Abstract
Protein kinases are key signaling nodes that regulate fundamental biological and disease processes. Illuminating kinase signaling from multiple angles can provide deeper insights into disease mechanisms and improve therapeutic targeting. While fluorescent biosensors are powerful tools for visualizing live-cell kinase activity dynamics in real time, new molecular tools are needed that enable recording of transient signaling activities for post hoc analysis and targeted manipulation. Here, we develop a light-gated kinase activity coupled transcriptional integrator (KINACT) that converts dynamic kinase signals into “permanent” fluorescent marks. KINACT enables robust monitoring of kinase activity across scales, accurately recording subcellular PKA activity, highlighting PKA signaling heterogeneity in 3D cultures, and identifying PKA activators and inhibitors in high-throughput screens. We further leverage the ability of KINACT to drive signaling effector expression to allow feedback manipulation of the balance of GαsR201C-induced PKA and ERK activation and dissect the mechanisms of oncogenic G protein signaling.
Introduction
Kinase signaling pathways stringently regulate cellular processes under normal physiological conditions, which are also central to tumorigenesis and cancer development when they are dysregulated. Thanks to the development of fluorescent biosensors for visualizing the activities of various target kinases, we are able to illuminate these biological signals and activities in situ in a real-time manner1. However, real-time biosensors face great challenges in several settings, particularly when it is desirable to remove the transiency of a real-time readout and convert a potentially transient cellular state into a “permanent” mark. For instance, when mapping the influence of complex tissue microenvironments on heterogeneous kinase activities, the ability to identify all cells across the entire tissue that experienced high kinase activity, even transiently, would be highly desirable, but is unfeasible through live imaging of biosensors in real time. Furthermore, being able to selectively manipulate cell function based on the activity state of the cell also calls for the ability to convert a potentially transient cellular state into a “permanent” mark. The concept of a calcium memory dye, which coverts calcium transients in single cells to their “permanent” fluorescence marking2, was realized for the study of neuronal activity in the brain3, 4. A similar design has been applied to the study of neuromodulator signaling5. These tools belong to a class of light-gated integrators where a specific sensing switch and a light-induced switch simultaneously control the recording of activity changes. Yet, such a light-gated integrator has been missing from the toolbox of available biosensors for illuminating kinase activity. Here, we present a light-gated kinase activity integrator design for converting kinase activity changes in live cells to a transcriptional change with high spatiotemporal resolution. This integrator displays robust performance in multiple research applications, including highlighting the heterogeneity of basal PKA activities within 3D cultures, screening potential PKA inhibitors/activators from small molecule libraries, and inducing effectors for signaling network manipulation in living cells.
Results
Developing a light-gated integrator of protein kinase A activity.
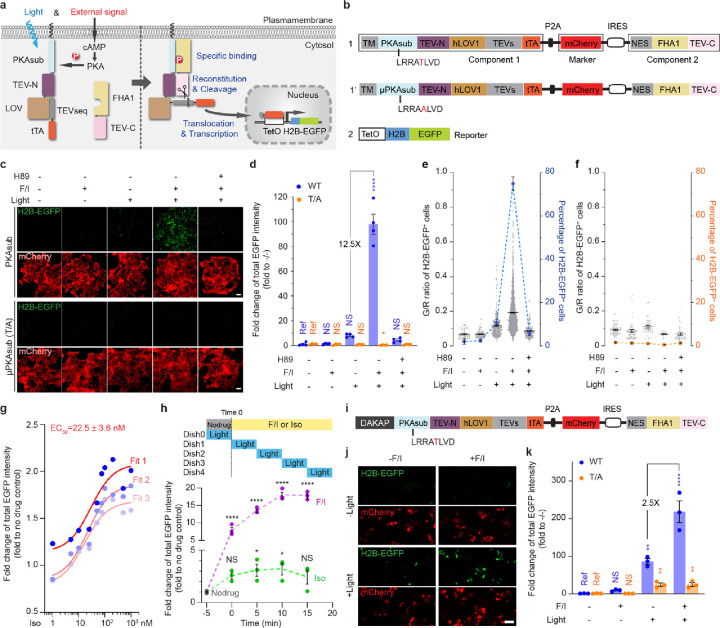
Light-gated, transcription-based activity integrators usually consist of three parts: a biochemical switch, an optical switch, and a transcriptional reporter. To construct a prototype kinase activity-coupled transcriptional integrator (KINACT) for memorizing protein kinase A (PKA) activity (A-KINACT), we used the PKA substrate (PKAsub; LRRATLVD) and FHA1 domain of AKAR6 as the biochemical switch to control reconstitution of split tobacco etch virus protease (TEVp). As the optical switch, we used a TEVp cleavage sequence (TEVseq) caged by a light-oxygen-voltage sensing (LOV) domain. Finally, we used a tetracycline-controlled transcriptional activator (tTA)/tetracycline operator (TetO) system as the transcriptional reporter. In the first component, PKAsub, the TEVp N-terminal fragment (TEVp-N; residues 1–118), the LOV domain-caged TEV-seq, and the tTA are fused in tandem and targeted to the plasma membrane using a transmembrane (TM) helix derived from platelet-derived growth factor receptor beta (PDGFRβ). The second component comprises a cytosolically expressed fusion of the FHA1 domain and TEVp C-terminal fragment (TEVp-C; residues 119–220) (Fig. 1a). Induction of PKA activity in cells expressing A-KINACT will lead to PKAsub phosphorylation and binding of the FHA1 domain, resulting in close proximity of TEVp-N and TEVp-C, favoring TEVp reconstitution. Concurrent blue light irradiation of the LOV domain will expose the caged TEVseq, allowing cleavage by reconstituted TEVp and subsequent release of the tTA to drive reporter gene expression in the nucleus (Fig. 1a). To facilitate imaging and analysis, we used EGFP-tagged Histone 2B (H2B-EGFP) as the reporter.
Figure 1 |. KINACT for cumulative PKA activity recording in live cells.
a, Schematic of PKA activity integrator and diagram of light- and activator-induced gene expression. b, The domain structure of A-KINACT (WT and T/A mutant). Construct 1 includes combines the two key components and a transfection marker, and construct 2 contains the H2B-EGFP reporter driven by TetO promoter. c, Snapshot imaging of cumulative PKA activity in A-KINACT (WT and T/A) dual-stable cells. Five treatment conditions were applied: −F/I/−light, +F/I/−light, −F/I/+light, +F/I/+light and H89 pretreat/+F/I/+light. d, Statistical quantification of total EGFP intensity under all conditions. P=0.0388 (T/A, +F/I/+light) and P<0.0001 (WT, +F/I/+light). e, The fraction and mean EGFP/mCherry (G/R) intensity ratio of H2B-EGFP+ cells stably expressing A-KINACT (WT). n = 52, n = 73, n = 285, n = 1975 and n = 154 cells. f, The fraction and G/R ratio of H2B-EGFP+ cells stably expressing A-KINACT (T/A). n = 52, n = 47, n = 37, n = 13 and n = 36 cells. g, The quantification of 3 replicates of isoproterenol (Iso) dose-response experiments in A-KINACT cells. The red lines represent 3 fitted logistic curves with an average fit value for the EC50. h, The quantification of light-gated experiments with full-time drug treatments (F/I, 50/100 μM or Iso, 100 nM) but 5-min time window of light illumination. Data points correspond to start of illumination (min after drug addition). P = 0.0417 (Iso, 5–10 min), P = 0.0333 (Iso, 10–15 min) and P < 0.0001 (F/I). i, Domain structure of outer mitochondrial membrane-targeted A-KINACT (WT) system. DAKAP was tagged at the N-terminus of component 1. j, Snapshot imaging of cumulative PKA activity near mitochondria. Four treatment conditions were applied: −F/I/−light, +F/I/−light, −F/I/+light and +F/I/+light. k, Statistical quantification of total EGFP intensity under all conditions. P = 0.0094 (WT, −F/I/+light), P < 0.0001 (WT, +F/I/+light), P = 0.0042 (T/A, −F/I/+light) and P = 0.0041 (T/A, +F/I/+light). For d-f, data from 4 independent experiments. For g, h and k, data from 3 independent experiments. For c and j, scale bars, 10 μm. For d, h and k, statistical analysis was performed using ordinary one-way ANOVA followed by Dunnett’s multiple-comparison test. NS, not significant. Data are mean ± s.e.m.
To minimize potential leaky reporter expression due to TEVp-mediated cleavage in the absence of either kinase activity or light, we evaluated several configurations featuring different combinations of LOV domain variants and TEVp-TEVseq pairs that had been previously evolved and optimized in various integrators such as iTango5, FLARE4 and SPARK7. By comparing the H2B-EGFP+ fraction in HEK293T cells lacking expression of Component 2 (Construct 7), we found that TEVseq with Gly preceding the cleavage site (TEVseq-G, Constructs 1 and 4; Supplementary Fig. 1) showed little cleavage by endogenous proteases, whereas TEVseq with Met preceding the cut site (TEVseq-M, Constructs 3 and 5) exhibited poor bio-orthogonality and a weaker LOV-dependent response. We then transfected both Components 1 (Constructs 1–5) and 2 (Construct 7) into HEK293T cells and compared the fraction of H2B-EGFP+ cells in the absence (untreated/dark) or presence of PKA activation using Fsk and IBMX (F/I) plus blue light (treated/light). hLOV1-tethered TEVseq-G (Construct 1) achieved the best performance in terms of contrast between OFF signal in the untreated/dark state (2.7% H2B-EGFP+), which was comparable to the negative control TEVseq-P (Construct 2), and ON signal (28.1% H2B-EGFP+) in the treated/light state. In contrast, iLID-tethered TEVseq-G (Construct 3) exhibited both higher leaky expression (4.5% H2B-EGFP+) in the untreated/dark state and lower ON signal (18.5% H2B-EGFP+) in the treated/light state. Notably, we also found that an alternative configuration based on recruitment of intact TEVp (Constructs 6 and 8) showed very high basal signal (55.1% H2B-EGFP+) in the untreated/dark state. Therefore, we selected Construct 1 and Construct 7 as the main components of A-KINACT (Supplementary Fig. 1).
To facilitate expression of Components 1 and 2 at an appropriate ratio, as well as provide a convenient internal marker for quantification, we combined the two main components plus an mCherry marker into a single polycistronic cassette (Fig. 1b). Specifically, we placed mCherry after a P2A sequence to indicate the expression level of Component 1, with Component 2 driven by an IRES to promote reduced expression relative to Component 1, thus minimizing spontaneous binding. Indeed, HEK293T cells transfected with the polycistronic construct expressed Components 1 and 2 at roughly a 10:1 ratio (Supplementary Fig. 2). Meanwhile, we generated a HEK293T line stably integrating the TetO-H2B-EGFP reporter construct to avoid variability from transient transfection. We then tested the performance of A-KINACT via epifluorescence microscopy under different conditions. Transiently expressing A-KINACT in TetO-H2B-EGFP cells produced low background fluorescence in the dark state (untreated, 0.7% H2B-EGFP+; treated, 0.9% H2B-EGFP+), with clear and varied signal increases – 18.6 fold (no F/I, 4.5% H2B-EGFP+) and 65.0 fold (F/I, 11.4% H2B-EGFP+) – upon illumination with blue light (460 nm) for 20 min (10 s on/50 s off) (Supplementary Fig. 3a–c, also see Methods). However, when testing whether the reporting behavior of A-KINACT was dependent on phosphorylation of the PKA substrate by generating a non-phosphorylatable mutant (T/A) A-KINACT (Fig. 1b), we noticed that the mutant integrator produced a detectable increase in H2B-EGFP expression - 8.4 fold (no F/I, 3.4% H2B-EGFP+) and 8.4 fold (F/I, 3.8% H2B-EGFP+) changes - under all illumination conditions versus the untreated/dark condition (Supplementary Fig. 3a–b, d). Given that H2B-EGFP expression did not increase upon F/I addition, we hypothesized that excessive A-KINACT expression in a fraction of cells, arising from transient transfection, may be driving spontaneous reconstitution of split TEVp and subsequent reporter expression. To test this possibility, we generated a TEV-only reporter system in which the PKAsub and FHA1 were omitted. Indeed, under blue light illumination, this TEV-only reporter induced a tenfold change in H2B-EGFP expression, similar to the T/A mutant A-KINACT (Supplementary Fig. 4).
Therefore, we established cell lines that stably express appropriate levels of each of the integrator variants (WT and T/A mutant A-KINACT) to avoid such overexpression-related artifacts in subsequent applications. In both the presence and absence of PKA stimulation, dual-stable A-KINACT cells exhibited similar low background (untreated, 2.0% H2B-EGFP+; treated, 2.5% H2B-EGFP+) in the dark, indicating tight control by light. Upon application of blue light, a large population (74.9%) of brightly H2B-EGFP+ cells were visible following F/I stimulation, representing a 98.0-fold change versus untreated/dark conditions (Fig. 1c–e). A small fraction (10.7%) of cells was also observed to express H2B-EGFP in the absence of F/I stimulation, representing a 7.8-fold change in H2B-EGFP expression versus untreated/dark conditions. To determine whether this EGFP+ cell population was still dependent on PKA activity, we pretreated cells with the PKA-specific inhibitor H89 prior to F/I and light application. We observed a smaller (5.8%) fraction of dim H2B-EGFP+ cells compared with the no-drug (light-only) condition (Fig. 1c–e), suggesting this EGFP+ cell population was at least partially dependent on basal PKA activity. In addition, A-KINACT (T/A) dual-stable cells presented extremely low background (untreated, 1.5% H2B-EGFP+; treated, 1.1% H2B-EGFP+) in the dark, and nonsignificant changes - 0.7 fold (no F/I, 1.0% H2B-EGFP+), 0.2 fold (F/I, 0.4% H2B-EGFP+) and 0.7 fold (H89+F/I, 1.2% H2B-EGFP+) - under all illumination conditions (Fig. 1c–d, f). Altogether, these results suggest that A-KINACT is an effective light-controlled tool for memorizing PKA activity changes.
Characterizing the sensitivity and spatiotemporal resolution of A-KINACT in live cells.
To characterize the sensitivity of A-KINACT, we performed dose-response experiments to evaluate the PKA activity enhancement reported by A-KINACT in response to increasing concentrations of isoproterenol (Iso, β-adrenoreceptor agonist). We recorded PKA activity changes during a 20-minute period after Iso addition and observed a graded increase in the H2B-EGFP signal corresponding to the applied Iso concentration (Fig. 1g). Using the resulting dose-response curve, we calculated an EC50 value of 22.5 ± 3.6 nM, which falls well within the previously reported EC50 range of 21–26 nM8 for Iso stimulation of endogenous β2-adrenoreceptors. Our results suggest that A-KINACT is sensitive and accurate enough to report low-amplitude, physiological PKA signaling events.
In addition to sensitivity, we also assessed whether A-KINACT can accurately recapitulate the temporal dynamics of PKA activity changes. We therefore performed experiments in which A-KINACT HEK293T cells received drug treatment for a full 20 min duration but were illuminated with blue light for only a 5 min (10 s on/10 s off) time window starting at varying times after drug addition (Fig. 1h). As expected, cells treated with F/I showed a sustained increase in the H2B-EGFP signal, whereas Iso stimulation only induced a transient increase in H2B-EGFP fluorescence. Tracing the accumulation of fluorescence during each time window reflects the dynamic changes in PKA activity induced under the different stimulation conditions, which are largely consistent with past results obtained using real-time PKA biosensors9, 10. These data reveal that the two components of the KINACT system can reversibly combine and separate in response to changes in kinase activity, thus providing an accurate readout of kinase activity levels during the corresponding recording window.
Similar to previous integrators3, 4, our initial KINACT implementation tethers Component 1 to the plasma membrane. Yet we also anticipated that the KINACT system can be applied to report kinase activity at other subcellular locations. We therefore targeted Component 1 of A-KINACT to the mitochondrial outer membrane using a peptide motif derived from DAKAP1 (see Methods) to sense and record PKA activities near the mitochondrial surface (Fig. 1i and Supplementary Fig. 2). Similar to the plasma membrane-targeted reporter, we observed an extremely low background signal in the dark and a significant (218-fold) induction of H2B-EGFP expression under concurrent F/I and light treatment (Fig. 1j–k and Supplementary Fig. 5). Compared with the plasma membrane-targeted version, mito-targeted A-KINACT reported a reduced PKA activity change from basal state to F/I stimulated state (2.5-fold versus 3.5-fold), which is consistent with previous results9. These data suggest that the KINACT system can be applied to monitor kinase activity within specific subcellular compartments.
General applicability of the KINACT system.
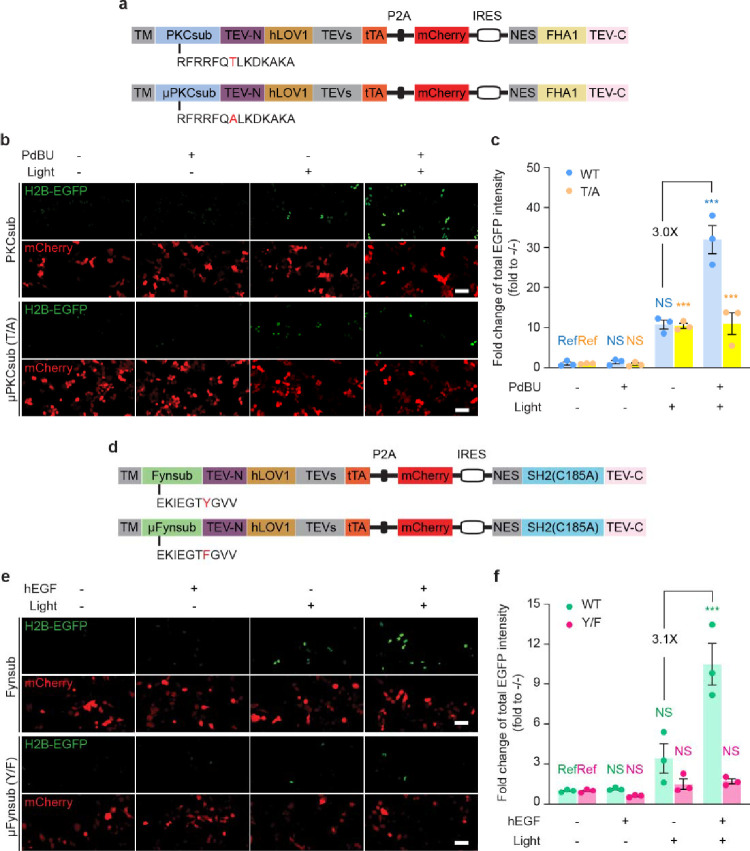
On the basis of traditional real-time biosensor toolbox design, we also expected KINACT to be broadly adaptable for recording various kinase activities. We first investigated whether KINACT could be generalized to other serine/threonine kinases by replacing the PKA substrate in A-KINACT with a protein kinase C (PKC)11 substrate (Fig. 2a). The resulting PKC integrator (C-KINACT) successfully recorded PKC activity changes when expressed in HEK293T cells (Fig. 2b and Supplementary Fig. 2). We observed a 32-fold change in H2B-EGFP expression when cells were treated with phorbol 12,13-Dibutyrate (PDBu) and blue light for 20 min. Conversely, cells expressing a T/A mutant C-KINACT showed no obvious response to PDBu treatment (Fig. 2c). To further investigate the general applicability of KINACT to record tyrosine kinase activity, we incorporated a Fyn kinase substrate and high-specificity SH2 domain12 into Components 1 and 2, respectively, to generate a Fyn integrator (F-KINACT) (Fig. 2d). WT F-KINACT, but not a non-phosphorylatable Y/F mutant, produced obvious H2B-EGFP expression increases upon human EGF stimulation and blue light illumination in HeLa TetO-H2B-EGFP cells (Fig. 2e and Supplementary Fig. 2). Quantification of total EGFP intensity revealed 10.5-fold change compared with no drug and no light conditions (Fig. 2f). Together, both C-KINACT and F-KINACT highlight the potential for developing a broad KINACT toolkit for recording kinase activities.
Fig. 2 |. General applicability of KINACT to different type of kinases.
a, Domain structures of C-KINACT (WT and T/A mutant). b, Representative images showing C-KINACT and mutant C-KINACT (T/A) reporting PKC activity changes induced by PdBU (200 nM) in HEK293T cells. c, Statistical quantification of total EGFP intensity under all conditions. P=0.0008 (WT, +PdBU/+light), P=0.0004 (T/A, −PdBU/+light), P=0.0005 (T/A, +PdBU/+light). d, Domain structures of F-KINACT (WT and Y/F mutant). e, Representative images showing F-KINACT reporting Fyn activity changes induced by human EGF (hEGF, 100 nM) in HeLa cells. f, Statistical quantification of total EGFP intensity under all conditions. P=0.0003 (WT, +hEGF/+light). n = 3 independent experiments. For b and e, scale bars, 10 μm. For c and f, statistical analysis was performed using ordinary one-way ANOVA followed by Dunnett’s multiple-comparison test. NS, not significant. Data are mean ± s.e.m.
A-KINACT reveals intra-organoid PKA activity heterogeneity.
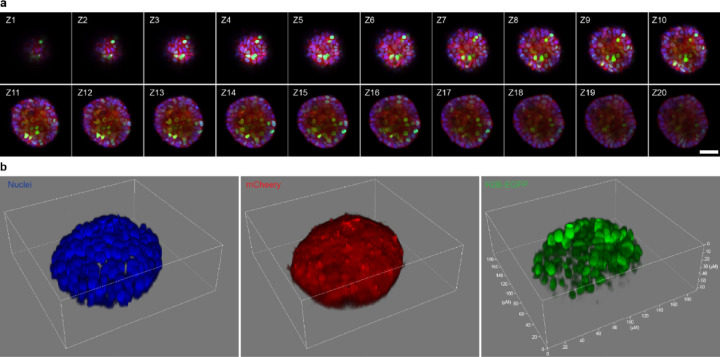
The capability of KINACT to enable post hoc imaging and analysis of recorded kinase activities motivated us to peer into the potential heterogeneity of PKA activity in 3D-cultured cells. Single cells stably expressing WT or T/A mutant A-KINACT were cultured into organoids with 5 or 6 cell layers on Matrigel, after which basal PKA activities were recorded during 20 min of blue light illumination. Under an epifluorescence microscope, 3D cultured HEK cells displayed obvious H2B-EGFP expression throughout the organoid, whereas normal 2D cultured cells under the same illumination condition showed very low basal PKA activity (Supplementary Fig. 6). These data suggest that cells within a 3D tissue environment have upregulated PKA activity in the basal state. To visualize the heterogeneity of this altered PKA activity, organoids were scanned along the Z-axis via confocal microscopy to construct a 3D image with multiple layers (Fig. 3a). We observed high H2B-EGFP expression across multiple cell layers of the A-KINACT organoids with differential expression in individual cells (Fig. 3b and Supplementary Fig. 7a), in contrast to extremely weak H2B-EGFP expression within H89-treated A-KINACT organoids or A-KINACT (T/A) organoids (Supplementary Fig. 7b–c). These data suggest that A-KINACT holds the potential to reveal the spatial heterogeneity of kinase activities inside complex, 3D tissue environments.
Fig. 3 |. 3D imaging of heterogeneous PKA activity in HEK293T organoids.
a, Triple-color confocal imaging of an A-KINACT dual-stable organoid in the Z-direction. 20-layer merged images of Hoechst-stained nuclei (blue), mCherry (red) and H2B-EGFP (green). Scale bar, 50 μm. b, 3D reconstruction of individual channels showing nuclear positions (left), uniform A-KINACT expression (middle) and heterogeneity of PKA activity within the organoid. n = 3 organoids (see Supplementary Fig 7a for Organoid 2 and 3).
High-throughput compound screening using A-KINACT.
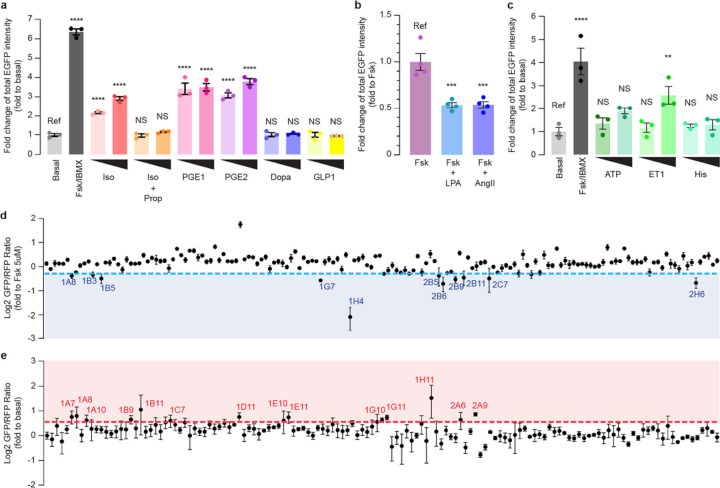
Given that KINACT is designed to integrate kinase activity into an amplified, transcriptional readout, we expected that A-KINACT would be capable of robustly detecting weak and transient modulators of PKA activity. Furthermore, in contrast to real-time recording, post hoc measurement is an important technological advantage of A-KINACT for screening compound libraries at various scales. As an initial proof of concept, we collected a small panel of well-known GPCR agonists known to activate, inhibit, or have no effect on PKA activity. Using epifluorescence microscopy, we found that HEK293T cells expressing A-KINACT responded strongly to several Gαs-linked agonists, such as Iso, PGE1, and PGE2, reporting dose-dependent activity changes (Fig. 4a). We also detected the suppression of Fsk-stimulated PKA activity in response to the Gαi-linked agonists lysophosphatidic acid (LPA) and Angiotensin II (Ang II) (Fig. 4b and Supplementary Fig. 8a). Interestingly, we found that high doses of the Gαq-linked agonist ET-1 (100 nM) led to increased PKA activity, which was successfully captured by A-KINACT (Fig. 4c and Supplementary Fig. 8b). One possible reason is that PKA was stimulated by these Gαq-linked agonists through parallel signaling such as calcium or PKC13. In addition, no obvious PKA activity was detected in response to treatment with Dopamine, GLP1, or Histamine, whose cognate receptors are known to be absent in HEK293T cells. Thus, A-KINACT accurately reported the effects of different GPCR agonists on PKA activity.
Fig. 4 |. A-KINACT for evaluating PKA responses to small molecule libraries.
a, Functional PKA responses to Gαs-coupled receptor agonists: Iso (100 nM, 1 μM); Iso (100 nM, 1 μM) with propranolol (Prop) (10 μM) costimulation; PGE1 (1 μM, 10 μM); PGE2 (1 μM, 10 μM); dopamine (Dopa) (1 μM, 10 μM); glucagon-like peptide 1 (GLP1) (10 nM, 30 nM). ****P<0.0001. b, Functional PKA responses to Gαi-coupled receptor agonists: Fsk (100 nM, 1 μM) costimulation with LPA (500 nM) or angiotensin II (Ang-II; 10 μM). P=0.0006 (LPA) and P=0.0007 (Ang-II). c, Functional PKA responses to Gαq-coupled receptor agonists: ATP (1 μM, 10 μM); endothelin 1 (ET1) (30 nM, 100 nM) and histamine (His) (1 μM, 10 μM). P=0.0083 (ET1, 100nM) and ****P<0.0001. d, High-throughput library screening of 160 kinase inhibitors to discover potential PKA inhibitors. Compounds with an average value below the 0.8-fold cut-off (blue dash line) were collected. e, High-throughput library screening of 137 marine natural products to discover potential PKA activators. Compounds with an average value above the 1.5-fold cut-off (red dash line) were collected. n = 3 independent experiments. For a-c, statistical analysis was performed using ordinary one-way ANOVA followed by Dunnett’s multiple-comparison test. NS, not significant. Data are mean ± s.e.m.
Next, to test the feasibility of measuring KINACT responses using an automated multi-well plate reader and thus facilitate high-throughput compound library screening, we performed a pilot experiment by measuring the responses of A-KINACT to a small panel of activators or inhibitors and found that A-KINACT provides a sensitive readout regardless of the detection modality (Supplementary Fig. 9). We then screened a library containing 160 known kinase inhibitors, which were applied to A-KINACT-stable HEK293T cells alongside 5 μM Fsk, as well as blue light illumination for 20 min. Screening on a plate reader revealed 11 inhibitors that diminished Fsk-stimulated induction of PKA activity by more than 20% (Fig. 4d and Supplementary Table 1). These potential PKA inhibitors included three AKT inhibitors, two Chk1 inhibitors, one PKC inhibitor, one ATR inhibitor, one PKD inhibitor, one AXL family inhibitor, one ETK/BMX inhibitor, and one broad-spectrum kinase inhibitor. Since most of these compounds show some intrinsic fluorescence, we chose to use a pair of red-shifted, real-time PKA biosensors, GR-AKARev14 and Booster-PKA15, to validate the inhibitory effects of these candidates. Of the 11 compounds, 10 showed inhibition of PKA activation by the maximum dose of F/I, largely in agreement with the A-KINACT data (Supplementary Fig. 10). One compound (1B3), which just cleared the cut-off in the A-KINACT results, showed a weak inhibitory effect in validation experiments but was not statistically significant. These data demonstrate the utility of A-KINACT in compound screens and further confirm the promiscuity of kinase inhibitors16.
Aside from inhibitors, identifying novel PKA activators can potentially enhance the therapeutic arsenal against cardiovascular17 and neurological diseases, while also helping screen out potential anti-cancer drug candidates that may produce adverse effects through unwanted PKA activation, thus creating a more effective therapeutic pipeline18. Marine natural products are often heralded as a promising reservoir for drug discovery. We therefore screened a collection of 137 marine natural products using A-KINACT and identified 14 compounds that led to an at least 1.5-fold induction of PKA activity relative to the basal signal (Fig. 4e and Supplementary Table 2). Among these, we took particular note of four interesting compounds that suggest the modulation of PKA activity by certain key pathways. For instance, Psammaplin A, derived from a marine sponge, inhibits class I histone deacetylase (HDAC1)19; Kalkitoxin induces cellular hypoxia by inhibiting the electron transport chain (ETC) complex 120; and Crossbyanol B and (7Z,9Z,12Z)-octadeca-7,9,12-trien-5-ynoic acid were reported as inhibitors of Cathepsin B21. We verified these compounds using real-time imaging in HEK293T cells expressing a sensitive PKA activity biosensor, AKAR3ev22, and indeed observed definite bioactivities toward PKA within 20 min of drug addition (Supplementary Fig. 11). Taken together, our screening results confirm that the KINACT system supports high-throughput functional compound screening with high sensitivity and reliability.
Applying KINACT to dissect oncogenic G protein signaling.
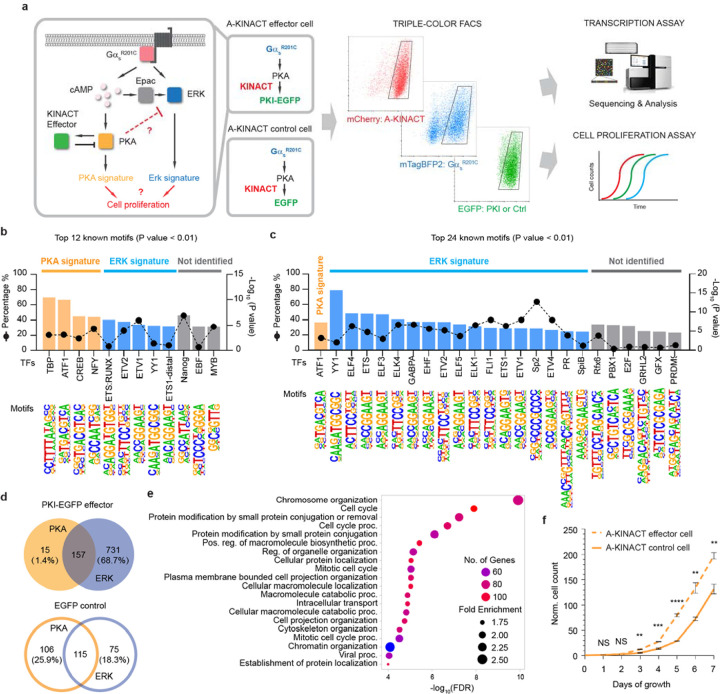
The stimulatory G protein α subunit (Gαs) regulates a variety of cell functions23, 24. In recent years, activating mutations in the Gαs-encoding gene GNAS have been detected in multiple cancer types, such as pancreatic and colorectal cancers25–27. The oncogenic Gαs R201C mutation leads to high PKA activity, which can be further stimulated via GPCR activation28. PKA is capable of triggering signaling cascades and regulating transcription of genes critical for cell proliferation. We recently discovered that Gαs can play an essential role in GPCR-mediated activation of the ERK pathway, which is known to play a critical role in regulating gene transcription and cell proliferation29. Given the complex crosstalk observed between the PKA and ERK signaling pathways30, it is challenging to dissect how GαsR201C reorganizes the PKA and ERK signaling networks to impact tumorigenesis, as well as the specific contributions of PKA versus ERK pathways. We therefore leveraged the innate ability of KINACT to translate cellular kinase activity into specific protein expression to engineer a KINACT effector that will exert negative-feedback control over dysregulated PKA signaling by GαsR201C-expressing (GαsR201C+) cells and thus allow more precise dissection of this oncogenic signaling network than would otherwise be possible. We generated two dual-stable cell lines: one A-KINACT effector cell line stably expressing A-KINACT and integrating TetO promoted EGFP-fused PKA inhibitory peptide (TetO-PKI-EGFP), and one control cell line stably expressing A-KINACT and integrating TetO-EGFP. Characterization of both revealed efficient responses to blue light illumination and PKA activator treatment, or GαsR201C-mediated high PKA activity (Supplementary Fig. 12). The A-KINACT effector memorizes elevated PKA activity and induces the expression of PKI-EGFP to inhibit PKA activity, which should perturb the balance between PKA and ERK signaling. A-KINACT-expressing HEK293T cells showing mTagBFP2-GαsR201C overexpression and A-KINACT-driven PKI-EGFP expression were identified and collected via triple-color fluorescence-activated cell sorting (FACS, Supplementary Fig. 13) to assess differences in transcription and cell proliferation versus control cells expressing A-KINACT-driven EGFP (Fig. 5a).
Figure 5 |. A-KINACT effector diverts GαsR201C-PKA signaling toward the ERK pathway.
a, Design of A-KINACT effector for manipulating GαsR201C-mediated PKA activity and scheme for identifying transcriptional and phenotypic changes. b, Top 12 enriched known TF motifs among 410 upregulated DEGs from light-induced A-KINACT control (EGFP) cells overexpressing GαsR201C. c, Top 24 enriched known TF motifs among 1064 upregulated DEGs from light-induced A-KINACT effector (PKI-EGFP) cells overexpressing GαsR201C. d, Venn diagrams comparing the PKA signature versus ERK signature from A-KINACT effector cells (upper) and A-KINACT control cells (lower). e, GO enrichment analysis of 731 ERK-upregulated DEGs for biological processes. f, Growth curves of light-reduced A-KINACT effector cells (dashed line) and A-KINACT control cells (solid line) with GαsR201C overexpression. n=3 independent experiments. P=0.005 (day 3), P=0.00099 (day 4), P=0.00007 (day 5), P=0.0042 (day 6) and P=0.0043 (day 7). Statistical analyses were performed using unpaired t tests. Data are mean ± s.e.m.
Analysis of differentially expressed genes (DEGs) from RNAseq data identified 1064 DEGs (Supplementary Table 3) that were upregulated in GαsR201C+/PKI-EGFP+ cells compared with GαsR201C−/PKI-EGFP− cells, whereas 410 DEGs (Supplementary Table 4) were upregulated in GαsR201C+/EGFP+ cells compared with GαsR201C−/EGFP− cells (Supplementary Fig. 14). Transcription factor (TF) motif enrichment analysis indicated that DEGs upregulated by GαsR201C contained promoter motifs that are predicted to bind PKA-activated TFs (e.g., TBP, ATF1, CREB and NFY31–34) or ERK-activated TFs (e.g., ETS:RUNX, ETV2, ETV1, ETS1-distal and YY135) (Fig. 5b). As expected, we observed that PKA-activated TFs were reduced (only ATF1) in cells where PKI negative feedback was activated. Strikingly, in the presence of the PKI negative feedback, many more ETS-family TFs, as well as YY1, sp2 and PR36, which are reported to be ERK effectors, were upregulated by GαsR201C (Fig. 5c). Looking into genes that were upregulated in PKI-expressing cells, we found that only 15 out of 172 ATF1-associated genes (1.4% of total DEGs) were regulated only by PKA, indicating that A-KINACT-mediated PKI induction robustly inhibited PKA activity. However, 731 genes (68.7%) were predicted to be upregulated by ERK alone (Fig. 5d). Conversely, in EGFP control cells, 106 genes (25.9%) were predicted to be upregulated by PKA, and 75 genes (18.3%) were predicted to be activated by ERK (Fig. 5d). Additionally, analyzing the 106 PKA-upregulated genes from GαsR201C cells with high PKA activity (i.e., EGFP only) highlighted genes that are known to negatively regulate the ERK cascade, such as ATF3, DUSP1, DUSP4 and DUSP10 (Supplementary Fig. 15). These findings suggest that GαsR201C upregulates both PKA- and ERK-mediated transcription and yet PKA activity antagonizes ERK signaling and suppresses the ERK-regulated transcriptome.
GO enrichment analysis of the 731 ERK-activated genes identified in PKI cells revealed many biological functions, including cell cycle modulation (Fig. 5e). Given the important role of ERK in cell proliferation, we hypothesize that normalizing PKA activity in GαsR201C cells with PKA-activity-driven feedback inhibition will effectively relieve the inhibition on the ERK pathway and further enhance ERK-mediated cell proliferation. To test this hypothesis, we performed a cell proliferation assay by counting cells for 7 days. Indeed, effector PKI-expressing cells showed enhanced proliferation compared with EGFP control cells (Fig. 5f), while no difference in cell growth rate was observed between the two conditions without light induction (Supplementary Fig. 16). Thus, GαsR201C is capable of strongly activating the ERK pathway, which can directly contribute to increased cell proliferation. This hyperactive ERK signaling is fortuitously suppressed by activated PKA, inhibition of which leads to further boosting of the pro-proliferative ERK signaling. These findings provide critical insights into the signaling effects of an oncogenic mutation that influences a key signaling network and have important implications for therapeutic interventions.
Discussion
Activity integrators were first conceived to address the challenges of using real-time biosensors to perform large-scale recording of neuronal activity to identify functional neural circuits in the brain2. While the earliest of these activity integrators were indeed developed and implemented to record Ca2+ (i.e., neuronal activity)3, 4 and neuromodulator dynamics5, the utility of these tools extends beyond neuroscience, leading to their generalization to monitor processes such as protein-protein interactions between cell-surface receptors and intracellular adaptors7, 37 and cell-cell contacts38. Here, we further demonstrate the generality of this powerful concept by reporting the development of KINACT, the first integrator for recording protein kinase activity. KINACT incorporates a dual, optical- and biochemical-switch design to enable recording of kinase activity during precisely defined windows. We show that KINACT is capable of sensitively and specifically memorizing changes in kinase activity in response to diverse stimuli and at different subcellular locations. Because its design features the same kinase-inducible switch, based on the interaction of a kinase-specific substrate and PAABD, found in real-time kinase sensors6, 11, 12, KINACT is also readily adapted to probe the activities of a multitude of kinases, as we demonstrate with the Ser/Thr kinases PKA (A-KINACT) and PKC (C-KINACT), as well as the Tyr kinase Fyn (F-KINACT). KINACT thus marks a substantial addition to the growing toolkit of activity integrators.
KINACT offers several notable advantages over real-time kinase sensors. First, KINACT accumulates activity signals over time, which are further amplified by the transcriptional readout. We believe that this feature creates new opportunities for robust and sensitive drug discovery of previously unknown kinase activators or inhibitors. Indeed, using A-KINACT, we were able to identify a number of kinase inhibitors with potential inhibitory effects against PKA. Eight of these inhibitors are reported to function as ATP-competitive inhibitors; thus, it is not surprising that they exhibit cross-activity with other kinases. Notably, Afuresertib (Akt) and Staurosporine (multiple kinases) inhibited PKA activity even more potently than H89, hinting at the need to exercise caution when applying these inhibitors in cells. On the other hand, the fact that three non-ATP-competitive inhibitors, namely, Miransertib (Akt), CRT0066101 (PKD) and AG879 (ETK/BMX), were also able to inhibit PKA activity may indicate the crosstalk between these kinases and PKA signaling. More excitingly, we discovered several novel PKA activators among a panel of marine natural products. Psammaplin A is known to inhibit class I histone deacetylase (HDAC1). A broad-spectrum HDAC inhibitor, Trichostatin A (TSA), has been reported to prevent the deacetylation of PKA catalytic subunits, thereby increasing PKA activity39. Future work will determine whether Psammaplin A modulates PKA activity in a similar fashion. In addition, treating cells with the ETC complex 1 inhibitor Kalkitoxin led to a robust induction of PKA activity within 20 min, suggesting a distinct, rapid pathway linking respiratory chain disruption to PKA signaling. Interestingly, two Cathepsin B inhibitors, Crossbyanol B and (7Z,9Z,12Z)-octadeca-7,9,12-trien-5-ynoic acid, were both identified using A-KINACT, implying that the molecular functions of Cathepsin B, such as the proteolysis of extracellular matrix components and disruption of intercellular communication, may modulate PKA activity. Complementing the high sensitivity, another advantage of KINACT is flexibility of the readout options. In addition to fluorescent proteins, the reporter can be easily substituted with luciferase37 or secreted alkaline phosphatase (SEAP)5. The latter two modalities would be more ideally suited for compound screening given the intrinsic fluorescence of many drug-like molecules.
Another major advantage of the KINACT system is that it allows post hoc analysis of kinase-activity-marked cell populations. Characterizing kinase activities in the complex tumor microenvironment is challenging yet critical for understanding the roles of various kinases in tumor-stroma-immune interactions, and guiding kinase-targeting therapeutics. KINACT provides a platform for addressing this challenge and for studying the heterogeneity of kinase activity within large tissues, such as tumors. Using this capability, we observed upregulated basal PKA activity in 3D versus 2D cultures. Future studies will investigate the underlying mechanisms, with the mechano-sensitivity of the PKA pathway as a possible avenue40.
Finally, the KINACT system offers the capacity for kinase-activity-driven manipulation of targeted cell populations. Using this capability, we engineered a feedback circuit to use the recorded high PKA activity to drive the expression of a PKA inhibitor to “normalize” cellular PKA activity levels. This circuit allowed us to dissect the functional effects of the oncogenic GαsR201C mutant via ERK signaling, which is antagonized by PKA. PKA has been shown to inhibit the ERK pathway in multiple ways, such as blocking Ras-dependent signals to ERK by blocking Raf-1 activation41, 42, or indirectly regulating EPAC through a feedback loop controlling the production and degradation of cAMP43, 44. We found that ATF3, DUSP1, DUSP4 and DUSP10 were upregulated by PKA, which could be involved in dephosphorylation and inactivation of the ERK family. This finding provides further insight into the interactions between PKA and ERK signaling pathways. It is worth noting that A-KINACT activates a feedback loop that exerts an inhibitory effect only in cells with high PKA activity. PKA activity levels are thus normalized to a relatively uniform level between cells, minimizing functional differences, which is difficult to achieve by simply overexpressing PKI. A previous study has shown that GαsR201C signals through a PKA-Salt Inducible Kinase (SIK) pathway to initiate pancreatic ductal adenocarcinoma27. Although PKA can promote tumorigenesis45, we show that neutralizing PKA activity can also increase cell proliferation by relieving inhibition of ERK signaling also induced by hyperactive GαsR201C. Similarly, a recent study showed blocking PKA signaling in mutant Gαs cells resulted in tumor aggression46. Thus, signaling activities driven by oncogenic mutations can be complex, and using KINACTs to advance our understanding of both the complete repertoire of signaling activities driven by oncogenic mutations and the complex cross-regulation between the major players would be critical for developing effective therapeutic strategies.
ONLINE METHODS
Generating KINACT.
A-KINACT components 1 and 2 were cloned based on blue-light-inducible TEV protease (BLITz) constructs (ref. 5) by Gibson assembly. Briefly, to generate A-KINACT constructs 1–5, CIBN in BLITz component 1 was replaced with a PKA substrate sequence (LRRATLVD). For constructs 1–3, hLOV1 domain-tethered TEVseq-G, TEVseq-P or TEVseq-M were introduced to replace the iLID-TEVseq-G in BLITz component 1. For construct 5, eLOV-TEVseq-M amplified by PCR from FLARE (ref. 4) was used to replace iLID-TEVseq-G. For construct 6, TEVp-N was removed from construct 1. To generate A-KINACT construct 7, CRY2PHR in BLITz component 2 was replaced with an FHA1 domain. A-KINACT construct 8 was generated based on construct 7 by replacing TEVp-C with full-length TEVp. Mitochondrial outer membrane-targeted A-KINACT component 1 was generated by replacing the PDGFβ transmembrane helix with the N-terminal 30 amino acids from DAKAP1(MAIQLRSLFPLALPGMALLGWWWFFSRKK). C-KINACT and F-KINACT were generated based on A-KINACT by replacing the PKA substrate with PKC or Fyn substrates, respectively, as well as replacing FHA1 with SH2 domain (for F-KINACT). Non-phosphorylatable negative-control substrate mutants were generated by PCR. Polycistronic A-KINACT, C-KINACT and F-KINACT constructs were assembled following the order component 1-P2A-mCherry-IRES-component 2. Lentiviral backbones containing Bleomycin (FUGW, Addgene #14883) or Hygromycin (w117–1, Addgene #17454) resistance genes were used to generate lentiviral constructs of A-KINACT and A-KINACT (T/A). The TetO-H2B-EGFP and TetO-PKI-EGFP reporter constructs were cloned by replacing EGFP in pTRE-EGFP (Addgene # 89871) with H2B-EGFP and PKI-EGFP, respectively, via Gibson assembly. A lentiviral backbone containing a Puromycin (w118–1, Addgene #17452) resistance gene was used to generate lentiviral reporter constructs. The DNA or protein sequences of all constructs are provided in the Supplement.
Other plasmids.
ExRai-AKAR29, GR-AKARev (ref. 14), Booster-PKA (ref. 15), and AKAR3ev (ref. 22) were described previously. mTagBFP2-GNAS(R201C) was generated in a pcDNA3 backbone by Gibson assembly. All constructs were verified by Sanger sequencing (Genewiz).
Cell culture and transfection.
HEK293T and HeLa cells were cultured in Dulbecco’s modified Eagle medium (DMEM; Gibco) and supplemented with 10% (v/v) fetal bovine serum (FBS, Sigma) and 1% (v/v) penicillin-streptomycin (Pen-Strep, Sigma-Aldrich). All cells were maintained at 37 °C in a humidified atmosphere of 5% CO2. For imaging experiments, cells were plated onto sterile 35-mm glass-bottomed dishes (Cellvis) and grown to 50% confluence. Transient transfection was performed using PolyJet (SignaGen) for HEK cells or Lipofectamine 2000 (Invitrogen) for HeLa cells, after which cells were cultured for an additional 24 h before blue light illumination or imaging.
Stable cell lines.
To package the lentivirus containing TetO-H2B-EGFP, TetO-EGFP, TetO-PKI-EGFP or A-KINACT, each pLenti-construct was mixed with psPAX2 and pMD2.G-VSV-g (3:2:1) and co-transfected into HEK293T cells. The medium containing packaged lentivirus was collected after 48 h and then concentrated using Lenti-X™ Concentrator (Clontech). To generate TetO-H2B-EGFP, TetO-EGFP and TetO-PKI-EGFP stable cell lines, concentrated solution containing lentiviral particles was added to HEK293T or HeLa cells. After 48 h, cells were diluted and plated in 10-cm cell culture dishes and then treated with Puromycin (1 μg ml−1) to select transduced cells. Single colonies were picked after a week and characterized by transfecting tTA to induce TetO-promoted gene expression. To generate A-KINACT or A-KINACT (T/A) plus TetO-gene dual-stable cell lines, concentrated lentiviral particles containing A-KINACT or A-KINACT (T/A) were added to established TetO-H2B-EGFP, TetO-EGFP and TetO-PKI-EGFP stable HEK293T cells. A-KINACT+TetO-H2B-EGFP dual-stable cells were selected with Puromycin (2 μg ml−1) and Zeocin (Invitrogen, 300 μg ml−1). A-KINACT (T/A)+TetO-H2B-EGFP, A-KINACT+TetO-EGFP and A-KINACT+TetO-PKI-EGFP dual-stable cells were selected with Puromycin (1 μg ml−1) and Hygromycin (Invitrogen, 150 μg ml−1). Single colonies were picked after a week and characterized by Fsk/IBMX and blue light treatment to induce reporter gene expression.
Blue-light illumination, snapshot imaging and image analysis.
Blue-light illumination was performed using a 465-nm-wavelength blue LED array controlled by a high-accuracy electronic timer (GraLab, model 451). Glass-bottom 35-mm dishes or 96-well plates were raised 1.5 cm above the LED source to avoid potential undesirable heating caused by direct contact with the LED. After 24-h expression, cells were stained with Hoechst 33342 for 20 min at 37 °C and washed once with Hanks’s balanced salt solution (HBSS, Gibco) prior to imaging. Cells were imaged in HBSS on a Zeiss AxioObserver Z1 microscope equipped with a 20×/0.8 NA objective and a Hamamatsu Flash 4.0 sCMOS camera, controlled using a modified version of the open-source MATscope suite47 implemented in MATLAB 2017a (Mathworks Inc.). Hoechst 33342 was imaged using a 420DF20 excitation filter, a 455DRLP dichroic mirror, and a 470DF40 emission filter. EGFP was imaged using a 495DF10 excitation filter, a 515DRLP dichroic mirror, and a 535DF25 emission filter. mCherry was imaged using a 568DF55 excitation filter, a 600DRLP dichroic mirror, and a 653DF95 emission filter.
Images were processed using Cell Profiler and segmented using DAPI channel images. To calculate normalized total EGFP fluorescence for each condition, the integrated EGFP intensity of all H2B-EGFP+ cells in a well was summed and then divided by the number of mCherry+ (i.e., KINACT-expressing) cells. To calculate the G/R ratio of individual cells, the mean EGFP intensity of each H2B-EGFP+ cell was divided by its own mean mCherry intensity.
HEK293T organoid culture and 3D imaging.
Matrigel (Growth Factor Reduced Matrigel, BD Biosciences) was plated onto 35-mm glass-bottom dishes and allowed to solidify for 30 min at 37°C. Dual-stable HEK293T cells were trypsinized, resuspended in media supplemented with 2% Matrigel and then plated on top of Matrigel. After 5 days in culture, organoids were induced by blue light for 20 min and cultured for another 24 h before imaging. Z-stack imaging was performed on a Leica SP8 confocal equipped with a 20×/0.75 NA objective over a total range of 60 μm (3-μm step per Z-position). The 405 nm, 488 nm and 561 nm laser bands were chosen for excitation of DAPI, H2B-EGFP and mCherry, respectively. DAPI, H2B-EGFP and mCherry emission were collected using 420–460 nm band-pass, 500–540 nm band-pass and 600–650 nm band-pass filters, respectively. 3D images were reconstructed using Leica software LAS X.
Fluorescence microplate screening.
Black-walled, glass-bottom 96-well assay plates (Costar) were coated with 0.1 mg ml−1 of poly-D-lysine in boric acid buffer (pH 8.5) for 30 min at 37°C and then seeded with 5 × 104 A-KINACT+TetO-H2B-EGFP dual-stable HEK293T cells per well for 24-h culturing. For PKA inhibitor screening, cells were treated with kinase inhibitor library (Cayman chemical, #10505–0577971, 10 μM each drug) and blue light for 20 min (10 s on/50 s off), followed by 24-h expression. For PKA activator screening, cells were treated with marine natural products (1 μg ml−1 per drug) and blue light for 20 min (10 s on/50 s off), followed by 24-h expression.
Fluorescence intensity was read on a Spark 20M fluorescence plate reader using SparkControl Magellan 1.2.20 software (TECAN). Cells were washed once and then placed in HBSS to acquire an RFP-GFP sequential read. mCherry was read at 555-nm excitation/640-nm emission and EGFP was read at 485-nm excitation/535-nm emission. Five regions were read per well. Raw green and red fluorescence values from each region were corrected by subtracting the background fluorescence intensity of a nontransfected well and then averaged. G/R ratio in each well was calculated by dividing the average EGFP intensity by the average mCherry intensity. Three replicates were performed.
Time-lapse epifluorescence imaging.
HEK293T cells expressing ExRai-AKAR2, GR-AKARev, Booster-PKA or AKAR3ev were washed twice with HBSS and subsequently imaged in HBSS in the dark at RT. Forskolin, IBMX, H89, LPA, Angiotensin II (Ang II), ATP, endothelin 1 (ET-1), Histamine (His), 11 screened kinase inhibitors and 4 selected marine natural products were added as indicated. Cells were imaged on a Zeiss AxioObserver Z1 microscope (Carl Zeiss) equipped with a 40×/1.3 NA objective and a Photometrics Evolve 512 EMCCD (Photometrics) controlled by METAFLUOR 7.7 software (Molecular Devices). For ExRai-AKAR2, dual GFP excitation-ratio imaging was performed using 480DF30 and 405DF40 excitation filters, a 505DRLP dichroic mirror and a 535DF45 emission filter. For GR-AKARev, dual green/red emission ratio imaging was performed using a 480DF30 excitation filter, a 505DRLP dichroic mirror and two emission filters (535DF45 for green fluorescent protein and ET605DF52 for red fluorescent protein). For Booster-PKA, dual orange/red emission ratio imaging was performed using a 555DF25 excitation filter, a 568DRLP dichroic mirror and two emission filters (605DF52 for orange fluorescent protein and 650DF100 for red fluorescent protein). For AKAR3ev, dual cyan/yellow emission ratio imaging was performed using a 420DF20 excitation filter, a 450DRLP dichroic mirror and two emission filters (475DF40 for cyan fluorescent protein and 535DF25 for yellow fluorescent protein). Filter sets were alternated by a Lambda 10–2 filter-changer (Sutter Instruments). Exposure times ranged between 50 and 500 ms, with EM gain 10, and images were acquired every 30 s. For the time-series analysis, all values were normalized to the time point prior to drug addition by dividing each value by the basal ratio value prior to drug addition.
Triple-color cell sorting by flow cytometry.
A-KINACT-PKI-EGFP induced cells and A-KINACT-EGFP control cells were collected and washed once with 1×DPBS, resuspended in HBSS buffer containing 1% FBS and strained (FALCON) to prevent clogging. Both A-KINACT-PKI-EGFP and A-KINACT-EGFP cells were sorted for mTagBFP2 at Ex. 405 nm (FL1 450/50 emission filter), EGFP at Ex. 488 nm (FL2 525/50 emission filter) and mCherry at Ex. 560 nm (FL3 600/60 emission filter) on a SONY SH800S Cell Sorter running Cell Sorter Software (Ver 2.1.6). Debris, dead cells, and cell aggregates were gated out before fluorescence interrogation by monitoring the forward and side scatter (Supplementary Fig. 13). Approximately 500,000 single cells were collected for each sample. Sorting was repeated three times.
RNA-sequencing and data analysis.
Total RNA from one aliquot of sorted cells was extracted using an RNA extraction kit (Zymol), and 20 μl of 50 ng μl−1 sample was sent to Novogene following the company’s instruction. Samples that passed quality control were proceeded to paired-end mRNA sequencing. Raw data were analyzed using the web-based platform Galaxy (https://usegalaxy.org) and open-source R software (version 4.1.0, https://www.r-project.org). Briefly, high-quality raw reads were mapped to the reference human genome hg38 using HISAT2 (version 2.2.1), and aligned reads were counted using htseq-count (version 0.9.1). The R package DESeq2 (version 1.34.0) was used to conduct differentially expressed gene analysis. Genes with fold change (> 2) and p-adj < 0.05 were assigned as significantly altered genes. Within each set of differentially expressed genes, downregulated and upregulated genes were separately subjected to motif enrichment analysis using Homer (version 4.11). Gene ontology (GO) enrichment analysis was performed using the online tool ShinyGO 0.77 (http://bioinformatics.sdstate.edu/go).
Cell proliferation assay.
One aliquot of the sorted cells from FACS was seeded in 12-well plates at 10,000 cells per well (Day 0). Cell numbers were quantified using a Countess II cell counter (Life Technologies) each day for 7 days.
Statistics and reproducibility.
All experiments were independently repeated as noted in the figure legends. Statistical analyses were performed using GraphPad Prism 8. For Gaussian data, pairwise comparison of two parametric data sets was performed using Student’s t test. For comparing three or more sets of data, ordinary one-way ANOVA followed by the indicated multiple comparisons test was done. Statistical significance was defined as P < 0.05 with a 95% confidence interval.
Supplementary Material
Acknowledgments
The authors are grateful to all members of the Zhang lab for various technical assistance and thoughtful discussions. We especially thank Dr Eric. C. Greenwald (now at Pfizer) for assembling the LED blue light array. We also wish to thank Dr. Jason Z. Zhang (now at U of Washington) for generating mTagBFP2-GNAS(R201C) and Dr. Evgenia Glukhov for preparing marine natural products library. Work in J.Z.’s laboratory is supported by the National Institutes of Health (NIH) (R35 CA197622, R01 DK073368 and R01 CA262815). Work in Y.W.’s laboratory is supported by the National Institutes of Health (R01 EB029122, R35 GM140929, R01 HD107206 and R01 CA262815). The MNP library from W.H.G.’s laboratory is supported by the National Institutions of Health (R01 GM107550) and the Chancellor of UC San Diego. This work is also supported by the UCSD Microscopy Core (P30 NS047101).
Footnotes
Competing Interests
The authors declare no competing interests.
Code availability
Custom MATLAB code used for image acquisition and analysis is available at https://github.com/jinzhanglab-ucsd/MatScopeSuite (https://doi.org/10.5281/zenodo.5908008).
Data availability
All data supporting the findings of this study are available upon reasonable request. Source data are provided with this paper.
References
- 1.Greenwald E.C., Mehta S. & Zhang J. Genetically Encoded Fluorescent Biosensors Illuminate the Spatiotemporal Regulation of Signaling Networks. Chem. Rev. 118, 11707–11794 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsien R.Y. Very long-term memories may be stored in the pattern of holes in the perineuronal net. Proc. Natl. Acad. Sci. U. S. A. 110, 12456–12461 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee D., Hyun J.H., Jung K., Hannan P. & Kwon H.B. A calcium- and light-gated switch to induce gene expression in activated neurons. Nat. Biotechnol. 35, 858–863 (2017). [DOI] [PubMed] [Google Scholar]
- 4.Wang W. et al. A light- and calcium-gated transcription factor for imaging and manipulating activated neurons. Nat. Biotechnol. 35, 864–871 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee D. et al. Temporally precise labeling and control of neuromodulatory circuits in the mammalian brain. Nat. Methods 14, 495–503 (2017). [DOI] [PubMed] [Google Scholar]
- 6.Zhang J., Hupfeld C.J., Taylor S.S., Olefsky J.M. & Tsien R.Y. Insulin disrupts beta-adrenergic signalling to protein kinase A in adipocytes. Nature 437, 569–573 (2005). [DOI] [PubMed] [Google Scholar]
- 7.Kim M.W. et al. Time-gated detection of protein-protein interactions with transcriptional readout. Elife 6, e30233 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Copik A.J. et al. Isoproterenol acts as a biased agonist of the alpha-1A-adrenoceptor that selectively activates the MAPK/ERK pathway. PLoS ONE 10, e0115701 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang J.F. et al. An ultrasensitive biosensor for high-resolution kinase activity imaging in awake mice. Nat. Chem. Biol. 17, 39–46 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greenwald E. et al. GPCR Signaling Measurement and Drug Profiling with an Automated Live-Cell Microscopy System. ACS Sens 8, 19–27 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herbst K.J., Allen M.D. & Zhang J. Luminescent kinase activity biosensors based on a versatile bimolecular switch. J. Am. Chem. Soc. 133, 5676–5679 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ouyang M. et al. Sensitive FRET Biosensor Reveals Fyn Kinase Regulation by Submembrane Localization. ACS Sens 4, 76–86 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Y. et al. Endogenous Galphaq-Coupled Neuromodulator Receptors Activate Protein Kinase A. Neuron 96, 1070–1083.e5 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mo G.C.H., Posner C., Rodriguez E.A., Sun T. & Zhang J. A rationally enhanced red fluorescent protein expands the utility of FRET biosensors. Nat. Commun. 11, 1848 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Watabe T., Terai K., Sumiyama K. & Matsuda M. Booster, a Red-Shifted Genetically Encoded Forster Resonance Energy Transfer (FRET) Biosensor Compatible with Cyan Fluorescent Protein/Yellow Fluorescent Protein-Based FRET Biosensors and Blue Light-Responsive Optogenetic Tools. ACS Sens 5, 719–730 (2020). [DOI] [PubMed] [Google Scholar]
- 16.Hanson S.M. et al. What Makes a Kinase Promiscuous for Inhibitors? Cell Chemical Biology 26, 390–399.e5 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Y., Chen J., Fontes S.K., Bautista E.N. & Cheng Z. Physiological and pathological roles of protein kinase A in the heart. Cardiovasc. Res. 118, 386–398 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rukoyatkina N. et al. Protein kinase A activation by the anti-cancer drugs ABT-737 and thymoquinone is caspase-3-dependent and correlates with platelet inhibition and apoptosis. Cell Death Dis 8, e2898 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim D.H., Shin J. & Kwon H.J. Psammaplin A is a natural prodrug that inhibits class I histone deacetylase. Exp. Mol. Med. 39, 47–55 (2007). [DOI] [PubMed] [Google Scholar]
- 20.Morgan J.B. et al. Kalkitoxin inhibits angiogenesis, disrupts cellular hypoxic signaling, and blocks mitochondrial electron transport in tumor cells. Mar. Drugs 13, 1552–1568 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Phan V.V. et al. Discovery of pH-Selective Marine and Plant Natural Product Inhibitors of Cathepsin B Revealed by Screening at Acidic and Neutral pH Conditions. ACS Omega 7, 25346–25352 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Komatsu N. et al. Development of an optimized backbone of FRET biosensors for kinases and GTPases. Mol. Biol. Cell 22, 4647–4656 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dorsam R.T. & Gutkind J.S. G-protein-coupled receptors and cancer. Nat. Rev. Cancer 7, 79–94 (2007). [DOI] [PubMed] [Google Scholar]
- 24.Neves S.R., Ram P.T. & Iyengar R. G protein pathways. Science 296, 1636–1639 (2002). [DOI] [PubMed] [Google Scholar]
- 25.O’Hayre M. et al. The emerging mutational landscape of G proteins and G-protein-coupled receptors in cancer. Nat. Rev. Cancer 13, 412–424 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu J. et al. Recurrent GNAS mutations define an unexpected pathway for pancreatic cyst development. Sci Transl Med 3, 92ra66 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patra K.C. et al. Mutant GNAS drives pancreatic tumourigenesis by inducing PKA-mediated SIK suppression and reprogramming lipid metabolism. Nat. Cell Biol. 20, 811–822 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wheeler E.C. et al. Integrative RNA-omics Discovers GNAS Alternative Splicing as a Phenotypic Driver of Splicing Factor-Mutant Neoplasms. Cancer Discov 12, 836–855 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kwon Y. et al. Non-canonical beta-adrenergic activation of ERK at endosomes. Nature 611, 173–179 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stork P.J.S. & Schmitt J.M. Crosstalk between cAMP and MAP kinase signaling in the regulation of cell proliferation. Trends Cell Biol. 12, 258–266 (2002). [DOI] [PubMed] [Google Scholar]
- 31.Cluck M.W., Murphy L.O., Olson J., Knezetic J.A. & Adrian T.E. Amylin gene expression mediated by cAMP/PKA and transcription factors HNF-1 and NFY. Mol. Cell. Endocrinol. 210, 63–75 (2003). [DOI] [PubMed] [Google Scholar]
- 32.Kinane T.B., Shang C., Finder J.D. & Ercolani L. cAMP regulates G-protein alpha i-2 subunit gene transcription in polarized LLC-PK1 cells by induction of a CCAAT box nuclear binding factor. J. Biol. Chem. 268, 24669–24676 (1993). [PubMed] [Google Scholar]
- 33.Baler R., Covington S. & Klein D.C. The rat arylalkylamine N-acetyltransferase gene promoter. cAMP activation via a cAMP-responsive element-CCAAT complex. J. Biol. Chem. 272, 6979–6985 (1997). [DOI] [PubMed] [Google Scholar]
- 34.Cote F. et al. Involvement of NF-Y and Sp1 in basal and cAMP-stimulated transcriptional activation of the tryptophan hydroxylase (TPH ) gene in the pineal gland. J. Neurochem. 81, 673–685 (2002). [DOI] [PubMed] [Google Scholar]
- 35.Zheng H., Chu J., Zeng Y., Loh H.H. & Law P.Y. Yin Yang 1 phosphorylation contributes to the differential effects of mu-opioid receptor agonists on microRNA-190 expression. J. Biol. Chem. 285, 21994–22002 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zassadowski F., Rochette-Egly C., Chomienne C. & Cassinat B. Regulation of the transcriptional activity of nuclear receptors by the MEK/ERK1/2 pathway. Cell. Signal. 24, 2369–2377 (2012). [DOI] [PubMed] [Google Scholar]
- 37.Kim C.K., Cho K.F., Kim M.W. & Ting A.Y. Luciferase-LOV BRET enables versatile and specific transcriptional readout of cellular protein-protein interactions. Elife 8, e43826 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cho K.F. et al. A light-gated transcriptional recorder for detecting cell-cell contacts. Elife 11, e70881 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Y. et al. Protein acetylation derepresses Serotonin Synthesis to potentiate Pancreatic Beta-Cell Function through HDAC1-PKA-Tph1 signaling. Theranostics 10, 7351–7368 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McKenzie A.J., Svec K.V., Williams T.F. & Howe A.K. Protein kinase A activity is regulated by actomyosin contractility during cell migration and is required for durotaxis. Mol. Biol. Cell 31, 45–58 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cook S.J. & Mccormick F. Inhibition by Camp of Ras-Dependent Activation of Raf. Science 262, 1069–1072 (1993). [DOI] [PubMed] [Google Scholar]
- 42.Schmitt J.M. & Stork P.J.S. Cyclic AMP-mediated inhibition of cell growth requires the small G protein Rap1. Mol. Cell. Biol. 21, 3671–3683 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iwami G. et al. Regulation of Adenylyl-Cyclase by Protein-Kinase-A. J. Biol. Chem. 270, 12481–12484 (1995). [DOI] [PubMed] [Google Scholar]
- 44.Bauman A.L. et al. Dynamic regulation of cAMP synthesis through anchored PKA-Adenylyl cyclase V/VI complexes. Mol. Cell 23, 925–931 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ramms D.J. et al. Galphas-Protein Kinase A (PKA) Pathway Signalopathies: The Emerging Genetic Landscape and Therapeutic Potential of Human Diseases Driven by Aberrant Galphas-PKA Signaling. Pharmacol. Rev. 73, 155–197 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hoy J.J., Parra N.S., Park J., Kuhn S. & Iglesias-Bartolome R. Protein kinase A inhibitor proteins (PKIs) divert GPCR-G alpha s-cAMP signaling toward EPAC and ERK activation and are involved in tumor growth. FASEB J. 34, 13900–13917 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schmitt D.L. et al. Spatial regulation of AMPK signaling revealed by a sensitive kinase activity reporter. Nat. Commun. 13, 3856 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data supporting the findings of this study are available upon reasonable request. Source data are provided with this paper.