Abstract
Background:
Chronic arsenic exposure has been associated with an increased risk of cardiovascular disease; diabetes; cancers of the lung, pancreas and prostate; and all-cause mortality in American Indian communities in the Strong Heart Study.
Objective:
The Strong Heart Water Study (SHWS) designed and evaluated a multilevel, community-led arsenic mitigation program to reduce arsenic exposure among private well users in partnership with Northern Great Plains American Indian Nations.
Methods:
A cluster randomized controlled trial (cRCT) was conducted to evaluate the effectiveness of the SHWS arsenic mitigation program over a 2-y period on a) urinary arsenic, and b) reported use of arsenic-safe water for drinking and cooking. The cRCT compared the installation of a point-of-use arsenic filter and a mobile Health (mHealth) program (3 phone calls; SHWS mHealth and Filter arm) to a more intensive program, which included this same program plus three home visits (3 phone calls and 3 home visits; SHWS Intensive arm).
Results:
A 47% reduction in urinary arsenic [ creatinine] was observed from baseline to the final follow-up when both study arms were combined. By treatment arm, the reduction in urinary arsenic from baseline to the final follow-up visit was 55% in the mHealth and Filter arm ( creatinine) and 30% in the Intensive arm ( creatinine). There was no significant difference in urinary arsenic levels by treatment arm at the final follow-up visit comparing the Intensive vs. mHealth and Filter arms: GM ratio of 1.21 (95% confidence interval: 0.77, 1.90). In both arms combined, exclusive use of arsenic-safe water from baseline to the final follow-up visit significantly increased for water used for cooking (17% to 53%) and drinking (12% to 46%).
Discussion:
Delivery of the interventions for the community-led SHWS arsenic mitigation program, including the installation of a point-of-use arsenic filter and a mHealth program on the use of arsenic-safe water (calls only, no home visits), resulted in a significant reduction in urinary arsenic and increases in reported use of arsenic-safe water for drinking and cooking during the 2-y study period. These results demonstrate that the installation of an arsenic filter and phone calls from a mHealth program presents a promising approach to reduce water arsenic exposure among private well users. https://doi.org/10.1289/EHP12548
Introduction
In the United States, individuals use private wells with arsenic levels above the US Environmental Protection Agency (EPA) guideline for drinking water ().1 Arsenic is a highly toxic and carcinogenic element, classified as the top hazardous substance by the Agency for Toxic Substances and Disease Registry.2 The US EPA mandates for potable water regulate only public water supplies; therefore, the burden lies with private well users to ensure drinking water safety. Arsenic in drinking water disproportionately affects rural populations relying on private wells, including American Indian communities.3–7 The Strong Heart Study (SHS), conducted in partnership with American Indian communities, found an association between arsenic exposure and cardiovascular disease8–10; diabetes prevalence and control11; albuminuria and kidney disease12,13; lung function measures14; neurological outcomes15,16; cancers of the lung, pancreas and prostate17; and all-cause mortality.18 These findings demonstrate the urgent need for interventions to reduce arsenic exposure in these communities.
Despite the growing evidence on the health implications of chronic arsenic exposure, there has been limited research focused on developing and evaluating effective interventions to reduce arsenic exposure for private well users in the United States. Most previous studies have focused on evaluating the effectiveness of arsenic filters in reducing water arsenic exposure, but not on developing approaches to ensure sustained use of filters over time in a way that will effectively reduce arsenic exposure.19–23 Some studies, mostly cross-sectional, have investigated the psychosocial factors associated with an individual’s or household’s decision to use an arsenic-safe drinking water source.19–28 However, few of these studies, existing in Bangladesh only, took the important next step of developing interventions targeting the psychosocial factors associated with using arsenic-safe water.29–31 Furthermore, the only randomized controlled trials (RCTs) of arsenic interventions were conducted in South Asia, and they have mostly focused on switching from arsenic-contaminated to alternative, arsenic-safe drinking water sources.32–35 One such trial in Bangladesh of arsenic filters made from sand and iron found small, nonsignificant reductions in urinary arsenic 1 month after installation, which attenuated thereafter.35 Research is needed on the design and implementation of evidence-based arsenic interventions for private well users in the United States.
The objective of the Strong Heart Water Study (SHWS) was to design and evaluate a multilevel, community-led arsenic mitigation program to reduce arsenic exposure among private well users in partnership with American Indian communities that participated in the SHS in the Northern Great Plains. As part of the SHWS program, a water arsenic testing program of 440 wells in the community found that 29% of private wells within SHS partner communities had arsenic levels of .36 The interventions delivered as part of the SHWS program were designed through formative research that included semi-structured interviews, community workshops, and a pilot study, described in detail by Thomas et al.37 A community-led approach for intervention design and implementation ensured the intervention targeted the needs of the community. The SHWS program interventions included water arsenic testing, installation of a point-of-use (POU) arsenic filter, a mobile Health (mHealth) program (phone call reminders), video testimonials from community members, home visits, and intervention promotional materials designed to facilitate arsenic-safe water use for all drinking and cooking. In the pilot study of the program, the intervention had high acceptability among participants.37 In addition, the installed POU arsenic filters performed well over the 9-month pilot period, with no filter failures.38
This present study evaluated the impact of the interventions delivered as part of the SHWS arsenic mitigation program on the following main outcomes: a) urinary arsenic (a biomarker of arsenic exposure and internal dose), and b) self-reported use of arsenic-safe water for drinking and cooking. We hypothesized that delivery of the SHWS interventions would reduce arsenic exposure and internal dose among private well users.
Methods
Study Design
Complete study methods have been previously described.39–41 In summary, a two-arm cluster RCT (cRCT) was conducted in a Northern Great Plains American Indian Nation from July 2018 to May 2021, where a cluster was a household (ClinicalTrials.gov Identifier: NCT03725592). Households were recruited through a community-led water arsenic testing program.36,38 The cRCT compared the installation of a POU arsenic filter with an accompanying mHealth program of three phone calls (mHealth and Filter arm) to a more intensive program, which included this same intervention plus three home visits, four video testimonials from community members, and intervention materials (e.g., mug, tankard) (Intensive arm). The SHWS intervention was designed to be delivered over a 12-month period. Informed consent was obtained for all participants. The ethical review boards of the Johns Hopkins Bloomberg School of Public Health, the Great Plains Indian Health Service, and the Tribal Research Review Board approved study procedures.
Participants
Households residing in an American Indian Nation in the Northern Great Plains who used a private well for drinking were eligible to have their well tested for arsenic and uranium to identify households eligible to receive a POU arsenic filter. The SHWS is a separate study occurring in the same communities and via the same networks as the SHS but which enrolled additional new households and participants. Only four households participated in both the SHWS and the SHS. From April 2014 to May 2021, drinking water from the kitchen faucet of 440 households was tested for arsenic and uranium.36 This sample size was based on the number of households interested in water quality testing of private wells at our study site. All water arsenic analyses were performed by inductively coupled plasma mass spectrometry (ICP-MS). To be eligible for the SHWS cRCT, households had to a) have water samples above the maximum contaminant level (MCL) of for arsenic and below the MCL of for uranium, b) have adult () of age eligible and willing to participate, c) have indoor plumbing and a permanent heat source, d) have household member(s) self-identify as American Indian, and e) be willing to grant study members access to the interior of their household for eligibility screening, filter installation, and data collection. In addition, participants had to plan to reside in the household for the next 12 months and reside in the residence for year-round (i.e., in all seasons). Uranium was included in the water quality analysis because the selected POU arsenic filters did not remove uranium from water, and elevated uranium levels were a concern in our study setting.36 Households with elevated uranium were referred to their local Tribal Water Resource Department, given that these households needed to receive a separate water treatment device from the one offered by the SHWS program. Households were enrolled in the SHWS cRCT between July 2018 and November 2019.
Randomization and Masking
Block randomization was performed at the household level, stratified by the presence of a 10- to 17-y-old child in the household, after baseline activities were completed and the POU arsenic filter was installed. Randomly varying block sizes of 2, 4, and 6 were used to reduce bias and achieve overall balance of this age group in the allocation of the households to the two study arms. The study biostatistician (L.H.M.) provided the randomization list, which was produced using a pseudo-random number generator. Sealed opaque envelopes including the randomization assignment for each household were given to the project coordinator (T.Z.). The sealed envelopes were opened in the order provided only after baseline activities were completed and the POU arsenic filter was installed in the household.
Intervention Procedures
The formative research conducted to develop the SHWS arsenic mitigation program and a detailed description of intervention development is published elsewhere.37,38 In brief, the SHWS arsenic mitigation was developed through community-centered participatory formative research using a theory-driven approach and guided by an ecological conceptual framework.42,43 The SHWS intervention focused on four key behaviors: a) obtaining an arsenic test for private wells, b) installing a POU arsenic filter, c) drinking and cooking with arsenic-safe water, and d) changing the POU arsenic filter cartridge after 12 months.
Households who enrolled in the study received a Multipure (model CB-As-SB) POU filtration system, which was installed under the kitchen sink/faucet area. The Multipure Drinking Water System is a POU system designed to remove arsenic from water using an adsorptive media filter. An adsorptive media filter was selected because of concerns raised by community members during formative research on how reverse osmosis could impact the taste of drinking water.37 The specific filter was also selected owing to considerations of cost, ease of use and installation, and the limited change to the taste of the water. The Tribal Housing Authority through funding from Indian Health Service oversaw the installation of the POU arsenic filter. The filter was installed under the kitchen sink connected to a filter faucet alongside the kitchen faucet for individuals to use for drinking and cooking water. The regular kitchen faucet could still be used for nonpotable water purposes, such as washing dishes, washing hands, and household cleaning, to reduce the use burden of the filter device and increase its life span. Tribal Housing Authority plumbers from the community installed the POU arsenic filters and provided households with a replacement cartridge and the manufacturer’s instruction manual on how to use the device (e.g., collect water from the filter faucet and replace the cartridge). Three households requested and had their refrigerator filter and icemaker connected to the arsenic filter.
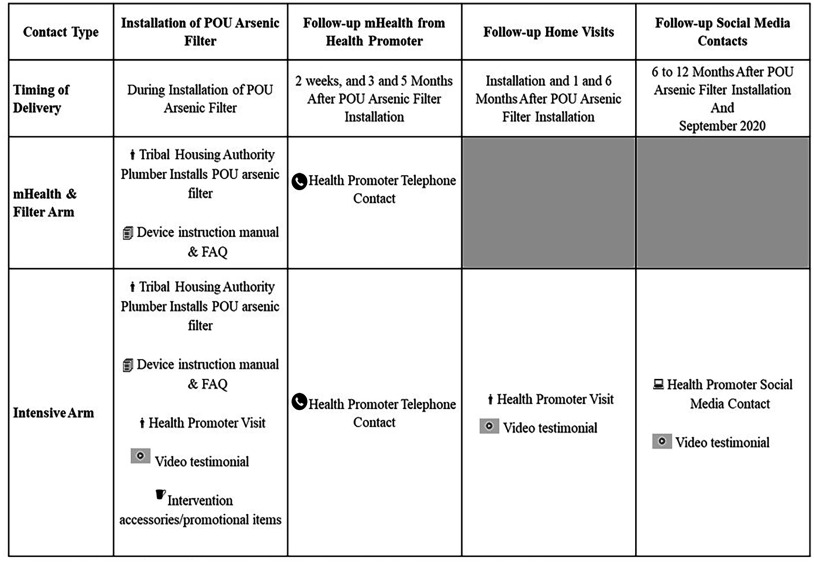
Both study arms (the mHealth and Filter arm and the Intensive arm) received the POU arsenic filter, replacement cartridge, and the manufacturer’s instruction manual (Figure 1). Both arms also received a Frequently Asked Questions document with information on arsenic exposure and the POU filter, and the number for the toll-free wellcare hotline (https://www.wellcarehotline.org). Health promoters from Missouri Breaks Industries Research Inc., a local American Indian-owned and run research organization, performed calls and conducted home visits for SHWS program delivery. The mHealth and Filter arm received phone calls from a health promoter at 2 wks, 3 months, and 5 months post-installation (3 calls) to encourage use of the filter faucet for all water used for drinking and cooking inside the home, and to remind households when to change their filter cartridge. These time points were selected based on our previous formative research.37 The objective of this series of contacts was to encourage adoption and maintenance of an arsenic-safe water source for all drinking and cooking, as well as to address challenges that the household encountered.
Figure 1.
Overview of Strong Heart Water Study (SHWS) program activities. The SHWS took place between July 2018 and May 2021. A total of 84 total participants were enrolled. Note: FAQ, frequently asked questions; mHealth, mobile Health; POU, point-of-use.
The Intensive arm also received the same mHealth program with phone calls scheduled at 2 wks, 3 months, and 5 months post-installation, with the addition of home visits within 2 wks of device installation and 1- and 6-months post-installation (3 home visits and 3 phone calls), video testimonials, and intervention materials. During home visits, health promoters showed three videos promoting the use of arsenic-safe water. These videos were developed by our research team in collaboration with community partners during our formative research, and all videos included community members. The first video was shown when the POU arsenic filter was installed in the home and included testimonials from community elders and a tribal leader, who were also private well users, discussing arsenic toxicity and the importance of using arsenic-safe water for drinking and cooking. The second video was delivered during the home visit at 1-month post-installation and included testimonials from POU arsenic filter recipients on overcoming challenges they had encountered with drinking and cooking with arsenic-safe water. The third video, delivered at 6-months post-installation during home visits and through text messages and social media (Facebook), was on how to change the arsenic filter cartridge. During the COVID-19 pandemic, in September 2020, a fourth video was created encouraging households to continue using their POU arsenic filter throughout the pandemic. This fourth video was delivered through text messages and Facebook to all enrolled Intensive arm households, regardless of the follow-up time point. Last, Intensive arm households also received intervention promotional materials (mugs, water bottles, window clings, and a tankard) as cues to drink and cook with arsenic-safe water. Health communication encouraged households to use arsenic-safe water from the filter faucet for preparing all drinks and food where water was required (e.g., tea, coffee, rice, and soup).
Evaluation Procedures
Prior to the installation of the POU arsenic filter, household members received a baseline evaluation visit, which included a study questionnaire, clinical exam, and urine collection. The study questionnaire collected information on individual and household demographic factors (e.g., age, smoking history), drinking and cooking water use and sources, and health history. The clinical exam included weight, height, and blood pressure measurements. A follow-up visit was planned 6 months and 2 y after the installation of the POU arsenic filter. The COVID-19 pandemic, however, delayed follow-up visits. During follow-up visits, participants were administered another questionnaire on demographic factors, drinking and cooking water use and sources, and health history. A urine sample was collected during follow-up visits.
Arsenic Measurements
Spot urine samples were collected at baseline and at 6 months to 2 y after enrollment using acid-washed containers.7,44 The urine samples were put in cooler boxes with ice packets immediately upon collection, transported to our local laboratory at Missouri Breaks Industries Research Inc. and frozen at within 2 h of collection. Samples were shipped on dry ice to Columbia University for arsenic analysis. In brief, the following procedures were carried out with urine samples from the SHWS using urine certified reference materials (CRMs), and method blanks; further details are provided in the Supplemental Material in “Supplementary File S1.” An aliquot of urine () was treated with hydrogen peroxide at 60°C for 30 min, before dilution to the 5-fold volume. Samples were analyzed using high-performance liquid chromatography (HPLC) coupled to elemental detection using ICP triple quadrupole MS (ICPMS/MS). Separation of individual arsenic species was performed on an Agilent 1260 Infinity-II BioInert series system using a Hamilton PRP-X100 (, particles) analytical column under isocratic elution conditions applying an aqueous mobile phase containing phosphate, nitrate, and 0.5% vol acetonitrile adjusted to pH 9.1. Detection of individual arsenic species was performed on an Agilent 8900 series ICPMS/MS system after HPLC separation. Arsenic species were detected in oxygen reaction mode by monitoring the mass transitions m/z 75 to 91 (75As), 77 to 93 (77Se and 40Ar37Cl), 82 to 98 (82Se), and 53 to 69 (40Ar13C). Quantification was performed after external calibration in matrix-matched mixed arsenic species standards for arsenobetaine (AB), dimethylarsinic acid (DMA), monomethylarsonic acid (MMA), and arsenate [As(V)]. The minimum detection limits (MDLs) for this method were determined to be As L-1 urine (AB, DMA, and MMA) and As L-1 urine [As(V)]; minimum quantitation limits (MQLs) for this method were As L-1 urine (AB and MA), As L-1 urine (DMA), and As L-1 urine [As(V)]. Accuracy of the method for arsenic speciation analysis was determined based on the five urinary CRMs ( each) and resulted in accuracies [] of , , , and for AB, DMA, MMA, and As(V), respectively. Intraday and interday coefficients of variation (CVs) were calculated based on the five certified reference urines ( each) and resulted in mean intraday CVs of 1.7%, 1.9%, 2.5%, and 2.1% and interday CVs of 2.9%, 3.2%, 4.0%, and 3.8% for AB, DMA, MA, and As(V), respectively. A total of one monomethylarsonic acid (MMA) and four inorganic arsenic (iAs) samples were between the MQL and the MDL and were calculated as the mean (MQL to MDL). One sample was below the MDL for iAs, and it was calculated as the MDL divided by the square root of 2.
Urinary arsenic was defined as the sum of iAs (in micrograms per gram creatinine), MMA, and DMA measurements. Arsenic speciation allowed for distinguishing inorganic arsenic (iAs, the sum of arsenite and arsenate), MMA, DMA from organic arsenicals such as arsenobetaine and other arsenic cations, which are considered to be nontoxic. To assess arsenic metabolism (a measure of how the body is transforming iAs into methylated species, with MMA being considered a more toxic intermediate species and DMA a less toxic species that is easier to eliminate from the body), the proportions of urine iAs, MMA, and DMA over the sum of inorganic and methylated species was used to calculate iAs%, MMA%, and DMA% (which sum to 100%). Finally, urinary creatinine was measured using the Mindray BS-200 Chemistry Analyzer (to account for measurement error due to urine dilution) at the Biomarker, Biochemistry, and Biorepository Core at Medstar Health. The testing value range was creatinine in urine.
Outcomes
Primary outcomes were a) changes in urinary arsenic from baseline to the final follow-up visit (before and after installation of the filter) in household members pooled and by study arm, and b) urinary arsenic at the final follow-up visit by study arm. The secondary outcomes were related to a) arsenic metabolism—change in iAs%, MMA%, and DMA% [95% confidence interval (CI)] from baseline to the final follow-up visit (before and after installation of the filter) in household members pooled and by study arm—and iAs%, MMA%, and DMA% at the final follow-up visit by study arm; and b) use of arsenic-safe water for drinking, cooking, and both drinking and cooking—change in exclusive use of arsenic-safe water from baseline to the final follow-up visit in household members pooled and by study arm—and exclusive use of arsenic-safe water at the final follow-up visit pooled and by study arm. Exclusive use was defined as a) exclusive use of arsenic-safe water for drinking, b) exclusive use of arsenic-safe water for cooking, and c) exclusive use of arsenic-safe water for both drinking and cooking. Arsenic-safe water sources included use of the POU arsenic filter faucet, bottled water, or the municipal water system, and arsenic-unsafe sources included use of the kitchen faucet, bathroom faucet, and refrigerator filter or icemaker (if not reported to be connected to the POU arsenic filter).
Power Calculation
Based on our pilot data, we estimated that 520 households of our partner in the Northern Great Plains American Indian Nation would be using private wells that exceeded the US EPA arsenic limit of . We estimated that 55% of these households would be eligible and willing to participate. We thus expected to have a sample size of 300 households for the study (150 per arm). Based on SHS follow-up data at the time the study started, we anticipated a 10% loss to follow-up, resulting in 135 households per study arm. We planned to recruit up to two individuals per household (owing to budgetary constraints). Assuming an intra-cluster correlation of 0.5 in urinary arsenic concentrations within households and a SD of in total urinary arsenic (A. Navas-Acien, personal communication), and type 1 error of 0.05, we estimated that we would have 80% power to detect a difference in mean urinary total arsenic concentrations of between the two study arms. Within study arms, assuming a within-person correlation of 0.5 and within-household correlation of 0.5 for urinary total arsenic and similar assumptions as above, we would have 90% power to detect a mean difference in urinary total arsenic between baseline and follow-up. Similarly, for secondary outcomes, we anticipated having 80% power to detect differences in distributions of continuous outcomes of 0.3 SD when measured among all participants.
Statistical Analysis
The main analysis for the primary outcomes of changes in urinary arsenic from baseline to the final follow-up visit and comparing participants in the mHealth and Filter arm vs. the Intensive arm at the final follow-up visit was performed on the modified intent-to-treat ITT (mITT) population: those household members with baseline and follow-up urine samples. We also report all descriptive results for all participants at baseline even if we did not obtain their follow-up urine samples. Linear regression models were performed to evaluate the change in log-transformed urinary arsenic per gram urine creatinine and percentages of iAs, MMA, and DMA from baseline to the final follow-up visit and between study arms at the final follow-up visit. For the model using log-transformed urinary arsenic per gram urinary creatinine, we exponentiated the beta coefficient to estimate the GM ratio, which represents the relative difference between time points and study arms and is easier to interpret than a mean difference in log units. For the percentages of iAs, MMA, and DMA, the beta coefficients are interpreted as mean differences. The change in self-reported exclusive arsenic-safe water use (for items used for cooking, items used for drinking, and a combination of both cooking and drinking items) in the home from baseline to the final follow-up visit and between study arms at the final follow-up visit was evaluated with logistic regression.
Generalized estimating equations with an exchangeable correlation matrix were used for all regression analyses to account for the within-household correlation. Urinary arsenic study outcomes are shown adjusted for sociodemographic factors (sex and age) and lifestyle factors [ever smoking and baseline body mass index (BMI)]. Selection of covariates was completed during the development of the Data Analysis Plan prior to analysis based on previous studies of urinary arsenic levels in American Indian communities.7,45,46 The final follow-up visit for all participants was used to evaluate study outcomes. Analysis was completed using SAS statistical software (version 9.4; SAS Institute, Inc.) and figures were produced in R (R 4.2.1 Development Core Team 2022).
Results
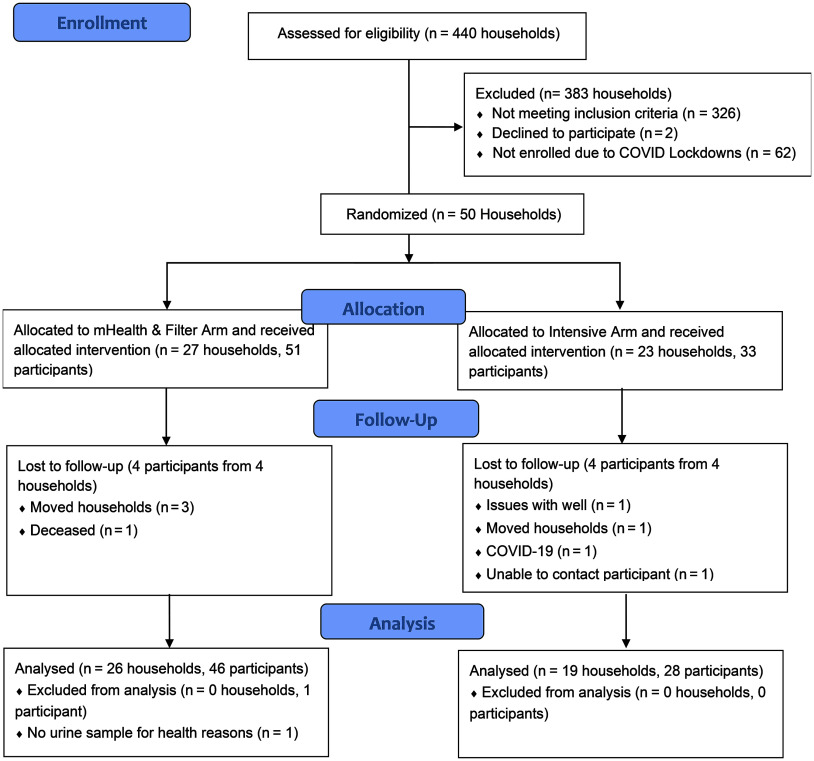
Between July 2018 and March 2020, 114 households were identified as eligible to participate in the study because they had kitchen faucet water arsenic concentrations of (26% of the 440 households screened for arsenic) and uranium concentrations of (Figure 2). Forty-four percent (50/114) of households were enrolled prior to the COVID-19 lockdown in March 2020, which prematurely ended our study recruitment activities. Among the 84 participants residing in the 50 households enrolled in the study, 51 participants from 27 households were randomized to the mHealth and Filter arm and 33 participants from 23 households were randomized to the Intensive arm. The (minimum–maximum) household size at baseline was (1–8) individuals. The age for study participants was , with 6 participants 12–17 years of age (Table 1). Fifty-four percent (45/84) of participants were female, 82% (69/84) reported ever smoking, 49% (41/84) had hypertension, and 38% (32/84) had a mean BMI . The (minimum–maximum) follow-up duration from baseline to the final follow-up visit for study participants was (8.8–32.5) months. Participants in the mHealth and Filter arm vs. the Intensive arm reported higher levels of education (53% vs. 30% more than a high school education) and had higher BMI (31 vs. ). Baseline characteristics by quartile of water arsenic is reported in the Excel Supplemental Material in Supplementary Table S1.
Figure 2.
The Strong Heart Water Study randomized controlled trial profile and analysis population for primary outcomes, June 2016–May 2021. Household assessment for eligibility began in June 2016, and the trial completed in May 2021. Note: mHealth, mobile Health.
Table 1.
Baseline Strong Heart Water Study randomized controlled trial participant characteristics overall and by study arm ().
| Characteristic | Overall | mHealth and Filter arm | Intensive arm |
|---|---|---|---|
| Participants | 84 | 51 | 33 |
| Households | 50 | 27 | 23 |
| Age (y) | |||
| 12–17 | 6 (7) | 4 (8) | 2 (6) |
| 78 (93) | 47 (92) | 31 (94) | |
| Female | 45 (54) | 27 (52) | 18 (55) |
| Education | |||
| High school or less | 47 (56) | 24 (47) | 23 (70) |
| More than high school | 37 (44) | 27 (53) | 10 (30) |
| Ever smoked | 69 (82) | 41 (80) | 28 (85) |
| BMI () | |||
| Overweight (BMI ) | 59 (71) | 36 (70) | 23 (70) |
| Obesity (BMI ) | 32 (38) | 23 (45) | 9 (27) |
| Hypertensiona | 41 (49) | 23 (45) | 18 (55) |
| Systolic bp | |||
| Diastolic bp | |||
| Household water arsenic () [ (min–max)]b | (6.0–210.0) | (6.0–210.0) | (8.5–22.8) |
Note: Values given as , (% of total), or mean (standard deviation). BMI, body mass index; bp, blood pressure; max, maximum; min, minimum; SD, standard deviation.
Based on average systolic bp or diastolic bp during three consecutive readings.
Household water arsenic reported at the household level.
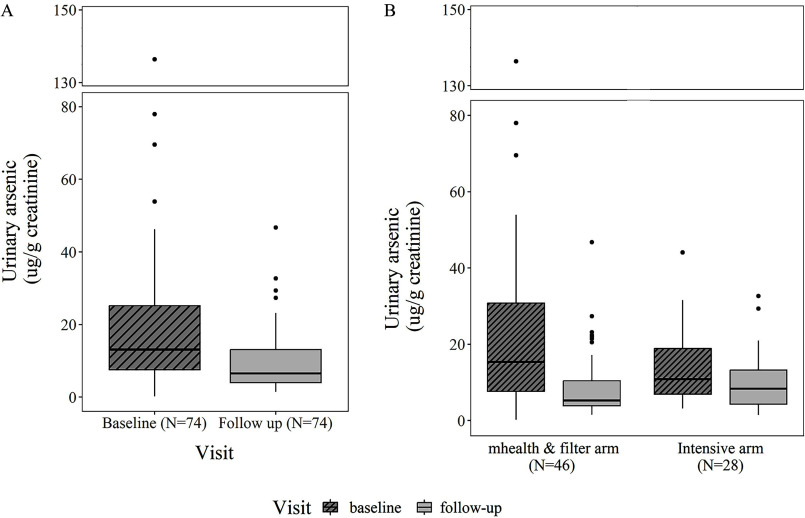
Eighty-two participants had a urine sample available at baseline and 74 had a urine sample from a follow-up visit. Participants in the highest urinary arsenic quartile had higher water arsenic, were older, were more educated, had lower BMIs, and reported less water use outside home compared with the other quartiles (Excel Supplemental Material, Supplementary Table S2). The geometric mean (GM) for urinary arsenic at baseline was creatinine (Table 2 and Figure 3A), with no significant difference at baseline by study arm (). A 47% reduction in GM for urinary arsenic per gram creatinine was observed from baseline to the final follow-up when both study arms were combined (13.2 to ). This reduction was 55% in the mHealth and Filter arm ( creatinine) and 30% in the Intensive arm ( creatinine) (Figure 3B). There was a significant decline in mean MMA% from baseline to the final follow-up visit when both study arms were combined [percentage point change: (95% CI: , )], and for the Intensive arm [ (95% CI: , )] (Table 3). There was also a significant decline in mean iAs% from baseline to final follow-up in the mHealth and Filter arm [ (95% CI: , )]. There was no statistically significant difference in total urinary arsenic levels per gram creatinine between study arms at the final follow-up visit [GM ratio of Intensive to mHealth and Filter: 1.21 (95% CI: 0.77, 1.90)] (Table 4).
Table 2.
Geometric mean (GM) and adjusted GM ratio of urinary arsenic ( creatinine) overall and by study arm comparing baseline [before Strong Heart Water Study (SHWS) intervention] to final follow-up for participants in the SHWS randomized controlled trial ().
| Arm | Urinary arsenic GM (range) | Adjusted linear regressiona | ||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Follow-up | GM ratio | (95% CI) | -Value | ||||
| Overall | 82 | 13.2 (0.21–136.4) | 74 | 7.00 (1.45–46.7) | 74 | 0.52 | (0.39, 0.69) | |
| mHealth and Filter arm | 50 | 14.6 (0.21–136.4) | 46 | 6.55 (1.51–46.7) | 46 | 0.43 | (0.28, 0.66) | |
| Intensive arm | 32 | 11.2 (3.17–44.1) | 28 | 7.82 (1.45–32.7) | 28 | 0.71 | (0.55, 0.92) | 0.011 |
Note: BMI, body mass index; CI, confidence interval; GM, geometric mean.
Urinary arsenic was defined as the sum of inorganic arsenic (iAs) ( creatinine), monomethylarsonic acid (MMA), and dimethylarsinic acid (DMA) measurements, with GMs reported. Adjusted for sociodemographic factors (sex and age) and lifestyle factors (ever smoking and baseline BMI), and study arm. Urinary arsenic is log-transformed for analysis. Regression analyses were performed using generalized estimating equations to account for clustering at the household level using an exchangeable correlation.
Figure 3.
(A) Urinary arsenic ( creatinine) at baseline and final follow-up visit for all participants in the Strong Heart Water Study (SHWS) randomized controlled trial. Urinary arsenic corresponds to the sum of inorganic and methylated arsenic species. Samples were collected between July 2018 and May 2021. Data corresponding to this figure is found in Table 2. (B) Urinary arsenic ( creatinine) at baseline and final follow-up visit by study arm visit for participants in the SHWS randomized controlled trial. Urinary arsenic corresponds to the sum of inorganic and methylated arsenic species. Samples were collected between July 2018 and May 2021. Data corresponding to this figure is found in Table 2.
Table 3.
Mean and adjusted mean difference of urinary arsenic metabolites (percentage species over the sum of inorganic and methylated arsenic species) comparing baseline (before intervention) to final follow-up overall and by study arm for participants in the Strong Heart Water Study randomized controlled trial ().
| Study arm | Mean (range) | Adjusted mean differencea | ||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Follow-up | (95% CI) | -Value | |||||
| Overall | 82 | 74 | 74 | |||||
| iAs% | 15.5 (1.62–47.7) | 14.5 (1.40–80.3) | (, 2.51) | 0.686 | ||||
| MMA% | 13.2 (2.19–23.6) | 11.8 (3.26–23.1) | (, ) | 0.013 | ||||
| DMA% | 71.3 (35.9–96.2) | 73.8 (16.5–93.1) | 1.86 | (, 5.31) | 0.290 | |||
| mHealth and Filter arm | 50 | 46 | 46 | |||||
| iAs% | 14.5 (1.62–47.7) | 11.8 (3.68–30.6) | (, ) | 0.037 | ||||
| MMA% | 12.5 (2.19–23.6) | 11.8 (3.26–23.1) | (, 0.60) | 0.345 | ||||
| DMA% | 73.0 (35.9–96.2) | 76.3 (53.5–93.1) | 3.17 | (, 6.41) | 0.055 | |||
| Intensive arm | 32 | 28 | 28 | |||||
| iAs% | 17.2 (6.55–34.8) | 18.8 (1.40–80.3) | 2.50 | (, 9.39) | 0.478 | |||
| MMA% | 14.3 (4.48–22.0) | 11.6 (3.29–18.9) | (, ) | 0.004 | ||||
| DMA% | 68.5 (46.4–88.9) | 69.7 (16.5–90.3) | (, 6.98) | 0.951 | ||||
Note: BMI, body mass index; CI, confidence interval; GM, geometric mean; iAs%, percentage inorganic arsenic species; MMA%, percentage of monomethylated arsenic; DMA%, percentage dimethylated arsenic.
Regression adjusted for sociodemographic factors (sex and age) and lifestyle factors (ever smoking and baseline BMI). Regression analyses were performed using generalized estimating equations to account for clustering at the household level using an exchangeable correlation. Participants without urinary arsenic data () were excluded.
Table 4.
Urinary arsenic ( creatinine) and arsenic metabolites (percentage species) at the final follow-up visit comparing Intensive arm to mHealth and Filter only arm participants in the Strong Heart Water Study randomized controlled trial ().
| Arsenic levels and metabolite | mHealth and Filter arm () | Intensive arm () | Intensive arm vs. mHealth and Filter arm ()a | mHealth and Filter arm () | Intensive arm () | Intensive arm vs. mHealth and Filter arm ()a | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| GM (range) | GM (range) | GM ratio | (95% CI) | -Value | Mean (range) | Mean (range) | MD | (95% CI) | -Value | |
| Urinary arsenic ( creatinine) | 6.55 (1.51–46.7) | 7.82 (1.45–32.7) | 1.21 | (0.77, 1.90) | 0.413 | — | — | — | — | — |
| Arsenic metabolites | ||||||||||
| iAs% | — | — | — | — | — | 11.8 (3.68–30.6) | 18.8 (1.40–80.3) | 4.89 | (, 11.08) | 0.122 |
| MMA% | — | — | — | — | — | 11.8 (3.26–23.1) | 11.6 (3.29–18.9) | (, 0.38) | 0.145 | |
| DMA% | — | — | — | — | — | 76.3 (53.5–93.1) | 69.7 (16.5–90.3) | (, 2.40) | 0.230 | |
Note: Urinary arsenic was defined as the sum of inorganic arsenic (iAs), monomethylarsonic acid (MMA), and dimethylarsinic acid (DMA) measurements, with GMs reported. —, Not applicable; CI, confidence interval; DMA%, percentage dimethylated arsenic; GM, geometric mean; iAs%, percentage inorganic arsenic species; MD, mean difference; %, percentages of species over the sum of inorganic and methylated arsenic species; MMA%, percentage of monomethylated arsenic.
Adjusted for sociodemographic factors (sex and age) and lifestyle factors (ever smoking and baseline BMI). Urinary arsenic was log-transformed for analysis. Regression analyses were performed using generalized estimating equations to account for clustering at the household level using an exchangeable correlation. Participants without urinary arsenic data were excluded.
Participants who reported exclusive use of arsenic-safe water for cooking, drinking, and cooking and drinking combined at the final follow-up visit had significantly lower urinary arsenic levels per gram creatinine at this time point compared with those who did not report exclusive use (Excel Supplemental Material, Supplementary Table S3). Participants reported increases in exclusive use of arsenic-safe water from baseline to the final follow-up visit for drinking (12% to 46%), for cooking (17% to 53%), and when this measure was combined (11% to 42%) (Table 5). There was no statistically significant difference between study arms in exclusive use of arsenic-safe water for drinking, cooking, or both drinking and cooking at the final follow-up visit (Table 6). At the final follow-up visit, 53% (24/45) of participants in the mHealth and Filter arm and 52% (14/27) of participants in the Intensive arm reported exclusive arsenic-safe water use for cooking (OR = 0.94; 95% CI: 0.31, 2.82), 47% (21/45) and 44% (12/27) for drinking (OR = 0.91; 95% CI: 0.29, 2.90), and 42% (19/45) and 41% (11/27) for both drinking and cooking (OR = 0.94; 95% CI: 0.30, 2.96), respectively. For drink items prepared at home using water, reported use of arsenic-safe water for making tea increased from 20% at baseline to 72% at the final follow-up visit, for making juices increased from 18% to 75%, for making ice increased from 13% to 62%, and for making powdered milk increased from 15% to 60% (Excel Supplemental Material, Supplementary Table S4). For changes in food items prepared at home using water, reported use of arsenic-safe water for making rice increased from 16% to 82%, for making baked goods increased from 17% to 85%, for making soup increased from 15% to 79%, for making gravy increased from 19% to 77%, and for making pasta increased from 15% to 76%. Findings were similar when stratified by study arm (Excel Supplemental Material, Supplementary Table S5).
Table 5.
Change in exclusive arsenic-safe water use between baseline and follow-up for participants in the Strong Heart Water Study randomized controlled trial (N = 84).
| Exclusive arsenic-safe water usea | Overall () | |||
|---|---|---|---|---|
| Baseline | Follow-up | OR (95% CI) | -Value | |
| (%) | (%) | |||
| Overall () | ||||
| Cooking | 14 (17) | 38 (53) | 5.33 (2.29, 12.42) | |
| Drinking | 10 (12) | 33 (46) | 6.21 (2.18, 17.70) | |
| Cooking and drinking | 9 (11) | 30 (42) | 5.98 (2.07, 17.26) | |
| mHealth and Filter arm () | ||||
| Cooking | 6 (12) | 24 (53) | 9.6 (2.01, 36.60) | |
| Drinking | 4 (8) | 21 (47) | 12.8 (2.32, 71.02) | 0.004 |
| Cooking and drinking | 4 (8) | 19 (42) | 10.7 (1.99, 57.63) | 0.006 |
| Intensive arm () | ||||
| Cooking | 8 (24) | 14 (52) | 2.69 (0.92, 7.85) | 0.070 |
| Drinking | 6 (18) | 12 (44) | 2.93 (0.81, 10.66) | 0.102 |
| Cooking and drinking | 5 (15) | 11 (41) | 3.16 (0.81, 12.30) | 0.097 |
Note: CI, confidence interval; OR, odds ratio; POU, point-of-use.
Exclusive use is defined as no arsenic-unsafe source is consumed. Arsenic-safe source options included use of the POU arsenic filter faucet, bottled water, or the municipal water system, and arsenic-unsafe sources included use of the kitchen faucet, bathroom faucet, and refrigerator filter or icemaker (if not reported to be connected to the POU arsenic filter faucet). Regression analyses were performed using generalized estimating equations to account for clustering at the household level using an exchangeable correlation.
Table 6.
Exclusive arsenic-safe water use at final follow-up visit comparing Intensive arm to mHealth and Filter only arm participants () in the Strong Heart Water Study randomized controlled trial.
| Exclusive arsenic-safe water usea | mHealth and Filter arm () | Intensive arm () | Intensive arm vs. mHealth and Filter arm | ||
|---|---|---|---|---|---|
| Exclusive use [ (%)] | Exclusive use [ (%)] | OR | (95% CI) | -Value | |
| Cooking | 24 (53) | 14 (52) | 0.94 | (0.31, 2.82) | 0.916 |
| Drinking | 21 (47) | 12 (44) | 0.91 | (0.29, 2.90) | 0.880 |
| Cooking and drinking | 19 (42) | 11 (41) | 0.94 | (0.30, 2.96) | 0.917 |
Note: Participants without final follow-up visit are not included. CI, confidence interval; OR, odds ratio; POU, point-of-use.
Exclusive use is defined as no arsenic-unsafe source is consumed. Arsenic-safe source options included use of the POU arsenic filter faucet, bottled water, or the municipal water system, and arsenic-unsafe sources included use of the kitchen faucet, bathroom faucet, and refrigerator filter or icemaker (if not reported to be connected to the POU arsenic filter faucet). Regression analyses were performed using generalized estimating equations to account for clustering at the household level using an exchangeable correlation.
Discussion
In our RCT, delivery of the SHWS arsenic mitigation program significantly reduced urinary arsenic and MMA% and increased reported exclusive use of arsenic-safe water for drinking and cooking during the 2-y follow-up period. There was no statistically significant difference between delivery of the SHWS program with installation of a POU arsenic filter and mHealth compared with the more intensive intervention with home visits by a health promoter. This result shows that reductions in arsenic exposure are possible without home visits by a health promoter for program delivery and suggests that mHealth and the installation of a POU arsenic filter may present a promising approach to deliver health promotion for household arsenic mitigation programs. To the best of our knowledge, this is the first RCT of an arsenic mitigation program in the Americas. Our findings demonstrate that the SHWS program can be delivered to households to reduce arsenic exposure in our partner American Indian communities.
We attribute the success of the SHWS program to the community-led, participatory design for program development and implementation. The participatory approach for intervention development engaged community members through community workshops, interviews, and a pilot study.37 Interviews helped to identify the facilitators and barriers to using arsenic-safe water in the community. During workshops, community members ranked their priorities related to water quality and their preferred communication channels for program delivery and selected and finalized intervention content. The pilot study then allowed us to determine acceptability and operability of the program in the community and identify areas for further refinement before program implementation. To ensure a community-led approach for program implementation, community members employed by Missouri Breaks Industries Research Inc. led the implementation of the water arsenic testing program and delivered the intervention content during phone calls and home visits, and community plumbers from the Tribal Housing Authority installed POU arsenic filters. Our qualitative evaluation of the SHWS program found that this community-led approach allowed for social ties to be leveraged in the community for program delivery and built community trust in the SHWS program.40 Additional results from the SHWS, reported elsewhere, found that the program significantly increased perceived vulnerability to arsenic exposure and self-efficacy to use the arsenic filter and that these psychosocial factors were associated with higher exclusive use of arsenic-safe water at follow-up.41 This suggests that increased perceived vulnerability and self-efficacy following delivery of the SHWS program were likely important contributors to the high use of arsenic-safe water and subsequent reductions in urinary arsenic at the final follow-up visit, further emphasizing the importance of our tailored intervention design centered around the community.
The 47% reduction in urinary arsenic from baseline to the final follow-up visit with delivery of the SHWS program is supported by the water arsenic findings from this RCT, where we found that at the final household visit 93% of POU arsenic filters were effective in reducing water arsenic below the US EPA MCL of .39 In addition to reducing arsenic exposure, we found a reduction in MMA%, an arsenic metabolite that has been associated with higher risk of cardiovascular disease and cancer.47 Although significant changes were not observed in DMA% and iAs% for the overall study population, these three main arsenic species are interrelated, and therefore the impact of arsenic metabolism may be better observed in one species, with the additional impact split between the other two species.47 This finding is consistent with a previous analysis in Northern Chile, at much higher arsenic levels, where an intervention reducing arsenic exposure also decreased MMA%.48 Overall, our results demonstrate that the adsorptive media POU arsenic filter selected for the SHWS program had a high efficacy in reducing arsenic exposure during the 2-y follow-up period. Previous studies have evaluated the efficacy of POU arsenic filters; however, these studies have mostly focused on reverse osmosis systems or sand filters,20,22,35,49,50 and some systems have not shown the ability to remove arsenic from water over an extended period of time in real-world scenarios.51–53 Even when arsenic filters are effective at removing arsenic from water, challenges with maintenance and continued use of POU water treatment systems have been substantial.20,23,35,54 For example, previous studies in Bangladesh found that POU arsenic filters resulted in only short-term reductions () in urinary arsenic owing to a lack of sustained filter use,35,54 making the continued efficacy of our filter after a 2-y period notable. Future studies should evaluate the efficacy of adsorptive media POU arsenic filters in reducing water and urinary arsenic for a longer duration than 2 y, and in other settings globally, to determine the potential sustained impact of this arsenic mitigation option.
The mHealth and Filter arm of the SHWS program led to a 55% reduction in urinary arsenic from baseline to follow-up during the 2-y follow-up period. The addition of home visits did not significantly change the reduction in urinary arsenic achieved by the mHealth and Filter intervention of the SHWS program. This finding demonstrates that home visits by promoters are not needed for successful SHWS program delivery. Therefore, installation of an effective filter together with an mHealth component was as effective as the intensive program. Water, sanitation, and hygiene (WASH) interventions delivered through in-person visits are often expensive and difficult to implement in rural settings. mHealth is a low-cost, innovative intervention that has been shown to improve disease prevention practices.55–58 mHealth programs are particularly beneficial in the present time, where the COVID-19 pandemic has demonstrated limitations with home visits for intervention delivery globally. This is the first study to date that we are aware of that implemented an mHealth program for an arsenic mitigation intervention. Beyond the present study, there is only one RCT of a WASH mHealth program published to date, to our knowledge.59 Our research group conducted this RCT in Bangladesh of the Cholera Hospital-based Intervention for 7 Days (CHoBI7) WASH mHealth program, an intervention focused on water treatment through chlorination and boiling and handwashing with soap. In this trial, we found that delivery of the CHoBI7 WASH mHealth program with a single in-person visit was effective in significantly reducing diarrheal disease and stunting among young children.59 These findings suggest that mHealth is a promising intervention approach for water treatment in multiple settings. Future studies are needed to evaluate the effectiveness of mHealth programs focused on reducing arsenic exposure in affected settings globally.
The SHWS study greatly increased exclusive arsenic-safe water use for both drinking and cooking over the 2-y study period in both study arms, with the greatest impact in the mHealth and Filter arm. We also found arsenic-safe water use was higher for preparing both drink items and food items. Some participants, however, reported continued use of arsenic-unsafe water for making ice. Plumbers only connected the refrigerator icemaker (a main source of ice) to the filter faucet at the request of households. Future studies should prioritize connecting the icemaker to the water filter and include specific reminders about ice in mHealth materials. Overall, safe water use for food and drink items was high; however, exclusive use was not. These findings indicate that more emphasis needs to be placed in our health communication materials on promoting exclusive use of arsenic-safe water for food and drink items prepared using water in the home. We observed that self-reported exclusive use of arsenic-safe water for drinking and cooking was associated with lower urinary arsenic compared with those reporting nonexclusive use. This finding provides evidence to support that self-reported exclusive use of arsenic-safe water can be used as a proxy measure of arsenic exposure in our study setting.
This study has several strengths. The first strength of this study is that we measured inorganic urinary arsenic and arsenic metabolites at baseline and follow-up, allowing us to assess reduction in arsenic exposure and internal dose and changes in arsenic methylation efficiency. This approach builds on previous water arsenic filter interventions that have focused solely on self-reported use of arsenic-safe water.29,31,33,60 Second, there was a long duration of household follow-up, with the mean follow-up duration being 24 months. Previous RCTs of arsenic interventions had follow-up periods of 3–12 months in duration.31–33,35 Finally, the community-led design for intervention development and implementation ensured that the intervention was tailored to our partner communities. Most previous arsenic intervention programs have focused solely on providing arsenic mitigation technologies, rather than taking a participatory approach that engages communities in tailoring arsenic mitigation programs to address their needs.35,54
This study also has some limitations. First, we lacked a control arm that did not receive an arsenic filter or mHealth program. Given the extensive literature demonstrating the health implications of chronic arsenic exposure, not providing an intervention to reduce this exposure among eligible households was viewed as unethical by both the community and other study investigators. Second, we lacked a study arm that received the filter only (i.e., no mHealth program or home visits). Including this additional arm would have been valuable to determine the added benefit of the WASH mHealth component of the study. This arm was not included due to budgetary constraints and because it was also considered preferable to provide the filter together with some level of support to the households to adequately use it. Third, although having repeated urine samples during the follow-up period for each participant would have been ideal, this was difficult because of the COVID-19 pandemic. However, urinary arsenic levels are known to reflect arsenic exposure in water after accounting for urine dilution if the source of water is consistent over time.7 Finally, we did not quantify POU filter water usage. This information will be important to collect in future studies.
Conclusion
Millions of people are exposed to elevated arsenic in potable water globally, and American Indian communities are disproportionally affected.61,62 The community-led SHWS arsenic mitigation program was effective in significantly reducing arsenic exposure among American Indian communities in the Northern Great Plains. A single visit for the installation of a POU arsenic filter followed by delivery of an mHealth program through calls only was sufficient to significantly reduce urinary arsenic over the 2-y study period. We attribute the success of this program to the community-led intervention design and implementation. The SHWS presents a model that can be used for the design, implementation, and evaluation of arsenic mitigation programs globally.
Supplementary Material
Acknowledgments
We thank all Strong Heart Water Study (SHWS) and Strong Heart Study participants, our partner Nations, and other study partners who have made this work possible. We also thank the Indian Health Service for their technical and logistical assistance, including James Begeman, Kris Neset, and Mike Boland. We thank Rae O’Leary for preparing the videos used for the Intensive intervention, and Joseph Yracheta, Marie Gross, Allison Barlow, and Martha Powers for their role in intervention development of the SHWS arsenic mitigation program during our formative research phase. We also thank Barbara Howard for her guidance and support throughout the SHWS.
The research was supported by the National Institute of Environmental Health Sciences (NIEHS), National Institutes of Health (NIH; R01ES025135, to C.M.G.). T.Z., K.J.S., R.G., M.O., and A.N.A. were supported by P42ES033719, IDOELMBI39893, and K.J.S., R.G., and A.N.A. are supported by P30ES009089 (to A.N.A.) from the NIEHS/NIH.
Conclusions and opinions are those of the individual authors and do not necessarily reflect the policies or views of EHP Publishing or the National Institute of Environmental Health Sciences.
References
- 1.Ayotte JD, Medalie L, Qi SL, Backer LC, Nolan BT. 2017. Estimating the high-arsenic domestic-well population in the conterminous United States. Environ Sci Technol 51(21):12443–12454, PMID: , 10.1021/acs.est.7b02881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.US EPA (US Environmental Protection Agency). 2022. Toxicological profile for arsenic. https://hero.epa.gov/hero/index.cfm/reference/details/reference_id/657856 [accessed 14 August 2022].
- 3.Ryker S. 2001. Mapping arsenic in groundwater. Geotimes 46(11):34–36. [Google Scholar]
- 4.US EPA. 2001. National primary drinking water regulations; arsenic and clarifications to compliance and new source contaminants monitoring; final rule. Docket No. WH-FRL-6934-9, RIN 2040-AB75. Fed Reg 66:6975–7066. https://www.govinfo.gov/content/pkg/FR-2001-01-22/pdf/01-1668.pdf [accessed 22 October 2022]. [Google Scholar]
- 5.Welch AH, Helsel DR, Focazio MJ, Watkins SA. 1999. Arsenic in ground water supplies of the United States. In: Arsenic Exposure and Health Effects III: Proceedings of the Third International Conference on Arsenic Exposure and Health Effects. Chappell WR, Abernathy CO, Calderon RL, eds. July 12–15 July 1998. Amsterdam, the Netherlands: Elsevier, 9–17. [Google Scholar]
- 6.Focazio MJ, Tipton D, Dunkle Shapiro S, Geiger LH. 2006. The chemical quality of self-supplied domestic well water in the United States. Ground Water Monit Remediat 26(3):92–104, 10.1111/j.1745-6592.2006.00089.x. [DOI] [Google Scholar]
- 7.Navas-Acien A, Umans JG, Howard BV, Goessler W, Francesconi KA, Crainiceanu CM, et al. 2009. Urine arsenic concentrations and species excretion patterns in American Indian communities over a 10-year period: the Strong Heart Study. Environ Health Perspect 117(9):1428–1433, PMID: , 10.1289/ehp.0800509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moon KA, Guallar E, Umans JG, Devereux RB, Best LG, Francesconi KA, et al. 2013. Association between exposure to low to moderate arsenic levels and incident cardiovascular disease: a prospective cohort study. Ann Intern Med 159(10):649–659, PMID: , 10.7326/0003-4819-159-10-201311190-00719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pichler G, Grau-Perez M, Tellez-Plaza M, Umans J, Best L, Cole S, et al. 2019. Association of arsenic exposure with cardiac geometry and left ventricular function in young adults. Circ Cardiovasc Imaging 12(5):e009018, PMID: , 10.1161/CIRCIMAGING.119.009018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mateen FJ, Grau-Perez M, Pollak JS, Moon KA, Howard BV, Umans JG, et al. 2017. Chronic arsenic exposure and risk of carotid artery disease: the Strong Heart Study. Environ Res 157:127–134, PMID: , 10.1016/j.envres.2017.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gribble MO, Howard BV, Umans JG, Shara NM, Francesconi KA, Goessler W, et al. 2012. Arsenic exposure, diabetes prevalence, and diabetes control in the Strong Heart Study. Am J Epidemiol 176(10):865–874, PMID: , 10.1093/aje/kws153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng LY, Umans JG, Tellez-Plaza M, Yeh F, Francesconi KA, Goessler W, et al. 2013. Urine arsenic and prevalent albuminuria: evidence from a population-based study. Am J Kidney Dis 61(3):385–394, PMID: , 10.1053/j.ajkd.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng LY, Umans JG, Yeh F, Francesconi KA, Goessler W, Silbergeld EK, et al. 2015. The association of urine arsenic with prevalent and incident chronic kidney disease: evidence from the Strong Heart Study. Epidemiology 26(4):601–612, PMID: , 10.1097/EDE.0000000000000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Powers M, Sanchez TR, Grau-Perez M, Yeh F, Francesconi KA, Goessler W, et al. 2019. Low-moderate arsenic exposure and respiratory in American Indian communities in the Strong Heart Study. Environ Health 18(1):104, PMID: , 10.1186/s12940-019-0539-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carroll CR, Noonan C, Garroutte EM, Navas-Acien A, Verney SP, Buchwald D. 2017. Low-level inorganic arsenic exposure and neuropsychological functioning in American Indian elders. Environ Res 156:74–79, PMID: , 10.1016/j.envres.2017.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suchy-Dicey A, Noonan C, Burduli E, Mateen FJ, Longstreth WT Jr, Buchwald D, et al. 2020. Urinary arsenic and cadmium associations with findings from cranial MRI in American Indians: data from the Strong Heart Study. Environ Health Perspect 128(12):127009, PMID: , 10.1289/EHP6930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.García-Esquinas E, Pollán M, Umans JG, Francesconi KA, Goessler W, Guallar E, et al. 2013. Arsenic exposure and cancer mortality in a US-based prospective cohort: the Strong Heart Study. Cancer Epidemiol Biomarkers Prev 22(11):1944–1953, PMID: , 10.1158/1055-9965.EPI-13-0234-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuo CC, Balakrishnan P, Gribble MO, Best LG, Goessler W, Umans JG, et al. 2022. The association of arsenic exposure and arsenic metabolism with all-cause, cardiovascular and cancer mortality in the Strong Heart Study. Environ Int 159:107029, PMID: , 10.1016/j.envint.2021.107029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.George CM, Smith AH, Kalman DA, Steinmaus CM. 2006. Reverse osmosis filter use and high arsenic levels in private well water. Arch Environ Occup Health 61(4):171–175, PMID: , 10.3200/AEOH.61.4.171-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang Q, Flanagan SV, Chillrud S, Ross J, Zeng W, Culbertson C, et al. 2020. Reduction in drinking water arsenic exposure and health risk through arsenic treatment among private well households in Maine and New Jersey, USA. Sci Total Environ 738:139683, PMID: , 10.1016/j.scitotenv.2020.139683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Slotnick MJ, Meliker JR, Nriagu JO. 2006. Effects of time and point-of-use devices on arsenic levels in southeastern Michigan drinking water, USA. Sci Total Environ 369(1–3):42–50, PMID: , 10.1016/j.scitotenv.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 22.Walker M, Seiler RL, Meinert M. 2008. Effectiveness of household reverse-osmosis systems in a Western U.S. region with high arsenic in groundwater. Sci Total Environ 389(2–3):245–252, PMID: , 10.1016/j.scitotenv.2007.08.061. [DOI] [PubMed] [Google Scholar]
- 23.Spayd SE, Robson MG, Buckley BT. 2015. Whole-house arsenic water treatment provided more effective arsenic exposure reduction than point-of-use water treatment at New Jersey homes with arsenic in well water. Sci Total Environ 505:1361–1369, PMID: , 10.1016/j.scitotenv.2014.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flanagan SV, Spayd SE, Procopio NA, Chillrud SN, Braman S, Zheng Y. 2016. Arsenic in private well water part 1 of 3: impact of the New Jersey Private Well Testing Act on household testing and mitigation behavior. Sci Total Environ 562:999–1009, PMID: , 10.1016/j.scitotenv.2016.03.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flanagan SV, Marvinney RG, Zheng Y. 2015. Influences on domestic well water testing behavior in a Central Maine area with frequent groundwater arsenic occurrence. Sci Total Environ 505:1274–1281, PMID: , 10.1016/j.scitotenv.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shaw WD, Walker M, Benson M. 2005. Treating and drinking well water in the presence of health risks from arsenic contamination: results from a U.S. hot spot. Risk Anal 25(6):1531–1543, PMID: , 10.1111/j.1539-6924.2005.00698.x. [DOI] [PubMed] [Google Scholar]
- 27.Severtson DJ, Baumann LC, Brown RL. 2006. Applying a health behavior theory to explore the influence of information and experience on arsenic risk representations, policy beliefs, and protective behavior. Risk Anal 26(2):353–368, PMID: , 10.1111/j.1539-6924.2006.00737.x. [DOI] [PubMed] [Google Scholar]
- 28.Jones-Hughes T, Peters J, Whear R, Cooper C, Evans H, Depledge M, et al. 2013. Are interventions to reduce the impact of arsenic contamination of groundwater on human health in developing countries effective? A systematic review. Environ Evid 2(1):11, 10.1186/2047-2382-2-11. [DOI] [Google Scholar]
- 29.Inauen J, Mosler HJ. 2014. Developing and testing theory-based and evidence-based interventions to promote switching to arsenic-safe wells in Bangladesh. J Health Psychol 19(12):1483–1498, PMID: , 10.1177/1359105313493811. [DOI] [PubMed] [Google Scholar]
- 30.Inauen J, Tobias R, Mosler HJ. 2014. The role of commitment strength in enhancing safe water consumption: mediation analysis of a cluster-randomized trial. Br J Health Psychol 19(4):701–719, PMID: , 10.1111/bjhp.12068. [DOI] [PubMed] [Google Scholar]
- 31.George CM, Inauen J, Perin J, Tighe J, Hasan K, Zheng Y. 2017. Behavioral determinants of switching to arsenic-safe water wells: an analysis of a randomized controlled trial of health education interventions coupled with water arsenic testing. Health Educ Behav 44(1):92–102, PMID: , 10.1177/1090198116637604. [DOI] [PubMed] [Google Scholar]
- 32.George CM, van Geen A, Slavkovich V, Singha A, Levy D, Islam T, et al. 2012. A cluster-based randomized controlled trial promoting community participation in arsenic mitigation efforts in Bangladesh. Environ Health 11:41, PMID: , 10.1186/1476-069X-11-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.George CM, Inauen J, Rahman SM, Zheng Y. 2013. The effectiveness of educational interventions to enhance the adoption of fee-based arsenic testing in Bangladesh: a cluster randomized controlled trial. Am J Trop Med Hyg 89(1):138–144, PMID: , 10.4269/ajtmh.12-0664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barnwal P, van Geen A, von der Goltz J, Singh CK. 2017. Demand for environmental quality information and household response: evidence from well-water arsenic testing. J Environ Econ Manage 86:160–192, 10.1016/j.jeem.2017.08.002. [DOI] [Google Scholar]
- 35.Milton AH, Smith W, Dear K, Ng J, Sim M, Ranmuthugala G, et al. 2007. A randomised intervention trial to assess two arsenic mitigation options in Bangladesh. J Environ Sci Health A Tox Hazard Subst Environ Eng 42(12):1897–1908, PMID: , 10.1080/10934520701567197. [DOI] [PubMed] [Google Scholar]
- 36.Sobel M, Sanchez TR, Zacher T, Mailloux B, Powers M, Yracheta J, et al. 2021. Spatial relationship between well water arsenic and uranium in Northern Plains native lands. Environ Pollut 287:117655, PMID: , 10.1016/j.envpol.2021.117655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thomas ED, Gittelsohn J, Yracheta J, Powers M, O’Leary M, Harvey DE, et al. 2019. The Strong Heart Water Study: informing and designing a multi-level intervention to reduce arsenic exposure among private well users in Great Plains Indian Nations. Sci Total Environ 650(pt 2):3120–3133, PMID: , 10.1016/j.scitotenv.2018.09.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Powers M, Yracheta J, Harvey D, O’Leary M, Best LG, Black Bear A, et al. 2019. Arsenic in groundwater in private wells in rural North Dakota and South Dakota: water quality assessment for an intervention trial. Environ Res 168:41–47, PMID: , 10.1016/j.envres.2018.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zacher T, Endres K, Richards F, Bear Robe L, Powers M, Yracheta J, et al. 2023. Evaluation of water arsenic filter treatment in a participatory intervention to reduce arsenic exposure in American Indian communities: the Strong Heart Water Study. Sci Total Environ 862:160217, PMID: , 10.1016/j.scitotenv.2022.160217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anderson DM, Black Bear A, Zacher T, Endres K, Saxton R, Richards F, et al. 2023. Implementing a community-led arsenic mitigation intervention for private well users in American Indian communities: a qualitative evaluation of the Strong Heart Water Study program. Int J Environ Res Public Health 20(3):2681, PMID: , 10.3390/ijerph20032681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Endres K, Zacher T, Richards F, Bear Robe L, Powers M, Yracheta J, et al. 2023. Behavioral determinants of arsenic-safe water use among Great Plains Indian Nation private well users: results from the community-led Strong Heart Water Study arsenic mitigation program. Environ Health 22(1):42, PMID: , 10.1186/s12940-023-00965-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mosler HJ. 2012. A systematic approach to behavior change interventions for the water and sanitation sector in developing countries: a conceptual model, a review, and a guideline. Int J Environ Health Res 22(5):431–449, PMID: , 10.1080/09603123.2011.650156. [DOI] [PubMed] [Google Scholar]
- 43.Dreibelbis R, Winch PJ, Leontsini E, Hulland KRS, Ram PK, Unicomb L, et al. 2013. The Integrated Behavioural Model for Water, Sanitation, and Hygiene: a systematic review of behavioural models and a framework for designing and evaluating behaviour change interventions in infrastructure-restricted settings. BMC Public Health 13:1015, PMID: , 10.1186/1471-2458-13-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bozack AK, Hall MN, Liu X, Ilievski V, Lomax-Luu AM, Parvez F, et al. 2019. Folic acid supplementation enhances arsenic methylation: results from a folic acid and creatine supplementation randomized controlled trial in Bangladesh. Am J Clin Nutr 109(2):380–391, PMID: , 10.1093/ajcn/nqy148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nigra AE, Olmedo P, Grau-Perez M, O’Leary R, O’Leary M, Fretts AM, et al. 2019. Dietary determinants of inorganic arsenic exposure in the Strong Heart Family Study. Environ Res 177:108616, PMID: , 10.1016/j.envres.2019.108616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seto EYW, Soller JA, Colford JM Jr.. 2007. Strategies to reduce person-to-person transmission during widespread Escherichia coli O157:H7 outbreak. Emerg Infect Dis 13(6):860–866, PMID: , 10.3201/eid1306.061264. [DOI] [PubMed] [Google Scholar]
- 47.Kuo CC, Moon KA, Wang SL, Silbergeld E, Navas-Acien A. 2017. The association of arsenic metabolism with cancer, cardiovascular disease, and diabetes: a systematic review of the epidemiological evidence. Environ Health Perspect 125(8):087001, PMID: , 10.1289/EHP577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hopenhayn-Rich C, Biggs ML, Kalman DA, Moore LE, Smith AH. 1996. Arsenic methylation patterns before and after changing from high to lower concentrations of arsenic in drinking water. Environ Health Perspect 104(11):1200–1207, PMID: , 10.1289/ehp.961041200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Flanagan SV, Marvinney RG, Johnston RA, Yang Q, Zheng Y. 2015. Dissemination of well water arsenic results to homeowners in Central Maine: influences on mitigation behavior and continued risks for exposure. Sci Total Environ 505:1282–1290, PMID: , 10.1016/j.scitotenv.2014.03.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lothrop N, Wilkinson ST, Verhougstraete M, Sugeng A, Loh MM, Klimecki W, et al. 2015. Home water treatment habits and effectiveness in a rural Arizona community. Water (Basel) 7(3):1217–1231, PMID: , 10.3390/w7031217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chiew H, Sampson ML, Huch S, Ken S, Bostick BC. 2009. Effect of groundwater iron and phosphate on the efficacy of arsenic removal by iron-amended BioSand filters. Environ Sci Technol 43(16):6295–6300, PMID: , 10.1021/es803444t. [DOI] [PubMed] [Google Scholar]
- 52.Thomas MA, Ekberg M. 2016. The Effectiveness of Water-Treatment Systems for Arsenic Used in 11 Homes in Southwestern and Central Ohio, 2013. Reston, VA: US Geological Survey. [Google Scholar]
- 53.Barnaby R, Liefeld A, Jackson BP, Hampton TH, Stanton BA. 2017. Effectiveness of table top water pitcher filters to remove arsenic from drinking water. Environ Res 158:610–615, PMID: , 10.1016/j.envres.2017.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sanchez TR, Levy D, Shahriar MH, Uddin MN, Siddique AB, Graziano JH, et al. 2016. Provision of well-water treatment units to 600 households in Bangladesh: A longitudinal analysis of urinary arsenic indicates fading utility. Sci Total Environ 563–564:131–137, PMID: , 10.1016/j.scitotenv.2016.04.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zurovac D, Sudoi RK, Akhwale WS, Ndiritu M, Hamer DH, Rowe AK, et al. 2011. The effect of mobile phone text-message reminders on Kenyan health workers’ adherence to malaria treatment guidelines: a cluster randomised trial. Lancet 378(9793):795–803, PMID: , 10.1016/S0140-6736(11)60783-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cole-Lewis H, Kershaw T. 2010. Text messaging as a tool for behavior change in disease prevention and management. Epidemiol Rev 32(1):56–69, PMID: , 10.1093/epirev/mxq004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Free C, Phillips G, Galli L, Watson L, Felix L, Edwards P, et al. 2013. The effectiveness of mobile-health technology-based health behaviour change or disease management interventions for health care consumers: a systematic review. PLoS Med 10(1):e1001362, PMID: , 10.1371/journal.pmed.1001362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Higgs ES, Goldberg AB, Labrique AB, Cook SH, Schmid C, Cole CF, et al. 2014. Understanding the role of mHealth and other media interventions for behavior change to enhance child survival and development in low- and middle-income countries: an evidence review. J Health Commun 19(suppl 1):164–189, PMID: , 10.1080/10810730.2014.929763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.George CM, Monira S, Zohura F, Thomas ED, Hasan MT, Parvin T, et al. 2020. Effects of a water, sanitation, and hygiene mobile health program on diarrhea and child growth in Bangladesh: a cluster-randomized controlled trial of the Cholera Hospital-based Intervention for 7 Days (CHoBI7) mobile health program. Clin Infect Dis 73(9):e2560–e2568, PMID: , 10.1093/cid/ciaa754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Inauen J, Mosler HJ. 2016. Mechanisms of behavioural maintenance: long-term effects of theory-based interventions to promote safe water consumption. Psychol Health 31(2):166–183, PMID: , 10.1080/08870446.2015.1085985. [DOI] [PubMed] [Google Scholar]
- 61.Spaur M, Lombard MA, Ayotte JD, Harvey DE, Bostick BC, Chillrud SN, et al. 2021. Associations between private well water and community water supply arsenic concentrations in the conterminous United States. Sci Total Environ 787:147555, PMID: , 10.1016/j.scitotenv.2021.147555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nigra AE, Chen Q, Chillrud SN, Wang L, Harvey D, Mailloux B, et al. 2020. Inequalities in public water arsenic concentrations in counties and community water systems across the United States, 2006–2011. Environ Health Perspect 128(12):127001, PMID: , 10.1289/EHP7313. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.