Abstract
Aims
Heart failure (HF) is a chronic disease affecting 64 million people worldwide and places a severe burden on society because of its mortality, numerous re‐hospitalizations and associated costs. HeartLogic™ is an algorithm programmed into implanted devices incorporating several biometric parameters which aims to predict HF episodes. It provides an index which can be monitored remotely, allowing pre‐emptive treatment of congestion to prevent acute decompensation. We aim to assess the impact and security of pre‐emptive HF management, guided by the HeartLogic™ index.
Methods and results
The HeartLogic™ France Cohort Study is an investigator‐initiated, prospective, multi‐centre, non‐randomized study. Three hundred ten patients with a history of HF (left ventricular ejection fraction ≤40%; or at least one episode of clinical HF with elevated NT‐proBNP ≥450 ng/L) and implanted with a cardioverter defibrillator enabling HeartLogic™ index calculation will be included across 10 French centres. The HeartLogic™ index will be monitored remotely for 12 months and in the event of a HeartLogic™ index ≥16, the local investigator will contact the patient for assessment and adjust HF treatment as necessary. The primary endpoint is unscheduled hospitalization for HF. Secondary endpoints are all‐cause mortality, cardiovascular death, HF‐related death, unscheduled hospitalizations for ventricular or atrial arrhythmia and HeartLogic™ index evolution over time. Blood samples will be collected for biobanking, and quality of life will be assessed. Finally, the safety of a HeartLogic™‐triggered strategy for initiating or increasing diuretic therapy will be assessed. A blind and independent committee will adjudicate the events.
Conclusions
The HeartLogic™ France Cohort Study will provide robust real‐world data in a cohort of HF patients managed with the HeartLogic™ algorithm allowing pre‐emptive treatment of heart failure exacerbations.
Keywords: Connected devices, Heart failure, Hospitalization, Mortality, Pre‐emptive action, Prediction, Remote monitoring
Introduction
Heart failure (HF) affects >50 million people worldwide and is associated with significant morbidity 1 and a mortality in excess of 40% 5 years after diagnosis. 2 HF is associated with a high readmission rate, which is estimated to be 25% within the first 30 days after diagnosis, 3 accounts for 1 to 2% of all hospitalizations and is the major economic burden related to HF. 4
Remote monitoring of patients with HF is a promising development which may enable treatment of subclinical HF decompensation to prevent overt clinical deterioration and rehospitalization. HeartLogic™ is an algorithm currently only available on Boston Scientific implantable cardioverter defibrillators designed to predict acute episodes of HF in advance which can be monitored remotely. It uses various biometric data (nocturnal heart rate, S1 and S3 heart sounds, respiratory rate, relative tidal volume, activity level and chest impedance) to compute an index. In the event of a rise in HeartLogic™ index, ambulatory therapeutic adjustments can be implemented, potentially avoiding an unscheduled hospitalization for HF. Initial experience with this algorithm has been evaluated in the Multisense study, which found that HeartLogic™ index ≥16 predicted acute episodes of HF an average of 34 days in advance with sensitivity of 70% and specificity of 85.7%. 5 Other studies have assessed the HeartLogic™ algorithm, but were carried out without the goal of pre‐emptive therapeutic adjustment to reduce HF events in case of a HeartLogic™ alert. 6 , 7 , 8 , 9
The aim of the HeartLogic™ France Cohort Study is to provide real‐world data on the impact and security of pre‐emptive HF management, guided by the HeartLogic™ index.
Study design
Design
The HeartLogic™ France Cohort Study is a prospective single‐arm observational study with the main goal of assessing the annual rate of unscheduled hospitalizations for HF in a cohort of patients with HF, managed with the HeartLogic™ algorithm. The study design is represented in Figure 1 . The study was approved by the Medical Ethics Committee ‘Centre de Protection des Personnes Sud Mediterranée IV’ under the registration ID CRB 20202‐A01835‐34. The study is conducted in accordance with the Declaration of Helsinki and registered at ClinicalTrials.gov with ID NCT04619888.
Figure 1.
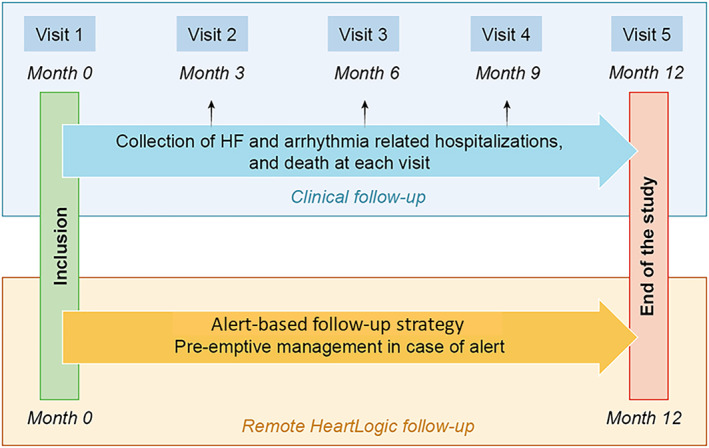
Design of the study. Clinical follow‐up will be scheduled every 3 months to collect clinical events. Remote HeartLogic™ index follow‐up is alert‐based.
Inclusion criteria
Patients aged ≥18 years old;
Patients implanted with a cardiac defibrillator, with or without resynchronization, utilizing the HeartLogic™ algorithm (Resonate device family, Boston Scientific);
History of HF (left ventricular ejection fraction ≤40%; or at least one episode of clinical HF with NT‐pro brain natriuretic peptide (NT‐proBNP) blood concentration ≥450 ng/L);
Patient without guardianship, conservatorship, or subordination;
Patients who benefit from a public health insurance or who benefit from it through a third party;
Patients having signed their free and informed consent.
Exclusion criteria
Patients with a concomitant HF device other than cardiac resynchronization, such as a ventricular assist device or cardiac contractility modulation device;
Planned heart transplant, or patients with a heart transplant;
Glomerular filtration rate <30 mL/min/m2 or dialysis;
Life expectancy ≤6 months;
Remote monitoring of HeartLogic™ not possible;
Non‐compliance with HF medications;
Patient with a mechanical heart valve;
Pregnant or lactating women, women of childbearing age without effective contraception;
Patients with reinforced protection, namely: minors, persons deprived of their liberty by a judicial or administrative decision, persons staying in a health or social institution, adults under legal protection.
Study protocol
At inclusion
Patients will be enrolled in the HeartLogic™ France Cohort Study during Visit 1 within the month following cardioverter defibrillator implantation. Demographic data, medical history such as cardiac aetiology, type of defibrillator implanted (single‐, dual‐chamber, and resynchronization) and drug treatment will be collected. Baseline investigations will consist of an electrocardiogram, and echocardiogram to assess left ventricular ejection fraction and dimensions, atrial volume, and diastolic function. New York Heart Association (NYHA) class will be assessed. Baseline blood results will be collected, including haemoglobin, creatinine, and NT‐proBNP levels. In addition, a biobank, consisting in a collection of 21 mL of blood samples will be carried out and will be made available for further assays. Finally, the Kansas City Cardiomyopathy Questionnaire will be administered to participants. 10
Clinical follow‐up
After inclusion, patients will be followed at 3, 6, 9, and 12 months for visits 2, 3, 4, and 5 respectively (Figure 1 : Clinical Follow‐Up). Unscheduled hospitalization for HF, atrial and ventricular arrhythmia as well as medication history will be collected at each visit. In addition to clinical data, NT‐proBNP level will be assessed at visits 3 and 5, and left ventricular ejection fraction will be collected at visit 5. Visits 2 and 4 can be done remotely. The study will conclude at visit 5 after 12 months of follow‐up.
Remote follow‐up
For each patient, the HeartLogic index will be monitored remotely on the Latitude platform. According to previous guidelines and previous publication, the follow up strategy will be alert‐based (Figure 1 : Remote HeartLogic™ Follow‐Up). 8 , 11
An agreed uniform protocol has been established to avoid heterogeneous management of HeartLogic™ alerts across centres and to foster pre‐emptive treatment of congestion (Figure 2 ). Briefly, in case of index value ≥16, the investigator will contact the patient to assess the patient's clinical status within 1 week. A clinical evaluation will be undertaken, assessing dyspnoea, peripheral oedema, fatigue and orthopnoea. Moreover, a cause for false alert, such as an active infection, will be sought. If this not the case, a trigger for HF deterioration, such as arrhythmia or a chest pain evoking a coronary syndrome will be ruled out. If there is no obvious reason for a false alert, diuretics will be increased by 50% to 100% during 3 to 5 days, even in asymptomatic patients. Re‐evaluation of clinical status, the HeartLogic™ index and renal function is recommended after 3 to 5 days of diuretic increase. In case of alert, the reason for non‐modification or the details of the treatment adjustment will be collected.
Figure 2.
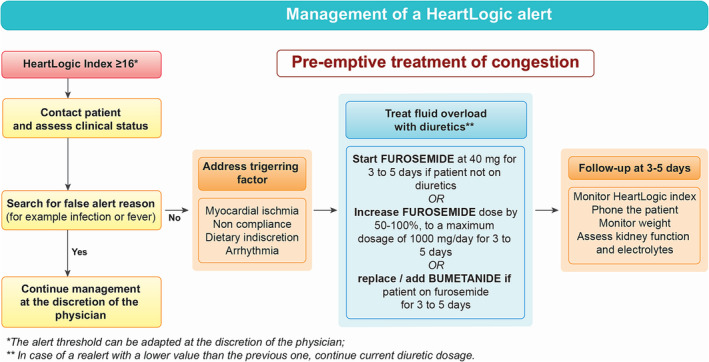
Management of a HeartLogic™ Alert. In case of HeartLogic™ ≥ 16, the patient will be contacted, and false positive alerts will be evaluated and discarded. In the absence of false alert, an increase of diuretic dosage is recommended for 3 to 5 days.
Study endpoints
Primary endpoint
The primary endpoint is the occurrence of an unscheduled hospitalization for ≥1 day due to HF decompensation, defined by the presence of HF signs or symptoms with evidence of HF on diagnostic evaluation on at least one test (elevated natriuretic peptides, radiological, or echocardiographic evidence) requiring an increase in diuretic treatment, whether intravenous or oral. 12 Scheduled hospitalization for routine follow‐up, pre‐transplant assessment, or iron injection is not considered as an unscheduled hospitalization.
Secondary endpoints
The secondary endpoints are
All causes of death;
Death from cardiovascular causes defined as sudden unexpected death, HF‐related death, myocardial infarction‐related death, and other cardiovascular deaths (including pulmonary embolism, peripheral thromboembolism, stroke, deaths related to a vascular procedure, or major cardiovascular event not otherwise specified). Sudden unexpected death includes observed arrhythmic deaths and sudden deaths not attributable to acute myocardial infarction or any other identifiable cause;
Death from HF, defined as patients with intractable HF, even if the terminal event was arrhythmia;
Unplanned hospitalizations for ventricular arrhythmia (treated or not by the implantable cardiac defibrillator);
Unplanned hospitalizations for atrial arrhythmia;
Number of days of hospitalization for HF, ventricular or atrial arrhythmia;
Patient quality of life using the Kansas City Cardiomyopathy Questionnaire;
Weekly‐averaged HeartLogic™ index over a 12‐month period.
Safety endpoint combining a creatinine level increase ≥30%, a potassium level change ≥30%, a sodium level decrease ≥30% between the HeartLogic™ alert and the end of the diuretic treatment, or the occurrence of symptomatic hypotension related to the diuretic treatment initiation or increase.
The primary endpoint and the secondary endpoints (death‐ and hospitalization ‐related) will be collected at least once every 3 months by consulting the patient's hospital medical record, or by telephone contact with the patient or their attending physician. Weekly averaged HeartLogic™ index will be collected once a week by the team in charge of the HeartLogic™ remote follow‐up.
Quality of life
To describe quality of life impairment, the longer version (23 items) of The Kansas City Cardiomyopathy Questionnaire will be administered at baseline and at the end of follow‐up. Scores are represented on a 0‐to‐100‐point scale where lower scores represent more severe symptoms and/or limitations and scores of 100 indicate no symptoms, no limitations, and excellent quality of life. Changes of 5, 10, and 15 points are considered as small, moderate, and large clinical changes. 10
Data management and study organization
Outcomes will be adjudicated in a blinded fashion according to pre‐specified definitions by two independent cardiologists who are members of the clinical events committee. Relevant information will be collected and supporting documentation will be sent to the two clinical events committee members. The supporting documentation will consist of copies of case record forms, autopsy reports, death certificates, hospital notes, electrocardiogram recordings and device electrogram tracings. If the two clinical events committee members disagree about the mode of death, they will be requested to obtain final agreement.
Data management and statistical analyses will be performed by the research data coordinating centre at the University hospital of Poitiers, Poitiers, France.
The HeartLogic™ France Cohort Study included the first patient on March 15th, 2021, and the enrollment phase has been terminated in July 2023. As of now, 10 centres, composed of University hospitals (n = 7), tertiary centres (n = 1), and private clinics (n = 2), are participating.
Statistical considerations
From the Paradigm and Castle‐AF study, we can assume an incidence of rehospitalization for HF of 10% for 1 year of follow‐up. 13 , 14 With absolute precision of 3.5% and first order risk of 5%, inclusion of 282 patients is required to provide sufficient statistical power for the study. Considering a 10% loss of follow‐up, we planned to include a total of 310 patients.
For the probability of first unscheduled rehospitalization for HF and first unscheduled rehospitalization for ventricular/atrial arrhythmia, cumulative incidence curves will be constructed. The 1‐year probabilities of first rehospitalization for HF, first rehospitalization for atrial arrhythmia and first rehospitalization for ventricular arrhythmia will be expressed as annual incidence and 95% confidence interval.
One‐year all‐cause, cardiovascular, and HF‐related mortality rates will be expressed as a percentage. All causes of death, cardiovascular, and HF‐related death will be assessed using Kaplan–Meier survival curves. Prespecified subgroup analyses on outcomes will include (1) Reduced ejection fraction HF versus preserved ejection fraction HF. (2) Resynchronization therapy versus non‐resynchronization therapy. (3) Permanent atrial fibrillation versus non‐permanent atrial fibrillation or no atrial fibrillation. (4) Males versus females. (5) Age ≥75 versus age <75 years. Subgroups will be compared using chi‐square test for re‐hospitalization for HF, ventricular and atrial arrhythmias and using the log‐rank test for all‐cause of death and cardiovascular death. To test the effect of the HeartLogic™ algorithm implementation on quality of life, groups with The Kansas City Cardiomyopathy Questionnaire score change <5, 5 to 9 and ≥10 will be compared using chi‐square test.
All patients included in the study will be included in the data analysis if they have not withdrawn consent. No interim analysis is planned. Missing data will be described but no imputation will be performed.
Discussion
This study presents a unique opportunity to assess the HeartLogic™ algorithm with pre‐emptive treatment of congestion in a real‐world and contemporary HF cohort, in patients with reduced and preserved ejection fraction.
Data from the Paradigm Heart Failure group demonstrated a rehospitalization rate for HF between 7% and 15% and cardiovascular mortality rate between 6 and 7% at 1 year. 13 The HeartLogic™ France Cohort Study project will assess those outcomes from real world usage in patients treated with contemporaneous guideline‐directed pharmacological therapy including sodium glucose co‐transporter 2 inhibitors and angiotensin receptor‐neprilysin inhibitors. 15
HF‐related hospitalizations are mainly caused by increasing signs and symptoms of congestion. 16 Even though congestion often develops over an extended period of time before acute presentation, in the absence of symptoms at time of HeartLogic™ alert some physicians may not alter the patients management. Indeed, till now, diuretics are indicated in patients with signs and/or symptoms of congestion and their use can be associated with worsening renal function and electrolyte disturbances. 15 We propose an algorithm (Figure 2 ) for HeartLogic™ alert management, which aims to be practical, and allows uniform management of patients across centres. This study is the first to implement a standardized protocol for diuretic therapy's modifications in response to HeartLogic™ alerts. Indeed, in previous studies the diuretic dosing and timing of the intervention in patients with device alerts was not performed in a predetermined manner but only according to each centre clinical practice. 17 , 18 , 19
The implementation of an algorithm intended to avoid HF‐related hospitalization based on pre‐emptive increase of diuretics is novel, and if its usefulness is confirmed, may change current paradigm in HF management. The concept of Near‐Term Prevention, that emerged a few years ago, 20 is now becoming increasingly important in the field of sudden cardiac death management with the exponential use of connected devices collecting biometric data and the use of artificial intelligence. 21 , 22 The general functioning of HeartLogic™ remote monitoring is summarized in Figure 3 . Implementing pre‐emptive treatment of HF‐based short‐term prediction of events is not yet advocated in current international guidelines, even though it may significantly improve the prognosis of patients with HF and lead to high‐quality outpatient care (Figure 4 ). In addition, reducing the hospitalization rate will significantly reduce the economic burden on secondary care.
Figure 3.
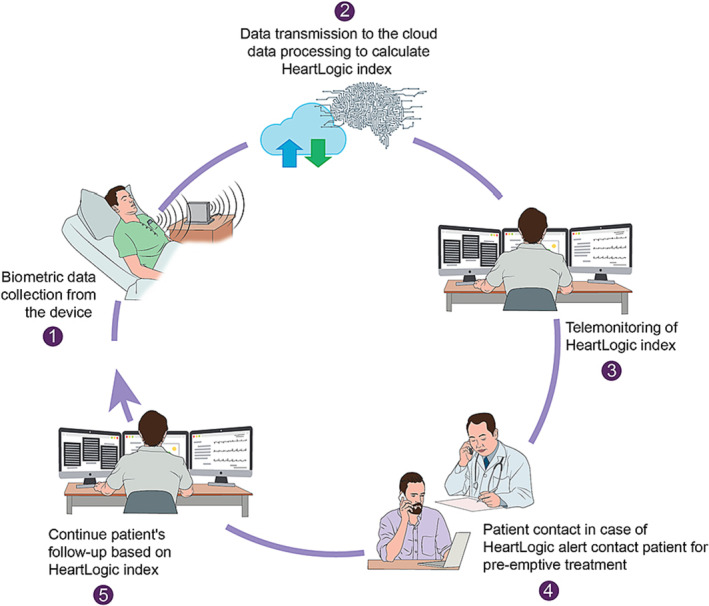
HeartLogic™ Remote Monitoring. Biometric data are collected by the device (1) and transmitted to the Cloud to compute the HeartLogic™ index (2). The HeartLogic™ index is monitored by local teams (3) and in case of a true alert, the patient is contacted and treatment initiated, even in absence of symptoms (4). The patient will then be followed‐up remotely using the HeartLogic™ index.
Figure 4.
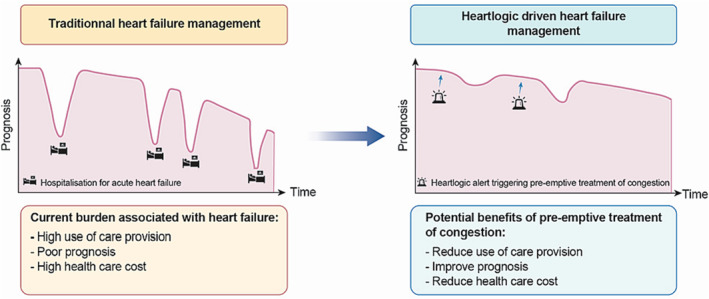
Central figure. Potential benefits of pre‐emptive treatment of congestion.
Several limitations might be discussed. Firstly, alerts can lead to a significant workload for the remote monitoring team. Capucci et al. found a HeartLogic™ alert rate of 0.99 alerts/patient per year. 6 Nevertheless, the economic impact of this increase in workload may be offset if the HeartLogic™ based strategy allows significant reductions in the number of hospitalizations and improves patient's prognosis. Patients with glomerular filtration rate <30 mL/min/m2 or dialysis were excluded from the study because thoracic impedance and repiratory rate may render the HeartLogic™ index inaccurate due to chronic fluid overload in those patients. Similarly, patients with mechanical valves were excluded because mechanical S3 heart sounds may also mislead the HeartLogic™ algorithm. 6 , 19 Moreover, only arrhythmia triggering hospitalizations will be collected to avoid an important burden of non‐specific episodes and to catch only those that are significant. Finally, the design of this study did not comprise a control group without HeartLogic™ management, and as such, the ascertainment of HF‐related hospitalization reduction due to HeartLogic™ implementation will not be possible. Further research, using a randomized controlled trial, need to investigate whether such pre‐emptive HF management, guided by the HeartLogic™ index can reduce heart‐failure related hospitalization.
Conclusions
HeartLogic™ algorithm offers the possibility to implement pre‐emptive treatment of congestion in HF treatment strategy. The HeartLogic™ France Cohort Study is a prospective multi‐centre, study aiming at assessing the impact and safety of pre‐emptive HF management, guided by the HeartLogic™ index.
Funding
This work was supported by Boston Scientific through an Investigator‐Sponsored Research Program. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the manuscript, and its final contents.
Conflict of interest
R Garcia received lecture fees and research grants from Boston Scientific. S Boveda is consultant for Medtronic, Boston Scientific, Microport, and Zoll. A Bisson received lecture fees and served as a consultant for Medtronic. J Mansourati received research and expertise fees from Abbott, Biotronik, Boston Scientific, Medtronic and Microport. P Jacon consultant for Boston Scientific, Medtronic, Microport and Abott. E Marijon is consultant for Boston Scientific. B Pierre served as a consultant for Abbott, Boston Scientific, Biotronik, Microport. D Gras received consultancy fees from Boston Scientific, Abbott, Biotronik, Zoll. A Li received moderate unrestricted research grant and travel grants from Abbott and speaker fees from Baylis Medical.
Acknowledgements
We thank Céline Delétage and Oualid Ayad for coordinating the study.
Garcia, R. , Gras, D. , Mansourati, J. , Defaye, P. , Bisson, A. , Boveda, S. , Gandjbakhch, E. , Gras, M. , Gueffet, J.‐P. , Himbert, C. , Jacon, P. , Khattar, P. , Lequeux, B. , Li, A. , Mansourati, V. , Minois, D. , Marijon, E. , Pierre, B. , Probst, V. , Degand, B. , and the HeartLogic™ France Cohort Study Investigators (2024) Pre‐emptive treatment of heart failure exacerbations in patients managed with the HeartLogic™ algorithm. ESC Heart Failure, 11: 1228–1235. 10.1002/ehf2.14624.
Study registration: ClinicalTrials.gov ID NCT04619888.
References
- 1. James SL, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the global burden of disease study 2017. Lancet 2018;392:1789–1858. doi: 10.1016/S0140-6736(18)32279-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jones NR, Roalfe AK, Adoki I, Hobbs FDR, Taylor CJ. Survival of patients with chronic heart failure in the community: A systematic review and meta‐analysis. Eur J Heart Fail 2019;21:1306–1325. doi: 10.1002/ejhf.1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dharmarajan K, Hsieh AF, Lin Z, Bueno H, Ross JS, Horwitz LI, et al. Diagnoses and timing of 30‐day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA 2013;309:355–363. doi: 10.1001/jama.2012.216476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Urbich M, Globe G, Pantiri K, Heisen M, Bennison C, Wirtz HS, et al. A systematic review of medical costs associated with heart failure in the USA (2014‐2020). Pharmacoeconomics 2020;38:1219–1236. doi: 10.1007/s40273-020-00952-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boehmer JP, Hariharan R, Devecchi FG, Smith AL, Molon G, Capucci A, et al. A multisensor algorithm predicts heart failure events in patients with implanted devices: Results from the MultiSENSE study. JACC Heart Fail 2017;5:216–225. doi: 10.1016/j.jchf.2016.12.011 [DOI] [PubMed] [Google Scholar]
- 6. Capucci A, Santini L, Favale S, Pecora D, Petracci B, Calò L, et al. Preliminary experience with the multisensor HeartLogic algorithm for heart failure monitoring: A retrospective case series report. ESC Heart Fail 2019;6:308–318. doi: 10.1002/ehf2.12394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Treskes RW, Beles M, Caputo M‐L, Cordon A, Biundo E, Maes E, et al. Clinical and economic impact of HeartLogic™ compared with standard care in heart failure patients. ESC Heart Fail 2021;8:1541–1551. doi: 10.1002/ehf2.13252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Santini L, D'Onofrio A, Dello Russo A, Calò L, Pecora D, Favale S, et al. Prospective evaluation of the multisensor HeartLogic algorithm for heart failure monitoring. Clin Cardiol 2020;43:691–697. doi: 10.1002/clc.23366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Calò L, Bianchi V, Ferraioli D, Santini L, Dello Russo A, Carriere C, et al. Full list of participant centers and investigators. Multiparametric implantable cardioverter‐defibrillator algorithm for heart failure risk stratification and management: An analysis in clinical practice. Circ Heart Fail 2021;14:e008134. doi: 10.1161/CIRCHEARTFAILURE.120.008134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Spertus JA, Jones PG, Sandhu AT, Arnold SV. Interpreting the Kansas City cardiomyopathy questionnaire in clinical trials and clinical care: JACC state‐of‐the‐art review. J Am Coll Cardiol 2020;76:2379–2390. doi: 10.1016/j.jacc.2020.09.542 [DOI] [PubMed] [Google Scholar]
- 11. Ferrick AM, Raj SR, Deneke T, Kojodjojo P, Lopez‐Cabanillas N, Abe H, et al. 2023 HRS/EHRA/APHRS/LAHRS Expert Consensus Statement on Practical Management of the Remote Device Clinic. Europace. 2023;25:euad123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hicks KA, Mahaffey KW, Mehran R, Nissen SE, Wiviott SD, Dunn B, et al. Standardized Data Collection for Cardiovascular Trials Initiative (SCTI). 2017 Cardiovascular and Stroke Endpoint Definitions for Clinical Trials. Circulation. 2018;137:961–972. [DOI] [PubMed] [Google Scholar]
- 13. McMurray JJV, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, et al. Angiotensin‐neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371:993–1004. doi: 10.1056/NEJMoa1409077 [DOI] [PubMed] [Google Scholar]
- 14. Marrouche NF, Brachmann J, Andresen D, Siebels J, Boersma L, Jordaens L, et al. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med 2018;378:417–427. doi: 10.1056/NEJMoa1707855 [DOI] [PubMed] [Google Scholar]
- 15. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021;42:3599–3726. doi: 10.1093/eurheartj/ehab368 [DOI] [PubMed] [Google Scholar]
- 16. Chioncel O, Mebazaa A, Harjola V‐P, Coats AJ, Piepoli MF, Crespo‐Leiro MG, et al. ESC heart failure long‐term registry investigators. Clinical phenotypes and outcome of patients hospitalized for acute heart failure: The ESC heart failure long‐term registry. Eur J Heart Fail 2017;19:1242–1254. doi: 10.1002/ejhf.890 [DOI] [PubMed] [Google Scholar]
- 17. de Juan Bagudá J, Gavira Gómez JJ, Pachón Iglesias M, Cózar León R, Escolar Pérez V, González Fernández Ó, et al. Remote heart failure management using the HeartLogic algorithm. RE‐HEART registry. Rev Esp Cardiol (Engl Ed) 2022;75:709–716. doi: 10.1016/j.rec.2021.09.015 [DOI] [PubMed] [Google Scholar]
- 18. Hernandez AF, Albert NM, Allen LA, Ahmed R, Averina V, Boehmer JP, et al. Multiple cArdiac seNsors for mAnaGEment of heart failure (MANAGE‐HF) ‐ phase I evaluation of the integration and safety of the HeartLogic multisensor algorithm in patients with heart failure. J Card Fail 2022;28:1245–1254. doi: 10.1016/j.cardfail.2022.03.349 [DOI] [PubMed] [Google Scholar]
- 19. Guerra F, D'Onofrio A, De Ruvo E, Manzo M, Santini L, Giubilato G, et al. Decongestive treatment adjustments in heart failure patients remotely monitored with a multiparametric implantable defibrillators algorithm. Clin Cardiol 2022;45:670–678. doi: 10.1002/clc.23832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Marijon E, Uy‐Evanado A, Dumas F, Karam N, Reinier K, Teodorescu C, et al. Warning symptoms are associated with survival from sudden cardiac arrest. Ann Intern Med 2016;164:23–29. doi: 10.7326/M14-2342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Marijon E, Garcia R, Narayanan K, Karam N, Jouven X. Fighting against sudden cardiac death: Need for a paradigm shift‐adding near‐term prevention and pre‐emptive action to long‐term prevention. Eur Heart J 2022;43:ehab903. doi: 10.1093/eurheartj/ehab903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Garcia R, Warming PE, Narayanan K, Defaye P, Guedon‐Moreau L, Blangy H, et al. Dynamic changes in nocturnal heart rate predict short‐term cardiovascular events in patients using the wearable cardioverter‐defibrillator: From the WEARIT‐France cohort study. Europace 2023;25:euad062. doi: 10.1093/europace/euad062 [DOI] [PMC free article] [PubMed] [Google Scholar]


