Significance
This work describes a general approach of anchoring earth-abundant metal single atoms to improve the battery performance of covalent organic framework (COF) electrodes. Single atoms induce pronounced electronic interaction with COF, which activates the COF for ion intercalation. The assembled Li and K ion batteries display enhanced capacity and rate performance. Extensive characterizations confirm the multiple positive effects of single atoms including modulation of the COF bandgap structure, optimization of binding affinity between Li/K ions and active sites, and acceleration of the electrochemical reaction kinetics. This work provides a different route to engineer organic electrodes for metal-ion batteries.
Keywords: covalent organic frameworks, lithium-ion batteries, metal single atoms, electronic metal-support interaction, potassium-ion batteries
Abstract
Organic electrodes mainly consisting of C, O, H, and N are promising candidates for advanced batteries. However, the sluggish ionic and electronic conductivity limit the full play of their high theoretical capacities. Here, we integrate the idea of metal-support interaction in single-atom catalysts with π–d hybridization into the design of organic electrode materials for the applications of lithium (LIBs) and potassium-ion batteries (PIBs). Several types of transition metal single atoms (e.g., Co, Ni, Fe) with π–d hybridization are incorporated into the semiconducting covalent organic framework (COF) composite. Single atoms favorably modify the energy band structure and improve the electronic conductivity of COF. More importantly, the electronic interaction between single atoms and COF adjusts the binding affinity and modifies ion traffic between Li/K ions and the active organic units of COFs as evidenced by extensive in situ and ex situ characterizations and theoretical calculations. The corresponding LIB achieves a high reversible capacity of 1,023.0 mA h g−1 after 100 cycles at 100 mA g−1 and 501.1 mA h g−1 after 500 cycles at 1,000 mA g−1. The corresponding PIB delivers a high reversible capacity of 449.0 mA h g−1 at 100 mA g−1 after 150 cycles and stably cycled over 500 cycles at 1,000 mA g−1. This work provides a promising route to engineering organic electrodes.
Human society is at the dawn of the energy transition from fossil fuel to renewable electricity. Lithium-ion batteries (LIBs) as portable power sources currently take a lion’s share and are expected to seize a vital role in distributed energy storage stations (1–5). Meanwhile, there is increasing interest in developing batteries based on other alkali metals (i.e., Na, K) with low cost and high crust abundance. Nonetheless, the large ionic radius (e.g., K+, 1.33 Å) causes serious volume changes at the anode during the charge/discharge process, resulting in low reversible capacities and inferior cycling stability. To date, various carbon materials such as graphite, graphene, carbon nanotube, and soft and hard carbons have been reported as anode materials in sodium and potassium ion batteries (SIBs, PIBs) (6–10). However, these carbon-based electrodes were obtained mainly by post-modifying carbons or pyrolysis of porous polymers, which is difficult to yield controllable heteroatom-doping at the atomic level and porous structure. Recently, organic electrodes have been receiving more attention for storing alkali ions. Due to their attractive properties, including low mass density, well-defined structures, and the tunable highest occupied molecular orbital (HOMO) or the lowest unoccupied molecular orbital (LUMO) energetics, it is easy to regulate at the molecular level (11–16). Nonetheless, organic electrode materials have several critical drawbacks including inferior electronic conductivity, dissolution in organic electrolytes, and inefficiency of redox-active site utilization, which limit the full play of their high theoretical capacities.
Covalent organic frameworks (COFs) are crystalline polymers with a regular porous organic framework, which can be constructed with atomic precision (17–21). COFs are ideal for electrochemical energy storage devices due to several advantages. First, COFs are insoluble and maintain structural integrity in most organic electrolytes under polarized conditions. Second, COFs have ordered porous channels that are accessible for ion transportation. Third, the porous skeleton of COF can be easily incorporated with functional groups owning alkali-ion storage capacity (22–26). To date, electronic structure engineering has emerged as an effective route for enhancing battery capacity. Past research mainly focused on introducing new organic functional groups or foreign materials with electron-donating or withdrawing effects, which complicates the synthesis (27–29). A facile strategy for crafting the electronic properties of COFs is still lacking.
Single-atom catalysts (SACs) recently emerged as a new frontier in catalysis and energy storage (30–38). One major feature of SACs is pronounced electronic metal support interaction as compared to conventional nanoparticle catalysts. Such interaction has been regarded as an important factor governing the electrochemical properties of metal single atoms. Recently, single atoms have been found the ability to in turn modulate the electronic properties of the support (39, 40). Lou and coworkers reported the lattice-confined Pt single atoms, which can impact not only adjacent atoms but also atoms on the second and third shell in the carbon support (41). Zhang et al. prepared Au single atoms supported by NiFe layered double hydroxide. Au donates electrons to the neighboring Fe atoms, which further improves the oxygen evolution reaction (OER) activity of Fe (42). Pan et al. synthesized Zn single atoms and Co nanoparticles co-implanted carbon nanosheets. Dual active sites strongly confine the polysulfides and catalyze their reactions by decreasing the energy barrier of the composite, which effectively promotes the electrochemical performance of lithium–sulfur batteries (LSBs) (43). These results provide hints that incorporating metal single atoms with π–d hybridization into COFs may modulate the electronic properties of COFs, thereby impacting the ion storage capacity. Although there have been reports on COF-based SACs, the metal single atoms act as an active center for catalysis (44–48). Little attention has been paid to employing metal single atoms in COFs for rechargeable ion batteries.
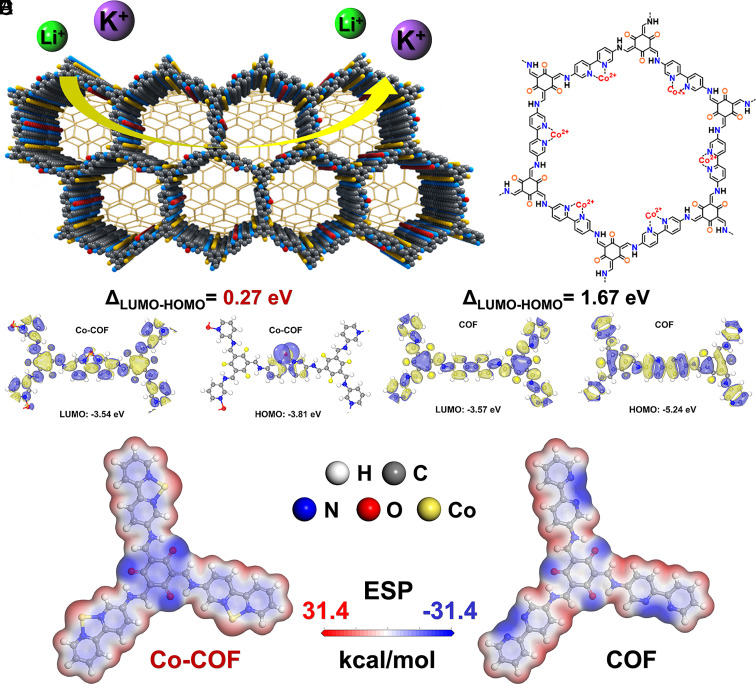
Herein, we demonstrate the first example of integrating metal single atoms into a COF to enhance Li+/K+ storage performance. Fig. 1 A and B show the schematic of the representative hybrid structure of Co-COF@CNT. Density function theory (DFT) calculations suggest that the incorporation of Co single atoms can effectively modulate the energy bandgap of the COF consisting of bipyridine units (Fig. 1 C and D) due to the π–d hybridization. The narrow gap between HOMO and LUMO of the COF enhances electronic conductivity, and the uniform distribution in the molecular electrostatic potential (MESP, blue stands for the electronegative parts and red stands for the electropositive parts) in Fig. 1E also indicates the tuned electronic structure of total molecules. Other transition metal single atoms (e.g., Fe, Ni) incorporated COFs were calculated with the reduced gap, suggesting the generality of this method. Inspired by the theoretical results, the COFs inserted with metal single atoms (i.e., M-COF@CNT, M = Co, Fe, Ni) were synthesized and hybridized with multi-walled carbon nanotubes (CNT). The CNT as the support improves the dispersion of active COF materials and further enhances the conductivity. The LIBs based on M-COF@CNT are assembled, which show enhanced capacity and cycling performance. Extensive ex situ and in situ characterizations together with in-depth DFT calculations unravel the active functional groups for Li+ and K+ storage in the COFs as well as the promotive role of metal single atoms.
Fig. 1.
Molecular simulations and chemical structure of samples. (A) Schematic of Co single atoms assisted Li+/K+ storage in the TP–BPY–COF supported by CNT. (B) The chemical structure of Co-COF. Calculated HOMO and LUMO distribution and levels of (C) Co-COF and (D) TP–BPY–COF. (E) Simulated MESP distribution of Co-COF and TP–BPY–COF. Notes: blue stands for the electronegative parts and red stands for the electropositive parts.
Results
By using a solvothermal method, the 2,4,6-trihydroxybenzene-1,3,5-tricarbaldehyde (TP) and [2,2′-bipyridine]-5,5′-diamine (BPY) were converted into the TP–BPY–COF with conventional AA stacking (49, 50). Metal single atoms were anchored into the pore walls of TP–BPY–COF (abbreviated as Fe/Ni/Co-COF) via coordination with bipyridine molecules, using Co as the example. To increase the conductivity of COF, CNTs were added during COF synthesis. This composite structure, known as COF@CNT, was then utilized as a template to anchor Fe/Ni/Co ions, resulting in Fe/Ni/Co-COF@CNT. As shown in SI Appendix, Figs. S1–S3, the powder X-ray diffraction (PXRD) measurement was conducted to investigate the crystallinity of the as-prepared COFs. The peaks for Fe/Ni/Co-COF@CNT at 3.6° and 26.2° are from (100) and (001) facets of TP–BPY–COF, which suggest the retention of the framework from TP–BPY–COF. There is no obvious peak for Fe/Ni/Co ions due to the form of metal single atoms with nano size and a low loading amount in COFs. The nitrogen sorption isotherm measurements at 77 K revealed the Brunauer–Emmett–Teller (BET) surface areas for TP–BPY–COF, Co-COF, and Co-COF@CNT are 562, 135, and 190 m2 g−1, respectively (SI Appendix, Fig. S1B). The pore size distribution curves show that the pore size of COFs is between 1.2 and 2.0 nm (SI Appendix, Fig. S1C). Fourier transform infrared spectrometer (FT-IR) measurements (SI Appendix, Fig. S1D) were performed to investigate the as-prepared COFs. The vibrant peaks at ~1,657 and 1,580 cm−1 are due to the C=O and C=C groups of TP–BPY–COF. The peaks at ~1,610 and 1,395 cm−1 are attributed to the C=N and C–N linkage of bipyridine. With anchoring the Co2+ ions, the bands from C=O remain unchanged, whereas the peak of C=N attenuates and the bands from C–N show a pronounced blue shift, suggesting that the Co2+ has been successfully anchored on the bipyridine sites in the COFs. In addition, all the peaks of COFs can be clearly observed on the Co-COF@CNT. X-ray photoelectron spectroscopy (XPS) measurements (SI Appendix, Fig. S4) were then conducted. The peaks from Co 2p can be clearly identified for Co-COF and Co-COF@CNT, indicating the Co2+ ions have been anchored in the matrix of COFs. The Co mass loadings in Co-COF and Co-COF@CNT are calculated to be 13.6 and 7.9 wt.%, respectively, which are close to those results determined by inductively coupled plasma optical emission spectrometer (ICP-OES, 17.9 wt.% for Co-COF and 9.1 wt.% for Co-COF@CNT).
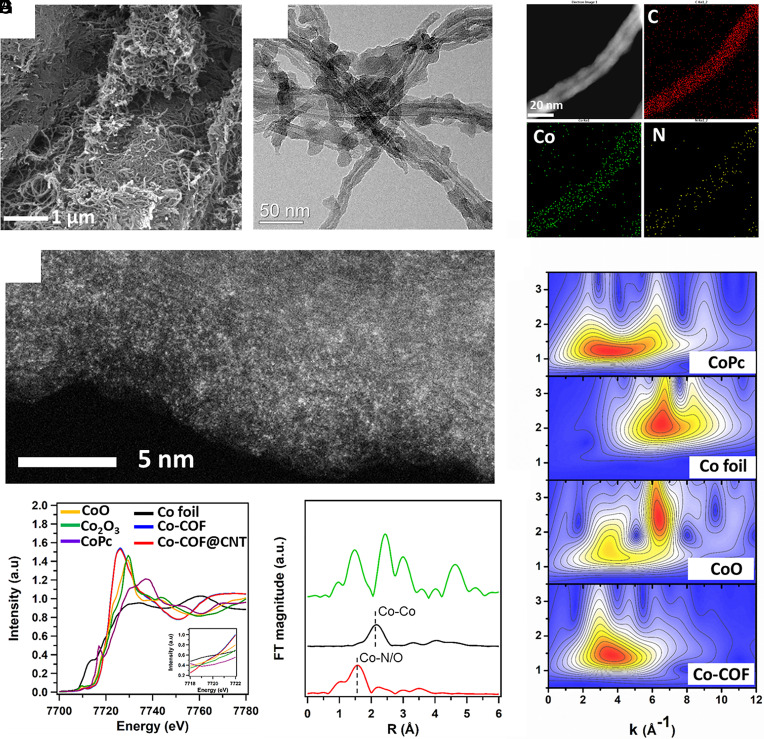
Scanning electron microscope (SEM) and transmission electron microscope (TEM) were applied to demonstrate the microstructure, respectively. Co-COF@CNT shares a similar nanofiber morphology with pristine CNT but has a rugged surface (Fig. 2 A and B), suggesting the successful coating of Co-COF on the CNT surface. Both COF and Co-COF have identical morphologies (SI Appendix, Fig. S5) since Co ions enter the pores without changing the morphology of COF. The Co-COF coating with a few nanometer thicknesses has curved lattice fringes (SI Appendix, Fig. S6). EDS mapping (Fig. 2C) shows that Co was homogeneously distributed throughout the nanofiber structure of Co-COF@CNT. The atomic structure of Co-COF and Co-COF@CNT was investigated by abbreviation-corrected high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) images with sub-Ångström resolution (Fig. 2D). Densely populated bright dots (i.e., Co single atoms) can be clearly observable (SI Appendix, Figs. S7 and S8).
Fig. 2.
Morphology characterizations and coordination structure of Co-COF@CNT. (A) SEM, (B) TEM, (C) STEM with corresponding EDS mapping, and (D) HAADF-STEM images of Co-COF@CNT. (E) Co K-edge XANES spectra and (F) EXAFS fitting curves for Co-COF. (G) WT-EXAFS of the CoPc, Co foil, CoO, and Co-COF.
The chemical state and coordination environment of Co single atoms were further probed by X-ray absorption near-edge structure spectroscopy (XANES) and extended X-ray absorption fine structure (EXAFS) spectroscopy. Fig. 2E shows the Co K-edge XANES spectrum. The absorption edge positions of Co-COF and Co-COF@CNT lie between that of CoO and Co2O3, suggesting that the valence of Co single atoms is between +2 and +3. The Fourier-transformed (FT) k3-weighted EXAFS spectrum (Fig. 2F) of Co-COF shows a prominent peak at ~1.5 Å, which can be ascribed to the first shell Co–N/O coordination. The Co–Co coordination peak at ~2.2 Å is weak, suggesting that Co species predominantly exist as single atoms. Quantitively, EXAFS fitting was carried out to determine the structural parameters. Based on the fitting results (SI Appendix, Table S1), Co single atoms are proposed to bond with two nitrogen and four oxygen atoms in the first shell. This generally agrees with our structure model (Fig. 1B), in which Co single atoms are embedded in the COF matrix in the form of Co–N2O4. The extra four Co–O might be contributed by oxygen-containing species existing in the pores of COF. WT-EXAFS further reveals that the Co species in the Co-COF are different from those of CoPc, Co foil, and CoO. Wavelet transform (WT) of the Co K-edge spectrum was also performed to further analyze the bonding environment. Fig. 2G shows the contour maps of Co-COF and the reference CoPc, CoO, and Co foil. There is one pronounced peak at 3.5 Å−1 in the WT map of Co-COF. The position of its intensity maximum coincides with that of CoPc (contributed by Co–N coordination) and CoO (contributed by Co–O coordination), which indicates the first shell coordination is a mixture of Co–O and Co–N. This generally agrees with the fitting results of the FT-EXAFS spectrum.
The electrochemical properties of the Co-COF@CNT electrode in LIBs were explored by cyclic voltammetry (CV) from 0.01 to 3.0 V (vs. Li/Li+) at the scan rate of 0.1 mV s−1. As shown in SI Appendix, Fig. S9, the obvious irreversible peak at ~0.6 V could be observed in the first scan, which could be ascribed to the formation of SEI film. By comparing the CV curve of Co-COF@CNT with TP–BPY–COF, Co-COF, and COF@CNT, it can be clearly observed that the reduction peak at ~0.5 V and 1.3 V which are ascribed to the reduction of the bipyridine rings and O=C–C=C–NH units in the Co-COF@CNT electrode. Due to the improved binding affinity of lithium ions and enhanced conductivity of Co-COF@CNT, the material can be effectively activated and then fully participate in the redox reactions. The peak displayed in CV curves well corresponds to the discharging and charging platforms shown in SI Appendix, Fig. S10.
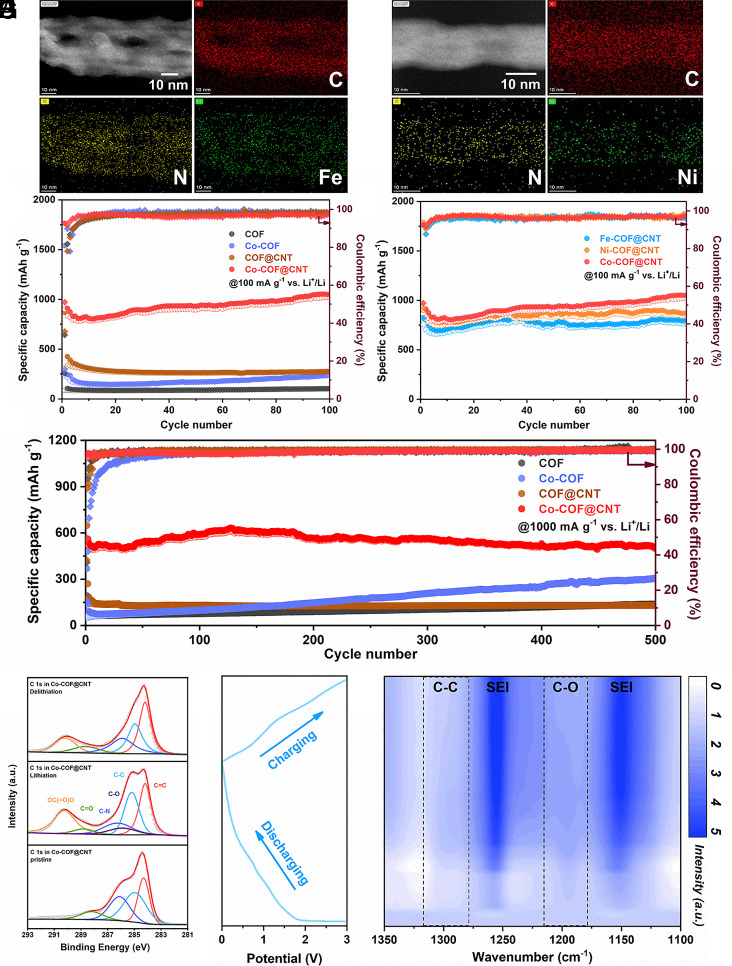
To confirm the generality of metal single atoms in the electrode, the samples with other metal single atoms (Fe-COF@CNT and Ni-COF@CNT) were obtained using an identical preparation procedure. Fe and Ni elements achieve atomic dispersion over the COF@CNT nanofiber as EDS mapping images shown in Fig. 3 A and B, which also suggests the generality of the preparation strategy for anchoring metal single atoms into COFs. The electrochemical properties of TP–BPY–COF, Co-COF, and COF@CNT electrodes in LIBs were further studied at the current density of 100 mA g−1. In Fig. 3C and SI Appendix, Fig. S11, the initial charge and discharge capacities of TP–BPY–COF are ~86.4 and 256.4 mA h g−1. With the introduction of Co2+ and CNT into the frameworks, the capacities increase to ~239.0/1540.0 (Co-COF) and 308.4/867.6 mA h g−1 (COF@CNT), respectively. The Fe-COF@CNT- and Ni-COF@CNT-based LIBs display reversible capacities of ~765.7 and 854.5 mA h g−1 at 100 mA g−1 after 100 cycles (Fig. 3D), respectively. The lithium-ion storage behavior is obviously improved in Co-COF@CNT. Due to the π–d hybridization of Co single atoms in COFs, the well-distributed charge in the molecule promotes the activation and full utilization of interior active sites, then boosting the obvious increase of capacity during cycling (51, 52). The Co-COF@CNT electrode has a large reversible capacity of 1,023.0 mA h g−1 after 100 cycles. Notably, the reversible capacity is only 205.3 mA h g−1 after 50 cycles for bare CNTs (SI Appendix, Fig. S12), which indicates that the synergistic effect of π–d hybridization and π–π interaction among Co single metals, COFs, and CNTs is critical for the enhanced cycling properties. The rate performance of all electrodes was evaluated at current densities ranging from 100 to 5,000 mA g−1 (SI Appendix, Fig. S13). The measured capacities of Co-COF@CNT are ~894.5, 685.8, 608.0, 518.6, 416.3, and 307.9 mA h g−1 at current densities of 100, 200, 500, 1,000, 2,000, and 5,000 mA g−1, respectively. When the current density is set back to 100 mA g−1, the capacity remains 807.1 mA h g−1. In addition, the rate capacities for Fe-COF@CNT and Ni-COF@CNT electrodes from 100 to 5,000 mA g−1 (SI Appendix, Fig. S14) are ~760.4/864.5, 606.6/681.1, 494.7/570.3, 412.4/478.2, 282.6/367.9, and 129.5/188.9 mA h g−1, respectively. It can indicate that the Co-COF@CNT electrode shows superior properties than Fe-COF@CNT and Ni-COF@CNT electrodes, especially at high current densities. The long-term stability of LIBs with different electrodes was studied at the large current density of 1,000 mA g−1 (Fig. 3E). The reversible capacity remains 501.1 mA h g−1 after 500 cycles for Co-COF@CNT, much higher and more stable than other samples.
Fig. 3.
HAADF-STEM images, electrochemical properties, and mechanism characterizations for LIBs. HAADF-STEM image and the corresponding EDS mapping images of (A) Fe-COF@CNT and (B) Ni-COF@CNT. Cycling performance at (C and D) 100 mA g–1 and (E) 1,000 mA g–1. Notes: The capacities were all calculated based on the total weight of the electrodes. (F) Ex situ XPS results of the C 1 s spectrum during lithiation and delithiation process. (G) Corresponding discharging and charging curves and (H) in situ FT-IR contour map for the Co-COF@CNT electrode.
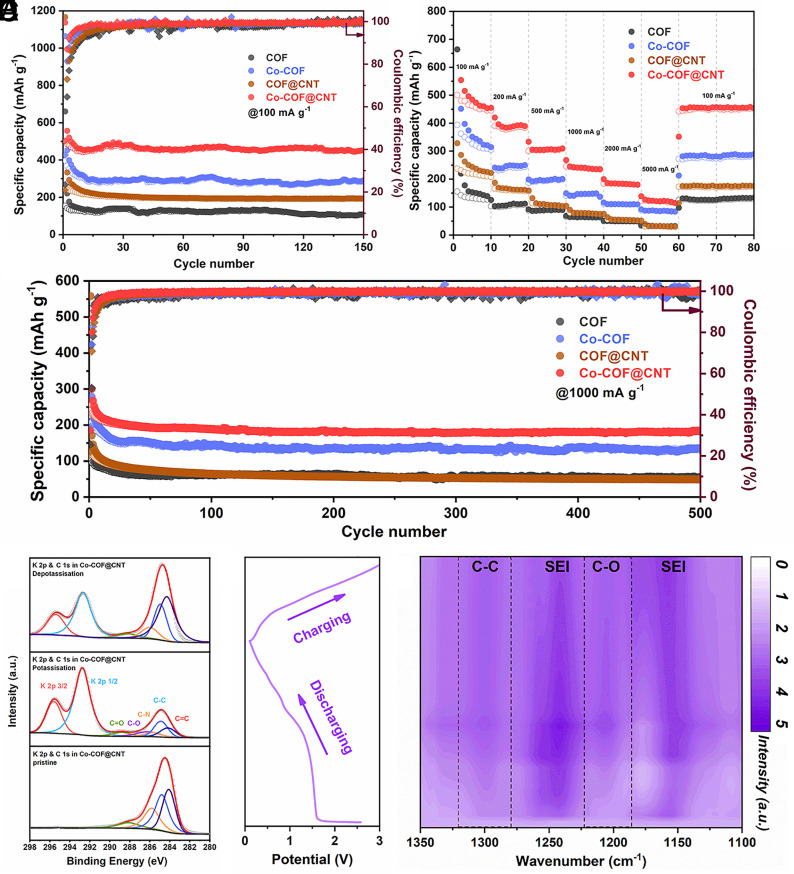
The lithium storage mechanism for as-prepared Co-COF@CNT was explored by ex situ XPS and in situ FT-IR measurements. As Fig. 3F shows, the high-resolution C 1 s spectrum of Co-COF@CNT can be deconvoluted into five peaks including C=C (284.2 eV), C–C (284.9 eV), C–O (285.8 eV), C–N (286.2 eV) and C = O (288.5 eV). The peak area ratio of C–C/C=C and C–O/C=O increases in the lithiation process while decreases in the next delithiation process, indicating the reversible reaction during discharging and charging process. The peak at 290.2 eV can be assigned to the irreversible formation of OC(=O)O units from SEI (53–55). The steady peak of Co 2p in SI Appendix, Fig. S15 indicates the stability of Co single ions and the maintenance of the π–d hybridization conjugated framework of Co-COF@CNT, which is beneficial for the charge transfer and intrinsic conductivity of molecules. The reversible change of peaks at ~1,300 cm−1 (C–C groups) and 1,200 cm−1 (C–O groups) in Fig. 3 G and H and SI Appendix, Fig. S16 gives more evidence of the reversible redox reactions between Li+ and the conjugated β-ketoenamine units (O=C–C=C–NH). It is worth mentioning that the irreversible peaks that appeared at ~1,255 cm−1 and 1,150 cm−1 can be attributed to the C–C and OC(=O)O bonds from the decomposition of the electrolyte and the formation of the SEI.
To confirm the Li+ storage kinetics of the COF-based electrodes for LIBs, CV measurements at different scan rates ranging from 0.1 to 1.0 mV s−1 were carried out (SI Appendix, Figs. S17 and S18). It can be observed that the area of CV curves in Co-COF@CNT is much larger than in others (based on the same weight ratio of all electrodes), which indicates the efficient redox reaction of the Co-COF@CNT electrode. The capacitive contribution (SI Appendix, Fig. S18C) of Co-COF@CNT at 1.0 mV s−1 is 85.0%, higher than COF, Co-COF, COF@CNT, Fe-COF@CNT, and Ni-COF@CNT electrodes. The electrochemical impedance spectroscopy (EIS) measurement (0.01 to 100,000 Hz) was also implemented to probe the charge transfer process at the interface of electrodes and electrolytes for the Co-COF@CNT electrode. As shown in SI Appendix, Fig. S18D, the Rct value could be calculated to be ~218.0, 168.8, 155.0, 157.4, and 132.2 Ω for TP–BPY–COF, Co-COF, COF@CNT, Fe-COF@CNT, and Ni-COF@CNT, respectively. However, the Rct value of Co-COF@CNT is only ~112.5 Ω, which is the smallest among all samples, suggesting the incorporation of Co single atoms and CNT with π–d hybridization and π–π interaction could significantly accelerate the charge transfer of the COF based electrode.
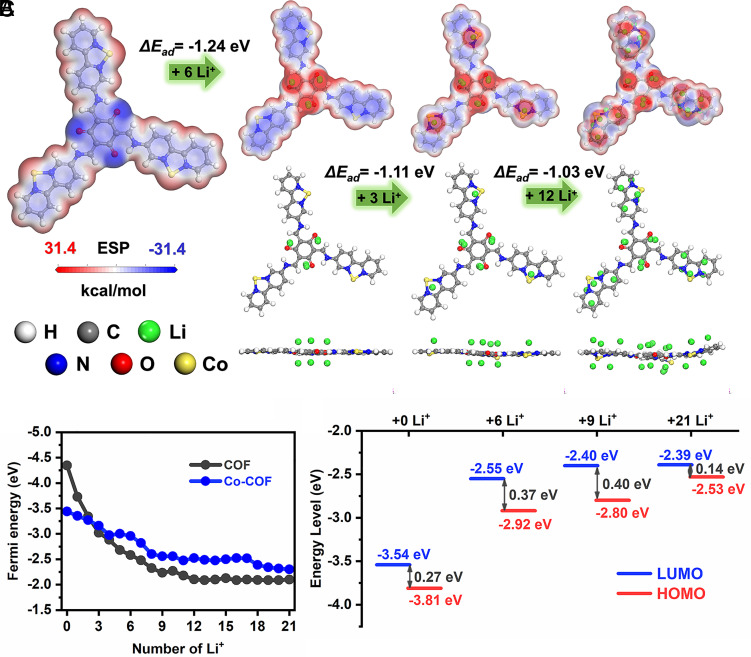
Furthermore, theoretical simulations of the adsorption abilities of COF and Co-COF were conducted to investigate the excellent lithium storage capability of the samples by the Fukui function for nucleophilic sites (SI Appendix, Fig. S19) and the MESP distribution (Fig. 1E, blue for negative and red for positive) of COF and Co-COF, it can be detected that the nucleophilic sites and electronegative parts of molecule get well-distributed due to the π–d hybridization. It confirms that the π–d hybridization between single metal atoms and the COFs is beneficial for the full utilization of conjugated active redox sites. Lithium atoms are sequentially placed at different sites of Co-COF, and the optimized geometries are obtained by the calculation in Fig. 4A. The blue parts with the most concentrated negative charge are the most probable sites for accepting lithium ions. Therefore, the first 6 lithium ions can be set at three conjugated β-ketoenamine units (O=C–C=C–NH) with the adsorption energy of −1.24 eV after the optimization for each COF unit. Then, the second possible part is going to be at the linkage of bipyridine units with the adsorption energy of −1.11 eV for further accepting 3 lithium ions. Finally, six unsaturated C=C groups in bipyridine units can further accept 12 lithium ions with the adsorption energy of −1.03 eV after the optimization (53, 56). In addition, the Fermi energy after accepting different numbers of lithium ions was simulated as shown in Fig. 4B. The lower value of Fermi energy stands for the stronger ability of accepting lithium ions. It can be clearly detected that it is easier for Co-COF to accept metal ions than COF generally because the binding ability of Li+ on Co-COF is much stronger than COF after accepting one lithium-ion. It is worth mentioning that the Fermi energy of COF at the initial state is much lower than Co-COF before accepting one lithium-ion, which can be due to the strong electronegative N atoms in bipyridine units of COF. Moreover, the increased HOMO energy value from −3.81 eV to −2.53 eV after accepting different numbers of lithium ions in Fig. 4C indicates the improved oxidizability. The enhanced oxidizability also facilitates the next reversible delithiation process of the material. On the contrary, the increased LUMO energy value from −3.54 eV to −2.39 eV indicates the reduced reduction ability of the simulated molecule during the lithiation process. The LUMO energy value remains stable after accepting 21 lithium ions, which means that the lithiation process is approaching the end.
Fig. 4.
Simulated adsorption configurations and theoretical calculations. (A) The MESP distribution (blue and red stand for the electronegative and positive parts respectively), (B) Fermi energy, and (C) HOMO–LUMO energy of Co-COF with accepting different numbers of Li+. Notes: The size of Li+ was enlarged to better visualize their spatial distribution.
PIBs were assembled using the obtained COF composites as electrode materials. The electrochemical properties of the Co-COF electrode in PIBs were investigated by cyclic voltammetry (CV) from 0.01 to 3.0 V (vs. K/K+) at the scan rate of 0.1 mV s−1. As shown in SI Appendix, Fig. S20, two obvious peaks at ~0.6 V and 1.1 V could be observed in the first scan can be ascribed to the formation of solid electrolyte interface (SEI) film. The CV peak in PIBs is not as obvious as in LIBs, which can be attributed to the weak interaction between the large-radius potassium ions and the functional groups in COFs. The electrochemical performance of TP–BPY–COF, Co-COF, COF@CNT, and Co-COF@CNT electrodes in PIBs was further studied at the current density of 100 mA g−1. As shown in Fig. 5A, the initial charge capacity of TP–BPY–COF can be 154.4 mA h g−1 (black curves). With the introduction of the Co and CNT into the frameworks, the charge capacity obviously increases to 499.4 mA h g−1 (red curves). After 150 cycles, the Co-COF@CNT electrode still has a larger reversible capacity of 449.0 mA h g−1. However, the capacity for TP–BPY–COF, Co-COF, and COF@CNT is only 107.8, 287.4, and 192.6 mA h g−1, respectively, about 24.0%, 64.0%, and 42.9% of that from Co-COF@CNT. It could be concluded that the Co-COF@CNT electrode shows the best cycling performance among all electrodes, which could be attributed to the synergistic effect of COF, CNT, and Co single atoms with π–d hybridization and π–π interaction. The rate performance of TP–BPY–COF, Co-COF, COF@CNT, and Co-COF@CNT electrodes was evaluated at current densities ranging from 100 to 5,000 mA g−1 (Fig. 5B). The measured capacities of Co-COF@CNT can be 500.7, 390.9, 300.7, 245.6, 184.0, and 122.5 mA h g−1 at current densities of 100, 200, 500, 1,000, 2,000, and 5,000 mA g−1, respectively. The capacity recovers to 442.2 mA h g−1 when the current density returns to 100 mA g−1. Importantly, the Co-COF@CNT electrode shows better rate performance than the control samples and reported COF-based electrodes at the same range of current densities as summarized in SI Appendix, Table S2.
Fig. 5.
Electrochemical properties and mechanism characterizations for PIBs. (A) Cycling performance at 100 mA g−1, (B) rate capabilities, and (C) cycling performance at 1,000 mA g–1. Notes: The capacities were all calculated based on the total weight of the electrodes. (D) Ex situ XPS results of the C 1 s spectrum during the potassiation and de-potassiation process. (E) Corresponding discharging and charging curves and (F) in situ FT-IR contour map for the Co-COF@CNT electrode.
The reversible capacity for bare CNT can be only 76.0 mA h g−1 for 50 cycles at 100 mA g−1 (SI Appendix, Fig. S21), which further confirms the critical role of Co-COF on the CNTs. The long-term stability of PIBs with different electrodes was studied at a large current density of 1,000 mA g−1, as shown in Fig. 5C. The reversible capacity is 133.4 mA h g−1 for Co-COF, while the capacity is 55.4 mA h g−1 for TP–BPY–COF at 500 cycles. The improved K+ storage ability with anchoring Co could be attributed to the lower band gap and higher electron conductivity due to the π–d hybridization between Co single atoms and conjugated structure from COF. The electron conductivity of COFs can be further improved by covering Co-COF on the CNT surface, and the resulting Co-COF@CNT electrode achieves a high reversible capacity of 184.9 mA h g−1 after 500 cycles. Compared with the increased trend of capacity in LIBs, the cycling performance remains stable in PIBs. It can be attributed to the more difficult insertion of potassium ions with large radius into the interior active sites of COFs than lithium ions (57). Moreover, the excellent performance (SI Appendix, Fig. S22) of Fe, Ni, and Co-COF@CNT with π–d hybridization indicates the universality of the strategy of introducing single metal atoms into the COF structure. In addition, it is worth noting that the electrochemical properties of Co-COF@CNT are outstanding compared to those carbon-based electrodes in SI Appendix, Table S3.
The potassium storage mechanism of prepared Co-COF@CNT was investigated by ex situ XPS measurements. As shown in Fig. 5D, there is no peak for K in the as-prepared Co-COF@CNT. The high-resolution K 2p spectrum shows that two peaks of K 2p 1/2 and K 2p 3/2 appear with potassiation, which significantly attenuates in the following de-potassiation process. The high-resolution C 1 s spectrum of Co-COF@CNT can be deconvoluted into five peaks including C=C (284.0 eV), C–C (284.8 eV), C–N (285.8 eV), C–O (286.2 eV), and C=O (288.2 eV), after the first potassiation process. The irreversible peak at 292.8 and 295.5 eV belongs to K 2p 1/2 and K 2p 3/2, respectively. In the potassiation process, the peak of C=C, C–N, and C=O weakens, while the peak of C–C and C–O gets strengthened. During the following de-potassiation process, the peak of C=C, C–N, and C=O recovers, indicating the reversible reaction during potassiation and de-potassiation. The result of stable Co 2p in SI Appendix, Fig. S23 for PIBs corresponds to it in LIBs, which also demonstrates the maintenance of intrinsic conductivity due to the π–d hybridization conjugated system. The active site for K+ storage of Co-COF@CNT was also confirmed by the in situ FT-IR contour map and spectra (Fig. 5 E and F and SI Appendix, Fig. S24). In the potassiation process, the intensity of peaks at 1,300 cm−1 (C–C units) and 1,210 cm−1 (C–O units) increases, indicating the successful intercalation of K+ ions with conjugated β-ketoenamine units (O=C–C=C–NH). The intensity of C–C and C–O bonds can be recovered after de-potassiation, suggesting the reversible charge and discharge process. The irreversible peaks at 1,240 cm−1 and 1,150 cm−1 are consistent with those in LIBs, indicating the formation of SEI during the decomposition of the electrolyte.
To further understand the K+ storage kinetics of the COF-based electrodes for PIBs, CV measurements at different scan rates ranging from 0.2 to 1.0 mV s−1 were also carried out (SI Appendix, Fig. S25). To quantitatively understand the diffusion and capacitive contributions, we calculate the distinguished contributions according to the formula: i = k1v + k2v0.5, where k1 and k2 are constants while k1v and k2v0.5 stand for the capacitive and diffusion contributions, respectively. Accordingly, the storage performance can be attributed to diffusion and captive storage at low scan rates. With increasing scan rates from 0.2 to 1.0 mV s−1, the capacitive-controlled capacities increased from 71.2 to 97.1% for the Co-COF@CNT electrode (SI Appendix, Fig. S26), which means the capacitive process plays a more important role in storage kinetics as the rise of the scan rates. For TP–BPY–COF and Co-COF, the capacitive contribution at 0.2 mV s−1 is 33.1% and 36.2%, indicating the K+ storage at the low scan rate is mainly from the diffusion process. The capacitive contribution for COF@CNT increases to 52.1% at 0.2 mV s−1, suggesting the enhanced capacitive storage ability.
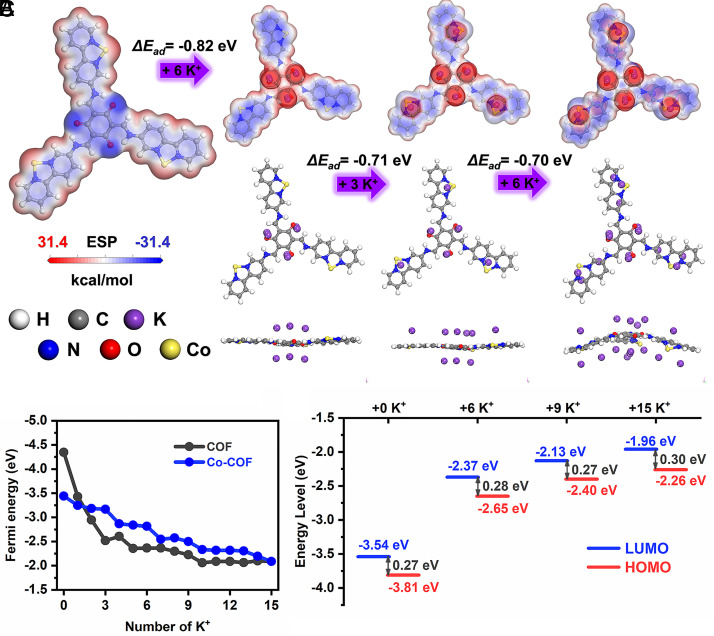
To explore the superior K+ storage capability of the Co-COF, relative K+ adsorption abilities on COF and Co-COF configurations were simulated. Potassium atoms are sequentially placed at different sites of Co-COF, and the optimized geometries are obtained by calculation in Fig. 6A. The first six potassium ions are set at three conjugated β-ketoenamine units (O=C–C=C–NH) with the adsorption energy of −0.82 eV after the optimization, corresponding to the result from LIBs. Then, the second site is going to be at the conjugated linkage near N atoms of bipyridine units with the adsorption energy of −0.71 eV for further accepting three potassium ions. Finally, six unsaturated pyridinic rings accept six potassium ions with an adsorption energy of −0.70 eV after the optimization of the simulated COF unit. The lower Fermi energy of Co-COF after accepting one potassium ion in Fig. 6B also suggests its stronger ability to accept potassium ions due to the π–d hybridization from single metal atoms. In addition, the result from HOMO–LUMO energy levels in Fig. 6C is consistent with this in LIBs, which indicates the improved capacity and reversibility of Co-COF. The stable energy gap between HOMO and LUMO energy levels also illustrates the excellent conductivity of Co-COF, due to the well-distributed nucleophilic sites and electronegative parts of the molecule with π–d hybridization.
Fig. 6.
Simulated adsorption configurations and theoretical calculations. (A) The MESP distribution (blue and red stand for the electronegative and positive parts respectively), (B) Fermi energy, and (C) HOMO–LUMO energy of Co-COF with accepting different numbers of K+. Notes: The size of K+ was enlarged to better visualize their spatial distribution.
Such adsorption configuration benefits the ion traffics when intercalating numerous K+ ions at high rates and the uniform adsorption energy for K+ ions gives rise to the stable and ultrafast charge/discharge ability of Co-COF with π–d hybridization, which is consistent with the adsorption of Li+. The calculated Fermi energy of Co-COF is much more stable than pure COF for adsorbing Li+ or K+ ions, which indicates the introduction of the metal single atoms into COFs can facilitate the electrochemical properties in both LIBs and PIBs. Furthermore, the reduced adsorption energy for both Li+ and K+ also demonstrates the universal and effective capacitive ability of Co-COF for energy storage.
Discussion
In summary, the concept of single-atom catalysts (Co, Ni, and Fe) is applied to design organic electrodes in LIBs and PIBs. Densely populated Co single atoms can be homogeneously incorporated into the porous skeleton of TP–BPY–COF with π–d hybridization, which significantly alters the electrochemical properties of TP–BPY–COF. Co single atoms play multiple positive roles in lithium and potassium storage, including engineering the HOMO and LUMO levels, tuning binding affinity between Li/K ions and active sites in TP–BPY–COF (e.g., β-ketoenamine units), and improving the ion transportation as revealed by in situ and ex situ characterizations as well as DFT calculations. Consequently, the Co-COF electrode supported by CNTs with π–π interaction displays a large reversible capacity of 1,023.0 mA h g−1 after 100 cycles for LIBs and 449.0 mA h g−1 after 150 cycles for PIBs at 100 mA g−1. This work also indicates that anchoring earth-abundant metal single atoms can be a general approach to improve the performance of organic electrodes. The synergy between electronically functional metal single atoms, COFs with abundant redox-active groups and conductive carbon, endows the assembled LIB and PIB with better capacity and rate performance than that of reported COF and carbon-based batteries. This work provides an insight to engineer organic electrodes for rechargeable metal-ion organic batteries.
Materials and Methods
Synthesis of TP–BPY–COF.
TP–BPY–COF and Co-COF were synthesized according to the previous report (58). A mixture of DMAC/oDCB (0.9 mL/0.3 mL), Tp (12.6 mg), Bpy (16.7 mg), and aqueous acetic acid solution (6 m, 0.12 mL) was ultrasonicated for several minutes and then degassed in a Pyrex tube (10 mL) by three freeze–pump–thaw cycles. The tube was sealed off and heated at 120 °C for 3 d. The precipitate was collected by centrifugation, washed with THF, HAc (3 m) aqueous solution, and MeOH several times, and dried at 120 °C under vacuum overnight to obtain TP–BPY–COF in 82% yield.
Synthesis of Co-COF.
TP–BPY–COF (100.0 mg) was dispersed in EtOH and ultrasonicated for 2 h. Co(OAc)2 (60.0 mg) was then added to the solution and stirred overnight. The precipitate was collected by centrifugation, washed with EtOH, and dried under vacuum to obtain Co-COF. Fe-COF and Ni-COF were synthesized by the same method, except with different metal salts, Fe(OAc)2 and Ni(OAc)2, respectively.
Synthesis of Co-COF@CNT.
CNT (20.0 mg) was added to a mixture of DMAC/oDCB (0.9 mL/0.3 mL), and the mixture was ultrasonicated for 5 min. Then, Tp (12.6 mg), Bpy (16.7 mg), and aqueous acetic acid solution (6 m, 0.12 mL) were added and ultrasonicated for several minutes and then degassed in a Pyrex tube (10 mL) by three freeze–pump–thaw cycles. The tube was sealed off and heated at 120 °C for 3 d. The precipitate was collected by centrifugation, washed with THF, HAc (3 m) aqueous solution, and MeOH several times, and dried at 120 °C under vacuum overnight to obtain COF@CNT. Fe2+/Ni2+/Co2+ was then introduced into the COF@CNT to obtain Fe/Ni/Co-COF@CNT by the same method as the synthesis of Co-COF.
Fabrication Electrodes for LIBs/PIBs.
The electrochemical performance versus metallic lithium and potassium was tested in CR2032‐type coin cells. The working electrodes were prepared by mixing 60 wt.% active materials (TP–BPY–COF, Co-COF, Fe/Ni/Co-COF@CNT), 30 wt.% super P, and 10 wt.% PVDF (polyvinylidene fluoride) as the binder. The slurry was then coated onto copper foil and dried at 90 °C in a vacuum oven for 10 h to evaporate the solvent. Circular electrodes with a diameter of 13 mm were obtained using a punch machine and the average mass loading of active material was about ~0.6 mg cm−2. The coin cells were assembled in an argon‐filled glove box with excess metallic lithium/potassium applied as the anode. For LIBs, the porous polypropylene (Celgard 2500) was applied as the separator, and the electrolyte was a 1.0 m LiPF6 solution dissolved in ethylene carbonate/diethyl carbonate (1:1 by volume). As for PIBs, a Whatman GF/C glass fiber was used as the separator, and a 0.8 m KPF6 solution dissolved in ethylene carbonate/diethyl carbonate (1:1 by volume) was applied as the electrolyte. The galvanostatic charge/discharge tests were conducted within the voltage range of 0.01 to 3.0 V using a LAND‐CT2001A multichannel galvanostatic technique at room temperature (25 °C). The CV profiles were obtained within the voltage window of 0.01 to 3.0 V at various scan rates. In the in situ FT-IR analysis, the preparation method of the working electrode was basically the same as that of the ordinary working electrode. However, the copper foil was changed to nickel foam, and a special battery testing device containing a diamond window was used. The designed device consists of a bottom bracket with a diamond window, a sealing ring with titanium wire, a battery container, and a stainless steel with electrode sheet. The infrared light was converted to the material through the diamond window to detect the change of functional groups of the electrodes during the discharging and charging processes. The Nicolet iS50 FT-IR spectrometer (attenuated total reflection, ATR Mode) was connected to the LAND CT2001 battery test system for in situ FT-IR assembly, and the spectrogram was acquired every 10 min in the voltage range of 0.01 to 3.0 V. The initial state of the spectrogram for each electrode was set in the background to clearly show the change during the cycling process (59).
Characterizations.
The powder XRD pattern of all the samples was recorded on a powder X-ray diffractometer (Ultima IV) with a scanning rate of 8° per minute. The chemical state of the elements was analyzed by an XPS spectrometer (Thermo Scientific K-Alpha) using an Al Kα X-ray source. The porosity and surface area of the samples were obtained by analyzing the nitrogen sorption isotherms at 77 K recorded on a gas sorption analyzer (TriStar II, Micromeritics). The pore volume was deduced based on the non-local density functional theory (NLDFT) model. The functional groups in the samples were probed by the FT-IR spectrometer (Nicolet iS50). The high-resolution electron images were collected by HR-TEM (FEI Tecnai G2), and the element distribution was obtained by an EDS spectrometer (Oxford) equipped on the Tecnai (60).
Simulations and Calculations.
We have employed the DMol3 to perform all the spin-polarized density functional theory (DFT) calculations within the generalized gradient approximation (GGA) using the Perdew–Burke–Ernzerhof (PBE) formulation (61). We have chosen the DFT semi-core pseudopotentials to describe the core electrons and take valence electrons into account using a double numerical basis set including p-polarization function (DNP) with the orbital cutoff of 4.7 Å (62). The electronic energy was considered self-consistent when the energy change was smaller than 10−6 eV. Geometry optimization was considered convergent when the energy change was smaller than 10−5 eV. Dispersion corrected DFT (DFT-D) scheme was used to describe the dispersion interactions among all the atoms in adsorption models of interest. All the calculations were performed with a 3 × 3 × 1 Monkhorst-Pack k-point grid (63). The structural optimization parameters are the following: Energy, Max. force, and Max. displacement was set to 1.0e−5 Ha, 0.002 Ha/Å, and 0.002 Å, respectively. To achieve accurate electronic convergence, a smearing value of 0.02 Ha was applied.
In this calculation, the adsorption energy of Li+/K+ was calculated with the flowing equations: , where M is Li+ or K+ and n is the number of Li+/K+ adsorbed on the COF model, the ECOF+nM, ECOF, and EM stands for the energy of the model with n Li+/K+, without Li+/K+, and the Li+/K+, respectively (64).
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
We acknowledge the support from the National Natural Science Foundation of China (Nos. 52073170, 52272289, and 22065017), the Eastern Scholar Research Fund Shanghai Institutions of Higher Learning, the Innovation Program of Shanghai Municipal Education Commission (Innovative Project 2019-01-07-00-09-E00021 and 2021-01-07-00-03-E00109), Jiangxi Provincial Natural Science Foundation (20224BAB214019), the Fundamental Research Funds for the Central Universities, the DHU Distinguished Young Professor Program, and the Shanghai Innovative Research Team for Local High-level Universities. This work was also supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2022R1C1C1010025), the New Renewable Energy Core Technology Development Project of the Korea Institute of Energy Technology Evaluation and Planning (KETEP) that granted financial resource from the Ministry of Trade, Industry & Energy, Republic of Korea (No. 20223030010240).
Author contributions
Y.C., Q.X., X.L., and Y.W. conceptualized research; Y.T. provided TEM methodology; Y.C., Q.X., X.C., J.S., and T.Z. conducting research; Y.S., Y.X., S.Y., Z.J., and H.U. visualized research; X.L. and Y.W. supervised research; and Y.C., Q.X., X.L., and Y.W. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission.
Contributor Information
Xiaopeng Li, Email: xiaopeng.li@dhu.edu.cn.
Yong Wang, Email: yongwang@shu.edu.cn.
Data, Materials, and Software Availability
All study data are included in the article and/or SI Appendix.
Supporting Information
References
- 1.Cano Z. P., et al. , Batteries and fuel cells for emerging electric vehicle markets. Nat. Energy 3, 279–289 (2018). [Google Scholar]
- 2.Park M., Ryu J., Wang W., Cho J., Material design and engineering of next-generation flow-battery technologies. Nat. Rev. Mater. 2, 18 (2017). [Google Scholar]
- 3.Liu Y. Y., Zhu Y. Y., Cui Y., Challenges and opportunities towards fast-charging battery materials. Nat. Energy 4, 540–550 (2019). [Google Scholar]
- 4.Albertus P., Babinec S., Litzelman S., Newman A., Status and challenges in enabling the lithium metal electrode for high-energy and low-cost rechargeable batteries. Nat. Energy 3, 16–21 (2018). [Google Scholar]
- 5.Li M., et al. , Design strategies for nonaqueous multivalent-ion and monovalent-ion battery anodes. Nat. Rev. Mater. 5, 276–294 (2020). [Google Scholar]
- 6.Wu X., et al. , Advanced carbon-based anodes for potassium-ion batteries. Adv. Energy Mater. 9, 46 (2019). [Google Scholar]
- 7.Fan L., Ma R. F., Zhang Q. F., Jia X. X., Lu B. G., Graphite anode for a potassium-ion battery with unprecedented performance. Angew. Chem. Int. Ed. 58, 10500–10505 (2019). [DOI] [PubMed] [Google Scholar]
- 8.Hwang J. Y., Myung S. T., Sun Y. K., Recent progress in rechargeable potassium batteries. Adv. Funct. Mater. 28, 45 (2018). [Google Scholar]
- 9.Ding J., et al. , Sulfur-grafted hollow carbon spheres for potassium-ion battery anodes. Adv. Mater. 31, 9 (2019). [DOI] [PubMed] [Google Scholar]
- 10.Zhang W. L., et al. , A site-selective doping strategy of carbon anodes with remarkable K-ion storage capacity. Angew. Chem. Int. Ed. 59, 4448–4455 (2020). [DOI] [PubMed] [Google Scholar]
- 11.Wang J. D., et al. , Conjugated sulfonamides as a class of organic lithium-ion positive electrodes. Nat. Mater. 20, 665–673 (2021). [DOI] [PubMed] [Google Scholar]
- 12.Poizot P., et al. , Opportunities and challenges for organic electrodes in electrochemical energy storage. Chem. Rev. 120, 6490–6557 (2020). [DOI] [PubMed] [Google Scholar]
- 13.Huang J. H., Dong X. L., Guo Z. W., Wang Y. G., Progress of organic electrodes in aqueous electrolyte for energy storage and conversion. Angew. Chem. Int. Ed. 59, 18322–18333 (2020). [DOI] [PubMed] [Google Scholar]
- 14.Luo Z. Q., et al. , A microporous covalent-organic framework with abundant accessible carbonyl groups for lithium-ion batteries. Angew. Chem. Int. Ed. 57, 9443–9446 (2018). [DOI] [PubMed] [Google Scholar]
- 15.Song Z. P., et al. , Polyanthraquinone as a reliable organic electrode for stable and fast lithium storage. Angew. Chem. Int. Ed. 54, 13947–13951 (2015). [DOI] [PubMed] [Google Scholar]
- 16.Zhang C., et al. , Conjugated microporous polymers with tunable electronic structure for high-performance potassium-ion batteries. ACS Nano 13, 745–754 (2019). [DOI] [PubMed] [Google Scholar]
- 17.Huang N., Wang P., Jiang D. L., Covalent organic frameworks: A materials platform for structural and functional designs. Nat. Rev. Mater. 1, 19 (2016). [Google Scholar]
- 18.Wang D. G., et al. , Covalent organic framework-based materials for energy applications. Energy Environ. Sci. 14, 688–728 (2021). [Google Scholar]
- 19.Guan X. Y., Chen F. Q., Fang Q. R., Qiu S. L., Design and applications of three dimensional covalent organic frameworks. Chem. Soc. Rev. 49, 1357–1384 (2020). [DOI] [PubMed] [Google Scholar]
- 20.Geng K. Y., et al. , Covalent organic frameworks: Design, synthesis, and functions. Chem. Rev. 120, 8814–8933 (2020). [DOI] [PubMed] [Google Scholar]
- 21.Jiang J. C., Zhao Y. B., Yaghi O. M., Covalent chemistry beyond molecules. J. Am. Chem. Soc. 138, 3255–3265 (2016). [DOI] [PubMed] [Google Scholar]
- 22.Tao S. S., et al. , Confining H3PO4 network in covalent organic frameworks enables proton super flow. Nat. Commun. 11, 8 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu Q., Tao S. S., Jiang Q. H., Jiang D. L., Ion conduction in polyelectrolyte covalent organic frameworks. J. Am. Chem. Soc. 140, 7429–7432 (2018). [DOI] [PubMed] [Google Scholar]
- 24.Wang S., et al. , Exfoliation of covalent organic frameworks into few-layer redox-active nanosheets as cathode materials for lithium-ion batteries. J. Am. Chem. Soc. 139, 4258–4261 (2017). [DOI] [PubMed] [Google Scholar]
- 25.Gu S., et al. , Tunable redox chemistry and stability of radical intermediates in 2D covalent organic frameworks for high performance sodium ion batteries. J. Am. Chem. Soc. 141, 9623–9628 (2019). [DOI] [PubMed] [Google Scholar]
- 26.Xu J. S., et al. , An olefin-linked covalent organic framework as a flexible thin-film electrode for a high-performance micro-supercapacitor. Angew. Chem. Int. Ed. 58, 12065–12069 (2019). [DOI] [PubMed] [Google Scholar]
- 27.Lei Z. D., et al. , Boosting lithium storage in covalent organic framework via activation of 14-electron redox chemistry. Nat. Commun. 9, 13 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen X. D., Zhang H., Ci C. G., Sun W. W., Wang Y., Few-layered boronic ester based covalent organic frameworks/carbon nanotube composites for high-performance K-organic batteries. ACS Nano 13, 3600–3607 (2019). [DOI] [PubMed] [Google Scholar]
- 29.Zhang H., Sun W. W., Chen X. D., Wang Y., Few-layered fluorinated triazine-based covalent organic nanosheets for high-performance alkali organic batteries. ACS Nano 13, 14252–14261 (2019). [DOI] [PubMed] [Google Scholar]
- 30.Lang R., et al. , Single-atom catalysts based on the metal-oxide interaction. Chem. Rev. 120, 11986–12043 (2020). [DOI] [PubMed] [Google Scholar]
- 31.Ji S. F., et al. , Chemical synthesis of single atomic site catalysts. Chem. Rev. 120, 11900–11955 (2020). [DOI] [PubMed] [Google Scholar]
- 32.Zhao D., et al. , Atomic site electrocatalysts for water splitting, oxygen reduction and selective oxidation. Chem. Soc. Rev. 49, 2215–2264 (2020). [DOI] [PubMed] [Google Scholar]
- 33.Liu L. C., Corma A., Confining isolated atoms and clusters in crystalline porous materials for catalysis. Nat. Rev. Mater. 6, 244–263 (2021). [Google Scholar]
- 34.Fei H. L., et al. , Single atom electrocatalysts supported on graphene or graphene-like carbons. Chem. Soc. Rev. 48, 5207–5241 (2019). [DOI] [PubMed] [Google Scholar]
- 35.Wang Y. C., et al. , Single atom catalysts for fuel cells and rechargeable batteries: Principles, advances, and opportunities. ACS Nano 15, 210–239 (2021). [DOI] [PubMed] [Google Scholar]
- 36.Zhao C., et al. , A high-energy and long-cycling lithium-sulfur pouch cell via a macroporous catalytic cathode with double-end binding sites. Nat. Nanotechnol. 16, 224–224 (2021). [DOI] [PubMed] [Google Scholar]
- 37.Li Y. F., Kong M. H., Hu J. P., Zhou J. S., Carbon-microcuboid-supported phosphorus-coordinated single atomic copper with ultrahigh content and its abnormal modification to Na storage behaviors. Adv. Energy Mater. 10, 2000400 (2020). [Google Scholar]
- 38.Li Y. X., et al. , Mesoporous N-rich carbon with single-Ni atoms as a multifunctional sulfur host for Li-S batteries. Angew. Chem. Int. Ed. 61, e202212680 (2022). [DOI] [PubMed] [Google Scholar]
- 39.Yang J. R., Li W. H., Wang D. S., Li Y. D., Electronic metal-support interaction of single-atom catalysts and applications in electrocatalysis. Adv. Mater. 32, 29 (2020). [DOI] [PubMed] [Google Scholar]
- 40.Qi K., Chhowalla M., Voiry D., Single atom is not alone: Metal-support interactions in single-atom catalysis. Mater. Today 40, 173–192 (2020). [Google Scholar]
- 41.Zhang H. B., et al. , Dynamic traction of lattice-confined platinum atoms into mesoporous carbon matrix for hydrogen evolution reaction. Sci. Adv. 4, 9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang J. F., et al. , Single-atom Au/NiFe layered double hydroxide electrocatalyst: Probing the origin of activity for oxygen evolution reaction. J. Am. Chem. Soc. 140, 3876–3879 (2018). [DOI] [PubMed] [Google Scholar]
- 43.Wang R. R., et al. , Implanting single Zn atoms coupled with metallic Co nanoparticles into porous carbon nanosheets grafted with carbon nanotubes for high-performance lithium-sulfur batteries. Adv. Funct. Mater. 32, 2200424 (2022). [Google Scholar]
- 44.Lin S., et al. , Covalent organic frameworks comprising cobalt porphyrins for catalytic CO2 reduction in water. Science 349, 1208–1213 (2015). [DOI] [PubMed] [Google Scholar]
- 45.Diercks C. S., et al. , Reticular electronic tuning of porphyrin active sites in covalent organic frameworks for electrocatalytic carbon dioxide reduction. J. Am. Chem. Soc. 140, 1116–1122 (2018). [DOI] [PubMed] [Google Scholar]
- 46.Lu M., et al. , Stable dioxin-linked metallophthalocyanine covalent organic frameworks (COFs) as photo-coupled electrocatalysts for CO2 reduction. Angew. Chem. Int. Ed. 60, 4864–4871 (2021). [DOI] [PubMed] [Google Scholar]
- 47.Huang N., et al. , A stable and conductive metallophthalocyanine framework for electrocatalytic carbon dioxide reduction in water. Angew. Chem. Int. Ed. 59, 16587–16593 (2020). [DOI] [PubMed] [Google Scholar]
- 48.Zhu H. J., et al. , Efficient electron transmission in covalent organic framework nanosheets for highly active electrocatalytic carbon dioxide reduction. Nat. Commun. 11, 10 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aiyappa H. B., Thote J., Shinde D. B., Banerjee R., Kurungot S., Cobalt-modified covalent organic framework as a robust water oxidation electrocatalyst. Chem. Mater. 28, 4375–4379 (2016). [Google Scholar]
- 50.Chandra S., et al. , Interplaying intrinsic and extrinsic proton conductivities in covalent organic frameworks. Chem. Mater. 28, 1489–1494 (2016). [Google Scholar]
- 51.Sun W., et al. , Coordination-induced interlinked covalent- and metal-organic-framework hybrids for enhanced lithium storage. Adv. Mater. 31, 1903176 (2019). [DOI] [PubMed] [Google Scholar]
- 52.Wang L., et al. , Phenediamine bridging phthalocyanine-based covalent organic framework polymers used as anode materials for lithium-ion batteries. Phys. Chem. Chem. Phys. 25, 8050–8063 (2023). [DOI] [PubMed] [Google Scholar]
- 53.Cao Y., et al. , Alkynyl boosted high-performance lithium storage and mechanism in covalent phenanthroline framework. Angew. Chem. Int. Ed. 62, e202302143 (2023). [DOI] [PubMed] [Google Scholar]
- 54.Nie M., et al. , Silicon solid electrolyte interphase (SEI) of lithium ion battery characterized by microscopy and spectroscopy. J. Phys. Chem. C 117, 13403–13412 (2013). [Google Scholar]
- 55.Nie M., et al. , Lithium ion battery graphite solid electrolyte interphase revealed by microscopy and spectroscopy. J. Phys. Chem. C 117, 1257–1267 (2013). [Google Scholar]
- 56.Cao Y., et al. , Dendritic sp carbon-conjugated benzothiadiazole-based polymers with synergistic multi-active groups for high-performance lithium organic batteries. Angew. Chem. Int. Ed. 63, e202316208 (2024). [DOI] [PubMed] [Google Scholar]
- 57.Zhao L., et al. , Cobalt coordinated cyano covalent-organic framework for high-performance potassium-organic batteries. ACS Appl. Mater. Interfaces 13, 48913–48922 (2021). [DOI] [PubMed] [Google Scholar]
- 58.Xu Q., et al. , Boosting lithium-sulfur battery performance by integrating a redox-active covalent organic framework in the separator. ACS Appl. Energy Mater. 2, 5793–5798 (2019). [Google Scholar]
- 59.Cao Y., et al. , Rational construction of yolk-shell bimetal-modified quinonyl-rich covalent organic polymers with ultralong lithium-storage mechanism. ACS Nano 16, 9830–9842 (2022). [DOI] [PubMed] [Google Scholar]
- 60.Guo Y., et al. , Hierarchical confinement of PtZn alloy nanoparticles and single-dispersed Zn atoms on COF@MOF-derived carbon towards efficient oxygen reduction reaction. J. Mater. Chem. A 9, 13625 (2021). [Google Scholar]
- 61.Perdew J. P., Burke K., Ernzerhof M., Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996). [DOI] [PubMed] [Google Scholar]
- 62.Ma C., Shao X., Cao D., Nitrogen-doped graphene nanosheets as anode materials for lithium ion batteries: A first-principles study. J. Mater. Chem. 22, 8911–8915 (2012). [Google Scholar]
- 63.Das D., Hardikar R. P., Han S. S., Lee K.-R., Singh A. K., Monolayer BC2: An ultrahigh capacity anode material for Li ion batteries. Phys. Chem. Chem. Phys. 19, 24230–24239 (2017). [DOI] [PubMed] [Google Scholar]
- 64.Zhang J., et al. , Graphene-like carbon-nitrogen materials as anode materials for Li-ion and Mg-ion batteries. Appl. Surf. Sci. 487, 1026–1032 (2019). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
All study data are included in the article and/or SI Appendix.