Abstract
Background & Aims
Hepatocellular carcinoma (HCC) is a male-dominant disease, but targeted sex hormone therapies have not been successful. Bile acids are a potential liver carcinogen and are biomolecules with hormone-like effects. A few studies highlight their potential sex dimorphism in physiology and disease. We hypothesized that bile acids could be a potential molecular signature that explains sex disparity in HCC.
Methods & Results
We used the farnesoid X receptor knockout (FxrKO) mouse model to study bile acid-dependent HCC. Temporal tracking of circulating bile acids determined more than 80% of FxrKO females developed spontaneous cholemia (ie, serum total bile acids ≥40 μmol/L) as early as 8 weeks old. Opposingly, FxrKO males were highly resistant to cholemia, with ∼23% incidence even when 26 weeks old. However, FxrKO males demonstrated higher levels of deoxycholate than females. Compared with males, FxrKO females had more severe cholestatic liver injury and further aberrancies in bile acid metabolism. Yet, FxrKO females expressed more detoxification transcripts and had greater renal excretion of bile acids. Intervention with CYP7A1 (rate limiting enzyme for bile acid biosynthesis) deficiency or taurine supplementation either completely or partially normalized bile acid levels and liver injury in FxrKO females. Despite higher cholemia prevalence in FxrKO females, their tumor burden was less compared with FxrKO males. An exception to this sex-dimorphic pattern was found in a subset of male and female FxrKO mice born with congenital cholemia due to portosystemic shunt, where both sexes had comparable robust HCC.
Conclusions
Our study highlights bile acids as sex-dimorphic metabolites in HCC except in the case of portosystemic shunt.
Keywords: Hepatocellular Carcinoma, Cholestasis, Farnesoid X Receptor, Portosystemic Shunt
Summary.
Our study found bile acids to be sex-dimorphic metabolites in a HCC mouse model. We suggest bile acids as potential therapeutic targets and biomarkers for HCC cases. This study supports for advancements in sex-personalized therapies to treat liver cancer.
Hepatocellular carcinoma (HCC) is the most prevalent primary liver cancer, and recent statistics estimate it to be the third leading cause of cancer mortality worldwide.1 A sequence of liver injury, chronic inflammation, fibrosis, and cirrhosis normally precedes HCC. However, the mechanism(s) behind HCC pathogenesis is complex and varies depending on its risk factor(s). For instance, it is well-known that aflatoxins drive liver carcinogenesis by the formation of mutagenic DNA adducts and induction of oxidative stress.2,3 Moreover, hepatitis B virus (HBV)-mediated HCC is determined by the viral regulatory protein HBx, which disrupts the p53-dependent tumor suppressive pathway and promotes telomerase expression in cancer cells.4 Unlike the aforementioned risk factors, another HCC determinant is sex disparity, but the driving mechanism(s) is not well-established.
Epidemiologic surveillance with sex-specific rates demonstrates a male predominance in HCC. For every 1 female patient, there is an average ratio of 2–4 male patients with HCC, and mortality rates are at least 2-fold greater in the male population.1 Previous research has investigated chromatin-modifying proto-oncogenes and sex hormones as possible mechanisms behind sex disparity in HCC. Several Y-linked proto-oncogenes, including testis specific protein Y-encoded and RNA-binding motif gene on Y chromosome, have been identified as positive HCC biomarkers.5,6 Alongside, the X-chromosome gene TSPY homologue X, known to promote proteasomal degradation of the HBx oncoprotein, was found to be significantly down-regulated in HCC tumors.7 These findings have been positively pursued for diagnostic and prognostic applications in HCC cases. Investigation with targetable sex hormones and their receptors has suggested male-dominant androgens to be pathologic by accelerating cell proliferation, whereas female-dominant estrogens protected from HCC by inducing cell apoptosis and anti-tumor immune responses.8, 9, 10 However, high variability of estrogen receptor expression and failed survival benefits of anti-androgen agents have questioned hormonal therapy for HCC.11,12 Accordingly, the objective of this study was to identify molecular signature(s) that could explain sex disparity and be a relevant therapeutic target in HCC.
The liver is responsible for the detoxification of xenobiotics/endobiotics via redox and conjugation reactions that make the biotic less toxic and water soluble for excretion.13 Bile acids are one such metabolite that undergo hydroxylation, glucuronidation, and sulfation reactions to prevent their accumulation toward toxic liver injury levels.14,15 Bile acids are detergent-like metabolites produced in the liver that aid in the assimilation of dietary lipids. Sex differences in bile acid metabolism are sparsely noted, but a few reports conducted in hamsters and rats suggest that females have increased expression for enzymes in bile acid detoxification at physiological conditions.16,17 Bile acid levels are also regulated by the farnesoid X receptor (FXR), a major bile acid sensor, where its activation leads to tissue-specific suppression of bile acid biosynthesis.18 In cases where bile acid metabolism is dysregulated, hepatic diseases such as cholestasis and HCC can result from the pro-inflammatory effects of bile acids.19, 20, 21, 22, 23 A well-established example is reported with FXR knockout (FxrKO) mice that develop cholemia (ie, elevated bile acids) and spontaneous HCC at 16 months of age.24,25 Interestingly, the majority of reports with FxrKO mice have focused only on males,26, 27, 28, 29, 30 probably because of the convention that liver pathologies, such as HCC, are outwardly more severe in males than females.
We generated the hypothesis that sex differences in bile acid metabolism could drive sex disparity in HCC. Using FxrKO mice as a bile acid-dependent HCC model, we found females to have a higher prevalence of spontaneous cholemia (serum total bile acids ≥40 μmol/L) at both precancerous and cancerous time points. Comparatively, the male counterparts were more resistant to spontaneous cholemia irrespective of age. Despite the toxic accumulation of bile acids in FxrKO females, these mice had lower tumor burden than FxrKO males, suggesting an inverse relationship between spontaneous cholemia and HCC severity. This pattern might be attributed to the increased expression of bile acid detoxification transcripts and renal excretion of bile acids in FxrKO females. Noteworthy, we stumbled on an exception to the sex dimorphism paradigm where a subset of male and female FxrKO mice had congenital cholemia due to a developmental vascular defect called portosystemic shunt (PSS). Both male and female FxrKO mice with congenital cholemia exhibited equally robust HCC, negating the sex dimorphism effects we originally observed. Overall, our study highlights bile acids as targetable, sex-dimorphic metabolites with therapeutic potential in HCC.
Results
Cholemia and Cholestatic Liver Injury Are Sexually Dimorphic in FxrKO Mice
FXR is a nuclear receptor predominantly localized in the liver and ileum. On activation, FXR inhibits bile acid biosynthesis in a tissue-specific manner.18 Hepatic FXR induces the expression of small heterodimer partner (SHP), which inhibits hepatocyte nuclear factor 4α (HNF4α); HNF4α normally positively regulates 2 bile acid biosynthetic genes, cholesterol 7α-hydroxylase (Cyp7a1) and Cyp8b1. In the ileum, FXR induces fibroblast growth factor 15 (FGF15), and FGF15 promotes hepatic FGFR4-ERK1/2 signaling that suppresses Cyp7a1 and Cyp8b1 expression. FxrKO mice have high bile acids and hypercholesterolemia at an early age, and they develop HCC later in life.24,25,31 Reports with the FxrKO mouse model have mostly focused on males because of the convention that liver pathologies such as HCC are outwardly more severe in males than females. In this study, we investigated for potential sex-specific alterations in bile acid metabolism that could explain HCC disparity in males vs females.
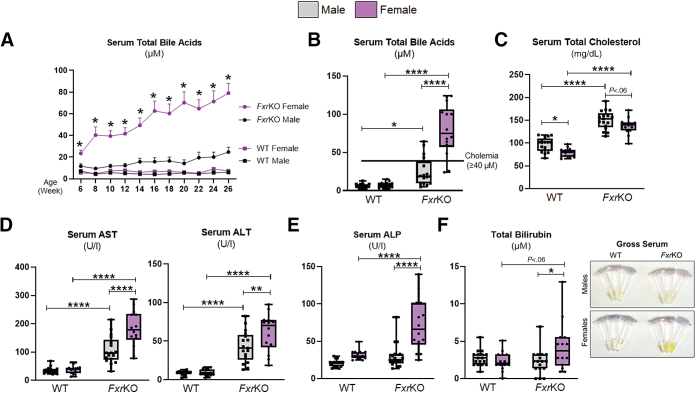
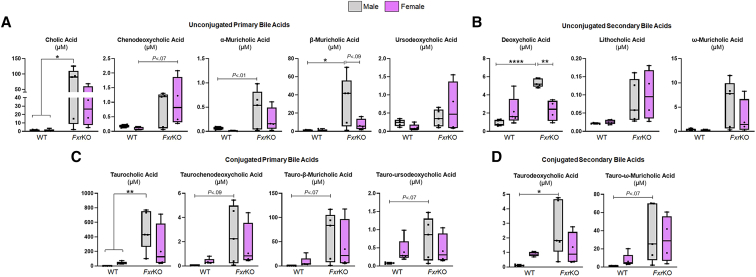
Our first objective was to determine a possible sex disparity in bile acid levels at the precancerous age in FxrKO mice. As an initial reference, normal levels of serum total bile acids (TBA) in wild-type (WT) mice are between 1–5 μmol/L. Temporal tracking depicted surprisingly higher serum TBA levels in FxrKO females than males (mean TBA, females, 23.5 μmol/L vs males, 11.6 μmol/L) when 6 weeks of age (Figure 1A). TBA levels continued to rapidly increase in FxrKO females as they aged, whereas males were resistant to spontaneous increases in TBA levels (Figure 1A). When aged to 26 weeks (ie, 6 months old), 12 of 14 female FxrKO mice reached cholemia levels (TBA ≥40 μmol/L), but only 4 of 17 male FxrKO mice surpassed the cholemia threshold, which gave this cohort an approximate cholemia incidence of 85% in females and 23% in males (Figure 1A and B). The cholemia benchmark was determined on the basis of reports with intrahepatic cholestasis of pregnancy that fetal complications occurred when TBA levels were ≥40 μmol/L.32 Serum metabolomic analysis suggested some variation in individual bile acids between male and female FxrKO mice (Figure 2A–D). One notable difference was FxrKO males had a significant increase in deoxycholic acid compared with their female counterparts (P = .0024). As expected, both sexes of FxrKO mice had hypercholesterolemia when compared with WT mice; however, FxrKO females had lower serum total cholesterol than their male counterparts (Figure 1C). These results demonstrate spontaneous cholemia is most prevalent in female FxrKO mice.
Figure 1.
Female FxrKO mice are more susceptible to cholemia and cholestatic liver injury. (A) Male and female WT and FxrKO mice were bled every 2 weeks from 6–26 weeks of age and analyzed for serum total bile acid levels. Serum levels of (B) total bile acids, (C) total cholesterol, (D) AST and ALT, (E) ALP, and (F) total bilirubin and gross serum (depicting hyperbilirubinemia from the yellow fluorescent jaundice) were analyzed when WT and FxrKO mice were 26 weeks of age. Sample sizes: WT (male, N = 17; female, N = 14) and FxrKO (male, N = 17; female, N=14). Results are expressed as means ± SEM. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001.
Figure 2.
FxrKO male mice have greater levels of deoxycholic acid and β-muricholic acid in circulation. (A–D) Serum samples were collected from 16-week-old male and female WT and FxrKO mice and sent for bile acid profiling via metabolomics. (A) Unconjugated primary bile acids (cholic acid, chenodeoxycholic acid, α-muricholic acid, β-muricholic acid, and ursodeoxycholic acid), (B) unconjugated secondary bile acids (deoxycholic acid, lithocholic acid, and ω-muricholic acid), (C) conjugated primary bile acids (taurocholic acid, taurochenodeoxycholic acid, tauro-β-muricholic acid, and tauro-ursodeoxycholic acid), and (D) conjugated secondary bile acids (taurodeoxycholic acid and tauro-ω-muricholic acid). Sample sizes: WT (male, N = 5; female, N = 5) and FxrKO (male, N = 5; female, N = 4). Results are expressed as means ± SEM. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001.
Because bile acids can be hepatotoxic agents, we next measured clinical parameters of cholestatic liver injury when FxrKO mice were 26 weeks old. Compared with WT mice, both sexes of FxrKO mice had transaminitis, ie, elevated aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels (Figure 1D). In accord with the sex disparity in serum TBA levels, FxrKO females had a higher degree of elevation in AST (P < .0001) and ALT (P = .01) compared with males (Figure 1D). Impressively, the cholestasis-specific marker alkaline phosphatase (ALP) was significantly increased only in FxrKO females (P < .0001), whereas ALP levels in FxrKO males were comparable with WT mice (Figure 1E). Visualization of the serum color showed most (albeit not all) FxrKO females also had jaundice with high serum bilirubin (Figure 1F). Altogether, our profound findings demonstrate female FxrKO mice to have cholemia predominance and increased cholestatic liver injury.
Female FxrKO Mice Have Exacerbated Bile Acid Dysmetabolism
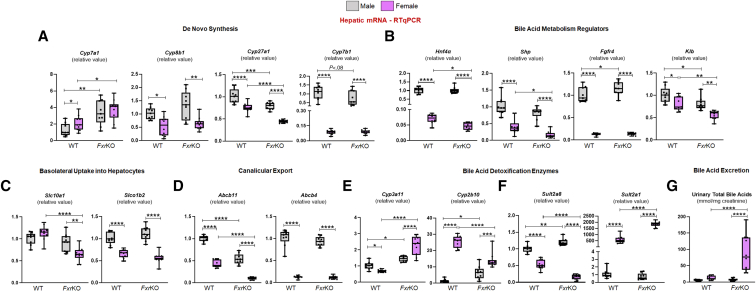
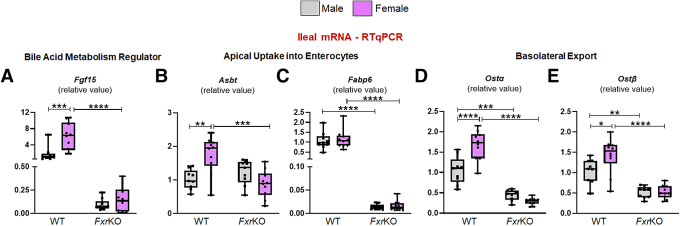
To discern the possible reason behind cholemia dominance in female FxrKO mice, a panel of bile acid metabolism genes were quantitated at sites of FXR signaling, ie, liver and ileum. When analyzing for the major bile acid biosynthesis genes (Figure 3A), the hepatic gene Cyp7a1, which encodes the rate limiting enzyme, was equally up-regulated in both sexes of FxrKO mice compared with WT mice. Opposingly, female but not male WT and FxrKO mice had a depression in Cyp8b1 levels (P = .001), suggesting females might produce less cholic acid. Correspondingly, FxrKO females had the most significant down-regulation of Cyp27a1 and Cyp7b1 transcripts (P < .0001), the latter of which is related to chenodeoxycholic acid production. Measuring the expression levels of bile acid regulators (Figure 3B) demonstrated FxrKO females, but not males, to have repression in Hnf4α and Shp, two Cyp7a1 regulators. Similarly, the hepatic membrane receptor complex that mediates ileum FXR-FGF15 signaling, ie, fibroblast growth factor receptor 4 (Fgfr4) and β-Klotho (Klb), were significantly suppressed in FxrKO females. Yet, there was an equal down-regulation of ileal Fgf15 seen in male and female FxrKO mice (Figure 4A). These data suggest that part of the reason for cholemia dominance in female FxrKO mice is the greater lack of negative feedback regulation of bile acid biosynthesis.
Figure 3.
Enhanced bile acid dysmetabolism and detoxification could explain the cholemia disparity in female FxrKO mice. (A–F) Liver samples from 16-week-old male and female WT and FxrKO mice were analyzed by qRT-PCR) for transcripts related to bile acid de novo synthesis, regulators, transporters, and detoxification. Relative expressions of genes for bile acid biosynthesis (A) Cyp7a1, Cyp8b1, Cyp27a1, and Cyp7b1; bile acid regulators (B) Hnf4α, Shp, Fgfr4, and Klb; bile acid uptake transporters (C) Slc10a1 and Slco1b2; bile acid canicular exporters (D) Abcb11 and Abcb4; bile acid hydroxylation (E) Cyp3a11 and Cyp2b10; and bile acid sulfonation (F) Sult2a8 and Sult2a1. 36b4 was used as internal control for qRT-PCR. (G) Urinary total bile acid levels (normalized to creatinine) in 16-week-old male and female WT and FxrKO mice. Sample sizes: WT (male, N = 9; female, N = 10) and FxrKO (male, N = 9; female, N = 10). Results are expressed as means ± SEM. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001.
Figure 4.
FxrKO mice, irrespective of sex, have equal suppression of ileal transcripts related to bile acid regulation and reabsorption. (A–E) Ileal samples from 16-week-old male and female WT and FxrKO mice were analyzed by qRT-PCR for transcripts related to bile acid metabolism. Relative expressions of (A) Fgf15, (B) Asbt, (C) Fabp6, (D) Ostα, and (E) Ostβ. 36b4 was used as internal control for qRT-PCR. Sample sizes: WT (male, N = 9; female, N = 10) and FxrKO (male, N = 9; female, N = 10). Results are expressed as means ± SEM. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001.
We next asked whether there could be a compromise in enterohepatic bile acid circulation to explain the sex disparity in cholemia. Indeed, FxrKO females had lessened expression of hepatic sodium-dependent taurocholate co-transport peptide (NTCP encoded by Slc10a1) and sodium-independent organic anion transport peptide (OATP1B2 encoded by Slco1b2) for bile acid uptake into hepatocytes (Figure 3C). We also found that the expression of bile salt exporter pump (BSEP) (encoded by Abcb11) was further diminished in FxrKO females (P < .0001), and only the female groups had a suppression of multidrug resistant protein 2 (encoded by Abcb4) (Figure 3D). Interestingly, male and female FxrKO mice had equal down-regulation in transcripts related to bile acid reabsorption in the ileum, ie, apical sodium-dependent bile salt transporter (Asbt) (Figure 4B), fatty acid binding protein 6 (Fabp6) (Figure 4C), and sinusoidal bile acid efflux transporters, organic solute transporter α and β (Ostα and Ostβ) (Figure 4D and E). These data suggest that lowered bile acid secretion into the biliary tree could be another reason for cholemia dominance in female FxrKO mice.
Female FxrKO Mice Exhibit Increased Expression for Bile Acid Detoxification
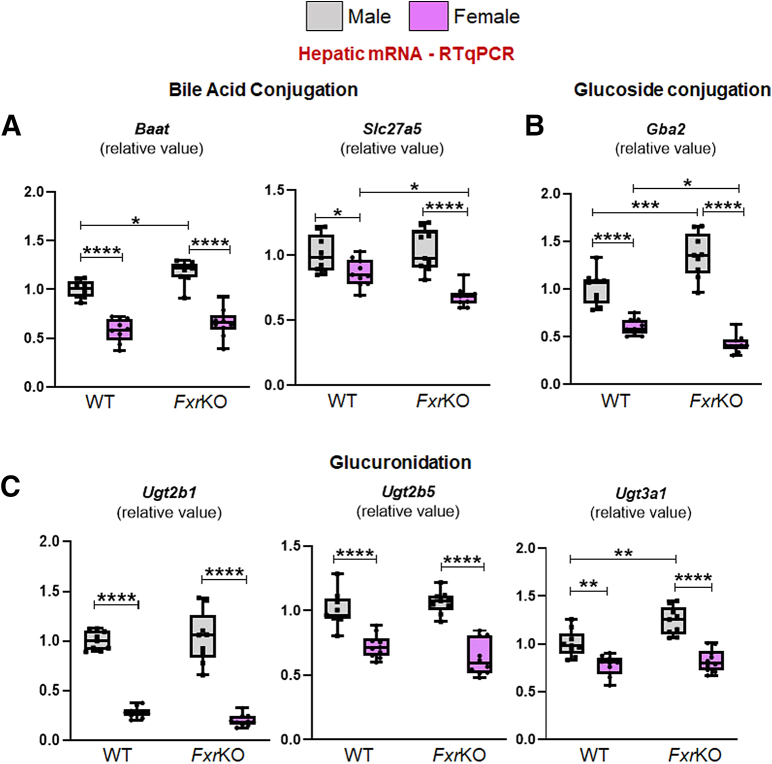
Several studies highlight the notion that females have better bile acid detoxification because of elevated xenobiotics-processing CYP450 hydroxylating enzymes and sulfotransferases.16,17,33,34 When analyzing bile acid hydroxylating genes (Figure 3E), both sexes of FxrKO mice had increased Cyp3a11 expression compared with WT mice, but the levels were substantially higher in FxrKO females (P < .0001). Moreover, FxrKO females had greater Cyp2b10 expression than FxrKO males (P = .0005). Of note, Cyp2b10 expression in WT females was elevated, even more than FxrKO mice, compared with WT males. When analyzing sulfotransferase transcripts (Figure 3F), FxrKO males had a greater expression in the male-dominant Sult2a8 compared with WT males, whereas both female groups had reduced mRNA levels. Comparatively, when measuring the female-dominant Sult2a1, WT females had around 500-fold increase, and FxrKO females had around 1500-fold increase in mRNA expression than their respective male groups. Of note, analysis of amidation (Baat, Slc27a5, or Bacs), glucuronidation (Ugt2b1, 2b5, 3a1), and glucoside genes (Gba2) were decreased in FxrKO females (Figure 5A–C). Hydroxylation and sulfonation detoxify bile acids by promoting their renal excretion, and indeed FxrKO females had the highest urinary TBA levels (P < .0001) (Figure 3G). These data emphasize cholemia dominance in female FxrKO mice is associated with an up-regulation in bile acid detoxification and excretion.
Figure 5.
Female mice have lowered hepatic expression of glucuronidation and glucoside genes. (A–C) Liver samples from 16-week-old male and female WT and FxrKO mice were analyzed by qRT-PCR for transcripts related to bile acid conjugation (A) Baat and Slc27a5; glucoside formation (B) Gba2; and glucuronidation (C) Ugt2b1, Ugt2b5, and Ugt3a1. 36b4 was used as internal control for qRT-PCR. Sample sizes: WT (male, N = 9; female, N = 10) and FxrKO (male, N = 9; female, N = 10). Results are expressed as means ± SEM. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001.
Deletion of CYP7A1 Normalizes TBA Levels and Liver Injury in Female FxrKO Mice
Our next goal was to find a relevant intervention that can restore bile acid metabolism in FxrKO mice with emphasis to females. We attempted cholestyramine intervention that sequesters luminal bile acids, enhances their fecal excretion, and stimulates bile acid biosynthesis.35 Introducing dietary cholestyramine to FxrKO females at time points before (Figure 6A–C) or after (Figure 6D–F) cholemia did not lower TBA or liver injury. Similarly, we attempted treating with the HMG-CoA reductase inhibitor atorvastatin to reduce substrate availability (ie, cholesterol) for bile acid biosynthesis, but FxrKO females still retained spontaneous cholemia and liver injury (Figure 7A–H). Because the gut microbiota is responsible for generating secondary bile acids, we also administered broad-spectrum antibiotics orally to FxrKO females with no success to reduce TBA and liver injury (Figure 8A–C). All of these data indicate and confirm that bile acid metabolism is dysregulated in FxrKO mice.
Figure 6.
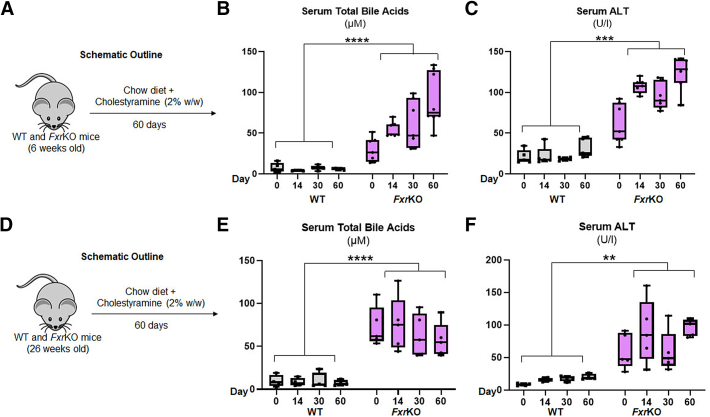
Cholestyramine does not lower bile acids or reduce liver injury in female FxrKO mice. (A–C) Grain-based chow diet (LabDiet 5001) was supplemented with cholestyramine (2% w/w, Sigma-Aldrich) and given to 6-week-old female WT (N = 5) and FxrKO (N = 7) mice for 60 days. (A) Schematic outline, (B) serum total bile acids, and (C) serum ALT. (D–F) Grain-based chow diet (LabDiet 5001) was supplemented with cholestyramine (2% w/w, Sigma-Aldrich) and given to 26-week-old female WT (N = 4) and FxrKO (N = 5) mice for 60 days. (D) Schematic outline, (E) serum total bile acids, and (F) serum ALT. Results are expressed as means ± SEM. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001.
Figure 7.
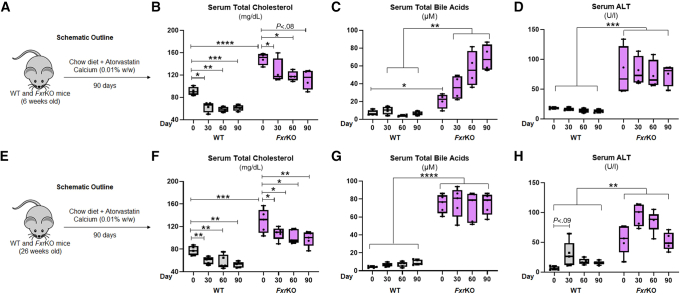
Atorvastatin does not lower bile acids or liver injury during either delayed onset or established cholemia in female FxrKO mice. (A–D) Grain-based chow diet (LabDiet 5001) was supplemented with atorvastatin calcium (0.01% w/w, MilliporeSigma) and given to 6-week-old female WT (N = 4) and FxrKO (N = 4) mice for 90 days. (A) Schematic outline, (B) serum total cholesterol, (C) serum total bile acids, and (D) serum ALT. (E–H) Grain-based chow diet (LabDiet 5001) was supplemented with atorvastatin calcium (0.01% w/w, MilliporeSigma) and given to 26-week-old female WT (N = 5) and FxrKO (N = 5) mice for 90 days. (E) Schematic outline, (F) serum total cholesterol, (G) serum total bile acids, and (H) serum ALT. Results are expressed as means ± SEM. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001.
Figure 8.
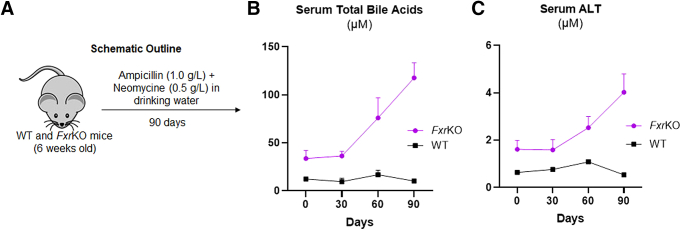
Antibiotics do not inhibit delayed onset cholemia or liver injury in female FxrKO mice. (A–C) Broad-spectrum antibiotics (ampicillin [1.0 g/L] + neomycin [0.5 g/L]; Sigma-Aldrich) were given to 6-week-old female WT (N = 4) and FxrKO (N = 4) mice in the drinking water for 90 days. (A) Schematic outline, (B) serum total bile acids, and (C) serum ALT. Results are expressed as means ± SEM. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001.
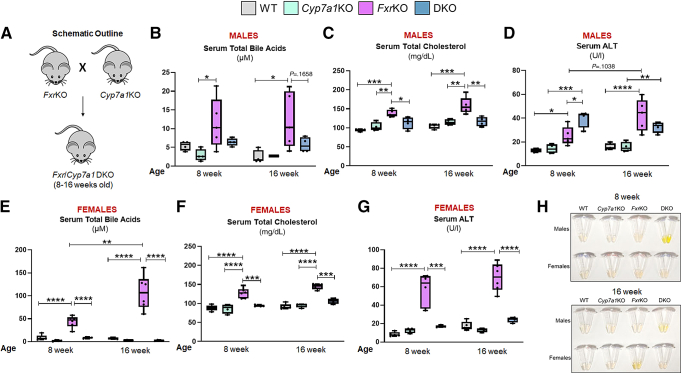
For a more direct and genetic approach, we crossed FxrKO mice with Cyp7a1-deficient (Cyp7a1KO) mice to generate double knockouts (DKO) (Figure 9A). Cyp7a1KO mice are reported to have only half the bile acid biosynthesis rate typically found in WT mice.36 Impressively, 8- and 16-week-old male (P = .1658) and female (P < .0001) DKO mice had normalized TBA levels to that seen in their sex-matched WT counterparts (Figure 9B and E), possibly due in part to their lowered cholesterol levels (Figure 9C and F). It must be emphasized that this intervention was most remarkable in the females because the TBA shift was from an average of 107.3 μmol/L in FxrKO to 2.7 μmol/L in DKO mice compared with the TBA reduction from 12.2 μmol/L to 5.6 μmol/L in male FxrKO and DKO mice, respectively. Even though TBA was normalized, serum ALT levels in DKO males were higher than in FxrKO males (P = .0121), and DKO males had hyperbilirubinemia (Figure 9D and H). Comparatively, DKO females had significantly reduced ALT levels to that found in WT females and were protected from jaundice (Figure 9G and H). These results demonstrate that female FxrKO mice better responded to the intervention of Cyp7a1 deficiency with restoration toward low TBA and ALT levels.
Figure 9.
Bile acids and liver injury are normalized in female FxrKO mice on CYP7A1 deletion. (A–H) FxrKO mice were crossed with Cyp7a1KO mice to generate DKO and were analyzed for cholemia and liver injury markers when 8 and 16 weeks old. (A) Schematic outline. Serum (B) total bile acids, (C) total cholesterol, and (D) ALT levels in the male mice. Serum (E) total bile acids, (F) total cholesterol, and (G) ALT levels in the female mice. (H) Gross serum (depicting hyperbilirubinemia from the yellow fluorescent jaundice). Sample sizes: WT (male, N = 4; female, N = 5), Cyp7a1KO (male, N = 4; female, N = 4), FxrKO (male, N = 5; female, N = 6), and DKO (male, N = 4; female, N = 4). Results are expressed as means ± SEM.∗P < .05, ∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001.
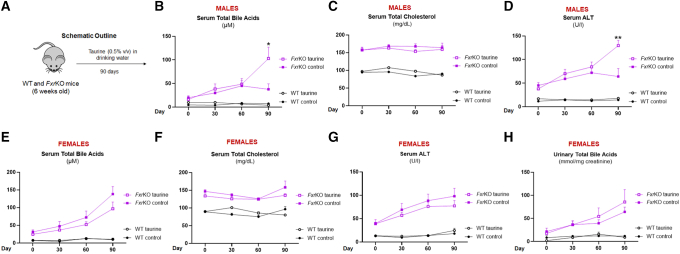
Cholemia in Male FxrKO Mice Is Accelerated by Taurine Supplementation
Taurine is a sulfur-containing non-proteinaceous amino acid that can conjugate with bile acids, which increases their solubility and biliary excretion.37 Bile acid conjugation enzyme BAAT (bile acid-CoA: amino acid N-acyltransferase) deficiency is known to cause hypercholanemia in mice.38 We found that female WT and FxrKO mice had significantly lowered Baat mRNA expression (Figure 5A). We hypothesized that taurine supplementation (Figure 10A) could serve as a clinically relevant approach to prevent cholemia and liver injury in FxrKO mice. Taurine feeding to FxrKO females resulted in a modest reduction in serum TBA and ALT levels that was associated with increased urinary TBA excretion but no changes in serum cholesterol (Figure 10E–H). Unexpectedly, we observed FxrKO males to have an acceleration in spontaneous cholemia where 7 of 8 males fed taurine reached cholemia by 18 weeks of age compared with the 3 of 8 control FxrKO males (Figure 10B). The rapid cholemia development after taurine also resulted in a significant increase in serum ALT levels for FxrKO males (Figure 10D) but no changes in cholesterol levels (Figure 10C) or urinary TBA levels (data not shown). Overall, taurine seems to have sex-dependent effects on cholemia and liver injury where males exhibit more pathologic indices.
Figure 10.
Taurine supplementation lessens cholemia development in female FxrKO mice but accelerates cholemia in male FxrKO mice. (A–H) Drinking water containing 0.5% v/v of taurine (Sigma-Aldrich) was given to 6-week-old WT and FxrKO mice for 90 days, and mice were tracked for changes in cholemia and liver injury markers. Serum (B) total bile acids, (C) total cholesterol, and (D) ALT levels in the male mice. Serum (E) total bile acids, (F) total cholesterol, and (G) ALT levels in the female mice. (F) Urinary total bile acid levels (normalized to creatinine) in taurine-fed female WT and FxrKO mice. Sample sizes: control WT (male, N = 5; female, N = 5), taurine WT (male, N = 5; female, N = 5), control FxrKO (male, N = 7; female, N = 7), and taurine FxrKO (male, N = 8; female, N=8). Results are expressed as means ± SEM. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001.
Sex Heterogeneity in Cholemia Is Inversely Associated With HCC Severity
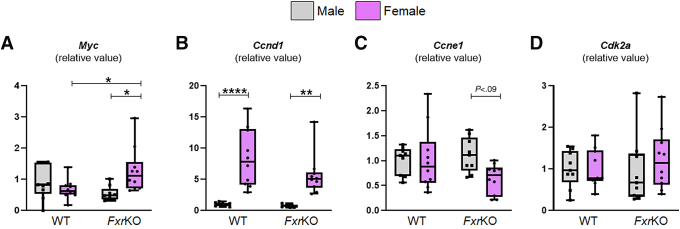
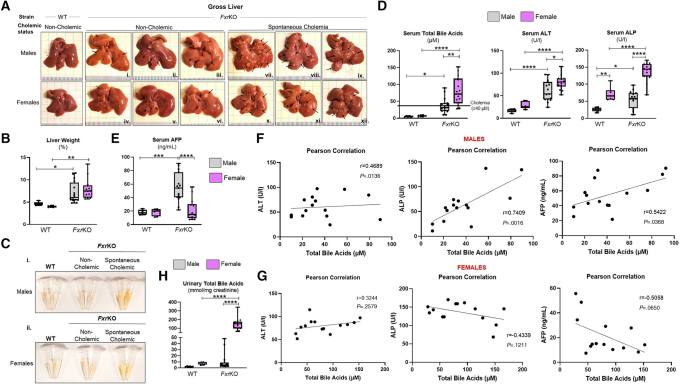
Our next objective was to determine whether sex-dependent spontaneous cholemia at the precancerous age reflects HCC severity in FxrKO mice when 16 months of age. Our initial thought was FxrKO females could have worse HCC because of their high TBA, and they had altered mRNA expression of the oncogene Myc and cell cycle regulators at the precancerous stage (Figure 11A–D). At the cancer time point, male and female FxrKO mice who never developed cholemia (TBA <40 μmol/L) had the least HCC severity, whereas tumor burden increased in both sexes for FxrKO mice with spontaneous cholemia (TBA ≥40 μmol/L) (Figure 12A). There were no apparent differences in HCC severity when comparing male and female FxrKO mice without cholemia. However, the few FxrKO males that became cholemic had aggravated tumor burden compared with FxrKO females with prevalent cholemia (Figure 12A). Both male and female FxrKO mice had equivalent intensity of hepatomegaly and jaundice (Figure 12B and C), but females still dominated with the highest levels of TBA (P = .0015), ALT (P = .0192), and ALP (P < .0001) (Figure 12D). When measuring the widely used serologic biomarker for HCC, α-fetoprotein (AFP), FxrKO males had sustainably high levels, whereas FxrKO females barely had an elevation compared with their WT counterparts (Figure 12E). Pearson correlation demonstrated that TBA levels in FxrKO males had a positive correlation with ALT, ALP, and AFP (Figure 12F). Alternately, TBA levels in FxrKO females had a modest positive relationship with serum ALT but negative correlations with ALP and AFP (Figure 12G). Urinary TBA levels were over 10-fold more in FxrKO females compared with FxrKO males (Figure 12H), suggesting females have more efficient detoxification. Collectively, the summation of all data to this point underscores female FxrKO mice are more susceptible to spontaneous cholemia but are substantially protected from HCC severity because of potential adaptions for better bile acid detoxification.
Figure 11.
Female FxrKO mice have increased hepatic expression of Myc and Ccnd1, whereas Ccne1 and Cdk2a are comparable. (A–D) Liver samples from 16-week-old male and female WT and FxrKO mice were analyzed by qRT-PCR for transcripts related to oncogenes and cell cycle regulators. (A) Myc, (B) Ccnd1, (C) Ccne1, and (D) Cdk2a. 36b4 was used as internal control for qRT-PCR. Sample sizes: WT (male, N = 9; female, N = 10) and FxrKO (male, N = 9; female, N = 10). Results are expressed as means ± SEM. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001.
Figure 12.
Heterogeneity in cholestasis and HCC is positively associated with bile acids in male FxrKO mice. (A–H) Male and female WT and FxrKO mice were aged until 16 months and assessed for liver cancer. (A) Gross livers, (B) % liver weight, (C) gross serum (depicting hyperbilirubinemia from the yellow fluorescent jaundice), serum (D) total bile acids, ALT, ALP, and (E) AFP levels. Pearson correlations for (F) male FxrKO mice comparing serum total bile acids and ALT, ALP, and AFP. Pearson correlations for (G) female FxrKO mice comparing serum total bile acids and ALT, ALP, and AFP. (H) Urinary total bile acid levels (normalized to creatinine) in WT and FxrKO mice. Sample sizes: WT (male, N = 6; female, N = 6) and FxrKO (male, N = 15; female, N = 14). Results are expressed as means ± SEM. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001.
Portosystemic Shunt Is an Exception to the Sex-dependent Effects of Cholemia on HCC
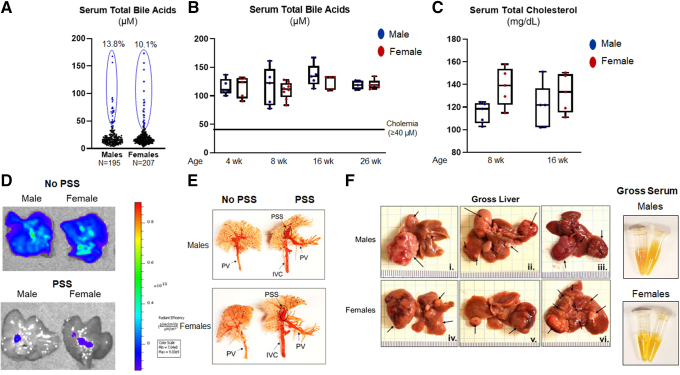
We stumbled on an unexpected exception to the sex disparity that has been highlighted. When screening FxrKO mice at the pre-weaning age (16–18 days old), we found a subset of males (13.8%) and females (10.1%) exhibited congenital cholemia with mostly similar TBA levels (Figure 13A). Essentially, these FxrKO mice are born with high TBA concentrations, and they remain elevated throughout their lifespan (Figure 13B). Recently, we reported the presence of congenital cholemia in BL6 WT mice who had hypocholesterolemia (serum total cholesterol <75 mg/dL),39 but surprisingly, FxrKO mice with congenital cholemia still retained high cholesterol levels above 100 mg/dL (Figure 13C). We previously denoted the reason for congenital cholemia in WT mice was from PSS, which is an abnormal blood vessel architecture that “shunts” blood returning from the intestine directly to the heart, thus bypassing the liver.40 To assess whether FxrKO mice with congenital cholemia also have PSS, we first injected 4 kDa fluorescein isothiocyanate (FITC)–dextran into the portal vein and imaged the livers by an in vivo imaging system (IVIS). In male and female FxrKO mice with congenital cholemia, we observed FITC–dextran was not retained in the liver and rather entered the inferior vena cava (Figure 13D), which supported our hypothesis that these mice have PSS. To better confirm anatomically, resin casting was performed, and we could visualize the shunt architecture in male and female FxrKO mice with congenital cholemia (Figure 13E). Last, we aged the mice to the HCC time point and found that male and female FxrKO mice with congenital cholemia/PSS had equally aggravated tumor burden and jaundice (Figure 13F). The tumor burden in mice with congenital cholemia/PSS was more severe than the prior results of spontaneous, delayed onset cholemia in FxrKO mice without PSS (Figure 12). Congenital cholemia in FxrKO mice also accelerated mortality in several mice toward 12 months of age instead of the 16-month cancer time point (data not shown). Overall, PSS is an exception to the sex-dependent effects of cholemia on HCC pathogenesis in FxrKO mice.
Figure 13.
Congenital cholemia due to portosystemic shunt is an exception to the sex dimorphism and HCC severity relationship in FxrKO mice. (A) Pre-weaned male (N = 195) and female (N = 207) FxrKO mice (16–18 days old) were bled, and serum total bile acid levels were measured to identify congenital cholemia. (B) Male (n = 5) and female (n = 5) FxrKO with congenital cholemia were tracked for their bile acid levels from 4–26 weeks of age. (C) Male (n = 5) and female (n = 5) FxrKO with congenital cholemia were tracked for their total cholesterol levels at 8 and 16 weeks of age. (D and E) Two methods were used to assess PSS in FxrKO mice. (D) 4 kDa FITC–dextran (Sigma-Aldrich, 0.136 mg/g body weight) was injected into the portal vein, and then the liver was extracted and imaged via IVIS at excitation 490 nm and emission 520 nm. (E) Mercox II resin (Ladd Research) was injected into the portal vein with 30-gauge, 0.5-inch needle at necropsy/dissection. Resin cast was immersed in 15% KOH overnight, rinsed with water, and followed by visual inspection. (F) Male (n = 5) and female (n = 5) FxrKO mice with congenital cholemia were aged to 16 months and assessed for liver cancer. Gross liver (3 representative images per sex) and serum (depicting hyperbilirubinemia from the yellow fluorescent jaundice). Results are expressed as means ± SEM. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001.
Discussion
Sex dimorphism is an unavoidable feature in a plethora of diseases including HCC, and it can dictate susceptibility, severity, outcomes, and response to therapy. Data mining and case studies of HCC specimens have identified several Y-linked proto-oncogenes41 and possible epigenetic modifications such as histone methylation42 to discern sex differences. Sex-biased molecular signatures in HCC have focused on sex hormones, ie, androgen and estrogen. Much research over the last 20 years provides the concept that androgen is pro-carcinogenic by accelerating cell proliferation via androgen receptor signaling, whereas estrogen is tumor suppressive by inducing cell apoptosis and immune responses.43 Of note, a novel estrogen-related gene prognostic signature was recently discovered to predict patient survival and immunotherapy response of HCC patients.44 Overall, these markers have provided diagnostic and prognosis potential and suggest that genetic and/or hormonal mechanisms could explain male predominance in HCC.
Translational research has focused on targeting sex hormones for HCC treatment. On a therapeutic level, inhibiting androgen receptor signaling has not yielded clinical benefits, but new approaches such as proteolysis-targeting chimeras are being studied.12 Feminization via estrogen administration in mouse and zebrafish HCC models has demonstrated regressed tumor progression.9,10 However, there is concern in exogenous estrogenic exposure to humans. In the Liver Cancer Pooling Project, exogenous estrogenic exposures such as oral contraceptives were not associated with increased risk for HCC,45 but there was 62% increased risk for intrahepatic cholangiocarcinoma, the second most common type of liver cancer.46 Moreover, a recent report demonstrated that loss of hepatocyte-specific histone deacetylase 3 in female mice reduced FOXA1/2 and promoted HCC development in an estrogen-dependent manner.47 Collectively, the literature supports the drive for our current study to discover new sex-based, molecular targets that are clinically relevant for HCC patients.
Substantial evidence emphasizes bile acids as a liver oncogenic metabolite,23 and new findings continue to accumulate.48,49 In accord, odds ratio by multivariable conditional logistic regression models demonstrated individuals with high circulating TBA to have at least 2-fold increase in HCC risk.50 Moreover, several reported molecular signatures and computational models based on bile acid metabolism-related genes were found to effectively predict the prognosis of HCC patients and their immunotherapeutic response.50, 51, 52, 53, 54 A few available reports hint the possibility that bile acid metabolism could explain sex divergences in liver disease pathologies. One 2007 study highlights a female-specific resistance in hepatotoxic and cholestasis mouse models due to their better bile acid detoxification.55 Two consecutive publications in 2012 and 2017 also suggested that male-specific liver adenomas in mice were associated with changes in xenobiotic-processing cytochrome p450 (Cyp) genes, including those for bile acid detoxification.56,57 As such, we hypothesized that physiological sex deviations in bile acid metabolism could explain the pathology differences observed between males and females with HCC.
Using the FxrKO mouse model, we determined that bile acids are a sex-dimorphic molecule in HCC. Our findings demonstrate that FxrKO females have greater incidence and severity of cholemia but have lower tumor burden when compared with males. This is an important observation because the majority of reports using the FxrKO mouse model for liver cancer investigation did not compare males vs females. The literature demonstrates only males were used, or sometimes the sex was not specified.26, 27, 28, 29, 30 The first 2 publications using the FxrKO mouse model did analyze HCC in both males and females but did not highlight potential factors underlying the difference in tumor burden between the sexes.24,25 Of note, one study that investigated HBV-induced hepatocarcinogenesis with both sexes did observe FxrKO females to have worsened HCC than males,58 which emphasizes that etiology can influence sex-dependent effects in HCC. As such, other HCC models such as diethylnitrosamine,21,59,60 high-fat and high-cholesterol,61 alcohol,62 and circadian dysfunction (jet lag),63,64 which are noted with pathologic changes in bile acid pathways, should be better investigated for possible sex variations. Regardless, our study herein supports the future exploration for possible polymorphisms in bile acid genes, including FXR, that may explain sex dimorphism of HCC in humans.
Physiological sex differences in bile acid metabolism are sparsely noted in the literature, where the first line of evidence from the late l970s uncovered heterogeneity in bile acid sulfonation with significantly increased activity in female hamsters and rats.16,17 Additional studies in hamsters and rats found females to have a higher rate of bile acid hydroxylation,33,34 which is another method of bile acid detoxification along with sulfonation. We believe that our observation of lessened hepatocarcinogenesis in cholemic FxrKO female mice could be due to adaptations that promote bile acid detoxification. The up-regulated expressions of Cyp3a11, Cyp2b10, and the female-dominate Sult2a1 and the stark increase of urinary TBA excretion in FxrKO females support the possible concept of better bile acid detoxification. Notably, a recent analysis from the Gene Expression Omnibus found the CYP3A4 gene (human ortholog of Cyp3a11) to be down-regulated in male HCC patients.65 A prior study highlights a female-specific resistance in hepatotoxic and cholestasis mouse models due to their better bile acid detoxification55; however, we observed increased cholestatic liver injury for cholemic FxrKO females. We believe this could be partially attributed to their lowered hepatic Cyp7b1 expression, a male-dominate gene, where this compromise would increase oxidized steroid intermediates that can induce liver injury.66 Furthermore, it is possible that dysregulation in Cyp7b1 could result in high levels of 27-hydroxycholesterol and thus cause abnormalities in estrogen-mediated gene expression67 that may change HCC risk.
Our study provides the groundwork for follow-up studies to expand on the possible mechanisms behind the sex-specific role of bile acids in HCC. One of the future steps includes performing unbiased gene expression analysis such as RNA sequencing. Our quantitative reverse transcription-polymerase chain reaction (qRT-PCR) results demonstrate that FxrKO females, rather than males, have increased Myc expression and changes in cell cycle regulation at the precancerous age. Interestingly, FxrKO males are reported to have elevated Myc expression at the cancerous time point,26 which could explain their worsened HCC. Besides oncogenes such as Myc, other known mechanisms for how bile acids promote HCC development, eg, activating inflammasome,19 inducing an immunosuppressive environment,48,68 and altering the gut microbiota69 should be further researched for possible sex variations. Another mechanistic approach includes studying how individual bile acids activate inflammatory and/or oncogenic pathways. On the other hand, some individual bile acids such as ursodeoxycholic acid are noted to reduce transforming growth factor-β activity and promote anti-tumor immune responses.70 Metabolomic analyses in our study delineated FxrKO males had significantly higher deoxycholic acid. This secondary bile acid is known to provoke the malignant behavior of senescence in hepatic stellate cells via induction of interleukin 8 and transforming growth factor-β.71,72 It would be important to next test whether blocking secondary bile acid production via a bile salt hydrolase inhibitor could be of therapeutic relevance in the FxrKO mouse model.
Growing evidence for sex-dependent variability in treatment responses has encouraged for sex-specific therapies as a new line of personalized medicine, including for liver diseases.73 There are current approaches to use anti-tumorigenic FGF19 (human ortholog of Fgf15) and other FXR agonists to protect against HCC,74, 75, 76 but whether these interventions have sex-dependent effects is unknown. Of note, a recent study found that Fgf15 overexpression in healthy male mice can cause a phenotypical switch toward a female pattern of drug metabolizing enzymes.77 Administering FGF15/19 could be thought as one therapeutic idea for FxrKO mice, but studies demonstrate FGF15/FGFR4 signaling to have pro-HCC effects.78,79 An alternative intervention is to introduce a xenobiotic-responsive receptor agonist that targets constitutive androstane receptor (CAR). However, although the CAR-specific agonist ligand 1,4-bis-[2-(3,5-dichloropyridyloxy)]benzene (TCPOBOP) can strongly enrich xenobiotic and drug metabolism better in female mice, TCPOBOP exposure up-regulates hepatic oncogenes in males.80 Furthermore, another CAR activator called phenobarbital is well-documented to be a liver tumor promoter.81
In this study we sought to therapeutically intervene by Cyp7a1 deficiency or taurine supplementation instead. Interestingly, our intervention studies of Cyp7a1 deletion and taurine supplementation had opposing sex-dependent effects in FxrKO mice. These treatments either completely or partially alleviated cholemia and liver injury in FxrKO females, but males had an unexpected acceleration in spontaneous cholemia and worsened liver injury. It is plausible that taurine-mediated detoxification in FxrKO males lowered TBA below physiological levels, and that inadvertently triggered more production. Alongside, taurine restoration may have caused a bloom in sulfidogenic bacteria38 that could contribute to the unexpected increase in cholemia. As such, further studies are required to test other possible therapies. One option is targeting the pregnane X receptor (PXR). However, there is controversial knowledge about the role of PXR in cell cycle regulation,81 which must be kept in mind when interpreting results of such an intervention.
Intriguingly, we did find a sex-independent etiologic factor of PSS in male and female FxrKO mice that resulted in congenital cholemia and comparable aggravation in HCC pathogenesis. This observation matches our recent publication that a subset of male and female C57BL/6 mice have PSS and were equally prone to HCC only when fed a compositionally defined diet (alias purified diet).39 In essence, mice with PSS are experimental outliers and should be statistically accounted for appropriate sample sizing. Regardless, the continual exposure of high TBA levels from birth is probably why FxrKO mice with congenital cholemia have the worst HCC compared with FxrKO mice with delayed onset, spontaneous cholemia. It would be interesting to determine whether TBA levels in congenital cholemic FxrKO mice are resistant to interventions such as lactulose, which is used to prevent hepatic encephalopathy for liver cirrhosis patients who surgically undergo transjugular intrahepatic portosystemic shunt.82
Overall, our study newly identifies bile acids as sex-dimorphic metabolites in HCC with the exception of PSS cases. This study advocates for the rigorous incorporation of sex as a biological variable and bile acids to be a possible screening biomarker for HCC. The continual evaluation of sex-dependent markers in HCC will help to determine personalized medicines, including for those undergoing or completed gender-affirming hormone therapy. Moving forward, future studies should expand on the possible mechanisms behind the sex-specific role of bile acids in HCC pathogenesis and their clinical implications.
Materials and Methods
Mice
FxrKO mice on C57BL/6J background31 were a gift from Dr Frank J. Gonzalez (National Cancer Institute, NIH). Homozygous FxrKO and cholesterol 7α-hydroxylase-deficient (Cyp7a1KO, C57BL/6J background83) mice were crossed to generate the heterozygous F1 generation, and continual breeding resulted in DKO mice. In our studies, second to fourth generation male and female offspring were used. The above mice were bred with C57BL/6J WT mice in our colony to generate their WT littermates. All mice were bred and maintained under specific pathogen-free conditions at the University of Toledo. Housing was in cages (N = 5 mice/cage) containing corncob bedding (Bed-O-Cob, The Andersons Co) and nestlets (cat# CABFM00088, Ancare) at 23°C under a 12-hour light/dark phase cycle. Mice were fed ad libitum (LabDiet 5020 for breeders and LabDiet 5001 for weaned mice) and given unrestricted access to regular, non-acidified drinking water. Custom diets were generated and supplied by Cincinnati Lab Supply (Certified LabDiet Dealer). Using an alpha of 0.05 (two-sided) and a power of 0.80, we calculated 5–7 mice per genotype (WT, Cyp7a1KO, FxrKO, and DKO) and per intervention as the minimum number necessary to get statistically significant data. All animal studies were conducted per institutional animal care and use committee (IACUC) approvals obtained from University of Toledo.
Serum and Urine Bile Acid Tracking
Submandibular blood was collected from male and female WT and FxrKO mice using a BD microtainer (Becton, Dickinson). Hemolysis-free sera were obtained after centrifugation (10,000 rpm for 10 minutes at 4°C) and stored at –80°C until further analysis for serum TBA. Mice were bled biweekly from 6–26 weeks of age. Urine was also collected for tracking TBA excretion. Same approaches were applied for another study with male and female WT, Cyp7a1KO, FxrKO, and Fxr/Cyp7a1 DKO mice from 8 and 16 weeks of age. In another independent study, pre-weaned FxrKO mice (16–18 days old) were bled, and TBA levels were measured to identify congenital cholemia and then tracked from 4–26 weeks of age. The cholemia threshold (pathologic TBA levels) was determined on the basis of reports in intrahepatic cholestasis of pregnancy where fetal complications occurred when TBA levels were ≥40 μmol/L.32
Total Bile Acid Quantification
TBA in hemolysis-free sera and urine were measured using a TBA assay kit (enzyme cycling method; Diazyme Laboratories) according to the manufacturer’s protocol. Urinary TBA was normalized to creatinine measured by the creatinine kit (Randox).
Bile Acid Quantitation by Ultra-High Performance Liquid Chromatography-Tandem Mass Spectrometry
Bile acid levels were measured using a published protocol84 with minor modification. Targeted analysis of bile acids in serum was performed using a Vanquish UHPLC system coupled with a TSQ Quantis Triple Quadrupole mass spectrometer (Thermo Fisher Scientific) with an ACQUITY C8 BEH UPLC column (2.1 ×100 mm, 1.7 μm) (Waters). Sera (50 μL) were extracted with 150 μL ice-cold methanol containing 0.5 μmol/L deuterated internal standards. After 20 minutes of incubation at –20°C, the samples were centrifuged (12,000g, 4°C, and 10 minutes), 150 μL of supernatants was transferred to autosampler vials. Bile acids were detected by multiple reaction monitoring and selected ion monitoring modes. The results were quantified by comparing integrated peak areas against standard curves with Skyline (MacCoss Lab Software).
PSS Detection
Two methods were used to assess PSS in FxrKO mice with congenital cholemia. First, we injected Mercox II resin (Ladd Research) into the portal vein with a 30-gauge, 0.5-inch needle at necropsy/dissection. Resin cast was immersed in 15% KOH overnight, rinsed with water, and followed by visual inspection. Second, 4 kDa FITC–dextran (Sigma-Aldrich, 0.136 mg/g body weight) was injected into the portal vein, and then the liver was extracted and imaged via IVIS at excitation 490 nm and emission 520 nm.
Intervention With Dietary Cholestyramine
Cholestyramine is a large cationic exchange resin polymer that sequesters bile acids and enhances their fecal excretion.35 Grain-based chow diet (LabDiet 5001) was supplemented with cholestyramine (2% w/w, Sigma-Aldrich) and given to female WT and FxrKO mice starting either at 6 weeks of age (before cholemia) or at 26 weeks of age (after cholemia). Mice were fed cholestyramine for 60 days and tracked for changes in serum TBA levels and liver injury markers.
Atorvastatin Supplemented Diet Intervention
Atorvastatin, an inhibitor of HMG-CoA reductase, lowers cholesterol levels. LabDiet 5001 was supplemented with atorvastatin calcium (0.01% w/w, MilliporeSigma) and fed to female WT and FxrKO mice starting either at 6 weeks of age (before cholemia) or at 26 weeks of age (after cholemia). Mice were fed atorvastatin diet for 90 days and tracked for changes in serum TBA levels and liver injury markers.
Antibiotics Administration for Gut Microbiota Ablation
Broad-spectrum antibiotics (ampicillin [1.0 g/L] + neomycin [0.5 g/L]; Sigma-Aldrich) were given to 6-week-old female WT and FxrKO mice in the drinking water, as described previously,39 for 90 days. Antibiotics were changed once weekly. Mice were tracked for changes in serum TBA levels and liver injury markers.
Taurine Treatment
Taurine is a sulfur-containing, non-proteinaceous amino acid that can conjugate with bile acids, which increases their excretion rate.37 Drinking water containing 0.5% v/v of taurine (Sigma-Aldrich) was given to 6-week-old female WT and FxrKO mice for 90 days, and mice were tracked for changes in serum TBA levels and liver injury markers.
Liver Cancer Study
FxrKO mice develop HCC at 15–16 months of age, and this happens spontaneously without the introduction of a hepatocarcinogen or dietary intervention.24,25 Accordingly, male and female WT and FxrKO mice were aged until 16 months and assessed for spontaneous HCC.
Euthanasia With Tissue and Serum Collection
At the end of each bile acid tracking and intervention studies, male and female WT and FxrKO mice underwent 5-hour fasting, were euthanized via CO2 asphyxiation, and their blood was collected using a BD microtainer (Becton, Dickinson) via cardiac puncture. Hemolysis-free sera were obtained after centrifugation (10,000 rpm for 10 minutes at 4°C) and stored at –80°C until further analysis of cholestatic injury and liver cancer markers as described below. For the bile acid tracking study, livers from 16-week-old WT and FxrKO mice were processed for qRT-PCR. For the 16-month liver cancer study, livers were collected for organ weight and gross images.
Serum Lipids and Transaminases Measurement
Serum total cholesterol, AST, ALT, ALP, and total bilirubin were measured by using biochemical kits from Randox Laboratories (Kearneysville, WV).
Enzyme-linked Immunosorbent Assay
Serum AFP was measured using the Duoset ELISA kit (R&D Systems; Minneapolis, MN) according to the manufacturer’s protocol.
Quantitative Reverse-Transcription Polymerase Chain Reaction
Liver samples from 16-week-old male and female WT and FxrKO mice were analyzed for the relative expression of genes encoding for bile acid de novo synthesis, conjugation, regulators, transporters, and detoxification. Moreover, select oncogenes and cell cycle regulator genes were analyzed. RNA was extracted from liver tissue using TRI reagent (Sigma-Aldrich) as per the manufacturer’s protocol. The cDNA was synthesized from 800 ng of purified RNA using the qScript cDNA Synthesis Kit (Quanta BioSciences). QRT-PCR was performed using the Step One Plus Real-Time PCR System (Applied Biosystems) in a 10 μL reaction mixture containing cDNA, SYBR Green Master Mix (Quanta BioSciences), and mouse-specific oligonucleotides (Supplementary Table 1). The 36B4 gene was used as the housekeeping gene. The data were expressed as a relative fold-change in comparison with the WT male as control.
Statistical Analysis
All data are presented as mean ± standard error of the mean (SEM). Statistical significance between 2 groups was calculated using unpaired, two-tailed Student t test. Data from more than 2 groups were compared using a one-way analysis of variance, followed by Tukey’s multiple comparison test (to compare the mean of each column with the mean of every other column). Pearson correlation was used to establish the association of 2 variables. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, and ∗∗∗∗P < .0001 were considered statistically significant. All statistical analyses were performed with the GraphPad Prism 9.0 program (GraphPad).
Acknowledgments
The authors thank Mrunmayee Kandalgaonkar and Sareh Zeydabadinejad for their technical assistance.
CRediT Authorship Contributions
Rachel M. Golonka (Conceptualization: Lead; Data curation: Lead; Formal analysis: Lead; Methodology: Lead; Visualization: Lead; Writing – original draft: Lead; Writing – review & editing: Lead)
Beng San Yeoh (Data curation: Equal; Formal analysis: Equal; Writing – review & editing: Equal)
Piu Saha (Data curation: Equal; Formal analysis: Equal; Writing – review & editing: Equal)
Yuan Tian (Data curation: Supporting; Formal analysis: Supporting)
John Y. L. Chiang (Formal analysis: Supporting; Writing – review & editing: Supporting)
Andrew D. Patterson (Formal analysis: Supporting; Writing – review & editing: Supporting)
Andrew T. Gewirtz (Formal analysis: Supporting; Funding acquisition: Supporting)
Bina Joe (Formal analysis: Supporting; Writing – review & editing: Supporting)
Matam Vijay-Kumar, PhD (Conceptualization: Equal; Formal analysis: Equal; Funding acquisition: Lead; Investigation: Lead; Resources: Lead; Validation: Lead; Writing – review & editing: Equal)
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding Matam Vijay-Kumar is supported by R01 grant from the National Institutes of Health (NIH) [grant number CA219144]. Rachel M. Golonka is supported by the National Cancer Institute of the NIH under award number F31CA260842. Beng San Yeoh is supported by Postdoctoral Fellowship from American Heart Association (AHA) under award ID 831112 and the American Liver Foundation’s Liver Scholar Award. Piu Saha is supported by Crohn's and Colitis Foundation (CCF) and AHA Career Development Awards, grant numbers #854385 and #855256, respectively.
Data Availability The data generated in this study are available upon request from the corresponding author. All authors had access to the study data and had received and approved the final manuscript.
Note: To access the supplementary material accompanying this article, go to the full text version at http://doi.org/10.1016/j.jcmgh.2024.01.011.
Supplementary Material
References
- 1.Rumgay H., Arnold M., Ferlay J., et al. Global burden of primary liver cancer in 2020 and predictions to 2040. J Hepatol. 2022;77:1598–1606. doi: 10.1016/j.jhep.2022.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cao W., Yu P., Yang K., et al. Aflatoxin B1: metabolism, toxicology, and its involvement in oxidative stress and cancer development. Toxicol Mech Methods. 2022;32:395–419. doi: 10.1080/15376516.2021.2021339. [DOI] [PubMed] [Google Scholar]
- 3.Coskun E., Jaruga P., Vartanian V., et al. Aflatoxin-guanine DNA adducts and oxidatively induced DNA damage in aflatoxin-treated mice in vivo as measured by liquid chromatography-tandem mass spectrometry with isotope dilution. Chem Res Toxicol. 2019;32:80–89. doi: 10.1021/acs.chemrestox.8b00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chidambaranathan-Reghupaty S., Fisher P.B., Sarkar D. Hepatocellular carcinoma (HCC): epidemiology, etiology and molecular classification. Adv Cancer Res. 2021;149:1–61. doi: 10.1016/bs.acr.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li S., Mo C., Huang S., et al. Over-expressed testis-specific protein Y-encoded 1 as a novel biomarker for male hepatocellular carcinoma. PLoS One. 2014;9 doi: 10.1371/journal.pone.0089219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsuei D.J., Hsu H.C., Lee P.H., et al. RBMY, a male germ cell-specific RNA-binding protein, activated in human liver cancers and transforms rodent fibroblasts. Oncogene. 2004;23:5815–5822. doi: 10.1038/sj.onc.1207773. [DOI] [PubMed] [Google Scholar]
- 7.Kido T., Lo R.C., Li Y., et al. The potential contributions of a Y-located protooncogene and its X homologue in sexual dimorphisms in hepatocellular carcinoma. Hum Pathol. 2014;45:1847–1858. doi: 10.1016/j.humpath.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 8.Nakatani T., Roy G., Fujimoto N., et al. Sex hormone dependency of diethylnitrosamine-induced liver tumors in mice and chemoprevention by leuprorelin. Jpn J Cancer Res. 2001;92:249–256. doi: 10.1111/j.1349-7006.2001.tb01089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naugler W.E., Sakurai T., Kim S., et al. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317:121–124. doi: 10.1126/science.1140485. [DOI] [PubMed] [Google Scholar]
- 10.Li Y., Li H., Spitsbergen J.M., et al. Males develop faster and more severe hepatocellular carcinoma than females in kras(V12) transgenic zebrafish. Sci Rep. 2017;7 doi: 10.1038/srep41280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sukocheva O.A. Estrogen, estrogen receptors, and hepatocellular carcinoma: are we there yet? World J Gastroenterol. 2018;24:1–4. doi: 10.3748/wjg.v24.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang H., Spencer K., Burley S.K., et al. Toward improving androgen receptor-targeted therapies in male-dominant hepatocellular carcinoma. Drug Discov Today. 2021;26:1539–1546. doi: 10.1016/j.drudis.2021.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marin J.J.G., Serrano M.A., Monte M.J., et al. Role of genetic variations in the hepatic handling of drugs. Int J Mol Sci. 2020;21 doi: 10.3390/ijms21082884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Camilleri M. Bile acid detergency: permeability, inflammation, and effects of sulfation. Am J Physiol Gastrointest Liver Physiol. 2022;322:G480–G488. doi: 10.1152/ajpgi.00011.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kastrinou Lampou V., Poller B., Huth F., et al. Novel insights into bile acid detoxification via CYP, UGT and SULT enzymes. Toxicol In Vitro. 2023;87 doi: 10.1016/j.tiv.2022.105533. [DOI] [PubMed] [Google Scholar]
- 16.Barnes S., Burhol P.G., Zander R., et al. Enzymatic sulfation of glycochenodeoxycholic acid by tissue fractions from adult hamsters. J Lipid Res. 1979;20:952–959. [PubMed] [Google Scholar]
- 17.Hammerman K.J., Chen L.J., Fernandez-Corugedo A., et al. Sex differences in hepatic sulfation of taurolithocholate in the rat. Gastroenterology. 1978;75:1021–1025. [PubMed] [Google Scholar]
- 18.Kong B., Wang L., Chiang J.Y., et al. Mechanism of tissue-specific farnesoid X receptor in suppressing the expression of genes in bile-acid synthesis in mice. Hepatology. 2012;56:1034–1043. doi: 10.1002/hep.25740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen W., Ding M., Ji L., et al. Bile acids promote the development of HCC by activating inflammasome. Hepatol Commun. 2023;7 doi: 10.1097/HC9.0000000000000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ohtaki Y., Hida T., Hiramatsu K., et al. Deoxycholic acid as an endogenous risk factor for hepatocarcinogenesis and effects of gomisin A, a lignan component of Schizandra fruits. Anticancer Res. 1996;16:751–755. [PubMed] [Google Scholar]
- 21.Sun L., Beggs K., Borude P., et al. Bile acids promote diethylnitrosamine-induced hepatocellular carcinoma via increased inflammatory signaling. Am J Physiol Gastrointest Liver Physiol. 2016;311:G91–G104. doi: 10.1152/ajpgi.00027.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamada S., Takashina Y., Watanabe M., et al. Bile acid metabolism regulated by the gut microbiota promotes non-alcoholic steatohepatitis-associated hepatocellular carcinoma in mice. Oncotarget. 2018;9:9925–9939. doi: 10.18632/oncotarget.24066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li T., Apte U. Bile acid metabolism and signaling in cholestasis, inflammation, and cancer. Adv Pharmacol. 2015;74:263–302. doi: 10.1016/bs.apha.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim I., Morimura K., Shah Y., et al. Spontaneous hepatocarcinogenesis in farnesoid X receptor-null mice. Carcinogenesis. 2007;28:940–946. doi: 10.1093/carcin/bgl249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang F., Huang X., Yi T., et al. Spontaneous development of liver tumors in the absence of the bile acid receptor farnesoid X receptor. Cancer Res. 2007;67:863–867. doi: 10.1158/0008-5472.CAN-06-1078. [DOI] [PubMed] [Google Scholar]
- 26.Takahashi S., Tanaka N., Fukami T., et al. Role of farnesoid X receptor and bile acids in hepatic tumor development. Hepatol Commun. 2018;2:1567–1582. doi: 10.1002/hep4.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Degirolamo C., Modica S., Vacca M., et al. Prevention of spontaneous hepatocarcinogenesis in farnesoid X receptor-null mice by intestinal-specific farnesoid X receptor reactivation. Hepatology. 2015;61:161–170. doi: 10.1002/hep.27274. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y.D., Chen W.D., Li C., et al. Farnesoid X receptor antagonizes JNK signaling pathway in liver carcinogenesis by activating SOD3. Mol Endocrinol. 2015;29:322–331. doi: 10.1210/me.2014-1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anakk S., Bhosale M., Schmidt V.A., et al. Bile acids activate YAP to promote liver carcinogenesis. Cell Rep. 2013;5:1060–1069. doi: 10.1016/j.celrep.2013.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wolfe A., Thomas A., Edwards G., et al. Increased activation of the Wnt/beta-catenin pathway in spontaneous hepatocellular carcinoma observed in farnesoid X receptor knockout mice. J Pharmacol Exp Ther. 2011;338:12–21. doi: 10.1124/jpet.111.179390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sinal C.J., Tohkin M., Miyata M., et al. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell. 2000;102:731–744. doi: 10.1016/s0092-8674(00)00062-3. [DOI] [PubMed] [Google Scholar]
- 32.Glantz A., Marschall H.U., Mattsson L.A. Intrahepatic cholestasis of pregnancy: relationships between bile acid levels and fetal complication rates. Hepatology. 2004;40:467–474. doi: 10.1002/hep.20336. [DOI] [PubMed] [Google Scholar]
- 33.Carlson S.E., Mitchell A.D., Goldfarb S. Sex-related differences in diurnal activities and development of hepatic microsomal 3-hydroxy-3-methylglutaryl coenzyme A reductase and cholesterol 7alpha-hydroxylase. Biochim Biophys Acta. 1978;531:115–124. doi: 10.1016/0005-2760(78)90188-1. [DOI] [PubMed] [Google Scholar]
- 34.Kuroki S., Muramoto S., Kuramoto T., et al. Sex differences in gallbladder bile acid composition and hepatic steroid 12 alpha-hydroxylase activity in hamsters. J Lipid Res. 1983;24:1543–1549. [PubMed] [Google Scholar]
- 35.Riaz S., John S. StatPearls; FL: 2022. Cholestyramine resin. Treasure Island. [PubMed] [Google Scholar]
- 36.Schwarz M., Russell D.W., Dietschy J.M., et al. Alternate pathways of bile acid synthesis in the cholesterol 7alpha-hydroxylase knockout mouse are not upregulated by either cholesterol or cholestyramine feeding. J Lipid Res. 2001;42:1594–1603. [PubMed] [Google Scholar]
- 37.Bellentani S., Pecorari M., Cordoma P., et al. Taurine increases bile acid pool size and reduces bile saturation index in the hamster. J Lipid Res. 1987;28:1021–1027. [PubMed] [Google Scholar]
- 38.Alrehaili B.D., Lee M., Takahashi S., et al. Bile acid conjugation deficiency causes hypercholanemia, hyperphagia, islet dysfunction, and gut dysbiosis in mice. Hepatol Commun. 2022;6:2765–2780. doi: 10.1002/hep4.2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yeoh B.S., Saha P., Golonka R.M., et al. Enterohepatic shunt-driven cholemia predisposes to liver cancer. Gastroenterology. 2022;163:1658–1671 e16. doi: 10.1053/j.gastro.2022.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Franchi-Abella S., Gonzales E., Ackermann O., et al. Congenital portosystemic shunts: diagnosis and treatment. Abdom Radiol (NY) 2018;43:2023–2036. doi: 10.1007/s00261-018-1619-8. [DOI] [PubMed] [Google Scholar]
- 41.Heydari R., Jangravi Z., Maleknia S., et al. Y chromosome is moving out of sex determination shadow. Cell Biosci. 2022;12:4. doi: 10.1186/s13578-021-00741-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chlamydas S., Markouli M., Strepkos D., et al. Epigenetic mechanisms regulate sex-specific bias in disease manifestations. J Mol Med (Berl) 2022;100:1111–1123. doi: 10.1007/s00109-022-02227-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang L., Wu J., Wu Q., et al. Sex steroid axes in determining male predominance in hepatocellular carcinoma. Cancer Lett. 2023;555 doi: 10.1016/j.canlet.2022.216037. [DOI] [PubMed] [Google Scholar]
- 44.Gao B., Wang Y., Li C., et al. Estrogen-related genes influence immune cell infiltration and immunotherapy response in hepatocellular carcinoma. Front Immunol. 2023;14 doi: 10.3389/fimmu.2023.1114717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McGlynn K.A., Sahasrabuddhe V.V., Campbell P.T., et al. Reproductive factors, exogenous hormone use and risk of hepatocellular carcinoma among US women: results from the Liver Cancer Pooling Project. Br J Cancer. 2015;112:1266–1272. doi: 10.1038/bjc.2015.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Petrick J.L., McMenamin U.C., Zhang X., et al. Exogenous hormone use, reproductive factors and risk of intrahepatic cholangiocarcinoma among women: results from cohort studies in the Liver Cancer Pooling Project and the UK Biobank. Br J Cancer. 2020;123:316–324. doi: 10.1038/s41416-020-0835-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu Y., Zhu Y., Wu Z., et al. Hepatocyte-specific HDAC3 ablation promotes hepatocellular carcinoma in females by suppressing Foxa1/2. BMC Cancer. 2023;23:906. doi: 10.1186/s12885-023-11393-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun R., Zhang Z., Bao R., et al. Loss of SIRT5 promotes bile acid-induced immunosuppressive microenvironment and hepatocarcinogenesis. J Hepatol. 2022;77:453–466. doi: 10.1016/j.jhep.2022.02.030. [DOI] [PubMed] [Google Scholar]
- 49.Wang L., Luo Q., Zeng S., et al. Disordered farnesoid X receptor signaling is associated with liver carcinogenesis in Abcb11-deficient mice. J Pathol. 2021;255:412–424. doi: 10.1002/path.5780. [DOI] [PubMed] [Google Scholar]
- 50.Stepien M., Lopez-Nogueroles M., Lahoz A., et al. Prediagnostic alterations in circulating bile acid profiles in the development of hepatocellular carcinoma. Int J Cancer. 2022;150:1255–1268. doi: 10.1002/ijc.33885. [DOI] [PubMed] [Google Scholar]
- 51.Shi Q., Yuan X., Xue C., et al. Establishment and validation of a novel risk score for hepatocellular carcinoma based on bile acid and bile salt metabolism-related genes. Int J Mol Sci. 2023:24. doi: 10.3390/ijms24108597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang W., Zhang Y., Wan Y., et al. A bile acid-related prognostic signature in hepatocellular carcinoma. Sci Rep. 2022;12 doi: 10.1038/s41598-022-26795-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cui H., Lian J., Xu B., et al. Identification of a bile acid and bile salt metabolism-related lncRNA signature for predicting prognosis and treatment response in hepatocellular carcinoma. Sci Rep. 2023;13 doi: 10.1038/s41598-023-46805-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang G., Guan J., Yang Q., et al. Development of a bile acid-related gene signature for predicting survival in patients with hepatocellular carcinoma. Comput Math Methods Med. 2022;2022 doi: 10.1155/2022/9076175. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 55.Uppal H., Saini S.P., Moschetta A., et al. Activation of LXRs prevents bile acid toxicity and cholestasis in female mice. Hepatology. 2007;45:422–432. doi: 10.1002/hep.21494. [DOI] [PubMed] [Google Scholar]
- 56.Bojkowska K., Aloisio F., Cassano M., et al. Liver-specific ablation of Kruppel-associated box-associated protein 1 in mice leads to male-predominant hepatosteatosis and development of liver adenoma. Hepatology. 2012;56:1279–1290. doi: 10.1002/hep.25767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cassano M., Offner S., Planet E., et al. Polyphenic trait promotes liver cancer in a model of epigenetic instability in mice. Hepatology. 2017;66:235–251. doi: 10.1002/hep.29182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Niu Y., Xu M., Slagle B.L., et al. Farnesoid X receptor ablation sensitizes mice to hepatitis B virus X protein-induced hepatocarcinogenesis. Hepatology. 2017;65:893–906. doi: 10.1002/hep.28924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cameron R.G., Imaida K., Tsuda H., et al. Promotive effects of steroids and bile acids on hepatocarcinogenesis initiated by diethylnitrosamine. Cancer Res. 1982;42:2426–2428. [PubMed] [Google Scholar]
- 60.Xing L., Zhang Y., Li S., et al. A dual coverage monitoring of the bile acids profile in the liver-gut axis throughout the whole inflammation-cancer transformation progressive: reveal hepatocellular carcinoma pathogenesis. Int J Mol Sci. 2023:24. doi: 10.3390/ijms24054258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chun H.J., Shim Y.J., Kwon Y.H. Cholic acid supplementation accelerates the progression of nonalcoholic fatty liver disease to the procarcinogenic state in mice fed a high-fat and high-cholesterol diet. J Nutr Biochem. 2022;100 doi: 10.1016/j.jnutbio.2021.108869. [DOI] [PubMed] [Google Scholar]
- 62.Chen W., Zhang Q., Ding M., et al. Alcohol triggered bile acid disequilibrium by suppressing BSEP to sustain hepatocellular carcinoma progression. Chem Biol Interact. 2022;356 doi: 10.1016/j.cbi.2022.109847. [DOI] [PubMed] [Google Scholar]
- 63.Kettner N.M., Voicu H., Finegold M.J., et al. Circadian homeostasis of liver metabolism suppresses hepatocarcinogenesis. Cancer Cell. 2016;30:909–924. doi: 10.1016/j.ccell.2016.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Padilla J., Osman N.M., Bissig-Choisat B., et al. Circadian dysfunction induces NAFLD-related human liver cancer in a mouse model. J Hepatol. 2023 doi: 10.1016/j.jhep.2023.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu Y., Yao N., Feng Y., et al. Identification and characterization of sexual dimorphism-linked gene expression profile in hepatocellular carcinoma. Oncol Rep. 2019;42:937–952. doi: 10.3892/or.2019.7217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Setchell K.D., Schwarz M., O’Connell N.C., et al. Identification of a new inborn error in bile acid synthesis: mutation of the oxysterol 7alpha-hydroxylase gene causes severe neonatal liver disease. J Clin Invest. 1998;102:1690–1703. doi: 10.1172/JCI2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Umetani M., Domoto H., Gormley A.K., et al. 27-Hydroxycholesterol is an endogenous SERM that inhibits the cardiovascular effects of estrogen. Nat Med. 2007;13:1185–1192. doi: 10.1038/nm1641. [DOI] [PubMed] [Google Scholar]
- 68.Xia J.K., Tang N., Wu X.Y., et al. Deregulated bile acids may drive hepatocellular carcinoma metastasis by inducing an immunosuppressive microenvironment. Front Oncol. 2022;12 doi: 10.3389/fonc.2022.1033145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shen R., Ke L., Li Q., et al. Abnormal bile acid-microbiota crosstalk promotes the development of hepatocellular carcinoma. Hepatol Int. 2022;16:396–411. doi: 10.1007/s12072-022-10299-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shen Y., Lu C., Song Z., et al. Ursodeoxycholic acid reduces antitumor immunosuppression by inducing CHIP-mediated TGF-beta degradation. Nat Commun. 2022;13:3419. doi: 10.1038/s41467-022-31141-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nguyen P.T., Kanno K., Pham Q.T., et al. Senescent hepatic stellate cells caused by deoxycholic acid modulates malignant behavior of hepatocellular carcinoma. J Cancer Res Clin Oncol. 2020;146:3255–3268. doi: 10.1007/s00432-020-03374-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yoshimoto S., Loo T.M., Atarashi K., et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature. 2013;499:97–101. doi: 10.1038/nature12347. [DOI] [PubMed] [Google Scholar]
- 73.Durazzo M., Belci P., Collo A., et al. Gender specific medicine in liver diseases: a point of view. World J Gastroenterol. 2014;20:2127–2135. doi: 10.3748/wjg.v20.i9.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cariello M., Peres C., Zerlotin R., et al. Long-term administration of nuclear bile acid receptor FXR agonist prevents spontaneous hepatocarcinogenesis in Abcb4(-/-) mice. Sci Rep. 2017;7 doi: 10.1038/s41598-017-11549-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gadaleta R.M., Scialpi N., Peres C., et al. Suppression of hepatic bile acid synthesis by a non-tumorigenic FGF19 analogue protects mice from fibrosis and hepatocarcinogenesis. Sci Rep. 2018;8 doi: 10.1038/s41598-018-35496-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shi L., Zhao T., Huang L., et al. Engineered FGF19(DeltaKLB) protects against intrahepatic cholestatic liver injury in ANIT-induced and Mdr2-/- mice model. BMC Biotechnol. 2023;23:43. doi: 10.1186/s12896-023-00810-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rizzolo D., Kong B., Piekos S., et al. Effects of overexpression of fibroblast growth factor 15/19 on hepatic drug metabolizing enzymes. Drug Metab Dispos. 2022;50:468–477. doi: 10.1124/dmd.121.000416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cui G., Martin R.C., Jin H., et al. Up-regulation of FGF15/19 signaling promotes hepatocellular carcinoma in the background of fatty liver. J Exp Clin Cancer Res. 2018;37:136. doi: 10.1186/s13046-018-0781-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lin B.C., Desnoyers L.R. FGF19 and cancer. Adv Exp Med Biol. 2012;728:183–194. doi: 10.1007/978-1-4614-0887-1_12. [DOI] [PubMed] [Google Scholar]
- 80.Lodato N.J., Melia T., Rampersaud A., et al. Sex-differential responses of tumor promotion-associated genes and dysregulation of novel long noncoding RNAs in constitutive androstane receptor-activated mouse liver. Toxicol Sci. 2017;159:25–41. doi: 10.1093/toxsci/kfx114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yoshinari K., Shizu R. Distinct roles of the sister nuclear receptors PXR and CAR in liver cancer development. Drug Metab Dispos. 2022;50:1019–1026. doi: 10.1124/dmd.121.000481. [DOI] [PubMed] [Google Scholar]
- 82.de Wit K., Schaapman J.J., Nevens F., et al. Prevention of hepatic encephalopathy by administration of rifaximin and lactulose in patients with liver cirrhosis undergoing placement of a transjugular intrahepatic portosystemic shunt (TIPS): a multicentre randomised, double blind, placebo controlled trial (PEARL trial) BMJ Open Gastroenterol. 2020;7 doi: 10.1136/bmjgast-2020-000531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ferrell J.M., Boehme S., Li F., et al. Cholesterol 7alpha-hydroxylase-deficient mice are protected from high-fat/high-cholesterol diet-induced metabolic disorders. J Lipid Res. 2016;57:1144–1154. doi: 10.1194/jlr.M064709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tian Y., Cai J., Allman E.L., et al. Quantitative analysis of bile acid with UHPLC-MS/MS. Methods Mol Biol. 2021;2194:291–300. doi: 10.1007/978-1-0716-0849-4_15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.