Abstract
Background
New patterns of progression under immune-oncology (IO) antibodies (mAb) have been described such as pseudoprogression. Except for melanoma, variations between studies reveal difficulties to establish their prevalence.
Methods
This retrospective study enrolled patients participating in IO phase I trials at Gustave Roussy cancer center for solid tumors excluding melanoma. Radiological assessment according to iRECIST was correlated with prospectively registered patient characteristics and outcomes. Pseudoprogression (PsPD) was defined as RECIST-defined progression followed by stabilization or decrease at the next imaging, and dissociated response (DisR) as concomitant decrease in some tumor lesions and increase in others at a same timepoint.
Results
Among 360 patients included, 74% received IO mAb combination: 45% with another IO mAb, 20% with targeted therapy and 10% with radiotherapy. The overall response rate was 19.7%. PsPD were observed in 10 (2.8%) patients and DisR in 12 (3.3%) patients. Atypical responses (AR), including PsPD and DisR, were not associated with any patient’s baseline characteristics. Compare with typical responder patients, patients experiencing AR presented a shorter iPFS (HR 0.34; p < 0.001) and OS (HR 0.27; p = 0.026). Among the 203 patients who progressed in 12 weeks, 80 (39.4%) patients were treated beyond progression. PD was confirmed in 80% of cases, while 10% of patients presented a response.
Conclusion
Pseudoprogression and dissociated response are uncommon patterns of progression. Their prevalence should be balanced with the rate of real progressing patients treated beyond progression. Prognosis or on-treatment biomarkers are needed to identify early patients who will benefit from immunotherapy.
Electronic supplementary material
The online version of this article (10.1007/s00262-020-02647-z) contains supplementary material, which is available to authorized users.
Keywords: Immune checkpoint inhibitors, Immunotherapy, Pseudoprogression, Imaging evaluation, Phase I
Introduction
Immune-oncology (IO) antibodies (mAb) have led to a paradigm shift in cancer treatment and its assessment. Anti CTLA-4 (cytotoxic T-lymphocyte antigen-4), anti PD-1 (programmed death 1) and anti PDL-1 (programmed death ligand 1) antibodies have demonstrated their efficacy [1] and were approved for the treatment of many advanced solid tumors such as melanoma, non-small-cell lung carcinoma (NSCLC), renal cell carcinoma, urothelial carcinoma and head and neck squamous cell carcinoma, Merkel carcinoma, and microsatellite instability-high (MSI) cancers [2]. By their mechanisms of action, IO mAb led to additional radiological patterns of response and progression, challenging tumor assessment by accurate radiological response criteria, the Response Evaluation Criteria in Solid Tumours (RECIST) v 1.1 guidelines. RECIST guidelines were developed as a standardized method for tumor response assessment in advanced solid cancers treated with cytotoxic chemotherapy, based on the change in tumor burden [3, 4]. The tumor shrinkage is reflecting the benefit and the efficacy of treatment. First described in melanoma with ipilimumab, an anti CTLA-4 antibody, some patients experienced an initial increase size of tumor lesions at first evaluation with a subsequent decreased or stabilized tumor burden in a second time [5]. This transient tumor flare, termed as pseudoprogression (PsPD), was also observed with anti PD-1 and anti PDL-1 [6]. Other patterns have been described such as dissociated responses [7, 8], hyperprogressive disease (HPD) [9], and more recently fast progression (FastPD) [10]. On the basis of these observations, RECIST 1.1 criteria has been modified in RECIST 1.1 for immune-based therapeutics (iRECIST) [11]. It requires a confirmation of progressive disease (PD) with a 4 weeks later assessment. These new criteria may capture equivocal progression such as pseudoprogression and may help to surrogate survival benefit [12].
Atypical responses were observed in about 10% of metastatic melanoma patients treated with ipilimumab and were associated with improved survival [5]. Whether these patterns of response have been previously characterized in more depth in melanoma, variations between studies reveal difficulties to establish the real frequency of these new patterns and their particular outcomes in other solid tumors.
The main objective of this study was to assess the occurrence of the different patterns of progression with IO mAb phase I trials and their correlation with patient outcomes in solid tumors excluding melanoma. Secondly, we investigated the potential clinical benefit of treatment beyond unconfirmed PD, according to iRECIST, in patients experiencing an initial progression in the 12 weeks of drug exposure.
Materials and methods
Study design and patients
We retrospectively reviewed all cases of patients with advanced solid tumor, enrolled between September 2015 and November 2017 in IO mAb phase I trials in the Drug Development Department (DITEP) at Gustave Roussy Cancer Centre. Patients with hematological malignancies, melanoma or receiving intratumoral injection were excluded of our study. Only patients who received at least one dose of therapy were included. Clinical and biological characteristics at treatment initiation, the first 6-month treatment follow-up CT-scans, the nadir radiological assessment, and patient outcomes (collected prospectively by the trial investigator) were recorded until June 2018. The Royal Marsden Hospital (RMH) [13] score, the Gustave Roussy Immune Score (GRIm-Score) [14] and the lung immune prognostic index (LIPI) [15] score were calculated at treatment initiation (see Supplementary Methods). The study was conducted in accordance with the principles of the Helsinki Declaration and retrospective data collection was approved by ethical review board.
Tumor assessment
Tumor response was centrally assessed with iRECIST by an immune-oncology radiologist. As per protocols, all patients were evaluated every 6 or 8 weeks. Stable disease (SD), partial responses (PRs) and complete responses (CRs) were identical for RECIST and iRECIST criteria. For progressing patients evaluated per the iRECIST, unconfirmed progressive disease (iUPD) should be confirmed by 4 or 8 weeks later (iCPD) for patients clinically stable. If the progression is not confirmed at 4 or 8 weeks, the patient remains in iUPD.
Atypical responses were identified and proper characterized by a senior radiologist. According to iRECIST guidelines, pseudoprogression (PsPD) was defined by an increase ≥ 20% in the sum of longest diameter of target lesions, and/or an unequivocal progression in non-target lesions and/or appearance of new lesions following by a decrease or stability noticed in the next imaging assessment. We defined dissociated response (DisR) as a concomitant relative decrease greater than 30% in some tumor lesions and relative increase greater than 20% in others (significant increase ≥ 5 mm in the sum of measures).The overall response rate (ORR) was defined as the best tumor response (complete or partial response) during the clinical trial.
Statistics analysis
Patients characteristics were reported descriptively in this retrospective analysis. Progression-free survival (iPFS) was defined as the time from treatment initiation to confirmed progressive disease (iCPD) or death from any cause or lost to follow up, and overall survival (OS) was defined as the time from treatment initiation to death from any cause or lost to follow up. We identified fast progression (FastPD) as an overall survival shorter than 12 weeks [10, 16]. PFS and OS were analyzed by the Kaplan–Meier method. Median follow-up was calculated by the inverse Kaplan–Meier method. Patients who presented a clinical progression and were withdrawn from trial before the first evaluation were not included in objective response assessment but were included for survival analysis. Prognosis factors for predicting response were tested with logistic regression in univariate analyses. To investigate the potential benefit from treatment in patients experiencing an initial progression (iUPD) according to iRECIST, a landmark for post-iUPD survival analyses was set at the time of the first progression assessment. Hazard ratios (HR) were assessed by Cox model. Statistical analyses were performed using GraphPad Prism version 7 and R version 3.4.4. All statistical tests were two-tailed, and p values < 0.05 were considered statistically significant.
Results
Patients and treatment
A total of 360 patients, enrolled in IO mAb phase I trials between September 2015 and November 2017, were included in our study. The median age was 60 years (range 25–88). Patients received a median of two previous line of therapy for their advanced disease and only eight patients (2.2%) were previously treated by a previous IO mAb. The main tumor types were non-small cell lung cancer (NSCLC) (13.6%), colorectal adenocarcinoma (13.6%), urothelial carcinoma (13.1%), renal cell carcinoma (8.3%), breast cancer (6.9%) and head and neck carcinoma (6.7%). Patients characteristics are summarized in Table 1.
Table 1.
Patients characteristics
| No (%) | ||||
|---|---|---|---|---|
| All patients (n = 360) | FastPD (n = 45) | PsPD (n = 10) | DisR (n = 12) | |
| Median age | ||||
| Years (range) | 59.8 (24.6–88.3) | 59.5 (27.2–73) | 59.7 (33.9–74.7) | 59.6 (34.3–88.3) |
| Gender | ||||
| Male | 214 (59.4%) | 26 (57.8%) | 7 (70%) | 7 (58.3%) |
| Female | 146 (40.6%) | 19 (42.2%) | 3 (30%) | 5 (41.7%) |
| RMH score | ||||
| 0 | 137 (38.1%) | 5 (11.1%) | 5 (50%) | 5 (41.7%) |
| 1 | 137 (38.1%) | 10 (22.2%) | 1 (10%) | 6 (50%) |
| 2 | 70 (19.4%) | 22 (48.9%) | 4 (40%) | 1 (8.3%) |
| 3 | 16 (4.4%) | 8 (17.8%) | 0 | 0 |
| GRIm score | ||||
| 0 | 163 (45.3%) | 5 (11.1%) | 5 (50%) | 5 (41.7%) |
| 1 | 130 (36.1%) | 17 (37.8%) | 3 (30%) | 6 (50%) |
| 2 | 52 (14.4%) | 16 (35.6%) | 2 (20%) | 1 (8.3%) |
| 3 | 15 (4.2%) | 7 (15.6%) | 0 | 0 |
| LIPI score | ||||
| 0 | 160 (44.4%) | 9 (20%) | 6 (60%) | 4 (33.3%) |
| 1 | 161 (44.7%) | 10 (22.2%) | 4 (40%) | 8 (66.7%) |
| 2 | 39 (10.8%) | 16 (35.6%) | 0 | 0 |
| Histology | ||||
| Colorectal | 49 (13.6%) | 7 (15.6%) | 1 (10%) | 1 (6.7%) |
| NSCLC | 49 (13.6%) | 4 (8.9%) | 1 (10%) | 2 (13.3%) |
| Urothelial | 47 (13.1%) | 10 (22.2%) | 2 (20%) | 2 (13.3%) |
| Colorectal MSS | 39 (10.8%) | 7 (15.6%) | 0 | 1 (6.7%) |
| Renal | 30 (8.3%) | 0 | 2 (20%) | 1 (6.7%) |
| Breast | 25 (6.9%) | 3 (6.7%) | 0 | 0 |
| Head and neck | 24 (6.7%) | 3 (6.7%) | 0 | 0 |
| Cervix | 20 (5.6%) | 0 | 1 (10%) | 2 (13.3%) |
| Gastric, oesophagus | 19 (5.3%) | 4 (8.9%) | 0 | 0 |
| Hepatocarcinoma | 14 (3.9%) | 2 (4.4%) | 1 (10%) | 0 |
| Pancreas | 14 (3.9%) | 2 (4.4%) | 0 | 0 |
| Prostate | 12 (3.3%) | 3 (6.7%) | 0 | 0 |
| Cavum | 10 (2.8%) | 2 (4.4%) | 0 | 0 |
| Colorectal MSI-high | 10 (2.8%) | 0 | 1 (10%) | 0 |
| Ovarian | 9 (2.5%) | 2 (4.4%) | 0 | 1 (6.7%) |
| Mesothelioma | 8 (2.2%) | 1 (2.2%) | 0 | 1 (6.7%) |
| Endometrium | 7 (1.9%) | 1 (2.2%) | 0 | 1 (6.7%) |
| Thyroid | 5 (1.4%) | 0 | 1 (10%) | 0 |
| Ileum | 4 (1.1%) | 0 | 0 | 0 |
| Merkel carcinoma | 4 (1.1%) | 0 | 0 | 0 |
| Cholangiocarcinoma | 3 (0.8%) | 1 (2.2%) | 0 | 0 |
| Thymic | 3 (0.8%) | 0 | 1 (10%) | 1 (6.7%) |
| Adnexial carcinoma | 1 (0.3%) | 0 | 0 | 0 |
| Penis | 1 (0.3%) | 0 | 0 | 0 |
| Sarcoma | 1 (0.3%) | 0 | 0 | 0 |
| Vagina | 1 (0.3%) | 0 | 0 | 0 |
| Previous ICI | ||||
| Yes | 8 (2.2%) | 1 (2.2%) | 0 | 0 |
| No | 352 (97.8%) | 44 (97.8%) | 10 (100%) | 12 (80%) |
| Previous lines: median(range) | 2 (0–10) | 2 (1–9) | 1 (0–3) | 1 (0–7) |
FastPD fast progression, PsPD Pseudo-progression, DisR dissociated response, IO mAb immune-oncology antibodies
Patients were enrolled in 36 trials and 74% received IO mAb combination: 44% received an anti PD-1/anti-PDL-1 with another IO mAb, 20% an anti-PD-1 with targeted therapy and 10% an anti-PDL-1 with radiotherapy (Supplementary Table 1). Sixty-two patients (17.5%) received an anti PD-1 or anti PDL-1 in monotherapy and thirty (8.5%) patients another IO mAb.
Outcomes and efficacy
With a median duration of follow-up of 14.1 months (95% CI 12.7–15.4), the median PFS was 3.4 months (95% CI 2.8–3.9) and median OS 12.8 months (95% CI 10.2–14.4). In the overall population, 13 patients were withdrawn from trial because of an unacceptable toxicity.
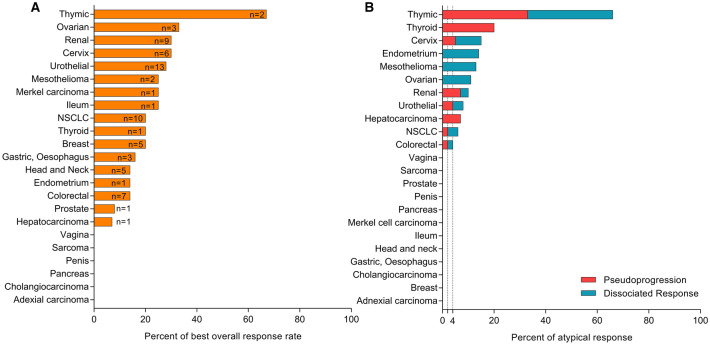
Three hundred thirty-eight patients (94%) were evaluable for first radiological assessment. The overall response rate (ORR) was 19.7% (95% CI 16.9–24.2) with 62 (17.2%) partial response (PR) and 9 (2.5%) complete response (CR). The ORR according to the histology are described in Fig. 1a. The median time to the best response was 5.5 months (95% CI 4.5–6.1). Stable disease as best response was observed in 32.8% of patients.
Fig. 1.
Response rate (a) and atypical response rate (b) according to tumor histology
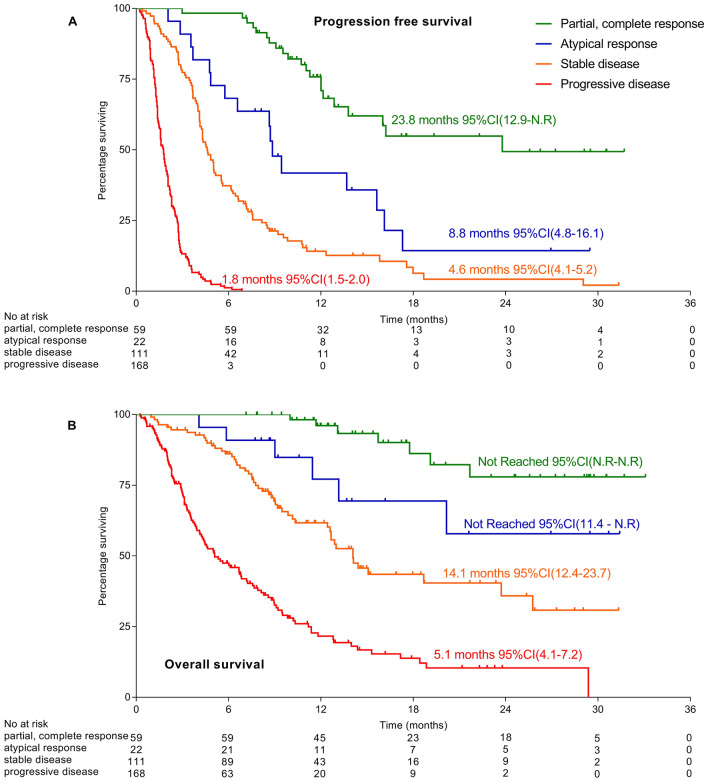
No event (progression or death) occurred in patients achieving complete response. The median iPFS was significantly longer in patients achieving a PR compare to patients with a SD as best response (median, 15.6 versus 4.8 months; hazard ratio (HR), 0.2; 95% CI 0.2–0.4; p = 0.001). Similar results were observed for OS (PR vs SD: not reached (N.R.) versus 14.1 months; HR, 0.2; 95% CI 0.7–.4; p < 0.001). Patients who experienced a PD presented a median PFS of 1.8 months (95% CI 1.6–2.0) and a median OS of 5.1 months (95% CI 4.1–7.2) (Supplementary Fig. 1).
Among patients with a SD, prognostic scores were correlated with OS. Patients with a low RMH score and GRIM score presented a longer OS than patients with a high score (HR for RMH, 0.3; 95% CI 0.2–0.6; HR for GRIM, 0.3; 95% CI 0.1–0.5), whereas no significant difference was observed for LIPI score (HR 1.1; 95% CI 0.7–1.7). Among responder patients, prognostic scores were not correlated with OS (p = 0.3 for RMH score; p = 0.4 for GRIM score; p = 0.3 for LIPI score) (Supplementary Fig. 2).
Pseudoprogression
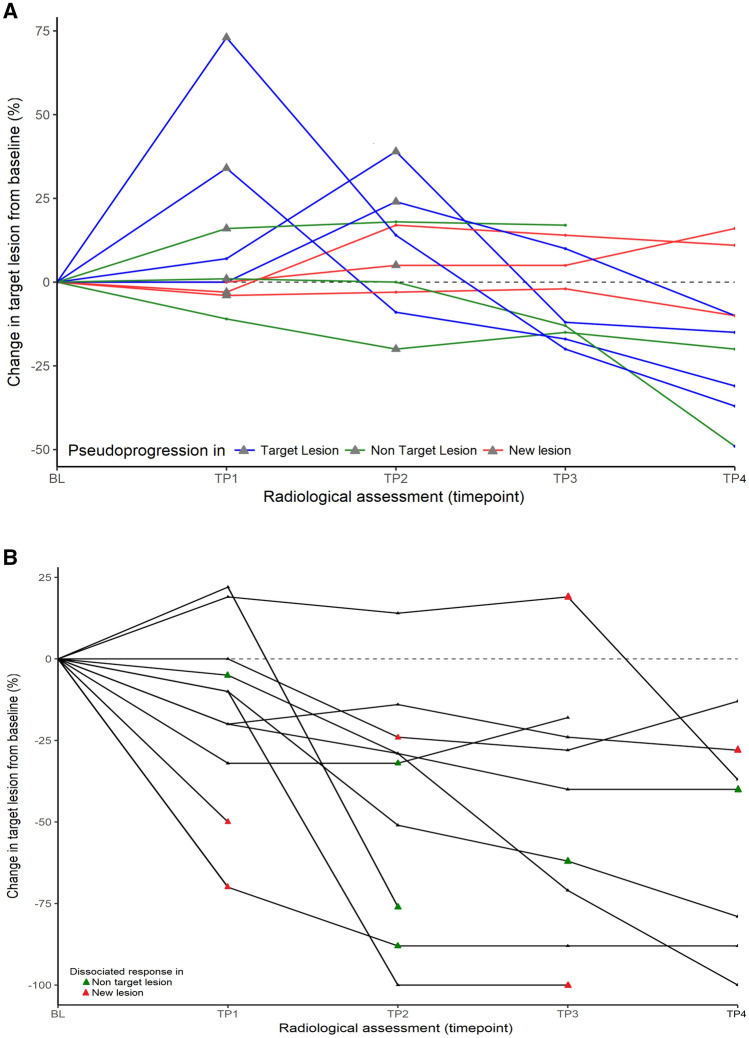
We observed pseudoprogression (PsPD) in 10 (2.8%) patients. Among histology most represented, one (10%) PsPD were reported for microsatellite instability (MSI) high genotype colorectal adenocarcinoma, two (7%) for renal cell carcinoma, two (4%) for urothelial carcinoma and one (2%) for NSCLC (Fig. 1b and Supplementary Table 2). No differences in prognostic scores were observed between PsPD and real progressive patients: for RMH score, OR = 0.7 (95% CI 0.3–1.4, p = 0.3); for GRIm score, OR = 0.6 (95% CI 0.3–1.5, p = 0.3); and for LIPI score, OR = 0.3 (95% CI 0.1–1.1, p = 0.06). Of these patients, three received an anti PD-1/PDL-1, four an IO mAb combination with another IO mAb and two an anti PD-1 combined with targeted therapy. The median time to PsPD was 7.4 weeks (95% CI 6.3–4.7). No specific type of PsPD was observed: 40% presented a progression in target lesion (TL), 30% in non-target lesion (NTL) and 30% presented an appearance of new lesion (NL) (Fig. 2a). The site of PsPD was preferentially lymph node (n = 3) and pulmonary metastasis (n = 2), but PsPD was also observed in primitive tumor localization for 2 patients (examples in Supplementary Fig. 3a, b). After a median time of 6.0 months (95% CI 4.6–19.3), one patient achieved a complete response, four a partial response and five a stable disease (examples in Supplementary Fig. 1a, b). In total, patients experiencing a PsPD presented a median iPFS of 9.4 months (95% CI 4.8–N.R.).
Fig. 2.
Percent change from baseline in target lesion per iRECIST in patients with a pseudoprogression (n = 10/360) and b dissociated response (n = 12/360)
Dissociated response
We observed dissociated responses (DisR) in 12 (3.3%) of patients. The median time to DisR was 11.3 weeks (95% CI 10.3–21.4). Among histology most represented, two (4%) of DisR were reported for NSCLC, two (4%) for urothelial carcinoma, one (3%) for microsatellite stable genotype colorectal adenocarcinoma and one (3%) for renal cell carcinoma (Fig. 1b and Supplementary Table 2). Of these patients, three received an anti PD-1/PDL-1, three with another IO mAb and six with targeted therapy. Dissociated response was observed in the first 12 weeks of treatment for 7 patients. Increasing of NTL (50%) and NL (50%) were reported in lymph nodes, cerebral metastasis, liver metastasis, peritoneal carcinomatosis and primitive tumor localization (Fig. 2b). For 7 out of 12 patients, concomitant response was observed with a reduction in the size of pulmonary metastasis (example in Supplementary Fig. 3c). No differences were observed between DisR and classical RECIST patterns of response: for RMH score, OR = 0.7 (95% CI 0.3–1.5, p = 0.3); for GRIm score, OR = 0.9 (95% CI 0.4–1.7, p = 0.6); and for LIPI score, OR = 1.0 (95% CI 0.4–2.4, p = 0.99). After the dissociated response reported, one (8%) patient achieved a complete response, 3 (35%) a partial response with a median time of 5.4 months (95% CI 3.0–8.2), and 6 (50%) patients were considered as progressor after dissociated response reported. For two patients, dissociated response consisted in a single progressing cerebral lesion. As both patients were irradiated (stereotactic radiotherapy), they had been considered as dissociated response (and not pseudoprogression), before achieving CR and PR.
In total, patients experiencing a dissociated response presented a median iPFS of 6.6 months (95% CI 2.9–15.6).
Atypical responses and outcomes
Because of the limited number of patients, no significant difference was found in outcomes of patients experiencing an PsPD or DisR and patients without (Supplementary Fig. 4). Pooled together, patients experiencing an atypical response (AR) presented a significantly shorter iPFS than patients achieving PR or CR without AR (median PFS CR, PR vs AR: 23.8 months vs 8.8 months; HR 0.34, 95% CI 0.18–0.65; p < 0.001) but significantly longer than patients achieving SD without AR (SD vs AR: 4.6 months vs 8.8 months; HR, 2.2; 95% CI 1.29–3.65; p = 0.004) (Fig. 3a). Median OS were not reached in patients with AR (95% CI 11.4–N.R.) and significantly shorter than patients with PR or CR (CR–PR vs AR: HR 0.27; 95% CI 0.09–0.85; p = 0.026). No difference was observed in OS with patients achieving SD without atypical response (SD vs AR: 14.1 months vs N.R.; HR 2.46; 95% CI 0.44–0.96; p = 0.06) (Fig. 3b). Patients experiencing atypical response had significantly longer OS than patients experiencing a true progression (AR vs PD: HR 0.18; 95% CI 0.12–0.27; p < 0.001).
Fig. 3.
Survivals according to radiological assessment: a confirmed progression free-survival (PFS) and b overall survival (OS)
Fast progression
Forty-five (12.5%) patients presented an OS shorter than 12 weeks, defined as fast progression (FastPD). Of these, 13 (29%) patients were withdrawn from trial for clinical progression before the first radiological assessment and 6 (13%) patients presented a clinical progression with a stable disease according to iRECIST. FastPD was observed in many histology subtypes (Table 1 and Supplementary Table 2). FastPD patients presented a higher punctuation in all the prognostic scores in comparison with non-FastPD patients who experienced a PD: for RMH score, OR = 3.2 (95% CI 1.9–5.2, p = 0.001); for GRIm score, OR = 2.8 (95% CI 1.8–4.4, p = 0.001); for LIPI score, OR = 2.7 (95% CI 0.08–0.3, p = 0.001).
FastPD patients presented a median PFS shorter than non-FastPD patients who experienced PD (1.1 months, (95% CI 0.9–1.3) vs 2.3 months (95% CI 2.0–2.6), p = 0.001) and a shorter OS (1.6 months (95% CI 1.3–2.0) versus 8.1 months (95% CI 6.7–9.5), p = 0.001).
Treatment beyond initial progression
Among the 203 patients who experienced an initial progression (iUPD) in the 12 weeks of drug exposure, 81 (39.4%) patients were treated beyond progression, including 35% at first assessment (n = 55/158) and 57% at second assessment (n = 26/45). Patients characteristics at baseline are described in Supplementary Table 3.
Among these patients treated beyond iUPD, progression was confirmed with 4 or 8 weeks later CT-scan assessment (iCPD) in 80% of cases (Supplementary Table 4). Eight (10%) patients achieved a partial (n = 6) and a completed response (n = 2) after receiving treatment beyond progression. Eight (10%) patients achieved a stable disease for a median time of 2.4 months (95% CI 0.9–12.2) after receiving treatment beyond progression. The median time from iUPD to the best response was 4.2 months (range 2.9–16.6). Overall, the median duration of treatment after iUPD was 1.6 months (95% CI 1.4–1.9).
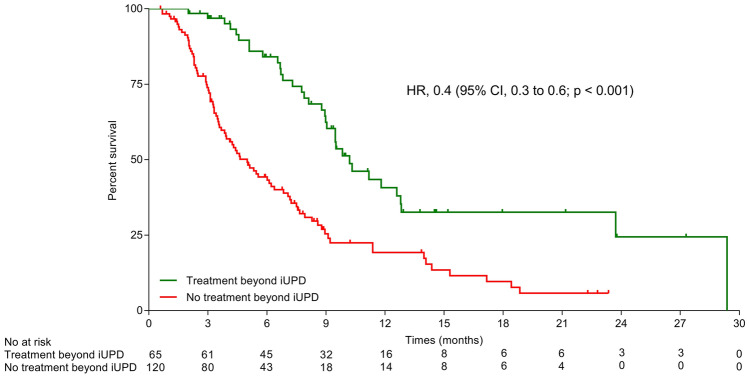
Among real progressive patients (excluding patients experiencing AR), patients treated beyond progression presented a significant longer OS compare to patients not treated beyond progression (Fig. 4). The median post-iUPD OS was 10.1 months (95% CI 7.8–N.R.) for patients treated beyond progression vs 3.0 months (95% CI 2.3–4.6) with an HR of 0.4 (95% CI 0.3–0.6; p < 0.001).
Fig. 4.
Post-progression OS according in patients treated beyond progression and not treated beyond progression among real progressive patients (excluding patients experiencing atypical response). A landmark analysis was set at the time of initial progression
Discussion
This study assessed the prevalence of new patterns of progression under immune-oncology antibodies in a large cohort of immune-naive patients (excepted melanoma) enrolled in phase I trials. In this retrospective study, we reported 6.1% of atypical response with less than 3% of pseudoprogression. The occurrence of these unconventional responses is still unclear, never exceeding 10% but varying among histology subtypes [17]—range of 7.3% in melanoma [12] to 1.5% in urothelial cancer [18]. A recent systematic review of 38 studies [19] found 6% of atypical responses (151 on 2400 patients) treated with anti PD-1. Variation are also observed between clinical trials: in NSCLC patients, PsPD were observed in 6.7% in nivolumab phase 3 trial [20] but none was observed in a real-life retrospective study [21, 22]. Theses variations could be explained by different hypotheses. Firstly, pseudoprogression may result from recruitment and infiltration of activated T cells in the tumor generating edema, leading to a transient increase of tumor size [6, 23]. Pseudoprogression could correspond also to a slow antitumor response, the imaging assessment is capturing the natural growing of the tumor before the effective immune activation. Thus, first timepoint assessment might misevaluate the rate of true PsPD. Therefore, only histology biopsy can support the diagnosis and differentiate from true progression. Our study captured an estimation of these patterns among various cancers and various IO trials, confirming a class effect in monotherapy as in combination.
Dissociated response (DisR), or mixed response, corresponds to different radiological patterns of response at the same timepoint. Mixed response is a real clinical routine challenge although it is not defined in radiological criteria guidelines, and only a small number of studies described it [5, 7, 24]. Tazdait et al. reported 7.5% of DisR among 160 patients with advanced NSCLC patients treated with an anti PD-1 or PD-L1 [25]. Half of them presented a clinical benefit from treatment. After DisR reported in our cohort, 50% of patients presented a real progression, illustrating difficulties to define a single entity of patients who benefit from treatment. We also observed different rates of DisR among cancer types with a low level of sensitivity for anti-PD-(l)1 monotherapy as MSS colorectal, ovarian and endometrium cancer. Dissociated responses may reflect the tumoral heterogeneity and illustrate the influence of tissue-specific tumor microenvironments on response [26]. Thus, capturing different patterns of response and progression at the same time point remains a pitfall of iRECIST criteria. For instance, we found one dissociated response and one pseudoprogression out of three patients with thymic carcinoma, while none was described in the pembrolizumab phase 2 [27]. Dissociated response could be misclassified as progression by iRECIST. In our study, two patients underwent a brain stereotactic radiation before achieving response. Recently, in a cohort of NSCLC patients treated with immune checkpoint inhibitors, Hendricks et al. observed a dissociated cranial-extracranial response in 12.7% of patients with brain metastasis and 0.8% brain pseudoprogression [28]. Therefore, focal treatment of a single progressive lesion for dissociated response should be considered, especially for brain and spinal metastasis [29].
Neither clinical prognostic scores (RMH, GRIm and LIPI score) nor type of progression is relevant to identify these patterns, confirming that circulating biomarkers such as LDH and derived neutrophil to lymphocyte ratio (NLR) are not associated with type of progression [22]. A retrospective analysis conducted on 356 patients with various cancer type in phase I and II trial found that pseudoprogression was associated with a high likehood of 1-year survival compared to patients experiencing typical responses [30]. In our cohort, we showed that pseudoprogression is not associated with better outcomes compare to responder patients without PsPD. Recently, Fujimoto et al. reported similar results among advanced NSCLC patients treated with nivolumab [31]. Patients who experienced PsPD presented a significant shorter median PFS than patients with a typical response (p < 0.001). We confirmed here that the most important feature for prediction of long duration of response is the best overall response, as demonstrated previously [32].
In contrast of these atypical responses, we observed 12.5% of patients with OS less than 12 weeks, defined as fast progression. This rate is comparable with what has been observed in other phase I cohort [10] and phase III trial [16]. The emergence of this new concept could overcome difficulties to identify hyperprogressive disease (HPD) as no consensus exists on the quantitative definition for assessing tumor growth [9, 22, 33–35]. FastPD could be a surrogate marker of HPD to pick out prognostic scores for selecting patients who will not benefit from IO mAb.
Our study highlights the clinical implication of treating beyond progression according iRECIST criteria. Among 40% of patients treated beyond progression, 80% underwent another CT scan at 1 month confirming PD. These results were similarly reported in unresecable melanoma in a pooled analysis of FDA clinical trials. Among 2624 patients receiving immunotherapy on clinical trials allowing for treating beyond RECIST-defined progression, only 14% of the patients had a 30% or more decrease in tumor burden, representing 4% of all patients [36]. Benefit from treatment beyond progression is also supported by observation that progression free-survival cannot adequately capture the benefit of IO mAbs [37]. Meanwhile, our results highlight that responses after initial progression is uncommon. Whether atypical responses support a rational for treating beyond progression, given the risk of delaying an efficient treatment and exposing to immune adverse events, this observation reinforce that treating beyond RECIST1.1 progression should be limited to a number of patients [38].
Prognosis or on-treatment biomarkers are needed to identify early patients who should (or not) continue IO mAb therapy once a first progression is evidenced. Using molecular patterns, ctDNA could be an early tool for the assessment of tumor responsiveness, as it has been demonstrated for chemotherapy [39]. A recent study showed, on 125 melanoma patients on anti PD-1 alone or in combination with ipilimumab, that ctDNA profiles can accurately differentiate pseudoprogression from real progression [40]. An approach integrating hybrid imaging may be considered to discriminate these patterns. An approach integrating hybrid imaging may be considered to discriminate these patterns. Upon the radiological assessment, the complete metabolic response assessed by FDG-PET imaging may better predict long-term outcomes [41]; or the radiomic signature may predict the immune phenotype of tumors and, therefore, potential responders [42].
This study presents a number of limitations. Firstly, it is the retrospective value of our work. We identified retrospectively atypical responses based on data collected prospectively in phase I trials. Another limitation is the lack of biological biomarker analysis such as PD-L1 expression or tumor-infiltrating lymphocytes. For now, no relationship has been demonstrated between displaying an atypical response and PD-L1 expression [12]. The heterogeneity of the population might misestimate the real prevalence among rare histology subtypes. Though, in this wide spectrum of cancers, atypical responses were only described among the subgroup of tumors that are known to be sensitive to anti-PD(L)1 therapies, known as ‘PD-Loma’ [1]. The heterogeneity of therapies might also be a bias in our study, as some IO mAbs combinations have demonstrated to be detrimental [43]. Thus, the percentage of fast progression could be overestimated.
Conclusion
In conclusion, despite this phase I trial cohort might not reflect the clinical routine of patients treated with immunotherapy, these results highlight that outcomes of patients experiencing atypical responses differ from true responder and dissociated responses should be better characterized in clinical trials. Pseudoprogression provides rationale for modifying radiological criteria of response, as iRECIST are proper to capture long-term benefit from IO mAb therapies. However, the low prevalence of patients displaying atypical response should be balanced with the rate of patients treated beyond progression without radiological response.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank all the patients, their families who participated in the trials. We thank also the ESMO annual meeting abstract.
Abbreviations
- CTLA-4
Cytotoxic T-lymphocyte antigen-4
- DisR
Dissociated response
- FastPD
Fast progression
- GRIm score
Gustave Roussy immune score
- HPD
Hyperprogressive disease
- HR
Hazard ratios
- iCPD
Confirmed progressive disease
- IO
Immune-oncology
- iUPD
Unconfirmed progressive disease
- LIPI score
Lung immune prognostic index
- mAb
Antibodies
- NSCLC
Non-small-cell lung carcinoma
- ORR
Overall response rate
- OS
Overall survival
- PD
Progression
- PD-1
Programmed death 1
- PDL-1
Programmed death ligand 1
- PFS
Progression-free survival
- PsPD
Pseudoprogression
- RECIST
Response evaluation criteria in solid tumours
- RMH score
Royal Marsden Hospital score
Author contribution
Conception and study design: ABT, CB, CM. Acquisition, analysis, or interpretation of data: ABT, CB, EC, CM, SA. Statistical analysis: ABT, EC. Drafting and revising the manuscript: ABT, CB, EC, AH, SPV, JMM, AM, JCS, CM, SA. All authors read and approved the final manuscript.
Funding
This research did not receive any specific Grant from funding agencies in the public, commercial, or not-for-profit sectors.
Compliance with ethical standards
Ethical approval
The study was conducted in accordance with the principles of the Helsinki Declaration. All patients included in this retrospective study were treated in clinical trials approved by the French health agency ANSM and an ethical committee. Informed consent was obtained from all participants in the study.
Conflict of interest
CB: personal fees: Sanofi, BMS, Abbvie. EC declares travel grants from Astra Zeneca, BMS, MSD, and Roche and consulting/advisory role for Astra Zeneca, BMS, MSD, and Roche, all outside of the scope of this work. PM: Research Grants from Astrazeneca, BMS, Boehringer Ingelheim, Janssen Cilag, Merck, Novartis, Pfizer, Roche, Sanofi. Non-financial support (drug supplied) from Astrazeneca, Bayer, BMS, Boringher Ingelheim, Johnson & Johnson, Lilly, Medimmune, Merck, NH TherAGuiX, Pfizer, Roche. AH: Principal/sub Investigator of Clinical Trials for A bbvie, A gios P harmaceuticals, Amgen, Argen X Bvba, Arno Therapeutics, Astex Pharmaceuticals, Astra Zeneca, Aveo, Bayer Healthcare Ag, Bbb Technologies Bv, Blueprint Medicines, Boehringer Ingelheim, Bristol Myers Squibb, Celgene Corporation, Chugai Pharmaceutical Co., Cl ovis Oncology, Daiichi Sankyo, Debiopharm S.A., Eisai, Eli Lilly, Exelixis, Forma, Gamamabs, Genentech, Inc., Glaxosmithkline, Gristone Oncology, H3 Biomedicine, Inc, Hoffmann La Roche Ag, Innate Pharma, Iris Servier, Janssen Cilag, Kyowa Kirin Pharm. Dev. Dev., Inc., Loxo Oncology, Lytix Biopharma As, Medimmune, Menarini Merck Serono, Merck Sharp & Dohme Chibret, Merrimack Pharmaceuticals, Merus, Millennium Pharmaceuticals, Nanobiotix, Nektar Therapeutics, Novartis Pharma, Octimet Oncology Nv, Oncoeth ix, Onyx Therapeutics, Orion Pharma, Oryzon Genomics, Pfizer, Pharma Mar, Pierre Fabre, Roche, Sanofi Aventis, Taiho Pharma, Tesaro, Inc, Xencor. Consultant/Advisory role for Amgen, Spectrum Pharmaceuticals, Lilly. Travel and accommodation expenses from Servier, Amgen, Lilly. Courses, trainings for Bayer. SPV: Research Grants from Astrazeneca, BMS, Boehringer Ingelheim, Janssen Cilag, Merck, Novartis, Pfizer, Roche, Sanofi; As part of her research activity, SPV has received research funding from Boehringer Ingelheim, Roche and Merck KGaA for research projects unrelated to this manuscript. SPV has participated to advisory boards for Merck KGaA and has benefited from non-financial support (travel paid and congress registration) for attending symposia from AstraZeneca. JMM personal fees: Bristol-Myers Squibb, AstraZeneca, Janssen. non-financial support: AstraZeneca, Roche, Novartis, Gilead, Celgene, Bristol-Myers Squibb. VR: consultant Gilead, Infinity, MSD, BMS, Epizyme, Nanostring, Incyte, Roche, AstraZeneca, Servier. Research funding from ArgenX. Dr Varga reported advisory board membership for Bristol-Myers Squibb, Pfizer, Roche, AstraZeneca, and Celgene. Dr Champiat reported honoraria from AstraZeneca, Bristol-Myers Squibb, Janssen, MSD, Novartis, and Roche. Dr Marabelle reported scientific advisory board membership for Merck Serono, eTheRNA, Lytix, Kyowa Kirin, Bayer, Novartis, BMS, Symphogen, Genmab, Amgen, Biothera, Nektar, GlaxoSmithKline, Oncovir, Pfizer, Seattle Genetics, Flexus Bio, Roche/Genentech, OSE, Transgene, and Gritstone. JCS: Over the last 5 years, Dr Soria has received consultancy fees from AstraZeneca, Astex, Clovis, GSK, GamaMabs, Lilly, MSD, Mission Therapeutics, Merus, Pfizer, PharmaMar, Pierre Fabre, Roche/Genentech, Sanofi, Servier, Symphogen, and Takeda. Dr Soria has been a full-time employee of AstraZeneca since September 2017. He is a shareholder of AstraZeneca and Gritstone. CM: Consultant/AdvisoryfeesfromAmgen, Astellas, Astra Zeneca, Bayer, BeiGene, BMS, Celgene, Debiopharm, Genentech, Ipsen, Janssen, Lilly, MedImmune, MSD, Novartis, Pfizer, Roche, Sanofi, Orion. Principal/sub-Investigatorof ClinicalTrials for Abbvie, Aduro, Agios, Amgen, Argen-x, Astex, AstraZeneca, Aveopharmaceuticals, Bayer, Beigene, Blueprint, BMS, BoeringerIngelheim, Celgene, Chugai, Clovis, DaiichiSankyo, Debiopharm, Eisai, Eos, Exelixis, Forma, Gamamabs, Genentech, Gortec, GSK, H3 biomedecine, Incyte, InnatePharma, Janssen, Kura Oncology, Kyowa, Lilly, Loxo, Lysarc, LytixBiopharma, Medimmune, Menarini, Merus, MSD, Nanobiotix, NektarTherapeutics, Novartis, Octimet, Oncoethix, OncopeptidesAB, Orion, Pfizer, Pharmamar, Pierre Fabre, Roche, Sanofi, Servier, Sierra Oncology, Taiho, Takeda, Tesaro, Xencor. The rest of the authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hirsch L, Zitvogel L, Eggermont A, Marabelle A. PD-Loma: a cancer entity with a shared sensitivity to the PD-1/PD-L1 pathway blockade. Br J Cancer. 2019;120:3–5. doi: 10.1038/s41416-018-0294-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gong J, Chehrazi-Raffle A, Reddi S, Salgia R. Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: a comprehensive review of registration trials and future considerations. J Immunother Cancer. 2018;6:8. doi: 10.1186/s40425-018-0316-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 4.Nishino M. Tumor response assessment for precision cancer therapy: response evaluation criteria in solid tumors and beyond. Am Soc Clin Oncol Educ Book. 2018 doi: 10.1200/EDBK_201441. [DOI] [PubMed] [Google Scholar]
- 5.Wolchok JD, Hoos A, O’Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412–7420. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 6.Chiou VL, Burotto M. Pseudoprogression and immune-related response in solid tumors. J Clin Oncol. 2015;33:3541–3543. doi: 10.1200/JCO.2015.61.6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferté C, Marabelle A. iRECIST: a clarification of tumour response assessment in the immunotherapy era. Eur J Cancer. 2017;77:165–167. doi: 10.1016/j.ejca.2017.02.015. [DOI] [PubMed] [Google Scholar]
- 8.Borcoman E, Kanjanapan Y, Champiat S, et al. Novel patterns of response under immunotherapy. Ann Oncol Off J Eur Soc Med Oncol. 2019;30:385–396. doi: 10.1093/annonc/mdz003. [DOI] [PubMed] [Google Scholar]
- 9.Champiat S, Ferrara R, Massard C, et al. Hyperprogressive disease: recognizing a novel pattern to improve patient management. Nat Rev Clin Oncol. 2018;15:748–762. doi: 10.1038/s41571-018-0111-2. [DOI] [PubMed] [Google Scholar]
- 10.Massard C, Segal NH, Cho DC, et al. 439P prospective validation of prognostic scores to improve patient selection for immuno-oncology trials. Ann Oncol. 2018 doi: 10.1093/annonc/mdy279.426. [DOI] [Google Scholar]
- 11.Seymour L, Bogaerts J, Perrone A, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18:e143–e152. doi: 10.1016/S1470-2045(17)30074-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hodi FS, Hwu W-J, Kefford R, et al. Evaluation of immune-related response criteria and RECIST v1.1 in patients with advanced melanoma treated with pembrolizumab. J Clin Oncol. 2016;34:1510–1517. doi: 10.1200/JCO.2015.64.0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arkenau H-T, Olmos D, Ang JE, et al. Clinical outcome and prognostic factors for patients treated within the context of a phase I study: the Royal Marsden Hospital experience. Br J Cancer. 2008;98:1029–1033. doi: 10.1038/sj.bjc.6604218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bigot F, Castanon E, Baldini C, et al. Prospective validation of a prognostic score for patients in immunotherapy phase I trials: the Gustave Roussy Immune Score (GRIm-Score) Eur J Cancer. 2017;84:212–218. doi: 10.1016/j.ejca.2017.07.027. [DOI] [PubMed] [Google Scholar]
- 15.Mezquita L, Auclin E, Ferrera R, et al. Association of the lung immune prognostic index with immune checkpoint inhibitor outcomes in patients with advanced non-small cell lung cancer. JAMA Oncol. 2018;4:351–357. doi: 10.1001/jamaoncol.2017.4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gandara DR, Reck M, Morris S, et al. LBA1Fast progression in patients treated with a checkpoint inhibitor (cpi) vs chemotherapy in OAK, a phase III trial of atezolizumab (atezo) vs docetaxel (doc) in 2L + NSCLC. Ann Oncol. 2018 doi: 10.1093/annonc/mdy511. [DOI] [Google Scholar]
- 17.Borcoman E, Nandikolla A, Long G, et al. Patterns of response and progression to immunotherapy. Am Soc Clin Oncol Educ Book. 2018 doi: 10.1200/EDBK_200643. [DOI] [PubMed] [Google Scholar]
- 18.Powles T, Eder JP, Fine GD, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515:558–562. doi: 10.1038/nature13904. [DOI] [PubMed] [Google Scholar]
- 19.Queirolo P, Spagnolo F. Atypical responses in patients with advanced melanoma, lung cancer, renal-cell carcinoma and other solid tumors treated with anti-PD-1 drugs: a systematic review. Cancer Treat Rev. 2017;59:71–78. doi: 10.1016/j.ctrv.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 20.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non–small-cell lung cancer. N Engl J Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nishino M, Ramaiya NH, Chambers ES, et al. Immune-related response assessment during PD-1 inhibitor therapy in advanced non-small-cell lung cancer patients. J Immunother Cancer. 2016;4:84. doi: 10.1186/s40425-016-0193-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferrara R, Mezquita L, Texier M, et al. Hyperprogressive disease in patients with advanced non-small cell lung cancer treated with PD-1/PD-L1 inhibitors or with single-agent chemotherapy. JAMA Oncol. 2018;4:1543. doi: 10.1001/jamaoncol.2018.3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Di Giacomo AM, Danielli R, Guidoboni M, et al. Therapeutic efficacy of ipilimumab, an anti-CTLA-4 monoclonal antibody, in patients with metastatic melanoma unresponsive to prior systemic treatments: clinical and immunological evidence from three patient cases. Cancer Immunol Immunother CII. 2009;58:1297–1306. doi: 10.1007/s00262-008-0642-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Velasco G, Krajewski KM, Albiges L, et al. Radiologic heterogeneity in responses to anti-PD-1/PD-L1 therapy in metastatic renal cell carcinoma. Cancer Immunol Res. 2016;4:12–17. doi: 10.1158/2326-6066.CIR-15-0197. [DOI] [PubMed] [Google Scholar]
- 25.Tazdait M, Mezquita L, Lahmar J, et al. Patterns of responses in metastatic NSCLC during PD-1 or PDL-1 inhibitor therapy: comparison of RECIST 1.1, irRECIST and iRECIST criteria. Eur J Cancer. 2018;88:38–47. doi: 10.1016/j.ejca.2017.10.017. [DOI] [PubMed] [Google Scholar]
- 26.Binnewies M, Roberts EW, Kersten K, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med. 2018;24:541–550. doi: 10.1038/s41591-018-0014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giaccone G, Kim C, Thompson J, et al. Pembrolizumab in patients with thymic carcinoma: a single-arm, single-centre, phase 2 study. Lancet Oncol. 2018;19:347–355. doi: 10.1016/S1470-2045(18)30062-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hendriks LEL, Henon C, Auclin E, et al. Outcome of patients with non-small cell lung cancer and brain metastases treated with checkpoint inhibitors. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer. 2019;14:1244–1254. doi: 10.1016/j.jtho.2019.02.009. [DOI] [PubMed] [Google Scholar]
- 29.Klemen ND, Wang M, Feingold PL, et al. Patterns of failure after immunotherapy with checkpoint inhibitors predict durable progression-free survival after local therapy for metastatic melanoma. J Immunother Cancer. 2019;7:196. doi: 10.1186/s40425-019-0672-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kurra V, Sullivan RJ, Gainor JF, et al. Pseudoprogression in cancer immunotherapy: rates, time course and patient outcomes. J Clin Oncol. 2016;34:6580. doi: 10.1200/JCO.2016.34.15_suppl.6580. [DOI] [Google Scholar]
- 31.Fujimoto D, Yoshioka H, Kataoka Y, et al. Pseudoprogression in previously treated patients with non-small cell lung cancer who received nivolumab monotherapy. J Thorac Oncol. 2019;14:468–474. doi: 10.1016/j.jtho.2018.10.167. [DOI] [PubMed] [Google Scholar]
- 32.Gauci M-L, Lanoy E, Champiat S, et al. Long-term survival in patients responding to anti-PD-1/PD-L1 therapy and disease outcome upon treatment discontinuation. Clin Cancer Res Off J Am Assoc Cancer Res. 2019;25:946–956. doi: 10.1158/1078-0432.CCR-18-0793. [DOI] [PubMed] [Google Scholar]
- 33.Champiat S, Dercle L, Ammari S, et al. Hyperprogressive disease is a new pattern of progression in cancer patients treated by anti-PD-1/PD-L1. Clin Cancer Res. 2017;23:1920–1928. doi: 10.1158/1078-0432.CCR-16-1741. [DOI] [PubMed] [Google Scholar]
- 34.Kato S, Goodman A, Walavalkar V, et al. Hyperprogressors after Immunotherapy: analysis of genomic alterations associated with accelerated growth rate. Clin Cancer Res. 2017;23:4242–4250. doi: 10.1158/1078-0432.CCR-16-3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saâda-Bouzid E, Defaucheux C, Karabajakian A, et al. Hyperprogression during anti-PD-1/PD-L1 therapy in patients with recurrent and/or metastatic head and neck squamous cell carcinoma. Ann Oncol. 2017;28:1605–1611. doi: 10.1093/annonc/mdx178. [DOI] [PubMed] [Google Scholar]
- 36.Beaver JA, Hazarika M, Mulkey F, et al. Patients with melanoma treated with an anti-PD-1 antibody beyond RECIST progression: a US Food and Drug Administration pooled analysis. Lancet Oncol. 2018;19:229–239. doi: 10.1016/S1470-2045(17)30846-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gyawali B, Hey SP, Kesselheim AS. A comparison of response patterns for progression-free survival and overall survival following treatment for cancer with PD-1 inhibitors: a meta-analysis of correlation and differences in effect sizes. JAMA Netw Open. 2018;1:e180416. doi: 10.1001/jamanetworkopen.2018.0416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Borcoman E, Kanjanapan Y, Champiat S, et al. Novel patterns of response under immunotherapy. Ann Oncol. 2019 doi: 10.1093/annonc/mdz003. [DOI] [PubMed] [Google Scholar]
- 39.Cabel L, Proudhon C, Romano E, et al. Clinical potential of circulating tumour DNA in patients receiving anticancer immunotherapy. Nat Rev Clin Oncol. 2018;15:639–650. doi: 10.1038/s41571-018-0074-3. [DOI] [PubMed] [Google Scholar]
- 40.Lee JH, Long GV, Menzies AM, et al. Association between circulating tumor DNA and pseudoprogression in patients with metastatic melanoma treated with anti-programmed cell death 1 antibodies. JAMA Oncol. 2018 doi: 10.1001/jamaoncol.2017.5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tan AC, Emmett L, Lo S, et al. FDG-PET response and outcome from anti-PD-1 therapy in metastatic melanoma. Ann Oncol. 2018 doi: 10.1093/annonc/mdy330. [DOI] [PubMed] [Google Scholar]
- 42.Sun R, Limkin EJ, Vakalopoulou M, et al. A radiomics approach to assess tumour-infiltrating CD8 cells and response to anti-PD-1 or anti-PD-L1 immunotherapy: an imaging biomarker, retrospective multicohort study. Lancet Oncol. 2018;19:1180–1191. doi: 10.1016/S1470-2045(18)30413-3. [DOI] [PubMed] [Google Scholar]
- 43.Shrimali RK, Ahmad S, Verma V, et al. Concurrent PD-1 blockade negates the effects of OX40 agonist antibody in combination immunotherapy through inducing T-cell apoptosis. Cancer Immunol Res. 2017;5:755–766. doi: 10.1158/2326-6066.CIR-17-0292. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.