Abstract
Metabolic (dysfunction)-associated fatty liver disease (MAFLD) has emerged as a significant global health concern, representing a major cause of liver disease worldwide. This condition spans a spectrum of histopathologic stages, beginning with simple fatty liver (MAFL), characterized by over 5% fat accumulation, and advancing to metabolic (dysfunction)-associated steatohepatitis, potentially leading to hepatocellular carcinoma. Despite extensive research, there remains a substantial gap in effective therapeutic interventions. This condition’s progression is closely tied to micronutrient levels, crucial for biological functions like antioxidant activities and immune efficiency. The levels of these micronutrients exhibit considerable variability among individuals with MAFLD. Moreover, the extent of deficiency in these nutrients can vary significantly throughout the different stages of MAFLD, with disease progression potentially exacerbating these deficiencies. This review focuses on the role of micronutrients, particularly vitamins A, D, E, and minerals like iron, copper, selenium, and zinc, in MAFLD’s pathophysiology. It highlights how alterations in the homeostasis of these micronutrients are intricately linked to the pathophysiological processes of MAFLD. Concurrently, this review endeavors to harness the existing evidence to propose novel therapeutic strategies targeting these vitamins and minerals in MAFLD management and offers new insights into disease mechanisms and treatment opportunities in MAFLD.
Keywords: metabolic (dysfunction)-associated fatty liver disease, vitamins, minerals, nutritional assessment, therapeutic strategy
1. Introduction
Non-alcoholic fatty liver disease (NAFLD) represents a spectrum of liver disorders, ranging from simple steatosis to more severe conditions like steatohepatitis with fibrosis, and ultimately, cirrhosis. Recognizing its association with hepatic steatosis, obesity, T2DM, and hypertriglyceridemia, NAFLD has been renamed Metabolic (Dysfunction)-Associated Fatty Liver Disease (MAFLD), highlighting its metabolic underpinnings (1–3).
MAFLD can lead to hepatocellular carcinoma in its more advanced stages, a malignancy known for its high mortality rate. Recent epidemiological studies reveal that MAFLD’s global prevalence has reached approximately 30% (4), and this trend shows no signs of abating. Most MAFLD patients initially have a benign condition, MAFL, with over 5% of hepatocytes containing lipid droplets (5). However, 20–30% progress to metabolic (dysfunction)-associated steatohepatitis (6, 7), characterized by significant steatosis, inflammation, and cellular ballooning, primarily in the liver’s alveolar zone 3 (8). Alarmingly, up to 38% of MASH patients with fibrosis may develop cirrhosis, and 2.4–12.8% of these individuals are at risk of hepatocellular carcinoma (HCC) (7). Both cirrhosis and hepatocellular carcinoma linked to MAFLD are associated with poor prognoses, highlighting the urgency for timely and effective management strategies in MAFLD patients.
Vitamins and minerals, essential micronutrients predominantly sourced from our diet, play a crucial role in normal body functioning through their antioxidant properties, enzyme activities, and immune system modulation (9). Recent research has brought to light the significant role of certain trace elements, particularly vitamins A, D, E, and minerals like iron, copper, selenium, and zinc. This article delves into their involvement in immune-inflammatory and metabolic processes (10, 11). The destabilization of these micronutrients has been linked to a variety of metabolic diseases (12), including MAFLD (13). Globally, vitamin and mineral deficiencies are widespread (14), and MAFLD patients frequently face similar challenges. These deficiencies are often tied to the dietary choices (15) of the individuals and a reduction in vitamin production due to altered intestinal flora (16). Despite the prevalence of MAFLD, current medical treatments remain inadequate. However, observations of micronutrient imbalances in MAFLD patients and animal models (17), along with the improvements seen in targeted therapies, open up new avenues for treating this condition. The ability of vitamins and trace minerals to positively impact the mechanisms at the core of MAFLD offers promising prospects for its pharmacological treatment (18, 19). This insight, focusing on correcting micronutrient imbalances, could pave the way for innovative strategies in managing and potentially mitigating the progression of MAFLD.
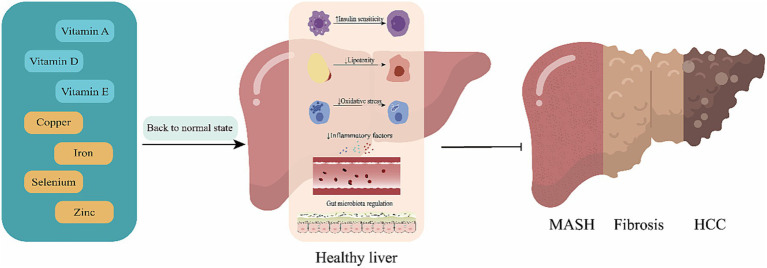
The review aims to provide the latest summary on the pathophysiologic pathways linking micronutrients to the development of MAFLD and to focus on new data from clinical trials exploring the safety and efficacy of vitamin and mineral supplementation on liver outcomes in patients with MAFLD (See Figure 1).
Figure 1.
Mechanisms of action for the effect of micronutrients. Replenishment of deficient micronutrients plays a pivotal role in reducing the risk and progression of metabolic (dysfunction)-associated fatty liver disease (MAFLD). By restoring these essential nutrients to optimal levels, there is a marked improvement in insulin sensitivity, a reduction in lipotoxicity, a decrease in inflammatory mediators, and a regulation of the intestinal microbiota. These changes collectively contribute to slowing the progression from metabolic (dysfunction)-associated fatty liver (MAFL) to metabolic (dysfunction)-associated steatohepatitis, fibrosis, and potentially hepatocellular carcinoma.
2. Pathogenesis of MAFLD and current therapeutics
2.1. Pathogenesis of MAFLD
The pathogenesis of MAFLD is not well defined, and the “multi-hit theory” is more widely recognized. MAFLD is a complex disease characterized by interactions between the environment and the susceptible polygenic host background that determine the phenotype and progression of the disease, MBOAT7, and other variants in the genes are strongly and consistently associated with MAFLD (20). On this basis, modern high-fat diet and unhealthy lifestyle habits act as triggers for impaired hepatic fat metabolism, hepatocellular fat accumulation producing lipotoxicity (21), endoplasmic reticulum stress (22), increased synthesis of reactive oxygen species, synthesis of adipokines, activation of inflammatory cells and release of inflammatory factors triggering intrahepatic inflammation (23), disruption of hepatic homeostasis, and comorbid insulin resistance (24). Gut microecological changes (25), accelerating the transformation of MAFL to MASH, liver fibrosis and cirrhosis (See Table 1).
Table 1.
Effect of vitamins and minerals in MAFLD.
| Author (reference) | Treatment and control | Experimental model | Treatment dosage and administration | Findings |
|---|---|---|---|---|
| Tang et al. (26) | Treatment: retinoic acid receptor β2 agonist(AC261066) Negative controls:- Positive controls:- |
High-fat diet (HFD) induced wild-type (wt) male C57BL/6 mice mouse | 3 mg/100 mL drinking water, oral for 2 months |
|
| Trasino et al. (27) | Treatment: retinoic acid receptor β2 agonist(AC261066) Negative control: RARγ agonist (CD1530) Positive control: no treatment |
High-fat diet (HFD) induced Wild type (wt) male C57BL/6 mice | 15 IU/vitamin A-acetate/gram, oral for 3 months |
|
| Zarei et al. (28) | Treatment: atRA Negative control:- Positive control: - |
High-fat diet (HFD) induced male New Zealand rabbits | 5 mg/kg/day, oral for 30 days |
|
| Kim et al. (29) | Treatment: atRA Negative control: - Positive control: - |
WD-fed C57BL/6 mice | Corn oil containing atRA (15 mg/kg/day), oral for 7 days |
|
| Berry et al. (30) | Treatment: atRA Negative control: - Positive control: - |
High-fat/high-sucrose diet C57BL/6Ntac mice | Subcutaneously implanted with an RA pellet or mock pelleted by using a 10-gauge precision trochar |
|
| Tsuchiya et al. (31) | Treatment: ATRA Negative control: - Positive control:- |
High-fat, high-fructose diet-induced C57BL/6 J mice | 50 mg/kg ATRA, oral for 4 weeks |
|
| Li et al. (18) | Treatment: 1,25 (15)2D3 Negative control: - Positive control: - |
High-fat diet (HFD) induced male C57BL/6 mice | 2.5 ng/g, three times per week for 4 weeks, i.p. |
|
| Dabbaghmanesh et al. (32) | Treatment: cholecalciferol & calcitriol Negative control: placebo Positive control: no treatment |
MAFLD patient | 50,000 U vitamin D3 pearl/week for 3 months, oral or0.25 mg calcitriol pearl/day for 3 months, oral |
|
| Wenclewska et al. (33) | Treatment: cholecalciferol Negative control: no treatment Positive control: no treatment |
Metabolic Disorder patients | 2000 International Unit (11) cholecalciferol/day oral for three months |
|
| El Amrousy et al. (34) | Treatment: vitamin D Negative control: placebo Positive control: no treatment |
100 children with biopsy-proven MAFLD | 2000 IU/day orally for 6 months |
|
| Mosca et al. (19) | Treatment: vitamin E & hydroxytyrosol Negative control: placebo Positive control: no treatment |
Children with MAFLD | 3.75 mg of hydroxytyrosol plus 5 mg of Vitamin E/day, oral for 16 weeks |
|
| Scorletti et al. (35) | Treatment: vitamin E Negative control: no treatment Positive control: no treatment |
MAFLD patients | Not for sure |
|
| Vilar-Gomez et al. (36) | Treatment: vitamin E Negative control: no treatment Positive control: no treatment |
MASH patients | 800 international units/day of vitamin E for ≥2 years |
|
| Podszun et al. (37) | Treatment:vitamin E Negative control: no treatment Positive control: no treatment |
MAFLD patients | 200–800 IU/d, oral for 24 weeks |
|
| Doboszewskaet al. (38) | Treatment: Zinc Negative controls: Zinc-deficient (ZnD)diet Positive controls: Zinc-adequate (ZnA) diet |
Male Sprague-Dawley rats | 50 mg Zn/kg or 3 mg Zn/kg, for 4 or 6 weeks |
|
| Ma et al. (39) | Treatment: high-iron (HI) diets Negative controls: low-iron (LI) diets Positive controls: - |
Male db/db mice | High-iron (HI) diets (1,000 mg/kg chow) or low-iron (LI) diets (12 mg/kg), oral for 9 weeks |
|
| Fujiwara et al. (40) | Treatment: high-iron Negative controls: Western diet Positive controls: Western diet + high-iron |
Male F344/DuCrlCrlj rats | 6% of blending iron citrate (FeC6H5O7・5H2O), oral for 26 weeks |
|
| Wang et al. (41) | Treatment: Se-enriched spirulina Negative controls: normal diet with Se-enriched spirulina Positive controls: HFD with Se-enriched spirulina |
High-fat diet (HFD) induced C57BL/6 mice | Se content 0.45 mg/kg, oral 12 weeks |
|
| Xu et al. (42) | Treatment: sodium selenate, Negative controls: - Positive controls: - |
Male APP/PS1 transgenic mice | 12 μg/mL sodium selenate, oral for 2 months |
|
| Zhang et al. (43) | Treatment: Se Negative controls: - Positive controls: - |
free fatty acid (FFA) induced primary rat hepatocytes | 0.1 μM Se, cell cultivation |
|
| Miyataet al. (6) | Treatment: Selenoneine Negative controls: - Positive controls: - |
Fxr-null mice | 0.3 mg Se/kg selenoneine-containing diet oral for 4 months |
|
2.2. Current therapeutics
Current treatment strategies for Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD) are limited in effectiveness. Clinical guidelines primarily advocate for lifestyle interventions (44) and, in cases where obesity has advanced significantly, bariatric surgery (45) to facilitate weight loss. For lean MAFLD patients, the recommended approach is lifestyle modification coupled with a reduction in fructose and sugar-sweetened beverages, aiming for a modest weight loss of 3–5% (46). Other pharmacological treatments, such as metformin, thiazolidinediones, and liraglutide, are generally reserved for patients with concurrent diabetes mellitus. However, their efficacy specifically for MAFLD is not conclusively proven, and they have shown potential side effects or unintended results in animal studies (47).
Research into MAFLD patients’ micronutrient levels reveals complex interactions and trends. Vitamins A, E, Zinc, and Copper are often reduced, while Vitamin D varies and Iron increases. These micronutrients interact within MAFLD, complicating disease understanding and progression. This interplay presents challenges yet offers new therapeutic opportunities. Current research focuses on understanding these interactions to develop targeted MAFLD treatments, marking a shift towards more effective management approaches.
3. Vitamins and MAFLD
3.1. Vitamins deficiency status in MAFLD patients
Vitamin deficiency is a global health issue with widespread impact (48). In metabolic diseases related to obesity, most vitamins are found to be deficient (49). Specifically, in MAFLD, the primary vitamins affected are the fat-soluble ones: A, D, and E. MAFLD patients, often consuming diets low in nutrients, rich in high-fat meats/proteins, and high in sodium (50), are prone to lower levels of these vitamins without additional supplementation. Furthermore, alterations in the intestinal microecology significantly influence vitamin absorption, contributing to the vitamin deficiencies observed in MAFLD patients (51, 52). Additionally, vitamin deficiencies play a role in low-intensity inflammation, exacerbated by the release of inflammatory adipokines from adipose tissue, which further aggravates the condition of MAFLD patients. This complex interplay underscores the importance of addressing vitamin deficiencies in managing and improving the health outcomes for those with MAFLD (49).
3.2. Role of vitamins in pathogenesis of MAFLD
3.2.1. Vitamin A and MAFLD
Vitamin A, essential for various physiological functions in the human body, relies exclusively on dietary intake. Its primary active form, retinoic acid (RA), plays a pivotal role by binding to retinoic acid receptors to facilitate biological signal transduction (53). Normally, vitamin A, being fat-soluble, is stored in hepatic stellate cells (54, 55). In patients with MAFLD, circulating concentrations of retinoic acid are observed to be lower (56). There is a notable correlation between diminished levels of Vitamin A and the severity of hepatic fibrosis, as well as an increase in liver-related mortality (57). Furthermore, in patients with metabolic (dysfunction)-associated Steatohepatitis (MASH), high expression of hepatic AKR1B10 is linked to reduced hepatic retinoid levels, exacerbating the progression from MASH to Hepatocellular Carcinoma (HCC) (58). This highlights the critical role of Vitamin A in liver health and its potential implications in the progression of liver diseases.
Vitamin A contributes to the management of MAFLD through various mechanisms, including the modulation of lipid metabolism (26, 30, 59), antioxidant effects (28, 60), anti-inflammatory properties (27), and enhancing insulin sensitivity (30, 31). Notably, the retinoic acid receptor β2 agonist AC261066 has been shown to induce changes in the transcriptome and metabolome of hepatocytes (26), reduce the TGF-β1 inflammatory response in Kupffer cells, and alleviate liver fibrosis (27, 61). Dietary supplementation with all-trans retinoic acid (ATRA) notably improved insulin sensitivity in MAFLD model mice (C57BL/6J) (31). Additionally, ATRA acts on the retinoic acid receptor (62) to decrease PPAR-γ2 expression, thereby reducing fat accumulation in the liver (29).
Despite these promising findings, the clinical application of vitamin A in MAFLD treatment is constrained by its narrow therapeutic window and the limited number of clinical trials. This highlights the need for further research to fully understand and harness Vitamin A’s potential in MAFLD treatment while ensuring safety and efficacy in human applications.
3.2.2. Vitamin D and MAFLD
Vitamin D, primarily produced in the skin via sunlight exposure, is vital for both skeletal and extra-skeletal health. Clinical guidelines recommend keeping human serum 25(OH)D levels above 50 nmoL/L. Despite this, about 7% of the global population has vitamin D levels below this threshold (63). Studies indicate that vitamin D deficiency is nonlinearly linked to increased MAFLD severity and higher all-cause mortality (64). Lower 25(OH) vitamin D levels are associated with increased MAFLD prevalence and liver fibrosis (65), while higher levels reduce fibrosis risk in MAFLD patients (66).
In a Western diet rat model, Vitamin D deficiency exacerbated MAFLD, potentially via toll-like receptor activation and endotoxin exposure (67). This deficiency also caused insulin resistance, increased hepcidin expression, and heightened inflammation and oxidative stress genes. Key to this severity in vitamin D-deficient MAFLD patients might be the activation of MAPK and NF-κB pathways (68).
In the realm of treating MAFLD with vitamin D, significant strides have been made. Studies across various regions (69, 70) and populations (71, 72) have shown that increased vitamin D levels may help prevent MAFLD. Different dosages of vitamin D exhibit varying degrees of improvement in MAFLD (33, 73).
Vitamin D induces autophagy (18) in mice, primarily by upregulating ATG16L1, thereby inhibiting the p53 pathway to prevent hepatocyte senescence and apoptosis (74). It also reduces inflammation via the enterohepatic axis, underscoring the importance of timely supplementation (75). Phototherapy-enhanced active vitamin D3 in mice mitigates hepatocyte apoptosis, inflammation, fibrosis, and insulin/leptin resistance caused by a CDAA diet (76). Additionally, vitamin D treatment curbs MAFLD induced by a high-fat diet (HFD), involving gut microbiota (77) and metabolic regulation (78), and modulates lipid metabolism through the PPARa signaling pathway (79). Vitamin D-regulated miRNAs are implicated in MAFLD pathogenesis, though more research is needed (80). It also exhibits antifibrotic effects by countering TGF-β signaling in hepatic stellate cells (81).
Contrastingly, some studies have found no correlation between plasma vitamin D levels and insulin resistance, hepatic fat accumulation, or MASH severity (82–85). Similarly, randomized trials using vitamin D supplements for MAFLD treatment have not consistently shown benefits (73, 86). Polymorphisms in the Vitamin D receptor gene could explain the varied outcomes observed. While Vitamin D ameliorates liver damage in MAFLD, early expression of its receptor in MAFLD patients’ livers and decreased lipid accumulation in mice lacking this receptor gene point to its intricate involvement in MAFLD’s development and progression (87).
These findings highlight vitamin D’s potential in MAFLD treatment but also reveal its multifaceted and context-dependent nature. The genetics and epigenetics of MAFLD may influence vitamin D’s regulatory mechanisms, necessitating further research to elucidate these intricate relationships.
3.2.3. Vitamin E and MAFLD
Vitamin E, currently the only medication recommended by guidelines for treating MASH, is valued for its antioxidant and anti-inflammatory properties (88, 89). It has been observed that patients with MAFLD often have reduced serum levels of both vitamin E and A (90). In a non-randomized, propensity score-adjusted study, a daily intake of 800 IU of vitamin E was associated with significant reductions in total mortality and hepatic decompensation in patients with MASH-induced bridging fibrosis and cirrhosis, both in diabetic and non-diabetic individuals (36). Moreover, vitamin E has been effective in lowering AST and ALT levels in adult patients with MAFLD (91). It inhibits oxidative stress, which reduces de novo lipogenesis (DNL) and intrahepatic triglyceride (IHTG) accumulation, thereby disrupting the cycle between oxidative stress and the MAFLD process (92). Histological improvements in MAFLD patients have also been noted with vitamin E treatment, demonstrating its therapeutic potential (92–95).
However, the effectiveness of vitamin E in altering the histological course of MASH in patients with Type 2 Diabetes Mellitus (T2DM) has not been significant (96). Additionally, its use is limited in the treatment of common comorbidities associated with MAFLD (96). While vitamin E shows promise in MAFLD treatment, its role and efficacy may vary depending on specific patient conditions and comorbidities, indicating the need for a nuanced approach in its clinical application.
4. Minerals and MAFLD
4.1. Minerals deficiency status in MAFLD patients
Mineral deficiencies are widely acknowledged as a significant public health issue worldwide, often leading to increased susceptibility to infections. By replenishing these deficient trace minerals to their recommended levels, we can enhance immune function, bolster resistance to infection, and facilitate quicker recovery from such illnesses. While epidemiological data on the connection between trace mineral deficiencies and the onset and advancement of MAFLD are scant, the role of inflammation as a key contributor to MAFLD, coupled with the dietary habits commonly observed in individuals with MAFLD, suggests a potential close link between these mineral deficiencies and the disease’s development and progression.
4.2. Role of minerals deficiency in the process of MAFLD progression
4.2.1. Major minerals
Calcium, phosphorus, and magnesium, as major minerals, play important roles in MAFLD. These minerals are key factors in the inflammatory processes related to MAFLD, participating in signaling mechanisms, hepatocyte injury and regeneration, and the regulation of inflammatory factors (62, 97–99). The intricate roles of these major minerals in the human body and their specific associations with MAFLD have been extensively discussed in other reviews (100) and studies (101), and thus fall outside the primary focus of this paper. Instead, this review concentrates on trace minerals, including zinc, iron, copper, and selenium, exploring their relationship with MAFLD and their impact on the progression and management of the disease.
4.2.2. Zinc and MAFLD
Zinc, a crucial trace element, plays vital roles in antioxidant, anti-inflammatory, and anti-apoptotic functions in the human body (102, 103). The risk of zinc deficiency increases with age (104). There is growing evidence linking zinc deficiency to the development of MAFLD (105, 106). In patients with biopsy-proven MAFLD, a J-shaped correlation exists between serum zinc levels and the severity of hepatic necroinflammation (107, 108). Serum zinc deficiency, commonly associated with oxidative stress, endoplasmic reticulum stress, apoptosis, and inflammation, has been noted in MAFLD mouse models (109, 110).
Furthermore, zinc supplementation has been shown to alleviate disorders in lipid and glucose metabolism caused by high-fat diets (111). In diet-induced MAFLD mice, zinc supplementation not only improves liver weight and morphology but also helps prevent hepatic failure (112).
Recent studies reveal zinc’s mechanisms in improving MAFLD: in mouse models, PLZF, relying on SIRT1, regulates hepatic lipid and glucose homeostasis (113). The HDAC3/β-catenin pathway promotes lipolysis and inhibits adipogenesis (114). ZHX2 activation of PTEN protects against MASH progression (115). Zinc alpha2 glycoprotein in hepatocytes impacts triglyceride accumulation and key gene expressions (116). The ADA/XO/UA pathway and caspase 3 signaling show potential in liver rescue (116). Zinc oxide nanoparticles in mice reduce hepatic steatosis via the AMPK axis (117), while the Zn2+/MTF-1/PPARa pathway aids in reducing lipid deposition (118). These findings collectively highlight zinc’s multifaceted role in addressing various aspects of MAFLD pathogenesis and progression.
Despite that, the specific studies and recommended zinc dosages for clinical treatment of MAFLD still require further exploration and validation.
4.2.3. Iron and MAFLD
The increasingly recognized causal link between iron overload and the progression of MAFLD (119, 120) suggests that dietary iron overload may worsen inflammation and lipid metabolism disorders, akin to human dietary iron overload syndrome (DIOS) (40). Hyperferritinemia the main manifestation of disturbed iron homeostasis often portends more severe metabolic dysfunction and liver injury (121, 122). In the hypoxic intestinal environment, HIF-2alpha plays a crucial role in regulating iron absorption by affecting the DMT1 gene (123). Abnormal iron-induced hepcidin release, influenced by natural genetic variants may enhance iron absorption (124). Additionally, excess free fatty acids (FFAs) disrupt hepatic iron metabolism, encouraging iron uptake via IRP1 and TfR-1 (125). Iron overload contributes to ferroptosis, initiating inflammation in nonalcoholic steatohepatitis and leading to oxidative DNA damage (126, 127). This condition can be exacerbated by a high-fat diet, which aggravates lipid metabolism disorders, hepatic injury, and oxidative stress (128). Iron-containing extracellular vesicles from hepatocytes induce liver steatosis and fibrosis in mice on a Western diet, causing iron deficiency in hepatocytes and overload in hepatic stellate cells (129). The complex interaction between gut microflora and the host not only impacts MAFLD progression but also influences iron balance (130, 131). This situation results in a detrimental cycle where iron overload increases lipid deposition through oxidative stress-induced mitochondrial dysfunction and activation of the HIF1α-PPARγ pathway (129, 132).
While numerous studies have indicated that bloodletting to address iron overload can improve insulin resistance in patients with MAFLD and hyperferritinemia (133–135), other findings suggest that lowering ferritin through phlebotomy does not necessarily improve liver enzymes, liver fat, or insulin resistance in MAFLD patients (136). This discrepancy highlights the need for more detailed research to unravel these complex interactions and effects.
4.2.4. Copper and MAFLD
Copper, a vital cofactor in numerous physiological redox reactions, has a complex relationship with MAFLD. In vivo bioluminescence imaging has shown copper deficiency in a mouse model of MAFLD (137). Concurrently, both hair and hepatic copper concentrations in MAFLD patients are significantly lower and correlate with increased hepatic steatosis, MASH severity, and metabolite alterations (138). Limiting copper intake in mice has been shown to induce hepatic steatosis and insulin resistance, leading to the development of MAFLD (17, 139).
Research exploring the link between copper and lipid metabolism indicates a negative correlation (140). Restoration of intrahepatic copper, achieved by down-regulating copper cyanin, enhances lipolysis through the assembly of copper-loaded SCO1-LKB1-AMPK complexes, showing improvements in MAFLD conditions in mice (141). A case-control study found that high levels of copper significantly improved MAFL in males, highlighting copper’s protective role in MAFLD treatment (140, 142).
However, studies also point out the harmful effects of copper overload on lipid metabolism (143, 144) and increased MAFLD risk and severity (145). Moreover, there is a noticeable gap in clinical research exploring the relationship between copper and MAFLD, and the potential toxicology of copper also warrants special attention. Despite these complexities, the intricate link between copper and MAFLD presents a potential avenue for breakthroughs in MAFLD treatment.
4.2.5. Selenium and MAFLD
Selenium, a crucial micronutrient, plays diverse roles in the human body, including antioxidant activities, cancer prevention, and immunomodulation, thanks to its structural and enzymatic functions (146–148). It also has significant implications in metabolic diseases (149). The relationship between selenium and MAFLD is complex and appears to be dose-dependent (150).
Studies have shown that lower blood selenium levels are associated with a higher incidence of advanced liver fibrosis (151). Conversely, higher blood selenium levels (above ~130 μg/L) have been positively correlated with both MAFLD and ghrelin, indicating a dose–response relationship (150, 152). In experimental settings, selenium supplementation in MAFLD mice models has demonstrated beneficial effects, such as mitigating hepatic injury, reducing oxidative stress, lowering insulin resistance, and decreasing inflammation (6, 41, 43, 153).
However, the use of selenium in MAFLD treatment necessitates careful consideration of its delicate balance between therapeutic efficacy and toxicity. Determining the appropriate dosage of selenium is critical and remains a subject of ongoing research and debate in the context of MAFLD treatment. This nuanced understanding of selenium’s role underscores the importance of precise dosing in its potential application as a therapeutic agent for MAFLD.
4.3. Relationship between micronutrients in MAFLD
While individual trace elements’ roles in MAFLD have been detailed, research on their complex interrelationships is scarce. Zinc and selenium have been linked to reduced cardiovascular risk (109), and Vitamin D and zinc both enhance immune function (154). Additionally, copper and ascorbic acid can interfere with non-heme iron absorption (155). These findings indicate a delicate balance among trace elements, crucial for maintaining overall body homeostasis.
5. Conclusion and outlook
In this review, we examine recent research on the impact of various vitamins and trace minerals on MAFLD. Most studies suggest that deficiencies in vitamins and minerals negatively affect MAFLD. Timely and appropriate supplementation could aid in disease recovery or slow its progression, potentially improving patient prognosis. However, there are also contrasting views or skepticism regarding the causal link between these deficiencies and MAFLD, an aspect this review critically explores.
Currently, there’s no unified approach to the pharmacological treatment of MAFLD. Lifestyle interventions and bariatric surgery have shown relative effectiveness, but their success is often limited by patient compliance and eligibility criteria. Hence, their widespread application among MAFLD patients is restricted. The search for effective drugs targeting MAFLD’s pathogenesis continues. Vitamins and minerals, crucial in regulating oxidative stress, inflammation, and lipid metabolism, offer promising directions for MAFLD treatment. The need for a drug that can improve the course and prognosis of MAFLD, provided in the necessary amounts for normal body function, is urgent.
Given the complex pathophysiology of MAFLD, the effectiveness of single-agent treatments observed in various studies suggests that individualized combination regimens might be necessary for optimal management of MAFLD. This review seeks to shed light on these multifaceted approaches and the potential of vitamins and minerals in the treatment landscape of MAFLD.
Author contributions
YL: Writing – original draft. XQ: Writing – original draft. TC: Writing – original draft. MC: Writing – original draft. LW: Writing – review & editing. BH: Supervision, Writing – review & editing.
Funding Statement
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The study is supported by Administration of Traditional Chinese Medicine of Zhejiang Province (No. 2023ZL419) and the Zhejiang Provincial Natural Science Foundation of China (No. LGF22H290001).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.European Association for the Study of the Liver (EASL) . EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. (2016) 64:1388–402. doi: 10.1016/j.jhep.2015.11.004 [DOI] [PubMed] [Google Scholar]
- 2.Eslam M, Sanyal AJ, George J. MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. (2020) 158:1999–2014.e1. doi: 10.1053/j.gastro.2019.11.312, PMID: [DOI] [PubMed] [Google Scholar]
- 3.Tilg H, Effenberger M. From NAFLD to MAFLD: when pathophysiology succeeds. Nat Rev Gastroenterol Hepatol. (2020) 17:387–8. doi: 10.1038/s41575-020-0316-6, PMID: [DOI] [PubMed] [Google Scholar]
- 4.Younossi ZM, Golabi P, Paik JM, Henry A, Van Dongen C, Henry L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): a systematic review. Hepatology. (2023) 77:1335–47. doi: 10.1097/hep.0000000000000004, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown GT, Kleiner DE. Histopathology of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Metabolism. (2016) 65:1080–6. doi: 10.1016/j.metabol.2015.11.008, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miyata M, Matsushita K, Shindo R, Shimokawa Y, Sugiura Y, Yamashita M. Selenoneine ameliorates hepatocellular injury and hepatic steatosis in a mouse model of NAFLD. Nutrients. (2020) 12:12. doi: 10.3390/nu12061898, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calzadilla Bertot L, Adams LA. The natural course of non-alcoholic fatty liver disease. Int J Mol Sci. (2016) 17:17. doi: 10.3390/ijms17050774, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yeh MM, Brunt EM. Pathological features of fatty liver disease. Gastroenterology. (2014) 147:754–64. doi: 10.1053/j.gastro.2014.07.056 [DOI] [PubMed] [Google Scholar]
- 9.Maggini S, Pierre A, Calder P. Immune function and micronutrient requirements change over the life course. Nutrients. (2018) 10:10. doi: 10.3390/nu10101531, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Norris SA, Frongillo EA, Black MM, Dong Y, Fall C, Lampl M, et al. Nutrition in adolescent growth and development. Lancet. (2022) 399:172–84. doi: 10.1016/s0140-6736(21)01590-7 [DOI] [PubMed] [Google Scholar]
- 11.Berger MM, Shenkin A, Schweinlin A, Amrein K, Augsburger M, Biesalski H-K, et al. ESPEN micronutrient guideline. Clin Nutr. (2022) 41:1357–424. doi: 10.1016/j.clnu.2022.02.015 [DOI] [PubMed] [Google Scholar]
- 12.Dubey P, Thakur V, Chattopadhyay M. Role of minerals and trace elements in diabetes and insulin resistance. Nutrients. (2020) 12:12. doi: 10.3390/nu12061864, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pickett-Blakely O, Young K, Carr RM. Micronutrients in nonalcoholic fatty liver disease pathogenesis. Cell Mol Gastroenterol Hepatol. (2018) 6:451–62. doi: 10.1016/j.jcmgh.2018.07.004, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGuire S, International Food Policy Research Institute . Washington, DC: global nutrition report 2014: actions and accountability to accelerate the world's progress on nutrition. Adv Nutr. (2014) 6:278–9. doi: 10.3945/an.115.008599, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yasutake K, Kohjima M, Kotoh K, Nakashima M, Nakamuta M, Enjoji M. Dietary habits and behaviors associated with nonalcoholic fatty liver disease. World J Gastroenterol. (2014) 20:1756–67. doi: 10.3748/wjg.v20.i7.1756, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barone M, D’Amico F, Brigidi P, Turroni S. Gut microbiome-micronutrient interaction: the key to controlling the bioavailability of minerals and vitamins? Biofactors. (2022) 48:307–14. doi: 10.1002/biof.1835, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou J, Liu C, Francis M, Sun Y, Ryu MS, Grider A, et al. The causal effects of blood Iron and copper on lipid metabolism diseases: evidence from phenome-wide Mendelian randomization study. Nutrients. (2020) 12:12. doi: 10.3390/nu12103174, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li R, Guo E, Yang J, Li A, Yang Y, Liu S, et al. 1, 25(OH)(2) D (3) attenuates hepatic steatosis by inducing autophagy in mice. Obesity. (2017) 25:561–71. doi: 10.1002/oby.21757, PMID: [DOI] [PubMed] [Google Scholar]
- 19.Mosca A, Crudele A, Smeriglio A, Braghini MR, Panera N, Comparcola D, et al. Antioxidant activity of hydroxytyrosol and vitamin E reduces systemic inflammation in children with paediatric NAFLD. Dig Liver Dis. (2021) 53:1154–8. doi: 10.1016/j.dld.2020.09.021, PMID: [DOI] [PubMed] [Google Scholar]
- 20.Trépo E, Valenti L. Update on NAFLD genetics: from new variants to the clinic. J Hepatol. (2020) 72:1196–209. doi: 10.1016/j.jhep.2020.02.020, PMID: [DOI] [PubMed] [Google Scholar]
- 21.Dong J, Viswanathan S, Adami E, Singh BK, Chothani SP, Ng B, et al. Hepatocyte-specific IL11 cis-signaling drives lipotoxicity and underlies the transition from NAFLD to NASH. Nat Commun. (2021) 12:66. doi: 10.1038/s41467-020-20303-z, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ajoolabady A, Kaplowitz N, Lebeaupin C, Kroemer G, Kaufman RJ, Malhi H, et al. Endoplasmic reticulum stress in liver diseases. Hepatology. (2023) 77:619–39. doi: 10.1002/hep.32562, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huby T, Gautier EL. Immune cell-mediated features of non-alcoholic steatohepatitis. Nat Rev Immunol. (2022) 22:429–43. doi: 10.1038/s41577-021-00639-3, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khan RS, Bril F, Cusi K, Newsome PN. Modulation of insulin resistance in nonalcoholic fatty liver disease. Hepatology. (2019) 70:711–24. doi: 10.1002/hep.30429 [DOI] [PubMed] [Google Scholar]
- 25.Bauer KC, Littlejohn PT, Ayala V, Creus-Cuadros A, Finlay BB. Nonalcoholic fatty liver disease and the gut-liver axis: exploring an undernutrition perspective. Gastroenterology. (2022) 162:1858–1875.e2. doi: 10.1053/j.gastro.2022.01.058, PMID: [DOI] [PubMed] [Google Scholar]
- 26.Tang XH, Melis M, Lu C, Rappa A, Zhang T, Jessurun J, et al. A retinoic acid receptor β2 agonist attenuates transcriptome and metabolome changes underlying nonalcohol-associated fatty liver disease. J Biol Chem. (2021) 297:101331. doi: 10.1016/j.jbc.2021.101331, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trasino SE, Tang XH, Jessurun J, Gudas LJ. A retinoic acid receptor β2 agonist reduces hepatic stellate cell activation in nonalcoholic fatty liver disease. J Mol Med. (2016) 94:1143–51. doi: 10.1007/s00109-016-1434-z, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zarei L, Farhad N, Abbasi A. All-trans retinoic acid (at RA) effectively improves liver steatosis in a rabbit model of high fat induced liver steatosis. Arch Physiol Biochem. (2022) 128:1010–5. doi: 10.1080/13813455.2020.1743725, PMID: [DOI] [PubMed] [Google Scholar]
- 29.Kim SC, Kim CK, Axe D, Cook A, Lee M, Li T, et al. All-trans-retinoic acid ameliorates hepatic steatosis in mice by a novel transcriptional cascade. Hepatology. (2014) 59:1750–60. doi: 10.1002/hep.26699, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berry DC, Noy N. All-trans-retinoic acid represses obesity and insulin resistance by activating both peroxisome proliferation-activated receptor beta/delta and retinoic acid receptor. Mol Cell Biol. (2009) 29:3286–96. doi: 10.1128/mcb.01742-08, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsuchiya H, Ikeda Y, Ebata Y, Kojima C, Katsuma R, Tsuruyama T, et al. Retinoids ameliorate insulin resistance in a leptin-dependent manner in mice. Hepatology. (2012) 56:1319–30. doi: 10.1002/hep.25798, PMID: [DOI] [PubMed] [Google Scholar]
- 32.Dabbaghmanesh MH, Danafar F, Eshraghian A, Omrani GR. Vitamin D supplementation for the treatment of non-alcoholic fatty liver disease: a randomized double blind placebo controlled trial. Diabetes Metab Syndr. (2018) 12:513–7. doi: 10.1016/j.dsx.2018.03.006 [DOI] [PubMed] [Google Scholar]
- 33.Wenclewska S, Szymczak-Pajor I, Drzewoski J, Bunk M, Śliwińska A. Vitamin D supplementation reduces both oxidative DNA damage and insulin resistance in the elderly with metabolic disorders. Int J Mol Sci. (2019) 20:20. doi: 10.3390/ijms20122891, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.El Amrousy D, Abdelhai D, Shawky D. Vitamin D and nonalcoholic fatty liver disease in children: a randomized controlled clinical trial. Eur J Pediatr. (2022) 181:579–86. doi: 10.1007/s00431-021-04243-4, PMID: [DOI] [PubMed] [Google Scholar]
- 35.Scorletti E, Creasy KT, Vujkovic M, Vell M, Zandvakili I, Rader DJ, et al. Dietary vitamin E intake is associated with a reduced risk of developing digestive diseases and nonalcoholic fatty liver disease. Am J Gastroenterol. (2022) 117:927–30. doi: 10.14309/ajg.0000000000001726, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vilar-Gomez E, Vuppalanchi R, Gawrieh S, Ghabril M, Saxena R, Cummings OW, et al. Vitamin E improves transplant-free survival and hepatic decompensation among patients with nonalcoholic steatohepatitis and advanced fibrosis. Hepatology. (2020) 71:495–509. doi: 10.1002/hep.30368 [DOI] [PubMed] [Google Scholar]
- 37.Podszun MC, Alawad AS, Lingala S, Morris N, Huang WA, Yang S, et al. Vitamin E treatment in NAFLD patients demonstrates that oxidative stress drives steatosis through upregulation of de-novo lipogenesis. Redox Biol. (2020) 37:101710. doi: 10.1016/j.redox.2020.101710, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Doboszewska U, Szewczyk B, Sowa-Kućma M, Noworyta-Sokołowska K, Misztak P, Gołębiowska J, et al. Alterations of bio-elements, oxidative, and inflammatory status in the zinc deficiency model in rats. Neurotox Res. (2016) 29:143–54. doi: 10.1007/s12640-015-9571-7, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ma W, Feng Y, Jia L, Li S, Li J, Wang Z, et al. Dietary Iron modulates glucose and lipid homeostasis in diabetic mice. Biol Trace Elem Res. (2019) 189:194–200. doi: 10.1007/s12011-018-1446-3, PMID: [DOI] [PubMed] [Google Scholar]
- 40.Fujiwara S, Izawa T, Mori M, Atarashi M, Yamate J, Kuwamura M. Dietary iron overload enhances Western diet induced hepatic inflammation and alters lipid metabolism in rats sharing similarity with human DIOS. Sci Rep. (2022) 12:21414. doi: 10.1038/s41598-022-25838-3, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Y, Liu B, Wu P, Chu Y, Gui S, Zheng Y, et al. Dietary selenium alleviated mouse liver oxidative stress and NAFLD induced by obesity by regulating the KEAP1/NRF2 pathway. Antioxidants. (2022) 11:e349. doi: 10.3390/antiox11020349, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu X, Qi P, Zhang Y, Sun H, Yan Y, Sun W, et al. Effect of selenium treatment on central insulin sensitivity: a proteomic analysis in β-amyloid precursor protein/Presenilin-1 transgenic mice. Front Mol Neurosci. (2022) 15:931788. doi: 10.3389/fnmol.2022.931788, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang Z, Li S, Jiang H, Liu B, Lv Z, Guo C, et al. Effects of selenium on apoptosis and abnormal amino acid metabolism induced by excess fatty acid in isolated rat hepatocytes. Mol Nutr Food Res. (2017) 61:61. doi: 10.1002/mnfr.201700016, PMID: [DOI] [PubMed] [Google Scholar]
- 44.Younossi ZM, Corey KE, Lim JK. AGA clinical practice update on lifestyle modification using diet and exercise to achieve weight loss in the Management of Nonalcoholic Fatty Liver Disease: expert review. Gastroenterology. (2021) 160:912–8. doi: 10.1053/j.gastro.2020.11.051 [DOI] [PubMed] [Google Scholar]
- 45.Lassailly G, Caiazzo R, Ntandja-Wandji LC, Gnemmi V, Baud G, Verkindt H, et al. Bariatric surgery provides Long-term resolution of nonalcoholic steatohepatitis and regression of fibrosis. Gastroenterology. (2020) 159:1290–1301.e5. doi: 10.1053/j.gastro.2020.06.006, PMID: [DOI] [PubMed] [Google Scholar]
- 46.Long MT, Noureddin M, Lim JK. AGA clinical practice update: diagnosis and management of nonalcoholic fatty liver disease in lean individuals: expert review. Gastroenterology. (2022) 163:764–774.e1. doi: 10.1053/j.gastro.2022.06.023, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wattacheril JJ, Abdelmalek MF, Lim JK, Sanyal AJ. AGA clinical practice update on the role of noninvasive biomarkers in the evaluation and management of nonalcoholic fatty liver disease: expert review. Gastroenterology. (2023) 165:1080–8. doi: 10.1053/j.gastro.2023.06.013, PMID: [DOI] [PubMed] [Google Scholar]
- 48.Rautiainen S, Manson JE, Lichtenstein AH, Sesso HD. Dietary supplements and disease prevention – a global overview. Nat Rev Endocrinol. (2016) 12:407–20. doi: 10.1038/nrendo.2016.54, PMID: [DOI] [PubMed] [Google Scholar]
- 49.Thomas-Valdés S, Tostes M, Anunciação PC, da Silva BP, Sant'Ana HMP. Association between vitamin deficiency and metabolic disorders related to obesity. Crit Rev Food Sci Nutr. (2017) 57:3332–43. doi: 10.1080/10408398.2015.1117413 [DOI] [PubMed] [Google Scholar]
- 50.Kim CH, Kallman JB, Bai C, Pawloski L, Gewa C, Arsalla A, et al. Nutritional assessments of patients with non-alcoholic fatty liver disease. Obes Surg. (2010) 20:154–60. doi: 10.1007/s11695-008-9549-0 [DOI] [PubMed] [Google Scholar]
- 51.De Filippis F, Pellegrini N, Vannini L, Jeffery IB, La Storia A, Laghi L, et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut. (2016) 65:1812–21. doi: 10.1136/gutjnl-2015-309957, PMID: [DOI] [PubMed] [Google Scholar]
- 52.Ghosh TS, Rampelli S, Jeffery IB, Santoro A, Neto M, Capri M, et al. Mediterranean diet intervention alters the gut microbiome in older people reducing frailty and improving health status: the NU-AGE 1-year dietary intervention across five European countries. Gut. (2020) 69:1218–28. doi: 10.1136/gutjnl-2019-319654, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Blomhoff R, Green MH, Berg T, Norum KR. Transport and storage of vitamin A. Science. (1990) 250:399–404. doi: 10.1126/science.2218545 [DOI] [PubMed] [Google Scholar]
- 54.Blaner WS. Vitamin a signaling and homeostasis in obesity, diabetes, and metabolic disorders. Pharmacol Ther. (2019) 197:153–78. doi: 10.1016/j.pharmthera.2019.01.006, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fortuna VA, Martucci RB, Trugo LC, Borojevic R. Hepatic stellate cells uptake of retinol associated with retinol-binding protein or with bovine serum albumin. J Cell Biochem. (2003) 90:792–805. doi: 10.1002/jcb.10703 [DOI] [PubMed] [Google Scholar]
- 56.Liu Y, Chen H, Wang J, Zhou W, Sun R, Xia M. Association of serum retinoic acid with hepatic steatosis and liver injury in nonalcoholic fatty liver disease. Am J Clin Nutr. (2015) 102:130–7. doi: 10.3945/ajcn.114.105155, PMID: [DOI] [PubMed] [Google Scholar]
- 57.Song J, Jiang ZG. Low vitamin a levels are associated with liver-related mortality: a nationally representative cohort study. Hepatol Commun. (2023) 7:7. doi: 10.1097/hc9.0000000000000124, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pettinelli P, Arendt BM, Teterina A, McGilvray I, Comelli EM, Fung SK, et al. Altered hepatic genes related to retinol metabolism and plasma retinol in patients with non-alcoholic fatty liver disease. PLoS One. (2018) 13:e0205747. doi: 10.1371/journal.pone.0205747, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim EJ, Yoon YS, Hong S, Son HY, Na TY, Lee MH, et al. Retinoic acid receptor-related orphan receptor α-induced activation of adenosine monophosphate-activated protein kinase results in attenuation of hepatic steatosis. Hepatology. (2012) 55:1379–88. doi: 10.1002/hep.25529, PMID: [DOI] [PubMed] [Google Scholar]
- 60.Geng C, Xu H, Zhang Y, Gao Y, Li M, Liu X, et al. Retinoic acid ameliorates high-fat diet-induced liver steatosis through sirt 1. Sci China Life Sci. (2017) 60:1234–41. doi: 10.1007/s11427-016-9027-6, PMID: [DOI] [PubMed] [Google Scholar]
- 61.Cassim Bawa FN, Xu Y, Gopoju R, Plonski NM, Shiyab A, Hu S, et al. Hepatic retinoic acid receptor alpha mediates all-trans retinoic acid's effect on diet-induced hepatosteatosis. Hepatol Commun. (2022) 6:2665–75. doi: 10.1002/hep4.2049, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ali ES, Girard D, Petrovsky N. Impaired ca (2+) signaling due to hepatic steatosis mediates hepatic insulin resistance in Alström syndrome mice that is reversed by GLP-1 analog treatment. Am J Physiol Cell Physiol. (2021) 321:C187–98. doi: 10.1152/ajpcell.00020.2021, PMID: [DOI] [PubMed] [Google Scholar]
- 63.Bouillon R, Manousaki D, Rosen C, Trajanoska K, Rivadeneira F, Richards JB. The health effects of vitamin D supplementation: evidence from human studies. Nat Rev Endocrinol. (2022) 18:96–110. doi: 10.1038/s41574-021-00593-z, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang JJ, Yu HC, Li Y, Zhang YB, Geng TT, Lu Q, et al. Association between serum 25-hydroxy vitamin D concentrations and mortality among individuals with metabolic dysfunction-associated fatty liver disease: a prospective cohort study. Am J Clin Nutr. (2022) 116:1409–17. doi: 10.1093/ajcn/nqac260, PMID: [DOI] [PubMed] [Google Scholar]
- 65.Ciardullo S, Muraca E, Cannistraci R, Perra S, Lattuada G, Perseghin G. Low 25 (OH) vitamin D levels are associated with increased prevalence of nonalcoholic fatty liver disease and significant liver fibrosis. Diabetes Metab Res Rev. (2023) 39:e3628. doi: 10.1002/dmrr.3628, PMID: [DOI] [PubMed] [Google Scholar]
- 66.Ji Y, Wei CB, Gu W, Hou LL. Relevance of vitamin D on NAFLD and liver fibrosis detected by vibration controlled transient elastography in US adults: a cross-sectional analysis of NHANES 2017-2018. Ann Med. (2023) 55:2209335. doi: 10.1080/07853890.2023.2209335, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Roth CL, Elfers CT, Figlewicz DP, Melhorn SJ, Morton GJ, Hoofnagle A, et al. Vitamin D deficiency in obese rats exacerbates nonalcoholic fatty liver disease and increases hepatic resistin and toll-like receptor activation. Hepatology. (2012) 55:1103–11. doi: 10.1002/hep.24737 [DOI] [PubMed] [Google Scholar]
- 68.Nelson JE, Roth CL, Wilson LA, Yates KP, Aouizerat B, Morgan-Stevenson V, et al. Vitamin D deficiency is associated with increased risk of non-alcoholic steatohepatitis in adults with non-alcoholic fatty liver disease: possible role for MAPK and NF-κB? Am J Gastroenterol. (2016) 111:852–63. doi: 10.1038/ajg.2016.51, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yuan S, Larsson SC. Inverse association between serum 25-Hydroxyvitamin D and nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. (2023) 21:398–405.e4. doi: 10.1016/j.cgh.2022.01.021, PMID: [DOI] [PubMed] [Google Scholar]
- 70.Zhang R, Wang M, Wang M, Zhang L, Ding Y, Tang Z, et al. Vitamin D level and vitamin D receptor genetic variation were involved in the risk of non-alcoholic fatty liver disease: a case-control study. Front Endocrinol. (2021) 12:648844. doi: 10.3389/fendo.2021.648844, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Heo NJ, Park HE, Yoon JW, Kwak MS, Yang JI, Chung SJ, et al. The association between vitamin D and nonalcoholic fatty liver disease assessed by controlled attenuation parameter. J Clin Med. (2021) 10:10. doi: 10.3390/jcm10122611, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stepan MD, Vintilescu ȘB, Streață I, Podeanu MA, Florescu DN. The role of vitamin D in obese children with non-alcoholic fatty liver disease and associated metabolic syndrome. Nutrients. (2023) 15:15. doi: 10.3390/nu15092113, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lukenda Zanko V, Domislovic V, Trkulja V, Krznaric-Zrnic I, Turk-Wensveen T, Krznaric Z, et al. Vitamin D for treatment of non-alcoholic fatty liver disease detected by transient elastography: a randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab. (2020) 22:2097–106. doi: 10.1111/dom.14129, PMID: [DOI] [PubMed] [Google Scholar]
- 74.Liu Y, Wang M, Xu W, Zhang H, Qian W, Li X, et al. Active vitamin D supplementation alleviates initiation and progression of nonalcoholic fatty liver disease by repressing the p 53 pathway. Life Sci. (2020) 241:117086. doi: 10.1016/j.lfs.2019.117086, PMID: [DOI] [PubMed] [Google Scholar]
- 75.Jahn D, Dorbath D, Kircher S, Nier A, Bergheim I, Lenaerts K, et al. Beneficial effects of vitamin D treatment in an obese mouse model of non-alcoholic steatohepatitis. Nutrients. (2019) 11:11. doi: 10.3390/nu11010077, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nakano T, Cheng YF, Lai CY, Hsu LW, Chang YC, Deng JY, et al. Impact of artificial sunlight therapy on the progress of non-alcoholic fatty liver disease in rats. J Hepatol. (2011) 55:415–25. doi: 10.1016/j.jhep.2010.11.028 [DOI] [PubMed] [Google Scholar]
- 77.Zhang XL, Chen L, Yang J, Zhao SS, Jin S, Ao N, et al. Vitamin D alleviates non-alcoholic fatty liver disease via restoring gut microbiota and metabolism. Front Microbiol. (2023) 14:1117644. doi: 10.3389/fmicb.2023.1117644, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yin Y, Yu Z, Xia M, Luo X, Lu X, Ling W. Vitamin D attenuates high fat diet-induced hepatic steatosis in rats by modulating lipid metabolism. Eur J Clin Investig. (2012) 42:1189–96. doi: 10.1111/j.1365-2362.2012.02706.x, PMID: [DOI] [PubMed] [Google Scholar]
- 79.Du T, Xiang L, Zhang J, Yang C, Zhao W, Li J, et al. Vitamin D improves hepatic steatosis in NAFLD via regulation of fatty acid uptake and β-oxidation. Front Endocrinol. (2023) 14:1138078. doi: 10.3389/fendo.2023.1138078, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang Z, Moon R, Thorne JL, Moore JB. NAFLD and vitamin D: evidence for intersection of micro RNA-regulated pathways. Nutr Res Rev. (2023) 36:120–39. doi: 10.1017/s095442242100038x, PMID: [DOI] [PubMed] [Google Scholar]
- 81.Beilfuss A, Sowa JP, Sydor S, Beste M, Bechmann LP, Schlattjan M, et al. Vitamin D counteracts fibrogenic TGF-β signalling in human hepatic stellate cells both receptor-dependently and independently. Gut. (2015) 64:791–9. doi: 10.1136/gutjnl-2014-307024, PMID: [DOI] [PubMed] [Google Scholar]
- 82.Bril F, Maximos M, Portillo-Sanchez P, Biernacki D, Lomonaco R, Subbarayan S, et al. Relationship of vitamin D with insulin resistance and disease severity in non-alcoholic steatohepatitis. J Hepatol. (2015) 62:405–11. doi: 10.1016/j.jhep.2014.08.040, PMID: [DOI] [PubMed] [Google Scholar]
- 83.Zhang Z, Burrows K, Fuller H, Speliotes EK, Abeysekera KWM, Thorne JL, et al. Non-alcoholic fatty liver disease and vitamin D in the UK biobank: a two-sample bidirectional Mendelian randomisation study. Nutrients. (2023) 15:15. doi: 10.3390/nu15061442, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ha Y, Hwang SG, Rim KS. The association between vitamin D insufficiency and nonalcoholic fatty liver disease: a population-based study. Nutrients. (2017) 9:9. doi: 10.3390/nu9080806, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pittas AG, Dawson-Hughes B, Sheehan P, Ware JH, Knowler WC, Aroda VR, et al. Vitamin D supplementation and prevention of type 2 diabetes. N Engl J Med. (2019) 381:520–30. doi: 10.1056/NEJMoa1900906, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kitson MT, Pham A, Gordon A, Kemp W, Roberts SK. High-dose vitamin D supplementation and liver histology in NASH. Gut. (2016) 65:717–8. doi: 10.1136/gutjnl-2015-310417, PMID: [DOI] [PubMed] [Google Scholar]
- 87.Tourkochristou E, Mouzaki A, Triantos C. Gene polymorphisms and biological effects of vitamin D receptor on nonalcoholic fatty liver disease development and progression. Int J Mol Sci. (2023) 24:24. doi: 10.3390/ijms24098288, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cusi K, Isaacs S, Barb D, Basu R, Caprio S, Garvey WT, et al. American Association of Clinical Endocrinology Clinical Practice Guideline for the diagnosis and Management of Nonalcoholic Fatty Liver Disease in primary care and endocrinology clinical settings: co-sponsored by the American Association for the Study of Liver Diseases (AASLD). Endocr Pract. (2022) 28:528–62. doi: 10.1016/j.eprac.2022.03.010 [DOI] [PubMed] [Google Scholar]
- 89.Wallert M, Börmel L, Lorkowski S. Inflammatory diseases and vitamin E-what do we know and where do we go? Mol Nutr Food Res. (2021) 65:e2000097. doi: 10.1002/mnfr.202000097, PMID: [DOI] [PubMed] [Google Scholar]
- 90.Erhardt A, Stahl W, Sies H, Lirussi F, Donner A, Häussinger D. Plasma levels of vitamin E and carotenoids are decreased in patients with nonalcoholic steatohepatitis (NASH). Eur J Med Res. (2011) 16:76–8. doi: 10.1186/2047-783x-16-2-76, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang LL, Zhang PH, Yan HH. Functional foods and dietary supplements in the management of non-alcoholic fatty liver disease: a systematic review and meta-analysis. Front Nutr. (2023) 10:1014010. doi: 10.3389/fnut.2023.1014010, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sanyal AJ. ACP journal Club: vitamin E, but not pioglitazone, improved nonalcoholic steatohepatitis in nondiabetic patients. Ann Intern Med. (2010) 153:Jc3-12. doi: 10.7326/0003-4819-153-6-201009210-02012 [DOI] [PubMed] [Google Scholar]
- 93.Poonyam P, Kritsanaviparkporn C, Chommaitree P, Soodcharoen A. The effects of combined vitamin E and C for treatment of non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis of randomized controlled trials. Asian Pac J Cancer Prev. (2022) 23:2891–9. doi: 10.31557/apjcp.2022.23.9.2891, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sato K, Gosho M, Yamamoto T, Kobayashi Y, Ishii N, Ohashi T, et al. Vitamin E has a beneficial effect on nonalcoholic fatty liver disease: a meta-analysis of randomized controlled trials. Nutrition. (2015) 31:923–30. doi: 10.1016/j.nut.2014.11.018 [DOI] [PubMed] [Google Scholar]
- 95.Armstrong MJ, Houlihan DD, Rowe IA. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. (2010) 363:1185. doi: 10.1056/NEJMc1006581 [DOI] [PubMed] [Google Scholar]
- 96.Bril F, Biernacki DM, Kalavalapalli S, Lomonaco R, Subbarayan SK, Lai J, et al. Role of vitamin E for nonalcoholic steatohepatitis in patients with type 2 diabetes: a randomized controlled trial. Diabetes Care. (2019) 42:1481–8. doi: 10.2337/dc19-0167 [DOI] [PubMed] [Google Scholar]
- 97.Yang C, Wu S, Lan Y, Chen S, Zhang D, Wang Y, et al. Association between blood calcium, magnesium, and non-alcoholic fatty liver disease in adults: a cohort-based case-control study. Biol Trace Elem Res. (2023) 201:4625–36. doi: 10.1007/s12011-022-03543-6, PMID: [DOI] [PubMed] [Google Scholar]
- 98.Shin JY, Kim MJ, Kim ES, Mo EY, Moon SD, Han JH, et al. Association between serum calcium and phosphorus concentrations with non-alcoholic fatty liver disease in Korean population. J Gastroenterol Hepatol. (2015) 30:733–41. doi: 10.1111/jgh.12832, PMID: [DOI] [PubMed] [Google Scholar]
- 99.Eshraghian A, Nikeghbalian S, Geramizadeh B, Malek-Hosseini SA. Serum magnesium concentration is independently associated with non-alcoholic fatty liver and non-alcoholic steatohepatitis. United European Gastroenterol J. (2018) 6:97–103. doi: 10.1177/2050640617707863, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ali ES, Petrovsky N. Calcium signaling as a therapeutic target for liver steatosis. Trends Endocrinol Metab. (2019) 30:270–81. doi: 10.1016/j.tem.2019.02.005 [DOI] [PubMed] [Google Scholar]
- 101.Wilson CH, Ali ES, Scrimgeour N, Martin AM, Hua J, Tallis GA, et al. Steatosis inhibits liver cell store-operated Ca2+ entry and reduces ER Ca2+ through a protein kinase C-dependent mechanism. Biochem J. (2015) 466:379–90. doi: 10.1042/bj20140881, PMID: [DOI] [PubMed] [Google Scholar]
- 102.Sakiyama H, Fujiwara N, Yoneoka Y, Yoshihara D, Eguchi H, Suzuki K. Cu, Zn-SOD deficiency induces the accumulation of hepatic collagen. Free Radic Res. (2016) 50:666–77. doi: 10.3109/10715762.2016.1164856, PMID: [DOI] [PubMed] [Google Scholar]
- 103.Varin A, Larbi A, Dedoussis GV, Kanoni S, Jajte J, Rink L, et al. In vitro and in vivo effects of zinc on cytokine signalling in human T cells. Exp Gerontol. (2008) 43:472–82. doi: 10.1016/j.exger.2007.12.008 [DOI] [PubMed] [Google Scholar]
- 104.Haase H, Mocchegiani E, Rink L. Correlation between zinc status and immune function in the elderly. Biogerontology. (2006) 7:421–8. doi: 10.1007/s10522-006-9057-3 [DOI] [PubMed] [Google Scholar]
- 105.Abdallah AAM, Abdelrahman MM, Attia H, Hafez A, Anwar Rashed S, Amin YA, et al. Decreased serum zinc, selenium, and vitamin E as possible risk factors of hepatic fibrosis in non-alcoholic fatty liver disease. Nutr Health. (2022):2601060221103032. doi: 10.1177/02601060221103032, PMID: [DOI] [PubMed] [Google Scholar]
- 106.Kim MC, Lee JI, Kim JH, Kim HJ, Cho YK, Jeon WK, et al. Serum zinc level and hepatic fibrosis in patients with nonalcoholic fatty liver disease. PLoS One. (2020) 15:e0240195. doi: 10.1371/journal.pone.0240195, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kosari F, Jamali R, Ramim T, Mosavi Jahan Abad E. The correlation between serum zinc level and liver histology in non-alcoholic steatohepatitis. Iran J Pathol. (2019) 14:17–25. doi: 10.30699/ijp.14.1.17, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chen SD, Zhang H, Rios RS, Li YY, Zhu PW, Jin Y, et al. J-shaped relationship between serum zinc levels and the severity of hepatic necro-inflammation in patients with MAFLD. Nutr Metab Cardiovasc Dis. (2022) 32:1259–65. doi: 10.1016/j.numecd.2022.01.035, PMID: [DOI] [PubMed] [Google Scholar]
- 109.Mousavi SN, Faghihi A, Motaghinejad M, Shiasi M, Imanparast F, Amiri HL, et al. Zinc and selenium co-supplementation reduces some lipid peroxidation and angiogenesis markers in a rat model of NAFLD-fed high fat diet. Biol Trace Elem Res. (2018) 181:288–95. doi: 10.1007/s12011-017-1059-2 [DOI] [PubMed] [Google Scholar]
- 110.Cui J, Xu T, Lv H, Guo MY. Zinc deficiency causes oxidative stress, endoplasmic reticulum stress, apoptosis and inflammation in hepatocytes in grass carp. Fish Shellfish Immunol. (2023) 139:108905. doi: 10.1016/j.fsi.2023.108905, PMID: [DOI] [PubMed] [Google Scholar]
- 111.Qi Y, Zhang Z, Liu S, Aluo Z, Zhang L, Yu L, et al. Zinc supplementation alleviates lipid and glucose metabolic disorders induced by a high-fat diet. J Agric Food Chem. (2020) 68:5189–200. doi: 10.1021/acs.jafc.0c01103 [DOI] [PubMed] [Google Scholar]
- 112.Gatiatulina ER, Sheina EA, Nemereshina ON, Popova EV, Polyakova VS, Agletdinov EF, et al. Effect of Zn supplementation on trace element status in rats with diet-induced non-alcoholic fatty liver disease. Biol Trace Elem Res. (2020) 197:202–12. doi: 10.1007/s12011-019-01985-z, PMID: [DOI] [PubMed] [Google Scholar]
- 113.Hu H, Sun N, Du H, He Y, Pan K, Liu X, et al. Mouse promyelocytic leukemia zinc finger protein (PLZF) regulates hepatic lipid and glucose homeostasis dependent on SIRT1. Front Pharmacol. (2022) 13:1039726. doi: 10.3389/fphar.2022.1039726, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Xu YC, Zheng H, Hogstrand C, Tan XY, Zhao T, Song YF, et al. Novel mechanism for zinc inducing hepatic lipolysis via the HDAC3-mediated deacetylation of β-catenin at lysine 311. J Nutr Biochem. (2023) 121:109429. doi: 10.1016/j.jnutbio.2023.109429, PMID: [DOI] [PubMed] [Google Scholar]
- 115.Zhao Y, Gao L, Jiang C, Chen J, Qin Z, Zhong F, et al. The transcription factor zinc fingers and homeoboxes 2 alleviates NASH by transcriptional activation of phosphatase and tensin homolog. Hepatology. (2022) 75:939–54. doi: 10.1002/hep.32165 [DOI] [PubMed] [Google Scholar]
- 116.Xiao X, Li H, Qi X, Wang Y, Xu C, Liu G, et al. Zinc alpha 2 glycoprotein alleviates palmitic acid-induced intracellular lipid accumulation in hepatocytes. Mol Cell Endocrinol. (2017) 439:155–64. doi: 10.1016/j.mce.2016.06.003, PMID: [DOI] [PubMed] [Google Scholar]
- 117.Dogra S, Kar AK, Girdhar K, Daniel PV, Chatterjee S, Choubey A, et al. Zinc oxide nanoparticles attenuate hepatic steatosis development in high-fat-diet fed mice through activated AMPK signaling axis. Nanomedicine. (2019) 17:210–22. doi: 10.1016/j.nano.2019.01.013, PMID: [DOI] [PubMed] [Google Scholar]
- 118.Wei CC, Luo Z, Hogstrand C, Xu YH, Wu LX, Chen GH, et al. Zinc reduces hepatic lipid deposition and activates lipophagy via Zn (2+)/MTF-1/PPARα and ca (2+)/CaMKKβ/AMPK pathways. FASEB J. (2018) 32:6666. doi: 10.1096/fj.201800463 [DOI] [PubMed] [Google Scholar]
- 119.Brunet S, Thibault L, Delvin E, Yotov W, Bendayan M, Levy E. Dietary iron overload and induced lipid peroxidation are associated with impaired plasma lipid transport and hepatic sterol metabolism in rats. Hepatology. (1999) 29:1809–17. doi: 10.1002/hep.510290612, PMID: [DOI] [PubMed] [Google Scholar]
- 120.He H, Liao S, Zeng Y, Liang L, Chen J, Tao C. Causal relationships between metabolic-associated fatty liver disease and iron status: two-sample Mendelian randomization. Liver Int. (2022) 42:2759–68. doi: 10.1111/liv.15455, PMID: [DOI] [PubMed] [Google Scholar]
- 121.Wang Q, Zhu M, Li H, Chen P, Wang M, Gu L, et al. Hyperferritinemia correlates to metabolic dysregulation and steatosis in Chinese biopsy-proven nonalcoholic fatty liver disease patients. Diabetes Metab Syndr Obes. (2022) 15:1543–52. doi: 10.2147/dmso.S361187, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Barros RK, Cotrim HP, Daltro CH, Oliveira YA. Hyperferritinemia in patients with nonalcoholic fatty liver disease. Rev Assoc Med Bras. (1992, 2017) 63:284–9. doi: 10.1590/1806-9282.63.03.284, PMID: [DOI] [PubMed] [Google Scholar]
- 123.Mastrogiannaki M, Matak P, Keith B, Simon MC, Vaulont S, Peyssonnaux C. HIF-2alpha, but not HIF-1alpha, promotes iron absorption in mice. J Clin Invest. (2009) 119:1159–66. doi: 10.1172/jci38499, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rametta R, Dongiovanni P, Baselli GA, Pelusi S, Meroni M, Fracanzani AL, et al. Impact of natural neuromedin-B receptor variants on iron metabolism. Am J Hematol. (2020) 95:167–77. doi: 10.1002/ajh.25679, PMID: [DOI] [PubMed] [Google Scholar]
- 125.Dongiovanni P, Lanti C, Gatti S, Rametta R, Recalcati S, Maggioni M, et al. Correction: high fat diet subverts hepatocellular iron uptake determining dysmetabolic iron overload. PLoS One. (2015) 10:e0120457. doi: 10.1371/journal.pone.0120457, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tsurusaki S, Tsuchiya Y, Koumura T, Nakasone M, Sakamoto T, Matsuoka M, et al. Hepatic ferroptosis plays an important role as the trigger for initiating inflammation in nonalcoholic steatohepatitis. Cell Death Dis. (2019) 10:449. doi: 10.1038/s41419-019-1678-y, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Fujita N, Miyachi H, Tanaka H, Takeo M, Nakagawa N, Kobayashi Y, et al. Iron overload is associated with hepatic oxidative damage to DNA in nonalcoholic steatohepatitis. Cancer Epidemiol Biomarkers Prev. (2009) 18:424–32. doi: 10.1158/1055-9965.Epi-08-0725, PMID: [DOI] [PubMed] [Google Scholar]
- 128.Zhang L, Dai X, Wang L, Cai J, Shen J, Shen Y, et al. Iron overload accelerated lipid metabolism disorder and liver injury in rats with non-alcoholic fatty liver disease. Front Nutr. (2022) 9:961892. doi: 10.3389/fnut.2022.961892, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gao H, Jin Z, Bandyopadhyay G, Wang G, Zhang D, Rocha KCE, et al. Aberrant iron distribution via hepatocyte-stellate cell axis drives liver lipogenesis and fibrosis. Cell Metab. (2022) 34:1201–1213.e5. doi: 10.1016/j.cmet.2022.07.006, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Seyoum Y, Baye K, Humblot C. Iron homeostasis in host and gut bacteria – a complex interrelationship. Gut Microbes. (2021) 13:1–19. doi: 10.1080/19490976.2021.1874855, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mayneris-Perxachs J, Moreno-Navarrete JM, Fernández-Real JM. The role of iron in host-microbiota crosstalk and its effects on systemic glucose metabolism. Nat Rev Endocrinol. (2022) 18:683–98. doi: 10.1038/s41574-022-00721-3, PMID: [DOI] [PubMed] [Google Scholar]
- 132.Song CC, Pantopoulos K, Chen GH, Zhong CC, Zhao T, Zhang DG, et al. Iron increases lipid deposition via oxidative stress-mediated mitochondrial dysfunction and the HIF1α-PPARγ pathway. Cell Mol Life Sci. (2022) 79:394. doi: 10.1007/s00018-022-04423-x, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Valenti L, Fracanzani AL, Dongiovanni P, Bugianesi E, Marchesini G, Manzini P, et al. Iron depletion by phlebotomy improves insulin resistance in patients with nonalcoholic fatty liver disease and hyperferritinemia: evidence from a case-control study. Am J Gastroenterol. (2007) 102:1251–8. doi: 10.1111/j.1572-0241.2007.01192.x, PMID: [DOI] [PubMed] [Google Scholar]
- 134.Valenti L, Fracanzani AL, Dongiovanni P, Rovida S, Rametta R, Fatta E, et al. A randomized trial of iron depletion in patients with nonalcoholic fatty liver disease and hyperferritinemia. World J Gastroenterol. (2014) 20:3002–10. doi: 10.3748/wjg.v20.i11.3002, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jaruvongvanich V, Riangwiwat T, Sanguankeo A, Upala S. Outcome of phlebotomy for treating nonalcoholic fatty liver disease: a systematic review and meta-analysis. Saudi J Gastroenterol. (2016) 22:407–14. doi: 10.4103/1319-3767.195551, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Adams LA, Crawford DH, Stuart K, House MJ, St Pierre TG, Webb M, et al. The impact of phlebotomy in nonalcoholic fatty liver disease: a prospective, randomized, controlled trial. Hepatology. (2015) 61:1555–64. doi: 10.1002/hep.27662, PMID: [DOI] [PubMed] [Google Scholar]
- 137.Heffern MC, Park HM, Au-Yeung HY, Van de Bittner GC, Ackerman CM, Stahl A, et al. In vivo bioluminescence imaging reveals copper deficiency in a murine model of nonalcoholic fatty liver disease. Proc Natl Acad Sci USA. (2016) 113:14219–24. doi: 10.1073/pnas.1613628113, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lee SH, Kim MJ, Kim YS, Chun H, Won BY, Lee JH, et al. Low hair copper concentration is related to a high risk of nonalcoholic fatty liver disease in adults. J Trace Elem Med Biol. (2018) 50:28–33. doi: 10.1016/j.jtemb.2018.06.001, PMID: [DOI] [PubMed] [Google Scholar]
- 139.Aigner E, Strasser M, Haufe H, Sonnweber T, Hohla F, Stadlmayr A, et al. A role for low hepatic copper concentrations in nonalcoholic fatty liver disease. Am J Gastroenterol. (2010) 105:1978–85. doi: 10.1038/ajg.2010.170, PMID: [DOI] [PubMed] [Google Scholar]
- 140.Blades B, Ayton S, Hung YH, Bush AI, La Fontaine S. Copper and lipid metabolism: a reciprocal relationship. Biochim Biophys Acta Gen. (2021) 1865:129979. doi: 10.1016/j.bbagen.2021.129979, PMID: [DOI] [PubMed] [Google Scholar]
- 141.Xie L, Yuan Y, Xu S, Lu S, Gu J, Wang Y, et al. Downregulation of hepatic ceruloplasmin ameliorates NAFLD via SCO1-AMPK-LKB1 complex. Cell Rep. (2022) 41:111498. doi: 10.1016/j.celrep.2022.111498, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lan Y, Wu S, Wang Y, Chen S, Liao W, Zhang X, et al. Association between blood copper and nonalcoholic fatty liver disease according to sex. Clin Nutr. (2021) 40:2045–52. doi: 10.1016/j.clnu.2020.09.026, PMID: [DOI] [PubMed] [Google Scholar]
- 143.Zhong CC, Zhao T, Hogstrand C, Chen F, Song CC, Luo Z. Copper (cu) induced changes of lipid metabolism through oxidative stress-mediated autophagy and Nrf2/PPARγ pathways. J Nutr Biochem. (2022) 100:108883. doi: 10.1016/j.jnutbio.2021.108883, PMID: [DOI] [PubMed] [Google Scholar]
- 144.Song M, Vos MB, McClain CJ. Copper-fructose interactions: a novel mechanism in the pathogenesis of NAFLD. Nutrients. (2018) 10:10. doi: 10.3390/nu10111815, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chen C, Zhou Q, Yang R, Wu Z, Yuan H, Zhang N, et al. Copper exposure association with prevalence of non-alcoholic fatty liver disease and insulin resistance among US adults (NHANES 2011-2014). Ecotoxicol Environ Saf. (2021) 218:112295. doi: 10.1016/j.ecoenv.2021.112295, PMID: [DOI] [PubMed] [Google Scholar]
- 146.Rayman MP. The importance of selenium to human health. Lancet. (2000) 356:233–41. doi: 10.1016/s0140-6736(00)02490-9 [DOI] [PubMed] [Google Scholar]
- 147.Hariharan S, Dharmaraj S. Selenium and selenoproteins: it's role in regulation of inflammation. Inflammopharmacology. (2020) 28:667–95. doi: 10.1007/s10787-020-00690-x, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Barchielli G, Capperucci A, Tanini D. The role of selenium in pathologies: an updated review. Antioxidants. (2022) 11:11. doi: 10.3390/antiox11020251, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gupta M, Gupta S. An overview of selenium uptake, metabolism, and toxicity in plants. Front Plant Sci. (2016) 7:2074. doi: 10.3389/fpls.2016.02074, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wang X, Seo YA, Park SK. Serum selenium and non-alcoholic fatty liver disease (NAFLD) in U.S. adults: National Health and nutrition examination survey (NHANES) 2011-2016. Environ Res. (2021) 197:111190. doi: 10.1016/j.envres.2021.111190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Liu J, Tan L, Liu Z, Shi R. The association between non-alcoholic fatty liver disease (NAFLD) and advanced fibrosis with blood selenium level based on the NHANES 2017-2018. Ann Med. (2022) 54:2258–67. doi: 10.1080/07853890.2022.2110277, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wu J, Zeng C, Yang Z, Li X, Lei G, Xie D, et al. Association between dietary selenium intake and the prevalence of nonalcoholic fatty liver disease: a cross-sectional study. J Am Coll Nutr. (2020) 39:103–11. doi: 10.1080/07315724.2019.1613271 [DOI] [PubMed] [Google Scholar]
- 153.Zhu M, Niu Q, Zhang J, Yu Y, Wang H, Zhu T, et al. Amorphous selenium nanodots alleviate non-alcoholic fatty liver disease via activating VEGF receptor 1 to further inhibit phosphorylation of JNK/p38 MAPK pathways. Eur J Pharmacol. (2022) 932:175235. doi: 10.1016/j.ejphar.2022.175235, PMID: [DOI] [PubMed] [Google Scholar]
- 154.Gombart AF, Pierre A, Maggini S. A review of micronutrients and the immune system-working in harmony to reduce the risk of infection. Nutrients. (2020) 12:12. doi: 10.3390/nu12010236, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Olivares M, Figueroa C, Pizarro F. Acute copper and ascorbic acid supplementation inhibits non-heme Iron absorption in humans. Biol Trace Elem Res. (2016) 172:315–9. doi: 10.1007/s12011-015-0605-z, PMID: [DOI] [PubMed] [Google Scholar]