Graphical abstract

Keywords: Myocardial infarction, Ischemic stroke, Atherosclerotic cardiovascular disease, Lipid profiles, Prognosis
Highlights
-
•
In this nationwide primary prevention cohort study, about two-thirds of the subjects had a 10-year ASCVD risk of <5 %.
-
•
J-shaped associations were observed between MI risk, IS risk, and LDL cholesterol levels.
-
•
Associations between MI and IS risks and LDL cholesterol levels were influenced by ASCVD risk and obesity.
-
•
A J-shaped association was observed between hs-CRP level and LDL cholesterol level.
-
•
Therefore, low LDL cholesterol levels with no statin medication do not warrant safety from ASCVD.
Abstract
Introduction
Low-density lipoprotein (LDL) cholesterol-lowering treatment is beneficial for the secondary or primary prevention of high-risk atherosclerotic cardiovascular disease (ASCVD). However, the prognostic implications of low LDL cholesterol levels in patients without previous ASCVD and without statin use remain elusive.
Methods
From a nationwide cohort, 2,432,471 participants without previous ASCVD or statin use were included. For myocardial infarction (MI) and ischemic stroke (IS), participants were followed-up from 2009 to 2018. They were stratified according to 10-year ASCVD risk (<5 %, 5 %–<7.5 %, 7.5 %–<20 %, and ≥20 %) and LDL cholesterol level (<70, 70–99, 100–129, 130–159, 160–189, and ≥190 mg/dL).
Results
The relationship between LDL cholesterol levels and ASCVD events exhibited a J-shaped curve for both MI and IS. After classification according to the ASCVD risk, this J-shaped relationship was consistently observed for the composite of MI and IS. Participants with an LDL cholesterol level <70 mg/dL showed a higher MI risk than those with a level of 70–99 mg/dL or 100–129 mg/dL in the low-ASCVD risk group. The J-shaped curve between LDL cholesterol levels and MI risk was attenuated across ASCVD risk groups. For IS, participants with an LDL cholesterol level <70 mg/dL demonstrated increased risks compared with those with a level of 70–99 mg/dL, 100–129 mg/dL, or 130–159 mg/dL in the borderline, intermediate, and high ASCVD risk groups, respectively. In contrast, a linear association was observed in participants taking statins. Interestingly, a J-shaped association was observed between LDL cholesterol and high-sensitivity C-reactive protein (hs-CRP) levels; the mean hs-CRP level and the proportion of individuals with increased hs-CRP levels were relatively high among individuals with an LDL cholesterol level <70 mg/dL.
Conclusions
Although high LDL cholesterol levels increase the risk of ASCVD, low LDL cholesterol levels do not warrant safety from ASCVD. Therefore, individuals with low LDL cholesterol levels should be carefully monitored.
Introduction
Atherosclerotic cardiovascular disease (ASCVD) poses a substantial burden worldwide [1], [2]. Owing to its high prevalence and case fatality rate, various attempts have been made to improve the clinical outcomes of ASCVD. Based on these efforts, several studies have reported advances in ASCVD outcomes in recent decades [1], [3]. However, it remains largely responsible for morbidity and mortality [1], suggesting the need for better risk stratification and tailored management.
Elevated low-density lipoprotein (LDL) cholesterol levels is a critical risk factor for ASCVD, and its pivotal role in atherosclerosis has been extensively investigated in animal experiments and human cohort studies [4]. Randomized controlled trials have demonstrated that lowering LDL cholesterol levels could provide cardiovascular benefits in patients with previous ASCVD [5], [6], [7], [8], diabetes mellitus (DM) [9], high ASCVD risk [10], [11], and high LDL cholesterol levels [10], [12]. Therefore, current guidelines recommend lowering LDL cholesterol levels to prevent recurrent ASCVD, as well as for primary prevention in individuals at high risk of ASCVD [13], [14], [15].
Despite the remarkable benefits of lowering LDL cholesterol, it remains uncertain whether low levels of LDL cholesterol, specifically in individuals not taking lipid-lowering medications, are associated with lower ASCVD risks. In these patients, the relationship between low LDL cholesterol levels and myocardial infarction (MI) risk remains unknown. There are contradictory reports regarding the relationship between LDL cholesterol levels and ischemic stroke (IS) [16], [17], [18]. Hence, it is reasonable to hypothesize that the benefits of LDL cholesterol-lowering treatment differ from the association between MI and IS risk and LDL cholesterol levels. As few studies have shown the therapeutic effects of primary prevention in individuals with a low ASCVD risk [19], [20], we postulated that this association could be influenced by the calculated ASCVD risk. If a low LDL cholesterol level cannot guarantee safety from incidental ASCVD, there is a need to explain this counterintuitive phenomenon.
In this study, we aimed to explore the prognostic implications of low LDL cholesterol levels on the risk of MI and IS among individuals without previous ASCVD and with no statin medication. We unveiled the relationship between MI, IS risk, and LDL cholesterol levels based on the 10-year American College of Cardiology/American Heart Association (ACC/AHA) ASCVD risk [21] using a nationwide primary prevention cohort.
Materials and Methods
Compliance with ethics requirements
This study was conducted in compliance with the principles of the Declaration of Helsinki. This study was approved by the Institutional Review Board (IRB) of our institute (IRB No. E-2101-012-1184). After approval from the National Health Insurance Service (NHIS), data were made available from designated terminals.
Data sources and population selection
This nationwide, population-based, retrospective cohort study was conducted using data from the Korean NHIS database. A summary of the NHIS database elsewhere [22]. The NHIS, a single public insurer, covers the entire Korean population. Biannual general health check-ups provided by the NHIS are recommended for all Korean adults. The medical information for each individual was recorded based on the International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM).
Fig. 1 visualize the flowchart of the study. We identified 4,234,339 individuals who underwent general health checkups in 2009. This study included 3,600,292 adult participants (aged 30–75 years). We excluded participants who were previously diagnosed with ASCVD, including those with peripheral artery disease, ischemic heart disease, heart failure, ischemic/hemorrhagic stroke, and transient ischemic attack (n = 878,524) (Supplemental Table 1). Individuals with missing data or triglyceride levels of ≥400 mg/dL were also excluded (n = 197,004). Among the 2,524,764 individuals screened, 2,432,471 who were not taking statin medications were included. The participants were followed-up until December 2018.
Fig. 1.
Flowchart of study population. The flow of the study is described in this section. Among the 2,524,764 individuals who underwent general health checkups in 2009, we identified 2,432,471 individuals without previous ASCVD and no statin medication. They were stratified according to 10-year ASCVD risk (<5 %, 5 %-<7.5 %, 7.5 %-<20 %, and ≥20 %) and LDL cholesterol level (<70, 70–99, 100–129, 130–159, 160–189, and ≥190 mg/dL). ACC/AHA, American College of Cardiology/American Heart Association; ASCVD, atherosclerotic cardiovascular disease; LDL, low-density lipoprotein.
ACC/AHA ASCVD risk evaluation
The risk calculation equation reported in the ACC/AHA guidelines was adopted [21]. Individuals were classified as low risk (10-year ASCVD risk <5 %), borderline risk (10-year ASCVD risk 5 %-<7.5 %), intermediate risk (10-year ASCVD risk 7.5 %-<20 %), and high risk (10-year ASCVD risk ≥20 %) [15]. The participants were further categorized into six groups according to their LDL cholesterol levels: < 70 mg/dL, 70–99 mg/dL, 100–129 mg/dL, 130–159 mg/dL, 160–189 mg/dL, and ≥190 mg/dL (Fig. 1) [23], [24]. Based on clinical guidelines [14], [15], [24], participants with an LDL cholesterol level <70 mg/dL were included in the reference group.
Data collection
Demographic data and histories of hypertension, DM, cancer, and liver disease were collected. In addition, data on lifestyle behaviors, statin use, systolic and diastolic blood pressures, laboratory examinations of lipid profiles, glomerular filtration rates, and fasting glucose levels were collected. These data were assessed during the index general health checkup. LDL cholesterol levels were derived using the Friedewald formula for total cholesterol, high-density lipoprotein (HDL) cholesterol, and triglyceride levels [25].
We jointly defined newly diagnosed ASCVD with MI and IS as the study endpoints. Supplemental Table 1 provides a detailed definition of the endpoints and comorbidities. The time interval between the index health checkup and the first occurrence of the aforementioned study endpoints was defined as the follow-up duration.
High-sensitivity C-reactive protein (hs-CRP) level
Since general health checkups performed by the NHIS did not examine hs-CRP levels, a representative marker for inflammation, we additionally analyzed data from general health checkups at the Seoul National University Hospital Healthcare System Gangnam Center from May 2015 to October 2021 and data from health checkups collected from the Korea National Health and Nutrition Examination Survey (KNHNES) between January 2015 and December 2018. A summary of the KNHNES database has been previously reported [26]. We excluded patients with previous ASCVD and those taking statins in these two cohorts.
Statistics
For continuous variables, data are presented as mean ± standard deviation or median (interquartile range). Categorical variables are presented as numbers and frequencies. The chi-square test was used to compare categorical variables. When comparing continuous variables between three or more groups, one-way analysis of variance was adopted. We present the annual event incidence rate (IR) according to the estimated number of endpoints per 1,000 person-years. We calculated the area under the curve (AUC) for LDL cholesterol levels using receiver operating characteristic (ROC) curve analysis to predict the occurrence of MI and IS. Kaplan–Meier curves were plotted and compared using the log-rank test according to the LDL cholesterol levels. We collected hazard ratios (HRs) and corresponding 95 % confidence intervals (CIs) using Cox regression models. After classifying participants into six subgroups of LDL cholesterol: 70 mg/dL, 70–99 mg/dL, 100–120 mg/dL, 130–159 mg/dL, and 160–189 mg/dL, and ≥190 mg/dL, we analyzed participants with an LDL cholesterol level of <70 mg/dL as a reference. Cox multivariate analyses included age, sex, obesity evaluated using body mass index (BMI), hypertension, DM, current smoking, alcohol consumption habits, regular exercise, and glomerular filtration rate. Nonlinearities were evaluated for associations between LDL cholesterol levels and MI and IS risks using restricted cubic splines as covariates. Subgroup analyses were conducted based on the BMI, which reflects the health effects of obesity [27]. For the sensitivity analyses, we performed Cox regression analyses after excluding individuals with cancer, liver disease, or chronic kidney disease. The mean high-sensitivity C-reactive protein (hs-CRP) level and proportion of patients with increased hs-CRP levels (≥2 mg/L) were assessed. Statistical significance was defined as a two-sided P-value <0.05. SAS version 9.5 (SAS Institute, Cary, North Carolina, USA) was used for this study.
Results
Baseline features of the study participants
In total, 2,432,471 participants (mean age 46.3 ± 10.6 years; men, 1,419,042 [58.3 %]) were analyzed in this study. Of the included patients, 464,097 (19.1 %) had hypertension; 136,002 (5.6 %) had DM; 129,514 (5.3 %) had chronic kidney disease; 26,212 (1.1 %) had cancer; and 98,960 (4.1 %) had liver disease.
We categorized the participants into four groups according to their 10-year ASCVD risk; the baseline features of each group are provided (Table 1). Approximately two-thirds of the participants were classified into the low-risk ASCVD group. Approximately 5 % of the participants were classified into the high-ASCVD risk group. Compared with individuals in the low ASCVD risk group, those in the high ASCVD risk group were older, had a male preponderance, and had a history of hypertension, DM, chronic kidney disease, cancer, and liver disease. Participants in the high-risk group showed increased systolic/diastolic blood pressure and triglyceride levels and decreased high-density lipoprotein (HDL) cholesterol levels.
Table 1.
Clinical characteristics of statin non-users according to 10-year ASCVD risk.
| 10-year ASCVD risk |
|||||
|---|---|---|---|---|---|
| <5 % (n = 1,566,755) |
5 to <7.5 % (n = 308,642) |
7.5 to <20 % (n = 435,553) |
≥ 20 % (n = 121,521) |
p value | |
| Demographics | |||||
| Age, years | 40.7 ± 6.6 | 50.0 ± 6.4 | 57.7 ± 7.1 | 68.2 ± 5.1 | <0.0001 |
| Sex, % | <0.0001 | ||||
| Male | 857,337 (54.7) | 193,756 (62.8) | 283,640 (65.1) | 84,417 (69.5) | |
| Female | 709,418 (45.3) | 114,886 (37.2) | 151,913 (34.9) | 37,104 (30.5) | |
| BMI, kg/m2 | 23.3 ± 3.1 | 24.4 ± 3.0 | 24.3 ± 3.0 | 23.9 ± 3.1 | <0.0001 |
| Comorbidities, % | |||||
| Hypertension | 115,919 (7.4) | 85,058 (27.6) | 184,166 (42.3) | 78,954 (65.0) | <0.0001 |
| Diabetes mellitus | 25,843 (1.7) | 20,278 (6.6) | 56,187 (12.9) | 33,694 (27.7) | <0.0001 |
| Chronic kidney disease | 67,111 (4.3) | 15,775 (5.1) | 31,805 (7.3) | 14,823 (12.2) | <0.0001 |
| Cancer | 12,322 (0.8) | 3,706 (1.2) | 7,261 (1.7) | 2,923 (2.4) | <0.0001 |
| Liver disease | 54,442 (3.5) | 14,270 (4.6) | 23,378 (5.4) | 6,870 (5.7) | <0.0001 |
| Social history | |||||
| Current Smoking | 392,429 (25.1) | 111,234 (36.0) | 148,775 (34.2) | 41,074 (33.8) | <0.0001 |
| Alcohol consumption | <0.0001 | ||||
| Non-drinker | 742,171 (47.4) | 148,749 (48.2) | 229,692 (52.7) | 71,529 (58.9) | |
| Mild-drinker | 710,107 (45.3) | 129,198 (41.9) | 162,901 (37.4) | 38,474 (31.7) | |
| Heavy-drinker | 114,477 (7.3) | 30,695 (10.0) | 42,960 (9.9) | 11,518 (9.5) | |
| Regular physical activity | 263,401 (16.8) | 60,915 (19.7) | 92,994 (21.4) | 27,056 (22.3) | <0.0001 |
| Vital sign | |||||
| Systolic blood pressure | 117.3 ± 12.1 | 126.6 ± 13.3 | 130.4 ± 15.4 | 136.4 ± 17.5 | <0.0001 |
| Diastolic blood pressure | 73.9 ± 9.0 | 79.5 ± 9.8 | 80.9 ± 10.7 | 81.7 ± 11.1 | <0.0001 |
| Laboratory results | |||||
| Glomerular filtration rates (mL/min/1.73 m2) |
89.8 ± 51.5 | 85.8 ± 37.6 | 84.6 ± 34.9 | 82.2 ± 34.0 | <0.0001 |
| Fasting blood glucose (mg/dL) | 92.5 ± 15.1 | 98.7 ± 23.1 | 103.0 ± 28.8 | 109.5 ± 34.6 | <0.0001 |
| Total cholesterol (mg/dL) | 191.0 ± 33.2 | 206.6 ± 35.7 | 206.5 ± 37.2 | 202.1 ± 38.2 | <0.0001 |
| LDL cholesterol (mg/dL) | 111.4 ± 34.9 | 124.8 ± 35.5 | 124.9 ± 37.2 | 122.4 ± 36.7 | <0.0001 |
| HDL cholesterol (mg/dL) | 57.6 ± 26.1 | 53.4 ± 20.5 | 52.6 ± 19.7 | 50.1 ± 13.8 | <0.0001 |
| Triglyceride (mg/dL) | 100.9 (100.8–101.0) | 127.0 (126.7–127.2) | 128.9 (128.7–129.1) | 130.3 (123.0–130.6) | <0.0001 |
ASCVD, atherosclerotic cardiovascular disease; BMI, body mass index; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Differential association between ASCVD and LDL cholesterol level according to ACC/AHA 10-year ASCVD risk
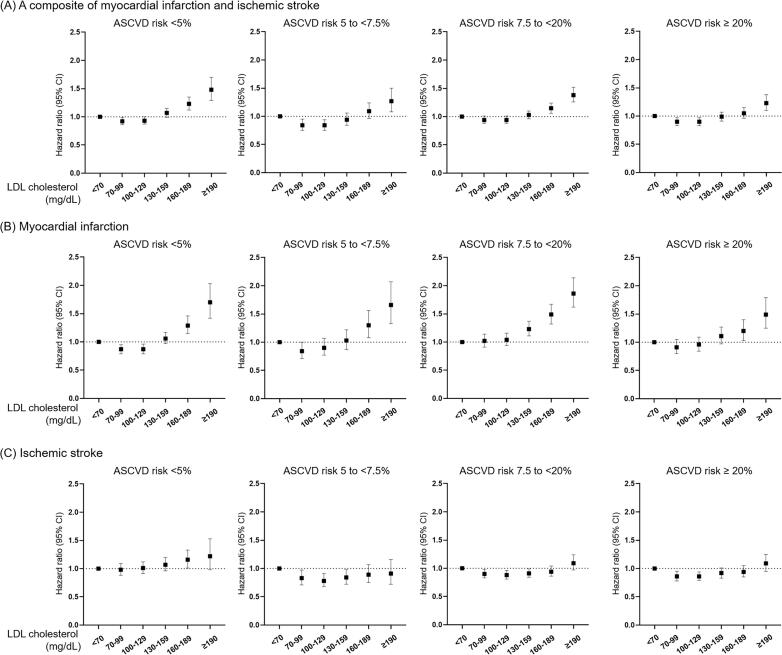
In this study, 24,708 cases of MI and 30,039 cases of IS occurred within the follow-up of 9.3 (9.1–9.6) years. First, we explored the predictive value of LDL cholesterol levels for the occurrence of MI and IS by ROC curve analysis, but we found low predictive values suggesting the complex relationship between LDL cholesterol levels and risks of ASCVD (Supplemental Table 2). We found a J-shaped association between the risk of MI and LDL cholesterol levels and between the risk of IS and LDL cholesterol levels (Fig. 2). After classification of individuals according to ASCVD risk, this J-shaped association was observed for the composite of MI and IS in individuals with low ASCVD risk, whereas statistical significance was attenuated in intermediate-risk participants (Fig. 3A and Supplemental Table 3). This J-shaped association was also observed in the Kaplan–Meier survival curves (Supplemental Fig. 1). In the low ASCVD risk individuals, participants with LDL cholesterol levels of 70–99 mg/dL and 100–129 mg/dL had lower MI risks compared to the reference group (HR, 0.87; 95 % CI: 0.79–0.95 and HR, 0.87; 95 % CI: 0.80–0.96, respectively), whereas those with an LDL cholesterol level of 160–189 mg/dL and ≥190 mg/dL showed upraised MI risks (Fig. 3B and Supplemental Table 4). In the other ASCVD risk groups, the J-shaped association was attenuated. Regarding IS, participants with an LDL cholesterol level <70 mg/dL demonstrated increased risks compared to those with level of 70–99 mg/dL, 100–129 mg/dL, or 130–159 mg/dL in the borderline, intermediate, and high ASCVD risk groups, but not in those with low ASCVD risk (Fig. 3C and Supplemental Table 5). In contrast, neither a J-shaped association between MI and LDL cholesterol levels nor between IS and LDL cholesterol levels was found in participants taking statins (Supplemental Fig. 2A and B). Remarkably, the high ASCVD risk group taking statins showed equivalent MI and IS risks across LDL cholesterol levels. These different associations might be induced by decreased LDL cholesterol levels in participants with high ASCVD risk and, therefore, those taking high-dose statins.
Fig. 2.
Restricted cubic splines showing J-shaped relation across myocardial infarction, ischemic stroke, and LDL cholesterol. J-shaped curves between myocardial infarction risk and LDL cholesterol level (left panel), and between ischemic stroke risk and LDL cholesterol level (right panel) are shown. The hazard ratios (solid lines) and 95 % confidence intervals (shaded areas) are shown in both panels. CI, confidence interval; HR, hazard ratio; LDL, low-density lipoprotein.
Fig. 3.
Association between myocardial infarction, ischemic stroke, and LDL cholesterol level according to 10-year ASCVD risk. Risks for a composite of myocardial infarction and ischemic stroke (A), myocardial infarction (B), and ischemic stroke (C) according to LDL cholesterol levels in each 10-year ASCVD risk group. Briefly, the J-shaped curve between LDL cholesterol levels and MI risk was attenuated across ASCVD risks. For IS, participants with an LDL cholesterol level <70 mg/dL demonstrated increased risks compared with those with a level of 70–99 mg/dL, 100–129 mg/dL, or 130–159 mg/dL in the borderline, intermediate, and high ASCVD risk groups, respectively. Hazard ratios (HRs) with 95 % CIs are presented as dot and whisker plots after adjusting for covariates. ASCVD, atherosclerotic cardiovascular disease; CI, confidence interval; LDL, low-density lipoprotein. *Adjusted for age, sex, body mass index, history of hypertension, diabetes mellitus, current smoking, heavy alcohol consumption, regular exercise, and glomerular filtration rate.
When we explored the association between MI, IS, and LDL levels according to ASCVD risk after excluding chronic diseases, the aforementioned phenomenon was consistently observed (Supplemental Fig. 3A and B).
Subgroup analyses according to BMI
For BMI stratification, participants were classified as having a BMI of <18.5 kg/m2, 18.5-<25 kg/m2, and ≥25 kg/m2 (Supplemental Fig. 4A and B). The association between LDL cholesterol levels and the risk of MI and IS differed across these subgroups. The risks of both MI and IS increased when LDL cholesterol levels increased in participants with a BMI of ≥25 kg/m2. Counterintuitively, the relationship between MI, IS, and LDL cholesterol levels was complex rather than linear among participants with BMI <18.5 kg/m2; the reference group did not have the lowest event rates.
High hs-CRP level in participants who have diminished LDL cholesterol level
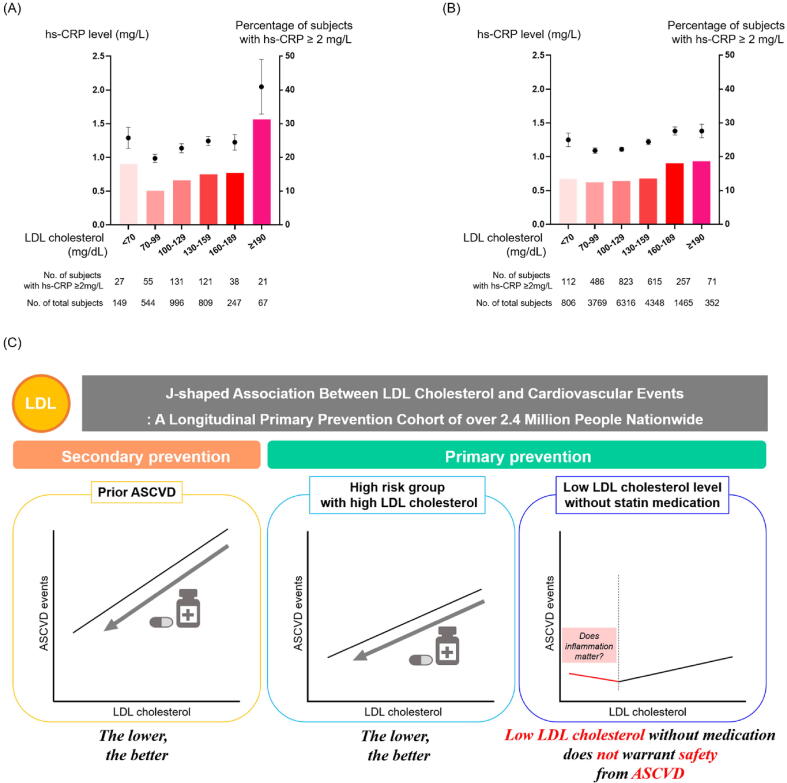
To verify whether inflammation could play a key role in linking LDL cholesterol level and ASCVD risk, we analyzed hs-CRP levels according to LDL cholesterol levels in two independent datasets: data from general health checkups at Seoul National University Hospital Healthcare System Gangnam Center and data from health checkups at KNHNES. A J-shaped association between LDL cholesterol level and hs-CRP level was consistently observed; participants with an LDL cholesterol level of <70 mg/dL not only showed relatively higher hs-CRP levels but also had a higher proportion of participants with upraised hs-CRP (≥2 mg/L) than those of other groups with higher LDL cholesterol levels (Fig. 4 A-B and Supplemental Table 6). Similarly, there was a J-shaped association between white blood cell count and LDL cholesterol levels (Supplemental Fig. 5).
Fig. 4.
Relationship between LDL cholesterol and hs-CRP levels, and a summarizing schematic of the overall study. Relationships between high-sensitivity C-reactive protein and LDL cholesterol levels in Seoul National University Hospital Healthcare System Gangnam Center (A) and the Korea National Health and Nutrition Examination Survey (B). In both cohorts, a J-shaped association was observed between LDL cholesterol and hs-CRP levels. A schematic of the overall study (C) for the association between myocardial infarction, ischemic stroke, and LDL cholesterol according to 10-year ASCVD risk. We found that low LDL cholesterol levels do not warrant safety from ASCVD. For LDL cholesterol levels (A and B), data are presented as mean ± standard error. hs-CRP, high-sensitivity C-reactive protein; LDL, low-density lipoprotein.
Discussion
We investigated the association of MI, IS, and LDL cholesterol levels with the risk of ASCVD (Fig. 4C). The main findings of this study are as follows. First, approximately two-thirds of the included participants showed a 10-year ASCVD risk of <5 % in a large nationwide population-based primary prevention cohort. Second, J-shaped associations were observed between MI risk and LDL cholesterol levels and between IS risk and LDL cholesterol levels. Participants with LDL cholesterol levels <70 mg/dL did not show the lowest MI or IS risks. In contrast, a linear association was observed in participants taking statins. Third, the associations between MI and IS risk and LDL cholesterol levels were influenced by ASCVD risk and obesity. Finally, a J-shaped association was observed between hs-CRP and LDL cholesterol levels; the mean hs-CRP level and the proportion of participants with elevated hs-CRP levels were relatively high among individuals with LDL cholesterol levels <70 mg/dL.
LDL cholesterol contributes to the initiation and aggravation of atherosclerosis, and high LDL cholesterol levels are associated with an increased risk of ASCVD [4]. With robust evidence, the use of statins has become a milestone in the management of ASCVD. Statin use significantly decreases ASCVD recurrence in patients with a history of ASCVD [8], [28], [29]. Statins have also proven beneficial for primary prevention in various high-risk groups [9], [11], [30]. Accordingly, the hypothesis that ‘the lower, the better’ is a valid strategy in clinical practice [31]. However, the clinical implications of low LDL levels for primary prevention remain elusive. We found that low LDL cholesterol levels (<70 mg/dL) without medication were not safe for ASCVD. To the best of our knowledge, this is the first study to show a J-shaped relationship between LDL cholesterol levels and MI risk in participants without previous ASCVD. For IS, our results are in line with those of the Ibaraki Prefectural Health Study and Atherosclerosis Risk in Communities (ARIC) study, which implied an increased risk of IS in participants with diminished LDL cholesterol levels in primary prevention [16], [17]. After adjusting for covariates, a J-shaped relationship between LDL cholesterol level and MI risk and a U-shaped association between LDL cholesterol level and IS risk were found. Furthermore, we verified that both MI and IS risks increased in participants with low LDL cholesterol levels in a nationwide primary prevention cohort. Taken together, the authors suggest that the association between MI and IS risk and LDL cholesterol levels in patients without previous ASCVD differs from the robust benefits of LDL cholesterol-lowering treatment in patients with prior ASCVD. The authors agree that this J-shaped association might seem awkward, because high LDL cholesterol levels should be lowered in patients with a high ASCVD risk. Nevertheless, considering that bioelectronics made of nanomaterials, which had been unfamiliar to clinical fields, are now actively studied in cardiovascular disease models [32] based on substantially accumulated data [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], it is important to derive valuable clinical implications from this J-shaped association.
The authors attempted to explore plausible explanations for the complex associations among MI, IS risk, and LDL cholesterol levels. Inflammation plays a key role (Fig. 4). Inflammation has essential implications for atherosclerosis [43], and hs-CRP, a representative inflammatory marker, has prognostic implications for predicting future ASCVD risks [44], [45], [46]. Notably, Ridker et al. reported that individuals with low LDL cholesterol levels accompanied by elevated hs-CRP levels showed a higher risk of ASCVD than those with increased LDL cholesterol but diminished hs-CRP levels [43]. Other studies also demonstrated that those with low LDL cholesterol (<130 mg/dL and <70 mg/dL) and elevated hs-CRP levels (≥2 mg/L) had higher risks of ASCVD compared to individuals with high LDL cholesterol but low hs-CRP levels [47], [48]. For IS, those with diminished LDL cholesterol and elevated hs-CRP levels showed an increased risk of recurrent attacks compared to individuals with elevated LDL cholesterol but diminished hs-CRP levels [49]. Considering these reports and our data, the authors postulated that inflammation could provide a notable answer to the complicated J-shaped association.
Additionally, there is a differential association between BMI, LDL cholesterol levels, and ASCVD risk. Obese individuals have high LDL cholesterol levels, whereas underweight individuals have low LDL cholesterol levels [50], [51]. However, both overweight and underweight individuals are known to have a high ASCVD risk compared with normal-weight individuals [52], although underweight individuals have low LDL cholesterol levels. Similarly, in our nationwide cohort, a linear relationship between LDL cholesterol levels and incident ASCVD was observed in individuals with obesity, whereas a complex association was observed in the underweight group (Supplemental Fig. 4). Therefore, it is not counterintuitive that LDL cholesterol and ASCVD could have a complicated relationship.
Our study has several strengths. We demonstrated the clinical value of determining hs-CRP levels. Previous trials have reported the therapeutic efficacy of anti-inflammatory treatments such as colchicine and canakinumab in reducing ASCVD events [53], [54]. Considering our findings, measuring hs-CRP levels could be more actively considered, especially in individuals who have diminished low LDL cholesterol levels in clinics. Further, we carefully suggest that individuals with decreased LDL cholesterol and elevated hs-CRP levels may receive substantial benefits from anti-inflammatory treatment in reducing ASCVD risks. Our study has valuable implications for the management of patients with low LDL cholesterol levels without statin medication. We determined that patients with LDL cholesterol levels <70 mg/dL may have increased MI and IS risks compared with those with higher LDL cholesterol levels, and we first showed a J-shaped association between LDL cholesterol levels and MI Risk. Interestingly, patients with low LDL cholesterol levels showed relatively high levels of inflammation based on hs-CRP levels. Thus, statin therapy, which is known to have anti-inflammatory and lipid-lowering effects, can be considered in real-world practice for patients with low LDL cholesterol levels and increased hs-CRP levels. Further studies are required to verify the benefits of anti-inflammatory treatments and statin medications in patients with low LDL cholesterol and high hs-CRP levels.
This was a retrospective observational study; therefore, although it was a large and well-controlled nationwide study, there may have been confounding factors. For example, we performed sensitivity analyses to rule out the confounding effects of chronic diseases (Supplemental Fig. 3); however, there could be other variables that may influence the results. Secondly, this study was conducted using data mainly from an Asian population, and careful interpretation is necessary to extrapolate these results to other ethnicities [55]. Because of the absence of hs-CRP examinations in the general health checkups provided by the NHIS, we investigated the association between LDL cholesterol levels and hs-CRP levels in two separate cohorts. However, because we recruited study participants with the same inclusion and exclusion criteria in these two cohorts, which were also based on the Korean population, they may be representative subgroups of the original NHIS cohort. In addition, the mechanisms underlying the inverse relationship between LDL cholesterol and hs-CRP levels at low LDL cholesterol levels could not be investigated. Finally, because we analyzed anonymous data provided by the Korean NHIS, it is not feasible to present a specific case that could recapitulate the contents of the manuscript.
Conclusion
Although high LDL cholesterol levels are associated with an increased risk of MI and IS, the relationship between LDL cholesterol levels and ASCVD is complex rather than simply linear. Interestingly, low LDL cholesterol levels without statin medication do not warrant safety against ASCVD, and these individuals require careful medical attention to prevent incident ASCVD.
Funding sources
Han-Mo Yang received grants from the Korea Health Technology R&D Project “Korea Research-Driven Hospital (HI14C1277)” through the Korea Health Industry Development Institute (KHIDI) funded by the Korea Government (MHW)) and of the National Research Foundation of Korea (NRF) grant (2020R1A2C1011311) funded by the Korea government (MSIT).
Compliance with Ethics Requirements
All study was performed according to the principles of the Declaration of Helsinki. The institutional Review Board of our medical institute approved this study (IRB No. E-2101-012-1184). After approval of National Health Insurance Serve (NHIS), data were available from designated terminals.
CRediT authorship contribution statement
Chan Soon Park: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Validation, Visualization, and Writing - original draft. Han-Mo Yang: Conceptualization, Data curation, Formal analysis, Funding acquisition, Project administration, Resources, Supervision, and Writing - review & editing. Kyungdo Han: Data curation, Formal analysis, Investigation, Methodology, Resources, Software, and Writing - review & editing. Hee-Sun Lee: Conceptualization, Data curation, Methodology, Supervision, and Writing - review & editing. Jeehoon Kang: Conceptualization, Supervision, and Writing - review & editing. Jung-Kyu Han: Conceptualization, Supervision, and Writing - review & editing. Kyung Woo Park: Conceptualization, Supervision, and Writing - review & editing. Hyun-Jae Kang: Conceptualization, Supervision, and Writing - review & editing. Bon-Kwon Koo: Conceptualization, Supervision, and Writing - review & editing. Hyo-Soo Kim: Conceptualization, Supervision, and Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jare.2023.05.003.
Contributor Information
Han-Mo Yang, Email: hanname@hanmail.net.
Kyungdo Han, Email: hkd0917@naver.com.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Virani S.S., Alonso A., Aparicio H.J., Benjamin E.J., Bittencourt M.S., Callaway C.W., et al. Heart disease and stroke statistics-2021 update: a report from the American Heart Association. Circulation. 2021;143:e254–e743. doi: 10.1161/CIR.0000000000000950. [DOI] [PubMed] [Google Scholar]
- 2.Townsend N., Kazakiewicz D., Lucy Wright F., Timmis A., Huculeci R., Torbica A., et al. Epidemiology of cardiovascular disease in Europe. Nat Rev Cardiol. 2022;19(2):133–143. doi: 10.1038/s41569-021-00607-3. [DOI] [PubMed] [Google Scholar]
- 3.Koton S., Schneider A.L.C., Rosamond W.D., Shahar E., Sang Y., Gottesman R.F., et al. Stroke incidence and mortality trends in US communities, 1987 to 2011. JAMA. 2014;312(3):259. doi: 10.1001/jama.2014.7692. [DOI] [PubMed] [Google Scholar]
- 4.Borén J., Chapman M.J., Krauss R.M., Packard C.J., Bentzon J.F., Binder C.J. Low-density lipoproteins cause atherosclerotic cardiovascular disease: pathophysiological, genetic, and therapeutic insights: a consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2020;41(24):2313–2330. doi: 10.1093/eurheartj/ehz962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cannon C.P., Blazing M.A., Giugliano R.P., McCagg A., White J.A., Theroux P., et al. Ezetimibe Added to Statin Therapy after Acute Coronary Syndromes. N Engl J Med. 2015;372(25):2387–2397. doi: 10.1056/NEJMoa1410489. [DOI] [PubMed] [Google Scholar]
- 6.Sabatine M.S., Giugliano R.P., Keech A.C., Honarpour N., Wiviott S.D., Murphy S.A., et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376(18):1713–1722. doi: 10.1056/NEJMoa1615664. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz G.G., Steg P.G., Szarek M., Bhatt D.L., Bittner V.A., Diaz R., et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379(22):2097–2107. doi: 10.1056/NEJMoa1801174. [DOI] [PubMed] [Google Scholar]
- 8.Sacks F.M., Pfeffer M.A., Moye L.A., Rouleau J.L., Rutherford J.D., Cole T.G., et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med. 1996;335(14):1001–1009. doi: 10.1056/NEJM199610033351401. [DOI] [PubMed] [Google Scholar]
- 9.Colhoun H.M., Betteridge D.J., Durrington P.N., Hitman G.A., W Neil H.A., Livingstone S.J., et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364(9435):685–696. doi: 10.1016/S0140-6736(04)16895-5. [DOI] [PubMed] [Google Scholar]
- 10.Cholesterol Treatment Trialists C., Baigent C., Blackwell L., Emberson J., Holland L.E., Reith C., et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670–1681. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ridker P.M., Danielson E., Fonseca F.A.H., Genest J., Gotto A.M., Kastelein J.J.P., et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359(21):2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 12.Besseling J., Hovingh G.K., Huijgen R., Kastelein J.J.P., Hutten B.A. Statins in familial hypercholesterolemia: consequences for coronary artery disease and all-cause mortality. J Am Coll Cardiol. 2016;68(3):252–260. doi: 10.1016/j.jacc.2016.04.054. [DOI] [PubMed] [Google Scholar]
- 13.Arnett D.K., Blumenthal R.S., Albert M.A., Buroker A.B., Goldberger Z.D., Hahn E.J., et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;74(10):1376–1414. doi: 10.1016/j.jacc.2019.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mach F., Baigent C., Catapano A.L., Koskinas K.C., Casula M., Badimon L., et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41(1):111–188. doi: 10.1093/eurheartj/ehz455. [DOI] [PubMed] [Google Scholar]
- 15.Grundy S.M., Stone N.J., Bailey A.L., Beam C., Birtcher K.K., Blumenthal R.S., et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73(24):e285–e350. doi: 10.1016/j.jacc.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 16.Noda H., Iso H., Irie F., Sairenchi T., Ohtaka E., Doi M., et al. Low-density lipoprotein cholesterol concentrations and death due to intraparenchymal hemorrhage: the Ibaraki Prefectural Health Study. Circulation. 2009;119(16):2136–2145. doi: 10.1161/CIRCULATIONAHA.108.795666. [DOI] [PubMed] [Google Scholar]
- 17.Shahar E., Chambless L.E., Rosamond W.D., Boland L.L., Ballantyne C.M., McGovern P.G., et al. Plasma lipid profile and incident ischemic stroke: the Atherosclerosis Risk in Communities (ARIC) study. Stroke. 2003;34(3):623–631. doi: 10.1161/01.STR.0000057812.51734.FF. [DOI] [PubMed] [Google Scholar]
- 18.Sun L., Clarke R., Bennett D., Guo Y.u., Walters R.G., Hill M., et al. Causal associations of blood lipids with risk of ischemic stroke and intracerebral hemorrhage in Chinese adults. Nat Med. 2019;25(4):569–574. doi: 10.1038/s41591-019-0366-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cholesterol Treatment Trialists C., Mihaylova B., Emberson J., Blackwell L., Keech A., Simes J., et al. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet. 2012;380:581–590. doi: 10.1016/S0140-6736(12)60367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yusuf S., Bosch J., Dagenais G., Zhu J., Xavier D., Liu L., et al. Cholesterol lowering in intermediate-risk persons without cardiovascular disease. N Engl J Med. 2016;374(21):2021–2031. doi: 10.1056/NEJMoa1600176. [DOI] [PubMed] [Google Scholar]
- 21.Goff D.C., Jr., Lloyd-Jones D.M., Bennett G., Coady S., D'Agostino R.B., Sr., Gibbons R., et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2935–2959. doi: 10.1016/j.jacc.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheol Seong S., Kim Y.Y., Khang Y.H., Heon Park J., Kang H.J., Lee H., et al. Data resource profile: the national health information database of the National Health Insurance Service in South Korea. Int J Epidemiol. 2017;46:799–800. doi: 10.1093/ije/dyw253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sathiyakumar V., Park J., Golozar A., Lazo M., Quispe R., Guallar E., et al. Fasting versus nonfasting and low-density lipoprotein cholesterol accuracy. Circulation. 2018;137(1):10–19. doi: 10.1161/CIRCULATIONAHA.117.030677. [DOI] [PubMed] [Google Scholar]
- 24.Committee for the Korean guidelines for the management of D Korean guidelines for the management of dyslipidemia: executive Summary (English Translation) Korean Circ J. 2015;2016(46):275–306. doi: 10.4070/kcj.2016.46.3.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Friedewald W.T., Levy R.I., Fredrickson D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 26.Kweon S., Kim Y., Jang M.-j., Kim Y., Kim K., Choi S., et al. Data resource profile: the Korea National Health and Nutrition Examination Survey (KNHANES) Int J Epidemiol. 2014;43(1):69–77. doi: 10.1093/ije/dyt228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ng M., Fleming T., Robinson M., Thomson B., Graetz N., Margono C., et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9945):766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amarenco P., Bogousslavsky J., Callahan A., 3rd, Goldstein L.B., Hennerici M., Rudolph A.E., et al. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355:549–559. doi: 10.1056/NEJMoa061894. [DOI] [PubMed] [Google Scholar]
- 29.Heart Protection Study Collaborative G Randomized trial of the effects of cholesterol-lowering with simvastatin on peripheral vascular and other major vascular outcomes in 20,536 people with peripheral arterial disease and other high-risk conditions. J Vasc Surg. 2007;45 doi: 10.1016/j.jvs.2006.12.054. [DOI] [PubMed] [Google Scholar]
- 30.Sever P.S., Dahlöf B., Poulter N.R., Wedel H., Beevers G., Caulfield M., et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial-Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet. 2003;361(9364):1149–1158. doi: 10.1016/S0140-6736(03)12948-0. [DOI] [PubMed] [Google Scholar]
- 31.Lüscher T.F. ‘The lower the better’ revisited: low-density lipoprotein and lipoprotein(a) Eur Heart J. 2018;39:2509–2512. [Google Scholar]
- 32.Sunwoo S.-H., Cha M.-J., Han S.I., Kang H., Cho Y.S., Yeom D.-H., et al. Ventricular tachyarrhythmia treatment and prevention by subthreshold stimulation with stretchable epicardial multichannel electrode array. Sci Adv. 2023;9(13):eadf6856. doi: 10.1126/sciadv.adf6856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mahdi M.A., Yousefi S.R., Jasim L.S., Salavati-Niasari M. Green synthesis of DyBa2Fe3O7.988/DyFeO3 nanocomposites using almond extract with dual eco-friendly applications: photocatalytic and antibacterial activities. Int J Hydrog. Energy. 2022;47 [Google Scholar]
- 34.Yousefi S.R., Ghanbari M., Amiri O., Marzhoseyni Z., Mehdizadeh P., Hajizadeh‐Oghaz M., et al. Dy2BaCuO5/Ba4DyCu3O9.09 S-scheme heterojunction nanocomposite with enhanced photocatalytic and antibacterial activities. J Am Ceram Soc. 2021;104(7):2952–2965. [Google Scholar]
- 35.Yousefi S.R., Alshamsi H.A., Amiri O., Salavati-Niasari M. Synthesis, characterization and application of Co/Co3O4 nanocomposites as an effective photocatalyst for discoloration of organic dye contaminants in wastewater and antibacterial properties. J Mol Liq. 2021;337 [Google Scholar]
- 36.Yousefi S.R., Sobhani A., Alshamsi H.A., Salavati-Niasari M. Green sonochemical synthesis of BaDy2NiO5/Dy2O3 and BaDy2NiO5/NiO nanocomposites in the presence of core almond as a capping agent and their application as photocatalysts for the removal of organic dyes in water. RSC Adv. 2021;11(19):11500–11512. doi: 10.1039/d0ra10288a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yousefi S.R., Amiri O., Salavati-Niasari M. Control sonochemical parameter to prepare pure Zn(0.35)Fe(2.65)O(4) nanostructures and study their photocatalytic activity. Ultrason Sonochem. 2019;58 doi: 10.1016/j.ultsonch.2019.104619. [DOI] [PubMed] [Google Scholar]
- 38.Yousefi S.R., Masjedi-Arani M., Morassaei M.S., Salavati-Niasari M., Moayedi H. Hydrothermal synthesis of DyMn2O5/Ba3Mn2O8 nanocomposite as a potential hydrogen storage material. Int J Hydrog Energy. 2019;44(43):24005–24016. [Google Scholar]
- 39.Yousefi S.R., Sobhani A., Salavati-Niasari M. A new nanocomposite superionic system (CdHgI4/HgI2): synthesis, characterization and experimental investigation. Adv Powder Technol. 2017;28(4):1258–1262. [Google Scholar]
- 40.Yousefi S.R., Ghanbari D., Salavati-Niasari M., Hassanpour M. Photo-degradation of organic dyes: simple chemical synthesis of Ni(OH)2 nanoparticles, Ni/Ni(OH)2 and Ni/NiO magnetic nanocomposites. J Mater Sci: Mater Electron. 2016;27(2):1244–1253. [Google Scholar]
- 41.Yousefi S.R., Ghanbari D., Salavati-Niasari M. Hydrothermal synthesis of nickel hydroxide nanostructures and flame retardant poly vinyl alcohol and cellulose acetate nanocomposites. J Nanostruct. 2016;6:77–82. [Google Scholar]
- 42.Mehdizadeh P., Jamdar M., Mahdi M.A., Abdulsahib W.K., Jasim L.S., Raheleh Yousefi S., et al. Rapid microwave fabrication of new nanocomposites based on Tb-Co-O nanostructures and their application as photocatalysts under UV/Visible light for removal of organic pollutants in water. Arabian J Chem. 2023;16(4):104579. [Google Scholar]
- 43.Epstein F.H., Ross R. Atherosclerosis–an inflammatory disease. N Engl J Med. 1999;340(2):115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 44.Emerging Risk Factors C., Kaptoge S., Di Angelantonio E., Pennells L., Wood A.M., White I.R., et al. C-reactive protein, fibrinogen, and cardiovascular disease prediction. N Engl J Med. 2012;367:1310–1320. doi: 10.1056/NEJMoa1107477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ridker P.M., Rifai N., Rose L., Buring J.E., Cook N.R. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347(20):1557–1565. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 46.Arroyo-Espliguero R., Viana-Llamas M.C., Silva-Obregon A., Avanzas P. The role of C-reactive protein in patient risk stratification and treatment. Eur Cardiol. 2021;16:e28. doi: 10.15420/ecr.2020.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ridker P.M., Danielson E., Fonseca F.AH., Genest J., Gotto A.M., Kastelein J.JP., et al. Reduction in C-reactive protein and LDL cholesterol and cardiovascular event rates after initiation of rosuvastatin: a prospective study of the JUPITER trial. Lancet. 2009;373(9670):1175–1182. doi: 10.1016/S0140-6736(09)60447-5. [DOI] [PubMed] [Google Scholar]
- 48.Yang E.Y., Nambi V., Tang Z., Virani S.S., Boerwinkle E., Hoogeveen R.C., et al. Clinical implications of JUPITER (Justification for the Use of statins in Prevention: an Intervention Trial Evaluating Rosuvastatin) in a U.S. population insights from the ARIC (Atherosclerosis Risk in Communities) study. J Am Coll Cardiol. 2009;54(25):2388–2395. doi: 10.1016/j.jacc.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li J., Pan Y., Xu J., Li S., Wang M., Quan K., et al. Residual inflammatory risk predicts poor prognosis in acute ischemic stroke or transient ischemic attack patients. Stroke. 2021;52(9):2827–2836. doi: 10.1161/STROKEAHA.120.033152. [DOI] [PubMed] [Google Scholar]
- 50.Sangha R., Jones K., Havstad S., Wegienka G. Association between body mass index and patient-centered outcomes after hysterectomy. J Reprod Med. 2015;60:392–396. [PubMed] [Google Scholar]
- 51.Shamai L., Lurix E., Shen M., Novaro G.M., Szomstein S., Rosenthal R., et al. Association of body mass index and lipid profiles: evaluation of a broad spectrum of body mass index patients including the morbidly obese. Obes Surg. 2011;21(1):42–47. doi: 10.1007/s11695-010-0170-7. [DOI] [PubMed] [Google Scholar]
- 52.Hansel B., Roussel R., Elbez Y., Marre M., Krempf M., Ikeda Y., et al. Cardiovascular risk in relation to body mass index and use of evidence-based preventive medications in patients with or at risk of atherothrombosis. Eur Heart J. 2015;36(40):2716–2728. doi: 10.1093/eurheartj/ehv347. [DOI] [PubMed] [Google Scholar]
- 53.Tardif J.-C., Kouz S., Waters D.D., Bertrand O.F., Diaz R., Maggioni A.P., et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med. 2019;381(26):2497–2505. doi: 10.1056/NEJMoa1912388. [DOI] [PubMed] [Google Scholar]
- 54.Ridker P.M., Everett B.M., Thuren T., MacFadyen J.G., Chang W.H., Ballantyne C., et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377(12):1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 55.Kaplan H., Thompson R.C., Trumble B.C., Wann L.S., Allam A.H., Beheim B., et al. Coronary atherosclerosis in indigenous South American Tsimane: a cross-sectional cohort study. Lancet. 2017;389(10080):1730–1739. doi: 10.1016/S0140-6736(17)30752-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.