Abstract
Background
To facilitate the shift from risk-factor management to primordial prevention of cardiovascular disease, the American Heart Association developed guidelines to score and track cardiovascular health (CVH). How the prevalence and trajectories of a high level of CVH across the life course compare among high- and lower-income countries is unknown.
Methods
Nationally representative survey data with CVH variables (physical activity, cigarette smoking, body mass index, blood pressure, blood glucose, and total cholesterol levels) were identified in Ethiopia, Bangladesh, Brazil, England, and the US for adults (aged 18–69 years and not pregnant). Data were harmonized, and CVH metrics were scored using the American Heart Association guidelines, as high (2), moderate (1), or low (0), with the prevalence of high scores (better CVH) across the life course compared across countries.
Results
Among 28,092 adults (Ethiopia n = 7686, 55.2% male; Bangladesh n = 6731, 48.4% male; Brazil n = 7241, 47.9% male; England n = 2691, 49.5% male, and the US n = 3743, 50.3% male), the prevalence of high CVH scores decreased as country income level increased. Declining CVH with age was universal across countries, but differences were already observable in those aged 18 years. Excess body weight appeared to be the main driver of poor CVH in higher-income countries, and the prevalence of current smoking was highest in Bangladesh.
Conclusions
Our findings suggest that CVH decline with age may be universal. Interventions to promote and preserve CVH throughout the life course are needed in all populations, tailored to country-specific time courses of the decline. In countries where the level of CVH remains relatively high, protection of whole societies from risk-factor epidemics may still be feasible.
Résumé
Contexte
Afin de faciliter la transition de la prise en charge des facteurs de risque vers la prévention primordiale des maladies cardiovasculaires, l’American Heart Association a élaboré des lignes directrices en vue de mesurer la santé cardiovasculaire (SCV) et d’en faire le suivi. On ignore dans quelle mesure la prévalence et la trajectoire d’un niveau élevé de SCV au cours d’une vie se comparent entre les pays à revenu élevé et les pays à plus faible revenu.
Méthodologie
Des résultats de sondages représentatifs des pays concernant les variables de la SCV (activité physique, tabagisme, indice de masse corporelle, pression artérielle, glycémie et taux de cholestérol total) ont été obtenus de l’Éthiopie, du Bangladesh, du Brésil, de l’Angleterre et des États-Unis, pour des adultes âgés de 18 à 69 ans, excluant les femmes enceintes. Les données ont été harmonisées, et la SCV a été mesurée conformément aux lignes directrices de l’American Heart Association, et notée en fonction des scores suivants : élevée (2), modérée (1) ou faible (0). La prévalence de scores élevés, soit une meilleure SCV tout au long de la vie, a été comparée entre les pays.
Résultats
Parmi 28 092 adultes (Éthiopie, n = 7 686, 55,2 % de sexe masculin; Bangladesh, n = 6 731, 48,4 % de sexe masculin; Brésil, n = 7 241, 47,9 % de sexe masculin; Angleterre, n = 2 691, 49,5 % de sexe masculin, et États-Unis, n = 3 743, 50,3 % de sexe masculin), la prévalence de scores correspondant à une SCV élevée diminuait à mesure que le niveau de revenu du pays augmentait. La diminution de la SCV avec l’âge était universelle dans tous les pays, mais des différences étaient déjà observables chez les personnes âgées de 18 ans. Un surplus de poids corporel semblait être le principal facteur d’une faible SCV dans les pays à revenu plus élevé; la prévalence d’un tabagisme actuel était la plus élevée au Bangladesh.
Conclusions
Nos observations laissent croire que le déclin de la SCV avec l’âge pourrait être universel. Il est nécessaire de mener des interventions adaptées à la progression du déclin dans chacun des pays en vue de favoriser et de préserver la SCV tout au long de la vie, et ce, dans toutes les populations. Dans les pays où le niveau de SCV demeure relativement élevé, il pourrait être encore possible de protéger des sociétés entières contre des épidémies liées aux facteurs de risque.
Globally, cardiovascular diseases (CVD) remain the leading cause of mortality and an important contributor to morbidity.1 In terms of years of life lost, diminished quality of life, as well as direct and indirect medical costs, the burden of CVD is enormous.2 As a result of ongoing epidemiologic transitions, almost 80% of global CVD-related deaths occur in low-income countries (LICs) and middle-income countries (together, LMICs).1 Given that health systems are overwhelmed, shifts to primary prevention (risk-factor modification), and ultimately primordial prevention (avoidance of risk-factor onset), are increasing, as a means to maintain good cardiovascular health (CVH) for as long as possible and reduce the burden of CVD.2
In 2010, the American Heart Association defined ideal CVH as a combination of 7 metrics— “Life’s Simple 7” (LS7: dietary quality, physical activity [PA], exposure to cigarette smoke, body mass index [BMI], blood pressure [BP], blood glucose, and total cholesterol). All metrics can be characterized as either ideal (2 points), intermediate (1 point), or poor (0 points), to generate a combined CVH score range of 0-14 points, with good CVH defined as having 5-7 metrics at an ideal level.2 In June 2020, the American Heart Association introduced an updated CVH score—"Life’s Essential 8” (LE8), which revised assessment methods for some components of LS7, added an 8th new metric (sleep), and revised the scoring system (with scores 0-100) for each metric and overall CVH.3
Previous studies have shown that ideal CVH is strongly associated with a lower risk of CVD morbidity and mortality, and with all-cause mortality in young, middle-aged, and older adults.4, 5, 6, 7, 8, 9, 10 However, the prevalence of ideal CVH typically decreases with age, and by adulthood, it is generally low, although evidence from LMICs is lacking.10,11 To better understand the differences in CVD burden between high-income countries (HICs) and LMICs, population-level data are needed that capture the CVH metrics of interest, including health behaviours and sociodemographic profiles. Large population health surveys, as opposed to clinical data, have the potential to inform countries’ health planning and promotion strategies, and their understanding of the future burden of poor CVH, while identifying subgroups that are at particular risk.12
A nationally representative, population-based survey in Venezuela showed that two-thirds of the population had poor or only intermediate CVH,13 and an analysis of population CVH using the Brazilian Health Survey suggested that over half (56.7%) of the population had ideal CVH.14 However, differences in the use and application of the CVH score in these neighbouring countries obscures country comparisons. Additionally, much of the prior research investigating CVH has originated from HICs, including the US, where less than 20% of adults are reported to have ideal CVH.15 Therefore, this research sought to explore the availability and comparability of nationally representative adult CVH data from 5 countries, ranging in income level from high to low, and to compare the CVH prevalence and patterns of decline with age. The aim was to test the hypothesis that the decline in CVH with age is universal, although this decline may differ in starting level and rate of change, potentially revealing influential factors and inflection points that may be discernible as points of intervention.
Methods
Data sources and study design
We purposely selected 5 countries that were diverse along dimensions of geographic location, per-capita income, burden of disease, educational systems, and health-system capacity. Population surveys were assessed for the presence of the 8 CVH metrics. The World Health Organization (WHO) STEPwise (STEPS) surveys were an important source of data covering over 120 countries from across all WHO world regions.16 Datasets from Bangladesh and Ethiopia were selected for analysis, representing LICs and LMICs from Africa and South Asia.
The suitability of the WHO-STEPS survey for CVH assessment is perhaps unsurprising, as it was designed as a standardized noncommunicable disease (NCD) risk surveillance tool, with a manual for collecting, analyzing, and disseminating data.17 As such, WHO-STEPS data are designed to be directly comparable among implementing countries, many of which are LICs. To undertake a comparison of these data with data from upper-middle-income countries and HICs, the US National Health and Nutrition Examination Survey (NHANES), the Health Survey for England (HSE). and the Brazilian Pesquisa Nacional de Saúde (PNS) were identified as nationally representative surveys with CVH data available. Together, these 5 countries span a range from low to high income as defined by the World Bank,18 with England and the US being HICs, Brazil being an upper-middle-income country, Bangladesh being a lower-middle-income country, and Ethiopia being an LIC.
Permission was requested from respective data owners, and access was granted to cross-sectional survey data from these 5 countries: WHO-STEPS surveys from Bangladesh (2018) and Ethiopia (2015)16,17,19; US NHANES (pre-pandemic) 2017-202020; HSE 201621; and PNS from Brazil, 2013.14,22 These 5 countries also were selected as examples with varying structural systems that may influence lifetime CVH. For example, Bangladesh has only 5 years of compulsory education; Ethiopia has 8 years; England has 11 years; the US has 12 years; and Brazil has 14 years.23 Health expenditure also varies, from $71 per capita in Ethiopia to almost $11,000 per capita in the US.24 The causes of mortality in the 5 countries also vary, with the percentage of deaths from maternal and child health, nutrition, or communicable disease at 45% of deaths in Ethiopia and 5% in the US.25
All surveys obtained ethical permission from relevant national and/or institutional ethics committees before implementation. For this specific secondary data analysis, ethical approval was granted from the University of the Witwatersrand Medical Human Research Ethics Committee [Ref: M220437].
Surveys and participants
In combination, the 5 selected surveys included 49,774 participants, distributed as follows: STEPS Bangladesh (2018)26—8185 adults (aged 18-69 years); STEPS Ethiopia 201519—9251 adolescents and adults (aged 15-69 years); NHANES (US) 2017-202020—15,560 children and adults (aged 0-80 years); HSE (England) 201627—7826 children and adults (aged 0-90 years); and PNS (Brazil) 201322—8952 adults (aged 18-104 years). For both the NHANES and HSE, data collection occurred in stages. The HSE included a home interview and a nurse visit, and participants in the NHANES undertook home interviews, health assessments at a mobile examination centre, clinical examinations, and laboratory investigations. Not all participants are selected for or completed all stages. Only those participants with survey, health and clinical examination, and lab analysis (as evidenced by data on total cholesterol, fasting blood glucose or glycated hemoglobin (HbA1c), BMI, BP, PA, and smoking) from the NHANES were included in this analysis. For the HSE, only participants with a home interview and nurse visit who agreed to give blood were included. Participants who were pregnant, were aged under 18 or over 69 years, or had missing data, were excluded.
Cardiovascular health data and harmonization
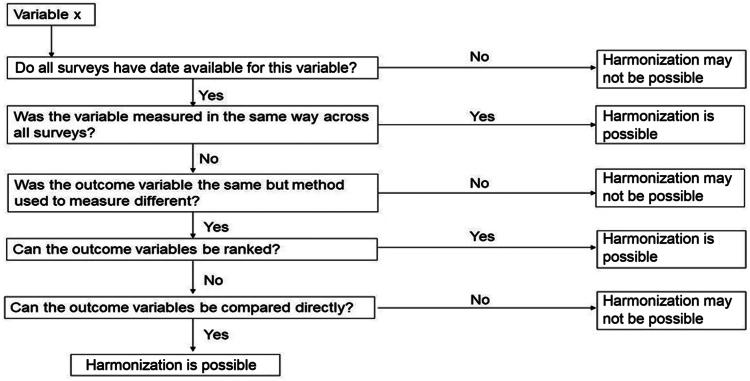
Availability of CVH variables (dietary quality, sleep, PA, cigarette smoking, BMI, BP, blood glucose or HbA1c, and total cholesterol levels) and the methods of data collection were compared across surveys to make decisions regarding harmonization (Fig. 1). The data collection methods for each of the CVH metrics are reviewed briefly in Supplemental Table S1, with the full methodology for each survey described elsewhere.14,16,17,19, 20, 21, 22,26, 27, 28, 29 Data that were considered sufficiently comparable were harmonized (Supplemental Table S1). Harmonized variables were also created for education and current medication use prescribed to reduce BP, glucose, or cholesterol (Supplemental Fig. S1), with age and sex data extracted from each survey.
Figure 1.
Harmonization decision matrix. Adapted from Schaap et al.30 under Creative Commons Attribution 2.0 Generic (CC BY 2.0 DEED) license.
Data analysis
The data available on dietary quality and sleep could not be harmonized. The 6 remaining CVH metrics (PA, cigarette smoking, BMI, BP, blood glucose and/or HbA1c, and total cholesterol levels) were harmonized and categorized as shown in Table 1 (and Supplemental Table S1), based on both the LS7 and LE8 guidelines.2,3 This secondary data analysis was conceived under the LS7 paradigm; however, during the process of analysis, the LE8 criteria were published, and therefore, we utilized elements of both LS7 and LE8 that best fit with our datasets. A score of 2 was allocated for each CVH metric rated as being in the “high” category; 1 was assigned for “moderate,” and 0 for “low.” Scores were summed to create a combined CVH score, categorized as follows: for 6 CVH metrics, a score of 9-12 was high CVH; a score of 6-8 was moderate CVH; and a score of < 6 was low CVH. For 5 CVH metrics, a score of 8-10 was high CVH, 5-7 was moderate CVH, and < 5 was low CVH. Statistical analyses were performed with SPSS, version 28 (IBM, Armonk, NY). Participant characteristics were described by country, with results reported as number of participants (n) or a weighted percentage (%), using weights developed by each of the surveys as described elsewhere.19,26,31 Normality of data was determined with visual inspection of histograms. The prevalence of a high level of CVH was determined for each country, comparing categorical variables using χ2 tests and comparing weighted prevalence (arithmetic mean ± standard deviation) of each CVH metric across countries using analysis of variance. To create CVH trajectories, age was categorized into 5-year bands, and the median of each age band was plotted against the weighted age-specific prevalence of high CVH rates.
Table 1.
Cardiovascular health (CVH) scores for adults aged 18-69 years, based on Lloyd-Jones et al. (2010 and 2022)2,3
| CVH metric, by CVH score (high, moderate, low) | CVH scores | ||
|---|---|---|---|
| Life’s Simple 7 (LS7) | Life’s Essential 8 (LE8)∗ | Adapted/combined† | |
| Body mass index, kg/m2 | |||
| High | <25 | < 25 | As LS7 & LE8 |
| Moderate | 25–29.9 | 25–29.9 | As LS7 & LE8 |
| Low | ≥ 30 | ≥ 30 | As LS7 & LE8 |
| Blood pressure, mmHg | |||
| High | <120/80 (– meds) | < 120/80 (+/–) | As LS7 |
| Moderate | 120–139/80–89 (– meds) or TTG | 120–129/ < 80 (+/–) or 130–139/80–89 (–) | 120–139/80–89 (–) or < 140/90 (+) |
| Low | ≥ 140/90 (+/– meds) | 130–139/80–89 (+) or ≥ 140/90 (+/–) | As LS7 |
| Cholesterol level, mg/dL (blood lipids) | |||
| High | Total cholesterol ˂ 200 (–) | Non-HDL cholesterol < 130 (+/–) | As LS7 |
| Moderate | 200–239 (–) or TTG | 130–159 (–) | 200–239 (–) or < 240 (+) |
| Low | ≥ 240 | 130–159 (+) or ≥ 160 (+/–) | ≥ 240 (+/–) |
| Fasting blood glucose, mg/dL | |||
| High | <100 (–) | < 100 and no history of diabetes | As LS7 |
| Moderate | 100–125 (–) or TTG | 100–125 and no diabetes | 100–125 (–) or < 126 (+) |
| Low | ≥ 126 | ≥ 126 or diabetes | ≥ 126 (+/–) |
| HbA1c, % | |||
| High | NA | No history of diabetes and < 5.7 | < 5.7 (–) |
| Moderate | NA | No diabetes and 5.7–6.4 | 5.7-6.4 (–) or < 6.5 (+)‡ |
| Low | NA | ≥ 6.5 or diabetes | ≥ 6.5 (+/–) |
| Nicotine exposure (self-reported use) | |||
| High | Never smoked or quit > 12 mo | Never used combustible tobacco or inhaled NDSs | As LS7 |
| Moderate | Former ≤ 12 mo | Former ≥ 1 y or if living with active indoor smoker at home, former ≥ 5 y | As LS7 |
| Low | Current smoker | Current combustible tobacco or NDS use or former < 1 y | As LS7 |
| Physical activity, self-reported min/wk | |||
| High | ≥ 150 MVPA or ≥ 75 VPA | ≥ 90 MVPA | As LS7 |
| Moderate | 1–149 MVPA or 1–74 VPA | 60–89 MVPA | As LS7 |
| Low | None | < 60 MVPA | As LS7 |
| Healthy diet (self-reported) | |||
| High | 4–5 components | ≥ 75th percentile (populations) or MEPA score 12–16 (individuals) | NI |
| Moderate | 2–3 components | 50 – 74th percentile or MEPA score 8–11 | NI |
| Low | 0–1 components | < 50th percentile or MEPA score < 8 | NI |
| Sleep health (self-reported average hours of sleep per night) | |||
| High | NI | 7 - < 10 h | NI |
| Moderate | NI | 6 - < 7 h | NI |
| Low | NI | < 6 or ≥ 10 h | NI |
(+), (–), and (+/–) indicate with, without, and with or without medicines, respectively.
HbA1c, glycated hemoglobin; HDL, high-density lipoprotein; MEPA, Mediterranean Eating Pattern for Americans questionnaires; MPA, moderate physical activity; MVPA, moderate-vigorous physical activity; NA, not applicable; NDS, nicotine delivery system; NI, not included; TTG, treated to goal; VPA, vigorous physical activity.
LE8 is scored on a 0–100 scale, with scores of 80–100 considered a high level of CVH, 50–79 considered a moderate level of CVH, and 0–49 points considered a low level of CVH.
The CVH score was created from either a combination of LS7 and LE8 guidelines or adapted to be consistent with LS7 and/or LE8 guidelines, as indicated.
Glycemia assessed using either fasting glucose or HbA1c. Moderate HbA1c scoring applied to include TTG to align with analysis of fasting blood glucose data.
Results
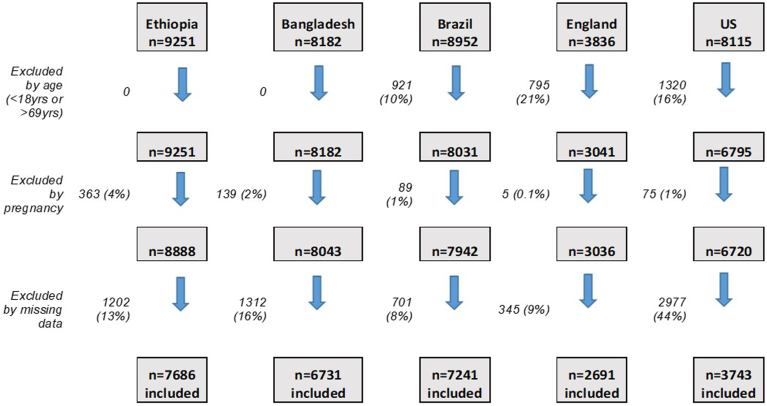
Among the 49,774 participants (aged 0-104 years) across the surveys, 28,092 adults (aged 18-69 years) met the inclusion criteria for this secondary data analysis (Fig. 2). Exclusion by age ranged from 0% in the STEPS surveys, to 21% of the survey population in the HSE; exclusion for pregnancy was ≤ 4% across all surveys. The most frequently observed missing data in the WHO-STEPS and PNS surveys were the glycemia and cholesterol measures (Bangladesh, both glycemia and cholesterol 14% missing; Ethiopia, glycemia 10%, and cholesterol 9% missing; Brazil, both glycemia and cholesterol 4% missing). For BMI and BP, respectively, the percentage of cases with missing data were as follows: Bangladesh, 2% and 0.3%; Ethiopia, 5% and 1%; and Brazil, 1% and 1%. For smoking and PA, respectively, the percentage of cases with missing data were generally low (Bangladesh, 0% and 2%; Ethiopia, 0.1% and 3%; and Brazil, 0.1% and 1%). Following exclusion of participants based on age and/or pregnancy, the percentage of cases excluded due to missing data ranged from 8% of the survey sample in Brazil to 44% in the US. Missing data in the US and England were due primarily to missing information on current treatment use for BP (US, 47%), cholesterol (US, 47%) or glycemia (US, 22%) and/or measurements of BP (England, 38%), cholesterol (England, 52%) or glycemia (England, 53%), and/or missing sociodemographic data (US: age and sex, 50%; education, 52%; England: education, 22%).
Figure 2.
Consort diagram of participants included in the analysis.
Included participants for each country were as follows: Ethiopia n = 7686 (55.2% male); Bangladesh n = 6731 (48.4% male); Brazil n = 7241 (47.9% male); England n = 2691 (49.5% male); and the US n = 3743 (50.3% male; Table 2). Education level varied significantly between LMICs and HICs, with 88% of participants having attended primary school or less in Ethiopia, compared to just 3% at the same educational level in the US. The age distribution of the surveys also varied, with more younger adults in the lower-income than in the higher-income countries (% of sample aged 18-35 years: Ethiopia, 60%; Bangladesh, 48.7%; Brazil, 33.5%; England, 25.0%; US, 29.6%). Significant differences among countries occurred in the population levels of all weighted mean CVH metrics. The highest reported levels of PA were in Ethiopia. Including both those on treatment and those with no treatment, the lowest systolic BP was observed in the US, the lowest diastolic BP was observed in England, the lowest BMI, cholesterol, and glucose levels were observed in Ethiopia, and the lowest HbA1c level was observed in Brazil. The level of medication use for BP, glycemia, and cholesterol was typically higher in England, the US, and Brazil than in Ethiopia and Bangladesh, although the prevalence of treatment use for hypertension was lower in England, compared to both Brazil and the US. Treatment levels for hyperlipidemia and for diabetes generally tracked with the mean population levels of these metrics, ie, treatment increased as population mean levels increased. The exceptions to this finding were the US, which showed the second-highest mean total cholesterol level, but the highest percentage on treatment for lipids (more than twice that of England), and Brazil, which showed the lowest mean HbA1c levels and the highest prevalence of treatment for diabetes (twice that of England and the US).
Table 2.
Characteristics of the adult participants included in the analysis for each country
| Characteristic | Ethiopia (n = 7686) | Bangladesh (n = 6731) | Brazil (n = 7241) | England (n = 2691) | USA (n = 3743) | P |
|---|---|---|---|---|---|---|
| Age, y, range | 18 – 69 | 18 – 69 | 18 – 69 | 18 – 69 | 18 – 69 | < 0.001 |
| 18–24 | 1388 (26.9) | 717 (20.7) | 540 (9.20) | 153 (8.40) | 428 (11.5) | |
| 25–34 | 2442 (33.1) | 1739 (28.0) | 1502 (24.3) | 408 (16.6) | 577 (18.1) | |
| 35–44 | 1794 (18.8) | 1904 (18.9) | 1806 (22.7) | 523 (20.5) | 612 (17.3) | |
| 45–54 | 1119 (12.2) | 1356 (13.6) | 1626 (21.0) | 614 (22.7) | 728 (19.6) | |
| 55–64 | 654 (6.60) | 812 (13.4) | 1268 (16.7) | 669 (21.6) | 985 (23.8) | |
| 65–69 | 298 (2.60) | 203 (5.30) | 499 (6.10) | 324 (10.2) | 413 (9.6) | |
| Male sex | 3219 (55.2) | 3073 (48.4) | 3042 (47.9) | 1194 (49.5) | 1826 (50.3) | < 0.001 |
| Highest education level | < 0.001 | |||||
| Primary school or less | 6791 (88.4) | 5282 (78.0) | 3737 (45.9) | NA | 245 (3.00) | |
| Secondary or high school | 493 (6.90) | 1050 (17.3) | 2370 (36.1) | 610 (21.4) | 1205 (32.1) | |
| College or university | 7 (0.10) | 399 (4.70) | 1134 (18.0) | 1699 (65.3) | 2143 (62.3) | |
| Body mass index, kg/m2 | 20.4 ± 2.97 (a; e; f; g) | 22.5 ± 4.35 (a; b; c; d) | 26.5 ± 5.15 (d; g; i; j) | 27.1 ± 5.29 (c; f; h; j) | 30.0 ± 7.54 (b; e; h; i) | < 0.001 |
| Underweight | 1775 (23.5) | 909 (14.8) | 169 (2.4) | 33 (1.4) | 56 (1.6) | |
| Healthy weight | 5120 (70.0) | 3855 (60.1) | 2818 (40.1) | 955 (37.3) | 871 (24.5) | |
| Overweight / obese | 791 (6.5) | 1967 (25.1) | 4254 (57.5) | 1703 (61.3) | 2816 (73.4) | |
| Systolic BP, mmHg | 121 ± 17.94 (a; e; f; g) | 121 ± 18.29 (a; b; c; d) | 124 ± 17.85 (d; g; i) | 124 ± 15.12 (c; f; h) | 119 ± 15.95 (b; e; h; i) | < 0.001 |
| Diastolic BP, mmHg | 78 ± 11.70 (a; e; f) | 78 ± 11.78 (a; b; c) | 78 ± 11.36 (i; j) | 73 ± 10.57 (c; f; j) | 74 ± 10.84 (b; e; i) | < 0.001 |
| On treatment for BP | 120 (1.1) | 545 (6.0) | 1125 (15.1) | 303 (9.5) | 1030 (23.1) | < 0.001 |
| Total cholesterol, mg/dL | 131 ± 31.95 (a; e; f; g) | 170 ± 37.48 (a; b; c; d) | 184 ± 38.62 (d; g; j) | 198 ± 41.68 (c; f; h; j) | 186 ± 41.48 (b; e; h) | < 0.001 |
| On treatment for lipids | 8 (0.0) | 76 (0.8) | 648 (3.7) | 279 (8.2) | 738 (18.2) | < 0.001 |
| Glucose, mg/dL | 79.6 ± 20.24 (a; e; f; g) | 97.5 ± 31.67 (a; b; c; d) | NA | NA | NA | < 0.001 |
| HbA1c, % | NA | NA | 5.47 ± 0.95 (d; g) | 5.53 ± 0.76 (c; f) | 5.76 ± 1.10 (b; e) | < 0.001 |
| On treatment for diabetes | 51 (0.5) | 243 (2.9) | 284 (8.1) | 111 (4.1) | 191 (3.9) | < 0.001 |
| Total active min/wk | 2111 ± 1839 (a; e; f; g) | 1462 ± 1983 (a; b; c; d) | 71 ± 174 (d; g; i; j) | 521 ± 688 (c; f; h; j) | 897 ± 1232 (b; e; h; i) | < 0.001 |
Values are n (%), or mean (± standard deviation), unless otherwise indicated. Weighted values are reported for mean ± standard deviation and for percentages. For smoking prevalence, see Table 3 (low = current smoker, moderate = former smoker, high = never smoker). Letters in italics denote statistical significance at P < 0.001 between the groups sharing the same letter (eg, a indicates the significance between Bangladesh and Ethiopia, b indicates significance between Bangladesh and USA, etc).
BP, blood pressure; HbA1c, glycated hemoglobin; NA, data not available.
The mean CVH score for each country and the prevalence of high CVH (for all 6 metrics combined and for each metric) are shown in Table 3. Based on the combination of all 6 metrics, Brazil had the lowest mean CVH score (7.7 of 12), with 38.7% of the population having a high CVH score, and Ethiopia had the highest (10.5 of 12), with 91.2% of the population having a high CVH score. An examination of each of the CVH metrics showed clear patterns among countries, regarding income level, BMI, and cholesterol—as country income level increased, both BMI and cholesterol scores decreased. Glycemia showed a similar trend, with less-prevalent low glycemia scores (≥ 126 mg/dL fasting blood glucose or ≥ 6.5% HbA1c) in the lowest-income country (Ethiopia, 2%) and the highest prevalence in the US (12.6%). However, Bangladesh, England, and Brazil showed similar levels, ranging from 5.2% to7.1%, with low scores for glycemia.
Table 3.
Prevalence of high, moderate, and low cardiovascular health (CVH) scores by country study population
| CVH metric, by CVH score (low, moderate, high) | Ethiopia (n = 7686) | Bangladesh (n = 6731) | Brazil (n = 7241) | England (n = 2691) | US (n = 3743) | P |
|---|---|---|---|---|---|---|
| BMI | ||||||
| Low | 179 (1.2) | 446 (5.1) | 1607 (22.0) | 710 (24.7) | 1704 (43.2) | < 0.001 |
| Moderate | 612 (5.3) | 1521 (20.0) | 2647 (35.5) | 993 (36.6) | 1112 (30.8) | |
| High | 6895 (93.5) | 4764 (74.9) | 2987 (42.5) | 988 (38.7) | 927 (26.1) | |
| BP | ||||||
| Low | 1599 (19.4) | 1520 (19.5) | 1475 (21.7) | 487 (16.3) | 692 (14.7) | < 0.001 |
| Moderate | 2942 (38.4) | 2397 (35.5) | 2923 (41.3) | 499 (17.4) | 1583 (41.8) | |
| High | 3145 (42.2) | 2814 (45.0) | 2843 (37.0) | 1705 (66.3) | 1468 (43.5) | |
| Total cholesterol | ||||||
| Low | 104 (0.7) | 363 (4.6) | 636 (8.3) | 520 (16.8) | 359 (10.6) | < 0.001 |
| Moderate | 369 (3.1) | 1157 (16.0) | 2118 (28.0) | 1017 (33.4) | 1388 (36.4) | |
| High | 7213 (96.2) | 5211 (79.4) | 4487 (63.7) | 1154 (49.7) | 1996 (53.0) | |
| Glycemia | ||||||
| Low | 199 (2.0) | 568 (7.1) | 486 (6.5) | 143 (5.2) | 656 (12.6) | < 0.001 |
| Moderate | 844 (9.3) | 1493 (20.8) | 1588 (20.8) | 606 (19.3) | 1084 (25.0) | |
| High | 6643 (88.7) | 4670 (72.1) | 5167 (72.7) | 1942 (75.5) | 2003 (62.4) | |
| Smoking | ||||||
| Low | 435 (4.8) | 1585 (24.2) | 899 (13.4) | 450 (16.7) | 561 (13.3) | < 0.001 |
| Moderate | 165 (2.2) | 518 (7.1) | 178 (2.0) | 935 (33.4) | 177 (4.2) | |
| High | 7086 (93.0) | 4628 (68.7) | 6164 (84.5) | 1306 (49.9) | 3005 (82.5) | |
| PA | ||||||
| Low | 461 (3.3) | 412 (6.3) | 5121 (67.9) | 444 (15.9) | 809 (18.0) | < 0.001 |
| Moderate | 607 (7.6) | 668 (9.5) | 980 (15.0) | 386 (14.1) | 801 (22.2) | |
| High | 6618 (89.1) | 5651 (84.3) | 1140 (17.0) | 1861 (70.0) | 2133 (59.8) | |
| Combined CVH score, including PA | ||||||
| Mean ± SD | 10.5 ± 1.40 | 9.3 ± 1.82 | 7.7 ± 2.06 | 8.3 ± 2.28 | 7.8 ± 2.28 | |
| High | 7011 (91.2) | 4813 (71.5) | 2804 (38.7) | 1351 (50.2) | 1513 (40.4) | < 0.001 |
| Combined CVH score, excluding PA | ||||||
| Mean ± SD | 8.7 ± 1.24 | 7.6 ± 1.71 | 7.2 ± 1.84 | 6.7 ± 2.01 | 6.4 ± 2.02 | |
| High | 7400 (96.3) | 5709 (84.4) | 4071 (56.2) | 1756 (65.3) | 2101 (56.1) | < 0.001 |
| Combined CVH score, excluding underweight adults | ||||||
| Mean ± SD, including —PA | 10.4 ± 1.44 | 9.2 ± 1.84 | 7.7 ± 2.06 | 8.2 ± 2.28 | 7.7 ± 2.27 | |
| High, including PA | 5319 (90.0) | 4008 (68.8) | 2690 (38.0) | 1329 (50.0) | 1470 (39.9) | < 0.001 |
| Mean ± SD, excluding PA | 8.6 ± 1.27 | 7.4 ± 1.73 | 7.2 ± 1.84 | 6.7 ± 2.01 | 6.4 ± 2.01 | |
| High, excluding PA | 5654 (95.7) | 4838 (83.1) | 3933 (55.6) | 1728 (65.0) | 2051 (55.6) | < 0.001 |
Values are n (%), unless otherwise indicated.
CVH score (range: 0–12) was the result of the sum of points (0, 1, or 2) for 6 metrics, including physical activity (PA) or 5 metrics, excluding PA (range: 0–10). Bold values denote statistical significance between the categories (P < 0.001). Weighted values are reported for all percentages, except for high CVH score.
BMI, body mass index; BP, blood pressure; DBP, diastolic BP; MVPA, moderate-vigorous PA; SBP, systolic BP; SD, standard deviation.
BP was also more similar across the countries, with 14.7%-21.7% of individuals scoring low (BP ≥ 140/90 mm Hg). However, the pattern in England appeared to be different, with fewer individuals in the moderate BP category (prehypertensive or hypertensive and treated to goal), and relatively more in the high CVH BP category (< 120/80 mm Hg untreated), compared to other countries. To investigate this finding further, we revisited the medication use for BP in England and found that, although 303 (9.5% of adults) were taking medication prescribed for BP, a higher number of adults (n = 371; 11.6% of adults) reported taking medications that were known to influence BP yet were not prescribed specifically for BP. This situation may have affected the recorded BPs but would not be a factor in the classification of BP for the CVH score, potentially inflating the number of individuals classified as scoring high for BP. Other surveys did not have such data.
Although in Ethiopia, Brazil, and the US, over 80% of the population had never smoked (high scores), this percentage was lower in England (49%) and in Bangladesh (69%). In England, this finding was due to a high prevalence of former smokers (33.4%), whereas in Bangladesh, this was due to a higher prevalence of current smokers (24.2%).
The prevalence of low PA scores ranged from 3% to 18% in all countries, except for Brazil (67.9%). To further investigate this large difference, we again reviewed the PA self-reported instruments used. Further examination of the methodology revealed that the PA instrument used in Brazil queried about only leisure-time activity, whereas both the Global Physical Activity Questionnaire (GPAQ) and the International Physical Activity Questionnaire (IPAQ) used in other countries queried about PA across multiple domains, including work, leisure time, and travel. Due to the risk that PA results were not comparable, country median CVH scores and percentage ideal CVH were recalculated, excluding PA. Following the removal of the PA metric, the mean CVH scores decreased for all countries, with Ethiopia still having the highest mean score (8.7 of 10), but with the US moving to the lowest overall CVH scoring position (6.4 of 10). Additionally, the percentage of individuals classified as having a high CVH score based on these 5 metrics increased for all countries, ranging from 56% in both the US and Brazil to 96% in Ethiopia.
Examining the BMI data further, we investigated the percentage of individuals who were scored as having a high level of CVH but had an underweight BMI (< 18.5 kg/m2). This percentage was particularly high in Ethiopia, with underweight individuals making up one quarter (25.7%; n = 1775) of those adults scoring high for BMI (< 25 kg/m2). An analysis that excluded those individuals classified as underweight did not significantly affect the mean country CVH scores (Ethiopia 10.5 ± 1.40, including all adults; 10.4 ± 1.44, excluding underweight adults) or the percentage of adults scoring high for CVH (Ethiopia 91.2%, including all adults; 90.0%, excluding underweight adults).
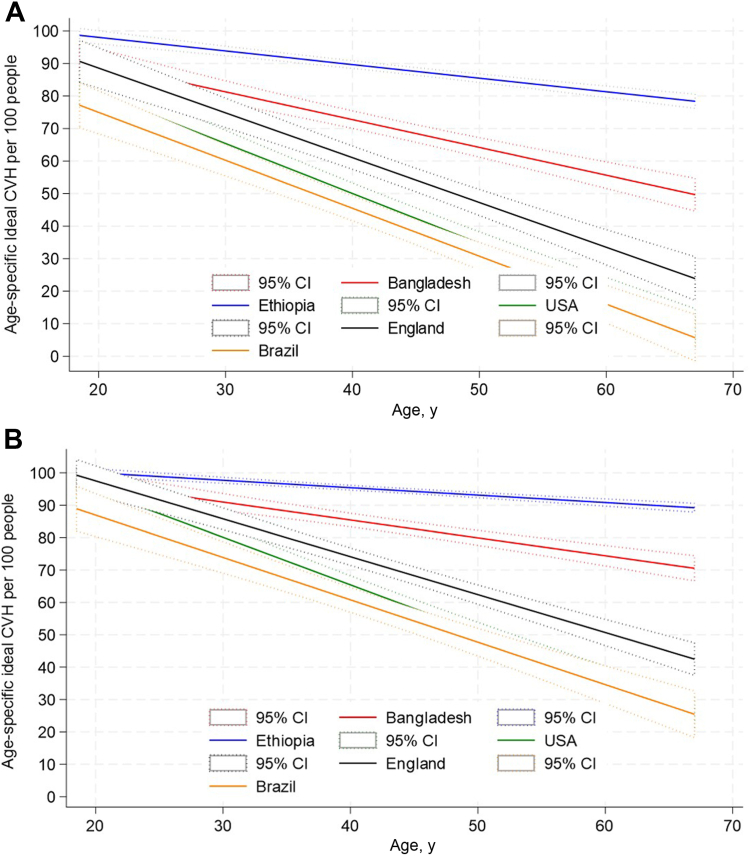
The prevalence and trajectories of a high level of CVH by age, starting in adolescence (age 18 years), were then plotted by country, both including and excluding the PA metric, for comparison (Fig. 3, A and B). In all countries, a decline in high CVH score with aging was observed, as was our initial hypothesis. The levels of high CVH score observed in adolescents also varied by country, as per our initial hypothesis. When PA was included, levels of high CVH scores in adolescence were highest in Ethiopia, followed by Bangladesh, England, the US, and then Brazil. This ordering was maintained across the adult age span, but with steeper declines observed in Brazil, the US, and England, compared to Bangladesh and Ethiopia, such that marked differences occurred in the levels of high CVH score by age 69 years among countries, and the LICs appeared to perform much better than the HICs.
Figure 3.
(A) Age-specific ideal cardiovascular health (CVH) rates for the 5 countries (adults aged 18–69 years)—based on 6 metrics (blood pressure, body mass index, cholesterol, glycemia, smoking, physical activity) (B) Age-specific ideal CVH ratesfor the 5 countries (adults aged 18–69 years)—based on 5 metrics (blood pressure, body mass index, cholesterol, glycemia, smoking). CI, confidence interval
With PA excluded, Ethiopia continued to have a high starting point and a shallower decline in high CVH score across the life course, although the relative advantage among adolescents appeared also in Bangladesh and the US, and to a lesser extent England, with Brazil starting at a lower point than other countries, indicating that relatively fewer adolescents from Brazil enter adulthood with high CVH scores. The steepest declines in CVH were observed in the highest-income countries and followed the order of country income level—that is, the US, then England and Brazil, followed by Bangladesh, and last, Ethiopia. However, the overall pattern in all countries and in both sets of analyses (Fig. 3, A and B) was the same, showing a decreased prevalence of high CVH score as age increased.
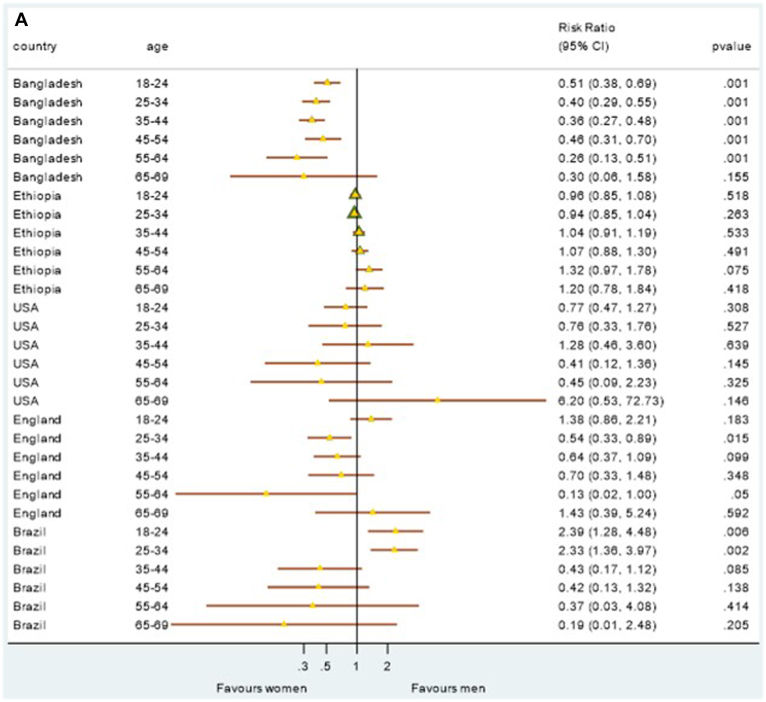
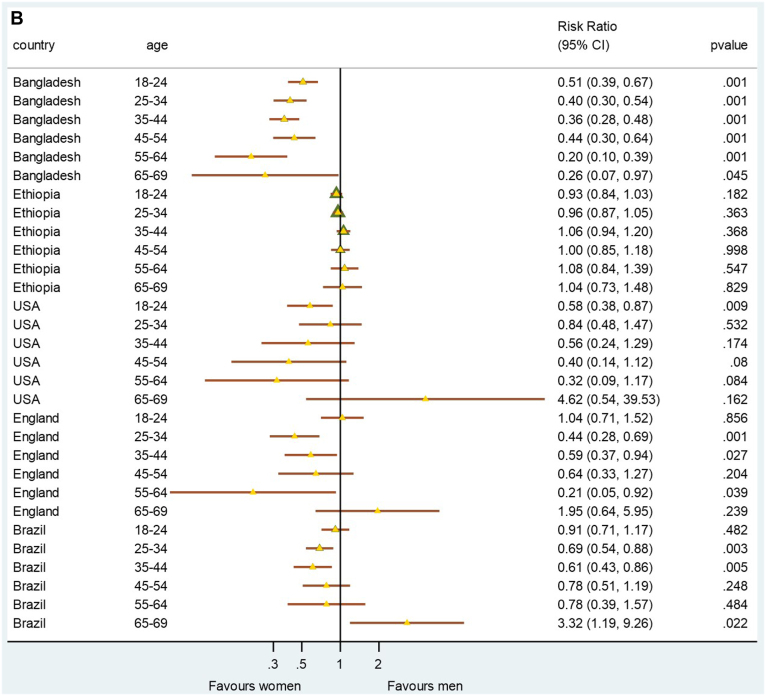
The prevalence of sex-specific CVH risk was plotted to determine whether sex-specific CVH differences exist and/or are consistent across higher-income, lower-middle-income and lower-income countries, both including and excluding the PA metric for comparison (Fig. 4, A and B). When analysis was conducted on all 6 CVH metrics (including PA), younger men presented with significantly better CVH scores in Brazil than their female counterparts (Fig. 4A). When PA was excluded from the analysis, with the exception of Ethiopia, women generally had a better CVH score than men did, in most countries and especially at younger ages (Fig. 4B).
Figure 4.
(A) Sex-specific ideal cardiovascular health risk by age group for each country—based on 6 metrics (blood pressure, body mass index, cholesterol, glycemia, smoking, physical activity). (B) Sex-specific ideal cardiovascular health risk by age group for each country—based on 5 metrics (blood pressure, body mass index, cholesterol, glycemia, smoking). CI, confidence interval.
Discussion
This research sought to harmonize, analyze, and compare CVH data from nationally representative adult surveys of 5 diverse countries ranging in income from high to low, to test the hypothesis that the decline in CVH with age is universal. Although comparable data were lacking for the diet and sleep metrics, analysis of the remaining CVH metrics (BP, glycemia, BMI, smoking, cholesterol, and PA) supported our hypothesis and showed that the prevalence of high CVH scores declined across the life course in all 5 countries. Further, our hypothesis that the starting point and the tempo of decline may differ among HICs, middle-income countries, and LICs was also supported within this analysis.
Our findings that 56.2% of adults in Brazil had high CVH scores, using 5 CVH metrics, are similar to those previously published from the PNS (2013). Dias Moreira et al. reported that among 4585 Brazilian adults from the PNS (mean age 43.2 years ± standard deviation 23.7), 56.7% had ideal CVH scores, based on their having 4 or more of LS7 CVH metrics in the ideal range.14 The results from Brazil were similar to those in the US NHANES sample (56.1% of adults scored high for CVH, or ≥ 8 of 10, based on the 5 CVH metrics). Although our analysis used the 2017-2020 pre-pandemic NHANES dataset, analysis using earlier NHANES data suggests a much lower prevalence of ideal CVH (17%-18% of adults aged ≥ 20 years, with ≥ 5 of the LS7 metrics at ideal levels; 5% with ≥ 6 ideal CVH metrics; and ≤ 2% with all of the LS7 CVH metrics at ideal levels).4,32,33 However, this finding may reflect differences in scoring, such as in counts of the number of individual metrics classified as ideal, which typically generates lower prevalence estimates of good or high CVH scores, compared to the allocation of scores to each metric that are summed to give an overall high, moderate, or low CVH rating. More recent analysis in NHANES data employing this latter approach with all of the LE8 CVH metrics produces more similar estimates, with a mean score of 65.2 of 100 in US adults aged ≥ 20 years,34 compared to our observed mean CVH scores of 6.4 of 10 or 7.8 of 12. Additionally, as we have shown, the prevalence of a high CVH score increases as the number of metrics used decreases, such that with 6 metrics, we found 40.4% of US adults scored high (≥ 9 of 12), whereas with 5 metrics, this increased to 56.1% of US adults with high CVH scores (≥ 8 of 10). This finding is similar to data from a larger US adult sample showing 43.5% having a high CVH score when using 6 metrics (≥ 5 of 6, n = 177,421).35 Among the US sample, we did observe that the BMI metric had particularly low scores, with 74% of adults scored as moderate or low (overweight or obese). This finding is consistent with previous NHANES analysis showing 56% as being overweight or obese in 1998-1994, increasing to 68% in 2005-2010.4
Previous analysis conducted in Bangladesh, also using the 2018 STEPS survey, showed that 43% of adults had at least 5 of the LS7 metrics at ideal levels,36 corresponding to twice the level reported in US adults (17%-18%) using the same approach.32,33 Although we did not find quite this magnitude of difference, possibly because diet was not evaluated, we did observe that Bangladesh had a higher total CVH score than the US, using either 6 or 5 metrics, and a higher score on each CVH metric individually, with the exception of smoking, with Bangladesh having the highest proportion of current smokers (24%). In contrast, England had the lowest prevalence of high CVH scores for smoking, due primarily to a higher prevalence of former smokers (33%). These HSE data were collected in 2016, post-implementation of stringent United Kingdom (UK) tobacco-control policies and may reflect the success of these.37 However, our finding that 40% of UK adults in 2016 achieved a high CVH score, based on 6 metrics, is much higher than the 14% found earlier in over 300,000 adults from the UK Biobank study (2006-2010) using a similar scoring method and all of the LS7 metrics.38 This difference may be due to both the inclusion of diet, for which fewer adults score well, and the higher age of the Biobank sample (37-73 years).
We were unable to find evidence of the previous application of the CVH score in Ethiopia for comparison. Our prevalence of overweight and obesity (6.5%) in Ethiopia is lower than many previous findings that suggest a pooled prevalence of overweight and obesity at approximately 24%.39 This difference may be due in part to sampling strategy (national sampling vs urban samples alone, which tend to show higher BMI) and sample size. This difference also may be influenced by the high number of underweight individuals in our study that were classified as having high CVH scores for BMI, owing to the CVH score including all individuals with BMI < 25 kg/m2. Although we did evaluate the impact of this on the total CVH score and found it to be negligible overall, application of a lower threshold (BMI 18.5 kg/m2, based on the WHO classification for underweight) for the high BMI CVH category may be needed, especially as being underweight may confer additional risk for CVD.40
Furthermore, when applying scores for BMI in populations in other geographic locations, or with different ethnicities, the BMI cutoffs may need to be revised, as the level that is predictive of cardiometabolic risk may be lower.41 Thus, our analysis for Bangladesh especially may present a more optimistic situation than is actually the case, although the lower levels of obesity (defined as BMI < 25 kg/m2) in Ethiopia and Bangladesh also were associated with lower levels of hyperglycemia and hypercholesterolemia, potentially explaining the apparent higher prevalence of high CVH scores in these LICs. Comparing other health behaviours across countries is important, as PA also varied widely and, with the exception of Brazil, which had a different PA data collection method, generally decreased as country income level increased. This finding may reflect the reductions in energy expenditure associated with occupation, transport, and domestic activities observed in HICs over the past decades that are not (yet) observed to the same extent in LICs.42
We also found that just 5% of adults in Ethiopia use tobacco, compared to 13%-17% in HICs and 24% in Bangladesh. This finding may explain in part why the percentage of deaths from NCD, including CVD, may follow the overall CVH scoring pattern and decline as country income level declines (88% in England and the US, 75% in Brazil, 70% in Bangladesh, and 43% in Ethiopia25), whereas in Bangladesh, NCD mortality rates are more similar to those in the HICs than to those in Ethiopia. Additionally, the percentage of deaths from maternal and child health, nutrition, or communicable disease remains high, at 45% in Ethiopia, compared to 5% in the US.25 In addition to differing causes of mortality across the countries, differences were observed in access to health screening and treatment, with lower rates of detection and treatment for hypertension in lower-income compared to higher-income countries. These findings reinforce previous calls for the strengthening of health systems in LMICs, to prevent a range of chronic diseases.43
Furthermore, other variables not yet captured in the CVH scoring may influence relative NCD mortality and morbidity rates. For example, UK adults consume 11.5 liters of alcohol per capita, whereas in Bangladesh, this figure is below 0.05 liters per capita.44 Evidence is also growing on the impact of both positive and negative psychosocial factors on CVH and disease (including optimism, mindfulness, resilience, and hope, as well as depression, perceived stress at home or work, low locus of control, and major life events) across the range of LICs and HICs.45,46 In addition, acknowledgement should be made that among children and adults living in LICs, rheumatic heart disease is a common cause of acquired heart disease.47,48 Given this, the LS7 and LE8 metrics, which focus on risk factors for atherosclerotic CVD, may not fully capture the true level of CVH in these settings.
Limitations
The results of this secondary data analysis should be viewed within the limitations of this study. For example, surveys were not all conducted at the same time, with the year of data collection ranging from 2013 (Brazil) up to pre-pandemic 2020 (US). The lack of availability of both sleep and dietary data is a further limitation in this analysis. The challenges in harmonizing dietary data collected from such different countries may have been expected, as a previous secondary data analysis showed that this could not be achieved for population surveys from 6 countries within Europe,49 despite the recommendation for simple dietary metrics in the initial LS7 CVH scoring.2 The revised LE8 CVH scoring guidelines propose a new method for assessing dietary quality that could be employed for both rapid individual assessment in clinical settings and for population-level surveys,3 although this approach has yet to be widely deployed, especially in LMICs. Future surveys could also include the simple sleep-assessment question proposed by LE8: “On average, how many hours of sleep do you get per night?”. However, the validity of simple sleep questions, in comparison to objectively measured sleep data, has proven to be questionable in the past,50,51 and this area may need further research, especially across different regions, languages, and cultures.
A further limitation lies in the PA analysis, with Brazil using the Physical Activity Vital Sign (PAVS), a PA questionnaire that differed from the approaches used in other countries. The lower PA levels reported using PAVS, compared to those found with instruments that explore multiple activity domains or types, have been reported previously, and the PAVS was found on average to record 86 less minutes of moderate-vigorous PA per week.52 Brazil reported lower moderate-vigorous PA in our study; possibly, Brazil does have a lower incidence of achieving the PA recommendations. For example, a national study in Brazil using a PA instrument that queried about multiple activity domains found that in 2013, 50% of the population met the PA guidelines53 and therefore would have met the criteria for high CVH PA categorization. Although this level is higher than the 17% high CVH PA categorization we observed, it remains lower than that for the other 4 countries included in this analysis.
Another limitation is the wide variation in missing data across surveys, with the NHANES presenting the largest amount of missing data. We described the NHANES design earlier in the paper, indicating that staged subsampling was used to select those with full examination data; we subsequently describe similarity between those with a complete examination (the analytic sample) and the total study population. An analysis comparing the NHANES analytical sample with eligible NHANES participants who were excluded due to missing data showed that no significant differences were present in sex or education levels, but a significant difference in age was present between these groups, with excluded participants being younger (mean age in analytical sample, 46 ± 15.0 years, mean age in the excluded sample, 42 ± 15.1 years [P < 0.001]). This finding may be due, in part, to how cholesterol medication is recorded in this survey. For example, to understand treatment use, all countries ask questions that generally follow the care cascade (have you been measured, diagnosed, and prescribed treatment, and are you taking the treatment?). However, in the US, primary and secondary prevention of CVD does not require high cholesterol levels to indicate statin use; statins are used according to cardiovascular risk and age, not just per cholesterol levels.54 Indeed, this difference was observed in our data; the US showed the second-highest mean total cholesterol level among the 5 countries but the highest percentage on treatment for lipids (more than twice that of England). The reduced analytic sample resulting from the study design is distinct from “missing data” in the usual sense of loss of expected data because of failure to collect these data.
A strength of this analysis is the comparison across a highly diverse group of 5 low- to high-income countries, including a total combined analytical sample of over 28,000 adults, with selected countries at varying stages of urban, economic, and nutritional transition. Although over 80% of people in Brazil, the US, and England live in urban areas, in Bangladesh and Ethiopia, urban dwellers still form less than one quarter of the population.55 Despite increasing urbanization, relatively little has changed in the food supply in Ethiopia over the past 50 years.56 A further strength in this analysis is the general agreement between our findings and those of the Global Burden of Disease Study (GBD, 2019).57 For example, our findings of the apparent high level of CVH in Ethiopia are consistent with GBD data for the Ethiopia country profile.
The significant differences among countries in population age structure, although representative of national demographics, do present a challenge for such comparative analysis. However, the patterns observed in our data tables and figures appear to agree that CVH assessed in these five countries and using these methods declines in populations as country income level increases. Further analysis is needed across a greater number of countries to confirm this finding.
Future recommendations
Despite this apparent alignment, the utility and potential adaptation of CVH scoring for use across multiple countries and regions require further exploration. The formulation of a single protocol for data collection that considers the contextual challenges of low-resource settings would facilitate this analysis. A single questionnaire that includes all relevant questions for CVH assessment, with methods that can be applied across high- and low-income settings, would allow validations of this approach in different countries and regions, as well as support global comparisons. Harmonized collection of data on dietary quality across countries is likely to require special consideration.
Although important differences among the included countries were found, our analysis suggests that the decline in levels of CVH seen from adolescence across the adult life course may be universal. Differences observed among countries and already occurring in those aged 18 years may indicate that strategies to maintain higher CVH levels in this young-adult age range may be more successful sooner in lower-income than in higher-income countries, where CVH is already reduced at this age. This finding indicates the need for earlier life interventions in HICs, with a major focus on attaining and maintaining a healthy body weight from childhood across the life course. Our data on the individual CVH metrics across the countries may be useful to inform other country-specific intervention strategies. For example, efforts in Bangladesh to reduce tobacco use could utilize learnings from effective tobacco-control policies observed in the UK. In Ethiopia, however, policies envisioned by Strasser, Popkin and Ng, and others58,59 aimed at protecting the whole of society as transitions occur to support the primordial prevention of cardiovascular risk are likely to be beneficial. Although approximately 80% of global CVD-related deaths occur in LMICs, based on our analysis, the age-specific CVH rates are higher in LMICs, compared to HICs. Access to and quality of care may partly explain this mortality disparity, but future studies need to assess the association of CVH with cause-specific mortality in these populations.
Conclusions
Our harmonization of data on CVH metrics from nationally representative population surveys in 5 widely diverse countries, ranging in income from low to high, reveals a consistent pattern of decline in overall CVH with age, supporting our hypothesis that such decline may be universal across countries. Differences in the prevalence of a high level of CVH, already evident at age 18 years, suggests that important differences be considered—in the starting age levels (potentially from birth), tempos of decline, and inflection points—in creating CVH promotion strategies in each country. Lack of dietary data suitable for harmonization remains a critical problem to be resolved as new standardized methods for population studies of CVH are designed. Given the rapid urban, economic, and nutrition transitions taking place in LMICs, targeted and contextually relevant interventions to change CVH trajectories are needed now to increase the prevalence of a high level of CVH at all ages, globally.
Acknowledgements
This paper uses data from the Bangladesh 2018 and Ethiopia 2015 STEPS surveys, implemented by the Bangladesh National Institute of Preventive and Social Medicine (NIPSOM) and the Ethiopian Public Health Institute (EPHI), respectively, with the support of the World Health Organization.
Ethics Statement
All surveys obtained ethical permission from relevant national and/or institutional ethics committees before implementation. For this specific secondary data analysis, ethical approval was granted from the University of the Witwatersrand Medical Human Research Ethics Committee [Ref: M220437]. In order to conduct the national surveys, each country obtained ethical approval from their local ethics committee. All countries used a multistage cluster sampling technique and randomly selected participants to take part in the surveys. Each participant provided informed consent before surveys commenced. Detailed information about the various study populations and designs are available on each of the participating countries’ reports.16,17,19, 20, 21, 22
Patient Consent
The authors confirm that patient consent is not applicable to this article. This study made use of nationally representative data that did not include any identifiable information from participants; therefore, patient/participant consent was not required.
Funding Sources
This work was conducted with funding support from the Institute for Global Health, Northwestern University, United States (Catalyzer Award No. 1005); from the Department of Science Innovation (DSI)-National Research Foundation (NRF) Centre of Excellence in Human Development hosted at the University of the Witwatersrand in South Africa, and the support of the University of the Witwatersrand Research Office, South Africa. Kavita Singh is supported by the Fogarty International Centre, US National Institutes of Health (NIH) (grant award: 1K43TW011164).
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
See page 594 for disclosure information.
To access the supplementary material accompanying this article, visit CJC Open at https://www.cjcopen.ca/ and at https://doi.org/10.1016/j.cjco.2023.11.021.
Supplementary Material
References
- 1.Mensah G.A., Roth G.A., Fuster V. The global burden of cardiovascular diseases and risk factors: 2020 and beyond. J Am Coll Cardiol. 2019:742529–742532. doi: 10.1016/j.jacc.2019.10.009. [DOI] [PubMed] [Google Scholar]
- 2.Lloyd-Jones D.M., Hong Y., Labarthe D., et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s Strategic Impact Goal through 2020 and beyond. Circulation. 2010;121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 3.Lloyd-Jones D.M., Allen N.B., Anderson C.A., et al. Life’s essential 8: updating and enhancing the American Heart Association’s construct of cardiovascular health: a presidential advisory from the American Heart Association. Circulation. 2022;146:e18–e43. doi: 10.1161/CIR.0000000000001078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang Q., Cogswell M.E., Flanders W.D., et al. Trends in cardiovascular health metrics and associations with all-cause and CVD mortality among US adults. JAMA. 2012;307:1273–1283. doi: 10.1001/jama.2012.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Folsom A.R., Yatsuya H., Nettleton J.A., et al. Community prevalence of ideal cardiovascular health, by the American Heart Association definition, and relationship with cardiovascular disease incidence. J AmColl Cardiol. 2011;57:1690–1696. doi: 10.1016/j.jacc.2010.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gaye B., Canonico M., Perier M.-C., et al. Ideal cardiovascular health, mortality, and vascular events in elderly subjects: the three-city study. J Am Coll Cardiol. 2017;69:3015–3026. doi: 10.1016/j.jacc.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 7.Chomistek A.K., Chiuve S.E., Eliassen A.H., et al. Healthy lifestyle in the primordial prevention of cardiovascular disease among young women. J Am Coll Cardiol. 2015;65:43–51. doi: 10.1016/j.jacc.2014.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong C., Rundek T., Wright C.B., et al. Ideal cardiovascular health predicts lower risks of myocardial infarction, stroke, and vascular death across Whites, Blacks, and Hispanics: the northern Manhattan study. Circulation. 2012;125:2975–2984. doi: 10.1161/CIRCULATIONAHA.111.081083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ford E.S., Greenlund K.J., Hong Y. Ideal cardiovascular health and mortality from all causes and diseases of the circulatory system among adults in the United States. Circulation. 2012;125:987–995. doi: 10.1161/CIRCULATIONAHA.111.049122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Younus A., Aneni E.C., Spatz E.S., et al. A systematic review of the prevalence and outcomes of ideal cardiovascular health in US and non-US populations. Mayo Clin Proc. 2016;91:649–670. doi: 10.1016/j.mayocp.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 11.Virani S.S., Alonso A., Aparicio H.J., et al. Heart disease and stroke statistics—2021 update: a report from the American Heart Association. Circulation. 2021;143:e254–e743. doi: 10.1161/CIR.0000000000000950. [DOI] [PubMed] [Google Scholar]
- 12.Manuel D.G., Tuna M., Bennett C., et al. Development and validation of a cardiovascular disease risk-prediction model using population health surveys: the Cardiovascular Disease Population Risk Tool (CVDPoRT) CMAJ. 2018;190:E871–E882. doi: 10.1503/cmaj.170914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.González-Rivas J.P., Mechanick J.I., Ugel E., et al. Cardiovascular health in a national sample of Venezuelan subjects assessed according to the AHA score: the EVESCAM. Global Heart. 2019;14:285–293. doi: 10.1016/j.gheart.2019.06.006. [DOI] [PubMed] [Google Scholar]
- 14.Dias Moreira A., Saar Gomes C., Machado Í Eloah, Carvalho Malta D., Santos Felisbino-Mendes M. Cardiovascular health and validation of the self-reported score in Brazil: analysis of the National Health Survey. Ciên Saúde Colet. 2020;25:4259–4268. doi: 10.1590/1413-812320202511.31442020. [DOI] [PubMed] [Google Scholar]
- 15.Michos E.D., Khan S.S. Further understanding of ideal cardiovascular health score metrics and cardiovascular disease. Expert Rev Cardiovasc Ther. 2021;19:607–617. doi: 10.1080/14779072.2021.1937127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riley L., Guthold R., Cowan M., et al. The World Health Organization STEPwise approach to noncommunicable disease risk-factor surveillance: methods, challenges, and opportunities. Am J Public Health. 2016;106:74–78. doi: 10.2105/AJPH.2015.302962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization . WHO STEPS Surveillance Manual: The WHO STEPwise approach to chronic disease risk factor surveillance. World Health Organization; Geneva: 2005. https://iris.who.int/bitstream/handle/10665/43376/?sequence=1 Available at: [Google Scholar]
- 18.World Bank Country and Lending Groups: Country classification. https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups Available at:
- 19.Ethiopian Health and Nutrition Research Institute Ethiopia STEPS report on risk factors for non-communicable diseases and prevalence of selected NCDs. https://cdn.who.int/media/docs/default-source/ncds/ncd-surveillance/data-reporting/ethiopia/steps/ethiopia-2015-steps-report.pdf Available at:
- 20.Chen T.C., Clark J., Riddles M.K., Mohadjer L.K., Fakhouri T.H. National Health and Nutrition Examination Survey, 2015− 2018: sample design and estimation procedures. Vital Health Stat. 2020;2(184):1–35. [PubMed] [Google Scholar]
- 21.Craig R., Mindell J., Hirani V. Health Survey for England. Health and Social Care Information Centre; 2013. https://dam.ukdataservice.ac.uk/media/262778/healthusermeeting_craig_11july13.pdf Available at: [Google Scholar]
- 22.Szwarcwald C.L., Malta D.C., Pereira C.A., et al. National Health Survey in Brazil: design and methodology of application. Cien Saude Colet. 2014;19:333. doi: 10.1590/1413-81232014192.14072012. [DOI] [PubMed] [Google Scholar]
- 23.National Center for Education Statistics: Comparative Indicators of Education in the United States and Other G-20 Countries, 2015. Available at: https://nces.ed.gov/pubs2016/2016100/app_a3.asp. Accessed February 1, 2023.
- 24.World Health Organization Global health expenditure database. http://apps.who.int/nha/database Available at:
- 25.World Health Organization, The Global Health Obsrvatory Global Health Estimates: Life expectancy and leading causes of death and disability. https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates/ghe-leading-causes-of-death Available at:
- 26.World Health Organization National STEPS survey for non-communicable diseases risk factors in Bangladesh 2018. https://apps.who.int/iris/handle/10665/332886 Available at:
- 27.National Health Service (NHS) England NHS Digitl: Health Survey for England 2016 Quick Guide. 2017. https://files.digital.nhs.uk/publication/m/e/hse2016-quick-guide.pdf Available at:
- 28.Szwarcwald C.L., Malta D.C., de Souza Junior P.R.B., et al. Laboratory exams of the National Health Survey: methodology of sampling, data collection and analysis. Rev Bras Epidemiol. 2019;22(Suppl 2) doi: 10.1590/1980-549720190004.supl.2. SUPL.2. [DOI] [PubMed] [Google Scholar]
- 29.Velasquez-Melendez G., Felisbino-Mendes M.S., Matozinhos F.P., et al. Ideal cardiovascular health prevalence in the Brazilian population—National Health Survey (2013) Rev Bras Epidemiol. 2015;18(Suppl 2):97–108. doi: 10.1590/1980-5497201500060009. [DOI] [PubMed] [Google Scholar]
- 30.Schaap L.A., Peeters G.M., Dennison E.M., et al. European Project on Osteoarthritis (EPOSA): methodological challenges in harmonization of existing data from five European population-based cohorts on aging. BMC Musculoskelet Disord. 2011;12:272. doi: 10.1186/1471-2474-12-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akinbami L.J., Chen T.-C., Davy O., et al. National Health and Nutrition Examination Survey, 2017–March 2020 Prepandemic File: Sample Design, Estimation, and Analytic Guidelines. Vital Health Stat 1. 2022;(190):1–36. [PubMed] [Google Scholar]
- 32.Virani S.S., Alonso A., Benjamin E.J., et al. Heart disease and stroke statistics—2020 update: a report from the American Heart Association. Circulation. 2020;141:e139–e596. doi: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 33.Benjamin E.J., Muntner P., Alonso A., et al. Heart disease and stroke statistics—2019 update: a report from the American Heart Association. Circulation. 2019;139:e56–e528. doi: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 34.Tsao C.W., Aday A.W., Almarzooq Z.I., et al. Heart disease and stroke statistics—2023 update: a report from the American Heart Association. Circulation. 2023;147:e93–e621. doi: 10.1161/CIR.0000000000001123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tibuakuu M., Okunrintemi V., Savji N., et al. Nondietary cardiovascular health metrics with patient experience and loss of productivity among US adults without cardiovascular disease: the Medical Expenditure Panel Survey 2006 to 2015. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.120.016744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gupta R.D., Tamanna R.J., Hashan M.R., et al. Prevalence and associated factors with ideal cardiovascular health metrics in Bangladesh: analysis of the nationally representative STEPS 2018 Survey. Epidemiologia (Basel) 2022;3:533–543. doi: 10.3390/epidemiologia3040040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levy D.T., Currie L., Clancy L. Tobacco control policy in the UK: blueprint for the rest of Europe? Eur J Public Health. 2012;23:201–206. doi: 10.1093/eurpub/cks090. [DOI] [PubMed] [Google Scholar]
- 38.Xu C., Zhang P., Cao Z. Cardiovascular health and healthy longevity in people with and without cardiometabolic disease: a prospective cohort study. eClinicalMedicine. 2022;45 doi: 10.1016/j.eclinm.2022.101329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kassie A.M., Abate B.B., Kassaw M.W. Prevalence of overweight/obesity among the adult population in Ethiopia: a systematic review and meta-analysis. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2020-039200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park D., Lee J.H., Han S. Underweight: another risk factor for cardiovascular disease?: a cross-sectional 2013 Behavioral Risk Factor Surveillance System (BRFSS) study of 491,773 individuals in the USA. Medicine (Baltimore) 2017;96 doi: 10.1097/MD.0000000000008769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Razak F., Anand S.S., Shannon H., et al. Defining obesity cut points in a multiethnic population. Circulation. 2007;115:2111–2118. doi: 10.1161/CIRCULATIONAHA.106.635011. [DOI] [PubMed] [Google Scholar]
- 42.Ozemek C., Lavie C.J., Rognmo Ø. Global physical activity levels–need for intervention. Prog Cardiovasc Dis. 2019;62:102–107. doi: 10.1016/j.pcad.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 43.Samb B., Desai N., Nishtar S., et al. Prevention and management of chronic disease: a litmus test for health-systems strengthening in low-income and middle-income countries. Lancet. 2010;376:1785–1797. doi: 10.1016/S0140-6736(10)61353-0. [DOI] [PubMed] [Google Scholar]
- 44.World Health Organization, The Global Health Observatory: Indicators: Alcohol, total per capita (15+ years) consumption (in litres of pure alcohol) https://www.who.int/data/gho/data/indicators/indicator-details/GHO/alcohol-total-per-capita-(15-years)-consumption-(in-litres-of-pure-alcohol) Available at:
- 45.Teo K.K., Rafiq T. Cardiovascular risk factors and prevention: a perspective from developing countries. Can J Cardiol. 2021;37:733–743. doi: 10.1016/j.cjca.2021.02.009. [DOI] [PubMed] [Google Scholar]
- 46.Labarthe D.R., Hernandez R. In: Fuster and Hurst's The Heart. 15th ed. Fuster V., Narula J., Vaishnava P., et al., editors. McGraw-Hill Education; New York: 2022. Psychological factors in cardiovascular health and disease. [Google Scholar]
- 47.Rothenbühler M., O'Sullivan C.J., Stortecky S., et al. Active surveillance for rheumatic heart disease in endemic regions: a systematic review and meta-analysis of prevalence among children and adolescents. Lancet Global Health. 2014;2:e717–e726. doi: 10.1016/S2214-109X(14)70310-9. [DOI] [PubMed] [Google Scholar]
- 48.Watkins D.A., Johnson C.O., Colquhoun S.M., et al. Global, regional, and national burden of rheumatic heart disease, 1990–2015. N Engl J Med. 2017;377:713–722. doi: 10.1056/NEJMoa1603693. [DOI] [PubMed] [Google Scholar]
- 49.Beer-Borst S., Hercberg S., Morabia A., et al. Dietary patterns in six European populations: results from EURALIM, a collaborative European data harmonization and information campaign. Eur J Clin Nutr. 2000;54:253–262. doi: 10.1038/sj.ejcn.1600934. [DOI] [PubMed] [Google Scholar]
- 50.Girschik J., Fritschi L., Heyworth J., Waters F. Validation of self-reported sleep against actigraphy. J Epidemiol. 2012;22:462–468. doi: 10.2188/jea.JE20120012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee P.H. Validation of the National Health and Nutritional Survey (NHANES) single-item self-reported sleep duration against wrist-worn accelerometer. Sleep Breath. 2022;26:2069–2075. doi: 10.1007/s11325-021-02542-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ball T.J. Concurrent validity of a self-reported physical activity “vital sign” questionnaire with adult primary care patients. Prev Chronic Dis. 2016;13:E16. doi: 10.5888/pcd13.150228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ferrari G., Dulgheroff P.T., Claro R.M., Rezende L.F., Azeredo C.M. Socioeconomic inequalities in physical activity in Brazil: a pooled cross-sectional analysis from 2013 to 2019. Int J Equity Health. 2021;20:1–9. doi: 10.1186/s12939-021-01533-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Heller D.J., Coxson P.G., Penko J., et al. Evaluating the impact and cost-effectiveness of statin use guidelines for primary prevention of coronary heart disease and stroke. Circulation. 2017;136:1087–1098. doi: 10.1161/CIRCULATIONAHA.117.027067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ritchie H., Roser M. Urbanization. https://ourworldindata.org/urbanization Available at:
- 56.Sheehy T., Carey E., Sharma S., Biadgilign S. Trends in energy and nutrient supply in Ethiopia: a perspective from FAO food balance sheets. Nutr J. 2019;18:46. doi: 10.1186/s12937-019-0471-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gouda H.N., Charlson F., Sorsdahl K., et al. Burden of non-communicable diseases in sub-Saharan Africa, 1990–2017: results from the Global Burden of Disease Study 2017. Lancet Glob Health. 2019;7:e1375–e1387. doi: 10.1016/S2214-109X(19)30374-2. [DOI] [PubMed] [Google Scholar]
- 58.Popkin B.M., Ng S.W. The nutrition transition to a stage of high obesity and noncommunicable disease prevalence dominated by ultra-processed foods is not inevitable. Obes Rev. 2022;23 doi: 10.1111/obr.13366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Strasser T. Reflections on cardiovascular diseases. Interdisc Sci Rev. 1978;3:225–230. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.