Abstract
Ty1 retrotransposition, like retroviral replication, is a complex series of events requiring reverse transcription of an RNA intermediate, RNA-primed minus- and plus-strand DNA synthesis, multiple strand transfers, and precise cleavages of the template and primers by RNase H. In this report, we examine the structure of in vivo Ty1 replication intermediates, specifically with regard to the behavior of reverse transcriptase upon reaching template ends and to the precision with which RNase H might generate these ends. While the expected 3′ termini were always identified, terminal nontemplated bases were also observed at all of the RNA and DNA template ends examined. Nontemplated A residues were most common at all 3′ ends, although C residues were preferentially added to minus-strand termini paused at the 5′ end of capped Ty1 RNA. In addition, we observed that RNase H removal of the tRNA primer and of the polypurine tract was not always precise or efficient. Finally, we noted numerous instances of Ty1 reverse transcriptase transferring from normal Ty1 template ends to various tRNA templates, with continued synthesis to specific modified bases. A similar pattern was obtained for Ty2, indicating that template ends offer unique opportunities for these two related reverse transcriptases to generate errors.
Ty1, a yeast retrotransposon which is structurally and mechanistically related to vertebrate retroviruses (20), replicates via reverse transcription of an RNA template. Like retroviruses, Ty1 transcription is initiated inside the left long terminal repeat (LTR) and is terminated within the right LTR, resulting in an RNA template truncated at both the 5′ and 3′ ends, relative to the full-length double-stranded DNA element. During transposition, Ty1 reverse transcriptase (RT) must carry out a complex replication process involving multiple strand transfers, RNase H cleavage of the RNA template, and removal of both plus- and minus-strand RNA primers in order to reconstitute a full-length double-stranded DNA copy (Fig. 1). Integration of the replicated Ty1 element requires two complete LTRs with precise end structures (16, 33, 44).
FIG. 1.
Schematic diagram of the Ty1 replication cycle, based on the retroviral model. (A) Structures of Ty1 double-stranded DNA and the RNA transcribed from that DNA. Positions of GAG and POL open reading frames are indicated beneath the diagram of Ty1 genomic DNA. (B) Minus-strand strong-stop synthesis primed by IMT, with associated RNase H cleavage of the RNA template (dashed wavy line). (C) Minus-strand nearly full-length DNA paused at the 5′ end of RNase H-cleaved RNA, with associated RNase H cleavage of the RNA template. (D) Minus-strand nearly full-length DNA paused at the 5′ end of the full-length capped Ty1 transcript, with associated RNase H cleavage of the RNA template. (E) PPT1-primed plus-strand strong-stop DNA synthesis, using either intra- or intermolecularly transferred minus-strand DNA as the template. Two PPT1-primed plus-strand DNAs are depicted: one extends to the first modified base of the IMT primer, 2 bases beyond the region of PBS complementarity (dd), and the other extends to the 5′ end of the minus-strand template, determined by RNase H cleavage of the IMT primer (see text for details). (F) Plus-strand strong-stop DNA transfer to minus-strand DNA paused at the 5′ end of RNase H-cleaved RNA, or to minus-strand DNA paused at the 5′ end of the full-length capped Ty1 transcript. Note that RNase H presumably removes both the PPT1 plus-strand primer and the IMT minus-strand primer. (G) Full-length, unintegrated double-stranded DNA. rrr represents ribonucleotides remaining after incomplete RNase H removal of either the PPT plus-strand primer or the IMT minus-strand primer. ddd represents deoxyribonucleotides templated by RNA remaining after incomplete removal of plus- and/or minus-strand primers. The termini examined in this study are boxed, and (x) refers to sequence changes found at specific intermediates. Sequence changes previously described at the 3′ end of nearly full-length minus-strand DNA paused at the RNase H-generated RNA template end near the U5/PBS border (34) are depicted in diagram C and are not boxed.
In vitro studies have shown that DNA polymerases, particularly those lacking detectable 3′-to-5′ exonuclease activity (e.g., retroviral RTs), can add extra bases beyond template ends (11, 12, 37, 38). We have previously reported the sequence analysis of 29 independent Ty1 transposition events which revealed a concentration of errors in the left LTR coinciding with known template ends encountered during retrotransposition (22). Subsequently, we demonstrated that sequence changes often occurred at the 3′ ends of intramolecularly transferred minus-strand DNA intermediates paused at heterogeneous RNase H cleavage sites upstream of the U5/primer binding site (PBS) border in vivo, consistent with nontemplated base addition (34). Similarly, Kulpa et al. (27) observed nontemplated bases at the 3′ ends of murine leukemia virus (MuLV) minus strands paused at the 5′ end of the RNA template. These in vivo data, as well as the observation of extra bases at retroviral and retrotransposable element LTR circle junctions (17, 45, 48), suggest that reverse transcription at template ends may be particularly prone to sequence changes.
Based on the retroviral replication model, six Ty1 DNA intermediates are predicted to pause at various template ends: (i) minus-strand strong-stop DNA (Fig. 1B) and (ii) intermolecularly transferred minus-strand DNA (Fig. 1D) both pause at the capped 5′ end of full-length Ty1 RNA; (iii) intramolecularly transferred minus-strand DNA pauses at the U5/PBS border (Fig. 1C); (iv) plus-strand strong-stop DNA initiates at polypurine tract 1 (PPT1), just upstream of U3, and pauses at the first modified base within the 3′ end of the minus-strand primer, tRNAiMet (IMT) (Fig. 1E) before transfer to paused minus-strand DNA intermediates (Fig. 1F); (v) the correct 3′ end of full-length minus-strand DNA is determined by the 5′ end of plus-strand DNA, from which the PPT1 RNA primer has been cleaved (Fig. 1G); and finally, (vi) the correct 3′ end of full-length plus-strand DNA is determined by the 5′ end of minus-strand DNA, from which the tRNA primer has been cleaved (Fig. 1G). However, this standard model does not fully account for all of the observed Ty1 replication intermediates. For example, the 3′ termini of intramolecularly transferred minus-strand DNAs (Fig. 1C) have been mapped to multiple sites upstream of the U5/PBS border, secondary to heterogeneous RNase H cleavage of the template RNA during minus-strand strong-stop synthesis (34). An abundant, nonessential 2.3-kb plus-strand intermediate has been identified whose synthesis is primed from an internal PPT (referred to as PPT2) (24, 35, 40). Furthermore, it appears that the expected and observed ∼330-base plus-strand strong-stop intermediate which extends from PPT1 to the first tRNA-modified base, 12 bases into the IMT, is not a direct intermediate in Ty1 retrotransposition (28–30).
In order to gain a perspective on the terminal structures actually generated in vivo during Ty1 replication, we used our previously developed ligation-mediated PCR (LM-PCR) approach (34) to map, at the nucleotide level, the termini of the major Ty1 replication intermediates. In this report, we define the precise 5′ end of the Ty1 full-length RNA and demonstrate that inappropriate base addition can occur at the 3′ ends of all Ty1 and Ty2 DNA intermediates. We present indirect evidence that RNase H cleavage can be imprecise during removal of both the tRNA and the PPT primer. Finally, we report numerous examples of Ty1 RT strand transferring from template ends to the 3′ ends of various tRNAs. These unexpected in vivo-generated cDNA structures, which, for the most part, are probably dead-end products, indicate that Ty replication is an inefficient and error-prone process.
MATERIALS AND METHODS
Plasmids and yeast strains.
All of the yeast strains used, except YH10, contained plasmids carrying wild-type or mutant Ty1 or Ty2 elements downstream of the galactose-inducible promoter, GAL1 (Table 1). Expression of endogenous genomic Ty1 elements is suppressed in YH51-derived strains due to the spt3-202 mutation (5). Strain YH10 lacks plasmid Ty1 elements and is wild type with regard to SPT3.
TABLE 1.
Yeast strains and plasmids used in this study
| Yeast strain | Host genotype | Plasmid(s) | Description of plasmid(s) | Reference |
|---|---|---|---|---|
| AG51 | YH51 (MATaGAL+ ura3-52 spt3-202 his4-539 lys2-801) | pJEF724 (pGTy1-H3) | 2μm URA3-marked plasmid containing galactose-inducible wild-type Ty1-H3 | 21 |
| AG53 | YH51 | pGTyΔSal (pGTy1-H3ΔTYB) | Same as pJEF724 but with most of POL open reading frame deleted | 21 |
| AG1583 | YH51 | pJEF1076 | Same as pJEF724 but containing Ty1-173 from XhoI to BstEII | 4 |
| AG1585 | YH51 | pJEF1510 | Same as pJEF724 but Ty2-H556 | 3a |
| AG331 | MATα ura3-52 trp1Δ1 leu2-3,112 imt1::TRP1 imt2::TRP1 imt3::TRP1 imt4::TRP1 | pKC35 + pKC66 | Same as pJEF724 but containing PBS mutations and a neor marker + cen plasmid containing wild-type IMT4 | 10 |
| YH10 | MATa GAL+ ura3-52 his4-539 lys2-801 | None | 54 |
VLP preparations.
Virus-like particle (VLP) production was induced, and VLPs were partially purified through sucrose gradients as previously described (6, 34).
Nucleic acids from VLPs.
For decapping and LM-PCR and after endogenous primer extension experiments, aliquots of VLP suspensions were incubated for 1 h in 50-μg/ml proteinase K, 25 mM EDTA (pH 8.0), 0.1% sodium dodecyl sulfate (SDS) at 25°C, extracted twice with phenol (equilibrated to pH 7.9)-chloroform (1:1), and ethanol precipitated. For in vitro primer extension experiments, RNA was directly extracted from VLPs with hot acidic phenol (1) as previously described (34).
Determination of the Ty1 RNA 5′ terminus.
RNA was decapped with tobacco acid pyrophosphatase as recommended by the manufacturer (Epicentre Technologies, Inc.) and then ligated with the RNA linker RAG347 as previously described (34). Note that we were never able to recover tobacco acid pyrophosphatase-decapped linker ligation products by using acid phenol-purified RNA. Subsequent reverse transcription was primed by a Ty1 minus-strand primer (RAG199 or RAG321, Table 2) and then subjected to PCR amplification (primed by plus-strand primer RAG198 and minus-strand primer RAG199 or RAG233), subcloning, and sequence analysis as previously described (34).
TABLE 2.
Oligonucleotide primers used in this study
| Primer | Sequence (5′–3′) | Derivationa | Strand | Function (restriction site on 5′ end) |
|---|---|---|---|---|
| RAG198 | GGGGTACCGGAACGCTTCACGAA | Complementary to RNA linker cDNA and DNA linker | Linker PCR primer (KpnI) | |
| RAG199 | CATGGAGCTCTGAGGAGAGGCATGA | Ty1 GAG, bp 497–511 | Minus | RT and PCR primer for decapped full-length RNA, PCR primer for full-length (or paused) minus-strand DNA (SacI) |
| RAG208 | TTCGTGAAGCGTTCCGGTACCCCGC | DNA linker (5′ phosphorylated, 3′ blocked) | ||
| RAG228 | AAAAGAGCTCGACGCAAATGATGAGAAATAG | Ty1 U3, bp 5664–5684 | Plus | PCR primer for plus-strand strong-stop (includes full-length and PPT2-primed) DNA (SacI) |
| RAG233 | AAAAGAGCTCAGAATTGGGTGAATGTTGAG | Ty1 U5, bp 313–332 | Minus | PCR primer for decapped full-length RNA, minus-strand strong-stop (includes full-length) DNA (SacI) |
| RAG266 | AAAAGAGCTCCGAGACCAAGAAGAACATTG | Ty1 POL, bp 5475–5494 | Plus | PCR primer for plus-strand full-length (and PPT2-primed) DNA (SacI) |
| RAG321 | GCTGAAACGTCTAACGGATC | Ty1 GAG, bp 384–403 (gel purified) | Minus | RT primer for decapped full-length RNA |
| RAG322 | AGAATTGGGTGAATGTTGAG | Ty1 U5, bp 313–332 (gel purified) | Minus | Primer extension primer for U3/R region |
| RAG340 | ACACGGTACCGGGGGGGGGG | Complementary to poly(C)-tailed templates | TdT-PCR poly(G) primer (KpnI) | |
| RAG347 | GGAACGCUUCACGAA | RNA linker (gel purified) | ||
| RAG400 | CATGGAGCTCAAGCAGGTTGAGGAG | Ty2 GAG, bp 503–517 | Minus | PCR primer for full-length or paused minus-strand Ty2 DNA (SacI) |
| RAG478 | TACTCGAGACACGGTACCCCCCCCCC | Complementary to poly(G)-tailed templates | TdT-PCR poly(C) primer (KpnI) |
Numbering is based on the standard Ty1-H3 sequence (4). Except for RAG199, which is specific for Ty1, and RAG400, which is specific for Ty2, these sequences are identical for Ty1 and Ty2.
Primer extension.
Primer extensions were carried out with avian myeloblastosis virus (AMV) RT (in vitro) or within VLPs with Ty1 RT (endogenous). Primer RAG322, corresponding to the U5 minus strand (Table 2), was gel purified, end labeled, and extended as previously described (34).
LM-PCR.
Full-length 5′-phosphorylated RNA ends exposed by decapping and 3′ DNA ends (either Ty replication intermediates or primer extension cDNAs) were ligated to RNA (RAG347) and DNA (RAG208) linkers, respectively, by using T4 RNA ligase. PCR amplification of ligation products (directly for DNA or after cDNA synthesis for RNA ligation products) was primed by a linker-specific oligonucleotide, RAG198, and different Ty-specific oligonucleotides, depending on which transposition intermediate was being investigated (Table 2). PCR products were subcloned and sequenced. All procedures were performed as previously described (34).
TdT-mediated PCR.
3′ tailing reactions of VLP DNA, using calf thymus terminal deoxynucleotidyl transferase (TdT), were performed as recommended by the enzyme manufacturer (Boehringer Mannheim). Subsequent PCR and cloning conditions were as previously described (34). Oligonucleotide RAG340 or RAG478 was used to anneal to TdT-generated polynucleotide tails, and PCR amplification was carried out with specific Ty1 primers, depending on the region analyzed (Table 2).
RESULTS
Ty1 transcription initiates precisely at bp 241.
In our previous study of replication errors generated during a single cycle of Ty1 retrotransposition (22), we observed a T to G transversion at base 240, which is at or near the left U3/R border (Fig. 1). We wanted to determine whether this substitution could be ascribed to nontemplated base addition by Ty1 RT after copying the 5′ end of capped Ty1 RNA (as shown in Fig. 1D). However, we first needed to determine the precise site(s) of transcription initiation of the GAL1-driven Ty1-H3 construct. Previous primer extension studies had suggested that GAL1-induced, plasmid-borne Ty1 RNAs and endogenous Ty1 RNAs both have the same 5′ termini, consisting of a pair of adjacent terminal primer extension bands (8a).
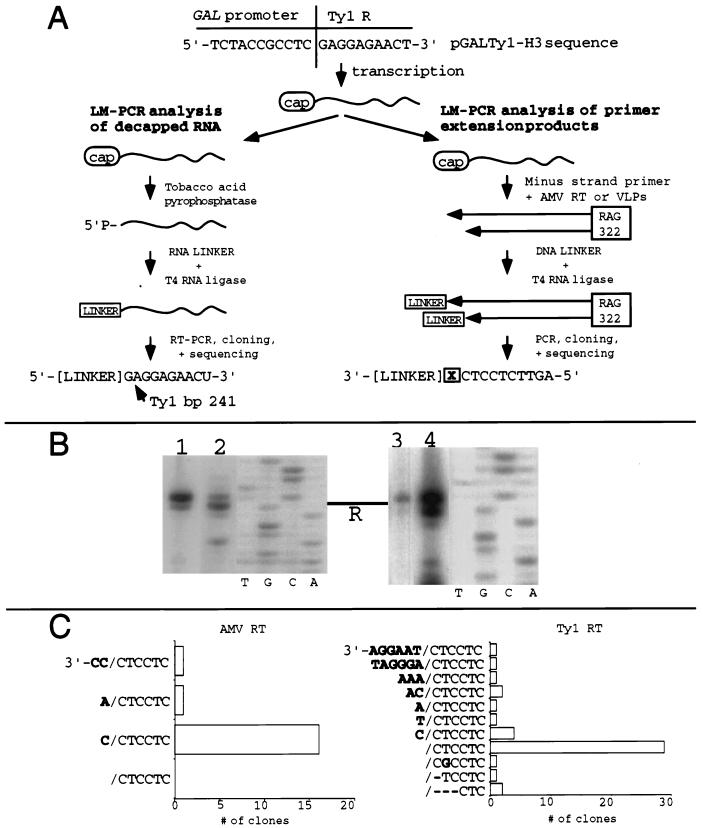
We used LM-PCR to define the precise site of Ty1 transcription initiation (Fig. 2A). We first extracted RNA from VLPs derived from a strain (AG331) bearing a galactose-inducible Ty1 element with several silent substitutions in its PBS which disrupt base pairing to its IMT primer (10). Consequently, VLPs should contain intact RNA but no replication-associated cleaved RNA or cDNA products. After decapping this RNA, we ligated an RNA linker to the resulting 5′-monophosphorylated end, reverse transcribed and then PCR amplified the resulting cDNA, and finally subcloned the PCR products. If decapping was omitted from this procedure, no PCR products of the size representing full-length Ty1 RNA were obtained (34 and data not shown), indicating that full-length Ty1 RNA in VLPs is normally 5′ capped. Sequence data from 50 clones demonstrated that initiation of GAL1-Ty1 transcription is typically at Ty1 base 241 (43 [86%] of 50 clones) (Fig. 2A). Seven remaining clones were ligated to the linker at nonspecific locations, providing no evidence of alternative transcription start sites. Thus, the base substitution observed in our previous study was 1 base upstream of the normal transcription initiation site, consistent with nontemplated base addition.
FIG. 2.
Determination of the 5′ end of full-length Ty1 transcript and evidence for in vitro and endogenous nontemplated base addition. (A) Schemes for determining the 5′ end of the GAL1-induced Ty1 transcript by using LM-PCR or primer extension analysis. Primer extension cDNAs were obtained by extending RAG322, positioned just upstream of the U5/PBS border for maximum resolution at the 5′ end of the Ty1 transcript. The boxed X represents bases present in the cDNAs beyond the RNA 5′ end. (B) Primer extension of 5′-end-labeled RAG322 along Ty1-H3 RNA. Lanes: 1, in vitro reaction using AMV RT and RNA extracted from AG51 (wild-type) VLPs; 2, endogenous reaction using AG51 VLP-associated RT and RNA; 3, in vitro reaction using AMV RT and genomic Ty1 RNA extracted from YH10, an SPT3 wild-type strain lacking any plasmid-based Ty1-H3; 4, in vitro reaction using AMV RT and RNA associated with AG53 (ΔRT) VLPs, under endogenous reaction conditions. Sequencing reactions using a RAG322 primer and pJEF724 as a template (4) were run as size markers. The bar above R indicates the 5′ end of the Ty1 transcript. (C) Frequency distribution of the primer extension cDNA 3′ ends ligated to the 5′ phosphorylated, 3′ blocked DNA linker RAG208, as derived from sequence analysis of cloned PCR products. In vitro primer extension was performed by using Ty1 RNA extracted from either AG53 (ΔRT) or AG331 (Ty1-H3 PBSmutant) VLP and AMV RT. Products were ligated to the DNA linker (RAG208), PCR amplified with RAG198 and RAG233, and subcloned. Similarly, endogenous primer extension products from a reaction using AG51 (wild-type) VLPs with their associated RNA and Ty1 RT were ligated, amplified, and subcloned. Nontemplated bases are in boldface type, and / represents the beginning of R.
To further investigate this possibility, we performed primer extension analysis of the 5′ end of galactose-induced Ty1 RNA by using a gel-purified, end-labeled U5 minus-strand primer (RAG322) and either AMV RT (in vitro primer extension) or Ty1 RT (endogenous primer extension) (Fig. 2A). As shown in Fig. 2B, lanes 1 and 2, we observed two major products when using either RT source. One product was the size predicted for a transcript beginning at base 241, while the other was 1 base longer. Comparing the two lanes, we noted an inverse relationship between the band intensities. To determine whether the observed difference between the two different RTs was due to the reaction conditions employed, we carried out AMV RT-catalyzed primer extension reactions under endogenous primer extension conditions, using Ty1 RNA within VLPs from a strain lacking RT activity. Under these conditions, AMV RT can extend the primer which is complementary to the Ty1 RNA present in the VLPs. As shown in Fig. 2B, lane 4, the band pattern is similar to that seen with AMV using its own ideal reaction conditions. Therefore, the relative intensities of the two bands are likely a function of the specific RT source used to carry out the reaction. Finally, we asked at what position endogenous Ty1 elements initiate transcription. We therefore performed primer extension with the same minus-strand primer and whole-cell RNA derived from a strain in which endogenous Ty1 elements are transcriptionally active. As shown in Fig. 2B, lane 3, the AMV RT-generated primer extension pattern is the same as that derived from experiments using plasmid-based GAL1-induced Ty1 RNA, indicating that both sources of Ty1 initiate transcription from the same position.
Nontemplated base addition accounts for longer primer extension products.
Since we had already determined that galactose-induced Ty1 transcription was initiated from a single site, we were interested to know how the abundant longer primer extension product shown in Fig. 2B was generated. We used the LM-PCR method to examine the 3′ ends of in vitro and endogenous reaction products, as depicted in Fig. 2A. A total of 18 clones derived from the AMV RT primer extension products were sequenced, and all exhibited nontemplated bases at their 3′ ends (Fig. 2C). Sixteen (88%) of these clones contained an added C at the 3′ terminus of the minus strand; one had an added A, and one had two added C residues. No clone had a correct termination at the beginning of the Ty1 transcript. We performed a similar LM-PCR analysis of endogenous Ty1 RT-directed primer extension products. Forty-two clones of extension products terminating near the U3/R border were sequenced (Fig. 2C). Twenty-eight (67%) of these primer extension products terminated at the precise 5′ end of the Ty1 transcript. Five clones were extended by a single base, two clones by 2 bases, one clone by 3 bases, and two clones by 6 bases. Three additional clones terminated just downstream of the transcription start site, and a final clone exhibited a single base substitution 1 base downstream of the 5′ end of the RNA. These data indicate that the primer extension products observed in Fig. 2B consist of a combination of cDNAs correctly terminated at the 5′ end of the Ty1 transcript plus those extended by nontemplated bases. The LM-PCR findings are consistent with the observed inverse relationship of the band intensities for the two RTs (Fig. 2B).
In vivo minus-strand replication intermediates paused at the 5′ end of the Ty1 transcript frequently contain nontemplated terminal base additions.
We previously used LM-PCR and TdT-PCR to examine the 3′ termini of minus-strand DNAs paused near the U5/PBS region and observed sequence changes that occurred during in vivo reverse transcription (34). Having analyzed the in vitro and endogenous primer extension products associated with the 5′ end of the Ty1 RNA, we next applied LM-PCR to the in vivo-derived Ty1 replication intermediates paused at this site, i.e., minus-strand strong-stop DNA and intermolecularly transferred minus-strand nearly full-length DNA prior to plus-strand strong-stop transfer (Fig. 1B and D). We examined 18 clones of PCR products representing either minus-strand strong-stop or nearly full-length DNA (RAG198 and RAG233) and 39 clones representing only nearly full-length DNA (RAG198 and RAG199) (Fig. 3A). As the distribution of termini was similar for both experiments, the results were combined. The majority (60% [34 of 57]) of the sequenced clones ended, as expected, precisely at the 5′ end of the Ty1 RNA (Fig. 3B). This result was confirmed by using TdT as an alternative method of marking the 3′ cDNA termini prior to PCR. In parallel TdT-PCR studies, 59% (10 of 17) of the minus-strand DNA intermediates ended at the Ty1 transcription initiation site (Fig. 3B).
FIG. 3.
The 3′ termini of minus-strand cDNAs paused, in vivo, at the 5′ end of Ty1-H3 full-length RNA are heterogeneous. (A) Schematic diagram of the LM-PCR approach, showing the two replication intermediates predicted to pause at the 5′ end of the Ty1 transcript. By positioning the minus-strand PCR primer upstream of the PBS (RAG233), both minus-strand strong-stop and transferred minus-strand nearly full-length DNA intermediates were amplified. A minus-strand PCR primer positioned downstream of the PBS (RAG199) amplified transferred minus-strand nearly full-length DNA intermediates only; short arrows represent PCR primers. (B) Frequency distribution of the Ty1 minus-strand DNA 3′ bases, paused in vivo at the 5′ end of the Ty1 transcript, combining cloned PCR products which were amplified by minus-strand primers upstream or downstream of the PBS. Open bars represent LM-PCR-derived data; filled bars represent TdT-PCR-derived data. The A in the uppermost Ty1 sequence points out a G-to-A substitution in the GAL1 promoter sequence. (C) Frequency distribution of the Ty2 minus-strand DNA 3′ bases, paused in vivo at the 5′ end of the Ty2 transcript. Cloned PCR products were amplified by RAG400, downstream of the PBS (see Table 2). Nontemplated bases are in boldface type and / represents the beginning of R.
However, in addition to correctly templated termini paused at the beginning of the Ty1 transcript, we observed numerous errors generated in vivo among cloned LM-PCR and TdT-PCR products (Fig. 3B). Twenty-seven percent (20 of 74) of the PCR products exhibited a terminal nontemplated base addition of either a C (13 of 20) or an A (7 of 20). Five clones were extended from 2 to 9 bases, while five other clones showed substitutions or deletions downstream of the beginning of R. One of the eight-base additions (3′-ACTCTTAA-5′) represents the sequence at the 3′ end of U5 for the Ty1-H3 plus strand, consistent with strand transfer to the U5 region of a minus-strand cDNA. The other eight-base extension and the nine-base extension are very similar to the GAL1 promoter sequence upstream of R (note the GAL1 sequence in Fig. 2A), suggesting that aberrantly initiated transcripts may have served as templates for these cDNAs. In a parallel LM-PCR analysis of 18 clones derived from nearly full-length minus-strand DNAs paused at the 5′ end of the Ty2 transcript, we observed a comparable pattern of correct termination and frequent nontemplated base additions (Fig. 3C), with a preference for addition of C. These in vivo data are consistent with our initial primer extension results and indicate that nontemplated base addition can occur when Ty1 minus-strand cDNA comes to the 5′ end of the full-length Ty1 transcript.
The 3′ ends of Ty1 and Ty2 full-length minus-strand DNA do not always exhibit precise integration-competent ends.
Plus-strand strong-stop DNA is primed by the RNase H-generated PPT oligoribonucleotide just upstream of the beginning of U3 (Fig. 1E). Subsequent RNase H removal of this RNA primer generates a plus-strand DNA with a precise 5′ end at the first base of U3. When plus-strand strong-stop DNA transfers to the 3′ end of paused minus-strand intermediates (Fig. 1F), it provides a DNA template for completion of minus-strand synthesis. Full-length minus-strand DNA synthesis is completed when RT reaches the 5′ end of the plus-strand DNA template (Fig. 1G). Experimental evidence suggests that successful integration of full-length Ty1 elements depends upon accurate generation of a blunt-ended double-stranded terminal structure with the correct terminal sequences (16, 33, 44). This requires generation of a precise 5′-end structure of the plus-strand DNA, as well as termination of minus-strand DNA synthesis upon reaching that template end. However, given our previous findings on the imprecise behavior of Ty1 and Ty2 RTs upon reaching RNA template ends, we asked whether these terminal full-length structures are also subject to nontemplated base addition.
To investigate these specific ends, we PCR amplified DNA linker-ligation products by using a primer complementary to the linker and a minus-strand primer positioned downstream of the PBS (RAG199). We then cloned the ∼545-bp product representing the 3′ end of full-length minus-strand DNA (Fig. 4A). Sixteen LM-PCR clones of Ty1-H3 minus-strand full-length DNA were sequenced, and eight (50%) ended correctly at the first base of U3 (Fig. 4B). However, the other eight full-length minus-strand clones had unexpected sequences at their 3′ ends. Like minus-strand intermediates paused at RNA template ends (see above and reference 34), four full-length DNA 3′ ends exhibited single base additions (A in two clones and T in two clones). A fifth clone extended 8 bases into the polypurine tract, suggesting failure of RNase H to cleave the plus-strand RNA primer from this particular template (Fig. 4B and D, g). Finally, three clones contained tRNA sequences joined to the beginning of U3 (described below). As in previous experiments, independent TdT-PCR results supported the LM-PCR data (Fig. 4B). Ten (53%) of 19 full-length minus-strand TdT-PCR clones ended, as expected, at the first base of U3. Three additional clones exhibited nontemplated addition of T. Two clones contained PPT1 sequences beyond the first base of U3. In one of these clones, the sequence extended 10 bases upstream of PPT1. The second clone contained seven bases of PPT1, followed by two 3′-terminal mismatched bases. One TdT-PCR clone revealed a tRNA sequence 3′ of the Ty1 full-length minus-strand sequence (see below).
FIG. 4.
Ty1 and Ty2 full-length minus-strand DNAs generated in vivo can exhibit aberrant 3′ termini. (A) After ligation of RAG208 to the 3′ ends of VLP-derived nucleic acids, amplification using a linker-specific primer (RAG198) and a primer positioned downstream of the PBS (RAG199 for Ty1 and RAG400 for Ty2) generated (among other products representing nearly full-length minus-strand intermediates paused at RNA template ends [Fig. 1B and C and reference 34]) a 545-bp product corresponding to full-length minus-strand DNA. Arrows represent PCR primers. (B) Frequency distribution of Ty1 minus-strand full-length DNA 3′ ends. Open bars represent LM-PCR-derived data; filled bars represent TdT-PCR-derived data. The AG at the extreme 3′ end of one Ty1 clone points out a two-base mismatch with the presumed PPT1 template. Minus-strand sequences are presented in the 3′-to-5′ direction. The beginning of U3 is indicated by /. The boldface sequence represents unexpected 3′ ends of minus-strand full-length DNAs. (C) Frequency distribution of Ty2 minus-strand full-length DNA 3′ ends. (D) Proposed models of PPT1 generation by RNase H, priming of plus-strand DNA synthesis, and subsequent extension of minus-strand full-length DNAs to the extreme 5′ end of the plus-strand template. (a) Transfer of minus-strand strong-stop DNA to the 3′ end of the RNA template, followed by extension. The large open arrowheads represent expected RNase H cleavage sites on either side of the PPT1 sequence. The smaller open arrowhead represents a proposed alternative RNase H cleavage site 1 base upstream of the PPT1/U3 border, generating a PPT1 truncated at the 3′ end. (b) Correct generation of the PPT1 plus-strand primer by RNase H and extension of plus-strand DNA with a correct 5′ end. The large open arrowhead represents expected RNase H cleavage of PPT1 from plus-strand strong-stop DNA during synthesis. The smaller open arrowhead represents a proposed alternative site of RNase H cleavage of PPT1 from plus-strand strong-stop DNA 1 base upstream of the PPT1/U3 border, generating a plus strand with a 5′-terminal ribonucleotide A. (c) Plus-strand DNA extension after RNase H cleavage of the PPT1 sequence 1 base upstream of the PPT1/U3 border (see a), generating a plus-strand DNA with an additional deoxynucleotide A on the extreme 5′ end. (d) Correct removal of the PPT1 plus-strand primer, followed by accurate minus-strand full-length extension to the first base of U3. (e) Extension of minus-strand full-length DNA 1 base beyond the beginning of U3 after inaccurate removal of PPT1 by RNase H, leaving one ribonucleotide A at the 5′ end of the plus-strand template (see b). (f) Extension of minus-strand full-length DNA 1 base beyond the beginning of U3 after RNase H removal of a 3′-truncated PPT1 primer generated a plus strand with an additional deoxynucleotide A at the 5′ end (see c). (g) Extension of minus-strand full-length DNA to the 5′ end of PPT1 after failure of RNase H to remove PPT1. RNA sequences are in lowercase, and DNA sequences are in uppercase. PPT1 sequences are underlined. The vertical line indicates the PPT1/U3 border. Plus and minus strands are indicated at their 5′ ends.
We observed a similar pattern in an LM-PCR analysis of 17 Ty2 full-length minus-strand DNA clones (Fig. 4C). Eleven clones (65%) ended correctly at the first base of U3. An additional four clones revealed nontemplated T’s beyond the first base of U3. Taken together with the Ty1 results, unexpected T residues at the 3′ termini of full-length minus-strand DNAs make up the majority (9 of 11) of the single base additions. This excess may, in fact, be due to inaccurate generation of the PPT1 RNA primer by Ty RNase H or its subsequent incomplete removal. In the first scenario, when generating the PPT1 primer during minus-strand extension, RNase H may cleave the PPT1 sequence 1 base upstream of the beginning of U3 (Fig. 4D, a). Ty RT, primed by this 3′-truncated PPT1, would incorporate a deoxy A just upstream of U3 that is resistant to subsequent RNase H removal (Fig. 4D, c). In the second case, RNase H may fail to completely remove the RNA primer from the 5′ end of the plus-strand DNA, leaving a ribonucleotide A (Fig. 4D, b). In either case, the T’s observed upstream of the first base of U3 would actually be templated by a 5′ ribo- or deoxyribonucleotide (Fig. 4D, e and f).
Two Ty2 clones were extended beyond the first base of U3 by 5 and 6 nontemplated bases (Fig. 4C). These clones were similar to three clones observed in the TdT-PCR Ty1-H3 experiment, which showed four, six, and seven extra bases beyond the first base of U3 (Fig. 4B). The sequences of all five multiple base additions are similar in that they contain 3′-GGT-5′, the complement of the posttranscriptionally added 5′-CCA-3′ at the 3′ end of all tRNAs. These will be discussed with other tRNA sequences below. Our combined data regarding the structure of the 3′ ends of full-length minus-strand DNAs imply that the generation of precise 5′ ends required for integration of Ty1 and Ty2 transposable elements can be subverted by nontemplated base addition, imprecise removal or generation of the plus-strand RNA primer by RNase H, and strand transfer to inappropriate, i.e., tRNA, templates.
A plus-strand “intermediate” which copies the 12 most 3′ bases of the IMT primer is the major product when LM-PCR includes PPT1-primed plus-strand DNA.
Models of retroviral replication predict that during plus-strand strong-stop DNA synthesis, RT copies the minus-strand template, including the minus-strand tRNA primer, stopping when it reaches a specific modified tRNA base (23). This results in precise copying of the PBS sequence, allowing complementary base pairing of plus-strand strong-stop DNA to nearly full-length minus-strand DNA paused at the U5/PBS border, followed by continuation of both plus-strand and minus-strand DNA synthesis (Fig. 1F). However, this simple model cannot explain plus-strand strong-stop synthesis for yeast retrotransposons, where the analogous plus-strand DNA extends 2 bases beyond the region of complementarity to the PBS, resulting in a two-base terminal mismatch after strand transfer (28).
Using a plus-strand primer in U3 (Fig. 5A), we found that for 14 (48%) of 29 examined clones, the 3′ end of Ty1-H3 plus strands was precisely the 12-base extended product expected from copying of the tRNA template to the first modified base (Fig. 5B). A comparable analysis of Ty2 plus strands revealed similar findings (Fig. 5C), with 14 (67%) of 21 clones extended 12 bases into the tRNA primer. Furthermore, both elements showed numerous examples of nontemplated base addition beyond the 12th templated base. In fact, of 50 clones examined, we found only 1 (in Ty2) in which the linker was ligated at the precise 3′ end of the PBS (i.e., 10 bases beyond the end of U5). We repeated this approach by using a plus-strand primer upstream of PPT1, thereby excluding plus strands primed by PPT1 (Fig. 6A) and, instead, selecting for either full-length plus-strand products or products primed at PPT2. Under these circumstances, clones representing 12-base extensions into the tRNA (with or without nontemplated bases) constituted only 10% of the 3′ ends present (see below). These findings confirm those of others that Ty1 RT normally does continue copying the tRNA primer sequence until the first modified base is encountered (28–30, 52). Further, the minus-strand tRNA sequences are generally, albeit inefficiently, cleaved during PPT1-primed plus-strand synthesis.
FIG. 5.
Ty1 and Ty2 plus-strand strong-stop DNAs generated in vivo usually extend to the first modified base of the IMT minus-strand primer. (A) Scheme of LM-PCR analysis of plus-strand 3′ ends. The Ty-specific plus-strand primer used to generate LM-PCR products was positioned downstream of PPT1 (RAG228). Amplification products potentially included plus-strand strong-stop, full-length, and PPT2-primed plus-strand 3′ ends. The small arrows represent PCR primers. Frequency distribution of Ty1 (B) and Ty2 (C) plus-strand 3′ ends presented in the 5′-to-3′ direction. The symbol / represents the U5/PBS border. GC represents the complement to the two IMT bases beyond the PBS. The boldface letters represent unexpected sequences at 3′ termini.
FIG. 6.
The 3′ ends of full-length (or PPT2-generated) plus-strand DNAs generated in vivo often exhibit the PBS sequence and nontemplated bases. (A) LM-PCR scheme showing that by amplifying the DNA-linker ligation product with a Ty plus-strand primer positioned upstream of PPT1 (RAG266 and RAG198), only plus-strand full-length (or PPT2-generated) DNA 3′ ends will be included. (B) Depiction of plus-strand strong-stop synthesis, during which RNase H removes the IMT minus-strand primer from the 5′ end of the minus strand. The 3′ end of the plus-strand full-length DNA is determined by the precision of primer removal. The IMT sequence is in lowercase and underlined. Plus and minus strands are indicated at their respective 5′ ends, and arrows represent the direction of synthesis. The vertical line indicates the U5/PBS border. The 3′ termini of full-length double-stranded Ty elements are shown for several cases in which the tRNA is completely or incompletely removed. Frequency distribution of Ty1 (C) and Ty2 (D) plus-strand full-length (or PPT2-generated) 3′ DNA ends presented in the 5′-to-3′ direction. The symbol / represents the U5/PBS border. GC represents the complement to the two IMT bases beyond the PBS. The boldface letters represent unexpected sequences at 3′ termini.
Full-length plus-strand DNA (or plus-strand DNA primed by PPT2) shows evidence of incomplete removal of the tRNA primer by RNase H.
During retroviral plus-strand strong-stop synthesis, an RNA-DNA hybrid is formed between the 3′ end of the tRNA minus-strand primer and plus-strand strong-stop DNA (Fig. 1E and 6B). Accurate RNase H removal of the tRNA primer and subsequent cessation of full-length plus-strand synthesis at the 5′ end of the minus strand, i.e., at the last base of U5, generate the precise end of the element necessary for integration. When the linker ligation product was PCR amplified by primers RAG266 (upstream of PPT1) and RAG198, the plus-strand ends that were observed represented transferred full-length plus-strand DNA or plus-strand DNA primed by PPT2 (Fig. 6A). In either case, tRNA removal during plus-strand strong-stop synthesis would be expected to have defined the appropriate plus-strand 3′ end of the element (Fig. 6B). However, of 22 Ty1-H3 clones generated by this specific amplification, only 8 (36%) exhibited a plus-strand 3′ end precisely at the predicted last base of U5 (Fig. 6C). Most of the remaining clones contained 1 to 12 (usually 3) bases complementary to the 3′ end of IMT, with or without additional nontemplated bases. As at other ends, clones with non-IMT tRNA sequences were identified. Results for Ty2 were comparable, except that none of the clones exhibited the expected termination at the last base of U5.
These results for full-length or PPT2-generated plus-strand 3′ ends suggest that RNase H cleavage of the tRNA primer during plus-strand strong-stop synthesis can be imprecise or, in some cases, nonexistent. As with the left end of Ty elements, these aberrant full-length plus-strand ends, generated by multiple mechanisms, including copying of uncleaved tRNA sequences, copying of other tRNA templates, and/or addition of nontemplated terminal bases, are predicted to result in Ty elements which are defective for integration.
Plus- and minus-strand DNAs sometimes add heterogeneous tRNA sequences to 3′ ends.
We observed that Ty DNA transposition intermediates are sometimes extended beyond template ends by a variety of non-IMT tRNA sequences (Table 3). These tRNA sequences share several important features, suggesting that they are products of continued reverse transcription beyond the Ty template ends. All tRNA sequences (except one) are complements of the precise tRNA 3′ ends, copied in the orientation expected (i.e., 3′ to 5′) if the tRNA 3′ end served as the template for RT. The single exceptional clone (EM 2516) copies a tRNAGlnUUG, not from the extreme 3′ end, but starting from base 37. The orientation of this sequence, however, was also consistent with reverse transcription using the tRNA as a template. Of 16 clones with tRNA sequences, 14 extended from the extreme 3′ end of the tRNA template to a modified base. The inability of RTs to copy specific modified tRNA bases is widely assumed to account for the generation of retroviral plus-strand strong-stop DNAs terminated precisely at the 3′ end of the PBS (2, 7, 42, 55). This property also likely accounts for our observation that the major Ty1 or Ty2 plus-strand strong-stop intermediate ended just before the 2′-O-ribosylated adenosine at position 64 within IMT (Fig. 5B and C). As for the two exceptions, the sequence of one clone (EM 2081) extended to within 1 base of the first 1-methyladenosine before adding two apparent nontemplated 3′ bases. Clone EM 2516 copies tRNAGlnUUG from base 37 to base 1. The base modifications of this yeast tRNA have not, to our knowledge, been experimentally determined. In all but three cases, the modified base evidently responsible for blocking continued reverse transcription is a conserved 1-methyladenosine at position 58. Three clones (OU154, EM 6156, and EM2122), all showing extension along tRNAAspGUC, ended at a 1-methylguanosine at position 37. Interestingly, Ty RT was able to copy several other tRNA-modified bases: pseudouridine, found in the 3′ ends of both tRNALysUUU and tRNAAspGUC, as well as ribosylthymine and 5-methylcytidine, found in the 3′ end of tRNAAspGUC. Five clones showed evidence of nontemplated base addition, either before transfer to the tRNA template (EM 2815, EM 2041, and OU 154) or at the end of the tRNA sequence (EM 2081 and EM 2491), consistent with Ty RT behavior at other template ends. Five full-length minus-strand DNAs, described above (Fig. 4B and C), exhibited 4- to 7-base extensions beyond their 3′ ends which could be interpreted as truncated copies of tRNAs. These data support the notion that tRNAs participate in promiscuous strand transfer from legitimate Ty1 and Ty2 templates.
TABLE 3.
tRNA sequences linked to Ty termini
| Replication intermediate (most 3′ Ty sequence) | tRNA sequencea | tRNA, anticodon (bases)b | Next modified base (base no.)c | Ty elementd | Clone |
|---|---|---|---|---|---|
| Minus strand full length (beginning of U3) | 3′-linker-CGGGGGATGTCC[delC]GAGGTT/ACAACCTT…-5′ | LYS, CUU (59–76) | m1a (58) | Ty1-H3 | EM 2815 |
| 3′-linker-TAGGGGGATGTCCCGAGGT/ACAACCTT…-5′ | LYS, CUU (60–76) | m1a (58) | Ty1-H3 | EM 2081 | |
| 3′-linker-CGGGGGATACTCCTCGGTT/ACAACCTT…-5′ | LYS, UUU (59–76) | m1a (58) | Ty1-H3 | EM 2041 | |
| 3′-CCCCCGCACGGTCTAGCCCCAAGTTAAGGGGCAGCGCCTCGGTT/ACAACCTT…-5′ | ASP, GUC (38–75) | m1g (37) | Ty1-H3 | OU 154 | |
| Minus strand nearly full length (beginning of PBS) | 3′-linker-GGGGGTAGCACTCACGGT/ACCATCGCGG…-5′ | ARG, UCU (58–75) | m1a (57) | Ty2 | EM 5953 |
| PPT2-primed or full-length plus strand (end of U5) | 5′-…TTCTCA/TCAAAACCGAAAGTGATAACCACTACACTATAGGACC-linker-3′ | GLN, UUG (37–1) | Ty1-H3 | EM 2516 | |
| 5′-…TTCTCA/TGGAGCCCTGTAGGGGGC-linker-3′ | LYS, CUU (76–59) | m1a (58) | Ty1-H3 | EM 2513 | |
| 5′-…TTCTCA/TGGCTCCGCGACGGGGAATTGAACCCCGATCTGGCACG-linker-3′ | ASP, GUC (75–38) | m1g (37) | Ty1-173/Ty1-H3 | EM 6156 | |
| 5′-…TCTACA/TGGCTTCCCCGCCAGGAC-linker-3′ | ARG, ACG (76–59) | m1a (58) | Ty2 | EM 6189 | |
| PPT1- or PPT2-primed or full-length plus strand (end of U5) | 5′-…TTCTCA/TGGCTCCGCGACGGGGAATTGAACCCCGATCTGGCACG-linker-3′ | ASP, GUC (75–38) | m1g (37) | Ty1-H3 | EM 2122 |
| 5′-…TTCTCA/TGGCACTCACGATGGGGG-linker-3′ | ARG, UCU (75–58) | m1a (57) | Ty1-H3 | EM 2502 | |
| 5′-…TTCTCA/TGGCTCCTCATAGGGGGCC-linker-3′ | LYS, UUU (76–59) | m1a (58) | Ty1-H3 | EM 2491 | |
| 5′-…TTCTCA/TGGCTTCCCCGCCAGGAC-linker-3′ | ARG, ACG (76–59) | m1a (58) | Ty1-173/Ty1-H3 | EM 5966 | |
| 5′-…TTCTCA/TGGCTCCTCATAGGGGGC-linker-3′ | LYS, UUU (76–59) | m1a (58) | Ty1-173/Ty1-H3 | EM 5719 | |
| 5′…TTCTCA/TGGCGTCACAGACAGGAT-linker-3′ | SER, GCU (85–68) | m1a (67) | Ty1-173/Ty1-H3 | EM 5721 | |
| 5′-…TTCTCA/TGGAGCCCTGTAGGGGGC-linker-3′ | LYS, CUU (76–59) | m1a (58) | Ty1-173/Ty1-H3 | EM 5962 |
The tRNA sequence is separated from the Ty sequence by the symbol /. For minus-strand intermediates, the tRNA sequence is to the left of the /. For plus-strand intermediates, the tRNA sequence is to the right of the /. Nontemplated bases are underlined.
Numbers indicate specific tRNA bases added to Ty intermediate 3′ ends.
m1a is 1-methyladenosine; m1g is 1-methylguanosine.
Ty1-H3, wild-type Ty1 element in yeast strain AG51; Ty2, wild-type Ty2 element in yeast strain AG 1585; Ty1-173/Ty1-H3, hybrid Ty1 element combining the 5′ end of Ty1-173 (R region through the beginning of POL) with the 3′ end of Ty1-H3 (most of POL through the 3′ LTR) in yeast strain AG 1583 (see Table 1).
We have shown that plus-strand DNAs are often extended beyond the last base of U5 by 5′-TGG-3′, templated by the minus-strand primer (IMT) 3′ end (Fig. 5 and 6). Because all tRNAs end with 3′-ACC-5′, this sequence extension at the 3′ end of the Ty plus strand suggests a model for strand transfer from the minus-strand 5′ end to the complementary 3′ end of available tRNA templates. Eleven of the 16 clones showing extension by tRNA sequences were derived from plus-strand intermediates. Similarly, one Ty2 nearly full-length minus-strand clone (EM 5953), extended beyond the U5/PBS border by tRNAArgUCU, could be explained by strand transfer mediated by a two-base-pair complementarity between the 3′ end of the Ty2 minus-strand intermediate paused 2 bases upstream of the U5/PBS border (3′-GT-5′) and the two most 3′ tRNA bases (5′-CA-3′). In the case of the 3′ end of minus-strand full-length DNA, we found that the most common “nontemplated” base added is a T and postulated that this may result from aberrant cleavage of the PPT1 primer sequence (Fig. 4). This could provide a single base of complementarity to the 3′ terminus of any tRNA. Thus, strand transfer between the growing 3′ DNA end and the 3′ tRNA template end may be mediated by limited terminal complementarity.
DISCUSSION
In this paper, we examine, at the nucleotide level, the distribution of 3′ ends generated in vivo by the various replication intermediates during Ty1 and Ty2 retrotransposition. We had previously determined that the 3′ end of minus-strand nearly full-length DNA, paused upstream of the U5/PBS border, is heterogeneous and subject to terminal nontemplated base addition (34). In the present work, we examined the universality of our model in which terminal nontemplated bases are added at natural template ends during in vivo Ty1 and Ty2 replication (22). Our data demonstrate that inappropriate bases are present in a sizable fraction of all observed intermediates paused at examined template ends [(x) regions in Fig. 1]. We cannot, however, determine the true ratio of expected to unexpected transposition intermediates generated in VLPs, since normal intermediates are likely to proceed to completion and therefore be underrepresented (see below). In addition to the anticipated nontemplated terminal bases, we observed other aberrant 3′-terminal sequences which most likely resulted from imprecise RNase H removal and/or generation of RNA primers. Finally, we observed strand transfers from the template ends of both plus- and minus-strand DNA intermediates to various tRNA templates. These results indicate that during Ty1 and Ty2 retrotransposition, defective cDNA ends can be generated by multiple mechanisms.
We observed addition of 3′ nontemplated bases to paused replication intermediates at both RNA and DNA template ends. In addition to base addition subsequent to pausing at the physical end of a template, we noted nontemplated base addition when RT extension was blocked by specific tRNA base modifications, e.g., 2′-O-ribosylated adenosine in IMT and 1-methyladenosine in various other tRNAs (Fig. 5 and Table 3). Base addition is not random and depends, to a large extent, on the specific template end. For example, the predominance of C residues added to minus-strand DNAs paused at the 5′ end of Ty1 and Ty2 transcripts is likely due to the presence of the 5′ RNA cap (see below). The preponderance of added T residues at the 3′ end of full-length minus-strand DNA (five of seven Ty1 and four of four Ty2 clones with single base additions in Fig. 4B and C) could be explained by imprecise generation or removal of PPT1 at the 5′ end of the plus strand (Fig. 4D). In both of these cases, the added bases may not, strictly speaking, be nontemplated. On the other hand, A residues are added frequently to all 3′ ends, including those at the RNase H-generated RNA template ends near the U5/PBS border (34). This predilection for nontemplated A addition is similar to in vitro experiments showing that human immunodeficiency virus (HIV) RT inserts A most efficiently beyond template ends (37, 38) and at abasic sites (9).
The capped 5′ end of full-length Ty1 RNA is the template terminus during both minus-strand strong-stop synthesis and intermolecular nearly full-length minus-strand synthesis. Using LM-PCR, we determined the Ty1 transcription initiation site to be at position 241 (in the Ty1-H3 reference sequence [4]) and resolved the ambiguity resulting from the mapping of this site by primer extension. Primer extension analysis revealed a doublet, mapping to bases 240 and 241, and these products were observed when we used either VLP-derived RNA from galactose-induced Ty1-H3 elements or cellular RNA from a yeast strain expressing endogenous Ty elements (compare Fig. 2B, lanes 1 and 3). Further, the same two bands were detected when we used either AMV RT (in vitro reaction) or Ty1 RT (endogenous reaction) as the primer extension polymerase. By sequencing the resulting cDNAs, as well as the in vivo Ty1 intermediates, we found that the longer primer extension product correlated, in each case, with addition of nontemplated bases (usually C residues) to minus-strand DNA paused at the 5′ end of the Ty1 transcript (see Fig. 2C and 3B and C). Terminal nontemplated addition of C residues during in vitro primer extension of capped RNAs with AMV RT has previously been noted and ascribed to copying of the 5′-terminal cap G residue (25, 50). These findings provide a warning that primer extension determination of transcription initiation sites may be confounded by this apparently common, if not universal, behavior of RTs at capped ends.
Kulpa et al. (27) observed the in vivo appearance of plus-strand G residues at the U3/R border of 10% of newly integrated MuLV proviruses, indicating that such terminal changes can proceed to integration. However, these substitutions failed to accumulate, implying that viruses with this U3/R border error do not to replicate efficiently. We previously observed a C-to-G substitution in the left U3/R border in 1 of 29 newly transposed Ty1 elements (22). Similarly, the appearance of bilateral G substitutions at both the left and right U3/R borders of a different newly transposed Ty1 element has been observed (3a). Thus, nontemplated addition of C residues at the U3/R border during replication is an in vivo phenomenon shared by Ty1 and retroviral RTs. Is there evidence in the yeast genome that elements with these changes are stably inherited? Of the 45 full-length Ty1 and Ty2 elements present in the complete sequenced Saccharomyces cerevisiae genome, only a single Ty2 element (on chromosome 4) contains a G, rather than the usual C or T, just upstream of the left U3/R border (1 [1.1%] of 90 U3/R borders). Moreover, among the ∼85 Ty1 and Ty2 solo LTRs in the complete genome with >70% nucleotide identity to the standard Ty1-H3 LTR (26), 6 contained a G residue at the U3/R border (7%). Solo LTRs are thought to be the consequence of recombination between the left and right LTRs of full-length Ty elements. These observations suggest that despite their apparently high level within VLPs, the terminally mismatched 3′ C ends seldom go on to generate stable, integration-competent, full-length Ty elements. Those that do integrate are more likely lost (i.e., converted to solo LTRs) than propagated. This stringent selection at the U3/R border is in contrast to the high degree of polymorphism we previously observed just upstream of the U5/PBS border, a region also subject to nontemplated base addition (34). The result implies that the presence of a G residue just upstream of the transcription initiation site in some way blocks subsequent transposition.
The plus-strand transfer step of Ty1 replication is distinct from the analogous step in retroviral replication (3, 29). Our LM-PCR analysis of the 3′ terminus of plus-strand DNA confirms previous reports that a plus-strand product which copies the 12 most 3′ bases of the IMT primer is the major product when PCR amplification includes PPT1-primed plus-strand DNA (24, 30, 52). This is the expected product if Ty1 RT copies the bound IMT template until encountering the modified 2′-O-ribosylated adenosine at IMT position 64. However, strand transfer and base pairing of this plus-strand strong-stop DNA to the minus-strand PBS (Fig. 1F) would result in a two-base terminal mismatch and would consequently be blocked for further plus-strand synthesis (28). Lauermann and Boeke observed that a single substitution at the seventh base of the PBS, which creates a mismatch with the 3′ IMT sequence, is consistently maintained after reverse transcription, even if the two bases downstream of the PBS were experimentally altered to remove the terminal mismatch. This implies that the 12-base extended plus-strand strong-stop intermediate does not directly participate in continuation of plus-strand synthesis (28, 29). Based on their observations, those authors proposed a plus-strand primer recycling model. In this scheme, the 12-base extended plus-strand strong-stop species serves only to provide the RNA-DNA hybrid required for RNase H removal of the tRNA primer. Recycled PPT1 (i.e., cleaved from the 5′ end of plus-strand strong-stop DNA by RNase H) then creates a second plus-strand strong stop, extended only to the end of U5, which does accomplish strand transfer.
The primer recycling model for Ty1 plus-strand synthesis requires a functional RNase H to cleave both the tRNA and PPT1. While our data are consistent with the model, many of the unexpected minus- and plus-strand full-length 3′ ends we observed are most easily explained by inefficient and/or heterogeneous RNase H cleavage of these two RNA primers. For example, we observed several clones in which the full-length minus-strand 3′ end was extended into (and, in one case, beyond) PPT1 (Fig. 4B). Moreover, the most frequently observed nontemplated base at the 3′ end of the full-length minus strand was T, which is complementary to the 3′-most base of PPT1. Wilhelm et al. (52) described similar Ty3 full-length minus-strand 3′ ends. We observed numerous examples of plus strands extended from 1 to 5 (usually 3) bases into the PBS region (Fig. 5B and C and 6B and C). Examples of similar 3′ plus-strand ends were also reported by Wilhelm et al. (52). Such extended plus-strand ends most likely result from prior RNase H cleavage of the tRNA primer beyond the expected cleavage site at the junction of RNA and DNA. Extension of plus-strand strong-stop DNA into the PBS region due to incomplete removal of the tRNA primer would tend to promote successful strand transfer. Just as RNase H cleavage of the RNA template 3 to 6 bases upstream of the U5/PBS border during minus-strand strong-stop synthesis lengthens the template for intramolecularly transferred nearly full-length minus-strand DNA (Fig. 1B and reference 34), tRNA sequences downstream of U5 could serve the same function. The result would be an expanded region of mutual complementarity between the 3′ ends of the two DNA strands. It is of note that this strand transfer model is compatible with observations of Lauermann and Boeke regarding the failure of Ty1 RT to copy the seventh base of IMT, since our observed plus-strand 3′ ends rarely extend more than 5 bases into the tRNA sequence.
Incomplete and/or imprecise cleavage of the RNA primers is not unique to Ty elements. Analysis of in vivo-generated circle junctions from both HIV (45, 51) and MuLV (13) indicate the presence of extra terminal bases most likely derived from uncleaved primer sequences. By using in vitro model substrates and either HIV or MuLV RT, it has been shown that RNase H cleavage of the tRNA primer leaves a single ribonucleotide A attached to the 5′ end of the full-length minus-strand DNA (19, 41, 43, 46). A recent study of HIV preintegration complexes revealed heterogeneous U5 3′ ends, consistent with either nontemplated base addition or inefficient removal of the minus-strand primer (31). What are the potential effects of these extra terminal bases on subsequent integration? Retroviral integrases normally recognize specific subterminal sequences and utilize an end-processing activity to remove terminal 3′ bases and reveal the essential recessed CA 3′-OH. Because of end processing, retroviral integrases can, within certain limits, accommodate extra terminal bases and remain integration competent (8, 15, 31, 42, 49). In contrast, Ty1 integrase appears to lack a 3′-end processing activity and, instead, preferentially integrates blunt-end substrates with a terminal CA 3′ sequence (16, 33, 44). Consequently, aberrant Ty RNase H removal of RNA primer sequences will likely lead to full-length products which are not substrates for integration, thus limiting the element’s capacity for successful Ty1 transposition. Note that it is conceivable that the observed RNase H cleavage patterns in our VLP-derived transposition intermediates are influenced by cellular RNase H activities. We are in the process of constructing specific Ty1 RNase H mutants to see whether plus- and minus-strand primer removal is determined solely by Ty1 RNase H activity.
What is the basis for the replication intermediates extending beyond minus- and plus-strand template ends into various tRNA sequences? We found these unusual extensions associated with several different intermediates and with Ty1 and Ty2, as well as a hybrid Ty1 element. The common thread in all but one of these plus- and minus-strand clones is that the junctions occurred between known Ty-specific pause sites and the 3′ ends of various tRNAs. Therefore, the most likely basis for these products is Ty RT strand transfer from Ty template ends to the 3′ ends of tRNA. A similar phenomenon was observed by Colicelli and Goff (14), who analyzed an MuLV two-LTR circle junction with a glycine tRNA sequence between the LTRs. They attributed this product to unorthodox minus-strand priming by glycine tRNA rather than by the wild-type proline tRNA. While we could invoke similar spurious minus-strand priming by tRNAs lacking complementarity to the PBS as a mechanism for the generation of the aberrant plus-strand intermediates (which represented 11 of 16 clones), it cannot account for the tRNA sequences observed at the 3′ ends of minus-strand DNAs, which represent the same range of tRNA species (5 of 16 clones). These minus-strand-derived clones are similar in structure to another reported circle junction insertion consisting of 15 bases of the 3′ end of IMT, described for the Drosophila LTR-containing retrotransposon copia (18). The orientation of the copia insertion is inconsistent with aberrant minus-strand priming. Aberrant priming of plus-strand synthesis by a tRNA instead of normal PPT1 seems highly unlikely due to the complete lack of DNA complementarity between the 3′ ends of tRNAs and the sequence of Ty minus-strand DNA just upstream of U3 or, in one case (EM5953), upstream of the U5/PBS border. Thus, an association between template termini and tRNA sequences may be a universal phenomenon for retroelements in vivo, reflecting some special affinity of these RNA molecules to replicating retroelements.
Although we repeatedly identified certain tRNAs, i.e., tRNALysCUU, tRNALysUUU, tRNAArgUCU, tRNAArgACG, and tRNAAspGUC, linked to intermediate ends, it is unclear why these particular tRNAs were overrepresented. In the only study of its kind, Pochart et al. (39) reported that in addition to the expected IMT, only tRNASerAGA and the rare tRNASerGCU were selectively packaged and present in significant amounts in Ty1 VLPs. Consistent with this, we did observe the 3′ end of tRNASerGCU in one of our clones (EM 5721). However, the other tRNAs which we identified were detected in VLPs in only trace amounts. Conversely, other tRNAs that were detected in VLPs (tRNALeuU*mAA, tRNALeuCAA, and tRNATyr) were not represented in our clones. We examined the sequences of the tRNAs found at Ty DNA 3′ ends with regard to four short sequences (box 0, box 1, box 2.1, and the PBS) which have been implicated in VLP packaging of the IMT primer (53). No pattern suggesting that these tRNAs take advantage of the same packaging mechanism was observed. Data from the sequence analysis of these yeast tRNAs indicated that they do not share any specific base modifications. tRNALysCUU, tRNALysUUU, tRNAArgUCU, and tRNAAspGUC are all found in multiple copies in the yeast genome (12, 7, 11, and 15 copies, respectively), but other multicopy tRNAs were not seen, and the tRNAGlnUUG gene is present in only 1 copy. The only obvious similarity among these tRNAs is that they all code for charged amino acids. Perhaps these tRNAs are aminoacylated within the VLPs and are put into proximity to RT, paused at template ends, by some nonspecific ionic interaction.
In this study, we marked the 3′ ends of specific Ty1 single-stranded DNA intermediates with either a linker by using T4 RNA ligase or a mononucleotide run by using TdT. After PCR amplification, subcloning, and sequencing, we were able to describe a frequency distribution of ends associated with specific steps in Ty1 replication. Although we were restricted by the necessarily limited number of clones which could reasonably be sequenced, the distribution of ends observed for Ty1 and Ty2, as detected by either LM-PCR or TdT-PCR, was similar and, where applicable, reflected observed primer extension patterns. We therefore conclude that our results reflect a true sample of 3′ ends found within VLPs (see also reference 34). However, several factors may have influenced the observed distribution of specific transposition intermediates. We isolated replication intermediates from VLPs after galactose induction of Ty transposition. It is possible that such overexpression affected the availability of factors potentially necessary for accurate Ty replication. In addition, a bias is introduced by virtue of the fact that Ty1 DNA intermediates which lack 3′-end complementarity to expected templates are less likely to complete replication than those with no terminal mismatches. We suspect that these errant intermediates accumulate in VLPs relative to their correct counterparts and are therefore preferentially available for ligation to our linker (or to act as a substrate for mononucleotide addition by TdT). Our sample, therefore, is undoubtedly weighted toward discovery of terminal errors. Because of this inherent bias, we cannot estimate the rate at which Ty1 RT generates correct versus aberrant replication intermediates. On the other hand, we have described in vivo data from integrated Ty1 elements (34) and retroviruses (27), as well as corroborating circle junction analyses (14, 45), which indicate that terminal changes due to nontemplated base addition, errors in formation or removal of RNA primers, and terminal association with nonpriming tRNA sequences are universal retroelement phenomena.
Why have error-prone transposable elements evolved? For Ty1 and Ty2, this mutational propensity appears to be the combined effect of RT-mediated nontemplated base addition, imprecise RNase H cleavages, and inappropriate strand transfers. The generation of frequent errors at the 3′ termini of replication intermediates creates the potential for introduction of sequence changes to both internal and terminal regions of Ty elements. Multiple aspects of the Ty life cycle may be affected by such sequence changes, including the amino acid composition of its encoded proteins, regulation of transcription, ability to complete the steps of replication, and the ultimate frequency of integration. A selective advantage may be gained by genomic variability generated via this mechanism. From a replication standpoint, Ty1 plus-strand DNA transfer to paused minus-strand DNA may be facilitated by an increased region of 3′ end complementarity generated by imprecise RNase H cleavage of the RNA template upstream of the U5/PBS border and imprecise RNase H removal of the IMT minus-strand primer. The linear “dead-end” products, not destined for integrative transposition, can still serve a role in DNA recombination (36, 44) and repair (32, 47). Finally, it is interesting to speculate that the frequent creation of nonintegratable ends may be a means to down-regulate the number of potentially deleterious transposition events and thereby adapt the transposon to the host cell environment. In this regard, the relationship of a retrotransposon to its host is quite different from that of a pathogenic virus to its host.
ACKNOWLEDGMENTS
This work was supported in part by grant AI39201 from the NIH, by a Lucille P. Markey Scholar Award in Biomedical Science, and by a Busch Memorial Fund grant.
We thank R. Bambara, J. Boeke, J. Dougherty, G. Keith, T. Heyman, M. Roth, S. Hughes, and F. Wilhelm for useful discussions and J. Boeke and A. Bystrom for communicating unpublished data.
REFERENCES
- 1.Ausubel F M, Brent R, Kingston R L, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1994. [Google Scholar]
- 2.Ben-Artzi H, Shemesh J, Zeelon E, Amit B, Kleiman L, Gorecki M, Panet A. Molecular analysis of the second template switch during reverse transcription of the HIV RNA template. Biochemistry. 1996;35:10549–10557. doi: 10.1021/bi960439x. [DOI] [PubMed] [Google Scholar]
- 3.Berwin B, Barklis E. Retrovirus mediated insertion of expressed and non-expressed genes at identical chromosomal locations. Nucleic Acids Res. 1993;21:2399–2407. doi: 10.1093/nar/21.10.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3a.Boeke, J. D. Personal communication.
- 4.Boeke J D, Eichinger D, Castrillon D, Fink G R. The Saccharomyces cerevisiae genome contains functional and nonfunctional copies of transposon Ty1. Mol Cell Biol. 1988;8:1432–1442. doi: 10.1128/mcb.8.4.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boeke J D, Styles C A, Fink G R. Saccharomyces cerevisiae SPT3 gene is required for transposition and transpositional recombination of chromosomal Ty elements. Mol Cell Biol. 1986;6:3575–3581. doi: 10.1128/mcb.6.11.3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Braiterman L T, Manokian G M, Eichinger D J, Merbs S L, Gabriel A, Boeke J D. In-frame linker insertion mutagenesis of yeast transposon Ty1: phenotypic analysis. Gene. 1994;139:19–26. doi: 10.1016/0378-1119(94)90518-5. [DOI] [PubMed] [Google Scholar]
- 7.Burnett B P, McHenry C S. Posttranscriptional modification of retroviral primers is required for late stages of DNA replication. Proc Natl Acad Sci USA. 1997;94:7210–7415. doi: 10.1073/pnas.94.14.7210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bushman F D, Craigie R. Activities of human immunodeficiency virus (HIV) integration protein in vitro: specific cleavage and integration of HIV DNA. Proc Natl Acad Sci USA. 1991;88:1339–1343. doi: 10.1073/pnas.88.4.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8a.Bystrom, A., and J. Boeke. Personal communication.
- 9.Cai H, Bloom L B, Eritja R, Goodman M F. Kinetics of deoxyribonucleotide insertion and extension at abasic template lesions in different sequence contexts using HIV-1 reverse transcriptase. J Biol Chem. 1993;268:23567–23572. [PubMed] [Google Scholar]
- 10.Chapman K B, Byström A S, Boeke J D. Initiator methionine tRNA is essential for Ty1 transposition in yeast. Proc Natl Acad Sci USA. 1992;89:3236–3240. doi: 10.1073/pnas.89.8.3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clark J M. Novel non-templated nucleotide addition reactions catalyzed by procaryotic and eucaryotic DNA polymerases. Nucleic Acids Res. 1988;16:9677–9686. doi: 10.1093/nar/16.20.9677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clark J M, Joyce C M, Beardsley G P. Novel blunt-end addition reactions catalyzed by DNA polymerase I of Escherichia coli. J Mol Biol. 1987;198:123–127. doi: 10.1016/0022-2836(87)90462-1. [DOI] [PubMed] [Google Scholar]
- 13.Colicelli J, Goff S P. Sequence and spacing requirements of a retrovirus integration site. J Mol Biol. 1988;199:47–59. doi: 10.1016/0022-2836(88)90378-6. [DOI] [PubMed] [Google Scholar]
- 14.Colicelli J, Goff S P. Structure of a cloned circular retroviral DNA containing a tRNA sequence between the terminal repeats. J Virol. 1986;57:674–677. doi: 10.1128/jvi.57.2.674-677.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Craigie R, Fujiwara T, Bushman F. The IN protein of Moloney murine leukemia virus processes the viral DNA ends and accomplishes their integration in vitro. Cell. 1990;62:829–837. doi: 10.1016/0092-8674(90)90126-y. [DOI] [PubMed] [Google Scholar]
- 16.Eichinger D J, Boeke J D. A specific terminal structure is required for Ty1 transposition. Genes Dev. 1990;4:324–330. doi: 10.1101/gad.4.3.324. [DOI] [PubMed] [Google Scholar]
- 17.Feuerbach F, Drouaud J, Lucas H. Retrovirus-like end processing of the tobacco Tnt1 retrotransposon linear intermediates of replication. J Virol. 1997;71:4005–4015. doi: 10.1128/jvi.71.5.4005-4015.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flavell A J, Ish-Horowicz D. The origin of extrachromosomal circular copia elements. Cell. 1983;34:415–419. doi: 10.1016/0092-8674(83)90375-6. [DOI] [PubMed] [Google Scholar]
- 19.Furfine E S, Reardon J E. Reverse transcriptase-RNase H from the human immunodeficiency virus. Relationship of the DNA polymerase and RNA hydrolysis activities. J Biol Chem. 1991;266:406–412. [PubMed] [Google Scholar]
- 20.Gabriel A, Boeke J D. Retrotransposon reverse transcription. In: Skalka A M, Goff S P, editors. Reverse transcriptase. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1993. pp. 275–328. [Google Scholar]
- 21.Gabriel A, Boeke J D. Reverse transcriptase encoded by a retrotransposon from the trypanosomatid Crithidia fasciculata. Proc Natl Acad Sci USA. 1991;88:9794–9798. doi: 10.1073/pnas.88.21.9794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gabriel A, Willems M, Mules E H, Boeke J D. Replication infidelity during a single cycle of Ty1 retrotransposition. Proc Natl Acad Sci USA. 1996;93:7767–7771. doi: 10.1073/pnas.93.15.7767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gilboa E, Mitra S W, Goff S, Baltimore D. A detailed model of reverse transcription and a test of crucial aspects. Cell. 1979;18:93–100. doi: 10.1016/0092-8674(79)90357-x. [DOI] [PubMed] [Google Scholar]
- 24.Heyman T, Agoutin B, Friant S, Wilhelm F X, Wilhelm M L. Plus-strand DNA synthesis of the yeast retrotransposon Ty1 is initiated at two sites, PPT1 next to the 3′ LTR and PPT2 within the pol gene. PPT1 is sufficient for Ty1 transposition. J Mol Biol. 1995;253:291–303. doi: 10.1006/jmbi.1995.0553. [DOI] [PubMed] [Google Scholar]
- 25.Hirzmann J, Lou D, Hahnen J, Hobom G. Determination of messenger RNA 5′-ends by reverse transcription of the cap structure. Nucleic Acids Res. 1993;21:3597–3598. doi: 10.1093/nar/21.15.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim J M, Vanguri S, Boeke J D, Gabriel A, Voytas D F. Transposable elements and genome organization: a comprehensive survey of retrotransposons revealed by the Saccharomyces cerevisiae genome sequence. Genome Res. 1998;8:464–478. doi: 10.1101/gr.8.5.464. [DOI] [PubMed] [Google Scholar]
- 27.Kulpa D, Topping R, Telesnitsky A. Determination of the site of first strand transfer during Moloney murine leukemia virus reverse transcription and identification of strand transfer-associated reverse transcriptase errors. EMBO J. 1997;16:856–865. doi: 10.1093/emboj/16.4.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lauermann V, Boeke J D. Plus strand strong-stop DNA transfer in yeast Ty retrotransposons. EMBO J. 1997;16:6603–6612. doi: 10.1093/emboj/16.21.6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lauermann V, Boeke J D. The primer tRNA sequence is not inherited during Ty1 retrotransposition. Proc Natl Acad Sci USA. 1994;91:9847–9851. doi: 10.1073/pnas.91.21.9847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lauermann V, Nam K, Trambley J, Boeke J D. Plus-strand strong-stop DNA synthesis in retrotransposon Ty1. J Virol. 1995;69:7845–7850. doi: 10.1128/jvi.69.12.7845-7850.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller M D, Farnet C M, Bushman F D. Human immunodeficiency virus type 1 preintegration complexes: studies of organization and composition. J Virol. 1997;71:5382–5390. doi: 10.1128/jvi.71.7.5382-5390.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moore J K, Haber J E. Capture of retrotransposon DNA at the sites of chromosomal double-strand breaks. Nature. 1996;383:644–646. doi: 10.1038/383644a0. [DOI] [PubMed] [Google Scholar]
- 33.Moore S P, Powers M, Garfinkel D J. Substrate specificity of Ty1 integrase. J Virol. 1995;69:4683–4692. doi: 10.1128/jvi.69.8.4683-4692.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mules E H, Uzun O, Gabriel A. Replication errors during in vivo Ty1 transposition are linked to heterogeneous RNase H cleavage sites. Mol Cell Biol. 1998;18:1094–1104. doi: 10.1128/mcb.18.2.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Müller F, Laufer W, Pott U, Ciriacy M. Characterization of products of Ty1-mediated reverse transcription in Saccharomyces cerevisiae. Mol Gen Genet. 1991;226:145–153. doi: 10.1007/BF00273598. [DOI] [PubMed] [Google Scholar]
- 36.Nevo-Caspi Y, Kupiec M. cDNA-mediated Ty recombination can take place in the absence of plus-strand cDNA synthesis, but not in the absence of the integrase protein. Curr Genet. 1997;32:32–40. doi: 10.1007/s002940050245. [DOI] [PubMed] [Google Scholar]
- 37.Patel P H, Preston B D. Marked infidelity of human immunodeficiency virus type 1 reverse transcriptase at RNA and DNA template ends. Proc Natl Acad Sci USA. 1994;91:549–553. doi: 10.1073/pnas.91.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peliska J A, Benkovic S J. Mechanism of DNA strand transfer reactions catalyzed by HIV-1 reverse transcriptase. Science. 1992;258:1112–1118. doi: 10.1126/science.1279806. [DOI] [PubMed] [Google Scholar]
- 39.Pochart P, Agoutin B, Keith G, Heyman T. A very poorly expressed tRNASer is highly concentrated together with replication primer initiator tRNAMet in the yeast Ty1 virus-like particles. Nucleic Acids Res. 1993;21:1517–1521. doi: 10.1093/nar/21.7.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pochart P, Agoutin B, Rousset S, Chanet R, Doroszkiewicz V, Heyman T. Biochemical and electron microscope analyses of the DNA reverse transcripts present in the virus-like particles of the yeast transposon Ty1. Identification of a second origin of Ty1 DNA plus strand synthesis. Nucleic Acids Res. 1993;21:3513–3520. doi: 10.1093/nar/21.15.3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pullen K A, Ishimoto L K, Champoux J J. Incomplete removal of RNA primer for minus-strand DNA synthesis by human immunodeficiency virus type 1 reverse transcriptase. J Virol. 1992;66:367–373. doi: 10.1128/jvi.66.1.367-373.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roth M J, Schwartzberg P L, Goff S P. Structure of the termini of DNA intermediates in the integration of retroviral DNA: dependence on IN function and terminal DNA sequence. Cell. 1989;58:47–54. doi: 10.1016/0092-8674(89)90401-7. [DOI] [PubMed] [Google Scholar]
- 43.Schultz S J, Whiting S H, Champoux J J. Cleavage specificities of Moloney murine leukemia virus RNase H implicated in the second strand transfer during reverse transcription. J Biol Chem. 1995;270:24135–24145. doi: 10.1074/jbc.270.41.24135. [DOI] [PubMed] [Google Scholar]
- 44.Sharon G, Burkett T J, Garfinkel D J. Efficient homologous recombination of Ty1 element cDNA when integration is blocked. Mol Cell Biol. 1994;14:6540–6551. doi: 10.1128/mcb.14.10.6540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith J S, Kim S, Roth M J. Analysis of long terminal repeat circle junctions of human immunodeficiency virus type 1. J Virol. 1990;64:6286–6290. doi: 10.1128/jvi.64.12.6286-6290.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith J S, Roth M J. Specificity of human immunodeficiency virus-1 reverse transcriptase-associated ribonuclease H in removal of the minus-strand primer, tRNALys3. J Biol Chem. 1992;267:15071–15079. [PubMed] [Google Scholar]
- 47.Teng S-C, Kim B, Gabriel A. Retrotransposon reverse transcriptase-mediated repair of chromosomal breaks. Nature. 1996;383:641–644. doi: 10.1038/383641a0. [DOI] [PubMed] [Google Scholar]
- 48.Van Beveren C, Rands E, Chattopadhyay S K, Lowy D R, Verma I M. Long terminal repeat of murine retroviral DNAs: sequence analysis, host-proviral junctions, and preintegration site. J Virol. 1982;41:542–556. doi: 10.1128/jvi.41.2.542-556.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vink C, van Gent D C, Elgersma Y, Plasterk R H A. Human immunodeficiency virus integrase protein requires a subterminal position of its viral DNA recognition sequence for efficient cleavage. J Virol. 1991;65:4636–4644. doi: 10.1128/jvi.65.9.4636-4644.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Volloch V Z, Schweitzer B, Rits S. Transcription of the 5′-terminal cap nucleotide by RNA-dependent DNA polymerase: possible involvement in retroviral reverse transcription. DNA Cell Biol. 1995;14:991–996. doi: 10.1089/dna.1995.14.991. [DOI] [PubMed] [Google Scholar]
- 51.Whitcomb J M, Kumar R, Hughes S H. Sequence of the circle junction of human immunodeficiency virus type 1: implications for reverse transcription and integration. J Virol. 1990;64:4903–4906. doi: 10.1128/jvi.64.10.4903-4906.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wilhelm M, Heyman T, Friant S, Wilhelm F-X. Heterogeneous terminal structure of Ty1 and Ty3 reverse transcripts. Nucleic Acids Res. 1997;25:2161–2166. doi: 10.1093/nar/25.11.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wilhelm M, Wilhelm F X, Keith G, Agoutin B, Heyman T. Yeast Ty1 retrotransposon: the minus-strand primer binding site and a cis-acting domain of the Ty1 RNA are both important for packaging of primer tRNA inside virus-like particles. Nucleic Acids Res. 1994;22:4560–4565. doi: 10.1093/nar/22.22.4560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu H, Boeke J D. Inhibition of Ty1 transposition by mating pheromones in Saccharomyces cerevisiae. Mol Cell Biol. 1991;11:2736–2743. doi: 10.1128/mcb.11.5.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Youvan D C, Hearst J E. Reverse transcriptase pauses at N2-methylguanine during in vitro transcription of Escherichia coli 16S ribosomal RNA. Proc Natl Acad Sci USA. 1979;76:3751–3754. doi: 10.1073/pnas.76.8.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]