ABSTRACT
Two Wolbachia strains, wMel and wAlbB, have been transinfected into Aedes aegypti mosquitoes for population replacement with the aim of reducing dengue transmission. Epidemiological data from various endemic sites suggest a pronounced decrease in dengue transmission after implementing this strategy. In this study, we investigated the impact of the Wolbachia strains wMel and wAlbB on Ae. aegypti fitness in a common genetic background. We found that Ae. aegypti females infected with the wMel strain exhibited several significant differences compared with those infected with the wAlbB strain. Specifically, wMel-infected females laid significantly fewer eggs, ingested a lower amount of blood, had a reduced egg production rate, and exhibited a decreased Wolbachia density at a later age compared with mosquitoes infected with the wAlbB strain. Conversely, the wAlbB strain showed only mild negative effects when compared with Wolbachia-uninfected specimens. These differential effects on Ae. aegypti fitness following infection with either wMel or wAlbB may have important implications for the success of population replacement strategies in invading native Ae. aegypti populations in endemic settings. Further research is needed to better understand the underlying mechanisms responsible for these differences in fitness effects and their potential impact on the long-term efficacy of Wolbachia-based dengue control programs.
IMPORTANCE
The transmission of arboviruses such as dengue, Zika, and chikungunya is on the rise globally. Among the most promising strategies to reduce arbovirus burden is the release of one out of two strains of Wolbachia-infected Aedes aegypti: wMel and wAlbB. One critical aspect of whether this approach will succeed involves the fitness cost of either Wolbachia strains on mosquito life history traits. For instance, we found that wMel-infected Ae. aegypti females laid significantly fewer eggs, ingested a lower amount of blood, had a reduced egg production rate, and exhibited a decreased Wolbachia density at a later age compared with mosquitoes infected with the wAlbB strain. Conversely, the wAlbB strain showed only mild negative effects when compared with Wolbachia-uninfected specimens. These differential effects on mosquito fitness following infection with either wMel or wAlbB may have important implications for the success of population replacement strategies in invading native Ae. aegypti populations.
KEYWORDS: Aedes aegypti, Wolbachia, vectorial capacity, disease transmission, blocking, life history traits
INTRODUCTION
Arthropods usually host a wide variety of microorganisms, some of which live in an intimate and long-term biological interaction with their host. The interactions between insects and viruses, bacteria, and fungi can be classified into a gradient ranging from benefits to both parties involving mutualism (1) to antagonistic interactions resulting in parasitism (2, 3), both of which are driven by evolutionary changes in the hosts and the microbes (4, 5). Microorganisms are important modulators of host phenotypes, providing heritable variation acted upon by natural selection (6). Host-parasite interactions represent one of the strongest selection pressures in nature, with considerable impact on the ecology and evolution of the microbes and thus on disease epidemiology (7). These biological interactions in a changing environment form part of a complex ecosystem shaping disease transmission.
Due to epidemiological impacts on human health, it is of paramount importance to understand host-parasite interactions within the context of vector-borne diseases. Among mosquitoes, special interest has been given to Aedes aegypti, the primary vector of arboviruses such as dengue (DENV), Zika (ZIKV), and chikungunya (CHIKV). DENV is organized in four distinct serotypes (DENV-1, -2, -3, and -4), with an estimated infection of 390 million people per year globally (8–10). In 2013, ZIKV rapidly disseminated from the Pacific islands to Southern America and became a global public health emergence due to its association with microcephaly in newborns (11). At least two CHIKV lineages have spread over the world (ECSA—the East-Central-South Africa and the Asian lineage), causing severe outbreaks in sites where Ae. aegypti was abundant (12, 13).
In the absence of widely applicable, effective vaccines for most arboviruses, mitigation efforts rely on suppressing and maintaining vector populations below a threshold at which outbreaks are unlikely to occur (14). Traditional vector control methods have had limited success in managing arbovirus outbreaks, due to factors such as widespread insecticide resistance in vector populations and the huge effort required in targeting key breeding sites in urban settings in the long term (15–17). Therefore, new strategies to supplement traditional vector control methods need to be developed to manage mosquito-borne diseases. One innovative approach currently undertaken in at least 14 countries is the release of Ae. aegypti carrying Wolbachia, an endosymbiont naturally present in around 40% of insect species but naturally absent from Ae. aegypti (18). Wolbachia can be successfully established in Ae. aegypti using embryo cytoplasm microinjection (19). So far, two artificially Wolbachia-infected strains of Ae. aegypti have been released in the field: wMel (transinfected from Drosophila melanogaster) and wAlbB (transinfected from Ae. albopictus). Wolbachia strains in Ae. aegypti can induce density-dependent blocking of DENV, ZIKV, and CHIKV, which is favored by the high density of this bacterium in salivary glands (20–22). The spread of Wolbachia in the mosquito population following release is facilitated by two attributes: maternal transmission to offspring, coupled with cytoplasmic incompatibility, wherein Wolbachia-infected males induce sterility in wild females, producing a frequency-dependent fitness advantage for Wolbachia-infected females (23, 24). So far, wMel or wAlbB strains have been released in countries such as Australia, Brazil, Indonesia, Malaysia, and Vietnam to replace natural populations highly competent to arbovirus by Wolbachia-infected specimens with reduced vector competence (25–28).
Although available data point to a reduction in dengue incidence due to Wolbachia deployment (28–30), a number of important issues remain unresolved regarding both the short- and long-term interaction between the bacterium and Ae. aegypti mosquitoes. For example, Wolbachia is known to affect invertebrate fitness and reproduction and generate several physiological changes in mosquitoes (31–34), but so far, there are limited data on how the released strains (wMel and wAlbB) affect Ae. aegypti reproductive strategies. Trade-offs within an organism’s life history result from allocation of a fixed resource budget among growth, survival, and reproduction (35, 36). For instance, wMel-infected Ae. aegypti have a significant decrease in egg hatching, which could likely limit the spread of this strain within a native Ae. aegypti population (26, 37). There remains a gap in understanding whether the two Wolbachia strains currently deployed can impact Ae. aegypti reproductive tactics, particularly on genetic backgrounds from areas where the strategy is being deployed. Therefore, this study aimed to analyze the effect of Wolbachia infection on Ae. aegypti with a Brazilian genetic background for different fitness-related mosquito traits, including wing length, wing shape, blood meal size, and fecundity.
MATERIALS AND METHODS
Wild mosquitoes and backcrossing
We used three Ae. aegypti strains in this study. To represent a Wolbachia-uninfected field population, we sampled eggs in the neighborhood of Urca (22°56′44″S, 43°09′42″W). Previous reports highlighted that insecticide resistance in Wolbachia-carrying mosquitoes (wMel strain) plays a role in the bacterium being able to invade into wild Ae. aegypti populations. Mosquitoes from Rio de Janeiro are resistant to pyrethroids, and insecticide resistance is a widely spread phenomenon in Brazil (16, 26, 38–41), highlighting the importance of controlling the genetic background. Urca is located 12 km away from the southern geographic limit of the area in which Wolbachia has been released in Rio de Janeiro. Thus, Wolbachia-infected individuals are not expected to be present in this neighborhood (42). We sampled eggs in Urca using 60 ovitraps with wooden paddles replaced weekly for ~2 months until we obtained a minimum of 10,000 eggs to capture local genetic diversity. Eggs were hatched in plastic containers with 3 L of tap water and yeast in the week following their collection in the field. Larvae were fed daily with fish food (4.5 mg) until the pupae stage. Pupae were transferred to 30 × 30 × 30 cm cages (BugDorms, Taichung, Taiwan) to allow adult emergence and mating. Two cages with at least 500 Ae. aegypti females in each were established, with females blood fed twice a week to obtain eggs.
The wMel and wAlbB strains were originally created through microinjection into Ae. aegypti eggs of an Australian background (43, 44). By backcrossing Ae. aegypti females from the lines to field-collected males for five consecutive generations, we ensured that the material used in experiments has a similar genetic background to those of field mosquitoes, allowing us to isolate phenotypic effects specifically associated with Wolbachia. After colonies were closed, i.e., when the backcrossing was concluded, genetic variation in the colonies of Wolbachia lines and genetic similarity to the Urca population was maintained by ensuring that around 50% of males were from the Wolbachia-uninfected Urca-derived colony every two generations (26). By doing so, we refreshed the genetic background of infected colonies, ensuring that mosquitoes from the three strains have a similar genetic background. All colonies were maintained with around 500 females each in an insectary at 27 ± 2°C, 14:10 light:dark photoperiod, >60% relative humidity. For the experiments reported herein, we used eggs derived from two independent cages of each colony, i.e., in duplicate. The egg hatching and larval rearing methods were the same as stated above.
Blood feeding
Adult mosquitoes were supplied with 8% sucrose diet ad libitum until 24 h before blood feeding. A total of six cages was used in this experiment, two for each mosquito strain, with males and females mixed from the two cages. Each cage had approximately 250 Ae. aegypti females. A blood meal was offered for one cage of each strain when Ae. aegypti females were approximately 1 week old (7–8 days after emergence). Females in the second cage of a strain received their first blood meal when they were 3 weeks old (21–23 days after emergence). Given what is known about Ae. aegypti reproductive biology, we expected that after 1 week, all females would have been inseminated. Blood feeding was carried out with expired human blood group 0 purchased as blood bags from local blood banks. The blood donor’s private information is unknown, i.e., ethical approval is not required. The blood was offered with a Hemotek Membrane Feeding System (Hemotek Ltd., Blackburn, UK), and females were allowed to feed for half an hour. Visually completely engorged females were individualized in 50-mL plastic vials containing moistened cotton overlaid with filter paper as oviposition substrate on the bottom and a sugar solution 8% ad libitum on the top. Tubes were covered on the top with mosquito netting.
Blood meal size and egg production
Females remained inside the vials for 1 week after blood feeding in the same insectary conditions as the colonies described above. After this period, mosquitoes were killed by freezing and stored for wing removal and Wolbachia quantification. Eggs laid on the filter paper were counted with the aid of a stereomicroscope at 10× magnification (Leica M205 C, Leica Microsystems, Wetzlar, Germany), recorded, and later discarded for estimating the blood meal size. We measured the blood meal size by quantifying the hematin from mosquito feces on the filter paper. The filter papers were added to a 2-mL cuvette (Sarstedt, Nümbrecht, Germany) containing 1 mL of a 1% lithium carbonate (Sigma Aldrich, Burlington, USA) solution to dilute the feces. A standard line was prepared by diluting known amounts of blood (0, 0.8, 1.6, 2.4, and 3.2 µL) and measuring the corresponding hematin in a spectrophotometer (Thermo BioMate 3) with an absorbance at 387 nM (45–48). The standard line had an R2 of 0.9832 in a linear regression. By dividing the number of eggs laid per female by the amount of blood ingested (transformed using the standard), we were able to evaluate the individual efficiency of egg production with a known amount of blood, a ratio expressed as eggs/ug.
Wing shape and length
The right and left wings of each specimen were removed and mounted under a cover slip (15 × 15 mm) with Euparal (Carl Roth, Karlsruhe, Germany). Pictures of each wing were taken with a stereomicroscope (as above) at 20× magnification. Fiji (49), a bioscience package of ImageJ (50), was used to digitize 18 landmarks. The landmark selection was in accordance with other studies using geometric morphometrics to analyze the wing shape of mosquitoes (51–53). The wing length was measured as the distance from the axillary incision to the apical margin excluding the fringe (54).
Wolbachia quantification
Wolbachia was quantified on the whole body of each specimen without the wings. Wolbachia DNA was extracted with a DNeasy Blood & Tissue Kits (Qiagen, Hilden, Germany), following the manufacturer’s instructions and previous experiments (44, 55, 56). Detection of Wolbachia wMel strain was based on amplification of the WD0513 gene. The following primers were used to amplify a fragment of 110 bp: TM513-F: 5′-CAAATTGCTCTTGTCCTGTGG-3′ and TM513-R: 5′-GGGTGTTAAGCAGAGTTACGG-3′, and the probe 5′-FAM-TGAAATGGAAAAATTGGCGAGGTGTAGG-3BHQ1-3′. In the same reaction, a ribosomal gene from Ae. aegypti (RPS17) with the length of 68 bp was amplified with the two primers: RPS17-F: 5′- TCCGTGGTATCTCCATCAAGCT-3′ and RPS-R: 5′- CACTTCCGGCACGTAGTTGTC-3′ and the probe RPS17: 5′-HEX-CAGGAGGAGGAACGTGAGCGCAG-BHQ1-3′. The amplification was carried out on a Qiagen Rotor-Gene Q using Taqman Universal PCR Master Mix (Thermo Scientific, Waltham, USA) following the manufacturer’s instructions. The relative quantification of wMel strain relative quantification was performed according the procedure described elsewhere (55). The Wolbachia wAlbB strain was detected by high-resolution melting polymerase chain reaction (qPCR-HRM) (56) with 1:10 diluted DNA using the following wAlbB1-specifc primers: wAlbB1-F (5′-CCTTACCTCCTGCACAACAA-3′) and wAlbB1-R (5′-GGATTGTCCAGTGGCCTTA-3′), as well as universal mosquito primers: mRpS6_F (5′-AGTTGAACGTATCGTTTCCCGCTAC-3′) and mRpS6_R (5′-GAAGTGACGCAGCTTGTGGTCGTCC-3′), which target the conserved region of the RpS6 gene, and Ae. aegypti primers aRpS6-F (5′-ATCAAGAAGCGCCGTGTCG-3′) and aRpS6-R (5′-CAGGTGCAGGATCTTCATGTATTCG-3′), which target the Ae. aegypti-specific polymorphisms within RpS6 and do not amplify Ae. albopictus. Reactions were run as 384-well plates in a LightCycler 480 II (Roche, Basel, Switzerland). qPCR-HRM was performed following the same cycling conditions as described elsewhere (28). Samples were considered positive for Wolbachia when the Tm for the amplicon produced by the Ae. aegypti primers was at least 84°C and the Tm for the Wolbachia-primer amplicon was around 80°C. Differences between the Crossing points (Cp) of the Wolbachia and Ae. aegypti markers were transformed by 2n to obtain approximate estimates of Wolbachia density. Negative and positive controls were used in all reactions as non-infected strain and lab colony samples that tested positive in previous assays (37, 57).
Data analysis
The data set comprised six variables: number of eggs (count data); wing length, blood meal size, and Wolbachia density (continuous variables); age (1st or 3rd week); and strain (Wolbachia-uninfected, wMel, and wAlbB) as categorical variables. Variables were treated as response or explanatory variables depending on the research hypothesis tested. Depending on the normality of the response variable, comparisons within categories of interest were performed using one-way analysis of variance (ANOVA), followed by Tukey-Kramer post test, or Kruskal Wallis (KW) followed by pairwise Wilcoxon test when the null hypothesis of equal means or medians across treatments was rejected. In both cases, the Bonferroni-adjusted significance level was adopted. To verify the effects of explanatory variables on the response variables with count or continuous data, we performed Generalized Linear Model (GLM) analyses and followed a model selection approach. The GLMs were developed as follows. First, we tested the normality and homoscedasticity of variances within the count and continuous variables using the Shapiro-Wilk test. The family distribution of the response variable was assessed through the Cullen and Fray graph, and dispersion was tested using the “RT4Bio” and “fitdistrplus” packages [v1.1; (58, 59)]. The effects of Wolbachia density (for the wMel and wAlbB groups), blood meal size, wing length, female age, and strain on the number of eggs laid per Ae. aegypti female were analyzed with GLM with a negative binomial distribution. This distribution was preferred over the traditionally used Poisson distribution, because data exhibited overdispersion (i.e., variance was larger than the mean), confirmed by Pearson’s chi-squared test and dispersion statistic > 1. Blood meal size and Wolbachia density as response variable were analyzed by GLMs with a Gaussian distribution. The variance inflation factor of the models were accessed using the “vcd” package [v1.4–11 (60)]. Variables were removed when multicollinearity was detected, i.e., when GVIF > 5. The most informative and parsimonious model was selected through delta Akaike’s Information Criteria scores corrected for small sample sizes (ΔAICc). Akaike weights were used to assess the uncertainty of model selection, which quantifies the probability that the model is the best among all models built for that response variable (61, 62). For each set of models created for a given response variable, we selected the best model(s) for interpretation of its parameters if ΔAICc was less than 2.0 (61, 62). Multicollinearity was checked again in the best model. Finally, the assumptions of the best model were examined by checking heteroscedasticity, residual dispersion, and the presence of outliers with the package “DHARMa” [v.0.4.6 (63)]. All analyses were done in the R environment (64).
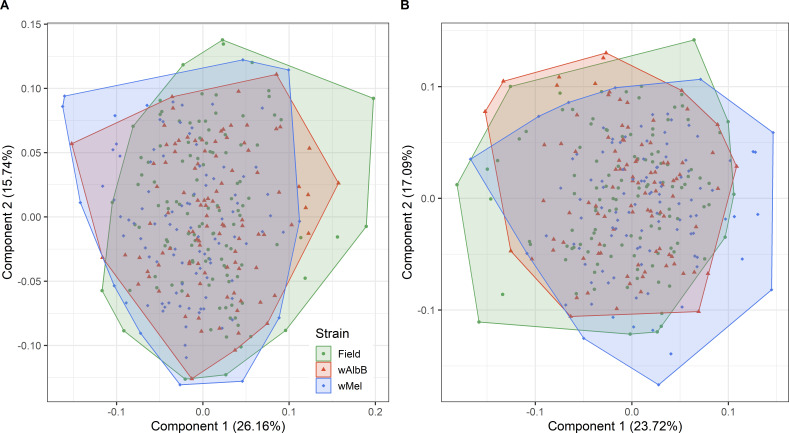
Path analysis or Structural Equation Modeling (SEM) is a multivariate regression technique that allows the direction and magnitude of each of the direct effects (path coefficients) on the response variable (65) to be obtained. The main purpose of SEM is to confirm an agreement between specific causal hypotheses and empirical data, which is assessed through a goodness-of-fit statistic between the observed and expected correlations (65, 66). We established a causal relation model to assess whether Ae. aegypti age, wing length, blood meal size, and Wolbachia density (except for field strain) affect the number of eggs laid by female mosquitoes (Fig. 1). A principal component analysis (PCA) using the package “psych” in R (67) showed the data differ among the three strains, with no overlapping clusters between Wolbachia-infected strains and the Wolbachia-uninfected strain (Fig. S1). Therefore, we built a path diagram based on the SEM approach for each strain. Our global model used mosquito age as an exogenous variable and wing length, blood meal size, Wolbachia density (when possible), and the number of eggs as endogenous variables. Alternative path diagrams were tested by comparing coefficients of non-determination from reduced models to those of the full model using the same model selection approach described above. Total-effect coefficients (the sum of the direct and indirect effects of one variable on another) were calculated for each of the endogenous variables in the path diagram. We assessed the model fit of the selected path diagram for each strain using the following model fit index: chi-square test (χ2), Comparative Fit Index (CFI), Tucker-Lewis Index (TLI), Root Mean Square Error of Approximation (RMSEA), and the Standardized Root Mean Square Residual (SRMSR). All SEM analyses were done in R with the “lavaan” package [v 0.6–15 (64, 68)].
Fig 1.
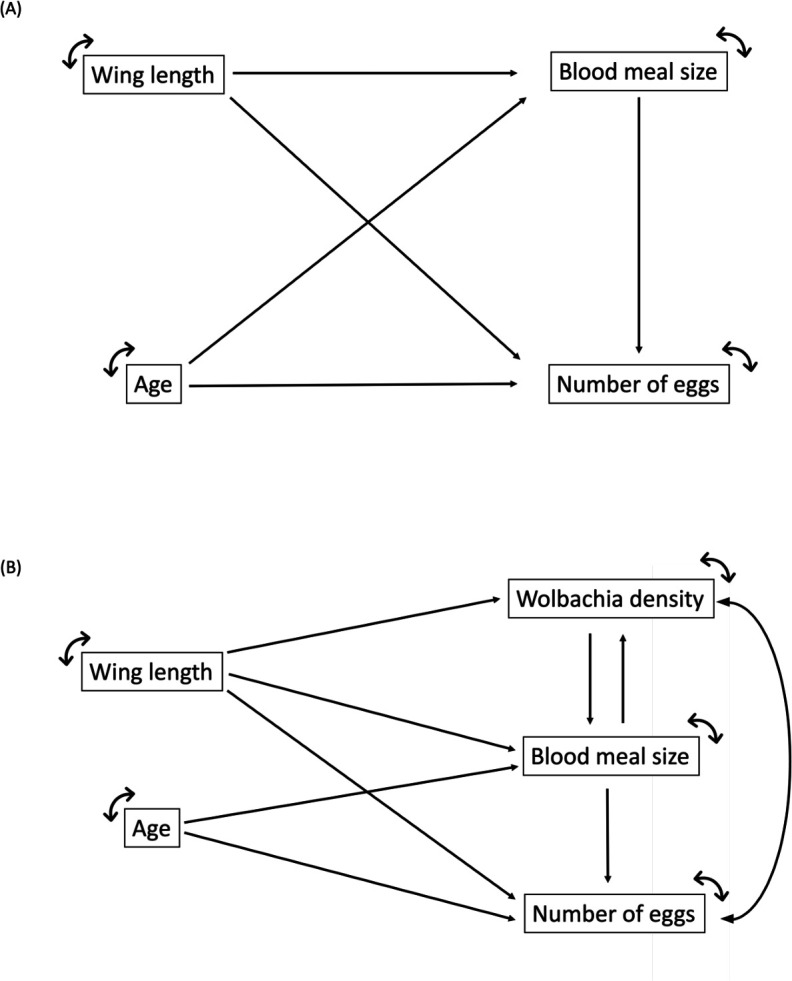
Hypothesized path diagram considering Ae. aegypti age, wing length, blood meal size, and the number of eggs. (A) The Wolbachia-uninfected field strain; (B) the two strains with Wolbachia (wMel and wAlbB strains). The notations of the path diagram are as follows: age is an exogenous variable, whereas wing length, blood meal size, Wolbachia density, and the number of eggs are endogenous variables. The arrows represent the directional relationship, and circled double arrows represent the variance and the residual errors of exogenous and endogenous variables, respectively.
The two-dimensional landmark coordinates of the 18 vein crosses were used to analyze the wing shape of the mosquitoes. The landmark coordinates were aligned by a Generalized Procrustes Analysis using the R package geomorph (version 4.0.1) (69). The wing shape variation between specimens was visualized with a PCA. The differences in the wing shape between the three strains (Urca, wMel, and wAlbB) were statistically compared by a Procrustes ANOVA using the proc.D.lm function (with 1,000 permutations). In addition, we calculated the strain-specific morphological disparity with the “morphol.disparity” function. This function calculates the Procrustes variance for the three different strains using the residuals of a linear model and can, thus, provide information about morphological diversity within the strains. The statistical significance of the calculated Procrustes variance between the three strains was checked pairwise using a randomized permutation test with 499 iterations. All wing shape analyses were conducted separately for the right and the left wing to check the consistency of the results and to avoid duplicated measurements per specimen in the same analysis (70).
RESULTS
Only three mosquitoes died over the period that insects were monitored. Thus, we monitored the blood meal size, wing length, and number of eggs in a total of 417 Ae. aegypti females, 140 from the Wolbachia-free strain, 139 from wMel, and 138 from wAlbB. The three deaths were recorded in mosquitoes that were blood fed in their 3rd week post emergence. All three strains had 75 Ae. aegypti females blood feeding in their 1st week, whereas in the 3rd week, we had 65 insects from the Wolbachia-free strain, 64 from wMel, and 63 from wAlbB.
Number of eggs
From the 417 Ae. aegypti females, 47 (11.2%) females had not laid any eggs 1 week after blood feeding (8 from the field, 21 from wMel, and 18 from wAlbB strain). The maximum number of eggs laid per female was 134. The number of eggs laid by Ae. aegypti females varied significantly among strains (KW: χ2: 65.56, df = 2, P < 0.001), but not with age (KW: χ2: 2.88, df = 1, P = 0.089) (Fig. 2A), with Ae. aegypti females from wMel laying significantly fewer eggs than the field Wolbachia-uninfected strain (P < 0.001). There was no detectable difference between the number of eggs laid by wAlbB and the field strain (P = 0.21).
Fig 2.
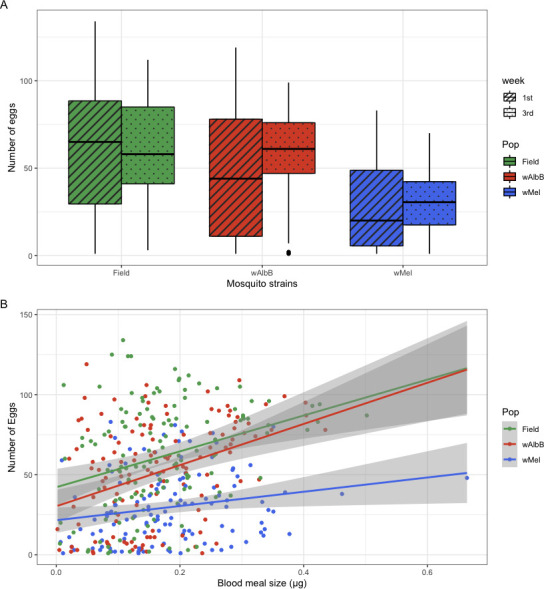
Fecundity of Aedes aegypti females from the three tested strains and its association with (A) age in weeks and (B) blood meal size. The shaded area in B represents the 95% confidence level interval for predictions from a linear model.
When we used a GLM with a negative binomial distribution to examine various effects on the number of eggs laid by Ae. aegypti females, both infected strains had lower fecundity than the Wolbachia-uninfected field strain (Table 1). The blood meal size was positively associated with the number of eggs laid (Table 1). However, the strength of the association was not the same among the three strains, with wAlbB mosquitoes presenting a trend similar to females from the uninfected strain and wMel mosquitoes showing a weaker association than for the other two strains (Fig. 2B).
TABLE 1.
Results of the Generalized Linear Model (negative binomial) of the number of eggs laid by Aedes aegypti females from three different strains
| Term | Estimate | SE | z-value | P valuea |
|---|---|---|---|---|
| Strain (wAlbB) | −0.225 | 0.0968 | 2.946 | 0.0601 |
| Strain (wMel) | −0.715 | 0.0956 | −2.325 | < 0.001 |
| Age (3rd) | −0.129 | 0.0879 | −7.484 | 0.1403 |
| Blood meal size | 1.951 | 0.4699 | −1.475 | < 0.001 |
| Wing length | 0.695 | 0.2417 | 2.877 | 0.0040 |
P values under 0.05 are bold.
Blood meal size
We estimated the blood meal size by quantifying the hematin present on the filter paper. Aedes aegypti females from the three strains ingested more blood when fed in the 3rd than in the 1st week (ANOVA, F1,392 = 106.6, P < 0.001) (Fig. 3A). However, the amount of blood ingested did not vary according to the strain (ANOVA, F1,391 = 1.169, P = 0.312).
Fig 3.
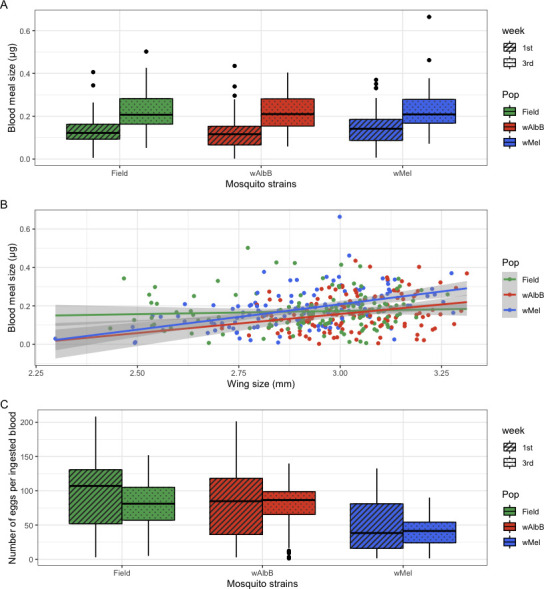
The effects of explanatory variables on the blood meal size of Aedes aegypti mosquitoes from three different populations. (A) Blood meal size variation according to mosquito strain, (B) effects of wing length on the amount of blood ingested by Aedes aegypti females, (C) egg production based on the amount of blood meal size by Aedes aegypti from a Wolbachia-uninfected field population, wAlbB, and wMel strains, expressed as egg/μg. The shaded area in B represents the 95% confidence level interval for predictions from a linear model.
The best Gaussian GLM considering tblood meal size as the response variable involved no effect of the mosquito strain on the amount of blood ingested by Ae. aegypti females. The wing length of mosquitoes was positively correlated with blood meal size (Table 2), although the association varied among strains (Fig. 3B). It is worth noting that the Wolbachia-free strain ingested similar amounts of blood regardless of wing length, whereas Wolbachia-infected groups behaved similarly, with wMel and wAlbB females ingesting more blood when they were bigger. However, the egg production rate significantly varied among strains (KW: χ2: 73.71, df = 2, P < 0.001), with Ae. aegypti infected by the wMel strain producing less egg per blood ingested when compared with wAlbB and the Wolbachia-uninfected strain (P < 0.001 for both paired comparison). The Wolbachia-uninfected and wAlbB strains had similar egg production (P = 0.12). When combined with fecundity and blood meal size variation within age and mosquito strain, the results show that mosquitoes had similar fecundity over ages tested but ingested more blood in the 3rd week, with an overall loss in egg production relative to the amount of blood ingested (Fig. 3C). These loss effects were stronger in the wMel females.
TABLE 2.
Results of the Generalized Linear Model (Gaussian) of the blood meal size of Aedes aegypti females from three different strains
| Term | Estimate | SE | t-value | P valuea |
|---|---|---|---|---|
| Strain (wAlbB) | 0.6295 | 0.3844 | 1.637 | 0.1023 |
| Strain (wMel) | −0.0552 | 0.2857 | −0.193 | 0.8468 |
| Age (3rd) | 0.2931 | 0.1459 | 2.009 | 0.0452 |
| Wing | 0.2087 | 0.0794 | 2.628 | 0.0089 |
| Strain(wAlbB)*wing | −0.2142 | 0.1284 | −1.668 | 0.0961 |
| Strain(wMel)*wing | 0.0384 | 0.0938 | 0.391 | 0.6960 |
| Age(3rd)*wing | −0.0913 | 0.0499 | −1.828 | 0.0683 |
P values under 0.05 are bold.
Wolbachia density
The Wolbachia density was quantified through Reverse transcription-quantitative polymerase chain reaction (RT-qPCR), and wAlbB was present in higher densities in Ae. aegypti mosquitoes than wMel (ANOVA, F1,276 = 251.8, P < 0.001) (Fig. 4A). At the later age tested, the density of wAlbB increased, but for wMel, it decreased (ANOVA, F1,276 = 8.938, P = 0.003) (Fig. 4B). The best model considering Wolbachia density as the response variable included an influence of mosquito strain, age, and an interaction between these two independent variables (Table 3).
Fig 4.
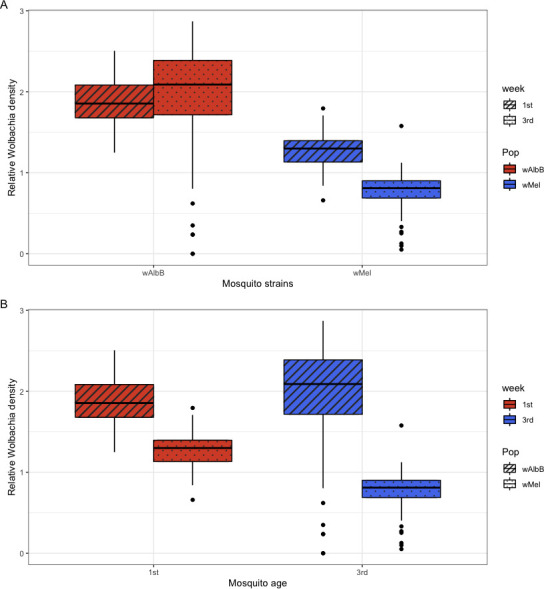
The effects of explanatory variables on Wolbachia density considering (A) mosquito populations and (B) mosquito age.
TABLE 3.
Results of the Generalized Linear Model (Gaussian) of the Wolbachia density of Aedes aegypti females from two different strains (wMel and wAlbB)
| Term | Estimate | SE | t-value | P valuea |
|---|---|---|---|---|
| Strain (wMel) | −4.5889 | 2.0129 | −2.280 | 0.0234 |
| Age (3rd) | 0.1437 | 0.0826 | 1.739 | 0.0832 |
| Wing length | −1.1810 | 0.5569 | −2.121 | 0.0349 |
| Blood meal size | −19.471 | 9.5248 | −2.044 | 0.0419 |
| Strain(wMel)*age (3rd) | −0.6838 | 0.1161 | −5.890 | < 0.001 |
| Strain(wMel)*wing | 1.3145 | 0.6765 | 1.943 | 0.0531 |
| Strain(wMel)*blood meal size | 19.276 | 11.3378 | 1.700 | 0.0903 |
| wing*blood meal size | 6.3156 | 3.0957 | 2.040 | 0.0424 |
| Strain(wMel)*wing*blood meal size | −6.1904 | 3.7341 | −1.658 | 0.0986 |
P values under 0.05 are bold.
Wing size and shape
The wing size varied among strains (KW: χ2: 38.65, df = 2, P < 0.001), with wAlbB bigger than wMel (P < 0.001) and the Wolbachia-uninfected strain (P < 0.001). A marginally non-significant difference was observed between the wing length of wMel and Wolbachia-uninfected mosquitoes (P = 0.053). The shape of the left wings did not differ significantly between the three strains (ANOVA, F2,316 = 1.5171, R2 = 0.0095, P = 0.078). In contrast, a small but significant difference was observed for the right wings of the three strains (ANOVA, F2,319 = 5.358, R2 = 0.0325, P < 0.001) (Fig. 5). The Procrustes variance of the right wings was 0.00125 for the Wolbachia-uninfected strain, 0.00123 for the wAlbB-infected strain, and 0.00126 for the wMel-infected strain. For the left wings, the Procrustes variance was 0.00122 for the field strain, 0.00119 for the wAlbB-infected strain, and 0.00119 for the wMel-infected strain. For both wing sides, pairwise comparisons of the Procrustes variance between the three strains did not reveal statistically significant differences, indicating a similar morphological diversity in the wings of the three strains (Fig. 5).
Fig 5.
Principal component analysis showing the wing shape variation of the specimens in the left wings (A) and right wings (B). Green points indicate the specimens from the field population. Red triangles indicate the wAlbB-infected population, and blue squares indicate the wMel-infected population.
Path analysis
The full model for each strain was classified as overidentified, i.e., with degrees of freedom ≥ 1 (Fig. S2). However, the path diagram of the best model differed for each strain. The best model for the Wolbachia-uninfected field strain was the full model, whereas for wMel and wAlbB, the best models were reduced versions of the full model, suggesting that metabolic pathways of egg production might be indirectly affected by Wolbachia presence (Fig. 6; Table 4). When we identified the best path models, some of the variables included in the full model were omitted for wMel and wAlbB. Fecundity in Wolbachia-uninfected mosquitoes was positively correlated with blood meal size and wing length but decreased with Ae. aegypti at an older age. By comparing the path diagrams of wMel and wAlbB mosquitoes, we observed a positive statistically significant effect of wing length and age on blood meal size for both strains but only in wMel did the blood meal size significantly affect fecundity. Unlike for uninfected specimens, females carrying either wMel or wAlbB showed a non-significant effect of wing size on fecundity. Regarding the presence of Wolbachia, the most evident difference between wMel and wAlbB was their effect on fecundity: a positive relation regarding wAlbB and a negative relationship for wMel. On the other hand, in both path diagrams, the effects of wing length and blood meal size on Wolbachia density was removed to increase model fit.
Fig 6.
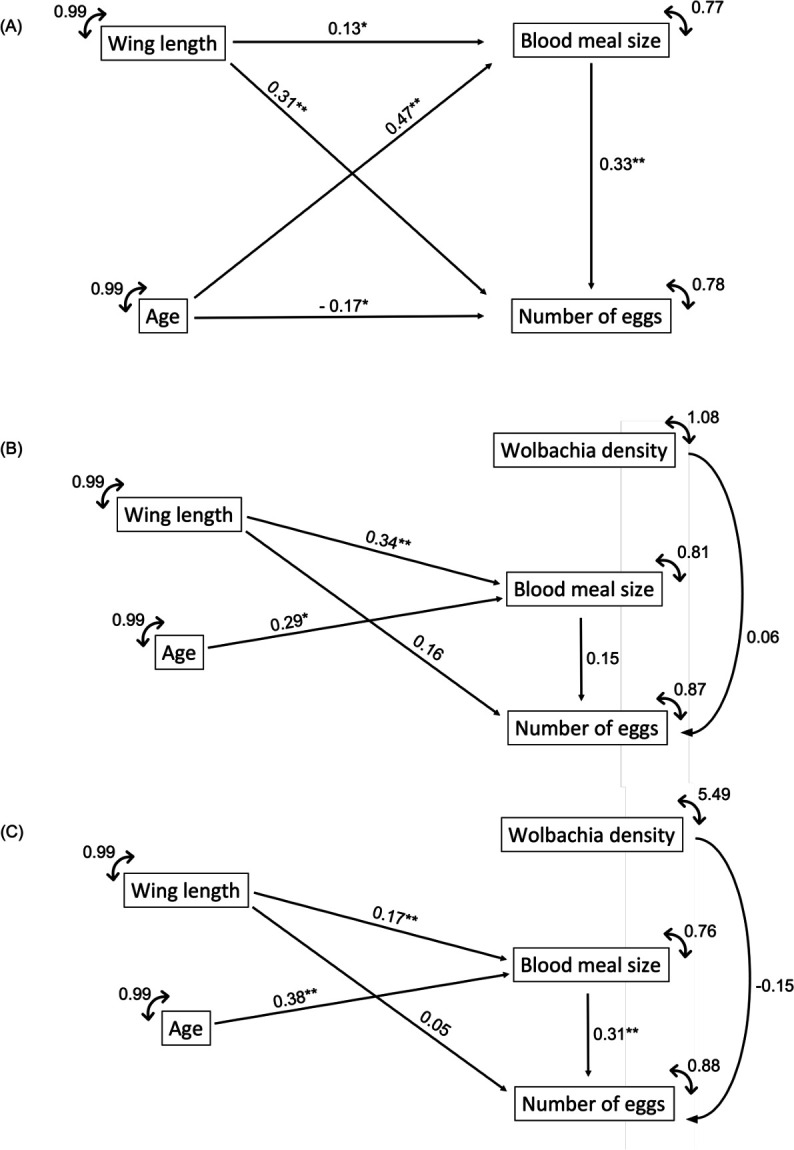
Best model path diagrams for (A) Wolbachia-uninfected field population, (B) wAlbB, and (C) wMel. Numbers over the arrows are the path coefficients and their statistical significance is indicated as follows: *P < 0.05 and **P < 0.01.
TABLE 4.
Model fit of full (initial model) and the simplified best path model determining the number of eggs of Aedes aegypti females carrying one of the two Wolbachia strains (wMel or wAlbB) or in the absence of this bacteriuma
| Population | Structural equation model | df | χ2 | CFI | TLI | RMSEA | SRMSR | AIC | ΔAIC |
|---|---|---|---|---|---|---|---|---|---|
| Field (full = best model) | EGG ~ AGE + WNG + BLD, BLD ~ AGE + WNG, AGE ~~ AGE, WNG ~~ WNG, BLD ~~ BLD, EGG ~~ EGG | 1 | 0.143 | 0.981 | 0.88 | 0.093 | 0.048 | 1,446.97 | - |
| wMel (full model) | EGG ~ AGE + WNG + BLD + WB, BLD ~ AGE + WNG + WB, WB ~ WNG + BLD, AGE ~~ AGE, BLD ~~ BLD, EGG ~~ EGG, WB ~~ WB | 1 | <0.001 | 0.877 | 0.23 | 0.395 | 0.144 | 1,530.97 | - |
| wMel (best model) | EGG ~ WNG + BLD + WB, BLD ~ AGE + WNG, BLD ~~ BLD, EGG ~~ EGG | 2 | 0.065 | 0.922 | 0.726 | 0.122 | 0.034 | 624.57 | 906.4 |
| wAlbB (full model) | EGG ~ AGE + WNG + BLD + WB, BLD ~ AGE + WNG + WB, WB ~ WNG + BLD, AGE ~~ AGE, BLD ~~ BLD, EGG ~~ EGG, WB ~~ WB | 1 | <0.001 | 0.669 | 0.238 | 0.399 | 0.112 | 1,636.52 | - |
| wAlbB (best model) | EGG ~ WNG + BLD + WB, BLD ~ AGE + WNG, BLD ~~ BLD, EGG ~~ EGG | 2 | 0.494 | 0.988 | 0.954 | <0.001 | 0.024 | 632.06 | 1,004.46 |
EGG, number of eggs; AGE, age of mosquito when blood fed; WNG, wing length; BLD, blood meal size; WB, Wolbachia density; χ2, chi-square; CFI, Comparative Fit Index; TLI, Tucker-Lewis Index; RMSEA, Root Mean Square Error of Approximation; SRMSR, Standardized Root Mean Square Residual; AIC, Akaike Information Criteria; DAIC, difference in AIC between the full and best models.
DISCUSSION
The use of Wolbachia to mitigate arbovirus transmission is on the rise in the last few years, with approximately 15 countries conducting release interventions simultaneously. Wolbachia population replacement and suppression are influenced by the Wolbachia strain considered, mosquito host factors, and environmental variables (24, 71–73). Nowadays, there are two Wolbachia strains transinfected into Ae. aegypti mosquitoes that are used for population replacement, and for both strains, data suggest a significant reduction in dengue transmission (28, 30, 74). In general, Wolbachia infection in Ae. aegypti causes a decrease in host fecundity and egg hatching, likely affecting the long-term Wolbachia stability under some specific field conditions, particularly those involving high temperatures (37, 75–77). Herein, we adopted a causal relation model followed by structural equation models to determine a path diagram determining how mosquito age, wing length, blood meal size, and Wolbachia density affects Ae. aegypti fecundity. We observed different path diagrams in the presence of wMel and wAlbB, which could potentially affect their success in invading native Ae. aegypti populations in endemic settings (26, 78, 79).
By testing a substantial sample size of three strains of Ae. aegypti (Wolbachia-uninfected Ae. aegypti, wMel-infected mosquitoes, and wAlbB-infected mosquitoes) in a Brazilian genetic background, we compared the impact of both Wolbachia strains on mosquito life history traits. We observed that wMel-infected mosquitoes laid significantly fewer eggs than control mosquitoes, whereas wAlbB-infected Ae. aegypti had a similar fecundity with control insects. There is evidence in the literature for the detrimental effects of both Wolbachia strains on Ae. aegypti fitness on host fecundity and fertility, although these effects are not always consistently observed (26, 34, 37, 75, 80, 81). The presence of Wolbachia may alter metabolic and physiological processes of Ae. aegypti leading to a trade-off impacting mosquito fecundity. For instance, the presence of wMel regulates proteins involved in reactive oxygen species production and regulates humoral immune response and antioxidant production in the ovaries and salivary glands of Ae. aegypti females (32, 33). On the other hand, we have not seen any effect of age on fecundity, although Ae. aegypti mosquitoes have a trend of reducing the number of eggs laid when aging (46, 82–84). One factor that could explain the lack of aging on the number of eggs laid in our experiment is based on the fact mosquitoes only received one single blood meal and were killed 1 week after for egg counting. Many adult mosquito females will take multiple blood meals during their lifespan, resulting in regular exposure to toxins and blood-meal induced oxidative stress through the secretion of proteolytic enzymes and peritrophic matrix components from midgut epithelial cells, uptake of amino acids, oligopeptides, and lipids through membrane-bound transporter proteins (85–87). Thus, blood feeding-induced mortality in mosquitoes is well characterized, and these mortality/feeding effects need to be considered when testing mosquito lifespan impacts.
Blood meal size, or the amount of blood taken in by female mosquitoes, is believed to regulate several aspects of their biology including host-seeking behavior and fecundity. An intriguing pattern observed for all the three strains is that Ae. aegypti females took in larger blood meals when they were 21–23 days old. The effects of aging on Ae. aegypti blood meal size have been explored in at least two studies, and in both of them, the amount of blood ingested over time remained stable (46, 88). A strain infected by Wolbachia wMelPop had decreased blood-feeding success, i.e., increased the number of attempted bites and reduced the blood meal size. Furthermore, a behavior termed as “bendy proboscis” was observed in wMelPop-infected mosquitoes after aging, highlighting potential negative effects of aging on mosquito traits (88). By analyzing the number of eggs produced per microliter of ingested blood (in a ratio expressed as eggs/μg), mosquitoes with either wMel or wAlbB exhibited reduced egg production compared with Wolbachia-uninfected mosquitoes, with a strong effect for wMel and weak effect for wAlbB particularly at 3 weeks. Taking the fecundity and blood meal data together, the loss in egg production in older Wolbachia mosquitoes seems to be mostly due to an increase in blood meal size rather than a decrease in fecundity, since the number of eggs laid was not affected by mosquito age. One important limitation of our study is that aging and senescence are continuous variables and our aging observations were based on only two points of this continuous distribution, when Ae. aegypti females were 7–8 or 21–23 days old. One additional limitation that must be addressed refers to the reduced number of replicates adopted in this experiment. We used eggs derived from two independent cages of each colony. Increasing the number of replicates allows increasing confidence and credibility of the results by reducing the chances of false positives, sampling bias, or measurement error. Thus, our data should be viewed with caution and extrapolations to field must be avoided.
A previous study demonstrated that wing shape can quickly change over just a few generations in Ae. aegypti, suggesting microevolutionary adaption (53). Changes in wing shape can be influenced by various environmental and genetic factors (89). For instance, Jaramillo et al. (90) founded that the wing shape of female Ae. aegypti was correlated with insecticide resistance levels, which was in turn associated with reduced fecundity and survival. In our study, Wolbachia infections had little influence on wing shape variation. In addition, we observed no influence on morphological diversity, i.e., Procrustes variance within the strains. Thus, the wing shape analyses did not indicate a difference in phenotypic variation due to the Wolbachia infection. The extent of Wolbachia-mediated virus blocking is heterogeneous (91, 92) and depends on factors such as virus serotype, host genetic background, and rearing conditions and the method of infection. Thus, methodological differences between studies may produce different outcomes (27, 55, 93–95). Virus blocking has been positively linked to Wolbachia density, with a higher blocking phenotype being verified in specimens with a high density of this bacterium (96). Therefore, a critical trait for the long-term applicability and stability of Wolbachia in mitigating arbovirus transmission is its density under natural conditions, especially under fluctuating temperatures (77, 94, 97, 98). Recent data regarding the effects of Wolbachia in reducing DENV and CHIKV transmission in Rio de Janeiro revealed a strong seasonal effect on Wolbachia introgression in a native population, with a lower frequency of Wolbachia-infected mosquitoes during the warmer months (74). However, no information was available for the Wolbachia density over the study period. Field data gathered in Cairns, Australia, show the detrimental effects of high temperatures on the stability of Wolbachia and its Ae. aegypti interaction, affecting maternal transmission, cytoplasmic incompatibility, and likely the potential of Wolbachia to mitigate arbovirus transmission (94). Thus, the mosquito-Wolbachia density association under a gradient of temperature regimes needs to be carefully investigated for both strains to understand the likely long-term stability of Wolbachia as a disease control tool.
Path analysis has been used in ecological studies to describe a myriad of relationships among traits by estimating the reciprocal magnitude of direct and indirect effects (path coefficients) on the response variable (35, 66, 99–101). By combining path analysis and model selection approaches, we explored multiple direct and indirect paths that connect variables involved in blood ingestion and mosquito fecundity for Ae. aegypti females transinfected with either wMel or wAlbB and uninfected individuals. The best model for the Wolbachia-uninfected strain was the full model, whereas reduced models presented better fit for both Wolbachia-infected strains. The best model selected for each strain, additionally to resulting in a lower AIC, also provided the best fit according to conventional cutoffs for the model fit indices, enhancing our confidence to interpret model parameter estimates (102). Overall, the relationships revealed by the path analysis were in accordance with generalized linear models. The most intriguing conclusion is that the presence of Wolbachia promotes a reshaping of trait pathways regardless of the strain. Noteworthy was the fact the blood meal size, wing length, the number of eggs have no effect on the Wolbachia density, a trend observable for both the wMel and wAlbB strains. Wolbachia is known to affect the reproductive traits of their hosts (31), leading to several physiological and behavioral changes in mosquito biology. Considering both wMel and wAlbB strains have been released in several dengue endemic areas of the globe and the well-known fitness costs associated with Wolbachia in terms of mosquito fecundity and egg fertility (37, 75), other traits involved in the Ae. aegypti-Wolbachia interaction could be investigated to better comprehend invasion patterns in endemic settings. For example, it remains unknown to what extension the loss observed in egg fertility and changes in the direct and indirect pathways between fitness traits as reported here through path diagrams can affect the long-term stability of invasions. Determining a path network that includes the infection with an arbovirus like DENV will add new insights into this symbiotic interaction.
In conclusion, our study provides insights into the effects of Wolbachia strains wMel and wAlbB on various life history traits of Ae. aegypti mosquitoes with a controlled genetic background. We observed differential effects on fecundity, blood meal size, and wing shape, which may have implications for the ease with which Wolbachia invasion happens in endemic settings (26, 78). For example, a reduction in fecundity of wMel-infected Ae. aegypti and a lower production of eggs with a set amount of ingested blood relative to uninfected individuals could slow invasion into native populations. Furthermore, due to egg hatching issues, the native Wolbachia-free population can produce a larger egg bank in dry months that synchronously hatch when wet summer conditions start, whereas the eggs from Wolbachia-infected eggs (particularly those infected by wAlbB) can lose their viability a few weeks after being laid in natural breeding sites (103–105). The interaction between Wolbachia and Ae. aegypti is complex and influenced by multiple factors, including environmental conditions and mosquito age. To enhance the long-term stability of Wolbachia as a tool for mitigating arbovirus transmission, further research is needed to explore additional traits and the influence of virus infections on Wolbachia-host interactions. Understanding the intricacies of these interactions will undoubtedly aid in the design and implementation of effective strategies for controlling vector-borne diseases.
ACKNOWLEDGMENTS
We appreciate the efforts of Marcelo Celestino, Mauro Muniz, Unchana Lange, and Anucha Ponyiam in providing technical support.
This work was supported by the Deutsche Forschungsgemeinschaft (DFG) (grant number MA 9541/1-1), the Federal Ministry of Education and Research of Germany (BMBF) (grant number NEED 01Kl2022), and Fundação Carlos Chagas Filho de Amparo à Pesquisa no Estado do Rio de Janeiro (grant number E-14/2019). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributor Information
Rafael Maciel-de-Freitas, Email: freitas@ioc.fiocruz.br.
Pradip Sen, CSIR - Institute of Microbial Technology, Chandigarh, India.
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/spectrum.00128-24.
Figures S1 and S2.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Gorton AJ, Heath KD, Pilet-Nayel M-L, Baranger A, Stinchcombe JR. 2012. Mapping the genetic basis of symbiotic variation in legume-rhizobium interactions in Medicago truncatula. G3 (Bethesda) 2:1291–1303. doi: 10.1534/g3.112.003269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lambrechts L, Fellous S, Koella JC. 2006. Coevolutionary interactions between host and parasite genotypes. Trends Parasitol 22:12–16. doi: 10.1016/j.pt.2005.11.008 [DOI] [PubMed] [Google Scholar]
- 3. Schmid-Hempel P, Ebert D. 2003. On the evolutionary ecology of specific immune defence. Trends Ecol Evol 18:27–32. doi: 10.1016/S0169-5347(02)00013-7 [DOI] [Google Scholar]
- 4. Agrawal A, Lively CM. 2002. Infection genetics: gene-for-gene versus matching-alleles models and all points in between. Evol Ecol Res 4:79–90. [Google Scholar]
- 5. Hamilton WD, Axelrod R, Tanese R. 1990. Sexual reproduction as an adaptation to resist parasites (a review). Proc Natl Acad Sci U S A 87:3566–3573. doi: 10.1073/pnas.87.9.3566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brinker P, Fontaine MC, Beukeboom LW, Falcao Salles J. 2019. Host, symbionts, and the microbiome: the missing tripartite interaction. Trends Microbiol 27:480–488. doi: 10.1016/j.tim.2019.02.002 [DOI] [PubMed] [Google Scholar]
- 7. Bose J, Schulte RD. 2014. Testing GxG interactions between coinfecting microbial parasite genotypes within hosts. Front Genet 5:124. doi: 10.3389/fgene.2014.00124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Holmes EC, Burch SS. 2000. The causes and consequences of genetic variation in dengue virus. Trends Microbiol 8:74–77. doi: 10.1016/s0966-842x(99)01669-8 [DOI] [PubMed] [Google Scholar]
- 9. Rico-Hesse R. 2003. Microevolution and virulence of dengue viruses, p 315–341. In Advances in virus research [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers MF, George DB, Jaenisch T, Wint GRW, Simmons CP, Scott TW, Farrar JJ, Hay SI. 2013. The global distribution and burden of dengue. Nature 496:504–507. doi: 10.1038/nature12060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Barreto ML, Barral-Netto M, Stabeli R, Almeida-Filho N, Vasconcelos PFC, Teixeira M, Buss P, Gadelha PE. 2016. Zika virus and microcephaly in Brazil: a scientific agenda. Lancet 387:919–921. doi: 10.1016/S0140-6736(16)00545-6 [DOI] [PubMed] [Google Scholar]
- 12. Rezza G, Nicoletti L, Angelini R, Romi R, Finarelli AC, Panning M, Cordioli P, Fortuna C, Boros S, Magurano F, Silvi G, Angelini P, Dottori M, Ciufolini MG, Majori GC, Cassone A, CHIKV study group . 2007. Infection with chikungunya virus in Italy: an outbreak in a temperate region. Lancet 370:1840–1846. doi: 10.1016/S0140-6736(07)61779-6 [DOI] [PubMed] [Google Scholar]
- 13. Nunes MRT, Faria NR, de Vasconcelos JM, Golding N, Kraemer MUG, de Oliveira LF, Azevedo R do S da S, da Silva DEA, da Silva EVP, da Silva SP, Carvalho VL, Coelho GE, Cruz ACR, Rodrigues SG, Vianez J da S, Nunes BTD, Cardoso JF, Tesh RB, Hay SI, Pybus OG, Vasconcelos P da C. 2015. Emergence and potential for spread of chikungunya virus in Brazil. BMC Med 13:102. doi: 10.1186/s12916-015-0348-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Focks DA, Brenner RJ, Hayes J, Daniels E. 2000. Transmission thresholds for dengue in terms of Aedes aegypti pupae per person with discussion of their utility in source reduction efforts. Am J Trop Med Hyg 62:11–18. [PubMed] [Google Scholar]
- 15. Maciel-de-Freitas R, Avendanho FC, Santos R, Sylvestre G, Araújo SC, Lima JBP, Martins AJ, Coelho GE, Valle D. 2014. Undesirable consequences of insecticide resistance following Aedes aegypti control activities due to a dengue outbreak. PLoS One 9:e92424. doi: 10.1371/journal.pone.0092424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Garcia G de A, David MR, Martins A de J, Maciel-de-Freitas R, Linss JGB, Araújo SC, Lima JBP, Valle D. 2018. The impact of insecticide applications on the dynamics of resistance: the case of four Aedes aegypti populations from different Brazilian regions. PLoS Negl Trop Dis 12:e0006227. doi: 10.1371/journal.pntd.0006227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moyes CL, Vontas J, Martins AJ, Ng LC, Koou SY, Dusfour I, Raghavendra K, Pinto J, Corbel V, David J-P, Weetman D. 2017. Contemporary status of insecticide resistance in the major Aedes vectors of arboviruses infecting humans. PLoS Negl Trop Dis 11:e0005625. doi: 10.1371/journal.pntd.0005625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zug R, Hammerstein P. 2012. Still a host of hosts for Wolbachia: analysis of recent data suggests that 40% of terrestrial Arthropod species are infected. PLoS One 7:e38544. doi: 10.1371/journal.pone.0038544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McMeniman CJ, Lane RV, Cass BN, Fong AWC, Sidhu M, Wang Y-F, O’Neill SL. 2009. Stable introduction of a life-shortening Wolbachia infection into the mosquito Aedes aegypti. Science 323:141–144. doi: 10.1126/science.1165326 [DOI] [PubMed] [Google Scholar]
- 20. Dutra HLC, Rocha MN, Dias FBS, Mansur SB, Caragata EP, Moreira LA. 2016. Wolbachia blocks currently circulating Zika virus isolates in Brazilian Aedes aegypti mosquitoes. Cell Host Microbe 19:771–774. doi: 10.1016/j.chom.2016.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Moreira LA, Iturbe-Ormaetxe I, Jeffery JA, Lu G, Pyke AT, Hedges LM, Rocha BC, Hall-Mendelin S, Day A, Riegler M, Hugo LE, Johnson KN, Kay BH, McGraw EA, van den Hurk AF, Ryan PA, O’Neill SL. 2009. A Wolbachia symbiont in Aedes aegypti limits infection with dengue, chikungunya, and Plasmodium. Cell 139:1268–1278. doi: 10.1016/j.cell.2009.11.042 [DOI] [PubMed] [Google Scholar]
- 22. Aliota MT, Walker EC, Uribe Yepes A, Velez ID, Christensen BM, Osorio JE. 2016. The wMel strain of Wolbachia reduces transmission of chikungunya virus in Aedes aegypti. PLoS Negl Trop Dis 10:e0004677. doi: 10.1371/journal.pntd.0004677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Turelli M. 1994. Evolution of incompatibility-inducing microbes and their hosts. Evolution 48:1500. doi: 10.2307/2410244 [DOI] [PubMed] [Google Scholar]
- 24. Ross PA, Turelli M, Hoffmann AA. 2019. Evolutionary ecology of Wolbachia releases for disease control. Annu Rev Genet 53:93–116. doi: 10.1146/annurev-genet-112618-043609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ferguson NM, Kien DTH, Clapham H, Aguas R, Trung VT, Chau TNB, Popovici J, Ryan PA, O’Neill SL, McGraw EA, Long VT, Dui LT, Nguyen HL, Chau NVV, Wills B, Simmons CP. 2015. Modeling the impact on virus transmission of Wolbachia-mediated blocking of dengue virus infection of Aedes aegypti. Sci Transl Med 7:279ra37. doi: 10.1126/scitranslmed.3010370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Garcia G de A, Sylvestre G, Aguiar R, da Costa GB, Martins AJ, Lima JBP, Petersen MT, Lourenço-de-Oliveira R, Shadbolt MF, Rašić G, Hoffmann AA, Villela DAM, Dias FBS, Dong Y, O’Neill SL, Moreira LA, Maciel-de-Freitas R. 2019. Matching the genetics of released and local Aedes aegypti populations is critical to assure Wolbachia invasion. PLoS Negl Trop Dis 13:e0007023. doi: 10.1371/journal.pntd.0007023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. King JG, Souto-Maior C, Sartori LM, Maciel-de-Freitas R, Gomes MGM. 2018. Variation in Wolbachia effects on Aedes mosquitoes as a determinant of invasiveness and vectorial capacity. Nat Commun 9:1483. doi: 10.1038/s41467-018-03981-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nazni WA, Hoffmann AA, NoorAfizah A, Cheong YL, Mancini MV, Golding N, Kamarul GMR, Arif MAK, Thohir H, NurSyamimi H, et al. 2019. Establishment of Wolbachia strain wAlbB in Malaysian populations of Aedes aegypti for dengue control. Curr Biol 29:4241–4248. doi: 10.1016/j.cub.2019.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. O’Neill SL, Ryan PA, Turley AP, Wilson G, Retzki K, Iturbe-Ormaetxe I, Dong Y, Kenny N, Paton CJ, Ritchie SA, Brown-Kenyon J, Stanford D, Wittmeier N, Anders KL, Simmons CP. 2018. Scaled deployment of Wolbachia to protect the community from dengue and other Aedes transmitted arboviruses. Gates Open Res 2:36. doi: 10.12688/gatesopenres.12844.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Utarini A, Indriani C, Ahmad RA, Tantowijoyo W, Arguni E, Ansari MR, Supriyati E, Wardana DS, Meitika Y, Ernesia I, Nurhayati I, Prabowo E, Andari B, Green BR, Hodgson L, Cutcher Z, Rancès E, Ryan PA, O’Neill SL, Dufault SM, Tanamas SK, Jewell NP, Anders KL, Simmons CP. 2021. Efficacy of Wolbachia-infected mosquito deployments for the control of dengue. N Engl J Med 384:2177–2186. doi: 10.1056/NEJMoa2030243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Werren JH, Baldo L, Clark ME. 2008. Wolbachia: master manipulators of invertebrate biology. Nat Rev Microbiol 6:741–751. doi: 10.1038/nrmicro1969 [DOI] [PubMed] [Google Scholar]
- 32. Ramos LFC, Martins M, Murillo JR, Domont GB, de Oliveira DMP, Nogueira FCS, Maciel-de-Freitas R, Junqueira M. 2022. Interspecies isobaric labeling-based quantitative proteomics reveals protein changes in the ovary of Aedes aegypti coinfected with ZIKV and Wolbachia. Front Cell Infect Microbiol 12:1–16. doi: 10.3389/fcimb.2022.900608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Martins M, Ramos LFC, Murillo JR, Torres A, de Carvalho SS, Domont GB, de Oliveira DMP, Mesquita RD, Nogueira FCS, Maciel-de-Freitas R, Junqueira M. 2021. Comprehensive quantitative proteome analysis of Aedes aegypti identifies proteins and pathways involved in Wolbachia pipientis and Zika virus interference phenomenon. Front Physiol 12:1–19. doi: 10.3389/fphys.2021.642237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ross PA, Hoffmann AA. 2022. Fitness costs of Wolbachia shift in locally‐adapted Aedes aegypti mosquitoes. Environ Microbiol 24:5749–5759. doi: 10.1111/1462-2920.16235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Frankino WA, Juliano SA. 1999. Costs of reproduction and geographic variation in the reproductive tactics of the mosquito Aedes triseriatus. Oecologia 120:59–68. doi: 10.1007/s004420050833 [DOI] [PubMed] [Google Scholar]
- 36. Schwartz A, Koella JC. 2001. Trade-offs, conflicts of interest and manipulation in Plasmodium–mosquito interactions. Trends Parasitol 17:189–194. doi: 10.1016/s1471-4922(00)01945-0 [DOI] [PubMed] [Google Scholar]
- 37. Petersen MT, Couto-Lima D, Garcia GA, Pavan MG, David MR, Maciel-de-Freitas R. 2023. Dengue exposure and Wolbachia wMel strain affects the fertility of quiescent eggs of Aedes aegypti. Viruses 15:952. doi: 10.3390/v15040952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. David MR, Garcia GA, Valle D, Maciel-de-Freitas R. 2018. Insecticide resistance and fitness: the case of four Aedes aegypti populations from different Brazilian regions. Biomed Res Int 2018:6257860. doi: 10.1155/2018/6257860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pavan MG, Garcia GA, David MR, Maciel-de-Freitas R. 2023. The double-edged sword effect of expanding Wolbachia deployment in Dengue endemic settings. Lancet Reg Health - Am 27:100610. doi: 10.1016/j.lana.2023.100610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Brito LP, Carrara L, de Freitas RM, Lima JBP, Martins AJ. 2018. Levels of resistance to pyrethroid among distinct kdr alleles in Aedes aegypti laboratory lines and frequency of kdr alleles in 27 natural populations from Rio de Janeiro, Brazil. Biomed Res Int 2018:2410819. doi: 10.1155/2018/2410819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Maciel-de-Freitas R. 2023. Listen to the shopkeeper. Elife 12:10–12. doi: 10.7554/eLife.87366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gesto JSM, Pinto SB, Dias FBS, Peixoto J, Costa G, Kutcher S, Montgomery J, Green BR, Anders KL, Ryan PA, Simmons CP, O’Neill SL, Moreira LA. 2021. Large-scale deployment and establishment of Wolbachia into the Aedes aegypti population in Rio de Janeiro, Brazil. Front Microbiol 12:711107. doi: 10.3389/fmicb.2021.711107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Walker T, Johnson PH, Moreira LA, Iturbe-Ormaetxe I, Frentiu FD, McMeniman CJ, Leong YS, Dong Y, Axford J, Kriesner P, Lloyd AL, Ritchie SA, O’Neill SL, Hoffmann AA. 2011. The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature 476:450–453. doi: 10.1038/nature10355 [DOI] [PubMed] [Google Scholar]
- 44. Ross PA, Gu X, Robinson KL, Yang Q, Cottingham E, Zhang Y, Yeap HL, Xu X, Endersby-Harshman NM, Hoffmann AA. 2021. A wAlbB Wolbachia transinfection displays stable phenotypic effects across divergent Aedes aegypti mosquito backgrounds. Appl Environ Microbiol 87:e0126421. doi: 10.1128/AEM.01264-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Benkova I, Volf P. 2007. Effect of temperature on metabolism of Phlebotomus papatasi (Diptera: Psychodidae). J Med Entomol 44:150–154. doi: 10.1093/jmedent/41.5.150 [DOI] [PubMed] [Google Scholar]
- 46. Petersen MT, Silveira ID da, Tátila-Ferreira A, David MR, Chouin-Carneiro T, Van den Wouwer L, Maes L, Maciel-de-Freitas R. 2018. The impact of the age of first blood meal and Zika virus infection on Aedes aegypti egg production and longevity. PLoS One 13:e0200766. doi: 10.1371/journal.pone.0200766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hauser G, Thiévent K, Koella JC. 2019. The ability of Anopheles gambiae mosquitoes to bite through a permethrin-treated net and the consequences for their fitness. Sci Rep 9:8141. doi: 10.1038/s41598-019-44679-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Briegel H. 1980. Determination of uric acid and hematin in a single sample of excreta from blood-fed insects. Experientia 36:1428–1428. doi: 10.1007/BF01960142 [DOI] [Google Scholar]
- 49. Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez J-Y, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. 2012. Fiji: an open-source platform for biological-image analysis. Nat Methods 9:676–682. doi: 10.1038/nmeth.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675. doi: 10.1038/nmeth.2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wilke ABB, Christe R de O, Multini LC, Vidal PO, Wilk-da-Silva R, de Carvalho GC, Marrelli MT. 2016. Morphometric wing characters as a tool for mosquito identification. PLoS ONE 11:e0161643. doi: 10.1371/journal.pone.0161643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sauer FG, Jaworski L, Erdbeer L, Heitmann A, Schmidt-Chanasit J, Kiel E, Lühken R. 2020. Geometric morphometric wing analysis represents a robust tool to identify female mosquitoes (Diptera: Culicidae) in Germany. Sci Rep 10:17613. doi: 10.1038/s41598-020-72873-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Louise C, Vidal PO, Suesdek L. 2015. Microevolution of Aedes aegypti. PLoS One 10:e0137851. doi: 10.1371/journal.pone.0137851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Harbach RE, Knight KL. 1980. Taxonomist’s glossary of mosquito anatomy, p 1–54. Plexus Publications Co. [Google Scholar]
- 55. Souto-Maior C, Sylvestre G, Braga Stehling Dias F, Gomes MGM, Maciel-de-Freitas R. 2018. Model-based inference from multiple dose, time course data reveals Wolbachia effects on infection profiles of type 1 dengue virus in Aedes aegypti. PLoS Negl Trop Dis 12:e0006339. doi: 10.1371/journal.pntd.0006339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lee SF, White VL, Weeks AR, Hoffmann AA, Endersby NM. 2012. High-throughput PCR assays to monitor Wolbachia infection in the dengue mosquito (Aedes aegypti) and Drosophila simulans. Appl Environ Microbiol 78:4740–4743. doi: 10.1128/AEM.00069-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. de Oliveira S, Villela DAM, Dias FBS, Moreira LA, Maciel de Freitas R. 2017. How does competition among wild type mosquitoes influence the performance of Aedes aegypti and dissemination of Wolbachia pipientis? PLoS Negl Trop Dis 11:e0005947. doi: 10.1371/journal.pntd.0005947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Reis R, Oliveira M de, Borges GRA. 2015. R tools for biologists (RT4Bio)
- 59. Delignette-Muller ML, Dutang C. 2015. fitdistrplus: an R package for fitting distributions. J Stat Softw 64:1–34. doi: 10.18637/jss.v064.i04 [DOI] [Google Scholar]
- 60. Meyer D, Zeileis A, Hornik K. 2020. vcd: visualizing categorical data
- 61. Burnham KP, Anderson DR, Huyvaert KP. 2011. AIC model selection and multimodel inference in behavioral ecology: some background, observations, and comparisons. Behav Ecol Sociobiol 65:23–35. doi: 10.1007/s00265-010-1029-6 [DOI] [Google Scholar]
- 62. Burnham KP, Anderson DR. 2015. Model selection and multimodel inference: a practical information-theoretic approach. 2nd ed. Springer, New York. [Google Scholar]
- 63. Hartig F. 2002. DHARMa: residual diagnostics for hierarchical (multi-level / mixed) regression models
- 64. R Development Core Team . 2020. R: a language and environment for statistical computing. R Foundation for Statistical Computing. [Google Scholar]
- 65. Mitchell RJ. 2020. Path analysis: pollination, p 445. In Design and analysis of ecological experiments. Chapman and Hall/CRC. [Google Scholar]
- 66. Carbone LM, Aguilar R. 2021. Abiotic and biotic interactions as drivers of plant reproduction in response to fire frequency. Arthropod Plant Interact 15:83–94. doi: 10.1007/s11829-020-09792-3 [DOI] [Google Scholar]
- 67. Revelle W. 2023. psych: procedures for psychological, psychometric, and personality research
- 68. Rosseel Y. 2012. lavaan: an R package for structural equation modeling. J Stat Softw 48:1–36. doi: 10.18637/jss.v048.i02 [DOI] [Google Scholar]
- 69. Adams D, Collyer M, Kaliontzopoulou A, Baken E. 2023. Geometric morphometric analyses of 2D and 3D landmark data
- 70. Lorenz C, Almeida F, Almeida-Lopes F, Louise C, Pereira SN, Petersen V, Vidal PO, Virginio F, Suesdek L. 2017. Geometric morphometrics in mosquitoes: what has been measured? Infect Genet Evol 54:205–215. doi: 10.1016/j.meegid.2017.06.029 [DOI] [PubMed] [Google Scholar]
- 71. Garcia GA, Hoffmann AA, Maciel-de-Freitas R, Villela DAM. 2020. Aedes aegypti insecticide resistance underlies the success (and failure) of Wolbachia population replacement. Sci Rep 10:63. doi: 10.1038/s41598-019-56766-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ryan PA, Turley AP, Wilson G, Hurst TP, Retzki K, Brown-Kenyon J, Hodgson L, Kenny N, Cook H, Montgomery BL, Paton CJ, Ritchie SA, Hoffmann AA, Jewell NP, Tanamas SK, Anders KL, Simmons CP, O’Neill SL. 2019. Establishment of WMel Wolbachia in Aedes aegypti mosquitoes and reduction of local dengue transmission in Cairns and surrounding locations in northern Queensland, Australia. Gates Open Res 3:1547. doi: 10.12688/gatesopenres.13061.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Nguyen TH, Nguyen HL, Nguyen TY, Vu SN, Tran ND, Le TN, Vien QM, Bui TC, Le HT, Kutcher S, et al. 2015. Field evaluation of the establishment potential of wmelpop Wolbachia in Australia and Vietnam for dengue control. Parasit Vectors 8:563. doi: 10.1186/s13071-015-1174-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ribeiro Dos Santos G, Durovni B, Saraceni V, Souza Riback TI, Pinto SB, Anders KL, Moreira LA, Salje H. 2022. Estimating the effect of the wMel release programme on the incidence of dengue and chikungunya in Rio de Janeiro: a spatiotemporal modelling study. Lancet Infect Dis 22:1587–1595. doi: 10.1016/S1473-3099(22)00436-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Lau M-J, Ross PA, Hoffmann AA. 2021. Infertility and fecundity loss of Wolbachia-infected Aedes aegypti hatched from quiescent eggs is expected to alter invasion dynamics. PLoS Negl Trop Dis 15:e0009179. doi: 10.1371/journal.pntd.0009179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Dutra HLC, Lopes da Silva V, da Rocha Fernandes M, Logullo C, Maciel-de-Freitas R, Moreira LA. 2016. The influence of larval competition on Brazilian Wolbachia-infected Aedes aegypti mosquitoes. Parasit Vectors 9:282. doi: 10.1186/s13071-016-1559-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Foo I-H, Hoffmann AA, Ross PA. 2019. Cross-generational effects of heat stress on fitness and Wolbachia density in Aedes aegypti mosquitoes. Trop Med Infect Dis 4:13. doi: 10.3390/tropicalmed4010013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ross PA, Elfekih S, Collier S, Klein MJ, Lee SS, Dunn M, Jackson S, Zhang Y, Axford JK, Gu X, Home JL, Nassar MS, Paradkar PN, Tawfik EA, Jiggins FM, Almalik AM, Al-Fageeh MB, Hoffmann AA. 2023. Developing Wolbachia-based disease interventions for an extreme environment. PLoS Pathog 19:e1011117. doi: 10.1371/journal.ppat.1011117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Maciel-de-Freitas R, Aguiar R, Bruno RV, Guimarães MC, Lourenço-de-Oliveira R, Sorgine MHF, Struchiner CJ, Valle D, O’Neill SL, Moreira LA. 2012. Why do we need alternative tools to control mosquito-borne diseases in Latin America? Mem Inst Oswaldo Cruz 107:828–829. doi: 10.1590/s0074-02762012000600021 [DOI] [PubMed] [Google Scholar]
- 80. Axford JK, Ross PA, Yeap HL, Callahan AG, Hoffmann AA. 2016. Fitness of wAlbB Wolbachia infection in Aedes aegypti: parameter estimates in an outcrossed background and potential for population invasion. Am J Trop Med Hyg 94:507–516. doi: 10.4269/ajtmh.15-0608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Fraser JE, De Bruyne JT, Iturbe-Ormaetxe I, Stepnell J, Burns RL, Flores HA, O’Neill SL. 2017. Novel Wolbachia-transinfected Aedes aegypti mosquitoes possess diverse fitness and vector competence phenotypes. PLoS Pathog 13:e1006751. doi: 10.1371/journal.ppat.1006751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Javed N, Bhatti A, Paradkar PN. 2021. Advances in understanding vector behavioural traits after infection. Pathogens 10:1376. doi: 10.3390/pathogens10111376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. da Silveira ID, Petersen MT, Sylvestre G, Garcia GA, David MR, Pavan MG, Maciel-de-Freitas R. 2018. Zika virus infection produces a reduction on Aedes aegypti lifespan but no effects on mosquito fecundity and oviposition success. Front Microbiol 9:3011. doi: 10.3389/fmicb.2018.03011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Maciel-de-Freitas R, Koella JC, Lourenço-de-Oliveira R. 2011. Lower survival rate, longevity and fecundity of Aedes aegypti (Diptera: Culicidae) females orally challenged with dengue virus serotype 2. Trans R Soc Trop Med Hyg 105:452–458. doi: 10.1016/j.trstmh.2011.05.006 [DOI] [PubMed] [Google Scholar]
- 85. Isoe J, Collins J, Badgandi H, Day WA, Miesfeld RL. 2011. Defects in coatomer protein I (COPI) transport cause blood feeding-induced mortality in yellow fever mosquitoes. Proc Natl Acad Sci U S A 108:E211–E217. doi: 10.1073/pnas.1102637108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Day JF, Edman JD, Scott TW. 1994. Reproductive fitness and survivorship of Aedes aegypti (Diptera: Culicidae) maintained on blood, with field observations from Thailand. J Med Entomol 31:611–617. doi: 10.1093/jmedent/31.4.611 [DOI] [PubMed] [Google Scholar]
- 87. Oliver SV, Brooke BD. 2014. The effect of multiple blood-feeding on the longevity and insecticide resistant phenotype in the major malaria vector Anopheles arabiensis (Diptera: Culicidae). Parasit Vectors 7:390. doi: 10.1186/1756-3305-7-390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Turley AP, Moreira LA, O’Neill SL, McGraw EA. 2009. Wolbachia infection reduces blood-feeding success in the dengue fever mosquito, Aedes aegypti. PLoS Negl Trop Dis 3:e516. doi: 10.1371/journal.pntd.0000516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Dujardin J-P. 2008. Morphometrics applied to medical entomology. Infect Genet Evol 8:875–890. doi: 10.1016/j.meegid.2008.07.011 [DOI] [PubMed] [Google Scholar]
- 90. Jaramillo-O N, Fonseca-González I, Chaverra-Rodríguez D. 2014. Geometric morphometrics of nine field isolates of Aedes aegypti with different resistance levels to lambda-cyhalothrin and relative fitness of one artificially selected for resistance. PLoS One 9:e96379. doi: 10.1371/journal.pone.0096379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Carrington LB, Tran BCN, Le NTH, Luong TTH, Nguyen TT, Nguyen PT, Nguyen CVV, Nguyen HTC, Vu TT, Vo LT, Le DT, Vu NT, Nguyen GT, Luu HQ, Dang AD, Hurst TP, O’Neill SL, Tran VT, Kien DTH, Nguyen NM, Wolbers M, Wills B, Simmons CP. 2018. Field- and clinically derived estimates of Wolbachia-mediated blocking of dengue virus transmission potential in Aedes aegypti mosquitoes. Proc Natl Acad Sci U S A 115:361–366. doi: 10.1073/pnas.1715788115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Caragata EP, Rocha MN, Pereira TN, Mansur SB, Dutra HLC, Moreira LA. 2019. Pathogen blocking in Wolbachia-infected Aedes aegypti is not affected by Zika and dengue virus co-infection. PLoS Negl Trop Dis 13:e0007443. doi: 10.1371/journal.pntd.0007443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Wilson AJ, Harrup LE. 2018. Reproducibility and relevance in insect-arbovirus infection studies. Curr Opin Insect Sci 28:105–112. doi: 10.1016/j.cois.2018.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Ross PA, Ritchie SA, Axford JK, Hoffmann AA. 2019. Loss of cytoplasmic incompatibility in Wolbachia-infected Aedes aegypti under field conditions. PLoS Negl Trop Dis 13:e0007357. doi: 10.1371/journal.pntd.0007357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Dodson BL, Andrews ES, Turell MJ, Rasgon JL. 2017. Wolbachia effects on rift valley fever virus infection in Culex tarsalis mosquitoes. PLoS Negl Trop Dis 11:e0006050. doi: 10.1371/journal.pntd.0006050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Martinez J, Longdon B, Bauer S, Chan Y-S, Miller WJ, Bourtzis K, Teixeira L, Jiggins FM. 2014. Symbionts commonly provide broad spectrum resistance to viruses in insects: a comparative analysis of Wolbachia strains. PLoS Pathog 10:e1004369. doi: 10.1371/journal.ppat.1004369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Ulrich JN, Beier JC, Devine GJ, Hugo LE. 2016. Heat sensitivity of wMel Wolbachia during Aedes aegypti development. PLoS Negl Trop Dis 10:e0004873. doi: 10.1371/journal.pntd.0004873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Ross PA, Wiwatanaratanabutr I, Axford JK, White VL, Endersby-Harshman NM, Hoffmann AA. 2017. Wolbachia infections in Aedes aegypti differ markedly in their response to cyclical heat stress. PLOS Pathog 13:e1006006. doi: 10.1371/journal.ppat.1006006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Feriche M, Reguera S, Santos X, Mociño-Deloya E, Setser K, Pleguezuelos JM. 2016. Female reproduction in Thamnophis scaliger: the significance of parturition timing. J Herpetol 50:209–215. doi: 10.1670/15-005 [DOI] [Google Scholar]
- 100. Cohen C, Einav M, Hawlena H. 2015. Path analyses of cross-sectional and longitudinal data suggest that variability in natural communities of blood-associated parasites is derived from host characteristics and not interspecific interactions. Parasit Vectors 8:429. doi: 10.1186/s13071-015-1029-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Messika I, Garrido M, Kedem H, China V, Gavish Y, Dong Q, Fuqua C, Clay K, Hawlena H. 2017. From endosymbionts to host communities: factors determining the reproductive success of arthropod vectors. Oecologia 184:859–871. doi: 10.1007/s00442-017-3906-4 [DOI] [PubMed] [Google Scholar]
- 102. Beaujean AA. 2014. Latent variable modeling using R. Routledge. https://www.taylorfrancis.com/books/9781317970736. [Google Scholar]
- 103. Maciel-de-Freitas R, Marques WA, Peres RC, Cunha SP, de Oliveira RL. 2007. Variation in Aedes aegypti(Diptera: Culicidae) container productivity in a slum and a suburban district of Rio de Janeiro during dry and wet seasons. Mem Inst Oswaldo Cruz 102:489–496. doi: 10.1590/s0074-02762007005000056 [DOI] [PubMed] [Google Scholar]
- 104. Carbajo AE, Cardo MV, Guimarey PC, Lizuain AA, Buyayisqui MP, Varela T, Utgés ME, Giovacchini CM, Santini MS. 2018. Is autumn the key for dengue epidemics in non endemic regions? The case of Argentina. PeerJ 6:e5196. doi: 10.7717/peerj.5196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Ritchie SA, Townsend M, Paton CJ, Callahan AG, Hoffmann AA. 2015. Application of wMelPop Wolbachia strain to crash local populations of Aedes aegypti. PLoS Negl Trop Dis 9:e0003930. doi: 10.1371/journal.pntd.0003930 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1 and S2.