Abstract
Falls are the leading cause of injury, disability, and injury-related mortality in the older adult population. Older adults with Alzheimer disease (AD) are over twice as likely to experience a fall compared to cognitively normal older adults. Intrinsic and extrinsic fall risk factors may influence falls during symptomatic AD; intrinsic factors include changes in cognition and impaired functional mobility, and extrinsic factors include polypharmacy and environmental fall hazards. Despite many known fall risk factors, the high prevalence of falls, and the presence of effective fall prevention interventions for older adults without cognitive impairment, effective fall prevention interventions for older adults with AD to date are limited and inconclusive. Falls may precede AD-related cognitive impairment during the preclinical phase of AD, though a narrow understanding of fall risk factors and fall prevention interventions for older adults with preclinical AD limits clinical treatment of falls among cognitively normal older adults with preclinical AD. This mini review explores fall risk factors in symptomatic AD, evidence for effective fall prevention interventions in symptomatic AD, and preclinical AD as an avenue for future falls research, including recommendations for future research directions to improve our understanding of falls and fall risk during preclinical AD. Early detection and tailored interventions to address these functional changes are needed to reduce the risk of falls for those at risk for developing AD. Concerted efforts should be dedicated to understanding falls to inform precision fall prevention strategies for this population.
Keywords: Alzheimer disease, aging, intervention, older adults
INTRODUCTION
Falls among community-dwelling older adults are a public health crisis. Falls are the leading cause of injury, disability, and injury-related mortality in the older adult population [1]. Approximately 30% of community-dwelling older adults experience one or more falls annually [2,3]. Consequences of falls include fractures, anxiety, and limited activity participation [4,5]. Older adults with neurodegenerative disorders, including Alzheimer disease (AD), Parkinson disease, dementia with Lewy bodies, and Huntington disease, are at a greater risk for falls compared to older adults without neurodegenerative disorders [6]. Given the growing prevalence and significant impact on global health systems, falls among older adults with AD pose a particularly pressing concern [7,8]. Older adults with AD are more than twice as likely (60%–80%) to experience a fall compared to cognitively normal (CN) older adults [9,10]. Older adults with AD who fall experience greater incidence of injurious falls [11,12] and are five times more likely to be institutionalized compared to older adults with AD who do not fall [4,13]. Approximately one in nine people aged 65 and older are currently living with AD, and this prevalence is expected to increase exponentially as the number of older adults worldwide continues to rise [14,15]. Therefore, an improved understanding of falls in AD is a crucial step toward precision fall prevention strategies for this population. A growing body of evidence suggests that an increased risk of falls and functional mobility impairments, such as slowing gait and worsening balance, may precede AD-related cognitive decline during the preclinical phase of AD [16–19]. It is important to understand falls and fall risk factors across the AD continuum, particularly during preclinical AD, as this may enable early preventative treatment of falls prior to the onset of cognitive impairment. Therefore, the objective of this mini review is to provide a concise yet informative update on current evidence related to falls among older adults with AD. The mini review format is ideal to address this topic, as it offers a focused approach to a specific research area and provides an easily accessible summary of relevant findings that may benefit both researchers and healthcare professionals working with older adults across the AD continuum [20]. In this mini review, we will first provide an overview of AD symptoms that can lead to an increased risk of falls, followed by a review of interventions to address falls among older adults with symptomatic AD. Finally, we will examine the preclinical phase of AD as a potential target for future fall prevention research.
THE ALZHEIMER DISEASE CONTINUUM
AD is a progressive, degenerative brain disorder. Pathological changes of symptomatic AD include abnormal accumulation of proteins, such as beta-amyloid and tau, in the brain that leads to neuronal damage and a decrease in overall brain volume over time [21]. The AD continuum is defined by three phases: preclinical AD, mild cognitive impairment, and dementia due to symptomatic AD [8]. The hallmark symptoms of AD include memory loss, behavioral changes, and safety concerns, including an increased likelihood of falling [8,13,22]. An area that holds promise for understanding the AD-related pathologic and functional changes that precede cognitive decline is the preclinical phase of AD [23]. Individuals with preclinical AD are CN but have brain pathology consistent with symptomatic AD [24]. The preclinical phase of AD can last for years or even decades before progression to symptomatic AD, and it is estimated that 30% of older adults are living with preclinical AD [25]. Advances in neuroimaging and biomarker research have provided valuable insights into the neurophysiological changes that occur during this period of AD [26–29]; in addition to a greater risk of falls [16,17], the presence of AD biomarkers, consistent with preclinical AD, have been associated with functional impairments in complex activities [30], greater dual task interference [31], poorer driving performance [32,33], and sleep disturbances [34]. Therefore, subtle brain changes that begin during the preclinical phase of AD may contribute to an increased risk of falls that persists as AD progresses [16]. Understanding changes that occur in this clinically silent period of AD may improve screening for the progression from preclinical to symptomatic AD.
INTRINSIC AND EXTRINSIC RISK FACTORS FOR FALLS IN SYMPTOMATIC ALZHEIMER DISEASE
Falls during symptomatic AD are associated with multiple intrinsic and extrinsic risk factors [35]. The risk of falls increases with advancing cognitive impairment [36]. Progressive challenges, including impaired judgment, memory, and executive functioning, may undermine an individual’s capacity to maintain safety awareness or manage complex situations [36]. Delirium, confusion, and disorientation can further compromise the individual’s ability to discern potential fall risks within their physical environment [37,38]. These symptoms also often contribute to wandering behaviors, diminished spatial awareness, and slowed reaction times [38,39]. Dual tasking, or the concurrent execution of a motor-motor or motor-cognitive task [40], is often impaired in older adults with AD and has been linked to an increased risk of falling [41]. This may be attributed to prioritizing the cognitive demands of a secondary task, such as calling a phone number or sustaining a conversation; it may also be due to decreased gait performance, such as decreased cadence and stride length, while attempting to negotiate the task for older adults with AD [42,43]. Additionally, mental health concerns such as depression and anxiety are common among older adults with AD and often contribute to reduced attentional control, fatigue, weakness, agitation, and psychomotor symptoms that impede reaction times, all of which are risk factors for falls [44–46].
The systems and areas of the brain that impact cognition are closely related to those that impact motor function [47,48]. Impairment in cognition, especially higher-level cognition such as executive function, is thought to impact functional mobility (i.e., gait and balance) and muscle strength through impaired motor planning, motor control, attention, and sensory integration [47,48]. AD pathology may influence motor function by disrupting the neural networks that are integral for planning and executing various motor behaviors [49]. Gait parameters, such as decreased stride speed and length, become increasingly impaired as AD progresses [42]. Additionally, declines in physical strength and body composition are associated with AD progression and increase one’s risk of falls [22,49]. Sensory loss is also common in symptomatic AD and contributes to impairments in gait and balance and an increased risk of falls; of note, impaired visual acuity and perception, as well as peripheral sensation, are frequently impaired in AD [50,51]. Dynamic balance (i.e., ability to maintain balance while moving) and dual task conditions require the interaction of cognition and motor functions and are particularly compromised in AD [47,52–54]. In fact, declines in motor and functional mobility have been shown to occur prior to the onset of cognitive impairment in AD and increase older adults’ risk of falls [16,18,50,53,55].
Several extrinsic fall risk factors exist for older adults with AD. They are often prescribed medications for symptoms associated with AD such as depression, anxiety, sleep irregularities, and problematic behaviors, many of which alone have side effects known to increase risk of falls [35,56]. For example, the side effects of psychotropic drugs (antidepressants, antipsychotics, sedatives), acetylcholinesterase inhibitors (cognitive enhancers), and benzodiazepines (sedatives) include weakness, motor disturbances, dizziness, vertigo, depression, and falls [56–58]. In addition, polypharmacy, or taking four or more prescription medications, is a known risk factor for falls due to increasing drug-drug interactions and likelihood of experiencing side effects among community-dwelling older adults [59], including those with AD [60]. Environmental hazards are another type of extrinsic fall risk factor for older adults with AD [38]. Many falls among older adults with AD occur within the home environment [38]. Environmental hazards that may contribute to falls in the home for this population include slippery or uneven flooring, stairs without railings, or furniture of inappropriate heights (e.g., beds, toilets) [38,61]. Furthermore, inadequate lighting or improper footwear may lead to falls among individuals with cognitive impairment, particularly among older adults with AD who have visual impairments or decreased sensation [22,62].
Collectively, the above intrinsic and extrinsic factors can increase the risk of falling for older adults with AD. These factors often interact to increase fall risk even more. In reality, one’s risk of falling is often multifaceted rather than attributable to a single factor [63,64]. For example, an older adult with AD with impaired judgment and lower extremity weakness may experience a fall while descending stairs with no handrail. To address multifaceted fall risks, interventions that are robust to target multiple domains of risk factors are necessary.
FALL PREVENTION INTERVENTIONS FOR OLDER ADULTS WITH ALZHEIMER DISEASE
Despite many known fall risk factors, the high prevalence of falls, and the presence of effective fall prevention interventions for older adults without cognitive impairment, effective fall prevention interventions for older adults with AD to date are limited and inconclusive [10,53,65–68]. Some studies suggest that the critical components of adherence to fall prevention interventions, such as structured home-based exercise programs and home hazard removal programs, for older adults with AD include support from clinicians, particularly occupational and physical therapists, and caregivers [69–72]. Exercise interventions often target major fall risk factors of poor balance and muscle weakness. Exercise interventions are feasible and improve balance among older adults with AD [72,73], but there is limited evidence on their effectiveness in reducing falls in this population [66,74–76]. In addition, interventions to reduce home hazards or fall risks in the environment (i.e., tripping hazards, cluttered pathways, slippery floors) are effective among older adults at risk of falling [77], especially when they are delivered by an occupational therapist with community-dwelling older adults [78]. However, the effectiveness of home hazard removal and home modifications is less well-established among older adults with AD. Insufficient evidence indicates that home modifications alone are sufficient to reduce the incidence of falls among older adults with AD, though they may have greater effectiveness in reducing falls when combined with other approaches, such as exercise [68,79], but not with Vitamin D supplementation, for cognitively impaired older adults [76]. Home-based systems, including bed-exit alarm systems and automatic path lighting, have demonstrated effectiveness in reducing the risk and incidence of falls [79,80]. Additionally, efforts to limit wandering, such as monitoring devices, concealed doorways, and wander gardens, may hold promise for effectively reducing falls among older adults with AD, though these findings have been limited to individuals in residential or institutional settings [79]. While medication review and reducing the number of interacting prescription medications can be effective in reducing falls among CN older adults [59], further investigation into polypharmacy and falls is warranted among older adults with AD pathology to determine best practices for prescribing fall-risk-increasing medications within this population [81].
Interventions that target multiple fall risk factors have been more commonly tested in a clinical trial setting with mixed results [61,67,76,82]. For example, an intervention that included exercise and tailored home hazard removal among community-dwelling older adults with cognitive impairment [61] and an intervention that included medication review, vision correction, exercise, and home hazard modification in people with cognitive impairment did not reduce falls [82]. However, a recent review of fall prevention interventions found that those targeting strength, balance, and cognition (executive function) can improve postural stability and may reduce the risk of falling among older adults with AD [67]; however, additional research is needed to examine the effectiveness of multicomponent fall prevention interventions for older adults with AD.
The evidence on effective fall prevention interventions among community-dwelling older adults with symptomatic AD is limited. The exclusion of older adults with cognitive impairment in clinical trials greatly limits knowledge in this area, and, among studies that include those with symptomatic AD, clinical implications are tempered due to weak study designs [10,53,65–68]. Common challenges specific to symptomatic AD are difficulty controlling for and assessing stage of AD, confounding factors, and the accelerated declines associated with AD, as well as the general aging process and comorbidities. Testing fall prevention interventions in an earlier stage of AD prior to onset of hallmark memory symptoms, such as preclinical AD, may be a particularly promising direction to better understand the effectiveness of fall prevention interventions during the progression of AD.
PRECLINICAL ALZHEIMER DISEASE: AN AVENUE FOR FUTURE FALLS RESEARCH?
The preclinical phase of AD has gained much attention in recent years as a potential target for intervention for slowing or preventing progression to symptomatic AD [26,83]. Older adults with preclinical AD show no symptoms of cognitive impairment through traditional screening measures but have evidence of AD pathology [26]. Core biomarker abnormalities, including abnormal accumulation of beta-amyloid and tau proteins, may present decades before symptomatic AD [84]. AD biomarkers are typically evaluated through positron emission tomography, magnetic resonance imaging, cerebrospinal fluid, or, in recent developments, blood plasma samples [28,29]. These biomarkers can be used to stage progression from preclinical to symptomatic AD [85]; however, screening for AD biomarkers is costly and time consuming and, thus, is not part of routine clinical care for older adults [86]. As a result, additional work is needed to improve screening and detection of preclinical AD in order for this phase to be a robust target for fall prevention interventions for people with AD.
Examining falls may be a simple and inexpensive way to screen for preclinical AD. Emerging research examining falls during preclinical AD has identified potential relationships between increased falls and AD pathology. The presence of AD biomarkers has been associated with greater numbers of falls and decreased time to experiencing a fall compared to older adults without preclinical AD biomarkers [16,17]. Additionally, evidence suggests that worsening balance and slowing gait precede cognitive decline by several years and are linked to AD pathology [18,19,87]. Therefore, balance and gait speed assessments should be integrated into routine clinical evaluations of older adults to identify those who may be at an increased risk of cognitive decline and AD [87]. Dual-task gait may better predict future cognitive decline for older adults with preclinical AD than gait speed alone, although further research is needed to determine whether differences in dual tasking arise during the preclinical phase of AD or exclusively in symptomatic AD [88]. Other sensorimotor fall risk factors, including balance, sensation, strength, and vision, have not been investigated specifically in relation to preclinical AD; therefore, it is difficult to determine whether these risk factors precede or arise following AD-related cognitive impairment. Figure 1 presents a conceptual diagram highlighting fall risk factors that are present in symptomatic AD. It is unknown whether an increased risk of falling during preclinical AD is the result of biological abnormalities, behavioral changes, or a combination of these factors. Gaining a deeper understanding of the mechanisms underlying falls during preclinical AD holds great promise for early detection and intervention strategies.
Figure 1. Fall risk factors in the preclinical phase of Alzheimer disease (AD).
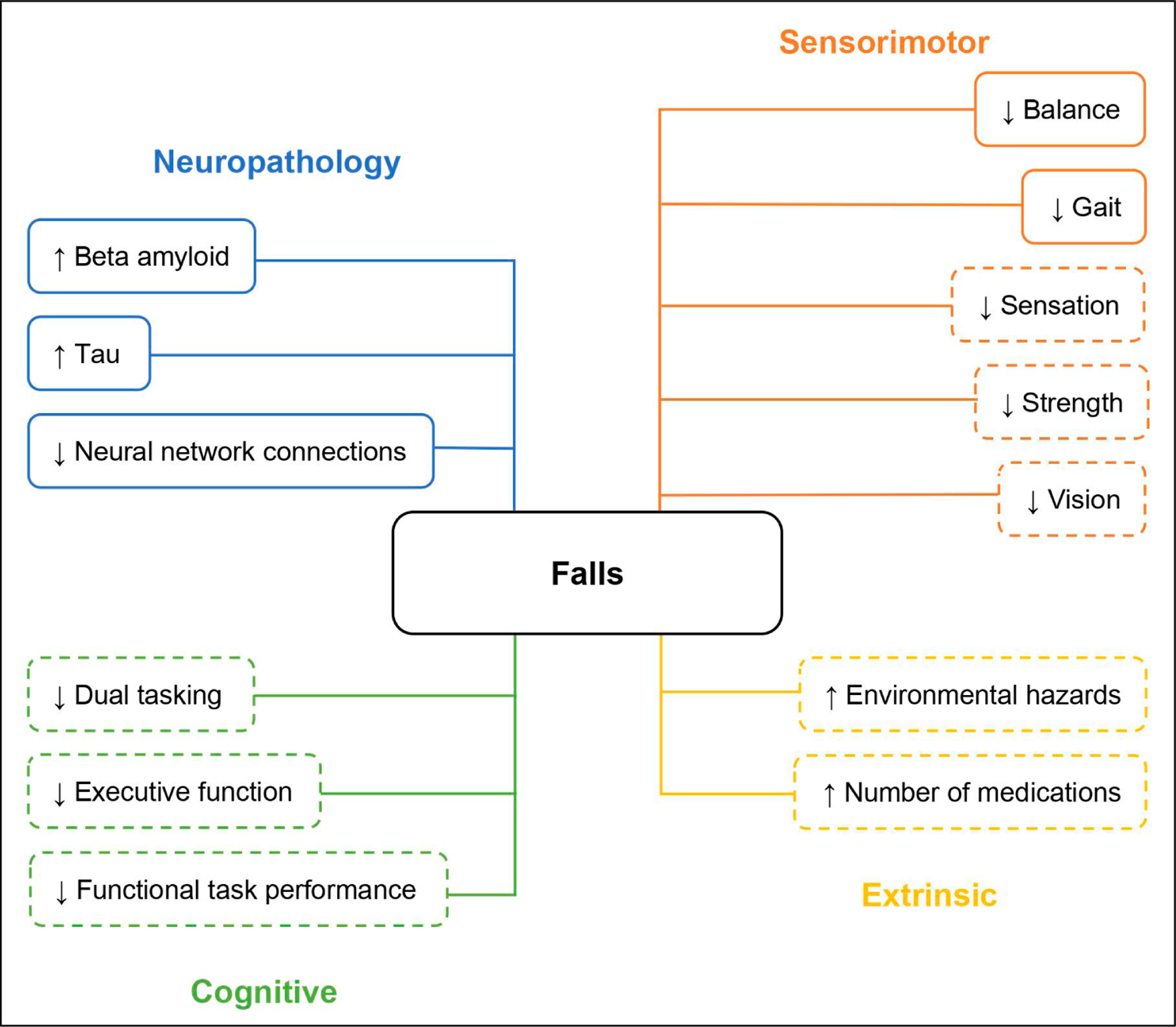
Note: Solid lines represent well-established risk factors for falls in preclinical AD. Dashed lines represent factors requiring further investigation to determine their roles in influencing falls risk in preclinical AD.
While addressing the preclinical phase of AD holds potential for falls research, several evidence gaps remain. To our knowledge, no studies have reported fall risk factors specific to the preclinical phase of AD. Knowledge of the intrinsic and extrinsic factors that increase one’s risk of falling may improve targeted screening for preclinical AD. Additionally, the ways in which the circumstances of falls, such as where, when, or how they occur, differ between older adults with and without preclinical AD remain unknown. It is also unclear where falls begin during the progression from preclinical to symptomatic AD, and this knowledge could inform targeted fall prevention interventions.
Understanding falls and changes in functional mobility that begin during preclinical AD may improve detection and inform precision fall prevention interventions for older adults with preclinical AD. Future efforts to understand falls during the preclinical phase of AD should help identify fall risk factors during this phase, which will allow for comparisons of fall risk factors during preclinical and symptomatic AD. In addition, future studies should examine dual-task gait as an improved predictor of future cognitive decline compared to traditional gait speed assessments. While polypharmacy remains a fall risk factor for older adults with and without AD, future research should attempt to elucidate relationships among polypharmacy, falls, and preclinical AD, as the pathologic changes during preclinical AD may interact with prescription medications and increase one’s risk of falls. Longitudinal studies incorporating comprehensive assessments of both cognitive and motor function are needed to better understand the relationship between AD and falls during the preclinical phase. These studies should report circumstantial information about falls, including where, when, and how falls occur, as this information is important to design tailored approaches to fall prevention. These studies should also focus on identifying when falls occur in the progression from preclinical to symptomatic AD. Finally, interventions that prevent or reduce falls, including exercise programs, home fall hazard removal, and multicomponent approaches, warrant further investigation to determine their efficacy among older adults with preclinical AD.
CONCLUSIONS
Understanding the complex relationship between falls and AD is of utmost importance, as the older adult population is expected to rise exponentially over the next 30 years [15]. Growing evidence suggests that subtle cognitive changes, functional impairments, and falls may precede cognitive decline during the preclinical phase of AD [16,17,30,33]. Early detection of falls and tailored interventions to address functional changes are needed to reduce fall risk for those at risk for developing AD. Future studies should examine fall risk factors, circumstances of falls, and efficacy of fall prevention interventions, such as exercise and home hazard removal, among CN older adults with preclinical AD. Falls among older adults with AD represent a significant public health concern; therefore, concerted efforts should be dedicated to understanding falls to inform precision fall prevention strategies for this population.
FUNDING
This project was supported by the National Institutes of Health Award Number 5R01AG057680-05 “Falls: a marker of preclinical Alzheimer disease” and the National Center for Advancing Translational Sciences of the National Institutes of Health Award Number TL1TR002344.
ABBREVIATIONS
- AD
Alzheimer disease
- CN
cognitively normal
Footnotes
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
DATA AVAILABILITY
Data sharing is not applicable to this article, as no datasets were generated or analyzed during the current study.
REFERENCES
- 1.Moreland B, Kakara R, Henry A. Trends in Nonfatal Falls and Fall-Related Injuries Among Adults Aged ≥65 Years - United States, 2012–2018. Morb Mortal Wkly Rep 2020;69(27):875–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ganz DA, Latham NK. Prevention of Falls in Community-Dwelling Older Adults. N Engl J Med 2020;382(8):734–43. [DOI] [PubMed] [Google Scholar]
- 3.Salari N, Darvishi N, Ahmadipanah M, Shohaimi S, Mohammadi M. Global prevalence of falls in the older adults: a comprehensive systematic review and meta-analysis. J Orthop Surg Res 2022;17(1):334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tinetti ME, Speechley M, Ginter SF. Risk Factors for Falls among Elderly Persons Living in the Community. N Engl J Med 1988;319(26):1701–7. [DOI] [PubMed] [Google Scholar]
- 5.American Geriatrics Society. Guideline for the prevention of falls in older persons. American Geriatrics Society, British Geriatrics Society, and American Academy of Orthopaedic Surgeons Panel on Falls Prevention. J Am Geriatr Soc 2001;49(5):664–72. [PubMed] [Google Scholar]
- 6.Minta K, Colombo G, Taylor WR, Schinazi VR. Differences in fall-related characteristics across cognitive disorders. Front Aging Neurosci 2023;15:1171306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016;388(10053):1545–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.2023 Alzheimer’s disease facts and figures. Alzheimers Dement 2023. Apr;19(4):1598–1695. [DOI] [PubMed] [Google Scholar]
- 9.Allan LM, Ballard CG, Rowan EN, Kenny RA. Incidence and prediction of falls in dementia: a prospective study in older people. PLoS One 2009;4(5):e5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Booth V, Logan P, Harwood R, Hood V. Falls prevention interventions in older adults with cognitive impairment: A systematic review of reviews. Int J Ther Rehabil 2015;22(6):289–96. [Google Scholar]
- 11.Zhang L, Wang J, Dove A, Yang W, Li X, Qi X, et al. Injurious falls before, during, and after dementia diagnosis: A population-based study. Alzheimers Dement 2023;19(S5):e062830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oleske DM, Wilson RS, Bernard BA, Evans DA, Terman EW. Epidemiology of Injury in People with Alzheimer’s Disease. J Am Geriatr Soc 1995;43(7):741–6. [DOI] [PubMed] [Google Scholar]
- 13.Morris JC, Rubin EH, Morris EJ, Mandel SA. Senile Dementia of the Alzheimer’s Type: An Important Risk Factor for Serious Falls. J Gerontol 1987;42(4):412–7. [DOI] [PubMed] [Google Scholar]
- 14.Alzheimer’s disease facts and figures. Alzheimers Dement 2022. Apr;18(4):700–89. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization. Ageing and health: World Health Organization; 2022. Available from: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health#:~:text=At%20this%20time%20the%20share,2050%20to%20reach%20426%20million. Acessed 2024 Feb 11.
- 16.Stark SL, Roe CM, Grant EA, Hollingsworth H, Benzinger TL, Fagan AM, et al. Preclinical Alzheimer disease and risk of falls. Neurology 2013;81(5):437–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keleman A, Wisch JK, Bollinger RM, Grant EA, Benzinger TL, Morris JC, et al. Falls Associate with Neurodegenerative Changes in ATN Framework of Alzheimer’s Disease. J Alzheimers Dis 2020;77(2):745–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Skillbäck T, Blennow K, Zetterberg H, Skoog J, Rydén L, Wetterberg H, et al. Slowing gait speed precedes cognitive decline by several years. Alzheimers Dement 2022;18(9):1667–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keleman AA, Nicosia J, Bollinger RM, Wisch JK, Hassenstab J, Morris JC, et al. Precipitating Mechanisms of Falls in Preclinical Alzheimer’s Disease. J Alzheimers Dis Rep 2023;7(1):739–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elfar JC. Introduction to mini-review. Geriatr Orthop Surg Rehabil 2014;5(2):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rajmohan R, Reddy PH. Amyloid-Beta and Phosphorylated Tau Accumulations Cause Abnormalities at Synapses of Alzheimer’s disease Neurons. J Alzheimers Dis 2017;57(4):975–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lach HW, Harrison BE, Phongphanngam S. Falls and Fall Prevention in Older Adults With Early-Stage Dementia: An Integrative Review. Res Gerontol Nurs 2017;10(3):139–48. [DOI] [PubMed] [Google Scholar]
- 23.Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 2011;7(3):280–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hanseeuw BJ, Betensky RA, Jacobs HIL, Schultz AP, Sepulcre J, Becker JA, et al. Association of Amyloid and Tau With Cognition in Preclinical Alzheimer Disease: A Longitudinal Study. JAMA Neurol 2019;76(8):915–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dubois B, Hampel H, Feldman HH, Scheltens P, Aisen P, Andrieu S, et al. Preclinical Alzheimer’s disease: Definition, natural history, and diagnostic criteria. Alzheimers Dement 2016;12(3):292–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jack CR Jr, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement 2018;14(4):535–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aschenbrenner AJ, Balota DA, Fagan AM, Duchek JM, Benzinger TL, Morris JC. Alzheimer Disease Cerebrospinal Fluid Biomarkers Moderate Baseline Differences and Predict Longitudinal Change in Attentional Control and Episodic Memory Composites in the Adult Children Study. J Int Neuropsychol Soc 2015;21(8):573–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schindler SE, Gray JD, Gordon BA, Xiong C, Batrla-Utermann R, Quan M, et al. Cerebrospinal fluid biomarkers measured by Elecsys assays compared to amyloid imaging. Alzheimers Dement 2018;14(11):1460–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schindler SE, Bollinger JG, Ovod V, Mawuenyega KG, Li Y, Gordon BA, et al. High-precision plasma β-amyloid 42/40 predicts current and future brain amyloidosis. Neurology 2019;93(17):e1647–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keleman AA, Bollinger RM, Wisch JK, Grant EA, Benzinger TL, Ances BM, et al. Assessment of Instrumental Activities of Daily Living in Preclinical Alzheimer Disease. OTJR (Thorofare N J) 2022;42(4):277–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whitson HE, Potter GG, Feld JA, Plassman BL, Reynolds K, Sloane R, et al. Dual-Task Gait and Alzheimer’s Disease Genetic Risk in Cognitively Normal Adults: A Pilot Study. J Alzheimers Dis 2018;64(4):1137–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roe CM, Babulal GM, Head DM, Stout SH, Vernon EK, Ghoshal N, et al. Preclinical Alzheimer’s disease and longitudinal driving decline. Alzheimers Dement 2017;3(1):74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roe CM, Barco PP, Head DM, Ghoshal N, Selsor N, Babulal GM, et al. Amyloid Imaging, Cerebrospinal Fluid Biomarkers Predict Driving Performance Among Cognitively Normal Individuals. Alzheimer Dis Assoc Disord 2017;31(1):69–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lucey BP. It’s complicated: The relationship between sleep and Alzheimer’s disease in humans. Neurobiol Dis 2020;144:105031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fernando E, Fraser M, Hendriksen J, Kim CH, Muir-Hunter SW. Risk Factors Associated with Falls in Older Adults with Dementia: A Systematic Review. Physiother Can 2017;69(2):161–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muir SW, Gopaul K, Montero Odasso MM. The role of cognitive impairment in fall risk among older adults: a systematic review and meta-analysis. Age Ageing 2012;41(3):299–308. [DOI] [PubMed] [Google Scholar]
- 37.Sillner AY, Holle CL, Rudolph JL. The Overlap Between Falls and Delirium in Hospitalized Older Adults: A Systematic Review. Clin Geriatr Med 2019;35(2):221–36. [DOI] [PubMed] [Google Scholar]
- 38.Kato-Narita EM, Radanovic M. Characteristics of falls in mild and moderate Alzheimer’s disease. Dement Neuropsychol 2009;3(4):337–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Agrawal AK, Gowda M, Achary U, Gowda GS, Harbishettar V. Approach to Management of Wandering in Dementia: Ethical and Legal Issue. Indian J Psychol Med 2021;43(Suppl 5):S53–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leland A, Tavakol K, Scholten J, Mathis D, Maron D, Bakhshi S. The role of dual tasking in the assessment of gait, cognition and community reintegration of veterans with mild traumatic brain injury. Mater Sociomed 2017;29(4):251–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ansai JH, Andrade LP, Rossi PG, Takahashi ACM, Vale FAC, Rebelatto JR. Gait, dual task and history of falls in elderly with preserved cognition, mild cognitive impairment, and mild Alzheimer’s disease. Braz J Phys Ther 2017;21(2):144–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Melo Coelho FG, Stella F, de Andrade LP, Barbieri FA, Santos-Galduróz RF, Gobbi S, et al. Gait and risk of falls associated with frontal cognitive functions at different stages of Alzheimer’s disease. Aging Neurpsychol Cogn 2012;19(5):644–56. [DOI] [PubMed] [Google Scholar]
- 43.Gonçalves J, Ansai JH, Masse FAA, Vale FAC, Takahashi ACM, Andrade LP. Dual-task as a predictor of falls in older people with mild cognitive impairment and mild Alzheimer’s disease: a prospective cohort study. Braz J Phys Ther 2018;22(5):417–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Botto R, Callai N, Cermelli A, Causarano L, Rainero I. Anxiety and depression in Alzheimer’s disease: a systematic review of pathogenetic mechanisms and relation to cognitive decline. Neurol Sci 2022;43(7):4107–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iaboni A, Flint AJ. The complex interplay of depression and falls in older adults: a clinical review. Am J Geriatr Psychiatry 2013;21(5):484–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luo Y, Miyawaki CE, Valimaki MA, Tang S, Sun H, Liu M. Symptoms of anxiety and depression predicting fall-related outcomes among older Americans: a longitudinal study. BMC Geriatr 2022;22(1):749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang W, Low L-F, Schwenk M, Mills N, Gwynn JD, Clemson L. Review of gait, cognition, and fall risks with implications for fall prevention in older adults with dementia. Dement Geriatr Cogn Disord 2019;48(1–2):17–29. [DOI] [PubMed] [Google Scholar]
- 48.Sheridan PL, Hausdorff JM. The role of higher-level cognitive function in gait: executive dysfunction contributes to fall risk in Alzheimer’s disease. Dement Geriatr Cogn Disord 2007;24(2):125–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Buchman AS, Bennett DA. Loss of motor function in preclinical Alzheimer’s disease. Expert Rev Neurother 2011;11(5):665–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Albers MW, Gilmore GC, Kaye J, Murphy C, Wingfield A, Bennett DA, et al. At the interface of sensory and motor dysfunctions and Alzheimer’s disease. Alzheimers Dement 2015;11(1):70–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Okoye SM, Fabius CD, Reider L, Wolff JL. Predictors of falls in older adults with and without dementia. Alzheimers Dement 2023;19(7):2888–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ansai JH, de Andrade LP, Masse FAA, Gonçalves J, de Medeiros Takahashi AC, Vale FAC, et al. Risk factors for falls in older adults with mild cognitive impairment and mild Alzheimer disease. J Geriatr Phys Ther 2019;42(3):E116–E21. [DOI] [PubMed] [Google Scholar]
- 53.Chantanachai T, Sturnieks DL, Lord SR, Payne N, Webster L, Taylor ME. Risk factors for falls in older people with cognitive impairment living in the community: systematic review and meta-analysis. Ageing Res Rev 2021;71:101452. [DOI] [PubMed] [Google Scholar]
- 54.Suttanon P, Hill KD, Said CM, LoGiudice D, Lautenschlager NT, Dodd KJ. Balance and mobility dysfunction and falls risk in older people with mild to moderate Alzheimer disease. Am J Phys Med Rehabil 2012;91(1):12–23. [DOI] [PubMed] [Google Scholar]
- 55.Grande G, Triolo F, Nuara A, Welmer A-K, Fratiglioni L, Vetrano DL. Measuring gait speed to better identify prodromal dementia. Exp Gerontol 2019;124:110625. [DOI] [PubMed] [Google Scholar]
- 56.Epstein NU, Guo R, Farlow MR, Singh JP, Fisher M. Medication for Alzheimer’s disease and associated fall hazard: a retrospective cohort study from the Alzheimer’s Disease Neuroimaging Initiative. Drugs Aging 2014;31:125–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Orsel K, Taipale H, Tolppanen A-M, Koponen M, Tanskanen A, Tiihonen J, et al. Psychotropic drugs use and psychotropic polypharmacy among persons with Alzheimer’s disease. Eur Neuropsychopharmacol 2018;28(11):1260–9. [DOI] [PubMed] [Google Scholar]
- 58.Zdanys KF, Carvalho AF, Tampi RR, Steffens DC. The treatment of behavioral and psychological symptoms of dementia: weighing benefits and risks. Curr Alzheimer Res 2016;13(10):1124–33. [DOI] [PubMed] [Google Scholar]
- 59.Richardson K, Bennett K, Kenny RA. Polypharmacy including falls risk-increasing medications and subsequent falls in community-dwelling middle-aged and older adults. Age Ageing 2014;44(1):90–6. [DOI] [PubMed] [Google Scholar]
- 60.Esumi S, Ushio S, Zamami Y. Polypharmacy in Older Adults with Alzheimer’s Disease. Medicina 2022;58(10):1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Taylor ME, Wesson J, Sherrington C, Hill KD, Kurrle S, Lord SR, et al. Tailored exercise and home hazard reduction program for fall prevention in older people with cognitive impairment: the i-FOCIS randomized controlled trial. J Gerontol A 2021;76(4):655–65. [DOI] [PubMed] [Google Scholar]
- 62.Clemson L, Cumming RG, Roland M. Case-control study of hazards in the home and risk of falls and hip fractures. Age Ageing 1996;25(2):97–101. [DOI] [PubMed] [Google Scholar]
- 63.Li Y, Hou L, Zhao H, Xie R, Yi Y, Ding X. Risk factors for falls among community-dwelling older adults: A systematic review and meta-analysis. Front Med (Lausanne) 2022;9:1019094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lusardi MM, Fritz S, Middleton A, Allison L, Wingood M, Phillips E, et al. Determining Risk of Falls in Community Dwelling Older Adults: A Systematic Review and Meta-analysis Using Posttest Probability. J Geriatr Phys Ther 2017;40(1):1–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li F, Harmer P, Eckstrom E, Ainsworth BE, Fitzgerald K, Voit J, et al. Efficacy of exercise-based interventions in preventing falls among community-dwelling older persons with cognitive impairment: is there enough evidence? An updated systematic review and meta-analysis. Age Ageing 2021;50(5):1557–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hauer K, Becker C, Lindemann U, Beyer N. Effectiveness of physical training on motor performance and fall prevention in cognitively impaired older persons: a systematic review. Am J Phys Med Rehabil 2006;85(10):847–57. [DOI] [PubMed] [Google Scholar]
- 67.Adzhar MA, Manlapaz D, Singh DKA, Mesbah N. Exercise to improve postural stability in older adults with Alzheimer’s disease: A systematic review of randomized control trials. Int J Environ Res Public Health 2022;19(16):10350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jensen LE, Padilla R. Effectiveness of Interventions to Prevent Falls in People With Alzheimer’s Disease and Related Dementias. Am J Occup Ther 2011;65(5):532–40. [DOI] [PubMed] [Google Scholar]
- 69.Meyer C, Hill S, Dow B, Synnot A, Hill K. Translating falls prevention knowledge to community-dwelling older PLWD: a mixed-method systematic review. Gerontologist 2015;55(4):560–74. [DOI] [PubMed] [Google Scholar]
- 70.Suttanon P, Hill KD, Said CM, Byrne KN, Dodd KJ. Factors influencing commencement and adherence to a home-based balance exercise program for reducing risk of falls: perceptions of people with Alzheimer’s disease and their caregivers. Int Psychogeriatr 2012;24(7):1172–82. [DOI] [PubMed] [Google Scholar]
- 71.Wesson J, Clemson L, Brodaty H, Lord S, Taylor M, Gitlin L, et al. A feasibility study and pilot randomised trial of a tailored prevention program to reduce falls in older people with mild dementia. BMC Geriatr 2013;13:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Taylor ME, Lord SR, Brodaty H, Kurrle SE, Hamilton S, Ramsay E, et al. A home-based, carer-enhanced exercise program improves balance and falls efficacy in community-dwelling older people with dementia. Int Psychogeriatr 2017;29(1):81–91. [DOI] [PubMed] [Google Scholar]
- 73.Padala KP, Padala PR, Lensing SY, Dennis RA, Bopp MM, Roberson PK, et al. Home-based exercise program improves balance and fear of falling in community-dwelling older adults with mild Alzheimer’s disease: a pilot study. J Alzheimers Dis 2017;59(2):565–74. [DOI] [PubMed] [Google Scholar]
- 74.Suttanon P, Hill K, Said C, Dodd K. Can balance exercise programmes improve balance and related physical performance measures in people with dementia? A systematic review. Eur Rev Aging Phys Act 2010;7(1):13–25. [Google Scholar]
- 75.Taylor ME, Close JC, Lord SR, Kurrle SE, Webster L, Savage R, et al. Pilot feasibility study of a home-based fall prevention exercise program (StandingTall) delivered through a tablet computer (iPad) in older people with dementia. Australas J Ageing 2020;39(3):e278–87. [DOI] [PubMed] [Google Scholar]
- 76.Guo JL, Tsai YY, Liao JY, Tu HM, Huang CM. Interventions to reduce the number of falls among older adults with/without cognitive impairment: an exploratory meta-analysis. Int J Geriatr Psychiatry 2014;29(7):661–9. [DOI] [PubMed] [Google Scholar]
- 77.Clemson L, Stark S, Pighills AC, Fairhall NJ, Lamb SE, Ali J, et al. Environmental interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev 2019; 2019(2): CD013258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cumming RG, Thomas M, Szonyi G, Salkeld G, O’Neill E, Westbury C, et al. Home Visits by an Occupational Therapist for Assessment and Modification of Environmental Hazards: A Randomized Trial of Falls Prevention. J Am Geriatr Soc 1999;47(12):1397–402. [DOI] [PubMed] [Google Scholar]
- 79.Jensen L, Padilla R. Effectiveness of Environment-Based Interventions That Address Behavior, Perception, and Falls in People With Alzheimer’s Disease and Related Major Neurocognitive Disorders: A Systematic Review. Am J Occup Ther 2017;71(5):7105180030p1–p10. [DOI] [PubMed] [Google Scholar]
- 80.Tchalla AE, Lachal F, Cardinaud N, Saulnier I, Rialle V, Preux P-M, et al. Preventing and managing indoor falls with home-based technologies in mild and moderate Alzheimer’s disease patients: pilot study in a community dwelling. Dement Geriatr Cogn Disord 2013;36(3–4):251–61. [DOI] [PubMed] [Google Scholar]
- 81.Harris CM, Lykina T. Fall-Risk-Increasing Drugs in People With Dementia Who Live in a Residential Aged Care Facility: A Pilot Study. Cureus 2022;14(4):e24559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shaw FE, Bond J, Richardson DA, Dawson P, Steen IN, McKeith IG, et al. Multifactorial intervention after a fall in older people with cognitive impairment and dementia presenting to the accident and emergency department: randomised controlled trial. BMJ 2003;326(7380):73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sperling R, Mormino E, Johnson K. The Evolution of Preclinical Alzheimer’s Disease: Implications for Prevention Trials. Neuron 2014;84(3):608–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Price JL, Morris JC. Tangles and plaques in nondemented aging and “preclinical” Alzheimer’s disease. Ann Neurol 1999;45(3):358–68. [DOI] [PubMed] [Google Scholar]
- 85.Jack CR Jr, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, et al. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol 2013;12(2):207–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lee SA, Sposato LA, Hachinski V, Cipriano LE. Cost-effectiveness of cerebrospinal biomarkers for the diagnosis of Alzheimer’s disease. Alzheimers Res Ther 2017;9(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Verghese J. Gait and Cognitive Declines in Dementia-Double or Nothing. JAMA Netw Open 2022;5(5):e2214654. [DOI] [PubMed] [Google Scholar]
- 88.Ramírez F, Gutiérrez M. Dual-Task Gait as a Predictive Tool for Cognitive Impairment in Older Adults: A Systematic Review. Front Aging Neurosci 2021. Dec 24:13:769462. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article, as no datasets were generated or analyzed during the current study.


