Abstract
Invasive alien species (IAS) pose a severe threat to global agriculture, with their impact projected to escalate due to climate change and expanding international trade. The fall armyworm (FAW), Spodoptera frugiperda (J. E. Smith), a native of the Americas, has rapidly spread across various continents, causing significant damage to several food crops, especially maize. Integrated pest management (IPM) programs are vital for sustainable FAW control, combining multiple strategies for sustainable results. Over three consecutive years, 2019–20, 2020–21 and 2021–22, the field demonstrations were conducted in semiarid regions of India, testing a four-component IPM approach viz., pheromone traps, microbial, botanicals and ETL based applications of insecticides against farmers' practices (sole insecticide application). IPM implementation led to substantial reductions in FAW infestation. Furthermore, egg mass and larvae infestations were significantly lower in IPM-adopted villages compared to conventional practices. Pheromone-based monitoring demonstrated a consistent reduction in adult moth populations. The lowest technology gap (10.42), extension gap (8.33) and technology index (12.25) was recorded during 2020–21. The adoption of IPM led to increased maize yields (17.49, 12.62 and 24.87% over control), higher net returns (919, 906.20 and 992.93 USD), and favourable benefit–cost ratios (2.74, 2.39 and 2.33) compared to conventional practices respectively during 2019–20, 2020–21 and 2021–22. The economic viability of IPM strategies was evident across three consecutive years, confirming their potential for sustainable FAW management in the semiarid region of India. These strategies hold promise for adoption in other parts of the world sharing similar climatic conditions.
Keywords: Fall armyworm, Invasive pests, IPM, Metarhizium anisopliae, Pheromone traps, Spodoptera frugiperda, Technology gap
Subject terms: Ecology, Plant sciences, Zoology, Environmental sciences
Introduction
Invasive alien species (IAS) constitute a serious threat to agricultural products, and this threat is expected to grow in response to climate change and growing global trade1. The IAS also supplant native species, harm biodiversity, and alter ecosystems, resulting in significant economic losses1–4. The fall armyworm (FAW), Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae), is a native species to the tropical and subtropical areas of the Americas5. After being first identified in West Africa6 it quickly spread to sub-Saharan Africa7 the Asian subcontinent8–10. The FAW has a wide host range of over 353 plants from 76 families, primarily from the Poaceae (106), Asteraceae (31), and Fabaceae (31)11,12. The infestations of FAW have been shown to reduce maize yields by 15–73%9. Due to the high consumption of these cereal crops, mainly maize, in smallholder diets, FAW could substantially impact global food security. Further the damage on maize, may aggravates the dependent industries like bio-ethanol, poultry, and animal husbandry9.
The management of FAW is challenging mainly because of higher reproduction potential, polyphagous feeding habitat, strong flight capability, absence of diapause, potential displacement of rival species and evolved resistance to an array of pesticide classes. In addition, mature larvae's cryptic eating habits frequently render chemical insecticides inefficient. The farmers have attempted to control this damaging pest using several physical, chemical, and biological methods, but most of these have been ineffective when used individually13. So, for long-term FAW control, Integrated Pest Management (IPM) programmes comprising a variety of supplementary approaches are required. IPM is an ecosystem-based strategy that focuses on long-term prevention of pests or their damage through a combination of techniques such as biological control, habitat manipulation, modification of cultural practices, and use of resistant varieties. For early identification of pest, and to make timely and effective management decisions, pheromone-based monitoring, surveillance, and scouting using flying moths are crucial and essential tools7,14.
The effectiveness of pest control techniques in mitigating crop losses is contingent on farmers' comprehension, attitudes, and practices related to pest management. Therefore, it is hypothesized that evaluating farmers' knowledge concerning insect pests, yield damage, and efficient management strategies through surveys is imperative. Additionally, current agricultural pest management practices require a robust scientific foundation, and the rationale behind farmers' adoption of these practices may be uncertain. Farmers have the potential to employ crop rotation, intercropping, and resistant crop varieties to minimize infestations13. Biological control, involving the use of natural enemies like parasitoids, predators, and entomopathogens, can significantly reduce pest populations when integrated with compatible pest management techniques8. Moreover, the controlled and prudent use of insecticides, coupled with appropriate safety measures to prevent adverse impacts on human health and the environment, may not be environmentally harmful. These methods aim to enhance the resilience of the agro ecosystem and reduce the pest's reproductive potential and dispersal. Early inspection and monitoring of crops with pheromone traps, have proven effective in managing the fall armyworm in the northeastern states. It is imperative to provide periodic awareness training for maize growers and enhance the capacity of extension officers and input dealers in early scouting, surveillance, and monitoring of FAW incidence to achieve similar success in other regions. The hypothesis regarding Integrated Pest Management (IPM)-based strategies for FAW suggests that such approaches can efficiently control the pest and enhance crop productivity compared to conventional pesticide applications. IPM strategies encompass various techniques including cultural, biological, mechanical, and behavioral methods such as crop rotation, resistant varieties, natural enemies, pheromone traps, and bio-pesticides. In India, researchers have explored different modules, including bio-intensive, IPM, and synthetic insecticide-based practices for managing S. frugiperda15. While initially, the exclusive use of insecticides proved effective, S. frugiperda has since developed resistance to commonly used insecticides like cypermethrin16, lambda cyhalothrin17 and profenophos18,19 rendering them ineffective at the recommended field doses. Similarly, IPM results have been satisfactory, it's noted that these approaches may not be universally applicable across all agro-ecological regions of India due to variations in inputs and challenges in availability. Successful IPM implementation requires a participatory approach involving farmers, extension agents, researchers, and policymakers to ensure adoption and sustainability. Given the location-specific nature of IPM strategies influenced by prevailing weather conditions, it's crucial to develop and validate location-specific models for effective FAW management in maize crops. In order to validate the hypothesis, interviews were carried out with farmers to discern their preferred FAW control methods, based on which compatible components of IPM were selected and evaluated under farmers' field conditions.
Results
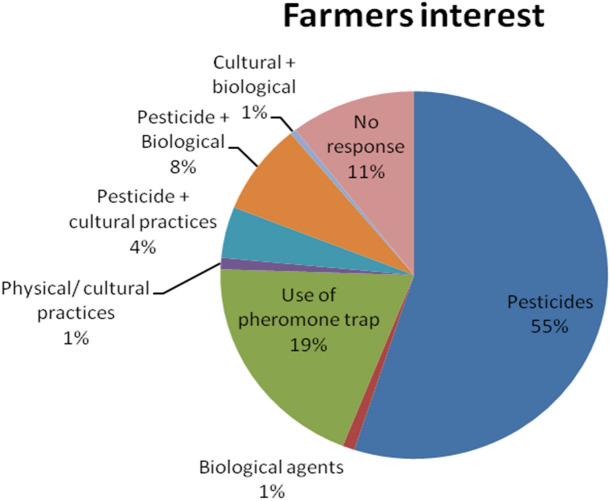
A total of 200 farmers were interviewed for their preference towards the choice of control methods for FAW management. Among the options listed, pesticides were the most preferred choice, with 55% of the farmers expressing interest in using them for FAW management. Biological pest control, which utilizes natural enemies of S. frugiperda, garnered minimal interest, with only 1% of farmers showing a preference for this approach (Fig. 1). Interestingly, use of pheromone traps captured the attention of 19.5% of the farmers. Some farmers (1%) expressed interest in physical or cultural practices. Combining pesticides with cultural practices or biological agents gained interest from 4.5 and 8% of the farmers, respectively. Surprisingly, only 0.5% of the farmers showed interest in combining cultural practices with biological agents. Additionally, 10.5% of the farmers did not respond, suggesting a lack of awareness or uncertainty regarding the various FAW control methods.
Figure 1.
Preference of farmers towards different management practices for FAW management.
The mean per cent infestation of S. frugiperda, before and after the application of different chemicals in IPM adopted villages during 2019–20, 2020–21 and 2021–22 were analyzed and presented in Tables 1, 2 and 3, respectively. During 2019–20, application of all three chemicals viz., Azadirachtin, Emamectin benzoate, and Metarhizium anisopliae in IPM-adopted villages led to a significant reduction in FAW infestation. Use of azadirachtin decreased mean infestation from 34.87 to 18.06% (t value = 7.06**). Similarly, application of M. anisopliae (Fig. 2) effectively brought the infestation from 35.50 to 18.68% (t value = 16.19**). The application of EB resulted in the most effective reduction of FAW infestation from 28.52 to 10.56 (t value = 11.64**). A similar trend in population regulation was observed during 2020–21. However, the per cent infestation was significantly higher. The application of Azadirachtin, emamectin benzoate and M. anisopliae resulted in a decrease of S. frugiperda infestation from 41.06, 52.84 and 48.55 to 20.01 (t value = 1.51**), 10.70 (t value = 18.24**) and 21.32 (t value = 19.60**), respectively. During 2021–22, the efficacy of Azadirachtin, emamectin benzoate and M. anisopliae in reducing the FAW infestation respectively as follows 45.56 to 20.21% (t value = 16.56**), 55.97 to 11.08% (t value = 16.56**) and 51.83 to 26.87% (t value = 16.66**).
Table 1.
The mean percent infestation of FAW, S. frugiperda before and after application of different chemicals in IPM adopted villages during 2019–20.
| Village | Azadirachtin | Emamectin benzoate | Metarhizium anisopliae | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Before | After | T statistics | Before | After | T statistics | Before | After | T statistics | |
| IPM1 | 34.45 | 19.27 | 5.59** | 28.67 | 10.26 | 14.37** | 36.2 | 15.7 | 14.07** |
| IPM2 | 28.9 | 11.26 | 6.56** | 22.84 | 7.68 | 20.28** | 34.8 | 18.6 | 9.47** |
| IPM3 | 41.28 | 22.18 | 5.09** | 32.88 | 12.98 | 16.38** | 35.9 | 16.8 | 12.10** |
| IPM4 | 33.89 | 18.22 | 3.78** | 28.61 | 11.12 | 16.81** | 34.6 | 18.7 | 8.26** |
| IPM5 | 31.78 | 18.19 | 4.15** | 27.89 | 9.18 | 21.65** | 35.1 | 19.6 | 8.18** |
| IPM6 | 38.9 | 19.26 | 4.23** | 30.21 | 12.16 | 16.00** | 36.4 | 22.7 | 7.03** |
| Mean | 34.87 | 18.06 | 7.06** | 28.52 | 10.56 | 11.64** | 35.50 | 18.68 | 16.19** |
**Samples are significantly different at both 5% and 1% level of significance.
Table 2.
The mean percent infestation of FAW, S. frugiperda before and after application of different chemicals in IPM adopted villages during 2020–21.
| Village | Azadirachtin | Emamectin benzoate | Metarhizium anisopliae | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Before | After | T statistics | Before | After | T statistics | Before | After | T statistics | |
| IPM1 | 40.45 | 20.27 | 10.79** | 54.17 | 10.66 | 40.08** | 46.2 | 20.5 | 13.25** |
| IPM2 | 36.78 | 16.26 | 14.01** | 44.9 | 9.68 | 36.82** | 48.9 | 21.9 | 12.98** |
| IPM3 | 43.44 | 23.43 | 9.58** | 58.19 | 12.98 | 35.92** | 50.7 | 18.2 | 18.99** |
| IPM4 | 46.89 | 22.67 | 11.65** | 58.67 | 11.12 | 43.55** | 51.3 | 25.1 | 11.11** |
| IPM5 | 38.87 | 18.19 | 12.55** | 47.89 | 9.51 | 39.61** | 44.8 | 20.8 | 12.62** |
| IPM6 | 39.9 | 19.26 | 11.69** | 53.21 | 10.26 | 41.30** | 49.4 | 21.4 | 13.93** |
| Mean | 41.06 | 20.01 | 1.51** | 52.84 | 10.70 | 18.24** | 48.55 | 21.32 | 19.60** |
**Samples are significantly different at both 5% and 1% level of significance.
Table 3.
The mean percent infestation of FAW, S. frugiperda before and after application of different chemicals in IPM adopted villages during 2021–22.
| Village | Azadirachtin | Emamectin benzoate | Metarhizium anisopliae | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Before | After | T statistics | Before | After | T statistics | Before | After | T statistics | |
| IPM1 | 43.89 | 19.29 | 12.56** | 52.45 | 9.78 | 42.75** | 52.5 | 31.1 | 7.85** |
| IPM2 | 39.78 | 20.16 | 10.44** | 60.59 | 13.44 | 34.93** | 51.8 | 26.9 | 10.39** |
| IPM3 | 46.44 | 21.43 | 12.08** | 53.29 | 12.98 | 28.97** | 50.7 | 24.6 | 11.25** |
| IPM4 | 44.49 | 20.17 | 13.01** | 54.17 | 8.78 | 37.26** | 52.2 | 27.3 | 10.06** |
| IPM5 | 48.87 | 19.89 | 15.92** | 57.45 | 11.23 | 32.11** | 50.9 | 22.7 | 13.15** |
| IPM6 | 49.9 | 20.31 | 15.36** | 57.89 | 10.26 | 41.91** | 52.9 | 28.6 | 9.22** |
| Mean | 45.56 | 20.21 | 16.56** | 55.97 | 11.08 | 16.56** | 51.83 | 26.87 | 16.66** |
**Samples are significantly different at both 5% and 1% level of significance.
Figure 2.
Mummification of Metarhizium anisopliae infected larvae of S. frugiperda.
The results on number of egg mass and larvae per plant in different villages and years, comparing farmers' practice (FP) with IPM strategies, show that the egg mass/plant was lower in the IPM villages compared to the FP villages, indicating the effectiveness of IPM in reducing S. frugiperda infestation. The mean number of egg mass/plant on FP ranged from 0.95–1.2, 0.8–1.3 and 0.75- 1.35 during 2019–20, 2020–21 and 2021–22, respectively. However, the corresponding values in IPM villages show that the mean egg masses are 0.4–0.65, 0.4–0.6 and 0.6–0.8/ plant (Fig. 3). Similarly, the larvae/plant was significantly lower in IPM-adopted villages than in FP. The mean numbers of larvae per plant in FP villages are 1.26–1.63, 1.2–1.45 and 1.41–1.7, respectively, during 2019–20, 2020–21 and 2021–22. However, corresponding values on the mean number of larvae per plant in IPM-adopted villages are 1.05–1.25, 0.85–1.15 and 0.65–1.24 (Fig. 4). The results indicate the average trap catches in different IPM villages over the specified years. The results show that the trap catches varied among the different IPM villages across three years. There is some variation in trap catches within each village yearly. Interestingly, the adults trapped each week show a decreasing trend in all the observed locations across the years. The number of adults trapped each year ranged from 24.30–32.43, 31.21–37.94 and 30.20–38.72 months, respectively, during 2019–20 (Fig. 5), 2020–21 (Fig. 6) and 2021–22 (Fig. 7).
Figure 3.
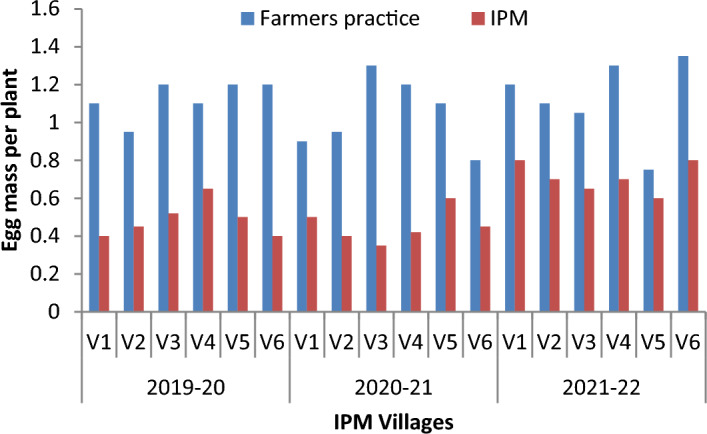
Mean number of egg mass/ plant observed in different villages (Mean of 5, 6, 7, 8 and 9 weeks observation).
Figure 4.
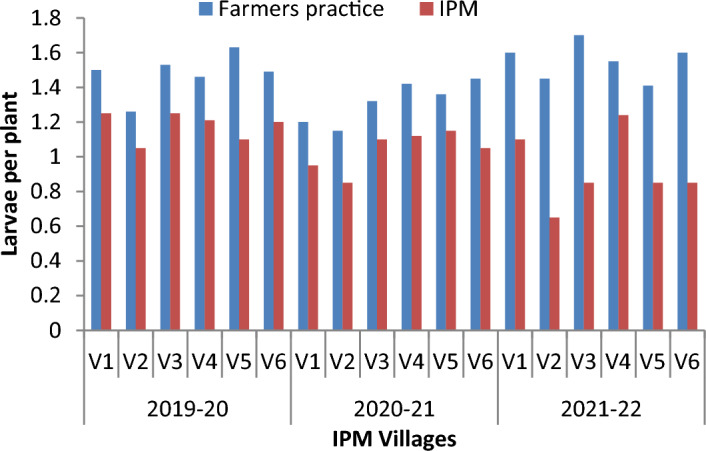
Mean number of larvae/ plant observed in different villages (Mean of 5, 6, 7, 8 and 9 weeks observation).
Figure 5.
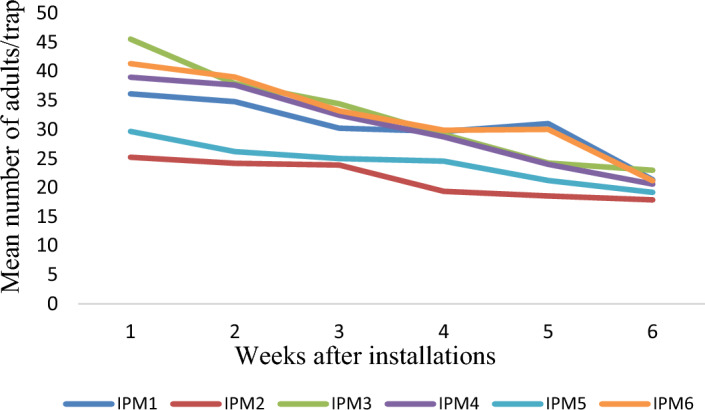
Mean number of adults/trap in different IPM villages during 2019–20.
Figure 6.
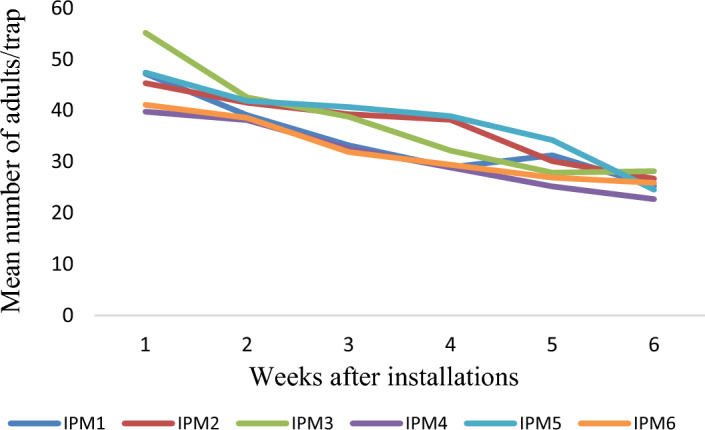
Mean number of adults/trap in different IPM villages during 2020–21.
Figure 7.
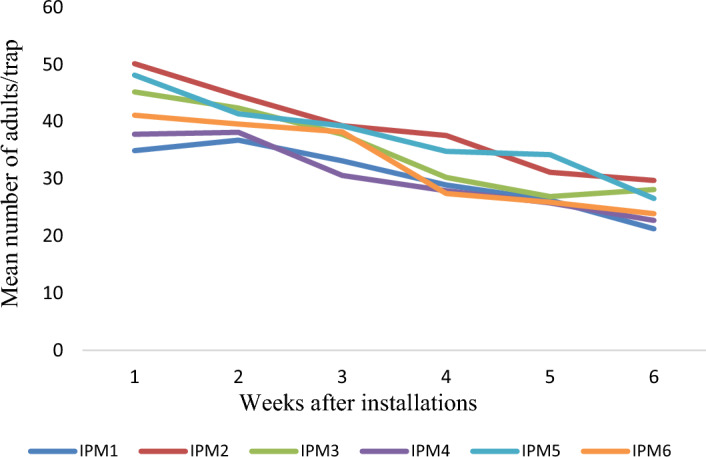
Mean number of adults/trap in different villages during 2021–22.
The technology gap, extension gap, and technology index in adopting Integrated Pest Management (IPM) strategies under the demonstration for three consecutive years (2019–20, 2020–21, and 2021–22) were presented in Fig. 8. In 2019–20, the technology gap was 23.33, representing the difference between the potential yield of maize (8500 kg (85 quintal)/ ha) and the actual yield obtained by farmers implementing IPM strategies. In 2020–21, the technology gap was reduced to 10.42. However, in 2021–22, the technology gap remained relatively stable at 10.50. Similarly, the extension gap was 9.17 during 2019–20, indicating the difference between the actual yield obtained by farmers implementing IPM strategies and the yield achieved under the demonstration. In 2020–21, the extension gap decreased to 8.33. However, in 2021–22, the extension gap increased to 14.82. The technology index was 27.45 in 2019–20, representing the extent farmers adopted and successfully implemented IPM strategies compared to the potential yield. In 2020–21, the technology index improved to 12.25. However, in 2021–22, the technology index slightly increased to 12.35.
Figure 8.
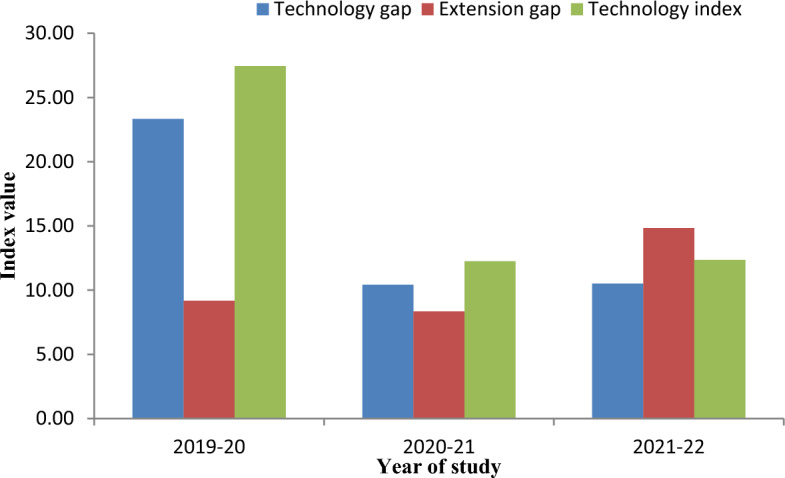
Technology gap, extension gap and technology index in adopting IPM strategies under demonstrated farmer’s fields. (Potential yield of maize = 85 q/ha in experimental area).
The data shows that implementing IPM practices improved yields, increased gross and net returns, and favorable benefit–cost ratios compared to the traditional Farmer's Practice (FP). These results highlight the economic advantages of employing effective FAW management strategies in maize cultivation. In 2019–20, the adoption of IPM for FAW management (D) resulted in a higher yield of 61.67 q/ha compared to the FP yield of 52.5 q/ha. This represents a yield increase of 9.17 q/ha, translating to a percentage increase of 17.49% over the FP. The net return in the D scenario was USD 919.00, which was higher than the FP's net return of USD 659.96 (Table 4). Moreover, the B:C ratio for the D scenario was 2.74, indicating a favorable return on investment compared to the FP's ratio of 2.16. Moving to 2020–21, the D scenario continued to show positive results. The yield increased to 74.58 q/ha, with a yield increase of 8.33 q/ha (12.62% increase over the FP). The B:C ratio for the D scenario stood at 2.39, indicating a profitable outcome compared to the FP's ratio of 1.94. The D scenario demonstrated even greater success in the most recent year, 2021–22. The yield increased significantly to 74.49 q/ha, resulting in a substantial yield increase of 14.82 q/ha (24.87% increase over the FP), with the B:C ratio for the D scenario was 2.33 compared to the FP's ratio of 1.77 (Table 4).
Table 4.
Economics of management of fall armyworm in maize during different growing years.
| Year | Yield (q/ha) | Yield increase over FP | % increase over FP | Gross returns (USD) | Cost of cultivation (USD) | Net return (USD) | B:C ratio | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FP | D | FP | D | FP | D | FP | D | FP | D | |||
| 2019–20 | 52.5 | 61.67 | 9.17 | 17.49 | 1232 | 1447.11 | 572.04 | 528.11 | 659.96 | 919 | 2.16 | 2.74 |
| 2020–21 | 66.25 | 74.58 | 8.33 | 12.62 | 1387.67 | 1562.23 | 715 | 656.01 | 672.67 | 906.20 | 1.94 | 2.39 |
| 2021–22 | 59.675 | 74.49 | 14.82 | 24.87 | 1395 | 1741.52 | 790.99 | 748.59 | 604.00 | 992.93 | 1.77 | 2.33 |
Exchange rate used for conversion of INR to USD in the respective years.
Discussion
The fall armyworm, S. frugiperda, is a devastating pest that threatened maize production within a year of its invasion in India and spread throughout the maize growing regions of India15. Owing to favorable climatic conditions and also a wide range of crops being grown in the Indian subcontinent, the impact of the FAW invasion in India is everlasting. The farmers have been grappling with the challenges posed by FAW infestation, which can lead to significant yield losses if not effectively managed. To manage this pest, Indian farmers have used various methods, including chemical insecticides. The data on farmer’s interviews highlights that most farmers (55%) prefer using pesticides as their primary approach for fall armyworm control. However, there is also some interest in alternative methods, such as pheromone traps and combining pesticides with cultural practices or biological agents. These findings underscore the need to promote sustainable pest management practices and provide farmers with information and resources to make informed decisions regarding FAW control methods.
Integrating IPM components resulted in the sustainable management of S. frugiperda. Installation of pheromone traps helps monitor and mass-trapping adults of fall armyworm20. The results of the present study shows that the pheromone trap catches varied between the different IPM fields, interestingly, the number of adults trapped decreased with each meteorological week. Further, the number of egg masses was also less in the IPM-adopted villages showing the efficacy of traps in bringing down both the adults and larval populations. Further, water-pan traps baited with different lures placed at 1.5 m and 2 m above the ground captured the highest number of moths21. They recommended it for decision-making regarding pest control. Cruz et al.21 found that the number of trap-caught moths was the best means of deciding on insecticide application in maize to control the fall armyworm. The reduction in egg masses seen in IPM fields is largely attributed to the amalgamation of compatible strategies. Notably, pheromone traps efficiently ensnare adult male moths, while the application of selective insecticides based on ETL effectively curtails future populations21. Moreover, the use of these selective insecticides poses minimal risk to natural enemies. Consequently, populations of natural enemies, particularly egg parasitoids, thrive, further diminishing egg masses. Remarkably, farmer training in the collection and destruction of egg masses significantly contributes to the decline of S. frugiperda in IPM-managed fields22. Moreover, the presence of bird perches and clean cultivation practices also played a role in reducing pest incidence within the IPM fields. FAW adults prefer to lay eggs in farmer practice fields despite the application of a cocktail of insecticides. It might be due to the farmers not adopting pheromone traps and differential egg placement i.e., if the FAW populations are high or if the crop foliage remains soft (as during the first nine weeks of crop establishment), moths will lay eggs higher up on the plant or on surrounding vegetation20. In such cases, even if insecticides are applied directly to the crop, they may not effectively reach these higher areas where egg deposition occurs.
Applying azadirachtin 1500 ppm decreased mean infestation from 34.87 to 18.06, 41.06–20.01 and 45.56 to 20.21% during the study period. Azadirachtin is a naturally occurring compound with insecticidal properties derived from the neem tree (Azadirachta indica)23. It is commonly used in organic farming as a biopesticide and found to be effective against a variety of insect pests, including the FAW that infests maize crops. Tiwari and Boppu24 discuss various bio-safe alternatives for controlling FAW and recommended application methods of azadirachtin against FAW in maize. King and Ataholo25 also evaluated the effectiveness of azadirachtin 1500 ppm. They reported high efficacy against FAW larvae and recommended to be used as components for integrated pest management (IPM) plans for FAW under smallholder farmer conditions.
In our study, the efficacy of M. anisopliae on S. frugiperda was found effective in reducing the damage percentage throughout the investigation period, i.e., from 35.50 to 18.68, 48.55 to 21.32 and 51.83 to 26.87%, respectively during the three consecutive years. Further, we observed a high level of incidence during November this is mainly due to the favorable weather conditions, which enables the rapid multiplication of M. anisopliae. Similar findings were reported by Kumar and Singh4 and Keerthi et al.26. Further, Sharma and Thakur27 explore the effectiveness of M. anisopliae against FAW and evaluate farmers' perception of this biopesticide. The study highlights the positive impact of using M. anisopliae on FAW control and the willingness of farmers to adopt this approach, emphasizing its potential as an eco-friendly and sustainable tool for FAW control. Fernandes et al.28 conducted field trials in Brazil, they found that a formulation of M. anisopliae applied alone or in combination with an insecticide effectively controlled FAW in maize. Elzen et al.29 investigated using M. anisopliae with a sex pheromone lure for fall armyworm control. They found that combining the fungal pathogen and the lure significantly reduced fall armyworm damage to maize plants. However, it is important to note that the actual effectiveness of this biological control agent can vary depending on various factors30, including climatic conditions31, application methods32 and population dynamics of the pest33.
In the present study, Emamectin benzoate was chosen purposefully as per the DPPQS made an ad-hoc recommendation and also, the farmers opined that it is giving a consistent result in reducing the incidence of FAW. Our results show that the incidence of FAW reduced from 28.52 to 10.56, 52.84 to 10.70 and 55.97 to 11.08% during the study period. The findings are in accordance with the study conducted by Wankhede et al.34 assessed the efficacy of different insecticides, including emamectin benzoate, and they found that Emamectin benzoate provided excellent control of the pest, with significantly higher grain yield compared to untreated control plots35,36. It is important to note that while emamectin benzoate is effective against fall armyworms, farmers need to follow recommended application rates and safety guidelines to minimize any potential negative impacts on the environment and non-target organisms37. The outcome of the farmer’s interview showed that the primary intention of applying insecticide is to achieve a higher yield. While synthetic insecticides play a role in pest management, relying solely on chemical control is unsustainable and can harm beneficial insects and ecosystems38. Initially, widespread use of synthetic pesticides was common in smallholder farming systems to combat FAW. However, farmers faced challenges with the effectiveness of these pesticides against FAW, leading to increased costs and risks associated with their intensive use39. To address these challenges, farmers need access to diversified integrated pest management strategies, improved extension services, and research-based recommendations tailored to their specific contexts. However, the results of the present study showed that adopting IPM resulted in maximum grain yield (61.67 q/ha) and monetary return (2.74 rupees/ rupee invested) compared to the sole application of insecticides. Reddy et al.40 reported that the benefit–cost ratio was significantly higher in the recommended approach (2.51) when compared to the farmer’s practice (2.12). The higher grain output and better market pricing of the produce may be the causes of the maize demonstration's higher net returns and B:C ratio37,40. The lower yield in the farmer’s field might be attributed to the use of insecticides, in which FAW known to developed resistance against most of them for instance cypermethrin16, lambda cyhalothrin17 and profenophos18,19. This is supported by the highest average number of egg masses and larvae per plant observed in the farmers practices examined in our study. The implementation of IPM techniques, such as mechanical removal and destruction of egg masses, trapping adult moths with pheromone traps, and employing self-sustaining entomopathogen alongside multi-modal azadirachtin, likely played a role in achieving the highest crop yields and net profits for the farmers20,24,33.
The technology index, as defined by Jeengar et al.41, serves as a measure of the suitability of advanced technology implementation in farmers' fields, with a lower score indicating higher feasibility. The collaboration of farmers in conducting such demonstrations, resulting in positive outcomes in subsequent years, is reflected in the fluctuating trend of the technology gap, ranging from 10.42 to 23.33. Variations in soil fertility, nutrient enrichment, organic manure application, and environmental factors such as rainfall and temperature, as noted by Dhandhalya and Shiyani42, likely contribute to observed discrepancies in the technology gap. Over the study period, there is a consistent upward trend in the extension gap, ranging from 9.17 to 14.32 q ha−1, highlighting the efforts of scientists to educate farmers on Integrated Pest Management (IPM) practices. Notably, a lower technological index value signifies greater practicality of technological implementation on farms. Throughout the experimental duration, the substantial fluctuation in the technological index, spanning from 12.25 to 27.4 percent, may be attributed to variances in soil fertility status and meteorological conditions.
Methods
Farmer’s interview and the study location
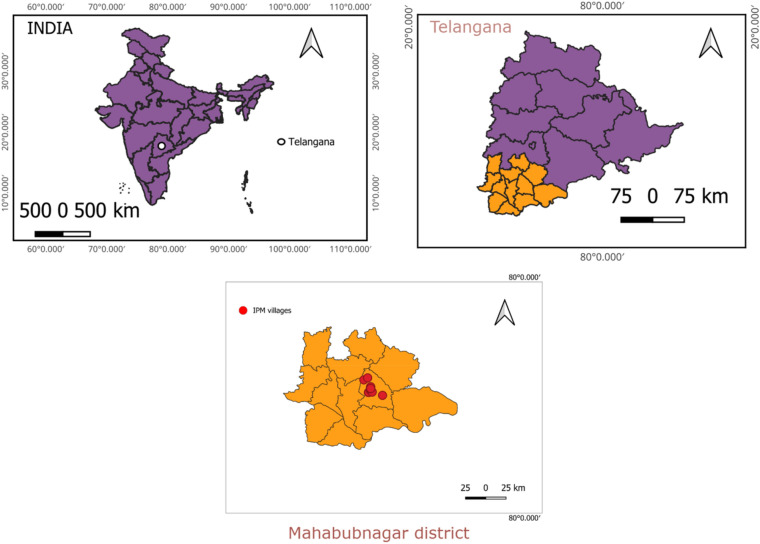
The farmers (n = 200) of study locations were interviewed (1-on-1) for their preference for pest management practices. The questionnaire used in the survey was provided in the supplementary material. We secured the necessary permissions for conducting the interview from our host institution, Professor Jayashankar Telangana State Agricultural University (PJTSAU), Telangana, India. The interviews were carried out in accordance with relevant guidelines and in alignment with the technical program approved by the institute research committee (IRC). Further, we also obtained informed consent from the farmers or their guardians before proceeding with the interviews. The selection of IPM components was determined by both the control methods preferred by farmers and the availability of biological inputs in the study area. The Krishi Vigyan Kendra (KVK), Palem, Nagarkurnool of PJTSAU, Telangana, India has conducted 18 On-farm Testing (OFTs) on “Assessment of IPM modules against invasive Fall armyworm, S. frugiperda in Maize”, under real irrigated farming situations during Rabi season for consecutive three years 2019–20, 2020–21 and 2021–22 at different adopted villages (n = 18) (Fig. 9). The GPS coordinate of the study locations provided with the figure. All 18 on-farm demonstrations were conducted in semi-arid areas of the southern Telangana zone of Telangana state, India. The main objective is to distribute and integrate the appropriate IPM knowledge to manage fall armyworm on maize effectively among all farmers.
Figure 9.
GIS maps depicting the study location and IPM practicing villages (Year 2019–20: IPM1—16.5078734, 78.231732; IPM2—16.5082880, 78.228844; IPM3—16.623484, 78.1588717; IPM4—16.6463967, 78.202408; IPM5—16.4793954, 78.210666; IPM6-n16.4460255, 78.372663. Year 2020–21: IPM1—16.480701596, 78.2149810; IPM2—16.48102895, 78.21498102, IPM3—16.48056361, 78.21398727, IPM4—16.54407863, 78.23688299, IPM5—16.54439100, 78.23664425, IPM6—16.54378814, 78.23687441. Year 2021–22: IPM1—16.5067690, 78.2334704; IPM2—16.5052449, 78.2334493; IPM3—16.4866143, 78.2544631; IPM4—16.4836707, 78.2544438; IPM5—16.5233138, 78.2327685; IPM6—16.5244808, 78.2370171.)
Treatment details
The farmer's meeting and diagnostic field visits to train the farmers were conducted during the crop period to create awareness of IPM modules to manage FAW effectively. Four IPM components were selected based on the farmers interview consisting of pheromone traps (8 traps with 40 lures Spodoptera frugiperda ® Pheromone chemicals, Hyderabad, Telangana, India), Azadirachtin 1500 ppm (Neem Gold ® Foliage Crop Solutions Private Limited, Chennai, Tamil Nadu, India) @ 1 L, Emamectin benzoate 5% SG (Proclaim ® Crystal Crop Protection Limited, Telangana, India) @ 100 g and Metarhizium anisopliae (® Biological control laboratory, PJTSAU, Rajendranagar, Hyderabad, Telangana, India) @ 1 kg/ acre. The chosen farmers received guidance and practical demonstrations on various topics, such as installing pheromone traps and applying plant protection chemicals at the appropriate times based on the ETL level of the pest (Table 5). The procedures, including the selection of sites and farmers, the establishment of demonstration layouts, and active farmer participation, were implemented in accordance with the methods given by Choudhary43. Each IPM module and farmer's practice covered an area of 0.4 hectares (equivalent to 1 acre), and a minimum of 3 villages with a total of 6 locations. The treatment plan comprises several key components: the deployment of pheromone traps starting from the early vegetative stage (when the crop is 7 days old) and continuing until harvest, with lure replacement every 21 days. Additionally, Azadiractin at 1500 ppm and Emamectin benzoate at 5% SG are applied when the pest population reaches the Economic Threshold Level (ETL). ETL is stage specific and the ETL thresholds for S. frugiperda are as follows: (a) 1–2 larvae per whorl, (b) 5% of seedlings being cut, (c) 15% of whorls infested in young plants within the first 30 days, and (d) Initiation of management practices if pheromone traps capture more than 2–3 adult moths for three consecutive days44.
Table 5.
Information on treatments.
| On Farm Testing/ IPM practices | Farmers practice |
|---|---|
| 1. Avoid staggered sowing of maize | 1. Staggered sowing of maize |
| 2. Installation of pheromone traps @ 8 per acre | 2. Not installing pheromone traps |
| 3. Clean cultivation balanced application of fertilizers | 3. No application of Azadiractin 1500 PPM at the early stage of the crop |
| 4. Erection of bird perches @10/acre | 4. Spray of following synthetic pesticides twice at weekly interval |
| 5. Mechanical/ manual collection and destruction of egg masses | 5. Initial spray with Profenophos 50% EC @400 ml/acre, cost is: 680/- |
| 6. Spraying of Azadiractin (1500 ppm) to deter and repel the egg laying of FAW | 6. 2nd spray is with Lambda cyhalothrin 5% EC@ 250 ml/acre, cost is: 590/- |
| 7. Trap catches > 3 adult moths for three consecutive days need based whorl application of Emamectin benzoate 5% SG @ 0.5 g/L of water | 7. 3rd spray with Chlorantraniliprole 18.5% SC @ 60 ml/acre, cost is: 920/- |
| 8. Spraying with bio fungicide Metarhizium anisopliae @ 5 g/L of water | 8. 4th spray with Cypermethrin 25% EC @ 100 ml/ acre, cost is: 490/- |
In order to ensure the effectiveness of bio-fungicide, Metarhizium anisopliae (5 g/L), farmers who have adopted this approach were advised to abstain from any other synthetic chemical spraying for at least two weeks when facing severe FAW infestations. This is because M. anisopliae has a self-propagating nature and exhibits effective dispersal abilities, making it a reliable choice in such circumstances. Furthermore, farmers underwent training sessions aimed at identifying egg masses. This involved meticulous inspection of maize plants, wherein they searched for egg masses on the leaves or stems. This inspection was conducted by walking in a "W" pattern across the field, leaving out 3–4 outer rows. As they proceeded along the first straight line, they selected groups of 10 plants and tallied the number of egg masses. This counting method continued in a “W” fashion, gathering data from at least 40 plants. Additionally, they were advised to examine damaged whorls and record the number of larvae present in infested whorls at 15-day intervals45. Both expressed in mean egg mass/ plants and mean larvae/ plants, respectively. In contrast, the farmers’ practice includes spraying synthetic pesticides (Table 5) twice weekly. For duration of three years, all essential inputs were consistently distributed to each farmer as part of a demonstration46. Observations on the incidence of FAW were made based on the number of whorl damage in 50 randomly selected maize plants47,48. The whorls were manually opened and inspected for FAW larvae. The infestation ratio was determined by counting infected whorls and then represented as a percentage. The total number of male adult moths in each trap was counted at weekly intervals and later presented as a mean number of moths trapped up to crop harvesting. To evaluate the bioefficacy of azadirachtin, emamectin benzoate and M. anisopliae, the number of FAW larvae in each 50 m2 area (minimum of 50 plants) was counted before and after the application of insecticides. FAW incidence before and after treatment of pesticides and some plant protection sprays was recorded in On Farm Testing (OFT)/ IPM practicing and non-practicing farmer's field plots49. The per cent reduction in pest population was calculated using
where Xi—number of larvae before insecticide application and Xo—number of larvae after insecticide application50. The two-sample t-test was performed to study the significant difference between the before and after application of respective biopesticides, and insecticides using an online statistical software WASP-Web Agri Stat Package 2.0 (https://ccari.icar.gov.in/wasp2.0/index.php).
Economics of IPM module
The economics of IPM module and farmers practice were worked and qualitative data were converted into quantitative form and expressed in terms of per cent increase in yield40. Finally, the extension gap, technology gap, technology index along with benefit cost ratio was worked out51,52 by using following formula:
Ethics approval
The collection of insects and insect data, as well as experimental research and field studies on integrated pest management (IPM) techniques were conducted in accordance with pertinent institutional, national, and international regulations. The required necessary permissions were obtained from the host institution, Professor Jayashankar Telangana State Agricultural University (PJTSAU), Telangana, India for conducting the interview.
Conclusion
Field demonstrations conducted over three consecutive years in semiarid regions of India revealed the efficacy of a four-component IPM approach in significantly reducing FAW infestations compared to conventional practices reliant solely on insecticide applications. Notably, IPM implementation led to substantial reductions in egg mass and larvae infestations, highlighting its effectiveness in curbing FAW populations at different growth stages. IPM consistently outperformed traditional Farmer's Practices (FP) in reducing egg masses and larvae per plant. Moreover, adopting IPM led to increased yields (9.17, 8.33 and 14.82% over farmers practice), gross and net returns, and favourable benefit–cost ratios (2.74, 2.39, and 2.33), reinforcing the economic benefits of strategic FAW management in maize cultivation. These findings underscore the significance of IPM in enhancing agricultural sustainability and profitability. For effective management of FAW in maize crops, government policies primarily revolve around implementing preventive measures, providing farmers with the necessary information, and supporting research and development efforts. Cooperative efforts between agricultural extension services and farmers can help build trust, provide ongoing support, and address any concerns or challenges farmers may face while implementing the IPM strategies against S. frugiperda in India.
Supplementary Information
Acknowledgements
Authors would like to express sincere gratitude to the Professor Jayashankar Telangana State Agricultural University (PJTSAU), Rajendranagar, Hyderabad for invaluable support in conduction of this research. Authors also appreciate the assistance of the ICAR- ATARI, ZONE-X, Hyderabad for technical guidance and constructive feedback throughout the experiment. We would like to express our sincere thanks to all the farmers who have supported us in planning and execution of experiments.
Author contributions
M.R. and K.M.C conceived the idea and designed methodology. M.R., B.R., T.P.R., K.V., V.S. and O.S. collected the field data. K.M.C. performed the statistical analyses. K.M.C and M.R. performed data analysis and interpreted the results. M.R., K.M.C., B.R., T.P.R., K.R., K.V., V.S., O.S., M.J.M.R., E.S., A.S., A.J. and S.S.R prepared the original draft of the manuscript. All authors contributed to the preparation of the final version of the manuscript and approved its submission for publication.
Data availability
All data have been provided in the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mandla Rajashekhar, Email: razshekarm@gmail.com.
Keerthi Manikyanahalli Chandrashekara, Email: keerthimanikya@gmail.com.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-57860-y.
References
- 1.Pratt CF, Constantine KL, Murphy ST. Economic impacts of invasive alien species on African smallholder livelihoods. Glob. Food Sec. 2017;14:31–37. doi: 10.1016/j.gfs.2017.01.011. [DOI] [Google Scholar]
- 2.Kenis M, Rozenberg MA, Roques A, Timms L, Péré C, Cock MJW, Settele J, Augustin S, Vaamonde CL. Ecological effects of invasive alien insects. Biol. Invasions. 2009;11:21–45. doi: 10.1007/s10530-008-9318-y. [DOI] [Google Scholar]
- 3.Fortuna TM, Gall LP, Mezdour S, Calatayud PA. Impact of invasive insects on native insect communities. Curr. Opin. Insect. Sci. 2022;51:100904. doi: 10.1016/j.cois.2022.100904. [DOI] [PubMed] [Google Scholar]
- 4.Kumar P, Singh JS. Invasive alien plant species: Their impact on environment, ecosystem services and human health. Ecol. Indic. 2020;111:106020. doi: 10.1016/j.ecolind.2019.106020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sparks AN. A review of the biology of the fall armyworm. FL. Entomol. 1979;62:276–283. doi: 10.2307/3494083. [DOI] [Google Scholar]
- 6.Goergen G, Kumar PL, Sankung SB, Togola A, Tamò M. First report of outbreaks of the fall armyworm Spodoptera frugiperda (J E Smith) (Lepidoptera, Noctuidae), a new alien invasive pest in West and Central Africa. PLoS ONE. 2016;11:1–9. doi: 10.1371/journal.pone.0165632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stokstad E. New crop pest takes Africa at lightning speed. Science. 2017;356:473–474. doi: 10.1126/science.356.6337.473. [DOI] [PubMed] [Google Scholar]
- 8.Keerthi MC, Sravika A, Mahesha HS, Gupta A, Bhargavi HA, Ahmed S. Performance of the native predatory bug, Eocanthecona furcellata (Wolff) (Hemiptera: Pentatomidae), on the fall armyworm, Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae), and its limitation under field condition. Egypt. J. Biol. Pest Control. 2020;30(1):1–4. doi: 10.1186/s41938-020-00272-7. [DOI] [Google Scholar]
- 9.Guo J, Zhao J, He K, Zhang F, Wang Z. Potential invasion of the crop devastating insect pest fall armyworm Spodoptera frugiperda to China. Plant Prot. 2018;44:1–10. [Google Scholar]
- 10.Ullah NU, Ansari MA, Iqbal N, Saeed S. First authentic report of Spodoptera frugiperda (JE Smith) (Noctuidae: Lepidoptera) an alien invasive species from Pakistan. Appl. Sci. Bus. Econ. 2019;6:1–3. [Google Scholar]
- 11.Dumas P, Legeai F, Lemaitre C, Scaon E, Orsucci M, Labadie K, Gimenez S, Clamens AL, Henri H, Vavre F. Spodoptera frugiperda (Lepidoptera: Noctuidae) host-plant variants: Two host strains or two distinct species? Genetica. 2015;143:305–316. doi: 10.1007/s10709-015-9829-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Montezano D, Specht A, G’omez SD, Specht RV, Silva SJ, Moraes PS, Peterson J, Hunt T. Host plants of Spodoptera frugiperda (Lepidoptera: Noctuidae) in the Americas. Afr. Entomol. 2018;26:286–300. doi: 10.4001/003.026.0286. [DOI] [Google Scholar]
- 13.Hailu G, Niassy S, Zeyaur KR, Ochatum N, Subramanian S. Maize–legume intercropping and push–pull for management of fall armyworm, stem borers, and striga in Uganda. Agron. J. 2018;110:2513–2522. doi: 10.2134/agronj2018.02.0110. [DOI] [Google Scholar]
- 14.Hendrichs J, Pereira R, Vreysen MJ. Area-Wide Integrated Pest Management: Development and Field Application. Taylor & Francis; 2021. p. 1028. [Google Scholar]
- 15.Rwomushana, I. Spodoptera frugiperda (fall armyworm). CABI Compendium, CABI Compendium (2022). 10.1079/cabicompendium.29810
- 16.Samanta S, Barman M, Thakur H, Chakraborty S, Upadhyaya G, Roy D, Tarafdar J. Evidence of population expansion and insecticide resistance mechanism in invasive fall armyworm (Spodoptera frugiperda) BMC Biotechnol. 2023;23(1):17. doi: 10.1186/s12896-023-00786-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodriguez GID, Omoto C. Inheritance of lambda-cyhalothrin resistance in Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae) Neotrop. Entomol. 2001;30:311–316. doi: 10.1590/S1519-566X2001000200016. [DOI] [Google Scholar]
- 18.Carvalho RA, Omoto C, Field LM, Williamson M, Bass C. Investigating the molecular mechanisms of organophosphate and pyrethroid resistance in the fall armyworm, Spodoptera frugiperda. PLoS ONE. 2013;8:e62268. doi: 10.1371/journal.pone.0062268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sisay B, Sevgan S, Weldon CW, Kruger K, Torto B, Tamiru A. Responses of the fall armyworm (Spodoptera frugiperda) to different host plants: Implications for its management strategy. Pest Manag. Sci. 2022;79:845–856. doi: 10.1002/ps.7255. [DOI] [PubMed] [Google Scholar]
- 20.Sisay BA, Subramanian DS, Weldon CW, Krüger K, Khamis F, Tefera T, Torto B, Tamiru A. Evaluation of pheromone lures, trap designs and placement heights for monitoring the fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae) in maize fields of Kenya. Pre print. 2023;8:1–30. [Google Scholar]
- 21.Cruz I, Figueiredo MDLC, Silva RBD, Silva IFD, Paula CD, Foster JE. Using sex pheromone traps in the decision-making process for pesticide application against fall armyworm (Spodoptera frugiperda [Smith] [Lepidoptera: Noctuidae]) larvae in maize. Int. J. Pest Manag. 2012;58:83–90. doi: 10.1080/09670874.2012.655702. [DOI] [Google Scholar]
- 22.Niassy S, Agbodzavu MK, Kimathi E, Mutune B, Abdel-Rahman EFM, Salifu D, Subramanian S. Bio-ecology of fall armyworm Spodoptera frugiperda (JE Smith), its management and potential patterns of seasonal spread in Africa. PloS One. 2021;16(6):e0249042. doi: 10.1371/journal.pone.0249042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kilani-Morakchi S, Morakchi-Goudjil H, Sifi K. Azadirachtin-based insecticide: Overview, risk assessments, and future directions. Front. Agron. 2021;3:676208. doi: 10.3389/fagro.2021.676208. [DOI] [Google Scholar]
- 24.Tiwari S, Boppu S. Bio-safe alternatives against fall armyworm (Spodoptera frugiperda) infesting maize crop: A review. J. Pharmacogn. Phytochem. 2020;9:692–697. [Google Scholar]
- 25.King ABS, Ataholo MO. Comparative efficacy of synthetic insecticides and neem seed kernel extracts against the infestation of maize (Zea mays L.) by fall armyworm (Spodoptera frugiperda J.E. Smith) Ethiop. J. Agric. Sci. 2020;29:111–125. [Google Scholar]
- 26.Keerthi MC, Suroshe SS, Doddachowdappa S, Shivakumara KT, Mahesha HS, Rana VS, Gupta A, Murukesan A, Casini R, Elansary HO, Shakil NA. Bio-intensive tactics for the management of invasive fall armyworm for organic maize production. Plants. 2023;12(3):685. doi: 10.3390/plants12030685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharma L, Thakur N. Effect of Metarhizium anisopliae on the control of fall armyworm (Spodoptera frugiperda) in maize cultivation and farmers' perception. Indian J. Agric. Sci. 2019;89:11–15. [Google Scholar]
- 28.Fernandes ÉK, Rangel DE, Braga GU, Roberts DW, Bittencourt VR. Combined use of the entomopathogenic fungus Metarhizium anisopliae with rates of the insecticide chlorantraniliprole for control of the fall armyworm, Spodoptera frugiperda. Biol. Control. 2018;116:23–29. [Google Scholar]
- 29.Elzen GW, Lacey LA, Lacey CM. Plant-mediated effects on clonization and establishment of the entomopathogenic fungus, Metarhizium anisopliae, in Corn (Zea mays) root system. Biol. Control. 2014;71:34–44. [Google Scholar]
- 30.Souza CSF, Silveira LCP, Souza BHS, Nascimento PT, Damasceno NCR, Mendes SM. Efficiency of biological control for fall armyworm resistant to the protein Cry1F. Braz. J. Biol. 2020;81:154–163. doi: 10.1590/1519-6984.224774. [DOI] [PubMed] [Google Scholar]
- 31.Baudron F, Zaman-Allah MA, Chaipa I, Chari N, Chinwada P. Understanding the factors influencing fall armyworm (Spodoptera frugiperda JE Smith) damage in African smallholder maize fields and quantifying its impact on yield. A case study in Eastern Zimbabwe. Crop. Prot. 2019;120:141–150. doi: 10.1016/j.cropro.2019.01.028. [DOI] [Google Scholar]
- 32.Abbas A, Ullah F, Hafeez M, Han X, Dara MZN, Gul H, Zhao CR. Biological control of fall armyworm, Spodoptera frugiperda. Agronomy. 2022;12:2704–2714. doi: 10.3390/agronomy12112704. [DOI] [Google Scholar]
- 33.Tavares CJ, Pazini WC, Vargas BLR, Schrank A, Vainstein MH. Biocontrol potential of different formulations and concentrations of Metarhizium anisopliae against fall armyworm larvae. J. Pest Sci. 2019;92:1067–1079. [Google Scholar]
- 34.Wankhede G, et al. Management of fall armyworm, Spodoptera frugiperda (J. E. Smith) through bio-pesticides. Int. J. Chem. Stud. 2019;7:2462–2465. [Google Scholar]
- 35.Rakate B, Bhale U. Evaluation of different insecticides against fall armyworm (Spodoptera frugiperda J.E. Smith) on maize. J. Entomol. Zool. Stud. 2018;7:1689–1693. [Google Scholar]
- 36.Ghimire MN, Mulenga RM, Misinzo G, Kehoe M, Wang Q. Potential efficacy of selected insecticides against fall armyworm (Spodoptera frugiperda) inmaize A. Rev. Plants. 2020;9:629. [Google Scholar]
- 37.Huang X, Chen W, Yu Z, Chen C, Qi H, Hu Q. Efficacy evaluation and resistance risk assessments of emamectin benzoate against Spodoptera frugiperda and Helicoverpa armigera. Pestic. Biochem. Physiol. 2019;161:83–90. [Google Scholar]
- 38.Pathak VM, Verma VK, Sharma A, Dewali S, Kumari R, Mohapatra A, Cunill JM. Current status of pesticide effects on environment, human health and it’s eco-friendly management as bioremediation: A comprehensive review. Front. Microbiol. 2022;13:962619. doi: 10.3389/fmicb.2022.962619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tambo JA, Kansiime MK, Mugambi I, Rwomushana I, Kenis M, Day RK, Lamontagne-Godwin J. Understanding smallholders' responses to fall armyworm (Spodoptera frugiperda) invasion: Evidence from five African countries. Sci. Total Environ. 2020;740:140015. doi: 10.1016/j.scitotenv.2020.140015. [DOI] [PubMed] [Google Scholar]
- 40.Reddy BKK, Jyothi VS, Sadhineni M, Johnson M, Swamy GN. Evaluation of IPM modules against fall armyworm in maize through frontline demonstration and its economic impact. Int. J. Environ. Clim. 2023;13:24–29. [Google Scholar]
- 41.Jeengar KL, Panwar P, Pareek OP. Front line demonstration on maize in Bhilwara District of Rajasthan. Curr. Agric. 2006;30(1/2):115–116. [Google Scholar]
- 42.Dhandhalya MG, Shiyani RL. Production potentials, yield gaps and research prioritization of production constraints in major oilseed crops of Saurashtra region. Indian J. Agric. Res. 2009;43(1):18–25. [Google Scholar]
- 43.Choudhary BN. Krishi Vigyan Kendra—A Guide for KVK Managers. Publication, Division of Agricultural Extension, ICAR; 1999. pp. 73–78. [Google Scholar]
- 44.Kushwaha UKS. A cost-efficient and alternative technique of managing fall armyworm, Spodoptera frugiperda (JE Smith) larvae in maize crop. Sci. Rep. 2022;12:6741. doi: 10.1038/s41598-022-10982-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singh S, Raghuraman M, Keerthi MC, Das A, Kar SK, Das B, Acharya GC. Occurrence, distribution, damage potential, and farmers’ perception on fall armyworm, Spodoptera frugiperda (JE Smith): Evidence from the eastern Himalayan region. Sustainability. 2023;15(7):5681. doi: 10.3390/su15075681. [DOI] [Google Scholar]
- 46.Bateman ML, Day RK, Luke B, Edgington S, Kuhlmann U, Cock MJ. Assessment of potential biopesticide options for managing fall armyworm (Spodoptera frugiperda) in Africa. J. Appl. Entomol. 2018;142:805–819. doi: 10.1111/jen.12565. [DOI] [Google Scholar]
- 47.Rajashekhar M, Rajashekar B, Reddy TP, Ramakrishna K, Shankar AJA, Reddy MJM. IPM: An ecofriendly and low cost technology in arresting pest complex for higher net returns in cotton. J. Entomol. Zool. Stud. 2020;8:1726–1728. [Google Scholar]
- 48.Keerthi MC, Mahesha HS, Manjunatha N, Gupta A, Saini RP, Shivakumara KT, Bhargavi HA, Gupta G, Kulkarni NS. Biology and oviposition preference of fall armyworm, Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae) on fodder crops and its natural enemies from Central India. Int. J. Pest Manag. 2021;6:1–10. [Google Scholar]
- 49.Rajashekhar M, Reddy TP, Keerthi MC, Rajashekar B, Reddy MJR, Ramakrishna K, Satyanarayana E, Shankar A, Jahan A, Kumar P, Shravika L. Evaluation of integrated pest management module for Pink bollworm, Pectinophora gossypiella (Saunders) and its economic analysis under farmer’s field conditions. Int. J. Pest Manag. 2022;68:1–10. doi: 10.1080/09670874.2022.2096269. [DOI] [Google Scholar]
- 50.Ramadevi A, Kumar YP, Charan GS, Raghuveer M, Kumar MS, Poshadri A, Reddy RU. Impact of extension activities on pink bollworm management in Bt-cotton in tribal areas of Adilabad district. J. Entomol. Zool. Stud. 2020;8:1683–1687. [Google Scholar]
- 51.Narasimha RS, Satish P, Samuel G. Productivity improvement in soybean (Glycine max L. Merrill) through technological interventions. J. Oilseeds Res. 2007;24:271–273. [Google Scholar]
- 52.Samui SK, Maitra S, Roy DK, Mondal AK, Saha D. Evaluation on front line demonstration on groundnut (Arachis hypogea L.) J. Indian. Soc. Coast. Agric. Res. 2000;18:180–183. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data have been provided in the manuscript.