Abstract
Background: Dolutegravir (DTG) is a cornerstone of global antiretroviral (ARV) therapy (ART) due to its high efficacy and favorable tolerability. However, limited data exist regarding the risk of emergent integrase strand transfer inhibitor (INSTI) drug-resistance mutations (DRMs) in individuals receiving DTG-containing ART. Methods: We performed a PubMed search using the term “Dolutegravir”, last updated 18 December 2023, to estimate the prevalence of VF with emergent INSTI DRMs in people living with HIV (PLWH) without previous VF on an INSTI who received DTG-containing ART. Results: Of 2131 retrieved records, 43 clinical trials, 39 cohorts, and 6 cross-sectional studies provided data across 6 clinical scenarios based on ART history, virological status, and co-administered ARVs: (1) ART-naïve PLWH receiving DTG plus two NRTIs; (2) ART-naïve PLWH receiving DTG plus lamivudine; (3) ART-experienced PLWH with VF on a previous regimen receiving DTG plus two NRTIs; (4) ART-experienced PLWH with virological suppression receiving DTG plus two NRTIs; (5) ART-experienced PLWH with virological suppression receiving DTG and a second ARV; and (6) ART-experienced PLWH with virological suppression receiving DTG monotherapy. The median proportion of PLWH in clinical trials with emergent INSTI DRMs was 1.5% for scenario 3 and 3.4% for scenario 6. In the remaining four trial scenarios, VF prevalence with emergent INSTI DRMs was ≤0.1%. Data from cohort studies minimally influenced prevalence estimates from clinical trials, whereas cross-sectional studies yielded prevalence data lacking denominator details. Conclusions: In clinical trials, the prevalence of VF with emergent INSTI DRMs in PLWH receiving DTG-containing regimens has been low. Novel approaches are required to assess VF prevalence with emergent INSTI DRMs in PLWH receiving DTG in real-world settings.
Keywords: HIV, epidemiology, systematic review, treatment
1. Introduction
Among integrase strand transfer inhibitor (INSTI)-naïve people living with HIV (PLWH), antiretroviral (ARV) therapy (ART) with the second-generation INSTIs dolutegravir (DTG) or bictegravir (BIC) has been associated with low rates of virological failure (VF) and emergent INSTI-associated drug-resistance mutations (DRMs). Of these two INSTIs, DTG is instrumental in the World Health Organization’s (WHO’s) efforts to improve global viral suppression rates because DTG-containing regimens are recommended in multiple clinical scenarios, including first-line ART, programmatic transition for individuals being treated with a first-line nonnucleoside reverse transcriptase inhibitor (NNRTI)-based regimen, and second-line ART following VF on a first-line NNRTI-based regimen [1]. By 2022, an estimated 22.2 million PLWH in low- and middle-income countries (LMICs) were receiving a DTG-containing regimen [2].
We recently published a systematic review that identified 36 publications reporting 99 INSTI-naïve PLWH with emergent VF and INSTI DRMs on a DTG-containing regimen [3]. However, this review did not estimate the risk of emergent INSTI DRMs in INSTI-naïve PLWH because most individuals with INSTI DRMs were reported in studies for which the total number of PLWH receiving DTG was unknown. Additionally, this review’s search identified only studies reporting INSTI DRMs and not those in which DTG was received but INSTI DRMs were not reported.
Therefore, we performed a scoping review to estimate the risk of VF with emergent INSTI DRMs in PLWH without a history of VF on a previous INSTI-based regimen according to whether (1) they were ART-naïve or experienced, (2) they were virologically suppressed when DTG-containing ART was initiated, and (3) the ARVs co-administered with DTG. Our review included clinical trials, observational cohort studies, and cross-sectional studies in which PLWH underwent genotypic resistance testing (GRT). We confined our analysis to PLWH without a history of VF on a first-generation INSTI because such VF is often associated with DTG cross-resistance or a marked reduction in the genetic barrier to DTG resistance [4]. Additionally, first-generation INSTIs have rarely been used in LMICs, where the concern about emergent DTG resistance is the greatest.
2. Materials and Methods
This rapid scoping review evaluated the risk of VF with emergent INSTI DRMs in PLWH without VF on a first-generation INSTI. The review adhered to the Preferred Reporting in Systematic Review and Meta-Analysis (PRISMA) extension for Scoping Reviews [5]. One author (RWS) reviewed the results of a PubMed query using the search term “Dolutegravir” from database inception through 18 December 2023, to identify clinical trials, cohort studies, and cross-sectional studies containing data on the prevalence of emergent INSTI DRMs in populations without previous VF on an INSTI-containing regimen who received a DTG-containing regimen.
Publications were excluded for any of the following reasons: (i) absence of GRT data; (ii) inclusion of individuals with previous VF on a first-generation INSTI whose data could not be distinguished from those who were previously INSTI-naïve; (iii) lack of essential information such as ART history, viral replication status at the initiation of DTG, or the ARVs co-administered with DTG; (iv) studies with fewer than 30 individuals; (v) studies not reporting individual-level VF data; (vi) studies with data previously reported in an already included study; or (vii) studies not involving individuals treated with DTG.
Clinical trials, cohort studies, and cross-sectional studies that included PLWH who had previously received a first-generation INSTI were eligible only if the study subjects had been virologically suppressed at the time DTG-containing ART was begun and had not previously experienced VF on an INSTI-containing regimen. Studies that reported no instances of VF were included despite the fact that GRT was not performed.
The following data were extracted from published studies meeting eligibility criteria: (1) geographic region; (2) study population characteristics including age range, pregnancy, and whether rifampin-containing antituberculosis (anti-TB) treatment was co-administered; (3) ART history; (4) whether virological suppression (VS) was present at the time DTG-containing ART was begun; (5) the ARVs co-administered with DTG; (6) the number of individuals receiving a DTG-containing regimen; (7) the proportion of individuals with protocol-defined VF at specific time points such as weeks 24, 48, and 96; (8) the definition of author-defined VF; (9) the proportion of individuals undergoing GRT; and (10) the proportion of individuals with emergent INSTI DRMs. Data extraction was performed primarily by CC and RWS with the assistance of VK, AA, JS, and PMG.
The results are presented as the proportions of PLWH experiencing VF, undergoing GRT, and developing INSTI DRMs. Clinical trials and observational studies meeting the search criteria were grouped into six clinical scenarios based on the population’s ART experience, the presence of VS prior to initiating DTG-containing ART, and the ARVs co-administered with DTG.
Complete HIV-1 sequences were rarely reported by authors or submitted to GenBank. Therefore, we relied on the reports of integrase mutations in each published study. INSTI-associated DRMs were defined as the following nonpolymorphic mutations: H51Y, T66A/I/K, E92G/Q, G118R, F121Y, E138A/K/T, G140A/C/S, Y143C/H/R/S, S147G, Q148H/R/K, S153Y/F, N155H, S230R, and R263K [3].
3. Results
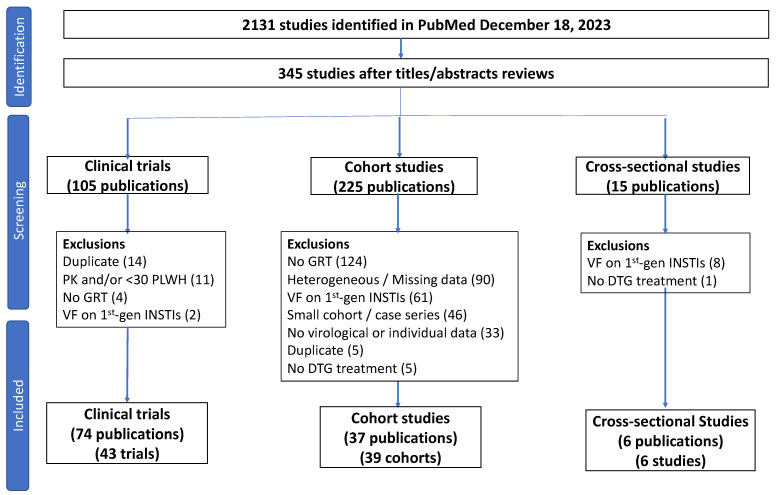
As of 18 December 2023, 2131 publications were retrieved from PubMed. Following the title and abstract review, 345 publications were selected for full-text review. Of 105 publications describing clinical trials, 32 were excluded because they (i) did not contain GRT data; (ii) included individuals with previous VF on a first-generation INSTI; (iii) contained pharmacokinetic data only; (iv) contained fewer than 30 individuals; or (iv) contained the same information reported in another study (Figure 1).
Figure 1.
Flow chart summarizing the review process. Of 2131 publications identified in the PubMed search, 345 were read in their entirety following an initial review of titles and abstracts. Exclusions for clinical trials and cross-sectional studies were usually based on a single exclusion criterion. Exclusions for cohort studies were usually based on more than one exclusion criterion. Two publications contained descriptions of two cohorts. Abbreviations: PK—pharmacokinetic study; PLWH—people living with HIV; GRT—genotypic resistance testing; VF—virological failure; 1st-gen INSTI—previous VF on a 1st-generation integrase strand transfer inhibitor (raltegravir or elvitegravir). Heterogeneous/Missing data—indicates that the study described different subsets of individuals with different ART histories, levels of virus suppression, and DTG-containing regimens but that the virological and/or GRT outcomes were not provided for the different subsets.
Of 225 publications describing clinical cohorts, 186 were excluded because they (i) did not contain GRT data; (ii) were missing critical data such as ART history and virological status (i.e., whether patients were virologically suppressed) prior to DTG initiation; (iii) included individuals with previous VF on a first-generation INSTI; (iv) contained fewer than 30 individuals; (v) did not contain individual-level VF data; (vi) contained the same information reported in another study; and/or (vii) did not include individuals who received DTG (Figure 1).
Of 15 publications describing cross-sectional studies of GRT in PLWH with VF after receiving one or more INSTIs, 9 were excluded because they (i) did not distinguish individuals who experienced VF on DTG alone versus those who received DTG following VF on a first-generation INSTI or (ii) did not include persons receiving a DTG-containing regimen (Figure 1).
Following full-text review, 74 publications describing 43 clinical trials, 37 publications describing 39 cohort studies, and 6 publications describing cross-sectional studies met study eligibility criteria. When grouped according to population ART history, level of virus replication, and ARVs co-administered with DTG, the studies were classified into six clinical scenarios: (1) ART-naïve PLWH receiving initial therapy with DTG plus two nucleoside RT inhibitors (NRTIs); (2) ART-naïve PLWH receiving initial therapy with DTG plus lamivudine (3TC); (3) ART-experienced PLWH with previous VF on an NNRTI-containing regimen receiving DTG plus two NRTIs or an optimized background regimen; (4) ART-experienced PLWH with VS switching to a regimen of DTG plus two NRTIs; (5) ART-experienced PLWH with VS switching to a two-drug DTG-containing regimen; and (6) ART-experienced PLWH with VS switching to DTG monotherapy. The number of clinical trials, cohort studies, and cross-sectional studies describing populations belonging to each of these scenarios is shown in Table 1.
Table 1.
Number of clinical trials, cohort studies, and cross-sectional studies belonging to each of the six clinical scenarios.
| Clinical Scenario | ART History |
Viral Load Prior to DTG |
DTG-Containing ART |
Clinical Trials (Number Trials) |
Clinical Trials (Number PLWH) |
Cohort Studies (Number Studies) |
Cohort Studies (Number PLWH) |
Cross-Sectional Studies (Number Studies) |
Cross-Sectional Studies (Number PLWH) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Naïve | Viremic | DTG + 2 NRTIs | 16 | 4585 | 7 | 2698 | 1 | 113 |
| 2 | Naïve | Viremic | DTG + 3TC | 4 | 1075 | 3 | 581 | 0 | 0 |
| 3 | Experienced | Viremic | DTG + 2 NRTIs | 6 | 1428 | 5 | 3630 | 4 | 218 |
| 4 | Experienced | Suppressed | DTG + 2 NRTIs | 3 | 877 | 3 | 2204 | 0 | 0 |
| 5 | Experienced | Suppressed | DTG + 2nd ARV | 10 | 1894 | 18 | 5930 | 0 | 0 |
| 6 | Experienced | Suppressed | DTG monotherapy | 4 | 276 | 3 | 123 | 0 | 0 |
Abbreviations: ARV—antiretroviral; ART—antiretroviral therapy; DTG—dolutegravir; PLWH—people living with HIV.
3.1. ART-Naïve PLWH (Scenarios 1 and 2)
DTG plus two NRTIs: Table 2 summarizes the population characteristics, prevalence of VF, and GRT results in 16 clinical trials of 4636 ART-naïve PLWH treated with DTG plus two NRTIs [6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34]. The trials included seven multicenter registration trials in which participants were evaluated at week 96 (and week 144 in one trial), two trials from Sub-Saharan Africa in which participants were evaluated at week 96, two trials in which participants were evaluated at week 48, and five trials of special populations. The latter trials included PLWH who were also receiving anti-TB therapy, pregnant women, individuals with acute HIV-1 infection, and individuals co-infected with hepatitis B virus. VF was defined as a confirmed plasma HIV-1 RNA level ≥ 50 copies/mL in eight trials, ≥200 copies/mL in three trials, ≥400 copies/mL in three trials, and ≥1000 copies/mL in two trials.
The median proportion of PLWH experiencing VF in the 16 trials was 4.9% (IQR: 2.8–6.1% range: 2.0–10.4%). At the time of VF, GRT was performed on 147 individuals (3.2% of all participants) receiving DTG and only one (0.02% of all participants and 0.7% of those with VF) developed an INSTI DRM. This individual was 1 of 405 women treated during the second trimester of pregnancy and evaluated 50 weeks post-partum [30]. The emergent INSTI DRMs included S147G, N155H, and S230R. This individual and at least four other individuals acquired the 3TC/emtricitabine (FTC)-associated reverse transcriptase (RT) mutation, M184VI.
Seven cohort studies described 2698 ART-naïve PLWH treated with DTG plus two NRTIs including two studies from the U.S. [35,36], two from Italy [37,38], and one each from Spain [39], South Korea [40], and Tanzania [41] (Supplementary Table S1). One of the two studies from the U.S. included just pregnant women [35]. The median duration of follow-up ranged from 9 to 26 months, and the proportions with VF ranged from 0% to 23%. None of these studies reported new instances of emergent INSTI DRMs.
One cross-sectional study from Brazil reported that among 113 previously ART-naïve PLWH with confirmed VF while receiving DTG plus tenofovir disoproxil fumarate (TDF)/3TC, seven (6.2%) individuals had major INSTI DRMs [42] (Supplementary Table S1). However, the size of the population receiving DTG plus TDF/3TC and the total number with VF was not reported in this study.
Table 2.
Virological failure (VF) and prevalence of emergent INSTI-associated DRMs in clinical trials of ART-Naïve PLWH receiving a first-line DTG-containing regimen.
| Trial | Regions | Population | DTG-Containing Regimen | # PLWH |
Weeks | # (%) VF on DTG 1 |
# (%) Undergoing GRT 2 |
# (%) with INSTI DRMs 3 |
|---|---|---|---|---|---|---|---|---|
| DTG plus 2 NRTIs | ||||||||
| SPRING-1 2012–2013 [6,7] 4 |
Europe, North America | Adults; without DRMs | DTG + 2 NRTIs |
51 | 96 | 2 (3.9%) |
0 (0%) |
0 (0%) |
| SPRING-2 2013 [8,9] |
Europe, North America | Adults; without DRMs | DTG + 2 NRTIs |
411 | 96 | 22 (5.4%) |
22 (5.4%) |
0 (0%) |
| SINGLE 2013, 2015 [10,11] |
Europe, North America | Adults; without DRMs | DTG + ABC/3TC |
414 | 144 | 43 (10.4%) |
39 (9.4%) |
0 (0%) |
| FLAMINGO 2014, 2015 [12,13] |
Europe, North America | Adults; without DRMs | DTG + 2 NRTIs |
242 | 96 | 19 (7.9%) |
2 (0.8%) |
0 (0%) |
| ARIA 2017 [14] |
Europe, North America, South America, Asia, Africa | Adult women; without DRMs | DTG + ABC/3TC |
248 | 48 | 16 (6.5%) |
6 (2.4%) |
0 (0%) |
| ADVANCE 2019, 2020 [15,16,17] 5 |
South Africa | Adults/Adolescents | DTG + TXF/FTC |
702 | 96 | 25 (3.6%) |
28 (4.0%) |
0 (0%) |
| NAMSAL 2019, 2020 [18,19] 6 |
Cameroon | Adults | DTG + TDF/FTC |
310 | 96 | 8 (2.6%) |
3 (1.0%) |
0 (0%) |
| GS-US-3801490 2017, 2019 [20,21,22] |
Europe, North America | Adults | DTG + TAF/FTC |
325 | 96 | 9 (2.8%) |
6 (1.8%) |
0 (0%) |
| GS-US-3801489 2017, 2019 [23,24] |
Europe, North America | Adults; without DRMs | DTG + ABC/3TC |
315 | 96 | 7 (2.2%) |
5 (1.6%) |
0 (0%) |
| SYMTRI 2022 [25] |
Spain | Adults | DTG + ABC/3TC |
155 | 48 | 6 (3.9%) |
Not reported | 0 (0%) |
| GEMINI-1/GEMINI-2 2019, 2020, 2022 [26,27,28] |
Europe, North America, South America, Asia, Africa | Adults without known major DRMs | DTG + TDF/FTC |
717 | 96 | 14 (2.0%) |
7 (1.0%) |
0 (0%) |
| IMPAACT 2010/VESTED 2021, 2023 [29,30] |
Sub-Saharan Africa, Thailand, Brazil, India, U.S. | Pregnant women (14–28 weeks) | DTG + TXF/FTC |
405 | 50 post-partum | 20 (4.9%) |
15 (3.7%) |
1 (0.2%) |
| OPTIMPRIM2 2022 [31] |
France | Adults with primary HIV (1 had M184VI) | DTG + TDF/FTC |
49 | 48 | 3 (6.1%) |
Not reported | 0 (0%) |
| ALLIANCE 2023 [32] |
Europe, North America, Asia | Adults with HBV Infection | DTG + TDF/FTC |
122 | 48 | 7 (5.7%) |
6 (4.9%) |
0 (0%) |
| INSPIRING 2020 [33] |
Europe, South America, Africa, Asia | Adults; Anti-TB therapy ≤ 8 weeks | DTG BID + 2 NRTIs |
69 | 48 | 2 (2.9%) |
2 (2.9%) |
0 (0%) |
| RADIANT-TB 2023 [34] 7 |
South Africa | Adults; Anti-TB therapy ≤ 12 weeks | DTG + TDF/3TC |
52 | 48 | 3 (5.8%) |
3 (5.8%) |
0 (0%) |
| DTG BID + TDF/3TC |
49 | 48 | 3 (6.1%) |
3 (6.1%) |
0 (0%) |
|||
| DTG plus 3TC | ||||||||
| GEMINI-1/GEMINI-2 2019, 2020, 2022 [26,27,28] |
Europe, North America, South America, Asia, Africa | VL ≤ 500,000; no major DRMs |
DTG/3TC | 716 | 96 | 22 (3.1%) |
10 (1.4%) |
0 (0%) |
| ACTG A5353 2018, 2019 [43,44] |
United States | VL ≤ 500,000; no major DRMs | DTG/3TC | 120 | 48 | 6 (5.0%) |
4 (3.3%) |
1 (0.8) |
| STAT 2021, 2023 [45,46] 8 |
United States | Newly diagnosed individuals | DTG/3TC | 131 | 48 | 19 (14.5%) |
2 (1.5%) |
0 (0%) |
| DOLAVI 2022 [47] 9 |
Spain | Newly diagnosed individuals | DTG/3TC | 88 | 48 | 12 (13.7%) |
1 (1.1%) |
0 (0%) |
1 VF was defined as a confirmed plasma HIV-1 RNA level (VL) ≥ 50 copies/mL at the indicated time point for SPRING-2, SINGLE, ADVANCE, GS-US-3801490, GS-US-3801489, SYMTRI, OPTIMPRIM2, ALLIANCE, and in each of the DTG/3TC trials; as a confirmed VL ≥ 200 for GEMINI, FLAMINGO, and IMPAACT 2010/VESTED; as a confirmed VL ≥ 400 for SPRING-1, ARIA and INSPIRING; and as a confirmed VL ≥ 1000 for NAMSAL and RADIANT-TB. IMPAACT 2010/VESTED contained an initial phase in which VL was measured at delivery. At this time, 10 of 405 participants undergoing VL testing experienced VF but GRT results were not reported. In NAMSAL, 20 individuals who discontinued therapy because of withdrawal or loss of follow-up were classified as having VF. The higher rates of VF in RADIANT-TB were considered to largely reflect nonadherence. 2 Although the number undergoing genotypic resistance testing (GRT) in SYMTRI and OPTIMPRIM2 was not noted, the papers specifically indicated that no individual developed INSTI-associated DRMs. 3 At least six individuals developed M184VI including both individuals who developed INSTI DRMs as well as two individuals in ADVANCE, one in ALLIANCE, and one in RADIANT-TB. 4 SPRING-1 was a dose-finding trial. The data shown were from the persons receiving DTG 50 mg per day. 5 In ADVANCE, 351 participants received TDF, and 351 received TAF. Individuals with VF (i.e., VL ≥ 50) were allowed to continue therapy unless the VL was ≥1000. As a result, several individuals with a VL ≥ 50 copies/mL at week 48 attained a VL < 50 in longer-term follow-up following adherence counseling. 6 In NAMSAL, five individuals receiving DTG-based therapy had M184V at baseline. 7 RADIANT-TB also included individuals who had previously interrupted a first-line regimen. 8 In STAT, individuals who were required to change therapy because they were found to be co-infected with HBV or had transmitted M184VI were considered to have experienced VF. 9 In the DOLAVI trial, individuals who were lost to follow-up were also counted among those with VF. Abbreviations: 3TC—lamivudine; ABC—abacavir; ART—antiretroviral therapy; DTG—dolutegravir; DRMs—drug-resistance mutations; GRT—genotypic resistance testing; INSTI—integrase strand transfer inhibitor; NRTIs—nucleoside/nucleotide reverse transcriptase inhibitors; PLWH—people living with HIV; TAF—tenofovir alafenamide; TDF—tenofovir disoproxil fumarate; TXF—TAF or TDF; TB—tuberculosis; VF—virological failure.
DTG plus 3TC (Scenario 2): Two phase 3, one phase 2, and one open-label non-randomized clinical trial examined the prevalence of VF with emergent INSTI DRMs in ART-naïve individuals receiving a first-line DTG plus 3TC regimen (Table 2) [26,27,28,43,44,45,46,47,48]. In each trial, VF was defined as either a single or confirmed VL ≥ 50 copies/mL at week 96 in the GEMINI trials and at week 48 in the remaining trials. Of the 1055 PLWH in these trials, 59 (5.6%) experienced VF, and 17 (1.6%) underwent GRT. One individual (0.1% of the total) developed an INSTI DRM, R263K, in combination with the 3TC-associated DRM M184VI.
Three cohort studies included 581 PLWH who received DTG plus 3TC and were followed for a median of 48 weeks [39,49,50] (Supplementary Table S2). Two studies were from Spain, and one was from China. Four individuals (0.7%) were considered to have VF. GRT was performed on samples from two individuals (0.3%) across the studies, and neither individual had an INSTI DRM.
3.2. ART-Experienced PLWH with a History of VF on an NNRTI-Containing Regimen (Scenario 3)
Table 3 summarizes data from five clinical trials of DTG plus two NRTIs and one trial of DTG plus an optimized background regimen for treating PLWH with a history of VF on a previous NNRTI-containing regimen [51,52,53,54,55,56,57,58,59,60,61,62,63,64]. Four trials were conducted on adults and/or adolescents [51,52,53,54,55,56,57,58,59] and two on infants, children, and/or adolescents [60,61,62,63,64]. VF was defined as a plasma HIV-1 RNA level ≥ 50 copies/mL at week 24 in the ARTIST trial and ≥400 copies/mL at week 48 or 96 in the remaining trials.
Table 3.
Virological failure (VF) and prevalence of emergent INSTI DRMs in clinical trials of ART-experienced PLWH with active virus replication receiving DTG plus 2 NRTIs.
| Trial | Regions | Population 1 | DTG-Containing Regimen 2 | # PLWH |
Weeks | # (%) VF 3 |
# (%) Undergoing GRT | # (%) with INSTI DRMs 4 |
|---|---|---|---|---|---|---|---|---|
| SAILING 2013, 2015 [51,52] |
Europe, North America, South America, Asia, Oceania, Africa | Adults; VL ≥ 400; INSTI-naïve, ≥2-class resistance | DTG + OBR | 354 | 48 | 21 (5.9%) |
9 (2.5%) |
2 (0.6%) |
| DAWNING 2019, 2022 [53,54] |
Europe, South America, Asia, Africa | Adults; VL ≥ 400 on a first-line NNRTI-containing regimen | DTG + 2 NRTIs (≥1 fully active) | 312 | 48 | 11 (3.5%) |
11 (3.5%) |
3 (1.0%) |
| NADIA 2021, 2022 [55,56] 5 |
Uganda, Kenya, Zimbabwe | Adults/Adolescents; VL ≥ 1000 on a first-line NNRTI-containing regimen | DTG + TDF/3TC or AZT/3TC |
235 | 96 | 24 (10.2%) |
21 (8.9%) |
9 (3.8%) |
| ARTIST 2021, 2023 [57,58,59] |
South Africa | Adults; VL ≥ 400 while on a first-line NNRTI-containing regimen | DTG + TDF/3TC |
135 | 24 | 21 (15.6%) |
4 (3.0%) |
0 (0%) |
| DTG BID + TDF/3TC | 64 | 24 | 9 (14.1%) |
3 (4.7%) |
0 (0%) |
|||
| IMPAACT P1093 2015, 2020, 2022 [60,61,62] |
North America, South America, Asia, Africa | Infants/children/adolescents with VL ≥ 1000; most ART-experienced | DTG + 2 NRTIs |
142 | 48 | 36 (25.3%) |
36 (25.3%) |
5 (3.5%) |
| ODYSSEY 2021, 2022 [63,64] |
Europe, Asia, Africa | Children and adolescents with V ≥ 500 on a 1st- or 2nd-line ART regimen | DTG + 2 NRTIs |
196 | 96 | 31 (15.8%) |
29 (14.7%) |
4 (2.0%) |
1 The ODYSSEY trial enrolled 350 participants of whom 154 were ART-naïve and 196 were ART-experienced. For IMPACT P1093, the number shown includes those who were ART-experienced. 2 In NADIA, individuals were randomized 1:1 to receive TDF/3TC or AZT/3TC but the numbers undergoing GRT in each arm were not available. For ODYSSEY and IMPACT P1093, the dose of DTG was based on participant weight. 3 For SAILING, DAWNING, NADIA, IMPACT P1093, and ODYSSEY, VF was defined as a confirmed VL ≥ 400 copies/mL. For ARTIST, VF was defined as a VL ≥ 50 copies/mL. 4 Complete sequences were available only for participants in the DAWNING and IMPACT P1093 trials. For the remaining trials, the table indicates only those DRMs reported by the authors. In the SAILING trial, one individual developed the polymorphic accessory INSTI DRM V151I. After week 48, three additional persons developed DRMs including N155H, N155H + T97A, and R263K + S230R. In the DAWNING trial, four additional individuals developed INSTI DRMs during 100 additional weeks of follow-up including G118R, G118GR + E138EK + Q148QR + R263RK, G118R + T66TI, and Q148H + N155H + E138K + G140S. In IMPACT P1093, the individual with R263RK at week 48 later developed A49G, E138T, and S147G by week 136, while another developed G118R + L74M by week 168. 5 For NADIA the cumulative proportion with VF and those undergoing GRT at week 96 is shown. Among those with INSTI DRMs by week 96, 3 received TDF/3TC and 6 received AZT/3TC. Abbreviations: 3TC—lamivudine; AZT—zidovudine; ART—antiretroviral therapy; DTG—dolutegravir; DRMs—drug-resistance mutations; GRT—genotypic resistance testing; INSTI—integrase strand transfer inhibitor; NRTIs—nucleoside/nucleotide reverse transcriptase inhibitors; OBR—optimized background regimen; PLWH—people living with HIV; TDF—tenofovir disoproxil fumarate; VF—virological failure; VL—plasma virus load measured in copies/mL.
In the SAILING trial, baseline GRT was used to select the ARVs co-administered with DTG to create an optimized background regimen. In the DAWNING trial, baseline GRT was used to exclude individuals in whom no NRTI was predicted to retain antiviral activity. In contrast, in the remaining trials, baseline GRT results were neither available in real time nor used to guide therapy. Retrospective analysis of baseline samples in the NADIA and ARTIST trials indicated high levels of baseline NRTI-associated DRMs. In the NADIA trial, approximately 85% of the baseline samples had the 3TC-resistance mutation M184VI, whereas about 50% had a TDF-resistance mutation at RT position 65 [55].
In the six trials, 153 (10.6%) of the 1428 participants developed VF by week 24 (ARTIST), 48 (SAILING, DAWNING, and IMPAACT P1093), or 96 (NADIA and ODYSSEY). The median proportion with VF was 12.7%, ranging from 3.5% to 25.3%. Overall, 113 (7.9% of the total) had samples undergoing GRT at the time of VF. Among these, 23 (1.6% of the total, or 20.4% of those tested) had samples containing one or more major non-polymorphic INSTI DRMs. Additionally, seven participants (in SAILING, DAWNING, and IMPAACT P1093) were later identified with emergent INSTI DRMs at subsequent time points, specifically weeks 60, 72 (two individuals), 108, 120, and 168 (two individuals) [52,54,62].
Among the 30 individuals that eventually developed INSTI DRMs, the patterns of DRMs were R263K alone (n = 8), G118R alone (n = 5), N155H alone (n = 2), Q148R/K alone (n = 2), R263K + G118R, R263K + S230R, G118R + H51Y + E138K, G118R + T66I, G118R + E92Q, G118R + T66I + E138K, G118R + T66A + E138K, G118R + T66I + E138K + G149A, G118R + T66A + E138K + G149A, G118R + E138K, G118R + E138A + G140A, Q148H + N155H + E138K + G140S, and Q148R + E138A + G140A. Complete integrase sequences were available only for the 12 sequences in the DAWNING and IMPAACT trials.
Of the nine participants with emergent INSTI DRMs in the NADIA trial, six were randomized to zidovudine (AZT) plus 3TC while three were randomized to TDF plus 3TC. The three TDF/3TC recipients with emergent INSTI DRMs each acquired R263K, which was associated with approximately two-fold reduced DTG susceptibility [3]. In contrast, the six AZT/3TC recipients included five who acquired multiple mutations including G118R or Q148R that were predicted to be associated with a high level of reduced DTG susceptibility.
Five cohort studies described the use of DTG plus two NRTIs in PLWH with a history of VF on an NNRTI-containing regimen (Supplementary Table S3). Two were conducted in upper-income countries [65,66] and three in Sub-Saharan Africa [67,68,69]. The two upper-income country cohorts included 374 individuals with variable ART histories of whom approximately 70% were virologically suppressed prior to receiving DTG. Seven (1.9% of the total) individuals underwent GRT at the time of VF, and two developed the INSTI DRM R263K.
The three African cohorts included two cohorts containing 3117 individuals of whom approximately 95% were virologically suppressed on their first-line NNRTI-containing regimen [67,68] and one cohort of 139 individuals of whom just 10% were virologically suppressed [69]. In two cohorts, the proportions of those who were not suppressed at baseline and who developed VF were estimated to be 8% in one study and 10% in the other study [67,69]. GRT was performed in at least 20 individuals in the three cohorts. Two individuals with baseline resistance to TDF and 3TC developed INSTI DRMs–R263K in one individual and G118R in another [67].
Five cross-sectional studies from Sub-Saharan Africa examined the prevalence of INSTI DRMs in PLWH with VF while receiving DTG plus two NRTIs (Supplementary Table S3). The populations in these studies included mixtures of individuals with different ART histories and different proportions with VS prior to receiving DTG. The proportions of individuals with VF undergoing GRT also varied between studies. In a study from Malawi, GRT was performed on 27 samples from more than 6400 individuals with VF [70]. In a study from Nigeria, GRT was performed on 33 samples from 281 individuals with VF [71]. In three studies from Tanzania, GRT was performed in nearly all of the 181 individuals with VF [72,73,74]. Among the estimated 214 individuals in these five studies undergoing GRT, 19 (8.9%) developed an INSTI DRM—most commonly G118R and R263K.
3.3. ART-Experienced PLWH with VS (Scenarios 4, 5, and 6)
DTG plus two NRTIs (Scenario 4): Three clinical trials examined the efficacy of DTG plus two NRTIs in 877 ART-experienced PLWH with VS for six or more months [75,76,77,78] (Table 4). The NEAT022 and STRIIVING trials enrolled individuals with no history of VF or NRTI-associated DRMs. In contrast, the 2SD trial enrolled individuals who were on a second-line ritonavir-boosted protease inhibitor-containing regimen. These individuals were, therefore, likely to have harbored a high proportion of viruses with NRTI-resistance DRMs as a result of VF on their previous first-line regimen.
The proportion of individuals with VF defined as a confirmed plasma HIV-1 RNA level ≥ 50 copies/mL by week 48 in STRIIVING and 2SD and by week 96 in NEAT022 ranged from 0% to 5.0%. Three individuals in NEAT022 and none in STRIIVING or 2SD underwent GRT. In the 2SD trial, 19 of the 20 individuals with VF had a plasma HIV-1 RNA level below 200 copies/mL, and virus amplification was unsuccessful in the one individual with an RNA level above 200 copies/mL.
Three European cohort studies described 2204 PLWH with VS for six or more months on a wide variety of past ART regimens who changed their therapy to DTG plus two NRTIs [79,80,81] (Supplementary Table S4). The proportion with a history of VF prior to VS ranged from 11% to 80%. The proportion with M184VI on a historical GRT ranged from 8% to 80%. VF was reported in 52 (2.4%) individuals after a median of 9 to 21 months. A history of M184VI prior to starting DTG-containing ART was not reported to be associated with an increased risk of VF in any study. Of 15 individuals with VF undergoing GRT, none developed INSTI DRMs.
DTG plus one additional ARV (Scenario 5): Ten clinical trials examined the efficacy of a two-drug DTG-containing regimen in PLWH with VS for six or more months including six trials of DTG/3TC [82,83,84,85,86,87,88,89,90], one trial of DTG/FTC [91], one trial of DTG/rilpivirine [92,93,94], and two trials of DTG plus ritonavir-boosted darunavir (DRV/r) [95,96,97] (Table 4). Among the seven trials of DTG/3TC or DTG/FTC, six enrolling 987 individuals excluded those with a history of VF or major DRMs. In these six trials, 21% to 78% of participants were on an INSTI prior to starting dual therapy, 8 (0.8%) experienced VF and six (0.6%) underwent GRT, but none developed INSTI DRMs. One pilot trial of 41 individuals stratified its enrollment to include 21 individuals with a history of M184VI [88,89,90]. None of the participants in this trial had a history of receiving an INSTI. By week 96, the four individuals with VF in this trial included three with a history of M184VI. None of the four individuals developed emergent INSTI DRMs.
Among the 513 SWORD trial participants randomized to switch to DTG/RPV, the cumulative proportion with VF was 2.7% at week 144. Seven individuals met the criteria for GRT and none developed INSTI DRMs. At least four individuals acquired NNRTI-associated DRMs. Among the 477 control participants who underwent a deferred switch to DTG/rilpivirine, 10 (2.1%) experienced VF by week 48. The one individual undergoing GRT did not acquire INSTI DRMs.
DUALIS and SMILE assessed the efficacy of DTG plus DRV/r at maintaining VS in PLWH with diverse ART histories before attaining VS. SMILE was a study of predominantly perinatally infected children and adolescents. The prevalence of VF at week 48 was 3.8% in DUALIS and 5.1% in SMILE. Six SMILE participants underwent GRT. No participant in either trial acquired an INSTI DRM.
Eighteen observational studies described 5930 PLWH with VS for six or more months on a wide variety of previous ART regimens who changed their therapy primarily to DTG/3TC or DTG/rilpivirine (Supplementary Table S5) [81,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114]. Sixteen studies were from Western Europe, one was from Turkey, and one was from South Korea. The median proportion with a history of VF prior to starting DTG was 25% in the eight studies reporting this information. The median proportion with a history of M184VI was 4.1% in the six studies reporting this information. VF, which was usually defined as a confirmed plasma HIV-1 RNA level ≥ 50 copies/mL or a single RNA level ≥ 1000 copies/mL, was reported in 203 (2.9%) individuals after a median of 12 months. Of the 41 PLWH undergoing GRT in eleven of the studies, three developed emergent INSTI DRMs including R263K; G118R and R263K; and T66A, G118R, and E138K [109,111,113].
DTG monotherapy (Scenario 6): Table 4 also summarizes data from four clinical trials of DTG monotherapy that enrolled 276 PLWH with VS for six or more months [115,116,117,118,119,120,121]. The proportion with a history of receiving an INSTI ranged from 15% and 17% in DOMONO and DOLAM to 60% in EARLY SIMPLIFIED and 100% in MONCAY. Participants in DOMONO and EARLY SIMPLIFIED were required to have no history of VF, while those in DOLAM and MONCAY were required to have no history of VF on an INSTI-containing regimen. Participants in EARLY SIMPLIFIED were required to have initiated ART within six months of their primary HIV-1 infection. VF was defined as a confirmed plasma HIV-1 RNA level ≥ 200 copies/mL in DOMONO and ≥50 copies/mL in the remaining trials.
Table 4.
Virological failure (VF) and prevalence of emergent INSTI-associated DRMs in clinical trials of ART-experienced PLWH with virological suppression (VS) receiving a DTG-containing regimen.
| Trial | Regions | Population | DTG-Containing Regimen | # PLWH |
Weeks | # (%) VF 1 |
# (%) Undergoing GRT 2 |
# (%) INSTI DRMs |
|---|---|---|---|---|---|---|---|---|
| DTG plus 2 NRTIs | ||||||||
| NEAT022 2017, 2019 [75,76] 3 |
Europe | <50 copies x ≥ 6 months on a boosted PI regimen; no h/o VF or NRTI DRMs | DTG + 2 NRTIs | 205 | 96 | 5 (2.4%) |
3 (1.5%) |
0 (0%) |
| STRIIVING 2017 [77] 3 |
North America | <50 copies x ≥ 6M on an NNRTI-, boosted PI, or 1st-generation INSTI regimen; no h/o VF | DTG + ABC/3TC | 275 | 48 | 0 (0%) |
0 (0%) |
0 (0%) |
| 2SD 2023 [78] 4 |
Kenya | <50 copies x ≥ 3M on a 2nd-line boosted PI regimen | DTG + 2 NRTIs | 397 | 48 | 20 (5.0%) |
0 (0%) |
0 (0%) |
| DTG plus a second ARV | ||||||||
| TANGO 2020, 2022 [82,83] |
Europe, North America, Asia, Oceania | <50 copies/mL x ≥ 6 months on a TAF-containing regimen (78% on an INSTI); no h/o of VF or DRMs | DTG/3TC | 369 | 144 | 1 (0.3%) |
0 (0%) |
0 (0%) |
| SALSA 2023 [84] |
Europe, North America, South America, Asia, Africa | <50 copies/mL x ≥ 6 months; 40% on an INSTI; no h/o of VF or DRMs | DTG/3TC | 246 | 48 | 1 (0.4%) |
0 (0%) |
0 (0%) |
| DOLAM–Phase B 2021 [85] 5 |
Spain | <50 copies/mL x ≥ 12 months; 47% on an INSTI; no h/o of VF or DRMs on an XTC- or INSTI-containing regimen | DTG/3TC | 131 | 48 | 3 (2.3%) |
3 (2.3%) |
0 (0%) |
| LAMIDOL 2019 [86] |
France | <50 copies/mL x ≥ 24 months; 21% on an INSTI; no h/o of VF or DRMs | DTG/3TC | 104 | 48 | 1 (1.0%) |
1 (1.0%) |
0 (0%) |
| ASPIRE 2018 [87] |
United States | <50 copies/mL x ≥ 6 months; 37% on an INSTI; no h/o of VF or DRMs | DTG/3TC | 44 | 24 | 1 (2.3%) |
1 (2.3%) |
0 (0%) |
| ART-PRO 2020, 2021, 2022 [88,89,90] |
Spain | <50 copies/mL x ≥ 12 months; 21 had a history of M184VI; single arm pilot trial. | DTG/3TC | 41 | 48 | 0 (0%) |
0 (0%) |
0 (0%) |
| SIMPL’HIV 2020 [91] |
Switzerland | <50 copies/mL x ≥ 6 months; 63% on an INSTI; no h/o VF or DRMs; | DTG/FTC | 93 | 48 | 1 (1.1%) |
1 (1.1%) |
0 (0%) |
| SWORD-1 and 2 2018, 2019, 2020 [92,93,94] 5 |
Europe, North America, Asia, Oceania | <50 copies/mL x ≥ 6 months on a 1st or 2nd ART regimen; 20% on an INSTI; no h/o of VF or DRMs | DTG/RPV | 513 | 144 | 14 (2.7%) |
7 (1.4%) |
0 (0%) |
| DUALIS 2020 [95,96] |
Germany | <50 copies/mL x ≥ 6 months receiving boosted DRV + 2 NRTIs; 7% with prior INSTI use; no h/o DTG or DRV DRMs | DTG/DRV/r | 131 | 48 | 5 (3.8%) |
Not reported |
0 (0%) |
| SMILE 2023 [97] 6 |
Uganda, South Africa, Thailand, Europe, Latin America | Perinatally infected children/adolescents; <50 copies/mL x ≥ 12 months; no h/o DTG or DRV DRMs | DTG/DRV/r | 158 | 48 | 8 (5.1%) |
6 (3.8%) |
0 (0%) |
| DTG monotherapy | ||||||||
| DOMONO 2017, 2018, 2019 7 |
Netherlands | VL < 50 x ≥ 6 months; plasma HIV-1 RNA zenith <105 copies/mL and CD4 count nadir > 200 cells/mm3; 15% INSTI experienced; no h/o VF. | DTG monotherapy | 99 | 48 | 10 (10.1%) |
8 (8.1%) |
4 (4.1%) |
| DOLAM 2018 [118] 8 |
Spain | VL < 50 x ≥ 12 months; CD4 count nadir > 200 cells/mm3; 17% INSTI experienced; no h/o VF on an INSTI regimen. | DTG monotherapy | 31 | 24 | 2 (6.5%) |
2 (6.5%) |
2 (6.5%) |
| EARLY SIMPLIFIED 2019, 2023 [119,120] |
Switzerland | VL < 50 x ≥ 12 months; ART began within 6 months of HIV infection; 60% INSTI experienced; no h/o VF. | DTG monotherapy | 68 | 96 | 0 (0%) |
0 (0%) |
0 (0%) |
| MONCAY 2019 [121] 9 |
France | VL < 50 x ≥ 12 months and on DTG/ABC/3TC x ≥ 1 month; CD4 count nadir > 100 cells/mm3; no h/o VF on an INSTI regimen. | DTG monotherapy | 78 | 48 | 7 (9.0%) |
7 (9.0%) |
2 (2.6%) |
1 VF was defined as a confirmed VL ≥ 50 in each trial except for DOLAM phase B which required a confirmed VL ≥ 50 or a single VL ≥ 1000; LAMIDOL, ASPIRE, and SMILE which required a confirmed VL ≥50, and DOMONO which required a confirmed VL ≥ 200. In STRIIVING, VF did not include individuals who discontinued DTG-containing ART for reasons other than loss of viral efficacy. 2 In STRIIVING, no individual met the criteria for GRT which was two consecutive VL ≥ 400. Only one of 20 individuals with VF in 2SD met the criteria for GRT, VL ≥ 200. But this sample could not be successfully amplified. 3 In both NEAT022 and STRIIVING, participants initially randomized to continue their original ART were subsequently offered a deferred switch to the DTG-containing regimen. The number of individuals undergoing deferred switch and their duration of follow-up was not available, although it was noted that none developed emergent INSTI-associated DRMs. 4 In 2SD, there were 3 NRTI combinations: TDF/3TC (52%), AZT/3TC (44%), and ABC/3TC (4%). 5 In the SWORD trials, there was an immediate switch group of 513 individuals and a late switch group of 477 individuals that switched after one year. Ten (2.0%) of the late switch group experienced VF. Two underwent successful GRT and one was found to have the polymorphic accessory INSTI-resistance mutation V151I. 6 In SMILE, the first five participants randomized to the INSTI arm received elvitegravir; the remaining 153 received DTG. Whether any of those with VF had received elvitegravir was not reported. 7 The DOMONO trial included a pilot study group of four individuals, an immediate switch group of 50 individuals, and a delayed switch group of 45 individuals. The 10 individuals with VF included 6 in the immediate switch, 2 in the delayed switch, and 2 in the pilot study. All four of those with emergent INSTI DRMs had been INSTI-naïve. One of the eight individuals who underwent GRT was found to have 3′ polypurine tract mutations. This individual did not have DRMs in integrase and was not counted among the four with INSTI DRMs. 8 The DOLAM study had two phases. The first phase (phase A, reported in this table) included a monotherapy arm. In phase B, this arm was discontinued. The two individuals in the DOLAM trial reported to have developed INSTI DRMs were INSTI-naïve. 9 Among the 7 individuals with VF, 1 had previously received raltegravir. This individual was not reported to develop INSTI DRMs. One individual developing R263K also had a 3′ polypurine tract mutation. Abbreviations: 3TC—lamivudine; ABC—abacavir; ART—antiretroviral therapy; DTG—dolutegravir; DRMs—drug-resistance mutations; DRV/r—ritonavir-boosted darunavir; FTC—emtricitabine; GRT—genotypic resistance testing; INSTI—integrase strand transfer inhibitor; NRTIs—nucleoside/nucleotide reverse transcriptase inhibitors; PI—protease inhibitor; PLWH—people living with HIV; RPV—rilpivirine; TAF—tenofovir alafenamide; TDF—tenofovir disoproxil fumarate; VF—virological failure; VL—virus load (plasma HIV RNA copies/mL) vs.—virological suppression; XTG—3TC or FTC.
Overall, 19 (6.9%) of 276 individuals experienced VF by week 24 or 48, 17 (6.2%) underwent GRT, and 8 (2.9%) developed one or more INSTI DRMs. INSTI DRMs emerged in one or more participants in each of the trials except for EARLY SIMPLIFIED. The eight individuals with emergent INSTI DRMs acquired the following mutations: R263K (n = 2), N155H, S230R, N155H + E92Q, N155H + S147G, N155H + E138K + G140S, and N155H + Q148R + S147G. Complete integrase sequences were not available for any of the samples undergoing GRT.
One of the eight individuals who underwent GRT in the DOMONO trial was reported to have developed multiple 3′ polypurine tract mutations without DRMs in the integrase gene. One of the seven individuals who underwent GRT in the MONCAY trial was found to have a single 3′ polypurine tract mutation in combination with R263K.
Three small European observational studies described 123 PLWH with VS for six or more months who were treated with DTG monotherapy (Supplementary Table S6) [122,123,124]. Within 24 months, five cases of VF and emergent INSTI DRMs were reported.
4. Discussion
Estimating the prevalence of VF with emergent INSTI DRMs in PLWH receiving a DTG-containing regimen is crucial for updating HIV-1 treatment guidelines, developing strategies to monitor acquired DTG resistance, and determining potential indications for GRT in LMICs, where GRT is not routinely available or recommended. Three of the six clinical scenarios addressed in this review are relevant to the global expansion of DTG-containing regimens in LMICs: ART-naïve PLWH receiving a first-line regimen containing DTG plus two NRTIs (scenario 1); ART-experienced PLWH with VF on a first-line NNRTI-containing regimen switching to a second-line regimen comprising DTG plus two NRTIs (scenario 3); and ART-experienced PLWH with VS switching to a regimen containing DTG plus two NRTIs (scenario 4). In contrast, the use of two-drug regimens for initial ART (scenario 2) or maintenance ART for PLWH with VS (scenario 5) is recommended almost exclusively in upper-income countries while DTG monotherapy (scenario 6) is not recommended in any scenario outside of a clinical trial. For each of the clinical scenarios, Table 5 contains summary data for the 43 clinical trials including the median and interquartile range for the number of participants, the proportion of PLWH with virological failure, the proportion of PLWH undergoing genotypic resistance testing, and the proportion of PLWH with emergent INSTI-associated drug-resistance mutations.
Table 5.
The median prevalence of the proportions of clinical trial participants experiencing virological failure (VF), undergoing genotypic resistance testing (GRT), and developing emergent INSTI-associated DRMs in six clinical scenarios.
| Clinical Scenario | ART History |
Viral Load Prior to DTG |
DTG-Containing ART |
# Clinical Trials | Median (IQR) # Participants |
Median (IQR) % with VF 1 |
Median (IQR) % with GRT 2 |
Median (IQR) % with INSTI-DRMs |
|---|---|---|---|---|---|---|---|---|
| 1 | Naïve | Viremic | DTG + 2 NRTIs | 16 | 279 (106–410) |
4.4 (2.8–6.1) |
2.7 (1.0–5.0) |
0 (0–0) |
| 2 | Naïve | Viremic | DTG + 3TC | 4 | 126 (96–570) |
9.4 (3.6–14.3) |
1.5 (1.2–2.9) |
0 (0–0.6) |
| 3 | Experienced | Viremic | DTG + 2 NRTIs | 6 | 217 (183–323) |
12.7 (5.3–18.2) |
6.6 (3.3–17.4) |
1.5 (0.5–3.6) |
| 4 | Experienced | Suppressed | DTG + 2 NRTIs | 3 | 275 (205–397) |
2.4 (0–5.0) |
0 (0–1.5) |
0 (0–0) |
| 5 | Experienced | Suppressed | DTG + 2nd ARV | 10 | 131 (81–277) |
1.7 (0.4–3.0) |
1.1 (0–2.3) |
0 (0–0) |
| 6 | Experienced | Suppressed | DTG monotherapy | 4 | 73 (40–93) |
7.8 (1.6–9.8) |
7.3 (1.6–8.8) |
3.4 (0.7–5.9) |
1 The definitions of VF differed across and to a lesser extent within each clinical scenario. The definitions are provided in Table 2, Table 3 and Table 4. 2 The criteria for GRT was VF and a plasma HIV-1 RNA considered high enough for successful HIV-1 sequencing, which was usually about 1000 copies/mL. Abbreviations: 3TC—lamivudine; ART—antiviral therapy; ARV—antiretroviral; DTG—dolutegravir; DRMs—drug-resistance mutations; GRT—genotypic resistance testing; INSTI—integrase strand transfer inhibitor; IQR—interquartile range; NRTI—nucleoside reverse transcriptase inhibitor; VF—virological failure.
Of the three scenarios relevant to LMICs, a discernable risk of VF and emergent INSTI DRMs was observed only in clinical scenario 3. The median prevalence of VF and emergent DRMs in this scenario, at 1.5% over a period of 48 to 96 weeks in clinical trials, was lower than anticipated considering that most participants probably had viruses resistant to 3TC and that a significant proportion likely had viruses with reduced TDF susceptibility. However, in three of these trials, a continued risk of VF with emergent INSTI DRMs was observed in the second and third year of follow-up [52,54,62]. Preliminary observations based on limited data from these trials suggest that the risk of VF and emergent INSTI DRMs may be higher in children compared with adults and in those receiving AZT/3TC compared with those receiving TDF/3TC.
Further studies are required to elucidate the factors that influence the risk of VF and emergent INSTI DRMs in this scenario. The often-present reduced susceptibility to the NRTIs combined with DTG raises concerns that some PLWH may be effectively undergoing what is termed functional DTG monotherapy. It is, therefore, critical to investigate whether the risk of VF and INSTI DRMs will escalate with time or if it will plateau after viral suppression has been attained.
In two scenarios, we were able to estimate the prevalence of INSTI DRMs in the subset of individuals developing VF and undergoing GRT. Among ART-naïve PLWH receiving a first-line regimen of DTG plus two NRTIs, 1 (0.7%) of 147 with VF undergoing GRT developed INSTI DRMs. This is much lower than the prevalence of NNRTI-resistance mutations in PLWH with VF on a first-line NNRTI-containing regimen. For example, combined 48- and 96-week data from the SINGLE [11], ADVANCE [16], and NAMSAL [19] trials demonstrated that 25 (58.0%) of 43 PLWH with VF on a first-line EFV-containing regimen developed NNRTI-associated DRMs. The infrequent detection of INSTI DRMs in PLWH with VF on a first-line DTG-containing regimen suggests that incomplete adherence rather than emergent drug resistance is a more significant contributor to VF in those receiving a first-line DTG-containing regimen compared with a first-line NNRTI-containing regimen. This suggests that in PLWH with confirmed VF on a first-line DTG-containing regimen enhancing adherence support may prove more beneficial than transitioning to a second-line ART regimen. In contrast, among ART-experienced PLWH receiving a second-line regimen of DTG plus two NRTIs, 23 (20.4%) of 113 individuals with VF undergoing GRT developed INSTI DRMs suggesting a need to investigate a potential role of GRT in this setting to inform treatment changes.
The 43 clinical trials analyzed in this review described populations that were well-defined in terms of their ART history, plasma HIV-1 RNA level at the time their DTG-containing regimen was initiated, and the ARVs co-administered with DTG. These trials also uniformly reported the proportion of individuals with VF, the proportion undergoing GRT, and the proportion with emergent INSTI DRMs at specific time points. However, clinical trials may report lower estimates of VF with emergent drug resistance because trial participants are more likely to be adherent to ART than PLWH in real-world settings and because, in a trial, GRT is performed promptly following the detection of VF, reducing the time HIV replicates while an individual is receiving suboptimal therapy. Moreover, participants in a clinical trial of first-line ART are usually required to be ART-naïve. In contrast, in real-world settings, a significant proportion of individuals presenting for first-line ART are re-initiating therapy and may harbor acquired NRTI-associated DRMs [125,126,127].
Two additional clinical trials of DTG-containing ART in adults with VF on an NNRTI-containing regimen were reported at scientific meetings. In the VISEND trial, 10% of 208 PLWH treated with DTG plus TDF/3TC and 14% treated with DTG plus tenofovir alafenamide (TAF)/FTC experienced VF at week 48 [128]. In the D2EFT trial, 22% of 291 PLWH treated with DTG plus TDF/3TC or TDF/FTC experienced VF at week 48 [129]. Data on emergent INSTI DRMs in these trials have yet to be reported.
Observational cohorts theoretically provide a more cost-effective approach for estimating the prevalence of VF with emergent INSTI DRMs in real-world settings. However, we found that, in practice, few published cohort studies contributed relevant quantitative data because all but four were from upper-income countries where ART histories were highly variable and where decisions about whether to switch to a DTG-containing regimen and ARVs to administer with DTG were highly individualized. Moreover, in the clinical cohorts, GRT was performed at the discretion of care providers rather than according to well-defined criteria and may, therefore, have underestimated the prevalence of emergent INSTI-associated DRMs.
Cross-sectional studies in which GRT is performed in persons with confirmed VF on a DTG-containing regimen provide an alternate mechanism for assessing the risk of emergent DTG resistance. We described six such studies including one from Brazil of 113 PLWH with confirmed VF on a first-line regimen of DTG plus two NRTIs [42] and five from Sub-Saharan Africa. The studies from Sub-Saharan Africa included approximately 214 individuals, most of whom had prior ART experience before initiating DTG treatment [70,71,72,73,74]. The study from Brazil found that approximately six percent of the samples tested were found to have INSTI DRMs, whereas in the studies from Sub-Saharan Africa, approximately nine percent of the samples tested had INSTI DRMs. However, in these studies, either the total number of persons receiving DTG-containing ART or their past ART histories were unknown, making it impossible to estimate the prevalence of VF with emergent INSTI DRMs in any particular clinical scenario.
Our review has several limitations. First, the substantial heterogeneity observed in the study populations, methodologies, definitions of VF, availability of GRT, and follow-up durations precluded a meta-analysis. Additionally, variations such as whether PLWH were administered single- or multi-tablet DTG-containing regimens could have impacted the risk of VF and the development of INSTI DRMs. Second, our literature search was confined to the PubMed database; however, during the examination of 345 full-text publications, we encountered no references to relevant data outside of this database. Third, to maintain conciseness in our tables, we excluded data from 14 clinical trials and 46 cohort studies that either focused on pharmacokinetics or included fewer than 30 PLWH, as they reported no emergent INSTI DRMs.
With the rising global adoption of DTG-based antiretroviral therapy (ART) and prolonged treatment durations for PLWH, the development of uniform protocols to surveil for acquired DTG resistance is crucial. The WHO has devised several survey models [130], which have been complemented by additional methodologies implemented by the U.S. Centers for Disease Control and Prevention (CDC) [131,132,133,134,135]. These cost-effective, cross-sectional studies focus on PLWH with VF during DTG-based ART, as opposed to more resource-intensive longitudinal cohort studies. Recent presentations at a scientific meeting highlighted four CDC collaborative studies in Sub-Saharan Africa [132,133,134,135]. The number of samples tested per study ranged from 65 to 512 and the proportion of samples with an INSTI DRM ranged from 3% to 21%. To improve the impact of such studies, integrating estimates of the total population under treatment and comprehensive ART histories would provide a more accurate projection of resistance risk across the treated demographic.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/v16030399/s1, Table S1: Virological Failure (VF) and Prevalence of Emergent INSTI-Associated DRMs in Cohorts of ART-Naïve PLWH Receiving DTG Plus Two NRTIs; Table S2: Virological Failure (VF) and Emergent INSTI-Associated DRMs in Cohorts of ART-Naïve PLWH Receiving DTG Plus 3TC; Table S3: Virological Failure (VF) and Emergent INSTI-Associated DRMs in Cohort Studies Describing the Use of DTG Plus Two NRTIs in PLWH With Previous VF Who Were Not Uniformly Virologically Suppressed at DTG Initiation; Table S4: Virological Failure (VF) and Emergent INSTI-Associated DRMs in Cohorts of ART-Experienced PLWH with VS Receiving DTG Plus Two NRTIs; Table S5: Virological Failure (VF) and Emergent INSTI-Associated DRMs in Cohorts of ART-Experienced PLWH with VS Receiving DTG Plus a 2nd ARV; Table S6: Virological Failure (VF) and Emergent INSTI-Associated DRMs in Cohorts of ART-Experienced PLWH with VS Receiving DTG Monotherapy.
Author Contributions
Conceptualization: R.W.S. and M.R.J.; Literature review: R.W.S., C.C., K.T., V.K., A.A., J.S., P.M.G. and S.-Y.R.; Writing: R.W.S., C.C., M.R.J., S.M.M. and R.L.M. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study does not involve human participants.
Conflicts of Interest
Robert W. Shafer has received honoraria for participation in advisory boards from Gilead Sciences and GlaxoSmithKline and speaking honoraria from Gilead Sciences and ViiV Healthcare.
Funding Statement
This work was funded by a grant from the National Institutes of Health: 2R24AI13661806. The funder played no role in this review.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.World Health Organization Consolidated Guidelines on HIV Prevention, Testing, Treatment, Service Delivery and Monitoring: Recommendations for a Public Health Approach. [(accessed on 17 August 2022)]. Available online: https://www.who.int/publications-detail-redirect/9789240031593. [PubMed]
- 2.Clinton Health Access Initiative . The State of HIV Treatment, Testing, and Prevention in Low- and Middle-Income Countries. Clinton Health Access Initiative; Boston, MA, USA: 2023. HIV Market Report 2023. [Google Scholar]
- 3.Tao K., Rhee S.-Y., Chu C., Avalos A., Ahluwalia A.K., Gupta R.K., Jordan M.R., Shafer R.W. Treatment Emergent Dolutegravir Resistance Mutations in Individuals Naïve to HIV-1 Integrase Inhibitors: A Rapid Scoping Review. Viruses. 2023;15:1932. doi: 10.3390/v15091932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tzou P.L., Rhee S.-Y., Descamps D., Clutter D.S., Hare B., Mor O., Grude M., Parkin N., Jordan M.R., Bertagnolio S., et al. Integrase Strand Transfer Inhibitor (INSTI)-Resistance Mutations for the Surveillance of Transmitted HIV-1 Drug Resistance. J. Antimicrob. Chemother. 2020;75:170–182. doi: 10.1093/jac/dkz417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tricco A.C., Lillie E., Zarin W., O’Brien K.K., Colquhoun H., Levac D., Moher D., Peters M.D.J., Horsley T., Weeks L., et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018;169:467–473. doi: 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- 6.van Lunzen J., Maggiolo F., Arribas J.R., Rakhmanova A., Yeni P., Young B., Rockstroh J.K., Almond S., Song I., Brothers C., et al. Once Daily Dolutegravir (S/GSK1349572) in Combination Therapy in Antiretroviral-Naive Adults with HIV: Planned Interim 48 Week Results from SPRING-1, a Dose-Ranging, Randomised, Phase 2b Trial. Lancet Infect. Dis. 2012;12:111–118. doi: 10.1016/S1473-3099(11)70290-0. [DOI] [PubMed] [Google Scholar]
- 7.Stellbrink H.-J., Reynes J., Lazzarin A., Voronin E., Pulido F., Felizarta F., Almond S., St Clair M., Flack N., Min S., et al. Dolutegravir in Antiretroviral-Naive Adults with HIV-1: 96-Week Results from a Randomized Dose-Ranging Study. AIDS. 2013;27:1771–1778. doi: 10.1097/QAD.0b013e3283612419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raffi F., Rachlis A., Stellbrink H.-J., Hardy W.D., Torti C., Orkin C., Bloch M., Podzamczer D., Pokrovsky V., Pulido F., et al. Once-Daily Dolutegravir versus Raltegravir in Antiretroviral-Naive Adults with HIV-1 Infection: 48 Week Results from the Randomised, Double-Blind, Non-Inferiority SPRING-2 Study. Lancet. 2013;381:735–743. doi: 10.1016/S0140-6736(12)61853-4. [DOI] [PubMed] [Google Scholar]
- 9.Raffi F., Jaeger H., Quiros-Roldan E., Albrecht H., Belonosova E., Gatell J.M., Baril J.-G., Domingo P., Brennan C., Almond S., et al. Once-Daily Dolutegravir versus Twice-Daily Raltegravir in Antiretroviral-Naive Adults with HIV-1 Infection (SPRING-2 Study): 96 Week Results from a Randomised, Double-Blind, Non-Inferiority Trial. Lancet Infect. Dis. 2013;13:927–935. doi: 10.1016/S1473-3099(13)70257-3. [DOI] [PubMed] [Google Scholar]
- 10.Walmsley S.L., Antela A., Clumeck N., Duiculescu D., Eberhard A., Gutiérrez F., Hocqueloux L., Maggiolo F., Sandkovsky U., Granier C., et al. Dolutegravir plus Abacavir–Lamivudine for the Treatment of HIV-1 Infection. N. Engl. J. Med. 2013;369:1807–1818. doi: 10.1056/NEJMoa1215541. [DOI] [PubMed] [Google Scholar]
- 11.Walmsley S., Baumgarten A., Berenguer J., Felizarta F., Florence E., Khuong-Josses M.-A., Kilby J.M., Lutz T., Podzamczer D., Portilla J., et al. Brief Report: Dolutegravir Plus Abacavir/Lamivudine for the Treatment of HIV-1 Infection in Antiretroviral Therapy-Naive Patients: Week 96 and Week 144 Results From the SINGLE Randomized Clinical Trial. JAIDS J. Acquir. Immune Defic. Syndr. 2015;70:515–519. doi: 10.1097/QAI.0000000000000790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clotet B., Feinberg J., van Lunzen J., Khuong-Josses M.-A., Antinori A., Dumitru I., Pokrovskiy V., Fehr J., Ortiz R., Saag M., et al. Once-Daily Dolutegravir versus Darunavir plus Ritonavir in Antiretroviral-Naive Adults with HIV-1 Infection (FLAMINGO): 48 Week Results from the Randomised Open-Label Phase 3b Study. Lancet. 2014;383:2222–2231. doi: 10.1016/S0140-6736(14)60084-2. [DOI] [PubMed] [Google Scholar]
- 13.Molina J.-M., Clotet B., van Lunzen J., Lazzarin A., Cavassini M., Henry K., Kulagin V., Givens N., de Oliveira C.F., Brennan C. Once-Daily Dolutegravir versus Darunavir plus Ritonavir for Treatment-Naive Adults with HIV-1 Infection (FLAMINGO): 96 Week Results from a Randomised, Open-Label, Phase 3b Study. Lancet HIV. 2015;2:e127–e136. doi: 10.1016/S2352-3018(15)00027-2. [DOI] [PubMed] [Google Scholar]
- 14.Orrell C., Hagins D.P., Belonosova E., Porteiro N., Walmsley S., Falcó V., Man C.Y., Aylott A., Buchanan A.M., Wynne B., et al. Fixed-Dose Combination Dolutegravir, Abacavir, and Lamivudine versus Ritonavir-Boosted Atazanavir plus Tenofovir Disoproxil Fumarate and Emtricitabine in Previously Untreated Women with HIV-1 Infection (ARIA): Week 48 Results from a Randomised, Open-Label, Non-Inferiority, Phase 3b Study. Lancet HIV. 2017;4:e536–e546. doi: 10.1016/S2352-3018(17)30095-4. [DOI] [PubMed] [Google Scholar]
- 15.Venter W.D.F., Moorhouse M., Sokhela S., Fairlie L., Mashabane N., Masenya M., Serenata C., Akpomiemie G., Qavi A., Chandiwana N., et al. Dolutegravir plus Two Different Prodrugs of Tenofovir to Treat HIV. N. Engl. J. Med. 2019;381:803–815. doi: 10.1056/NEJMoa1902824. [DOI] [PubMed] [Google Scholar]
- 16.Venter W.D.F., Sokhela S., Simmons B., Moorhouse M., Fairlie L., Mashabane N., Serenata C., Akpomiemie G., Masenya M., Qavi A., et al. Dolutegravir with Emtricitabine and Tenofovir Alafenamide or Tenofovir Disoproxil Fumarate versus Efavirenz, Emtricitabine, and Tenofovir Disoproxil Fumarate for Initial Treatment of HIV-1 Infection (ADVANCE): Week 96 Results from a Randomised, Phase 3, Non-Inferiority Trial. Lancet HIV. 2020;7:e666–e676. doi: 10.1016/S2352-3018(20)30241-1. [DOI] [PubMed] [Google Scholar]
- 17.Pepperrell T., Venter W.D.F., McCann K., Bosch B., Tibbatts M., Woods J., Sokhela S., Serenata C., Moorhouse M., Qavi A., et al. Participants on Dolutegravir Resuppress Human Immunodeficiency Virus RNA After Virologic Failure: Updated Data from the ADVANCE Trial. Clin. Infect. Dis. 2021;73:e1008–e1010. doi: 10.1093/cid/ciab086. [DOI] [PubMed] [Google Scholar]
- 18.Kouanfack C., The NAMSAL ANRS 12313 Study Group Dolutegravir-Based or Low-Dose Efavirenz–Based Regimen for the Treatment of HIV-1. N. Engl. J. Med. 2019;381:816–826. doi: 10.1056/NEJMoa1904340. [DOI] [PubMed] [Google Scholar]
- 19.Calmy A., Tovar Sanchez T., Kouanfack C., Mpoudi-Etame M., Leroy S., Perrineau S., Lantche Wandji M., Tetsa Tata D., Omgba Bassega P., Abong Bwenda T., et al. Dolutegravir-Based and Low-Dose Efavirenz-Based Regimen for the Initial Treatment of HIV-1 Infection (NAMSAL): Week 96 Results from a Two-Group, Multicentre, Randomised, Open Label, Phase 3 Non-Inferiority Trial in Cameroon. Lancet HIV. 2020;7:e677–e687. doi: 10.1016/S2352-3018(20)30238-1. [DOI] [PubMed] [Google Scholar]
- 20.Sax P.E., Pozniak A., Montes M.L., Koenig E., DeJesus E., Stellbrink H.-J., Antinori A., Workowski K., Slim J., Reynes J., et al. Coformulated Bictegravir, Emtricitabine, and Tenofovir Alafenamide versus Dolutegravir with Emtricitabine and Tenofovir Alafenamide, for Initial Treatment of HIV-1 Infection (GS-US-380–1490): A Randomised, Double-Blind, Multicentre, Phase 3, Non-Inferiority Trial. Lancet. 2017;390:2073–2082. doi: 10.1016/S0140-6736(17)32340-1. [DOI] [PubMed] [Google Scholar]
- 21.Sax P.E., DeJesus E., Crofoot G., Ward D., Benson P., Dretler R., Mills A., Brinson C., Peloquin J., Wei X., et al. Bictegravir versus Dolutegravir, Each with Emtricitabine and Tenofovir Alafenamide, for Initial Treatment of HIV-1 Infection: A Randomised, Double-Blind, Phase 2 Trial. Lancet HIV. 2017;4:e154–e160. doi: 10.1016/S2352-3018(17)30016-4. [DOI] [PubMed] [Google Scholar]
- 22.Stellbrink H.-J., Arribas J.R., Stephens J.L., Albrecht H., Sax P.E., Maggiolo F., Creticos C., Martorell C.T., Wei X., Acosta R., et al. Co-Formulated Bictegravir, Emtricitabine, and Tenofovir Alafenamide versus Dolutegravir with Emtricitabine and Tenofovir Alafenamide for Initial Treatment of HIV-1 Infection: Week 96 Results from a Randomised, Double-Blind, Multicentre, Phase 3, Non-Inferiority Trial. Lancet HIV. 2019;6:e364–e372. doi: 10.1016/S2352-3018(19)30080-3. [DOI] [PubMed] [Google Scholar]
- 23.Gallant J., Lazzarin A., Mills A., Orkin C., Podzamczer D., Tebas P., Girard P.-M., Brar I., Daar E.S., Wohl D., et al. Bictegravir, Emtricitabine, and Tenofovir Alafenamide versus Dolutegravir, Abacavir, and Lamivudine for Initial Treatment of HIV-1 Infection (GS-US-380-1489): A Double-Blind, Multicentre, Phase 3, Randomised Controlled Non-Inferiority Trial. Lancet. 2017;390:2063–2072. doi: 10.1016/S0140-6736(17)32299-7. [DOI] [PubMed] [Google Scholar]
- 24.Wohl D.A., Yazdanpanah Y., Baumgarten A., Clarke A., Thompson M.A., Brinson C., Hagins D., Ramgopal M.N., Antinori A., Wei X., et al. Bictegravir Combined with Emtricitabine and Tenofovir Alafenamide versus Dolutegravir, Abacavir, and Lamivudine for Initial Treatment of HIV-1 Infection: Week 96 Results from a Randomised, Double-Blind, Multicentre, Phase 3, Non-Inferiority Trial. Lancet HIV. 2019;6:e355–e363. doi: 10.1016/S2352-3018(19)30077-3. [DOI] [PubMed] [Google Scholar]
- 25.Podzamczer D., Micán R., Tiraboschi J., Portilla J., Domingo P., Llibre J.M., Ribera E., Vivancos M.J., Morano L., Masiá M., et al. Darunavir/Cobicistat/Emtricitabine/Tenofovir Alafenamide Versus Dolutegravir/Abacavir/Lamivudine in Antiretroviral-Naive Adults (SYMTRI): A Multicenter Randomized Open-Label Study (PReEC/RIS-57) Open Forum Infect. Dis. 2022;9:ofab595. doi: 10.1093/ofid/ofab595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cahn P., Madero J.S., Arribas J.R., Antinori A., Ortiz R., Clarke A.E., Hung C.-C., Rockstroh J.K., Girard P.-M., Sievers J., et al. Dolutegravir plus Lamivudine versus Dolutegravir plus Tenofovir Disoproxil Fumarate and Emtricitabine in Antiretroviral-Naive Adults with HIV-1 Infection (GEMINI-1 and GEMINI-2): Week 48 Results from Two Multicentre, Double-Blind, Randomised, Non-Inferiority, Phase 3 Trials. Lancet. 2019;393:143–155. doi: 10.1016/S0140-6736(18)32462-0. [DOI] [PubMed] [Google Scholar]
- 27.Cahn P., Madero J.S., Arribas J.R., Antinori A., Ortiz R., Clarke A.E., Hung C.-C., Rockstroh J.K., Girard P.-M., Sievers J., et al. Durable Efficacy of Dolutegravir Plus Lamivudine in Antiretroviral Treatment–Naive Adults with HIV-1 Infection: 96-Week Results from the GEMINI-1 and GEMINI-2 Randomized Clinical Trials. JAIDS J. Acquir. Immune Defic. Syndr. 2020;83:310–318. doi: 10.1097/QAI.0000000000002275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cahn P., Sierra Madero J., Arribas J.R., Antinori A., Ortiz R., Clarke A.E., Hung C.-C., Rockstroh J.K., Girard P.-M., Sievers J., et al. Three-Year Durable Efficacy of Dolutegravir plus Lamivudine in Antiretroviral Therapy—Naive Adults with HIV-1 Infection. AIDS. 2022;36:39–48. doi: 10.1097/QAD.0000000000003070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lockman S., Brummel S.S., Ziemba L., Stranix-Chibanda L., McCarthy K., Coletti A., Jean-Philippe P., Johnston B., Krotje C., Fairlie L., et al. Efficacy and Safety of Dolutegravir with Emtricitabine and Tenofovir Alafenamide Fumarate or Tenofovir Disoproxil Fumarate, and Efavirenz, Emtricitabine, and Tenofovir Disoproxil Fumarate HIV Antiretroviral Therapy Regimens Started in Pregnancy (IMPAACT 2010/VESTED): A Multicentre, Open-Label, Randomised, Controlled, Phase 3 Trial. Lancet. 2021;397:1276–1292. doi: 10.1016/S0140-6736(21)00314-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chinula L., Ziemba L., Brummel S., McCarthy K., Coletti A., Krotje C., Johnston B., Knowles K., Moyo S., Stranix-Chibanda L., et al. Efficacy and Safety of Three Antiretroviral Therapy Regimens Started in Pregnancy up to 50 Weeks Post Partum: A Multicentre, Open-Label, Randomised, Controlled, Phase 3 Trial. Lancet HIV. 2023;10:e363–e374. doi: 10.1016/S2352-3018(23)00061-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chéret A., Bauer R., Meiffrédy V., Lopez P., Ajana F., Lacombe K., Morlat P., Lascoux C., Reynes J., Calin R., et al. Once-Daily Dolutegravir versus Darunavir plus Cobicistat in Adults at the Time of Primary HIV-1 Infection: The OPTIPRIM2-ANRS 169 Randomized, Open-Label, Phase 3 Trial. J. Antimicrob. Chemother. 2022;77:2506–2515. doi: 10.1093/jac/dkac207. [DOI] [PubMed] [Google Scholar]
- 32.Avihingsanon A., Lu H., Leong C.L., Hung C.-C., Koenig E., Kiertiburanakul S., Lee M.-P., Supparatpinyo K., Zhang F., Rahman S., et al. Bictegravir, Emtricitabine, and Tenofovir Alafenamide versus Dolutegravir, Emtricitabine, and Tenofovir Disoproxil Fumarate for Initial Treatment of HIV-1 and Hepatitis B Coinfection (ALLIANCE): A Double-Blind, Multicentre, Randomised Controlled, Phase 3 Non-Inferiority Trial. Lancet HIV. 2023;10:e640–e652. doi: 10.1016/S2352-3018(23)00151-0. [DOI] [PubMed] [Google Scholar]
- 33.Dooley K.E., Kaplan R., Mwelase N., Grinsztejn B., Ticona E., Lacerda M., Sued O., Belonosova E., Ait-Khaled M., Angelis K., et al. Dolutegravir-Based Antiretroviral Therapy for Patients Coinfected with Tuberculosis and Human Immunodeficiency Virus: A Multicenter, Noncomparative, Open-Label, Randomized Trial. Clin. Infect. Dis. 2020;70:549–556. doi: 10.1093/cid/ciz256. [DOI] [PubMed] [Google Scholar]
- 34.Griesel R., Zhao Y., Simmons B., Omar Z., Wiesner L., Keene C.M., Hill A.M., Meintjes G., Maartens G. Standard-Dose versus Double-Dose Dolutegravir in HIV-Associated Tuberculosis in South Africa (RADIANT-TB): A Phase 2, Non-Comparative, Randomised Controlled Trial. Lancet HIV. 2023;10:e433–e441. doi: 10.1016/S2352-3018(23)00081-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grayhack C., Sheth A., Kirby O., Davis J., Sibliss K., Nkwihoreze H., Aaron E., Alleyne G., Laguerre R., Rana A., et al. Evaluating Outcomes of Mother–Infant Pairs Using Dolutegravir for HIV Treatment during Pregnancy. AIDS. 2018;32:2017–2021. doi: 10.1097/QAD.0000000000001931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rhee S.-Y., Clutter D., Hare C.B., Tchakoute C.T., Sainani K., Fessel W.J., Hurley L., Slome S., Pinsky B.A., Silverberg M.J., et al. Virological Failure and Acquired Genotypic Resistance Associated with Contemporary Antiretroviral Treatment Regimens. Open Forum Infect. Dis. 2020;7:ofaa316. doi: 10.1093/ofid/ofaa316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mondi A., Cozzi-Lepri A., Tavelli A., Rusconi S., Vichi F., Ceccherini-Silberstein F., Calcagno A., De Luca A., Maggiolo F., Marchetti G., et al. Effectiveness of Dolutegravir-Based Regimens as Either First-Line or Switch Antiretroviral Therapy: Data from the Icona Cohort. J. Int. AIDS Soc. 2019;22:e25227. doi: 10.1002/jia2.25227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Armenia D., Bouba Y., Gagliardini R., Gori C., Bertoli A., Borghi V., Gennari W., Micheli V., Callegaro A.P., Gazzola L., et al. Evaluation of Virological Response and Resistance Profile in HIV-1 Infected Patients Starting a First-Line Integrase Inhibitor-Based Regimen in Clinical Settings. J. Clin. Virol. 2020;130:104534. doi: 10.1016/j.jcv.2020.104534. [DOI] [PubMed] [Google Scholar]
- 39.Suárez-García I., Alejos B., Hernando V., Viñuela L., Vera García M., Rial-Crestelo D., Pérez Elías M.J., Albendín Iglesias H., Peraire J., Tiraboschi J., et al. Effectiveness and Tolerability of Dolutegravir/Lamivudine for the Treatment of HIV-1 Infection in Clinical Practice. J. Antimicrob. Chemother. 2023;78:1423–1432. doi: 10.1093/jac/dkad102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chin B.S., Lee J.H., Kim G. Similar Durability of Two Single Tablet Regimens, Dolutegravir/Abacavir/Lamivudine and Elvitegravir/Cobicistat/Tenofovir/Emtricitabine: Single Center Experience. J. Korean Med. Sci. 2020;35:e235. doi: 10.3346/jkms.2020.35.e235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ntamatungiro A.J., Eichenberger A., Okuma J., Vanobberghen F., Ndege R., Kimera N., Francis J.M., Kagura J., Weisser M., for the Kilombero and Ulanga Antiretroviral Cohort (KIULARCO) Study Group Transitioning to Dolutegravir in a Programmatic Setting: Virological Outcomes and Associated Factors Among Treatment-Naive Patients with HIV-1 in the Kilombero and Ulanga Antiretroviral Cohort in Rural Tanzania. Open Forum Infect. Dis. 2023;10:ofad321. doi: 10.1093/ofid/ofad321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Diaz R.S., Hunter J.R., Camargo M., Dias D., Galinskas J., Nassar I., De Lima I.B., Caldeira D.B., Sucupira M.C., Schechter M. Dolutegravir-Associated Resistance Mutations after First-Line Treatment Failure in Brazil. BMC Infect. Dis. 2023;23:347. doi: 10.1186/s12879-023-08288-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taiwo B.O., Zheng L., Stefanescu A., Nyaku A., Bezins B., Wallis C.L., Godfrey C., Sax P.E., Acosta E., Haas D., et al. ACTG A5353: A Pilot Study of Dolutegravir Plus Lamivudine for Initial Treatment of Human Immunodeficiency Virus-1 (HIV-1)–Infected Participants with HIV-1 RNA <500000 Copies/mL. Clin. Infect. Dis. 2018;66:1689–1697. doi: 10.1093/cid/cix1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nyaku A.N., Zheng L., Gulick R.M., Olefsky M., Berzins B., Wallis C.L., Godfrey C., Sax P.E., Acosta E.P., Haas D.W., et al. Dolutegravir plus Lamivudine for Initial Treatment of HIV-1-Infected Participants with HIV-1 RNA <500 000 Copies/mL: Week 48 Outcomes from ACTG 5353. J. Antimicrob. Chemother. 2019;74:1376–1380. doi: 10.1093/jac/dky564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rolle C.-P., Berhe M., Singh T., Ortiz R., Wurapa A., Ramgopal M., Leone P.A., Matthews J.E., Dalessandro M., Underwood M.R., et al. Dolutegravir/Lamivudine as a First-Line Regimen in a Test-and-Treat Setting for Newly Diagnosed People Living with HIV. AIDS. 2021;35:1957–1965. doi: 10.1097/QAD.0000000000002979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rolle C.-P., Berhe M., Singh T., Ortiz R., Wurapa A., Ramgopal M., Jayaweera D.T., Leone P.A., Matthews J.E., Cupo M., et al. Sustained Virologic Suppression with Dolutegravir/Lamivudine in a Test-and-Treat Setting through 48 Weeks. Open Forum Infect. Dis. 2023;10:ofad101. doi: 10.1093/ofid/ofad101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hidalgo-Tenorio C., Pasquau J., Vinuesa D., Ferra S., Terrón A., SanJoaquín I., Payeras A., Martínez O.J., López-Ruz M.Á., Omar M., et al. DOLAVI Real-Life Study of Dolutegravir Plus Lamivudine in Naive HIV-1 Patients (48 Weeks) Viruses. 2022;14:524. doi: 10.3390/v14030524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cahn P., Rolón M.J., Figueroa M.I., Gun A., Patterson P., Sued O. Dolutegravir–Lamivudine as Initial Therapy in HIV-1 Infected, ARV-Naive Patients, 48-Week Results of the PADDLE (Pilot Antiretroviral Design with Dolutegravir LamivudinE) Study. J. Int. AIDS Soc. 2017;20:21678. doi: 10.7448/IAS.20.01.21678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cabello-Ubeda A., de Quirós J.C.L.B., Carbonero L.M., Sanz J., Vergas J., Mena Á., Torralba M., Segurado M.H., Pinto A., Tejerina F., et al. 48-Week Effectiveness and Tolerability of Dolutegravir (DTG) + Lamivudine (3TC) in Antiretroviral-Naïve Adults Living with HIV: A Multicenter Real-Life Cohort. PLoS ONE. 2022;17:e0277606. doi: 10.1371/journal.pone.0277606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li J., Chen D., Wen Z., Du Y., Huang Z., Zhong H., Wang Y., Yin S. Real-World Efficacy and Safety of Dolutegravir plus Lamivudine versus Tenofovir plus Lamivudine and Efavirenz in ART-Naïve HIV-1-Infected Adults. Medicine. 2022;101:e31100. doi: 10.1097/MD.0000000000031100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cahn P., Pozniak A.L., Mingrone H., Shuldyakov A., Brites C., Andrade-Villanueva J.F., Richmond G., Buendia C.B., Fourie J., Ramgopal M., et al. Dolutegravir versus Raltegravir in Antiretroviral-Experienced, Integrase-Inhibitor-Naive Adults with HIV: Week 48 Results from the Randomised, Double-Blind, Non-Inferiority SAILING Study. Lancet. 2013;382:700–708. doi: 10.1016/S0140-6736(13)61221-0. [DOI] [PubMed] [Google Scholar]
- 52.Underwood M. Euro Resistance Wk: Resistance Post Week 48 in ART-Experienced, Integrase Inhibitor-Naive Subjects with Dolutegravir (DTG) vs. Raltegravir (RAL) in SAILING (ING111762); Proceedings of the 13th European HIV & Hepatitis Workshop; Barcelona, Spain. 3–5 June 2015. [Google Scholar]
- 53.Aboud M., Kaplan R., Lombaard J., Zhang F., Hidalgo J.A., Mamedova E., Losso M.H., Chetchotisakd P., Brites C., Sievers J., et al. Dolutegravir versus Ritonavir-Boosted Lopinavir Both with Dual Nucleoside Reverse Transcriptase Inhibitor Therapy in Adults with HIV-1 Infection in Whom First-Line Therapy Has Failed (DAWNING): An Open-Label, Non-Inferiority, Phase 3b Trial. Lancet Infect. Dis. 2019;19:253–264. doi: 10.1016/S1473-3099(19)30036-2. [DOI] [PubMed] [Google Scholar]
- 54.Underwood M., Horton J., Nangle K., Hopking J., Smith K., Aboud M., Wynne B., Sievers J., Stewart E.L., Wang R. Integrase Inhibitor Resistance Mechanisms and Structural Characteristics in Antiretroviral Therapy-Experienced, Integrase Inhibitor-Naive Adults with HIV-1 Infection Treated with Dolutegravir plus Two Nucleoside Reverse Transcriptase Inhibitors in the DAWNING Study. Antimicrob. Agents Chemother. 2022;66:e0164321. doi: 10.1128/AAC.01643-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Paton N.I., Musaazi J., Kityo C., Walimbwa S., Hoppe A., Balyegisawa A., Kaimal A., Mirembe G., Tukamushabe P., Ategeka G., et al. Dolutegravir or Darunavir in Combination with Zidovudine or Tenofovir to Treat HIV. N. Engl. J. Med. 2021;385:330–341. doi: 10.1056/NEJMoa2101609. [DOI] [PubMed] [Google Scholar]
- 56.Paton N.I., Musaazi J., Kityo C., Walimbwa S., Hoppe A., Balyegisawa A., Asienzo J., Kaimal A., Mirembe G., Lugemwa A., et al. Efficacy and Safety of Dolutegravir or Darunavir in Combination with Lamivudine plus Either Zidovudine or Tenofovir for Second-Line Treatment of HIV Infection (NADIA): Week 96 Results from a Prospective, Multicentre, Open-Label, Factorial, Randomised, Non-Inferiority Trial. Lancet HIV. 2022;9:e381–e393. doi: 10.1016/S2352-3018(22)00092-3. [DOI] [PubMed] [Google Scholar]
- 57.Keene C.M., Griesel R., Zhao Y., Gcwabe Z., Sayed K., Hill A., Cassidy T., Ngwenya O., Jackson A., van Zyl G., et al. Virologic Efficacy of Tenofovir, Lamivudine and Dolutegravir as Second-Line Antiretroviral Therapy in Adults Failing a Tenofovir-Based First-Line Regimen. AIDS. 2021;35:1423–1432. doi: 10.1097/QAD.0000000000002936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Keene C.M., Cassidy T., Zhao Y., Griesel R., Jackson A., Sayed K., Omar Z., Hill A., Ngwenya O., Van Zyl G., et al. Recycling Tenofovir in Second-Line Antiretroviral Treatment with Dolutegravir: Outcomes and Viral Load Trajectories to 72 Weeks. JAIDS J. Acquir. Immune Defic. Syndr. 2023;92:422–429. doi: 10.1097/QAI.0000000000003157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhao Y., Griesel R., Omar Z., Simmons B., Hill A., van Zyl G., Keene C., Maartens G., Meintjes G. Initial Supplementary Dose of Dolutegravir in Second-Line Antiretroviral Therapy: A Noncomparative, Double-Blind, Randomized Placebo-Controlled Trial. Clin. Infect. Dis. 2023;76:1832–1840. doi: 10.1093/cid/ciad023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Viani R.M., Alvero C., Fenton T., Acosta E.P., Hazra R., Townley E., Steimers D., Min S., Wiznia A., on Behalf of the P1093 Study Team Safety, Pharmacokinetics and Efficacy of Dolutegravir in Treatment-Experienced HIV-1 Infected Adolescents: Forty-Eight-Week Results from IMPAACT P1093. Pediatr. Infect. Dis. J. 2015;34:1207–1213. doi: 10.1097/INF.0000000000000848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Viani R.M., Ruel T., Alvero C., Fenton T., Acosta E.P., Hazra R., Townley E., Palumbo P., Buchanan A.M., Vavro C., et al. Long-Term Safety and Efficacy of Dolutegravir in Treatment-Experienced Adolescents with Human Immunodeficiency Virus Infection: Results of the IMPAACT P1093 Study. J. Pediatr. Infect. Dis. Soc. 2020;9:159–165. doi: 10.1093/jpids/piy139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vavro C., Ruel T., Wiznia A., Montañez N., Nangle K., Horton J., Buchanan A.M., Stewart E.L., Palumbo P. Emergence of Resistance in HIV-1 Integrase with Dolutegravir Treatment in a Pediatric Population from the IMPAACT P1093 Study. Antimicrob. Agents Chemother. 2022;66:e0164521. doi: 10.1128/AAC.01645-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Turkova A., White E., Mujuru H.A., Kekitiinwa A.R., Kityo C.M., Violari A., Lugemwa A., Cressey T.R., Musoke P., Variava E., et al. Dolutegravir as First- or Second-Line Treatment for HIV-1 Infection in Children. N. Engl. J. Med. 2021;385:2531–2543. doi: 10.1056/NEJMoa2108793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Amuge P., Lugemwa A., Wynne B., Mujuru H.A., Violari A., Kityo C.M., Archary M., Variava E., White E., Turner R.M., et al. Once-Daily Dolutegravir-Based Antiretroviral Therapy in Infants and Children Living with HIV from Age 4 Weeks: Results from the below 14 Kg Cohort in the Randomised ODYSSEY Trial. Lancet HIV. 2022;9:e638–e648. doi: 10.1016/S2352-3018(22)00163-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lepik K.J., Harrigan P.R., Yip B., Wang L., Robbins M.A., Zhang W.W., Toy J., Akagi L., Lima V.D., Guillemi S., et al. Emergent Drug Resistance with Integrase Strand Transfer Inhibitor-Based Regimens. AIDS. 2017;31:1425–1434. doi: 10.1097/QAD.0000000000001494. [DOI] [PubMed] [Google Scholar]
- 66.Sörstedt E., Carlander C., Flamholc L., Hejdeman B., Svedhem V., Sönnerborg A., Gisslén M., Yilmaz A. Effect of Dolutegravir in Combination with Nucleoside Reverse Transcriptase Inhibitors (NRTIs) on People Living with HIV Who Have Pre-Existing NRTI Mutations. Int. J. Antimicrob. Agents. 2018;51:733–738. doi: 10.1016/j.ijantimicag.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 67.Schramm B., Temfack E., Descamps D., Nicholas S., Peytavin G., Bitilinyu-Bangoh J.E., Storto A., Lê M.P., Abdi B., Ousley J., et al. Viral Suppression and HIV-1 Drug Resistance 1 Year after Pragmatic Transitioning to Dolutegravir First-Line Therapy in Malawi: A Prospective Cohort Study. Lancet HIV. 2022;9:e544–e553. doi: 10.1016/S2352-3018(22)00136-9. [DOI] [PubMed] [Google Scholar]
- 68.Brown J.A., Nsakala B.L., Mokhele K., Rakuoane I., Muhairwe J., Urda L., Amstutz A., Tschumi N., Klimkait T., Labhardt N.D. Viral Suppression after Transition from Nonnucleoside Reverse Transcriptase Inhibitor- to Dolutegravir-Based Antiretroviral Therapy: A Prospective Cohort Study in Lesotho (DO-REAL Study) HIV Med. 2022;23:287–293. doi: 10.1111/hiv.13189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Semengue E.N.J., Fokam J., Etame N.-K., Molimbou E., Chenwi C.A., Takou D., Mossiang L., Meledie A.P., Yagai B., Nka A.D., et al. Dolutegravir-Based Regimen Ensures High Virological Success despite Prior Exposure to Efavirenz-Based First-LINE ART in Cameroon: An Evidence of a Successful Transition Model. Viruses. 2023;15:18. doi: 10.3390/v15010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.van Oosterhout J.J., Chipungu C., Nkhoma L., Kanise H., Hosseinipour M.C., Sagno J.B., Simon K., Cox C., Hoffman R., Steegen K., et al. Dolutegravir Resistance in Malawi’s National HIV Treatment Program. Open Forum Infect. Dis. 2022;9:ofac148. doi: 10.1093/ofid/ofac148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Abdullahi A., Kida I.M., Maina U.A., Ibrahim A.H., Mshelia J., Wisso H., Adamu A., Onyemata J.E., Edun M., Yusuph H., et al. Limited Emergence of Resistance to Integrase Strand Transfer Inhibitors (INSTIs) in ART-Experienced Participants Failing Dolutegravir-Based Antiretroviral Therapy: A Cross-Sectional Analysis of a Northeast Nigerian Cohort. J. Antimicrob. Chemother. 2023;78:2000–2007. doi: 10.1093/jac/dkad195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kamori D., Barabona G., Rugemalila J., Maokola W., Masoud S.S., Mizinduko M., Sabasaba A., Ruhago G., Sambu V., Mushi J., et al. Emerging Integrase Strand Transfer Inhibitor Drug Resistance Mutations among Children and Adults on ART in Tanzania: Findings from a National Representative HIV Drug Resistance Survey. J. Antimicrob. Chemother. 2023;78:779–787. doi: 10.1093/jac/dkad010. [DOI] [PubMed] [Google Scholar]
- 73.Khamadi S.A., Bahemana E., Dear N., Mavere C., George F., Kapene R., Papianus G., Willoughby W., Chambers J., Ganesan K., et al. Factors Associated with Viral Suppression and Drug Resistance in Children and Adolescents Living with HIV in Care and Treatment Programs in Southern Tanzania. J. Pediatr. Infect. Dis. Soc. 2023;12:353–363. doi: 10.1093/jpids/piad040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bwire G.M., Aiko B.G., Mosha I.H., Kilapilo M.S., Mangara A., Kazonda P., Swai J.P., Swalehe O., Jordan M.R., Vercauteren J., et al. High Viral Suppression and Detection of Dolutegravir-Resistance Associated Mutations in Treatment-Experienced Tanzanian Adults Living with HIV-1 in Dar Es Salaam. Sci. Rep. 2023;13:20493. doi: 10.1038/s41598-023-47795-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gatell J.M., Assoumou L., Moyle G., Waters L., Johnson M., Domingo P., Fox J., Martinez E., Stellbrink H.-J., Guaraldi G., et al. Switching from a Ritonavir-Boosted Protease Inhibitor to a Dolutegravir-Based Regimen for Maintenance of HIV Viral Suppression in Patients with High Cardiovascular Risk. AIDS. 2017;31:2503–2514. doi: 10.1097/QAD.0000000000001675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gatell J.M., Assoumou L., Moyle G., Waters L., Johnson M., Domingo P., Fox J., Martinez E., Stellbrink H.-J., Guaraldi G., et al. Immediate Versus Deferred Switching from a Boosted Protease Inhibitor–Based Regimen to a Dolutegravir-Based Regimen in Virologically Suppressed Patients with High Cardiovascular Risk or Age ≥50 Years: Final 96-Week Results of the NEAT022 Study. Clin. Infect. Dis. 2019;68:597–606. doi: 10.1093/cid/ciy505. [DOI] [PubMed] [Google Scholar]
- 77.Trottier B., Lake J.E., Logue K., Brinson C., Santiago L., Brennan C., Koteff J.A., Wynne B., Hopking J., Granier C., et al. Dolutegravir/Abacavir/Lamivudine versus Current ART in Virally Suppressed Patients (STRIIVING): A 48-Week, Randomized, Non-Inferiority, Open-Label, Phase IIIb Study. Antivir. Ther. 2017;22:295–305. doi: 10.3851/IMP3166. [DOI] [PubMed] [Google Scholar]
- 78.Ombajo L.A., Penner J., Nkuranga J., Mecha J., Mburu M., Odhiambo C., Ndinya F., Aksam R., Njenga R., Wahome S., et al. Second-Line Switch to Dolutegravir for Treatment of HIV Infection. N. Engl. J. Med. 2023;388:2349–2359. doi: 10.1056/NEJMoa2210005. [DOI] [PubMed] [Google Scholar]
- 79.Olearo F., Nguyen H., Bonnet F., Yerly S., Wandeler G., Stoeckle M., Cavassini M., Scherrer A., Costagiola D., Schmid P., et al. Impact of the M184V/I Mutation on the Efficacy of Abacavir/Lamivudine/Dolutegravir Therapy in HIV Treatment-Experienced Patients. Open Forum Infect. Dis. 2019;6:ofz330. doi: 10.1093/ofid/ofz330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jary A., Marcelin A.-G., Charpentier C., Wirden M., Lê M.P., Peytavin G., Descamps D., Calvez V. M184V/I Does Not Impact the Efficacy of Abacavir/Lamivudine/Dolutegravir Use as Switch Therapy in Virologically Suppressed Patients. J. Antimicrob. Chemother. 2020;75:1290–1293. doi: 10.1093/jac/dkaa019. [DOI] [PubMed] [Google Scholar]
- 81.Borghetti A., Alkhatib M., Dusina A., Duca L., Borghi V., Zazzi M., Di Giambenedetto S. Virological Outcomes with Dolutegravir plus Either Lamivudine or Two NRTIs as Switch Strategies: A Multi-Cohort Study. J. Antimicrob. Chemother. 2022;77:740–746. doi: 10.1093/jac/dkab429. [DOI] [PubMed] [Google Scholar]
- 82.van Wyk J., Ajana F., Bisshop F., De Wit S., Osiyemi O., Portilla Sogorb J., Routy J.-P., Wyen C., Ait-Khaled M., Nascimento M.C., et al. Efficacy and Safety of Switching to Dolutegravir/Lamivudine Fixed-Dose 2-Drug Regimen vs. Continuing a Tenofovir Alafenamide–Based 3- or 4-Drug Regimen for Maintenance of Virologic Suppression in Adults Living with Human Immunodeficiency Virus Type 1: Phase 3, Randomized, Noninferiority TANGO Study. Clin. Infect. Dis. 2020;71:1920–1929. doi: 10.1093/cid/ciz1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Osiyemi O., De Wit S., Ajana F., Bisshop F., Portilla J., Routy J.P., Wyen C., Ait-Khaled M., Leone P., Pappa K.A., et al. Efficacy and Safety of Switching to Dolutegravir/Lamivudine Versus Continuing a Tenofovir Alafenamide–Based 3- or 4-Drug Regimen for Maintenance of Virologic Suppression in Adults Living with Human Immunodeficiency Virus Type 1: Results through Week 144 From the Phase 3, Noninferiority TANGO Randomized Trial. Clin. Infect. Dis. 2022;75:975–986. doi: 10.1093/cid/ciac036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Llibre J.M., Brites C., Cheng C.-Y., Osiyemi O., Galera C., Hocqueloux L., Maggiolo F., Degen O., Taylor S., Blair E., et al. Efficacy and Safety of Switching to the 2-Drug Regimen Dolutegravir/Lamivudine Versus Continuing a 3- or 4-Drug Regimen for Maintaining Virologic Suppression in Adults Living with Human Immunodeficiency Virus 1 (HIV-1): Week 48 Results from the Phase 3, Noninferiority SALSA Randomized Trial. Clin. Infect. Dis. 2023;76:720–729. doi: 10.1093/cid/ciac130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rojas J., de Lazzari E., Negredo E., Domingo P., Tiraboschi J., Ribera E., Abdulghani N., Puig J., Mateo M.G., Podzamczer D., et al. Efficacy and Safety of Switching to Dolutegravir plus Lamivudine versus Continuing Triple Antiretroviral Therapy in Virologically Suppressed Adults with HIV at 48 Weeks (DOLAM): A Randomised Non-Inferiority Trial. Lancet HIV. 2021;8:e463–e473. doi: 10.1016/S2352-3018(21)00100-4. [DOI] [PubMed] [Google Scholar]
- 86.Joly V., Burdet C., Landman R., Vigan M., Charpentier C., Katlama C., Cabié A., Benalycherif A., Peytavin G., Yeni P., et al. Dolutegravir and Lamivudine Maintenance Therapy in HIV-1 Virologically Suppressed Patients: Results of the ANRS 167 Trial (LAMIDOL) J. Antimicrob. Chemother. 2019;74:739–745. doi: 10.1093/jac/dky467. [DOI] [PubMed] [Google Scholar]
- 87.Taiwo B.O., Marconi V.C., Berzins B., Moser C.B., Nyaku A.N., Fichtenbaum C.J., Benson C.A., Wilkin T., Koletar S.L., Colasanti J., et al. Dolutegravir Plus Lamivudine Maintains Human Immunodeficiency Virus-1 Suppression Through Week 48 in a Pilot Randomized Trial. Clin. Infect. Dis. 2018;66:1794–1797. doi: 10.1093/cid/cix1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.De Miguel Buckley R., Rial-Crestelo D., Montejano R., Pinto A., Jimenez-Gonzalez M., Lagarde M., Esteban-Cantos A., Aranguren-Rivas P., Cadiñanos J., Bisbal O., et al. Long-Term Evaluation of Residual Viremia in a Clinical Trial of Dolutegravir Plus Lamivudine as Maintenance Treatment for Participants with and without Prior Lamivudine Resistance. Open Forum Infect. Dis. 2022;9:ofac610. doi: 10.1093/ofid/ofac610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rial-Crestelo D., de Miguel R., Montejano R., Dominguez-Dominguez L., Aranguren-Rivas P., Esteban-Cantos A., Bisbal O., Santacreu-Guerrero M., Garcia-Alvarez M., Alejos B., et al. Long-Term Efficacy of Dolutegravir plus Lamivudine for Maintenance of HIV Viral Suppression in Adults with and without Historical Resistance to Lamivudine: Week 96 Results of ART-PRO Pilot Study. J. Antimicrob. Chemother. 2021;76:738–742. doi: 10.1093/jac/dkaa479. [DOI] [PubMed] [Google Scholar]
- 90.De Miguel R., Rial-Crestelo D., Dominguez-Dominguez L., Montejano R., Esteban-Cantos A., Aranguren-Rivas P., Stella-Ascariz N., Bisbal O., Bermejo-Plaza L., Garcia-Alvarez M., et al. Dolutegravir plus Lamivudine for Maintenance of HIV Viral Suppression in Adults with and without Historical Resistance to Lamivudine: 48-Week Results of a Non-Randomized, Pilot Clinical Trial (ART-PRO) EBioMedicine. 2020;55:102779. doi: 10.1016/j.ebiom.2020.102779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sculier D., Wandeler G., Yerly S., Marinosci A., Stoeckle M., Bernasconi E., Braun D.L., Vernazza P., Cavassini M., Buzzi M., et al. Efficacy and Safety of Dolutegravir plus Emtricitabine versus Standard ART for the Maintenance of HIV-1 Suppression: 48-Week Results of the Factorial, Randomized, Non-Inferiority SIMPL’HIV Trial. PLoS Med. 2020;17:e1003421. doi: 10.1371/journal.pmed.1003421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Llibre J.M., Hung C.-C., Brinson C., Castelli F., Girard P.-M., Kahl L.P., Blair E.A., Angelis K., Wynne B., Vandermeulen K., et al. Efficacy, Safety, and Tolerability of Dolutegravir-Rilpivirine for the Maintenance of Virological Suppression in Adults with HIV-1: Phase 3, Randomised, Non-Inferiority SWORD-1 and SWORD-2 Studies. Lancet. 2018;391:839–849. doi: 10.1016/S0140-6736(17)33095-7. [DOI] [PubMed] [Google Scholar]
- 93.Aboud M., Orkin C., Podzamczer D., Bogner J.R., Baker D., Khuong-Josses M.-A., Parks D., Angelis K., Kahl L.P., Blair E.A., et al. Efficacy and Safety of Dolutegravir–Rilpivirine for Maintenance of Virological Suppression in Adults with HIV-1: 100-Week Data from the Randomised, Open-Label, Phase 3 SWORD-1 and SWORD-2 Studies. Lancet HIV. 2019;6:e576–e587. doi: 10.1016/S2352-3018(19)30149-3. [DOI] [PubMed] [Google Scholar]
- 94.van Wyk J., Orkin C., Rubio R., Bogner J., Baker D., Khuong-Josses M.-A., Parks D., Angelis K., Kahl L.P., Matthews J., et al. Brief Report: Durable Suppression and Low Rate of Virologic Failure 3 Years After Switch to Dolutegravir + Rilpivirine 2-Drug Regimen: 148-Week Results From the SWORD-1 and SWORD-2 Randomized Clinical Trials. J. Acquir. Immune Defic. Syndr. 2020;85:325–330. doi: 10.1097/QAI.0000000000002449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Spinner C.D., Kümmerle T., Schneider J., Cordes C., Heiken H., Stellbrink H.-J., Krznaric I., Scholten S., Jensen B., Wyen C., et al. Efficacy and Safety of Switching to Dolutegravir with Boosted Darunavir in Virologically Suppressed Adults with HIV-1: A Randomized, Open-Label, Multicenter, Phase 3, Noninferiority Trial: The DUALIS Study. Open Forum Infect. Dis. 2020;7:ofaa356. doi: 10.1093/ofid/ofaa356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wolf E., Boesecke C., Balogh A., Bidner H., Cordes C., Heiken H., Krznaric I., Kümmerle T., Stellbrink H.-J., Schneider J., et al. Virologic Outcomes of Switching to Boosted Darunavir plus Dolutegravir with Respect to History of Drug Resistance. AIDS Res. Ther. 2021;18:58. doi: 10.1186/s12981-021-00384-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Compagnucci A., Chan M.K., Saïdi Y., Cressey T.R., Bamford A., Riault Y., Coelho A., Nolan A., Chalermpantmetagul S., Morkunaite G., et al. Nucleoside/Nucleotide Reverse Transcriptase Inhibitor Sparing Regimen with Once Daily Integrase Inhibitor plus Boosted Darunavir Is Non-Inferior to Standard of Care in Virologically-Suppressed Children and Adolescents Living with HIV—Week 48 Results of the Randomised SMILE Penta-17-ANRS 152 Clinical Trial. EClinicalMedicine. 2023;60:102025. doi: 10.1016/j.eclinm.2023.102025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gantner P., Cuzin L., Allavena C., Cabie A., Pugliese P., Valantin M.-A., Bani-Sadr F., Joly V., Ferry T., Poizot-Martin I., et al. Efficacy and Safety of Dolutegravir and Rilpivirine Dual Therapy as a Simplification Strategy: A Cohort Study. HIV Med. 2017;18:704–708. doi: 10.1111/hiv.12506. [DOI] [PubMed] [Google Scholar]
- 99.Baldin G., Ciccullo A., Borghetti A., Di Giambenedetto S. Virological Efficacy of Dual Therapy with Lamivudine and Dolutegravir in HIV-1-Infected Virologically Suppressed Patients: Long-Term Data from Clinical Practice. J. Antimicrob. Chemother. 2019;74:1461–1463. doi: 10.1093/jac/dkz009. [DOI] [PubMed] [Google Scholar]
- 100.Casado J.L., Monsalvo M., Fontecha M., Vizcarra P., Rodriguez M.A., Vivancos M.J., Moreno S. Dolutegravir plus Rilpivirine as Dual Regimen in Virologically Suppressed HIV-1 Infected Patients in a Clinical Setting. HIV Res. Clin. Pract. 2019;20:64–72. doi: 10.1080/15284336.2019.1628460. [DOI] [PubMed] [Google Scholar]
- 101.Castagna A., Rusconi S., Gulminetti R., Bonora S., Mazzola G., Quiros-Roldan M.E., De Socio G.V., Ladisa N., Carosella S., Cattelan A., et al. Switch to Dolutegravir and Unboosted Atazanavir in HIV-1 Infected Patients with Undetectable Viral Load and Long Exposure to Antiretroviral Therapy. AIDS. 2019;33:1256–1260. doi: 10.1097/QAD.0000000000002188. [DOI] [PubMed] [Google Scholar]
- 102.Calza L., Colangeli V., Borderi M., Testi D., Granozzi B., Bon I., Re M.C., Viale P. Simplification to Dual Therapy Containing Lamivudine and Raltegravir or Dolutegravir in HIV-Infected Patients on Virologically Suppressive Antiretroviral Therapy. J. Antimicrob. Chemother. 2020;75:3327–3333. doi: 10.1093/jac/dkaa319. [DOI] [PubMed] [Google Scholar]
- 103.Ciccullo A., Borghi V., Giacomelli A., Cossu M.V., Sterrantino G., Latini A., Giacometti A., De Vito A., Gennari W., Madeddu G., et al. Five Years with Dolutegravir Plus Lamivudine as a Switch Strategy: Much More Than a Positive Finding. JAIDS J. Acquir. Immune Defic. Syndr. 2021;88:234. doi: 10.1097/QAI.0000000000002787. [DOI] [PubMed] [Google Scholar]
- 104.Ciccullo A., Baldin G., Borghi V., Cossu M.V., Giacomelli A., Lagi F., Farinacci D., Iannone V., Passerotto R.A., Capetti A., et al. Analysing the Efficacy and Tolerability of Dolutegravir plus Either Rilpivirine or Lamivudine in a Multicentre Cohort of Virologically Suppressed PLWHIV. J. Antimicrob. Chemother. 2023;78:117–121. doi: 10.1093/jac/dkac362. [DOI] [PubMed] [Google Scholar]
- 105.Ergen P., Bektas B., Aydın Ö., Keskin H., Üçışık A.C., Karadağ F.Y., Cağ Y. Evaluation of Treatment Efficacy after Switching to Dolutegravir-Lamivudine Dual Therapy in People Living with HIV. Afr. Health Sci. 2022;22:426–435. doi: 10.4314/ahs.v22i3.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lee K.H., Kim J., Lee J.A., Kim C.H., Ahn J.Y., Jeong S.J., Ku N.S., Choi J.Y., Yeom J.-S., Song Y.G., et al. Real-World Effectiveness, Tolerability, and Safety of Dolutegravir/Lamivudine in Korea. Viruses. 2022;14:2558. doi: 10.3390/v14112558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Maggiolo F., Gulminetti R., Pagnucco L., Digaetano M., Cervo A., Valenti D., Callegaro A., Mussini C. Long-Term Outcome of Lamivudine/Dolutegravir Dual Therapy in HIV-Infected, Virologically Suppressed Patients. BMC Infect. Dis. 2022;22:782. doi: 10.1186/s12879-022-07769-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Troya J., Dueñas C., Irazola I., de los Santos I., de la Fuente S., Gil D., Hernández C., Galindo M.J., Gómez J., Delgado E., et al. Dolutegravir plus Rilpivirine: Benefits beyond Viral Suppression: DORIPEX Retrospective Study. Medicine. 2022;101:e29252. doi: 10.1097/MD.0000000000029252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bowman C., Ambrose A., Kanitkar T., Flores K., Simoes P., Hart J., Hunter A., Akodu J., Barber T.J. Real World Use of Dolutegravir Two Drug Regimens. AIDS. 2023;37:785–788. doi: 10.1097/QAD.0000000000003480. [DOI] [PubMed] [Google Scholar]
- 110.Buzón L., Dueñas C., Pedrero R., Iribarren J.A., de los Santos I., Díaz de Santiago A., Morán M.Á., Pousada G., Moreno E., Ferreira E., et al. Dolutegravir Plus 3TC in Virologically Suppressed PLWHIV: Immunological Outcomes in a Multicenter Retrospective Cohort in Spain during the COVID-19 Pandemic. Viruses. 2023;15:322. doi: 10.3390/v15020322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Palmier E., De Miguel R., Montejano R., Busca C., Micán R., Ramos L., Cadiñanos J., Serrano L., Bernardino J.I., Pérez-Valero I., et al. Three-Year Efficacy of Switching to Dolutegravir plus Lamivudine: A Real-World Study. HIV Med. 2023;24:1013–1019. doi: 10.1111/hiv.13500. [DOI] [PubMed] [Google Scholar]
- 112.Calza L., Colangeli V., Legnani G., Cretella S., Bon I., Viale P. Efficacy and Safety of Switching to Dolutegravir/Lamivudine in Virologically Suppressed People Living with HIV-1 Aged over 65 Years. AIDS Res. Hum. Retroviruses. 2023;40:73–79. doi: 10.1089/aid.2023.0046. [DOI] [PubMed] [Google Scholar]
- 113.Knobel H., Cañas-Ruano E., Guelar A., Knobel P., Villar-García J., González-Mena A., Canepa C., Arrieta-Aldea I., Marcos A., Abalat-Torrres A., et al. Switching to Dolutegravir/Lamivudine or Bictegravir/Emtricitabine/Tenofovir Alafenamide. A Comparative Real-World Study. HIV Res. Clin. Pract. 2023;24:2239564. [PubMed] [Google Scholar]
- 114.Poliseno M., Mazzitelli M., Narducci A., Ferrara S.M., Resnati C., Gervasoni C., Cattelan A.M., Lo Caputo S. Doravirine Plus Integrase Strand Transfer Inhibitors as a 2-Drug Treatment–Switch Strategy in People Living with HIV: The Real-Life DORINI Multicentric Cohort Study. JAIDS J. Acquir. Immune Defic. Syndr. 2023;94:235–243. doi: 10.1097/QAI.0000000000003248. [DOI] [PubMed] [Google Scholar]
- 115.Wijting I., Rokx C., Boucher C., van Kampen J., Pas S., de Vries-Sluijs T., Schurink C., Bax H., Derksen M., Andrinopoulou E.-R., et al. Dolutegravir as Maintenance Monotherapy for HIV (DOMONO): A Phase 2, Randomised Non-Inferiority Trial. Lancet HIV. 2017;4:e547–e554. doi: 10.1016/S2352-3018(17)30152-2. [DOI] [PubMed] [Google Scholar]
- 116.Wijting I.E.A., Lungu C., Rijnders B.J.A., van der Ende M.E., Pham H.T., Mesplede T., Pas S.D., Voermans J.J.C., Schuurman R., van de Vijver D.A.M.C., et al. HIV-1 Resistance Dynamics in Patients with Virologic Failure to Dolutegravir Maintenance Monotherapy. J. Infect. Dis. 2018;218:688–697. doi: 10.1093/infdis/jiy176. [DOI] [PubMed] [Google Scholar]
- 117.Wijting I., Rutsaert S., Rokx C., Burger D., Verbon A., van Kampen J., Boucher C., Rijnders B., Vandekerckhove L. Predictors of Virological Failure in HIV-1-Infected Patients Switching to Dolutegravir Maintenance Monotherapy. HIV Med. 2019;20:63–68. doi: 10.1111/hiv.12675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Blanco J.L., Rojas J., Paredes R., Negredo E., Mallolas J., Casadella M., Clotet B., Gatell J.M., de Lazzari E., Martinez E., et al. Dolutegravir-Based Maintenance Monotherapy versus Dual Therapy with Lamivudine: A Planned 24 Week Analysis of the DOLAM Randomized Clinical Trial. J. Antimicrob. Chemother. 2018;73:1965–1971. doi: 10.1093/jac/dky093. [DOI] [PubMed] [Google Scholar]
- 119.Braun D.L., Turk T., Tschumi F., Grube C., Hampel B., Depmeier C., Schreiber P.W., Brugger S.D., Greiner M., Steffens D., et al. Noninferiority of Simplified Dolutegravir Monotherapy Compared to Continued Combination Antiretroviral Therapy That Was Initiated During Primary Human Immunodeficiency Virus Infection: A Randomized, Controlled, Multisite, Open-Label, Noninferiority Trial. Clin. Infect. Dis. 2019;69:1489–1497. doi: 10.1093/cid/ciy1131. [DOI] [PubMed] [Google Scholar]
- 120.West E., Zeeb M., Grube C., Kuster H., Wanner K., Scheier T., Neumann K., Jörimann L., Hampel B., Metzner K.J., et al. Sustained Viral Suppression with Dolutegravir Monotherapy over 192 Weeks in Patients Starting Combination Antiretroviral Therapy during Primary HIV Infection (EARLY-SIMPLIFIED): A Randomized, Controlled, Multi-Site, Non-Inferiority Trial. Clin. Infect. Dis. 2023;77:1012–1020. doi: 10.1093/cid/ciad366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hocqueloux L., Raffi F., Prazuck T., Bernard L., Sunder S., Esnault J.-L., Rey D., Le Moal G., Roncato-Saberan M., André M., et al. Dolutegravir Monotherapy Versus Dolutegravir/Abacavir/Lamivudine for Virologically Suppressed People Living with Chronic Human Immunodeficiency Virus Infection: The Randomized Noninferiority MONotherapy of TiviCAY Trial. Clin. Infect. Dis. 2019;69:1498–1505. doi: 10.1093/cid/ciy1132. [DOI] [PubMed] [Google Scholar]
- 122.Rojas J., Blanco J.L., Marcos M.A., Lonca M., Tricas A., Moreno L., Gonzalez-Cordon A., Torres B., Mallolas J., Garcia F., et al. Dolutegravir Monotherapy in HIV-Infected Patients with Sustained Viral Suppression. J. Antimicrob. Chemother. 2016;71:1975–1981. doi: 10.1093/jac/dkw078. [DOI] [PubMed] [Google Scholar]
- 123.Oldenbuettel C., Wolf E., Ritter A., Noe S., Heldwein S., Pascucci R., Wiese C., Krosigk A.V., Jaegel-Guedes E., Jaeger H., et al. Dolutegravir Monotherapy as Treatment De-Escalation in HIV-Infected Adults with Virological Control: DoluMono Cohort Results. Antivir. Ther. 2017;22:169–172. doi: 10.3851/IMP3082. [DOI] [PubMed] [Google Scholar]
- 124.Tebano G., Soulié C., Schneider L., Blanc C., Agher R., Seang S., Valantin M.A., Palich R., Tubiana R., Peytavin G., et al. Long-Term Follow-up of HIV-Infected Patients on Dolutegravir Monotherapy. J. Antimicrob. Chemother. 2020;75:675–680. doi: 10.1093/jac/dkz478. [DOI] [PubMed] [Google Scholar]
- 125.Gregson J., Kaleebu P., Marconi V.C., Van Vuuren C., Ndembi N., Hamers R.L., Kanki P., Hoffmann C.J., Lockman S., Pillay D., et al. Occult HIV-1 Drug Resistance to Thymidine Analogues Following Failure of First-Line Tenofovir Combined with a Cytosine Analogue and Nevirapine or Efavirenz in Sub Saharan Africa: A Retrospective Multi-Centre Cohort Study. Lancet Infect. Dis. 2017;17:296–304. doi: 10.1016/S1473-3099(16)30469-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.World Health Organization . HIV Drug Resistance Report 2021. WHO; Geneva, Switzerland: 2021. [Google Scholar]
- 127.Benade M., Maskew M., Juntunen A., Flynn D.B., Rosen S. Prior Exposure to Antiretroviral Therapy among Adult Patients Presenting for HIV Treatment Initiation or Reinitiation in Sub-Saharan Africa: A Systematic Review. BMJ Open. 2023;13:e071283. doi: 10.1136/bmjopen-2022-071283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mulenga L.B., Fwoloshi S., Mweemba A., Siwingwa M., Sivile S., Kampamba D., Engamba D.C., Mbewe N., Phiri H., Shibemba A., et al. Dolutegravir with Recycled Nrtis Is Noninferior to Pi-Based Art: Visend Trial; Proceedings of the 29th Conference on Retroviruses and Opportunistic Infections; Virtual. 12–16 February 2022. [Google Scholar]
- 129.Matthews G., Borok M., Eriobou N., Kaplan R., Kumarasamy N., Avihingsanon A., Losso M.H., Azwa I.S., Karyana M., Dao S., et al. D2EFT: Dolutegravir and Darunavir Evaluation in Adults Failing First-Line Hiv Therapy; Proceedings of the 30th Conference on Retroviruses and Opportunistic Infections; Seattle, WA, USA. 19–22 February 2023. [Google Scholar]
- 130.World Health Organization . Sentinel Surveys of Acquired HIV Resistance to Dolutegravir among People Receiving Dolutegravir Containing Antiviral Therapy. WHO; Geneva, Switzerland: 2022. [Google Scholar]
- 131.da Silva J., Pals S., Chang J., Hackett S., Godfrey C., Raizes E. Monitoring Emerging Human Immunodeficiency Virus Drug Resistance in Sub-Saharan Africa in the Era of Dolutegravir. J. Infect. Dis. 2022;225:364–366. doi: 10.1093/infdis/jiab382. [DOI] [PubMed] [Google Scholar]
- 132.Bhatt N., Ismail N., Magule C., Hussein C., Meque I., Couto A., Nhangave A., Muteerwa A., Bonou A., Ramos A., et al. HIV Drug Resistance Profile in Clients Experiencing Treatment Failure after the Transition Fo a Dolutegravir-Based First-Line Antiretroviral Treatment Regimen in Mozambique [Abstract 51]; Proceedings of the International Workshop on HIV Drug Resistance and Treatment Strategies; Cape Town, South Africa. 20–22 September 2023. [Google Scholar]
- 133.Kalata N., Pals S., Bighignoli B., Kabaghe A., Mkungudza J., Panja L., Raizes E., Chitenje M., Kampira E., Maida A., et al. HIV Drug Resistance to Dolutegravir Is Uncommon among Adults Investigated for Treatment Failure in Malawi [Abstract 53]; Proceedings of the International Workshop on HIV Drug Resistance and Treatment Strategies; Cape Town, South Africa. 20–22 September 2023. [Google Scholar]
- 134.Namayanja G., Watera C., Pais S., Asio J., Ssemwanga D., Sanyu G., Zheng D., Zeh C., Hackett S., Ntali J., et al. Cyclic Acquired HIV Drug Resistance to Dolutegravir among People Living with HIV in Uganda. National Remnant Samples Surveillance 2022 [Abstract 50]; Proceedings of the International Workshop on HIV Drug Resistance and Treatment Strategies; Cape Town, South Africa. 20–22 September 2023. [Google Scholar]
- 135.Parikh A., Ochleng C., Almas S., Habib P., Towett J., Daud I., Maswal J., Owuoth J., Singoel V., Bahamena E., et al. HIV Drug Resistance in the Dolutegravir Era: Preliminary Results from East Africa [Abstract 56]; Proceedings of the International Workshop on HIV Drug Resistance and Treatment Strategies; Cape Town, South Africa. 20–22 September 2023. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.