Abstract
Purpose of review
The management of hypertension in frail older adults remains controversial, as these patients are underrepresented in clinical trials and practice guidelines. Overtreatment may cause harm while undertreatment may lead to greater risk of cardiovascular events. Our research aims to examine this controversy and provide guidance regarding deprescribing decisions in frail older adults.
Results
Current evidence suggests that there may be minimal cardiovascular benefit and significant harm of antihypertensive medication in the frail older adult population. A minority of hypertension guidelines provide sufficient recommendations for frail older adults, and there are limited tools available to guide clinical decision-making.
Conclusion
Randomized controlled trials and well-designed observational studies are needed to confirm the benefit-to-harm relationship of antihypertensive medication in frail older adults. Decision tools that comprehensively address antihypertensive deprescribing would be advantageous to help clinicians with hypertension management in this population. Clinicians should engage in shared decision-making with the patient and family to ensure that decisions regarding antihypertensive deprescribing best meet the needs of all involved.
Keywords: Deprescribing, Blood pressure, Hypertension, Antihypertensive agents, Frailty, Older adults
1. Introduction
Treatment of high blood pressure has become standard practice in countries worldwide. The benefit of treating blood pressure is clear, with multiple studies finding that such treatment reduces cardiovascular events in populations [1]. However, it is unclear whether the cardiovascular benefit of reduced blood pressure extends to the frail older adult population.
Frail older adults comprise an estimated 15 % to upwards of 20 % of the over–65-year-old population in North America [2], [3], and >25 % are on antihypertensive medication for blood pressure [4]. Frailty can be defined as having increased vulnerability to external stresses, where even a common cold can result in morbidity and mortality [5]. There are many models used to assess frailty; a popular one adopted in clinical practice and integrated into a number of guidelines is the Clinical Frailty Model by Rockwood et al., which defines and illustrates stages from fit to frail [6].
There have been several recent narrative reviews on blood pressure targets in the frail older adult population that have summarized evidence of blood pressure management in frail older adults [7], [8], [9]. Overall, they found that for the frail older adult population: 1) There is a lack of evidence on the cardiovascular and cognitive benefit of antihypertensive medication; 2) Further well-designed observational and randomized controlled trials with clinical outcomes are needed to help determine blood pressure targets, blood pressure thresholds, and deprescribing; and 3) Treatment decisions should be made on a case-by-case basis in the frail older adult population. However, the reviews did not consider antihypertensive medication in frail older adults through a deprescribing lens, nor did they include a comprehensive review of potential harms and deprescribing tools, which is particularly important in this population given the trajectory of declining blood pressure in the final years of life [10].
Our narrative review sets out to examine the evidence on the benefits and harms of antihypertensive medication to provide guidance on deprescribing decisions. We also discuss available tools to deprescribe antihypertensive medication including guidelines, medication appropriateness tools, and decision aids.
2. Benefits of antihypertensive medication in frail older adults
The principal benefit of antihypertensive medication is a reduction in cardiovascular events.
This benefit was examined in two pivotal randomized controlled trials in older adults, Systolic Blood Pressure Intervention Trial (SPRINT) [11] and Hypertension in the Very Elderly Trial (HYVET) [12], [13]. SPRINT enrolled older adults ≥75 years old and targeted a systolic blood pressure (SBP) of <120 mmHg in its intervention group and < 140 mmHg in its control group; HYVET enrolled older adults ≥80 years old and targeted a blood pressure of 150/80 in the intervention group. In both studies, approximately 30 % of older adults were considered frail (frailty index >0.21) and the subset of frail older adults had similar outcomes as the fit/less fit older adults. However, the internal validity of SPRINT is highly questionable [14] and the generalizability of HYVET and SPRINT findings to frail older adults ≥80 years old is unclear.
In SPRINT, older adults who had a life expectancy of <3 years, diabetes, congestive heart failure with an ejection fraction <35 %, and dementia were excluded, while for HYVET, exclusions included dementia, a requirement of nursing care, and taking antihypertensive medication for congestive heart failure. Sheppard et al. found that of patients in British general practices ≥80 years old with a frailty index of >0.21 only 1.5 % and 22.4 % would be eligible to participate in HYVET and SPRINT, respectively [15].
While there are other studies that have included older adults, such as the recent Strategy of blood pressure intervention in the Elderly Hypertensive Patients (STEP), these trials have included a range of chronological ages, but failed to identify any measures of frailty. [16] Including ambulatory community-dwelling older adults does not exclude the possibility of a frail senior, but it does make it far less likely. Given the availability of validated frailty measures, this could be integrated into future hypertension trials. Prescribing simply on chronological age is not an adequate indicator of frailty.
There are also several randomized controlled trials examining the impact deprescribing antihypertensive medication has cardiovascular events or mortality in the frail older adult population. There were two studies that focused on frail older adults in the 2020 Cochrane systematic review on withdrawal of antihypertensives in older adults, Burr 1977 (N = 141) and Myers 1982 (N = 77) [17], [18], [19]. Both found deprescribing diuretics had no impact on cardiovascular events and mortality. Furthermore, COmmunication, Systematic pain assessment and treatment, Medication review, Organization of activities and Safety (COSMOS) (N = 295) was a cluster randomized trial using an educational intervention to increase antihypertensive deprescribing in Norwegian long-term care facilities [20]. The authors did not report cardiovascular events or mortality but did find that hospitalizations were significantly higher in the control group in month four (7 residents vs 14 residents) and between months 4 and 9 (7 residents vs 12 residents). In addition, the results of the Optimizing Treatment for Mild Systolic Hypertension in the Elderly (OPTIMISE) trial are insightful, even though it was not focused on the frail older adult population and did not have a primary outcome of cardiovascular events or mortality [21]. The OPTIMISE trial found that a reduction of antihypertensive medication in older adults ≥80 years living in the community was not associated with a significant change in blood pressure or adverse events.
In addition, several well-designed observational studies have suggested that treatment of blood pressure in frail older adults may not improve cardiovascular outcomes.
-
1.
The Predictive Values of Blood Pressure and Arterial Stiffness in Institutionalized Very Aged Population (PARTAGE) study (N = 1127), a longitudinal study based in French long-term care facilities [22]. This study found that low SBP (SBP <130 mmHg) and ≥ 2 antihypertensive medications were associated with increased mortality. The exposed group had a higher rate of cardiovascular disease (72.2 % vs 46.9 %) compared with the other unexposed group; however, the possibility of reverse causality is minimized given 1) the hazard ratios incorporated cofactors including cardiovascular risk, activities of daily living (ADL), and Charleston Comorbidity index, and 2) excess mortality was still present with propensity score matching.
-
2.
Boockvar 2019 (N = 255,670), a retrospective cohort study in US long-term care facilities [23]. This study examined the association between an increased intensity of antihypertension treatment (1, 2 vs ≥3 antihypertensives) and hospitalization, mortality, and ADL. In comparison with the PARTAGE study, the selection criteria were less restrictive: The study included long-term care residents with a diagnosis of hypertension, 66 years old, and on at least one antihypertensive. Both adjusted and unadjusted odds ratios (OR) showed an increased intensity of antihypertensive treatment associated with a marginal increase in hospitalization, 0.24 % difference per additional medication (95 % CI: 0.03–0.45 %); a marginal increase in cardiovascular hospitalization, 0.30 % (95 % CI: 0.21–0.39 %); and a marginal decline in ADL, −0.46 % (95 % CI: −0.67 to −0.25 %). In addition, increased intensity of antihypertension medication was associated with a decrease in mortality, but this association was not present with adjusted results (−0.05 %, CI: −0.23-0.13 %). Multiple cofactors were considered including cardiovascular disease and life expectancy.
-
3.
Stessman 2017 (N = 480), a prospective observational study of 90-year-old adults in Jerusalem [24]. Participants were divided into three groups: normotensive group (not treated); hypertensive group (untreated); and hypertensive group (treated). Some characteristics in the hypertensive (treated) group were different from the hypertensive (not treated) group (e.g., depression, heart failure, and ischemic heart disease) and these were accounted for in the adjusted hazard ratios. The unadjusted and adjusted hazard ratios (HR) were similar and untreated hypertension was not associated with increased mortality risk but rather a trend toward decreased mortality: hypertensive group (untreated) unadjusted HR 1.38 (95 % CI: 0.89–2.15) and adjusted HR 1.39 (95 % CI: 0.83–2.33); hypertensive group (treated) unadjusted HR 0.70 (95 % CI: 0.37–1.31) and adjusted HR: 0.67 (95 % CI: 0.31–1.45). These results persisted with sensitivity analysis separating groups based on grip strength as a proxy for frailty and comorbidities.
The lack of evidence from randomized controlled trials, together with the observational trial evidence, suggests that it is unclear whether antihypertensive medication reduces cardiovascular events and mortality or rather the contrary: Antihypertensive medication may lead to worse outcomes such as increased mortality. It is important for health care professionals to consider that the benefit of cardiovascular risk reduction is uncertain when making antihypertensive prescribing and deprescribing decisions in the frail older adult population.
3. Harms of antihypertensives in frail older adults
There are many potential harms of antihypertensive medication in frail older adults, including ones more directly related to antihypertensive medication and ones related to polypharmacy in general. In this review we have focused on the direct harms and highlighted those most clinically relevant.
3.1. Falls
Falls are a significant concern for frail older adults leading to fractures, hospitalizations, and reduced quality of life [25], [26]. However, the evidence that antihypertensives directly contribute to falls in frail older adults is still far from certain.
There have been three recent systematic reviews and meta-analysis (all published in 2018) examining antihypertensive medication impact on fall risk in older adults (>60 years of age) [28], [29], [30]. Overall, there was little relationship observed between fall risk and antihypertensive medication and the relationship that was observed was inconsistent: de Vries et al., found that diuretics significantly increase the risk of falls; Ang et al. [30], observed ACE inhibitors, calcium channel blockers, and beta blockers associated with a lower risk of falls causing injury; and Kahlaee et al., noted a significant increase in risk of falls within the first 24 h of adding antihypertensives. The inconsistency is perhaps secondary to 1) differing literature searches and eligibility criteria among the systemic reviews and 2) the methodology of studies in the systematic reviews often not adjusting for confounding factors; having heterogenous populations; not considering dosage; and listing medications by class instead of by individual medication. In addition, even if the results are valid, it is questionable whether they are applicable to frail older adults given that the majority of the studies recruited older adults from the community.
We have summarized the studies included in the three recent systematic reviews specific to long-term care (n = 25) so that we could focus on the relationship between antihypertensives and falls in frail older adults (see Table 1). The median publication date of these studies was 2003 (interquartile range, IQR 1994–2011). Based on the Newcastle-Ottawa Scale used in the systematic reviews, 12 studies were low quality; 8 studies were moderate quality; and 5 studies were high quality (low quality 0–4; moderate quality 5–6; high quality 7–10) [56].
Table 1.
Observational studies in long-term care facilities with antihypertensive medication (exposure) and falls (outcome).
| Study | Study design | Location | Mean age (SD) | N | Exposure | Outcome | Qualitya | Impactb |
|---|---|---|---|---|---|---|---|---|
| Berry 2012 [31] | Case-crossover | USA | 88 (8) | 1181 | Diuretics, ACE + BB | Falls (one day after change in prescription) | high | Unadjusted OR: all diuretics 2.08 (95 % CI: 0.89–4.86); loop diuretics 2.46 (95 % CI: 1.02–5.92); ACE + BB 0.30 (95 % CI: 0.04–2.34) |
| Baranzini 2009 [32] | Cohort | Italy | Injured fallers: 84.6 (8.2); non-injured fallers: 84.8 (7.7) | 293 | Diuretics + AH | Injurious falls | high | Adjusted OR: 1.3 (95 % CI: 0.78–2.17) |
| Bozat-Emre 2015 [33] | Case-control | Canada | Cases: 96 % ≥ 75 years; controls: 97 % ≥ 75 years (mean and SD not given) | 3014 | Diuretics + AH | Falls | low | Chi-squared (used for cases and controls among nonuser, intermittent user, current user): P = .003 |
| Fisher 2003 [34] | Case-control | Australia | 87 (on AH 4, not on AH 3) | 119 | Diuretics, AH, ACE, CCB, BB, thiazide, potassium sparing | Falls | low | Unadjusted OR: diuretics 0.6 (95 % CI: 0.3–1.4); AH 0.8 (95 % CI: 0.4–1.6); ACE 1.0 (95 % CI: 0.5–2.3); CCB 1.4 (95 % CI: 0.6–3.2); BB 1.2 (95 % CI: 0.5–3.4); thiazide 0.4 (95 % CI: 0.1–1.5); potassium-sparing 0.2 (95 % CI: 0.04–1.0) |
| Granek 1987 [35] | Case-control | USA | Cases: 83; controls: 81 (median ages) | 368 | AH | Falls | mod | Chi-squared: P = .29 |
| Hasegawa 2010 [36] | Cohort, prospective | Japan | Non-fallers: 82.4 (8.5); fallers: 82.8 (8.5) | 1082 | AH (besides CCB and ACE), CCB, ACE | Falls, injurious falls, recurrent falls | low | Adjusted HR: AH (except for CCB and ACE): 1.24 (95 % CI: 0.88–1.76); CCB 1.2 (95 % CI: 0.92–1.56); unadjusted HR: ACE 0.9 (95 % CI: 0.62–1.37) |
| Jäntti 1993 [37] | Case-control | Finland | Cases: 84 (7); controls: 85 (5) | 301 | Diuretics | Falls | low | Chi-squared: no significant difference |
| Kerman 1990 [38] | Case-control | USA | Fall: 87 (5); without fall: 86 (7) | 147 | Diuretics, AH | Falls | mod | Unadjusted OR: diuretics 0.64 (95 % CI: 0.29–1.40); AH 1.96 (95 % CI: 0.55–7.06) |
| Lundin-Olsson 2003 [39] | Cohort, prospective | Sweden | Cases: 84.1 (7.3); controls: 82.4 (6.2) | 208 | Diuretics | Falls | low | Chi-squared: P = .10 |
| Luukinen 1995 [40] | Cohort, prospective | Finland | 81 (6) | 93 | Diuretics | Recurrent falls | low | Unadjusted RR: 1.1 (95 % CI: 0.74–1.59) |
| Makhlouf 2000 [41]c | Cross-sectional | Egypt | Average age 73 | 165 | Diuretics | Falls | low | Unknown |
| Maurer 2005 [42] | Cohort, prospective | USA | 88 (7) | 139 | Loop diuretic, ACE, CCB (no dihydropyridines) | Falls | low | Unadjusted HR: loop diuretics 1.72 (95 % CI: 0.96–3.07); ACE 2.08 (95 % CI: 1.18–3.68); CCB 2.18 (95 % CI: 0.98–4.85) |
| Mustard 1997 [43] | Case-control | Canada | 91.6 % ≥75 (mean and SD not given) | 2972 | Diuretics, AH, CCB, BB | Injurious falls | mod | Adjusted OR: diuretics: 0.97 (95 % CI: 0.82–1.15); AH 0.91 (95 % CI: 0.68–1.26); CCB 1.02 (95 % CI: 0.68–1.51); BB 1.04 (95 % CI: 0.64–1.63) |
| Myers 1994 [44] | Cohort, prospective | USA | ≥65 (mean and SD not given) | 242 | Diuretics, AH | Falls; injurious falls | low | Falls: unadjusted RR diuretics 1.22 (95 % CI: 0.80–1.85); AH 0.98 (95 % CI: 0.54–1.80) Injurious falls: unadjusted RR Diuretics 2.20 (95 % CI: 1.01–4.76); AH 1.47 (95 % CI: 0.54–4.01) |
| Neutel 2002 [45] | Cohort, prospective | Canada | 76 % ≥ 80 (mean and SD not given) | 227 | Diuretics | Falls | mod | Adjusted OR: 1.0 (95 % CI: 0.5–1.9) |
| Pelaez 2015 [46] | Cohort, prospective | Spain | Fall: 85 (7); without fall: 82 (8) | 74 | AH | Falls | mod | Chi-squared: AH P = .006, Wald test: AH combined with statins, platelets 0.80 (95 % CI: 0.14–4.71) |
| Pellfolk 2009 [47] | Cohort, prospective | Sweden | 84 (7) | 160 | Diuretics, ACE, CCB, BB | Falls | low | Chi-squared: Diuretic P = .281; ACE P = .406 (chi-squared with Yates correction); CCB P = .882; BB P = .198 |
| Reardon 2012 [48] | Cohort, prospective | USA | 81 (0.51) | 632 | Diuretics, BB | Fall, recurrent falls | mod | Adjusted OR: diuretics 1.25 (95 % CI: 0.78–1.99), BB 1.16 (95 % CI: 0.74–1.82) |
| Sieri 2004 [49] | Cross-sectional | Italy | Fallers: 88 (8); non-fallers 86 (5) | 40 | Diuretics | Falls | low | 23 % of fallers on diuretics and 60 % of non-fallers on diuretics (chi-square P = .019)d |
| Simonson 2011 [50] | Cross-sectional | USA | 82 (SD not given) | 7272 | AH, BB | Falls, injurious falls | low | Adjusted OR: AH 1.08 (95 % CI: 0.71–1.71); BB 1.14 (95 % CI: 1.04–1.2) |
| Sobel 1983 [51] | Case-control | USA | Fallers: 84; controls: 81 (SD not given)4 | 75 | Potassium sparing ± thiazide, thiazide ± potassium sparing, loop diuretic, BB, alpha 1 blocker, alpha 2 blocker, central monoamine depleting-agent ± other | Falls | high | Chi-squared: loop diuretic (P < .05) |
| Sterke 2012 [52] | Cohort, retrospective | Nether-lands | Injurious fall: 83 (7); without injurious fall: 81 (8) | 248 | AH | Falls, injurious falls | mod | Adjusted HR: use/no use 1.39 (95 % CI: 0.79–2.47); dose response 1.15 (95 % CI: 0.91–1.46) |
| Wells 1985 [53] | Case-control | USA | Cases: 82; controls; 80 (SD not given) | 77 | Diuretics, AH (all), AH (excluding diuretics) | Falls | mod | Chi-squared: diuretics not significant; AH (p = .02) |
| Whitney 2012 [54] | Cohort, prospective | UK | 85 (8) | 109 | AH, ACE+ARB | Falls | high | Unadjusted RR: AH 1.01 (95 % CI: 0.70–1.46); ACE+ARB 0.77 (95 % CI: 0.53–1.10) |
| Yip 1994 [55] | Case-control | Australia | 82 (SD not given) | 126 | Diuretic, AH | Falls | high | Adjusted OR: diuretics 0.92 (95 % CI: 0.34–2.49); AH 1.12 (95 % CI: 0.37–3.41) |
SD: standard deviation; AH: all antihypertensive medication (does not include diuretics); ACE: ACE inhibitors; ARB: angiotensin receptor blocker; BB: beta blockers; CCB: calcium channel blockers; diuretics: when study did not specify subcategory of diuretic (ie, loop, potassium sparing, and/or thiazide)
Mod: moderate.
Quality assessment from de Vries 2018 systematic review and Ang 2018 systematic review. Both used the Newcastle-Ottawa Scale.
Adjusted: statistical analysis adjusted for confounders; unadjusted: statistical analysis not adjusted for cofounders.
Unable to locate Makhlouf 2000, so data about this study is from the table in Ang 2018 systematic review.
Calculated chi-square based on values in Table 1 of study.
The 25 studies included in total 13 medication classes: diuretics (15 studies); all antihypertensives as a group (14 studies); angiotensin converting enzyme (ACE) inhibitors–angiotensin receptor blockers (6 studies); beta blockers (5 studies); calcium channel blockers (5 studies); thiazide diuretics (2 studies); potassium sparing diuretics (2 studies); loop diuretics (2 studies); and 5 other medications (1 study). In addition, there was no medication class that clearly showed a stronger association between antihypertensives and falls than another medication class.
Among the 25 studies, the following associations were found:
-
•
Seven studies: statistically significant association between antihypertensive medication and falls [31], [33], [42], [44], [46], [50], [51], only one of these studies accounted for confounders [50]
-
•
Nine studies: no significant association but a trend toward increased risk (OR, HR, and/or relative risk (RR) close to 1 and a confidence interval skewed to >1) [32], [36], [40], [43], [45], [48], [52], [54], [55], only 2 these studies did not adjust for confounders [40], [54]
-
•
Four studies: no significant association, unable to determine trend as utilized chi-squared for analysis [35], [37], [39], [47]
-
•
Three studies: inconsistent results among medication classes [34], [38], [53]
- •
The evidence infers that antihypertensive medication may increase the fall risk in frail older adults. Therefore, it is important to consider this possible harm in prescribing and deprescribing, especially given the impact a fall has on the function and quality of life of a frail older adult.
3.2. Cognitive impairment
The impact of antihypertensive medication on cognition in frail older adults is important given that cognitive impairment is prevalent in frail older adults and can significantly impact quality of life, disability, and mortality in this population [57], [58].
Longitudinal studies convincingly suggest that antihypertensive medication in middle age hypertensive adults reduces the risk of cognitive impairment in later life [59], [60]. However, this relationship is less clear in older adults. A 2020 systematic review and meta-analysis focused on older adults >60 years old, including 9 trials with a mean follow-up of 5 years, and found a small but statistically significant reduction in cognition decline with antihypertensive medication (standard mean difference of change in cognition: -0.049 (95 % CI: 0.078–0.019)) [61]. Another 2020 systematic review and meta-analysis, with the mean age of participants of 69 years and a follow-up period of 4.1 years, noted this same relationship: Among the 7 trials that referenced criteria for a dementia diagnosis, the odds and absolute risk reduction of developing dementia on antihypertensive medication was 0.87 (95 % CI: 0.78–0.97) and 0.2 % (95 % CI: 0.05–0.7 %), respectively [62]. By contrast, a 2021 Cochrane systematic review that included 12 studies with the majority of participants ≥60 years (9/12 studies) found that there was insufficient evidence to make this claim [63]. They attributed this to short duration of studies; studies not powered to assess cognition (i.e., cognition was not a primary outcome); and lack of appropriate cognitive outcome measures.
In the subset of older adults who are frail, there is even less evidence and, to our knowledge, only observational studies [64], [65], [66], [67], [68]. The majority of these studies found higher SBP to be associated with improved cognition; however, they provide little conclusive evidence, given that the extent of baseline frailty of the participants is often unclear; the exposure is often to blood pressure rather than antihypertensive medication; and often frailty/multimorbidity are not included as confounders.
The observational study that provides the best evidence on this is the Leiden 85-plus study. It is ingeniously designed to differentiate the impact of low blood pressure vs antihypertensive medication and accounts for frailty [69]. The study divided older adults into two groups (1 group on antihypertensive and 1 group not on antihypertensives) and compared the cognitive decline of older adults in the lowest quintile for blood pressure (SBP <140 mmHg) to the highest quintile for blood pressure (SBP >170 mmHg) for both groups. Older adults on antihypertensive medication in the lowest quintile had accelerated decline compared with those in the highest quintile (−1.1 points on the MMSE vs 0.1 point MMSE, P = .022). Older adults not on antihypertensive medication did not have a difference in cognitive decline between quintiles. The study used grip strength as a proxy for frailty and found that in the antihypertensive group, older adults with low grip strength had a more rapid cognitive decline than those with high grip strength, but this relationship was not found in the group not taking antihypertensives. This study suggests that antihypertensive medication in frail older adults with an SBP <140 mmHg may accelerate cognitive decline.
Although the evidence is limited, given that the best available evidence suggests that antihypertensive medication may worsen cognition in frail older adults, it would be prudent to factor this possible harm into decisions of whether to stop or start antihypertensive medication in the frail older adult population.
3.3. Orthostatic hypotension
Orthostatic hypotension is defined as a decrease in SBP of at least 20 mmHg and/or a decrease in diastolic blood pressure (DBP) of at least 10 mmHg within 3 min of standing [70]. It is prevalent in frail older adults [71], common symptoms include light-headedness, fatigue, and blurred vision [72], and it has been associated with falls [73] and cognitive decline [74].
Literature, including intervention and observational studies, consistently suggests that among the antihypertensive drug classes, it is the beta-blockers, alpha-blockers, and diuretics that increase the risk of orthostatic hypotension [75], [76]. There has also been one deprescribing trial evaluating the effect of deprescribing antihypertensive medication on orthostatic hypotension in community-dwelling older adults with mild cognitive impairment (N = 162). In per protocol analysis, there was a statistically significant decrease in orthostatic hypotension in the arm that had fully discontinued antihypertensive medication [77]. Intention-to-treat analysis in comparison showed a non-significant decrease, but this was attributed to not all intervention participants being deprescribed due to safety issues. In addition, this trial was not able to provide evidence on the effect between antihypertensive classes due to insufficient power. For frail older adults, there is sparse literature available. Two observational studies did not find an association between antihypertensive medication and orthostatic hypotension; however, both studies also did not account for confounders [78], [79].
Although there is limited evidence in frail older adults, the evidence available for adults and older adults suggests that some classes of antihypertensive medication increases the risk of orthostatic hypotension. Thus, it is reasonable to consider this as a possible harm when prescribing/deprescribing antihypertensive medication in frail older adults.
3.4. Pressure ulcers
Pressure ulcers are defined as “a localized injury to the skin and/or underlying tissue, as a result of pressure or pressure in combination with shear” [80]. Degenholz et al. found that pressure ulcers decreased feelings of autonomy and functional competence in long-term care residents [81]. A qualitative study by Goreccki et al. found that pressure ulcers were painful and pressure-relieving mattresses were often uncomfortable [82]. In addition, pressure ulcers are associated with increased cost of care [83].
Frail older adults are, unfortunately, a perfect storm for pressure ulcers given that they often have risk factors associated with pressure ulcers including reduced mobility, incontinence, decreased sensation, aged skin, and malnutrition [84]. Intuitively, it makes sense that antihypertensive medication may also be a risk factor given that decreased blood pressure can reduce blood flow to the skin. However, there is sparse literature on this. We are aware only of two cohort studies, both from Asia (N = 259 and N = 157) that examined the association between hypotension/antihypertensive medication and pressure ulcer formation in older adults (>60 years of age) [85], [86]. These found, after adjusting for confounders, that hypotension was statistically significantly associated with pressure ulcer formation. One of these studies, Man et al. also looked at the association between the use of antihypertensive medication and the development of pressure ulcers and did not find an association.
There is a clear lack of evidence on the association between antihypertensive medication and pressure ulcers; however, given both the plausibility of the association and extensive impact of pressure ulcers in the frail older adult population, this harm should be considered in antihypertensive-management decisions.
4. Guidelines
Guidelines are an important tool that allow practitioners to base their decisions on the best available evidence and expert opinion. Hypertension guidelines are no exception, and many jurisdictions/countries have published their own guidelines that provide recommendations on when to start hypertension treatment (threshold blood pressure) and if hypertension treatment is started, what the goal blood pressure should be (target blood pressure).
It appears that most guidelines now acknowledge there should be separate blood pressure thresholds and targets for older adults. The 2021 Bogaerts, et al. review of global guidelines found that 46 % of guidelines included targets for older adults while another 2021 review found that 81 % included targets for older adults [87], [88].
In addition, a growing number of guidelines also consider frailty: in a 2021 review of antihypertensive guidelines in older adults 18 out of 34 guidelines adapted recommendations for frailty/comorbidities (Table 2) [88]. However, the validity and reliability of these recommendations is questionable given the following:
-
•
Lack of frailty discussion in guidelines: Only 7 guidelines provided a definition of frailty.
-
•
Lack of high-quality evidence: The majority of guidelines that listed the evidence level primarily indicated C-level evidence (consensus of opinion of the experts and/or small studies, retrospective, or registries).
-
•
Lack of quality among guidelines: Only 8 guidelines were rated as moderate-to-high quality.
-
•
Lack of specific target and thresholds: Only 2 guidelines (2014 Canadian guidelines and 2019 Chinese guidelines) provide specific threshold and targets for frail older adults, neither of which are the mainstream guidelines in their respective countries [90], [95]. The primary Canadian guideline (Hypertension Canada), updated annually, recommends in general a lower threshold and target in older adults (>75 years of age) due to higher risk of cardiovascular disease [107]. The primary Chinese guideline, published in 2018, indicates that the “very elderly” can still benefit from hypertension treatment and subdivides older adults based on chronological age [93].
Table 2.
Hypertension guidelines that mention frailty.
| Author | Country | Association/Society | Frailty defined | Blood pressure threshold for frail older adultsa | Blood pressure target for frail older adultsa | Recommendationb/level of evidencec | Quality of guidelined |
|---|---|---|---|---|---|---|---|
| Feitosa 2019 [89] | Brazil | Brazilian Society of Cardiology and Brazilian Geriatrics and Gerontology Society | yes | Did not indicate | Adapted | Considered treatment of choicee/C | low |
| Hua 2019 [90] | China | Hypertension Branch of Chinese Geriatrics Society, National Clinical Research Centre of the Geriatric Diseases–Chinese Alliance of Geriatric Cardiovascular Disease | yes | ≥ 160/90 mmHg | 130–150 mmHg (systolic) | Should be considered/C | low |
| Kinoshita 2017 [91] | Japan | Japan Atherosclerosis Society | yes | Did not indicate | Did not indicate | Not given | low |
| Lee 2019 [92] | South Korea | Korean Society of Hypertension | no | >160 mmHg (systolic) | Did not indicate | Should be performed/A | low |
| Liu 2018 [93] | China | Chinese Hypertension League, Chinese Society of Cardiology, Hypertension Committee of the Chinese Medical doctor Association, Hypertension Branch of the China Association for the Promotion of International Exchanges of Health Care and Hypertension Branch of the Chinese Geriatrics Society | no | Adapted | Adapted | Not given | low |
| Malachias 2016 [94] | Brazil | Brazilian Society of Cardiology, Brazilian Society of Hypertension and Brazilian Society of Nephrology | no | Did not indicate | Adapted | Not given | low |
| Mallory 2014 [95] | Canada | Dalhouse Academic Detailing Service and the Palliative and Therapeutic Harmonization program | yes | ≥160 mmHg | 140–160 mmHg, severely frail 160–190 mmHg (systolic) | Not given | low |
| MsH, MOH 2018 [96] | Malaysia | Malaysian Society of Hypertension, Ministry of Health Malaysia, Academy of Medicine of Malaysia | yes | Did not indicate | Adapted | C | moderate - high |
| Nice 2019 [97] | UK | National Institute for Health and Care Excellence | yes | Adapted | Adapted | Not given | moderate - high |
| Piepoli 2016 [98] | Europe | European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice | no | Did not indicate | Adapted | May be considered/B (target) | moderate - high |
| Shah 2019 [99] | India | The Association of Physicians in India, Cardiological Society of India, Indian College of Physicians, Hypertension Society of India | no | Did not indicate | ≥120/70 mmHg | Not given | low |
| SIGN 2017 [100] | Scotland | Scottish Intercollegiate Guidelines Network | no | Did not indicate | Adapted | Not given | moderate - high |
| Tay 2017 [101] | Singapore | Ministry of Health, Chapter of Family Medicine Physicians Academy of Medicine, Singapore, Chapter of Endocrinologists College of Physicians, Singapore, College of Family Physicians, Singapore, Singapore Hypertension Society | no | Did not indicate | Adapted | Af | low |
| Tykarski 2019 [102] | Poland | Polish Society of Hypertension | no | Adapted | Did not indicate | Not given | low |
| Umemura 2019 [103] | Japan | Japanese Society of Hypertension | yes | Adapted | Adapted | weak, C (target) | moderate - high |
| US Department of Defense 2020 [104] | USA | Department of Veteran Affairs and Department of Defense | no | Did not indicate | Adapted | Not given | moderate - high |
| Whelton 2017 [105] | USA | American College of Cardiology/American Heart Association Task Force | no | Adaptedg | Adaptedg | Can be beneficial, C | moderate - high |
| Williams 2018 [106] | Europe | European Society of Cardiology and European Society of Hypertension | no | Adapted | Adapted | May be considered/B3 (threshold) | moderate - high |
Used the term “adapted” to encompass the varying terminology used to indicate that recommendations can be modified for frailty/comorbidities without mentioning a specific threshold or target (eg, exceptions, individualized, use clinical judgment, consider benefits vs harm/clinical situation, etc.)
Guidelines provided either definition, level (strong, medium, high), and/or suggested wording for recommendation grade. For this table, used suggested wording as it is the most concise, and if this were not provided, then used level.
All studies had the same basic levels of evidence, but the terminology used to describe the levels varied between studies. The one provided here is the version from Hau 2019. A: data derived from multiple randomized clinical trials or meta-analyses. B: data derived from a single randomized clinical trial or large non-randomized studies. C: consensus of opinion of the experts and or/small studies, retrospective studies, registries.
From Bogaerts et al. systematic review [88]. Assessed with AGREE 11 instrument.
From 2010 guideline as updated 2019; did not provide class of recommendation.
Provided recommendation grade as well (1+). However, the explanation of what each recommendation grade meant was unclear.
Recommendation appears to pertain only to a subset of frail older adults with a high burden of comorbidities, limited life expectancy, non-ambulatory and not living in the community.
Just as guidelines need to provide recommendations of when to start antihypertensive medication, it is also pertinent to provide recommendations of when antihypertensive medication should be discontinued. Of the guidelines in the 2021 systematic review, only 3 guidelines mentioned deprescribing antihypertensive medication for frail older adults [95], [96], [103]. The 2014 guidelines by Mallory et al. provide the most comprehensive guidance on stopping antihypertensive medication [95]. However, it still only includes three points on deprescribing and lacks details including which antihypertensive classes should be deprescribed first; how to reduce the dosage safely (percentage and time frame); and how to engage the individual, the family, and the team in deprescribing.
In summary, only a portion of the guidelines provide recommendations for frail older adults and of those that do discuss frail older adults, the discussion is often brief and based on low levels of evidence. In addition, only a minority of guidelines mention deprescribing antihypertensive medication, which is a continuum of prescribing. The lack of guidance and consistency across guidelines can make it difficult for practitioners to know when to treat, what target to treat to, and when to consider deprescribing for a frail older adult under their care.
The guideline that provides the best recommendation for frail older adults is the 2014 consensus guideline by Mallory et al.; it not only explicitly discusses frailty and provides targets/thresholds for two degrees of frailty, but it also includes deprescribing. [95] We recommend that this guideline be followed for frail older adults while waiting for evidence to become available to allow mainstream guidelines to include explicit recommendations for both prescribing and deprescribing in frail older adults.
In the future, it may also be more efficient for guidelines to move to simply providing recommendations for fit and frail adults rather than several recommendations based on chronological age and then an additional recommendation for frailty. In addition, as suggested by Ben-Eltriki et al., it may be advantageous to consider one single independent global hypertensive guideline with addenda for local use to allow for a consistent, evidence-based approach to hypertension management worldwide [108].
5. Tools to assess medication appropriateness
Given the number of adverse drug events; polypharmacy; pharmacokinetic and pharmacodynamic changes with ageing; and the complexity of managing medications in multimorbid older adults, clinicians have often depended on tools that can guide decision-making related to prescribing. There are both implicit and explicit tools to measure medication appropriateness in older adults [109].
Implicit tools require judgment, which improves with years of experience. For example, the Medication Appropriateness Index (MAI) is a tool developed over 30 years ago that provides a list of 10 questions that the clinician (or researcher, prescriber, or student) may use to score each medication the patient is taking [110]. Examples of the questions include if the directions are correct or if the duration of treatment is appropriate. There are notable limitations with an implicit tool, primarily interrater reliability [109]. The tool also takes time to apply 10 questions to each medication, which can be time-consuming when dealing with a patient on a long regimen. The MAI also was not designed to address prescribing omissions.
In contrast, explicit tools have had far greater integration with clinical practice due to their more straightforward nature. The ideal tool provides a concrete statement or guiding score that explicitly tells the prescriber (or clinician or student) if a medication is appropriate for older adults in general. These tools offer guidance on medication overuse (eg, not a valid indication) and medication misuse (eg, incorrect choice of medication) [111]. There are a number of explicit tools that have been published and used in research and practice, primarily based on their country of origin. The 5 tools we will discuss include the American Geriatric Society (AGS) Beers Criteria®; STOPP/START (Screening Tool of Older Persons' Prescriptions/Screening Tool to Alert to Right Treatment); STOPP Frail; STOPPFall and the FORTA (Fit fOR The Aged) classification [96], [97], [98], [99], [100].
The AGS Beers Criteria were first published in 1991 and were developed by expert consensus for medications deemed inappropriate for a nursing home setting [112]. Since 2011, the American Geriatrics Society has taken responsibility for updating these criteria. The process expanded to include more experts, interprofessional experts, and integration of medication safety evidence versus expert opinion only. The original criteria in 1991 included 4 antihypertensive statements: hydrochlorothiazide should be avoided at doses over 50 mg/d, and methyldopa, propranolol, and reserpine should be avoided [112]. The most recent 2019 Criteria includes avoiding peripheral alpha-1 blockers; central alpha-agonists (including clonidine); and nifedipine immediate release for treatment of hypertension [113]. The 2019 Beers Criteria also lists medications that are potentially inappropriate in older adults based on the drug-drug interactions, including combining RAS-I (Renin-angiotensin-aldosterone system) inhibitors (ACE inhibitors, ARBs) or potassium-sparing diuretics that could increase the risk of hyperkalemia, ACE inhibitors, and loop diuretics interacting with lithium as well as peripheral alpha-1 blockers interacting with loop diuretics. The final recommendations include criteria based on renal function; both spironolactone and triamterene should be avoided if creatinine clearance is <30 mL/min [96].
The STOPP/START criteria were developed with experts from 13 European countries and reflect medications and practices from that setting [114]. These criteria were updated from the 2008 original publication [115] and include a START component that addresses gaps in care where prescribing omissions are common in older adults. The START criteria states that antihypertensive medication should be initiated when SBP is consistently >160 mmHg or diastolic blood pressure is consistently >90 mmHg, or in an older adult who has diabetes, >140/90 mmHg [114].
There are other START recommendations for cardiovascular medications for purposes besides hypertension (eg, heart failure, ischemic heart disease). Medications in the STOPP criteria include beta blockers, verapamil, and diltiazem if the patient has heart block; thiazide diuretics with hypokalemia, hyponatremia, or hypercalcemia; and ACE inhibitors or ARBs in patients with hyperkalemia. Loop diuretics are also to be avoided for first-line hypertension treatment and with urinary incontinence. Centrally acting antihypertensives (eg, methyldopa, clonidine) are to be avoided, and aldosterone antagonists (eg, spironolactone) should be avoided with other potassium-sparing medications (eg, ACE inhibitors) without potassium monitoring [114].
A variation on the STOPP criteria was published in 2017 [116]. These criteria were specifically developed for guidance with older adults who are frail or with limited life expectancy. The only antihypertension statement is positioned under the medication class of alpha-blockers, noting that stringent blood pressure control is not required in very frail older people, and alpha-blockers cause marked vasodilation and risks for falls and injuries [116]. STOPPFrail version 2 was also designed to support deprescribing and has been validated in this context.
The STOPPFall is also a Delphi-consensus developed tool that focuses on medications that are potentially inappropriate in older adults with high fall risk. [117]. The final tool contains 14 drug classes, including 4 cardiovascular drug classes (centrally acting antihypertensives, vasodilators, and alpha-blocker antihypertensives). What is progressive about this tool is that the supplemental content includes deprescribing algorithms for all the drug classes identified, the withdrawal strategy, monitoring and follow-up for these patients.
FORTA was developed in Germany, designed to address both over- and undertreatment providing 4 categories for medication following ABCD (absolutely, beneficial, careful, do not). The experts involved in the updates are from Germany, Austria, and Switzerland [99]. These criteria were first developed in 2012 and have recently been updated in 2021. This most-updated version of the criteria had the most changes related to hypertension [118]. RAS-I, long-acting calcium channel blockers (eg, amlodipine) and indapamide are labeled A. Diuretics are the only category listed as B. Alpha-blockers and spironolactone are included as C. Centrally acting clonidine, minoxidil, verapamil, and atenolol are in the D category.
While there are other explicit tools to guide prescribing decisions for medication appropriateness, we have highlighted the most common tools used in practice. They each have slightly different designs and include medications unique to particular countries, but there are some consistencies, including the identification of centrally acting and vasodilating medications as being inappropriate.
Overall, the tools provide little guidance on stopping regular antihypertensive medication in frail older adults, aSTOPPFall is an exception; however, it applies only to those at very high risk for falls and does not provide guidance on the most common classes of antihypertensive medication. The lack of guidance integrated into medication appropriateness tools is understandable given the lack of evidence on benefits and harms of antihypertensive medication; however, this leaves practitioners without a medication appropriateness tool to guide them in deprescribing antihypertensive medication in frail older adults. The STOPPFrail is perhaps the ideal tool to expand to include guidance on deprescribing antihypertensive medication in the future.
6. Clinical decision support
Two tools were identified for this review that were designed specifically to guide decisions on antihypertensive medication for older adults. A tool from Australia acknowledges the complexity of treating a condition that is associated with morbidity and mortality while integrating comorbidities and frailty [119]. This decision framework is designed around 5 main steps. The first is to decide therapeutic goals involving shared decision-making. The authors also emphasize the importance of addressing comorbidities that can worsen hypertension (eg, obstructive sleep apnea) and implementing non-pharmacologic interventions if possible. The second step is to estimate absolute cardiovascular event risk, which could be done with a tool such as the Framingham Risk Score Calculator for Coronary Heart Disease, although it should be noted that its tools are based on clinical trial evidence and most trials have excluded older adults, particularly those over 80 years of age. This step also factors in other competing causes of death that may play more of a role in life expectancy than hypertension. The third step is to accurately measure blood pressure, including using validated instruments, measuring orthostatic blood pressures, and even taking overnight blood pressure readings.
The fourth step includes identifying the threshold and target blood pressures. The factors that guide this step include age (especially age 85 and above, which is associated with a decline in blood pressure). Other considerations in this step are comorbidity burden, frailty, and cognition. Notably, these patients are often excluded from clinical trials, but the authors of this framework note that what scant evidence there is points to the risk for harm, including falls, fractures, and renal insufficiency. The final and fifth step includes considering situations for deprescribing. The authors provide criteria for patients who are most likely to benefit at particular targets, such as those who should not have an SBP <130 mmHg. Those most likely to be harmed by antihypertensives medication to <140 mmHg are those over 80 years of age without CV disease; severe frailty; functional limitations; cognitive impairment; labile blood pressure; history of orthostasis, syncope, or falls; and end-stage disease with life expectancy <12 months.
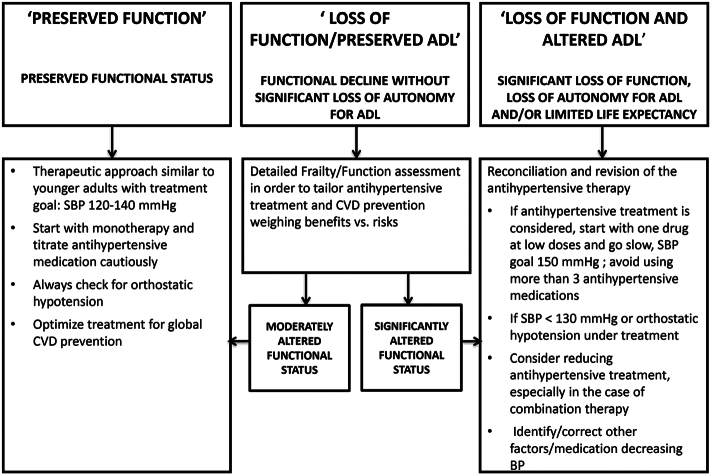
The second tool was developed by a team from the EU [60] and guides decision-making using 3 patient profiles: preserved function, loss of some activities, and loss of function affecting daily living (Fig. 1). This tool requires a similar preliminary approach as the tool by Scott et al. [119], including the appropriate measurement of blood pressure and determining functional status and frailty while considering patient autonomy. Indeed, each tool references the same studies (eg, HYVET, SPRINT), and provides similar guidance. The first category involves older adults with preserved function who are recommended to be treated similarly to younger patients, with SBP goals of 120 mm–140 mmHg. The second category, with some functional loss, was excluded from many clinical trials and poses more of a challenge for decision-making. The algorithm recommends categorizing this group into “moderately altered functional status” and “significantly altered functional status.” For those who are only moderately altered, it is recommended to treat as if they have preserved function, and for those with significant loss to treat as the third category. Factoring in multiple comorbidities and geriatric syndromes and scoring on the Clinical Frailty Scale is recommended in order to guide decisions [6]. The final category of those with significant functional loss mostly includes those 85 years of age and older and suggests SBP of 150 mmHg and avoiding 3 or more antihypertensive medications. In fact, this group is specifically mentioned for deprescribing attempts.
Fig. 1.
Decisional algorithm for management of hypertension in older adults ≥80 years old from Benetos 2019 [60].
Overall, we found very few clinical decision supports to guide deprescribing antihypertensive medications. Both of the tools we identified emphasized taking time to establish goals and correct measurements before making decisions. The integration of function and frailty, including cognitive impairment, play the greatest role in decision-making.
7. Conclusion
While the benefit of antihypertensive medication in the fit older adult population is clear, there is a paucity of evidence about both the benefits and harms of antihypertensive medication in the frail older population. The current evidence suggests that the cardiovascular benefit is uncertain and antihypertensive medication may lead to increased harm in the frail older adult population. In addition, guidelines, medication appropriateness tools and decision aids do not yet provide sufficient guidance for hypertension management and deprescribing in frail older adults.
It will take time for evidence to become available to confirm the benefit and harms of antihypertensives, time for this evidence to be integrated into guidelines, and time to modify and develop medication appropriateness tools and clinical decision supports. In the interim, given the potential of harm of antihypertensive medication in frail older adults, it is imperative that medical professionals do not just wait for further evidence and tools to become available, but rather take the time to engage in shared decision-making with the patient and/or their family to explain the uncertainty, provide the options, explore their preferences, and together make the decision of whether deprescribing antihypertensive medication is appropriate for their situation.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Roni Kraut: Conceptualization, Methodology, Formal analysis, Investigation, Data curation, Writing – original draft, Project administration. Carina Lundby: Writing – review & editing. Oksana Babenko: Formal analysis, Writing – review & editing. Ahmad Kamal: Investigation. Cheryl A. Sadowski: Conceptualization, Methodology, Formal analysis, Investigation, Writing – original draft, Project administration.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Roni Kraut, Email: rkraut@ualberta.ca.
Carina Lundby, Email: Carina.Lundby.Olesen@rsyd.dk.
Oksana Babenko, Email: obabenko@ualberta.ca.
Ahmad Kamal, Email: aakamal@ualberta.ca.
Cheryl A. Sadowski, Email: cherylas@ualberta.ca.
References
- 1.Blood Pressure Lowering Treatment Trialists' Collaboration Pharmacological blood pressure lowering for primary and secondary prevention of cardiovascular disease across different levels of blood pressure: an individual participant-level data meta-analysis. Lancet. 2021;397:1625–1636. doi: 10.1016/S0140-6736(21)00590-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Song X., Mitnitski A., Rockwood K. Prevalence and 10-year outcomes of frailty in older adults in relation to deficit accumulation. J. Am. Geriatr. Soc. 2010;58:681–687. doi: 10.1111/j.1532-5415.2010.02764.x. [DOI] [PubMed] [Google Scholar]
- 3.Bandeen-Roche K., Seplaki C.L., Huang J., Buta B., Kalyani R.R., Varadhan R., et al. Frailty in older adults: a nationally representative profile in the United States. J. Gerontol. A Biol. Sci. Med. Sci. 2015;70:1427–1434. doi: 10.1093/gerona/glv133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Welsh T., Gladman J., Gordon A.L. The treatment of hypertension in care home residents: a systematic review of observational studies. JAMDA. 2014;15:8–16. doi: 10.1016/j.jamda.2013.06.012. (Online) Cited15 March 2022. [DOI] [PubMed] [Google Scholar]
- 5.Clegg A., Young J., Iliffe S., Rikkert M.O., Rockwood K. Frailty in elderly people. Lancet. 2013;381:752–762. doi: 10.1016/S0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rockwood K., Song X., MacKnight C., Bergman H., Hogan D.B., McDowell I., et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489–495. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rivasi G., Tortù V., D'Andria M.F., Turrin G., Ceolin L., Rafanelli M., et al. Hypertension management in frail older adults: a gap in evidence. J. Hypertens. 2021;39:400–407. doi: 10.1097/HJH.0000000000002685. [DOI] [PubMed] [Google Scholar]
- 8.Masoli J.A.H., Delgado J. Blood pressure, frailty and dementia. Exp. Gerontol. 2021;155:155:111557. doi: 10.1016/j.exger.2021.111557. [DOI] [PubMed] [Google Scholar]
- 9.Vu M., Schleiden L.J., Harlan M.L., Thorpe C.T. Hypertension management in nursing homes: review of evidence and considerations for care. Curr. Hypertens. Rep. 2020;22:8. doi: 10.1007/s11906-019-1012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delgado João. Blood pressure trajectories in the 20 years before death. JAMA Intern. Med. 2018;178:93–99. doi: 10.1001/jama.2016.7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williamson J.D., Supiano M.A., Applegate W.B., et al. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥75 years: a randomized clinical trial. JAMA. 2016;315:2673–2682. doi: 10.1001/jama.2016.7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beckett N.S., Peters R., Fletcher A.E., Staessen J.A., Liu L., Dumitrascu D., Stoyanovsky V., Antikainen R.L., Nikitin Y., Anderson C., Belhani A., Forette F., Rajkumar C., Thijs L., Banya W., Bulpitt C.J., HYVET Study Group Treatment of hypertension in patients 80 years of age or older. N. Engl. J. Med. 2008;358(18):1887–1898. doi: 10.1056/NEJMoa0801369. (May 1) [DOI] [PubMed] [Google Scholar]
- 13.Warwick J., Falaschetti E., Rockwood K., Mitnitski A., Thijs L., Beckett N., et al. No evidence that frailty modifies the positive impact of antihypertensive treatment in very elderly people: an investigation of the impact of frailty upon treatment effect in the HYpertension in the very elderly trial (HYVET) study, a double-blind, placebo-controlled study of antihypertensives in people with hypertension aged 80 and over. BMC Med. 2015;78 doi: 10.1186/s12916-015-0328-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nordmann A., Fernandes M., Olde Rikkert M.G.M. Look before you SPRINT: look at the data and look at the consequences. Swiss Med. Wkly. 2018;1 doi: 10.4414/smw.2018.14629. [DOI] [PubMed] [Google Scholar]
- 15.Sheppard J.P., Lown M., Burt J., Temple E., Lowe R., Ashby H., et al. Generalizability of blood pressure lowering trials to older patients: cross-sectional analysis. J. Am. Geriatr. Soc. 2020;68:2508–2515. doi: 10.1111/jgs.16749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang W., Zhang S., Deng Y., Wu S., Ren J., Sun G., et al. Trial of intensive blood-pressure control in older patients with hypertension. N. Engl. J. Med. 2021;385:1268–1279. doi: 10.1056/NEJMoa2111437. [DOI] [PubMed] [Google Scholar]
- 17.Reeve E., Jordan V., Thompson W., Sawan M., Todd A., Gammie T.M., et al. Withdrawal of antihypertensive drugs in older people. Cochrane Database Syst. Rev. 2020;6 doi: 10.1002/14651858.CD012572.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burr M.L., King S., Davies H.E., Pathy M.S. The effects of discontinuing long-term diuretic therapy in the elderly. Age Ageing. 1977;6:38–45. doi: 10.1093/ageing/6.1.38. [DOI] [PubMed] [Google Scholar]
- 19.Myers M.G., Weingert M.E., Fisher R.H., Gryfe C.I., Shulman H.S. Unnecessary diuretic therapy in the elderly. Age Ageing. 1982;11:213–221. doi: 10.1093/ageing/11.4.213. [DOI] [PubMed] [Google Scholar]
- 20.Gulla C., Flo E., Kjome R.L., Husebo B.S. Deprescribing antihypertensive treatment in nursing home patients and the effect on blood pressure. J. Geriatr. Cardiol. 2018;15:275–283. doi: 10.11909/j.issn.1671-5411.2018.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sheppard J.P., Burt J., Lown M., et al. Effect of antihypertensive medication reduction vs usual care on short-term blood pressure control in patients with hypertension aged 80 years and older: the OPTIMISE randomized clinical trial. JAMA. 2020;323:2039–2051. doi: 10.1001/jama.2020.4871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benetos A., Labat C., Rossignol P., Fay R., Rolland Y., Valbusa F., et al. Treatment with multiple blood pressure medications, achieved blood pressure, and mortality in older nursing home residents: the PARTAGE study. JAMA Intern. Med. 2015;175:989–995. doi: 10.1001/jamainternmed.2014.8012. [DOI] [PubMed] [Google Scholar]
- 23.Boockvar K.S., Song W., Lee S., Intrator O. Hypertension treatment in US long-term nursing home residents with and without dementia. J. Am. Geriatr. Soc. 2019;67:2058–2064. doi: 10.1111/jgs.16081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stessman J., Bursztyn M., Gershinsky Y., Hammerman-Rozenberg A., Jacobs J.M. Hypertension and its treatment at age 90 years: is there an association with 5-year mortality? J. Am. Med. Dir. Assoc. 2017;218 doi: 10.1016/j.jamda.2016.12.076. e13-277. [DOI] [PubMed] [Google Scholar]
- 25.de Jong M.R., Van der Elst M., Hartholt K.A. Drug-related falls in older patients: implicated drugs, consequences, and possible prevention strategies. Ther. Adv. Drug Saf. 2013;4:147–154. doi: 10.1177/2042098613486829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Joode S.G.C.J., Kalmet P.H.S., Fiddelers A.A.A., Poeze M., Blokhuis T.J. Long-term functional outcome after a low-energy hip fracture in elderly patients. J. Orthop. Traumatol. 2019;20:20. doi: 10.1186/s10195-019-0529-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Vries M., Seppala L.J., Daams J.G., Masud T., van der Velde N., van de Glind E.M.M., EUGMS Task and Finish Group on Fall-Risk-Increasing Drugs Fall-risk-increasing drugs: a systematic review and meta-analysis: I. Cardiovascular drugs. J. Am. Med. Dir. Assoc. 2018;19 doi: 10.1016/j.jamda.2017.12.013. e1-371.e9. [DOI] [PubMed] [Google Scholar]
- 29.Kahlaee H.R., Latt M.D., Schneider C.R. Association between chronic or acute use of antihypertensive class of medications and falls in older adults. A systematic review and meta-analysis. Am. J. Hypertens. 2018;31:467–479. doi: 10.1093/ajh/hpx189. [DOI] [PubMed] [Google Scholar]
- 30.Ang H.T., Lim K.K., Kwan Y.H., Tan P.S., Yap K.Z., Banu Z., et al. A systematic review and meta-analyses of the association between anti-hypertensive classes and the risk of falls among older adults. Drugs Aging. 2018;35:625–635. doi: 10.1007/s40266-018-0561-3. [DOI] [PubMed] [Google Scholar]
- 31.Berry S.D., Mittleman M.A., Zhang Y., et al. New loop diuretic prescriptions may be an acute risk factor for falls in the nursing home. Pharmacoepidemiol. Drug Saf. 2012;21:560–563. doi: 10.1002/pds.3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baranzini F., Diurni M., Ceccon F., et al. Fall-related injuries in a nursing home setting: is polypharmacy a risk factor? BMC Health Serv. Res. 2009;9 doi: 10.1186/1472-6963-9-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bozat-Emre S., Doupe M., Kozyrskyj A.L., Grymonpre R., Mahmud S.M. Atypical antipsychotic drug use and falls among nursing home residents in Winnipeg, Canada. Int. J. Geriatr. Psychiatry. 2015;30:842–850. doi: 10.1002/gps.4223. [DOI] [PubMed] [Google Scholar]
- 34.Fisher A.A., McLean A.J., Davis M.W., Le Couteur D.G. A multicenter, case-control study of the effects of antihypertensive therapy on orthostatic hypotension, postprandial hypotension, and falls in octo- and nonagenarians in residential care facilities. Curr. Ther. Res. Clin. Exp. 2003;64:206–214. doi: 10.1016/S0011-393X(03)00023-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Granek E., Baker S.P., Abbey H., Robinson E., Myers A.H., Samkoff J.S., et al. Medications and diagnoses in relation to falls in a long-term care facility. J. Am. Geriatr. Soc. 1987;35:503–511. doi: 10.1111/j.1532-5415.1987.tb01395.x. [DOI] [PubMed] [Google Scholar]
- 36.Hasegawa J., Kuzuya M., Iguchi A. Urinary incontinence and behavioral symptoms are independent risk factors for recurrent and injurious falls, respectively, among residents in long-term care facilities. Arch. Gerontol. Geriatr. 2010;50:77–81. doi: 10.1016/j.archger.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 37.Jäntti P.O., Pyykkö V.I., Hervonen A.L. Falls among elderly nursing home residents. Public Health. 1993;107:89–96. doi: 10.1016/s0033-3506(05)80404-4. [DOI] [PubMed] [Google Scholar]
- 38.Kerman M., Mulvihill M. The role of medication in falls among the elderly in a long-term care facility. Mt. Sinai J. Med. 1990;57:343–347. doi: 10.1177/0898264310381277. PMID: 1981925. [DOI] [PubMed] [Google Scholar]
- 39.Lundin-Olsson L., Jensen J., Nyberg L., Gustafson Y. Predicting falls in residential care by a risk assessment tool, staff judgement, and history of falls. Aging Clin. Exp. Res. 2003;15:51–59. doi: 10.1007/BF03324480. [DOI] [PubMed] [Google Scholar]
- 40.Luukinen H., Koski K., Laippala P., Kivela S.L. Risk factors for recurrent falls in the elderly in long-term institutional care. Public Health. 1995;109:57–65. doi: 10.1016/s0033-3506(95)80076-x. [DOI] [PubMed] [Google Scholar]
- 41.Makhlouf M., Ayoub A. Falls among institutionalized elderly in Alexandria. J. Egypt. Public Health Assoc. 2000;75:507–528. [PubMed] [Google Scholar]
- 42.Maurer M.S., Burcham J., Cheng H. Diabetes mellitus is associated with an increased risk of falls in elderly residents of a long-term care facility. J. Gerontol. A Biol. Sci. Med. Sci. 2005;60:1157–1162. doi: 10.1093/gerona/60.9.1157. [DOI] [PubMed] [Google Scholar]
- 43.Mustard C.A., Mayer T. Case-control study of exposure to medication and the risk of injurious falls requiring hospitalization among nursing home residents. Am. J. Epidemiol. 1997;145:738–745. doi: 10.1093/aje/145.8.738. [DOI] [PubMed] [Google Scholar]
- 44.Myers A.H., Van Natta M., Robinson E.G., Baker S.P. Can injurious falls be prevented? J. Long Term Care Adm. 1994;22:26–29. [PubMed] [Google Scholar]
- 45.Neutel C.I., Perry S., Maxwell C. Medication use and risk of falls. Pharmacoepidemiol. Drug Saf. 2002;11:97–104. doi: 10.1002/pds.686. [DOI] [PubMed] [Google Scholar]
- 46.Peláez V.C., Ausín L., Mambrilla M.R., Gonzalez-Sagrado M., Pérez Castrillón J.L. Prospective observational study to evaluate risk factors for falls in institutionalized elderly people: the role of cystatin C. Aging Clin. Exp. Res. 2015;27:419–424. doi: 10.1007/s40520-014-0304-1. [DOI] [PubMed] [Google Scholar]
- 47.Pellfolk T., Gustafsson T., Gustafson Y., Karlsson S. Risk factors for falls among residents with dementia living in group dwellings. Int. Psychogeriatr. 2009;21:187–194. doi: 10.1017/S1041610208007837. [DOI] [PubMed] [Google Scholar]
- 48.Reardon G., Pandya N., Bailey R.A. Falls in nursing home residents receiving pharmacotherapy for anemia. Clin. Interv. Aging. 2012;7:397–407. doi: 10.2147/CIA.S34789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sieri T., Beretta G. Fall risk assessment in very old males and females living in nursing homes. Disabil. Rehabil. 2004;26:718–723. doi: 10.1080/09638280410001704304. [DOI] [PubMed] [Google Scholar]
- 50.Simonson W., Han L.F., Davidson H.E. Hypertension treatment and outcomes in US nursing homes: results from the US National Nursing Home Survey. J. Am. Med. Dir. Assoc. 2011;12:44–49. doi: 10.1016/j.jamda.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 51.Sobel K.G., McCart G.M. Drug use and accidental falls in an intermediate care facility. Drug Intell. Clin. Pharm. 1983;17:539–542. doi: 10.1177/106002808301700708. [DOI] [PubMed] [Google Scholar]
- 52.Sterke C.S., Ziere G., van Beeck E.F., Looman C.W., van der Cammen T.J. Dose-response relationship between selective serotonin re-uptake inhibitors and injurious falls: a study in nursing home residents with dementia. Br. J. Clin. Pharmacol. 2012;73:812–820. doi: 10.1111/j.1365-2125.2011.04124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wells B.G., Middleton B., Lawrence G., Lillard D., Safarik J. Factors associated with the elderly falling in intermediate care facilities. Drug Intell. Clin. Pharm. 1985;19:142–145. doi: 10.1177/106002808501900212. [DOI] [PubMed] [Google Scholar]
- 54.Whitney J., Close J.C., Jackson S.H., Lord S.R. Understanding risk of falls in people with cognitive impairment living in residential care. J. Am. Med. Dir. Assoc. 2012;13:535–540. doi: 10.1016/j.jamda.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 55.Yip Y.B., Cumming R.G. The association between medications and falls in Australian nursing-home residents. Med. J. Aust. 1994;160:14–18. [PubMed] [Google Scholar]
- 56.Cheng Y.Z., Huang Z.Z., Shen Z.F., Wu H.Y., Peng J.X., Waye M.M., Rao S.T., Yang L. ACE inhibitors and the risk of fractures: a meta-analysis of observational studies. Endocrine. 2017;55:732–740. doi: 10.1007/s12020-016-1201-5. [DOI] [PubMed] [Google Scholar]
- 57.Alberta long-term care resident profile 2016/2017. 2022. https://open.alberta.ca/dataset/90c128a6-3a8e-4c6e-8591-58e88fe6b6f9/resource/894a3a9c-8999-4487-b7e5-2850b3bb1a2e/download/cc-ltc-resident-profile-2017.pdf Cited 21 March.
- 58.Sugimoto T., Sakurai T., Ono R., Kimura A., Saji N., Niida S., et al. Epidemiological and clinical significance of cognitive frailty: a mini review. Ageing Res. Rev. 2018;44:1–7. doi: 10.1016/j.arr.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 59.Qiu C., Winblad B., Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia. Lancet Neurol. 2005;4:487–499. doi: 10.1016/S1474-4422(05)70141-1. [DOI] [PubMed] [Google Scholar]
- 60.Benetos A., Petrovic M., Strandberg T. Hypertension management in older and frail older patients. Circ. Res. 2019;124:1045–1060. doi: 10.1161/CIRCRESAHA.118.313236. [DOI] [PubMed] [Google Scholar]
- 61.Gupta A., Perdomo S., Billinger S., Beddhu S., Burns J., Gronseth G. Treatment of hypertension reduces cognitive decline in older adults: a systematic review and meta-analysis. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2020-038971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hughes D., Judge C., Murphy R., Loughlin E., Costello M., Whiteley W., et al. Association of blood pressure lowering with incident dementia or cognitive impairment: a systematic review and meta-analysis. JAMA. 2020;323:1934–1944. doi: 10.1001/jama.2020.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cunningham E.L., Todd S.A., Passmore P., Bullock R., McGuinness B. Pharmacological treatment of hypertension in people without prior cerebrovascular disease for the prevention of cognitive impairment and dementia. Cochrane Database Syst. Rev. 2021;5 doi: 10.1002/14651858.CD004034.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nilsson S.E., Read S., Berg S., Johansson B., Melander A., Lindblad U. Low systolic blood pressure is associated with impaired cognitive function in the oldest old: longitudinal observations in a population-based sample 80 years and older. Aging Clin. Exp. Res. 2007;19:41–47. doi: 10.1370/afm.2367. [DOI] [PubMed] [Google Scholar]
- 65.Stewart R., Xue Q.L., Masaki K., Petrovitch H., Ross G.W., White L.R., et al. Change in blood pressure and incident dementia: a 32-year prospective study. Hypertension. 2009;54:233–240. doi: 10.1161/HYPERTENSIONAHA.109.128744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Heikinheimo R.J., Haavisto M.V., Kaarela R.H., Kanto A.J., Koivunen M.J., Rajala S.A. Blood pressure in the very old. J. Hypertens. 1990;8:361–367. doi: 10.1097/00004872-199004000-00010. [DOI] [PubMed] [Google Scholar]
- 67.Corrada M.M., Hayden K.M., Paganini-Hill A., Bullain S.S., DeMoss J., Aguirre C., et al. Age of onset of hypertension and risk of dementia in the oldest-old: the 90+ study. Alzheimers Dement. 2017;13:103–110. doi: 10.1016/j.jalz.2016.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Szewieczek J., Dulawa J., Gminski J., Kurek A., Legierska K., Francuz T., et al. Better cognitive and physical performance is associated with higher blood pressure in centenarians. J. Nutr. Health Aging. 2011;15:618–622. doi: 10.1111/j.1532-5415.2009.02264.x. [DOI] [PubMed] [Google Scholar]
- 69.Streit S., Poortvliet R.K.E., Gussekloo J. Lower blood pressure during antihypertensive treatment is associated with higher all-cause mortality and accelerated cognitive decline in the oldest old—data from the Leiden 85-plus study. Age Ageing. 2018;47:545–550. doi: 10.1093/ageing/afy072. [DOI] [PubMed] [Google Scholar]
- 70.Consensus statement on the definition of orthostatic hypotension, pure autonomic failure, and multiple system atrophy. The Consensus Committee of the American Autonomic Society and the American Academy of Neurology. Neurology. 1996;46:1470. doi: 10.1212/wnl.46.5.1470. [DOI] [PubMed] [Google Scholar]
- 71.Saedon N.I., Pin Tan M., Frith J. The prevalence of orthostatic hypotension: a systematic review and meta-analysis. J. Gerontol. A Biol. Sci. Med. Sci. 2020;75:117–122. doi: 10.1093/gerona/gly188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Joseph A., Wanono R., Flamant M., Vidal-Petiot E. Orthostatic hypotension: a review. Nephrol. Ther. 2017;13:S55–S67. doi: 10.1016/j.nephro.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 73.Mol A., Bui Hoang P.T.S., Sharmin S., Reijnierse E.M., van Wezel R.J.A., Meskers C.G.M., et al. Orthostatic hypotension and falls in older adults: a systematic review and meta-analysis. J. Am. Med. Dir. Assoc. 2019;20:589–597.e5. doi: 10.1016/j.jamda.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 74.Peters R., Anstey K.J., Booth A., Beckett N., Warwick J., Antikainen R. Orthostatic hypotension and symptomatic subclinical orthostatic hypotension increase risk of cognitive impairment: an integrated evidence review and analysis of a large older adult hypertensive cohort. Eur. Heart J. 2018;39:3135–3143. doi: 10.1093/eurheartj/ehy418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bhanu C., Nimmons D., Petersen I., Orlu M., Davis D., Hussain H., et al. Drug-induced orthostatic hypotension: a systematic review and meta-analysis of randomised controlled trials. PLoS Med. 2021;18 doi: 10.1371/journal.pmed.1003821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rivasi G., Rafanelli M., Mossello E., Brignole M., Ungar A. Drug-Related Orthostatic Hypotension: Beyond Anti-Hypertensive Medications. Vol. 37. 2020. pp. 725–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Moonen J.E., Foster-Dingley J.C., de Ruijter W., van der Grond J., de Craen A.J., van der Mast R.C. Effect of discontinuation of antihypertensive medication on orthostatic hypotension in older persons with mild cognitive impairment: the DANTE study Leiden. Age Ageing. 2016;45:249–255. doi: 10.1093/ageing/afv199. [DOI] [PubMed] [Google Scholar]
- 78.Valbusa F., Labat C., Salvi P., Vivian M.E., Hanon O., Benetos A., et al. Orthostatic hypotension in very old individuals living in nursing homes. J. Hypertens. 2012;30:53–60. doi: 10.1097/HJH.0b013e32834d3d73. [DOI] [PubMed] [Google Scholar]
- 79.Ooi W.L., Barrett S., Hossain M., Kelley-Gagnon M., Lipsitz L.A. Patterns of orthostatic blood pressure change and their clinical correlates in a frail, elderly population. JAMA. 1997;16:1299–1304. doi: 10.1001/jama.1997.03540400049030. [DOI] [PubMed] [Google Scholar]
- 80.European Pressure Ulcer Advisory Panel, National Pressure Injury Advisory Panel, Pan Pacific Pressure Injury Alliance . In: Prevention and Treatment of Pressure Ulcers/Injuries: Clinical Practice Guideline. The International Guideline. Haesler Emily., editor. EPUAP/NPIAP/PPPIA; 2019. [Google Scholar]
- 81.Degenholtz H.B., Rosen J., Castle N., Mittal V., Liu D. The association between changes in health status and nursing home resident quality of life. Gerontologist. 2008;48:584–592. doi: 10.1093/geront/48.5.584. [DOI] [PubMed] [Google Scholar]
- 82.Gorecki C., Nixon J., Madill A., Firth J., Brown J.M. What influences the impact of pressure ulcers on health-related quality of life? A qualitative patient-focused exploration of contributory factors. J. Tissue Viability. 2012;21:3–12. doi: 10.1016/j.jtv.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 83.Severens J.L., Habraken J.M., Duivenvoorden S., Frederiks C.M. The cost of illness of pressure ulcers in the Netherlands. Adv. Skin Wound Care. 2002;15:72–77. doi: 10.1097/00129334-200203000-00008. [DOI] [PubMed] [Google Scholar]
- 84.Braden B., Bergstrom N. A conceptual schema for the study of the etiology of pressure sores. Rehabil. Nurs. 1987;12:8–12. doi: 10.1002/j.2048-7940.1987.tb00541.x. [DOI] [PubMed] [Google Scholar]
- 85.Man S.P., Au-Yeung T.W. Hypotension is a risk factor for new pressure ulcer occurrence in older patients after admission to an acute hospital. J. Am. Med. Dir. Assoc. 2013;14(627) doi: 10.1016/j.jamda.2013.05.003. e1-5. [DOI] [PubMed] [Google Scholar]
- 86.Gong X., Chen H.L., Shen J.H., Zhu B.F. Hypotension at emergency department admission and hospital-acquired pressure ulcers in older patients: prospective study. J. Wound Care. 2019;28:527–531. doi: 10.12968/jowc.2019.28.8.527. [DOI] [PubMed] [Google Scholar]
- 87.Philip R., Beaney T., Appelbaum N., Gonzalvez C.R., Koldeweij C., Golestaneh A.K., et al. Variation in hypertension clinical practice guidelines: a global comparison. BMC. 2021;19:117. doi: 10.1186/s12916-021-01963-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bogaerts J.M.K., von Ballmoos L.M., Achterberg W.P., Gussekloo J., Streit S., van der Ploeg M.A., et al. Do we AGREE on the targets of antihypertensive drug treatment in older adults: a systematic review of guidelines on primary prevention of cardiovascular diseases? Age Ageing. 2022;51 doi: 10.1093/ageing/afab192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Feitosa-Filho G.S., Peixoto J.M., Pinheiro J.E.S., Afiune Neto A., Albuquerque A.L.T., Cattani Á.C., et al. Updated geriatric cardiology guidelines of the brazilian Society of Cardiology - 2019. Arq. Bras. Cardiol. 2019;112:649–705. doi: 10.5935/abc.20190086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hua Q., Fan L., Li J., Joint Committee for Guideline Revision 2019 Chinese guideline for the management of hypertension in the elderly. J. Geriatr. Cardiol. 2019;16:67–99. doi: 10.5603/KP.2017.0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kinoshita M., Yokote K., Arai H., Iida M., Ishigaki Y., Ishibashi S., et al. Japan atherosclerosis society (JAS) guidelines for prevention of atherosclerotic cardiovascular diseases 2017. J. Atheroscler. Thromb. 2018;25:846–984. doi: 10.5551/jat.GL2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lee H.Y., Shin J., Kim G.H., Park S., Ihm S.H., Kim H.C., et al. 2018 Korean Society of Hypertension Guidelines for the management of hypertension: part II-diagnosis and treatment of hypertension. Clin. Hypertens. 2019;25:20. doi: 10.1186/s40885-019-0124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Joint Committee for Guideline Revision Chinese guidelines for prevention and treatment of hypertension-a report of the revision Committee of Chinese Guidelines for prevention and treatment of hypertension. J. Geriatr. Cardiol. 2018;16(2019):182–241. doi: 10.11909/j.issn.1671-5411.2019.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Malachias M.V. 7th brazilian guideline of arterial hypertension: presentation. Arq. Bras. Cardiol. 2016;107 doi: 10.5935/abc.20160140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mallery L.H., Allen M., Fleming I., Kelly K., Bowles S., Duncan J., Moorhouse P. Promoting higher blood pressure targets for frail older adults: a consensus guideline from Canada. Cleve. Clin. J. Med. 2014;81:427–437. doi: 10.3949/ccjm.81a.13110. [DOI] [PubMed] [Google Scholar]
- 96.Malaysian Society of Hypertension, Ministry of Health Malaysia, Academy of Medicine of Malaysia Management of hypertension 5th edition of clinical practice guidelines. 2018. http://www.acadmed.org (online) Cited 15 March 22.
- 97.National Institute for Health and Care Excellence Hypertension in adults: diagnosis and management. 2019. https://www.nice.org.uk/guidance/ng136 Cited 15 March 2022. [PubMed]
- 98.Piepoli M.F., Hoes A.W., Agewall S., Albus C., Brotons C., Catapano A.L., et al. European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR) Eur. Heart J. 2016;37(2016):2315–2381. doi: 10.1093/eurheartj/ehw106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shah S.N., Munjal Y.P., Kamath S.A., Wander G.S., Mehta N., Mukherjee S., et al. Indian guidelines on hypertension-IV (2019) J. Hum. Hypertens. 2020;34:745–758. doi: 10.1038/s41371-020-0349-x. [DOI] [PubMed] [Google Scholar]
- 100.SIGN Guideline 149: risk estimation and the prevention of cardiovascular disease. 2017. https://wwwsignacuk/assets/sign149pdf (Online) Cited15 March 2022.
- 101.Tay J.C., Sule A.A., Chew E.K., Tey J.S., Lau T., Lee S., et al. Ministry of health clinical practice guidelines: hypertension. Singap. Med. J. 2018;59:17–27. doi: 10.11622/smedj.2018007. [DOI] [PubMed] [Google Scholar]
- 102.Tykarski A., Filipiak K.J., Januszewicz A., et al. guidelines for the management of hypertension. Arterial Hypertens. 2019;23(2019):41–87. doi: 10.5603/AH.a2019.0008. [DOI] [Google Scholar]
- 103.Umemura S., Arima H., Arima S., Asayama K., Dohi Y., Hirooka Y., et al. The Japanese Society of Hypertension guidelines for the management of hypertension (JSH 2019) Hypertens. Res. 2019;42:1235–1481. doi: 10.1038/s41440-019-0284-9. [DOI] [PubMed] [Google Scholar]
- 104.U.S. Department of Veterans Affairs, U.S. Department of Defense VA/DoD clinical practice guideline for diagnosis and management of hypertension in the primary care setting (online) 2022. https://wwwhealthqualityvagov/guidelines/CD/htn/VADoDHypertensionCPG508Corrected792020pdf Cited 15 March.
- 105.Whelton P.K., Carey R.M., Aronow W.S., Casey D.E., Jr., Collins K.J., Dennison Himmelfarb C., et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71 doi: 10.1161/HYP.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 106.Williams B., Mancia G., Spiering W., Agabiti Rosei E., Azizi M., Burnier M. ESC Scientific Document Group. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur. Heart J. 2018;39:3021–3104. doi: 10.1093/eurheartj/ehy339. [DOI] [PubMed] [Google Scholar]
- 107.Rabi D.M., McBrien K.A., Sapir-Pichhadze R., Nakhla M., Ahmed S.B., Dumanski S.M. Hypertension Canada's 2020 comprehensive guidelines for the prevention, diagnosis, risk assessment, and treatment of hypertension in adults and children. Can. J. Cardiol. 2020;36:596–624. doi: 10.1016/j.cjca.2020.02.086. [DOI] [PubMed] [Google Scholar]
- 108.Ben-Eltriki M., Cassels A., Erviti J., et al. Why we need a single independent international hypertension clinical practice guideline. Hypertens. Res. 2021;44:1037–1039. doi: 10.1038/s41440-021-00666-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Spinewine A., Schmader K.E., Barber N., Hughes C., Lapane K.L., Swine C. Appropriate prescribing in elderly people: how well can it be measured and optimised? Lancet. 2007;(370):173–184. doi: 10.1016/S0140-6736(07)61091-5. [DOI] [PubMed] [Google Scholar]
- 110.Hanlon J.T., Schmader K.E., Samsa G.P., Weinberger M., Uttech K.M., Lewis I.K., et al. A method for assessing drug therapy appropriateness. J. Clin. Epidemiol. 1992;45:1045–1051. doi: 10.1093/ageing/afx005. [DOI] [PubMed] [Google Scholar]
- 111.Curtin D., Gallagher P.F., O'Mahony D. Explicit criteria as clinical tools to minimize inappropriate medication use and its consequences. Ther. Adv. Drug Saf. 2019;10 doi: 10.1177/2042098619829431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Beers M.H., Ouslander J.G., Rollingher I., Reuben D.B., Brooks J., Beck J.C. Explicit criteria for determining inappropriate medication use in nursing home residents. UCLA Division of Geriatric Medicine. Arch. Intern. Med. 1991;151:1825–1832. [PubMed] [Google Scholar]
- 113.By the 2019 American Geriatrics Society Beers Criteria® Update Expert Panel American Geriatrics Society 2019 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J. Am. Geriatr. Soc. 2019;67:674–694. doi: 10.1111/jgs.15767. e1-371.e9. [DOI] [PubMed] [Google Scholar]
- 114.O'Mahony D., O'Sullivan D., Byrne S., O'Connor M.N., Ryan C., Gallagher P. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing. 2015;44:213–218. doi: 10.1093/ageing/afu145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gallagher P., Ryan C., Byrne S., Kennedy J., O'Mahony D. STOPP (Screening Tool of Older Person's Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment). Consensus validation. Int. J. Clin. Pharmacol. Ther. 2008;46:72–83. doi: 10.5414/cpp46072. [DOI] [PubMed] [Google Scholar]
- 116.Lavan A.H., Gallagher P., Parsons C., O'Mahony D. STOPPFrail (Screening Tool of Older Persons Prescriptions in Frail adults with limited life expectancy): consensus validation. Age Ageing. 2017;46 doi: 10.1093/ageing/afx005. [DOI] [PubMed] [Google Scholar]
- 117.Seppala L.J., Petrovic M., Ryg J., et al. STOPPFall (Screening Tool of Older Persons Prescriptions in older adults with high fall risk): a Delphi study by the EuGMS Task and Finish Group on Fall-Risk-Increasing Drugs. Age Ageing. 2021;50:1189–1199. doi: 10.1093/ageing/afaa249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pazan F., Weiss C., Wehling M., FORTA The FORTA (Fit fOR The Aged) list 2021: fourth version of a validated clinical aid for improved pharmacotherapy in older adults. Drugs Aging. 2022;39:245–247. doi: 10.1007/s40266-022-00922-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Scott I.A., Hilmer S.N., Le Couteur D.G. Going beyond the guidelines in individualising the use of antihypertensive drugs in older patients. Drugs Aging. 2019;36:675–685. doi: 10.1007/s40266-019-00683-8. [DOI] [PubMed] [Google Scholar]