Abstract
It has been suggested that Fusobacterium nucleatum (Fn) may differentially impact tumor immune responses according to microsatellite instability (MSI) status in colorectal cancers (CRCs). We aimed to reveal the detailed relationship between intratumoral Fn and immune microenvironmental features in MSI-high CRCs. A total of 126 MSI-high CRCs were subjected to analyses for intratumoral Fn DNA load using quantitative PCR and for densities of tumor-infiltrating immune cells, including CD3+ T cells, CD4+ T cells, CD8+ T cells, FoxP3+ T cells, CD68+ macrophages, CD163+ macrophages, and CD177+ neutrophils, at invasive margin (IM) and center of tumor (CT) areas using computational image analysis of immunohistochemistry. Based on the Fn load, the 126 MSI-high CRCs were classified into Fn-high, -low, and -negative subgroups. The Fn-high subset of MSI-high CRCs was significantly correlated with larger tumor size and advanced invasion depth (p = 0.017 and p = 0.034, respectively). Compared with the Fn-low/negative subgroup, Fn-high tumors demonstrated significantly lower density of FoxP3+ cells in both IM and CT areas (p = 0.002 and p = 0.003, respectively). Additionally, Fn-high was significantly associated with elevated CD163+ cell to CD68+ cell ratio in only CT areas of MSI-high CRCs (p = 0.028). In conclusion, the Fn-enriched subset of MSI-high CRCs is characterized by increased tumor growth and invasion and distinct immune microenvironmental features, including decreased FoxP3+ T cells throughout the tumor and increased proportion of M2-polarized macrophages in the tumor center. These findings collectively support that Fn may be linked to pro-tumoral immune responses in MSI-high CRCs.
Electronic supplementary material
The online version of this article (10.1007/s00262-020-02657-x) contains supplementary material, which is available to authorized users.
Keywords: Colorectal carcinoma, Gut microbiota, Gut microbiome, Tumor immunity, Tumor immunology
Introduction
Emerging evidence has revealed various implications of gut microbiota in tumorigenesis, tumor microenvironment, patient prognosis, and treatment responses in malignancies [1]. Gut microbiota in humans includes more than 100 trillion microorganisms, some of which may be involved in modulating host metabolism and immunity [2]. Fusobacterium nucleatum (Fn), a bacterial species mainly found in the human oral cavity, has been reported to be specifically enriched in a subset of human colorectal cancers (CRCs) and to be associated with the development and progression of CRCs [3–5]. Experimentally, Fn can promote CRC tumorigenesis by modulating E-cadherin/β-catenin signaling pathways [4]. In addition, in the mouse model, Fn can also contribute to intestinal tumor progression through modulating expansion and recruitment of specific immune cells [5]. These results indicate that Fn may facilitate initiation and progression of human CRCs through affecting not only tumor cell-intrinsic pathways but also the tumor immune microenvironment.
In terms of the immunologic implications of Fn in CRCs, there have been recent important progresses in related studies. In experimental models, it was suggested that Fn could inhibit antitumor immune responses in CRC cells [6]. It has also been reported that Fn can modulate the tumor immune microenvironment by promoting M2 polarization of tumor-associated macrophages, which may play tumor-promoting roles [7]. In a similar context with these experimental evidences, a clinical study using human CRC cohorts demonstrated that intratumoral Fn abundance is associated with a lower density of tumor-infiltrating lymphocytes (TILs) in CRC tissues [8]. These recent experimental and clinical data have collectively suggested that Fn may be involved in direct or indirect suppression of antitumor immune responses in CRCs. However, detailed evidence of the precise relationship between Fn and tumor immunity in CRCs is still insufficient, and a comprehensive landscape of interactions between intratumoral Fn and the tumor immune microenvironment in human CRCs has not yet been fully elucidated. Moreover, the impacts of Fn on the immune microenvironment may be confounded by underlying molecular status in CRCs. For example, Hamada et al. recently reported that Fn might influence host immune responses differentially by microsatellite instability (MSI) status in CRCs [9]. According to the study, high Fn loads were significantly correlated with decreased TILs specifically in the MSI-high molecular subtype of CRCs, but not in microsatellite-stable (non-MSI-high) CRCs [9].
Therefore, to more exactly confirm associations between Fn and local tumor immunity in an MSI-high subtype of CRCs, we decided to investigate comprehensive correlations between intratumoral Fn DNA load and intratumoral region-specific densities of various tumor-infiltrating immune cells (TIICs), including CD3+, CD8+ CD4+, and FoxP3+ T cells, CD68+ and CD163+ macrophages, and CD177+ neutrophils, in a large series of MSI-high CRC tissues. Through our intensive analyses, this study aimed to provide detailed data regarding in situ associations between Fn and TIICs in MSI-high CRCs.
Materials and methods
Tissue samples
Initially, 153 MSI-high CRCs were retrospectively collected from the pathology archive of Seoul National University Hospital, Seoul, Korea. All the tissues were obtained from surgical specimens of patients who underwent surgical resection at Seoul National University Hospital between 2014 and 2018. All the study samples were previously confirmed to be MSI-high tumors by DNA fragment analysis using five DNA microsatellite markers (BAT-25, BAT-26, D5S346, D17S250, and D2S123) according to the Bethesda Guideline [10]. Among the 153 samples, 18 cases were excluded due to insufficiency of their remaining tissues. Thus, 135 cases were subjected to quantitative polymerase chain reaction (qPCR) analysis for Fn. Among them, nine samples determined as invalid from the qPCR analysis, as described subsequently, were excluded. Ultimately, a total of 126 MSI-high CRC cases were included in this study. The Institutional Review Board of our hospital approved this study (IRB No. 1805-018-944).
Clinicopathologic data
Clinical data of the 126 MSI-high CRCs were retrospectively collected by review of electronic medical records. The data included age, sex, tumor location, and clinical evidence of distant metastasis (cM category). Data regarding disease-free survival (DFS) and overall survival (OS), including tumor recurrence and patient death, were also collected. Among the 126 MSI-high CRCs, the survival data were established only for 78 cases with the clinical follow-up period of three years or more. The pathologic features were evaluated by gastrointestinal pathologists (J.A.L. and J.H.K.). The assessed pathologic parameters included gross tumor type, tumor size, depth of invasion (pT category), lymph node metastasis (pN category), distant metastasis (pM category), lymphatic/venous/perineural invasion, tumor differentiation (WHO tumor grade), mucinous histology, medullary histology, signet ring cell histology, and peritumoral tertiary lymphoid structure (TLS). To assess TLS activity, a representative indicator of peritumoral antitumor lymphoid responses, we used Ueno’s criteria (measuring the maximum diameter of the largest TLS) as previously described [11, 12].
Immunohistochemistry
Multicore tissue microarray (TMA) blocks of the 126 MSI-high CRCs were constructed. In detail, four representative tumor areas, two of which were from the center of tumor (CT) area and two of which were from the invasive margin (IM) area, on formalin-fixed, paraffin-embedded (FFPE) tissue blocks of each CRC case were selected and extracted as TMA cores (2 mm in diameter). Immunohistochemistry (IHC) for CD3 (2GV6 clone, Ventana RTU, Roche, Basel, Switzerland) and CD8 (SP57 clone, Ventana RTU, Roche), major components for immunoscoring of antitumor TILs in CRCs [13], was conducted on a whole slide of a representative tumor section of each case (Supplementary Fig. S1a). IHC for FoxP3 (236A/E7 clone, Abcam, Cambridge, UK), CD4 (SP35 clone, Ventana RTU, Roche), CD68 (DAKO KP1 clone, Agilent Technologies, Santa Clara, CA, USA), CD163 (Cell Marque MRQ-26 clone, MilliporeSigma, Burlington, MA, USA), CD177 (HPA041820 clone, Atlas antibodies, Bromma, Sweden), PD-L1 (E1L3N clone; Cell Signaling Technology, Danvers, MA, USA), MLH1 (M1 clone, Ventana RTU, Roche), MSH2 (Invitrogen FE11 clone, ThermoFisher Scientific, Waltham, MA, USA), MSH6 (Cell Marque 44 clone, MilliporeSigma), and PMS2 (Cell Marque MRQ-28 clone, MilliporeSigma) was conducted on TMA sections of the 126 MSI-high CRC samples (four cores per each case; Supplementary Fig. S1b). All IHC procedures were performed using automated immunostainers (Ventana BenchMark XT, Roche or Bond-III, Leica Biosystems, Wetzlar, Germany).
Computational image analysis for quantification of TIICs
Quantification of TIICs was undertaken by counting staining-positive cells on IHC sections stained for each TIIC marker. All whole slides (CD3 or CD8 stained) or TMA slides (FoxP3, CD4, CD68, CD163, or CD177 stained) were scanned by an Aperio AT2 slide scanner (Leica biosystems) at 20 × magnification with a resolution of 0.5 μm per pixel. Enumeration of each TIIC was conducted using ‘positive cell detection’ functionality of QuPath software, a validated open-source software for digital pathology image analysis [14, 15]. Cell positivity was determined based on the mean optical density of 3,3′-diaminobenzidine (DAB) intensity at the nuclear compartment. Due to varying staining quality, we adjusted the positivity threshold of each TIIC marker so that detected cells corresponded to those deemed positive by the two pathologists (J.A.L. and S.Y.Y.). As a result, cells with mean DAB optical density greater than 0.3 were counted as positive for CD4, CD68, CD163, and CD177. In contrast, the threshold value of 0.4 was adopted for CD3 and CD8, and 0.7 for FoxP3. Density of each TIIC in each region (IM or CT) was defined as the number of positive cells divided by the field area (cells/mm2). For whole-slide image analysis of CD3 and CD8 IHC, a region of interest (ROI) was manually delineated to encompass the entire tumor and peritumoral stroma within 1-mm distance while excluding the area of necrosis, abscess, mucin pools, and tertiary lymphoid structures. The ROI was segmented into 1 mm × 1 mm tiles; the frontmost tiles were designated as invasive margin and the tiles that were not IM assigned were referred to as CT (Supplementary Fig. S1a). For example, if A is the number of IM tiles in a CD3 IHC slide and X is the total number of positive cells identified within the A IM tiles, then CD3+ T cell density in IM is calculated as X/A cells/mm2 since the area of each tile equals to 1 mm2. For TMA slides, the number of positive cells per mm2 in each core was obtained following the TMA analysis instruction provided by the developer (https://github.com/qupath/qupath/wiki). Since two slides were available for each TIIC marker in each region, TIIC density in a given region was calculated by calculating the average of the two slides values (Supplementary Fig. S1b). Although TIL assessment guidelines, such as that of the International Immuno-Oncology Biomarkers Working Group, recommend to report the abundance of TILs in terms of an overall percentage of the intratumoral stromal area covered by immune cells [16], we argue that the guideline rooted from the limitation of semiquantitative assessment based on visual inspection of hematoxylin and eosin (H&E)-stained sections. Since we used IHC instead of H&E sections, we were able to introduce image analysis-based precision quantification of each TIIC marker-stained cells. Additionally, we confirmed significantly strong positive correlations between TIIC count-based densities (cells/mm2) and matched TIIC area-based densities (%; the area occupied by positive cells divided by the field area), indicating that the “counting” methodology and the “percentage of area” methodology produce similar results.
Fn qPCR
Genomic DNA was extracted from FFPE tissues of the 135 CRCs, and subsequent qPCR for Fn using the 135 tumor DNA samples was conducted. Primers, probes, and PCR conditions used in this study were modified from our previous methodology referring to other study [17–19]. In brief, the following primers and probes targeting the 16S rRNA gene DNA sequence of Fn and a reference gene (prostaglandin transporter, PGT), were used: Fn forward primer, 5′-CGGTGGAGCATGTGGTTTAA-3′; Fn reverse primer, 5′-TTCCTAAGATGTCAAACGCTGG-3′; Fn probe, 5′-FAM-TCGACGCAACGCGAGGAACCTT-BHQ1-3′; PGT forward primer, 5′-ATCCCCAAAGCACCTGGTTT-3′; PGT reverse primer, 5′-AGAGGCCAAGATAGTCCTGGTAA-3′; PGT probe, 5′-VIC-CCATCCATGTCCTCATCTC-MGBNFQ-3′. The PCR conditions were 95 °C for 10 min, followed by 45 cycles of 95 °C for 15 s and 59 °C for 1 min. To compare the Fn DNA amounts between MSI-high CRC samples, the relative values () calculated from the threshold cycle (Ct) values for Fn normalized to PGT were used. The qPCR method was validated using serially diluted standard genomic DNA samples of Fn (25586D-5; ATCC, Manassas, VA, USA). Among the samples of the initial 135 cases subjected to Fn qPCR analysis, nine cases were determined as failed or inadequate, based on non-evaluable or high Ct values of PGT. Thus, 126 cases were ultimately included in this study. The Fn-positive CRCs were further classified into two subgroups (Fn-high or Fn-low) using a cut-off median value of of all Fn-positive cases. The qPCR experiment of each sample was performed independently in triplicate, and the mean of from the triplicate results was used as the value for Fn DNA load of each case.
DNA methylation and KRAS/BRAF mutational analyses
DNA analyses for determination of CpG island methylator phenotype (CIMP) and KRAS/BRAF mutations were performed in tumor DNA samples of the 126 MSI-high CRCs as previously described [20].
Statistical analysis
Statistical analyses in this study were performed using SPSS ver. 23 (IBM Corp., Armonk, NY, USA) and GraphPad Prism ver. 8.3 (GraphPad Software, San Diego, CA, USA). Comparison analyses between parametric or non-parametric categorical variables were conducted using the Chi square test or Fisher’s exact test. Mean comparisons between parametric or non-parametric independent continuous variables were conducted using the Student’s t test or Mann–Whitney U test. Comparisons between non-parametric paired continuous variables were performed using the Wilcoxon matched-pairs signed-rank test. Survival analyses were carried out using the Kaplan–Meier method with the log-rank test. All p-values were two-sided and considered to be statistically significant if less than 0.05.
Results
Fn-associated clinicopathologic and molecular features in MSI-high CRCs
Among the 126 MSI-high CRCs, 114 were determined as Fn-positive cases by Fn qPCR. Using the median value of , the 114 Fn-positive MSI-high CRCs were classified into Fn-high and -low subgroups (each n = 57). First, we analyzed clinicopathologic and molecular associations of Fn subgroups in MSI-high CRCs. The results are summarized in Table 1. Among the 15 major clinicopathologic parameters, tumor size and depth of invasion were identified as factors significantly associated with Fn subgroups in MSI-high CRCs. In detail, Fn-high tumors were significantly correlated with larger tumor size than Fn-low/negative tumors (p = 0.017; Table 1). Compared with Fn-low/negative tumors, the Fn-high subgroup was also significantly associated with advanced invasion beyond the proper muscle layer (pT3 or pT4) (p = 0.034; Table 1). All the seven major molecular parameters, including MLH1/MSH2/MSH6/PMS2 expression, CIMP, and KRAS/BRAF mutations, showed no significant associations with Fn subgroups in MSI-high CRCs (Table 1). Survival analyses revealed that there was no prognostic significance of the Fn subgroups in MSI-high CRCs (Supplementary Fig. S2a, b).
Table 1.
Clinicopathologic and molecular features of MSI-high CRCs according to intratumoral Fn status
| Variable | Fn-high (n = 57) | Fn-low/negative (n = 69) | p-value |
|---|---|---|---|
| Age | |||
| Older (≥ 65 years) | 37 (65%) | 35 (51%) | 0.109 |
| Younger ( 65 years) | 20 (35%) | 34 (49%) | |
| Sex | |||
| Male | 24 (42%) | 40 (58%) | 0.076 |
| Female | 33 (58%) | 29 (42%) | |
| Tumor location | |||
| Proximal colon | 40 (70%) | 56 (81%) | 0.15 |
| Distal colon or rectum | 17 (30%) | 13 (19%) | |
| Gross tumor type | |||
| Polypoid or fungating | 34 (60%) | 40 (58%) | 0.849 |
| Ulceroinfiltrative | 23 (40%) | 29 (42%) | |
| Tumor size | |||
| Larger (≥ 6.3 cm) | 31 (54%) | 23 (33%) | 0.017 |
| Smaller (< 6.3 cm) | 26 (46%) | 46 (67%) | |
| AJCC/UICC cancer stage | |||
| Stage I/II | 43 (75%) | 47 (68%) | 0.365 |
| Stage III/IV | 14 (25%) | 22 (32%) | |
| Depth of invasion (pT) | |||
| Submucosa or proper muscle (pT1/pT2) | 4 (7%) | 14 (20%) | 0.034 |
| Beyond the proper muscle (pT3/pT4) | 53 (93%) | 55 (80%) | |
| Lymph node metastasis (pN) | |||
| Absent (pN0) | 43 (75%) | 50 (72%) | 0.705 |
| Present (pN1/pN2) | 14 (25%) | 19 (28%) | |
| Distant metastasis (pM or cM) | |||
| Absent (M0) | 53 (93%) | 63 (91%) | 1 |
| Present (M1) | 4 (7%) | 6 (9%) | |
| Early recurrencea | |||
| Absent | 52 (91%) | 65 (94%) | 0.73 |
| Present | 5 (9%) | 4 (6%) | |
| Lymphatic invasion | |||
| Absent | 41 (72%) | 47 (68%) | 0.642 |
| Present | 16 (28%) | 22 (32%) | |
| Venous invasion | |||
| Absent | 49 (86%) | 60 (87%) | 0.871 |
| Present | 8 (14%) | 9 (13%) | |
| Perineural invasion | |||
| Absent | 42 (74%) | 53 (77%) | 0.685 |
| Present | 15 (26%) | 16 (23%) | |
| Tumor grade (differentiation) | |||
| Low grade (well to moderately differentiated) | 37 (65%) | 45 (65%) | 0.971 |
| High grade (poorly differentiated) | 20 (35%) | 24 (35%) | |
| Mucinous histology | |||
| Non-mucinous (< 50%) | 41 (72%) | 52 (75%) | 0.663 |
| Mucinous (≥ 50%) | 16 (28%) | 17 (25%) | |
| Medullary histology | |||
| Non-medullary (< 50%) | 47 (82%) | 58 (84%) | 0.81 |
| Medullary (≥ 50%) | 10 (18%) | 11 (16%) | |
| Signet ring cell histology | |||
| Absent | 49 (86%) | 59 (86%) | 0.942 |
| Present (≥ 5%) | 8 (14%) | 10 (14%) | |
| MLH1 expression | |||
| Loss | 36 (63%) | 46 (67%) | 0.681 |
| Retained | 21 (37%) | 23 (33%) | |
| MSH2 expression | |||
| Loss | 9 (16%) | 9 (13%) | 0.661 |
| Retained | 48 (84%) | 60 (87%) | |
| MSH6 expression | |||
| Isolated loss | 9 (16%) | 7 (10%) | 0.344 |
| Retained or non-isolated loss | 48 (84%) | 62 (90%) | |
| PMS2 expression | |||
| Isolated loss | 5 (9%) | 6 (9%) | 1 |
| Retained or non-isolated loss | 52 (91%) | 63 (91%) | |
| CIMP | |||
| CIMP-high | 18 (32%) | 23 (33%) | 0.834 |
| CIMP-low/negative | 39 (68%) | 46 (67%) | |
| KRAS mutation | |||
| Absent | 32 (56%) | 50 (72%) | 0.056 |
| Present | 25 (44%) | 19 (28%) | |
| BRAF mutationb | |||
| Absent | 51 (91%) | 62 (91%) | 1 |
| Present | 5 (9%) | 6 (9%) | |
MSI-high microsatellite instability-high, CRCs colorectal cancers, Fn Fusobacterium nucleatum, AJCC/UICC American Joint Committee on Cancer/Union for International Cancer Control, CIMP CpG island methylator phenotype
aEarly recurrence was defined as clinically found and/or pathologically confirmed tumor recurrence within two years after curative surgery
bTwo cases were excluded in BRAF mutation analysis results due to suboptimal quality or quantity of their isolated DNA samples
Fn-associated immune microenvironmental features in MSI-high CRCs
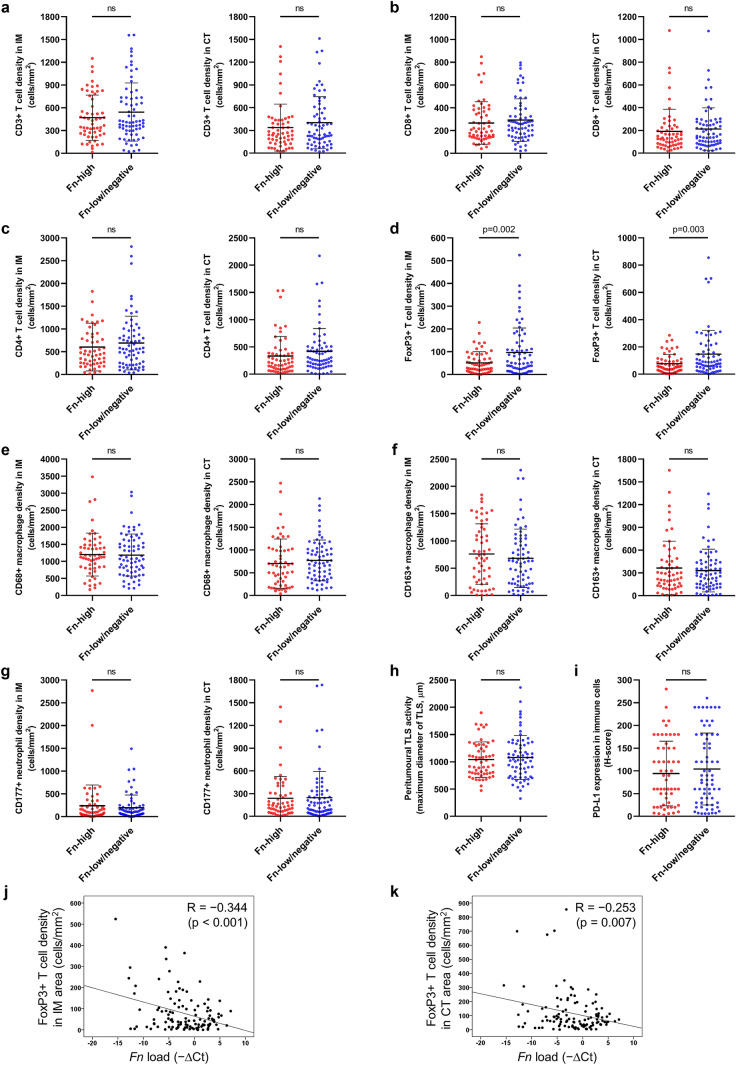
To comprehensively analyze the quantitative landscape of the tumor immune microenvironment of MSI-high CRCs, densities of tumor-infiltrating immune cells, including CD3+ T cells, CD8+ T cells, CD4+ T cells, FoxP3+ T cells, CD68+ macrophages, CD163+ macrophages, and CD177+ neutrophils, in the 126 MSI-high CRCs were quantified using a validated computational image analysis tool (QuPath). We obtained each immune cell density separately in the IM and CT areas and compared those values between Fn-high and Fn-low/negative subgroups. The results are presented in Fig. 1. Among the 14 parameters of seven major immune cell types (CD3+ cells in IM or CT, CD8+ cells in IM or CT, CD4+ cells in IM or CT, FoxP3+ cells in IM or CT, CD68+ cells in IM or CT, CD163+ cells in IM or CT, CD177+ cells in IM or CT; Fig. 1a–g), only the mean densities of FoxP3+ T cells in IM and CT areas displayed significant differences between Fn-high and Fn-low/negative subgroups. In detail, FoxP3+ T cell densities in both IM and CT areas of Fn-high tumors were significantly lower than those of Fn-low/negative tumors (p = 0.002 (IM) and p = 0.003 (CT), respectively; Fig. 1d). In addition to tumor-infiltrating immune cell densities, peritumoral TLS activity and PD-L1 expression status were also analyzed as major immune microenvironmental factors representing peritumoral antitumor immune reaction and immune checkpoint activity, respectively. However, there were no significant differences in those immune factors between Fn-high and Fn-low/negative tumors (Fig. 1h, i).
Fig. 1.
Comprehensive analysis of tumor-infiltrating immune cells and immune responses according to Fn status in MSI-high CRCs. a Comparisons of CD3+ T cell densities of IM (left) or CT (right) area between Fn-high and Fn-low/negative MSI-high CRCs. b Comparisons of CD8+ T cell densities of IM (left) or CT (right) area between Fn-high and Fn-low/negative MSI-high CRCs. c Comparisons of CD4+ T cell densities of IM (left) or CT (right) area between Fn-high and Fn-low/negative MSI-high CRCs. d Comparisons of FoxP3+ T cell densities of IM (left) or CT (right) area between Fn-high and Fn-low/negative MSI-high CRCs. e Comparisons of CD68+ macrophage densities of IM (left) or CT (right) area between Fn-high and Fn-low/negative MSI-high CRCs. f Comparisons of CD163+ macrophage densities of IM (left) or CT (right) area between Fn-high and Fn-low/negative MSI-high CRCs. g Comparisons of CD177+ neutrophil densities of IM (left) or CT (right) area between Fn-high and Fn-low/negative MSI-high CRCs. h A comparison of peritumoral TLS activity (maximum diameter of the largest TLS) between Fn-high and Fn-low/negative MSI-high CRCs. i A comparison of PD-L1 expression in immune cells (H-score of PD-L1 immunohistochemical staining in immune cells) between Fn-high and Fn-low/negative MSI-high CRCs. (j, k) Correlation scatter plots between Fn load and FoxP3+ T cell density of the IM (j) or CT (k) area in MSI-high CRCs
We further tested the potential inverse correlation between intratumoral Fn amount and FoxP3+ T cell density in MSI-high CRCs. As expected, both FoxP3+ T cell densities in IM and CT areas showed significant negative correlations with Fn DNA loads (r = − 0.344, p < 0.001 (IM) and r = − 0.253, p = 0.007 (CT), respectively; Fig. 1j, k). In contrast to the significant association between Fn and FoxP3+ T cell, there were no significant correlations between Fn loads and other immune cell densities, including CD3+, CD8+, CD4+, CD68+, CD163+, and CD177+ cell densities (Supplementary Fig. S3a–f).
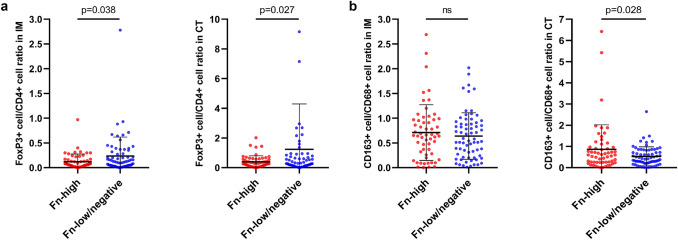
Next, since some immune cell types are subsets of other immune cell types, we investigated whether there are differences of relative densities of TIICs between Fn-high and Fn-low/negative tumors. For example, FoxP3+ cells are generally regarded as regulatory T (Treg) cells (CD3+CD4+FoxP3+ cells) and are a subset of CD3+ pan-T cells and of CD4+ helper T (Th) cells. Moreover, CD163+ cells represent M2-polarized macrophages and are a subset of CD68+ pan-macrophages. Therefore, we comprehensively analyzed immune cell ‘ratio’ parameters, including CD8+ cell density to CD3+ cell density ratio in IM and CT areas, CD4+ cell density to CD3+ cell density ratio in IM and CT areas, FoxP3+ cell density to CD3+ cell density ratio in IM and CT areas, FoxP3+ cell density to CD4+ cell density ratio in IM and CT areas, and CD163+ cell density to CD68+ cell density ratio in IM and CT areas. Among these factors, FoxP3+ cell density to CD4+ cell density ratio in IM and CT areas and CD163+ cell density to CD68+ cell density ratio in CT area demonstrated significant differences between Fn-high and Fn-low/negative tumors (Fig. 2a, b). In detail, Fn-high tumors were significantly associated with lower FoxP3+ cell to CD4+ cell ratio in both IM and CT areas compared with Fn-low/negative tumors (p = 0.038 (IM) and p = 0.027 (CT), respectively; Fig. 2a). Although there were tendencies towards low FoxP3+ cell to CD3+ cell ratios at both IM and CT areas in an Fn-high subgroup of MSI-high CRCs, these relationships were not statistically significant (Supplementary Fig. S4a, b). Interestingly, Fn-low/negative tumors showed significantly higher CD163+ cell to CD68+ cell ratio in only the CT area compared with Fn-low/negative tumors (p = 0.028; Fig. 2b), whereas there was no significant difference of CD163+ cell to CD68+ cell ratio in IM area between Fn-high and Fn-low/negative MSI-high CRCs (Fig. 2b).
Fig. 2.
Differential immune cell density ratios according to Fn status in MSI-high CRCs. a Comparisons of FoxP3+ T cell density to CD4+ T cell density ratios of the IM (left) or CT (right) area between Fn-high and Fn-low/negative MSI-high CRCs. b Comparisons of CD163+ macrophage density to CD68+ macrophage density ratios of the IM (left) or CT (right) area between Fn-high and Fn-low/negative MSI-high CRCs
Discussion
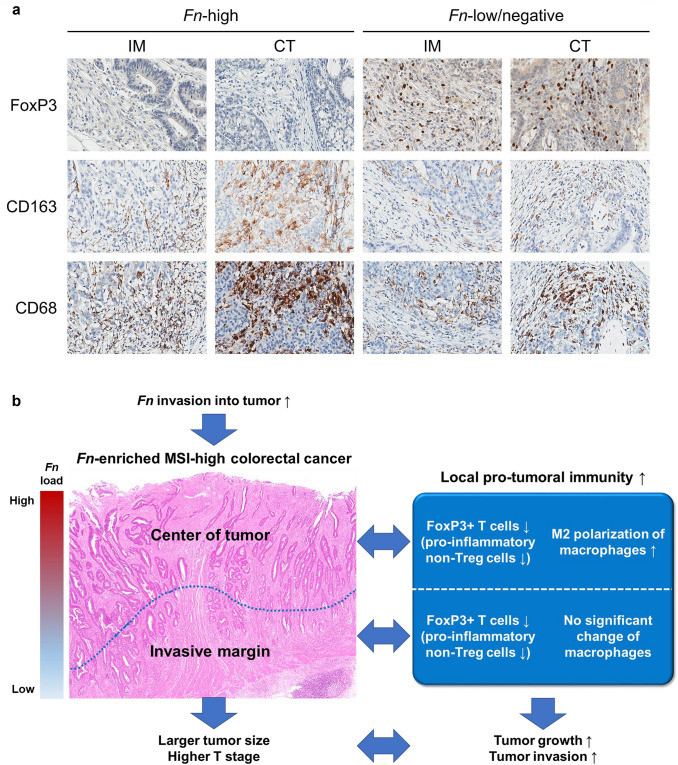
Previous investigations have suggested that tumor-invasive Fn can affect various biologic and clinical aspects of CRC, including tumorigenesis, host immune responses, patient prognosis, and treatment responses [4, 5, 21–23]. Focusing on the relationship between Fn and tumor immunity, several experimental and clinical results indicated that Fn might promote or disrupt specific immune cell infiltration in CRCs [5–8]. However, a recent study by Hamada et al. reported that features of Fn-associated immune responses were contrasting between MSI subtypes of CRCs [9]. In addition, we previously found that Fn was associated with differential prognostic impacts according to MSI status in CRCs treated with adjuvant chemotherapy [17]. These findings proposed that MSI status may be a critical confounding factor for understanding authentic effects of Fn on immune responses, prognosis or treatment responses in CRC. Therefore, we decided to investigate the association between intratumoral Fn load and immune microenvironmental features in only an MSI-high subtype of CRCs. In this study, we especially focused on the potential differential associations between Fn load and immune responses depending on different intratumoral regions. Thus, we analyzed densities of seven major TIICs separately in IM and CT regions of each MSI-high CRC case. Through this intensive analysis, our study successfully identified the negative correlation between Fn load and FoxP3+ T cell density in the whole tumor area of MSI-high CRCs. The main results of our study are graphically summarized in Fig. 3.
Fig. 3.
Characterization of the Fn-associated immune microenvironment in MSI-high CRC. a Representative photomicrographs of FoxP3, CD163, and CD68 IHC according to combined Fn (Fn-high and Fn-low/negative) and region (IM and CT) status. In contrast to Fn-low/negative tumors, there is scanty infiltration of FoxP3+ cells in both IM and CT areas of Fn-high tumors. Note the feature that most CD68+ cells are nearly overlap with CD163+ cells in the same CT area of an Fn-high tumor, indicating the high proportion of M2 macrophages among tumor-infiltrating macrophages. b A graphical summary of this study
In the clinicopathologic aspect, Fn-high status was significantly associated with large tumor size and deep invasion level of the tumor (Table 1). These findings are moderately consistent with previous results. According to the data by Yamaoka et al., the mean tumor size of Fn-high CRCs was significantly larger than that of Fn-low CRCs [24]. Several previous studies have also reported tendencies toward advanced invasion depth of tumors (pT3/pT4) in Fn-high CRCs compared with Fn-low CRCs, with or without statistical significance [17, 21, 24]. Interestingly, among the TNM staging categories, although high pT category (invasion depth) was determined to be related to Fn-high status in MSI-high CRCs, the pN (nodal metastasis) or p/cM (distant metastasis) categories demonstrated no significant association with Fn subgroups in MSI-high CRCs (Table 1). These findings indicate that intratumoral Fn abundance may be directly or indirectly promoting local tumor growth and invasion but may not have much effect on the metastatic potential of primary MSI-high CRCs. Other pathologic factors supporting this interpretation, including lymphatic invasion, venous invasion, and perineural invasion, which are well-known risk factors for nodal or distant metastasis, were not related to Fn status in MSI-high CRCs (Table 1).
The most notable finding of this study was the significant correlation between intratumoral Fn abundance and low density of tumor-infiltrating FoxP3+ T cells in MSI-high CRCs (Fig. 1d, j, k). To construe clinicopathologic impacts of decreased FoxP3+ T cells in Fn-high MSI-high CRCs, implications of FoxP3+ T cells in the immune context of MSI-high CRCs should be understood. FoxP3+ T cells have been generally regarded as Treg cells, which have a major role in immune regulation by immune suppression [25]. Therefore, it has been conceptually expected that tumor-infiltrating FoxP3+ T cells may suppress antitumor immune responses and may consequently be linked to tumor progression and aggressiveness. Many previous studies have reported that high density of tumor-infiltrating FoxP3+ T cells is significantly associated with decreased cytotoxic TILs and poor prognosis in a variety of human tumors such as breast cancer, lung cancer, renal cell cancer, ovarian cancer, and cervical cancer [26]. However, in contrast to other malignancies, CRC is one of the unusual cancer types frequently showing the correlation between increased FoxP3+ T cells and favorable prognosis [26–28]. Interestingly, recent investigations have suggested that not all of the FoxP3+ T cells are functionally immune-suppressive Treg cells [29, 30]. In detail, CD4+FoxP3+ T cells can be categorized into three subpopulations, including Fr-I naive Treg cells (FoxP3lowCD45RA+), Fr-II immune-suppressive effector Treg cells (FoxP3highCD45RA−), and Fr-III pro-inflammatory non-Treg cells (FoxP3lowCD45RA−) [30]. According to the study by Saito et al., subtype “B” CRCs, in which Fr-III pro-inflammatory non-Treg cells were dominant among tumor-infiltrating FoxP3+ cells, showed significantly upregulated immune-related genes and better prognosis, indicating that type B CRCs may considerably overlap with MSI-high CRCs [30]. In addition, type B CRCs demonstrated an intratumoral abundance of intestinal microbiota such as Fn [30], consistent with previous findings regarding the significant correlation between Fn-high and MSI-high statuses in overall CRCs [21]. To elucidate the implications of FoxP3+ T cells in MSI-high CRCs, we performed additional analyses. As a result, CD3+ pan-T cell density, CD8+ cytotoxic T cell density, and peritumoral TLS activity, which are representative indicators of antitumor lymphocytic responses, were significantly higher in MSI-high CRCs with a high density of FoxP3+ T cells (FoxP3-high subgroup) than those with a low density of FoxP3+ T cells (FoxP3-low subgroup) (Supplementary Fig. S5a–c). There was also a significant positive correlation between CD3+ T cell density and FoxP3+ T cell density in MSI-high CRCs (Supplementary Fig. S5d). Clinicopathologically, FoxP3-low MSI-high CRCs were significantly associated with larger tumor size, higher cancer stage, deeper tumor invasion, and presence of distant metastasis compared with FoxP3-high MSI-high CRCs (Supplementary Table S1). There was also a tendency toward a favorable prognosis of the FoxP3-high subgroup in MSI-high CRCs (Supplementary Fig. S6). Although statistical significances of differences in DFS and OS between FoxP3-high and FoxP3-low MSI-high CRCs were not observed, tumor recurrence or patient death was found only in the FoxP3-low subgroup in MSI-high CRCs (Supplementary Fig. S6a, b). These data collectively support that FoxP3+ T cells in MSI-high CRCs may have roles in facilitating antitumor immune responses and suppressing tumor progression. Taken together, we inferred that the dominant type of tumor-infiltrating FoxP3+ T cells in MSI-high CRCs may be pro-inflammatory non-Treg cells. Therefore, the low density of tumor-infiltrating FoxP3+ T cells in an Fn-high subset of MSI-high CRCs indicates the decrease of pro-inflammatory non-Treg FoxP3+ cells and subsequent alteration of tumor immune microenvironment toward pro-tumoral immunity (Fig. 3a, b).
To further elucidate whether relative compositions of tumor-infiltrating immune cells significantly correlate with intratumoral Fn abundance in MSI-high CRCs, we additionally analyzed associations between Fn status and various immune cell density ratios. As a result, low FoxP3+ cell to CD4+ cell ratios at both IM and CT areas and high CD163+ cell to CD68+ cell ratio at CT area were significantly associated with the Fn-high subgroup in MSI-high CRCs (Fig. 2a, b). The decreased FoxP3+ T cell composition among CD4+ T cells can be interpreted as a relative increase of CD4+FoxP3− cell subsets, including other Th cells. Previous studies have suggested that the balance between Treg cells and IL17-producing Th (Th17) cells may be important for maintaining immune defence mechanism against microbiota in gut mucosa [31]. In addition, FoxP3+ T cells have been known to be able to suppress Th17 cells that can act as a pro-tumoral immune cells, and a study suggested that a low ratio of Treg cells to Th17 cells was associated with poor prognosis in CRCs [32, 33]. Therefore, the decreased FoxP3+ cell to CD4+ cell ratio observed in the Fn-high subset of our MSI-high CRC cohort may reflect disrupted homeostasis between FoxP3+ T cells and Th17 cells, and this feature may increase the chance of bacterial invasion into tumor cells and may also promote pro-tumoral immune responses.
Another interesting finding in the immune cell ratio analyses was the significant association between Fn-high status and a high ratio of CD163+ cells to CD68+ cells at CT area in MSI-high CRCs (Fig. 2b). In fact, relationships between Fn and macrophages in CRCs have been suggested. In detail, several recent studies have reported that Fn can recruit macrophages and can promote M2 polarization of macrophages in CRC [7, 18]. Consistent with these findings, the high ratio of CD163+ cells to CD68+ cells in Fn-high MSI-high CRCs found in our present study may reflect the increased polarization of CD68+ pan-macrophages into a CD163+ M2 subset, indicating the augmentation of pro-tumoral immunity (Fig. 3a, b). Interestingly, our finding was significant only in the CT area, but not in the IM area. This phenomenon may be based on the regional heterogeneity of intratumoral Fn load in CRCs. According to a study by Bullman et al., intratumoral Fn was more enriched in superficial areas than in deeper portions of CRCs [34]. In addition, we separately analyzed Fn DNA loads of paired CT and IM areas of representative 18 cases (10 Fn-high and 8 Fn-low) and found that Fn loads of IM areas were significantly lower than those of matched CT areas (Supplementary Fig. S7a, b). Thus, the local effect of Fn on M2 polarization is possibly tenuous in the deep IM region of CRCs (Fig. 3b).
In addition to the alteration of innate or adaptive immune cell responses, including decreased infiltration of FoxP3+ T cells and increased polarization of M2 macrophages, recent investigations have suggested that the chemokine network may also play an important role in inducing pro-tumoral immunity in Fn-enriched tumors [35]. Yamamura et al. previously investigated Fn-associated expression pathways in esophageal cancer tissues using Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis, and found that the “cytokine–cytokine receptor interaction” was the most significantly activated pathway in Fn-positive esophageal cancers [36]. Specifically, CCL20 was identified as a key chemokine associated with intratumoral Fn abundance in esophageal cancers [36]. CCL20 and its receptor, CCR6, regulate intratumoral recruitment of CCR6+ Th17 and Treg cells, and promote tumor growth and aggressiveness [35]. Therefore, it can be inferred that pro-tumoral immune microenvironmental features in Fn-enriched MSI-high CRCs could be mediated by the activated CCL20/CCR6 chemokine axis. Further studies are warranted to confirm this.
Bullman et al. found that when patient-derived xenograft tumors with Fn-positivity were treated with metronidazole, a lethal antibiotic against Fusobacteria, there were significant decreases in Fn load, tumor growth, and tumor cell proliferation [34]. This finding suggests that intratumoral Fn removal could potentially be a treatment strategy in Fn-high CRCs. However, the underlying mechanism of tumor growth suppression by intratumoral Fn load reduction remains unclear. Based on the previously reported tumorigenesis-promoting or immune-modulating effects of Fn [4, 5, 7], both tumor-intrinsic and immune-mediated mechanisms are possible. Importantly, accumulating data, including our present study, have suggested that high Fn loads in tumor tissues are significantly associated with pro-tumoral immune responses. Thus, reducing intratumoral Fn may result in weakening local pro-tumoral immunity and subsequently dampening tumor development in Fn-high MSI-high CRCs. Whether Fn load reduction induces an increase in FoxP3+ pro-inflammatory non-Treg cells and a decrease in M2 polarization in MSI-high tumors remains to be elucidated. If this hypothesis is confirmed, the Fn-immunity axis in the tumor microenvironment will be one of the potential immunotherapeutic targets in MSI-high CRCs.
According to the recent data by Gurjao et al., immune cell composition analysis from RNA sequencing data of an MSI-high CRC case showing intrinsic resistance to immune checkpoint inhibitor (ICI) demonstrated characteristic immune cell infiltration patterns, including high M2 macrophages and activated NK cells and low Treg cells [37]. These findings indicate that Fn-enriched MSI-high CRCs, which are characterized by low FoxP3+ cells and a high proportion of M2 macrophages, may be potentially associated with poor response to ICI-based immunotherapy. Thus, the association of intratumoral Fn load with immunotherapy response in patients with MSI-high CRCs should be further evaluated in the future clinical studies. Moreover, investigations regarding modulation of gut microbiota including Fn for improving response to immunotherapy in CRCs are also necessary.
In summary, our study performed comprehensive analyses of relationships between Fn load and immune cell infiltrates in a large series of MSI-high CRCs. We successfully unveiled that intratumoral Fn abundance is significantly correlated with decreased FoxP3+ T cells throughout tumor area and increased proportion of M2 macrophages at CT area in MSI-high CRCs (Fig. 3a, b). Based on the accumulating evidence, the majority of FoxP3+ T cells may be pro-inflammatory non-Treg cells in MSI-high CRCs. Thus, decreased pro-inflammatory non-Treg cells and increased polarization of macrophages into immune-suppressive M2 phenotype collectively indicate that pro-tumoral immune microenvironment is promoted in Fn-enriched MSI-high CRCs (Fig. 3b). Ultimately, this Fn-associated immune microenvironment is likely to contribute to local tumor growth and invasion in MSI-high CRCs, which were represented by a significantly large tumor size and advanced pT stage in the Fn-high subset of MSI-high CRCs.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by the National Research Foundation of Korea funded by the Korea government (Ministry of Science and ICT) under Grants NRF-2016R1C1B2010627 and NRF-2019R1F1A1059535. The biospecimens for this study were partly provided by the Seoul National University Hospital Cancer Tissue Bank (SNUH CTB). All samples derived from the SNUH CTB were obtained with informed consent under institutional review board-approved protocols. We also thank those who have developed and shared QuPath, an open-source software for digital pathology analysis.
Abbreviations
- CRC
Colorectal cancer
- CIMP
CpG island methylator phenotype
- CT
Center of tumor
- DFS
Disease-free survival
- FFPE
Formalin-fixed, paraffin-embedded
- Fn
Fusobacterium nucleatum
- ICI
Immune checkpoint inhibitor
- IHC
Immunohistochemistry
- IM
Invasive margin
- MSI
Microsatellite instability
- OS
Overall survival
- qPCR
Quantitative polymerase chain reaction
- Th
Helper T cell
- TIIC
Tumor-infiltrating immune cell
- TIL
Tumor-infiltrating lymphocyte
- TLS
Tertiary lymphoid structure
- TMA
Tissue microarray
- Treg
Regulatory T cell
Author contributions
Conceptualization: JHK; Formal analysis: JAL, SYY, SJ, NYC, JHK; Funding acquisition: JHK; Investigation: JAL, SYY, JHK; Methodology: JAL, SYY, HJO, SJ, NYC, JHK; Project administration: GHK, JHK; Resources: JAL, HJO; Supervision: GHK, JHK; Validation: JAL, SYY, JHK; Visualization: JAL, SYY, JHK; Writing—original draft: JAL, SYY, JHK; Writing—review & editing: GHK, JHK.
Data and/or code availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
This study was approved by the institutional review board of Seoul National University Hospital (IRB No. 1805-018-944). This study was performed in accordance with the Declaration of Helsinki.
Informed consent
All patients were provided written consent for the research or molecular diagnostic use of residual tumor tissues obtained during their surgical treatment.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ji Ae Lee and Seung-Yeon Yoo have contributed equally to this work.
References
- 1.Wieczorska K, Stolarek M, Stec R. The role of the gut microbiome in colorectal cancer: where are we? Where are we going? Clin Colorectal Cancer. 2019 doi: 10.1016/j.clcc.2019.07.006. [DOI] [PubMed] [Google Scholar]
- 2.Louis P, Hold GL, Flint HJ. The gut microbiota, bacterial metabolites and colorectal cancer. Nat Rev Microbiol. 2014;12:661–672. doi: 10.1038/nrmicro3344. [DOI] [PubMed] [Google Scholar]
- 3.Brennan CA, Garrett WS. Fusobacterium nucleatum—symbiont, opportunist and oncobacterium. Nat Rev Microbiol. 2019;17:156–166. doi: 10.1038/s41579-018-0129-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rubinstein MR, Wang X, Liu W, Hao Y, Cai G, Han YW. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/beta-catenin signaling via its FadA adhesin. Cell Host Microbe. 2013;14:195–206. doi: 10.1016/j.chom.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kostic AD, Chun E, Robertson L, Glickman JN, Gallini CA, Michaud M, et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe. 2013;14:207–215. doi: 10.1016/j.chom.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nosho K, Sukawa Y, Adachi Y, Ito M, Mitsuhashi K, Kurihara H, et al. Association of Fusobacterium nucleatum with immunity and molecular alterations in colorectal cancer. World J Gastroenterol. 2016;22:557–566. doi: 10.3748/wjg.v22.i2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen T, Li Q, Wu J, Wu Y, Peng W, Li H, et al. Fusobacterium nucleatum promotes M2 polarization of macrophages in the microenvironment of colorectal tumours via a TLR4-dependent mechanism. Cancer Immunol Immunother. 2018;67:1635–1646. doi: 10.1007/s00262-018-2233-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mima K, Sukawa Y, Nishihara R, Qian ZR, Yamauchi M, Inamura K, et al. Fusobacterium nucleatum and T cells in colorectal carcinoma. JAMA Oncol. 2015;1:653–661. doi: 10.1001/jamaoncol.2015.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamada T, Zhang X, Mima K, Bullman S, Sukawa Y, Nowak JA, et al. Fusobacterium nucleatum in colorectal cancer relates to immune response differentially by tumor microsatellite instability status. Cancer Immunol Res. 2018;6:1327–1336. doi: 10.1158/2326-6066.CIR-18-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boland CR, Thibodeau SN, Hamilton SR, Sidransky D, Eshleman JR, Burt RW, et al. A National Cancer Institute Workshop on microsatellite instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998;58:5248–5257. [PubMed] [Google Scholar]
- 11.Ueno H, Hashiguchi Y, Shimazaki H, Shinto E, Kajiwara Y, Nakanishi K, et al. Objective criteria for crohn-like lymphoid reaction in colorectal cancer. Am J Clin Pathol. 2013;139:434–441. doi: 10.1309/AJCPWHUEFTGBWKE4. [DOI] [PubMed] [Google Scholar]
- 12.Kim JH, Kim KJ, Bae JM, Rhee YY, Cho NY, Lee HS, et al. Comparative validation of assessment criteria for Crohn-like lymphoid reaction in colorectal carcinoma. J Clin Pathol. 2015;68:22–28. doi: 10.1136/jclinpath-2014-202603. [DOI] [PubMed] [Google Scholar]
- 13.Pages F, Mlecnik B, Marliot F, Bindea G, Ou S, Bifulco C, et al. International validation of the consensus immunoscore for the classification of colon cancer: a prognostic and accuracy study. Lancet. 2018;391:2128–2139. doi: 10.1016/S0140-6736(18)30789-X. [DOI] [PubMed] [Google Scholar]
- 14.Bankhead P, Loughrey MB, Fernandez JA, Dombrowski Y, McArt DG, Dunne PD, et al. QuPath: open source software for digital pathology image analysis. Sci Rep. 2017;7:16878. doi: 10.1038/s41598-017-17204-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loughrey MB, Bankhead P, Coleman HG, Hagan RS, Craig S, McCorry AMB, et al. Validation of the systematic scoring of immunohistochemically stained tumour tissue microarrays using QuPath digital image analysis. Histopathology. 2018;73:327–338. doi: 10.1111/his.13516. [DOI] [PubMed] [Google Scholar]
- 16.Hendry S, Salgado R, Gevaert T, Russell PA, John T, Thapa B, et al. Assessing tumor-infiltrating lymphocytes in solid tumors: a practical review for pathologists and proposal for a standardized method from the International Immuno-Oncology Biomarkers Working Group: Part 2: TILs in melanoma, gastrointestinal tract carcinomas, non-small cell lung carcinoma and mesothelioma, endometrial and ovarian carcinomas, squamous cell carcinoma of the head and neck, genitourinary carcinomas, and primary brain tumors. Adv Anat Pathol. 2017;24:311–335. doi: 10.1097/PAP.0000000000000161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oh HJ, Kim JH, Bae JM, Kim HJ, Cho NY, Kang GH. Prognostic impact of Fusobacterium nucleatum depends on combined tumor location and microsatellite instability status in stage II/III colorectal cancers treated with adjuvant chemotherapy. J Pathol Transl Med. 2019;53:40–49. doi: 10.4132/jptm.2018.11.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park HE, Kim JH, Cho NY, Lee HS, Kang GH. Intratumoral Fusobacterium nucleatum abundance correlates with macrophage infiltration and CDKN2A methylation in microsatellite-unstable colorectal carcinoma. Virchows Arch. 2017;471:329–336. doi: 10.1007/s00428-017-2171-6. [DOI] [PubMed] [Google Scholar]
- 19.Leishman SJ, Ford PJ, Do HL, Palmer JE, Heng NC, West MJ, et al. Periodontal pathogen load and increased antibody response to heat shock protein 60 in patients with cardiovascular disease. J Clin Periodontol. 2012;39:923–930. doi: 10.1111/j.1600-051X.2012.01934.x. [DOI] [PubMed] [Google Scholar]
- 20.Bae JM, Kim JH, Oh HJ, Park HE, Lee TH, Cho NY, et al. Downregulation of acetyl-CoA synthetase 2 is a metabolic hallmark of tumor progression and aggressiveness in colorectal carcinoma. Mod Pathol. 2017;30:267–277. doi: 10.1038/modpathol.2016.172. [DOI] [PubMed] [Google Scholar]
- 21.Mima K, Nishihara R, Qian ZR, Cao Y, Sukawa Y, Nowak JA, et al. Fusobacterium nucleatum in colorectal carcinoma tissue and patient prognosis. Gut. 2016;65:1973–1980. doi: 10.1136/gutjnl-2015-310101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu T, Guo F, Yu Y, Sun T, Ma D, Han J, et al. Fusobacterium nucleatum promotes chemoresistance to colorectal cancer by modulating autophagy. Cell. 2017;170(548–563):e516. doi: 10.1016/j.cell.2017.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geller LT, Barzily-Rokni M, Danino T, Jonas OH, Shental N, Nejman D, et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science. 2017;357:1156–1160. doi: 10.1126/science.aah5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamaoka Y, Suehiro Y, Hashimoto S, Hoshida T, Fujimoto M, Watanabe M, et al. Fusobacterium nucleatum as a prognostic marker of colorectal cancer in a Japanese population. J Gastroenterol. 2018;53:517–524. doi: 10.1007/s00535-017-1382-6. [DOI] [PubMed] [Google Scholar]
- 25.Takeuchi Y, Nishikawa H. Roles of regulatory T cells in cancer immunity. Int Immunol. 2016;28:401–409. doi: 10.1093/intimm/dxw025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ladoire S, Martin F, Ghiringhelli F. Prognostic role of FOXP3 + regulatory T cells infiltrating human carcinomas: the paradox of colorectal cancer. Cancer Immunol Immunother. 2011;60:909–918. doi: 10.1007/s00262-011-1046-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu P, Fan W, Zhang Z, Wang J, Wang P, Li Y, et al. The clinicopathological and prognostic implications of FoxP3(+) regulatory T cells in patients with colorectal cancer: a meta-analysis. Front Physiol. 2017;8:950. doi: 10.3389/fphys.2017.00950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shang B, Liu Y, Jiang SJ, Liu Y. Prognostic value of tumor-infiltrating FoxP3 + regulatory T cells in cancers: a systematic review and meta-analysis. Sci Rep. 2015;5:15179. doi: 10.1038/srep15179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhuo C, Xu Y, Ying M, Li Q, Huang L, Li D, et al. FOXP3 + Tregs: heterogeneous phenotypes and conflicting impacts on survival outcomes in patients with colorectal cancer. Immunol Res. 2015;61:338–347. doi: 10.1007/s12026-014-8616-y. [DOI] [PubMed] [Google Scholar]
- 30.Saito T, Nishikawa H, Wada H, Nagano Y, Sugiyama D, Atarashi K, et al. Two FOXP3(+)CD4(+) T cell subpopulations distinctly control the prognosis of colorectal cancers. Nat Med. 2016;22:679–684. doi: 10.1038/nm.4086. [DOI] [PubMed] [Google Scholar]
- 31.Omenetti S, Pizarro TT. The Treg/Th17 axis: a dynamic balance regulated by the gut microbiome. Front Immunol. 2015;6:639. doi: 10.3389/fimmu.2015.00639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ward-Hartstonge KA, Kemp RA. Regulatory T-cell heterogeneity and the cancer immune response. Clin Transl Immunol. 2017;6:e154. doi: 10.1038/cti.2017.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Q, Feng M, Yu T, Liu X, Zhang P. Intratumoral regulatory T cells are associated with suppression of colorectal carcinoma metastasis after resection through overcoming IL-17 producing T cells. Cell Immunol. 2014;287:100–105. doi: 10.1016/j.cellimm.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 34.Bullman S, Pedamallu CS, Sicinska E, Clancy TE, Zhang X, Cai D, et al. Analysis of Fusobacterium persistence and antibiotic response in colorectal cancer. Science. 2017;358:1443–1448. doi: 10.1126/science.aal5240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Borroni EM, Qehajaj D, Farina FM, Yiu D, Bresalier RS, Chiriva-Internati M, et al. Fusobacterium nucleatum and the immune system in colorectal cancer. Curr Colorectal Canc R. 2019;15:149–156. doi: 10.1007/s11888-019-00442-2. [DOI] [Google Scholar]
- 36.Yamamura K, Baba Y, Nakagawa S, Mima K, Miyake K, Nakamura K, Sawayama H, Kinoshita K, Ishimoto T, Iwatsuki M, Sakamoto Y, Yamashita Y, Yoshida N, Watanabe M, Baba H. Human microbiome Fusobacterium nucleatum in esophageal cancer tissue is associated with prognosis. Clin Cancer Res. 2016;22:5574–5581. doi: 10.1158/1078-0432.Ccr-16-1786. [DOI] [PubMed] [Google Scholar]
- 37.Gurjao C, Liu D, Hofree M, AlDubayan SH, Wakiro I, Su MJ, Felt K, Gjini E, Brais LK, Rotem A, Rosenthal MH, Rozenblatt-Rosen O, Rodig S, Ng K, Van Allen EM, Corsello SM, Ogino S, Regev A, Nowak JA, Giannakis M. Intrinsic resistance to immune checkpoint blockade in a mismatch repair-deficient colorectal cancer. Cancer Immunol Res. 2019;7:1230–1236. doi: 10.1158/2326-6066.CIR-18-0683. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its supplementary information files.