Abstract
Background:
Team-based care (TBC), a team of two or more healthcare professionals working collaboratively toward a shared clinical goal, is a recommended strategy to manage blood pressure (BP). However, the most effective and cost-effective TBC strategy is unknown.
Methods:
A meta-analysis of clinical trials in US adults (aged ≥20 years) with uncontrolled hypertension (≥140/90 mmHg) was performed to estimate the systolic BP (SBP) reduction for TBC strategies vs. usual care at 12 months. TBC strategies were stratified by the inclusion of a non-physician team member who could titrate antihypertensive medications. The validated BP Control Model-Cardiovascular Disease (CVD) Policy Model was used to project the expected BP reductions out to 10 years, and simulate CVD events, direct healthcare costs, quality-adjusted life years (QALYs), and cost-effectiveness of TBC with physician and non-physician titration.
Results:
Among 19 studies comprising 5,993 participants, the 12-month SBP change vs. usual care was −5.0 (95% confidence interval: −7.9 to −2.2) mmHg for TBC with physician titration and −10.5 (−16.2 to −4.8) mmHg for TBC with non-physician titration. Relative to usual care at 10 years, TBC with non-physician titration was estimated to cost $95 (95% uncertainty interval: -$563 to $664) more per patient and gain 0.022 (0.003 to 0.042) QALYs, costing $4,400/QALY gained. TBC with physician titration was estimated to cost more and gain fewer QALYs than TBC with non-physician titration.
Conclusions:
TBC with non-physician titration yields superior hypertension outcomes compared with other strategies and is a cost-effective way to reduce hypertension-related morbidity and mortality in the US.
Keywords: Hypertension, blood pressure, team-based care, meta-analysis, cost-effectiveness
INTRODUCTION
Blood pressure (BP) control rates worsened by 10% from 2013-2014 to 2017-2018 despite the availability of effective antihypertensive medications.1 In order to improve BP control and reduce associated cardiovascular disease (CVD) morbidity and mortality, it is important to understand the most efficient and effective care delivery strategies to guide program design and health policy development.1
Team-based care (TBC), i.e., healthcare services delivered by two or more providers working collaboratively, is a hypertension control strategy recommended by the 2017 American College of Cardiology (ACC)/American Heart Association (AHA) BP guideline and the 2020 US Surgeon General’s Call to Action to Control Hypertension.2-4 TBC has been associated with improved rates of BP control compared with usual primary care.5, 6 Additionally, TBC with non-physician team members who can manage medications (e.g., pharmacists, nurse practitioners), is associated with greater BP reductions compared with programs where only physicians can manage medications.6 However, training and deploying non-physician team members to manage antihypertensive medications may be costly to clinics and healthcare systems.
Reversing recent BP control trends and meeting quality benchmarks may require healthcare organizations to substantially change usual hypertension care processes.7 However, the most clinically effective and cost-effective TBC design, including TBC impact on hypertension care processes (e.g., provider visit frequency, clinical inertia, and optimal length of the program) is unknown. We therefore performed a meta-analysis and simulation to estimate the effectiveness and cost-effectiveness of TBC program strategies for hypertension management.
METHODS
The data that support the findings of the meta-analysis are available from Dr. Bellows upon reasonable request. The simulation model and key inputs used for the cost-effectiveness analysis are available on reasonable request and approval by the research team. Interested researchers will be asked to submit a 1- to 2-page research proposal and collaboration plan to Dr. Bellows and will be required to sign a Creative Commons agreement.
Meta-Analysis
Data Searches and Study Inclusion
Randomized controlled trials were identified through a literature search performed using PubMed and from prior systematic reviews and meta-analyses (Supplemental Methods).5, 6, 8 Studies were included if they were (1) performed in the US, (2) published after 1/1/2003, (3) had a duration of ≥6 months, (4) included a TBC arm, and (5) included participants with uncontrolled BP at baseline, defined as systolic BP (SBP) ≥140 mmHg or ≥130 mmHg in those with diabetes or chronic kidney disease. Studies were excluded if they did not report sufficient data on BP outcomes, did not report outcomes at six months, or did not report information such as standard deviations or confidence intervals for BP outcomes.
Data Extraction
Data extraction was performed using a standardized tool in the Covidence systematic review software (Veritas Health Innovation, Melbourne, Australia). Data were extracted by authors KBB, ASR, LPC, and BKB, with all data extracted verified by a second team member. All data extracted are described in the Supplemental Methods.
Outcomes
The primary outcomes were SBP change at six and twelve months for TBC vs. usual care (Supplemental Methods). Secondary outcomes included diastolic BP (DBP) change and BP control at six and twelve months. All outcomes assessed compared with usual care, which was generally defined across studies as hypertension management by a primary care physician without non-physician team member support.
Quality Assessment
We used the Cochrane Risk of Bias 2 tool for randomized controlled trials to assess the quality of the data collected.9 Each paper was assessed by two independent reviewers (KBB, ASR, LPC, and BKB), with differences resolved by consensus with a third reviewer.
Statistical Analyses
The meta-analysis adhered to practices recommended in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (Table S1). The meta-analysis was performed using Stata version 16.1 (StataCorp LLC, College Station, TX) using random effects models to generate weighted mean differences of SBP and DBP outcomes and percent difference in BP control. Analyses were stratified by whether a physician or non-physician team (e.g., pharmacist, nurse practitioner) member performed antihypertensive medication titration. When there were two or more intervention arms meeting inclusion criteria within a given study, they were each included in the meta-analysis, and labeled sequentially. Funnel plots were used to examine the potential for publication bias. A “leave-one-out” sensitivity analysis was performed to examine the impact of excluding each study from the analysis one at a time to identify studies that might have skewed the results. Meta-regression was used to examine heterogeneity by baseline SBP.
Cost-Effectiveness Analysis
Model Overview
The BP Control-CVD Policy Model (BP-CVDPM) is a hybrid, discrete event simulation model combining the independently validated BP Control Model and CVD Policy Model (Figure S1).10 The BP-CVDPM predicts long-term BP outcomes by simulating modifiable hypertension care management processes and subsequent effect on CVD events, direct healthcare costs, survival, and quality-adjusted survival (Supplemental Methods).
Population
The model simulated a nationally representative cohort of 100,000 US adults (aged ≥20 years) with uncontrolled BP (SBP/DBP ≥140/90 mmHg) identified from the National Health and Nutrition Examination Survey (NHANES) 1999-2018 cycles. Included individuals from NHANES had lifetime CVD risk factor trajectories multiply imputed from participants in the National Heart, Lung, and Blood Institute Pooled Cohorts Study (Supplemental Methods).10-14
Comparators
In the primary analysis, three strategies were compared: usual care, TBC with physician antihypertensive medication titration, and TBC with non-physician antihypertensive medication titration (Table S2). The usual care strategy was the same as previously published.7, 10, 15 All TBC visits were assumed to be in addition to usual care visits. For TBC with physician antihypertensive medication titration, it was assumed individuals had TBC visits with a registered nurse where BP was measured and hypertension counseling, including medication adherence, was provided. If BP was uncontrolled at these visits, it was assumed that this was communicated to the physician who then determined if treatment should be intensified. TBC visits with non-physician medication titration were assumed to be the same except that care was provided by a pharmacist who could also intensify treatment if BP was uncontrolled.
In the primary analysis, TBC visits were assumed to occur for twelve months, matching the meta-analysis; this was explored in sensitivity analysis. After TBC visits had ended, individuals were assumed to return to usual care. Both TBC arms were assumed to have beneficial effects on medication adherence (Supplemental Methods), which decreased linearly over one year after the end of TBC visits until no adherence benefits over usual care remained.10, 15
Model Inputs
In the primary analysis, the frequency of physician office visits (once every 11.5 weeks) and TBC visits (once every six weeks) matched the frequency from the meta-analysis intervention during the first twelve months (Table 1). After the first twelve months, TBC visits ended, and usual care visit frequency was dependent upon BP control and patient and visit characteristics.7, 10
Table 1.
Selected BP-CVDPM team-based care inputs.
| Team-Based Care or Self-Monitoring Intervention Components |
Base-case | Lower Range |
Upper Range |
Source |
|---|---|---|---|---|
| Processes of Hypertension Care | ||||
| Weeks between visits during intervention | Current meta-analysis | |||
| TBC visit | 6.0 | 2.0 | 12.0 | |
| Physician visit | 11.5 | - | - | |
| Probability of treatment intensification for TBC with non-physician titration when SBP | Bryant et al.,10 calibrated | |||
| 140-149 mmHg | 0.713 | 0.330 | 1.000 | |
| ≥150 mmHg | 0.950 | 0.330 | 1.000 | |
| Probability of treatment intensification by physician when BP uncontrolled | Bellows et al.7 | |||
| First antihypertensive medication | ||||
| ≥160 mmHg without diabetes or CKD, ≥140/90 mmHg with diabetes or CKD | 0.333 | 0.313 | 0.440 | |
| BP otherwise uncontrolled | 0.208 | 0.207 | 0.310 | |
| All subsequent medication intensifications | 0.130 | 0.065 | 0.195 | |
| Relative reduction in non-adherence with intervention | 0.400 | - | - | Calibrated, Assumption |
| Costs (2021 USD) | ||||
| Minutes per first TBC visit | 42.5 | 25.0 | 60.0 | Bryant et al.10 |
| Minutes per TBC visit when measured BP | ||||
| <140/90 mmHg | 15.0 | 12.5 | 45.0 | |
| ≥140/90 mmHg | 30.0 | 15.0 | 52.5 | |
| Pharmacist hourly wage | $60 | $41 | $79 | Bureau of Labor Statistics |
| Nurse hourly wage | $38 | $26 | $56 | |
| Physician review following TBC with physician titration | $23 | $20 | $30 | CMS Physician Fee Schedule, CPT 99211 |
| Employee benefit rate | 30.0% | - | - | Assumption |
BP – blood pressure, CKD – chronic kidney disease, SBP – systolic blood pressure, TBC – team-based care, USD – United States dollars.
Notes: The table shows the mean base-case values, and the upper and lower values used in sensitivity analysis, for selected model inputs related to the TBC interventions.
Costs for TBC visits were calculated using the national median hourly wage for registered nurses and pharmacists, derived from the US Bureau of Labor Statistics, and duration of the visit derived from interviews with providers in prior analyses, and an assumed 30% rate for the cost of employee benefits (Table 1).10, 16, 17 For TBC with physician titration, an additional cost was added for physicians to review notes and determine if treatment intensification was needed using (CPT code: 99211). All other model inputs, including other costs, were derived from published literature and analysis of publicly available databases (Table S3).
Outcomes
The primary outcomes were total direct healthcare costs (2021 USD), quality-adjusted life years (QALYs), and incremental cost-effectiveness ratios (ICERs) at 10 years from a healthcare sector perspective.18 All future costs and QALYs were discounted 3% annually.18 TBC was classified as highly cost-effective when the ICER was <$50,000 per QALY gained and at least intermediately cost-effective when <$150,000 per QALY gained.19 Secondary outcomes included CVD events, survival, SBP changes, proportion with controlled BP (<140/90 mmHg), disaggregated costs, and disaggregated QALYs.
Statistical Analyses
As in prior analyses, the BP-CVDPM was calibrated to match contemporary US CVD event and mortality rates and reproduce the difference in SBP with TBC interventions vs. usual care at six and twelve months from the meta-analysis (Supplemental Methods, Figures S2-S3, Table S4).10-12, 15 The analyses adhered to practices recommended in the Consolidated Health Economic Evaluation Reporting Standards (Table S5).20 The BP-CVDPM was programmed in TreeAge Pro 2021 (TreeAge Software Inc, Williamstown, MA) with additional analyses performed using R version 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria). To ensure stable cost-effectiveness estimates, 100,000 individuals were simulated in all analyses. The mean and uncertainty interval (UI) for all outcomes were derived from 100 probabilistic iterations, where input parameters were randomly sampled from pre-specified statistical distributions. Uncertainty in the ICER was reported as the proportion of iterations with an ICER <$50,000 and <$150,000 per QALY gained.
Sensitivity Analyses
One-way sensitivity analyses were used to examine the effect of independently varying the model inputs shown in Table 1 across plausible ranges on the cost-effectiveness of TBC, while holding all other inputs at their base-case values (Supplemental Methods). Additional analyses examined the impact of extending the duration over which TBC visits occurred to 5 and 10 years, with the same visit frequency as occurred in the first year. Separately, a “light touch” scenario was simulated in which individuals only had one TBC visit per year after the initial twelve months with 5- and 10-year durations of TBC visits. As costs beyond those required to directly provide patient care may be incurred with TBC, a scenario analysis included administrative costs of TBC (Supplemental Methods).10
RESULTS
Meta-Analysis
A total of 19 studies were identified from the prior meta-analyses and updated literature search, comprising 5,993 individual participants (Figure S4).17, 21-39 The mean (SD) baseline age was 58.9 (6.8), 35.3% were female, 37.7% were Black, and mean baseline SBP was 148.1 (10.3) mmHg (Tables S6-S7). The components included in TBC (e.g., web-based communications, self-monitoring of BP) and workflow for hypertension management varied across trials (Table S7). The overall risk of bias was rated as low in 63% of the studies, some concerns of bias in 32%, and high in 5% (Figure S5).
A total 12 studies (3,322 participants) and 7 studies (3,128 participants) were included in the meta-analyses of SBP change at six and twelve months, respectively. At six and twelve months, TBC overall had 7.2 mmHg (95% confidence interval [CI] −10.6, −3.8) and 8.1 mmHg (95% CI −12.1, −4.1) greater reductions in SBP than usual care (Figure 1), respectively. SBP reductions vs. usual care varied by the team member titrating antihypertensive mediations; physician titration resulted in 4.7 mmHg (95% CI −8.1, −1.4) and 5.0 mmHg (95% CI −7.9, −2.2) greater reductions, and non-physician titration in 11.5 mmHg (95% CI −15.7, −7.4) and 10.5 mmHg (95% CI −16.2, −4.8) greater reductions, at six and twelve months, respectively.
Figure 1. Systolic blood pressure changes with team-based care vs. usual care.
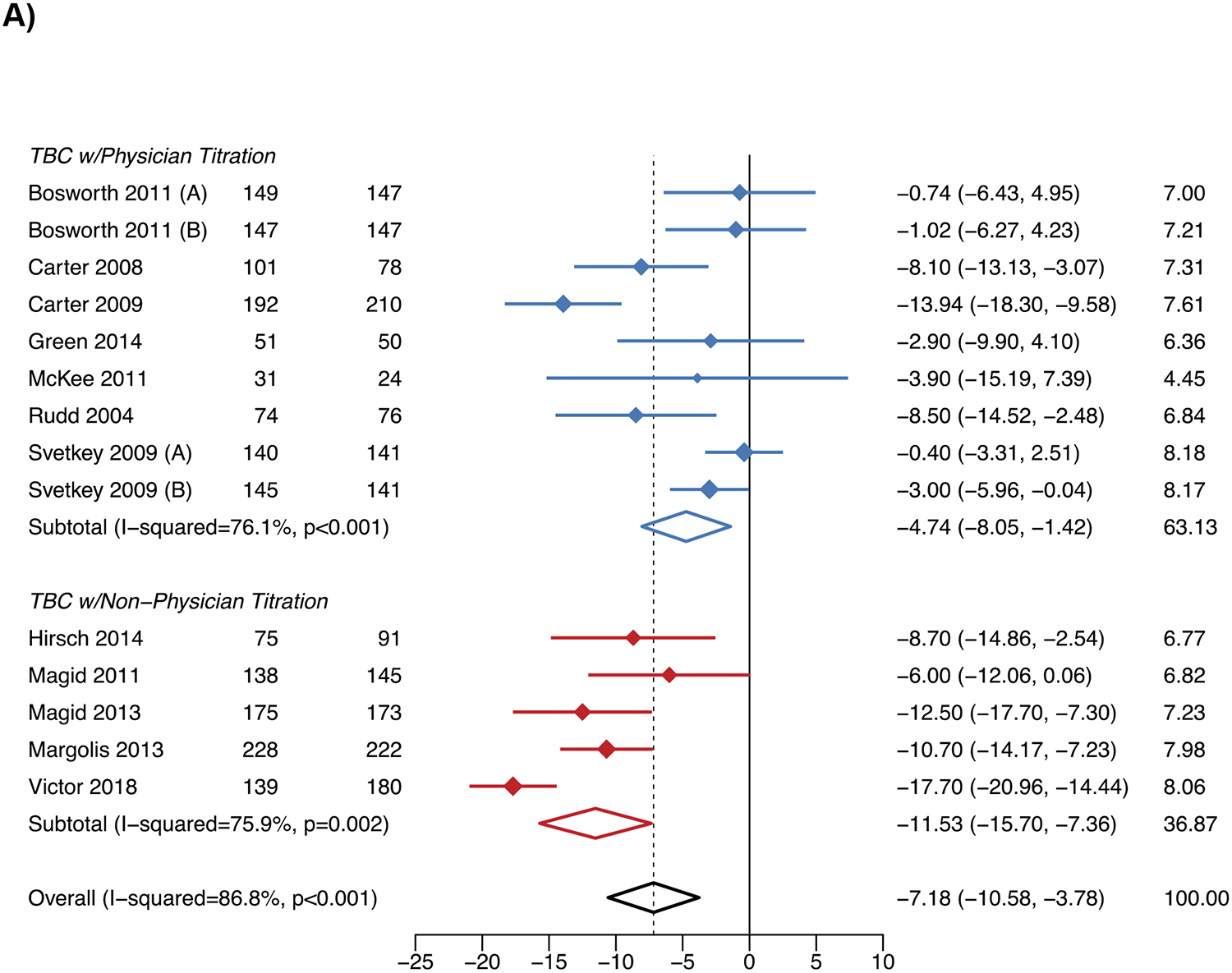
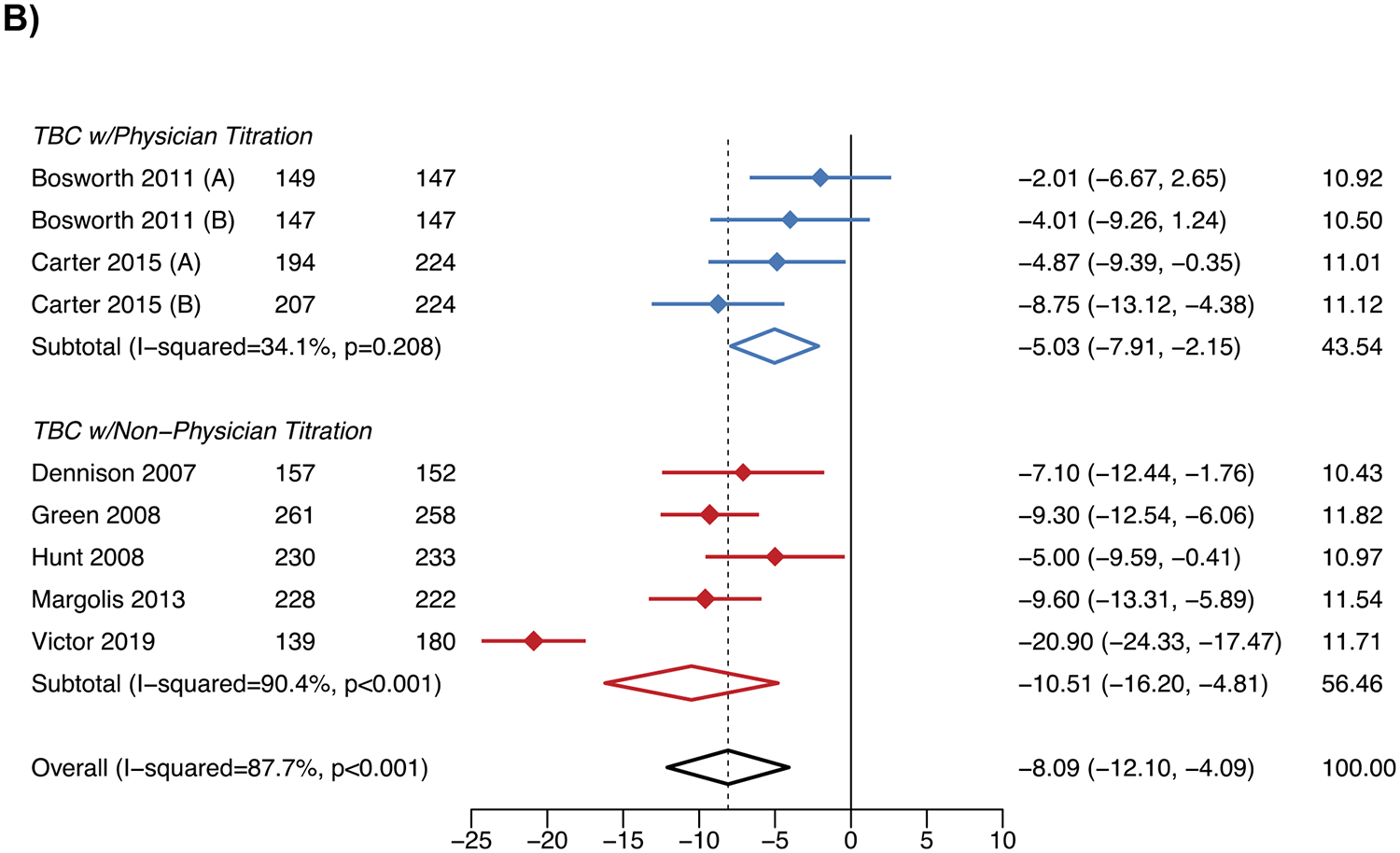
A) Six months
B) Twelve months
WMD – weighted mean difference.
Notes: The figure shows the results of the meta-analysis for the change in systolic blood pressure for team-based care vs. usual care at 6 months in Panel A and 12 months in Panel B.
The results for DBP and BP control followed the same patterns as SBP (Figures S6-S7). For the outcomes of SBP change and BP control at 6 months, there did not appear to be evidence of publication bias. However, for other outcomes, asymmetry in the funnel plots was observed (Figure S8). The “leave-one-out” sensitivity analysis indicated the SBP reduction at twelve months for TBC with non-physician titration was sensitive to the inclusion of the Victor et al. 2019 study, decreasing to −8.3 mmHg (95% CI −10.3, −6.3) when removed (Figure S9).17 The meta-regression showed that greater reductions in SBP at six and twelve months with TBC vs. usual care were associated with higher baseline SBP (Figure S10).
Cost-effectiveness
Primary Analysis
The mean (SD) baseline age of the simulated population of US adults with uncontrolled hypertension was 59.7 (16.4) years, 50.1% were female, and mean baseline SBP was 150.6 (14.3) mmHg (Table S8). At 10 years, the mean SBP with usual care was projected to be 137.9 (95% UI 136.6, 139.9) mmHg; twelve months of TBC was estimated to reduce SBP relative to usual care at 10 years by 0.8 (95% UI −1.0, −0.7) mmHg with physician titration and 3.3 (95% UI −3.9, −2.6) mmHg with non-physician titration (Figure 2, Table 2). TBC was estimated to reduce the number of individuals with ≥1 CVD event by 5 (95% UI 3, 8) per 1000 treated with physician titration and 11 (95% UI 8, 14) with non-physician titration.
Figure 2. Projected systolic blood pressure outcomes and cost-effectiveness of team-based care.
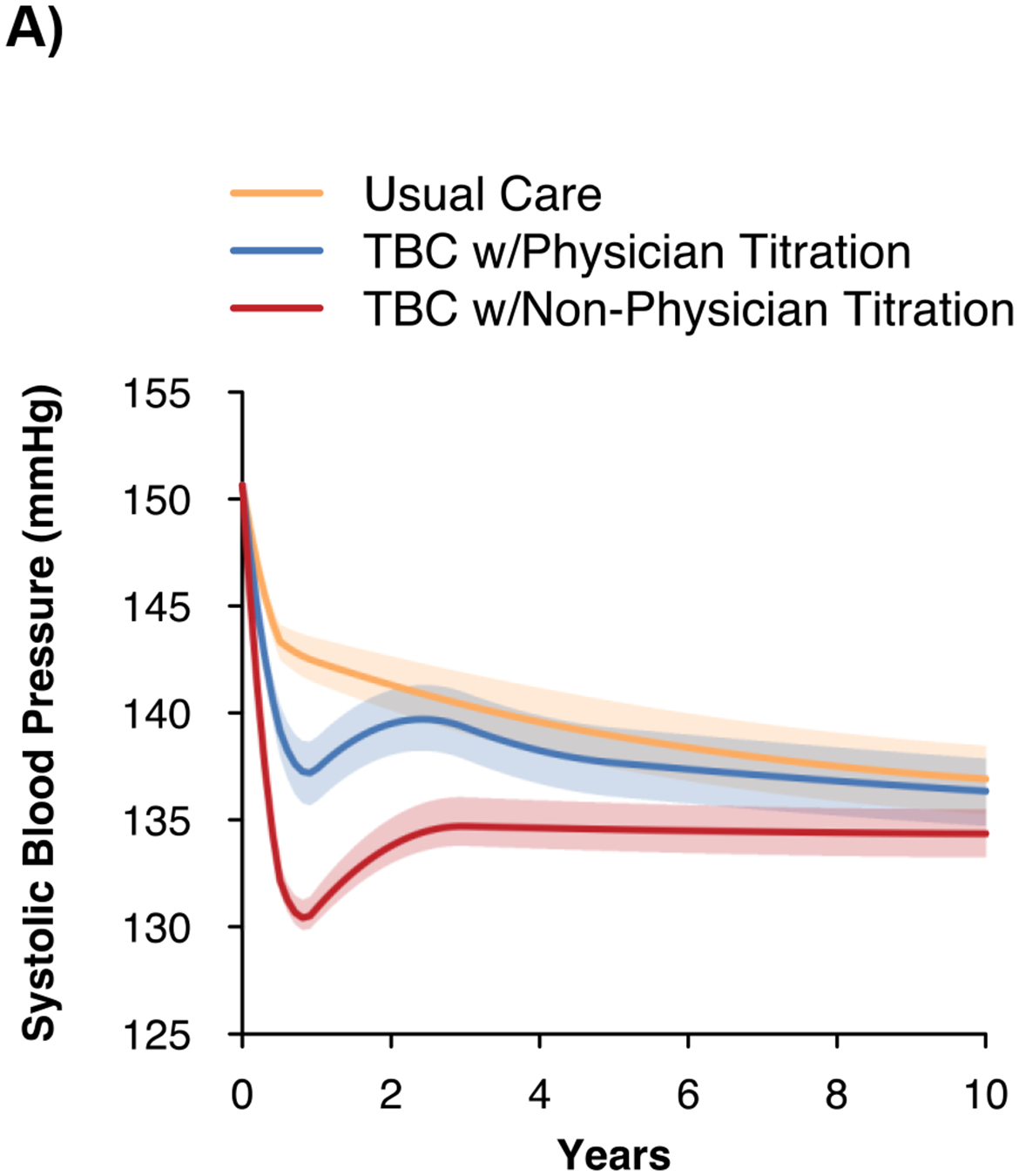
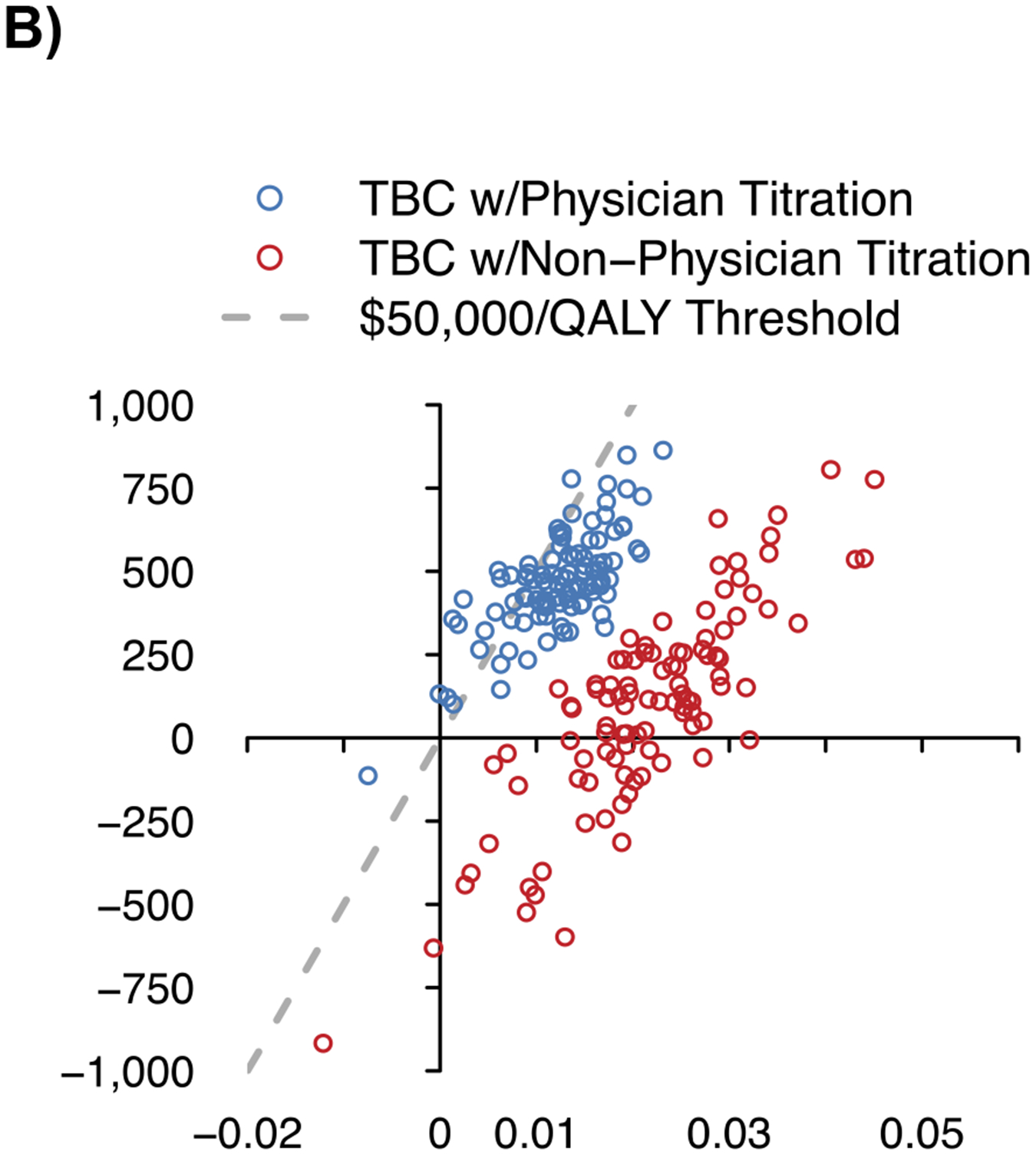
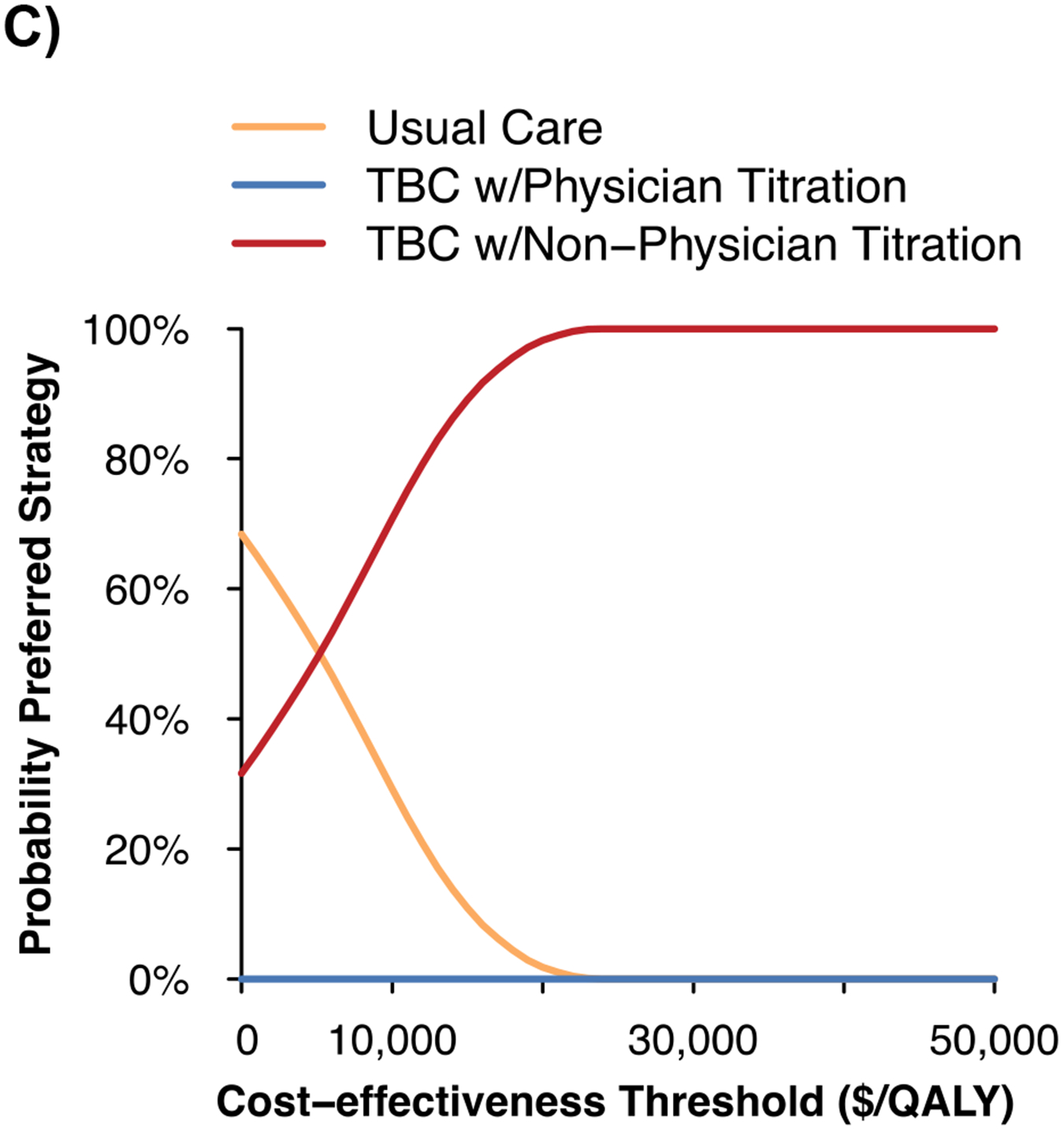
A) Projected systolic blood pressure over time
B) Incremental costs and quality-adjusted life years vs. usual care
C) Cost-effectiveness acceptability curve
QALY – quality-adjusted life years, SBP – systolic blood pressure, TBC – team-based care, USD – United States dollars.
Notes: The figure shows the projected changes in SBP with each strategy over the simulated time horizon in Panel A, the incremental costs and incremental QALYs for TBC vs. usual care in Panel B, and the probability of TBC and usual care being the preferred strategy as the cost-effectiveness threshold is varied in Panel C. The shaded region in Panel A shows the 95% uncertainty interval from 100 probabilistic iterations of the model.
Table 2.
Clinical and cost-effectiveness outcomes at 10 years.
| Usual Care | TBC with Physician Titration |
TBC with Non- physician Titration |
|
|---|---|---|---|
| Clinical Outcomes | |||
| SBP (mmHg) | 137.9 (136.3, 139.5) | 137.0 (135.5, 138.7) | 134.6 (133.5, 135.8) |
| Difference | Ref | −0.8 (−1.0, −0.7) | −3.3 (−3.9, −2.6) |
| BP control | 66.6% (57.8%, 75.4%) | 70.0% (61.2%, 78.8%) | 80.6% (73.5%, 86.7%) |
| Difference | Ref | 3.4% (2.9%, 3.9%) | 13.9% (10.8%, 17.0%) |
| Incident CVD events | 16.9% (14.2%, 20.1%) | 16.4% (13.6%, 19.5%) | 15.3% (12.7%, 18.1%) |
| Difference | Ref | −0.5% (−0.8%, −0.3%) | −1.1% (−1.4%, −0.8%) |
| Cost-effectiveness Outcomes | |||
| Mean total direct healthcare costs (2021 USD) | $105,085 ($99,127, $110,330) | $105,548 ($99,798, $110,760) | $105,180 ($99,731, $110,359) |
| Difference | Ref | $463 ($126, $770) | $95 (-$563, $664) |
| TBC visit costs* | Ref | $373 ($369, $378) | $231 ($226, $235) |
| Medication costs* | Ref | $105 ($94, $117) | $388 ($340, $436) |
| CVD costs* | Ref | −$123 (-$212, -$24) | −$430 (-$586, -$304) |
| Mean QALYs | 6.116 (5.926, 6.292) | 6.128 (5.937, 6.3) | 6.138 (5.945, 6.314) |
| Difference | Ref | 0.012 (0.001, 0.021) | 0.022 (0.003, 0.042) |
| ICER ($/QALY gained) | Ref | Dominated** | $4,390 |
| Probability preferred strategy at cost-effectiveness threshold of $50,000/QALY | 0% | 0% | 100% |
BP – blood pressure, CVD – cardiovascular disease, ICER – incremental cost-effectiveness ratio, QALY – quality-adjusted life year, Ref – reference group, SBP – systolic blood pressure, TBC – team-based care, USD – United States dollars.
Only selected cost types are shown and thus do not sum to the overall difference. Additional costs include, background (non-hypertension related) healthcare, non-TBC physician office visits, and adverse events.
TBC with physician titration is dominated by TBC with non-physician titration, meaning it costs more and is less effective.
Notes: The table shows the projected clinical and economic outcomes of usual care, TBC with physician titration of medications, and TBC with non-physician titration of medications at 10 years. TBC was assumed to occur during the first year of the simulation. After the first year, hypertension care processes (e.g., visit frequency, probability of medication titration, medication adherence) were assumed to return to usual care. Values are mean and 95% uncertainty interval (2.5th to 97.5th percentile) derived from 100 probabilistic iterations of 100,000 individuals.
The estimated per-patient cost for TBC visits over twelve months was $373 (95% UI $369, $378) with physician titration and $231 (95% UI $226, $235) with non-physician titration. Relative to usual care at 10 years, TBC with non-physician titration was estimated to cost $4,400/QALY gained and was the preferred strategy in 100% of model iterations at a cost-effectiveness threshold of <$50,000/QALY gained (Figure 2). TBC with non-physician titration was estimated to dominate (i.e., cost less and be more effective) TBC with physician titration in 30% of probabilistic analyses.
Sensitivity Analysis
The cost-effectiveness of TBC with non-physician titration was most sensitive to the probability of non-physician medication titration when BP was uncontrolled and frequency of TBC visits (Figure S11). Relative to usual care, TBC with non-physician titration remained highly cost-effective even with only one TBC visit every 12 weeks. In the scenario analyses with increased duration of TBC to 5 and 10 years, TBC with physician titration continued to be dominated by TBC with non-physician titration, which was highly or intermediately cost-effective vs. usual care (Table S9). In the “light touch” scenarios with 5 and 10 years of TBC but with only one visit per year in subsequent years, TBC with non-physician titration continued to be highly cost-effective relative to usual care. In the scenario analysis including administrative costs, TBC with non-physician titration remained highly cost-effective with an ICER $17,400/QALY gained and continued to dominate TBC with physician titration. If TBC with non-physician titration could be implemented for <$1,100 or <$3,300 per patient enrolled, it would remain highly or intermediately cost-effective, respectively.
DISCUSSION
Hypertension TBC with a non-physician team member (e.g., pharmacist) titrating antihypertensive medications yields better BP outcomes than usual care or TBC with physician medication titration. Additionally, TBC with a non-physician team member titrating medications is highly cost-effective vs. usual care (ICER $4,400/QALY gained) and is estimated to cost less and be more effective than TBC with physician titration. Health care organizations that have capacity to deploy non-physicians to titrate antihypertensive medications as part of a TBC program may face up-front implementation costs, but our analysis suggests that this approach will yield health and economic value over time.
The greater BP reduction with TBC with non-physician medication titration that we estimated is consistent with prior meta-analyses upon which our analysis expanded.5, 6 Few prior economic analyses have estimated the impact of TBC for hypertension management. One analysis of US adults estimated that 1-year of hypertension TBC delivered by a pharmacist or nurse would decrease SBP by 8.1 mmHg relative to usual care at a per-patient cost of $1,046 (inflated to 2021 USD) for office and telephone visits.40 Another analysis of a pharmacist-led home BP telemonitoring intervention found a decrease in SBP by 9.7 mmHg relative to usual care at 1 year with a total per-patient cost of $1,457 (inflated to 2021 USD). This estimate did not substantially differ from usual care costs and included pharmacist visits, telemonitoring costs, and savings in medical care but did not include longer-term projections or impact on quality of life.35, 41 The current study builds upon these analyses by estimating the impact of varying the processes of care driving changes in BP, which may be useful for health systems in designing and implementing TBC that fits into existing frameworks.
Healthcare systems are often reimbursed relative to their success in controlling BP, among other quality metrics, introducing financial incentives to optimize care delivery. Our analysis indicates that use of non-physician team members with the ability to prescribe and titrate antihypertensive medications, e.g., clinical pharmacists under a collaborative practice agreement, may improve BP and CVD outcomes in a cost-effective manner. However, health systems may encounter barriers to implementing these programs in a sustainable fashion due to challenges with team member availability, variability in state laws allowing collaborative practice agreements between physicians and pharmacist, insufficient reimbursement for pharmacist services, and high upfront training and implementation costs. However, successful TBC programs demonstrate that these obstacles can be overcome.35, 42, 43 With persistent decline in BP control rates on a national scale and disruptions to care because of the COVID-19 pandemic, establishing a framework for TBC for hypertension control is imperative to improve CVD outcomes.
This work is strengthened by use of the BP-CVDPM and the ability to simulate processes of hypertension care that drive changes in BP. However, these results should be considered in the context of the following limitations. There is a lack of representation of racial and ethnic minority populations in the original trials, as most studies in our meta-analysis had a majority white population, with few exceptions.17, 27 Additionally, the impacts of social determinants of health were not captured in our analysis. We were therefore unable to explore TBC designs that account for different outcomes that may result from differences in access to care, affordable medications, adequate nutrition, and safe, stable environments. Future research should examine the impact of these factors and how TBC may be implemented in ways that promote health equity and decrease disparities.44 Cost data from individual trials were not available, trials may not have intended to perform cost-effectiveness analyses, and cost-effectiveness components were not necessarily systematically reported; therefore, national estimates were used in our analysis. Though our analysis indicates TBC with a non-physician who can titrate medications improves BP control, detailed workflows and intervention components (e.g., inclusion of self-monitoring of BP) varied across trials and their contribution to BP lowering with TBC were not assessed in our analysis. Meta-analyses show the intensity of a self-monitoring of BP intervention is positively associated with BP lowering.8 There may be a synergistic effect on BP lowering achieved by combining TBC with self-monitoring of BP.8, 15 As uptake of self-monitoring increases both because of improved guideline adherence and transition to and persistence of telemedicine in the wake of the COVID-19 pandemic, more data are likely to be available to better understand the impact of self-monitoring of BP on TBC.
This work was conducted from a healthcare sector perspective, which includes direct healthcare costs regardless of the payer, and used national averages for costs of providers and medications, which may not be applicable to local health systems. Training and licensing costs for non-physicians were not included and other costs of implementing TBC (e.g., time for scheduling TBC visits, BP measurement equipment) may not be fully accounted for in our analysis. However, incorporating administrative costs for TBC in our analysis did not alter cost-effectiveness conclusions.
Supplementary Material
NOVELTY AND RELEVANCE.
What Is New?
Hypertension care provided by a team of providers that includes a non-physician who can titrate antihypertensive medications cost-effectively reduces systolic blood pressure and cardiovascular disease risk
What Is Relevant?
Hypertension control has worsened in the US over the last decade and team-based care is recommended by hypertension treatment guidelines to improve control
Clinical Implications?
Health systems providing team-based care for hypertension should prioritize inclusion of non-physician providers who can titrate antihypertensive medications
PERSPECITVES.
TBC with non-physician titration is a clinically effective and cost-effective strategy to improve hypertension control and help reverse the current national trends. These results should be used to inform policy with the potential to restructure reimbursement to facilitate implementation. Clinics and health systems actively implementing TBC can use these results to guide program design.
ACKNOWLEDGEMENTS
This work would not have been possible without the original trial participants and dedicated researchers.
SOURCES OF FUNDING
This analysis was funded by R01HL130500 (Dr. Moran) and K01HL140170 (Dr. Bellows) from National Heart, Lung, and Blood Institute (NHLBI; Bethesda, MD). Additionally, Dr. Kronish was supported by R01HL152699, Dr. Fontil was supported by K23HL136899, and Dr. Zhang was supported by R01HL158790, both from the NHLBI (Bethesda, MD). The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, approval of the manuscript; or decision to submit for publication.
NON-STANDARD ABBREVIATIONS
- ACC
American College of Cardiology
- AHA
American Heart Association
- BP-CVDPM
Blood Pressure Control-Cardiovascular Disease Policy Model
- ICER
incremental cost-effectiveness ratio
- NHANES
National Health and Nutrition Examination Survey
- QALY
quality-adjusted life year
- TBC
team-based care
- UI
uncertainty interval
Footnotes
DISCLOSURES
The meta-analysis was not prospectively registered.
REFERENCES
- 1.Muntner P, Hardy ST, Fine LJ, Jaeger BC, Wozniak G, Levitan EB, et al. Trends in blood pressure control among us adults with hypertension, 1999-2000 to 2017-2018. JAMA. 2020;324:1190–1200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mitchell PH, Matthew W, Golden R, Mcnellis B, Okun S, Webb Edwin C, et al. Core principles & values of effective team-based health care. 2012:1–32 [Google Scholar]
- 3.Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, Dennison Himmelfarb C, et al. 2017. acc/aha/aapa/abc/acpm/ags/apha/ash/aspc/nma/pcna guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: A report of the american college of cardiology/american heart association task force on clinical practice guidelines. Hypertension. 2018;71:e13–e115 [DOI] [PubMed] [Google Scholar]
- 4.Surgeon general’s call to action to control hypertension. 2020
- 5.Proia KK, Thota AB, Njie GJ, Finnie RK, Hopkins DP, Mukhtar Q, et al. Team-based care and improved blood pressure control: A community guide systematic review. Am J Prev Med. 2014;47:86–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mills KT, Obst KM, Shen W, Molina S, Zhang HJ, He H, et al. Comparative effectiveness of implementation strategies for blood pressure control in hypertensive patients: A systematic review and meta-analysis. Ann Intern Med. 2018;168:110–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bellows BK, Ruiz-Negron N, Bibbins-Domingo K, King JB, Pletcher MJ, Moran AE, et al. Clinic-based strategies to reach united states million hearts 2022 blood pressure control goals. Circ Cardiovasc Qual Outcomes. 2019;12:e005624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tucker KL, Sheppard JP, Stevens R, Bosworth HB, Bove A, Bray EP, et al. Self-monitoring of blood pressure in hypertension: A systematic review and individual patient data meta-analysis. PLoS Med. 2017;14:e1002389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. Rob 2: A revised tool for assessing risk of bias in randomised trials. Bmj. 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- 10.Bryant KB, Moran AE, Kazi DS, Zhang Y, Penko J, Ruiz-Negron N, et al. Cost-effectiveness of hypertension treatment by pharmacists in black barbershops. Circulation. 2021;143:2384–2394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kohli-Lynch CN, Bellows BK, Thanassoulis G, Zhang Y, Pletcher MJ, Vittinghoff E, et al. Cost-effectiveness of low-density lipoprotein cholesterol level-guided statin treatment in patients with borderline cardiovascular risk. JAMA Cardiol. 2019;4:969–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kohli-Lynch CN, Bellows BK, Zhang Y, Spring B, Kazi DS, Pletcher MJ, et al. Cost-effectiveness of lipid-lowering treatments in young adults. J Am Coll Cardiol. 2021;78:1954–1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zeki Al Hazzouri A, Vittinghoff E, Zhang Y, Pletcher MJ, Moran AE, Bibbins-Domingo K, et al. Use of a pooled cohort to impute cardiovascular disease risk factors across the adult life course. Int J Epidemiol. 2019;48:1004–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oelsner EC, Balte PP, Cassano PA, Couper D, Enright PL, Folsom AR, et al. Harmonization of respiratory data from 9 us population-based cohorts: The nhlbi pooled cohorts study. Am J Epidemiol. 2018;187:2265–2278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bryant KB, Sheppard JP, Ruiz-Negrón N, Kronish IM, Fontil V, King JB, et al. Impact of self-measured blood pressure monitoring and management on processes of hypertension care and long-term blood pressure. J Am Heart Assoc. 2020;9:e016174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.US Bureau of Labor Statistics. 29-1141 registered nurses. Occupational Employment and Wages. 2021;2022 [Google Scholar]
- 17.Victor RG, Blyler CA, Li N, Lynch K, Moy NB, Rashid M, et al. Sustainability of blood pressure reduction in black barbershops. Circulation. 2019;139:10–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanders GD, Neumann PJ, Basu A, Brock DW, Feeny D, Krahn M, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: Second panel on cost-effectiveness in health and medicine. JAMA. 2016;316:1093–1103 [DOI] [PubMed] [Google Scholar]
- 19.Anderson JL, Heidenreich PA, Barnett PG, Creager MA, Fonarow GC, Gibbons RJ, et al. Acc/aha statement on cost/value methodology in clinical practice guidelines and performance measures: A report of the american college of cardiology/american heart association task force on performance measures and task force on practice guidelines. Circulation. 2014;129:2329–2345 [DOI] [PubMed] [Google Scholar]
- 20.Husereau D, Drummond M, Augustovski F, de Bekker-Grob E, Briggs AH, Carswell C, et al. Consolidated health economic evaluation reporting standards 2022 (cheers 2022) statement: Updated reporting guidance for health economic evaluations. Value Health. 2022;25:3–9 [DOI] [PubMed] [Google Scholar]
- 21.Borenstein JE, Graber G, Saltiel E, Wallace J, Ryu S, Archi J, et al. Physician-pharmacist comanagement of hypertension: A randomized, comparative trial. Pharmacotherapy. 2003;23:209–216 [DOI] [PubMed] [Google Scholar]
- 22.Bosworth HB, Powers BJ, Olsen MK, McCant F, Grubber J, Smith V, et al. Home blood pressure management and improved blood pressure control: Results from a randomized controlled trial. Arch Intern Med. 2011;171:1173–1180 [DOI] [PubMed] [Google Scholar]
- 23.Carter BL, Ardery G, Dawson JD, James PA, Bergus GR, Doucette WR, et al. Physician and pharmacist collaboration to improve blood pressure control. Arch Intern Med. 2009;169:1996–2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carter BL, Bergus GR, Dawson JD, Farris KB, Doucette WR, Chrischilles EA, et al. A cluster randomized trial to evaluate physician/pharmacist collaboration to improve blood pressure control. J Clin Hypertens (Greenwich). 2008;10:260–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carter BL, Coffey CS, Ardery G, Uribe L, Ecklund D, James P, et al. Cluster-randomized trial of a physician/pharmacist collaborative model to improve blood pressure control. Circ Cardiovasc Qual Outcomes. 2015;8:235–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dennison CR, Post WS, Kim MT, Bone LR, Cohen D, Blumenthal RS, et al. Underserved urban african american men: Hypertension trial outcomes and mortality during 5 years. Am J Hypertens. 2007;20:164–171 [DOI] [PubMed] [Google Scholar]
- 27.Edelman D, Fredrickson SK, Melnyk SD, Coffman CJ, Jeffreys AS, Datta S, et al. Medical clinics versus usual care for patients with both diabetes and hypertension: A randomized trial. Ann Intern Med. 2010;152:689–696 [DOI] [PubMed] [Google Scholar]
- 28.Green BB, Anderson ML, Cook AJ, Catz S, Fishman PA, McClure JB, et al. E-care for heart wellness: A feasibility trial to decrease blood pressure and cardiovascular risk. Am J Prev Med. 2014;46:368–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Green BB, Cook AJ, Ralston JD, Fishman PA, Catz SL, Carlson J, et al. Effectiveness of home blood pressure monitoring, web communication, and pharmacist care on hypertension control: A randomized controlled trial. JAMA. 2008;299:2857–2867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hill MN, Han HR, Dennison CR, Kim MT, Roary MC, Blumenthal RS, et al. Hypertension care and control in underserved urban african american men: Behavioral and physiologic outcomes at 36 months. Am J Hypertens. 2003;16:906–913 [DOI] [PubMed] [Google Scholar]
- 31.Hirsch JD, Steers N, Adler DS, Kuo GM, Morello CM, Lang M, et al. Primary care-based, pharmacist-physician collaborative medication-therapy management of hypertension: A randomized, pragmatic trial. Clin Ther. 2014;36:1244–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hunt JS, Siemienczuk J, Pape G, Rozenfeld Y, MacKay J, LeBlanc BH, et al. A randomized controlled trial of team-based care: Impact of physician-pharmacist collaboration on uncontrolled hypertension. J Gen Intern Med. 2008;23:1966–1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Magid DJ, Ho PM, Olson KL, Brand DW, Welch LK, Snow KE, et al. A multimodal blood pressure control intervention in 3 healthcare systems. Am J Manag Care. 2011;17:e96–103 [PubMed] [Google Scholar]
- 34.Magid DJ, Olson KL, Billups SJ, Wagner NM, Lyons EE, Kroner BA. A pharmacist-led, american heart association heart360 web-enabled home blood pressure monitoring program. Circ Cardiovasc Qual Outcomes. 2013;6:157–163 [DOI] [PubMed] [Google Scholar]
- 35.Margolis KL, Asche SE, Bergdall AR, Dehmer SP, Groen SE, Kadrmas HM, et al. Effect of home blood pressure telemonitoring and pharmacist management on blood pressure control: A cluster randomized clinical trial. Jama. 2013;310:46–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McKee MD, Fletcher J, Sigal I, Giftos J, Schechter C, Walker EA. A collaborative approach to control hypertension in diabetes: Outcomes of a pilot intervention. J Prim Care Community Health. 2011;2:148–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rudd P, Miller NH, Kaufman J, Kraemer HC, Bandura A, Greenwald G, et al. Nurse management for hypertension. A systems approach. Am J Hypertens. 2004;17:921–927 [DOI] [PubMed] [Google Scholar]
- 38.Svetkey LP, Pollak KI, Yancy WS Jr., Dolor RJ, Batch BC, Samsa G, et al. Hypertension improvement project: Randomized trial of quality improvement for physicians and lifestyle modification for patients. Hypertension. 2009;54:1226–1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Victor RG, Lynch K, Li N, Blyler C, Muhammad E, Handler J, et al. A cluster-randomized trial of blood-pressure reduction in black barbershops. N Engl J Med. 2018;378:1291–1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dehmer SP, Baker-Goering MM, Maciosek MV, Hong Y, Kottke TE, Margolis KL, et al. Modeled health and economic impact of team-based care for hypertension. Am J Prev Med. 2016;50:S34–S44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dehmer SP, Maciosek MV, Trower NK, Asche SE, Bergdall AR, Nyboer RA, et al. Economic evaluation of the home blood pressure telemonitoring and pharmacist case management to control hypertension (hyperlink) trial. J Am Coll Clin Pharm. 2018;1:21–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jaffe MG, Lee GA, Young JD, Sidney S, Go AS. Improved blood pressure control associated with a large-scale hypertension program. Jama. 2013;310:699–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sim JJ, Handler J, Jacobsen SJ, Kanter MH. Systemic implementation strategies to improve hypertension: The kaiser permanente southern california experience. Can J Cardiol. 2014;30:544–552 [DOI] [PubMed] [Google Scholar]
- 44.Colvin CL, Kalejaiye A, Ogedegbe G, Commodore-Mensah Y. Advancing equity in blood pressure control: A response to the surgeon general's call-to-action. Am J Hypertens. 2022;35:217–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Y, Vittinghoff E, Pletcher MJ, Allen NB, Zeki Al Hazzouri A, Yaffe K, et al. Associations of blood pressure and cholesterol levels during young adulthood with later cardiovascular events. J Am Coll Cardiol. 2019;74:330–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Law MR, Wald NJ, Morris JK, Jordan RE. Value of low dose combination treatment with blood pressure lowering drugs: Analysis of 354 randomised trials. BMJ. 2003;326:1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: Meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ. 2009;338:b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther. 2001;23:1296–1310 [DOI] [PubMed] [Google Scholar]
- 49.Vrijens B, Vincze G, Kristanto P, Urquhart J, Burnier M. Adherence to prescribed antihypertensive drug treatments: Longitudinal study of electronically compiled dosing histories. BMJ. 2008;336:1114–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kronish IM, Edmondson D, Shimbo D, Shaffer JA, Krakoff LR, Schwartz JE. A comparison of the diagnostic accuracy of common office blood pressure measurement protocols. Am J Hypertens. 2018;31:827–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Turchin A, Goldberg SI, Shubina M, Einbinder JS, Conlin PR. Encounter frequency and blood pressure in hypertensive patients with diabetes mellitus. Hypertension. 2010;56:68–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xie XMD, Atkins EB, Lv JP, Bennett AB, Neal BP, Ninomiya TP, et al. Effects of intensive blood pressure lowering on cardiovascular and renal outcomes: Updated systematic review and meta-analysis. The Lancet (British edition). 2016;387:435–443 [DOI] [PubMed] [Google Scholar]
- 53.SPRINT Research Group, Wright JT Jr., Williamson JD, Whelton PK, Snyder JK, Sink KM, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103–2116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bress AP, Bellows BK, King JB, Hess R, Beddhu S, Zhang Z, et al. Cost-effectiveness of intensive versus standard blood-pressure control. N Engl J Med. 2017;377:745–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pletcher MJ, Vittinghoff E, Thanataveerat A, Bibbins-Domingo K, Moran AE. Young adult exposure to cardiovascular risk factors and risk of events later in life: The framingham offspring study. PLoS One. 2016;11:e0154288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McManus DD, Chinali M, Saczynski JS, Gore JM, Yarzebski J, Spencer FA, et al. 30-year trends in heart failure in patients hospitalized with acute myocardial infarction. Am J Cardiol. 2011;107:353–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Spencer FA, Meyer TE, Gore JM, Goldberg RJ. Heterogeneity in the management and outcomes of patients with acute myocardial infarction complicated by heart failure: The national registry of myocardial infarction. Circulation. 2002;105:2605–2610 [DOI] [PubMed] [Google Scholar]
- 58.Blumer V, Lemor A, Kittipibul V, Maning J, Chaparro S, Joyce E, et al. Predictors of 90-days readmissions for new onset heart failure after acute coronary syndrome. J Card Fail. 2019;25:S48 [Google Scholar]
- 59.Steg PG, Dabbous OH, Feldman LJ, Cohen-Solal A, Aumont MC, Lopez-Sendon J, et al. Determinants and prognostic impact of heart failure complicating acute coronary syndromes: Observations from the global registry of acute coronary events (grace). Circulation. 2004;109:494–499 [DOI] [PubMed] [Google Scholar]
- 60.Gerber Y, Weston SA, Enriquez-Sarano M, Berardi C, Chamberlain AM, Manemann SM, et al. Mortality associated with heart failure after myocardial infarction: A contemporary community perspective. Circ Heart Fail. 2016;9:e002460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Krumholz HM, Normand SL, Wang Y. Trends in hospitalizations and outcomes for acute cardiovascular disease and stroke, 1999-2011. Circulation. 2014;130:966–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ettehad D, Emdin CA, Kiran A, Anderson SG, Callender T, Emberson J, et al. Blood pressure lowering for prevention of cardiovascular disease and death: A systematic review and meta-analysis. Lancet. 2016;387:957–967 [DOI] [PubMed] [Google Scholar]
- 63.Howden LM, Meyer JA. 2010 census briefs: Age and sex composition. U.S. Department of commerce economics and statistics administration: United states census bureau. Washington, dc: 2023. 3. C2010br–03. . 2011 [Google Scholar]
- 64.Harris-Kojetin L, Sengupta M, Park-Lee E, Valverde R, Caffrey C, Rome V, et al. Long-term care providers and services users in the united states: Data from the national study of long-term care providers, 2013-2014. Vital Health Stat 3. 2016;3 x-xii; 1–105 [PubMed] [Google Scholar]
- 65.Moran AE, Forouzanfar MH, Roth GA, Mensah GA, Ezzati M, Murray CJ, et al. Temporal trends in ischemic heart disease mortality in 21 world regions, 1980 to 2010: The global burden of disease 2010 study. Circulation. 2014;129:1483–1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. Disability-adjusted life years (dalys) for 291 diseases and injuries in 21 regions, 1990-2010: A systematic analysis for the global burden of disease study 2010. Lancet. 2012;380:2197–2223 [DOI] [PubMed] [Google Scholar]
- 67.Moran AE, Forouzanfar MH, Roth GA, Mensah GA, Ezzati M, Flaxman A, et al. The global burden of ischemic heart disease in 1990 and 2010: The global burden of disease 2010 study. Circulation. 2014;129:1493–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.King JB, Shah RU, Bress AP, Nelson RE, Bellows BK. Cost-effectiveness of sacubitril-valsartan combination therapy compared with enalapril for the treatment of heart failure with reduced ejection fraction. JACC Heart Fail. 2016;4:392–402 [DOI] [PubMed] [Google Scholar]
- 69.Sullivan PW, Ghushchyan V. Preference-based eq-5d index scores for chronic conditions in the united states. Med Decis Making. 2006;26:410–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hutchins R, Pignone MP, Sheridan SL, Viera AJ. Quantifying the utility of taking pills for preventing adverse health outcomes: A cross-sectional survey. BMJ Open. 2015;5:e006505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hutchins R, Viera AJ, Sheridan SL, Pignone MP. Quantifying the utility of taking pills for cardiovascular prevention. Circ Cardiovasc Qual Outcomes. 2015;8:155–163 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


