Abstract
High glucose (HG) culture conditions in vitro and persistent exposure to hyperglycemia in diabetes patients are detrimental to stem cells, analogous to any other cell type in our body. It interferes with diverse signaling pathways, i.e. mammalian target of rapamycin (mTOR)-phosphoinositide 3-kinase (PI3K)-Akt signaling, to impact physiological cellular functions, leading to low cell survival and higher cell apoptosis rates. While elucidating the underlying mechanism responsible for the apoptosis of adipose tissue-derived mesenchymal stem cells (MSCs), a recent study has shown that HG culture conditions dysregulate mTOR-PI3K-Akt signaling in addition to mitochondrial malfunctioning due to defective mitochondrial membrane potential (MtMP) that lowers ATP production. This organelle-level dysfunction energy-starves the cells and increases oxidative stress and ultrastructural abnormalities. Disruption of the mitochondrial electron transport chain produces an altered mitochondrial NAD+/NADH redox state as evidenced by a low NAD+/NADH ratio that primarily contributes to the reduced cell survival in HG. Some previous studies have also reported altered mitochondrial membrane polarity (causing hyperpolarization) and reduced mitochondrial cell mass, leading to perturbed mitochondrial homeostasis. The hostile microenvironment created by HG exposure creates structural and functional changes in the mitochondria, altering their bioenergetics and reducing their capacity to produce ATP. These are significant data, as MSCs are extensively studied for tissue regeneration and restoring their normal functioning in cell-based therapy. Therefore, MSCs from hyperglycemic donors should be cautiously used in clinical settings for cell-based therapy due to concerns of their poor survival rates and increased rates of post engraftment proliferation. As hyperglycemia alters the bioenergetics of donor MSCs, rectifying the loss of MtMP may be an excellent target for future research to restore the normal functioning of MSCs in hyperglycemic patients.
Keywords: Adipose tissue, Apoptosis, Bioenergetics, Cell survival, Cell therapy, Hyperglycemia, Mitochondria, Mesenchymal stem cells, Stem cells
Core Tip: High glucose (HG) conditions, seen in vitro as well as in diabetic patients, adversely affect stem cells by disrupting mammalian target of rapamycin-phosphoinositide 3-kinase-Akt signaling, resulting in reduced cell survival and increased apoptosis. A recent study of adipose tissue-derived mesenchymal stem cells (MSCs) found dysregulation of this signaling pathway and defective mitochondrial membrane potential (MtMP) under HG conditions. This leads to decreased ATP production, heightened oxidative stress, and structural abnormalities, causing diminished cell survival. Altered mitochondrial NAD+/NADH redox state and disrupted mitochondrial homeostasis worsen the hostile microenvironment induced by HG exposure. These findings are a note of caution for using MSCs from hyperglycemic donors in cell-based therapy owing to their poor survival and proliferation rates. Future research targeting MtMP restoration may enhance the therapeutic efficacy of MSCs in hyperglycemic patients.
INTRODUCTION
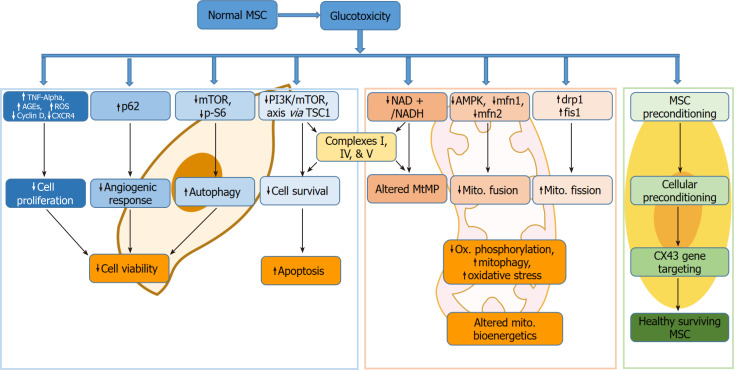
Chronic exposure to a high glucose (HG) microenvironment in vitro and in vivo is detrimental to cells and has physiological and pathological consequences (Figure 1). At the cellular level, the damaging effects of HG exposure for a prolonged period can cause glucose cytotoxicity that invariably affects every body cell, encompassing red blood cells to stem cells[1-3].
Figure 1.
Glucotoxicity in mesenchymal stem cells exposed to high glucose culture conditions and hyperglycemia. AGE: Advanced glycation end product; AMPK: AMP-activated protein kinase; CXCR: C-X-C chemokine receptor type 4; drp1: Dynamin-related protein 1; fis1: Fission protein 1. mfn1: Mitofusin 1; MSC: Mesenchymal stem cell; mTOR: Mammalian target of rapamycin; PI3K: Phosphoinositide 3-kinase; ROS: Reactive oxygen species; TNF: Tumor necrosis factor; TSC1: Tuberous sclerosis 1.
Insulin resistance, pancreatic beta cell damage, and decreased insulin production lead to hyperglycemia that drastically affects the whole body at the organ and cellular levels. An uncontrolled hyperglycemic state leads to chronic systemic inflammation that brings about morphological and functional changes in the body cells, including stem cells[4]. This persistent uncontrolled hyperglycemia also produces changes in the bone marrow (BM) microenvironment that cause functional impairment of stem cells[5]. Nguyen et al[6] reported a reduced proliferation rate and increased expression of stress-associated genes, activating transcription factor 4, and C/EBP homologous protein in mesenchymal stem cells (MSCs). On the same note, Kim et al[7] reported defective osteogenic differentiation but an increased adipogenic differentiation rate in BM-derived MSCs. MSCs from streptozotocin (STZ)-induced diabetic rats have a slow proliferation rate and poor myogenic potential[8]. These studies show that hyperglycemia causes changes in progenitor cell biology and affects their normal behavior and functions during tissue repair[9]. Hence, attempts have been made in some cases to predifferentiate MSCs into insulin-producing cells before transplantation in hyperglycemic experimental animal models[10].
Antihyperglycemic therapy to regain glucose homeostasis can also interfere with the quality and efficacy of MSC treatment. Hsiao et al[11] reported that metformin caused apoptosis of MSCs via the AMP-activated protein kinase (AMPK)-mammalian target of rapamycin (mTOR) pathway. Interestingly, the authors observed that hyperglycemia protected cells from metformin-induced apoptosis. In another study involving a rat model of diabetic cardiomyopathy, Ammar et al[12] observed impaired angiogenesis and higher myocardial fibrosis in response to concomitant treatment with metformin and MSCs compared to MSCs treated animals. These data were attributed to impaired MSC functionality in the presence of metformin treatment.
HG CULTURE- AND HYPERGLYCEMIA-INDUCED SIGNALING
Hyperglycemic conditions in vivo are simulated in vitro by culturing the cells in HG conditions to study the effects of hyperglycemia. HG culture conditions have been shown to cause rapid cellular dysfunction by promoting transcriptional changes[13]. Some of the essential mechanisms involved therein include the formation of advanced glycation products (AGEs), PKC activation, mTOR/Akt dysregulation, etc, that lead to elevated reactive oxygen species (ROS) stress, increased pro-inflammatory cytokines production, growth factors, abnormally high gas transmitters, altered cell bioenergetics, etc.
For example, Aguiari et al[14] reported that muscle-derived stem cells and adipose tissue-derived stem cells under HG culture conditions preferentially adopted adipogenic phenotype in response to ROS accumulation and activation of PKC-β in the cells. They supported their findings by treating the cells with oxidizing agents and silencing PKC-β in the cells to inhibit their adipogenic differentiation. In a subsequent study, culture of human aortic endothelial cells in HG was reported to cause significant pathway changes during the first 4 h, with distinct clusters of genes showing altered transcriptional profiles unique to HG conditions[13]. Temporal co-expression and causal network analysis showed a relationship between type 2 diabetes mellitus and activation of growth factor signaling pathways, including signal transducer and activator of transcription 3 and nuclear factor-kappa B. On the same note, MSCs in HG culture undergo senescence mediated by Akt/mTOR dysregulation[3]. However, some studies report that for the detrimental effects of HG culture conditions, the cells may need persistent long-term exposure because they may resist short-term exposure to HG culture conditions[15]. It is interesting to note that MSCs from healthy donors had shorter doubling time under HG culture conditions compared with MSCs from diabetic donors, thus implying that the difference in their responsiveness is more a function of the pathophysiology of diabetes. On the same note, changes observed in diabetes donor-derived MSCs respiration capacity were responsible for their compromised cellular functions[6]. There is reportedly a decreased angiogenic paracrine activity, which was evident from reduced secretion of pro-angiogenic growth factors, i.e. vascular endothelial growth factor-A (VEGF-A), angiopoietin-1 (Ang-1), and Ang-2, and VEGF-C in the HG MSCs[7].
Chronic HG culture conditions also drive glycation reactions through the receptor for advanced glycation end products (AGEs), resulting in the formation of AGEs and endogenous inflammatory mediators[16,17]. It has been reported that stimulation with AGE-bovine serum albumin induced the generation of ROS and attenuated the proliferation and migration of MSCs via activation of the ROS-p38 mediated pathway[18]. Another study reported that HG reduced the regeneration ability of BM-MSCs through the activation of glycogen synthase kinase-3beta, which plays a vital role in inhibiting the proliferation of BM-MSCs via the inhibition of C-X-C chemokine receptor type 4[19].
Continuing their efforts to study the effects of hyperglycemia on MSC functionality, Abu-El-Rub et al[20] reported interesting comparative data in vitro by culturing human adipose tissue-derived MSCs (AD-MSCs) under low glucose and HG conditions in a parallel set of experiments. It is pertinent to mention that the authors used in vitro culture conditions for exposure to HG. Hence, the term “hyperglycemia” in the aims, conclusion, and elsewhere in the manuscript does not reflect the experimental design. The authors have primarily focused on three endpoints, cell viability, cell apoptosis, and mitochondrial energetics, to share their findings supported by some mechanistic studies that will be discussed in the following sections.
CELL VIABILITY AND APOPTOSIS
In addition to cellular dysfunction and suppression of proliferation, an HG microenvironment activates signaling pathways that direct MSC apoptosis. However, these signaling pathways need to be studied and established further. Change of tumor necrosis factor-α expression significantly affected MSC proliferation and death in an STZ-induced type 1 diabetic mouse model[21]. In contrast, in another interesting study, human BM-MSCs in diabetic serum showed increased cellular death and decreased angiogenic response caused by the induction of autophagy signaling with a high level of p62 expression[22].
Endoplasmic reticulum stress-induced autophagy is another mechanism contributing to the inactivation of mTOR, which was shown to reduce p-S6 (a marker of mTOR activity)[23]. Building on these data, Abu-El-Rub et al[20] revealed higher apoptosis in human AD-MSCs (hAD-MSCs) cultured in HG using low glucose culture as the control. Elucidating the mechanism causing poor survival of MSCs in an HG microenvironment via impairment of the phosphoinositide 3-kinase (PI3K)/mTOR axis, they found significantly increased tuberous sclerosis 1 (TSC1) protein. It is now well established that mTOR is an essential regulator of mitochondrial dynamics via generating the required mitochondrial potential to produce ATP[24]. As a part of the mechanism, TSC1 binding inactivates mTOR, while PI3K, a known activator of mTOR, is needed to remove the inhibitory effects of TSC1[25]. Furthermore, the downregulation of mTOR significantly reduced complex I, IV, and V in HG-cultured hAD-MSCs. These molecular data suggest an impact on mitochondrial oxidative phosphorylation and induction of mitophagy and massive oxidative stress[26]. Although data from the Abu-El-Rub et al[20] provide a better understanding of the activation of proapoptotic signaling in hAD-MSCs in the HG microenvironment, it would have been interesting to see if similar signaling was activated in MSCs from other tissue sources as well as from other species to delineate any tissue or species-specific differential responsiveness to HG culture conditions. Also, the mechanistic data would have been more convincing if the authors had used gain-of-function and loss-of-function studies to establish a causal relationship between mTOR, PI3K, Akt, and TSC1. There is no mention of TSC2, which forms a physical and functional complex in vivo[27]. The evidence is based on western blotting alone, showing TSC1 expression with simultaneous loss of PI3K and mTOR in HG-cultured cells. There needs to be more evidence to prove their dependence/relationship with each other. Intriguingly, the authors designed the studies for stipulated time points of 3, 7, and 14 d; they provided data only for the day 7 time point. It would have been interesting to include day 3 and day 14 data in the results or at least as supplementary data to show how early these molecular and organelle-level changes occurred and continued in the HG culture. Similarly, it would have been interesting if the cells were returned to normoglycemic conditions at each time point to observe any possible reversibility of the changes at each time point. There are better methods to observe cell viability than the trypan blue dye exclusion method to exclude researcher bias. Another mechanism suggested by the authors for MSCs’ low viability was the drop in NAD+/NADH ratio in hAD-MSCs, correlated with impairment of the inner mitochondrial membrane potential (MtMP) that is discussed in the next section.
MITOCHONDRIAL CHANGES IN RESPONSE TO HYPERGLYCEMIC MICROENVIRONMENT
Before discussing the impairment of MtMP as a part of the cell’s response to hyperglycemia, readers need to understand the basic functioning of mitochondria. A continual, uninterrupted energy supply is critical for cellular processes, i.e. growth, repair, maintenance, etc, for which robust intracellular mechanisms are in place[28]. Mitochondria play a crucial role in supporting these cellular functions with ATP production during normal mitochondrial bioenergetics, along with contributing to other processes such as aging, ion homeostasis, and apoptosis[29]. The mitochondrial intermembranous space houses the enzymes involved in the electron transport chain, capturing energy carried by electrons in NADH and FADH2 to generate ATP. The flux of electrons creates a stable MtMP facilitated by proton pumps, i.e. complexes I, III, and IV. Contingent upon the cell’s energy needs, mitochondria undergo fusion or fission such that the process stimulates and inhibits ATP synthesis, respectively[30]. At the molecular level, AMPK enables mitochondrial fusion via mitofusin 1 (Mfn1), Mfn2, and optic atrophy 1, while dynamin-related protein 1 (Drp1) and fission protein 1 control mitochondrial fission. More recent studies have shown that mitochondrial functions go far beyond energy-producing organelle, i.e. cell differentiation and their regenerative potential[31-33].
HG culture conditions in vitro and hyperglycemia in diabetes patients cause mitochondrial dysfunction because of altered MtMP, thus lowering ATP production. A low NAD+/NADH ratio is observed in cardiac dysfunction in diabetic hearts. At the same time, it also changes mitochondrial membrane polarity and reduces mitochondrial cell mass, leading to perturbed mitochondrial homeostasis in human mononuclear cells[34,35]. Hyperglycemia also causes mitochondrial fragmentation with upregulation of Drp1 (promoting fission) or downregulation of Mfn1/2 (inhibiting fusion), thus further reducing mitochondrial ATP synthesis[35]. It creates structural and functional changes in the mitochondria, altering their bioenergetics and thus jeopardizing their survival[36-38]. There is also an increase in ROS stress in the cytosol and mitochondria[39]. Abu-El-Rub et al[20] have attributed reduced NAD+/NADH ratio in hAD-MSCs exposed to an HG environment as responsible for driving the cells toward apoptosis via dysregulation of mitochondrial complexes I, IV, and V. They have supported their findings with MtMP changes in hAD-MSCs assessed by the MtMP assay kit. All these factors confirm dysfunction in mitochondrial bioenergetics in the cells, resulting in low survival and higher apoptosis in HG culture conditions. Table 1 summarizes some of the studies from the published literature reporting the effect of HG culture conditions.
Table 1.
Summary of some studies from the published literature reporting the effect of high glucose culture conditions
|
Ref.
|
Cell type and source
|
Glucose concentration used
|
Mechanism
|
Findings
|
| Zhang et al[19], 2016 | Rat BM-MSCs | HG: 16.5 mM vs NG: 5.5 mM | Activation of GSK3β and suppression of CXCR-4, β-catenin, LEF-1, and cyclin D1 under HG culture conditions | The study related HG culture with the activation of GSK3β to affect the proliferation and migration of BM-MSCs in HG culture. The proliferation and migration ability of the cells were suppressed in HG culture. HG activated GSK3β but suppressed CXCR-4, β-catenin, LEF-1, and cyclin D1. Inhibition of GSK3β by lithium chloride led to increased levels of β-catenin, LEF-1, cyclin D1, and CXCR-4 expression |
| Abu-El-Rub et al[20], 2023 | hAD-MSCs | HG: 25 mM vs NG: 5.5 mM | Altered mitochondrial membrane potential, Low NAD+/NADH pool, reduced mTOR and PI3K | HG culture for 7 d showed reduced cell viability compared to NG cultured control. HG culture significantly reduced the mitochondrial membrane potential and NAD+/NADH ratio, showing dysregulated mitochondrial function. PI3K protein expression significantly decreased in HG-cultured cells MSCs with increased TSC1 and downregulation of mTOR protein. Mitochondrial complexes I, IV, and V were reduced in HG, leading to poor survival of MSCs in HG |
| Li et al[37], 2007 | Human BM-MSCs | HG: 25 mM vs NG: 5.6 mM | Molecular mechanism not explored | The effect of HG culture on human MSC in vitro was assessed using telomerase-immortalized MSC (hMSC-TERT) and primary MSC (hMSC). HG increased hMSC-TERT proliferation in long-term studies, while it remained unchanged for hMSCs. Apoptosis was not influenced by HG in both cell types. Moreover, HG culture conditions supported osteogenic differentiation of the cells |
| Hankamolsiri et al[40], 2016 | Human BM-MSCs and MSCs from gestational tissues | HG: 25 mM vs NG: 5.5 mM | HG-induced the expression of adipogenic gene PPARγ and LPL in BM-MSCs, as well as ADIPOQ and LPL genes in gestational tissue-derived MSCs | No change in surface markers’ expression. HG reduced proliferation but enhanced adipogenic differentiation of all MSCs examined. The expression of some adipogenic genes were also upregulated when MSCs were cultured in HG. Although HG transiently reduced some osteogenic genes, its effect on the osteogenic differentiation rate of the MSCs was not demonstrated |
| Al-Qarakhli et al[41], 2019 | Rat BM-MSCs | HG: 25 mM vs NG: 5.5 mM | HG culture conditions significantly reduced telomere length at 50 PDs and 100 PDs. Also attribute it to IGFs, TGF-β1, and BMPs | HG and NG cultured cells had similar morphology and growth characteristics. HG-cultured cells proliferated beyond 50 doublings, although with signs of senescence. The osteogenic and adipogenic differentiation rates were significantly reduced in HG-cultured cells. The effect of HG was more pronounced in advanced PDs |
| Khasawneh et al[42], 2023 | Human AD-MSCs | HG: 25 mM vs NG: 5.5 mM | Reduced AMPK and PFK-1 | Immunomodulation potential was lost in the hAD-MSCs under HG conditions and were detectable by immune cells. These changes were mediated by low IDO, IL-10, and complement factor H. AMPK and PFK-1, integral glycolysis regulators, were reduced in HG-cultured MSCs. These findings show the possibility of an immunomodulatory shift in MSCs under HG, leading to poor survival of the cells |
ADIPOQ: Adiponectin; AMPK: AMP-activated protein kinase; BM: Bone marrow; BMP: Bone morphogenic proteins; CXCR4: C-X-C chemokine receptor type 4; GSK-3β: Glycogen synthase kinase-3β; hAD-MSCs: Human adipose tissue-derived mesenchymal stem cells; HG: High glucose; IDO: Indoleamine 2,3-dioxygenase; IGF: Insulin-like growth factor; IL: Interleukin; LEF-1: Lymphoid enhancer binding factor-1; LPL: Lipoprotein lipase; MSC: Mesenchymal stem cell; mTOR: Mammalian target of rapamycin; NAD: Nicotinamide adenine dinucleotide; NG: Normal glucose; PD: Population doubling; PFK-1: Phosphofructokinase-1; PI3K: Phosphoinositide 3-kinase; PPARγ: Peroxisome proliferator-activated receptor gamma; TGF-β1: Transforming growth factor-β1; TSC1: Tuberous sclerosis 1.
One of the main limitations of the proposed mechanism is that there needs to be an attempt to extrapolate these data in vivo using experimental animal models. This is important before use as a novel target to improve the survival of MSC in diabetic patients. Moreover, it would have been interesting if the authors had used cellular preconditioning using preconditioning mimetics or a subcellular preconditioning approach to stabilize the MtMP, which can enhance cell survival and reduce apoptosis in HG culture conditions[43-45]. The authors have already successfully used subcellular preconditioning for cytoprotection of donor stem cells for heart-cell therapy to enhance their post engraftment survival[46,47]. Underscoring the mechanism, the authors have shown that mito-Cx43 gene targeting was cytoprotective via a shift of mitochondrial Bak and Bcl-xL balance.
CONCLUSION
In conclusion, it is evident that HG conditions have detrimental effects on different cell types, including cancer cells, and may also change their normal functions, i.e. migration potential and invasiveness[1,2,48-50]. Hence, understanding the mechanism of apoptosis by chronic exposure to HG, both in vitro and in vivo, will help efforts to combine preconditioning strategies, especially the subcellular preconditioning approach. That will go a long way in promoting donor cell post engraftment survival in diabetes patients and vice versa in clinical settings wherein MSCs have already progressed to advanced phases of assessment[51,52].
Footnotes
Conflict-of-interest statement: The authors declare that they have no conflicting interests.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: December 25, 2023
First decision: January 11, 2024
Article in press: January 29, 2024
Specialty type: Cell and tissue engineering
Country/Territory of origin: Saudi Arabia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Wang L, China; Zhou X, China S-Editor: Wang JJ L-Editor: Filipodia P-Editor: Xu ZH
Contributor Information
Muhammad Abdul Mateen, Basic Sciences, Sulaiman AlRajhi University, AlQaseem 52736, Saudi Arabia.
Nouralsalhin Alaagib, Basic Sciences, Sulaiman AlRajhi University, AlQaseem 52736, Saudi Arabia.
Khawaja Husnain Haider, Cellular and Molecular Pharmacology, Sulaiman AlRajhi Medical School, Al Bukairiyah 51941, Saudi Arabia. kh.haider@sr.edu.sa.
References
- 1.Rajab AM, Haider KhH. Hyperglycemia and RBCs: too sweet to survive. Int J Diabetes Dev Countries. 2018;38:357–365. [Google Scholar]
- 2.Rajab AM, Rahman S, Rajab TM, Haider KH. Morphology and Chromic Status of Red Blood Cells Are Significantly Influenced by Gestational Diabetes. J Hematol. 2018;7:140–148. doi: 10.14740/jh449w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang D, Lu H, Chen Z, Wang Y, Lin J, Xu S, Zhang C, Wang B, Yuan Z, Feng X, Jiang X, Pan J. High glucose induces the aging of mesenchymal stem cells via Akt/mTOR signaling. Mol Med Rep. 2017;16:1685–1690. doi: 10.3892/mmr.2017.6832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Musleh Ali AR, Al-Kasssas W, Haider KH. Fatty Acid Escape Hypothesis: The Pathway to Type-II Diabetes. Diabetes Res. 2019 [Google Scholar]
- 5.Fadini GP, Ferraro F, Quaini F, Asahara T, Madeddu P. Concise review: diabetes, the bone marrow niche, and impaired vascular regeneration. Stem Cells Transl Med. 2014;3:949–957. doi: 10.5966/sctm.2014-0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nguyen LT, Hoang DM, Nguyen KT, Bui DM, Nguyen HT, Le HTA, Hoang VT, Bui HTH, Dam PTM, Hoang XTA, Ngo ATL, Le HM, Phung NY, Vu DM, Duong TT, Nguyen TD, Ha LT, Bui HTP, Nguyen HK, Heke M, Bui AV. Type 2 diabetes mellitus duration and obesity alter the efficacy of autologously transplanted bone marrow-derived mesenchymal stem/stromal cells. Stem Cells Transl Med. 2021;10:1266–1278. doi: 10.1002/sctm.20-0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim H, Han JW, Lee JY, Choi YJ, Sohn YD, Song M, Yoon YS. Diabetic Mesenchymal Stem Cells Are Ineffective for Improving Limb Ischemia Due to Their Impaired Angiogenic Capability. Cell Transplant. 2015;24:1571–1584. doi: 10.3727/096368914X682792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jin P, Zhang X, Wu Y, Li L, Yin Q, Zheng L, Zhang H, Sun C. Streptozotocin-induced diabetic rat-derived bone marrow mesenchymal stem cells have impaired abilities in proliferation, paracrine, antiapoptosis, and myogenic differentiation. Transplant Proc. 2010;42:2745–2752. doi: 10.1016/j.transproceed.2010.05.145. [DOI] [PubMed] [Google Scholar]
- 9.Liu Y, Li Y, Nan LP, Wang F, Zhou SF, Wang JC, Feng XM, Zhang L. The effect of high glucose on the biological characteristics of nucleus pulposus-derived mesenchymal stem cells. Cell Biochem Funct. 2020;38:130–140. doi: 10.1002/cbf.3441. [DOI] [PubMed] [Google Scholar]
- 10.He X, Yang Y, Yao MW, Ren TT, Guo W, Li L, Xu X. Full title: High glucose protects mesenchymal stem cells from metformin-induced apoptosis through the AMPK-mediated mTOR pathway. Sci Rep. 2019;9:17764. doi: 10.1038/s41598-019-54291-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsiao CY, Chen TH, Huang BS, Chen PH, Su CH, Shyu JF, Tsai PJ. Comparison between the therapeutic effects of differentiated and undifferentiated Wharton's jelly mesenchymal stem cells in rats with streptozotocin-induced diabetes. World J Stem Cells. 2020;12:139–151. doi: 10.4252/wjsc.v12.i2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ammar HI, Shamseldeen AM, Shoukry HS, Ashour H, Kamar SS, Rashed LA, Fadel M, Srivastava A, Dhingra S. Metformin impairs homing ability and efficacy of mesenchymal stem cells for cardiac repair in streptozotocin-induced diabetic cardiomyopathy in rats. Am J Physiol Heart Circ Physiol. 2021;320:H1290–H1302. doi: 10.1152/ajpheart.00317.2020. [DOI] [PubMed] [Google Scholar]
- 13.Bayaraa O, Inman CK, Thomas SA, Al Jallaf F, Alshaikh M, Idaghdour Y, Ashall L. Hyperglycemic conditions induce rapid cell dysfunction-promoting transcriptional alterations in human aortic endothelial cells. Sci Rep. 2022;12:20912. doi: 10.1038/s41598-022-24999-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aguiari P, Leo S, Zavan B, Vindigni V, Rimessi A, Bianchi K, Franzin C, Cortivo R, Rossato M, Vettor R, Abatangelo G, Pozzan T, Pinton P, Rizzuto R. High glucose induces adipogenic differentiation of muscle-derived stem cells. Proc Natl Acad Sci U S A. 2008;105:1226–1231. doi: 10.1073/pnas.0711402105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weil BR, Abarbanell AM, Herrmann JL, Wang Y, Meldrum DR. High glucose concentration in cell culture medium does not acutely affect human mesenchymal stem cell growth factor production or proliferation. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1735–R1743. doi: 10.1152/ajpregu.90876.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aikawa E, Fujita R, Asai M, Kaneda Y, Tamai K. Receptor for Advanced Glycation End Products-Mediated Signaling Impairs the Maintenance of Bone Marrow Mesenchymal Stromal Cells in Diabetic Model Mice. Stem Cells Dev. 2016;25:1721–1732. doi: 10.1089/scd.2016.0067. [DOI] [PubMed] [Google Scholar]
- 17.Silva JC, Sampaio P, Fernandes MH, Gomes PS. The Osteogenic Priming of Mesenchymal Stem Cells is Impaired in Experimental Diabetes. J Cell Biochem. 2015;116:1658–1667. doi: 10.1002/jcb.25126. [DOI] [PubMed] [Google Scholar]
- 18.Yang K, Wang XQ, He YS, Lu L, Chen QJ, Liu J, Shen WF. Advanced glycation end products induce chemokine/cytokine production via activation of p38 pathway and inhibit proliferation and migration of bone marrow mesenchymal stem cells. Cardiovasc Diabetol. 2010;9:66. doi: 10.1186/1475-2840-9-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang B, Liu N, Shi H, Wu H, Gao Y, He H, Gu B, Liu H. High glucose microenvironments inhibit the proliferation and migration of bone mesenchymal stem cells by activating GSK3β. J Bone Miner Metab. 2016;34:140–150. doi: 10.1007/s00774-015-0662-6. [DOI] [PubMed] [Google Scholar]
- 20.Abu-El-Rub E, Almahasneh F, Khasawneh RR, Alzu'bi A, Ghorab D, Almazari R, Magableh H, Sanajleh A, Shlool H, Mazari M, Bader NS, Al-Momani J. Human mesenchymal stem cells exhibit altered mitochondrial dynamics and poor survival in high glucose microenvironment. World J Stem Cells. 2023;15:1093–1103. doi: 10.4252/wjsc.v15.i12.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ko KI, Coimbra LS, Tian C, Alblowi J, Kayal RA, Einhorn TA, Gerstenfeld LC, Pignolo RJ, Graves DT. Diabetes reduces mesenchymal stem cells in fracture healing through a TNFα-mediated mechanism. Diabetologia. 2015;58:633–642. doi: 10.1007/s00125-014-3470-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rezabakhsh A, Cheraghi O, Nourazarian A, Hassanpour M, Kazemi M, Ghaderi S, Faraji E, Rahbarghazi R, Avci ÇB, Bagca BG, Garjani A. Type 2 Diabetes Inhibited Human Mesenchymal Stem Cells Angiogenic Response by Over-Activity of the Autophagic Pathway. J Cell Biochem. 2017;118:1518–1530. doi: 10.1002/jcb.25814. [DOI] [PubMed] [Google Scholar]
- 23.Meng Y, Ji J, Tan W, Guo G, Xia Y, Cheng C, Gu Z, Wang Z. Involvement of autophagy in the procedure of endoplasmic reticulum stress introduced apoptosis in bone marrow mesenchymal stem cells from nonobese diabetic mice. Cell Biochem Funct. 2016;34:25–33. doi: 10.1002/cbf.3161. [DOI] [PubMed] [Google Scholar]
- 24.Morita M, Prudent J, Basu K, Goyon V, Katsumura S, Hulea L, Pearl D, Siddiqui N, Strack S, McGuirk S, St-Pierre J, Larsson O, Topisirovic I, Vali H, McBride HM, Bergeron JJ, Sonenberg N. mTOR Controls Mitochondrial Dynamics and Cell Survival via MTFP1. Mol Cell. 2017;67:922–935.e5. doi: 10.1016/j.molcel.2017.08.013. [DOI] [PubMed] [Google Scholar]
- 25.Zhang H, Cicchetti G, Onda H, Koon HB, Asrican K, Bajraszewski N, Vazquez F, Carpenter CL, Kwiatkowski DJ. Loss of Tsc1/Tsc2 activates mTOR and disrupts PI3K-Akt signaling through downregulation of PDGFR. J Clin Invest. 2003;112:1223–1233. doi: 10.1172/JCI17222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo C, Sun L, Chen X, Zhang D. Oxidative stress, mitochondrial damage and neurodegenerative diseases. Neural Regen Res. 2013;8:2003–2014. doi: 10.3969/j.issn.1673-5374.2013.21.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol. 2002;4:648–657. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- 28.Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. How cells obtain energy from food. In: Molecular Biology of the Cell. 4th ed. New York: Garland Science, 2002. [Google Scholar]
- 29.Chen W, Zhao H, Li Y. Mitochondrial dynamics in health and disease: mechanisms and potential targets. Signal Transduct Target Ther. 2023;8:333. doi: 10.1038/s41392-023-01547-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fu W, Liu Y, Yin H. Mitochondrial Dynamics: Biogenesis, Fission, Fusion, and Mitophagy in the Regulation of Stem Cell Behaviors. Stem Cells Int. 2019;2019:9757201. doi: 10.1155/2019/9757201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wanet A, Arnould T, Najimi M, Renard P. Connecting Mitochondria, Metabolism, and Stem Cell Fate. Stem Cells Dev. 2015;24:1957–1971. doi: 10.1089/scd.2015.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Q, Gao Z, Chen Y, Guan MX. The role of mitochondria in osteogenic, adipogenic and chondrogenic differentiation of mesenchymal stem cells. Protein Cell. 2017;8:439–445. doi: 10.1007/s13238-017-0385-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paliwal S, Chaudhuri R, Agrawal A, Mohanty S. Regenerative abilities of mesenchymal stem cells through mitochondrial transfer. J Biomed Sci. 2018;25:31. doi: 10.1186/s12929-018-0429-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chiao YA, Chakraborty AD, Light CM, Tian R, Sadoshima J, Shi X, Gu H, Lee CF. NAD(+) Redox Imbalance in the Heart Exacerbates Diabetic Cardiomyopathy. Circ Heart Fail. 2021;14:e008170. doi: 10.1161/CIRCHEARTFAILURE.120.008170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zheng Y, Luo A, Liu X. The Imbalance of Mitochondrial Fusion/Fission Drives High-Glucose-Induced Vascular Injury. Biomolecules. 2021;11 doi: 10.3390/biom11121779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rovira-Llopis S, Bañuls C, Diaz-Morales N, Hernandez-Mijares A, Rocha M, Victor VM. Mitochondrial dynamics in type 2 diabetes: Pathophysiological implications. Redox Biol. 2017;11:637–645. doi: 10.1016/j.redox.2017.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li YM, Schilling T, Benisch P, Zeck S, Meissner-Weigl J, Schneider D, Limbert C, Seufert J, Kassem M, Schütze N, Jakob F, Ebert R. Effects of high glucose on mesenchymal stem cell proliferation and differentiation. Biochem Biophys Res Commun. 2007;363:209–215. doi: 10.1016/j.bbrc.2007.08.161. [DOI] [PubMed] [Google Scholar]
- 38.Gong L, Liu FQ, Wang J, Wang XP, Hou XG, Sun Y, Qin WD, Wei SJ, Zhang Y, Chen L, Zhang MX. Hyperglycemia induces apoptosis of pancreatic islet endothelial cells via reactive nitrogen species-mediated Jun N-terminal kinase activation. Biochim Biophys Acta. 2011;1813:1211–1219. doi: 10.1016/j.bbamcr.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 39.Sen S, Domingues CC, Rouphael C, Chou C, Kim C, Yadava N. Genetic modification of human mesenchymal stem cells helps to reduce adiposity and improve glucose tolerance in an obese diabetic mouse model. Stem Cell Res Ther. 2015;6:242. doi: 10.1186/s13287-015-0224-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hankamolsiri W, Manochantr S, Tantrawatpan C, Tantikanlayaporn D, Tapanadechopone P, Kheolamai P. The Effects of High Glucose on Adipogenic and Osteogenic Differentiation of Gestational Tissue-Derived MSCs. Stem Cells Int. 2016;2016:9674614. doi: 10.1155/2016/9674614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Al-Qarakhli AMA, Yusop N, Waddington RJ, Moseley R. Effects of high glucose conditions on the expansion and differentiation capabilities of mesenchymal stromal cells derived from rat endosteal niche. BMC Mol Cell Biol. 2019;20:51. doi: 10.1186/s12860-019-0235-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khasawneh RR, Abu-El-Rub E, Almahasneh FA, Alzu'bi A, Zegallai HM, Almazari RA, Magableh H, Mazari MH, Shlool HF, Sanajleh AK. Addressing the impact of high glucose microenvironment on the immunosuppressive characteristics of human mesenchymal stem cells. IUBMB Life. 2023 doi: 10.1002/iub.2796. [DOI] [PubMed] [Google Scholar]
- 43.Ahmad N, Wang Y, Haider KH, Wang B, Pasha Z, Uzun O, Ashraf M. Cardiac protection by mitoKATP channels is dependent on Akt translocation from cytosol to mitochondria during late preconditioning. Am J Physiol Heart Circ Physiol. 2006;290:H2402–H2408. doi: 10.1152/ajpheart.00737.2005. [DOI] [PubMed] [Google Scholar]
- 44.Rodríguez-Sinovas A, Cabestrero A, López D, Torre I, Morente M, Abellán A, Miró E, Ruiz-Meana M, García-Dorado D. The modulatory effects of connexin 43 on cell death/survival beyond cell coupling. Prog Biophys Mol Biol. 2007;94:219–232. doi: 10.1016/j.pbiomolbio.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 45.Lu G, Haider HKh, Porollo A, Ashraf M. Mitochondria-specific transgenic overexpression of connexin-43 simulates preconditioning-induced cytoprotection of stem cells. Cardiovasc Res. 2010;88:277–286. doi: 10.1093/cvr/cvq293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lu G, Haider HK, Jiang S, Ashraf M. Sca-1+ stem cell survival and engraftment in the infarcted heart: dual role for preconditioning-induced connexin-43. Circulation. 2009;119:2587–2596. doi: 10.1161/CIRCULATIONAHA.108.827691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lu G, Jiang S, Ashraf M, Haider KH. Subcellular preconditioning of stem cells: mito-Cx43 gene targeting is cytoprotective via shift of mitochondrial Bak and Bcl-xL balance. Regen Med. 2012;7:323–334. doi: 10.2217/rme.12.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Su BL, Wang LL, Zhang LY, Zhang S, Li Q, Chen GY. Potential role of microRNA-503 in Icariin-mediated prevention of high glucose-induced endoplasmic reticulum stress. World J Diabetes. 2023;14:1234–1248. doi: 10.4239/wjd.v14.i8.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Duan XK, Sun YX, Wang HY, Xu YY, Fan SZ, Tian JY, Yu Y, Zhao YY, Jiang YL. miR-124 is upregulated in diabetic mice and inhibits proliferation and promotes apoptosis of high-glucose-induced β-cells by targeting EZH2. World J Diabetes. 2023;14:209–221. doi: 10.4239/wjd.v14.i3.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin CY, Lee CH, Huang CC, Lee ST, Guo HR, Su SB. Impact of high glucose on metastasis of colon cancer cells. World J Gastroenterol. 2015;21:2047–2057. doi: 10.3748/wjg.v21.i7.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Al-Khani AM, Khalifa MA, Haider KhH. Mesenchymal stem cells: How close we are to their routine clinical use? In: Haider KH. Handbook of Stem Cell Therapy. Singapore: Springer, 2022. [Google Scholar]
- 52.Al-Khani AM, Kalou Y, Haider KH. Response to Letter to the Editor: Comment on Bone Marrow Mesenchymal Stem Cells for Heart Failure Treatment: A Systematic Review and Meta-Analysis. Heart Lung Circ. 2023;32:e59–e60. doi: 10.1016/j.hlc.2023.07.001. [DOI] [PubMed] [Google Scholar]