Abstract
Whether circulating levels of specific cytokines at baseline link with treatment efficacy of immune checkpoint blockade (ICB) therapy in patients with non-small cell lung cancer remains unknown. In this study, serum samples were collected in two independent, prospective, multicenter cohorts before the initiation of ICB. Twenty cytokines were quantified, and cutoff values were determined by receiver operating characteristic analyses to predict non-durable benefit. The associations of each dichotomized cytokine status with survival outcomes were assessed. In the discovery cohort (atezolizumab cohort; N = 81), there were significant differences in progression-free survival (PFS) in accordance with the levels of IL-6 (log-rank test, P = 0.0014), IL-15 (P = 0.00011), MCP-1 (P = 0.013), MIP-1β (P = 0.0035), and PDGF-AB/BB (P = 0.016). Of these, levels of IL-6 and IL-15 were also significantly prognostic in the validation cohort (nivolumab cohort, N = 139) for PFS (log-rank test, P = 0.011 for IL-6 and P = 0.00065 for IL-15) and overall survival (OS; P = 3.3E-6 for IL-6 and P = 0.0022 for IL-15). In the merged cohort, IL-6high and IL-15high were identified as independent unfavorable prognostic factors for PFS and OS. The combined IL-6 and IL-15 status stratified patient survival outcomes into three distinct groups for both PFS and OS. In conclusion, combined assessment of circulating IL-6 and IL-15 levels at baseline provides valuable information to stratify the clinical outcome of patients with non-small cell lung cancer treated with ICB. Further studies are required to decipher the mechanistic basis of this finding.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-023-03453-z.
Keywords: Non-small cell lung cancer, Immune checkpoint inhibitor, Cytokine, IL-6, IL-15, Prognostic factor
Introduction
The development of antiprogrammed cell death protein 1 (PD-1)/programmed death-ligand 1 (PD-L1) inhibitory antibodies as immune checkpoint blockade (ICB) therapy has been a remarkable breakthrough in the treatment of non-small cell lung cancer (NSCLC) and improved patient outcome. However, clinical benefit for the single-agent therapy is only observed in a limited fraction of patients, and the response rates were approximately 20% in clinical trials [1, 2]. The efficacy of treatment is not completely satisfactory, even after the enrichment of patients by approved biomarkers such as tumor PD-L1 expression measured by immunohistochemistry and tumor mutation burden. For example, in the KEYNOTE-024 trial, more than half of NSCLC patients with PD-L1 tumor proportion score (TPS) ≥50% who were treated with the PD-1 inhibitor pembrolizumab in the first-line setting lacked a treatment response [3]. Additionally, although pembrolizumab was superior to standard chemotherapy in terms of survival in this trial, the survival curves for progression-free survival (PFS) highly overlapped in the initial 4 months from the initiation of therapy. Furthermore, overall survival (OS) was similar between pembrolizumab and chemotherapy in patients with PD-L1 TPS 1–49% in the KEYNOTE-042 trial [4], and the PFS curves of pembrolizumab and chemotherapy groups crossed across PD-L1 TPS cutoffs of 50%, 20%, and 1%, suggesting the existence of further determinants of response. Additionally, high tumor mutation burden was shown not to be a universal biomarker of response to ICB across cancer types [5]. Therefore, novel biomarkers are required to predict which patient is most likely to benefit from anti-PD-1/PD-L1 therapy and in which patient the therapy should not be prioritized.
ICB treatment enhances antitumor immunity by removing the co-inhibitory signaling in exhausted T cells in the tumor microenvironment [6]. Thus, the host immune status is an important determinant of treatment efficacy. Indeed, host factors that could affect the immune system, including nutritional status [7], systemic inflammation [8], and gut microbiota [9], have been associated with the treatment response to ICB. Cytokines are a broad category of small proteins including chemokines, interferons (IFNs), interleukins (ILs), tumor necrosis factors (TNFs), and growth factors. Cytokines are essential for immune cell autocrine and paracrine signaling and mediate crucial interactions between immune and non-immune cells in the tumor microenvironment [10, 11]. Cytokine production is tightly controlled to promote and amplify a range of pro- and anti-inflammatory immune responses. Cytokines exert powerful immunomodulatory effects, and therefore, they have been extensively explored as cancer targets and treatments in the context of antitumor immunity. Furthermore, the systemic levels of some cytokines have been shown to be associated with response and resistance to ICB [8, 12–18]. However, whether specific circulating cytokine levels are robustly linked with treatment efficacy of ICB in patients with advanced or recurrent NSCLC remains unknown.
In this study, we aimed to identify cytokines that may serve as determinants of survival outcomes in patients with NSCLC treated with PD1/PD-L1 inhibitors, using two independent, prospective, multicenter cohorts of patients treated with the PD-L1 inhibitor atezolizumab or the PD-1 inhibitor nivolumab.
Materials and methods
Study design and patients
Data were collected from patients with advanced or recurrent NSCLC enrolled in two independent, prospective, multicenter, observational studies conducted in Japan by our research group. The discovery cohort included 86 patients who were treated with atezolizumab monotherapy at 12 institutions between January 2019 and May 2020; the validation cohort included 200 patients who received single-agent nivolumab at 14 institutions between July 2016 and December 2018. The study design, eligibility criteria, and exclusion criteria for each cohort were described previously [19, 20]. In brief, patients in both cohorts had an Eastern Cooperative Oncology Group performance status (PS) of 0–2 and had histologically or cytologically proven unresectable stage III or IV or recurrent NSCLC. Molecular analyses for oncogenes such as EGFR and ALK were not mandatory and performed as part of the standard of care in both cohorts. PD-L1 protein expression in tumor cells was evaluated by the approved 22C3 immunohistochemistry assay as part of the standard of care; immunohistochemistry assay using E1L3N antibody (Cell Signaling Technology, Danvers, MA, USA) was used in a fraction of patients in the validation cohort before the approval of the 22C3 assay [20]. Data were analyzed from June 2022 to October 2022.
Treatment procedures and assessment of efficacy
In the discovery cohort, all patients received atezolizumab intravenously at a dose of 1200 mg on day 1 of each 21-day cycle. Before each treatment cycle, response was assessed by the treating investigators. In the validation cohort, all patients were treated with nivolumab at a dose of 3 mg/kg or a fixed dose of 240 mg on day 1 of each 14-day cycle. Therapeutic response was assessed every 8 weeks by the treating investigators. In both cohorts, surveillance was continued after completion of the treatment to define survival outcomes. Response was assessed following the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1.
Sample collection and laboratory assays
Peripheral blood was collected at baseline immediately before the initiation of atezolizumab or nivolumab therapy. The samples were centrifuged, and sera were aliquoted and cryopreserved at −80 °C until analysis. A customized MILLIPLEX Human Cytokine/Chemokine/Growth Factor Panel A Magnetic Based Panel (HCYTA-60 K-PX38, Merck, Darmstadt, Germany), which enables the simultaneous quantification of multiple cytokines, chemokines, and growth factors on a Luminex bead-based platform, was used to quantitatively evaluate circulating 20 analytes (epidermal growth factor [EGF], eotaxin, granulocyte–macrophage colony-stimulating factor [GM-CSF], IFN-α2, IL-1 receptor antagonist [IL-1RA], IL-4, IL-6, IL-7, IL-10, IL-13, IL-15, IL-17E/IL-25, IL-18, IFN-γ-inducible protein 10 [IP-10], monocyte chemoattractant protein-1 [MCP-1], monokine induced by IFN-γ [MIG], macrophage inflammatory protein-1β [MIP-1β], platelet-derived growth factor-AA [PDGF-AA], PDGF-AB/BB, and TNF-α). Assays were conducted in accordance with the manufacturer’s instructions. Samples were de-identified so that laboratory personnel were blinded to patient information. Fluorescent intensity was measured using the Bio-Plex 200 System (Bio-Rad Laboratories, Hercules, CA, USA). Data were analyzed using the Bio-Plex manage software version 6.1 (Bio-Rad Laboratories). Assay working ranges for each analyte per each assay were determined as a range of concentrations between the upper and lower limits of quantification that were defined as the maximum and minimum standard points where recovery rates fell between 70% and 130%, respectively. The recovery rate was calculated as follows: (an observed concentration)/(an expected concentration) × 100. When assay results were below the assay working range, the values were assigned to the midpoint between zero and the lower limit of the working range (applicable for EGF, GM-CSF, IL-1RA, IL-4, IL-6, IL-7, IL-10, IL-13, IL-15, IL-17E/IL-25, IL-18, MIP-1β, and TNF-α) [19, 21]. When the results fell above the assay working range, the values were assigned to the upper limit of the working range (applicable for PDGF-AB/BB).
Lung immune prognostic index (LIPI) score calculation
Pretreatment LIPI was calculated on the basis of the derived neutrophil-to-lymphocyte ratio [dNLR; neutrophils/(leukocytes minus neutrophils)] and lactate dehydrogenase (LDH) level. Factors dNLR greater than 3 and LDH greater than the upper limit of normal were scored as one, respectively, and the sum was calculated for each case (score 0, 1, or 2) [22].
Statistical analysis
The median follow-up time was estimated by the reverse Kaplan–Meier method. To determine the appropriate cutoff values to better categorize patients who poorly responded to therapy, receiver operating characteristic (ROC) curve analyses to predict non-durable benefit (NDB) were performed. The area under the curve (AUC) was calculated, where durable clinical benefit (DCB) was defined as partial response or stable disease with PFS ≥6 months, and NDB was defined as the others [23]. PFS and OS curves were estimated with the Kaplan–Meier method and compared by the log-rank test. The survival durations were defined as the time between the date of the first administration of atezolizumab or nivolumab and the date of progression or death from any cause for PFS, and the date of death from any cause for OS. Censoring was undertaken at the date of last contact. Hazard ratios (HRs) were estimated with Cox proportional hazards models. The Fisher exact test was used for categorical variables. Statistical tests were two-sided, and P < 0.05 was defined as statistically significant. Analyses were conducted using EZR statistical software version 1.55 (Saitama Medical Center, Jichi Medical University) [24] and GraphPad Prism version 8.4.3 (GraphPad Software, San Diego, CA, USA).
Results
Characteristics of patients in the discovery and validation cohorts
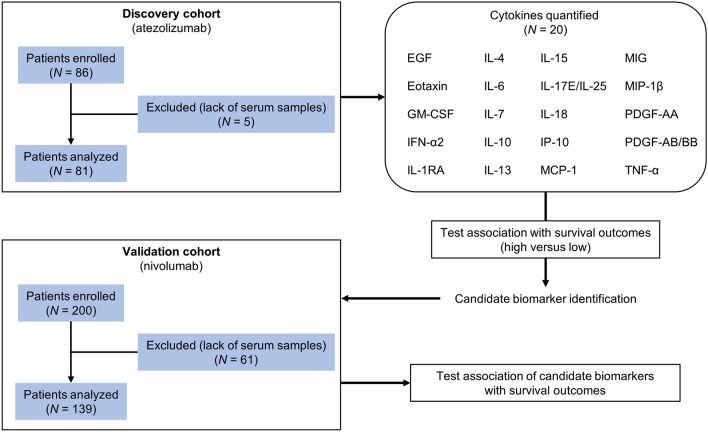
Of the 86 patients initially enrolled in the discovery cohort, 5 patients were excluded because of a lack of serum samples (Fig. 1). The demographic characteristics of the remaining 81 patients at baseline were reported previously [19] and are presented in Table 1. Most patients were male (64 [79.0%]) and had PS 0 or 1 (72 [88.9%]), a history of smoking (65 [80.2%]), and stage IV disease (61 [75.3%]). The median number of previous systemic therapies was 2 (range, 1–7), and 36 (44.4%) and 20 (24.7%) patients received atezolizumab as the second-line and third-line treatment, respectively. Among the 81 patients, 38 (46.9%) had LIPI of 0, 36 (44.4%) had LIPI of 1, and 7 (8.6%) had LIPI of 2. Ten patients (12.3%) had received prior ICI therapy. EGFR mutation status was evaluated in 68 patients (84.0%), and activating mutations were identified in 15 patients (18.5%). Tumor PD-L1 expression was assessed in 66 patients (81.5%), and 13 (16.9%) had PD-L1 ≥50%. The overall response rate was 13.6% (95% confidence interval [CI], 7.0–23.0) and the definition of DCB was fulfilled in 18 patients (22.2%). The median follow-up time was 25.7 months (95% CI, 21.2–28.0), and 72 PFS events and 54 deaths were recorded at the time of data cutoff (November 2021).
Fig. 1.
Overview of the study design. A flowchart of the study design to identify cytokines associated with treatment efficacy of anti-PD-1/PD-L1 immunotherapy in the discovery cohort (atezolizumab cohort) and the independent validation cohort (nivolumab cohort) is shown. EGF, epidermal growth factor; GM-CSF, granulocyte–macrophage colony-stimulating factor; IFN-α2, interferon-α2; IL-1RA, interleukin-1 receptor antagonist; IP-10, IFN-γ-inducible protein 10; MCP-1, monocyte chemoattractant protein-1; MIG, monokine induced by IFN-γ; MIP-1β, macrophage inflammatory protein-1β; PDGF-AA, platelet-derived growth factor-AA; PD-1, programmed cell death protein 1; PD-L1, programmed death-ligand 1; TNF-α, tumor necrosis factor-α
Table 1.
Patient and tumor characteristics
| Discovery cohort (atezolizumab cohort) | Validation cohort (nivolumab cohort) | |
|---|---|---|
| Characteristic | N (%) | N (%) |
| No. of patients | ||
| 81 | 139 | |
| Age, years | ||
| Median (range) | 70 (36–84) | 69 (43–83) |
| Sex | ||
| Male | 64 (79.0) | 112 (80.6) |
| Female | 17 (21.0) | 27 (19.4) |
| ECOG performance status | ||
| 0 | 48 (59.3) | 71 (51.1) |
| 1 | 24 (29.6) | 62 (44.6) |
| 2 | 9 (11.1) | 6 (4.3) |
| Smoking status | ||
| Never | 16 (19.8) | 20 (14.4) |
| Current or former | 65 (80.2) | 119 (85.6) |
| Histology | ||
| Adenocarcinoma | 55 (67.9) | 76 (54.7) |
| Squamous cell carcinoma | 20 (24.7) | 55 (39.6) |
| Other | 6 (7.4) | 8 (5.8) |
| Stage at treatment | ||
| III | 13 (16.0) | 28 (20.1) |
| IV | 61 (75.3) | 101 (72.7) |
| Recurrence | 7 (8.6) | 10 (7.2) |
| No. of previous systemic therapies* | ||
| Median (range) | 2 (1–7) | 1 (1–8) |
| Previous treatment with PD-1/PD-L1 inhibitors | ||
| Nivolumab | 5 (6.2) | 0 |
| Pembrorizumab | 4 (4.9) | 0 |
| Duruvaumab | 1 (1.2) | 0 |
| None | 71 (87.7) | 139 (100) |
| EGFR mutation | ||
| Presence | 15 (18.5) | 11 (7.9) |
| Absence | 53 (65.4) | 97 (69.8) |
| Unknown | 13 (16.0) | 31 (22.3) |
| ALK rearrangement | ||
| Presence | 0 | 1 (0.7) |
| Absence | 57 (70.4) | 100 (71.9) |
| Unknown | 24 (29.6) | 38 (27.3) |
*Includes (neo)adjuvant therapy
ALK, anaplastic lymphoma kinase, ECOG Eastern Cooperative Oncology Group, EGFR epidermal growth factor receptor, PD-1 programmed cell death protein 1, PD-L1 programmed death-ligand 1
In the validation cohort, 200 patients were initially enrolled. After excluding 61 patients because of a lack of serum samples, 139 patients were analyzed (Fig. 1). The patient characteristics at baseline are listed in Table 1. Age, sex, PS, smoking history, and disease stage were almost similar to those in the discovery cohort. The proportion of patients with adenocarcinoma was smaller in the validation cohort than the discovery cohort. Among the 139 patients, 74 (53.2%) and 38 (27.3%) patients received nivolumab as the second-line and third-line treatment, respectively. No patients had a history of prior ICI treatment, and 11 patients (7.9%) had EGFR mutations. In the validation cohort, 49 (35.3%), 65 (46.8%), and 25 (18.0%) patients had LIPI of 0, 1, and 2, respectively. PD-L1 expression status was available in all patients, as evaluation of PD-L1 expression using archived tumor samples was mandatory in this cohort [20], and 16 (11.5%) were revealed as high expressors (≥50%). The overall response rate was 21.6% (95% CI, 15.1–29.4). The median follow-up time was 29.7 months (95% CI, 25.8–33.1), and 121 PFS events and 94 deaths were documented at the time of data cutoff (December 2019).
Association of circulating cytokines with survival outcomes in the discovery cohort
First, we quantified 20 cytokines in sera collected at baseline in the discovery cohort (Figure 1). ROC analyses identified cutoff values to predict NDB for each analyte as follows: EGF, 44.100 pg/mL (AUC, 0.52; 95% CI, 0.37–0.67); eotaxin, 113.110 pg/mL (AUC, 0.62; 95% CI, 0.48–0.76); GM-CSF, 8.490 pg/mL (AUC, 0.50; 95% CI, 0.36–0.65); IL-1RA, 0.821 pg/mL (AUC, 0.58; 95% CI, 0.42–0.74); IL-4, 2.200 pg/mL (AUC, 0.52; 95% CI, 0.36–0.67); IL-6, 8.300 pg/mL (AUC, 0.54; 95% CI, 0.40–0.67; Supplementary Figure S1a); IL-7, 12.180 pg/mL (AUC, 0.60; 95% CI, 0.45–0.75); IL-10, 6.080 pg/mL (AUC, 0.56; 95% CI, 0.40–0.71); IL-13, 2.412 pg/mL (AUC, 0.52; 95% CI, 0.38–0.67); IL-15, 6.590 pg/mL (AUC, 0.56; 95% CI, 0.42–0.71; Supplementary Figure S1b); IL-17E/IL-25, 260.530 pg/mL (AUC, 0.59; 95% CI, 0.43–0.74); IL-18, 106.160 pg/mL (AUC, 0.55; 95% CI, 0.41–0.69); IP-10, 229.280 pg/mL (AUC, 0.46; 95% CI, 0.31–0.62); MCP-1, 536.800 pg/mL (AUC, 0.56; 95% CI, 0.41–0.70); MIG, 5695.190 pg/mL (AUC, 0.56; 95% CI, 0.40–0.72); MIP-1β, 38.080 pg/mL (AUC, 0.67; 95% CI, 0.54–0.79); PDGF-AA, 3742.510 pg/mL (AUC, 0.56; 95% CI, 0.42–0.71); PDGF-AB/BB, 42647.980 pg/mL (AUC, 0.62; 95% CI, 0.46–0.78); and TNF-α, 21.900 pg/mL (AUC, 0.59; 95% CI, 0.44–0.74). IFN-α2 was excluded from the analysis because only one sample showed a value above the lower limit of the working range. Among the other 19 analytes, more than half of the samples had lower values than the individual lower limits for GM-CSF (N = 71, 87.7%), IL-10 (N = 46, 56.8%), IL-13 (N = 63, 77.8%), and IL-15 (N = 51, 63.0%).
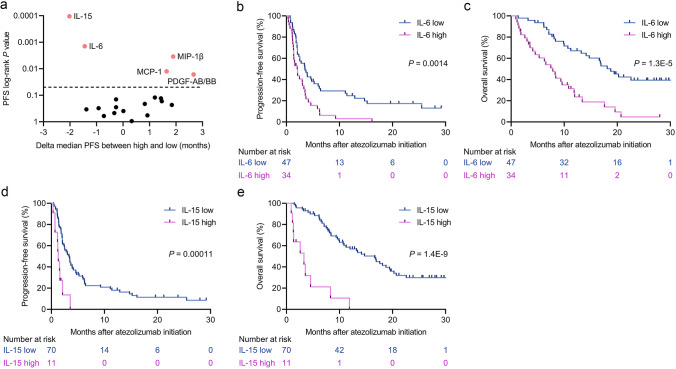
We next tested whether survival curves for PFS and OS were stratified by the levels of the 19 analytes using the cutoff values. There were significant differences in PFS on the basis of levels of IL-6, IL-15, MCP-1, MIP-1β, and PDGF-AB/BB (Figure 2a). The median PFS and OS were 2.0 months (95% CI, 1.3–3.3) and 7.9 months (95% CI, 4.1–10.8), respectively, for IL-6high patients in comparison with 3.5 months (95% CI, 2.1–5.2) and 18.8 months (95% CI, 13.2 to not reached [NR]), respectively, for IL-6low patients (Figure 2b, c). Similarly, the median PFS in the IL-15high patients was shorter compared with that in the IL-15low patients (1.3 months [95% CI, 0.7–2.1] versus 3.3 months [95% CI, 2.1–3.9]); similar results were observed for OS (3.3 months [95% CI, 1.1–4.5] versus 16.6 months [95% CI, 10.7–19.4]; Figure 2d, e). The prognostic impact of MCP-1, MIP-1β, and PDGF-AB/BB in PFS was not translated into OS (Supplementary Figure S2a–c).
Fig. 2.
Identification of circulating cytokines associated with progression-free survival (PFS) and overall survival (OS) at baseline before atezolizumab initiation in the discovery cohort. a Log-rank P values for PFS and the delta median PFS of individual serum cytokines are plotted (N = 19). Delta median PFS values were calculated as follows: median PFS in the high group minus that in the low group for each of the 19 cytokines. Each dot represents one factor, and a dashed line represents a PFS log-rank P value of 0.05. The statistically significant results (P < 0.05) from the log-rank analysis are shown in orange. b Kaplan–Meier survival curves for PFS on the basis of IL-6 status (log-rank, P = 0.0014). c Kaplan–Meier survival curves for OS on the basis of IL-6 status (log-rank, P = 1.3E-5). d Kaplan–Meier survival curves for PFS on the basis of IL-15 status (log-rank, P = 0.00011). e Kaplan–Meier survival curves for OS on the basis of IL-15 status (log-rank, P = 1.4E-9)
Validation in an independent, prospectively collected, multicenter patient cohort
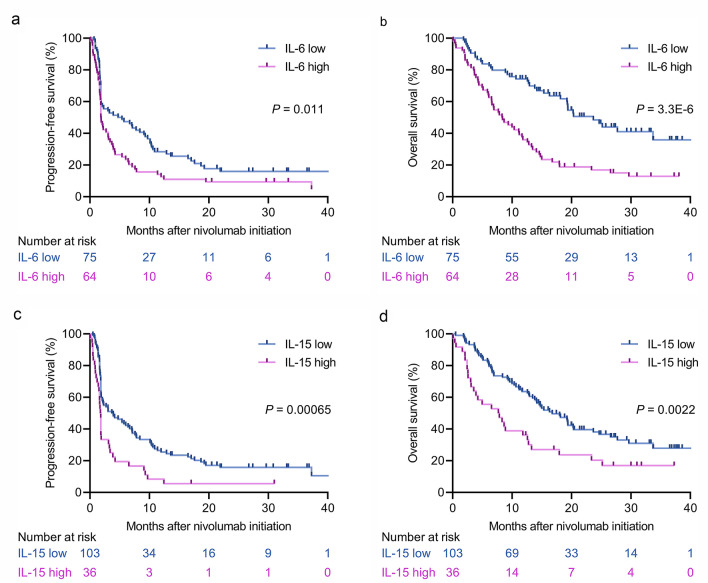
The robustness of the above results was evaluated using an independent external validation cohort using the same cutoffs as defined in the discovery cohort (Fig. 1). Survival curves for both PFS and OS were significantly separated by IL-6 (log-rank test, P = 0.011 for PFS; P = 3.3E-6 for OS; Fig. 3a, b) and IL-15 (log-rank test, P = 0.00065 for PFS; P = 0.0022 for OS; Fig. 3c, d) as observed in the discovery cohort. The median PFS and OS were 1.9 months (95% CI, 1.7–3.1) and 8.4 months (95% CI, 6.1–11.7), respectively, for IL-6high patients in comparison with 5.4 months (95% CI, 1.8–9.3) and 23.7 months (95% CI, 18.1 to NR), respectively, for IL-6low patients. For IL-15high patients, the median PFS and OS were 1.8 months (95% CI, 1.4–1.9) and 7.8 months (95% CI, 3.1–12.6), respectively, whereas the median PFS and OS in IL-15low patients were 3.9 months (95% CI, 1.9–6.7) and 16.8 months (95% CI, 13.4–20.4), respectively. To further evaluate the consistency of IL-6 and IL-15 values to discriminate the efficacy of PD-1/PD-L1 inhibitors, ROC analyses were carried out in the validation cohort to identify cutoff values to predict NDB (Supplementary Figure S1c and d). The determined cutoff values for IL-6 (6.740 pg/mL) and IL-15 (5.520 pg/mL) were very close to those in the discovery cohort. In contrast to the findings for IL-6 and IL-15, PFS curves were highly overlapped in accordance with the levels of MCP-1 (Supplementary Figure S3a), MIP-1β (Supplementary Figure S3b), and PDGF-AB/BB (Supplementary Figure S3c).
Fig. 3.
Validation of the prognostic effects of baseline IL-6 and IL-15 levels before nivolumab initiation in the independent validation cohort. a Kaplan–Meier survival curves for PFS on the basis of IL-6 status (log-rank, P = 0.011). b Kaplan–Meier survival curves for OS on the basis of IL-6 status (log-rank, P = 3.3E-6). c Kaplan–Meier survival curves for PFS on the basis of IL-15 status (log-rank, P = 0.00065). d Kaplan–Meier survival curves for OS on the basis of IL-15 status (log-rank, P = 0.0022)
Multivariable Cox regression analysis and prognostic stratification in accordance with levels of IL-6 and IL-15
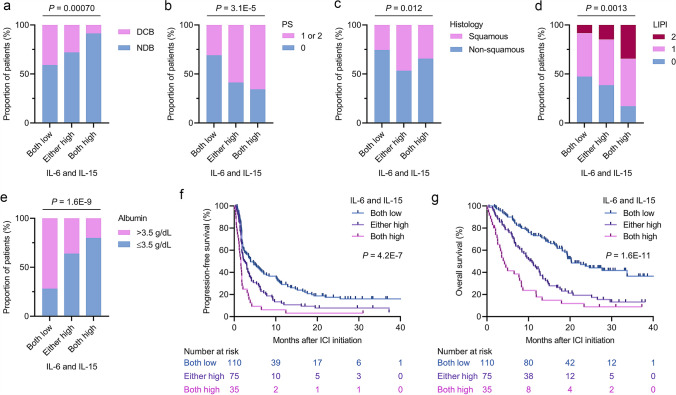
Our results showed that IL-6 and IL-15 had prognostic effects for PFS and OS in both the discovery and validation cohorts. To further explore the prognostic impact of IL-6 and IL-15 status, we combined the cohorts and categorized patients into the following three groups: both IL-6 and IL-15 low group; either IL-6 or IL-15 high group; and both IL-6 and IL-15 high group. The proportions of patients who derived DCB were significantly reduced when either factor or both factors were elevated (P = 0.00070; Fig. 4a). Additionally, the proportions of patients with poorer PS (P = 3.1E-5; Fig. 4b), squamous histology (P = 0.012; Fig. 4c), and greater LIPI (P = 0.0013; Fig. 4d) were significantly increased in accordance with IL-6high and/or IL-15high status. Furthermore, patients with low serum albumin values (≤3.5 g/dL) were significantly enriched when IL-6 and IL-15 were elevated (P = 1.6E-9; Fig. 4e). In contrast, there were no significant relationships of IL-6 and IL-15 status with smoking history (Supplementary Figure S4a), tumor PD-L1 expression (Supplementary Figure S4b), and EGFR mutation status (Supplementary Figure S4c). Despite the above relationships of IL-6 and IL-15 status with PS, histology, LIPI, and serum albumin status, multivariable analyses adjusted for relevant clinical prognostic factors including these factors revealed that IL-6high and IL-15high were independent prognostic factors for PFS and OS (Table 2). Tumor PD-L1 expression status and EGFR mutation status were not included in the analysis because information was not available in 15 (6.8%) and 44 (20%) patients, respectively. Notably, survival curves for PFS (Fig. 4f) and OS (Fig. 4g) were clearly stratified into the three distinct groups, with the worst survival outcomes in the IL-6high and IL-15high patients and patients in the either IL-6 or IL-15 high group showing worse survival outcomes than IL-6low and IL-15low patients. The median PFS and OS were 3.9 months (95% CI, 2.3–6.7) and 20.3 months (95% CI, 18.1–33.7), respectively, for the both IL-6 and IL-15 low group, 2.5 months (95% CI, 1.8–3.5) and 10.8 months (95% CI, 7.9–12.8), respectively, for the either IL-6 or IL-15 high group, and 1.6 months (95% CI, 1.2–1.8) and 4.2 months (95% CI, 2.6–7.9), respectively, for the both IL-6 and IL-15 high group.
Fig. 4.
Patient characteristics and survival outcomes on the basis of baseline IL-6 and IL-15 status in the merged cohort. a Proportion of patients who experienced DCB or NDB on the basis of IL-6 and IL-15 levels classified as both low, either high, or both high. P value was calculated by the Fisher exact test. b Proportion of patients with PS of ≥1 or 0 on the basis of IL-6 and IL-15 levels classified as both low, either high, or both high. P value was calculated by the Fisher exact test. c Proportion of patients with squamous cell carcinoma or non-squamous histology on the basis of IL-6 and IL-15 levels classified as both low, either high, or both high. P value was calculated by the Fisher exact test. d Proportion of patients with an LIPI of 2, 1, or 0 on the basis of IL-6 and IL-15 levels classified as both low, either high, or both high. P value was calculated by the Fisher exact test. e Proportion of patients with a baseline serum albumin level of >3.5 g/dL or ≤3.5 g/dL on the basis of IL-6 and IL-15 levels classified as both low, either high, or both high. P value was calculated by the Fisher exact test. f Kaplan–Meier survival curves for progression-free survival on the basis of IL-6 and IL-15 levels classified as both low, either high, or both high (log-rank, P = 4.2E-7). g Kaplan–Meier survival curves for overall survival on the basis of IL-6 and IL-15 levels classified as both low, either high, or both high (log-rank, P = 1.6E-11). DCB, durable clinical benefit; LIPI, lung immune prognostic index; NDB, non-durable benefit; PS, performance status
Table 2.
Univariable and multivariable Cox regression analyses of progression-free survival and overall survival in the merged cohort (N = 220)
| Variable | Progression-free survival | Overall survival | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariable | Multivariable | Univariable | Multivariable | |||||||||
| HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value | |
| Age (≥75 versus <75 years) | 0.94 | 0.68–1.29 | 0.69 | 1.07 | 0.75–1.52 | 0.70 | 0.80 | 0.55–1.15 | 0.23 | 0.83 | 0.56–1.25 | 0.38 |
| Sex (male versus female) | 0.69 | 0.49–0.98 | 0.038 | 0.71 | 0.46–1.10 | 0.13 | 1.01 | 0.68–1.50 | 0.95 | 1.07 | 0.64–1.78 | 0.79 |
| ECOG-PS (≥1 versus 0) | 1.34 | 1.01–1.78 | 0.046 | 0.96 | 0.70–1.31 | 0.80 | 1.71 | 1.23–2.37 | 0.0013 | 1.15 | 0.81–1.64 | 0.42 |
| Smoking history (ever versus never) | 0.52 | 0.36–0.76 | 0.00074 | 0.54 | 0.34–0.85 | 0.0079 | 0.85 | 0.56–1.30 | 0.46 | 0.58 | 0.34–0.99 | 0.046 |
| Disease stage (IV versus other) | 1.26 | 0.91–1.74 | 0.17 | 1.07 | 0.75–1.51 | 0.71 | 1.39 | 0.95–2.03 | 0.090 | 1.14 | 0.76–1.71 | 0.53 |
| Histology (non-squamous versus squamous) | 0.86 | 0.64–1.15 | 0.31 | 0.88 | 0.64–1.20 | 0.41 | 0.66 | 0.48–0.93 | 0.016 | 0.68 | 0.48–0.98 | 0.037 |
| LIPI (≥1 versus 0) | 1.28 | 0.96–1.71 | 0.098 | 1.11 | 0.82–1.51 | 0.50 | 1.89 | 1.33–2.68 | 0.00037 | 1.60 | 1.11–2.31 | 0.013 |
| Albumin (≤3.5 g/dL versus >3.5 g/dL) | 1.37 | 1.03–1.82 | 0.031 | 1.15 | 0.83–1.59 | 0.39 | 2.09 | 1.51–2.91 | 1.0E-5 | 1.40 | 0.95–2.07 | 0.090 |
| IL-6 serum level (high versus low) | 1.75 | 1.31–2.33 | 0.00013 | 1.67 | 1.19–2.36 | 0.0033 | 2.76 | 1.99–3.85 | 1.7E-9 | 2.05 | 1.37–3.07 | 0.00046 |
| IL-15 serum level (high versus low) | 2.09 | 1.49–2.94 | 2.3E-5 | 1.82 | 1.26–2.61 | 0.0012 | 2.33 | 1.61–3.37 | 6.8E-6 | 1.57 | 1.06–2.32 | 0.026 |
CI confidence interval, ECOG Eastern Cooperative Oncology Group, HR hazard ratio, IL-6, interleukin-6, IL-15 interleukin-15, LIPI lung immune prognostic index, PS performance status
Discussion
An accurate prediction of survival benefit from ICB in patients with NSCLC is crucial for treatment decision-making, and novel determinants of treatment efficacy beyond the approved biomarkers such as tumor PD-L1 expression and TMB are required. Given their essential and powerful roles in orchestrating the antitumor immune response via inflammatory and immunosuppressive signaling networks [11], cytokines have shown promise as quantitative candidate biomarkers for cancer immunotherapy. In this study, we screened a panel of cytokines and found that higher levels of circulating IL-6 and IL-15 were robust and independent factors associated with poor survival outcomes in patients with NSCLC receiving PD-1/PD-L1 inhibitors. Notably, the combined assessment of IL-6 and IL-15 status enabled precise and clinically meaningful stratification of patient survival outcomes.
IL-6 is a pro-inflammatory and tumor-promoting cytokine produced in chronic inflammatory conditions and various types of cancers [13, 25, 26]. In many pathogenic conditions including cancer, the IL-6/JAK/STAT3 signaling pathway plays a major immunoregulatory role [27]. In the context of antitumor immunity, IL-6 attenuates CD4+ T cell-derived IFNγ production and PD-1/PD-L1 ligation on tumor-associated macrophages [28]. Furthermore, IL-6 induces tumor angiogenesis [10] and promotes tumor growth by inhibition of apoptosis [29]. Moreover, the survival of cancer cells exhibiting chromosomal instability has been demonstrated to depend on a cGAS-mediated inflammatory response, and IL-6 is a pivotal component of this signaling cascade [30]. In line with these pleiotropic functions of IL-6, multiple studies have reported that elevated circulating IL-6 is an unfavorable prognostic factor in patients with a variety of types of cancer who were treated with ICB [8, 13, 16–18] or who did not receive ICB [31–33]. These findings together with our work support the concept that targeting IL-6 with ICB could have therapeutic benefits, as proposed elsewhere [28, 34].
IL-15 is a cytokine that functions in innate and adaptive immunity and preferentially promotes the generation, proliferation, and activity of antitumor NK cells and CD8+ T cells but not of Treg cells [35]. Multiple trials of immunotherapy targeting IL-15 have been conducted, but the beneficial effects on circulating NK cells and CD8+ T cells were not translated into clinically meaningful efficacy as monotherapy [36, 37]. However, the combination with nivolumab, an IL-15 super-agonist ALT-803, which targets the shared IL-2 and IL-15Rβγ pathway, showed promising clinical activity with an objective response rate of 28.6% (6 of 21) and median PFS of 9.4 months in a non-randomized, open-label, phase 1b trial in patients with previously treated NSCLC [38]. Another study showed that exercise promotes the accumulation of tumor-infiltrating IL-15Rα+ CD8+ T cells in murine models of pancreatic ductal adenocarcinoma, resulting in antitumor effects [39]. The study also showed that an IL-15 super-agonist sensitized pancreatic tumors to PD-1 blockade therapy. Our result that a higher level of circulating IL-15 was independently associated with poor survival outcomes in NSCLC patients receiving ICB, however, stands in contrast to the role of IL-15 as a stimulator of NK cells and effector T cells. This could be, at least in part, exemplified by a paradoxical effect of continuous IL-15 treatment on NK cells, which results in decreased viability and a functional change consistent with exhaustion [40]. Given the powerful functions of IL-15 on immune cells, mechanistic links of increased IL-15 expression with leukemogenesis and myelomagenesis have been demonstrated [41, 42], with implications for prognosis in hematological malignancies [43]. Additionally, in colorectal cancer cells, IL-15 was reported to promote cell proliferation and invasion with downregulation of p21WAF1 and BAX and upregulation of BCL-2, phospho-AKT, and VEGF [44]. These findings indicate possibilities that the unfavorable prognostic effects of IL-15 in our study were governed through the exhaustion of immune cells by continuous exposure to IL-15, through the direct promotion of aggressiveness of tumor cells, or through both scenarios.
In the present study, increased circulating levels of IL-6 and IL-15 were significantly associated with poorer PS, higher LIPI scores, and lower serum albumin levels, suggesting potential associations with impaired general conditions such as cancer-induced cachexia, although the prognostic effects of IL-6 and IL-15 were independent from those factors. Indeed, IL-6 causes cellular metabolic reprogramming and promotes cachexia in patients with cancer [10]. Moreover, the IL-15 gene is highly expressed in skeletal muscle, where IL-15 regulates metabolism and maintains muscle fiber growth [45]. IL-15 was shown to decrease proteolysis [46]. Thus, increased circulating IL-15 might be a result of increased production and release from decreased skeletal muscle in the condition of IL-6-induced cachexia. Whether such a regulatory feedback loop between IL-6-induced cachexia or sarcopenia and a compensatory increase of muscle-derived IL-15 exists warrants future investigation.
This retrospective study using two independent, prospective, multicenter cohorts has several major limitations. First, the sample sizes in both cohorts were not designed specifically to identify serum biomarkers associated with predetermined patient outcomes. Second, while serum collection was prespecified, samples were not available in 5.8% and 30.5% of patients initially enrolled in the discovery and validation cohorts, respectively. Third, the relatively high frequencies of EGFR mutations (18.5%) and a history of prior ICB (12.3%) in the discovery cohort might lead to the relatively low response rate of 13.6%. However, in the validation cohort, EGFR mutations were identified in only 7.9% of patients and no patients had received ICB before nivolumab therapy. Fourth, the association between changes in cytokine levels during treatment and the clinical response was not assessed in this study. Although dynamic measures on treatment would be useful, we believe that pretreatment biomarkers are more actionable for guiding clinical decision. Fifth, when IL-6 and IL-15 values were assessed as continuous variables in the multivariable Cox regression analysis, IL-15 alone remained an independent factor associated with poor survival outcomes (Supplementary Table S1). While the role of IL-6 levels in predicting the response and promoting resistance to ICB therapy in cancer patients has been shown in multiple studies [8, 13, 16–18], whether the dichotomized IL-6 status separated at an optimal cutoff point serves as a prognostic factor or whether there is a quantitative correlation between IL-6 concentrations and survival outcomes in patients receiving ICB therapy should be clarified in future studies. Finally, this study lacks the same analysis in patients treated with chemotherapy as a non-immunotherapy control. Thus, no conclusions can be drawn as to whether the findings in this study are specific for patients with NSCLC treated with ICB or general for NSCLC regardless of therapeutic types offered.
In conclusion, from the investigation of two independent cohorts, we showed, for the first time, that combined assessment of circulating IL-6 and IL-15 levels at therapy baseline would provide valuable information to stratify the clinical outcome of patients with NSCLC treated with PD-1/PD-L1 inhibitors. Further studies are warranted to confirm our results and decipher the mechanistic basis underlying why this combination is prognostic in the context of immunotherapy, whether these factors are prognostic for other malignancies, and whether these factors are useful specifically for patients receiving ICB or for cancer patients in general.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary Figure S1. Receiver operating characteristic (ROC) curve analyses of serum IL-6 and IL-15 levels for the prediction of non-durable benefit. a ROC curve analysis of IL-6 levels in the discovery cohort. b ROC curve analysis of IL-15 levels in the discovery cohort. c ROC curve analysis of IL-6 levels in the validation cohort. d ROC curve analysis of IL-15 levels in the validation cohort. (TIF 869 KB)
Supplementary Figure S2. Survival outcomes on the basis of baseline MCP-1, MIP-1β, or PDGF-AB/BB status in the discovery cohort. a Kaplan–Meier survival curves for PFS (left) and OS (right) on the basis of MCP-1 status (log-rank, P = 0.013 for PFS and P = 0.11 for OS). b Kaplan–Meier survival curves for PFS (left) and OS (right) on the basis of MIP-1β status (log-rank, P = 0.0035 for PFS and P = 0.42 for OS). c Kaplan–Meier survival curves for PFS (left) and OS (right) on the basis of PDGF-AB/BB status (log-rank, P = 0.016 for PFS and P = 0.41 for OS). MCP-1, monocyte chemoattractant protein-1; MIP-1β, macrophage inflammatory protein-1β; OS, overall survival; PDGF-AB/BB, platelet-derived growth factor-AB/BB; PFS, progression-free survival. (TIF 1303 KB)
Supplementary Figure S3. Progression-free survival on the basis of baseline MCP-1 (a), MIP-1β (b), or PDGF-AB/BB (c) status in the validation cohort. P values were calculated by the log-rank test. (TIF 690 KB)
Supplementary Figure S4. Patient and tumor characteristics on the basis of baseline IL-6 and IL-15 status in the merged cohort. a Proportion of patients who ever smoked or never smoked on the basis of IL-6 and IL-15 levels classified as both low, either high, or both high. P value was calculated by the Fisher exact test. b Proportion of patients with PD-L1 expression of ≥50% or <50% on the basis of IL-6 and IL-15 levels classified as both low, either high, or both high. P value was calculated by the Fisher exact test. c Proportion of patients harboring tumors with EGFR mutations or without on the basis of IL-6 and IL-15 levels classified as both low, either high, or both high. P value was calculated by the Fisher exact test. Among the patients in the merged cohort (N = 220), information regarding PD-L1 expression and EGFR mutations was available in 205 and 176 patients, respectively. EGFR, epidermal growth factor receptor; PD-L1, programmed death-ligand 1. (TIF 700 KB)
Acknowledgements
The authors would like to thank the patients, their families, and all the investigators who participated in this study. We thank Gabrielle White Wolf, PhD, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Abbreviations
- ALK
Anaplastic lymphoma kinase
- AUC
Area under the curve
- CI
Confidence interval
- DCB
Durable clinical benefit
- EGF
Epidermal growth factor
- EGFR
Epidermal growth factor receptor
- GM-CSF
Granulocyte–macrophage colony-stimulating factor
- HR
Hazard ratio
- ICB
Immune checkpoint blockade
- IFN
Interferon
- IL
Interleukin
- IL-1RA
IL-1 receptor antagonist
- IP-10
IFN-γ-inducible protein 10
- LIPI
Lung immune prognostic index
- MCP-1
Monocyte chemoattractant protein-1
- MIG
Monokine induced by IFN-γ
- MIP-1β
Macrophage inflammatory protein-1β
- NDB
Non-durable benefit
- OS
Overall survival
- PDGF
Platelet-derived growth factor
- PD-1
Programmed cell death protein 1
- PD-L1
Programmed death-ligand 1
- PFS
Progression-free survival
- PS
Performance status
- ROC
Receiver operating characteristic
- TNF
Tumor necrosis factor
- TPS
Tumor proportion score
Author contributions
YI was involved in conceptualization, acquisition of data, data curation, formal analysis, investigation, methodology, project administration, resources, supervision, validation, visualization, writing—original draft, and writing—review and editing. NI was involved in conceptualization, acquisition of data, data curation, funding acquisition, investigation, methodology, project administration, resources, supervision, validation, visualization, writing—original draft, and writing—review and editing. MK, KA, MF, SM, TU, DH, TM, MI, HY, HH, YS, KF, NE, TF and TS were responsible for acquisition of data, data curation, supervision, validation, writing—review and editing.
Funding
This work was supported by Chugai Pharmaceutical Co., Ltd. (Tokyo, Japan). The funder had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
The authors have no conflicts to declare.
Consent to participate
All patients provided written informed consent to participate in the study.
Ethical approval
This study was approved by the institutional review board at each site (Hamamatsu University School of Medicine, #18–164 for the discovery cohort and E16-051 for the validation cohort) and was conducted in accordance with the International Council for Harmonisation Good Clinical Practice guidelines and the Declaration of Helsinki. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines. This study was registered at the UMIN Clinical Trials Registry as UMIN000035616 and UMIN000022505.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. New Engl J Med. 2016;375:1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 4.Mok TSK, Wu YL, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393:1819–1830. doi: 10.1016/S0140-6736(18)32409-7. [DOI] [PubMed] [Google Scholar]
- 5.McGrail DJ, Pilie PG, Rashid NU, et al. High tumor mutation burden fails to predict immune checkpoint blockade response across all cancer types. Ann Oncol. 2021;32:661–672. doi: 10.1016/j.annonc.2021.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A. 2002;99:12293–12297. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karayama M, Inoue Y, Yoshimura K, et al. Association of the geriatric nutritional risk index with the survival of patients with non-small cell lung cancer after nivolumab therapy. J Immunother. 2022;45:125–131. doi: 10.1097/CJI.0000000000000396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kauffmann-Guerrero D, Kahnert K, Kiefl R, et al. Systemic inflammation and pro-inflammatory cytokine profile predict response to checkpoint inhibitor treatment in NSCLC: a prospective study. Sci Rep. 2021;11:10919. doi: 10.1038/s41598-021-90397-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gopalakrishnan V, Helmink BA, Spencer CN, Reuben A, Wargo JA. The influence of the gut microbiome on cancer, immunity, and cancer immunotherapy. Cancer Cell. 2018;33:570–580. doi: 10.1016/j.ccell.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Briukhovetska D, Dorr J, Endres S, Libby P, Dinarello CA, Kobold S. Interleukins in cancer: from biology to therapy. Nat Rev Cancer. 2021;21:481–499. doi: 10.1038/s41568-021-00363-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Propper DJ, Balkwill FR. Harnessing cytokines and chemokines for cancer therapy. Nat Rev Clin Oncol. 2022;19:237–253. doi: 10.1038/s41571-021-00588-9. [DOI] [PubMed] [Google Scholar]
- 12.Lim SY, Lee JH, Gide TN, et al. Circulating cytokines predict immune-related toxicity in melanoma patients receiving anti-PD-1-based immunotherapy. Clin Cancer Res. 2019;25:1557–1563. doi: 10.1158/1078-0432.CCR-18-2795. [DOI] [PubMed] [Google Scholar]
- 13.Laino AS, Woods D, Vassallo M, et al. Serum interleukin-6 and C-reactive protein are associated with survival in melanoma patients receiving immune checkpoint inhibition. J Immunother Cancer. 2020;8:e000842. doi: 10.1136/jitc-2020-000842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schalper KA, Carleton M, Zhou M, et al. Elevated serum interleukin-8 is associated with enhanced intratumor neutrophils and reduced clinical benefit of immune-checkpoint inhibitors. Nat Med. 2020;26:688–692. doi: 10.1038/s41591-020-0856-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yuen KC, Liu LF, Gupta V, et al. High systemic and tumor-associated IL-8 correlates with reduced clinical benefit of PD-L1 blockade. Nat Med. 2020;26:693–698. doi: 10.1038/s41591-020-0860-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang DH, Park CK, Chung C, et al. Baseline serum interleukin-6 levels predict the response of patients with advanced non-small cell lung cancer to PD-1/PD-L1 inhibitors. Immune Netw. 2020;20:e27. doi: 10.4110/in.2020.20.e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huseni MA, Wang L, Klementowicz JE, et al. CD8(+) T cell-intrinsic IL-6 signaling promotes resistance to anti-PD-L1 immunotherapy. Cell Rep Med. 2023;4:100878. doi: 10.1016/j.xcrm.2022.100878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu C, Yang L, Xu H, et al. Systematic analysis of IL-6 as a predictive biomarker and desensitizer of immunotherapy responses in patients with non-small cell lung cancer. BMC Med. 2022;20:187. doi: 10.1186/s12916-022-02356-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inoue Y, Inui N, Karayama M et al (2022) Serum immune modulators associated with immune-related toxicities and efficacy of atezolizumab in patients with non-small cell lung cancer. J Cancer Res Clin Oncol (in press) [DOI] [PMC free article] [PubMed]
- 20.Inoue Y, Yoshimura K, Nishimoto K, et al. Evaluation of programmed death ligand 1 (PD-L1) gene amplification and response to nivolumab monotherapy in non-small cell lung cancer. JAMA Netw Open. 2020;3:e2011818. doi: 10.1001/jamanetworkopen.2020.11818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mukama T, Fortner RT, Katzke V, et al. Prospective evaluation of 92 serum protein biomarkers for early detection of ovarian cancer. Br J Cancer. 2022;126:1301–1309. doi: 10.1038/s41416-021-01697-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mezquita L, Auclin E, Ferrara R, et al. Association of the lung immune prognostic index with immune checkpoint inhibitor outcomes in patients with advanced non-small cell lung cancer. JAMA Oncol. 2018;4:351–357. doi: 10.1001/jamaoncol.2017.4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wei C, Wang M, Gao Q, et al. Dynamic peripheral blood immune cell markers for predicting the response of patients with metastatic cancer to immune checkpoint inhibitors. Cancer Immunol Immunother. 2022;72:23–37. doi: 10.1007/s00262-022-03221-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013;48:452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lutgendorf SK, Weinrib AZ, Penedo F, et al. Interleukin-6, cortisol, and depressive symptoms in ovarian cancer patients. J Clin Oncol. 2008;26:4820–4827. doi: 10.1200/JCO.2007.14.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knupfer H, Preiss R. Serum interleukin-6 levels in colorectal cancer patients–a summary of published results. Int J Colorectal Dis. 2010;25:135–140. doi: 10.1007/s00384-009-0818-8. [DOI] [PubMed] [Google Scholar]
- 27.Johnson DE, O'Keefe RA, Grandis JR. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev Clin Oncol. 2018;15:234–248. doi: 10.1038/nrclinonc.2018.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsukamoto H, Fujieda K, Miyashita A, et al. Combined blockade of IL6 and PD-1/PD-L1 signaling abrogates mutual regulation of their immunosuppressive effects in the tumor microenvironment. Cancer Res. 2018;78:5011–5022. doi: 10.1158/0008-5472.CAN-18-0118. [DOI] [PubMed] [Google Scholar]
- 29.Leu CM, Wong FH, Chang C, Huang SF, Hu CP. Interleukin-6 acts as an antiapoptotic factor in human esophageal carcinoma cells through the activation of both STAT3 and mitogen-activated protein kinase pathways. Oncogene. 2003;22:7809–7818. doi: 10.1038/sj.onc.1207084. [DOI] [PubMed] [Google Scholar]
- 30.Hong C, Schubert M, Tijhuis AE, et al. cGAS-STING drives the IL-6-dependent survival of chromosomally instable cancers. Nature. 2022;607:366–373. doi: 10.1038/s41586-022-04847-2. [DOI] [PubMed] [Google Scholar]
- 31.Hoejberg L, Bastholt L, Johansen JS, Christensen IJ, Gehl J, Schmidt H. Serum interleukin-6 as a prognostic biomarker in patients with metastatic melanoma. Melanoma Res. 2012;22:287–293. doi: 10.1097/CMR.0b013e3283550aa5. [DOI] [PubMed] [Google Scholar]
- 32.Ashizawa T, Okada R, Suzuki Y, et al. Clinical significance of interleukin-6 (IL-6) in the spread of gastric cancer: role of IL-6 as a prognostic factor. Gastric Cancer. 2005;8:124–131. doi: 10.1007/s10120-005-0315-x. [DOI] [PubMed] [Google Scholar]
- 33.Martin F, Santolaria F, Batista N, et al. Cytokine levels (IL-6 and IFN-gamma), acute phase response and nutritional status as prognostic factors in lung cancer. Cytokine. 1999;11:80–86. doi: 10.1006/cyto.1998.0398. [DOI] [PubMed] [Google Scholar]
- 34.Hailemichael Y, Johnson DH, Abdel-Wahab N, et al. Interleukin-6 blockade abrogates immunotherapy toxicity and promotes tumor immunity. Cancer Cell. 2022;40:509–523. doi: 10.1016/j.ccell.2022.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Waldmann TA. Cytokines in cancer immunotherapy. Cold Spring Harb Perspect Biol. 2018;10(12):a028472. doi: 10.1101/cshperspect.a028472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Conlon KC, Lugli E, Welles HC, et al. Redistribution, hyperproliferation, activation of natural killer cells and CD8 T cells, and cytokine production during first-in-human clinical trial of recombinant human interleukin-15 in patients with cancer. J Clin Oncol. 2015;33:74–82. doi: 10.1200/JCO.2014.57.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller JS, Morishima C, McNeel DG, et al. A first-in-human phase i study of subcutaneous outpatient recombinant human IL15 (rhIL15) in adults with advanced solid tumors. Clin Cancer Res. 2018;24:1525–1535. doi: 10.1158/1078-0432.CCR-17-2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wrangle JM, Velcheti V, Patel MR, et al. ALT-803, an IL-15 superagonist, in combination with nivolumab in patients with metastatic non-small cell lung cancer: a non-randomised, open-label, phase 1b trial. Lancet Oncol. 2018;19:694–704. doi: 10.1016/S1470-2045(18)30148-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kurz E, Hirsch CA, Dalton T, et al. Exercise-induced engagement of the IL-15/IL-15Ralpha axis promotes anti-tumor immunity in pancreatic cancer. Cancer Cell. 2022;40:720–737. doi: 10.1016/j.ccell.2022.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Felices M, Lenvik AJ, McElmurry R, et al. Continuous treatment with IL-15 exhausts human NK cells via a metabolic defect. JCI Insight. 2018;3:e96219. doi: 10.1172/jci.insight.96219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tinhofer I, Marschitz I, Henn T, Egle A, Greil R. Expression of functional interleukin-15 receptor and autocrine production of interleukin-15 as mechanisms of tumor propagation in multiple myeloma. Blood. 2000;95:610–618. doi: 10.1182/blood.V95.2.610. [DOI] [PubMed] [Google Scholar]
- 42.Williams MT, Yousafzai Y, Cox C, et al. Interleukin-15 enhances cellular proliferation and upregulates CNS homing molecules in pre-B acute lymphoblastic leukemia. Blood. 2014;123:3116–3127. doi: 10.1182/blood-2013-05-499970. [DOI] [PubMed] [Google Scholar]
- 43.Wang H, Zhu JY, Liu CC, et al. Increased serum levels of interleukin-15 correlate with negative prognostic factors in extranodal NK/T cell lymphoma. Med Oncol. 2015;32:370. doi: 10.1007/s12032-014-0370-4. [DOI] [PubMed] [Google Scholar]
- 44.Kuniyasu H, Oue N, Nakae D, et al. Interleukin-15 expression is associated with malignant potential in colon cancer cells. Pathobiology. 2001;69:86–95. doi: 10.1159/000048761. [DOI] [PubMed] [Google Scholar]
- 45.Argiles JM, Lopez-Soriano FJ, Busquets S. Therapeutic potential of interleukin-15: a myokine involved in muscle wasting and adiposity. Drug Discov Today. 2009;14:208–213. doi: 10.1016/j.drudis.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 46.Busquets S, Figueras MT, Meijsing S, et al. Interleukin-15 decreases proteolysis in skeletal muscle: a direct effect. Int J Mol Med. 2005;16:471–476. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1. Receiver operating characteristic (ROC) curve analyses of serum IL-6 and IL-15 levels for the prediction of non-durable benefit. a ROC curve analysis of IL-6 levels in the discovery cohort. b ROC curve analysis of IL-15 levels in the discovery cohort. c ROC curve analysis of IL-6 levels in the validation cohort. d ROC curve analysis of IL-15 levels in the validation cohort. (TIF 869 KB)
Supplementary Figure S2. Survival outcomes on the basis of baseline MCP-1, MIP-1β, or PDGF-AB/BB status in the discovery cohort. a Kaplan–Meier survival curves for PFS (left) and OS (right) on the basis of MCP-1 status (log-rank, P = 0.013 for PFS and P = 0.11 for OS). b Kaplan–Meier survival curves for PFS (left) and OS (right) on the basis of MIP-1β status (log-rank, P = 0.0035 for PFS and P = 0.42 for OS). c Kaplan–Meier survival curves for PFS (left) and OS (right) on the basis of PDGF-AB/BB status (log-rank, P = 0.016 for PFS and P = 0.41 for OS). MCP-1, monocyte chemoattractant protein-1; MIP-1β, macrophage inflammatory protein-1β; OS, overall survival; PDGF-AB/BB, platelet-derived growth factor-AB/BB; PFS, progression-free survival. (TIF 1303 KB)
Supplementary Figure S3. Progression-free survival on the basis of baseline MCP-1 (a), MIP-1β (b), or PDGF-AB/BB (c) status in the validation cohort. P values were calculated by the log-rank test. (TIF 690 KB)
Supplementary Figure S4. Patient and tumor characteristics on the basis of baseline IL-6 and IL-15 status in the merged cohort. a Proportion of patients who ever smoked or never smoked on the basis of IL-6 and IL-15 levels classified as both low, either high, or both high. P value was calculated by the Fisher exact test. b Proportion of patients with PD-L1 expression of ≥50% or <50% on the basis of IL-6 and IL-15 levels classified as both low, either high, or both high. P value was calculated by the Fisher exact test. c Proportion of patients harboring tumors with EGFR mutations or without on the basis of IL-6 and IL-15 levels classified as both low, either high, or both high. P value was calculated by the Fisher exact test. Among the patients in the merged cohort (N = 220), information regarding PD-L1 expression and EGFR mutations was available in 205 and 176 patients, respectively. EGFR, epidermal growth factor receptor; PD-L1, programmed death-ligand 1. (TIF 700 KB)
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.