Abstract
Background
Targeting interleukin-23 (IL-23) is an important therapeutic strategy for Crohn’s disease (CD).
Aims
This systematic review and meta-analysis assessed the efficacy and safety of selective IL-23p19 and IL-12/23p40 inhibitors in patients with moderate-to-severe CD.
Methods
MEDLINE, Embase, and the Cochrane library (CENTRAL) were searched from inception to May 24, 2023, for randomized, placebo- or active comparator-controlled induction and/or maintenance trials of selective IL-23p19 and IL-12/23p40 inhibitors in pediatric and adult patients with CD. The primary outcome was the proportion of patients in clinical remission. Secondary outcomes were clinical response, endoscopic remission, endoscopic response, and safety. Data were pooled using a random-effects model. Risk of bias and certainty of evidence were assessed using the Cochrane risk of bias tool and the GRADE criteria, respectively.
Results
Eighteen trials (n = 5561) were included. Most studies were rated as low risk of bias. Targeting IL-23 was significantly superior to placebo for inducing clinical (risk ratio [RR] = 1.87, 95% confidence interval [CI] 1.58–2.21) and endoscopic (RR = 3.20, 95%CI 2.17–4.70) remission and maintaining clinical remission (RR = 1.39, 95%CI 1.10–1.77) (GRADE high certainty evidence for all outcomes). Subgroup analysis showed that targeting IL-23 was superior to placebo for inducing clinical remission in biologic-naïve (RR = 2.20, 95%CI 1.46–3.32, I2 = 0%, p = 0.39) and biologic-experienced patients (RR = 1.82, 95%CI 1.27–2.60, I2 = 56.5%, p = 0.01). Targeting IL-23 was associated with a decreased risk of serious adverse events in induction (RR = 0.55, 95%CI 0.44–0.73) and maintenance (RR = 0.72, 95%CI 0.53–0.98) trials compared to placebo (high certainty evidence).
Conclusion
Targeting IL-23 is effective and safe for inducing and maintaining clinical and endoscopic remission in patients with moderate-to-severe CD.
Keywords: Interleukin-23 inhibitors, Ustekinumab, Biologic therapy, Crohn’s disease, Inflammatory bowel disease, Risankizumab
Introduction
Crohn’s disease (CD) is a chronic immune-mediated inflammatory disease (IMID) resulting from complex environmental interactions in genetically susceptible individuals. The introduction of infliximab as the first tumor necrosis factor alpha (TNF-α) antagonist nearly 25 years ago revolutionized the management of moderate-to-severely active CD [1]. While TNF-α antagonists are highly effective, approximately one-third of patients are primary non-responders to induction therapy, half of patients who have an initial response may lose response over time, and most patients do not achieve the guideline-recommended therapeutic target of endoscopic remission with anti-TNF therapy [1–3]. Thus, new approaches are needed.
Interleukin (IL)-23 is a critical inflammatory mediator, responsible for differentiation and expansion of the proinflammatory Th17 subset of CD4 + T-cells. In genome-wide association studies, IL-23 receptor (IL-23R) variants are strongly associated with the development of CD [4] and a recent study showed that patients refractory to TNF-α antagonists demonstrate immunological escape through increased expression of IL-23R on mucosal TNFR2 expressing CD4 + cells, indicating a potential therapeutic role for targeting IL-23 in this population [5]. IL-23 has 2 subunits (p40 and p19). Monoclonal antibodies targeting the shared p40 subunit block both IL-12 and IL-23 [6]. Ustekinumab was the first biologic targeting IL-12/23p40 approved for CD treatment, after pivotal phase III trials demonstrated superiority of ustekinumab over placebo for achieving and maintaining clinical remission (UNITI I and II and IM-UNITI) [7]. A subsequent head-to-head randomized controlled trial (RCT) of ustekinumab compared with adalimumab showed no difference in clinical remission rates at week 52 in patients with biologic-naïve CD [8]. However, in other IMIDs, such as psoriasis, targeting IL-23 specifically via the p19 subunit has resulted in significantly higher response rates compared to either TNF-α antagonists or ustekinumab [9, 10]. Accordingly, there has been substantial interest in developing IL-23p19 antagonists for CD, several RCTs investigating these agents have been reported, and the first agent in this class has recently been approved for CD (risankizumab) [11].
Given the expanding therapeutic armamentarium in CD and to better understand the efficacy and safety of IL-12/23p40 and IL-23p19 antagonists, we conducted a systematic review and meta-analysis of all RCTs evaluating these agents in moderate-to-severe CD.
Methods
This systematic review is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement [12].
Selection Criteria
We included phase II and III RCTs of pediatric and adult patients with moderate-to-severe CD that compared anti-IL-12/23p40 (e.g., ustekinumab, briakinumab, apilimod mesylate) or anti-IL-23p19 (e.g., brazikumab, risankizumab, guselkumab, mirikizumab) to placebo or an active comparator. Clinical, endoscopic, biomarker, quality of life, and safety outcome data were collected for both induction and maintenance studies.
Data Sources, Search Strategy, and Study Selection
MEDLINE, Embase, and the Cochrane CENTRAL Register of Controlled Trials were searched to May 24, 2023 (Supplementary Appendix 1). Two authors (SKV and AZ) independently performed title and abstract review to identify relevant studies. Full-text review determined eligibility according to pre-specified criteria. Discrepancies were resolved through discussion with a third author (JKM). The bibliographies of included studies, relevant review articles, and abstracts from conference proceedings (2010–2023) were manually searched for additional studies.
Data Abstraction and Quality Assessment
Data pertaining to study characteristics, participants, interventions, comparators, and outcomes were extracted by 2 independent investigators (SKV and AZ). Discrepancies were resolved through discussion with a third author (JKM). Risk of bias was assessed using the Cochrane risk of bias tool [13]. The GRADE approach was used to assess the certainty of evidence for primary and secondary outcomes [14]. Results from RCTs were initially considered high quality, but potentially downgraded due to risk of bias, indirectness of evidence, unexplained heterogeneity, publication bias, or sparse data/imprecision.
Outcomes
The primary outcome was the proportion of patients achieving or maintaining clinical remission at study endpoint, as defined by the original studies. If data from multiple time points were reported, data were extracted at 8 weeks for induction (range: week 6–16) and 52 weeks for maintenance (range: week 24–52) trials. Secondary outcomes included the proportion of patients achieving or maintaining clinical response, patient-reported outcome (PRO)-defined response or remission, endoscopic response, endoscopic remission, and ulcer-free endoscopy (i.e., mucosal healing), as defined by the original trial. Quality of life, adverse events (AEs), serious adverse events (SAEs), and withdrawal due to adverse events were also secondary outcomes. Subgroup analyses based on IL-12/23p40 vs. IL-23p19 inhibitors and prior exposure to biologics were performed.
Statistical Analysis
Pooled risk ratios (RRs) and corresponding 95% confidence intervals (CIs) were calculated using a random-effects model to account for between- and within-study heterogeneity, given differences in trial design and patient populations. Effect sizes were only pooled if there were 3 or more studies available per outcome. Data were analyzed on an intention-to-treat basis; patients lost to follow-up or excluded for other reasons were deemed treatment failures. Between-study heterogeneity was assessed using the I2 statistic [15]. All analyses were performed using the ‘metafor’ package R (version 4.0.1).
Results
Search Results and Included Studies
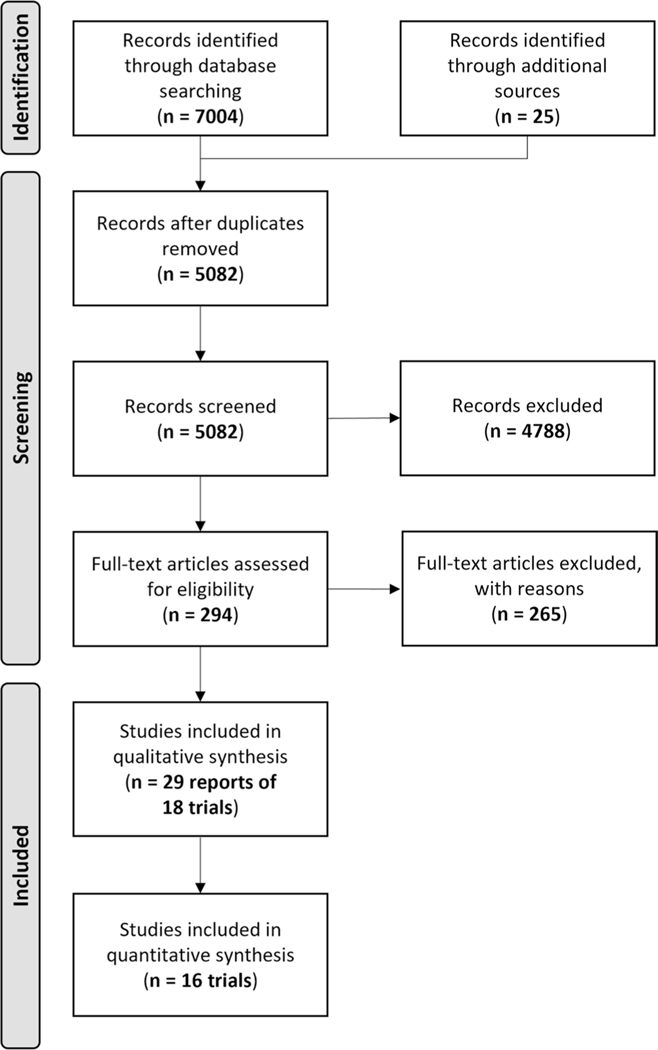
After removing duplicates, 5082 records were screened and 294 citations were selected for full-text review. A total of 29 records reporting data from 18 RCTs (n = 5561) were included (Fig. 1).
Fig. 1.
PRISMA flow diagram
Study Characteristics and Outcomes
Characteristics of the included studies are reported in Table 1. Ten of the included studies evaluated IL-12/23p40 inhibitors (ustekinumab, briakinumab, and apilimod mesylate) and 8 studies evaluated IL-23p19 inhibitors (brazikumab, risankizumab, guselkumab, and mirikizumab). Two trials were not placebo controlled and thus, were not included in the quantitative analysis [8, 16]. Of the remaining 16 RCTs, 8 were induction studies [7, 17–21], 2 were induction responder re-randomization maintenance studies [7, 22], and 6 studies included both induction and maintenance phases [23–28]. In the maintenance phase of the SERENITY study, all patients received both placebo and the study drug in a double-dummy design to maintain study blinding; hence, the maintenance data were not included in the quantitative analysis. Of the 18 trials, 7 recruited pre-dominantly biologic-experienced patients (proportion of biologic-experienced patients: 91–100%) [7, 16, 19, 21, 25, 27], 10 recruited both biologic-experienced and biologic-naïve patients (29–76%) [7, 17, 18, 20, 21, 24, 26, 28], and 1 trial recruited exclusively biologic-naïve patients [8]. Nine studies permitted previous exposure to TNF-α antagonists and 5 studies permitted previous exposure to either TNF-α antagonists or anti-integrin agents. Only the phase III risankizumab trials allowed previous exposure to ustekinumab [21, 22].
Table 1.
Baseline characteristics of the included studies
| Study ID | Number of participants | Intervention (n) comparator (n) | Trial phase | Induction/ maintenance | Sex | Disease location Ileum/ colon/ ileocolonic | Concomitant steroids | Concomitant immunosuppressants | Prior biologic exposure |
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Mannon 2004 [17] | Total = 79 BRA:63 PBO:16 |
Briakinumab Placebo | II | Induction | M:33% F: 67% |
BRI: 22%/32%/46% PBO: 50%/25%/25% |
BRI: 25% PBO: 37.5% |
BRI: 38% PBO: 12.5% |
BRI: NA PBO: NA |
| Sandborn 2008 [18] | Total = 104 UST:51 PBO:53 |
Ustekinumab Placebo | II | Induction | M:55% F:45% |
UST: 78.4%/55%/ PBO:75.4%/64%/- |
UST:33.3% PBO:30.2% |
UST:29.4% PBO:37.7% |
UST:41% PBO:51% |
| Sands 2010 [24] | Total = 220 AM:137 PBO:73 |
Apilimod mesylate Placebo |
II | Induction Maintenance | M:39.5% F:60.5% |
AM:NA PBO:NA |
AM:11.5% PBO:19% |
AM:4.0% PBO:1.0% |
AM:62.5% PBO:52% |
| Sandborn 2012 [25] (CERTIFI) | Total = 526 UST:394 PBO:132 |
Ustekinumab Placebo | II | Induction Maintenance | M:41.3% F:58.8% |
UST:NA PBO:NA |
UST:48% PBO:55.3% |
UST:24.4% PBO:22.7% |
UST:100% PBO:100% |
| Panaccione 2015 [26] | Total = 246 BRI:200 PBO:46 |
Briakinumab Placebo | II | Induction maintenance | M:33.7% F:66.3% |
BRI:71.7%/63%/- PBO:67.4%/58.7%/- |
BRI:46.2% PBO:47.8% |
BRI:20% PBO:21.7% |
BRI:73.9% PBO:75.5% |
| NCT02574637 [23] | Total = 29 BRA:24 PBO:5 |
Brazikumab Placebo | II | Induction maintenance | M:42.8% F:57.2% |
BRA:NA PBO:NA |
BRA:NA PBO:NA |
BRA:NA PBO:NA |
BRA:100% PBO:100% |
| Feagan 2016 [7] (UNITI-1) | Total = 741 UST: 494 PBO:247 |
Ustekinumab Placebo | III | Induction maintenance | M: 42.8% F:57.2% |
UST:15.2%/15.4%/69.2% PBO: 11.4%/19.5%/67.5% |
UST:46.3% PBO:44.9% |
UST:30.7% PBO:32.8% |
UST:99.0% PBO:99.6% |
| Feagan 2016 [7] (UNITI-2) | Total = 628 UST:418 PBO:210 |
Ustekinumab Placebo | III | Induction maintenance | M:46.6% F:53.4% |
UST:24.5%/20.8%/54.2% PBO:21%/17.6%/61.4% |
UST:41.2% PBO:35.7% |
UST:35% PBO:34.8% |
UST:29.2% PBO:37.6% |
| Feagan 2016 [7] (IM-UNITI) | Total = 397 UST:264 PBO:133 |
Ustekinumab Placebo | III | Maintenance | M:43.5% F:56.5% |
UST:17%/19.7%/63.2% PBO:14.3%/21.1%/64.6% |
UST:46.2% PBO:44.4% |
UST:36.3% PBO:35.3% |
UST:60.2% PBO:60.9% |
| Feagan 2017 [19] | Total = 121 RIS:82 PBO:39 |
Risankizumab Placebo | II | Induction | M:38.8% F:61.2% |
RIS:20%/50%/29% PBO:13%/41%/46% |
RIS:20% PBO:15% |
RIS:15% PBO:21% |
RIS:92.7% PBO:95% |
| Sands 2017 [27] | Total = 121 BRA:60 PBO:61 |
Brazikumab Placebo | II | Induction maintenance | M:37.8% F:62.2% |
BRA:23.7%/27.1%/47.5% PBO:30%/30%/40% |
BRA:40.7% PBO:40% |
BRA:30.5% PBO:23.3% |
BRA:100% PBO:100% |
| Sands 2022 [28] (SERENITY) | Total = 191 MIR:127 PBO:64 |
Mirikizumab Placebo | II | Induction maintenance | M:48.7% F:51.3% |
MIR:16%/39.4%/43.3% PBO:17.2%/39.1%/43.8% |
MIR:28.3% PBO:32.8% |
MIR:33.8% PBO:29.7% |
MIR:60.6% PBO:67.2% |
| Sandborn 2022 [20] (GALAXI-1) | Total = 309 GUS:185 PBO:61 UST:63 |
Guselkumab Placebo | II | Induction | M:59.2% F:40.8% |
GUS:32.4%/41.1%/26.5% PBO:26.2%/42.6%/69.9% |
GUS:34.1% PBO:39.3% |
GUS:31.4% PBO:42.6% |
GUS:60% PBO:68.9% |
| D’Haens 2022 [21] (ADVANCE) | Total = 850 RIS:675 PBO:175 |
Risankizumab Placebo | III | Induction | M:54% F:46% |
RIS:15%/36%/50% PBO:11%/40%/49% |
RIS:30% PBO:29% |
RIS:24% PBO:24% |
RIS:58% PBO:55% |
| D’Haens 2022 [21] (MOTIVATE) | Total = 569 RIS:382 PBO:187 |
Risankizumab Placebo | III | Induction | M:51% F:49% |
RIS:14%/39%/47% PBO:14%/39%/47% |
RIS:34% PBO:36% |
RIS:23% PBO:21% |
RIS:100% PBO:100% |
| Rosh 2021 [16] (UNISTAR) | Total = 44 UST (3 mg/ kg): UST (9 mg/ kg): |
Ustekinumab (3 mg/kg) Ustekinumab (9 mg/kg) |
I | Induction maintenance | M:41% F:59% |
UST (3 mg/ kg):17%/35%/48% UST (9 mg/ kg):5%/30%/65% |
UST (3 mg/ kg):30% UST (9 mg/ kg):33% |
UST (3 mg/ kg):30% UST (9 mg/ kg):48% |
UST (3 mg/ kg):91% UST (9 mg/ kg):91% |
| Sands 2022 [8] (SEAVUE) | Total = 386 UST:191 ADA:195 |
Ustekinumab Adalimumab | III | Induction maintenance | M:48% F:52% |
UST:32%/14%/54% ADA:28%/17%/53% |
UST:22% ADA:24% |
UST:NA ADA:NA |
UST:0% ADA:0% |
| Ferrante 2022 [22] (FORTIFY) | Total = 462 RIS:292 PBO:164 |
Risankizumab Placebo | III | Maintenance | M:51.5% F:48.5% |
RIS:10%/43.2%/46.6% PBO:14%/38%/48% |
RIS:31.2% PBO:31% |
RIS:21.2% PBO:24% |
RIS:72.2% PBO:75% |
ADA adalimumab, AM apilimod mesylate, BRA brazikumab, BRI briakinumab; GUS guselkumab, MIR mirikizumab, NA not applicable, PBO placebo, RIS risankizumab, UST ustekinumab
Clinical remission (CDAI score < 150) was assessed at weeks 6 to 16 in the induction studies and at weeks 24 to 52 in the maintenance studies. Among the 7 induction studies that reported on endoscopic outcomes, all except 1 used SES-CD-based definitions. Most commonly, an SES-CD score ≤ 2 was used to define endoscopic remission and a ≥ 50% reduction from baseline was used to define endoscopic response.
Risk of Bias and Overall Quality of Evidence
All the studies were rated as having low or unclear risk of bias, except for NCT02574637 [23], which was rated as high risk of bias for “other sources of bias” (study was terminated early and only descriptive efficacy endpoints were reported, Supplementary Table 2). The results of the GRADE analyses are reported in Supplementary Tables 3 and 4.
Efficacy of IL-12/23p40 and IL-23p19 Antagonists as Induction Therapy
Clinical Outcomes
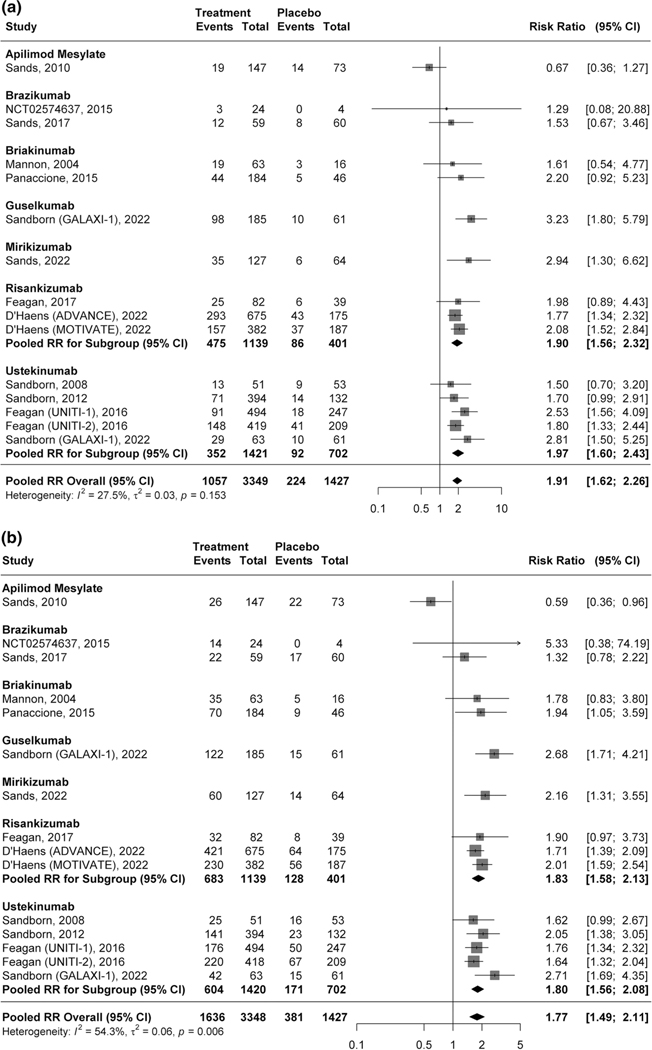
A total of 31.5% (1057/3349) of patients receiving an IL-12/23p40 or IL-23p19 inhibitor achieved clinical remission compared to 15.7% (224/1427) of patients assigned to placebo (RR 1.91, 95% CI 1.62–2.26, 15 studies, I2 = 27.5%, high certainty evidence; Fig. 2a). On subgroup analysis, there was no significant difference in the proportion of participants treated with an IL-12/23p40 antagonist achieved clinical remission (23.9%, 434/1815) compared to participants receiving an IL-23p19 inhibitor (37.6%, 1057/3349) (RR 0.87, 95% CI 0.61–1.24, p = 0.43; Supplementary Fig. 1; Supplementary Table 5).
Fig. 2.
a Pooled efficacy of IL-12/23p40 and IL-23p19 antagonists for inducing clinical remission. b Pooled efficacy of IL-12/23p40 and IL-23p19 antagonists for inducing clinical response
Forty-nine percent (1636/3348) of patients treated with an IL-12/23p40 or IL-23p19 antagonist had a clinical response (> 100-point reduction in CDAI score from baseline or a CDAI score < 150) compared with 27% of patients receiving placebo (381/1427). This difference was statistically significant (RR 1.77, 95% CI 1.49–2.11, 14 studies, I2 = 54.3%, moderate certainty of evidence; Fig. 2b). On subgroup analysis, clinical response was achieved by 40.5% (735/1814) and 48.8% (1636/3348) of patients treated with an IL-12/23p40 and IL-23p19 antagonist, respectively (RR 0.87, 95% CI 0.62–1.21, p = 0.41; Supplementary Fig. 2; Supplementary Table 5).
In subgroup analysis based on prior exposure to biologics, IL-12/23p40 and IL-23p19 antagonists were superior to placebo for inducing clinical remission (RR 2.20, 95% CI 1.46–3.32, I2 = 0%, p = 0.39; high certainty evidence; Supplementary Fig. 3) and clinical response (RR 1.39, 95% CI 1.05–1.83, I2 = 45.6%; high certainty evidence; Supplementary Fig. 4) in biologic-naïve patients. Similarly, IL-12/23p40 and IL-23p19 agents were superior to placebo for inducing clinical remission (RR 1.82, 95% CI 1.27–2.60, I2 = 56.5%; moderate certainty evidence; Supplementary Fig. 5) and response (RR 1.85, 95% CI 1.64–2.09, I2 = 41.1%; moderate certainty evidence; Supplementary Fig. 6) in biologic-experienced patients.
The UNISTAR study was the only pediatric RCT identified. This was a phase I pharmacokinetic study evaluating 2 doses of ustekinumab. At 16 weeks, 22% of patients in the low-dose arm (3 mg/kg or 130 mg) and 29% of patients in the high-dose arm (9 mg/kg or 390 mg) achieved clinical remission.
Endoscopic Outcomes
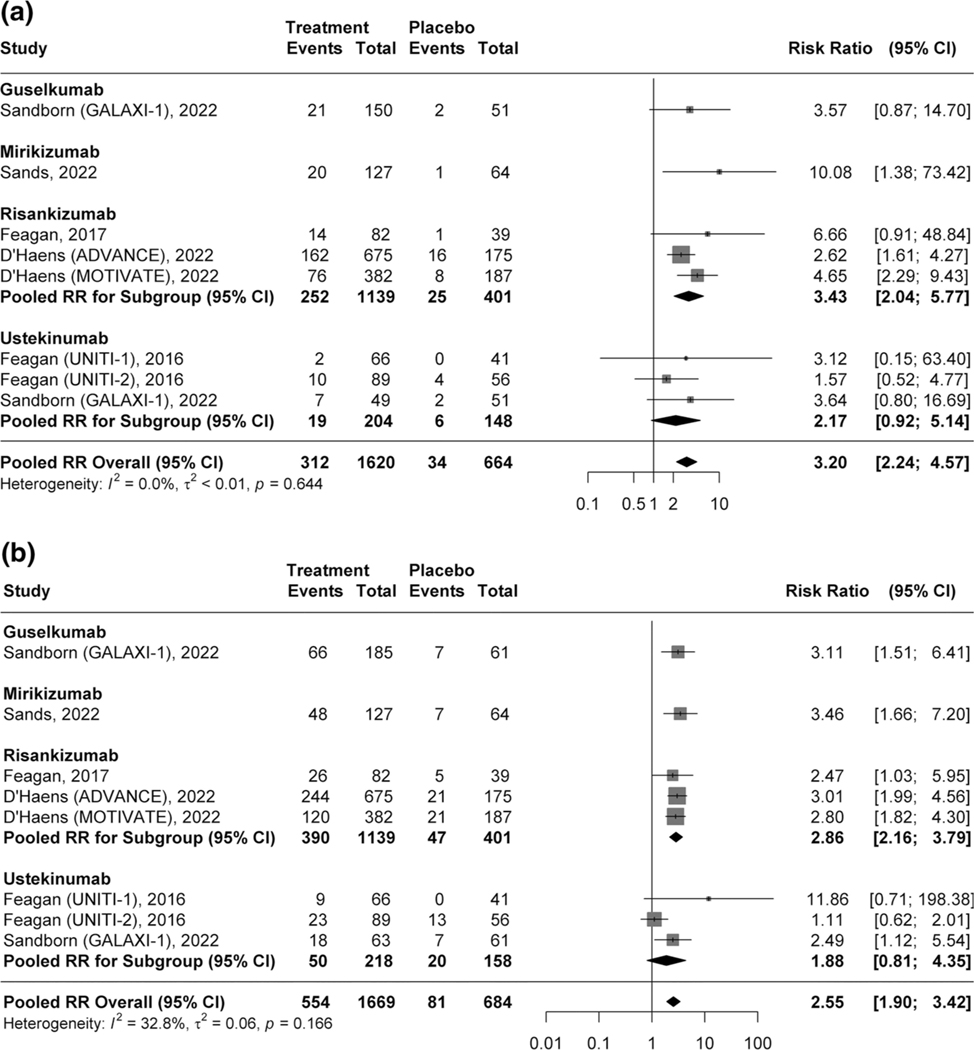
Overall, 19.2% (312/1620) of patients receiving an IL-12/23p40 or IL-23p19 inhibitor achieved endoscopic remission compared to 5.1% (34/664) patients receiving placebo (RR 3.20, 95% CI 2.24–4.57, 7 studies, I2 = 0%, high certainty evidence; Fig. 3a). The pooled analysis showed 33.2% (554/1669) and 15.8% (242/1534) had endoscopic response (RR 2.55, 95% CI 1.90–3.42, I2 = 32.8%, high certainty evidence; Fig. 3b) and ulcer-free endoscopy, respectively (RR 2.77, 95% CI 1.93–3.98, I2 = 0%, moderate certainty evidence; Supplementary Fig. 7) compared to 11.8% (81/684) and 5.1% (31/609) in patients receiving placebo.
Fig. 3.
a Pooled efficacy of IL-12/23p40 and IL-23p19 antagonists for inducing endoscopic remission. b Pooled efficacy of IL-12/23p40 and IL-23p19 antagonists for inducing endoscopic response
Treatment with IL-12/23p40 antagonists was not superior to placebo for inducing endoscopic remission or response, whereas treatment with IL-23p19 antagonists was significantly better than placebo for inducing all endoscopic outcomes. However, there was no significant difference between IL-12/23p40 and IL-23p19 antagonists for inducing endoscopic remission (RR 0.60, 95% CI 0.23–1.59, p = 0.30; Supplementary Table 5).
Efficacy of IL-12/23p40 and IL-23p19 Antagonists as Maintenance Therapy
Clinical Outcomes
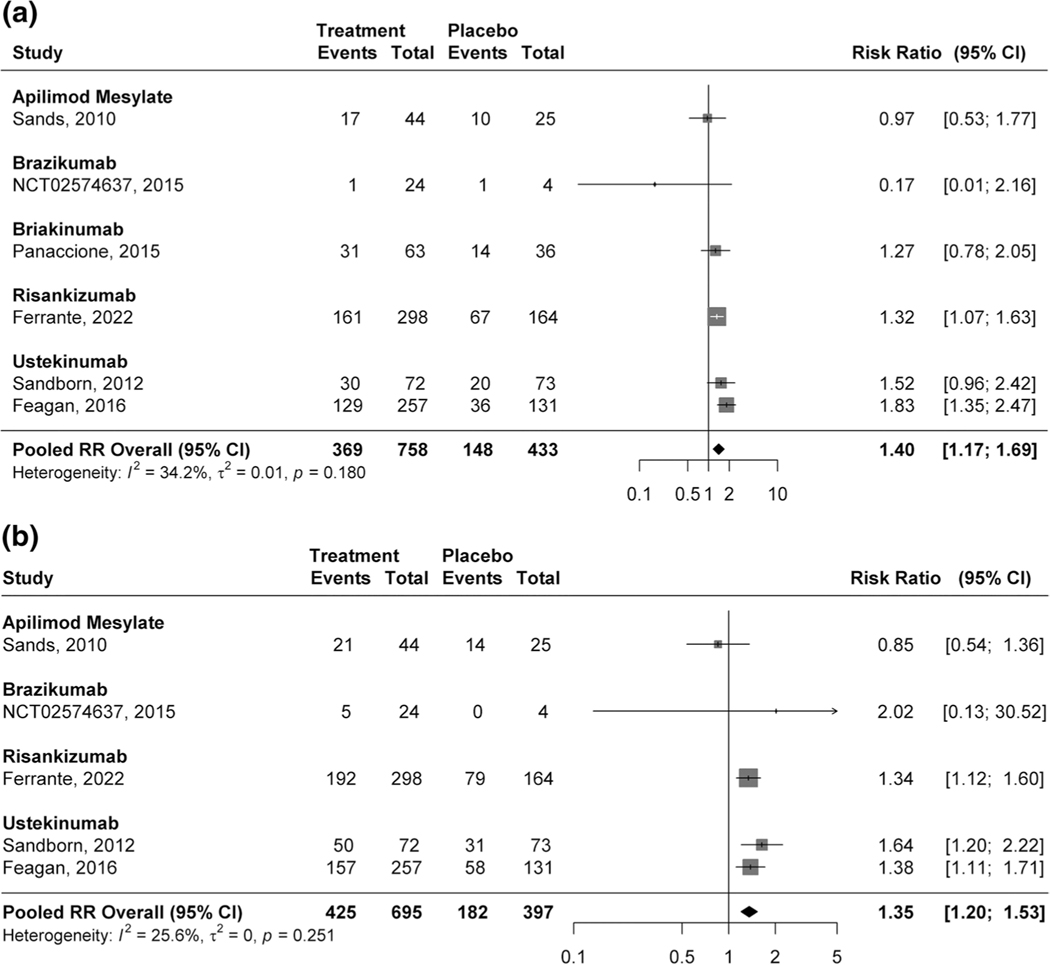
Forty-nine percent (369/758) of participants treated with IL-12/23p40 or IL-23p19 antagonists maintained remission compared with 34.2% (148/433) of patients randomized to placebo (RR 1.40, 95% CI 1.17–1.69, 6 studies, I2 = 34.2%, high certainty evidence; Fig. 4a). Clinical response was maintained in 61.1% (425/695) of patients treated with IL-12/23p40 or IL-23p19 agents compared with 45.8 (182/397) of participants receiving placebo (RR 1.35, 95% CI 1.20–1.53, 5 studies, I2 = 25.6%, Fig. 4b).
Fig. 4.
a Pooled efficacy of IL-12/23p40 and IL-23p19 antagonists for maintaining clinical remission. b Pooled efficacy of IL-12/23p40 and IL-23p19 antagonists for maintaining clinical response
Data on clinical remission stratified by prior biologic exposure were available for brazikumab, risankizumab, and ustekinumab. Pooled analysis demonstrated overall superiority in biologic-experienced patients (RR 1.39, 95% CI 1.1–1.77, 3 studies, I2 = 28%; Supplementary Fig. 8). Two studies reported maintenance of clinical remission in biologic-naïve patients [7, 22]. There was a numerically higher clinical remission rate among patients receiving active treatment compared to placebo in the IM-UNITI (60.9% vs 49%) and FORTIFY (68.7% vs 58.5%) studies.
Endoscopic Outcomes
Three maintenance studies reported endoscopic outcomes [7, 22, 23]. Pooled analyses showed that IL-12/23p40 and IL-23p19 agents were superior to placebo for maintaining endoscopic remission (RR 2.61, 95% CI 1.72–3.96, I2 = 0%, moderate certainty evidence; Supplementary Fig. 9) and response (RR 2.17, 95% CI 1.60–2.95, I2 = 0%, moderate certainty evidence; Supplementary Fig. 10). Among the individual agents, ustekinumab and brazikumab were not associated with better endoscopic outcomes compared to placebo. Risankizumab was superior to placebo for maintaining endoscopic response, remission, and ulcer-free endoscopy.
Patient-Reported Outcomes
Patients treated with IL-12/23p40 or IL-23p19 antagonists achieved statistically superior induction of Inflammatory Bowel Disease Questionnaire (IBDQ remission (29.7% vs 14.2%, RR 2.01, 95% CI 1.57–2.58, 6 studies, I2 = 33.1%; Supplementary Fig. 11), IBDQ improvement (RR 1.49, 95% CI 1.39–1.61, 7 studies, I2 = 0%; Supplementary Fig. 12), and PRO2 remission compared to placebo (RR 2.06, 95% CI 1.72–2.47, 6 studies, I2 = 0%; Supplementary Fig. 13) with high certainty evidence for all the 3 outcomes. In addition, treatment with IL-12/23p40 and IL-23p19 antagonists was superior to placebo for maintenance of IBDQ improvement (RR 1.36, 95% CI 1.20–1.53, 3 studies, I2 = 0%, high certainty evidence; Supplementary Fig. 14).
Safety Outcomes
Fifty-nine percent (2031/3418) of patients treated with an IL-12/23p40 or IL-23p19 antagonist experienced any AE compared to 65.1% (932/1431) of patients receiving placebo (RR 0.91, 95% CI 0.87–0.96, I2 = 0%; high certainty evidence Supplementary Fig. 15). Similar results were observed for SAEs (RR 0.55, 95% CI 0.44–0.73, I2 = 0%, high certainty evidence Supplementary Fig. 16). For maintenance trials, there was no statistically significant difference in AEs (RR 0.94, 95% CI 0.89–1.00, 6 studies, I2 = 0%, high certainty evidence; Supplementary Fig. 17) and a significantly lower risk of serious AEs (RR 0.72, 95% CI: 0.53–0.98, I2 = 0%, moderate certainty evidence; Supplementary Fig. 18) in patients treated with anti-IL-12/23p40 or anti-IL-23p19 agents compared to placebo. Patients receiving treatment were also less likely to withdraw due to AEs compared to patients receiving placebo during induction (RR 0.44, 95% CI 0.30–0.67, I2 = 11.3%; Supplementary Fig. 19), and this trend persisted but was not statistically significant during maintenance therapy (RR 0.53, 95% CI 0.23–1.19, I2 = 35.4%; Supplementary Fig. 20).
Discussion
IL-12 and IL-23 play important roles in both homeostasis and the inflammatory process. IL-12 mediates Th1 CD4 + T-cell differentiation [29, 30], whereas IL-23 is the primary pathogenic driver of Th17-dominant inflammatory pathways [31]. Key findings of our analysis include moderate-to-high certainty evidence supporting the superiority of IL-12/23p40 and IL-23p19 antagonists compared to placebo for inducing and maintaining clinical, endoscopic, PRO, and quality of life outcomes in biologic-naïve and biologic-experienced patients. Furthermore, we show that treatment with agents blocking IL-23 in RCT settings is associated with fewer SAEs and AEs requiring treatment discontinuation compared to placebo. Taken together, these findings can help clinicians place IL-23-targeted agents in treatment algorithms for CD.
We found similar clinical efficacy with ustekinumab and IL-23p19 antagonists, relative to placebo. However, in other IMIDs, targeting p19 compared to p40 has shown superior efficacy. Although both classes inhibit pathogenic IL-23, targeting p19 is generally associated with more specific and higher affinity binding [32]. For example, in the phase III UltIMMa-1 and UltIMMa-2 RCTs, approximately 30% more patients treated with risankizumab achieved 90% improvement in the Psoriasis Area Severity Index at week 16 compared to patients treated with ustekinumab (adjusted treatment differences 27.6–33.5%, p < 0.0001 in both trials) [9, 33]. In patients with CD the relative efficacy of IL-23p19 antagonists and ustekinumab have been indirectly compared. First, in the GALAXI-I trial, similar clinical remission (53.0% pooled guselkumab doses vs. 46.0% ustekinumab), PRO2 remission (42.7% vs. 39.7%), endoscopic response (35.7% vs. 28.6%), and clinical biomarker response (47.0% vs. 46.0%) rates were observed between the guselkumab and ustekinumab reference arm at week 12 [20]. Similar results for clinical and PRO2 remission between guselkumab and ustekinumab at week 48 have been reported [34]. Second, 2 independently conducted network meta-analyses found that treatment with risankizumab may be more likely to induce clinical remission in patients with moderate-to-severe CD compared to ustekinumab, although this difference was not statistically significant [3, 35].
While the relative risk of achieving clinical remission compared to placebo was similar between ustekinumab and anti-IL-23-p19 agents in our analysis, we observed numerically higher rates of remission and achievement of endoscopic outcomes with anti-IL-23p19 treatment. Specific targeting of IL-23 may achieve better endoscopic outcomes. In a sub-study from the UNITI trials, there was no statistically significant difference between ustekinumab and placebo for achieving week 8 endoscopic response (20.6% vs. 13.4%, p = 0.14), endoscopic remission (7.7% vs. 4.1%, p = 0.25), or ulcer-free mucosal healing (9.0% vs. 4.1%, p = 0.14) [36]. In contrast, phase III trials of risankizumab showed that treatment with either 600 mg or 1200 mg was associated with significantly higher rates of endoscopic response (29–40% vs. 11–12%), endoscopic remission (19–24% vs. 4–9%), and ulcer-free endoscopy (14–21% vs. 4–8%) at week 12 (p < 0.001 for all comparisons in both trials), and these differences were maintained at week 52 in the FORTIFY study [22]. These trials also enrolled difficult-to-treat patients with CD who failed multiple prior biologic therapies. However, it should be noted that comparing endoscopic outcomes across CD trials is challenging and definitions of endoscopic remission vary [37]. The head-to-head SEQUENCE trial (NCT04524611) comparing risankizumab to ustekinumab using a primary endoscopic outcome at 1 year will provide more definitive answers for whether targeting IL-23p19 is a superior treatment strategy to targeting IL-12/23p40 in CD.
Our analysis confirms that IL-12/23p40 and IL-23p19 antagonists are effective in biologic-naïve and biologic-exposed populations. We found a lower risk of SAEs and AEs requiring treatment withdrawal compared to placebo in patients treated with anti-IL-12/23p40 or anti-IL-23p19 agents, which likely relates to fewer AEs from worsening CD [38]. Although RCTs are generally underpowered for detecting rare AEs, five-year safety data in CD support the favorable safety profile of long-term ustekinumab [39]. Furthermore, a recent meta-analysis of head-to-head cohort studies suggests that ustekinumab is associated with approximately half the risk of serious infections compared to TNF-α antagonists [40]. Although long-term real world and registry-based data for IL-23p19 antagonists in CD is still required, integrated safety analyses in psoriasis and psoriatic arthritis have not identified any new or concerning safety signals [41, 42].
For patients with prior biologic failure, a network meta-analysis by Barberio et al. [35] has suggested that anti-IL-23 therapy may be the most effective strategy. It should be acknowledged that overall, patients enrolled in more recent IL-23p19 trials had more refractory disease, failed more prior biologics, and often demonstrated failure to multiple mechanisms of action beyond TNF-α antagonists alone. Therapeutic options in this difficult-to-treat population are relatively limited: although some patients with prior TNF-α antagonist failure may benefit from trialing a different anti-TNF-α agent, response rates are generally low [43] and in the GEMINI-3 trial, vedolizumab was not more effective than placebo for inducing clinical remission at week 6 in patients with CD and prior TNF-α antagonist failure [44].
Our study has some important strengths. We summarize all the phase II and III clinical trial data for targeting IL-23 in adult patients and generate estimates of treatment efficacy and safety across different disease populations by biologic exposure. These data will help inform the relative positioning of IL-23 antagonists in clinical care. However, we also acknowledge some limitations. First, although there was low statistical heterogeneity for most outcomes, there were differences in trial design, inclusion criteria, and outcome definitions. Therefore, we generated conservative effect size estimates using random-effects rather than fixed-effects models. Nevertheless, we recognize that differences in baseline populations are likely to persist. For example, even though recent trials enrolled patients using endoscopy, the baseline endoscopic requirements varied from an SES-CD ≥ 3 to ≥ 7 for ileocolonic disease. Additionally, PROs have been recently introduced for enrollment and outcome assessment, although our analyses of clinical remission defined by CDAI and PROs were consistent. Second, there were insufficient data on biomarkers, such as fecal calprotectin and C-reactive protein. Third, except for risankizumab, most data for anti-IL-23p19 agents were from phase II trials.
In conclusion, biologics targeting IL-23 are effective and safe for inducing and maintaining clinical and endoscopic remission and for improving patient quality of life. These therapies have an important role in the management of biologic-naïve and biologic-experienced patients with CD, but future head-to-head controlled studies are required to better inform the relative positioning of these drugs for the management of CD.
Supplementary Material
Abbreviations
- ADA
Adalimumab
- AM
Apilimod mesylate
- BRA
Brazikumab
- BRI
Briakinumab
- CD
Crohn’s disease
- CDAI
Crohn’s Disease Activity Index CDEIS Crohn’s Disease Endoscopic Index of Severity
- CENTRAL
Cochrane Central Register of Controlled Trials
- CI
Confidence interval
- GUS
Guselkumab
- IBD
Inflammatory bowel disease
- IBDQ
Inflammatory Bowel Disease Questionnaire
- IL-23
Interleukin-23
- IL-23R
IL-23 receptor
- MD
Mean difference
- MIR
Mirikizumab
- PASI
Psoriasis Area Severity Index
- PBO
Placebo
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PRO2
Patient-reported outcome-2
- RCT
Randomized controlled trial
- RIS
Risankizumab
- RR
Risk ratio
- SES-CD
Simple endoscopic score for Crohn’s disease SF Stool frequency
- TNF
Tumor necrosis factor
- UST
Ustekinumab
Footnotes
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s10620-023-08014-z.
Declarations
Conflict of interest SKV and VS: None. MH, JKM, AZ, and CEP are employees of Alimentiv Inc. NN has received honoraria from Janssen, Abbvie, Takeda, Pfizer, Merck, Sandoz, Novartis, and Ferring. SS has received grant/research support from Pfizer and AbbVie and consulting fees from Pfizer. BES has received consulting and/or speaking from AbbVie; Abivax, Adiso Therapeutics, Alimentiv Inc., Amgen; Arena Pharmaceuticals, Artizan Biosciences, Artugen Therapeutics, AstraZeneca, Bacainn Therapeutics, Boehringer-Ingelheim, Boston Pharmaceuticals, Bristol Myers Squibb, Calibr, Celltrion Healthcare, ClostraBio, Cytoki Pharma, Connect Biopharma, Entera, Evommune, Fresenius Kabi, Galapagos, Genentech, Gilead Sciences, GlaxoSmith-Kline, Gossamer Bio, Imhotex, Immunic, Index Pharmaceuticals, Inotrem, Innovation Therapeutics, Ironwood Pharmaceuticals, Janssen, Kaleido, Kallyope, Lilly Pfizer, MiroBio, Morphic Therapeutics, MRM Health, OSE Immunotherapeutics, Progenity, Prometheus Biosciences, Protagonist Therapeutics, Q32 Bio, Redhill Biopharma, Sun Pharma, Surrozen, Synlogic, Takeda, Target RWE, Teva, Theravance Biopharma, TLL Pharmaceutical, USWM Enterprises, VielaBio, and VTA Labs; and consulting fees and stock options from Ventyx Biosciences. RP has received consulting fees from Abbott, AbbVie, Alimentiv Inc., Amgen, Arena Pharmaceuticals, AstraZeneca, Biogen, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Celltrion, Cosmos Pharmaceuticals, Eisai, Elan, Eli Lilly, Ferring, Galapagos, Fresenius Kabi, Genentech, Gilead Sciences, Glaxo-Smith Kline, JAMP Bio, Janssen, Merck, Mylan, Novartis, Oppilan Pharma, Organon, Pandion Pharma, Pendopharm, Pfizer, Progenity, Protagonist Therapeutics, Roche, Sandoz, Satisfai Health, Shire, Sublimity Therapeutics, Takeda Pharmaceuticals, Theravance Biopharma, Trellus, Viatris, and UCB. BGF has received grant/research support from Millennium Pharmaceuticals, Merck, Tillotts Pharma AG, AbbVie, Novartis Pharmaceuticals, Centocor Inc., Elan/Biogen, UCB Pharma, Bristol-Myers Squibb, Genentech, ActoGenix, and Wyeth Pharmaceuticals Inc.; consulting fees from Millennium Pharmaceuticals, Merck, Centocor Inc., Elan/Biogen, Janssen-Ortho, Teva Pharmaceuticals, Bristol Myers Squibb, Celgene, UCB Pharma, AbbVie, Astra Zeneca, Serono, Genentech, Tillotts Pharma AG, Unity Pharmaceuticals, Albireo Pharma, Given Imaging Inc., Salix Pharmaceuticals, Novonordisk, GSK, Actogenix, Prometheus Therapeutics and Diagnostics, Athersys, Axcan, Gilead, Pfizer, Shire, Wyeth, Zealand Pharma, Zyngenia, Gi-Care Pharma Inc., and Sigmoid Pharma; and speaker’s fees from UCB, AbbVie, and J&J/Janssen. VJ has received consulting/advisory board fees from AbbVie, Alimentiv Inc., Arena pharmaceuticals, Asahi Kasei Pharma, Asieris, Astra Zeneca, Bristol Myers Squibb, Celltrion, Eli Lilly, Ferring, Flagship Pioneering, Fresenius Kabi, Galapagos, GlaxoSmithKline, Genentech, Gilead, Janssen, Merck, Mylan, Pandion, Pendopharm, Pfizer, Protagonist, Reistone Biopharma, Roche, Sandoz, Second Genome, Takeda, Teva, Topivert, Ventyx, and Vividion and speaker’s fees from, Abbvie, Ferring, Galapagos, Janssen Pfizer Shire, Takeda, and Fresenius Kabi. CM has received consulting fees from AbbVie, Alimentiv Inc., Amgen, AVIR Pharma Inc., Bio-JAMP, Bristol Myers Squibb, Celltrion, Ferring, Fresenius Kabi, Janssen, McKesson, Mylan, Takeda, Pendopharm, Pfizer, Roche; speaker’s fees from AbbVie, Amgen, AVIR Pharma Inc., Alimentiv Inc., Bristol Myers Squibb, Ferring, Fresenius Kabi, Janssen, Takeda, Pendopharm, and Pfizer; royalties from Springer Publishing; and research support from Ferring and Pfizer. Alimentiv Inc. is an academic gastrointestinal contract research organization, operating under the Alimentiv Health Trust. CM, BGF, and VJ are consultants to Alimentiv Inc. and have a primary academic appointment; they are not employees of Alimentiv and do not hold equity positions or shares in Alimentiv Inc.
Data availability
All relevant data are included within the article and/ or its supplementary materials.
References
- 1.Hanauer SB, Feagan BG, Lichtenstein GR et al. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet. 2002;359:1541–1549. [DOI] [PubMed] [Google Scholar]
- 2.Gisbert JP, Marín AC, McNicholl AG, Chaparro M. Systematic review with meta-analysis: the efficacy of a second anti-TNF in patients with inflammatory bowel disease whose previous anti-TNF treatment has failed. Aliment Pharmacol Ther. 2015;41:613–623. [DOI] [PubMed] [Google Scholar]
- 3.Singh S, Murad MH, Fumery M et al. Comparative efficacy and safety of biologic therapies for moderate-to-severe Crohn’s disease: a systematic review and network meta-analysis. Lancet Gastroenterol Hepatol. 2021;6:1002–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duerr RH, Taylor KD, Brant SR et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006;314:1461–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmitt H, Billmeier U, Dieterich W et al. Expansion of IL-23 receptor bearing TNFR2+ T cells is associated with molecular resistance to anti-TNF therapy in Crohn’s disease. Gut. 2019;68:814–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lupardus PJ, Garcia KC. The structure of interleukin-23 reveals the molecular basis of p40 subunit sharing with interleukin-12. J Mol Biol. 2008;382:931–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feagan BG, Sandborn WJ, Gasink C et al. Ustekinumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2016;375:1946–1960. [DOI] [PubMed] [Google Scholar]
- 8.Sands BE, Irving PM, Hoops T et al. Ustekinumab versus adalimumab for induction and maintenance therapy in biologic-naive patients with moderately to severely active Crohn’s disease: a multicentre, randomised, double-blind, parallel-group, phase 3b trial. Lancet. 2022;399:2200–2211. [DOI] [PubMed] [Google Scholar]
- 9.Gordon KB, Strober B, Lebwohl M et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet. 2018;392:650–661. [DOI] [PubMed] [Google Scholar]
- 10.Diels J, Thilakarathne P, Cameron C, McElligott S, Schubert A, Puig L. Adjusted treatment COMPArisons between guSelkumab and uStekinumab for treatment of moderate-to-severe plaque psoriasis: the COMPASS analysis. Br J Dermatol. 2020;183:276–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Almradi A, Hanzel J, Sedano R et al. Clinical Trials of IL-12/IL-23 Inhibitors in Inflammatory Bowel Disease. BioDrugs. 2020;34:713–721. [DOI] [PubMed] [Google Scholar]
- 12.Page MJ, McKenzie JE, Bossuyt PM et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Br Med J. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higgins JP, Altman DG, Gøtzsche PC et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. Br Med J. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guyatt GH, Oxman AD, Vist GE et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. Br Med J. 2008;336:924–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deeks JJ, Higgins JP, Altman DG, on behalf of the Cochrane Statistical Methods Group. Analysing data and undertaking meta-analyses. In: Cochrane Handbook for Systematic Reviews of Interventions. 2019:241–284. [Google Scholar]
- 16.Rosh JR, Turner D, Griffiths A et al. Ustekinumab in paediatric patients with moderately to severely active Crohn’s disease: Pharmacokinetics, safety, and efficacy results from UniStar, a phase 1 study. J Crohns Colitis. 2021;15:1931–1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mannon PJ, Fuss IJ, Mayer L et al. Anti-interleukin-12 antibody for active Crohn’s disease. N Engl J Med. 2004;351:2069–2079. [DOI] [PubMed] [Google Scholar]
- 18.Sandborn WJ, Feagan BG, Fedorak RN et al. A randomized trial of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with moderate-to-severe Crohn’s disease. Gastroenterology. 2008;135:1130–1141. [DOI] [PubMed] [Google Scholar]
- 19.Feagan BG, Sandborn WJ, D’Haens G et al. Induction therapy with the selective interleukin-23 inhibitor risankizumab in patients with moderate-to-severe Crohn’s disease: a randomised, double-blind, placebo-controlled phase 2 study. Lancet. 2017;389:1699–1709. [DOI] [PubMed] [Google Scholar]
- 20.Sandborn WJ, D’Haens GR, Reinisch W et al. Guselkumab for the treatment of Crohn’s disease: Induction results from the phase 2 GALAXI-1 study. Gastroenterology. 2022;162:1650–1664.e1658. [DOI] [PubMed] [Google Scholar]
- 21.D’Haens G, Panaccione R, Baert F et al. Risankizumab as induction therapy for Crohn’s disease: Results from the phase 3 ADVANCE and MOTIVATE induction trials. Lancet. 2022;399:2015–2030. [DOI] [PubMed] [Google Scholar]
- 22.Ferrante M, Panaccione R, Baert F et al. Risankizumab as maintenance therapy for moderately to severely active Crohn’s disease: results from the multicentre, randomised, double-blind, placebo-controlled, withdrawal phase 3 FORTIFY maintenance trial. Lancet. 2022;399:2031–2046. [DOI] [PubMed] [Google Scholar]
- 23.Evaluation of efficacy and safety of brazikumab (MEDI2070) in participants with active, moderate to severe Crohn’s disease, 2021. Available at: https://clinicaltrials.gov/ct2/show/NCT02574637. Accessed December 31, 2022.
- 24.Sands BE, Jacobson EW, Sylwestrowicz T et al. Randomized, double-blind, placebo-controlled trial of the oral interleukin-12/23 inhibitor apilimod mesylate for treatment of active Crohn’s disease. Inflamm Bowel Dis. 2010;16:1209–1218. [DOI] [PubMed] [Google Scholar]
- 25.Sandborn WJ, Gasink C, Gao LL et al. Ustekinumab induction and maintenance therapy in refractory Crohn’s disease. N Engl J Med. 2012;367:1519–1528. [DOI] [PubMed] [Google Scholar]
- 26.Panaccione R, Sandborn WJ, Gordon GL et al. Briakinumab for treatment of Crohn’s disease: Results of a randomized trial. Inflamm Bowel Dis. 2015;21:1329–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sands BE, Chen J, Feagan BG et al. Efficacy and safety of MEDI2070, an antibody against interleukin 23, in patients with moderate to severe Crohn’s disease: A phase 2a study. Gastroenterology. 2017;153:77–86.e76. [DOI] [PubMed] [Google Scholar]
- 28.Sands BE, Peyrin-Biroulet L, Kierkus J et al. Efficacy and safety of mirikizumab in a randomized phase 2 study of patients with Crohn’s disease. Gastroenterology. 2022;162:495–508. [DOI] [PubMed] [Google Scholar]
- 29.Zhu J, Paul WE. Peripheral CD4+ T-cell differentiation regulated by networks of cytokines and transcription factors. Immunol Rev. 2010;238:247–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haabeth OA, Lorvik KB, Hammarström C et al. Inflammation driven by tumour-specific Th1 cells protects against B-cell cancer. Nat Commun. 2011;2:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moschen AR, Tilg H, Raine T. IL-12, IL-23 and IL-17 in IBD: Immunobiology and therapeutic targeting. Nat Rev Gastroenterol Hepatol. 2019;16:185–196. [DOI] [PubMed] [Google Scholar]
- 32.Singh S, Kroe-Barrett RR, Canada KA et al. Selective targeting of the IL23 pathway: Generation and characterization of a novel high-affinity humanized anti-IL23A antibody. MAbs. 2015;7:778–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Strober B, Menter A, Leonardi C et al. Efficacy of risankizumab in patients with moderate-to-severe plaque psoriasis by baseline demographics, disease characteristics and prior biologic therapy: an integrated analysis of the phase III UltIMMa-1 and UltIMMa-2 studies. J Eur Acad Dermatol Venereol. 2020;34:2830–2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Danese S, Panaccione R, Rubin DT et al. Clinical efficacy and safety of guselkumab maintenance therapy in patients with moderately to severely active Crohn’s Disease: Week 48 analyses from the phase 2 GALAXI 1 study. J Crohns Colitis. 2022;16:i026–i027. [Google Scholar]
- 35.Barberio B, Gracie DJ, Black CJ, Ford AC. Efficacy of biological therapies and small molecules in induction and maintenance of remission in luminal Crohn's disease: Systematic review and network meta-analysis. Gut. 2022. [DOI] [PubMed] [Google Scholar]
- 36.Rutgeerts P, Gasink C, Chan D et al. Efficacy of ustekinumab for inducing endoscopic healing in patients with Crohn’s disease. Gastroenterology. 2018;155:1045–1058. [DOI] [PubMed] [Google Scholar]
- 37.Ma C, Hanzel J, Panaccione R et al. CORE-IBD: A multidisciplinary international consensus initiative to develop a core outcome set for randomized controlled trials in inflammatory bowel disease. Gastroenterology. 2022;163:950–964. [DOI] [PubMed] [Google Scholar]
- 38.Ma C, Panaccione NR, Nguyen TM et al. Adverse events and nocebo effects in inflammatory bowel disease: A Systematic review and meta-analysis of randomized controlled trials. J Crohns Colitis. 2019;13:1201–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sandborn WJ, Rebuck R, Wang Y et al. Five-year efficacy and safety of ustekinumab treatment in Crohn’s disease: The IM-UNITI trial. Clin Gastroenterol Hepatol. 2022;20:578–590.e574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Solitano V, Facciorusso A, Jess T et al. Comparative risk of serious infections with biologic agents and oral small molecules in inflammatory bowel diseases: A systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2022. [DOI] [PubMed] [Google Scholar]
- 41.Gordon KB, Lebwohl M, Papp KA et al. Long-term safety of risankizumab from 17 clinical trials in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2022;186:466–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rahman P, Ritchlin CT, Helliwell PS et al. Pooled safety results through 1 year of 2 phase III trials of guselkumab in patients with psoriatic arthritis. J Rheumatol. 2021;48:1815–1823. [DOI] [PubMed] [Google Scholar]
- 43.Sandborn WJ, Rutgeerts P, Enns R et al. Adalimumab induction therapy for Crohn disease previously treated with infliximab: a randomized trial. Ann Intern Med. 2007;146:829–838. [DOI] [PubMed] [Google Scholar]
- 44.Sands BE, Feagan BG, Rutgeerts P et al. Effects of vedolizumab induction therapy for patients with Crohn’s disease in whom tumor necrosis factor antagonist treatment failed. Gastroenterology. 2014;147:618–627.e613. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data are included within the article and/ or its supplementary materials.