Keywords: astrocytes, spasticity, spinal cord injury, synapse
Abstract
Spasticity is a chronic neurological complication associated with spinal cord injury (SCI), characterized by increased muscle tone and stiffness. A physiological sign of spasticity is hyperreflexia, evident by the loss of evoked rate-dependent depression (RDD) in the H-reflex. Although previous work has shown that SCI-induced astrogliosis contributes to hyperexcitability disorders, including neuropathic pain and spasticity, it is unclear how reactive astrocytes can modulate synaptic transmission within the injured spinal cord. To study astrocytes’ role in post-SCI hyperreflexia, we examined glutamate transporter-1 (GLT-1) and postsynaptic density protein 95 (PSD-95) proteins in astrocytes and neurons, respectively, within the ventral horn (lamina IX) below the level of injury (spinal segment L4-5). The close juxtaposition of GLT-1 and PSD-95 markers is a molecular correlate of tripartite synapses and is thought to be a key element in the astrocyte-induced plasticity of neuronal synapses. Our study compared animals with and without SCI-induced hyperreflexia and spasticity and investigated potential synaptic abnormalities associated with astrocyte involvement. As expected, 4 wk after SCI, we observed a loss in evoked H-reflex RDD in hindlimb electromyogram recordings, i.e., hyperreflexia, in contrast to uninjured sham. Importantly, our main findings show a significant increase in the presence of GLT-1–PSD-95 tripartite synapses in the ventral spinal cord motor regions of animals exhibiting SCI-induced hyperreflexia. Taken together, our study suggests the involvement of astrocyte-neuron synaptic complexes in the plasticity-driven progression of chronic spasticity.
NEW & NOTEWORTHY The role of astrocytes in H-reflex hyperexcitability following SCI remains understudied. Our findings establish a relationship between GLT-1 expression, its proximity to neuronal PSD-95 in the spinal cord ventral horn, and the loss of H-reflex RDD, i.e., hyperreflexia. Our findings provide a new perspective on synaptic alterations and the development of SCI-related spasticity.
INTRODUCTION
Spasticity, a debilitating consequence of spinal cord injury (SCI), manifests as heightened muscle tone and stiffness, leading to compromised motor function and coordination and a range of discomforting sensations including pain (1, 2). Moreover, this condition substantially diminishes the quality of life by restricting mobility, independence, and overall well-being (3–6). The key driver of spasticity after SCI is hyperexcitability within H-reflex (7). Both clinical trials and preclinical studies have suggested the involvement of reactive astrocytes in various disorders. However, their exact role in synaptic transmission after SCI remains elusive.
The concept of a tripartite synapse describes the dynamic relationship between the pre- and postsynaptic neurons, and an adjacent astrocyte (8, 9). Astrocytes in particular perform a key functional role in the tripartite complex, modifying synaptic structure and function, and influencing overall neuronal circuit activity (10, 11). Recent data has shown that the close juxtaposition of glutamate transporter-1 (GLT-1) and postsynaptic density protein 95 (PSD-95) protein markers expressed in astrocytes and neurons, respectively, serves as a robust indicator for the presence of active tripartite synapses (12). Glutamate transporter GLT-1 (also known as EAAT2) contributes to the homeostatic clearance and recycling of glutamate from the synaptic cleft (13). Postsynaptic density-95 marker, PSD-95, primarily expressed within the postsynaptic membrane, serves as a protein marker for mature dendritic spines, the microscopic protrusions along the dendritic branch of the postsynaptic neuron (14).
The role of astrocytes in H-reflex hyperexcitability following SCI remains understudied. To further understand the involvement of astrocytes in synaptic physiology and circuit pathology associated with hyperreflexia after SCI, we performed a histological study of tripartite synapse expression in the ventral horn concomitantly with electromyogram (EMG) studies of H-reflex function. Our findings establish a possible relationship between increased GLT-1 expression, its proximity to neuronal PSD-95 in lamina IX of the spinal cord ventral horn (indicative of tripartite synapses), and the loss of H-reflex RDD, i.e., hyperreflexia. Collectively, our study suggests that astrocyte-neuron synaptic complexes in the ventral spinal cord may correlate with the development of hyperreflexia and spasticity. Such findings offer a fresh perspective that could help develop molecular-based therapies aimed at modifying synaptic-level alterations to effectively treat spasticity.
METHODS
Animals
Experiments were performed in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. All animal protocols were approved by the Yale University and Veterans Affairs Institutional Animal Use Committee (IACUC). Animals were housed under a 12-h light-dark cycle with food and water provided ad libitum. Eight- to ten-week-old male and female mice (c57/bl6) underwent either sham or SCI surgery. A total of 11 animals were used between sham n = 5 (female n = 2, male n = 3), SCI n = 6 (female n = 4, male n = 2) (see Study Design in Fig. 1). To determine the specificity of the GLT-1 antibody labeling, tissue was collected from animals with glial fibrillary acidic protein (GFAP)-Cre conditional expression of tdTomato. These animals were bred in-house by crossing Gt(ROSA)26Sortm9(CAG-tdTomato)Hze/J (Jackson laboratories, Stock No. 007909) with GFAP-Cre (Jackson, Stock No. 024098).
Figure 1.
Study design. Schematic detailing the timeline from when mice grouped into sham and SCI conditions underwent the appropriate surgery. Mice underwent weekly BMS testing and then EMG recording occurred before histology and analysis of the spinal cord tissue. BMS, Basso Mouse Scale; EMG, electromyogram; SCI, spinal cord injury.
Spinal Cord Injury
To produce our SCI-spasticity model, we performed a laminectomy at the 11th thoracic vertebra (T11), which exposed the dorsal L1-L2 spinal cord segment as previously published (15). All surgeries were performed on mice deeply anesthetized with vaporized 1–3% isoflurane (SomnoSuite anesthesia system, Kent Scientific). An Infinite Horizons Impactor (Precision Systems and Instrumentation, LLC) was used to create a mildly severe SCI contusion injury (15), in which a metal rod (tip diameter: 1.3 mm) was applied to the exposed dorsal surface with an impact force of 50kDyn. Sham animals underwent the same procedures without SCI. Following surgeries, muscle, fascia and skin were closed in layers with 6-0 monofilament sutures. Postoperative treatments included postoperative injection of 0.9% saline solution (3.0 mL sc), Baytril (antibiotic) (0.3 mL, 3.5 mg/kg), 3 days of buprenorphine (0.05 mg/kg twice per day) and meloxicam (5 mg/kg once per day) for pain management. Animals with SCI were monitored and received manual bladder expression twice until bladder function returned.
Behavioral Assays
To confirm equivalent injury across SCI animals, blinded investigators monitored animals using the Basso Mouse Scale (BMS). The BMS grades locomotor function with 0 to 9 scores, where a 0 indicates complete paralysis of the hindlimb, and a 9 indicates normal locomotor capability (16). Animal hindlimb ambulatory functions were scored separately and then averaged. BMS scoring occurred at baseline (before SCI) and weekly following SCI or Sham surgery. In statistical comparisons, the average BMS score collected from each weekly test was compared across SCI and Sham cohorts.
EMG Reflex Testing
To assess hindlimb H-reflex function, sham and SCI animals underwent longitudinal electromyogram (EMG) testing while under anesthesia. Based on previous studies anesthesia was induced using a mixture of ketamine and xylazine (17, 18). These studies have shown that hyperreflexia is readily detectable in SCI animals anesthetized with ketamine/xylazine mixture (17, 18, 19). To minimize any possible effect on anesthesia on H-reflex, all animals were anesthetized with the same 100/10 mg/kg intraperitoneal dose of ketamine and xylazine and maintained on ketamine alone (20 mg/kg ip). To record EMG-evoked spinal motor-reflex data–including the motor response (M wave) and the H reflex–we used a percutaneous needle preparation (15, 20–24). This procedure is an analogous method to that used to study H-reflex in humans (22, 25) and does not confound gross locomotor function (15, 19).
For H-reflex stimulus, a pair of Teflon-insulated stainless steel wire electrodes (0.002 in. diameter; A-M Systems, Inc., Carlsborg, WA) were threaded through a 32 G syringe needle, and their ends were bent into sharp barbs. The insulation was removed with heat (exposed tips ∼1 mm), which also sterilized the exposed tips. The needle-barb of the stimulating electrode was transcutaneously inserted into the gastrocnemius/soleus muscle groups, near the tibial branch of the sciatic nerve. The wire remained in place once the needle was retracted. A second electrode was inserted similarly ∼2 mm away from the first electrode (17–19). Stimulating electrode placement was adjusted until square wave stimulating pulses (0.2 ms duration given at a rate of 1 every 3 s) elicited visible motor twitch responses, i.e., plantar flexion (21, 24). To record EMG data (e.g., plantar reflex), an electrode was inserted into the plantar muscles within the hind paw palmar/ventral surface, proximal to the ankle. A reference electrode was placed subcutaneously in the dorsolateral hind paw surface. EMG responses were filtered, amplified, and analyzed offline using Spike 2 software (v. 7.08; CED Software, Cambridge, UK). Threshold (T) was defined as the minimum intensity required for an M-wave response ∼50% of the time. We used a stimulation intensity that produced consistent M- and H-wave responses (∼1.4–1.8 T). To measure rate-dependent depression (RDD) of the H-reflex, we applied a paired-pulse stimulation paradigm: a control pulse and test pulse (0.2 ms2) separated by a range of interpulse intervals (10-2,000 ms). Three trials (10 sweeps/trial) were recorded for each interpulse interval. We quantified the M- and H-wave amplitudes from rectified and averaged waveforms (26, 27). For comparisons, the maximum amplitudes of the H and M response to the test pulse were converted into a percentage of the maximum amplitude response to the control pulse (test/control × 100). M and H waveforms were measured from baseline-to-peak amplitude. We calculated the H/M ratio by dividing the %H-reflex by the %M-wave for each interpulse interval. All H-reflex testing was performed acutely (i.e., recordings lasted <1 h per animal), and all animals underwent similar H-reflex testing protocols.
Immunohistochemistry
At the experimental endpoint, 4 wk post-SCI/sham surgery, all animals were placed under deep anesthesia (100/10 mg/kg ip) and euthanized via intracardial perfusion with ice-cold phosphate-buffered saline (0.01 M PBS) followed by paraformaldehyde (4% PFA in 0.01 M PBS). Lumbar enlargement spinal cord tissues (L4–L5) were removed and postfixed in 4% PFA at 4°C overnight and subsequently embedded in gelatin (10% dissolved in water) for vibratome sectioning. Coronal tissue sections (thickness: 35 µm) of the lumbar enlargement caudal to the lesion were sectioned. For immunohistochemistry (IHC), free-floating sections were permeabilized with 0.05% Triton- X-100 in PBS for 5 min. Sections were mounted on Superfrost glass slides (Fisher Scientific). Sections were then blocked at 1 h room temperature with gentle shaking in 10% normal donkey serum and 0.05% Triton in PBS. After blocking, sections were incubated with polyclonal GP X Anti-Glutamate Transporter Antibody GLT-1 (1:1,000, AB1783, EMD Millipore Corp.) and rabbit monoclonal to PSD-95 (1:100; AB238135, Abcam). Tissue sections were washed in with 0.05% Triton- X-100 in PBS and incubated in appropriate secondary antibodies (donkey anti-guinea pig, 647; donkey anti-rabbit, Alexa 568) for 2 h. Coverslips were applied using anti-fade gel/mount (BioMeda, Foster City, CA).
Image Analysis and Tripartite Synapse Identification
Images were captured using an Andor Dragonfly spinning disk confocal microscope (Andor, Oxford Instruments) and an SR Apo TIRF 100X oil immersion Nikon objective with consistent acquisition settings across all groups. Images were acquired at a step size of 0.1 μm creating a 20 μm Z-stack. An equivalent area and volume of the ventral spinal cord was analyzed across all samples and the analysis was conducted by blinded investigators using Imaris software (Andor, Oxford Instruments).
To analyze PSD-95 puncta expression, we applied the Imaris function “detect spots” to detect PSD-95 fluorescently labeled Z-stacks. The detect spots function creates a representative sphere for each PSD-95 puncta, which includes size, intensity, and topographical positional information.
As part of the spots detect function, both a minimum and maximum fluorescence intensity filter was set to exclude both background noise and oversaturated artifacts, e.g., dust and autofluorescence. The variability in fluorescence levels of PSD95 immunolabeled tissues necessitated the setting of threshold values individually for each image. These values were determined by a blinded experimenter who measured the intensity of the nonspecific background fluorescence of the tissue and the intensity of autofluorescent artifacts. Importantly, we did not observe a significant difference between the average intensity of PSD95 spots between sham and SCI (data not shown).
As part of the spots detect function, both a minimum and maximum fluorescence intensity filter with a minimum threshold set between 4,000 and 6,000 was set to exclude both background noise and oversaturated artifacts, e.g., dust and autofluorescence.
To analyze GLT-1 expression, we used the “detect surface” function in Imaris to render a three-dimensional (3-D) model of GLT-1. The same settings were used across all images, including setting a minimum fluorescent intensity threshold and using 1.21 μm diameter seed points. A comparison of PSD-95 spheres and the GLT-1 3-D surface model allowed us to define the spatial relationship between PSD-95 and GLT-1.
We identified tripartite synapses, using the schema above, as a PSD-95 spot within a distance of less than 0.35 μm of the edge of GLT-1 3 D model (denoted as “Spot Close”; more details below). The rationale for this approach is based on published studies of tripartite synaptic cleft structure, which estimate the average distance from astrocyte processes to postsynaptic elements within the synaptic cleft to be ∼0.1 μm based on stimulated emission depletion (STED) microscopy (28, 29). In our pilot studies, PSD-95 puncta had an average radius of 0.21 μm. To determine the relationship between GLT-1 and PSD-95, we used the Imaris function “Find Spots Close To Surface,” which measured from the center point of each PSD-95 puncta to the nearest GLT-1 3-D model. Taken together, this process function divided all PSD-95 spheres into two categories: 1) “Spot Close” or 2) “Spot Far.” “Spots Close” were defined as any PSD-95 sphere in which their center point was at or within 0.35 μm distance to the nearest edge of a GLT-1 3-D model surface. All spots outside this 0.35 μm boundary were “Spots Far” from GLT-1 surface.
Statistical Analysis
All statistical tests were performed at the α-level of the significance of 0.05 by two-tailed analyses using the parametric test. Normality assumptions of each dataset were determined using a Shapiro–Wilk test. Sham and SCI groups were compared using Student’s t test. Data management, statistical analyses, and graph generation were performed using GraphPad Prism 9.5.1 and Microsoft Office Excel. All data are visually presented as box plots with overlaid scatter plots showing all data points. Data written in text is means ± SD.
RESULTS
Disrupted Gross Locomotor Function following SCI
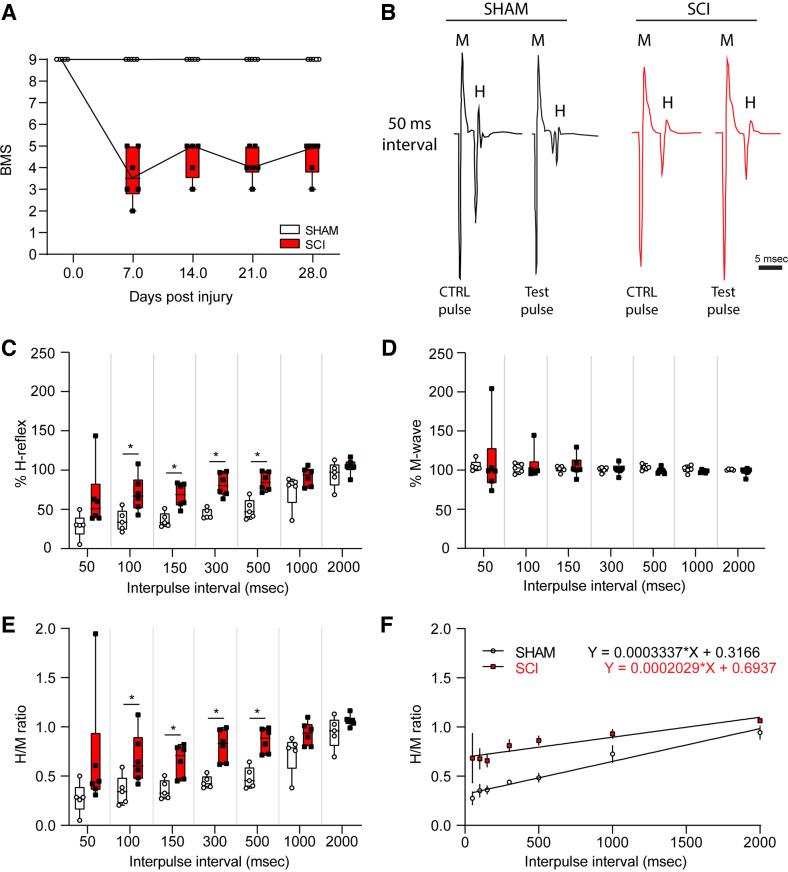
To assess gross motor function recovery, mice underwent weekly open-field testing using the Basso Mouse Scale (BMS) until the experimental endpoint at 4 wk post-SCI (Fig. 2A). As expected, before injury (baseline), all mice showed a score of 9, indicating no locomotor impairment. Similarly, sham animals continued to show no impairments through our experimental testing period. In contrast, mice with SCI displayed functional impairment over the 4 wk with an average BMS score of 4.50 ± 0.342 (15). This observation confirmed the durability of our SCI model, but that importantly, animals retained some hindlimb function, i.e., SCI did not lead to paralysis.
Figure 2.
Mice with SCI demonstrated significant movement impairments. A: BMS testing showed that all mice initially started with perfect motility. However, after surgery SCI mice showed significant impairment and weekly testing indicated no significant recovery over the period of 4 wk. B: example EMG recordings showing that although the M wave was similar for both groups, the H-reflex was hyperactive in the SCI group, indicating that these mice display spasticity. C: H-reflex recordings at various time intervals showed that SCI mice displayed significantly higher %H-reflex (amplitude of the H-reflex test pulse/control pulse × 100) to stimulation with interpulse intervals between 50 ms and 500 ms. D: percent M-wave (amplitude of the M-wave test pulse/control pulse × 100) recording indicates that the animals’ initial response to stimulation was the same for both groups. E: to normalize for changes in muscle response, we compared H-reflex and M-wave ratios (%H-reflex/%M-wave). SCI mice showed a significant increase in H/M ratio compared with sham mice during interpulse intervals between 50 and 500 ms. F: SCI increased the H/M ratio as indicated by a flatter linear regression trend line and a higher y-intercept. Data represented as box plot with individual data points, sham n = 5, SCI n = 6. Scale bar in B = 5 ms and applies to all traces. BMS, Basso Mouse Scale; EMG, electromyogram; SCI, spinal cord injury.
Contusion SCI Contributes to the Development of Hyperreflexia
n normal conditions, electrically evoked H-reflex response decreases with shortening interpulse intervals, resulting in an overall decrease in amplitude in EMG recording. This phenomenon of rate-dependent depression (RDD) can be seen in healthy subjects and may be disrupted in neurological disorders. A loss of RDD is indicative of hyperreflexia and spasticity and is observed after SCI in humans and animals (30). To determine that SCI animals had hyperreflexia after SCI, we performed EMG recordings to measure evoked M-wave and H-reflex response following percutaneous stimulus of the tibial nerve (19) (Fig. 2, B–F). To measure RDD, we used an established paired-pulse paradigm that consisted of a control and test stimulus, separated by interpulse intervals ranging from 50 to 2,000 ms.
Representative traces from baseline and after SCI demonstrate that the stimulation protocol produced rate-dependent changes and H-reflex amplitude responses (Fig. 2B). For example, at experimental baseline, short interpulse intervals (i.e., 50 ms) resulted in a loss of H-wave amplitude response following the test pulse as compared with the control pulse (Fig. 2B). After SCI, however, and in agreement with our previous studies (15, 19), SCI animals displayed an exaggerated %H-reflex response at 50, 100, 150, 300, and 500 ms interpulse intervals compared with sham, indicating hyperreflexia 4 wk post-SCI [sham vs. SCI; at 50 ms: 29.03 ± 15.84% vs. 64.36 ± 40.28%, t(9) = 1.833, P = 0.100: at 100 ms: 35.71 ± 13.70% vs. 70.06 ± 22.98%, t(9) = 2.922, P = 0.017; at 150 ms: 36.37 ± 9.41% vs. 68.12 ± 15.44%, t(9) = 3.998, P = 0.003; at 300 ms 44.24 ± 6.43% vs. 81.61 ± 14.76%, t(9) = 5.226, P < 0.001; at 500 ms: 50.17 ± 12.83% vs. 85.38 ± 11.79%, t(9) = 4.741, P = 0.001; at 1,000 ms: 73.88 ± 21.49% vs. 91.63 ± 11.97%, t(9) = 1.737, P = 0.116; at 2,000 ms: 94.54 ± 16.52% vs. 104.43 ± 9.58%, t(9) = 1.243, P = 0.245; unpaired t test] (Fig. 2C).
As a measure of the direct activation of spinal motor neurons, we also measured the M-wave. Here, we observed no difference in %M-wave at any interpulse interval between baseline, sham, and SCI [sham vs. SCI; at 50 ms: 105.52 ± 7.03% vs. 110.90 ± 47.019%, t(9) = 0.251, P = 0.807; at 100 ms: 102.52 ± 6.14% vs. 106.01 ± 18.99%, t(9) = 0.391, P = 0.705; at 150 ms: 101.46 ± 3.94% vs. 104.88 ± 13.77%, t(9) = 0.534, P = 0.606; at 300 ms: 100.78 ± 3.41% vs. 101.14 ± 6.86%, t(9) = 0.107, p = 0.917; at 500 ms: 103.33 ± 3.37% vs. 99.36 ± 3.75%, t(9) = 1.830, P = 0.101; at 1,000 ms: 101.03 ± 4.50% vs. 98.48 ± 1.71%, t(9) = 1.292, P = 0.229; at 2,000 ms: 100.68 ± 0.81% vs. 97.98 ± 4.84%, t(9) = 1.221, P = 0.253; unpaired t test] (Fig. 2D).
The H/M ratio, which normalizes any changes in muscle response, further confirmed the localized functional loss of RDD within the spinal cord following SCI as compared with sham. SCI leads to a higher H/M ratio at 100, 150, 300, and 500 ms interpulse intervals compared with sham [sham vs. SCI; at 50 ms: 0.28 ± 0.16% vs. 0.68 ± 0.63%, t(9) = 1.406, P = 0.193, at 100 ms: 0.35 ± 0.15% vs. 0.68 ± 0.26%, t(9) = 2.45, P = 0.037, at 150 ms: 0.36 ± 0.10% vs. 0.66 ± 0.16%, t(9) = 3.529, P = 0.006, at 300 ms: 0.44 ± 0.06% vs. 0.81 ± 0.16%, t(9) = 4.807, P = 0.001, at 500 ms: 0.48 ± 0.11% vs. 0.86 ± 0.125%, t(9) = 5.265, P = 0.001, at 1,000 ms: 0.73 ± 0.20% vs. 0.93 ± 0.12%, t(9) = 2.114, P = 0.097, at 2,000 ms: 0.94 ± 0.16% vs. 1.07 ± 0.06%, t(9) = 1.715, P = 0.120; unpaired t test] (Fig. 2E).
A linear regression of the H/M ratio across all interpulse intervals reveals the loss of RDD in SCI animals, as indicated by a shallower slope of the linear regression coefficient (sham vs. SCI; Y = 0.0003337 × X + 0.3166 vs. Y = 0.0002029 × X + 0.6937) (Fig. 2F).
Expression of PSD-95 and GLT-1 in Ventral Spinal Cord
To determine whether there were any differences in the relationship PSD-95 and GLT-1 expression after SCI, spinal cord tissue sections were fluorescently labeled with antibodies specific for PSD-95 and GLT1. To confirm the specificity of GLT-1 antibody labeling to astrocytes, spinal cord tissue sections from GFAP-tdTomato reporter animals were labeled (Fig. 3A). As expected, GLT-1 was present throughout spinal cord astrocytes, including astrocytic end processes, which form part of the tripartite synapse (Fig. 3A). Imaris software (v. 9.5, Andor, Oxford Instruments) was used to analyze the expression and 3-D spatial relationship between PSD-95 spots and GLT-1 surface object after SCI within the ventral horn of the spinal cord. Analysis of the overall number of PSD-95 puncta found no difference between SCI and sham groups [sham vs. SCI: 101,200 ± 5,745 vs. 110,097 ± 19,399 PSD-95 puncta, t(6) = 0.880, P = 0.413: unpaired t test] (Fig. 3C). In addition, no differences were found in mean intensity of PSD-95 puncta between sham and SCI animals (data not shown). The ventral horn GLT-1 expression was analyzed by creating a 3-D surface model of the GLT-1 fluorescent antibody labeling using Imaris 9.5 software (see methods). This analysis revealed that SCI induced a significant increase in the total volume of GLT-1 in the ventral spinal cord compared with sham [sham vs. SCI: 18,266 ± 3,158 vs. 44,641 ± 12,072 µm3, t(6) = 4.227, P = 0.006; unpaired t test] (Fig. 3D). However, there was no difference in the average mean GLT-1 intensity between groups (sham vs. SCI: 8,432 ± 3,052 vs. 6,778 ± 4,774 mean intensity, t(6) = 0.584, P = 0.581; unpaired t test] (Fig. 3E).
Figure 3.
Analysis of PSD-95 puncta and GLT-1 surface in the ventral horn. A: colocalization of GLT-1 immunofluorescence with GFAP conditional tdTomato expression, confirming the specificity of GLT-1 antibody labeling to astrocytes. B: an example image of a sham and SCI mouse. The leftmost images show the channel for PSD-95. The middle images show the channel for GLT-1. The rightmost images show the two channels merged. C: the number of PSD-95 puncta in the ventral horn was not different between groups. D: the volume of the GLT-1 surface was significantly increased after SCI. E: there was no significant difference in GLT-1 surface intensity between groups. Data represented as box plot with individual data points, sham n = 4, SCI n = 4. Scale bars in A = 10 µm and B = 30 µm. SCI, spinal cord injury.
Identification and Analysis of Tripartite Synapses
To determine whether there were any significant differences in tripartite synapses between sham and SCI groups, we assessed the number of PSD-95 spots within close proximity to the GLT-1 (Fig. 4A). PSD-95 puncta were considered to be associated with a tripartite structure if they were found near the surface of the GLT-1 3-D model, such that their center point was at or within 0.35 μm of the nearest GLT-1 surface. Animals with SCI had a significant increase in PSD-95 puncta near to a GLT-1 surface compared with the sham group [sham vs. SCI: 48,046 ± 7,759 vs. 78,822 ± 17,939 PSD-95 puncta, t(6) = 3.149, P = 0.019; unpaired t test] (Fig. 4B). Conversely, the sham group showed a significant increase in number of PSD-95 puncta far from a GLT-1 surfaces [sham vs. SCI: 53,154 ± 7,690 vs. 31,274 ± 8,470, t(6) = 3.825, P = 0.009; unpaired t test] (Fig. 4C). The ratio of synapses thought to be associated with tripartite function to the total number of synapses increased significantly after SCI [sham vs. SCI; 47.458 ± 7.185 vs. 71.250 ± 7.925; t(6) = 4.448, P = 0.004; unpaired t test] whereas the ratio of nontripartite synapses decreased [sham vs. SCI; 52.542 ± 7.185 vs. 28.750 ± 7.925; t(6) = 4.448, P = 0.004; unpaired t test] (Fig. 4D).
Figure 4.
Analysis of tripartite synapses. A: example of a zoomed-in segment in the ventral horn, showing that there was no overlap between the PSD-95 puncta and the GLT-1 surface. B: the number of tripartite synapses increased significantly after SCI. C: there was a significant decrease in the number of nontripartite synapses after SCI. D: the ratio of tripartite synapses to nontripartite synapses reversed after SCI. Data represented as box plot with individual data points, sham n = 4, SCI n = 4. Scale bar = 2 µm and applies to all images. SCI, spinal cord injury.
DISCUSSION
Spasticity is a chronic complication following SCI with limited clinical treatments, some of which carry safety or tolerability risks with long-term use, e.g., Botox and baclofen (31). Although many factors have been linked to the development of spasticity after SCI, the exact mechanism by which spasticity develops is still not firmly understood. This study investigated the involvement of astrocytes in post-spinal cord injury (SCI) hyperreflexia by examining the expression of GLT-1 and PSD-95 proteins in astrocytes and neurons, respectively, within the ventral horn (lamina IX) of the lumbar enlargement (L4-L5). We performed a histological study to assess the presence of tripartite synapses in the ventral horn of the spinal cord and EMG studies to measure H-reflex pathology. Our primary findings reveal two key observations. First, we observed an elevation in GLT-1 expression, which closely interacts with neuronal PSD-95 in lamina IX ventral horn motor pools following spinal cord injury (SCI). This suggests an increased presence of tripartite synapses in ventral motor-reflex circuits that innervate the hindlimb musculature. Second, we noted a corresponding reduction in H-reflex RDD, indicating the presence of hyperreflexia when compared to uninjured animals (sham group) (15, 19). Together, our findings suggest the involvement of astrocytes in modulating synaptic organization and its implications for SCI-induced hyperreflexia.
Astrocytes are a structurally and functionally diverse class of glia, and through their interactions with neurons and other glial, astrocytes influence neuronal excitability (32). In SCI or other insults to the central nervous system (CNS) [e.g., multiple sclerosis (MS), amyotrophic lateral sclerosis (ALS)], reactive astrocytes contribute to the development and maintenance of hyperexcitability disorders, including central sensitization and hyperreflexia (33–35). At the astroglial-neuronal interface at the synaptic cleft, astrocytes have a key role in the homeostatic regulation of excitatory neurotransmitter signaling (36, 37). They perform this function in part by expressing glutamate transporters such as GLT-1, which are localized to the perisynaptic astrocytic processes (PAPs) that interact with both pre- and postsynaptic elements and regulate synaptic transmission (38). The expression of GLT-1 in astrocytes can vary depending on the CNS region and is regulated by neuronal activity. Relevant to our study, GLT-1 expression and distribution could be altered in response to injury, which may have functional implications for local synaptic transmission and hyperexcitability-related disorders. To analyze the interaction between astrocytes and neuronal synapses, we immunolabeled GLT-1 as a proxy for the astrocytic perisynaptic process. Because PSD-95 is considered an established marker of mature, excitatory postsynaptic sites (39, 40), we used the expression of PSD-95 to label putative postsynaptic elements. Importantly, PSD-95 influences synaptic strength and interacts with astrocytic proteins for bidirectional synapse-astrocyte communication (41), playing a pivotal role in synaptic structure and function (42, 43). The presence of both GLT-1 and PSD-95 in close proximity is considered a molecular correlate of tripartite synapses and is thought to be a key element in astrocyte-induced plasticity of neuronal synapses, e.g., pre- and postsynaptic reorganization (44).
In our present study, we observed an increase in PSD-95–GLT-1 tripartite complexes after SCI, which agrees with other published findings from a number of neurological CNS disorders, including Amyotrophic lateral sclerosis (ALS), multiple sclerosis (MS), and epilepsy (45–47). To accurately identify tripartite synapses within our imaging equipment's optical diffraction limit, we set a boundary distance of less than 0.35 μm as the criterion for determining the proximity of a single PSD-95 puncta to GLT-1 astrocyte surface expression. This approach aligned well with other studies which demonstrated that the dynamic interactions between astrocytes and neuronal elements within the synapse typically occur within a range of 0.1 μm to 0.3 μm, varying by CNS region (28, 29).
Our research reveals a potentially important functional consequence of increased GLT-1 expression with its close association with neighboring PSD-95 puncta after SCI, particularly in the presentation of spinal motor-reflex hyperexcitability. We observed an increase in the volume of GLT-1 immunolabeling after injury, with no accompanying increase in overall fluorescence intensity. This suggests that changes in GLT-1 may result from an expansion in the size or quantity (or both) of astrocytes within the ventral horn of the spinal cord. Consequently, the expression of GLT-1 at individual synapses is likely comparable between sham and SCI animals. We hypothesize that the increase in GLT-1/PSD95 contacts may represent a typical astrocytic response to heightened synaptic excitability post-SCI. Because stringent regulation of glutamatergic transmissions is crucial for maintaining synaptic transmission fidelity (48, 49), the increased number of tripartite complexes might contribute to enhanced synaptic efficacy. This enhancement following traumatic injury could potentially amplify motor neuron excitability and partly explain the development of hyperreflexia. Hence, the increased formation of GLT-1/PSD-95 tripartite complexes might act as a morphological marker of strengthened synaptic efficacy following injury (50). It is plausible that an increase in the expression of GLT-1 at individual synapses would be required to mitigate overall excitability.
Our study cannot preclude the possible influence of peripheral afferent plasticity, which may contribute to spinal reorganization following SCI (27). Indeed, our PSD-95 labeling measures only provide information about the postsynaptic element. Moreover, due to this limitation, we have not determined whether the increased presence of astrocytic tripartite synapses occurs equally across all peripheral inputs to motor neurons or is specific to synapses within individual neuronal circuits following SCI. However, the large increase in the number PSD-95 puncta over a broad area of the ventral horn and within proximity to astrocytic GLT-1 after SCI, suggests that changes in a tripartite organization are not neuronal subtype-specific.
We note an increase in GLT-1 post-SCI, yet previous research has associated neurodegeneration and neuropathic pain with a GLT-1 decrease (51, 52). The apparent contradiction might be due to injury type, e.g., SCI, CNS location. For example, Kabdesh et al. (53), reported decreased GLT-1 near the SCI lesion but increased levels more distal from the injury site. This latter finding supports our observations where we examined and documented that a GLT-1 increase up to three spinal segments below the injury site.
In conclusion, our study suggests a disruption in the interaction between astrocytes and neuronal synapses in the progression of post-SCI chronic spasticity. These findings indicate a possible role of astrocytic function in synaptic organization and hyperreflexia. However, these results also underscore the need for further investigations to fully unravel molecular and cellular mechanisms underpinning this phenomenon.
DATA AVAILABILITY
Data will be made available upon reasonable request.
GRANTS
The work is supported by grants from the Paralyzed Veterans of America (PVA), the Department of Veterans Affairs (VA) Medical Research Service and Rehabilitation Research Service (B9253-C and 1 I01 RX002969-01A2), and The Taylor Foundation for Chronic Disease. The Center for Neuroscience and Regeneration Research is a Collaboration of the Paralyzed Veterans of America with Yale University.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.A.B. and A.M.T. conceived and designed research; C.A.B., J.F.K., and S.D.K. performed experiments; C.A.B., J.F.K., and S.D.K. analyzed data; C.A.B., J.F.K., S.D.K., and A.M.T. interpreted results of experiments; C.A.B. and J.F.K. prepared figures; C.A.B., J.F.K., and A.M.T. drafted manuscript; C.A.B., J.F.K., S.D.K., S.G.W., and A.M.T. edited and revised manuscript; C.A.B., S.G.W., and A.M.T. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Jennifer Carrara and Pamela Zwinger for excellent technical assistance. The graphical abstract was created with BioRender.com.
REFERENCES
- 1. Ghai A, Garg N, Hooda S, Gupta T. Spasticity—Pathogenesis, prevention and treatment strategies. Saudi J Anaesth 7: 453–460, 2013. doi: 10.4103/1658-354X.121087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sáinz-Pelayo MP, Albu S, Murillo N, Benito-Penalva J. Espasticidad en la patología neurológica. Actualización sobre mecanismos fisiopatológicos, avances en el diagnóstico y tratamiento [Spasticity in neurological pathologies. An update on the pathophysiological mechanisms, advances in diagnosis and treatment]. Rev Neurol 70: 453–460, 2020. doi: 10.33588/rn.7012.2019474. [DOI] [PubMed] [Google Scholar]
- 3. Holtz KA, Lipson R, Noonan VK, Kwon BK, Mills PB. Prevalence and effect of problematic spasticity after traumatic spinal cord injury. Arch Phys Med Rehabil 98: 1132–1138, 2017. doi: 10.1016/j.apmr.2016.09.124. [DOI] [PubMed] [Google Scholar]
- 4. Sköld C, Levi R, Seiger A. Spasticity after traumatic spinal cord injury: nature, severity, and location. Arch Phys Med Rehabil 80: 1548–1557, 1999. doi: 10.1016/s0003-9993(99)90329-5. [DOI] [PubMed] [Google Scholar]
- 5. Walter JS, Sacks J, Othman R, Rankin AZ, Nemchausky B, Chintam R, Wheeler JS. A database of self-reported secondary medical problems among VA spinal cord injury patients: its role in clinical care and management. J Rehabil Res Dev 39: 53–61, 2002. [Erratum in J Rehabil Res Dev 39: 623, 2002]. [PubMed] [Google Scholar]
- 6. Zörner B, Hostettler P, Meyer C, Killeen T, Gut P, Linnebank M, Weller M, Straumann D, Filli L. Prognosis of walking function in multiple sclerosis supported by gait pattern analysis. Mult Scler Relat Disord 63: 103802, 2022. Jul. doi: 10.1016/j.msard.2022.103802. [DOI] [PubMed] [Google Scholar]
- 7. Lance JW. The control of muscle tone, reflexes, and movement: Robert Wartenberg Lecture. Neurology 30: 1303–1313, 1980. doi: 10.1212/wnl.30.12.1303. [DOI] [PubMed] [Google Scholar]
- 8. Savtchouk I, Volterra A. Gliotransmission: beyond black-and-white. J Neurosci 38: 14–25, 2018. doi: 10.1523/JNEUROSCI.0017-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ventura R, Harris KM. Three-dimensional relationships between hippocampal synapses and astrocytes. J Neurosci 19: 6897–6906, 1999. doi: 10.1523/JNEUROSCI.19-16-06897.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Andersen JV, Markussen KH, Jakobsen E, Schousboe A, Waagepetersen HS, Rosenberg PA, Aldana BI. Glutamate metabolism and recycling at the excitatory synapse in health and neurodegeneration. Neuropharmacology 196: 108719, 2021. doi: 10.1016/j.neuropharm.2021.108719. [DOI] [PubMed] [Google Scholar]
- 11. Blanco-Suárez E, Caldwell AL, Allen NJ. Role of astrocyte-synapse interactions in CNS disorders. J Physiol 595: 1903–1916, 2017. doi: 10.1113/JP270988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Broadhead MJ, Bonthron C, Arcinas L, Bez S, Zhu F, Goff F, Nylk J, Dholakia K, Gunn-Moore F, Grant SGN, Miles GB. Nanostructural diversity of synapses in the mammalian spinal cord. Sci Rep 10: 8189, 2020. [Erratum in Sci Rep 11: 23160, 2021]. doi: 10.1038/s41598-020-64874-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Peterson AR, Binder DK. Astrocyte glutamate uptake and signaling as novel targets for antiepileptogenic therapy. Front Neurol 11: 1006, 2020. doi: 10.3389/fneur.2020.01006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Craven SE, El-Husseini AE, Bredt DS. Synaptic targeting of the postsynaptic density protein PSD-95 mediated by lipid and protein motifs. Neuron 22: 497–509, 1999. doi: 10.1016/s0896-6273(00)80705-9. [DOI] [PubMed] [Google Scholar]
- 15. Benson CA, Olson KL, Patwa S, Reimer ML, Bangalore L, Hill M, Waxman SG, Tan AM. Conditional RAC1 knockout in motor neurons restores H-reflex rate-dependent depression after spinal cord injury. Sci Rep 11: 7838, 2021. doi: 10.1038/s41598-021-87476-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Basso DM, Fisher LC, Anderson AJ, Jakeman LB, McTigue DM, Popovich PG. Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J Neurotrauma 23: 635–659, 2006. doi: 10.1089/neu.2006.23.635. [DOI] [PubMed] [Google Scholar]
- 17. Ho SM, Waite PM. Effects of different anesthetics on the paired-pulse depression of the h reflex in adult rat. Exp Neurol 177: 494–502, 2002. doi: 10.1006/exnr.2002.8013. [DOI] [PubMed] [Google Scholar]
- 18. Hosoido T, Motoyama S, Goto M, Mori F, Tajima T, Hirata H, Wada N. Characteristics of H- and M-waves recorded from rat forelimbs. Neurosci Lett 450: 239–241, 2009. doi: 10.1016/j.neulet.2008.11.045. [DOI] [PubMed] [Google Scholar]
- 19. Bandaru SP, Liu S, Waxman SG, Tan AM. Dendritic spine dysgenesis contributes to hyperreflexia after spinal cord injury. J Neurophysiol 113: 1598–1615, 2015. doi: 10.1152/jn.00566.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Boulenguez P, Vinay L. Strategies to restore motor functions after spinal cord injury. Curr Opin Neurobiol 19: 587–600, 2009. doi: 10.1016/j.conb.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 21. Lee HJ, Jakovcevski I, Radonjic N, Hoelters L, Schachner M, Irintchev A. Better functional outcome of compression spinal cord injury in mice is associated with enhanced H-reflex responses. Exp Neurol 216: 365–374, 2009. doi: 10.1016/j.expneurol.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 22. Schieppati M. The Hoffmann reflex: a means of assessing spinal reflex excitability and its descending control in man. Prog Neurobiol 28: 345–376, 1987. doi: 10.1016/0301-0082(87)90007-4. [DOI] [PubMed] [Google Scholar]
- 23. Thompson FJ, Reier PJ, Lucas CC, Parmer R. Altered patterns of reflex excitability subsequent to contusion injury of the rat spinal cord. J Neurophysiol 68: 1473–1486, 1992. doi: 10.1152/jn.1992.68.5.1473. [DOI] [PubMed] [Google Scholar]
- 24. Valero-Cabré A, Forés J, Navarro X. Reorganization of reflex responses mediated by different afferent sensory fibers after spinal cord transection. J Neurophysiol 91: 2838–2848, 2004. doi: 10.1152/jn.01177.2003. [DOI] [PubMed] [Google Scholar]
- 25. Palmieri RM, Ingersoll CD, Hoffman MA. The Hoffmann reflex: methodologic considerations and applications for use in sports medicine and athletic training research. J Athl Train 39: 268–277, 2004. [PMC free article] [PubMed] [Google Scholar]
- 26. Boulenguez P, Liabeuf S, Bos R, Bras H, Jean-Xavier C, Brocard C, Stil A, Darbon P, Cattaert D, Delpire E, Marsala M, Vinay L. Down-regulation of the potassium-chloride cotransporter KCC2 contributes to spasticity after spinal cord injury. Nat Med 16: 302–307, 2010. doi: 10.1038/nm.2107. [DOI] [PubMed] [Google Scholar]
- 27. Tan AM, Chakrabarty S, Kimura H, Martin JH. Selective corticospinal tract injury in the rat induces primary afferent fiber sprouting in the spinal cord and hyperreflexia. J Neurosci 32: 12896–12908, 2012. doi: 10.1523/JNEUROSCI.6451-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Arizono M, Inavalli VVGK, Panatier A, Pfeiffer T, Angibaud J, Levet F, Ter Veer MJT, Stobart J, Bellocchio L, Mikoshiba K, Marsicano G, Weber B, Oliet SHR, Nägerl UV. Structural basis of astrocytic Ca2+ signals at tripartite synapses. Nat Commun 11: 1906, 2020. [Erratum in Nat Commun 11: 2541, 2020]. doi: 10.1038/s41467-020-15648-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Panatier A, Arizono M, Nägerl UV. Dissecting tripartite synapses with STED microscopy. Philos Trans R Soc Lond B Biol Sci 369: 20130597, 2014. doi: 10.1098/rstb.2013.0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nielsen JB, Crone C, Hultborn H. The spinal pathophysiology of spasticity–from a basic science point of view. Acta Physiol (Oxf) 189: 171–180, 2007. doi: 10.1111/j.1748-1716.2006.01652.x. [DOI] [PubMed] [Google Scholar]
- 31. Sezer N, Akkuş S, Uğurlu FG. Chronic complications of spinal cord injury. World J Orthop 6: 24–33, 2015. doi: 10.5312/wjo.v6.i1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol 119: 7–35, 2010. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hefferan MP, Kucharova K, Kinjo K, Kakinohana O, Sekerkova G, Nakamura S, Fuchigami T, Tomori Z, Yaksh TL, Kurtz N, Marsala M. Spinal astrocyte glutamate receptor 1 overexpression after ischemic insult facilitates behavioral signs of spasticity and rigidity. J Neurosci 27: 11179–11191, 2007. doi: 10.1523/JNEUROSCI.0989-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ji RR, Donnelly CR, Nedergaard M. Astrocytes in chronic pain and itch. Nat Rev Neurosci 20: 667–685, 2019. doi: 10.1038/s41583-019-0218-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Verhoog QP, Holtman L, Aronica E, van Vliet EA. Astrocytes as guardians of neuronal excitability: mechanisms underlying epileptogenesis. Front Neurol 11: 591690, 2020. doi: 10.3389/fneur.2020.591690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chung WS, Allen NJ, Eroglu C. Astrocytes control synapse formation, function, and elimination. Cold Spring Harb Perspect Biol 7: a020370, 2015. doi: 10.1101/cshperspect.a020370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Farhy-Tselnicker I, Allen NJ. Astrocytes, neurons, synapses: a tripartite view on cortical circuit development. Neural Dev 13: 7, 2018. doi: 10.1186/s13064-018-0104-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rimmele TS, Rosenberg PA. GLT-1: The elusive presynaptic glutamate transporter. Neurochem Int 98: 19–28, 2016. doi: 10.1016/j.neuint.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jeyifous O, Lin EI, Chen X, Antinone SE, Mastro R, Drisdel R, Reese TS, Green WN. Palmitoylation regulates glutamate receptor distributions in postsynaptic densities through control of PSD95 conformation and orientation. Proc Natl Acad Sci USA 113: E8482–E8491, 2016. doi: 10.1073/pnas.1612963113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ayuso-Dolado S, Esteban-Ortega GM, Vidaurre ÓG, Díaz-Guerra M. A novel cell-penetrating peptide targeting calpain-cleavage of PSD-95 induced by excitotoxicity improves neurological outcome after stroke. Theranostics 11: 6746–6765, 2021. doi: 10.7150/thno.60701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Clifton NE, Trent S, Thomas KL, Hall J. Regulation and function of activity-dependent homer in synaptic plasticity. Mol Neuropsychiatry 5: 147–161, 2019. doi: 10.1159/000500267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Béïque JC, Andrade R. PSD-95 regulates synaptic transmission and plasticity in rat cerebral cortex. J Physiol 546: 859–867, 2003. Feb 1 doi: 10.1113/jphysiol.2002.031369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sheng M. Molecular organization of the postsynaptic specialization. Proc Natl Acad Sci USA 98: 7058–7061, 2001. doi: 10.1073/pnas.111146298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Palpagama TH, Waldvogel HJ, Faull RLM, Kwakowsky A. The role of microglia and astrocytes in Huntington's Disease. Front Mol Neurosci 12: 258, 2019. doi: 10.3389/fnmol.2019.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lalo U, Koh W, Lee CJ, Pankratov Y. The tripartite glutamatergic synapse. Neuropharmacology 199: 108758, 2021. Nov 1. doi: 10.1016/j.neuropharm.2021.108758. [DOI] [PubMed] [Google Scholar]
- 46. Rudy CC, Hunsberger HC, Weitzner DS, Reed MN. The role of the tripartite glutamatergic synapse in the pathophysiology of Alzheimer's disease. Aging Dis 6: 131–148, 2015. doi: 10.14336/AD.2014.0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yang Y, Gozen O, Watkins A, Lorenzini I, Lepore A, Gao Y, Vidensky S, Brennan J, Poulsen D, Won Park J, Li Jeon N, Robinson MB, Rothstein JD. Presynaptic regulation of astroglial excitatory neurotransmitter transporter GLT1. Neuron 61: 880–894, 2009. doi: 10.1016/j.neuron.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lau A, Tymianski M. Glutamate receptors, neurotoxicity and neurodegeneration. Pflugers Arch 460: 525–542, 2010. doi: 10.1007/s00424-010-0809-1. [DOI] [PubMed] [Google Scholar]
- 49. Zhou Y, Danbolt NC. Glutamate as a neurotransmitter in the healthy brain. J Neural Transm 121: 799–817, 2014. doi: 10.1007/s00702-014-1180-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tan AM, Stamboulian S, Chang YW, Zhao P, Hains AB, Waxman SG, Hains BC. Neuropathic pain memory is maintained by Rac1-regulated dendritic spine remodeling after spinal cord injury. J Neurosci 28: 13173–13183, 2008. doi: 10.1523/JNEUROSCI.3142-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pajarillo E, Rizor A, Lee J, Aschner M, Lee E. The role of astrocytic glutamate transporters GLT-1 and GLAST in neurological disorders: potential targets for neurotherapeutics. Neuropharmacology 161: 107559, 2019. doi: 10.1016/j.neuropharm.2019.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sheldon AL, Robinson MB. The role of glutamate transporters in neurodegenerative diseases and potential opportunities for intervention. Neurochem Int 51: 333–355, 2007. doi: 10.1016/j.neuint.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kabdesh IM, Mukhamedshina YO, Arkhipova SS, Sabirov DK, Kuznecov MS, Vyshtakalyuk AB, Rizvanov AA, James V, Chelyshev YA. Cellular and molecular gradients in the ventral horns with increasing distance from the injury site after spinal cord contusion. Front Cell Neurosci 16: 817752, 2022. doi: 10.3389/fncel.2022.817752. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available upon reasonable request.