Abstract
Human T-cell leukemia virus type 1 (HTLV-1) Tax targets I-κBα and I-κBβ for phosphorylation, ubiquitination, and proteasome-mediated degradation, causing the nuclear translocation of NF-κB/Rel proteins and transcription induction of many cellular genes. The mechanism by which a nuclear protein such as Tax stimulates I-κB phosphorylation and degradation remains unclear. Here, we describe two cytoplasmic mutants of Tax, designated TaxΔN81 and TaxΔN109, from which the domains important for cyclic AMP response element binding factor (CREB) and serum response factor (SRF) binding and nuclear transport have been removed. These mutants were unable to trans activate from the HTLV-1 21-bp repeats or the serum response element in the c-fos promoter. In contrast, they activated NF-κB reporters, suggesting that activation of NF-κB by Tax occurs in the cytoplasm. Incorporation of the nuclear localization signal (NLS) of the simian virus 40 large T antigen into TaxΔN81 and TaxΔN109 redirected both proteins predominantly to the nucleus yet did not restore trans activation via CREB or SRF. The NLS fusion had little effect on TaxΔN81 but reduced NF-κB trans activation by TaxΔN109, possibly because of its proximity to the NF-κB-activating domain of Tax. In contrast to wild-type Tax, the cytoplasmic TaxΔN mutants are not cytotoxic. Stable expression of TaxΔN109 in HeLa cells resulted in a significant reduction in the intracellular level of I-κBα, with the constitutive presence of NF-κB in the nucleus and concomitant activation of the NF-κB enhancer. These results are suggestive of a potential application of the TaxΔN109-like mutants in targeting I-κB degradation and NF-κB activation. Interestingly, a Tax species with a molecular mass similar to that of TaxΔN109 was identified in many HTLV-1-transformed T cells, suggesting that TaxΔN109-like species might play a role in HTLV-1-induced leukemogenesis.
Members of the NF-κB/Rel family of transcription factors use a conserved Rel homology domain of approximately 300 amino acids to form homo- and heterodimers and bind the κB DNA motif, GGGRNNYYCC, to activate transcription (for reviews, see references 4–6, 35, and 47). They function as inducible trans activators of viruses such as human immunodeficiency virus (HIV) and many cellular genes involved in immune or inflammatory responses (reviewed in references 4–6, 35, and 47). Of the NF-κB/Rel family members, RelA (p65) homodimer and RelA/NF-κB1 (p50) heterodimer are the most abundantly and ubiquitously expressed. In resting or unstimulated cells, NF-κB/Rel factors are sequestered in the cytoplasm through interactions with inhibitory molecules, principally I-κBα and I-κBβ (reviewed in references 4–6, 35, and 47). Upon activation by mitogens, cytokines, or physical stress, I-κBα and I-κBβ become serine phosphorylated (10, 11, 14) and targeted for degradation through the ubiquitin-proteasome pathway (12). The degradation of I-κB allows NF-κB to be released for nuclear transport and transcriptional activation. Via multiple κB motifs in the transcriptional control region of the I-κBα gene, NF-κB greatly stimulates I-κBα mRNA expression (51). The newly synthesized I-κBα, in turn, down-modulates NF-κB activity and restores the autoregulatory loop (2, 4–6, 35, 47, 51). Dysregulation and/or hyperactivation of the NF-κB/I-κB regulatory pathway caused by chromosomal translocation (40), oncogene transduction (reviewed in references 18 and 19), or targeted gene disruption (7, 30) leads to cancers of the hematopoietic cells or chronic inflammatory diseases.
The diseases caused by human T-cell leukemia virus type 1 (HTLV-1), adult T-cell leukemia (24, 44) and tropical spastic paraparesis-HTLV-1-associated myelopathy (16, 42), have their etiologies in the dysregulated proliferation of virus-infected T cells. The molecular basis for T-cell transformation by HTLV-1 is not well understood. HTLV-1 does not transduce cellular oncogenes or activate proto-oncogenes by site-specific integration (46). It is generally thought that the virally encoded trans activator, Tax, is responsible for HTLV-1 leukemogenesis. Tax exerts pleiotropic effects on virus-infected cells by interacting directly with key cellular transcription factors, including the cyclic AMP response element binding protein (CREB); activating transcription factor 1 (ATF-1) (3, 52, 57, 60, 61); CREB binding protein and its homolog, p300 (31); serum response factor (SRF) (15); and components of the NF-κB/I-κB signaling pathway (10, 21, 25–29, 32, 37, 50, 53, 54) or the proteasome components (45).
Here, we report several novel forms of Tax that exclusively activate NF-κB at a high level. These mutants were derived based on trypsin-sensitive sites in Tax. These tryptic sites appear to approximate the borders of the various domains of Tax that are involved in CREB binding and nuclear transport, subunit dimerization and NF-κB activation, and HTLV-1 trans activation. Removal of the NH2-terminal 80 and 108 amino acid residues of Tax produced two mutants, TaxΔN81 and TaxΔN109, respectively. Due to a loss of the CREB-binding and nuclear transport domains, these mutants were unable to activate transcription via CREB or SRF and became localized to the cytoplasm predominantly. However, they continued to activate NF-κB. When fused with the nuclear localization signal (NLS) of the simian virus 40 (SV40) large T antigen, both mutants became transported to the nucleus but were unable to trans activate via CREB or SRF. While NLS-TaxΔN81 was able to activate NF-κB, NLS-TaxΔN109 was not able to. In contrast to wild-type Tax, which induces apoptosis and is highly cytotoxic (13, 58), TaxΔN81 and TaxΔN109 mutants exhibited little or no cytotoxicity. HeLa cells stably transfected with a TaxΔN109-expressing vector showed constitutive I-κBα degradation and NF-κB activation at a low level of TaxΔN109 expression. These results are consistent with the proposal that Tax directly alters cellular signaling pathways in the cytoplasm to affect NF-κB activation (10, 28). Interestingly, Tax species with molecular masses similar to that of TaxΔN109 (28 kDa) can be detected in many HTLV-1-transformed cell lines.
MATERIALS AND METHODS
Expression vectors and reporter plasmids.
A murine leukemia virus (MuLV)-based retroviral vector, pBabe-puro (38), was used as the backbone to express wild-type Tax and Tax mutants. The U3 region of the MuLV 5′ long terminal repeat (LTR) in pBabe-puro was replaced with the cytomegalovirus (CMV) enhancer-promoter. The puromycin resistance gene was removed from all Tax expression plasmids used in this study, as shown in Fig. 2. Coding sequences for the Tax truncation mutants were derived by PCR with the Vent polymerase (Biolabs) and confirmed by DNA sequence analyses. The upstream primers for TaxΔN109, TaxΔN81, NLS-TaxΔN109, and NLS-TaxΔN81 were 5′-CCATGGGCAAATACTCC-3′, 5′-CCATGGGAACCTCTAAGACC-3′, 5′-CGCGGATCCATGCCCAAAAAGAAACGGAAGGGCAAATACTCCCCCTTCCGA-3′, and 5′-CGCGGATCCATGCCCAAAAAGAAACGGAAGGGAACCTCTAAGACCCTCAAG-3′, respectively. A common downstream primer, 5′-GCCGTCGACTCAGACTTCTGTTTCTCGG-3′, was used for all constructs. The reporter constructs used for chloramphenicol acetyltransferase (CAT) assays, 218 CAT (HTLV-1 LTR) (17), c-fos-CAT, and 204K17 (an HIV LTR with mutated SP1 sites) (kindly provided by K. T. Jeang), have been previously described (8).
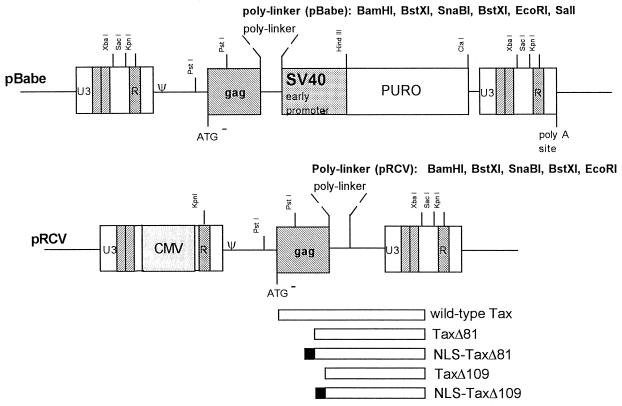
FIG. 2.
Construction of Tax expression vectors. The MuLV-based retroviral vector pBabe (38) was used as the backbone to generate a series of expression plasmids for Tax and its truncation mutants. Several modifications of the pBabe vector were made. First, the SV40 promoter-puromycin resistance gene cassette contained within a HindIII-ClaI fragment was deleted. Second, the CMV enhancer was used to replace the U3 region residing between the SacI and XbaI restriction sites in the 5′ LTR. Finally, the coding sequence of the wild-type Tax gene was cloned at the EcoRI site, and those of the respective truncation mutants were inserted between the BamHI and EcoRI sites.
Cell culture conditions and derivation of TaxΔN109 cell lines.
HeLa, HeLa-TaxΔN109, and CV1 cells were routinely cultured in Dulbecco’s minimum essential medium (DMEM) supplemented with 5% heat-inactivated fetal calf serum (FCS), 100 U of penicillin/ml, and 100 μg of streptomycin/ml. HTLV-1-transformed cells were maintained in RPMI 1640 supplemented with 10% FCS and antibiotics at the same concentrations as listed above. To derive Tax- or TaxΔN109-expressing cell lines, HeLa cells were cotransfected by electroporation with a mixture of 30 μg of DNA containing the wild-type Tax (29 μg) or the TaxΔN109 (29 μg) expression plasmid, each with 1 μg of a plasmid that carries the puromycin resistance gene under the control of the SV40 promoter. Two and a half million HeLa cells were suspended in 300 μl of serum-free DMEM and mixed with the plasmid DNA solution in 30 μl of 10 mM Tris (pH 7.0)–0.1 M NaCl. The cells were then electroporated with a BTX Electro cell manipulator at 250 V, 800 μF, and 13 Ω. After selection in DMEM containing 5% FCS and 2 μg of puromycin/ml for 2 weeks, colonies were picked and grown in the absence of puromycin. No colonies that expressed Tax were observed following transfection of HeLa cells with the wild-type Tax plasmid. In contrast, cell lines were readily obtained with the TaxΔN109 DNA.
Partial proteolysis of HTLV-1 Tax.
Purification of Tax from an Escherichia coli expression system has been previously reported (61). For partial trypsin digestion, approximately 10 μg of purified Tax in 50 μl of a buffer containing 20 mM HEPES (pH 7.9), 150 mM KCl, 0.2 mM EDTA, 0.5 mM dithiothreitol (DTT), and 20% glycerol was mixed with 50 μl of the same buffer containing 0.1 U of trypsin and 2.5 mM CaCl2 and incubated at 37°C for 2 min. The reaction was quenched by adding an equal volume of 2× sodium dodecyl sulfate (SDS) gel loading buffer (80 mM Tris [pH 6.8], 2% SDS, 15% glycerol, 100 mM DTT) followed by heating at 100°C for 5 min. Tryptic fragments were then resolved by SDS–12% polyacrylamide gel electrophoresis (PAGE), transferred to Immobilon, and visualized by Coomassie brilliant blue staining or by immunoblotting with Tax-C antibody (directed against the carboxyl-terminal 33 residues of Tax). Immobilon membrane slices containing the tryptic fragments were then directly used for amino acid sequence determination as described previously (36).
Immunofluorescence studies.
HeLa cells were transfected by using Lipofectamine (Gibco-BRL) according to the manufacturer’s instructions. At 24 h posttransfection, cells were seeded on eight-well chamber slides (Nunc) and incubated for an additional 24 h at 37°C. The monolayer was then washed twice with warm phosphate-buffered saline solution (PBS) and fixed with methanol overnight at −20°C. After three washes with cold PBS, the cells were incubated overnight at 4°C with the Tax-C antibody that had been diluted 1/5,000 in Tween buffer (0.5 M NaCl, 5% milk, 5 mM sodium phosphate [pH 6.5], 0.5% Tween 20) and preabsorbed overnight at 4°C on HeLa cells transfected with the RCV vector. The cells were then washed four times with cold PBS containing 0.3% Triton X-100 and incubated with the secondary fluorescein isothiocyanate-conjugated antibody (1/100 in PBS containing 0.05% Tween) for 2 h at 4°C. The secondary antibody was then removed by four 5-min washes with cold PBS, two 5-min washes with cold PBS containing 0.3% Triton X-100, and one final 10-min wash with cold PBS containing 0.05% Tween. The slides were mounted with SlowFade (Molecular Probes), kept at 4°C in the dark, and examined 24 h later under a fluorescent microscope.
Immunoblot analyses.
Cytoplasmic and nuclear extracts were prepared from 107 cells by a procedure previously reported (39). Protein concentrations were determined by the Bradford assay and confirmed by Coomassie blue staining. Fifty micrograms of protein extract was loaded for Western blotting. Immunoblotting was carried out with various specific primary antibodies, and blots were incubated with a secondary, horseradish peroxidase-conjugated antibody and developed with the chemiluminescence supersignal detection system from Pierce. All antibodies except Tax-C antibody were purchased from Santa Cruz Biotechnologies, Inc.
DNA transfection and CAT assays.
Calcium phosphate-mediated DNA transfection of HeLa cells and CAT assays were carried out as described before (17). The dose-dependent NF-κB reporter assays were carried out with CV1 cells, which exhibit low basal reporter activity. CV1 cells were transfected by using Lipofectamine according to the manufacturer’s instructions. The amounts of plasmids used in transfection are indicated in the legend to Fig. 4. For each set of CAT assays, extracts containing equal amounts of proteins (100 μg) as determined by the Bradford method were used. At least three independent transfections were performed for each data set to ensure reproducibility.
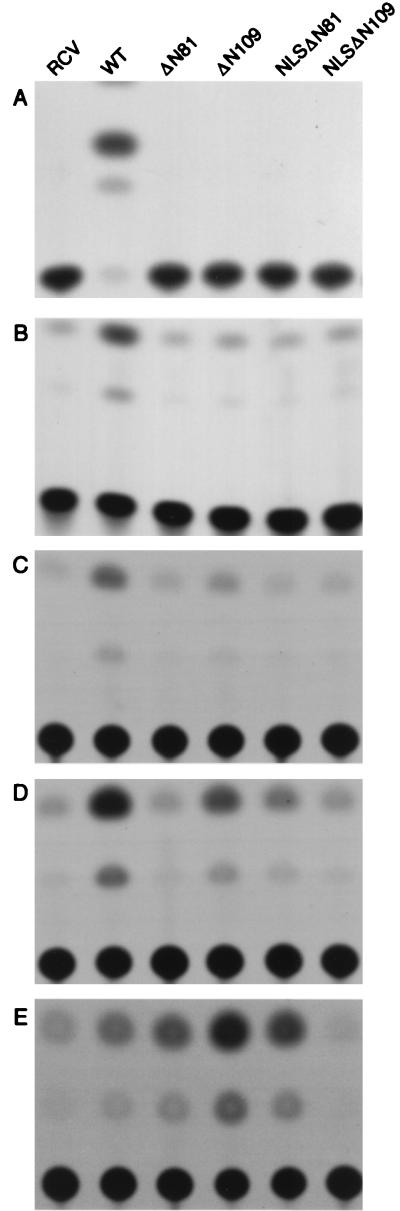
FIG. 4.
Trans activation phenotypes of Tax and Tax mutants. (A and B) HeLa cells were transiently transfected with RCV control (4 μg) (RCV), Tax (4 μg) (wild type [WT]), and Tax mutants (4 μg) (ΔN81, ΔN109, NLSΔN81, and NLSΔN109) and either the HTLV-1 LTR-CAT (2 μg) (A) or c-fos-CAT (4 μg) (B) reporter construct by the calcium phosphate method. (C, D, and E) An NF-κB reporter construct, 204K17 (0.25 μg), together with 0.25 (C), 0.5 (D), or 1 (E) μg of each Tax construct was transfected into CV1 cells by using Lipofectamine. Protein concentrations of the cell extracts were determined by Bradford assay. CAT assays were performed on extracts containing 100 μg of protein. The results shown are representative of three independent transfections.
RESULTS
Mapping protease-sensitive sites in Tax.
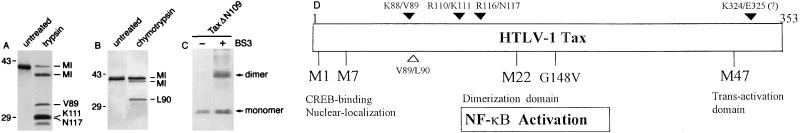
In an effort to derive stable protein modules of Tax to study its various biological activities, purified Tax was subjected to partial proteolysis by trypsin (Fig. 1). Immunoblot analyses of the tryptic fragments with a rabbit antibody directed against the carboxyl-terminal 33 amino acid residues of Tax (Tax-C antibody [23]) revealed multiple protein species of approximately 38, 31, 28 (a doublet), and 27 (a minor species) kDa (Fig. 1A). Amino acid sequence analysis of these fragments revealed trypsin cleavage sites at residues K88 and V89 for the 31-kDa fragment and at residues R110 and K111 as well as R116 and N117 for the 29-kDa doublet. The NH2-terminal sequence of the 38-kDa protein is identical to that of full-length Tax, suggesting that for this protein species trypsin cleavage occurred at the COOH terminus. Given the primary sequence of Tax, we think that the 38-kDa protein originated from a tryptic digestion of the peptide bond between K324 and E325 and most likely retained an epitope (FNEK; residues 321 to 324) that reacted with the polyclonal Tax-C antibody. Partial proteolysis of Tax by chymotrypsin also revealed residues V89 and L90 to be susceptible to cleavage (Fig. 1B) and confirmed that residues 88 to 90 are exposed and highly sensitive to proteolysis. These results are in general agreement with results of previous epitope mapping showing that residues 106 to 125, 316 to 335, 331 to 350, and 336 to 353 of Tax are reactive to patient sera (33).
FIG. 1.
Partial proteolysis of Tax. (A) For each proteolyzed fragment, the first amino acid residue and its position in the p40 Tax sequence are indicated. “MI” denotes an intact amino terminus. Tryptic cleavages occur at Lys-88 and Val-89, at Arg-110 and Lys-111, at Arg-116 and Asn-117, and possibly at Lys-324 and Glu-325. (B) Chymotrypsin cleaves between residues Val-89 and Leu-90 (L90) and between undetermined residues at the COOH terminus. Both panels A and B are immunoblots probed with Tax-C antibody, an antibody generated against a peptide corresponding to the COOH-terminal 33 residues of Tax. Removal of a short peptide from the COOH terminus of Tax by either of the proteases did not affect reactivity to Tax-C antibody (see the band marked “MI” which migrated slightly faster than the full-length protein in the lanes marked “trypsin” [A] and “chymotrypsin” [B]). The Tax-C antibody in effect served as an end label for probing the proteolyzed fragments. The bands corresponding to the tryptic or chymotryptic fragments were transferred to Immobilon, visualized by Coomassie blue staining, and sequenced. (C) TaxΔN109 forms a dimer. The coding sequence of TaxΔN109 (Tax amino acid residues 109 to 353) was generated by PCR with appropriate primers and inserted into the pET-11d vector for phage T7 promoter-driven expression. E. coli-derived TaxΔN109 was purified by Ni2+-nitrilotriacetic acid-Sepharose column chromatography by virtue of a COOH-terminal hexahistidine extension (marked with a minus sign). Treatment of TaxΔN109 with a chemical cross-linker, bis-sulfosuccinimidyl suberate (BS3), produced a protein species of approximately 60 kDa corresponding to a cross-linked dimer (marked with a plus sign). TaxΔN109 was also eluted as a 60-kDa protein in a Pharmacia Superose 12 H/R molecular sieve column (protein not shown). Immunoblots of the purified and cross-linked TaxΔN109 are shown. (D) Schematic summary of the domain organization of Tax. Arrowheads above and below the construct denote trypsin and chymotrypsin cleavage sites, respectively. M1 (H3S) and M7 (C29A-P30S) mutations abolish CREB binding, and M22 (T130A-L131S) and G148V mutations abrogate NF-κB activation (1, 48, 59). The M22 mutation also weakens Tax dimerization (56). The M47 (L319R and L320S) mutation abolishes HTLV-1 LTR trans activation but does not significantly affect CREB binding or Tax dimerization (1, 57).
Construction of Tax mutants.
Based on the trypsin cleavage sites described above, two Tax mutants with deletions of NH2-terminal amino acid residues 1 to 80 (TaxΔN81) and 1 to 108 (TaxΔN109) were generated by PCR amplification with the Vent polymerase, and the DNA sequences were confirmed by automated sequencing. Both TaxΔN81 and TaxΔN109 were stable when expressed in E. coli and could be readily purified as soluble proteins. Mutations with deletions beyond the R116 and N117 tryptic cleavage sites (TaxΔN120 and TaxΔN154, respectively) resulted in proteins that became rapidly degraded upon expression in E. coli (data not shown). TaxΔN109 remained dimeric, as evidenced by the formation of a 60-kDa protein species after treatment of the purified protein with the chemical cross-linker bis-sulfosuccinimidyl suberate (Fig. 1C). As detailed below, the protease cleavage sites appear to fall within or at the boundaries of distinct Tax domains that are involved in various protein-protein interactions. These domains of Tax are tentatively assigned as illustrated in Fig. 1D. The NLS and the CREB-binding domain of Tax have been localized to its NH2-terminal region previously (49). As TaxΔN81 and TaxΔN109 mutants lack this region, two additional mutants were made by fusing the NLS sequence (Pro-Lys-Lys-Lys-Arg-Lys-Val) of the SV40 large T antigen (34) to the NH2 termini of TaxΔN81 (NLS-TaxΔN81) and TaxΔN109 (NLS-TaxΔN109). These mutants were expressed from a chimeric CMV-MuLV promoter construct in which the enhancer in the MuLV LTR was replaced with the CMV immediate-early enhancer (Fig. 2).
Tax mutants exhibit distinct trans activation phenotypes.
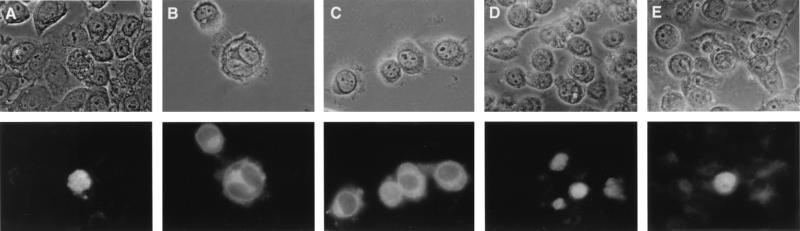
Indirect immunofluorescence studies of HeLa cells transiently transfected with the various Tax expression constructs indicated that, unlike wild-type Tax, which localized principally in the nucleus (Fig. 3), TaxΔN81 and TaxΔN109 were located predominantly in the cytoplasm (Fig. 3). Incorporation of the NLS of SV40 T antigen redirected NLS-TaxΔN81 and NLS-TaxΔN109 to the nucleus (Fig. 3). To determine the effects of the TaxΔN mutants on CREB/ATF-1-, SRF-, and NF-κB-mediated trans activation, HeLa or CV1 cells were transiently cotransfected with each of the Tax expression constructs and CAT reporter plasmids driven by the HTLV-1 LTR, the c-fos promoter, or the HIV LTR construct 204K17 (Fig. 4). In contrast to wild-type Tax, none of the truncated mutants were able to activate CREB/ATF-1(HTLV-1 LTR-CAT) or SRF (c-fos-CAT)-responsive reporters, irrespective of their cellular locations (Fig. 4A and B). This is consistent with previous reports that the NH2-terminal region of Tax is required for CREB and possibly SRF binding (1). Dose-response analyses of the various Tax constructs showed that for the activation of the NF-κB reporter, wild-type Tax is more active than the three mutants (TaxΔN81, TaxΔN109, and NLS-TaxΔN81) at low concentrations (Fig. 4C and D), but it becomes less active when a larger DNA amount is used (Fig. 4E). We think that at higher concentrations wild-type Tax is cytotoxic and that this cytotoxicity indirectly reduces the levels of NF-κB activation. The lower activities of TaxΔ81, TaxΔ109, and NLS-TaxΔ81 than of wild-type Tax at low concentrations may be due to their levels of expression and/or their stability in the cells. In any event, these results indicate that the ability to trans activate the NF-κB reporter remains with TaxΔ81 and TaxΔ109, supporting the notion that amino acid residues 1 to 108 of Tax are dispensable for NF-κB activation (Fig. 4C). The activation of NF-κB appears to be a cytoplasmic function of Tax, since both TaxΔ81 and TaxΔ109 are localized to the cytoplasm primarily and induce significant NF-κB activities. Somewhat unexpectedly, fusion of the SV40 T antigen NLS to the TaxΔ81 did not significantly alter its NF-κB-activating function. In this respect, NLS-TaxΔN81 may be similar to wild-type Tax, which activates NF-κB potently and yet is predominantly nuclear in location. We think that the reason that both NLS-TaxΔ81 and wild-type Tax activate NF-κB is because even though both proteins reside in the nucleus primarily, a fraction of either form makes its way to or remains in the cytoplasm to effect NF-κB activation. NH2-terminal NLS fusion abolished the NF-κB-activating function of TaxΔN109 (Fig. 4C to E, lanes NLS-TaxΔN109). We think that this is not due to the nuclear targeting but rather to the site of NLS fusion being too close to the domain important for NF-κB activation and thus posing a block and/or altering the conformation of this region, rendering it inactive.
FIG. 3.
Subcellular localization of Tax mutants. HeLa cells were transiently transfected with 1 μg of each Tax-expressing vector by using Lipofectamine. Subcellular localization of the respective Tax proteins (wild-type Tax [A], ΔN81 [B], NLS-ΔN81 [C], ΔN109 [D], and NLS-ΔN109 [E]) was analyzed 48 h later by indirect immunofluorescence with a rabbit serum antibody directed against the carboxyl-terminal region of Tax (Tax-C antibody [23]) and a fluorescein isothiocyanate-conjugated secondary antibody.
Stable expression of TaxΔN109 in HeLa cells.
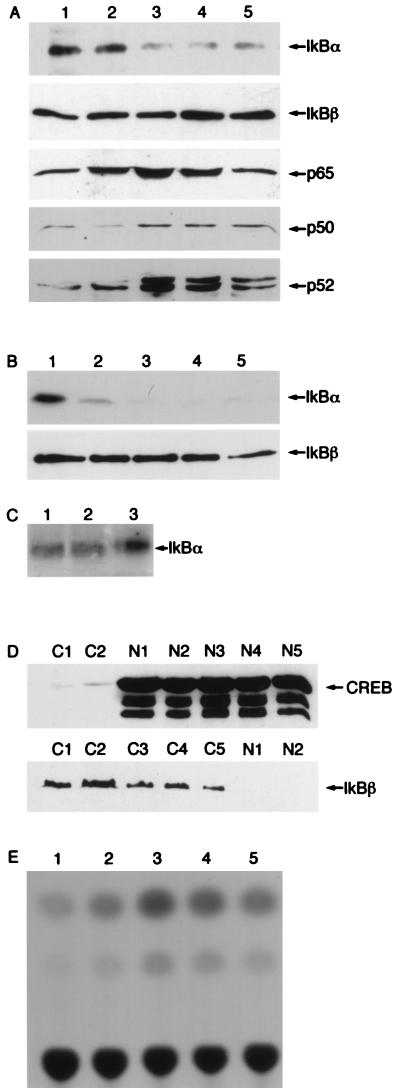
One striking feature of the HTLV-1-transformed cell lines is the complete absence of both I-κBα and I-κBβ from these cells, coincident with the constitutive nuclear presence of NF-κB. This property has recently been demonstrated to be due to the ability of Tax to target I-κB for phosphorylation and ubiquitin- and proteasome-mediated degradation (10, 21, 28, 37, 50). While HTLV-1-transformed T-cell lines, such as HUT102, MT2, and C91/PL, express high levels of Tax protein, stable cell lines constitutively expressing wild-type Tax have been very difficult to establish, probably because of Tax-induced apoptosis (13, 58). As TaxΔN109 activated NF-κB and lacked most other biochemical and biological activities of wild-type Tax, we hypothesized that it might be without the apoptotic and cytotoxic effects of Tax and thus might be used to target cellular I-κB destruction. To this end, HeLa cells were electroporated in duplicate with wild-type Tax or with TaxΔN109 expression plasmids (pBabe-CMV-Tax and pBabe-CMV-TaxΔN109) together with a plasmid (pBabe-CMV-puro) containing the puromycin resistance gene at a molar ratio of 29:1. While pBabe-CMV-puro alone or the combination of pBabe-CMV-puro and pBabe-CMV-TaxΔN109 yielded many transfectants when introduced into HeLa cells, multiple cotransfections of HeLa cells with pBabe-CMV-puro and pBabe-CMV-Tax followed by puromycin selection yielded no transfectants expressing full-length Tax. This result is consistent with the notion that the wild-type Tax protein is highly cytotoxic compared to TaxΔN109. HeLa cells stably transfected with TaxΔN109 DNA (HeLa-109 cells) were cloned, and then populations of the clones were expanded in culture and analyzed. As shown in Fig. 5A (lanes 2 to 5), in agreement with previous reports that Tax triggers I-κB phosphorylation and degradation, a significant reduction in the levels of I-κBα, but not I-κBβ, could be seen in HeLa-109 clones. The clonal variation in I-κBα levels is likely due to the levels of TaxΔN109 expression, as shown by the different levels in transcriptional activation after transient transfection (Fig. 5E). The reduction in I-κB levels was accompanied by a significant increase in the nuclear presence of p65-RelA, p50-NF-κB1, and p52-NF-κB2 (Fig. 5A, lanes 2 to 5). To ensure that no cross-contamination between the cytoplasmic and nuclear extracts occurred during fractionation of the cells, nuclear fractions were probed with an I-κBβ antibody. As shown in Fig. 5D, cytoplasmic I-κBβ was not detectable in the nuclear extracts. Likewise, an antibody against CREB, an exclusively nuclear protein, detected CREB only in the nuclear fractions. Because de novo I-κBα synthesis is induced at a high level following NF-κB activation, we treated HeLa-109 clones with the protein synthesis inhibitor cycloheximide to accentuate the effects Tax exerts over I-κBα turnover (Fig. 5B, lanes 2 to 5). Indeed, cycloheximide-treated HeLa-109 cells had barely detectable or undetectable levels of I-κBα. In contrast, the control HeLa cells that had been similarly treated retained significant levels of I-κBα (Fig. 5B, lane 1). A serine protease inhibitor, N-tosyl-l-phenylalanine chloromethyl ketone (TPCK), has been shown to block I-κBα degradation by inhibiting the phosphorylation required for the ubiquitin- and proteasome-mediated degradation pathway (22). Indeed, TPCK treatment restored I-κBα levels in HeLa-109 clones (Fig. 5A and B, lanes 4 and 5) to that seen in the control HeLa cells (Fig. 5C, lane 1). This is consistent with the notion that TPCK acts at or downstream of the step of I-κB metabolism that is affected by Tax, most likely the phosphorylation of I-κB (Fig. 5C). To demonstrate constitutive NF-κB activation in HeLa-109 cells, HeLa-109 clones were transiently transfected with HIV LTR-CAT as a reporter construct (Fig. 5E). Consistent with nuclear expression of p50, p52, and p65 (Fig. 5A), significant induction of the reporter was observed in HeLa-109 cells (Fig. 5E, lanes 2 to 5) compared to the control HeLa cells (lane 1). The levels of reporter activity also correlated with the degrees of I-κBα degradation (Fig. 5A, B, D, and E). These results demonstrate that TaxΔN109 induces NF-κB activation and is devoid of the cytotoxicity of wild-type Tax.
FIG. 5.
Constitutive NF-κB activation in HeLa cells stably transfected with TaxΔN109. (A) Immunoblot analyses were carried out with 50 μg of proteins from HeLa cells (lane 1) or HeLa-109 clones (lanes 2 to 5). Immunoblotting for I-κBα and I-κBβ was performed with cytoplasmic extracts, and that for p65, p50, and p52 was performed with nuclear extracts. (B) HeLa and HeLa-109 cells were treated with 50 μg of cycloheximide/ml for 3 h prior to immunoblotting for I-κBα and I-κBβ. (C) Following cycloheximide treatment, TPCK (50 μM final concentration) was added to the cells for 30 min. Cell lysates were then analyzed by immunoblotting for I-κBα. Only HeLa-109 clones (corresponding to lanes 4 and 5 in panel A) were used. (D) Cytoplasmic (C) and nuclear (N) fractions were subjected to Western blot analysis with CREB and I-κBβ antibodies as nuclear and cytoplasmic markers, respectively. Immunoblots of cytoplasmic and nuclear extracts from HeLa cells (C1 and N1) and from HeLa-109 clones (C2 through 5 and N2 through 5) are shown. (E) Five micrograms of the HIV LTR-CAT reporter construct was transfected into control HeLa cells (lane 1) or HeLa-109 clones (the same as those used for panels A and B) by the calcium phosphate method, and the extent of NF-κB activation was determined by CAT assays. Results shown are representative of three independent transfections. The levels of activation are 1-, 1.5-, 4-, 3.5-, and 3-fold, respectively, for lanes 1 to 5.
HTLV-1-transformed cell lines express a TaxΔN109-like protein.
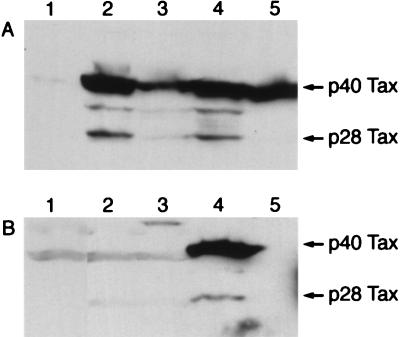
To determine if TaxΔN109-like protein might be expressed in HTLV-1-transformed cell lines, immunoblot analyses of cells from four such lines, HUT102, MT4, MT2, and C91/PL, were carried out with the Tax-C antibody. Freshly grown cells were harvested and boiled in sample buffer for SDS-PAGE containing SDS and DTT. Indeed, in addition to the 40-kDa full-length Tax protein, HUT102, MT4, and MT2 cells each expressed a 28-kDa (p28) and a 35-kDa (p35) protein species that reacted with Tax-C antibody. A leukemic T-cell line, Jurkat, which is unrelated to HTLV-1, did not express any Tax-C antibody-reactive protein species (Fig. 6A, lane 1). C91/PL cells appeared to produce only the 40-kDa Tax species, with little or no expression of the 28-kDa Tax species (Fig. 6A, lane 5). Intriguingly, despite significant reductions in the levels of I-κBα in HeLa-109 cells (Fig. 5A and B), the amounts of TaxΔN109 in these cells, while detectable, were low (Fig. 6B, lanes 2 and 3). We estimated them to be no more than a few percent of that seen in HUT102 or MT2 cells.
FIG. 6.
Detection of a TaxΔN109-like protein in HTLV-1-transformed T cells. (A) Whole-cell extracts from 107 cells of each of four HTLV-1-transformed cell lines, HUT102, MT4, MT2, and C91/PL (lanes 2 to 5, respectively), and a control T-cell line, Jurkat (lane 1), were probed by immunoblotting with Tax-C antibody. (B) Immunoblots of cytoplasmic extracts from 107 control HeLa cells (lane 1) and HeLa-109 cells (lanes 2 and 3; the same clones as those shown in lanes 3 and 4, Fig. 5E). Whole-cell extracts from HUT102 cells were included for comparison (lane 4).
DISCUSSION
In this report, we show that the protein species TaxΔN81 and TaxΔN109, containing amino acid residues 81 to 353 and 109 to 353 of Tax, respectively, activate I-κB degradation, NF-κB nuclear translocation, and trans activation via the NF-κB enhancer. Lacking the domains for nuclear transport and CREB binding, both Tax mutants localized to the cytoplasm predominantly and did not trans activate via the CRE containing HTLV-1 21-bp repeats or the serum response element containing the c-fos promoter. In-depth analyses were performed on TaxΔN109 because it contains less of the Tax amino acid sequence and appears to trans activate NF-κB better than TaxΔN81. In contrast to wild-type Tax, TaxΔN109 can be expressed in HeLa cells, where it potently induces I-κBα degradation and NF-κB nuclear translocation. The constitutively reduced levels of I-κBα in TaxΔN109-expressing cell lines occurred despite the surge of NF-κB-induced de novo I-κBα synthesis, which apparently failed to compensate for the degradation triggered by TaxΔN109. Although I-κBβ levels remained the same in the TaxΔN109-expressing cells, this did not affect the constitutive nuclear presence and activation of NF-κB in these cells. It should be noted that I-κB degradation with NF-κB activation by both TaxΔN81 and TaxΔN109 has also been observed in Jurkat T cells in transient-transfection assays (data not shown). The 28- and the 35-kDa Tax species were detected in many HTLV-1-transformed cell lines. Because p28 and p35 reacted specifically with the antibody raised against the COOH-terminal end of Tax, we think that they lack the NH2-terminal region. It is possible that p28 resembles TaxΔN109 structurally and functionally and therefore might function as an inducer of I-κB degradation and NF-κB activation, like TaxΔN109. While we cannot rule out the possibility that these Tax species derived from proteolytic degradation of full-length Tax during extract preparation, we think that the proteolysis most likely occurred intracellularly, because the cell extracts used in this analysis were prepared by lysis of freshly grown cells in SDS-PAGE sample buffer with minimal manipulation. Several studies have indicated that NF-κB activation is required for cellular transformation mediated by Tax. Thus, it is possible that p28 and/or p35 might play a role in HTLV-1 pathogenesis. The fact that cytoplasmic variants of Tax are trans activators of NF-κB supports the notion that Tax activates NF-κB by stimulating I-κB phosphorylation and degradation (10, 28) which occur in the cytoplasm. These results are also consistent with earlier reports showing that a fraction of wild-type Tax is localized in the cytoplasmic compartments of HTLV-1-infected cells (20). In agreement with the idea that Tax stimulates I-κB phosphorylation, chemical agents, such as TPCK, which had been shown to inhibit I-κBα phosphorylation, effectively blocked TaxΔN109-induced I-κBα degradation.
Transient transfection of CV1 cells with the mutants indicated that TaxΔN109 and NLS-TaxΔN81 activate NF-κB in a dose-dependent manner, although TaxΔN81 required a larger amount of DNA to show a comparable level of activation. This may be due to the instability of TaxΔN81 in the cells. While the mechanism by which Tax accelerates I-κBα phosphorylation and degradation remains to be elucidated, because only a small quantity of Tax is sufficient to produce a significant effect on I-κBα turnover, it is possible that Tax directly targets cellular signaling pathways as suggested previously (10, 28). We think that the inability of NLS-TaxΔN109 to activate NF-κB is not caused by the nuclear targeting of the fusion protein. Rather, the NLS fusion is most likely positioned too close to the domain important for NF-κB activation and thus poses a block and/or alters the conformation of this region, rendering it inactive. In agreement with this conclusion, at least two Tax mutations, G148V (59) and T130A-L131S (48), that abolish NF-κB activating function, have been localized to this region.
Because the 109th codon of the Tax-coding sequence is AUG, it is possible that internal translational initiation at codon 109 might yield the same protein species as TaxΔN109. Further, since the Tax/Rex mRNA is derived from a double-splicing event where the AUG codon of env together with another G residue (in the second exon) become spliced in frame to the Tax-coding sequence, alternative mRNA splicing that bypasses the second exon would produce a pX mRNA species lacking the p40 Tax initiation codon. Such a singly spliced mRNA species, termed pX-p21rex mRNA, has been described (9, 41). For the pX-p21rex mRNA, again, translational initiation at the AUG codon for Met-109 might produce TaxΔN109. A plasmid containing the cDNA of the pX-p21rex mRNA placed under the control of the CMV enhancer and promoter, however, failed to trans activate the NF-κB reporter (data not shown). Therefore, we think that the submolecular Tax species in HTLV-1-infected or -transformed cells result most likely from proteolysis of full-length Tax. The exact amino acid sequences of these protein species and any role they might play in HTLV-1 pathogenesis remain to be demonstrated.
ACKNOWLEDGMENTS
We thank G. Franchini for the HTLV-1-transformed cell line C91/PL and B. Cullen and K. T. Jeang for Tax antibodies and the NF-κB reporter construct 204K17.
This work was supported by grants RO1 CA48709 and RO1 CA75638 from the National Institutes of Health.
REFERENCES
- 1.Adya N, Giam C-Z. Distinct regions in human T-cell lymphotropic virus type I Tax mediate interactions with activator protein CREB and basal transcription factors. J Virol. 1995;69:1834–1841. doi: 10.1128/jvi.69.3.1834-1841.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arenzana-Seisdedos F, Thompson J, Rodriguez M S, Bachelerie F, Thomas D, Hay R T. Inducible nuclear expression of newly synthesized IκBα negatively regulates DNA-binding and transcriptional activities of NF-κB. Mol Cell Biol. 1995;15:2689–2696. doi: 10.1128/mcb.15.5.2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armstrong A P, Franklin A A, Uittenbogaard M N, Giebler H A, Nyborg J K. Pleiotropic effect of the human T-cell leukemia virus Tax protein on the DNA binding activity of eukaryotic transcription factors. Proc Natl Acad Sci USA. 1993;90:7303–7307. doi: 10.1073/pnas.90.15.7303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baeuerle P A, Baltimore D. NF-kappa B: ten years after. Cell. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- 5.Baldwin A S., Jr The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 6.Beg A A, Baldwin A S. The I kappa B proteins: multifunctional regulators of Rel/NF-kappa B transcription factors. Genes Dev. 1993;7:2064–2070. doi: 10.1101/gad.7.11.2064. [DOI] [PubMed] [Google Scholar]
- 7.Beg A A, Sha W C, Bronson R T, Baltimore D. Constitutive NF-kappa B activation, enhanced granulopoiesis, and neonatal lethality in I kappa B alpha-deficient mice. Genes Dev. 1995;9:2736–2746. doi: 10.1101/gad.9.22.2736. [DOI] [PubMed] [Google Scholar]
- 8.Berkhout B, Jeang K-T. Functional roles for the TATA promoter and enhancers in basal and Tat-induced expression of the human immunodeficiency virus type 1 long terminal repeat. J Virol. 1992;66:139–149. doi: 10.1128/jvi.66.1.139-149.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berneman Z N, Gartenhaus R B, Reitz M S J, Blattner W A, Manns A, Hanchard B, Ikehara O, Gallo R C, Klotman M E. Expression of alternatively spliced human T-lymphotropic virus type I pX mRNA in infected cell lines and in primary uncultured cells from patients with adult T-cell leukemia/lymphoma and healthy carriers. Proc Natl Acad Sci USA. 1992;89:3005–3009. doi: 10.1073/pnas.89.7.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brockman J A, Scherer D C, McKinsey T A, Hall S M, Qi X, Lee W Y, Ballard D W. Coupling of a signal response domain in IκBα to multiple pathways for NF-κB activation. Mol Cell Biol. 1995;15:2809–2818. doi: 10.1128/mcb.15.5.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown K, Gerstberger S, Carlson L, Franzoso G, Siebenlist U. Control of I kappa B-alpha proteolysis by site-specific, signal-induced phosphorylation. Science. 1995;267:1485–1488. doi: 10.1126/science.7878466. [DOI] [PubMed] [Google Scholar]
- 12.Chen Z, Hagler J, Palombella V J, Melandri F, Scherer D, Ballard D, Maniatis T. Signal-induced site-specific phosphorylation targets I kappa B alpha to the ubiquitin-proteasome pathway. Genes Dev. 1995;9:1586–1597. doi: 10.1101/gad.9.13.1586. [DOI] [PubMed] [Google Scholar]
- 13.Chlichlia K, Moldenhauer G, Daniel P T, Busslinger M, Gazzolo L, Schirrmacher V, Khazaie K. Immediate effects of reversible HTLV-1 tax function: T-cell activation and apoptosis. Oncogene. 1995;10:269–277. [PubMed] [Google Scholar]
- 14.Finco T S, Baldwin A S. Mechanistic aspects of NF-kappa B regulation: the emerging role of phosphorylation and proteolysis. Immunity. 1995;3:263–272. doi: 10.1016/1074-7613(95)90112-4. [DOI] [PubMed] [Google Scholar]
- 15.Fujii M, Tsuchiya H, Chuhjo T, Akizawa T, Seiki M. Interaction of HTLV-1 Tax with p67SRF causes the aberrant induction of cellular immediate early genes through CArG boxes. Genes Dev. 1992;6:2066–2076. doi: 10.1101/gad.6.11.2066. [DOI] [PubMed] [Google Scholar]
- 16.Gessain A, Barin F, Vernant J C, Gout O, Maurs L, Calender A, de The G. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet. 1985;ii:407–410. doi: 10.1016/s0140-6736(85)92734-5. [DOI] [PubMed] [Google Scholar]
- 17.Giam C Z, Xu Y L. HTLV-I tax gene product activates transcription via pre-existing cellular factors and cAMP responsive element. J Biol Chem. 1989;264:15236–15241. [PubMed] [Google Scholar]
- 18.Gilmore T D. Role of rel family genes in normal and malignant lymphoid cell growth. Cancer Surv. 1992;15:69–87. [PubMed] [Google Scholar]
- 19.Gilmore T D. Regulation of Rel transcription complexes. In: Goodburn S, editor. Frontiers in molecular biology: eukaryotic gene transcription. Oxford, England: Oxford University Press; 1995. pp. 104–133. [Google Scholar]
- 20.Goh W C, Sodroski J, Rosen C, Essex M, Haseltine W A. Subcellular localization of the product of the long open reading frame of human T-cell leukemia virus type I. Science. 1985;227:1227–1228. doi: 10.1126/science.2983419. [DOI] [PubMed] [Google Scholar]
- 21.Good L, Sun S-C. Persistent activation of NF-κB/Rel by human T-cell leukemia virus type 1 Tax involves degradation of IκBβ. J Virol. 1996;70:2730–2735. doi: 10.1128/jvi.70.5.2730-2735.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guesdon F, Ikebe T, Stylianou E, Warwick-Davies J, Haskill S, Saklatvala J. Interleukin 1-induced phosphorylation of MAD3, the major inhibitor of nuclear factor kappa B of HeLa cells. Interference in signalling by the proteinase inhibitors 3,4-dichloroisocoumarin and tosylphenylalanyl chloromethylketone. Biochem J. 1995;307:287–295. doi: 10.1042/bj3070287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanly S M, Rimsky L T, Malim M H, Kim J H, Hauber J, Duc Dodon M, Le S Y, Maizel J V, Cullen B R, Greene W C. Comparative analysis of the HTLV-I Rex and HIV-1 Rev trans-regulatory proteins and their RNA response elements. Genes Dev. 1989;3:1534–1544. doi: 10.1101/gad.3.10.1534. [DOI] [PubMed] [Google Scholar]
- 24.Hinuma Y, Nagata K, Hanaoka M, Nakai M, Matsumoto T, Kinoshita K I, Shirakawa S, Miyoshi I. Adult T-cell leukemia: antigen in an ATL cell line and detection of antibodies to the antigen in human sera. Proc Natl Acad Sci USA. 1981;78:6476–6480. doi: 10.1073/pnas.78.10.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirai H, Fujisawa J, Suzuki T, Ueda K, Muramatsu M, Tsuboi A, Arai N, Yoshida M. Transcriptional activator Tax of HTLV-1 binds to the NF-kappa B precursor p105. Oncogene. 1992;7:1737–1742. [PubMed] [Google Scholar]
- 26.Hirai H, Suzuki T, Fujisawa J, Inoue J, Yoshida M. Tax protein of human T-cell leukemia virus type I binds to the ankyrin motifs of inhibitory factor kappa B and induces nuclear translocation of transcription factor NF-kappa B proteins for transcriptional activation. Proc Natl Acad Sci USA. 1994;91:3584–3588. doi: 10.1073/pnas.91.9.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kanno T, Brown K, Franzoso G, Siebenlist U. Kinetic analysis of human T-cell leukemia virus type I Tax-mediated activation of NF-κB. Mol Cell Biol. 1994;14:6443–6451. doi: 10.1128/mcb.14.10.6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kanno T, Brown K, Siebenlist U. Evidence in support of a role for human T-cell leukemia virus type I Tax in activating NF-kappa B via stimulation of signaling pathways. J Biol Chem. 1995;270:11745–11748. doi: 10.1074/jbc.270.20.11745. [DOI] [PubMed] [Google Scholar]
- 29.Kanno T, Franzoso G, Siebenlist U. Human T-cell leukemia virus type I Tax-protein-mediated activation of NF-kappa B from p100 (NF-kappa B2)-inhibited cytoplasmic reservoirs. Proc Natl Acad Sci USA. 1994;91:12634–12638. doi: 10.1073/pnas.91.26.12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klement J F, Rice N R, Car B D, Abbondanzo S J, Powers G D, Bhatt H, Chen C-H, Rosen C A, Stewart C L. IκBα deficiency results in a sustained NF-κB response and severe widespread dermatitis in mice. Mol Cell Biol. 1996;16:2341–2349. doi: 10.1128/mcb.16.5.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kwok R P, Laurance M E, Lundblad J R, Goldman P S, Shih H, Connor L M, Marriott S J, Goodman R H. Control of camp-regulated enhancers by the viral transactivator tax through creb and the co-activator cbp. Nature. 1996;380:642–646. doi: 10.1038/380642a0. [DOI] [PubMed] [Google Scholar]
- 32.Lacoste J, Petropoulos L, Pépin N, Hiscott J. Constitutive phosphorylation and turnover of IκBα in human T-cell leukemia virus type I-infected and Tax-expressing T cells. J Virol. 1995;69:564–569. doi: 10.1128/jvi.69.1.564-569.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lal R B, Giam C Z, Coligan J E, Rudolph D L. Differential immune responsiveness to the immunodominant epitopes of regulatory proteins (tax and rex) in human T cell lymphotropic virus type I-associated myelopathy. J Infect Dis. 1994;169:496–503. doi: 10.1093/infdis/169.3.496. [DOI] [PubMed] [Google Scholar]
- 34.Lanford R E, Kanda P, Kennedy R C. Induction of nuclear transport with a synthetic peptide homologous to the SV40 T antigen transport signal. Cell. 1986;46:575–582. doi: 10.1016/0092-8674(86)90883-4. [DOI] [PubMed] [Google Scholar]
- 35.Liou H C, Baltimore D. Regulation of the NF-kappa B/rel transcription factor and I kappa B inhibitor system. Curr Opin Cell Biol. 1993;5:477–487. doi: 10.1016/0955-0674(93)90014-h. [DOI] [PubMed] [Google Scholar]
- 36.Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987;262:10035–10038. [PubMed] [Google Scholar]
- 37.McKinsey T A, Brockman J A, Scherer D C, Al-Murrani S W, Green P L, Ballard D W. Inactivation of IκBβ by the Tax protein of human T-cell leukemia virus type 1: a potential mechanism for constitutive induction of NF-κB. Mol Cell Biol. 1996;16:2083–2090. doi: 10.1128/mcb.16.5.2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morgenstern J P, Land H. Advanced mammalian gene transfer: high titer retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 1990;18:3587–3596. doi: 10.1093/nar/18.12.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muller M M, Schreiber E, Schaffner W, Matthias P. Rapid test for in vivo stability and DNA binding of mutated octamer binding proteins with ‘mini-extracts’ prepared from transfected cells. Nucleic Acids Res. 1989;17:6420. doi: 10.1093/nar/17.15.6420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neri A, Chang C C, Lombardi L, Salina M, Corradini P, Maiolo A T, Chaganti R S, Dalla Favera R. B cell lymphoma-associated chromosomal translocation involves candidate oncogene lyt-10, homologous to NF-kappa B p50. Cell. 1991;67:1075–1087. doi: 10.1016/0092-8674(91)90285-7. [DOI] [PubMed] [Google Scholar]
- 41.Orita S, Kobayashi H, Aono Y, Saiga A, Maeda M, Igarashi H. p21X mRNA is expressed as a singly spliced pX transcript from defective provirus genomes having a partial deletion of the pol-env region in human T-cell leukemia virus type 1-infected cells. Nucleic Acids Res. 1993;21:3799–3807. doi: 10.1093/nar/21.16.3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Osame M, Matsumoto M, Usuku K, Izumo S, Ijichi N, Amitani H, Tara M, Igata A. Chronic progressive myelopathy associated with elevated antibodies to human T-lymphotropic virus type I and adult T-cell leukemialike cells. Ann Neurol. 1987;21:117–122. doi: 10.1002/ana.410210203. [DOI] [PubMed] [Google Scholar]
- 43.Petropoulos L, Lin R, Hiscott J. Human T-cell leukemia virus type 1 tax protein increases NF-kappa B dimer formation and antagonizes the inhibitory activity of the I kappa B alpha regulatory protein. Virology. 1996;225:52–64. doi: 10.1006/viro.1996.0574. [DOI] [PubMed] [Google Scholar]
- 44.Poiesz B J, Ruscetti F W, Gazdar A F, Bunn P A, Minna J D, Gallo R C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci USA. 1980;77:7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rousset R, Desbois C, Bantignies F, Jalinot P. Effects on NF-kappa B1/p105 processing of the interaction between the HTLV-1 transactivator tax and the proteasome. Nature. 1996;381:328–331. doi: 10.1038/381328a0. [DOI] [PubMed] [Google Scholar]
- 46.Seiki M, Eddy R, Shows T B, Yoshida M. Nonspecific integration of the HTLV provirus genome into adult T-cell leukaemia cells. Nature. 1984;309:640–642. doi: 10.1038/309640a0. [DOI] [PubMed] [Google Scholar]
- 47.Siebenlist U, Franzoso G, Brown K. Structure, regulation and function of NF-kappa B. Annu Rev Cel Biol. 1994;10:405–455. doi: 10.1146/annurev.cb.10.110194.002201. [DOI] [PubMed] [Google Scholar]
- 48.Smith M R, Greene W C. Identification of HTLV-I tax trans-activator mutants exhibiting novel transcriptional phenotypes. Genes Dev. 1990;4:1875–1885. doi: 10.1101/gad.4.11.1875. [DOI] [PubMed] [Google Scholar]
- 49.Smith M R, Greene W C. Characterization of a novel nuclear localization signal in the HTLV-I tax transactivator protein. Virology. 1992;187:316–320. doi: 10.1016/0042-6822(92)90320-o. [DOI] [PubMed] [Google Scholar]
- 50.Sun S-C, Elwood J, Béraud C, Greene W C. Human T-cell leukemia virus type I Tax activation of NF-κB/Rel involves phosphorylation and degradation of IκBα and RelA (p65)-mediated induction of the c-rel gene. Mol Cell Biol. 1994;14:7377–7384. doi: 10.1128/mcb.14.11.7377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sun S C, Ganchi P A, Ballard D W, Greene W C. NF-kappa B controls expression of inhibitor I kappa B alpha: evidence for an inducible autoregulatory pathway. Science. 1993;259:1912–1915. doi: 10.1126/science.8096091. [DOI] [PubMed] [Google Scholar]
- 52.Suzuki T, Fujisawa J I, Toita M, Yoshida M. The trans-activator tax of human T-cell leukemia virus type 1 (HTLV-1) interacts with cAMP-responsive element (CRE) binding and CRE modulator proteins that bind to the 21-base-pair enhancer of HTLV-1. Proc Natl Acad Sci USA. 1993;90:610–614. doi: 10.1073/pnas.90.2.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Suzuki T, Hirai H, Murakami T, Yoshida M. Tax protein of HTLV-1 destabilizes the complexes of NF-kappa B and I kappa B-alpha and induces nuclear translocation of NF-kappa B for transcriptional activation. Oncogene. 1995;10:1199–1207. [PubMed] [Google Scholar]
- 54.Suzuki T, Hirai H, Yoshida M. Tax protein of HTLV-1 interacts with the Rel homology domain of NF-kappa B p65 and c-Rel proteins bound to the NF-kappa B binding site and activates transcription. Oncogene. 1994;9:3099–3105. [PubMed] [Google Scholar]
- 55.Thompson J E, Phillips R J, Erdjument-Bromage H, Tempst P, Ghosh S. I kappa B beta regulates the persistent response in a biphasic activation of NF-kappa B. Cell. 1995;80:573–582. doi: 10.1016/0092-8674(95)90511-1. [DOI] [PubMed] [Google Scholar]
- 56.Tie F, Adya N, Greene W C, Giam C-Z. Interaction of the human T-lymphotropic virus type 1 Tax dimer with CREB and the viral 21-base-pair repeat. J Virol. 1996;70:8368–8374. doi: 10.1128/jvi.70.12.8368-8374.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wagner S, Green M R. HTLV-I Tax protein stimulation of DNA binding of bZIP proteins by enhancing dimerization. Science. 1993;262:395–399. doi: 10.1126/science.8211160. [DOI] [PubMed] [Google Scholar]
- 58.Yamada T, Yamaoka S, Goto T, Nakai M, Tsujimoto Y, Hatanaka M. The human T-cell leukemia virus type I Tax protein induces apoptosis which is blocked by the Bcl-2 protein. J Virol. 1994;68:3374–3379. doi: 10.1128/jvi.68.5.3374-3379.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yamaoka S, Inoue H, Sakurai M, Sugiyama T, Hazama M, Yamada T, Hatanaka M. Constitutive activation of NF-kappa B is essential for transformation of rat fibroblasts by the human T-cell leukemia virus type I Tax protein. EMBO J. 1996;15:873–887. [PMC free article] [PubMed] [Google Scholar]
- 60.Zhao L J, Giam C Z. Interaction of the human T-cell lymphotropic virus type I (HTLV-I) transcriptional activator Tax with cellular factors that bind specifically to the 21-base-pair repeats in the HTLV-I enhancer. Proc Natl Acad Sci USA. 1991;88:11445–11449. doi: 10.1073/pnas.88.24.11445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao L J, Giam C Z. Human T-cell lymphotropic virus type I (HTLV-I) transcriptional activator, Tax, enhances CREB binding to HTLV-I 21-base-pair repeats by protein-protein interaction. Proc Natl Acad Sci USA. 1992;89:7070–7074. doi: 10.1073/pnas.89.15.7070. [DOI] [PMC free article] [PubMed] [Google Scholar]