Abstract
Background
Medically unexplained physical symptoms (MUPS) are physical symptoms for which no adequate medical explanation can be found after proper examination. The presence of MUPS is the key feature of conditions known as 'somatoform disorders'. Various psychological and physical therapies have been developed to treat somatoform disorders and MUPS. Although there are several reviews on non‐pharmacological interventions for somatoform disorders and MUPS, a complete overview of the whole spectrum is missing.
Objectives
To assess the effects of non‐pharmacological interventions for somatoform disorders (specifically somatisation disorder, undifferentiated somatoform disorder, somatoform disorders unspecified, somatoform autonomic dysfunction, pain disorder, and alternative somatoform diagnoses proposed in the literature) and MUPS in adults, in comparison with treatment as usual, waiting list controls, attention placebo, psychological placebo, enhanced or structured care, and other psychological or physical therapies.
Search methods
We searched the Cochrane Depression, Anxiety and Neurosis Review Group's Specialised Register (CCDANCTR) to November 2013. This register includes relevant randomised controlled trials (RCTs) from The Cochrane Library, EMBASE, MEDLINE, and PsycINFO. We ran an additional search on the Cochrane Central Register of Controlled Trials and a cited reference search on the Web of Science. We also searched grey literature, conference proceedings, international trial registers, and relevant systematic reviews.
Selection criteria
We included RCTs and cluster randomised controlled trials which involved adults primarily diagnosed with a somatoform disorder or an alternative diagnostic concept of MUPS, who were assigned to a non‐pharmacological intervention compared with usual care, waiting list controls, attention or psychological placebo, enhanced care, or another psychological or physical therapy intervention, alone or in combination.
Data collection and analysis
Four review authors, working in pairs, conducted data extraction and assessment of risk of bias. We resolved disagreements through discussion or consultation with another review author. We pooled data from studies addressing the same comparison using standardised mean differences (SMD) or risk ratios (RR) and a random‐effects model. Primary outcomes were severity of somatic symptoms and acceptability of treatment.
Main results
We included 21 studies with 2658 randomised participants. All studies assessed the effectiveness of some form of psychological therapy. We found no studies that included physical therapy.
Fourteen studies evaluated forms of cognitive behavioural therapy (CBT); the remainder evaluated behaviour therapies, third‐wave CBT (mindfulness), psychodynamic therapies, and integrative therapy. Fifteen included studies compared the studied psychological therapy with usual care or a waiting list. Five studies compared the intervention to enhanced or structured care. Only one study compared cognitive behavioural therapy with behaviour therapy.
Across the 21 studies, the mean number of sessions ranged from one to 13, over a period of one day to nine months. Duration of follow‐up varied between two weeks and 24 months. Participants were recruited from various healthcare settings and the open population. Duration of symptoms, reported by nine studies, was at least several years, suggesting most participants had chronic symptoms at baseline.
Due to the nature of the intervention, lack of blinding of participants, therapists, and outcome assessors resulted in a high risk of bias on these items for most studies. Eleven studies (52% of studies) reported a loss to follow‐up of more than 20%. For other items, most studies were at low risk of bias. Adverse events were seldom reported.
For all studies comparing some form of psychological therapy with usual care or a waiting list that could be included in the meta‐analysis, the psychological therapy resulted in less severe symptoms at end of treatment (SMD ‐0.34; 95% confidence interval (CI) ‐0.53 to ‐0.16; 10 studies, 1081 analysed participants). This effect was considered small to medium; heterogeneity was moderate and overall quality of the evidence was low. Compared with usual care, psychological therapies resulted in a 7% higher proportion of drop‐outs during treatment (RR acceptability 0.93; 95% CI 0.88 to 0.99; 14 studies, 1644 participants; moderate‐quality evidence). Removing one outlier study reduced the difference to 5%. Results for the subgroup of studies comparing CBT with usual care were similar to those in the whole group.
Five studies (624 analysed participants) assessed symptom severity comparing some psychological therapy with enhanced care, and found no clear evidence of a difference at end of treatment (pooled SMD ‐0.19; 95% CI ‐0.43 to 0.04; considerable heterogeneity; low‐quality evidence). Five studies (679 participants) showed that psychological therapies were somewhat less acceptable in terms of drop‐outs than enhanced care (RR 0.93; 95% CI 0.87 to 1.00; moderate‐quality evidence).
Authors' conclusions
When all psychological therapies included this review were combined they were superior to usual care or waiting list in terms of reduction of symptom severity, but effect sizes were small. As a single treatment, only CBT has been adequately studied to allow tentative conclusions for practice to be drawn. Compared with usual care or waiting list conditions, CBT reduced somatic symptoms, with a small effect and substantial differences in effects between CBT studies. The effects were durable within and after one year of follow‐up. Compared with enhanced or structured care, psychological therapies generally were not more effective for most of the outcomes. Compared with enhanced care, CBT was not more effective. The overall quality of evidence contributing to this review was rated low to moderate.
The intervention groups reported no major harms. However, as most studies did not describe adverse events as an explicit outcome measure, this result has to be interpreted with caution.
An important issue was that all studies in this review included participants who were willing to receive psychological treatment. In daily practice, there is also a substantial proportion of participants not willing to accept psychological treatments for somatoform disorders or MUPS. It is unclear how large this group is and how this influences the relevance of CBT in clinical practice.
The number of studies investigating various treatment modalities (other than CBT) needs to be increased; this is especially relevant for studies concerning physical therapies. Future studies should include participants from a variety of age groups; they should also make efforts to blind outcome assessors and to conduct follow‐up assessments until at least one year after the end of treatment.
Keywords: Adult, Humans, Cognitive Behavioral Therapy, Psychotherapy, Psychotherapy/methods, Randomized Controlled Trials as Topic, Somatoform Disorders, Somatoform Disorders/therapy, Waiting Lists
Plain language summary
Talking therapies and physical therapies for medically unexplained physical symptoms: a review of the evidence
Who may be interested in this review?
People with unexplained physical symptoms (somatoform disorders) and their family and friends.
Professionals working with people with somatoform disorders or working in chronic pain services.
General practitioners.
Why is this review important?
Up to one in three people consulting their doctor about physical symptoms have medically unexplained physical symptoms (MUPS) that have no clear cause. MUPS are a key feature of health problems called somatoform disorders. MUPS and somatoform disorders often cause significant distress and cause people spending a lot of time consulting doctors and health professionals to try to find the cause of their symptoms and the correct treatment.
Talking therapies for MUPS are recommended to help with mental health problems that exist alongside the physical symptoms, and to help people change the way they think about their physical symptoms. Physical therapies for MUPS aim to help people improve their physical functioning through various types of exercise. This review aimed to examine the evidence for talking therapies and physical therapies for MUPS and somatoform disorders.
What questions does this review aim to answer?
What is the quality of current research on talking therapies and physical therapies for MUPS?
Are talking therapies an effective treatment for MUPS compared with usual treatment or waiting list?
Which types of talking therapies are most effective?
Are physical therapies an effective treatment for MUPS?
How acceptable are talking therapies and physical therapies to people with MUPS?
Which studies were included in the review?
We used search databases to find all studies of talking therapies and physical therapies for people with somatoform disorders published to November 2013. To be included in the review, studies had to compare talking therapies or physical therapies with either usual treatment, waiting list, enhanced or structured care (where a doctor offered structured appointments to the person but no specific therapy for MUPS), or other talking or physical therapies. We included studies if they had adults aged over 18 years with a clear diagnosis of somatoform disorders or main presenting problem of MUPS.
We included 21 studies in the review with 2658 participants.
What does the evidence from the review tell us?
We rated the quality of current research as low to moderate. Fourteen out of the 21 studies focused on cognitive behavioural therapy, which is a specific form of talking therapy based on the idea that thoughts and thinking can influence emotions and behaviours.
Cognitive behavioural therapy was more effective than usual care in reducing the severity of MUPS. For other types of therapy, we found only one or two studies giving insufficient evidence for conclusions.
Cognitive behavioural therapy was no more effective than enhanced care provided by the person's doctor.
No studies of physical therapy met the criteria to be included in the review.
Talking therapies were acceptable to people and few people dropped out of the trials; however, this may not reflect real clinical practice as the study participants were people with somatoform disorders or MUPS who were willing to try talking therapies. In clinical practice, a high proportion of people may not be willing to accept these treatments.
What should happen next?
The review authors suggest that future high‐quality trials should be carried out to find out more about which groups of people benefit most from cognitive behavioural therapy and how it can be most effectively delivered. They also suggest that more studies are needed of other talking therapies, and a particular focus should be on high‐quality studies of physical therapies.
Summary of findings
Summary of findings for the main comparison. Psychological therapy compared with usual care or waiting list for somatoform disorders and medically unexplained physical symptoms.
| Psychological therapy compared with usual care for somatoform disorders and medically unexplained physical symptoms | ||||||
| Patient or population: people with somatoform disorders and medically unexplained physical symptoms Settings: all settings Intervention: psychological therapy Comparison: usual care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Usual care | Psychological therapy | |||||
| Severity of somatic symptoms at end of treatment Various instruments | The mean severity of somatic symptoms at end of treatment ranged across control groups from 0.5 to 48.71 using varying scales1 | The mean severity of somatic symptoms at end of treatment in the intervention groups was 0.34 standard deviations lower (0.53 to 0.16 lower) | ‐ | 1081 (10 studies2) | ⊕⊕⊝⊝ low3,4,5 | A difference of 0.34 SMD was considered to be 'small to medium' |
| Acceptability 1 ‐ proportion of participants withdrawing during treatment | 896 per 1000 | 833 per 1000 (788 to 887) | RR 0.93 (0.88 to 0.99) | 1644 (14 studies6) | ⊕⊕⊕⊝ moderate7,8 | Excluding the outlier (see footnote) (70 participants) reduced I2 statistic from 70% to 33% |
| Dysfunctional cognitions, emotions, or behaviours (participant rated) at end of treatment Whitely Index | The mean dysfunctional cognitions, emotions, or behaviours (participant rated) at end of treatment in the control groups was 7.3 on the Whitely Index | The mean dysfunctional cognitions, emotions, or behaviours (participant rated) at end of treatment in the intervention groups was 0.11 standard deviations lower (0.37 lower to 0.16 higher) | ‐ | 440 (3 studies9) | ⊕⊕⊕⊝ moderate10 | A difference of 0.11 SMD was considered to be 'small' |
| Treatment response at end of treatment CGI‐improvement/Global impression of change | 157 per 1000 | 517 per 1000 (326 to 816) | RR 3.30 (2.08 to 5.21) | 391 (4 studies11) | ⊕⊕⊝⊝ low12,13 | ‐ |
| Functional disability/quality of life at end of treatment Various instruments | ‐ | The mean functional disability/quality of life at end of treatment in the intervention groups was 0.17 standard deviations higher (0.03 to 0.32 higher) | ‐ | 730 (7 studies14) | ⊕⊕⊝⊝ low3,13 | A difference of 0.17 SMD was considered to be small |
| Healthcare use Various measures, participant or physician assessed < 1 year after end of treatment Follow‐up: 6‐11 months | ‐ | The mean healthcare use in the intervention groups was 0.09 standard deviations lower (0.31 lower to 0.12 higher) | ‐ | 532 (4 studies15) | ⊕⊕⊕⊝ moderate12 | Difference small and not statistically significant |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CGI: Clinical Global Impression; CI: confidence interval; RR: risk ratio; SMD: standardised mean difference | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Measured with different instruments using different scales. 2 Analysis 1.1. 3 Quality of evidence downgraded one point for each of the following study limitations (present in most studies): lack of blinding and incomplete outcome data (loss to follow up) 4 I2 = 49%. 5 95% CI crossed effect size of 0.5. 6 Analysis 1.4. 7 Quality downgraded by one point as studies not blinded. As acceptability and loss to follow‐up are interrelated, we decided not to downgrade the evidence for loss to follow‐up. 8 I2 = 70%. One outlier explained most of the heterogeneity (Kashner 1995). 9 Analysis 1.15. 10 Due to lack of blinding in all studies and loss to follow‐up in one study. 11 Analysis 1.18. 12 Due to lack of blinding in all studies and loss to follow‐up > 20% in 2 studies. 13 < 300 events. 14 Analysis 1.21. 15 Analysis 1.25.
Summary of findings 2. Psychological therapy compared with enhanced or structured care for somatoform disorders and medically unexplained physical symptoms.
| Patient or population: somatoform disorders and medically unexplained physical symptoms Settings: all settings Intervention: psychological therapies Comparison: enhanced or structured care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Enhanced or structured care | Psychological therapies | |||||
| Severity of somatic symptoms at end of treatment | ‐ | The mean severity of somatic symptoms at end of treatment in the intervention groups was 0.19 standard deviations lower (0.43 lower to 0.04 higher) | ‐ | 624 (5 studies1) | ⊕⊕⊕⊝ low2, 11 | 95% CI excluded large effect (> 0.5 SMD) |
| Acceptability 1 ‐ proportion of participants withdrawing during treatment | 904 per 1000 | 841 per 1000 (787 to 904) | RR 0.93 (0.87 to 1) | 679 (5 studies3) | ⊕⊕⊕⊝ moderate4 | ‐ |
| Dysfunctional cognitions, emotions, or behaviours at end of treatment Whitely Index (different forms) | ‐ | The mean dysfunctional cognitions, emotions, or behaviours at end of treatment in the intervention groups was 0.09 standard deviations lower (0.29 lower to 0.1 higher) | ‐ | 499 (4 studies5) | ⊕⊕⊕⊝ moderate6 | 95% CI excluded clinically relevant effect |
| Treatment response at end of treatment | Study population | Not estimable | 0 (0) | See comment | No studies reported on this outcome (see text) | |
| See comment | See comment | |||||
| Moderate | ||||||
| ‐ | ‐ | |||||
| Functional disability/quality of life at end of treatment Various instruments | ‐ | The mean functional disability/quality of life at end of treatment in the intervention groups was 0.13 standard deviations higher (0.05 lower to 0.3 higher) | ‐ | 497 (4 studies7) | ⊕⊕⊕⊝ moderate6 | 95% CI excluded clinically relevant effect |
| Healthcare use within 1 year after treatment | ‐ | The mean healthcare use within 1 year after treatment in the intervention groups was 0.24 standard deviations lower (0.46 to 0.01 lower) | ‐ | 319 (2 studies8) | ⊕⊕⊝⊝ low9,10 | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; SMD: standardised mean difference. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Analysis 2.1. 2 I2 = 53% 3 Analysis 2.4. 4 Quality of evidence downgraded by one point as studies not blinded. As acceptability and loss to follow‐up are interrelated, we decided not to downgrade the evidence for loss to follow‐up. 5 Analysis 2.8. 6 Assessment of quality of evidence downgraded by one point as studies were not blinded. 7 Analysis 2.11. 8 Analysis 2.15. 9 In addition to both studies not being blinded, high loss to follow‐up in one study. We therefore downgraded our assessment of the quality of the evidence by two points. 10 Only 2 studies with < 400 analysed participants.
11 No blinding (all studies) and >20% loss to follow up (2 studies)
Background
Description of the condition
Medically unexplained physical symptoms (MUPS) are physical symptoms for which adequate evidence of an underlying pathophysiological process cannot be identified after appropriate examination and investigation. MUPS are common in all healthcare settings. Up to one‐third of all people presenting with physical symptoms have MUPS (Kirmayer 2004). The presence of MUPS is the key feature of conditions known as somatoform disorders. The Diagnostic and Statistical Manual of Mental Disorders (DSM; APA 2000) and International Classification of Diseases (ICD; WHO 2004) describe four somatoform diagnostic categories that include MUPS as their main indication. These categories are: somatisation disorder, (persistent somatoform) pain disorder, undifferentiated somatoform disorder, and unspecified somatoform disorder. The ICD also describes a fifth category: somatoform autonomic dysfunction disorder. All these disorders are established through a validated psychiatric diagnostic interview. Many different diagnostic revisions of somatoform disorders have been suggested and used in research since the early 2000s. Examples of proposed revised diagnoses include abridged somatisation disorder (Escobar 1998), multisomatoform disorder (Kroenke 1997), bodily distress disorder (Fink 2007), and complex somatic symptom disorder (Dimsdale 2009). These alternative diagnoses have their own diagnostic criteria, mainly based on symptom counts. Finally, in some fields, MUPS are not described as a feature of a specific disorder, but as a health problem in their own right. As a result, the treatment of MUPS in general is also described in literature, for example, in primary care research. Assessing the presence of MUPS is usually based on the combination of a validated somatic symptom scale, the duration of symptoms, and clinical judgement by the physician.
In some health care settings the term 'somatoform' is falling out of favour, as people can find this term offensive. In these settings the term is being replaced with other terms such as 'functional'.
Somatoform disorders and MUPS may lead to functional impairment, high levels of psychological distress, a reduced quality of life, and a troubled doctor‐patient relationship (Escobar 1987; Gureje 1997; Ring 2004; Zoccolillo 1986). Furthermore, chronic MUPS may lead to absence from work, fragmented and high utilisation of health care, and the associated high costs for society (Konnopka 2012; Kroenke 1989; Smith 1986).
DSM 5 describes the 'somatic symptom disorder' (SSD), which requires explicit cognitive criteria (e.g. excessive and disproportionate thoughts, feelings, and behaviours regarding symptoms) (APA 2013). The diagnosis does not require the somatic symptoms to be medically unexplained.
Description of the intervention
In previous decades, many pharmacological and non‐pharmacological interventions for somatoform disorders and MUPS were developed. The use of antidepressants, in particular, as pharmacological agents for syndromes of MUPS (Ford 2009; Pae 2009), or chronic pain (Saarto 2007), was tested. The most relevant groups of antidepressants are the tricyclic antidepressants, selective serotonin reuptake inhibitors, and selective serotonin and noradrenaline (norepinephrine) reuptake inhibitors. In addition to antidepressants, antiepileptic drugs are also commonly used for somatoform disorders (Moore 2014; Silberstein 2002), although they are not advised in guidelines. Pharmacological interventions will be described in a separate forthcoming Cochrane review (published protocol: Kleinstäuber 2013) and this review only focuses on non‐pharmacological interventions.
Most non‐pharmacological interventions for MUPS focus on addressing cognitions, behaviour, coping styles, and functional consequences of symptoms. These interventions include psychological therapies as well as physical therapies. Psychological therapies are mostly used to tackle underlying psychological disorders and problems, and aim to change the way that people perceive their symptoms in order to help them to manage their symptoms. Cognitive behavioural therapy (CBT) appears to be a promising treatment in this category, if people accept the treatment (Kroenke 2000). Physical therapies usually concern physical activity treatments, which aim to improve physical function by expanding physical activity and thereby reducing symptoms. In the paragraph below, we described examples of several frequently studied forms of psychological and physical therapies.
How the intervention might work
Psychological therapies ‐ cognitive behavioural therapy
The first and most commonly used and investigated psychological therapy for MUPS is CBT, which is based on the cognitive behavioural model (Deary 2007). This model proposes that MUPS are caused by a self perpetuating multi‐factorial cycle, based on the interaction of different factors in several domains, including somatic (physical) aspects, cognitions (thoughts), behaviour, emotions, and environment (Sharpe 1992). This model provides a framework to incorporate people's personal predisposing, precipitating, and perpetuating factors according to their symptoms. CBT, as a consequence, focuses on addressing or changing cognitions and behaviours that people have in interaction with their symptoms.
Reattribution is a specific form of CBT (Goldberg 1989). This method aims to encourage people to reattribute their MUPS to physiological or psychosocial causes rather than to somatic causes. Reattribution consists of three stages: 1. making the person feel understood; 2. changing the agenda of the person, and the doctor, and their mutual agenda during the consultations; and 3. making the link between physical symptoms and psychosocial problems.
This self propagating disadvantageous situation is illustrated by the case of a person with low back pain who moves less freely and more stiffly because of the pain; this, in turn, causes the low back pain to continue and possibly worsen, and creates more stress. Reattribution might focus on making the person realise that stress caused by work may be responsible for causing or perpetuating the low back pain.
Problem‐solving treatment is another form of CBT that has been used for people with MUPS and somatoform disorders. The aim is to reduce complaints associated with unresolved problems in daily life by enhancing a person's problem‐solving capacities in a step‐by‐step manner. This therapy has a positive effect on mental and physical health problems in general (Malouff 2007).
Psychological therapies ‐ behavioural therapy
Behavioural therapy, the second group, aims to constructively change a person's behaviour towards their symptoms using operant conditioning ‐ also known as instrumental conditioning ‐ in which a response in a certain context is followed by a reinforcing stimulus or consequence, thereby increasing the likelihood that the same response will follow in future. Biofeedback therapy is an important behavioural intervention relevant to this review. In this therapy, one or more physiological measures (such as heart rate, respiratory rate, or muscle tension) are thought to relate to the person's physical symptoms, and people are taught to control these measures voluntarily. As a result, they develop a personal strategy for controlling them (Nanke 2003b; Schwartz 2003). Other forms of behavioural therapy include relaxation therapy (Loew 2000), and psycho‐education (Guerney 1971).
Other psychological therapies
A third group of psychological therapies, more aimed at increasing insight, such as:
third‐wave cognitive behavioural therapy (i.e. the development of a new attitude towards symptoms, based on self regulation of attention and acceptance) (van Ravensteijn 2013);
psychodynamic therapies, a form of depth psychology, which focusses on revealing the unconscious content of a person's psyche in order to alleviate psychological of physical tension (Noyes 2008). This might include group therapy, in which people regularly come together to discuss their symptoms, supervised by a trained group leader. The therapy aims to stimulate an active search for causes and perpetuating factors of symptoms, and to treat them by remedial education aiming at insight;
humanistic therapies, focusing on self development, growth, and responsibilities. Treatment aims to help individuals recognise their strengths, creativity, and choices in the 'here and now'. An example is person‐centred therapy, which specifically focusses on an individual's self esteem and values; and
integrative therapies, which integrate components from several theoretical schools, e.g. cognitive analytical therapy, which aims to work with the person to identify procedural sequences, chains of events, thoughts and emotions that explain how a target problem (e.g. a physical symptom) is established and maintained, and other psychodynamic therapies (Noyes 2008).
Enhanced care
Another group of therapies offered to people with MUPS is enhanced care. Within these therapies people receive care as usual (mostly by their general practitioners (GP)), enhanced with, for example, participant education, structured counselling moments, a psychiatric interview, or a reattribution training of the doctor (Rosendal 2013). Within these therapies, there is no specific treatment agenda or structure, the aim is to offer the person some tools to assist in the recovery process, stimulating self management. This category of treatment was not mentioned in the protocol for this current review, but added post‐hoc when we found that it had been used as a comparator in several studies.
Physical therapies ‐ physical activity training
Several studies have indicated that mental health, including mood, pain thresholds, and sleep, can be improved by low‐ or moderate‐intensity activity (Weyerer 1994). Graded activity training is an operant‐conditioning behavioural approach in which physical activity is expanded step by step, based on a predetermined time schedule. It focusses on changing the fear‐avoidance behaviour that people with MUPS may have for particular physical activities (Lindström 1992). Supervised aerobic exercise training has beneficial effects on the physical capacity and symptoms of people with fibromyalgia (Busch 2007). A similar effect was found for graded activity training in people with chronic fatigue syndrome (Edmonds 2004;Yancey 2012). In non‐specific low back pain, exercise appeared to be slightly effective at decreasing pain and improving function (Hayden 2005). However, for graded activity the evidence is lacking (Van der Giessen 2012).
Other physical therapies
Other examples of physical therapies for somatoform disorders and MUPS include activation therapy, where physical and behavioural activation is increased in a step‐wise fashion, and running therapy, where running is used therapeutically, mainly to influence the level of stress.
Why it is important to do this review
Although there are several reviews on non‐pharmacological interventions for somatoform disorders and MUPS, a complete overview of the whole spectrum is missing. Some reviews did not include a meta‐analysis (Edwards 2010; Sumathipala 2007), while other reviews included only specific treatment types (Kroenke 2000; Nezu 2001), or applied restrictions on diagnostic types of MUPS or on treatment setting (Allen 2002; Edwards 2010; Kleinstäuber 2011; Rosendal 2013). Furthermore, currently there are no reviews that evaluate variations in treatment effects on the basis of diagnosis and severity of symptoms at baseline, setting, or healthcare provider.
In this review, we aimed to give an overview of the evidence for non‐pharmacological interventions for somatoform disorders and MUPS. This will help healthcare providers and patients to make optimal treatment decisions. In addition, the results of this review will provide insight into gaps in the evidence that merit future research. This review will complement the existing portfolio of four Cochrane reviews covering somatoform disorders (Hoedeman 2010; Ipser 2009; Ruddy 2005; Thompson 2007), and also the Cochrane review on pharmacological interventions for somatoform disorders, which is currently being developed (see Kleinstäuber 2013 for protocol).
Objectives
To assess the effects of non‐pharmacological interventions for somatoform disorders (specifically somatisation disorder, undifferentiated somatoform disorder, somatoform disorder unspecified, somatoform autonomic dysfunction, pain disorder, and alternative somatoform diagnoses proposed in the literature) and MUPS in adults in comparison with treatment as usual, waiting list controls, attention placebo, psychological placebo, enhanced or structured care, and other psychological or physical therapies.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) and cluster randomised controlled trials (CRCTs). We also planned to include data from the first phase of cross‐over trials, but we identified no such trials that met our inclusion criteria.
We excluded quasi‐randomised trials (e.g. allocation to the study group by day of the week).
Types of participants
Participant characteristics
Participants had to be at least 18 years old. We applied no maximum age, as the condition can be present at any age. We placed no restriction on gender or culture.
Diagnosis
Participants had to meet the criteria for a somatoform disorder according to DSM III (APA 1980), DSM IV‐TR (APA 2000), ICD‐9 (WHO 1975), or ICD‐10 (WHO 2004), or the criteria for one of the alternative somatoform diagnoses proposed in the literature. The primary diagnosis (a somatoform disorder) had to be made on the basis of a structured clinical interview such as the Structured Clinical Interview for Mental Disorders (SCID; First 2002; Spitzer 1990), the World Health Organization (WHO) Composite International Diagnostic Interview (CIDI; WHO 1990), the MINI International Neuropsychiatric Interview Plus (Mini‐Plus; Sheehan 1998), or diagnostic checklists such as the International Diagnostic Checklists (IDCL; Janca 1996). The secondary diagnosis of an alternative somatoform diagnosis needed to be based on the criteria that characterise that specific disorder, for example, abridged somatisation disorder needed to be established through use of the Somatic Symptom Index (SSI; Escobar 1998).
Participants were characterised with MUPS as their primary problem, on the basis of a validated scale for the assessment of MUPS, such as the Screening for Somatoform Disorders (Screening für Somatoforme Störungen) (SOMS; Rief 1997), the Bradford Somatic Inventory (Mumford 1991), or component subscales of validated standardised instruments for the assessment of general psychopathology or general health status, such as the Patient Health Questionnaire‐15 (PHQ‐15; Kroenke 2002), the subscale 'Somatisation' of the Four Dimensional Symptom Questionnaire (4DSQ; Terluin 2006), the subscale 'Somatisation' of the Symptom Checklist‐90‐R (SCL‐90R; Derogatis 1986), or the Brief Symptom Inventory (BSI; Derogatis 1983).
As the subdivision of these two diagnostic concepts (somatoform disorders and MUPS) is based on differences in selection methods used in different research settings rather than on differences between individual people, it is possible that the nature and severity of symptoms may show a certain overlap between the two groups.
We disregarded the DSM‐5 criteria for somatoform disorders for this version of the review. In the DSM‐5, one category, the SSD, replaced the four diagnostic categories of the somatoform disorders (APA 2013). This disorder was diagnosed on the basis of explicit psychological criteria (e.g. excessive and disproportionate thoughts, feelings, and behaviours regarding symptoms), and diagnosis did not require the somatic symptoms to be medically unexplained. However, as the publication of the DSM‐5 was recent, there are currently no instruments available to establish the diagnosis. As a result, to our knowledge, no trials have been performed that use these criteria. We will add the SSD diagnosis to the list of conditions in future updates of the review, if more information about diagnostic instruments becomes available and once studies that use these criteria have been performed.
See Table 3 for an overview of all diagnostic categories of somatoform disorders and MUPS, and a clear indication of whether or not they were eligible for the current review.
1. Diagnostic categories of somatoform disorders and medically unexplained physical symptoms and their eligibility for the current review.
| Eligible for this review? | |||
| DSM‐IV | ICD‐10 | YES | NO |
| Somatisation disorder | Somatisation disorder | x | ‐ |
| Undifferentiated somatoform disorder (duration > 6 months) | Undifferentiated somatoform disorder (duration > 6 months) | x | ‐ |
| ‐ | Somatoform autonomic dysfunction | x | ‐ |
| Pain disorder | Persistent somatoform pain disorder | x | ‐ |
| Somatoform disorders, unspecified | Somatoform disorders unspecified | x | ‐ |
| Hypochondriasis | Hypochondriacal disorder | ‐ | x |
| ‐ | Other somatoform disorders | ‐ | x |
| Body dysmorphic disorder | Body dysmorphic disorder | ‐ | x |
| Conversion disorder | Dissociative and conversion disorders | ‐ | x |
| YES | NO | ||
| Alternative somatoform diagnoses (such as abridged somatisation disorder or multisomatoform disorder) | x | ‐ | |
| Chronic MUPS (duration ≥ 6 months) | x | ‐ | |
| Functional somatic syndromes | ‐ | x | |
| Specific functional somatic symptoms | ‐ | x | |
DSM: Diagnostic and Statistical Manual of Mental Disorders; ICD: International Classification of Diseases.
Co‐morbidities
As we aimed to summarise interventions for multiple symptoms, we excluded studies that examined participants diagnosed with only one specific functional syndrome or symptom (e.g. fibromyalgia or fatigue). Moreover, existing Cochrane reviews address specific syndromes and complaints (Bernardy 2013; Price 2008; Zijdenbos 2009).
Setting
We place no restrictions on the type of setting.
Subsets of participants
Some studies could include 'eligible' participants as well as 'ineligible' participants for this review, for example when an age cut‐off was used that was different to the cut‐off of this review. When no detailed information was available about these subsets of participants, we requested the data from the trial authors. If this did not yield any further information, we included the study only if at least 80% of the sample population had the characteristic of interest (e.g. aged 18 years or over). The Characteristics of included studies and Characteristics of excluded studies tables document the decisions about the eligibility of these subsets of participants. We assessed the impact of these decisions using sensitivity analysis.
Types of interventions
Experimental interventions
Eligible studies included one or more of the following experimental interventions.
-
Psychological therapies:
CBT (e.g. reattribution therapy and problem‐solving therapy);
behavioural therapy (e.g. classical CBT, biofeedback therapy, relaxation therapy, and psycho‐education);
third‐wave CBT (e.g. mindfulness);
psychodynamic therapies (e.g. group therapy);
humanistic therapies (e.g. person‐centred therapy);
integrative therapies (e.g. cognitive analytical therapy).
-
Physical therapies:
physical activity training (e.g. graded activity training);
other physical therapies (e.g. activation therapy or running therapy).
We excluded interventions based on complementary medicine from this review. In addition, pharmacological interventions and consultation letter interventions were beyond the scope of this review; they were evaluated in other Cochrane reviews (Hoedeman 2010; Kleinstäuber 2013). However, in several of the studies, in both study arms a consultation letter was sent to the primary care physician after baseline assessment, in addition to the planned psychological therapy or comparison condition. Post‐hoc, we decided that this was not a reason for exclusion, and we categorised these studies according to the main comparison (We conducted a sensitivity analysis to explore this decision).
Comparator interventions
We accepted the following comparator interventions.
Normal/usual treatment (e.g. treatment according to (multidisciplinary) guidelines or common practice in primary or secondary care) or waiting list procedures.
Attention or psychological placebo (an attention placebo was regarded as being inactive by both participants and researchers in a trial, while a psychological placebo was regarded as active by participants but inactive by researchers).
Enhanced or structured care (e.g. care as usual by physician trained in reattribution, or structured appointments with a physician without a specific treatment being performed). This comparator was not foreseen at the protocol stage, but added afterwards (see Differences between protocol and review).
Other psychological therapies (as per the list of experimental interventions above).
Other physical therapies (as per the list of experimental interventions above).
Types of outcome measures
We included studies that met the inclusion criteria described above regardless of whether they reported on the following outcomes.
Primary outcomes
(Outcomes marked with an asterisk were included in the 'Summary of findings' tables).
1. Severity/intensity of somatic symptoms*
If a validated self report scale was used for symptom severity, we extracted the results for this instrument for the meta‐analysis. Validated scales for the assessment of MUPS considered for this review were already described in Criteria for considering studies for this review above. Where multiple visual analogue self report scales or unvalidated scales were used, the first two review authors (NvD, MdB) decided which scale most closely approximates MUPS. This was supervised by two other review authors (HvdH, BT), who were experts on somatoform disorders, MUPS, and clinical diagnostics, but who were not directly involved in the process of study selection or data extraction and management, so that they were blinded to the results. We examined clinician‐rated severity of MUPS separately and it was not combined with self report outcomes into one effect‐size index.
2. Acceptability*
We measured acceptability by the complement of the proportion of trial participants who dropped out during the trial from either the experimental or the comparator intervention.
Secondary outcomes
3. Depression and anxiety
We distinguished between validated clinician‐rated instruments (e.g. the Hamilton Depression Rating Scale (HDRS) (Hamilton 1960), the Hamilton Anxiety Rating Scale (HARS) (Hamilton 1959)), and participant self report instruments (e.g. BDI (Beck 1961), and the Beck Anxiety Inventory (BAI) (Beck 1988)).
4. Dysfunctional cognitions, emotions, or behaviours (participant‐rated)*
We measured dysfunctional cognitions, emotions, or behaviours by validated self report scales (e.g. the Whitely Index (WI) (Pilowsky 1967), Illness Attitude Scales (IAS) (Kellner 1986), and the Scale for the Assessment of Illness Behavior (SAIB (Rief 2003)).
5. Adverse events
We expected that the frequency of adverse events of non‐pharmacological interventions would be low, but not absent. Forms of psychotherapy may lead to psychological decompensation, while an intervention such as running therapy may lead to certain injuries. When possible, we described the most common adverse effects for the included studies narratively (defined as effects that occur in at least 10% of participants receiving the specific therapy), as well as significant differences in the rate of occurrence of adverse events between intervention and control groups. However, it must be noted that RCTs and CRCTs are not the optimal study design for obtaining information about rare or long‐term adverse outcomes, limiting our interpretation of adverse effect data.
6. Treatment response (responder versus non‐responder)*
We measured treatment response using the clinician‐rated Clinical Global Impression Scale (CGI) ‐ Improvement item (Guy 1976); this scale defined responders as those with a score of "1 = very much improved" or "2 = much improved". Alternatively, we used the number of participants who responded to the treatment according to the author's definition. We calculated response rates out of the total number of randomised participants.
7. Functional disability and quality of life*
We assessed functional disability and quality of life through validated clinician‐rated scales (e.g. the Global Assessment of Functioning (GAF) (Hall 1995)) or validated self report instruments (e.g. the 36‐item Short Form Questionnaire (SF‐36) (Ware 1992); or the Sheehan Disability Scale (SDS) (Sheehan 1983)).
8. Healthcare use*
We assessed healthcare use as measured by direct measurements (e.g. consultation counts), or through participant‐rated measurements (e.g. healthcare use diary or Trimbos/iMTA questionnaire for Costs associated with Psychiatric Illness (TiC‐P; Hakkaart‐van Roijen 2002).
Hierarchy of outcome measures
If there were multiple instruments measuring the same outcome, we preferred whichever instrument was most commonly used from those listed above. Several studies used both the Physical Component Scale (PCS) and the Mental Component Scale (MCS) of the SF‐36, and sometimes also the subdomains. In these cases, we restricted ourselves to the main domains and combined PCS and MCS into one outcome.
Timing of outcome assessment
We analysed primary and secondary outcomes at the following time points, if available:
immediately post treatment;
within 12 months after treatment ending; and
more than 12 months after treatment ended.
Search methods for identification of studies
Electronic searches
The Cochrane Depression, Anxiety and Neurosis Review Group's Specialized Register (CCDANCTR)
The Cochrane Depression, Anxiety and Neurosis Group (CCDAN) maintains two clinical trials registers at the editorial base in Bristol, UK: a references register and a studies‐based register. The CCDANCTR‐References Register contains over 36,000 reports of RCTs in depression, anxiety and neurosis. Approximately 60% of these references have been tagged to individual, coded trials. The coded trials are held in the CCDANCTR‐Studies Register and records are linked between the two registers through the use of unique Study ID tags. Coding of trials is based on the EU‐Psi coding manual, using a controlled vocabulary. Reports of trials for inclusion in the Group's registers are collated from routine (weekly), generic searches of MEDLINE (1950 to date), EMBASE (1974 to date), and PsycINFO (1967 to date); quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL), and review‐specific searches of additional databases. Reports of trials are also sourced from international trials registers c/o the WHO trials portal (the International Clinical Trials Registry Platform (ICTRP)), pharmaceutical companies, and handsearching of key journals, conference proceedings, and other (non‐Cochrane) systematic reviews and meta‐analyses.
Details of CCDAN's generic search strategies (used to identify RCTs) can be found on the Group's website.
1. We searched the CCDANCTR (Studies and References Registers) on 29 November 2013 using the following free‐text terms: (somatization or somatisation or somatoform or hysteri* or briquet or polysymptom* or multisomatoform or somatizer* or (somatic NEAR symptom*) or (MUPS or “medical* unexplained” or "unexplained medical*" or (unexplained NEAR (symptom* or syndrom*)) or "frequent attend*" or (multiple NEAR (“physical symptom*” or “symptom diagnos*”)) OR neurastheni*)
We screened the records retrieved manually for non‐pharmacological interventions.
2. We conducted complementary searches on the following bibliographic databases using relevant subject headings (controlled vocabularies) and search syntax, appropriate to each resource:
Cochrane Central Register of Controlled Trials (CENTRAL) (all years) (Appendix 1);
Web of Science (from 1945 onwards, cited references search only (April 2014)).
3. To identify ongoing trials, we searched the ClinicalTrials.gov register (clinicaltrials.gov/), the Current Controlled Trials metaRegister of Controlled Trials ‐ active registers (mRCT; www.controlled‐trials.com/mrct/), and the WHO International Clinical Trials Registry Platform Search Portal (www.who.int/trialsearch).
We applied no restrictions regarding date, language, or publication status to the searches. We will revise the search strategies for future updates, as these will include studies that use DSM‐5 criteria.
Searching other resources
Grey literature
We searched the ProQuest Dissertation & Theses Database (www.proquest.com), National Guideline Clearing House (guideline.gov/) and Open Grey (www.opengrey.eu/) for grey literature.
Handsearching
We searched the conference proceedings for the following associations from 2009 if available (for titles not already indexed in EMBASE or PsycINFO, or already handsearched within The Cochrane Collaboration).
Annual meeting of the American Psychiatric Association (APA);
International Congress of Behavioral Medicine (ICBM);
European Conference on Psychosomatic Research (ECPR);
Annual Meeting of the European Association for Consultation‐Liaison Psychiatry and Psychosomatics (EACLPP);
Annual congress of the Dutch Network on Unexplained Physical Symptoms (NOLK).
Reference lists
We checked the reference lists of all included studies and relevant systematic reviews to identify additional studies that might have been missed from the original electronic searches (e.g. references that were unpublished or in press). We also conducted a cited reference search on the Web of Science (15 April 2014) for citations to primary reports of included studies.
Correspondence
We contacted researchers and subject experts for information on unpublished or ongoing studies or to request additional trial data.
Data collection and analysis
Selection of studies
In the first step, two review authors (NvD, MdB) independently screened the titles and abstracts of reports identified from the literature search. We discarded studies that obviously did not fulfil the inclusion criteria at this stage of the screening process. Two review authors (NvD, MdB) retrieved eligible or potentially eligible articles for full‐text assessment. We identified and excluded duplicate records and we collated multiple reports that related to the same study so that each study ‐ rather than each report ‐ was the unit of interest in the review. After full‐text assessment, the review authors identified studies for inclusion and exclusion. We recorded reasons for exclusion of studies, and resolved disagreements by consensus ‐ if necessary with the involvement of a third review author (JvdW). We listed studies for which additional information was required in order to determine their suitability for inclusion in the review as 'Studies awaiting assessment'. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (Moher 2009), and Characteristics of included studies and Characteristics of excluded studies tables.
Data extraction and management
We used a data collection form, piloted on one study in the review, to extract study characteristics and outcome data. Independently, four review authors (NvD, MdB, HvdW, HvM) extracted study characteristics and outcome data from included studies. If necessary, we contacted the authors of trial reports for clarification or for additional information. We organised data using the most recent version of Review Manager 5 software (RevMan 2012). We negotiated disagreements with another review author. We extracted data on the following study characteristics.
Trial characteristics: first author, publication year, status of publication, language of publication.
Details of methodology: study design, study setting, total duration of study, details of any 'run‐in' period, number of study centres and location, withdrawals, lost to follow‐ups.
Participants' characteristics: source of sample, size of sample, mean age, age range, gender, severity and duration of MUPS at baseline, diagnostic criteria, inclusion and exclusion criteria, selection instrument (e.g. interview), presence of a somatoform diagnosis, co‐morbidity, and previous treatments for MUPS.
Intervention characteristics: intervention, category of non‐pharmacological intervention, healthcare provider performing the intervention, intervention frequency and duration, comparison, concomitant MUPS‐related interventions (e.g. pharmacotherapy) and number of drop‐outs due to adverse effects or inefficacy of treatment.
Outcome measures: primary and secondary outcome measures (as specified in Primary outcomes; Secondary outcomes), summary statistics of continuous data (mean, standard deviation (SD)) and dichotomous data (number of responders), timing of outcome assessments, intention‐to‐treat (ITT) analysis (with last observation carried forward (LOCF)) or observed cases/completer analysis and other methods of estimating the outcome for participants who dropped out (e.g. mixed‐effect analyses).
Notes: source of funding for trial and any notable conflicts of interest for trial authors.
We noted in the Characteristics of included studies table whether outcome data were reported in a usable way. We resolved disagreements by consensus. Two review author (NvD, HvdW) entered data into Review Manager 5 for analysis (RevMan 2012). We double‐checked that data had been entered correctly by comparing the data presented in the systematic review with the data in the study reports. A third review author (MdB) spot‐checked study characteristics for accuracy against the trial reports.
Main comparisons
We aimed to stratify comparisons according to treatment classes as described in Types of interventions when the available data allowed. For physical therapies, we planned to perform a specific analysis for physical activity training, all other therapies would be combined into one group. We planned the following main comparisons for each class of non‐pharmacological treatment.
Treatment versus care as usual or waiting list procedures.
Treatment versus attention or psychological placebo.
Treatment versus another non‐pharmacological treatment.
After inclusion of eligible articles, we concluded that all studies addressed some form of psychological therapy; no studies addressed 'treatment versus attention or psychological placebo'; and no studies addressed any form of physical therapy. In addition, as mentioned above (How the intervention might work), we found several studies comparing a psychological therapy to a form of enhanced or structured care. As a result of these findings, we decided to add the comparison 'psychological therapy versus enhanced care'. So the final categorisation of comparisons was as follows:
psychological therapy versus usual care (or waiting list procedures);
psychological therapy versus enhanced (or structured) care;
psychological therapy versus another psychological therapy.
If in future updates of this review we find new studies not fitting into this categorisation (e.g. comparing some physical therapy with psychological therapy), we will update the categorisations.
Assessment of risk of bias in included studies
Independently, two review authors (NvD, MdB) assessed the risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreements by discussion or by involving another review author (HvM, HvdW). We assessed the risk of bias for the following domains.
Random sequence generation: was the method used to generate the sequence of randomised allocation adequate for the production of comparable groups?
Allocation concealment: was the allocation adequately concealed so that intervention allocations could not have been foreseen in advance of, or during, enrolment?
-
Blinding:
was knowledge of the allocation of treatment by the participants and study personnel adequately prevented during the study?
was knowledge of the allocation of treatment by the outcome assessor(s) adequately prevented during the study?
Incomplete outcome data: were incomplete outcome data adequately addressed? Was the completeness of outcome data described, including attrition and exclusions from analyses? If there were attritions and exclusions in the treatment and control groups, they were reported, along with the underlying reasons? We also reported whether the review authors conducted any re‐inclusions in their analyses.
Selective outcome reporting: were reports of the study free of suggestion of selective outcome reporting?
Other sources of bias: was the study apparently free of other sources that could produce risk of bias?
Treatment fidelity: were therapies based on protocols or manuals? Were therapy sessions adequately monitored to assess whether they adhered to treatment protocols and manuals?
Researcher allegiance: could researchers be expected to have a preference for one of the treatment modalities?
Therapist qualifications: were the therapists qualified to perform the interventions?
In order to assess risk of bias in the following specific types of study design, we made additional judgements.
Multiple‐intervention studies: were data presented for each of the groups to which participants were randomised?
-
Cross‐over trials:
was it clear that the order of receiving a treatment was randomised?
were unbiased data from the first treatment period available?
-
CRCTs:
were individuals recruited to the trial after the clusters had been randomised?
were methods of stratified or pair‐matched randomisations of clusters used?
were adequate statistical analyses (taking clustering into account) used?
We judged each potential source of bias as to be of high, low, or unclear risk and provided a supporting quotation from the study as justification for our judgement in the 'Risk of bias' table. We summarised the risk of bias judgements across different studies for each of the domains by means of a 'Risk of bias' figure. Where information on risk of bias related to unpublished data or correspondence with researchers, we noted this in the 'Risk of bias' table. When considering treatment effects, we took into account the risk of bias for the studies that contributed to each outcome.
Measures of treatment effect
Dichotomous data
For dichotomous outcomes, we used risk ratio (RR) as the summary statistic, together with 95% confidence intervals (CI). If relevant, we calculated the number needed to treat for an additional beneficial outcome (NNTB) for every class of non‐pharmacological treatment for which we found a statistically significant treatment effect. We used the RR estimate and the control risk from the pooled control groups for this calculation.
Continuous data
As different measures were used to assess the same outcome, we pooled data using the standardised mean difference (SMD); we calculated 95% CI. Specific attention was paid to the secondary outcome 'functional disability and quality of life', as the direction of scales for these outcomes can differ. An increase on a scale of functional disability usually indicates deterioration, while an increase on a scale for quality of life often indicates improvement. We ensured that it was appropriate to pool the data (e.g. different instruments measuring the same underlying concept). Using the SMD does not correct for such differences in the direction of scales. In this case, we multiplied the mean values from the smaller set of studies by ‐1 to ensure that all the scales pointed in the same direction.
Unit of analysis issues
Cluster‐randomised controlled trials
In order to avoid unit‐of‐analysis errors for trials in which incorrect statistical analyses were conducted, we performed approximate analyses for continuous outcomes based on inflating standard errors. Before we entered data into Review Manager 5 for meta‐analytic calculations (RevMan 2012), we multiplied the standard error of the effect estimate (from an analysis that did not take clustering into account) by the square root of the design effect. The design effect was 1+ ((M‐1)* ICC), where M was the mean cluster size and ICC was the intracluster correlation coefficient. We assumed a common design effect across intervention groups. If the ICC was not available in the published report, we used an external estimate (0.03) obtained from Campbell 2005. We meta‐analysed these inflated variances using Review Manager 5 (RevMan 2012), and the generic inverse‐variance method. For dichotomous data, we divided both the number of events and the group sizes by the design effect (Higgins 2011, Chapter 16.3).
Studies with multiple treatment groups
In trials with more than two relevant non‐pharmacological treatment arms, we managed data as follows.
If the different experimental treatments were of the same class of treatments (e.g. psychotherapy), we summarised them into a single group and compared it with the control group. For continuous data, we pooled means and SDs across all of the treatment arms as a function of the number of participants in each arm (Higgins 2011, Table 7.7.a). For dichotomous outcomes, we summed both the sample sizes and the numbers of participants with events across groups.
If the different treatments were of different classes (e.g. a form of psychotherapy versus a physical activity intervention), we included each pair‐wise comparison separately, but divided the 'shared control‐group' into two or more smaller groups (according to the number of intervention groups). For dichotomous outcomes, we divided both the number of events and the total number of participants over these groups. For continuous outcomes, we divided only the total number of participants over these groups and left the means and SDs unchanged. Although this method only partially overcomes the unit‐of‐analysis error, the advantage of this approach is that it permits investigation of heterogeneity across intervention arms.
Cross‐over trials
We found no cross‐over trials. For future updates of our review, we will only include cross‐over trials for meta‐analysis if relevant data are available, or can be obtained, from the first treatment period for both the treatment and control group(s).
Dealing with missing data
We attempted to contact trial authors in order to verify key study characteristics and obtain outcome data that were not reported in the articles. When we could not retrieve missing data, we managed the data as follows.
Dichotomous data
We managed missing dichotomous data through ITT analysis, in which we assumed that participants who dropped out after randomisation had a negative outcome. We conducted sensitivity analyses for dichotomous data according to Gamble and Hollis (Gamble 2005): we calculated best‐case/worst‐case scenarios for the clinical response outcome, in which we assumed, for the best‐case scenario, that drop‐outs in the active treatment group had positive outcomes and those in the control group had negative outcomes; and, for the worst‐case scenario, that drop‐outs in the active treatment group had negative outcomes and those in the control group had positive outcomes. This method provided boundaries for the observed treatment effect.
Continuous data
Where continuous data were missing, we performed analysis on an endpoint basis, including only participants with a final assessment, or by using LOCF to the final assessment where reported by the trial authors. If necessary, we performed sensitivity analyses where we compared outcomes with the observed case data versus the LOCF data. When using LOCF data, we used the baseline SD as a more conservative approach. If SDs were missing, we calculated them from t‐, F‐, P, or CI values (Higgins 2011), or standard errors (Altman 1996).
Assessment of heterogeneity
We assessed the groups for clinical similarities including elements such as age, gender, and setting.
First, we assessed statistical heterogeneity visually by inspecting forest plots of standardised mean effect sizes and of relative risks. We used the I2 statistic as a second test: I2 describes the percentage of variability in effect estimates that is due to heterogeneity rather than chance.
We used conventions of interpretation defined by Higgins (Higgins 2011). In the case of substantial levels (i.e. where I2 = 50% to 90%) and considerable levels (I2 = 75% to 100%) of heterogeneity, we explored data further by means of subgroup and sensitivity analyses (see below). However, these were not clear‐cut criteria, as the importance of the observed I2 also depends on the magnitude and direction of treatment effects and the strength of evidence for heterogeneity (Deeks 2008; Higgins 2003); for example: if the I2 value fell slightly below 50% (e.g. 45%) and the direction and magnitude of treatment effects suggested important heterogeneity, we investigated the data further.
Assessment of reporting biases
We created funnel plots (treatment effect versus standard error of the effect size), if we included at least 10 trials in a meta‐analysis, according to the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011; Sterne 2011). When analysing and interpreting a funnel plot, we considered all potential reasons for asymmetry, not just publication bias (e.g. differences in methodological quality, true heterogeneity in intervention effects).
Data synthesis
If we found two or more included studies in a comparison category (see Data extraction and management) that used the same outcome construct, we performed a meta‐analysis of the results. Two authors (NvD, JvdW) entered data into Review Manager 5 software (RevMan 2012). We expected to find high heterogeneity in non‐pharmacological therapy approaches and in symptom severity, duration of symptoms and co‐morbidities among the various study populations. Therefore, we analysed dichotomous and continuous treatment effects using a random‐effects model. For studies of which data could not be combined, we summarised the results narratively.
Subgroup analysis and investigation of heterogeneity
If analysis of heterogeneity indicated significant heterogeneity, we performed subgroup analysis in order to explore whether methodological and clinical differences between the trials had produced systematic differences observed in the treatment outcomes. If we found statistically significant differences between subgroups, we reported the results of the corresponding subgroup meta‐analysis. If subgroup analysis provided no explanation for heterogeneity, we did not report the results of meta‐analysis.
When available data allowed (at least 10 studies), we performed subgroup analyses based on the following factors (only for the primary outcomes).
Severity, based on symptom count, and duration of MUPS at baseline.
Diagnosis at baseline.
Somatoform disorders versus alternative somatoform diagnoses versus MUPS.
Psychiatric and somatic co‐morbidity: as known from various mental disorders, co‐morbid psychological and somatic problems can modify the efficacy of an intervention. Therefore, we compared the effects of non‐pharmacological interventions for people with somatoform disorders or MUPS with or without co‐morbid mental or somatic disorders.
Primary care versus secondary care and tertiary care, including healthcare provider performing the intervention.
Treatment as usual versus a waiting list procedure as a control intervention.
Sensitivity analysis
We planned sensitivity analyses to evaluate the robustness of the conclusions in conjunction with decisions made during the review process. Sensitivity analyses would inform that the results of the review did not depend on specific decisions that were made during the review process. In case of sufficient data (i.e. at least 10 studies), we planned these analyses to examine the effects of the following options, restricted to the primary outcome.
Exclusion of CRCTs.
Exclusion of studies with unclear allocation concealment, or only a subset of relevant participants.
Exclusion of studies with unclear methods of sequence generation.
Exclusion of trials where missing data have been imputed.
Exclusion of studies with a drop‐out rate higher than 20%.
'Summary of findings' tables
We added 'Summary of main results' tables for the two main comparisons to create a structured overview of the main review results for the two subgroup comparisons with the largest number of studies: CBT versus usual care and CBT versus enhanced care. The table only includes information about the outcome measures marked with an asterisk (see Primary outcomes; Secondary outcomes), as these were considered the most important for the current review. For the same reason, the selected time point was end of therapy, except for healthcare use, where we thought the period within one year after end of treatment was more meaningful. For dichotomous outcomes (acceptability, treatment response), we reported an assumed and corresponding absolute risk with 95% CI as well as a relative risk ‐ corresponding to the RR with 95% CI obtained from meta‐analysis. For continuous outcomes, in the column of assumed and corresponding risk, we presented a difference in means or SMD with 95% CI. We used footnotes in order to specify the source or rationale for each assumed and corresponding risk. In order to assess the quality of body of evidence for each outcome, we used the GRADE approach (GRADEpro software; Schünemann 2008), classifying the quality of evidence for each study as 'high', 'moderate', 'low', or 'very low'. We explained judgements other than 'high' quality in footnotes or the Comments column in the 'Summary of main results' table.
Results
Description of studies
Results of the search
Searches were conducted up to November 2013 (CCDAN registers) and April 2014 (cited reference searches). Figure 1 shows the PRISMA flow diagram of the study selection.
1.
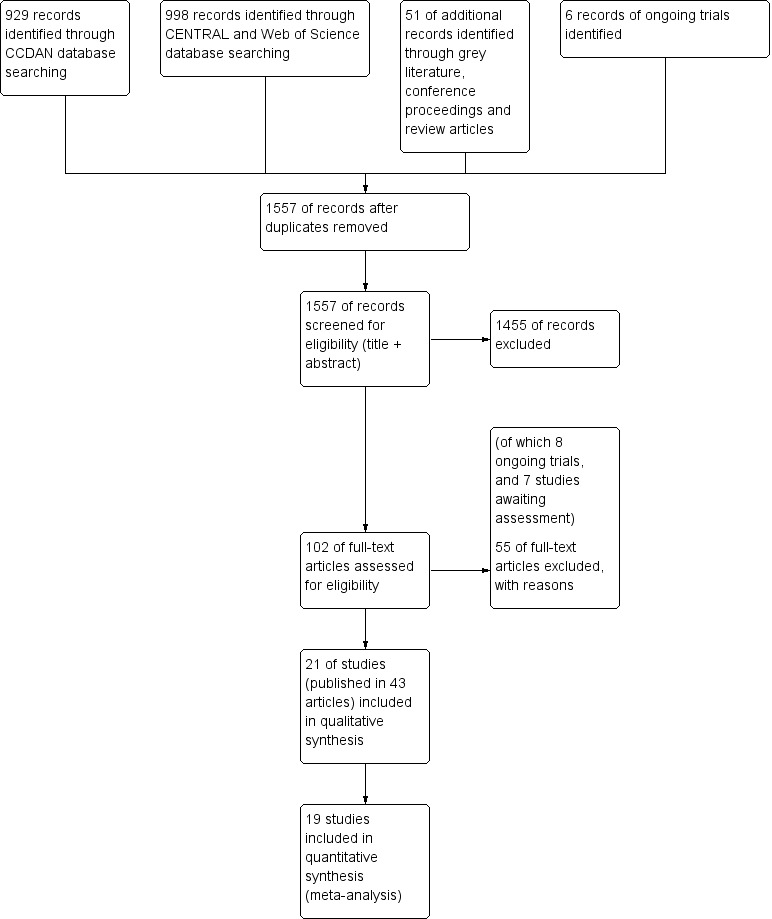
6 Study flow diagram.
Literature database searches
In the search of the CDANCTR‐Studies and CCDANCTR‐Reference Register (from now on referred to as CCDAN database), we found 929 abstracts after de‐duplication. We excluded 842 records, based on the title and abstract, leaving 82 references (65 studies) selected for full‐text retrieval. After reading the full‐text, we judged 27 studies (49 articles) eligible for inclusion in this review. We excluded 35 studies (38 articles) and six articles are still awaiting assessment, for example, due to unavailability of a full‐text article or difficulties in contacting authors.
The search of CENTRAL database found 995 records. After removing duplicates from the CCDAN search, there were 568 new references. After title and abstract screening, we excluded 560 references, and selected eight articles for full‐text reading. After full‐text reading, we excluded five articles, and judged three articles eligible for inclusion; however, all three articles described studies already included in the review (e.g. long‐term follow‐up results) (Gili 2014; Schröder 2013; Zonneveld 2012). As the Schröder article reported a more detailed trial methodology and higher number of participants, we decided to use this article as the main reference of this study (Schröder 2013) instead of Zaby 2008, which was retrieved from the CCDAN search.
We performed a cited reference search on the Web of Science, for citations to primary reports of all studies expected to be included in this review. When handsearching the retrieved articles, we identified three additional relevant references. After full‐text reading, we included one new study (Burton 2012), and excluded one article due to randomisation method (Rembold 2011). One article (Chernyak 2014) described an already included study (Sattel 2012).
Grey literature
We performed searches for grey literature in the databases of www.guideline.gov (36 results), www.opengrey.eu (eight results), and www.proquest.com (no results). We found no new articles. We screened the conference proceedings of the International Congress of Behavioral Medicine (ICBM, 2010 and 2012), the Annual Meeting of the European Association for Consultation‐Liaison Psychiatry and Psychosomatics and the European Conference on Psychosomatic Research (EACLPP/ECPR, 2009, 2010, 2011, 2012), the Annual Meeting of the American Psychiatric Association (APA, abstracts and syllabus of 2009‐2013), and the Annual congress of the Dutch Network on Unexplained Physical Symptoms (NOLK, 2013) and found no new articles.
Systematic reviews
We found 14 reviews about (specific) non‐pharmacological interventions for somatoform disorders or MUPS. After title screening in the reference lists of the reviews, we selected seven additional articles for screening of abstract. After abstract reading, we excluded four articles, and selected three articles for full‐text reading. However, none of these three articles were eligible due to lack of randomisation or inappropriate selection method (Hellmann 1990; Lupke 1996; Tschuschke 2007).
Trial registers
We performed ongoing trial searches in the databases of www.clinicaltrials.gov, www.controlled‐trials.com, and www.who.int/trialsearch. We found six potentially eligible ongoing trials. As full details of the design and study results were not available, we could not included these studies in the review (see Characteristics of ongoing studies table).
Contacting authors
We tried to contact 10 trial authors for missing information regarding the eligibility of studies; four responded and provided the desired information (Lidbeck 1997; Gottschalk 2011; Pols 2008; Steel 2011). The other six trials are listed in the Studies awaiting classification table.
We contacted authors of 20 of the included studies for additional information regarding study design and outcomes, of which 10 provided requested data.
Included studies
We included 21 studies, reported in 43 publications, in this review (see Characteristics of included studies table). All included studies concerned psychological interventions.
Design
Twenty of the included 21 studies had a parallel‐group, individually randomised design (RCT). One study had a cluster‐randomised design (Schaefert 2013). We found not trials with a cross‐over design.
Sample size
The total number of randomised participants was 2658, a mean number of 127 per study (range 32 to 328). Two studies included fewer than 25 participants per arm (Burton 2012; Katsamanis 2011). Most studies reported 25 to 75 participants per arm. Three studies included 75 to 100 participants per arm (Escobar 2007; Schilte 2001; Sumathipala 2008), and two studies included more than 100 participants per arm (Sattel 2012; Schaefert 2013). The largest study was Schaefert 2013, with 328 randomised participants.
Setting
Eight studies recruited participants in primary care only (Burton 2012; Escobar 2007; Moreno 2013; Schaefert 2013; Schilte 2001; Sumathipala 2000; Sumathipala 2008; Van Ravesteijn 2013). Only two studies recruited in secondary care (e.g. outpatient clinics) (Sattel 2012;Speckens 1995), and one study recruited inpatients in hospitals (Schweickhardt 2007). Seven studies recruited via medical settings as well as the open population (e.g. through advertisements) (Allen 2006; Kashner 1995; Katsamanis 2011; Kolk 2004; Martin 2007; Schröder 2013; Zonneveld 2012). Three studies recruited via primary care as well as secondary care (Fjorback 2013; Lidbeck 1997; Schröder 2012).
In one study, treatment was performed in group sessions by GPs in primary care who were trained in the specific psychological technique, combined with a psychosomatic specialist (Schaefert 2013). In six other studies, treatment took place at a department of psychiatry or psychology (Allen 2006; Escobar 2007; Fjorback 2013; Katsamanis 2011; Kolk 2004; Van Ravesteijn 2013). Another six studies treated participants in other outpatient clinics (Kashner 1995; Lidbeck 1997; Schröder 2012; Sumathipala 2000; Sumathipala 2008; Zonneveld 2012). Five studies treated participants in specific outpatient symptom clinics or outpatient clinics for psychosomatics (Burton 2012; Martin 2007; Sattel 2012; Schröder 2013; Speckens 1995). One study treated participants as inpatients (they were admitted to a ward) (Schweickhardt 2007). One study treated participants at home (Schilte 2001). Finally, in one study the treatment setting was unknown (Moreno 2013).
Five of 21 studies were carried out in the Netherlands (Kolk 2004; Schilte 2001; Speckens 1995; Van Ravesteijn 2013; Zonneveld 2012). Five studies were carried out in Germany (Martin 2007; Sattel 2012; Schaefert 2013; Schröder 2013; Schweickhardt 2007). Other studies were carried out in the USA (Allen 2006; Escobar 2007; Kashner 1995; Katsamanis 2011), Denmark (Fjorback 2013;Schröder 2012), Spain (Moreno 2013), Sri Lanka (Sumathipala 2000; Sumathipala 2008), Scotland (Burton 2012), and Sweden (Lidbeck 1997). Only two studies were carried out in a developing country (Sri Lanka) (Sumathipala 2000; Sumathipala 2008).
Several of the included studies were performed by the same research groups, or at least had an overlap of authors. This was the case for the studies of Allen 2006, Escobar 2007, and Katsamanis 2011, for the studies of Fjorback 2013 and Schröder 2012, for the studies of Sumathipala (Sumathipala 2000; Sumathipala 2008), and for the studies of Speckens 1995 and Van Ravesteijn 2013.
Participants
Proportion of women
Most studies recruited women than men, as found in epidemiological studies (Fink 1999; Fink 2004). Only one study reported more men in the intervention group (56%, Speckens 1995). The proportion of women among all participants in all treatment groups ranged between 66% (Burton 2012) and 89% (Allen 2006;Escobar 2007).
Age
The mean age was 43 years in all included studies, ranging from 35 years (Kolk 2004;Sumathipala 2008) to 49 years (Martin 2007;Schaefert 2013). One study did not provide the mean age of participants (Katsamanis 2011), but only provided data about the proportion of participants under and over 40 years old.
Diagnosis
Diagnostic criteria and inclusion criteria varied widely between studies. Fourteen studies used standardised diagnostic interviews (such as CIDI or SCID) to establish the diagnosis, the other seven studies used standardised questionnaires (such as SOMS or PHQ‐15). In nine studies, symptoms were referred to as medically unexplained symptoms or unexplained physical symptoms. Three studies used the diagnoses of somatisation disorder and somatoform disorder to describe the symptoms (Allen 2006; Kashner 1995;Lidbeck 1997). Two studies used only the term somatisation (Schilte 2001; Schweickhardt 2007). One study spoke of abridged somatisation disorder (Moreno 2013), and two other studies spoke of multiple somatoform symptoms (Martin 2007; Schröder 2013). The other four studies used individual terminologies such as bodily distress syndrome (Fjorback 2013; Katsamanis 2011; Sattel 2012; Schröder 2012).
Exclusion criteria varied between studies, but often included dementia, severe psychopathology such as psychosis, active suicidal thoughts, alcohol dependence, pregnancy, and current psychological therapy.
Eleven studies reported severity of symptoms at baseline in terms of number of symptoms. This number varied widely, ranging from a lifetime number of seven symptoms (Martin 2007), to a current number of 32 symptoms (Schröder 2012). Other studies used somatic symptom scales or did not report baseline severity at all. Only nine studies reported exact duration of symptoms, all of them reported a duration of at least several years, ranging from 3.5 years (Sumathipala 2008) to 25 years (Allen 2006). Four additional studies reported a minimal duration of symptoms, the other eight did not report on symptom duration at baseline.
Seven studies reported on psychiatric co‐morbidity in general, percentages of participants with a current co‐morbid axis 1 disorder varied between 41% (Zonneveld 2012) and 92% (Escobar 2007). Depression and anxiety were the co‐morbidities reported most often. Twelve studies reported symptoms or disorders of depression and anxiety, methods of establishment and percentages varied widely. Only two studies reported physical co‐morbidities such as hypertension, arthrosis, or asthma (Schaefert 2013; Van Ravesteijn 2013). Four studies did not report any co‐morbidities (Kashner 1995; Sattel 2012; Sumathipala 2000; Sumathipala 2008).
Interventions
As described in the Types of interventions section, we aimed to select studies investigating psychological therapies, as well as studies on physical therapies. However, we found no studies on physical therapies that were eligible for inclusion. All 21 included studies evaluated a form of psychological therapy. We classified psychological therapies into six subcategories, as pre‐defined by the Cochrane Depression, Anxiety and Neurosis Review Group: CBT, behaviour therapy, or other therapies such as third‐wave CBT, psychodynamic therapy, humanistic therapy, or integrative therapies. Fourteen studies described certain forms of CBT. Two studies evaluated behaviour therapies (Katsamanis 2011;Schilte 2001). Two studies described third‐wave CBT (mindfulness) (Fjorback 2013; Van Ravesteijn 2013), and two studies described psychodynamic therapies (Sattel 2012; Schaefert 2013). In the study of Kolk et al., participants received CBT, client‐centred or eclectic therapy, depending on the therapist the participant was assigned to (Kolk 2004); we classified this as integrative therapy. None of the included studies described humanistic therapies.
Table 4 provides a summary overview of the main characteristics of the interventions of the included studies. In eight studies, the participants received group therapy, and in 11 studies they received individual therapy. In one study, participants received both (Schröder 2012), and in one study there were two intervention groups of which one group received group CBT, and one group received personal CBT (Moreno 2013).
2. Interventions in included studies sorted by number of sessions.
| Intervention | Group/alone | Duration | Number of sessions | Therapist | Comparison | duration of follow‐up | |
| Martin 2007 | CBT | group | once | 1 | psychologist and psychotherapeutic specialist | usual care | 6 months |
| Burton 2012 | psychological therapy in symptoms clinic | alone | 3 months | 4 | experienced specialised GP | usual care | 3 months |
| Schweickhardt 2007 | psychotherapy | alone | 2 weeks | 5 | psychotherapists | psychoeducational reading material | 6 months |
| Sumathipala 2000 | CBT | alone | 3 months | 6 | research psychiatrist | usual care | 3 months |
| Sumathipala 2008 | CBT | alone | 3 months | 6 | trained primary care physician | enhanced treatment as usual | 12 months |
| Schröder 2013 | CBT | group | 2 months | 8 | psychotherapist | progressive muscle relaxation | 6 months |
| Van Ravensteijn 2013 | mindfullness‐based cognitive therapy | group | 2 months | 8 | experienced mindfullness trainer | enhanced treatment as usual | 9 months |
| Fjorback 2013 | mindfullness | group | 3 months | 8 | experienced psychiatrist | enhanced treatment as usual | 15 months |
| Kashner 1995 | group psychological intervention + CL | group | 4 months | 8 | master level clinician | consultation letter | 12 months |
| Lidbeck 1997 | CBT | group | 3 months | 9 | trained physician | waiting list | 9 months |
| Schroder 2012 | stress intervention (psychotherapy + letter etc) | group + alone | 4 months | 9 | consultant or senior resident psychiatry | enhanced treatment as usual | 16 months |
| Katsamanis 2011 | psychophysiologic treatment + psychiatric consultation | alone | 10 weeks | 10 | psychologist or biofeedback clinician | psychiatric consultation | 10 weeks |
| Moreno 2013 | CBT + Letter (1) CBT + Letter (2) | alone (1) group (2) | 10 weeks | 10 | psychologist | usual care + letter | 12 months |
| Allen 2006 | CBT + psychiatric consultation | alone | 3 months | 10 | trained and experienced psychologist | psychiatric consultation | 15 months |
| Escobar 2007 | CBT + psychiatric consultation letter | alone | 3 months | 10 | therapist | usual care + PCL | 9 moths |
| Sattel 2012 | psychodynamic interpersonal psychotherapy | alone | 3 months | 12 | psychologist or physician with psychotherapy experience | enhanced treatment as usual | 9 months |
| Schaefert 2013 | GP training in MUPS + interpersonal psychodynamically based therapy | group | 9 months | 12 | psychosomatic specialist | GP training in MUPS | 12 months |
| Zonneveld 2012 | CBT | group | 13 weeks | 13 | psychologist | waiting list | 13 weeks |
| Schilte 2001 | disclosure intervention | alone | unclear | unclear | trained disclosure doctor | usual care | 24 months |
| Speckens 1995 | CBT | alone | 6 months | 6‐16 | trained physician and behavioural therapist | enhanced treatment as usual | 12 months |
| Kolk 2004 | psychological intervention (varied) | alone | max 6 months | max 12 | trained therapist | usual care | 12 months |
CBT: cognitive behavioural therapy; GP: general practitioner; max: maximum; MUPS: medically unexplained physical symptoms.
The duration of treatment ranged from one day (one single session) (Martin 2007) to nine months (Schaefert 2013), most often between one and three months.
The mean number of sessions varied among studies and ranged from one session (Martin 2007) to 13 sessions (Zonneveld 2012). Thirteen studies used five to 10 sessions. Four studies used one to five sessions (Burton 2012; Martin 2007; Schilte 2001; Schweickhardt 2007). Four studies used more than 10 sessions (Sattel 2012; Schaefert 2013; Speckens 1995; Zonneveld 2012).
All studies performed follow‐up assessment, but one did not report the outcomes of all follow‐ups (Schilte 2001). Reported duration of follow‐up varied between two weeks (Schweickhardt 2007) and 24 months (Schilte 2001).
Comparisons
As described in the Types of interventions section, we aimed to select the following comparator interventions: usual treatment or waiting list, attention or psychological placebo, and other psychological/physical therapies. Fifteen studies compared an intervention to usual treatment or a waiting list. One of these studies had two intervention groups (receiving psychological therapy) and one control group (receiving usual care) (Moreno 2013). None of the included studies described a placebo comparator intervention, but five included studies compared an intervention with enhanced or structured care (i.e. more than just usual care or a waiting list condition) (Fjorback 2013; Sattel 2012; Schröder 2012; Speckens 1995; Sumathipala 2000). We had not foreseen this comparator at the protocol stage, so we added this later as an additional comparison. Examples of the enhanced care control condition were a basic training for GPs in the detection and management of psychiatric disorders (Speckens 1995). See Characteristics of included studies for details.
One study used compared two psychological therapies (Schröder 2013). This study also included a waiting list group, but we excluded data from this group from our analysis as participants were not randomly assigned to this group (see Other potential sources of bias section for details). We found no studies that compared psychological interventions with physical therapies.
In one study, GPs in both study arms were trained in diagnosis and management of medically unexplained symptoms (Schaefert 2013). In addition, the GPs in the intervention group conducted group sessions for people with MUPS, together with a psychosomatic specialist.
In six studies, in both study arms a consultation letter was sent to the primary care physician after baseline assessment, in addition to the planned psychological therapy (Allen 2006; Escobar 2007; Fjorback 2013; Katsamanis 2011; Moreno 2013; Schröder 2012). This was not a reason for exclusion, and we categorised these studies according to the main comparison. In sensitivity analyses, we assessed the effect of the interventions excluding these studies.
Scale used to measure outcomes
As described in the section Types of outcome measures, we aimed to retrieve data about our primary outcomes: severity of somatic symptoms and acceptability and on our secondary outcomes: depression and anxiety, dysfunctional cognitions, emotions or behaviours, adverse events, treatment response, functional disability and quality of life, and healthcare use.
Severity or intensity of somatic symptoms (or both) was most often measured using the PHQ‐15 (Kroenke 2002, five studies), the subscale 'Somatisation' of the SCL‐90R (Derogatis 1986, five studies), the SOMS (Rief 1997, three studies), the BSI (Derogatis 1983, three studies), and the Clinical Global Impession Scale for Somatoform Disorders (CGI‐SD; APA 2000, three studies). Two studies used more than one instrument for severity or intensity of somatic symptoms (or both) (Escobar 2007; Martin 2007). Two other studies did not report severity or intensity of somatic symptoms (or both) (Kashner 1995; Lidbeck 1997). Acceptability was calculated from provided data about the total number of randomised participants in the study groups and the total number of participants who completed assessments at end of treatment (also in the control group). For anxiety and depressive symptoms, studies mostly used Hospital Anxiety and Depression Scale (HADS) subscales (Zigmond 1983, five studies), SCL‐90R subscales (Derogatis 1986; three studies), and PHQ‐9 (Kroenke 2001, three studies). Dysfunctional cognitions, emotions, and behaviours were mostly operationalised as health anxiety, and measured by the WI (Pilowsky 1967, seven studies). Withdrawals due to adverse events were incidentally registered by the authors. Treatment response was mainly registered using the clinician‐rated CGI (Guy 1976, three studies). Functional disability was mainly measured using the SF‐36 (Ware 1992, 10 studies) and the Short Form 12 Questionnaire (SF‐12; a shortened version of the SF‐36, three studies). No standardised questionnaire was used to measure healthcare use, and mostly consultation counts (as counted by physicians or participants) were reported.
Excluded studies
Reasons for exclusion of studies were recorded for all studies that were read in full‐text. Common reasons included the absence of a randomisation procedure or a participant selection method that did not include a standardised interview or questionnaire. We also excluded three studies in which GPs were trained to provide some psychological therapy (Blankenstein 2001; Toft 2010;Van der Feltz‐Cornelis 2006); these studies were included in another Cochrane review (Rosendal 2013). The Characteristics of excluded studies table provides an overview of all excluded studies.
Ongoing studies
Searches of trial databases and personal communication identified eight ongoing studies that appear to meet the inclusion criteria for this review (Agger 2012; Hassett 2007; Olde Hartman 2013; Rief 2013; Schröder 2014; Sitnikova 2014; Steel 2011; Zimmermann 2014). No findings have yet been reported or obtained from these studies. The Characteristics of ongoing studies table provides an overview of these ongoing studies.
Studies awaiting classification
There are six studies awaiting classification, mostly due to unavailability of the full‐text articles. We attempted to contact all study authors and searched for the articles in various sources. All studies awaiting classification are potentially eligible studies that could not yet be incorporated into the review (see Characteristics of studies awaiting classification table).
Risk of bias in included studies
We classified the methodological quality of the 21 studies according to The Cochrane Collaboration's tool for assessing the risk of bias (see above). The final ratings are reported in the Characteristics of included studies table. A risk of bias summary graph (Figure 2) and summary figure (Figure 3) are also presented.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
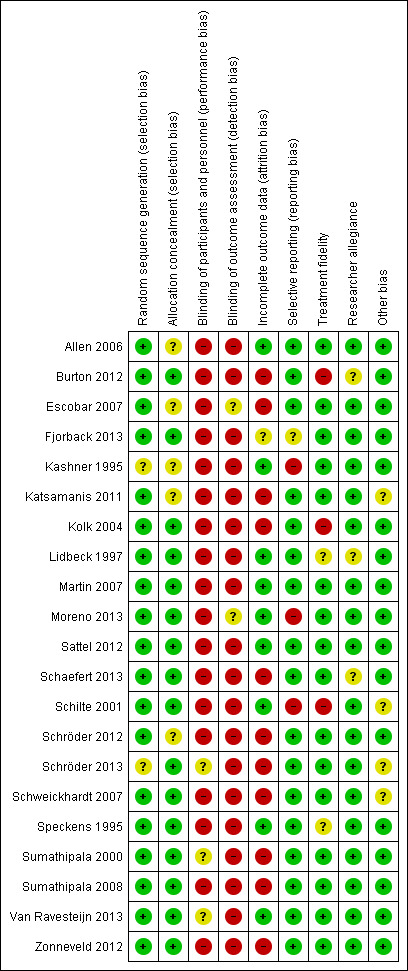
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Sequence generation
While all studies specified that participants were randomly allocated to conditions (or GP practices randomised to treatment or control conditions), there were two studied that did not describe how sequence generation was performed (Kashner 1995; Schröder 2013). Therefore, we rated them as 'uncertain'. We rated the other studies as 'low risk' as they all used random sequence generation methods, whether by computer or non‐digital, for example, using random number tables (Kolk 2004; Martin 2007), or a sequence of labelled cards in envelopes or bags (Fjorback 2013; Schilte 2001; Schweickhardt 2007; Sumathipala 2000).
Allocation concealment
For five studies, it was unclear who performed allocation, or whether the person allocating participants to the trial groups was independent. Therefore, we rated these studies 'unclear' (Allen 2006; Escobar 2007; Kashner 1995; Katsamanis 2011; Schröder 2013). We rated the remaining 16 studies 'low risk of bias' as there was an adequate description of the person performing allocation or the relation to the researchers and therapists(e.g. "randomisation was carried out independently by a nurse who was not participating in the study") (Lidbeck 1997).
Blinding
Blinding of participants and personnel
In 18 studies, blinding of participants and personnel was not possible, due to the nature of the interventions (e.g. psychological group therapy versus waiting list). As this may have influenced the judgement, we rated almost all studies 'high risk'. We rated two studies 'unclear' because one of the two groups (participants or personnel) was blinded and the other was not (Sumathipala 2000; Van Ravesteijn 2013). One study did not describe blinding of personnel (Schröder 2013), and, therefore, we rated it 'unclear'.
Blinding of outcome assessment
In 19 studies, blinding of outcome assessment was not possible as most outcomes were participant reported. In one study, outcomes were assessed by blinded interviewers, but they did this together with the participants (who were not blinded) (Escobar 2007). We rated this study 'unclear'. One study mainly used clinician‐rated instruments (Moreno 2013). The outcome assessor was blinded, but, as there also were a few participant report instruments (and participants were not blinded), we rated this study 'unclear'.
Incomplete outcome data
All studies reported follow‐up rates; nine (43%) studies reported a loss to follow‐up of 20% or less. We rated these studies 'low risk'. We rated one study 'unclear', because it had a high loss to follow‐up, but corrected for this statistically by multiple imputation (Fjorback 2013). The remaining 11 studies reported high loss to follow‐up (greater than 20%) and, therefore, we rated them 'high risk'.
Selective reporting
Seventeen studies reported all intended outcomes and, therefore, we rated them 'low risk'. For one study, a protocol was lacking, therefore it was impossible to evaluate the possibility of selective outcome reporting (Fjorback 2013). We rated this study 'unclear'. We rated the remaining three studies 'high risk'. In Kashner 1995, the outcome 'days in bed' was described as assessed, but was not reported in the article. In Moreno 2013, healthcare use and CGI were mentioned as outcomes in the protocol, but they were not reported. Schilte 2001 performed follow‐up measurements at six, 12, and 24 months, but only reported outcomes of the last follow‐up moment.
Treatment fidelity
Sixteen studies used a treatment manual or protocol for studied treatments. We rated them 'low risk'. Three studies did not apply a structured intervention according to a protocol (Burton 2012; Kolk 2004; Schilte 2001), therefore, we rated them 'high risk'. The two remaining studies did provide information about a form of structure in treatment, but did not mention a protocol or manual for this. We rated them 'unclear'.
Researcher allegiance
In 18 studies, researchers did not report to have a preference for one of the treatment modalities. In the studies of Burton 2012, Lidbeck 1997, and Schaefert 2013, an author was also (one of) the therapist(s), which may have caused some bias. Therefore, we rated these studies 'unclear'.
Other potential sources of bias
We included two multiple intervention studies (Moreno 2013; Schröder 2013). In the first study, data were presented for each groups to which participants were randomised, so no other potential sources of bias were found (rating: 'low risk'). In the second study, participants were randomised for CBT or progressive muscle relaxation (PMR) using random sequences. When both groups were full, newly included participants were allocated to the waiting list group. In a later stage, these participants were included in both intervention groups. As participants were their own controls due to this method, we decided to exclude data from the waiting list group from analysis. For this reason, we rated this study 'unclear'.
One of the studies was a CRCT (Schaefert 2013). GPs were randomised, after which individuals were recruited. We considered the randomisation method and statistical analysis appropriate for the study design.
In the studies of Schilte 2001 and Katsamanis 2011, we found considerable baseline imbalances. In the study of Schweickhardt 2007, a high percentage (29%) of participants from the control group became involved in psychotherapy. This may have influenced the results, although this study provided data for only one outcome (acceptability) and the effects were in the same order of magnitude as in other studies. We rated these three studies 'unclear'.
Effects of interventions
For the description of the results, we stratified the comparisons in the following way (as per the categories of therapies presented in Types of interventions, where data allowed):
-
Psychological therapies versus usual care or waiting list:
CBT versus usual care or waiting list;
behavioural therapy versus usual care or waiting list;
third‐wave CBT versus usual care or waiting list;
psychodynamic therapy versus usual care or waiting list;
integrative therapies versus usual care or waiting list;
-
Psychological therapies versus enhanced or structured care:
CBT versus enhanced or structured care;
third‐wave CBT versus enhanced or structured care;
psychodynamic therapy versus enhanced or structured care;
-
Psychological therapy versus another psychological therapy:
CBT versus behavioural therapy.
Most studies provided data for some of the outcomes. One study did not provide any data that were suitable for meta‐analysis, because the authors only reported change scores (Schilte 2001). Across all comparisons, outcomes, and time points, we created 44 forest plots. Most of these included data from only a limited number of studies: 25 of the forest plots included three or fewer studies, only two included 10 or more studies. Below, we present the results of the meta‐analyses. We also give attention to the subgroups that included a considerable proportion of the studies contributing to the overall comparisons: CBT versus usual care or waiting list and CBT versus enhanced or structured care, because these subgroups were more homogeneous in terms of type of intervention than the overall comparisons. In terms of risk of bias, the studies that provided outcomes for the meta‐analyses were representative for the whole group (i.e. covered the broad spectrum of risk of bias assessments across items).
1. Pschological therapy versus usual care or waiting list
Fifteen studies, with 1805 randomised participants, compared some form of psychological therapy with usual care or waiting list controls. They addressed the following psychological therapies:
CBT versus usual care or waiting list: 10 studies, 1037 randomised participants (Allen 2006; Burton 2012; Escobar 2007; Kashner 1995; Lidbeck 1997; Martin 2007; Moreno 2013; Schweickhardt 2007; Sumathipala 2000; Zonneveld 2012);
behavioural therapy versus usual care or waiting list: two studies, 209 randomised participants (Katsamanis 2011; Schilte 2001);
third‐wave CBT versus usual care or waiting list: one study, 125 randomised participants (Van Ravesteijn 2013);
psychodynamic therapy versus usual care or waiting list: one study, 328 randomised participants (Schaefert 2013);
integrative therapies versus usual care or waiting list: one study, 106 randomised participants (Kolk 2004).
In four of these studies, this was combined with a consultation letter sent to the primary care physician after baseline assessment, in both treatment arms (Allen 2006; Escobar 2007; Katsamanis 2011; Moreno 2013). A consultation letter provided recommendations for the primary care physician tailored to the individual person's diagnosis, symptoms, and problems. In one study, the GPs in both treatment groups were trained in diagnosis and management of MUPS (Schaefert 2013).
Below we describe the main results, sorted by outcomes. Graded evidence (i.e. using the GRADE approach) is only described for the outcomes that were selected for the 'Summary of findings' tables. Apart from CBT, for each of the other types of psychological therapy only one study provided outcomes. Hence, for each of these separate treatment types there was insufficient evidence. Below, we described results for the whole group and for the subgroup of studies that compared CBT with usual treatment.
Primary outcomes
1.1 Severity of somatic symptoms
Combining all studies that compared some psychological therapy with usual care or waiting list, psychological therapies were significantly more effective at end of treatment, though the effect was small (SMD ‐0.34; 95% CI ‐0.53 to ‐0.16; 10 studies, 1081 analysed participants, Analysis 1.1). Heterogeneity was moderate (I2 = 49%), and the overall quality of the evidence was low (Table 1). Compared with usual care, the subgroup of studies that used CBT were also significantly more effective in reducing severity of symptoms at end of treatment (SMD ‐0.37; 95% CI ‐0.69 to ‐0.05; 6 studies, 593 participants, random‐effects model; Analysis 1.1, subgroup 1.1.1). Heterogeneity was substantial (I2 = 70%), and the overall quality of the evidence was low. The point estimates of all but one of the studies favoured the CBT group. The two studies with the smallest effects offered low‐intensity CBT (Burton 2012; Martin 2007). A post‐hoc analysis without these two studies provided an SMD of ‐0.58 (a moderate effect size) (95% CI ‐0.77 to ‐0.38) and reduced heterogeneity (I2 = 0%.
1.1. Analysis.

Comparison 1 Psychological therapies versus usual care or waiting list, Outcome 1 Severity of somatic symptoms at end of treatment.
At follow‐up, measurements within one year of follow‐up, the effect of psychological therapies remained significant (SMD ‐0.24; 95% CI ‐0.37 to ‐0.11; 7 studies, 950 participants; I2 = 0%). The same was the case for the subgroup of CBT studies (SMD ‐0.29; 95% CI ‐0.49 to ‐0.09; 4 studies, 496 participants; Analysis 1.2, subgroup 1.2.1). Heterogeneity was low (I2 = 17%).
1.2. Analysis.
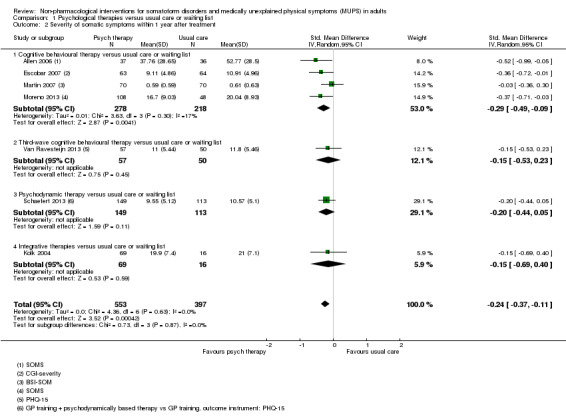
Comparison 1 Psychological therapies versus usual care or waiting list, Outcome 2 Severity of somatic symptoms within 1 year after treatment.
Only two studies (all of CBT) with 228 participants provided data for this severity of symptoms beyond one year of follow‐up (SMD ‐0.52; 95% CI ‐0.80 to ‐0.24; Analysis 1.3). Heterogeneity was low (I2 = 0%).
1.3. Analysis.
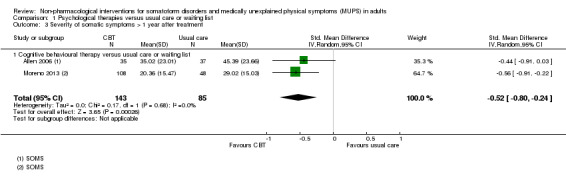
Comparison 1 Psychological therapies versus usual care or waiting list, Outcome 3 Severity of somatic symptoms > 1 year after treatment.
1.2 Acceptability
Compared with usual care, psychological therapies resulted in a higher proportion of drop‐outs (RR acceptability 0.93; 95% CI 0.88 to 0.99 favouring usual care; 14 studies, 1644 participants; Analysis 1.4). Heterogeneity was moderate (I2 = 70%). For the studies comparing CBT with usual care, results were of the same magnitude but no longer statistically significant (RR acceptability 0.93; 95% CI 0.85 to 1.01 favouring usual care; 10 studies, 1037 participants; Analysis 1.4, subanalysis 1.4.1). Heterogeneity was considerable (I2 = 78%). See under Subgroup analysis and investigation of heterogeneity for results of a subgroup analysis. The overall quality of the evidence for this outcome was moderate.
1.4. Analysis.
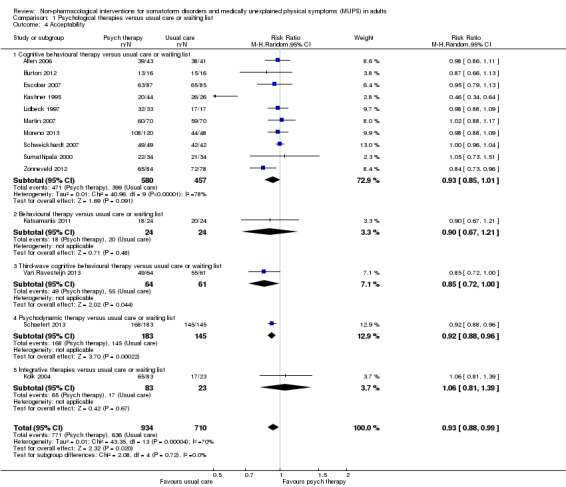
Comparison 1 Psychological therapies versus usual care or waiting list, Outcome 4 Acceptability.
Secondary outcomes
1.3 Severity of anxiety or depressive symptoms (or both)
For participant‐rated anxiety symptoms, there was no significant difference at end of treatment (SMD 0.06; 95% CI ‐0.20 to 0.32, 4 studies, 270 participants; Analysis 1.5). For the studies comparing CBT with usual care, results were similar (SMD 0.07; 95% CI ‐0.22 to 0.37; 3 studies, 185 participants; Analysis 1.5). Within one year of follow‐up only two studies were available (SMD 0.18; 95% CI ‐0.22 to 0.58; 134 participants; Analysis 1.7).
1.5. Analysis.
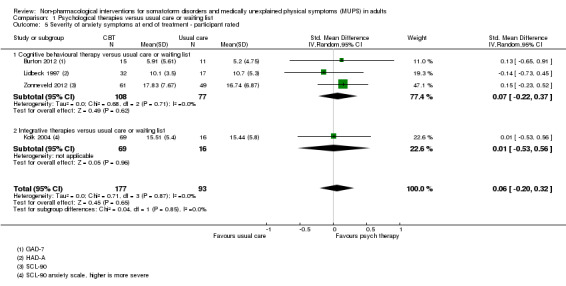
Comparison 1 Psychological therapies versus usual care or waiting list, Outcome 5 Severity of anxiety symptoms at end of treatment ‐ participant rated.
1.7. Analysis.

Comparison 1 Psychological therapies versus usual care or waiting list, Outcome 7 Severity of anxiety symptoms within 1 year after treatment ‐ participant rated.
For clinician‐rated anxiety symptoms at end of treatment, there was a statistically significant difference at end of treatment in favour psychological therapies (SMD ‐0.40; 95% CI ‐0.63 to ‐0.17; 3 studies, 320 participants; Analysis 1.6). Within and beyond one year of follow‐up, differences remained statistically significant (within one year: SMD ‐0.66; 95% CI ‐1.15 to ‐0.18, 2 studies both CBT, 251 participants; Analysis 1.8; beyond one year: SMD ‐0.91; 95% CI ‐1.26 to ‐0.55; 1 study, 156 participants; Analysis 1.9).
1.6. Analysis.
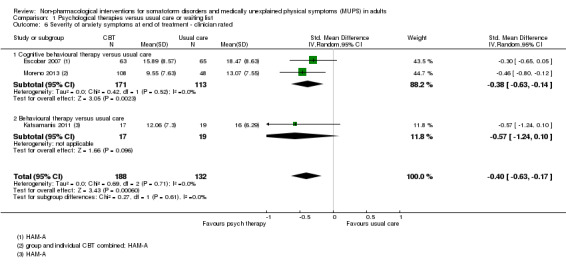
Comparison 1 Psychological therapies versus usual care or waiting list, Outcome 6 Severity of anxiety symptoms at end of treatment ‐ clinician rated.
1.8. Analysis.

Comparison 1 Psychological therapies versus usual care or waiting list, Outcome 8 Severity of anxiety symptoms within 1 year after treatment ‐ clinician rated.
1.9. Analysis.
Comparison 1 Psychological therapies versus usual care or waiting list, Outcome 9 Severity of anxiety symptoms > 1 year after treatment ‐ clinician rated.
For participant‐rated depressive symptoms, there was no significant difference at end of treatment (SMD ‐0.03; 95% CI ‐0.22 to 0.16; 6 studies, 661 participants; Analysis 1.10). Similar results were found for the studies that compared CBT with usual care (SMD 0.09; 95% CI ‐0.13 to 0.31; 4 studies, 325 participants; Analysis 1.10), and for outcomes after not more than one year of follow‐up (SMD 0.04; 95% CI ‐0.34 to 0.42; four studies, 535 participants; Analysis 1.12).
1.10. Analysis.
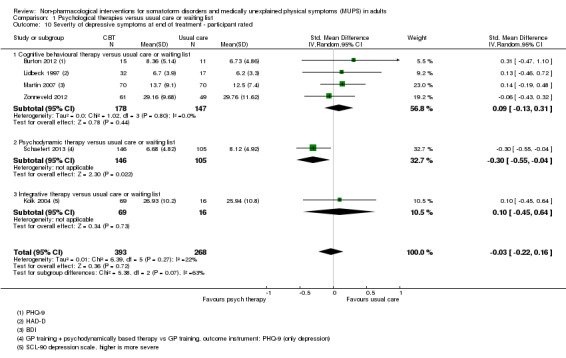
Comparison 1 Psychological therapies versus usual care or waiting list, Outcome 10 Severity of depressive symptoms at end of treatment ‐ participant rated.
1.12. Analysis.

Comparison 1 Psychological therapies versus usual care or waiting list, Outcome 12 Severity of depressive symptoms within 1 year after treatment ‐ participant rated.
For clinician‐rated depressive symptoms, there was a statistically significant difference at end of treatment in favour of p
sychological therapies (SMD ‐0.25; 95% CI ‐0.48 to ‐0.02; 3 studies, 316 participants; Analysis 1.11). Within one year of follow‐up, the difference was no longer statistically significant (SMD ‐0.55; 95% CI ‐1.17 to 0.07; 2 studies, 251 participants; Analysis 1.13). Only one study reported on this outcome beyond one year after treatment (SMD ‐0.81; 95% CI ‐1.16 to ‐0.46; 156 participants; Analysis 1.14).
1.11. Analysis.
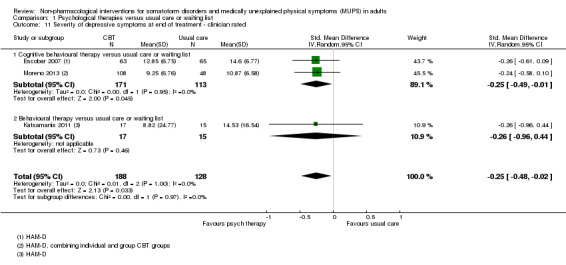
Comparison 1 Psychological therapies versus usual care or waiting list, Outcome 11 Severity of depressive symptoms at end of treatment ‐ clinician rated.
1.13. Analysis.
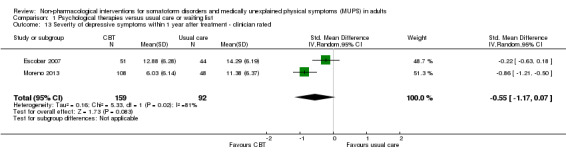
Comparison 1 Psychological therapies versus usual care or waiting list, Outcome 13 Severity of depressive symptoms within 1 year after treatment ‐ clinician rated.
1.14. Analysis.

Comparison 1 Psychological therapies versus usual care or waiting list, Outcome 14 Severity of depressive symptoms > 1 year after treatment ‐ clinician rated.
1.4 Dysfunctional cognitions, emotions, and behaviours
Three studies, two of which compared CBT with usual care, with 440 participants, reported on dysfunctional cognitions, emotions, and behaviours. At end of treatment, there was no significant difference between the two groups (SMD ‐0.11; 95% CI ‐0.37 to 0.16; Analysis 1.15). The quality of the evidence was moderate. At follow‐up within one year, differences remained non‐significant (SMD ‐0.16; 95% CI ‐0.38 to 0.07; Analysis 1.16).
1.15. Analysis.
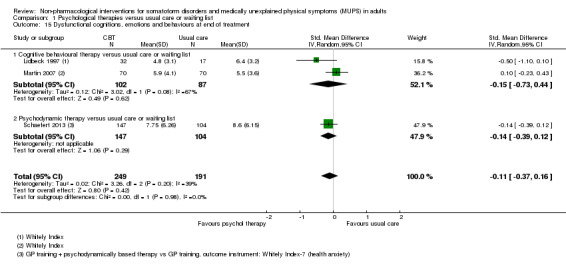
Comparison 1 Psychological therapies versus usual care or waiting list, Outcome 15 Dysfunctional cognitions, emotions and behaviours at end of treatment.
1.16. Analysis.
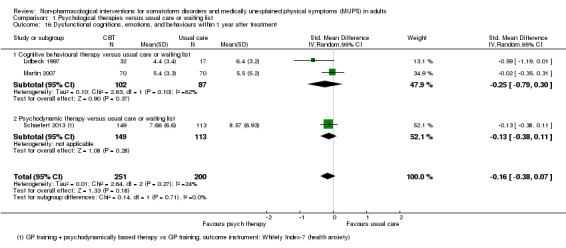
Comparison 1 Psychological therapies versus usual care or waiting list, Outcome 16 Dysfunctional cognitions, emotions, and behaviours within 1 year after treatment.
1.5 Adverse events
Only three studies, all comparing CBT with usual care, reported on adverse events during the treatment period. One study could not be included in the meta‐analysis, because no adverse events were found in both groups. The pooled result of the other two studies also showed no significant differences between both conditions (RR 1.31; 95% CI 0.47 to 3.66; 445 participants; I2 = 0%; Analysis 1.17).
1.17. Analysis.
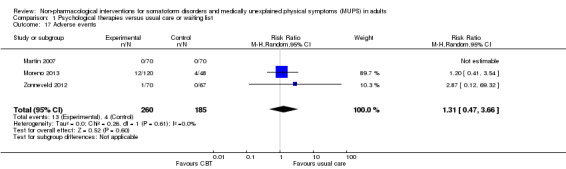
Comparison 1 Psychological therapies versus usual care or waiting list, Outcome 17 Adverse events.
1.6 Treatment response (clinician rated)
All four studies addressing clinician‐rated treatment response comparing CBT with usual care. At end of treatment, results strongly favoured the treatment group (RR 3.30; 95% CI 2.08 to 5.21; 4 studies, 391 participants; I2 = 19%; Analysis 1.18). We considered the quality of the evidence to be low for this outcome. Three studies provided data for clinician‐rated treatment response within one year after end of treatment, still in favour of the treatment group (RR 2.53; 95% CI 1.25 to 5.10; 332 participants; I2 = 59%; Analysis 1.19). At longer follow up (greater than one year after treatment) only two studies reported outcomes, highly favouring the treatment group (RR 10.31; 95% CI 2.95 to 36.02; 240 participants; Analysis 1.20).
1.18. Analysis.

Comparison 1 Psychological therapies versus usual care or waiting list, Outcome 18 Treatment response at end of treatment.
1.19. Analysis.

Comparison 1 Psychological therapies versus usual care or waiting list, Outcome 19 Treatment response within 1 year after treatment.
1.20. Analysis.
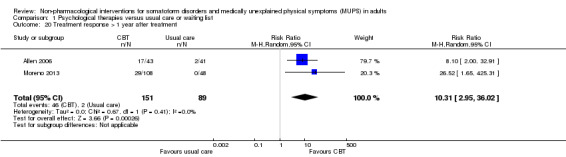
Comparison 1 Psychological therapies versus usual care or waiting list, Outcome 20 Treatment response > 1 year after treatment.
1.7 Functional disability and quality of life
Seven studies, of which four addressing CBT reported on functional disability and quality of life, using a variety of instruments. At the end of treatment, a statistically significant effect was found favouring the psychological therapies (SMD 0.17; 95% CI 0.03 to 0.32; 7 studies, 730 participants; I2 = 0%; Analysis 1.21). We judged the evidence to be moderate. At follow‐up within one year after treatment, differences were similar but no longer significant (less than one year: SMD 0.16; 95% CI ‐0.01 to 0.33; 4 studies, 526 participants; I2 = 0%; Analysis 1.22). After one year, only one study provided data for functional disability and quality of life (Analysis 1.23).
1.21. Analysis.

Comparison 1 Psychological therapies versus usual care or waiting list, Outcome 21 Functional disability and quality of life at end of treatment.
1.22. Analysis.
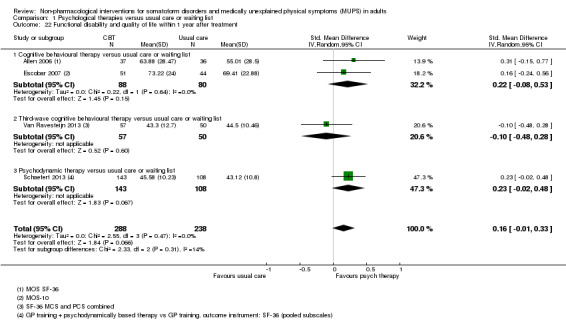
Comparison 1 Psychological therapies versus usual care or waiting list, Outcome 22 Functional disability and quality of life within 1 year after treatment.
1.23. Analysis.
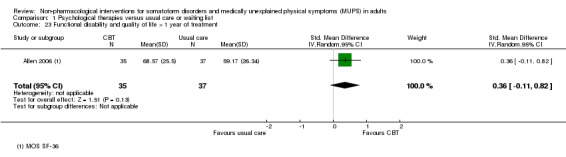
Comparison 1 Psychological therapies versus usual care or waiting list, Outcome 23 Functional disability and quality of life > 1 year of treatment.
Four studies compared CBT with usual care. At end of treatment, a non‐significant difference was found favouring CBT (SMD 0.15; 95% CI ‐0.06 to 0.37; 4 studies, 341 participants; I2 = 0%; Analysis 1.21, subanalysis 1.21.1).
1.8 Healthcare use
Six studies assessed healthcare use, operationalised in different ways, with moderate quality of evidence. During the treatment phase, two studies found a significant difference in the number of participant‐initiated doctor visits and medication usage in favour of CBT (SMD ‐0.68; 95% CI ‐1.06 to ‐0.30; 117 participants; Analysis 1.24). In the period less than one year after treatment, perhaps a more relevant timeframe, four studies found no clear evidence of a difference (SMD ‐0.09; 95% CI ‐0.31 to 0.12; 532 participants; I2 = 20%; Analysis 1.25). We judged the quality of the evidence to be moderate. For one of the studies, the effect was in the opposite direction, that is, favouring the control group (Kolk 2004). No study provided data for healthcare use beyond one year after treatment. See footnotes of analyses for details about the way healthcare use was assessed.
1.24. Analysis.
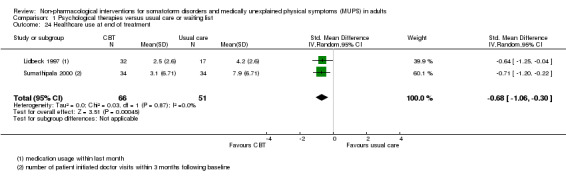
Comparison 1 Psychological therapies versus usual care or waiting list, Outcome 24 Healthcare use at end of treatment.
1.25. Analysis.
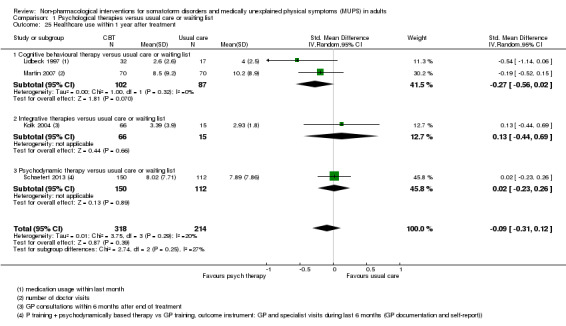
Comparison 1 Psychological therapies versus usual care or waiting list, Outcome 25 Healthcare use within 1 year after treatment.
2. Psychological therapy versus enhanced or structures care
Five studies with 680 randomised participants compared a certain psychological therapy with enhanced or structured care (see Characteristics of included studies for details). They addressed the following treatments:
CBT versus enhanced or structured care: three studies, 349 randomised participants (Schröder 2012; Speckens 1995;Sumathipala 2008);
third‐wave CBT versus enhanced or structured care: one study, 120 randomised participants (Fjorback 2013);
psychodynamic therapy versus enhanced or structured care: one study, 211 randomised participants (Sattel 2012).
In two of these studies, treatment was combined with a consultation letter sent to the primary care physician after baseline assessment, in both treatment arms (Fjorback 2013; Schröder 2012).
Below we describe the main results, sorted by outcomes. Apart from CBT, only one or two trials provided data for each of the three other types of psychological therapy; hence, for each of these other treatment types there was insufficient evidence. Below, we do not describe the results for these subgroups separately. The reader is referred to the combined forest plots for each outcome.
Primary outcomes
2.1 Severity of somatic symptoms
Five studies (with 624 analysed participants) assessed severity of somatic symptoms comparing some psychological therapy versus enhanced care (pooled SMD ‐0.19; 95% CI ‐0.43 to 0.04; I2 = 53%; Analysis 2.1). We considered the quality of the evidence to be low (Table 2). Within one year of follow‐up, this effect was similar but now statistically significant (SMD ‐0.21; 95% CI ‐0.40 to ‐0.02; 5 studies, 593 participants; I2 = 25%; Analysis 2.2). Only two studies each comparing a different psychological therapy to enhanced care, assessed severity of somatic symptoms beyond one year after treatment (SMD ‐0.32; 95% CI ‐0.73 to 0.10; 172 participants; Analysis 2.3). The subgroup of studies comparing CBT with enhanced care showed similar results. Heterogeneity was substantial at the end of treatment (I2 = 62%) and moderate within one year after treatment (I2 = 39%).
2.1. Analysis.
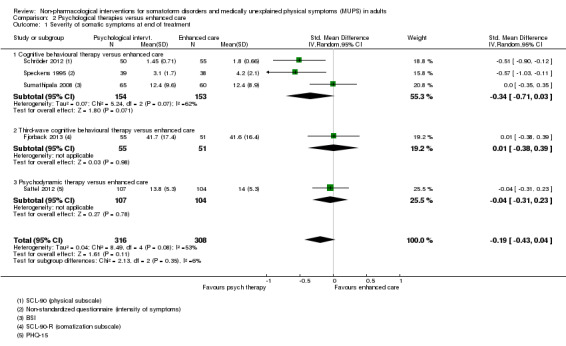
Comparison 2 Psychological therapies versus enhanced care, Outcome 1 Severity of somatic symptoms at end of treatment.
2.2. Analysis.

Comparison 2 Psychological therapies versus enhanced care, Outcome 2 Severity of somatic symptoms within 1 year after treatment.
2.3. Analysis.
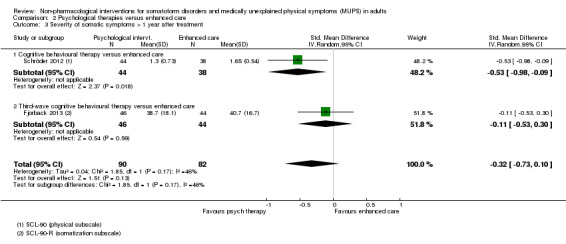
Comparison 2 Psychological therapies versus enhanced care, Outcome 3 Severity of somatic symptoms > 1 year after treatment.
2.2 Acceptability
Five studies, with 679 analysed participants, showed that psychological therapies were less acceptable in terms of drop‐outs than enhanced care (RR 0.93; 95% CI 0.87 to 1.00; Analysis 2.4). Heterogeneity was moderate (I2 = 36%), and we judged the quality of the evidence to be moderate. The largest subgroup was CBT. Compared with enhanced care, moderate‐quality evidence showed that there was no clear difference between CBT and enhanced or structured care (RR 0.91; 95% CI 0.82 to 1.02; 3 studies, 331 participants; Analysis 2.4, subgroup 2.4.1). Heterogeneity was moderate to considerable (I2 = 50%).
2.4. Analysis.
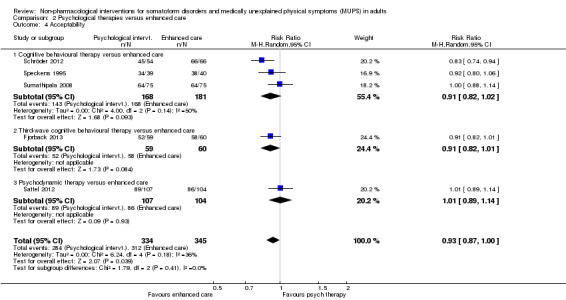
Comparison 2 Psychological therapies versus enhanced care, Outcome 4 Acceptability.
Secondary outcomes
2.3 Severity of anxiety or depressive symptoms (or both)
Five studies assessed severity of anxiety or depressive symptoms (or both) at end of treatment (SMD ‐0.14; 95% CI ‐0.30 to 0.02; 624 analysed participants; I2 = 0%; Analysis 2.5), showing no clear difference. Similar results were found within one year after treatment (SMD ‐0.13; 95% CI ‐0.29 to 0.03; 5 studies, 593 participants; Analysis 2.6) and beyond one year after treatment (SMD ‐0.26; 95% CI ‐0.55 to 0.03; 2 studies, 184 participants; Analysis 2.7).
2.5. Analysis.
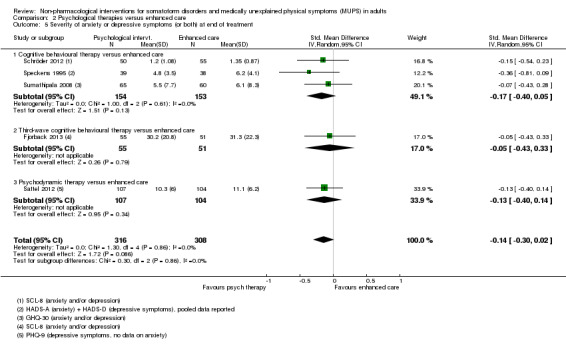
Comparison 2 Psychological therapies versus enhanced care, Outcome 5 Severity of anxiety or depressive symptoms (or both) at end of treatment.
2.6. Analysis.
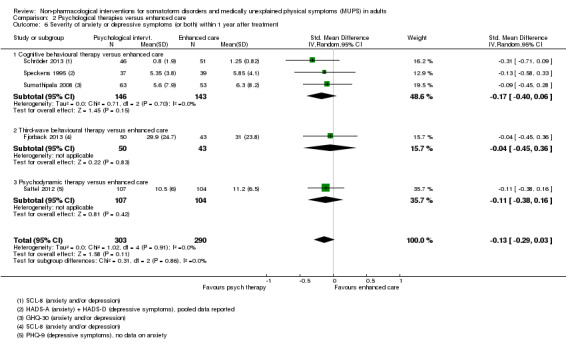
Comparison 2 Psychological therapies versus enhanced care, Outcome 6 Severity of anxiety or depressive symptoms (or both) within 1 year after treatment.
2.7. Analysis.
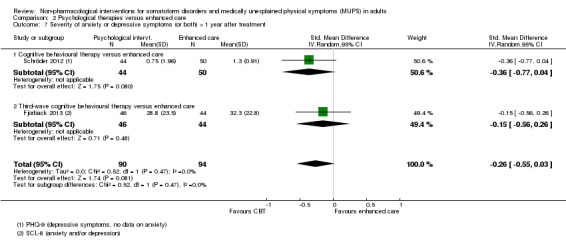
Comparison 2 Psychological therapies versus enhanced care, Outcome 7 Severity of anxiety or depressive symptoms (or both) > 1 year after treatment.
The studies investigating CBT showed no significant difference in level of anxiety and depressive symptoms between CBT and enhanced care at end of treatment (SMD ‐0.17; 95% CI ‐0.40 to 0.05; 3 studies. 307 participants; Analysis 2.5, subanalysis 2.5.1) and within one year after treatment (SMD ‐0.17; 95% CI ‐0.40 to 0.06; 3 studies, 289 participants; Analysis 2.6, subanalysis 2.6.1). Heterogeneity was low (I2 = 0% at end of treatment and within one year after treatment). Only one CBT study reported on severity of anxiety or depressive symptoms (or both) beyond one year after treatment (Analysis 2.7).
2.4 Dysfunctional cognitions, emotions, and behaviours
Four studies, with 499 analysed participants, provided data for dysfunctional cognitions, emotions, and behaviours at end of treatment, showing no clear evidence of a difference between psychological therapy and enhanced care (SMD ‐0.09; 95% CI ‐0.29 to 0.10; I2 = 14%; Analysis 2.8). We judged quality of the evidence to be moderate. At follow‐up within one year after treatment, the difference was statistically significant (P value = 0.05), favouring the psychological therapy over enhanced care (SMD ‐0.24; 95% CI ‐0.49 to 0.00; 4 studies, 477 participants; I2 = 42%, Analysis 2.9). Beyond one year of follow‐up, only two studies reported on dysfunctional cognitions, emotions, and behaviours and showed no significant difference (SMD ‐0.58; 95% CI ‐1.27 to 0.11; 2 studies, 184 participants; I2 = 82%; Analysis 2.10).
2.8. Analysis.

Comparison 2 Psychological therapies versus enhanced care, Outcome 8 Dysfunctional cognitions, emotions, and behaviours at end of treatment.
2.9. Analysis.
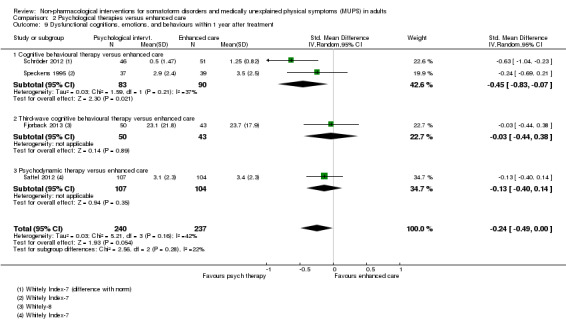
Comparison 2 Psychological therapies versus enhanced care, Outcome 9 Dysfunctional cognitions, emotions, and behaviours within 1 year after treatment.
2.10. Analysis.

Comparison 2 Psychological therapies versus enhanced care, Outcome 10 Dysfunctional cognitions, emotions, and behaviours > 1 year after treatment.
The two studies comparing CBT with enhanced care showed no clear evidence of a difference in dysfunctional cognitions, emotions, and behaviours at end of treatment (SMD ‐0.28; 95% CI ‐0.57 to 0.01; 2 studies, 182 participants; Analysis 2.8). Heterogeneity was low (I2 = 0%). However, within one year after treatment, levels of dysfunctional cognitions, emotions, and behaviours were significantly lower for CBT (SMD ‐0.45; 95% CI ‐0.83 to ‐0.07; 2 studies, 173 participants; Analysis 2.9), though more heterogeneous (I2 = 37%). This effect was even more significant at more than one year after treatment, although this comparison only included one study (SMD ‐0.94; 95% CI ‐1.36 to ‐0.51; 94 participants; Analysis 2.10) (Schröder 2012).
2.5 Adverse events
None of the studies comparing psychological therapy versus enhanced or structures care reported information about adverse events.
2.6 Treatment response
None of the included studies comparing psychological therapy versus enhanced or structures care reported about treatment response using a standardised method as described in Secondary outcomes. One study reported about treatment response, but for this outcome measure the SF‐36 (Ware 1992) was used (Schröder 2012). In this review, we used the outcomes of this questionnaire in the analyses of functional disability. Another study reported about participants' perceived change in symptoms (Speckens 1995). At all measurement moments after baseline, participants were asked if their symptoms were "recovered", "improved", "the same", or "worse" since the previous measurement, using a non‐standardised questionnaire. At the end of treatment, 32 (82%) participants in the intervention group declared that symptoms were improved or recovered versus 24 (64%) participants in the control group. Six months after treatment, 27 (73%) participants of intervention group reported recovery or improvement versus 23 (59%) participants of the control group.
2.7 Functional disability and quality of life
At end of treatment, four studies with 497 analysed participants reporting on functional disability and quality of life, found no significant difference (SMD 0.13; 95% CI ‐0.05 to 0.30; I2 = 0%; Analysis 2.11). We considered the quality of the evidence to be moderate. Within one year of follow‐up, there was a small effect in favour of psychological therapies (SMD 0.20; 95% CI 0.02 to 0.38; 5 studies, 727 participants; I2 = 0%; Analysis 2.12). Only two studies reported on functional disability and quality of life beyond one year of follow‐up and there was no clear evidence of a difference between the interventions (SMD 0.22; 95% CI ‐0.16 to 0.60; 2 studies, 184 participants; Analysis 2.13).
2.11. Analysis.
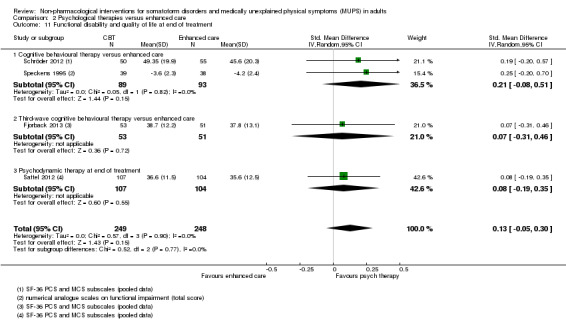
Comparison 2 Psychological therapies versus enhanced care, Outcome 11 Functional disability and quality of life at end of treatment.
2.12. Analysis.

Comparison 2 Psychological therapies versus enhanced care, Outcome 12 Functional disability and quality of life within 1 year after treatment.
2.13. Analysis.

Comparison 2 Psychological therapies versus enhanced care, Outcome 13 Functional disability and quality of life > 1 year of treatment.
For the studies comparing CBT with enhanced care, at end of treatment, moderate‐quality evidence showed no significant difference in terms of level of function/quality of life, with a large CI but homogeneous population (SMD 0.21; 95% CI ‐0.08 to 0.51; 2 studies, 182 participants; I2 = 0%; Analysis 2.11). There was a small but significant difference in favour of CBT within one year after treatment (SMD 0.30; 95% CI 0.00 to 0.60; 2 studies, 173 participants; Analysis 2.12). At this time point, heterogeneity was low (I2 = 0%). After one year of follow‐up, only one study provided data. In this study, CBT resulted in a significantly higher level of function compared with enhanced care (SMD 0.42; 95% CI 0.01 to 0.83; 94 participants; Analysis 2.13).
2.8 Healthcare use
Only two studies provided usable data for this analysis and quality of the evidence was low (Sumathipala 2008; Sattel 2012). There were no significant differences healthcare use between psychological therapies and enhanced care, neither at end of treatment (Analysis 2.14), nor within one year after end of treatment (Analysis 2.15). See footnotes of analyses for details about the way healthcare use was assessed.
2.14. Analysis.
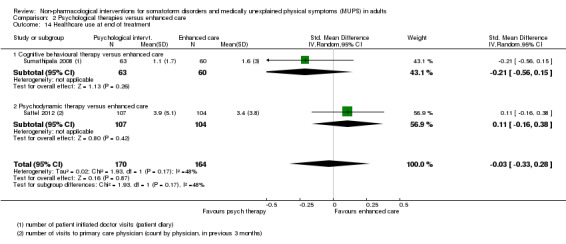
Comparison 2 Psychological therapies versus enhanced care, Outcome 14 Healthcare use at end of treatment.
2.15. Analysis.
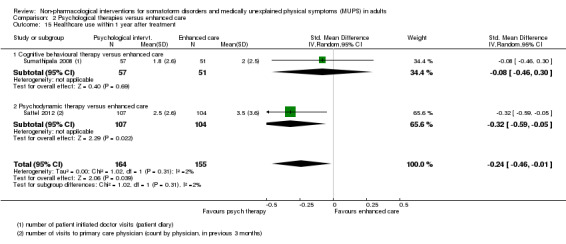
Comparison 2 Psychological therapies versus enhanced care, Outcome 15 Healthcare use within 1 year after treatment.
3. Psychological therapy versus other psychological therapy
Only one included study addressed psychological therapy versus other psychological therapy (Schröder 2013, 173 randomised participants). The study compared CBT with PMR therapy. The study also included a waiting list group, but we excluded this group from analyses as participants in the waiting list group were not randomly assigned (for details, see the Other potential sources of bias section).
Primary outcomes
3.1 Severity of somatic symptoms
No significant difference was found for severity of somatic symptoms between CBT and PMR at end of treatment (SMD 0.10; 95% CI ‐0.33 to 0.53; 84 participants; Analysis 3.1).
3.1. Analysis.

Comparison 3 Cognitive behavioural therapy versus behavioural therapy, Outcome 1 Severity of somatic symptoms at end of treatment.
3.2 Acceptability
There was no significant difference in drop‐out rates between CBT and PMR during treatment (SMD 0.98; 95% CI 0.83 to 1.15; 90 participants; Analysis 3.2).
3.2. Analysis.

Comparison 3 Cognitive behavioural therapy versus behavioural therapy, Outcome 2 Acceptability.
Secondary outcomes
3.3 Severity of anxiety or depressive symptoms (or both) at end of treatment
There was no significant difference in level of depression and anxiety between CBT and PMR at end of treatment (SMD 0.01; 95% CI ‐0.42 to 0.44; 84 participants; Analysis 3.3).
3.3. Analysis.

Comparison 3 Cognitive behavioural therapy versus behavioural therapy, Outcome 3 Severity of anxiety or depressive symptoms (or both) at end of treatment.
3.4 Dysfunctional cognitions, emotions, and behaviours
The study did not report about dysfunctional cognitions, emotions, and behaviours.
3.5 Adverse events
The study comparing CBT with PMR did not report about adverse events.
3.6 Treatment response
The study comparing CBT with PMR did not report about treatment response.
3.7 Functional disability and quality of life
There was no significant difference in level of function between CBT and PMR at end of treatment (SMD 0.28; 95% CI ‐0.15 to 0.71; 84 participants; Analysis 3.4).
3.4. Analysis.

Comparison 3 Cognitive behavioural therapy versus behavioural therapy, Outcome 4 Functional disability and quality of life at end of treatment.
3.8 Healthcare use
The study comparing CBT with PMR did not report about healthcare use.
Subgroup analyses and investigation of heterogeneity
For most comparisons and outcomes, we considered the number of studies to be too small to explore possible sources of heterogeneity.
Sensitivity analyses
Although we stated in our protocol that we would only perform sensitivity analyses when at least 10 studies were available, we performed some analyses with fewer studies, in response to peer reviewers' requests.
Consultation letters
For our first comparison, we repeated the analysis for the primary outcomes excluding the studies that included consultation letters in both study arms. For symptom severity at end of treatment, the original analysis included 10 studies with 1081 participants (pooled SMD ‐0.34; 95% CI ‐0.53 to ‐0.16; I2 = 49%; Analysis 1.1). Excluding the three studies with consultation letters resulted in seven remaining studies with 722 participants, and a pooled of SMD ‐0.22 (95% CI ‐0.40 to ‐0.03; I2 = 24%) (Allen 2006; Escobar 2007;Moreno 2013).
At follow‐up measurements within one year of follow‐up, the original analysis showed that the effect of psychological therapies compared with usual care CBT remained significant (SMD ‐0.24; 95% CI ‐0.37 to ‐0.11; 7 studies, 950 participants; Analysis 1.2). Heterogeneity was low (I2 = 0%). Excluding the same three studies with a consultation letter as above resulted in four remaining studies with 594 participants, and a pooled SMD of ‐0.14 (95% CI ‐0.31 to 0.02) and also low heterogeneity (I2 = 0%).
Outliers
For our first comparison, with 14 studies, the outcome acceptability showed considerable heterogeneity (I2 = 70%; Analysis 1.4), the effect was small but statistically significant (RR 0.93; 95% CI 0.88 to 0.99). One of the studies was a clear outlier (Kashner 1995), with only 20/44 (45%) participants in the CBT group completing the treatment phase versus all of the 26 participants in the control group (100% acceptability) (see also the funnel plot; Figure 4). Removing this study from the analysis (a post hoc choice) reduced the heterogeneity from 70% to 33%. The pooled results for the remaining studies resulted in a somewhat smaller effect (RR 0.95; 95% CI 0.92 to 0.99; 13 studies, 1574 analysed participants). As the outlying study had an extreme low acceptability in the intervention group, publication bias was not an issue.
4.
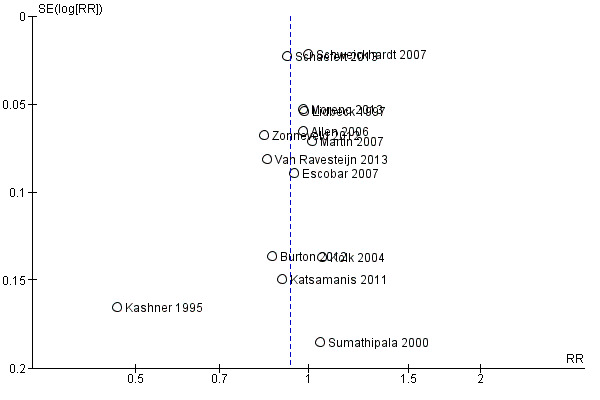
Funnel plot of comparison: 1 Psychological therapies versus usual care or waiting list, outcome: 1.4 Acceptability.
Reporting bias
In few studies, we considered reporting bias to be a serious problem. However, our assessment was suboptimal, as for most of the studies no protocol was available, so we could not compare the published outcomes with the planned outcomes. One study evidently assessed more outcomes than initially reported (Moreno 2013), but this was at least partially overcome by a later publication, and by additional outcome data provided by the authors on request. The only funnel plot we were able to produce (because enough studies were available) did reveal one outlier, but this did not suggest any publication bias (see Figure 4).
Discussion
Summary of main results
We added two 'Summary of findings' tables to create a structured overview of the main review results, using the GRADE methodology (Table 1; Table 2). These tables only addressed the most important outcome measures (marked with an asterisk in the section 'Types of outcome measures') of the two main comparisons. A summary of the main findings for all comparisons follows below.
Psychological therapy versus usual care
Fifteen studies compared some form of psychological therapy with usual care or a waiting list. Combining 10 of these studies, the psychological therapy was significantly more effective on symptom severity at end of treatment, though the effect was small. Heterogeneity was considerable and the overall quality of the evidence was low. Six of the 10 studies compared CBT with usual care; for this subgroup it was also apparent that CBT was more effective in reducing severity of symptoms at the end of treatment. The treatment effect of psychological therapies as a whole was also noted within one year of follow‐up (seven studies). After one year, the evidence was limited to two studies (both CBT), but still in favour of the psychological therapy. Results for treatment response, one of our secondary outcomes, supported the findings for symptom severity, with moderate‐quality evidence.
Regarding the other primary outcome, acceptability, we found a 7% difference in drop‐outs, favouring the usual care group. The quality of the evidence was moderate. After we removed an apparent outlier, the result was smaller (5%), but still statistically significant. There was no significant difference in drop‐out rates between CBT and usual care.
For participant‐rated symptoms of depression and anxiety, there was no significant difference at the end of treatment or at follow‐up. However, in three studies using clinician‐rated instruments, the level of anxiety and depression was slightly lower in the psychological therapy groups at the end of treatment. For anxiety, this difference became larger at follow‐up. For clinician‐rated depressive symptoms, this effect fluctuated during follow‐up (no effect within one year (two studies) and a large effect after one year of follow‐up (one study)). Only three studies reported adverse effects and dysfunctional cognitions, emotions, and behaviours. There was no clear evidence of a difference on these outcomes. There was a small difference in functional disability at the end of treatment favouring psychological therapies. This effect was not apparent during follow‐up. Two studies (both on CBT) found a small difference in favour of psychological therapies on healthcare use during treatment, four studies found no effect within one year of follow‐up. Due to the small number of studies, these results should be considered with caution.
Only two studies compared behavioural therapy with usual care, of which only one provided relevant data (Katsamanis 2011). In this study, there were no significant differences for any of the outcomes. Only one study compared third‐wave CBT (mindfulness therapy) with usual care (Van Ravesteijn 2013). In this study, mindfulness was more acceptable than usual care, but no evidence of differences was found with respect to other outcomes. One study compared a variety of psychological therapies with usual care (therapy depended on the orientation of the 15 participating therapists) (Kolk 2004). In this study, there was no evidence of differences with respect to any of the outcomes. This comparison had a high external validity as it emulated the way the referral process normally works.
Psychological therapy versus enhanced or structured care
Five studies compared a certain psychological therapy with enhanced or structured care. The quality of the evidence was moderate for most outcomes. At the end of treatment, there was no clear evidence of a difference for symptom severity, but there was a small statistically significant difference within one year after end of treatment. The psychological therapy groups had a 7% higher drop‐out rate than the control groups.
There was no clear evidence of a difference between the groups in terms of severity of anxiety or depressive symptoms (or both) at the end of treatment and within one year after treatment. There was no clear evidence of a difference between the groups in terms of dysfunctional cognitions, emotions, and behaviours at end of treatment, but at follow‐up within one year of treatment there was a small effect in favour of psychological therapy over enhanced care. None of the studies in this comparison reported information about adverse events or treatment response in a standardised way. For functional disability and quality of life, there was no clear evidence of a difference at the end of treatment, but there was a small significant difference within one year of follow‐up. There were no significant differences in healthcare use between psychological therapies and enhanced care.
Three of the studies compared CBT with enhanced or structured care. For symptom severity, CBT showed similar results as the whole group. There were no differences in drop‐out rates. In addition, there were no significant differences in levels of anxiety and depressive symptoms at the end of treatment and within one year after treatment. Only one study reported data after one year. At the end of treatment, CBT did not result in lower levels of dysfunctional cognitions, emotions, and behaviours, compared with enhanced care. However, within one year of treatment, these levels were lower for CBT (two studies). Only one study reported beyond one year of treatment. The level of functional disability at the end of treatment was comparable for CBT and enhanced care. Within and after one year of treatment there was a small difference in favour of CBT, although only a few studies were included in these analyses. Only one CBT study reported data about healthcare use and found no evidence of difference.
Psychological therapy versus another psychological therapy
Only one study compared two forms of psychological therapy (CBT versus PMR). There were no differences between the groups for any of the outcomes.
Overall completeness and applicability of evidence
The aim of this review was to assess the effects of non‐pharmacological interventions for somatoform disorders (specifically somatisation disorder, undifferentiated somatoform disorder, somatoform disorders unspecified, somatoform autonomic dysfunction, pain disorder, and alternative somatoform diagnoses proposed in literature) and MUPS in adults in comparison with treatment as usual, waiting list controls, attention placebo, psychological placebo, enhanced care, and other psychological or physical therapies.
Studies
A thorough literature search in electronic databases and many other resources such as conference proceedings, international trial registers, grey unpublished literature, and reference lists resulted in 21 studies that could be included in this review. In comparison to other existing reviews about non‐pharmacological interventions for MUPS or somatoform disorders (e.g. Kleinstäuber 2011; Kroenke 2007; Rosendal 2013), this number of eligible studies is quite high. Hence, a considerable number of studies was available in order to address our questions. However, only a few studies contributed to most of the outcomes. In addition, due to the small number of studies, we were unable to consider the effect of study characteristics (setting, severity, chronicity) on the outcomes.
We believe that the included studies cover a broad spectrum of settings, and both RCTs and CRCTs were included. Participants were recruited in various ways and from various healthcare settings, including primary care, secondary care, tertiary care, and the open population. In the included studies, therapists had different backgrounds (e.g. GPs, psychologists, and other physicians) and different levels of experience. A limitation of the included studies was the relatively low number of included participants per study as most studies only included 25 to 75 participants per study arm.
A critical issue concerns the fact that somatoform disorders are conceptually overlapping with certain functional somatic syndromes, which we excluded from the current review when studies only focused on one of these (e.g. chronic fatigue syndrome and irritable bowel syndrome). However, the results of the current review should be interpreted as part of a portfolio of various Cochrane reviews covering somatoform disorders (Hoedeman 2010; Ipser 2009; Ruddy 2005; Thompson 2007); the Cochrane review on pharmacological interventions for somatoform disorders, which is currently being developed (see Kleinstäuber 2013 for protocol) and Cochrane reviews focusing on different functional syndromes (e.g. Moore 2014).
It is debatable whether our inclusion criterion that selection of participants should include a diagnostic interview or questionnaire to establish MUPS or somatoform disorder was not too strict. We excluded several potentially relevant randomised trials because they did not fulfil this criterion. It is unclear whether these excluded studies included participants of similar severity as the included studies.
Participants
With only two exceptions (Sumathipala 2000; Sumathipala 2008), studies were performed in developed countries (Western Europe and USA). Most studies randomised more women than men. This is in line with existing reviews, as MUPS and somatoform disorders are more common among women. Included studies cover a broad age range. However, as the mean age of participants was in the 30s or 40s in most of the studies, it may be possible that younger and older people were relatively underpresented. Severity of MUPS at baseline was mostly analysed based on the number of symptoms or duration (or both) of symptoms. The number of symptoms at baseline varied widely, ranging from a lifetime number of symptoms of seven (Martin 2007) to a current number of symptoms of 32 (Schröder 2012). Baseline duration, only reported in nine studies, ranged on average from four to 25 years. This suggests that most of the included participants may have had chronic symptoms at baseline. Included studies also reported high psychiatric co‐morbidity rates, percentages of participants with a current co‐morbid axis 1 disorder varied between 41% (Zonneveld 2012) and 92% (Escobar 2007). Taking these findings together, we can say that a limitation may be that participants of included studies were people with relatively severe forms of somatoform disorders and MUPS. The milder forms, with lower levels of co‐morbidity may have been underpresented. In contrast, people with milder symptoms may need less intensive therapy.
Interventions
Fourteen of the included studies compared CBT with another intervention. As a result, relatively robust conclusions could be drawn about the effectiveness of CBT. The number of studies describing other psychological therapies (such as behavioural therapies, third‐wave CBT, or psychodynamic therapies) was too low to draw conclusions about these forms of therapy. Duration and number of treatment sessions varied widely between the included studies.
It is especially remarkable that we found no studies on physical therapies (such as running therapy). We believe that there is a clear need for this type of research.
Many included studies used forms of enhanced care or other forms of therapy as the control treatment. A limitation of this method may be an underestimation of the treatment effect, due to small inter‐group differences. This is illustrated since these studies found fewer and smaller effects than studies comparing a treatment with usual care.
Outcomes
In this review, the outcome of functional impairment introduced a problem, as a certain number of studies used SF‐36 subscales as the outcome measure. As a result, we reported physical functioning and mental functioning or even subdomains separately. We decided to pool the two main domains into one outcome, but this led to the limitation that differences in effects for physical and mental functioning, as found in some studies (e.g. Zonneveld 2012), disappeared.
Another problem of the current review was that, with one exception, there were not enough studies to assess reporting bias with funnel plots. According to recommendations of the Cochrane Handbook for Systematic Reviews of Interventions, there should be at least 10 studies to perform this (Sterne 2011). In future updates of this review, the addition of new studies may enable us to produce funnel plots for more comparisons and outcomes.
Adverse effects were very infrequently reported and various ways of reporting were used. Therefore, it was impossible to extract these in a standardised way in order to include them in our meta‐analytical calculations, except for the first comparison (psychological therapy versus usual care or waiting list). We also have to emphasise again that RCTs and CRCTs are not sufficient to gain information about the more rare or longer‐term (or both) adverse events.
The difference in effect between participant‐reported symptoms of anxiety and depression and physician‐reported symptoms is interesting. In most studies, both participants and physicians were aware of the treatment that was provided, but physicians may have been more biased towards active treatment than participants. In contrast, we should realise that the studies that asked participants to rate their symptoms were not always the same as the studies that asked physicians, so it is possible that other study characteristics have caused these differences.
Quality of the evidence
According to the first quality criterion risk of bias defined by the guidelines by GRADE, Figure 2 and Figure 3 showed that in regard to different types of biases most of our included studies showed a low risk. However, a few specific domains were often rated as being at high risk of bias across the studies. Especially for blinding of the outcome measurement, we identified a high risk of bias in most of the included studies. Most studies could not blind the outcome reporters, mostly the participants, due to the nature of the intervention. A high risk of bias in blinding of participants and personnel was found for the same reason. Nine studies (43% of studies) reported incomplete outcomes, defined as a loss to follow‐up of more than 20%. Reasons for loss to follow‐up were not systematically described. Another study aspect that affected the quality of the evidence was the generally low number of included participants per study. It has to be taken into account that several of the included studies were performed before the publication and implementation of current quality criteria for conducting and reporting RCTs.
The small number of studies did not allow us to assess the effects in subgroups of participants or interventions. Apart from CBT, all other comparisons between specific therapies and usual care or enhanced care, the number of studies was too small (often only one study). We did not consider indirectness (a GRADE item comparing the interventions and outcomes in which we are interested to what was actually studied in the included studies) and publication bias to be important sources of risk of bias, publication bias because of our thorough search process; the overall completeness of reporting, and the fact that several studies that did not find an effect.
Potential biases in the review process
This review has several methodological strengths. The quality of meta‐analyses depends on the robustness of the search methods used. In this review, the electronic search was thorough and large in scale with broad parameters. We evaluated published and unpublished studies. The selection criteria were broad, which led to the selection of a relatively high number of studies. We also included non‐English studies. As a result, it seems likely that all or almost all evidence in the searched databases that should have been included was included. However, as we did not search Asian databases, this may have led to a potential bias.
The study was performed according to a pre‐published protocol. Different review authors performed evaluation of studies for selection, extract data, and assess risk of bias, with the possibility of consulting another review author to resolve disputes. However, due to the fact that not all choices that had to be made were foreseen, there were also post hoc decisions. Excluding studies that trained GPs to deliver some psychological therapy was one of these decisions. In addition, we performed the allocation of the included studies to the different groups of treatment for analyses post hoc. Another post hoc decision was the addition of enhanced or structured care as a comparator. We made decisions very carefully and included achieving consensus between several review authors with specific knowledge in the field. However, some studies were difficult to categorise, as, for example, treatments included elements of different treatment categories. Therefore, allocation of these studies remained slightly arbitrary. Another post hoc decision was to combine the physical component scale and the mental component scale of the SF‐36 into one outcome. Other post hoc decisions were to carry out sensitivity analyses by excluding studies that included consultation letters in both study groups, and by excluding studies with the least intensive interventions.
Although we attempted to obtain missing data from the authors of included studies, it was not possible in every case to obtain these data, and, therefore, the included studies were not represented fully in the meta‐analyses. This may also have led to a certain form of bias, although it is difficult to say in what direction this bias would be.
As described in the section Types of outcome measures, we aimed to retrieve data about severity/intensity of MUPS; acceptability; depression and anxiety; dysfunctional cognitions, emotions, or behaviours; adverse events; treatment response; functional disability; and quality of life. However, the number of studies reporting on many of our outcomes was relatively low. Results about depression; anxiety; dysfunctional cognitions, emotions, and behaviours; adverse events; and treatment response were frequently lacking. Therefore, we could not draw robust conclusions about these outcome measures.
Although acceptability was a primary outcome of our study, we restricted this to the period from randomisation to the end of treatment. We did not take into account the acceptability of the interventions in the recruitment phase. Participants for whom the intervention or control condition was unattractive probably did not participate. This affects the external validity of study findings.
In this review, we used point estimates at all follow‐up periods to evaluate treatment effect, instead of scores based on change from baseline. We chose this method as these results were retrievable from most of the studies, and combining follow‐up outcome data with change from baseline data was considered inappropriate given our choice of SMDs due to the variety of outcome measures that had to be combined (Higgins 2011, Chapter 9.4.5.2). However, pooling the results of follow‐up measurements has the disadvantage that baseline values (and possible baseline differences) are not taken into account. As data were pooled in most analyses, we believe that distortions such as these are generally corrected by the other studies in the analyses. Some studies only reported data about change from baseline (without the actual baseline data) (e.g. Burton 2012; Fjorback 2013). These data could not be used in this review. We contacted authors in order to be obtain the required data, and were successful in many cases though not all.
Agreements and disagreements with other studies or reviews
Several systematic reviews have addressed non‐pharmacological treatments for participants with some form of somatoform disorder or MUPS (Allen 2002; Blankenstein 2001 (thesis, chapter 2); Guthrie 1996; Hofmann 2012; Huibers 2007; Kleinstäuber 2011; Koelen 2014; Kroenke 2000; Kroenke 2007; Looper 2002; Nezu 2001; Rosendal 2013; Sumathipala 2007). As many of the included studies in our review were published after 2005, we focused this discussion on the systematic reviews that were published after 2005.
In general, we can say that the results of this review are in line with results of existing reviews. In most reviews, the majority of included studies concerned CBT in some form, and small effect sizes were found. In other reviews, also limited evidence was found for other forms of psychological therapies. Studies investigating physical therapies for somatoform disorders or MUPS were also hardly reported in other reviews.
Hofmann et al. evaluated the effectiveness of CBT for a broad range of conditions, including somatoform disorders and chronic pain and fatigue (Hofmann 2012). Within the somatoform disorders category of DSM‐IV, meta‐analyses primarily examined the efficacy of psychological interventions for hypochondriasis and body dysmorphic disorder, which were both excluded from this review. For chronic fatigue and pain, the effect of a range on treatments was reviewed, including relaxation techniques, mindfulness‐based techniques, acceptance‐based techniques, biofeedback, psycho‐education, and behavioural and cognitive behavioural treatments. Results of these meta‐analyses revealed varying effect sizes for these treatments depending on the type of chronic pain targeted; however, CBT treatments for chronic pain were consistently in the small‐to‐medium effect size range. For fatigue, effect sizes were medium.
Huibers et al. published one Cochrane review focusing on psychosocial interventions provided by GPs (Huibers 2007). They included 10 studies, addressing five distinct disorders or health complaints, one of which was somatisation, for which three RCTs were found. One of these was also included in the current review (Lidbeck 1997). The authors of the Cochrane review concluded that there was little evidence available for the use of psychosocial interventions by GPs, while the conclusions of the three separate studies were slightly more positive, indicating that the model of training GPs in psychosocial interventions could be beneficial and with modifications might be useful in practice.
Kleinstäuber et al. evaluated the effect of short‐term psychotherapy on multiple medically unexplained symptoms (Kleinstäuber 2011). They included a relatively high number of studies (27 studies), showing a considerable overlap with this review (nine studies also in our review). Most of the 18 studies that we did not include in our review were either not randomised or used a participant selection procedure that did not fulfil our criteria. Like our review, they found small but stable effect sizes of CBT on severity of symptoms versus usual care. For quality of life and medical healthcare utilisation, non‐significant effects were found directly after psychotherapy, which increased to significant small effects at follow‐up assessment. For depression and anxiety, a small effect was found, which disappeared over time. The authors concluded that current psychotherapeutic concepts do not sufficiently focus on co‐morbid depression or anxiety.
Rosendal et al. published one Cochrane review focusing on enhanced care for people with functional somatic symptoms and disorders, performed by generalists in primary care (Rosendal 2013). Seven studies fulfilled their inclusion criteria, three of which were also potential candidates for inclusion in this review (Blankenstein 2001;Larisch 2004;Toft 2010, see Excluded studies). For most outcomes, they found small and non‐significant overall effect sizes. They concluded that current evidence does not answer the question whether enhanced care delivered by frontline primary care professionals has an effect or not on the outcome of people with functional somatic symptoms. They pointed out that enhanced care may have an effect when delivered per protocol to well‐defined groups of people with functional disorders, but that this needs further investigation.
Sumathipala searched for systematic reviews and trials published since 2000, addressing treatments for people with medically unexplained symptoms (Sumathipala 2007). He found six systematic reviews and 14 new trials. He concluded that CBT was efficacious for either symptom syndromes or for the broader category of medically unexplained symptoms, reducing physical symptoms, psychological distress, and disability. He found limited level II evidence for other interventions such as collaborative care models, consultation letters, reattribution therapy, and bioenergetics exercise. Sumathipala 2007 included a relatively low number of studies in primary care.
In 2014, one systematic review was published on psychotherapy compared with treatment as usual for severe somatoform disorders (Koelen 2014). Ten randomised and six non‐randomised trials were included. Of the randomised trials, two were also included in our review. One of the other randomised trials may also be relevant for our review and was listed under Studies awaiting classification, to be examined for a future update (Nickel 2006). The authors of this systematic review concluded that psychotherapy was effective, but further studies are needed to examine specific interventions and mechanisms of change.
Authors' conclusions
Implications for practice.
The overall quality of the evidence provided by 21 randomised controlled trials was low to moderate. All psychological therapies combined were superior to usual care or waiting list condition for symptom severity, our first primary outcome, but effect sizes were small. As a single treatment, only cognitive behavioural therapy (CBT) was adequately studied to allow conclusions for practice. Compared with usual care or waiting list conditions, CBT reduced somatic symptoms, with a small effect and substantial differences in effects between CBT studies. The effects were durable within and after one year of follow‐up.
Compared with enhanced or structured care, psychological therapies generally were not more effective for most of the outcomes. CBT was also not superior to enhanced care. The question remains how specific CBT is over structured improvements of care. No major adverse events were reported in the intervention groups, although most studies did not describe adverse events as an explicit outcome measure. Apart from CBT, neither psychological nor other non‐pharmacological therapies have been adequately studied.
In daily practice, a substantial percentage of people with medically unexplained physical symptoms (MUPS) may not be willing to accept psychologically oriented treatments. Whether such acceptance is associated with the effect of psychological treatments for the total MUPS population was not clear. Due to the small number of studies, we could not draw conclusions about the effect of characteristics such as a profession and experience of the therapist, about treatment intensity and treatment location, on treatment efficacy.
Further optimisation of CBT to target optimal participant profiles and match treatment providers, treatment characteristics, and participants could improve outcomes. Motivating and preparing people for CBT is important for this participant group (Timmer 2006). As drop‐out rates were not much lower than in control groups, this indicates that when a person has accepted involvement in the treatment, the prospects that the treatment will be completed are good.
Implications for research.
Based on the findings in this review, we can make several recommendations for future research. The number of studies investigating various treatment modalities other than CBT needs to increase to build a broader and more varied evidence‐base for the treatment of somatoform disorders and MUPS. As physical therapies may offer a more acceptable starting point for treatment for these people than psychological approaches, investigating the effectiveness of physical therapies is to be considered. We found no such studies.
Most studies in our review focused on chronic manifestations of physical symptoms, often of considerable severity. It is conceivable that interventions were more effective in people with milder symptoms, or of shorter duration, but this needs further testing. A related conceptual issue is that chronic conditions deserve a World Health Organization chronic care or chain care approach as acute treatments will not suffice. Preventing symptoms from become chronic may be a relevant outcome to be added in future studies.
In future research, more attention should be paid to the impact of interventions on risk factors for recurrence and persistence of symptoms in somatoform disorders and MUPS. These factors include anxiety; depression; and dysfunctional cognitions, emotions, and behaviours. Most included studies in this review did not report on all of these factors. Specific attention to the effect of treatment duration and number of treatment sessions is also needed. In the studies included in this review, duration and number of sessions varied widely, and it is yet unclear which treatment intensities are effective for which participants.
Psychological treatments were not superior to enhanced care. It could be argued that an active comparator such as enhanced care underestimates treatment effects. However, as this comparative treatment is probably cheaper than more intensive psychological interventions, it would deserve further study (cf. Rosendal 2013).
In our view, teaching people how to tolerate uncertainty and deal with their bodily symptoms can be problematic and will probably always involve high levels of clinical skills. One potential intermediate factor is the amount of trust that people have in their therapist or physician, a factor to be taken into account in the design of new studies (van der Feltz‐Cornelis 2004).
There is a clear need for developing and testing strategies for motivating and preparing people for CBT. CBT is the only evidence‐based psychological treatment available at the moment.
A more structural question is, how psychological therapies for participants with somatoform disorders can be better integrated into the healthcare system. Can the healthcare system be restructured in such a way that it facilitates the access of people with somatoform disorders to psychological therapies?
As the cost of treatment can be substantial, but also the cost of the disorder in terms of absenteeism and healthcare use, cost‐effectiveness needs to be addressed in future studies.
Future studies should include more participants, preferably use a uniform set of validated outcome measurements, and extend follow‐up assessments beyond one year after treatment.
Finally, as newer‐generation antidepressants and particularly natural products also reduce somatic symptoms, a preference‐led or profile‐led approach may be possible (Unutzer 2002). The aim would be to evaluate to what extent an intervention (consisting of a choice between non‐pharmacological and pharmacological therapy combined with chain care), would improve symptoms over usual care.
What's new
| Date | Event | Description |
|---|---|---|
| 4 November 2014 | Amended | Contact details updated. |
Acknowledgements
We thank the following authors for providing additional information about their studies: Nettie Blankenstein, Chris Burton, Javier Escobar, Lone Fjorback, Kurt Fritsche, Margalida Gili, Gonzalo Grandes, Jan Lidbeck, Alexandra Martin, Winfried Rief, Bert Schilte, Andreas Schröder, Anette Schröder, Anne Speckens, and Lyonne Zonneveld. We thank the referees for their thorough and constructive comments on the draft review. Special thanks to the Cochrane Depression, Anxiety and Neurosis Review Group's editorial base team for assistance in the development of the review, for their support in the development of the search strategies, and their excellent feedback on draft versions of the review.
Funding for this review was provided by the National Institute for Health Research (NIHR) Cochrane Incentive Award Scheme 2014.
The Department of General Practice and Elderly Care Medicine, EMGO Institute of Health and Care Research, VU University Medical Center, Amsterdam provided in‐kind funding for most of the authors of the review.
CRG Funding Acknowledgement: The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Depression, Anxiety and Neurosis Group.
Disclaimer: The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, the National Health Service (NHS) or the Department of Health.
Appendices
Appendix 1. Additional search strategies: CENTRAL (all years)
The Cochrane Central Register of Controlled Trials (CENTRAL) was searched using the following terms:
#1 MeSH descriptor: [Somatoform Disorders] explode all trees #2 MeSH descriptor: [Psychophysiologic Disorders] this term only #3 somatization or somatisation or somatoform or somatizer* or multisomatoform or (somatic NEAR/2 (symptom* or syndrom*)) #4 MUPS or "medical* unexplained" or "unexplained medical*" or (unexplained near/2 symptom*) #5 neurastheni* #6 (#1 or #2 or #3 or #4 or #5) #7 SR‐DEPRESSN or HS‐DEPRESSN #8 (#6 not #7)
Data and analyses
Comparison 1. Psychological therapies versus usual care or waiting list.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Severity of somatic symptoms at end of treatment | 10 | 1081 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐0.53, ‐0.16] |
| 1.1 Cognitive behavioural therapy versus usual care or waiting list | 6 | 593 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.37 [‐0.69, ‐0.05] |
| 1.2 Behavioural therapy versus usual care or waiting list | 1 | 37 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.87, 0.43] |
| 1.3 Third‐wave cognitive behavioural therapy versus usual care or waiting list | 1 | 114 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.68, 0.06] |
| 1.4 Psychodynamic therapy versus usual care or waiting list | 1 | 252 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.39 [‐0.64, ‐0.13] |
| 1.5 Integrative therapies versus usual care or waiting list | 1 | 85 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.69, 0.40] |
| 2 Severity of somatic symptoms within 1 year after treatment | 7 | 950 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.37, ‐0.11] |
| 2.1 Cognitive behavioural therapy versus usual care or waiting list | 4 | 496 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.49, ‐0.09] |
| 2.2 Third‐wave cognitive behavioural therapy versus usual care or waiting list | 1 | 107 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.53, 0.23] |
| 2.3 Psychodynamic therapy versus usual care or waiting list | 1 | 262 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.44, 0.05] |
| 2.4 Integrative therapies versus usual care or waiting list | 1 | 85 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.69, 0.40] |
| 3 Severity of somatic symptoms > 1 year after treatment | 2 | 228 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.52 [‐0.80, ‐0.24] |
| 3.1 Cognitive behavioural therapy versus usual care or waiting list | 2 | 228 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.52 [‐0.80, ‐0.24] |
| 4 Acceptability | 14 | 1644 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.88, 0.99] |
| 4.1 Cognitive behavioural therapy versus usual care or waiting list | 10 | 1037 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.85, 1.01] |
| 4.2 Behavioural therapy versus usual care or waiting list | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.9 [0.67, 1.21] |
| 4.3 Third‐wave cognitive behavioural therapy versus usual care or waiting list | 1 | 125 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.72, 1.00] |
| 4.4 Psychodynamic therapy versus usual care or waiting list | 1 | 328 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.88, 0.96] |
| 4.5 Integrative therapies versus usual care or waiting list | 1 | 106 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.81, 1.39] |
| 5 Severity of anxiety symptoms at end of treatment ‐ participant rated | 4 | 270 | Std. Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.20, 0.32] |
| 5.1 Cognitive behavioural therapy versus usual care or waiting list | 3 | 185 | Std. Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.22, 0.37] |
| 5.2 Integrative therapies versus usual care or waiting list | 1 | 85 | Std. Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.53, 0.56] |
| 6 Severity of anxiety symptoms at end of treatment ‐ clinician rated | 3 | 320 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐0.63, ‐0.17] |
| 6.1 Cognitive behavioural therapy versus usual care | 2 | 284 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐0.63, ‐0.14] |
| 6.2 Behavioural therapy versus usual care | 1 | 36 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.57 [‐1.24, 0.10] |
| 7 Severity of anxiety symptoms within 1 year after treatment ‐ participant rated | 2 | 134 | Std. Mean Difference (IV, Random, 95% CI) | 0.18 [‐0.22, 0.58] |
| 7.1 Cognitive behavioural therapy versus usual care or waiting list | 1 | 49 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.59, 0.59] |
| 7.2 Integrative therapies versus usual care or waiting list | 1 | 85 | Std. Mean Difference (IV, Random, 95% CI) | 0.33 [‐0.21, 0.88] |
| 8 Severity of anxiety symptoms within 1 year after treatment ‐ clinician rated | 2 | 251 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.66 [‐1.15, ‐0.18] |
| 9 Severity of anxiety symptoms > 1 year after treatment ‐ clinician rated | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 10 Severity of depressive symptoms at end of treatment ‐ participant rated | 6 | 661 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.22, 0.16] |
| 10.1 Cognitive behavioural therapy versus usual care or waiting list | 4 | 325 | Std. Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.13, 0.31] |
| 10.2 Psychodynamic therapy versus usual care or waiting list | 1 | 251 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.55, ‐0.04] |
| 10.3 Integrative therapy versus usual care or waiting list | 1 | 85 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.45, 0.64] |
| 11 Severity of depressive symptoms at end of treatment ‐ clinician rated | 3 | 316 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.48, ‐0.02] |
| 11.1 Cognitive behavioural therapy versus usual care or waiting list | 2 | 284 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.49, ‐0.01] |
| 11.2 Behavioural therapy versus usual care or waiting list | 1 | 32 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.96, 0.44] |
| 12 Severity of depressive symptoms within 1 year after treatment ‐ participant rated | 4 | 535 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.34, 0.42] |
| 12.1 Cognitive behavioural therapy versus usual care or waiting list | 2 | 189 | Std. Mean Difference (IV, Random, 95% CI) | 0.21 [‐0.07, 0.50] |
| 12.2 Psychodynamic therapy versus usual care or waiting list | 1 | 261 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.35 [‐0.59, ‐0.10] |
| 12.3 Integrative therapy versus usual care or waiting list | 1 | 85 | Std. Mean Difference (IV, Random, 95% CI) | 0.31 [‐0.24, 0.85] |
| 13 Severity of depressive symptoms within 1 year after treatment ‐ clinician rated | 2 | 251 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.55 [‐1.17, 0.07] |
| 14 Severity of depressive symptoms > 1 year after treatment ‐ clinician rated | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 15 Dysfunctional cognitions, emotions and behaviours at end of treatment | 3 | 440 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.37, 0.16] |
| 15.1 Cognitive behavioural therapy versus usual care or waiting list | 2 | 189 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.73, 0.44] |
| 15.2 Psychodynamic therapy versus usual care or waiting list | 1 | 251 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.39, 0.12] |
| 16 Dysfunctional cognitions, emotions, and behaviours within 1 year after treatment | 3 | 451 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐0.38, 0.07] |
| 16.1 Cognitive behavioural therapy versus usual care or waiting list | 2 | 189 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.79, 0.30] |
| 16.2 Psychodynamic therapy versus usual care or waiting list | 1 | 262 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.38, 0.11] |
| 17 Adverse events | 3 | 445 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.47, 3.66] |
| 18 Treatment response at end of treatment | 4 | 391 | Risk Ratio (M‐H, Random, 95% CI) | 3.30 [2.08, 5.21] |
| 19 Treatment response within 1 year after treatment | 3 | 332 | Risk Ratio (M‐H, Random, 95% CI) | 2.53 [1.25, 5.10] |
| 20 Treatment response > 1 year after treatment | 2 | 240 | Risk Ratio (M‐H, Random, 95% CI) | 10.31 [2.95, 36.02] |
| 21 Functional disability and quality of life at end of treatment | 7 | 730 | Std. Mean Difference (IV, Random, 95% CI) | 0.17 [0.03, 0.32] |
| 21.1 Cognitive behavioural therapy versus usual care or waiting list | 4 | 341 | Std. Mean Difference (IV, Random, 95% CI) | 0.15 [‐0.06, 0.37] |
| 21.2 Behavioural therapy versus usual care or waiting list | 1 | 34 | Std. Mean Difference (IV, Random, 95% CI) | 0.16 [‐0.51, 0.83] |
| 21.3 Third‐wave cognitive behavioural therapy versus usual care or waiting list | 1 | 111 | Std. Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.29, 0.46] |
| 21.4 Psychodynamic therapy versus usual care or waiting list | 1 | 244 | Std. Mean Difference (IV, Random, 95% CI) | 0.24 [‐0.01, 0.50] |
| 22 Functional disability and quality of life within 1 year after treatment | 4 | 526 | Std. Mean Difference (IV, Random, 95% CI) | 0.16 [‐0.01, 0.33] |
| 22.1 Cognitive behavioural therapy versus usual care or waiting list | 2 | 168 | Std. Mean Difference (IV, Random, 95% CI) | 0.22 [‐0.08, 0.53] |
| 22.2 Third‐wave cognitive behavioural therapy versus usual care or waiting list | 1 | 107 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.48, 0.28] |
| 22.3 Psychodynamic therapy versus usual care or waiting list | 1 | 251 | Std. Mean Difference (IV, Random, 95% CI) | 0.23 [‐0.02, 0.48] |
| 23 Functional disability and quality of life > 1 year of treatment | 1 | 72 | Std. Mean Difference (IV, Random, 95% CI) | 0.36 [‐0.11, 0.82] |
| 24 Healthcare use at end of treatment | 2 | 117 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.68 [‐1.06, ‐0.30] |
| 25 Healthcare use within 1 year after treatment | 4 | 532 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.31, 0.12] |
| 25.1 Cognitive behavioural therapy versus usual care or waiting list | 2 | 189 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.56, 0.02] |
| 25.2 Integrative therapies versus usual care or waiting list | 1 | 81 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.44, 0.69] |
| 25.3 Psychodynamic therapy versus usual care or waiting list | 1 | 262 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.23, 0.26] |
Comparison 2. Psychological therapies versus enhanced care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Severity of somatic symptoms at end of treatment | 5 | 624 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.43, 0.04] |
| 1.1 Cognitive behavioural therapy versus enhanced care | 3 | 307 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐0.71, 0.03] |
| 1.2 Third‐wave cognitive behavioural therapy versus enhanced care | 1 | 106 | Std. Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.38, 0.39] |
| 1.3 Psychodynamic therapy versus enhanced care | 1 | 211 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.31, 0.23] |
| 2 Severity of somatic symptoms within 1 year after treatment | 5 | 593 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.40, ‐0.02] |
| 2.1 Cognitive behavioural therapy versus enhanced care | 3 | 289 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.60, ‐0.00] |
| 2.2 Third‐wave cognitive behavioural therapy versus enhanced care | 1 | 93 | Std. Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.36, 0.46] |
| 2.3 Psychodynamic therapy versus enhanced care | 1 | 211 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.47, 0.07] |
| 3 Severity of somatic symptoms > 1 year after treatment | 2 | 172 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.73, 0.10] |
| 3.1 Cognitive behavioural therapy versus enhanced care | 1 | 82 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐0.98, ‐0.09] |
| 3.2 Third‐wave cognitive behavioural therapy versus enhanced care | 1 | 90 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.53, 0.30] |
| 4 Acceptability | 5 | 679 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.87, 1.00] |
| 4.1 Cognitive behavioural therapy versus enhanced care | 3 | 349 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.82, 1.02] |
| 4.2 Third‐wave cognitive behavioural therapy versus enhanced care | 1 | 119 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.82, 1.01] |
| 4.3 Psychodynamic therapy versus enhanced care | 1 | 211 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.89, 1.14] |
| 5 Severity of anxiety or depressive symptoms (or both) at end of treatment | 5 | 624 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.30, 0.02] |
| 5.1 Cognitive behavioural therapy versus enhanced care | 3 | 307 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.40, 0.05] |
| 5.2 Third‐wave cognitive behavioural therapy versus enhanced care | 1 | 106 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.43, 0.33] |
| 5.3 Psychodynamic therapy versus enhanced care | 1 | 211 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.40, 0.14] |
| 6 Severity of anxiety or depressive symptoms (or both) within 1 year after treatment | 5 | 593 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.29, 0.03] |
| 6.1 Cognitive behavioural therapy versus enhanced care | 3 | 289 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.40, 0.06] |
| 6.2 Third‐wave behavioural therapy versus enhanced care | 1 | 93 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.45, 0.36] |
| 6.3 Psychodynamic therapy versus enhanced care | 1 | 211 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.38, 0.16] |
| 7 Severity of anxiety or depressive symptoms (or both) > 1 year after treatment | 2 | 184 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.55, 0.03] |
| 7.1 Cognitive behavioural therapy versus enhanced care | 1 | 94 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.36 [‐0.77, 0.04] |
| 7.2 Third‐wave cognitive behavioural therapy versus enhanced care | 1 | 90 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.56, 0.26] |
| 8 Dysfunctional cognitions, emotions, and behaviours at end of treatment | 4 | 499 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.29, 0.10] |
| 8.1 Cognitive behavioural therapy versus enhanced care | 2 | 182 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.57, 0.01] |
| 8.2 Third‐wave cognitive behavioural therapy versus enhanced care | 1 | 106 | Std. Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.32, 0.44] |
| 8.3 Psychodynamic therapy versus enhanced care | 1 | 211 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.27, 0.27] |
| 9 Dysfunctional cognitions, emotions, and behaviours within 1 year after treatment | 4 | 477 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.49, 0.00] |
| 9.1 Cognitive behavioural therapy versus enhanced care | 2 | 173 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐0.83, ‐0.07] |
| 9.2 Third‐wave cognitive behavioural therapy versus enhanced care | 1 | 93 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.44, 0.38] |
| 9.3 Psychodynamic therapy versus enhanced care | 1 | 211 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.40, 0.14] |
| 10 Dysfunctional cognitions, emotions, and behaviours > 1 year after treatment | 2 | 184 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.58 [‐1.27, 0.11] |
| 10.1 Cognitive behavioural therapy versus enhanced care | 1 | 94 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.94 [‐1.36, ‐0.51] |
| 10.2 Third‐wave cognitive behavioural therapy versus enhanced care | 1 | 90 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.64, 0.19] |
| 11 Functional disability and quality of life at end of treatment | 4 | 497 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.05, 0.30] |
| 11.1 Cognitive behavioural therapy versus enhanced care | 2 | 182 | Std. Mean Difference (IV, Random, 95% CI) | 0.21 [‐0.08, 0.51] |
| 11.2 Third‐wave cognitive behavioural therapy versus enhanced care | 1 | 104 | Std. Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.31, 0.46] |
| 11.3 Psychodynamic therapy at end of treatment | 1 | 211 | Std. Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.19, 0.35] |
| 12 Functional disability and quality of life within 1 year after treatment | 4 | 476 | Std. Mean Difference (IV, Random, 95% CI) | 0.20 [0.02, 0.38] |
| 12.1 Cognitive behavioural therapy versus enhanced care | 2 | 173 | Std. Mean Difference (IV, Random, 95% CI) | 0.30 [0.00, 0.60] |
| 12.2 Third‐wave cognitive behavioural therapy versus enhanced care | 1 | 92 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.31, 0.51] |
| 12.3 Psychodynamic therapy versus enhanced care | 1 | 211 | Std. Mean Difference (IV, Random, 95% CI) | 0.16 [‐0.11, 0.43] |
| 13 Functional disability and quality of life > 1 year of treatment | 2 | 184 | Std. Mean Difference (IV, Random, 95% CI) | 0.22 [‐0.16, 0.60] |
| 13.1 Cognitive behavioural therapy versus enhanced care | 1 | 94 | Std. Mean Difference (IV, Random, 95% CI) | 0.42 [0.01, 0.83] |
| 13.2 Third‐wave cognitive behavioural therapy versus enhanced care | 1 | 90 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.39, 0.44] |
| 14 Healthcare use at end of treatment | 2 | 334 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.33, 0.28] |
| 14.1 Cognitive behavioural therapy versus enhanced care | 1 | 123 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.56, 0.15] |
| 14.2 Psychodynamic therapy versus enhanced care | 1 | 211 | Std. Mean Difference (IV, Random, 95% CI) | 0.11 [‐0.16, 0.38] |
| 15 Healthcare use within 1 year after treatment | 2 | 319 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.46, ‐0.01] |
| 15.1 Cognitive behavioural therapy versus enhanced care | 1 | 108 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.46, 0.30] |
| 15.2 Psychodynamic therapy versus enhanced care | 1 | 211 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.59, ‐0.05] |
Comparison 3. Cognitive behavioural therapy versus behavioural therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Severity of somatic symptoms at end of treatment | 1 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2 Acceptability | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3 Severity of anxiety or depressive symptoms (or both) at end of treatment | 1 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4 Functional disability and quality of life at end of treatment | 1 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Allen 2006.
| Methods | Study design: randomised controlled trial | |
| Participants |
Diagnosis: DSM‐IV diagnosis of somatisation disorder Method of diagnosis: participants (18‐70 years) with English fluency and literacy, a DSM‐IV diagnosis for somatoform disorder and a CGI‐SD score ≥ 4 were included Exclusion criteria: unstable major medical condition, medication regimen that has not been stable for at least 2 months prior to baseline, active suicidal ideation, history of psychosis, current psychoactive substance dependence, pregnant women or attempting to conceive, participants in psychotherapy concurrent with the period between baseline and 3 months appointments Total number randomised: 84 Age: for intervention group, M = 45.5 (SD = 8.5); for control group, M = 47.9 (SD = 11) Sex: 89% women; 11% men for intervention group 84% women (n = 36), for control group 95% (n = 39) Severity of symptoms at baseline: not reported Duration of symptoms at baseline: for intervention group M = 24.95 (SD = 11.54) years, for control group M = 25 (SD = 15.12) years Setting: participants were recruited through medical clinics and through advertisements in the community (70% referred by physician), after informed consent they received a telephone screening interview. Treatment: department of Psychiatry of medical school Location: New Jersey, USA Number of treatment centres: 1 Co‐morbidities: 65% current co‐morbid DSM‐IV axis I disorder; 70% (n = 30) for intervention group, 59% (n = 24) for control group Adjunctive therapy: not mentioned Adjunctive medication: not mentioned |
|
| Interventions | Participants were randomly assigned to either 1. CBT + PCI (n = 43) Duration: 10 sessions during a period of 3 months Treatment protocol: CBT: manualised intervention for people with somatoform disorder, with detailed guidelines for each session, focused on stress management, activity regulation, emotional awareness, cognitive restructuring, and interpersonal communication (Allen 2006, refs 5 and 6 for details) PCI: standard consultation letter sent to the treating physician including recommendations for the ongoing treatment (Allen 2006, Table 1 for details) Therapist: 4 therapists, master‐ or doctoral‐level psychologists with at least 3 years of supervised training in CBT. All received a special training in CBT for SD before the trial 2. PCI alone (n = 41) Duration: NA Treatment protocol: standard consultation letter sent to the treating physician including recommendations for the ongoing treatment (Allen 2006, Table 1 for details). Therapist/face‐to‐face contact: none |
|
| Outcomes |
Time points for assessment: baseline and 3 months, 9 months, 15 months after baseline Primary outcome: 1. severity of somatisation (CGI‐SD) 2. improvement (CGI‐SD) Secondary outcome: 1. participants' rating of physical functioning (MOS‐36) 2. severity of somatic symptoms (SSS) 3. healthcare utilisation (medical records) Potential mediator 1. participant expectations of improvement |
|
| Notes |
Date of study: September 1999 ‐ April 2003 Funding source: National Institute of Mental Health Declarations of interest among the primary researchers: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number sequence was used |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and therapists were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Study personnel were masked to participants treatment condition (independent evaluators), but participants were not blinded, and most outcomes were participant reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | < 20% missing per follow‐up moment |
| Selective reporting (reporting bias) | Low risk | All intended outcomes reported |
| Treatment fidelity | Low risk | The treatment was described in a manual containing detailed guidelines for the conduct of each session (Allen 2006, page 1513) |
| Researcher allegiance | Low risk | No indication that researchers had a preference for 1 of the treatment modalities |
| Other bias | Low risk | No other sources of bias |
Burton 2012.
| Methods | Study design: randomised controlled trial | |
| Participants |
Diagnosis: medically unexplained symptoms (according to a PHQ‐14 score > 10 and a GP check) Method of diagnosis: adults aged 18‐65 years and registered with participating practices were eligible if they met all 3 criteria: 1. they had been referred at least twice to specialists in the preceding 3 years; 2. they currently reported multiple physical symptoms (PHQ > 10); and 3. their GP believed that their symptoms were unlikely to be adequately explained by physical disease Exclusion criteria: participants who were unable to leave the house independently, other health or social problems precluded an invitation to take part in a study, thoughts of self harm more than a few times in a week (PHQ‐9), current self reported alcohol or drug problems, and current or planned engagement in psychological therapy Total number randomised: 32 Age: for intervention group, M = 45.9 (SD = 12.7); for control group, M = 49.2 (SD = 10.1) Sex: for intervention group 56.3% women (n = 9), 43.7% men (n = 7); for control group 75% women (n = 12), 25% men (n = 4) Severity of symptoms at baseline: PHQ‐14 score for intervention group M = 13.9 (SD = 3.3), for control group M = 14.7 (SD = 2.6) Duration of symptoms at baseline: not reported Setting: selection in 7 participating practices in primary care (selection based on database search, postal questionnaire, and check for exclusion criteria by GP), treatment in a secondary care outpatient symptoms clinic Location: North‐east Edinburgh, Scotland Number of treatment centres: 1 Co‐morbidities: Participants with PHQ‐9 score > 10 (indicating major depressive disorder: for intervention group 31% (n = 5); for control group 56% (n = 9) Participants with GAD‐7 score > 10 (indicating generalised anxiety disorder): for intervention group 19% (n = 3); for control group 31% (n = 5) Adjunctive therapy: participants in both arms continued to receive usual care from their registered general practice. This included referral for investigation or treatment of symptoms as the GP deemed appropriate Adjunctive medication: none reported |
|
| Interventions | Participants were randomly assigned to either 1. Treatment in the symptoms clinic (n = 16) Duration: 4 sessions, the first session of 1 hour and further sessions of 20 minutes, during a period of 3 months Treatment protocol: the consultations were structured to first hear the participant's experience of illness then to propose and negotiate constructive explanations of physical symptoms. These explanations were used as the basis for simple cognitive and behavioural actions to modify symptoms and their impact. No specific attempt was made to screen for common mental disorders; however, participants were encouraged to describe their emotional responses to symptoms and other events, and diagnostic labels such as depression were discussed collaboratively with the participant rather than imposed by the doctor (see Burton 2012, ref 14 for theoretical basis). Therapist: an experienced GP with special interest in MUS 2. Usual care (n = 16) Duration: NA Treatment protocol: NA Therapist: NA |
|
| Outcomes |
Time points for assessment: baseline and 3 months after baseline Primary outcome: (systematic identification of participants, trial recruitment and retention, acceptability to participants (Client Satisfaction Questionnaire)) 1. number and severity of physical symptoms (PHQ‐14) 2. subjective physical and mental health (SF‐12) 3. depressive symptoms (PHQ‐9) 4. generalised anxiety (GAD‐7) |
|
| Notes |
Date of study: August 2009 ‐ May 2010 Funding source: Chief Scientist Office Declarations of interest among the primary researchers: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was carried out by automated telephone system using blocked allocation with variable block size |
| Allocation concealment (selection bias) | Low risk | Participants were randomised to either usual care or intervention by the researcher (WN). Treatments were performed by a doctor (CB) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel could not be blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Participants were not blinded, and most outcomes were participant reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | In the intervention group, outcome data were analysed for 11 out of 16 participants. Missing: 31.25% (> 20%) |
| Selective reporting (reporting bias) | Low risk | All intended outcomes reported |
| Treatment fidelity | High risk | The consultations were structured, but no protocol or manual has been used (Burton 2012, page 3) |
| Researcher allegiance | Unclear risk | The author was also the therapist performing the intervention in the intervention group |
| Other bias | Low risk | No other sources of bias |
Escobar 2007.
| Methods | Study design: randomised controlled trial | |
| Participants |
Diagnosis: MUS (abridged somatisation disorder) Method of diagnosis: clinicians referred participants when they thought symptoms were a source of distress OR suspected they had a psychiatric origin. Participants were then interviewed using PHQ and PRIME‐MD, participants were eligible if they had ≥ 4 unexplained symptoms for men and ≥ 6 for women Exclusion criteria: severe psychiatric diagnosis, requiring more intensive intervention of major physical disorder that explains any of the symptoms Total number randomised: 172 Age: for intervention group, M = 41.0 (SD = 12.7); for control group, M = 39.6 (SD = 13.4) Sex: 88% women; 12% men; 86.2% women in intervention group (n = 75); 89.4% women in control group (n = 76). Severity of symptoms at baseline:baseline PHQ‐15 score for intervention group M = 14.17 (95% CI = 13.03 to 15.32), for control group M = 13.98 (95% CI = 12.82 to 15.13) Duration of symptoms at baseline: not reported Setting: recruited from 2 university based primary care clinics, intervention at Psychiatry department Location: New Brunswick, New Jersey, USA Number of treatment centres: 1 Co‐morbidities: 92% had a current co‐morbid DSM‐IV axis I disorder; 92.0% of intervention group (n = 80), 91.8% of control group (n = 78) Adjunctive therapy: not mentioned Adjunctive medication: not mentioned |
|
| Interventions | Participants were randomly assigned to either 1. CBT + psychiatric consultation letter (n = 87) Duration: 10 sessions of 50 minutes (first session 90 minutes) during 10‐20 weeks (mean of 3 months) Treatment protocol: standardised CBT intervention according to manual, focusing on reduction of reduction of physical distress and somatic pre‐occupation, through training in relaxation techniques, activity regulation, facilitation of emotional awareness, cognitive restructuring and interpersonal communication. Details in book Woolfolk et al. (Woolfolk 2007, ref 18). Consultation letter: a standard consultation letter was sent to the treating primary care physician, originally developed by Smith et al. (Escobar 2007, ref 13), including recommendations about taking care of people with MUPS Therapist: therapists received training on the intervention protocol from 2 of the authors. Therapists' treatment adherence to the study protocol was rated routinely during the study from evaluations of taped sessions 2. Usual clinical care + psychiatric consultation intervention (n = 85) Duration: NA Treatment protocol: a standard consultation letter was sent to the treating primary care physician (see above) Therapist/face‐to‐face contact: none (other than usual care) |
|
| Outcomes |
Time points for assessment: baseline and 3 months, 9 months, after baseline Primary outcome: 1. severity of somatisation (CGI + PHQ‐15) 2. improvement of physical symptoms (CGI ‐ improvement) Secondary outcome: 1. participants' rating of physical functioning (physical subscale of MOS‐10) 2. severity of somatic symptoms (VAS) 3. anxiety and depression (HAM‐A and HAM‐D) |
|
| Notes |
Date of study: recruitment took place from January 2001 through to February 2005, follow‐up until the end of 2006 Funding source: National Institute of Mental Health Declarations of interest among the primary researchers: none reported See Allen 2006 (similar study, same research group) Additional data provided by Escobar (June 2014) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number sequence |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessors were blinded (but had to ask participants…) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 21‐24% loss to follow‐up directly after treatment; 41‐48% loss to follow‐up 6 months later |
| Selective reporting (reporting bias) | Low risk | CGI scores not reported (but provided later by first author) |
| Treatment fidelity | Low risk | Treatment sessions followed a manual with step‐by‐step guidelines for each session (Escobar 2007, ref 18) |
| Researcher allegiance | Low risk | No indication that researchers had a preference for 1 of the treatment modalities |
| Other bias | Low risk | No other sources of bias |
Fjorback 2013.
| Methods | Study design: randomised controlled trial | |
| Participants |
Diagnosis: chronic multi‐organ BDS Method of diagnosis: participants received a 5‐7 hour bio‐psycho‐social assessment, including a laboratory screening battery, schedules for clinical assessment in neuropsychiatry (SCAN)‐diagnostic interview, as well as a physical and neurological examination. Participants (aged 20‐50 years) with chronic (≥ 2 years) multi‐organ type BDS and moderate‐to‐severe impairment in daily living were eligible for inclusion Exclusion criteria: severe psychiatric morbidity, current alcohol or drug abuse, pregnancy, non‐Scandinavian origin, no informed consent Total number randomised: 120 Age: for intervention group, M = 38 (SD = 9); for control group, M = 40 (SD = 8) Sex: 80% women; 20% men in both study groups, 47 women in intervention group, 48 in control group Severity of symptoms at baseline: all participants in both study groups had a somatisation disorder according to ICD‐10 codes. In the intervention group, 95% had a somatisation disorder according to the DSM‐IV codes (n = 56), in the control group 95% (n = 57). 5% of intervention group (n = 3) and 5% of control group (n = 3) had an undifferentiated somatoform disorder (DSM‐IV codes) Duration of symptoms at baseline: for intervention group M = 12 (SD = 10.6), for control group M = 15 (SD = 12.6) years Setting: primary care physicians and hospital wards referred participants both from rural and urban areas. Intervention took place at the Research Clinic for Funcional Disorders and Psychosomatics in Aarhus University Hospital Location: Arhus region, Denmark Number of treatment centres: 1 Co‐morbidities: 71% of intervention group (n = 42) and 62 % (n = 37) of control group had lifetime psychiatric co‐morbidity. 22% of intervention group (n = 13) and 20% of control group (n =12) had a current major depressive disorder (DSM‐IV codes). 24% of intervention group (n = 14) and 23% of control group (n = 14) had a current anxiety disorder (DSM‐IV codes) Adjunctive therapy/medication: consultation letter; letter to social authorities, when needed; advise to taper off morphine derivatives and benzodiazepines (all also in comparison group) |
|
| Interventions | Participants were randomly assigned to either 1. Mindfulness therapy (n = 59) Duration: 8 weekly session and 1 in week 12 (3.5 hours each) during a period of 12 weeks Treatment protocol: participants received information about the nature, course, and treatment of BDS, a treatment manual, 9 group treatment modules based on a mindfulness‐based stress reduction and a cognitive‐behavioural approach (closely following the manual by Jon Kabat Zinn (Fjorback 2013, ref 22, 36, 36). A consultation letter was sent to participants' primary care physicians, and letters to social authorities were sent when needed (see Fjorback 2013 Table 1 for details) Therapist: 2 psychiatrists with experience in BDS and CBT and in psychotherapy of meditation 2. Enhanced TAU (n = 60) (see Fjorback 2013 Figure 1 for details) Duration: 1 x 2‐hour session Treatment protocol: participants received information about the nature, course, and treatment of BDS, a consultation letter to participants' primary care physicians, letters to social authorities when needed, an individual CBT treatment plan according to a manual and an individual psychiatric consultation within the first month after clinical assessment (see Fjorback 2013 Figure 1 for details) Therapist: psychiatrist |
|
| Outcomes |
Time points for assessment: baseline and 3 months, 9 months, 15 months after baseline Primary outcome: 1. mean change in SF‐36 PCS between baseline and 15 months Secondary outcome: 1. change in other health‐related quality of life measures of the SF‐36 2. illness worry (WI‐8) 3. physical symptoms (SCL‐90‐R Somatisation Subscale) 4. severity of depression and anxiety (SCL‐8) 5. participants reporting improvement (greater than half a SD) |
|
| Notes |
Date of study: recruitment took place between April 2007 and September 2008, follow‐up until the end of 2009 Funding source: Danish Agency for Science Technology and Innovation, Aase and Ejnar Danielsens Fund, Trygfonden Declarations of interest among the primary researchers: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was prepared by a statistician |
| Allocation concealment (selection bias) | Low risk | They used pre‐defined concealed random numbers tied to consecutive assessments of participants resulting in opaque envelopes numbered in succession containing assigned treatment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not blinded, given difference in treatment content, intensity, and duration |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | All outcomes were participant reported, and these were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | > 20% no data at follow up, but multiple imputation may have solved this problem |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. Secondary outcome probability of change > 0.5 SD predefined? |
| Treatment fidelity | Low risk | Treatment followed a model based on a manual (Fjorback 2013, table 1 and ref 39) |
| Researcher allegiance | Low risk | No indication that researchers had a preference for 1 of the treatment modalities |
| Other bias | Low risk | No other sources of bias |
Kashner 1995.
| Methods | Study design: randomised controlled trial | |
| Participants |
Diagnosis: somatisation disorder Method of diagnosis: inclusion criteria were diagnosis of somatisation disorder, history of ≥ 13 unexplained symptoms, the first beginning < 30 years of age, severe enough to seek treatment, take medication, or reduce functioning. This was determined by a research psychiatrist, using a checklist of 37 items that made up diagnostic criteria of somatisation disorder, according to DSM‐III‐R criteria Exclusion criteria: the absence of a primary care provider who agreed to allow the research team to see the participant, lack of transportation to the medical centre, indication of moving out of town during study Total number randomised: 70 Age: mean age 44.2 years (no SD provided) Sex: 84% women (n = 59); 16% men (n = 11) Severity of symptoms at baseline: inclusion criterion: ≥ 13 unexplained symptoms Duration of symptoms at baseline: inclusion criterion: 1 symptom starting before age 30 years Setting: recruitment: internists, GPs, and general population (through advertisements in local media). Treatment: at medical centre Location: Central Arkansas, USA Number of treatment centres: 1 Co‐morbidities: unknown Adjunctive therapy: not mentioned Adjunctive medication: not mentioned Co‐intervention: none reported |
|
| Interventions | Participants were randomly assigned to either 1. Group therapy intervention + consultation letter (n = 44) Duration: 8 small group sessions of 2 hours every other week, during a 4‐month period Treatment protocol: the overall goals were to develop a source of peer support, share methods of coping with physical problems, enable participants to increase their ability to perceive and express emotion, and enjoy the experience of participating in the group. Treatment in groups of 4‐6, according to a structured protocol, with a class atmosphere, including didactic presentations, small group discussions, therapy exercises, and group discussions. See Kashner 1995 p.464‐5 for details Co‐intervention (both groups): a standard psychiatric consultation letter was sent to the primary care physician, diagnosing the participant with somatisation disorder and including recommendations for its management Therapist: master's level clinicians 2. Consultation letter only (n = 26) Co‐intervention (both groups): standard psychiatric consultation letter sent to primary care physician, diagnosing the participant with somatisation disorder and including recommendations for its management Duration: NA Treatment protocol: NA Therapist: NA |
|
| Outcomes |
Time points for assessment: baseline and 4 months, 8 months and 12 months after baseline Outcomes: (unclear what is primary and what is secondary) 1. RAND Health Status (4 domains) 2. days in bed (count) 3. healthcare utilisation (costs) |
|
| Notes |
Date of study: started in 1987, end unclear (healthcare costs were adjusted for inflation using 1990 dollar rates) Funding sources: Robert Wood Johnson Foundation, National Institute of Mental Health, VA Health Services Research and Development Program for Mental Health Declarations of interest among the primary researchers: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel not blinded to intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessors did not know whether participant was in experimental or control group, but most outcomes were participant reported and these were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed study |
| Selective reporting (reporting bias) | High risk | Days in bed described as assessed but no results reported |
| Treatment fidelity | Low risk | Treatment was based on a structured protocol developed before the study (available from the authors) |
| Researcher allegiance | Low risk | No indication that researchers had a preference for 1 of the treatment modalities |
| Other bias | Low risk | No other sources of bias |
Katsamanis 2011.
| Methods | Study design: randomised controlled trial | |
| Participants |
Diagnosis: subthreshold somatisation disorder (abridged somatisation) Method of diagnosis: participants (18‐70 years old) were eligible if no major medical illness explained symptoms after detailed physical and laboratory assessment; individuals must have met criteria for ≥ 4 MUPS out of the 42 somatic symptoms listed in the CIDI rated as currently present if males and at least 6 if female, according to a diagnostic interview Exclusion criteria: history of alcohol/drug abuse (within last 12 months), bipolar or psychotic, unstable medical condition, pregnancy, active suicidal ideation Total number randomised: 48 (10 dropped out, baseline data reported for only 38) Age: intervention group 83% < 40 years (n = 15); control group 40% < 40 years (n = 8) Sex: 79% women; 21% men; for intervention group 83.3% women (n = 15), for control group 75.0% (n = 15) Severity of symptoms at baseline: unclear, participants in both groups met a CGI rating of 4, which equals moderate somatisation Duration of symptoms at baseline: not described Setting: participants were recruited from primary medical clinics and community (advertisements). Treatment: department of psychiatry of a medical school Location: New Jersey, USA Number of treatment centres: 1 Co‐morbidities: 55% met criteria for severe depression (n = 21, HAM‐D 17 criteria), 40% for mild‐to‐moderate depression (n = 15, HAM‐D 17 criteria) and 85% for significant anxiety (n = 23, HAM‐A criteria) Adjunctive therapy: not mentioned Adjunctive medication: psychotropic medication were allowed (Katsamanis 2011, table 3) |
|
| Interventions | Participants were randomly assigned to either 1. Psychophysiological treatment + psychiatric consultation intervention (PCI) (n = 24) Duration: 10 weekly sessions during a period of 10 weeks Treatment protocol: psychophysiological treatment: treatment consisted of a manualised intervention, described to participants as an intervention which comprises of a set 'self regulation' techniques that are specifically targeted at particular symptoms of body systems, aiming to assist in coping with physical discomfort and stress (Katsamanis 2011, page 221 for details) PCI: a standard consultation letter was sent to the principal treating physician making recommendations for the ongoing treatment (Katsamanis 2011, Table 1 for details) Therapist: 4 therapists, either master or doctoral level psychologists, 3 of them certified as biofeedback clinicians with at least 3 years of supervised training in psychophysiological treatment 2. PCI alone (n = 24) Duration: NA Treatment protocol: standard consultation letter sent to the treating physician including recommendations for the ongoing treatment (Katsamanis 2011, table 1 for details). Therapist: NA |
|
| Outcomes |
Time points for assessment: baseline, halfway the intervention (5 weeks) and at the end of treatment (10 weeks) Primary outcome: 1. severity of somatisation (CGI‐SD, clinician rated) Secondary outcome: 1. level of depression and anxiety (HAM‐D and HAM‐A) 2. participants' rating of physical functioning (MOS, SF‐36) 3. participants' rating of mental functioning (MOS, SF‐36) |
|
| Notes |
Date of study: the study took place between June 2006 and August 2008 Funding source: National Institue of Mental Health Declarations of interest among the primary researchers: design based on Allen. Cross‐over group (receiving treatment after wait condition) not used |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number sequence |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Personnel and participants not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessors were masked but almost all outcomes were participant reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 10/48 dropped out and for several outcomes even more (> 20%) |
| Selective reporting (reporting bias) | Low risk | All intended outcomes reported |
| Treatment fidelity | Low risk | Treatment was based on a manual, containing guidelines, developed before the study (available from the authors) |
| Researcher allegiance | Low risk | No indication that researchers had a preference for 1 of the treatment modalities |
| Other bias | Unclear risk | Large difference in age between groups suggests randomisation failed |
Kolk 2004.
| Methods | Study design: randomised controlled trial | |
| Participants |
Diagnosis: multiple medically unexplained symptoms Method of diagnosis: participants with relatively recent onset (3‐12 months) of symptoms were tested for eligibility was assessed with a standardised interview and had to fit to 3 additional criteria: 1. the GP confirmed the medically unexplained nature of their symptoms presented at enrolment, 2. between 18‐60 years of age, 3. sufficient understanding of the Dutch language Exclusion criteria: current psychotherapy or a primary diagnosis of mood, anxiety or psychotic disorder requiring treatment Total number randomised: 106 (4:1) Age: for intervention group, M = 35.5 (SD = 9.4); for control group, M = 35.0 (SD = 8.9) Sex: 69% women; 31% men; for intervention group 67% women (n = 54), for control group 78% women, (n = 14) Severity of symptoms at baseline: not described Duration of symptoms at baseline: not described Setting: recruited in general practices and via advertisements in open population (about 50/50 from each source). Treatment: department of Clinical Psychology of a university Location: Amsterdam, the Netherlands Number of treatment centres: 1 Co‐morbidities: in intervention group, participants had M = 2.34 (SD = 1.9) chronic diseases, in control group, M = 1.89 (SD = 1.7) Adjunctive therapy: not mentioned Adjunctive medication: not mentioned |
|
| Interventions | Participants were randomly assigned to either 1. Psychological intervention (n = 83) Duration: maximum 12 x 1‐hour sessions once a week or every 2 weeks during a maximum period of 6 months Treatment protocol: depending on therapist (1 of 15 qualified therapists): cognitive behavioural, client‐centred or eclectic therapy, reflecting usual treatment practice Therapist/face‐to‐face contact: 1 out of 15 trained therapists 2. Usual care (n = 23) Duration: NA Treatment protocol: after the intake, participants were referred back to the GP for care as usual, intake reports were also sent to GP Therapist: 1 of 15 trained therapists |
|
| Outcomes |
Time points for assessment: baseline and 6 and 12 months after baseline Primary outcome: 1. severity of somatisation (somatisation scale of the SCL‐90) Secondary outcome: 1. self reported psychological symptoms (anxiety and depression: SCL‐90) 2. registered unexplained and explained symptoms in general practice (database) 3. GP consultations (database) |
|
| Notes |
Date of study: unclear Funding source: Nationaal Fonds Geestelijke Volksgezondheid / ZAO Zorgverzekeringen Declarations of interest among the primary researchers: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes, opened after pretest |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel could not be blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Self report questionnaires mostly |
| Incomplete outcome data (attrition bias) All outcomes | High risk | > 20% drop‐outs |
| Selective reporting (reporting bias) | Low risk | All intended outcomes reported |
| Treatment fidelity | High risk | Treatment depended on therapist, no manual or protocol was used |
| Researcher allegiance | Low risk | No indication that researchers had a preference for 1 of the treatment modalities |
| Other bias | Low risk | No other sources of bias |
Lidbeck 1997.
| Methods | Study design: randomised controlled trial | |
| Participants |
Diagnosis: somatisation disorder Method of diagnosis: participants received an interview and a physical examination. Participants (30‐60 years old) were eligible in case of presence of somatisation disorder in accordance with the definition of functional somatic symptoms and ≥ 1 symptoms fulfilling the criteria of specific functional disorders as outlined in the International Classification of Health Problems for Primary Care‐2. Exclusion criteria: major mental disorder, current psychological of psychiatric treatment, acute or transient functional symptoms, drug abuse, chronic pain disorder, inability to speak Swedish fluently Total number randomised: 50 Age: for intervention group, M = 43.8 (SD = 9.3); for control group, M = 44.6 (SD = 7.4) Sex: 84% women; 16% men; for intervention group 84.8% (n = 28) female, for control group 82.4% female (n = 14) Severity of symptoms at baseline: in intervention group 14/33 participants had ≥ 4 symptoms (42.4%), in the control group 9/17 participants had ≥ 4 symptoms (52.9%). Duration of symptoms at baseline: in the intervention group the mean duration of illness was 9.2 years, in the control group 6.6 years. Setting: recruitment: GPs and hospital doctors; treatment: outpatient clinic of Preventive Medicine Unit at Helsingborg County Hospital Location: Helsingborg, Sweden Number of treatment centres: 1 Co‐morbidities: 19/50 (38%) of all participants were previously treated by psychologist/psychiatrist Adjunctive therapy: not mentioned Adjunctive medication: allowed and recorded |
|
| Interventions | Participants were randomly (2 : 1) assigned to either 1. Group therapy using a short cognitive‐behavioural treatment model (n = 43) Duration: 8 weekly sessions of 3 hours, and 1 final session after 3 months Treatment protocol: group sessions of CBT focused on reducing dread of somatic diseases and included the following items: 1. thorough physical examination, 2. education to explain stress symptoms in order to enable cognitive restructuring, 3. relaxation training (Lidbeck 1997, table 2 for details) Therapist: a physician (the author), who was specialised in internal, family, and social medicine and who had received training in stress relaxation 2. Waiting list control group (n = 17) Duration: NA Treatment protocol: NA Therapist: NA |
|
| Outcomes |
Time points for assessment: baseline and 3 months, 9 months after baseline Outcomes: 1. social problems (SPQ) 2. illness behaviour (IBQ) 3. anxiety and depression (HADS) 4. sleep disturbance (SDI) 5. medication usage (questionnaire) |
|
| Notes |
Date of study: unclear (probably early 1990s) Funding source: not mentioned Declarations of interest among the primary researchers: not mentioned Lidbeck 2003 provides 1.5‐year follow‐up data, but only for intervention group |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "using pair‐wise grouping, i.e. subjects with similar symptoms, duration of symptoms, age etc, were randomly allotted to either the treatment or the control group" (personal communication with Lidbeck) |
| Allocation concealment (selection bias) | Low risk | "randomization was carried out independently by a nurse who was not participating in the study"(Lidbeck 1997, p.17) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Participants filled in questionnaires and were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 1 drop‐out, no missing data |
| Selective reporting (reporting bias) | Low risk | All intended outcomes reported |
| Treatment fidelity | Unclear risk | Treatment was structured, but no information provided about a manual |
| Researcher allegiance | Unclear risk | The author was also the therapist performing the intervention in the intervention group |
| Other bias | Low risk | No other sources of bias |
Martin 2007.
| Methods | Study design: randomised controlled trial | |
| Participants |
Diagnosis: multiple somatoform symptoms Method of diagnosis: participants were selected after presentation of ≥ 2 MUPS in the last 6 months, leading to significant clinical distress, according to their GP or to a screening questionnaire. A diagnostic interview (IDCL and Mini‐DIPS) was used to assess study criteria and DSM‐5 psychiatric diagnoses Exclusion criteria: severe current medical condition, chronic medical disease explaining the symptoms, psychotic symptoms, substance dependence Total number randomised: 140 Age: for intervention group, M = 45.7 (SD = 13.6); for control group, M = 51.7 (SD = 15,9) (significant difference at P value < 0.05) Sex: for intervention group 68.6% female (n = 48); for control group, 81.4% female (n = 57) Severity of symptoms at baseline: number of somatoform symptoms, lifetime: for intervention group M = 7.6 (SD = 3.9), for control group M = 6.6 (SD = 3.6); DSM‐IV Somatoform Disorder: for intervention group 91.4% (n = 64), for control group 87.1% (n = 61) Duration of symptoms at baseline:years from onset of somatoform symptoms: for intervention group M = 9.2 (SD = 11.2), for control group M = 11.8 (SD = 12.8) Setting: participants were recruited in primary care practices, general population (news reports), or via 'other ways', treatment took place at an outpatient treatment centre in the university hospital (secondary care) Location: Marburg, Germany Number of treatment centres: 1 Co‐morbidities: affective disorder: for intervention group 66.7% (n = 46); for control group 52.2% (n = 36) DSM‐IV anxiety disorder: for intervention group 41.4% (n = 29); for control group 32.9% (n = 22) Adjunctive therapy: none reported Adjunctive medication: none reported |
|
| Interventions | Participants were randomly assigned to either 1. CBT (n = 70) Duration: 1 session of 3‐4 hours, 2 weeks after baseline Treatment protocol: treatment consisted of 1 session in groups of 2‐4 participants. Treatment followed a structured manual with 5 central modules: psychophysiological explanation of symptoms, relaxation, importance of cognition, activity instead of avoidance behaviour, and treatment options and healthcare utilisation (Martin 2007, page 296 for details). Therapist: clinical psychologist or medical specialist for psychotherapeutic medicine, both licences CBT specialists 2. Standard medical care (n = 70) Duration: NA Treatment protocol: NA Therapist: NA |
|
| Outcomes |
Time points for assessment: baseline, 4 weeks, and 6 months after baseline Primary outcome: 1. healthcare utilisation (structured interview, not at 4 weeks) 2. number and severity of somatoform symptoms (BSI and SOMS‐7) Secondary outcome: 1. health anxiety (WI) 2. general psychopathological symptoms (GSI) 3. Depressive symptoms (BDI) 4. health‐related internal control (KKG) |
|
| Notes |
Date of study: trial conducted between August 2001 ‐ December 2002 Funding source: German Ministry of Research, Education and Science Declarations of interest among the primary researchers: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was based on a predefined list of binary variables, using blocking procedures to ensure comparable sample sizes (page 295) |
| Allocation concealment (selection bias) | Low risk | Study assistants enrolled and assigned participants to the groups according to the randomisation list |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel could not be blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Participants were not blinded, and most outcomes were participant reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up at 6 months 15.7% in both groups (< 20%) |
| Selective reporting (reporting bias) | Low risk | All intended outcomes are reported |
| Treatment fidelity | Low risk | The treatment followed a structured manual with 5 central modules (in German), available on request (Martin 2007, page 296) |
| Researcher allegiance | Low risk | No indication that researchers had a preference for 1 of the treatment modalities |
| Other bias | Low risk | No other sources of bias |
Moreno 2013.
| Methods | Study design: randomised controlled trial | |
| Participants |
Diagnosis: DSM‐IV diagnosis of abridged somatisation disorder Method of diagnosis: participants (aged 18‐65 years) were eligible if they were able to understand and read Spanish and fulfilled the criteria for ASD (somatic symptom indexes 4 (men) and 6 (women)). In addition, they had to be stable on pharmacotherapy in the previous month and needed to sign informed consent. The Othmer‐DeSouza test was used as a screening tool Exclusion criteria: any primary psychiatric diagnosis other than somatoform disorders, severe personality disorder, non‐white (not mentioned in design paper), inability to attend intervention sessions Total number randomised: 168 Age: for individual CBT group, M = 43.1 (SD = 11.4); for group CBT group, M = 49.2 (SD 8.6); TAU control group, M = 44.1 (SD = 11.7) Sex: 86% women; 14% men; for TAU group 87.5% women (n = 42), for individual CBT group 82.14% women (n = 46), for group CBT group 89.06% women, n = 57. Severity of symptoms at baseline: not described Duration of symptoms at baseline: most participants had symptoms for > 2 years: for TAU group 79.16% (n = 38), for individual CBT group 87.5% (n = 49), for group CBT group 87.5% (n = 56) Setting: recruitment in primary healthcare centres. Treatment setting not mentioned, but carried out by 2 psychologists Location: provinces of Zaragoza, Mallorca, Huesca in Spain Number of treatment centres: unknown Co‐morbidities: approximately 10% had a co‐morbid depressive disorder, 40% had a co‐morbid anxiety disorder, and 30% had a depressive and anxiety co‐morbid disorder Adjunctive therapy: not mentioned Adjunctive medication: not mentioned |
|
| Interventions | Participants were randomly assigned to either 1. Individual CBT + standardised letter to family doctor (n = 56) Duration: 10 weekly sessions of 1 hour, during a period of 10 weeks Treatment protocol: CBT: muscle relaxation training, behaviour modification, emotional mindfulness, cognitive restructuring, social skills, based on Escobar 1998; standardised letter with treatment advice for GP based on Smith 1991 Therapist: psychologists 2. Group CBT + standardised letter to family doctor (n = 64) Duration: 10 weekly sessions of 2 hours, during a period of 10 weeks Treatment protocol: CBT: muscle relaxation training, behaviour modification, emotional mindfulness, cognitive restructuring, social skills, based on Escobar 1998; standardised letter with treatment advice for GP based on Smith 1991 Therapist: psychologists 3. TAU: standardised letter to family doctor only (n = 48) Duration: NA Treatment protocol: standardised letter with treatment advice for GP based on Smith 1991 Therapist: NA |
|
| Outcomes |
Time points for assessment: baseline, after 10 weeks (immediately after treatment) and 6 and 12 months after treatment (i.e. about 9 and 15 months after baseline) Primary outcome: 1. severity of somatisation (Screening for Somatic Disorders and SSS scale) 2. anxiety and depression (HADS) Secondary outcome: 1. quality of life (SF‐36: reported in Gili 2014) 2. self declared health services (according to design paper (Magallon 2008), but not reported) 3. global improvement (CGI) (according to design paper (Magallon 2008), but not reported) |
|
| Notes |
Date of study: data collection began in March 2008 and ended in June 2010 Funding source: Red de Investigacion en Actividades de Prvencion y Promocion de la Salud Declarations of interest among the primary researchers: authors declared that they had no competing interests |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number sequence (Maggalon 2008, p. 4) |
| Allocation concealment (selection bias) | Low risk | Allocation was carried out by an independent person who was not involved in the study (p.602) Using central telephone. Sequence will be concealed until interventions are assigned (Moreno 2013, Magallon 2008, p. 4) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Personnel and participants could not be blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Study personnel that carried our the measurements were unaware of which treatment the participant was given. GPs were also kept blind to intervention, as participants were asked not to reveal their treatment condition. However, some outcomes were participant rated and participants could not be blinded (Moreno 2013, p. 602) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | < 20% drop‐out, and intention‐to‐treat analysis with last observation carried forward |
| Selective reporting (reporting bias) | High risk | Healthcare use and CGI were mentioned in protocol but not reported Quality of life reported in Gili 2014 |
| Treatment fidelity | Low risk | There were 2 different treatment conditions following the same protocol: individual and group formats (Moreno 2013, ref 23) |
| Researcher allegiance | Low risk | No indication that researchers had a preference for 1 of the treatment modalities |
| Other bias | Low risk | No other sources of bias |
Sattel 2012.
| Methods | Study design: randomised controlled trial | |
| Participants |
Diagnosis: DSM‐IV diagnosis of multisomatoform disorder Method of diagnosis: participants were screened with the PHQ‐15 and SF‐36, then SCID, modified to check for presence of multisomatoform disorder (at least 3 current somatoform symptoms that are functionally disabling and that an organic disease or another mental disorder cannot sufficiently explain, and a history of somatoform symptoms over at least 2 years, resulting in healthcare use Exclusion criteria: insufficient cognitive abilities, severe, chronic and disabling somatic disease or severe co‐morbid mental disorder that caused major impairment in social functioning (e.g. schizophrenia, severe forms of bipolar disorder or substance misuse), risk of suicide, people undergoing psychotherapy at the time of the screening or inability to speak German. In addition, a small number of people with a DSM‐IV diagnosis of hypochondriasis were excluded Total number randomised: 211 Age: for intervention group, M = 47.9 (SD = 10.8); for control group, M = 48.0 (SD = 12.4) Sex: 66% women; 34% men; for intervention group 63% women (n = 67), for control group 69% women (n = 72) Severity of symptoms at baseline:number of SCID somatoform symptoms: for intervention group M = 10.0 (SD = 3.9), for control group M = 10.6 (SD = 4.0) Duration of symptoms at baseline: for intervention group M = 10.4 years (SD = 5.5), for control group M = 10.8 (SD = 5.5) Setting: recruited from outpatients departments of neurology and internal medicine as well as from pain treatment centres and an orthopaedics private clinic. Treatment setting: outpatient departments of psychosomatic medicine Location: southern Germany (Munich, Düsseldorf, Hannover, Heidelberg, Münster and Regensburg) Number of treatment centres: 6 Co‐morbidities: unknown Adjunctive therapy: not mentioned Adjunctive medication: not mentioned |
|
| Interventions | Participants were randomly assigned to either 1. Psychodynamic interpersonal psychotherapy (n = 107) Duration: 12 weekly sessions, 1st session 90 minutes, other sessions 45 minutes, during a period of 12 weeks Treatment protocol: manualised treatment consisted of 3 phases: 1. emphasis lay on building a therapeutic relationship with underscoring the legitimacy of bodily complaints and relaxation was introduced, 2. clarifying the participant's emotions, 3. concentrates on termination issues (Sattel 2012, ref 20) Therapist: 4 psychologists and 4 physicians with at least 3 years of training in psychotherapy, who were trained in the use of the manual 2. Enhanced medical care (n = 104) Duration: 3 approximately 30‐minute sessions at 6‐week intervals Treatment protocol: participants received education and counselling regarding therapeutic alternatives based on evidence‐based guidelines for the treatment of somatoform disorders/functional somatic syndromes in primary and somatic specialist care Therapist: physicians specifically trained in EMC |
|
| Outcomes |
Time points for assessment: baseline, 3 months (end of treatment), and 9 months after end of treatment Primary outcome: 1. SF‐36 ‐ PCS at 9 months' follow‐up Secondary outcome: 1. SF‐36 ‐ MCS 2. PHQ‐15 somatisation module 3. PHQ‐9 depression module 4. Health anxiety: WI 5. doctor visits, use of antidepressants, and use of psychotherapy |
|
| Notes |
Date of study: between June 2006 and December 2007 participants were selected, follow‐up continued until the beginning of 2009 Funding source: German Research Foundation Declarations of interest among the primary researchers: the authors report no conflicting interests |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was computer generated |
| Allocation concealment (selection bias) | Low risk | After receiving informed consent, the authors submitted a randomisation request, which was received within 24 hours |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Participant administered questionnaires: blinding impossible Healthcare use: blinding unclear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | < 20% drop‐outs, multiple imputation, and intention to treat |
| Selective reporting (reporting bias) | Low risk | All intended outcomes reported |
| Treatment fidelity | Low risk | The used treatment manual was developed jointly and pilot‐tested over the course of 3 years (Sattel 2012, page 61) |
| Researcher allegiance | Low risk | No indication that researchers had a preference for 1 of the treatment modalities |
| Other bias | Low risk | No other sources of bias |
Schaefert 2013.
| Methods | Study design: cluster‐randomised controlled trial | |
| Participants |
Diagnosis: MUS Method of diagnosis: participants were eligible in case of: 1. persistent (> 6 months) bodily complaints without sufficient explanatory peripheral organ pathology (according to GP), 2. MUS as the main treatment issue, 3. PHQ‐15 score of ≥ 5, 4. relevant health anxiety on the WI‐7 (score ≥ 4), or a combination of these Exclusion criteria: age < 18 or > 70 years, living further than 20 miles away from the respective practice; ongoing psychotherapy; substance abuse; severe psychiatric disorder (e.g. major depression, psychosis, dementia); severe organic disease; inability to complete the questionnaire, or ongoing litigation due to disability, pension or compensation for personal suffering Total number randomised: 328 Age: for intervention group, M = 50.8 (SD = 12.0); for control group, M = 46.6 (SD = 12.9) Sex: for intervention group 75.3% women (n = 128), 24.7% men (n = 42); for control group 74.6% women (n = 100), 25.6% men (n = 34) Severity of symptoms at baseline: somatic symptom severity according to PHQ‐15‐score: LOW (0‐9): for intervention group 28.2% (n = 48) for control group 30.6% (n = 41) MEDIUM (10‐14): for intervention group 38.2% (n = 65) for control group 35.8% (n = 48) HIGH (15‐30): for intervention group 33.5% (n = 57) for control group 33.6% (n = 45) Duration of symptoms at baseline: for intervention group M = 6.74 years (SD = 5.4), for control group M = 5.00 years (SD = 4.6) Setting: participants were recruited and treated by GPs in primary care Location: Heidelberg area, Germany Number of treatment centres: 35 GPs (from 34 practices) Co‐morbidities: Depressive symptoms: for intervention group 33.5% (n = 57); for control group 43.3% (n = 58) Generalised anxiety: for intervention group 16.6% (n = 28); for control group 21.8% (n = 29) Panic disorder: for intervention group 21.8% (n = 29); for control group 17.3% (n = 23) Musculoskeletal system disorders: for intervention group 45%; for control group 51% Hypertension: for intervention group 39%; for control group 39% Endocrine/alimentary/metabolic disorders: for intervention group 34%; for control group 34% Gastrointestinal system disorders: for intervention group 25%; for control group 29% Adjunctive therapy: none reported Adjunctive medication: none reported |
|
| Interventions | Participants were randomly assigned to either 1. GP training in diagnosis and management of MUPS and group leading (GP level) + an interpersonal approach of psychodynamically based therapy (participant level) (n = 183) Duration: GP level: 4 training sessions, in total 15.5 hours (diagnosis + management of MUPS) + 3 sessions, in total 12 hours (group leading) participant level: 10 weekly sessions of 90 minutes + 2 booster sessions 3 and 9 months later Treatment protocol: GP level: guideline based curriculum for training of GPs in diagnosis and management of MUPS, consisting of lectures, discussions and role plays (Schaefert 2013, ref 42 for details) + GP training in group leading (methodology not described) Participant level: manualised group intervention consisting of an interpersonal approach of psychodynamically based therapy, with embedded cognitive behavioural elements (Schaefert 2013, table 1 and ref 43 for details) Therapist: GP level: the investigators; participant level: GP + 1‐3 psychosomatic specialists (with ≥ 3 years of training in psychosomatic therapy) 2.GP training in diagnosis and management of MUPS (n = 145) Duration: GP level: 4 training sessions, in total 15.5 hours (diagnosis + management of MUPS) Treatment protocol: guideline based curriculum for training of GPs in diagnosis and management of MUPS, consisting of lectures, discussions and role plays (Schaefert 2013, ref 42 for details) Therapist: GP level: the investigators |
|
| Outcomes |
Time points for assessment: baseline and 6 months, 12 months after baseline Primary outcome: Quality of life (SF‐36, PCS and MCS) Secondary outcome: Somatic symptom severity (PHQ‐15) Depression (PHQ‐9) Anxiety and panic (PHQ anxiety and panic, only at baseline) Stress (PHQ stress) Health anxiety (WI) Healthcare utilisation (GP documentation and self report) |
|
| Notes |
Date of study: participant recruitment started in November 2007 in both groups and ended in September 2008 in the intervention group and in December 2009 in the control group, follow‐up continued until the end of 2010 Funding source: German Federal Ministry of Education and Research Declarations of interest among the primary researchers: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | GPs were unit of randomisation, randomisation was performed in a data and co‐ordinating centre |
| Allocation concealment (selection bias) | Low risk | Blinded randomisation was performed by a statistician under independent management. The GPs were informed by a research assistant |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants and personnel possible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Participants were not blinded, and most outcomes were participant reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Incomplete data in intervention group at 6 months 20.8%, at 12 months 21.9% Incomplete data in control group at 6 months 31.8%, at 12 months 25.6% |
| Selective reporting (reporting bias) | Low risk | All intended outcomes reported |
| Treatment fidelity | Low risk | The participating GPs were trained with a guideline‐based curriculum in the diagnosis and management of MUS (Schaefert 2013, ref 41, 42) |
| Researcher allegiance | Unclear risk | Some of the authors were also providing the intervention |
| Other bias | Low risk | No other sources of bias |
Schilte 2001.
| Methods | Study design: randomised controlled trial | |
| Participants |
Diagnosis: somatisation Method of diagnosis: participants of 20‐45 years who had ≥ 15 contacts in the past 3 years and who had ≥ 5 somatisation symptoms according to a somatisation scale (based on all symptoms listed in DSM‐IIIR) Exclusion criteria: cancer, AIDS, rheumatoid arthritis, multiple sclerosis, dementia, schizophrenia, mental disorder, and psychosis. Included people with other chronic diseases, such as asthma, osteoarthritis, or cardiovascular diseases Total number randomised: 161 Age: for intervention group M = 38 (IQR = 33‐41), for control group M = 39 (IQR = 36‐41) Sex: for intervention group 80% female (n = 61), for control group 78% female (n = 59) Severity of symptoms at baseline: mean somatisation score (scale 0‐48): for intervention group M = 20 (IQR = 16‐25), for control group M = 22 (IQR = 17‐22) Duration of symptoms at baseline: unknown Setting: participants were recruited in the registration network of primary physicians (primary care). the intervention took place at participants' homes Location: Maastricht area, the Netherlands Number of treatment centres: 77 participants' homes Co‐morbidities: of the 77 participants who actually received the intervention, 34 had an active depressive or anxiety disorder (16 depressive, 30 anxiety) according to the DSM‐IV screening. 2 participants fulfilled criteria of DSM‐IV hypochondriasis and 18 of a DSM‐IV chronic benign pain syndrome Adjunctive therapy: none reported Adjunctive medication: none reported |
|
| Interventions | Participants were randomly assigned to either 1.Disclosure intervention (n = 81): Duration: unknown Treatment protocol: treatment consisted of 2 meetings with a disclosure doctor + an optional meeting with the doctor and the GP. They were invited to disclose emotionally important events in their life. If not mentioned spontaneously, the doctor asked questions about family life, health, work situation, and childhood Therapist: a trained disclosure doctor and in the third session also the GP 2.Usual care (n = 80): Duration: NA Treatment protocol: NA Therapist: NA (care as usual by own GP) |
|
| Outcomes |
Time points for assessment: baseline, 6, 12 and 24 months after baseline Primary outcome: 1. Healthcare use (number of visits to all health care) Secondary outcome: 1. Subjective health (scale) 2. Severity of symptoms (SCL‐90 subscore) 3. Depressive symptoms (SCL‐90 subscore) 4. Anxiety (SCL‐90 subscore) 5. Sick leave (number of weeks of sick leave over the preceding 6 months) |
|
| Notes |
Date of study: unknown, published in 2001 Funding source: Netherlands Organisation for Scientific research Declarations of interest among the primary researchers: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation was stratified (1 stratum per practice), using a sequence of labelled cards in opaque, sealed, numbered envelopes |
| Allocation concealment (selection bias) | Low risk | An independent person produced the randomisation envelopes, and the research assistant, who did not apply the intervention, executed the randomisation procedure |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Although the general practitioners knew which participant received the intervention, they were not told which participants participated as controls. Participants could not be blinded for the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Participants were not blinded, and most outcomes were participant reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | In the intervention group 70/81 (86.4%) participants completed the trial (and questionnaire at 2 years follow‐up) In the control group 67/80 (83.7%) participants completed the trial (and questionnaire at 2 years' follow‐up) (Schilte 2001, page 87) (loss to follow‐up < 20%) |
| Selective reporting (reporting bias) | High risk | Only results at 24 months of follow‐up are reported |
| Treatment fidelity | High risk | No protocol or manual for treatment (Schilte 2001, page 323) |
| Researcher allegiance | Low risk | No indication that researchers had a preference for 1 of the treatment modalities |
| Other bias | Unclear risk | Results were not corrected for baseline imbalances |
Schröder 2012.
| Methods | Study design: randomised controlled trial | |
| Participants |
Diagnosis: BDS Method of diagnosis: participants received a thorough biopsychosocial assessment including a review of all clinical records, a SCAN interview, a physical and neurological examination, and a laboratory screening battery were performed. Participants aged 20‐45 years with chronic (i.e. ≥ 2 years' duration) BDS of the severe multi‐organ type, which requires functional somatic symptoms from at least 3 of 4 bodily systems, and moderate to severe impairment in daily living (Schröder 2012, ref 18 and 19 for details) Exclusion criteria: severe psychiatric morbidity (psychotic and bipolar disorders, alcohol or drug misuse), people involved in litigation, those who were pregnant and those who were not fluent in the Danish language (operationalised as non‐Scandinavian origin) Total number randomised: 120 Age: for intervention group, M = 35.4 (SD = 6.3); for control group, M = 36.2 (SD = 6.5) Sex: for intervention group 74% women (n = 40), 26% men (n = 14); for control group 83% women (n = 55), 17% men (n = 11) Severity of symptoms at baseline: number of functional somatic symptoms: for intervention group M = 32.3 (SD = 7.5), for control group M = 32.6 (SD = 10.0). Duration of symptoms at baseline: for intervention group M = 6.7 (IQR = 3‐14), for control group M = 9.5 (IQR = 4‐15) Setting: the intervention took place in cooperation with the university general hospital in Aarhus, Denmark. Participants were referred from all primary care physicians and hospital wards in the western part of Denmark.(primary and secondary care) Location: Western part of Denmark (Jutland), which covers a population of approximately 2 million persons living in both urban and rural areas. Number of treatment centres: unclear Co‐morbidities: Major depressive disorder: for intervention group 17% (n = 9); for control group 21% (n = 14) Dysthymia: for intervention group 4% (n = 2); for control group 5% (n = 3) Anxiety disorder: for intervention group 19% (n = 10); for control group 18% (n = 12) At least 1 of the above diagnoses: for intervention group 30% (n = 16); for control group 36 (n = 24) Lifetime psychiatric co‐morbidity: for intervention group 57% (n = 31); for control group 61% (n = 40) Adjunctive therapy: none reported Adjunctive medication: none reported |
|
| Interventions | Participants were randomly assigned to either 1.STreSS intervention (n = 54) Duration: 9 x 3.5 hour sessions during a period of 4 months Treatment protocol: 1. Comprehensive lifetime review of case notes and clinical records; 2. Comprehensive biopsychosocial assessment and individualised information about the nature,course and treatment options for the symptoms; 3. Letter to participant's primary care physician and referring doctor (if not the primary care physician) regarding diagnosis and illness history as well as treatment recommendations in case of co‐morbid depression or anxiety; 4. 'Usual care' delivered by primary care physician and specialists; 5. 9 modules of manualised psychotherapy, based on a cognitive‐behavioural approach. Each participant was allowed to receive 2 supplemental individual consultations in case of new important physical symptoms or major psychiatric problems; 6. Letter with management recommendations for functional somatic symptoms sent to primary care physician; 7. Treatment manual, including schedule, symptom diary, educational material, worksheets and homework assignment for the 9 treatment modules; 8. Consultancy service by telephone for primary care physicians and specialists; 9. Close cooperation with social authorities or the participant's employer, when needed (see Schröder 2012a, figure 1 for details) Therapist: consultants or senior residents in psychiatry with at least 2 years of training in cognitive‐behavioural treatment, experience with group treatment and expertise in the field of functional somatic syndromes. The senior residents were supervised. 2.Enhanced usual care (n = 66) Duration: 4 months Treatment protocol: 1. Comprehensive lifetime review of case notes and clinical records; 2. Comprehensive biopsychosocial assessment and individualised information about the nature,course and treatment options for the symptoms; 3. Letter to participant's primary care physician and referring doctor (if not the primary care physician) regarding diagnosis and illness history as well as treatment recommendations in case of co‐morbid depression or anxiety; 4. 'Usual care' delivered by primary care physician and specialists (see Schröder 2012a, figure 1 for details) Therapist/face‐to‐face contact: GP, occasionally assisted by a mental care specialist |
|
| Outcomes |
Time points for assessment: baseline, 4 months, 10 months, 16 months after baseline Primary outcome: 1. treatment response (improvement in the SF‐36 aggregate score 'physical functioning', 'bodily pain' and 'vitality') 2. PCS (subscore SF‐36) Secondary outcome: 1. health anxiety (WI) 2. subjective physical symptoms (physical subscale SCL‐90) 3. mental well‐being (SCL‐8, severity of depression and anxiety) |
|
| Notes |
Date of study: between March 2005 and December 2006 the case notes of all participants referred were screened for eligibility, follow‐up continued until 16 months after Funding source: Central Denmark Region, the Aarhus University Hospital Research Initiative, the A.P. Møller Foundation for the Advancement of Medical Science and the Medical Association for the County of Aarhus Declarations of interest among the primary researchers: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | In a block randomisation protocol participants were randomised by means of a computer algorithm that used predefined concealed random numbers and stratified for gender and psychiatric lifetime co‐morbidity status. We used the ratio 9 : 11 (STreSS vs. enhanced usual care) because we expected a higher attrition rate in those allocated to enhanced usual care |
| Allocation concealment (selection bias) | Unclear risk | It was not described who performed the allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Referring doctors and therapists were aware of the assignment, as were the participants |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Questionnaires were sent by post and administered by independent research assistants who were unaware of the allocation of participants. However, participants were not blinded, and most outcomes were participant reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Incomplete data in intervention group at total of all time points 20.3% (11 out of 54 participants) Incomplete data in control group at total of all time points 36.4% (24 out of 66 participants) (see Schröder 2012, figure 2 for details) |
| Selective reporting (reporting bias) | Low risk | All intended outcomes reported |
| Treatment fidelity | Low risk | Treatment was based on manualised modules (Schröder 2012, appendix) |
| Researcher allegiance | Low risk | No indication that researchers had a preference for 1 of the treatment modalities |
| Other bias | Low risk | No other sources of bias |
Schröder 2013.
| Methods | Study design: randomised controlled trial | |
| Participants |
Diagnosis: multiple somatoform symptoms Method of diagnosis: participants ≥ 18 years of age with at least 2 somatoform symptoms according to IDCL (for DSM‐IV). For each recorded symptom, the researchers checked whether there was substantial suffering or impairment. A medical evaluation was performed by a physician to exclude medically explained symptoms Exclusion criteria: medically explained symptoms, serious concentration or language problems, suicidal tendencies, psychotic symptoms Total number randomised: 173 (134 actually started treatment, lower numbers in Zaby 2008) Age: for intention‐to‐treat sample (n = 134) M = 48.02 years (SD = 12.34) (no baseline imbalances between study groups) Sex: for intention‐to‐treat sample (n = 134) 76.9% women (n = 103), 23.1% men (n = 31) (no baseline imbalances between study groups) Severity of symptoms at baseline: number of symptoms M = 9.9 (SD = 5.5) Duration of symptoms at baseline: ≥ 6 months Setting: participants were consecutively recruited in co‐operation with primary care physicians, psychotherapists, and by advertisements in local newspapers in the southwest of Germany Treatments were conducted in the outpatient treatment centre for psychological intervention at the University of Landau and the University of Mannheim, Germany Location: Landau and Mannheim region, Germany Number of treatment centres: 2 Co‐morbidities: Panic disorder: for intention‐to‐treat sample 20.1% (n = 27) Social phobia: for intention‐to‐treat sample 9.0% (n = 12) Specific phobia: for intention‐to‐treat sample 6.0% (n = 8) Generalised anxiety: for intention‐to‐treat sample 10.4% (n = 14) Major depressive disorder: for intention‐to‐treat sample 18.7% (n = 25) Adjunctive therapy: participants were free to seek further care Adjunctive medication: participants were free to seek further care |
|
| Interventions | Participants were randomly assigned to either 1. CBT (n = 49) Duration: manualised group training with 8 weekly sessions of 90 minutes in 8 groups of 4‐11 members Treatment protocol: the CBT used in this study was developed on the basis of theoretical considerations (Deary et al. 2007) and standardised guidelines for psychological therapy of somatoform disorders, as published elsewhere (Rief 1999; Rief 2002; Sharpe 1992). The manual contained detailed guidelines for conducting each session. Aim of treatment was to create a model to understand bodily discomfort, with integrated biological, psychological and social factors (see Zaby 2008, table 1 for extended information about the group sessions). Therapist: psychological psychotherapists who were supervised regularly 2. Progressive muscle relaxation (n = 41) Duration: manualised group training with 8 weekly sessions of 90 minutes in 11 groups of 4‐11 members Treatment protocol: the progressive muscle relaxation treatment was based on modifications of Jacobson's original program by Bernstein and Borkovec, following a manual (Zaby 2008). It involved learning to tense and relax groups of muscles beginning with a large number of small groups (in this case 16) and then proceeding in steps to a smaller number of large groups (first 7, then 4 groups) (for details, see Schröder 2013, page 299) Therapist/face‐to‐face contact: psychological psychotherapists who were supervised regularly 3. Waiting list control (excluded from analysis, as participants in waiting list group were not randomly assigned) Duration: NA Treatment protocol: participants with a waiting time for the intervention longer than 4 weeks, less than 3 months were included in the waiting list group Therapist/face‐to‐face contact: NA |
|
| Outcomes |
Time points for assessment: baseline, directly post‐treatment (8 weeks) and 6 months after baseline Primary outcome: 1. Intensity and number of symptoms (SOMS‐7) Secondary outcome: 1. Depression (HADS‐D) 2. Anxiety (HADS‐A) 3. Physical and mental health (SF‐12, PWB, and MWB subscale) 4. Medical care utilisation in previous 6 months (interview at baseline and 6 months) |
|
| Notes |
Date of study: participants were recruited from April 2005 until May 2006. Treatments were conducted between April 2005 and October 2008 Funding source: the study was supported by a grant awarded to Annette Schröder by the German Research Foundation (DFG) Declarations of interest among the primary researchers: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomised for CBT or PMR using random sequences, exact method not described. When both groups were full, newly included participants were included in the waiting list group. As this group was not randomly assigned we excluded data from this group from analysis |
| Allocation concealment (selection bias) | Low risk | The randomisation was performed by a person who was not involved in assessment or treatment delivery |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants could not be blinded. Blinding of personnel not described |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Participants were not blinded, and most outcomes were participant reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | In both intervention groups 23% of participants dropped out before the first treatment (> 20%) |
| Selective reporting (reporting bias) | Low risk | All intended outcomes reported |
| Treatment fidelity | Low risk | CBT and PMR were conducted as a manualised group training (Schröder 2013, treatment conditions) |
| Researcher allegiance | Low risk | No indication that researchers had a preference for 1 of the treatment modalities |
| Other bias | Unclear risk | Participants were randomised for CBT or PMR. When both groups were full, newly included participants were included in the waiting list group. In a later stage, these participants were included in both intervention groups. (source: email contact with author) As participants are their own control participant due to this method, we decided to exclude data from the waiting list group from analysis |
Schweickhardt 2007.
| Methods | Study design: randomised controlled trial | |
| Participants |
Diagnosis: somatisation Method of diagnosis: participants (18‐65 years old) were included if they screened positive for the SOMS‐2 and the GHQ‐12 and if the attending hospital physician was not able to provide a clear physical explanation for the complaints. The screening criteria for SOMS were 4 somatoform symptoms for men and 6 for women, and the cut‐off for the GHQ was ≥ 2. Other inclusion criteria were: persistent symptoms for at least 3 months, ≥ 5 annual doctor's visits or 2 hospitalisations during the past year as a result of the respective symptoms and availability for ≥ 6 months Exclusion criteria: severe mental disorders, e.g. major depression with suicidal ideation, eating disorders, alcohol or substance abuse, an organic disease deemed responsible for most of the symptoms, psychotherapy ‐ ongoing or completed during the past 3 years, pregnancy and low intellectual capacity Total number randomised: 91 Age: for intervention group, M = 44.43 (SD = 13.329); for control group, M = 49.22 (SD = 11.084) Sex: for intervention group 69.4% women (n = 34), for control group 71.4% women (n = 30) Severity of symptoms at baseline: 55 of the participants (92%) had a somatoform disorder Duration of symptoms at baseline: unknown Setting: general hospital (inpatients). Data were collected in the Departments of Neurology, Internal Medicine, General Medicine and Orthopedics of the University Hospital. A research assistant visited the participating units 3 times per week and systematically examined all new participants Location: Freiburg, Germany Number of treatment centres: 1 (although 4 different departments) Co‐morbidities: diagnostic interviews were conducted with 60 participants (66%). 24 (40%) were had depression and 18 (32%) had an anxiety disorder Adjunctive therapy: none reported Adjunctive medication: none reported |
|
| Interventions | Participants were randomly assigned to either 1. Short‐term psychotherapeutic intervention (n = 49) Duration: 5 sessions of approximately 50 minutes, during a period of 2 weeks Treatment protocol: treatment consisted of a program predominantly based on the reattribution model, the World Health Organization training package for primary care physicians, cognitive behavioural techniques and a psychodynamic approach (see Schweickhardt 2007, table 1 for the session topics and ref 29 for the treatment manual) Therapist: licensed psychotherapists (3 physicians and 2 psychologists), who have been working with somatising participants for several years 2. Psychoeducational reading material (n = 42) Duration: NA Treatment protocol: psychoeducational reading material consisting of 9 pages describing the aetiology, course, and treatment recommendation of somatoform symptoms Therapist/face‐to‐face contact: NA |
|
| Outcomes |
Time points for assessment: baseline, 2 weeks, 3 months and 6 months after baseline Primary outcome: 1. Changes regarding motivation for psychotherapy (FPTM) 2. Contact with a psychotherapist Secondary outcome: 1. number and intensity of somatoform symptoms (SOMS‐7) 2. changes regarding emotional distress (HADS, GHQ) 3. quality of life (SF‐12) |
|
| Notes |
Date of study: participants were recruited between June 2002 and May 2004. The last follow‐up assessments were performed in November 2004 Funding source: this clinical trial was supported by grants from the German Research Association (Deutsche Forschungsgemeinschaft) Declarations of interest among the primary researchers: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Envelopes containing the allocation information were created in a random order. Participants who had fulfilled all inclusion criteria were assigned to the 2 different groups |
| Allocation concealment (selection bias) | Low risk | Randomisation was performed by an independent statistician. He was not aware of the therapy allocation or the details of the study design |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not blinded due to the study conditions. Data were collected by independent research assistants, it is unknown if they were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Participants were not blinded, and most outcomes were participant reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Missing data at 6‐month follow‐up for psychotherapy motivation and the secondary outcomes were up to 28.5% in the intervention group and 23.8% in the control group (> 20%) |
| Selective reporting (reporting bias) | Low risk | All intended outcomes reported |
| Treatment fidelity | Low risk | Treatment was based on a manual regarding therapy goals, basic concepts and operationalisation of the individual therapy steps (Schweickhardt 2007, ref 29) |
| Researcher allegiance | Low risk | No indication that researchers had a preference for 1 of the treatment modalities |
| Other bias | Unclear risk | A high percentage (29%) of the participants from the control group became involved in psychotherapy. This might have influenced the results |
Speckens 1995.
| Methods | Study design: randomised controlled trial | |
| Participants |
Diagnosis: MUS Method of diagnosis: participants (aged 18‐64, Dutch natives) with no explanation for their symptoms according to their internist were interviewed by the researchers. Those with a mean intensity of symptoms score of ≥ 5 (88%) or a HADS anxiety/depression score of ≥ 10 and ≥ 5 points on functional impairment score (12%) were eligible Exclusion criteria: organic psychiatric disorders (e.g. dementia) or with chronic alcoholism, psychosis, or suicidal ideas and those currently having psychological or psychiatric treatment Total number randomised: 79 Age: for intervention group, M = 36.4 (SD = 12.4); for control group, M = 37.8 (SD = 12.8) Sex: for intervention group 46% women (n = 18), 54% men (n = 21); for control group 53% women (n = 21), 47% men (n = 19) Severity of symptoms at baseline: number of symptoms for intervention group M = 12.9 (SD = 6.7), for control group M = 13.0 (SD = 7.5) Duration of symptoms at baseline: unknown Setting: general medical outpatient clinic of Leiden university hospital (recruitment and treatment) Location: Leiden, the Netherlands Number of treatment centres: 1 Co‐morbidities: psychiatric disorders (based on caseness in the present state examination at baseline): 46% (n = 18) in intervention group; 28% (n = 11) in control group Adjunctive therapy: none reported Adjunctive medication: none reported |
|
| Interventions | Participants were randomly assigned to either 1. CBT (n = 39) Duration: 6‐16 x 1‐hour sessions during a maximum period of 6 months Treatment protocol: a broad CBT approach was used in view of the heterogeneous nature of the participants' problems. The main therapeutic techniques included identification and modification of dysfunctional automatic thoughts and behavioural experiments aimed at breaking the vicious cycle of the symptoms and their consequences. The methods used were similar to those described by Salkovskis and Sharpe et al. (for details: Speckens 1995, ref 2 and 12) Therapist: a physician trained in CBT and a behavioural therapist 2. Control group (n = 40) Duration: 1 x 90‐minute session every 3 months (for doctors) Treatment protocol: treatment consisted of optimised medical care. The quality of care was enhanced by basic training by 3 researchers in the detection and management of psychiatric disorders Therapist: the researchers |
|
| Outcomes |
Time points for assessment: baseline 6 months and 12 months after baseline Outcomes: 1. change in physical symptoms (perceived change, frequency and intensity according to non‐standardised questionnaire) 2. psychological distress (GHQ and HADS anxiety and depression subscale) 3. functional impairment (SIP subscales and numerical analogue scales on functional impairment) 4. hypochondriacal beliefs (IAS subscales health anxiety and illness behaviour and WI) 5. healthcare use (frequency of visits to GP, based on visit counts by participant and doctor, only at 12 months) |
|
| Notes |
Date of study: from March 1992 to March 1993 participants were recruited, follow‐up continued until 1 year after Funding source: Dutch Ministry of Education and Sciences and the Ministry of Welfare, Public Health Care and Culture Declarations of interest among the primary researchers: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed using computerised randomisation in blocks of 4 (confirmed by email) |
| Allocation concealment (selection bias) | Low risk | Randomisation was performed by an independent person (confirmed by email) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Participants were not blinded, and most outcomes were participant reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐out rates were low at all follow‐ups (< 20%) |
| Selective reporting (reporting bias) | Low risk | All intended outcomes reported |
| Treatment fidelity | Unclear risk | A certain structure in methodology, but no manual or protocol was used (Speckens 1995, p. 1329) |
| Researcher allegiance | Low risk | No indication that researchers had a preference for 1 of the treatment modalities |
| Other bias | Low risk | No other sources of bias |
Sumathipala 2000.
| Methods | Study design: randomised controlled trial | |
| Participants |
Diagnosis: medically unexplained complaints Method of diagnosis: participants (16‐65 years old) with ≥ 5 medically unexplained symptoms and repeated consultations during the last 6 months underwent a physical examination by a GP and a research psychiatrist plus a re‐assessment through 2 questions by the research psychiatrist: 1. What are your symptoms/problems, why are you here today? and, 2. Are there any other symptoms/problems? Finally 1 question was asked about how many visits participants had in last 6 months according to current symptoms, to make sure that they fitted inclusion criteria. After inclusion the standardised BSI questionnaire was filled out by all participants. Exclusion criteria: dementia, psychosis, active suicidal thoughts, alcohol dependence or current treatment for a psychiatric disorder Total number randomised: 68 Age: for intervention group, M = 38.1 (SD = 13); for control group, M = 38.7 (SD = 14) Sex: for intervention group 64.7% women (n = 22), 35.3% men (n = 12); for control group 76.5% women (n = 26), 23.5% men (n = 8) Severity of symptoms at baseline: number of symptoms: for intervention group M = 7.8 (SD = 1.7), for control group M = 8.2 (SD = 1.9) Duration of symptoms at baseline: 57% was > 2 years ill, in the intervention group 20.6% (n = 7) had < 6 months symptoms, in the control group 14.7% (n = 5) Setting: participants were selected by GPs and treated in general outpatient clinic of a general hospital where participants initiate their own visits without prior appointments (primary care) Location: Colombo, Sri Lanka Number of treatment centres: 1 Co‐morbidities: not described Adjunctive therapy: none described Adjunctive medication: none described |
|
| Interventions | Participants were randomly assigned to either 1. CBT (n = 34) Duration: 6 x 30‐minute structured sessions over a period of 3 months Treatment protocol: treatment consisted of structured regular visits to 1 professional carer thereby hoping to reduce unstructured visits to different practitioners and co‐ordinating care. Treatment was based on the principles of CBT, using modifications of that described by Salkovskis and Sharpe et al. and Goldberg et al.'s reattribution technique for details Sumathipala 2000, ref Salkovskis, Sharpe and Goldberg). Through structured sessions, participants were made aware of the psychological component of their condition, and helped to reduce unnecessary medical consultations and investigations. When possible, 1 non‐professional carer, usually the spouse, was involved. The intervention group was managed by a research psychiatrist using the above specified intervention strategy. A treatment manual was prepared to keep the therapeutic sessions uniform Therapist: research psychiatrist 2. Control group (n = 34) Duration: NA Treatment protocol: the controls received assessments but no intervention in terms of a structured therapy. They continued to receive care from their usual carers and could visit the doctors of their choice. The controlled group received appointments for a follow‐up assessment after 3 months Therapist/face‐to‐face contact: NA |
|
| Outcomes |
Time points for assessment: baseline and 3 months after baseline Outcomes: 1. level of distress/psychiatric morbidity (GHQ‐30) 2. number and severity of symptoms (BSI + 2 open‐ended questions) 3. number of participant initiated doctor visits (diary) 4. participants perceived satisfaction with previous treatment (VAS scale) |
|
| Notes |
Date of study: participant recruitment took place from consecutive participants attending the clinic from 15 December 1997 to the end of March 1998, the treatment and re‐assessments continued until the end of June 1998 Funding source: The Wellcome Trust (international programme) provided a project grant Declarations of interest among the primary researchers: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A list of treatment assignments was prepared in advance using simple randomisation by random numbers generated from a calculator. These were available in 68 sealed opaque envelops bearing sequential registration numbers on the outside of the envelope |
| Allocation concealment (selection bias) | Low risk | An epidemiologist who did not take part in the data collection was responsible for randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The first research psychiatrist opened the envelope to reveal the random treatment allocation. The non‐clinical research assistant and the second psychiatrist remained blind to the group status throughout the study. Participants could not be blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Participants were not blinded, and most outcomes were participant reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | At 3 months, loss to follow‐up was 30% in the intervention group and 38% in control group (> 20%) |
| Selective reporting (reporting bias) | Low risk | All intended outcomes reported |
| Treatment fidelity | Low risk | Treatments were structured. A treatment manual was prepared to keep the therapeutic sessions uniform. (Sumathipala 2000, p. 750) |
| Researcher allegiance | Low risk | No indication that researchers had a preference for 1 of the treatment modalities |
| Other bias | Low risk | No other sources of bias |
Sumathipala 2008.
| Methods | Study design: randomised controlled trial | |
| Participants |
Diagnosis: MUS Method of diagnosis: participants (16‐65 years old) with ≥ 5 MOS and repeated consultations during the last 6 months were selected by the primary care doctors. They underwent an extensive physical examination by trial co‐ordinator and trial physician plus an independent reassessment through 2 questions: 1. What are your symptoms/problems, why are you here today? and, 2. Are there any other symptoms/problems? to elicit the number of symptoms and visits in the previous 6 months. After inclusion the standardised BSI questionnaire was filled out by all participants Exclusion criteria: dementia, psychosis or alcohol dependence current treatment for a psychiatric disorder Total number randomised: 150 Age: aged 16‐58 years, M 35 years Sex: for intervention group 79% women (n = 59), 21% men (n = 16); for control group 77% women (n = 58), 23% men (n = 17) Severity of symptoms at baseline: number of symptoms M = 8.6 (SD = 2.2) Duration of symptoms at baseline: M = 42.0 months (SD = 40) Setting: a general outpatient clinic of a general hospital where participants initiate their own visits without prior appointments (primary care) Location: Colombo, Sri Lanka Number of treatment centres: 1 Co‐morbidities: not described Adjunctive therapy: none described Adjunctive medication: none described |
|
| Interventions | Participants were randomly assigned to either 1. CBT (n = 75) Duration: 6 x 30‐minute structured sessions over a period of 3 months (of which 3 mandatory) Treatment protocol: treatment consisted of structured regular visits to 1 professional carer thereby aiming to reduce unstructured visits to different practitioners and co‐ordinating care. Treatment was based on the principles of CBT, using modifications of that described by Salkovskis and Sharpe et al. and Goldberg et al.'s reattribution technique for details Sumathipala 2000, ref Salkovskis, Sharpe and Goldberg) Treatment started with a SEMI interview by 1 of the authors: results, summary, and formulation based on findings were passed on to primary care physician, they were also trained to use this information to inform the strategy for their CBT intervention. Participants in the intervention group completed a diary, which was used during training. The CBT training was a short course consisting of 5 sessions covering the basis of MUS; the relevance of the explanatory model, elicited by the SEMI, to the CBT model of such symptoms; and the CBT treatment approach. Training was accomplished through lectures, supplemented by case vignettes and role‐play of therapeutic sessions by simulated participants based on case scenarios from the pilot trial, all with reference to the intervention manual (for details: Sumathipala 2008, ref 30) Therapist: trained primary care physicians 2. Structured care (n = 75) Duration: 6 x 30‐minute structured sessions over a period of 3 months (of which 3 mandatory) Treatment protocol: treatment started with a SEMI interview by 1 of the authors: only results were passed on to primary care physician, they were not trained to use this information to inform the strategy for their CBT intervention No CBT was given, but during the 6 sessions the physicians were free to manage the participants as they wished within the sessions. No training or supervision was provided for these doctors, and the intervention was not manualised Therapist: untrained primary care physicians |
|
| Outcomes |
Time points for assessment: baseline, 3 months, 6 months, 9 months and 12 months after baseline Outcomes: 1. number of symptoms (as counted by researcher) 2. level of distress/psychiatric morbidity (GHQ‐30) 3. number and severity of symptoms (BSI + 2 open‐ended questions) 4. number of participant initiated doctor visits (diary) |
|
| Notes |
Date of study: not described Funding source: The Wellcome Trust (international programme) provided a project grant Declarations of interest among the primary researchers: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Trial participants were first randomised to the 2 intervention groups using a random permuted block design, with a block size of 4. Next, participants were randomly allocated to 1 of the 3 doctors selected to deliver the intervention to which they had been allocated |
| Allocation concealment (selection bias) | Low risk | Randomisation codes were generated by a statistician in the UK and passed on to the independent epidemiologist (M.R.N.A.) in Sri Lanka, who executed the random allocation of treatment condition |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Neither the primary care doctors who delivered the interventions nor the participants who received them could be masked to their allocation because of the nature of the interventions |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Participants were not blinded, and most outcomes were participant reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow‐up was highest after 6 and 12 months: 20% at both time points in the intervention group and 28% (6 months) and 30% (12 months) in the structured care group (> 20%) |
| Selective reporting (reporting bias) | Low risk | All intended outcomes are reported |
| Treatment fidelity | Low risk | Treatments were structured. A treatment manual was prepared to keep the therapeutic sessions uniform (Sumathipala 2000, p. 750) |
| Researcher allegiance | Low risk | No indication that researchers had a preference for 1 of the treatment modalities |
| Other bias | Low risk | No other sources of bias |
Van Ravesteijn 2013.
| Methods | Study design: randomised controlled trial | |
| Participants |
Diagnosis: MUS Method of diagnosis: men and women (aged 18‐70 years) belonging to the 10% most frequently attending in the past year, who had physical symptoms for at least 6 months that were not (fully) explained by a physical disease or by substance abuse (according to the GP) were selected. They had to experience functional impairment due to these physical symptoms and they had to be eligible according to a MINI SCID interview Exclusion criteria: frequent attendance for other reasons than physical symptoms, physical symptoms fully explained by somatic diseases, no significant distress or functional impairment due to the symptoms, psychosis or bipolar disorder in medical history, current alcohol or drug abuse, cognitive impairment, problems with the Dutch language, and previous mindfullness‐based cognitive therapy Total number randomised: 125 Age: for intervention group, M = 47.6 (SD = 11); for control group, M = 46.5 (SD = 12) Sex: for intervention group 80% women (n = 49), 20% men (n = 12); for control group 68% women (n = 38), 32% men (n = 18) Severity of symptoms at baseline:somatisation disorder 13% in intervention group (n = 8), 13% in control group (n =7); pain disorder 18% (n =11) in intervention group, 21% (n = 12) in control group Duration of symptoms at baseline: not described Setting:primary care, potential participants were selected from the digital databases, screened for exclusion criteria by the GP, invited by letter and interviewed by telephone Location: area of Nijmegen, a medium‐sized city in the Netherlands, general practices were located in neighbourhoods with both low and higher socioeconomic standards Number of treatment centres: unknown Co‐morbidities: hypochondriasis 3% (n = 2) in intervention group, 2% (n = 1) in control group; depressive disorder 15% in intervention group (n = 9), 23% in control group (n = 13); anxiety disorder 25% in intervention group (n = 15), 25% in control group (n = 14); hypertension 30% in intervention group (n = 18), 20% in control group (n = 11), arthrosis 18% in intervention group (n = 11), 14% in control group (n = 8), asthma/bronchitis 18% in intervention group (n = 11), 11% in control group (n = 6); diabetes mellitus type II 10% in intervention group (n = 6), 9% in control group (n = 5) Adjunctive therapy: none reported Adjunctive medication: none reported |
|
| Interventions | Participants were randomly assigned to either 1. Mindfullness‐based cognitive therapy (n = 64) Duration: 8 weekly group sessions of 2.5 hours + 1 silent day (6 hours) during a period of 8 weeks Treatment protocol: the programme protocol was based on the mindfullness‐based cognitive therapy format for people with recurrent depression (see Van Ravesteijn 2013, ref 11) consisted of formal meditation exercises such as body scan, sitting meditation, walking meditation, and mindful movement. Participants were also encouraged to cultivate awareness of everyday activities, such as eating or taking a shower. In addition, the programme included cognitive techniques such as psychoeducation, monitoring and scheduling of activities, identification of negative automatic thoughts, and devising a relapse prevention plan. In the section on psychoeducation, information about respecting physical and mental boundaries and dealing with impairments was included. The silent day was included to give participants the opportunity to deepen their mindfulness practice. To support home practice, participants received a folder with information about the individual sessions, homework assignments, and forms to keep a record of their practice, together with CDs with guided meditations and movement exercises Therapist: 2 experienced mindfulness trainers who had both participated in a 2‐year mindfulness training programme and who had many years of practice experience and experience with courses (both over 30 courses) 2. Enhanced usual care (n = 61) Duration: NA Treatment protocol: participants in the enhanced usual care condition received usual care provided by their GP and other healthcare professionals. The term 'enhanced usual care' was considered appropriate as all participants received a psychiatric interview. The GP was explicitly informed about the psychiatric diagnoses resulting from the interview Therapist: NA |
|
| Outcomes |
Time points for assessment: baseline, end of treatment and 9 months after baseline Primary outcome: 1. general health status (VAS of EuroQoL 5D) Secondary outcome: 1. mental and physical function (SF‐36, MCS and PCS subscales) 2. physical and mental symptoms (PHQ‐15 and PHQ‐9) 3. health anxiety (WI) 4. healthcare utilisation (contact count by participant) |
|
| Notes |
Date of study: participants were recruited from December 2009 to August 2010, follow‐up continued until 9 months after Funding source: the study was funded by research grants from The Netherlands Organization for Health Research and Development (ZonMW) Declarations of interest among the primary researchers: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer‐generated permuted‐block randomisation table was used, with block size 20. The participant identification number was matched with the corresponding name to inform each participant about the allocation |
| Allocation concealment (selection bias) | Low risk | The assistant performing allocation was blinded (participants had unique identification numbers) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The research assistant was blinded for interview data. However, other personnel and participants could not be blinded due to the nature of the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Participants were not blinded and most outcomes were participant reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up was < 20% in both research groups, at all follow‐up times |
| Selective reporting (reporting bias) | Low risk | All intended outcomes reported |
| Treatment fidelity | Low risk | Treatment followed a structured training protocol (Van Ravesteijn 2013, p. 198) |
| Researcher allegiance | Low risk | No indication that researchers had a preference for 1 of the treatment modalities |
| Other bias | Low risk | No other sources of bias |
Zonneveld 2012.
| Methods | Study design: randomised controlled trial | |
| Participants |
Diagnosis: UPS (according to SCID interview) Method of diagnosis: participants (aged 18‐65 years old, who were able to speak, read, and write Dutch) were eligible if their UPS persisted ≥ 6 months and if their UPS was classified as undifferentiated somatoform disorder or chronic pain disorder according to the criteria of the Structured Clinical Interview for DSM‐IV Axis I Disorders/ Patient edition (SCID‐I/P) Exclusion criteria: UPS not being the principal somatic disease; undifferentiated somatoform disorder or chronic pain disorder not being the principal DSM‐IV‐TR classification; handicaps like cognitive mental impairment with or without blindness impending the participant to participate in the training Total number randomised: 162 Age: for intervention group, M = 46 (IQR = 38‐53); for control group, M = 44 (SD = 35‐52) Sex: for intervention group 79.8% women (n = 67), 20.2% men (n = 17); for control group 82.1% women (n = 64), 17.9% men (n = 14) Severity of symptoms at baseline: undifferentiated somatoform disorder 38.1% for intervention group (n = 32) 39.7% for control group (n = 31); chronic pain disorder 61.9% for intervention group (n = 52), 60.3% for control group (n = 47) Duration of symptoms at baseline: for intervention group M = 8 years, for control group M = 9.5 years Setting: primary care, outpatient clinic and secondary community mental‐health service physicians' received periodical postcards informing them when and how to refer patients. Participants were also recruited via announcements in local newspapers and on websites of patients' associations. Treatment setting unclear Location: Rotterdam area, the Netherlands Number of treatment centres: unknown Co‐morbidities: for intervention group 45.2% had ≥ 1 DSM‐IV axis I disorders (n = 38), for control group this was 37.2% (n = 29) (for details about specific axis I and axis II disorders, see Zonneveld 2012, table 2) Adjunctive therapy: not mentioned Adjunctive medication: not mentioned |
|
| Interventions | Participants were randomly assigned to either 1. Group training (n = 84) Duration: 13 weekly 2‐hour sessions in groups of 5‐9 participants (mean 6), in a period of 13 weeks Treatment protocol: treatment consisted of cognitive‐behavioural therapy based on the consequences model. In this model, psychological and social factors, are labelled as consequences of UPS. In the long term, these consequences might produce self perpetuating vicious circles that maintain or aggravate UPS. By changing and reducing the consequences, beliefs are addressed indirectly, after which the beliefs can still be addressed directly. Focus is on improvement of quality of life. Based on this tailored cognitive‐behavioural model, a manual was developed for a group training called 'Coping with the consequences of unexplained physical symptoms' (for details Zonneveld 2012, ref 38). Sessions concern psychoeducation on arousal, habits, activity, emotions, beliefs, physical fitness, information processing, breathing and relaxation, and relapse prevention Therapist: 6 psychologists with a Master's degree, 4 of whom had had at least 3 years' post‐Master's experience with group therapy or CBT, or both and who familiarised themselves with this method 2. Waiting list (n = 78) Duration: 13 weeks Treatment protocol: NA Therapist/face‐to‐face contact: NA |
|
| Outcomes |
Time points for assessment: baseline and directly post‐treatment (further follow‐ups are without a control group) Primary outcome: 1. improvement in quality of life (SF‐36, PCS and MCS) Secondary outcome: 1. improvement in quality of life (SF‐36, 8 individual subscales) 2. intensity of psychological problems (SCL‐90‐R, 8 subscales including depression and anxiety) |
|
| Notes |
Date of study: participants were recruited between February 2005 and September 2008. The follow‐up ended in December 2009; 1 year after the intervention group of the last randomisation had completed the training Funding source: the study was funded by RIAGG Rijnmond Declarations of interest among the primary researchers: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were assigned to the training or to the waiting list according to a computer generated randomisation list |
| Allocation concealment (selection bias) | Low risk | The randomisation was performed by an investigator who had no clinical involvement in the trial and was working in a different building that the building were assessment and enrolment were done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and trainers could not be blinded for group assignment, as the control condition was a simple waiting list. The data were imported and analysed after participants had completed the trial |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Participants were not blinded and most outcomes were participant reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Of the 84 participants in the intervention group, 23 dropped out (27.4%), of the 78 participants in the control group, 6 dropped out (7.7%) (intervention group loss to follow‐up > 20%) |
| Selective reporting (reporting bias) | Low risk | All intended outcomes reported, no means/SDs reported, but on our request these were provided for the meta‐analysis |
| Treatment fidelity | Low risk | The training was manual‐based (Zonneveld 2012, ref 24, 28) |
| Researcher allegiance | Low risk | No indication that researchers had a preference for 1 of the treatment modalities |
| Other bias | Low risk | No other sources of bias |
AIDS: acquired immune deficiency syndrome; BDS: bodily distress syndrome; BSI: Brief Symptom Inventory; CBT: cognitive behavioural therapy; CGI‐SD: Clinical Global Impession Scale for Somatoform Disorders; CIDI: Composite International Diagnostic Interview; DSM: Diagnostic and Statistical Manual of Mental Disorders; GAD: Generalised Anxiety Disorder Scale; GHQ: General Health Questionnaire; GP: general practitioner; GSI: General Symptom Index; HADS: Hospital Anxiety and Depression Scale; HAM‐A: Hamilton Anxiety Rating Scale; HAM‐D: Hamilton Depression Rating Scale; IAS: Illness Attitude Scales; IBQ: Illness Behaviour Questionnaire; ICD: International Classification of Diseases; IDCL: International Diagnostic Checklists; IQR: interquartile range; KKG: Krankheit und Gesundheit; M: mean; MCS: Mental Component Scale; Mini‐DIPS: mini‐Diagnostische Interview bei psychischen Störungen; MOS: Medical Outcomes Study; MUS: medically unexplained symptoms; MWB: Mental Well‐Being; n: number; NA: not available; PCI: psychiatric consultation intervention; PCS: Physical Component Score; PHQ: Patient Health Questionnaire; PRIME‐MD: Primary Care Evaluation of Mental Disorders; PWB: Psychological Well‐Being; ref: reference; SCAN: Schedules for Clinical Assessment in Neuropsychiatry; SCID: Structured Clinical Interview for Mental Disorders; SCL: Symptom Checklist; SD: standard deviation; SDI: Sleep Debt Index; SF‐12: 12‐item Short Form; SIP: Session Initiation Protocol; SPQ: Social Problem Questionnaire; SSS: Severity of Somatic Symptoms; TAU: treatment as usual; UPS: unexplained physical symptoms; VAS: visual analogue scale; WI: Whitely Index.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aiarzaguena 2007 | Compared 2 forms of CBT |
| Arnold 2009 | This study was not randomised |
| Barsky 2013 | No separate analysis for participants with somatisation (only for total group, including people with hypochondriacal health anxiety as their main problem) |
| Bernal 1995 | A non‐pharmacological intervention (relaxation therapy) was combined with a pharmacological one (antidepressants) |
| Blankenstein 2001 | GP training study |
| Bleichhardt 2004 | Compared CBT with CBT+ BT |
| Cano‐Vindel 2013 | Treatment as usual in the control group included pharmacological treatment |
| Detaille 2013 | Not MUPS or somatoform disorder |
| Gottschalk 2011 | This (pilot) study was not randomised |
| Grepmair 2007 | The study did not concern people with MUPS of somatoform disorders explicitly |
| Gyllensten 2003 | No separate analysis for people with somatisation/MUPS |
| Hellmann 1990 | Participant selection procedure did not include a diagnostic interview or questionnaire to establish MUPS or somatoform disorders |
| Hiller 2003 | This study was not randomised |
| Hiller 2004 | This study was not randomised |
| Houtveen 2013 | The study had a prospective study design (no randomised controlled study) |
| Klapow 2001 | Participants were not required to have medically unexplained symptoms or somatoform disorders |
| Kocken 2008 | Participant selection procedure did not include a diagnostic interview or questionnaire to establish MUPS or somatoform disorders |
| Larisch 2004 | GP training study |
| Lopez‐Garcia‐Franco 2012 | Participant selection procedure did not include a diagnostic interview or questionnaire to establish MUPS or somatoform disorders |
| Lupke 1996 | The study was not randomised |
| McLeod 1997 | Participant selection procedure did not include a diagnostic interview or questionnaire to establish MUPS or somatoform disorders |
| Morriss 2007 | Participant selection procedure did not include a diagnostic interview or questionnaire to establish MUPS or somatoform disorders |
| Nanke 2003a | Compared 2 forms of BT |
| Payne 2009 | The study was not randomised |
| Peters 2002 | Participant selection procedure did not include a diagnostic interview or questionnaire to establish MUPS or somatoform disorders |
| Pols 2008 | Treatment was focused on depressive symptoms, not on MUPS |
| Rasmussen 2006 | Participants include high healthcare utilisers, the participant selection procedure did not include a diagnostic interview or questionnaire to establish MUPS or somatoform disorders |
| Rembold 2011 | The study was not randomised |
| Rosendal 2007 | No separate analysis for people with somatisation/MUPS |
| Rost 1994 | The intervention consisted of a consultation letter |
| Ryan 2004 | Participant selection procedure did not include a diagnostic interview or questionnaire to establish MUPS or somatoform disorders |
| Schade 2011 | Participant selection procedure did not include a diagnostic interview or questionnaire to establish MUPS or somatoform disorders |
| Schwarz 2012 | The study was not randomised |
| Sharpe 2011 | Participant selection procedure did not include a diagnostic interview or questionnaire to establish MUPS or somatoform disorders |
| Smith 2006 | Participant selection procedure did not include a diagnostic interview or questionnaire to establish MUPS or somatoform disorders and the intervention included pharmacological treatment |
| Smith 2009 | Participant selection procedure did not include a diagnostic interview or questionnaire to establish MUPS or somatoform disorders and for a certain percentage of the participants intervention included pharmacological treatment |
| Toft 2010 | GP training study |
| Tschuschke 2007 | The study was not randomised |
| Tyrer 2011 | The study was not randomised |
| Van der Feltz‐Cornelis 2006 | Intervention aimed at psychiatric consultation model, combined with GP training |
| Whitehead 2002 | Only people with chronic fatigue syndrome |
| Wiefferink 1997 | Participant selection procedure did not include a diagnostic interview or questionnaire to establish MUPS or somatoform disorders |
BT: behavioural therapy; CBT: cognitive behavioural therapy; GP: general practitioner; MUPS: medically unexplained physical symptoms.
Characteristics of studies awaiting assessment [ordered by study ID]
Burwell‐Walsh 2002.
| Methods | Randomised controlled trial (cross‐over?) |
| Participants | Couples in which 1 of the partners met criteria for somatoform disorder or undifferentiated somatoform disorder as determined by the SOMS and who scored ≤ 101 on the Dyadic Adjustment Scale participated in this study |
| Interventions | Emotion focused couples therapy versus waiting list controls |
| Outcomes | Unclear. Abstract only reports on reported symptoms |
| Notes | Study included in Kleinstäuber 2011 |
Crompton 2003.
| Methods | Phase 1 ‐ the development of a client manual that will be used as an adjunct to therapy Phase 2 ‐ randomised trial in primary care |
| Participants | People with MUPS |
| Interventions | Reattribution therapy |
| Outcomes | Modified Social Adjustment Scale (primary outcome measure) Illness Perception Questionnaire (IPQ), Hospital Anxiety and Depression Scale (HADS), Ecomomis Questionnaire (EQ), SCL‐90‐R (Measures psychological and somatic symptoms) and number of contacts with primary and secondary health care services (derived from participant questionnaire and case notes) |
| Notes | Registration in research register, article untraceable |
Gournay 1998.
| Methods | Randomised clinical trial in primary health care |
| Participants | People with medically unexplained problems |
| Interventions | Intervention group: CBT by F‐grade community mental health nurses (advice) Control group: CBT by F‐grade community mental health nurses (treatment) |
| Outcomes | Unknown |
| Notes | Registration in research register, article untraceable |
Lopez‐Garcia‐Franco 2009.
| Methods | Cluster‐randomised controlled trial in primary care |
| Participants | People with symptoms of somatisation |
| Interventions | Intervention group: cognitive behavioural group therapy Control group: care as usual |
| Outcomes | Primary outcome measures: ‐ Perceived quality of life (SF‐12) ‐ Questionnaires on Global Clinical Impression (PGI and CGI questionnaire) ‐ Number of examinations (number of requested and programmed doctor's examinations during the period of study) ‐ Prescribed medicine ‐ Temporary labor disability (TLD) |
| Notes | Registration in research register, article untraceable |
Mussgay 2006.
| Methods | Randomised controlled trial in inpatients |
| Participants | Inpatients with anxiety and somatisation disorders |
| Interventions | Intervention group: standard treatment + additional exercise training regimen Control group: standard treatment |
| Outcomes | ‐ Respiration, weight, and body fat ‐ Anxiety and depression (Hospital and Depression Scale, HADS), general state of health (SF‐36), and complaints (Symptom Check List, SCL‐90‐R, and the Giessener Beschwerdebogen, GBB), amount of everyday activity and sport activity (Freiburger Fragebogen fuer Koerperliche Aktivitaet, FFKA), and a Screening for Somatoform Disorders instrument (SOMS). ‐an ergometric test was used to establish individual aerobic fitness (aerob/anaerob threshold based on lactate measurement) and training heart rate |
| Notes | Article untraceable |
Nickel 2006.
| Methods | Randomised trial |
| Participants | Turkish immigrants in a tertiary care German hospital |
| Interventions | Bioenergetic training, gymnastics, gestalt therapy, behavioural therapy, social therapy |
| Outcomes | Symptom severity, depression, anxiety, somatisation, aggressiveness and others |
| Notes |
Woolfolk 2007.
| Methods | Clinical trial |
| Participants | People with somatisation |
| Interventions | Affective cognitive behavioural therapy versus treatment as usual |
| Outcomes | Unknown |
| Notes | Article (book chapter) ‐ untraceable |
CBT: cognitive behavioural; therapy; CGI: Clinical Global Impression Scale; MUPS: medically unexplained physical symptoms.
Characteristics of ongoing studies [ordered by study ID]
Agger 2012.
| Trial name or title | Treatment of Multi‐organ Bodily Distress Syndrome. A Randomized Controlled Trial of the Effects of Acceptance and Commitment Therapy Given as Group Therapy or Workshop Compared to Standard Treatment (Stress‐4) |
| Methods | Randomised controlled trial, 3‐arm parallel study |
| Participants | First‐time referred participants fulfilling diagnostic criteria for BDS multi‐organ type with symptoms for more than 3 of 4 symptom categories; moderate or severe impact on daily life; symptoms lasting for at least 2 years Aged 20‐50 years; born in Denmark or have Danish parents. The participant understands, speaks, writes and read Danish |
| Interventions | 1. Experimental: Group Therapy: ACT given as conventional group therapy in groups of 7‐8 participants 3,5 hours each session, 9 sessions during 3 month 2. Experimental: Workshop. ACT given as a one‐day workshop with 15 participants with a following individual consultation 3. Standard treatment; one single advisory consultation given 2 weeks after randomisation |
| Outcomes | Primary Outcome Measures:Global Clinical Improvement Scale [Time Frame: 14 month after randomisation ] [ Designated as safety issue: No ]Questionnaire, participant‐rated improvement of health since the beginning of the study Secondary Outcome Measures:SF‐36 [ Time Frame: Before randomisation, and at 6, 14 and 20 months after randomisation ] [ Designated as safety issue: No ]Questionnaire, participant‐rated. Assessment of physical, social and mental functioning Visual Analogue Scale for pain and worst symptom [ Time Frame: Before randomisation, and at 6, 14 and 20 month after randomisation ] [ Designated as safety issue: No ] Symptom Checklist (SCL) [ Time Frame: Before randomisation, and at 6, 14 and 20 month after randomisation ] [ Designated as safety issue: No ]Questionnaire, participant‐rated. Assessment of physical, social and mental functioning WHODAS II [ Time Frame: Before randomisation, and at 6, 14 and 20 month after randomisation ] [ Designated as safety issue: No ]Questionnaire, participant‐rated. Assessment of physical, social and mental functioning |
| Starting date | January 2012, planned end date March 2015 |
| Contact information | jlag@clin.au.dk (Johanne Agger) |
| Notes | ClinicalTrials.gov Identifier: NCT01518647 |
Hassett 2007.
| Trial name or title | A Computer‐Based Intervention for Medically Unexplained Physical Symptoms |
| Methods | Randomised controlled trial |
| Participants | Participants aged 18‐75 seeking medical care for physical symptoms (i.e., persistent fatigue, pain complaints, and gastrointestinal, cardiovascular or musculoskeletal symptoms). No major medical illness to explain symptom(s) is found after detailed physical and laboratory assessment. Participants with common disorders such as hypertension, asthma, diabetes, low back strain, etc., will be included if in the opinion of the physician the presenting physical symptoms are not due to the underlying disorder. In order to enter the study, participants must meet criteria for at least 4 medically unexplained symptoms out of the 42 somatic symptoms listed in the Composite International Diagnostic Interview (CIDI) rated as currently present if males and at least 6 symptoms if females (Escobar's abridged criteria). Participants without ready access to a computer and the Internet will be excluded from participating. Also excluded will be individuals with life threatening medical illness, communicative disorder, lack of fluency in English, illiteracy, and major psychiatric conditions including psychoses, bipolar disorder, and alcohol or drug abuse. Participants will be required to add no medications to their regimen during the study period (approximately 6 weeks) |
| Interventions | Intervention: Computer‐based exercises to be executed at home Comparator: Sham computer‐based exercises to be executed at home |
| Outcomes | Quick Inventory of Depressive Symptoms Positive and Negative Affect Scale Satisfaction with Life Scale |
| Starting date | 2007 |
| Contact information | afton@med.umich.edu |
| Notes | ClinicalTrials.gov identifier: NCT00468013 According to trial register completed in 2009. Contacted investigator (17 July 2014) |
Olde Hartman 2013.
| Trial name or title | Psychosomatic Therapy, Feasibility and Cost Analysis (PsySom) |
| Methods | Randomised pilot study consisting of participants with MUS in primary care. Participants will be followed for 1 year |
| Participants | People with MUS |
| Interventions | Participants will be randomised to intervention (usual care and additional psychosomatic therapy) or control condition (usual care alone) |
| Outcomes | Primary outcome measures are: the number of participants identified and recruited, perceived symptom severity, measured on a Visual Analogue Scale (VAS) and participants' self rated symptoms of distress, depression, anxiety and somatisation (4DSQ: The Four Dimensional Symptom Questionnaire). Other primary outcome measures are the time needed to include the eligible participants, the number of withdrawals in the intervention and control group, compliance in the therapy group and the number of participants who complete the questionnaires Secondary outcome measures are: symptoms of hyperventilation (NHL: Nijmegen Hyperventilation List), physical and mental health status and quality of life (SF‐36), and level of functioning (MAF: measure of general functioning). Participant satisfaction with the received therapy is rated on a 5‐point Likert‐type scale. Medical consumption will be measured by the Cost Diary for medical consumption |
| Starting date | April 2013 |
| Contact information | Dr T.C. olde Hartman, Radboud University, email: Tim.OldeHartman@radboudumc.nl |
| Notes | ClinicalTrials.gov Identifier: NCT01935258. Results expected Octiver 2014 |
Rief 2013.
| Trial name or title | Enriching Cognitive‐Behavioral Therapy With Emotion Regulation Training in Patients With Chronic Multiple Somatoform Symptoms (ENCERT): A Randomized Controlled Trial |
| Methods | Randomised controlled trial |
| Participants | Participants 18‐69 years with inclusion Criteria based on DSM‐V diagnosis "somatic symptom disorder [SSD] 300.82" |
| Interventions | ENCERT contains 1. psychoeducation (session 1), 2. relaxation techniques for coping with stress (sessions 2‐4), 3. non‐judgmental awareness of body perceptions, (sessions 5‐7), 4. modifying illness behaviour and accepting unpleasant body perceptions (sessions 8‐13), 5. attention defocusing on positive perceptions plus emotional self support (sessions 14‐15), 6. analysing interpretation processes to understand situational cues (sessions 16‐17), and 7. change of behaviour and interpretations (sessions 18‐20). The innovative elements of ENCERT are: improving the awareness for the association of somatic symptoms with emotions, learning non‐judgmental awareness and acceptance of unpleasant body perceptions, achieving high‐frequent skill exercising with the emotion regulation audio training. Comparator: CBT. This arm is based on traditional cognitive‐behavioural therapy that can be considered the current "treatment of choice", being the only intervention with an evidence grade 1a (Kroenke 2007). As such, it presents the reference of efficacy and safety for new regimen. The strictly manualised program includes the following components focusing on the special needs of people with chronic somatoform disorders: psychoeducation providing a framework for psychotherapy, attention defocusing, reduction of over‐interpretation of symptoms, increase of physical activity, stress reduction |
| Outcomes | Primary Outcome Measures:Change in somatic symptom severity (Screening for Somatoform Disorders, SOMS‐7T) from pre‐assessment to four in‐between assessments to post‐assessment to follow‐up [Time Frame: From pre‐assessment (admission) to four in‐between assessments (9, 13, 17, 21 weeks after admission) to post‐assessment (25 weeks after admission) to follow‐up (12 months after admission) ] [ Designated as safety issue: No ]Assessment of somatic symptom severity during the last 7 days (self rating) Secondary Outcome Measures:Change in depressive symptoms (Beck Depression Inventory‐II, BDI‐II) from pre‐assessment to one in‐between assessment to post‐assessment to follow‐up [ Time Frame: From pre‐assessment (admission) to one in‐between assessment (13 weeks after admission) to post‐assessment (25 weeks after admission) to follow‐up (12 months after admission) ] [ Designated as safety issue: No ]Assessment of depressive symptoms (self rating) Change in emotion regulation skills (Emotion Regulation Skills Questionnaire, ERSQ) from pre‐assessment to one in‐between assessment to post‐assessment to follow‐up [ Time Frame: From pre‐assessment (admission) to one in‐between assessment (13 weeks after admission) to post‐assessment (25 weeks after admission) to follow‐up (12 months after admission) ] [ Designated as safety issue: No ]Assessment of emotion regulation skills (self rating) Change in symptom‐focused coping strategies (Pain Coping Questionnaire, FESV; Geissner, 2003) from pre‐assessment to one in‐between assessment to post‐assessment to follow‐up [ Time Frame: From pre‐assessment (admission) to one in‐between assessment (13 weeks after admission) to post‐assessment (25 weeks after admission) to follow‐up (12 months after admission) ] [ Designated as safety issue: No ]Assessment of symptom‐focused coping strategies (self rating) Change in general psychopathological symptoms (Symptom Checklist‐90, SCL‐90) from pre‐assessment to one in‐between assessment to post‐assessment to follow‐up [ Time Frame: From pre‐assessment (admission) to one in‐between assessment (13 weeks after admission) to post‐assessment (25 weeks after admission) to follow‐up (12 months after admission) ] [ Designated as safety issue: No ]Assessment of general psychopathological symptoms (self rating) Change in symptom‐caused disability (Pain Disability Index, PDI) from pre‐assessment to one in‐between assessment to post‐assessment to follow‐up [ Time Frame: From pre‐assessment (admission) to one in‐between assessment (13 weeks after admission) to post‐assessment (25 weeks after admission) to follow‐up (12 months after admission) ] [ Designated as safety issue: No ]Assessment of symptom‐caused disability in different areas of life (self rating) Change in health‐related quality of life (EuroQoL‐5D, EQ‐5D) from pre‐assessment to one in‐between assessment to post‐assessment to follow‐up [ Time Frame: From pre‐assessment (admission) to one in‐between assessment (13 weeks after admission) to post‐assessment (25 weeks after admission) to follow‐up (12 months after admission) ] [ Designated as safety issue: No ]Assessment of health‐related quality of life (self rating) Change in health anxiety (Whiteley Index, WI) from pre‐assessment to one in‐between assessment to post‐assessment to follow‐up [ Time Frame: From pre‐assessment (admission) to one in‐between assessment (13 weeks after admission) to post‐assessment (25 weeks after admission) to follow‐up (12 months after admission) ] [ Designated as safety issue: No ]Assessment of health anxiety (self rating) Change in social competence, emotion regulation, relaxation abilities, stress management, etc., in different areas of life (The Operationalized Assessment of Abilities, OFD) from pre‐assessment to post‐assessment to follow‐up [ Time Frame: From pre‐assessment (admission) to post‐assessment (25 weeks after admission) to follow‐up (12 months after admission) ] [ Designated as safety issue: No ]Observer‐based assessment of scores for social competence, emotion regulation, relaxation abilities, stress management, etc., in different areas of life (job, family, leisure) Change in healthcare utilisation and indirect costs (Structured Interview for the Assessment of Health Care Utilization, HCU) from pre‐assessment to follow‐up [ Time Frame: From pre‐assessment (admission) to follow‐up (12 months after admission) ] [ Designated as safety issue: No ]Observer‐based assessment of HCU and indirect costs. HCU will be transformed to costs using health economy tables Inventory of the Assessment of Negative Effects of Psychotherapy (INEP) at post‐assessment [ Time Frame: Post‐assessment (25 weeks after admission) ] [ Designated as safety issue: No ]Assessment of psychotherapy‐induced side effects (self rating) Inventory of the Assessment of Negative Effects of Psychotherapy (INEP) at follow‐up [ Time Frame: Follow‐up (12 months after admission) ] [ Designated as safety issue: No ]Assessment of psychotherapy‐induced side effects (self rating) |
| Starting date | October 2013 |
| Contact information | Winfried Rief, Ph.D. riefw@uni‐marburg.de Maria Kleinstäuber maria.kleinstaeuber@Staff.Uni‐Marburg.de |
| Notes | ClinicalTrials.gov identifier: NCT01908855. Results expected 2016 |
Schröder 2014.
| Trial name or title | How effective is a walking training for somatoform disorders? A randomized controlled study |
| Methods | Randomised controlled study |
| Participants | Participants fulfilling as primary diagnosis the criteria of 1 of the 5 somatoform disorders in the DSM‐IV focusing on multiple somatoform symptoms; participants with co‐morbid disorders are eligible; attestation on exclusion of medical causes and physical fitness by a physician; participants with acute suicidality, psychotic symptoms, and who already exercising sufficiently at the beginning of the study (due to expected ceiling effects) will be excluded |
| Interventions | Intervention: walking training based on a walking program by Bös 2006 (see Notes). Participants will be instructed by a qualified coach. The walking training comprises a weekly session of 90 minutes over 12 weeks. The program manual includes a detailed description of each training session. Every session builds on 7 sequences: 1. Introduction, 2. warming‐up, 3. walking, 4. weight and strength training/stretching, 5. relaxation, 6. exchange of experiences, 7. movement‐/health‐related information Comparator: waiting list |
| Outcomes | All of the following primary and secondary outcomes are assessed at baseline and week 12. Primary outcomes: Screening for Somatoform Disorders (SOMS‐7T; baseline, week 12); Short Form 36 Questionnaire (SF‐36) ‐ subscale Physical Health; physiological parameters (physical constitution, heart rate variability; Freiburg Questionnaire of Physical Activity Secondary outcomes: Hospital Anxiety and Depression Scale (HADS‐D); Cognitions About Body and Health (CABAH); Whiteley Index (WI); Short Form 36 Questionnaire (SF‐36) ‐ subscale Mental Health; items healthcare use developed by study authors; body mass index Weekly assessment in the walking group: Symptom Diary of the SOMS‐7T; Short Form 12 Questionnaire (SF‐12) ‐ subscales Physical and Mental Health; protocol on training frequency |
| Starting date | June 2011 |
| Contact information | Prof. Dr. Annette Schröder, University Koblenz‐Landau, Germany: Schroede@uni‐landau.de; Dr. Jens Heider, University Koblenz‐Landau, Germany: heider@uni‐landau.de |
| Notes | Provided by Maria Kleinstäuber (design paper in German) Literature: Bös K, Tiemann M, Brehm W, Mommert‐Jauch P. Walking and more ‐ reaching fitness step by step [Walking und mehr ‐ Schritt für Schritt zur Fitness] (2nd ed.). Aachen, Germany: Meyer & Meyer. 2006 |
Sitnikova 2014.
| Trial name or title | CIPRUS ‐ Cognitive‐behavioural intervention in primary care for undifferentiated somatoform disorder |
| Methods | Randomised controlled study in general practice |
| Participants | Inclusion criteria:
1) Being 18 years of age or older 2) Meeting the criteria for undifferentiated somatoform disorder according to DSM IV: a) The presence of ≥ 1 medically unexplained physical symptoms b) The symptoms last at least 6 months c) The symptoms significantly impair functioning/quality of life Exclusion criteria: 1) Having a medical disorder that explains the symptoms 2) Having a severe psychiatric disorder (i.e. psychosis‐related disorders, dementia and bipolar disorder) 3) Having a handicap such as cognitive mental impairment and/or blindness 4) Being unable to speak or read Dutch |
| Interventions | Mental health nurse practitioners (MHNP) will offer intervention participants a short structured intervention based on cognitive‐behavioural (CB) principles, in addition to usual GP care, to teach participants how to cope with the consequences of their symptoms. In up to 6 sessions participants will be provided with psycho‐education, problem solving techniques, relaxation techniques, and activity scheduling. The consequences model of somatoform complaints has successfully been used in previous Dutch intervention studies and focuses on the consequences or problems that arise due to somatoform complaints and on their aggravating effects, rather than on causes of somatoform complaints. This model will be used as the treatment rationale. The focus is not so much on treating the symptoms, but rather on producing beneficial changes in (physical) functional outcome and quality of life. The MHNPs provide the CB approach of problem solving treatment (PST) as a means to learn to tackle and cope with the identified consequences. PST teaches problem‐solving styles and skills. Several steps to problem solving have been described which will be practised during the sessions: 1. explanation of treatment rationale and 'contracting', 2. identification and clarification of problems, 3. the setting of clear goals, 4. formulation of alternative solutions, 5. selection of preferred solutions, 6. clarification of the necessary steps to implement solutions, and 7. evaluation of progress. In addition, activity scheduling and progressive relaxation techniques will be provided as these are important general features of CBT for somatoform complaints. Participants in the control group will not be offered a specific additional intervention other than the care they would usually receive from the GP |
| Outcomes | The primary clinical outcome is the development in physical functioning along the total follow‐up period as measured by the physical component summary (PCS) of the RAND‐36 The primary outcome measure for the economic evaluation is quality of life as measured by the EuroQol/EQ‐5D. Direct and indirect costs will be assessed with the TIC‐P 20 and data on healthcare use extracted from the electronic medical records of the GPs. Direct costs will be based on the Dutch standard cost prices and the indirect costs will be estimated based on the average of the population Secondary outcome measures are the severity of somatisation (PHQ‐15) and depressive/anxiety symptoms (HADS) |
| Starting date | 2014 |
| Contact information | Kate Sitnikova, VUmc Amsterdam, Netherlands, email e.sitnikova@vumc.nl, phone +31204448032 |
| Notes | Dutch Trial Register, identifier NTR4686 |
Steel 2011.
| Trial name or title | The management of medically unexplained symptoms in primary care settings in Viet Nam: A clinical trial of cognitive behavior therapy |
| Methods | Randomised controlled trial, 3 parallel arms |
| Participants | Primary care participants 18‐65 yrs presenting with ≥ 5 or medically unexplained symptoms; participants with co‐morbid medically unexplained symptoms and depression and/or anxiety |
| Interventions | Arm 1: mood enhanced Cognitive Behaviour Therapy for medically unexplained symptoms. Arm 2: Structured Care. Both interventions are administered using once‐weekly 30‐minute sessions of one‐on‐one therapy with a primary care physician over 4 consultations with an option for 2 additional consultations. Arm 1, Cognitive Behaviour Therapy includes an initial ethnographic illness interview and then metaphor based cognitive restructuring to reduce abnormal illness behaviour, breathing retraining for anxiety symptoms, and activity scheduling for depression symptoms, with sleep hygiene instruction. Arm 2, Structured Care involves an initial ethnographic illness interview followed by time matched clinical sessions with a primary care physician without the CBT content Arm 3: Treatment as Usual will involve short primary care consultations consistent with current practice within Vietnam The duration of the study for any participant will conclude after the 12‐month follow‐up assessment, resulting in participation duration of 14 months |
| Outcomes | Primary Outcome 1: number of reported MUS as recorded by non‐treating Primary Care Physician Primary Outcome 2: Phan Vietnamese Psychiatric Rating Scale: Somatisation Score Secondary Outcome 1: Vietnamese Psychiatric Rating Scale: Depression Score Secondary Outcome 2: Phan Vietnamese Psychiatric Rating Scale: Anxiety Score Secondary Outcome 3: Medical Outcomes Survey Short Form 12 WHO‐QOL BREF |
| Starting date | Not yet recruiting (July 2014) |
| Contact information | z.steel@unsw.edu.au (Zachary Steel) |
| Notes | Australian Trial Register Identifier ACTRN12611000946910 |
Zimmermann 2014.
| Trial name or title | Effectiveness of a primary care based complex intervention to promote self‐management in patients presenting psychiatric symptoms: study protocol of a cluster‐randomized controlled trial |
| Methods | Cluster‐randomised controlled trial in primary care |
| Participants | 340 participants will be enrolled in the study, 170 in either arm. Inclusion criteria are: a PHQ ≥ 5 on the anxiety, depression or somatoform scale, an age of 18‐65 years old, German literacy, fully able to give consent, sufficient auditory and visual capabilities and no current psychotherapeutic treatment |
| Interventions | Intervention group: a complex, low‐threshold intervention by an Advanced Practice Nurse (APN) using a mixture of case management and counselling techniques to promote self management Control group: usual care |
| Outcomes | Primary outcome: self efficacy, measured by the General Self‐Efficacy Scale (GSE), here used as a proxy for self management Secondary outcomes: PHQ‐D symptom load and questionnaires regarding coping with illness and health related quality of life. Outcome assessments will be applied 8 weeks and 12 months after the baseline assessment |
| Starting date | 2014 |
| Contact information | t.zimmermann@uke.uni‐hamburg.de |
| Notes | Design paper: Zimmermann T, Puschmann E, Ebersbach M, Daubmann A, Steinmann S, Scherer M. Effectiveness of a primary care based complex intervention to promote self management in participants presenting psychiatric symptoms: study protocol of a cluster‐randomised controlled trial. BMC Psychiatry 2014;14:2. doi: 10.1186/1471‐244X‐14‐2 Trial registration: Clinicaltrials.gov Identifier: NCT01726387. Results expected: 2015 |
Differences between protocol and review
Although the protocol was thought through very thoroughly, we had to make a few post‐hoc changes to procedures, to improve the quality of the review. First, in the included studies, we found almost no information regarding previous treatments for MUPS. Therefore, we did not include this item in the 'Characteristics of included studies' table.
A second post‐hoc change also regarded the 'Characteristics of included studies' table. In all included studies, therapists had received a certain form of training before they performed the interventions. There was a great variation in background, experience, and intensity and duration of extra training. We found it problematic to define criteria to assess the amount of training. Therefore, we decided not to categorise the therapists based on experience, but to describe their extent of experience narratively in the 'Characteristics of included studies' table.
Although not specifically addressed in the protocol, for brevity's sake we chose to combine anxiety and depression outcomes for three of five comparisons: Fjorback 2013; Schröder 2012; Sumathipala 2008. We decided to do this, as different outcome instruments were used in these studies, some measuring anxiety and depression separately, some measuring them altogether reporting one combination score. By combining all scores, we made it possible to make study results more comparable. For the same reason, we chose to combine SF‐36 subscales (mainly physical component scores and mental component scores), in order to be able to pool these with other scales measuring function or quality of life.
We had not anticipated that several studies used consultation letters, in both study arms, in addition to an intervention or usual care. We categorised these studies under the main comparison (CBT versus usual care or waiting list conditions), as the consultation letter only concerned a co‐intervention. We did not conduct a sensitivity analysis.
Finally, several studies compared a psychological therapy with some form of enhanced or structured care, a comparison we had not foreseen at the protocol stage. Therefore, we added this comparison.
Contributions of authors
Nikki van Dessel: developed and drafted the article, performed database searches and study selection, performed analyses under supervision of Johannes C. van der Wouden.
Madelon den Boeft: assisted in drafting the article, performed database searches and study selection, and gave feedback on the draft version of the review.
Johannes C. van der Wouden: supervised database searches, performed analyses, supervised the development of the article, gave feedback on the draft version of the review, and had a leading role in processing the editorial feedback.
Harm van Marwijk: launched the original idea, supervised development of the article, and gave feedback on draft versions of the protocol and review.
Stephanie S Leone, Berend Terluin, Maria Kleinstauber, Mattijs Numans, and Henriëtte E van der Horst: supported preparation of the review and gave feedback on draft versions of the review.
Sources of support
Internal sources
VU University Medical Centre Amsterdam, Netherlands.
External sources
-
National Institute for Health Research, UK.
Funding for this review was provided by the NIHR Cochrane Incentive Award Scheme 2014
Declarations of interest
Nikki van Dessel: none known.
Madelon den Boeft: none known.
Johannes C van der Wouden: none known.
Stephanie S Leone: none known.
Berend Terluin: none known.
Maria Kleinstauber: none known.
Mattijs Numans: none known.
Henriëtte E van der Horst: none known.
Harm WJ van Marwijk: none known
Edited (no change to conclusions)
References
References to studies included in this review
Allen 2006 {published data only}
- Allen L. A new treatment for somatization disorder. Proceedings of the World Psychiatric Association, International Congress; 2006 Jul 12‐16; Istanbul, Turkey. 2006.
- Allen LA, Woolfolk RL, Escobar JI, Gara MA, Hamer RM. Cognitive‐behavioral therapy for somatization disorder: a randomized controlled trial. Archives of Internal Medicine 2006;166:1512‐8. [DOI] [PubMed] [Google Scholar]
Burton 2012 {published and unpublished data}
- Burton C, Weller D, Marsden W, Worth A, Sharpe M. A primary care symptoms clinic for patients with medically unexplained symptoms: pilot randomised trial. BMJ Open 2012;2:e000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
Escobar 2007 {published and unpublished data}
- Escobar JI. An effective intervention for medically unexplained symptoms in primary care. Proceedings of the World Psychiatric Association, International Congress; 2006 Jul 12‐16; Istanbul, Turkey. 2006.
- Escobar JI, Gara M, Alex I, Allen L, Diaz‐Martinez A, Warman M. Treatment of patients presenting with unexplained physical symptoms in primary care. Neuropsychopharmacology 2007;29 Suppl 1:S101. [Google Scholar]
- Escobar JI, Gara MA, Diaz‐Martinez AM, Interian A, Warman M, Allen LA, et al. Effectiveness of a time‐limited cognitive behavior therapy type intervention among primary care patients with medically unexplained symptoms. Annals of Family Medicine 2007;5:328‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fjorback 2013 {published and unpublished data}
- Fjorback L, Arendt M, Carstensen T, Fink P, Oernboel E, Rehfeld E, et al. Mindfulness therapy for Bodily distress syndrome: Economic evaluation alongside a randomized trial. Journal of Psychosomatic Research 2012;72(6):480. [Google Scholar]
- Fjorback L, Arendt M, Fink P, Oernboel E, Rehfeld E, Schroder A, et al. Mindfulness therapy for Bodily distress syndrome ‐ randomized trial, one‐year follow‐up, active control. Journal of Psychosomatic Research 2012;72(6):480. [Google Scholar]
- Fjorback L, Schroder A, Ornbol E, Rehfeld E, Arendt M, Fink P. Mindfulness therapy for bodily distress syndrome ‐ a randomized controlled trial abstract. Journal of Psychosomatic Research 2011;70:580‐623. [Google Scholar]
- Fjorback LO, Arendt M, Ornbøl E, Walach H, Rehfeld E, Schröder A, et al. Mindfulness therapy for somatization disorder and functional somatic syndromes: randomized trial with one‐year follow‐up. Journal of Psychosomatic Research 2013;74:31‐40. [DOI] [PubMed] [Google Scholar]
- Fjorback LO, Carstensen T, Arendt M, Ornbøl E, Walach H, Rehfeld E, et al. Mindfulness therapy for somatization disorder and functional somatic syndromes: analysis of economic consequences alongside a randomized trial. Journal of Psychosomatic Research 2013;74:41‐8. [DOI] [PubMed] [Google Scholar]
Kashner 1995 {published data only}
- Kashner TM, Rost K, Cohen B, Anderson M, Smith GR Jr. Enhancing the health of somatization disorder patients. Effectiveness of short‐term group therapy. Psychosomatics 1995;36:462‐70. [DOI] [PubMed] [Google Scholar]
Katsamanis 2011 {published data only}
- Katsamanis M, Lehrer PM, Escobar JI, Gara MA, Kotay A, Liu R. Psychophysiologic treatment for patients with medically unexplained symptoms: a randomized controlled trial. Psychosomatics 2011;52:218‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kolk 2004 {published data only}
- Kolk AM, Schagen S, Hanewald GJ. Multiple medically unexplained physical symptoms and health care utilization: outcome of psychological intervention and patient‐related predictors of change. Journal of Psychosomatic Research 2004;57:379‐89. [DOI] [PubMed] [Google Scholar]
Lidbeck 1997 {published and unpublished data}
- Lidbeck J. Group therapy for somatization disorders in general practice: effectiveness of a short cognitive‐behavioural treatment model. Acta Psychiatrica Scandinavica 1997;96:14‐24. [DOI] [PubMed] [Google Scholar]
- Lidbeck J. Group therapy for somatization disorders in primary care: maintenance of treatment goals of short cognitive‐behavioural treatment one‐and‐a‐half‐year follow‐up. Acta Psychiatrica Scandinavica 2003;107:449‐56. [DOI] [PubMed] [Google Scholar]
Martin 2007 {published and unpublished data}
- Martin A, Rauh E, Fichter M, Rief W. A one‐session treatment for patients suffering from medically unexplained symptoms in primary care: a randomized clinical trial. Psychosomatics 2007;48:294‐303. [DOI] [PubMed] [Google Scholar]
Moreno 2013 {published and unpublished data}
- Gili M, Magallón R, López‐Navarro E, Roca M, Moreno S, Bauzá N, et al. Health related quality of life changes in somatising patients after individual versus group cognitive behavioural therapy: a randomized clinical trial. Journal of Psychosomatic Research 2014;76:89‐93. [DOI] [PubMed] [Google Scholar]
- Magallón R, Gili M, Moreno S, Bauzá N, García‐Campayo J, Roca M, et al. Cognitive‐behaviour therapy for patients with abridged somatization disorder (SSI 4,6) in primary care: a randomized, controlled study. BMC Psychiatry 2008;8:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno S, Gili M, Magallón R, Bauzá N, Roca M, Hoyo YL, Garcia‐Campayo J. Effectiveness of group versus individual cognitive‐behavioral therapy in patients with abridged somatization disorder: a randomized controlled trial. Psychosomatic Medicine 2013;75:600‐8. [DOI] [PubMed] [Google Scholar]
Sattel 2012 {published data only}
- Chernyak N, Sattel H, Scheer M, Baechle C, Kruse J, Henningsen P, et al. Economic evaluation of brief psychodynamic interpersonal therapy in patients with multisomatoform disorder. PLoS One 2014;9(1):e83894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahmann C, Sack M, Ronel J. PISO ‐ an evidence‐based approach in the therapy of somatoform disorders [PISO ‐ Ein evidenzbasierter Ansatz zur manualisierten Therapie somatoformer Störungen]. PDP Psychodynamische Psychotherapie 2007;6(3):131‐9. [Google Scholar]
- Sattel H, Lahmann C, Gündel H, Guthrie E, Kruse J, Noll‐Hussong M, et al. Brief psychodynamic interpersonal psychotherapy for patients with multisomatoform disorder: randomised controlled trial. British Journal of Psychiatry 2012;200:60‐7. [DOI] [PubMed] [Google Scholar]
Schaefert 2013 {published data only}
- Schaefert R, Kaufmann C, Wild B, Schellberg D, Boelter R, Faber R, et al. Specific collaborative group intervention for patients with medically unexplained symptoms in general practice: a cluster randomized controlled trial. Psychotherapy and Psychosomatics 2013;82:106‐19. [DOI] [PubMed] [Google Scholar]
Schilte 2001 {published and unpublished data}
- Schilte AF, Portegijs PJ, Blankenstein AH, Horst HE, Latour MB, Eijk JT, et al. Randomised controlled trial of disclosure of emotionally important events in somatisation in primary care. BMJ 2001;323:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilte AF, Portegijs PJM, Blankenstein AH, Latour MBF, Eijk JThM Knottnerus JA. Indicators of childhood adversity in somatisation in general practice. Scandinavian Journal of Primary Health Care 2001;19:232‐6. [DOI] [PubMed] [Google Scholar]
- Schilte B. Somatisation in General Practice: Clinical Assessment and the Effectiveness of Disclosing Emotionally Important Events [thesis]. Maastricht, the Netherlands: Maastricht University, 2001. [Google Scholar]
Schröder 2012 {published and unpublished data}
- Schröder A, Rehfeld E, Oernboel E, Sharpe M, Licht RW, Fink P. A novel treatment approach for people with severe functional somatic syndromes (STreSS‐1): randomized trial conference abstract. Journal of Psychosomatic Research 2010;6:664. [Google Scholar]
- Schröder A, Rehfeld E, Ornbøl E, Sharpe M, Licht RW, Fink P. Cognitive‐behavioural group treatment for a range of functional somatic syndromes: randomised trial. British Journal of Psychiatry 2012;200:499‐507. [DOI] [PubMed] [Google Scholar]
Schröder 2013 {published and unpublished data}
- Schröder A, Heider J, Zaby A, Göllner R. Cognitive behavioral therapy versus progressive muscle relaxation training for multiple somatoform symptoms: results of a randomized controlled trial. Cognitive Therapy and Research 2013;37:296‐306. [Google Scholar]
- Zaby A. Kognitiv‐Behaviorale Gruppentherapie bei Patientinnen und Patienten mit multiplen somatoformen Symptomen. Eine randomisierte kontrollierte Interventionsstudie [Thesis]. Koblenz‐Laundau: Zaby, 2009. [Google Scholar]
- Zaby A, Heider J, Schröder A. Waiting, relaxation, or cognitive‐behavioral therapy ‐ how effective is outpatient group therapy for somatoform symptoms? [Warten, Entspannung oder Verhaltenstherapie. Wie effektiv sind ambulante Gruppenbehandlungen bei multiplen somatoformen Symptomen]. Zeitschrift für Klinische Psychologie und Psychotherapie 2008;37:15‐23. [Google Scholar]
Schweickhardt 2007 {published data only}
- Schweickhardt A, Larisch A, Fritzsche K. Psychotherapeutic interventions for somatizing patients in the general hospital. Journal of Psychosomatic Research 2006;60:659. [DOI] [PubMed] [Google Scholar]
- Schweickhardt A, Larisch A, Wirsching M, Fritzsche K. Short‐term psychotherapeutic interventions for somatizing patients in the general hospital: a randomized controlled study. Psychotherapy and Psychosomatics 2007;76:339‐46. [DOI] [PubMed] [Google Scholar]
Speckens 1995 {published and unpublished data}
- Speckens AE, Hemert AM, Spinhoven P, Hawton KE, Bolk JH, Rooijmans HG. Cognitive behavioural therapy for medically unexplained physical symptoms: a randomised controlled trial. BMJ 1995;311:1328‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speckens AEM, Hemert AM, Spinhoven P, Hawton KE, Bolk JH, Rooijmans HGM. Favourable effects of cognitive behavioural therapy on unexplained physical symptoms: A randomized trial [Gunstige effecten van cognitieve gedragstherapie voor onverklaarde lichamelijke klachten; een gerandomiseerd onderzoek]. Nederlands Tijdschrift voor Geneeskunde 1996;140:1227‐1232. [Google Scholar]
Sumathipala 2000 {published data only}
- Sumathipala A, Hewege S, Hanwella R, Mann AH. Randomized controlled trial of cognitive behaviour therapy for repeated consultations for medically unexplained complaints: a feasibility study in Sri Lanka. Psychological Medicine 2000;30:747‐57. [DOI] [PubMed] [Google Scholar]
Sumathipala 2008 {published data only}
- Sumathipala A, Siribaddana S, Abeysingha MR, Silva P, Dewey M, Prince M, et al. Cognitive‐behavioural therapy v. structured care for medically unexplained symptoms: randomised controlled trial. British Journal of Psychiatry 2008;193:51‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Ravesteijn 2013 {published data only}
- Ravesteijn H, Grutters J, olde Hartman T, Lucassen P, Bor H, Weel C, Wilt GJ, Speckens A. Mindfulness‐based cognitive therapy for patients with medically unexplained symptoms: a cost‐effectiveness study. Journal of Psychosomatic Research 2013;74:197‐205. [DOI] [PubMed] [Google Scholar]
- Ravesteijn H, Lucassen P, Bor H, Weel C, Speckens A. Mindfulness‐based cognitive therapy for patients with medically unexplained symptoms: a randomized controlled trial. Psychotherapy and Psychosomatics 2013;82:299‐310. [DOI] [PubMed] [Google Scholar]
- Ravesteijn H, Lucassen P, Speckens A. Mindfulness training for patients with medically unexplained symptoms in primary care, an RCT. Journal of Psychosomatic Research 2012;72:506. [Google Scholar]
Zonneveld 2012 {published and unpublished data}
- Zonneveld LN, Rood YR, Kooiman CG, Timman R, 't Spijker A, Busschbach JJ. Predicting the outcome of a cognitive‐behavioral group training for patients with unexplained physical symptoms: a one‐year follow‐up study. BMC Public Health 2012;12:848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zonneveld LN, Rood YR, Timman R, Kooiman CG, Van't Spijker A, Busschbach JJ. Effective group training for patients with unexplained physical symptoms: a randomized controlled trial with a non‐randomized one‐year follow‐up. PLoS One 2012;7:e42629. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Aiarzaguena 2007 {published data only}
- Aiarzaguena JM, Grandes G, Gaminde I, Salazar A, Sánchez A, Ariño J. A randomized controlled clinical trial of a psychosocial and communication intervention carried out by GPs for patients with medically unexplained symptoms. Psychological Medicine 2007;37:283‐94. [DOI] [PubMed] [Google Scholar]
Arnold 2009 {published data only}
- Arnold IA, Waal MWM, Eekhof JAH, Assendelft WJJ, Spinhoven P, Hemert AM. Medically unexplained physical symptoms in primary care: a controlled study on the effectiveness of cognitive‐behavioral treatment by the family physician. Psychosomatics 2009;50:515‐24. [DOI] [PubMed] [Google Scholar]
Barsky 2013 {published data only}
- Barsky AJ, Ahern DK, Bauer MR, Nolido N, Orav EJ. A randomized trial of treatments for high‐utilizing somatizing patients. Journal of General Internal Medicine 2013;28(11):1396‐404. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bernal 1995 {published data only}
- Bernal I, Cercos A, Fuste I, Vallverdu R, Urbieta Solana R, Montesinos Molina I. Relaxation therapy in patients with anxiety and somatoform disorders in primary care [Tratamiento de relajacion en pacientes con trastornos de ansiedad y somatoformes en atencion primaria]. Atencion primaria / Sociedad Espanola de Medicina de Familia y Comunitaria 1995;15(8):499‐504. [PubMed] [Google Scholar]
Blankenstein 2001 {published and unpublished data}
- Blankenstein AH. Somatisation in General Practice. Reattribution, a Promising Approach [thesis]. Amsterdam, the Netherlands: VU University Medical Center, 2001. [Google Scholar]
- Blankenstein AH, Horst HE, Schilte AF, Vries D, Zaat JO, Knottnerus JA, et al. Development and feasibility of a modified reattribution model for somatising patients, applied by their own general practitioners. Patient Education and Counseling 2002;47:229‐35. [DOI] [PubMed] [Google Scholar]
Bleichhardt 2004 {published data only}
- Bleichhardt G, Timmer B, Rief W. Cognitive‐behavioural therapy for patients with multiple somatoform symptoms ‐ a randomised controlled trial in tertiary care. Journal of Psychosomatic Research 2004;56:449‐54. [DOI] [PubMed] [Google Scholar]
- Timmer B, Bleichhardt G, Rief W. Effectiveness of an cognitive‐behavioral group therapy for somatization: results of a randomized controlled trial in tertiary care [Effektivität einer stationären Gruppentherapie für somatoforme Störungen ‐ Ergebnisse einer kontrolliert‐randomisierten Therapieevaluationstudie]. Zeitschrift fur Klinische Psychologie 2004;33(1):24‐32. [Google Scholar]
Cano‐Vindel 2013 {published data only}
- Cano‐Vindel A. A pilot study to treat anxiety, depression and somatisations in Spanish primary care services with psychological techniques in seven group sessions: comparison of results using psychological treatment versus usual primary care treatment, with follow‐up assessments after 3, 6 and 12 months ‐ a randomised controlled trial. www.controlled‐trials.com/ISRCTN58437086/ (accessed 27 October 2014).
Detaille 2013 {published data only}
- Detaille SI, Heerkens YF, Engels JA, Gulden JWJ, Dijk FJH. Effect evaluation of a self‐management program for Dutch workers with a chronic somatic disease: a randomized controlled trial. Journal of Occupational Rehabilitation 2013;23:189‐99. [DOI] [PubMed] [Google Scholar]
Gottschalk 2011 {published data only}
- Gottschalk JM, Bleichhardt G, Hiller W, Berking M, Rief W. Cognitive‐behavioral therapy enriched with emotion regulation training (ENCERT) in patients with multiple somatoform symptoms: preliminary findings. Verhaltenstherapie 2011;21 Suppl 1:1‐39. [Google Scholar]
- Gottschalk JM, Bleichhardt G, Kleinstäuber M, Berking M, Rief W. Enriching cognitive behavioral therapy with emotion regulation training in patients with multiple somatoform symptoms: Results of a controlled pilot study [Erweiterung der kognitiven Verhaltenstherapie um Emotionsregulationstraining bei Patienten mit multiplen somatoformen Symptomen:Ergebnisse einer kontrollierten Pilotstudie]. Philipps‐Universität Marburg 2011; Vol. Personal communication.
Grepmair 2007 {published data only}
- Grepmair L, Mitterlehner F, Loew T, Bachler E, Rother W, Nickel M. Promoting mindfulness in psychotherapists in training influences the treatment results of their patients: a randomized, double‐blind, controlled Study. Psychotherapy and Psychosomatics 2007;76:332‐8. [DOI] [PubMed] [Google Scholar]
Gyllensten 2003 {published data only}
- Gyllensten AL, Hansson L, Ekdahl C. Outcome of basic body awareness therapy. A randomized controlled study of patients in psychiatric outpatient care. Advances in Physiotherapy 2003;5:179‐90. [Google Scholar]
Hellmann 1990 {published data only}
- Hellman CJC, Budd M, Borysenko J, McClelland DC, Benson H. A study of the effectiveness of two group behavioral medicine Interventions for patients with psychosomatic complaints. Behavioural Medicine 1990;16(4):165‐73. [DOI] [PubMed] [Google Scholar]
Hiller 2003 {published data only}
- Hiller W, Fichter MM, Rief W. A controlled treatment study of somatoform disorders including analysis of healthcare utilization and cost‐effectiveness. Journal of Psychosomatic Research 2003;54:369‐80. [DOI] [PubMed] [Google Scholar]
Hiller 2004 {published data only}
- Hiller W, Kroymann R, Leibbrand R, Cebulla M, Korn HJ, Rief W, et al. Effects and cost‐effectiveness analysis of inpatient treatment for somatoform disorders [Wirksamkeit und Kosten‐Nutzen‐Effekte der stationären Therapie somatoformer Störungen]. Fortschritte der Neurologie ‐ Psychiatrie 2004;72:136‐46. [DOI] [PubMed] [Google Scholar]
Houtveen 2013 {published data only}
- Houtveen JH, Lintmeijer L, Broeckhuysen S. The clinical effectiveness of a long‐term multidisciplinary integrative psychological treatment with a focus on body‐related mentalisation for patients with severe somatoform disorder. Journal of Psychosomatic Research 2013;74(6):547. [Google Scholar]
Klapow 2001 {published data only}
- Klapow JC, Schmidt SM, Taylor LA, Roller P, Li Q, Calhoun JW, et al. Symptom management in older primary care patients: feasibility of an experimental, written self‐disclosure protocol. Annals of Internal Medicine 2001;134:901‐11. [DOI] [PubMed] [Google Scholar]
Kocken 2008 {published data only}
- Kocken PL, Joosten‐van Zwanenburg E, Hoop T. Effects of health education for migrant females with psychosomatic complaints treated by general practitioners: a randomised controlled evaluation study. Patient Education and Counseling 2008;70:25‐30. [DOI] [PubMed] [Google Scholar]
Larisch 2004 {published data only}
- Fritzsche K, Larisch A. Treating patients with functional somatic symptoms. A treatment guide for use in general practice. Scandinavian Journal of Primary Health Care 2003;21:132‐5. [DOI] [PubMed] [Google Scholar]
- Larisch A, Schweickhardt A, Wirsching M, Fritzsche K. Psychosocial interventions for somatizing patients by the general practitioner: a randomized controlled trial. Journal of Psychosomatic Research 2004;57:507‐14. [DOI] [PubMed] [Google Scholar]
Lopez‐Garcia‐Franco 2012 {published data only}
- Lopez‐Garcia‐Franco A, del‐Cura‐Gonzalez MI, Caballero‐Martinez L, Sanz‐Cuesta T, Diaz‐Garcia MI, Rodriguez‐Monje MT, et al. Effectiveness of a cognitive behavioral intervention in patients with medically unexplained symptoms: cluster randomized trial. BMC Family Practice 2012;13:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lupke 1996 {published data only}
- Lupke U, Ehlert U, Hellhammer D. Behavioural medicine at the general hospital: evaluation of treatment for patients with somatoform Disorders [Verhaltensmedizin im Allgemeinkrankenhaus: Verlaufsuntersuchung an Patienten mit Somatoformen Störungen]. Verhaltenstherapie 1996;6:22‐32. [Google Scholar]
McLeod 1997 {published data only}
- McLeod CC, Budd MA, McClelland DC. Treatment of somatization in primary care. General Hospital Psychiatry 1997;19(4):251‐8. [DOI] [PubMed] [Google Scholar]
Morriss 2007 {published data only}
- Morriss R, Dowrick C, Salmon P, Peters S, Dunn G, Rogers A, et al. Cluster randomised controlled trial of training practices in reattribution for medically unexplained symptoms. British Journal of Psychiatry 2007;191:536‐42. [DOI] [PubMed] [Google Scholar]
- Morriss R, Dowrick C, Salmon P, Peters S, Rogers A, Dunn G, et al. Turning theory into practice: rationale, feasibility and external validity of an exploratory randomized controlled trial of training family practitioners in reattribution to manage patients with medically unexplained symptoms (the MUST). General Hospital Psychiatry 2006;28:343‐51. [DOI] [PubMed] [Google Scholar]
Nanke 2003a {published data only}
- Nanke A, Rief W. Biofeedback in the treatment of somatoform disorders [Biofeedback‐Therapie bei somatoformen Störungen]. Verhaltenstherapie 2000;10:238‐48. [Google Scholar]
- Nanke A, Rief W. Biofeedback‐based interventions in somatoform disorders: a randomized controlled trial. Acta Neuropsychiatrica 2003;15:249‐56. [DOI] [PubMed] [Google Scholar]
Payne 2009 {published data only}
- Payne H. Pilot study to evaluate dance movement psychotherapy (the body mind approach) in patients with medically unexplained symptoms: participant and facilitator perceptions and a summary discussion. Body, Movement and Dance in Psychotherapy 2009;4(2):77‐94. [Google Scholar]
Peters 2002 {published data only}
- Peters S, Stanley I, Rose M, Kaney S, Salmon P. A randomized controlled trial of group aerobic exercise in primary care patients with persistent, unexplained physical symptoms. Family Practice 2002;19(6):665‐74. [DOI] [PubMed] [Google Scholar]
Pols 2008 {published data only}
- Pols RG, Battersby M, Searcy D, Hawkins R, Mahoney S, Allen K, et al. A controlled trial of coordinated care for somatisation disorders. Proceedings of the WPA, Section of Epidemiology and Community Psychiatry Symposium; 1997 Oct 19‐22; Sydney, Australia. 1997.
- Pols RG, Battersby MW. Coordinated care in the management of patients with unexplained physical symptoms: depression is a key issue. Medical Journal of Australia 2008;188(12):S133‐7. [DOI] [PubMed] [Google Scholar]
Rasmussen 2006 {published data only}
- Rasmussen NH, Furst JW, Swenson‐Dravis DM, Agerter DC, Smith AJ, Baird MA, et al. Innovative reflecting interview: effect on high‐utilizing patients with medically unexplained symptoms. Disease Management 2006;9(6):349‐59. [DOI] [PubMed] [Google Scholar]
Rembold 2011 {published data only}
- Rembold SM. Somatoform disorders in general practice: construction and evalisation of a psychosocial group program [Somatoforme Störungen in der Hausarztpraxis: Konstruktion und Evaluation eines psychosozialen Gruppenprogramms]. Gruppenpsychotherapie & Gruppendynamik 2011;47:2‐12. [Google Scholar]
Rosendal 2007 {published data only}
- Rosendal M, Olesen F, Fink P, Toft T, Sokolowski I, Bro F. A randomized controlled trial of brief training in the assessment and treatment of somatization in primary care: effects on patient outcome. General Hospital Psychiatry 2007;29(4):364‐73. [DOI] [PubMed] [Google Scholar]
Rost 1994 {published data only}
- Rost K, Kashner TM, Smith RG Jr. Effectiveness of psychiatric intervention with somatization disorder patients: improved outcomes at reduced costs. General Hospital Psychiatry 1994;16(6):381‐7. [DOI] [PubMed] [Google Scholar]
Ryan 2004 {published data only}
- Ryan M, Gevirtz R. Biofeedback‐based psychophysiological treatment in a primary care setting: an initial feasibility study. Applied Psychophysiology and Biofeedback 2004;29(2):79‐93. [DOI] [PubMed] [Google Scholar]
Schade 2011 {published data only}
- Schade N, Torres P, Beyebach M. Cost‐efficiency of a brief family intervention for somatoform patients in primary care. Families, Systems & Health 2011;29(3):197‐205. [DOI] [PubMed] [Google Scholar]
Schwarz 2012 {published data only}
- Schwarz MJ, Hennings A, Riemer S, Stapf T, Selberdinger V, Gil FP, et al. Effect of physical exercise on psychoneuroimmunological parameters in patients with depression and patients with somatoform disorder. Proceeding of the 11th Psychoimmunology Expert Meeting; 2012 Mar 8‐11; Gunzburg, Germany. 2012.
Sharpe 2011 {published data only}
- Sharpe M, Walker J, Williams C, Stone J, Cavanagh J, Murray G, et al. Guided self‐help for functional (psychogenic) symptoms: a randomized controlled efficacy trial. Neurology 2011;77(6):564‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 2006 {published data only}
- Smith RC, Lyles JS, Gardiner JC, Sirbu C, Hodges A, Collins C, et al. Primary care clinicians treat patients with medically unexplained symptoms: a randomized controlled trial. Journal of General Internal Medicine 2006;21(7):671‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 2009 {published data only}
- Smith RC, Gardiner JC, Luo Z, Schooley S, Lamerato L, Rost K. Primary care physicians treat somatization. Journal of General Internal Medicine 2009;24(7):829‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Toft 2010 {published data only}
- Toft T, Rosendal M, Ørnbøl E, Olesen F, Frostholm L, Fink P. Training general practitioners in the treatment of functional somatic symptoms: effects on patient health in a cluster‐randomised controlled trial (the Functional Illness in Primary Care study). Psychotherapy and Psychosomatics 2010;79:227‐37. [DOI] [PubMed] [Google Scholar]
Tschuschke 2007 {published data only}
- Tschuschke V, Weber R, Horn E, Kiencke P, Tress W. Short ambulant psychodynamic group therapy for patients with somatoform disorders [Ambulante psychodynamische Kurzgruppen psychotherapie bei Patienten mit somatoformen Störungen]. Zeitschrift für Psychiatrie, Psychologie und Psychotherapie 2007;55(2):87‐95. [Google Scholar]
Tyrer 2011 {published data only}
- Tyrer H, Tyrer P, Lovett I. Adapted cognitive‐behavior therapy for medically unexplained symptoms in secondary care reduces hospital contacts. Psychosomatics: Journal of Consultation Liaison Psychiatry 2011;52(2):194‐7. [DOI] [PubMed] [Google Scholar]
Van der Feltz‐Cornelis 2006 {published data only}
- Feltz‐Cornelis CM, Oppen P, Ader H, Dyk R. Psychiatric consultation for somatoform disorder in primary care. Proceedings of the 157th Annual Meeting of the American Psychiatric Association; 2004 May 1‐6; New York. 2004.
- Feltz‐Cornelis CM, Oppen P, Adèr HJ, Dyck R. Randomised controlled trial of a collaborative care model with psychiatric consultation for persistent medically unexplained symptoms in general practice. Psychotherapy and Psychosomatics 2006;75:282‐9. [DOI] [PubMed] [Google Scholar]
Whitehead 2002 {published data only}
- Whitehead L, Campion P. Can general practitioners manage chronic fatigue syndrome? A controlled trial. Journal of Chronic Fatigue Syndrome 2002;10:55‐64. [Google Scholar]
Wiefferink 1997 {published data only}
- Wiefferink CH, Moleman N, Wijkel D. Effect of a psychiatric intervention, aimed at the family situation, at the consumption of care by somatic patients [Het effect van een psychiatrische interventie, gericht op de gezinssituatie, op de zorgconsumptie van somatiserende patienten]. Tijdschrift voor Sociale Gezondheidszorg 1997;1:31. [Google Scholar]
References to studies awaiting assessment
Burwell‐Walsh 2002 {unpublished data only}
- Burwell‐Walsh SR. Emotion‐Focused Couples Therapy as a Treatment of Somatoform Disorders: an Outcome Study [Doctoral dissertation]. Blacksburg, VA: Virginia Polytechnic Institute and State University, 2002. [Google Scholar]
Crompton 2003 {published data only}
- Crompton N. To investigate the effectiveness of (manualised) reattribution therapy in the treatment of medically unexplained symptoms (MUS) within the general hospital. www.controlled‐trials.com/ISRCTN28015467 (accessed 27 October 2014).
Gournay 1998 {published data only}
- Gournay K. CBT in primary health care (the relative efficacy of two forms of cognitive behavioural psychotherapy CBT delivered by community mental health nurses CMHNs in the treatment of medically unexplained problems). National Research Register (www.sheffield.ac.uk/library/cdfiles/nrr) 1998.
Lopez‐Garcia‐Franco 2009 {published data only}
- Lopez‐Garcia‐Franco A. Effectiveness of a cognitive behavioral intervention in patients with symptoms somatization, as measure quality of life, front the clinical practice usual action in primary health care. A controlled clinical trial with parallel groups. clinicaltrials.gov/show/NCT01484223 (accessed 27 October 2014).
Mussgay 2006 {published data only}
- Mussgay L, Schmidt F, Morad E, Rueddel H. Effects of aerobic exercise on autonomic dysregulation in patients with anxiety and somatization disorders. In: Jäckel WH, Bengel J, Herdt J editor(s). Research in Rehabilitation: Results from a Research Network in Southwest Germany. Stuttgart, Germany: Schattauer, 2006:183‐98. [Google Scholar]
Nickel 2006 {published data only}
- Nickel M, Cangoez B, Bachler E, Muehlbacher M, Lojewski N, Mueller‐Rabe N, et al. Bioenergetic exercises in inpatient treatment of Turkish immigrants in tertiary care. Journal of Psychosomatic Research 2006;61:507‐13. [DOI] [PubMed] [Google Scholar]
Woolfolk 2007 {published data only}
- Woolfolk RL, Allen LA. Clinical trial assessing the efficacy of affective cognitive‐behavioral therapy. Treating Somatization: a Cognitive‐Behavioral Approach. New York: Guilford Press, 2007:165‐72. [Google Scholar]
References to ongoing studies
Agger 2012 {published data only}
- Agger JL. Treatment of multi‐organ bodily distress syndrome. A randomized controlled trial of the effects of acceptance and commitment therapy given as group therapy or workshop compared to standard treatment (Stress‐4). clinicaltrials.gov/show/NCT01518647 (accessed 27 October 2014).
Hassett 2007 {published data only}
- Hassett AL. A computer‐based intervention for medically unexplained physical symptoms. clinicaltrials.gov/ct2/show/NCT00468013 (accessed 27 October 2014).
Olde Hartman 2013 {published data only}
- Olde Hartman T. Psychosomatic therapy, feasibility and cost analysis (PsySom). clinicaltrials.gov/ct2/show/NCT01935258 (accessed 27 October 2014).
Rief 2013 {published data only}
- Rief W, Kleinstauber M. Enriching cognitive‐behavioral therapy with emotion regulation training in patients with chronic multiple somatoform symptoms (ENCERT): a randomized controlled trial. clinicaltrials.gov/ct2/show/NCT01908855 (accessed 27 October 2014).
Schröder 2014 {unpublished data only}
- Schröder A. How effective is a walking training for somatoform disorders? A randomized controlled study. Personal communication, provided by Maria Kleinstäuber.
Sitnikova 2014 {published data only}
- Sitnikova E. CIPRUS ‐ Cognitive‐behavioural intervention in primary care for undifferentiated somatoform disorder. (http://www.emgo.nl/research/mental‐health/research‐projects/1463/) 2014. [DOI] [PubMed]
- Sitnikova K. Cost‐effectiveness of a short‐term cognitive‐behavioural intervention by mental health nurse practitioners for primary care patients with an undifferentiated somatoform disorder ‐ CIPRUS. http://www.trialregister.nl/trialreg/admin/rctview.asp?TC=4686 (accessed 27 October 2014). [Google Scholar]
Steel 2011 {published data only}
- Steel Z. Randomised controlled trial of cognitive behavior therapy, structured care and treatment as usual in the management of adult primary care patients presenting with 5 or more chronic medically unexplained symptoms in Ho Chi Minh City, Vietnam. http://www.anzctr.org.au/ACTRN12611000946910.aspx (accessed 27 October 2014).
Zimmermann 2014 {published data only}
- Zimmermann T, Puschmann E, Ebersbach M, Daubmann A, Steinmann S, Scherer M. Effectiveness of a primary care based complex intervention to promote self‐management inpatients presenting psychiatric symptoms: study protocol of a cluster‐randomized controlled trial. BMC Psychiatry 2014;14:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Allen 2002
- Allen LA, Escobar JI, Lehrer PM, Gara MA, Woolfolk RL. Psychosocial treatments for multiple unexplained physical symptoms: a review of the literature. Psychosomatic Medicine 2002;64:939‐50. [DOI] [PubMed] [Google Scholar]
Altman 1996
- Altman DG, Bland JM. Detecting skewness from summary information. BMJ 1996;313:1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
APA 1980
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Health Disorders. 1st Edition. Washington DC: American Psychiatric Association, 1980. [Google Scholar]
APA 2000
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Health Disorders. 4th Edition. Washington DC: American Psychiatric Association, 2000. [Google Scholar]
APA 2013
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Health Disorders. 5th Edition. Washington DC: American Psychiatric Association, 2013. [Google Scholar]
Beck 1961
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry 1961;4(6):561‐71. [DOI] [PubMed] [Google Scholar]
Beck 1988
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. Journal of Consulting and Clinical Psychology 1988;56(6):893‐97. [DOI] [PubMed] [Google Scholar]
Bernardy 2013
- Bernardy K, Klose P, Busch AJ, Choy EH, Häuser W. Cognitive behavioural therapies for fibromyalgia. Cochrane Database of Systematic Reviews 2013, Issue 9. [DOI: 10.1002/14651858.CD009796.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Busch 2007
- Busch AJ, Barber KA, Overend TJ, Peloso PM, Schachter CL. Exercise for treating fibromyalgia syndrome. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD003786.pub2] [DOI] [PubMed] [Google Scholar]
Campbell 2005
- Campbell MK, Fayers PM, Grimshaw JM. Determinants of the intracluster correlation coefficient in cluster randomized trials: the case of implementation research. Clinical Trials 2005;2:99‐107. [DOI] [PubMed] [Google Scholar]
Chernyak 2014
- Chernyak N, Sattel H, Scheer M, Baechle C, Kruse J, Henningsen P, et al. Economic evaluation of brief psychodynamic interpersonal therapy in patients with multisomatoform disorder. PLoS One 2014;22(9):e83894. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deary 2007
- Deary V, Chalder T, Sharpe M. The cognitive behavioural model of medically unexplained symptoms: a theoretical and empirical review. Clinical Psychology Review 2007;27:781‐97. [DOI] [PubMed] [Google Scholar]
Deeks 2008
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis. In: Egger M, Smith GD, Altman DG editor(s). Systematic Reviews in Health Care: Meta‐Analysis in Context. 2nd Edition. London: BMJ Publishing Group, 2008. [Google Scholar]
Derogatis 1983
- Derogatis LR, Melisaratos N. The Brief Symptom Inventory: an introductory report. Psychological Medicine 1983;13:596‐605. [PubMed] [Google Scholar]
Derogatis 1986
- Derogatis LR. The SCL‐90‐R: Administration, Scoring, and Procedures Manual II. Towson MD: Clinical Psychometric Research, 1986. [Google Scholar]
Dimsdale 2009
- Dimsdale J, Creed F. The proposed diagnosis of somatic symptom disorders in DSM‐V to replace somatoform disorders in DSM‐IV ‐ a preliminary report. Journal of Psychosomatic Research 2009;66:473‐6. [DOI] [PubMed] [Google Scholar]
Edmonds 2004
- Edmonds M, McGuire H, Price J. Exercise therapy for chronic fatigue syndrome. Cochrane Database of Systematic Reviews 2004, Issue 3. [DOI: 10.1002/14651858.CD003200.pub2] [DOI] [PubMed] [Google Scholar]
Edwards 2010
- Edwards TM, Stern A, Clarke DD, Ivbijaro G, Kasney LM. The treatment of patients with medically unexplained symptoms in primary care: a review of the literature. Mental Health in Family Medicine 2010;7:209‐21. [PMC free article] [PubMed] [Google Scholar]
Escobar 1987
- Escobar J, Burnam M. Somatization in the community. Archives of General Psychiatry 1987;44:713‐8. [DOI] [PubMed] [Google Scholar]
Escobar 1998
- Escobar JI, Waitzkin H, Silver RC, Gara M, Holman A. Abridged somatization: a study in primary care. Psychosomatic Medicine 1998;60:466‐72. [DOI] [PubMed] [Google Scholar]
Fink 1999
- Fink P, Sørensen L, Engberg M, Holm M, Munk‐Jørgensen P. Somatization in primary care. Prevalence, health care utilization, and general practitioner recognition. Psychosomatics 1999;40:330‐8. [DOI] [PubMed] [Google Scholar]
Fink 2004
- Fink P, Hansen MS, Oxhøj ML. The prevalence of somatoform disorders among internal medical inpatients. Journal of Psychosomatic Research 2004;56:413‐8. [DOI] [PubMed] [Google Scholar]
Fink 2007
- Fink P, Toft T, Hansen MS, Ørnbøl E, Olesen F. Symptoms and syndromes of bodily distress: an exploratory study of 978 internal medical, neurological, and primary care patients. Psychosomatic Medicine 2007;69:30‐9. [DOI] [PubMed] [Google Scholar]
First 2002
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM‐IV‐TR Axis I Disorders, Research Version, Patient Edition. New York: Biometrics Research, New York State Psychiatric Institute, 2002. [Google Scholar]
Ford 2009
- Ford AC, Talley NJ, Schoenfeld PS, Quigley EM, Moayyedi P. Efficacy of antidepressants and psychological therapies in irritable bowel syndrome: systematic review and meta‐analysis. Gut 2009;58:367‐78. [DOI] [PubMed] [Google Scholar]
Gamble 2005
- Gamble C, Hollis S. Uncertainty method improved on best‐worst case analysis in a binary meta‐analysis. Journal of Clinical Epidemiology 2005;58:579‐88. [DOI] [PubMed] [Google Scholar]
Gili 2014
- Gili M, Magallón R, López‐Navarro E, Roca M, Moreno S, Bauzá N, et al. Health related quality of life changes in somatising patients after individual versus group cognitive behavioural therapy: a randomized clinical trial. Journal of Psychosomatic Research 2014;76:89‐93. [DOI] [PubMed] [Google Scholar]
Goldberg 1989
- Goldberg D, Gask L, O'Dowd T. The treatment of somatization: teaching techniques of reattribution. Journal of Psychosomatic Research 1989;33:689‐95. [DOI] [PubMed] [Google Scholar]
Guerney 1971
- Guerney B, Stollak G, Guerney L. The practicing psychologist as educator ‐ an alternative to the medical practitioner model. Professional Psychology 1971;2:271‐2. [Google Scholar]
Gureje 1997
- Gureje O, Simon GE, Ustun TB, Goldberg DP. Somatization in cross‐cultural perspective: a World Health Organization study in primary care. American Journal of Psychiatry 1997;154:989‐95. [DOI] [PubMed] [Google Scholar]
Guthrie 1996
- Guthrie E. Emotional disorder in chronic illness: psychotherapeutic interventions. British Journal of Psychiatry 1996;168:265‐73. [DOI] [PubMed] [Google Scholar]
Guy 1976
- Guy W. 028 CGI Clinical Global Impressions. ECDEU Assessment Manual for Psychopharmacology ‐ Revised. Rockville, MD: Psychopharmacology Research Branch, Division of Extramural Research Programs, US Department of Health, Education, and Welfare, Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration, 1976:218‐22. [Google Scholar]
Hakkaart‐van Roijen 2002
- Hakkaart‐van Roijen L. Trimbos/iMTA Questionnaire for Costs Associated with Psychiatric Illness (TiC‐P). Rotterdam: Institute for Medical Technology Assessment, Erasmus University, 2002. [Google Scholar]
Hall 1995
- Hall RC. Global assessment of functioning. A modified scale. Psychosomatics 1995;36(3):267‐75. [DOI] [PubMed] [Google Scholar]
Hamilton 1959
- Hamilton M. The assessment of anxiety states by rating. British Journal of Medical Psychology 1959;32:50‐5. [DOI] [PubMed] [Google Scholar]
Hamilton 1960
- Hamilton M. A rating scale for depression. Neurosurgery and Psychiatry 1960;23:56‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hayden 2005
- Hayden JA, Tulder MW, Malmivaara A, Koes BW. Exercise therapy for treatment of non‐specific low back pain. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD000335.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hoedeman 2010
- Hoedeman R, Blankenstein AH, Feltz‐Cornelis CM, Krol B, Stewart R, Groothoff JW. Consultation letters for medically unexplained physical symptoms in primary care. Cochrane Database of Systematic Reviews 2010, Issue 12. [DOI: 10.1002/14651858.CD006524.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hofmann 2012
- Hofmann SG, Asnaani A, Vonk IJ, Sawyer AT, Fang A. The efficacy of cognitive behavioral therapy: a review of meta‐analyses. Cognitive Therapy and Research 2012;36(5):427‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Huibers 2007
- Huibers MJ, Beurskens A, Bleijenberg G, Schayck CP. Psychosocial interventions by general practitioners. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD003494.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ipser 2009
- Ipser JC, Stein DJ. Pharmacotherapy and psychotherapy for body dysmorphic disorder. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD005332] [DOI] [PMC free article] [PubMed] [Google Scholar]
Janca 1996
- Janca A, Hiller W. ICD‐10 checklists ‐ a tool for clinicians' use of the ICD‐10 classification of mental and behavioral disorders. Comprehensive Psychiatry 1996;37(3):180‐7. [DOI] [PubMed] [Google Scholar]
Kellner 1986
- Kellner R. Somatization and Hypochondriasis. London: Greenwood, 1986. [Google Scholar]
Kihlstrom 1985
- Kihlstrom JF. Hypnosis. Annual Review of Psychology 1985;36:385‐418. [DOI] [PubMed] [Google Scholar]
Kirmayer 2004
- Kirmayer LJ, Groleau D, Looper KJ, Dao MD. Explaining medically unexplained symptoms. Canadian Journal of Psychiatry 2004;49:663‐72. [DOI] [PubMed] [Google Scholar]
Kleinstäuber 2011
- Kleinstäuber M, Witthöft M, Hiller W. Efficacy of short‐term psychotherapy for multiple medically unexplained physical symptoms: a meta‐analysis. Clinical Psychology Review 2011;31:146‐60. [DOI] [PubMed] [Google Scholar]
Kleinstäuber 2013
- Kleinstäuber M, Witthöft M, Steffanowski A, Lambert M, Meinhardt G, Lieb K, et al. Pharmacological interventions for somatoform disorders in adults. Cochrane Database of Systematic Reviews 2013, Issue 7. [DOI: 10.1002/14651858.CD010628] [DOI] [PMC free article] [PubMed] [Google Scholar]
Koelen 2014
- Koelen JA, Houtveen JH, Abbass A, Luyten P, Eurelings‐Bontekoe EH, van Broeckhuysen‐Kloth, et al. Effectiveness of psychotherapy for severe somatoform disorders. British Journal of Psychiatry 2014;204:12‐9. [DOI] [PubMed] [Google Scholar]
Konnopka 2012
- Konnopka A, Schaefert R, Heinrich S, Kaufmann C, Luppa M, Herzog W, et al. Economics of medically unexplained symptoms: a systematic review of the literature. Psychotherapy and Psychosomatics 2012;81:265‐75. [DOI] [PubMed] [Google Scholar]
Kroenke 1989
- Kroenke K, Mangelsdorff AD. Common symptoms in ambulatory care: incidence, evaluation, therapy, and outcome. American Journal of Medicine 1989;86:262‐6. [DOI] [PubMed] [Google Scholar]
Kroenke 1997
- Kroenke K, Spitzer RL, deGruy FV, Hahn SR, Linzer M, Williams JB, et al. Multisomatoform disorder: an alternative to undifferentiated somatoform disorder for the somatizing patient in primary care. Archives of General Psychiatry 1997;54:352‐8. [DOI] [PubMed] [Google Scholar]
Kroenke 2000
- Kroenke K, Swindle R. Cognitive‐behavioral therapy for somatization and symptom syndromes: a critical review of controlled clinical trials. Psychotherapy and Psychosomatics 2000;69:205‐15. [DOI] [PubMed] [Google Scholar]
Kroenke 2001
- Kroenke K, Spitzer RL, Williams JBW. The PHQ‐9: validity of a brief depression severity measure. Journal of General Internal Medicine 2001;16(9):606‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kroenke 2002
- Kroenke K, Spitzer RL, Williams JBW. The PHQ‐15: validity of a new measure for evaluating the severity of somatic symptoms. Psychosomatic Medicine 2002;64:258‐66. [DOI] [PubMed] [Google Scholar]
Kroenke 2007
- Kroenke K. Efficacy of treatment for somatoform disorders: a review of randomized controlled trials. Psychosomatic Medicine 2007;69:881‐8. [DOI] [PubMed] [Google Scholar]
Lindström 1992
- Lindström I, Öhlund C, Eek C, Wallin L, Peterson LE, Fordyce WE, et al. Mobility of graded activity on patients with subacute low back pain: a randomised prospective clinical study with operant‐conditioning behavioural approach. Physical Therapy 1992;4:279‐93. [DOI] [PubMed] [Google Scholar]
Loew 2000
- Loew TH, Sohn R, Martus P, Tritt K, Rechlin T. Functional relaxation as a somatopsychotherapeutic intervention: a prospective controlled study. Alternative Therapies in Health and Medicine 2000;6(6):70‐5. [PubMed] [Google Scholar]
Looper 2002
- Looper KJ, Kirmayer LJ. Behavioral medicine approaches to somatoform disorders. Journal of Consulting and Clinical Psychology 2002;70:810‐27. [PubMed] [Google Scholar]
Malouff 2007
- Malouff JM, Thorsteinsson EB, Schutte NS. The efficacy of problem solving therapy in reducing mental and physical health problems: a meta‐analysis. Clinical Psychology Review 2007;27(1):46‐57. [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG. The PRISMA Group (2009). Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA Statement. BMJ 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2014
- Moore RA, Wiffen PJ, Derry S, Toelle T, Rice AS. Gabapentin for chronic neuropathic pain and fibromyalgia in adults. Cochrane Database of Systematic Reviews 2014, Issue 4. [DOI: 10.1002/14651858.CD007938.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mumford 1991
- Mumford DB. The Development and Validation of the Bradford Somatic Inventory: an Investigation of Functional Somatic Symptoms in Britain and Pakistan [MD Thesis]. Bristol, UK: University of Bristol, 1991. [Google Scholar]
Nanke 2003b
- Nanke A, Rief W. Biofeedback‐based interventions in somatoform disorders: a randomized controlled trial. Acta Neuropsychiatrica 2003;15:249‐56. [DOI] [PubMed] [Google Scholar]
Nezu 2001
- Nezu AM, Nezu CM, Lombardo ER. Cognitive‐behavior therapy for medically unexplained symptoms: a critical review of the treatment literature. Behavior Therapy 2001;32:537‐83. [Google Scholar]
Noyes 2008
- Noyes R, Stuart SP, Watson DB. A reconceptualization of the somatoform disorders. Psychosomatics 2008;49:14‐22. [DOI] [PubMed] [Google Scholar]
Pae 2009
- Pae CU, Marks DM, Patkar AA, Masand PS, Luyten P, Serretti A. Pharmacological treatment of chronic fatiguesyndrome: focusing on the role of antidepressants. Expert Opinion on Pharmacotherapy 2009;10:1561‐70. [DOI] [PubMed] [Google Scholar]
Pilowsky 1967
- Pilowsky I. Dimensions of hypochondriasis. British Journal of Psychiatry 1967;113:89‐93. [DOI] [PubMed] [Google Scholar]
Price 2008
- Price JR, Mitchell E, Tidy E, Hunot V. Cognitive behaviour therapy for chronic fatigue syndrome in adults. Cochrane Database of Systematic Reviews 2008, Issue 3. [DOI: 10.1002/14651858.CD001027.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Rief 1997
- Rief W, Hiller W, Heuser J. SOMS: Das Screening für Somatoforme Störungen [SOMS: Screening for Somatoform Disorders]. Bern: Huber, 1997. [Google Scholar]
Rief 2003
- Rief W, Ihle D, Pilger F. A new approach to assess illness behaviour. Journal of Psychosomatic Research 2003;54(5):405‐14. [DOI] [PubMed] [Google Scholar]
Ring 2004
- Ring A, Dowrick C, Humphris G, Salmon P. Do patients with unexplained physical symptoms pressurise general practitioners for somatic treatment? A qualitative study. BMJ 2004;328(7447):1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rosendal 2013
- Rosendal M, Burton C, Blankenstein AH, Fink P, Kroenke K, Sharpe M, et al. Enhanced care by generalists for functional somatic symptoms and disorders in primary care. Cochrane Database of Systematic Reviews 2013, Issue 10. [DOI: 10.1002/14651858.CD008142] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ruddy 2005
- Ruddy R, House A. Psychosocial interventions for conversion disorder. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD005331.pub2] [DOI] [PubMed] [Google Scholar]
Saarto 2007
- Saarto T, Wiffen PJ. Antidepressants for neuropathic pain. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD005454] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schwartz 2003
- Schwartz MD, Andrasik F. Biofeedback: A Practitioner's Guide. 3rd Edition. New York: Guilford Publications, 2003. [Google Scholar]
Schünemann 2008
- Schünemann HJ, Oxman AD, Brozek J, Glasziou P, Jaeschke R, Vist GE, et al for the GRADE Working Group. Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ 2008;336(7653):1106‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sharpe 1992
- Sharpe M, Peveler R, Mayou R. The psychological treatment of patients with functional somatic symptoms: a practical guide. Journal of Psychosomatic Research 1992;36(6):515‐29. [DOI] [PubMed] [Google Scholar]
Sheehan 1983
- Sheehan DV. The Anxiety Disease. New York: Charles Scribner and Sons, 1983. [Google Scholar]
Sheehan 1998
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini‐International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM‐IV and ICD‐10. Journal of Clinical Psychiatry 1998;59 Suppl. 20:22‐33. [PubMed] [Google Scholar]
Silberstein 2002
- Silberstein SD, Peres MFP, Hopkins MM, Shechter AL, Young WB, Rozen TD. Olanzapine in the treatment of refractory migraine and chronic daily headache. Headache 2002;45:515‐8. [DOI] [PubMed] [Google Scholar]
Smith 1986
- Smith GR, Monson RA, Ray DC. Patients with multiple unexplained symptoms: their characteristics, functional health, and health care utilization. Archives of Internal Medicine 1986;146:69‐72. [PubMed] [Google Scholar]
Smith 1991
- Smith GR. Somatization Disorder in the Medical Setting. Washington DC: American Psychiatric Press, 1991. [Google Scholar]
Spitzer 1990
- Spitzer RL, Williams JBW, Gibbon M, First MB. Structured Clinical Interview for DSM‐III‐R. Patient/non‐patient. Washington DC: American Psychiatric Press, 1990. [Google Scholar]
Sterne 2011
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta‐analyses of randomised controlled trials. BMJ 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
Sumathipala 2007
- Sumathipala A. What is the evidence for the efficacy of treatments for somatoform disorders? A critical review of previous intervention studies. Psychosomatic Medicine 2007;69:889‐900. [DOI] [PubMed] [Google Scholar]
Terluin 2006
- Terluin B, Marwijk HWJ, Adèr HJ, Vet HCW, Penninx BWJH, Hermens MLM, et al. The Four‐Dimensional Symptom Questionnaire (4DSQ): a validation study of a multidimensional self‐report questionnaire to assess distress, depression, anxiety and somatization. BMC Psychiatry 2006;6:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thompson 2007
- Thomson AB, Page LA. Psychotherapies for hypochondriasis. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD006520.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Timmer 2006
- Timmer B, Bleichhardt G, Rief W. Importance of psychotherapy motivation in patients with somatization syndrome. Psychotherapy Research 2006;16:348‐56. [Google Scholar]
Unutzer 2002
- Unutzer J, Katon W, Callahan CM, Williams JW, Hunkeler E, Harpole L, et al. Collaborative care management of late‐life depression in the primary care setting: a randomized controlled trial. JAMA 2002;288:2836‐45. [DOI] [PubMed] [Google Scholar]
van der Feltz‐Cornelis 2004
- Feltz‐Cornelis CM, Oppen P, Marwijk HWJ, Beurs E, Dyck R. A patient‐doctor relationship questionnaire (PDRQ‐9) in primary care: development and psychometric evaluation. General Hospital Psychiatry 2004;26:115‐20. [DOI] [PubMed] [Google Scholar]
Van der Giessen 2012
- Giessen RN, Speksnijder CM, Helders PJ. The effectiveness of graded activity in patients with non‐specific low‐back pain: a systematic review. Disability Rehabilitation 2012;34:1070‐6. [DOI] [PubMed] [Google Scholar]
van Ravensteijn 2013
- Ravensteijn H, Lucassen P, Bor H, Weel C, Speckens A. Mindfulness‐based cognitive therapy for patients with medically unexplained symptoms: a randomized controlled trial. Psychotherapy and Psychosomatics 2013;82:299‐310. [DOI] [PubMed] [Google Scholar]
Ware 1992
- Ware JE, Sherbourne CD. The RAND‐36 Short‐form Health Status Survey: 1. Conceptual framework and item selection. Medical Care 1992;30(6):473‐81. [PubMed] [Google Scholar]
Weyerer 1994
- Weyerer S, Kupfer B. Physical exercise and psychological health. Sports Medicine 1994;17(2):108‐16. [DOI] [PubMed] [Google Scholar]
WHO 1975
- World Health Organization. International Statistical Classification of Diseases and Related Health Problems. 1st Edition. Geneva: World Health Organization, 1975. [Google Scholar]
WHO 1990
- World Health Organization. Composite International Diagnostic Interview. 1st Edition. Geneva: World Health Organization, 1990. [Google Scholar]
WHO 2004
- World Health Organization. International Statistical Classification of Diseases and Related Health Problems. 2nd Edition. Vol. 1, Geneva: World Health Organization, 2004. [Google Scholar]
Yancey 2012
- Yancey JR, Thomas SM. Chronic fatigue syndrome: diagnosis and treatment. American Family Physician 2012;86(8):741‐6. [PubMed] [Google Scholar]
Zaby 2008
- Zaby A, Heider J, Schröder A. Waiting, relaxation, or cognitive‐behavioral therapy ‐ how effective is outpatient group therapy for somatoform symptoms? [Warten, Entspannung oder Verhaltenstherapie. Wie effektiv sind ambulante Gruppenbehandlungen bei multiplen somatoformen Symptomen]. Zeitschrift für Klinische Psychologie und Psychotherapie 2008;37:15‐23. [Google Scholar]
Zigmond 1983
- Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatrica Scandinavia 1983;67:361‐70. [DOI] [PubMed] [Google Scholar]
Zijdenbos 2009
- Zijdenbos IL, Wit NJ, Heijden GJ, Rubin G, Quartero AO. Psychological treatments for the management of irritable bowel syndrome. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD006442.pub2] [DOI] [PubMed] [Google Scholar]
Zoccolillo 1986
- Zoccolillo M, Cloninger CR. Somatization disorder: psychologic symptoms, social disability, and diagnosis. Comprehensive Psychiatry 1986;27(1):65‐73. [DOI] [PubMed] [Google Scholar]


