Highlights
-
•
Brain temperature within motor cortex is regulated in a narrower homeostatic range when compared to body rectal temperature.
-
•
Bilateral motor cortical activity was suppressed with exertional and passive hyperthermia.
-
•
Global and sensorimotor brain blood flow were lowered with passive hyperthermia.
-
•
Poorer inhibitory control observed after passive heat exposure could have real-world implications such as causing an increase in work-related errors.
-
•
Ice ingestion is a viable strategy to limit hyperthermia and avoid heat-related neural deficits.
Keywords: Brain functional activity, Cognition, Heat stress, Hyperthermia, Motor function
Abstract
Background
Excessive heat exposure can lead to hyperthermia in humans, which impairs physical performance and disrupts cognitive function. While heat is a known physiological stressor, it is unclear how severe heat stress affects brain physiology and function.
Methods
Eleven healthy participants were subjected to heat stress from prolonged exercise or warm water immersion until their rectal temperatures (Tre) attained 39.5°C, inducing exertional or passive hyperthermia, respectively. In a separate trial, blended ice was ingested before and during exercise as a cooling strategy. Data were compared to a control condition with seated rest (normothermic). Brain temperature (Tbr), cerebral perfusion, and task-based brain activity were assessed using magnetic resonance imaging techniques.
Results
Tbr in motor cortex was found to be tightly regulated at rest (37.3°C ± 0.4°C (mean ± SD)) despite fluctuations in Tre. With the development of hyperthermia, Tbr increases and dovetails with the rising Tre. Bilateral motor cortical activity was suppressed during high-intensity plantarflexion tasks, implying a reduced central motor drive in hyperthermic participants (Tre = 38.5°C ± 0.1°C). Global gray matter perfusion and regional perfusion in sensorimotor cortex were reduced with passive hyperthermia. Executive function was poorer under a passive hyperthermic state, and this could relate to compromised visual processing as indicated by the reduced activation of left lateral-occipital cortex. Conversely, ingestion of blended ice before and during exercise alleviated the rise in both Tre and Tbr and mitigated heat-related neural perturbations.
Conclusion
Severe heat exposure elevates Tbr, disrupts motor cortical activity and executive function, and this can lead to impairment of physical and cognitive performance.
Graphical Abstract

1. Introduction
Climate change and rapid urbanization have led to rising global temperatures. Excessive heat exposure constitutes a major issue for subtropical and tropical countries where hard-labor workers and athletes are susceptible to heat stress as intense physical exertion exacerbates heat-related deficits.1,2 Moreover, the deleterious impact of heat stress on sports performance and health has been underscored in major sporting events such as the 2015 International Association of Athletics Federations (IAAF) World Athletics Championships3 and the 2021 Tokyo Olympics.4 Extreme heat events are becoming more common globally5 and may lead to increased prevalence of heat-related illnesses in competing athletes. Thus, it is paramount for us to profile and understand the physiological impact of heat stress on the human body to formulate effective heat management strategies.
When exercising in the heat, endurance performance of athletes is markedly impaired.6, 7, 8 Premature fatigue experienced while exercising in the heat is traditionally associated with peripheral physiological factors, such as increased thermoregulatory and cardiovascular strain.9, 10, 11 However, there is increasing evidence implicating the role of the central nervous system in an earlier onset of fatigue during hyperthermia. For instance, force production during sustained maximal voluntary contractions and central motor drive were reduced by exertional hyperthermia.12 Moreover, a study showed that male cyclists exercising in the heat exhibited elevated α/β index as assessed by electroencephalogram, indicating suppressed arousal.13 Brain electrical activity is shown to be altered by hyperthermia but not dehydration.14 Our previous work demonstrated that exertional hypohydration did not influence motor cortical activity, suggesting heat may play a greater role in central fatigue.15 Thus, we propose that brain functional activity is impaired by hyperthermia, compromising both motor and cognitive functions.
To date, evidence on central fatigue during hyperthermia has been indirect, and the underlying central alterations that hinder optimal neural function in the heat remain unclear. Efforts at assessing brain temperature and function have been challenging due to the relatively inaccessible nature of human brain tissue. With excellent spatial resolution and versatility, magnetic resonance imaging (MRI) presents a useful non-invasive tool to examine the human brain. While MRI has been employed to investigate the brain functional connectivity in humans exposed to passive hyperthermia,16 no studies have examined real-time brain activity changes during hyperthermia in relation to a physical or cognitive task.
The purpose of the present study was to investigate alterations in brain properties and functional activity in healthy volunteers exposed to exertional or passive hyperthermia. Using MRI-based evaluation of brain temperature, resting cerebral perfusion, and task-based brain activity, we identified the implicated brain regions and neural changes in hyperthermic humans. We further explored ice ingestion as a potential heat mitigation strategy.
2. Methods
2.1. Participants
Eleven healthy males between 21 and 35 years of age were recruited. Participant characteristics were as follows: age = 23 ± 2 years; body mass = 64.0 ± 6.4 kg; height = 1.70 ± 0.04 m; body fat percentage = 10% ± 2%; peak oxygen consumption (VO2peak) = 59 ± 5 mL/kg/min (mean ± SD). All participants self-reported to be right handed. Individuals with medical implants, non-MRI-compatible dentures, and claustrophobia were excluded. All protocols were approved by the institutional review board of the National University of Singapore (B-15-123; NUS 2700). Details of trial procedures, risks involved, precautions taken, and special instructions or risks pertaining to MRI were made clear to participants before written informed consent. All participants completed a health declaration form and were certified fit for trials by a physician prior to enrollment.
2.2. Experimental trials
The study comprised 1 familiarization trial and 4 experimental trials: (a) exertional hyperthermia, (b) passive hyperthermia, (c) exertional hyperthermia with cooling, and (d) control (Fig. 1A). These randomized controlled trials were conducted in a crossover manner, with participants serving as their own controls. Each trial was spaced at least 7 days apart for recovery. A preliminary session for anthropometric measurements and VO2peak assessment preceded the trials.
Fig. 1.
Experimental design and task-based fMRI protocols. (A) For EX trial, participants were given ambient temperature drink after their first MRI scan, followed by a treadmill run until they achieved 39.5°C. The trial ended with a second MRI scan after the run. PA trial shares the same protocol as EX except participants were passively heated in a water bath at 41°C until they achieved 39.5°C. Exertional hyperthermia with CL shares the same protocol as EX except participants ingested blended ice instead of ambient temperature (25°C) drinks. CT excludes any hyperthermia-inducing interventions (exercise/immersion) where participants rested in a sitting position and ingested ambient temperature drinks. (B) Insulative thermal inner wear to limit heat loss and absorb sweat. (C) Water-perfused thermosuit with circulating water at 41°C to restrict heat loss in scanner. (D) External water pump with insulated tubes. (E) Step ergometer (denoted by red box) for the conduct of plantarflexion task in MRI scanner. (F) Motor task-based fMRI scan where participants were asked to perform 10 sets of plantarflexion with rest intervals of 1 min, repeated for 12 blocks. (G) Cognitive task-based fMRI scan where participants performed the Stroop color-naming task for 1 min followed by 1-min fixation task, repeated for 4 blocks. CL = cooling trial; CT = control trial; EX = exertional hyperthermia trial; fMRI = functional magnetic resonance imaging; MRI = magnetic resonance imaging; MVC = maximal voluntary contraction; PA = passive hyperthermia trial; VO2peak = peak oxygen consumption.
VO2peak was measured using an incremental exercise protocol. Each participant ran at 4 different speeds on a treadmill (Mercury; h/p/cosmos, Bayern, Germany), starting at a speed 1 km/h slower than his expected pace for a 10 km race, with increments of 1 km/h every 3 min, for a total of 12 min. Heart rate (HR) and ratings of perceived exertion (Borg scale17) were recorded during the last 10 s of each 3-min stage. Oxygen uptake (VO2) was assessed over the final minute of each stage with a metabolic cart (Parvomedics, Salt Lake City, UT, USA). After 5–10 min rest, the participant ran at a fixed speed of moderate intensity (determined based on the preceding run) with an initial gradient of 1%, which increased by 1% every min until volitional exhaustion. VO2peak was recorded as the mean VO2 over the last min before termination, and the data was used to determine running intensity in subsequent trials.
Prior to each experimental trial, participants’ baseline body mass was measured, and pre-trial blood sample (10 mL) was taken by a phlebotomy-trained nurse. Each trial consisted of 2 MRI scans: a first scan (baseline; S1) before each trial, and a second scan after the completion of intervention (post; S2). After the post scan, the body mass was recorded, and a post-trial blood sample (10 mL) was taken.
2.2.1. Familiarization/Exertional hyperthermia trial (EX)
After the baseline scan, participants were given 6 aliquots of ambient temperature water (25°C; portioned from a volume equivalent to 8 g/kg body mass), with each aliquot given at 5-min intervals over 30 min. After the drinking phase, the participants ran at 70% VO2peak on a treadmill for up to 60 min, while wearing a disposable raincoat to retain body heat, until the rectal temperature (Tre) reached 39.5°C. HR (A300 heart rate monitor; Polar Electro, Kempele, Finland) and Tre (fiber optic sensor; Opsens, Quebec City, Canada) were monitored and recorded at 5-min intervals. VO2, ratings of perceived exertion, and ratings of thermal sensation (Young et al.18) were recorded at 15-min intervals. Ambient water (1.5 g/kg body mass) was given every 15 min. After post-run body mass measurement, they proceeded with the second scan. Prior to this, the participant donned thermal insulative clothing (Heat-tech; Uniqlo Co. Ltd., Tokyo, Japan) and woolen socks in addition to a water-perfused thermosuit (Med-Eng, Ottawa, Canada) with circulating water maintained at 41°C by an external heater (GR08-1-A; uCoolz, Singapore) to minimize heat loss (Fig. 1B–1D).
2.2.2. Passive hyperthermia trial (PA)
The protocol was similar to EX trial except that participants were passively heated by immersing (up to shoulder level in a seated position) in a water tub maintained at 41°C by an external heater. Immersion was terminated when Tre hit 39.5°C.
2.2.3. Cooling trial (CL)
The protocol was similar to EX trial except that well-blended ice (–1°C) was given instead of ambient temperature (25°C) water. The mass of ice slurry given was identical to that of water provided in other trials.
2.2.4. Control trial (CT)
The protocol was similar to PA trial with the exception of warm water immersion. Participants rested in a sitting position during the 60-min period (or shorter, depending on prior exercise/passive heating duration) after the drinking phase.
2.3. MRI data acquisition
The MRI protocols included T1-weighted imaging, 1H magnetic resonance spectroscopy (MRS), arterial spin labeling (ASL), and blood oxygenation level-dependent (BOLD) contrast imaging. All scans were recorded using a 3T Magnetom TrioTim (Siemens Medical Systems, Erlangen, Germany) and a 32-channel head coil. The image protocols were:
-
(a)
High-resolution isotropic T1-weighted magnetization prepared rapid gradient recalled echo (MPRAGE); 176 slices, 1 mm thickness, sagittal acquisition, field of view = 256 mm × 256 mm, matrix size = 256 × 256, repetition time = 1950 ms, echo time = 3.06 ms, inversion time = 900 ms, flip angle = 9°.
-
(b)
Single voxel spectroscopy with weak water suppression; bandwidth = 2000 Hz, acquisition duration = 1024 ms, repetition time = 1500 ms, echo time = 30 ms, flip angle = 90°, 32 acquisitions. The single voxel was centered at primary motor cortex.
-
(c)
Pseudo-continuous ASL with multi-slice single-shot echo planar imaging sequence; 21 slices with 4 mm thickness, field of view = 256 mm × 256 mm, matrix size = 64 × 64, repetition time = 4000 ms, echo time = 9.2 ms, flip angle = 90°, 20 label/control pairs, scanning time = 6 min, label offset = 124 mm and post-label delay = 1500 ms.
-
(d)
Task (motor)-based functional MRI (fMRI) with BOLD contrast using a gradient-echo echo planar imaging pulse sequence; 36 slices with 4-mm slice thickness, repetition time = 2000 ms, echo time = 30 ms, field of view = 256 mm × 256 mm, matrix size = 64 × 64. During imaging, the participants were placed in a supine position with the head immobilized. The right foot was placed on a pedal ergometer (Trispect module; Ergospect, Innsbruck, Austria), where participants performed dorsi- and plantarflexion (Fig. 1E). Each task lasted for 20 s, with each flexion performed at 2-s intervals with verbal instructions, and was followed by a rest period of 40 s. This constitutes 1 task block (1 min), and the process was repeated for 12 blocks (12 min). The pedal resistance was pseudo-randomly assigned at 20%, 40%, 60%, 80% of their pre-determined maximal voluntary contraction (Fig. 1F).
-
(e)
Task (cognitive)-based fMRI with BOLD contrast using a gradient-echo echo planar imaging pulse sequence; 36 slices with 4 mm slice thickness, repetition time = 2000 ms, echo time = 30 ms, field of view = 256 mm × 256 mm, matrix size = 64 × 64. During imaging, the participant performed the Stroop color-naming task.19 A color word was presented on the projector screen, where the participant was instructed to select for the ink color of the word, instead of its semantic meaning, using a 4-button box (Lumina LU444-RH; Cedrus, San Pedro, CA, USA) with their right hand. Stimuli consisted of words printed in 1 of 4 colors (red, blue, green, or yellow), and the congruent or incongruent stimuli were presented in random sequence. The proportion of congruent or incongruent stimuli presented was approximately equal. The stimuli were presented centrally for 1 min and repeated for 4 cycles. A solid black cross was presented on a white background as control stimulus for 1 min prior to each Stroop cycle, as intervals between the cycles, and at the end of the 4 cycles (total duration = 9 min; Fig. 1G). Single subject level analysis was performed using general linear model (GLM) analysis with the motion regressor (on/off). The hemodynamic response function was modeled during the presentation of stimuli, and was compared to the baseline signal when the control cross was displayed.
2.4. MRI data analysis
Raw Digital Imaging and Communications in Medicine (DICOM) data were converted to Neuroimaging Informatics Technology Initiative (NIfTI) using MCverter (Lewis Centre for Neuroimaging, University of Oregon, Eugene, OR, USA).
For 1H MRS, Siemens Twix data were analyzed using in-house built Matlab scripts, which included 2 Hz exponential line broadening and phase correction. The brain temperature (Tbr) was estimated for each individual acquisition from the frequency shift between the water and N-acetylaspartate (NAA) peaks using an equation from Cady et al.20 (Tbr = 286.9 – 94.0 ΔH20-NAA) and then averaged across individual spectra. The data were compared to Tre as recorded during the scan corresponding to the same timepoint at which the 1H MRS scan was conducted.
Individual subjects’ MPRAGE images were run through the Freesurfer longitudinal analysis pipeline21,22 to parcellate the brain for coregistration and further analysis. For ASL, image preprocessing was performed with Matlab R2014a Package (FIL Methods Group, London, UK) and Statistical Parametric Mapping version 12.0 (SPM12). ASL data were split into label/non-label pairs before motion correction and averaging. Label and non-label images were coregistered to the MPRAGE images using Advanced Normalization Tools23 based on the non-label coregistration parameters, before projection to the cortical surface and application of surface smoothing (10 mm). These smoothed cortical signals were used to generate the difference and perfusion images for further analysis by normalizing with relaxation-corrected label-off image and performing quantification correction.24 To remove motion artifacts, a second set of data was generated without motion correction, and a comparison was made. Where significant motion was seen in the motion parameters, or where the non-motion corrected data were significantly affected by motion, label/non-label data pairs containing motion were removed and regeneration of cerebral blood flow images were performed on the truncated set. GLM results were obtained using a 1-sample group mean GLM with vertexwise threshold p < 0.05 and cluster correction at p < 0.05, correcting for 2 spaces (left and right hemispheres). Extraction of regions of interest averaged data was performed using in-house built Matlab scripts, and regions of interest generated from Freesurfer parcellation (surfaces transformed to volume space). To obtain the normalized values accounting for circadian-related changes, the % perfusion values were subtracted with time-matched CT values, and comparisons were made between baseline and post scans. Similar analysis was applied for task response accuracy and reaction time. fMRI data were analyzed using the Freesurfer Functional Analysis STream (FS-FAST, http://www.surfer.nmr.mgh.harvard.edu). The pipeline involves motion correction of fMRI data to the middle time-point of each run, registration of the anatomical and fMRI images, intensity then spatial normalization (to Freesurfer “fsaverage”) using a 6-parameter affine transformation and smoothing (5-mm full width at half maximum). Individual participant/visit scans were analyzed, using a GLM, convolving the task regressor with a canonical hemodynamic response function (γ function with delay δ = 2.25 s and decay time constant τ = 1.2525).
For the ergometer paradigm, an event-related design was used with 4 contrasts (20%, 40%, 60%, and 80% maximal voluntary contraction) and rest. Positive clusters were extracted for 60% + 80% vs. rest using vertexwise threshold (p < 0.001), with clusterwise threshold (p < 0.05) and correcting for 2 spaces. Individual participant's % signal change was extracted from the activated clusters. Data were normalized with time-matched CT values and compared between baseline and post scans.
For Stroop-task fMRI analysis, the comparison across all trials was performed for (a) all stimuli vs. neutral stimuli, and (b) incongruent stimuli vs. congruent stimuli to generate the task-induced regions with vertexwise (p < 0.001) and clusterwise (p < 0.05) thresholds. For trial-to-trial comparison (PA S2 vs. CT S2), voxelwise paired comparisons were made between scans with the clusterwise threshold set to 0.05.
2.5. Statistical analysis
Statistical Package for Social Sciences (SPSS) Version 22.0 (IBM Corp., Armonk, NY, USA) was used to compute all data, and 2-tailed paired Student's t tests were performed to test for statistical differences between trial conditions. For time-by-trial interaction effects, 2-way repeated-measures analysis of variance was performed to test for significance between trial conditions. Where significant interaction effects were established, pairwise differences were identified using the Bonferroni post hoc analysis adjusted for multiple comparisons. Significance level was set at p < 0.05. All values were expressed as mean ± SD.
Power analyses were computed to detect power of 0.8 at an α level of 0.05 using a 2-tailed paired t test. For BOLD changes, the power analysis was conducted using data from a previous study examining % signal change in the primary motor cortex with finger flexion.26 Using the calculated effect size (1.2) between performing precise and simple finger movement, it was determined that a sample size of n = 8 was required (G*Power 3.1.9.4, gpower.hhu.de). For cognitive performance changes, 11 subjects were required based upon the calculated effect size (0.43) from previous motor coordination task data following ∼3% body mass loss27 compared to an exertional heat stress trial (estimated within-measures correlation of 0.5).
3. Results
3.1. Physiological and subjective parameters
Resting Tre (36.8°C ± 0.1°C) and HR (62 ± 2 bpm; Fig. 2A–2D) were similar across trials. All participants achieved a hyperthermic state during EX and PA trials (EX Tre = 39.4°C ± 0.2°C; PA Tre = 39.3°C ± 0.2°C; Fig. 2A). EX trial induced greater physiological strain (assessed using physiological strain index28) and higher perceived exertion and thermal sensation compared to CT (Fig. 2E–2G). All participants began the trials euhydrated, and there was no excessive body mass loss beyond 3% after each intervention (Fig. 2H and 2I). Relative to EX, PA resulted in lower physiological strain (p < 0.001), but perceived exertion and thermal sensation were similar. Ingestion of blended ice before exercise lowered resting Tre by 0.6°C ± 0.3°C, while the mean end Tre after running was lower than EX by 0.4°C ± 0.3°C (p = 0.001), thus demonstrating the cooling efficacy of ice ingestion (Fig. 2C). While participants felt cooler after ingesting ice before exercise (2 units lower, p < 0.001), the ratings of perceived exertion and thermal sensation remained similar to those in the EX trial during exercise (Fig. 2F and 2G). With lowered Tre and HR after ice ingestion, the physiological strain was lower in CL compared to EX (p = 0.020).
Fig. 2.
Physiological responses to heating interventions. (A) Tre profiling during the drinking phase and the intervention phase (exercise/immersion/rest). EX, PA, CL, and CT trials denoted by red, yellow, blue, and green lines, respectively. Each point represented the mean ± SD. (B) HR profiling during the drinking phase and the intervention phase. (C) Mean Tre recorded at baseline, at pre-intervention (after drinking phase), and post-intervention. Bars represent the mean, and lines represent the SD. (D) Mean HR recorded at baseline, at pre-intervention, and post-intervention. (E) Modified physiological strain index for each trial. (F) Ratings of perceived exertion at pre- and post-intervention. (G) Ratings of thermal sensation at pre- and post-intervention. (H) Percentage change in mean body mass at baseline and post scan. Red line denotes –3% body mass. (I) Serum osmolality at baseline and post scan. Red line denotes the euhydrated threshold defined at 295 mOsmol/kg. (J) Tre profiling (mean ± SD) during the MRI scans. Blue lines denote the baseline profiles while red lines denote the post-intervention profiles. The color bar below the graph shows the sequence of scans performed across the duration of the study. Significant difference between trials is denoted by asterisk (* p < 0.05, ** p < 0.01, *** p < 0.001). The symbol Δ represents significant difference (p < 0.05) from the rest of the trials. ASL = arterial spin labeling; bpm = beats per minute; CL = cooling trial; CT = control trial; EX = exertional hyperthermia trial; fMRI = functional magnetic resonance imaging; HR = heart rate; Int = Intervention; MPRAGE = magnetization prepared rapid gradient recalled echo; MRI = magnetic resonance imaging; MRS = magnetic resonance spectroscopy; PA = passive hyperthermia trial; Tre = rectal temperature.
During the MRI scans, a hyperthermic state was maintained in EX and PA trials (Tre = 38.5°C ± 0.1°C; Fig. 2J). For CL and CT trials, the Tre was maintained lower, at 37.7°C ± 0.1°C and 36.3°C ± 0.1°C, respectively.
3.2. Brain temperature
At baseline, Tbr was consistently higher by ∼0.6°C than Tre (Tbr = 37.3°C ± 0.4°C vs. Tre = 36.7°C ± 0.2°C, p < 0.05) (Fig. 3A–3D). For the EX and CL trials, post-intervention Tbr corresponded with the hyperthermic Tre (EX: p = 0.887, CL: p = 0.574) (Fig. 3A and 3C), showing that Tbr is elevated similarly to Tre. However, in the PA trial (Fig. 3B), post-intervention Tbr was lower than Tre (p < 0.05), with a lower magnitude of temperature elevation from baseline (+0.9°C ± 0.5°C vs. +2.1°C ± 0.2°C). In CT trials (Fig. 3D), post-intervention Tbr remained consistently higher than resting Tre (p < 0.01).
Fig. 3.
Tbr and cerebral perfusion under hyperthermia. Tbr and Tre measurements (mean ± SD) during pre- and post-intervention MRI scans for (A) EX, (B) PA, (C) CL, and (D) CT trials. Tbr is denoted by solid lines while Tre is denoted by dotted lines. (E) Cerebral perfusion changes across the cortical surface in all trials. Color scale represents the cluster significance (-log(p)) where 4.00 denotes p < 0.0001, 1.33 denotes p < 0.05; red gradient denotes increased perfusion while blue gradient denotes decreased perfusion. Normalized % change in cerebral perfusion across trials in (F) whole brain (restricted to gray matter); (G) precentral gyrus, (H) postcentral gyrus, (I) insula, and (J) thalamus. Bars represent the mean, and lines represent the SD. Each symbol represents a value from an individual participant (n = 11). Significant difference between trials is denoted by asterisk (* p < 0.05, ** p < 0.01, *** p < 0.001). CL = cooling trial; CT = control trial; EX = exertional hyperthermia trial; GM = gray matter; MRI = magnetic resonance imaging; PA = passive hyperthermia trial; Tbr = brain temperature; Tre = rectal temperature.
3.3. Cerebral blood flow
Reductions in cerebral perfusion (p < 0.0001) were observed across parietal and frontal areas in EX trials (Fig. 3E). These deficits appeared greater and more distributed across cortical areas in PA trials. In contrast, the reduced cerebral perfusion in CL and CT trials was confined to smaller cortical regions, and it was less pronounced in CT trials (Fig. 3E). Global gray matter perfusion was reduced in the post scans of PA trials as compared with baseline (p = 0.016; Fig. 3F). Regional perfusion in precentral and postcentral gyri were lowered in the post scans of PA, but not for CT and CL trials (precentral: p = 0.009; postcentral: p = 0.035; Fig. 3G and 3H). No differences in perfusion were observed for subcortical regions (insula and thalamus) across scans (Fig. 3I and 3J).
3.4. Cortical motor activity
Motor task-related BOLD analysis was used to compare functional brain activity during plantarflexion at higher intensities (60% + 80% maximal voluntary contraction) with that of no-task baseline (refer to Supplementary Fig. 1 for combined analysis from all intensities). Voxelwise task-activated brain clusters from data at all timepoints are shown in Fig. 4A. All activated clusters are expected regions of interest related to task except for left lateral-orbitofrontal and right medial-orbitofrontal clusters, which could be a consequence of slight motion during plantarflexion. However, we found no significant differences in movement between baseline and post scans when assessed from both the maximum displacement and the rate of change of displacement. For the left hemispheric motor-related cluster (precentral gyrus; Fig. 4B), the relative percent BOLD values in the post scans were lower than baseline for EX (p = 0.009) and PA (p = 0.006) but not for CL trials. The left paracentral cluster (Fig. 4C) presented a lower relative percent BOLD value in the post scans for EX (p = 0.014). A precentral gyrus cluster was observed in the right hemisphere (ipsilateral to leg movement; Fig. 4F) with relative percent BOLD values reduced from baseline to post scans for both EX (p = 0.0009) and PA (p = 0.026).
Fig. 4.
Functional activation (BOLD) during plantarflexion motor task. (A) Task-related activation (60% + 80% MVC vs. no-task). Activated brain clusters include precentral gyrus, superior frontal gyrus, paracentral lobule, medial orbitofrontal cortex, lateral orbitofrontal cortex, and superior parietal gyrus. Color scale represents the cluster significance (-log(p)) where 4.00 denotes p < 0.0001, 1.33 denotes p < 0.05; red gradient denotes increased activity while blue gradient denotes decreased activity. Normalized percent BOLD change in baseline (S1) and post-intervention (S2) scans in LH: (B) precentral, (C) paracentral, (D) superior frontal, and (E) lateral orbitofrontal clusters; in RH: (F) precentral and (G) superior parietal clusters. Each symbol represents a value from an individual participant (n = 11). Significant difference between trials is denoted by asterisk (* p < 0.05, ** p < 0.01, *** p < 0.001). BOLD = blood oxygenation level-dependent; CL = cooling trial; EX = exertional hyperthermia trial; LH = left hemisphere; MVC = maximal voluntary contraction; PA = passive hyperthermia trial; RH = right hemisphere.
Other clusters observed to exhibit significant changes included the left superior-frontal and right superior-parietal clusters. Similar to other clusters, the left hemispheric superior-frontal cluster (Fig. 4D) showed a lower relative percent BOLD value in the post scan for PA trial (p = 0.020), and the right hemisphere superior-parietal cluster similarly reduced relative percent BOLD values in the post scans for both EX (p = 0.003) and PA (p = 0.012) trials (Fig. 4G). While no significant changes were seen in the right medial-orbitofrontal cluster, the left hemispheric lateral-orbitofrontal cluster (Fig. 4E) showed lower relative percent BOLD values than the baseline scans (p < 0.05).
3.5. Executive function and associated brain activity
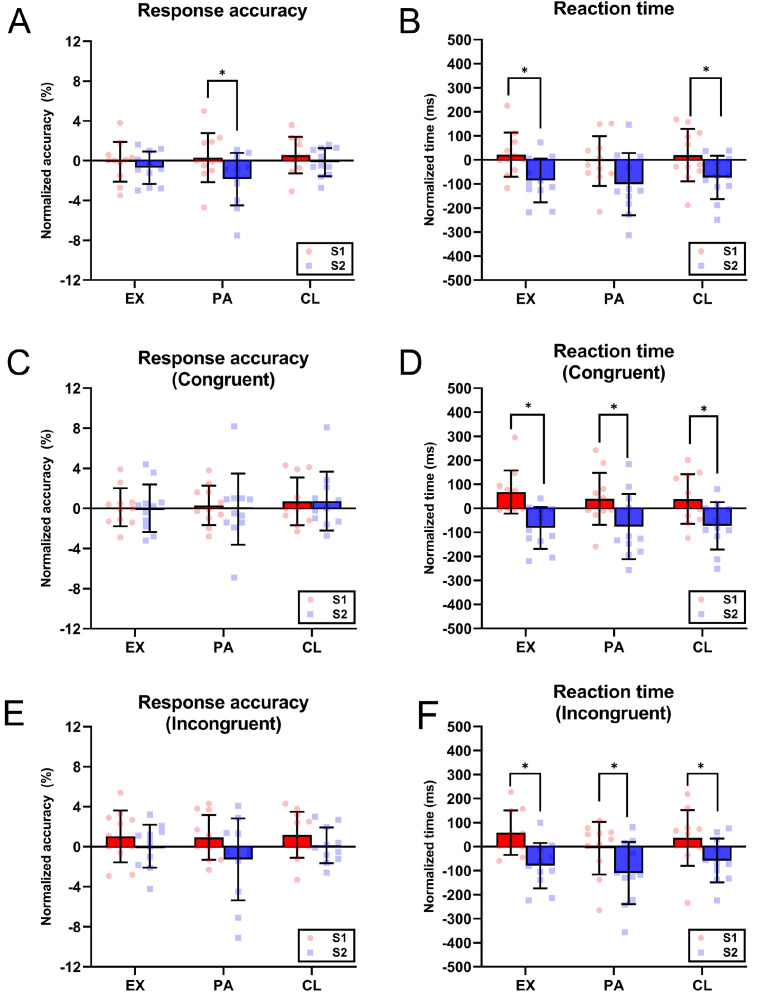
Faster reaction times were observed in the post scans of both EX and CL trials compared to baseline (EX: p = 0.045; CL: p = 0.048; Fig. 5B), though a reduction in response accuracy was noted exclusively in the post scans of PA trials (p = 0.017) (Fig. 5A).
Fig. 5.
Response accuracy and reaction time for Stroop color-naming task. Normalized change in baseline (S1) and post-intervention (S2) scans in overall (A) Stroop response accuracy and (B) reaction time for EX, PA, and CL trials. Independent stimulus response analysis for Stroop task shows the normalized change in S1 and S2 scans in response accuracy and reaction time for (C and D) congruent stimuli and (E and F) incongruent stimuli, respectively. Bars represent the mean, and lines represent the SD. Each symbol represents a value from individual participant (n = 11). Significant difference between trials is denoted by asterisk (* p < 0.05). CL = cooling trial; EX = exertional hyperthermia trial; PA = passive hyperthermia trial.
The reduced task accuracy could be attributed to either attentional deficit or impaired conflicting resolving ability, thus the responses to congruent or incongruent stimuli were analyzed independently (refer to Supplementary Table 1 for overall Stroop interference scores). The % change in accuracy for congruent stimuli was similar across trials (Fig. 5C). However, the % change in accuracy for incongruent stimuli (Fig. 5E) tended to be lower in PA trials (p = 0.083), which could account for the overall lowered response accuracy. The reaction times, for both congruent and incongruent stimuli, were faster in the post scans of EX, CL, and PA trials (p < 0.05) (Fig. 5D and 5F).
To assess changes in brain function, task-activated BOLD clusters were extracted (Fig. 6A). No significant changes were found for EX or PA trials relative to baseline; however, increased activities in left and right superior-temporal sulci were observed in CL trials (left: p = 0.014; right: p = 0.032; Fig. 6B). No clusters were found when comparing between congruent and incongruent stimuli across trials. To investigate the neural changes underlying the lower task accuracy in PA trials, paired voxelwise comparison was performed between the post scans of PA and CT. For task-related activation, PA trials showed greater activity in the precentral gyrus and depressed activities in superior-temporal and superior-parietal gyri (Fig. 6C). In terms of specific comparison between congruent and incongruent stimuli, lower BOLD activity was found in the left lateral occipital cortex in PA trials (Fig. 6D).
Fig. 6.
Functional activation (BOLD) during Stroop color-naming task. (A) Task-related activation (task vs. no-task). Activated brain clusters include superior-parietal gyrus, supramarginal gyrus, superior-frontal gyrus, caudal middle frontal gyrus, rostral middle frontal gyrus, lateral orbitofrontal cortex, medial orbitofrontal cortex, lateral occipital cortex, superior temporal sulcus, isthmus cingulate cortex, and pars opercularis. (B) Normalized percent BOLD change (relative to time-matched CT values) in baseline (S1) and post-intervention (S2) scans in left and right hemispheres: superior-temporal clusters. Bars represent the mean, and lines represent the SD. Each symbol represents a value from an individual participant (n = 11). Significant difference between trials is denoted by asterisk (* p < 0.05). (C) PA vs. CT post scans comparison for task-related activation (task vs. no-task). Significant brain clusters (p < 0.05) include precentral gyrus, superior parietal gyrus, superior temporal gyrus. (D) PA vs. CT post scans comparison for incongruent vs. congruent stimuli. Significant brain cluster in left hemisphere – lateral occipital cortex. The color scale in A, C, and D represents the cluster significance (-log(p)) where 4.00 denotes p < 0.0001, 1.33 denotes p < 0.05; red gradient denotes increased activity while blue gradient denotes decreased activity. BOLD = blood oxygenation level-dependent; CL = cooling trial; CT = control trial; EX = exertional hyperthermia trial; LH = left hemisphere; PA = passive hyperthermia trial; RH = right hemisphere.
4. Discussion
To date, our understanding of the relationship between Tbr and systemic body temperature is largely informed by clinical studies examining patients with brain injuries.29, 30, 31 In healthy individuals, Tbr is traditionally assessed with indirect measurements such as the tympanic membrane temperature.32 However, the validity of this method has been challenged because tympanic temperature can be influenced by ambient air temperature and skin temperature of the head.33 In our study, 1H MRS thermometry allowed for non-invasive Tbr estimation by measuring the chemical shift of water molecules, whose frequency shifts linearly with temperature34 in reference to NAA with a temperature-independent chemical shift. This enabled us to reliably assess Tbr in hyperthermic volunteers while relating it to Tre in real time.
The resting Tbr was observed to be consistently higher than Tre, which corroborates the findings of a recent study comparing resting Tbr to oral temperature in healthy humans.35 A higher resting Tbr could be attributed to the relatively greater metabolic rate of the brain, which accounts for 20% of our body oxygen consumption at rest despite representing only ∼2% of body mass. Brain metabolism has been shown to be remarkably constant over time36 and could account for the maintained Tbr despite a lowered Tre in the post scans for CT trials. Fluctuations in temperature can affect the properties of ion channels and neuronal excitability.37 Hence, the regulation of resting Tbr within a tighter range (between 37.2°C and 37.4°C) could be critical in maintaining optimal neural function despite changes in body temperature.
Tbr was found to be elevated with the development of body hyperthermia. This concurs with the past finding33 showing a concomitant increase in jugular venous temperature (indicative of Tbr) and esophageal temperature with exercise. For EX and CL trials, Tbr was elevated to similar levels as Tre but not higher. Interestingly, Tbr was lower than Tre with passive hyperthermia. Since the PA trial did not involve motor-related activity, the lower Tbr could be due to a less active motor cortex with lower metabolic heat production. Moreover, the reduced motor cortical perfusion in the PA trial could have limited heat transfer from blood circulation since heat was introduced externally via warm water immersion. Dovetailing a higher basal Tbr with the convergence of Tbr and Tre under hyperthermia, Tbr could be regulated in a narrower homeostatic range relative to Tre. The distinction between the rate of rise of Tbr and Tre under hyperthermia suggests potential mechanisms supporting selective brain cooling in humans.38 During hyperthermia, the Tbr could be selectively lowered to confer neuroprotection from the excessive rise in temperature and to preserve neural function,39 though the exact mechanism(s) by which this could be mediated is unclear and, thus, warrants further investigation.
In conjunction with an elevated Tbr, the cortical motor activity was suppressed by hyperthermia during execution of high-intensity motor tasks. However, a similar deficit was absent with the ingestion of ice. High force exertion has been shown to associate with a stronger BOLD signal in the motor-related cortices as more cortical neurons participate in generating descending motor commands.40 Here, the diminished BOLD signal likely indicates a downstream impairment to motor function, though the extent of physical deficit remains to be determined. Functional activity in other motor-related regions was also affected by heat stress, including the paracentral lobule implicated for lower extremity movement,41 the superior-parietal gyrus involved in visuomotor integration and control of movements,42 and the superior-frontal cluster encompassing the premotor area for execution of skilled action sequences.43 Lower superior-frontal cortex activity under passive hyperthermia could affect motor coordination as lesions in the same region have been associated with deficits in complex and bilateral motor movements.44 Last, the reduced lateral-orbitofrontal activity could relate to changes in affective responses during heat exposure, which modulate exercise tolerance.45
We postulate that the suppressed functional activity in motor-related brain regions could result from altered excitability or transient neuronal dysfunction with elevated Tbr. While the effects of temperature fluctuations on human neural function have not been well-characterized, variation of Tbr in animal models by 1°C–3°C has been reported to affect neural function at multiple levels, including transmembrane ionic transport, action potential generation, and properties of passive membrane.46 Furthermore, heat stroke is known to cause neurological dysfunction in humans, underscoring the adverse impact on neuronal function.47
Hyperthermia is also known to impair brain blood flow during prolonged exercise in a hot environment.48,49 In our study, the global cerebral perfusion was depressed in the PA trial, which could be associated with the vasoconstriction of cerebral arteries triggered by a fall in arterial carbon dioxide tension with hyperthermia-induced hyperventilation.50 Given the lower production of metabolic carbon dioxide, passive hyperthermia can elicit a greater magnitude of hypocapnic response and, thus, a larger perfusion deficit relative to exertional hyperthermia.51 Interestingly, subcortical perfusion was well-preserved whereas perfusion in cortical areas was reduced. This finding concurs with another study16 that demonstrated similar reductions in sensorimotor perfusion after passive hyperthermia. This could be a physiological defense mechanism where perfusion is redistributed to critical brain areas involved in thermoregulatory behavior, such as the thalamus, insula, and cingulate cortex.52
Cognition was also altered by hyperthermia, specifically the domains of selective attention and executive function. Executive function here refers to a set of cognitive processes and mental skills crucial for the cognitive control of behavior such as (but not limited to) working memory, cognitive flexibility, attentional control, and inhibitory control.53 The latter 2 components were assessed with the Stroop task in this study. During the Stroop task, reaction times were quicker with induced hyperthermia. Higher muscle temperatures can improve muscle contractility due to its thermal dependence,54 thus facilitating faster response times. A hyperthermic state could also have contributed to anxiety and discomfort, which may have prompted quicker responses. Despite faster reaction times, task accuracy was not compromised in EX and CL trials, indicating that executive control was well-preserved under exertional hyperthermia. The absence of cognitive deficits in exertional trials could be attributed to the elevated arousal brought about by exercise, which benefits cognition.55 In contrast, the faster reaction time in PA trials was coupled with a lowered task accuracy specific to incongruent stimuli. Given that the task accuracy for congruent stimuli was maintained, attentional capacity was likely preserved, but the ability to resolve conflict related to Stroop interference was compromised. Moreover, the response times were quicker, suggesting an absence of speed–accuracy trade off (i.e., slower response times to ensure accuracy). This could be due to aberrant decision-making or a deficit in response inhibition. Shibasaki et al.56 reported similar findings where passive heat exposure led to an increased error rate in a Go/No-go task, suggesting an impairment in executive function. In real-world settings, this reduced inhibitory control could result in a greater likelihood of work-related errors or unsafe work behavior when workers experience high heat strain from excessive heat exposure. In contrast, it could be possible that manual workers who carry out exertional work under heat may be less affected cognitively and, instead, may benefit from enhanced reaction time.
The Stroop task-activated brain regions (e.g., medial frontal gyrus, inferior prefrontal areas, parietal cortex) were similar to those found in other MRI studies.57,58 Increased bilateral superior-temporal sulcus activity during the post scans in CL trials could be due to the heightened arousal induced by exercise that was less counteracted by heat strain. Nonetheless, there was no functional gain from the increase in BOLD activity, as indicated by the unaltered Stroop task performance. When comparing PA to CT post scans (incongruent vs. congruent stimuli), the left hemispheric lateral occipital cortex was less active in PA trials. This region corresponds to Brodmann Area 19 (V3/V3a), which is thought to be linked with visual fatigue.59 Thus, a possible deficit in visual processing of stimuli could partially account for the reduced task accuracy.
Several limitations in this study should be noted. Firstly, a higher Tre (>38.5°C) could not be maintained during the scans due to the efficient thermoregulatory responses in humans and the cold temperature of the scanner room. The observed functional deficits would likely be exacerbated if Tre was further elevated. Second, the participants lost modest amounts of body fluids due to sweating (<3% body mass loss) during the scan. Ingestion of water during MRI scan is complicated to execute as the drinking action would introduce unnecessary noise; hence, no fluids were provided. Nonetheless, the post-scan serum osmolality was only marginally above 295 mOsmol/kg, indicating a minor contribution (if any) of dehydration to the results. In this study, only male participants were recruited, and thus it is pertinent to consider that there could be sex differences in brain properties and function60,61 that may modulate differential brain responses to heating. Last, the difficulty of replicating dynamic exercise within the scanner restricts our ability to investigate neural changes at the “point of fatigue” in a realistic exercise setting.
5. Conclusion
Our study demonstrated for the first time that resting Tbr is regulated in a narrower homeostatic range than Tre, which was disrupted by hyperthermia. Importantly, we also showed that this led to the suppression of motor cortical activity and impaired inhibitory control in humans. This emphasizes the vulnerability of the human brain to heat stress and highlights the importance of future strategies to lower or maintain Tbr within an optimal range for the preservation of neural function. Furthermore, the identified brain regions with neural changes may be exploited for early detection of over-exertion under heat (e.g., detecting heat-deficit signatures with wearable devices using functional near-infrared spectroscopy62). Poorer executive function under passive heat exposure is particularly relevant to occupational settings where heat strain may impair decision making, thereby causing occupational hazards. Limiting the increase in body temperature with a preemptive cooling measure (ice ingestion) is a viable strategy for preventing heat-related deficits. This study enhances our understanding of cerebral responses to heat stress, paving the way for the development of novel heat mitigation strategies and better heat management in sporting and occupational settings.
Acknowledgments
We would like to thank the participants for their efforts and commitment to the study and the radiographers and nurses for their rendered assistance in MRI scans. We thank Ivan Teng Po Wen for his invaluable help with the design and conduct of Stroop Test during the fMRI scans. We express our sincere gratitude to the students (Lemuel Teo Wei En, Yuen Wing Yee, and Tan Shi Pei) who helped with the conduct of trials and the collection of data. This research was supported by Defence Innovative Research Program (DIRP) Grant (PA No. 9015102335) from Defence Research & Technology Office, Ministry of Defence, Singapore.
Authors’ contributions
ICCL, TWS, and JKWL were involved in experimental conception and design, interpretation of study results, and critical revision of manuscript; XRT, SBA, and KWZL carried out data collection, data analysis, interpretation of results, and drafted the manuscript; MCS supported the MRI scans and MRI data analysis, and critically revised the manuscript. All authors have read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Peer review under responsibility of Shanghai University of Sport.
Supplementary materials associated with this article can be found in the online version at doi:10.1016/j.jshs.2023.09.001.
Contributor Information
Jason K.W. Lee, Email: phsjlkw@nus.edu.sg.
Ivan C.C. Low, Email: phsilcc@nus.edu.sg.
Supplementary materials
References
- 1.Spector JT, Sheffield PE. Re-evaluating occupational heat stress in a changing climate. Ann Occup Hyg. 2014;58:936–942. doi: 10.1093/annhyg/meu073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kjellstrom T, Briggs D, Freyberg C, Lemke B, Otto M, Hyatt O. Heat, human performance, and occupational health: A key issue for the assessment of global climate change impacts. Annu Rev Public Health. 2016;37:97–112. doi: 10.1146/annurev-publhealth-032315-021740. [DOI] [PubMed] [Google Scholar]
- 3.Périard JD, Racinais S, Timpka T, et al. Strategies and factors associated with preparing for competing in the heat: A cohort study at the 2015 IAAF World Athletics Championships. Br J Sports Med. 2017;51:264–270. doi: 10.1136/bjsports-2016-096579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soligard T, Palmer D, Steffen K, et al. New sports, COVID-19 and the heat: Sports injuries and illnesses in the Tokyo 2020 summer Olympics. Br J Sports Med. 2023;57:46–54. doi: 10.1136/bjsports-2022-106155. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y, Li Q, Ge Y, Du X, Wang H. Growing prevalence of heat over cold extremes with overall milder extremes and multiple successive events. Commun Earth Environ. 2022;3:73. doi: 10.1038/s43247-022-00404-x. [DOI] [Google Scholar]
- 6.González-Alonso J, Teller C, Andersen SL, Jensen FB, Hyldig T, Nielsen B. Influence of body temperature on the development of fatigue during prolonged exercise in the heat. J Appl Physiol (1985) 1999;86:1032–1039. doi: 10.1152/jappl.1999.86.3.1032. [DOI] [PubMed] [Google Scholar]
- 7.Cheuvront SN, Kenefick RW, Montain SJ, Sawka MN. Mechanisms of aerobic performance impairment with heat stress and dehydration. J Appl Physiol (1985) 2010;109:1989–1995. doi: 10.1152/japplphysiol.00367.2010. [DOI] [PubMed] [Google Scholar]
- 8.Ely BR, Cheuvront SN, Kenefick RW, Sawka MN. Aerobic performance is degraded, despite modest hyperthermia, in hot environments. Med Sci Sports Exerc. 2010;42:135–141. doi: 10.1249/MSS.0b013e3181adb9fb. [DOI] [PubMed] [Google Scholar]
- 9.González-Alonso J, Calbet JAL, Nielsen B. Metabolic and thermodynamic responses to dehydration-induced reductions in muscle blood flow in exercising humans. J Physiol. 1999;520:577–589. doi: 10.1111/j.1469-7793.1999.00577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hargreaves M. Physiological limits to exercise performance in the heat. J Sci Med Sport. 2008;11:66–71. doi: 10.1016/j.jsams.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 11.Sawka MN, Cheuvront SN, Kenefick RW. High skin temperature and hypohydration impair aerobic performance. Exp Physiol. 2012;97:327–332. doi: 10.1113/expphysiol.2011.061002. [DOI] [PubMed] [Google Scholar]
- 12.Nybo L, Nielsen B. Hyperthermia and central fatigue during prolonged exercise in humans. J Appl Physiol (1985) 2001;91:1055–1060. doi: 10.1152/jappl.2001.91.3.1055. [DOI] [PubMed] [Google Scholar]
- 13.Nybo L, Nielsen B. Perceived exertion is associated with an altered brain activity during exercise with progressive hyperthermia. J Appl Physiol (1985) 2001;91:2017–2023. doi: 10.1152/jappl.2001.91.5.2017. [DOI] [PubMed] [Google Scholar]
- 14.van den Heuvel AMJ, Haberley BJ, Hoyle DJR, Taylor NAS, Croft RJ. Hyperthermia, but not dehydration, alters the electrical activity of the brain. Eur J Appl Physiol. 2020;120:2797–2811. doi: 10.1007/s00421-020-04492-5. [DOI] [PubMed] [Google Scholar]
- 15.Tan XR, Low ICC, Stephenson MC, et al. Altered brain structure with preserved cortical motor activity after exertional hypohydration: A MRI study. J Appl Physiol (1985) 2019;127:157–167. doi: 10.1152/japplphysiol.00081.2019. [DOI] [PubMed] [Google Scholar]
- 16.Qian S, Jiang Q, Liu K, et al. Effects of short-term environmental hyperthermia on patterns of cerebral blood flow. Physiol Behav. 2014;128:99–107. doi: 10.1016/j.physbeh.2014.01.028. [DOI] [PubMed] [Google Scholar]
- 17.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14:377–381. [PubMed] [Google Scholar]
- 18.Young AJ, Sawka MN, Epstein Y, Decristofano B, Pandolf KB. Cooling different body surfaces during upper and lower body exercise. J Appl Physiol. 1987;63:1218–1223. doi: 10.1152/jappl.1987.63.3.1218. [DOI] [PubMed] [Google Scholar]
- 19.Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychol. 1935;18:643–662. [Google Scholar]
- 20.Cady EB, D'Souza PC, Penrice J, Lorek A. The estimation of local brain temperature by in vivo 1h magnetic resonance spectroscopy. Magn Reson Med. 1995;33:862–867. doi: 10.1002/mrm.1910330620. [DOI] [PubMed] [Google Scholar]
- 21.Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 22.Reuter M, Schmansky NJ, Rosas HD, Fischl B. Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage. 2012;61:1402–1418. doi: 10.1016/j.neuroimage.2012.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Avants BB, Tustison NJ, Song G, Cook PA, Klein A, Gee JC. A reproducible evaluation of ants similarity metric performance in brain image registration. Neuroimage. 2011;54:2033–2044. doi: 10.1016/j.neuroimage.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Z, Aguirre GK, Rao H, et al. Empirical optimization of asl data analysis using an asl data processing toolbox: Asltbx. Magn Reson Imaging. 2008;26:261–269. doi: 10.1016/j.mri.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dale AM, Buckner RL. Selective averaging of rapidly presented individual trials using fMRI. Hum Brain Mapp. 1997;5:329–340. doi: 10.1002/(SICI)1097-0193(1997)5:5<329::AID-HBM1>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 26.Carey JR, Greer KR, Grunewald TK, et al. Primary motor area activation during precision-demanding versus simple finger movement. Neurorehabil Neural Repair. 2006;20:361–370. doi: 10.1177/1545968306289289. [DOI] [PubMed] [Google Scholar]
- 27.Cian C, Koulmann N, Barraud PA, Raphel C, Jimenez C, Melin B. Influences of variations in body hydration on cognitive function: Effect of hyperhydration, heat stress, and exercise-induced dehydration. J Psychophysiol. 2000;14:29–36. [Google Scholar]
- 28.Moran DS, Shitzer A, Pandolf KB. A physiological strain index to evaluate heat stress. Am J Physiol. 1998;275:R129–R134. doi: 10.1152/ajpregu.1998.275.1.R129. [DOI] [PubMed] [Google Scholar]
- 29.Childs C, Vail A, Protheroe R, King AT, Dark PM. Differences between brain and rectal temperatures during routine critical care of patients with severe traumatic brain injury. Anaesthesia. 2005;60:759–765. doi: 10.1111/j.1365-2044.2005.04193.x. [DOI] [PubMed] [Google Scholar]
- 30.Rumana CS, Gopinath SP, Uzura M, Valadka AB, Robertson CS. Brain temperature exceeds systemic temperature in head-injured patients. Crit Care Med. 1998;26:562–567. doi: 10.1097/00003246-199803000-00032. [DOI] [PubMed] [Google Scholar]
- 31.Karaszewski B, Carpenter TK, Thomas RG, et al. Relationships between brain and body temperature, clinical and imaging outcomes after ischemic stroke. J Cereb Blood Flow Metab. 2013;33:1083–1089. doi: 10.1038/jcbfm.2013.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mariak Z, Lewko J, Luczaj J, Połocki B, White MD. The relationship between directly measured human cerebral and tympanic temperatures during changes in brain temperatures. Eur J Appl Physiol Occup Physiol. 1994;69:545–549. doi: 10.1007/BF00239873. [DOI] [PubMed] [Google Scholar]
- 33.Nybo L, Secher NH, Nielsen B. Inadequate heat release from the human brain during prolonged exercise with hyperthermia. J Physiol. 2002;545:697–704. doi: 10.1113/jphysiol.2002.030023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Corbett RJ, Laptook AR, Tollefsbol G, Kim B. Validation of a noninvasive method to measure brain temperature in vivo using 1h NMR spectroscopy. J Neurochem. 1995;64:1224–1230. doi: 10.1046/j.1471-4159.1995.64031224.x. [DOI] [PubMed] [Google Scholar]
- 35.Rzechorzek NM, Thrippleton MJ, Chappell FM, et al. A daily temperature rhythm in the human brain predicts survival after brain injury. Brain. 2022;145:2031–2048. doi: 10.1093/brain/awab466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raichle ME, Gusnard DA. Appraising the brain's energy budget. Proc Natl Acad Sci U S A. 2002;99:10237–10239. doi: 10.1073/pnas.172399499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sarria I, Ling J, Gu JG. Thermal sensitivity of voltage-gated Na+ channels and A-type K+ channels contributes to somatosensory neuron excitability at cooling temperatures. J Neurochem. 2012;122:1145–1154. doi: 10.1111/j.1471-4159.2012.07839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.White MD, Greiner JG, McDonald PLL. Point: Humans do demonstrate selective brain cooling during hyperthermia. J Appl Physiol (1985) 2011;110:569–571. doi: 10.1152/japplphysiol.00992.2010. [DOI] [PubMed] [Google Scholar]
- 39.Marino FE. The critical limiting temperature and selective brain cooling: Neuroprotection during exercise? Int J Hyperthermia. 2011;27:582–590. doi: 10.3109/02656736.2011.589096. [DOI] [PubMed] [Google Scholar]
- 40.Dai TH, Liu JZ, Sahgal V, Brown RW, Yue GH. Relationship between muscle output and functional MRI-measured brain activation. Exp Brain Res. 2001;140:290–300. doi: 10.1007/s002210100815. [DOI] [PubMed] [Google Scholar]
- 41.Jiang T, Wu W, Wang X, Weng C, Wang Q, Guo Y. Activation of brain areas following ankle dorsiflexion versus plantar flexion: Functional magnetic resonance imaging verification. Neural Regen Res. 2012;7:501–505. doi: 10.3969/j.issn.1673-5374.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iacoboni M. Visuo-motor integration and control in the human posterior parietal cortex: Evidence from TMS and fMRI. Neuropsychologia. 2006;44:2691–2699. doi: 10.1016/j.neuropsychologia.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 43.Solopchuk O, Alamia A, Zénon A. The role of the dorsal premotor cortex in skilled action sequences. J Neurosci. 2016;36:6599–6601. doi: 10.1523/JNEUROSCI.1199-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martino J, Gabarrós A, Deus J, et al. Intrasurgical mapping of complex motor function in the superior frontal gyrus. Neuroscience. 2011;179:131–142. doi: 10.1016/j.neuroscience.2011.01.047. [DOI] [PubMed] [Google Scholar]
- 45.Robertson CV, Marino FE. A role for the prefrontal cortex in exercise tolerance and termination. J Appl Physiol (1985) 2016;120:464–466. doi: 10.1152/japplphysiol.00363.2015. [DOI] [PubMed] [Google Scholar]
- 46.Kiyatkin EA, Brown PL, Wise RA. Brain temperature fluctuation: A reflection of functional neural activation. Eur J Neurosci. 2002;16:164–168. doi: 10.1046/j.1460-9568.2002.02066.x. [DOI] [PubMed] [Google Scholar]
- 47.Yang M, Li Z, Zhao Y, et al. Outcome and risk factors associated with extent of central nervous system injury due to exertional heat stroke. Medicine (Baltimore) 2017;96:e8417. doi: 10.1097/MD.0000000000008417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nybo L, Nielsen B. Middle cerebral artery blood velocity is reduced with hyperthermia during prolonged exercise in humans. J Physiol. 2001;534:279–286. doi: 10.1111/j.1469-7793.2001.t01-1-00279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nybo L, Møller K, Volianitis S, Nielsen B, Secher NH. Effects of hyperthermia on cerebral blood flow and metabolism during prolonged exercise in humans. J Appl Physiol (1985) 2002;93:58–64. doi: 10.1152/japplphysiol.00049.2002. [DOI] [PubMed] [Google Scholar]
- 50.Lassen NA, Christensen MS. Physiology of cerebral blood flow. Br J Anaesth. 1976;48:719–734. doi: 10.1093/bja/48.8.719. [DOI] [PubMed] [Google Scholar]
- 51.Nelson MD, Haykowsky MJ, Stickland MK, et al. Reductions in cerebral blood flow during passive heat stress in humans: Partitioning the mechanisms. J Physiol. 2011;589:4053–4064. doi: 10.1113/jphysiol.2011.212118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Flouris AD. Functional architecture of behavioural thermoregulation. Eur J Appl Physiol. 2011;111:1–8. doi: 10.1007/s00421-010-1602-8. [DOI] [PubMed] [Google Scholar]
- 53.Chan RC, Shum D, Toulopoulou T, Chen EY. Assessment of executive functions: Review of instruments and identification of critical issues. Arch Clin Neuropsychol. 2008;23:201–216. doi: 10.1016/j.acn.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 54.Bennett AF. Thermal dependence of muscle function. Am J Physiol. 1984;247:R217–R229. doi: 10.1152/ajpregu.1984.247.2.R217. [DOI] [PubMed] [Google Scholar]
- 55.Tomporowski PD. Effects of acute bouts of exercise on cognition. Acta Psychol (Amst) 2003;112:297–324. doi: 10.1016/s0001-6918(02)00134-8. [DOI] [PubMed] [Google Scholar]
- 56.Shibasaki M, Namba M, Oshiro M, Kakigi R, Nakata H. Suppression of cognitive function in hyperthermia; from the viewpoint of executive and inhibitive cognitive processing. Sci Rep. 2017;7:43528. doi: 10.1038/srep43528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Parris BA, Wadsley MG, Hasshim N, Benattayallah A, Augustinova M, Ferrand L. An fMRI study of response and semantic conflict in the Stroop task. Front Psychol. 2019;10:2426. doi: 10.3389/fpsyg.2019.02426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Song Y, Hakoda Y. An fMRI study of the functional mechanisms of Stroop/reverse-Stroop effects. Behav Brain Res. 2015;290:187–196. doi: 10.1016/j.bbr.2015.04.047. [DOI] [PubMed] [Google Scholar]
- 59.Tanaka M, Sadato N, Okada T, et al. Reduced responsiveness is an essential feature of chronic fatigue syndrome: A fMRI study. BMC Neurol. 2006;6:9. doi: 10.1186/1471-2377-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xin J, Zhang Y, Tang Y, Yang Y. Brain differences between men and women: Evidence from deep learning. Front Neurosci. 2019;13:185. doi: 10.3389/fnins.2019.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cosgrove KP, Mazure CM, Staley JK. Evolving knowledge of sex differences in brain structure, function, and chemistry. Biol Psychiatry. 2007;62:847–855. doi: 10.1016/j.biopsych.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Morren G, Wolf M, Lemmerling P, et al. Detection of fast neuronal signals in the motor cortex from functional near infrared spectroscopy measurements using independent component analysis. Med Biol Eng Comput. 2004;42:92–99. doi: 10.1007/BF02351016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.