Abstract
Background
In clinical practice, some patients with advanced intrahepatic cholangiocarcinoma (ICC) cannot tolerate or refuse chemotherapy due to the toxicity, necessitating alternative treatments. PD-1 blockade combined with lenvatinib showed promising results in phase II studies with small sample size, but there is a lack of data on the routine use with this regimen. This study aimed to evaluate the effectiveness and safety of the regimen in patients with advanced ICC, and to identify predictors for treatment response and prognosis.
Methods
We conducted a retrospective cohort study of patients treated with PD-1 inhibitors plus lenvatinib for advanced ICC between July 2017 and August 2022. The study endpoints were progression-free survival (PFS), overall survival (OS), objective response rate (ORR), disease control rate (DCR), and safety. Biomarker analysis for CA19-9 and PD-L1 expression was performed. Exploratory analysis for genetic alternation was conducted.
Results
The study included 103 patients. It demonstrated a median PFS of 5.9 months and a median OS of 11.4 months. ORR was 18.4% and DCR was 80.6%. The incidence of grade 3 or 4 adverse events was 50.5%. Positive PD-L1 expression (TPS ≥ 1%) was associated with higher ORR (P = 0.013) and prolonged PFS (P = 0.023). Elevated CA19-9 (> 37 U/ml) was associated with decreased ORR (P = 0.019), poorer PFS (P = 0.005) and OS (P = 0.034). Patients with IDH1 mutations exhibited a favorable response to the treatment (P = 0.011), and patients with TP53 mutations tended to have worse OS (P = 0.031).
Conclusions
PD-1 blockade plus lenvatinib is effective and safe in routine practice. PD-L1 expression and CA19-9 level appear to predict the treatment efficacy. IDH1 mutations might indicate a better treatment response.
Clinical trial registration: NCT03892577.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-023-03523-2.
Keywords: Liver cancer, Biliary tract cancer, Targeted therapy, Immunotherapy, Programmed death 1
Introduction
Intrahepatic cholangiocarcinoma (ICC) is a rare malignancy, accounting for 15% of hepatic malignancies and 3% of gastrointestinal malignancies [1]. As a subtype of biliary tract cancer (BTC), ICC is different from others in terms of anatomical location, epidemiology, cellular origin, and molecular landscape [2]. The incidence of ICC has been increasing globally over the decades [3]. Unfortunately, approximately 70% to 80% of ICC patients present with locally advanced or metastatic diseases, which results in a dismal prognosis with a 5‐year overall survival (OS) of less than 10% [1]. Systemic chemotherapy represented by GemCis (gemcitabine plus cisplatin) and FOLFOX (folinic acid, fluorouracil and oxaliplatin) is a key component for the treatment of advanced ICC, but it causes significant toxic effects. In well-known phase III trials (ABC-02, ABC-06, TOPAZ-1, and KEYNOTE-966), more than 10% of patients who received chemotherapy experienced treatment discontinuation due to adverse events (AEs) [4–7]. In clinical practice, some patients cannot tolerate chemotherapy or refuse it for the fear of toxicity, highlighting the need for alternative treatments.
The tumor microenvironment in ICC is characterized by immunosuppressive features, and high PD-L1 expression supports the rationale of using PD-1 blockade immunotherapy in ICC [8]. Prior studies of PD-1 blockade monotherapy in refractory BTC demonstrated modest benefits, but did not lead to regulatory approval [9, 10]. Lenvatinib, a tyrosine kinase inhibitor, are known to exert immunomodulatory effects and reshape the immunosuppressive microenvironment [11, 12]. Lenvatinib inhibits the vascular endothelial growth factor (VEGF) pathway by targeting VEGF receptors 1–3, which promotes vascular normalization and enhances the delivery of cytotoxic T cells, resulting in increased accumulation of T cells within the tumor microenvironment [12]. Lenvatinib also induces dendritic cell maturation and reduces Treg infiltration [11]. On the other hand, PD-1 blockade inhibits the PD-1/PD-L1 pathway to boost T cell-mediated antitumor immunity by “releasing the brake”. Consequently, a strong rationale supports the combination of lenvatinib and PD-1 blockade [13]. LEAP-005 study evaluated pembrolizumab plus lenvatinib in 31 patients with previously treated BTC, which demonstrated a median progression-free survival (PFS) of 6.1 months, and manageable toxicity with grade 3 or 4 AEs reported in 48% of patients [14]. As a result, the National Comprehensive Cancer Network (NCCN) guidelines (Biliary Tract Cancers, version 1.2023) and the Chinese Society of Clinical Oncology (CSCO) guidelines (Biliary Tract Carcinoma, version 2022) both recommend pembrolizumab plus lenvatinib as a subsequent-line option for patients with unresectable or metastatic BTC. A phase II study assessed toripalimab (PD-1 inhibitor) plus lenvatinib as first-line treatment for 31 patients with advanced ICC, which showed promising antitumor activity with an ORR of 32.3% and a reasonable safety profile [15]. Notably, the above clinical trials adhered to strict inclusion and exclusion criteria (e.g., only included patients with good physical conditions), which may compromise their representativeness due to the small sample size. In contrast, real-world studies employ more flexible inclusion and exclusion criteria, which utilizes real-world data collected during routine practice and permits to evaluate medications across a broader patient population [16]. There is currently no data confirming the effectiveness and safety of PD-1 blockade plus lenvatinib in real-world settings. To address this issue, we conducted a retrospective real-world cohort study of patients with advanced ICC who treated with PD-1 blockade plus lenvatinib, and identified predictive biomarkers for tumor response and prognosis.
Materials and methods
Patients
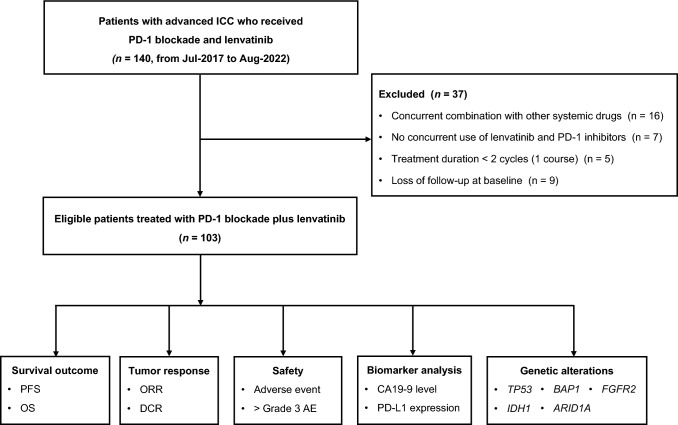
Patients with advanced ICC who received PD-1 inhibitors and lenvatinib between July 2017 and August 2022 at Peking Union Medical College Hospital (PUMCH) were retrospectively screened. The inclusion criteria were: (i) histologically confirmed unresectable locally advanced or metastatic ICC, (ii) disease measurable per Response Evaluation Criteria in Solid Tumors (RECIST) v1.1, (iii) treated with PD-1 inhibitors and lenvatinib at least one dose, (iv) Eastern Cooperative Oncology Group performance status (ECOG PS) 0–2, and (v) at least 18 years old. The exclusion criteria were (i) concurrent combination with other systemic drugs, (ii) no concurrent use of lenvatinib and PD-1 inhibitors, and (iii) treatment duration of fewer than two cycles (one course).
This study was performed in accordance with the Declaration of Helsinki and approved by the Ethics Committee of PUMCH (No. JS-1391). The study was registered at ClinicalTrials.gov (NCT03892577).
Treatment, assessment, and endpoints
Patients received a combined regimen of PD-1 blockade and lenvatinib. PD-1 inhibitors encompassed pembrolizumab, nivolumab, camrelizumab, sintilimab, tislelizumab, and toripalimab, all of which are available in mainland China. PD-1 inhibitors were administrated intravenously every 3 weeks with a fixed dose of 200 mg (pembrolizumab, nivolumab, camrelizumab, sintilimab, and tislelizumab) or 240 mg (toripalimab). Lenvatinib was given orally once daily at a dose of 8 mg (for body weight < 60 kg) or 12 mg (for body weight ≥ 60 kg).
Tumor assessments were performed every 6–9 weeks using CT or MRI and evaluated by experienced radiologists per RECIST v1.1. Safety profiles included monitoring of vital signs, physical exams, hematological and biochemical parameters, etc. AEs were assessed at every contact with the patient and were graded by investigators according to National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) v5.0.
The study endpoints were PFS, OS, ORR, disease control rate (DCR), and safety. PFS was defined as the time from initiation of the combination regimen to either radiological progression or death. OS was defined as the time from the start of combination therapy to death. ORR was defined as the proportion of patients with complete response (CR) or partial response (PR). DCR was defined as the proportion of patients with disease control (CR, PR, or stable disease [SD]). Subgroup analyses stratified by treatment lines (first-line and subsequent-line) were performed to evaluate the effectiveness in different settings.
Biomarker analysis
Biomarkers analyses were conducted to identify predictors associated with tumor response and prognosis. Carbohydrate antigen 19-9 (CA19-9) is recommended by guidelines as tumor marker for early detection and diagnosis of ICC, and CA19-9 level may predict treatment response and prognosis [17, 18]. CA19-9 levels at baseline were acquired from electronic medical records, and stratified as ≤ 37 U/ml and > 37 U/ml (the normal upper limit of CA19-9 is 37 U/mL). PD-L1 expression was evaluated using immunohistochemistry (IHC) with a PD-L1 IHC 22C3 pharmDx kit (Agilent Technologies, USA) on formalin-fixed, paraffin-embedded (FFPE) tumor specimens. PD-L1 expression was reported as tumor proportion score (TPS), defined as the percentage of tumor cells with membranous PD-L1 staining. TPS were classified as TPS ≥ 1% and < 1%, as suggested by previous reports [19–21]. Microsatellite instability/mismatch repair (MSI/MMR) status was determined from tumor samples by IHC detection of MMR-related proteins (MLH1/MSH2/MSH6/PMS2) or computational analysis of tumor microsatellite loci using next-generation sequencing (NGS) data [22–24]. Tumor genetic alterations were revealed using targeted panel sequencing on NGS platforms provided by OrigiMed (China) [25], Genecast (China) [26], and 3D Medicines (China) [27]. Exploratory biomarker analysis was performed based on mutated genes, and genes with mutation frequencies greater than 15% in our cohort were included.
Statistical analysis
Continuous variables were presented as median and range. Categorical variables were shown as frequency and percentage and were compared by chi-square test or Fisher’s exact test as appropriate. Survival analyses were conducted using the Kaplan–Meier method and compared by the log-rank test. Univariate and multivariate Cox regression was applied to identify risk factors for PFS and OS and to estimate hazard ratios (HR) and 95% confidence intervals (95% CI). A two-sided P < 0.05 was considered statically significant. All statistical analyses were done with SPSS version 27.
Results
Patient characteristics
A total of 103 patients were included in this study (Fig. 1). The median age of patients was 57 years old (range, 30–82), 38.8% of patients (40/103) were female, and 77.7% of patients (80/103) had an Eastern Cooperative Oncology Group performance status (ECOG PS) of 1 or 2. Additionally, 64.1% of patients (66/103) presented with distant metastasis. Forty-four patients (42.7%) were in the first-line setting. Fifty-nine patients (57.3%) experienced prior systemic treatments, among whom 41 (69.5%) received chemotherapy. Baseline characteristics were summarized in Table 1.
Fig. 1.
Flowchart of the study
Table 1.
Baseline characteristics
| Total (n = 103) | 1st line (n = 44) | ≥ 2nd line (n = 59) | |
|---|---|---|---|
| Median age (range), yr | 57 (30–82) | 59 (33–77) | 57 (30–73) |
| Female, n (%) | 40 (38.8%) | 14 (31.8%) | 26 (44.1%) |
| ECOG PS, n (%) | |||
| 0 | 23 (22.3%) | 10 (22.7%) | 13 (22.0%) |
| 1 | 61 (59.2%) | 26 (59.1%) | 35 (59.3%) |
| 2 | 19 (18.4%) | 8 (18.2%) | 11 (18.6%) |
| Metastatic site, n (%) | |||
| Liver | 87 (84.5%) | 38 (86.4%) | 49 (83.1%) |
| Lymph nodes | 83 (80.6%) | 35 (79.5%) | 48 (81.4%) |
| Lung | 25 (24.3%) | 6 (13.6%) | 19 (32.2%) |
| Bone | 24 (23.3%) | 10 (22.7%) | 14 (23.7%) |
| Extent of disease, n (%) | |||
| Locally advanced | 37 (35.9%) | 16 (36.4%) | 21 (35.6%) |
| Distant metastasis | 66 (64.1%) | 28 (63.6%) | 38 (64.4%) |
| Treatment line, n (%) | |||
| 1 | 44 (42.7%) | 44 (100%) | – |
| 2 | 52 (50.5%) | – | 52 (88.1%) |
| ≥ 3 | 7 (6.8%) | – | 7 (11.9%) |
| Prior treatment, n (%) | |||
| Surgery | 37 (35.9%) | 12 (27.3%) | 25 (42.4%) |
| Locoregional therapy | 48 (46.6%) | 12 (27.3%) | 36 (61.0%) |
| Systemic therapy | 59 (57.3%) | – | 59 (100%) |
Effectiveness
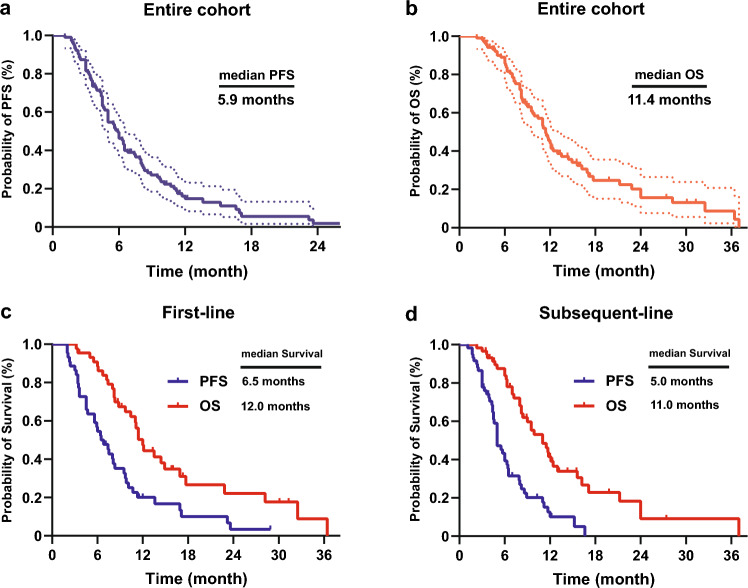
At the data cutoff of analysis (February 1, 2023), median follow-up time was 17.3 months (range, 2.3 to 37.3). In the overall population (n = 103), median PFS (mPFS) was 5.9 months (95% CI 5.1–6.7), and the 6-, and 12-month PFS rates were 46.2% and 14.7%, respectively (Fig. 2a). Median OS (mOS) was 11.4 months (95% CI 10.1–12.7), and the 6-, 12-, and 24-month OS rates were 85.8%, 44.1%, and 15.7%, respectively (Fig. 2b). Among 104 patients, 19 (18.4%) achieved an objective response, all of which were PRs, resulting in an ORR of 18.4%. Disease control was achieved in 83 patients (80.6%), with 47 patients (45.6%) experiencing continued disease control for 6 months or longer (Table 2).
Fig. 2.
Survival curves for overall population and subgroups a Progression free-survival of the entire cohort. b Overall survival of the entire cohort. Survival curves of c patients in first-line, and d patients in subsequent-line
Table 2.
Tumor response
| Total (n = 103) | 1st line (n = 44) | ≥ 2nd line (n = 59) | |
|---|---|---|---|
| Objective response, n (%) | 19 (18.4%) | 10 (22.7%) | 9 (15.3%) |
| Complete response, n (%) | 0 (0) | 0 (0) | 0 (0) |
| Partial response, n (%) | 19 (18.4%) | 10 (22.7%) | 9 (15.3%) |
| Stable disease, n (%) | 64 (62.1%) | 26 (59.1%) | 38 (64.4%) |
| Disease control rate, n (%) | 83 (80.6%) | 36 (81.8%) | 47 (79.7%) |
| Continued disease control, n (%) | |||
| ≥ 3 months | 89 (86.4%) | 39 (88.6%) | 50 (84.7%) |
| ≥ 6 months | 47 (45.6%) | 25 (56.8%) | 22 (37.3%) |
| ≥ 9 months | 23 (22.3%) | 14 (31.8%) | 9 (15.3%) |
| Progressive disease, n (%) | 20 (19.4%) | 8 (18.2%) | 12 (20.3%) |
In first-line (n = 44), mPFS was 6.5 months (95% CI 4.8–8.2), and mOS was 12.0 months (95% CI 10.7–13.3) (Fig. 2c). The 6-, and 12-month PFS rates were 54.5% and 20.1%, respectively. The 6-, 12-, and 24-month OS rates were 88.5%, 47.3%, and 22.1%, respectively. ORR and DCR were 22.7% (10/44) and 81.8% (36/44), respectively (Table 2).
In subsequent-line (n = 59), mPFS was 5.0 months (95% CI 4.3–5.7), and mOS was 11.0 months (95%CI, 8.3 to 13.7) (Fig. 2d). The 6-, and 12-month PFS rates were 39.3% and 10.1%, respectively. The 6-, 12-, and 24-month OS rates were 83.8%, 41.5%, and 9.1%, respectively. ORR and DCR were 15.3% (9/59) and 79.7% (47/59), respectively (Table 2).
Safety
All patients experienced AEs of various grade (Table 3). The most common AEs were fatigue (60.2%), hypertension (51.4%), elevated alanine aminotransferase (ALT) or aspartate alanine aminotransferase (AST) (45.6%), decreased appetite (43.7%), and hypothyroidism (34.0%). The incidence of grade 3 or 4 AEs was 50.5%. The most common grade 3 or 4 AEs were hypertension (12.6%), fatigue (6.8%), increased blood bilirubin (6.8%), diarrhea (5.8%), and elevated ALT or AST (4.9%). Grade 5 AEs occurred in 2 patients (1.9%), including pneumonia and arrhythmia (one each). Ten patients (9.6%) discontinued the treatment due to AEs.
Table 3.
Most common adverse events
| Any grade | Grade 3 or 4 | |
|---|---|---|
| Total | 103 (100%) | 52 (50.5%) |
| Fatigue | 62 (60.2%) | 7 (6.8%) |
| Hypertension | 53 (51.4%) | 13 (12.6%) |
| Elevated ALT or AST | 47 (45.6%) | 5 (4.9%) |
| Decreased appetite | 45 (43.7%) | 4 (3.9%) |
| Hypothyroidism | 35 (34.0%) | 1 (1.0%) |
| Proteinuria | 32 (31.1%) | 3 (2.9%) |
| Increased blood bilirubin | 31 (30.1%) | 7 (6.8%) |
| Rash | 30 (29.1%) | 4 (3.9%) |
| Diarrhea | 29 (28.2%) | 6 (5.8%) |
| Abdominal pain | 28 (27.2%) | 3 (2.9%) |
| Thrombocytopenia | 25 (24.3%) | 4 (3.9%) |
| Vomiting | 23 (22.3%) | 2 (1.9%) |
| Decreased weight | 22 (21.4%) | 0 |
| Nausea | 19 (18.4%) | 0 |
| Palmar-plantar erythrodysesthesia | 18 (17.5%) | 3 (2.9%) |
| Hypoalbuminemia | 18 (17.5%) | 2 (1.9%) |
| Anemia | 16 (15.5%) | 2 (1.9%) |
| Dysphonia | 13 (12.6%) | 0 |
| Upper gastrointestinal hemorrhage | 10 (9.7%) | 3 (2.9%) |
Biomarker analysis
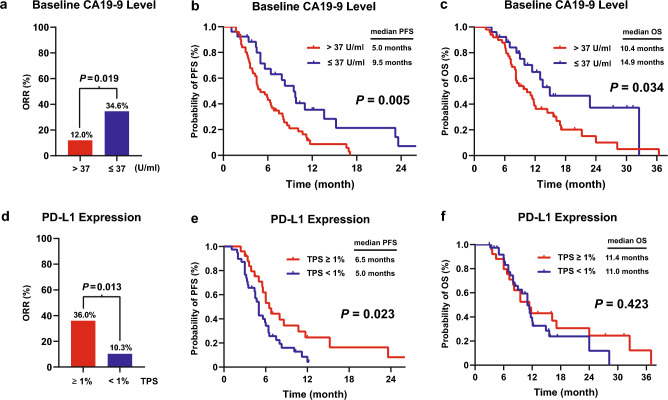
Seventy-six patients had records of baseline CA19-9 levels, among whom 50 patients (65.8%) had CA19-9 higher than 37 U/ml. Patients with CA19-9 ≤ 37 U/ml achieved a higher ORR than those with CA19-9 > 37 U/ml (34.6% vs. 12.0%, P = 0.019; Fig. 3a). Moreover, patients with CA19-9 ≤ 37 U/ml showed longer PFS and OS than those with CA19-9 > 37 U/ml (PFS, P = 0.005; OS, P = 0.034; Fig. 3b, c). Evaluation of PD-L1 expression was performed in 64 patients, and 25 patients (39.1%) were classified as TPS ≥ 1%. Patients with TPS ≥ 1% experienced a higher ORR than those with TPS < 1% (36.0% vs. 10.3%, P = 0.013; Fig. 3d). Patients with TPS ≥ 1% displayed a longer PFS than those with TPS < 1% (P = 0.023; Fig. 3e). Although the difference in OS between the two subgroups was not significant (P = 0.423; Fig. 3f), the TPS ≥ 1% subgroup showed an improvement in the tail of the OS curve. Detection of MSI/MMR status was performed in 42 patients. Most of the patients (92.9%) were presented with MSS/MSI-L or pMMR, and only 3 patients (7.1%) were MSI-H/dMMR. Given the small number of patients with MSI-H/dMMR, it would be improper and inaccuracy to evaluate the predictive power of MSI/MMR status, and a further attempt at biomarker analysis for MSI/MMR status was abolished.
Fig. 3.
Treatment-related biomarkers a–c ORR, PFS, and OS for patients with CA19-9 > 37 U/ml and ≤ 37 U/ml. d–f ORR, PFS, and OS for patients with TPS ≥ 1% and < 1%
Cox regression analyses were done to identify prognostic factors (Supplementary material Table 4). Univariate analyses indicated that CA19-9 level (> 37 vs. ≤ 37 U/ml) and PD-L1 expression (TPS ≥ 1% vs. < 1%) were significantly associated with PFS (P < 0.05). Multivariate analyses demonstrated that positive PD-L1 expression (TPS ≥ 1%) was a favorable prognostic factor for PFS (HR = 0.46, P = 0.047). Moreover, CA19-9 > 37 U/ml and distant metastasis were independent predictors for worse OS (CA19-9 level: HR = 2.42, P = 0.008; Extent of disease: HR = 2.46, P = 0.039).
Exploratory analysis on genetic alterations
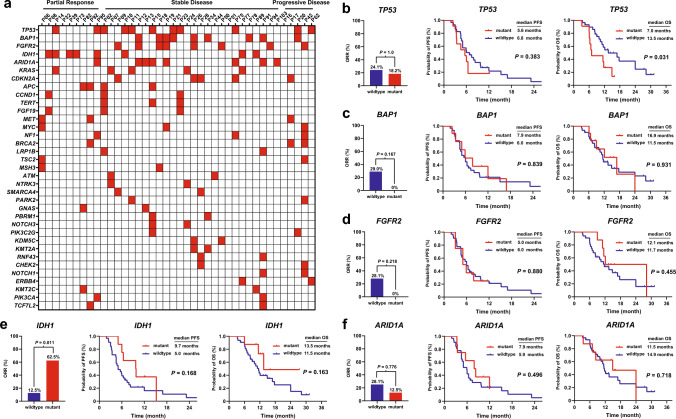
Profiles of genetic alterations were available in 40 patients (Fig. 4a). TP53 mutations were the most frequently observed alterations (11/40, 27.5%), followed by BAP1 mutations (9/40, 22.5%), FGFR2 fusions/rearrangement (8/40, 20%), IDH1 mutations (8/40, 20%), ARID1A mutations (8/40, 20%), KRAS mutations (5/40, 12.5%), and CDKN2A mutations (5/40, 12.5%). More than half of the patients with PR harbored IDH1 mutations (5/9, 55.6%). Patients with SD frequently exhibited alterations in BAP1 (8/26, 30.8%), TP53 (7/26, 26.9%), FGFR2 (7/26, 26.9%), and ARID1A (7/26, 26.9%). TP53 mutations were identified in 2 out of 5 (40%) patients with progressive disease. We conducted biomarker analysis for the top 5 altered genes. Patients with mutant TP53 had a similar ORR and PFS compared to those with wildtype TP53, but showed a worse OS (P = 0.031) (Fig. 4b). Patients harboring genetic alterations of BAP1, FGFR2, or ARID1A tended to obtain a higher ORR, but with no statistical difference. There was no difference in survival between the mutated and the wildtype of the three genes. Intriguing, none of the patients with alterations in BAP1 or FGFR2 responded to the regimen (Fig. 4c, d). Moreover, patients with IDH1 mutations exhibited a higher ORR when compared to those with wildtype IDH1 (62.5% vs. 12.5%, P = 0.011), and appeared to have a prolonged PFS and OS, though the difference was not statistically significant (Fig. 4e).
Fig. 4.
Exploratory analysis for genetic alternations a Profiles of genetic alternations. ORR, PFS, and OS stratified by mutation status for b TP53, c BAP1, d FGFR2, e IDH1, and f ARID1A
Discussion
The application of PD-1 blockade combined with lenvatinib for ICC has not been comprehensively assessed in real-world settings. In this study, we evaluated the experience in the use of this therapeutic option in a retrospective real-world cohort, focusing on effectiveness and safety, as well as treatment-related biomarkers. We were able to confirm that the combination treatment is an effective and safe option in patients with advanced ICC. Our study has three strengths: (i) the study population is the largest sample size cohort to date (a total of 103 patients); (ii) a long observation period (from 2017 to 2022); (iii) a comprehensive biomarker analysis.
In the entire cohort, PD-1 inhibitors plus lenvatinib demonstrated a mPFS of 5.9 months, a mOS of 11.4 months, an ORR of 18.4%, and a DCR of 80.6%. To better interpret the efficacy data and compare with previous reports, the cohort was divided based on treatment lines. In the first-line setting, this regimen demonstrated a mPFS of 6.5 months, a mOS of 12.0 months, an ORR of 22.7%, and a DCR of 81.8%. Only one phase II study (preliminary results reported as an abstract) evaluated toripalimab plus lenvatinib as a first-line treatment for 31 patients with advanced ICC [15]. The ORR and DCR was 32.3% and 74.2%, and 6-months OS rate was 87.1%. Besides, our efficacy data was comparable to GemCis in the TOPAZ-1 study (mPFS 5.7 months, mOS 11.5 months, ORR 18.7%, and DCR 82.6%), though showed a slight numerical differences compared to GemCis plus durvalumab (mPFS 7.2 months, mOS 12.8 months, ORR 26.7%, and DCR 85.3%) [6]. As a subsequent-line treatment, PD-1 inhibitors plus lenvatinib achieved a mPFS of 5.0 months, a mOS of 11.0 months, an ORR of 15.3%, and a DCR of 79.7%. Lin and his colleagues reported pembrolizumab plus lenvatinib as a subsequent-line therapy in 32 patients with BTC, which demonstrated a mPFS of 4.9 months, a mOS of 11.0 months, an ORR of 25%, and a DCR of 78.1% [28]. The phase II LEAP-005 study assessed lenvatinib plus pembrolizumab in a second-line setting for 31 patients with advanced BTC, demonstrating a mPFS of 6.1 months, a mOS of 8.6 months, an ORR of 10%, and a DCR of 68% [14]. Ding et al. conducted a retrospective study investigating sintilimab plus lenvatinib as second-line therapy for 41 patients with advanced ICC [21]. The median time to progression was 6.6 months, and mOS was 16.6 months. Strikingly, 46.3% of patients achieved an objective response, which might owe to that 80.5% of the patients were positive PD-L1 expression. Our results were similar to these prior studies, especially in terms of survival. In addition, when compared to the FOLFOX regimen in the ABC-06 study (the only phase III trial in second-line) [5], which only achieved a mPFS of 4.0 months, a mOS of 6.2 months, an ORR of 4.9%, and a DCR of 33%, PD-1 blockade combined with lenvatinib exhibited conspicuous advantages. Although direct comparisons between studies may be inappropriate because patient characteristics are different in each study, but helpful in interpreting results. Collectively, these results strongly support the efficacy and reliability of PD-1 blockade plus lenvatinib for advanced ICC. It holds promise as a viable first-line treatment, especially for patients who cannot tolerate or refuse chemotherapy. Moreover, it represents a potential subsequent-line option preferred for patients who experience progression after chemotherapy. To validate our findings, high-level evidence from well-designed trials is still needed. Several ongoing prospective studies (NCT04361331, NCT03797326, NCT04211168) are expected to provide more robust evidence.
Safety profiles are another emphasis. In our cohort, 50.5% of the patients experienced grade 3 or 4 AEs. The vast majority of AEs could be well managed. Fatigue and decreased appetite could cause low quality of life. Some AEs such as gastrointestinal bleeding, pneumonia, hepatitis, myocarditis, and enteritis could be life-threatening and require periodic monitoring and timely management. With the accumulation of experience, general and severe AEs management will gradually improve. PD-1 blockade plus lenvatinib have been widely used in HCC [29]. Oncologists can leverage their experience from treating HCC to promptly detect and manage AEs during the administration of this regimen in ICC.
To aid clinical decision-making, we identified several treatment-related biomarkers. PD-L1 expression is the most widely used biomarker for immunotherapy. High PD-L1 expression is generally considered to associate with better response to immunotherapy and prolonged survival [30]. In our study, positive PD-L1 expression was associated with higher ORR and prolonged PFS, consistent with previous studies [21, 28]. There are few reports exploring the influence of genetic alteration on immunotherapy for ICC. IDH1 mutations are common molecular alterations of ICC, detected in approximately 20% of the patients [31, 32]. Mutation of IDH1 results in the accumulation of the oncometabolite 2-hydroxyglutarate, and the 2-hydroxyglutarate is involved in carcinogenesis and immunoevasion [33]. In our cohort, patients with IDH1 mutations were found to have higher ORR and a tendency towards longer survival. It seems that IDH1 mutations might predict a better response to immunotherapy. Genetic alterations of BAP1 and FGFR2 occur in 15–20% of patients with ICC [31, 32]. Interestingly, in our cohort, none of the patients with alterations in BAP1 or FGFR2 responded to the regimen. BAP1 is a tumor suppressor gene, and its mutations have been identified in various tumors, including ICC [34]. Downregulation or inactivation of BAP1 can accelerate tumor onset, invasion, recurrence, and progression. FGFR2 alterations, such as gain-of-function mutations, amplifications, and chromosomal translocations, can activate FGFR2 signaling, leading to tumorigenesis and progression by promoting proliferation and survival of tumor cells [35]. The presence of genetic alterations in BAP1 and FGFR2 may contribute to an aggressive biological behavior of tumors, which could partly explain why the regimen was less effective in patients with these altered genes. The underlying molecular mechanisms are fascinating and require further investigation before drawing generalizable conclusions [36]. Besides, considering that lenvatinib inhibits the VEGF/VEGFR pathway by targeting VEGFR1/2/3, future investigation will be conducted to determine whether VEGFR expression could serve as a potential biomarker.
The study has some limitations. Firstly, its retrospective nature may introduce potential bias. Multicenter prospective studies are needed to confirm our results. Secondly, multiple kinds of PD-1 inhibitors may introduce heterogeneity. It is unavoidable in complex real-world situations because many kinds of PD-1 inhibitors are currently available, and various factors may influence patient choices such as economic considerations, insurance coverage, charitable donation and preferences. Thirdly, a proportion of patients received the regimen as first-line treatment instead of standard chemotherapy (e.g., GemCis), which requires explanation. This is because these patients explicitly refused chemotherapy during the visit. The treatment decision was made with patient consent and regulatory approval. It was compliant with ethical guidelines and the principle of compassionate use.
In conclusion, PD-1 blockade plus lenvatinib is effective and safe in routine practice. It presents as a viable treatment option for advanced ICC. PD-L1 expression and baseline CA19-9 level appear to predict the treatment efficacy. IDH1 mutations might predict a better response to the regimen.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Dr. Jianzhen Lin (the First Affiliated Hospital of Nanjing Medical University) for his sincere assistance.
Abbreviations
- AE
Adverse event
- BTC
Biliary tract cancer
- CA19-9
Carbohydrate antigen 19-9
- CI
Confidence interval
- CR
Complete response
- CSCO
Chinese Society of Clinical Oncology
- CTCAE
Common Terminology Criteria for Adverse Events
- DCR
Disease control rate
- ECOG PS
Eastern Cooperative Oncology Group performance status
- ICC
Intrahepatic cholangiocarcinoma
- IHC
Immunohistochemistry
- MMR
Mismatch repair
- MSI-H/L
Microsatellite instability-high/low
- NCCN
National Comprehensive Cancer Network
- NGS
Next-generation sequencing
- ORR
Objective response rate
- OS
Overall survival
- PD-1
Programmed death 1
- PFS
Progression-free survival
- PR
Partial response
- PUMCH
Peking Union Medical College Hospital
- RECIST
Response evaluation criteria in solid tumors
- SD
Stable disease
- TPS
Tumor proportion score
Author contributions
HZ and LL contributed to the conception and design of the study. All authors participated in patient follow-up and contributed to data collection. JC, SW, and HW collated the data and performed analysis. JC, SW, and HW drafted the manuscript. All authors contributed to reviewing or revising the manuscript and approved the final version.
Funding
This work was supported by the National High Level Hospital Clinical Research Funding (2022-PUMCH-B-128), CAMS Innovation Fund for Medical Sciences (2019-I2M-5-035, 2021-I2M-1-061, 2022-I2M-C&T-A-003), CSCO-Hengrui Cancer Research Fund (Y-HR2019-0239, Y-HR2020QN-0414), CSCO-MSD Cancer Research Fund (Y-MSDZD2021-0213), and the National Ten-thousand Talent Program.
Data availability
The data that support the findings of our study are available from the corresponding author upon request.
Declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
The study was approved by the Ethics Committee of Peking Union Medical College Hospital (No. JS-1391), and was conducted in accordance with the Declaration of Helsinki. Informed consent was obtained from all patients.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jiashuo Chao, Shanshan Wang and Hao Wang have contributed equally to this work.
Contributor Information
Ling Lu, Email: lvling@njmu.edu.cn.
Haitao Zhao, Email: zhaoht@pumch.cn.
References
- 1.Moris D, Palta M, Kim C, Allen PJ, Morse MA, Lidsky ME. Advances in the treatment of intrahepatic cholangiocarcinoma: An overview of the current and future therapeutic landscape for clinicians. CA Cancer J Clin. 2023;73:198–222. doi: 10.3322/caac.21759. [DOI] [PubMed] [Google Scholar]
- 2.Valle JW, Kelley RK, Nervi B, Oh DY, Zhu AX. Biliary tract cancer. Lancet. 2021;397:428–444. doi: 10.1016/S0140-6736(21)00153-7. [DOI] [PubMed] [Google Scholar]
- 3.Florio AA, Ferlay J, Znaor A, Ruggieri D, Alvarez CS, Laversanne M, et al. Global trends in intrahepatic and extrahepatic cholangiocarcinoma incidence from 1993 to 2012. Cancer. 2020;126:2666–2678. doi: 10.1002/cncr.32803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–1281. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 5.Lamarca A, Palmer DH, Wasan HS, Ross PJ, Ma YT, Arora A, et al. Second-line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer (ABC-06): a phase 3, open-label, randomised, controlled trial. Lancet Oncol. 2021;22:690–701. doi: 10.1016/S1470-2045(21)00027-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oh D-Y, Ruth He A, Qin S, Chen L-T, Okusaka T, Vogel A, et al. Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer. NEJM Evid. 2022;1:EVIDoa200015. doi: 10.1056/EVIDoa2200015. [DOI] [PubMed] [Google Scholar]
- 7.Kelley RK, Ueno M, Yoo C, Finn RS, Furuse J, Ren Z, et al. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2023;401:1853–1865. doi: 10.1016/S0140-6736(23)00727-4. [DOI] [PubMed] [Google Scholar]
- 8.Job S, Rapoud D, Dos Santos A, Gonzalez P, Desterke C, Pascal G, et al. Identification of four immune subtypes characterized by distinct composition and functions of tumor microenvironment in intrahepatic cholangiocarcinoma. Hepatology. 2020;72:965–981. doi: 10.1002/hep.31092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim RD, Chung V, Alese OB, El-Rayes BF, Li D, Al-Toubah TE, et al. A phase 2 multi-institutional study of nivolumab for patients with advanced refractory biliary tract cancer. JAMA Oncol. 2020;6:888–894. doi: 10.1001/jamaoncol.2020.0930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Piha-Paul SA, Oh DY, Ueno M, Malka D, Chung HC, Nagrial A, et al. Efficacy and safety of pembrolizumab for the treatment of advanced biliary cancer: results from the KEYNOTE-158 and KEYNOTE-028 studies. Int J Cancer. 2020;147:2190–2198. doi: 10.1002/ijc.33013. [DOI] [PubMed] [Google Scholar]
- 11.Torrens L, Montironi C, Puigvehí M, Mesropian A, Leslie J, Haber PK, et al. Immunomodulatory effects of Lenvatinib plus anti-programmed cell death protein 1 in mice and rationale for patient enrichment in hepatocellular carcinoma. Hepatology. 2021;74:2652–2669. doi: 10.1002/hep.32023. [DOI] [PubMed] [Google Scholar]
- 12.Deng H, Kan A, Lyu N, Mu L, Han Y, Liu L, et al. Dual vascular endothelial growth factor receptor and fibroblast growth factor receptor inhibition elicits antitumor immunity and enhances programmed cell death-1 checkpoint blockade in hepatocellular carcinoma. Liver Cancer. 2020;9:338–357. doi: 10.1159/000505695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fukumura D, Kloepper J, Amoozgar Z, Duda DG, Jain RK. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat Rev Clin Oncol. 2018;15:325–340. doi: 10.1038/nrclinonc.2018.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Villanueva L, Lwin Z, Chung HC, Gomez-Roca C, Longo F, Yanez E, et al. Lenvatinib plus pembrolizumab for patients with previously treated biliary tract cancers in the multicohort phase II LEAP-005 study. J Clin Oncol. 2021;39:321–321. doi: 10.1200/JCO.2021.39.3_suppl.321. [DOI] [Google Scholar]
- 15.Jian Z, Fan J, Shi G-M, Huang X-Y, Wu D, Liang F, et al. Lenvatinib plus toripalimab as first-line treatment for advanced intrahepatic cholangiocarcinoma: a single-arm, phase 2 trial. J Clin Oncol. 2021;39:4099–4099. doi: 10.1200/JCO.2021.39.15_suppl.4099. [DOI] [Google Scholar]
- 16.Chodankar D. Introduction to real-world evidence studies. Perspect Clin Res. 2021;12:171–174. doi: 10.4103/picr.picr_62_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kubo S, Shinkawa H, Asaoka Y, Ioka T, Igaki H, Izumi N, et al. Liver cancer study group of japan clinical practice guidelines for intrahepatic cholangiocarcinoma. Liver Cancer. 2022;11:290–314. doi: 10.1159/000522403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vogel A, Bridgewater J, Edeline J, Kelley RK, Klümpen HJ, Malka D, et al. Biliary tract cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023;34:127–140. doi: 10.1016/j.annonc.2022.10.506. [DOI] [PubMed] [Google Scholar]
- 19.Dietel M, Savelov N, Salanova R, Micke P, Bigras G, Hida T, et al. Real-world prevalence of programmed death ligand 1 expression in locally advanced or metastatic non-small-cell lung cancer: the global, multicenter EXPRESS study. Lung Cancer. 2019;134:174–179. doi: 10.1016/j.lungcan.2019.06.012. [DOI] [PubMed] [Google Scholar]
- 20.Chen X, Wu X, Wu H, Gu Y, Shao Y, Shao Q et al (2020) Camrelizumab plus gemcitabine and oxaliplatin (GEMOX) in patients with advanced biliary tract cancer: a single-arm, open-label, phase II trial. J Immunother Cancer 8 [DOI] [PMC free article] [PubMed]
- 21.Ding X, Li G, Sun W, Shen Y, Teng Y, Xu Y, et al. Sintilimab combined with lenvatinib for advanced intrahepatic cholangiocarcinoma in second-line setting-a multi-center observational study. Front Oncol. 2022;12:907055. doi: 10.3389/fonc.2022.907055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maio M, Ascierto PA, Manzyuk L, Motola-Kuba D, Penel N, Cassier PA, et al. Pembrolizumab in microsatellite instability high or mismatch repair deficient cancers: updated analysis from the phase II KEYNOTE-158 study. Ann Oncol. 2022;33:929–938. doi: 10.1016/j.annonc.2022.05.519. [DOI] [PubMed] [Google Scholar]
- 23.Kautto EA, Bonneville R, Miya J, Yu L, Krook MA, Reeser JW, et al. Performance evaluation for rapid detection of pan-cancer microsatellite instability with MANTIS. Oncotarget. 2017;8:7452–7463. doi: 10.18632/oncotarget.13918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salipante SJ, Scroggins SM, Hampel HL, Turner EH, Pritchard CC. Microsatellite instability detection by next generation sequencing. Clin Chem. 2014;60:1192–1199. doi: 10.1373/clinchem.2014.223677. [DOI] [PubMed] [Google Scholar]
- 25.Cao J, Chen L, Li H, Chen H, Yao J, Mu S, et al. An accurate and comprehensive clinical sequencing assay for cancer targeted and immunotherapies. Oncologist. 2019;24:e1294–e1302. doi: 10.1634/theoncologist.2019-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang L, Chen Y, Wang H, Xu Z, Wang Y, Li S et al (2021) Massive PD-L1 and CD8 double positive TILs characterize an immunosuppressive microenvironment with high mutational burden in lung cancer. J Immunother Cancer 9 [DOI] [PMC free article] [PubMed]
- 27.Xiao J, Li W, Huang Y, Huang M, Li S, Zhai X, et al. A next-generation sequencing-based strategy combining microsatellite instability and tumor mutation burden for comprehensive molecular diagnosis of advanced colorectal cancer. BMC Cancer. 2021;21:282. doi: 10.1186/s12885-021-07942-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin J, Yang X, Long J, Zhao S, Mao J, Wang D, et al. Pembrolizumab combined with lenvatinib as non-first-line therapy in patients with refractory biliary tract carcinoma. Hepatobiliary Surg Nutr. 2020;9:414–424. doi: 10.21037/hbsn-20-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang X, Chen B, Wang Y, Wang Y, Long J, Zhang N et al (2023) Real-world efficacy and prognostic factors of lenvatinib plus PD-1 inhibitors in 378 unresectable hepatocellular carcinoma patients. Hepatol Int 1–11 [DOI] [PMC free article] [PubMed]
- 30.Yi M, Jiao D, Xu H, Liu Q, Zhao W, Han X, et al. Biomarkers for predicting efficacy of PD-1/PD-L1 inhibitors. Mol Cancer. 2018;17:129. doi: 10.1186/s12943-018-0864-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tomczak A, Springfeld C, Dill MT, Chang DH, Kazdal D, Wagner U, et al. Precision oncology for intrahepatic cholangiocarcinoma in clinical practice. Br J Cancer. 2022;127:1701–1708. doi: 10.1038/s41416-022-01932-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boerner T, Drill E, Pak LM, Nguyen B, Sigel CS, Doussot A, et al. Genetic determinants of outcome in intrahepatic cholangiocarcinoma. Hepatology. 2021;74:1429–1444. doi: 10.1002/hep.31829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu MJ, Shi L, Dubrot J, Merritt J, Vijay V, Wei TY, et al. Mutant IDH inhibits IFNγ-TET2 signaling to promote immunoevasion and tumor maintenance in cholangiocarcinoma. Cancer Discov. 2022;12:812–835. doi: 10.1158/2159-8290.CD-21-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen XX, Yin Y, Cheng JW, Huang A, Hu B, Zhang X, et al. BAP1 acts as a tumor suppressor in intrahepatic cholangiocarcinoma by modulating the ERK1/2 and JNK/c-Jun pathways. Cell Death Dis. 2018;9:1036. doi: 10.1038/s41419-018-1087-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mahipal A, Tella SH, Kommalapati A, Anaya D, Kim R. FGFR2 genomic aberrations: achilles heel in the management of advanced cholangiocarcinoma. Cancer Treat Rev. 2019;78:1–7. doi: 10.1016/j.ctrv.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 36.Munugala N, Maithel SK, Shroff RT. Novel biomarkers and the future of targeted therapies in cholangiocarcinoma: a narrative review. Hepatobiliary Surg Nutr. 2022;11:253–266. doi: 10.21037/hbsn-20-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of our study are available from the corresponding author upon request.