Abstract
Aims
The microbiome is a critical factor in health throughout human development. The aims of this scoping review are to (i) elucidate the differences between the youth (post-natal day 21–65 for rodents, 2–7 years for non-human primates, and 10–25 years for humans) microbiome with other life stages and (ii) identify youth-specific microbial changes associated with substance use.
Methods
Peer-reviewed studies published up to May 2023 were identified in PubMed and SCOPUS and included gut and oral microbiome studies from rodents, non-human primates, and humans (N = 1733). Twenty-six articles were determined eligible based on inclusion criteria (aim 1: n = 19, aim 2: n = 7).
Results
The adolescent and young adult oral and gut microbiomes are distinct compared to other life stages, within both non-human and human models. While there is limited research in this area, the microbiome appears to be vulnerable to substance use exposure earlier in life, including substances commonly initiated and escalated during adolescence and young adulthood (i.e. alcohol, cannabis, and tobacco).
Conclusions
Studies across the lifespan indicate that adolescence and young adulthood are distinct periods of development, where the microbiome is sensitive to exposures, including substance use. There is a need for more studies focused on the adolescent and young adult microbiome and substance use, as well as focused on the oral microbiome during this developmental period. Understanding the gut and oral microbiome during adolescence and young adulthood may provide insight into the pathophysiology of substance use disorders.
Keywords: microbiome, adolescence, development, gut, oral, substances
Short Summary: The adolescent and young adult microbiome is different from the child and adult microbiome. This period may be sensitive to exposures, including substance use. Future youth-specific gut and oral microbiome studies are needed to further elucidate the pathophysiology of alcohol and substance use disorders.
Introduction
The development and maturation of the human brain primarily takes place during adolescence and is not fully complete until the age of ~25 (Arain et al. 2013). Importantly, initiation and escalation of alcohol, cannabis, nicotine, and other drugs often occurs during this developmental period, potentially enhancing vulnerability to the negative consequences of substance use. Research shows that earlier initiation of substance use is associated with a wide range of adverse outcomes, including increased risk for substance use disorder (SUD) diagnosis (Ellickson et al. 2003, Dawson et al. 2008, Lopez-Quintero et al. 2011). Interventions during this heightened period of vulnerability may improve outcomes, but current treatment options for this age group are mainly psychosocial and limited in efficacy (Fadus et al. 2019).
Past efforts to study the pathophysiology of SUDs have mainly focused on central nervous system specific mechanisms and effects; however, it is important to consider peripheral biological systems to fully understand how youth substance use may lead to long-term consequences. Growing preclinical and clinical adult evidence suggests that the human microbiome, a collection of microbes that live in and on our bodies, may play a key role in the etiology of SUDs. Given this, modulation of the microbiome has recently been proposed as a novel treatment option for SUDs (Leclercq et al. 2019, Chinna Meyyappan et al. 2020, Verma et al. 2020, Pizarro et al. 2021). Targeting the microbiome during a window of neural and microbial plasticity may help mitigate substance-induced effects; however, little is known about how the adolescent and young adult microbiome compares to other life stages or how it is influenced by substances. More youth (ages 10–25) focused studies on the microbiome and substance use are needed given the numerous biological changes that occur during this period. Furthermore, youth substance use patterns can differ greatly from that of adults, with most youth using less frequently but in much higher quantities (SAMHSA 2021). Therefore, this scoping review provides a critical overview of (i) the differences between the adolescent and young adult microbiome with other life stages and (ii) youth-specific microbial changes associated with substance use. The goal of this scoping review is to highlight the importance of studying the youth microbiome to further elucidate the pathophysiology of SUDs.
General overview of the microbiome
Humans have evolved in the context of their microbial communities, which consist of bacteria, archaea, fungi, and viruses/phages. Collectively, these trillions of microorganisms are called the microbiota and their combined genomes are referred to as the microbiome (Ursell et al. 2012). Importantly, the microbiome plays a key role in brain development and function. Studies show that germ-free mice (mice devoid of all microorganisms) experience deficits in areas important for adolescent brain development, including myelination, neurotransmission, synaptic plasticity, microglia function, neurogenesis, dendritic growth, and blood—brain-barrier permeability (Cryan et al. 2019). Growing evidence also indicates that the microbiome can affect behavior. For example, in both rodents and humans, administration of certain strains of bacteria is associated with reductions in anxiety and depression behaviors, which are commonly comorbid with substance use (Mckernan et al. 2010, Bravo Javier et al. 2011, Tillisch et al. 2013, Savignac et al. 2014, Akkasheh et al. 2016, Allen et al. 2016, Pinto-Sanchez et al. 2017, Li et al. 2018, Morshedi et al. 2018, Chahwan et al. 2019, Liao et al. 2019, Marotta et al. 2019, Murray et al. 2019, Sun et al. 2019, Wei et al. 2019, Stenman et al. 2020). Together, the involvement of the microbiome in brain development and function, and the data supporting the manipulation of behaviors via the microbiome, highlights the potential for interventions in the context of substance use disorders.
Gut microbiome and substance use in adults
Most studies investigating the microbiome and the brain have focused on the gut which contains the largest bacterial ecosystem in the human body (Proctor et al. 2019). Gut microbiota and the brain can communicate with each other through the microbiota—gut—brain axis, a complex bidirectional communication network composed of a variety of routes, including cranial nerves, neuroendocrine pathways, immune pathways, and microbial metabolites (Cryan et al. 2019). A growing body of literature suggests that the microbiota—gut—brain axis may play a role in substance use disorders. Several studies have reported that adult substance use alters the diversity and abundances of microbes in the gut, as already reviewed elsewhere (Meckel and Kiraly 2019, Salavrakos et al. 2021, Simpson et al. 2022). Recent studies indicate that these differences in the gut microbiome may not only be a consequence of substance use but may also play a role in the development and maintenance of SUDs. Alterations in gut permeability and microbiome composition have been associated with craving, a central feature of SUDs (Leclercq et al. 2012, Leclercq et al. 2014). Administration of antibiotics, which depletes the microbiome, has been shown to decrease voluntary alcohol drinking, reward, withdrawal symptoms, behavioral responses to cocaine, and opioid tolerance (Kiraly et al. 2016, Kang et al. 2017, Lee et al. 2018, Angoa-Pérez and Kuhn 2021). Likewise, transplantation of gut microbiota has been shown to influence alcohol withdrawal, alcohol preference, alcohol craving, methamphetamine reward behavior, and opioid withdrawal (Xiao et al. 2018, Zhao et al. 2020, Bajaj et al. 2021, Thomaz et al. 2021, Wolstenholme et al. 2022, Wang et al. 2023).
Oral microbiome and substance use in adults
Until recently, the majority of microbiome research often disregarded another key environment that is part of the digestive tract: the oral microbiome. As the second largest and diverse microbial community in humans, the oral microbiome has also been shown to play a prominent role in several systemic diseases, including neurological and psychiatric disorders (Bowland and Weyrich 2022, Peng et al. 2022). Furthermore, both oral inflammation and poor oral health are associated with SUDs, suggesting the importance of understanding the oral microbiome to support a comprehensive knowledge of the biological underpinnings and consequences of such disorders (Baghaie et al. 2017). Epidemiological and experimental evidence suggest that the oral microbiota may affect the brain through many of the same routes as the gut including cranial nerves and circulating blood (Peng et al. 2022). Oral microbes have also been shown to invade and colonize the gut which can lead to several negative outcomes, including inflammation and increased intestinal permeability, potentially allowing bacterial components to reach systemic circulation (Iwauchi et al. 2019, Li et al. 2019, Huh and Roh 2020). In a recent study, seven genera (Actinomyces, Bifidobacterium, Dialister, Granulicatella, Lactobacillus, Megasphaera, and Veillonella) were shown to be present in both fecal and oral samples of patients with alcohol use disorder (AUD), but not in healthy individuals from the Human Microbiome Project (HMP), suggesting there might be more oral to gut transmission in individuals with AUD (Ames et al. 2020). Although less researched than the gut, recent studies on the oral microbiome and substance use in adults show promising complementary results. Alterations in the diversity and abundances of oral microbes have been associated with use of alcohol (Fan et al. 2018, Yussof et al. 2020, Barb et al. 2022, Li et al. 2022, Liao et al. 2022, Maley et al. 2022), amphetamine (Kosciolek et al. 2021), opioid (Kosciolek et al. 2021), cannabis (Luo et al. 2021), and nicotine (Wu et al. 2016, Lin et al. 2019, Pushalkar et al. 2020, Chopyk et al. 2021, Wang et al. 2022).
Current review aims
Overall, the adult literature suggests a relationship between substance use and both the gut and oral microbiomes; however, it is important to study microbial changes in the context of adolescence and young adulthood, as changes during this period may be related to initiation and escalation of substance use, affect the developing brain, and influence SUD development and maintenance. The current scoping review advances the literature by providing a critical overview and synthesis of what is known about (i) the adolescent and young adult microbiome in both animals and humans and (ii) youth-specific microbial changes associated with substance use. It also identifies and discusses gaps in the literature to help future research studies.
Methods
The current review was conducted using published guidelines for scoping studies (Arksey and O’malley 2005). Studies were searched in May 2023 using SCOPUS and PubMed. Backward reference searching of relevant articles (e.g. citations in included studies and reviews) was conducted.
Separate searches were performed for each of the aims. To produce critical overview and synthesis of adolescent and young adult microbiome, we used the following search terms: ‘adolescent,’ ‘adolescence,’ ‘youth,’ ‘teen,’ ‘teenager,’ ‘young adult,’ AND ‘microbiome,’ ‘microbiota,’ ‘microbes,’ and ‘bacteria’. This search yielded 1267 results, 301 results from SCOPUS, 963 from PubMed, and 3 through backward reference searching. To relate adolescent microbiome to substance use, we added to the search terms the following AND-clauses ‘alcohol,’ ‘ethanol,’ ‘alcohol use,’ ‘binge drinking,’ ‘alcoholic beverage,’ ‘alcohol use disorder,’ ‘alcohol dependence,’ ‘alcohol abuse,’ ‘alcoholism,’ ‘nicotine,’ ‘tobacco,’ ‘cigarette,’ ‘cannabis,’ ‘cannabis use disorder,’ ‘marijuana,’ ‘cocaine,’ ‘opioid,’ ‘crack,’ ‘methamphetamine,’ ‘amphetamine,’ ‘opioid,’ ‘codeine,’ ‘oxycodone,’ ‘heroin,’ ‘benzodiazepine,’ ‘sedatives,’ ‘hypnotics,’ ‘anxiolytics,’ ‘PCP,’ ‘LSD,’ ‘hallucinogen,’ ‘inhalants,’ ‘psilocybin,’ ‘illegal drug,’ ‘illicit drug,’ ‘street drug,’ ‘substance related disorder,’ ‘substance abuse,’ ‘substance dependence,’ ‘drug abuse,’ ‘drug dependence,’ and ‘addiction’. This search yielded 466 results, 48 from SCOPUS, 416 from PubMed, and 2 through backward reference searching.
Studies were reviewed in Covidence (Innovation n.d.) for inclusion. For aim 1, studies were included if they compared the microbiome of healthy adolescents and young adults [post-natal day (PND) 21–65 for rodents, 2–7 years for non-human primates, and 10–25 years for humans] to healthy individuals in another life stage (e.g. childhood or adulthood). For aim 2, studies were included if microbial effects of substance use during adolescence or young adulthood [post-natal day (PND) 21–65 for rodents, 2–7 years for non-human primates, and 10–25 years for humans] was assessed. In addition, both aims required studies to meet the following inclusion criteria: (i) examined gut or oral microbiota; (ii) included rodent, non-human primates, or humans; (iii) written in English; and (iv) published in peer-reviewed journals between January 1990 and May 2023. Any questions about eligibility criteria were discussed and resolved within the research team (n = 4). Across both searches, a total of 26 articles were determined eligible: 19 publications for aim 1 and 7 publications for aim 2. One study was included for both aims (Vetreno et al. 2021). Figure 1 provides a flow chart of the selection process.
Figure 1.
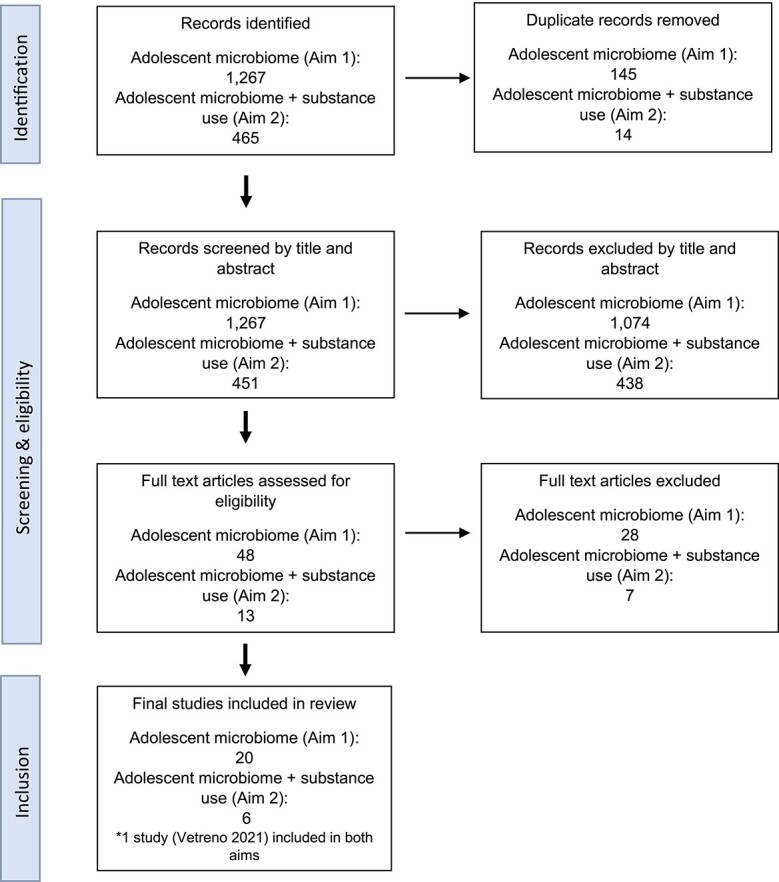
PRISMA flow diagram for the scoping review. Aim 1: the adolescent microbiome compared other stages of the lifespan. Aim 2: associations between the adolescent microbiome and substance use.
Results
Aim 1: the adolescent microbiome is distinct compared to other stages of the lifespan
Nineteen studies were included for aim 1. Study methods varied greatly in terms of population, study design, microbiome type, sequencing method, taxonomic identification method, and microbiome analyses (Table 1). The studies were conducted in humans (n = 7), rodents (n = 7; rats n = 6, mice n = 1), and non-human primates (n = 5) and utilized either cross-sectional (n = 15) or longitudinal (n = 4) microbiome assessments. Most of the studies compared the adolescent period to adulthood (n = 15), and four studies investigated changes between adolescents and children. Only two studies (one adolescent and young adult vs. adult, one adolescent and young adult vs. child) examined the oral microbiome and all others focused on the gut (n = 17). Most studies utilized 16S rRNA sequencing; however, different variable regions and taxonomic identification methods were used. All but one study assessed differences in the abundance of taxa through a variety of statistical methods and spanned the species, genus, family, order, class, and phylum levels. Studies also examined the diversity of the microbiome by determining whether there were differences in the number or distribution of bacteria (alpha diversity; n = 14) and/or the similarity or dissimilarity in microbiota communities between groups (beta diversity; n = 14). Metrics used to assess diversity varied widely across studies. For alpha diversity, the Shannon index was the most widely utilized (n = 13), followed by observed species/operational taxonomic units (OTUs)/amplicon sequence variants (ASVs; n = 7), phylogenetic diversity (n = 5), Chao1 (n = 5), Simpson (n = 4), ACE (n = 1), Good’s coverage (n = 1), and evenness (n = 2). Distance/dissimilarity metrics included unweighted UniFrac (n = 5), Bray—Curtis (n = 5), weighted UniFrac (n = 4), Jaccard (n = 2), and Euclidean (n = 2). Only four studies assessed differences in microbial function or metabolic potential between the microbiomes of adolescents and adults. These are detailed in Table 1.
Table 1.
Summary of included studies.
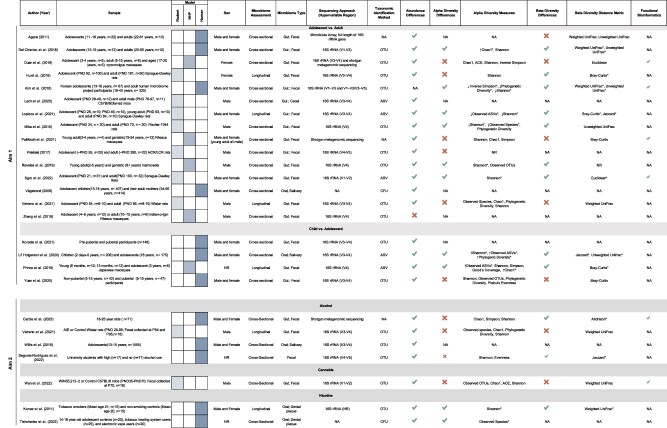
|
Abundance and diversity measures are only included in this table if they were assessed statistically. OTU: operational taxonomic unit; ASV: amplicon sequence variant; *significantly different.
Most of our current understanding of the adolescent microbiome has been extrapolated from adult studies under the assumption that the microbiome is stable by adolescence; however, based on the studies identified in this review, the adolescent microbiome has a distinct microbial profile compared to the adult and child microbiomes (Figs 2 and 3). Of the 19 identified studies, 17 reported differences in relative abundance of taxa (1 null finding, 1 not assessed), 8 in alpha diversity (6 null findings, 5 not assessed), and 9 in beta diversity (5 null findings, 5 not assessed).
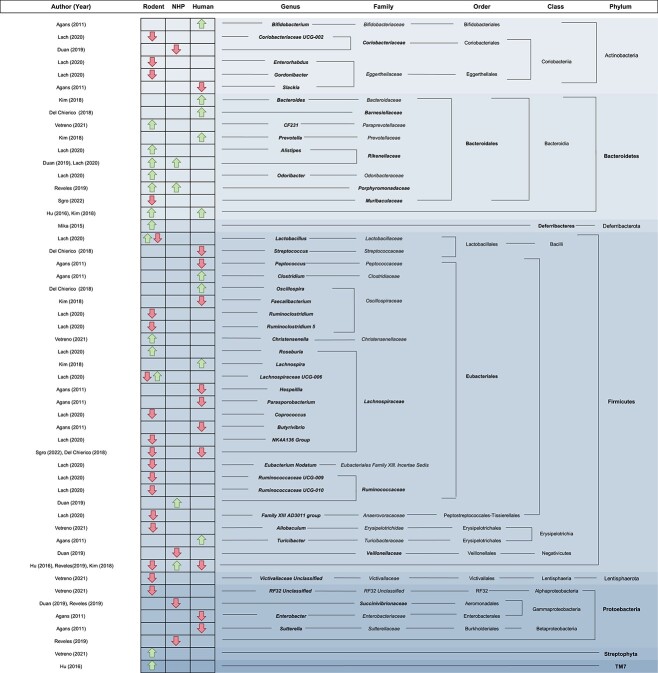
Figure 2.
Significantly different taxa reported between adolescents and adults. Taxa specifically reported in the text are in bold while associated taxonomic levels are not. *Identifies oral microbiome studies while all others are gut microbiome studies. This table only includes significantly different taxa; therefore, some identified studies in aim 1 are not included. NHP: non-human primate.
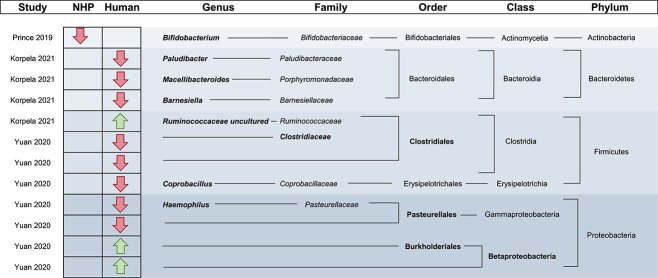
Figure 3.
Significantly different taxa reported between adolescents and children. Taxa specifically reported in the text are in bold while associated taxonomic levels are not. This table only includes significantly different taxa identified in both males and females; therefore, some identified studies in aim 1 are not included.
Adolescent and young adult versus adult
In the 15 adolescent and young adult versus adult studies, differences in 52 taxa spanning the genus, family, order, class, and phylum levels were identified. Most of the differences in the identified studies belonged to the phylum Firmicutes (Bacillota; 52%); however, these findings were also the most variable with both higher and lower levels reported at several taxonomic ranks. The three studies that directly assessed differences in Firmicutes between adolescents and adults were also conflicting, with two reporting decreases and one reporting increases (Fig. 2) (Hu et al. 2016, Reveles et al. 2019). In addition to Firmicutes, differences in four other taxa, including Bacteroidetes, Rikenellaceae, Lachnospiraceae, and Succinivibrionaceae, were reported by multiple studies (Hu et al. 2016, Del Chierico et al. 2018, Kim et al. 2018, Duan et al. 2019, Reveles et al. 2019, Lach et al. 2020, Sgro et al. 2022). While few studies reported differences in the same taxa, there seems to be consistencies when grouping these taxa by their associated phyla with the exception of Firmicutes. For example, when classifying significantly different species, genus, family, and order level taxa at the associated phylum level, 13 of 14 studies reported higher abundances of Bacteroidetes (Bacteroidota), 5 of 6 studies reported lower levels of Actinobacteria (Actinomycetota), and all 5 studies reported lower Proteobacteria (Pseudomonadota) in adolescents compared to adults (Fig. 2) (Kim et al. 2018, Duan et al. 2019, Lach et al. 2020, Korpela et al. 2021, Vetreno et al. 2021). These findings are consistent with the directions reported in studies directly analyzing these phyla, including increased Bacteroidetes and decreased Proteobacteria (Hu et al. 2016, Kim et al. 2018, Reveles et al. 2019). For alpha diversity, six studies found significant differences between adolescents and adults, five found no significant differences, and four did not assess alpha diversity or did not include statistical measures. Of those with significant differences, most reported lower alpha diversity in adolescents/young adults compared to adults (five out of six), and one study reported higher Chao1 in adolescents (Del Chierico et al. 2018). For beta diversity, seven studies reported statistically significant differences between adolescents/young adults and adults, four found no statistically significant differences, and four did not assess for beta diversity and did not include statistical measures. Only four studies assessed microbial function using bioinformatics (Kim et al. 2018, Duan et al. 2019, Pallikkuth et al. 2021, Sgro et al. 2022).
In addition to the unique composition and diversity of the adolescent microbiome, several findings suggest that the adolescent microbiome is more susceptible to environmental and physiological stimuli. Multiple studies in rodents reported that exposure to different environmental stimuli, such as chemicals, early life stress, alcohol, exercise, or antibiotics during adolescence led to long-lasting effects on microbiota composition, but this did not occur when the exposure was introduced in adulthood (Mika et al. 2015, Hu et al. 2016, Lach et al. 2020, Lopizzo et al. 2021, Vetreno et al. 2021). In humans, the gut microbial profiles of healthy adolescents and adults as well as those with obesity differed; however, the main differences in microbiota composition were found when comparing adolescents with obesity to healthy control adolescents which might indicate that obesity has a larger influence on microbial profiles during adolescence than in adulthood (Del Chierico et al. 2018). Findings from these studies suggest that the adolescent and young adult microbiome is not as stable as the adult microbiome, and environmental insults during this period may lead to long-term consequences.
Adolescent and young adult versus child
In children versus adolescents and young adults, all four included studies reported differences in the relative abundance of taxa. When classifying significantly different species, genus, family, and order level taxa at the phylum level, two studies reported decreases in the Bacteroidetes phylum, while the results for Firmicutes and Proteobacteria varied (Fig. 3) (Yuan et al. 2020, Korpela et al. 2021). Figure 3 only reports significant differences that are present in both males and females; however, sex-specific effects were identified (Korpela et al. 2021). In females, the gut microbiome became more adult-like as puberty progressed; however, the same development was not observed in males, possibly due to the later onset of puberty. Another study not only found differences in the abundances of microbes of pre-pubertal and pubertal individuals, but the abundances of several genera, including Adlercreutzia, Dorea, Ruminococcus, Clostridium, and Parabacteroides, were also associated with levels of testosterone (Yuan et al. 2020). For diversity, two studies identified higher alpha and beta diversity in the adolescent gut microbiome compared to the gut microbiome of children (Prince et al. 2019, Lif Holgerson et al. 2020); however, another included study did not identify any significant diversity differences between the two groups (Yuan et al. 2020).
Aim 2: the adolescent microbiome is different in those engaged in substance use versus controls
Only seven studies investigating the relationship between substance use and the microbiome during adolescence and young adulthood were identified (Table 1). All seven studies reported significant microbial differences in youth who used substances compared to controls.
Alcohol
Four studies reported microbial differences associated with alcohol use during adolescence (Willis et al. 2018, Vetreno et al. 2021, Segovia-Rodríguez et al. 2022, Carbia et al. 2023). The studies were conducted in humans (n = 3) and rats (n = 1) and utilized either cross-sectional (n = 3) or longitudinal (n = 1) microbiome assessments. Only one study examined the oral microbiome and all others focused on the gut (n = 3). All studies assessed differences in the abundance of taxa. Three out of four studies assessed differences in alpha and beta diversity. These three studies utilized the Shannon index to assess differences in alpha diversity. Other alpha diversity metrics included Simpson (n = 1), Chao1 (n = 2), observed species (n = 1), phylogenetic diversity (n = 1), and evenness (n = 1). Distance/dissimilarity metrics included weighted UniFrac (n = 1), Jaccard (n = 1), and Aitchison (n = 1). Only one study used functional bioinformatics. See Table 1 for details.
The longitudinal gut microbiome study in rats indicated that adolescent intermittent alcohol (AIE) treatment, a model of adolescent binge drinking, led to both immediate and lasting changes in the microbiome, and that the lasting microbial changes were also associated with alterations of enteric neurotransmitters (Vetreno et al. 2021). Immediate changes included decreases in the relative abundance of several microbes such as Dehalobacterium, Lachnospiraceae, CF231, Paraprevotella, Prevotella, and Actinobacteria and increases in the relative abundance of other microbes, including Allobaculum, Bifidobacterium, and Butyricimonas. Most of these alterations returned to control levels by adulthood due to time and/or abstinence, but the decrease of Dehalobacterium and CF231 persisted, suggesting lasting AIE-induced microbial changes. In addition, AIE treatment resulted in some microbial alterations that did not manifest until adulthood, including increases in the relative abundance of Christensenella, Streptococcus, and Rothia. No differences were found in alpha or beta diversity across aging or AIE treatment.
One of the cross-sectional human studies found associations between binge drinking and alterations in microbiome composition (Carbia et al. 2023). Importantly, this study focused on binge drinking in the absence of AUD to identify potential early microbiome markers of change. While no differences in alpha diversity were identified between young binge-drinkers and controls, beta diversity differences were related to the number of drinks per drinking session. There were also several differences in the abundances of bacteria, such as lower species of the genus Alistipes and higher Veillonella, associated with binge drinking. Recent binge drinking was associated with even more widespread changes, including alterations in Bacteroides spp., Alistipes spp., Blautia wexlerae, Ruminococcus lactaris, and Coprococcus euctactus, in addition to others. Furthermore, reductions in Ruthenibacterium lactiformans were associated with craving both at baseline and the 3-month follow-up.
Segovia-Rodríguez et al. also identified alcohol-related associations in the gut microbiome of university students (Segovia-Rodríguez et al. 2022). Similar to Carbia et al., no differences were identified in alpha diversity; however, there were differences between individuals who engage in heavy episodic drinking and controls in beta diversity. In addition, all taxonomic levels of Actinobacteria besides genus and species were increased in youth who used alcohol compared to controls.
The other cross-sectional oral microbiome study in humans did not look specifically at alcohol, but rather identified alcohol as one of the factors that impacts the oral microbiome during adolescence among other lifestyle, diet, hygiene, socioeconomic, and environmental parameters (Willis et al. 2018). Alcohol consumption during adolescence was associated with high levels of several bacterial genera including Mycoplasma, Filifactor, Treponema, and Desulfobulbus. Diversity measures were not assessed in this study.
Cannabis
One study investigated the impact of repeated cannabis exposure in adolescent mice and found significant differences in composition of the microbiome in adulthood (Wan et al. 2022); however, the direct effects of cannabis on the microbiome were not assessed during adolescence. In the WIN55,21-2 group, several genera (Acetoanaerobium, Dehalobacter, Desnuesiella, Jeotgalibacillus, Lysobacter, Mobilitalea, Prevotella, Rikenella, and Thermosyntropha) and species (Prevotella baroniae, Clostridium bolteae, Rikenella microfusus, Prevotella dentasini, Butyricicoccus faecihominis, Desnuesiella massiliensis, Blautia hydrogenotrophica, Mobilitalea sibirica, Acetoanaerobium sticklandii, Jeotgalibacillus malaysiensis, Thermosyntropha tengcongensis, Paraprevotella xylaniphila, Lysobacter oligotrophicus, and Dehalobacter restrictus) had significantly higher relative abundances than the control group. In contrast, eight genera (Adlercreutzia, Akkermansia, Dorea, Eisenbergiella, Gellertiella, Hungatella, Photobacterium, and Phyllobacterium) and nine species (Eisenbergiella massiliensis, Dorea formicigenerans, Hungatella hathewayi, Adlercreutzia muris, Blautia hominis, Gellertiella hungarica, Photobacterium damselae, Phyllobacterium loti, and Akkermansia muciniphila) had lower abundances in the WIN55,212-2 group. No differences in alpha or beta diversity were found.
Tobacco
Two studies investigating tobacco use during adolescence were identified (Kumar et al. 2011, Tishchenko et al. 2022). Both studies reported differences in microbial composition and diversity associated with tobacco use. In particular, opportunistic pathogens belonging to Fusobacterium, Cardiobacterium, Synergistes, Selenomonas, Haemophilus, Pseudomonas, and Streptococcus were higher in youth who used tobacco compared to controls. Higher alpha diversity was also associated with tobacco use in both studies, although different diversity metrics were utilized.
Discussion
This scoping review highlights differences in the adolescent and young adult gut and oral microbiomes compared to other stages of the lifespan (aim 1), as well as the effects of substance use on the gut and oral microbiomes during adolescence and young adulthood (aim 2). Accounting for adolescence/young adulthood is an important consideration for future microbiome research as most studies investigate the microbiome of children or adults, missing a critical piece of the microbiome development. This may be due to previous studies claiming that the gut microbiome achieves an adult-like composition as early as 3 years of age (Yatsunenko et al. 2012); however, the studies identified in this review indicate that both the gut and oral microbiomes are still developing during adolescence and young adulthood and may be particularly vulnerable to certain exposures. Several studies identified in aim 1 also suggest an association between sex and the microbiome (Del Chierico et al. 2018, Lif Holgerson et al. 2020, Yuan et al. 2020, Korpela et al. 2021, Lopizzo et al. 2021). Females and males differ slightly in their vulnerability to psychiatric disorders, such as substance use disorders. Increased understanding of sex differences in the microbiome, especially during adolescence when sex differences and psychiatric disorders typically emerge, may lead to advances in the understanding of risk for psychiatric disorders and lead to better treatments.
Previous studies have started to investigate the link between SUDs and the microbiome in adulthood (Temko et al. 2017, Kosciolek et al. 2021, Russell et al. 2021). Some of our adolescent and young adult substance use findings are consistent with what is reported in adult studies; however, key differences were identified as well. All three adolescent alcohol studies that assessed differences in gut alpha diversity reported null findings. This is different from adult studies that mostly report decreases in gut alpha diversity. The findings on gut beta diversity are conflicting with two studies reporting significant differences (Segovia-Rodríguez et al. 2022, Carbia et al. 2023) and one reporting null findings (Vetreno et al. 2021). It is possible these differences are due to the model species, as the two studies reporting significant findings are in humans and the one with null findings is in rats. Finally, all four studies identified differences in the abundances of several bacteria associated with alcohol use in adolescence. When classifying all significant taxa at the phylum level, differences were reported in Actinobacteria, Bacteroidetes, Firmicutes, and Desulfotomaculota. Actinobacteria was directly assessed in two studies and both reported increases with alcohol use (Vetreno et al. 2021, Segovia-Rodríguez et al. 2022), which is in line with the adult alcohol literature (Bull-Otterson et al. 2013, Lowe et al. 2017). Multiple other findings, including increases in Rothia and Allobaculum and decreases in Paraprevotella, Prevotella, and Lachnospiraceae, are also reported in adults (Bull-Otterson et al. 2013, Dubinkina et al. 2017, Puri et al. 2018, Xiao et al. 2018, Bjørkhaug et al. 2019, Ames et al. 2020, Piacentino et al. 2021, Du et al. 2022). Importantly, several of the gut microbes identified as being influenced by alcohol in adolescence were also identified in aim 1 as being different between adolescents and adults (Bifidobacterium, Prevotella, CF231, Lachnospiraceae, Christensenella, Allobaculum, Streptococcus; Fig. 4). Similar to the gut, some adolescent oral microbiome findings were comparable to those of adults while some differed. An adult AUD study reported similar findings to Willis et al. (2018) for several genera (Barb et al. 2022). Desulfobulbus was identified in AUD patients but not in control participants, and abstinence from alcohol was associated with decreases in Filifactor. However, the study reported opposite findings for Treponema, which was highly abundant in HMP participants but represented at less than 0.2% average abundance in patients with AUD (Barb et al. 2022).
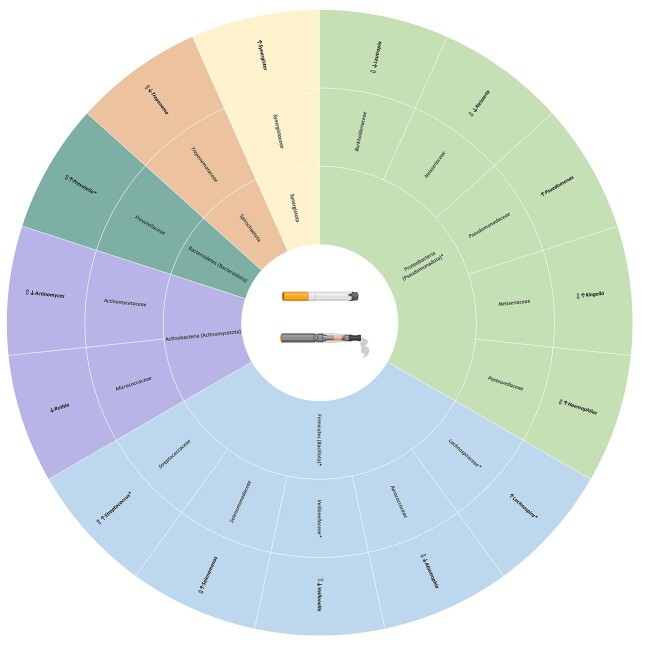
Figure 4.
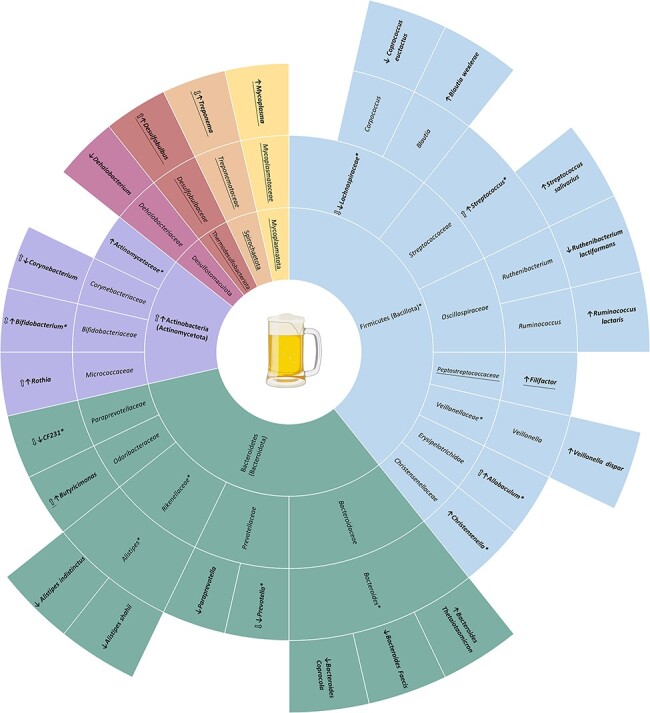
Alcohol-related microbial differences in adolescents and young adults. Taxa specifically reported in the text are in bold while associated taxonomic levels are not. Filled in arrows indicate the direction of abundance in substance using adolescents compared to adolescent controls. Arrows with no fill represent the direction of abundance reported in adult literature (Kirpich et al. 2008, Bull-Otterson et al. 2013, Tsuruya et al. 2016, Dubinkina et al. 2017, Lowe et al. 2017, Fan et al. 2018, Puri et al. 2018, Xiao et al. 2018, Bjørkhaug et al. 2019, Ames et al. 2020, Piacentino et al. 2021, Rodríguez-González et al. 2021, Yang et al. 2021, Barb et al. 2022, Du et al. 2022). Taxa marked with * were identified in aim 1 as being different in adolescent and young adults compared to adults. Underlined taxa represent oral taxa while all others are gut taxa.
Associations between cannabis use and the microbiome are vastly understudied, in both adolescents/young adults and adults (Fig. 5). The adolescent cannabis study reported increases in Prevotella, which is conflicting with previous reports in adults of decreased Prevotella (Panee et al. 2018). Prevotella was identified in aim 1 as a differentially abundant genera between adolescents and adults. In addition to this, the decrease in taxa A. muciniphila in the adolescent cannabis study is inconsistent with findings from another study in diet-induced obese mice treated with THC during adulthood, suggesting a possible age-dependent effect (Cluny et al. 2015). The adolescent study included in this review used a synthetic cannabinoid, WIN55,212-2, which is a potent CB receptor agonist instead of natural THC exposure which may also contribute to the differences in results.
Figure 5.
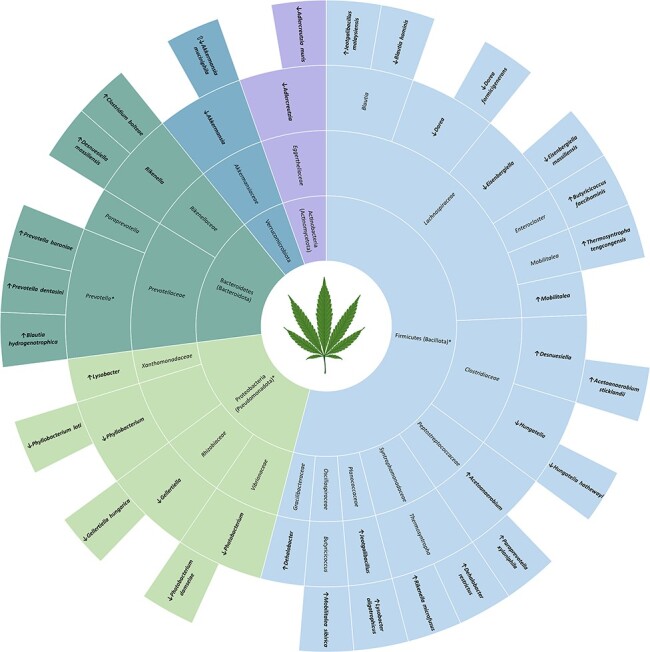
Cannabis-related gut microbial differences in adolescents and young adults. Taxa specifically reported in the text are in bold while associated taxonomic levels are not. Filled in arrows indicate the direction of abundance in substance using adolescents compared to adolescent controls. Arrows with no fill represent the direction of abundance reported in adult literature (Cluny et al. 2015, Panee et al. 2018). Taxa marked with * were identified in aim 1 as being different in adolescent and young adults compared to adults.
Finally, the findings from the adolescent tobacco study are consistent with findings in adult humans showing tobacco use impacts the oral microbiome, particularly increased diversity and levels of opportunistic pathogens (Fig. 6) (Mason et al. 2015, Wu et al. 2016). Increased Streptococcus is reported in both adolescent/young adult and adult tobacco use despite being one of the genera identified in aim 1 as different between adolescents and adults. There were also several genera uniquely associated with tobacco use in the adolescent studies compared to those in adults. Differences in these findings may be due to age; however, they could also be due to heated tobacco rather than burning through conventional methods.
Figure 6.
Tobacco-related oral microbial differences in adolescents and young adults. Taxa specifically reported in the text are in bold while associated taxonomic levels are not. Filled in arrows indicate the direction of abundance in substance using adolescents compared to adolescent controls. Arrows with no fill represent the direction of abundance reported in adult literature (Wu et al. 2016, Vallès et al. 2018, Al-Zyoud et al. 2019, Chopyk et al. 2021, Jia et al. 2021, Suzuki et al. 2022). Taxa marked with * were identified in aim 1 as being different in adolescent and young adults compared to adults.
Gaps in literature
This review identified a substantial gap in the literature on how substance use during adolescence/young adulthood influences the microbiome. Across rodents, non-human primates, and humans, only seven studies on the adolescent and young adult microbiome and substance use were identified (four on alcohol use, one on cannabis use, and two on tobacco use), which highlights the fact that more research on this age group is needed. In addition, many of the microbes that were associated with substance use during adolescence/young adulthood (aim 2) were also identified as being different between adolescents/young adults and adults (aim 1).
Another gap identified in this scoping review is the lack of studies assessing the oral microbiome. Out of the 26 studies identified through both searches, only 5 investigated changes in the oral microbiome. Compared to the gut microbiota, the oral microbiota is understudied, especially regarding SUDs. Several studies have documented extensive transmission of microbes from the mouth to the gut (Schmidt et al. 2019), and oral-derived bacteria have been shown to colonize the intestines (Atarashi et al. 2017, Du Teil Espina et al. 2019). The oral cavity is a promising target for future microbiome studies as the oral cavity is the start of the gastrointestinal tract, is linked both physically and chemically to the gut, and has close proximity to the brain. In addition, given the ease and flexibility of collection, the oral microbiome may be a favorable alternative to classic stool collection, but much additional research is needed.
Finally, this review points to the inconsistencies among studies in microbiome methods. For identification of microbes, most studies (n = 21) utilized 16S rRNA sequencing; however, several different variable regions were used. Given this inconsistency, it is hard to generalize the results across studies as there may be inaccuracies in reported bacterial community compositions due to amplification bias of the targeted hypervariable region. This limitation could be avoided by using shotgun metagenomic sequencing which involves sequencing random fragments of DNA that contain a mixture of both bacterial and host DNA. This method is preferred as it allows for taxonomic profiling, metabolic function profiling, and antibiotic resistance gene profiling (Jovel et al. 2016, Laudadio et al. 2018); however, it is more expensive than amplicon sequencing and requires a large amount of input DNA, the availability of reference genome sequences, and more rigorous IRB approval for human studies. In addition to using different variable regions, some studies identified taxa using sequence-based clustering (producing OTUs), while others used denoising methods (producing ASVs). ASV approaches allow for more precise identification of microbes, and therefore, there are many arguments that the field should be moving away from OTU-based analyses (Callahan et al. 2017). Other methods for bacterial identification and quantification included microbiota array- and culture-based counting methods. While these methods eliminate the need for high-throughput sequencing and are more convenient, efficient, and affordable, they are not as precise and may not be effective at identifying the presence of novel microbes or known but unculturable microbes. Finally, several different diversity metrics were used throughout the studies. Each diversity measure has unique characteristics, advantages, and disadvantages. The wide range of methods and indices used among the studies in this scoping review limits the generalizability of the findings. Expert recommendations and guidelines for the analysis and reporting of the microbiome should be referenced to guide future work (Mirzayi et al. 2021, Bastiaanssen et al. 2022).
Limitations
This scoping review provides a comprehensive summary of studies comparing the adolescent/young adult and young adult microbiome with other stages of the lifespan, as well as the impact of substance use on the microbiome during adolescence and young adulthood. However, this review should be read within the context of certain caveats. First, there is no agreed upon age range for adolescence/young adulthood. For this review, we defined adolescence and young adults as ages 10–25 years in humans, 2–7 years in non-human primates, and PND 21–65 in rodents to be comprehensive; however, this led to some studies with overlapping age ranges (Table 1). In addition, it is a strength that we included studies in multiple model species including rodents, non-human primates, and humans to synthesize all the available data; however, it is not completely clear how the timepoints of rodents and non-human primates translate into human developmental stages. It is also important to consider that host genetics influence the microbiome and may limit cross-species comparisons. The studies identified in this review also varied in study design and protocols which may hinder the identification of consistent changes. Only 13 of the 26 included studies included both sexes, and of those only 8 controlled for sex or investigated sex-specific effects. Out of the 12 human studies, 9 controlled for antibiotic use, 7 controlled for or examined diet, and 1 controlled for psychiatric disorders. Future longitudinal studies that control for these variables, incorporate species level data, and include multi-omic techniques such as transcriptomics, proteomics, and/or metabolomics will answer important questions on trajectories of normal microbial development and different factors that may lead to microbial changes. Finally, as a scoping review, there was no formal assessment of the methodological quality of the studies included. The goal of this review was not to provide a systematic review of the available literature, but rather provide an up-to-date integration of two different topic areas, including (i) differences in the microbiome between youth and other life stages and (ii) substance-associated microbial changes in adolescence and young adulthood to draw attention to this understudied area of research.
Conclusion
The adolescent and young adult microbiome is an understudied area of research, especially in the context of youth substance use. The studies reviewed here suggest that the adolescent and young adult oral and gut microbiomes contain distinct microbial profiles compared to other stages of development. In addition, the adolescent and young adult microbiome appears to be particularly sensitive to different stimuli, including several commonly used substances during adolescence. Given the differences identified in this review, it is vital to tailor future microbiota research to adolescent and young adult populations rather than generalizing from child and adult literature.
Microbiome manipulation may be a potential treatment or adjunctive therapy for neuropsychiatric disorders such as SUDs (Chinna Meyyappan et al. 2020, Verma et al. 2020, Pizarro et al. 2021); however, it is first important to understand how the microbiome is influenced by substances during the adolescent/young adult period. There are currently only seven microbiome-related studies, across species, investigating substance use during adolescence and young adulthood and there are no human adolescent or young adult studies with a longitudinal design. Future studies should aim to characterize changes associated with the oral and gut microbiome during adolescence/young adulthood to determine if there are consistent changes. If consistent changes are noted across studies, certain bacterial strains can be targeted and tested, as probiotics or prebiotics could potentially be given to promote the growth of certain bacteria. Pathogenic bacteria can also be eliminated through techniques like antibiotic administration or phage therapy. In addition to characterizing microbial signatures of change, emphasis should also be placed on the functional profiles of microbes as changes may be taxonomically distinct but function in similar ways or vice versa. In this case, therapeutic development may focus on targeting microbiome-related metabolomic products, administration of microbial products, and/or genetic engineering of microbes for certain functions. While much work still needs to be done, understanding the connection between the adolescent/young adult microbiome and SUDs may further elucidate the pathophysiology of SUDs and offer an avenue for future therapeutic prevention and intervention options.
Conflict of interest: None declared.
Contributor Information
Brittney D Browning, Department of Psychiatry and Behavioral Sciences, Medical University of South Carolina, 67 President St., Charleston, SC 29425, United States; Department of Neuroscience, Medical University of South Carolina, 173 Ashley Ave., Charleston, SC 29425, United States.
Anna E Kirkland, Department of Psychiatry and Behavioral Sciences, Medical University of South Carolina, 67 President St., Charleston, SC 29425, United States.
Rejoyce Green, Department of Psychiatry and Behavioral Sciences, Medical University of South Carolina, 67 President St., Charleston, SC 29425, United States.
Melinda Engevik, Department of Regenerative Medicine and Cell Biology, Medical University of South Carolina, 173 Ashley Ave., Charleston SC, 29425, United States.
Alexander V Alekseyenko, Department of Public Health Sciences, Biomedical Informatics Center, Medical University of South Carolina, 135 Cannon St., Charleston, SC 29425, United States.
Lorenzo Leggio, Clinical Psychoneuroendocrinology and Neuropsychopharmacology Section, Translational Addiction Medicine Branch, National Institute on Drug Abuse Intramural Research Program and National Institute on Alcohol Abuse and Alcoholism Division of Intramural Clinical and Biological Research, National Institutes of Health, Baltimore, Maryland, USA.
Rachel L Tomko, Department of Psychiatry and Behavioral Sciences, Medical University of South Carolina, 67 President St., Charleston, SC 29425, United States.
Lindsay M Squeglia, Department of Psychiatry and Behavioral Sciences, Medical University of South Carolina, 67 President St., Charleston, SC 29425, United States.
Funding
B.B. is supported by F31AA030920. L.L. is supported by the NIH Intramural Research Program (NIDA/NIAAA).
Data availability
All acquired data is included in the article and is publicly available in the original articles.
References
- (SAMHSA), S A A M H S A . Reports and Detailed Tables from the 2021 National Survey on Drug Use and Health. Rockville: Substance Abuse and Mental Health Administration, 2021. [Google Scholar]
- Akkasheh G, Kashani-Poor Z, Tajabadi-Ebrahimi M. et al. Clinical and metabolic response to probiotic administration in patients with major depressive disorder: a randomized, double-blind, placebo-controlled trial. Nutrition 2016;32:315–20. 10.1016/j.nut.2015.09.003. [DOI] [PubMed] [Google Scholar]
- Allen AP, Hutch W, Borre YE. et al. Bifidobacterium longum 1714 as a translational psychobiotic: modulation of stress, electrophysiology and neurocognition in healthy volunteers. Transl Psychiatry 2016;6:e939–9. 10.1038/tp.2016.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Zyoud W, Hajjo R, Abu-Siniyeh A. et al. Salivary microbiome and cigarette smoking: a first of its kind investigation in Jordan. Int J Environ Res Public Health 2019;17:1–20. 10.3390/ijerph17010256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames NJ, Barb JJ, Schuebel K. et al. Longitudinal gut microbiome changes in alcohol use disorder are influenced by abstinence and drinking quantity. Gut Microbes 2020;11:1608–31. 10.1080/19490976.2020.1758010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angoa-Pérez M, Kuhn DM. Evidence for modulation of substance use disorders by the gut microbiome: hidden in plain sight. Pharmacol Rev 2021;73:571–96. 10.1124/pharmrev.120.000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arain M, Haque M, Johal L. et al. Maturation of the adolescent brain. Neuropsychiatr Dis Treat 2013;9:449–61. 10.2147/NDT.S39776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arksey H, O’malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol 2005;8:19–32. 10.1080/1364557032000119616. [DOI] [Google Scholar]
- Atarashi K, Suda W, Luo C. et al. Ectopic colonization of oral bacteria in the intestine drives TH1 cell induction and inflammation. Science 2017;358:359–65. 10.1126/science.aan4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baghaie H, Kisely S, Forbes M. et al. A systematic review and meta-analysis of the association between poor oral health and substance abuse. Addiction 2017;112:765–79. 10.1111/add.13754. [DOI] [PubMed] [Google Scholar]
- Bajaj JS, Gavis EA, Fagan A. et al. A randomized clinical trial of fecal microbiota transplant for alcohol use disorder. Hepatology 2021;73:1688–700. 10.1002/hep.31496. [DOI] [PubMed] [Google Scholar]
- Barb JJ, Maki KA, Kazmi N. et al. The oral microbiome in alcohol use disorder: a longitudinal analysis during inpatient treatment. J Oral Microbiol 2022;14:2004790. 10.1080/20002297.2021.2004790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastiaanssen TF, Quinn TP, Loughman A. Treating bugs as features: a compositional guide to the statistical analysis of the microbiome-gut-brain axis. 2022; arXiv preprint arXiv:2207.12475.
- Bjørkhaug ST, Aanes H, Neupane SP. et al. Characterization of gut microbiota composition and functions in patients with chronic alcohol overconsumption. Gut Microbes 2019;10:663–75. 10.1080/19490976.2019.1580097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowland GB, Weyrich LS. The oral-microbiome-brain axis and neuropsychiatric disorders: an anthropological perspective. Front Psych 2022;13:1–12. 10.3389/fpsyt.2022.810008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo Javier A, Forsythe P, Chew Marianne V. et al. Ingestion of lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci 2011;108:16050–5. 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull-Otterson L, Feng W, Kirpich I. et al. Metagenomic analyses of alcohol induced pathogenic alterations in the intestinal microbiome and the effect of Lactobacillus rhamnosus GG treatment. PloS One 2013;8:e53028. 10.1371/journal.pone.0053028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan BJ, Mcmurdie PJ, Holmes SP. Exact sequence variants should replace operational taxonomic units in marker-gene data analysis. ISME J 2017;11:2639–43. 10.1038/ismej.2017.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbia C, Bastiaanssen TFS, Iannone LF. et al. The microbiome-gut-brain axis regulates social cognition & craving in young binge drinkers. EBioMedicine 2023;89:104442. 10.1016/j.ebiom.2023.104442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahwan B, Kwan S, Isik A. et al. Gut feelings: a randomised, triple-blind, placebo-controlled trial of probiotics for depressive symptoms. J Affect Disord 2019;253:317–26. 10.1016/j.jad.2019.04.097. [DOI] [PubMed] [Google Scholar]
- Chinna Meyyappan A, Forth E, Wallace CJK. et al. Effect of fecal microbiota transplant on symptoms of psychiatric disorders: a systematic review. BMC Psychiatry 2020;20:299. 10.1186/s12888-020-02654-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopyk J, Bojanowski CM, Shin J. et al. Compositional differences in the oral microbiome of E-cigarette users. Front Microbiol 2021;12:599664. 10.3389/fmicb.2021.599664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cluny NL, Keenan CM, Reimer RA. et al. Prevention of diet-induced obesity effects on body weight and gut microbiota in mice treated chronically with Δ9-tetrahydrocannabinol. PloS One 2015;10:e0144270. 10.1371/journal.pone.0144270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covidence Systematic Review Software [Online]. Veritas Health Innovation. Melbourne, Australia. www.covidence.org (accessed June 1, 2023). [Google Scholar]
- Cryan JF, O’riordan KJ, Cowan CSM. et al. The microbiota-gut-brain axis. Physiol Rev 2019;99:1877–2013. 10.1152/physrev.00018.2018. [DOI] [PubMed] [Google Scholar]
- Dawson DA, Goldstein RB, Patricia Chou S. et al. Age at first drink and the first incidence of adult-onset DSM-IV alcohol use disorders. Alcohol Clin Exp Res 2008;32:2149–60. 10.1111/j.1530-0277.2008.00806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Chierico F, Abbatini F, Russo A. et al. Gut microbiota markers in obese adolescent and adult patients: age-dependent differential patterns. Front Microbiol 2018;9:1–12. 10.3389/fmicb.2018.01210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Teil Espina M, Gabarrini G, Harmsen HJM. et al. Talk to your gut: the oral-gut microbiome axis and its immunomodulatory role in the etiology of rheumatoid arthritis. FEMS Microbiol Rev 2019;43:1–18. 10.1093/femsre/fuy035. [DOI] [PubMed] [Google Scholar]
- Du Y, Li L, Gong C. et al. The diversity of the intestinal microbiota in patients with alcohol use disorder and its relationship to alcohol consumption and cognition. Front Psych 2022;13:1–12. 10.3389/fpsyt.2022.1054685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan J, Yin B, Li W. et al. Age-related changes in microbial composition and function in cynomolgus macaques. Aging 2019;11:12080–96. 10.18632/aging.102541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubinkina VB, Tyakht AV, Odintsova VY. et al. Links of gut microbiota composition with alcohol dependence syndrome and alcoholic liver disease. Microbiome 2017;5:141. 10.1186/s40168-017-0359-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellickson PL, Tucker JS, Klein DJ. Ten-year prospective study of public health problems associated with early drinking. Pediatrics 2003;111:949–55. 10.1542/peds.111.5.949. [DOI] [PubMed] [Google Scholar]
- Fadus MC, Squeglia LM, Valadez EA. et al. Adolescent substance use disorder treatment: an update on evidence-based strategies. Curr Psychiatry Rep 2019;21:96. 10.1007/s11920-019-1086-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X, Peters BA, Jacobs EJ. et al. Drinking alcohol is associated with variation in the human oral microbiome in a large study of American adults. Microbiome 2018;6:59. 10.1186/s40168-018-0448-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Raikhel V, Gopalakrishnan K. et al. Effect of postnatal low-dose exposure to environmental chemicals on the gut microbiome in a rodent model. Microbiome 2016;4:26–6. 10.1186/s40168-016-0173-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh JW, Roh TY. Opportunistic detection of Fusobacterium nucleatum as a marker for the early gut microbial dysbiosis. BMC Microbiol 2020;20:208. 10.1186/s12866-020-01887-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwauchi M, Horigome A, Ishikawa K. et al. Relationship between oral and gut microbiota in elderly people. Immun Inflamm Dis 2019;7:229–36. 10.1002/iid3.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y-J, Liao Y, He Y-Q. et al. Association between oral microbiota and cigarette smoking in the Chinese population. Front Cell Infect Microbiol 2021;11:1–10. 10.3389/fcimb.2021.658203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovel J, Patterson J, Wang W. et al. Characterization of the gut microbiome using 16S or shotgun metagenomics. Front Microbiol 2016;7:459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang M, Mischel RA, Bhave S. et al. The effect of gut microbiome on tolerance to morphine mediated antinociception in mice. Sci Rep 2017;7:42658. 10.1038/srep42658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JW, Lee JS, Kim JH. et al. Comparison of microbiota variation in Korean healthy adolescents with adults suggests notable maturity differences. Omics 2018;22:770–8. 10.1089/omi.2018.0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiraly DD, Walker DM, Calipari ES. et al. Alterations of the host microbiome affect behavioral responses to cocaine. Sci Rep 2016;6:35455. 10.1038/srep35455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirpich IA, Solovieva NV, Leikhter SN. et al. Probiotics restore bowel flora and improve liver enzymes in human alcohol-induced liver injury: a pilot study. Alcohol 2008;42:675–82. 10.1016/j.alcohol.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korpela K, Kallio S, Salonen A. et al. Gut microbiota develop towards an adult profile in a sex-specific manner during puberty. Sci Rep 2021;11:23297. 10.1038/s41598-021-02375-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosciolek T, Victor TA, Kuplicki R. et al. Individuals with substance use disorders have a distinct oral microbiome pattern. Brain Behav Immun - Health 2021;15:100271. 10.1016/j.bbih.2021.100271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar PS, Matthews CR, Joshi V. et al. Tobacco smoking affects bacterial acquisition and colonization in oral biofilms. Infect Immun 2011;79:4730–8. 10.1128/IAI.05371-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lach G, Fülling C, Bastiaanssen TFS. et al. Enduring neurobehavioral effects induced by microbiota depletion during the adolescent period. Transl Psychiatry 2020;10:382. 10.1038/s41398-020-01073-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laudadio I, Fulci V, Palone F. et al. Quantitative assessment of shotgun metagenomics and 16S rDNA amplicon sequencing in the study of human gut microbiome. OMICS J Integr Biol 2018;22:248–54. 10.1089/omi.2018.0013. [DOI] [PubMed] [Google Scholar]
- Leclercq S, Cani PD, Neyrinck AM. et al. Role of intestinal permeability and inflammation in the biological and behavioral control of alcohol-dependent subjects. Brain Behav Immun 2012;26:911–8. 10.1016/j.bbi.2012.04.001. [DOI] [PubMed] [Google Scholar]
- Leclercq S, Matamoros S, Cani PD. et al. Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc Natl Acad Sci 2014;111:E4485–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclercq S, Stärkel P, Delzenne NM. et al. The gut microbiota: a new target in the management of alcohol dependence? Alcohol 2019;74:105–11. 10.1016/j.alcohol.2018.03.005. [DOI] [PubMed] [Google Scholar]
- Lee K, Vuong HE, Nusbaum DJ. et al. The gut microbiota mediates reward and sensory responses associated with regimen-selective morphine dependence. Neuropsychopharmacology 2018;43:2606–14. 10.1038/s41386-018-0211-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Wang Q, Wang Y. et al. Oral probiotics ameliorate the behavioral deficits induced by chronic mild stress in mice via the gut microbiota-inflammation axis. Front Behav Neurosci 2018;12:266. 10.3389/fnbeh.2018.00266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Ge Y, Cheng L. et al. Oral bacteria colonize and compete with gut microbiota in gnotobiotic mice. Int J Oral Sci 2019;11:10. 10.1038/s41368-018-0043-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Zhao K, Chen J. et al. Diurnal changes of the oral microbiome in patients with alcohol dependence. Front Cell Infect Microbiol 2022;12:1–13. 10.3389/fcimb.2022.1068908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao JF, Hsu CC, Chou GT. et al. Lactobacillus paracasei PS23 reduced early-life stress abnormalities in maternal separation mouse model. Benef Microbes 2019;10:425–36. 10.3920/BM2018.0077. [DOI] [PubMed] [Google Scholar]
- Liao Y, Tong X-T, Jia Y-J. et al. The effects of alcohol drinking on oral microbiota in the Chinese population. Int J Environ Res Public Health 2022;19:5729. 10.3390/ijerph19095729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lif Holgerson P, Esberg A, Sjödin A. et al. A longitudinal study of the development of the saliva microbiome in infants 2 days to 5 years compared to the microbiome in adolescents. Sci Rep 2020;10:9629. 10.1038/s41598-020-66658-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D, Hutchison KE, Portillo S. et al. Association between the oral microbiome and brain resting state connectivity in smokers. Neuroimage 2019;200:121–31. 10.1016/j.neuroimage.2019.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Quintero C, Pérez De Los Cobos J, Hasin DS. et al. Probability and predictors of transition from first use to dependence on nicotine, alcohol, cannabis, and cocaine: results of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). Drug Alcohol Depend 2011;115:120–30. 10.1016/j.drugalcdep.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopizzo N, Marizzoni M, Begni V. et al. Social isolation in adolescence and long-term changes in the gut microbiota composition and in the hippocampal inflammation: implications for psychiatric disorders – Dirk Hellhammer award paper 2021. Psychoneuroendocrinology 2021;133:105416. 10.1016/j.psyneuen.2021.105416. [DOI] [PubMed] [Google Scholar]
- Lowe PP, Gyongyosi B, Satishchandran A. et al. Alcohol-related changes in the intestinal microbiome influence neutrophil infiltration, inflammation and steatosis in early alcoholic hepatitis in mice. PloS One 2017;12:e0174544. 10.1371/journal.pone.0174544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Z, Fitting S, Robinson C. et al. Chronic cannabis smoking-enriched oral pathobiont drives behavioral changes, macrophage infiltration, and increases β-amyloid protein production in the brain. EBioMedicine 2021;74:103701. 10.1016/j.ebiom.2021.103701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maley S, Hovey K, Wactawski-Wende J. et al. Alcohol consumption and the diversity of the oral microbiome in postmenopausal women. Curr Dev Nutr 2022;6:927–7. 10.1093/cdn/nzac067.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marotta A, Sarno E, Del Casale A. et al. Effects of probiotics on cognitive reactivity, mood, and sleep quality. Front Psych 2019;10:164–4. 10.3389/fpsyt.2019.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MR, Preshaw PM, Nagaraja HN. et al. The subgingival microbiome of clinically healthy current and never smokers. ISME J 2015;9:268–72. 10.1038/ismej.2014.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mckernan DP, Fitzgerald P, Dinan TG. et al. The probiotic Bifidobacterium infantis 35624 displays visceral antinociceptive effects in the rat. Neurogastroenterol Motil 2010;22:1029–e268. 10.1111/j.1365-2982.2010.01520.x. [DOI] [PubMed] [Google Scholar]
- Meckel KR, Kiraly DD. A potential role for the gut microbiome in substance use disorders. Psychopharmacology (Berl) 2019;236:1513–30. 10.1007/s00213-019-05232-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mika A, Van Treuren W, González A. et al. Exercise is more effective at altering gut microbial composition and producing stable changes in lean mass in juvenile versus adult male F344 rats. PloS One 2015;10:e0125889. 10.1371/journal.pone.0125889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzayi C, Renson A, Zohra F. et al. Reporting guidelines for human microbiome research: the STORMS checklist. Nat Med 2021;27:1885–92. 10.1038/s41591-021-01552-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morshedi M, Valenlia KB, Hosseinifard ES. et al. Beneficial psychological effects of novel psychobiotics in diabetic rats: the interaction among the gut, blood and amygdala. J Nutr Biochem 2018;57:145–52. 10.1016/j.jnutbio.2018.03.022. [DOI] [PubMed] [Google Scholar]
- Murray E, Sharma R, Smith KB. et al. Probiotic consumption during puberty mitigates LPS-induced immune responses and protects against stress-induced depression- and anxiety-like behaviors in adulthood in a sex-specific manner. Brain Behav Immun 2019;81:198–212. 10.1016/j.bbi.2019.06.016. [DOI] [PubMed] [Google Scholar]
- Pallikkuth S, Mendez R, Russell K. et al. Age associated microbiome and microbial metabolites modulation and its association with systemic inflammation in a rhesus macaque model. Front Immunol 2021;12:748397. 10.3389/fimmu.2021.748397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panee J, Gerschenson M, Chang L. Associations between microbiota, mitochondrial function, and cognition in chronic marijuana users. J Neuroimmune Pharmacol 2018;13:113–22. 10.1007/s11481-017-9767-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng X, Cheng L, You Y. et al. Oral microbiota in human systematic diseases. Int J Oral Sci 2022;14:14. 10.1038/s41368-022-00163-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piacentino D, Grant-Beurmann S, Vizioli C. et al. Gut microbiome and metabolome in a non-human primate model of chronic excessive alcohol drinking. Transl Psychiatry 2021;11:609. 10.1038/s41398-021-01728-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto-Sanchez MI, Hall GB, Ghajar K. et al. Probiotic Bifidobacterium longum NCC3001 reduces depression scores and alters brain activity: a pilot study in patients with irritable bowel syndrome. Gastroenterology 2017;153:448–459.e8. 10.1053/j.gastro.2017.05.003. [DOI] [PubMed] [Google Scholar]
- Pizarro N, Kossatz E, González P. et al. Sex-specific effects of synbiotic exposure in mice on addictive-like behavioral alterations induced by chronic alcohol intake are associated with changes in specific gut bacterial taxa and brain tryptophan metabolism. Front Nutr 2021;8:1–20. 10.3389/fnut.2021.750333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince AL, Pace RM, Dean T. et al. The development and ecology of the Japanese macaque gut microbiome from weaning to early adolescence in association with diet. Am J Primatol 2019;81:e22980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor L, Lotempio J, Marquitz A. et al. A review of 10 years of human microbiome research activities at the US National Institutes of Health, fiscal years 2007-2016. Microbiome 2019;7:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri P, Liangpunsakul S, Christensen JE. et al. The circulating microbiome signature and inferred functional metagenomics in alcoholic hepatitis. Hepatology 2018;67:1284–302. 10.1002/hep.29623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pushalkar S, Paul B, Li Q. et al. Electronic cigarette aerosol modulates the oral microbiome and increases risk of infection. iScience 2020;23:100884. 10.1016/j.isci.2020.100884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reveles K, Patel S, Forney L. et al. Age-related changes in the marmoset gut microbiome: REVELES. Am J Primatol 2019;81:e22960. 10.1002/ajp.22960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-González A, Vitali F, Moya M. et al. Effects of alcohol binge drinking and oleoylethanolamide pretreatment in the gut microbiota. Front Cell Infect Microbiol 2021;11:731910. 10.3389/fcimb.2021.731910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell JT, Zhou Y, Weinstock GM. et al. The gut microbiome and substance use disorder. Front Neurosci 2021;15:1–8. 10.3389/fnins.2021.725500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salavrakos M, Leclercq S, De Timary P. et al. Microbiome and substances of abuse. Prog Neuropsychopharmacol Biol Psychiatry 2021;105:110113. 10.1016/j.pnpbp.2020.110113. [DOI] [PubMed] [Google Scholar]
- Savignac HM, Kiely B, Dinan TG. et al. Bifidobacteria exert strain-specific effects on stress-related behavior and physiology in BALB/c mice. Neurogastroenterol Motil 2014;26:1615–27. 10.1111/nmo.12427. [DOI] [PubMed] [Google Scholar]
- Schmidt TS, Hayward MR, Coelho LP. et al. Extensive transmission of microbes along the gastrointestinal tract. Elife 2019;8:1–18. 10.7554/eLife.42693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segovia-Rodríguez L, Echeverry-Alzate V, Rincón-Pérez I. et al. Gut microbiota and voluntary alcohol consumption. Transl Psychiatry 2022;12:146. 10.1038/s41398-022-01920-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sgro M, Iacono G, Yamakawa GR. et al. Age matters: microbiome depletion prior to repeat mild traumatic brain injury differentially alters microbial composition and function in adolescent and adult rats. PloS One 2022;17:e0278259. 10.1371/journal.pone.0278259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson S, Mclellan R, Wellmeyer E. et al. Drugs and bugs: the gut-brain axis and substance use disorders. J Neuroimmune Pharmacol 2022;17:33–61. 10.1007/s11481-021-10022-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenman LK, Patterson E, Meunier J. et al. Strain specific stress-modulating effects of candidate probiotics: a systematic screening in a mouse model of chronic restraint stress. Behav Brain Res 2020;379:112376. 10.1016/j.bbr.2019.112376. [DOI] [PubMed] [Google Scholar]
- Sun Y, Geng W, Pan Y. et al. Supplementation with Lactobacillus kefiranofaciens ZW3 from Tibetan kefir improves depression-like behavior in stressed mice by modulating the gut microbiota. Food Funct 2019;10:925–37. 10.1039/C8FO02096E. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Nakano Y, Yoneda M. et al. The effects of cigarette smoking on the salivary and tongue microbiome. Clin Exp Dent Res 2022;8:449–56. 10.1002/cre2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temko JE, Bouhlal S, Farokhnia M. et al. The microbiota, the gut and the brain in eating and alcohol use disorders: a “ménage à ’trois’? Alcohol Alcohol 2017;52:403–13. 10.1093/alcalc/agx024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomaz AC, Iyer V, Woodward TJ. et al. Fecal microbiota transplantation and antibiotic treatment attenuate naloxone-precipitated opioid withdrawal in morphine-dependent mice. Exp Neurol 2021;343:113787. 10.1016/j.expneurol.2021.113787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillisch K, Labus J, Kilpatrick L. et al. Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology 2013;144:1394–1401.e4. 10.1053/j.gastro.2013.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tishchenko OV, Kryvenko LS, Gargina VV. Influence of smoking heating up tobacco products and e-cigarettes on the microbiota of dental plaque. Pol Merkur Lekarski 2022;50:16–20. [PubMed] [Google Scholar]
- Tsuruya A, Kuwahara A, Saito Y. et al. Ecophysiological consequences of alcoholism on human gut microbiota: implications for ethanol-related pathogenesis of colon cancer. Sci Rep 2016;6:27923. 10.1038/srep27923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursell LK, Metcalf JL, Parfrey LW. et al. Defining the human microbiome. Nutr Rev 2012;70:S38–44. 10.1111/j.1753-4887.2012.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallès Y, Inman CK, Peters BA. et al. Types of tobacco consumption and the oral microbiome in the United Arab Emirates Healthy Future (UAEHFS) pilot study. Sci Rep 2018;8:11327. 10.1038/s41598-018-29730-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma H, Phian S, Lakra P. et al. Human gut microbiota and mental health: advancements and challenges in microbe-based therapeutic interventions. Indian J Microbiol 2020;60:405–19. 10.1007/s12088-020-00898-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetreno RP, Massey V, Crews FT. Long-lasting microbial dysbiosis and altered enteric neurotransmitters in adult rats following adolescent binge ethanol exposure. Addict Biol 2021;26:e12869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan X, Eguchi A, Qu Y. et al. Gut–microbiota–brain axis in the vulnerability to psychosis in adulthood after repeated cannabis exposure during adolescence. Eur Arch Psychiatry Clin Neurosci 2022;272:1297–309. 10.1007/s00406-022-01437-1. [DOI] [PubMed] [Google Scholar]
- Wang X, Mi Q, Yang J. et al. Effect of electronic cigarette and tobacco smoking on the human saliva microbial community. Braz J Microbiol 2022;53:991–1000. 10.1007/s42770-022-00721-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Guo X, Yue Q. et al. Exploring the role and mechanism of gut microbiota in methamphetamine addiction using antibiotic treatment followed by fecal microbiota transplantation. Anat Rec 2023;306:1149–64. 10.1002/ar.25055. [DOI] [PubMed] [Google Scholar]
- Wei CL, Wang S, Yen JT. et al. Antidepressant-like activities of live and heat-killed Lactobacillus paracasei PS23 in chronic corticosterone-treated mice and possible mechanisms. Brain Res 2019;1711:202–13. 10.1016/j.brainres.2019.01.025. [DOI] [PubMed] [Google Scholar]
- Willis JR, González-Torres P, Pittis AA. et al. Citizen science charts two major “stomatotypes” in the oral microbiome of adolescents and reveals links with habits and drinking water composition. Microbiome 2018;6:218. 10.1186/s40168-018-0592-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstenholme JT, Saunders JM, Smith M. et al. Reduced alcohol preference and intake after fecal transplant in patients with alcohol use disorder is transmissible to germ-free mice. Nat Commun 2022;13:6198. 10.1038/s41467-022-34054-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Peters BA, Dominianni C. et al. Cigarette smoking and the oral microbiome in a large study of American adults. ISME J 2016;10:2435–46. 10.1038/ismej.2016.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao HW, Ge C, Feng GX. et al. Gut microbiota modulates alcohol withdrawal-induced anxiety in mice. Toxicol Lett 2018;287:23–30. 10.1016/j.toxlet.2018.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Chen W, Sun Y. et al. Effects of cadmium on organ function, gut microbiota and its metabolomics profile in adolescent rats. Ecotoxicol Environ Saf 2021;222:112501. 10.1016/j.ecoenv.2021.112501. [DOI] [PubMed] [Google Scholar]
- Yatsunenko T, Rey FE, Manary MJ. et al. Human gut microbiome viewed across age and geography. Nature 2012;486:222–7. 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X, Chen R, Zhang Y. et al. Gut microbiota: effect of pubertal status. BMC Microbiol 2020;20:334–4. 10.1186/s12866-020-02021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yussof A, Yoon P, Krkljes C. et al. A meta-analysis of the effect of binge drinking on the oral microbiome and its relation to Alzheimer’s disease. Sci Rep 2020;10:19872. 10.1038/s41598-020-76784-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Hu Y, Li C. et al. Transplantation of fecal microbiota from patients with alcoholism induces anxiety/depression behaviors and decreases brain mGluR1/PKC ε levels in mouse. Biofactors 2020;46:38–54. 10.1002/biof.1567. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All acquired data is included in the article and is publicly available in the original articles.