Abstract
Background & Aims
Micronutrient deficiency (MND) (ie, lack of vitamins and minerals) during pregnancy is a major public health concern. Historically, studies have considered micronutrients in isolation; however, MNDs rarely occur alone. The impact of co-occurring MNDs on public health, mainly in shaping mucosal colonization by pathobionts from the Enterobacteriaceae family, remains undetermined due to lack of relevant animal models.
Methods
To establish a maternal murine model of multiple MND (MMND), we customized a diet deficient in vitamins (A, B12, and B9) and minerals (iron and zinc) that most commonly affect children and women of reproductive age. Thereafter, mucosal adherence by Enterobacteriaceae, the associated inflammatory markers, and proteomic profile of intestines were determined in the offspring of MMND mothers (hereafter, low micronutrient [LM] pups) via bacterial plating, flow cytometry, and mass spectrometry, respectively. For human validation, Enterobacteriaceae abundance, assessed via 16s sequencing of 3-month-old infant fecal samples (n = 100), was correlated with micronutrient metabolites using Spearman’s correlation in meconium of children from the CHILD birth cohort.
Results
We developed an MMND model and reported an increase in colonic abundance of Enterobacteriaceae in LM pups at weaning. Findings from CHILD cohort confirmed a negative correlation between Enterobacteriaceae and micronutrient availability. Furthermore, pro-inflammatory cytokines and increased infiltration of lymphocyte antigen 6 complex high monocytes and M1-like macrophages were evident in the colons of LM pups. Mechanistically, mitochondrial dysfunction marked by reduced expression of nicotinamide adenine dinucleotide (NAD)H dehydrogenase and increased expression of NAD phosphate oxidase (Nox) 1 contributed to the Enterobacteriaceae bloom.
Conclusion
This study establishes an early life MMND link to intestinal pathobiont colonization and mucosal inflammation via damaged mitochondria in the offspring.
Keywords: Enterobacteriaceae, Maternal Micronutrient Deficiencies, Mitochondrial Dysfunction, Subclinical Inflammation
Graphical abstract
Summary.
A novel mouse model of co-existing micronutrient deficiencies in pregnant mothers demonstrated the transgenerational consequences of micronutrient availability on colonization by pathobionts and sub-clinical inflammation in the gut.
An estimated 33% of the world population suffers from some form of malnutrition, defined as a dietary imbalance in the intake of nutrients including macronutrients (proteins, fats, and carbohydrates) or micronutrients (vitamins and minerals). Micronutrient deficiency (MND) rarely occurs alone and more frequently presents as multiple co-occurring deficiencies of vitamins and minerals.1,2 MNDs of the greatest public concerns, due to their significant impact on morbidity and mortality, are vitamin A, vitamin D, iron, zinc, folate (vitamin B9), vitamin B12, and iodine, notably among women of reproductive age and children under the age of 5 years.1 Although MNDs are more prevalent in low- and middle-income countries, industrialized nations are also susceptible as well due to the imbalanced diets, which are calorically high with low nutrient value.2, 3, 4, 5, 6, 7, 8 MND during pregnancy carries significant risks to the overall health of both the mother and the unborn fetus.9 Pregnant women are at increased risk of MNDs, as the estimated average requirement for most micronutrients increase substantially to support fetal growth and development.10, 11, 12 Furthermore, the developmental origins of health and disease theory suggests that perturbation in maternal nutrition such as MND during early life (first 2 years from conception) may skew gut microbial composition of the offspring towards an inflammatory phenotype.13 Whether maternal MNDs can affect intestinal colonization by pathobionts, such as Enterobacteriaceae, in the offspring remains to be explored.
Enterobacteriaceae is a family of bacteria encompassing potentially pathogenic commensals, also known as pathobionts.14 Enrichment of Enterobacteriaceae members is a known hallmark of dysbiotic intestines in malnourished children.15 Increased colonization by Enterotoxigenic E. coli, Shigella, and Salmonella spp. contributed to villous blunting, epithelial damage, and increased intestinal permeability in stunted Zambian children (<2 years old).16 Likewise, increased Enterobacteriaceae colonization in children from rural Zimbabwe resulted in environmental enteric dysfunction, a disease marked by intestinal inflammatory damage, loss of intestinal barrier, and microbial translocation to systemic sites.17 Furthermore, enhanced intestinal epithelial injury and turnover due to colonization by Campylobacter jejuni and Enteroaggregative E. coli may have contributed to reduced weight- and length-for-age ratios in children from Enteric Infections and Malnutrition and the Consequences for Child Health and Development (MAL-ED) cohort.17,18 Because most of the previous studies have focused on the contribution of either macronutrient malnourishment or MND in isolation, it remains unexplored whether early-life maternal MNDs (MMNDs) can shape the mucosal colonization of Enterobacteriaceae members and thus regulate intestinal inflammation in the offspring. Unfortunately, high-level investigation of MMNDs in humans is impossible for ethical reasons. Therefore, animal models can provide a biologically relevant alternative to investigate these intricacies.
This study describes a novel murine clinical model of MMND. We have further determined the impact of maternal micronutrients on the mucosal blooming of Enterobacteriaceae and on the intestinal inflammatory status of the offspring (F1) at weaning. In a validation study, we also confirmed a negative correlation between Enterobacteriaceae and micronutrient availability in human infants.
Results
Development of a Novel Maternal Murine MMND Model
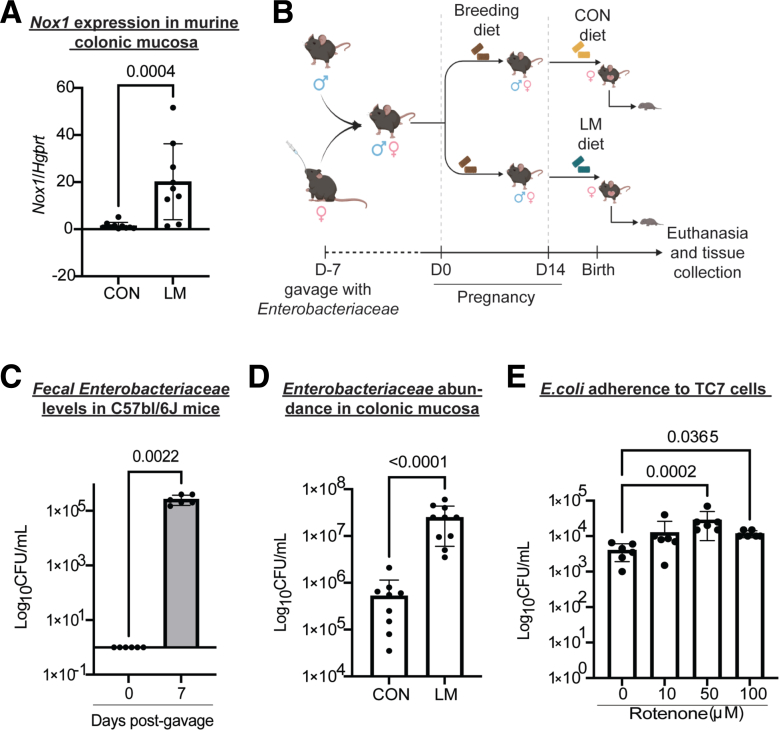
Co-occurring MMNDs during pregnancy are undeniably detrimental to child growth and development. Here, we developed a murine maternal MMND model using multiple micronutrients of public concern: vitamin A, vitamin B12, vitamin B9, zinc, and iron, via 2 different approaches (in Materials & Methods section and Figure 1A). For model 1, we noticed that feeding or administering the low micronutrient (LM) diets to female mice prior to mating led to either spontaneous abortion or cannibalization of pups soon after delivery (Table 1), underscoring the importance of micronutrient sufficiency in pregnant mothers. For model 2 (Figure 1A), we were able to successfully breed the mice on an LM diet, by first supplementing with a standard breeding diet, followed by the experimental diets at day 14 of pregnancy. The timeline of the newly developed model (ie, late gestation through weaning) was rationally adapted to remain appropriate for studying the host-microbe interactions at intestinal mucosa.19, 20, 21 Phenotypically, LM pups were characterized by reduced body hair with unnoticeable differences in weight (Figure 1B) and tail length (Figure 1C; as marker of stunting) compared with control (CON) pups. Although the role of malnourishment in reducing fertility rate or fecundity in mothers remains to be established,22 the specific role of maternal multiple MNDs on offspring health outcomes has not been explored either. In the newly developed MMND model, we determined lower fecundity in LM mothers with significantly smaller average litter size (∼3.5 pups) compared with CON mothers (∼5.5 pups) (Figure 1D). Overall, we showed a successful approach to developing a maternal murine MMND model to study host-microbe interactions.
Figure 1.
Development and characterization of murine model of MMNDs.A, A schematic of the model. A bar graph representing weight in grams (B), tail length in mm (C), and average litter size (D) of pups at weaning in LM (∼31% males and 69% females) and CON (∼40% males and 60% females) groups. D, Litter size was ascertained by- ‘total pups/total mothers in respective CON or LM group’ for 4 different experiments. Data are mean ± standard deviation (n = 13–20 mice/group; 2 independent experiments) and P < .05 (Mann-Whitney test) was considered significant.
Table 1.
Pregnancy Outcome in MMND Model 1
| Group | Bred | Aborted | Cannibalized |
|---|---|---|---|
| CON | 6 of 6 | 0 of 6 | 0 of 6 |
| LM | 6 of 6 | 2 of 6 | 4 of 6 |
CON, Control; LM, low micronutrient; MMND, maternal murine model of multiple micronutrient deficiencies.
MMND Promotes Enterobacteriaceae Abundance in Colons of LM Offspring
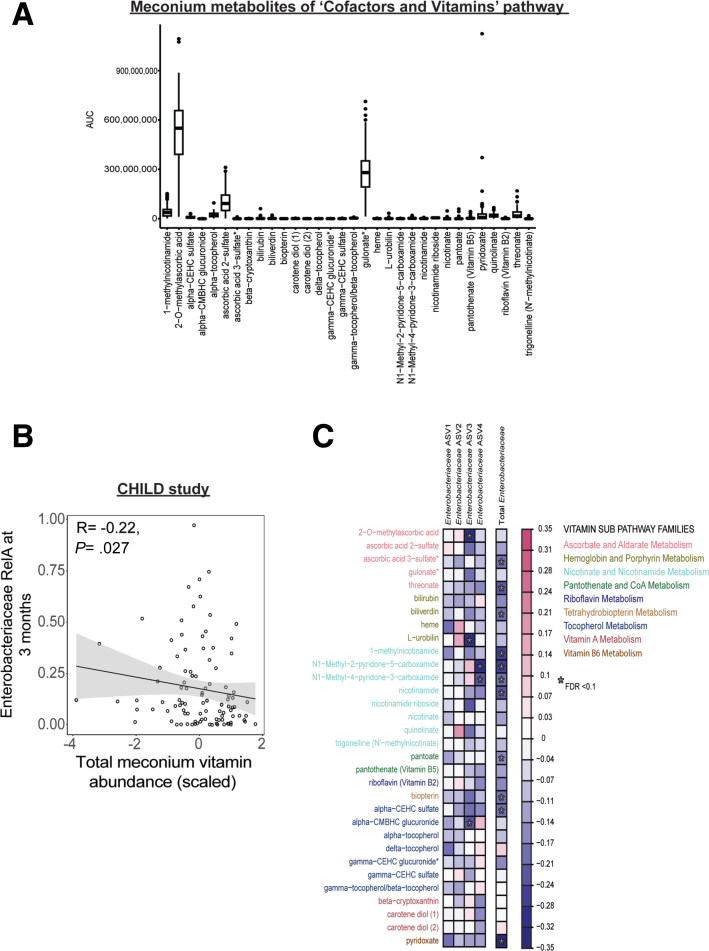
Our lab has previously shown that malnourished diet deficient in proteins and fats increases barrier permeability and small intestinal abundance of Enterobacteriaceae in mice model of environmental enteropathy.23 However, the specific intergenerational role of MMND in promoting Enterobacteriaceae colonization in the offspring is understudied.18 In the newly developed MMND model, we determined an increase in the colonic mucosa-associated and fecal Enterobacteriaceae levels in the LM pups compared with CON pups at weaning (Figure 2A and 2B, respectively). Additionally, bacterial families including, Atopobiaceae, Gastranaerophilales, Lactobacillaceae, Peptostreptococcaceae, Ruminococcaceae, and Tannerellaceae were increased, whereas Bacteroidaceae, Enterococcaceae, and Lachnospiraceae decreased in the feces of LM vs CON pups (Figure 2C and Table 2). Next, using a subset of 100 children from the CHILD cohort, we sought to validate whether micronutrient availability within the gut has downstream impacts on Enterobacteriaceae blooming. We determined individual abundances of the 32 detected metabolites that belonged to co-factors and vitamins family (Table 3 and Figure 3A). Supporting our animal findings, we observed statistically significant negative correlations for total or some individual vitamin levels (r = −0.22; P = .027) in the meconium and Enterobacteriaceae abundance at 3 months of age (Figure 3B and 3C, respectively). Previous multi-cohort studies in children with moderate acute malnutrition have found elevated serum markers of inflammation, such as cytokines and C-reactive protein, with no overt signs of diseases.24,25 Similarly, we determined a sub-clinical inflammation in the intestine of LM pups characterized by no signs of gross pathology (Figure 4A); however, the colon length was relatively shorter than CON pups (Figure 4B). Furthermore, pro-inflammatory cytokines Tnf-α and KC, but not Il-6, expressions were enhanced in the colonic mucosa (Figure 4C). Next, we measured cytokine production in the blood plasma of LM and CON mice as a proxy for systemic inflammation. There was an increase in TNF-α and IL-6, a decrease in anti-inflammatory IL-10, and no change in monocyte chemoattractant protein-1, IFN-γ, and IL-12p70 cytokines in LM vs CON pups (Figure 4D). Interestingly, gross histopathology (Figure 5A), Enterobacteriaceae abundance (Figure 5B), and pro-inflammatory cytokine expressions (Figure 5C) were not statistically different in LM vs CON ilea. In conclusion, this novel MMND model primarily showed increased colonic abundance of Enterobacteriaceae, which was accompanied by a constitutive subclinical inflammation in the offspring.
Figure 2.
MMNDs lead to mucosal colonization by Enterobacteriaceae in the colon of LM offspring.A–B, Bar plots representing Enterobacteriaceae (A) colony forming unit (CFU) count in colonic mucosa as log10 CFU/mL, normalized to total tissue weight (n = 10–17 mice/group; 2 independent experiments), and B, relative abundance as derived via 16S rRNA sequencing of fecal pellets (n = 5–7 mice/group, repeated once) in CON and LM pups. C, A stacked bar plot representing top 10 differentially abundant bacterial families in LM and CON pups (n = 5–7 mice/group). Data are mean ± standard deviation, and P (Mann-Whitney test) or q (Benjamini-Hochberg correction) < .05 was considered significant.
Table 2.
Differentially Abundant Bacterial Families in Feces of LM vs CON Pups
| Family | Association | P value | q value |
|---|---|---|---|
| Lachnospiraceae | Negative | .003560818 | 0.008011841 |
| Tannerellaceae | Positive | .023902671 | 0.041117848 |
| Ruminococcaceae | Positive | 4.95E-09 | 4.46E-08 |
| Enterococcaceae | Negative | .025127574 | 0.041117848 |
| Eggerthellaceae | Positive | 1.38E-18 | 2.49E-17 |
| Enterobacteriaceae | Positive | .002521347 | 0.006483465 |
| Peptostreptococcaceae | Positive | 4.38E-08 | 2.63E-07 |
| Lactobacillaceae | Positive | 7.48E-04 | 0.002243123 |
| Rikenellaceae | Negative | .413549236 | 0.46524289 |
| Erysipelotrichaceae | Negative | .363649339 | 0.436379207 |
| Erysipelatoclostridiaceae | Negative | .21994483 | 0.28278621 |
| Bacteroidaceae | Negative | .024269377 | 0.041117848 |
| Akkermansiaceae | Positive | .044168527 | 0.065596702 |
| Clostridiaceae | Negative | .047375396 | 0.065596702 |
| Sutterellaceae | Negative | .832988526 | 0.832988526 |
| Muribaculaceae | Positive | .78237105 | 0.828392877 |
| Atopobiaceae | Positive | 5.63E-05 | 2.03E-04 |
| Gastranaerophilales | Positive | 1.04E-05 | 4.70E-05 |
CON, Control; LM, low micronutrient.
Table 3.
List of Metabolites From Cofactors and Vitamins Super Pathway
| Biochemical | Sub Pathway |
|---|---|
| Pyridoxate | Vitamin B6 metabolism |
| Beta-cryptoxanthin | Vitamin A metabolism |
| Carotene diol (1) | Vitamin A metabolism |
| Carotene diol (2) | Vitamin A metabolism |
| Alpha-CEHC sulfate | Tocopherol metabolism |
| Alpha-CMBHC glucuronide | Tocopherol metabolism |
| Alpha-tocopherol | Tocopherol metabolism |
| Delta-tocopherol | Tocopherol metabolism |
| Gamma-CEHC glucuronide | Tocopherol metabolism |
| Gamma-CEHC sulfate | Tocopherol metabolism |
| Gamma-tocopherol/beta-tocopherol | Tocopherol metabolism |
| Biopterin | Tetrahydrobiopterin metabolism |
| Riboflavin (vitamin B2) | Riboflavin metabolism |
| Pantoate | Pantothenate and CoA metabolism |
| Pantothenate (vitamin B5) | Pantothenate and CoA metabolism |
| 1-methylnicotinamide | Nicotinate and nicotinamide metabolism |
| N1-Methyl-2-pyridone-5-carboxamide | Nicotinate and nicotinamide metabolism |
| N1-Methyl-4-pyridone-3-carboxamide | Nicotinate and nicotinamide metabolism |
| Nicotinamide | Nicotinate and nicotinamide metabolism |
| Nicotinamide riboside | Nicotinate and nicotinamide metabolism |
| Quinolinate | Nicotinate and nicotinamide metabolism |
| Trigonelline (N'-methylnicotinate) | Nicotinate and nicotinamide metabolism |
| Bilirubin | Hemoglobin and porphyrin metabolism |
| Biliverdin | Hemoglobin and porphyrin metabolism |
| Heme | Hemoglobin and porphyrin metabolism |
| L-urobilin | Hemoglobin and porphyrin metabolism |
| 2-O-methylascorbic acid | Ascorbate and aldarate metabolism |
| Ascorbic acid 2-sulfate | Ascorbate and aldarate metabolism |
| Ascorbic acid 3-sulfate | Ascorbate and aldarate metabolism |
| Gulonate | Ascorbate and aldarate metabolism |
| Threonate | Ascorbate and aldarate metabolism |
Note: Super pathway for all metabolites = cofactors and vitamins.
Figure 3.
Micronutrient-associated metabolites in meconium negatively correlate with the fecal Enterobacteriaceae abundance in CHILD cohort.A, A box plot representing AUCs for all the 32 metabolites from CHILD study. B, A scatter plot depicting a negative correlation between total vitamin levels in meconium of children at birth, and Enterobacteriaceae levels in feces at 3 months of age in CHILD cohort. C, A heat map representing correlations among vitamins/vitamin-associated metabolites and multiple amplicon sequencing variants (ASVs) of Enterobacteriaceae. A Spearman correlation test was performed, and P < .05 or q < 0.1 was considered significant (n = 100).
Figure 4.
MMNDs lead to subclinical inflammation in the colon of LM offspring.A, H&E microphotography of colons of CON and LM pups at 60X magnification (scale bar = 100 μm). B, A bar graph showing length of colons (in mm) from LM and CON pups at weaning. C–D, Expression of inflammatory cytokines in colons (C) and blood plasma (D) of CON and LM pups were determined by qPCR (C) or CBA bead-based assay (D). C, Data were normalized to Hgprt housekeeping gene. D, The data were quantitated by flow cytometry and analyzed using FlowJo software. Data are mean ± standard deviation (n = 10–17 mice/group; 2 independent experiments), and P < .05 (Mann-Whitney test) was considered significant.
Figure 5.
Maternal MNDs do not promote Enterobacteriaceae colonization or inflammation in the ileum of the pups.A, H&E microphotography of ileums of CON and LM pups at 60× magnification (scale bar = 100 μm). B, A bar graph representing Enterobacteriaceae count in ileal mucosa of CON and LM pups as log10 CFU/mL, normalized to total tissue weight. C, Expression of pro-inflammatory cytokines (Tnf-α, KC, and Il-6) in ileums of CON and LM offspring as determined by qPCR. Data were normalized to Hgprt housekeeping gene. Data are mean ± standard deviation (n = 8–11 mice/group), and P < .05 (Mann-Whitney test) was considered significant.
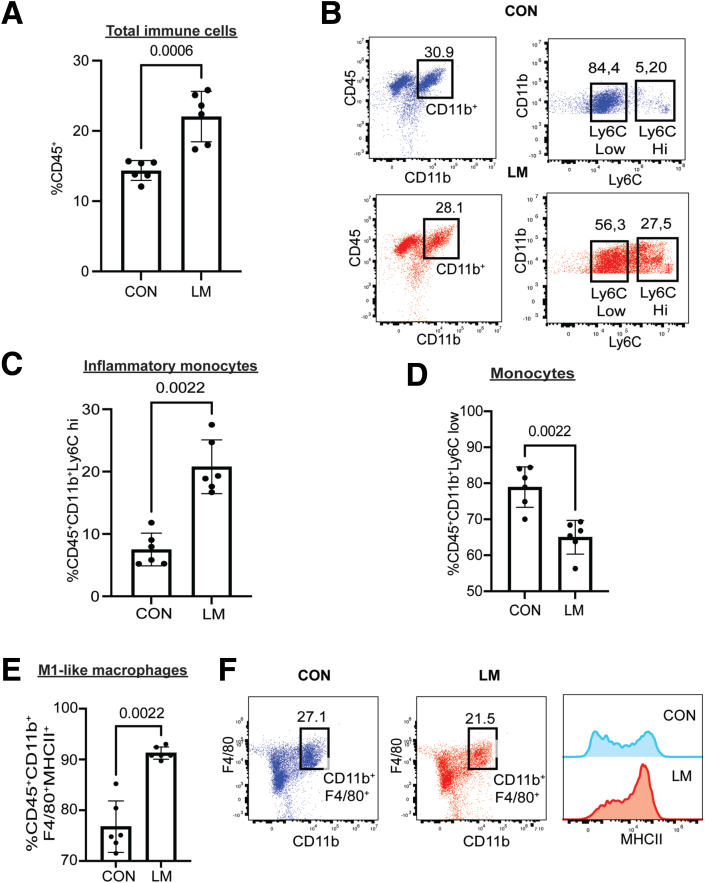
Ly6C ii Monocytes and M1-like Macrophages are Recruited in the Colon of LM Pups
Since we observed increased colonization by Enterobacteriaceae, specifically in LM colons, we next determined the immune cell profile at the colonic mucosa in the newly developed MMND model. We noticed an overall increase in colonic infiltration by immune cells (CD45+) (Figure 6A), suggesting a constitutive inflammatory stimulus in LM colon. Poor activation ability of helper (CD4+) and killer (CD8+) T-cells, reduced antibody productions, and increased pro-inflammatory monocyte/macrophage populations have routinely been reported in malnourished individuals.26, 27, 28 Of interest, MNDs may promote differentiation of monocytic cells into pro-inflammatory phenotypes such as high Lymphocyte antigen 6 complex (Ly6C hi)-expressing cells, which are commonly recruited to the target tissue during chronic inflammation by a chemokine monocyte chemoattractant protein-1.29, 30, 31 In the novel MMND model, we noticed an increase in colonic proportions of pro-inflammatory monocytes (CD45+CD11b+Ly6C hi) with a corresponding decrease in Ly6C low monocyte population (CD45+CD11b+Ly6C mild) in LM vs CON pups (Figure 6B–D). Furthermore, a concomitant increase in colonic mucosal proportion of macrophages (CD45+CD11b+F4/80+) expressing major histocompatibility factor II (pro-inflammatory M1-like macrophages) was evident (Figure 6E–F). Additionally, we found that proportions of natural killer cells (CD45+CD3-NK1.1+), which are important in mounting anti-bacterial defense against enteric gram-negative bacteria,32 were increased in colons of LM vs CON pups (Figure 7A). Interestingly, populations of adaptive immune cells, including antibody-producing B cells (CD45+CD19+) (Figure 7B), total T-cells (CD45+CD3+) (Figure 7C), CD4 T-cells (CD45+CD3+CD4+CD8-) (Figure 7D), and CD8 T-cells (CD45+CD3+CD4-CD8+) (Figure 7E) remained relatively unchanged in LM pups compared with CON pups. Together, LM pups had a chronic inflammatory phenotype characterized by an increase in mainly innate immune cell types, mostly Ly6C hi monocytes and M1-like macrophages in the colonic mucosa.
Figure 6.
MMNDs increase recruitment of total immune cells, Ly6C hi monocytes, and inflammatory M1 macrophages in the colons of the pups.A–F, Dot plots and graphs showing relative proportions of CD45+ (A), CD45+CD11b+Ly6C hi (B and C), CD45+CD11b+Ly6C low (B and D), and CD45+CD11b+F4/80+MHCII+ (E and F) cells in colonic mucosa of LM and CON pups. The data were quantitated by flow cytometry and analyzed using FlowJo software. Data are mean ± standard deviation (n = 6 mice/group), and P < .05 (Mann-Whitney test) was considered significant.
Figure 7.
MMNDs modulate recruitment of various immune cells in the colons of the LM pups.A–H, Graphs showing relative proportions of, CD45+CD3-NK1.1+ (A), CD45+CD19+ (B), CD45+CD3+ (C), CD45+CD3+CD4+CD8- (D), and CD45+CD3+CD4+CD8+ (E) cells in colonic mucosa of LM and CON pups. The data were quantitated by flow cytometry and analyzed using FlowJo software. Data are mean ± standard deviation (n = 6 mice/group), and P < .05 (Mann-Whitney test) was considered significant.
Mitochondrial Dysfunction in the Colon Contributes to the Mucosal Blooming of Enterobacteriaceae in LM Pups
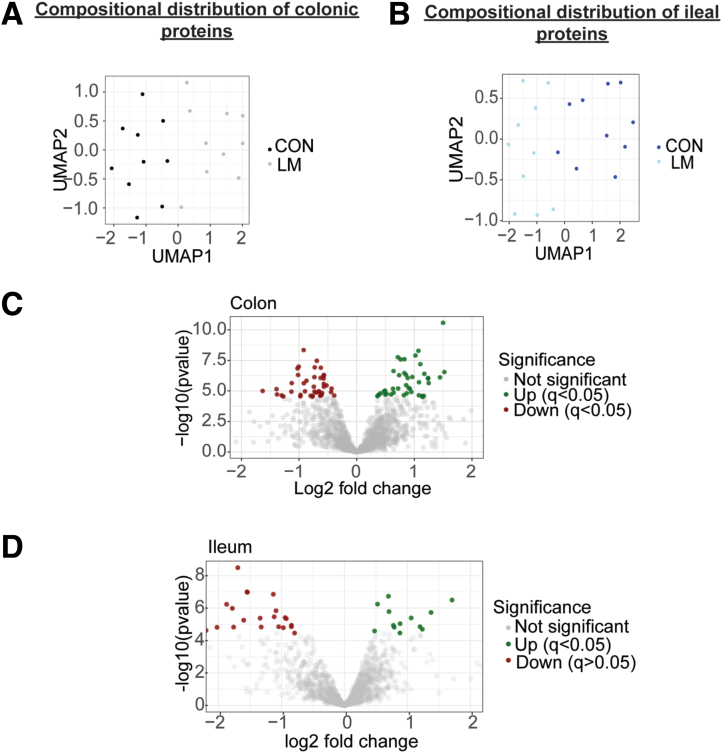
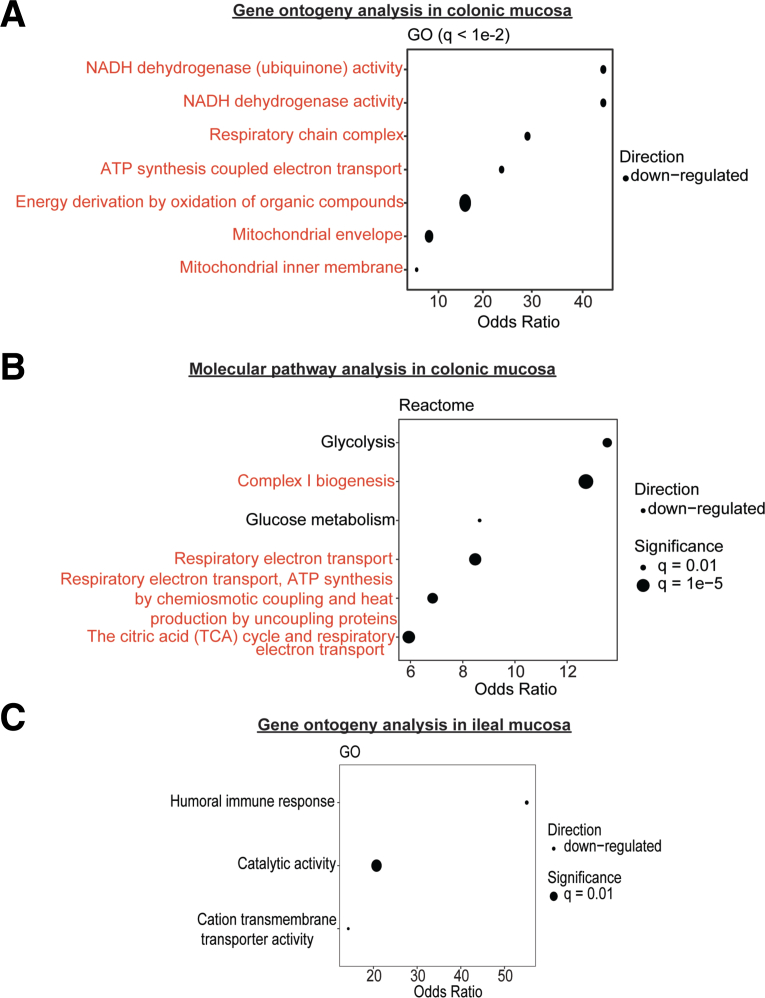
Next, we performed data-dependent acquisition proteomics to determine potential mechanisms of enhanced colonization by Enterobacteriaceae in the newly developed MMND model. We observed a distinct pattern of clustering for CON and LM groups on Uniform Manifold Approximation and Projection density plots for both colon and ileal proteins, confirming tissue-specific protein profiles (Figure 8A–B). Of 85 differentially abundant colonic proteins (q < 0.05), approximately one-half of them (42) were downregulated in the LM group (Figure 8C and Table 4). On the other hand, there were only 41 differentially abundant ileal proteins, of which 14 were upregulated and 27 were downregulated in LM vs CON groups (Figure 8D and Table 5). Gene ontogeny (GO) analysis identified that most protein clusters downregulated in LM colons belonged to mitochondrial functioning and bioenergetics, particularly related to nicotinamide adenine dinucleotide hydrogen (NADH) dehydrogenase/mitochondrial complex I and the electron transport chain (Figure 9A). Reactome-based molecular pathway analysis further confirmed dysfunctional mitochondrial and oxidative phosphorylation signaling in LM pups (Figure 9B). Although GO analysis in LM ilea only identified a total of 3 terms that passed FDR correction (Figure 9C), and there were no significant reactome-based molecular pathways, further suggesting that our novel MMND model has tissue-specific effects. Poor NADH dehydrogenase/mitochondrial complex I functioning can significantly enhance mitochondrial reactive oxygen species (ROS) production.33,34 Furthermore, mitochondrial ROS accumulation can activate nicotinamide adenine dinucleotide phosphate oxidase 1 (NOX1) enzyme, which is widely expressed in the plasma membrane of the intestinal epithelium and enhances oxygen-free radical synthesis in colonic cytoplasm and lumen.35 We confirmed that Nox1 expression was upregulated in LM vs CON colonic mucosa (Figure 10A). Because increased ROS generation in gut lumen can selectively promote intestinal abundance of Enterobacteriaceae family,36,37 we tested whether mitochondrial dysfunction, and hence ROS generation, may promote attachment of commensal bacteria using two different models: (1) in vivo in C57BL/6J mice which are characterized by a spontaneous mutation in nicotinamide nucleotide transhydrogenase enzyme and abnormal ROS production38; and (2) in vitro in human colonic TC7 epithelial cells. We determined that C57Bl/6J mice were successfully colonized at day 7 post gavage with Enterobacteriaceae isolated from the feces of C57Bl/6N mice (Figure 10B–C). Next, we found that C57Bl/6J LM pups had strikingly higher levels of colonic mucosa-associated Enterobacteriaceae (∼2 log folds) (Figure 10D). Similarly, we found that inhibition of mitochondrial complex I by rotenone pre-treatment enhanced attachment of commensal E. coli to TC7 cells in a concentration-dependent manner (Figure 10E). Because micronutrient supplementation or fortification are commonly employed strategies to tackle MNDs in an affected population, we wanted to understand if a persistent nutrient-rich environment could reinstate mitochondrial functioning and prevent pathobiont colonization in LM pups. To do this, we developed a diet-reversal model, wherein the LM pups were transferred to the CON diet for 4 weeks after weaning (Figure 11A). We found that colonic Nox 1 expression (Figure 11B) reverted to dCON level in LMCON pups. Similarly, colon length and pro-inflammatory cytokine Il-6 expression in LMCON pups were statistically similar to dCON pups (Figure 11C–D, respectively), whereas expressions of Tnf-a and KC were not detected (Figure 11D). However, the relative abundance of Enterobacteriaceae was still higher in feces of LMCON vs dCON pups (Figure 12A), albeit to a lesser extent than LM C57Bl/6N pups at weaning (Figure 1B). Additionally, we noticed significant differences in abundance of other bacterial families, which are listed in Figure 12B and Table 6. Next, we isolated colonic crypts from LM pups and developed organoids ex vivo in a nutrient-rich media to simulate physiological environment. Though Nox1 expression was consistent (Figure 12C), no quantitative difference in the E. coli attachment was evident in LM vs CON pups-derived organoid cultures (Figure 12D), which was contrary to the in vivo reversal experiment. Overall, poor maternal micronutrient availability damages mitochondria in the offspring by reducing NADH dehydrogenase expression. Consequently, luminal ROS synthesizing Nox1 enzyme expression increases in the epithelium, which may enhance colonic abundance of Enterobacteriaceae in the offspring. Furthermore, persistent nutrient availability partially reverses the deleterious effects of MMND by reducing inflammation and minimizing Enterobacteriaceae colonization.
Figure 8.
MMNDs lead to altered global protein expressions in the intestines of the LM offspring.A–B, Uniform Manifold Approximation and Projection scatter plots showing spatial distribution of CON and LM groups based on proteomics profiling of colon (A) and ileum (B). B–C, Volcano plots depicting upregulated (green), downregulated (red), and unaltered (gray) proteins in colon (B) and ileum (C) of pups born to LM mother compared with corresponding CON controls. Data are mean ± standard deviation (n = 10 mice/group), and q value < 0.05 (limma package in R and Benjamini-Hochberg correction) was considered significant.
Table 4.
A List of Differentially Abundant Colonic Proteins in LM Pups
| Protein ID | Gene name | Effect size (log2) | P-value | q-value | Direction of change |
|---|---|---|---|---|---|
| Q9DC69 | Ndufa9 | −1.0171818698351500 | 4.9176643570658E-07 | 7.90268662180475E-04 | Down-regulated |
| Q8BH04 | Pck2 | −0.5698744019772810 | 4.5456137025968E-06 | 0.007213888946021120 | Down-regulated |
| P62889 | Rpl30 | 0.38498391842565500 | 1.98783985519615E-05 | 0.031050058538163900 | Up-regulated |
| Q9DC16 | Ergic1 | −0.7290496384786000 | 1.17047415233173E-07 | 1.89148623016808E-04 | Down-regulated |
| Q91YT0 | Ndufv1 | −1.3020189692086000 | 2.33146903187949E-05 | 0.03627765813604490 | Down-regulated |
| E9Q616 | Ahnak | 0.94758354213423000 | 1.21340442893264E-05 | 0.019086851667110500 | Up-regulated |
| E9Q9C6 | Fcgbp | 1.2391346856646700 | 8.8119032098072E-07 | 0.0014090233232481700 | Up-regulated |
| Q91VS7 | Mgst1 | 0.5169999137432440 | 1.71667750067879E-05 | 0.026866002885623100 | Up-regulated |
| O35074 | Ptgis | −1.0277880624131500 | 1.43606521713443E-07 | 2.31780926045498E-04 | Down-regulated |
| O35295 | Purb | −0.7443545869244500 | 4.53387529502636E-06 | 0.00719979396850186 | Down-regulated |
| O55126 | Gbas | −0.6252080318095650 | 2.35180144316423E-05 | 0.03657051244120380 | Down-regulated |
| O70400 | Pdlim1 | 0.6393353942556060 | 1.4123260318744E-05 | 0.022159395440109300 | Up-regulated |
| O70456 | Sfn | 0.901495129389748 | 5.64221767132574E-06 | 0.00894855722672263 | Up-regulated |
| O88329 | Myo1a | 1.0781662909397500 | 2.26912480894703E-05 | 0.035330273275305200 | Up-regulated |
| O88342 | Wdr1 | 0.3587833745556320 | 2.68767102640811E-05 | 0.041578270778533400 | Up-regulated |
| P00329 | Adh1 | 1.144143516850340 | 3.17051783309298E-05 | 0.04892109016462470 | Up-regulated |
| P00920 | Ca2 | 0.9217420465877650 | 6.65085042176938E-06 | 0.010521645367239200 | Up-regulated |
| P05202 | Got2 | −0.39110976834573700 | 2.35933415415862E-05 | 0.036664052755625000 | Down-regulated |
| P06151 | Ldha | 0.7619493983424560 | 2.60699784756597E-08 | 4.22333651305687E-05 | Up-regulated |
| P08207 | S100a10 | 0.8172296887581710 | 2.22777437329802E-05 | 0.03470872473598320 | Up-regulated |
| P09411 | Pgk1 | 0.63829507239324 | 2.34658440979959E-07 | 3.78504065300674E-04 | Up-regulated |
| P0DP28 | Calml3 | 0.9650128495014000 | 7.18148488529362E-07 | 0.0011519101756011000 | Up-regulated |
| P10639 | Txn | 1.0709637307592300 | 5.17442677382889E-09 | 8.40326908069812E-06 | Up-regulated |
| P11688 | Itga5 | −0.7660890165059910 | 2.94283621103085E-05 | 0.04543739109831640 | Down-regulated |
| P14824 | Anxa6 | −0.601833921491811 | 1.46513193834636E-05 | 0.022973268793271000 | Down-regulated |
| P16110 | Lgals3 | 0.8671999751166040 | 1.57784847876247E-05 | 0.02470910717742030 | Up-regulated |
| P16460 | Ass1 | −1.273999972640000 | 2.8363554950416E-05 | 0.043821692398392800 | Down-regulated |
| P16858 | Gapdh;Gm3839 | 0.49179961132435600 | 9.34289493363338E-06 | 0.014761773995140700 | Up-regulated |
| P17182 | Eno1 | 0.4141117279066980 | 1.57279240854768E-05 | 0.024645657041942100 | Up-regulated |
| P17751 | Tpi1 | 0.4769660851008110 | 1.0744454679598E-05 | 0.016944005029726100 | Up-regulated |
| P19324 | Serpinh1 | −0.8716923425011680 | 1.47080155918761E-06 | 0.0023473992884634200 | Down-regulated |
| P22437 | Ptgs1 | −1.1283606740510700 | 2.28642441206065E-06 | 0.003642274088412610 | Down-regulated |
| P24270 | Cat | −1.3831652655920200 | 1.93923318169876E-05 | 0.030329606961768500 | Down-regulated |
| P31786 | Dbi | 1.5202170499579300 | 2.85414602571919E-07 | 4.60088339345933E-04 | Up-regulated |
| P42125 | Eci1 | −0.5755705444302770 | 5.83628353829163E-07 | 9.36723507895806E-04 | Down-regulated |
| P47856 | Gfpt1 | 1.019288940373460 | 1.24927660913274E-08 | 2.02757593662244E-05 | Up-regulated |
| P48036 | Anxa5 | −0.6099245258318020 | 2.06676215445047E-05 | 0.032241489609427300 | Down-regulated |
| P48758 | Cbr1 | 0.6292103260257740 | 6.59438582685158E-06 | 0.010438912763906100 | Up-regulated |
| P48962 | Slc25a4 | −0.6590987072353540 | 1.21812478751988E-05 | 0.019148921659812500 | Down-regulated |
| P52196 | Tst | −0.7320663029720220 | 1.12555269406664E-05 | 0.01772745493154960 | Down-regulated |
| P52480 | Pkm | 0.7138800492018810 | 1.74193542393247E-08 | 2.82541925761846E-05 | Up-regulated |
| P54071 | Idh2 | −0.5235004273740410 | 3.52845918071537E-06 | 0.005606721638156720 | Down-regulated |
| P56391 | Cox6b1 | 1.1037399123232500 | 6.44934726972134E-08 | 1.04350438824091E-04 | Up-regulated |
| P84089 | Erh | 1.248695986080910 | 2.27717533297001E-06 | 0.0036298174807542000 | Up-regulated |
| P99027 | Rplp2 | 1.4979517453160000 | 2.63240828100999E-11 | 4.28292827320326E-08 | Up-regulated |
| P99028 | Uqcrh | 1.4452349299947400 | 7.77795280650818E-07 | 0.0012460280396026100 | Up-regulated |
| Q3UQ44 | Iqgap2 | 1.237715263384300 | 9.73624453263659E-07 | 0.0015548782518620600 | Up-regulated |
| Q91V92 | Acly | 1.1646027714965200 | 2.55782944481409E-05 | 0.039620778100170300 | Up-regulated |
| Q68FD5 | Cltc | 0.8273326749240670 | 2.53546579080991E-08 | 4.10999004690286E-05 | Up-regulated |
| Q62351 | Tfrc | 3.3549532892756100 | 4.49519458491449E-09 | 7.30918639507096E-06 | Up-regulated |
| Q80X90 | Flnb | 0.8841179150567020 | 8.74911304281274E-07 | 0.0013998580868500400 | Up-regulated |
| Q8BFR5 | Tufm | −0.4368504158444770 | 6.4626739489949E-06 | 0.010236875535207900 | Down-regulated |
| Q8BH59 | Slc25a12 | −0.6233638809428940 | 1.26535189211404E-07 | 2.04354330576417E-04 | Down-regulated |
| Q8BMD8 | Slc25a24 | −0.574032620457047 | 9.61895563488708E-07 | 0.001537109110454960 | Down-regulated |
| Q8BMK4 | Ckap4 | −0.9781753610744740 | 2.78969823242334E-05 | 0.043128734673264800 | Down-regulated |
| Q8BWT1 | Acaa2 | −0.692756315751157 | 3.383847483537E-08 | 5.4784490758464E-05 | Down-regulated |
| Q9WUA3 | Pfkp | 0.7189705613722490 | 6.29890048358158E-06 | 0.009983757266476800 | Up-regulated |
| Q8K0C9 | Gmds | 0.7356656555620930 | 5.12874086820523E-07 | 8.23675783433759E-04 | Up-regulated |
| Q8K3J1 | Ndufs8 | −1.0061373325053900 | 1.02419070768762E-07 | 1.65611637433088E-04 | Down-regulated |
| Q8QZT1 | Acat1 | −0.571845237423549 | 2.66429838502315E-06 | 0.00423889873057183 | Down-regulated |
| Q8R0Y6 | Aldh1l1 | 1.173311316002950 | 3.97439816102578E-07 | 6.39878103925151E-04 | Up-regulated |
| Q91V61 | Sfxn3 | −0.9213072890036610 | 4.51384641994155E-09 | 7.33500043240502E-06 | Down-regulated |
| Q91VD9 | Ndufs1 | −1.3910446323582500 | 7.10073967628065E-06 | 0.011226269428199700 | Down-regulated |
| Q91WD5 | Ndufs2 | −1.6323070402188000 | 1.00580034534228E-05 | 0.015881587452954600 | Down-regulated |
| Q91WT7 | Akr1c14 | 1.1302618764599300 | 2.57428488310453E-05 | 0.039849929990458100 | Up-regulated |
| Q922Q1 | Marc2 | −0.7085594828559300 | 1.37694752110807E-05 | 0.021618076081396700 | Down-regulated |
| Q99JY0 | Hadhb | −0.6265326237259230 | 8.66169998318574E-07 | 0.0013867381673080400 | Down-regulated |
| Q99KI0 | Aco2 | −0.6557037199393300 | 1.06588726954952E-05 | 0.016819701113491400 | Down-regulated |
| Q99L13 | Hibadh | −0.4454361110506540 | 1.24809544353259E-05 | 0.019607579417897000 | Down-regulated |
| Q99LC3 | Ndufa10 | −0.9184319316054970 | 2.35818943668034E-06 | 0.0037542375831951100 | Down-regulated |
| Q99N15 | Hsd17b10 | −0.5685713190996870 | 4.89526166226531E-07 | 7.87158075292262E-04 | Down-regulated |
| Q9CPQ8 | Atp5l | 0.4954045300547160 | 2.02182453626773E-05 | 0.031560681011139300 | Up-regulated |
| Q9CQA3 | Sdhb | −0.9755228267565400 | 2.10735449918714E-05 | 0.032853656642327500 | Down-regulated |
| Q9CQC7 | Ndufb4 | −0.7660451147218130 | 2.46096055910836E-05 | 0.03819410787736170 | Down-regulated |
| Q9D0K2 | Oxct1 | −0.7341814642884000 | 7.40434837410179E-07 | 0.0011869170443685200 | Down-regulated |
| Q9D154 | Serpinb1a | 0.369172207048607 | 2.43904252245649E-05 | 0.03787833037374930 | Up-regulated |
| Q9D819 | Ppa1 | 0.8554100243039610 | 4.79262454698919E-07 | 7.7113328961056E-04 | Up-regulated |
| Q9DCS9 | Ndufb10 | −0.6338937050514080 | 2.47728623173612E-05 | 0.03842270945422720 | Down-regulated |
| Q9DCT2 | Ndufs3 | −1.1335976585544300 | 1.14749848015516E-05 | 0.018061626077642300 | Down-regulated |
| Q9EQ20 | Aldh6a1 | −0.7998924145386340 | 2.48728290588783E-05 | 0.03855288504126140 | Down-regulated |
| Q9ERS2 | Ndufa13 | −0.8978429192454760 | 1.07889170036085E-05 | 0.017003333197686900 | Down-regulated |
| Q9JKF1 | Iqgap1 | 0.8249988991999700 | 3.40303292750374E-07 | 5.48228604620852E-04 | Up-regulated |
| Q9QUH0 | Glrx | 0.5952811334929240 | 1.97719899559554E-05 | 0.030903620301158200 | Up-regulated |
| Q9R100 | Cdh17 | 0.8577598326879210 | 3.38306900834554E-06 | 0.005379079723269400 | Up-regulated |
| Q9Z1Q9 | Vars | 1.080446902068260 | 1.9482174423313E-06 | 0.0031074068205184200 | Up-regulated |
LM, Low micronutrient.
Table 5.
A List of Differentially Abundant Ileal Proteins in LM Pups
| Protein ID | Gene name | Effect size (log2) | P-value | q-value | Direction of change |
|---|---|---|---|---|---|
| Q60605 | Myl6 | 1.2370989878864900 | 2.01281730362787E-05 | 0.028883928307060000 | Up-regulated |
| Q91YT0 | Ndufv1 | −0.8433293806317380 | 1.40842026326072E-05 | 0.020323504398852200 | Down-regulated |
| E9Q509 | Pklr | −1.1300153338196000 | 1.40375777454968E-07 | 2.05790889748983E-04 | Down-regulated |
| Q6ZWZ6 | Rps12 | 0.698585731327696 | 1.85930237916802E-07 | 2.72387798548114E-04 | Up-regulated |
| F8VPQ6 | Alpi | −4.004047567210490 | 1.83343230426363E-05 | 0.02632808788922580 | Down-regulated |
| F8VPT3 | Lct | −4.202408985704460 | 2.06092072667672E-06 | 0.0029986396573146300 | Down-regulated |
| G3UZP7 | H2-D1;H2-L | 1.2005892358631700 | 1.40594705102753E-05 | 0.020301875416837600 | Up-regulated |
| Q9R0P3 | Esd | −1.7605285136884600 | 1.49006931828689E-05 | 0.021456998183331200 | Down-regulated |
| O55142 | Rpl35a | 0.7074803931822540 | 1.65422220916579E-06 | 0.0024102017587545600 | Up-regulated |
| O70475 | Ugdh | −2.751201326462120 | 3.4236213696455E-09 | 5.03272341337889E-06 | Down-regulated |
| O88312 | Agr2 | 0.790212914668354 | 1.51591376337715E-05 | 0.02181399905499720 | Up-regulated |
| P03930 | Mtatp8 | 1.0597763357486900 | 4.05621400904059E-06 | 0.005889622741126940 | Up-regulated |
| P12787 | Cox5a | 0.8836041756435050 | 9.14052870878679E-06 | 0.013235485570323300 | Up-regulated |
| P24270 | Cat | −1.693930533094730 | 3.22382719790435E-09 | 4.7422498081173E-06 | Down-regulated |
| P24549 | Aldh1a1 | −1.0447106634966300 | 1.41518172923822E-05 | 0.020406920535615200 | Down-regulated |
| P28271 | Aco1 | −0.7917686970933590 | 3.45645356990867E-05 | 0.04946185058539300 | Down-regulated |
| P28843 | Dpp4 | −0.9711436627995890 | 1.62290410551764E-05 | 0.023321131996288500 | Down-regulated |
| P29758 | Oat | −0.9232173792610570 | 4.47535539220895E-06 | 0.0064892653187029700 | Down-regulated |
| P34884 | Mif | 0.8806908186675840 | 3.43356753663742E-05 | 0.0491686871246479 | Up-regulated |
| P34914 | Ephx2 | −2.93616928671433 | 2.83950119037278E-07 | 4.15702974270575E-04 | Down-regulated |
| P48758 | Cbr1 | −1.3223961841851400 | 1.48001184186236E-05 | 0.02132697064123650 | Down-regulated |
| P49290 | Epx | −2.646435499246080 | 5.74808605994743E-07 | 8.39795373358319E-04 | Down-regulated |
| P51660 | Hsd17b4 | −0.8419867889953800 | 1.1426957744384E-05 | 0.016523380898379300 | Down-regulated |
| P52825 | Cpt2 | −1.7806838624063500 | 1.03831899808693E-06 | 0.00151490741820883 | Down-regulated |
| P54869 | Hmgcs2 | −2.6987032108217300 | 1.09994149071065E-05 | 0.01591615337058310 | Down-regulated |
| Q921G7 | Etfdh | −1.0869424581697400 | 1.45685514603955E-06 | 0.0021240948029256600 | Down-regulated |
| Q7M758 | Naaladl1 | −1.8716158073509200 | 5.8252018817094E-07 | 8.50479474729572E-04 | Down-regulated |
| Q8BW75 | Maob | −1.5478150075831100 | 1.00016052348323E-07 | 1.46823564847338E-04 | Down-regulated |
| W4VSN6 | Defa22;Defa21 | 2.425464679176370 | 1.2267908162544E-08 | 1.80215570907771E-05 | Up-regulated |
| Q8K157 | Galm | −1.59980361971755 | 5.58192983560826E-06 | 0.008088216331796370 | Down-regulated |
| Q8VC30 | Dak | −1.5423677136468100 | 1.06035568786154E-07 | 1.55554179409288E-04 | Down-regulated |
| Q921I1 | Tf | 1.7093276560266400 | 3.1771181971545E-07 | 4.64812392243703E-04 | Up-regulated |
| Q9CPQ8 | Atp5l | 0.7772787473575950 | 1.16516349550101E-05 | 0.01683661250998960 | Up-regulated |
| Q9CQ19 | Myl9 | 1.3754825179014200 | 1.86302361070536E-06 | 0.002712562377187010 | Up-regulated |
| Q9CZX8 | Rps19 | 0.5251096537064370 | 5.69304993283564E-07 | 8.32323900180571E-04 | Up-regulated |
| Q9DBM2 | Ehhadh | −0.942097479342048 | 3.87778797764859E-06 | 0.005634425931523400 | Down-regulated |
| Q9EST5 | Anp32b | 0.47853237702428100 | 2.58439135165197E-05 | 0.03703432806917270 | Up-regulated |
| Q9JIL4 | Pdzk1 | −2.02425223337327 | 1.53678379104192E-05 | 0.022098950915182800 | Down-regulated |
LM, low micronutrient.
Figure 9.
MMNDs induce mitochondrial dysfunction in the colons but not ilea of the LM pups.A–B, GO (top 7 groups) (A) and Reactome (B) analyses showing gene groups (A) and molecular pathways (B) that were relatively downregulated in the colons of LM pups compared with the CON group. Mitochondria-related terms are highlighted in red. C, GO analysis showing differentially downregulated gene groups in the ilea of LM vs CON groups. Data are mean ± standard deviation (n = 10 mice/group), and q value < 0.05 (hypergeometric test and Benjamini-Hochberg correction) was considered significant.
Figure 10.
Intestinal mitochondrial dysfunction contributes to E. coli attachment to the epithelium.A, Nox1 gene expression was quantified in colons of C57Bl/6N LM and CON pups using qPCR. Hgprt was used as housekeeping control. B, A schematic of the MMND experiment with C57Bl/6J mice. C–D, Bar plots representing Enterobacteriaceae colony forming unit (CFU) count in colonic mucosa of C57Bl/6J female parents at Day 0 and 7 post-gavage (C) and C57Bl/6J CON and LM pups at weaning (D) as log10 CFU/mL, normalized to total tissue weight. E, Human colonic TC7 cells were pre-treated with rotenone at variable doses (as indicated) for 3 hours, followed by infection with commensal E. coli for 2 hours (n = 2, in triplicates). A bar graph representing absolute count of E. coli as log10 CFU/mL. Data are mean ± standard deviation (n = 9–10 mice/group, unless mentioned otherwise in respective figure), and P value < .05 (Mann-Whitney or Kruskal-Wallis test) was considered significant.
Figure 11.
Reversal of MMNDs lead to normalization of Nox1 expression and inflammatory markers in the colons of LM offspring.A, A schematic of the diet reversal model in mice. B, Nox1 gene expression was quantified in colons of dCON and LMCON pups using qPCR. C, A bar graph showing length of colons (in mm) from dCON and LMCON mice. D, Expression of pro-inflammatory cytokines Il-6 , Tnf-α, and KC in colons of dCON and LMCON mice as determined by qPCR. B and D, Hgprt was used as housekeeping control. Data are mean ± standard deviation (n = 8α10 mice/group), and P-value < .05 (Mann-Whitney test) was considered significant.
Figure 12.
Reversal of MMNDs did not normalize increased Enterobacteriaceae in the colon of LM offspring but reversed E.coli attachment in colonic organoids.A, A bar graph representing relative abundance of Enterobacteriaceae derived via 16S rRNA sequencing of fecal pellets from dCON and LMCON pups. B, A stacked bar plot representing top 10 differentially abundant bacterial families in dCON and LMCON pups. C and D, Nox1 gene expression (C), and absolute count of E. coli as log10 CFU/mL (D) in colonic organoids from LM and CON pups (n = 4–6 mice/group). Hgprt was used as housekeeping control. Data are mean ± standard deviation (n = 8–10 mice/group, unless mentioned otherwise in respective figure), and P- or q-value < .05 (Mann-Whitney or Kruskal-Wallis test) was considered significant.
Table 6.
Differentially Abundant Bacterial Families in Feces of LMCON vs dCON Pups
| Family | Association | P value | q_value |
|---|---|---|---|
| Rikenellaceae | Negative | .293886374 | 0.391848499 |
| Streptococcaceae | Negative | .087369472 | 0.145615786 |
| Tannerellaceae | Negative | .533401548 | 0.561475314 |
| Enterobacteriaceae | Negative | 4.18E-06 | 2.79E-05 |
| Peptostreptococcaceae | Negative | .01571997 | 0.034933266 |
| Marinifilaceae | Positive | .567382059 | 0.567382059 |
| Lactobacillaceae | Positive | .021072556 | 0.042145113 |
| Staphylococcaceae | Negative | 1.29E-19 | 2.57E-18 |
| Deferribacteraceae | Positive | 1.41E-04 | 5.64E-04 |
| Enterococcaceae | Negative | 1.65E-08 | 1.65E-07 |
| Erysipelatoclostridiaceae | Positive | .476459053 | 0.561475314 |
| Bacteroidaceae | Positive | .005359202 | 0.013398006 |
| Akkermansiaceae | Positive | .2113315 | 0.325125385 |
| Bifidobacteriaceae | Positive | 9.59E-04 | 0.00319821 |
| Clostridiaceae | Positive | 3.29E-05 | 1.64E-04 |
| Sutterellaceae | Positive | .513729959 | 0.561475314 |
| Erysipelotrichaceae | Positive | .520469479 | 0.561475314 |
| Lachnospiraceae | Positive | .003475805 | 0.009930871 |
| Eggerthellaceae | Positive | .032376401 | 0.058866183 |
| Muribaculaceae | Negative | .25724017 | 0.367485957 |
dCON, CON pups continued on CON diet; LMCON, LM pups transferred to CON diet.
Discussion
In this study, we developed a model of early life MMNDs, suitable for studying host-microbe interactions. Although we chose to focus on offspring microbiome and gut inflammation, this model is well-poised to aid the scientific community in future studies concerning the role of early life MND in adult metabolic diseases. We showed that inadequate maternal micronutrient availability increased the intestinal Enterobacteriaceae colonization and the associated gut and systemic inflammatory signature in the LM offspring. The inflammation was characterized by increased cytokine production and colonic infiltration of chronic Ly6C hi monocytes and pro-inflammatory M1-like macrophages. Lastly, mitochondrial dysfunction, particularly decreased in NADH dehydrogenase/mitochondrial complex I production, and increased ROS-producing Nox1 enzyme expression coincided with Enterobacteriaceae blooming in the offspring colons.
Our model was based on a previous study from our lab, which characterized the role of micronutrients in host growth, metabolism, and gut microbiome in mice.39 Micronutrients interact to influence absorption and bioavailability of each other, and hence, co-existing and increased deficiencies in minerals and vitamins are likely.1,39, 40, 41, 42, 43 However, previous maternal animal models involving micronutrients are based on deficiency/supplementation of only 1 or 2 micronutrient(s) and are unable to interrogate the deleterious outcomes of multiple co-existing MNDs. A recent maternal model of single MND shows that gestational vitamin C deficiency induces aberrant DNA methylation in fetal germ-cell development, resulting in lower oocyte numbers and reduced fecundity rate in the female offspring.44 Likewise, a model of gastric intrinsic factor (Gif)-deficient C57Bl/6 mice, which lack the capacity to absorb vitamin B12, has previously been developed to study gut immunological and developmental changes in the offspring.45 Our MMND model not only combines multiple micronutrient deficiencies, but also captures the intergenerational effects of maternal malnutrition on pathobiont colonization and enteric inflammation. Furthermore, the LM pups did not exhibit any overt signs of stunting, such as reduced tail length and body weight, perhaps due to the specific time frame of the model and the absence of more severe forms of malnutrition.39,46
MMND substantially contributed to the intestinal blooming of Enterobacteriaceae and subclinical inflammation in the offspring. Pathobionts and colitogenic bacteria from the Enterobacteriaceae family, such as Salmonella and Escherichia spp., are often over-represented in the intestines of children from malnourished settings.1,37 It has been inferred from the MAL-ED cohort that the prevalence of pathobionts in non-diarrheal stool of children from undernourished countries increases by more than 2-fold from birth to 8 to 24 months of age.18 Likewise, a recent re-analysis of the Global Enteric Multi-center Study (GEMS) has shown a strong association between diarrhea-causing members of Enterobacteriaceae family, such as E. coli, and poor nutritional status of the children (<5 years) from countries in Africa and Asia.47 We observed Enterobacteriaceae tissue-specific (colon vs ileum) colonization patterns, which we hypothesize may be attributed to the interactions among different commensals in an MND environment.48, 49, 50 Previous work from our lab has shown that Enterobacteriaceae and Bacteroidales spp. grow cooperatively in a protein and iron-deficient environment.23,51 Additionally, increased expression of defensins antimicrobial peptides in ileal mucosa (refer to Table 5) may have limited the growth of Enterobacteriaceae in LM ilea. Such site-specific increase in Enterobacteriaceae colonization enhanced the infiltration of immune cells, expression of pro-inflammatory cytokines (TNF-α and IL-6), and reduction in anti-inflammatory (IL-10) cytokine expression in colons of LM pups, perhaps via an altered ‘weaning reaction.52 Furthermore, the increased recruitment of Ly6C hi monocytes and M1-like macrophages may prime a chronic pro-inflammatory response at colonic mucosa by making the host permissive to co-infections, hence elevating the risk of chronic disorders in adulthood. Giardia lamblia persistently colonizes intestines of protein-malnourished mice, thereby promoting mucosal recruitment of cd11b+ monocyte/macrophage populations and co-infection by enteroaggregative E. coli.53
Enteric pathobionts are rampant in children from impoverished and undernourished nations. The molecular mechanisms for increased susceptibility to pathogens remain poorly understood but could be related to mitochondrial dysfunction. Malnutrition has long been known to negatively regulate mitochondrial functions.35,54,55 A low-protein diet, where 1% of total calories come from protein sources, reduces mitochondrial complexes I, IV, and V expression in mice, causing hepatic steatosis by inhibiting nicotinamide adenine dinucleotide-dependent sirtunin activity.56 Mitochondrial dysfunction in the offspring may arise from inefficient vertical transfer of nutrients or via inheritance of epigenetic changes occurring during pregnancy.54,57, 58, 59, 60 In a recent human study conducted on mother-infant pairs in Germany, maternal vitamin B12 and folic acid levels significantly correlated with corresponding micronutrients in the offspring.61 Vitamin B12 and folate are important mediators in synthesis of S-adenosylmethionine, an indispensable methyl donor for DNA methylation reactions during epigenetic modifications.62 Both maternal zinc and folic acid levels regulate methylation of long interspersed element-1 (LINE-1; a surrogate for assessing global DNA methylation) in the offspring,63,64 which seems to be positively associated with the birth weight and neurological function.64,65 Malnutrition affects mitochondrial function, which disturbs the balance between ROS and antioxidant productions.35,54,55,66 Blood samples from children (<5 years) with clinically severe acute malnutrition have relatively lower antioxidant and higher mitochondrial DNA levels.67 We observed reduced mitochondrial NADH dehydrogenase levels and increased ROS synthesizing Nox1 enzyme expression in LM pup colons, which may have contributed to the colonization of Enterobacteriaceae. Moreover, inherent abnormality of mitochondrial functioning in C57Bl/6J mice may have further contributed to the increased the levels of colonic mucosa-associated Enterobacteriaceae in the corresponding LM pups. This suggests that mitochondria may play a critical role in shaping and establishing the intestinal microbiome. Previous studies have shown that mitochondrial ROS accumulation enhances cytoplasmic NOX1 activity, and hence, increases oxygen free radical generation in human embryonic kidney 293T cells.68 Interestingly, facultative anaerobic bacteria, like members of Enterobacteriaceae, can utilize H2O2 to facilitate intimate attachment to intestinal epithelium and subsequent colonization of mucosa.34 Citrobacter rodentium anaerobically reduces NOX1 generated H2O2 via cytochrome c peroxidase, to outcompete resident commensals and attach to colonic epithelium in mice during early infection.69 It is possible that utilization of ROS by Enterobacteriaceae, along with increased abundance of short-chain fatty acid producing bacteria, such as Lactobacillaceae and Enterococcaceae, may have played a role in minimizing intestinal damage caused by the oxidative environment in LM pups.70, 71, 72 Furthermore, despite normalization of Nox1 expression upon feeding of CON diet, the abundance of intestinal Enterobacteriaceae remained high. Our findings align with the ‘window of opportunity’ and developmental origins of health and disease hypotheses, indicating that the gut microbiota composition is particularly responsive to early-life environmental factors like diet, with reduced plasticity in the microbiome thereafter.73,74 Such differences in Enterobacteriaceae attachment were not evident in ex vivo cultures, perhaps attributed to the normal Nox1 expression and inter-individual differences in the procedure for growing organoids, which likely overshadowed the phenotype.
The newly developed MMND model is highly relevant and broadly applicable to the undernourished children in low- and middle-income countries; however, there are a few limitations. First, pectin was used in LM diet to minimize reabsorption of vitamin B12, which can modify microbial abundances in the gut.75 We compensated for this by supplementing CON diet with cellulose, another type of fiber known to affect the microbiome.76 In our studies of the gut microbiome, we only focused here on Enterobacteriaceae, which are poor fiber fermenters.77 Second, meconium samples were used as a proxy for maternal nutritional status from the CHILD study due to the inaccessibility of blood and fecal samples from pregnant mothers. Third, although relative abundances represent important information regarding the composition of the microbiome,78 it is possible that changes in Enterobacteriaceae measured in the CHILD samples could be due to inhibited growth of other, less abundant bacteria. Lastly, while no overt pathology was seen in the small intestine (primary site for micronutrients absorption) of LM pups; however, the observed proteomic changes in the ilea of LM pups suggest that the MMND model could be used to further interrogate mechanistic changes in the tissue as a result of malnutrition.
Taken together, we developed an intriguing early life MMND model that showed mitochondrial dysfunction as a major contributor to the intestinal blooming of Enterobacteriaceae in the offspring. This novel MMND model facilitates research into MMNDs, shedding light on their potential public health implications, which were previously difficult to investigate in human populations.
Materials and Methods
Ethics Statement
All studies in mice were conducted as per the Canadian Council on Animal Care regulations and were approved by the University of British Columbia Animal Care Committee (A22-0057/A22-0168). The CHILD Study protocols were approved by the human clinical research ethics boards (H07-03120) at all universities and institutions directly involved with the CHILD cohort (McMaster University, University of British Columbia, the Hospital for Sick Children, University of Manitoba, and University of Alberta).
Murine Model and Tissue Processing
Seven-week-old C57Bl/6N and C57Bl/6J littermate male and female mice were purchased from Charles Rivers and the Jackson Laboratory (Jax), respectively. C57Bl/6J mice were acquired from the maximum barrier facility at Jax. Based on previous findings from our lab,39 2 experimental approaches were undertaken to develop the early-life MMND model using C57Bl/6N mice: In model 1, mice were randomly assigned to either a CON or an LM diet deficient in vitamin A, vitamin B12, vitamin B9, zinc, and iron (#D18062501I and #D19041709I, respectively; Research Diets Inc) (Table 7). Because gut resident bacteria can synthesize vitamin B12 and mice are coprophagic, pectin fiber was added to LM diet to prevent reabsorption of vitamin B12, whereas cellulose fiber was added to CON diet as a corresponding control.79 Next, the mice were mated in trios (2 females/male), and all the pregnant mice remained on their respective diets for a total of 42 ± 2 days, starting from the day of mating until weaning. In model 2, mice were mated in a trio while on a breeder’s diet (#0007689; PicoLab Mouse Diet 20 5058, LabDiet). At day 14 of pregnancy (counted from the day a viscous vaginal plug was visible to confirm mating), mice were randomized and switched to either CON or LM treatment diet. All pregnant mice remained on their respective diets for a total of 28 ± 2 days (from day 14 of pregnancy until weaning). For C57Bl/6J mice experiments, model 2 was followed. In agreement with previous findings from our lab, C57Bl/6J mice were determined to lack culturable levels of commensal Enterobacteriaceae in their intestines,80 possibly due to variation in hygienic conditions among different barrier facilities at Jax. Microbial shifts during animal transportation and the diverse facility conditions found at various institutes and universities may also impact gut microbiota composition.81,82 Subsequently, female C57Bl/6J mice were orally administered Enterobacteriaceae (5 × 108 CFU/100 uL; one gavage 7 days prior to mating) isolated from female C57Bl/6N fecal pellets via plating onto McConkey agar. Mice were given ad libitum access to the diet throughout the experiment. At weaning, murine pups were weighed (g), tail lengths (mm) measured, and later sacrificed (∼ 3 weeks old) to ascertain colon length (mm) and harvest tissues for downstream analysis. For the diet-reversal experiment, model 2 was employed except that, after weaning, both LM and CON pups were put on CON diet (hereafter, LMCON and dCON pups, respectively) for 4 weeks before being euthanized for tissue collection.
Table 7.
Murine Diet Compositions
| Product # | D18062501 |
D19041709 |
||
|---|---|---|---|---|
| CON diet |
LMCON w/ pectin |
|||
| gm% | kcal% | gm% | kcal% | |
| Protein | 18.9 | 20 | 19.2 | 20 |
| Carbohydrate | 63.1 | 65 | 61.7 | 62 |
| Fat | 6.5 | 15 | 6.6 | 15 |
| Total | 100 | 100 | ||
| kcal/gm | 3.77 | 3.77 | ||
| Ingredient | gm | kcal | gm | kcal |
|---|---|---|---|---|
| Casein | 0 | 0 | 0 | 0 |
| Egg white | 203 | 812 | 203 | 812 |
| L-cystine | 0 | 0 | 0 | 0 |
| Corn starch | 346 | 1384 | 332.7 | 1330.8 |
| Maltodextrin 10 | 45 | 180 | 45 | 180 |
| Dextrose | 250 | 1000 | 250 | 1000 |
| Sucrose | 0 | 0 | 0 | 0 |
| Cellulose, BW200 | 75 | 0 | 22 | 0 |
| Inulin | 25 | 25 | 25 | 25 |
| Pectin, tic gums | 0 | 0 | 53 | 53 |
| Soybean oil | 70 | 630 | 70 | 630 |
| Mineral mix S10026 | 10 | 0 | 0 | 0 |
| Mineral mix S19427 (No Ca, P, K, Zn, or Fe) | 0 | 0 | 10 | 0 |
| Dicalcium phosphate | 13 | 0 | 13 | 0 |
| Calcium carbonate | 5.5 | 0 | 5.5 | 0 |
| Potassium citrate, 1 H2O | 16.5 | 0 | 16.5 | 0 |
| Ferric citrate (17.4% Fe) | 0 | 0 | 0.029 | 0 |
| Zinc carbonate (52.1% Zn) | 0 | 0 | 0.004 | 0 |
| Vitamin mix V10001 | 10 | 40 | 0 | 0 |
| Vitamin mix V15927 (No vitamin A, folate, or B12) | 0 | 0 | 10 | 40 |
| Vitamin mix V15928 (350 IU A, 3 ug B12, 0.11 mg folate) | 0 | 0 | 0 | 0 |
| Biotin, 1% | 0.4 | 0 | 0.4 | 0 |
| Choline bitartrate | 2 | 0 | 2 | 0 |
| Pure red dye #40 | 0 | 0 | 0 | 0 |
| Pure blue dye #1 | 0 | 0 | 0.05 | 0 |
| Pure yellow dye #5 | 0.05 | 0 | 0 | 0 |
| Total | 1071.45 | 4071 | 1058.183 | 4071 |
CON, Control; LM, low-micronutrient; LMCON, LM pups transferred to CON diet.
16S rRNA Sequencing of Murine Feces
DNA was extracted using the QIAamp PowerFecal Pro DNA kit (51804, Qiagen) as recommended. The V4 region of bacterial 16s rDNA was amplified using indexed, barcoded primers (515F: GTGCCAGCMGCCGCGGTAA; 806R: GGACTACHVHHHTWTCTAAT). Samples were then pooled, and the library was sequenced on an Illumina MiSeq platform using a NextSeq2000 600 cycle P1 kit. Raw reads were quality-filtered and processed using DADA2 implemented in QIIME2.83,84 Taxonomy was assigned using a Naive Bayes classifier trained on the SILVA 138 database of the 515/806 region at 100% amplicon sequence variants (ASV) cutoffs.85 Further filtration was performed in R using the phyloseq package to remove singletons and rarefy samples to a uniform sequencing depth of 8000 reads.86 Differential abundance analysis was conducted on unrarefied samples using DESeq2 (version 1.34.0).87 Results were visualized using the ggplot288 package in R.
Determination of Enterobacteriaceae in Intestinal Mucosa
Both colon and ileum were aseptically removed, followed by perfusion with sterile phosphate buffered saline (PBS) without Ca2+ and Mg2+ (1x; Thermo Fisher Scientific; hereafter PBS) and later, distal tissues (∼1–2 cm) were homogenized in PBS (50 mg/mL). Colonies were grown and quantitated on MacConkey agar (#B212123; BD Biosciences). Data were log-transformed and represented as log10 colony forming units (CFU/mL).
RNA Isolation and Gene Transcription of Pro-inflammatory Mediators
RNA was extracted using RNeasy kit (74104, Qiagen) as recommended. Quantitative polymerase chain reaction (qPCR) was performed using SYBR Green (#208054; Qiagen) as recommended by manufacturer. The primers used for qPCR are listed in Table 8.89, 90, 91, 92 Hypoxanthine-guanine phosphoribosyl transferase (Hgprt) was used as an internal control for normalization.
Table 8.
List of Primers Used in the Study
| Gene | Forward | Reverse |
|---|---|---|
| Tnf-a | GCCTCTTCTCATTCCTGCTTG | CTGATGAGAGGGAGGCCATT |
| Il-6 | ACGGCCTTCCCTACTTCACA | CATTTCCACGATTTCCCAGA |
| KC | AAAAGGTGTCCCCAAGTA | AAGCAGAACTGAACTACCATCG |
| Nox 1 | CCTCCTGACTGTGCCAAAGG | ATTTGAACAACAGCACTCACCAA |
| Hgprt | TCAGTCAACGGGGGACATAAA | GGGGCTGTACTGCTTAACCAG |
Cytometric Bead Array Assay
Cytokines were measured in serum samples by flow cytometry using a BD Cytometric Bead Array (CBA) Mouse Inflammation Kit (552364; BD Biosciences) according to the manufacturer’s instructions.
Histology
Distal ileum and colon tissues were fixed in 10% neutral-buffered formalin and embedded in paraffin. Slides were prepared with 5-micron thick sections and stained commercially at Wax-it Histology Services Inc. Hematoxylin-eosin (H&E) images were taken using BX53 light microscope (Olympus) at 60× magnification in tile-format.
Intestinal Immune Cell Isolation and Flow Cytometry
Intestinal immune cells were isolated as described previously.93 Briefly, intestinal epithelial cells were dissociated from tissues (37 ˚C for 45 minutes) in PBS containing 5 mM Ethylenediaminetetraacetic acid (EDTA; #324506; EMD Millipore) and passed through 70 μM filter for use in proteomics study. Then, the intact pieces of tissue were digested using 5% fetal bovine serum (FBS) (#A3160502; Gibco) in PBS with calcium and magnesium and 0.5 mg/mL collagenase from Clostridium histolyticum (#C2139; Sigma-Aldrich). Single cells were collected through 70 μM filters via centrifugation (800 × g, 4 °C, 10 minutes), and red blood cells were lysed using ammonium–chloride–potassium lysing buffer (#A1049201; Gibco). For flow cytometry, isolated immune cells were stained with respective antibodies (Table 9) diluted 1:500 in column buffer (ie, 2 mM EDTA, 10 mM HEPES [#15630080; Thermo Fisher Scientific]), 5% (v/v) FBS in PBS (20 minutes at 4 ˚C). After staining, cells were fixed overnight in a 1:1 fix solution of column buffer: 4% PFA. Flow cytometry data were collected with a CytoFLEX (Beckman Coulter) machine and analyzed with FlowJo (Version 10) software.
Table 9.
List of Antibodies Used in this Study
| Cell marker | Fluorophore | Source | Clone | Catalog # |
|---|---|---|---|---|
| CD45 | BV605 | BioLegend | 30-F11 | 103139 |
| CD3 | e450 | BioLegend | 17A2 | 100214 |
| CD4 | e506 | ThermoFischer | RM4-5 | 69-0042-82 |
| CD8 | PE610 | ThermoFischer | 53-6.7 | 61-0081-82 |
| NK1.1 | APCe780 | ThermoFischer | PK136 | 47-5941-82 |
| CD19 | SB780 | ThermoFischer | 1D3 | 78-0193-82 |
| CD11b | e506 | ThermoFischer | M1/70 | 69-0112-82 |
| F4/80 | e450 | ThermoFischer | BM8 | 48-4801-82 |
| MHCII | PerCp | BioLegend | M5/114.15.2 | 107624 |
| Ly6c | PE | ThermoFischer | AL-21 | 12-5932-82 |
Proteomics
Ten micrograms of protein lysate were reduced and alkylated94 before running on 10% SDS-PAGE. Proteins in the entire lane were digested overnight in gel with a total of 0.3 μg trypsin-ultra-MS grade (#P8101S; New England BioLabs Inc).95 Resulting peptides were cleaned using C-18 STop and Go Extraction (STAGE) tips.96 Peptide concentrations were measured using NanoDrop One (Thermo Fisher Scientific), and then approximately 100 ng of peptides from each sample were loaded on Orbitrap Exploris 480 (Thermo Fisher Scientific) coupled to easy nLC 1200 (Thermo Fisher Scientific) with ionopticks’ Aurora series 25 cm × 75 μm C18 1.6-μm column heated to 40 °C. Forty-five minutes of separation was set from 3% to 25% Buffer B (80% acetonitrile, 0.1% formic acid), followed by an additional 15 minutes to reach 35% concentration of buffer B before the necessary column wash. Buffer A consisted of 2% acetonitrile and 0.1% formic acid, and LC flow rate was set at 250 nL/min. Instrument settings were as follows: Instrument polarity was set to positive mode, the spray voltage was set to 1900 V, the ion transfer tube temperature was set to 290 °C, the expected peak width to 15 seconds, the RF lens was set to 50%, and FAIMS was not enabled with data acquisition. Data-dependent mode was set for 20 scans, and the orbitrap resolution was set to 120,000 for full MS and 15,000 for fragment MS, with AGC target set to 100% with 20 ms injection time (IT) for full MS and 50% with auto IT for fragment MS. Profile data type was acquired at full MS level, and centroid at fragment MS level. Intensity threshold for fragmentation was set at 8,000, isolation window for fragmentation at 2 m/z, with normalized collision energy at 28%. Dynamic exclusion was enabled to exclude after 1 time for 45 seconds. Sample batch was randomized before injection.
Acquired data were searched on MaxQuant version 2.1.0.097 against Uniprot’s murine sequences (UP000000589, downloaded April 12, 2019) and common contaminant sequences. Label-free quantitation, iBAQ, and match-between-run options were enabled using peptide mass tolerance of 4.5 ppm, fixed modification of carbamidomethylation of cysteines, and variable modifications of oxidation of methionines and acetylation of protein N-termini. Specific proteolytic cleavages after arginine and lysine with up to 2 missed cleavages were set. The data were filtered for 1% false discovery at protein, peptide, and PSM levels using revert decoy search mode.
TC7 Cell Culture Experiment
The human colonic epithelial cell line, TC7 (SCC209; Sigma-Aldrich) was cultured in Dulbecco’s Modified Eagle’s Media (DMEM; Thermo Fisher Scientific) and supplemented with 10% FBS, 1% GlutMax (35050-061; Thermo Fisher Scientific) and 1% non-essential amino acids (11140050; Thermo Fisher Scientific) in a humidified environment at 37 °C with 5% CO2. For experiments, cells were treated with Rotenone (R-8875; Sigma-Aldrich) at variable doses (10–100 μM) for 3 hours at 37 °C, followed by infection with E. coli Nissle 1917 (isolated from probiotic Mutaflor and later, validated by Sanger sequencing; hereafter E. coli). E. coli cultures (5 mL) were grown aerobically overnight (∼16 hours) in a shaker incubator (225 rpm at 37 °C) and then, sub-cultured (1:100) before infecting cells at multiplicity of infection (MoI) = 1, for 2 hours. Then, cells were washed, resuspended in 0.1% Triton X-100 (T-9284; Sigma-Aldrich) and plated on Luria-Bertani (LB)-agar medium (#244520; BD Biosciences). Bacterial colonies were counted, log-transformed, and represented as log10 CFU/mL.
CHILD Birth Cohort
The CHILD cohort is a prospective, longitudinal study that enrolled pregnant mothers from 4 cities (Vancouver, Edmonton, Winnipeg, and Toronto) across Canada in their second trimester from 2008 to 2011.98 Of 3405 recruited to the General cohort, 77 were ineligible at birth, and 32 withdrew before any maternal or child data were collected, leaving 3296 eligible children with some maternal data. Thirty-two additional subjects withdrew before childbirth, leaving 3264 eligible children commencing the study.
Inclusion criteria:
-
(1)
Pregnant women aged 18 years and older (19 in Vancouver);
-
(2)
Residence in reasonable proximity to the delivery hospital;
-
(3)
Able to read, write, and speak English;
-
(4)
Willing to provide informed consent;
-
(5)
Willing to consent to cord blood collection for the study;
-
(6)
Planning to give birth at a designated recruitment center participating hospital;
-
(7)
Infants born at or after 35 weeks;
-
(8)
Able to provide name, address, and telephone numbers of two alternate contact individuals.
Exclusion criteria:
-
(1)
Children born with major congenital abnormalities or respiratory distress syndrome (RDS);
-
(2)
Expectation of moving away from a recruitment area within 1 year;
-
(3)
Children of multiple births;
-
(4)
Children resulting from in vitro fertilization;
-
(5)
Children who will not spend at least 80% of nights in the index home;
-
(6)
Children born before 35 weeks gestation
In the CHILD cohort protocol, the first stool passed was collected within 24 hours of birth as the meconium sample. Meconium samples were obtained from a larger cohort that already contained 16s sequencing data as part of a study focused on the impact of prenatal exposures on early infant microbiome maturation and the development of atopic sensitization.99 Since meconium begins accumulating by gestational week 16, it offers an excellent snapshot of exposures and nutrients received by the infant from the mother.100 Furthermore, comprised of ingested materials, including skin and gut cells, amniotic fluid, vernix and lanugo hair, as well as excreted fetal metabolites, meconium also provides the initial nutrient niche for pioneering bacteria that will establish the nascent microbiota.101 Thus, we used meconium to assess intestinal vitamin levels in the children. In this study, we analyzed the meconium metabolome and 3-month infant stool microbiota in a subset of 100 infants.99
Human Stool 16s Sequencing and Preprocessing
The gut microbiota of infants in the CHILD cohort was derived as previously described.99,102 Briefly, the V4 hypervariable region of the 16s rRNA gene was sequenced using universal primers (V4-515f: V4-806r). Paired-end sequences were pre-processed using Dada2 in Qiime2 v.2018.6 (www.qiime2.org).83 Taxonomic identity was assigned to the resulting ASVs by alignment to the Greengenes reference (v13.8) database at 99% sequence similarity. Sequences were further filtered in R (version 4.2.1) to remove samples with less than 8000 bp reads and ASVs with less than 10% prevalence before being normalized by relative abundance for downstream analyses.
Human Meconium Metabolomics
Meconium metabolic profiles were characterized in the CHILD cohort as previously described.99 Briefly, 100 mg of meconium from 100 infants were stored at −80 °C and sent to Metabolon, Inc for non-targeted metabolic profiling via their mView Global Metabolomics Profiling Platform using Ultrahigh Performance Liquid Chromatography-Tandem Mass Spectroscopy. A total of 714 different metabolites, including those specific to vitamin metabolism, were identified by automated comparison of the meconium samples to an in-house library of chemical standards that included retention time, molecular weight (m/z), and associated MS spectra and were visually inspected and curated for quality control using software developed at Metabolon. Raw units for each vitamin/co-factor metabolite are defined by area under the curve (AUC).
Correlating Human Meconium Vitamin Abundance and Enterobacteriaceae Colonization
Of the 714 meconium metabolites quantified by Metabolon, we identified 32 metabolites as belonging to ‘Cofactors and Vitamins’ super pathway (Table 3). Individual and cumulative (‘total vitamins’) raw abundances of metabolites were correlated to specific and aggregated ASVs (by Phyloseq version 1.40.086) of Enterobacteriaceae. For the ‘total vitamins’ approach, raw abundances of individual metabolites were first scaled to ensure that higher and lower abundant metabolites had similar influences on the total sum as described previously.99 The cumulative AUC sum of all 32 vitamin metabolites were then scaled again to ensure a more succinct range while still reflecting the spread within the 100 samples. Spearman correlations and scatter plot were calculated and visualized via ggpubr (version 0.5.0) and ggplot2 (version 3.4.0).
Organoid Culture
Murine colonic organoid cultures were grown as previously described.103 Briefly, murine colons were chopped into pieces (∼1 cm), and crypts were isolated. The crypts were cultured as hanging drop in Matrigel matrix (356231; Corning) in IntestiCult Organoid Growth Medium (06005; STEMCELL Technologies) for 10 days until the colonoid morphology was apparent. During the experiment, organoids were mechanically disrupted in TrypLE Express Enzyme solution (12604013; Thermo Fisher; 37 °C for 5 minutes) and then plated and cultured in Matrigel-coated (1:49 in PBS) 96-well plate for 7 days. qPCR and E. coli attachment assays were performed as described above. In all cases, the organoid media was replaced every 2 to 3 days.
Statistical Analyses
Analytical data represented as histograms were recorded as mean ± standard deviation and compared using non-parametric 2-sided Mann-Whitney test or Kruskal-Wallis 1-way analysis of variance for multiple group comparisons to control group. Prior to statistical analyses of proteomics data, protein intensity values were log-transformed with zeros converted to missing values. Differential expression analysis was conducted using the limma package in R to test for significant abundance differences between LM and CON pups. To assess the functional roles of the significant proteins, an over-representation analysis was conducted (hypergeometric test) using annotations from GO (Gene Ontology Consortium 2004) and Reactome.104 Mouse GO annotations were taken from the org.Mm.eg.db R package (version 3.13.0). GO over-representation was calculated using the GOStats R package. Reactome annotations were taken from the ReactomePA R package (version 1.36.0).105 All multiple comparisons were adjusted using the Benjamini-Hochberg correction (q-value), and a P- or q-value of < .05 was considered significant. All statistical analyses were performed with Graph Pad Prism software (Graph Pad version 9.1.0), except for proteomics and CHILD data, where R (version 4.2.1) was used.
Acknowledgments
B. Brett Finlay is a University of British Columbia Peter Wall Distinguished Professor. The authors thank the CHILD Cohort Study (CHILD) participant families for their dedication and commitment to advancing health research. CHILD was initially funded by CIHR and AllerGen NCE. Visit CHILD at childstudy.ca. Mass spectrometry infrastructure used here was supported by the Canada Foundation for Innovation and the BC Knowledge Development Fund. The authors would like to thank MBF husbandry technicians at UBC for their help with mouse experiments.
CRediT Authorship Contributions
Ravi Holani, PhD (Conceptualization: Lead; Data curation: Lead; Formal analysis: Lead; Investigation: Lead; Methodology: Lead; Writing – original draft: Lead)
Paula T. Littlejohn, PhD (Conceptualization: Supporting; Methodology: Supporting; Writing – review & editing: Supporting)
Karlie Edwards, BA (Methodology: Supporting; Writing – review & editing: Supporting)
Charisse Petersen, PhD (Methodology: Supporting; Software: Equal; Writing – review & editing: Supporting)
Kyung-Mee Moon, BS (Methodology: Supporting; Software: Lead; Writing – review & editing: Supporting)
Richard G. Stacey, PhD (Data curation: Equal; Software: Equal; Writing – review & editing: Supporting)
Tahereh Bozorgmehr, MS (Methodology: Supporting; Writing – review & editing: Supporting)
Zachary J. Gerbec, PhD (Methodology: Supporting; Writing – review & editing: Supporting)
Antonio Serapio-Palacios, PhD (Methodology: Supporting; Writing – review & editing: Supporting)
Zakhar Krekhno, PhD (Formal analysis: Equal; Writing – review & editing: Supporting)
Katherine Donald (Methodology: Equal; Writing – review & editing: Supporting)
Leonard J. Foster, PhD (Funding acquisition: Supporting; Methodology: Supporting; Resources: Supporting; Writing – review & editing: Supporting)
Stuart E. Turvey, MBBS, DPhil (Funding acquisition: Supporting; Resources: Supporting; Writing – review & editing: Supporting)
B. Brett Finlay, PhD (Conceptualization: Equal; Funding acquisition: Lead; Methodology: Supporting; Resources: Lead; Supervision: Lead; Writing – review & editing: Supporting)
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding Work in the Finlay and Foster lab was supported by Canadian Institutes of Health Research (CIHR) (FDN-159935) and Genome Canada/Genome BC (264PRO), respectively.
Data availability The raw proteomics data has been uploaded on ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD041389 (Username: reviewer_pxd041389@ebi.ac.uk; Password: Rb20t8AZ). Demultiplexed 16s sequencing data used in CHILD study are deposited into the Sequence Read Archive of NCBI and can be accessed via accession number PRJNA657821. Metabolomic data created by Metabolon at MetaboLights can be located at accession number MTBLS3476. Remaining study materials will be made available to other researchers upon request.
References
- 1.Stevens G.A., Beal T., Mbuya M.N.N., et al. Global Micronutrient Deficiencies Research Group Micronutrient deficiencies among preschool-aged children and women of reproductive age worldwide: a pooled analysis of individual-level data from population-representative surveys. Lancet Glob Health. 2022;10:e1590–e1599. doi: 10.1016/S2214-109X(22)00367-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bird J.K., Murphy R.A., Ciappio E.D., McBurney M.I. Risk of deficiency in multiple concurrent micronutrients in children and adults in the United States. Nutrients. 2017;9:655. doi: 10.3390/nu9070655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Semba R.D. The historical evolution of thought regarding multiple micronutrient nutrition. J Nutr. 2012;142:143S–156S. doi: 10.3945/jn.110.137745. [DOI] [PubMed] [Google Scholar]
- 4.Wallace T.C., McBurney M., Fulgoni V.L., 3rd Multivitamin/mineral supplement contribution to micronutrient intakes in the United States, 2007-2010. J Am Coll Nutr. 2014;33:94–102. doi: 10.1080/07315724.2013.846806. [DOI] [PubMed] [Google Scholar]
- 5.Committee on World Food Security. High Level Panel of Experts. HLPE#12 R. A report by high level panel of experts on food security and nutrition. 2017. https://www.fao.org/fileadmin/user_upload/hlpe/hlpe_documents/HLPE_Reports/HLPE-Report-12_EN.pdf. Accessed December 15, 2023.
- 6.Bailey R.L., West K.P., Jr., Black R.E. The epidemiology of global micronutrient deficiencies. Ann Nutr Metab. 2015;66(Suppl 2):22–33. doi: 10.1159/000371618. [DOI] [PubMed] [Google Scholar]
- 7.Kaganov B., Caroli M., Mazur A., et al. Suboptimal micronutrient intake among children in Europe. Nutrients. 2015;7:3524–3535. doi: 10.3390/nu7053524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mensink G.B., Fletcher R., Gurinovic M., et al. Mapping low intake of micronutrients across Europe. Br J Nutr. 2013;110:755–773. doi: 10.1017/S000711451200565X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keats E.C., Haider B.A., Tam E., Bhutta Z.A. Multiple-micronutrient supplementation for women during pregnancy. Cochrane Database Syst Rev. 2019;3:CD004905. doi: 10.1002/14651858.CD004905.pub6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cetin I., Buhling K., Demir C., et al. Impact of micronutrient status during pregnancy on early nutrition programming. Ann Nutr Metab. 2019;74:269–278. doi: 10.1159/000499698. [DOI] [PubMed] [Google Scholar]
- 11.Hansu K., Cikim I.G. Vitamin and mineral levels during pregnancy. Rev Assoc Med Bras (1992) 2022;68:1705–1708. doi: 10.1590/1806-9282.20220769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maykondo B.K., Horwood C., Haskins L., et al. A qualitative study to explore dietary knowledge, beliefs, and practices among pregnant women in a rural health zone in the Democratic Republic of Congo. J Health Popul Nutr. 2022;41:51. doi: 10.1186/s41043-022-00333-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stinson L.F. Establishment of the early-life microbiome: a DOHaD perspective. J Dev Orig Health Dis. 2020;11:201–210. doi: 10.1017/S2040174419000588. [DOI] [PubMed] [Google Scholar]
- 14.Chandra H., Sharma K.K., Tuovinen O.H., et al. Pathobionts: mechanisms of survival, expansion, and interaction with host with a focus on Clostridioides difficile. Gut Microbes. 2021;13 doi: 10.1080/19490976.2021.1979882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones H.J., Bourke C.D., Swann J.R., Robertson R.C. Malnourished microbes: host-microbiome interactions in child undernutrition. Annu Rev Nutr. 2023;43:327–353. doi: 10.1146/annurev-nutr-061121-091234. [DOI] [PubMed] [Google Scholar]
- 16.Amadi B., Zyambo K., Chandwe K., et al. Adaptation of the small intestine to microbial enteropathogens in Zambian children with stunting. Nat Microbiol. 2021;6:445–454. doi: 10.1038/s41564-020-00849-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gough E.K., Moulton L.H., Mutasa K., et al. Sanitation Hygiene Infant Nutrition Efficacy (SHINE) Trial Team. Effects of improved water, sanitation, and hygiene and improved complementary feeding on environmental enteric dysfunction in children in rural Zimbabwe: a cluster-randomized controlled trial. PLoS Negl Trop Dis. 2020;14 doi: 10.1371/journal.pntd.0007963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MAL-ED Network Investigators Relationship between growth and illness, enteropathogens and dietary intakes in the first 2 years of life: findings from the MAL-ED birth cohort study. BMJ Glob Health. 2017;2 doi: 10.1136/bmjgh-2017-000370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chae S.A., Son J.S., de Avila J.M., et al. Maternal exercise improves epithelial development of fetal intestine by enhancing apelin signaling and oxidative metabolism. Am J Physiol Regul Integr Comp Physiol. 2022;323:R728–R738. doi: 10.1152/ajpregu.00128.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiao Z., Liu S., Li Z., et al. The maternal microbiome programs the m(6)A epitranscriptome of the mouse fetal brain and intestine. Front Cell Dev Biol. 2022;10 doi: 10.3389/fcell.2022.882994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gregorieff A., Stange D.E., Kujala P., et al. The ets-domain transcription factor Spdef promotes maturation of goblet and paneth cells in the intestinal epithelium. Gastroenterology. 2009;137:1333–1345.e1–3. doi: 10.1053/j.gastro.2009.06.044. [DOI] [PubMed] [Google Scholar]
- 22.Gardner D.S., Ozanne S.E., Sinclair K.D. Effect of the early-life nutritional environment on fecundity and fertility of mammals. Philos Trans R Soc Lond B Biol Sci. 2009;364:3419–3427. doi: 10.1098/rstb.2009.0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown E.M., Wlodarska M., Willing B.P., et al. Diet and specific microbial exposure trigger features of environmental enteropathy in a novel murine model. Nat Commun. 2015;6:7806. doi: 10.1038/ncomms8806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cichon B., Fabiansen C., Yaméogo C.W., et al. Children with moderate acute malnutrition have inflammation not explained by maternal reports of illness and clinical symptoms: a cross-sectional study in Burkina Faso. BMC Nutrition. 2016;2 [Google Scholar]
- 25.Patterson G.T., Manthi D., Osuna F., et al. Environmental, metabolic, and inflammatory factors converge in the pathogenesis of moderate acute malnutrition in children: an observational cohort study. Am J Trop Med Hyg. 2021;104:1877–1888. doi: 10.4269/ajtmh.20-0963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Corware K., Yardley V., Mack C., et al. Protein energy malnutrition increases arginase activity in monocytes and macrophages. Nutr Metab (Lond) 2014;11:51. doi: 10.1186/1743-7075-11-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Michael H., Langel S.N., Miyazaki A., et al. Malnutrition decreases antibody secreting cell numbers induced by an oral attenuated human rotavirus vaccine in a human infant fecal microbiota transplanted gnotobiotic pig model. Front Immunol. 2020;11:196. doi: 10.3389/fimmu.2020.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodriguez L., Gonzalez C., Flores L., et al. Assessment by flow cytometry of cytokine production in malnourished children. Clin Diagn Lab Immunol. 2005;12:502–507. doi: 10.1128/CDLI.12.4.502-507.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song P., Zhang J., Zhang Y., et al. Hepatic recruitment of CD11b+Ly6C+ inflammatory monocytes promotes hepatic ischemia/reperfusion injury. Int J Mol Med. 2018;41:935–945. doi: 10.3892/ijmm.2017.3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pang J., Maienschein-Cline M., Koh T.J. Enhanced proliferation of Ly6C(+) monocytes/macrophages contributes to chronic inflammation in skin wounds of diabetic mice. J Immunol. 2021;206:621–630. doi: 10.4049/jimmunol.2000935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arts R.J., Blok B.A., van Crevel R., et al. Vitamin A induces inhibitory histone methylation modifications and down-regulates trained immunity in human monocytes. J Leukoc Biol. 2015;98:129–136. doi: 10.1189/jlb.6AB0914-416R. [DOI] [PubMed] [Google Scholar]
- 32.Castleman M.J., Dillon S.M., Purba C., et al. Enteric bacteria induce IFNgamma and granzyme b from human colonic group 1 innate lymphoid cells. Gut Microbes. 2020;12 doi: 10.1080/19490976.2019.1667723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seo B.B., Marella M., Yagi T., Matsuno-Yagi A. The single subunit NADH dehydrogenase reduces generation of reactive oxygen species from complex I. FEBS Lett. 2006;580:6105–6108. doi: 10.1016/j.febslet.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 34.Crowley S.M., Vallance B.A. Microbial respiration in the colon: using H(2)O(2) to catch your breath. Cell Host Microbe. 2020;28:771–773. doi: 10.1016/j.chom.2020.11.006. [DOI] [PubMed] [Google Scholar]
- 35.Rodriguez-Cano A.M., Calzada-Mendoza C.C., Estrada-Gutierrez G., et al. Nutrients, mitochondrial function, and perinatal health. Nutrients. 2020;12:2166. doi: 10.3390/nu12072166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dikalov S. Cross talk between mitochondria and NADPH oxidases. Free Radic Biol Med. 2011;51:1289–1301. doi: 10.1016/j.freeradbiomed.2011.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nabwera H.M., Espinoza J.L., Worwui A., et al. Interactions between fecal gut microbiome, enteric pathogens, and energy regulating hormones among acutely malnourished rural Gambian children. EBioMedicine. 2021;73 doi: 10.1016/j.ebiom.2021.103644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vozenilek A.E., Vetkoetter M., Green J.M., et al. Absence of nicotinamide nucleotide transhydrogenase in C57BL/6J mice exacerbates experimental atherosclerosis. J Vasc Res. 2018;55:98–110. doi: 10.1159/000486337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Littlejohn P.T., Bar-Yoseph H., Edwards K., et al. Multiple micronutrient deficiencies alter energy metabolism in host and gut microbiome in an early-life murine model. Front Nutr. 2023;10 doi: 10.3389/fnut.2023.1151670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sandstrom B. Micronutrient interactions: effects on absorption and bioavailability. Br J Nutr. 2001;85(Suppl 2):S181–S185. [PubMed] [Google Scholar]
- 41.Gu T., Jia X., Shi H., et al. An evaluation of exposure to 18 toxic and/or essential trace elements exposure in maternal and cord plasma during pregnancy at advanced maternal age. Int J Environ Res Public Health. 2022;19 doi: 10.3390/ijerph192114485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jiang T., Christian P., Khatry S.K., et al. Micronutrient deficiencies in early pregnancy are common, concurrent, and vary by season among rural Nepali pregnant women. J Nutr. 2005;135:1106–1112. doi: 10.1093/jn/135.5.1106. [DOI] [PubMed] [Google Scholar]
- 43.Pathak P., Kapil U., Kapoor S.K., et al. Prevalence of multiple micronutrient deficiencies amongst pregnant women in a rural area of Haryana. Indian J Pediatr. 2004;71:1007–1014. doi: 10.1007/BF02828117. [DOI] [PubMed] [Google Scholar]
- 44.DiTroia S.P., Percharde M., Guerquin M.J., et al. Maternal vitamin C regulates reprogramming of DNA methylation and germline development. Nature. 2019;573:271–275. doi: 10.1038/s41586-019-1536-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mottram L., Speak A.O., Selek R.M., et al. Infection susceptibility in gastric intrinsic factor (vitamin B12)-defective mice is subject to maternal influences. mBio. 2016;7 doi: 10.1128/mBio.00830-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hidalgo-Villeda F., Million M., Defoort C., et al. Prolonged dysbiosis and altered immunity under nutritional intervention in a physiological mouse model of severe acute malnutrition. iScience. 2023;26 doi: 10.1016/j.isci.2023.106910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu J., Platts-Mills J.A., Juma J., et al. Use of quantitative molecular diagnostic methods to identify causes of diarrhoea in children: a reanalysis of the GEMS case-control study. Lancet. 2016;388:1291–1301. doi: 10.1016/S0140-6736(16)31529-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Foster K.R., Schluter J., Coyte K.Z., Rakoff-Nahoum S. The evolution of the host microbiome as an ecosystem on a leash. Nature. 2017;548:43–51. doi: 10.1038/nature23292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Coyte K.Z., Rakoff-Nahoum S. Understanding competition and cooperation within the mammalian gut microbiome. Curr Biol. 2019;29:R538–R544. doi: 10.1016/j.cub.2019.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Coyte K.Z., Schluter J., Foster K.R. The ecology of the microbiome: networks, competition, and stability. Science. 2015;350:663–666. doi: 10.1126/science.aad2602. [DOI] [PubMed] [Google Scholar]
- 51.Huus K.E., Bauer K.C., Brown E.M., et al. Commensal bacteria modulate immunoglobulin A binding in response to host nutrition. Cell Host Microbe. 2020;27:909–921.e5. doi: 10.1016/j.chom.2020.03.012. [DOI] [PubMed] [Google Scholar]
- 52.Al Nabhani Z., Dulauroy S., Marques R., et al. A weaning reaction to microbiota is required for resistance to immunopathologies in the adult. Immunity. 2019;50:1276–1288.e5. doi: 10.1016/j.immuni.2019.02.014. [DOI] [PubMed] [Google Scholar]
- 53.Bartelt L.A., Bolick D.T., Mayneris-Perxachs J., et al. Cross-modulation of pathogen-specific pathways enhances malnutrition during enteric co-infection with Giardia lamblia and enteroaggregative Escherichia coli. PLoS Pathog. 2017;13 doi: 10.1371/journal.ppat.1006471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bomer N., Pavez-Giani M.G., Grote Beverborg N., et al. Micronutrient deficiencies in heart failure: Mitochondrial dysfunction as a common pathophysiological mechanism? J Intern Med. 2022;291:713–731. doi: 10.1111/joim.13456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reusens B., Theys N., Remacle C. Alteration of mitochondrial function in adult rat offspring of malnourished dams. World J Diabetes. 2011;2:149–157. doi: 10.4239/wjd.v2.i9.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hu G., Ling C., Chi L., et al. The role of the tryptophan-NAD + pathway in a mouse model of severe malnutrition induced liver dysfunction. Nat Commun. 2022;13:7576. doi: 10.1038/s41467-022-35317-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Luo S., Valencia C.A., Zhang J., et al. Biparental inheritance of mitochondrial DNA in humans. Proc Natl Acad Sci U S A. 2018;115:13039–13044. doi: 10.1073/pnas.1810946115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zuccarello D., Sorrentino U., Brasson V., et al. Epigenetics of pregnancy: looking beyond the DNA code. J Assist Reprod Genet. 2022;39:801–816. doi: 10.1007/s10815-022-02451-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hales C.N., Barker D.J. The thrifty phenotype hypothesis. Br Med Bull. 2001;60:5–20. doi: 10.1093/bmb/60.1.5. [DOI] [PubMed] [Google Scholar]
- 60.Koemel N.A., Skilton M.R. Epigenetic aging in early life: role of maternal and early childhood nutrition. Curr Nutr Rep. 2022;11:318–328. doi: 10.1007/s13668-022-00402-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reischl-Hajiabadi A.T., Garbade S.F., Feyh P., et al. Maternal vitamin B(12) deficiency detected by newborn screening-evaluation of causes and characteristics. Nutrients. 2022;14:3767. doi: 10.3390/nu14183767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mentch S.J., Locasale J.W. One-carbon metabolism and epigenetics: understanding the specificity. Ann N Y Acad Sci. 2016;1363:91–98. doi: 10.1111/nyas.12956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rerkasem A., Nantakool S., Wilson B.C., et al. Associations between maternal plasma zinc concentrations in late pregnancy and LINE-1 and Alu methylation loci in the young adult offspring. PLoS One. 2022;17 doi: 10.1371/journal.pone.0279630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fryer A.A., Nafee T.M., Ismail K.M., et al. LINE-1 DNA methylation is inversely correlated with cord plasma homocysteine in man: a preliminary study. Epigenetics. 2009;4:394–398. doi: 10.4161/epi.4.6.9766. [DOI] [PubMed] [Google Scholar]
- 65.Joubert B.R., den Dekker H.T., Felix J.F., et al. Maternal plasma folate impacts differential DNA methylation in an epigenome-wide meta-analysis of newborns. Nat Commun. 2016;7 doi: 10.1038/ncomms10577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Harvey A.J. Mitochondria in early development: linking the microenvironment, metabolism and the epigenome. Reproduction. 2019;157:R159–R179. doi: 10.1530/REP-18-0431. [DOI] [PubMed] [Google Scholar]
- 67.Saha D., Mehndiratta M., Aaradhana, et al. Oxidative stress, mitochondrial dysfunction, and premature ageing in severe acute malnutrition in under-five children. Indian J Pediatr. 2022;89:558–562. doi: 10.1007/s12098-021-03981-5. [DOI] [PubMed] [Google Scholar]
- 68.Lee S.B., Bae I.H., Bae Y.S., Um H.D. Link between mitochondria and NADPH oxidase 1 isozyme for the sustained production of reactive oxygen species and cell death. J Biol Chem. 2006;281:36228–36235. doi: 10.1074/jbc.M606702200. [DOI] [PubMed] [Google Scholar]
- 69.Miller B.M., Liou M.J., Zhang L.F., et al. Anaerobic respiration of NOX1-derived hydrogen peroxide licenses bacterial growth at the colonic surface. Cell Host Microbe. 2020;28:789–797.e5. doi: 10.1016/j.chom.2020.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Caetano M.A.F., Castelucci P. Role of short chain fatty acids in gut health and possible therapeutic approaches in inflammatory bowel diseases. World J Clin Cases. 2022;10:9985–10003. doi: 10.12998/wjcc.v10.i28.9985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Deleu S., Machiels K., Raes J., et al. Short chain fatty acids and its producing organisms: an overlooked therapy for IBD? EBioMedicine. 2021;66 doi: 10.1016/j.ebiom.2021.103293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fusco W., Lorenzo M.B., Cintoni M., et al. Short-chain fatty-acid-producing bacteria: key components of the human gut microbiota. Nutrients. 2023;15:2211. doi: 10.3390/nu15092211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Thriene K., Michels K.B. Human gut microbiota plasticity throughout the life course. Int J Environ Res Public Health. 2023;20:1463. doi: 10.3390/ijerph20021463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Littlejohn P., Finlay B.B. When a pandemic and an epidemic collide: COVID-19, gut microbiota, and the double burden of malnutrition. BMC Med. 2021;19:31. doi: 10.1186/s12916-021-01910-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Blanco-Perez F., Steigerwald H., Schulke S., et al. The dietary fiber pectin: health benefits and potential for the treatment of allergies by modulation of gut microbiota. Curr Allergy Asthma Rep. 2021;21:43. doi: 10.1007/s11882-021-01020-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kim Y., Hwang S.W., Kim S., et al. Dietary cellulose prevents gut inflammation by modulating lipid metabolism and gut microbiota. Gut Microbes. 2020;11:944–961. doi: 10.1080/19490976.2020.1730149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Williams B.A., Grant L.J., Gidley M.J., Mikkelsen D. Gut fermentation of dietary fibres: physico-chemistry of plant cell walls and implications for health. Int J Mol Sci. 2017;18:2203. doi: 10.3390/ijms18102203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zeng M.Y., Inohara N., Nunez G. Mechanisms of inflammation-driven bacterial dysbiosis in the gut. Mucosal Immunol. 2017;10:18–26. doi: 10.1038/mi.2016.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ghosh S., Sinha J.K., Putcha U.K., Raghunath M. Severe but not moderate vitamin B12 deficiency impairs lipid profile, induces adiposity, and leads to adverse gestational outcome in female C57BL/6 mice. Front Nutr. 2016;3:1. doi: 10.3389/fnut.2016.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Serapio-Palacios A., Woodward S.E., Vogt S.L., et al. Type VI secretion systems of pathogenic and commensal bacteria mediate niche occupancy in the gut. Cell Rep. 2022;39 doi: 10.1016/j.celrep.2022.110731. [DOI] [PubMed] [Google Scholar]
- 81.Montonye D.R., Ericsson A.C., Busi S.B., et al. Acclimation and Institutionalization of the mouse microbiota following transportation. Front Microbiol. 2018;9:1085. doi: 10.3389/fmicb.2018.01085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cazares-Olivera M., Miroszewska D., Hu L., et al. Animal unit hygienic conditions influence mouse intestinal microbiota and contribute to T-cell-mediated colitis. Exp Biol Med (Maywood) 2022;247:1752–1763. doi: 10.1177/15353702221113826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bolyen E., Rideout J.R., Dillon M.R., et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol. 2019;37:852–857. doi: 10.1038/s41587-019-0209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Callahan B.J., McMurdie P.J., Rosen M.J., et al. DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods. 2016;13:581–583. doi: 10.1038/nmeth.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pruesse E., Quast C., Knittel K., et al. SILVA: a comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Res. 2007;35:7188–7196. doi: 10.1093/nar/gkm864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.McMurdie P.J., Holmes S. phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS One. 2013;8 doi: 10.1371/journal.pone.0061217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Love M.I., Huber W., Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wickham H. Springer-Verlag; 2016. ggplot2: elegant graphics for data analysis. [Google Scholar]
- 89.Yamakawa I., Kojima H., Terashima T., et al. Inactivation of TNF-alpha ameliorates diabetic neuropathy in mice. Am J Physiol Endocrinol Metab. 2011;301:E844–E852. doi: 10.1152/ajpendo.00029.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.De Filippo K., Henderson R.B., Laschinger M., Hogg N. Neutrophil chemokines KC and macrophage-inflammatory protein-2 are newly synthesized by tissue macrophages using distinct TLR signaling pathways. J Immunol. 2008;180:4308–4315. doi: 10.4049/jimmunol.180.6.4308. [DOI] [PubMed] [Google Scholar]
- 91.Eissa N., Kermarrec L., Hussein H., et al. Appropriateness of reference genes for normalizing messenger RNA in mouse 2,4-dinitrobenzene sulfonic acid (DNBS)-induced colitis using quantitative real time PCR. Sci Rep. 2017;7 doi: 10.1038/srep42427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Moll F., Walter M., Rezende F., et al. NoxO1 controls proliferation of colon epithelial cells. Front Immunol. 2018;9:973. doi: 10.3389/fimmu.2018.00973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Boutin R.C., Petersen C., Woodward S.E., et al. Bacterial-fungal interactions in the neonatal gut influence asthma outcomes later in life. Elife. 2021;10 doi: 10.7554/eLife.67740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Goodman J.K., Zampronio C.G., Jones A.M.E., Hernandez-Fernaud J.R. Updates of the in-gel digestion method for protein analysis by mass spectrometry. Proteomics. 2018;18 doi: 10.1002/pmic.201800236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shevchenko A., Wilm M., Vorm O., Mann M. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal Chem. 1996;68:850–858. doi: 10.1021/ac950914h. [DOI] [PubMed] [Google Scholar]
- 96.Ishihama Y., Rappsilber J., Andersen J.S., Mann M. Microcolumns with self-assembled particle frits for proteomics. J Chromatogr A. 2002;979:233–239. doi: 10.1016/s0021-9673(02)01402-4. [DOI] [PubMed] [Google Scholar]
- 97.Tyanova S., Temu T., Cox J. The MaxQuant computational platform for mass spectrometry-based shotgun proteomics. Nat Protoc. 2016;11:2301–2319. doi: 10.1038/nprot.2016.136. [DOI] [PubMed] [Google Scholar]
- 98.Moraes T.J., Lefebvre D.L., Chooniedass R., et al. CHILD Study Investigators The Canadian healthy infant longitudinal development birth cohort study: biological samples and biobanking. Paediatr Perinat Epidemiol. 2015;29:84–92. doi: 10.1111/ppe.12161. [DOI] [PubMed] [Google Scholar]
- 99.Petersen C., Dai D.L.Y., Boutin R.C.T., et al. A rich meconium metabolome in human infants is associated with early-life gut microbiota composition and reduced allergic sensitization. Cell Rep Med. 2021;2 doi: 10.1016/j.xcrm.2021.100260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Skelly C.L., Zulfiqar H., Sankararaman S. StatPearls Publishing; 2023. Meconium. [PubMed] [Google Scholar]
- 101.Houghteling P.D., Walker W.A. Why is initial bacterial colonization of the intestine important to infants' and children's health? J Pediatr Gastroenterol Nutr. 2015;60:294–307. doi: 10.1097/MPG.0000000000000597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fehr K., Moossavi S., Sbihi H., et al. Breastmilk feeding practices are associated with the co-occurrence of bacteria in mothers' milk and the infant gut: the CHILD Cohort Study. Cell Host Microbe. 2020;28:285–297.e4. doi: 10.1016/j.chom.2020.06.009. [DOI] [PubMed] [Google Scholar]
- 103.Holani R., Babbar A., Blyth G.A.D., et al. Cathelicidin-mediated lipopolysaccharide signaling via intracellular TLR4 in colonic epithelial cells evokes CXCL8 production. Gut Microbes. 2020;12 doi: 10.1080/19490976.2020.1785802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gillespie M., Jassal B., Stephan R., et al. The reactome pathway knowledgebase 2022. Nucleic Acids Res. 2022;50:D687–D692. doi: 10.1093/nar/gkab1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yu G., He Q.Y. ReactomePA: an R/Bioconductor package for reactome pathway analysis and visualization. Mol Biosyst. 2016;12:477–479. doi: 10.1039/c5mb00663e. [DOI] [PubMed] [Google Scholar]