:173–182. doi: 10.1097/CMR.0000000000000816. [DOI] [PubMed] [Google Scholar]
- 41.Becco P, Gallo S, Poletto S, Frascione MPM, Crotto L, Zaccagna A, Paruzzo L, Caravelli D, Carnevale-Schianca F, Aglietta M. Melanoma Brain Metastases in the Era of Target Therapies: An Overview. Cancers. 2020;12:1640. doi: 10.3390/cancers12061640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garcia-Alvarez A, Papakonstantinou A, Oliveira M. Metastases in HER2-Positive Breast Cancer: Current and Novel Treatment Strategies. Cancers. Brain. 2021;13:2927. doi: 10.3390/cancers13122927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hackshaw MD, Danysh HE, Henderson M, Wang E, Tu N, Islam Z, Ladner A, Ritchey ME, Salas M. Prognostic factors of brain metastasis and survival among HER2-positive metastatic breast cancer patients: a systematic literature review. Bmc Cancer. 2021;21:967. doi: 10.1186/s12885-021-08708-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murthy RK, Loi S, Okines A, Paplomata E, Hamilton E, Hurvitz SA, Lin NU, Borges V, Abramson V, Anders C, Bedard PL, Oliveira M, Jakobsen E, Bachelot T, Shachar SS, Müller V, Braga S, Duhoux FP, Greil R, Cameron D, Carey LA, Curigliano G, Gelmon K, Hortobagyi G, Krop I, Loibl S, Pegram M, Slamon D, Palanca-Wessels MC, Walker L, Feng W, Winer EP. Tucatinib, Trastuzumab, and Capecitabine for HER2-Positive Metastatic Breast Cancer. N Engl J Med. 2020;382:597–609. doi: 10.1056/NEJMoa1914609. [DOI] [PubMed] [Google Scholar]
- 45.Cortés J, Kim S-B, Chung W-P, Im S-A, Park YH, Hegg R, Kim MH, Tseng L-M, Petry V, Chung C-F, Iwata H, Hamilton E, Curigliano G, Xu B, Huang C-S, Kim JH, Chiu JWY, Pedrini JL, Lee C, Liu Y, Cathcart J, Bako E, Verma S, Hurvitz SA. Trastuzumab Deruxtecan versus Trastuzumab Emtansine for Breast Cancer. N Engl J Med. 2022;386:1143–1154. doi: 10.1056/NEJMoa2115022. [DOI] [PubMed] [Google Scholar]
- 46.Bartsch R, Berghoff AS, Furtner J, Marhold M, Bergen ES, Roider-Schur S, Starzer AM, Forstner H, Rottenmanner B, Dieckmann K, Bago-Horvath Z, Haslacher H, Widhalm G, Ilhan-Mutlu A, Minichsdorfer C, Fuereder T, Szekeres T, Oehler L, Gruenberger B, Singer CF, Weltermann A, Puhr R, Preusser M. Trastuzumab deruxtecan in HER2-positive breast cancer with brain metastases: a single-arm, phase 2 trial. Nat Med. 2022;28:1840–1847. doi: 10.1038/s41591-022-01935-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Modi S, Jacot W, Yamashita T, Sohn J, Vidal M, Tokunaga E, Tsurutani J, Ueno NT, Prat A, Chae YS, Lee KS, Niikura N, Park YH, Xu B, Wang X, Gil-Gil M, Li W, Pierga J-Y, Im S-A, Moore HCF, Rugo HS, Yerushalmi R, Zagouri F, Gombos A, Kim S-B, Liu Q, Luo T, Saura C, Schmid P, Sun T, Gambhire D, Yung L, Wang Y, Singh J, Vitazka P, Meinhardt G, Harbeck N, Cameron DA. Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. N Engl J Med. 2022;387:9–20. doi: 10.1056/NEJMoa2203690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.LeCompte MC, McTyre ER, Strowd RE, Lanier C, Soike MH, Hughes RT, Masters AH, Cramer CK, Farris M, Ruiz J, Watabe K, Laxton AW, Tatter SB, Winkfield KM, Chan MD. Impact of diabetes mellitus on outcomes in patients with brain metastasis treated with stereotactic radiosurgery. J Radiosurgery Sbrt. 2018;5:285–291. [PMC free article] [PubMed] [Google Scholar]
- 49.Hurria A, Togawa K, Mohile SG, Owusu C, Klepin HD, Gross CP, Lichtman SM, Gajra A, Bhatia S, Katheria V, Klapper S, Hansen K, Ramani R, Lachs M, Wong FL, Tew WP. Predicting Chemotherapy Toxicity in Older Adults With Cancer: A Prospective Multicenter Study. J Clin Oncol. 2011;29:3457–3465. doi: 10.1200/JCO.2011.34.7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bellera CA, Rainfray M, Mathoulin-Pélissier S, Mertens C, Delva F, Fonck M, Soubeyran PL. Screening older cancer patients: first evaluation of the G-8 geriatric screening tool. Ann Oncol. 2012;23:2166–2172. doi: 10.1093/annonc/mdr587. [DOI] [PubMed] [Google Scholar]
- 51.Ilic I, Faron A, Heimann M, Potthoff A-L, Schäfer N, Bode C, Borger V, Eichhorn L, Giordano FA, Güresir E, Jacobs AH, Ko Y-D, Landsberg J, Lehmann F, Radbruch A, Herrlinger U, Vatter H, Schuss P, Schneider M. Combined Assessment of Preoperative Frailty and Sarcopenia Allows the Prediction of Overall Survival in Patients with Lung Cancer (NSCLC) and Surgically Treated Brain Metastasis. Cancers. 2021;13:3353. doi: 10.3390/cancers13133353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Furtner J, Berghoff AS, Schöpf V, Reumann R, Pascher B, Woitek R, Asenbaum U, Pelster S, Leitner J, Widhalm G, Gatterbauer B, Dieckmann K, Höller C, Prayer D, Preusser M. Temporal muscle thickness is an independent prognostic marker in melanoma patients with newly diagnosed brain metastases. J Neurooncol. 2018;140:173–178. doi: 10.1007/s11060-018-2948-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement 1 Items assessed for ds-GPA, score calculation, and estimated survival
Supplement 2 Overview of systemic treatments per patient
Supplement 3 Patient-specific course of disease and treatment