Abstract
We have previously demonstrated that hepatitis B virus (HBV) replication and gene expression are abolished in the livers of HBV transgenic mice by cytotoxic T lymphocytes (CTLs) and during lymphocytic choriomeningitis virus (LCMV) infection, stimuli that trigger the production of alpha/beta interferon, gamma interferon, and tumor necrosis factor alpha in the liver. We now report that hepatic HBV replication and gene expression are inhibited by the local induction of these cytokines during adenovirus- and murine cytomegalovirus (MCMV)-induced hepatitis. Further, we show that MCMV also blocks HBV replication and gene expression in the proximal convoluted tubules of the kidney by causing interstitial nephritis and inducing the same cytokines in the renal parenchyma. These results suggest that inflammatory cytokines probably contribute to viral clearance during acute viral hepatitis in humans, and they imply that induction of these cytokines in the liver and other infected tissues of chronically infected patients might have therapeutic value.
Hepatitis B virus (HBV) is a noncytopathic, enveloped virus that causes acute and chronic hepatitis and hepatocellular carcinoma (7). The cellular immune response to HBV antigens is thought to play a critical role in the pathogenesis of the disease and in clearance of the infection. We have previously reported that adoptively transferred HBV-specific cytotoxic T lymphocytes (CTLs) abolish HBV replication and gene expression in the liver of HBV transgenic mice (19). This effect is achieved by two distinct mechanisms following antigen recognition: first, the CTLs kill a small fraction of the hepatocytes; and second, they secrete gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α), which noncytolytically interrupt the viral life cycle in all of the hepatocytes. Based on these observations, we have suggested that this cytokine-mediated curative CTL function may contribute substantially to viral clearance during HBV infection (17).
If this argument is correct, HBV should be susceptible to control by nonspecific stimuli that induce antiviral cytokines in infected tissues. Indeed, we have recently demonstrated that HBV replication can be abolished in these animals during lymphocytic choriomeningitis virus infection (16) and following the administration of interleukin-12 (5). The present study was undertaken to determine if other viruses, including murine cytomegalovirus (MCMV) and a replication-defective recombinant adenovirus developed to deliver foreign genes to the liver, can induce sufficient quantities of these cytokines to suppress HBV replication. These viruses were chosen because they are hepatotropic (43, 47) and because CD8+ CTLs and natural killer (NK) cells that produce large amounts of these cytokines play a pivotal role in resolving their respective infections (27, 32, 54).
MATERIALS AND METHODS
HBV transgenic mice.
The HBV transgenic mouse lineage 1.3.32 (official designation, Tg[HBV 1.3 genome]Chi32) used in this study has been described previously (21). These mice replicate HBV at high levels in the liver and kidney without any evidence of cytopathology. Lineage 1.3.32 was expanded by repetitive backcrossing against the C57BL/6 parental strain and then bred one generation against BALB/c mice to produce the F1 hybrids used in all experiments described here. Mice matched for age (6 to 10 weeks), sex (male), and levels of hepatitis B surface antigen (HBsAg) in the serum (determined by using a commercially available kit from Abbott Laboratories, Abbott Park, Ill.) were used.
Adenovirus infection.
A recombinant, replication-deficient adenovirus designated Ad.CBlacZ (28) was kindly provided by James Wilson (University of Pennsylvania Medical Center, Philadelphia). This virus is based on human adenovirus type 5, in which sequences spanning the E1a and E1b genes (from 1.0 to 9.2 map units) were deleted and replaced with a minigene cassette of the Escherichia coli lacZ gene, encoding β-galactosidase, driven by a cytomegalovirus-enhanced β-actin promoter. It also contains a small deletion in the E3 region (150 bp within the 14.6-kDa protein). Mice infected with this virus develop a strong CD8+ CTL response to adenovirus proteins, resulting in hepatitis (54, 56).
Stocks of Ad.CBlacZ were grown in 293 cells (12) and were purified by two rounds of CsCl density centrifugation as previously described (9). Viral titers were determined by plaque assay on 293 cells, and a single stock was used throughout this study. Mice were infected with various doses of Ad.CBlacZ diluted in 200 μl of sterile 0.9% NaCl (saline) solution via the tail vein. Control mice were injected with the same volume of saline. Animals were sacrificed at multiple time points following infection, and their livers and kidneys were harvested for histological, immunohistochemical, and histochemical analyses (see below), or they were snap frozen in liquid nitrogen and stored at −80°C for subsequent DNA and RNA analyses.
MCMV infection.
The Smith strain of MCMV (American Type Culture Collection [ATCC] VR-194; ATCC, Rockville, Md.) was used in this study. Acute infection with MCMV leads to widespread growth of the virus in virtually all organ systems (36) and can also result in severe hepatitis (43). A stock of MCMV was prepared as a 10% (wt/vol) homogenate of the submaxillary salivary glands from infected BALB/c mice (41). These mice, at 21 days of age, were injected intraperitoneally with 1.0 × 106 PFU of tissue culture-passaged virus (grown in and titered on NIH 3T3 cells [ATCC CRL1658]), and their salivary glands were harvested, pooled, and homogenized 14 days later. This salivary gland-passaged MCMV stock was titered by standard plaque assay on NIH 3T3 cells. Mice were injected intraperitoneally with various doses of MCMV diluted in 300 μl of sterile saline and were sacrificed at multiple time points postinfection. Control mice were injected with a dilution of a salivary gland homogenate prepared from uninfected BALB/c mice. The livers and kidneys harvested at autopsy were processed exactly as those described for the adenovirus-infected animals. In addition, liver and kidney were frozen in media at −80°C for quantitation of infectious MCMV. For this purpose, the right frontal liver lobe and one-half of one kidney (cut longitudinally) were weighed and homogenized, and MCMV was quantitated by plaque assay on NIH 3T3 monolayers. The limit of detection was 10 PFU/ml of tissue homogenate (10%, wt/vol).
Tissue DNA and RNA analyses.
Frozen liver (left lobe) and kidney tissues were mechanically pulverized under liquid nitrogen, and total genomic DNA and RNA were isolated for Southern and Northern blot analyses exactly as previously described (21). Nylon membranes were analyzed for HBV DNA, HBV RNA, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and 2′5′-oligoadenylate synthetase (2′5′-OAS) as described elsewhere (16). Quantitation of cytokine and T-lymphocyte marker mRNAs was performed by RNase protection assay exactly as previously described (19, 24).
Serum HBV DNA analysis.
Quantitation of HBV DNA in the serum of transgenic animals was assessed by dot blot analysis as previously described (21), using 400 μl of serum pooled from mice within the same treatment group. Pooled serum from age- and sex-matched saline-injected HBV transgenic mice was used as a positive control, and pooled serum from C57BL/6 × BALB/c nontransgenic mice was used as a negative control.
β-Galactosidase histochemistry.
The in vivo expression of β-galactosidase in the livers and kidneys of Ad.CBlacZ-infected animals was quantitated by 5-bromo-4-chloro-3-indolyl-β-d-galactosidase (X-Gal) histochemistry. The left liver lobe and one-half of one kidney were embedded in OCT (optimum cryosectioning temperature) compound and frozen on dry ice. Fresh frozen tissue sections (6 μm) were prepared and fixed in 0.5% glutaraldehyde for 5 min, rinsed in phosphate-buffered saline (PBS; pH 7.3) three times for 10 min each, and incubated overnight in 1 mg of X-Gal per ml, 5 mM K3Fe(CN)6, 5 mM K4Fe(CN)6, and 1 mM MgCl2 in PBS as described previously (57).
Biochemical and histological analysis.
The extent of hepatocellular injury during Ad.CBlacZ or MCMV infection was monitored by measuring serum alanine aminotransferase (sALT) activity at multiple time points after infection. sALT activity was measured in a Paramax chemical analyzer (Baxter Diagnostics Inc., McGaw Park, Ill.) exactly as previously described (19). For histological analysis, liver and kidney tissue samples were fixed in 10% zinc-buffered formalin (Anatech, Battle Creek, Mich.), embedded in paraffin, sectioned (3 μm), and stained with hematoxylin and eosin. The intracellular distribution of hepatitis B core antigen (HBcAg) was assessed by the labeled avidin-biotin detection procedure exactly as described previously (20).
Anticytokine antibodies.
Hamster monoclonal antibody (MAb) TN3 19.12 specific for murine TNF-α (44) was a generous gift from Robert Schreiber (Washington University, St. Louis, Mo.). Polyclonal sheep immunoglobulin (Ig) to murine IFN-α/β (13) was kindly provided by Ion Gresser (Institut de Recherches Scientifiques sur le Cancer, Villejuif, France). These antibodies were administered to animals intraperitoneally 6 h before Ad.CBlacZ or MCMV infection. Purified hamster IgG (Jackson ImmunoResearch, West Grove, Pa.) and normal sheep Ig were used as control antibodies. The hamster MAb was diluted to 250 μg/200 μl/mouse with nonpyrogenic PBS (GIBCO BRL, Gaithersburg, Md.) immediately prior to administration, and the sheep antiserum was used undiluted (300 μl/mouse) as previously described (16).
RESULTS
Adenovirus infection inhibits HBV replication and cytoplasmic nucleocapsid content in the livers of HBV transgenic mice.
The replication-defective recombinant adenovirus (Ad.CBlacZ) used in this study is capable of infecting murine hepatocytes and expressing the introduced transgene (in this case the E. coli lacZ gene), but it does not replicate in these cells (28). Expression of β-galactosidase and adenoviral proteins in the infected hepatocytes triggers a specific cellular immune response which causes a prolonged necroinflammatory liver disease and eventually clears the infection (54–56, 58–60).
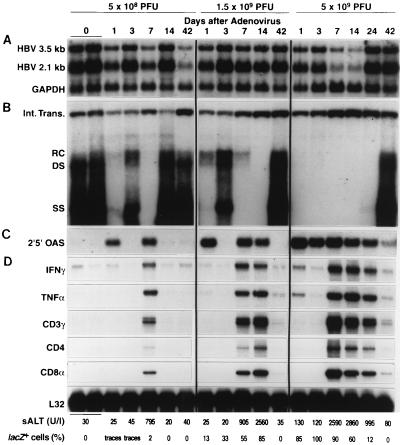
Eighteen age-, sex-, and serum HBsAg-matched transgenic mice were infected intravenously with 5.0 × 108, 1.5 × 109, or 5.0 × 109 PFU of Ad.CBlacZ, and groups of three mice were sacrificed on days 1, 3, 7, 14, 24, and 42 after infection. The number of lacZ-positive hepatocytes detectable corresponded to the infectious inoculum and was time dependent (shown at the bottom of Fig. 1), indicating that all animals were successfully infected. These animals developed a dose-dependent necroinflammatory liver disease that was detectable histologically (Fig. 2C) and biochemically as elevated sALT activity (Fig. 1, bottom) starting 3 to 7 days after inoculation and lasting 1 to 6 weeks, until lacZ-positive hepatocytes were no longer detectable. The severity and duration of the liver disease was dependent on the dose of infecting virus, as illustrated at the bottom of Fig. 1 (the upper limit of normal is approximately 50 U/liter).
FIG. 1.
Adenovirus infection inhibits hepatic HBV replication and induces the expression of cytokine genes and T-cell markers in the liver. Age-, sex-, and serum HBsAg-matched HBV transgenic mice were injected intravenously (via the lateral tail vein) with 5 × 108, 1.5 × 109, or 5.0 × 109 PFU of Ad.CBlacZ, and livers were harvested from each of three mice sacrificed on days 1, 3, 7, 14, 24, and 42 after infection, as indicated. Northern blot analysis (A) was performed with 20 μg of total liver RNA from one representative mouse per group. The membrane was cohybridized with 32P-labeled HBV- and GAPDH-specific DNA probes. The steady-state HBV and GAPDH mRNA content was compared with total hepatic RNA from two saline-injected control mice (time zero). Bands corresponding to the 3.5- and 2.1-kb HBV mRNAs are indicated. Southern blot analysis (B) was performed with 30 μg of total liver DNA isolated from the same mice. All DNA samples were treated with RNase A before their concentrations were determined. Bands corresponding to the integrated transgene (Int. Trans.) and the relaxed circular (RC), double-stranded (DS) linear, and single-stranded (SS) linear HBV DNA replicative forms are indicated. The integrated transgene can be used to normalize the amount of DNA bound to the membrane. The membrane was hybridized with a 32P-labeled HBV-specific DNA probe. Total liver RNA (20 μg) from the same mice was analyzed by Northern blot analysis for the expression of 2′5′-OAS (C), a marker of IFN-α/β induction. The housekeeping enzyme GAPDH was used to normalize the amount of RNA loaded in each lane, and its expression was uniform in all mice (not shown). Total hepatic RNA (10 μg) from the same Ad.CBlacZ-infected transgenic mice was also analyzed by RNase protection (D) for the expression of IFN-γ and TNF-α cytokine transcripts and for the expression of CD3γ, CD4, and CD8α, as indicated. The mRNA encoding the ribosomal protein L32 was used to normalize the amount of RNA loaded in each lane. The sALT activity, measured at the time of autopsy, is indicated for each mouse and is expressed in units/liter. Fresh frozen liver sections from each animal were stained for β-galactosidase activity, and the percentage of lacZ-positive hepatocytes in each section is indicated.
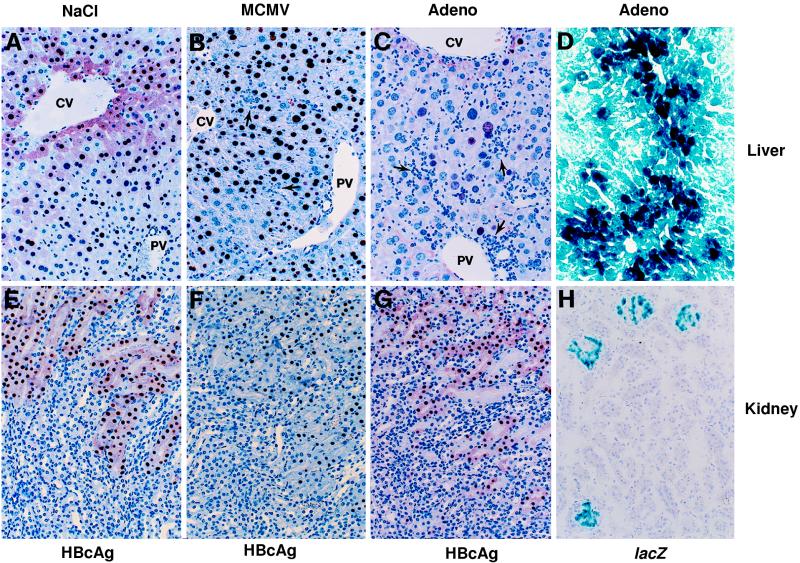
FIG. 2.
Expression of HBcAg in the livers and kidneys of HBV transgenic mice after adenovirus and MCMV infections, and lacZ expression in the same tissues of adenovirus-infected mice. Immunohistochemical analysis of HBcAg expression in liver (A to C) and kidney (E to G) sections from a saline-injected control animal (A and E) and from animals sacrificed 3 days after infection with 2.0 × 104 PFU of MCMV (B and F) or 7 days after infection with 1.5 × 109 PFU of Ad.CBlacZ (C and G) was performed. X-Gal histochemistry of a frozen liver section (D) from an animal sacrificed 1 day after infection with 5.0 × 109 PFU of Ad.CBlacZ and of a frozen kidney section (H) from a mouse sacrificed 7 days after infection with the same dose is also shown. CV, central vein; PV, portal vein.
Southern blot analysis was performed to compare the levels of hepatic HBV replicative DNA forms in adenovirus-infected mice and in age- and sex-matched transgenic mice that had been injected with saline. At all three doses of Ad.CBlacZ, HBV DNA disappeared from the liver within 1 day of infection (Fig. 1B) and before the onset of liver disease, indicated by normal sALT values shown at the bottom of Fig. 1 and normal liver histology (not shown). These results indicate that HBV replication was not inhibited as a consequence of hepatocellular regeneration because there was no evidence of liver cell injury or turnover in the liver at this time point. Importantly, the antiviral effect coincided with the induction of 2′5′-OAS mRNA (Fig. 1C), an IFN-α/β-inducible gene mRNA, and it preceded the induction of mRNAs encoding CD3, CD4, and CD8 T-cell markers and IFNγ (Fig. 1D), although traces of TNF-α mRNA were induced by the highest dose of adenovirus at the 1-day time point.
At the two lower doses of Ad.CBlacZ, HBV replication reappeared on day 3 postinfection, coinciding with the disappearance of 2′5′-OAS mRNA in the liver and in the absence of the T-cell, IFN-γ, and TNF-α mRNAs (Fig. 1). At these doses, HBV replication disappeared a second time by day 7 postinfection, coinciding with the reinduction of 2′5′-OAS mRNA and the appearance of CD3, CD4, CD8, IFN-γ, and TNF-α mRNAs (Fig. 1). Corresponding with the influx of T cells, sALT activity was clearly elevated on day 7 (Fig. 1), reflecting the onset of an inflammatory liver disease which was also detectable histologically (Fig. 2C), as previously described (50, 54–56, 58–60). The low-dose Ad.CBlacZ infection was cleared by day 14, reflected by the disappearance of lacZ-positive hepatocytes, normalization of sALT activity, and the disappearance of T-cell and cytokine markers (Fig. 1). Corresponding with these events, HBV replication resumed at this time (Fig. 1). The liver disease was more prolonged and severe in mice infected with intermediate and high doses of adenovirus, and it was associated with more sustained induction of 2′5′-OAS, T-cell, and cytokine mRNAs in these animals (Fig. 1). Accordingly, HBV replication was inhibited for up to 6 weeks until the adenovirus infection resolved, and the T-cell and cytokine mRNAs disappeared from the liver, whereupon HBV replication resumed (Fig. 1).
HBV DNA replication occurs inside HBcAg-positive nucleocapsid particles in the cytoplasm of centrilobular hepatocytes in these transgenic mice (Fig. 2A) (21). HBV nucleocapsid particles are also detectable in the vast majority of hepatocyte nuclei in these animals (Fig. 2A), although these intranuclear core particles do not contain replicating viral genomes (21). After adenovirus infection, HBcAg disappeared from the cytoplasm of the centrilobular hepatocytes (Fig. 2C) with the same kinetics as the disappearance of HBV replicative DNA intermediates from the liver and circulating HBV DNA from the serum of these transgenic mice (not shown). It is noteworthy that only traces of β-galactosidase were detectable in the liver 1 day after low-dose Ad.CBlacZ infection, and only 2% of hepatocytes were β-galactosidase positive at the peak of disease on day 7, yet cytoplasmic HBV capsids and replicative forms disappeared from all of the centrilobular hepatocytes. These results indicate that suppression of HBV replication is not due to direct competition between adenovirus and HBV within the hepatocyte. Rather, the local induction of antiviral cytokines by the adenovirus infection is likely to mediate the observed effect. Interestingly, the intranuclear capsid antigen content was unaffected in these animals, consistent with the stability of hepatic HBV RNA content in most of the livers, as we will now discuss.
Adenovirus infection does not inhibit HBV gene expression in the livers of HBV transgenic mice.
We have recently reported that adoptively transferred HBsAg-specific CTLs abolish HBV gene expression as well as HBV replication in the same lineage of transgenic mice (19). In addition, we have demonstrated that both HBV gene expression and replication are inhibited in the livers of transgenic mice during acute and chronic LCMV infection (16). As shown in Fig. 1A, however, the hepatic steady-state levels of the 3.5-kb pregenomic and 2.1-kb envelope HBV mRNAs were relatively unchanged in the livers of the adenovirus-infected HBV transgenic mice despite the induction of cytokines known to inhibit HBV gene expression following CTL injection or LCMV infection. These results indicate that HBV capsids and replicative intermediates are more susceptible to cytokine-induced inhibition than HBV RNA and that suppression of HBV replication is not due to a decrease in transcriptional template under these conditions.
MCMV infection inhibits HBV replication and gene expression in the livers of HBV transgenic mice.
MCMV infects most visceral organs (36) and can cause acute hepatitis (43) in susceptible strains, including BALB/c and C57BL/6 (6, 14, 42). During acute MCMV infection, visceral lesions are induced both by the virus and by immunopathological mechanisms (15). In acutely infected mice, an early NK cell response (2, 4, 46) and a later CTL response (27) ultimately control the infection. To examine the effects of MCMV infection on hepatic HBV replication and gene expression, groups of matched transgenic mice were infected intraperitoneally with 2.0 × 104 PFU of virulent salivary gland-passaged MCMV, and their livers were harvested on days 1, 3, 5, 7, and 14 after infection. By Southern blot analysis, total hepatic DNA was compared to DNA isolated from transgenic control animals receiving a salivary gland homogenate from uninfected mice.
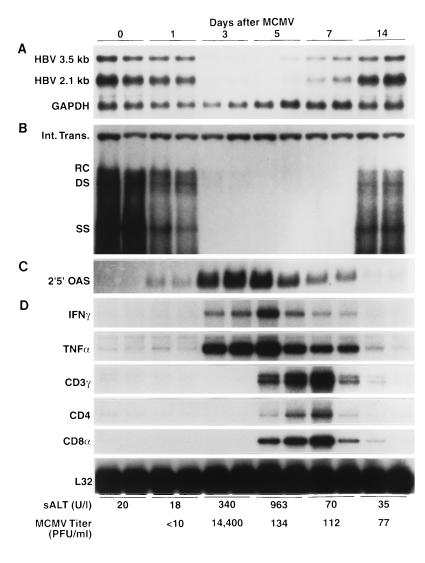
As shown in Fig. 3, hepatic HBV replicative DNA forms were significantly reduced by the first day after MCMV infection in the absence of histological (not shown) and biochemical evidence of liver disease (sALT activity 18 U/l), indicating that it was not due to the destruction or turnover of hepatocytes. At this time point, 2′5′-OAS mRNA was detectable in the liver, while IFN-γ, TNF-α, and the T-cell markers were absent (Fig. 3). HBV DNA completely disappeared from the liver by the third day after infection. This was accompanied by moderately elevated sALT activity (340 U/liter), increased expression of 2′5′-OAS mRNA, and the induction of IFN-γ and TNF-α in the liver (Fig. 3) in the absence of T-cell markers, probably reflecting the presence of NK cells (32, 38) as well as the activation and recruitment of other inflammatory cells (e.g., Kupffer cells and monocytes). In contrast to adenovirus infection, MCMV profoundly suppressed the hepatic steady-state content of the 3.5- and 2.1-kb HBV mRNAs by the third day after infection (Fig. 3A), similar to the cytokine-mediated suppression of HBV gene expression and replication following CTL injection and LCMV infection (16). The reason why the same cytokines fail to inhibit hepatic HBV gene expression during adenovirus infection is currently unclear, especially since they were induced to comparable degrees in both infections (not shown) and for a much longer period of time during adenovirus infection (Fig. 1 and 3).
FIG. 3.
MCMV infection inhibits hepatic HBV replication and gene expression and induces the expression of cytokine genes and T-cell markers in the liver. HBV transgenic mice were injected intraperitoneally with 2.0 × 104 PFU of salivary gland-passaged MCMV, and livers were harvested from each of three mice sacrificed on days 1, 3, 5, 7, and 14 after infection, as indicated. Total hepatic RNA and DNA were extracted, analyzed by Northern and Southern blotting, respectively, for the expression of HBV mRNAs, 2′5′-OAS mRNA, and HBV DNA replicative forms, and compared with total liver RNA and DNA from two mice injected with a salivary gland homogenate from uninfected BALB/c mice (time zero). Northern blot (A and C) and Southern blot (B) analyses were performed exactly as described in the legend to Fig. 1 for two representative mice per group. Total RNA (10 μg) from the same livers was analyzed by RNase protection (D) for the expression of IFN-γ and TNF-α cytokine transcripts and for the expression of CD3γ, CD4, and CD8α, as indicated. The mRNA encoding the ribosomal protein L32 was used to normalize the amount of RNA loaded in each lane. The mean sALT activity, measured at the time of autopsy, is indicated for each group and is expressed in units/liter. A 10% (wt/vol) liver homogenate was prepared, and MCMV titers were quantitated by plaque assay on NIH 3T3 cell monolayers. The mean MCMV titers in the liver are shown for each group and are expressed in PFU/milliliter of tissue homogenate. Abbreviations are as defined in the legend to Fig. 1.
By day 5 after MCMV infection, T-cell markers (especially CD8) were strongly induced in the liver (Fig. 3), corresponding with further elevation of sALT activity (963 U/liter) and marking the beginning of an MCMV-specific T-cell response. Both HBV replication and HBV gene expression remained suppressed at this time, corresponding with the continued expression of all three cytokines (Fig. 3). The T-cell response continued at a reduced level on day 7, and the level of cytokine gene expression and the suppression of HBV gene expression were reduced to a commensurate degree. By day 14, MCMV was almost completely cleared from the liver and sALT activity, cytokines, and T-cell markers returned to baseline levels, as did the hepatic content of HBV DNA and HBV RNA. As in the adenovirus-infected animals, MCMV induced the disappearance of HBV nucleocapsids in the cytoplasm of the centrilobular hepatocytes (Fig. 2B) and virions from the serum (data not shown), with the same kinetics as that observed for the disappearance of HBV replicative DNA forms from the liver.
MCMV titers and liver pathology in MCMV-infected HBV transgenic mice.
MCMV productively infects hepatocytes and replicates to high levels in the liver, reaching peak titers on day 3 after intraperitoneal infection (43). As expected, MCMV titers in the liver (shown at the bottom of Fig. 3) peaked at 1.44 × 104 PFU/ml of tissue homogenate on the third day after infection. Surprisingly, this high level of hepatic MCMV replication was associated with only a slight elevation in sALT activity (340 U/liter). This finding indicates that direct viral damage to the liver was minimal at this time, suggesting that the profound reduction in HBV replication and gene expression observed in these livers was not the result of extensive hepatocyte destruction. This inference was confirmed by histological examination of the same livers on day 3 after MCMV infection, which showed small, scattered necroinflammatory foci containing infiltrating mononuclear cells and apoptotic hepatocytes (Fig. 2B, arrows) together with enlarged hepatocytes whose nuclei contained distinct intranuclear inclusions, indicative of MCMV replication. By day 5 postinfection, sALT activity rose to 963 U/liter, and widespread areas of inflammation containing mononuclear cells and necrotic and apoptotic hepatocytes were visible in the liver. At this time, MCMV titers in the liver fell more than 100-fold (134 PFU/ml of tissue homogenate), and T-cell markers and cytokine genes were strongly induced in the liver (Fig. 3), presumably reflecting the MCMV-specific cell-mediated immune response. By day 7, sALT activity dropped to 70 U/liter, the inflammatory foci were greatly reduced, and the MCMV titer remained low (112 PFU/ml of liver homogenate), suggesting that the MCMV infection was resolving. As expected, consistent with the diminished inflammatory response and reduced expression of antiviral cytokines, the level of HBV gene expression began to increase in the liver. By day 14, sALT activity was normal, only small and isolated inflammatory foci were visible in the liver, and cytokine and T-cell markers were barely detectable, at which point HBV DNA and HBV RNA returned to preinfection levels.
MCMV infects the kidney and inhibits renal HBV replication and gene expression, but adenovirus does not.
HBV replicates to high levels in the proximal convoluted tubules of the kidney in these transgenic mice as well as in the liver (21). In a recent study, we showed that interleukin-12 can abolish HBV replication in the kidney as well as in the liver in similar transgenic mice (5). To examine the effects of adenovirus and MCMV infections on renal HBV replication and gene expression, the kidneys of transgenic mice infected with Ad.CBlacZ or MCMV were analyzed as described above.
In this analysis, we found that MCMV productively infects the kidney, where, by day 5, it induces a mild interstitial inflammatory infiltrate composed of IFN-γ- and TNF-α-producing T cells (not shown). This inflammatory infiltrate became more prominent by day 7 after infection, and renal inflammation at this time consisted of a glomerular as well as an interstitial nephritis. As early as day 3, however, HBV DNA replicative forms and cytoplasmic HBcAg were eliminated from the kidney (Fig. 2F and 4) in the absence of histological evidence of kidney disease. Also on day 3, HBV DNA and cytoplasmic HBcAg disappeared from the livers of the same animals; however, this suppression occurred in the presence of liver disease (described above). MCMV titers peaked in both tissues by day 3 after infection (1.44 × 104 PFU/ml of liver homogenate; 3.75 × 103 PFU/ml of kidney homogenate), and mRNAs encoding 2′5′-OAS (Fig. 3 and 4), TNF-α, and IFN-γ (Fig. 3 and data not shown for kidney) were detected in both tissues. In addition, the steady-state content of HBV RNA was dramatically reduced in the liver (Fig. 3) at this time point but was only slightly reduced in the kidney (data not shown). Histologically, there was no evidence of inflammation in the kidneys until the fifth day after infection, when T-cell markers were first detectable and interstitial nephritis was evident. At this time, renal HBV RNA content was further reduced (not shown), and renal MCMV titers dropped to 1.73 × 103 PFU/ml, marking the onset of the T-cell-mediated immune response in the kidney. Also as in the liver, HBV replication and gene expression were suppressed in the kidney from days 3 to 7 postinfection, returning to baseline levels by day 14, when the cytokine genes and T-cell markers were no longer detectable in the kidney and the MCMV infection was cleared from this tissue (13 PFU/ml).
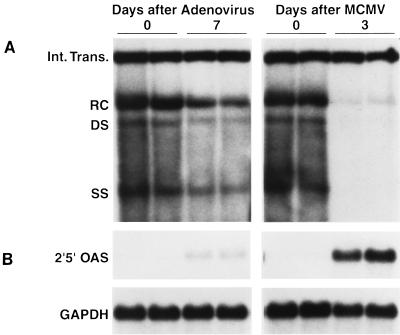
FIG. 4.
Infection with MCMV, but not adenovirus, inhibits renal HBV replication. Kidneys were harvested from each of three to four HBV transgenic mice sacrificed 7 days after infection with 5.0 × 109 PFU of Ad.CBlacZ or 3 days after infection with 2.0 × 104 PFU of MCMV. Total renal DNA and RNA were extracted from one kidney of each animal, analyzed by Southern and Northern blotting, respectively, for the expression of HBV DNA replicative forms and 2′5′-OAS mRNA, and compared with total renal DNA and RNA from mock-infected control mice (time zero). Southern (A) and Northern (B) blot analyses were performed exactly as described in the legend to Fig. 1 for two representative mice per group. Abbreviations are as defined in the legend to Fig. 1.
In contrast to MCMV, the kidney is not readily infected by Ad.CBlacZ since only the glomeruli were β-galactosidase positive (Fig. 2H) whereas nearly all of the hepatocytes were positive (Fig. 2D). To determine if glomerular infection is sufficient to inhibit HBV replication in the tubular epithelial cells, total cellular DNA was extracted from the kidney and Southern blot analysis was performed (Fig. 4). Although HBV replication was profoundly suppressed in the livers of these mice (Fig. 1), there was little change in HBV replication in the kidney (Fig. 4). Consistent with these results, there were no changes in cytoplasmic HBcAg in the proximal convoluted tubules of the kidney, no histological evidence of inflammation in the kidneys of the adenovirus-infected mice (Fig. 2G), and only minimal expression of 2′5′-OAS (Fig. 4), TNF-α, IFN-γ, or the T-cell marker mRNAs (data not shown). These results reflect the fact that the kidney is not readily infected by this recombinant adenovirus.
Early suppression of HBV replication by adenovirus and MCMV is mediated by IFN-α/β and TNF-α.
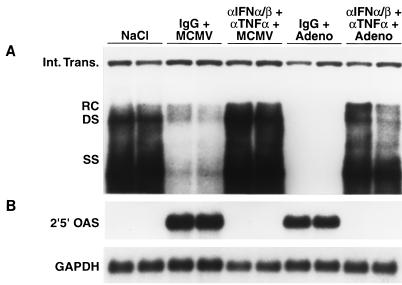
Since IFN-α/β and TNF-α mRNA were detectable in the liver within 1 day after adenovirus (Fig. 1) and MCMV (Fig. 3) infections, we monitored the ability of neutralizing antibodies to these cytokines to modulate HBV replication and the expression of 2′5′-OAS mRNA 24 h after infection. Groups of age- and sex-matched transgenic mice were injected intraperitoneally with a mixture of sheep antiserum against murine IFN-α/β (13) and MAb against murine TNF-α (44) 6 h before infection with either 1.5 × 109 PFU of Ad.CBlacZ or 5.0 × 104 PFU of MCMV. Control mice were injected with a mixture of irrelevant hamster IgG and normal sheep serum. Twenty-four hours after infection, their livers were harvested, total hepatic DNA was extracted, and a Southern blot analysis was performed (Fig. 5A). As expected, HBV replication was abolished in the adenovirus-infected control mice and was greatly reduced in the MCMV-infected control mice, coinciding with the strong expression of 2′5′-OAS mRNA (Fig. 5B). Both of these events were blocked by the cocktail of anticytokine antibodies, indicating that IFN-α/β and/or TNF-α are primarily responsible for the early, noncytolytic suppression of HBV replication following adenovirus and MCMV infections.
FIG. 5.
Early suppression of HBV replication by adenovirus and MCMV infections is mediated by IFN-α/β and TNF-α. Groups of three HBV transgenic mice were intraperitoneally injected with a combination of antibodies to IFN-α/β and TNF-α or with control antibodies 6 h before infection with 5.0 × 104 PFU of MCMV or 1.5 × 109 PFU of Ad.CBlacZ and were sacrificed 24 h after infection. Total hepatic DNA and RNA were extracted, analyzed by Southern and Northern blotting, respectively, for the expression of HBV DNA replicative forms and 2′5′-OAS mRNA, and compared with total hepatic DNA and RNA from two saline-injected control mice (lanes NaCl). Southern (A) and Northern (B) blot analyses were performed exactly as described in the legend to Fig. 1 for two representative mice per group. Abbreviations are as defined in the legend to Fig. 1.
DISCUSSION
In this study, we demonstrate that inflammatory cytokines induced during adenovirus and cytomegalovirus infection suppress HBV replication in two distinct waves in infected tissues. The first wave occurs during the first 24 h of infection and is characterized by the disappearance of cytoplasmic HBV nucleocapsids and replicative DNA intermediates in response to the antiviral effects of IFN-α/β induced by the infecting virus. Since there is no liver disease or hepatocellular regeneration at this time point, the antiviral effect cannot be ascribed to the lysis or turnover of infected cells. The second wave is mediated by IFN-α/β, IFN-γ, and TNF-α produced during the cellular immune response to each virus, beginning between 3 to 5 days after infection and continuing until the incoming virus is cleared. Since tissue injury is evident during this period, theoretically the inhibition of HBV gene expression and replication observed at these time points could be due, at least in part, to the destruction and regeneration of HBV-positive hepatocytes. This is unlikely, however, because we have previously reported that HBV gene expression and replication are not suppressed during hepatocyte turnover, provided that certain cytokines are absent (18).
These observations extend our understanding of the host-virus relationship during HBV infection at several important levels. For example, this is the first report that HBV gene expression and replication can be inhibited by hepatocytotropic DNA viruses, like HBV, providing additional support to the notion that similar antiviral events can limit HBV infection during acute viral hepatitis in humans. Next, this is the first demonstration of the biphasic nature of the antiviral effect of a hepatotropic viral infection, emphasizing the importance of the initial, nonspecific, innate response to infection. Furthermore, this report provides the first evidence that certain viruses can inhibit HBV replication in tissues other than the liver. Specifically, we showed that HBV replication is suppressed in the kidney during MCMV infection but not during adenovirus infection, apparently because MCMV infects the kidney much more efficiently than adenovirus. Finally, this report illustrates that HBV replication is more sensitive to cytokine-mediated control than HBV gene expression. Furthermore, since HBV gene expression is inhibited during MCMV infection but not during adenovirus infection, even though IFN-α/β, TNF-α, and IFN-γ are induced to comparable degrees for similar periods of time in both infections, the present data suggest that these cytokines may be necessary but not sufficient to eliminate HBV RNA from the liver. Rather, the data are consistent with a model in which the inhibition of HBV RNA expression is mediated by two independent but parallel mechanisms: one induced by IFN-α/β, TNF-α, and IFN-γ, and the other induced by an unknown factor or factors. According to this model, both pathways must be induced to inhibit HBV gene expression. If this is correct, it is possible that MCMV infection induces both pathways whereas adenovirus infection induces only the first. Alternatively, it is theoretically possible that the two viruses can trigger both pathways, with adenovirus infection also inducing a third pathway that inhibits one of the others.
Since the inflammatory disease induced by both of these viruses is histologically similar to viral hepatitis in humans, and because of the extraordinary efficiency of these antiviral processes, the present data suggest that inflammatory cytokines can contribute to viral clearance during acute HBV infection. In view of these results, it is interesting that HBV infection has been shown to resolve during hepatitis A virus (8) and hepatitis C virus (45) superinfection of chronic HBV carriers, perhaps by a process similar to that described herein. The failure of these mechanisms to clear HBV in chronically infected patients may be due to the relative mildness of the inflammatory response in those individuals (7). Alternatively, the quality of the intrahepatic cytokine profile may differ during acute and chronic hepatitis. This curative intracellular viral inactivation process may greatly amplify the protective effects of the immune response and may be particularly important in massive infections of vital organs where the host must eliminate the infecting virus while preserving the tissue. The intracellular viral inactivation pathways that are responsible for this remarkable effect are undefined.
These observations raise the possibility that inflammatory cytokines contribute to the control of other viral infections. For example, during MCMV infection, IFN-γ and TNF-α are thought to be required to clear virus from persistently infected salivary glands (30, 33) and dramatically alter the morphogenesis of MCMV nucleocapsids, profoundly reducing virus replication (29). Indeed, neutralization of IFN-α/β, IFN-γ, or TNF-α during MCMV infection results in a significant increase in virus burden in several organs, including the liver (23, 31). TNF-α has also been reported to inhibit the replication of adenovirus, and IFN-γ synergizes with TNF-α to further reduce viral replication (53). In addition, IFN-α/β, IFN-γ, and TNF-α have also been shown to control other viral infections in vivo, including lymphocytic choriomeningitis virus (34), vaccinia virus (26, 39, 51), measles virus (10), herpes simplex virus (3, 37), influenza virus (49), and mouse hepatitis virus (61). The mechanisms responsible for the antiviral effects of these cytokines are not well defined, however, and they may do so primarily by facilitating development of the immune response against these infections rather than by exerting a direct antiviral effect on virus replication in these systems. Further studies are needed to clarify this important issue.
In keeping with the concept of intracellular viral inactivation, a number of viruses encode proteins that have the potential to inhibit the antiviral actions of interferons (11), including adenovirus, where the E1A proteins block the activation of interferon response genes by inhibiting the function of a cellular transcription factor (ISGF3) that is activated when IFN-α/β binds to its receptor (22, 25, 35). Viruses may also have evolved additional strategies for circumventing host antiviral defenses by encoding receptor analogues for IFN-α/β, IFN-γ, and TNF-α (1, 40, 48), thereby neutralizing the antiviral activity of these cytokines. In fact, adenoviruses encode three proteins (E3-14.7K, E3-10.4K/14.5K, and E1B-19K) that protect the infected cell against the antiviral effects of TNF-α (52). At present, none of the seven proteins encoded by the HBV genome (precore, core, polymerase, large envelope, middle envelope, major envelope, and X proteins) have been shown to block the antiviral actions of host cytokines.
It is possible, therefore, that the curative properties of inflammatory cytokines produced by virus-specific T cells, NK cells, and macrophages play a pivotal role in the outcome of many viral infections in addition to HBV. If this concept is correct, it is likely that each virus will exhibit its own individual cytokine sensitivity profile, and some viruses may resist cytokine-mediated control. Future analysis of the molecular basis for viral sensitivity and resistance to cytokine-mediated control are clearly warranted, not only to provide better insight into the immunopathogenesis of these viral infections but also to facilitate the development of therapeutic strategies to deliver or induce the appropriate antiviral cytokines in infected tissue.
ACKNOWLEDGMENTS
We thank James Wilson for providing the recombinant adenovirus Ad.CBlacZ; Ann Campbell for providing the tissue culture-passaged MCMV; Ion Gresser for providing the sheep antiserum to murine IFN-α/β; Robert Schreiber for providing the hamster MAb to murine TNF-α; Monte Hobbs for providing the cytokine gene and T-cell marker probe sets used in the RNase protection assays; Margie Chadwell for preparation and staining of tissue sections for HBcAg expression; Rick Koch, Brent Matzke, and Josan Chung for excellent technical assistance; and Jennifer Newmann and Pamela Fagan for help with manuscript preparation.
This work was supported by grants R37 CA40489 and AI40696 from the National Institutes of Health.
Footnotes
Manuscript no. 10943-MEM from the Scripps Research Institute.
REFERENCES
- 1.Alcami A, Smith G L. Vaccinia, cowpox, and camelpox viruses encode soluble gamma interferon receptors with novel broad species specificity. J Virol. 1995;69:4633–4639. doi: 10.1128/jvi.69.8.4633-4639.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bancroft G J, Shellam G R, Chalmer J E. Genetic influences on the augmentation of natural killer (NK) cells during murine cytomegalovirus infection: correlation with patterns of resistance. J Immunol. 1981;126:988–994. [PubMed] [Google Scholar]
- 3.Bouley D M, Kanangat S, Wire W, Rouse B T. Characterization of herpes simplex virus type-1 infection and herpetic stromal keratitis development in IFN-γ knockout mice. J Immunol. 1995;155:3964–3971. [PubMed] [Google Scholar]
- 4.Bukowski J F, Woda B A, Welsh R M. Pathogenesis of murine cytomegalovirus infection in natural killer cell-depleted mice. J Virol. 1984;52:119–128. doi: 10.1128/jvi.52.1.119-128.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cavanaugh V J, Guidotti L G, Chisari F V. Interleukin-12 inhibits hepatitis B virus replication in HBV transgenic mice. J Virol. 1997;71:3236–3243. doi: 10.1128/jvi.71.4.3236-3243.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chalmer J E, Mackenzie J S, Stanley N F. Resistance to murine cytomegalovirus linked to the major histocompatibility complex of the mouse. J Gen Virol. 1977;37:107–114. doi: 10.1099/0022-1317-37-1-107. [DOI] [PubMed] [Google Scholar]
- 7.Chisari F V, Ferrari C. Hepatitis B virus immunopathogenesis. Annu Rev Immunol. 1995;13:29–60. doi: 10.1146/annurev.iy.13.040195.000333. [DOI] [PubMed] [Google Scholar]
- 8.Davis G L, Hoofnagle J H, Waggoner J G. Acute type A hepatitis during chronic hepatitis B virus infection: association of depressed hepatitis B virus replication with appearance of endogenous alpha interferon. J Med Virol. 1984;14:141–147. doi: 10.1002/jmv.1890140208. [DOI] [PubMed] [Google Scholar]
- 9.Engelhardt J F, Yang Y, Stratford-Perricaudet L D, Allen E D, Kozarsky K, Perricaudet M, Yankaskas J R, Wilson J M. Direct gene transfer of human CFTR into human bronchial epithelia of xenografts with E1-deleted adenoviruses. Nat Genet. 1993;4:27–34. doi: 10.1038/ng0593-27. [DOI] [PubMed] [Google Scholar]
- 10.Finke D, Brinckmann U G, ter Meulen V, Liebert U G. Gamma interferon is a major mediator of antiviral defense in experimental measles virus-induced encephalitis. J Virol. 1995;69:5469–5474. doi: 10.1128/jvi.69.9.5469-5474.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gooding L R. Virus proteins that counteract host immune defenses. Cell. 1992;71:5–7. doi: 10.1016/0092-8674(92)90259-f. [DOI] [PubMed] [Google Scholar]
- 12.Graham F L, Smiley J, Russell W C, Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977;36:59–72. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- 13.Gresser I, Tovey M G, Bandu M T, Maury C, Brouty-Boye D. Role of interferon in the pathogenesis of virus diseases in mice as demonstrated by the use of anti-interferon serum. I. Rapid evolution of encephalomyocarditis virus infection. J Exp Med. 1976;144:1305–1315. doi: 10.1084/jem.144.5.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grundy (Chalmer) J E, Mackenzie J S, Stanley N F. Influence of H-2 and non-H-2 genes on resistance to murine cytomegalovirus infection. Infect Immun. 1981;32:277–286. doi: 10.1128/iai.32.1.277-286.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grundy J E, Shanley J D, Griffiths P D. Is cytomegalovirus interstitial pneumonitis in transplant recipients an immunopathological condition? Lancet. 1987;ii:996–999. doi: 10.1016/s0140-6736(87)92560-8. [DOI] [PubMed] [Google Scholar]
- 16.Guidotti L G, Borrow P, Hobbs M V, Matzke B, Gresser I, Oldstone M B A, Chisari F V. Viral cross talk: intracellular inactivation of the hepatitis B virus during an unrelated viral infection of the liver. Proc Natl Acad Sci USA. 1996;93:4589–4594. doi: 10.1073/pnas.93.10.4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guidotti L G, Chisari F V. To kill or to cure: options in host defense against viral infection. In: Zinkernagel R, Bloom B, editors. Current opinion in immunology. London, England: Current Biology, Ltd.; 1996. pp. 478–483. [DOI] [PubMed] [Google Scholar]
- 18.Guidotti L G, Matzke B, Chisari F V. Hepatitis B virus is cell cycle independent during liver regeneration in transgenic mice. J Virol. 1997;71:4804–4808. doi: 10.1128/jvi.71.6.4804-4808.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guidotti L G, Ishikawa T, Hobbs M V, Matzke B, Schreiber R, Chisari F V. Intracellular inactivation of the hepatitis B virus by cytotoxic T lymphocytes. Immunity. 1996;4:25–36. doi: 10.1016/s1074-7613(00)80295-2. [DOI] [PubMed] [Google Scholar]
- 20.Guidotti L G, Martinez V, Loh Y T, Rogler C E, Chisari F V. Hepatitis B virus nucleocapsid particles do not cross the hepatocyte nuclear membrane in transgenic mice. J Virol. 1994;68:5469–5475. doi: 10.1128/jvi.68.9.5469-5475.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guidotti L G, Matzke B, Schaller H, Chisari F V. High-level hepatitis B virus replication in transgenic mice. J Virol. 1995;69:6158–6169. doi: 10.1128/jvi.69.10.6158-6169.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gutch M J, Reich N C. Repression of the interferon signal transduction pathway by the adenovirus E1A oncogene. Proc Natl Acad Sci USA. 1991;88:7913–7917. doi: 10.1073/pnas.88.18.7913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heise M T, Virgin H W., IV The T-cell-independent role of gamma interferon and tumor necrosis factor alpha in macrophage activation during murine cytomegalovirus and herpes simplex virus infections. J Virol. 1995;69:904–909. doi: 10.1128/jvi.69.2.904-909.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hobbs M V, Weigle W O, Noonan D J, Torbett B E, McEvilly R J, Koch R J, Cardenas G J, Ernst D N. Patterns of cytokine gene expression by CD4+ T cells from young and old mice. J Immunol. 1993;150:3602–3614. [PubMed] [Google Scholar]
- 25.Kalvakolanu D V R, Bandyopadhyay S K, Harter M L, Sen G C. Inhibition of interferon-inducible gene expression by adenovirus E1A proteins; block in transcriptional complex formation. Proc Natl Acad Sci USA. 1991;88:7459–7463. doi: 10.1073/pnas.88.17.7459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kohonen-Corish M R J, King N J C, Woodhams C E, Ramshaw I A. Immunodeficient mice recover from infection with vaccinia virus expressing interferon-γ. Eur J Immunol. 1990;20:157–161. doi: 10.1002/eji.1830200123. [DOI] [PubMed] [Google Scholar]
- 27.Koszinowski U H, Del Val M, Reddehase M J. Cellular and molecular basis of the protective immune response to cytomegalovirus infection. Curr Top Microbiol Immunol. 1990;154:189–220. doi: 10.1007/978-3-642-74980-3_8. [DOI] [PubMed] [Google Scholar]
- 28.Kozarsky K, Wilson J M. Gene therapy: adenovirus vectors. Curr Opin Genet Dev. 1993;3:499–503. doi: 10.1016/0959-437x(93)90126-a. [DOI] [PubMed] [Google Scholar]
- 29.Lucin P, Jongic S, Messerle M, Polic B, Hengel H, Koszinowski U H. Late phase inhibition of murine cytomegalovirus replication by synergistic action of interferon-gamma and tumour necrosis factor. J Gen Virol. 1994;75:101–110. doi: 10.1099/0022-1317-75-1-101. [DOI] [PubMed] [Google Scholar]
- 30.Lucin P, Pavic I, Polic B, Jonjic S, Koszinowski U H. Gamma interferon-dependent clearance of cytomegalovirus infection in salivary glands. J Virol. 1992;66:1977–1984. doi: 10.1128/jvi.66.4.1977-1984.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Orange J S, Biron C A. Characterization of early IL-12, IFN-αβ, and TNF effects on antiviral state and NK cell responses during murine cytomegalovirus infection. J Immunol. 1996;156:4746–4756. [PubMed] [Google Scholar]
- 32.Orange J S, Wang B, Terhorst C, Biron C A. Requirement for natural killer cell-produced interferon γ in defense against murine cytomegalovirus infection and enhancement of this defense pathway by interleukin 12 administration. J Exp Med. 1995;182:1045–1056. doi: 10.1084/jem.182.4.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pavic I, Polic B, Crnkovic I, Lucin P, Jonjic S, Koszinowski U H. Participation of endogenous tumour necrosis factor α in host resistance to cytomegalovirus infection. J Gen Virol. 1993;74:2215–2223. doi: 10.1099/0022-1317-74-10-2215. [DOI] [PubMed] [Google Scholar]
- 34.Planz O, Ehl S, Furrer E, Horvath E, Bründler M-A, Hengartner H, Zinkernagel R M. A critical role for neutralizing-antibody-producing B cells, CD4+ T cells, and interferons in persistent and acute infections of mice with lymphocytic choriomeningitis virus: implications for adoptive immunotherapy of virus carriers. Proc Natl Acad Sci USA. 1997;94:6874–6879. doi: 10.1073/pnas.94.13.6874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reich N, Pine R, Levy D, Darnell J E., Jr Transcription of interferon-stimulated genes is induced by adenovirus particles but is suppressed by E1A products. J Virol. 1988;62:114–119. doi: 10.1128/jvi.62.1.114-119.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reynolds R P, Rahija R J, Schenkman D I, Richter C B. Experimental murine cytomegalovirus infection in severe combined immunodeficient mice. Lab Anim Sci. 1993;43:291–295. [PubMed] [Google Scholar]
- 37.Rossol-Voth R, Rossol S, Schütt K H, Corridori S, de Cian W, Falke D. In vivo protective effect of tumor necrosis factor α against experimental infection with herpes simplex virus type I. J Gen Virol. 1991;72:143–147. doi: 10.1099/0022-1317-72-1-143. [DOI] [PubMed] [Google Scholar]
- 38.Salazar-Mather T P, Ishikawa R, Biron C A. NK cell trafficking and cytokine expression in splenic compartments after IFN induction and viral infection. J Immunol. 1996;157:3054–3064. [PubMed] [Google Scholar]
- 39.Sambhi S K, Kohonen-Corish M R J, Ramshaw I A. Local production of tumor necrosis factor encoded by recombinant vaccinia virus is effective in controlling viral replication in vivo. Proc Natl Acad Sci USA. 1991;88:4025–4029. doi: 10.1073/pnas.88.9.4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schreiber M, McFadden G. The myxoma virus TNF-receptor homologue (T2) inhibits tumor necrosis factor-α in a species-specific fashion. Virology. 1994;204:692–705. doi: 10.1006/viro.1994.1585. [DOI] [PubMed] [Google Scholar]
- 41.Selgrade M K, Nedrud J G, Collier A M, Gardner D E. Effects of cell source, mouse strain, and immunosuppressive treatment on production of virulent and attenuated murine cytomegalovirus. Infect Immun. 1981;33:840–847. doi: 10.1128/iai.33.3.840-847.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shanley J D. Host genetic factors influence murine cytomegalovirus lung infection and interstitial pneumonitis. J Gen Virol. 1984;65:2121–2128. doi: 10.1099/0022-1317-65-12-2121. [DOI] [PubMed] [Google Scholar]
- 43.Shanley J D, Biczak L, Forman S J. Acute murine cytomegalovirus infection induces lethal hepatitis. J Infect Dis. 1993;164:264–269. doi: 10.1093/infdis/167.2.264. [DOI] [PubMed] [Google Scholar]
- 44.Sheehan K C F, Ruddle N H, Schreiber R D. Generation and characterization of hamster monoclonal antibodies that neutralize murine tumor necrosis factor. J Immunol. 1989;142:3884–3893. [PubMed] [Google Scholar]
- 45.Sheen I-S, Liaw Y-F, Lin D-Y, Chu C-M. Role of hepatitis C and delta viruses in the termination of chronic hepatitis B surface antigen carrier state: a multivariate analysis in a longitudinal follow-up study. J Infect Dis. 1994;170:1358–1361. doi: 10.1093/infdis/170.2.358. [DOI] [PubMed] [Google Scholar]
- 46.Shellam G R, Allan J E, Papadimitriou J M, Bancroft G J. Increased susceptibility to cytomegalovirus infection in beige mutant mice. Proc Natl Acad Sci USA. 1981;78:5104–5108. doi: 10.1073/pnas.78.8.5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stratford-Perricaudet L D, Levrero M, Chasse J F, Perricaudet M, Briand P. Evaluation of the transfer and expression in mice of an enzyme-encoding gene using a human adenovirus vector. Hum Gene Ther. 1990;1:241–256. doi: 10.1089/hum.1990.1.3-241. [DOI] [PubMed] [Google Scholar]
- 48.Symons J A, Alcami A, Smith G L. Vaccinia virus encodes a soluble type I interferon receptor of novel structure and broad species specificity. Cell. 1995;81:551–560. doi: 10.1016/0092-8674(95)90076-4. [DOI] [PubMed] [Google Scholar]
- 49.Topham D J, Tripp R A, Sarawar S R, Sangster M Y, Doherty P C. Immune CD4+ T cells promote the clearance of influenza virus from major histocompatibility complex class II −/− respiratory epithelium. J Virol. 1996;70:1288–1291. doi: 10.1128/jvi.70.2.1288-1291.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tripathy S K, Black H B, Goldwasser E, Leiden J M. Immune responses to transgene-encoded proteins limit the stability of gene expression after injection of replicative-defective adenovirus vectors. Nat Med. 1996;2:545–550. doi: 10.1038/nm0596-545. [DOI] [PubMed] [Google Scholar]
- 51.van den Broek M F, Müller U, Huang S, Aguet M, Zinkernagel R M. Antiviral defense in mice lacking both alpha/beta and gamma interferon receptors. J Virol. 1995;69:4792–4796. doi: 10.1128/jvi.69.8.4792-4796.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wold W S M, Gooding L R. Region E3 of adenovirus: a cassette of genes involved in host immunosurveillance and virus-cell interactions. Virology. 1991;184:1–8. doi: 10.1016/0042-6822(91)90815-s. [DOI] [PubMed] [Google Scholar]
- 53.Wong G H W, Goeddel D V. Tumor necrosis factor α and β inhibit virus replication and synergize with interferons. Nature. 1986;323:819–822. doi: 10.1038/323819a0. [DOI] [PubMed] [Google Scholar]
- 54.Yang Y, Ertl H C J, Wilson J M. MHC class I-restricted cytotoxic T lymphocytes to viral antigens destroy hepatocytes in mice infected with E1-deleted recombinant adenoviruses. Immunity. 1994;1:433–442. doi: 10.1016/1074-7613(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 55.Yang Y, Li Q, Ertl H C J, Wilson J M. Cellular and humoral immune responses to viral antigens create barriers to lung-directed gene therapy with recombinant adenoviruses. J Virol. 1995;69:2004–2015. doi: 10.1128/jvi.69.4.2004-2015.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang Y, Nunes F A, Berencsi K, Furth E E, Gönczöl E, Wilson J M. Cellular immunity to viral antigens limits E1-deleted adenoviruses for gene therapy. Proc Natl Acad Sci USA. 1994;91:4407–4411. doi: 10.1073/pnas.91.10.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang Y, Raper S E, Cohn J A, Englehardt J F, Wilson J M. An approach for treating the hepatobiliary disease of cystic fibrosis by somatic gene transfer. Proc Natl Acad Sci USA. 1993;90:4601–4605. doi: 10.1073/pnas.90.10.4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang Y, Su Q, Wilson J M. Role of viral antigens in destructive cellular immune responses to adenovirus vector-transduced cells in mouse lungs. J Virol. 1996;70:7209–7212. doi: 10.1128/jvi.70.10.7209-7212.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang Y, Wilson J M. Clearance of adenovirus-infected hepatocytes by MHC class I-restricted CD4+ CTLs in vivo. J Immunol. 1995;155:2564–2570. [PubMed] [Google Scholar]
- 60.Yang Y, Ziang Z, Ertl H C J, Wilson K M. Upregulation of class I major histocompatibility complex antigens by interferon γ is necessary for T-cell-mediated elimination of recombinant adenovirus-infected hepatocytes in vivo. Proc Natl Acad Sci USA. 1995;92:7257–7261. doi: 10.1073/pnas.92.16.7257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang X, Hinton D R, Cua D J, Stohlman S A, Lai M M C. Expression of interferon-γ by a coronavirus defective-interfering RNA vector and its effect on viral replication, spread, and pathogenicity. Virology. 1997;233:327–338. doi: 10.1006/viro.1997.8598. [DOI] [PMC free article] [PubMed] [Google Scholar]