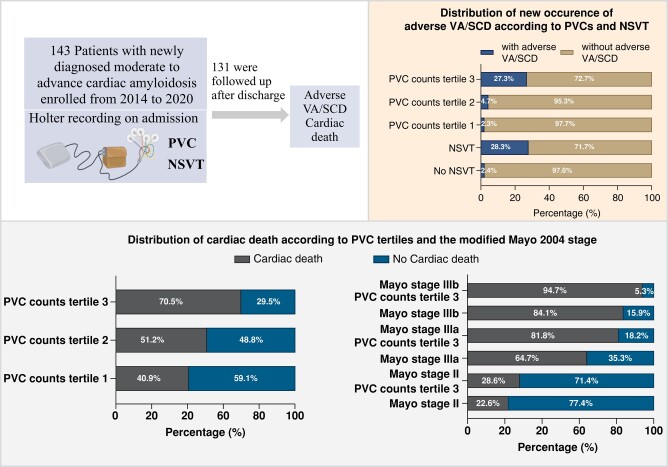
Abstract
Aims
Premature ventricular contractions (PVC) and non-sustained ventricular tachycardia (NSVT) are commonly observed in light chain cardiac amyloidosis (AL-CA), but their association with prognosis is still unclear. We aimed to evaluate the prognostic value of PVCs and NSVT in patients with moderate-to-advanced AL-CA.
Methods and results
We retrospectively included patients with AL-CA at modified 2004 Mayo stages II-IIIb between February 2014 and December 2020. Twenty-four-hour Holter recordings were assessed on admission. The outcomes included (i) new onset of adverse ventricular arrhythmia (VA) or sudden cardiac death (SCD) and (ii) cardiac death during follow-up. Of the 143 patients studied (60.41 ± 11.06 years, male 64.34%), 132 (92.31%) had presence of PVC, and 50 (34.97%) had NSVT on Holter. Twelve (8.4%) patients died in hospital and 131 patients were followed up (median 24.4 months), among whom 71 patients had cardiac death, and 15 underwent adverse VA/SCD. NSVT [hazard ratio (HR): 13.57, 95% confidence interval (CI): 3.06–60.18, P < 0.001], log-transformed PVC counts (HR: 1.46, 95%CI: 1.15–1.86, P = 0.002) and PVC burden (HR: 1.43 95%CI:1.14–1.80, P = 0.002) were predictive of new onset of adverse VA/SCD. The highest tertile of PVC counts (HR: 2.33, 95%CI: 1.27–4.28, P = 0.006) and PVC burden (HR: 2.58, 95%CI: 1.42–4.69, P = 0.002), rather than NSVT (HR: 1.16, 95%CI: 0.67–1.98, P = 0.603), was associated with cardiac death. Higher PVC counts/burden provided incremental value on modified 2004 Mayo stage in predicting cardiac death, with C index increasing from 0.681 to 0.712 and 0.717, respectively (P values <0.05).
Conclusion
PVC count, burden, and NSVT significantly correlated with adverse VA/SCD during follow-up in patients with AL-CA. Higher PVC counts/burdens added incremental value for predicting cardiac death.
Keywords: Cardiac amyloidosis, Risk stratification, Ventricular arrhythmia, Sudden death, Outcome
Graphical Abstract
Graphical abstract.
What’s new?
Higher burden of PVC and proportion of NSVT were observed in patients with cardiac amyloidosis (AL-CA) who experienced adverse ventricular arrhythmias (VA) or sudden cardiac death (SCD).
PVC counts, PVC burden, and NSVT may assist in identifying patients who experience new onset of VA/SCD and may demonstrate better predictive performance than the Mayo stage.
Higher PVC counts and PVC burden were independent predictors for cardiac death, in addition to predicting adverse VA/SCD. Higher PVC counts and PVC burden add incremental value for predicting cardiac death.
Twenty-four-hour Holter may be a cost-effective tool to improve risk stratification for adverse VA/SCD and cardiac death in patients with moderate-to-advanced AL-CA.
Introduction
Cardiac amyloidosis (CA), which is characterized by extracellular deposition of insoluble amyloid fibrils in the myocardium, is often associated with adverse clinical outcomes.1,2 There are two main forms of disease, namely light chain amyloidosis (AL) and transthyretin amyloidosis (ATTR), both of which provide a substrate for electro-mechanical remodeling3,4 and various arrhythmias.5 Compared with patients with the ATTR subtype, those with AL-CA often develop complex ventricular arrhythmias (VA) and have a poorer prognosis.1,3 It was reported that 5–27% of AL-CA patients present with a non-sustained ventricular tachycardia (NSVT),6,7 and premature ventricular contractions (PVCs) were found in 72% patients.8 Progressive heart failure and sudden cardiac death (SCD) are two major causes of cardiac death in patients with AL-CA,9 and identifying those at risk is a clinically important but challenging endeavour. Although shorter life expectancy in AL-CA and the reported association of arrhythmic death to pulseless electrical activity may limit the benefit of implantable cardioverter defibrillator (ICD) implantation in these patients, 8–30% sustained VTs, ventricular fibrillation (VF) and one-third appropriate therapy rate have been reported in device recipients.10,11 Therefore, identifying the patients at high risk for adverse VA and SCD is also important. Cardiac biomarkers, such as N-terminal prohormone of brain natriuretic peptide (NT-proBNP) and troponin, and prolonged His-ventricular (HV) intervals in electrophysiological testing are known to be associated with overall mortality and cardiac death, but their predictive value for life-threatening VAs and SCD in AL-CA seems to be limited.12 The role of NSVTs and PVC burden in predicting SCD or cardiac death in AL-CA is still not yet well-defined. Small retrospective studies suggest a potential predictive clinical significance of these arrhythmias in recipients of ICDs.8,10 However, studies with larger sample sizes demonstrated controversial results regarding the prognostic value of NSVT on survival among AL-CA patients.13,14
In this study, we aim to fulfil this gap by evaluating the prognostic impact of NSVTs and PVCs in a large cohort of patients with moderate-to-advanced newly diagnosed AL-CA.
Methods
Study population and data collection
Consecutive inpatients who were diagnosed with AL-CA with Mayo stages II-III,15 at the Fuwai Hospital in Beijing from February 2014 to December 2020 and underwent Holter monitoring during the initial hospitalization were included in this study. The diagnosis of AL-CA was made either from endomyocardial biopsy or by non-invasive imaging criteria in combination with a positive extracardiac biopsy.16 We excluded patients aged below 18 years and those who were previously treated with chemotherapy and/or autologous stem cell transplant (ASCT) for over three months. The patients were also excluded if they did not have Holter recordings or if the interval between the Holter and disease diagnosis exceeded 7 days. Detailed clinical history and laboratory tests at diagnosis were collected from institutional medical records. Echocardiography was performed using standard techniques on admission by experienced echocardiographers. Interventricular septum thickness, left ventricular posterior wall thickness (LVPW), LV ejection fraction (LVEF), and LV end-diastolic diameter (LVEDD) were collected. Patients were further classified as stages II, IIIa, and IIIb according to the modified Mayo stage (based on NT-proBNP cut-off level of 8500 pg/L).15 The study complied with the Declaration of the Helsinki and was approved by the local institutional review committee (Approval No. 2022–1887). Written informed consent was obtained from all patients.
Holter monitoring
All patients underwent Holter electrocardiogram (ECG) monitoring for a duration of 24-h, which is routinely performed in our institution, often within the first three days of hospital admission. If a patient underwent more than one Holter during the hospitalization, the results of the first exam were analyzed. PVC burden was determined as the percentage of the total number of PVCs divided by total number of heart beats. NSVT was defined as three or more consecutive ventricular beats with wide QRS complex in the absence of aberration, with a mean inter-beat (RR) interval of ≤600 ms and lasting less than 30 s.17 Holter analyses were performed using a digitized Holter analyzer (BI9800, Biomedical Instruments Co., Ltd., Osaka, Japan) and all results were confirmed by an experienced electrophysiologist.
Outcome definition and follow-up
Adverse VAs (life-threatening VAs) included documented spontaneous sustained ventricular tachycardia (VT) and VF causing haemodynamic compromise or appropriate ICD therapies.18 The cardiac death included SCD and heart failure-related death. SCD was defined as witnessed unexpected sudden collapse occurring within one hour of new cardiac symptoms among patients in a stable clinical condition from fatal VA (documented on ECG), or in case of out-of-hospital SCD, death following an unexpected, sudden collapse without pulse or respiration in the absence of an obvious non-cardiac reason.19,20 Heart-failure-related death was considered in the context of progressive cardiac decompensation with exacerbation of heart failure signs/symptoms, which frequently required hospitalization, especially when complicated by pulmonary oedema.21 Survival analysis was calculated among patients alive at the time of discharge, with the date of the Holter recording, regarded as the first day. The primary outcome was defined as new onset of any adverse VA/SCD during follow-up. Successful resuscitation after cardiac arrest was considered equivalent to SCD in calculating the primary outcome. The secondary outcome of the study was cardiac death.
Statistical analysis
Continuous variables with normal distribution are presented as mean ± standard deviation, otherwise as median (interquartile range). Categorical variables are described as numbers (percentage). Student's t-test or Mann–Whitney U test was applied for evaluating the differences of continuous variables with normal and non-normal distributions as appropriate. The Chi-square test or Fisher's exact test was used for assessing the difference of discrete variables between groups. Univariate logistic regression analyses were conducted to evaluate the association between clinical parameters, PVCs and NSVT and the overall presence of adverse VAs or SCD. PVCs or NSVT was introduced into the multivariate regression separately with adjustment of the significant clinical variables. The receiver-operating characteristic (ROC) curves were used to evaluate the accuracy of overall presence and new occurrence of adverse VA/SCD prediction between multiple measures, including the PVC counts, PVC burden, NSVT, and the Mayo stage. The maximum value of the Youden index was used as the best cut-off to calculate sensitivity and specificity. Survival analysis was performed among the alive patients at discharge using the Kaplan-Meier method and log-rank test. Considering the relatively low number of adverse VA/SCD events during follow-up, univariate Cox regression was applied to assess the association between PVCs, NSVT, and new-onset of adverse VA/SCD, while multivariable Cox proportional hazards model was applied to assess the association between PVCs and NSVT and cardiac death using forward stepwise selection and the results were presented with hazard ratio (HR) [95% confidence interval (CI)]. The proportional hazard assumption was assessed with graphical checks and Schoenfeld residuals–based tests. Sensitive analyses were conducted to determine the stability and reliability of the results by truncating the PVC counts values that exceeded 95 and 99 percentiles and in the subgroups of patients without a history of adverse VA/SCD. Statistical significance was defined as a two-sided P-value of <0.05. Statistical analyses were performed using R software, version 4.1.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline clinical characteristics and holter-derived findings
A total of 143 patients were included in the study. Baseline characteristics are described in Table 1. The mean age at diagnosis was 60.41 years and the majority of the patients were male (64.34%). The mean recording time of Holter exam was 22.9 ± 1.3 h. PVCs were present in 132 (92.31%) patients. Five hundred or more PVCs were present in 16 (11.19%) patients. PVCs with different morphologies were found in 108 (75.52%) patients. Fifty patients (34.97%) had NSVTs, with a median of two episodes (range 1–526) and lasting a median of 5 beats (range: 3–33 beats). The median rate of NSVT was 131 bpm (range 101–202 bpm). Among these patients, ten had a history of SCD or adverse VA, and five had been implanted with an ICD.
Table 1.
Patient characteristics
| Variables | All patients (n = 143) |
|---|---|
| Age, yrs | 60.41 ± 11.06 |
| Male, n (%) | 92 (64.34%) |
| BMI, kg/m2 | 22.96 ± 3.09 |
| NYHA class, n (%) | |
| I-II | 20 (13.99%) |
| III-IV | 123 (86.01%) |
| Modified 2004 Mayo stage | |
| II, n (%) | 60 (41.96%) |
| IIIa, n (%) | 36 (25.17%) |
| IIIb, n (%) | 47 (32.87%) |
| History of syncope, n (%) | 23 (16.08%) |
| History of adverse VA/SCD, n (%) | 10 (6.99%) |
| History of chemotherapy, n (%) | 13 (9.09%) |
| Comorbidities | |
| Hypertension, n (%) | 36 (25.17%) |
| CAD, n (%) | 53 (37.06%) |
| Diabetes mellitus, n (%) | 18 (12.59%) |
| Hyperlipidemia, n (%) | 42 (29.37%) |
| CKD, n (%) | 53 (37.06%) |
| Aortic valve disease, n (%) | 19 (13.29%) |
| Atrial flutter/fibrillation, n (%) | 42 (29.37%) |
| Biomarkers | |
| cTNI, μg/L | 0.13 (0.04–0.25) |
| eGFR, mL/(min 1.73 m2) | 73.52 (54.12–96.35) |
| NT-proBNP, pg/mL | 7753.00 (3191.40–13 170.00) |
| Serum creatinine, μmol/L | 96.12 (74.94–118.31) |
| BUN, mmol/L | 8.37 (6.50–10.43) |
| D-dimer, ng/mL | 1.06 (0.58–2.42) |
| dFLC, mg/dL | 30.60 (14.87–63.68) |
| Echocardiogram | |
| LVEF, % | 52.05 ± 10.55 |
| LVEDD, mm | 43.09 ± 5.32 |
| Interventricular septum thickness, mm | 15.25 ± 3.33 |
| LVPW thickness, mm | 13.53 ± 2.36 |
| Holter findings | |
| Average heart rate, bpm | 77 (67–87) |
| Presence of PVC, n (%) | 132 (92.31%) |
| PVC counts, n | 41 (9–168) |
| PVC burden, % | 0.04 (0.01–0.15) |
| PVC counts ≥100, n (%) | 43 (30.07%) |
| PVC counts ≥500, n (%) | 16 (11.19%) |
| Multi-morphological PVC, n (%) | 108 (75.52%) |
| NSVT, n (%) | 50 (34.97%) |
| NSVT episodesa | 2 (1–3) |
| ≥2 NSVT episodes | 33 (23.08%) |
| NSVT length, beatsa | 5 (3–7) |
| NSVT rate, bpma | 131 (113–151) |
| Chemotherapy regimens, n (%) | 50 (34.96%) |
| Dexamethasone, n (%) | 48 (33.57%) |
| Bortezomib, n (%) | 45 (31.47%) |
| Melphalan, n (%) | 2 (1.40%) |
| Daratumumab, n (%) | 21 (14.69%) |
| Immunomodulatory drug, n (%) | 6 (4.20%) |
| ASCT, n (%) | 2 (1.40%) |
| Palliative care, n (%) | 93 (65.03%) |
| CIED treatments, n (%) | 19 (13.29%) |
| Pacemaker, n (%) | 14 (9.79%) |
| ICD, n (%) | 5 (3.50%) |
NYHA, New York Heart association; BMI, body mass index; VT, ventricular tachycardia; VF, ventricular fibrillation; SCD, sudden cardiac death; cTnI, cardiac troponin I; NT-proBNP, N-terminal pro-B-type natriuretic peptide; BUN, blood urea nitrogen; PVC, premature ventricular contraction; NSVT, non-sustained ventricular tachycardia; dFLC, difference in free light chain; eGFR, estimated glomerular filtration rate; LVEF, left ventricular ejection fraction; LVEDD, left ventricular end diastolic diameter; LVPW, left ventricular posterior wall; CAD, coronary artery disease; CKD, chronic kidney disease; ASCT, autologous stem-cell transplantation; CIED, cardiac implantable electrical device; ICD, implantable cardiac defibrillator.
aSummarized as median with interquartile range in patients with presence of non-sustained- ventricular tachycardia.
Relation of clinical factors, PVC, and NSVT to cardiac events
During the initial hospitalization, 12 of the 143 patients died due to adverse VA/SCD (n = 7) or end-stage heart failure (n = 5). The remaining 131 patients were discharged and over a median follow-up of 24.36 months (95% confidence interval: 21.44–27.28 months), 71 of these patients suffered from cardiac death, 15 patients experienced adverse VA/SCD, two of whom had history of adverse VA/SCD on diagnosis. Based on the clinical history, events during the initial hospitalization and follow-up, the patients were analyzed in three groups, in which 28 patients had suffered at least once VA/SCD (Group III) and 61 patients had cardiac death without undergoing any adverse VA/SCD events (Group II), while the other 54 had neither adverse VA/SCD nor cardiac death (Group I). The clinical characteristics of these three groups of patients are shown in Table 2. Compared with Group I, those in Group II and Group III were in more advanced Mayo stages, more likely to have atrial flutter/fibrillation and coronary artery disease (CAD), had higher levels of cTNI and NT-proBNP and thicker LVPW, but no differences regarding the above parameters were observed between Group II and Group III. On the other hand, compared with Group II, those in Group III were more likely to have history of syncope (28.57% vs. 11.48%, P = 0.045) and larger LVEDD (45.04 ± 6.58 vs. 42.56 ± 4.73, P = 0.046). The Holter findings between the three groups are presented in Figure 1.
Table 2.
Comparison of clinical features among AL-CA patients with different clinical outcomes
| Group I (n = 54) | Group II (n = 61) | Group III (n = 28) | P-value Group I vs. Group II | P-value Group I vs. Group III | P-value Group II vs. Group III | |
|---|---|---|---|---|---|---|
| Age | 59.26 ± 11.70 | 62.06 ± 11.26 | 59.07 ± 9.07 | 0.194 | 0.943 | 0.222 |
| Male | 37 (68.52%) | 38 (62.30%) | 17 (60.71%) | 0.484 | 0.480 | 0.887 |
| BMI | 23.54 ± 3.30 | 22.58 ± 2.81 | 22.68 ± 3.18 | 0.093 | 0.261 | 0.875 |
| NYHA III-IV | 43 (79.63%) | 56 (91.80%) | 24 (85.71%) | 0.060 | 0.499 | 0.454 |
| Adjusted 2004 Mayo stage | <0.001 | <0.001 | 0.660 | |||
| II | 39 (72.22%) | 14 (22.95%) | 7 (25.00%) | |||
| IIIa | 9 (16.67%) | 17 (27.87%) | 10 (35.71%) | |||
| IIIb | 6 (11.11%) | 30 (49.18%) | 11 (39.29%) | |||
| History of syncope | 8 (14.81%) | 7 (11.48%) | 8 (28.57%) | 0.596 | 0.136 | 0.045 |
| Atrial flutter/fibrillation | 10 (18.52%) | 21 (34.43%) | 11 (39.29%) | 0.055 | 0.041 | 0.657 |
| Hypertension | 10 (18.52%) | 17 (27.87%) | 9 (32.14%) | 0.238 | 0.166 | 0.681 |
| CAD | 14 (25.93%) | 26 (42.62%) | 13 (46.43%) | 0.061 | 0.010 | 0.737 |
| Diabetes mellitus | 8 (14.81%) | 6 (9.84%) | 4 (14.29%) | 0.415 | 0.949 | 0.537 |
| Aortic valve disease | 8 (14.81%) | 10 (16.39%) | 1 (3.57%) | 0.816 | 0.156 | 0.088 |
| CKD | 20 (37.04%) | 21 (34.43%) | 12 (42.86%) | 0.770 | 0.608 | 0.444 |
| Hyperlipidemia | 17 (31.48%) | 18 (29.51%) | 7 (25.00%) | 0.818 | 0.541 | 0.660 |
| cTNI | 0.05 (0.02–0.12) | 0.20 (0.11–0.36) | 0.17 (0.10–0.33) | <0.001 | <0.001 | 0.539 |
| eGFR | 82.48 (60.50–105.92) | 71.10 (54.85–89.86) | 62.64 (51.26–91.95) | 0.088 | 0.091 | 0.437 |
| NT-proBNP | 3191.40 (1469.00–7895.75) | 9752.00 (6912.00–13 756.00) | 9135.94 (5752.25–17 922.50) | <0.001 | <0.001 | 0.860 |
| D-dimer | 0.76 (0.42–1.44) | 1.72 (0.97–2.96) | 0.88 (0.58–2.54) | <0.001 | 0.110 | 0.123 |
| Serum Creatinine | 86.18 (71.60–112.76) | 97.71 (80.25–116.04) | 107.03 (81.85–121.00) | 0.140 | 0.102 | 0.453 |
| BUN | 7.77 (6.10–9.76) | 8.74 (7.15–11.00) | 8.97 (6.94–10.62) | 0.105 | 0.120 | 0.996 |
| LVEDD | 42.69 ± 5.11 | 42.56 ± 4.73 | 45.04 ± 6.58 | 0.889 | 0.078 | 0.046 |
| LVEF | 55.17 ± 9.44 | 49.51 ± 10.58 | 51.57 ± 11.30 | 0.003 | 0.131 | 0.405 |
| Interventricular septum thickness | 15.24 ± 4.04 | 15.41 ± 2.99 | 14.93 ± 2.48 | 0.798 | 0.710 | 0.460 |
| LVPW thickness | 12.72 ± 2.14 | 14.20 ± 2.40 | 13.64 ± 2.26 | <0.001 | 0.074 | 0.307 |
Group I: Patients did not suffer from adverse VA/SCD or cardiac death, Group II: Cardiac death without experience of adverse VA/SCD event, Group III: with presence of adverse VA/SCD.
NYHA, New York Heart association; BMI, body mass index; VT, ventricular tachycardia; VF, ventricular fibrillation; SCD, sudden cardiac death; cTnI, cardiac troponin I; NT-proBNP, N-terminal pro-B-type natriuretic peptide; BUN, blood urea nitrogen; eGFR, estimated glomerular filtration rate; LVEF, left ventricular ejection fraction; LVEDD, left ventricular end diastolic diameter; CAD, coronary artery disease; CKD, chronic kidney disease; LVPW, left ventricular posterior wall.
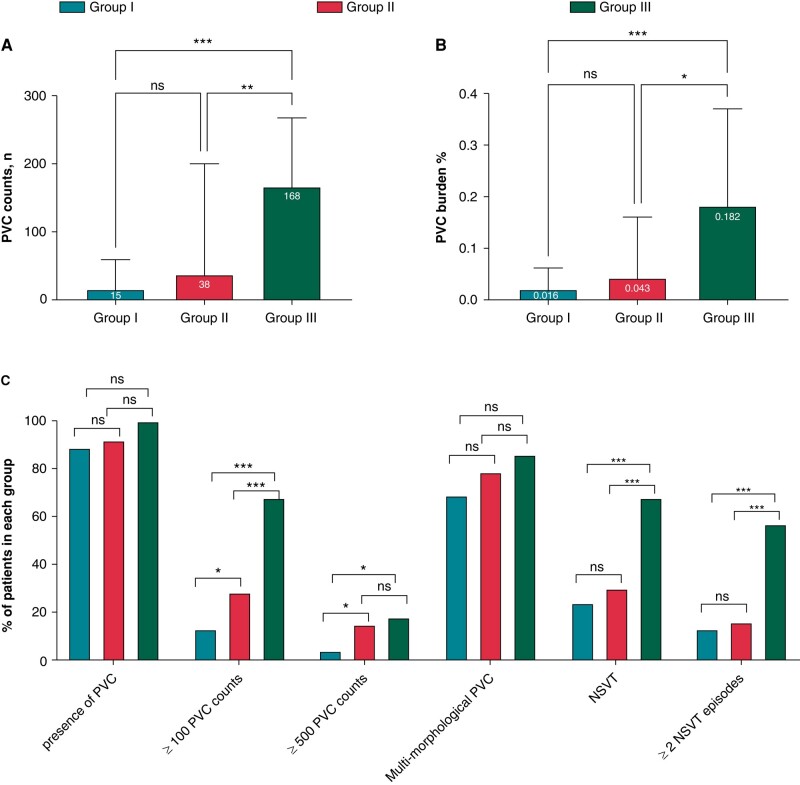
Figure 1.
Comparison of PVCs and NSVT parameters among AL-CA patients with different clinical outcomes. Notes: Comparison of PVC counts (A) PVC burden (B) among patients with the three groups. Prevalence of VA derived from Holter among the three groups. Group I: Patients did not suffer from adverse VA/SCD or cardiac death, Group II: Cardiac death without experience of adverse VA/SCD event, Group III: with presence of adverse VA/SCD; *P < 0.05, **P < 0.01, ***P < 0.001, ns P > 0.05.
Compared with Group I and II, the NSVT and PVCs on Holter recordings were significantly more prevalent in Group III (PVC counts [median (IQR): Group I, 15.50 (4.00–58.25); Group II, 38.00 (9.00–176.00); Group III, 167.50 (40.00–264.75); Group I vs. Group III P < 0.001, Group II vs. group III, P = 0.007], PVC burden [median (IQR): Group I, 0.02 (0.00–0.06)%; Group II, 0.04 (0.01–0.16)%; Group III, 0.18 (0.04–0.35)%; Group I vs. Group III P < 0.001; Group II vs. Group III, P = 0.009], NSVT [Group I, 24.07%; Group II, 29.51%; Group III, 67.86%; Group I vs. Group III P < 0.001; Group II vs. Group III, P < 0.001]). However, the differences were not significant between Group I and Group II. On the other hand, patients in Group II had a higher proportion of PVC counts more than 100 (P = 0.05) and more than 500 PVCs (P = 0.044), as compared with Group I. There was no significant difference with respect to presence (or absence) of PVCs and PVCs with different morphologies between the three study groups.
Predictive value of PVC, NSVT for occurrence of VA/SCD
Among the total study population, the clinical parameters including history of syncope, Mayo stage, NT-proBNP levels, and LVEDD were significantly associated with overall presence of adverse VA/SCD (Table 3). Moreover, significant association between PVC counts (odds ratio (OR): 1.39, 95%CI: 1.13–1.72), PVC burden (OR: 1.37, 95%CI: 1.12–1.67), and NSVT (OR: 5.72, 95%CI: 2.34–13.98) and odds of overall presence of VA/SCD was also observed in univariate logistic regression analysis (Figure 2).
Table 3.
Association between clinical parameters and VA/SCD
| Parameters | Presence of VT/VF/SCD | ||
|---|---|---|---|
| OR | 95% CI | P value | |
| Male | 0.82 | 0.35–1.93 | 0.656 |
| Age | 0.99 | 0.95–1.02 | 0.474 |
| BMI | 0.96 | 0.84–1.10 | 0.592 |
| History of syncope | 2.67 | 1.00–7.13 | 0.050 |
| Adjusted 2004 Mayo stage | |||
| II | 1 | ||
| IIIa | 2.91 | 1.00–8.52 | 0.051 |
| IIIb | 2.31 | 0.82–6.53 | 0.113 |
| NYHA III-IV | 0.97 | 0.30–3.17 | 0.959 |
| Atrial flutter/fibrillation | 1.75 | 0.74–4.16 | 0.202 |
| Hypertension | 1.54 | 0.63–3.81 | 0.346 |
| CAD | 1.63 | 0.70–3.75 | 0.255 |
| Diabetes mellitus | 1.20 | 0.36–3.98 | 0.763 |
| Aortic valve disease | 0.20 | 0.03–1.56 | 0.125 |
| CKD | 1.35 | 0.58–3.14 | 0.480 |
| eGFR | 0.99 | 0.98–1.01 | 0.195 |
| cTNI | 2.27 | 0.34–15.35 | 0.401 |
| D-Dimer | 1.04 | 0.89–1.21 | 0.639 |
| NT-proBNP (ln) | 1.55 | 1.02–2.35 | 0.039 |
| Serum creatinine | 1.00 | 0.99–1.01 | 0.681 |
| BUN | 1.05 | 0.97–1.12 | 0.235 |
| LVEDD | 1.08 | 1.01–1.17 | 0.035 |
| LVEF | 0.99 | 0.96–1.03 | 0.789 |
| Interventricular septum thickness | 0.96 | 0.84–1.10 | 0.566 |
| Posterior LV thickness | 1.03 | 0.86–1.22 | 0.780 |
| Multi-morphological PVC | 4.32 | 0.57–32.85 | 0.158 |
NYHA, New York Heart association; BMI, body mass index; VT, ventricular tachycardia; VF, ventricular fibrillation; SCD, sudden cardiac death; cTnI, cardiac troponin I; NT-proBNP, N-terminal pro-B-type natriuretic peptide; BUN, blood urea nitrogen; eGFR, estimated glomerular filtration rate; LVEF, left ventricular ejection fraction; LVEDD, left ventricular end diastolic diameter; CAD, coronary artery disease; CKD, chronic kidney disease; LVPW, left ventricular posterior wall; PVC, premature ventricular contraction; OR, Odds ratio; CI, confidence interval.
Figure 2.
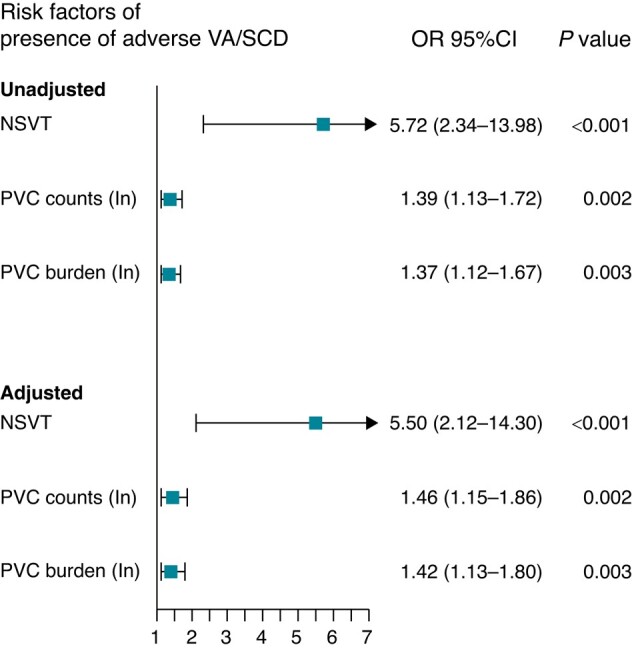
Association between NSVT, PVC and presence of adverse VA/SCD. OR, Odds ratio; CI, confidence interval; Adjusted OR from multivariate analysis included Mayo stage, history of syncope, LVEDD, NT-proBNP levels with stepwise selection. PVC, premature ventricular contraction; NSVT, non-sustained ventricular tachycardia.
In multivariate models that included significant clinical parameters, such associations were still significant. In addition, the presence of NSVT, PVC counts, and PVC burden provided modest discriminability for estimating overall presence of adverse VA/SCD, with area under the curve (AUC) of 0.70 (95CI%: 0.61–0.80), 0.73 (95%CI: 0.64–0.83) and 0.72 (95%CI: 0.63–0.82), respectively, which were better than the performance of the Mayo stage (AUC: 0.59, 95% CI: 0.49–0.70) (Figure 3A). The presence of NSVT provided a sensitivity of 0.68 and a specificity of 0.73. With a cut-off value of 103 and 0.102%, respectively, PVC counts and PVC burdens had sensitivity of 0.68 and 0.64, as well as specificity of 0.79 and 0.78, respectively (see Supplementary material online, Table S1). Furthermore, the three parameters also demonstrated decent performance for predicting new onset of adverse VAs or SCD during follow-up, with NSVT demonstrating highest AUC of 0.79 (95% CI: 0.69–0.89), and PVC counts and burden holding the same AUC of 0.77 (both 95%CI: 0.67–0.88) (Figure 3B).
Figure 3.
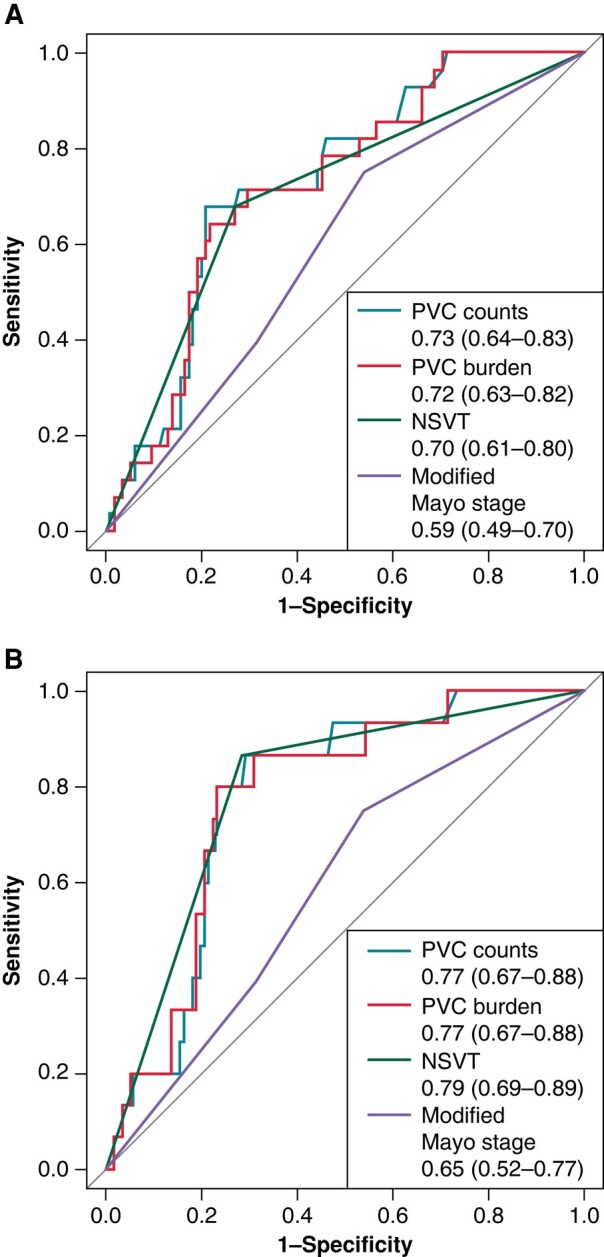
ROC curves for predicting adverse VA/SCD. Note: The AUC presented the accuracy of PVC, NSVT and Mayo stage of predicting presence of adverse VA/SCD (A) and new-onset of adverse VA/SCD during follow-up (B).
Association between PVC, NSVT, and outcomes during follow-up
In univariate Cox regression analysis, the presence of NSVT (HR: 13.57, 95%CI: 3.06–60.18), PVC counts (HR:1.46, 95%CI: 1.15–1.86) and PVC burden (HR: 1.43, 95%CI: 1.14–1.80) were significantly associated with the new onset of adverse VA or SCD, while among the clinical metrics, only modified Mayo stages and LVEDD demonstrated significant associations (see Supplementary material online, Table S2). The patients were further divided into three groups by tertiles of PVC counts (<14, ≥ 14 to <81, and ≥ 81) or PVC burden (<0.0142%, ≥ 0.0142% to <0.0848%, and ≥ 0.0848%). Incidence of adverse VA/SCD was significantly higher among patients with presence of NSVT (Figure 4A) and highest tertile of PVC counts (Figure 4B) and PVC burden (Figure 4C). However, regarding occurrence of cardiac death, the overall median survival of patients with (17.8 months) and without NSVT (23.4 months) was not significant (log-rank P = 0.07, Figure 5A). Elevated risk of cardiac death and short survival time was found in patients with highest tertile of PVC counts (tertile1:33 months, tertile2:19.5 months, tertile3: 14.6 months) and PVC burden (tertile1: 23.8 months, tertile2: 20.7 months, tertile3: 13.8 months) (Table 4, Figure 5B and C). After excluding the extreme values over 95 or 99, the hazard ratios of the highest tertile group for both the new occurrence of adverse VA/SCD and cardiac death did not display any pronounced change (see Supplementary material online, Table S3). Similar findings regarding the association of PVC, NSVT, and risk of adverse VA/SCD or cardiac death were also observed in patients without previous history of adverse VA/SCD (see Supplementary material online, Figure S1), and the subgroup of patients in Mayo 2004 stage III without history of adverse VA/SCD (see Supplementary material online, Figure S2).
Figure 4.
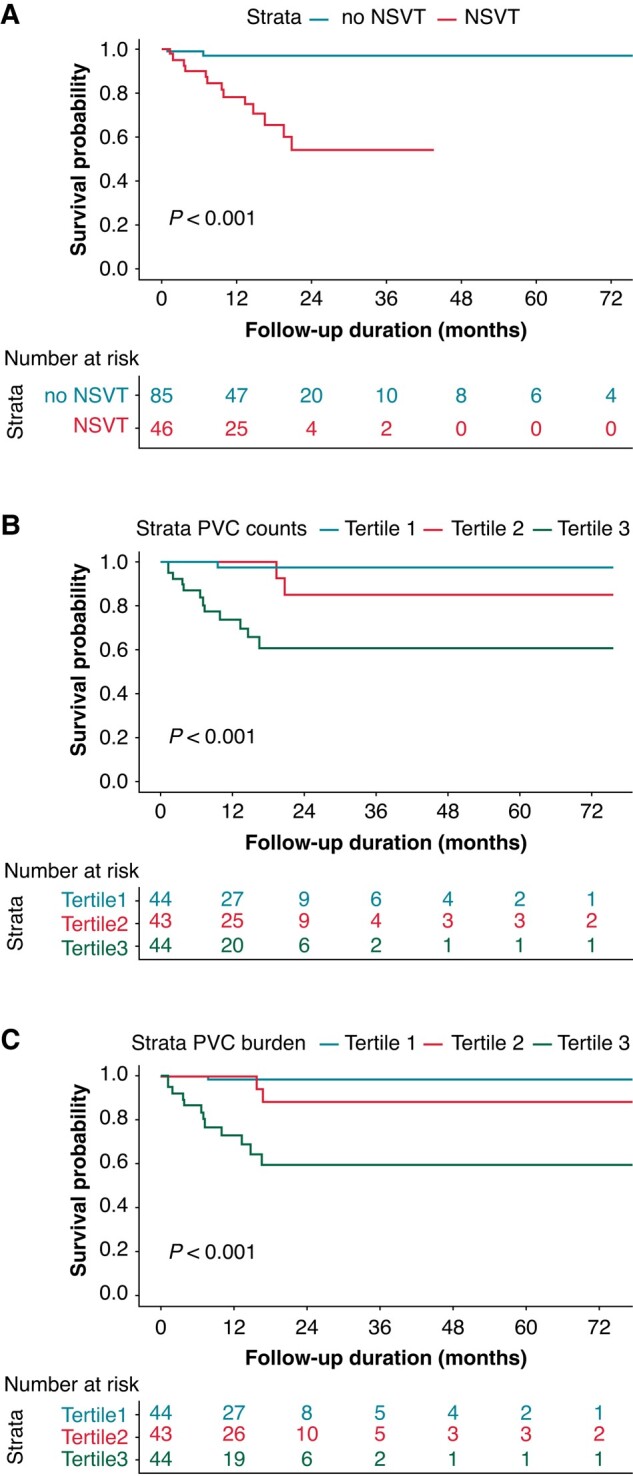
Kaplan-Meier curves of survival free from new occurrence of adverse VA/SCD during follow-up. Note: Kaplan-Meier curve showing survival free from new-onset of adverse VA/SCD according to presence of NSVT (A) tertiles of PVC counts (B) and PVC burden (C).
Figure 5.
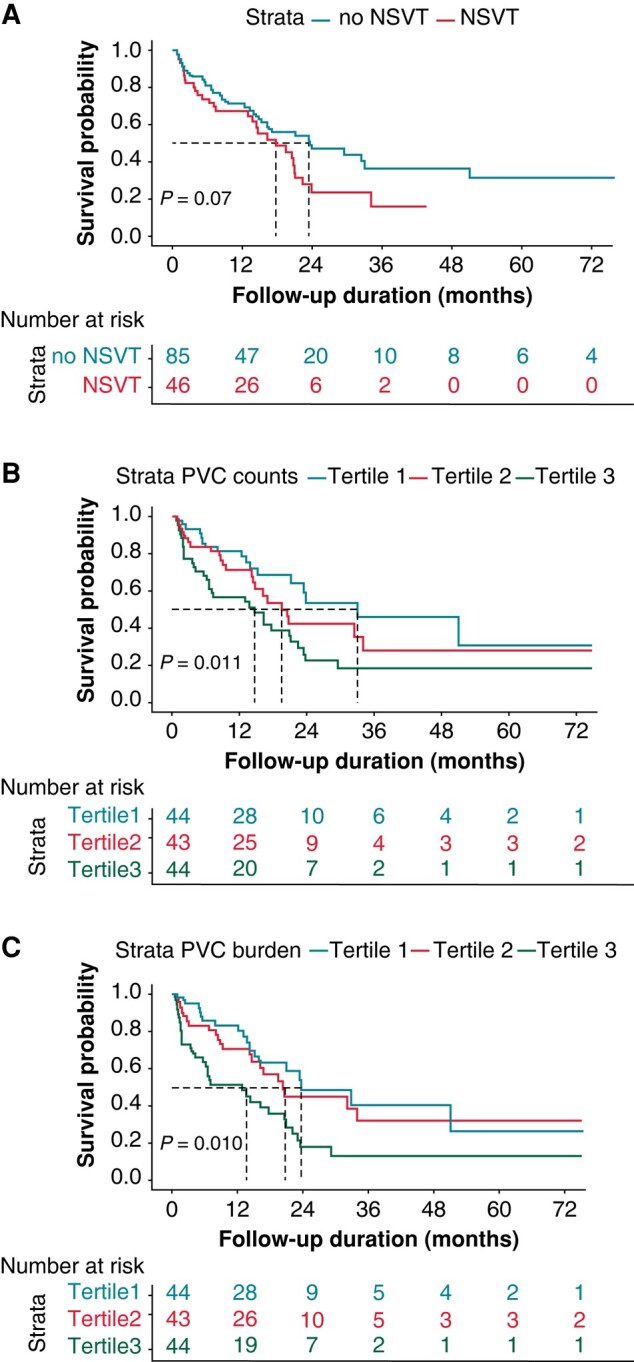
Kaplan-Meier curves of survival free from cardiac death. Kaplan-Meier curve showing survival free from cardiac death according to presence of NSVT (A) tertiles of PVC counts (B) and PVC burden (C).
Table 4.
Prognostic value of NSVT and PVCs on new occurrence of VA/SCD and cardiac death
| New onset of adverse VA/SCD (n = 15) | Cardiac death (n = 71) | |||||
|---|---|---|---|---|---|---|
| Univariate | Univariate | Multivariate | ||||
| HR (95%CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | |
| NSVT | 13.57 (3.06–60.18) | <0.001 | 1.55 (0.96–2.51) | 0.073 | 1.16 (0.67–1.98) | 0.603 |
| PVC counts (ln) | 1.46 (1.15–1.86) | 0.002 | 1.09 (1.01–1.17) | 0.022 | 1.10 (1.02–1.19) | 0.019 |
| PVC burden (ln) | 1.43 (1.14–1.80) | 0.002 | 1.09 (1.01–1.17) | 0.022 | 1.10 (1.02–1.19) | 0.017 |
| Categorical PVC counts | ||||||
| Tertile 1st | Ref | Ref | Ref | |||
| Tertile 2nd | 2.18 (0.20–24.05) | 0.525 | 1.44 (0.76–2.71) | 0.262 | 1.42 (0.72–2.79) | 0.309 |
| Tertile 3rd | 15.37 (2.00–118.28) | 0.009 | 2.37 (1.31–4.29) | 0.004 | 2.33 (1.27–4.28) | 0.006 |
| Categorical PVC burden | ||||||
| Tertile 1st | Ref | Ref | Ref | |||
| Tertile 2nd | 2.10 (0.19–23.21) | 0.544 | 1.21 (0.64–2.27) | 0.557 | 1.41 (0.73–2.69) | 0.306 |
| Tertile 3rd | 15.78 (2.05–121.46) | 0.008 | 2.25 (1.25–4.02) | 0.007 | 2.58 (1.42–4.69) | 0.002 |
HR, Hazard ratio; CI, confidence interval; PVC, premature ventricular contraction; NSVT, non-sustained-ventricular tachycardia. Multivariate analysis included significant clinical parameters in univariate analysis (age, BMI, modified Mayo stage 2004, LVEF, cTnI, NT-proBNP, Serum Creatinine, BUN, LVPW thickness, eGFR, NYHA class III-IV) with a stepwise selection.
Incremental value of PVC for predicting cardiac death
After adjustments of the prognostic variables for cardiac death derived from univariate Cox analysis (see Supplementary material online, Table S2), the PVC counts and PVC burden as continuous variables were still associated with elevated risks of cardiac death. Compared with the tertile1, the highest tertile of PVC counts and PVC burden remained significantly associated with marked risk of cardiac death independent of Mayo stage and other significant clinical parameters, with an adjusted HR of 2.33 (95%CI: 1.27–4.28) and 2.58 (95%CI: 1.42–4.69), respectively (Table 4). By adding the PVC counts or burden as a categorical variable to the modified Mayo staging system, the C index for predicting cardiac death increased from 0.681 to 0.712 and 0.717, respectively, with an improvement in reclassification as seen using the integrated discrimination improvement (IDI) (0.134 and 0.140, respectively, after adding PVC counts tertile or PVC burden tertiles) and overall net reclassification index (NRI) (0.720 and 0.701, respectively, after adding PVC counts tertile or PVC burden tertiles) (Table 5).
Table 5.
Incremental prognostic value of PVCs over modified mayo stages
| C index | P value | IDI (95%CI) | P value | Overall NRI(95%CI) | P value | |
|---|---|---|---|---|---|---|
| Modified Mayo stage | 0.681 | Ref | Ref | Ref | ||
| PVC counts tertile + modified Mayo stage | 0.712 | 0.046 | 0.134 (0.027–0.240) | 0.016 | 0.720 (0.121–1.105) | 0.026 |
| PVC burden tertile + modified Mayo stage | 0.717 | 0.022 | 0.140 (0.034–0.245) | 0.018 | 0.701 (0.088–1.051) | 0.026 |
CI, confidence interval; C-index, Harrell’s C concordance index; IDI, integrated discrimination improvement; NRI, net reclassification index.
Discussion
This is the first study, to our knowledge, to comprehensively assess the association between 24-h Holter-derived PVC counts, PVC burden, and NSVT with the risk of adverse VA/SCD and cardiac mortality in AL-CA patients. The main results of this study may be summarized as follows: First, patients who experienced adverse VA/SCD tended to have significantly higher PVC counts, PVC burden, and presence of NSVT, as compared to the other patient groups. Second, PVC counts, PVC burden, and NSVT were independent predictors for identifying patients who later developed VA/SCD and demonstrated better performance than the Mayo stage. Third, higher PVC counts and PVC burden were also independent predictors for cardiac death, in addition to predicting adverse VA/SCD. Moreover, higher PVC counts and PVC burden added incremental value for predicting cardiac death.
Since CA has gained more clinical recognition in the field of heart failure,22,23 various arrhythmias ranging from brady- and tachyarrhythmias accompanying this disease have also been increasingly addressed.5 In particular, VAs and their potential link to adverse clinical outcomes have been a matter of concern. However, identification of patients with CA who may benefit from ICD therapy remains to be a challenge. It remains unclear whether ICD prevents SCD in CA, given the fact that pulseless electrical activity is a common cause of SCD in these patients. Patients with AL-CA have a higher prevalence of VA and incidence of adverse VA/SCD,24,25 as compared with the ATTR-CA, and therefore, the risk for adverse VA/SCD deserves further investigation.
Previous studies have reported a large disparity regarding the prevalence of PVCs and NSVT in AL-CA, possibly due to differences in sample size, study population, and type of rhythm monitoring. Goldsmith et al described NSVT in all of the 24 AL-CA patients studied,26 whereas Palladini et al. observed NSVT in 18% and PVCs in 72% in 51 patients (13 of whom with cardiac involvement), respectively.8 The above-mentioned studies were limited by rather small sample sizes. By contrast, Murtagh et al. reported only 1% NSVT and 13% PVC occurrence in 127 AL-CA patients with routine 12 lead ECG data,6 but the authors did not provide continuous ECG monitoring data. More recently, Sidana et al. reported 25.1% NSVT and 78.8% PVC occurrence based on 24-h Holter recordings among 239 patients with AL. In their study population, 35.5% NSVT and 86.5% PVC were reported among patients with AL-CA. In our study, we observed a similar prevalence of NSVT (34.5%) and PVC (92.3%) on Holter monitoring at baseline among 143 AL-CA patients with Mayo stages II-III, reinforcing the frequent occurrence of VAs in these patients.
A significant finding of our study was the role of PVC count, PVC burden, and presence of NSVTs in predicting adverse VA/SCD during follow-up. Previous work from Brandon et al. reported that NSVT was associated with an increased risk of adverse VA in smaller cohort of ICD recipients with AL-CA.10 Palladini et al. demonstrated that the presence of couplets was an independent predictors of sudden death.8 In line with these observations, our findings add clinically relevant information regarding the importance of Holter-derived PVC count, PVC burden, and presence of NSVTs on adverse VA/SCD risk in patients with moderate-to-advanced AL-CA. On the other hand, we also demonstrated similar levels of cTNI and NT-proBNP in patients who suffered from cardiac death without adverse VA/SCD (Group II) and in those who experienced at least one adverse VA/SCD during the disease course (Group III). This observation suggests that the traditional cardiac biomarkers as well as the Mayo staging system, which are known to demonstrate good performance for predicting overall mortality or cardiac death, still lack the capability to identify patients who might suffer from adverse VA/SCD. In contrast, PVC counts, PVC burden, and NSVT occurrence on Holter monitoring were shown to be powerful predictors of adverse VA/SCD in our study. Therefore, Holter monitoring may not only be helpful in improving risk stratification but also may play an important role in identifying patients at risk and to prevent such patients from SCD/VAs due to improvement of treatment which would need further and prospective studies.
In AL-CA, the predictive value of NSVT for major arrhythmic events and mortality has been a matter of debate.27 Sidana et al14 reported that NSVT was an independent predictor of overall mortality after adjusting for age and Mayo stage, but in their study, only 59% of the patients were with cardiac involvement and early stage (median NT-proBNP of 824 pg/mL) and 21.9% patients were in Mayo stage III, with most of the patients undergoing stem cell therapy. Although their study did not distinguish the cause of death, one can speculate among the patients with early stage of AL-CA and stem cell therapy, SCD rather than worsening heart failure as the major cause of death, as previously reported.28 Differing from the findings of Sidana et al., in a recent study including 51 AL-CA patients, Alberto et al found that the presence of NSVT on 24-h ECG monitoring did not confer a higher risk of the combined end-point of death and HF hospitalizations.29 Our study partly supports their findings, with the observation that neither the presence of NSVT nor higher episodes of NSVT yielded higher risk of cardiac death. Furthermore, as complementary to the results of these previous studies, our study, based on a relatively larger sample size population, distinguished the adverse VA/SCD events during follow-up and showed that NSVT in 24-hour Holter was associated with more than 12 fold-increase in the risk of new occurrence of adverse VA/SCD, emphasizing the potential clinical value of the NSVT for identifying arrhythmic risks in patients with AL-CA.
In our study, we also found that PVC counts and PVC burden rather than the presence of the PVC are of clinical importance. Although there is no significant difference in the proportion of PVC presence among the survivors without undergoing adverse VA/SCD and those who suffered from adverse VA/SCD, PVC counts and PVC burden were significantly higher in the latter group. Survival analysis further suggested that patients within the upper tertile had a more than 12-fold in risk of adverse VA/SCD. Moreover, PVC counts and PVC burden predicted the risk of cardiac death, which was also independent of the Mayo stage. Such observations might not only be due to their association with SCD but also the potential association with heart failure, which has also been reported in other cardiovascular diseases.30,31 By combining the PVC count or PVC burden with the Mayo stage, a higher percentage of patients who are at risk for cardiac death can be identified.
Our study has some limitations. First, this is a single-centre, retrospective study, and possible referral bias might exist since the patients were from a tertiary cardiovascular centre. Second, the limited number of adverse VA/SCD events did not allow further adjustment in the Cox regression. Our exploratory results only suggest that higher PVC burden is associated with poor prognosis in AL-CA, which might serve as an excellent background for hypothesis-generation rather than giving a definite cut-off value of the PVCs. Prospective studies with larger sample sizes are warranted to validate our results and determine the exact burden for clinical relevance in these patients. Moreover, we did not explore the impact of therapy on possible change of VA occurrence using serial or 7 day-Holter monitoring. Therefore, future studies are needed to elucidate the impact of changes in PVC count and burden during disease progression in patients with AL-CA. Finally, due to the relatively small sample size with monomorphic PVCs and lack of electrophysiologic study data, we consider that the available data are insufficient to explore the clinical significance of PVC morphology or origin in determining the prognosis of AL-CA. However, these are important issues, which warrant further studies in the future.
Conclusions
PVC count, PVC burden, and NSVT occurrence significantly correlated with adverse VA or SCD during follow-up in patients with AL-CA. Moreover, higher PVC counts and burden added incremental value for predicting cardiac death. Overall, our results suggest that Holter ECG recording may be a cost-effective tool to improve risk stratification in patients with AL-CA.
Supplementary Material
Acknowledgements
The authors would like to thank Dr Yingxuan Zhu for the valuable advice on statistical analyses.
Contributor Information
Zhongli Chen, State Key Laboratory of Cardiovascular Disease, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Fuwai Hospital, No. 167 North Lishi Road, Xicheng District, Beijing 100037, China; Cardiac Arrhythmia Center, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Fuwai Hospital, No. 167 North Lishi Road, Xicheng District, Beijing 10037, China.
Anteng Shi, State Key Laboratory of Cardiovascular Disease, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Fuwai Hospital, No. 167 North Lishi Road, Xicheng District, Beijing 100037, China.
Hongbin Dong, Department of Radiology, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Fuwai Hospital, No. 167 North Lishi Road, Xicheng District, Beijing 10037, China.
Natallia Laptseva, Division of Heart Failure, Department of Cardiology, University Heart Center, Rämistrasse 100, Zurich CH-8091, Switzerland.
Feng Chen, State Key Laboratory of Cardiovascular Disease, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Fuwai Hospital, No. 167 North Lishi Road, Xicheng District, Beijing 100037, China; Cardiac Arrhythmia Center, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Fuwai Hospital, No. 167 North Lishi Road, Xicheng District, Beijing 10037, China.
Jiandu Yang, State Key Laboratory of Cardiovascular Disease, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Fuwai Hospital, No. 167 North Lishi Road, Xicheng District, Beijing 100037, China; Cardiac Arrhythmia Center, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Fuwai Hospital, No. 167 North Lishi Road, Xicheng District, Beijing 10037, China.
Xiaogang Guo, State Key Laboratory of Cardiovascular Disease, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Fuwai Hospital, No. 167 North Lishi Road, Xicheng District, Beijing 100037, China; Cardiac Arrhythmia Center, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Fuwai Hospital, No. 167 North Lishi Road, Xicheng District, Beijing 10037, China.
Firat Duru, Center for Translational and Experimental Cardiology, University of Zurich, Rämistrasse 100, Zurich CH-8091, Switzerland; Division of Cardiac Arrhythmias, Department of Cardiology, University Heart Center, Rämistrasse 100, Zurich CH-8091, Switzerland.
Keping Chen, State Key Laboratory of Cardiovascular Disease, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Fuwai Hospital, No. 167 North Lishi Road, Xicheng District, Beijing 100037, China; Cardiac Arrhythmia Center, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Fuwai Hospital, No. 167 North Lishi Road, Xicheng District, Beijing 10037, China.
Liang Chen, State Key Laboratory of Cardiovascular Disease, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Fuwai Hospital, No. 167 North Lishi Road, Xicheng District, Beijing 100037, China; Center for Translational and Experimental Cardiology, University of Zurich, Rämistrasse 100, Zurich CH-8091, Switzerland.
Supplementary material
Supplementary material is available at Europace online.
Authors contributions
L.C. and K.C. designed the work, contributed to supervision, funding, and resource acquisition. F.D. contributed to the conceptualization, interpretation, and revision of the work. Z.C. performed the research, analyzed data, and drafted the manuscript. A.S. assisted in data analysis and follow-up. H.D. contributed to data collection, diagnosis confirmation and manuscript revision. F.C., J.Y., and X.G. provided help and advice on the data interpretation and assisted in event confirmation during patient’s follow-up. N.L. and F.D. contributed to the interpretation of the findings and manuscript editing and revision.
Funding
This study was supported by National Natural Science Foundation of China (82100377), Beijing Nova Program (Z211100002121046), and National High Level Hospital Clinical Research Funding (2023-GSP-RC-01, 2023-GSP-ZD-2, 2022-GSP-GG-31).
Data availability
The authors declare that the data that support the findings of this study are available from the corresponding author.
References
- 1. Hartnett J, Jaber W, Maurer M, Sperry B, Hanna M, Collier P et al. Electrophysiological manifestations of cardiac amyloidosis. JACC CardioOncol 2021;3:506–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arbelo E, Protonotarios A, Gimeno JR, Arbustini E, Barriales-Villa R, Basso C et al. 2023 ESC guidelines for the management of cardiomyopathies. Eur Heart J 2023;44:3503–626. [DOI] [PubMed] [Google Scholar]
- 3. Khanna S, Lo P, Cho K, Subbiah R. Ventricular arrhythmias in cardiac amyloidosis: a review of current literature. Clin Med Insights Cardiol 2020;14:1179546820963055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Orini M, Graham AJ, Martinez-Naharro A, Andrews CM, de Marvao A, Statton B et al. Noninvasive mapping of the electrophysiological substrate in cardiac amyloidosis and its relationship to structural abnormalities. J Am Heart Assoc 2019;8:e012097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Laptseva N, Rossi VA, Sudano I, Schwotzer R, Ruschitzka F, Flammer AJ et al. Arrhythmic manifestations of cardiac amyloidosis: challenges in risk stratification and clinical management. J Clin Med 2023;12:2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Murtagh B, Hammill SC, Gertz MA, Kyle RA, Tajik AJ, Grogan M. Electrocardiographic findings in primary systemic amyloidosis and biopsy-proven cardiac involvement. Am J Cardiol 2005;95:535–7. [DOI] [PubMed] [Google Scholar]
- 7. Sayed RH, Rogers D, Khan F, Wechalekar AD, Lachmann HJ, Fontana M et al. A study of implanted cardiac rhythm recorders in advanced cardiac AL amyloidosis. Eur Heart J 2015;36:1098–105. [DOI] [PubMed] [Google Scholar]
- 8. Palladini G, Malamani G, Cò F, Pistorio A, Recusani F, Anesi E et al. Holter monitoring in AL amyloidosis: prognostic implications. Pacing Clin Electrophysiol 2001;24:1228–33. [DOI] [PubMed] [Google Scholar]
- 9. John RM. Arrhythmias in cardiac amyloidosis. J Innov Card Rhythm Manag 2018;9:3051–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Varr BC, Zarafshar S, Coakley T, Liedtke M, Lafayette RA, Arai S et al. Implantable cardioverter-defibrillator placement in patients with cardiac amyloidosis. Heart Rhythm 2014;11:158–62. [DOI] [PubMed] [Google Scholar]
- 11. Lin G, Dispenzieri A, Kyle R, Grogan M, Brady PA. Implantable cardioverter defibrillators in patients with cardiac amyloidosis. J Cardiovasc Electrophysiol 2013;24:793–8. [DOI] [PubMed] [Google Scholar]
- 12. Reisinger J, Dubrey SW, Lavalley M, Skinner M, Falk RH. Electrophysiologic abnormalities in AL (primary) amyloidosis with cardiac involvement. J Am Coll Cardiol 1997;30:1046–51. [DOI] [PubMed] [Google Scholar]
- 13. Dubrey SW, Cha K, Anderson J, Chamarthi B, Reisinger J, Skinner M et al. The clinical features of immunoglobulin light-chain (AL) amyloidosis with heart involvement. QJM 1998;91:141–57. [DOI] [PubMed] [Google Scholar]
- 14. Sidana S, Tandon N, Brady PA, Grogan M, Gertz MA, Dispenzieri A et al. Prognostic significance of holter monitor findings in patients with light chain amyloidosis. Mayo Clin Proc 2019;94:455–64. [DOI] [PubMed] [Google Scholar]
- 15. Wechalekar AD, Schonland SO, Kastritis E, Gillmore JD, Dimopoulos MA, Lane T et al. A European collaborative study of treatment outcomes in 346 patients with cardiac stage III AL amyloidosis. Blood 2013;121:3420–7. [DOI] [PubMed] [Google Scholar]
- 16. Garcia-Pavia P, Rapezzi C, Adler Y, Arad M, Basso C, Brucato A et al. Diagnosis and treatment of cardiac amyloidosis: a position statement of the ESC working group on myocardial and pericardial diseases. Eur Heart J 2021;42:1554–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines and the Heart Rhythm Society. Circulation 2018;138:e272–391. [DOI] [PubMed] [Google Scholar]
- 18. Raafs AG, Verdonschot JAJ, Henkens MTHM, Adriaans BP, Wang P, Derks K et al. The combination of carboxy-terminal propeptide of procollagen type I blood levels and late gadolinium enhancement at cardiac magnetic resonance provides additional prognostic information in idiopathic dilated cardiomyopathy—a multilevel assessment of myocardial fibrosis in dilated cardiomyopathy. Eur J Heart Fail 2021;23:933–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Maron BJ, Olivotto I, Spirito P, Casey SA, Bellone P, Gohman TE et al. Epidemiology of hypertrophic cardiomyopathy-related death: revisited in a large non-referral-based patient population. Circulation 2000;102:858–64. [DOI] [PubMed] [Google Scholar]
- 20. Kitai T, Miyakoshi C, Morimoto T, Yaku H, Murai R, Kaji S et al. Mode of death among Japanese adults with heart failure with preserved, midrange, and reduced ejection fraction. JAMA Netw Open 2020;3:e204296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Melacini P, Basso C, Angelini A, Calore C, Bobbo F, Tokajuk B et al. Clinicopathological profiles of progressive heart failure in hypertrophic cardiomyopathy. Eur Heart J 2010;31:2111–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Parker AM, Ahmed MM. Early is better than late, but late is better than never: referral to advanced heart failure cardiology. Cardiovasc Innov Appl 2023;8:953. [Google Scholar]
- 23. Vilaro JR. Heart failure with preserved ejection fraction: important things to know about the stiff heart. Cardiovasc Innov Appl 2023;8:954. [Google Scholar]
- 24. Donnellan E, Wazni OM, Hanna M, Saliba W, Jaber W, Kanj M. Primary prevention implantable cardioverter-defibrillators in transthyretin cardiac amyloidosis. Pacing Clin Electrophysiol 2020;43:1401–3. [DOI] [PubMed] [Google Scholar]
- 25. Hamon D, Algalarrondo V, Gandjbakhch E, Extramiana F, Marijon E, Elbaz N et al. Outcome and incidence of appropriate implantable cardioverter-defibrillator therapy in patients with cardiac amyloidosis. Int J Cardiol 2016;222:562–8. [DOI] [PubMed] [Google Scholar]
- 26. Goldsmith YB, Liu J, Chou J, Hoffman J, Comenzo RL, Steingart RM. Frequencies and types of arrhythmias in patients with systemic light-chain amyloidosis with cardiac involvement undergoing stem cell transplantation on telemetry monitoring. Am J Cardiol 2009;104:990–4. [DOI] [PubMed] [Google Scholar]
- 27. Martini N, Sinigiani G, De Michieli L, Mussinelli R, Perazzolo Marra M, Iliceto S et al. Electrocardiographic features and rhythm disorders in cardiac amyloidosis. Trends Cardiovasc Med 2023:S1050-1738(23)00024-5. (EPUB ahead of print: Feb 24) [DOI] [PubMed] [Google Scholar]
- 28. Kharoubi M, Bodez D, Bézard M, Zaroui A, Galat A, Guendouz S et al. Describing mode of death in three major cardiac amyloidosis subtypes to improve management and survival. Amyloid 2022;29:79–91. [DOI] [PubMed] [Google Scholar]
- 29. Cappelli F, Cipriani A, Russo D, Tini G, Zampieri M, Zocchi C et al. Prevalence and prognostic role of nonsustained ventricular tachycardia in cardiac amyloidosis. Amyloid 2022;29:211–2. [DOI] [PubMed] [Google Scholar]
- 30. Kim YG, Choi YY, Han K-D, Min KJ, Choi HY, Shim J et al. Premature ventricular contraction increases the risk of heart failure and ventricular tachyarrhythmia. Sci Rep 2021;11:12698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dukes JW, Dewland TA, Vittinghoff E, Mandyam MC, Heckbert SR, Siscovick DS et al. Ventricular ectopy as a predictor of heart failure and death. J Am Coll Cardiol 2015;66:101–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that the data that support the findings of this study are available from the corresponding author.