Abstract
Engineered materials are ubiquitous in biomedical applications ranging from systemic drug delivery systems to orthopedic implants, and their actions unfold across multiple time- and length-scales. The efficacy and safety of biologics, nanomaterials, and macroscopic implants are all dictated by the same general principles of pharmacology as apply to small molecule drugs, comprising how the body affects materials (pharmacokinetics, PK) and conversely how materials affect the body (pharmacodynamics, PD). Imaging technologies play an increasingly insightful role in monitoring both of these processes, often simultaneously: translational macroscopic imaging modalities such as MRI and PET/CT offer whole-body quantitation of biodistribution and structural or molecular response, while ex vivo approaches and optical imaging via in vivo (intravital) microscopy reveal behaviors at subcellular resolution. In this review, the authors survey developments in imaging the in situ behavior of systemically and locally administered materials, with a particular focus on using microscopy to understand transport, target engagement, and downstream host responses at a single-cell level. The themes of microenvironmental influence, controlled drug release, on-target molecular action, and immune response, especially as mediated by macrophages and other myeloid cells are examined. Finally, the future directions of how new imaging technologies may propel efficient clinical translation of next-generation therapeutics and medical devices are proposed.
Keywords: engineered materials, material pharmacology, pharmacokinetics, pharmacodynamics, in vivo Imaging, intravital microscopy
Graphical Abstract
The efficacy and safety of engineered materials, such as biologics, nanoparticles, and biomedical implants, are jointly dictated by their pharmacokinetic and pharmacodynamic properties in the body. In vivo imaging techniques, especially intravital microscopy, are invaluable tools to dissect the interaction of such materials with biological processes over time and in physiologically relevant contexts.
1. Introduction
Broadly defined, components of engineered healthcare materials range in size from small molecules to macroscopic orthopedic implants, they act over timescales from seconds to years, and they exhibit wide diversity in material composition and physicochemical properties. Yet despite these differences, the action and fate of materials in the body are influenced by many shared biological and physicochemical processes. All materials and drugs are governed by the dynamics of transport and degradation or transformation through the body — their pharmacokinetics[1,2]. The intended action of a material or its therapeutic payload depends on its ability to reach its intended target, to stably remain in place (especially if implanted), and/or to safely degrade at a prescribed rate. Recurring themes have also emerged in the biology of how materials impact the body — that is, their pharmacodynamics — with involvement of endothelial, fibrotic, cytostatic/cytotoxic, and immune responses, especially of macrophages and other phagocytic myeloid cells. These common host responses are critical factors in determining whether or not a material succeeds in the clinic[1–3]. For studying both pharmacokinetics and pharmacodynamics (PK/PD), imaging technologies are continually increasing in their spatial and temporal resolution, ability to simultaneously monitor multiple features (multiplexing), and penetration through tissue at anatomically relevant sites (Fig. 1).
Figure 1. Imaging the pharmacology of materials at multiple scales.
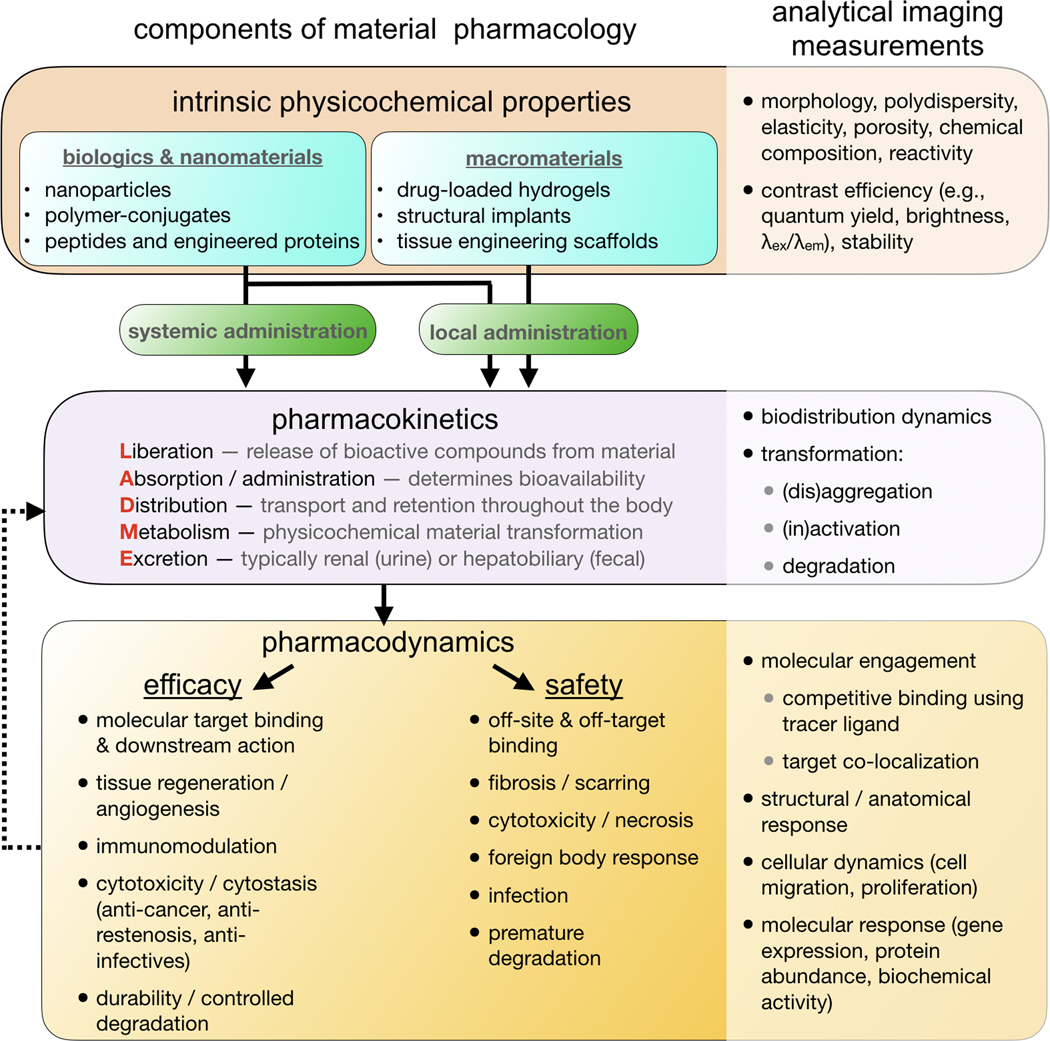
Various advanced materials, including biologics, nanomaterials, and macromaterials, have been developed for healthcare and therapeutic purposes. These materials can be systemically administered or introduced locally. The pharmacokinetics of these materials, including liberation, absorption, distribution, metabolism, and excretion processes, are dependent on both intrinsic properties of the materials and the biological processes inside the body. Pharmacokinetics ultimately impact how these materials affect the biological systems, or material pharmacodynamics, which determines the efficacy and safety of these materials. Various in vivo and ex vivo imaging techniques can be used to measure the physicochemical properties, pharmacokinetics, and pharmacodynamics of the materials. These imaging techniques have been crucial in helping us understand how healthcare materials affect the human body and vice versa.
Substantial research is invested in optimizing the L-ADME components of material pharmacokinetics (Liberation, Absorption, Distribution, Metabolism, and Excretion)[4]. These concepts are perhaps self-explanatory for biodegradable drug delivery systems, especially in oncology where materials are used to mitigate side-effects of highly cytotoxic drugs. Examples include therapeutic nanoparticles (TNPs) like liposomal doxorubicin (DOXIL, FDA-approved for Kaposi’s Sarcoma, multiple myeloma, and refractory ovarian cancer)[5], antibody-drug conjugates like ado-trastuzumab emtansine (Kadcyla, FDA-approved for metastatic HER2+ breast cancer) [6], and drug-eluting materials such as carmustine wafers (Gliadel, FDA-approved for glioblastoma)[7]. However, even relatively inert materials such as titanium alloys used in orthopedic implants can undergo dynamic L-ADME processes. For instance, metal-on-metal implant wear and corrosion can generate metallic NPs that exert both local and systemic reactions in patients, and such effects have had enormous clinical and economic ramifications[8]. Generally speaking, the pharmacokinetics of materials are more complex than those of small-molecule drugs, since often the latter are an encapsulated component of the former, the larger size of materials amplify spatially heterogeneous transport (even for nanometer-sized biologics), and they can operate over extended time-scales. For these reasons, imaging — particularly done in a multiplexed, longitudinal manner in patients and live-animal models of disease — is especially informative.
Imaging approaches to study material pharmacology are by nature interdisciplinary, and have benefited from convergent advances in genetic engineering, optics, computer science, and synthetic chemistry. In particular, in vivo microscopy (intravital microscopy, IVM) has emerged as a powerful suite of techniques for monitoring dynamic cellular processes in live animal models of disease[9]. In vivo confocal, multiphoton, and multichannel imaging enable multiple cellular, molecular, and material properties to be simultaneously and quantitatively monitored over time at subcellular resolution. Recent studies empowered by IVM have uncovered new cellular-level mechanisms of material transport and action at a single-cell level[10–13], for instance in the study of biologics[14,15], nanomedicines[10,16] and implanted tissue engineering scaffolds[17]. In this review, we focus in particular on IVM, and survey advances in complementary imaging approaches including translational radiologic modalities and ex vivo multiplexed and/or whole-tissue optical methods. We describe recent IVM developments, including with respect to microscopy equipment, fluorescently-conjugated materials and payloads, and fluorescent reporters of pharmacodynamic response. We then discuss how imaging has been used to examine the single-cell PK/PD of advanced materials in major organs and sites of disease, and highlight corresponding biological insights. For material pharmacokinetics, we concentrate on how physiochemical properties of materials and the in vivo microenvironment affect the material transport. With respect to pharmacodynamics, our discussion includes findings of material effects on molecular targeting, immunomodulation, angiogenesis/tissue regeneration, foreign body response, and cytotoxicity (Fig. 1).
2. Technology for imaging material pharmacology
2.1. In vivo imaging overview
A diverse toolkit of imaging techniques is available for clinicians and scientists to visualize biological processes in the living body. In this section, we will survey these diverse imaging techniques. Although this review primarily focuses on optical microscopy approaches, other imaging modalities play important roles in visualizing the in vivo delivery and action of materials, especially in the clinic and at the whole-body scale. X-ray computed tomography (CT) and magnetic resonance imaging (MRI) are the most widely used approaches for cross-sectional clinical imaging, and intrinsic physical material properties can be leveraged to generate magnetic or x-ray image contrast. Materials incorporating transition metals, such as gold NPs, can be imaged by CT, with their high density providing contrast against soft tissue attenuation of the surrounding tissue, thus providing anatomical context, biodistribution, and pharmacokinetics[18]. Calcium and iodine likewise offer CT contrast, and differentially attenuate photons of different energies. This property allows dual- or multi-energy CT to differentiate materials and metals such as gadolinium and gold from each other and with other high density materials such as bone. CT imaging agents can be engineered to enhance the contrast of targeted tissues. For instance, a pH sensitive nanocomposite and an upconverting nanoparticle have been designed to enhance the CT contrast between bone and osteosarcoma, which are traditionally difficult to distinguish due to their similarly high CT density values[19,20]. The susceptibility to magnetization of iron-oxide forms the basis for their extensive use in MRI[21], and more recently, magnetic particle imaging (MPI)[22]. Iron-oxide is usually associated with negative-contrast when imaged with commonly used MRI sequences, which can be difficult to interpret when surrounded by anatomical structures also presenting with negative contrast signal (e.g. blood vessels). Recent development of ultra-short echo-time sequences enable positive-contrast imaging of iron-oxide particles in vivo, potentially alleviating this issue[23]. Furthermore, the magnetic properties of iron oxide materials can be sensitive to their physicochemical environment, for instance allowing processes such as cellular uptake and drug release to be monitored by MRI. We refer the reader to other excellent reviews on CT and MRI for further details[24–26], including the use of materials incorporating other MRI-imageable isotopes (e.g. 19F)[27].
Ultrasound imaging provides unique advantages compared to other clinically relevant modalities, including low cost, no use of ionizing radiation, portability and real-time imaging. Ultrasound can be used to visualize sonoluminescent NPs[28]. Materials such as perfluorocarbon provide good echogenic contrast compared to the surrounding tissue[29]. Microbubbles also offer good echogenic properties and can be used to deliver drugs[30]. High intensity focused ultrasound can burst microbubbles in vivo, enabling targeted delivery of drugs[31]. We refer readers to a recently published excellent review for further details on ultrasound imaging[32].
Materials are frequently labeled with a reporter when their native properties offer insufficient contrast. Given the high sensitivity offered by ionizing radiation, many agents have been developed for visualization using positron emission tomography (PET) and single-photon emission computed tomography (SPECT). The choice of radioisotope is dictated by several factors, including its radioactive half-life and the type of chemical reaction available to link it to the material without significantly altering the material properties. For example, we have recently developed a polyglucose-NP, Macrin, for imaging macrophages. The NP size was tuned for several applications. A 5 nm hydrodynamic sized Macrin was developed to visualize cardiac and plaque macrophages[33]. Given its fast renal excretion, it was labelled with fluorine-18 (t ½ = 120 minutes), enabling excellent PET contrast. By comparison, imaging extravascular macrophages in solid tumors required a longer circulating NP, thus a 20nm sized Macrin was developed to maximize tissue penetration and macrophage uptake[34]. Concordantly, these NPs were labelled with Cu64 (t½ = 12.7 hours). Materials have also been labelled for imaging with other modalities. Gadolinium (Gd) is often used for MRI[35], but low sensitivity typically requires high levels of Gd to be attached to achieve sufficient signal.
Multimodal imaging combines the advantages offered by individual modalities together, and several groups have sought to develop materials with multimodal functionality[36], or to develop hybrid imaging instrumentation to combine information from different modalities. For example, PET/CT is now the standard modality in clinical nuclear medicine, allowing the visualization of PET agents within the anatomical context provided by CT. PET/MR has also been developed to combine soft tissue contrast with the exquisite sensitivity of PET[37]. Recent development of total body PET/CT systems with up to 40x the sensitivity of standard PET systems may allow visualization of very small amounts of materials in vivo or the tracking of materials over a much longer time period than is currently possible[38]. Nanoparticles have also been designed as contrast agents for dual MR/CT[39,40] and dual MR/fluorescent imaging[41]. Indeed, there has been a long history of development for these multimodal imaging agents, and a detailed discussion on this topic is outside the scope of this review.
While lacking the depth penetration offered by the imaging modalities described above, optical techniques are increasingly being adopted for in vivo imaging, especially in intra-operative and endoscopic settings. Endoscopy in the visible light range is routinely used clinically to evaluate the GI tract. Fluorescently labelled antibodies/nanoprobes or vascular markers have been used in endoscopic and intra-operative settings to delineate dysplasia and tumor margins in patients[42]. Whole body biodistribution of fluorescently-labeled probes — in small animal models — can also be provided with fluorescence reflectance imaging or fluorescence molecular tomography (FMT)[43], while Cerenkov luminescence imaging has been used to optically image Cerenkov radiation emitted from β-emitting isotopes[44]. Raman spectroscopy, based upon the inelastic scattering of photons, has been adapted for biomedical imaging purposes[45]. Coherent anti-Stokes Raman spectroscopy (CARS) is especially used to image C-H bonds in lipids, providing information on subcellular structures. Since the inherent Raman signal is relatively weak, amplification of the Raman signal has been developed by adsorbing molecules onto a metal surface, such as metallic NPs, enhancing the signal intensity up to 1015 fold. This surface-enhanced Raman spectroscopy (SERS) technique can be multiplexed with different NP surface functionalizations, potentially allowing several biological targets of interest to be probed simultaneously. Single-walled carbon nanotubes (SWNT) have an endogenous Raman signature, and have been used to image tumor targeting strategies in vivo. Several other optical imaging contrast mechanisms have also been explored to image materials in vivo, including optical coherence tomography (OCT)[46], multispectral optoacoustic tomography (MSOT)[47] and terahertz imaging[48]. These and other optical techniques can also be combined with advanced computational algorithms and new microscopy hardware to improve the spatial and temporal resolution for in vivo imaging applications.
Intravital microscopy (IVM) comprises a group of techniques that enable researchers to observe and image live animals, and in some cases patients, at high resolution. For more than 20 years, IVM has enabled the study of biological and pharmacological processes in physiologically relevant contexts[49–51]. IVM setups typically consist of a laser-scanning or spinning-disk confocal microscope to acquire the microscopy images, mouse window chamber to allow the visualization of tissues in vivo, fluorescent reporters of drug action for visualizing PD, and fluorescent material and companion imaging drugs for tracking PK. Indeed, IVM has become an indispensable tool in understanding the complex biology that governs the PK and PD of various pharmaceutical agents. Unlike other imaging modalities such as MRI, CT, and PET, IVM offers a unique capability to assess the material PK and PD at a single-cell resolution. In the following sections, we will showcase various components of the IVM toolkit.
2.2. Intravital microscopy models
IVM can be performed non-invasively on the skin of live animals[52] and endoscopically [53], ophthalmologically, or intraoperatively [54] during surgery in patients. Mammalian embryos and lower organisms such as zebrafish and C. elegans are small and transparent enough to be internally imaged by confocal microscopy without surgery[55]. Zebrafish in particular have been used to examine the in vivo efficacy of anti-tumor chemotherapy[55], as well as sustained in vivo activity of implanted materials[56,57]. Nonetheless, mouse models of disease generally require surgical manipulation to access internal tissues. Imaging in mice can typically be performed for >4 hours under general anesthesia during a terminal surgical procedure, as has been successfully performed to image pancreatic islets, malignancy in the peritoneal cavity (ovarian cancer)[21,58–60], and mammary fat pad (breast cancer)[61,62]. For repeated imaging across days or weeks, surgically implanted optical window chambers (WCs) have been useful, and under proper technique are well tolerated with minimal inflammation or signs of pain or distress on the subjects [63]. WCs frequently interface with stabilization brackets on the microscope, and target disease sites can be immobilized to their surface for spatial co-registration across multiple imaging sessions. Window frames providing Cartesian reference positions are also helpful in co-registration[64].
2.2.1. Dorsal Skinfold Window Chambers
Increasingly sophisticated WC platforms for in vivo imaging of different organ sites in mice have been developed, some of which take advantage of image-guided 3D printing. The first type of WC developed, and perhaps the easiest to experimentally prepare, is the dorsal skinfold window chamber (SWC) (Fig. 2A). This model establishes a transparent SWC on the skin of the back of the animal. This surgery is relatively easy to perform and carries minimal risk of infection, thus allowing the SWC to remain viable for potentially over a month[65]. The biological processes inside the skin layer can therefore be readily observed over time through the use of confocal laser scanning or multiphoton microscopy. This widely popular SWC model has been used to study wound healing[66], angiogenesis [67,68], and various properties of the tumor microenvironment[69]. In the latter, tumor cells are injected and grown under the skin fascia inside the chamber, allowing the longitude tracking of biological processes, drug delivery, and therapeutic response. Macromolecular materials are often used to measure transport properties of the tumor. For instance, Harney et al. have used the SWC, in combination with two-photon microscopy, to study the interaction of cancer cells with the tumor microenvironment during the intravasation process, a key steps of metastasis, at sub-cellular resolution[70] (Fig. 2A). They were able to observe a dynamic interaction between cancer cells, endothelial cells, and TIE2hi macrophages, which results in transient vascular permeability (leakage of a fluorescent dextran into the tumor interstitium) and the transmigration of cancer cells into blood vessels. More recently, Gruionu et al. have described an improvement over the traditional SWC by implanting a biocompatible and optically-clear tissue isolation chamber fabricated with polydimethylsiloxane (PDMS) within the SWC. This tissue isolation chamber can partially confine tumors within a pre-set geometry, facilitating the study of tumor architectures in a controlled and reproducible manner[71]. SWC tumor models have also been utilized to study the PK/PD of small molecule drugs[62], therapeutic NPs[10], and antibodies[14,15].
Figure 2: Window chamber models for intravital microscopy.

(A) Skinfold WC for IVM of tumors or wound-healing responses. Tumors were implanted in the SWC (upper left). Confocal microscopy can be used to visualize tumor cell (green) intravasation into dextran (white) labeled tumor blood vessels (red). Adapted with permission.[70] Copyright 2015, American Association for Cancer Research. (B) Cranial window chamber (CWC) for IVM of the brain (upper left). A piece of skull was removed and replaced with a coverslip or PDMS membrane that allowed visualization of the cranial tissues (lower left). Confocal microscopy visualizes the GFP-expressing microglia in situ in the brain (right). Adapted with permission.[83] Copyright 2016, Nature Publishing Group. (C) Lung window chamber (LWC) allows visualization of lung tissue with IVM (left). LWC was used to track the extravasation of tumor cells (green) from lung microvasculature (red, labelled with dextran) into lung tissue space over time (right). Adapted with permission.[64] Copyright 2018, Springer Nature Limited (D) Window chambers can be established in the mammary tissue of the mouse (mammary window chamber, MWC, top). Breast tumors can be implanted into the MWC to observe their behaviors in the orthotopic site. IVM was used to image the interaction of breast cancer cells (green) with blood vessels (blue) and collagen ECM (purple, imaged via SHG) in the mammary tissue (bottom). Adapted with permission.[85,419] Copyright 2008, Springer Nature Limited. Adapted with permission.[419] Copyright 2015, The Optical Society. (E) Abdominal window chamber (AWC) for IVM of liver (top) and pancreas (middle). IVM and AWC were used to observe the loss of β cells (green) in a mouse model of diabetes (bottom). Adapted with permission.[92] Copyright 2019, Nature Publishing Group. Adapted with permission.[420] Copyright 2017, Elsevier (F) Bone window chamber (BWC) can be established by implanting tissue engineering scaffolds that induce bone or bone marrow formation in the SWC. As an example, bone marrow niche (top left) can be formed in the dorsal skin, with organized collagen structure (top right), vessels (red, bottom), and bone marrow stromal cells (green, bottom). IVM was used to image metastasis of cancer cells into this engineered bone marrow niche. Adapted with permission.[94] Copyright 2019, Springer Nature Limited.
2.2.2. Cranial Window Chambers
The thinned skull window (TSW)[72] and chronic cranial window (CCW)[65] are two types of cranial windows commonly used to image the brain (Fig. 2B). For the TSW model, a micro-drill is used to thin the skull and allow imaging access to the brain surface immediately beneath via two-photon microscopy. This cranial window model avoids the implantation of coverglass. However, the thinned skull impedes light penetration and imaging resolution. Furthermore, skull regrowth requires the thinning procedure to be periodically repeated. Consequently, this model can be difficult to use for long-term studies[72] compared to the CCW. The CCW involves removal of a piece of the skull via craniotomy and replacement with a glass coverslip glued permanently in place. The skull functions as a solid support for the coverslip, and the CCW allows repeated long-term imaging of brain surface for up to a year. Since no skull material exists in between the microscope objective and brain surface, high resolution imaging deep within the brain can be performed[65]. However, a disadvantage of the CCW is that the implantation of glass coverslip, a foreign object, often results in a mild inflammation in the brain for a month after surgery[73]. The CCW has been used in IVM studies of biological processes such as neuronal activities, cerebral blood circulation[74], and neuronal structural plasticity[75]. It has also been heavily utilized to study diseases such as brain cancer[76], traumatic brain injury[77], and stroke[78]. As with the SWC, the CCW has been used to study the permeability of tumor blood vessels by visualizing extravasation of fluorescently labeled materials. More recently, photoacoustic microscopy has been utilized with the CCW to image blood oxygenation and flow deep within the brain, as it has higher penetration compared to multiphoton microscopy[79]. Cranial WCs have also been used to track hematopoietic stem cell localization and vascular changes in calvarial bone marrow cavities over time[80,81].
Recent reports have introduced improvements to the designs and experimental procedures of the traditional CCW. For example, Goldey et al. described a surgical method that allows periodic removal and replacement of cranial windows[82]. This surgical method gives the researchers physical access to the brain surface, and thus allows application of reagents to the brain during the course of long-term experiments. Recently, Heo et al. described a novel PDMS cranial WC, in which the glass coverslip is replaced with a flexible, optically transparent, and elastic PDMS membrane[83] (Fig. 2B). The flexible nature of the PDMS membrane allows the WC to take the curved shape of the skull and cover a larger area. Moreover, since PDMS is elastic, this PDMS window allows the insertion of a micropipette and microelectrode through the PDMS membrane onto the brain surface without any subsequent fluid leakage. The authors used this PDMS WC to image microglia and hemodynamic activities in a Cx3Cr1+/GFP transgenic reporter mouse expressing GFP+ microglia.
2.2.3. Lung Window Chambers
Entenberg et al. recently described a permanent lung WC for studying pulmonary metastasis[64](Fig. 2C). This WC is designed to firmly embed within the thoracic cavity by sutures and glues, replacing a part of the chest wall in the mouse. A small part of muscle and rib cage in the animal are resected, exposing the lung tissue underneath. A glass coverslip separates the chest cavity and the lung tissue from the outside environment, allowing easy imaging access to the lung surface. The authors were able to demonstrate that mice carrying these WCs can breathe independently and remain viable for up to 2 weeks. They also designed fiducial marks on the WC to identify the same imaging location across repeated imaging sessions. Entenberg et al. used these chambers to track the dynamic movement of intravenously injected breast cancer cells and fluorescent dextran in the lung. The approach allowed visualization, for the first time, of important steps of metastasis, including cell adhesion to endothelium, extravasation, and micro-metastatic growth, in real-time at a sub-cellular resolution in vivo. Hence, this newly developed WC is a promising tool for understanding the biology of metastasis and evaluating the efficacies of anti-metastatic therapies in a physiologically relevant context.
2.2.4. Mammary Window Chambers
Mammary WCs are designed to study breast tissues and the biology of breast cancer, and are implanted by suturing and gluing a plastic or titanium window frame between the mammary gland and the skin. A transparent glass coverslip separates the exposed mammary gland from the outside environment[63] (Fig. 2D). IVM of the mouse mammary gland has been used to monitor the formation and transport of milk lipid droplets in the mammary duct [84], as well as xenograft or MMTV-PyMT models of breast cancer, the latter of which is a spontaneously developing genetically driven mouse tumor model that mimics the natural progression and development of human disease. For example, Kedrin et al. have used the mammary WC and a photo-switchable fluorescent protein to study the migration of orthotopically implanted MTLn3 rat breast tumor cells in vivo[85] (Fig. 2D). They observed that MTLn3 cancer cells residing in vascularized areas had a higher migration speed than cancer cells in avascular areas, suggesting the tumor microenvironment impacts the metastatic potential of cancer cells. They also imaged the interaction between cancer cells and the extracellular matrix (ECM) within breast tumor tissues with second harmonic generation (SHG). The same group of authors later used the mammary WC and Confetti technology to trace the lineage of cancer stem cells residing in the MMTV-PyMT tumors[86]. More recently, the mammary WC has been employed to study the mechanisms of metastasis in HER2+ breast cancer[87]. It has also been used to image oxygen saturation and blood perfusion in a patient derived xenograft model of breast cancer[88].
2.2.5. Abdominal Window Chambers
Abdominal WCs have been developed to visualize the small intestine, kidney, spleen, pancreas, liver, and ovary [63] (Fig. 2E). These WCs consist of a titanium ring with a side groove, onto which the opened abdominal wall can be sutured and tightened. A glass coverslip is affixed in the titanium ring, creating a permanent seal for the opened abdomen. Two common problems associated with abdominal WCs are the movement of internal organs in the abdomen and a higher chance of infection at the implantation site[89]. The abdominal WC has been used to study colon cancer development, cancer cell metastasis to liver[90], intestinal stem cell regeneration[91], and diabetes [92]. As an example, Reissaus et al. have successfully utilized the abdominal WC to track the shrinkage of pancreas and the destruction of β-cells over a period of one month in a mouse model of diabetes induced by multi-low-dose streptozotocin[92] (Fig. 2E). Their time-lapse experiment has shown that a threshold level of loss in β-cell volume is required before the rise of blood glucose level and the development of diabetes. They also demonstrated the ability to image β-cell responses to reactive oxygen species and calcium signaling by inducing the expression of fluorescent biosensors.
2.2.6. Bone Window Chambers
With the exception of the cranium, it has traditionally been difficult to perform long-term IVM of the bone microenvironment, due to its poor accessibility for WC placement. Yet, bone is a major site of metastasis, and a technique that enables the longitudinal observation of cancer cell-bone tissue interaction in real-time can greatly benefit our understanding of bone metastasis and the potential therapeutic strategies to prevent their formation. To address this need, Dondossola et al. created an engineered bone microenvironment in the SWC by implanting a polycaprolactone scaffold functionalized with bone morphogenetic protein 7 (BMP7) and calcium phosphate[93]. The BMP7 in the scaffold induced formation of ectopic ellipsoidal bone with mature bone marrow within a month. The author then used this engineered bone WC to image the growth of prostate cancer within the bone microenvironment. They discovered that tumors that were established on the interface between cortical bone and marrow could induce osteolysis by activating osteoclasts. More recently, Carpenter et al. have described a strategy to create a bone marrow microenvironment by implanting an inverted colloidal crystal hydrogel scaffold seeded with human bone marrow stromal cells (hBMSC) on the back of a mouse[94] (Fig. 2F). A vascularized bone marrow microenvironment can quickly be developed from the implanted scaffold, which can then be used to image the spontaneous metastasis of prostate cancer cells to the bone marrow. The authors used these engineered bone marrow niches to study the establishment of metastatic tumors in the bone.
2.2.7. Ophthalmic imaging
The eye is naturally amenable to optical imaging. As such, IVM of retina does not require WCs, and has been used to measure blood flow[95], track immune cell trafficking[96,97], and study hematopoietic stem cell localization[98] in the retina. More recently, Chitinis et al. used IVM to evaluate the delivery of fluorescently-labeled mesenchymal stem cells to the suprachoroidal space (SCS) inside the retina by a newly designed resistance-sensing mechanical injector. They found good co-localization of the stem cells with the collagen ECM (imaged by SHG) in the SCS. This site-specific delivery was also evaluated with μCT[99] (Fig. 3). Optical coherence tomography (OCT) is routinely used in the clinic to image the eye, and has been used to evaluate the interaction between intravitreally injected PLGA micromaterials[100] or surgically introduced Argus II medical implants[101,102] with the retina (Fig. 4).
Figure 3: Imaging the local delivery and transport of injected material.
μ-CT and IVM monitored the ability of a resistance-sensing mechanical injector to precisely deliver therapies to the suprachoroidal space (SCS) of the eye. (A) The mechanical injector delivered DiD-labeled mesenchymal stem cells (MSCs) into the SCS. (B) The presence of MSCs in this space was visualized by IVM (collagen imaged with SHG). (C) CT contrast agent was injected into the SCS, and its diffusion was observed over time by μ-CT. Adapted with permission.[99]Copyright 2019, Springer Nature Limited.
Figure 4: Imaging anatomical placement and toxicity of surgically implanted materials in the eye.
(A-B) OCT imaging reveals localization of intravitreally injected PLGA microparticles in the retina (A), with evidence of retinal detachment in some cases as a response (B). Adapted with permission.[100] Copyright 2017, Association for Research in Vision and Ophthalmology (ARVO) (C-E) OCT can also be used to observe the localization of electrode array from the Argus® prosthesis system (C-D) in the retina. Electrode was observed to touch the retinal surface (E). Adapted with permission.[101] Copyright 2018, Dove Medical Press. Adapted with permission.[102] Copyright 2016, Elsevier.
2.3. Image stabilization and correction techniques
Cardiopulmonary and smooth muscle body movement is a major challenge for IVM that can be addressed through a variety of means. Tissue can be immobilized through bracketing the WC to the microscope stage. In addition to or in absence of the WC, tissue can be further restrained by clamping, as has been successfully implemented to reduce the motion of the liver and kidney[103,104] (Fig. 5A). Tissue can also be suctioned by vacuum to the microscope objective, using an objective adaptor designed to create a gentle negative pressure on the tissue of interest to hold it in place during image acquisition[105]. In contrast, the rhythmic movement of vital organs, such as lung and heart, cannot be so easily immobilized. The effect of these movements on image quality can be mitigated by synchronizing image acquisition frequency with the frequency of breathing and heartbeat (Fig. 5B-D). Both retrospective and prospective gating have been successfully performed, including with mechanically paced ventilation and paced heartbeat, and have enabled IVM image acquisition at subcellular resolution in the beating heart and breathing lung [104,106,107].
Figure 5: Stabilization and synchronization techniques for IVM.
(A) A metallic clamp constrains and stabilizes dynamic tissues for IVM. Adapted with permission.[104] Copyright 2012, SPIE. (B) Schematic of cardiac gating systems to synchronize cardiac IVM with heartbeat. The acquisition rate of the laser scanning microscope (LSM) was aligned with the pacemaker signal (P). Tissue stabilizer (TS) added to the stability of the tissue during image acquisition. (C) The waveform of imaging acquisition (Frame, blue) and echocardiogram (ECG, black) before (top) and after (bottom) synchronization. Adapted with permission.[106] Copyright 2014, National Academy of Sciences.(D) Representative cardiac IVM with different stabilization and synchronization conditions. Adapted with permission.[104] Copyright 2012, SPIE.
Finally, confocal and two-photon IVM exhibit an inherently limited ability to image deep into tissue due to light scattering. As the sampling depth increases, the signal quality decreases due to the optical aberration contributed by tissue samples. Various groups have introduced hardware and software upgrades, such as deformable mirrors[108], adaptive optics[109], and objective correction collars[110] to reduce optical aberration. Limited penetration depth of optical imaging can be improved by materials such as upconverting NPs (UCNPs), which can be excited with photons at the near infrared spectrum (~800–900 nm) [111]. At this long wavelength, light can penetrate deeper into the biological samples, allowing imaging of tissue 3–4 cm below the surface[112]. The UCNPs have also been used to reduce the background autofluorescence and improve the resolution of IVM[113]. Another technique used to perform non-invasive imaging of deep tissue is photoacoustic imaging, an imaging modality in which near infrared laser pulse delivered to the tissues is converted to ultrasonic emission[114]. This imaging modality has been used to detect gastric acid secretion in the stomach[115]. All these methods are broadening in their commercial availability — many UCNPs are commercially available, as are adaptive optics components including deformable mirrors and wavefront sensors.
2.4. Ex vivo microscopy to complement IVM
Although IVM is a powerful tool to monitor high resolution in vivo dynamics, it carries limitations in imaging depth, resolution, multiplexing, and fluorescent labeling requirements. Despite specialized approaches to monitor broad fields of view in tissue[116], penetration depth is typically <1cm with specialized approaches, and often <300 μm for single-photon confocal imaging. IVM setups are often capable of imaging 3–4 fluorescence species, which can be extended to ~10 with deconvolution approaches [117]. However, this multiplexing is modest compared to many -omic scale methods, and live-cell imaging typically precludes many immunostaining, in situ hybridization, and mass spectrometry approaches. Spatial resolution can also be limited in vivo by tissue movement and practical constraints in accommodating live animals on the microscope stage.
To overcome these limitations, IVM can be complemented by a growing array of ex vivo imaging methods. For instance, the limited penetration depth of IVM can be addressed by tissue clearing methods[118]. Tissue clearing comprises a variety of protocols that render whole fixed tissues or organs transparent by chemically modifying or removing components of the tissues to homogenize their refractive index, for instance by removing light scattering lipids[119]. Many clearing reagents and methods (CUBIC, iDISCO, CLARITY, among others) have been developed, and each one of them has its advantages and drawbacks, which are extensively reviewed elsewhere [120–123]. In general, these approaches allow the imaging of fluorescent signals deep through tissue or entire organs at high resolution, such that diffusive penetration of staining reagents into tissue, and the working distance of the objective, rather than light scattering in tissue, can become the limiting factors in imaging depth. As an example, Cuccarese et al. and Kim et al. have recently used a modified CUBIC clearing solution to facilitate the visualization of macrophage composition and heterogeneity, as well as the distribution of NPs, in disseminated tumors through entire intact lungs of mice[124]. Specifically, Kim et al. performed multi-modal imaging of the polyglucose NP (Macrin) biodistribution by co-registering PET/CT images with fluorescent confocal images obtained from the clarified lung (Fig. 6). Hence, these studies demonstrated the potential power of tissue clearing in clarifying global microanatomical structure of tissue and its relationship to material transport and cellular uptake.
Figure 6: Multi-modal imaging of the biodistribution of macrophage-avid NPs.
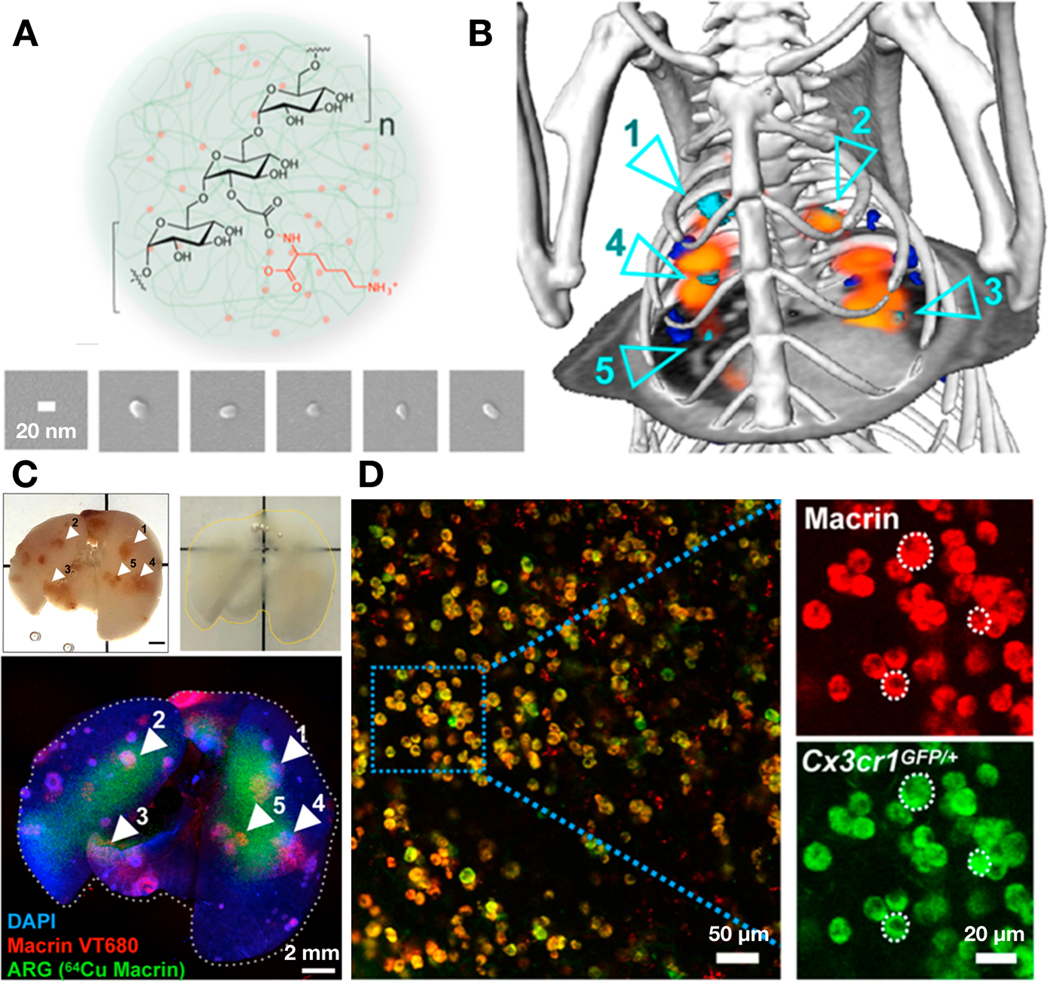
(A) A polyglucose NP (Macrin) ~17 nm in size was designed to target macrophages in the tumor tissues. Macrin can be labeled with 64Cu or fluorescent dyes to assess its localization in the body at whole-organ or single-cell level using PET or microscopy, respectively. (B) Representative in vivo PET/CT of 64Cu Macrin (orange) showing its localization in the lung of the mouse bearing orthotopic tumors (segmented as cyan/blue). (C) Whole-organ tissue clearing (modified-CUBIC) technique was used to image Macrin distribution within the entire lung. Fluorescently-labeled macrin (Macrin VT680, imaged via confocal microscopy) and 64Cu-labeled Macrin (imaged via autoradiography/ARG, also in B) were both shown to accumulate in lung tumor tissues. (D) Single-cell confocal imaging of the cleared lung showed almost exclusive accumulation of Macrin in Cx3cr1+ phagocytes including tumor-associated macrophages. Adapted with permission.[34] Copyright 2018, American Chemical Society.
Multiplexed tissue imaging (MTI) technologies are rapidly developing as methods to more comprehensively profile the landscape of diverse cell types and the coordinated activities of multiple gene expression or signaling pathways. MTI is a group of techniques that enable the assessment of multiple target proteins, materials, or nucleic acids, or other relevant species in a single histological section of tissue [125–128]. In principle, the approach is capable of preserving the spatial information of material distribution, which can be correlated with detailed maps of biological response and co-registered with data collected from IVM. Diverse innovations are converging to improve MTI, with the most promising techniques relying on high-resolution multichannel fluorescence microscopy and image-cycling for repeated rounds of molecular labeling. In general, image-cycling (including in situ sequencing) comprises iterations of sample imaging, labeling with multiple antibodies or nucleotides, re-imaging, and processing to remove the labels. MTI technologies have now made it possible to image dozens of proteins and potentially thousands of transcripts, all while preserving spatial localization in tissue and within single-cells[129]. Particularly relevant to the imaging of materials, imaging mass cytometry can visualize the spatial distribution of drugs, metabolites, and even elements not naturally found in the body[130,131]. This latter approach is commercialized for multiplexed immunostaining with lanthanide-series metal conjugated antibodies[132], and similar (mass cytometry) approaches have been used to monitor the biodistribution of administered metal-tagged NPs[133]. As with genomics, MTI data and associated software are increasingly made available through online repositories. Thus, these technologies are poised to generate a flood of high-content data. Finally, various imaging methods, including super resolution microscopy, lattice light sheet microscopy, expansion microscopy, and electron microscopy, offer higher-resolution imaging than conventional confocal laser scanning microscopy. These imaging methods can be used to track the sub-cellular localization of material and its payload. A detailed review of these microscopy methods is outside the scope of this review. However, we compare and contrast the image resolution, penetration depth, and acquisition speed of these microscopy methods with other in vivo imaging modality described in the prior sections. For a detailed description of each imaging method, please refer to references listed in Table 1.
Table 1.
Resolution +: >10−2 mm; ++: 10−2 ≈ 10−3 mm; +++: 10−3 ≈ 10−4 mm; ++++: 10−4 ≈ 10−5 mm; +++++: 10−5 ≈ 10−6 mm. Penetration +: <10−2 mm; ++: 10−2 ≈ 10−1 mm; +++: 10−1 ≈ 10° mm; ++++: 10° ≈ 101 mm; +++++: >101 mm. Speed n/a: fix/frozen; ++: slow; +++: fast
| Imaging modality | Resolution | Penetration Depth | Acquisition Speed | Applications | References |
| positron emission tomography | + | +++++ | +++ | in vivo imaging | [421 ] |
| single photon emission computed tomography | + | +++++ | ++ | in vivo imaging | [422] |
| fluorescence molecular tomography | + | +++++ | ++ | in vivo imaging | [423] |
| sculpted light | + | +++ | +++ | in vivo imaging | [424,425] |
| ultrasound imaging | + | +++++ | +++ | in vivo imaging | [426,427] |
| computed tomography | ++ | +++++ | +++ | in vivo imaging | [428] |
| magnetic resonance imaging | ++ | +++++ | ++ | in vivo imaging | [429] |
| ultrasound lens imaging | ++ | ++ | +++ | in vivo imaging | [430] |
| optical coherence tomography | ++ | + | ++ | in vivo imaging | [431] |
| photoacoustic imaging | ++ | ++++ | +++ | in vivo imaging | [432,433] |
| remote axial scanning | ++ | ++ | +++ | in vivo imaging | [434] |
| epifluorescence | +++ | + | +++ | in vivo imaging | [435] |
| tissue clearing microscopy | +++ | ++++ | n/a | ex vivo imaging | [124,436,437] |
| multiphoton microscopy | +++ | +++ | ++ | in vivo imaging | [438] |
| confocal laser scanning | +++ | ++ | ++ | in vivo imaging | [435] |
| optical microscopy (serial sectioning) | +++ | +++++ | n/a | ex vivo imaging | [439] |
| light sheet | +++ | ++ | ++ | in vivo imaging | [440] |
| raman imaging | ++++ | ++ | ++ | in vivo imaging | [441] |
| expansion microscopy | ++++ | ++++ | n/a | ex vivo imaging | [442,443] |
| lattice light sheet | ++++ | ++ | ++ | in vivo imaging | [444] |
| super resolution microscopy | ++++ | + | ++ | in vivo imaging | [445,446] |
| electron microscopy (serial sectioning) | +++++ | +++++ | n/a | ex vivo imaging | [447,448] |
• Note: Many techniques can apply across a range of resolutions, penetrations, and speeds. Annotation here captures representative properties.
2.5. Fluorescent labeling of materials and payloads
2.5.1. Fluorescent labeling of macromolecular materials and scaffolds
Various different classes of nanomaterials have been developed to function as drug delivery vehicles. These nanomaterials include polymeric NPs (e.g. PLGA-PEG NP), liposomes, quantum dots (Qdots), metallic NPs, and carbon-based NPs (e.g. carbon nanotubes). To visualize nano-carriers in vivo, many different fluorescent labeling methods and schemes have been developed. For example, fluorophores can be covalently conjugated to polymers that are co-encapsulated within the material (such as with PLGA-PEG NPs stably loaded with fluorescent PLGA-BODIPY). Fluorescent lipophilic dyes, such as the cationic indocarbocyanine dyes DiI and DiO, are also frequently used for incorporation into the lipid bilayer of liposomes. Quantum dots are nanoscale semiconducting crystals that have ability to emit lights of various wavelengths based on their size and shape. These intrinsically fluorescent nanomaterials have been used for in vivo imaging and phototherapy[134–137]. However, Qdots can also be used to fluorescently label other NPs by encapsulating them into the nanocarriers[138–140]. Finally, gold NPs can be coupled to fluorophores via glycine-cystamine linker[141,142], while carbon nanotubes can be cross-linked to fluorescent probes using carbodiimide, a heterobifunctional cross-linker[143].
Macromaterials, such as tissue engineering scaffolds and drug-loaded hydrogels, are routinely visualized by IVM using material autofluorescence[144,145] or second harmonic generation (SHG) imaging[146]. Yet, these macromaterials have also been labeled with fluorescent dyes to assist in high-resolution imaging of scaffold-tissue interaction or scaffold degradation. Functionalized scaffolds composed of biological materials (i.e. collagen or fibrin) or synthetic polymers (i.e. PEG-amine) can be readily conjugated to fluorescent dyes with NHS-ester reactions[147,148]. As another example, the free amine group of chitosan can be reacted with isothiocyanate-based dyes (FITC or TRITC), thus covalently linking the chitosan to fluorescent molecules via isothiourea linkage[149]. Finally, biological scaffolds can be labelled with fluorescent-conjugated antibodies designed to target the main biological component of the scaffolds[150].
2.5.2. Fluorescent imaging of therapeutic payloads
Various fluorescent companion imaging drugs have been developed to visualize the biodistribution of the drug payloads at a single cell resolution. Fluorescent labeling of proteins and cells can often be done without dramatically impacting their PK/PD, however this is often not the case for small-molecule compounds. Therefore, although fluorophore-drug conjugates are designed to share similar PK and target affinity as their unlabeled parent drugs, the inevitable impact of fluorophore labeling must be taken into account when interpreting results. Indirect drug imaging using co-administered labeled and unlabeled drug has been one strategy to address this issue. Nonetheless, results from direct imaging of fluorescent drug conjugates have been useful in elucidating principles of in vivo material pharmacology. Recently published examples of fluorescent-drug conjugates include a fluorescently labeled platinum (Pt) prodrug of the widely used chemotherapeutic cisplatin, encapsulated in PLGA-PEG NPs[10]; a BODIPY-TMR-conjugate of the immunostimulatory agent resiquimod, which was developed to study the impact of nanoencapsulation on drug delivery [151]; BODIPY-FL-conjugated eribulin, a microtubule-targeting chemotherapeutic [152]; silicon-rhodamine-conjugated ibrutinib, a targeted BTK inhibitor[153]; BODIPY-FL-conjugated olaparib, a PARP inhibitor (fluorescent conjugated olaparib is now in clinical trials for cancer imaging, NCT03085147)[62]; BODIPY-FL-conjugated vemurafenib, a BRAF inhibitor[154]; and silicon-rhodamine-labeled taxane, an anti-microtubule agent[155]. Nucleic acids, antibodies, antibody drug conjugates, and small peptides, are more routinely labeled with fluorophores as extensively reviewed elsewhere[156,157].
2.6. Imaging reporters of drug action
2.6.1. Injectable reporters for cell identification
Fluorescent reagents can be injected into animals to label specific compartments or cell-types in vivo. Blood vessels are often labeled with fluorescently-tagged lectin, which binds tightly to glycocalyx expressed on the endothelial cell luminal surface[158]. High-molecular weight fluorescent dextran (70 kDa-500 kDa) and other long-circulating materials can be used to label functionally perfused vasculature. Upon injection, these materials stay within the endothelial lumen. However, over time, they extravasate from the blood vessels into tissue, and IVM can be used compute corresponding permeability coefficients and effective diffusion constants[159]. Targeted small molecules have been widely developed as probes for imaging cell populations or extracellular matrix in vivo. As one recent example, a silicone-rhodamine labeled small molecule probe of Mer, a receptor tyrosine kinase highly expressed on macrophages, was recently found to accumulate selectively in Mer+ tumor-associated macrophages in a mouse allograft tumor model[160]. Other examples include probes for imaging cancer cells that over-express certain proteins such as prostate-specific membrane antigen (PSMA)[161] or somatostatin receptors (SSTRs)[162], and such probes can also be conjugated to radionuclides for clinical radiotheranostic applications.
Certain fluorescently-tagged NPs, such as the FDA-approved, carboxymethyldextran-coated iron oxide NP ferumoxytol, and a recently developed polyglucose NP Macrin, can be injected to label phagocytes, such as macrophages and neutrophils in the mouse[34,163,164]. On the other hand, cancer cells can be labeled with recently developed telomerase-specific spherical nucleic acid probes[165]. To improve the specificity of labeling, fluorescent antibodies targeting markers expressed on the cell-types of interest can also be used. For example, a fluorescent anti-CD3 antibody has been used to label T cells, while an anti-CD11b antibody has been used to visualize circulating monocytes[166]. Although useful, fluorescent antibodies face limitations. The antibody-based labeling of even general cell types in the body can require multiple markers to be simultaneously used, antibody labeling can be transport limited in poorly perfused tissue (such as fibrotic tumors), and antibodies can be trafficked in complex manners by both Fab- and Fc-region binding activities. These issues are in part addressed by nanobodies, which are VHH fragments of full length antibodies. These nanobodies are small enough to penetrate deep within the tissue, while maintaining the specificity for marker of interest. Fang et al. have recently used these nanobodies to immunolabel astrocytes and microglia deep within the brain[167]. Fluorescent antibodies can also be used to label subcellular structures. For instance, Gonda et al. have used a Qdot-conjugated antibody targeting PAR1, a membrane-bound protein, to track cell membrane fluidity in vivo [168].
2.6.2. Transgenic fluorescent reporter mouse models
Genetically engineered fluorescent reporter mice have been developed to image cell populations, protein levels, and enzyme activities. Many are available commercially, chiefly through Jackson Labs (jax.org). In general, many useful models involve fluorescent protein knock-in at the gene of interest, such that the fluorescent protein expression functions as a surrogate for endogenous gene expression[169]. For example, the IL12-eYFP reporter mouse was developed by inserting an enhanced YFP (eYFP) gene under the control of the promoter for p40 subunit of IL12[170]. Therefore, eYFP is co-expressed with IL12 in heterozygous reporter mice, and they have been used to evaluate the immunostimulatory effects of therapies and vaccines. When the targeted promoter is predominantly activated in a specific cell type, these fluorescent reporters can be used to label cells of interest. For instance, Cx3cr1GFP/+ reporter mice express EGFP in Cx3cr1+ monocytes, dendritic cells, and macrophages[171]. The EGFP signals in these mice can thus be used to identify macrophages and dendritic cells during IVM[34]. Similarly, fluorescent reporters in FoxP3-mRFP[172], Col I-GFP[173], and Rag1-GFP[174] reporter mice are used to label T cells, myofibroblasts, and B cells, respectively. More recently, Brainbow[175] and Confetti[176] reporter mice are designed so that each cell in these mice expresses unique combination of CFP, GFP, and RFP. The different expression levels of these three fluorescent proteins produce different variations of colors for each cell, enabling researchers to barcode cells in vivo and perform lineage tracing experiments by IVM.
Besides labeling entire cells, various transgenic mouse models exist to label subcellular compartments with fluorescent proteins. For instance, several groups have engineered mice that express histone 2B fused with a fluorescent protein (H2B-mCherry)[177]. Since histones localize to the nucleus, these reporter mice have been used to visualize the cell nucleus and chromosomal morphology in vivo. Reporter mice expressing venus fluorescent protein fused to Lyn kinase, a protein predominantly expressed on the cell membrane, have been used to outline cell boundaries, facilitating the downstream segmentation of the cells during imaging analysis[177–179]. Moreover, transgenic mice expressing fluorescent proteins labeling the Golgi apparatus, microtubules, and mitochondria have been created to visualize the in vivo dynamics of these cell components[177,180,181].
Engineered transgenic reporter mice can be used to evaluate cell cycle and signaling states. For example, FUCCI (fluorescent ubiquitination-based cell cycle indicator) was engineered into transgenic mice and used to track cell-cycle phases in real-time in vivo. The expression of red fluorescent mKO2-Cdt1 fusion protein in the nucleus indicates G1 phase, while the expression of green fluorescent mAG-Geminin fusion protein indicates G2/M phase[182,183]. Reporter mice for vascular endothelial growth factor (VEGF) expression [184], NF-kB activity [185], and AP-1 activity [186] are also available and can be used to evaluate the effects of drug treatments on these cellular pathways in vivo.
2.6.3. Transgenic fluorescent reporter cell models
Transgenic fluorescent reporters can be stably introduced into cells via transfection or transduction in vitro, and reporter cells can then be grafted onto mice for IVM. For instance, a truncated 53BP1-mApple fusion protein reporter was optimized for IVM to monitor DNA damage responses in vivo at a single-cell level[187,188]. As 53BP1 proteins bind to the site of non-homologous end joining DNA repair, the accumulation of 53BP1-mApple reporter in nuclear foci can be used to measure the degree of DNA damage. Laughney et al. created a multi-drug resistance 1 (MDR1)-mApple fusion protein reporter, and used it to monitor cancer cell resistance to eribulin[152]. HER2-GFP fusion protein reporter has been used to evaluate the binding of fluorescent trastuzumab to HER2 in vivo at a sub-cellular resolution[15,189]. A variety of kinase translocation reporters (KTR) have been developed to monitor kinase activity in real time. Once transfected into the cells, these fluorescent reporters can translocate between nucleus and cytoplasm depending on the activity level of the kinase. These KTRs can thus be a useful tool to probe the heterogenous responses of cells to materials carrying kinase inhibitors[190].
Forster resonance energy transfer (FRET) sensors are also used to monitor post-translational regulation in cells. These sensors consist of two fluorescent proteins (donor and acceptor) linked in close proximity. When the donor fluorophore is excited, a FRET process occurs in which energy emitted from the donor fluorophore is transferred to the acceptor fluorophore, resulting in fluorescence emission of the accepter fluorophore. If the distance between the fluorescent protein increases, energy transfer and FRET activity decrease. FRET kinase sensors are designed so the distance between the two fluorophores is controlled by the kinase activity [191]. These FRET sensors have been used to evaluate the activities of ERK[192], CDK1[193], and RhoA[194] in response to therapeutic treatments via IVM.
Photoconvertible fluorescent reporters have been developed to aid in tagging and tracking of specific sets of cells during IVM. These photoconvertible fluorescent proteins, of which most commonly used is Dendra, are designed to switch fluorescent excitation and emission spectra when they undergo exposure to light (typically high energy ultraviolet light). Cells carrying these reporters have been grafted onto animals for in vivo studies[85,195,196], and mouse models expressing these reporters have also been generated[197]. Researchers can optically highlight a subgroup of cells in a tissue using a pinpoint UV laser, and track the movement of the photoconverted cells over time. The photoconvertible fluorescent reporters can be used to evaluate cancer cell migration in response to anti-metastatic drugs, migration of epithelial cells in the process of wound healing, or the movement of immune cells in response to biomaterial implants.
2.7. Label-free IVM
Certain molecules and structures within the mouse can be visualized by multi-photon confocal microscopy without the use of exogenous fluorescence labeling. Label-free IVM using second harmonic generation utilizes the variation in the intensity of second harmonic light generated by different biological samples to produce imaging contrasts. Second harmonic imaging enables the visualization of cellular and tissues structures, most notably the bundles of collagen fibers in the extracellular matrix, in fine detail[198]. Indeed, second harmonic imaging has been used to visualize the organization of collagen matrix in the tumor stroma[199], as well as the effects of the matrix organization and density on nanotherapy delivery[200]. In addition to second harmonic generation, certain metabolites such as NADH and FAD possess intrinsic fluorescence that can be detected via two-photon microscopy[201]. For example, NADH, which accumulates in the cells with enhanced glycolysis, can emit a blue fluorescent signal. In contrast, NAD, the oxidized product of NADH and an indicator of enhanced oxidative phosphorylation, is not autofluorescent. Thus, the autofluorescence of NADH can serve as an indicator of cellular metabolic state, and it can be evaluated by measuring the intensity or lifetime of the signals, the latter of which is a more quantitative measurement of NADH level[202]. Indeed, fluorescent life-time imaging microscopy (FLIM) has become a popular tool for label-free measurement of NADH and FAD levels, and many groups have used this method in IVM[203]. Excellent review of detailed principles behind NADH imaging can be found elsewhere[202].
3. Material Pharmacokinetics
Advanced materials, such as engineered biologics and nanomaterials, can be introduced into the body via systemic or local injection. On the other hand, engineered macromaterials, such as drug-loaded hydrogels, medical implants, and tissue engineering scaffolds, are usually locally introduced at the site of interest. Once entering the body, the pharmacokinetics of these materials, including liberation, absorption, distribution, metabolism, and excretion processes, are determined by their intrinsic physicochemical properties. Moreover, various biological processes in the body can also control material pharmacokinetics. In vivo imaging has been used to understand various aspects of material pharmacokinetics. In this section, we review recent advances in using imaging, especially IVM, to evaluate the transport and biodistribution of materials in various organs and diseased tissues at a single-cell level.
3.1. PK of systemically introduced materials
3.1.1. EPR effects and passive deposition of materials
Passive deposition of systematically delivered materials, such as macromolecular drugs and nanomaterials, is governed in part by the enhanced permeability and retention (EPR) effect and is extensively reviewed elsewhere [204–206]. The EPR effect is a bio-transport phenomenon frequently observed in solid tumors of mice and in a fraction of patients. EPR arises when the tumor microenvironment creates a condition that allows macromolecules (e.g. antibodies) and nanomaterials, but not small molecule drugs, to accumulate and be retained inside the tumor. EPR stems in part from the pathophysiology of tumor blood and lymphatic vessels. As the tumor grows, cancer cells secrete growth factors to recruit neovasculature into the tumor tissues. These tumor blood vessels are immature and tend to be more permeable than blood vessels in the normal tissues, thus enhancing the extravasation of macromolecules and NPs into the tumor interstitium. Normally, these macromolecules and nanomaterials can exit the tumor via lymphatic drainage. However, the growth of the solid tumors leads to the collapse of tumor lymphatic vessels. Therefore, the enhanced permeability of tumor blood vessels and suppressed lymphatic drainage lead to the passive accumulation of NPs and macromolecules in tumors[207,208]. EPR is also observed at sites of inflammation and wound healing, as inflammation can also result in hyper-permeabilized blood vessels and dysfunctional lymphatic vessels[209]. Although EPR has formed the basis of superior PK of nanomaterials and large macromolecules compared to small molecule drugs, the heterogeneity of this effect — especially in patients — leads to a variable impact on nanomedicine efficacy. Companion or complementary diagnostic approaches for imaging the EPR effect, for instance based on MRI of magnetic NPs, have recently been developed to predict the tumor accumulation and, in extension, the therapeutic efficacy of subsequently administered therapeutic NPs. It is hoped that such an approach could identify patients with tumors that exhibit EPR effects favorable to NP accumulation[21].
3.1.2. Imaging PK and biodistribution of materials in tumors
Various groups have used IVM to study the PK and biodistribution of nanomaterials and their drug payloads in the tumor tissues. For instance, IVM of tumors grown in SWC was used to quantify the intratumoral PK and PD of fluorescently-labelled PLGA-PEG NPs and their cytotoxic payload[10]. The NP and drug payload were labeled with spectrally distinct fluorophores, allowing the simultaneous tracking of both components over time. Moreover, cancer cells grown in the xenograft mouse model were engineered to express a 53BP1 fluorescent reporter of DNA damage response. This approach revealed that the PLGA-PEG nanocarrier extended the blood half-life of its cytotoxic Pt drug payload, and enhanced tumoral uptake of the payload was observed in part due to factors of the EPR effect. At a single cell level, both NP and payload were found to initially accumulate inside tumor-associated macrophages after they exited the blood vessels. Longitudinal tracking of NP and payload revealed that tumor-associated macrophages served as drug depots from which DNA-damaging Pt payloads could be slowly released into the surrounding tumor cells (Fig. 7). IVM has also been used to monitor prodrug activation within the tumor, for instance using a nano-encapsulated bio-orthogonal catalyst based on palladium. IVM visualized that sequentially administered PLGA-PEG NPs carrying palladium compounds and model pro-drugs accumulated and locally activated within the tumor microenvironment, while minimizing activation at potential sites of toxicity [210,211]. Similarly, other studies have shown that implanted Pd-conjugated resin microparticles induce site-specific catalysis of model pro-drugs[57] (Fig. 8). For imaging, caged fluorophores are typically used as model “pro-drugs” that become fluorescent upon palladium-triggered activation, and such IVM data are used to guide strategies to activate therapeutically relevant pro-drugs based on doxorubicin, monomethyl auristatin E (MMAE), and other cytotoxics.
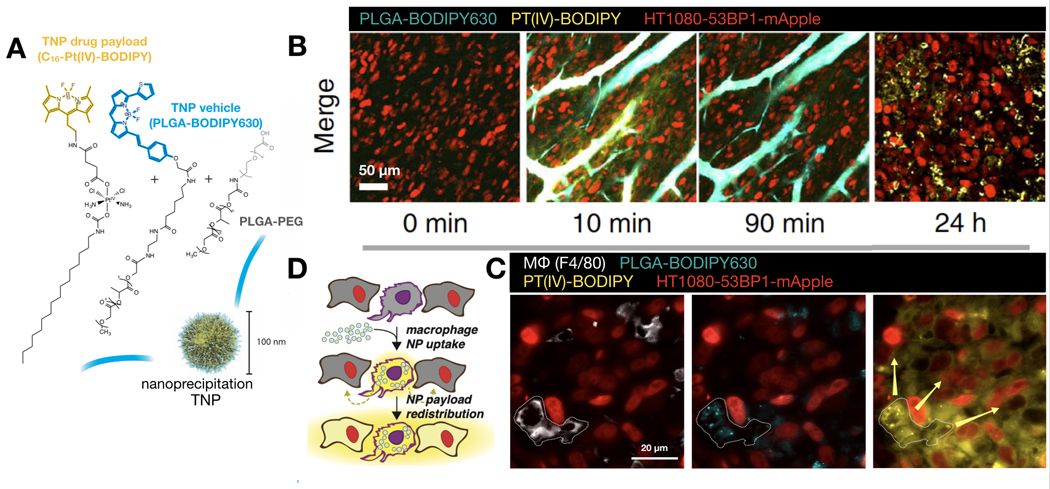
Figure 7: IVM shows tumor-associated macrophages function as slow-release drug depots for NP payloads.
(A) PLGA-PEG NPs and their Pt(IV) pro-drug payload were labeled with BODIPY630 and BODIPY fluorescent dyes, respectively. The dual-labeled therapeutic NPs were then administered to tumor-bearing mice and imaged by IVM using the SCW model. (B) Representative IVM shows the circulation and accumulation of NPs and their drug payload. (C-D) NPs accumulated at highest levels in tumor-associated macrophages (TAMs), while the pro-drug payload can be found in both TAMs and surrounding tumor cells. Adapted with permission.[10] Copyright 2015, Nature Publishing Group.
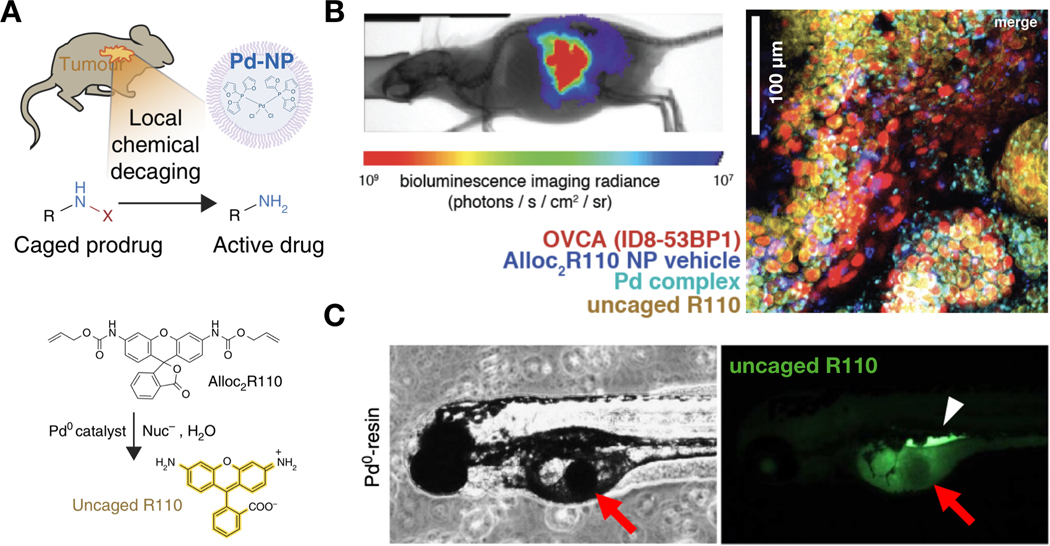
Figure 8: IVM monitors in situ bio-orthogonal activation of a model prodrug by transition metal catalysts.
(A) Schematic for using a nanoformulated palladium complex (Pd-NP) to locally convert nontoxic caged prodrugs into active cytotoxic compounds. In certain experiments, Alloc2R110 was used as a model prodrug, which becomes fluorescent upon allyl carbamate cleavage by palladium. (B) Confocal microscopy of an orthotopic model of ovarian cancer revealed co-localization of the palladium complex, and activation of Alloc2R110 to fluorescent R110. Adapted with permission.[210] Copyright 2017, Nature Publishing Group. (C) Locally implanted Pd-resin particles can perform site-specific conversion of non-fluorescent Alloc2R110 to fluorescent R110 in zebrafish. Adapted with permission.[57] Copyright 2014, Nature Publishing Group.
Besides polymeric NPs, other nanomaterials, such as liposomes[212], iron-oxide NPs[213], gold NPs[214], and carbon nanotubes[215], have been found to accumulate in monocytes, macrophages, and neutrophils in tumors by imaging. For instance, Smith et al. used IVM to monitor the PK of intravenously injected fluorescently-labeled carbon nanotubes[215]. They discovered that immediately after intravenous injection, the fluorescent nanotubes were found as discrete puncta traveling in the blood vessels. Since these puncta approximated the size of cell, the authors complemented the in vivo imaging data with flow cytometry analysis and found that carbon nanotubes were taken up by monocytes within the blood vessels. These carbon nanotubes were subsequently carried to the tumor site by active monocyte migration. They also found that functionalizing nanotubes with arginylglycylaspartic acid (RGD) ligand significantly enhanced the trafficking of nanotube-loaded monocytes to the tumors. Once reaching the tumor site and presumably after their release from monocytes, the RGD-nanotubes slowly bound to cancer cells over-expressing integrin. In a separate study, Choi et al. found that monocytes and macrophages in the tumor can phagocytose gold NPs, and high-power irradiation of these NPs can induce the apoptosis of monocytes/macrophages, as well as tumor cells surrounding them[214]. Finally, Zhao et al. used intravital FRET microscopy to image the trafficking of individual components of self-assembled lipid-Qdot NPs (SALNPs) in a SWC tumor model[216]. SALNPs comprised a Qdot core decorated with PEGylated lipid. In this study, the authors labeled the lipid with a fluorophore that functioned as a FRET pair with the Qdots. The proximity of the lipid, and hence the integrity of the SALNPs, could thus be monitored in real time by measuring the FRET signals. Of note, the authors found a significant difference in the biodistribution of the lipid and Qdot SALNP components: Qdot was predominantly found in phagocytes in the tumor tissues, while the lipid component was discovered in tumor draining lymph nodes, and later in the kidney and liver. These results suggest that the SALNPs can disintegrate in the tumor microenvironment, with both key components having different biodistribution and PK properties. Finally, in vivo imaging was used to discover that a RGD-functionalized peptide, ribonucleoprotein octamer, silicasome, and liposome can successfully deliver a variety of therapeutic payloads (i.e. siRNA and irinotecan) to cancers of pancreas, prostate, colon, and breast, respectively [217–220]
Although high-resolution imaging of antibody biodistribution has recently been demonstrated with ex vivo imaging of optically cleared tumor tissues [221] (Fig. 9A), a detailed understanding of the in vivo dynamics of monoclonal antibodies (mAb) has been lacking. IVM has allowed the evaluation of the trafficking of antibody therapies in tumors in real-time. For instance, Arlauckas et al. assessed the targeting of fluorescently-labeled anti-PD-1 to T-cells in the tumor microenvironment in vivo[14]. In vitro, these antibodies are known to bind specifically to PD-1, which is highly expressed on T cells. However, in vivo the authors discovered that most anti-PD-1 antibody administered to the tumor-bearing animals was found instead in tumor-associated macrophages, which did not express PD-1. A detailed IVM analysis showed transient binding of anti-PD-1 antibody to CD8+ T cells known to express PD1, followed by the capture of the antibody by tumor-associated macrophages within minutes (Fig. 9B-C). The authors further demonstrated that this capture was mediated by the binding of Fc𝛾 receptors on tumor-associated macrophages with Fc domain of the anti-PD1 antibody, and inhibiting this interaction enhanced the efficacy of anti-PD-1 therapy. Similarly, a recent IVM study reported that although the HER2-targeted therapeutic antibody trastuzumab can bind to HER2 expressed on cancer cells in vivo, the majority of administered antibody was found in tumor-associated phagocytes including macrophages. Over-expression of HER2 in tumor cells surrounding the macrophages could enhance the uptake of trastuzumab by these phagocytes[15] (Fig. 9D-E). In an independent study, tumor-associated macrophages have also been shown to take up a significant portion of intravenously delivered anti-CD30-vcMMAE, an antibody-drug conjugate (ADC)[222]. Taken together, these studies reveal the key roles that tumor-associated macrophages and monocytes play in determining the PK and PD of anti-tumor therapeutics, especially therapeutic NPs and antibodies. They also highlight the capability of IVM in revealing the spatial and temporal dynamics of material delivery, most importantly at a single-cell resolution. It is key to understand which cell types in the tumor microenvironment (stromal cells in addition to tumor cells) actually take up the systemically introduced materials, along with the associated molecular mechanisms underlying such uptake. The insights gleaned from IVM studies of material PK can be helpful in clarifying: 1) rate-limiting barriers to anti-tumor nanotherapies, which frequently show mixed results in clinical trials, 2) how to improve nanomaterial delivery to cancer cells, and 3) how to design the next generation of advanced materials to target different types of cells in tumor tissues.
Figure 9: High resolution imaging of monoclonal antibodies (mAbs) in tumor tissues.
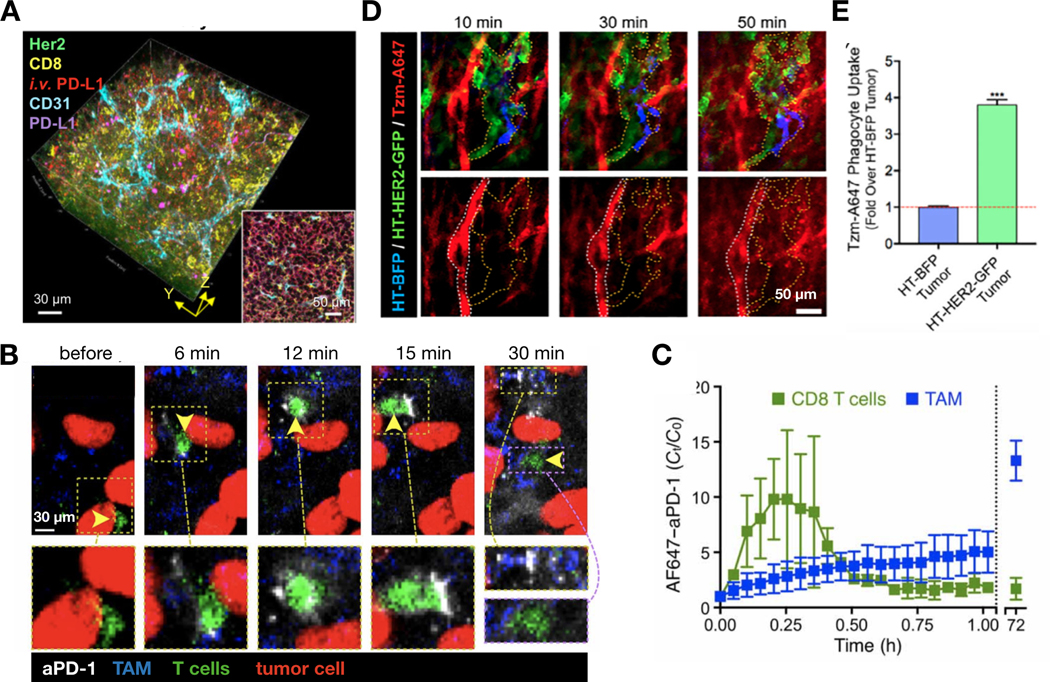
(A) Optical clearing of serially sectioned, immunostained tumor tissues can show the distribution of various molecular markers in high-resolution, as well as the ability of antibodies to reach those markers when administered in vivo. Adapted with permission.[221] Copyright 2019, American Association for Cancer Research. (B-C) IVM monitored the biodistribution of fluorescently-labeled anti-PD-1 antibody (aPD-1) in the tumor microenvironment at a single-cell level. IVM images (B) and corresponding quantification (C) showed transfer of aPD-1 antibody from T cells to macrophages over ~30 min. By 72 hrs, systemically injected aPD-1 was found in tumor associated macrophages (TAMs). Adapted with permission.[14] Copyright 2017, American Association for the Advancement of Science. (D-E) The biodistribution of fluorescently labeled, HER2-targeted trastuzumab (Tzm-A647) was evaluated with IVM in a mosaic tumor. These tumors comprised a mixture of HER2-overexpressing (HT-HER2-GFP) and HER2-low (HT-BFP) HT1080 cancer cells. IVM showed extravasation of Tzm from tumor blood vessels into interstitial space containing both tumor cell types (D). At 72 hrs, however, most Tzm was found in tumor-associated phagocytes. The over-expression of HER2 in surrounding cancer cells enhanced the phagocytic uptake of Tzm (E). Adapted with permission.[15] Copyright 2019, International Society for Advancement of Cytometry.
3.1.3. Imaging PK and biodistribution of materials in inflamed tissues
Inflammation is a natural process that occurs at sites of injury or infection. During inflammation, the blood vessels near the injured site can become dilated and hyper-permeable, thereby facilitating influx of immune cells and wound healing factors. This altered blood vessel physiology can result in EPR at the inflamed site. Nanomaterials have been designed to exploit the EPR effects to enhance the delivery of drugs to the injured or infected site. Some groups have used IVM to observe the uptake of these nanomaterials by cells that participate in inflammation process, especially neutrophils. Neutrophils are typically the first immune responders at the site of injury or infection, they are major producers of factors such as TNFα that are responsible for subsequent inflammatory responses, and they have been extensively studied for their interaction with nanomaterials. For example, Chu et al. used IVM to investigate the PK of fluorescently-labeled NPs made from denatured bovine serum albumin in mouse models of acute inflammation[223]. Immediately after intravenous injection, these albumin NPs were taken up by activated neutrophils that resided in the blood vessels at the injured site. The activated neutrophils subsequently carried the albumin NPs across the blood vessels and migrated toward the inflamed tissues. These results thus suggest that neutrophils can enable targeted delivery of NPs to inflamed tissue by active cellular transport. The authors were able to enhance the delivery of anti-inflammatory drug TPCA-1 and antibiotic cefoperazone acid (Cefo-A) to injured and inflamed sites, respectively, by encapsulating these drugs in albumin NPs. In another example, Gao et al. manufactured NPs using the cell membrane of neutrophils. IVM was able to show the adhesion of these NPs to inflamed vasculatures due to the interaction between β2 integrin on the neutrophil membrane and ICAM-1 on the inflamed vessels[224].
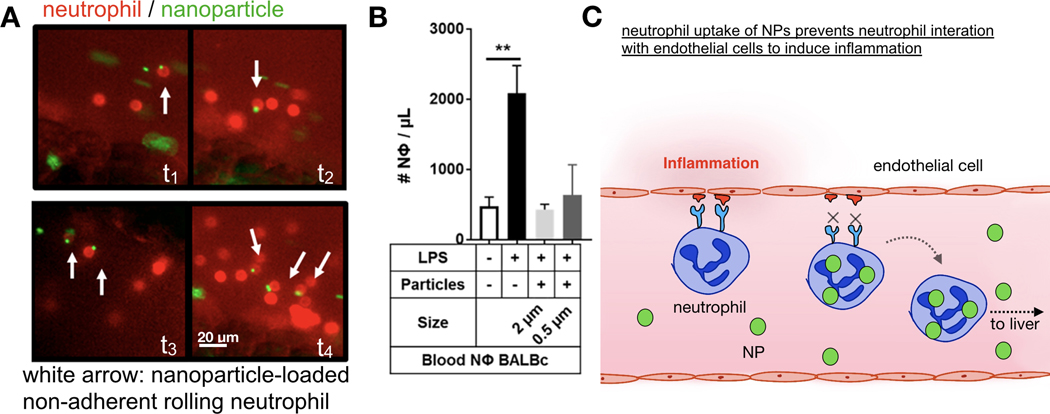
The interaction between nanomaterials and neutrophils can also have direct consequence on neutrophil function. For instance, Fromen et al. found that the uptake of carboxylate-modified polystyrene particles by neutrophils can reduce their binding to inflamed endothelium[225], as monitored by IVM of neutrophils rolling on inflamed mesentery vascular walls. Subsequent experiments demonstrated that this suppressed endothelial binding is the consequence of NP-mediated homing of neutrophils to liver, presumably since neutrophils are tasked with clearing these particles from the circulation (Fig. 10). In another study, Wang et al. showed that albumin NPs can selectively deliver piceatannol, a kinase that blocks β2 integrin, to neutrophils in the bloodstream[226]. The inhibition of β2 integrin in these NP-loaded neutrophils resulted in detachment of the cells from inflamed endothelium. The authors used this strategy to reduce the number of neutrophils at inflamed tissues, thus alleviating inflammation. Finally, many different types of biologics have been developed to treat inflammatory diseases, and some of these biologics have already been approved by the FDA for clinical uses. These include: anti-TNFα monoclonal antibody (Adalimumab, marketed as Humira), recombinant IL-1R antagonist (Anakinra, marketed as Kineret), PEGylated Fab fragment of anti-TNFα antibody (certolizumab, marketed as Cimzia), and anti-α4 integrin antibody (natalizumab, marketed as Tysabri)[227]. IVM can be useful in understanding the PK of these biologics in the body, and the above highlighted findings in oncology and with nanomaterials suggest potential opportunity in applying similar IVM approaches to anti-inflammatory biologics as well. In the future, as more transgenic fluorescent reporter mouse models become widely available, researchers can use IVM to investigate how different subsets of immune cells contribute to material delivery to the inflamed tissues. In addition, the developments of fluorescent sensors of pH[228,229] and mechanical force[230,231] can enable IVM studies of how physical properties of inflamed tissues affect material delivery and drug distribution.
Figure 10: IVM aids in understanding NP PK in inflamed tissues.
(A-B) IVM was used to track the rolling motion of NP (green)-loaded neutrophils (red) on blood vessels in LPS-treated mice. IVM quantification shows that the uptake of polymeric NPs reduced the adhesion of neutrophils on the LPS-treated inflamed blood vessels. (C) These data and further experiments suggested that the uptake of polymeric NPs can redirect neutrophils in the blood to the clearance organs, thus reducing inflammation by preventing their binding to the inflamed sites. Adapted with permission.[225] Copyright 2017, American Chemical Society.
3.1.4. Imaging PK and biodistribution of materials in brain
The blood-brain barrier (BBB) is a significant obstacle to drug delivery, and engineered nanomaterials represent a promising strategy to overcome this barrier[232,233]. Indeed, numerous IVM studies have been performed to evaluate the ability of nanomaterials to extravasate from brain endothelium and accumulate in the brain tissue[234]. For example, Lam et al. recently described a transferrin-functionalized PEGylated liposome (Tf-NP) as a delivery vehicle for glioblastoma treatment using temozolomide and a bromodomain inhibitor [235]. With IVM and the mouse cranial window model, the authors were able to show that Tf-NP can efficiently pass through the BBB, in part due to the high expression level of transferrin receptor on the brain capillary endothelial cells, and accumulate in the brain tumor (Fig. 11A). This enhanced accumulation translated to improvement in therapeutic efficacy. Transferrin-functionalized liposomes have also been used to deliver α-mangostin [236] and plasmid DNA[237] to the brain for Alzheimer's treatment and neuronal gene therapy, respectively. More recently, studies have shown that decorating NPs with angiopep-2 can assist their transmigration through the BBB. Angiopep-2 is a bifunctional peptide that can be transcytosed through the brain endothelial cells by binding to LDL related protein-1. Li et al. and Gao et al. have shown that polymeric NPs functionalized with angiopep-2 can penetrate the BBB and accumulate in brain striatum and cortex[238,239]. Yet another strategy to improve BBB extravasation is by coating the nanomaterials with polysorbate 80. Koffie et al. have previously demonstrated, using IVM, that poly(n-butyl cyanoacrylate) dextran NPs coated with polysorbate 80 can carry BBB-impermeable imaging agents through the brain endothelium[240]. They then utilized these NPs for whole brain MRI and the detection of amyloid plaque. A subsequent report later revealed that polysorbate coating allows for the adsorption of apolipoproteins on the NPs, which enhances the binding of NPs to the BBB by directly interacting with scavenger receptors and LDL expressed on the brain endothelium. Indeed, biologics engineered by fusing scavenger receptor-binding domain of apolipoproteins with lysosomal enzymes can enhance the delivery of these enzymes across the BBB[241]. These in vivo imaging studies thus demonstrate the utility of targeting receptors on the brain endothelium in improving the penetration of materials through BBB.
Figure 11: IVM quantifies material transport across the blood brain barrier.
(A) IVM has demonstrated that functionalizing liposomes with transferrin, which binds efficiently to transferrin receptors that are highly expressed on the brain endothelium, improved the extravasation of liposomes from the microvessels in brain (white arrow, left). Transferrin-functionalized liposomes can more efficiently deliver chemotherapeutics to brain tumors, resulting in an improved efficacy compared to non-targeting liposomal or solvent-based formulations (right). Adapted with permission.[235] Copyright 2018, Nature Publishing Group. (B) Imaging of the brain microvasculature showed that non-targeting polymeric NPs cannot extravasate from intact and healthy BBB. Adapted with permission.[243] Copyright 2015, Elsevier. (C) Diagram summarizing the superior PK and biodistribution of BBB-targeting NPs compared to non-targeting NPs.
Not all targeted materials can pass through the BBB. Medina et al. found that surface conjugation of PLGA NP with TAT peptide, while enhancing the targeting of NPs to the brain, did not allow the crossing of the particles through intact BBB[242]. In fact, high resolution imaging of the mouse brain showed that these particles were trapped inside the blood vessels (Fig. 11B-C). However, the authors were able to observe the diffusion of the particles into the central nervous system through regions of elevated BBB permeability in a mouse brain tumor model. BBB leakiness is frequently observed in patients with traumatic brain injury, stroke, and brain tumors. NPs have been used to exploit this BBB disruption for targeted delivery of drugs into the diseased brain tissues. For instance, nano-encapsulated copper/zinc superoxide dismutase (SOD1)[243] and brain-derived neurotrophic factor (BDNF)[244] were developed to mitigate the effects of ischemia on brain tissues following stroke. These nanomaterials were shown to accumulate at the site of brain damage due to the elevated BBB permeability. Similar results were observed with anti-VEGF antibody bevacizumab in a cranial WC model. Specifically, the authors discovered that in intact brain vessels with normal BBB functionality, bevacizumab was mostly trapped inside the vessels. However, mannitol-induced osmotic imbalance in the brain vessels led to BBB opening and diffusion of bevacizumab into the brain tissues[245]. NPs can also co-opt monocytes in the blood for the delivery of drugs across the BBB. As an example, liposomes carrying serotonin can be phagocytosed by monocytes, which then transmigrate across the BBB[246]. This liposome thus allows serotonin, which is normally BBB impermeable, to be delivered to the brain. Lastly, nanobodies have been shown, via imaging studies, to diffuse across the BBB due to their small size[247].
Nanomaterials derived from biological sources can also be used to deliver therapies across the BBB. IVM studies have shown that intravenously injected extracellular vesicles, which are lipid-bilayered NPs naturally released from cells, can home to brain vasculatures and cross the BBB[248,249]. Extracellular vesicles have previously been used to encapsulate small molecule drugs and nucleic acids[250]. IVM has also been used to study the molecules responsible for parasite transmigration across the BBB, and the targets identified in these studies could be conjugated to NPs to enhance their delivery into brain[251]. NPs fabricated from plant viruses including a potato virus have been designed, in part as adjuvants for oncology applications, and Shukla et al. used IVM to track the PK of fluorescently-labeled viral NPs in the brain of a mouse over the period of 3 wks. They found that repeated intravenous injection of viral NPs led to widely different behaviors after each injection. Most importantly, the NPs were uniformly distributed in the brain microvessels after first injection, while the NPs tended to aggregate after the second injection (Fig. 12). The authors attributed this phenomenon to the production of host antibody after the first injection, which led to the antibody-assisted clearance of the NPs upon second injection[252]. Finally, various techniques have been developed to image blood flow and neuromuscular coupling inside the brain[253]. Fluorescently-labeled Tobacco mosaic virus was recently used to monitor the blood flow inside the brain using two-photon microscopy[254]. More recently, a label-free functional photoacoustic IVM method was described to record the fast-changing fluid dynamic and oxygenation conditions in brain vasculatures[79]. Combined, these novel IVM techniques can thus aid in the evaluation of PK and biodistribution of materials targeting brain. Currently, the majority of PK studies for brain-targeting materials are focused on transport through the BBB, which is the largest barrier to drug delivery in the brain. Yet, multiple processes ultimately govern material delivery to targets within the brain, including diffusion through the brain parenchyma and penetration into the target cells. IVM, combined with a variety of cranial window chamber models, can help researchers gain a detailed understanding of how brain-targeting materials navigate each of these multi-step processes.
Figure 12: IVM reveals the impact of material immunogenicity on pharmacokinetics following repeated administration.
(A) Viral NPs were made from fluorescently labeled potato virus X (PVX-A647), visualized by transmission electron microscopy. (B-C) Viral NPs were injected intravenously into a mouse on a weekly schedule (B), and their blood circulation kinetics were observed in the brain microvasculature through a cranial window (C). (D) Representative IVM showing the behaviors of viral NPs in the brain vessels after each injection. The viral NPs were uniformly distributed into the blood vessels after the first injection. Aggregation of the NPs were observed after the second injection, in part due to adaptive immune recognition mediated by IgG and IgM. The third injection resulted in less particle aggregation, presumably due to the partial resolution of PVX immunogenicity. Adapted with permission.[252] Copyright 2016, American Chemical Society.
3.1.5. Imaging PK and biodistribution of materials in clearance organs
Besides accumulating in the targeted tissues, a large portion of materials can also be found in clearance organs such as the liver, kidney, and spleen. Typically, materials have been designed to maximize the biodistribution in target organs, while minimizing clearance organ uptake. Hence, understanding molecular and cellular mechanisms of clearance is crucial for material design. IVM has been used to track nanomaterial localization in kidney, liver, and spleen at single cell-level in real-time. For example, Jones et al. used IVM techniques to quantify the clearance of polymeric NPs from blood as well as the accumulation of these particles in various organs[255]. They were specifically interested in how NP clearance varies between mice of different strains and immune states. They found that a mouse strain naturally biased toward Th2 immune responses (i.e. BALB/c) cleared NPs faster than a strain biased toward Th1 responses (i.e. C57BL/6). Although a large amount of NPs were found in the liver and spleen of both strains, subtle differences were observed among the cellular distribution of NPs. IVM combined with flow cytometry analysis showed that within the spleen, dendritic cells, monocytes, neutrophils, and macrophages took up most particles, while uptake in T cells and B cells was minimal in both strains. However, monocyte uptake was higher in Th1 mice, and uptake in dendritic cells and neutrophils was higher in Th2 mice. Subsequent mechanistic studies revealed that subtle differences in immune cell phenotypes contributed to the difference in NP uptake. In a separate IVM study, Kai et al. found that the presence of the tumor can change the systematic clearance of hydrogel NPs by enhancing the ability of tissue-resident macrophages in the liver and spleen to take up nanomaterials[256]. These data clearly highlight the context-dependent nature of NP clearance.
Nanoparticle and antibody accumulation in the liver have been studied with different in vivo imaging modalities such as fluorescence reflectance imaging (FRI) [257], PET[258,259], and IVM[260], with the latter being instrumental in understanding the biodistribution at a single-cell level. For instance, Press et al. used IVM to evaluate the hepatic uptake of polymethine dye-coated PLGA NPs carrying short interference RNA payload[260]. The authors believe that polymethine dye, which binds to hepatic parenchymal uptake transporter, can aid in the selective uptake of NP by hepatocytes. Indeed, polymethine dye-coated NPs were found to accumulate mostly in hepatocytes, while the bare NPs were taken up mostly by Kupffer cells (liver-resident macrophages). The metabolic fate of the NPs was also tracked by IVM. The polymeric compound and RNAi cargo of the NP were found to be retained in the cytoplasm, while the polymethine dyes were excreted into the bile duct. In another study, IVM was used to show the preferential accumulation of cationic and anionic mesoporous silica NPs in hepatocytes and Kupffer cells, respectively[261]. These studies highlighted a strategy to manipulate cellular biodistribution of NPs via altering surface coating and charge of the nanomaterials. Single-cell hepatic distributions of PMMA NPs[262], liposomes[263], and silicone NPs[264] were studied by IVM. In particular, van de Ven et al. have used data obtained from IVM to construct computational models of NP biodistribution in an effort to minimize the hepatic uptake and maximize the tumor accumulation[249]. Indeed, research has shown that one strategy to increase tumor accumulation of NPs is to therapeutically remove Kupffer cells in the liver[265], and the phenotype of Kupffer cells can influence their NP uptake[266]. Furthermore, in vivo imaging was used to assess the targeting of steroid-tagged siRNA[267] and immunomodulatory polymeric nanoparticles[268] to liver.
Renal excretion is another major route of NP elimination, and in vivo imaging has been used to observe the accumulation of NPs in kidney. William et al. used IVM and the Cx3cr1GFP/+ transgenic mouse, which bears GFP+ macrophages, to study the uptake of a PLGA-PEG mesoscale (~300 nm) NPs in renal tissues[269]. They found that these mesoscale NPs preferentially accumulated in kidney compared to other organs. A closer examination revealed that these NPs were taken up by renal proximal tubular epithelial cells, not GFP-expressing phagocytes. Naumenko et al., on the other hand, used IVM to track the movement of intravenously injected PEGylated iron oxide NPs in kidney in real-time[270]. High-resolution time-lapse movies showed that NPs moved from renal peritubular capillaries to lumen for excretion by passing through tubular epithelial cells. Moreover, DNA-binding protein from starved cells, a naturally occurring nanostructure, can pass through glomerular barrier and accumulate in renal proximal tubules[271]. Another report has shown that repeated dosing of mineral-organic NPs can lead to the formation of kidney stones[272]. As a final example, IVM has revealed that angiotensin can promote the uptake of albumin by podocytes, cells that are important for the filtering of blood in the kidney[273]. Taken together, these studies clearly demonstrate the power of IVM in identifying physiologic and cellular-level mechanisms of material clearance. The scientific understanding gained from these IVM studies can potentially aid in the rational design of advanced materials to minimize their systemic clearance and extend their circulation half-lives.
3.1.6. Imaging PK and biodistribution of materials in the heart and lung
Whole-body in vivo imaging techniques, such as PET, MRI, SPECT/CT and FRI, have routinely been utilized to assess the PK of NPs targeting heart and lung [274–279]. On the other hand, the use of IVM to study the heart and lung is rarer, largely due to technical difficulties in preparing image setups that allow for long-term and stabilized observation. Recent advances in tissue stabilization and imaging synchronization techniques, as discussed earlier, have made IVM of heart and lung possible. As such, multiple groups have used IVM to determine the biodistribution of nanomaterials in heart and lung tissues at a single-cell resolution. For instance, Keliher et al. used IVM to observe the cellular uptake of polyglucose NPs in Cx3cr1GFP/+ reporter mice. They found that these NPs preferentially accumulated inside GFP+ cardiac macrophages, and myocardial infarction, which enriches macrophage contents in the heart, can significantly enhance the overall accumulation of these NPs in the infarcted tissues[33]. Two separate IVM studies have also demonstrated selected accumulation of dextran NPs and liposomal NPs in the infarcted heart[280,281]. These studies thus highlight the potential of using therapeutic nanomaterials to target infarcted tissues for the treatment of heart diseases. More recently, Ahmed et al. used IVM to show that cyclodextrin NPs accumulated in cardiac macrophages residing inside the atrioventricular node of the heart. Based on this observation, these nanocarriers were used to deliver amiodarone anti-arrhythmic therapy more efficiently to heart[282]. Not all NPs are taken up by macrophages. Miragoli et al. recently used IVM to discover that calcium phosphate NPs inhaled through lung were phagocytosed by cardiomyocytes rather than macrophages. Surprisingly, they also found that inhaled NPs reached cardiomyocytes faster than injected NPs[283]. Finally, IVM has been used to track the movement of inhaled nanoparticulates from the alveoli to the blood stream[284–286]. In particular, Detampel et al. found that silica NPs can form aggregates on the alveolar wall, resulting in the trapping of the NPs in the alveoli. Time-lapse imaging showed that a large portion of these aggregates were removed from the alveoli in 30 mins and found to accumulate subsequently inside the clearance organs. Subsequent TEM imaging revealed that these aggregates can naturally cross through the alveolar epithelium into the bloodstream[284]. In summary, these IVM studies clearly demonstrated that different routes of administration contribute to differential NP distribution at a single-cell level. Future IVM studies will continue to reveal the biological principles behind this differential distribution, and allow researchers to ascertain the optimal routes of administration for different types of NPs.
3.1.7. The effects of physical properties of nanomaterials on PK and biodistribution
NPs can be designed to have various sizes, shapes, charges, and compositions. The impacts of these physical properties on the PK and delivery of nanomaterials have been extensively studied[287–289], and many of these studies were carried out with IVM. Cabral et al., for instance, have compared the accumulation and penetration of fluorescently-labeled polymeric spherical NPs of different sizes, ranging from 30 nm to 100 nm in diameter, in tumors[290]. To capture high-resolution time-lapse movies of NP movement in an efficient manner, the authors used a confocal laser scanning microscope modified with a high-speed resonance scanner. They found that NPs of all sizes can penetrate CT26 colon tumor with ease, as this type of tumor has relatively sparse ECM that is considered quite permeable to macromolecules. In contrast, only particles with the size of 30 nm were able to penetrate into BxPC3 pancreatic tumors, due to the dense ECM in the tumor stroma. In these tumors, 70 nm particles tended to stay in or near the blood vessels. The authors further discovered that treating the tumor tissues with inhibitor targeting TGF-β1 could enhance the accumulation of 70 nm NPs in the pancreatic tumors, presumably via altering the tumor stromal properties. Another study showed that 100 nm gold NPs tend to accumulate at the tumor periphery, while 50 nm gold NPs can penetrate deep into tumor core[291]. Tumor penetration is a function of both extravasation efficiency and circulation half-life, since longer circulating materials have more time to extravasate. However, the relationship between particle size and clearance rate is not always straightforward. For instance, a recent report found that 15 nm gold NPs have longer blood circulation time compared to that of 150 nm gold particles, since the larger particles tend to rapidly accumulate in liver and spleen[292]. In contrast, IVM was used to compare the extravasation of PEG-PDLA (poly-D-lactide) polymeric micelles (~60 nm) to that of PEG-PDLA nanodrops (~230 nm)[293]. The polymer micelle was shown to have faster extravasation and shorter blood half-life when compared to nanodrop. The route of metabolism and excretion can also vary between NPs of different sizes. For instance, a study performed with functional MRI has revealed that small NPs (~5 nm) tend to be metabolized in kidney, while larger NPs (~10–20 nm) tend to be metabolized in liver and spleen [294]. Taken in sum, data obtained from in vivo imaging often suggest that smaller NPs generally have superior PK and tumoral penetration compared to larger ones.
The effects of particle geometry on PK and biodistribution of nanomaterials are harder to generalize. Smith et al. used IVM to compare the extravasation of high-aspect ratio carbon nanotubes and spherical NPs from blood vessels in different types of subcutaneously implanted tumors[12]. They were able to show that in an SKOV3 ovarian cancer xenograft, both types of nanomaterial failed to extravasate. However, spherical NPs were found to have enhanced ability to extravasate in LS174T colon tumor when compared to nanotubes. In contrast, nanotubes extravasated much more than spherical NPs in U87MG brain tumors. The authors were able to partially explain the observed phenomenon by demonstrating that nanotubes can diffuse faster through a 100 nm pore, which mimics the size of the vascular pores found in U87MG tumors, compared to spherical NPs. Similarly, another group has shown, using a subcutaneous HT1080 tumor model, that the elongated nano-worm has higher passive tumor-targeting ability than nano-spheres[295]. The ability of nano-rods to penetrate the tumor tissues was found to increase with size, while the opposite was true for nano-spheres[296]. These imaging data thus suggest that both the geometry of nanomaterials and the property of tumor microenvironment can play complex roles in determining their delivery.
The geometry of the nanomaterials can also affect clearance and circulation life-time. It is generally believed that, in contrast to spherical particles, non-spherical NPs tend to tumble and roll near the vascular wall as they travel through blood vessels. This characteristics of non-spherical NPs can thus enhance their interaction with the endothelium, which is thought to delay their liver or renal clearance[297]. Indeed, Zhao et al. recently demonstrated that nano-rods with high aspect ratio have longer blood circulation and lower renal clearance compared to nano-spheres or nano-rods with low aspect ratio[298]. Christian et al. and Geng et al. have also previously shown that filament-like nanostructures made from block copolymer can stay in the circulation for a week, while spherical nanostructures are cleared within a day[299,300]. Compared to taxane-loaded spherical nanostructures, taxane-loaded filament nanostructures demonstrated higher tumor uptake and lower toxicity in mice. This higher uptake is thought to be due to the enhanced interaction of non-spherical NPs with tumor vasculature, which increases the possibility of extravasation from permeable tumor blood vessels. Particle shape can also affect the endocytic uptake of nanomaterials by tumor cells and macrophages. Decuzzi et al. used a computational model to predict that particles with high aspect ratio have greater propensity to be internalized by cells compared to spherical particles[301]. This computational prediction was later verified by experimental data, which showed that the rod-shape design enhanced the specificity of HER2-targeting NPs[302].
The charge of nanomaterials can also impact their PK and biodistribution. In general, positively charged NPs tend to be taken up by non-phagocytic cells, while negatively charged NPs are taken up by phagocytic cells[303]. Due to their ability to disrupt cell membrane, positively charged NPs are considered more toxic than negatively charged particles[304]. This inherent toxicity of cationic NPs can be utilized for cancer treatment. For instance, using IVM, Wang et al. have shown that cationic PEG-b-PLA NPs are more efficacious in inhibiting tumor growth than anionic and neutral NPs, even though cationic NPs have inferior PK[305]. Han et al. later showed that positively charged NPs can penetrate deeper in early-stage tumors, while negatively charged particles can penetrate further in late-stage tumors[306]. This intriguing result suggests that the effects of charge on nanomaterial performance are context-dependent. Finally, the surface coating of the nanomaterials can also affect their PK. For instance, coating NPs with serum can enhance the uptake of the particles by macrophages[307]. In summary, IVM studies have revealed that the physical properties of nanomaterials can profoundly impact their PK. Yet, the relationship between these physical properties and material PK is complex and context-dependent. Indeed, an overarching principle that governs how nanomaterial size, shape, and charge affect delivery is currently unknown. Additional IVM studies that systematically profile the effects of these physical properties on NP PK can potentially uncover design principles to optimize nanomaterial delivery.
3.1.8. Priming strategies to enhance material deposition
The biodistribution and penetration of nanomaterials and biologics are critically dependent on microenvironmental factors at the target site of action. These inter-related factors include: 1) degree of vascularization, 2) permeability of the blood vessels, 3) the dynamics of interstitial fluid flow and pressure, 4) the density and composition of ECM, and 5) phagocyte accumulation [207,308,309]. Higher degree of vascularization and vessel permeability generally enhance the overall accumulation of NPs to their target organ. However, highly permeable blood vessels can also lead to a buildup of interstitial fluid pressure, reducing the diffusion of large NPs. Moreover, elevated interstitial flow, as the result of high interstitial fluid pressure, can flush small NPs and biologics out of the target organ. Dense ECM, on the other hand, decreases the diffusion of NPs and biologics via steric hinderance. The complexity of the NP and macromolecule biodistribution is evident by the spatial heterogeneity of their accumulation in tumors, which often reduces their anti-tumor efficacy. Although EPR effects are responsible for the improved overall uptake of NPs and biologics in the entire tumor, these materials often only reach the periphery, presumably due to the elevated interstitial pressure and ECM density that hinder penetration. These issues can be compounded by molecular targeting. Systemically administered antibodies are often found to preferentially bind to antigens near the tumor blood vessels or at tumor boundary, a phenomenon known as “binding site barrier”. This barrier, which is thought to primarily be the result of antigen binding near the tumor vessels, and therefore impeded transport of free antibody, severely limits the penetration of antibody into the tumor core[310]. A strategy to overcome this barrier, which is based on saturating the antigens around the tumor vessels, has recently been developed to improve the penetration of ado-trastuzumab emtansine[311]. Lastly, otherwise identical NPs or biologics can have distinct biodistribution in different types of tumors due to the difference in the microenvironment. In fact, certain types of tumors, such as pancreatic tumors, have microenvironment that is so unfavorable to drug delivery (lack of blood vessels and ultra-dense ECM) that even small molecule therapeutics fail to accumulate [312]. Therefore, strategies have been devised to improve the biodistribution and penetration of nanomaterials and biologics by altering the microenvironmental factors at the target organ[200]. These strategies have been evaluated with IVM, as it represents a unique tool to study the complexity of material distribution and penetration in a physiologically relevant context.
Altering blood vessel permeability and physiology
One of the most common methods to improve material deposition at the target organ is by enhancing the permeability of the blood vessels. This can be achieved via biochemical (growth factor and drug treatment) or biophysical (ultrasound treatment) means. For instance, Qiao et al. have reported, using SPECT/CT imaging, that pro-inflammatory cytokine TNFα, which is a known enhancer of vessel permeability, can drastically increase the tumoral uptake of radio-labeled liposome in a mouse model of glioma[313]. They also found that bacteria infection can enhance the uptake of liposomes, presumably by inducing an inflamed reaction and enhancing vessel permeability at the tumor site. Moreover, IVM was used to show that the inhibition of TGFβ1 receptor (Alk5) increased the permeability of tumor blood vessels to the iron oxide NP ferumoxytol[314]. On the other hand, systemic administration of IL-1β has been shown to enhance the transport of small molecules, but not liposomes, across healthy BBB in mice. However, IL-1β did enhance the permeability of tumor-associated BBB to liposomes[315]. More recently, Anraku et al. have shown, using IVM, an improved transmigration of glucose-decorated NPs through BBB following glycemic control[316] (Fig. 13A-B). They proposed that by fasting the animal and then administering a bolus of glucose, brain endothelial cells could be tricked to enhance the transmigration of glucose transporter-1, which can bind to glucose-conjugated NPs, from the luminal to abluminal membranes. IVM studies have also demonstrated that focused ultrasound can increase the permeability of BBB[317] or tumor blood vessels[318] to antibody-drug conjugates (ado-trastuzumab emtansine), liposomes, or polymeric NPs (Fig. 13C-D). This focused ultrasound treatment has advantage over biochemical treatment, as it can induce site-specific increase in permeability to enhance NP targeting. Finally, mild hyperthermia can also enhance vessel perfusion and permeability[319,320]. Specifically, Li et al. used IVM to show that site-specific heat treatment (42°C) can improve the diffusion of doxorubicin-loaded thermosensitive liposomes from the tumor blood vessels[321]. The heat treatment not only improved endothelial permeability, but also payload release from the liposomes. In this study, IVM was performed in sarcoma grown in a dorsal SWC, and the tumor-specific heating was accomplished by placing the WC directly over a heating coil. In another study, Bagley et al. showed that endothelial cells can develop tolerance to this thermo-therapy, making repeated round of this thermo-treatment less efficient in improving NP transport [322].
Figure 13: Microscopy monitors the delivery of nanomedicines and drugs enhanced by therapeutic manipulation of vascular permeability.
(A-B) IVM revealed that fasting followed by a systemic introduction of glucose can enhance the permeability of the blood brain barrier (BBB) to glucose-decorated NPs. Representative fluorescent IVM time-lapse imaging (A) showed efficient transport of fluorescent glucose-conjugated polymeric NPs from brain microvasculature following this glycemic control protocol. Quantification of fluorescent signals in brain parenchyma (white box) showed accumulation of glucose-decorated NPs, which corresponded to the increase in blood glucose level (B). Adapted with permission.[316] Copyright 2017, Nature Publishing Group. (C-D) IVM revealed focused high intensity ultrasound can enhance the permeability of blood vessels to drugs in brain tumors. Representative IVM time-lapse images (C) and quantification (D) showed ultrasound enhanced the accumulation of doxorubicin payload in the brain tumor tissues. Adapted with permission.[317] Copyright 2018, National Academy of Sciences.
Increasing the permeability of blood vessels can enhance the extravasation of materials; however, as discussed earlier, it may reduce the ability of nanomaterials and biologics to diffuse through the target tissues due to the increase in interstitial fluid pressure (IFP). To address this issue, elevated fluid pressure in the tumors may be potentially reduced by changing the phenotypes of tumor blood vessels so they are more similar to blood vessels in the normal tissues[323,324]. This vessel normalization can be accomplished by enhancing the maturity and pericyte coverage of tumor blood vessels[200]. Various therapeutic agents have been utilized for vessel normalization, and their ability to enhance NP penetration has been evaluated with IVM. For instance, Chauhan et al. have shown that pre-treatment of tumors with anti-VEGFR2 antibody DC101 enhanced the delivery of small NPs (size=12 nm) while decreased the delivery of large NPs (size=125 nm)[325]. Inhibiting VEGFR2 is known to normalize tumor blood vessels, as well as decrease IFP, by reducing the formation of fenestration on endothelium and preventing the leaking of blood plasma into the interstitial space[200]. The recruitment of the pericytes to the tumor blood vessels as the result of VEGFR2 inhibition also improves the barrier function of these vessels. Using IVM data and computational modeling, Chauhan et al. demonstrated that the increase in small particle penetration is due to decreased IFP, which results in the positive differential between microvascular pressure and interstitial fluid pressure that allows the effective convection of small particles from blood vessels into interstitial space. The decrease in large particle uptake is the result of reduced endothelial fenestration. Jiang et al. reported similar observation in a more recent publication. They also observed an enhanced diffusion and uniform distribution of small NPs as the result of DC101 therapy[326]. Furthermore, inhibiting VEGFR using selective tyrosine kinase inhibitor cediranib can also improve the delivery of NPs[327]. Of note, the beneficial effects of anti-VEGFR2 antibody DC101 to NP penetration are dose dependent, as excessive inhibition of VEGFR potentially closes the endothelium off to NP extravasation[328]. Therefore, the ability of vessel normalization to improve NP delivery depends on the properties of both the tumor microenvironment and NPs. Finally, the power of vessel normalization to enhance therapeutic delivery is not limited to nanomedicines. Indeed, various imaging studies have shown that vessel normalization brought about by VEGF inhibition can improve (or inhibit, depending on dose) the delivery of antibodies[329], oncolytic virus[330], bovine serum albumin [331], and fluorodeoxyglucose[324].
Altering macrophage physiology
Various IVM studies have shown that altering the content and physiology of phagocytes is a promising strategy to enhance nanomaterial deposition in the target organs. For instance, whole-organ tissue clearing and confocal imaging has been used to systematically compare macrophage accumulation and infiltration in tumors treated with different types of neoadjuvant therapies[34]. In this example, it was found that oxaliplatin/cyclophosphamide and radiotherapy, unlike paclitaxel/carboplatin, significantly enhanced the amount of tumor-associated macrophages (TAMs) in the lung tumor tissues. This enhanced accumulation of macrophages translated to increases in the uptake of PLGA-PEG NPs in treated tumors. A closer examination of the images also revealed that neoadjuvant treatment enhanced the infiltration of TAMs into the tumor mass, which led to an improved penetration of PLGA-PEG NPs into the tumor core. Similarly, a prior publication also showed that inhibiting CSF-1R, rather than decreasing TAM recruitment into tumor, enhanced the infiltration of TAMs into tumor core[124]. These studies thus demonstrate that modulating the content and localization of macrophages can improve the delivery and biodistribution of nanomaterials.
Besides changing the amount of macrophages, tumor irradiation can also alter the interaction between TAMs and tumor vasculature. IVM has previously indicated that TAMs over-expressing Tie2 are often found proximal to tumor endothelium. These Tie2-high TAMs produce VEGFA, which can lead to transient opening of vascular junctions and increased vascular permeability[70]. Utilizing high-resolution time-lapse IVM, Matsumoto et al. and Miller et al. have independently shown that transient increases in vascular permeability can result in a burst of NP extravasation from the tumor blood vessels[212,332]. Particularly, Matsumoto et al. demonstrated that this vascular burst is more important for the delivery of 70 nm NPs compared to 30 nm NPs. Miller et al. discovered that local tumor irradiation enriched the amount of perivascular TAMs, thus leading to increased vascular bursting and nanomaterial delivery [212]. Depleting perivascular TAMs abrogated radiation-enhanced NP accumulation and efficacy in that study [212]. Finally, a more recent report demonstrated that neutrophil extravasation can enhance liposome delivery by reducing the vessel barrier to diffusion[333]. These results thus demonstrate that NP deposition can be altered by modulating the vasculature-macrophage/neutrophil interface in the target tissues.
Altering ECM density and composition
Since ECM can sterically hinder NP transport, modifying ECM density and composition has been proposed as a viable strategy for enhancing NP deposition. IVM can aid in the evaluation of this strategy, as SHG imaging enables high-resolution real-time assessment of the structure and density of collagen matrix in the tissues. For example, Diop-Firmpong et al. and Chauhan et al. used SHG imaging to demonstrate that the dense collagen network in the tumor can decrease the penetration of NPs[13,334]. They also found that the expression of hyaluronan by stromal cells can compress tumor vessels, thus further hindering NP delivery. To address this issue, Liu et al. treated an orthotopic breast tumor with a TGFβ neutralizing antibody[335]. They discovered that TGFβ blockade reduced the production of collagen matrix by the tumor-associated fibroblasts, leading to an enhanced delivery of intravenously dosed PEGylated liposomal doxorubicin (Doxil). Similarly, losartan, a clinically approved angiotensin II inhibitor, has been shown by IVM to reduce tumor fibrosis and enhance the penetration of NPs of various sizes[13,334]. Indeed, losartan can reduce fibroblast and collagen density in the tumor tissues, as well as the production of TGFβ and hyaluronan. Since losartan targets various aspects of tumor ECM that hinder NP delivery, it represents a promising treatment strategy to improve NP penetration in tumors. The positive effects of losartan on drug delivery can also extend to small molecule drugs and macromolecules[13,336]. More recently, Papageorgis et al. have reported that tranilast, a common anti-fibrotic drug, can enhance nanotherapy delivery in breast tumors[337]. Finally, systemic introduction of PEGylated hyaluronidase (PEGPH20) and inhibitor of hyaluronic acid synthesis (methylumbelliferone) can both increase the distribution of nanomedicines in tumors[338,339]. All in all, IVM has been crucial in discovering various strategies to promote material deposition in targeted organs. Future IVM studies can continue to identify novel strategies to enhance the delivery of NPs and biologics to targeted organs, which could have profound implications in clinical feasibility of these materials.
3.2. PK of locally introduced materials
3.2.1. Locally introduced nanomaterials and biologics
Besides systemic delivery through intravenous injection, therapeutic materials can be introduced locally via subcutaneous, intratumoral, intraperitoneal, or other local injection or surgical implantation. These methods can enhance the specific accumulation of drugs at the target tissue while minimizing the systemic toxicity. Alternatively, materials can be locally administered as depots for controlled release of payloads into systemic circulation[340]. The advantages of local delivery especially apply to nanotherapies, since NPs, compared to small molecule drugs, transport in more constrained patterns from the injection site, particularly to the draining lymph node, and IVM and other in vivo imaging methods has been utilized to evaluate the PK of these locally injected nanotherapies. For instance, Lammers et al. used in vivo scintigraphy to compare the biodistribution of intravenously or intratumorally injected radio-labeled N-(2-hydroxypropyl)methacrylamide (HPMA) copolymer nanomaterial in a mouse model of subcutaneous tumor[341]. Through scintigrams, the authors observed that most HPMA copolymers introduced via intratumoral injection were still trapped in the tumor 24 hrs post-injection. Yet, in the animals that received intravenous injection, most copolymers were discovered in the clearance organs such as spleen and liver. Similar results were obtained by Yook et al., who used SPECT/CT imaging to show the trapping of locally delivered gold NPs at the tumor site even 48 hrs post injection[342]. Besides using radio-imaging to look at drug distribution at a whole organ/tumor level, researchers have used transmission electron microscopy (TEM) to evaluate the biodistribution of intratumorally injected NPs at a single-cell level. Specifically, Giustini et al. found that iron oxide NPs administered intratumorally can be rapidly internalized by the tumor cells, since 91.1% of injected NPs were found inside the cells 4 hrs post injection[343].
Locally injected materials trapped at the injection site can also function as controlled-release depots, from which small molecule drugs can be slowly introduced into the systemic circulation in a safe manner. For instance, Dong et al. injected micelles carrying ivermectin, an anti-parasitic drug, subcutaneously[344]. The authors found that although the blood plasma level of ivermectin introduced via this method was lower when compared to that of intravenous injection, the side-effects were much lower for subcutaneous injection. In another study, Jogala et al. found that subcutaneously delivered NPs can enable the sustained release of low molecular weight heparin[345]. Compared to a bolus injection, this locally delivered formulation improved the blood circulation half-life of heparin. Finally, the PK of locally introduced antibodies, cytokines, and small molecules have been assessed with a variety of in vivo imaging modalities. For example, Maeda et al. used IVM to observe the homing of small molecular probes carrying a bone-targeting moiety to osteoclasts in vivo[346]. Momin et al., on the other hand, used FRI to track the localization of intratumorally administered IL12 cytokine that was fused to lumican, a collagen binding protein. They found that lumican fusion enhanced the retention of IL12 in the tumor tissues, which improved the efficacy and reduced the toxicity of IL12 treatment[347]. Collagen-binding antibodies have also been engineered to improve the delivery of anti-TNFα antibody[348,349] and anti-EGFR antibody[350] to inflamed tissues and tumors, respectively. All in all, imaging has been instrumental in dissecting the in vivo kinetics of these engineered biologics.
Intraperitoneal injection is clinically performed to deliver medicine to the peritoneal cavity, especially since peritoneal cavity is frequently the metastatic site for disseminated ovarian cancer[351]. The drugs injected at this site have access to systemic circulation. Dogra et al. compared the PK of mesoporous silica NPs delivered via intravenous injection and intraperitoneal injection using SPECT/CT imaging[352]. They discovered that although immediately after intraperitoneal injection (<0.5 hr), most NPs were observed in the abdomen, by 5 hr post injection, the organ-level biodistribution resembled that of i.v. injection. The authors also found that particles of larger sizes can stay within the peritoneal cavity for longer periods of time compared to smaller particles.
3.2.2. Locally introduced macromaterials
In vivo imaging has also been used to observe the PK of therapies delivered by locally implanted hydrogels. As an example, Appel et al. used whole-animal IVM to evaluate the release and biodistribution of model polymeric NPs and hydrophilic drugs carried by hydroxypropylmethylcellulose hydrogels[353]. The authors found that while both polymeric NPs and hydrophilic drugs were predominantly found near the hydrogels, hydrophilic drugs diffused further than polymeric NPs. IVM was also used to demonstrate the local delivery of doxorubicin by zinc phthalocyanine-incorporated hydrogels[354]. Lastly, interest has grown in hydrogels or materials carrying immunomodulatory agents. Chen et al. designed a fibrin hydrogel to locally deliver anti-CD47 antibody at the tumor site[355]. The anti-CD47 antibody can promote anti-tumor immunity by inhibiting anti-phagocytic “don’t eat me” signals produced by cancer cells. The authors used intravital fluorescent imaging to show sustained retention of anti-CD47 antibody at tumor region over time. Moreover, PLGA microparticles carrying toll-like receptor-3 ligand poly(inosinic:cytidylic acid), poly(I:C), were implanted in the lymph node via intranodal injection[356]. The authors found that local delivery of poly(I:C) significantly enhanced its accumulation in lymph nodes, as well as antigen presenting cells, compared to systemic delivery. This intranodal delivery resulted in sustained dendritic cell activation and immune responses. Altogether, these studies demonstrate the utility of in vivo imaging in evaluating the PK of locally introduced therapeutic materials.
Most biomaterials implanted in vivo are designed to be degraded by the host cells over time, which can be imaged by assessing changes in implant structure and shape over time. As an example, Zhou et al. used multiple in vivo imaging modalities (radiography, μCT, and endoscopic OCT) to observe the degradation of a heart stent over a period of 24 months (Fig. 14)[357]. Researchers have used whole-body optical FRI to evaluate the degradation of fluorescently-labelled biomaterials over time. For instance, Artzi et al. tracked the loss of fluorescent signals to compare the biodegradability of PEG-dextran hydrogels of different shapes[148]. They discovered that a mesh cylindrical shape conferred the fastest erosion compared to a disk or block shape, presumably due to the high surface area of the mesh cylinder. In contrast, Berdichevski et al. used FRI to monitor the distribution of fluorescently-labeled degradation products in the tissue surrounding PEG-fibrinogen implants[147]. By monitoring the degradation products using FRI, the authors found a biphasic temporal pattern: the initial phase of rapid release of the biomaterials from the implant, and a later phase of slow resorption of degradation products into host tissues and blood stream. Finally, IVM [358] and ex vivo microscopy [149] have been used to track the degradation of starch microspheres and chitosan membranes, respectively. The positioning and functioning of the biomaterial and biomedical implants can also be monitored with in vivo imaging, such as μCT, echocardiography, and OCT[359,360] (Fig. 15). Taken together, the studies presented in this section clearly demonstrate the utility of IVM and other imaging modalities in assessing the PK of locally administered NPs, biologics, and drug-loaded macromaterials. Future IVM studies can be used to understand how the microenvironment of the injection site affects the PK of locally administered materials, and what properties of these materials govern their ability to enter systemic circulation. These understandings can allow researchers to design materials with tunable PK characteristics for diverse medical applications.
Figure 14: Translational imaging of coronary stent degradation kinetics.
A biodegradable Zn-Cu stent was inserted into a porcine coronary artery. (A) Angiography was used to show degradation of the stent 18 month after implantation. (B) 3D μ-CT imaging (green: degradation product, white: residual zinc and degradation product) showing 70% degradation 2 years after implantation with little local buildup of degradation products toward the vessel lumen. (C) Endoscopic OCT of the stent showing endothelialization after implantation. (D) 3D OCT reconstruction shows good integration of the stent with the blood vessels, leaving the vessels with smooth surface. Adapted with permission.[357] Copyright 2019, Elsevier.
Figure 15: Assessing the functional interaction between cardiac implants and tissue.
(A-C) Imaging was used to visualize the implantation of refillable drug-loaded gels for local delivery of therapeutic to the heart (A). μ-CT imaging (B) and CT reconstruction (C) show the positioning of the implants directly on the rat heart tissues. The implant was loaded with contrast agent mimicking therapeutics (blue). Adapted with permission.[360] Copyright 2018, Springer Nature Limited. (D-E) Intracardiac echocardiography (ICE) was used to track the morphology of a tissue engineered heart valve in vivo (D). (E) The ICE showed the morphology (left), valve regurgitation (middle), and valve computation length over the period of 24 wks. Adapted with permission.[359] Copyright 2018, American Association for the Advancement of Science.
4. Material pharmacodynamics
Advanced materials can be designed to modulate molecular target binding, tissue regeneration, angiogenesis, immune reactions, cytotoxicity, and cell migration in the host body. These materials can also induce unintended biological responses that are not within their design parameters. These unwanted responses include off-target molecular binding, infection, uncontrolled inflammation, fibrosis, and foreign body responses. The effects of advanced materials, both intended and unwanted effects, are collectively termed material pharmacodynamics. Material pharmacodynamics determine the efficacy and safety of advanced materials, and they can be observed with a variety of in vivo imaging techniques. In vivo imaging, especially IVM, can be used to analyze material-induced molecular target engagement, structural and anatomical changes in the target tissues, cell migration and proliferation, and biochemical activities. In the following section, we review recent studies that utilize imaging to study the effects of materials on biological tissues, with particular focus on IVM.
4.1. Molecular targeting
Nanomaterials and hydrogels can be designed to carry small molecule inhibitors or antibodies that target specific biological molecules. The binding of these payloads and their effects on the target molecule (pharmacodynamics) can be observed in vivo at a single-cell level with IVM. For instance, Kim et al. and Turetsky et al. tested the binding of fluorescently-labeled ibrutinib, a clinical Bruton’s tyrosine kinase (BTK) inhibitor, to mCherry-BTK fusion protein in vivo[153,361]. In these studies, the mCherry-BTK fusion protein was transduced into cancer cells that were implanted in SWC models. The authors successfully observed the delivery of fluorescent ibrutinib into the tumor tissues, as well as the co-localization of fluorescent ibrutinib with fluorescent BTK at a sub-cellular level. More recently, a microscopy technique with polarized light has been used to assess the in vivo binding of unlabeled BTK and poly (ADP-ribose) polymerase (PARP) inhibitors to their molecular targets[362,363]. This technique is based on the competition between the unlabeled drug and its fluorescently-labeled counterpart for the target binding. Therefore, the binding of fluorescent-labeled companion to the target, which is detected by polarized fluorescent microscopy, can serve as a surrogate inverse readout for target binding of co-administered unlabeled drug. Fluorescent drug binding alone can also be used to estimate behavior of the unlabeled parent compound. Combining this technique with IVM, Dubach et al. were able to measure the target-engagement of ibrutinib (BTK inhibitor) and olaparib (PARP inhibitor) and produce in vivo dose-response curves. For both inhibitors, the authors observed that the heterogeneity in single-cell target-engagement is larger in vivo than in vitro. Finally, a variety of radio-labeled analogs of small molecule kinase inhibitors have been used to evaluate the target binding and clinical potential of their parent compounds via PET or SPECT imaging[364–367]. They are also used in the clinic to monitor changes in kinase receptor status in patients as the results of disease progression or therapeutic treatment[368].
IVM can also be utilized to visualize drug effects on the activities of the targeted molecules. Using IVM and a fluorescent fusion reporter of drug efflux pump p-glycoprotein (P-gp; also known as multi-drug resistant protein, MDR1), Laughney et al. demonstrated that tumor cells expressing high levels of P-gp take up less fluorescently-tagged eribulin, an anti-microtubule drug, because these cells have enhanced ability to efflux it[152]. Using the same IVM approach, it was found that compared to solvent formulated P-gp inhibitor HM30181, nano-formulated HM30181 significantly enhanced the uptake of eribulin by resistant cells (cells with high P-gp expression). This result demonstrated that nanotherapy can be utilized to overcome tumor drug resistance and improve the delivery of drugs such as HM30181 that exhibit sub-optimal PK. Intravital fluorescent lifetime microscopic imaging has been used, in combination with a FRET biosensor for Src activity, to evaluate the pharmacodynamics of Src inhibitor dasatinib for cancer treatment[369]. The authors demonstrated that, in the liver mets, dasatinib successfully inhibited the Src activity at tumor rim but not the tumor core. Similarly, IVM and a FRET biosensor were used to evaluate the activity of ERK in response to the BRAF inhibitor PLX4720[370]. The investigators found that BRAF inhibitor failed to reduce ERK activity of tumor cells in regions with dense stromal ECM, as assessed by SHG imaging. Further mechanistic study revealed that dense ECM mechanically activated ERK via integrin β1/FAK signaling, which protected tumor cells from BRAF inhibition. These studies clearly demonstrate the power of IVM in assessing pharmacodynamics of targeted therapy in tissue environments. As more transgenic fluorescent reporter mouse and cell models become available, IVM may routinely be used to test the target specificity of drug payloads carried by engineered nanomaterials and hydrogels in physiologically relevant contexts. Furthermore, such efforts have potential to shed light on cellular mechanisms of drug resistance as they may arise.
4.2. Immunomodulation
NPs have been designed to deliver immunomodulatory payloads to various target organs, and the ability of these nanotherapies to affect the immune cells at the target sites can be evaluated by various in vivo imaging techniques, especially IVM. For instance, Rodell et al. designed cyclodextrin NPs carrying resiquimod, a TLR7/8 agonist [151]. IVM was used to evaluate not only the biodistribution of the NP and its payload, but also the ability of NPs to enhance the expression of IL12-YFP in the tumor tissues in p40-IRES-eYFP IL12 reporter mice. It was found that both fluorescently-labelled cyclodextrin NP and the resiquimod payload accumulated in dextran+ macrophages, and that IL12-YFP expression levels were enhanced in these macrophages. These results thus indicate that tumor-associated macrophages can readily take up resiquimod-loaded NPs in vivo, which then enhances polarization toward a pro-inflammatory M1-like phenotype by up-regulating IL12 production. A separate study has also shown that T-cell targeting PLGA NPs carrying resiquimod can produce a pro-inflammatory tumor immune microenvironment[371]. Therefore, both of these studies demonstrate that the anti-tumor immunity can be augmented by NPs carrying immunostimulant, and can synergistically combine with anti-PD-1 immune blockade therapy (Fig. 16A-D). In another example, lipopolysaccharide (LPS) has been loaded into PLGA NPs to produce an immunostimulatory nanotherapy. Choe et al. have used intravital fluorescence and reflectance spectral imaging of SWCs to monitor the recruitment of macrophages by this nanotherapy, as well as the resulting changes in blood oxygen level [372] (Fig. 16E). The degree of oxygenation in blood was determined by reflectance spectroscopy, which measures the amount of light absorption by blood. The authors found that locally injected LPS NPs recruited macrophages into the injection site, resulting in the loss of blood vessel oxygenation, and in the extreme case produced a necrosis region where no oxygenation was detected. In vivo imaging has also been used to observe pharmacodynamics of nano-formulated vaccines. Xiang et al. designed an upconversion polymeric NP decorated with ovalbumin as a model antigen for boosting immune responses [373]. This NP was engulfed by dendritic cells (DCs), and ovalbumin on the particle stimulated DC maturation and a pro-inflammatory reaction. The authors used luminescence imaging and confocal microscopy to discover that NP-loaded DCs can home to the draining lymph nodes for antigen presentation. Lastly, IVM has been used to study the mechanisms of action of NPs that can disrupt the interaction between neutrophils and cancer cells[374]. A DNase I coated NP was shown to inhibit the formation of neutrophil extracelluar traps (NETs) that can promote the seeding of metastatic cancer cells at secondary sites. IVM and fluorescent histology of LysM-EGFP mice, which express GFP in neutrophils, were used to observe NET formation, interaction between NETs and cancer cells, and the ability of DNase I to degrade NETs in vivo.
Figure 16: Pharmacodynamics of immunomodulatory materials at a single-cell level.
(A-D) IVM was used to assess the ability of resiquimod (R848)-loaded cyclodextrin NPs (CDNPs) to induce the expression of pro-inflammatory cytokine IL12 in tumor-associated macrophages labeled with fluorescent ferumoxytol. H2B-mApple expressing MC38 tumors were established in the SWC installed on IL12-YFP reporter mice, in which IL12 expression level is correlated with YFP signal intensity (A). Representative IVM (B) and quantification (C) showed CDNP-R848 induced a significant increase in IL12 YFP expression compared to empty NP (CDNP) or soluble R848. Quantification of changes in tumor size (D) demonstrated that CDNP-R848 improved the efficacy of anti-PD-1 therapy by promoting pro-inflammatory phenotype of tumor-associated macrophages. Adapted with permission.[151] Copyright 2018, Springer Nature Limited. (E) IVM of SWC showed accumulation of LPS-loaded particles can enhance the accumulation of macrophages over time. Nanoparticle treatment also resulted in an initial decrease, followed by a slow recovery, of blood oxygen level (hemoglobin saturation, Hb Sat) at the site of particle accumulation. Adapted with permission.[372] Copyright 2010, Elsevier. (F) Representative in vivo bioluminescence imaging showing immune-modulatory urinary bladder matrix (UBM) scaffolds can lead to shrinkage of melanoma. Adapted with permission.[377] Copyright 2019, American Association for the Advancement of Science.
Apart from NPs, hydrogels loaded with immunomodulatory compounds have also shown therapeutic promise, and in vivo imaging has evaluated their performance in physiologically relevant environments. Olingy et al. used IVM and Cx3cr1GFP/+ reporter mice to evaluate the immunomodulatory function of subcutaneously implanted PLGA thin films loaded with sphingosine-1-phosphate receptor (S1PR) agonist FTY720[375]. They found that this immunomodulatory implant can recruit pro-wound healing M2-like, GFP+ macrophages, which then attract the formation of blood vessels into ischemic muscle. Shah et al. designed a cryogel that released bone morphogenetic protein-2 (BMP-2) for stromal cell recruitment and notch ligand delta-like ligand-4 (DLL-4) for differentiation of recruited hematopoietic progenitor cells into T cells[376]. The authors used IVM and μ-CT imaging to demonstrate successful integration of this T-cell generating biomaterial implant into the host. In another example, Wolf et al. found that urinary bladder matrix (UBM), which is typically used to promote wound healing, can potentiate the anti-tumor immunity elicited via PD-1 and PD-L1 inhibitors[377] (Fig. 16F). In this study, FRI imaging was used to directly track the growth of fluorescently-tagged tumor cells in vivo.
IVM has also been used to investigate the responses of the immune system to antibody-based immunotherapy, for instance to monitor infiltration and migration of T cells [378–380], macrophage phagocytosis [14,381], and DC trafficking and activation [382]. More broadly speaking, IVM has demonstrated its utility in assessing a wide variety of immune processes[383,384], including: 1) neutrophil recruitment to the site of inflammation[385,386], 2) changes in immune cell composition in tumor and lymph nodes in response to treatments [117,387,388], 3) movement of immune cells in tissues and lymph nodes[389–391], 4) T cell behaviors in tumors[392], and 5) T cell interaction with antigen-presenting cells[393]. Various nanomaterials have also been utilized as in vivo imaging agents for macrophages[34], T cells[394], and DCs[395]. In sum, imaging represents a powerful tool to study how nanomaterials, implants, and biologics affect the spatial-temporal dynamics of heterogenous immune systems in vivo. Since the dynamic processes of immune cell trafficking, cell-cell interaction, and phagocytosis can all underly immunotherapy efficacy, IVM can undoubtably help guide strategies that manipulate these processes, especially using advanced materials carrying immunotherapeutic payloads.
4.3. Vascular modulation, angiogenesis, and tissue regeneration
In vivo imaging has enabled dynamic, real-time, and high-resolution observation of the biological effects of a wide range of materials for tissue engineering and regenerative medicine. One frequent objective of these materials is to promote angiogenesis, which is often monitored by imaging. For instance, Gniesmer et al. used a femur WC to evaluate different designs of grafts for rotator cuff repair[396]. Specifically, they used IVM to determine which design resulted in the largest amount of FITC dextran-labeled blood vessels and the least amount of tissue inflammation in the implanted grafts. They discovered that chitosan-decorated electrospun polycaprolactone (PCL) fiber mats promoted more angiogenesis of host vasculature compared to bare electrospun PCL fiber mats and a commonly used porous polyurethane (PUR)-based scaffold. They also quantified inflammatory responses to implants by measuring the amount of adherent leukocytes and the speed of leukocyte rolling in and around the implant. They found that bare PCL fiber mats induced significantly more inflammatory responses compared to chitosan-decorated PCL fiber mats and a porous PUR-based scaffold. In another study, Güc et al. used IVM to study how VEGF-C loaded fibrin promotes angiogenesis of lymphatic vessels at wound healing sites[17]. They were able to demonstrate that a fibrin-VEGF-C implant, in contrast to free VEGF-C, can stimulate the formation of new lymphatic vessels visualized by fluorescent Lyve-1 antibodies. Furthermore, the physiological function of these neo-lymphatic vessels was also tested using IVM. By measuring the diffusion of TRITC dextran, they discovered that neo-lymphatic vessels resulting from fibrin-VEGF-C implants had similar fluid clearance ability as neo-lymphatic vessels resulting from bare fibrin gels. However, the homing of dendritic cells to draining lymph nodes was enhanced in neo-vessels in fibrin-VEGF-C implants compared to bare implants (Fig. 17A-E). Finally, IVM has been used in conjunction with laser speckle imaging to measure the permeability of blood vessels in response to topical application of optical clearing agents on the mouse ear[397]. These studies collectively demonstrate that IVM can measure not only the degree of vessel formation, but also their functions with respect to permeability, perfusion, and cell trafficking.
Figure 17: The effects of tissue engineering scaffolds on angiogenesis.
(A-E) IVM was used to evaluate the effects of a VEGF-C-functionalized fibrin gel on the formation and function of lymphatic vessels. Representative images (A) and quantification (C) demonstrated that functionalizing the fibrin scaffold with VEGF-C enhanced lymphatic vessel regeneration compared to a control fibrin scaffold or to locally delivered VEGF-C. Although functionalizing fibrin with VEGF-C did not affect the clearance of 155 kDa dextran compared to control fibrin (B and D), it did enhance the amount of CD11c dendritic cells in the lymph nodes (E). Adapted with permission.[17] Copyright 2017, Elsevier. (F) Doppler perfusion IVM showed VEGF-conjugated NPs enhanced the blood perfusion, and hence vascularization, in the ischemic limb of the mouse. Adapted with permission.[402] Copyright 2017, Springer Nature Limited.
Besides observing neo-vasculature, imaging techniques can also be used to directly visualize tissue repair and regeneration. Zhang et al. used μ-CT, fluorescent imaging of tissue slides, and confocal imaging of whole tissues to understand the mechanisms behind the ability of magnesium-loaded implants to induce bone formation in the femur[398]. By perturbing the biological systems with shRNA, the authors were able to demonstrate that local delivery of magnesium at the bone fracture site can enhance neuronal production of calcitonin gene-related polypeptide-α, which induced osteogenesis. Stiers et al. used IVM and femur WCs to evaluate the ability of a tissue engineering construct to promote osteogenesis[399]. This tissue engineering construct consisted of fibroblast growth factor 2 (FGF2)-pretreated murine periosteum-derived cells (mPDCFGF2) seeded in collagen I gels. mPDC cells were transduced with a fluorescent DsRed reporter under the control of the promoter for collagen I, allowing the visualization of osteoblast differentiation in implanted cells. The authors found that the implant successfully recruited blood vessels (labeled by FITC-dextran) from the host, and the recruitment of blood vessels coincided with the expression of osteoblast markers (collagen I expression) in the implanted mPDC cells. In another study, Shvartsman et al. used IVM to evaluate the capability of a VEGF-loaded alginate hydrogel to promote axon regrowth and maintenance after ischemic injury in skeletal muscles[400]. Transgenic Thy1-YFP-16 reporter mice, which express YFP in certain neurons including motor neurons, were used to track neuron physiology in the injured muscle. By analyzing time-lapse images produced by IVM, the authors found that ischemic injury can induce axon degeneration, visualized by the retraction of YFP-expressing motor neurons. On the other hand, VEGF released by the hydrogel protected axons from ischemia-induced degeneration. The authors further demonstrated, using a neutralizing antibody, that this VEGF-induced effect in motor neurons was mediated by nerve growth factor (NGF) and glial-derived neurotrophic factor (GDNF). Lastly, SHG microscopy has been used to study how collagen structures within a de-cellularized organ affect its ability to induce tissue regeneration[401].
Nanomedicines have been designed to modulate vascular functions either for tissue regeneration or cancer treatment. For instance, VEGF-conjugated NPs have been shown to improve blood perfusion in hypoxic tissues via laser Doppler perfusion imaging[402](Fig. 17F). This imaging technique utilizes a laser beam and the Doppler effect to measure the relative speed of moving blood cells. Recently, polymer-lipid-peptide NPs were developed to deliver anti-platelet antibody to tumor sites. These NPs can deplete platelets in the tumor vasculature, thereby enhancing the permeability of blood vessels to co-injected doxorubicin[403]. Finally, IVM has also been used to evaluate the anti-angiogenic effects of various anti-tumor therapies[404,405]. Altogether, the studies described above demonstrate that IVM is an ideal tool for understanding how engineering biomaterials and nanomaterials affect the multi-step processes of angiogenesis and tissue regeneration. Currently, majority of these imaging studies focus on how engineering materials affect the morphology and protein expression of regenerated tissues. However, since the ultimate goal of regenerative medicine is to restore the normal function of a damaged tissue, future IVM studies should also focus on how advanced materials affect the physiological functions of regenerated tissues.
4.4. Foreign body responses
Implanted biomaterials, medical devices, and tissue engineering constructs frequently elicit foreign body response. This response is the result of the host recognizing the implanted materials as foreign objects, which then leads to the induction of inflammation that can ultimately result in material enclosure within a fibrotic capsule. Foreign body response is harmful to both the implant and the host. For instance, the fibrotic capsule prevents the recruitment of host blood vessels or stem cells into the tissue engineering constructs, thus reducing the abilities of these constructs to induce tissue regeneration. The low-grade chronic inflammation resulting from the foreign body response can also damage the host tissues surrounding the implants.Therefore, understanding how implanted materials interact with the host tissues is critical in designing methods to reduce the harmful effects of foreign body responses. Dondossola et al. used IVM to observe the foreign body responses to 3D electrospun calcium-coated polycaprolactone (mPCL-CaP) biomaterial scaffold subcutaneously implanted in the dorsal skin fold WC[146]. Specifically, the recruitment of host cells and the formation of blood vessels surrounding the implanted scaffold were tracked over time. To this end, the mPCL-CaP scaffold was visualized with second harmonic and third harmonic generation (SHG and THG) multi-photon imaging. The authors used C57BL/6-GFP transgenic reporter mice, which express GFP in all host cells, in the study. Over the period of 2 weeks after implantation, they observed several steps of foreign body responses within the implanted area, including: 1) infiltration of GFP+ host myeloid cells, 2) fusion of GFP+ host myeloid cells into multi-nucleated giant cells, 3) formation of neovasculature as visualized with fluorescent dextran, and 4) deposition of collagen fibers as visualized by SHG imaging. They found that depleting macrophages with clodronate treatment and neutralizing VEGF with a VEGF molecular trap reduced the foreign body response as evident by decreases in accumulation of host myeloid cells, neovascularization, and fibrosis (Fig. 18). In a yet another IVM study, Doloff et al. discovered that macrophages attracted to implanted alginate as the result of a foreign body response can secrete CXCL13 to recruit B cells to the implanted sites, which promoted fibrosis[406]. It should be noted that B cell migration to the implant was tracked by imaging a CCR6-EGFP reporter mouse, which expresses EGFP in B cells.
Figure 18: Evaluating the foreign-body response following scaffold implantation.
(A) PCL scaffold was implanted in the SWC installed on a C57BL/6-GFP mouse, in which all host cells are labeled with GFP. (B) Schematic of the window chamber setup. (C) IVM was used to image scaffold structure (via THG), collagen density (via SHG), host cell (GFP+) invasion, and blood vessel recruitment (via fluorescent dextran labeling) at the implanted region. (D) Quantification of images in (C) showed increase in host cell invasion, angiogenesis, and collagen deposition over the period of 2 wks after scaffold implantation. Adapted with permission.[146] Copyright 2016, Springer Nature Limited.
IVM has also been used to study suture-induced foreign body responses in zebrafish. Gurevich et al. compared the ability of nylon and vicryl sutures to produce immune reaction and foreign body responses in Tg(mpx:GFP);Tg(mpeg:mcherry) transgenic zebrafish which express GFP and mCherry in neutrophils and macrophages, respectively [56]. They found that vicryl sutures were more immunogenic, as they induced pronounced and persistent neutrophil/macrophage recruitment compared to nylon sutures. The sutures’ abilities to induce pro-inflammatory cytokine production and modulate vascularization were observed in Tg(tnfa:GFP) and Tg(fli:GFP) transgenic zebrafish, respectively. In Tg(tnfa:GFP) zebrafish, the expression of GFP is correlated with the production of TNFα. Blood vessels are labeled with GFP in Tg(fli:GFP) zebrafish. The authors found that vicryl sutures induced more TNFα expression than nylon sutures. Although both suture types initially induced comparable amounts of vascular retraction, vasculatures healed faster in the fishes implanted with nylon sutures(Fig. 19). The biocompatibility of sutures has also been evaluated via IVM in mouse WC models[407].
Figure 19: Imaging the foreign-body response to suture implantation.

(A) Zebrafish was used as a model organism for comparing the responses of host tissues to nylon and vicryl sutures. IVM was used to track various aspects of foreign body responses in transgenic zebrafish. (B-D) IVM images (B) and quantification showed vicryl sutures induced a higher level of TNFα expression (C) and macrophage accumulation (D) compared to a nylon suture or sham control. (E-F) Vicryl sutures enhanced neutrophil recruitment (E) and reduced the formation of neovasculature (F) compared to nylon sutures. Adapted with permission.[56] Copyright 2019, The Company of Biologists.
Finally, transparent graphene electrodes have recently been developed to enable the simultaneous recording of electrophysiology and in vivo imaging for studies of brain and neuronal physiology[408,409]. In these studies, the electrode is implanted directly in the cranial WC to apply electrical stimulation or record electrical signals, while fluorescence IVM is used to record changes in signaling, such as with the GCaMP6f calcium signaling reporter mouse model. Although these intracortical neuronal probes have greatly improved our understanding of brain functions, they frequently induce inflammation and foreign body responses, which decrease their performance. Kozai et al. discovered, via IVM of Cx3cr1GFP/+ reporter mice, that treating the animals with anti-inflammatory steroid dexamethasone can significantly reduce the recruitment of Cx3cr1GFP/+ macrophages to the implantation site, thus reducing the foreign body response near the electrodes[410]. In summary, IVM and other imaging techniques have already allowed us to gain insights into how implanted materials elicit foreign body responses. Foreign body responses are one of the primarily reasons for the failure of materials both clinically and pre-clinically. In the future, as IVM technologies become more widely available, we envision that in vivo imaging can become a routine technique in evaluating the translational feasibility of engineered materials. Scientific studies performed with IVM can also lead to novel strategies in reducing the foreign body responses, thus extending the operating lifespan of implanted materials.
4.5. Cytotoxic and cytostatic effects
Most anti-tumor nanotherapies are designed to carry cytostatic and cytotoxic payloads. Besides measuring the inhibition in tumor growth, the cytotoxic and cytostatic effects of therapeutic nanomaterials can be evaluated by microscopic imaging of fluorescent reporters of drug action at a single-cell level, thus allowing the observation of any potential heterogeneity in cellular responses to drugs. This is particularly useful for identifying subpopulations of drug-resistant cells, or understanding the spatial heterogeneity in drug response. For instance, as described previously, IVM has been used to assess PK of polymeric NPs loaded with a cytotoxic Pt(IV) prodrug[10]. In the same study, the PD of this nanoformulation, namely the ability of Pt prodrug to elicit DNA damage, was simultaneously quantified with IVM by using tumor cells expressing 53BP1-mApple fusion reporter. Upon DNA damage manifested as double strand break, 53BP1-mApple fusion protein, a DNA repair protein, focally concentrates at the site of the break, creating fluorescent puncta. This study found that increasing the dosage of Pt-loaded polymeric NPs led to formation of more 53BP1-mApple puncta in the tumor cells. More interestingly, corresponding to the observation that TAMs function as depots from which cytotoxic Pt payload can be released, the study found higher levels of DNA damage response around NP-loaded TAMs. This result thus suggests the functional consequences of the drug depot effect. IVM has also been used to evaluate the cytotoxicity of chemotherapy to cancer cells in liver mets, for instance by examining fragmentation and condensation of fluorescent signals from cancer cells expressing cytoplasmic RFP[411].
Anti-mitotic drug effects can be monitored in vivo over time by IVM. In on example, Orth et al. developed an imaging strategy in which cancer cells co-expressing histone H2B-mCherry and tubulin-EGFP fusion proteins were implanted into a SWC[11]. The tubulin-EGFP fusion reporter was used to assess microtubule structure, while the H2B-mCherry fusion reporter monitored the shape of the chromatin as an indicator for mitotic behavior. For instance, histone H2B-mCherry signals reported on mononucleated, multinucleated, mitotic, bipolar, multipolar, and arrested cells, which are relevant phenotypes of responses to drugs targeting mitosis and microtubules. In a subsequent study, imaging of H2B combined with FUCCI reporters enabled direct cell cycle profiling in cells treated with anti-mitotic drugs (eribulin, paclitaxel, and ispinesib)[412]. To assist in the analysis of large sets of single cell microscopy data, a computational machine learning framework was used to automatically perform 3D segmentation and quantification of the FUCCI reporter intensity. In another example, Janssen et al. simultaneously imaged mitotic and apoptosis states of cancer cells in vivo following docetaxel treatment[413]. In this study, the mitotic state was assessed by quantifying the morphology of the fluorescently-labeled chromatin, while apoptosis was measured by using a caspase-3 FRET activity sensor. They found that the ability of docetaxel to induce apoptosis was dependent on its ability to induce mitotic arrest in vitro, but not in vivo. Combined, these IVM studies clearly demonstrate that pharmacodynamics of anti-mitotic or cytostatic drugs are complex and context dependent, motivating the need for more physiologically relevant studies. As an example, future detailed IVM monitoring can reveal how the tumor microenvironment contributes to the resistance of tumor cells to these cytostatic and anti-mitotic drugs. IVM can also be used to assess whether same cytostatic or anti-mitotic drug elicits different responses (i.e. cell death via autophagy vs. apoptosis) in different parts of the tumor.
4.6. Anti-metastatic effects
Nanotherapies can be designed to carry anti-metastatic drugs to tumor cells. In vivo imaging can be used to measure the pharmacodynamics of anti-metastatic drugs by measuring the effects of these drugs on various aspects of the metastatic cascade (i.e. migration, intravasation, extravasation, seeding, and proliferation). For instance, IVM has been used to study the anti-migratory effects of c-MET inhibitor altiratinib[88], FAK inhibitor PF-562271, and SRC inhibitor dasatinib[414] by using cancer cells expressing the photo-convertible fluorescent reporter Dendra. In another study, the effect of paclitaxel on cancer cells expressing pro-metastatic protein MENA and MENAINV in mice was assessed by tracking the migration speed of fluorescently labeled cancer cells using IVM[415]. The authors found that MENA and MENAINV protected cancer cells against anti-migratory effects of paclitaxel. Furthermore, Karagiannis et al. recently discovered that paclitaxel paradoxically enhanced cancer cell intravasation by increasing the permeability of tumor blood vessels[416]. It should be noted that in this study, cancer cell intravasation was quantified using IVM by counting the amount of GFP-expressing cancer cells crossing the lectin-labeled blood vessels. The authors further discovered decreasing the permeability of blood vessels using TIE2 inhibitor rebastinib could reduce this paclitaxel-induced intravasation. Lastly, the extravasation of cancer cells and the growth of cancer cell bone mets have been visualized with IVM of a lung WC[64] and SWC containing tissue engineering bone constructs[93], respectively. The spatiotemporal resolutions of techniques like IVM are required to capture such dynamic and microscopic metastatic processes, and IVM thus is useful in revealing how therapies can affect various aspects of the metastatic cascade with high cellular detail.
5. Future directions
Diverse in vivo imaging tools have shed light on how materials interact with the body across multiple length and time scales, and will continue to play increasingly important roles as imaging technology improves and material designs grow in sophistication. Materials designed for immunomodulation promise to amplify the already groundbreaking results seen in oncology with immune checkpoint blockade and adoptive cell therapies. Both microenvironmental context and cellular dynamics are central to understanding the success, failure, and potential toxicity of immunomodulatory materials. These properties underscore the value in approaches like time-lapse IVM, which can be used to understand the heterogeneity of antibody-drug conjugate distribution in vivo, to guide optimization of material-based adoptive-cell therapies, and to quantify the ability of vaccine approaches in eliciting robust adaptive immune responses. Indeed, IVM has unique capability to assess PK and PD from organ level down to sub-cellular level, and it has already been used to study the pharmacology of engineered materials with various biological functionality. In the future, IVM can continue to provide invaluable insights into how various aspects of the tissue microenvironment affect material PK and PD. For instance, IVM can be used to study how physical factors in the tissue, such as elevated interstitial pressure and local pH environment, influence the transport and efficacy of engineered materials. In addition, whether the communication between different cell types affects the in vivo action of these materials can be profiled by IVM. IVM could also be used to differentiate malignant from benign lesions based on the anatomical structure and cellular morphology of the tumors. Finally, IVM also provides adequate resolution for the accurate assessment of the effects of intracellular microenvironment, such as cellular redox state, organelle structure, and kinase activity, on the PK and PD of engineered materials at a subcellular resolution.
Moreover, with the recent developments in wearable electronics and implantable bio-electronic devices, imaging is poised to become an invaluable tool to evaluate the safety and function of these devices in vivo. Furthermore, in vivo imaging can be used to assess the design of nanomaterials for gene therapy and understand the spatial-temporal dynamics of these therapeutic genetic materials. Finally, it has become increasingly clear that complex patient heterogeneity may be better treated by the personalized design of therapeutic materials[417,418]. For instance, the composition of a drug delivery material may be customized to improve its PK/PD based on the molecular, cellular, and anatomical properties of a patient’s disease. Similarly, the size and shape of biomaterial implants can be customized to match the needs of patients at the individual level. Clinical in vivo imaging modalities would thus be critical tools in guiding the personalization of these materials and evaluating their efficacy and safety.
Technical challenges need to be overcome to unlock the full potential of imaging in evaluating material pharmacology. Since every imaging technique has its shortcomings in terms of resolution, penetration depth, or acquisition speed, the simultaneous use of multiple imaging modalities in a single study can enable different imaging techniques to complement each other. Moreover, with the rising popularity of single-cell RNA sequencing and the widespread availability of -omic level data, there is an increasing appreciation that materials design and evaluation will benefit from close integration with these datasets. New highly multiplexed imaging technologies such as in situ sequencing offer one promising avenue to bridge context-dependent and spatially defined in vivo material action with -omic scale readouts of biological effects, and efforts are ongoing to bridge in vivo imaging with highly multiplexed ex vivo analyses. Additionally, the penetration depth, especially of optical in vivo imaging, should be improved to enable visualization of material pharmacology deeper within the tissues. Finally, single-cell microscopy can generate large sets of data that are impossible to analyze manually. Computational imaging analysis pipelines, combined with machine learning algorithms, can enable artificial intelligence-assisted automatic analysis of these large data sets as they have begun to do in clinical radiology and pathology applications. In vivo imaging offers unique insights into material behaviors that are impossible to obtain with other analytical methods, and promises to help usher new development and biological understanding of advanced healthcare materials.
Acknowledgement
This work was in part supported by the NIH UO1CA206997, R00CA207744, T32CA079443, an American Thyroid Association / Thyroid Cancer Survivors’ Association Research Grant, and a Radiological Society of North America R&E Foundation Resident Research Grant.
R.W. is a co-founder of T2Biosystems and Lumicell, serves as a scientific advisor for ModeRNA Therapeutics, Tarveda Therapeutics, and Alivio Therapeutics. None of these activities are related to the manuscript.
Abbreviation list
- BBB
blood brain barrier
- BMP
bone morphogenetic protein
- BTK
bruton’s tyrosine kinase
- CARS
coherent anti-Stokes Raman scattering
- CCW
chronic cranial window
- CT
computed tomography
- DLL-4
delta-like ligand-4
- ECM
extracellular matrix
- EPR
enhanced permeability and retention
- FAK
focal adhesion kinase
- FGF
fibroblast growth factor
- FITC
fluorescein isothiocyanate
- FMT
fluorescence molecular tomography
- FRI
fluorescence reflectance imaging
- GDNF
glial cell-derived neurotrophic factor
- GFP
green fluorescent protein
- HPMA-N
(2-hydroxypropyl) methacrylamide
- IFP
interstitial fluid pressure
- IVM
intravital microscopy
- IVM
intravital microscopy
- LPS
lipopolysaccharide
- MDR1
multidrug resistance-1
- MENA
mammalian-enabled protein
- MRI
magnetic resonance imaging
- MSC
mesenchymal stem cell
- NGF
nerve growth factor
- NP
nanoparticles
- OCT
optical coherence tomography
- PARP
poly (ADP-ribose) polymerase
- PCL
polycaprolactone
- Pd
palladium
- PD
pharmacodynamcs
- PDMS
polydimethylsiloxane
- PEG
polyethylene glycol
- PET
positron emission tomography
- PK
pharmacokinetics
- PLGA
poly(lactic-co-glycolic acid)
- Pt
platinum
- PUR
polyurethane
- Qdots
quantum dots
- RFP
red fluorescent protein
- RGD
arginylglycylaspartic acid
- S1PR
sphingosine-1-phosphate receptor
- SCS
suprachoroidal space
- SERS
surface enhanced Raman spectroscopy
- SHG
second harmonic generation
- SPECT
single-photon emission computed tomography
- SWC
skinfold window chamber
- SWNT
single-walled nanotubes
- TNP
therapeutic nanoparticles
- TRITC
tetramethylrhodamine
- TSW
thinned skull window
- UBM
urinary bladder matrix
- UCNPs
upconverting nanoaprticles
- VEGF
vascular endothelial growth factor
- WC
widow chamber
- YFP
yellow fluorescent protein
- μCT
micro CT
Biographies
Ran Li received his Ph.D. in Biological Engineering from Massachusetts Institute of Technology. He is currently a postdoctoral fellow at Center for Systems Biology, Massachusetts General Hospital. His research focuses on using in vivo microscopy and whole organ clearing and imaging techniques to understand how tissue microenvironment affects the biodistribution of nanomaterials at a single-cell level.

Dr. Ralph Weissleder is the Thrall Professor of Radiology and Professor of System Biology at Harvard Medical School, Director of the Center for Systems Biology at Massachusetts General Hospital (MGH), and Attending Clinician (Interventional Radiology) at MGH. The focus of his research lab is to obtain a deeper understanding of human biology in health and disease, to translate new biological understanding into clinically useful diagnostics and to identify new therapeutic approaches and drug targets.

Miles Miller is a Principal Investigator at the Center for Systems Biology within the Massachusetts General Hospital Research Institute, and is an Assistant Professor of Radiology at Harvard Medical School. He received a PhD in Biological Engineering from Massachusetts Institute of Technology and an A.B. in Chemistry from Princeton University. He specializes in parsing mechanisms of cell signaling and drug action from a quantitative network-level perspective, with training in imaging, nanotechnology, computational modeling, cell signaling biology, and drug delivery.

Footnotes
Disclosure
The other authors declare that they have no competing interests.
Reference:
- [1].Miller MA, Weissleder R. Nat Rev Cancer 2017,17,399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Davies JE. Occupational and Environmental Medicine 2007,64,e2. [Google Scholar]
- [3].Oliva N, Carcole M, Beckerman M, Seliktar S, Hayward A, Stanley J, Parry NM, Edelman ER, Artzi N. Sci Transl Med 2015,7,272ra11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Balani SK, Miwa GT, Gan LS, Wu JT, Lee FW. Curr Top Med Chem 2005,5,1033. [DOI] [PubMed] [Google Scholar]
- [5].Barenholz Y. J Control Release 2012,160,117. [DOI] [PubMed] [Google Scholar]
- [6].Lambert JM, Chari RV. J Med Chem 2014,57,6949. [DOI] [PubMed] [Google Scholar]
- [7].Lin SH, Kleinberg LR. Expert Rev Anticancer Ther 2008,8,343. [DOI] [PubMed] [Google Scholar]
- [8].Att W, Hori N, Takeuchi M, Ouyang J, Yang Y, Anpo M, Ogawa T. Biomaterials 2009,30,5352. [DOI] [PubMed] [Google Scholar]
- [9].Choi M, Kwok SJ, Yun SH. Physiology (Bethesda) 2015,30,40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Miller MA, Zheng YR, Gadde S, Pfirschke C, Zope H, Engblom C, Kohler RH, Iwamoto Y, Yang KS, Askevold B, Kolishetti N, Pittet M, Lippard SJ, Farokhzad OC, Weissleder R. Nat Commun 2015,6,8692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Orth JD, Kohler RH, Foijer F, Sorger PK, Weissleder R, Mitchison TJ. Cancer Res 2011,71,4608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Smith BR, Kempen P, Bouley D, Xu A, Liu Z, Melosh N, Dai H, Sinclair R, Gambhir SS. Nano Lett 2012,12,3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Chauhan VP, Martin JD, Liu H, Lacorre DA, Jain SR, Kozin SV, Stylianopoulos T, Mousa AS, Han X, Adstamongkonkul P, Popović Z, Huang P, Bawendi MG, Boucher Y, Jain RK. Nat Commun 2013,4,2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Arlauckas SP, Garris CS, Kohler RH, Kitaoka M, Cuccarese MF, Yang KS, Miller MA, Carlson JC, Freeman GJ, Anthony RM, Weissleder R, Pittet MJ. Sci Transl Med 2017,9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Li R, Attari A, Prytyskach M, Garlin MA, Weissleder R, Miller MA. Cytometry A 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Smith BR, Zavaleta C, Rosenberg J, Tong R, Ramunas J, Liu Z, Dai H, Gambhir SS. Nano Today 2013,8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Güç E, Briquez PS, Foretay D, Fankhauser MA, Hubbell JA, Kilarski WW, Swartz MA. Biomaterials 2017,131,160. [DOI] [PubMed] [Google Scholar]
- [18].Popovtzer R, Agrawal A, Kotov NA, Popovtzer A, Balter J, Carey TE, Kopelman R. Nano Lett 2008,8,4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Meng X, Zhang H, Zhang M, Wang B, Liu Y, Wang Y, Fang X, Zhang J, Yao Z, Bu W. Adv Sci (Weinh) 2019,6,1901214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Jin Y, Ni D, Gao L, Meng X, Lv Y, Han F, Zhang H, Liu Y, Yao Z, Feng X, Bu W, Zhang J. Advanced Functional Materials 2018,28,1802656. [Google Scholar]
- [21].Miller MA, Gadde S, Pfirschke C, Engblom C, Sprachman MM, Kohler RH, Yang KS, Laughney AM, Wojtkiewicz G, Kamaly N, Bhonagiri S, Pittet MJ, Farokhzad OC, Weissleder R. Sci Transl Med 2015,7,314ra183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Zhou XY, Jeffris KE, Yu EY, Zheng B, Goodwill PW, Nahid P, Conolly SM. Phys Med Biol 2017,62,3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wang L, Zhong X, Qian W, Huang J, Cao Z, Yu Q, Lipowska M, Lin R, Wang A, Yang L, Mao H. J Magn Reson Imaging 2014,40,1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Seeram E. Radiol Technol 2018,89,279CT. [PubMed] [Google Scholar]
- [25].Geethanath S, Vaughan JT. J Magn Reson Imaging 2019,49,e65. [DOI] [PubMed] [Google Scholar]
- [26].Ni D, Bu W, Ehlerding EB, Cai W, Shi J. Chem Soc Rev 2017,46,7438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Zhang C, Moonshi SS, Wang W, Ta HT, Han Y, Han FY, Peng H, Král P, Rolfe BE, Gooding JJ, Gaus K, Whittaker AK. ACS Nano 2018,12,9162. [DOI] [PubMed] [Google Scholar]
- [28].Perlman O, Azhari H MRI and Ultrasound Imaging of Nanoparticles for Medical Diagnosis. Nanotechnology Characterization Tools for Biosensing and Medical Diagnosis. Berlin, Heidelberg: Springer Berlin Heidelberg; 2018. p. 333–65. [Google Scholar]
- [29].Li X, Sui Z, Li X, Xu W, Guo Q, Sun J, Jing F. Int J Nanomedicine 2018,13,3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kiessling F, Fokong S, Koczera P, Lederle W, Lammers T. J Nucl Med 2012,53,345. [DOI] [PubMed] [Google Scholar]
- [31].Kim YS, Rhim H, Choi MJ, Lim HK, Choi D. Korean J Radiol 2008,9,291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Zlitni A, Gambhir SS. Curr Opin Chem Biol 2018,45,113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Keliher EJ, Ye YX, Wojtkiewicz GR, Aguirre AD, Tricot B, Senders ML, Groenen H, Fay F, Perez-Medina C, Calcagno C, Carlucci G, Reiner T, Sun Y, Courties G, Iwamoto Y, Kim HY, Wang C, Chen JW, Swirski FK, Wey HY, Hooker J, Fayad ZA, Mulder WJ, Weissleder R, Nahrendorf M. Nat Commun 2017,8,14064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kim HY, Li R, Ng TSC, Courties G, Rodell CB, Prytyskach M, Kohler RH, Pittet MJ, Nahrendorf M, Weissleder R, Miller MA. ACS Nano 2018,12,12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Yang C-T, Padmanabhan P, Gulyás BZ. RSC Advances 2016,6,60945. [Google Scholar]
- [36].Tu C, Ng TS, Jacobs RE, Louie AY. J Biol Inorg Chem 2014,19,247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ng TS, Bading JR, Park R, Sohi H, Procissi D, Colcher D, Conti PS, Cherry SR, Raubitschek AA, Jacobs RE. J Nucl Med 2012,53,1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Cherry SR, Jones T, Karp JS, Qi J, Moses WW, Badawi RD. J Nucl Med 2018,59,3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ni D, Zhang J, Bu W, Zhang C, Yao Z, Xing H, Wang J, Duan F, Liu Y, Fan W, Feng X, Shi J. Biomaterials 2016,76,218. [DOI] [PubMed] [Google Scholar]
- [40].Ni D, Ehlerding EB, Cai W. Angew Chem Int Ed Engl 2019,58,2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Ni D, Shen Z, Zhang J, Zhang C, Wu R, Liu J, Yi M, Wang J, Yao Z, Bu W, Shi J. ACS Nano 2016,10,3783. [DOI] [PubMed] [Google Scholar]
- [42].Mahmood U, Weissleder R. Mol Cancer Ther 2003,2,489. [PubMed] [Google Scholar]
- [43].Cao J, Zhou M. Methods Mol Biol 2016,1444,27. [DOI] [PubMed] [Google Scholar]
- [44].Ciarrocchi E, Belcari N. EJNMMI Phys 2017,4,14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Zhang Y, Hong H, Cai W. Curr Pharm Biotechnol 2010,11,654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Al Rawashdeh W, Kray S, Pich A, Pargen S, Balaceanu A, Lenz M, Spöler F, Kiessling F, Lederle W. Journal of Nanoparticle Research 2012,14 [Google Scholar]
- [47].Cong Z, Yang F, Cao L, Wen H, Fu T, Ma S, Liu C, Quan L, Liao Y. Int J Nanomedicine 2018,13,8549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Oh SJ, Kang J, Maeng I, Suh JS, Huh YM, Haam S, Son JH. Opt Express 2009,17,3469. [DOI] [PubMed] [Google Scholar]
- [49].Nobis M, Warren SC, Lucas MC, Murphy KJ, Herrmann D, Timpson P. J Cell Sci 2018,131 [DOI] [PubMed] [Google Scholar]
- [50].Amornphimoltham P, Masedunskas A, Weigert R. Adv Drug Deliv Rev 2011,63,119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Beerling E, Ritsma L, Vrisekoop N, Derksen PW, van Rheenen J. J Cell Sci 2011,124,299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Rompolas P, Mesa KR, Greco V. Nature 2013,502,513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Canto MI, Anandasabapathy S, Brugge W, Falk GW, Dunbar KB, Zhang Z, Woods K, Almario JA, Schell U, Goldblum J, Maitra A, Montgomery E, Kiesslich R, E.F.B.E.O.C.E.F.B.E.C.E.B.E.T.G. Confocal. Gastrointest Endosc 2014,79,211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Fisher DT, Muhitch JB, Kim M, Doyen KC, Bogner PN, Evans SS, Skitzki JJ. Nat Commun 2016,7,10684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Yan C, Brunson DC, Tang Q, Do D, Iftimia NA, Moore JC, Hayes MN, Welker AM, Garcia EG, Dubash TD, Hong X, Drapkin BJ, Myers DT, Phat S, Volorio A, Marvin DL, Ligorio M, Dershowitz L, McCarthy KM, Karabacak MN, Fletcher JA, Sgroi DC, Iafrate JA, Maheswaran S, Dyson NJ, Haber DA, Rawls JF, Langenau DM. Cell 2019,177,1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Gurevich DB, French KE, Collin JD, Cross SJ, Martin P. J Cell Sci 2019,133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Weiss JT, Dawson JC, Macleod KG, Rybski W, Fraser C, Torres-Sánchez C, Patton EE, Bradley M, Carragher NO, Unciti-Broceta A. Nat Commun 2014,5,3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Rhodes GJ. Methods 2017,128,129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Leinster DA, Kulbe H, Everitt G, Thompson R, Perretti M, Gavins FN, Cooper D, Gould D, Ennis DP, Lockley M, McNeish IA, Nourshargh S, Balkwill FR. J Pathol 2012,227,136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Mohan JF, Kohler RH, Hill JA, Weissleder R, Mathis D, Benoist C. Proc Natl Acad Sci U S A 2017,114,E7776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Junankar S, Shay G, Jurczyluk J, Ali N, Down J, Pocock N, Parker A, Nguyen A, Sun S, Kashemirov B, McKenna CE, Croucher PI, Swarbrick A, Weilbaecher K, Phan TG, Rogers MJ. Cancer Discov 2015,5,35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Thurber GM, Yang KS, Reiner T, Kohler RH, Sorger P, Mitchison T, Weissleder R. Nat Commun 2013,4,1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Alieva M, Ritsma L, Giedt RJ, Weissleder R, van Rheenen J. Intravital 2014,3,e29917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Entenberg D, Voiculescu S, Guo P, Borriello L, Wang Y, Karagiannis GS, Jones J, Baccay F, Oktay M, Condeelis J. Nat Methods 2018,15,73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Brown E, Munn LL, Fukumura D, Jain RK. Cold Spring Harb Protoc 2010,2010,pdb.prot5452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Machado MJC, Mitchell CA. The Journal of Physiology 2011,589,4681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Biel NM, Lee JA, Sorg BS, Siemann DW. Vasc Cell 2014,6,17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Schreiter J, Meyer S, Schmidt C, Schulz RM, Langer S. GMS Interdiscip Plast Reconstr Surg DGPW 2017,6,Doc10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Jain RK, Munn LL, Fukumura D. Nat Rev Cancer 2002,2,266. [DOI] [PubMed] [Google Scholar]
- [70].Harney AS, Arwert EN, Entenberg D, Wang Y, Guo P, Qian BZ, Oktay MH, Pollard JW, Jones JG, Condeelis JS. Cancer Discov 2015,5,932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Gruionu G, Bazou D, Maimon N, Onita-Lenco M, Gruionu LG, Huang P, Munn LL. Lab Chip 2016,16,1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Yang G, Pan F, Parkhurst CN, Grutzendler J, Gan WB. Nat Protoc 2010,5,201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Xu HT, Pan F, Yang G, Gan WB. Nat Neurosci 2007,10,549. [DOI] [PubMed] [Google Scholar]
- [74].Mostany R, Portera-Cailliau C. J Vis Exp 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Holtmaat A, Bonhoeffer T, Chow DK, Chuckowree J, De Paola V, Hofer SB, Hübener M, Keck T, Knott G, Lee WC, Mostany R, Mrsic-Flogel TD, Nedivi E, Portera-Cailliau C, Svoboda K, Trachtenberg JT, Wilbrecht L. Nat Protoc 2009,4,1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Yuan F, Salehi HA, Boucher Y, Vasthare US, Tuma RF, Jain RK. Cancer Res 1994,54,4564. [PubMed] [Google Scholar]
- [77].Miyauchi T, Wei EP, Povlishock JT. J Neurotrauma 2013,30,1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Dreier JP, Victorov IV, Petzold GC, Major S, Windmüller O, Fernández-Klett F, Kandasamy M, Dirnagl U, Priller J. Stroke 2013,44,490. [DOI] [PubMed] [Google Scholar]
- [79].Yao J, Wang L, Yang JM, Maslov KI, Wong TT, Li L, Huang CH, Zou J, Wang LV. Nat Methods 2015,12,407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Vandoorne K, Rohde D, Kim HY, Courties G, Wojtkiewicz G, Honold L, Hoyer FF, Frodermann V, Nayar R, Herisson F, Jung Y, Désogère PA, Vinegoni C, Caravan P, Weissleder R, Sosnovik DE, Lin CP, Swirski FK, Nahrendorf M. Circ Res 2018,123,415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Wu JW, Runnels JM, Lin CP. Methods Mol Biol 2014,1185,247. [DOI] [PubMed] [Google Scholar]
- [82].Goldey GJ, Roumis DK, Glickfeld LL, Kerlin AM, Reid RC, Bonin V, Schafer DP, Andermann ML. Nat Protoc 2014,9,2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Heo C, Park H, Kim YT, Baeg E, Kim YH, Kim SG, Suh M. Sci Rep 2016,6,27818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Masedunskas A, Chen Y, Stussman R, Weigert R, Mather IH. Mol Biol Cell 2017,28,935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Kedrin D, Gligorijevic B, Wyckoff J, Verkhusha VV, Condeelis J, Segall JE, van Rheenen J. Nat Methods 2008,5,1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Zomer A, Ellenbroek SI, Ritsma L, Beerling E, Vrisekoop N, Van Rheenen J. Stem Cells 2013,31,602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Harper KL, Sosa MS, Entenberg D, Hosseini H, Cheung JF, Nobre R, Avivar-Valderas A, Nagi C, Girnius N, Davis RJ, Farias EF, Condeelis J, Klein CA, Aguirre-Ghiso JA. Nature 2016,540,588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Leung E, Xue A, Wang Y, Rougerie P, Sharma VP, Eddy R, Cox D, Condeelis J. Oncogene 2017,36,2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Ritsma L, Steller EJ, Ellenbroek SI, Kranenburg O, Borel Rinkes IH, van Rheenen J. Nat Protoc 2013,8,583. [DOI] [PubMed] [Google Scholar]
- [90].Ritsma L, Steller EJ, Beerling E, Loomans CJ, Zomer A, Gerlach C, Vrisekoop N, Seinstra D, van Gurp L, Schäfer R, Raats DA, de Graaff A, Schumacher TN, de Koning EJ, Rinkes IH, Kranenburg O, van Rheenen J. Sci Transl Med 2012,4,158ra145. [DOI] [PubMed] [Google Scholar]
- [91].Ritsma L, Ellenbroek SIJ, Zomer A, Snippert HJ, de Sauvage FJ, Simons BD, Clevers H, van Rheenen J. Nature 2014,507,362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Reissaus CA, Piñeros AR, Twigg AN, Orr KS, Conteh AM, Martinez MM, Kamocka MM, Day RN, Tersey SA, Mirmira RG, Dunn KW, Linnemann AK. Sci Rep 2019,9,8449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Dondossola E, Alexander S, Holzapfel BM, Filippini S, Starbuck MW, Hoffman RM, Navone N, De-Juan-Pardo EM, Logothetis CJ, Hutmacher DW, Friedl P. Sci Transl Med 2018,10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Carpenter RA, Kwak JG, Peyton SR, Lee J. Nat Biomed Eng 2018,2,915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Harris NR, Watts MN, Leskova W. J Vis Exp 201351110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Alt C, Runnels JM, Mortensen LJ, Zaher W, Lin CP. Invest Ophthalmol Vis Sci 2014,55,5314. [DOI] [PubMed] [Google Scholar]
- [97].Bremer D, Pache F, Günther R, Hornow J, Andresen V, Leben R, Mothes R, Zimmermann H, Brandt AU, Paul F, Hauser AE, Radbruch H, Niesner R. Front Immunol 2016,7,642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Alt C, Runnels JM, Teo GSL, Lin CP. IntraVital 2012,1,132. [Google Scholar]
- [99].Chitnis GD, Verma MKS, Lamazouade J, Gonzalez-Andrades M, Yang K, Dergham A, Jones PA, Mead BE, Cruzat A, Tong Z, Martyn K, Solanki A, Landon-Brace N, Karp JM. Nat Biomed Eng 2019,3,621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Thackaberry EA, Farman C, Zhong F, Lorget F, Staflin K, Cercillieux A, Miller PE, Schuetz C, Chang D, Famili A, Daugherty AL, Rajagopal K, Bantseev V. Invest Ophthalmol Vis Sci 2017,58,4274. [DOI] [PubMed] [Google Scholar]
- [101].Finn AP, Grewal DS, Vajzovic L. Clin Ophthalmol 2018,12,1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Luo YH, da Cruz L. Prog Retin Eye Res 2016,50,89. [DOI] [PubMed] [Google Scholar]
- [103].Cao L, Kobayakawa S, Yoshiki A, Abe K. PLoS One 2012,7,e33876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Lee S, Vinegoni C, Feruglio PF, Weissleder R. J Biomed Opt 2012,17,96018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Vinegoni C, Aguirre AD, Lee S, Weissleder R. Nat Protoc 2015,10,1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Aguirre AD, Vinegoni C, Sebas M, Weissleder R. Proc Natl Acad Sci U S A 2014,111,11257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Edelstein AD, Tsuchida MA, Amodaj N, Pinkard H, Vale RD, Stuurman N. J Biol Methods 2014,1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Caroline Müllenbroich M, McGhee EJ, Wright AJ, Anderson KI, Mathieson K. J Biomed Opt 2014,19,16021. [DOI] [PubMed] [Google Scholar]
- [109].Ji N, Sato TR, Betzig E. Proc Natl Acad Sci U S A 2012,109,22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Muriello PA, Dunn KW. Opt Commun 2008,281,1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Wen S, Zhou J, Zheng K, Bednarkiewicz A, Liu X, Jin D. Nat Commun 2018,9,2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Chen G, Shen J, Ohulchanskyy TY, Patel NJ, Kutikov A, Li Z, Song J, Pandey RK, Agren H, Prasad PN, Han G. ACS Nano 2012,6,8280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Kostyuk AB, Vorotnov AD, Ivanov AV, Volovetskiy AB, Kruglov AV, Sencha LM, Liang L, Guryev EL, Vodeneev VA, Deyev SM, Lu Y, Zvyagin AV. Nano Research 2019 [Google Scholar]
- [114].Hu S, Wang LV. J Biomed Opt 2010,15,011101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Li J, Xiao H, Yoon SJ, Liu C, Matsuura D, Tai W, Song L, O’Donnell M, Cheng D, Gao X. Small 2016,12,4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Yang W, Yuste R. Nat Methods 2017,14,349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Gerner MY, Kastenmuller W, Ifrim I, Kabat J, Germain RN. Immunity 2012,37,364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Richardson DS, Lichtman JW. Cell 2015,162,246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Tainaka K, Kuno A, Kubota SI, Murakami T, Ueda HR. Annu Rev Cell Dev Biol 2016,32,713. [DOI] [PubMed] [Google Scholar]
- [120].Tainaka K, Murakami TC, Susaki EA, Shimizu C, Saito R, Takahashi K, Hayashi-Takagi A, Sekiya H, Arima Y, Nojima S, Ikemura M, Ushiku T, Shimizu Y, Murakami M, Tanaka KF, Iino M, Kasai H, Sasaoka T, Kobayashi K, Miyazono K, Morii E, Isa T, Fukayama M, Kakita A, Ueda HR. Cell Rep 2018,24,2196. [DOI] [PubMed] [Google Scholar]
- [121].Gómez-Gaviro MV, Balaban E, Bocancea D, Lorrio MT, Pompeiano M, Desco M, Ripoll J, Vaquero JJ. Development 2017,144,2092. [DOI] [PubMed] [Google Scholar]
- [122].Du H, Hou P, Zhang W, Li Q. Exp Ther Med 2018,16,1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Renier N, Wu Z, Simon DJ, Yang J, Ariel P, Tessier-Lavigne M. Cell 2014,159,896. [DOI] [PubMed] [Google Scholar]
- [124].Cuccarese MF, Dubach JM, Pfirschke C, Engblom C, Garris C, Miller MA, Pittet MJ, Weissleder R. Nat Commun 2017,8,14293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Dixon AR, Bathany C, Tsuei M, White J, Barald KF, Takayama S. Expert Rev Mol Diagn 2015,15,1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Stack EC, Wang C, Roman KA, Hoyt CC. Methods 2014,70,46. [DOI] [PubMed] [Google Scholar]
- [127].Baron CS, van Oudenaarden A. Nat Rev Mol Cell Biol 2019,20,753. [DOI] [PubMed] [Google Scholar]
- [128].van den Brink SC, Sage F, Vértesy Á, Spanjaard B, Peterson-Maduro J, Baron CS, Robin C, van Oudenaarden A. Nat Methods 2017,14,935. [DOI] [PubMed] [Google Scholar]
- [129].Lin JR, Izar B, Wang S, Yapp C, Mei S, Shah PM, Santagata S, Sorger PK. Elife 2018,7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Aichler M, Walch A. Lab Invest 2015,95,422. [DOI] [PubMed] [Google Scholar]
- [131].Norris JL, Caprioli RM. Proteomics Clin Appl 2013,7,733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Han G, Spitzer MH, Bendall SC, Fantl WJ, Nolan GP. Nat Protoc 2018,13,2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Yang YS, Atukorale PU, Moynihan KD, Bekdemir A, Rakhra K, Tang L, Stellacci F, Irvine DJ. Nat Commun 2017,8,14069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Bera D, Qian L, Tseng T-K, Holloway PH. Materials 2010,3,2260. [Google Scholar]
- [135].Li J, Rao J, Pu K. Biomaterials 2018,155,217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Yong KT, Wang Y, Roy I, Rui H, Swihart MT, Law WC, Kwak SK, Ye L, Liu J, Mahajan SD, Reynolds JL. Theranostics 2012,2,681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Han G, Zhao J, Zhang R, Tian X, Liu Z, Wang A, Liu R, Liu B, Han MY, Gao X, Zhang Z. Angew Chem Int Ed Engl 2019,58,7087. [DOI] [PubMed] [Google Scholar]
- [138].Skajaa T, Zhao Y, van den Heuvel DJ, Gerritsen HC, Cormode DP, Koole R, van Schooneveld MM, Post JA, Fisher EA, Fayad ZA, de Mello Donega C, Meijerink A, Mulder WJ. Nano Lett 2010,10,5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Zhao MX, Zeng EZ. Nanoscale Res Lett 2015,10,171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Mukthavaram R, Wrasidlo W, Hall D, Kesari S, Makale M. Bioconjug Chem 2011,22,1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Chou LY, Chan WC. Adv Healthc Mater 2012,1,714. [DOI] [PubMed] [Google Scholar]
- [142].Lu Y, Dasog M, Leontowich AFG, Scott RWJ, Paige MF. The Journal of Physical Chemistry C 2010,114,17446. [Google Scholar]
- [143].Yoshimura SH, Khan S, Maruyama H, Nakayama Y, Takeyasu K. Biomacromolecules 2011,12,1200. [DOI] [PubMed] [Google Scholar]
- [144].Cheng NC, Estes BT, Young TH, Guilak F. Regen Med 2011,6,81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Amirikia M, Shariatzadeh SMA, Jorsaraei SGA, Mehranjani MS. Eur Biophys J 2018,47,573. [DOI] [PubMed] [Google Scholar]
- [146].Dondossola E, Holzapfel BM, Alexander S, Filippini S, Hutmacher DW, Friedl P. Nat Biomed Eng 2016,1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Berdichevski A, Simaan Yameen H, Dafni H, Neeman M, Seliktar D. Proc Natl Acad Sci U S A 2015,112,5147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Artzi N, Oliva N, Puron C, Shitreet S, Artzi S, bon Ramos A, Groothuis A, Sahagian G, Edelman ER. Nat Mater 2011,10,704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Cunha-Reis C, El Haj AJ, Yang X, Yang Y. J Tissue Eng Regen Med 2013,7,39. [DOI] [PubMed] [Google Scholar]
- [150].Zhao B, Sun X, Li X, Yang Q, Li Y, Zhang Y, Li B, Ma X. Neurosci Lett 2013,556,52. [DOI] [PubMed] [Google Scholar]
- [151].Rodell CB, Arlauckas SP, Cuccarese MF, Garris CS, Li R, Ahmed MS, Kohler RH, Pittet MJ, Weissleder R. Nat Biomed Eng 2018,2,578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Laughney AM, Kim E, Sprachman MM, Miller MA, Kohler RH, Yang KS, Orth JD, Mitchison TJ, Weissleder R. Sci Transl Med 2014,6,261ra152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Kim E, Yang KS, Kohler RH, Dubach JM, Mikula H, Weissleder R. Bioconjug Chem 2015,26,1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Mikula H, Stapleton S, Kohler RH, Vinegoni C, Weissleder R. Theranostics 2017,7,1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Pineda JJ, Miller MA, Song Y, Kuhn H, Mikula H, Tallapragada N, Weissleder R, Mitchison TJ. Proc Natl Acad Sci U S A 2018,115,E11406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Dorywalska M, Blanchard SC, Gonzalez RL, Kim HD, Chu S, Puglisi JD. Nucleic Acids Res 2005,33,182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Vira S, Mekhedov E, Humphrey G, Blank PS. Anal Biochem 2010,402,146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Debbage PL, Griebel J, Ried M, Gneiting T, DeVries A, Hutzler P. J Histochem Cytochem 1998,46,627. [DOI] [PubMed] [Google Scholar]
- [159].Honkura N, Richards M, Laviña B, Sáinz-Jaspeado M, Betsholtz C, Claesson-Welsh L. Nat Commun 2018,9,2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Miller MA, Kim E, Cuccarese MF, Plotkin AL, Prytyskach M, Kohler RH, Pittet MJ, Weissleder R. Chem Commun (Camb) 2017,54,42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Ceci F, Castellucci P, Fanti S. Q J Nucl Med Mol Imaging 2019,63,7. [DOI] [PubMed] [Google Scholar]
- [162].Cascini GL, Cuccurullo V, Tamburrini O, Rotondo A, Mansi L. Curr Radiopharm 2013,6,36. [DOI] [PubMed] [Google Scholar]
- [163].Weissleder R, Nahrendorf M, Pittet MJ. Nat Mater 2014,13,125. [DOI] [PubMed] [Google Scholar]
- [164].Tassa C, Shaw SY, Weissleder R. Acc Chem Res 2011,44,842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Liu Z, Zhao J, Zhang R, Han G, Zhang C, Liu B, Zhang Z, Han MY, Gao X. ACS Nano 2018,12,3629. [DOI] [PubMed] [Google Scholar]
- [166].Kilarski WW, Güç E, Teo JC, Oliver SR, Lund AW, Swartz MA. PLoS One 2013,8,e57135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Fang T, Lu X, Berger D, Gmeiner C, Cho J, Schalek R, Ploegh H, Lichtman J. Nat Methods 2018,15,1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Gonda K, Watanabe TM, Ohuchi N, Higuchi H. J Biol Chem 2010,285,2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Maggi A, Ciana P. Nat Rev Drug Discov 2005,4,249. [DOI] [PubMed] [Google Scholar]
- [170].Reinhardt RL, Hong S, Kang SJ, Wang ZE, Locksley RM. J Immunol 2006,177,1618. [DOI] [PubMed] [Google Scholar]
- [171].Jung S, Aliberti J, Graemmel P, Sunshine MJ, Kreutzberg GW, Sher A, Littman DR. Mol Cell Biol 2000,20,4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Fontenot JD, Dooley JL, Farr AG, Rudensky AY. J Exp Med 2005,202,901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Kalajzic I, Kalajzic Z, Kaliterna M, Gronowicz G, Clark SH, Lichtler AC, Rowe D. J Bone Miner Res 2002,17,15. [DOI] [PubMed] [Google Scholar]
- [174].Kuwata N, Igarashi H, Ohmura T, Aizawa S, Sakaguchi N. J Immunol 1999,163,6355. [PubMed] [Google Scholar]
- [175].Livet J, Weissman TA, Kang H, Draft RW, Lu J, Bennis RA, Sanes JR, Lichtman JW. Nature 2007,450,56. [DOI] [PubMed] [Google Scholar]
- [176].Snippert HJ, van der Flier LG, Sato T, van Es JH, van den Born M, Kroon-Veenboer C, Barker N, Klein AM, van Rheenen J, Simons BD, Clevers H. Cell 2010,143,134. [DOI] [PubMed] [Google Scholar]
- [177].Abe T, Kiyonari H, Shioi G, Inoue K, Nakao K, Aizawa S, Fujimori T. Genesis 2011,49,579. [DOI] [PubMed] [Google Scholar]
- [178].Morris SA, Teo RT, Li H, Robson P, Glover DM, Zernicka-Goetz M. Proc Natl Acad Sci U S A 2010,107,6364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Rhee JM, Pirity MK, Lackan CS, Long JZ, Kondoh G, Takeda J, Hadjantonakis AK. Genesis 2006,44,202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Pratt T, Sharp L, Nichols J, Price DJ, Mason JO. Dev Biol 2000,228,19. [DOI] [PubMed] [Google Scholar]
- [181].Shitara H, Kaneda H, Sato A, Iwasaki K, Hayashi J, Taya C, Yonekawa H. FEBS Lett 2001,500,7. [DOI] [PubMed] [Google Scholar]
- [182].Abe T, Sakaue-Sawano A, Kiyonari H, Shioi G, Inoue K, Horiuchi T, Nakao K, Miyawaki A, Aizawa S, Fujimori T. Development 2013,140,237. [DOI] [PubMed] [Google Scholar]
- [183].Sakaue-Sawano A, Miyawaki A. Cold Spring Harb Protoc 2014,2014 [DOI] [PubMed] [Google Scholar]
- [184].Tang K, Xia FC, Wagner PD, Breen EC. Respir Physiol Neurobiol 2010,170,16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Everhart MB, Han W, Sherrill TP, Arutiunov M, Polosukhin VV, Burke JR, Sadikot RT, Christman JW, Yull FE, Blackwell TS. J Immunol 2006,176,4995. [DOI] [PubMed] [Google Scholar]
- [186].Huang C, Ma WY, Dawson MI, Rincon M, Flavell RA, Dong Z. Proc Natl Acad Sci U S A 1997,94,5826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Yang KS, Kohler RH, Landon M, Giedt R, Weissleder R. Sci Rep 2015,5,10129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Miller MA, Askevold B, Yang KS, Kohler RH, Weissleder R. ChemMedChem 2014,9,1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Offterdinger M, Bastiaens PI. Traffic 2008,9,147. [DOI] [PubMed] [Google Scholar]
- [190].Kudo T, Jeknić S, Macklin DN, Akhter S, Hughey JJ, Regot S, Covert MW. Nat Protoc 2018,13,155. [DOI] [PubMed] [Google Scholar]
- [191].Roy R, Hohng S, Ha T. Nat Methods 2008,5,507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Muta Y, Fujita Y, Sumiyama K, Sakurai A, Taketo MM, Chiba T, Seno H, Aoki K, Matsuda M, Imajo M. Nat Commun 2018,9,2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Vennin C, Chin VT, Warren SC, Lucas MC, Herrmann D, Magenau A, Melenec P, Walters SN, Del Monte-Nieto G, Conway JR, Nobis M, Allam AH, McCloy RA, Currey N, Pinese M, Boulghourjian A, Zaratzian A, Adam AA, Heu C, Nagrial AM, Chou A, Steinmann A, Drury A, Froio D, Giry-Laterriere M, Harris NL, Phan T, Jain R, Weninger W, McGhee EJ, Whan R, Johns AL, Samra JS, Chantrill L, Gill AJ, Kohonen-Corish M, Harvey RP, Biankin AV, Australian APGI Pancreatic Cancer Genome Initiative, Evans TR, Anderson KI, Grey ST, Ormandy CJ, Gallego-Ortega D, Wang Y, Samuel MS, Sansom OJ, Burgess A, Cox TR, Morton JP, Pajic M, Timpson P. Sci Transl Med 2017,9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Nobis M, Herrmann D, Warren SC, Kadir S, Leung W, Killen M, Magenau A, Stevenson D, Lucas MC, Reischmann N, Vennin C, Conway JRW, Boulghourjian A, Zaratzian A, Law AM, Gallego-Ortega D, Ormandy CJ, Walters SN, Grey ST, Bailey J, Chtanova T, Quinn JMW, Baldock PA, Croucher PI, Schwarz JP, Mrowinska A, Zhang L, Herzog H, Masedunskas A, Hardeman EC, Gunning PW, Del Monte-Nieto G, Harvey RP, Samuel MS, Pajic M, McGhee EJ, Johnsson AE, Sansom OJ, Welch HCE, Morton JP, Strathdee D, Anderson KI, Timpson P. Cell Rep 2017,21,274. [DOI] [PubMed] [Google Scholar]
- [195].Fumagalli A, Drost J, Suijkerbuijk SJ, van Boxtel R, de Ligt J, Offerhaus GJ, Begthel H, Beerling E, Tan EH, Sansom OJ, Cuppen E, Clevers H, van Rheenen J. Proc Natl Acad Sci U S A 2017,114,E2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Torcellan T, Hampton HR, Bailey J, Tomura M, Brink R, Chtanova T. Proc Natl Acad Sci U S A 2017,114,5677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Pham AH, McCaffery JM, Chan DC. Genesis 2012,50,833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Chen X, Nadiarynkh O, Plotnikov S, Campagnola PJ. Nat Protoc 2012,7,654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Provenzano PP, Eliceiri KW, Campbell JM, Inman DR, White JG, Keely PJ. BMC Med 2006,4,38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Chauhan VP, Stylianopoulos T, Boucher Y, Jain RK. Annu Rev Chem Biomol Eng 2011,2,281. [DOI] [PubMed] [Google Scholar]
- [201].Vergen J, Hecht C, Zholudeva LV, Marquardt MM, Hallworth R, Nichols MG. Microsc Microanal 2012,18,761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Schaefer PM, Kalinina S, Rueck A, von Arnim CAF, von Einem B. Cytometry A 2019,95,34. [DOI] [PubMed] [Google Scholar]
- [203].Gómez CA, Sutin J, Wu W, Fu B, Uhlirova H, Devor A, Boas DA, Sakadžić S, Yaseen MA. PLoS One 2018,13,e0194578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. J Control Release 2000,65,271. [DOI] [PubMed] [Google Scholar]
- [205].Maeda H, Nakamura H, Fang J. Adv Drug Deliv Rev 2013,65,71. [DOI] [PubMed] [Google Scholar]
- [206].Torchilin V. Adv Drug Deliv Rev 2011,63,131. [DOI] [PubMed] [Google Scholar]
- [207].Heldin CH, Rubin K, Pietras K, Ostman A. Nat Rev Cancer 2004,4,806. [DOI] [PubMed] [Google Scholar]
- [208].Rosenblum D, Joshi N, Tao W, Karp JM, Peer D. Nat Commun 2018,9,1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Nehoff H, Parayath NN, Domanovitch L, Taurin S, Greish K. Int J Nanomedicine 2014,9,2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Miller MA, Askevold B, Mikula H, Kohler RH, Pirovich D, Weissleder R. Nat Commun 2017,8,15906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Miller MA, Mikula H, Luthria G, Li R, Kronister S, Prytyskach M, Kohler RH, Mitchison T, Weissleder R. ACS Nano 2018,12,12814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Miller MA, Chandra R, Cuccarese MF, Pfirschke C, Engblom C, Stapleton S, Adhikary U, Kohler RH, Mohan JF, Pittet MJ, Weissleder R. Sci Transl Med 2017,9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Saxena A, Kessinger CW, Thompson B, McCarthy JR, Iwamoto Y, Lin CP, Jaffer FA. Mol Imaging Biol 2013,15,282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Choi MR, Stanton-Maxey KJ, Stanley JK, Levin CS, Bardhan R, Akin D, Badve S, Sturgis J, Robinson JP, Bashir R, Halas NJ, Clare SE. Nano Lett 2007,7,3759. [DOI] [PubMed] [Google Scholar]
- [215].Smith BR, Ghosn EE, Rallapalli H, Prescher JA, Larson T, Herzenberg LA, Gambhir SS. Nat Nanotechnol 2014,9,481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Zhao Y, van Rooy I, Hak S, Fay F, Tang J, Davies CL, Skobe M, Fisher EA, Radu A, Fayad ZA, de Mello Donegá C, Meijerink A, Mulder WJ. ACS Nano 2013,7,10362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Lo JH, Hao L, Muzumdar MD, Raghavan S, Kwon EJ, Pulver EM, Hsu F, Aguirre AJ, Wolpin BM, Fuchs CS, Hahn WC, Jacks T, Bhatia SN. Molecular Cancer Therapeutics 2018,17,2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Tai W, Li J, Corey E, Gao X. Nat Biomed Eng 2018,2,326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Liu X, Jiang J, Chan R, Ji Y, Lu J, Liao YP, Okene M, Lin J, Lin P, Chang CH, Wang X, Tang I, Zheng E, Qiu W, Wainberg ZA, Nel AE, Meng H. ACS Nano 2019,13,38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Lu J, Liu X, Liao YP, Wang X, Ahmed A, Jiang W, Ji Y, Meng H, Nel AE. ACS Nano 2018,12,11041. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [221].Lee SS, Bindokas VP, Kron SJ. Mol Cancer Ther 2019,18,213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Li F, Ulrich M, Jonas M, Stone IJ, Linares G, Zhang X, Westendorf L, Benjamin DR, Law CL. Mol Cancer Ther 2017,16,1347. [DOI] [PubMed] [Google Scholar]
- [223].Chu D, Gao J, Wang Z. ACS Nano 2015,9,11800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224].Gao J, Chu D, Wang Z. J Control Release 2016,224,208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Fromen CA, Kelley WJ, Fish MB, Adili R, Noble J, Hoenerhoff MJ, Holinstat M, Eniola-Adefeso O. ACS Nano 2017,11,10797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [226].Wang Z, Li J, Cho J, Malik AB. Nat Nanotechnol 2014,9,204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Kotsovilis S, Andreakos E. Methods Mol Biol 2014,1060,37. [DOI] [PubMed] [Google Scholar]
- [228].Qi J, Liu D, Liu X, Guan S, Shi F, Chang H, He H, Yang G. Anal Chem 2015,87,5897. [DOI] [PubMed] [Google Scholar]
- [229].Tsai YT, Zhou J, Weng H, Shen J, Tang L, Hu WJ. Adv Healthc Mater 2014,3,221. [DOI] [PubMed] [Google Scholar]
- [230].Austen K, Ringer P, Mehlich A, Chrostek-Grashoff A, Kluger C, Klingner C, Sabass B, Zent R, Rief M, Grashoff C. Nat Cell Biol 2015,17,1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231].LaCroix AS, Lynch AD, Berginski ME, Hoffman BD. Elife 2018,7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [232].Dong X. Theranostics 2018,8,1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [233].Teleanu DM, Chircov C, Grumezescu AM, Volceanov A, Teleanu RI. Pharmaceutics 2018,10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [234].Ding H, Wu F, Nair MP. Drug Discov Today 2013,18,1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [235].Lam FC, Morton SW, Wyckoff J, Vu Han TL, Hwang MK, Maffa A, Balkanska-Sinclair E, Yaffe MB, Floyd SR, Hammond PT. Nat Commun 2018,9,1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [236].Chen ZL, Huang M, Wang XR, Fu J, Han M, Shen YQ, Xia Z, Gao JQ. Nanomedicine 2016,12,421. [DOI] [PubMed] [Google Scholar]
- [237].dos Santos Rodrigues B, Oue H, Banerjee A, Kanekiyo T, Singh J. Journal of Controlled Release 2018,286,264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [238].Gao S, Tian H, Xing Z, Zhang D, Guo Y, Guo Z, Zhu X, Chen X. J Control Release 2016,243,357. [DOI] [PubMed] [Google Scholar]
- [239].Li Y, Zheng X, Gong M, Zhang J. Oncotarget 2016,7,79401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [240].Koffie RM, Farrar CT, Saidi LJ, William CM, Hyman BT, Spires-Jones TL. Proc Natl Acad Sci U S A 2011,108,18837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [241].Wang D, El-Amouri SS, Dai M, Kuan CY, Hui DY, Brady RO, Pan D. Proc Natl Acad Sci U S A 2013,110,2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [242].Medina DX, Householder KT, Ceton R, Kovalik T, Heffernan JM, Shankar RV, Bowser RP, Wechsler-Reya RJ, Sirianni RW. J Control Release 2017,253,172. [DOI] [PubMed] [Google Scholar]
- [243].Jiang Y, Brynskikh AM, S-Manickam D, Kabanov AV. J Control Release 2015,213,36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [244].Harris NM, Ritzel R, Mancini NS, Jiang Y, Yi X, Manickam DS, Banks WA, Kabanov AV, McCullough LD, Verma R. Pharmacol Biochem Behav 2016,150–151,48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [245].Chu C, Jablonska A, Lesniak WG, Thomas AM, Lan X, Linville RM, Li S, Searson PC, Liu G, Pearl M, Pomper MG, Janowski M, Magnus T, Walczak P. J Control Release 2019 [DOI] [PubMed] [Google Scholar]
- [246].Afergan E, Epstein H, Dahan R, Koroukhov N, Rohekar K, Danenberg HD, Golomb G. J Control Release 2008,132,84. [DOI] [PubMed] [Google Scholar]
- [247].Li T, Bourgeois JP, Celli S, Glacial F, Le Sourd AM, Mecheri S, Weksler B, Romero I, Couraud PO, Rougeon F, Lafaye P. FASEB J 2012,26,3969. [DOI] [PubMed] [Google Scholar]
- [248].Cabral H. IFAC-PapersOnLine 2018,51,22. [Google Scholar]
- [249].van der Vos KE, Abels ER, Zhang X, Lai C, Carrizosa E, Oakley D, Prabhakar S, Mardini O, Crommentuijn MH, Skog J, Krichevsky AM, Stemmer-Rachamimov A, Mempel TR, El Khoury J, Hickman SE, Breakefield XO. Neuro Oncol 2016,18,58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [250].Walker S, Busatto S, Pham A, Tian M, Suh A, Carson K, Quintero A, Lafrence M, Malik H, Santana MX, Wolfram J. Theranostics 2019,9,8001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [251].De Niz M, Nacer A, Frischknecht F. Cell Microbiol 2019,21,e13024. [DOI] [PubMed] [Google Scholar]
- [252].Shukla S, Dorand RD, Myers JT, Woods SE, Gulati NM, Stewart PL, Commandeur U, Huang AY, Steinmetz NF. ACS Biomater Sci Eng 2016,2,829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [253].Shih AY, Driscoll JD, Drew PJ, Nishimura N, Schaffer CB, Kleinfeld D. J Cereb Blood Flow Metab 2012,32,1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [254].Niehl A, Appaix F, Boscá S, van der Sanden B, Nicoud JF, Bolze F, Heinlein M. Front Plant Sci 2015,6,1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [255].Jones SW, Roberts RA, Robbins GR, Perry JL, Kai MP, Chen K, Bo T, Napier ME, Ting JP, Desimone JM, Bear JE. J Clin Invest 2013,123,3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [256].Kai MP, Brighton HE, Fromen CA, Shen TW, Luft JC, Luft YE, Keeler AW, Robbins GR, Ting JP, Zamboni WC, Bear JE, DeSimone JM. ACS Nano 2016,10,861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [257].Peterson NC, Wilson GG, Huang Q, Dimasi N, Sachsenmeier KF. Comp Med 2016,66,90. [PMC free article] [PubMed] [Google Scholar]
- [258].Schneider DW, Heitner T, Alicke B, Light DR, McLean K, Satozawa N, Parry G, Yoo J, Lewis JS, Parry R. J Nucl Med 2009,50,435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [259].Sharma SK, Chow A, Monette S, Vivier D, Pourat J, Edwards KJ, Dilling TR, Abdel-Atti D, Zeglis BM, Poirier JT, Lewis JS. Cancer Res 2018,78,1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [260].Press AT, Traeger A, Pietsch C, Mosig A, Wagner M, Clemens MG, Jbeily N, Koch N, Gottschaldt M, Bézière N, Ermolayev V, Ntziachristos V, Popp J, Kessels MM, Qualmann B, Schubert US, Bauer M. Nat Commun 2014,5,5565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [261].Cheng SH, Li FC, Souris JS, Yang CS, Tseng FG, Lee HS, Chen CT, Dong CY, Lo LW. ACS Nano 2012,6,4122. [DOI] [PubMed] [Google Scholar]
- [262].Yildirim T, Matthäus C, Press AT, Schubert S, Bauer M, Popp J, Schubert US. Macromol Biosci 2017,17 [DOI] [PubMed] [Google Scholar]
- [263].Chen A, Dogdas B, Mehta S, Haskell K, Ng B, Keough E, Howell B, Meacham DA, Aslamkhan AG, Davide J, Stanton M, Bagchi A, Sepp-Lorenzino L, Tao W. Conf Proc IEEE Eng Med Biol Soc 2012,2012,3712. [DOI] [PubMed] [Google Scholar]
- [264].Tanei T, Leonard F, Liu X, Alexander JF, Saito Y, Ferrari M, Godin B, Yokoi K. Cancer Res 2016,76,429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [265].Tavares AJ, Poon W, Zhang YN, Dai Q, Besla R, Ding D, Ouyang B, Li A, Chen J, Zheng G, Robbins C, Chan WCW. Proc Natl Acad Sci U S A 2017,114,E10871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [266].MacParland SA, Tsoi KM, Ouyang B, Ma XZ, Manuel J, Fawaz A, Ostrowski MA, Alman BA, Zilman A, Chan WC, McGilvray ID. ACS Nano 2017,11,2428. [DOI] [PubMed] [Google Scholar]
- [267].Tai W, Gao X. Biomaterials 2018,178,720. [DOI] [PubMed] [Google Scholar]
- [268].Liu Q, Wang X, Liu X, Kumar S, Gochman G, Ji Y, Liao YP, Chang CH, Situ W, Lu J, Jiang J, Mei KC, Meng H, Xia T, Nel AE. ACS Nano 2019,13,4778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [269].Williams RM, Shah J, Tian HS, Chen X, Geissmann F, Jaimes EA, Heller DA. Hypertension 2018,71,87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [270].Naumenko V, Nikitin A, Kapitanova K, Melnikov P, Vodopyanov S, Garanina A, Valikhov M, Ilyasov A, Vishnevskiy D, Markov A, Golyshev S, Zhukov D, Alieva I, Abakumov M, Chekhonin V, Majouga A. J Control Release 2019,307,368. [DOI] [PubMed] [Google Scholar]
- [271].Uchida M, Maier B, Waghwani HK, Selivanovitch E, Pay SL, Avera J, Yun E, Sandoval RM, Molitoris BA, Zollman A, Douglas T, Hato T. J Clin Invest 2019,129,3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [272].Martel J, Wu CY, Young JD. Nanomedicine (Lond) 2016,11,2399. [DOI] [PubMed] [Google Scholar]
- [273].Schießl IM, Hammer A, Kattler V, Gess B, Theilig F, Witzgall R, Castrop H. J Am Soc Nephrol 2016,27,731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [274].Rossin R, Muro S, Welch MJ, Muzykantov VR, Schuster DP. J Nucl Med 2008,49,103. [DOI] [PubMed] [Google Scholar]
- [275].Ferreira MPA, Ranjan S, Kinnunen S, Correia A, Talman V, Mäkilä E, Barrios-Lopez B, Kemell M, Balasubramanian V, Salonen J, Hirvonen J, Ruskoaho H, Airaksinen AJ, Santos HA. Small 2017,13 [DOI] [PubMed] [Google Scholar]
- [276].Pellico J, Lechuga-Vieco AV, Almarza E, Hidalgo A, Mesa-Nuñez C, Fernández-Barahona I, Quintana JA, Bueren J, Enríquez JA, Ruiz-Cabello J, Herranz F. Sci Rep 2017,7,13242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [277].Black KC, Ibricevic A, Gunsten SP, Flores JA, Gustafson TP, Raymond JE, Samarajeewa S, Shrestha R, Felder SE, Cai T, Shen Y, Löbs AK, Zhegalova N, Sultan DH, Berezin M, Wooley KL, Liu Y, Brody SL. Biomaterials 2016,98,53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [278].Huang G, Zhang C, Li S, Khemtong C, Yang SG, Tian R, Minna JD, Brown KC, Gao J. J Mater Chem 2009,19,6367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [279].Yang L, Gradl R, Dierolf M, Möller W, Kutschke D, Feuchtinger A, Hehn L, Donnelley M, Günther B, Achterhold K, Walch A, Stoeger T, Razansky D, Pfeiffer F, Morgan KS, Schmid O. Small 2019. e1904112. [DOI] [PubMed] [Google Scholar]
- [280].Cui J, Kessinger CW, McCarthy JR, Sosnovik DE, Libby P, Thadhani RI, Jaffer FA. Arterioscler Thromb Vasc Biol 2015,35,189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [281].Dvir T, Bauer M, Schroeder A, Tsui JH, Anderson DG, Langer R, Liao R, Kohane DS. Nano Lett 2011,11,4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [282].Ahmed MS, Rodell CB, Hulsmans M, Kohler RH, Aguirre AD, Nahrendorf M, Weissleder R. Bioconjug Chem 2019,30,733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [283].Miragoli M, Ceriotti P, Iafisco M, Vacchiano M, Salvarani N, Alogna A, Carullo P, Ramirez-Rodríguez GB, Patrício T, Esposti LD, Rossi F, Ravanetti F, Pinelli S, Alinovi R, Erreni M, Rossi S, Condorelli G, Post H, Tampieri A, Catalucci D. Sci Transl Med 2018,10 [DOI] [PubMed] [Google Scholar]
- [284].Detampel P, Ganguly A, Tehranian S, Green F, Singha S, Santamaria P, Jeje AA, Cho CS, Petri B, Amrein MW. PLoS One 2019,14,e0223339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [285].Nurkiewicz TR, Porter DW, Hubbs AF, Cumpston JL, Chen BT, Frazer DG, Castranova V. Part Fibre Toxicol 2008,5,1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [286].Roller J, Laschke MW, Tschernig T, Schramm R, Veith NT, Thorlacius H, Menger MD. Nanomedicine 2011,7,753. [DOI] [PubMed] [Google Scholar]
- [287].Albanese A, Tang PS, Chan WC. Annu Rev Biomed Eng 2012,14,1. [DOI] [PubMed] [Google Scholar]
- [288].Jiang W, Kim BY, Rutka JT, Chan WC. Nat Nanotechnol 2008,3,145. [DOI] [PubMed] [Google Scholar]
- [289].Wang X, Chang CH, Jiang J, Liu Q, Liao YP, Lu J, Li L, Liu X, Kim J, Ahmed A, Nel AE, Xia T. Small 2019,15,e1901642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [290].Cabral H, Matsumoto Y, Mizuno K, Chen Q, Murakami M, Kimura M, Terada Y, Kano MR, Miyazono K, Uesaka M, Nishiyama N, Kataoka K. Nat Nanotechnol 2011,6,815. [DOI] [PubMed] [Google Scholar]
- [291].Huo S, Ma H, Huang K, Liu J, Wei T, Jin S, Zhang J, He S, Liang XJ. Cancer Res 2013,73,319. [DOI] [PubMed] [Google Scholar]
- [292].Dong YC, Hajfathalian M, Maidment PSN, Hsu JC, Naha PC, Si-Mohamed S, Breuilly M, Kim J, Chhour P, Douek P, Litt HI, Cormode DP. Sci Rep 2019,9,14912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [293].Rapoport N, Gupta R, Kim YS, O’Neill BE. J Control Release 2015,206,153. [DOI] [PubMed] [Google Scholar]
- [294].Song C, Meng X, Liu Y, Shen A, Shao C, Wang K, Cheng H, Fang X, Wang P, Bu W. Biomaterials 2020,230,119631. [DOI] [PubMed] [Google Scholar]
- [295].Park JH, von Maltzahn G, Zhang L, Derfus AM, Simberg D, Harris TJ, Ruoslahti E, Bhatia SN, Sailor MJ. Small 2009,5,694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [296].Agarwal R, Jurney P, Raythatha M, Singh V, Sreenivasan SV, Shi L, Roy K. Adv Healthc Mater 2015,4,2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [297].Decuzzi P, Pasqualini R, Arap W, Ferrari M. Pharm Res 2009,26,235. [DOI] [PubMed] [Google Scholar]
- [298].Zhao Y, Wang Y, Ran F, Cui Y, Liu C, Zhao Q, Gao Y, Wang D, Wang S. Sci Rep 2017,7,4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [299].Christian DA, Cai S, Garbuzenko OB, Harada T, Zajac AL, Minko T, Discher DE. Mol Pharm 2009,6,1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [300].Geng Y, Dalhaimer P, Cai S, Tsai R, Tewari M, Minko T, Discher DE. Nat Nanotechnol 2007,2,249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [301].Decuzzi P, Ferrari M. Biophys J 2008,94,3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [302].Barua S, Yoo JW, Kolhar P, Wakankar A, Gokarn YR, Mitragotri S. Proc Natl Acad Sci U S A 2013,110,3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [303].Fröhlich E. Int J Nanomedicine 2012,7,5577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [304].Huang YW, Cambre M, Lee HJ. Int J Mol Sci 2017,18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [305].Wang H-X, Zuo Z-Q, Du J-Z, Wang Y-C, Sun R, Cao Z-T, Ye X-D, Wang J-L, Leong KW, Wang J. Nano Today 2016,11,133. [Google Scholar]
- [306].Han D, Qi H, Huang K, Li X, Zhan Q, Zhao J, Hou X, Yang X, Kang C, Yuan X. Journal of Materials Chemistry B 2018,6,3331. [DOI] [PubMed] [Google Scholar]
- [307].Walkey CD, Olsen JB, Guo H, Emili A, Chan WC. J Am Chem Soc 2012,134,2139. [DOI] [PubMed] [Google Scholar]
- [308].Maeda H. Adv Drug Deliv Rev 2015,91,3. [DOI] [PubMed] [Google Scholar]
- [309].Xenaki KT, Oliveira S, van PMP Bergen En Henegouwen. Front Immunol 2017,8,1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [310].Thurber GM, Schmidt MM, Wittrup KD. Adv Drug Deliv Rev 2008,60,1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [311].Cilliers C, Menezes B, Nessler I, Linderman J, Thurber GM. Cancer Res 2018,78,758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [312].Stylianopoulos T, Munn LL, Jain RK. Trends Cancer 2018,4,292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [313].Qiao Y, Huang X, Nimmagadda S, Bai R, Staedtke V, Foss CA, Cheong I, Holdhoff M, Kato Y, Pomper MG, Riggins GJ, Kinzler KW, Diaz LA, Vogelstein B, Zhou S. Oncotarget 2011,2,59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [314].Daldrup-Link HE, Mohanty S, Ansari C, Lenkov O, Shaw A, Ito K, Hong SH, Hoffmann M, Pisani L, Boudreau N, Gambhir SS, Coussens LM. JCI Insight 2016,1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [315].Melnikov PA, Valikhov MP, Kuznetsov II, Grinenko NF, Sukhinich KK, Simbirtsev AS, Kekelidze ZI, Chekhonin VP. Bull Exp Biol Med 2019 [DOI] [PubMed] [Google Scholar]
- [316].Anraku Y, Kuwahara H, Fukusato Y, Mizoguchi A, Ishii T, Nitta K, Matsumoto Y, Toh K, Miyata K, Uchida S, Nishina K, Osada K, Itaka K, Nishiyama N, Mizusawa H, Yamasoba T, Yokota T, Kataoka K. Nat Commun 2017,8,1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [317].Arvanitis CD, Askoxylakis V, Guo Y, Datta M, Kloepper J, Ferraro GB, Bernabeu MO, Fukumura D, McDannold N, Jain RK. Proc Natl Acad Sci U S A 2018,115,E8717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [318].Yemane PT, Åslund A, Sæterbø KG, Bjørkøy A, Snipstad S, Van Wamel A, Berg S, Mørch Y, Hansen R, Angelsen B. 2018,2018 IEEE International Ultrasonics Symposium,1. [Google Scholar]
- [319].Motamarry A, Negussie AH, Rossmann C, Small J, Wolfe AM, Wood BJ, Haemmerich D. Int J Hyperthermia 2019,36,817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [320].Stapleton S, Dunne M, Milosevic M, Tran CW, Gold MJ, Vedadi A, Mckee TD, Ohashi PS, Allen C, Jaffray DA. ACS Nano 2018,12,7583. [DOI] [PubMed] [Google Scholar]
- [321].Li L, ten Hagen TL, Hossann M, Süss R, van Rhoon GC, Eggermont AM, Haemmerich D, Koning GA. J Control Release 2013,168,142. [DOI] [PubMed] [Google Scholar]
- [322].Bagley AF, Scherz-Shouval R, Galie PA, Zhang AQ, Wyckoff J, Whitesell L, Chen CS, Lindquist S, Bhatia SN. Cancer Res 2015,75,3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [323].Goel S, Duda DG, Xu L, Munn LL, Boucher Y, Fukumura D, Jain RK. Physiol Rev 2011,91,1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [324].Willett CG, Boucher Y, di Tomaso E, Duda DG, Munn LL, Tong RT, Chung DC, Sahani DV, Kalva SP, Kozin SV, Mino M, Cohen KS, Scadden DT, Hartford AC, Fischman AJ, Clark JW, Ryan DP, Zhu AX, Blaszkowsky LS, Chen HX, Shellito PC, Lauwers GY, Jain RK. Nat Med 2004,10,145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [325].Chauhan VP, Stylianopoulos T, Martin JD, Popović Z, Chen O, Kamoun WS, Bawendi MG, Fukumura D, Jain RK. Nat Nanotechnol 2012,7,383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [326].Jiang W, Huang Y, An Y, Kim BY. ACS Nano 2015,9,8689. [DOI] [PubMed] [Google Scholar]
- [327].Xiao W, Ruan S, Yu W, Wang R, Hu C, Liu R, Gao H. Mol Pharm 2017,14,3489. [DOI] [PubMed] [Google Scholar]
- [328].Huang Y, Stylianopoulos T, Duda DG, Fukumura D, Jain RK. Cancer Res 2013,73,7144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [329].Nakahara T, Norberg SM, Shalinsky DR, Hu-Lowe DD, McDonald DM. Cancer Res 2006,66,1434. [DOI] [PubMed] [Google Scholar]
- [330].Kurozumi K, Hardcastle J, Thakur R, Yang M, Christoforidis G, Fulci G, Hochberg FH, Weissleder R, Carson W, Chiocca EA, Kaur B. J Natl Cancer Inst 2007,99,1768. [DOI] [PubMed] [Google Scholar]
- [331].Tong RT, Boucher Y, Kozin SV, Winkler F, Hicklin DJ, Jain RK. Cancer Res 2004,64,3731. [DOI] [PubMed] [Google Scholar]
- [332].Matsumoto Y, Nichols JW, Toh K, Nomoto T, Cabral H, Miura Y, Christie RJ, Yamada N, Ogura T, Kano MR, Matsumura Y, Nishiyama N, Yamasoba T, Bae YH, Kataoka K. Nat Nanotechnol 2016,11,533. [DOI] [PubMed] [Google Scholar]
- [333].Naumenko VA, Vlasova KY, Garanina AS, Melnikov PA, Potashnikova DM, Vishnevskiy DA, Vodopyanov SS, Chekhonin VP, Abakumov MA, Majouga AG. ACS Nano 2019,13,12599. [DOI] [PubMed] [Google Scholar]
- [334].Diop-Frimpong B, Chauhan VP, Krane S, Boucher Y, Jain RK. Proc Natl Acad Sci U S A 2011,108,2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [335].Liu J, Liao S, Diop-Frimpong B, Chen W, Goel S, Naxerova K, Ancukiewicz M, Boucher Y, Jain RK, Xu L. Proc Natl Acad Sci U S A 2012,109,16618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [336].Zhao Y, Cao J, Melamed A, Worley M, Gockley A, Jones D, Nia HT, Zhang Y, Stylianopoulos T, Kumar AS, Mpekris F, Datta M, Sun Y, Wu L, Gao X, Yeku O, Del Carmen MG, Spriggs DR, Jain RK, Xu L. Proc Natl Acad Sci U S A 2019,116,2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [337].Papageorgis P, Polydorou C, Mpekris F, Voutouri C, Agathokleous E, Kapnissi-Christodoulou CP, Stylianopoulos T. Sci Rep 2017,7,46140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [338].Kohli AG, Kivimäe S, Tiffany MR, Szoka FC. J Control Release 2014,191,105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [339].Provenzano PP, Cuevas C, Chang AE, Goel VK, Von Hoff DD, Hingorani SR. Cancer Cell 2012,21,418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [340].Weiser JR, Saltzman WM. J Control Release 2014,190,664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [341].Lammers T, Peschke P, Kühnlein R, Subr V, Ulbrich K, Huber P, Hennink W, Storm G. Neoplasia 2006,8,788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [342].Yook S, Cai Z, Lu Y, Winnik MA, Pignol JP, Reilly RM. J Nucl Med 2016,57,936. [DOI] [PubMed] [Google Scholar]
- [343].Giustini AJ, Ivkov R, Hoopes PJ. Nanotechnology 2011,22,345101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [344].Dong J, Song X, Lian X, Fu Y, Gong T. Drug Deliv 2016,23,2220. [DOI] [PubMed] [Google Scholar]
- [345].Jogala S, Rachamalla SS, Aukunuru J. J Adv Pharm Technol Res 2015,6,58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [346].Maeda H, Kowada T, Kikuta J, Furuya M, Shirazaki M, Mizukami S, Ishii M, Kikuchi K. Nat Chem Biol 2016,12,579. [DOI] [PubMed] [Google Scholar]
- [347].Momin N, Mehta NK, Bennett NR, Ma L, Palmeri JR, Chinn MM, Lutz EA, Kang B, Irvine DJ, Spranger S, Wittrup KD. Sci Transl Med 2019,11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [348].Katsumata K, Ishihara J, Mansurov A, Ishihara A, Raczy MM, Yuba E, Hubbell JA. Sci Adv 2019,5,eaay1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [349].Sasaki K, Ishihara J, Ishihara A, Miura R, Mansurov A, Fukunaga K, Hubbell JA. Sci Adv 2019,5,eaaw6081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [350].Liang H, Li X, Wang B, Chen B, Zhao Y, Sun J, Zhuang Y, Shi J, Shen H, Zhang Z, Dai J. Sci Rep 2016,6,18205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [351].Tewari D, Java JJ, Salani R, Armstrong DK, Markman M, Herzog T, Monk BJ, Chan JK. J Clin Oncol 2015,33,1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [352].Dogra P, Adolphi NL, Wang Z, Lin YS, Butler KS, Durfee PN, Croissant JG, Noureddine A, Coker EN, Bearer EL, Cristini V, Brinker CJ. Nat Commun 2018,9,4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [353].Appel EA, Tibbitt MW, Webber MJ, Mattix BA, Veiseh O, Langer R. Nat Commun 2015,6,6295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [354].Wei C, Dong X, Liang J, Zhang Y, Zhu D, Kong D, Lv F. J Biomed Nanotechnol 2019,15,100. [DOI] [PubMed] [Google Scholar]
- [355].Chen Q, Wang C, Zhang X, Chen G, Hu Q, Li H, Wang J, Wen D, Zhang Y, Lu Y, Yang G, Jiang C, Wang J, Dotti G, Gu Z. Nat Nanotechnol 2019,14,89. [DOI] [PubMed] [Google Scholar]
- [356].Jewell CM, López SC, Irvine DJ. Proc Natl Acad Sci U S A 2011,108,15745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [357].Zhou C, Li HF, Yin YX, Shi ZZ, Li T, Feng XY, Zhang JW, Song CX, Cui XS, Xu KL, Zhao YW, Hou WB, Lu ST, Liu G, Li MQ, Ma JY, Toft E, Volinsky AA, Wan M, Yao XJ, Wang CB, Yao K, Xu SK, Lu H, Chang SF, Ge JB, Wang LN, Zhang HJ. Acta Biomater 2019,97,657. [DOI] [PubMed] [Google Scholar]
- [358].Ebert M, Ebert J, Berger G. Journal of Drug Delivery 2013,2013,1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [359].Emmert MY, Schmitt BA, Loerakker S, Sanders B, Spriestersbach H, Fioretta ES, Bruder L, Brakmann K, Motta SE, Lintas V, Dijkman PE, Frese L, Berger F, Baaijens FPT, Hoerstrup SP. Sci Transl Med 2018,10 [DOI] [PubMed] [Google Scholar]
- [360].Whyte W, Roche ET, Varela CE, Mendez K, Islam S, O’Neill H, Weafer F, Shirazi RN, Weaver JC, Vasilyev NV, McHugh PE, Murphy B, Duffy GP, Walsh CJ, Mooney DJ. Nat Biomed Eng 2018,2,416. [DOI] [PubMed] [Google Scholar]
- [361].Turetsky A, Kim E, Kohler RH, Miller MA, Weissleder R. Sci Rep 2014,4,4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [362].Dubach JM, Vinegoni C, Mazitschek R, Fumene Feruglio P, Cameron LA, Weissleder R. Nat Commun 2014,5,3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [363].Dubach JM, Kim E, Yang K, Cuccarese M, Giedt RJ, Meimetis LG, Vinegoni C, Weissleder R. Nat Chem Biol 2017,13,168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [364].Memon AA, Jakobsen S, Dagnaes-Hansen F, Sorensen BS, Keiding S, Nexo E. Cancer Res 2009,69,873. [DOI] [PubMed] [Google Scholar]
- [365].Wang JQ, Gao M, Miller KD, Sledge GW, Zheng QH. Bioorg Med Chem Lett 2006,16,4102. [DOI] [PubMed] [Google Scholar]
- [366].Samén E, Thorell JO, Fredriksson A, Stone-Elander S. Nucl Med Biol 2006,33,1005. [DOI] [PubMed] [Google Scholar]
- [367].Hicks JW, VanBrocklin HF, Wilson AA, Houle S, Vasdev N. Molecules 2010,15,8260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [368].Willmann JK, van Bruggen N, Dinkelborg LM, Gambhir SS. Nat Rev Drug Discov 2008,7,591. [DOI] [PubMed] [Google Scholar]
- [369].Nobis M, McGhee EJ, Morton JP, Schwarz JP, Karim SA, Quinn J, Edward M, Campbell AD, McGarry LC, Evans TR, Brunton VG, Frame MC, Carragher NO, Wang Y, Sansom OJ, Timpson P, Anderson KI. Cancer Res 2013,73,4674. [DOI] [PubMed] [Google Scholar]
- [370].Hirata E, Girotti MR, Viros A, Hooper S, Spencer-Dene B, Matsuda M, Larkin J, Marais R, Sahai E. Cancer Cell 2015,27,574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [371].Schmid D, Park CG, Hartl CA, Subedi N, Cartwright AN, Puerto RB, Zheng Y, Maiarana J, Freeman GJ, Wucherpfennig KW, Irvine DJ, Goldberg MS. Nat Commun 2017,8,1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [372].Choe SW, Acharya AP, Keselowsky BG, Sorg BS. Acta Biomater 2010,6,3491. [DOI] [PubMed] [Google Scholar]
- [373].Xiang J, Xu L, Gong H, Zhu W, Wang C, Xu J, Feng L, Cheng L, Peng R, Liu Z. ACS Nano 2015,9,6401. [DOI] [PubMed] [Google Scholar]
- [374].Park J, Wysocki RW, Amoozgar Z, Maiorino L, Fein MR, Jorns J, Schott AF, Kinugasa-Katayama Y, Lee Y, Won NH, Nakasone ES, Hearn SA, Küttner V, Qiu J, Almeida AS, Perurena N, Kessenbrock K, Goldberg MS, Egeblad M. Sci Transl Med 2016,8,361ra138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [375].Olingy CE, San Emeterio CL, Ogle ME, Krieger JR, Bruce AC, Pfau DD, Jordan BT, Peirce SM, Botchwey EA. Sci Rep 2017,7,447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [376].Shah NJ, Mao AS, Shih TY, Kerr MD, Sharda A, Raimondo TM, Weaver JC, Vrbanac VD, Deruaz M, Tager AM, Mooney DJ, Scadden DT. Nat Biotechnol 2019,37,293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [377].Wolf MT, Ganguly S, Wang TL, Anderson CW, Sadtler K, Narain R, Cherry C, Parrillo AJ, Park BV, Wang G, Pan F, Sukumar S, Pardoll DM, Elisseeff JH. Sci Transl Med 2019,11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [378].Lehmann S, Perera R, Grimm HP, Sam J, Colombetti S, Fauti T, Fahrni L, Schaller T, Freimoser-Grundschober A, Zielonka J, Stoma S, Rudin M, Klein C, Umana P, Gerdes C, Bacac M. Clin Cancer Res 2016,22,4417. [DOI] [PubMed] [Google Scholar]
- [379].Pentcheva-Hoang T, Simpson TR, Montalvo-Ortiz W, Allison JP. Cancer Immunol Res 2014,2,970. [DOI] [PubMed] [Google Scholar]
- [380].Ruocco MG, Pilones KA, Kawashima N, Cammer M, Huang J, Babb JS, Liu M, Formenti SC, Dustin ML, Demaria S. J Clin Invest 2012,122,3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [381].Grandjean CL, Montalvao F, Celli S, Michonneau D, Breart B, Garcia Z, Perro M, Freytag O, Gerdes CA, Bousso P. Sci Rep 2016,6,34382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [382].Garris CS, Arlauckas SP, Kohler RH, Trefny MP, Garren S, Piot C, Engblom C, Pfirschke C, Siwicki M, Gungabeesoon J, Freeman GJ, Warren SE, Ong S, Browning E, Twitty CG, Pierce RH, Le MH, Algazi AP, Daud AI, Pai SI, Zippelius A, Weissleder R, Pittet MJ. Immunity 2018,49,1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [383].Pittet MJ, Garris CS, Arlauckas SP, Weissleder R. Sci Immunol 2018,3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [384].Sumen C, Mempel TR, Mazo IB, von Andrian UH. Immunity 2004,21,315. [DOI] [PubMed] [Google Scholar]
- [385].Hampton HR, Bailey J, Tomura M, Brink R, Chtanova T. Nat Commun 2015,6,7139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [386].Li JL, Lim CH, Tay FW, Goh CC, Devi S, Malleret B, Lee B, Bakocevic N, Chong SZ, Evrard M, Tanizaki H, Lim HY, Russell B, Renia L, Zolezzi F, Poidinger M, Angeli V, St John AL, Harris JE, Tey HL, Tan SM, Kabashima K, Weninger W, Larbi A, Ng LG. J Invest Dermatol 2016,136,416. [DOI] [PubMed] [Google Scholar]
- [387].Le VH, Lee S, Lee S, Wang T, Hyuk Jang W, Yoon Y, Kwon S, Kim H, Lee SW, Hean Kim K. Sci Rep 2017,7,44097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [388].Lohela M, Casbon AJ, Olow A, Bonham L, Branstetter D, Weng N, Smith J, Werb Z. Proc Natl Acad Sci U S A 2014,111,E5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [389].Mempel TR, Moser C, Hutter J, Kuebler WM, Krombach F. J Vasc Res 2003,40,435. [DOI] [PubMed] [Google Scholar]
- [390].von Andrian UH. Microcirculation 1996,3,287. [DOI] [PubMed] [Google Scholar]
- [391].Murooka TT, Deruaz M, Marangoni F, Vrbanac VD, Seung E, von Andrian UH, Tager AM, Luster AD, Mempel TR. Nature 2012,490,283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [392].Mempel TR, Bauer CA. Clin Exp Metastasis 2009,26,311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [393].Marangoni F, Murooka TT, Manzo T, Kim EY, Carrizosa E, Elpek NM, Mempel TR. Immunity 2013,38,237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [394].Kircher MF, Allport JR, Graves EE, Love V, Josephson L, Lichtman AH, Weissleder R. Cancer Res 2003,63,6838. [PubMed] [Google Scholar]
- [395].Kim EJ, Bhuniya S, Lee H, Kim HM, Shin WS, Kim JS, Hong KS. ACS Appl Mater Interfaces 2016,8,10266. [DOI] [PubMed] [Google Scholar]
- [396].Gniesmer S, Brehm R, Hoffmann A, de Cassan D, Menzel H, Hoheisel AL, Glasmacher B, Willbold E, Reifenrath J, Wellmann M, Ludwig N, Tavassol F, Zimmerer R, Gellrich NC, Kampmann A. J Tissue Eng Regen Med 2019,13,1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [397].Kalchenko V, Meglinski I, Sdobnov A, Kuznetsov Y, Harmelin A. J Biomed Opt 2019,24,1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [398].Zhang Y, Xu J, Ruan YC, Yu MK, O’Laughlin M, Wise H, Chen D, Tian L, Shi D, Wang J, Chen S, Feng JQ, Chow DH, Xie X, Zheng L, Huang L, Huang S, Leung K, Lu N, Zhao L, Li H, Zhao D, Guo X, Chan K, Witte F, Chan HC, Zheng Y, Qin L. Nat Med 2016,22,1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [399].Stiers PJ, van Gastel N, Moermans K, Stockmans I, Carmeliet G. JBMR Plus 2018,2,92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [400].Shvartsman D, Storrie-White H, Lee K, Kearney C, Brudno Y, Ho N, Cezar C, McCann C, Anderson E, Koullias J, Tapia JC, Vandenburgh H, Lichtman JW, Mooney DJ. Mol Ther 2014,22,1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [401].Nichols JE, La Francesca S, Niles JA, Vega SP, Argueta LB, Frank L, Christiani DC, Pyles RB, Himes BE, Zhang R, Li S, Sakamoto J, Rhudy J, Hendricks G, Begarani F, Liu X, Patrikeev I, Pal R, Usheva E, Vargas G, Miller A, Woodson L, Wacher A, Grimaldo M, Weaver D, Mlcak R, Cortiella J. Sci Transl Med 2018,10 [DOI] [PubMed] [Google Scholar]
- [402].Kim J, Cao L, Shvartsman D, Silva EA, Mooney DJ. Nano Lett 2011,11,694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [403].Li S, Zhang Y, Wang J, Zhao Y, Ji T, Zhao X, Ding Y, Zhao X, Zhao R, Li F, Yang X, Liu S, Liu Z, Lai J, Whittaker AK, Anderson GJ, Wei J, Nie G. Nat Biomed Eng 2017,1,667. [DOI] [PubMed] [Google Scholar]
- [404].Kodack DP, Chung E, Yamashita H, Incio J, Duyverman AM, Song Y, Farrar CT, Huang Y, Ager E, Kamoun W, Goel S, Snuderl M, Lussiez A, Hiddingh L, Mahmood S, Tannous BA, Eichler AF, Fukumura D, Engelman JA, Jain RK. Proc Natl Acad Sci U S A 2012,109,E3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [405].Manning CS, Jenkins R, Hooper S, Gerhardt H, Marais R, Adams S, Adams RH, van Rheenen J, Sahai E. IntraVital 2013,2,e24790. [Google Scholar]
- [406].Doloff JC, Veiseh O, Vegas AJ, Tam HH, Farah S, Ma M, Li J, Bader A, Chiu A, Sadraei A, Aresta-Dasilva S, Griffin M, Jhunjhunwala S, Webber M, Siebert S, Tang K, Chen M, Langan E, Dholokia N, Thakrar R, Qi M, Oberholzer J, Greiner DL, Langer R, Anderson DG. Nat Mater 2017,16,671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [407].Dudenhöffer DW, Laschke MW, Giebels C, Karliova I, Schneider U, Menger MD, Schäfers HJ. Thorac Cardiovasc Surg 2018 [DOI] [PubMed] [Google Scholar]
- [408].Frank JA, Antonini MJ, Anikeeva P. Nat Biotechnol 2019,37,1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [409].Park DW, Ness JP, Brodnick SK, Esquibel C, Novello J, Atry F, Baek DH, Kim H, Bong J, Swanson KI, Suminski AJ, Otto KJ, Pashaie R, Williams JC, Ma Z. ACS Nano 2018,12,148. [DOI] [PubMed] [Google Scholar]
- [410].Kozai TDY, Jaquins-Gerstl AS, Vazquez AL, Michael AC, Cui XT. Biomaterials 2016,87,157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [411].Tanaka K, Okigami M, Toiyama Y, Morimoto Y, Matsushita K, Kawamura M, Hashimoto K, Saigusa S, Okugawa Y, Inoue Y, Uchida K, Araki T, Mohri Y, Mizoguchi A, Kusunoki M. Oncol Rep 2012,28,1822. [DOI] [PubMed] [Google Scholar]
- [412].Chittajallu DR, Florian S, Kohler RH, Iwamoto Y, Orth JD, Weissleder R, Danuser G, Mitchison TJ. Nat Methods 2015,12,577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [413].Janssen A, Beerling E, Medema R, van Rheenen J. PLoS One 2013,8,e64029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [414].Canel M, Serrels A, Miller D, Timpson P, Serrels B, Frame MC, Brunton VG. Cancer Res 2010,70,9413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [415].Oudin MJ, Barbier L, Schäfer C, Kosciuk T, Miller MA, Han S, Jonas O, Lauffenburger DA, Gertler FB. Mol Cancer Ther 2017,16,143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [416].Karagiannis GS, Pastoriza JM, Wang Y, Harney AS, Entenberg D, Pignatelli J, Sharma VP, Xue EA, Cheng E, D’Alfonso TM, Jones JG, Anampa J, Rohan TE, Sparano JA, Condeelis JS, Oktay MH. Sci Transl Med 2017,9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [417].Oliva N, Conde J, Wang K, Artzi N. Acc Chem Res 2017,50,669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [418].Oliva N, Unterman S, Zhang Y, Conde J, Song HS, Artzi N. Adv Healthc Mater 2015,4,1584. [DOI] [PubMed] [Google Scholar]
- [419].Leung HM, Schafer R, Gmitro A. CLEO: 2015 2015 [Google Scholar]
- [420].Gupta K, Li Q, Fan JJ, Fong ELS, Song Z, Mo S, Tang H, Ng IC, Ng CW, Pawijit P, Zhuo S, Dong CY, Low BC, Wee A, Dan YY, Kanchanawong P, So P, Viasnoff V, Yu H. J Hepatol 2017,66,1231. [DOI] [PubMed] [Google Scholar]
- [421].Sun X, Cai W, Chen X. Acc Chem Res 2015,48,286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [422].Chen D, Dougherty CA, Yang D, Wu H, Hong H. Tomography 2016,2,3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [423].Ale A, Ermolayev V, Herzog E, Cohrs C, de Angelis MH, Ntziachristos V. Nat Methods 2012,9,615. [DOI] [PubMed] [Google Scholar]
- [424].Cohen AE, Farhi SL. Nat Neurosci 2018,21,776. [DOI] [PubMed] [Google Scholar]
- [425].Prevedel R, Verhoef AJ, Pernía-Andrade AJ, Weisenburger S, Huang BS, Nöbauer T, Fernández A, Delcour JE, Golshani P, Baltuska A, Vaziri A. Nat Methods 2016,13,1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [426].Klibanov AL, Hossack JA. Invest Radiol 2015,50,657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [427].Lyon PC, Griffiths LF, Lee J, Chung D, Carlisle R, Wu F, Middleton MR, Gleeson FV, Coussios CC. J Ther Ultrasound 2017,5,28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [428].Robison L, Zhang L, Drout RJ, Li P, Haney CR, Brikha A, Noh H, Mehdi BL, Browning ND, Dravid VP, Cui Q, Islamoglu T, Farha OK. ACS Applied Bio Materials 2019,2,1197. [DOI] [PubMed] [Google Scholar]
- [429].Aggarwal M, Zhang J, Miller MI, Sidman RL, Mori S. Neuroscience 2009,162,1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [430].Kong L, Tang J, Little JP, Yu Y, Lämmermann T, Lin CP, Germain RN, Cui M. Nat Methods 2015,12,759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [431].M. T, M. M, C. M, P. R. Optik 2019,176,302. [Google Scholar]
- [432].Zou C, Wu B, Dong Y, Song Z, Zhao Y, Ni X, Yang Y, Liu Z. Int J Nanomedicine 2017,12,179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [433].Taruttis A, Ntziachristos V. Nature Photonics 2015,9,219. [Google Scholar]
- [434].Botcherby EJ, Smith CW, Kohl MM, Débarre D, Booth MJ, Juškaitis R, Paulsen O, Wilson T. Proc Natl Acad Sci U S A 2012,109,2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [435].Combs CA. Curr Protoc Neurosci 2010,Chapter 2,Unit2.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [436].Treweek JB, Chan KY, Flytzanis NC, Yang B, Deverman BE, Greenbaum A, Lignell A, Xiao C, Cai L, Ladinsky MS, Bjorkman PJ, Fowlkes CC, Gradinaru V. Nat Protoc 2015,10,1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [437].Zhang Y, Shin Y, Sung K, Yang S, Chen H, Wang H, Teng D, Rivenson Y, Kulkarni RP, Ozcan A. Sci Adv 2017,3,e1700553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [438].Hoover EE, Squier JA. Nat Photonics 2013,7,93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [439].Wang CW, Budiman Gosno E, Li YS. Sci Rep 2015,5,15051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [440].Power RM, Huisken J. Nat Methods 2017,14,360. [DOI] [PubMed] [Google Scholar]
- [441].Z. Y, H. H, C. W. Curr Pharm Biotechnol 2011,11,654. [Google Scholar]
- [442].Chen F, Tillberg PW, Boyden ES. Science 2015,347,543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [443].Wassie AT, Zhao Y, Boyden ES. Nat Methods 2019,16,33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [444].Chen BC, Legant WR, Wang K, Shao L, Milkie DE, Davidson MW, Janetopoulos C, Wu XS, Hammer JA, Liu Z, English BP, Mimori-Kiyosue Y, Romero DP, Ritter AT, Lippincott-Schwartz J, Fritz-Laylin L, Mullins RD, Mitchell DM, Bembenek JN, Reymann AC, Böhme R, Grill SW, Wang JT, Seydoux G, Tulu US, Kiehart DP, Betzig E. Science 2014,346,1257998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [445].Pujals S, Albertazzi L. ACS Nano 2019,13,9707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [446].Pujals S, Feiner-Gracia N, Delcanale P, Voets I, Albertazzi L. Nature Reviews Chemistry 2019,3,68. [Google Scholar]
- [447].Gordon RE. Methods Mol Biol 2014,1180,119. [DOI] [PubMed] [Google Scholar]
- [448].Mikula S, Denk W. Nat Methods 2015,12,541. [DOI] [PubMed] [Google Scholar]