Abstract
The widespread use of antimicrobials causes antibiotic resistance in bacteria. The use of butyric acid and its derivatives is an alternative tactic. This review summarizes the literature on the role of butyric acid in the body and provides further prospects for the clinical use of its derivatives and delivery methods to the animal body. Thus far, there is evidence confirming the vital role of butyric acid in the body and the effectiveness of its derivatives when used as animal medicines and growth stimulants. Butyric acid salts stimulate immunomodulatory activity by reducing microbial colonization of the intestine and suppressing inflammation. Extraintestinal effects occur against the background of hemoglobinopathy, hypercholesterolemia, insulin resistance, and cerebral ischemia. Butyric acid derivatives inhibit histone deacetylase. Aberrant histone deacetylase activity is associated with the development of certain types of cancer in humans. Feed additives containing butyric acid salts or tributyrin are used widely in animal husbandry. They improve the functional status of the intestine and accelerate animal growth and development. On the other hand, high concentrations of butyric acid stimulate the apoptosis of epithelial cells and disrupt the intestinal barrier function. This review highlights the biological activity and the mechanism of action of butyric acid, its salts, and esters, revealing their role in the treatment of various animal and human diseases. This paper also discussed the possibility of using butyric acid and its derivatives as surface modifiers of enterosorbents to obtain new drugs with bifunctional action.
Keywords: Butyric acid, sodium butyrate, butyrates, animal husbandry
INTRODUCTION
Animal husbandry is a staple of global agriculture that aims to provide the population with food. Economic growth, urbanization, and changing food consumption models in low- and middle-income countries will increase the demand for foods of animal origin by more than twofold in the next twenty years [1]. According to forecasts, South Asian and sub-Saharan African countries will show the highest growth rates of demand for animal products [2]. Nevertheless, more attention must be paid to the quantitative and qualitative indicators of food, including those related to food safety, to meet the global demand for food products. A significant problem is the accumulation of medicinal drugs and various toxicants or their metabolites in animal products [3,4,5]. Such substances can migrate along various food chains, which poses a real threat to humans and animals [6,7,8]. The ubiquitous use of antimicrobial medicines causes antibiotic resistance in bacteria. Thus, it is necessary to develop strategies for combating the resistance of pathogens to the applied drugs [9,10,11,12,13,14]. Moreover, it is important to develop new medicines and feed additives that regulate a microbiome and increase the natural resistance of animals.
Butyric acid and its derivatives are an alternative tactic in intensive animal farming [15,16,17,18]. In this regard, this review summarizes the literature on the role of butyric acid in the body and provides further prospects for the clinical use of its derivatives and methods of their delivery to an animal body. Previous reviews presented methods for producing butyric acid and its derivatives, including using microorganisms [17]. The feasibility of their use for the functional development of the gastrointestinal tract, increasing productivity [19,20], and preventing damage to the intestinal mucosa has been shown [21]. Banasiewicz et al. [22] discussed the physiological requirements of butyrate in animals and the possibility of increasing the doses used. The reviews presented do not focus on the issues related to the possibility of expanding the clinical use of butyric acid and its derivatives as part of complex preparations. The development of such preparations based on sorbents appears important in the context of a sharply increased environmental load on animals and humans. The action of these sorbents is based on the absorption of exotoxins and endotoxins, microorganisms, and potential allergens in the intestine. This also helps normalize the enzyme and bacterial composition of the gastrointestinal tract and changes the concentration of many biologically active substances in the intestinal tissues [23].
ROLE OF BUTYRIC ACID IN A BODY
Butyric acid is a monobasic carboxylic acid (butanoic acid, CH3-(CH2)2-COOH; molar mass, 81 g/mol). At room temperature, it is a colorless liquid with the pungent odor of rancid oil. The acid is volatile, unstable in aqueous solutions, and decomposes rapidly. The melting and boiling points are −5°C and 163°C, respectively [24,25].
Butyric acid has a special place among short-chain fatty acids [26]. In animals and humans, it is formed in the large intestine due to intestinal microflora activity, which ferments dietary fiber and indigestible carbohydrates. Bifidobacteria and lactobacilli are not the primary butyric acid producers. It is mainly produced by anaerobic bacteria, such as Eubacterium, Roseburia, Faecalibacterium, and Coprococcus, as well as Fusobacterium and non-pathogenic clostridium species [27,28,29]. Butyric acid is the main source of nutrition for epithelial cells (colonocytes) and supports intestinal homeostasis. The acid improves metabolism, enhances protective functions, and prevents various intestinal diseases by supplying epithelial cells of the intestinal mucosa with energy [30,31].
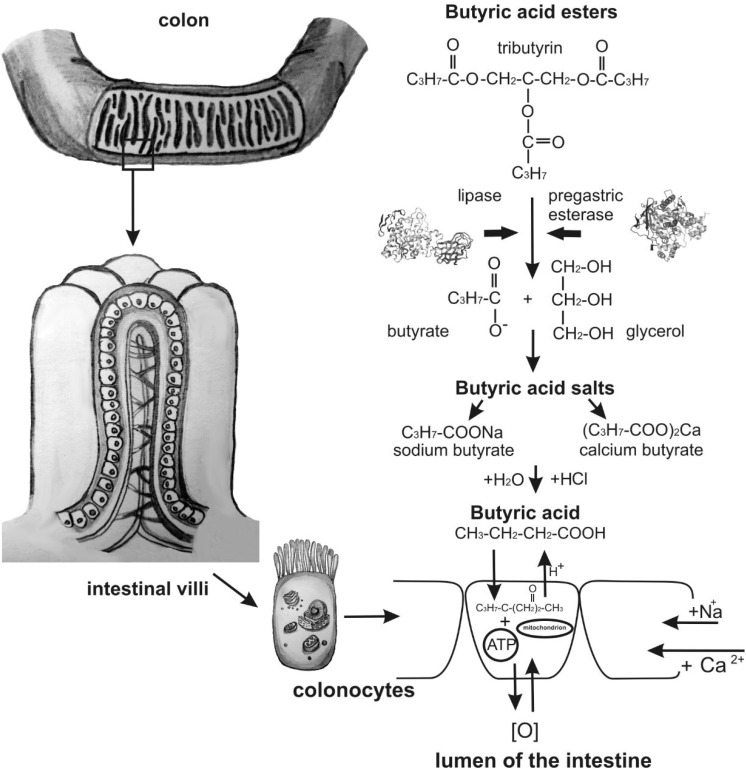
Butyric acid easily penetrates the cell membranes of enterocytes (colonocytes) and oxidizes to aldehyde and ATP molecules because it is a fat- and water-soluble compound with a relatively small molar mass [32,33]. Fig. 1 describes the absorption of butyric acid and its derivatives.
Fig. 1. Scheme of the absorption of butyric acid and its derivatives.
During oxidation, protons are released, and the pH balance of the cell decreases. Sodium enters a cell, and protons enter the intestinal lumen and affect the formation of an acidic environment because of the sodium–hydrogen exchange mechanism. This produces unfavorable conditions for the existence and development of opportunistic pathogens [24,34,35].
Butyric acid directly affects various metabolic processes in enterocytes, which contribute to the growth of the intestinal villi [36]. Bigger villi have a larger suction area, improving the digestion and assimilation of nutrients. All of these effects of butyric acid help increase the productivity of livestock [37].
Butyric acid stimulates a specific immune response and retards inflammation by suppressing the activation of nuclear factor. It reduces the formation of proinflammatory cytokines and nitric oxide. In addition, it activates the cholinergic anti-inflammatory pathway by acting through specific receptors [38,39]. The application of pure butyric acid is ineffective because most of it is inactivated in the stomach [40].
Pure butyric acid is volatile, resulting in active substance loss during feed storage. Another disadvantage is the extremely sharp, unpleasant odor that irritates the respiratory tract and provokes allergic reactions [25]. High-quality silage must not contain butyric acid. Its presence in silage indicates it was contaminated at the preparation stage, and protein breakdown processes have already begun. A large amount of butyric acid in cow diets causes severe ketosis. Therefore, silage containing more than 1% butyric acid in dry matter is undesirable and utterly unsuitable for feeding animals if there is 2%–3% [41].
BUTYRIC ACID DERIVATIVES AND THEIR PHYSICOCHEMICAL AND BIOLOGICAL PROPERTIES
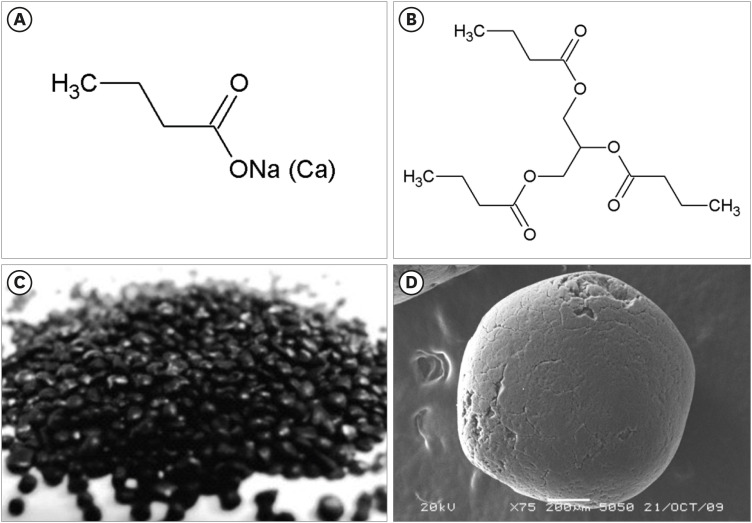
Butyric acid salts (butyrates) and glycerin and butyric acid esters (butyrins) are used in animal husbandry [17,42,43,44]. Fig. 2 presents the structural formulae of butyric acid derivatives: sodium (calcium) butyrate and tributyrin. Calcium butyrate has two anions, and sodium butyrate has only one (Fig. 2A). These differences significantly impact the interactions with water molecules and the dissociation rates.
Fig. 2. Structural formulae of sodium (calcium) butyrate (A) and tributyrin (B) and the form of the carbon intestinal sorbent Zoocarb (C) and an adsorbent pellet under a scanning electron microscope (D). Scale bar: 200 μm.
Sodium butyrate has pronounced hygroscopicity and an extremely high dissolution rate in a liquid medium [45,46]. Unlike the calcium salt, the sodium salt has higher biological activity because it interacts with the body cells directly in the active substance site of contact, and the solubility of sodium butyrate does not depend on the acidity of the medium. Sodium butyrate strongly affects pathogenic microflora because of the greater penetration of butyric acid into the cell membrane and a decrease in the pH of the internal environment. Sodium ions improve the absorption of amino acids and glucose in the intestine [47]. The reactivity and biological activity of the calcium salt are less pronounced because it is less soluble than the sodium salt, and it is directly dependent on the pH of the medium [46,48].
As bacterial resistance to antibiotics is widespread, the use of natural alternatives may be a promising option. Butyric acid derivatives induce the expression of antimicrobial peptides, the first line of defense of the mucous membranes against various microorganisms [49]. Butyrates reduce bacterial colonization and suppress inflammation [50] by modulating the expression and release of anti-inflammatory and proinflammatory cytokines [51]. Previous studies have shown the high efficiency of sodium and calcium salts of butyric acid. The choice of the active substance depends on the production tasks to be solved, the technology of feed production, and the personal preferences of experts in animal nutrition [24,45,46,47,48].
Butyrins are short–chain glycerides or lipids. Short-chain glycerides do not require a complex emulsion process. They are absorbed readily in the intestine, enter the portal vein, and move to the liver, bypassing the transformations in the intestinal wall [38]. Their typical representative is tributyrin, a structured trimolecular lipid of butyrate esterified with glycerol (Fig. 2B). In the stomach and small intestine, lipase hydrolyzes tributyrin to butyrate and glycerol (Fig. 1) [52]. Optimal hydrolysis occurs at pH 7.5–8 [53,54].
Tributyrin exhibits antibacterial and antifungal properties; it prevents thrombosis and accelerates wound-healing processes [55,56,57,58]. Experimental studies have shown that introducing tributyrin into the intestinal lumen increases the butyrate concentration in the portal vein and has a hepatoprotective effect [57]. In addition, the electrical activities of the duodenum and jejunum are stimulated when enteric administration is applied [21,59]. The antibacterial effectiveness of tributyrin against gram-negative bacteria is stronger than that of butyric acid itself [56]. Tributyrin has no adverse effects associated with the premature destruction and absorption of butyric acid in the anterior parts of the digestive system [60]. Tributyrin conditionally refers to low-toxic compounds (substance hazard category 4) [61].
Tributyrin production is based on the azeotropic esterification of glycerin and butanoic acid, where toluene acts as an azeotropic agent and orthophosphoric acid is the process catalyst. Excess butanoic acid must be distilled from the reaction under air pressure. The resulting product (tributyrin) is purified by vacuum distillation, achieving a tributyrin yield of 97–98 wt. % [62].
When choosing feed additives containing butyric acid derivatives, it is necessary to consider the amount of butyric acid that can reach the intestine. The dose of a feed additive and its effectiveness directly depends on the activity of the active substance.
APPLICATION OF BUTYRIC ACID DERIVATIVES IN ANIMAL HUSBANDRY AND CLINICAL PRACTICE
Many authors have indicated that organic acids affect the growth of poultry, its productivity, carcass yield, and digestibility of nutrients [63,64,65,66,67]. In one study, broiler chickens were fed a basic diet with or without the addition of protected calcium butyrate (0.2, 0.3, or 0.4 g/kg of prepared feed). Regardless of the dose, the feed conversion ratio improved [68]. Butyric acid is of particular interest because of its effect on animal physiology [69]. It is the preferred energy source for enterocytes and stimulates the proliferation of intestinal cells [70]. In this regard, it can be used as a component of diets for young pigs [71]. In that study [71], 160 weaned piglets ([Landrace × Yorkshire] × Duroc, 28 days old) received feed additives with different coated sodium butyrate contents (0.5 g/kg of feed; 1.5 g/kg of feed for the first three weeks, followed by a reduction to 0.75 g/kg of feed and 3 g/kg of feed during the next three weeks with a subsequent reduction to 1.5 g/kg of feed) [71]. Adding sodium butyrate increased the final body weight in a “gain: feed” ratio-dependent manner. At the same time, the amount of E. coli in the contents of the duodenum decreased, and the length of villi in the intestine increased. The research results confirmed the beneficial role of sodium butyrate in increasing the productivity and feed digestibility in weaned piglets.
Walia et al. [72] examined the role of feed additives containing sodium butyrate (3 kg/t) that had been administered to fattening pigs for four weeks before slaughter to reduce the bacterial transmission of salmonella in animals. The carriage of bacteria is characterized by prolonged persistence of the pathogen in the body without clinical manifestations of a disease but with the possibility of its manifestation under the influence of various factors [73]. The following plays an important role in the formation of bacterial carriage: genetic features of animals, antibacterial resistance of the pathogen, its anti-lysozyme activity and ability to destroy complement, and the features of the interaction of the pathogen with the immune system cells. Liver and gallbladder diseases contribute to the formation of a long-term bacterial carriage of salmonella because bile is a good nutrient medium for it. Salmonella can persist in the epithelium of the gallbladder and form biofilms that contribute to the chronification of infectious processes. When biofilms interact with bile components, their colloidal state is disturbed [74]. Animals carrying salmonella without symptoms are a serious concern because foods of animal origin often trigger salmonellosis in humans. Certain measures to control and prevent bacterial transmission are important for reducing the prevalence of this pathogen. The fecal excretion of salmonella decreased when feed additives with sodium butyrate were used, corresponding to lower seroprevalence in this group [72].
Feeding ruminants with butyrate plays an important role in rumen development. When administered to one-week-old calves, it triggers rumen papillae growth and increases pancreas secretion (especially chymotrypsin and lipase). This contributes to better absorption of nutrients and an increase in average daily weight gain [19,20]. Górka et al. [75,76,77] reported that adding butyrate to the milk substitute and the starter feed increased the rumen mass and stimulated papillae development. The butyrate-containing milk substitute had a more pronounced effect on the development of the small intestine, improved cell regeneration, and increased enzyme activity in the small intestine. Adding butyrate to the starter feed for calves before weaning prevented the development of diarrhea.
In the case of small pets, butyric acid has an energy-providing function and an anti-inflammatory effect in the intestines. Butyrates effectively prevent infectious and ulcerative colitis and irritable bowel syndrome and have oncoprotective properties [78]. When sodium butyrate unused by colonocytes enters the bloodstream, it has systemic effects, including increased tissue sensitivity to insulin [79].
Some authors reported that butyrate has cancer-protective properties [80,81,82]. On the other hand, the short biological half-life (six minutes) impedes its clinical use [83,84]. The use of tributyrin made it possible to overcome the pharmacokinetic disadvantages of butyrate. Kang et al. [85] reported the antitumor activity of tributyrin emulsion. They intended to produce an emulsion of tributyrin as a celecoxib carrier, a poorly water-soluble COX-2 inhibitor with antitumor effects. Currently, the use of celecoxib to prevent oncological disease development and progression has been validated theoretically. Tumor cells actively express COX-2, and prostaglandins synthesized by this enzyme play an essential role at all stages of oncogenesis.
Neoangiogenesis, a compulsory condition for the rapid proliferation of tumor tissue and its invasive growth, is also a COX-2-dependent process. The overexpression of COX-2 is associated with the active synthesis of thromboxane A2 by tumor cells, which plays an important role in metastasis and fixation of tumor thrombi in healthy tissues [86,87,88,89,90]. The use of a tributyrin emulsion can reduce the concentration of celecoxib necessary to suppress cancer cells by 50%, approximately 2–3 fold, possibly through the solubilizing ability and anticancer activity [85]. Earlier studies reported that both celecoxib and tributyrin inhibit the proliferation of cancer cells by inducing apoptosis [55,58]. This explains their additive effect and suggests the increased effectiveness of celecoxib/tributyrin in cancer treatment. In addition, butyric acid derivatives are histone deacetylase (HDAC) inhibitors. The aberrant activity of histone deacetylase is associated with the development of certain types of cancer in humans. In this regard, regulating aberrant genes via HDAC inhibitors is promising for preventing and treating cancers [91]. Recently, HDAC inhibitors have also been considered for use in the targeted therapy of mental disorders. Therefore, HDAC inhibitors, including butyric acid derivatives, can be used in the treatment of neuropsychiatric and neurodegenerative disorders [92,93].
Good performance in breeding poultry, which depends largely on the functional state of the digestive and reproductive systems, must be ensured for the successful development of poultry farming. Wang et al. [94] reported that feed additives containing tributyrin increase the quality of egg whites and reduce the expression of genes associated with ovarian proapoptosis, which improves reproductive function. On the other hand, a deterioration in ovarian function was noted in breeding individuals with lower egg production. This was confirmed by the lower antioxidant capacity and increased cell apoptosis rate. The concentration of reproductive hormones in the blood serum does not change significantly when tributyrin is added to the diet. Tributyrin reduces the cellular apoptosis rates and increases Bcl-2 gene expression associated with antiapoptosis. On the other hand, a diet containing tributyrin has a negligible effect on the concentration of reproductive hormones (estrogen, FSH, and progesterone) in the blood serum of birds. An increase in egg weight with a higher protein content is associated with the effect of tributyrin on the functional state of the intestine and the balance of the microbiota, which is extremely important for the sound absorption of nutrients and improving digestion. When tributyrin was used in pig breeding, piglets grew better after weaning, and there were fewer cases of diarrhea after transferring piglets to a nursery group without any additional medication [95].
Previous studies have shown that butyrins are effective in treating chickens infected with eimeriosis [96]. At the same time, they exhibit immunomodulatory activity amid the intensive growth of chickens. Moreover, a decrease in the severity of infection and suppression of oocyst formation were reported [96].
A decrease in the production of endogenous butyrate due to microbiome disruption and the development of various pathological conditions necessitates an increase in the intake of butyric acid to 30% of the daily requirements [22]. On the other hand, high concentrations of butyric acid stimulate the apoptosis of epithelial cells and disrupt the intestinal barrier function [97]. Nevertheless, one of the nine preclinical studies on the colitis model in mice showed no significant reduction in colon inflammation when using oral butyrate supplements [98]. At doses greater than 150 mmol/L, butyrate damages the mucous membrane of the colon and distal ileum in newborn rats [99]. The paradoxical effects on the intestine are that low butyrate concentrations increase the intestinal barrier function, and its excess provokes pronounced apoptosis of epithelial cells and destroys the intestinal barrier [97].
Butyric acid derivatives are also used as biologically active substances for treating various pathological conditions in humans. Positive effects have been noted in the treatment of inflammatory bowel diseases [100], colorectal cancer [101], eradication therapy of infection caused by Helicobacter pylori [102], irritable bowel syndrome [103], and functional constipation [104]. In addition, butyric acid and its derivatives have positive extraintestinal effects against the background of hemoglobinopathy, hypercholesterolemia, insulin resistance, and cerebral ischemia [105]. A randomized clinical trial confirmed the therapeutic effects of butyrate in childhood obesity [106]. At the same time, some studies reported a lack of pronounced effectiveness or conflicting results using butyric acid to treat several diseases [107,108,109].
The effect of butyrate on viral infections and the antiviral response has not been sufficiently studied. On the other hand, butyrate increases cell infection by viruses, including influenza virus, reovirus, HIV-1, human metapneumovirus, and vesicular stomatitis virus. This may be due to the inhibitory effect of butyrate on specific antiviral interferon-stimulated genes in human and mouse cells [110]. At the same time, there is evidence of changes in the intestinal microbiome and decreased butyrate production in patients with severe acute respiratory syndrome caused by coronavirus 2 (SARS-CoV-2) [111]. The butyrate-releasing drug (N-(1-carbamoyl-2-phenyl-ethyl) butyramide (FBA) positively modulates the most important aspects of infection in human small intestine biopsies and enterocytes. It reduces the expression of proinflammatory cytokines (interleukin-15, monocyte chemoattractant protein-1, and tumor necrosis factor-α) and regulates several genes involved in the antiviral response. The preventive effect of butyrate-releasing FBA against SARS-CoV-2 infections can be considered a possible strategy to limit the spread of coronavirus disease 2019 [112]. Nevertheless, further studies are needed on the clinical effectiveness of butyric acid and its derivatives in humans, necessitating comprehensive animal studies to determine the optimal doses, treatment duration, and delivery methods.
Microencapsulation during enzymatic synthesis of cyclodextrin has been proposed to increase the sensory qualities and bioavailability of butyric acid derivatives [113]. Cyclodextrins are obtained from starch or its derivatives using an enzymatic process catalyzed by cycledextringlycosyltransferase [114]. On the other hand, the product of enzymatic synthesis is a mixture of α-, β- and γ- cyclodextrins with unformed residues. Therefore, subsequent separation steps are necessary to obtain a specific cyclodextrin. This complex manufacturing process increases the cost of individual cyclodextrins and restricts their use range [115]. Li et al. [113] proposed to encapsulate tributyrin directly during the enzymatic synthesis of cyclodextrin. This approach eliminates complex isolation processes and allows using mixtures of reaction products as a potentially new material for making microcapsules.
Augustin et al. [116] proposed microencapsulation to transport a bioactive composition containing tributyrin to target organs quickly. At the same time, radioactive labels ([14C]-trilinolenin or [14C]-tributyrin and [3H]-resveratrol) were added to the bioactive composition to study their distribution in the digestive system, blood, and individual tissues of rats. Oral administration was used. Oil-in-water emulsions were stabilized with a heated mixture of milk protein, glucose, and modified starch. Microencapsulation led to an increase in the level of biologically active substances in the blood and liver and increased the bioavailability of the agents.
In recent years, developing long-acting veterinary medicines has become a relevant direction. Carbon sorbents are of particular interest in this field. The Department of Materials Science and Physicochemical Research Methods of the Center of New Chemical Technologies of the Federal Research Center Boreskov Institute of Catalysis of the Siberian Branch of the Russian Academy of Science (Center of New Chemical Technologies BIC, Russia) conducted long-term research on the production of carbon sorbent-based functional materials with preset biospecific properties for healthcare and veterinary medicine [117,118]. Organic acids hold a special place among modifiers. Nanoporous carbon sorbents modified with oligomers of lactic and glycolic acids were obtained [119]. The dependence of their detoxifying action on the nature of the modifier was proven experimentally [120].
The use of butyric acid derivatives as surface modifiers of the carbon intestinal sorbent Zoocarb (TU 9318-003-71069834-2016) is a promising approach. Fig. 2C and D present the carbon intestinal sorbent Zoocarb.
The advantages of this matrix include high chemical purity (at least 99.5% carbon), smooth surface relief, and spherical granules (size of 0.1–0.5 mm) to prevent injury to the mucous membrane of the gastrointestinal tract. The technology of sorbent production ensures an almost complete absence of dust on its surface and in the pores and guarantees high robustness of the granules. The mesoporous structure of the carbon sorbent contributes to its good low and medium molecular weight substances adsorption capacity, including that of butyric acid derivatives, which is necessary for the local delivery of active substances into the intestine and prolongation of the pharmacological effect.
Modification of the sorbent surface with oxygen-containing hydrophilic functional groups of butyrates and butyrins will allow highly effective preparations with improved sensory properties. These preparations can preserve the useful properties of the modifiers.
Research has been conducted to develop a method for modifying a carbon sorbent with butyric acid derivatives [121]. In particular, according to the results of adsorption studies, the optimal conditions were chosen for carbon sorbent modification with tributyrin. The sorbent/modifier ratio was 1/10; the duration of impregnation with ethanol solutions of tributyrin was 24 h at room temperature under static conditions. The sorbent was dried in air for 24 h and then in an inert atmosphere for 2 h at 105°C to remove ethanol vapor completely [121]. Physicochemical studies showed that the sorbent modified with the ethanol solution of tributyrin with an initial concentration of 227 g/L was of the greatest interest. A ~28 wt. % modifier was deposited onto this sorbent. This modified sample was characterized by a specific surface area of 16 m2/g and contained carboxylic and phenolic oxygen-containing groups on its surface (0.243 mmol/g). Desorption research has shown that upon contact with the physiological solution (0.9% aqueous solution of sodium chloride) and ethanol solution (96%), tributyrin deposited on the carbon support is gradually desorbed within 6 h. In this process, the pH of the model solutions decreased by 3–4 units within 30 min, and the amount of deposited modifier decreased from 28 to 6 wt. %. The specific surface area of the sample increased regularly from 16 to 195 m2/g [121]. Future studies will examine in more detail the desorption of the modifier from the samples of carbon sorbents in biorelevant media and describe the mechanisms of this process. The efficient and safe use of these modified carbon sorbents in animal husbandry and veterinary medicine will also be examined.
DISCUSSION
Butyric acid and its derivatives can improve the morpho-functional state of the intestine, activate digestive processes by increasing the size of the intestinal villi, strengthening the intestinal mucosal barrier, stimulating cellular immunity, and developing optimal conditions for beneficial microflora, which reduces the risk of infectious and invasive diseases significantly. This opens up great outlooks for developing new medicines based on butyrates and butyrins.
ACKNOWLEDGMENTS
The authors are very grateful to the editors and reviewers for their valuable comments and recommendations on the article.
Footnotes
Funding: This work was supported by the Ministry of Science and Higher Education of the Russian Federation within the governmental assignment for Boreskov Institute of Catalysis (project FWUR-2024-0039).
Conflict of Interest: The authors declare no conflicts of interest.
- Conceptualization: Gerunova LK, Gerunov TV.
- Data curation: P’yanova LG, Lavrenov AV, Fedorov YN.
- Formal analysis, Resources: Sedanova AV, Delyagina MS, Kornienko NV, Kryuchek YO, Tarasenko AA.
- Visualisation: Sedanova AV, Gerunov TV, Delyagina MS.
- Writing - original draft: Gerunova LK, Gerunov TV, Delyagina MS.
- Writing - review & editing: P’yanova LG, Lavrenov AV, Fedorov YN.
References
- 1.Alders RG, Campbell A, Costa R, Guèye EF, Ahasanul Hoque M, Perezgrovas-Garza R, et al. Livestock across the world: diverse animal species with complex roles in human societies and ecosystem services. Anim Front. 2021;11(5):20–29. doi: 10.1093/af/vfab047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Komarek AM, Dunston S, Enahoro D, Godfray HC, Herrero M, Mason-D’Croz D, et al. Income, consumer preferences, and the future of livestock-derived food demand. Glob Environ Change. 2021;70:102343. doi: 10.1016/j.gloenvcha.2021.102343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lebelo K, Malebo N, Mochane MJ, Masinde M. Chemical contamination pathways and the food safety implications along the various stages of food production: a review. Int J Environ Res Public Health. 2021;18(11):5795. doi: 10.3390/ijerph18115795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel T, Marmulak T, Gehring R, Pitesky M, Clapham MO, Tell LA. Drug residues in poultry meat: a literature review of commonly used veterinary antibacterials and anthelmintics used in poultry. J Vet Pharmacol Ther. 2018;41(6):761–789. doi: 10.1111/jvp.12700. [DOI] [PubMed] [Google Scholar]
- 5.Yamaguchi T, Okihashi M, Harada K, Konishi Y, Uchida K, Do MH, et al. Antibiotic residue monitoring results for pork, chicken, and beef samples in Vietnam in 2012–2013. J Agric Food Chem. 2015;63(21):5141–5145. doi: 10.1021/jf505254y. [DOI] [PubMed] [Google Scholar]
- 6.Becker-Algeri TA, Castagnaro D, de Bortoli K, de Souza C, Drunkler DA, Badiale-Furlong E. Mycotoxins in bovine milk and dairy products: a review. J Food Sci. 2016;81(3):R544–R552. doi: 10.1111/1750-3841.13204. [DOI] [PubMed] [Google Scholar]
- 7.Canton L, Lanusse C, Moreno L. Rational pharmacotherapy in infectious diseases: issues related to drug residues in edible animal tissues. Animals (Basel) 2021;11(10):2878. doi: 10.3390/ani11102878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ng CA, von Goetz N. The global food system as a transport pathway for hazardous chemicals: the missing link between emissions and exposure. Environ Health Perspect. 2017;125(1):1–7. doi: 10.1289/EHP168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoelzer K, Wong N, Thomas J, Talkington K, Jungman E, Coukell A. Antimicrobial drug use in food-producing animals and associated human health risks: what, and how strong, is the evidence? BMC Vet Res. 2017;13(1):211. doi: 10.1186/s12917-017-1131-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma F, Xu S, Tang Z, Li Z, Zhang L. Use of antimicrobials in food animals and impact of transmission of antimicrobial resistance on humans. Biosafety and Health. 2021;3(1):32–38. [Google Scholar]
- 11.Marshall BM, Levy SB. Food animals and antimicrobials: impacts on human health. Clin Microbiol Rev. 2011;24(4):718–733. doi: 10.1128/CMR.00002-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pokharel S, Shrestha P, Adhikari B. Antimicrobial use in food animals and human health: time to implement ‘One Health’ approach. Antimicrob Resist Infect Control. 2020;9(1):181. doi: 10.1186/s13756-020-00847-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sazykin IS, Khmelevtsova LE, Seliverstova EY, Sazykina MA. Effect of antibiotics used in animal husbandry on the distribution of bacterial drug resistance. Appl Biochem Microbiol. 2021;57(1):20–30. [Google Scholar]
- 14.Waluszewski A, Cinti A, Perna A. Antibiotics in pig meat production: restrictions as the odd case and overuse as normality? Experiences from Sweden and Italy. Humanit Soc Sci Commun. 2021;8(1):172. [Google Scholar]
- 15.Bedford A, Gong J. Implications of butyrate and its derivatives for gut health and animal production. Anim Nutr. 2018;4(2):151–159. doi: 10.1016/j.aninu.2017.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gomez-Osorio LM, Yepes-Medina V, Ballou A, Parini M, Angel R. Short and medium chain fatty acids and their derivatives as a natural strategy in the control of necrotic enteritis and microbial homeostasis in broiler chickens. Front Vet Sci. 2021;8:773372. doi: 10.3389/fvets.2021.773372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang L, Fu H, Yang HK, Xu W, Wang J, Yang ST. Butyric acid: applications and recent advances in its bioproduction. Biotechnol Adv. 2018;36(8):2101–2117. doi: 10.1016/j.biotechadv.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 18.Yang Q, Chen B, Robinson K, Belem T, Lyu W, Deng Z, et al. Butyrate in combination with forskolin alleviates necrotic enteritis, increases feed efficiency, and improves carcass composition of broilers. J Anim Sci Biotechnol. 2022;13(1):3. doi: 10.1186/s40104-021-00663-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Górka P, Kowalski ZM, Zabielski R, Guilloteau P. Invited review: Use of butyrate to promote gastrointestinal tract development in calves. J Dairy Sci. 2018;101(6):4785–4800. doi: 10.3168/jds.2017-14086. [DOI] [PubMed] [Google Scholar]
- 20.Niwińska B, Hanczakowska E, Arciszewski MB, Klebaniuk R. Review: Exogenous butyrate: implications for the functional development of ruminal epithelium and calf performance. Animal. 2017;11(9):1522–1530. doi: 10.1017/S1751731117000167. [DOI] [PubMed] [Google Scholar]
- 21.Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost FJ, Brummer RJ. Review article: the role of butyrate on colonic function. Aliment Pharmacol Ther. 2008;27(2):104–119. doi: 10.1111/j.1365-2036.2007.03562.x. [DOI] [PubMed] [Google Scholar]
- 22.Banasiewicz T, Domagalska D, Borycka-Kiciak K, Rydzewska G. Determination of butyric acid dosage based on clinical and experimental studies - a literature review. Prz Gastroenterol. 2020;15(2):119–125. doi: 10.5114/pg.2020.95556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gerunov TV, Drozdetskaya MS, Gerunova LK, P’yanova LG. Enterosorbents in veterinary: significance and prospects of new medicinal products for animal use. Innov Food Saf. 2017;3:17–24. [Google Scholar]
- 24.Gothals L, Gorbakova A. Stimulyatory rosta na osnove maslyanoj kisloty dlya svinovodstva. Kombikorma. 2015;9:92–95. [Google Scholar]
- 25.Luo H, Yang R, Zhao Y, Wang Z, Liu Z, Huang M, et al. Recent advances and strategies in process and strain engineering for the production of butyric acid by microbial fermentation. Bioresour Technol. 2018;253:343–354. doi: 10.1016/j.biortech.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 26.Mirzaei R, Afaghi A, Babakhani S, Sohrabi MR, Hosseini-Fard SR, Babolhavaeji K, et al. Role of microbiota-derived short-chain fatty acids in cancer development and prevention. Biomed Pharmacother. 2021;139:111619. doi: 10.1016/j.biopha.2021.111619. [DOI] [PubMed] [Google Scholar]
- 27.Hold GL, Schwiertz A, Aminov RI, Blaut M, Flint HJ. Oligonucleotide probes that detect quantitatively significant groups of butyrate-producing bacteria in human feces. Appl Environ Microbiol. 2003;69(7):4320–4324. doi: 10.1128/AEM.69.7.4320-4324.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feng J, Guo X, Cai F, Fu H, Wang J. Model-based driving mechanism analysis for butyric acid production in Clostridium tyrobutyricum . Biotechnol Biofuels Bioprod. 2022;15(1):71. doi: 10.1186/s13068-022-02169-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fu H, Yue Z, Feng J, Bao T, Yang ST, Cai Y, et al. Consolidated bioprocessing for butyric acid production from raw cassava starch by a newly isolated Clostridium butyricum SCUT620. Ind Crops Prod. 2022;187:115446 [Google Scholar]
- 30.Larsson SC, Wolk A. Meat consumption and risk of colorectal cancer: a meta-analysis of prospective studies. Int J Cancer. 2006;119(11):2657–2664. doi: 10.1002/ijc.22170. [DOI] [PubMed] [Google Scholar]
- 31.Litvak Y, Byndloss MX, Bäumler AJ. Colonocyte metabolism shapes the gut microbiota. Science. 2018;362(6418):eaat9076. doi: 10.1126/science.aat9076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee S, Knotts TA, Goodson ML, Barboza M, Wudeck E, England G, et al. Metabolic responses to butyrate supplementation in LF- and HF-fed mice are cohort-dependent and associated with changes in composition and function of the gut microbiota. Nutrients. 2020;12(11):3524. doi: 10.3390/nu12113524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salvi PS, Cowles RA. Butyrate and the intestinal epithelium: modulation of proliferation and inflammation in homeostasis and disease. Cells. 2021;10(7):1775. doi: 10.3390/cells10071775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boyen F, Haesebrouck F, Vanparys A, Volf J, Mahu M, Van Immerseel F, et al. Coated fatty acids alter virulence properties of Salmonella Typhimurium and decrease intestinal colonization of pigs. Vet Microbiol. 2008;132(3-4):319–327. doi: 10.1016/j.vetmic.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 35.Gurney MA, Laubitz D, Ghishan FK, Kiela PR. Pathophysiology of intestinal Na+/H+ exchange. Cell Mol Gastroenterol Hepatol. 2017;3(1):27–40. doi: 10.1016/j.jcmgh.2016.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Papadopoulos GA, Poutahidis T, Chalvatzi S, Kroustallas F, Karavanis E, Fortomaris P. Effects of a tributyrin and monolaurin blend compared to high ZnO levels on growth performance, faecal microbial counts, intestinal histomorphometry and immunohistochemistry in weaned piglets: a field study in two pig herds. Res Vet Sci. 2022;144:54–65. doi: 10.1016/j.rvsc.2022.01.011. [DOI] [PubMed] [Google Scholar]
- 37.Masmeijer C, Rogge T, van Leenen K, De Cremer L, Deprez P, Cox E, et al. Effects of glycerol-esters of saturated short- and medium chain fatty acids on immune, health and growth variables in veal calves. Prev Vet Med. 2020;178:104983. doi: 10.1016/j.prevetmed.2020.104983. [DOI] [PubMed] [Google Scholar]
- 38.Usami M, Kishimoto K, Ohata A, Miyoshi M, Aoyama M, Fueda Y, et al. Butyrate and trichostatin A attenuate nuclear factor kappaB activation and tumor necrosis factor alpha secretion and increase prostaglandin E2 secretion in human peripheral blood mononuclear cells. Nutr Res. 2008;28(5):321–328. doi: 10.1016/j.nutres.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 39.Vinolo MA, Rodrigues HG, Nachbar RT, Curi R. Regulation of inflammation by short chain fatty acids. Nutrients. 2011;3(10):858–876. doi: 10.3390/nu3100858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hansen VL, Kahl S, Proszkowiec-Weglarz M, Jiménez SC, Vaessen SF, Schreier LL, et al. The effects of tributyrin supplementation on weight gain and intestinal gene expression in broiler chickens during Eimeria maxima-induced coccidiosis. Poult Sci. 2021;100(4):100984. doi: 10.1016/j.psj.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cao Q, Zhang W, Lian T, Wang S, Dong H. Short chain carboxylic acids production and dynamicity of microbial communities from co-digestion of swine manure and corn silage. Bioresour Technol. 2021;320(Pt B):124400. doi: 10.1016/j.biortech.2020.124400. [DOI] [PubMed] [Google Scholar]
- 42.Guilloteau P, Martin L, Eeckhaut V, Ducatelle R, Zabielski R, Van Immerseel F. From the gut to the peripheral tissues: the multiple effects of butyrate. Nutr Res Rev. 2010;23(2):366–384. doi: 10.1017/S0954422410000247. [DOI] [PubMed] [Google Scholar]
- 43.Lum J, Sygall R, Felip JM. Comparison of tributyrin and coated sodium butyrate as sources of butyric acid for improvement of growth performance in Ross 308 broilers. Int J Poult Sci. 2018;17(6):290–294. [Google Scholar]
- 44.Roda A, Simoni P, Magliulo M, Nanni P, Baraldini M, Roda G, et al. A new oral formulation for the release of sodium butyrate in the ileo-cecal region and colon. World J Gastroenterol. 2007;13(7):1079–1084. doi: 10.3748/wjg.v13.i7.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Russo R, Santarcangelo C, Badolati N, Sommella E, De Filippis A, Dacrema M, et al. In vivo bioavailability and in vitro toxicological evaluation of the new butyric acid releaser N-(1-carbamoyl-2-phenyl-ethyl) butyramide. Biomed Pharmacother. 2021;137:111385. doi: 10.1016/j.biopha.2021.111385. [DOI] [PubMed] [Google Scholar]
- 46.Zhao H, Bai H, Deng F, Zhong R, Liu L, Chen L, et al. Chemically protected sodium butyrate improves growth performance and early development and function of small intestine in broilers as one effective substitute for antibiotics. Antibiotics (Basel) 2022;11(2):132. doi: 10.3390/antibiotics11020132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thorsen K, Drengstig T, Ruoff P. Transepithelial glucose transport and Na+/K+ homeostasis in enterocytes: an integrative model. Am J Physiol Cell Physiol. 2014;307(4):C320–C337. doi: 10.1152/ajpcell.00068.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gothals L, Gorbakova A. Sravnitel’naya harakteristiki butiratov. Kombikorma. 2014;5:43–46. [Google Scholar]
- 49.Sunkara LT, Achanta M, Schreiber NB, Bommineni YR, Dai G, Jiang W, et al. Butyrate enhances disease resistance of chickens by inducing antimicrobial host defense peptide gene expression. PLoS One. 2011;6(11):e27225. doi: 10.1371/journal.pone.0027225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smulikowska S, Czerwiński J, Mieczkowska A, Jankowiak J. The effect of fat-coated organic acid salts and a feed enzyme on growth performance, nutrient utilization, microflora activity, and morphology of the small intestine in broiler chickens. J Anim Feed Sci. 2009;18(3):478–489. [Google Scholar]
- 51.Zhang WH, Jiang Y, Zhu QF, Gao F, Dai SF, Chen J, et al. Sodium butyrate maintains growth performance by regulating the immune response in broiler chickens. Br Poult Sci. 2011;52(3):292–301. doi: 10.1080/00071668.2011.578121. [DOI] [PubMed] [Google Scholar]
- 52.Wächtershäuser A, Stein J. Rationale for the luminal provision of butyrate in intestinal diseases. Eur J Nutr. 2000;39(4):164–171. doi: 10.1007/s003940070020. [DOI] [PubMed] [Google Scholar]
- 53.Fernandes TV, Keesman KJ, Zeeman G, van Lier JB. Effect of ammonia on the anaerobic hydrolysis of cellulose and tributyrin. Biomass Bioenergy. 2012;47:316–323. [Google Scholar]
- 54.Wu HS, Tsai MJ. Kinetics of tributyrin hydrolysis by lipase. Enzyme Microb Technol. 2004;35(6-7):488–493. [Google Scholar]
- 55.Kang SN, Lee E, Lee MK, Lim SJ. Preparation and evaluation of tributyrin emulsion as a potent anti-cancer agent against melanoma. Drug Deliv. 2011;18(2):143–149. doi: 10.3109/10717544.2010.522610. [DOI] [PubMed] [Google Scholar]
- 56.Kovanda L, Zhang W, Wei X, Luo J, Wu X, Atwill ER, et al. In vitro antimicrobial activities of organic acids and their derivatives on several species of gram-negative and gram-positive bacteria. Molecules. 2019;24(20):3770. doi: 10.3390/molecules24203770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Miyoshi M, Sakaki H, Usami M, Iizuka N, Shuno K, Aoyama M, et al. Oral administration of tributyrin increases concentration of butyrate in the portal vein and prevents lipopolysaccharide-induced liver injury in rats. Clin Nutr. 2011;30(2):252–258. doi: 10.1016/j.clnu.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 58.Venkatesan P, Puvvada N, Dash R, Prashanth Kumar BN, Sarkar D, Azab B, et al. The potential of celecoxib-loaded hydroxyapatite-chitosan nanocomposite for the treatment of colon cancer. Biomaterials. 2011;32(15):3794–3806. doi: 10.1016/j.biomaterials.2011.01.027. [DOI] [PubMed] [Google Scholar]
- 59.Leonel AJ, Teixeira LG, Oliveira RP, Santiago AF, Batista NV, Ferreira TR, et al. Antioxidative and immunomodulatory effects of tributyrin supplementation on experimental colitis. Br J Nutr. 2013;109(8):1396–1407. doi: 10.1017/S000711451200342X. [DOI] [PubMed] [Google Scholar]
- 60.Li J, Hou Y, Yi D, Zhang J, Wang L, Qiu H, et al. Effects of tributyrin on intestinal energy status, antioxidative capacity and immune response to lipopolysaccharide challenge in broilers. Asian-Australas J Anim Sci. 2015;28(12):1784–1793. doi: 10.5713/ajas.15.0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.United Nations. Globally Harmonized System of Classification and Labelling of Chemicals (GHS Rev. 9, 2021) [Internet] New York: United Nations; [Accessed 2023 Sep 13]. http://2021 . https://unece.org/transport/standards/transport/dangerous-goods/ghs-rev9-2021. [Google Scholar]
- 62.Levanova SV, Krasnykh EL, Moiseeva SV, Safronov SP, Glazko IL. Scientific and technological features of synthesis of new ester plasticizers based on renewable raw materials. ChemChemTech. 2021;64(6):69–75. [Google Scholar]
- 63.Van Immerseel F, Boyen F, Gantois I, Timbermont L, Bohez L, Pasmans F, et al. Supplementation of coated butyric acid in the feed reduces colonization and shedding of Salmonella in poultry. Poult Sci. 2005;84(12):1851–1856. doi: 10.1093/ps/84.12.1851. [DOI] [PubMed] [Google Scholar]
- 64.Leeson S, Namkung H, Antongiovanni M, Lee EH. Effect of butyric acid on the performance and carcass yield of broiler chickens. Poult Sci. 2005;84(9):1418–1422. doi: 10.1093/ps/84.9.1418. [DOI] [PubMed] [Google Scholar]
- 65.Polycarpo GV, Andretta I, Kipper M, Cruz-Polycarpo VC, Dadalt JC, Rodrigues PH, et al. Meta-analytic study of organic acids as an alternative performance-enhancing feed additive to antibiotics for broiler chickens. Poult Sci. 2017;96(10):3645–3653. doi: 10.3382/ps/pex178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mustafa A, Bai S, Zeng Q, Ding X, Wang J, Xuan Y, et al. Effect of organic acids on growth performance, intestinal morphology, and immunity of broiler chickens with and without coccidial challenge. AMB Express. 2021;11(1):140. doi: 10.1186/s13568-021-01299-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ma J, Wang J, Mahfuz S, Long S, Wu D, Gao J, et al. Supplementation of mixed organic acids improves growth performance, meat quality, gut morphology and volatile fatty acids of broiler chicken. Animals (Basel) 2021;11(11):3020. doi: 10.3390/ani11113020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kaczmarek SA, Barri A, Hejdysz M, Rutkowski A. Effect of different doses of coated butyric acid on growth performance and energy utilization in broilers. Poult Sci. 2016;95(4):851–859. doi: 10.3382/ps/pev382. [DOI] [PubMed] [Google Scholar]
- 69.Mroz Z, Koopmans SJ, Bannink A, Partanen AK, Krasucki W, Øverland M, et al. In: Biology of Nutrition in Growing Animals. Mosenthin R, Zentek J, Zebrowska T, editors. Amsterdam: Elsevier; 2005. Carboxylic acids as bioregulators and gut growth promoters in non-ruminants; pp. 1–79. [Google Scholar]
- 70.Kien CL, Blauwiekel R, Bunn JY, Jetton TL, Frankel WL, Holst JJ. Cecal infusion of butyrate increases intestinal cell proliferation in piglets. J Nutr. 2007;137(4):916–922. doi: 10.1093/jn/137.4.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Upadhaya SD, Yang J, Kim YM, Lee KY, Kim IH. Coated sodium butyrate supplementation to a reduced nutrient diet enhanced the performance and positively impacted villus height and faecal and digesta bacterial composition in weaner pigs. Anim Feed Sci Technol. 2020;265:114534 [Google Scholar]
- 72.Walia K, Argüello H, Lynch H, Leonard FC, Grant J, Yearsley D, et al. Effect of feeding sodium butyrate in the late finishing period on Salmonella carriage, seroprevalence, and growth of finishing pigs. Prev Vet Med. 2016;131:79–86. doi: 10.1016/j.prevetmed.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 73.Molyneux DH. Control of human parasitic diseases: context and overview. Adv Parasitol. 2006;61:1–45. doi: 10.1016/S0065-308X(05)61001-9. [DOI] [PubMed] [Google Scholar]
- 74.Gonzalez-Escobedo G, Gunn JS. Gallbladder epithelium as a niche for chronic Salmonella carriage. Infect Immun. 2013;81(8):2920–2930. doi: 10.1128/IAI.00258-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Górka P, Kowalski ZM, Pietrzak P, Kotunia A, Jagusiak W, Holst JJ, et al. Effect of method of delivery of sodium butyrate on rumen development in newborn calves. J Dairy Sci. 2011;94(11):5578–5588. doi: 10.3168/jds.2011-4166. [DOI] [PubMed] [Google Scholar]
- 76.Górka P, Kowalski ZM, Pietrzak P, Kotunia A, Jagusiak W, Zabielski R. Is rumen development in newborn calves affected by different liquid feeds and small intestine development? J Dairy Sci. 2011;94(6):3002–3013. doi: 10.3168/jds.2010-3499. [DOI] [PubMed] [Google Scholar]
- 77.Górka P, Pietrzak P, Kotunia A, Zabielski R, Kowalski ZM. Effect of method of delivery of sodium butyrate on maturation of the small intestine in newborn calves. J Dairy Sci. 2014;97(2):1026–1035. doi: 10.3168/jds.2013-7251. [DOI] [PubMed] [Google Scholar]
- 78.Simpson JW. Diet and large intestinal disease in dogs and cats. J Nutr. 1998;128(12) Suppl:2717S–2722S. doi: 10.1093/jn/128.12.2717S. [DOI] [PubMed] [Google Scholar]
- 79.Gao Z, Yin J, Zhang J, Ward RE, Martin RJ, Lefevre M, et al. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes. 2009;58(7):1509–1517. doi: 10.2337/db08-1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kuwajima A, Iwashita J, Murata J, Abe T. The histone deacetylase inhibitor butyrate inhibits melanoma cell invasion of Matrigel. Anticancer Res. 2007;27(6B):4163–4169. [PubMed] [Google Scholar]
- 81.Marks PA, Richon VM, Rifkind RA. Histone deacetylase inhibitors: inducers of differentiation or apoptosis of transformed cells. J Natl Cancer Inst. 2000;92(15):1210–1216. doi: 10.1093/jnci/92.15.1210. [DOI] [PubMed] [Google Scholar]
- 82.Munshi A, Kurland JF, Nishikawa T, Tanaka T, Hobbs ML, Tucker SL, et al. Histone deacetylase inhibitors radiosensitize human melanoma cells by suppressing DNA repair activity. Clin Cancer Res. 2005;11(13):4912–4922. doi: 10.1158/1078-0432.CCR-04-2088. [DOI] [PubMed] [Google Scholar]
- 83.Heerdt BG, Houston MA, Anthony GM, Augenlicht LH. Initiation of growth arrest and apoptosis of MCF-7 mammary carcinoma cells by tributyrin, a triglyceride analogue of the short-chain fatty acid butyrate, is associated with mitochondrial activity. Cancer Res. 1999;59(7):1584–1591. [PubMed] [Google Scholar]
- 84.Su J, Ho PC. Preparation of tributyrin emulsion and characterization of the binding of the emulsion particles to low-density lipoprotein in vitro . J Pharm Sci. 2004;93(7):1755–1765. doi: 10.1002/jps.20092. [DOI] [PubMed] [Google Scholar]
- 85.Kang SN, Hong SS, Lee MK, Lim SJ. Dual function of tributyrin emulsion: solubilization and enhancement of anticancer effect of celecoxib. Int J Pharm. 2012;428(1-2):76–81. doi: 10.1016/j.ijpharm.2012.02.037. [DOI] [PubMed] [Google Scholar]
- 86.Gately S, Li WW. Multiple roles of COX-2 in tumor angiogenesis: a target for antiangiogenic therapy. Semin Oncol. 2004;31(2) Suppl 7:2–11. doi: 10.1053/j.seminoncol.2004.03.040. [DOI] [PubMed] [Google Scholar]
- 87.Grösch S, Maier TJ, Schiffmann S, Geisslinger G. Cyclooxygenase-2 (COX-2)-independent anticarcinogenic effects of selective COX-2 inhibitors. J Natl Cancer Inst. 2006;98(11):736–747. doi: 10.1093/jnci/djj206. [DOI] [PubMed] [Google Scholar]
- 88.Harris RE. Cyclooxygenase-2 (cox-2) and the inflammogenesis of cancer. Subcell Biochem. 2007;42:93–126. doi: 10.1007/1-4020-5688-5_4. [DOI] [PubMed] [Google Scholar]
- 89.Khan KN, Masferrer JL, Woerner BM, Soslow R, Koki AT. Enhanced cyclooxygenase-2 expression in sporadic and familial adenomatous polyposis of the human colon. Scand J Gastroenterol. 2001;36(8):865–869. doi: 10.1080/003655201750313405. [DOI] [PubMed] [Google Scholar]
- 90.Thun MJ, Henley SJ, Patrono C. Nonsteroidal anti-inflammatory drugs as anticancer agents: mechanistic, pharmacologic, and clinical issues. J Natl Cancer Inst. 2002;94(4):252–266. doi: 10.1093/jnci/94.4.252. [DOI] [PubMed] [Google Scholar]
- 91.Weisberg E, Catley L, Kujawa J, Atadja P, Remiszewski S, Fuerst P, et al. Histone deacetylase inhibitor NVP-LAQ824 has significant activity against myeloid leukemia cells in vitro and in vivo . Leukemia. 2004;18(12):1951–1963. doi: 10.1038/sj.leu.2403519. [DOI] [PubMed] [Google Scholar]
- 92.Dinarello CA, Fossati G, Mascagni P. Histone deacetylase inhibitors for treating a spectrum of diseases not related to cancer. Mol Med. 2011;17(5-6):333–352. doi: 10.2119/molmed.2011.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Haberland M, Montgomery RL, Olson EN. The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nat Rev Genet. 2009;10(1):32–42. doi: 10.1038/nrg2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang J, Zhang H, Bai S, Zeng Q, Su Z, Zhuo Y, et al. Dietary tributyrin improves reproductive performance, antioxidant capacity, and ovary function of broiler breeders. Poult Sci. 2021;100(11):101429. doi: 10.1016/j.psj.2021.101429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kotunia A, Woliński J, Laubitz D, Jurkowska M, Romé V, Guilloteau P, et al. Effect of sodium butyrate on the small intestine development in neonatal piglets fed [correction of feed] by artificial sow. J Physiol Pharmacol. 2004;55(Suppl 2):59–68. [PubMed] [Google Scholar]
- 96.Proszkowiec-Weglarz M, Miska KB, Schreier LL, Grim CJ, Jarvis KG, Shao J, et al. Research note: effect of butyric acid glycerol esters on ileal and cecal mucosal and luminal microbiota in chickens challenged with Eimeria maxima . Poult Sci. 2020;99(10):5143–5148. doi: 10.1016/j.psj.2020.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Peng L, He Z, Chen W, Holzman IR, Lin J. Effects of butyrate on intestinal barrier function in a Caco-2 cell monolayer model of intestinal barrier. Pediatr Res. 2007;61(1):37–41. doi: 10.1203/01.pdr.0000250014.92242.f3. [DOI] [PubMed] [Google Scholar]
- 98.Lee JG, Lee J, Lee AR, Jo SV, Park CH, Han DS, et al. Impact of short-chain fatty acid supplementation on gut inflammation and microbiota composition in a murine colitis model. J Nutr Biochem. 2022;101:108926. doi: 10.1016/j.jnutbio.2021.108926. [DOI] [PubMed] [Google Scholar]
- 99.Lin J, Nafday SM, Chauvin SN, Magid MS, Pabbatireddy S, Holzman IR, et al. Variable effects of short chain fatty acids and lactic acid in inducing intestinal mucosal injury in newborn rats. J Pediatr Gastroenterol Nutr. 2002;35(4):545–550. doi: 10.1097/00005176-200210000-00016. [DOI] [PubMed] [Google Scholar]
- 100.Ota S, Sakuraba H. Uptake and advanced therapy of butyrate in inflammatory bowel disease. Immuno. 2022;2(4):692–702. [Google Scholar]
- 101.Kaźmierczak-Siedlecka K, Marano L, Merola E, Roviello F, Połom K. Sodium butyrate in both prevention and supportive treatment of colorectal cancer. Front Cell Infect Microbiol. 2022;12:1023806. doi: 10.3389/fcimb.2022.1023806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Andreev DN, Kucheryavyy YA, Maev IV. Efficacy of butyric acid inclusion in eradication regimens for Helicobacter pylori infection: a meta-analysis of controlled trials. Ter Arkh. 2021;93(2):158–163. doi: 10.26442/00403660.2021.02.200608. [DOI] [PubMed] [Google Scholar]
- 103.Lewandowski K, Kaniewska M, Karłowicz K, Rosołowski M, Rydzewska G. The effectiveness of microencapsulated sodium butyrate at reducing symptoms in patients with irritable bowel syndrome. Prz Gastroenterol. 2022;17(1):28–34. doi: 10.5114/pg.2021.112681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pituch A, Walkowiak J, Banaszkiewicz A. Butyric acid in functional constipation. Prz Gastroenterol. 2013;8(5):295–298. doi: 10.5114/pg.2013.38731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Canani RB, Costanzo MD, Leone L, Pedata M, Meli R, Calignano A. Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J Gastroenterol. 2011;17(12):1519–1528. doi: 10.3748/wjg.v17.i12.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Coppola S, Nocerino R, Paparo L, Bedogni G, Calignano A, Di Scala C, et al. Therapeutic effects of butyrate on pediatric obesity: a randomized clinical trial. JAMA Netw Open. 2022;5(12):e2244912. doi: 10.1001/jamanetworkopen.2022.44912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Liu H, Wang J, He T, Becker S, Zhang G, Li D, et al. Butyrate: a double-edged sword for health? Adv Nutr. 2018;9(1):21–29. doi: 10.1093/advances/nmx009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.de Groot PF, Nikolic T, Imangaliyev S, Bekkering S, Duinkerken G, Keij FM, et al. Oral butyrate does not affect innate immunity and islet autoimmunity in individuals with longstanding type 1 diabetes: a randomised controlled trial. Diabetologia. 2020;63(3):597–610. doi: 10.1007/s00125-019-05073-8. [DOI] [PubMed] [Google Scholar]
- 109.Pietrzak A, Banasiuk M, Szczepanik M, Borys-Iwanicka A, Pytrus T, Walkowiak J, et al. Sodium butyrate effectiveness in children and adolescents with newly diagnosed inflammatory bowel diseases-randomized placebo-controlled multicenter trial. Nutrients. 2022;14(16):3283. doi: 10.3390/nu14163283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chemudupati M, Kenney AD, Smith AC, Fillinger RJ, Zhang L, Zani A, et al. Butyrate reprograms expression of specific interferon-stimulated genes. J Virol. 2020;94(16):e00326–e00e20. doi: 10.1128/JVI.00326-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gu S, Chen Y, Wu Z, Chen Y, Gao H, Lv L, et al. Alterations of the gut microbiota in patients with coronavirus disease 2019 or H1N1 influenza. Clin Infect Dis. 2020;71(10):2669–2678. doi: 10.1093/cid/ciaa709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Paparo L, Maglio MA, Cortese M, Bruno C, Capasso M, Punzo E, et al. A new butyrate releaser exerts a protective action against SARS-CoV-2 infection in human intestine. Molecules. 2022;27(3):862. doi: 10.3390/molecules27030862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Li C, Li Z, Liu T, Gu Z, Ban X, Tang X, et al. Encapsulating tributyrin during enzymatic cyclodextrin synthesis improves the solubility and bioavailability of tributyrin. Food Hydrocoll. 2021;113:106512 [Google Scholar]
- 114.Chen S, Li Z, Gu Z, Hong Y, Cheng L, Li C. Variants at position 603 of the CGTase from Bacillus circulans STB01 for reducing product inhibition. Int J Biol Macromol. 2019;136:460–468. doi: 10.1016/j.ijbiomac.2019.05.160. [DOI] [PubMed] [Google Scholar]
- 115.Wadhwa G, Kumar S, Chhabra L, Mahant S, Rao R. Essential oil–cyclodextrin complexes: an updated review. J Incl Phenom Macrocycl Chem. 2017;89(1–2):39–58. [Google Scholar]
- 116.Augustin MA, Abeywardena MY, Patten G, Head R, Lockett T, de Luca A, et al. Effects of microencapsulation on the gastrointestinal transit and tissue distribution of a bioactive mixture of fish oil, tributyrin and resveratrol. J Funct Foods. 2011;3(1):25–37. [Google Scholar]
- 117.Drozdov VA, P’yanova LG, Lavrenov AV, Likholobov VA, Kudrya EN, Luzyanina LS. State and properties of a carbon sorbent modified with a bioactive substance. Prot Met Phys Chem Surf. 2019;55(6):1035–1043. [Google Scholar]
- 118.P’yanova LG, Likholobov VA, Sedanova AV, Drozdetskaya MS. Fundamental technological approaches to the synthesis of carbon sorbents for medical and veterinary applications. Russ J Gen Chem. 2020;90(3):550–558. [Google Scholar]
- 119.Lavrenov AV, P’yanova LG, Leont’eva NN, Sedanova AV, Delyagina MS, Trenikhin MV. Physicochemical approach for the modification of medical nanoporous carbon sorbents. Adsorption. 2023;29(5-6):309–321. [Google Scholar]
- 120.Dorozhkin VI, Fedorov YN, Gerunova LK, P’yanova LG, Gerunov TV, Delyagina MS, et al. Therapeutic efficiency of sorbents modified by hydroxic acids during animal experimental poisoning with ivermectin. Sh Biol. 2020;55(2):394–405. [Google Scholar]
- 121.Sedanova AV, P’yanova LG, Delyagina MS, Kornienko NV, Ogurtsova DN, Leont’eva NN. Modification of porous carbon sorbent with tributyrin. Chem for Sust Develop. 2022;30(5):522–531. [Google Scholar]