Abstract
A deeper understanding of the nano and mesoscale structure of chromatographic adsorbents and the distribution of proteins within the media, is critical to a mechanistic understanding of separation processes using these materials. Characterisation of the media’s architecture at this scale and protein adsorption within, is challenging using conventional techniques. In this study, we propose a novel resin characterisation technique that enables in-situ measurement of the structure of the adsorbed protein layer within the resin, under typical chromatographic conditions. A quartz flow-through cell was designed and fabricated for use with Small Angle Neutron Scattering (SANS), in order to measure the nano- to mesoscale structures of a silica based protein A chromatography resin during the monoclonal antibody sorption process. We were able to examine the pore-to-pore (˜133nm) and pore size (˜63nm) correlations of the resin and the in-plane adsorbed antibody molecules (4.2nm) correlation at different protein loadings and washing buffers, in real time using a contrast matching approach. When 0.03M sodium phosphate with 1M urea and 10% isopropanol buffer, pH 8, was introduced into the system as a wash buffer, it disrupted the system’s order by causing partial unfolding of the adsorbed antibody, as evidenced by a loss of the in-plane protein correlation. This method offers new ways to investigate the nanoscale structure and ligand immobilisation within chromatography resins; and perhaps most importantly understand the in-situ behaviour of adsorbed proteins within the media under different mobile phase conditions within a sample environment replicating that of a chromatography column.
Keywords: Protein A, Small Angle Neutron Scattering, Antibody, Chromatography, Adsorption, Interface
1. Introduction
In commercial separations for therapeutic proteins, binding capacity is a critical factor. An example of such a process is protein A-based affinity chromatography, which is the crucial purification stage for monoclonal antibodies. Maximised binding capacity leads to improved productivity, which in turn leads to better process intensification (PI). Although PI is ultimately the goal for several scale-up procedures, product stability and quality must always be maintained. It is widely accepted that low pH in such processes leads to increased aggregation propensity [1–3]. However, pH is not the only parameter that can affect aggregation levels. Structural stability of the eluted IgG can be jeopardized by protein A, making at least a subpopulation of eluted IgG more prone to aggregation. This can be attributed to the protein A destabilising effect (conformational relaxation) on the upper portion of IgG’s second constant domain [4,5], in addition to the denaturing effects of low pH. Gagnon et al. have demonstrated that during the protein A chromatographic elution step, size and conformation of the IgG1, 150kDa, undergo significant changes due to protein A mediated, 42kDa, denaturation, pH, ionic strength and high IgG concentrations [6,7]. It is suggested that the decrease in hydrodynamic radius of the IgG1 molecules in solution arises predominantly from the propensity of IgG to adopt smaller conformations at higher concentrations, low pH and ionic strength. However, when binding to protein A, an increase in IgG size accompanied the loss of secondary structure. This change was attributed to the excess α-helices, extending the hydrodynamic axis of the protein. The high degree of changes in the secondary structure appeared to result from the dual-site interaction between both IgG heavy chains and distinct protein A molecules (one IgG is bound to two protein A molecules) [7]. Other studies from Shukla et al. and Mazzer et al. also support that adsorption / binding to the protein A resin has a destabilizing effect on the antibody molecules and promotes the formation of aggregation-prone species. They quantified the increase in aggregation rate following low pH elution from a protein A column, which was significantly higher when compared to the aggregation rates arising from low pH alone [8,9]. Kulsing also experimented using lysozyme adsorbed to a chromatography column at different temperatures. Conformational changes upon elution where measured ex-situ using Small Angle X-ray Scattering (SAXS) which was connected to the column’s outlet and showed that the protein was larger at higher concentrations. These conformational changes were also attributed to the ‘on-column residency effects rather than just detecting a temperature-induced shift’ [10].
The architecture of the media impacts desorption, adsorption, stability, retention and transport rates of the protein during the chromatographic process [11]. It is thus vital to not only characterise the architecture of the media, but also to understand its effect on protein behaviour within the material itself. However, this has proven to be challenging to characterise following traditional approaches. Imaging techniques, such as optical microscopy that can be used to visualise the macro and microstructure of these materials, lack the resolution required to reveal structural information. Methods with the required nanoscale resolution, such as atomic force microscopy and electron microscopy, often demand tailored sample preparation, for instance drying, and thus the output may not reflect the in situ conditions. Techniques like inverse size-exclusion chromatography (ISEC) that can measure surface area and pore distribution at these length scales do not provide information on the material’s architecture [11]. Hence, there is an opportunity for new characterisation techniques that not only measure the intrinsic structure of the media but also the nanoscale distribution of the protein under the same conditions as found during the chromatographic separation process. Therefore, novel techniques that can reveal the media’s nanoscale structure, and the spatial distribution of the protein within the media, are needed, which will eventually benefit the design of new media as well as improve the modelling of existing ones [11]. The work presented here aims to help address these issues.
Neutron and x-ray scattering techniques are powerful, non-destructive probes for studying the structure and dynamics of materials on the nano to micon scale and from picoseconds upwards. Neutrons are particularly useful for hydrogenous/soft materials under processing condition due to their high sensitivity to light elements, and hydrogen in particular, and to their high penetrating power allowing them to probe samples contained within a complex experimental apparatus, such as pressure cells and temperature regulators [13]. For example Mazzer et al. recently used Neutron Reflectivity to characterise the structure and orientation of adsorbed Immunoglobulin G (IgG) on a model surface designed to mimic the affinity chromatography surface [9,11]. Moreover, neutron scattering power differs significantly between isotopes allowing the judicial use of hydrogen/deuterium isotopic substitution to highlight one component in the presence of several others which are rendered invisible [12].
In the present work, we used Small-Angle Neutron Scattering (SANS) to measure protein adsorption and the resulting resin bound protein structuring on the chromatographic media in a flow-through cell. Even though this technique is widely used by the scientific community, to the best of our knowledge, there are no previous studies on protein adsorption in chromatographic media using a flow through cell with SANS to study the chromatographic process. Pozzo used SANS to probe the conformation of SDS-BSA protein surfactant complexes during electrophoresis in cross-linked polyacrylamide gels. This work demonstrated that SANS has the unique potential to probe nanoscale structures on complex systems that contain multiple components and that this technique is useful in understanding complex phenomena such as polyelectrolyte electrophoretic migration in hydrogels[14]. In another study by Plewka et al., a flow cell was used during SAXS experiments when studying IgG adsorption on MabSelect Sure resin. The authors state that ‘It was, therefore, possible for the first time to directly correlate the nanostructure changes inside the column, which is otherwise a black box, with the adsorption and elution process’ [15]. As described by Koshari et al., SANS is very well suited for this application as it can resolve spatial features from the micrometre to the nanometre length scale. It can also characterise the static, as well as the dynamic aspects of the structure, without being disruptive to the sample [11,16].
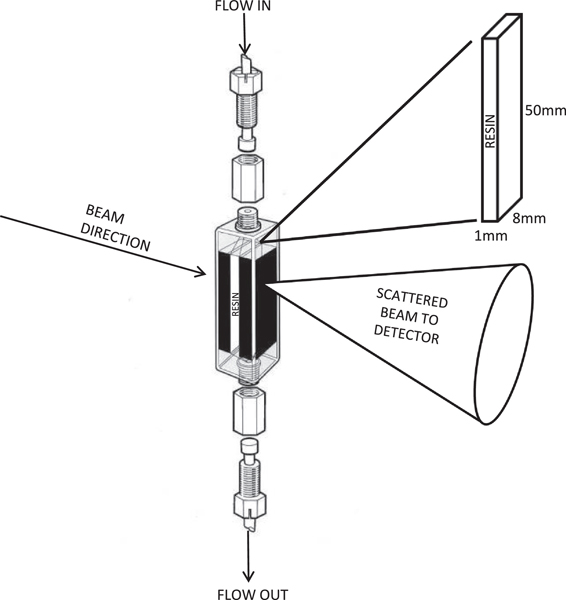
Here, we aim to establish SANS as a feasible characterisation technique to study protein adsorption in chromatographic media at the nano to mesoscale. We also wish for the first time to perform these experiments under real-time affinity chromatography conditions using SANS. For this purpose, a custom-made quartz flow-through cell was built mimicking the affinity chromatography columns, and the IgG1 was adsorbed onto Prosep Ultra Plus resin (Figure 1).
Figure 1:
Custom-made quartz flow through cell made by the Sample environment team at ISIS, RAL. Inlet and outlet are situated at the top and bottom of the cell respectively. The 1mL chamber (50mm x 8mm x 1mm) in the middle of the cell is packed with resin. The neutron beam is fired perpendicularly to the cell. Neutrons penetrate it and then are scattered to the detector. Measurements were performed at top position of the cell, where the antibody concentration is the most accurate, since the antibody concentration differs across the column. This column is built to closely resemble the real life chromatographic columns.
2. Materials and Methods
2.1. Materials
For protein A chromatography, a silica-based resin (Prosep Ultra Plus, Millipore, Hertfordshire, UK) was used. The model protein used for this study was IgG1, protein A purified humanised IgG1 produced in CHO cell culture as described in El-Sabbahy et al., 2018. It was dialysed into a running buffer (sodium phosphate, pH 7.2) with a final concentration of 1 mg/mL. For contrast matching experiments, the bare silica beads (Prosep Ultra Plus without the ligand) were kindly donated by Millipore. All buffer materials, sodium phosphate monobasic, sodium chloride, deuterium oxide at 99.8% atom D, sodium caprylate, urea, isopropanol, hydrochloric acid and sodium hydroxide were purchased from Sigma-Aldrich. Slide-A-Lyzer® 10K Dialysis Cassettes of 10000 MWCO and Spectrum Spectra/Por® molecular porous membrane tubing of 12–14000 MWCO were from ThermoFisher Scientific.
2.2. Equipment
SANS measurements at the NIST Center for Neutron Research (NCNR), National Institute of Standards and Technology (NIST), Gaithersburg, MD, USA, were performed on a 30-meter-long Small-Angle Neutron Scattering (SANS) instrument, NGB30. SANS experiments at ISIS Neutron and Muon Source, Science and Technology Facilities Council (STFC), Rutherford Appleton Laboratory (RAL), Didcot, UK were performed using the SANS2d instrument. Scanning electron microscopy was conducted using a JSM-6480LV, scanning electron microscope with a high resolution of 3.0nm at University College London, UK.
2.3. Methods
2.3.1. Sample preparation for SANS experiments
IgG1 was prepared by overnight dialysis into 60.5% D2O (the resin contrast match point found in preliminary studies) based 0.03M sodium phosphate buffer, pH 7.2 using Slide-A-Lyzer® 10K Dialysis Cassettes of 10000 MWCO and Spectrum Spectra/Por® molecular porous membrane tubing of 12–14000 MWCO (ThermoFisher Scientific). The resulting solutions were diluted to a concentration of 1mg/mL IgG1. The final concentration was determined by measuring the absorbance at 280nm with a NanoDrop Spectrophotometer using Beer’s law and an extinction coefficient of (experimentally determined by ThermoScientific, USA). Before injection into the flow-through cell, IgG1 was filtered through a 0.45 µm membrane.
For the static experiments, the resin, initially in the company’s (Millipore) solution, had undergone buffer exchange (60.5% D2O- based 0.03M sodium phosphate buffer, pH 7.2) in batches of 10mL. This was achieved by firstly centrifuging the resin for 2min. The excess liquid at the top was removed, and the tube was topped up to 10mL with 0.03M sodium phosphate buffer. It was then incubated on a roller for 10min. The process was repeated three times. After the final centrifuge round the tube was topped up to 5mL, ready to be used for the column packing.
For the static bead characterisation studies, 0.9g of bare silica beads, already in dry powder form, was dissolved in a 100% D2O- based 0.03M sodium phosphate buffer, pH 7.2. The beads were packed in a demountable quartz window sample cell with a path length (thickness) of 1mm.
2.3.2. Flow through cell packing
Firstly, the bottom filter, the outlet fittings and tubing of the column were fitted. The column was filled with 20% ethanol solution from the top, to remove all air. The diluted resin was gradually added into the column using a syringe until it was full and left overnight for the resin to settle using gravitational force. If needed, more resin was added to fill any empty gaps. Once full, the top filter and tubing of the column were fitted. Finally, the column was washed with either 20% ethanol or distilled water at a low flow rate (0.01mL/min) overnight to remove any air bubbles. The experimental buffer solution (0.03M sodium phosphate buffer, pH 7.2) was pumped onto the column prior to small angle scattering measurements.
2.3.3. Small-angle neutron scattering experiments
Hydrogen isotopes play an essential role in neutron scattering when studying biological materials. Neutrons interact very differently in the presence of 1H (protium) than when in the presence of 2H (deuterium). This property is known as scattering length, with Å units. Protium has a negative scattering length (−3.74 ×10−5 Å) whereas deuterium, on the other hand, has a very large scattering length (6.67 ×10−5 Å) [18]. When characterising large structures or bulk materials like proteins, their scattering length density (SLD) is calculated by adding up the molecules’ scattering lengths and then dividing by the total volume. Meaning that the scattering length density of H2O is negative (−0.56 ×10−6 Å−2) and that of D2O is positive (6.35×10−6 Å−2) [19]. By mixing D2O and H2O, the SLD can be manipulated.
The fact that hydrogen is in high abundance in nature makes it easy to exploit this contrast creating property, by either suspending the sample in a D2O-based mobile phase or by isotopically labelling the material of interest. These methods can create a high contrast between the suspension medium and the sample, or even between different subunits of a molecule [20].The components of the system are matched out based on the mixing of H2O and D2O. Neutron scattering only occurs if the SLD of the particles differs from its bulk solution. Therefore, matching one system component out, enables researchers to work out the distribution of another.
In the present study, we wished to render the resin invisible (remove all resin scattering) by matching its SLD to that of the solution. By removing the resin scattering, the scattering from the resin bound protein was revealed. The contrast matching point of the bare silica beads (the base matrix) was identified by suspending the beads in different H2O/D2O ratios. It was found that the contrast matching point of this silica matrix was 60.5% D2O. Any further experiments were therefore carried out under contrast matching conditions.
In summary, Small-Angle Neutron Scattering arises from variations in the scattering length density, which generally reflects structural heterogeneities in the sample. The intensity of scattered neutrons is recorded as a function of the angle, θ, from the incident beam at any given wavelength, λ. These counts are recorded as a function of Q, the scattering vector, also known as the momentum transfer vector, which is defined as:
| Eq. 1 |
Where λ is the neutrons’ wavelength. Q thus has units of inverse length (usually Å −1) and the data collected as a function of Q, I(Q), is said to be in reciprocal space [16]. This measured intensity provides insights about the structure of the sample. Q and the length scale being probed, L, are directly related by Bragg’s law [16],
| Eq. 2 |
Therefore, the real-space structures in the sample are inversely related to the features observed in the SANS profile.
At the NCNR each sample was measured with a fixed wavelength of 6 Å, using a 2 × 1 cm beam size, at three diffractometer settings, a high Q – 1m sample to detector distance (SDD), an intermediate Q – 4m SDD, and a low Q – 13m SDD. In some cases, an extra setting, using an 8.4 Å wavelength and a 13m SDD with lenses, was used to reach very low Q. The resulting maximum scattering range was 0.001 Å−1 < Q < 0.4 Å−1 with a wavelength spread, Δλ/λ, of 0.15. At ISIS, a 10 Hz pulsed neutron source, a time-of-flight small-angle neutron scattering instrument was used with a beam size of 6mm, utilising a 2.0 – 14.0 Å wavelength band. The sample-detector distance was also varied from 2 – 12 m to achieve a total scattering range of 0.004 Å−1 < Q < 1.77 Å-1. All experiments were performed at room temperature.
The column was placed on the sample stage and connected to an Aladdin syringe pump (NCNR) or a Knauer HPLC pump (ISIS), with valved tubing to avoid any air contamination during the buffer changes and IgG loading. The antibody solution was gradually added into the system at a flow rate of 1mL/min until the desired concentration was reached (10, 30, 50mg). The antibody was incubated for 10min and then washed by flowing buffer through it to remove any unbound molecules. The beam was directed towards the top area of the column to get a representative understanding of protein adsorption behaviour within particulate resin. For each concentration, measurements were taken under two different washing buffer conditions, 0.03M sodium phosphate, pH 7.2 and 0.03M sodium phosphate with 1M urea and 10% isopropanol, pH 8, resulting in 2 data sets for each concentration yielding a total of 6 data sets.
2.3.4. Data reduction
To obtain the corrected and radially averaged SANS scattering spectra for the NIST data, Igor Pro was used with the NCNR provide macros [21], and standard data reduction procedures were followed, whereas, for the ISIS data reduction, the reduction program, Mantid was employed [22]. In this case, the contrast matched resin in buffer SANS profile was used to subtract the background from the experimental data sets. Igor Pro was then used to obtain the averaged SANS spectra plots.
2.3.5. Modelling
A broad peak model was selected to fit the collected data using the SasView modelling software [23]. In this model the broad scattering peak from SANS data is represented by an empirical functional form calculated by [24]:
| Eq. 3 |
Where A and C are scaling factors, n is the Porod law exponent, m and ξ are the Lorentz exponent and screening length respectively and the peak position, Q0, is related to the d-spacing in the system as given in eq. 3. This basic model was used to translate the observed scattering peaks into real correlation lengths.
2.3.6. SEM
Bare silica beads (no ligand) were investigated using an SEM to visualise the inner structure of the resin. Beads were in a powder form already, thus eliminating the need for prior drying. The beads were placed on aluminium slabs and were sputter-coated with a gold layer. For the visualisation, multiple magnifications were used, from x250 to x100,000, which corresponds to length scales of 100μm to 0.1μm. SEM images were analysed by the ImageJ software [25] to obtain the average pore size as well as the average pore-to-pore correlation distance.
3. Results and discussion
3.1. Characterisation of bare silica beads
To obtain an internal structure overview of the bare silica beads (Prosep Ultra Plus resin beads before protein A coating) and characterise their pore size distribution, an SEM was utilised. The same beads were also examined via SANS, allowing for in situ structural characterisation of the chromatography resin. Since scattering measurements are performed in Fourier, or reciprocal, space ([26] whereas microscopy techniques are performed in real space (real world coordinates), the SEM images underwent Fourier Transformation (FT) in order to be able to compare the scattering and SEM data in the same reciprocal space. The FT-SEM image and the SANS profile exhibited a notable similarity, as expected. It was noted that both contained one broad peak at low scattering vector (q) Å−1 and one less distinct peak at mid q. The two features are likely to be broad peaks related to the resin’s pore-to-pore correlation distance, first peak, and the inter-pore correlation, second peak (Figure 2). The fact that the peak is broad indicates that there is a range of pore sizes in the resin. Prior X-ray Computed Tomography (CT) results were also used to validate the above findings [27]. The manufacturing specification states an average pore size of 70nm (Millipore, 2014), the silica average pore size obtained from SANS was approximately 104nm, whereas that reported from CT was 154nm. Note that CT has lower resolution and therefore only allowed the measurement of the larger pores in the structure, resulting in a larger average pore size measurement.
Figure 2:
A. Shows the SANS measurements of the bare silica beads at 100% D2O buffer composition (blue line) and Fourier Transform from the SEM image of the dry bare silica beads (red line). The red circles indicate the peak locations. B. Shows the SEM image of the dry bare silica beads at an x100,000 magnification. The pore-to-pore correlation (pore centre-to- pore centre distance) is also shown along with the pore size. Error bars have been removed for clarity but are commensurate with the scatter in the data.
3.2. Structural characterisation of resin-bound protein
Prosep Ultra Plus specifications state that the dynamic binding capacity of the resin is ~50mg/mL and this value was confirmed by performing a resin breakthrough experiment on an AKTA prior to the SANS experiments (data not shown as this is beyond the scope of this paper). Note that in some case the resin was loaded beyond its binding capacity (e.g. 69mg/mL and beyond) in order to ensure resin saturation of the protein A binding sites inside the column during the SANS experiment,.
Under contrast matching conditions, any observed scattering was solely due to the protein molecules in the system. However, there were two types of protein in the system, protein A ligand and IgG molecules. With the contrast matching approach, the resin could not be perfectly matched out due to the presence of bound protein A on the resin surface. Yet, the fact that the protein A distribution on the resin surface in-situ can be seen once the resin beads are matched out, is by itself novel and should be highlighted. This method opens the door to new ways of investigating ligands attached on affinity chromatography resins in-situ and consequently to their improvement.
Before injecting IgG into the column (Figure 4- Blue line), any measured scattering was due to the protein A ligand attached to the silica beads’ surface. The scattering profile contained one broad peak, visible at a low Q (0.0047 Å−1), which is correlates to a spacing of 133nm, reminiscent of the pore-to-pore correlation distance, suggesting protein A decoration of the resin surface, as expected.
Figure 4:
The experimental data gathered from different scattering vector ranges (Q). A. on the left-hand side is the low Q regime. The blue line is for the system with no IgG present displaying only one peak at 0.0048Å. The purple line shows the data after the addition of 69mg of IgG into the system, which gives rise to two peaks at 0.0047Å and 0.01Å (133nm and 63nm in real space respectively). The SEM image on top of the data shows the pore size and pore to pore correlation respectively which give rise to the scattering peaks. B. on the right-hand side is the higher Q regime taken after the addition of 30mg of IgG into the cell. At 0.1Å (4.2nm) a third peak is visible. On top of the data is an IgG molecule schematic, showing what the 4.2nmcorresponding in-plane repeat of IGg bound onto the resin surface.
After loading the column (up to 69mg IgG: Figure 4A – purple line), there were two peaks visible in the lower Q range data (0.001 – 0.5 Å−1). The first peak that corresponded to the pore to pore correlation of the silica beads, 133nm (0.0047 Å−1), and a second peak centred at 0.01 Å−1, which translates to D = 63nm. The second peak is attributed to the pore size observed in the resin data. These findings correspond well to the SEM images of the bare silica beads obtained previously (Figure 2).
Measuring the system at the higher Q range (0.005 – 1.2 Å−1), enabling the examination of smaller length scales approaching the IgG molecular size, allowed us to observe the interactions between the individual IgG molecules and the resin surface. Figure 4B shows the measurements taken after the addition of 30mg IgG into the system. An additional peak was observed at an even higher Q of 0.1 Å−1 corresponding to a repeat distance of 4.2nm. This feature was IgG related since it only appeared after the addition of IgG molecules in the column and the resin and water are matched meaning the scattering is only sensitive to protein structure. Based on the size of the IgG molecule, and previous reflectivity data[9] which suggested the densest bound protein region was close to the resin/water interface, we deduced that this peak corresponds to the repeat distance between IgG molecules bound onto the protein A surface.
3.3. Protein A – IgG binding
Protein A binding takes place at the Fc region of the IgG, between the CH2 and CH3 domains. Native Staphylococcus aureus protein A is a tetramer that, when stretched, has an approximate length of 10nm, and a single domain length of ~3nm (Protein Data Bank:1BDD [28]). Mazzer et al. suggested the binding is usually at a 2:1 ratio when used as an affinity chromatography ligand, whereas Plewka et al [15] estimated that antibodies bind to protein A molecules at a 1.2:1 ratio. They both agree however that the IgG molecules are bound to the protein A ligand at a tilted orientation near the resin surface [9,15]. Since the Fc regions of the IgG molecules are adsorbed onto protein A and stacked next to each other, they can be thought of as having little flexibility, thus making a dense protein layer. The Fab regions extended away from the surface, however, retaining extensive flexibility and making them a much less dense layer, with less order (no repeated distances). The system layers are described in more detail by Mazzer et al [9] who used neutron reflectivity where such data can be obtained using an idealised environment, with protein A ligands immobilised on silicon wafers instead of on native chromatography resins. In summary, they propose that during adsorption of the IgG, there are two distinct layers, an inner protein and an outer protein layer with volume fractions of 0.4 and 0.05 respectively, where the outer layer consist of the Fab arms extending outwards, forming a sparse protein layer [9]. Furthermore, based on the crystallographic data of IgG molecules, an IgG has a maximum distance of 15nm, a Fab distance of 7nm and an Fc region distance of roughly 5nm (Fig.5) [29]. Therefore, the 5nm is consistent with the 4.2 close packed spacing observed in the SANS measurements.
Figure 5:
A. shows the average IgG dimensions and B. shows the system layers (Silica beads activated with Protein- A ligand and the IgG molecules adsorbed to the protein A at the Fc region.
4.5. Characterisation of the solid/liquid interface under typical chromatographic conditions
SANS measurements performed at the higher q range, focusing on the IgG molecular scale, were taken at several IgG concentrations (10, 30 and 50mg/mL) adsorbed to the protein A resin under normal chromatographic conditions (0.03M sodium phosphate, pH 7.2). As seen in figure 6, all three concentrations showed an IgG correlation peak at the highest q (˜0.1Å). As indicated previously, the D spacing of this peak was approximately 4.2nm and represents the in-plane distance between the IgG molecules at the most densely packed protein layer. The peak was fit to a Broad peak model, as discussed in the methods section. At 30mg/mL IgG, a sharper peak was observed compared to 10mg/mL indicating a denser, more ordered layer. Antibody adsorption into the protein A resins follows a shrinking concentration behaviour [30] meaning that as the antibody concentration increases, the IgG molecules start to progressively occupy the resin surface from the outside and moving towards the centre of the bead. At higher concentrations, IgG molecules occupy any empty spaces left, thus creating a more compact and ordered layer. However, as the concentration increases, IgG molecules have poor stability and a tendency to form aggregates and thus the layer becomes less ordered, which could address the fact that the 50mg/mL peak is slightly broader than the previous one[31].F
Figure 6:
SANS measurements (at the higher q) were taken at increasing concentrations of IgG (10mg, 30mg and 50mg) into the system under normal chromatographic conditions, 0.03M sodium phosphate pH 7.2.
4.6. Urea buffer effect on the solid/liquid interface
In a final study, the buffer was changed to 0.03 M sodium phosphate plus 1 M of urea and 10% isopropanol based buffer, pH 8, to mimic what can be used as a wash buffer in order to remove non-specifically bound impurities deriving from the clarified cell culture supernatant an antibody is purified from. As a result, the peak located at the high Q region that corresponds to the protein clusters correlation peak, was no longer visible in any of the three concentrations (Figure 7). At first, it could be assumed that the urea buffer disrupted the protein A - IgG binding and removed the IgG molecules from the system altogether. When examining the system at the lower Q range under the same conditions, however, both pore-to-pore and pore-size correlation peaks were still present (Figure 8), which meant that the IgG molecules were still present, decorating the resin surface. These findings disprove our initial hypothesis. Instead, the absence of a visible protein peak indicates the buffer had disrupted the order of the system (no strong repeat distances) suggesting the urea buffer has had a destabilising effect on the protein clusters, causing partial unfolding of the antibody molecules. This has been observed in other related systems where harsher buffers cause partial unfolding and loss of native protein structure [32,33]. Urea at high concentrations, for example, 4M, acts as a chaotrope and is known to have a destabilising effect on proteins via both direct and indirect mechanisms [34]. As discussed by Bennion. et al., 2003, urea interacts directly with the peptide groups present in the protein and forms hydrogen bonds [34]. However, at low concentrations such as the 1M used in this study, urea is often used as an additive in the washing buffer to remove any non-specifically bound impurities without, it is assumed, disturbing the protein A – IgG attachment. Furthermore, isopropanol is an organic solvent that mitigates solvophobic interactions. During a study by Shukla et al., they found that adding a combination of 1M urea and 10% isopropanol to the washing buffer successfully removed a significant percentage of the impurities present. They showed that this was true for several Fc fusion proteins and monoclonal antibodies. This widely applicable wash condition can eradicate the need for a product-specific optimisation of wash conditions [35]. In the present work, we demonstrated that the urea buffer is disturbing and breaking up the antibody ordering without removing the protein of interest from the system.
Figure 7:
SANS measurements (at the higher q) were taken at increasing concentrations of IgG (10mg, 30mg and 50mg) into the system and in different washing buffers, 0.03M sodium phosphate pH 7.2 and 1M urea+ 10% isopropanol in sodium phosphate pH 8.
Figure 8:
SANS measurements (at the lower Q) of adsorbed antibody molecules in 0.03M sodium phosphate pH 8 and 1M urea+ 10% isopropanol in sodium phosphate pH 8.
4. Conclusions
Small-angle neutron scattering was performed with a custom-made flow-through cell designed to replicate typical protein A affinity chromatography conditions. All initial experiments were performed under typical chromatographic conditions with 0.03M sodium phosphate, pH7.2, as the washing buffer. Under off-contrast matching conditions (100% D2O), the bare silica beads appear to give two distinct correlation peaks arising from pore-to-pore and pore size correlations respectively. The contrast matching point of silica was measured to be 60.5% D2O, at which it renders the Prosep Ultra Plus resin beads invisible to neutrons and enables the investigation of only the protein molecules in the system, protein A and antibody molecules. All subsequent experiments were performed under these contrast matching conditions. Protein A had a very weak scattering signal and therefore, any observed excess scattering was attributed to the antibody molecules. Once the antibody molecules were added into the column, two correlation peaks appeared at the lower Q range (pore-to-pore and pore size), just as in the bare silica case, indicating the antibodies adsorbed to the surface of the resin. A third peak was also detectable at the higher Q range arising from the in-plane distance between the IgG clusters and was concentration-dependent indicating increased ordering accompanying the increased packing from the increased loading. The system was finally examined under a different washing buffer, 0.03M sodium phosphate with 1M urea and 10% isopropanol at pH8 which caused the disappearance of the highest q peak. This suggested that the urea disrupted the system’s order by causing partial unfolding of the adsorbed antibody thereby causing a loss of order on the surface. However, the molecules were still decorating the resin’s surface, so the pore-to-pore and pore size correlation peaks remained visible indicating the protein remained bound. In summary, the washing procedure seems to cause a disruption to these tightly packed proteins which could be a contributing factor to some of the increase in aggregation observed after passing through a column. The technique provides a unique way to investigate the nanoscale structure of chromatography resin and behaviour of the adsorbed protein within the media under different conditions. These findings will be beneficial when designing new chromatographic media, as well as improving upon the modelling of existing ones.
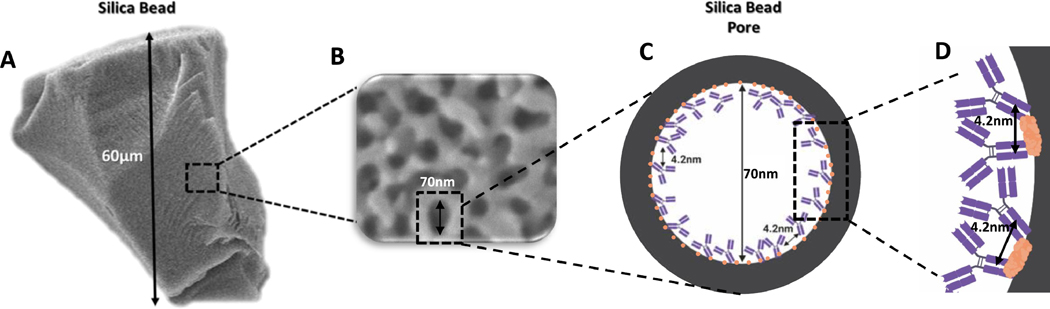
Figure 3:
Length scales involved in the affinity chromatography. A. SEM of an individual silica bead with size 60μm, B. Close-up of the silica bead surface showing the pores of an average size of 100nm, C. Single silica bead pore coated with protein A (red circles) and adsorbed IgG molecules with an average of 4.2nm distance between the antibody molecules. Figure not to scale.
5. Acknowledgements
This work was supported by the UK Engineering and Physical Sciences Research Council (EPSRC) Centre for Doctoral Training in Emergent Macromolecular Therapies: A National CDT linked to an EPSRC Centre for Innovative Manufacturing, grant EP/L015218/1) . We also acknowledge the support of the ISIS, Rutherford Appleton Laboratory, Harwell Science and Innovation Campus, UK, in providing the neutron research facilities used in this work (ISIS beamtime awards RB1810228) and the U.S. National Institute of Standards and Technology (NIST 70NANB12H239), for access to the NGB30 SANS instrument provided by the Center for High Resolution Neutron Scattering, a partnership between the National Institute of Standards and Technology and the National Science Foundation under Agreement No. DMR-1508249. The donation of bare silica beads by Millipore is gratefully acknowledged. Certain commercial equipment, instruments, or materials (or suppliers, or software, ...) are identified in this paper to foster understanding. Such identification does not imply recommendation or endorsement by the National Institute of Standards and Technology, nor does it imply that the materials or equipment identified are necessarily the best available for the purpose. This work benefited from the use of the SasView application, originally developed under NSF Award DMR-0520547. SasView also contains code developed with funding from the EU Horizon 2020 programme under the SINE2020 project Grant No 654000. Finally, unless otherwise stated, all uncertainties represent one standard deviation.
References
- [1].Fink AL, Protein aggregation: Folding aggregates, inclusion bodies and amyloid, Fold. Des. 3 (1998) 9–23. doi: 10.1016/S1359-0278(98)00002-9. [DOI] [PubMed] [Google Scholar]
- [2].Ptitsyn OB, Structures of folding intermediates, Curr. Opin. Struct. Biol. 5 (1995) 74–78. doi: 10.1016/0959-440X(95)80011-O. [DOI] [PubMed] [Google Scholar]
- [3].Latypov RF, Hogan S, Lau H, Gadgil H, Liu D, Elucidation of acid-induced unfolding and aggregation of human immunoglobulin IgG1 and IgG2 Fc, J. Biol. Chem. 287 (2012) 1381–1396. doi: 10.1074/jbc.M111.297697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Deisenhofer J, Crystallographic refinement and atomic models of a human Fc fragment and its complex with fragment B of protein A from Staphylococcus aureus at 2.9- and 2.8-A resolution, Biochemistry. 20 (1981) 2361–70. [PubMed] [Google Scholar]
- [5].Deisenhofer J, Jones TA, Huber R, Sjödahl J, Sjöquist J, Crystallization, crystal structure analysis and atomic model of the complex formed by a human Fc fragment and fragment B of protein A from Staphylococcus aureus., Hoppe. Seylers. Z. Physiol. Chem. 359 (1978) 975–85. doi: 10.1515/bchm2.1978.359.2.975. [DOI] [PubMed] [Google Scholar]
- [6].Gagnon P, Nian R, Leong D, Hoi A, Transient conformational modification of immunoglobulin G during purification by protein A affinity chromatography, J. Chromatogr. A. 1395 (2015) 136–142. doi: 10.1016/J.CHROMA.2015.03.080. [DOI] [PubMed] [Google Scholar]
- [7].Gagnon P, Nian R, Conformational plasticity of IgG during protein A affinity chromatography, J. Chromatogr. A. 1433 (2016) 98–105. doi: 10.1016/J.CHROMA.2016.01.022. [DOI] [PubMed] [Google Scholar]
- [8].Shukla AA, Gupta P, Han X, Protein aggregation kinetics during Protein A chromatography: Case study for an Fc fusion protein, J. Chromatogr. A. 1171 (2007) 22–28. doi: 10.1016/J.CHROMA.2007.09.040. [DOI] [PubMed] [Google Scholar]
- [9].Mazzer AR, Clifton LA, Perevozchikova T, Butler PD, Roberts CJ, Bracewell DG, Neutron reflectivity measurement of protein A–antibody complex at the solid-liquid interface, J. Chromatogr. A. 1499 (2017) 118–131. doi: 10.1016/J.CHROMA.2017.03.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kulsing C, Komaromy AZ, Boysen RI, Hearn MTW, On-line determination by small angle X-ray scattering of the shape of hen egg white lysozyme immediately following elution from a hydrophobic interaction chromatography column, Analyst. 141 (2016) 5810—5814. doi: 10.1039/c6an00851h. [DOI] [PubMed] [Google Scholar]
- [11].Koshari SHS, Wagner NJ, Lenhoff AM, Characterization of lysozyme adsorption in cellulosic chromatographic materials using small-angle neutron scattering, J. Chromatogr. A. 1399 (2015) 45–52. doi: 10.1016/J.CHROMA.2015.04.042. [DOI] [PubMed] [Google Scholar]
- [12].Clifton LA, Hall SCL, Mahmoudi N, Knowles TJ, Heinrich F, Lakey JH, Structural Investigations of Protein–Lipid Complexes Using Neutron Scattering, 2019. doi: 10.1007/978-1-4939-9512-7_11. [DOI] [PubMed] [Google Scholar]
- [13].Singh PS, Small-Angle Scattering Techniques (SAXS/SANS), Membr. Charact. (2017) 95–111. doi: 10.1016/B978-0-444-63776-5.00006-1. [DOI] [Google Scholar]
- [14].Pozzo DC, Neutron-scattering probe of complexes of sodium dodecyl sulfate and serum albumin during polyacrylamide gel electrophoresis, Langmuir. 25 (2009) 1558–1565. doi: 10.1021/la8039994. [DOI] [PubMed] [Google Scholar]
- [15].Plewka J, Silva GL, Tscheließnig R, Rennhofer H, Dias-Cabral C, Jungbauer A, Lichtenegger HC, Antibody adsorption in protein-A affinity chromatography – in situ measurement of nanoscale structure by small-angle X-ray scattering, J. Sep. Sci. 41 (2018) 4122–4132. doi: 10.1002/jssc.201800776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Koshari SHS, Wagner NJ, Lenhoff AM, Effects of Resin Architecture and Protein Size on Nanoscale Protein Distribution in Ion-Exchange Media, Langmuir. 34 (2018) 673–684. doi: 10.1021/acs.langmuir.7b03289. [DOI] [PubMed] [Google Scholar]
- [17].El-Sabbahy H, Ward D, Ogonah O, Deakin L, Jellum GM, Bracewell DG, The effect of feed quality due to clarification strategy on the design and performance of protein A periodic counter-current chromatography, Biotechnol. Prog. 34 (2018) 1380–1392. doi: 10.1002/btpr.2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Su TJ, Lu JR, Thomas RK, Cui ZF, Penfold J, The Adsorption of Lysozyme at the Silica–Water Interface: A Neutron Reflection Study, J. Colloid Interface Sci. 203 (1998) 419–429. doi: 10.1006/JCIS.1998.5545. [DOI] [PubMed] [Google Scholar]
- [19].Pynn R, Neutron Applications in Earth, Energy and Environmental Sciences, (2009) 1–29. doi: 10.1007/978-0-387-09416-8. [DOI] [Google Scholar]
- [20].Clifton LA, Neylon C, Lakey JH, Lipid-Protein Interactions, 2013. doi: 10.1007/978-1-62703-275-9. [DOI] [PubMed] [Google Scholar]
- [21].Kline SR, Reduction and analysis of SANS and USANS data using IGOR Pro, J. Appl. Crystallogr. 39 (2006) 895–900. doi: 10.1107/S0021889806035059. [DOI] [Google Scholar]
- [22].Arnold O, Bilheux JC, Borreguero JM, Buts A, Campbell SI, Chapon L, Doucet M, Draper N, Ferraz Leal R, Gigg MA, Lynch VE, Markvardsen A, Mikkelson DJ, Mikkelson RL, Miller R, Palmen K, Parker P, Passos G, Perring TG, Peterson PF, Ren S, Reuter MA, Savici AT, Taylor JW, Taylor RJ, Tolchenov R, Zhou W, Zikovsky J, Mantid - Data analysis and visualization package for neutron scattering and μ SR experiments, Nucl. Instruments Methods Phys. Res. Sect. A Accel. Spectrometers, Detect. Assoc. Equip. 764 (2014) 156–166. doi: 10.1016/j.nima.2014.07.029. [DOI] [Google Scholar]
- [23].Doucet M, Cho JH, Alina G, Bakker J, Bouwman W, Butler P, Campbell K, Gonzales M, Heenan R, Jackson A, Juhas P, King S, Kienzle P, Krzywon J, Markvardsen A, Nielsen T, O’Driscoll L, Potrzebowski W, Ferraz Leal R, Richter T, Rozycko P, Snow T, Washington A, SasView version 4.2, (2018). doi: 10.5281/ZENODO.1412041. [DOI] [Google Scholar]
- [24].Hammouda B, A new Guinier-Porod model, J. Appl. Crystallogr. 43 (2010) 716–719. doi: 10.1107/S0021889810015773. [DOI] [Google Scholar]
- [25].Schneider CA, Rasband WS, Eliceiri KW, NIH Image to ImageJ: 25 years of image analysis., Nat. Methods. 9 (2012) 671–5. http://www.ncbi.nlm.nih.gov/pubmed/22930834 (accessed October 13, 2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Mettus D, Michels A, Small-angle neutron scattering correlation functions of bulk magnetic materials, J. Appl. Crystallogr. 48 (2015) 1437–1450. doi: 10.1107/S1600576715013187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Johnson TF, Bailey JJ, Iacoviello F, Welsh JH, Levison PR, Shearing PR, Bracewell DG, Three dimensional characterisation of chromatography bead internal structure using X-ray computed tomography and focused ion beam microscopy, J. Chromatogr. A. (2018). doi: 10.1016/j.chroma.2018.06.054. [DOI] [PubMed] [Google Scholar]
- [28].Gouda H, Torigoe H, Arata Y, Shimada I, Saito A, Sato M, Three-Dimensional Solution Structure of the B Domain of Staphylococcal Protein A: Comparisons of the Solution and Crystal Structures, Biochemistry. (1992). doi: 10.1021/bi00155a020. [DOI] [PubMed] [Google Scholar]
- [29].Klein JS, Gnanapragasam PNP, Galimidi RP, Foglesong CP, West AP, Bjorkman PJ, Examination of the contributions of size and avidity to the neutralization mechanisms of the anti-HIV antibodies b12 and 4E10, Proc. Natl. Acad. Sci. 106 (2009) 7385–7390. doi: 10.1073/PNAS.0811427106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Bankston TE, Stone MC, Carta G, Theory and applications of refractive index-based optical microscopy to measure protein mass transfer in spherical adsorbent particles, J. Chromatogr. A. 1188 (2008) 242–254. doi: 10.1016/J.CHROMA.2008.02.076. [DOI] [PubMed] [Google Scholar]
- [31].Lowe D, Dudgeon K, Rouet R, Schofield P, Jermutus L, Christ D, Aggregation, stability, and formulation of human antibody therapeutics, Adv. Protein Chem. Struct. Biol. 84 (2011) 41–61. doi: 10.1016/B978-0-12-386483-3.00004-5. [DOI] [PubMed] [Google Scholar]
- [32].Jin W, Xing Z, Song Y, Huang C, Xu X, Ghose S, Li ZJ, Protein aggregation and mitigation strategy in low pH viral inactivation for monoclonal antibody purification., MAbs. (2019). doi: 10.1080/19420862.2019.1658493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Codina N, Hilton D, Zhang C, Chakroun N, Ahmad SS, Perkins SJ, Dalby PA, An Expanded Conformation of an Antibody Fab Region by X-Ray Scattering, Molecular Dynamics, and smFRET Identifies an Aggregation Mechanism, J. Mol. Biol. 431 (2019) 1409–1425. doi: 10.1016/j.jmb.2019.02.009. [DOI] [PubMed] [Google Scholar]
- [34].Bennion BJ, Daggett V, The molecular basis for the chemical denaturation of proteins by urea, Proc. Natl. Acad. Sci. 100 (2003) 5142–5147. doi: 10.1073/pnas.0930122100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Shukla AA, Hinckley P, Host cell protein clearance during protein a chromatography: Development of an improved column wash step, Biotechnol Prog. 24 (2008) 1115–1121. doi: 10.1021/bp.50. [DOI] [PubMed] [Google Scholar]