Abstract
Salivary gland damage and hypofunction result from various disorders, including autoimmune Sjögren's disease (SjD) and IgG4-related disease (IgG4-RD), as well as a side effect of radiotherapy for treating head and neck cancers. There are no therapeutic strategies to prevent the loss of salivary gland function in these disorders nor facilitate functional salivary gland regeneration. However, ongoing aquaporin-1 gene therapy trials to restore saliva flow show promise. To identify and develop novel therapeutic targets, we must better understand the cell-specific signaling processes involved in salivary gland regeneration. Transforming growth factor-β (TGF-β) signaling is essential to tissue fibrosis, a major endpoint in salivary gland degeneration, which develops in the salivary glands of patients with SjD, IgG4-RD, and radiation-induced damage. Though the deposition and remodeling of extracellular matrix proteins are essential to repair salivary gland damage, pathological fibrosis results in tissue hardening and chronic salivary gland dysfunction orchestrated by multiple cell types, including fibroblasts, myofibroblasts, endothelial cells, stromal cells, and lymphocytes, macrophages, and other immune cell populations. This review is focused on the role of TGF-β signaling in the development of salivary gland fibrosis and the potential for targeting TGF-β as a novel therapeutic approach to regenerate functional salivary glands. The studies presented highlight the divergent roles of TGF-β signaling in salivary gland development and dysfunction and illuminate specific cell populations in damaged or diseased salivary glands that mediate the effects of TGF-β. Overall, these studies strongly support the premise that blocking TGF-β signaling holds promise for the regeneration of functional salivary glands.
Keywords: Salivary glands, Hyposalivation, Salivary duct ligation model, Regeneration, Fibrosis, TGF-β signaling
Graphical abstract
1. Introduction
Hyposalivation, the objective and pathological loss of saliva production resulting from salivary gland damage, can arise due to autoimmune Sjögren's disease (SjD), IgG4-related disease (IgG4-RD), and collateral glandular damage caused by head and neck radiotherapy.1, 2, 3 Viral infections and other systemic autoimmune conditions, such as rheumatoid arthritis, can also result in salivary gland dysfunction.4,5 Additionally, medications such as antidepressants and anxiolytics, when used long term, can lead to chronic hyposalivation.6,7 Hyposalivation may lead to debilitating second-order effects such as problems with speech, decreased taste sensation, malnutrition, irritation of the oral mucosa, tooth enamel deterioration, and increased incidence of oral infections and gingivitis.8 Though promising approaches to promote salivary gland regeneration and restore saliva secretion continue to be investigated, including gene therapies,9,10 cell-based strategies,11, 12, 13, 14, 15, 16, 17 bioengineering approaches,18, 19, 20 and drug-based strategies that target regenerative signaling pathways,21,22 there remains no approved curative therapeutic strategy to restore salivary gland function in these disease processes.
Our understanding of tissue regeneration is heavily informed by our understanding of organogenesis. As will be discussed in this review, more than twenty years of research have demonstrated that transforming growth factor-β (TGF-β) signaling is required for overall development and is temporally critical to every stage of salivary gland development.23, 24, 25, 26 In addition to inflammation and acinar atrophy, fibrosis develops in all the salivary gland disorders discussed in this review. Though extracellular matrix remodeling is essential to wound healing and regeneration, fibrosis results in tissue hardening and loss of function that is often irreversible. TGF-β signaling is essential to fibrosis. Therefore, the TGF-β superfamily of ligands, their cognate receptors, and their complex regulatory network may offer numerous therapeutic targets to block glandular fibrosis and promote salivary gland regeneration. To better understand how to utilize this signaling network to promote regeneration, we must first understand the roles of TGF-β signaling in salivary gland injury and regenerative processes, as well as its roles in salivary gland development. In this review, we discuss evidence of a role for TGF-β in salivary gland fibrosis and regeneration, along with recent findings on the cellular specificity of the TGF-β-mediated responses involved and the potential that these studies will lead to a therapeutic approach to promote salivary gland regeneration in humans.
2. Salivary disorders
Many disorders impact the structure and function of the salivary glands. Three causes of hyposalivation that lead to tissue degeneration, and for which therapeutic regenerative therapies do not currently exist, include autoimmune Sjögren's disease and IgG4-related disease, and collateral salivary gland damage caused by radiotherapy for the treatment of head and neck cancers. This section introduces these salivary gland disorders and provides the context for discussing studies on how TGF-β regulates salivary gland regeneration.
2.1. Sjögren's disease (SjD)
Sjögren's disease (SjD) is a common autoimmune disease characterized by dry eye and dry mouth and lymphocytic infiltration of the lacrimal and salivary glands that leads to chronic inflammation. However, SjD is a systemic disease that affects other exocrine and non-exocrine tissues.27, 28, 29, 30 B cells play an essential role in the pathogenesis of SjD. Indicators of B cell hyperactivity in SjD include the production of autoantibodies (e.g., anti-Ro/SSA, anti-La/SSB), hypergammaglobulinemia, and the development of B cell lymphomas.31,32 SjD is highly sexually dimorphic, with an estimated ratio of affected women to men ranging from 9:1 to 20:1, and it has been demonstrated that SjD is X chromosome dose-dependent.33 Further, epigenetic modifications have been shown to alter transcription factor expression that enhances the expression of X chromosome-linked genes implicated in SjD.34 In addition to genetic factors,35 SjD is triggered by environmental factors, including various viral infections.36, 37, 38, 39, 40 Treatment options for oral and ocular pathologies in SjD primarily focus on symptom management, emphasizing the pressing need for alternative therapeutic approaches.
2.2. IgG4-related disease (IgG4-RD)
IgG4-RD is an autoimmune disorder characterized by mass formation in many tissues, including lacrimal and salivary glands, that can lead to permanent organ damage.41,42 The elevated presence of IgG4+ plasma cells and fibrosis in affected tissues are hallmarks of IgG4-RD.43 Not only does IgG4-RD's multi-organ nature confound diagnosis and treatment, but it is also an outlier among autoimmune disorders in that it manifests more often in males than females.42,44 IgG4-RD is believed to follow a biphasic progression, with the first stage characterized by inflammation followed by fibrosis. During the inflammatory phase, target tissues are infiltrated by antigen-experienced B and T lymphocytes that drive the secretion of pro-fibrotic cytokines such as IL-1β, IL-6, IFNγ, TGF-β, and others.45, 46, 47, 48
2.3. Radiation-induced hyposalivation
Globally, head and neck cancers account for almost 1 million new cancer cases annually, and radiotherapy (RT), along with surgical resection, are the current treatments of choice.49 RT for head and neck cancers is commonly delivered using a fractionated dosing strategy, where small daily doses (generally 2 Gy) to a total of 70 Gy are delivered, which damages surrounding tissues, including salivary glands.50 Hyposalivation is a common side effect of RT of the head and neck, with up to 60% loss in saliva output observed within days of treatment in nearly all patients.51 This RT-induced hyposalivation may last for weeks to months or persist indefinitely, leading to a significant decrease in the quality of life.52, 53, 54 Studies have demonstrated that the various major salivary glands, and even different regions of the same gland, are differentially radiosensitive.55,56 The causes of RT-induced hyposalivation are multifactorial and include the depletion of acinar cells and failure to regenerate secretory acini, chronic inflammation, and the development of fibrosis.57, 58, 59
3. TGF-β superfamily
The TGF-β superfamily is composed of numerous cytokines that can be divided into two major families. In mammals, the TGF-β/Nodal family includes TGF-β1, 2, and 3, Nodal, Activins (A, B, C, and E), six growth differentiation factors (GDFs; GDF1, 3, 8, 9, 10, and 11), Activin-inhibiting inhibins, and Lefty, which inhibits Nodal co-receptors. The bone morphogenetic protein (BMP) family includes BMPs, including inhibitory BMP3, along with three GDFs (GDF5, 6, and 7) and the anti-muellerian hormone (AMH) (Table 1).60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73 TGF-β subfamily members are pleiotropic cytokines secreted by fibroblasts and epithelial cells, whose function is tissue-specific and environment-dependent.74 TGF-β was originally discovered alongside transforming growth factor-α (TGF-α), which together were known as sarcoma growth factor (SGF).75 In mammals, there are three known isoforms of titular TGF-β (TGF-β1, TGF-β2, and TGF-β3) that have been shown to form hetero- and homodimers that bind to the same receptor complex.76,77 TGF-β1 has been the most extensively studied isoform, and although all three isoforms serve similar functions, TGF-β3 has shown opposing roles in certain tissue contexts.78 TGF-β isoforms are synthesized as pre-pro-proteins and rapidly dimerize after signal sequence cleavage. Post-translationally, sugar moieties are added to the amino-terminal pro-domain of TGF-βs that comprises latency-associated peptide (LAP). LAPs non-covalently bind to TGF-β via their carboxy-terminal growth factor domain and are required for proper folding and dimerization of TGF-β.79 Once dimerized, proteolytic cleavage at the LAP-binding domain produces a mature TGF-β dimer. Once formed, the LAPs remain non-covalently bound to the mature TGF-β dimer, forming the small latent complex (SLC). In the extracellular milieu, SLCs bind to various high molecular weight proteins called latent TGF-β binding proteins (LTBPs), forming large latent complexes (LLCs; i.e., TGF-β:LAP:LTBP complexes).80,81 LTBPs regulate the availability of TGF-β by sequestering TGF-β in LLCs, which retains TGF-β in the extracellular matrix.82 Although activation of mature TGF-β likely involves proteolytic cleavage of the LLCs mediated by thrombospondin, which subsequently releases TGF-β:LAPs from LTBPs, TGF-β activation also requires dissociation of the LAPs, which may occur via proteolysis by plasmin, matrix metalloproteases or bone morphogenic protein-1, or by physical disruption via integrin-mediated contraction.83,84
Table 1.
TGF-β superfamily ligands and receptors.
| Ligands | Type I receptor | Type II receptors | Co-receptors | R-SMADs |
|---|---|---|---|---|
| TGF-β1, TGF-β2, TGF-β3 | TGFBR1/TβRI/ALK5 | TGFBR2/TβRII | TGFBRIII/Betaglycan | SMAD 2/3 |
| Activin A, B, AB, AC | ACVR1B/ActR1B/ALK4, ACVR1C/ActR1C/ALK7 | ACVR2A/ActRII, ACVR2B/ActRIIb | SMAD 2/3 | |
| Activin C, E | ACVR1C/ActR1C/ALK7 | ACVR2A/ActRII, ACVR2B/ActRIIb | SMAD 2/3 | |
| Nodal | ACVR1B/ActR1B/ALK4, ACVR1C/ActR1C/ALK7 | ACVR2A/ActRII, ACVR2B/ActRIIb | Cripto/Tdgf1, Cryptic | SMAD 2/3 |
| GDF1 | ACVR1B/ActR1B/ALK4, ACVR1C/ActR1C/ALK7 | ACVR2A/ActRII, ACVR2B/ActRIIb | Cripto/Tdgf1, Cryptic | SMAD 2/3 |
| GDF3 | ACVR1B/ActR1B/ALK4, ACVR1C/ActR1C/ALK7 | ACVR2A/ActRII, ACVR2B/ActRIIb | Cripto/Tdgf1, Cryptic | SMAD 2/3 |
| GDF8/Myostatin | ACVR1B/ActR1B/ALK4, TGFBR1/TβRI/ALK5 | ACVR2A/ActRII, ACVR2B/ActRIIb | SMAD 2/3 | |
| GDF9 | TGFBR1/TβRI/ALK5 | BMPR2 | SMAD 2/3 | |
| GDF10 | ACVR1B/ActR1B/ALK4 | ACVR2B/ActRIIb | SMAD 2/3 | |
| GDF11 | ACVR1B/ActR1B/ALK4, TGFBR1/TβRI/ALK5, ACVR1C/ActR1C/ALK7 | ACVR2A/ActRII, ACVR2B/ActRIIb | SMAD 2/3 | |
| BMP2 | BMPR1A/ALK3, BMPR1B/ALK6 | BMPR2, ACVR2A/ActRII, ACVR2B/ActRIIb | repulsive guidance molecule (RGM) family | SMAD 1/5/8 |
| BMP4 | BMPR1A/ALK3, BMPR1B/ALK6 | BMPR2, ACVR2A/ActRII, ACVR2B/ActRIIb | RGMs | SMAD 1/5/8 |
| BMP5 | BMPR1A/ALK3, BMPR1B/ALK6, ACVR1A/ActR1A/ALK2 | BMPR2, ACVR2A/ActRII, ACVR2B/ActRIIb | RGMs | SMAD 1/5/8 |
| BMP6 | BMPR1A/ALK3, BMPR1B/ALK6, ACVR1A/ActR1A/ALK2 | BMPR2, ACVR2A/ActRII, ACVR2B/ActRIIb | RGMs | SMAD 1/5/8 |
| BMP7 | BMPR1A/ALK3, BMPR1B/ALK6, ACVR1A/ActR1A/ALK2 | BMPR2, ACVR2A/ActRII, ACVR2B/ActRIIb | SMAD 1/5/8 | |
| BMP8 | BMPR1A/ALK3, BMPR1B/ALK6, ACVR1A/ActR1A/ALK2 | BMPR2, ACVR2A/ActRII, ACVR2B/ActRIIb | SMAD 1/5/8 | |
| BMP8B | BMPR1A/ALK3, BMPR1B/ALK6, ACVR1A/ActR1A/ALK2 | BMPR2, ACVR2A/ActRII, ACVR2B/ActRIIb | SMAD 1/5/8 | |
| BMP9/GDF2 | ACVRL1/ALK1 | BMPR2, ACVR2A/ActRII, ACVR2B/ActRIIb | endoglin | SMAD 1/5/8 |
| BMP10 | ACVRL1/ALK1 | BMPR2, ACVR2A/ActRII, ACVR2B/ActRIIb | endoglin | SMAD 1/5/8 |
| BMP15 | BMPR1B/ALK6 | BMPR2 | SMAD 1/5/8 | |
| GDF5/BMP14 | BMPR1B/ALK6 | BMPR2, ACVR2A/ActRII, ACVR2B/ActRIIb | SMAD 1/5/8 | |
| GDF6/BMP13 | BMPR1B/ALK6 | BMPR2, ACVR2A/ActRII, ACVR2B/ActRIIb | SMAD 1/5/8 | |
| GDF7/BMP12 | BMPR1B/ALK6 | BMPR2, ACVR2A/ActRII, ACVR2B/ActRIIb | SMAD 1/5/8 | |
| AMH | AMHR2 | SMAD 1/5/8 | ||
| Inhibin A or B | ACVR2A/ActRII, ACVR2B/ActRIIb | TGFBRIII/Betaglycan | Inhibits Activins | |
| Lefty | Cripto/Tdgf1, Cryptic | Inhibits Nodal, SMAD2 and SMAD5 | ||
| BMP3 | BMPR1A/ALK3, ACVR1B/ActR1B/ALK4 | ACVR2B/ActRIIb | Inhibits BMPs |
TGF-β signaling is initiated by TGF-β ligands binding to cell surface TGF-β receptors (TGFβRs). There are seven type I (TGFβRI; ALK1-ALK7), five type II (TGFβRII; TGFβR2, BMPR2, ACVR2A, ACVR2B, and AMHR2), and one type III (TGFβRIII; β-glycan) receptors,85,86 each with varying affinities for TGF-β ligands (Table 1). Type I and type II TGFβRs contain a dual-specificity cytoplasmic kinase domain capable of both serine/threonine and tyrosine kinase activity.87 Downstream signaling is initiated upon binding of a mature TGF-β dimer to a heterotetrameric TGFβR complex formed between two type I and type II TGFβRs.88 Although TGFβRIII is the most widely expressed TGFβR, it does not possess kinase activity and serves as a coreceptor for type I and type II TGFβRs.89 Additionally, endoglin, a disulfide-linked dimeric glycoprotein, is a coreceptor for TGFβRI.90 As we will discuss in detail in this review, TGF-β superfamily members are essential for salivary gland development, injury, and regeneration. In the next section, we introduce canonical and non-canonical TGF-β signaling pathways before discussing the roles of TGF-β superfamily members in salivary gland fibrosis and regeneration in the subsequent sections.
4. TGF-β signaling
4.1. Canonical TGF-β signaling
The binding of TGF-βs to TGFβRs to activate downstream signaling pathways occurs in a stepwise manner, e.g., dimeric TGF-β binding induces a conformational change in TGFβRII that activates the receptor's kinase domain, which subsequently catalyzes the transphosphorylation of TGFβRI. This facilitates the binding of phospho-TGFβRI to the TGF-β:TGFβRII complex, thereby forming a tetrameric receptor:ligand complex capable of downstream signaling91, 92, 93 (Fig. 1). TGF-β signaling is also regulated at the receptor level by ubiquitylation and ubiquitin-mediated degradation of TGFβRs94 and sumoylation.95 In addition, TGF-β:TGFβR signaling is transduced by SMAD proteins (SMADs), transcription factors that are classified into three groups based on their function, i.e., receptor-regulated SMADs (R-SMADs; SMAD1, SMAD2, SMAD3, SMAD5, SMAD8), common SMAD (Co-SMAD; SMAD4), and inhibitory SMADs (I-SMADs; SMAD6 and SMAD7).96 Type I TGF-β receptors can be divided based on the R-SMAD they phosphorylate.85 In most cases, induction of the TGFβRI signaling pathway by TGF-β, or nodal and activin ligands, leads to phosphorylation of SMAD2 and SMAD3 (henceforth termed TGF-β/SMADs).85 In contrast, BMP ligand binding to TGFβRI induces the phosphorylation of SMAD1, SMAD5, and SMAD8 (henceforth termed BMP/SMADs).85 Both I-SMADs, SMAD6 and SMAD7, have been shown to inhibit phosphorylation of R-SMADs by forming complexes with TGFβRI97,98 as well as recruiting E3 ubiquitin ligases to induce degradation of TGFβRI.99, 100, 101, 102
Fig. 1.
Intracellular TGF-β Signaling Cascade Upon ligand (TGF-β or BMP) binding, an active tetrameric receptor:ligand complex forms (i.e., (P-TGFβRI)2:(TGFβRII)2; TGF-β/BMP) which binds SARA and/or R-SMAD proteins in ligand-specific or SMAD subtype-specific manners. R-SMAD proteins interact with the kinase domains of the active receptor:ligand complex, leading to SMAD activation via its phosphorylation and dissociation from the complexes. Once activated, R-SMADs localize to the nucleus by binding SMAD4, which binds to SMAD-binding elements to regulate the transcription of target genes. Also, SMAD6 and SMAD7 with Smurf2 act as accessory proteins to regulate the indicated steps in SMAD activation.
Figure created with Biorender.com.
R-SMADs and Co-SMAD consist of two globular domains called Mad-Homology-1 and -2 (MH1 and MH2) at their N-terminal and C-terminal, respectively.103 The MH1 domain contains a hairpin structure and DNA-binding capabilities, and the MH2 domain contains multiple hydrophobic patches and dictates interactions with adaptors and regulatory proteins. The only documented Co-SMAD in mammals is SMAD4.104 R-SMADs are activated upon phosphorylation by TGFβRI, leading to SMAD4 oligomerization with active R-SMADs. I-SMADs are activated by TGF-β family members and inhibit R-SMADs phosphorylation by competing for TGFβRI binding.105 Scaffold protein interactions have been shown to facilitate SMAD activation. SARA (SMAD anchor for receptor activation) has been demonstrated to bind to and retain un-phosphorylated (inactive) R-SMADs at the plasma membrane, which serves as a bridge between TGFβRI and R-SMADs, specifically SMAD2 and SMAD3.106 Other scaffold proteins, such as microtubules, filamin, and caveolin 1, have been shown to regulate R-SMAD signaling non-specifically.107,108 The Co-SMAD SMAD4, however, can be regulated in a specific manner by TGFβRI associated protein-1 (TRAP-1), which has been proposed to anchor SMAD4 to the plasma membrane proximal to the TGFβR heterotetramer, thereby facilitating its interaction with R-SMADs.109 Trimers of two R-SMADs and SMAD4 have been shown to be the functional units responsible for downstream transcriptional activation despite SMAD4 not being essential for nuclear localization.110,111 The I-SMADs, SMAD6 and SMAD7, serve inhibitory roles where SMAD6 predominantly inhibits BMP/SMADs signaling, and SMAD7 inhibits both BMP/SMADs and TGF-β/SMADs signaling. SMAD6 acts as a competitive antagonist of SMAD4, forming an inactive SMAD1/SMAD6 complex,112,113 whereas SMAD7 inhibits TGFβR-mediated phosphorylation as well as phosphorylation of SMAD2 and SMAD3.98,114,115 Interestingly, activation of TGFβRs by TGF-β1 has been shown to upregulate SMAD7 mRNA, hinting at a possible negative feedback loop.114
In their inactive state, monomeric SMADs are constitutively shuttled between the cytoplasm and the nucleus via nucleopores.116 However, in their active oligomeric form, SMAD complexes require nuclear import/export factors for transport.117 Once localized to the nucleus, active SMADs can negatively or positively regulate gene transcription. A conserved hairpin in the MH1 domains of SMADs 1, 3, 4, 5, and 8 binds with low affinity to palindromic repeats in SMAD-binding elements (SBEs) with a minimum sequence of 5′-AGAC-3’.48 Due to a difference in the positioning of the MH1 hairpin within SMAD1 compared to other SMADs, SMAD1 can also bind to GC-rich regions.118 With SBEs being found throughout the genome, as are GC-rich regions, one would expect to see many genes regulated by SMADs; however, SMAD-dependent transcriptional activity alone is insufficient for target gene expression due to the low binding affinity of SMAD complexes for SBEs. Additionally, since the MH1 DNA-binding hairpin is highly conserved among R-SMADs, there is a lack of selectivity among SMADs for SBE binding, with SMADs 1, 3, and 4 having similar affinities for SBEs.119,120 Thus, TGF-β-induced SMAD-mediated transcriptional regulation heavily depends on transcriptional coregulators that interact with nuclear-localized SMAD complexes. Indeed, lineage-specific coregulators have been shown to direct TGF-β- and BMP-induced transcriptional regulation in B cells, myeloid and erythroid precursors, and myoblasts.121,122
4.2. Non-canonical TGF-β signaling
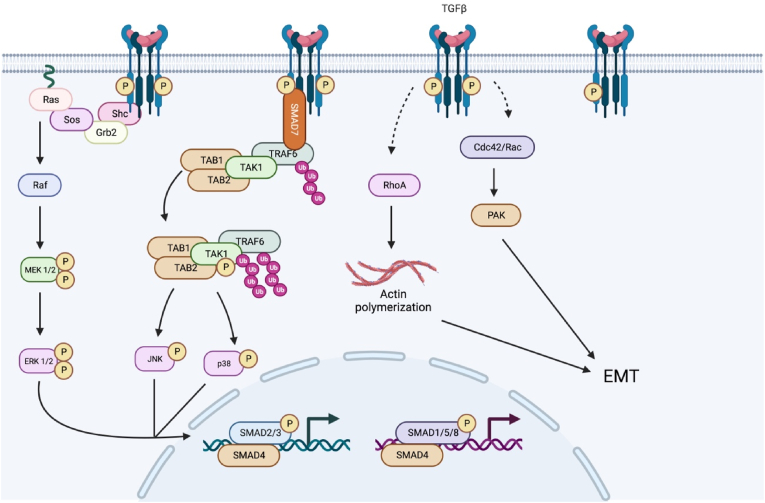
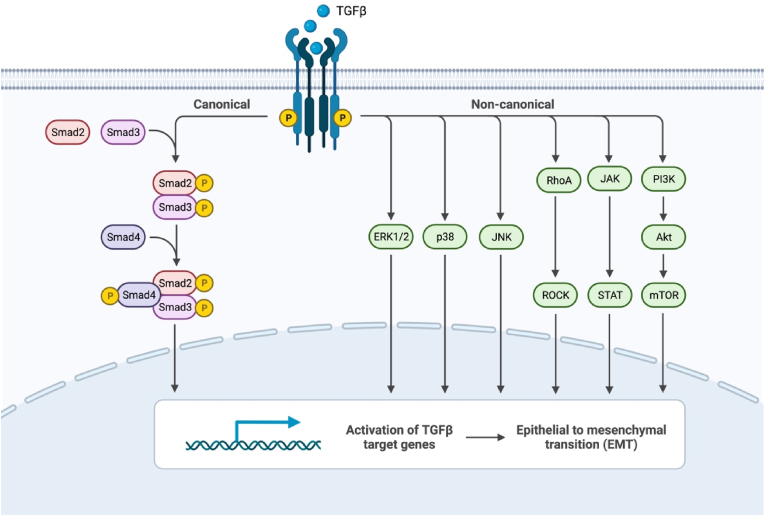
Despite the elegance of the SMAD signaling system, SMADs alone do not account for the plethora of biochemical processes that can be regulated by TGF-β signaling. Indeed, it is now recognized that TGF-β signaling can crosstalk with other signaling pathways and modulate diverse cell signaling events (Fig. 2). TGF-β signaling activates the mitogen-activated protein kinases (MAPKs) ERK1/2 in multiple cell types.123, 124, 125 TGFβRII can be phosphorylated on Y284 by Src, which serves as a docking site for Grb2 and ShcA.126 Additionally, activated TGFβRI can recruit and directly phosphorylate ShcA, thus promoting the formation of a ShcA/Grb2/Sos/Ras complex capable of activating Raf and serving as a scaffold for MAPK/ERK signaling.127 Through this pathway, rapid accumulation of phosphorylated p21/Ras occurs in rat intestines within minutes of treatment with TGF-β.128 Ras, a MAP4K, acts in this MAPK/ERK cascade to activate Raf, a MAP3K. Activated Raf localizes to the plasma membrane, where it activates MEK1/2, a MAP2K, and MEK1/2 subsequently activates the MAPK ERK1/2. In addition to this rapid response to TGF-β, latent ERK1/2 activation occurs on the order of hours in some cell lines and is SMAD4-dependent.129 This latent response to TGF-β promotes de novo synthesis of proteins in the MAPK/ERK phosphorylation cascade. Activation of ERK1/2 is a key regulator of the epithelial to mesenchyme transition (EMT).130, 131, 132 Although a normal part of embryonic development, EMT is associated with tumor metastasis and fibrosis.133 During EMT, cells lose their epithelial characteristics and acquire those of mesenchymal cells, including downregulation of adhesion proteins and increased matrix metalloprotease (MMP) levels. Additionally, ERK1/2 can phosphorylate SMADs 1, 2, and 3, indicating that TGF-β-MAPK crosstalk is bidirectional.134, 135, 136, 137
Fig. 2.
Non-canonical TGF-β Signaling. In addition to canonical SMAD-mediated signaling, TGF-β can activate diverse non-canonical signaling cascades in a cell type-specific manner. Figure created with Biorender.com.
In addition to the ERK1/2 MAPK cascade, TGF-β signaling can activate the JNK and p38 MAPK cascades. JNK and p38 are MAPKs downstream of MAP2K, MKK4, and MKK3/6, which can be regulated by the MAP3K TGF-β-activated kinase (TAK1).138 Studies utilizing dominant-negative SMAD4 demonstrated that SMAD4 is dispensable for TGF-β-mediated JNK activation, indicating that this crosstalk occurs independently of SMAD signaling.139,140 Embryos of TAK1-deficient mice exhibit a similar phenotype to TGFβRI or TGFβRIII loss-of-function mutants that show defects in vasculature formation.141 Studies on TRAF6, which plays a role in the interleukin (IL)-1R- and toll-like receptor (TLR)-mediated activation of TAK1, demonstrated that TRAF6 is indispensable for TAK1-mediated activation of the JNK and p38 MAPK cascades.142,143 Lysine-63-linked polyubiquitylation of TRAF6 was necessary to recruit and activate TAK1, which enables TAK1-mediated activation of JNK and p38.144 Further studies demonstrated that TRAF6 directly interacts with TGFβRII and that binding of TRAF6 to the activated TGFβR tetramer induces lysine-63-linked polyubiquitylation of TRAF6 and its association with TAK1.142,143 It was also shown that TGF-β-induced phosphorylation of p38 was dependent on SMAD7 expression, where SMAD7 is believed to act as an adapter protein, indicating that SMAD7's role in TGF-β signaling extends beyond its inhibition of R-SMAD phosphorylation.145
Alternative pathways for TGF-β signaling include the regulation of Rho-like GTPases and the PI3K/Akt and JAK/STAT pathways. Like the MAPK pathways, TGF-β-induced activation of RhoA is a critical regulator of EMT.146 Like TGF-β-induced activation of ERK1/2, some cells exhibit a latent activation of RhoA, indicating a biphasic response to TGF-β in part due to SMAD-mediated transcriptional activation.147 In addition to RhoA, TGF-β can activate PAK2 via Cdc42 GTPase activation.148 Physical interaction between Cdc42 and the TGFβR complex and associations with other members of the Cdc42-PAK2 signaling pathway have been demonstrated.149 Physical interactions between TGFβRI, TGFβRII, and the p85 regulatory subunit of PI3K, as well as TGF-β-induced activation of PI3K/Akt, indicate that TGF-β induces EMT via activation of the PI3K/Akt pathway.150, 151, 152
The JAK/STAT pathway is ubiquitous among vertebrates and is found in some lower-order organisms.153,154 The binding of TGF-β and subsequent activation of TGFβRs bring multiple JAKs in close proximity, enabling TGFβR-mediated transphosphorylation of key tyrosine residues in JAKs that promote their activation.155 Activated JAKs, in turn, phosphorylate key tyrosine residues in STATs, leading to their dimerization and nuclear localization. The JAK/STAT pathway provides transcription factors for genes containing consensus interferon-γ-activated sequence (GAS) recognition motifs, which regulate GAS-containing gene transcription. Additionally, crosstalk between the TGF-β and JAK/STAT pathways has been shown to negatively regulate canonical SMAD signaling via the upregulation of SMAD7.156
5. Salivary gland development
Our collective understanding of salivary gland development has provided a framework for investigating mechanisms that facilitate glandular regeneration. TGF-β1, TGF-β2, and TGF-β3 along with their cognate receptors, TGFβRI and TGFβRII, are all expressed during salivary gland development, suggesting their involvement in organogenesis.25 Indeed, TGF-β signaling is critical to salivary gland development,23 including ductal differentiation24 and branching morphogenesis.24, 25, 26 Expression of TGF-β signaling molecules is cell- and developmental stage-specific.25 For example, TGF-β1, TGF-β2, and TGF-β3 are expressed in the mesenchyme during the initial bud stage, but TGF-β3 is the sole isoform present in the mesenchyme during the pseudoglandular, canalicular, and terminal bud stages.25 Studies using global knockout of TGFβR signaling components have demonstrated that TGF-β is required for overall development, as embryonic or perinatal lethality was observed following deletion of TGF-β2,157 TGF-β3,158 TGFβRI,159 or TGFβRII.160 Interestingly, E16.5 and newborn mice lacking either TGF-β2 or TGF-β3 developed normal submandibular glands, suggesting that other TGFβR signaling components regulate TGF-β-dependent salivary gland development.25 To address this possibility, it was shown that global knockout of TGF-β1 in mice did not cause embryonic or perinatal lethality, and these mice developed normal salivary glands. However, the TGF-β1 knockout mice developed multiorgan focal lymphocytic infiltration, including in salivary glands, which resulted in death by four weeks of age.161,162 This lymphocytic accumulation in the salivary glands of TGF-β1 knockout mice mirrors the chronic inflammatory response observed in human SjD patients and mouse models of SjD.161,163 A later study using conditional Cre recombinase-mediated knockout of TGFβRI in mice (TGFβRI-coko mice) driven by mouse mammary tumor virus (MMTV) restricted TGFβRI knockout to the salivary and mammary glands. Results indicated that TGFβRI-coko mice were born normally with normal salivary gland development. Still, female TGFβRI-coko mice developed focal inflammation of the salivary and mammary glands and the heart, which proved lethal by 4–5 weeks of age.164 These results were similar to earlier findings in global TGF-β1 knockout mice.161,162 Female TGFβRI-coko mice also had increased levels of proinflammatory cytokines in the salivary glands, including IL-1, IL-2, IL-12, and IFNγ, indicative of a Th1-specific response.164 The female TGFβRI-coko mice also exhibited disruption of the wild-type aquaporin 5 distribution in submandibular glands, suggesting impairment of secretory function. However, male TGFβRI-coko mice did not develop inflammation and had a lifespan similar to wild-type mice. TGFβRI deletion using adenoviral vector-mediated Cre recombinase delivery directly into the submandibular gland (SMG) via the Wharton's excretory duct of adult TGFβRI-floxed mice (TGFβRIfl/fl mice) further demonstrated that female, but not male, TGFβRIfl/fl mice develop inflammatory foci in the SMG.164 These data support the conclusion that female mice are more susceptible to salivary gland inflammation and glandular dysfunction caused by impaired TGF-β signaling. Thus, TGF-β signaling during salivary gland development is sex- and tissue-dependent.
Though essential for organogenesis, elevated expression of TGF-β signaling components is also problematic for salivary gland development.165 The conditional overexpression of TGF-β1 in the mammary and salivary glands impeded normal salivary gland development in mice, with apparent disruption of proper branching, atrophy of granular convoluted ducts (GCDs) and acini, severe fibrosis, and salivary gland hypofunction.166 In vitro studies using a mixed salivary gland cell culture system to study epithelial-mesenchymal interactions have demonstrated that exogenous addition of TGF-βI results in acinar cell loss, inhibition of acinar differentiation, and increased expression of collagen, effects that could be abolished by inhibition of TGFβRI with SB525334.167 Therefore, proper TGF-β signaling regulation is essential for salivary gland development.
6. TGF-β in salivary gland disease
TGF-β signaling is involved in the pathogenesis of multiple diseases, including inflammatory diseases, cancer, and fibrosis.168, 169, 170, 171, 172, 173 TGF-β signaling is essential for the regulation of the fibrotic pathway, which has been studied in chronic kidney fibrosis, idiopathic pulmonary fibrosis, cystic fibrosis of the lung, and other tissues and fibrotic disorders.170, 171, 172, 173 TGF-β, secreted by senescent cells, can also mediate normal aging-related fibrosis.170 Further, TGF-β signaling is upregulated in salivary gland pathologies and disease models.174,175 For example, TGF-β is upregulated in the sera and salivary glands of patients with SjD, and SMAD-mediated mechanisms are active in SjD.175, 176, 177 TGF-β is also expressed in fibroblasts and lymphocytic foci in fibrotic lesions of labial salivary glands in SjD patients.178 Recent evidence shows that TGF-β induces EMT-dependent salivary gland fibrosis in primary SjD.176 TGF-β is also elevated in the parotid and submandibular glands of patients receiving radiotherapy for head and neck carcinomas179 compared with control glands. TGF-β staining in the collateral irradiated glands was concentrated in epithelial cells and myofibroblasts. As we discuss in the following sections, TGF-β also contributes to salivary gland fibrosis in a mouse salivary duct ligation-induced injury model.166,180
The regeneration of healthy salivary gland tissue following damage caused by inflammation or radiation exposure is often impeded by abundant extracellular matrix (ECM) deposition and fibrosis. TGF-β1 regulates ECM deposition by promoting the biosynthesis of collagen and fibronectin and the expression of protease inhibitors that reduce ECM degradation.166,181 TGF-β1 also enhances ECM deposition by inducing myofibroblast differentiation.182,183 Indeed, TGF-β signaling is essential to salivary gland fibrosis.166,180 The following two sections concern TGF-β signaling in salivary gland disease and tissue regeneration models.
7. Salivary gland injury and regeneration models
7.1. Salivary gland ductal obstruction and ligation models
Ligation followed by deligation of the main excretory ducts of salivary glands is a widely used model of salivary gland inflammation and subsequent regeneration, respectively, as deligated glands fully recover from the ligation-induced atrophic state. Experimental models of salivary gland ductal obstruction have been employed in mice, rats, cats, rabbits, and pigs to study the pathogenesis of sialopathies caused by obstruction, such as salivary gland inflammation (sialadenitis) and salivary gland stones (sialithiasis).174,184, 185, 186, 187, 188, 189 To obstruct ducts by ligation and investigate initiation of salivary gland dysfunction and degeneration, surgical sutures or clips are used, which enables easy deligation of the duct to monitor responses associated with recovery of normal salivary gland function. This approach has advantages over other models, such as radiation-induced salivary gland damage or sialoadenectomy, where full recovery is not obtainable. For the submandibular gland, ductal ligation-induced atrophy develops quickly with changes in epithelial acinar and ductal cell morphology and immune cell infiltration observable within 24 h. Upon ductal deligation, acinar and ductal epithelia recover within three days.190,191 Without ductal deligation, many saliva-secreting acinar cells succumb to apoptosis within seven days, and their debris becomes phagocytosed by intraepithelial macrophages.192 Another study demonstrates a progressive loss of approximately 85% of the salivary acini in rats, resulting in epithelial atrophy at two weeks post-ligation.193 Notably, ductal ligation has been employed to treat excessive salivation (sialorrhea).194, 195, 196
Using a ductal ligation model where the Wharton's SMG excretory duct was temporarily ligated, our laboratory was the first to report the upregulation of TGF-β signaling components following SMG ductal ligation in C57BL/6 mice that correlated with glandular fibrosis and acinar atrophy.174 Following a seven-day ligation, the ligature was removed, and gland regeneration was observed at 28 days following ductal deligation. In addition to functional restoration, histological changes, including loss of acini and fibrosis, were reversed following deligation. The elevated expression of both canonical and non-canonical TGF-β signaling components (TGF-β1, TGF-β3, TGFβRI, SMAD2/3, TAK1, TAB1, Snail, Slug) observed following ductal ligation was also reversed upon deligation. The fibrosis markers, collagen I and fibronectin, were highly expressed with ductal ligation and returned to near baseline levels following deligation. Significantly, in this study, in vivo treatment with the TGFβRI inhibitors, SB431542 and GW788388, blocked collagen I and fibronectin upregulation caused by ligation, supporting the notion that TGF-β signaling through TGFβRI is essential for fibrosis development observed in the ligation model. Woods et al. also noted that TGFβRI expression was restricted to acinar cell populations within ligated and control SMGs.174
Further studies reported similar findings in a rat model. Yasumitsu et al. utilized the rat SMG ligation model where the excretory duct was ligated for seven days, followed by removal of the ligature and collection of glands at 0, 1, 3, 7, and 14 days following deligation.197 Whereas in healthy glands, TGF-β1 expression was restricted to granular convoluted tubules and striated ducts and absent in acinar cell populations within SMGs, TGF-β1 staining was observed in connective tissue and ductal cell populations from 0 to 7 days following deligation, and returned to a non-ligated control staining pattern by 14 days.197
These studies demonstrate the involvement of TGF-β signaling in rodent salivary ductal ligation models of glandular fibrosis and the regeneration of normal salivary gland morphology and function upon ductal deligation. Further, these studies reveal cell-specific variation in the expression patterns and function of TGF-β signaling components in the ductal ligation model. Studies using single-cell omic technologies (e.g., transcriptomics, proteomics) to resolve the cell type-specific contributions to glandular fibrosis are needed. Some of the recent advances in this area are discussed in the next section.
7.2. Sialoadenectomy/resection models
Sialoadenectomy is a procedure that involves the partial or total surgical removal of salivary glands. Clinically, sialoadenectomy has been used to treat salivary gland inflammation, tumors, and cysts (sialoceles). However, it also serves as an approach to studying the effects of salivary gland injury on the submandibular, parotid, and sublingual glands. Sialoadenectomy of the submandibular gland (SMG), which produces 70% of human resting saliva, is a more commonly used murine model than sialoadenectomy of other salivary glands.198 This is likely because the SMG is easily accessible in mice, compared to the parotid and sublingual glands, and has a compact structure that makes the SMG easy to locate and remove. Sialoadenectomy of SMGs has been utilized in small rodent models such as mice,199 rats,200,201 and hamsters202and larger animal models such as dogs to study tissue regeneration after partial sialoadenectomy, infection, and tumor development. Additionally, sialoadenectomy has been used to investigate odontogenic responses beyond the salivary glands, such as the role of epidermal growth factor (EGF) in rodent saliva. Morris-Wiman et al. studied the role of EGF in the morphology and maintenance of taste buds using sialoadenectomized rats lacking both submandibular and sublingual glands.203 Their findings indicate that EGF secretion from these glands induces the formation and maintenance of fungiform taste buds and papillae. In contrast, EGF was dispensable for circumvallate papillae taste buds. Extending beyond the oral cavity, Helmrath et al. utilized sialoadenectomy of SMGs in mice to demonstrate that salivary gland-derived EGF was involved in bowel adaptation after small bowel resection.204
Only a few studies have utilized partial sialoadenectomy to investigate cell signaling responses to this injury. Instead, partial sialoadenectomy (alternatively referred to as partial resection or biopsy punch) is often used as a model for testing bioengineering approaches to salivary gland regeneration.205, 206, 207, 208 Using a partial salivary gland resection model, O'Keefe et al. demonstrated increased macrophage presence in the gland and concomitant elevated expression of both TGF-β1 and TGF-β3.198 A study utilizing a hamster sialoadenectomy model of the submandibular and sublingual glands determined that after sialoadenectomy, TGF-β1-expressing eosinophils infiltrated the wound during repair.209 Additional work is needed to better understand the cell signaling pathways involved in tissue regeneration following partial salivary gland resection.
7.3. Cancer and irradiation models
Despite a general decrease in tobacco smoking and the adoption of electronic cigarettes, data from the SEER program of the National Cancer Institute of the NIH shows an upward trend in the age-adjusted incidence of oral cancers between 2000 and 2019. The SEER program estimates over 54,000 new cases of oral cancer in 2023, representing 2.8% of all new cancer diagnoses. Notwithstanding the remarkable positive effects of immunotherapeutics such as pembrolizumab and nivolumab, surgical resection and radiotherapy remain first-line treatments for oral cancers.210 Radiotherapy for head and neck cancers causes collateral damage to adjacent odontogenic tissues, including salivary glands and gustatory tissue, leading to a significant decline in the patient's quality of life.211,212
Radiation-induced injury models have been used to study salivary gland regeneration, to identify the molecular and cellular processes governing acute and chronic damage caused by irradiation, and to identify therapeutics that may offer radioprotection and stimulate glandular regeneration following injury. Mouse models of radiation-induced injury utilize single (1–15 Gy) or fractionated (5 fractions of 1–6 Gy) dosing regimens targeted at the head and neck region, either using image-guided irradiation or by shielding the remainder of the body with lead shields.213, 214, 215, 216, 217 For a detailed review of the spatiotemporal effects of salivary gland irradiation in animal models, we refer the readers to the Jasmer et al. publication.214
It has been shown that following irradiation in humans, TGF-β1 expression is quickly elevated,218 and a study of patients receiving radiotherapy for the treatment of head and neck squamous cell carcinoma, demonstrated that post-irradiation plasma levels of TGF-β1 were predictive of late morbidities.219 Another study showed a 10-fold increase in TGF-β protein levels in parotid and submandibular gland samples from patients experiencing radiation-induced hyposalivation.179 These findings are consistent with results from other models of radiation-induced tissue damage, such as pulmonary pneumonitis due to thoracic radiotherapy. One study involving patients receiving pulmonary radiotherapy for the treatment of lung cancers revealed that though all patients had elevated plasma TGF-β levels following treatment, those that returned to baseline levels by the end of radiotherapy did not develop pulmonary pneumonitis. In contrast, those with sustained elevation of plasma TGF-β levels developed radiation-induced pneumonitis.220
8. Cell-specific TGF-β signaling in salivary gland regeneration
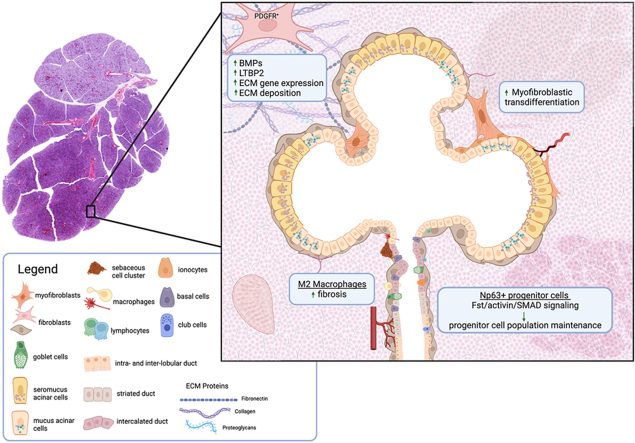
Extracellular matrix deposition and remodeling are essential to wound healing and regeneration, whereas fibrosis can result in tissue hardening and dysfunction.221 The development of fibrosis is mediated by several cell types, including tissue-resident fibroblasts, endothelial cells, myofibroblasts and their stromal precursors, and immune cells, including lymphocytes and macrophages.222, 223, 224 However, prior studies offer little insight into the contributions of specific cell types to salivary gland injury and regeneration in experimental models of glandular fibrosis, such as the ductal ligation model. This section will discuss different cell populations influencing salivary gland injury and regeneration through TGF-β signaling (Table 2).
Table 2.
Cell-specific Roles and Evidence of TGF-β signaling in salivary gland regeneration.
| Cell Type | Role(s) | Evidence | Reference |
|---|---|---|---|
| PDGFRα+/β+ stromal cells | Fibrosis: ECM deposition, collagen remodeling | Elevated expression (scRNA-seq data) of latent TGF-β binding protein-2 (Ltbp2) was found following a 14-day ductal ligation of SMGs and sublingual glands. | 226 |
| Stromal Cells | Not determined in study | Irradiated patient submandibular and parotid gland samples revealed upregulation of TGF-β specifically in stromal cells and myofibroblasts. | 179 |
| Macrophages (M2-like) | Fibrosis | Increased macrophage numbers and elevated expression of both TGF-β1 and TGF-β3 were found following a 14-day ductal ligation of SMGs and sublingual glands. | 239 |
| ΔNp63+ basal and myoepithelial progenitor cells | Proliferation and differentiation of salivary gland epithelial cells, primarily acini | By ablating ΔNp63+ progenitor cells in the salivary gland, it was determined that loss of ΔNp63 activates TGF-β signaling, and that Fst/activin/Smad signaling is responsible for the maintenance of the salivary gland stem/progenitor cell population. | 223 |
| Myofibroblasts | Transdifferentiation of fibroblasts into myofibroblasts | Irradiated patient submandibular and parotid gland samples revealed upregulation of TGF-β specifically in stromal cells and myofibroblasts. | 179 |
8.1. Stromal cells
Altreith et al. utilized ligation of Wharton's and Bartholin's ducts of the submandibular and sublingual glands, respectively, and observed glandular changes 7 and 14 days post-ligation.225,226 Consistent with previous reports, these studies showed that ductal ligation induced acinar cell atrophy, extracellular matrix accumulation, and collagen remodeling within seven days, which was significantly more advanced at 14 days following ductal ligation. Utilizing immunohistochemical analysis, Altreith et al. detected a three-fold expansion of PDGFRα+ stromal cells with ductal ligation. This response was previously reported to promote proacinar cell differentiation via a BMP-dependent mechanism and expression of multiple ECM genes in embryonic salivary gland organoids.227 These authors also used a lineage tracing mouse (Gli1-CreERT2;R26TdT) and demonstrated that the number of a subset of PDGFRα+/β+ stromal cells expressing glioma-associated oncogene 1 (Gli1) was significantly enhanced at 14-days post-ligation. Gli1 has been implicated in fibrosis development in multiple tissue types,228, 229, 230 and a population of Gli1+ cells has previously been identified in developing SMGs on embryonic day 16227. Following ductal ligation, the Gli1+ cells were shown to be located in the neurovascular regions of the SMG, where they contributed to ECM deposition and active collagen remodeling.225
With the advent of single-cell transcriptomics, we can now resolve cell type-specific gene expression changes caused by perturbations such as ductal ligation in an unbiased manner. Altreith et al. performed single-cell transcriptomic analyses of the submandibular and sublingual glands following a 14-day ligation of their main excretory ducts.225 This analysis revealed a limited number of Gli1+ cells but identified a subpopulation of fibrogenic PDGFRα+/β+ stromal cells that were expanded following ductal ligation. The differentially expressed genes in PDGFRα+/β+ stromal cells in the duct-ligated glands included several genes known to be essential for fibrosis development, including periostin (Postn), secreted phosphoprotein 1 (Spp1), and latent TGF-β binding protein-2 (Ltbp2).225 LTBPs interact with fibrillin microfibrils within the extracellular matrix,231 and, as discussed earlier, LAP, LTBP, and TGF-β combine to form a large latent complex (LLC). Other studies show that LTBP2 is secreted from lung myofibroblasts and is involved in idiopathic pulmonary fibrosis,232 while LTBP4 has been implicated in renal fibrosis.233 Thus, fibrogenic PDGFRα+/β+ stromal cells that are expanded by ductal ligation may be mediators of salivary gland fibrosis, partly by contributing to pro-fibrotic TGF-β signaling.
8.2. Macrophages
Altreith et al. also reported a 49- and 69-fold increase in F4/80+ macrophage staining in the SMG at 7- and 14-days post-ductal ligation, respectively. Macrophages, particularly those that are pro-fibrotic, known as M2-like macrophages, can influence the recruitment and proliferation of fibroblasts and their differentiation into myofibroblasts through the secretion of several factors, including TGF-β1.234, 235, 236 Macrophages can also indirectly contribute to TGF-β signaling by secretion of factors necessary to activate latent TGF-β1, such as metalloproteases.237 As mentioned earlier, using a partial salivary gland resection model that results in fibrosis, O'Keefe et al. reported increased macrophage presence in the gland and elevated expression of TGF-β1 and TGF-β3, along with several other markers associated with M2-like macrophages.198 Importantly, this study showed regional differences, with F4/80+ macrophage staining elevated globally across the gland at three days post-partial sialoadenectomy, but these macrophages remained elevated within fibrotic foci throughout the study duration of 56 days. Taken together, these studies indicate that macrophages may be an essential regulator of TGF-β-dependent salivary gland fibrosis.
8.3. Progenitor cells
In the submandibular gland, ΔNp63 is expressed in basal and myoepithelial cells that serve as progenitors capable of giving rise to all mature epithelial cell subtypes in the SMG.238 Using a ΔNp63 floxed (ΔNp63fl/fl) mouse crossed with a tamoxifen-inducible UBCCreERT2 mouse, Min et al. ablated ΔNp63 in adult mice by administering tamoxifen at 10–12 weeks of age.239 The SMGs of these global ΔNp63KO mice were smaller than wild-type mice, with reduced ductal structures and enlarged acini, decreased saliva production, and diminished K19 expression. Restricting the ablation of ΔNp63 to p63-expressing cells by crossing ΔNp63fl/fl mice with Trp63CreERT2 mice yielded SMG phenotypic changes that were similar between mice with global and restricted ablation of ΔNp63. Using bulk RNA-seq, multiple differentially expressed genes were identified between wild-type and ΔNp63KO mouse SMGs, including genes involved in TGF-β, Notch, MAPK, Hippo, and Wnt signaling.239 The elevated mRNA expression of multiple genes involved in TGF-β signaling in the ΔNp63KO mice suggests that loss of ΔNp63 can activate TGF-β signaling. Single-cell transcriptomic analysis was used to further evaluate progenitor cell-specific contributions in ΔNp63KO mice, which further demonstrated that ΔNp63 was essential for retaining the stem/progenitor cell populations in the SMG as basal/progenitor cells were absent in ΔNp63KO glands. Strikingly, ΔNp63KO glands had fewer acinar cell clusters, suggesting that overall gland maintenance, particularly of acini, was disrupted by the loss of ΔNp63-expressing progenitor cells. It was also shown that follistatin (Fst), which was highly expressed and co-expressed within the progenitor cell cluster with Trp63 in control SMGs, was highly downregulated in ΔNp63KO glands. Follistatin binds to and inhibits activin-mediated activation of SMAD2/3. In vitro studies also demonstrated that Fst was a key regulator of activin in salivary gland epithelial progenitor cells (SGEPCs) and that Fst/activin/SMAD signaling was responsible for the maintenance of salivary gland stem/progenitor cells.239 These studies revealed that TGF-β signaling, downstream of Fst and activin activation, governs the proliferation and differentiation of salivary gland epithelial cell populations, consistent with results using other models. For instance, Mou et al. found that in airway epithelium, TGF-β/BMP/SMAD signaling was suppressed in p63+ basal cells and that SMAD signaling promoted epithelial differentiation. In addition, inhibition of SMAD signaling resulted in an expansion of stem cell populations.240 A recent study in airway epithelium indicated that basal cells promote tissue regeneration via TGF-β following SO2-induced airway injury.241
Importantly, these studies reveal the requirement for ΔNp63 in SMG homeostasis in the absence of inflammation or salivary gland injury resulting from ductal ligation or irradiation. A recent publication from Song et al. utilized a unilateral ductal ligation model where the main excretory duct of the SMG was ligated for 14 days and deligated for 14 days, during which the SMG regenerated.242 These studies utilized Acta2CreERT2;ΔNp63fl/fl;Rosa26-TdTomato knockout (ΔNp63MECcKORFP) mice, which enabled visualization of myoepithelial cells in which ΔNp63 was ablated. Results determined that while ΔNp63 was essential for SMG homeostasis,239 its expression in SMG myoepithelial cells was not required for SMG regeneration following ductal ligation, as SMA+ myoepithelial cells compensated for the loss of ΔNp63 cells by contributing to the regeneration of both ductal and acinar cell populations.242 The contribution of TGF-β signaling in ΔNp63 salivary gland progenitor cells to salivary gland regeneration remains to be investigated. Further analysis of scRNA-seq data made publicly available by Altreith et al.225 may shed more light on the cell type-specific expression of TGF-β family genes following ductal ligation. However, the published data evaluated gene expression following ductal ligation, not during the regenerative process that follows deligation. As discussed in the discussion section, a subsequent preprint from Altreith et al.243 includes single-cell transcriptomic data from deligated samples, though the analysis is limited to endothelial cell populations.
8.4. Myofibroblasts
Generating bioactive TGF-β from latent stores requires localized coordination of proteases and integrins within fibrotic lesions.244 TGF-β targets various cell types, including fibroblasts, macrophages, epithelial cells, and vascular cells.244 TGF-β1 is critical for myofibroblast activation,245 though specific evidence for the involvement of myofibroblast activation in salivary gland fibrosis is not well documented. However, following radiotherapy, patient submandibular and parotid gland samples revealed that TGF-β upregulation is not uniformly distributed across specimens. TGF-β upregulation was not observed in parotid and submandibular gland acinar and ductal epithelial cells of radiotherapy patients, but rather, TGF-β expression was significantly elevated in stromal cells and myofibroblasts.179 In addition, the co-expression of α-SMA and TGF-β in myofibroblast populations was indicative of fibroblast transdifferentiation into myofibroblasts.179
9. Targeting TGF-β signaling to promote salivary gland regeneration
TGF-β superfamily members are pleiotropic, regulating diverse immune responses,246 development,23,247,248 wound healing and fibrosis,172,174 cellular maturation, and differentiation.249 Several studies have employed pharmacological inhibitors to target TGF-β signaling in vitro using standard and organoid cultures of salivary gland cells and in vivo using various glandular injury and disease models (Table 3). Targeting TGF-β signaling is complicated by the fact that both elevated and depleted TGF-β signaling are problematic for normal development, regeneration, and function of salivary glands.
Table 3.
Targeting TGF-β in models of salivary gland damage.
| Model/Cell Source | Inhibitor | Target(s) | Result | Reference | |
|---|---|---|---|---|---|
| In Vitro | |||||
| Embryonic murine salivary gland epidermis | RepSox | TGFβRI | RepSox increased the proliferation of p63+ epidermal progenitor cells. | 250 | |
| Heterogeneous adult mouse SMG preparations were grown on Matrigel | SB525334 | TGFβRI | SB525334 increased the expression of acinar cell markers (e.g., amy1a, aqp5, and zo1), and increased formation of acinar-like structures, irrespective of their proximity to mesenchymal cells. | 167 | |
| Homogenous SMG epithelial organoids | LDN19318 | BMP receptors, ALK2 and ALK3. | LDN19318 inhibited proacinar (AQP5) marker expression. | 227 | |
| 2D and 3D cultures of human salivary gland progenitor cells | A83-01 | TGFβRI | A83-01 reduced expression of the ductal marker keratin 7. | 252 | |
| Human salivary gland epithelial cultures from healthy subjects | SB431542 | TGFβRI | SB431542 reduced TGF-β1-induced expression of the fibrosis markers vimentin and collagen type I compared to cells treated with exogenous TGF-β1 alone. | 176 | |
| In Vivo | |||||
| SMG Wharton's duct ligation | SB431542 and GW788388 | TGFβRI | SB431542 and GW788388 blocked upregulation of the fibrosis markers, collagen I and fibronectin. | 174 | |
| SMG Wharton's duct ligation | SIS3 | SMAD3 | SIS3 reduced fibrosis, improved salivary function, reduced release of IL-1β and IL-6, reduced acinar atrophy, and preserved normal gland morphology. | 253 | |
Using a crude preparation of embryonic murine salivary gland epidermis, it was shown that the TGFβRI inhibitor, RepSox, increased the proliferation of p63+ epidermal progenitor cells without disrupting their ability to respond to Ca2+ addition.250 In another study, heterogeneous adult mouse SMG preparations grown in culture demonstrated cell-specific and temporal expression differences, as well as differential localization of TGF-β family members.167 Early passage epithelial cells, but not mesenchymal cells, showed high expression of TGF-β1 and TGFβRII localized to the cytoplasm, while TGFβRI was robustly expressed in both epithelial cytoplasm and nuclear area. Alternatively, both late-passage mesenchymal and epithelial cells highly expressed TGF-β1, TGFβRI, and TGFβRII. They also grew heterogeneous adult mouse SMG preparations on Matrigel with or without TGF-β1 and TGFβRI inhibitor, SB525334, to assess the role of TGF-β1 in salivary gland epithelial and mesenchymal differentiation.167 Acinar-like structures did not form near mesenchymal cells in the presence of TGF-β1 but did in Matrigel-only or cultures additionally treated with SB525334,167 revealing that TGFβRI inhibition promoted acinar-like structure formation. This study further showed differential effects of TGF-β1 signaling on epithelial and mesenchymal populations. TGF-β1 alone resulted in elevated collagen expression and inhibited the formation of acinar-like structures when epithelial cells were located near mesenchymal cells. In the presence of TGF-β1, the addition of SB525334 increased the expression of acinar cell markers (e.g., amy1a, aqp5, and zo1) and the formation of acinar-like structures, irrespective of proximity to mesenchymal cells. As addressed by Piraino et al., though both RepSox and SB525334 inhibit TGFβRI, comparing published results with these inhibitors is difficult, given differences in cell sources and preparations, culture conditions, and inhibitor affinities.251 Another in vitro study using the selective BMP receptor inhibitor, LDN19318, revealed that BMP signaling was necessary for proacinar marker (i.e., AQP5) expression in homogenous salivary gland epithelial organoid cultures.227 In both 2D and 3D cultures of human salivary gland progenitor cells, the TGFβRI inhibitor, A83-01, reduced expression of the ductal marker keratin 7 (K7).252 Studies with salivary gland progenitor cell cultures indicate that K7 expression is often a marker of cellular stress, which impedes hyaluronic acid (HA)-based hydrogel tissue engineering approaches to promote salivary gland regeneration.252 A study that treated human salivary gland epithelial cell cultures from healthy subjects with either TGF-β1 or TGF-β1 plus the TGFβRI inhibitor, SB-431542, demonstrated that TGFβRI inhibition causes a significant reduction in levels of the fibrosis markers vimentin, collagen type I, and E-cadherin, comparable to levels seen in untreated salivary gland epithelial cells from healthy subjects.176
The first in vivo evidence of a role for TGF-β signaling in salivary gland damage was provided by Woods et al., who utilized a 7-day SMG ductal ligation model (i.e., ligation of the Wharton's duct) to evaluate the effect of blocking TGF-β signaling with the TGFβRI inhibitors, SB431542 and GW788388.174 Results showed that collagen I and fibronectin mRNA levels were elevated at seven days following duct ligation and that the addition of the TGFβRI inhibitors decreased collagen I and fibronectin gene expression.174 A recent study demonstrated that the specific SMAD3 inhibitor, SIS3, reduced SMG fibrosis and dysfunction caused by a 7-day Wharton's duct ligation.253 This study further showed that SIS3 inhibited the release of the proinflammatory cytokines IL-1β and IL-6, reduced acinar atrophy, and preserved normal gland morphology.253 These in vivo studies clearly indicate that salivary gland damage due to ductal ligation is mediated by TGF-β signaling, though the role of TGF-β in gland regeneration post-deligation needs further investigation.
10. Further discussion
More than thirty years ago, TGF-β was identified as a key factor in the development of fibrosis, and subsequently, this role has been substantiated by many studies in multiple tissue and disease settings.171,172,180,254 In addition to the essential role of TGF-β signaling in salivary gland development,23 the role of TGF-β in promoting salivary gland damage, specifically fibrosis, has only been recently identified.174,253 As described in this review, convincing evidence for the cell-specific roles of TGF-β signaling in salivary gland regeneration following tissue damage is limited to investigations with a small number of cell types with scant data available on salivary glands. However, cell populations from other tissues where regeneration of cells damaged by fibrosis has been investigated provide insights into signaling pathways that may regulate salivary gland regeneration. In particular, we hypothesize that specific immune cell populations and endothelial cells play a role in the fibrosis of salivary epithelia and suggest that potential pathways discussed in this review may represent effective targets to regenerate salivary glands damaged by disease or other causes (e.g., cancer radiotherapy).
TGF-β signaling is known to regulate T cell differentiation, proliferation, and function.246,255,256 T cells have been implicated in the development of fibrosis, as has been supported by studies using various tissue fibrosis models and disease contexts.257 For example, studies of pulmonary fibrosis following thoracic irradiation have revealed a shift in CD4+ T lymphocytes from TH1 and TH17 in the early inflammatory (i.e., pneumonitic) phase to pro-fibrotic TH2 and TREG cells in the fibrotic phase.258 Whether this shift in CD4+ T cells is recapitulated in salivary gland fibrosis models remains to be determined. In addition to adaptive immune cell populations, innate lymphoid cells (ILCs) are key regulators of fibrosis.259, 260, 261 ILC classes (i.e., ILC1, ILC2, and ILC3) are either anti- or pro-fibrotic,259, 260, 261 and previous studies have identified a unique subset of ILCs present in mouse submandibular and sublingual but not parotid glands that are similar to anti-fibrotic, IFN-γ-producing ILC1s but don't produce significant amounts of IFN-γ.262,263 In a systemic sclerosis model, it was demonstrated that TGF-β, which is elevated in systemic sclerosis, indirectly promotes fibrosis through ILC2s by decreasing their expression of anti-fibrotic interleukin 10 (IL-10) and stimulating myofibroblast differentiation.264 As we work to fully understand the cellular landscape most appropriate for promoting salivary gland regeneration and define suitable pharmacological targets in the TGF-β signaling pathway for restoring salivary gland function, T lymphocytes and ILCs represent immune cell populations of significant interest.
The endothelial-specific role of TGF-β in fibrosis development has been reported in multiple tissues, including skin, lung, kidney, and liver.244 One compelling study in mice showed that TGF-β activation in endothelial cells resulted in skin, lung, and microvascular fibrosis development, associated with myofibroblast infiltration and endothelial-mesenchymal transition.265 In a chronic kidney disease mouse model, heterozygous knockout of TGFβRII in endothelial cells reduced fibrotic remodeling and endothelial-mesenchymal transition in kidney tissue.266 A recent preprint from Altreith et al. reported the results of single-cell transcriptomic analyses that compared submandibular and sublingual glands after 14 days of ductal ligation or after 14 days of ligation and five days of deligation, using homeostatic or mock glands as controls.243 Though there is much to consider from this robust dataset, the authors reported that after the 5-day deligation, the CellChat data analysis predicted that chemokine (C-X-C motif) and EPH signaling in endothelial cells promote salivary gland regeneration.243 These findings suggest that endothelial cells are relevant to the regenerative process and strongly support a role in tissue regeneration for TGF-β signaling in endothelial cells and perhaps other cell types that warrant further investigation.
As we interpret the findings described in this review, we should consider the limitations and context of the in vitro and in vivo models employed. For example, the ductal ligation model is most widely used to study salivary gland fibrosis and regeneration; however, optimum time courses for evaluating fibrosis development and tissue regeneration have not been uniformly employed. For example, data collected at 7 or 14 days after ligation are commonly used to assess fibrosis development, whereas data collected at 5, 7, or 14 days post-deligation have been used to evaluate the extent of tissue regeneration. Thus, we have included details on the time courses used in the studies reviewed. Still, we acknowledge that the optimal times to collect data likely vary widely based on tissue types and the experimental approaches used. When considering irradiation models of tissue injury that mimic collateral tissue damage caused by common radiotherapies in cancer patients, it is important to recognize that most experimental in vivo radiation exposure studies evaluate the effects of targeted irradiation of tissues, such as salivary glands, in the absence of a proximal tumor, which does not mimic the clinical setting for cancer patients. During tumor development, the immune and stromal microenvironment adjacent to salivary glands and other tissues is likely to be strikingly different than under healthy conditions, which could limit the significance of tissue irradiation studies done in the absence of tumors. Another consideration, especially as we investigate diseases that affect aging populations such as SjD, is the role TGF-β plays in normal physiologic aging and fibrotic development and distinguishing these roles from pathologic dysfunction and fibrosis. As mentioned earlier, TGF-β can mediate normal physiologic aging-related fibrosis,170 but the extent to which these contributions translate to salivary gland aging is unknown. One study utilizing mice of 12–18 months of age determined that inhibitory SMAD7 was protective of salivary gland function with age,267 as loss of SMAD7 resulted in more pronounced loss of function with age. These findings suggest that TGF-β signaling is important in salivary gland aging and diminished function, but more research is needed in this area.
Targeting TGF-β signaling components may be useful for promoting salivary gland regeneration following damage caused by myriad conditions. However, much more work is needed to elucidate the optimum therapeutic strategy for targeting this pathway in vivo and to further identify the cellular contributors to these responses and side effects that could limit the acceptance of a promising regenerative therapy.
Acknowledgement
This work was supported by National Institute of Dental and Craniofacial Research grants R01DE032032 (GAW and KJJ), R01DE029833 (GAW and KJJ), a University of Missouri Research Council Grant (KJJ), and the Wayne L. Ryan Fellowship from the Ryan Foundation (KMF).
References
- 1.Jensen S.B., Vissink A., Limesand K.H., et al. Salivary gland hypofunction and xerostomia in head and neck radiation patients. J Natl Cancer Inst Monogr. 2019;2019 doi: 10.1093/jncimonographs/lgz016. 2019/08/20. [DOI] [PubMed] [Google Scholar]
- 2.Turner M.D. Hyposalivation and xerostomia: etiology, complications, and medical management. Dent Clin. 2016;60:435–443. doi: 10.1016/j.cden.2015.11.003. 2016/04/05. [DOI] [PubMed] [Google Scholar]
- 3.Fragoulis G.E., Zampeli E., Moutsopoulos H.M. IgG4-related sialadenitis and Sjogren's syndrome. Oral Dis. 2017;23:152–156. doi: 10.1111/odi.12526. 2016/06/19. [DOI] [PubMed] [Google Scholar]
- 4.Zalewska A., Knas M., Waszkiewicz N., et al. Rheumatoid arthritis patients with xerostomia have reduced production of key salivary constituents. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;115:483–490. doi: 10.1016/j.oooo.2012.10.013. 2013/01/15. [DOI] [PubMed] [Google Scholar]
- 5.Proctor G.B., Shaalan A.M. Disease-induced changes in salivary gland function and the composition of saliva. J Dent Res. 2021;100:1201–1209. doi: 10.1177/00220345211004842. 2021/04/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murray Thomson W., Poulton R., Mark Broadbent J., et al. Xerostomia and medications among 32-year-olds. Acta Odontol Scand. 2006;64:249–254. doi: 10.1080/00016350600633243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolff A., Joshi R.K., Ekstrom J., et al. A guide to medications inducing salivary gland dysfunction, xerostomia, and subjective sialorrhea: a systematic review sponsored by the world workshop on oral medicine VI. Drugs R. 2017;17:1–28. doi: 10.1007/s40268-016-0153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rocchi C., Emmerson E. Mouth-watering results: clinical need, current approaches, and future directions for salivary gland regeneration. Trends Mol Med. 2020;26:649–669. doi: 10.1016/j.molmed.2020.03.009. 20200501. [DOI] [PubMed] [Google Scholar]
- 9.Baum B.J., Alevizos I., Zheng C., et al. Early responses to adenoviral-mediated transfer of the aquaporin-1 cDNA for radiation-induced salivary hypofunction. Proc Natl Acad Sci U S A. 2012;109:19403–19407. doi: 10.1073/pnas.1210662109. 2012/11/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alevizos I., Zheng C., Cotrim A.P., et al. Late responses to adenoviral-mediated transfer of the aquaporin-1 gene for radiation-induced salivary hypofunction. Gene Ther. 2017;24:176–186. doi: 10.1038/gt.2016.87. 2016/12/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lombaert I.M., Brunsting J.F., Wierenga P.K., et al. Rescue of salivary gland function after stem cell transplantation in irradiated glands. PLoS One. 2008;3 doi: 10.1371/journal.pone.0002063. 2008/05/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiao N., Lin Y., Cao H., et al. Neurotrophic factor GDNF promotes survival of salivary stem cells. J Clin Invest. 2014;124:3364–3377. doi: 10.1172/JCI74096. 2014/07/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pringle S., Maimets M., van der Zwaag M., et al. Human salivary gland stem cells functionally restore radiation damaged salivary glands. Stem Cell. 2016;34:640–652. doi: 10.1002/stem.2278. 2016/02/19. [DOI] [PubMed] [Google Scholar]
- 14.Lombaert I., Movahednia M.M., Adine C., et al. Concise review: salivary gland regeneration: therapeutic approaches from stem cells to tissue organoids. Stem Cell. 2017;35:97–105. doi: 10.1002/stem.2455. 2016/07/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hai B., Qin L., Yang Z., et al. Transient activation of hedgehog pathway rescued irradiation-induced hyposalivation by preserving salivary stem/progenitor cells and parasympathetic innervation. Clin Cancer Res. 2014;20:140–150. doi: 10.1158/1078-0432.CCR-13-1434. 2013/10/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peng X., Wu Y., Brouwer U., et al. Cellular senescence contributes to radiation-induced hyposalivation by affecting the stem/progenitor cell niche. Cell Death Dis. 2020;11:854. doi: 10.1038/s41419-020-03074-9. 2020/10/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Emmerson E., May A.J., Berthoin L., et al. Salivary glands regenerate after radiation injury through SOX2-mediated secretory cell replacement. EMBO Mol Med. 2018;10 doi: 10.15252/emmm.201708051. 2018/01/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hajiabbas M., D'Agostino C., Siminska-Stanny J., et al. Bioengineering in salivary gland regeneration. J Biomed Sci. 2022;29:35. doi: 10.1186/s12929-022-00819-w. 2022/06/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ozdemir T., Fowler E.W., Hao Y., et al. Biomaterials-based strategies for salivary gland tissue regeneration. Biomater Sci. 2016;4:592–604. doi: 10.1039/c5bm00358j. 2016/02/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nam K., Kim K., Dean S.M., et al. Using cell sheets to regenerate mouse submandibular glands. NPJ Regen Med. 2019;4:16. doi: 10.1038/s41536-019-0078-3. 2019/07/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morgan-Bathke M., Harris Z.I., Arnett D.G., et al. The Rapalogue, CCI-779, improves salivary gland function following radiation. PLoS One. 2014;9 doi: 10.1371/journal.pone.0113183. 2014/12/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hill G., Headon D., Harris Z.I., et al. Pharmacological activation of the EDA/EDAR signaling pathway restores salivary gland function following radiation-induced damage. PLoS One. 2014;9 doi: 10.1371/journal.pone.0112840. 2014/11/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McNairn A.J., Brusadelli M., Guasch G. Signaling moderation: TGF-beta in exocrine gland development, maintenance, and regulation. Eur J Dermatol. 2013 doi: 10.1684/ejd.2013.1979. 2013/04/17. [DOI] [PubMed] [Google Scholar]
- 24.Kahata K., Maturi V., Moustakas A. TGF-Beta family signaling in ductal differentiation and branching morphogenesis. Cold Spring Harbor Perspect Biol. 2018;10 doi: 10.1101/cshperspect.a031997. 2017/03/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jaskoll T., Melnick M. Submandibular gland morphogenesis: stage-specific expression of TGF-alpha/EGF, IGF, TGF-beta, TNF, and IL-6 signal transduction in normal embryonic mice and the phenotypic effects of TGF-beta2, TGF-beta3, and EGF-r null mutations. Anat Rec. 1999;256:252–268. doi: 10.1002/(SICI)1097-0185(19991101)256:3<252::AID-AR5>3.0.CO;2-6. 1999/10/16. [DOI] [PubMed] [Google Scholar]
- 26.Patel V.N., Rebustini I.T., Hoffman M.P. Salivary gland branching morphogenesis. Differentiation. 2006;74:349–364. doi: 10.1111/j.1432-0436.2006.00088.x. 2006/08/19. [DOI] [PubMed] [Google Scholar]
- 27.Malladi A.S., Sack K.E., Shiboski S.C., et al. Primary Sjogren's syndrome as a systemic disease: a study of participants enrolled in an international Sjogren's syndrome registry. Arthritis Care Res. 2012;64:911–918. doi: 10.1002/acr.21610. 2012/01/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mariette X., Criswell L.A. Primary sjogren's syndrome. N Engl J Med. 2018;378:931–939. doi: 10.1056/NEJMcp1702514. 2018/03/08. [DOI] [PubMed] [Google Scholar]
- 29.van Nimwegen J.F., van der Tuuk K., Liefers S.C., et al. Vaginal dryness in primary Sjogren's syndrome: a histopathological case-control study. Rheumatology. 2020;59:2806–2815. doi: 10.1093/rheumatology/keaa017. 2020/02/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Both T., Dalm V.A., van Hagen P.M., et al. Reviewing primary Sjogren's syndrome: beyond the dryness - from pathophysiology to diagnosis and treatment. Int J Med Sci. 2017;14:191–200. doi: 10.7150/ijms.17718. 2017/04/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nocturne G., Mariette X. Sjogren Syndrome-associated lymphomas: an update on pathogenesis and management. Br J Haematol. 2015;168:317–327. doi: 10.1111/bjh.13192. 2014/10/16. [DOI] [PubMed] [Google Scholar]
- 32.Kroese F.G., Abdulahad W.H., Haacke E., et al. B-cell hyperactivity in primary Sjogren's syndrome. Expet Rev Clin Immunol. 2014;10:483–499. doi: 10.1586/1744666X.2014.891439. 2014/02/26. [DOI] [PubMed] [Google Scholar]
- 33.Thorlacius G.E., Bjork A., Wahren-Herlenius M. Genetics and epigenetics of primary Sjogren syndrome: implications for future therapies. Nat Rev Rheumatol. 2023;19:288–306. doi: 10.1038/s41584-023-00932-6. 2023/03/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mougeot J.L., Noll B.D., Bahrani Mougeot F.K. Sjogren's syndrome X-chromosome dose effect: an epigenetic perspective. Oral Dis. 2019;25:372–384. doi: 10.1111/odi.12825. 2018/01/10. [DOI] [PubMed] [Google Scholar]
- 35.Khatri B., Tessneer K.L., Rasmussen A., et al. Genome-wide association study identifies Sjogren's risk loci with functional implications in immune and glandular cells. Nat Commun. 2022;13:4287. doi: 10.1038/s41467-022-30773-y. 2022/07/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.James J.A., Harley J.B., Scofield R.H. Role of viruses in systemic lupus erythematosus and Sjogren syndrome. Curr Opin Rheumatol. 2001;13:370–376. doi: 10.1097/00002281-200109000-00005. 2001/10/18. [DOI] [PubMed] [Google Scholar]
- 37.Igoe A., Scofield R.H. Autoimmunity and infection in Sjogren's syndrome. Curr Opin Rheumatol. 2013;25:480–487. doi: 10.1097/BOR.0b013e32836200d2. 2013/05/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weller M.L., Gardener M.R., Bogus Z.C., et al. Hepatitis delta virus detected in salivary glands of sjogren's syndrome patients and recapitulates a sjogren's syndrome-like phenotype in vivo. Pathog Immun. 2016;1:12–40. doi: 10.20411/pai.v1i1.72. 2016/06/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tanaka T., Warner B.M., Odani T., et al. LAMP3 induces apoptosis and autoantigen release in Sjogren's syndrome patients. Sci Rep. 2020;10:15169. doi: 10.1038/s41598-020-71669-5. 2020/09/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tanaka T., Warner B.M., Michael D.G., et al. LAMP3 inhibits autophagy and contributes to cell death by lysosomal membrane permeabilization. Autophagy. 2022;18:1629–1647. doi: 10.1080/15548627.2021.1995150. 2021/11/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shimizu Y., Yamamoto M., Naishiro Y., et al. Necessity of early intervention for IgG4-related disease--delayed treatment induces fibrosis progression. Rheumatology. 2013;52:679–683. doi: 10.1093/rheumatology/kes358. 20121219. [DOI] [PubMed] [Google Scholar]
- 42.Della-Torre E., Lanzillotta M., Doglioni C. Immunology of IgG4-related disease. Clin Exp Immunol. 2015;181:191–206. doi: 10.1111/cei.12641. 20150608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deshpande V., Zen Y., Chan J.K., et al. Consensus statement on the pathology of IgG4-related disease. Mod Pathol. 2012;25:1181–1192. doi: 10.1038/modpathol.2012.72. 20120518. [DOI] [PubMed] [Google Scholar]
- 44.Bledsoe J.R., Della-Torre E., Rovati L., et al. IgG4-related disease: review of the histopathologic features, differential diagnosis, and therapeutic approach. APMIS. 2018;126:459–476. doi: 10.1111/apm.12845. [DOI] [PubMed] [Google Scholar]
- 45.Mattoo H., Mahajan V.S., Maehara T., et al. Clonal expansion of CD4(+) cytotoxic T lymphocytes in patients with IgG4-related disease. J Allergy Clin Immunol. 2016;138:825–838. doi: 10.1016/j.jaci.2015.12.1330. 20160311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Della-Torre E., Feeney E., Deshpande V., et al. B-cell depletion attenuates serological biomarkers of fibrosis and myofibroblast activation in IgG4-related disease. Ann Rheum Dis. 2015;74:2236–2243. doi: 10.1136/annrheumdis-2014-205799. 20140820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Della-Torre E., Rigamonti E., Perugino C., et al. B lymphocytes directly contribute to tissue fibrosis in patients with IgG(4)-related disease. J Allergy Clin Immunol. 2020;145:968–981 e914. doi: 10.1016/j.jaci.2019.07.004. 20190715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lanzillotta M., Mancuso G., Della-Torre E. Advances in the diagnosis and management of IgG4 related disease. BMJ. 2020;369:m1067. doi: 10.1136/bmj.m1067. 20200616. [DOI] [PubMed] [Google Scholar]
- 49.Chow L.Q.M. Head and neck cancer. N Engl J Med. 2020;382:60–72. doi: 10.1056/NEJMra1715715. [DOI] [PubMed] [Google Scholar]
- 50.Cramer J.D., Burtness B., Le Q.T., et al. The changing therapeutic landscape of head and neck cancer. Nat Rev Clin Oncol. 2019;16:669–683. doi: 10.1038/s41571-019-0227-z. 2019/06/14. [DOI] [PubMed] [Google Scholar]
- 51.Dirix P., Nuyts S., Van den Bogaert W. Radiation-induced xerostomia in patients with head and neck cancer: a literature review. Cancer. 2006;107:2525–2534. doi: 10.1002/cncr.22302. [DOI] [PubMed] [Google Scholar]
- 52.Maria O.M., Eliopoulos N., Muanza T. Radiation-induced oral mucositis. Front Oncol. 2017;7:89. doi: 10.3389/fonc.2017.00089. 20170522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dirix P., Nuyts S., Vander Poorten V., et al. The influence of xerostomia after radiotherapy on quality of life: results of a questionnaire in head and neck cancer. Support Care Cancer. 2008;16:171–179. doi: 10.1007/s00520-007-0300-5. 20070706. [DOI] [PubMed] [Google Scholar]
- 54.Gupta N., Pal M., Rawat S., et al. Radiation-induced dental caries, prevention and treatment - a systematic review. Natl J Maxillofac Surg. 2015;6:160–166. doi: 10.4103/0975-5950.183870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Clark H., Hovan A., Moiseenko V., et al. Regional radiation dose susceptibility within the parotid gland: effects on salivary loss and recovery. Med Phys. 2015;42:2064–2071. doi: 10.1118/1.4915077. [DOI] [PubMed] [Google Scholar]
- 56.Konings A.W., Cotteleer F., Faber H., et al. Volume effects and region-dependent radiosensitivity of the parotid gland. Int J Radiat Oncol Biol Phys. 2005;62:1090–1095. doi: 10.1016/j.ijrobp.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 57.Avila J.L., Grundmann O., Burd R., et al. Radiation-induced salivary gland dysfunction results from p53-dependent apoptosis. Int J Radiat Oncol Biol Phys. 2009;73:523–529. doi: 10.1016/j.ijrobp.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Radfar L., Sirois D.A. Structural and functional injury in minipig salivary glands following fractionated exposure to 70 Gy of ionizing radiation: an animal model for human radiation-induced salivary gland injury. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;96:267–274. doi: 10.1016/s1079-2104(03)00369-x. [DOI] [PubMed] [Google Scholar]
- 59.Yu Z., Xu C., Song B., et al. Tissue fibrosis induced by radiotherapy: current understanding of the molecular mechanisms, diagnosis and therapeutic advances. J Transl Med. 2023;21:708. doi: 10.1186/s12967-023-04554-0. 20231009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Namwanje M., Brown C.W. Activins and inhibins: roles in development, physiology, and disease. Cold Spring Harbor Perspect Biol. 2016;8 doi: 10.1101/cshperspect.a021881. 2016/06/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Goebel E.J., Ongaro L., Kappes E.C., et al. The orphan ligand, activin C, signals through activin receptor-like kinase 7. Elife. 2022:11. doi: 10.7554/eLife.78197. 2022/1106/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vestal K.A., Kattamuri C., Koyiloth M., et al. Activin E is a TGFbeta ligand that signals specifically through activin receptor-like kinase 7. bioRxiv. 2023 doi: 10.1101/2023.09.25.559288. 2023/10/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Morvan F., Rondeau J.M., Zou C., et al. Blockade of activin type II receptors with a dual anti-ActRIIA/IIB antibody is critical to promote maximal skeletal muscle hypertrophy. Proc Natl Acad Sci U S A. 2017;114:12448–12453. doi: 10.1073/pnas.1707925114. 2017/11/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Aykul S., Parenti A., Chu K.Y., et al. Biochemical and cellular analysis reveals ligand binding specificities, a molecular basis for ligand recognition, and membrane association-dependent activities of cripto-1 and cryptic. J Biol Chem. 2017;292:4138–4151. doi: 10.1074/jbc.M116.747501. 2017/01/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Andersson O., Reissmann E., Ibanez C.F. Growth differentiation factor 11 signals through the transforming growth factor-beta receptor ALK5 to regionalize the anterior-posterior axis. EMBO Rep. 2006;7:831–837. doi: 10.1038/sj.embor.7400752. 2006/07/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Katagiri T., Watabe T. Bone morphogenetic proteins. Cold Spring Harbor Perspect Biol. 2016;8 doi: 10.1101/cshperspect.a021899. 2016/06/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Peng J., Li Q., Wigglesworth K., et al. Growth differentiation factor 9:bone morphogenetic protein 15 heterodimers are potent regulators of ovarian functions. Proc Natl Acad Sci U S A. 2013;110:E776–E785. doi: 10.1073/pnas.1218020110. 2013/02/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cadenas J., Pors S.E., Kumar A., et al. Concentrations of oocyte secreted GDF9 and BMP15 decrease with MII transition during human IVM. Reprod Biol Endocrinol. 2022;20:126. doi: 10.1186/s12958-022-01000-6. 2022/08/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Malinauskas T., Peer T.V., Bishop B., et al. Repulsive guidance molecules lock growth differentiation factor 5 in an inhibitory complex. Proc Natl Acad Sci U S A. 2020;117:15620–15631. doi: 10.1073/pnas.2000561117. 2020/06/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tian C., Liu J. Repulsive guidance molecules (RGMs) and neogenin in bone morphogenetic protein (BMP) signaling. Mol Reprod Dev. 2013;80:700–717. doi: 10.1002/mrd.22199. 2013/06/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Salmon R.M., Guo J., Wood J.H., et al. Molecular basis of ALK1-mediated signalling by BMP9/BMP10 and their prodomain-bound forms. Nat Commun. 2020;11:1621. doi: 10.1038/s41467-020-15425-3. 2020/04/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Massague J., Sheppard D. TGF-beta signaling in health and disease. Cell. 2023;186:4007–4037. doi: 10.1016/j.cell.2023.07.036. 2023/09/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zabala M., Lobo N.A., Antony J., et al. LEFTY1 is a dual-SMAD inhibitor that promotes mammary progenitor growth and tumorigenesis. Cell Stem Cell. 2020;27:284–299 e288. doi: 10.1016/j.stem.2020.06.017. 2020/07/22. [DOI] [PubMed] [Google Scholar]
- 74.Chaudhury A., Howe P.H. The tale of transforming growth factor-beta (TGFbeta) signaling: a soigne enigma. IUBMB Life. 2009;61:929–939. doi: 10.1002/iub.239. 2009/09/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.de Larco J.E., Todaro G.J. Growth factors from murine sarcoma virus-transformed cells. Proc Natl Acad Sci U S A. 1978;75:4001–4005. doi: 10.1073/pnas.75.8.4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cheifetz S., Weatherbee J.A., Tsang M.L., et al. The transforming growth factor-beta system, a complex pattern of cross-reactive ligands and receptors. Cell. 1987;48:409–415. doi: 10.1016/0092-8674(87)90192-9. [DOI] [PubMed] [Google Scholar]
- 77.Kumar R., Grinberg A.V., Li H., et al. Functionally diverse heteromeric traps for ligands of the transforming growth factor-beta superfamily. Sci Rep. 2021;11:18341. doi: 10.1038/s41598-021-97203-9. 2021/09/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Walton K.L., Johnson K.E., Harrison C.A. Targeting TGF-beta mediated SMAD signaling for the prevention of fibrosis. Front Pharmacol. 2017;8:461. doi: 10.3389/fphar.2017.00461. 2017/08/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gray A.M., Mason A.J. Requirement for activin A and transforming growth factor--beta 1 pro-regions in homodimer assembly. Science. 1990;247:1328–1330. doi: 10.1126/science.2315700. [DOI] [PubMed] [Google Scholar]
- 80.Miyazono K., Olofsson A., Colosetti P., et al. A role of the latent TGF-beta 1-binding protein in the assembly and secretion of TGF-beta 1. EMBO J. 1991;10:1091–1101. doi: 10.1002/j.1460-2075.1991.tb08049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sinha S., Nevett C., Shuttleworth C.A., et al. Cellular and extracellular biology of the latent transforming growth factor-beta binding proteins. Matrix Biol. 1998;17:529–545. doi: 10.1016/s0945-053x(98)90106-8. [DOI] [PubMed] [Google Scholar]
- 82.Taipale J., Miyazono K., Heldin C.H., et al. Latent transforming growth factor-beta 1 associates to fibroblast extracellular matrix via latent TGF-beta binding protein. J Cell Biol. 1994;124:171–181. doi: 10.1083/jcb.124.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Annes J.P., Chen Y., Munger J.S., et al. Integrin alphaVbeta6-mediated activation of latent TGF-beta requires the latent TGF-beta binding protein-1. J Cell Biol. 2004;165:723–734. doi: 10.1083/jcb.200312172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brown N.F., Marshall J.F. Integrin-mediated TGFbeta activation modulates the tumour microenvironment. Cancers. 2019:11. doi: 10.3390/cancers11091221. 20190821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hata A., Chen Y.G. TGF-Beta signaling from receptors to smads. Cold Spring Harbor Perspect Biol. 2016;8 doi: 10.1101/cshperspect.a022061. 2016/07/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Heldin C.H., Moustakas A. Signaling receptors for TGF-beta family members. Cold Spring Harbor Perspect Biol. 2016;8 doi: 10.1101/cshperspect.a022053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lawler S., Feng X.H., Chen R.H., et al. The type II transforming growth factor-beta receptor autophosphorylates not only on serine and threonine but also on tyrosine residues. J Biol Chem. 1997;272:14850–14859. doi: 10.1074/jbc.272.23.14850. [DOI] [PubMed] [Google Scholar]
- 88.Cheifetz S., Bassols A., Stanley K., et al. Heterodimeric transforming growth factor beta. Biological properties and interaction with three types of cell surface receptors. J Biol Chem. 1988;263:10783–10789. [PubMed] [Google Scholar]
- 89.Bilandzic M., Stenvers K.L. Betaglycan: a multifunctional accessory. Mol Cell Endocrinol. 2011;339:180–189. doi: 10.1016/j.mce.2011.04.014. 20110428. [DOI] [PubMed] [Google Scholar]
- 90.Nickel J., Ten Dijke P., Mueller T.D. TGF-beta family co-receptor function and signaling. Acta Biochim Biophys Sin. 2018;50:12–36. doi: 10.1093/abbs/gmx126. [DOI] [PubMed] [Google Scholar]
- 91.Shi Y., Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 92.Wrana J.L., Attisano L., Wieser R., et al. Mechanism of activation of the TGF-beta receptor. Nature. 1994;370:341–347. doi: 10.1038/370341a0. [DOI] [PubMed] [Google Scholar]
- 93.Tie Y., Tang F., Peng D., et al. TGF-beta signal transduction: biology, function and therapy for diseases. Mol Biomed. 2022;3:45. doi: 10.1186/s43556-022-00109-9. 20221219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kang J.S., Saunier E.F., Akhurst R.J., et al. The type I TGF-beta receptor is covalently modified and regulated by sumoylation. Nat Cell Biol. 2008;10:654–664. doi: 10.1038/ncb1728. 20080511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kerscher O., Felberbaum R., Hochstrasser M. Modification of proteins by ubiquitin and ubiquitin-like proteins. Annu Rev Cell Dev Biol. 2006;22:159–180. doi: 10.1146/annurev.cellbio.22.010605.093503. [DOI] [PubMed] [Google Scholar]
- 96.Song B., Estrada K.D., Lyons K.M. Smad signaling in skeletal development and regeneration. Cytokine Growth Factor Rev. 2009;20:379–388. doi: 10.1016/j.cytogfr.2009.10.010. 2009/11/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Imamura T., Takase M., Nishihara A., et al. Smad6 inhibits signalling by the TGF-beta superfamily. Nature. 1997;389:622–626. doi: 10.1038/39355. [DOI] [PubMed] [Google Scholar]
- 98.Hayashi H., Abdollah S., Qiu Y., et al. The MAD-related protein Smad7 associates with the TGFbeta receptor and functions as an antagonist of TGFbeta signaling. Cell. 1997;89:1165–1173. doi: 10.1016/s0092-8674(00)80303-7. [DOI] [PubMed] [Google Scholar]
- 99.Murakami G., Watabe T., Takaoka K., et al. Cooperative inhibition of bone morphogenetic protein signaling by Smurf1 and inhibitory Smads. Mol Biol Cell. 2003;14:2809–2817. doi: 10.1091/mbc.e02-07-0441. 20030404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Inoue Y., Imamura T. Regulation of TGF-beta family signaling by E3 ubiquitin ligases. Cancer Sci. 2008;99:2107–2112. doi: 10.1111/j.1349-7006.2008.00925.x. 20080918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hanyu A., Ishidou Y., Ebisawa T., et al. The N domain of Smad7 is essential for specific inhibition of transforming growth factor-beta signaling. J Cell Biol. 2001;155:1017–1027. doi: 10.1083/jcb.200106023. 20011210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Estrada K.D., Retting K.N., Chin A.M., et al. Smad6 is essential to limit BMP signaling during cartilage development. J Bone Miner Res. 2011;26:2498–2510. doi: 10.1002/jbmr.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nakao A., Imamura T., Souchelnytskyi S., et al. TGF-Beta receptor-mediated signalling through Smad2, Smad3 and Smad4. EMBO J. 1997;16:5353–5362. doi: 10.1093/emboj/16.17.5353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liu L., Li Q., Yang L., et al. SMAD4 feedback activates the canonical TGF-beta family signaling pathways. Int J Mol Sci. 2021:22. doi: 10.3390/ijms221810024. 2021/09/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Moustakas A., Souchelnytskyi S., Heldin C.H. Smad regulation in TGF-beta signal transduction. J Cell Sci. 2001;114:4359–4369. doi: 10.1242/jcs.114.24.4359. [DOI] [PubMed] [Google Scholar]
- 106.Tsukazaki T., Chiang T.A., Davison A.F., et al. SARA, a FYVE domain protein that recruits Smad2 to the TGFbeta receptor. Cell. 1998;95:779–791. doi: 10.1016/s0092-8674(00)81701-8. [DOI] [PubMed] [Google Scholar]
- 107.Sasaki A., Masuda Y., Ohta Y., et al. Filamin associates with Smads and regulates transforming growth factor-beta signaling. J Biol Chem. 2001;276:17871–17877. doi: 10.1074/jbc.M008422200. 20010226. [DOI] [PubMed] [Google Scholar]
- 108.Razani B., Zhang X.L., Bitzer M., et al. Caveolin-1 regulates transforming growth factor (TGF)-beta/SMAD signaling through an interaction with the TGF-beta type I receptor. J Biol Chem. 2001;276:6727–6738. doi: 10.1074/jbc.M008340200. 20001201. [DOI] [PubMed] [Google Scholar]
- 109.Ju Wurthner, Frank D.B., Felici A., et al. Transforming growth factor-beta receptor-associated protein 1 is a Smad4 chaperone. J Biol Chem. 2001;276:19495–19502. doi: 10.1074/jbc.M006473200. 20010220. [DOI] [PubMed] [Google Scholar]
- 110.De Bosscher K., Hill C.S., Nicolas F.J. Molecular and functional consequences of Smad4 C-terminal missense mutations in colorectal tumour cells. Biochem J. 2004;379:209–216. doi: 10.1042/BJ20031886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nicolas F.J., Hill C.S. Attenuation of the TGF-beta-Smad signaling pathway in pancreatic tumor cells confers resistance to TGF-beta-induced growth arrest. Oncogene. 2003;22:3698–3711. doi: 10.1038/sj.onc.1206420. [DOI] [PubMed] [Google Scholar]
- 112.Goto K., Kamiya Y., Imamura T., et al. Selective inhibitory effects of Smad6 on bone morphogenetic protein type I receptors. J Biol Chem. 2007;282:20603–20611. doi: 10.1074/jbc.M702100200. 20070510. [DOI] [PubMed] [Google Scholar]
- 113.Hata A., Lagna G., Massague J., et al. Smad6 inhibits BMP/Smad1 signaling by specifically competing with the Smad4 tumor suppressor. Genes Dev. 1998;12:186–197. doi: 10.1101/gad.12.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nakao A., Afrakhte M., Moren A., et al. Identification of Smad7, a TGFbeta-inducible antagonist of TGF-beta signalling. Nature. 1997;389:631–635. doi: 10.1038/39369. [DOI] [PubMed] [Google Scholar]
- 115.Souchelnytskyi S., Nakayama T., Nakao A., et al. Physical and functional interaction of murine and Xenopus Smad7 with bone morphogenetic protein receptors and transforming growth factor-beta receptors. J Biol Chem. 1998;273:25364–25370. doi: 10.1074/jbc.273.39.25364. [DOI] [PubMed] [Google Scholar]
- 116.Nicolas F.J., De Bosscher K., Schmierer B., et al. Analysis of Smad nucleocytoplasmic shuttling in living cells. J Cell Sci. 2004;117:4113–4125. doi: 10.1242/jcs.01289. 2004/07/29. [DOI] [PubMed] [Google Scholar]
- 117.Xu L., Kang Y., Col S., et al. Smad2 nucleocytoplasmic shuttling by nucleoporins CAN/Nup214 and Nup153 feeds TGFbeta signaling complexes in the cytoplasm and nucleus. Mol Cell. 2002;10:271–282. doi: 10.1016/s1097-2765(02)00586-5. [DOI] [PubMed] [Google Scholar]
- 118.Korchynskyi O., ten Dijke P. Identification and functional characterization of distinct critically important bone morphogenetic protein-specific response elements in the Id1 promoter. J Biol Chem. 2002;277:4883–4891. doi: 10.1074/jbc.M111023200. 20011129. [DOI] [PubMed] [Google Scholar]
- 119.Shi Y., Wang Y.F., Jayaraman L., et al. Crystal structure of a Smad MH1 domain bound to DNA: insights on DNA binding in TGF-beta signaling. Cell. 1998;94:585–594. doi: 10.1016/s0092-8674(00)81600-1. [DOI] [PubMed] [Google Scholar]
- 120.Zawel L., Dai J.L., Buckhaults P., et al. Human Smad3 and Smad4 are sequence-specific transcription activators. Mol Cell. 1998;1:611–617. doi: 10.1016/s1097-2765(00)80061-1. [DOI] [PubMed] [Google Scholar]
- 121.Mullen A.C., Orlando D.A., Newman J.J., et al. Master transcription factors determine cell-type-specific responses to TGF-beta signaling. Cell. 2011;147:565–576. doi: 10.1016/j.cell.2011.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Trompouki E., Bowman T.V., Lawton L.N., et al. Lineage regulators direct BMP and Wnt pathways to cell-specific programs during differentiation and regeneration. Cell. 2011;147:577–589. doi: 10.1016/j.cell.2011.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hartsough M.T., Mulder K.M. Transforming growth factor beta activation of p44mapk in proliferating cultures of epithelial cells. J Biol Chem. 1995;270:7117–7124. doi: 10.1074/jbc.270.13.7117. [DOI] [PubMed] [Google Scholar]
- 124.Frey R.S., Mulder K.M. TGFbeta regulation of mitogen-activated protein kinases in human breast cancer cells. Cancer Lett. 1997;117:41–50. doi: 10.1016/s0304-3835(97)00211-5. [DOI] [PubMed] [Google Scholar]
- 125.Mucsi I., Skorecki K.L., Goldberg H.J. Extracellular signal-regulated kinase and the small GTP-binding protein, Rac, contribute to the effects of transforming growth factor-beta1 on gene expression. J Biol Chem. 1996;271:16567–16572. doi: 10.1074/jbc.271.28.16567. [DOI] [PubMed] [Google Scholar]
- 126.Galliher A.J., Schiemann W.P. Src phosphorylates Tyr284 in TGF-beta type II receptor and regulates TGF-beta stimulation of p38 MAPK during breast cancer cell proliferation and invasion. Cancer Res. 2007;67:3752–3758. doi: 10.1158/0008-5472.CAN-06-3851. [DOI] [PubMed] [Google Scholar]
- 127.Lee M.K., Pardoux C., Hall M.C., et al. TGF-beta activates Erk MAP kinase signalling through direct phosphorylation of ShcA. EMBO J. 2007;26:3957–3967. doi: 10.1038/sj.emboj.7601818. 20070802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mulder K.M., Morris S.L. Activation of p21ras by transforming growth factor beta in epithelial cells. J Biol Chem. 1992;267:5029–5031. [PubMed] [Google Scholar]
- 129.Simeone D.M., Zhang L., Graziano K., et al. Smad4 mediates activation of mitogen-activated protein kinases by TGF-beta in pancreatic acinar cells. Am J Physiol Cell Physiol. 2001;281:C311–C319. doi: 10.1152/ajpcell.2001.281.1.C311. [DOI] [PubMed] [Google Scholar]
- 130.Zavadil J., Bitzer M., Liang D., et al. Genetic programs of epithelial cell plasticity directed by transforming growth factor-beta. Proc Natl Acad Sci U S A. 2001;98:6686–6691. doi: 10.1073/pnas.111614398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Davies M., Robinson M., Smith E., et al. Induction of an epithelial to mesenchymal transition in human immortal and malignant keratinocytes by TGF-beta1 involves MAPK, Smad and AP-1 signalling pathways. J Cell Biochem. 2005;95:918–931. doi: 10.1002/jcb.20458. [DOI] [PubMed] [Google Scholar]
- 132.Sisto M., Ribatti D., Lisi S. Organ fibrosis and autoimmunity: the role of inflammation in TGFbeta-dependent EMT. Biomolecules. 2021:11. doi: 10.3390/biom11020310. 20210218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Thiery J.P. Epithelial-mesenchymal transitions in development and pathologies. Curr Opin Cell Biol. 2003;15:740–746. doi: 10.1016/j.ceb.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 134.Kretzschmar M., Doody J., Massague J. Opposing BMP and EGF signalling pathways converge on the TGF-beta family mediator Smad1. Nature. 1997;389:618–622. doi: 10.1038/39348. [DOI] [PubMed] [Google Scholar]
- 135.Kretzschmar M., Doody J., Timokhina I., et al. A mechanism of repression of TGFbeta/Smad signaling by oncogenic Ras. Genes Dev. 1999;13:804–816. doi: 10.1101/gad.13.7.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Funaba M., Zimmerman C.M., Mathews L.S. Modulation of Smad2-mediated signaling by extracellular signal-regulated kinase. J Biol Chem. 2002;277:41361–41368. doi: 10.1074/jbc.M204597200. 20020821. [DOI] [PubMed] [Google Scholar]
- 137.Matsuura I., Wang G., He D., et al. Identification and characterization of ERK MAP kinase phosphorylation sites in Smad3. Biochemistry. 2005;44:12546–12553. doi: 10.1021/bi050560g. [DOI] [PubMed] [Google Scholar]
- 138.Weston C.R., Davis R.J. The JNK signal transduction pathway. Curr Opin Cell Biol. 2007;19:142–149. doi: 10.1016/j.ceb.2007.02.001. 20070215. [DOI] [PubMed] [Google Scholar]
- 139.Engel M.E., McDonnell M.A., Law B.K., et al. Interdependent SMAD and JNK signaling in transforming growth factor-beta-mediated transcription. J Biol Chem. 1999;274:37413–37420. doi: 10.1074/jbc.274.52.37413. [DOI] [PubMed] [Google Scholar]
- 140.Hocevar B.A., Brown T.L., Howe P.H. TGF-beta induces fibronectin synthesis through a c-Jun N-terminal kinase-dependent, Smad4-independent pathway. EMBO J. 1999;18:1345–1356. doi: 10.1093/emboj/18.5.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jadrich J.L., O'Connor M.B., Coucouvanis E. The TGF beta activated kinase TAK1 regulates vascular development in vivo. Development. 2006;133:1529–1541. doi: 10.1242/dev.02333. [DOI] [PubMed] [Google Scholar]
- 142.Yamashita M., Fatyol K., Jin C., et al. TRAF6 mediates Smad-independent activation of JNK and p38 by TGF-beta. Mol Cell. 2008;31:918–924. doi: 10.1016/j.molcel.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sorrentino A., Thakur N., Grimsby S., et al. The type I TGF-beta receptor engages TRAF6 to activate TAK1 in a receptor kinase-independent manner. Nat Cell Biol. 2008;10:1199–1207. doi: 10.1038/ncb1780. 20080831. [DOI] [PubMed] [Google Scholar]
- 144.Wang C., Deng L., Hong M., et al. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature. 2001;412:346–351. doi: 10.1038/35085597. [DOI] [PubMed] [Google Scholar]
- 145.Edlund S., Bu S., Schuster N., et al. Transforming growth factor-beta1 (TGF-beta)-induced apoptosis of prostate cancer cells involves Smad7-dependent activation of p38 by TGF-beta-activated kinase 1 and mitogen-activated protein kinase kinase 3. Mol Biol Cell. 2003;14:529–544. doi: 10.1091/mbc.02-03-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bhowmick N.A., Ghiassi M., Bakin A., et al. Transforming growth factor-beta1 mediates epithelial to mesenchymal transdifferentiation through a RhoA-dependent mechanism. Mol Biol Cell. 2001;12:27–36. doi: 10.1091/mbc.12.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Edlund S., Landstrom M., Heldin C.H., et al. Transforming growth factor-beta-induced mobilization of actin cytoskeleton requires signaling by small GTPases Cdc42 and RhoA. Mol Biol Cell. 2002;13:902–914. doi: 10.1091/mbc.01-08-0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wilkes M.C., Murphy S.J., Garamszegi N., et al. Cell-type-specific activation of PAK2 by transforming growth factor beta independent of Smad2 and Smad3. Mol Cell Biol. 2003;23:8878–8889. doi: 10.1128/MCB.23.23.8878-8889.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Barrios-Rodiles M., Brown K.R., Ozdamar B., et al. High-throughput mapping of a dynamic signaling network in mammalian cells. Science. 2005;307:1621–1625. doi: 10.1126/science.1105776. [DOI] [PubMed] [Google Scholar]
- 150.Vinals F., Pouyssegur J. Transforming growth factor beta1 (TGF-beta1) promotes endothelial cell survival during in vitro angiogenesis via an autocrine mechanism implicating TGF-alpha signaling. Mol Cell Biol. 2001;21:7218–7230. doi: 10.1128/MCB.21.21.7218-7230.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Shin I., Bakin A.V., Rodeck U., et al. Transforming growth factor beta enhances epithelial cell survival via Akt-dependent regulation of FKHRL1. Mol Biol Cell. 2001;12:3328–3339. doi: 10.1091/mbc.12.11.3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wilkes M.C., Mitchell H., Penheiter S.G., et al. Transforming growth factor-beta activation of phosphatidylinositol 3-kinase is independent of Smad2 and Smad3 and regulates fibroblast responses via p21-activated kinase-2. Cancer Res. 2005;65:10431–10440. doi: 10.1158/0008-5472.CAN-05-1522. [DOI] [PubMed] [Google Scholar]
- 153.Dearolf C.R. JAKs and STATs in invertebrate model organisms. Cell Mol Life Sci. 1999;55:1578–1584. doi: 10.1007/s000180050397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hombria J.C., Brown S. The fertile field of Drosophila Jak/STAT signalling. Curr Biol. 2002;12:R569–R575. doi: 10.1016/s0960-9822(02)01057-6. [DOI] [PubMed] [Google Scholar]
- 155.Rawlings J.S., Rosler K.M., Harrison D.A. The JAK/STAT signaling pathway. J Cell Sci. 2004;117:1281–1283. doi: 10.1242/jcs.00963. [DOI] [PubMed] [Google Scholar]
- 156.Ulloa L., Doody J., Massague J. Inhibition of transforming growth factor-beta/SMAD signalling by the interferon-gamma/STAT pathway. Nature. 1999;397:710–713. doi: 10.1038/17826. [DOI] [PubMed] [Google Scholar]
- 157.Sanford L.P., Ormsby I., Gittenberger-de Groot A.C., et al. TGFbeta2 knockout mice have multiple developmental defects that are non-overlapping with other TGFbeta knockout phenotypes. Development. 1997;124:2659–2670. doi: 10.1242/dev.124.13.2659. 1997/07/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kaartinen V., Voncken J.W., Shuler C., et al. Abnormal lung development and cleft palate in mice lacking TGF-beta 3 indicates defects of epithelial-mesenchymal interaction. Nat Genet. 1995;11:415–421. doi: 10.1038/ng1295-415. 1995/12/01. [DOI] [PubMed] [Google Scholar]
- 159.Larsson J., Goumans M.J., Sjostrand L.J., et al. Abnormal angiogenesis but intact hematopoietic potential in TGF-beta type I receptor-deficient mice. EMBO J. 2001;20:1663–1673. doi: 10.1093/emboj/20.7.1663. 2001/04/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Oshima M., Oshima H., Taketo M.M. TGF-beta receptor type II deficiency results in defects of yolk sac hematopoiesis and vasculogenesis. Dev Biol. 1996;179:297–302. doi: 10.1006/dbio.1996.0259. 1996/10/10. [DOI] [PubMed] [Google Scholar]
- 161.Shull M.M., Ormsby I., Kier A.B., et al. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature. 1992;359:693–699. doi: 10.1038/359693a0. 1992/10/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kulkarni A.B., Huh C.G., Becker D., et al. Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci U S A. 1993;90:770–774. doi: 10.1073/pnas.90.2.770. 1993/01/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.McCartney-Francis N.L., Mizel D.E., Redman R.S., et al. Autoimmune Sjogren's-like lesions in salivary glands of TGF-beta1-deficient mice are inhibited by adhesion-blocking peptides. J Immunol. 1996;157:1306–1312. 1996/08/01. [PubMed] [Google Scholar]
- 164.Nandula S.R., Amarnath S., Molinolo A., et al. Female mice are more susceptible to developing inflammatory disorders due to impaired transforming growth factor beta signaling in salivary glands. Arthritis Rheum. 2007;56:1798–1805. doi: 10.1002/art.22715. 2007/05/29. [DOI] [PubMed] [Google Scholar]
- 165.Mattingly A., Finley J.K., Knox S.M. Salivary gland development and disease. Wiley Interdiscip Rev Dev Biol. 2015;4:573–590. doi: 10.1002/wdev.194. 2015/05/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hall B.E., Zheng C., Swaim W.D., et al. Conditional overexpression of TGF-beta1 disrupts mouse salivary gland development and function. Lab Invest. 2010;90:543–555. doi: 10.1038/labinvest.2010.5. 2010/02/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Janebodin K., Buranaphatthana W., Ieronimakis N., et al. An in vitro culture system for long-term expansion of epithelial and mesenchymal salivary gland cells: role of TGF-beta1 in salivary gland epithelial and mesenchymal differentiation. BioMed Res Int. 2013;2013:815895. doi: 10.1155/2013/815895. 2013/07/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Batlle E., Massague J. Transforming growth factor-beta signaling in immunity and cancer. Immunity. 2019;50:924–940. doi: 10.1016/j.immuni.2019.03.024. 2019/04/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.David C.J., Massague J. Contextual determinants of TGFbeta action in development, immunity and cancer. Nat Rev Mol Cell Biol. 2018;19:419–435. doi: 10.1038/s41580-018-0007-0. 2018/04/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ren L.L., Miao H., Wang Y.N., et al. TGF-beta as A Master regulator of aging-associated tissue fibrosis. Aging Dis. 2023 doi: 10.14336/AD.2023.0222. 2023/05/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Stewart A.G., Thomas B., Koff J. TGF-beta: master regulator of inflammation and fibrosis. Respirology. 2018;23:1096–1097. doi: 10.1111/resp.13415. 2018/10/05. [DOI] [PubMed] [Google Scholar]
- 172.Meng X.M., Nikolic-Paterson D.J., Lan H.Y. TGF-beta: the master regulator of fibrosis. Nat Rev Nephrol. 2016;12:325–338. doi: 10.1038/nrneph.2016.48. 2016/04/26. [DOI] [PubMed] [Google Scholar]
- 173.Wei P., Xie Y., Abel P.W., et al. Transforming growth factor (TGF)-beta1-induced miR-133a inhibits myofibroblast differentiation and pulmonary fibrosis. Cell Death Dis. 2019;10:670. doi: 10.1038/s41419-019-1873-x. 2019/09/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Woods L.T., Camden J.M., El-Sayed F.G., et al. Increased expression of TGF-beta signaling components in a mouse model of fibrosis induced by submandibular gland duct ligation. PLoS One. 2015;10 doi: 10.1371/journal.pone.0123641. 2015/05/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Maslinska M., Paradowska-Gorycka A., Manczak M., et al. The level of TGF-beta in sera of patients with primary Sjogren's syndrome. Reumatologia. 2019;57:309–314. doi: 10.5114/reum.2019.91276. 2019/01/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Sisto M., Lorusso L., Ingravallo G., et al. The TGF-beta1 signaling pathway as an attractive target in the fibrosis pathogenesis of sjogren's syndrome. Mediat Inflamm. 2018;2018 doi: 10.1155/2018/1965935. 2019/01/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Sisto M., Ribatti D., Lisi S. SMADS-mediate molecular mechanisms in sjogren's syndrome. Int J Mol Sci. 2021:22. doi: 10.3390/ijms22063203. 20210321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Koski H., Konttinen Y.T., Gu X.H., et al. Transforming growth factor beta 2 in labial salivary glands in Sjogren's syndrome. Ann Rheum Dis. 1995;54:744–747. doi: 10.1136/ard.54.9.744. 1995/09/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Hakim S.G., Ribbat J., Berndt A., et al. Expression of Wnt-1, TGF-beta and related cell-cell adhesion components following radiotherapy in salivary glands of patients with manifested radiogenic xerostomia. Radiother Oncol. 2011;101:93–99. doi: 10.1016/j.radonc.2011.07.032. 2011/09/03. [DOI] [PubMed] [Google Scholar]
- 180.Zhang X., Yun J.S., Han D., et al. TGF-Beta pathway in salivary gland fibrosis. Int J Mol Sci. 2020:21. doi: 10.3390/ijms21239138. 2020/12/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Leask A., Abraham D.J. TGF-beta signaling and the fibrotic response. Faseb J. 2004;18:816–827. doi: 10.1096/fj.03-1273rev. 2004/05/01. [DOI] [PubMed] [Google Scholar]
- 182.Zavadil J., Bottinger E.P. TGF-beta and epithelial-to-mesenchymal transitions. Oncogene. 2005;24:5764–5774. doi: 10.1038/sj.onc.1208927. 2005/08/27. [DOI] [PubMed] [Google Scholar]
- 183.Al-Habeeb F., Aloufi N., Traboulsi H., et al. Human antigen R promotes lung fibroblast differentiation to myofibroblasts and increases extracellular matrix production. J Cell Physiol. 2021;236:6836–6851. doi: 10.1002/jcp.30380. 2021/04/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Carpenter G.H., Osailan S.M., Correia P., et al. Rat salivary gland ligation causes reversible secretory hypofunction. Acta Physiol. 2007;189:241–249. doi: 10.1111/j.1365-201X.2006.01662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Walker N.I., Gobe G.C. Cell death and cell proliferation during atrophy of the rat parotid gland induced by duct obstruction. J Pathol. 1987;153:333–344. doi: 10.1002/path.1711530407. [DOI] [PubMed] [Google Scholar]
- 186.Harrison J.D., Garrett J.R. The effects of ductal ligation on the parenchyma of salivary glands of cat studied by enzyme histochemical methods. Histochem J. 1976;8:35–44. doi: 10.1007/BF01004003. [DOI] [PubMed] [Google Scholar]
- 187.Ahn J.S., Camden J.M., Schrader A.M., et al. Reversible regulation of P2Y(2) nucleotide receptor expression in the duct-ligated rat submandibular gland. Am J Physiol Cell Physiol. 2000;279:C286–C294. doi: 10.1152/ajpcell.2000.279.2.C286. 2000/07/27. [DOI] [PubMed] [Google Scholar]
- 188.Maria O.M., Maria S.M., Redman R.S., et al. Effects of double ligation of Stensen's duct on the rabbit parotid gland. Biotech Histochem. 2014;89:181–198. doi: 10.3109/10520295.2013.832798. 20130923. [DOI] [PubMed] [Google Scholar]
- 189.Matsumoto S., Okumura K., Ogata A., et al. Isolation of tissue progenitor cells from duct-ligated salivary glands of swine. Clon Stem Cell. 2007;9:176–190. doi: 10.1089/clo.2006.0022. [DOI] [PubMed] [Google Scholar]
- 190.Woods L.T., Camden J.M., Batek J.M., et al. P2X7 receptor activation induces inflammatory responses in salivary gland epithelium. Am J Physiol Cell Physiol. 2012;303:C790–C801. doi: 10.1152/ajpcell.00072.2012. 2012/08/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Takahashi S., Schoch E., Walker N.I. Origin of acinar cell regeneration after atrophy of the rat parotid induced by duct obstruction. Int J Exp Pathol. 1998;79:293–301. doi: 10.1046/j.1365-2613.1998.710405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Takahashi S., Nakamura S., Suzuki R., et al. Apoptosis and mitosis of parenchymal cells in the duct-ligated rat submandibular gland. Tissue Cell. 2000;32:457–463. doi: 10.1016/s0040-8166(00)80002-6. [DOI] [PubMed] [Google Scholar]
- 193.Scott J., Liu P., Smith P.M. Morphological and functional characteristics of acinar atrophy and recovery in the duct-ligated parotid gland of the rat. J Dent Res. 1999;78:1711–1719. doi: 10.1177/00220345990780110801. [DOI] [PubMed] [Google Scholar]
- 194.Stern Y., Feinmesser R., Collins M., et al. Bilateral submandibular gland excision with parotid duct ligation for treatment of sialorrhea in children: long-term results. Arch Otolaryngol Head Neck Surg. 2002;128:801–803. doi: 10.1001/archotol.128.7.801. [DOI] [PubMed] [Google Scholar]
- 195.Dundas D.F., Peterson R.A. Surgical treatment of drooling by bilateral parotid duct ligation and submandibular gland resection. Plast Reconstr Surg. 1979;64:47–51. doi: 10.1097/00006534-197907000-00009. [DOI] [PubMed] [Google Scholar]
- 196.Scott I., Olsen J., Jattan R. Submandibular salivary gland ligation–Near-catastrophic post-operative complication. Ann Oral Maxillofac Surg. 2022;6 [Google Scholar]
- 197.Yasumitsu T., Shimizu O., Shiratsuchi H., et al. Distribution of aquaporin-5, transforming growth factor-beta(1) and laminin during regeneration of atrophic rat submandibular glands after duct ligation. J Oral Sci. 2018;60:595–600. doi: 10.2334/josnusd.17-0491. 2018/12/28. [DOI] [PubMed] [Google Scholar]
- 198.O'Keefe K.J., DeSantis K.A., Altrieth A.L., et al. Regional differences following partial salivary gland resection. J Dent Res. 2020;99:79–88. doi: 10.1177/0022034519889026. 2019/11/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Totti M.A., dos Santos E.B., de Almeida O.P., et al. Oral candidosis by Candida albicans in normal and xerostomic mice. Braz Oral Res. 2004;18:202–207. doi: 10.1590/s1806-83242004000300005. [DOI] [PubMed] [Google Scholar]
- 200.Saruta J., Lee T., Shirasu M., et al. Chronic stress affects the expression of brain-derived neurotrophic factor in rat salivary glands. Stress. 2010;13:53–60. doi: 10.3109/10253890902875167. [DOI] [PubMed] [Google Scholar]
- 201.Kattaia A.A.A., Selim A.O., Selim S.A., et al. Epidermal growth factor attenuates lingual papillae lesions in a rat model of sialoadenectomy. Tissue Cell. 2020;63:101319. doi: 10.1016/j.tice.2019.101319. 20191127. [DOI] [PubMed] [Google Scholar]
- 202.Yura Y., Harada K., Tsujimoto H., et al. Effects of sialoadenectomy and epidermal growth factor administration on 9,10-dimethyl-1,2-benzanthracene-induced tumor formation in hamster cheek pouch. Oral Surg Oral Med Oral Pathol. 1993;76:723–728. doi: 10.1016/0030-4220(93)90042-3. [DOI] [PubMed] [Google Scholar]
- 203.Morris-Wiman J., Sego R., Brinkley L., et al. The effects of sialoadenectomy and exogenous EGF on taste bud morphology and maintenance. Chem Senses. 2000;25:9–19. doi: 10.1093/chemse/25.1.9. [DOI] [PubMed] [Google Scholar]
- 204.Helmrath M.A., Shin C.E., Fox J.W., et al. Adaptation after small bowel resection is attenuated by sialoadenectomy: the role for endogenous epidermal growth factor. Surgery. 1998;124:848–854. [PubMed] [Google Scholar]
- 205.Pradhan-Bhatt S., Harrington D.A., Duncan R.L., et al. A novel in vivo model for evaluating functional restoration of a tissue-engineered salivary gland. Laryngoscope. 2014;124:456–461. doi: 10.1002/lary.24297. 2013/07/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Nam K., Wang C.S., Maruyama C.L.M., et al. L1 peptide-conjugated fibrin hydrogels promote salivary gland regeneration. J Dent Res. 2017;96:798–806. doi: 10.1177/0022034517695496. 2017/02/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Nam K., Maruyama C.L., Wang C.S., et al. Laminin-111-derived peptide conjugated fibrin hydrogel restores salivary gland function. PLoS One. 2017;12 doi: 10.1371/journal.pone.0187069. 2017/11/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Nam K., Dean S.M., Brown C.T., et al. Synergistic effects of laminin-1 peptides, VEGF and FGF9 on salivary gland regeneration. Acta Biomater. 2019;91:186–194. doi: 10.1016/j.actbio.2019.04.049. 2019/04/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Yang J., Tyler L.W., Donoff R.B., et al. Salivary EGF regulates eosinophil-derived TGF-alpha expression in hamster oral wounds. Am J Physiol. 1996;270:G191–G202. doi: 10.1152/ajpgi.1996.270.1.G191. 1996/01/01. [DOI] [PubMed] [Google Scholar]
- 210.Ellis M.A., Graboyes E.M., Wahlquist A.E., et al. Primary surgery vs radiotherapy for early stage oral cavity cancer. Otolaryngol Head Neck Surg. 2018;158:649–659. doi: 10.1177/0194599817746909. 20171219. [DOI] [PubMed] [Google Scholar]
- 211.Kelly C., Paleri V., Downs C., et al. Deterioration in quality of life and depressive symptoms during radiation therapy for head and neck cancer. Otolaryngol Head Neck Surg. 2007;136:108–111. doi: 10.1016/j.otohns.2006.06.1278. [DOI] [PubMed] [Google Scholar]
- 212.Epstein J.B., Robertson M., Emerton S., et al. Quality of life and oral function in patients treated with radiation therapy for head and neck cancer. Head Neck. 2001;23:389–398. doi: 10.1002/hed.1049. [DOI] [PubMed] [Google Scholar]
- 213.Liu X., Subedi K.P., Zheng C., et al. Mitochondria-targeted antioxidant protects against irradiation-induced salivary gland hypofunction. Sci Rep. 2021;11:7690. doi: 10.1038/s41598-021-86927-3. 2021/04/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Jasmer K.J., Gilman K.E., Munoz Forti K., et al. Radiation-induced salivary gland dysfunction: mechanisms, therapeutics and future directions. J Clin Med. 2020;9 doi: 10.3390/jcm9124095. 2020/12/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Grundmann O., Mitchell G.C., Limesand K.H. Sensitivity of salivary glands to radiation: from animal models to therapies. J Dent Res. 2009;88:894–903. doi: 10.1177/0022034509343143. 2009/09/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Limesand K.H., Avila J.L., Victory K., et al. Insulin-like growth factor-1 preserves salivary gland function after fractionated radiation. Int J Radiat Oncol Biol Phys. 2010;78:579–586. doi: 10.1016/j.ijrobp.2010.03.035. 2010/07/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Lombaert I.M.A., Patel V.N., Jones C.E., et al. CERE-120 prevents irradiation-induced hypofunction and restores immune homeostasis in porcine salivary glands. Mol Ther Methods Clin Dev. 2020;18:839–855. doi: 10.1016/j.omtm.2020.07.016. 2020/09/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Martin M., Lefaix J., Delanian S. TGF-beta1 and radiation fibrosis: a master switch and a specific therapeutic target? Int J Radiat Oncol Biol Phys. 2000;47:277–290. doi: 10.1016/s0360-3016(00)00435-1. 2000/05/10. [DOI] [PubMed] [Google Scholar]
- 219.Feltl D., Zavadova E., Pala M., et al. Post-treatment plasma transforming growth factor beta 1 (TGF-beta1) level predicts for late morbidity in patients with advanced head and neck cancer. Neoplasma. 2005;52:393–397. [PubMed] [Google Scholar]
- 220.Anscher M.S., Murase T., Prescott D.M., et al. Changes in plasma TGF beta levels during pulmonary radiotherapy as a predictor of the risk of developing radiation pneumonitis. Int J Radiat Oncol Biol Phys. 1994;30:671–676. doi: 10.1016/0360-3016(92)90954-g. 1994/10/15. [DOI] [PubMed] [Google Scholar]
- 221.Talbott H.E., Mascharak S., Griffin M., et al. Wound healing, fibroblast heterogeneity, and fibrosis. Cell Stem Cell. 2022;29:1161–1180. doi: 10.1016/j.stem.2022.07.006. 2022/08/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Wynn T.A. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199–210. doi: 10.1002/path.2277. 2007/12/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Gieseck R.L., 3rd, Wilson M.S., Wynn T.A. Type 2 immunity in tissue repair and fibrosis. Nat Rev Immunol. 2018;18:62–76. doi: 10.1038/nri.2017.90. 2017/08/31. [DOI] [PubMed] [Google Scholar]
- 224.Weiskirchen R., Weiskirchen S., Tacke F. Organ and tissue fibrosis: molecular signals, cellular mechanisms and translational implications. Mol Aspect Med. 2019;65:2–15. doi: 10.1016/j.mam.2018.06.003. 2018/07/01. [DOI] [PubMed] [Google Scholar]
- 225.Altrieth A.L., O'Keefe K.J., Gellatly V.A., et al. Identifying fibrogenic cells following salivary gland obstructive injury. bioRxiv. 2023 doi: 10.1101/2023.03.09.531751. 2023/03/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Altrieth A.L., O'Keefe K.J., Gellatly V.A., et al. Identifying fibrogenic cells following salivary gland obstructive injury. Front Cell Dev Biol. 2023;11 doi: 10.3389/fcell.2023.1190386. 2023/06/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Moskwa N., Mahmood A., Nelson D.A., et al. Single-cell RNA sequencing reveals PDFGRalpha+ stromal cell subpopulations that promote proacinar cell differentiation in embryonic salivary gland organoids. Development. 2022:149. doi: 10.1242/dev.200167. 2022/03/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Manshouri T., Veletic I., Li P., et al. GLI1 activates pro-fibrotic pathways in myelofibrosis fibrocytes. Cell Death Dis. 2022;13:481. doi: 10.1038/s41419-022-04932-4. 2022/05/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Schneider R.K., Mullally A., Dugourd A., et al. Gli1(+) mesenchymal stromal cells are a key driver of bone marrow fibrosis and an important cellular therapeutic target. Cell Stem Cell. 2018;23:308–309. doi: 10.1016/j.stem.2018.07.006. 2018/08/04. [DOI] [PubMed] [Google Scholar]
- 230.Cassandras M., Wang C., Kathiriya J., et al. Gli1(+) mesenchymal stromal cells form a pathological niche to promote airway progenitor metaplasia in the fibrotic lung. Nat Cell Biol. 2020;22:1295–1306. doi: 10.1038/s41556-020-00591-9. 2020/10/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Robertson I.B., Horiguchi M., Zilberberg L., et al. Latent TGF-beta-binding proteins. Matrix Biol. 2015;47:44–53. doi: 10.1016/j.matbio.2015.05.005. 2015/05/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Enomoto Y., Matsushima S., Shibata K., et al. LTBP2 is secreted from lung myofibroblasts and is a potential biomarker for idiopathic pulmonary fibrosis. Clin Sci (Lond) 2018;132:1565–1580. doi: 10.1042/CS20180435. 2018/07/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Su C.T., Jao T.M., Urban Z., et al. LTBP4 affects renal fibrosis by influencing angiogenesis and altering mitochondrial structure. Cell Death Dis. 2021;12:943. doi: 10.1038/s41419-021-04214-5. 2021/10/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Nikolic-Paterson D.J., Wang S., Lan H.Y. Macrophages promote renal fibrosis through direct and indirect mechanisms. Kidney Int Suppl. 2014;4:34–38. doi: 10.1038/kisup.2014.7. (2011) 2015/08/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Wang S., Meng X.M., Ng Y.Y., et al. TGF-beta/Smad3 signalling regulates the transition of bone marrow-derived macrophages into myofibroblasts during tissue fibrosis. Oncotarget. 2016;7:8809–8822. doi: 10.18632/oncotarget.6604. 2015/12/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Ueshima E., Fujimori M., Kodama H., et al. Macrophage-secreted TGF-beta(1) contributes to fibroblast activation and ureteral stricture after ablation injury. Am J Physiol Ren Physiol. 2019;317:F52–F64. doi: 10.1152/ajprenal.00260.2018. 2019/04/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Lee C.G., Homer R.J., Zhu Z., et al. Interleukin-13 induces tissue fibrosis by selectively stimulating and activating transforming growth factor beta(1) J Exp Med. 2001;194:809–821. doi: 10.1084/jem.194.6.809. 2001/09/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Song E.C., Min S., Oyelakin A., et al. Genetic and scRNA-seq analysis reveals distinct cell populations that contribute to salivary gland development and maintenance. Sci Rep. 2018;8:14043. doi: 10.1038/s41598-018-32343-z. 2018/09/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Min S., Oyelakin A., Gluck C., et al. p63 and its target follistatin maintain salivary gland stem/progenitor cell function through TGF-beta/activin signaling. iScience. 2020;23 doi: 10.1016/j.isci.2020.101524. 2020/09/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Mou H., Vinarsky V., Tata P.R., et al. Dual SMAD signaling inhibition enables long-term expansion of diverse epithelial basal cells. Cell Stem Cell. 2016;19:217–231. doi: 10.1016/j.stem.2016.05.012. 2016/06/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Kiyokawa H., Yamaoka A., Matsuoka C., et al. Airway basal stem cells reutilize the embryonic proliferation regulator, Tgfbeta-Id2 axis, for tissue regeneration. Dev Cell. 2021;56:1917–1929 e1919. doi: 10.1016/j.devcel.2021.05.016. 2021/06/16. [DOI] [PubMed] [Google Scholar]
- 242.Song E.C., Che M., Osinski J., et al. DeltaNp63 maintains the fidelity of the myoepithelial cell lineage and directs cell differentiation programs in the murine salivary gland. Cell Death Differ. 2023;30:515–526. doi: 10.1038/s41418-022-01101-0. 2022/12/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Altrieth A.L., Suarez E., Nelson D.A., et al. Single-cell transcriptomic analysis of salivary gland endothelial cells. bioRxiv. 2023 doi: 10.1101/2023.06.22.545817. 2023/07/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Frangogiannis N. Transforming growth factor-beta in tissue fibrosis. J Exp Med. 2020;217 doi: 10.1084/jem.20190103. 2020/10/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Zent J., Guo L.W. Signaling mechanisms of myofibroblastic activation: outside-in and inside-out. Cell Physiol Biochem. 2018;49:848–868. doi: 10.1159/000493217. 2018/09/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Sanjabi S., Oh S.A., Li M.O. Regulation of the immune response by TGF-beta: from conception to autoimmunity and infection. Cold Spring Harbor Perspect Biol. 2017;9 doi: 10.1101/cshperspect.a022236. 2017/01/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Vander Ark A., Cao J., Li X. TGF-beta receptors: in and beyond TGF-beta signaling. Cell Signal. 2018;52:112–120. doi: 10.1016/j.cellsig.2018.09.002. 2018/09/06. [DOI] [PubMed] [Google Scholar]
- 248.Peters S.B., Nelson D.A., Kwon H.R., et al. TGFbeta signaling promotes matrix assembly during mechanosensitive embryonic salivary gland restoration. Matrix Biol. 2015;43:109–124. doi: 10.1016/j.matbio.2015.01.020. 2015/02/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Liu C., Peng G., Jing N. TGF-beta signaling pathway in early mouse development and embryonic stem cells. Acta Biochim Biophys Sin. 2018;50:68–73. doi: 10.1093/abbs/gmx120. 2017/12/01. [DOI] [PubMed] [Google Scholar]
- 250.Suzuki D., Pinto F., Senoo M. Inhibition of TGF-beta signaling supports high proliferative potential of diverse p63(+) mouse epithelial progenitor cells in vitro. Sci Rep. 2017;7:6089. doi: 10.1038/s41598-017-06470-y. 2017/07/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Piraino L.R., Benoit D.S.W., DeLouise L.A. Salivary gland tissue engineering approaches: state of the art and future directions. Cells. 2021;10 doi: 10.3390/cells10071723. 2021/08/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Fowler E.W., van Venrooy E.J., Witt R.L., et al. A TGFbetaR inhibitor represses keratin-7 expression in 3D cultures of human salivary gland progenitor cells. Sci Rep. 2022;12:15008. doi: 10.1038/s41598-022-19253-x. 2022/09/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Li H., Wang G., Hu M., et al. Specific inhibitor of Smad3 (SIS3) alleviated submandibular gland fibrosis and dysfunction after dominant duct ligation in mice. J Dent Sci. 2023;18:865–871. doi: 10.1016/j.jds.2023.02.004. 2023/04/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Saadat S., Noureddini M., Mahjoubin-Tehran M., et al. Pivotal role of TGF-beta/smad signaling in cardiac fibrosis: non-coding RNAs as effectual players. Front Cardiovasc Med. 2020;7:588347. doi: 10.3389/fcvm.2020.588347. 2021/02/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Li M.O., Flavell R.A. TGF-beta: a master of all T cell trades. Cell. 2008;134:392–404. doi: 10.1016/j.cell.2008.07.025. 2008/08/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Li M.O., Wan Y.Y., Flavell R.A. T cell-produced transforming growth factor-beta1 controls T cell tolerance and regulates Th1- and Th17-cell differentiation. Immunity. 2007;26:579–591. doi: 10.1016/j.immuni.2007.03.014. 2007/05/08. [DOI] [PubMed] [Google Scholar]
- 257.Zhang M., Zhang S.T. Cells in fibrosis and fibrotic diseases. Front Immunol. 2020;11:1142. doi: 10.3389/fimmu.2020.01142. 2020/07/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Wirsdorfer F., Jendrossek V. The role of lymphocytes in radiotherapy-induced adverse late effects in the lung. Front Immunol. 2016;7:591. doi: 10.3389/fimmu.2016.00591. 2016/12/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Horsburgh S., Todryk S., Ramming A., et al. Innate lymphoid cells and fibrotic regulation. Immunol Lett. 2018;195:38–44. doi: 10.1016/j.imlet.2017.08.022. 2017/08/29. [DOI] [PubMed] [Google Scholar]
- 260.Mikami Y., Takada Y., Hagihara Y., et al. Innate lymphoid cells in organ fibrosis. Cytokine Growth Factor Rev. 2018;42:27–36. doi: 10.1016/j.cytogfr.2018.07.002. 2018/08/15. [DOI] [PubMed] [Google Scholar]
- 261.Zhang Y., Tang J., Tian Z., et al. Innate lymphoid cells: a promising new regulator in fibrotic diseases. Int Rev Immunol. 2016;35:399–414. doi: 10.3109/08830185.2015.1068304. 2015/07/30. [DOI] [PubMed] [Google Scholar]
- 262.Cortez V.S., Fuchs A., Cella M., et al. Cutting edge: salivary gland NK cells develop independently of Nfil3 in steady-state. J Immunol. 2014;192:4487–4491. doi: 10.4049/jimmunol.1303469. 2014/04/18. [DOI] [PubMed] [Google Scholar]
- 263.Cortez V.S., Cervantes-Barragan L., Robinette M.L., et al. Transforming growth factor-beta signaling guides the differentiation of innate lymphoid cells in salivary glands. Immunity. 2016;44:1127–1139. doi: 10.1016/j.immuni.2016.03.007. 2016/05/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Laurent P., Allard B., Manicki P., et al. TGFbeta promotes low IL10-producing ILC2 with profibrotic ability involved in skin fibrosis in systemic sclerosis. Ann Rheum Dis. 2021;80:1594–1603. doi: 10.1136/annrheumdis-2020-219748. 2021/07/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Wermuth P.J., Carney K.R., Mendoza F.A., et al. Endothelial cell-specific activation of transforming growth factor-beta signaling in mice induces cutaneous, visceral, and microvascular fibrosis. Lab Invest. 2017;97:806–818. doi: 10.1038/labinvest.2017.23. 2017/03/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Xavier S., Vasko R., Matsumoto K., et al. Curtailing endothelial TGF-beta signaling is sufficient to reduce endothelial-mesenchymal transition and fibrosis in CKD. J Am Soc Nephrol. 2015;26:817–829. doi: 10.1681/ASN.2013101137. 2014/12/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Hu M., Liu W., Ma P., et al. Smad7 attenuates TGF-beta-mediated aging-related hypofunction of submandibular glands. Exp Biol Med. 2021;246:1269–1273. doi: 10.1177/1535370221993430. 2021/03/02. [DOI] [PMC free article] [PubMed] [Google Scholar]