Abstract
The COVID-19 pandemic caused by the novel severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2), has emerged as a global public health concern and its sequels have barely started to outcrop. A good percentage of patients who suffered from COVID-19 are prone to develop long-COVID or post-COVID condition (PCC), a multisystemic, heterogeneous, chronic disorder. Patients with PCC may experience diverse manifestations, of which cardiovascular and neurological symptoms are among the most frequently reported. Indeed, dysautonomia presented as orthostatic intolerance has gained room following recent reports linking postural orthostatic tachycardia syndrome (POTS) with PCC. Disturbances in heart rate (HR) and blood pressure (BP) during postural changes are the cornerstones of orthostatic intolerance seen in patients suffering from PCC. A subtype of POTS, hyperadrenergic POTS, has been widely studied because of its association with mast cell activation syndrome (MCAS). Although a causative relationship between PCC, hyperadrenergic POTS, and MCAS remains unrevealed, these syndromes can overlap. We want to propose here a correlation produced by a close-loop mechanism with positive feedback established after SARS-CoV-2 infection in a previously healthy young patient.
Keywords: antihistamines, hyperadrenergic postural orthostatic tachycardia, hypertension, long COVID, mast cell activation syndrome, neuroinflammation, tachycardia
INTRODUCTION
Following remission of acute coronavirus disease 2019 (COVID-19), some patients may suffer from either a cluster of new symptoms or worsening of previous conditions, developing a syndrome known as post-COVID-19 condition (PCC). PCC (or long COVID) is defined as the persistence or appearance of new symptoms 3 months after the onset of COVID-19 with at least 2 months of duration [1]. Risk factors for PCC constitute: sex (female), age (>40 years), BMI (>30 kg/m2), and smoking. Notably, obesity and smoking represent the highest risk as both promote a generalized proinflammatory state, leading to multisystemic manifestations and their consequences [2].
PCC is a multisystemic disorder that can manifest heterogeneously. Symptoms may range from nonspecific (e.g. malaise, fatigue, discomfort) to well described symptoms (e.g. palpitations, dyspnea, diarrhea, rash, etc.). Particularly, the cardiovascular and neurological systems are among the most altered [3].
To some extent, PCC manifestations could be explained by autonomic dysfunction [4], a condition that has gained recognition because of the increasing incidence of orthostatic intolerance symptoms in these patients. Orthostatic intolerance includes predominantly: orthostatic hypotension and postural orthostatic tachycardia syndrome (POTS). Precisely, one of the most common symptoms associated with PCC is tachycardia, reported in 25–50% of patients, which could present as POTS [5].
POTS is characterized by an increase in more than 30 bpm in heart rate (HR) during orthostatism without a drop in blood pressure (BP) for at least 6 months of duration. It is further divided into hyperadrenergic, neuropathic, and hypovolemic POTS [6]. Hyperadrenergic POTS is defined as an increase greater than 30 bpm in HR when standing up, with a greater than 10 mmHg increase in SBP and the presence of elevated serum norepinephrine levels (>600 pg/ml) [7].
The pathophysiology of POTS remains incompletely understood, although several theories have been suggested. It has been proposed that mast cell activation syndrome (MCAS) may be present in hyperadrenergic POTS [8], as they share many symptoms and both have been found in postinfection viral syndromes [9].
Mast cells are myeloid-derived immune cells located in mucosal and connective tissues all over the body. They are capable of reacting to various stimuli, either endogenous or exogenous, because of the broad variety of receptors expressed on their surface [9]. MCAS refers to the clinical entity characterized by recurrent mast cell activation, in which mast cell mediators are released more abundantly and more frequently [10].
MCAS can be established when: typical symptoms arising from recurrent, acute, multiorgan mast cell activation are documented; mast cell mediators are increased in serum or urine from baseline; and remission of symptoms with pharmacological blockage of mast cell activation, mast cell mediators, mediator production, or their effects is achieved [11].
A causative connection between them remained hidden. To our knowledge, this is the first case where PCC, POTS, and MCAS converge at the same time, which opens up room for further investigations to fully understand the association of these entities.
CASE PRESENTATION
A previously healthy 26-year-old man was studied following 1 year of symptoms characterized by palpitations, presyncope, fatigue, postural dizziness, and brain fog, which began in March 2022, 2 months after recovery from mild COVID-19.
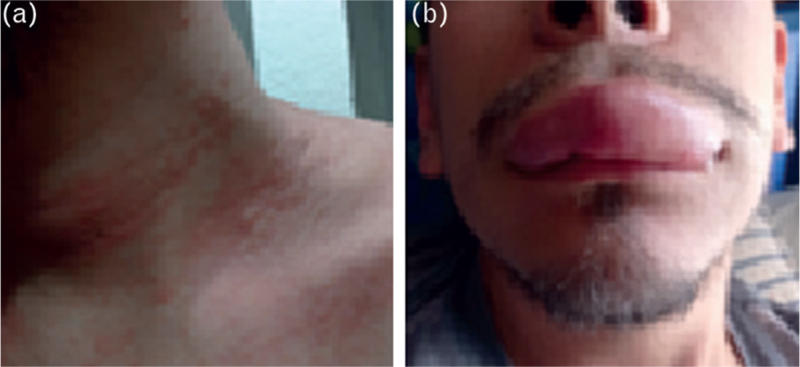
At the beginning, symptoms were intermittent and gradually progressed in severity throughout the months. In October 2022, subsequent to a presyncopal episode, he noticed for the first time the sudden onset of a generalized, pruritic, maculopapular rash, which remitted completely after self-medicating with chloropyramine, without seeking further medical attention. A few months later, the patient reported the appearance of angioedema after a presyncopal episode (Fig. 1). Various episodes of rash and angioedema occurred in the upcoming months, which appeared after orthostatic intolerance symptoms.
FIGURE 1.
Clinical manifestations registered by the patient, showing the first episode of hives (a) and angioedema (b), which appeared following an episode of orthostatic intolerance and palpitations on different occasions.
Shortly thereafter, gastrointestinal symptoms (diarrhea, abdominal pain, and nausea) began, followed by anxiety episodes, migraines, and sleep disturbances. Several diagnoses were considered, and diverse treatments were provided (including amiodarone, beta-blockers, and diphenidol) without any improvement.
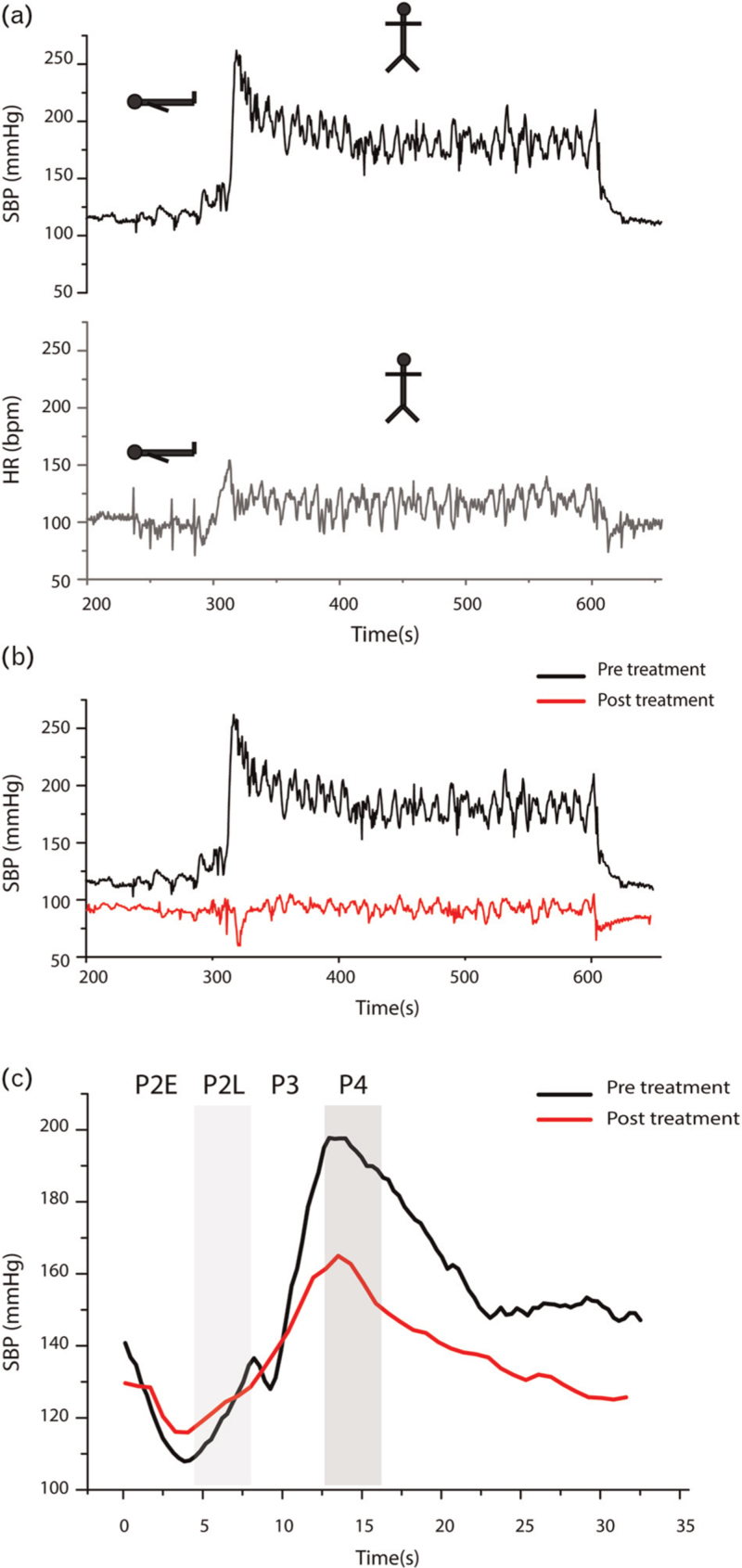
Finally, he was enrolled in the long-COVID follow-up program at the Instituto Nacional de Cardiología Ignacio Chavez, where a Head-Up tilt test (HUTT) (Fig. 2a) was performed, showing orthostatic tachycardia (from 85 bpm in supine to 120 bpm on standing) and hypertension (from 119 to 183 mmHg, a delta change of 33.7%), suggesting hyperadrenergic POTS with orthostatic hypertension.
FIGURE 2.
(a) First HUTT performed showing marked increase in SBP (in black) during orthostatism reaching more than 250 mmHg at the moment of standing-up (1) and maintaining an average of 180 mmHg (2) during orthostatism. There is also an increase in heart rate (HR) (in red) at the moment of standing-up (3) reaching almost 150 bpm and maintaining an average of 120 bpm during orthostatism (4). Both SBP and HR decreases when the patient changes to supine position (5). (b) Comparison between the pretreatment HUTT (in black) and the posttreatment HUTT (in green), performed 2 weeks after initiation of antihistamine. There is no increase at the moment of standing-up in the post-Tx HUTT (1) compared with the increase in the pre-Tx HUTT (2). During orthostatism, SBP maintains around 90–100 mmHg (3), compared with the increase showed in the pre-Tx HUTT (4). (c) Comparison between the changes in SBP during the Valsalva maneuver, showing significant reduction in SBP during the phase II-early and diminished overshoot in the phase IV in the post-Tx (in red) compared with the pre-Tx (in black) phase II-early and the overshoot in the phase IV.
Treatment with beta-blockers was started, which markedly worsened symptoms of presyncope, and was therefore discontinued. The patient was referred for further evaluation to the Autonomic Nervous System Laboratory at the Instituto Nacional de Ciencias Médicas y Nutrición, where MCAS was suspected based on a medical history of rash in accordance with orthostatic intolerance symptoms. A trial with a histamine blocker (Loratadine 10 mg/12 h) was initiated, achieving remission of symptoms after a few weeks. A HUTT was repeated, showing a significant reduction in blood pressure (Fig. 2b) on standing and during phases II-late and IV of the Valsalva maneuver (Fig. 2c) in comparison with the previous HUTT (Supplemental data file).
DISCUSSION
The physiopathology of PCC is not completely disclosed. Immune system misregulation could be a potential cause attributable to a switch from a pro-inflammatory state to a counterbalanced anti-inflammatory response, resulting in an immunosuppressive state in the post-COVID period [12]. Nevertheless, patients with mild to moderate and even asymptomatic COVID-19 have been identified to develop PCC, suggesting that misregulation of the immune system can emerge without clinical manifestations during acute COVID-19. Still, patients who had severe COVID-19 are more likely to develop PCC [2].
Diverse immune cells play crucial roles during and after viral infections, including mast cells. Mast cells can recognize SARS-CoV-2 through various pathways (e.g. the Toll-Like Receptor), and also express the constitutive receptor for SARS-CoV-2, the angiotensin-converting enzyme type 2 (ACE 2), [13]. Recently, it has been demonstrated that activation of mast cell is possible by direct binding to the spike protein of SARS-CoV-2 [14]. These mechanisms suggest an additive interaction with mast cells, which could drive that COVID-19, either acute or long, could be boosted because of mast cell activation.
SARS-CoV-2 can infect the central nervous system (CNS) using multiple pathways: olfactory nerve infiltration; haematogenous dissemination; transynaptically; and through the choroid plexus of the ventricular system [15]. Interestingly, the ACE-2 receptor is richly expressed in the brainstem, making it susceptible to viral invasion [16].
Matschke et al. showed in postmortem studies that the brainstem is a main target of the virus within the central nervous system. They found gliosis and astrogliosis in a patchy pattern near the nuclei that control sympathetic activity in patients deceased by COVID-19 [17]. Malfunction of these nuclei could give rise to sympathetic hyperactivity, producing HR and SBP disturbances during postural changes, as could be seen in hyperadrenergic POTS [7].
Sympathetic activity releases norepinephrine and substance P, among other substances, through sympathetic nerve endings, which share notable proximity to the mast cells [18]. Mast cells express different receptors, including adrenergic receptors and the recently discovered Mas-related G protein-coupled receptor X2 (MGRPRX2), which has been found to transduce mast cell activation through endogenous molecules, including substance P [19]. As a result, sympathetic release of NE and substance P can be sensed by mast cells, leading to direct interaction between mast cells and sympathetic nerves.
Mast cell degranulation evokes the liberation of diverse prestored mast cell mediators (e.g. histamine, bradykinin, tryptase) responsible for the multisystemic manifestations of MCAS, ranging from dermatologic (e.g. rash, angioedema), gastrointestinal (e.g. diarrhea), respiratory (e.g. bronchoconstriction), and cardiovascular (e.g. presyncope, palpitations) symptoms [13]. Mast cell implication in hyperadrenergic POTS can be explained predominantly by histamine effects, which is a strong vasodilator, as follows: upon vasodilation, blood redistribution to interstitial and third spaces occurs, diminishing venous return and cardiac output, which is sensed by high-pressure receptors within the arterial wall of the carotid and aorta, causing increased baroreflex activation and enhancing sympathetic activity through the blood vessels in order to re-stablish hemodynamic stability [8].
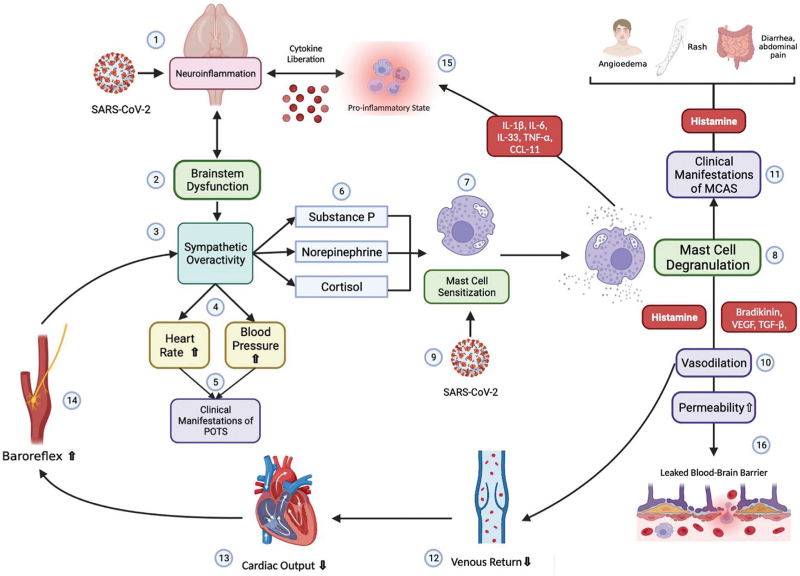
Hence, mast cell and sympathetic activity could create a closed-loop mechanism with positive feedback, as exemplified by the gradually progressing symptoms reported by patients. The more the mast cell is activated, the more sympathetic hyperactivity is produced, and vice versa (Fig. 3).
FIGURE 3.
Pathophysiology proposed modified from Shibao et al. The interaction of SARS-CoV-2 directly in the brainstem (1) could evoke an inflammatory response, causing brainstem dysfunction (2) manifested with sympathetic overactivity (3). Sympathetic activity will increase heart rate and blood pressure (4) giving place to clinical manifestations of POTS (5). Substance P, norepinephrine and cortisol will be released (6), sensitizing mast cells (7) and causing mast cell degranulation (8). In addition, direct interaction between SARS-CoV-2 and mast cells could enhance sensitization (9). Mast cell mediators (histamine, bradykinin, VEGF and TGF-β) will produce vasodilation (10) and clinical manifestations typical from MCAS (11). Vasodilation will provoke diminished venous return (12) and cardiac output (13), which will be sensed by the baroreceptors within the aorta and carotid artery, activating the baroreflex (14) resulting in more sympathetic activity (3). Mast cell mediators will potentiate a pro-inflammatory state (15) which along with augmented permeability, that can leak the blood–brain barrier (16), can perpetuate brainstem dysfunction (2).
Post-COVID condition treatment can be challenging because of the various manifestations presented from patient to patient. Moreover, there is not a standardized pharmacological approach that can be beneficial for all patients. Thus, symptomatic treatment along with nonpharmacological interventions (education, physiological support, physical activity, balanced diet, and rehabilitation) are the basis of the PCC treatment until now [18].
Similarly, treatment for POTS has targeted the symptoms rather than the cause. There have been several case reports suggesting diverse treatments, such as beta-blockers, alpha-blockers, fludrocortisone, ivabradine, midodrine, and methylphenidate, among others [20]. Nonetheless, it has been difficult to standardize treatment given POTS heterogeneity, comorbidities, and potential side effects of medications.
There are multiple studies evaluating the effect of mast cell-targeted therapy in PCC and POTS patients, including mast cell stabilizers (e.g. sodium cromoglycate), mast cell activation blockages (e.g. antihistamines), and mast cell mediator's blockers (e.g. antihistamines, antileukotryens) [13]. A specific group of patients suffering from hyperadrenergic POTS and MCAS in the PCC context can benefit from these pharmacological therapies. More studies are needed to determine the concrete treatment approach for these patients.
ACKNOWLEDGEMENTS
Conflicts of interest
There are no conflicts of interest.
Supplementary Material
Footnotes
Abbreviations: ACE 2, angiotensin-converting enzyme type 2; BP, blood pressure; CNS, central nervous system; COVID-19, coronavirus disease 2019; HR, heart rate; HUTT, head-up tilt test; long COVID, long coronavirus disease; MCAS, mast cell activation syndrome; MGRPRX2, Mas-related G protein-coupled receptor X2; PCC, post-COVID condition; POTS, postural orthostatic tachycardia syndrome; SARS-CoV 2, severe acute respiratory syndrome coronavirus type 2
Supplemental digital content is available for this article.
REFERENCES
- 1.Soriano JB, Murthy S, Marshall JC, Relan P, Diaz J. WHO Clinical Case Definition Working Group on Post-COVID-19 Condition. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis 2022; 22:e102–e107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsampasian V, Elghazaly H, Chattopadhyay R, Debski M, Naing TKP, Garg P, et al. Risk factors associated with post-COVID-19 condition: a systematic review and meta-analysis. JAMA Intern Med 2023; 183:566–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, et al. Postacute COVID-19 syndrome. Nat Med 2021; 27:601–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davis HE, McCorkell L, Vogel JM, Topol EJ. Author correction: long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol 2023; 21:408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ståhlberg M, Reistam U, Fedorowski A, Villacorta H, Horiuchi Y, Bax J, et al. Post-COVID-19 tachycardia syndrome: a distinct phenotype of post-acute COVID-19 syndrome. Am J Med 2021; 134:1451–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vernino S, Bourne KM, Stiles LE, Grubb BP, Fedorowski A, Stewart JM, et al. Postural orthostatic tachycardia syndrome (POTS): state of the science and clinical care from a 2019 National Institutes of Health Expert Consensus Meeting - Part 1. Auton Neurosci 2021; 235:102828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kanjwal K, Saeed B, Karabin B, Kanjwal Y, Grubb BP. Clinical presentation and management of patients with hyperadrenergic postural orthostatic tachycardia syndrome. A single center experience. Cardiol J 2011; 18:527–531. [DOI] [PubMed] [Google Scholar]
- 8.Shibao C, Arzubiaga C, Roberts LJ, 2nd, Raj S, Black B, Harris P, et al. Hyperadrenergic postural tachycardia syndrome in mast cell activation disorders. Hypertension 2005; 45:385–390. [DOI] [PubMed] [Google Scholar]
- 9.Arun S, Storan A, Myers B. Mast cell activation syndrome and the link with long COVID. Br J Hosp Med (Lond) 2022; 83:1–10. [DOI] [PubMed] [Google Scholar]
- 10.Valent P, Akin C, Nedoszytko B, Bonadonna P, Hartmann K, Niedoszytko M, et al. Diagnosis, classification and management of mast cell activation syndromes (MCAS) in the era of personalized medicine. Int J Mol Sci 2020; 21:9030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valent P, Hartmann K, Bonadonna P, Niedoszytko M, Triggiani M, Arock M, Brockow K. Mast cell activation syndromes: Collegium Internationale Allergologicum Update 2022. Int Arch Allergy Immunol 2022; 183:693–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Batiha GE, Al-Kuraishy HM, Al-Gareeb AI, Welson NN. Pathophysiology of post-COVID syndromes: a new perspective. Virol J 2022; 19:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Afrin LB, Weinstock LB, Molderings GJ. Covid-19 hyperinflammation and post-Covid-19 illness may be rooted in mast cell activation syndrome. Int J Infect Dis 2020; 100:327–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Theoharides TC, Kempuraj D. Role of SARS-CoV-2 spike-protein-induced activation of microglia and mast cells in the pathogenesis of neuro-COVID. Cells 2023; 12:688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bauer L, Laksono BM, de Vrij FMS, Kushner SA, Harschnitz O, van Riel D. The neuroinvasiveness, neurotropism, and neurovirulence of SARS-CoV-2. Trends Neurosci 2022; 45:358–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lukiw WJ, Pogue A, Hill JM. SARS-CoV-2 infectivity and neurological targets in the brain. Cell Mol Neurobiol 2022; 42:217–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matschke J, Lütgehetmann M, Hagel C, Sperhake JP, Schröder AS, Edler C, et al. Neuropathology of patients with COVID-19 in Germany: a postmortem case series. Lancet Neurol 2020; 19:919–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yong SJ. Long COVID or post-COVID-19 syndrome: putative pathophysiology, risk factors, and treatments. Infect Dis (Lond) 2021; 53:737–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Subramanian H, Gupta K, Ali H. Roles of Mas-related G protein-coupled receptor X2 on mast cell-mediated host defense, pseudoallergic drug reactions, and chronic inflammatory diseases. J Allergy Clin Immunol 2016; 138:700–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mallick D, Goyal L, Chourasia P, Zapata MR, Yashi K, Surani S. COVID-19 induced postural orthostatic tachycardia syndrome (POTS): a review. Cureus 2023; 15:e36955. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.