Abstract
The microbiome is in a symbiotic relationship with the host. Among the microbial consortia in the human body, that in the oral cavity is complex. Instead of repeatedly confirming biomarkers of oral and systemic diseases, recent studies have focused on a unified clinical diagnostic standard in microbiology that reduces the heterogeneity caused by individual discrepancies. Research has also been conducted on other topics of greater clinical importance, including bacterial pathogenesis, and the effects of drugs and treatments. In this review, we divide existing research into technology‐driven and hypothesis‐driven, according to whether there is a clear research hypothesis. This classification allows the demonstration of shifts in the direction of oral microbiology research. Based on the shifts, we suggested that establishing clear hypotheses may be the solution to major research challenges.
Keywords: hypothesis‐driven, metagenomics, oral microbiome, pathogenesis, technology‐driven
Genomics technologies have undoubtedly boosted the process of oral microbiology research which broadens people's understanding of diseases. Studies driven by genomics technologies tend to obtain a microbiological portrait of a specific population and then describe it. However, the recent studies share some goals even more concrete and definite, which symbolizes a critical turning point of global trend naturally from technology‐driven to hypothesis‐driven. Hypothesis‐driven studies have currently focused on the validation of pathogenic mechanisms and the effectiveness evaluation of therapeutics.
Highlights
Genomics technologies have undoubtedly boosted the process of oral microbiology research which broadens people's understanding of diseases.
Studies driven by genomics technologies tend to obtain a microbiological portrait of a specific population and then describe it.
However, the recent studies share some goals even more concrete and definite, which symbolizes a critical turning point of global trend naturally from technology‐driven to hypothesis‐driven.
Hypothesis‐driven studies have currently focused on the validation of pathogenic mechanisms and the effectiveness evaluation of therapeutics.
INTRODUCTION
The microbiome can be regarded as a second human genome, with which we are in a symbiotic relationship [1, 2]. We have just begun to reveal the nature and strength of this relationship, as well as its influence on physiology and pathology. Among the microbial consortia in the human body, that in the oral cavity is singularly complicated, with the second greatest diversity [3]. The oral cavity connects the outside with the digestive and respiratory tracts, and the microorganisms therein protect against harmful external factors. Microbiota dysbiosis is associated with oral diseases, such as periodontitis [4], peri‐implantitis [5], oral mucosal diseases [6], and dental caries [7], as well as systemic diseases, including gastrointestinal, endocrine, immune, and neurological diseases [8–11].
Due to the lack of specific driving factors (e.g., smoking, alcohol consumption, and human papillomavirus) for some diseases, scholarly attention is drawn to oral microorganisms as potential risk factors [12]. Furthermore, understanding the oral microbiome is essential for explaining the role of other risk factors in disease development.
The advent of next‐generation sequencing (NGS) has pushed the number of publications in the sequencing‐based oral microbiome to an unprecedented level, a breakthrough in human oral microbiology. To date, there has been a critical shift in research direction.
TECHNOLOGY‐DRIVEN RESEARCH PROGRESS
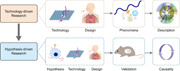
Describing the microbiology of a disease helps to infer its pathogenesis, namely extrapolating causality from phenomena (Figure 1). Technology‐driven research focuses on expanding databases, new advances and methods in sequencing technologies, as well as identifying new target populations and factors correlated with diseases.
Figure 1.

Technology‐driven studies refer to descriptive ones. That is, the study utilizes next‐generation sequencing technology to obtain a microbiological portrait of a specific population, and then describes it. Designing experiments to validate and thereby derive causality is called hypothesis‐driven research
Expanded database
The Human Oral Microbiome Database (HOMD) is based on 16S ribosomal RNA (rRNA) gene reference sequences and is a valuable resource. The expanded Human Oral Microbiome Database (eHOMD) covers numerous microbial species inhabiting the mouth and nose [13]. However, annotation or classification is hampered by the high conservation of 16S sequences among closely related species. For this reason, an intermediate taxonomic “superspecies level” was added between genus and species, which consisted of indistinguishable species sharing closely related sequences, reducing the error rate of short‐ and long‐read 16S rRNA [14].
However, the HOMD is derived from microbial culture data. Because of culture conditions and interactions of the microbiome with other factors, 20%–60% of oral microorganisms in the HOMD are unculturable [15]. Sequencing the metagenomes of bacteria isolated from the oral cavity enables the recognition of some unculturable oral microbes [16]. Using metagenomic shotgun data for 3346 oral metagenomics samples together with 808 published samples, 56,213 metagenome‐assembled genomes were obtained. Over 64% of the 3589 species‐level genome bins contained no publicly available genomes [17]. This study makes an important contribution to the replenishment of the database.
Using tongue and plaque samples, 790 nonredundant genomes were reconstructed, 43 of which belonged to TM7, forming six monophyletic clades [18]. The diversity of TM7s and their link to oral mucosal infectious diseases have been reported [19]. Moreover, 42 new sites (plaque vs. tongue) specific to TM7 were identified in 47 human samples, including the Saccharibacteria clades G3 and G6 [20]. The whole‐genome sequence of C17T was isolated from a child's oral cavity by Qi et al. [21] and an almost‐complete genome of a Tannerella sp. BU045 was detected by DNA amplification and sequencing from a single bacterial cell [22]. In a long‐read metagenomic study based on PromethION, 10 jumbo oral phages and prophages in human saliva were selected, the same as plasmid‐like components [23].
In addition to the discovery of new taxa, metagenome assembly can assemble the genomes of uncultured bacteria. Large‐scale metagenomic data from massive samples also allow the assembly of strains from important oral taxa, such as Porphyromonas and Neisseria [17]. In the assembled overlap, 50% of genes were singletons (unique to a single metagenomic sample). This clarified the unexplained heterogeneity in microbiome‐derived human phenotypes [16].
There are also previously isolated bacteria whose function is unclear, such as Streptococcus sp. A12. The molecular mechanisms by which this microbe resists antagonistic factors were identified by functional genomics, facilitating the development of biomarkers and therapeutics [24]. There is an association between the phylum Saccharibacteria and oral mucosal infectious diseases [19]. Interestingly, many oral communities contain homologs to gut bacteria encoding enzymes relating up to 41 human targeted drugs [17]. The HOMD is constantly updated with new discoveries (Figure 2).
Figure 2.

Human Oral Microbiome Database is constantly expanded and updated. Here is the database development from June 2018 to September 2021
New methods and technologies
Optimization and improvement of research methods are critical in this field. Yano et al. compared the human oral microbiome using four sample‐collection methods, which had a marked influence on the oral microbiome [25]. To overcome this variability, osmotic lysis followed by propidium monoazide treatment increases the yield of microbial DNA from human oral samples. It might be extended to other sample types [26].
A low‐cost sequencing method with higher resolution than 16S rRNA gene sequencing has been developed. The resolution of amplicon‐based microbiome identification was increased by merging high‐diversity marker gene sequencing (ribosomal 16–23S intergenic spacer region) and the DADA2 probabilistic error modeling based denoising algorithm [27].
Advances have been made in statistical methods for oral microbial genomics. As a mature method [28], co‐occurrence network analysis has been used in oral microbiology to evaluate the associations between the oral microbiome and other habitats, as well as the relationship between the oral microbiome and metabolites [20, 29]. Similarly, genome‐scale modeling has been used to identify microbiomes in a variety of tissues [30].
Machine learning algorithms can be used to recognize oral microbiome species from buccal and supragingival sites, representing those in subgingival plaque. This microbiota holds promise as a marker for the early diagnosis of periodontal disease [31].
Several novel techniques have been applied in oral microbiology. Based on 16S rRNA gene sequencing, the Human Oral Microbe Identification Microarray (HOMIM) can simultaneously detect about 300 of the most common oral bacterial species, including several nonculturable taxa [32]. HOMI NGS is a more comprehensive semiquantitative technique than HOMIM, allowing better characterization of oral microorganisms [33].
However, 16S rRNA gene sequencing is limited to 16S rRNA fragments, whereas identification of microorganisms should be based on the entire genome. Multiomics techniques (e.g., gene, transcription, proteomics) have been applied to oral microbial genomes [34]. Combining public genomes with Human Microbiome Project metagenomes, differences in the distribution of bacterial flora in the dorsal tongue, buccal mucosa, and supragingival plaque have been found [35]. Moreover, proteomics and metabolomics indicated systematic differences between the plaque and calculus microbiome, which was associated with biofilm physiology [36]. Multiomics will increasingly be used in microbiology studies.
The combination of traditional in vitro animal models and genomics has much potential. Adult Macaca mulatta were used to explore the interaction between oral microorganisms and gene expression profiles in autophagy, hypoxia, and apoptosis [37]. Fecal transplants have been used in studies of gut microorganisms [38], hinting at an innovative idea of transplanting oral bacteria from patients into germ‐free mice. Replication or transplantation of a healthy oral microbiota into patients has therapeutic potential for oral and systemic diseases. However, to date, no microbiological evaluation or clinical assessment has been performed.
Thus, new research methods and technologies facilitate the studies of microorganisms. The combination of omics with other technologies, or the application of advanced technologies in other fields, may trigger a new phase of microbiological research.
New research populations
The baseline levels of the oral microbiome in populations with different epidemiological characteristics have been reported, expanding our understanding of oral microorganisms (Figure 3).
Figure 3.

The investigation of the factors influencing oral microbiology continues, including age, smoking, alcohol, ethnicity, diet, water, delivery, breastfeeding, and so on. The studies included are all conducted using next‐generation sequencing technologies. It is evident that many studies are no longer limited to the observation of diversity and species distribution, but are gradually moving toward structural, mechanistic, or longitudinal perspectives, which will provide a clearer picture of the clinical significance of these factors.
Microbiome revealed a precise and perceptible association with age, including the number, abundance, and prevalence [39]. Microbial communities within different sites show a bell‐shaped trend in response to aging [40]. Human groups in different regions vary greatly in the degree of diversity among and within individuals [41]. The 28 species‐level operational taxonomic units vary considerably among different ethnic groups in Canada [42].
Smokers and e‐cigarette users were both rich in pathogens in their oral cavities, and the most significant effect was the changing of biofilm architecture [43]. Smoking also may lead to shifts in functional pathways that have an impact on smoking‐related diseases [44]. The level and type of drinking alcohol were associated with the overall microbial constitution and individual taxon abundance [45]. The Shannon diversity was lower in the oral microbiome than in the gut [46]. Drinking tap water might play a significant part in shaping oral microbiota, and the composition of tap water had a connection with the major modifications in the abundance of several bacterial genera [47]. Drinking high nitrate water increases oral nitrate‐reducing bacteria, which may lead to an increase in N‐nitroso compounds [48].
Differences in the composition of the salivary microbiota of vegetarians and omnivores exist at all taxonomic levels below the phylum level, including species associated with periodontal disease [49]. Oral and gut microbiota were correlated with specific dietary components, such as vegetable and sweets intake [50]. A relationship was confirmed between the mode of delivery and primitive bacterial content in the oral microbiome. During the first 2 years of age, shorter breastfeeding time and whether antibiotic‐treated were related to a distinct bacterial composition at a later age [51]. Oral microbiota differs between breast‐fed and formula‐fed infants at 3 months of age [52].
Human oral microbiological characteristics are influenced by multiple factors, but the underlying mechanism is unknown. For example, the salivary microbiome of obese subjects is distinct from that of nonobese subjects. Džunková et al. performed a longitudinal analysis of saliva samples from obese adults who underwent bariatric surgery. Various individual‐specific factors other than weight influenced the salivary microbiome distribution. It is reasonable for further studies to focus on the correlation between altered taste preferences and latent oral health deficit [53]. There are some other factors that affect the microbiome lack of causality. Various habits associated with modern lifestyles can reduce the diversity of the human oral microbiome, such as high‐sugar diets, alcohol consumption, smoking, and other factors [54]. For another instance, the pathological processes of mother‐to‐child microbiome transmission remain unclear [51, 55, 56]. In addition, the oral microbiota of smokers and e‐cigarette users contain pathogens, but with different bacterial compositions [43]. The studies reflect the unexplained heterogeneity in human microbiomes [16]. It is necessary to explain how such heterogeneities arise, which can be overcome by expanding research populations by including hitherto ignored groups, such as adolescents and babies [47].
New descriptions of disease
The association of some diseases with oral microorganisms has been updated, such as Alzheimer's disease (AD), Parkinson's disease (PD), and gestational diabetes (GDM).
There is a strong correlation between AD and the oral microbiota, and the impaired oral motor skills and inability to perform oral care as a result of cognitive impairment in patients with AD increases the risk of dental caries and periodontal disease [11]. Holmer et al. reported that the subgingival microbiota showed typical periodontal disease features in individuals with cognitive impairment or AD [57]. Fleury et al. sampled the microbiota of saliva and subgingival plaque and, using 16S rRNA gene amplicon sequencing, found that the oral microbiome changed in early and midterm PD patients, which might be related to local inflammation in the oral cavity [58]. Using 16S rDNA sequencing, Xu et al. discovered a link between GDM status in the third gestational period and oral microorganisms during pregnancy. The changes in the intestinal and oral microbial community might be used as noninvasive biomarkers for monitoring GDM in pregnancy [43, 59]. A common limitation of these studies is that they focus on correlation rather than causality. However, although the causality between these diseases and oral microbiota has not been confirmed, such correlations could help diagnose and prevent diseases.
There are limitations in studies of some other diseases, such as adverse pregnancy outcomes (APO). The clinical evidence of immunological activity in APO is dependent on a cross‐sectional case‐control studies. However, the gestation period includes proinflammatory and anti‐inflammatory phases, both of which are affected by fluctuations of female sex hormones [60]. This leaves such studies lacking in potential for future mechanistic exploration.
On the other hand, genomics technology has provided a clearer picture of human oral microbial genes, increasing our understanding of the disease. Human‐associated microbe pangenomes have been expanded by the thousands of microbial genomes of yet‐to‐be‐named species identified [61]. The first global snapshot of the healthy oral microbiome and its resistome was obtained from the data of 20 subjects by whole‐genome sequencing and microarray analysis [62]. For the first time, a catalog of microbiome genes from children with caries was constructed using samples from 25 3–5‐year‐old preschool children suffering from severe early childhood caries and 19 age‐matched healthy controls [63]. Also, 16S rRNA sequencing provided insight into the apical periodontitis and Down syndrome microbiome [64, 65]. With known findings previously into the biogeographic variation, an anterior‐to‐posterior gradient of subgingival community composition was determined [66]. However, most of these studies were small, and larger‐scale confirmatory studies are needed.
New diagnostic tools and models
Interindividual differences hamper generalization of the findings of microbiome studies. This means that it is not reliable to predict diseases only by the abnormal distribution of individual microorganisms unified standard is needed.
However, genetic diversity has not been completely quantified. Tierney et al. conducted a cross‐study meta‐analysis on metagenomes from two human‐body niches, with 3655 samples from 13 studies, and identified 23,961,508 oral and 22,254,436 gut nonredundant genes among a total of 45,666,334. The data were used to create a resource (https://microbial-genes.bio/) [16, 55].
Oral microbiological diagnostic tools and predictive models have been proposed based on large‐scale studies. The large sample size and several statistical methods reduce the interference of individual discrepancies in the results of those studies, namely the standardization process. With the standardization, the reliability of disease diagnosis tools and prediction models could be relatively trusted. The oral microbiota composition differed significantly between primary Sjögren syndrome and systemic lupus erythematosus patients, suggesting that it can be used to distinguish the two [67]. Xu et al. conducted a 1‐year longitudinal observation by 16S rDNA sequencing of the occurrence and development of caries in 144 3‐year‐old children, including 10 with caries and 19 healthy children as controls. The accuracy of their prediction model reached 93.1% [68]. Considering the high prevalence of dental caries, microbiological diagnostic tools are important, which requires a unified standardization of individual differences.
HYPOTHESIS‐DRIVEN RESEARCH PROGRESS
It is essential to formulate a potentially plausible hypothesis based on theoretical and scientific experience, which can be verified experimentally or clinically (Figure 1). The isolation of new bacteria, the recognition of new research populations, and links between diseases and the oral microbiome lay the foundation for hypothesis‐driven research.
By conducting confirmatory experiments, hypothesis‐driven research can lead to conclusive causality about the microbiological or immunological mechanisms of disease, the effectiveness of treatments, and so on.
Oral microbiome in disease pathogenesis
The complex metabolic and functional interactions within dental biofilms and between them and the host are involved in health‐maintaining mechanisms [69]. Wang et al. longitudinally tracked the reassembly of human oral biofilms after disturbance. The data revealed the resilience and long‐term stability of the oral microbial community and the critical time points and stages of the dramatic transformations in, and structural recovery of, the microflora [70].
There is an unambiguous relationship between oral microbial metabolism and oral diseases, such as dental caries [7], periodontitis [5], and oral mucosal diseases [71]. The theory of oral diseases has changed from a single pathogen to microecological dysbiosis. Dysbiosis is a result of the interactions of bacteria, fungi, and viruses in the community. For example, in severe early childhood caries etiopathogenesis, the activity of glucosyltransferases in plaque was significantly elevated by Candida albicans [72].
Genomics technology has been used in immunological research on pathogenic mechanisms, resulting in the discovery of putative pathogens and novel genes not previously linked to periodontitis [73]. A longitudinal study provided evidence that genes involved in carbohydrate‐related metabolism, such as methane metabolism, and energy‐metabolism‐related parameters were enriched in late‐stage oral squamous cell carcinoma, whereas those responsible for amino acid metabolism were significantly associated with the healthy controls [74]. Taste‐preference‐associated genes were found to have interrelationships with sucrose ingestion as well as allelic variation, using hierarchical cluster analysis of salivary microbiota groups [75]. Recent bioinformatics research has focused on gene clusters generating micromolecules, suggesting relationships between the microbiological community and signal molecules [76]. For instance, catechol siderophore synthesis gene clusters were detected in both Rothia mucilaginosa and Rothia dentocarios cultured in the presence of glycerol, which represented rich R. mucilaginosa in the saliva of children in health conditions [77]. Mohan et al. explored metatranscriptome data sets to assess the RNA regulatory mechanisms and metabolic shifts [78]. These gene‐level studies have provided insights on previously known pathways as they relate to oral cavities, with the aim of building a more comprehensive model of the oral microbial landscape.
There is a consensus that periodontal diseases are related to the oral microbiome. As the result of the host immune disorders, inflammation provides a suitable nutritional environment for pathogens and further promotes the persistence of dysbiosis. Thus, periodontitis is driven by a self‐sustainable cycle in which inflammation and dysbiosis positively strengthen each other. As an inflammatory response, periodontal disease is a typical dynamic process indicating the interaction between oral microorganisms and the host [79], and follow‐up studies should pay more attention to this dynamic nature to better understand the disease pathogenesis.
Oral dysbiosis is hypothesized to result in diseases of the gastrointestinal, endocrine, immune, and nervous systems [8–11]. Syndromic chronic periodontitis is a form of chronic periodontal damage that is considered a symptom of systemic disease as a result of defects in key genes that affect periodontal structures or immunity [80]. Dysbiosis can also cause periodontitis, likely resulting in bacteremia and the exacerbation of systemic diseases [81]. In conclusion, there is a two‐way interaction between oral microbiota and systemic diseases.
Systemic diseases are now in a reinforced causality with oral microbiome on the perspective of bacterial transplantation. Medini et al. evaluated cellular and soluble markers of inflammation and immune malfunction and found that the bacterial and fungal oral microbiome might be involved in chronic systemic immune activation in HIV‐infected patients [10]. Moentadj et al. researched the oral microbial community in a prospective cohort of rheumatoid arthritis (RA) patients, first‐degree relatives, and healthy controls (HC). Mild chronic arthritis in mice was induced by the streptococcal cell wall from RA and HC‐associated S. parasalivarius strains [82].
The oral microbiome may drive the development and exacerbation of systemic diseases via its action on the gut. For example, the oral microbiome is overrepresented in the lower intestinal tract in liver cirrhosis, potentially contributing to disease development and severity [83]. Intestinal inflammation is a causative factor for systemic diseases, including arthritis, psoriasis, and uveitis [84]. However, whether this is a result of oral inflammation or a direct effect of oral bacteria is unclear [85].
Periodontal inflammation reportedly contributes to gut inflammation in vivo. Human TH17 cell defects were correlated with reduced periodontal inflammation and bone loss [86]. Kitamoto et al. used mouse periodontitis and enteritis models to show that periodontitis produces oral phobia‐responsive Th17 cells which migrate to the gut and trigger colitis [8]. Also, Klebsiella spp. are strong inducers of T helper 1 cells in mice [87].
The oral cavity acts as a reservoir for gut bacteria [88], and oral‐intestinal microbial transfer is accepted. A multistage model of bacterial intestinal transport from the oral cavity emphasized the importance of the oral–intestinal axis microbial and immune compartments [86]. Hematogenous transmission is likely preferred according to Abed et al. in a study of oral fusobacteria translocating to colon tumors [89]. Ingestion or transplantation into the gut of Klebsiella and Enterobacter caused inflammasome activation in colonic mononuclear phagocytes and inflammation [8] (Figure 4).
Figure 4.

Potential mechanisms for the association of oral diseases with systemic diseases. A healthy oral cavity is maintained through resilience. Oral inflammation is self‐sustainable and bidirectionally driven to systemic disease. The mechanism may be that periodontal bacteria can enter the intestinal tract through the hematological circulation to trigger inflammation, or it can induce Th17, Th1, and other cells in the oral cavity to make their way to the intestinal tract. The inflammatory factors produced by inflammation lend themselves to an immune response to induce systemic disease
Metagenome sequencing has shown that the oral microbiome is significantly associated with cancer, suggesting novel prophylactic approaches. Possible mechanisms include upregulating putative virulence factors related to membrane biosynthesis, flagellum synthesis and assembly, iron transport, chemotaxis, hemolysin, and adhesins [90]. Other such studies have focused on oral [91–93], colorectal [89, 94, 95], pancreatic [96, 97], lung [98–100], and esophageal [101–103] cancer.
Multiple biological and environmental factors influence disease susceptibility, development, and severity, but longitudinal studies on these effects are scarce. Freire et al. analyzed the supragingival plaque microbiomes of dizygotic and monozygotic twins in a longitudinal study and found that oral microbiome variation was mainly caused by the environment [104]. Also, infants born by Cesarean section initially had abnormal levels of bacteria compared to those delivered vaginally, but this recovered with age. This finding revealed that oral microbiota development is an ecological succession [16].
Surana et al. delineated a bioinformatically straightforward approach to triangulate microbiota members likely to influence disease pathogenesis, enabling the sequencing methods to meet Koch's postulates [105]. Sanna et al. and Zhuang et al. employed bidirectional Mendelian randomization analyses to assess causality [106, 107]. These methods could be applied to demonstrate the causality of links between the oral microbiome and disease in the future.
Oral microbiome in disease therapy
Inflammatory bowel disease involves the oral intestinal microbial transfer pathway. Drug therapy is aimed at rectifying dysbiosis of inflammatory bowel disease. According to Simon‐Soro et al., drug effects, such as the intervention of thonzonium bromide in the oral cavity and gut, were impervious to each other. However, different oral flora could colonize the intestinal tract [9]. One possible explanation is that the drug intervention on the oral microbial community is in the original site of action, which affects other communities in the whole human body. A healthy oral microbiome could have therapeutic potential for gastrointestinal disease. In another study, the salivary microbiota colonized the gut, including Klebsiella strains resistant to several antibiotics. The study identified members of the healthy intestinal microbiome that resisted oral bacterial colonization, promoting the development of drugs active against resistant bacteria and for chronic inflammation [87].
Oral microorganisms can guide cancer treatment. A loss of alpha diversity was found in the stool and oral samples of acute myeloid leukemia (AML) patients receiving carbapenems [108]. In a prospective cohort analysis of 90 patients with AML, no association of Stenotrophomonas maltophilia infection with fecal relative abundance was found. Instead, the oral microbiome was predictive of S. maltophilia infection in chemotherapy patients with AML. Researchers also found that cumulative meropenem exposure was linked to an increased infection risk [109]. These conclusions will assist in balancing the efficacy and indirect damage of broad‐spectrum antibiotics in cancer treatment.
Oral mucositis (OM) is one of the most common complications of chemotherapy. In cancer therapy, a dynamic oral microbial community can trigger cancer therapy‐induced oral mucositis. In patients with locoregional squamous cell carcinoma of the head and neck, there was an association between the abundance of microbial genera and the occurrence of severe OM during treatment [110]. Hong et al. performed metagenomic sequencing of 49 participants undergoing 5‐fluorouracil or doxorubicin‐based chemotherapy and 30 healthy subjects longitudinally during one cycle. The results indicated that chemotherapy‐induced OM and dysbiosis were closely linked, and inflammation‐associated dysbiotic shifts could aggravate the injury to the oral epithelium. Controlling oral bacterial dysbiosis has the potential for improving oral mucositis [6].
In short, technology‐driven research has generated extensive databases, greatly broadening our knowledge of the oral microbiome. And hypothesis‐driven research focuses on scientific problems, transforming microbiological knowledge into useful clinical tools.
CONCLUSIONS
Oral microbiology research has reached a bottleneck. Instead of repeatedly confirming biomarkers of oral and systemic diseases, recent studies have focused on developing a unified clinical diagnostic standard in microbiology that reduces the effect of interindividual differences. Research has also focused on the mechanisms of bacterial pathogenesis and the effects of treatments. There are two major challenges in oral microbiology research.
One is the demand for hypothesis‐driven rather than technology‐driven research. Technology‐driven studies lack clear hypotheses but rather explore blank areas, resulting in contradictory or clinically nonsignificant conclusions. In contrast, hypothesis‐driven studies are likely to show greater clinical significance because of their concrete purpose and use of the databases created in the past two decades. Therefore, microbiome research should be hypothesis‐driven rather than technology‐driven. This is the first time for this classification method to be proposed. It clearly demonstrates a principal shift in the direction of oral microbiology research.
Another important challenge is the insufficient sample size. NGS has enabled the creation of large databases and has revealed marked interindividual variability. Therefore, standardization is important to deal with the data sets created. Most prior reviews of oral microbiology overlooked standardization, and others covered it only at the conceptual level. We emphasize the importance of standardization and the use of clear hypotheses in future studies.
The influence of oral microorganisms on tissues, organs, and systems may affect the whole body, thereby not only interfering with the judgment of the impact of a single disease but also providing new ideas for drug therapy. Thus, the oral microbiome is of great potential, whether in terms of disease prevention, diagnosis, or treatment. However, there is a lack of attention to the oral microbiome compared to the gut microbiome. Although they are correlated, phenomena linked to gut microbes may not be applicable to oral microbes. Therefore, the purpose of this review is to guide researchers who want to approach the study of the oral microbiome in the most reasoned and effective way, to speed up the process of oral microbial research.
AUTHOR CONTRIBUTIONS
Chuqi Gao, contributed to the conception, design, drafting, interpretation, and critical revision of the manuscript. Xiaole Zhao, contributed to drafting, interpretation, and revision of the manuscript. Xuantao Li, contributed to analysis, design, drafting, interpretation, and critical revision of the manuscript. Peiyue Yang, contributed to the analysis, drafting, interpretation, and revision of the manuscript. Xiao Wang, contributed to the design, drafting, interpretation, and critical revision of the manuscript. Xiaoli Chen, contributed to the design, drafting, interpretation, and critical revision the manuscript. Feng Chen and Ning Chen, contributed to the conception, design, and critical revision of the manuscript. All authors gave their final approval and agreed to be accountable for all aspects of the work.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
ACKNOWLEDGEMENT
This study is supported by the National Natural Science Foundation of China (grant number: 81991501), 2020‐SSDC‐05 and BMU2020KCL003.
Gao, Chuqi , Li Xuantao, Zhao Xiaole, Yang Peiyue, Wang Xiao, Chen Xiaoli, Chen Ning, and Chen Feng. 2022. “Standardized Studies of the Oral Microbiome: From Technology‐driven to Hypothesis‐driven.” iMeta 1, e19. 10.1002/imt2.19
Xuantao Li and Xiaole Zhao contributed equally to this study and should be considered the second authors.
Contributor Information
Ning Chen, Email: chenning79@139.com.
Feng Chen, Email: chenfeng2011@hsc.pku.edu.cn.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study. Supplementary information (graphical abstract, slides, videos, Chinese translated version, and updated materials) are available online at DOI or http://www.imeta.science/.
REFERENCES
- 1. Ding, Tao , and Schloss Patrick D.. 2014. “Dynamics and Associations of Microbial Community Types Across the Human Body.” Nature 509: 357–360. 10.1038/nature13178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Parida, Sheetal , Sharma Dipali. 2019. “The Microbiome–Estrogen Connection and Breast Cancer Risk.” Cells 8: 1642. 10.3390/cells8121642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Human Microbiome Project. March 31, 2021. https://www.hmpdacc.org.
- 4. Matsha, Tandi E. , Prince Y., Davids S., Chikte U., Erasmus Rajiv T., Kengne A. P., and Davison G. M.. 2020. “Oral Microbiome Signatures in Diabetes Mellitus and Periodontal Disease.” Journal of Dental Research 99: 658–665. 10.1177/0022034520913818 [DOI] [PubMed] [Google Scholar]
- 5. Ghensi, Paolo , Manghi Paolo, Zolfo Moreno, Armanini Federica, Pasolli Edoardo, Bolzan Mattia, Bertelle Alberto, et al. 2020. “Strong Oral Plaque Microbiome Signatures for Dental Implant Diseases Identified by Strain‐resolution Metagenomics.” NPJ Biofilms Microbiomes 6: 47. 10.1038/s41522-020-00155-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hong, Bo‐Young , Sobue Takanori, Choquette Linda, Dupuy Amanda K., Thompson Angela, Burleson Joseph A., Salner Andrew L., et al. 2019. “Chemotherapy‐Induced Oral Mucositis is Associated with Detrimental Bacterial Dysbiosis.” Microbiome 7: 66. 10.1186/s40168-019-0679-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Du, Qian , Ren Biao, He Jinzhi, Peng Xian, Guo Qiang, Zheng Liwei, Li Jiyao, et al. 2021. “ Candida albicans Promotes Tooth Decay by Inducing Oral Microbial Dysbiosis.” The ISME Journal 15: 894–908. 10.1038/s41396-020-00823-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kitamoto, Sho , Nagao‐Kitamoto Hiroko, Jiao Yizu, Gillilland Merritt G., Hayashi Atsushi, Imai Jin, Sugihara Kohei, et al. 2020. “The Intermucosal Connection between the Mouth and Gut in Commensal Pathobiont‐Driven Colitis.” Cell 182(447−462): e414. 10.1016/j.cell.2020.05.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Simon‐Soro, Aurea , Kim Dongyeop, Li Yong, Liu Yuan, Ito Tatsuro, Sims Kenneth R., Benoit Danielle S. W., et al. 2021. “Impact of the Repurposed Drug Thonzonium Bromide on Host Oral‐gut Microbiomes.” NPJ Biofilms Microbiomes 7: 7. 10.1038/s41522-020-00181-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Annavajhala Medini, K. , Khan Sabrina D., Sullivan Sean B., Shah Jayesh, Pass Lauren, Kister Karolina, Kunen Heather, et al. “Oral and Gut Microbial Diversity and Immune Regulation in Patients with HIV on Antiretroviral Therapy.” mSphere 5: e00798−00719. 10.1128/mSphere.00798-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sureda, Antoni , Daglia Maria, Castilla Sandro Argüelles, Sanadgol Nima, Nabavi Seyed Fazel, Khan Haroon, Belwal Tarun, et al. 2020. “Oral Microbiota and Alzheimer's Disease: Do all Roads Lead to Rome?.” Pharmacolgical Research 151: 104582. 10.1016/j.phrs.2019.104582 [DOI] [PubMed] [Google Scholar]
- 12. Ganly, Ian , Yang Liying, Giese Rachel A., Hao Yuhan, Nossa Carlos W., Morris Luc G. T., Rosenthal Matthew, et al. 2019. “Periodontal Pathogens are a Risk Factor of Oral Cavity Squamous Cell Carcinoma, Independent of Tobacco and Alcohol and Human Papillomavirus.” International Journal of Cancer 145: 775–784. 10.1002/ijc.32152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Escapa, Isabel F. , Chen Tsute, Huang Yanmei, Gajare Prasad, Dewhirst Floyd E., Lemon Katherine P., and Xu Jian. 2018. “New Insights into Human Nostril Microbiome from the Expanded Human Oral Microbiome Database (eHOMD): A Resource for the Microbiome of the Human Aerodigestive Tract.” mSystems 3: e00187−18. 10.1128/mSystems.00187-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Isabel, F. Escapa , Huang Yanmei, Chen Tsute, Lin Maoxuan, Kokaras Alexis, Dewhirst Floyd E., and Lemon Katherine P.. 2020. “Construction of Habitat‐Specific Training Sets to Achieve Species‐level Assignment In 16S RRNA Gene Datasets.” Microbiome 8: 65. 10.1186/s40168-020-00841-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Overview, Human Microbiome Project . March 31, 2021. https://commonfund.nih.gov/hmp/overview
- 16. Tierney, Braden T. , Yang Zhen, Luber Jacob M., Beaudin Marc, Wibowo Marsha C., Baek Christina, Mehlenbacher Eleanor, et al. 2019. “The Landscape of Genetic Content in the Gut and Oral Human Microbiome.” Cell Host Microbe 26(283–295): e288. 10.1016/j.chom.2019.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhu, Jie , Tian Liu, Chen Peishan, Han Mo, Song Liju, Tong Xin, Sun Xiaohuan, et al. 2021. “Over 50,000 Metagenomically Assembled Draft Genomes for the Human Oral Microbiome Reveal New Taxa.” Genomics, Proteomics & Bioinformatics. 10.1016/j.gpb.2021.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shaiber, Alon , Willis Amy D., Delmont Tom O., Roux Simon, Chen Lin‐Xing, Schmid Abigail C., Yousef Mahmoud, et al. 2020. “Functional and Genetic Markers of Niche Partitioning Among Enigmatic Members of the Human Oral Microbiome.” Genome Biology, 21: 292. 10.1186/s13059-020-02195-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bor, B. , Bedree J. K., Shi W., McLean J. S., and He X.. 2019. “Saccharibacteria (TM7) in the Human Oral Microbiome.” Journal of Dental Research, 98: 500–509. 10.1177/0022034519831671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Baker, Jonathon L. , Morton James T., Dinis Márcia, Alvarez Ruth, Tran Nini C., Knight Rob, and Edlund Anna. 2021. “Deep Metagenomics Examines the Oral Microbiome During Dental Caries, Revealing Novel Taxa and Co‐occurrences with Host Molecules.” Genome Research 31: 64–74. 10.1101/gr.265645.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Qi, He , Liu Defeng, Zou Yang, Wang Nan, Tian Han, and Xiao Chunling. 2021. “Description and Genomic Characterization of Streptococcus symci sp. nov., Isolated from a Child's Oropharynx.” Antonie Van Leeuwenhoek 114: 113–127. 10.1007/s10482-020-01505-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Beall Clifford, J. , Campbell Alisha G., Ann L. Griffen, Podar Mircea, Leys Eugene J., and Segata Nicola.“Genomics of the Uncultivated, Periodontitis‐Associated Bacterium Tannerella sp. BU045 (Oral Taxon 808).” mSystems 3: e00018. 10.1128/mSystems.00018-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yahara, Koji , Suzuki Masato, Hirabayashi Aki, Suda Wataru, Hattori Masahira, et al. 2021. “Long‐Read Metagenomics Using PromethION Uncovers Oral Bacteriophages and their Interaction with Host Bacteria.” Nature Communications 12: 27. 10.1038/s41467-020-20199-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee, K. , Walker A. R., Chakraborty B., Kaspar J. R., Nascimento M. M., Burne R. A., and Björkroth Johanna. 2019. “Novel Probiotic Mechanisms of the Oral Bacterium Streptococcus sp. A12 as Explored with Functional Genomics.” Applied and Environmental Microbiology 85: e01335−01319. 10.1128/AEM.01335-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yano, Yukiko , Hua Xing, Wan Yunhu, Suman Shalabh, Zhu Bin, Casey L. Dagnall, Hutchinson Amy, et al. “Comparison of Oral Microbiota Collected Using Multiple Methods and Recommendations for New Epidemiologic Studies.” mSystems 5: e00156−00120. 10.1128/mSystems.00156-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Marotz, Clarisse A. , Sanders Jon G., Zuniga Cristal, Zaramela Livia S., Knight Rob, and Zengler Karsten. 2018. “Improving Saliva Shotgun Metagenomics by Chemical host DNA Depletion.” Microbiome 6: 42. 10.1186/s40168-018-0426-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mukherjee, Chiranjit , Beall Clifford J., Griffen Ann L., and Leys Eugene J.. 2018. “High‐Resolution ISR Amplicon Sequencing Reveals Personalized Oral Microbiome.” Microbiome 6: 153. 10.1186/s40168-018-0535-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Org, Elin , Blum Yuna, Kasela Silva, Mehrabian Margarete, Kuusisto Johanna, Kangas Antti J., Soininen Pasi, et al. 2017. “Relationships Between Gut Microbiota, Plasma Metabolites, and Metabolic Syndrome Traits in the METSIM Cohort.” Genome Biology 18: 70. 10.1186/s13059-017-1194-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Carr, Victoria R. , Witherden Elizabeth A., Lee Sunjae, Shoaie Saeed, Mullany Peter, Proctor Gordon B., Gomez‐Cabrero David, et al. 2020. “Abundance and Diversity of Resistomes Differ Between Healthy Human Oral Cavities and Gut.” Nature Communications 11: 693. 10.1038/s41467-020-14422-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chowdhury, Shomeek , and Fong Stephen S.. 2020. “Computational Modeling of the Human Microbiome.” Microorganisms 8: 197. 10.3390/microorganisms8020197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Na, Hee S. , Si Y. Kim, Han Hyejung, Kim Hyun‐Joo, Lee Ju‐Youn, Lee Jae‐Hyung, and Chung Jin. 2020. “Identification of Potential Oral Microbial Biomarkers for the Diagnosis of Periodontitis.” Journal of Clinical Medicine 9: 1549. 10.3390/jcm9051549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Institute, The Forsyth . HOMINGS. March 31 2021. https://microbiome.forsyth.org
- 33. Mougeot, Jean‐Luc C. , Stevens Craig B., Cotton Sean L., Morton Darla S., Krishnan Keerthana, Brennan Michael T., Lockhart Peter B., et al. 2016. “Concordance of HOMIM and HOMINGS Technologies in the Microbiome Analysis of Clinical Samples.” Journal of Oral Microbiology 8: 30379. 10.3402/jom.v8.30379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Katy, Katy J. , Schwarzberg‐Lipson Karen, Garg Neha, Sean M. Gibbons, Caporaso J. Gregory, Slots Jørgen, and Cohen Chloe, et al. 2017. “Multi‐omics Analysis of Periodontal Pocket Microbial Communities Pre‐ and Posttreatment.” mSystems 2: e00016–e00017. 10.1128/mSystems.00016-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Utter, Daniel R. , Borisy Gary G., Murat Eren A., Cavanaugh Colleen M., Mark Welch Jessica L.. 2020. “Metapangenomics of the Oral Microbiome Provides Insights Into Habitat Adaptation and Cultivar Diversity.” Genome Biology 21: 293. 10.1186/s13059-020-02200-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Velsko, Irina M. , Fellows Yates James A., Aron Franziska, Hagan Richard W., Frantz Laurent A. F., Loe Louise, Rodriguez Martinez Juan Bautista, et al. 2019. “Microbial Differences Between Dental Plaque and Historic Dental Calculus are Related to Oral Biofilm Maturation Stage.” Microbiome 7: 102. 10.1186/s40168-019-0717-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ebersole, Jeffrey L. , Kirakodu Sreenatha S., and Gonzalez Octavio A.. 2021. “Oral Microbiome Interactions with Gingival Gene Expression Patterns for Apoptosis, Autophagy and Hypoxia Pathways in Progressing Periodontitis.” Immunology 162: 405−417. 10.1111/imm.13292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gopalakrishnan, Vancheswaran , Spencer Christine N., Nezi L., Reuben Alexandre, Andrews M. C., Karpinets T. V., Prieto P. A., et al. 2018. “Gut Microbiome Modulates Response to Anti–PD‐1 Immunotherapy in Melanoma Patients.” Science 359: 97–103. 10.1126/science.aan4236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chaudhari, Diptaraj S. , Dhotre Dhiraj P., Agarwal Dhiraj M., Gaike Akshay H., Bhalerao Devika, Jadhav Parmeshwar, Mongad Dattatray, et al. 2020. “Gut, Oral and Skin Microbiome of Indian Patrilineal Families Reveal Perceptible Association with Age.” Science Reports 10: 5685. 10.1038/s41598-020-62195-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Liu, Shili , Wang Yihua, Zhao Le, Sun Xiaoyuan, and Feng Qiang. 2020. “Microbiome Succession with Increasing Age in Three Oral Sites.” Aging 12: 7874–7907. 10.18632/aging.103108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gupta, Vinod K. , Paul Sandip, and Dutta Chitra. 2017. “Geography, Ethnicity or Subsistence‐Specific Variations in Human Microbiome Composition and Diversity.” Frontiers in Microbiology 81162. 10.3389/fmicb.2017.01162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Agnello, Melissa , Marques J., Cen L., Mittermuller B., Huang A., Chaichanasakul Tran N., Shi W., et al. 2017. “Microbiome Associated with Severe Caries in Canadian First Nations Children.” Journal of Dental Research 96: 1378–1385. 10.1177/0022034517718819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ganesan Sukirth, M. , Dabdoub Shareef M., Nagaraja Haikady N., Scott Michelle L., Pamulapati Surya, Berman Micah L., Shields Peter G., et al. “Adverse Effects of Electronic Cigarettes on the Disease‐naive Oral Microbiome.” Science Advances 6: eaaz0108. 10.1126/sciadv.aaz0108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wu, Jing , Peters Brandilyn A., Dominianni Christine, Zhang Yilong, Pei Zhiheng, Yang Liying, Ma Yingfei, et al. 2016. “Cigarette Smoking and the Oral Microbiome in a Large Study of American Adults.” The ISME Journal 10: 2435–2446. 10.1038/ismej.2016.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fan, Xiaozhou , Peters Brandilyn A., Jacobs Eric J., Gapstur Susan M., Purdue Mark P., Freedman Neal D., Alekseyenko Alexander V., et al. 2018. “Drinking Alcohol is Associated with Variation in the Human Oral Microbiome in a Large Study of American Adults.” Microbiome 6: 59. 10.1186/s40168-018-0448-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ames, Nancy J. , Barb Jennifer J., Schuebel Kornel, Mudra Sarah, Meeks Brianna K., Tuason Ralph Thadeus S., Brooks Alyssa T., et al. 2020. “Longitudinal Gut Microbiome Changes in Alcohol Use Disorder are Influenced By Abstinence and Drinking Quantity.” Gut Microbes 11: 1608−1631. 10.1080/19490976.2020.1758010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Willis, Jesse R. , González‐Torres Pedro, Pittis Alexandros A., Bejarano Luis A., Cozzuto Luca, Andreu‐Somavilla Nuria, Alloza‐Trabado Miriam, et al. 2018. “Citizen Science Charts Two Major “Stomatotypes” in the Oral Microbiome of Adolescents and Reveals Links With Habits and Drinking Water Composition.” Microbiome 6: 218. 10.1186/s40168-018-0592-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sinha, Rashmi , Zhao Ni, Goedert James J., Byrd Doratha A., Wan Yunhu, Hua Xing, Hullings Autumn G., et al. 2021. “Effects of Processed Meat and Drinking Water Nitrate on Oral and Fecal Microbial Populations in a Controlled Feeding Study.” Environmental Research 197: 111084. 10.1016/j.envres.2021.111084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hansen, Tue H. , Kern Timo, Bak Emilie G., Kashani Alireza, Allin Kristine H., Nielsen Trine, Hansen Torben, et al. 2018. “Impact of a Vegan Diet on the Human Salivary Microbiota.” Science Reports 8: 5847. 10.1038/s41598-018-24207-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Liu, Ke , Chen Siyu, Huang Jing, Ren Feihong, Yang Tingyu, Long Danfeng, Li Huan, et al. 2021. “Oral Microbiota of Children Is Conserved across Han, Tibetan and Hui Groups and Is Correlated with Diet and Gut Microbiota.” Microorganisms 9: 1030. 10.3390/microorganisms9051030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dzidic, Majda , Collado Maria C., Abrahamsson Thomas, Artacho Alejandro, Stensson Malin, Jenmalm Maria C., and Mira Alex. 2018. “Oral Microbiome Development During Childhood: An Ecological Succession Influenced by Postnatal Factors and Associated with Tooth Decay.” The ISME Journal 12: 2292–2306. 10.1038/s41396-018-0204-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Holgerson, Pernilla L. , Vestman Nelly R., Claesson Rolf, Ohman Carina, Domellöf Magnus, Tanner Anne C. R., Hernell Olle, et al. 2013. “Oral Microbial Profile Discriminates Breast‐fed From Formula‐fed Infants.” Journal of Pediatric Gastroenterology and Nutrition 56: 127–136. 10.1097/MPG.0b013e31826f2bc6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Džunková, Mária , Lipták Róbert, Vlková Barbora, Gardlík Roman, Čierny Michal, Moya Andrés, and Celec Peter. 2020. “Salivary Microbiome Composition Changes After Bariatric Surgery.” Science Reports 10: 20086. 10.1038/s41598-020-76991-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Freire, Marcelo , Nelson Karen E., and Edlund A.. 2021. “The Oral Host–Microbial Interactome: An Ecological Chronometer of Health? ” Trends in Microbiology 29: 551–561. 10.1016/j.tim.2020.11.004 [DOI] [PubMed] [Google Scholar]
- 55. Ferretti, Pamela , Pasolli Edoardo, Tett Adrian, Asnicar Francesco, Gorfer Valentina, Fedi Sabina, Armanini Federica, et al. 2018. “Mother‐to‐Infant Microbial Transmission from Different Body Sites Shapes the Developing Infant Gut Microbiome.” Cell Host Microbe 24(133‐145): e135. 10.1016/j.chom.2018.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wang, Jinfeng , Zheng Jiayong, Shi Wenyu, Du Nan, Xu Xiaomin, Zhang Yanming, Ji Peifeng, et al. 2018. “Dysbiosis of Maternal And Neonatal Microbiota Associated With Gestational Diabetes Mellitus.” Gut 67: 1614. 10.1136/gutjnl-2018-315988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Holmer, Jacob , Aho Velma, Eriksdotter Maria, Paulin Lars, Pietiäinen Milla, Auvinen Petri, Schultzberg Marianne, et al. 2021. “Subgingival Microbiota in a Population with and Without Cognitive Dysfunction.” Journal of Oral Microbiology 13: 1854552. 10.1080/20002297.2020.1854552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Fleury, Vanessa , Zekeridou Alkisti, Lazarevic Vladimir, Gaïa Nadia, Giannopoulou Catherine, Genton Laurence, Cancela José, et al. 2021. “Oral Dysbiosis and Inflammation in Parkinson's Disease.” Journal of Parkinson's Disease 11: 619–631. 10.3233/JPD-202459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Xu, Yajuan , Zhang Miao, Zhang Jingzhe, Sun Zongzong, Ran Limin, Ban Yanjie, Wang Biao, et al. 2019. “Differential Intestinal and Oral Microbiota Features Associated with Gestational Diabetes And Maternal Inflammation.” American Journal of Physiology‐Endocrinology and Metabolism 319: E247–E253. 10.1152/ajpendo.00266.2019 [DOI] [PubMed] [Google Scholar]
- 60. Bobetsis, Yiorgos A. , Graziani Filippo, Gürsoy Mervi, and Madianos Phoebus N.. 2020. “Periodontal Disease and Adverse Pregnancy Outcomes.” Periodontology 2000 83: 154−174. 10.1111/prd.12294 [DOI] [PubMed] [Google Scholar]
- 61. Pasolli, Edoardo , Asnicar Francesco, Manara Serena, Zolfo Moreno, Karcher Nicolai, Armanini Federica, Beghini Francesco, et al. 2019. “Extensive Unexplored Human Microbiome Diversity Revealed by Over 150,000 Genomes from Metagenomes Spanning Age, Geography, and Lifestyle.” Cell 176: 649–662. 10.1016/j.cell.2019.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Caselli, Elisabetta , Fabbri Chiara, D'Accolti Maria, Soffritti Irene, Bassi Cristian, Mazzacane Sante, and Franchi Maurizio. 2020. “Defining the Oral Microbiome by Whole‐genome Sequencing and Resistome Analysis: The Complexity of the Healthy Picture.” BMC Microbiology 20: 120. 10.1186/s12866-020-01801-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wang, Yuan , Wang Sa, Wu Chunyan, Chen Xi, Duan Zhuhui, Xu Qian, Jiang Wen, et al. 2019. “Oral Microbiome Alterations Associated with Early Childhood Caries Highlight the Importance of Carbohydrate Metabolic Activities.” mSystems 4: e0045000419. 10.1128/mSystems.00450-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Qian, Wenhao , Ma Ting, Ye Mao, Li Zhiyao, Liu Yuanhua, and Hao Pei. 2019. “Microbiota in the Apical Root Canal System of Tooth with Apical Periodontitis.” BMC Genomics 20: 189. 10.1186/s12864-019-5474-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Willis, Jesse R. , Iraola‐Guzmán Susana, Saus Ester, Ksiezopolska Ewa, Cozzuto Luca, Bejarano Luis A., and Andreu‐Somavilla Nuria, et al. 2021. “Oral Microbiome in Down Ayndrome and Its Implications on Oral Health.” Journal of Oral Microbiology 13: 1865690. 10.1080/20002297.2020.1865690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Proctor, Diana M. , Shelef Katie M., Gonzalez Antonio, Davis Clara L., Dethlefsen Les, Burns Adam R., Loomer Peter M., et al. 2020. “Microbial Biogeography and Ecology of the Mouth and implications forPeriodontal Diseases.” Periodontology 2000 82: 26–41. 10.1111/prd.12268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. van der Meulen, Taco A. , Harmsen Hermie J. M., Vich Vila Arnau, Kurilshikov Alexander, Liefers Silvia C., Zhernakova Alexandra, Fu Jingyuan, et al. 2019. “Shared Gut, But Distinct Oral Microbiota Composition in Primary Sjögren's Syndrome and Systemic Lupus Erythematosus.” J Autoimmun 97: 77−87. 10.1016/j.jaut.2018.10.009 [DOI] [PubMed] [Google Scholar]
- 68. Xu, He , Tian Jing, Hao Wenjing, Zhang Qian, Zhou Qiong, Shi Weihua, and Qin Man, et al. 2018. “Oral Microbiome Shifts From Caries‐Free to Caries‐Affected Status in 3‐Year‐Old Chinese Children: A Longitudinal Study.” Frontiers in Microbiology 9: 2009. 10.3389/fmicb.2018.02009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Rosier, B. T. , Marsh P. D., and Mira A.. 2017. “Resilience of the Oral Microbiota in Health: Mechanisms That Prevent Dysbiosis.” Journal of Dental Research 97: 371–380. 10.1177/0022034517742139 [DOI] [PubMed] [Google Scholar]
- 70. Wang, Jinfeng , Jia Zhen, Zhang Bing, Peng Lei, and Zhao Fangqing. 2020. “Tracing the Accumulation of in Vivo Human Oral Microbiota Elucidates Microbial Community Dynamics at The Gateway to the Gi Tract.” Gut 69: 1355. 10.1136/gutjnl-2019-318977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kirchner, Florian R. , and LeibundGut‐Landmann Salomé. 2021. “Tissue‐Resident Memory Th17 Cells Maintain Stable Fungal Commensalism in the Oral Mucosa.” Mucosal Immunology 14: 455–467. 10.1038/s41385-020-0327-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Xiao, Jin , Grier Alex, Faustoferri R. C., Alzoubi S., Gill Ann L., Feng C., Liu Y., et al. 2018. “Association between Oral Candida and Bacteriome in Children with Severe ECC.” Journal of Dental Research 97: 1468–1476. 10.1177/0022034518790941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Zhang, Shaoping , Yu Ning, Arce Roger M.. 2020. “Periodontal Inflammation: Integrating Genes and Dysbiosis.” Periodontology 2000 82: 129−142. 10.1111/prd.12267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Yang, Chia‐Yu , Yeh Yuan‐Ming, Yu Hai‐Ying, Chin Chia‐Yin, Hsu Chia‐Wei, Liu Hsuan, and Huang Po‐Jung. 2018. “Oral Microbiota Community Dynamics Associated with Oral Squamous Cell Carcinoma Staging.” Frontiers in Microbiology 9: 862. 10.3389/fmicb.2018.00862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Esberg, Anders , Haworth Simon, Hasslöf Pamela, Lif Holgerson Pernilla, and Johansson Ingegerd. 2020. “Oral Microbiota Profile Associates with Sugar Intake and Taste Preference Genes.” Nutrients 12: 681. 10.3390/nu12030681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Aleti, Gajender , Jonathon L. Baker, Tang Xiaoyu, Alvarez Ruth, Dinis Márcia, Nini C. Tran, Alexey V. Melnik, et al. “Identification of the Bacterial Biosynthetic Gene Clusters of the Oral Microbiome Illuminates the Unexplored Social Language of Bacteria during Health and Disease.” mBio 10: e00321−00319. 10.1128/mBio.00321-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Uranga Carla, C. , Arroyo Pablo, Brendan M. Duggan, William H. Gerwick, Edlund Anna, and David W. Cleary. “Commensal Oral Rothia mucilaginosa Produces Enterobactin, A Metal‐Chelating Siderophore.” mSystems 5: e00161−00120. 10.1128/mSystems.00161-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ram‐Mohan, Nikhil , and Meyer Michelle M.. 2020. “Comparative Metatranscriptomics of Periodontitis Supports a Common Polymicrobial Shift in Metabolic Function and Identifies Novel Putative Disease‐Associated ncRNAs.” Frontiers in Microbiology 11: 482. 10.3389/fmicb.2020.00482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Belibasakis, Georgios N. , and Hajishengallis George. 2019. “Advances in Oral Mucosal Immunity and the Microbiome.” Oral Mucosal Immunity and Microbiome 1197: 1–9. [DOI] [PubMed] [Google Scholar]
- 80. Kinane, Denis F. , Stathopoulou Panagiota G., and Papapanou Panos N.. 2017. “Periodontal Diseases.” Nature Reviews Disease Primers 3: 17038. 10.1038/nrdp.2017.38 [DOI] [PubMed] [Google Scholar]
- 81. Han, Yiping W. , and Wang Xiaowei. 2013. “Mobile Microbiome: Oral Bacteria in Extra‐Oral Infections and Inflammation.” Journal of Dental Research 92: 485–491. 10.1177/0022034513487559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Moentadj, Rabia , Wang Yiwen, Bowerman Kate, Rehaume Linda, Nel Hendrik, Cuiv Paraic O, Stephens Juliette, et al. 2021. “ Streptococcus species Enriched in the Oral Cavity of Patients With Ra are a Source of Peptidoglycan‐Polysaccharide Polymers that can Induce Arthritis In Mice.” Annals of the Rheumatic Diseases 80: 573. 10.1136/annrheumdis-2020-219009 [DOI] [PubMed] [Google Scholar]
- 83. Tilg, Herbert , Cani Patrice D., and Mayer Emeran A.. 2016. “Gut Microbiome and Liver Diseases.” Gut 65: 2035. 10.1136/gutjnl-2016-312729 [DOI] [PubMed] [Google Scholar]
- 84. Argollo, Marjorie , Gilardi Daniela, Peyrin‐Biroulet Carina, Chabot Jean‐Francois, Peyrin‐Biroulet Laurent, and Danese Silvio. 2019. “Comorbidities in Inflammatory Bowel Disease: A Call for Action.” The Lancet Gastroenterology & Hepatology 4: 643−654. 10.1016/S2468-1253(19)30173-6 [DOI] [PubMed] [Google Scholar]
- 85. Read, Emily , Curtis Michael A., and Neves Joana F.. 2021. “The Role of Oral Bacteria in Inflammatory Bowel Disease.” Nature Reviews Gastroenterology & Hepatology 18: 731−742. 10.1038/s41575-021-00488-4 [DOI] [PubMed] [Google Scholar]
- 86. Dutzan, Nicolas , Kajikawa Tetsuhiro, Abusleme Loreto, Greenwell‐Wild Teresa, Zuazo Carlos E., Ikeuchi Tomoko, and Brenchley Laurie, et al. 2018. “A Dysbiotic Microbiome Triggers TH17 Cells to Mediate Oral Mucosal Immunopathology In Mice and Humans.” Science Translational Medicine 10: eaat0797. 10.1126/scitranslmed.aat0797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Atarashi, Koji , Suda Wataru, Luo Chengwei, Kawaguchi Takaaki, Motoo Iori, Narushima Seiko, Kiguchi Yuya, et al. 2017. “Ectopic Colonization Of Oral Bacteria in the Intestine Drives TH1 Cell Induction and Inflammation.” Science 358: 359–365. 10.1126/science.aan4526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Schmidt, Thomas Sb , Hayward Matthew R., Coelho Luis P., Li Simone S., Costea Paul I., Voigt Anita Y., Wirbel Jakob, et al. 2019. “Extensive Transmission of Microbes Along the Gastrointestinal Tract.” Elife 8: e42693. 10.7554/eLife.42693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Abed, Jawad , Maalouf Naseem, Manson Abigail L., Earl Ashlee M., Parhi Lishay, Emgård Johanna E. M., and Klutstein Michael. 2020. “Colon Cancer‐Associated Fusobacterium nucleatum May Originate From the Oral Cavity and Reach Colon Tumors via the Circulatory System.” Frontiers in Cellular and Infection Microbiology 10: 400. 10.3389/fcimb.2020.00400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Yost, Susan , Stashenko Philip, Choi Yoonhee, Kukuruzinska Maria, Genco Caroline A., Salama Andrew, and Weinberg Ellen O., et al. 2018. “Increased Virulence of the Oral Microbiome in Oral Squamous Cell Carcinoma Revealed by Metatranscriptome Analyses.” International Journal of Oral Science 10: 32. 10.1038/s41368-018-0037-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Boot, Arnoud , Ng Alvin W. T., Chong Fui Teen, Ho Szu‐Chi, Yu Willie, Tan Daniel S. W., Gopalakrishna Iyer N., et al. 2020. “Characterization of Colibactin‐Associated Mutational Signature in an Asian Oral Squamous Cell Carcinoma and in Other Mucosal Tumor Types.” Genome Research 30: 803−813. 10.1101/gr.255620.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Su, Shih‐Chi , Chang Lun‐Ching, Huang Hsien‐Da, Peng Chih‐Yu, Chuang Chun‐Yi, Chen Yi‐Tzu, Lu Ming‐Yi, et al. 2021. “Oral Microbial Dysbiosis and its Performance in Predicting Oral Cancer.” Carcinogenesis 42: 127–135. 10.1093/carcin/bgaa062 [DOI] [PubMed] [Google Scholar]
- 93. Al‐hebshi, Nezar Noor , Nasher Akram Thabet, Maryoud Mohamed Yousef, Homeida Husham E., Chen Tsute, Idris Ali Mohamed, and Johnson Newell W.. 2017. “Inflammatory Bacteriome Featuring Fusobacterium nucleatum and Pseudomonas aeruginosa Identified in Association with Oral Squamous Cell Carcinoma.” Science Reports 7: 1834. 10.1038/s41598-017-02079-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Komiya, Yasuhiko , Shimomura Yumi, Higurashi Takuma, Sugi Yutaka, Arimoto Jun, Umezawa Shotaro, Uchiyama Shiori, et al. 2019. “Patients with Colorectal Cancer Have Identical Strains of Fusobacterium nucleatum in Their Colorectal Cancer and Oral Cavity.” Gut 68: 1335. 10.1136/gutjnl-2018-316661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Yu, Jun , Feng Qiang, Wong Sunny Hei, Zhang Dongya, Liang Qiao yi, Qin Youwen, Tang Longqing, et al. 2017. “Metagenomic Analysis of Faecal Microbiome as a Tool Towards Targeted Non‐invasive Biomarkers For Colorectal Cancer.” Gut 66: 70. 10.1136/gutjnl-2015-309800 [DOI] [PubMed] [Google Scholar]
- 96. Fan, Xiaozhou , Alekseyenko Alexander V., Wu Jing, Peters Brandilyn A., Jacobs Eric J., Gapstur Susan M., Purdue Mark P., et al. 2018. “Human oral microbiome and prospective risk for pancreatic cancer: a population‐based nested case‐control study.” Gut 67: 120. 10.1136/gutjnl-2016-312580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Olson, Sara H. , Satagopan Jaya, Xu Youming, Ling Lilan, Leong Siok, Orlow Irene, Saldia Amethyst, et al. 2017. “The Oral Microbiota in Patients with Pancreatic Cancer, Patients with IPMNS, and Controls: A Pilot Study.” Cancer Causes & Control 28: 959–969. 10.1007/s10552-017-0933-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Yan, Xinmin , Yang Mingxia, Liu Juan, Gao Ruichen, Hu Jihong, Li Jiong, Zhang Lijun, et al. 2015. “Discovery and Validation of Potential Bacterial Biomarkers For Lung Cancer.” American Journal of Cancer Research 5: 3111–3122. https://pubmed.ncbi.nlm.nih.gov/26693063https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4656734/ [PMC free article] [PubMed] [Google Scholar]
- 99. Hosgood, H. Dean , Cai Qiuyin, Hua Xing, Long Jirong, Shi Jianxin, Wan Yunhu, Yang Yaohua, et al. 2021. “Variation in Oral Microbiome is Associated with Future Risk of Lung Cancer Among Never‐Smokers.” Thorax 76: 256. 10.1136/thoraxjnl-2020-215542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Yang, Junjie , Mu Xiaofeng, Wang Ye, Zhu Dequan, Zhang Jiaming, Liang Cheng, and Chen Bin, et al. 2018. “Dysbiosis of the Salivary Microbiome Is Associated With Non‐smoking Female Lung Cancer and Correlated With Immunocytochemistry Markers.” Frontiers in Oncology 8: 520. 10.3389/fonc.2018.00520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Wang, Qian , Rao Yuting, Guo Xiaobing, Liu Na, Liu Shuxiu, Wen Peipei, Li Shuang, et al. 2019. “Oral Microbiome in Patients with Oesophageal Squamous Cell Carcinoma.” Science Reports 9: 19055. 10.1038/s41598-019-55667-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Peters, Brandilyn A. , Wu Jing, Pei Zhiheng, Yang Liying, Purdue Mark P., Freedman Neal D., Jacobs Eric J., et al. 2017. “Oral Microbiome Composition Reflects Prospective Risk for Esophageal Cancers.” Cancer Research 77: 6777–6787. 10.1158/0008-5472.CAN-17-1296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Yamamura, Kensuke , Baba Yoshifumi, Nakagawa Shigeki, Mima Kosuke, Miyake Keisuke, Nakamura Kenichi, Sawayama Hiroshi, et al. 2016. “Human Microbiome Fusobacterium Nucleatum in Esophageal Cancer Tissue is Associated with Prognosis.” Clinical Cancer Research 22: 5574–5581. 10.1158/1078-0432.CCR-16-1786 [DOI] [PubMed] [Google Scholar]
- 104. Freire, Marcelo , Moustafa Ahmed, Harkins Derek M., Torralba Manolito G., Zhang Yun, Leong Pamela, Saffery Richard, et al. 2020. “Longitudinal Study of Oral Microbiome Variation in Twins.” Science Reports 10: 7954. 10.1038/s41598-020-64747-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Surana, Neeraj K. , and Kasper Dennis L.. 2017. “Moving Beyond Microbiome‐wide Associations To Causal Microbe Identification.” Nature 552: 244–247. 10.1038/nature25019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Sanna, Serena , van Zuydam Natalie R., Mahajan Anubha, Kurilshikov Alexander, Vich Vila Arnau, Võsa Urmo, Mujagic Zlatan, et al. 2019. “Causal Relationships Among the Gut Microbiome, Short‐chain Fatty Acids and Metabolic Diseases.” Nat Genet 51: 600−605. 10.1038/s41588-019-0350-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Zhuang, Zhenhuang , Gao Meng, Yang Ruotong, Liu Zhonghua, Cao Weihua, and Huang Tao. 2021. “Causal Relationships Between Gut Metabolites and Alzheimer's Disease: A Bidirectional Mendelian Randomization Study.” Neurobiology of Aging 100: 119.e115–119.e118. 10.1016/j.neurobiolaging.2020.10.022 [DOI] [PubMed] [Google Scholar]
- 108. Galloway‐Peña, Jessica R. , Smith Daniel P., Sahasrabhojane Pranoti, Ajami Nadim J., Wadsworth W. Duncan, Daver Naval G., Chemaly Roy F., et al. 2016. “The Role of the Gastrointestinal Microbiome in Infectious Complications During Induction Chemotherapy for Acute Myeloid Leukemia.” Cancer 122: 2186–2196. 10.1002/cncr.30039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Aitken, Samuel L. , Sahasrabhojane Pranoti V., Kontoyiannis Dimitrios P., Savidge Tor C., Arias Cesar A., Ajami Nadim J., Shelburne Samuel A., et al. 2021. “Alterations of the Oral Microbiome and Cumulative Carbapenem Exposure are Associated with Stenotrophomonas maltophilia Infection in Patients With Acute Myeloid Leukemia Receiving Chemotherapy.” Clinical Infectious Diseases 72: 1507–1513. 10.1093/cid/ciaa778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Reyes‐Gibby, Cielito C. , Wang Jian, Zhang Liangliang, Peterson Christine B., Do Kim‐Anh, Jenq Robert R., Shelburne Samuel, et al. 2020. “Oral Microbiome and Onset of Oral Mucositis in Patients with Squamous Cell Carcinoma of the Head and Neck.” Cancer 126: 5124–5136. 10.1002/cncr.33161 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study. Supplementary information (graphical abstract, slides, videos, Chinese translated version, and updated materials) are available online at DOI or http://www.imeta.science/.


