Abstract
Reactivations of persistent viral infections pose a significant medical problem in immunocompromised cancer, transplant, and AIDS patients, yet little is known about how persistent viral infections are immunologically controlled. Here we describe a mouse model for investigating the role of the immune response in controlling a persistent retroviral infection. We demonstrate that, following recovery from acute Friend virus infection, a small number of B cells evade immunological destruction and harbor persistent virus. In vivo depletions of T-cell subsets in persistently infected mice revealed a critical role for CD4+ T cells in controlling virus replication, spread to the erythroid lineage, and induction of erythroleukemia. The CD4+ T-cell effect was independent of CD8+ T cells and in some cases was also independent of virus-neutralizing antibody responses. Thus, the CD4+ T cells may have had a direct antiviral effect. These results may have relevance for human immunodeficiency virus (HIV) infections where loss of CD4+ T cells is associated with an increase in HIV replication, reactivation of persistent viruses, and a high incidence of virus-associated cancers.
A wide range of viruses not only induce acute diseases in humans but also employ various methods to evade immunological destruction and establish persistent infections. These include hepatitis B virus, adenoviruses, rubella virus, measles virus, JC and BK polyomaviruses, several herpesviruses, human T-lymphotropic viruses, and human immunodeficiency virus (HIV). In most cases, persistent viral infections are innocuous and cause few serious clinical problems (14). However, if the host becomes immunocompromised due to drug therapies or infection with an immunosuppressive virus such as HIV, persistent viruses can reactivate and cause lethal diseases. For example, cytomegalovirus infections are a leading cause of mortality in AIDS patients and transplant patients, and a large proportion of the infections are due to reactivated viruses (15, 18, 22, 32). In addition, increased rates of cancer are also observed in immunocompromised patients, especially cancers associated with virus infections, such as non-Hodgkin’s lymphoma, Kaposi’s sarcoma, and adult T-cell leukemia (14). Although it is widely accepted that the cause of persistent virus reactivations is immunosuppression, the specific types of immune cells which normally control persistent viruses have not been identified.
In order to investigate this issue in vivo, we utilized mice persistently infected with Friend virus complex (FV). FV is an oncogenic complex of two retroviruses, a replication-competent virus known as Friend murine leukemia helper virus (F-MuLV) and a replication-defective component called spleen focus-forming virus (SFFV). Coinfection of cells by the two viruses allows SFFV to be packaged into particles formed from helper virus proteins. Infection of susceptible mice with FV induces an SFFV-dependent, polyclonal proliferation of erythroid precursors in the spleen, which leads to erythroleukemia unless the infection is controlled by immune responses (1, 13, 16). Mice of the strain chosen for these experiments mount potent immune responses and are able to recover from acute disease. Virus-specific T-helper, cytotoxic T-lymphocyte (CTL), and antibody responses are all required for such recovery (4, 12, 30). Although 95% of recovered mice show no clinical signs and have a normal life span, it has been shown previously that the animals remain persistently infected with low levels of virus in the spleen (3). Despite the inability of the immune system to totally eradicate FV infections, experiments have suggested that persistent FV is nevertheless under immune control. For example, it has been shown previously that spleen cell transfers from persistently infected mice to naive mice induce rapid acute disease (5), indicating that a host immune mechanism rather than virus mutation might be responsible for keeping persistent virus in check.
In this report, we show that the primary reservoir for persistent FV infection in the spleen is a small subset of B cells. In at least half of the mice, replication of virus and spread to other cell types are shown to be controlled by a subpopulation of T cells.
MATERIALS AND METHODS
Mice.
The mice used in this study were age- and sex-matched (C57BL/10 × A.BY)F1 mice of 3 to 6 months of age at experimental onset. Parental strains were obtained from Jackson Laboratories, and breeding of F1 strains was done at Rocky Mountain Laboratories. All animals were treated in accordance with the regulations of the National Institutes of Health and the Animal Care and Use Committee of Rocky Mountain Laboratories. Relevant Friend virus resistance genotypes in (C57BL/10 × A.BY)F1 mice are H-2b/b, Fv-1b/b, Fv-2r/s, and Rfv-3r/s.
Virus and virus infections.
The FV used in these experiments was a stock obtained from a 10% spleen cell homogenate from BALB/c mice infected 9 days previously. It is an FV-1 B-tropic, polycythemia-inducing strain originally obtained from Frank Lilly. Mice were injected intravenously with 0.5 ml of phosphate-buffered, balanced salt solution containing 2% fetal bovine serum and 1,500 spleen focus-forming U of FV.
Splenomegaly as a measure of Friend disease.
Palpation for splenomegaly is the standard procedure used to monitor the progression of Friend disease (8, 11, 28) and was used in the following manner: at weekly intervals, individual animals under general anesthesia were palpated in a blinded fashion and rated on a scale of 1+ to 4+. Spleens rated 1+ were palpated as less than twice normal size and weighed less than 0.4 g. Normal spleen weights ranged from 0.1 to 0.25 g. Spleens rated 2+ were palpated as more than twice normal size but not large enough to reach the ventral midline and weighed between 0.4 and 0.8 g. Spleens rated 3+ were large enough to reach the ventral midline and weighed between 0.8 and 1.6 g. Spleens rated 4+ were severely enlarged to more than 1.6 g, extended across the abdominal midline, and caused protrusion of the abdominal wall. Cross-checking of actual spleen weights against spleen sizes determined by palpations has shown that spleens weighing more than 0.4 g (2+) can accurately be differentiated from spleens in the normal weight range of 0.1 to 0.25 g (3, 12a). It should be noted that mice with sustained FV-induced splenomegaly for longer than 6 weeks generally have 3+ or 4+ spleens and that there is no ambiguity regarding their clinical status. Such mice display additional hallmarks of erythroleukemia such as hematocrits greater than 85% and high numbers of erythroid precursors in the blood. However, monitoring these attributes requires bleeding of the mice, which exacerbates Friend disease by stimulating erythropoiesis and is less accurate than spleen palpation at predicting clinical outcome.
T-cell depletions.
T-cell depletions were performed essentially as described elsewhere (6, 12, 25). Briefly, persistently infected mice were inoculated intraperitoneally with 0.5 ml of supernatant fluid obtained from tissue culture for monoclonal antibody (MAb) 169.4 (anti-CD8) or from artificial capillary cultures (Cellco, Germantown, Md.) for 191.1 (anti-CD4). Mice were inoculated three times per week for 2 weeks. The MAbs were both of the rat anti-mouse immunoglobulin G2b isotype. Blood samples from all mice were checked for T-cell depletion levels by flow cytometry at 7 to 10 days following the last injection of antibody. T-cell subset levels in mononuclear blood cells from depleted mice ranged from <1 to 3% of the nucleated peripheral blood cells, and levels of depletion did not correlate with relapse (data not shown).
Infectious center assays on specific cell lineages.
Single-cell suspensions from persistently infected mouse spleens were sorted with a FACStar flow cytometer (Becton Dickinson Immunocytometry Systems, San Jose, Calif.) modified for five-parameter analysis. The following tissue-specific MAbs were used for sorting and analyses: anti-Ter 119 (erythroid lineage) (17), anti-Mac-1 (M1/70.15.11.5.HL) (33), anti-CD4 (clone GK1.5) (9), anti-CD8 (169.4) (6), anti-B220 (RA3-6B2) (7), and anti-CD19 (19). Fluorescently labeled antibodies were obtained from Pharmingen, San Diego, Calif. In the first experiment reported in Table 1, T cells for the infectious center assay were enriched with T-cell subset enrichment columns (R&D Systems, Minneapolis, Minn.). Sorted and enriched populations were plated onto Mus dunni cells (20), cocultivated for 5 days, fixed with ethanol, and stained with F-MuLV envelope-specific MAb 720 (29), followed by goat anti-mouse antibody peroxidase conjugate (Cappel, West Chester, Pa.) and development with 3-amino-9-ethylcarbazole (AEC) substrate to detect foci.
TABLE 1.
Percentages of total spleen foci in separate cell lineages at different times postinfectiona
| Cell lineageb | % of total spleen foci at wk postinfection:
|
||||
|---|---|---|---|---|---|
| 1c
|
8–12
|
||||
| Expt 1 | Expt 2 | Expt 1 | Expt 2 | Expt 3 | |
| Erythroid | 61 | 20 | 7 | —d | —d |
| Monocyte/granulocyte | 10 | 44 | 1 | —d | —d |
| CD4+ T cell | 3 | <1 | <1 | —d | —d |
| CD8+ T cell | <1 | <1 | <1 | —d | —d |
| B cell | 25 | 35 | 94 | 89 | 97 |
Spleen cells were enriched for the various lineages as described in Materials and Methods and were then plated as infectious centers on susceptible M. dunni cells. The purities of the enriched subpopulations for experiment 1 were determined by flow cytometry.
Erythroid lineage cells were labeled with Ter 119 and were 95% pure. Monocytes/granulocytes were labeled with Mac-1 and were 78% pure. CD4+ cells were 91% pure, and CD8+ cells were 84%. B cells were labeled with B220 and were 93% pure. For experiment 1 at 8 to 12 weeks postinfection, Mac-1+ and B220+ cells were sorted from the spleen of one mouse, erythroid lineage cells were sorted from another mouse, and the T-cell lineages were sorted from two other mice, one for CD4+ T cells and one for CD8+ T cells. In experiment 2 at 8 to 12 weeks, the B220+ cells were 95% pure and the B220− cells were 90% B220 negative. Both subpopulations in experiment 3 were resorted to more than 99% purity.
Spleen cells were taken at 8 days postinfection, with one spleen per experiment.
Non-B-cell lineages all together formed 11 and 3% of the totals in experiments 2 and 3, respectively.
Viremia and virus-neutralizing antibody assays.
For viremia assays, freshly frozen plasma samples were titrated on susceptible M. dunni cells as described above for detection of spleen foci, except that the cells were pretreated with 4 μg of Polybrene per ml. To test plasma samples for virus-neutralizing antibodies, heat-inactivated (56°C, 10 min) samples at titrated dilutions were incubated with virus stock in the presence of complement at 37°C as previously described (23). Samples were then plated as described for the viremia assay for detection of foci.
Cytokine analyses.
Cytokine gene transcription was measured by an RNase protection assay with the RiboQuant system (Pharmingen). Assays were performed with 1 μg of mRNA isolated from CD4+ T cells from uninfected and persistently infected (C57BL/10 × A.BY)F1 mice. Spleens from six mice of each group were pooled, and >90% pure CD4+ T cells were obtained with T-cell subset enrichment columns (R&D Systems). Quantifications were done by phosphorimaging analyses.
CD4+ T-cell surface marker analyses.
Nucleated spleen cell suspensions were made from six uninfected mice and eight persistently infected mice. The cells were analyzed by flow cytometry with a FACStar flow cytometer modified for five-parameter analysis. CD4+ gated cells (104) were analyzed for expression of the CD69 activation marker (34) and the CD45RB memory marker (10). Labeled antibodies were obtained from Pharmingen. The data were analyzed by the Mann-Whitney test for statistical significance.
RESULTS
B cells are the primary reservoir of persistent F-MuLV.
The number of spleen cells infected with F-MuLV in persistently infected animals has been reported to range between 0.003 and 0.3% (3), but the types of cells involved in persistence have not been determined. For the current studies, it was important to establish the identity of these cells so that virus spread to other lineages following immunosuppression could be assessed. To address this issue, spleen cell suspensions from persistently infected mice were enriched for the major cell lineages. The enriched populations were then plated as infectious centers onto virus-susceptible indicator cells to produce foci. The first experiment revealed that B220+ cells accounted for the vast majority of the infectious centers (Table 1). Based on this finding, two subsequent experiments in which spleen cells from persistently infected mice were sorted on the basis of B220 expression were performed. In both cases, the B220-positive subpopulation contained almost all of the infectious centers (Table 1). When both the B220-positive and the B220-negative subpopulations were resorted to 99% purity before plating, 97% of the total infectivity was contained in the B220-positive population (Table 1). Since the B220 antigen (CD45R) is expressed on small populations of cells other than B cells, we also did similar studies with CD19+ cells. CD19 is reported elsewhere to be B cell specific (19), and in three persistently infected mice, we found more than 90% of the infectivity in the CD19+ fraction (data not shown). Thus, B cells were the major reservoir for persistent F-MuLV in the spleens of these mice.
These results contrasted with data obtained from mice at 1 week postinfection. In addition to B cells, cells of the erythroid and monocytic/granulocytic lineages were also heavily infected during acute infection (Table 1). Very little or no infection was associated with T cells in either acute or persistent infection. Furthermore, acutely infected mice had in the range of 1,000 times more infectious centers per spleen than persistently infected mice, and although large numbers of B cells were infected early, they did not make up the bulk of the infection (Fig. 1).
FIG. 1.
Infectious centers from spleens of persistently infected and acutely infected mice. Nucleated spleen cell suspensions were sorted by flow cytometry on the basis of B220 expression and plated as infectious centers as described for Table 1. The numbers of infectious centers per spleen were extrapolated from data from approximately 3 × 106 B cells and 6 × 106 total cells per mouse. These data are derived from the same mice analyzed for Table 1.
Induction of splenomegaly, virus spread, and erythroleukemia by CD4 depletion but not by CD8 depletion.
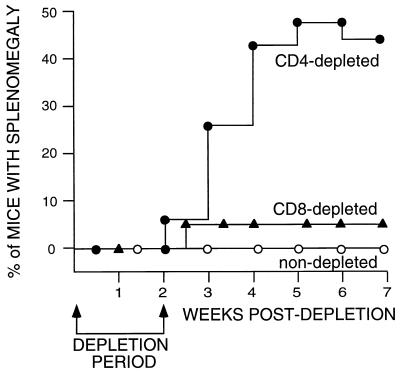
To determine whether host T-cell responses were responsible for keeping persistent virus in check, persistently infected mice were injected with specific MAbs to deplete T-cell subsets. The in vivo effectiveness of the CD8 depletion protocol was tested as described in the legend to Fig. 2. Only 1 of 19 animals depleted of CD8+ T cells became splenomegalic. This result was not statistically different from that for the nondepleted control group, and it is unknown whether the relapse was spontaneous or was induced by CD8 depletion. Two to five percent of untreated, persistently infected mice can be expected to relapse sometime during their life spans (5). The reasons for spontaneous relapses are unknown.
FIG. 2.
Splenomegaly in T-cell-depleted mice. Persistent FV infections were established and depletions were performed as described in Materials and Methods. Numbers of mice in the groups were as follows: CD4-depleted group, n = 27; CD8-depleted group, n = 19; nondepleted group, n = 34. The data were analyzed by Fisher’s exact test of contingency tables. There was a significant difference between the CD4-depleted group and the nondepleted group (P = 0.0049). The difference between CD4 depletion and CD8 depletion was also significant (P = 0.0027). There was no significant difference between CD8 depletion and no depletion (P = 0.3585). The efficacy of CD8 depletion was tested in a side-by-side control group of mice with acute FV infection. We have previously shown that acutely infected, CD8-depleted mice have poor recovery and develop erythroleukemia at a high rate (79%) (30). Six of eight CD8-depleted control mice developed erythroleukemia (75%) compared to 0 of 16 nondepleted mice. Dual depletion of both CD4 and CD8 did not increase relapse above the level with CD4 depletion alone (data not shown).
In contrast to the CD8 depletions, animals depleted of CD4+ T cells showed a marked increase in disease. Beginning at 2 weeks after the initiation of CD4 depletions, mice began to show grossly enlarged spleens indicative of FV-induced erythroproliferation (Fig. 2). The percentage of affected mice increased over the next month to approximately one-half of the treated group. No spontaneous relapses of splenomegaly were observed in the control group during this time.
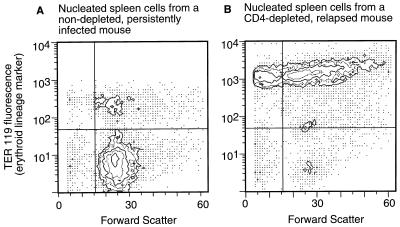
Flow cytometric analysis of an enlarged spleen from a CD4-depleted mouse revealed typical FV-induced erythroproliferation with more than 90% of the spleen cells expressing the Ter 119 erythroid lineage marker (Fig. 3). This was in sharp contrast to the profile for a typical nondepleted mouse, which showed less than 13% Ter 119+ cells. In addition, the Ter 119+ population from the CD4-depleted mouse showed a very abnormal size distribution with high percentages of both very small and very large cells. Infectious center assays of a CD4-depleted enlarged spleen revealed that the Ter 119+ cells accounted for 74% of the infectious centers in the spleen, indicating that there was massive infection as well as proliferation of erythroid cells (data not shown).
FIG. 3.
Flow cytometric analysis of nucleated spleen cells. Single-cell suspensions from spleens were labeled with biotin-Ter 119, stained with fluorescein isothiocyanate-avidin, and analyzed as described in Materials and Methods. Results are presented as contour plots generated with Consort 30 software. (A) Cells from a nondepleted, persistently infected mouse with no palpable splenomegaly. Ter 119-positive cells were 12.4% of the total. (B) Cells from a CD4-depleted, persistently infected mouse with gross splenomegaly. Ter 119-positive cells were 91.5% of the total.
One group of nine CD4-depleted mice which had four mice with splenomegaly was monitored over the long term. At 7 weeks postdepletion, one mouse recovered, possibly due to repopulation of its T-cell compartment. The other three mice developed fatal erythroleukemias. Thus, the disease induced by depletion of CD4+ T cells progressed from erythroproliferation to leukemia in most cases.
Increased virus replication in CD4-depleted, relapsed mice.
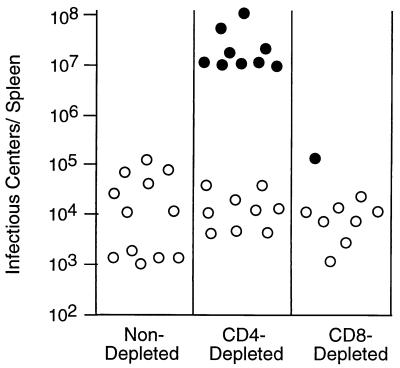
Infectious center assays were done to determine how T-cell depletions affected levels of virus replication in the spleen. Consistent with previous findings, the persistently infected, nondepleted mice had levels of infection that ranged between 0.001 and 0.2% of spleen cells (5). In contrast, CD4-depleted mice that developed palpable splenomegaly had mean infectious center titers more than 1,000-fold higher than titers from the other groups (Fig. 4).
FIG. 4.
Infectious center assays of virus-producing cells in the spleens of persistently infected mice. Spleen cell suspensions were plated onto indicator cells to produce infectious centers as described in Materials and Methods. Nonrelapsed mice are indicated by open circles, and relapsed splenomegalic mice are indicated by closed circles. There were significantly more infectious centers in CD4-depleted relapsed mice (mean = 3.4 × 107) than in the CD4-depleted nonleukemic group (mean = 1.9 × 104; two-tailed P < 0.0001 by Mann-Whitney test). The numbers of infectious centers in the CD4-depleted, nonleukemic group were not significantly different from those for the control group or the CD8-depleted group.
In addition to spleen infectious centers, CD4-depleted mice were also assayed for the presence of plasma viremia and virus-neutralizing antibodies. Previous studies with acutely infected mice have demonstrated that plasma viremia is controlled by virus-neutralizing antibodies (2, 4). Two-thirds of the CD4-depleted, relapsed mice had high titers of virus in their blood, while none of the nonrelapsed mice had detectable virus (Table 2). Virus-neutralizing antibody titers were reduced in the viremic mice, likely due to absorption of antibodies by free virus. Two mice relapsed with splenomegaly while maintaining virus-neutralizing antibodies which were still controlling viremia. These mice may have been at an earlier stage of disease at which virus replication had not yet outpaced antibody production. Induction of splenomegaly in the presence of virus-neutralizing antibodies indicated that antibody loss was not the reason for relapse in these two CD4-depleted mice.
TABLE 2.
Plasma viremia and virus-neutralizing antibody in persistently infected, CD4-depleted mice
| Mouse no. | Relapse of splenomegaly | Plasma viremia (titer/ml)a | Virus-neutralizing antibody titerb |
|---|---|---|---|
| 1 | No | <220 | 1/128 |
| 2 | No | <220 | 1/128 |
| 3 | No | <220 | 1/128 |
| 4 | No | <220 | 1/128 |
| 5 | No | <220 | 1/128 |
| 6 | No | <220 | 1/64 |
| 7 | No | <220 | 1/64 |
| 8 | Yes | <220 | 1/128 |
| 9 | Yes | <220 | 1/64 |
| 10 | Yes | 5.3 × 103 | 1/8 |
| 11 | Yes | 2.0 × 103 | 1/8 |
| 12 | Yes | 2.0 × 103 | 1/4 |
| 13 | Yes | 1.8 × 103 | 1/4 |
Viremia assays were performed on plasma samples taken after relapse of splenomegaly and on time-matched samples for nonrelapsed mice.
Antibody titers were obtained from the same plasma samples used to determine viremia. The neutralization titer was the dilution at which more than 75% of the input virus was inactivated by antibody plus complement.
In summary, depletion of CD4+ T cells in many cases allowed increased FV replication, permitted spread of virus from persistently infected B cells to the erythroid compartment, and significantly increased relapses of splenomegaly due to erythroproliferation. In most cases studied, virus spread to the blood and erythroproliferative disease progressed to erythroleukemia.
Differences in CD4+ T cells from persistently infected mice and those from uninfected mice.
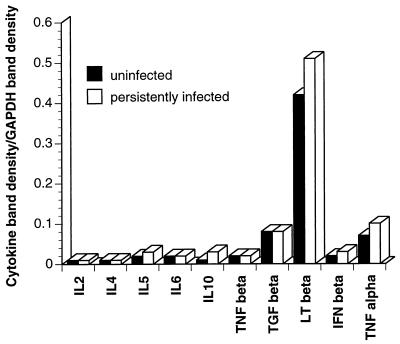
The mechanism of CD4+ T-cell-mediated control of persistent virus was investigated by analyzing cytokine mRNA levels by an RNase protection assay. Comparisons of mRNAs isolated from splenic CD4+ T cells showed no significant differences between uninfected and persistently infected mice (Fig. 5). Given that the virus-specific CD4+ T cells were likely a small percentage of the total splenic CD4+ T cells, this assay may not have been sensitive enough to detect a difference. Alternatively, the effector(s) could have been a cytokine or a factor not included in the assay.
FIG. 5.
Cytokine analyses. Levels of cytokine transcripts from purified CD4+ T cells derived from normal mice (solid bars) and persistently infected mice (open bars) were compared by RNase protection assays. The cells were not restimulated in vitro. Band densities are expressed as ratios of specific cytokine band density to the density of an internal housekeeping transcript band (glyceraldehyde-3-phosphate dehydrogenase [GAPDH]). Additional cytokine transcripts which were analyzed but did not give detectable bands were those of interleukin 9, interleukin 13, interleukin 15, transforming growth factor β2, and gamma interferon. Abbreviations: IL, interleukin; TNF, tumor necrosis factor; TGF, transforming growth factor; LT, lymphotoxin; IFN, interferon.
Flow cytometric analyses were also performed to determine if surface expression of memory or activation markers could be used to distinguish CD4+ T-cell subpopulations obtained from persistently infected mice from those obtained from uninfected mice. No significant differences were found in expression of the CD45RB memory marker (data not shown). However, persistently infected mice had a 4% increase in the number of splenic CD4+ T cells expressing the CD69 activation marker (Table 3). This increase in activated CD4+ T cells from persistently infected mice was statistically significant and may reflect the percentage of CD4+ T cells involved in virus control at a given time.
TABLE 3.
Expression of the CD69 activation marker on CD4+ T cells from spleens of uninfected and persistently FV-infected mice
| % of CD4+ T cells expressing CD69
|
||
|---|---|---|
| Uninfected mice | Infected mice | |
| 10.8 | 15.6 | |
| 9.1 | 14.0 | |
| 8.2 | 13.6 | |
| 8.3 | 11.9 | |
| 10.5 | 13.9 | |
| 8.3 | 12.1 | |
| 7.5 | 11.1 | |
| Mean ± SDa | 9.0 ± 1.2 | 13.2 ± 1.5 |
Means and standard deviations were determined by the Mann-Whitney test. The two-tailed P value in comparing the two columns was 0.0006.
DISCUSSION
(C57BL/10 × A.BY)F1 mice acutely infected with FV develop complex immune responses including CD4+ T helper cells, CD8+ CTLs, and virus-neutralizing antibodies, which collectively are able to clear infection from almost every cell, including most infected B cells. However, we found a very small subpopulation of B cells acting as a reservoir for persistent FV. We estimate that the number of B cells infected with FV ranges between 100 and 1,000 cells per spleen. The narrowing of FV infection to B cells during resolution of acute infection suggests an immunological escape mechanism specific to that cell type. It is unlikely that B cells are the major reservoir of persistent virus simply by chance because, within an order of magnitude, Mac-1+ cells account for as much infectivity during acute infection as do B220+ cells (Table 1), yet Mac-1+ cells were not observed to carry persistent virus. It is more likely that there is a unique microenvironment which is specific to a subset of B cells or which is induced by the virus in only a small fraction of infected B cells. Due to the very low percentage of B cells which are persistently infected, and because we have not observed surface expression of FV antigens on B cells from persistently infected mice, we have not yet been able to investigate some of the interesting questions about these cells.
Viruses have been reported elsewhere to employ a wide variety of methods to escape killing by CTLs (reviewed in reference 27), the main immunological effectors responsible for specifically eliminating virus-infected cells. Since the very presence of persistent virus indicates escape from CTL destruction, it was not totally unexpected that we found no significant role for CD8+ T cells in controlling persistent FV disease. For the lymphocytic choriomeningitis virus model of persistent viruses, CTL exhaustion, in which all reactive CTLs are induced during acute infection and subsequently disappear, has been described (24). This mechanism of immunological escape does not appear to be operating in persistent FV infections because rechallenge of persistently infected mice with FV induces strong CTL responses at 1 week postchallenge (data not shown). Thus, the animals still possess FV-specific CTLs, but those CTLs are ineffective at eliminating persistent virus from B cells.
In contrast to the lack of effect from CD8+ T cells, we were able to establish, for the first time, a critical in vivo role for CD4+ T cells in controlling persistent FV infections in many mice. It is known in the lymphocytic choriomeningitis virus model that CD4+ T cells are necessary for the maintenance of CD8+ CTL responses during chronic infections (21). However, in the FV model that we describe, CD8 depletions did not induce relapses, nor did dual depletions of both CD4+ and CD8+ cells have an additive effect (data not shown). Thus, the mechanism of CD4+ T-cell-mediated control appeared to be independent of CD8+ T cells. Two mice which maintained good titers of virus-neutralizing antibodies and controlled blood viremia, even after CD4 depletion and relapse of splenomegaly, were found. Thus, at least in some cases, a CD4+ T-cell function other than immunological help for CTLs or B cells may have been responsible for controlling virus replication and spread in the spleen. For most mice, it appeared that virus production rapidly swamped production of virus-neutralizing antibodies and that the animals became viremic as well as splenomegalic.
One of the most interesting questions raised by these studies is how CD4+ T cells control retrovirus replication and spread. While the current studies do not answer this question, the findings point to an antigen-specific interaction because the CD69 early activation marker is upregulated only in the presence of cognate antigen presented by major histocompatibility complex (MHC) class II molecules (34). B cells are one of the few types of cells which express MHC class II molecules and thus could serve as targets for recognition by CD4+ T cells. Clearly, these studies raise interesting questions regarding presentation of endogenous antigens by MHC class II molecule cells as well as questions regarding CD4+ T-cell control of persistent retroviruses.
In each T-cell depletion experiment that was performed, we observed induction of splenomegaly in only about half of the animals. Although antibody-mediated T-cell depletions are quite effective in reducing T-cell populations, residual CD4+ T cells could potentially have an immunological effect. This is especially true in the spleen, where it is more difficult than in the periphery to deplete T cells with antibody. Use of CD4+ T-cell knockout mice is not possible because such mice cannot recover from acute FV infection. Alternatively, the data may suggest that factors other than CD4+ T cells may help keep persistent virus in check. Another possibility is that the viral load of the animal may affect whether they relapse. It is evident from Fig. 4 that some animals have levels of persistent virus 10 to 100 times higher than those of other, identically treated mice. This extent of variability could impact the severity of the response to CD4 depletion, and it was not possible to assess the extent of persistent infection prior to treatment. Finally, it is possible that persistent infection in some of the mice consisted primarily of helper virus rather than the complex of both helper (F-MuLV) and SFFV. The assay we used detects only persistent F-MuLV, a necessary component for pathogenesis. It was previously shown that levels of persistent helper virus are 30-fold higher than levels of persistent complex (5). Since only complex is pathogenic in adult animals, animals with low levels of helper virus and even lower levels of cells infected by complex might not relapse.
The findings presented here have implications for vaccination against viruses able to establish persistent infections. We found massive infection of B cells as early as 1 week postinfection, indicating that persistence might be established quite early as well. Therefore, prevention of persistent FV would require either complete protection from infection or severe limitation of infection combined with rapid destruction of infected B cells. Little is known about the prevention of persistent infections, and it is doubtful whether immune responses can completely prevent infection. However, in lymphocytic choriomeningitis virus of mice, a vaccine which primes the CTL response has been shown to prevent persistent infection through rapid destruction of infected cells (26). Previous vaccine studies with Friend virus have demonstrated protection from acute disease but no protection from persistent infection, even though the vaccines primed CTL responses (11). Thus, protection from retroviral persistence may be more difficult to obtain than protection from viruses which do not integrate into the genome. Consideration of retroviral persistence may be a crucial aspect of vaccine development, especially for viruses such as HIV, which are immunosuppressive and have the potential to reactivate themselves by damaging the host’s resistance mechanisms. Recent studies show a critical role for CD4+ T cells in controlling HIV viremia (31). The FV model that we describe here provides the opportunity to study the basic mechanisms for both prevention and control of persistent retroviral infections.
REFERENCES
- 1.Ben-David Y, Bernstein A. Friend virus-induced erythroleukemia and the multistage nature of cancer. Cell. 1991;66:831–834. doi: 10.1016/0092-8674(91)90428-2. [DOI] [PubMed] [Google Scholar]
- 2.Britt W J, Chesebro B. Use of monoclonal anti-gp70 antibodies to mimic the effects of the Rfv-3 gene in mice with Friend virus-induced leukemia. J Immunol. 1983;130:2363–2367. [PubMed] [Google Scholar]
- 3.Chesebro B, Bloom M, Wehrly K, Nishio J. Persistence of infectious Friend virus in spleens of mice after spontaneous recovery from virus-induced erythroleukemia. J Virol. 1979;32:832–837. doi: 10.1128/jvi.32.3.832-837.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chesebro B, Wehrly K. Identification of a non-H-2 gene (Rfv-3) influencing recovery from viremia and leukemia induced by Friend virus complex. Proc Natl Acad Sci USA. 1979;76:425–429. doi: 10.1073/pnas.76.1.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chesebro B, Wehrly K, Stimpfling J. Host genetic control of recovery from Friend leukemia virus-induced splenomegaly. Mapping of a gene within the major histocompatibility complex. J Exp Med. 1974;140:1457–1467. doi: 10.1084/jem.140.6.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cobbold S P, Jayasuriya A, Nash A, Prospero T D, Waldmann H. Therapy with monoclonal antibodies by elimination of T-cell subsets in vivo. Nature (London) 1984;312:548–551. doi: 10.1038/312548a0. [DOI] [PubMed] [Google Scholar]
- 7.Coffman R L, Weissman I L. B220: a B cell-specific member of the T200 glycoprotein family. Nature (London) 1981;289:681–683. doi: 10.1038/289681a0. [DOI] [PubMed] [Google Scholar]
- 8.Corbin A, Sitbon M. Protection against retroviral diseases after vaccination is conferred by interference to superinfection with attenuated murine leukemia viruses. J Virol. 1993;67:5146–5152. doi: 10.1128/jvi.67.9.5146-5152.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dialynas D P, Quan Z S, Wall K A, Pierres A, Quintans J, Loken M R, Pierres M, Fitch F W. Characterization of the murine T cell surface molecule, designated L3T4, identified by monoclonal antibody GK 1.5: similarity of L3T4 to the human Leu-3/T4 molecule. J Immunol. 1983;131:2445–2451. [PubMed] [Google Scholar]
- 10.Dianzani U, Luqman M, Rojo J, Yagi J, Baron J L, Woods A, Janeway C A, Jr, Bottomly K. Molecular associations on the T cell surface correlate with immunological memory. Eur J Immunol. 1990;20:2249–2257. doi: 10.1002/eji.1830201014. [DOI] [PubMed] [Google Scholar]
- 11.Earl P L, Moss B, Morrison R P, Wehrly K, Nishio J, Chesebro B. T-lymphocyte priming and protection against Friend leukemia by vaccinia-retrovirus env gene recombinant. Science. 1986;234:728–731. doi: 10.1126/science.3490689. [DOI] [PubMed] [Google Scholar]
- 12.Hasenkrug K J, Brooks D M, Chesebro B. Passive immunotherapy for retroviral disease: influence of major histocompatibility complex type and T-cell responsiveness. Proc Natl Acad Sci USA. 1995;92:10492–10495. doi: 10.1073/pnas.92.23.10492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12a.Hasenkrug, K. J., D. M. Brooks, and U. Dittmer. Unpublished data.
- 13.Hasenkrug K J, Chesebro B. Immunity to retroviral infection: the Friend virus model. Proc Natl Acad Sci USA. 1997;94:7811–7816. doi: 10.1073/pnas.94.15.7811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haywood A M. Patterns of persistent viral infections. N Engl J Med. 1986;315:939–948. doi: 10.1056/NEJM198610093151506. [DOI] [PubMed] [Google Scholar]
- 15.Hirsch M S. Cytomegalovirus and its role in the pathogenesis of acquired immunodeficiency syndrome. Transplant Proc. 1991;23:118–121. [PubMed] [Google Scholar]
- 16.Hoatlin M E, Kabat D. Host-range control of a retroviral disease: Friend erythroleukemia. Trends Microbiol. 1995;3:51–57. doi: 10.1016/s0966-842x(00)88875-7. [DOI] [PubMed] [Google Scholar]
- 17.Ikuta K, Kina T, MacNeil I, Uchida N, Peault B, Chien Y H, Weissman I L. A developmental switch in thymic lymphocyte maturation potential occurs at the level of hematopoietic stem cells. Cell. 1990;62:863–874. doi: 10.1016/0092-8674(90)90262-d. [DOI] [PubMed] [Google Scholar]
- 18.Jacobson M A, Mills J. Serious cytomegalovirus disease in the acquired immunodeficiency syndrome (AIDS) Ann Intern Med. 1988;108:585–594. doi: 10.7326/0003-4819-108-4-585. [DOI] [PubMed] [Google Scholar]
- 19.Krop I, de Fougerolles A R, Hardy R R, Allison M, Schlissel M S, Fearon D T. Self-renewal of B-1 lymphocytes is dependent on CD19. Eur J Immunol. 1996;26:238–242. doi: 10.1002/eji.1830260137. [DOI] [PubMed] [Google Scholar]
- 20.Lander M R, Chattopadhyay S K. A Mus dunni cell line that lacks sequences closely related to endogenous murine leukemia viruses and can be infected by ecotropic, amphotropic, xenotropic, and mink cell focus-forming viruses. J Virol. 1984;45:695–698. doi: 10.1128/jvi.52.2.695-698.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matloubian M, Concepcion R J, Ahmed R. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J Virol. 1994;68:8056–8063. doi: 10.1128/jvi.68.12.8056-8063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meyers J D, Flournoy N, Wade J C, Hackman R C, McDougall J K, Neiman P E, Thomas E D. Biology of interstitial pneumonia after marrow transplantation. New York, N.Y: Alan R. Liss, Inc.; 1983. [Google Scholar]
- 23.Morrison R P, Earl P L, Nishio J, Lodmell D L, Moss B, Chesebro B. Different H-2 subregions influence immunization against retrovirus and immunosuppression. Nature (London) 1987;329:729–732. doi: 10.1038/329729a0. [DOI] [PubMed] [Google Scholar]
- 24.Moskophidis D, Lechner F, Pircher H, Zinkernagel R M. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature. 1993;362:758–761. doi: 10.1038/362758a0. . (Erratum, 364:262.) [DOI] [PubMed] [Google Scholar]
- 25.Nash A A, Jayasuriya A, Phelan J, Cobbold S P, Waldmann H, Prospero T. Different roles for L3T4+ and Lyt 2+ T cell subsets in the control of an acute herpes simplex virus infection of the skin and nervous system. J Gen Virol. 1987;68:825–833. doi: 10.1099/0022-1317-68-3-825. [DOI] [PubMed] [Google Scholar]
- 26.Oldstone M B, Tishon A, Eddleston M, de la Torre J C, McKee T, Whitton J L. Vaccination to prevent persistent viral infection. J Virol. 1993;67:4372–4378. doi: 10.1128/jvi.67.7.4372-4378.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oldstone M B A. How viruses escape from cytotoxic T lymphocytes: molecular parameters and players. Virology. 1997;234:179–185. doi: 10.1006/viro.1997.8674. [DOI] [PubMed] [Google Scholar]
- 28.Polsky D, Lilly F. Suppression of H-2b-associated resistance to Friend erythroleukemia virus by a class I gene from the H-2d major histocompatibility complex haplotype. Proc Natl Acad Sci USA. 1991;88:9243–9247. doi: 10.1073/pnas.88.20.9243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robertson M N, Miyazawa M, Mori S, Caughey B, Evans L H, Hayes S F, Chesebro B. Production of monoclonal antibodies reactive with a denatured form of the Friend murine leukemia virus gp70 envelope protein: use in a focal infectivity assay, immunohistochemical studies, electron microscopy and western blotting. J Virol Methods. 1991;34:255–271. doi: 10.1016/0166-0934(91)90105-9. [DOI] [PubMed] [Google Scholar]
- 30.Robertson M N, Spangrude G J, Hasenkrug K, Perry L, Nishio J, Wehrly K, Chesebro B. Role and specificity of T-cell subsets in spontaneous recovery from Friend virus-induced leukemia in mice. J Virol. 1992;66:3271–3277. doi: 10.1128/jvi.66.6.3271-3277.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosenberg E S, Billingsley J M, Caliendo A M, Boswell S L, Sax P E, Kalams S A, Walker B D. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science. 1997;278:1447–1450. doi: 10.1126/science.278.5342.1447. [DOI] [PubMed] [Google Scholar]
- 32.Singh N, Dummer J S, Kusne S, Breinig M K, Armstrong J A, Makowka L, Starzl T E, Ho M. Infections with cytomegalovirus and other herpesviruses in 121 liver transplant recipients: transmission by donated organ and the effect of OKT3 antibodies. J Infect Dis. 1988;158:124–131. doi: 10.1093/infdis/158.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Springer T, Galfre G, Sechler D S, Milstein C. Mac-1: a macrophage differentiation antigen identified by monoclonal antibody. Eur J Immunol. 1979;9:301–306. doi: 10.1002/eji.1830090410. [DOI] [PubMed] [Google Scholar]
- 34.Testi R, D’Ambrosio D, De Maria R, Santoni A. The CD69 receptor: a multipurpose cell-surface trigger for hematopoietic cells. Immunol Today. 1994;15:479–483. doi: 10.1016/0167-5699(94)90193-7. [DOI] [PubMed] [Google Scholar]