Abstract
Background
Patients with small-cell lung cancer (SCLC) have a dismal prognosis with limited overall survival (OS) despite a high response rate to chemotherapy. Recently, immune checkpoint inhibitors, combined with chemotherapy, as the first-line treatment for extensive-stage (ES)-SCLC have shown improvement in clinical outcomes.
Patients and methods
Real-world data from 68 Korean ES-SCLC patients, treated with atezolizumab, etoposide, and carboplatin at Yonsei Cancer Center between June 2019 and November 2020, were retrospectively analyzed to determine safety and efficacy using Cox regression analysis.
Results
The median follow-up was 11.6 months. The median progression-free survival was 4.6 months (95% confidence interval [CI] 4.0–5.2), and the median OS was 12.0 months (95% CI 7.4–16.6). Baseline bone metastasis, immune-related adverse events (IRAEs), and elevated LDH were related to OS (hazard ratio 2.18, 0.33, and 4.64; P = 0.05, 0.02, and 0.003, respectively). Among the 42 patients with disease progression, liver metastasis progression and baseline bone metastasis were associated with inferior OS, but without statistical significance (hazard ratio 2.47 and 1.97; P = 0.25 and 0.26, respectively). Overall, 61 (89.7%) patients experienced treatment-related adverse events (TRAEs), with hematologic toxicities as the most common grade 3–4 TRAEs. Twenty-two (32.4%) patients experienced IRAEs, with skin rash as the most common, and five (7.4%) patients had grade-3 IRAEs (pneumonitis, hyperglycemia, and aspartate aminotransferase elevation).
Conclusion
Atezolizumab, combined with etoposide and carboplatin, showed efficacy and safety in our real-world data. Further studies are needed to predict the response to immunotherapy in SCLC.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-021-03052-w.
Keywords: Real-world data, Asian, Korea, Immunotherapy, IMpower133 Study
Introduction
Lung cancer is one of the most frequently diagnosed malignancies and a leading cause of death worldwide [1]. Small-cell lung cancer (SCLC) is a unique disease entity, which accounts for approximately 14% of all lung cancer cases [2]. SCLC typically responds well to cytotoxic chemotherapy initially, but rapidly progresses thereafter, resulting in a poor prognosis at advanced stages. Most patients with SCLC experience a recurrence or death within 2 years after the initial diagnosis. In contrast to non-small cell lung cancer (NSCLC), which is well-known for its various oncogenic mutations and targeted therapies, there are no approved targeted agents for the treatment of SCLC. In recent decades, cytotoxic chemotherapy and radiotherapy have been the mainstream of SCLC treatment. Before the era of immune checkpoint inhibitors (ICIs), the objective response rate (ORR) of first-line treatment in extensive-stage SCLC (ES-SCLC) was 44–78%, median progression-free survival (mPFS) was 4.3–5.7 months, and median overall survival (mOS) was 7.5–10.9 months, with a 5-year survival rate of only 2.8% [3, 4].
The programmed death protein 1 (PD-1) inhibitors nivolumab and pembrolizumab showed a durable response in certain subgroups in the Checkmate-032, Keynote-028, and Keynote-158 trials, leading to their clinical use in the treatment of refractory ES-SCLC [5–7]. Global, multi-center, phase III clinical trials that combined ICIs with standard cytotoxic chemotherapy in the first-line treatment of ES-SCLC have also been conducted. In the IMpower133 study (NCT02763579), the programmed death-ligand 1 (PD-L1) inhibitor atezolizumab was compared with placebo in the treatment of 403 patients with ES-SCLC in a first-line setting, which was combined with standard etoposide and carboplatin chemotherapy. The mPFS and mOS significantly improved in the atezolizumab group compared with that in the placebo group (5.2 months versus 4.3 months and 12.3 months versus 10.3 months, respectively) [8]. The frequency and grade of treatment-related adverse events (TRAEs) were not significantly different between the two groups, although the health-related quality of life improvement was superior in the atezolizumab arm [9]. Based on these results, the US Food and Drug Administration approved atezolizumab, in combination with etoposide and carboplatin, for first-line treatment of ES-SCLC in 2019. In the CASPIAN study (NCT03043872), a randomized phase III trial of first-line treatment of ES-SCLC, another PD-L1 inhibitor, durvalumab, with or without tremelimumab, a cytotoxic T-lymphocyte antigen 4 inhibitor, was combined with standard chemotherapy. Durvalumab improved the mOS from 10.3 months to 13.0 months compared to the placebo despite no significant benefit in mPFS. However, the addition of tremelimumab did not significantly improve outcomes versus standard chemotherapy [10, 11]. In the Keynote-604 study (NCT03066778), pembrolizumab or placebo was added to the standard chemotherapy of ES-SCLC. Compared with placebo, pembrolizumab showed improvement in both mPFS and mOS, although mOS prolongation was not statistically significant [12].
Despite the promising findings of these clinical trials, real-world data are needed to supplement these results, since trials recruit only well-defined, selected patients and cannot reflect the complete heterogeneity of patients and the disease. Indeed, Asian patients accounted for only 17%, 14%, and 22.8% of all patients in the IMpower133, CASPIAN, and Keynote-604 studies, respectively. Therefore, it is meaningful to confirm whether the benefit of adding an ICI to a standard chemotherapy regimen can be generalized for patients with ES-SCLC.
Toward this end, we here share our experience at a single Korean institution, wherein we retrospectively evaluated the efficacy and safety of atezolizumab, etoposide, and carboplatin in the first-line treatment of ES-SCLC patients.
Materials and methods
Patients and data collection
This retrospective study included patients who were treated at Yonsei Cancer Center, Severance Hospital (Seoul, Korea) between June 2019 and November 2020. Eligible patients were those who had been diagnosed with ES-SCLC according to the definition of the Veterans Administration Lung Cancer Study Group or had recurrent disease in patients with limited-stage SCLC after definitive concurrent chemoradiation therapy (CCRT) without prior systemic treatment. Patients who had a history of autoimmune disease were excluded. The demographic and clinical characteristics of patients, such as age, sex, smoking history, performance status, baseline brain metastasis, previous treatment, and PD-L1 status, were collected through a review of electronic medical records. PD-L1 expression was assessed using the SP263 immunohistochemical assay (Ventana Medical Systems, Inc., Oro Valley, AZ, USA). The personal information of the patients was anonymized. The study was approved by the institutional review board at Severance Hospital (IRB number 4–2020-1151).
Treatment and response evaluation
Each patient was treated with four to eight cycles of etoposide (100 mg/m2 body surface area, administered intravenously on days 1 through 3 of each cycle), carboplatin (area under the curve of 5 mg mL−1 min–1, administered intravenously on day 1 of each cycle), and atezolizumab (1200 mg, administered intravenously on day 1 of each cycle), followed by atezolizumab maintenance every 3 weeks. Treatment was continued until disease progression, death, or unacceptable toxicity. The response was evaluated according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 [13]. Treating physicians evaluated the tumor response and they were unblinded to the clinical data. Computed tomography imaging was performed every 2 or 3 cycles initially and then every 4 cycles during atezolizumab maintenance. Treatment beyond disease progression was accepted if there was a clinical benefit. Prophylactic cranial irradiation was allowed during atezolizumab maintenance.
Endpoints
The primary endpoints were PFS and OS. PFS was defined as the time from treatment initiation to radiologically confirmed disease progression or death. OS was defined as the time from treatment initiation to death. Secondary endpoints were ORR and safety. ORR was defined as the proportion of patients with partial response (PR) or complete response (CR) according to RECIST version 1.1 [13]. The duration of response was defined as the length of time from the first PR or CR to disease progression. Safety was evaluated with Common Terminology Criteria for Adverse Events 4.0 [14]. The adverse events, their causal relationship with each drug, and the determination of immune-related adverse events (IRAEs) were based on the medical records by treating physicians.
Statistical analysis
For the analysis of PFS and OS, data for patients who had no disease progression or remained alive were censored on the day of their last outpatient clinic visit. PFS and OS were analyzed with the Kaplan–Meier method and are presented as the median value with the two-sided 95% confidence interval (CI). The hazard ratios (HRs) for PFS and OS were estimated with multivariable Cox proportional hazard regression analysis. For the analysis of poor prognostic factors, subgroup analysis of OS was performed for patients who experienced disease progression after first-line treatment and who had a PFS greater than 2 months. All statistical analyses were performed using the SPSS statistical software (version 25.0, IBM Corporation, USA) and GraphPad Prism (version 5, GraphPad Software Inc., USA).
Results
Patient characteristics
A total of 68 patients were treated with etoposide, carboplatin, and atezolizumab as the first-line treatment for ES-SCLC during the study period. Baseline characteristics of the patients are shown in Table 1. The median age was 68 (range 40–84) years. Majority of the patients were men (89.7%), who were current (55.9%) or former (39.7%) smokers. The Eastern Cooperative Oncology Group performance status (ECOG PS) score of patients was 0 (20.6%) or 1 (79.4%). Two patients (3.0%) had limited-stage SCLC and had previously undergone definitive CCRT. Seventeen patients (25.0%) had brain metastasis at baseline. Four patients (6.0%) underwent prophylactic cranial irradiation. Only two patients (3.0%) were PD-L1-positive, and the majority of patients were PD-L1-negative (66.2%) or had an unknown PD-L1 expression status (30.9%). Among the 68 patients, 10 (14.7%) patients received 5 to 8 cycles of etoposide and carboplatin chemotherapy. Compared with the IMpower133 study population, the patients in this study were slightly older (median age 68 vs. 64 years) and there was a higher proportion of poor ECOG PS (79.4% vs. 63.7%) and baseline brain metastasis (25.0% vs. 8.5%).
Table 1.
Patient characteristics
| Baseline characteristics (N = 68) | |
|---|---|
| Median age (range)—year | 68 (40–84) |
| Male sex, N (%) | 61 (89.7%) |
| ECOG performance status score—N (%) | |
| 0 | 14 (20.6%) |
| 1 | 54 (79.4%) |
| Smoking statusa—N (%) | |
| Never smoker | 3 (4.4%) |
| Current smoker | 38 (55.9%) |
| Former smoker | 27 (39.7%) |
| Brain metastases at baseline—N (%) | 17 (25.0%) |
| PCI | 4 (6.0%) |
| Previous treatment for LS-SCLC | 2 (3.0%) |
| PD-L1 statusb | |
| ≥ 1 | 2 (3.0%) |
| < 1 | 45 (66.2%) |
| Unknown | 21 (30.9%) |
ECOG Eastern Cooperative Oncology Group, PCI prophylactic cranial irradiation, LS-SCLC limited-stage small-Cell lung cancer, PD-L1 programmed death-ligand 1
aA current smoker was defined to be an individual who currently smokes or has quit for < 1 year, a former smoker was an individual who had stopped smoking for > 1 year, and a never smoker was an individual with a lifetime smoking history of < 100 cigarettes
bThe PD-L1 tumor proportion score was defined as the percentage of tumor cells with membranous PD-L1 expression
Efficacy
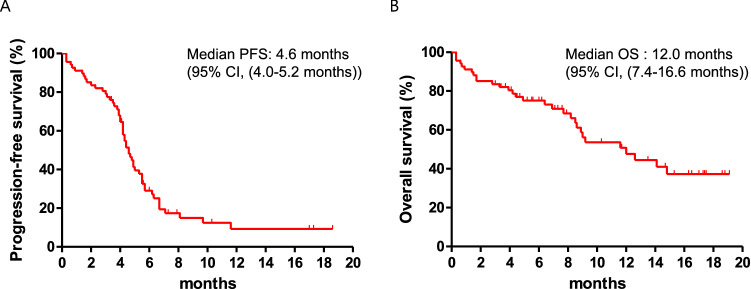
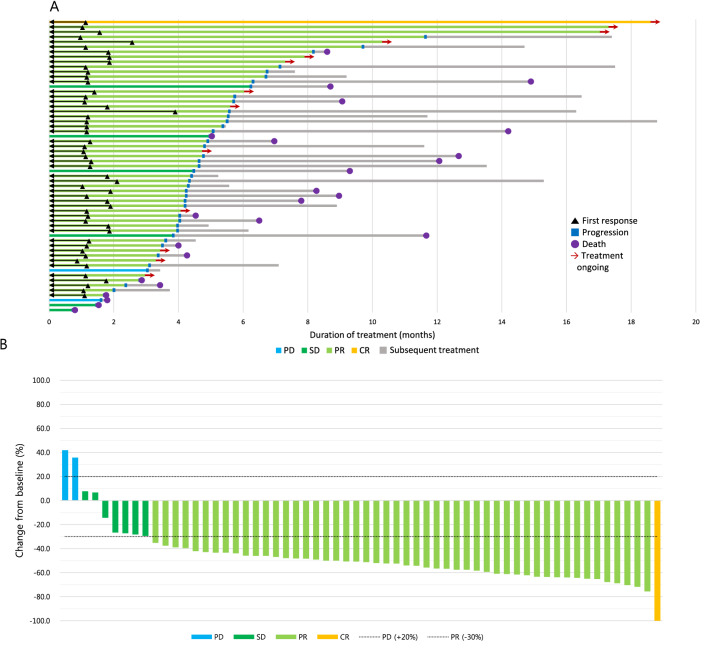
The median follow-up for all patients was 11.6 months (range 0.3–19.1 months). In total, 53 (77.9%) patients experienced disease progression and 30 (44.1%) patients died. The mPFS was 4.6 months (95% CI 4.0–5.2) and the mOS was 12.0 months (95% CI 7.4–16.6) (Fig. 1), which is similar to the values of 5.2 and 12.3 months, respectively, in the IMpower133 study. The swimmer’s plot in Fig. 2a shows that there were some long responders who maintained a response with this first-line therapy; contrastingly, other patients did not obtain a maximal benefit from this initial regimen with relatively less tumor shrinkage or shorter PFS, but had longer survival after disease progression with first-line treatment, marked by the several long gray bars in the graph.
Fig. 1.
Survival of total patients. The Kaplan–Meier estimates of a progression-free survival and b overall survival of total patients (N = 68) are shown (tick marks: censored data). CI confidence interval
Fig. 2.
Treatment outcomes. a Swimmer plot of patients with measurable lesions who had at least one response evaluation (N = 60). Time to first response, disease progression, death, and the duration of treatment are shown. b Waterfall plot of patients with measurable lesions who had at least one response evaluation (N = 60). Maximum percent change in tumor size from baseline is shown according to best response. CR complete response, PR partial response, SD stable disease, PD progressive disease
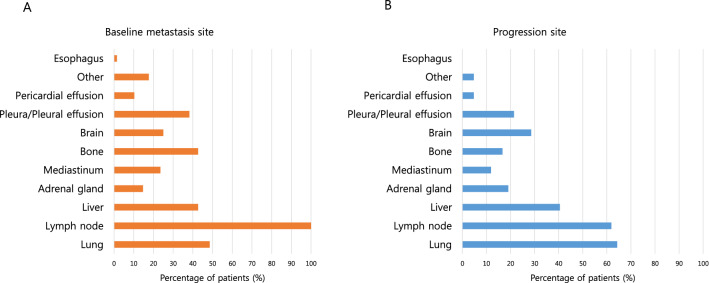
The 12-month and 18-month OS rates were 50.0% and 37.0%, respectively, as compared with a 1-year OS rate of 51.7% in the IMpower133 study. At the time of data cutoff, 15 (22.1%) patients had an ongoing response and continued treatment. Among the 68 patients, 1 (1.5%) achieved a CR, 50 (73.5%) showed a PR, and 7 (10.3%) had stable disease as their best treatment response (Fig. 2b). The ORR was 75.0% (51/68), and the disease control rate was 85.3% (58/68). The median duration of response was 3.3 months (range 0.6–17.5 months) (Table 2). Most patients had lymph node, lung, bone, or liver metastasis at the time diagnosed with metastatic or recurrent disease (Fig. 3a). The majority of patients experienced disease progression in the lung, lymph node, liver, and brain (Fig. 3b).
Table 2.
Response to treatment
| Response of treatment | |
|---|---|
| Total number of patients—68 | |
| Type of response—N (%) | |
| Complete response | 1 (1.5%) |
| Partial response | 50 (73.5%) |
| Stable disease | 7 (10.3%) |
| Progressive disease | 2 (3.0%) |
| Not evaluable | 8 (11.7%) |
| Objective response rate (%) | 75.0 |
| Median duration of response (range)—months | 3.3 (0.6–17.5) |
| Ongoing response at data cutoff—N (%) | 15 (22.1%) |
Fig. 3.
Metastases sites. a Baseline metastases sites of patients before treatment. b Progression sites after treatment with atezolizumab, etoposide, and carboplatin
In the univariate analysis, baseline bone metastasis, the presence of IRAEs, and elevated LDH were related to PFS. In the multivariable analysis, baseline brain metastasis (HR 2.44, P = 0.01), ECOG PS (HR 2.43, P = 0.02), baseline bone metastasis (HR 2.67, P = 0.003), the presence of IRAEs (HR 0.33, P = 0.001), and elevated LDH (HR 2.30, P = 0.01) were significantly associated with PFS (disease progression or death) (supplementary Table 1).
In the univariate analysis, baseline bone metastasis, the presence of IRAEs, and elevated LDH were related to OS, which remained significant in the multivariable analysis (HR 2.18, 0.33, and 4.64; P = 0.05, 0.02, and 0.003, respectively; supplementary Table 2). In the multivariable analysis for the subgroup of 42 patients with disease progression, liver metastasis progression and baseline bone metastasis were associated with inferior OS, but without statistical significance (HR 2.47 and 1.97; P = 0.25 and 0.26, respectively; supplementary Table 3).
Safety
The TRAEs are summarized in Table 3. Among the 68 patients, 61 (89.7%) experienced at least one TRAE, including 24 (35.3%) and 15 (22.1%) patients with grade-3 and grade-4 TRAEs, respectively. Four (5.9%) patients had grade-5 TRAEs, including colitis (2.9%) and pneumonia (2.9%). The most common grade-3–4 TRAEs were neutropenia (42.6%), anemia (22.1%), and thrombocytopenia (22.1%). Among the 68 patients, 22 (32.4%) experienced IRAEs, including 5 (7.4%) patients with grade-3 IRAEs such as pneumonitis (4.4%), hyperosmolar hyperglycemic state (1.5%), and aspartate aminotransferase elevation (1.5%). The most common IRAEs were skin rash (13.3%) and pruritus (8.9%) of grade 1 or 2 in all cases.
Table 3.
Treatment-related adverse events
| Adverse events related to treatment | |||
|---|---|---|---|
| Event | Grade 1 or 2 | Grade 3 or 4 | Grade 5 |
| Treatment-related adverse events | Number of patients (percent) | ||
| Neutropenia | 8 (11.7%) | 29 (42.6%) | |
| Anemia | 21 (30.9%) | 15 (22.1%) | |
| Anorexia | 4 (5.8%) | ||
| Nausea/Vomiting | 1 (1.5%) | ||
| Alopecia | 1 (1.5%) | ||
| Fatigue | 2 (2.9%) | 1 (1.5%) | |
| Thrombocytopenia | 14 (20.6%) | 15 (22.1%) | |
| Constipation | 5 (7.3%) | 1 (1.5%) | |
| Creatinine elevation | 2 (2.9%) | ||
| Febrile neutropenia | 6 (8.8%) | ||
| Colitis | 2 (2.9%) | ||
| Pneumonia | 1 (1.5%) | 2 (2.9%) | |
| Immune-related adverse events | |||
| Pruritus | 6 (8.9%) | ||
| Skin rash | 9 (13.3%) | ||
| Diarrhea | 2 (2.9%) | ||
| Adrenal insufficiency | 3 (4.4%) | ||
| Hypothyroidism | 3 (4.4%) | ||
| AST elevation | 2 (2.9%) | 1 (1.5%) | |
| ALT elevation | 2 (2.9%) | ||
| Hyperosmolar hyperglycemic state | 1 (1.5%) | ||
| Pneumonitis | 3 (4.4%) | ||
AST aspartate aminotransferase, ALT alanine aminotransferase
Discussion
With the increasing use of ICIs for several tumor types, the treatment outcomes, including duration of response, have markedly improved. Combining immunotherapy with conventional cytotoxic chemotherapies [15–18] or target agents [19–21] has also compensated for the shortcomings of immunotherapies, such as the low response rate or delayed initial response. Although SCLC has historically been associated with a dismal prognosis, the addition of ICIs to cytotoxic chemotherapy has resulted in better outcomes, similar to the effects for other solid tumors such as NSCLC.
This retrospective study evaluated the efficacy and safety of atezolizumab, in combination with etoposide and carboplatin, in the first-line treatment of SCLC in a single Korean institute. In our data, survival outcomes (mPFS 4.6 months and mOS 12.0 months) were numerically comparable to those of the IMpower133 (mPFS 5.2 months and mOS 12.3 months) [8] and CASPIAN (mPFS 5.1 months and mOS 13.0 months) [10] studies. Therefore, even though it was a retrospective study without comparator group, this study supported the value of adding ICI to the platinum-based doublet in ES-SCLC. There are some models to explain the improved outcome of an ICI and chemotherapy combination. Downregulation of major histocompatibility complex class I, low expression of PD-L1, poor tumor infiltration by effector T cells, and presence of myeloid-derived suppressor cells as well as regulatory T lymphocytes have been proposed to contribute to the mechanisms of resistance of immune response activation by ICIs in SCLC [22]. Carboplatin reportedly emerged as a candidate inducer of immunogenic cell death using a prediction algorithm [23]. Damage-associated molecular patterns, such as calreticulin or high-mobility group box 1, are exposed or released upon carboplatin treatment [24], thereby promoting anticancer immunity and exerting a synergist effect with ICIs.
In the multivariable analysis of PFS and OS, baseline bone metastasis was associated with decreased survival. Bone metastasis itself might be a poor prognostic factor [25] or it may deteriorate the treatment response to immunotherapy, as observed in NSCLC [26]. In contrast, IRAEs were related to better treatment outcomes and prolonged survival. The correlation between IRAEs and treatment efficacy has been discussed in several retrospective and prospective studies involving NSCLC patients treated with the anti-PD-1 and anti-PD-L1 inhibitors [27–30]. Elevated LDH is known as a poor prognostic marker in SCLC patients and predicts poor response to ICIs [31, 32]. In our study, elevated LDH was also associated with inferior PFS and OS.
Exploratory analyses in the IMpower133 study showed more long-term survivors, defined as patients who survived more than 18 months post-randomization, in the atezolizumab arm than in the placebo arm. Their median follow-up was 22.9 months. As the median follow-up was 11.6 months in our study and 15 (22.1%) patients had an ongoing response at the time of data cutoff, it can be expected that a longer duration of response and long-term survival may exist in the selected patients in our study. It is important to clarify the characteristics of certain patients that contribute to their longer responses or general benefit from immunotherapy. In our study, only two (3.0%) patients were PD-L1-positive and more than half of the patients were PD-L1-negative, which was consistent with the literature [7, 33, 34]. The two PD-L1 positive patients showed similar or shorter PFS and OS compared to the median value. Therefore, markers other than PD-L1 seem to be required in SCLC, such as the molecular subtypes proposed by Rudin et al. [35] or Gay et al. [36]. More follow-up and molecular analysis of our patients could identify the factors contributing to longer survival.
In the additional subgroup analysis of 42 patients with disease progression after treatment with the atezolizumab, etoposide, and carboplatin regimen, liver metastasis progression was related to an inferior OS, although this association was not statistically significant. Interestingly, baseline liver metastasis itself did not show any significant association with PFS or OS in this study. The progression of liver metastasis may have influenced ICI resistance, contributing to poor survival. In a recent study, Yu et al. [37] suggested that liver metastasis induced a decrease in circulating CD8+ T cells through the apoptosis of T cells after contact with immunosuppressive macrophages within the liver, and liver-directed radiotherapy eliminated macrophages to promote the antitumor efficacy of immunotherapy in preclinical models. If this hypothesis is confirmed in more prospective studies, close observation of liver metastasis progression and timely addition of local therapy to the liver metastasis would likely enhance the immunotherapy efficacy and contribute to improved survival outcomes.
The profile of prevalent adverse events in our study was similar to that in the IMpower133 trial. Although grade-5 treatment-related adverse events occurred in four (5.8%) patients, these were not IRAEs. Many patients showed hematologic toxicities, which were above grade 3 in 22.1–42.6% of the patients. Fortunately, these toxicities could be prevented through granulocyte colony-stimulating factor injection or transfusion. Most of the IRAEs were grade 1 or 2.
There are several limitations to this study. First, as it is a retrospective review and analysis from medical records, the incidence of some adverse events, such as nausea, vomiting, or alopecia, is likely underestimated. However, 33 (48.5%) patients received treatment before the approval of atezolizumab combined with chemotherapy for SCLC in Korea through an expanded access program. The adverse events of these patients were followed up and described in the electronic medical records at the time of treatment. Second, there were some practices that differed from those of pivotal clinical trials. Some patients had their first response evaluation after the initial third cycle of treatment, which may have led to a reduced duration of response. In addition, some patients (10/68, 14.7%) received more than five cycles of chemotherapy, which was not performed in the clinical trials. Third, the small number of patients and a limited follow-up period might have influenced the results. Therefore, subsequent studies with larger samples and longer follow-up are required.
Conclusion
Atezolizumab, combined with etoposide and carboplatin, showed efficacy and safety as first-line treatment for ES-SCLC in our real-world data. More follow-up is needed to confirm the durable response and effect of this regimen on long-term survival. Moreover, further studies are needed to evaluate molecular markers that can predict the response of immunotherapy in SCLC.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
None.
Abbreviations
- CRT
Concurrent chemoradiation therapy
- CI
Confidence interval
- CR
Complete response
- ES-SCLC
Extensive-stage small-cell lung cancer
- HR
Hazard ratio
- ICI
Immune checkpoint inhibitor
- IRAE
Immune-related adverse event
- mPFS
Median progression-free survival
- mOS
Median overall survival
- NSCLC
Non-small cell lung cancer
- ORR
Objective response rate
- OS
Overall survival
- PD-1
Programmed death protein-1
- PD-L1
Programmed death-ligand 1
- PFS
Progression-free survival
- PR
Partial response
- RECIST
Response Evaluation Criteria in Solid Tumors
- SCLC
Small-cell lung cancer
- TRAE
Treatment-related adverse event
Authors’ contributions
SL and MHH conceptualized and designed the study. SL, HSS, BCA, SML, HRK, BCC, and MHH were involved in data acquisition. SL and MHH analyzed and interpreted data. SL performed statistical analysis. The first draft of the manuscript was written by SL and MHH, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data Availability
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
Code availability
Not applicable.
Declarations
Conflicts of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Ethics approval
The study was approved by the institutional review board (IRB) at Severance Hospital (IRB number 4–2020-1151), and all experiments were performed in accordance with the guidelines and regulations of IRB of Severance Hospital. This study was given a formal waiver for the need of consent by the institutional review board of Severance Hospital in view of the retrospective nature of the study.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Footnotes
The study provides real-world data of Korean small-cell lung cancer patients showing comparable outcomes with IMpower133 study, in which Asians were underrepresented. Bone metastasis and liver metastasis progression suggested a poor prognosis.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Barta JA, Powell CA, Wisnivesky JP. Global epidemiology of lung cancer. Ann Glob Health. 2019 doi: 10.5334/aogh.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farago AF, Keane FK. Current standards for clinical management of small cell lung cancer. Transl Lung Cancer Res. 2018;7(1):69–79. doi: 10.21037/tlcr.2018.01.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schabath MB, Nguyen A, Wilson P, Sommerer KR, Thompson ZJ, Chiappori AA. Temporal trends from 1986 to 2008 in overall survival of small cell lung cancer patients. Lung Cancer. 2014;86(1):14–21. doi: 10.1016/j.lungcan.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ready N, Farago AF, de Braud F, Atmaca A, Hellmann MD, Schneider JG, et al. Third-line nivolumab monotherapy in recurrent SCLC: checkmate 032. J Thorac Oncol. 2019;14(2):237–244. doi: 10.1016/j.jtho.2018.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ott PA, Elez E, Hiret S, Kim DW, Morosky A, Saraf S, Piperdi B, Mehnert JM. Pembrolizumab in patients with extensive-stage small-cell lung cancer: results from the phase Ib KEYNOTE-028 study. J Clin Oncol. 2017;35(34):3823–3829. doi: 10.1200/jco.2017.72.5069. [DOI] [PubMed] [Google Scholar]
- 7.Chung HC, Piha-Paul SA, Lopez-Martin J, Schellens JHM, Kao S, Miller WH, Jr, et al. Pembrolizumab after two or more lines of previous therapy in patients with recurrent or metastatic SCLC: results from the KEYNOTE-028 and KEYNOTE-158 studies. J Thorac Oncol. 2020;15(4):618–627. doi: 10.1016/j.jtho.2019.12.109. [DOI] [PubMed] [Google Scholar]
- 8.Horn L, Mansfield AS, Szczęsna A, Havel L, Krzakowski M, Hochmair MJ, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018;379(23):2220–2229. doi: 10.1056/NEJMoa1809064. [DOI] [PubMed] [Google Scholar]
- 9.Mansfield AS, Każarnowicz A, Karaseva N, Sánchez A, De Boer R, Andric Z, et al. Safety and patient-reported outcomes of atezolizumab, carboplatin, and etoposide in extensive-stage small-cell lung cancer (IMpower133): a randomized phase I/III trial. Ann Oncol. 2020;31(2):310–317. doi: 10.1016/j.annonc.2019.10.021. [DOI] [PubMed] [Google Scholar]
- 10.Paz-Ares L, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet. 2019;394(10212):1929–1939. doi: 10.1016/s0140-6736(19)32222-6. [DOI] [PubMed] [Google Scholar]
- 11.Goldman JW, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, et al. Durvalumab, with or without tremelimumab, plus platinum-etoposide versus platinum-etoposide alone in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): updated results from a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2020 doi: 10.1016/s1470-2045(20)30539-8. [DOI] [PubMed] [Google Scholar]
- 12.Rudin CM, Awad MM, Navarro A, Gottfried M, Peters S, Csőszi T, et al. Pembrolizumab or placebo plus etoposide and platinum as first-line therapy for extensive-stage small-cell lung cancer: randomized, double-blind, phase III KEYNOTE-604 study. J Clin Oncol. 2020;38(21):2369–2379. doi: 10.1200/jco.20.00793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 14.Common Terminology Criteria for Adverse Events (CTCAE), version 4.0 (2009) National Cancer Institute. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf. Accessed 28 May 2009
- 15.Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378(22):2078–2092. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 16.Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gümüş M, Mazières J, et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. 2018;379(21):2040–2051. doi: 10.1056/NEJMoa1810865. [DOI] [PubMed] [Google Scholar]
- 17.Burtness B, Harrington KJ, Greil R, Soulières D, Tahara M, de Castro Jr. G, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019;394(10212):1915–1928. doi: 10.1016/s0140-6736(19)32591-7. [DOI] [PubMed] [Google Scholar]
- 18.Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379(22):2108–2121. doi: 10.1056/NEJMoa1809615. [DOI] [PubMed] [Google Scholar]
- 19.Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1116–1127. doi: 10.1056/NEJMoa1816714. [DOI] [PubMed] [Google Scholar]
- 20.Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 21.Choueiri TK, Powles T, Burotto M, Escudier B, Bourlon MT, Zurawski B, et al. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2021;384(9):829–841. doi: 10.1056/NEJMoa2026982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamilton G, Rath B. Immunotherapy for small cell lung cancer: mechanisms of resistance. Expert Opin Biol Ther. 2019;19(5):423–432. doi: 10.1080/14712598.2019.1592155. [DOI] [PubMed] [Google Scholar]
- 23.Galluzzi L, Humeau J, Buqué A, Zitvogel L, Kroemer G. Immunostimulation with chemotherapy in the era of immune checkpoint inhibitors. Nat Rev Clin Oncol. 2020;17(12):725–741. doi: 10.1038/s41571-020-0413-z. [DOI] [PubMed] [Google Scholar]
- 24.Golden EB, Frances D, Pellicciotta I, Demaria S, Helen Barcellos-Hoff M, Formenti SC. Radiation fosters dose-dependent and chemotherapy-induced immunogenic cell death. Oncoimmunology. 2014;3:e28518. doi: 10.4161/onci.28518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakazawa K, Kurishima K, Tamura T, Kagohashi K, Ishikawa H, Satoh H, Hizawa N. Specific organ metastases and survival in small cell lung cancer. Oncol Lett. 2012;4(4):617–620. doi: 10.3892/ol.2012.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Landi L, D'Incà F, Gelibter A, Chiari R, Grossi F, Delmonte A, et al. Bone metastases and immunotherapy in patients with advanced non-small-cell lung cancer. J Immunother Cancer. 2019;7(1):316. doi: 10.1186/s40425-019-0793-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ricciuti B, Genova C, De Giglio A, Bassanelli M, Dal Bello MG, Metro G, Brambilla M, Baglivo S, Grossi F, Chiari R. Impact of immune-related adverse events on survival in patients with advanced non-small cell lung cancer treated with nivolumab: long-term outcomes from a multi-institutional analysis. J Cancer Res Clin Oncol. 2019;145(2):479–485. doi: 10.1007/s00432-018-2805-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grangeon M, Tomasini P, Chaleat S, Jeanson A, Souquet-Bressand M, Khobta N, et al. Association between immune-related adverse events and efficacy of immune checkpoint inhibitors in non-small-cell lung cancer. Clin Lung Cancer. 2019;20(3):201–207. doi: 10.1016/j.cllc.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 29.Sato K, Akamatsu H, Murakami E, Sasaki S, Kanai K, Hayata A, et al. Correlation between immune-related adverse events and efficacy in non-small cell lung cancer treated with nivolumab. Lung Cancer. 2018;115:71–74. doi: 10.1016/j.lungcan.2017.11.019. [DOI] [PubMed] [Google Scholar]
- 30.Haratani K, Hayashi H, Chiba Y, Kudo K, Yonesaka K, Kato R, et al. Association of immune-related adverse events with nivolumab efficacy in non-small-cell lung cancer. JAMA Oncol. 2018;4(3):374–378. doi: 10.1001/jamaoncol.2017.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Z, Li Y, Yan X, Song Q, Wang G, Hu Y, Jiao S, Wang J. Pretreatment lactate dehydrogenase may predict outcome of advanced non small-cell lung cancer patients treated with immune checkpoint inhibitors: a meta-analysis. Cancer Med. 2019;8(4):1467–1473. doi: 10.1002/cam4.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang X, Guo M, Fan J, Lv Z, Huang Q, Han J, Wu F, Hu G, Xu J, Jin Y. Prognostic significance of serum LDH in small cell lung cancer: A systematic review with meta-analysis. Cancer Biomark. 2016;16(3):415–423. doi: 10.3233/cbm-160580. [DOI] [PubMed] [Google Scholar]
- 33.Schultheis AM, Scheel AH, Ozretić L, George J, Thomas RK, Hagemann T, Zander T, Wolf J, Buettner R. PD-L1 expression in small cell neuroendocrine carcinomas. Eur J Cancer. 2015;51(3):421–426. doi: 10.1016/j.ejca.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 34.Antonia SJ, López-Martin JA, Bendell J, Ott PA, Taylor M, Eder JP, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol. 2016;17(7):883–895. doi: 10.1016/s1470-2045(16)30098-5. [DOI] [PubMed] [Google Scholar]
- 35.Rudin CM, Poirier JT, Byers LA, Dive C, Dowlati A, George J, et al. Molecular subtypes of small cell lung cancer: a synthesis of human and mouse model data. Nat Rev Cancer. 2019;19(5):289–297. doi: 10.1038/s41568-019-0133-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gay CM, Stewart CA, Park EM, Diao L, Groves SM, Heeke S, et al. Patterns of transcription factor programs and immune pathway activation define four major subtypes of SCLC with distinct therapeutic vulnerabilities. Cancer Cell. 2021;39(3):346–360.e347. doi: 10.1016/j.ccell.2020.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu J, Green MD, Li S, Sun Y, Journey SN, Choi JE, et al. Liver metastasis restrains immunotherapy efficacy via macrophage-mediated T cell elimination. Nat Med. 2021;27(1):152–164. doi: 10.1038/s41591-020-1131-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
Not applicable.