Abstract
Purpose
Shorter time from symptoms recognition to diagnosis and timely treatment would be expected to improve the survival of patients with breast cancer (BC). This review identifies and summarizes evidence on time to diagnosis and treatment, and associated factors to inform an improved BC care pathways in Low- and Middle-Income Countries (LMICs).
Methods
A systematic search was conducted in electronic databases including Medline, Embase, PsycINFO and Global Health, covering publications between January 1, 2010, and November 6, 2023. Inclusion criteria encompassed studies published in English from LMICs that reported on time from symptoms recognition to diagnosis and/or from diagnosis to treatment, as well as factors influencing these timelines. Study quality was assessed independently by two reviewers using a standard checklist. Pre-contact, post-contact and treatment intervals and delays in these intervals are presented. Barriers and facilitators for shorter time to diagnosis and treatment found by individual studies after adjusting with covariates are summarized.
Results
The review identified 21 studies across 14 countries and found that BC cases took a longer time to diagnosis than to treatment. However, time to treatment also exceeded the World Health Organization (WHO) recommended period for optimal survival. There was inconsistency in terminology and benchmarks for defining delays in time intervals. Low socioeconomic status and place of residence emerged as frequent barriers, while initial contact with a private health facility or specialist was commonly reported as a facilitator for shorter time to diagnosis and treatment.
Conclusions
Guidelines or consensus recommendations are essential for defining the optimal time intervals to BC diagnosis and treatment. Our review supported WHO's Global Breast Cancer Initiative recommendations. Increasing public awareness, strengthening of healthcare professional's capacities, partial decentralization of diagnostic services and implementation of effective referral mechanisms are recommended to achieve a shorter time to diagnosis and treatment of BC in LMICs.
Keywords: Delay in diagnosis, Time to diagnosis, Time to treatment, Breast cancer, Low- and middle-income countries
Highlights
-
•
Standard guidelines defining time intervals and delays are required.
-
•
Time to diagnosis and treatment exceeded the recommended time in LMICs.
-
•
Low socioeconomic status was the most frequent barrier.
-
•
The common facilitator was the first point of contact being private facility or specialist.
-
•
Awareness, capacity building, and proper referral mechanisms can decrease delays.
Abbreviations
- BC
Breast Cancer
- LMICs
Low- and middle-income countries
- HDI
Human Development Index
- WHO
World Health Organization
- GBCI
Global Breast Cancer Initiative
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analysis
- PICOS
Population, Intervention/Comparison, Outcome, and Setting
1. Background
Breast cancer (BC) is the most frequently diagnosed cancer, accounting for 11.7% of all cancers globally in 2020 [1]. The estimated age-standardized incidence rate for BC is 47.8 per 100,000 [2]. Although the incidence rate is lower in developing (compared to developed) countries, mortality due to BC is disproportionately higher in developing countries [1]. BC incidence and mortality are projected to increase by 97.2% and 98.8%, respectively, in low Human Development Index (HDI) countries; in contrast in high HDI countries, it is expected that incidence and mortality will increase by 30.8% and 53.6% respectively between 2020 and 2040 [3]. If these projected rates remain the same, they will have a relatively higher impact in low- and middle-income countries (LMICs) [4]. In this context, the Global BC Initiative (GBCI) implementation framework developed by the World Health Organization (WHO) aims to decrease BC mortality in LMICs [4].
In order to develop targeted and efficient interventions, it is necessary to understand the underlying factors causing higher BC mortality in LMICs. The primary reasons for higher mortality were thought to be later diagnosis and limited access to cancer care, and delayed diagnosis and treatment [5,6]. Early diagnosis and treatment are expected to improve survival of patients with BC [7,8]. A recent review highlighted that even a month-long delay in treatment influences breast cancer survival [7].
A critical review [9] of published studies up to December 2013 provided insights on the time interval for BC diagnosis and treatment and factors associated with delayed BC treatment in developing countries. This review found a significant gap in the available literature on access barriers and the quality of care associated with delayed treatment in LMICs. In addition, it has been a decade since that review, during which many factors could have changed. Another systematic review on overall cancer in LMICs [10] with published studies to 2017, also reported the time intervals for diagnosis and treatment of BC but only reported factors which influenced the diagnosis interval. Hence, this review aims to systematically identify and summarize the most recent evidence on factors influencing time from symptom discovery to diagnosis and from diagnosis to treatment of BC in LMICs, as this information can be used to improve the BC care pathway in these countries.
2. Materials and methods
A systematic review was conducted using the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guideline [11]. The review protocol was registered in the PROSPERO database (CRD42022368305).
2.1. Literature search
The PICOS (Population, Intervention/Comparison, Outcome, and Setting) framework was employed to formulate the review question (Supplementary Table 1) and a search strategy (Supplementary Table 2) was developed. A comprehensive literature search was performed across four electronic databases (via Ovid): Medline, Embase, PsycINFO, and Global Health. The reference lists of selected studies were reviewed to identify any additional pertinent studies.
2.2. Inclusion and exclusion Criteria
Studies conducted in LMICs, as per the World Bank classification [12], and published in English between January 1, 2010, and October 13, 2022 were included in the review initially, with the search results being updated on November 6, 2023 to ensure that the most recent research was included. All publications that measured the time to diagnosis and/or treatment and reported on the factors influencing the time to diagnosis/treatment were included. We have included cross-sectional, cohort and pre/post intervention studies, and excluded qualitative studies, case studies, systematic reviews, and conference abstracts.
The time to diagnosis (diagnosis interval) refers to the time elapsed from the recognition of symptoms (self-discovered or screened positive) to the diagnosis of BC. Most of the studies divided this interval into two categories, the pre-contact interval, which refers to the time from symptom recognition to the first consultation with a medical facility, and the post-contact interval, which represents the time from the first consultation to the actual diagnosis. Studies that solely measured the pre-contact interval were excluded from the review, as they did not guarantee that the participants reached a diagnosis. However, studies that solely measure the post-contact interval were included, since they ensure that the patient reached the stage of diagnosis. The time to treatment (treatment interval) refers to the period between the diagnosis being made and the initiation of definitive treatment of BC.
2.3. Data collection and extraction
Study titles and abstracts identified by the search were screened based on pre-defined inclusion and exclusion criteria (Supplementary Table 3). The screening process was conducted independently by two reviewers, RS and AN. Full text checking of potentially relevant studies was independently performed by both reviewers with the reason for exclusion being recorded in an excel spreadsheet. Disagreements between the reviewers were resolved by discussion and consensus, or input from a third reviewer, XQY.
A data extraction tool adopted from Joanna Briggs Institute Reviewers' Manual [13] was used and RS extracted the information from the included studies. The information extracted included publication details (lead author name, country), participants' information (sample size, age, study setting, characteristics), study methods (objective, study year, study type, statistical analysis), key outcomes (median pre/post-contact intervals, time to treatment), interventions (facilitators and barriers to delay in diagnosis and treatment) and any reviewer's comments. The median times to diagnosis and treatment were recorded, analyzed, and reported across different countries. Furthermore, each country's definition of delay in diagnosis and treatment was extracted and summarized. To identify the facilitators for and barriers for time to diagnosis and treatment, statistically significant factors (after adjusting with other variables at a significance level of 0.05) obtained from individual studies were selected and described.
2.4. Assessment of the study quality
The quality of each study was independently assessed by two reviewers (RS and AN) using critical appraisal tools (Supplementary Tables 4 and 5) adopted from Standard Quality Assessment Criteria for Evaluating Primary Research Papers from various fields [14].
Among the 14 items of the tool for a quantitative study, nine were most relevant to the type of study included in this review. The remaining 5 items of the quality assessment tool were related to experimental study design (such as randomization, and blinding) and hence, were considered not applicable to this review. Each of these items was assigned a score: 2 for "yes," 1 for "partial," and 0 for "no," depending on the extent to which the specific criteria were met in each paper. The summary score of each study was obtained by summing the score in each item and dividing it by 18 (maximum possible score that a study can have). The mean summary score from the two reviewers was calculated by summing the summary score from each reviewer and dividing it by two. Studies with a mean summary score below 50% were deemed low quality and excluded from the review.
3. Results
Among the 1353 studies identified from the search, 40 were retrieved for full text review (Fig. 1) after removing duplicates, and screening titles and abstracts against exclusion and inclusion criteria. Following further exclusions (Fig. 1), 21 studies [[15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35]] were eligible for inclusion in the review. The reference lists of these studies were then searched but no additional relevant studies were found. Among the included 21 studies, six reported solely on time to diagnosis, seven studies reported solely on time to treatment, and eight reported on both.
Fig. 1.
PRISMA flow chart for selection of studies.
3.1. Geographical location of included studies
The geographical distribution of the studies included is shown in Table 1. Among the seven World Bank categories of regions, the studies were conducted in five regions, where the proportion of low- and middle-income countries was relatively high. The two studies from Sri Lanka and Sub-Saharan Africa (Zambia, Uganda, Nigeria and Namibia with two subsets; ‘black’ and ‘non-black’ in Namibia) had the same participants. These studies were counted separately as each study provided unique insight into factors influencing time to diagnosis and/or treatment, however, time interval was included only once for our analysis. The included studies were proportionately distributed in terms of income level according to the World Bank classifications.
Table 1.
Number of included studies by country and time to diagnosis and/or treatment.
| Region | Country | World Bank Classification | No of Studies Conducted |
Total No of Studies Conducted | |||
|---|---|---|---|---|---|---|---|
| Time to Diagnosis |
Time to Treatment | Both Time to Diagnosis & Treatment | |||||
| Pre-contact Interval | Post-contact Interval | ||||||
| Sub Saharan Africa | 5 | 2 | 2 | 5 | |||
| 1 | Mali [20] | LIC | 1 | 1 | 1 | 1 | 1 |
| 2 | Zambia [19,30] | LIC | 2a | 2a | 0 | 0 | 2a |
| 3 | Uganda [19,30] | LIC | 2a | 2a | 0 | 0 | 2a |
| 4 | Nigeria [19,30] | LMIC | 2a | 2a | 0 | 0 | 2a |
| 5 | Namibia [19,30] | HMIC | 2a | 2a | 0 | 0 | 2a |
| 6 | Rwanda [29] | LIC | 1 | 1 | 0 | 0 | 1 |
| 7 | Kenya [34] | LMIC | 1 | 1 | 1 | 1 | 1 |
| Middle East and North Africa | 1 | 1 | 1 | 1 | |||
| 8 | Syria [35] | LIC | 1 | 1 | 1 | 1 | 1 |
| East Asia and Pacific | 2 | 2 | 1 | 3 | |||
| 9 | Malaysia [25,28] | HMIC | 1 | 1 | 2 | 1 | 2 |
| 10 | Thailand [15] | HMIC | 1 | 1 | 0 | 0 | 1 |
| South Asia | 3 | 3 | 3 | 3 | |||
| 11 | Pakistan [24] | LMIC | 1 | 1 | 1 | 1 | 1 |
| 12 | Sri Lanka [21,22] | LMIC | 2b | 2b | 2b | 2b | 2b |
| Latin America and Caribbean | 3 | 7 | 1 | 9 | |||
| 13 | Brazil [[16], [17], [18],26,27,32,33] | HMIC | 0 | 2 | 6 | 1 | 7 |
| 14 | Mexico [23,31] | HMIC | 1 | 1 | 1 | 0 | 2 |
| Total | 14 | 15 | 8 | 21 | |||
LIC: low-income countries; LMIC: Low middle-income countries; HMIC: High middle-income countries.
Multicentric and two studies conducted with same participants (Both studies were counted but the time interval was counted/included only once).
Two studies conducted with the same participants.
3.2. Characteristics of the included studies
Table 2 presents the characteristics of the included studies. The majority (12) of studies [[15], [16], [17],19,21,22,24,28,29,31,33,34] were cross-sectional, four were retrospective cross-sectional [18,25,32,35], four were prospective cohort [20,26,27,30] and one study was a before and after intervention study [23]. The duration of these studies varied from four months up to eight years, while two studies did not clearly mention the study duration. Just over half (12) of the studies [[15], [16], [17],20,24,25,27,28,30,31,34,35] were conducted in urban settings, while six studies [19,21,22,30,32,33] were conducted in both urban and rural areas, two [18,29] were in rural areas. For one study [23], the setting was not clearly reported. All the studies were carried out in health facilities. The number of participants in the included studies varied from 64 to 78,527 BC cases. The data collection was primarily through reviewing medical records and patient interviews. The majority (66.7%) of the studies [[15], [16], [17], [18], [19], [20], [21], [22], [23],26,[30], [31], [32],34] recorded the mean age of the participants, ranging from 40 to 58 years, four studies [[27], [28], [29],34] provided the median age, ranging from 49 to 56 years, while four studies [24,25,33,35] reported only age categories of the participants.
Table 2.
Descriptive characteristics of included studies.
| Study No. | Lead Author | Published Year | Study Year/s | Study duration | Study Location | Setting | Study Design | Sample Size | Mean/Median Age (SD/IQR) |
|---|---|---|---|---|---|---|---|---|---|
| Studies for Diagnosis Interval Only (PICOS 1) | |||||||||
| 1 | Amornsak P et al. [15] | 2013 | 2009 (May–June) | 0.6 yrs | Thailand | Urban | Cross-sectional study | 180 | 50 yrs (11) |
| 2a | Foerster M et al. [19] | 2020 | Sept.2014–Sept.2017 | 3 yrs | Multinational Study (Namibia, Nigeria, Uganda, and Zambia) | Urban and rural | Cross-sectional study | 1429 | 50.1yrs (N/G) |
| 3 | Medeiros GC et al. [26] | 2019 | Oct. 2014–Oct. 2015 | 1 year | Brazil | Urban | Prospective Cohort study | 526 | 56 yrs (N/G) |
| 4 | Pace LE et al. [29] | 2015 | Nov. 2012–Feb. 2014 | 1.3 yrs | Rwanda | Rural | Cross-sectional study | 144 | 49 yrsb (N/G) |
| 5a | Togawa K et al. [30] | 2020 | Sept.2014–April.2017 May 2016-Sept. 2017 |
2.5 yrs 1.3 yrs |
Multinational Study (Namibia, Nigeria, Uganda) and (Zambia) | Urban and rural | Prospective Cohort study | 1541 | 50 yrs (13) |
| 6 | Unger-Saldana K et al. [31] | 2018 | July 2009–May 2011 | 1.8 yrs | Mexico | Urban | Cross-sectional study | 886 | 50.9 yrs (13.7) |
| Studies for Treatment Interval Only (PICOS 2) | |||||||||
| 7 | Cabral AL et al. [17] | 2019 | 2010–2013 | 3 yrs | Brazil 2 | Urban | Cross-sectional study | 715 | 57.2 yrs (N/G) |
| 8 | Alves Soares Ferreira N et al. [18] | 2017 | 2009–2011 | 2 yrs | Brazil 3 | Rural | Retrospective Cross-sectional study | 473 | 57.9 yrs (14.5) |
| 9 | Huerta-Gutiérrez R et al. [23] | 2021 | 2004–2009 | 5 yrs | Mexico 1 | N/A | Before and After Cross -sectional study | Before: 266 After: 309 |
Before: 52 yrs (13) After: 52.3 yrs (12) |
| 10 | Medeiros GC et al. [27] | 2021 | Oct. 2014–April 2015 | 0.5 yrs | Brazil 4 | Urban | Prospective Cohort | 470 | 56 yrsb (47–65) |
| 11 | Mastura M et al. [25] | 2013 | Jan. 2004–Dec. 2005 | 2 yrs | Malaysia | Urban | Retrospective Cross-sectional study | 648 | N/A |
| 12 | Jomar RT et al. [32] | 2023 | June 2013–Dec. 2019 | 7.5 yrs | Brazil 5 | Urban and rural | Retrospective Cross-sectional study | 12,100 | 57.2 yrs (12.9) |
| 13 | Nogueira MC et al. [33] | 2023 | 2019–2020 | 2 yrs | Brazil 6 | Urban and rural | Cross-sectional study | 78,527 | N/A |
| Studies for Both Diagnosis and Treatment Interval (PICOS 1 and 2) | |||||||||
| 14 | Dos Santos Andrade LS et al. [16] | 2021 | Oct.2016–March 2019 | 2.4 yrs | Brazil 1 | Urban | Cross-sectional study | 304 | 54.6 yrs (11.9) |
| 15 | Frie KG et al. [20] | 2018 | 2016 (Jan–April) | 0.4 yrs | Mali 1 | Urban | Prospective Cohort study | 64 | 45 yrs (N/G) |
| 16a | Hewage S et al. [21] | 2021 | N/A | N/A | Sri Lanka | Urban and rural | Cross-sectional study | 800 | 55.5 yrs (10.7) |
| 17a | Hewage SA et al. [22] | 2022 | N/A | N/A | Sri Lanka 1 | Urban and rural | Cross-sectional study | 800 | 55.5 yrs (10.7) |
| 18 | Imran M et al. [24] | 2021 | 2016–2018 | 2 yrs | Pakistan 1 | Urban | Cross-sectional study | 372 | N/A |
| 19 | Mohd Mujar NM et al. [28] | 2017 | 2012 (Jan–Dec) | 1 yr | Malaysia 1 | Urban | Cross-sectional study | 340 | 53 yrsb (23–74) |
| 20 | Daniel O et al. [34] | 2023 | 2018 (Jan–Dec) | 1 yr | Kenya | Urban | Mixed method (Cross-sectional) | 378 | 50 yrsb (25–88) |
| 21 | Hanafi I et al. [35] | 2023 | 2011–2019 | 8 yrs | Syria | Urban | Retrospective Cross-sectional | 519 | N/A |
SD: Standard Deviation; IQR: Inter-Quartile Range; PICOS: Population, Intervention/Comparison, Outcome, and Setting; N/G: Not Given; N/A: Information not available (age category given).
Bold letters in duration are for Zambia only.
1: Diagnosis and treatment interval both.
Study with same participants.
Only median age given.
3.3. Quality of included studies
The study-specific mean quality score from the two reviewers ranged from 0.67 to 1.0 (Supplementary Table 6). One study [36] was removed from the review due to its mean score being less than 50%. An additional reason for exclusion of the study was that the study had not conducted any statistical test to show the association of factors with time to diagnosis or treatment. The lower quality scores were due to various reasons. Some studies had small sample sizes [15,20,29], and some were conducted in a single tertiary hospital/pathology department/oncology clinic [15,18,20,24,25,29] that could limit the generalizability of their findings in other settings or areas. Some studies lacked important sociodemographic variables [17,18,25] or failed to appropriately categorize them [15]. A few studies did not present some of the major findings, such as two studies did not provide the median time interval [33,35] and only gave the proportion of cases with delay. Another study [32] reported the median interval according to year. It failed to report the combined median interval of all BC cases. Similarly, one study [25] did not describe the sociodemographic characteristics of the participants in the results and in another study [24] the association analysis was presented with limited variables. Additionally, the estimate of the variance (CI/SD/IQR) was not reported by four studies [15,18,24,31] and partly reported by one study [35]. Eleven studies [15,18,[20], [21], [22], [23], [24], [25],27,28,33] did not provide a brief overview of research findings in the conclusion or had no separate conclusion section. Other limitations included an insufficiently described objective [18], lack of justification of various statistical tests [16], and not reporting the variables that are adjusted/fitted in the logistic regression model [22]. Furthermore, one study had a low response rate of around 50% [28], which could introduce bias in the results. Lastly, in one study the effect of different participant characteristics before and after the intervention was implemented was not described in sufficient detail [23].
Despite these limitations, all studies identified the factors influencing time to diagnosis and/or treatment. However, using different methodologies and varying benchmarks to define delays in diagnosis and treatment can potentially lead to underestimation or overestimation of the associated variables.
3.4. Time from breast cancer symptoms to diagnosis
All of the 14 studies [15,16,[19], [20], [21], [22],24,26,[28], [29], [30], [31],34,35] that reported on time to diagnosis divided the total time to diagnosis into two categories: the first refers to the time interval from symptom recognition to the first medical consultation, while the second category refers to the time interval from the first medical consultation to BC diagnosis. In this review, the terms "pre-contact interval" and "post-contact interval" were adopted to refer to these two categories; and the term "time to diagnosis" refers to the total time from symptom recognition to diagnosis. However, the terminology used to refer to each category varied between the included studies. The pre-contact interval was referred to as patient interval, health seeking interval or presentation interval as the length was considered driven by patient-mediated factors. The post-contact interval was referred to as diagnosis interval, doctor interval, provider interval, physician interval, or system interval. Whatever term was used, it reflects the fact that the interval was driven by system-mediated factors. However, one of the studies [37] argued that each interval is an interplay between the factors in LMICs with limited universal access to healthcare (UHC) for each individual, unlike high-income countries where each individual has access to UHC. Therefore, the study referred to the intervals as pre-contact interval and post-contact interval, which aligns with the terminology adopted in this review.
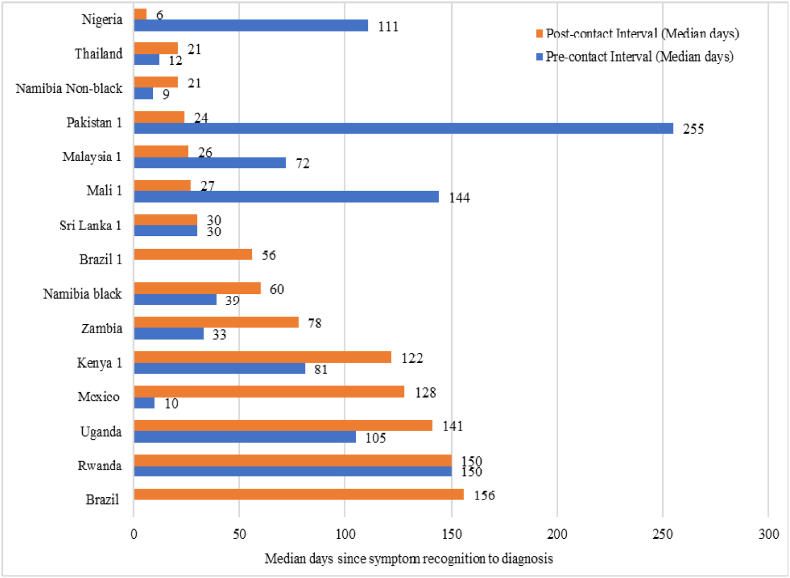
More than half of the studies reported the time interval in days; and thus, all the time intervals were converted into days to ensure uniformity. The pre-contact interval was not provided by two studies [16,26], while one study [35] had neither reported pre-contact nor post-contact interval but provided the proportion of participants with delay. The median pre-contact interval ranged from nine days in Namibia (non-black) to 255 days in Pakistan (Fig. 2). All 12 studies reported the post-contact interval, and the median duration ranged from six days in Nigeria to 156 days in Brazil. Four countries (Nigeria, Pakistan, Malaysia, Mali) reported a longer pre-contact interval than post-contact interval. Six countries (Thailand, Namibia (Black and Non-black), Zambia, Mexico, Kenya, Uganda) reported longer post-contact intervals, while in two countries (Sri Lanka and Rwanda) the pre-contact and post-contact intervals were the same.
Fig. 2.
Time to diagnosis of breast cancer reported across studies from low- and middle-income countries.
3.5. Time from breast cancer diagnosis to treatment
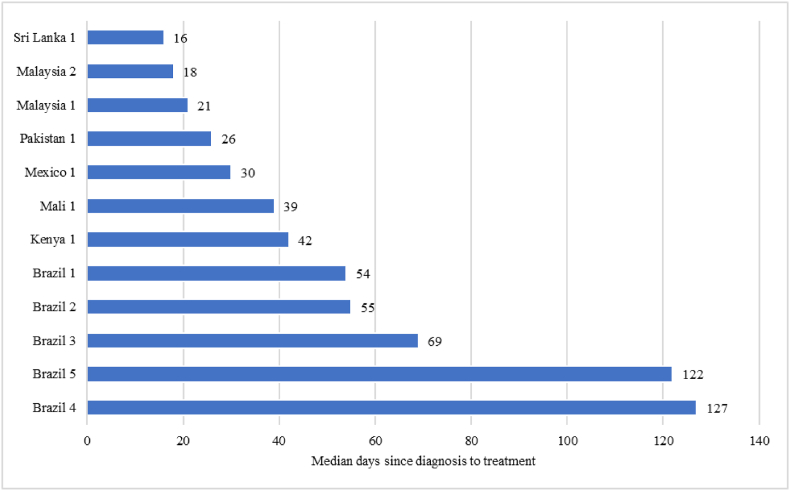
Among the 15 studies [[16], [17], [18],[20], [21], [22], [23], [24], [25],27,28,[32], [33], [34], [35]] which reported on time to treatment, the median time to treatment varied from 16 days in Sri Lanka to 127 days in Brazil (Fig. 3). Among these studies, one study [32] reported the median interval each individual year from 2013 till 2019, hence data for the latest year was considered. Two studies [33,35] did not report the median interval, however, they reported the proportion of BC cases with delay in treatment. In Brazil, the time to treatment varied from 54 days to 127 days. Among the studies that reported both time to diagnosis and treatment [16,[20], [21], [22],24,28,34], the median time to treatment after diagnosis was shorter than time to diagnosis.
Fig. 3.
Time to treatment of breast cancer reported across studies from low- and middle-income countries.
3.6. Determinants of longer time to diagnosis
Six studies [[19], [20], [21],24,31,34] included in this review compared the median pre-contact and post-contact intervals separately when identifying factors that contributed to longer pre and post-contact intervals. While six studies specifically defined and examined pre-contact delay and post-contact delay [15,29,30] [22,28,35], two studies defined the post-contact delay only [16,26]. However, the definition of pre-contact delay varied across countries, ranging from 14 days in Sri Lanka, to 90 days in Syria, Thailand, and Malaysia, and 180 in Rwanda (Table 3). Similarly, the definition of post-contact delay also varied, ranging from 30 days in Malaysia and Sri Lanka, 90 days in Syria, Thailand and Brazil, to 180 days in Rwanda (Table 3). There was a lack of consistency in defining and measuring the delay to diagnosis.
Table 3.
Summary of results showing barriers and facilitators for shorter time to diagnosis of breast cancer in low- and middle-income countries.
| Study No. | Lead Author | Delayed Pre-contact Interval (days) | Delayed Post-contact Interval (days) | Barriers to early diagnosisa | Facilitators of early diagnosisa |
|---|---|---|---|---|---|
| 1 | Amornsak P et al. [15] | >90b | >90b |
|
|
| 2c | Foerster M et al. [19] |
|
|
||
| 3 | Medeiros GC et al. [26] | >90 |
|
|
|
| 4 | Pace LE et al. [29] | >180b | >180b |
|
|
| 5c | Togawa K et al. [30] | >90b | >90b |
|
|
| 6 | Unger-Saldana K et al. [31] |
|
|||
| 14 | Dos Santos Andrade LS et al. [16] | >90 |
|
|
|
| 15 | Frie KG et al. [20] |
|
|||
| 16 | Hewage S et al. [21] |
|
|
||
| 17 | Hewage SA et al. [22] | >15b | >30b |
|
|
| 18e | Imran M et al. [24] |
|
|
||
| 19 | Mohd Mujar NM et al. [28] | >90b | >30b |
|
|
| 20 | Daniel O et al. [34] |
|
|||
| 21f | Hanafi I et al. [35] | >90b | >90b |
|
NGO: Non-governmental organization; BC: Breast Cancer.
Only the factors which are significant in multivariable analysis were included.
Time given in weeks/months converted to days.
Factors for total diagnosis time (from symptoms to diagnosis).
Residence compared to study area and other.
Factors for total time to treatment (symptom recognition to treatment).
Factors influencing any interval more than 90 days.
Except for five studies [19,24,30,35], all other studies provided the factors influencing pre-contact and post-contact interval separately. Among these five studies, three studies [19,24,30] showed the association of total time to diagnosis with various factors, one study [35] reported the factors associated with any interval which was more than 90 days and one study [34] reported the factors which influence longer time to diagnosis among women with different pre-contact intervals. This review considered all associated factors (pre-contact, post-contact, or total time to diagnosis) as the determinants of time to diagnosis. Table 3 shows the factors (barriers and facilitators) influencing time to diagnosis of BC.
Low socioeconomic status was identified as the most common barrier to a shorter time to diagnosis of BC in LMICs. Studies reported various measures of socioeconomic status, namely low socioeconomic position, socioeconomically disadvantaged, lower family income and low education attainment (illiterate or only primary education), to be associated with a longer time to diagnosis for BC. Additionally, lack of knowledge about BC and misinterpretation of symptoms were reported to significantly contribute to longer time to diagnosis. Another common factor for a longer time to diagnosis was the use of alternative medicine. People's belief and use of alternative medicine, first consultation with the ayurvedic doctor or traditional healer were identified as common barriers to a shorter duration for the diagnosis of BC. Similarly, multiple visits to health facilities or multiple consultations with health workers before diagnosis were also found to be a common factor influencing time to diagnosis. System-related factors such as limited accessibility to healthcare facilities (long distances and travel times), the quality of public health facilities, and negative perceptions of these facilities emerged as influential factors in prolonging the time to diagnosis. Furthermore, being employed was associated with a longer time to diagnosis due to the challenge in taking time off work. However, being unemployed was also associated with a longer time to diagnosis. Interestingly, both marital status and age were reported to have positive and negative influences on the time taken to diagnosis. The study also showed an interrelationship between residence in rural areas with lower income and lack of education. These factors are linked to inaccessible healthcare, resulting in a prolonged time to diagnosis which was more pronounced due to war like in Syria.
The most commonly reported facilitator for early diagnosis was having an initial consultation with a specialist and having a symptomatic breast lump as an initial symptom. Women who had knowledge about BC and perceived it as a prevalent issue experienced a shorter duration to diagnosis. Other factors that contributed to a shorter time to diagnosis include the availability of integrated health services, privately financed healthcare, and support from non-governmental organizations (NGOs). Residing in urban areas or a short distance from the health facility, was also shown to have a favourable association with the earlier diagnosis of BC.
3.7. Determinants of longer time to treatment
Among the 15 studies that described the factors influencing time from diagnosis to initiation of treatment, three studies [18,20,21] conducted in Brazil, Mali, and Sri Lanka compared the median time to treatment and reported associated factors. A study from Kenya [34] reported the factors influencing any interval longer than 90 days in the BC pathway. Another study from Pakistan [24] did not exclusively report the factors that influenced time to treatment. It reported the factors affecting the total treatment delay, which included the time from recognizing symptoms to receiving treatment. Since the time to treatment is part of the total treatment time, these factors were included in our analysis. The remaining ten studies [16,17,22,23,25,27,28,32,33,35] defined a longer time to treatment. However, the duration considered to represent delay varied greatly, ranging from 10 business days in Mexico, to 30 days in Malaysia and Sri Lanka, 60 days in Brazil, and 90 days in Syria (Table 4). Table 4 below summarizes the factors influencing time to treatment in LMICs.
Table 4.
Summary of barriers to and facilitators for shorter time to treatment of breast cancer in low- and middle-income countries.
| Study No. | Lead Author | Delayed Time to Treatment (days) | Barriers to early treatmenta | Facilitators of early treatmenta |
|---|---|---|---|---|
| 7 | Cabral AL et al. [17] | ≤60, 61–90, >90 |
|
|
| 8 | Alves Soares Ferreira N et al. [18] |
|
||
| 9 | Huerta-Gutiérrez R et al. [23] | >10 business days |
|
|
| 10 | Medeiros GC et al. [27] | >60 |
|
|
| 11 | Mastura M et al. [25] | >30b |
|
|
| 12 | Jomar RT et al. [32] | >60 |
|
|
| 13 | Nogueira MC et al. [33] | >60 |
|
|
| 14 | Dos Santos Andrade LS et al. [16] | >60 |
|
|
| 15 | Frie KG et al. [20] |
|
||
| 16d | Hewage S et al. [21] |
|
||
| 17d | Hewage SA et al. [22] | >30b |
|
|
| 18 | Imran M et al. [24] |
|
||
| 19 | Mohd Mujar NM et al. [28] | >30b |
|
|
| 20 | Daniel O et al. [34] |
|
|
|
| 21e | Hanafi I et al. [35] | >90b |
|
BC: Breast Cancer.
Only the factors which are significant in multivariable analysis were included.
Time given in weeks/months converted to days.
Residence compared to study area and other.
Total time to treatment from symptom recognition to treatment.
Factors influencing any interval more than 90 days.
Place of residence and distance from the health facilities were found to be the most common factors influencing longer time to treatment. Other commonly reported factors were unemployment and tumor characteristics. Women with a smaller tumor size and early-stage cancer had a longer time to treatment. Conversely, women with advanced-stage cancer also encountered more time to treatment in one study. Health system factors were identified as having an influence on time to treatment. Specifically, cases referred from a public health facility and those with first contact at a primary health facility experienced longer times to treatment than women whose first contact was a diagnostic service or private health facility. Other causes of longer time from diagnosis to treatment were related to sociodemographic characteristics such as older age, belonging to socially disadvantaged groups, being illiterate or having a low level of education, and specific ethnicities (specifically, Malays compared to Chinese in Malaysia).
Some of the identified facilitators for a shorter time to treatment in LMICs were individuals with a family history of BC, higher family income and privately financed diagnostic investigation. In addition, women who had been investigated with a core needle biopsy rather than fine-needle aspiration cytology and had undergone mammography prior to biopsy, experienced a shorter time from diagnosis to treatment.
4. Discussion
Our review is a timely and comprehensive evidence report on the time interval to diagnosis and the factors influencing time to diagnosis and treatment of BC in LMICs. Policymakers, healthcare professionals and other stakeholders can utilize the insights from this review to develop proactive strategies to mitigate factors contributing to delays in diagnosis and/or treatment of BC.
This review identified considerable variation in the definition and terminology used to describe the time intervals in the BC care pathway. Hence, we have reported three key intervals: pre- and post-contact intervals (time interval to diagnosis) and treatment interval. Similar reviews by Brand et al. [10] and Unger-Saldaña [9] had used different terminology or definitions of time interval compared to our review. To facilitate common understanding and enable cross-nation and intra-nation comparison, it is recommended that consistent definitions and terminology for these time intervals be established. While these intervals are influenced by both patient-mediated and system-mediated factors, especially in LMICs, where the UHC is limited [37], we recommend adopting the terminology “pre-contact interval” and “post-contact interval” rather than patient/presentation/health seeking or system/doctor/provider/diagnosis/physician intervals to describe the time from symptom recognition to first consultation (or contact with an element of the health system) and from the first consultation to diagnosis, respectively.
Most of the studies included in this review defined delays for the pre-contact interval, post-contact interval and treatment interval, however, the review identified inconsistencies in the definition of time delays. The complex relationship between individual behaviour, geographical factors and healthcare system structure were considered the main challenges in establishing a universally accepted benchmark for delays [9,38,39]. Standardized evidence-based guidelines are essential to ensure comparable and reliable results. The WHO GBCI, 2021 [4] set a benchmark of 60 days from BC symptom recognition to BC diagnosis in LMICs, providing a valuable reference for evaluating delays in diagnosis. Furthermore, a recent meta-analysis [7] emphasized that a 4-week delay in treatment after diagnosis increases the risk of mortality in BC, offering a reference for evaluating delays in treatment and underscoring the importance of timely treatment to improve BC survival.
We found substantial variation in time intervals in LMICs and that the median time to diagnosis was longer than the median time to treatment. The median pre-contact and post-contact intervals ranged from 9 to 255 days and 6–155 days respectively, while median time to treatment ranged from 16 to 127 days. The highest median pre-contact interval found in this review was 2.8 times higher than the interval reported by Unger-Saldaña (ranging from 10 to 90 days). This might be related to their exclusive focus on upper middle-income countries, while our review encompassed studies from upper-middle income (n = 4), lower-middle income (n = 5) and low-income countries (n = 5), with longer pre-contact intervals in the latter two categories, emphasizing the need for targeted efforts to reduce these intervals in such countries. Although the time to treatment was found to be shorter than the time to diagnosis in this review, the median time remains alarmingly high, especially considering that even a four-week delay in treatment post-diagnosis can significantly impact survival rates [7]. Given these findings, we advocate prioritizing the second pillar of the WHO GBCI [4], which emphasizes timely diagnosis through the establishment of accessible and swift diagnostic services along with a robust referral system for diagnostic processes.
This review highlights that socioeconomic status significantly affects the timely diagnosis and treatment of BC. Low socioeconomic status including social disadvantage, low family income and low level of education, was associated with longer time to diagnosis and treatment. This is further supported by studies finding a relationship between socioeconomic status and health outcomes [40,41]. People with low socioeconomic status tended to have poorer access to health services [42]. The internal variation in time to treatment in Brazil ranged from 54 to 127 days across four studies, could also be associated with the country's marked socioeconomic inequality. Brazil ranks as the fourth most unequal country in Latin America with a World Bank Gini index (a measure of wealth distribution inequality) of 52.9 in 2021 [43]. Consequently, it is plausible that the observed differences in time to treatment are linked to the socioeconomic development of specific regions within Brazil, as socioeconomically developed regions in Brazil seem to offer better BC care [42]. Therefore, addressing socioeconomic disparities in healthcare access is crucial for improving timely diagnosis and treatment, especially in LMICs.
The Place of residence of the individual with BC, whether in a rural area or within a shorter distance from healthcare facilities, has been found to be a barrier to achieving prompt diagnosis and treatment. This geospatial barrier is influenced by various factors, including financial constraints, logistic challenges within healthcare facilities and conflicting priorities such as work and family commitments [30]. Although establishing healthcare facilities in close proximity to a patients’ residence may not always be feasible, especially in LMICs, there are cost-effective strategies to overcome these challenges. One approach involves improving transportation options and alleviating the associated financial burden, such as implementing free hospital transportation, as has successfully been implemented in Namibia [30]. Additionally, a partially decentralized approach to diagnostic processes, such as conducting sample collection and screening mammography at lower levels, as is practised in Zambia [44] and recommended by the WHO [4] followed by effective feedback and referral for treatment after a positive diagnosis, could be another viable strategy for minimizing delays associated with geospatial barriers.
The review also reports that belief in and use of traditional medicine, including traditional healers or ayurvedic medicines, was also associated with prolonged time to diagnosis [19,21,28,29]. This phenomenon is widespread, particularly in low-income countries [45]. People often turn to traditional healers due to financial constraints [46,47], or a lack of adequate medical facilities in the primary healthcare system [46,48]. Traditional medicine is often more economically accessible than complementary alternatives [46,47]. Furthermore, misconceptions about complementary therapies, such as fears that surgery may disrupt cancer cells and exacerbate cancer growth, may also play a role [49]. It is important to increase public awareness about BC and to ensure accessible and adequate cancer diagnostic services. This could encourage individuals to choose conventional medical services over traditional healers, ultimately reducing the time needed to reach a diagnosis.
Initial presentation to a specialist was a facilitator for shorter time to BC diagnosis. The primary point of contact not only influences the time to diagnosis, but also impacts the time to treatment. BC patients whose first contact was a primary health facility were found to have a longer time to treatment. In LMICs, the contact person in a primary health facility was usually a general physician, a recently graduated medical doctor (not a specialist) or other health professional like a nurse or health assistants, the majority of whom might not be adequately trained or may not be familiar with BC diagnostic and treatment guidelines [9]. This review also identified that patients referred from a public health facility experienced longer time to treatment that could be related to the lack of expertise in the public health facility or proper guidelines for referrals. All of these findings signify the need to strengthen the capacity of primary/public healthcare professionals in the early detection of BC [50] and to develop a proper referral mechanism for BC diagnosis and treatment in LMICs.
There are some limitations to this review. The studies selected used different benchmarks for defining pre-contact, post-contact, and treatment delays, making it challenging to compare estimates and requiring careful interpretation of findings across studies. It was not appropriate to undertake meta-analysis due to heterogeneity of study design and defined outcomes. These differences between studies meant that while this review descriptively synthesized and summarized the findings reported by the studies, there might be limitations in the precision of the estimates for the time intervals and factors influencing them. Additionally, the review did not include qualitative studies within its scope, potentially overlooking abstract factors that require a profound understanding, such as socio-cultural and psychological perspectives. Similarly, the review contained a small number of studies from various regions, that might reduce the generalizability of the findings. However, despite these factors, this review has provided a snapshot of currently available evidence and identified relevant patterns. It has also identified knowledge gaps and proposed recommendations for improving BC care pathways in LMICs.
5. Conclusion
This systematic review suggests a need for uniform terminology and benchmarks for defining time intervals and delays, respectively, in BC pathways. The median time to diagnosis and treatment varied across studies reported in LMICs, nonetheless in some of the studies these were longer than the recommended time to facilitate early detection and improve BC outcomes. Low socioeconomic status (and, similarly, variables denoting social disadvantage) was frequently reported as a common barrier to timely diagnosis and treatment. Other important factors related to time to diagnosis and treatment include the place of residence, first point of contact and use of alternative medicine. Based on the findings from this review, early detection through increased public awareness, capacity enhancement of primary healthcare professionals in the early detection of BC, partial decentralization of diagnostic services and proper referral mechanisms are recommended to shorten the time for the diagnosis and treatment of BC in LMICs.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Ranjeeta Subedi: Writing – review & editing, Writing – original draft, Visualization, Project administration, Methodology, Formal analysis, Data curation, Conceptualization. Nehmat Houssami: Writing – review & editing, Visualization, Validation, Supervision, Methodology, Conceptualization. Carolyn Nickson: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Methodology, Conceptualization. Anant Nepal: Writing – review & editing, Visualization, Validation, Project administration, Methodology, Data curation, Conceptualization. Denise Campbell: Writing – review & editing, Visualization, Validation, Supervision, Project administration, Methodology, Data curation, Conceptualization. Michael David: Writing – review & editing, Visualization, Validation, Supervision, Methodology, Conceptualization. Xue Qin Yu: Writing – review & editing, Visualization, Validation, Supervision, Project administration, Methodology, Data curation, Conceptualization.
Declaration of competing interest
NH declares receiving funding from the National Breast Cancer Foundation (NBCF Australia) Chair in Breast Cancer Prevention grant (EC-21-001) and a National Health and Medical Research Council Investigator (Leader) grant (1194410). Other authors declare that no funds, grants, or other financial support were received during the preparation of this manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2024.103714.
Appendix A. Supplementary data
The following is/are the supplementary data to this article.
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.International Agency for Research on Cancer, World Health Organization Estimated age-standardized incidence rates (World) in 2020, World, both sexes, all ages (excl. NMSC) Global Cancer Observatory. 2020 https://gco.iarc.fr/ [Google Scholar]
- 3.Arnold M., Morgan E., Rumgay H., Mafra A., Singh D., Laversanne M., et al. Current and future burden of breast cancer: Global statistics for 2020 and 2040. Breast. 2022;66:15–23. doi: 10.1016/j.breast.2022.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization . World Health Organization; 2023. Global breast cancer initiative implementation framework: assessing, strengthening and scaling-up of services for the early detection and management of breast cancer.https://www.who.int/publications/i/item/9789240067134 [Google Scholar]
- 5.Rivera-Franco M.M., Leon-Rodriguez E. Delays in breast cancer detection and treatment in developing countries. Breast Cancer. 2018;12 doi: 10.1177/1178223417752677. 10.1177%2F1178223417752677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ginsburg O., Rositch A., Conteh L., Mutebi M., Paskett E., Subramanian S. Breast cancer disparities among women in low-and middle-income countries. Current Breast Cancer Reports. 2018;10:179–186. doi: 10.1007/s12609-018-0286-7. [DOI] [Google Scholar]
- 7.Hanna T.P., King W.D., Thibodeau S., Jalink M., Paulin G.A., Harvey-Jones E., et al. Mortality due to cancer treatment delay: systematic review and meta-analysis. BMJ. 2020;371 doi: 10.1136/bmj.m4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McLaughlin J.M., Anderson R.T., Ferketich A.K., Seiber E.E., Balkrishnan R., Paskett E.D. Effect on survival of longer intervals between confirmed diagnosis and treatment initiation among low-income women with breast cancer. J Clin Oncol. 2012;30(36):4493. doi: 10.1200/JCO.2012.39.7695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Unger-Saldaña K. Challenges to the early diagnosis and treatment of breast cancer in developing countries. World J Clin Oncol. 2014;5(3):465. doi: 10.5306/wjco.v5.i3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brand N.R., Qu L.G., Chao A., Ilbawi A.M. Delays and barriers to cancer care in low‐and middle‐income countries: a systematic review. Oncologist. 2019;24(12):e1371–e1380. doi: 10.1634/theoncologist.2019-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg. 2021;88 doi: 10.1016/j.ijsu.2021.105906. [DOI] [PubMed] [Google Scholar]
- 12.Hamadeh N., Rompaey V.R., Metreau E. World Bank Group country classifications by income level for FY24. World Bank Blog; 2023. https://blogs.worldbank.org/opendata/new-world-bank-group-country-classifications-income-level-fy24. [Accessed 24 August 2023].
- 13.Peters M.D., Godfrey C.M., McInerney P., Soares C.B., Khalil H., Parker D. 2015. The Joanna Briggs Institute reviewers' manual 2015: methodology for JBI scoping reviews. [Google Scholar]
- 14.Kmet L.M., Cook L.S., Lee R.C. Standard quality assessment criteria for evaluating primary research papers from a variety of fields. 2004. https://era.library.ualberta.ca/items/48b9b989-c221-4df6-9e35-af782082280e
- 15.Amornsak P., Supannee P., Duffy S.W., Parkin D.M. Factors associated with delayed diagnosis of breast cancer in Northeast Thailand. J Epidemiol. 2014;24(2):102–108. doi: 10.2188/jea.JE20130090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dos Santos Andrade L.S., De Melo Santos T.T., Case de Oliveira M.E., Lima Gomes K.A., Araújo Pereira Soares A.R., Almeida de Oliveira T., et al. Shorter delay to treatment by integrated diagnostic services and NGO-provided support among breast cancer patients in two Brazilian referral centres. J Public Health Res. 2021;10(3) doi: 10.4081/jphr.2021.1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cabral A.L., Giatti L., Casale C., Cherchiglia M.L. Social vulnerability and breast cancer: differentials in the interval between diagnosis and treatment of women with different sociodemographic profiles. Ciência Saúde Coletiva. 2019;24(2):613–622. doi: 10.1590/1413-81232018242.31672016. [DOI] [PubMed] [Google Scholar]
- 18.Alves Soares Ferreira N., Melo Figueiredo de Carvalho S., Engrácia Valenti V., Pinheiro Bezerra I.M., Melo Teixeira Batista H., de Abreu L.C., et al. Treatment delays among women with breast cancer in a low socio-economic status region in Brazil. BMC Wom Health. 2017;17(13):1–8. doi: 10.1186/s12905-016-0359-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foerster M., McKenzie F., Zietsman A., Galukande M., Anele A., Adisa C., et al. Dissecting the journey to breast cancer diagnosis in sub-Saharan Africa: findings from the multicountry ABC-DO cohort study. Int J Cancer. 2021;148(2):340–351. doi: 10.1002/ijc.33209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frie K.G., Kamate B., Traore C.B., Ly M., Malle B., Coulibaly B., et al. Factors associated with time to first healthcare visit, diagnosis and treatment, and their impact on survival among breast cancer patients in Mali. PLoS One. 2018;13(11) doi: 10.1371/journal.pone.0207928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hewage S., Samaraweera S., Joseph N., Kularatna S., Gunawardena N. Does the choice of care pathways matter in timely breast cancer care in Sri Lanka? Cancer Epidemiol. 2021;70 doi: 10.1016/j.canep.2020.101862. [DOI] [PubMed] [Google Scholar]
- 22.Hewage S.A., Samaraweera S., Joseph N., Kularatna S., Gunawardena N. Presentation, diagnosis and treatment delays in breast cancer care and their associations in Sri Lanka, a low-resourced country. Clin Oncol. 2022;34(9):598–607. doi: 10.1016/j.clon.2022.05.007. [DOI] [PubMed] [Google Scholar]
- 23.Huerta-Gutierrez R., Murillo-Zamora E., Angeles-Llerenas A., Ortega-Olvera C., Torres-Mejia G. Patient and treatment delays among Mexican women with breast cancer before and after the Seguro popular. Salud Publica Mex. Spanish. 2021;64(1):87–95. doi: 10.21149/12472. [DOI] [PubMed] [Google Scholar]
- 24.Imran M., Rana A., Anwar A.W., Rafique H.M., Faiza I. Diagnostic and treatment delays in breast cancer in association with multiple factors in Pakistan. East Mediterr Health J. 2021;27(1):23–32. doi: 10.26719/emhj.20.051. [DOI] [PubMed] [Google Scholar]
- 25.Mastura M., Maznah D., Cheng H., Nur Aishah T. Delays in time to primary treatment after a diagnosis of breast cancer: does it impact survival? Prev Med. 2013;56(3/4):222–224. doi: 10.1016/j.ypmed.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 26.Medeiros G.C., Thuler L.C.S., Bergmann A. Delay in breast cancer diagnosis: a Brazilian cohort study. Publ Health. 2019;167:88–95. doi: 10.1016/j.puhe.2018.10.012. [DOI] [PubMed] [Google Scholar]
- 27.Medeiros G.C., Thuler L.C.S., Bergmann A. Determinants of delay from cancer diagnosis to treatment initiation in a cohort of Brazilian women with breast cancer. Health Soc Care Community. 2021;29(6):1768–1778. doi: 10.1111/hsc.13284. [DOI] [PubMed] [Google Scholar]
- 28.Mohd Mujar NM., Dahlui M., Emran N.A., Abdul Hadi I., Wai Y.Y., Arulanantham S., et al. Complementary and alternative medicine (CAM) use and delays in presentation and diagnosis of breast cancer patients in public hospitals in Malaysia. PLoS One. 2017;12(4) doi: 10.1371/journal.pone.0176394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pace L.E., Mpunga T., Hategekimana V., Dusengimana J.M.V., Habineza H., Bigirimana J.B., et al. Delays in breast cancer presentation and diagnosis at two rural cancer referral centers in Rwanda. Oncologist. 2015;20(7):780–788. doi: 10.1634/theoncologist.2014-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Togawa K., Anderson B.O., Foerster M., Galukande M., Zietsman A., Pontac J., et al. Geospatial barriers to healthcare access for breast cancer diagnosis in sub-Saharan African settings: the African breast cancer-disparities in outcomes cohort study. Int J Cancer. 2020;148(9):2212–2226. doi: 10.1002/ijc.33400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Unger-Saldana K., Ventosa-Santaularia D., Miranda A., Verduzco-Bustos G. Barriers and explanatory mechanisms of delays in the patient and diagnosis intervals of care for breast cancer in Mexico. Oncologist. 2018;23(4):440–453. doi: 10.1634/theoncologist.2017-0431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jomar R.T., Velasco N.S., Mendes G.L., Guimarães R.M., Fonseca V.A., Meira K.C. Factors associated with time-to-treatment initiation of breast cancer. Ciência Saúde Coletiva. 2023;28:2155–2164. doi: 10.1590/1413-81232023287.14982022EN. [DOI] [PubMed] [Google Scholar]
- 33.Nogueira M.C., Atty A.T., Tomazelli J., Jardim B.C., Bustamante-Teixeira M.T., Azevedo e Silva G. Frequency and factors associated with delay in breast cancer treatment in Brazil, according to data from the Oncology Panel, 2019-2020. Epidemiol Serv Saude. 2023;32 doi: 10.1590/S2237-96222023000300004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Daniel O., Ashrafi A., Muthoni M.A., Njoki N., Eric H., Marilynn O., et al. Delayed breast cancer presentation, diagnosis, and treatment in Kenya. Breast Cancer Res Treat. 2023:1–13. doi: 10.1007/s10549-023-07067-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hanafi I., Alsalkini M., Husein S., Salamoon M. The delay of breast cancer diagnosis and management during the Syrian war. Cancer Epidemiol. 2023;82 doi: 10.1016/j.canep.2022.102290. [DOI] [PubMed] [Google Scholar]
- 36.Grosse Frie K., Kamaté B., Traoré C.B., Coulibaly B., Mallé B., Kantelhardt E.J. Health system organisation and patient pathways: breast care patients' trajectories and medical doctors' practice in Mali. BMC Publ Health. 2019;19:1–10. doi: 10.1186/s12889-019-6532-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Foerster M., McKenzie F., Zietsman A., Galukande M., Anele A., Adisa C., et al. Dissecting the journey to breast cancer diagnosis in sub‐Saharan Africa: findings from the multicountry ABC‐DO cohort study. Int J Cancer. 2021;148(2):340–351. doi: 10.1002/ijc.33209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Freitas A.G., Weller M. Patient delays and system delays in breast cancer treatment in developed and developing countries. Ciência Saúde Coletiva. 2015;20(10):3177–3189. doi: 10.1590/1413-812320152010.19692014.20:3177-89. [DOI] [PubMed] [Google Scholar]
- 39.Unger-Saldaña K., Infante-Castañeda C. Delay of medical care for symptomatic breast cancer: a literature review. Salud Publica Mex. 2009;51(suppl 2):s270–s285. doi: 10.1590/s0036-36342009000800018. [DOI] [PubMed] [Google Scholar]
- 40.Glymour M.M., Avendano M., Kawachi I. Socioeconomic status and health. Social epidemiology. 2014;2:17–63. [Google Scholar]
- 41.Adler N.E., Stewart J. Preface to the biology of disadvantage: socioeconomic status and health. Ann N Y Acad Sci. 2010;1186(1):1–4. doi: 10.1111/j.1749-6632.2009.05385.x. [DOI] [PubMed] [Google Scholar]
- 42.dos Santos Figueiredo F.W., Cardial D.T., do Carmo Almeida T.C., da Silva Cardial C., de Carvalho L.E., Adami F. Socioeconomic changes in Brazil impacted breast cancer indexes at the beginning of the 21st century? J Cancer Policy. 2018;16:39–42. doi: 10.1016/j.jcpo.2018.04.005. [DOI] [Google Scholar]
- 43.The World Bank. Gini index-Brazil. The World Bank Group; 2023. https://tinyurl.com/mmphzfz4. [Accessed 10 October 2023].
- 44.Cabanes A., Kapambwe S., Citonje-Msadabwe S., Parham G.P., Lishimpi K., Cruz T.A., et al. Challenges, opportunities, and priorities for advancing breast cancer control in Zambia: a consultative meeting on breast cancer control. J Glob Oncol. 2019;5:1–7. doi: 10.1200/JGO.18.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Muhamad M., Merriam S., Suhami N. Why breast cancer patients seek traditional healers. Int J Breast Cancer. 2012;2012 doi: 10.1155/2012/689168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pal S.K. Use of alternative cancer medicine in India. Lancet Oncol. 2002;3(7):394–395. doi: 10.1016/S1470-2045(02)00782-9. [DOI] [PubMed] [Google Scholar]
- 47.Tovey P., Chatwin J., Ahmad S. Toward an understanding of decision making on complementary and alternative medicine use in poorer countries: the case of cancer care in Pakistan. Integr Cancer Ther. 2005;4(3):236–241. doi: 10.1177/1534735405278641. [DOI] [PubMed] [Google Scholar]
- 48.Taib N.A., Yip C.H., Ibrahim M., Ng C., Farizah H. Breast cancer in Malaysia: are our women getting the right message? 10 year-experience in a single institution in Malaysia. Asian Pac J Cancer Prev APJCP. 2007;8(1):141. [PubMed] [Google Scholar]
- 49.Leong B., Chuah J., Kumar V., Rohamini S., Siti Z., Yip C. Trends of breast cancer treatment in Sabah, Malaysia: a problem with lack of awareness. Singapore Med J. 2009;50(8):772. [PubMed] [Google Scholar]
- 50.Sayed S., Ngugi A.K., Nwosu N., Mutebi M.C., Ochieng P., Mwenda A.S., et al. Training health workers in clinical breast examination for early detection of breast cancer in low‐and middle‐income countries. Cochrane Database Syst Rev. 2023;(4) doi: 10.1002/14651858.CD012515.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.