Abstract
Background:
Consistent evidence suggests diabetes-protective effects of dietary fiber intake. However, the underlying mechanisms, particularly the role of gut microbiota and host circulating metabolites, are not fully understood. We aimed to investigate gut microbiota and circulating metabolites associated with dietary fiber intake, and their relationships with type 2 diabetes (T2D).
Methods:
This study included up to 11394 participants from the Hispanic Community Health Study/Study of Latinos. Diet was assessed with two 24h dietary recalls at baseline. We examined associations of dietary fiber intake with gut microbiome measured by shotgun metagenomics (350 species/85 genera and 1958 enzymes, n=2992 at visit2) and serum metabolome measured by untargeted metabolomics (624 metabolites, n=6198 at baseline); and associations between fiber-related gut bacteria and metabolites (n=804 at visit 2). We examined prospective associations of serum microbial-associated metabolites (n=3579 at baseline) with incident T2D over 6 years.
Results:
We identified multiple bacterial genera, species and related enzymes associated with fiber intake. Several bacteria (e.g., Butyrivibrio, Faecalibacterium) and enzymes involved in fiber degradation (e.g., xylanase EC3.2.1.156) were positively associated with fiber intake, inversely associated with prevalent T2D, and favorably associated with T2D-related metabolic traits. We identified 159 metabolites associated with fiber intake, 47 of which were associated with incident T2D. We identified 18 of these 47 metabolites associated with the identified fiber-related bacteria, including several microbial metabolites (e.g., indolepropionate and 3-phenylpropionate) inversely associated with risk of T2D. Both Butyrivibrio and Faecalibacterium were associated with these favorable metabolites. The associations of fiber-related bacteria, especially Faecalibacterium and Butyrivibrio, with T2D were attenuated after further adjustment for these microbial metabolites.
Conclusion:
Among US Hispanics/Latinos, dietary fiber intake was associated with favorable profiles of gut microbiota and circulating metabolites for T2D. These findings advance our understanding of the role of gut microbiota and microbial metabolites in the relationship between diet and T2D.
Keywords: gut microbiota, circulating metabolomics, dietary fiber, type 2 diabetes, Diabetes, type 2, Diet and Nutrition, Epidemiology
Graphical Abstract

Introduction
Consistent evidence suggests diabetes-protective effects of dietary fiber intake1,2, but the underlying mechanisms are not well-elucidated. These mechanisms could potentially be related to gut microbiota and microbiota-derived metabolites which have been suggested to play important roles in human chronic diseases, including type 2 diabetes(T2D)3,4. Dietary fibers, though not susceptible to hydrolysis by human digestive enzymes, can be metabolized by specific gut bacteria which produce a spectrum of metabolites through fiber fermentation and other metabolism pathways3.
Recent studies have indicated the potential influence of dietary fiber intake on the human gut microbiota composition. Higher fiber intake has been associated with alterations in gut bacterial taxa (e.g., higher levels of Prevotella or Prevotella to Bacteroides ratio5, Roseburia6,7, Lachnospira6, and Eubacterium7) as well as in gut bacterial gene functions (e.g., higher levels of genes encoding xylanase, beta-glucanase, and other enzymes related to fiber degradation8). However, to what extent the specific fiber-associated gut microbiota taxonomic features and functional capacities may affect the host metabolic diseases such as T2D, and the underlying mechanisms, are not fully understood.
Microbial-derived metabolites can be absorbed into the host circulation and may affect host biological systems, and thus have been suggested as functional mediators linking gut microbiota and host metabolic health and disease3. While the majority of existing research has focused on short-chain fatty acids (SCFAs), well-known microbial metabolites through fiber fermentation9, there is also evidence suggesting other microbial metabolites associated with fiber intake and T2D, such as indolepropionate10. However, few studies have integrated host metabolomics and gut metagenomics data to investigate the relationships among dietary fiber intake, gut microbiota, host circulating metabolites and risk of T2D in population-based cohorts3. Data are even sparse in US Hispanics/Latinos, with westernized diet (e.g., reduced dietary intake of fiber-rich foods) and gut microbiome (e.g., reduced bacterial diversity) during acculturation5,8, who are disproportionally affected by T2D10.
In this study, using fecal shotgun metagenomics data, we aimed to identify gut microbial taxa and functional capacities associated with dietary fiber intake in the Hispanic Community Health Study/Study of Latinos (HCHS/SOL), and then linked these identified gut microbial features with prevalent T2D. We also performed a metabolome-wide analysis to identify serum metabolites associated with dietary fiber intake and examined whether these fiber-associated metabolites at baseline were associated with risk of incident T2D over a 6-year follow-up in the HCHS/SOL. Moreover, through a “Late Integration” strategy11, we linked both fiber-associated gut bacteria and fiber-associated metabolites to help better understand the role of gut microbiota and microbial metabolites in the relationship between dietary fiber intake and T2D.
Methods
Data Availability.
The gut microbiome shotgun metagenomics sequencing data in this study are deposited in QIITA (https://qiita.ucsd.edu/), ID 11666. HCHS/SOL has established a process for the scientific community to apply for access to participant data and materials, with such requests reviewed by the project’s Steering Committee. These policies are described at https://sites.cscc.unc.edu/hchs/. Please see the Major Resources Table in the Supplemental Material.
Study population
The HCHS/SOL is a prospective, population-based cohort study of 16,415 Hispanic/Latino adults aged 18–74 years living in four US metropolitan communities (Chicago, IL; Miami, FL; Bronx, NY; San Diego, CA). A comprehensive battery of interviews and a clinical assessment with blood draw were conducted at in-person clinic visits during 2008–2011(baseline) and 2014–2017 (visit 2)5,12. In this study, we included up to 11394 participants at baseline (including 6198 participants with serum metabolomics data), and 2992 participants in an ancillary gut microbiome study at visit 2. The specific sample size for each analysis is detailed in the respective sections. Information on demographics, behaviors, health status, medical histories, and medication use was collected using structured questionnaires12,13.
An expanded description of study populations, data assessments and statistical analyses is provided in Supplemental Methods. The study was approved by the institutional review boards of corresponding site institutions. Written informed consent was obtained from all participants.
Assessment of dietary intake
Dietary intake was collected using the multiple-pass methods of the Nutrition Data System for Research software (version 11) based on two 24-hour dietary recalls assessed ~6-week apart at baseline14. Dietary fiber intake assessment included the energy-adjusted total fiber intake (g/1000kcal per day), and separate estimates for soluble and insoluble fiber intake. We created a new diet score which comprised 7 non-fiber dietary factors (long chain omega-3 fatty acids, polyunsaturated fatty acids (PUFA), alcohol, sugar-sweetened drinks, red and processed meat, trans fats, and sodium).
Metagenomics sequencing, taxonomic and functional profiling
Shotgun metagenomics sequencing was performed on DNA extracted from fecal samples of 3035 participants at visit2, using Illumina NovaSeq platforms5,8. 2992 samples passed QC metrics were included in the analysis. Microbiome bioinformatics analyses, taxonomic assignment, and functional component identification were performed using the SHOGUN15 pipeline8. A total of 350 gut microbial species (presented in more than 20% of samples and average relative abundance ≥0.001%) and 1958 annotated known enzymes were included in the analysis. We conducted central log ratio transformation on these gut microbial features.
Metabolomic profiling
Serum metabolomic profiling was performed using an untargeted liquid chromatography-mass spectrometry(LC-MS) based protocol at Metabolon (Durham, North Carolina, USA), in 3972 randomly selected participants (at baseline), and 814 participants (at visit 2) who provided blood samples within one month of fecal sample collection16. Metabolomic profiling was performed in additional 2282 participants (at baseline) as a replication dataset. A total of 624 known metabolites with an undetectable rate of <20% were included in the analysis. Values of metabolites below detection were imputed by the half of the minimum value. We performed inverse-normal transformation on levels of metabolites.
Ascertainment of T2D and metabolic traits
T2D cases were identified if participants met at least one of the following criteria17: fasting glucose ≥7.0 mmol/L (126mg/dL); post 2h glucose ≥11.1 mmol/L (200 mg/dl); HbA1c ≥6.5%; or self-reported antidiabetic medication use. Participants free of diabetes at baseline who were identified as having T2D during the follow-up visits were deemed to be incident T2D. Metabolic traits, including body mass index (BMI), waist-hip ratio, systolic blood pressure (SBP), diastolic blood pressure (DBP), triglycerides (TG), high-density lipoprotein cholesterol (HDL), fasting glucose, post 2h glucose, HbA1c, and Homeostatic Model Assessment for Insulin Resistance (HOMA-IR),were measured following standardized methods and protocols17,18.
Statistical analysis
Dietary fiber intake and incident T2D.
Multivariable Poisson regression was used to examine the association between baseline dietary fiber intake and incident T2D over 6 years of follow-up, among 8185 participants who were free of diabetes, cardiovascular disease or cancer at baseline, controlling for age, sex, study center, education, family income, physical activity, smoking, drinking, antihypertensive medication, and lipid-lowering medication.
Dietary fiber intake, gut microbial taxa and prevalent T2D.
Among 2992 participants at visit 2, we examined associations of dietary fiber intake with microbial taxonomic features using multivariate linear regressions, adjusting for age, sex, study center, education, family income, physical activity, smoking, drinking, use of antibiotics, probiotics, antihypertensive medication, anti-diabetic medication, and lipid-lowering medication. We further adjusted for the diet score which comprised 7 non-fiber dietary factors to examine the potential influences of other dietary factors on the association results. We applied ANCOM219 to identify fiber-associated microbial taxa in a compositionally coherent manner. We performed logistic regressions to examine multivariable-adjusted associations of gut microbial taxonomic features with prevalent T2D and prediabetes, controlling for aforementioned covariates except for anti-diabetic medication use as this was used to define T2D. We constructed the integrated hierarchical phylogenetic tree using iTol20. Subsequently, we focused on those microbial taxa associated with both fiber intake and T2D, and examined their associations with T2D-relaetd metabolic traits using multivariate linear regressions, adjusting for aforementioned covariates.
Dietary fiber intake, gut microbial functional enzymes, and prevalent T2D.
We explored associations of dietary fiber intake with 1958 annotated microbial functional enzymes using multivariate linear regressions, adjusting for aforementioned covariates. An enrichment test was performed at enzyme category EC level II. We applied logistic regressions to examine multivariable-adjusted associations of the identified fiber-associated enzymes with prevalent T2D. We performed microbial genomic sequence-based-alignment analyses8 to examine potential contributions of specific bacterial taxa to these fiber-associated functional enzymes.
Dietary fiber intake, circulating metabolites, and incident T2D.
We examined associations of serum metabolites with dietary fiber intake using multivariate linear regressions among 3916 participants at baseline, adjusting for aforementioned covariates except antibiotics and probiotics. These associations were further examined in an additional dataset at baseline (n =2282). Then we evaluated the prospective associations of fiber-associated metabolites with incident T2D over a 6-year follow-up. Among 3916 participants, we excluded those with prevalent diabetes, cardiovascular disease, or cancer at baseline, and those who did not attend the second visit. The remaining 2010 participants were included in the multivariable Poisson regressions, to estimate rate ratios (RRs) of incident T2D per SD increment in metabolites. The same analysis was conducted in the replication dataset (n=1569). Results from two datasets were combined using fixed-effects meta-analysis. We further examined associations of selected metabolites with metabolic traits using multivariate linear regressions.
Integrated analyses of gut microbiota and circulating metabolites associated with fiber intake and T2D.
Among 804 participants with both omics data available at visit 2, we utilized partial Spearman correlation to assess correlations between the identified microbial taxa and metabolites which were associated with both fiber intake and T2D. Associations of 9 fiber-associated microbial genera with prevalent T2D were estimated using multivariable logistic regressions, adjusted for aforementioned covariates (Model 1); further adjusted for metabolites relevant to specific individual taxa (Model 2); and further adjusted for all 18 microbial-related metabolites (Model 3), to explore if these metabolites could partially explain the observed associations. In addition, we conducted a proxy association analysis21 to test potential prospective associations of these 9 bacterial genera with risk of T2D. The identified 18 microbial-related metabolites were used as proxies for these 9 bacterial genera. For each bacterial genus, we calculated a Spearman correlation coefficient between effect sizes (beta coefficients) from the cross-sectional associations of this genus with 18 microbial-related metabolites, and effects sizes (nature-log-transformed RRs) from the prospective associations of 18 microbial-related metabolites at baseline with risk of T2D. These effect sizes were standardized using z-score transformation to ensure comparability. A significant correlation between these two sets of effect sizes was considered as a significant proxy association.
Statistical analyses were performed using R 4.0.3. The Benjamini-Hochberg false discovery rate (FDR) method was used for multiple testing correction.
Results
Dietary fiber intake and incident T2D
We first examined the prospective association between dietary fiber intake and incident T2D among 8185 participants who were free of diabetes at baseline (participant characteristics are shown in Table.1). During an average 6-years of follow-up, 851 incident T2D cases were identified (Table.S1). After adjustment for multiple covariates, higher dietary fiber intake was significantly associated with lower risk of T2D (RR=0.95, 95% CI:0.90,0.99; per g/1000kcal per day; P=0.045). Compared to those in the lowest tertile of fiber intake (range 3.4–8.4 g/1000kcal per day), participants in the highest tertile (range 10.8–22.1 g/1000kcal per day) had a 29% (95% CI: 6–47%) lower risk of T2D (P-trend=0.023, Figure.S1).
Table 1.
Characteristics of study participants, free of diabetes at baseline (n=8185).
| Fiber intake | |||
|---|---|---|---|
|
|
|||
| Tertile 1 | Tertile 2 | Tertile 3 | |
|
| |||
| Age, year | 39.1(13.5) | 44.8(12.5) | 49.5(11.2) |
| Sex,% | |||
| female | 50.2 | 62.9 | 75.6 |
| male | 49.8 | 37.1 | 24.4 |
| Field Center,% | |||
| Bronx | 34.6 | 19.6 | 9.7 |
| Chicago | 13.9 | 25.9 | 39.1 |
| Miami | 40.7 | 26.5 | 9.2 |
| San Diego | 10.9 | 28 | 42 |
| Smoking,% | |||
| never | 58.7 | 64.1 | 71.2 |
| former | 15.6 | 19.6 | 19.1 |
| current | 13 | 9.2 | 6.5 |
| current heavy smoker | 12.7 | 7.1 | 3.2 |
| Alcohol consumption,% | |||
| never | 17.7 | 19.7 | 21.1 |
| former | 26.7 | 29.9 | 35.5 |
| current | 48 | 46.4 | 40.4 |
| current heavy drinker | 7.7 | 4 | 3 |
| Education,% | |||
| <high school | 26.7 | 31 | 43.6 |
| high school | 30.6 | 27.5 | 22.6 |
| >high school | 42.7 | 41.5 | 33.7 |
| Annually family income($),% | |||
| <30k | 69.7 | 65.9 | 66 |
| >=30k | 30.3 | 34.1 | 34 |
| Anti-hypertensive medication use,% | |||
| no | 92.4 | 91.5 | 88.3 |
| yes | 7.6 | 8.5 | 11.7 |
| Lipid-lowering medication use,% | |||
| no | 96.8 | 94.3 | 91.2 |
| yes | 3.2 | 5.7 | 8.8 |
| Obesity categories,% | |||
| normal | 25.0 | 21.4 | 19.3 |
| overweight | 36.9 | 41.2 | 42.7 |
| obese | 38.1 | 37.4 | 38.0 |
Data are mean(SD) for continuous variables or percentage for categorical variables.
Dietary fiber intake, gut microbial taxa, and prevalent T2D
We then examined associations of dietary fiber intake with individual gut microbial taxa at multiple taxonomic levels, among 2992 individuals (participant characteristics are shown in Table.S2 and Figure.S2). After controlling for multiple covariates, 24 of 85 predominant gut microbial genera were associated with fiber intake (FDR<0.05). As shown in the integrated phylogenetic tree (Figure.1A), 17 of these fiber-associated genera were under Firmicutes phylum, 4 under Actinobacteria, and 3 under Bacteroidetes. Associations between dietary fiber intake and these 24 gut microbial taxa remained significant after further adjustment for the diet score which comprised 7 non-fiber dietary factors (Figure.S3). Consistently, 23 of these 24 microbial genera were also identified to be associated with dietary fiber intake by ANCOM2 (Table.S3). Correlations among these 24 genera were generally weak, though a few moderate correlations were observed among 4 genera positively associated with fiber intake, and among 9 genera inversely associated with fiber intake (Figure.1B). We identified 99 microbial species within these 24 fiber-associated genera, and the species-fiber associations were generally consistent with genus level results (Figure.1C and Table.S4). For example, all of Roseburia, Butyrivibrio, Faecalibacterium species, and 20 of 26 Prevotella species, were positively associated with fiber intake.
Figure 1. Dietary fiber intake, gut microbial taxa, and prevalent T2D.

A. Integrated phylogenetic tree of gut microbial taxa associated with fiber intake (n= 2992). Taxa from inner to outer circle represent bacteria kingdom to genus level. The branch widths reflect the relative abundance of each taxon. Red/blue colors of the ring depict the significant positive/inverse associations with fiber intake (FDR <0.05) and the gradient colors reflect the beta coefficients estimated in linear regression models, after adjustment for age, sex, study center, education, family income, physical activity, smoking, drinking, use of antibiotics, probiotics, antihypertensive medication, anti-diabetic medication, and lipid-lowering medication. Among the 24 fiber-associated genera, 9 were significantly associated with prevalent T2D in multivariable logistic models, after adjustment for the aforementioned covariates except for anti-diabetic medication use. Red/blue stars depict the positive/inverse associations of genera with T2D (P <0.05).
B. Partial Spearman correlation heatmap for the 24 identified fiber-associated microbial genera. Red font highlights those genera positively associated with fiber intake. The pie pieces reflect the strength of the correlation. Results were adjusted for the aforementioned covariates.
C. Polar plot for associations of species-level microbial taxa with dietary fiber intake. The results for 99 predominant species (average relative abundance >0.001% and present in >20% samples) under the identified 24 fiber-associated genera are shown. The bar height represents −Log10(P) value. Bacterial species significantly associated with fiber intake are highlighted in red / blue (positive / inverse associations; P <0.05).
D. Associations of fiber-related genera with prevalent T2D. Data are odds ratios (ORs) and 95% confidence intervals (CIs) for T2D per increment of CLR-transformed abundance of gut bacterial genera, adjusting for the aforementioned covariates.
E. Associations of microbial genera with fiber intake and prevalent T2D. Data are beta coefficients estimated in regressions (for fiber intake) and natural logarithms of ORs estimated in logistic regressions (for T2D), after adjustment for the aforementioned covariates in panel A. Each dot represents a bacterial genus. Red dots highlight the 9 genera significantly associated with both fiber intake and T2D.
F. Associations of T2D-associated genera with metabolic traits. Data are beta coefficients estimated in linear regression models after adjustment for the aforementioned covariates in panel A (*P <0.05).
We also examined associations of insoluble and soluble fiber intake with these 24 identified microbial genera and results were similar (Figure S4). Among these 24 microbial genera, 21 and 15 genera were significantly associated with insoluble and soluble fiber intake respectively (14 genera associated with both), with consistent directions compared to results for the total fiber intake.
We thus focused on these 24 fiber-associated genera and examined their associations with prevalent T2D. After multivariable adjustment, 9 fiber-associated genera showed significant associations with T2D (Figure.1A and 1D). Higher levels of Butyrivibrio, Faecalibacterium, Roseburia, Ruminococcus and Marvinbryantia, all of which were positively associated with fiber intake, were associated with lower odds of T2D. Higher levels of Acidaminococcus, Erysipelatoclostridium, Hungatella and Lachnoclostridium were associated with lower fiber intake and higher odds of T2D (all P<0.05)(Figure.1D). For most of the other genera, we observed expected directions of the associations with fiber intake and T2D (i.e., positively associated with fiber intake and inversely associated with T2D), though these associations were not significant (Figure.1E). After further adjustment of obesity, associations between 9 microbial genera and T2D remained significant (Table.S5).
We observed generally consistent directions of associations of these 9 identified microbial genera with pre-diabetes and T2D (Figure.S5). In addition, several fiber-associated “beneficial” taxa, notably Butyrivibrio and Marvinbryantia, were linked with a favorable profile of metabolic traits. On the contrary, the “unbeneficial” taxa associated with lower fiber intake, such as Acidaminococcus and Lachnoclostridium, exhibited unfavorable associations with metabolic traits (Figure.1F).
Dietary fiber intake, gut microbial functional enzymes, and prevalent T2D.
After controlling for multiple covariates, we identified 211 enzymes associated with fiber intake (All FDR <0.05). Our enrichment tests at EC enzyme category level II indicated that fiber intake was associated with enrichment of enzymes belonging to specific categories (e.g.,EC3.2 Glycosylases; and EC2.5 Transferring alkyl or aryl groups; Table.S6).
In particular, we identified 17 enzymes under the glycosylases category associated with fiber intake (all FDR<0.05, Figure.2). The identified glycosylases formed two clusters, with enzymes within each cluster demonstrating high correlations with each other (Figure.2). The first cluster, which was positively associated with fiber intake and inversely associated with T2D, included several representative microbial fiber-degradation enzymes. For example, oligosaccharide reducing-end xylanase (EC3.2.1.156,K15531) is known as a high molecular mass xylanases which can degrade xylan, a type of dietary fibers found in plant cell walls22. The second cluster was inversely associated with fiber intake and positively associated with T2D. This cluster comprised microbial encoding enzymes related to metabolism of simple carbohydrates. In line with our results, one representative enzyme in this cluster, alpha-mannosidase (EC3.2.1.24, K01191), has been linked with insulin resistance23.
Figure 2. Fiber-associated gut microbial functional enzymes and prevalent T2D.

The partial Spearman correlation heatmap (the left panel) includes 17 microbial functional enzymes under the Glycosylases category that were significantly associated with fiber intake (all FDR <0.05) (n= 2992). For the associations of microbial functional enzymes with fiber intake (the middle-left panel), the gradient colors reflect the ranks of beta coefficients estimated in multivariate linear regressions, after adjustment for age, sex, study center, education, family income, physical activity, smoking, drinking, use of antibiotics, probiotics, antihypertensive medication, anti-diabetic medication, and lipid-lowering medication. For the associations of microbial functional enzymes with prevalent T2D (the middle-right panel), data are ORs and 95% CIs, estimated in multivariable logistic regressions, after adjustment for the aforementioned covariates except for anti-diabetic medication use. The partial Spearman correlation heatmap (the right panel) indicates correlations between these 17 microbial functional glycosylases and the 9 identified fiber-associated genera.
Besides the EC 3.2 Glycosylases, our results indicated that fiber intake was also associated with potential enrichment of enzymes in EC2.5, Transferring alkyl or aryl groups (Figure.S6). We identified 11 fiber-associated enzymes under this transferases category, all of which were associated with T2D in expected directions (Figure.S6). These 11 transferases showed weak-to-moderate correlations with the 17 enzymes under the glycosylases category (Figure.S7).
We then explored potential contributions of the 9 selected fiber-associated bacterial genera to these enzymes (Figure.2 and S6). Our genomic analyses provided further evidence supporting the presence of enzyme encoding-genes on the specific bacterial genomes (Table.S7). For example, we confirmed the presence of xylanase gene on representative Roseburia and Butyrivibrio genomes. These results expanded our previous findings8 and also consistent with other studies indicated that Roseburia species from the human gut displayed high xylanolytic activity22.
Dietary fiber intake, circulating metabolites, and incident T2D
After adjustment for multiple covariates, 164 out of 624 metabolites were significantly associated with fiber intake (FDR<0.05) in 3916 HCHS/SOL participants (Table.S8 and S9), and associations of 159 metabolites with fiber intake were validated in an additional dataset in HCHS/SOL (n=2282) (Table.S9 and Figure.3A).
Figure 3. Dietary fiber intake, circulating metabolites, and incident T2D.

A. Polar plot for associations of serum metabolites with fiber intake. Data are −Log10(P) values for 159 metabolites, which were significantly associated with fiber intake (FDR <0.05)(n=3916), and validated in an additional dataset (n=2282), from multivariate linear regressions, with adjustment for age, sex, study center, education, family income, physical activity, smoking, drinking, use of antibiotics, probiotics, antihypertensive medication, anti-diabetic medication, and lipid-lowering medication. Red/blue: positive/ inverse associations (FDR <0.05).
B. Prospective associations between fiber-related metabolites and incident T2D. Data are rate ratios (RRs) and 95% CIs, estimated by multivariable Poisson regressions, with adjustment for the aforementioned covariates except for anti-diabetic medication use. Results were from the discovery data (the upper panel) including 2010 participants free of diabetes at baseline, with 224 incident T2D cases over 6 years; from the replication analyses (middle panel) including 1569 participants free of diabetes at baseline with 204 incident T2D cases over 6 years; and combined (the lower panel) from both datasets using fixed-effects meta-analysis.
We then focused on these 159 fiber-associated metabolites and examined prospective associations of these metabolites with incident T2D among 2010 participants who were free of diabetes at baseline, with 224 incident T2D cases identified after 6 years of follow-up (Table.S10). We found that 69 fiber-associated metabolites were also associated with incident T2D (Table.S11). After further adjustment for obesity, the associations between metabolites and risk of T2D only changed slightly and 43 of the 69 remained significant (Figure.S8). In addition, we conducted stratified analyses based on baseline pre-diabetes status and results among participants with pre-diabetes were highly consistent with those observed in the overall sample (Figure.S9). This was expected as the majority incident T2D cases were identified among participants with pre-diabetes at baseline (Table.S12).
We further conducted replication analyses in the additional HCHS/SOL dataset (1569 participants free of diabetes at baseline with 204 incident T2D cases, Table.S10), and found that 44 out of 69 metabolites showed significant associations with risk of T2D (Table.S12). We then combined results from both datasets by meta-analysis, and 47 metabolites were associated with risk of T2D (Table.S11 and Figure.3B).
To explore potential relationships among these 47 metabolites which were associated with both fiber intake and T2D, we examined their correlations (Figure.S10) and performed network analysis (Figure.S11). Notably, 3-phenylpropionate, indolepropionate, and cinnamoylglycine, which were associated with higher fiber intake and lower risk of T2D, were clustered into the same module. This module also showed close relationships with beta-cryptoxanthin as well as oxalate. We also observed several modules that comprised metabolites positively associated with risk of T2D. As expected, the host kynurenine metabolites (kynurenine, kynurenate, and quinolinate) clustered into the same module. Another representative unfavorable metabolite module comprised gamma-glutamyl amino acids.
As shown in Figure.S12, among the 47 identified metabolites, those beneficial metabolites which were associated with lower risk of T2D, were generally linked with a favorable profile of metabolic traits; whereas unfavorable associations with metabolic traits were observed for metabolites positively associated with T2D, such as N-acetylglucosamine and hydroxyasparagine.
Integrated analyses of gut microbiota and circulating metabolites associated with fiber intake and T2D
We next examined associations between the microbial taxa and serum metabolites, among 804 participants at visit 2. After controlling for multiple covariates, we identified 18 potential microbial-related metabolites out of the 47 metabolites that were associated with both fiber intake and T2D (Figure.4A). Many of the fiber-associated genera were significantly correlated with multiple metabolites. For example, the “beneficial” taxa Butyrivibrio and Faecalibacterium, were correlated with 16 and 4 metabolites, respectively. Both of them demonstrated positive correlations with the recognized microbial metabolites, indolepropionate10 and 3-phenylpropionate24, suggesting that Butyrivibrio and Faecalibacterium could be potential contributors of these metabolites. In contrast, Lachnoclostridium, which was associated with lower fiber intake and higher odds of T2D, showed negative correlations with indolepropionate and 3-phenylpropionate, and positively linked with several unfavorable metabolites, including N-acetylglucosamine, a metabolite associated with insulin resistance and weight gain24; and hydroxyasparagine, a marker of the mild obesity-related diabetes25. Since fecal samples were collected after blood draw, with a median of 10 days (IQR:6–14 days), we conducted stratified analyses on the correlations between gut bacteria and serum metabolites by the sample collection time difference (≤10 days versus 10–30 days), and correlations between genera and metabolites were highly consistent (Figure.S13A and S13B).
Figure 4. Integrated analyses of gut microbiota and circulating metabolites associated with fiber intake and T2D.
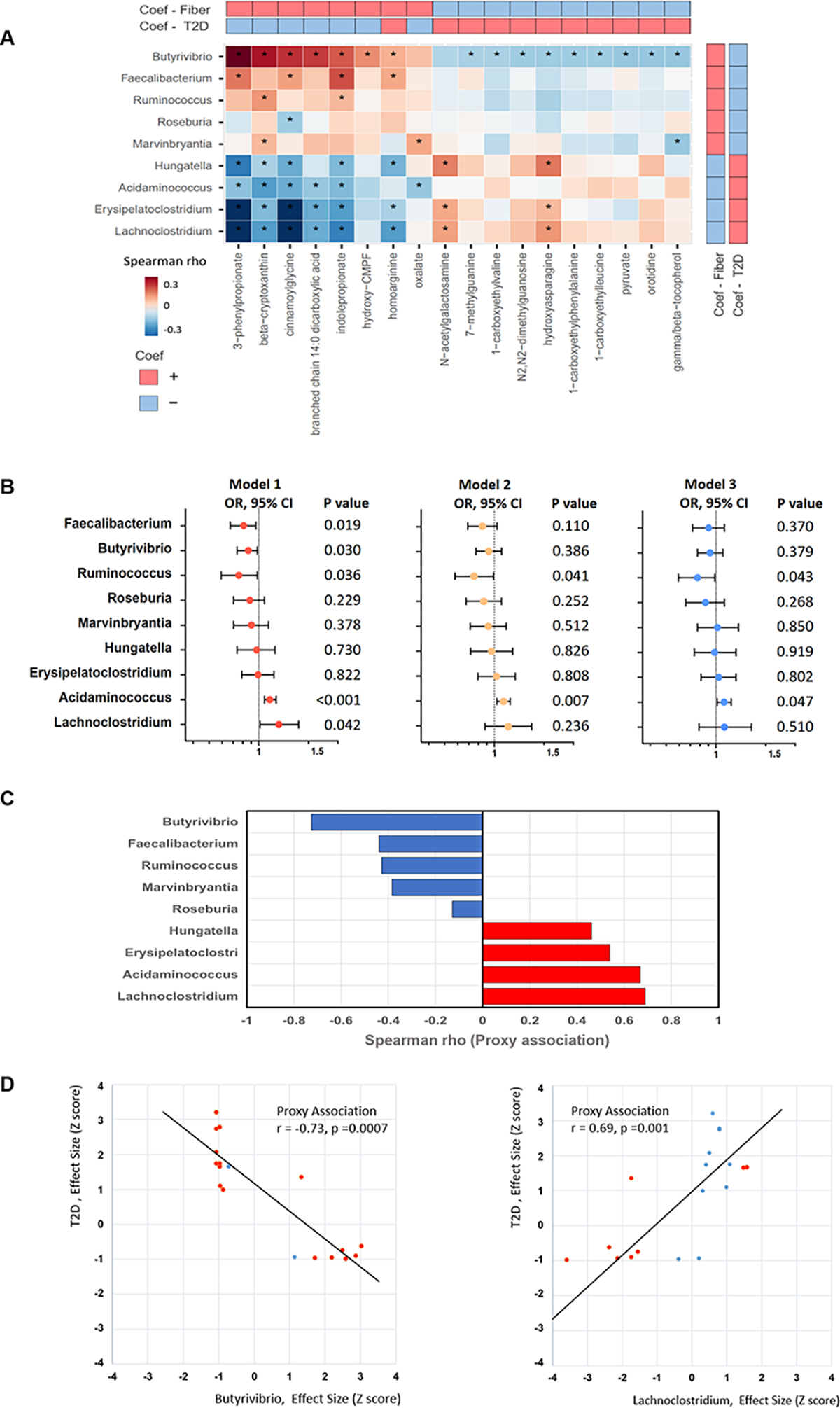
A. Correlation heatmap for the identified microbial taxa and serum metabolites associated with both fiber intake and T2D. Data are partial Spearman correlation coefficients among 804 participants after adjustment for age, sex, study center, education, family income, physical activity, smoking, drinking, use of antibiotics, probiotics, antihypertensive medication, anti-diabetic medication, and lipid-lowering medication. (*P <0.05)
B. Associations of fiber-related microbial genera with prevalent T2D with and without adjustment for microbial-related metabolites. Data are ORs and 95% CIs for T2D per increment of CLR-transformed abundance of gut bacterial genera, estimated in logistic regression models after adjustment for the aforementioned covariates (Model1); further adjustment for metabolites relevant to specific individual taxa (Model 2); and further adjustment for all 18 microbial-related metabolites (Model 3).
C. The proxy associations between fiber-related microbial genera and risk of T2D. The identified 18 microbial-related metabolites were used as proxies for these 9 bacterial genera. For each bacterial genus, we calculated a Spearman correlation coefficient between effect sizes (beta coefficients) from the associations of this genus with the microbial-related metabolites and effects sizes (nature-log-transformed RRs) from the associations of the microbial-related metabolites with risk of T2D. A significant correlation between these two sets of effect sizes was considered as a significant proxy association.
D. The representative proxy associations of Butyrivibrio (the left panel) and Lachnoclostridium (the right panel) with risk of T2D. Each dot represents a microbial-related metabolite. The x-axis shows effect sizes (beta coefficients) from the cross-sectional associations of Butyrivibrio or Lachnoclostridium with18 metabolites, and the y-axis shows effects sizes (nature-log-transformed RRs) from prospective associations of 18 metabolites at baseline with risk of T2D. These effect sizes were standardized using z-score transformation, to ensure comparability. Metabolites significantly associated with Butyrivibrio and Lachnoclostridium, respectively are highlighted with red.
To examine whether the identified microbial-related metabolites could partially explain the associations between microbial genera and T2D, we included these microbial-related metabolites as covariates in the regression models. Associations between four genera (Faecalibacterium, Butyrivibrio, Acidaminococcus, Lachnoclostridium) and T2D were attenuated or abolished after further adjusting for their corresponding taxa-related metabolites, and a similar or more pronounced attenuation was observed when including all these 18 microbial-related metabolites in the regression models (Figure.4B). In contrast, the association between Ruminococcus and T2D did not change materially after further adjusting for metabolites.
We also conducted a proxy association analysis21 to estimate potential prospective associations between these 9 gut bacteria and risk of T2D, using these 18 microbial-related metabolites measured at baseline as proxies for gut microbiota. Our analysis suggested four bacterial genera potentially associated with risk of T2D (all Spearman |r|>0.5 and P<0.05, represented by Butyrivibrio and Lachnoclostridium, Figure.4D and S14).
Discussion
Our integrative analyses shed light on the complex relationships among dietary fiber intake, gut microbiota, and circulating metabolites, offering insights into their potential roles in the development of T2D. We identified nine gut microbial genera associated with both fiber intake and T2D in a US Hispanic/Latino population. Further functional analysis highlighted specific microbial enzymes, particularly glycosylases involved in fiber degradation, which were enriched in individuals with higher fiber intake and exhibited inverse associations with T2D. Enhanced by longitudinal metabolomics data, we identified multiple microbial-related metabolites that could help explain the beneficial associations between specific fiber-associated bacterial genera and T2D.
Our study revealed a potential pathway/route through the fiber-Faecalibacterium-metabolite-T2D axis. Faecalibacterium is a Gram-positive anaerobe which is deemed as a symbiotic microorganism in human gastrointestinal tracts26. Faecalibacterium prausnitzii was the only predominant Faecalibacterium species identified in our study. Consistent with our results, Faecalibacterium has been associated with the high fiber diet27, and possesses the ability to metabolize various types of fibers and plant polysaccharides26. Although Faecalibacterium is known as a butyrate-producer, its anti-inflammatory and other beneficial effects could not be explained by butyrate alone28. Our study revealed that Faecalibacterium was associated with multiple potentially beneficial metabolites in serum, including indolepropionate, 3-phenylpropionate, and cinnamoylglycine, all linked to higher fiber intake and lower risk of T2D. Our findings are consistent with the reported beneficial role of indolepropionate in anti-inflammation, anti-oxidant activity, and amelioration of glucose metabolism10,29. The antimicrobial properties of 3-phenylpropionate may result in low production of inflammatory lipopolysaccharide and its antioxidant activities may contribute to insulin sensitivity24. Cinnamoylglycine is a marker of healthy gut microbiome, which inhibits the growth of pathogenic microorganisms and has potential metabolic health benefits in vitro30. Notably, the association between Faecalibacterium and T2D was greatly attenuated after adjusting for these Faecalibacterium-related metabolites, suggesting that the potentially protective effect of Faecalibacterium on T2D could be partially explained by these microbial metabolites.
Another representative route identified in our study was the fiber-Butyrivibrio-metabolite-T2D axis. Members of Butyrivibrio are known as important degraders of hemicelluloses and plant polysaccharides31. Our metagenomics data indicated that, among US Hispanics/Latinos, Butyrivibrio fibrisolvens was the most predominant species under Butyrivibrio genus. Our microbial genomic analysis detected the presence of several glycosylases on the representative Butyrivibrio fibrisolvens genome, including high molecular mass xylanases (K15531;EC3.2.1.156). In line with our results, Butyrivibrio species have been reported to grow on xylan32, a type of dietary fiber found in plant cell walls22,33. In addition, two recent pilot studies also observed decreased Butyrivibrio abundance in patients with diabetes using 16S amplicon sequencing34,35. Our integrative analysis linked Butyrivibrio with several potentially beneficial metabolites, including three aforementioned metabolites (i.e., indolepropionate, 3-phenylpropionate, and cinnamoylglycine) and β-cryptoxanthin, an antioxidant and provitamin A carotenoid associated with a reduced risk of T2D36. The protective association between Butyrivibrio and T2D might be partially explained by these microbial metabolites. In addition, we observed an inverse association between Butyrivibrio and circulating pyruvate, which might be related to its role in butyrate production via pyruvate fermentation37. Further studies are warranted to clarify the relationship between circulating and fecal pyruvate and their associations with gut butyrate-producers like Butyrivibrio.
Our study also identified several potentially pathogenic bacteria, including Lachnoclostridium and Acidaminococcus, associated with lower fiber intake and higher risk of T2D. Moreover, by integrating data on serum metabolomics, we found that Lachnoclostridium was positively associated with circulating metabolites implicated in T2D development, such as N-acetylglucosamine and hydroxyasparagine. Of note, N-acetylglucosamine is a recognized contributor to insulin resistance38 and has been linked with insulin resistance and weight gain in mouse model38,39. High levels of hydroxyasparagine were reported to play a role in obesity25. In addition, both Lachnoclostridium and Acidaminococcus were inversely associated with serum levels of three beneficial metabolites, indolepropionate, 3-phenylpropionate, and cinnamoylglycine. The associations between these two potentially pathogenic bacteria and T2D could be partially explained by their related microbial metabolites. Moreover, using metabolite signatures as proxies for gut microbiota, our results also support potential prospective associations of these two microbial taxa with increased risk of T2D.
The observed relationships between some fiber-associated genera (e.g.,Roseburia and Ruminococcus) and T2D might not be related to fiber-associated metabolites identified in this study. This may reflect the complexity of the microbiota-host crosstalk, and suggests that these bacterial may contribute to host metabolic health and disease through other microbial metabolites or metabolite-independent pathways. For example, Roseburia species, known butyrate-producers, have been reported to affect host metabolism through butyrate inhibiting NF-κB activation, or influence on T-cell proliferation40,41. Both our findings and previous results support the protective association between Roseburia and T2D42. In addition, our study found enriched Hungatella in individuals with T2D, and consistently, a decreased relative abundance of Hungatella in response to T2D treatment was observed in mouse model43.
Our results indicate that, both higher insoluble and soluble fiber intakes were associated with a favorable gut microbiota profile. Our findings aligned with recent in vitro studies showing that beneficial butyrogenic bacteria in Firmicutes preferentially utilize insoluble substrates to support their energy needs44,45. Additionally, insoluble fiber may affect the gut microbiota composition via other mechanisms, such as fecal bulking effect46 which can reduces the amount of time available for gut bacterial fermentation of non-digested foodstuff, and stimulates bacterial growth46. On the other hand, soluble fiber can be metabolized by gut bacteria efficiently, which produces many beneficial metabolites and thus offers health benefits47.
Our study also pinpointed certain circulating metabolites that were associated with both fiber intake and T2D, but not correlated with the identified T2D-related gut bacteria. Some of these associations could potentially be attributed to host factors. For instance, we found a set of gamma-glutamyl dipeptides associated with increased risk of T2D. These dipeptides are recognized as bioactive peptides, and in particular, gamma-glutamyl-leucine, has been extensively documented for its association with inflammation, oxidative stress, and T2D risk48. A recent genome-wide study also identified genetic variants in the host genome that may regulate levels of serum gamma-glutamyl-leucine48.
This study has several limitations. The ascertainment of dietary fiber intake was based on self-report data at baseline, which could potentially inject bias into our findings. The association between gut microbiota and T2D was examined in a cross-sectional dataset. However, using longitudinal metabolomics data, we identified specific gut microbial-related serum metabolites associated with incident T2D over 6 years. Our further analyses using microbial metabolites as proxies also supported a potential prospective relationship between gut microbiota and risk of T2D. Finally, given the observational nature of this study, our results should be interpreted with caution, and causal inference could not be established without further evidence.
In summary, in this study of US Hispanics/Latinos, we demonstrated that, higher dietary fiber intake was linked to favorable gut microbiota and circulating metabolite profiles for T2D. The relationships between some fiber-related gut bacteria and T2D could be partially explained by circulating microbial-related metabolites. Our findings provide new information that helps to better understand the relationships of dietary fiber intake with gut microbiota and circulating metabolites, and their roles in the development of T2D.
Supplementary Material
Novelty and Significance.
What Is Known?
Higher dietary fiber intake is associated with lower risk of type 2 diabetes (T2D); however, the underlying mechanisms are not well elucidated.
Dietary fiber can be metabolized by specific gut microbes. However, to what extent the specific fiber-associated gut microbiota taxonomic features and functional capacities may affect host T2), is not fully understood.
What New Information Does This Article Contribute?
Our integrated multi-omics analyses revealed the potential fiber-microbiota-metabolite-T2D route. We identified several bacterial genera (e.g., Butyrivibrio, Faecalibacterium) and functional capacities involved in fiber degradation (e.g., xylanase EC3.2.1.156) positively associated with fiber intake, and linked to lower odds of T2D.
Butyrivibrio and Faecalibacterium were positively associated with multiple beneficial serum metabolites, including indolepropionate, 3-phenylpropionate, and cinnamoylglycine, with the properties of anti-inflammation and anti-oxidant activity, and amelioration of glucose metabolism.
The protective associations of Butyrivibrio and Faecalibacterium with T2D could be partially explained by these microbial metabolites.
In summary, by leveraging integrative omics data in US Hispanics/Latinos, our study demonstrated that a higher intake of fiber was linked to beneficial patterns of gut microbiota taxonomic features, microbial functional enzymes, and circulating metabolites, all of which were associated with lower risk of T2D. Our analyses further revealed that, for certain fiber-associated taxa such as Butyrivibrio and Faecalibacterium, the potentially beneficial effects on T2D risk could be explained by microbial related metabolites, namely indolepropionate, 3-phenylpropionate and cinnamoylglycine. These findings contribute to our understanding of the complex relationships among dietary fiber intake, gut microbiota, and circulating metabolites, offering insights into their potential roles in the development of T2D. These insights facilitate the novel therapeutic strategies in precision nutrition and dietary intervention through modulating the gut microbiota and related microbial metabolites, potentially offering a more effective, precise way for T2D prevention.
Acknowledgments
We thank all investigators and participants of this study for their valuable contributions.
Sources of Funding
The Hispanic Community Health Study/Study of Latinos(HCHS/SOL) is a collaborative study supported by contracts from the National Heart, Lung, and Blood Institute(NHLBI) to the University of North Carolina(HHSN268201300001I/N01-HC-65233), University of Miami(HHSN268201300004I/N01-HC-65234), Albert Einstein College of Medicine(HHSN268201300002I/N01-HC-65235), University of Illinois at Chicago – HHSN268201300003I/N01-HC-65236 Northwestern University), and San Diego State University(HHSN268201300005I/N01-HC-65237). The following Institutes/Centers/Offices have contributed to the HCHS/SOL through a transfer of funds to the NHLBI: National Institute on Minority Health and Health Disparities, National Institute on Deafness and Other Communication Disorders, National Institute of Dental and Craniofacial Research, National Institute of Diabetes and Digestive and Kidney Diseases(NIDDK), National Institute of Neurological Disorders and Stroke, NIH Institution-Office of Dietary Supplements.
The present work is supported by R01-DK119268 and R01-DK126698 from the National Institute of Diabetes and Digestive and Kidney Diseases(NIDDK), and R01-MD011389 from the National Institute on Minority Health and Health Disparities. Other funding sources for this study include UM1-HG008898 from the National Human Genome Research Institute; R01-HL060712, R01-HL140976, and R01-HL136266 from the National Heart, Lung and Blood Institute(NHLBI); and R01-DK120870 and the New York Regional Center for Diabetes Translation Research(P30DK111022) from NIDDK.
Non-standard Abbreviations and Acronyms
- T2D
type 2 diabetes
- SCFAs
short-chain fatty acids
- HCHS/SOL
Hispanic Community Health Study/Study of Latinos
- PUFA
polyunsaturated fatty acids
- AHEI
Alternative Healthy Eating Index
- BMI
body mass index
- HOMA-IR
Homeostatic Model Assessment for Insulin Resistance
- SBP
systolic blood pressure
- DBP
diastolic blood pressure
- TG
triglycerides
- HDL
high-density lipoprotein cholesterol
Footnotes
Disclosures
None.
REFERENCES
- 1.McRae MP. Dietary Fiber Intake and Type 2 Diabetes Mellitus: An Umbrella Review of Meta-analyses. Journal of Chiropractic Medicine.2018;17:44–53. doi: 10.1016/j.jcm.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jenkins DJA, Kendall CWC, McKeown-Eyssen G, Josse RG, Silverberg J, Booth GL, Vidgen E, Josse AR, Nguyen TH, Corrigan S,et al. Effect of a low-glycemic index or a high-cereal fiber diet on type 2 diabetes: A randomized trial. JAMA - Journal of the American Medical Association.2008;300:2742–2753. doi: 10.1001/jama.2008.808. [DOI] [PubMed] [Google Scholar]
- 3.Myhrstad MCW, Tunsjø H, Charnock C, Telle-Hansen VH. Dietary fiber, gut microbiota, and metabolic regulation—current status in human randomized trials. Nutrients.2020;12(3): 859. doi: 10.3390/nu12030859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao L, Zhang F, Ding X, Wu G, Lam YY, Wang X, Fu H, Xue X, Lu C, Ma J,et al. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science.2018;359:1151–1156. doi: 10.1126/science.aao5774. [DOI] [PubMed] [Google Scholar]
- 5.Kaplan RC, Wang Z, Usyk M, Sotres-Alvarez D, Daviglus ML, Schneiderman N, Talavera GA, Gellman MD, Thyagarajan B,et al. Gut microbiome composition in the Hispanic Community Health Study/Study of Latinos is shaped by geographic relocation, environmental factors, and obesity. Genome Biol.2019;20: 219–z. doi: 10.1186/s13059-019-1831-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tuomainen M, Lindström J, Lehtonen M, Auriola S, Pihlajamäki J, Peltonen M, Tuomilehto J, Uusitupa M, De Mello VD, Hanhineva K. Associations of serum indolepropionic acid, a gut microbiota metabolite, with type 2 diabetes and low-grade inflammation in high-risk individuals. Nutrition and Diabetes.2018;8(1):35. doi: 10.1038/s41387-018-0046-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma W, Nguyen LH, Song M, Wang DD, Franzosa EA, Cao Y, Joshi A, Drew DA, Mehta R, Ivey KL,et al. Dietary fiber intake, the gut microbiome, and chronic systemic inflammation in a cohort of adult men. Genome Medicine.2021;13(1):102. doi: 10.1186/s13073-021-00921-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Z, Usyk M, Vázquez-Baeza Y, Chen G-, Isasi CR, Williams-Nguyen JS, Hua S, McDonald D, Thyagarajan B, Daviglus ML,et al. Microbial co-occurrence complicates associations of gut microbiome with US immigration, dietary intake and obesity. Genome Biol.2021;22(1):336. doi: 10.1186/s13059-021-02559-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koh A, De Vadder F, Kovatcheva-Datchary P, Bäckhed F. From dietary fiber to host physiology:Short-chain fatty acids as key bacterial metabolites. Cell.2016;165:1332–1345. doi: 10.1016/j.cell.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 10.Qi Q, Li J, Yu B, Moon J-, Chai JC, Merino J, Hu J, Ruiz-Canela M, Rebholz C, Wang Z,et al. Host and gut microbial tryptophan metabolism and type 2 diabetes:An integrative analysis of host genetics, diet, gut microbiome and circulating metabolites in cohort studies. Gut.2022;71(6):1095–1105. doi: 10.1136/gutjnl-2021-324053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cai Z, Poulos RC, Liu J, Zhong Q. Machine learning for multi-omics data integration in cancer. iScience.2022;25:103798. doi: 10.1016/j.isci.2022.103798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sorlie PD, Aviles-Santa LM, Wassertheil-Smoller S, Kaplan RC, Daviglus ML, Giachello AL, Schneiderman N, Raij L, Talavera G, Allison M,et al. Design and implementation of the Hispanic Community Health Study/Study of Latinos. Ann Epidemiol.2010;20:629–641. doi: 10.1016/j.annepidem.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arredondo EM, Sotres-Alvarez D, Stoutenberg M, Davis SM, Crespo NC, Carnethon MR, Castañeda SF, Isasi CR, Espinoza RA, Daviglus ML,et al. Physical Activity Levels in U.S. Latino/Hispanic Adults: Results From the Hispanic Community Health Study/Study of Latinos. Am J Prev Med.2016;50:500–508. doi: 10.1016/j.amepre.2015.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siega-Riz AM, Sotres-Alvarez D, Ayala GX, Ginsberg M, Himes JH, Liu K, Loria CM, Mossavar-Rahmani Y, Rock CL, Rodriguez B,et al. Food-group and nutrient-density intakes by Hispanic and Latino backgrounds in the Hispanic Community Health Study/Study of Latinos. Am J Clin Nutr.2014;99:1487–1498. doi: 10.3945/ajcn.113.082685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hillmann B, Al-Ghalith GA, Shields-Cutler RR, Zhu Q, Gohl DM, Beckman KB, Knight R, Knights D. Evaluating the Information Content of Shallow Shotgun Metagenomics. mSystems.2018;3(6):e00069–18. doi: 10.1128/mSystems.00069-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E, Lewis GD, Fox CS, Jacques PF, Fernandez C,et al. Metabolite profiles and the risk of developing diabetes. Nat Med.2011;17:448–453. doi: 10.1038/nm.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chai JC, Chen G-, Yu B, Xing J, Li J, Khambaty T, Perreira KM, Perera MJ, Vidot DC, Castaneda SF,et al. Serum Metabolomics of Incident Diabetes and Glycemic Changes in a Population With High Diabetes Burden:The Hispanic Community Health Study/Study of Latinos. Diabetes.2022;71:1338–1349. doi: 10.2337/db21-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qi Q, Strizich G, Merchant G, Sotres-Alvarez D, Buelna C, Castaneda SF, Gallo LC, Cai J, Gellman MD, Isasi CR,et al. Objectively Measured Sedentary Time and Cardiometabolic Biomarkers in US Hispanic/Latino Adults: The Hispanic Community Health Study/Study of Latinos (HCHS/SOL). Circulation.2015;132:1560–1569. doi: 10.1161/CIRCULATIONAHA.115.016938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mandal S, Van Treuren W, White RA, Eggesbø M, Knight R, Peddada SD. Analysis of composition of microbiomes: a novel method for studying microbial composition. Microb Ecol Health Dis.2015;26:27663. doi: 10.3402/mehd.v26.27663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Letunic I, Bork P. Interactive Tree Of Life(iTOL)v4:recent updates and new developments. Nucleic Acids Res.2019;47:W256–W259. doi: 10.1093/nar/gkz239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amin N, Liu J, Bonnechere B, MahmoudianDehkordi S, Arnold M, Batra R, Chiou Y, Fernandes M, Ikram MA, Kraaij R,et al. Interplay of Metabolome and Gut Microbiome in Individuals With Major Depressive Disorder vs Control Individuals. JAMA Psychiatry.2023;80(6):597–609. doi: 10.1001/jamapsychiatry.2023.0685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mirande C, Kadlecikova E, Matulova M, Capek P, Bernalier-Donadille A, Forano E, Bera-Maillet C. Dietary fibre degradation and fermentation by two xylanolytic bacteria Bacteroides xylanisolvens XB1A and Roseburia intestinalis XB6B4 from the human intestine. J Appl Microbiol.2010;109:451–460. doi: 10.1111/j.1365-2672.2010.04671.x. [DOI] [PubMed] [Google Scholar]
- 23.Lee S, Zhang C, Kilicarslan M, Piening BD, Bjornson E, Hallström BM, Groen AK, Ferrannini E, Laakso M, Snyder M,et al. Integrated Network Analysis Reveals an Association between Plasma Mannose Levels and Insulin Resistance. Cell Metab.2016;24:172–184. doi:S1550–4131(16)30248–0[pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McMurray F, Patten DA, Harper M-. Reactive Oxygen Species and Oxidative Stress in Obesity—Recent Findings and Empirical Approaches. Obesity. 2016;24:2301–2310. doi: 10.1002/oby.21654. [DOI] [PubMed] [Google Scholar]
- 25.Zaghlool SB, Halama A, Stephan N, Gudmundsdottir V, Gudnason V, Jennings LL, Thangam M, Ahlqvist E, Malik RA, Albagha OME,et al. Metabolic and proteomic signatures of type 2 diabetes subtypes in an Arab population. Nature Communications.2022;13(1):7121. doi: 10.1038/s41467-022-34754-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He X, Zhao S, Li Y. Faecalibacterium prausnitzii: A Next-Generation Probiotic in Gut Disease Improvement. Canadian Journal of Infectious Diseases and Medical Microbiology.2021;2021:6666114. doi: 10.1155/2021/6666114 [DOI] [Google Scholar]
- 27.Benno Y, Endo K, Miyoshi H, Okuda T. Effect of Rice Fiber on Human Fecal Microflora. Microbiol Immunol.1989;33:435–440. doi: 10.1111/j.1348-0421.1989.tb01992.x. [DOI] [PubMed] [Google Scholar]
- 28.Heinken A, Khan MT, Paglia G, Rodionov DA, Harmsen HJM, Thiele I. Functional metabolic map of Faecalibacterium prausnitzii, a beneficial human gut microbe.J Bacteriol.2014;196:3289–3302. doi: 10.1128/JB.01780-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dodd D, Spitzer MH, Van Treuren W, Merrill BD, Hryckowian AJ, Higginbottom SK, Le A, Cowan TM, Nolan GP, Fischbach MA,et al. A gut bacterial pathway metabolizes aromatic amino acids into nine circulating metabolites. Nature.2017;551:648–652. doi: 10.1038/nature24661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adisakwattana S Cinnamic acid and its derivatives:Mechanisms for prevention and management of diabetes and its complications. Nutrients.2017;9(2):163. doi: 10.3390/nu9020163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kelly WJ, Leahy SC, Altermann E, Yeoman CJ, Dunne JC, Kong Z, Pacheco DM, Li D, Noel SJ, Moon CD,et al. The glycobiome of the rumen bacterium butyrivibrio proteoclasticus B316T highlights adaptation to a polysaccharide-rich environment. PLoS ONE.2010;5(8):e11942. doi: 10.1371/journal.pone.0011942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palevich N, Kelly WJ, Ganesh S, Rakonjac J, Attwood GT. Butyrivibrio hungatei MB2003 competes effectively for soluble sugars released by Butyrivibrio proteoclasticus B316 T during growth on xylan or pectin. Appl Environ Microbiol.2019;85(3):e02056–18. doi: 10.1128/AEM.02056-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang T, Yang Y, Liang Y, Jiao X, Zhao C. Beneficial Effect of Intestinal Fermentation of Natural Polysaccharides. Nutrients.2018;10:1055. doi: 10.3390/nu10081055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kulkarni P, Devkumar P, Chattopadhyay I. Could dysbiosis of inflammatory and anti-inflammatory gut bacteria have an implications in the development of type 2 diabetes? A pilot investigation. BMC Research Notes.2021;14(1):52. doi: 10.1186/s13104-021-05466-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Talukdar R, Sarkar P, Jakkampudi A, Sarkar S, Aslam M, Jandhyala M, Deepika G, Unnisa M, Reddy DN. The gut microbiome in pancreatogenic diabetes differs from that of Type 1 and Type 2 diabetes. Scientific Reports.2021;11:10978. doi: 10.1038/s41598-021-90024-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Montonen J, Knekt P, Järvinen R, Reunanen A. Dietary Antioxidant Intake and Risk of Type 2 Diabetes. Diabetes Care.2004;27:362–366. doi: 10.2337/diacare.27.2.362. [DOI] [PubMed] [Google Scholar]
- 37.Anand S, Kaur H, Mande SS. Comparative in silico analysis of butyrate production pathways in gut commensals and pathogens. Frontiers in Microbiology.2016:7:1945. doi: 10.3389/fmicb.2016.01945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pedersen HK, Gudmundsdottir V, Nielsen HB, Hyotylainen T, Nielsen T, Jensen BA, Forslund K, Hildebrand F, Prifti E, Falony G,et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature.2016;535:376–381. doi: 10.1038/nature18646. [DOI] [PubMed] [Google Scholar]
- 39.Hesketh GG, Dennis JW. N-acetylglucosamine:More than a silent partner in insulin resistance. Glycobiology.2017;27:595–598. doi: 10.1093/glycob/cwx035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tamanai-Shacoori Z, Smida I, Bousarghin L, Loreal O, Meuric V, Fong SB, Bonnaure-Mallet M, Jolivet-Gougeon A. Roseburia spp.: a marker of health? Future Microbiol.2017;12:157–170. doi: 10.2217/fmb-2016-0130. [DOI] [PubMed] [Google Scholar]
- 41.Inan MS, Rasoulpour RJ, Yin L, Hubbard AK, Rosenberg DW, Giardina C. The luminal short-chain fatty acid butyrate modulates NF-κB activity in a human colonic epithelial cell line. Gastroenterology.2000;118:724–734. doi: 10.1016/S0016-5085(00)70142-9. [DOI] [PubMed] [Google Scholar]
- 42.Bakir-Gungor B, Bulut O, Jabeer A, Nalbantoglu OU, Yousef M. Discovering Potential Taxonomic Biomarkers of Type 2 Diabetes From Human Gut Microbiota via Different Feature Selection Methods. Frontiers in Microbiology.2021:12:628426. doi: 10.3389/fmicb.2021.628426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Silamiķele L, Silamiķelis I, Ustinova M, Kalniņa Z, Elbere I, Petrovska R, Kalniņa I, Kloviņš J. Metformin Strongly Affects Gut Microbiome Composition in High-Fat Diet-Induced Type 2 Diabetes Mouse Model of Both Sexes. Frontiers in Endocrinology.2021;12:626359. doi: 10.3389/fendo.2021.626359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lamothe LM, Cantu-Jungles TM, Chen T, Green S, Naqib A, Srichuwong S, Hamaker BR. Boosting the value of insoluble dietary fiber to increase gut fermentability through food processing. Food Funct.2021;12:10658–10666. doi: 10.1039/d1fo02146j. [DOI] [PubMed] [Google Scholar]
- 45.Baky MH, Salah M, Ezzelarab N, Shao P, Elshahed MS, Farag MA. Insoluble dietary fibers: structure, metabolism, interactions with human microbiome, and role in gut homeostasis. Critical Reviews in Food Science and Nutrition.2022;0:1–15. doi: 10.1080/10408398.2022.2119931. [DOI] [PubMed] [Google Scholar]
- 46.Abreu-Y-Abreu AT, Milke-García MP, Argüello-Arévalo GA, Calderón-de-la-Barca AM, Carmona-Sánchez RI, Consuelo-Sánchez A, Coss-Adame E, García-Cedillo MF,et al. Dietary fiber and the microbiota: A narrative review by a group of experts from the Asociación Mexicana de Gastroenterología. Rev Gastroenterol Mex (Engl Ed).2021;86:287–304. doi: 10.1016/j.rgmxen.2021.02.002. [DOI] [PubMed] [Google Scholar]
- 47.Guan Z, Yu E, Feng Q. Soluble Dietary Fiber, One of the Most Important Nutrients for the Gut Microbiota. Molecules.2021;26:6802. doi: 10.3390/molecules26226802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu Q, Li J, Zhu J, Sun X, He D, Li J, Cheng Z, Zhang X, Xu Y, Chen Q,et al. Gamma-glutamyl-leucine levels are causally associated with elevated cardio-metabolic risks. Front Nutr.2022; 9:936220. doi: 10.3389/fnut.2022.936220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, Stampfer MJ, Willett WC. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr.2012;142:1009–1018. doi: 10.3945/jn.111.157222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Langfelder P, Horvath S. WGCNA:An R package for weighted correlation network analysis. BMC Bioinform.2008;9:559. doi: 10.1186/1471-2105-9-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Otasek D, Morris JH, Bouças J, Pico AR, Demchak B. Cytoscape Automation: empowering workflow-based network analysis. Genome Biol.2019;20:185–4. doi: 10.1186/s13059-019-1758-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The gut microbiome shotgun metagenomics sequencing data in this study are deposited in QIITA (https://qiita.ucsd.edu/), ID 11666. HCHS/SOL has established a process for the scientific community to apply for access to participant data and materials, with such requests reviewed by the project’s Steering Committee. These policies are described at https://sites.cscc.unc.edu/hchs/. Please see the Major Resources Table in the Supplemental Material.


