Abstract
Background
At present, the diagnosis of post-traumatic stress disorder(PTSD) mainly relies on clinical symptoms and psychological scales, and finding objective indicators that are helpful for diagnosis has always been a challenge in clinical practice and academic research. Neuroimaging is a useful and powerful tool for discovering the biomarkers of PTSD,especially functional MRI (fMRI), structural MRI (sMRI) and Diffusion Weighted Imaging(DTI)are the most commonly used technologies, which can provide multiple perspectives on brain function, structure and its connectivity. Machine learning (ML) is an emerging and potentially powerful method, which has aroused people's interest because it is used together with neuroimaging data to define brain structural and functional abnormalities related to diseases, and identify phenotypes, such as helping physicians make early diagnosis.
Objectives
According to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) declaration, a systematic review was conducted to assess its accuracy in distinguishing between PTSD patients, TEHC(Trauma-Exposed Healthy Controls), and HC(healthy controls).
Methods
We searched PubMed, Embase, and Web of Science using common words for ML methods and PTSD until June 2023, with no language or time limits. This review includes 13 studies, with sensitivity, specificity, and accuracy taken from each publication or acquired directly from the authors.
Results
All ML techniques have an diagnostic accuracy rate above 70%,and support vector machine(SVM) are the most commonly used techniques. This series of studies has revealed significant neurobiological differences in key brain regions among individuals with PTSD, TEHC, and HC. The connectivity patterns of regions such as the Insula and Amygdala hold particular significance in distinguishing these groups. TEHC exhibits more normal connectivity patterns compared to PTSD, providing valuable insights for the application of machine learning in PTSD diagnosis.
Conclusion
In contrast to any currently available assessment and clinical diagnosis, ML techniques can be used as an effective and non-invasive support for early identification and detection of patients as well as for early screening of high-risk populations.
Keywords: Machine learning, Post-traumatic stress disorder, Support vector machine, Resting-state fMRI, sMRI, Multivariate pattern analysis
1. Introduction
PTSD is a condition that occurs after exposure to very stressful experiences [1]. The primary causes of this condition include abuse, sexual assaults, disasters, domestic, community, or school violence, medical trauma, and terrorist attacks [2].The symptoms of PTSD include intrusive thoughts, hyperarousal, flashbacks, nightmares, sleep disruptions, abnormalities in memory and attention, and startle reactions [3]. The Diagnostic and Statistical Manual of Mental Disorders is the current gold standard for PTSD diagnosis (updated version: fifth edition, or DSM-V). These procedures, like the DSM-IV used for the individuals in the current investigation, mainly rely on the subjective reports of patients. Considering the stigma associated with the diagnosis of specific groups or the difficulty of patients to express their symptoms, accurate diagnosis may be challenging. Therefore, a platform for objective diagnosis is widely desired [4].
Neuroimaging is a potent and effective technology for researching discriminating biomarkers in individuals with mental illnesses [5]. The most frequent diagnostic techniques used now are fMRI,sMRI and DTI which can be used to identify brain structures and functions with specific changes in patients, and to discover neurobiological markers with diagnostic and predictive value [6]. In a meta-analysis of functional and structural brain abnormalities in PTSD, In terms of facilitating the diagnosis, alterations in function did not appear to be specific, but structural aspects of the amygdala were of interest, and some regions, including the striatum, insula, primary visual, ACC/mPFC, auditory, and sensorimotor cortex showed structural abnormalities are more significant in PTSD [7]. Dissociative symptoms may be related with abnormalities in the transfrontal and parietal cortices, limbic system, and brainstem, according to functional and structural MRI studies of PTSD patients with dissociative symptoms [8]. According to these studies, the fMRI and sMRI are two techniques that commonly utilized to offer a variety of perspectives on brain function and structure. However, the majority of studies only provide methodological recommendations for further study, without providing practical and clinical valuable advice on how to identify and accurately. It is necessary to evaluate massive volumes of imaging data from MRI using automated techniques that can process data and determine the possibility of disorders with absolute accuracy [9]. ML,a field that focuses on the learning component of artificial intelligence(AI) by creating algorithms that best represent a collection of data, ML employs subsets of data to produce algorithms that may use innovative or different combinations of features and weights than can be deduced from first principles, in contrast to classical programming, where an algorithm may be explicitly implemented using known features. The four often employed learning techniques in ML, supervised, unsupervised, semi-supervised, and reinforcement learning, are each effective for tackling certain problems [10]. ML approaches also applied to neuroimaging data to discover features for translation into clinical practice for early diagnosis have received increased attentions [11]. The use of ML in neuroimaging to improve visual identification and achieve lower mistake rates than humans has also been a major driver of the rise of ML in medical imaging [12]. The idea behind ML is that by identifying patterns in data, computers may learn to carry out particular tasks without having to be trained to do so from specific input. Iterative learning algorithms are used in ML. To provide one example, it enables computers to locate information even when it is unknown without being specifically instructed to do so. The most common ML algorithms at the moment are support vector machines, linear regression, logistic regression, decision trees, and random forests [10].
ML approaches may be used to enhance the categorization of illnesses, predict risk factors and treatment results, and optimize the selection of treatments that are customized to each individual [13]. The discovery of a reasonably precise biological marker through machine learning may better enable understanding of the disease because PTSD is a condition with clinical and biological heterogeneity, which may be a barrier to understanding pathogenic mechanisms and developing ideal treatment and diagnostic tools. This study seeks to assess the data about the role of ML approaches in PTSD diagnosis discrimination.
According to PRISMA standards [14], the objective of this review was to evaluate the present state of the evidence on the use of ML approaches in diagnostic discrimination in PTSD patients , TEHCand HC utilizing neuroimaging data from fMRI and sMRI as input. Our ultimate aim is to succinctly summarize the existing applications of machine learning combined with neuroimaging in predicting and diagnosing PTSD. We strive to identify current research gaps and limitations, shedding light on challenges and areas for improvement in the current body of literature. Furthermore, we endeavor to propose potential directions for future research in this domain.
2. Materials and methods
2.1. Search strategy
Articles published 30 years before June 2023 in PubMed,Embase and Web of science,without time limits, were searched by using the following keywords: (Deep Learning OR DL OR Big data OR Artificial Intelligence OR Machine Learning OR ML OR Gaussian process OR Regularized logistic OR LDA OR Linear discriminant analysis OR Random forest OR Least Absolute selection shrinkage operator OR elastic net OR LASSO OR RVM OR relevance vector machine OR pattern recognition OR Computational Intelligence OR Machine Intelligence OR support vector OR SVM OR Pattern classification OR Deep learning) AND (PTSD OR Post-Traumatic Stress Disorder) AND (fMRI OR magnetic resonance imaging OR MRI OR functional MRI OR functional-MRI OR functional magnetic resonance imaging). Two researchers independently examined each of the studies that were picked.
2.2. Assessment of study quality
To assess the methodological quality of papers in this systematic review, the Jadad ranking system [15] was used. Jadad's technique allows for the qualification of a research based on the clarity with which the randomization, double-blinding procedure, and withdrawal and dropout data are described. The scale runs from 0 to 5. The inclusion criteria for this systematic review were a Jadad score of at least 3.
2.3. Selection criteria
We selected studies applying ML techniques with patients diagnosed with PTSD according to the DSM-IV, DSM-V criteria. Studies without a control group and trials involving patients with general medical conditions, neurological or psychiatric comorbidity, substance abuse or alcoholism, traumatic brain injuries resulting in loss of consciousness, and unclear or unreliable psychiatric diagnoses in accordance with the DSM criteria were excluded.
2.4. Data collection and extraction
Two authors(Jia and Yang) independently reviewed all of the article titles and abstracts that were gathered, as well as the complete texts of any publications that fit the requirements. When there was a dispute, a third researcher oversaw(Chen) and made the ultimate decision. Data from the article included the year it was published, the ML model and algorithm (e.g., SVM, MGPC), the sample size, the diagnoses the study evaluated, and statistical data (e.g., accuracy, sensitivity, and specificity).
3. Results
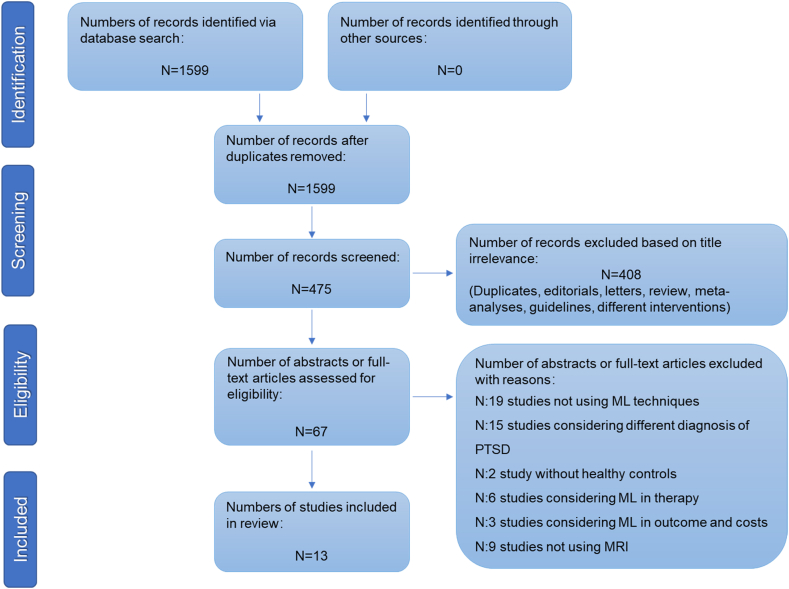
Initially, 1599 items were discovered, with 1124 articles being eliminated due to failure to meet inclusion requirements. The remaining 475 papers' abstracts were examined. Among of them, 408 papers were removed because they were editorials, letters to the editor, reviews, meta-analyses, case reports, or other interventions. Then, 54 of the 67 articles' manuscripts were eliminated since they did not meet the inclusion requirements. Finally, 13 studies were included in our study(Fig. 1). Among these 13 studies, 8 articles used SVM, 1 article used random vector machine (RVM), 3 articles used Multiclass Gaussian process classification(MGPC), and 1 article used multi-kernel learning(MKL)to study PTSD(Table 1)
Fig. 1.
PRISMA flowchart of included studies.
Table 1.
Summary of included studies classifying PTSD using ML.
| First author | Data preprocessing methods | Number of subjects | ML model | Most contributing brain regions | Best Accuracy | Other meatures | Commentary |
|---|---|---|---|---|---|---|---|
| Liu | rs-fMRI | PTSD:20 HC:20 Total:40 |
SVM | the limbic structure and pre-frontal cortex | 92.5% | Sensitivity:90%, Specificity:95% |
First time that PTSD patients have been identified at the individual level using resting-state fMRI data. |
| Zhang | rs-fMRI、sMRI | PTSD:17 TEC:20 HC:20 Total:57 | SVM | bilateral middle occipital gyrus, right inferior parietal lobule, left superior frontal gyrus et. | 90.0% | Sensitivity:76.47%, Specificity:100% |
Multimodal MRI approach improves the ability to classify PTSD |
| Zilcha-Mano | rs-fMRI | PTSD:51 PTSD + MDD:52 TEHCs:76 Total:179 | SVM | executive control network, prefrontal network, salience network and basal ganglia network | 76.7% | N.A. | The findings show that they are clinically useful in estimating symptomatology levels and treatment effectiveness. |
| Zhu,Z | rs-fMRI | PTSD:91 TEHCs:126 Total:217 | SVM + deep learning | default mode, central executive, and salience networks | 80.0% | sensitivity: 80.9%,specificity: 79.2% | These results show that DL based on graphical features is a potential technique to help with the diagnosis of PTSD. |
| Yang. | rs-fMRI | PTSD:33 HC:53 Total:86 | SVM + deep learning | Frontoparietal areas, cingulate cortex, and amygdala | 71.2% | sensitivity: 59.7%,specificity: 82.7% | Based on fMRI data, graphic topological metrics might add to imaging models of clinical usefulness in separating pediatric PTSD from HC. |
| Saba T | rs-fMRI | PTSD:14 HC:14 Total:28 | SVM KNN LR |
L-precuneus, R-precuneus, L-mPFC, and R-mPFC, amygdala, hippocampus, and thalamus | 99.2% | sensitivity: 96.6%,specificity:96.0% | The outcomes of the study might serve as a guideline for observing the in PTSD, and discriminating PTSD subjects using the recommended algorithms. |
| Q Gong. | sMRI | PTSD:50 TEHCs:50 HC:40, Total:140 |
SVM | volume of GM and WM | 91.0% | sensitivity: 97.0%,specificity:85.0% | Classification of PTST in SVM using structural and functional neuroimaging data is an integrated technique that has been effectively used to the study of mild cognitive impairment. |
| Suo | sMRI | PTSD:77 TEHCs:76 Total:153 | SVM | left uncinate fasciculus, right anterior thalamic radiation | 73.8% | Sensitivity:59.5%, Specificity:88.2% |
WM changes based on a tract-profile measurement method might be a biomarker for PTSD. |
| Harricharan | rs-fMRI | PTSD:84 PTSD + DS:49 HC:51 Total:184 | MGPC | Insula, frontal lobe | 80.4% | N.A. | machine learning algorithms can differentiate between PTSD and its dissociative variants using resting state connection patterns in the insula,. |
| Nicholson(2018) | rs-fMRI | PTSD:81 PTSD + SD:49 HC:51 Total:181 | MGPC | emotion regulation regions, amygdala,globus pallidus, and motor/somatosensory regions. | 91.63% | N.A. | For the first time Using machine learning to differentiate between PTSD and its dissociative subtypes |
| Nicholson(2020) | rs-fMRI | PTSD:81 PTSD + SD:49 HC:56 Total:186 | MGPC | Default mode network, Central executive network, Salience network | 80.0% | N.A. | Changes in the intrinsic connection network may explain the distinct psychopathology and symptom presentation of PTSD subtypes. |
| Nicholson(2022) | rs-fMRI | PTSD:14 HC:15 Total:29 | MKL | dmPFC, postcentral gyrus, amygdala/hippocampus, cingulate cortex, and temporal pole/middle and superior temporal gyri | 80.0% | N.A. | Findings show that EEG-NFB targeting brain networks associated with the PCC leads in acute reductions in symptoms over time. |
| Zhu,H | rs-fMRI | PTSD:57 TEHCs:59 Total:116 | RVM | default mode network (DMN), visual network (VIS), somatomotor network, limbic network, and dorsal attention network (DAN) | 89.2% | Sensitivity:86.5%, Specificity:92.0% |
Large-scale brain network-based rs-fMRI can both assist in clinical diagnosis and shed light on the underlying brain network processes of PTSD caused by natural disasters. |
3.1. Studies of classification methods using SVM
Two article employed a fusion of results from multiple data processing techniques to analyze data from resting-state fMRI and then combine the extracted features.
Liu et al. [16] proposed an innovative categorization framework for identifying PTSD patients and healthy controls, integrating features from amplitude of low frequency fluctuations (ALFF) and dynamic functional connectivity. The classification accuracy reached an impressive 92.5%, with a primary focus on limbic structures and the prefrontal cortex.In a study by Zhang et al. [17], a multimodal approach combining resting-state functional MRI (rs-fMRI) and structural MRI (sMRI) was employed. Features such as gray matter volume (GMV), ALFF, and regional homogeneity (ReHo) were fused using a multi-kernel combination approach. The accuracies for PTSD vs. HC, trauma-exposed healthy controls (TEHC) vs. HC, and PTSD vs. TEHC were reported as 89.19%, 90.00%, and 67.57%, respectively. The authors concluded that the multimodal feature combination technique, especially using multi-kernel support vector machines, outperformed single-feature approaches in classification performance. Zilcha-Mano et al. [18] utilized a within-network functional connectivity approach and achieved 70.6% accuracy in distinguishing individuals with PTSD from TEHCs. Additionally, they achieved 76.7% accuracy in distinguishing individuals with PTSD alone from those with PTSD comorbid with major depressive disorder (PTSD + MDD). Noteworthy regions contributing to classification accuracy included the executive control network, prefrontal network, and salience network.
Two studies by Zhu, Z et al. [19]and Yang et al. [20], combined deep learning and classical support vector machine (SVM) techniques. They employed graphic topological measures based on fMRI data to classify PTSD in adults vs. HC and PTSD in children vs. TEHC, achieving accuracies of 80% and 71.2%, respectively. The authors proposed a two-stage prediction pipeline technique for creating more accurate machine learning algorithms with potential clinical applications. The central executive network significantly contributed to the model's performance in both adults and children. Medial prefrontal cortex, amygdala, thalamus, hippocampus, and precuneus were chosen as the region of interests (ROIs) and compared the functional connections among them in another study. Saba T et al. [21] tested five machine learning algorithms and discovered that SVM had the greatest classification accuracy of 99.2%. The author concluded that this will identify the best algorithm for providing identification recommendations.
Gong et al. [22] compared gray and white matter volumes(prefrontal, temporal, parietal and occipital regions as well as subcortical structures) in PTSD patients and HC, with an accuracy of 91%. The author proposed the patterns of neuroanatomical alterations might be a possible biomarker for detecting structural brain abnormalities in people suffering from PTSD.
A further study suggested that a potential biomarker for PTSD is WM changes based on a tract-profile quantification method. Suo et al. [23] found PTSD had lower fractional anisotropy(FA) with greater radial diffusivity(RD) and mean diffusivity(MD) in the left uncinate fasciculus and lower FA with higher RD in the right anterior thalamic radiation compared to TEHC. Then the results of SVM revealed the axial diffusivity(AD) profile performed the best, with a mean balanced accuracy of 73.8%, sensitivity of 59.5%, and specificity of 88.2%.
3.2. Studies of classification methods using MGPC
All three investigations delved into the classification of individuals across distinct categories: those with post-traumatic stress disorder (PTSD), a subtype characterized by both PTSD and dissociative symptoms (PTSD + DS), and a control group of healthy individuals (HC). Harricharan et al. [24] used insula subregion connectivity patterns with 80.4% balanced accuracy. Using this methodology, the author identified that PTSD was associated with heightened bilateral posterior insula connections to subcortical regions, including the periaqueductal gray. In contrast, individuals with PTSD + DS exhibited more pronounced bilateral anterior and posterior insula connections with posterior cortices, specifically the left lingual gyrus and the left precuneus, in comparison to both PTSD and control groups. These findings underscore a discernible neurobiological distinction between PTSD and its dissociative subtype, particularly in terms of insula subregion functional connection patterns.
In the study by Nicholson et al., (2019) [25], it was revealed that the PTSD + DS group exhibited heightened activation in emotion regulation areas compared to the PTSD group, which, in turn, displayed increased activation in the amygdala, globus pallidus, and motor/somatosensory regions. The extracted features from both resting-state mean amplitude of low-frequency fluctuations (mALFF) (91.63% balanced accuracy) and amygdala complex connectivity maps (85.00% balanced accuracy) reliably predicted the categorization.
Furthermore, Nicholson et al., (2020) [26] employed alterations in intrinsic connectivity networks (ICN) to predict PTSD and its subtypes, achieving an impressive classification accuracy of 88.92%. The findings suggested that differences in intrinsic connectivity networks may serve as the foundation for distinct psychopathology and symptom presentation among PTSD subtypes. The findings of the author showed that differences in intrinsic connectivity networks may underpin distinct psychopathology and symptom presentation among PTSD subtypes.
3.3. Studies of classification methods using other method
Nicholson et al., (2022) [27] conducted a study exploring the effects of neurofeedback (NFB) training on symptoms related to trauma or stress word processing, specifically targeting the downregulation of the posterior cingulate cortex (PCC). Their investigation revealed immediate reductions in symptoms following the NFB training. Notably, they employed L1-Multiple Kernel Learning (MKL) Classification algorithms to achieve an 80% accuracy in classifying individuals with PTSD compared to healthy controls (HC).
In a similar vein, Zhu, H et al. [28] employed a different approach by extracting node-to-network functional connections. Utilizing Relevance Vector Machine (RVM), they achieved an impressive diagnostic accuracy of 89.2% in distinguishing between individuals with PTSD and trauma-exposed controls (TEC). The study highlighted the significance of various networks, such as the default mode network (DMN), visual network (VIS), somatomotor network, limbic network, and dorsal attention network (DAN), as being particularly influential in the categorization of PTSD.
4. Discussion
The study and implementation of artificial neural networks(ANNs) have contributed significantly to the advancement of ML [29],and the use of ML techniques is a promising strategy that could assist clinicians in the identification of mental illnesses and could be helpful in the classification of PTSD using MRI. The majority of the studies in the review had accuracy levels between 75% and 90%, with all of them achieving at least a minimum accuracy of about 70%.
The synthesis of findings from the 13 studies reveals distinct neural patterns associated with PTSD, TEHC, and HC. ML techniques applied across these investigations have elucidated specific brain regions critical for discriminating among these groups. At the forefront, the insula emerges as a key player in distinguishing between different trauma-related states. In PTSD patients compared to healthy controls, heightened connectivity between bilateral insular cortices and brainstem regions, notably the brainstem gray matter, was observed. This finding suggests aberrant responses to stress and emotional regulation in PTSD individuals. Further, in PTSD + DS, there was more pronounced connectivity between the anterior and posterior insular cortices and the posterior cingulate cortex, indicating nuanced neurobiological differences. The amygdala, a pivotal region in emotion regulation, exhibited increased activity in PTSD patients compared to trauma-exposed healthy controls. This heightened amygdala activation suggests specific neural activity patterns associated with processing emotional stimuli in PTSD individuals. Exploring intrinsic connectivity networks (ICN), the ML analyses demonstrated alterations in ICNs in PTSD patients, allowing for accurate prediction of PTSD. These findings underscore the potential of ML techniques to uncover neurobiological distinctions related to trauma exposure. Beyond specific brain regions, changes in the prefrontal cortex, hippocampus, and posterior cingulate cortex were identified as neuroimaging indicators contributing to the diagnosis of PTSD.
Importantly, the inclusion of TEHC as a distinct group plays a pivotal role in this investigation. ML applications revealed that TEHC exhibited more normalized connectivity patterns in these brain regions compared to PTSD patients and those with the dissociative subtype. This suggests that TEHC may represent a healthier emotional processing profile. Recognizing the significance of TEHC in distinguishing between trauma-exposed states enhances the clinical applicability and generalizability of ML techniques in the context of PTSD diagnosis and understanding its neurobiological underpinnings.
According to our review, SVM appears to be the preferred approach for classification investigations. SVM emerged as the preferred approach in more than half of the studies (8 out of 13), either used alone or in combination with other approaches to optimize the model across various datasets. Studies attempting to predict whether a person has PTSD appear to be well suited for the initial use of SVM, which addresses binary classification problems [30]. Several inputs have been employed to increase the technique's accuracy: changes in GM and WM (particularly in sMRI studies), anomalies in brain functional connectivity(FC)parameters (e.g., ReHo or ALFF), and networks or whole groups of functional connections. Previous MRI findings suggest that the medial and dorsolateral prefrontal cortex, orbitofrontal cortex, insula, amygdala, anterior and posterior cingulate, hippocampus and para-hippocampus cortex, precuneus, cuneus, syrinx, and lingual gyrus, as well as the white matter tracts connecting these brain regions, are relevant to PTSD pathophysiology. Of these, changes in the anterior cingulate, amygdala, hippocampus, and insula are highly repeatable across structural and functional MRI [31,32]. In the analysis of machine learning, particularly the prefrontal (e.g., DLPFC, prefrontal cortex of the eye sockets), amygdala, and posterior cingulate cortex, which appear to be most useful in predicting the diagnosis of PTSD. In addition the fusion of different inputs has contributed to improving the accuracy of classification techniques.
A MGPC machine learning study was performed within SPM12 utilizing the Pattern Recognition for Neuroimaging Toolbox (PRoNTo). Using fMRI feature sets, MGPC predicts group categorization across several classes [33]. The insula, amygdala, and limbic system are considered to be objective biomarkers with heterogeneity in the classification of PTSD dissociation subtypes, and the current study has important implications for advancing the application of machine learning with different algorithms in the field of psychiatry and the discovery of new biomarkers.
ML techniques exhibit the capability to handle large-scale, high-dimensional datasets, particularly crucial in neuroimaging research. They can capture complex nonlinear relationships, aiding in a comprehensive understanding of the neural mechanisms of PTSD [34]. In current literature, ML analysis can reveal drastically altered brain connections in PTSD subjects compared to healthy controls (e.g., default mode network, central executive network, visual network, and salience network, etc.). In other psychiatric disorders, such as schizophrenia(SCZ), ML analysis can also distinguish patients from healthy controls by altered brain connection [35,36],and alterations in brain connection overlap between different psychiatric disorders. So an integrated study employing biomarkers from various biological sources (e.g., fMRI, sMRI) should be studied to increase the ability to identify PTSD patients from HC and expedite diagnosis. From the extensive literature on traditional methods combining ML techniques for studying mental disorders, we can observe that, in comparison with traditional analytical approaches, ML has the ability to comprehensively explore patterns in data, thereby enhancing sensitivity to differences between PTSD patients and control groups. Traditional methods may rely on prior knowledge and assumptions, while ML methods engage in data-driven learning without predefined hypotheses. However, ML models often lack interpretability, making it challenging to understand the underlying mechanisms driving their predictions. This can be a drawback when aiming for a clear understanding of neural alterations in PTSD. Moreover, ML methods heavily rely on the quality and representativeness of the training data. Issues such as bias or noise in the data may impact the generalization and validity of the results. In summary, a synergistic approach combining ML and traditional analytical methods might prove effective. Traditional methods provide insights into specific biomarkers or brain regions based on prior knowledge, while ML methods uncover more complex and abstract relationships.
Within the scope of this review, the literature predominantly focuses on the diagnosis of PTSD and the prediction of its onset. Notably, Sheynin et al. [37] showcased a novel application of deep learning coupled with neuroimaging to predict the progression of PTSD symptoms at distinct time points post-occurrence. This represents a compelling facet of artificial intelligence, unveiling its potential beyond mere diagnostic capabilities. The ability to anticipate the trajectory of symptoms following PTSD onset, as demonstrated by Sheynin et al.'s study, presents a promising avenue for future research. This suggests a paradigm shift towards leveraging machine learning not only for early identification but also for forecasting symptom dynamics over time. Such predictive capabilities hold immense potential for clinicians to proactively intervene and tailor treatments based on specific time points and symptomatology. This nuanced approach aligns with the evolving landscape of artificial intelligence in mental health research, signifying a promising direction for advancing both diagnostic and therapeutic strategies in the clinical management of PTSD.
There are various restrictions that should be taken into account with this systematic review. The majority of the studies extracted categorical features using resting-state fMRI, with only one study using both diffusion MRI and fMRI data. While resting-state fMRI is a promising technique for measuring spontaneous brain activity, it lacks direct observation of anatomical connections. Future studies may focus on merging resting-state fMRI and diffusion MRI data, as well as imaging data with other clinical biological data to train more robust models. The role of the elapsed time since trauma emerged as a critical factor in understanding the applicability and effectiveness of ML techniques. Moving forward, researchers are encouraged to delve into the temporal dynamics of trauma effects, investigating how machine learning can discern patterns and alterations in the brain at different post-trauma intervals. This approach may unveil nuanced insights into the evolving nature of trauma's impact on neural mechanisms over time. Moreover,in the included studies, there were no longitudinal experimental designs, and all studies only differentiated between subgroups based on brain function or structure; future experimental designs may need to be prospectively designed and observe the process of treatment response and drug type/dose effects on PTSD brain structure or neural networks. The task completed by Nicholson et al., (2022)is the single study in this review that utilizes changes in neural networks during treatment to classify PTSD and healthy controls; the goal of further research is to provide as much evidence as possible for clinically targeted interventions in relevant brain regions. In addition to this, another important aspect is the need for early differentiation of HC from high-risk groups. In fact, it seems to be easier to distinguish patients from HC than from high-risk groups, and the application of simply distinguishing PTSD from HC is still limited in the real clinical field. Despite including comparisons between TEHC and non-TEHC groups in our incorporated studies, there is currently a lack of dedicated research specifically addressing individuals exposed to trauma without PTSD and normal controls. Future research efforts could concentrate on leveraging machine learning techniques for a more accurate differentiation between these two groups, exploring potential biological markers and neural mechanisms. This approach not only enhances our comprehension of the impact of trauma on the brain but also introduces new perspectives for precise diagnostic and treatment strategies. As a result, the goal will be to make ML more complex so that it can do not only category classifications but also dimensional diagnostics (eg, patients with prodromal symptoms of PTSD from HC).
5. Conclusion
In conclusion, the adoption of ML algorithms will be beneficial in automatically classifying patients with PTSD based on neuroimaging. These strategies, if consistently incorporated in the diagnosis process of patients with PTSD, might enable clinicians recognize individuals even in the early stages of the condition, offering a significant treatment advantage. We anticipate that the increased accuracy demonstrated by the various predictive models illustrated in this systematic review, as well as new models resulting from the integration of multiple ML techniques, will become increasingly important in the future for the early diagnosis and evaluation of treatment response, as well as determining the prognosis of patients with PTSD. To truly benefit patients, the next challenge will be to arrive at an accurate diagnosis not just through clinical examination but also with the assistance of ML algorithms.
Funding
This work was supported by the National Defense Science and Technology Special Zone Innovation Project (Contract Number:21-163-00-TS-018-007-01).
Data availability statement
No data associated to this study has been deposited into a publicly available repository.
Data will be made available on request.
CRediT authorship contribution statement
Y.L. Jia: Writing – review & editing, Writing – original draft. B.N. Yang: Data curation. Y.H. Yang: Methodology. W.M. Zheng: Methodology. L. Wang: Formal analysis. C.Y. Huang: Supervision. J. Lu: Supervision. N. Chen: Validation, Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We gratefully thank the editor and reviewers who took part in the review, and we appreciate your constructive remarks.
Abbreviation list
- PTSD
post-traumatic stress disorder
- fMRI
functional MRI: structural MRI
- DTI
Diffusion Weighted Imaging
- ML
Machine learning
- PRISMA
Preferred Reporting Items for Systematic reviews and Meta-Analyses
- SVM
support vector machine
- AI
artificial intelligence
- TEHC
trauma-exposed healthy controls
- HC
healthy controls
- RVM
random vector machine
- MGPC
Multiclass Gaussian process classification
- MKL
multi-kernel learning
- GMV
gray matter volume
- ALFF
amplitude of low frequency fluctuations
- ReHo
regional homogeneity
- PTSD + MDD
PTSD and major depressive disorder
- ROIs
region of interests
- FA
fractional anisotropy
- RD
radial diffusivity
- MD
mean diffusivity
- AD
axial diffusivity
- PTSD + DS
PTSD and dissociative subtypes
- ICN
intrinsic connectivity networks
- NFB
neurofeedback
- DMN
default mode network
- VIS
visual network
- DAN
dorsal attention network
- ANNs
artificial neural networks
- FC
functional connectivity
- SCZ
schizophrenia
References
- 1.Yehuda R., Hoge C.W., McFarlane A.C., Vermetten E., Lanius R.A., Nievergelt C.M., Hobfoll S.E., Koenen K.C., Neylan T.C., Hyman S.E. Post-traumatic stress disorder. Nat. Rev. Dis. Prim. 2015;1(1) doi: 10.1038/nrdp.2015.57. [DOI] [PubMed] [Google Scholar]
- 2.Beni Yonis O., Khader Y., Jarboua A., Al-Bsoul M.M., Al-Akour N., Alfaqih M.A., Khatatbeh M.M. Amarneh B: post-traumatic stress disorder among Syrian adolescent refugees in Jordan. J. Public Health. 2020;42(2):319–324. doi: 10.1093/pubmed/fdz026. [DOI] [PubMed] [Google Scholar]
- 3.Bremner J.D. Traumatic stress: effects on the brain. Dialogues Clin. Neurosci. 2006;8(4):445–461. doi: 10.31887/DCNS.2006.8.4/jbremner. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang J., Richardson J.D., Dunkley B.T. Classifying post-traumatic stress disorder using the magnetoencephalographic connectome and machine learning. Sci. Rep. 2020;10(1) doi: 10.1038/s41598-020-62713-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He Y., Chen Z.J., Evans A.C. Small-world anatomical networks in the human brain revealed by cortical thickness from MRI. Cerebr. Cortex. 2007;17(10):2407–2419. doi: 10.1093/cercor/bhl149. [DOI] [PubMed] [Google Scholar]
- 6.Pitman R.K., Rasmusson A.M., Koenen K.C., Shin L.M., Orr S.P., Gilbertson M.W., Milad M.R., Liberzon I. Biological studies of post-traumatic stress disorder. Nat. Rev. Neurosci. 2012;13(11):769–787. doi: 10.1038/nrn3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xiao S., Yang Z., Su T., Gong J., Huang L., Wang Y. Functional and structural brain abnormalities in posttraumatic stress disorder: a multimodal meta-analysis of neuroimaging studies. J. Psychiatr. Res. 2022;155:153–162. doi: 10.1016/j.jpsychires.2022.08.010. [DOI] [PubMed] [Google Scholar]
- 8.Lotfinia S., Soorgi Z., Mertens Y., Daniels J. Structural and functional brain alterations in psychiatric patients with dissociative experiences: a systematic review of magnetic resonance imaging studies. J. Psychiatr. Res. 2020;128:5–15. doi: 10.1016/j.jpsychires.2020.05.006. [DOI] [PubMed] [Google Scholar]
- 9.Veronese E., Castellani U., Peruzzo D., Bellani M., Brambilla P. Machine learning approaches: from theory to application in schizophrenia. Comput. Math. Methods Med. 2013;2013:867924. doi: 10.1155/2013/867924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi R.Y., Coyner A.S., Kalpathy-Cramer J., Chiang M.F., Campbell J.P. Introduction to machine learning, neural networks, and deep learning. Transl Vis Sci Technol. 2020;9(2):14. doi: 10.1167/tvst.9.2.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolfers T., Buitelaar J.K., Beckmann C.F., Franke B., Marquand A.F. From estimating activation locality to predicting disorder: a review of pattern recognition for neuroimaging-based psychiatric diagnostics. Neurosci. Biobehav. Rev. 2015;57:328–349. doi: 10.1016/j.neubiorev.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 12.Currie G., Hawk K.E., Rohren E., Vial A., Klein R., Learning Machine, Learning Deep. In medical imaging: intelligent imaging. J. Med. Imag. Radiat. Sci. 2019;50(4):477–487. doi: 10.1016/j.jmir.2019.09.005. [DOI] [PubMed] [Google Scholar]
- 13.Hahn T., Nierenberg A.A., Whitfield-Gabrieli S. Predictive analytics in mental health: applications, guidelines, challenges and perspectives. Mol. Psychiatr. 2017;22(1):37–43. doi: 10.1038/mp.2016.201. [DOI] [PubMed] [Google Scholar]
- 14.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gøtzsche P.C., Ioannidis J.P., Clarke M., Devereaux P.J., Kleijnen J., Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jadad A.R., Moore R.A., Carroll D., Jenkinson C., Reynolds D.J., Gavaghan D.J., McQuay H.J. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Contr. Clin. Trials. 1996;17(1):1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 16.Liu F., Xie B., Wang Y., Guo W., Fouche J.-P., Long Z., Wang W., Chen H., Li M., Duan X., et al. Characterization of post-traumatic stress disorder using resting-state fMRI with a multi-level parametric classification approach. Brain Topogr. 2015;28(2):221–237. doi: 10.1007/s10548-014-0386-2. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Q., Wu Q., Zhu H., He L., Huang H., Zhang J., Zhang W. Multimodal MRI-based classification of trauma survivors with and without post-traumatic stress disorder. Front. Neurosci. 2016:10. doi: 10.3389/fnins.2016.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zilcha-Mano S., Zhu X., Suarez-Jimenez B., Pickover A., Tal S., Such S., Marohasy C., Chrisanthopoulos M., Salzman C., Lazarov A., et al. Diagnostic and predictive neuroimaging biomarkers for posttraumatic stress disorder. BIOLOGICAL PSYCHIATRY-COGNITIVE NEUROSCIENCE AND NEUROIMAGING. 2020;5(7):688–696. doi: 10.1016/j.bpsc.2020.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu Z., Lei D., Qin K., Suo X., Li W., Li L., DelBello M.P., Sweeney J.A., Gong Q. Combining deep learning and graph-theoretic brain features to detect posttraumatic stress disorder at the individual level. Diagnostics. 2021;11(8) doi: 10.3390/diagnostics11081416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang J., Lei D., Qin K., Pinaya W.H.L., Suo X., Li W., Li L., Kemp G.J., Gong Q. Using deep learning to classify pediatric posttraumatic stress disorder at the individual level. BMC Psychiatr. 2021;21(1) doi: 10.1186/s12888-021-03503-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saba T., Rehman A., Shahzad M.N., Latif R., Bahaj S.A., Alyami J. Machine learning for post-traumatic stress disorder identification utilizing resting-state functional magnetic resonance imaging. Microsc. Res. Tech. 2022;85(6):2083–2094. doi: 10.1002/jemt.24065. [DOI] [PubMed] [Google Scholar]
- 22.Gong Q., Li L., Tognin S., Wu Q., Pettersson-Yeo W., Lui S., Huang X., Marquand A.F., Mechelli A. Using structural neuroanatomy to identify trauma survivors with and without post-traumatic stress disorder at the individual level. Psychol. Med. 2014;44(1):195–203. doi: 10.1017/S0033291713000561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suo X., Lei D., Li W., Sun H., Qin K., Yang J., Li L., Kemp G.J., Gong Q. Psychoradiological abnormalities in treatment-naive noncomorbid patients with posttraumatic stress disorder. Depress. Anxiety. 2022;39(1):83–91. doi: 10.1002/da.23226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harricharan S., Nicholson A.A., Thome J., Densmore M., McKinnon M.C., Théberge J., Frewen P.A., Neufeld R.W.J., Lanius R.A. PTSD and its dissociative subtype through the lens of the insula: anterior and posterior insula resting-state functional connectivity and its predictive validity using machine learning. Psychophysiology. 2020;57(1) doi: 10.1111/psyp.13472. [DOI] [PubMed] [Google Scholar]
- 25.Nicholson A.A., Densmore M., McKinnon M.C., Neufeld R.W.J., Frewen P.A., Théberge J., Jetly R., Richardson J.D., Lanius R.A. Machine learning multivariate pattern analysis predicts classification of posttraumatic stress disorder and its dissociative subtype: a multimodal neuroimaging approach. Psychol. Med. 2019;49(12):2049–2059. doi: 10.1017/S0033291718002866. [DOI] [PubMed] [Google Scholar]
- 26.Nicholson A.A., Harricharan S., Densmore M., Neufeld R.W.J., Ros T., McKinnon M.C., Frewen P.A., Theberge J., Jetly R., Pedlar D., et al. Classifying heterogeneous presentations of PTSD via the default mode, central executive, and salience networks with machine learning. NEUROIMAGE-CLINICAL. 2020:27. doi: 10.1016/j.nicl.2020.102262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nicholson A.A., Rabellino D., Densmore M., Frewen P.A., Steryl D., Scharnowski F., Théberge J., Neufeld R.W.J., Schmahl C., Jetly R., et al. Differential mechanisms of posterior cingulate cortex downregulation and symptom decreases in posttraumatic stress disorder and healthy individuals using real-time fMRI neurofeedback. Brain and Behavior. 2022;12(1) doi: 10.1002/brb3.2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu H., Yuan M., Qiu C., Ren Z., Li Y., Wang J., Huang X., Lui S., Gong Q., Zhang W., et al. Multivariate classification of earthquake survivors with post-traumatic stress disorder based on large-scale brain networks. Acta Psychiatr. Scand. 2020;141(3):285–298. doi: 10.1111/acps.13150. [DOI] [PubMed] [Google Scholar]
- 29.Liu N.T., Salinas J. Machine learning for predicting outcomes in trauma. Shock. 2017;48(5):504–510. doi: 10.1097/SHK.0000000000000898. [DOI] [PubMed] [Google Scholar]
- 30.Ramos-Lima L.F., Waikamp V., Antonelli-Salgado T., Passos I.C., Freitas L.H.M. The use of machine learning techniques in trauma-related disorders: a systematic review. J. Psychiatr. Res. 2020;121:159–172. doi: 10.1016/j.jpsychires.2019.12.001. [DOI] [PubMed] [Google Scholar]
- 31.Kunimatsu A., Yasaka K., Akai H., Kunimatsu N., Abe O. MRI findings in posttraumatic stress disorder. J. Magn. Reson. Imag. 2020;52(2):380–396. doi: 10.1002/jmri.26929. [DOI] [PubMed] [Google Scholar]
- 32.Zhou Y.G., Shang Z.L., Zhang F., Wu L.L., Sun L.N., Jia Y.P., Yu H.B., Liu W.Z. PTSD: past, present and future implications for China. Chin. J. Traumatol. 2021;24(4):187–208. doi: 10.1016/j.cjtee.2021.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schrouff J., Rosa M.J., Rondina J.M., Marquand A.F., Chu C., Ashburner J., Phillips C., Richiardi J., Mourão-Miranda J. PRoNTo: pattern recognition for neuroimaging toolbox. Neuroinformatics. 2013;11(3):319–337. doi: 10.1007/s12021-013-9178-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nielsen A.N., Barch D.M., Petersen S.E., Schlaggar B.L., Greene D.J. Machine learning with neuroimaging: evaluating its applications in psychiatry. Biol Psychiatry Cogn Neurosci Neuroimaging. 2020;5(8):791–798. doi: 10.1016/j.bpsc.2019.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Filippis R., Carbone E.A., Gaetano R., Bruni A., Pugliese V., Segura-Garcia C., De Fazio P. Machine learning techniques in a structural and functional MRI diagnostic approach in schizophrenia: a systematic review. Neuropsychiatric Dis. Treat. 2019;15:1605–1627. doi: 10.2147/NDT.S202418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steardo L., Jr., Carbone E.A., de Filippis R., Pisanu C., Segura-Garcia C., Squassina A., De Fazio P., Steardo L. Application of support vector machine on fMRI data as biomarkers in schizophrenia diagnosis: a systematic review. Front. Psychiatr. 2020;11:588. doi: 10.3389/fpsyt.2020.00588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sheynin S., Wolf L., Ben-Zion Z., Sheynin J., Reznik S., Keynan J.N., Admon R., Shalev A., Hendler T., Liberzon I. Deep learning model of fMRI connectivity predicts PTSD symptom trajectories in recent trauma survivors. Neuroimage. 2021;238 doi: 10.1016/j.neuroimage.2021.118242. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data associated to this study has been deposited into a publicly available repository.
Data will be made available on request.