Abstract
Abstract
Several CD19-targeting CAR-T cells are used to treat leukemias and lymphomas; however, relapsed and/or refractory (R/R) disease is still observed in a significant number of patients. Additionally, the success of CD19-CAR-T cell therapies is not uniform across hematological malignancies, particularly in chronic lymphocytic leukemia (CLL). In this study, we present the development of a novel CAR-T cell therapy targeting B-cell activating factor receptor (BAFF-R), a key regulator of B-cell proliferation and maturation. A new monoclonal antibody against BAFF-R was generated from a hybridoma clone and used to create a novel MC10029 CAR construct. Through a series of in vitro and in vivo models using the Nalm-6 cell line for leukemia and the Z138 cell line for lymphoma, we demonstrated the antigen-specific cytotoxicity of MC10029 CAR-T cells against tumor cells. Additionally, MC10029 CAR-T cells exhibited potent antitumor effects against CD19 knockout tumor cells, mimicking CD19-negative R/R disease. MC10029 CAR-T cells were specifically targeted to CLL, in which BAFF-R is nearly always expressed. The cytotoxicity of MC10029 CAR-T cells was first shown in the MEC-1 CLL cell line, before we turned our efforts to subject-derived samples. Using healthy donor-engineered MC10029 CAR-T cells against enriched primary tumor cells, followed by subject-derived MC10029 CAR-T cells against autologous tumor cells, we showed the efficacy of MC10029 CAR-T cells against CLL subject samples. With these robust data, we have advanced to the production of MC10029 CAR-T cells, using GMP lentivirus, and obtained an IND approval in preparation for a Phase 1 clinical trial.
Graphical abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-023-03537-w.
Keywords: Immunotherapy, CAR-T, BAFF-R, B-cell malignancies, Leukemia, Lymphoma
Introduction
The genetic engineering of T cells to express a surface chimeric antigen receptor (CAR) has revolutionized cancer therapeutics with B-cell malignancies providing an opportune landscape for this targeted therapy since B-cells possess tissue restricted antigens and B-cell aplasia can be medically managed. The expression of CD19 on most B-cell malignancies but not on hematopoietic stem cells positioned it as an antigen for CAR-T cell design and development [1]. Axicabtagene ciloleucel, tisagenlecleucel, lisocabtagene maraleucel, and brexucabtagene autoleucel have been approved for the treatment of various relapsed and/or refractory (R/R) B-cell malignancies that had failed systemic therapies [2–9]. Although all target the CD19 antigen, these CAR-T cells differ either in costimulatory domains or by their manufacturing processes [2–4]. These CD19 CAR-T cell therapies have become effective treatment options for some patients with R/R B-cell lymphoma, showing complete tumor eradication and prolonged remission for between 40 and 54% of patients [2–4]. Wanting higher (and more durable) remission rates, understanding these treatment failures remains an unmet need.
The desire to improve partial or complete remission(CR) rates for patients receiving CD19 CAR-T cell therapies applies to all B-cell malignancies; however, chronic lymphocytic leukemia (CLL) stands out as a particular need with an average CR of only 30% [10]. Although CD19 CAR-T cell therapy was pioneered as a therapy for CLL in 2011, the multiple refinements to treatment protocols in the subsequent years that included changes to the lymphodepletion chemotherapy protocol to increasing the dose of CAR-T cells to adding complementary agents (e.g., immune checkpoint inhibitors, Bruton tyrosine kinase inhibitor) did significantly enhance the success of CD19 CAR-T cells in treating B-cell malignancies but provided only modest improvements to the treatment outcomes for patients with CLL [10]. Thus, resolving R/R disease after CD19 CAR-T cell therapy, specifically for patients with CLL, remains an unmet medical need.
An effective strategy for addressing R/R disease involves the generation of CAR-T cells that target an alternative B-cell antigen. We propose B-cell activating factor receptor (BAFF-R) as an ideal target, since it is a B-cell marker specifically involved in B lymphocyte development and mature B-cell survival [11, 12]. BAFF-R and BCR work together to produce and maintain immunocompetent B-cells along the various stages of normal B-cell development via pathways that engage ERK, Akt, and NF-ĸB [13]. The functional linkage between BAFF-R and BCR allows for the selection and maturation of B-cell clones [13, 14]. BAFF-R has a highly restricted tissue specificity, is universally expressed in B-cell malignancies [12, 15], and plays a critical role in tumor survival [12, 16]. We were particularly intrigued in BAFF-R as a specific target antigen for CLL, due to not only the high expression of BAFF-R in CLL but also the localization of BAFF-R in germinal centers where B-cell survival is determined [15]. Hence, we first developed and characterized a novel BAFF-R targeted CAR, MC10029 CAR, before designed a series of in vitro, in vivo, and ex vivo experiments to assess the antigen-specific cytotoxicity of MC10029 CAR-T cells. Our experimental journey culminated in the generation of subject-derived MC10029 CAR-T cells that were then shown to be efficacious against autologous CLL tumor cells. These compelling data support the potential of MC10029 CAR-T cells as a promising treatment for CLL and the filing of an IND for MC10029 CAR-T cells for evaluation in a clinical trial.
Materials and methods
Cell lines
The cell lines of Nalm-6, MEC-1, and Z-138 were purchased from Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH (Braunschweig, Germany); Jurkat and 293FT cell lines were obtained from Thermo Fisher Scientific (Waltham, Massachusetts, USA) and American Type Culture Collection (Manassas, Virginia, USA), respectively. Cells were maintained in either 90% RPMI 1640, Iscove’s MDM, or 90% Dulbecco's Modified Eagle Medium (Thermo Fisher) supplemented with 10% heat-inactivated fetal bovine serum (Thermo Fisher). Cell lines were authenticated by flow cytometry. The following antigen knock out cells lines were generated: BAFF-R KO Nalm-6, CD19 KO Nalm-6, CD19 KO Z-138, and CD19 KO MEC-1. Luciferase expressing human cell lines were generated for in vivo experiments, as previously described [16].
PBMCs and Tn/mem isolation from health donors’ blood samples
Peripheral blood mononuclear cells (PBMCs) from healthy volunteer donors were isolated via leukapheresis using leukocyte reduction system (LRS) cones, by the Division of Transfusion Medicine, Mayo Clinic, Rochester, Minnesota, following current regulatory requirements and as previously described [17]. For the generation of CAR-T cells, naïve and memory T cell (Tn/mem) populations were isolated from PBMCs in a three-step procedure by negative selection of both CD14 and CD25 and positive selection of CD62L, using CD14, CD25, and CD62L microbeads, per the manufacturer protocol (Miltenyi Biotech, Germany).
Isolation of T cells and B-cells from blood samples from subject with CLL
Peripheral blood samples from subjects with CLL were procured per a biorepository protocol approved by the Institutional Review Board (IRB) of Mayo Clinic, Florida (20–010888) and followed the ethical principles of the Declaration of Helsinki. All subjects provided written informed consent. For primary cell analysis, 50 ml of blood was collected from subjects with a diagnosis of CLL. For CAR-T cell generation from CLL subjects, the T cells were isolated using the Pan T cell isolation kit (Miltenyi Biotec, Germany), following the instructions provided. The B-cells were isolated using the EasySep™ Direct Human B-cell Isolation Kit (Stemcell Technologies, Vancouver, Canada), per the manufacturer protocol.
CAR-T cell production
A second-generation BAFF-R-CAR was designed as described in the results. The CAR cDNA was cloned into a pHIV.7 lentiviral vector. CD19-CAR was generated similarly, replacing BAFF-R antibody single-chain variable fragment (scFv) with CD19 antibody scFv that was derived from a clinically tested and previously reported CD19-CAR [16]. Lentiviruses were produced in 293FT cells, concentrated, and titered with Jurkat cells. Tn/mem or subject T cells were isolated and activated with Human T-Activator CD3/CD28 beads (Life Technologies) for 24 h, followed by transduction with lentivirus encoding CAR at a multiplicity of infection (MOI) = 1. We used protamine sulfate as a transduction enhancer for lentivirus transduction to generate CAR-T cells. The optimal MOI was determined after testing the MOI range from 0.1 to 10 to maximize the CAR-T cell potency while minimizing residual lentivirus effect (WPRE and VSVG) in the CAR-T cells. The CAR-T cells were further activated with CD3/CD28 bead stimulation for seven days after which the beads are removed, and the CAR-T cells were expanded for an additional seven days. Non-CAR-T cells are non-transduced T cells from the same donor, expanded following the CAR-T cell protocol, and used as a control.
In vitro functional assays
Degranulation assay [16]
CAR-T cells were incubated with target cells at an effector-to-target (E:T) ratio of 2:1 in complete RPMI 1640 medium containing GolgiStop™ Protein Transport Inhibitor Reagent (BD Bioscience) and CD107a APC antibody (BD Biosciences) for 6 h. The cells were subsequently stained with anti-CD3 BV605 (BD Biosciences), anti-CD4 PE-Cy7 (BD Biosciences), anti-CD8 APC-Cy7 (BD Biosciences), and anti-EGFR BV421(BD Biosciences). Samples were evaluated on the Attune flow cytometer (Thermo Fisher Scientific) or the Fortessa flow cytometer (BD Biosciences); data were analyzed using FlowJo™ Version 10 software. Non-CAR T cells from the same patient were used as negative controls.
Granule release assay
CAR-T cells and target cells were co-incubated for 72 h at an E:T ratio of 4:1; the supernatant was collected after 72 h; and analytes were quantified using a customized U-PLEX Human ELISA kit from Meso Scale Diagnostics, following the manufacturer's instructions (Rockville, MD, USA).
Direct killing assay
To evaluate the cytolytic function of CAR-T cells on tumor cells, CAR-T cells were co-incubated with GFP-positive target cells at an E:T ratio of 20:1 for 24 h. Live dye staining with Sytox Blue (Thermo Fisher) identified the percentage of live GFP-positive tumor cells; the samples were run on the Attune flow cytometer.
In vivo modeling
NOD scid gamma (NSG) mouse breeding pairs were purchased from The Jackson Laboratory (stock no. 005557) to establish a breeding colony that was monitored in a pathogen-free animal facility at the Animal Resource Center at Mayo Clinic Florida, per institutional guidelines. Animal studies were approved by and in accordance with guidelines of the Institutional Animal Care and Use Committee (IACUC: 15020; protocol number A00005759). Mice (8–12 weeks old) received an intravenous (IV) challenge with a luciferase-expressing human tumor cell line (optimized in a separate experiment), randomized into test groups (5 mice per group), and treated with a single IV treatment dose, typically with non-CAR-T cell group receiving 10 × 106 cells total T cells and CAR-T cell groups receiving 2 × 106 cells CAR-T cells out of 10 × 106 total T cells. (Concentration is also specified in figure legends.) [16] The tumor burden was quantified weekly by bioluminescent signal intensity on isoflurane-anesthetized mice that received a subcutaneous injection of D-luciferin (150 μg luciferin/1 g mouse body weight) 10 min prior to IVIS® imaging (PerkinElmer, Waltham, MA). Survival data were presented and reported in Kaplan–Meier plots.
Statistical analysis
All statistical analyses were performed with GraphPad Prism software (San Diego, CA). Data are reported as means ± SD and analyzed by a student’s t test. Unpaired t test comparisons were performed with granule protein release data, and a log rank test was performed for the animal studies. Typical comparisons were between non-CAR T cells and antigen-specific CAR-T cells, with the following convention: * for p < 0.05; ** for p < 0.01; *** for p < 0.001.
Results
Generation of novel anti-BAFF-R MC10029 CAR
The MD Anderson Cancer Center Monoclonal Antibody Core Facility was contracted to generate an anti-BAFF-R monoclonal antibody (mAb) using BAFF-R expressing NIH/3T3 cells as the immunogen. (Supplementary Fig. 1a) Screening of candidate hybridoma clones against BAFF-R expressing 293FT cells was performed for identifying the lead antibody-producing clone. (Supplementary Fig. 1b) Remarkably, hybridoma clone 21 (C21) supernatants exhibited dose-dependent and antigen-specific binding patterns. (Supplementary Fig. 1c and 1d) This antigen-specific binding was further confirmed on the antibody purified from C21 hybridoma supernatant. (Supplementary Fig. 1e) Following confirmation of the lead hybridoma clone, the cDNA sequences of the heavy and light chain variable regions of the C21 mAb were identified for constructing the MC10029 CAR.
We generated a second generation BAFF-R CAR (MC10029 CAR) using a clinically approved lentiviral vector [18]. The construct includes the scFv of our new BAFF-R antibody with the following elements in tandem: IgG4 hinge, CD28 transmembrane domain, CD28 costimulatory domain, CD3ζ, and tEGFR. (Supplementary Fig. 2a) Our selection of the CD28 costimulatory domain was empirically determined by comparing the antigen-specific cytotoxicity of BAFF-R CAR constructs containing either CD28 or 4–1 BB (Supplementary Fig. 3a–c). Research grade MC10029 CAR-T cells were reproducibly generated with the specific requirements of cell quality, CAR-T cell specific characterization, and CAR-T cell fold expansion (Supplementary Fig. 2b–e) to ensure the quality of our experimental CAR-T cells.
MC10029 CAR-T cells exhibit antigen-specific cytotoxicity against acute lymphocytic leukemia (ALL) in both in vitro and in vivo models
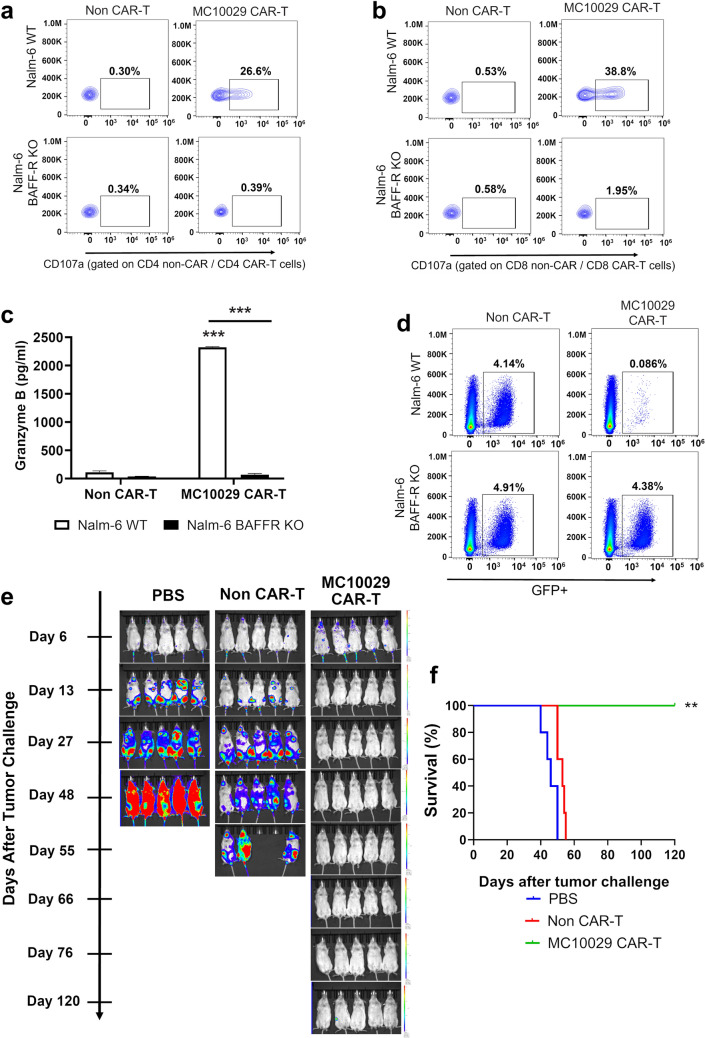
The surface expression of BAFF-R has been extensively documented across various types of B-cell malignancies [11, 19–21]. We confirmed BAFF-R expression in the cell lines that we used to characterize MC10029 CAR-T cell function (Supplementary Fig. 4a). After confirming BAFF-R expression on Nalm-6, an ALL cell line, we show the antigen-specific cytotoxic properties of MC10029 CAR-T cells against wild-type (WT) Nalm-6 but not BAFF-R KO Nalm-6 cells; the CD107a degranulation assay was either gated on CD4 or CD8 to highlight the activity of these specific MC10029 CAR-T cell populations (Fig. 1a, Supplementary Fig. 7a; Fig. 1b, and Supplementary Fig. 7b). Granzyme B release was only observed when MC10029 CAR-T cells were incubated with WT Nalm-6 (Fig. 1c). Cytolysis, as determined with the loss of target cells engineered to express green fluorescent protein (GFP), was observed when MC10029 CAR-T cells were incubated with the GFP-labeled, WT Nalm-6, but not BAFF-R KO Nalm-6 cells, again confirming antigen-specific cytotoxicity (Fig. 1d).
Fig. 1.
Antigen-specific cytotoxicity of MC10029 CAR-T cells against acute lymphocytic leukemia (ALL) cell lines in in vitro and in vivo models. a, b Flow cytometry contour plots of CAR T-cell functional potency, as measured by the surface expression of CD107a in a degranulation assay. MC10029 CAR-T cells were incubated with Nalm-6 WT or Nalm-6 BAFF-R KO cells at an E:T ratio of 2:1. Analysis was gated on CD4 + CAR-T cells (a) or gated on CD8 + CAR-T cells (b); gating on EGFR was used as a proxy for CAR expression in the CAR-T cells. Non-transduced T cells (Non-CAR-T) from the same donor were used as negative controls. (Statistical analysis, Supplementary Fig. 7a/b). c Granzyme B ELISA further confirmed the antigen-specific potency of MC10029 CAR-T cells against Nalm-6 cells. Non-CAR-T or MC10029 CAR-T cells generated from the same donor were co-incubated with either Nalm-6 WT or Nalm-6-BAFF-R KO cells at an E:T ratio of 4:1. After 72 h, the supernatants were collected and assayed in a granzyme B ELISA. Graphed data are means of quadruplicate sampling. The results shown are representative of three independent experiments. d Direct target cell cytolysis was measured by incubating GFP expressing Nalm-6 cells with CAR-T cells. Non-CAR-T cells or MC10029 CAR-T cells were co-incubated with either Nalm-6 WT or Nalm-6 BAFF-R KO cells at an E:T ratio of 20:1 for 24 h, followed by flow cytometry to quantify the GFP expression target cells. The results shown are representative from three independent experiments. e The in vivo therapeutic efficacy of MC10029 CAR-T cells was evaluated in an NSG mouse model in which the mice were challenged with luciferase labeled Nalm-6 tumor cells (0.25 × 106 cells/mouse). Bioluminescent imaging catalogs the changes of luciferase-expressing Nalm-6 tumors. Six days after tumor cell injection, tumor-bearing mice were randomized into three groups (N = 5 per group) to receive a single intravenous (IV) infusion of either vehicle (PBS), non-CAR T cells (10 × 106 total T cells), or MC10029 CAR-T cells (2 × 106 CAR-T cells out of 10 × 10.6 total T cells) that were generated from the same donor. Although imaged weekly, we share representative images along the time course to highlight changes due to treatment. These data are the representative of two independent experiments using different donor T cells. f A Kaplan–Meier plot of overall survival versus days after tumor challenge details the overall survival of the three treatment groups; log rank analysis identified statistical differences between the treatment groups. (**p < 0.01; ***p < 0.001)
The therapeutic efficacy of MC10029 CAR-T cells was assessed in NSG mice that were challenged with Nalm-6 tumor cells. Tumor changes were temporally monitored by bioluminescence imaging (Fig. 1e), and long-term survival was monitored to generate a Kaplan–Meier plot (Fig. 1f). Tumor growth and death were noted in the PBS treated mice within 42 days; the non-CAR-T cell treated group also showed advanced tumor progression. The MC10029 CAR-T cell treated mice displayed a considerable decrease in Nalm-6 tumor presence and a statistically significant survival rate to 120 days.
MC10029 CAR-T cells remained effective in models of CD19 antigen loss
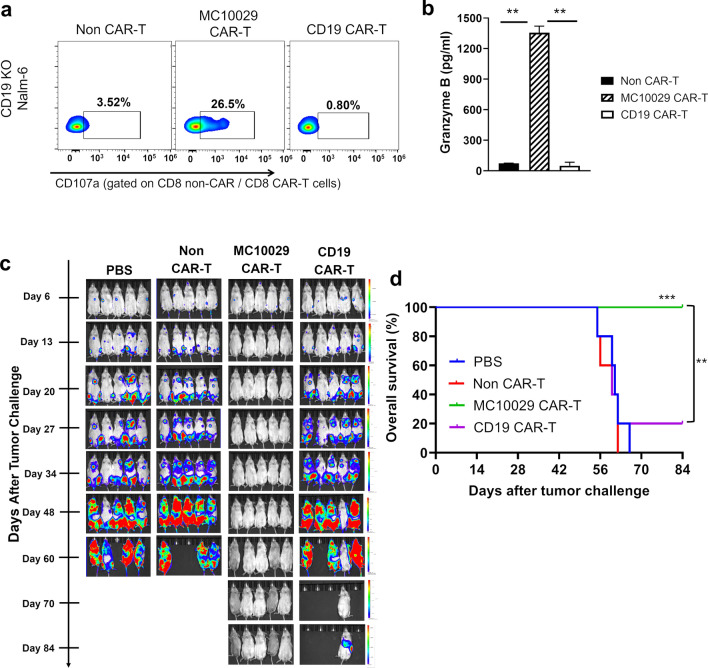
We generated a Nalm-6-based model deficient in CD19 to mimic antigen-escape disease. We showed that MC10029 CAR-T cells retain antigen-specific cytotoxic function against the CD19 KO Nalm-6 cell line, while only background activity was observed for non-CAR-T cells and CD19 CAR-T cells (Fig. 2a and Supplementary Fig. 7c). The robust MC10029 CAR-T cell activity against CD19-deficient tumor cells was confirmed by measuring granzyme B (Fig. 2b). The antitumor activity of MC10029 CAR-T cells against CD19-deficient tumors was consistently replicated in two additional CD19-deficient B-cell tumor models, namely CD19 KO Z-138 and CD19 KO MEC-1, which we generated (Supplementary Fig. 4b–f). This confirmation further supports our initial observation made in the CD19 KO Nalm-6 model. We next examined NSG mice that were challenged with CD19 KO Nalm-6 tumor cells followed by one of four therapies: PBS, Non-CAR-T cells, MC10029 CAR-T cells, or CD19 CAR-T cells. Bioluminescence imaging showed tumor progression (Fig. 2c), and a Kaplan–Meier curve plotted survival (Fig. 2d). The control groups of PBS and Non-CAR-T cells were euthanized around 60 days due to excessive tumor burden; 80% of the mice in the CD19 CAR-T cell treatment group showed similar tumor burden as the controls with one mouse surviving to the termination of the 84 day experiment. The MC10029 CAR-T cell treatment group displayed a considerable decrease in tumor presence and a statistically significant survival.
Fig. 2.
Characterization of in vitro and in vivo cytotoxicity of MC10029 CAR-T cells against CD19 deficient Nalm-6 cell line. a Flow cytometry contour plots show the functional potency of CAR-T cells against target cells by the surface expression CD107a in a degranulation assay. Non-CAR-T cells, MC10029 CAR-T cells, or CD19 CAR-T cells were generated from the same donor and incubated with CD19 KO-Nalm-6 cells at an E:T ratio of 2:1 to identify the cytotoxicity of MC10029 CAR-T cells against CD19-deficient tumor cells. Analysis was gated on CD8 + CAR-T cell populations. (Statistical analysis, Supplementary Fig. 7c). b Granzyme B ELISA shows functional potency of MC10029 CAR-T against CD19 KO Nalm-6 cells. Non-CAR-T cells, MC10029 CAR-T cells, or CD19 CAR-T cells were co-incubated with Nalm-6 CD19 KO cells at an E:T ratio of 4:1 for 72 h when the supernatants were harvested for subsequent ELISA. Graphed data are means of quadruplicate sampling. The data are representative of three independent experiments. c Bioluminescent imaging catalogs the changes of luciferase-expressing CD19 deficient-Nalm-6 model (CD19 KO- Nalm-6-Luc, 0.25 × 106 cells/mouse) in NSG mice followed one of four treatments. Six days after tumor cell injection, tumor-bearing mice were randomized into four groups (N = 5 per group). On day seven, mice received single infusion treatment with either PBS (vehicle), Non-CAR-T cells (10 × 106 total T cells), MC10029 CAR T-cells (2 × 106 CAR-T cells out of 10 × 106 total T cells), or CD19 CAR-T cells (2 × 106 CAR-T cells out of 10 × 10.6 total T cells). Tumor burden was quantified by bioluminescence intensity. d The Kaplan–Meier plot shows overall survival versus days after tumor challenge; log rank analysis identified statistical differences between the treatment groups. (**p < 0.01; ***p < 0.001)
MC10029 CAR-T cells show antigen-specific cytotoxicity of against lymphoma in both in vitro and in vivo models
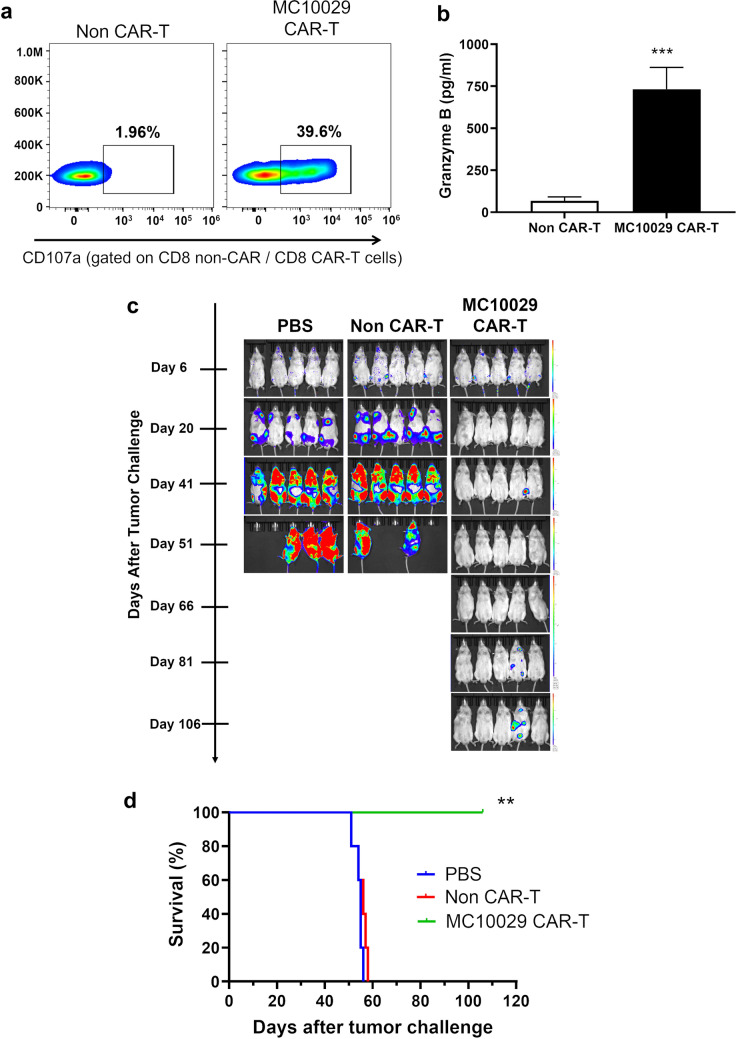
Z-138 is a non-Hodgkin lymphoma (NHL) cell line that expresses BAFF-R (Supplementary Fig. 4b). Antigen-specific cytotoxicity of MC10029 CAR-T cells against Z-138 was confirmed by using a CD107a degranulation assay (Fig. 3a and Supplementary Fig. 7d) and by measuring granzyme B release (Fig. 3b). We assessed the therapeutic efficacy of MC10029 CAR-T cells in NSG mice that were injected with Z-138 tumor cells followed by one of three treatments. Bioluminescence imaging of the mice showed tumor progression (Fig. 3c), and survival was plotted (Fig. 3d). Excessive tumor burden led to the euthanasia of the control groups (PBS and Non-CAR-T cells) within 56 days. The MC10029 CAR-T cell treatment group showed a considerable decrease in tumor presence and a statistically significant survival rate.
Fig. 3.
Characterization of in vitro and in vivo cytotoxicity of MC10029 CAR-T cells against Z-138, a lymphoma cell line. a Flow cytometry contour plots show the functional potency of CAR-T cells against target cells by the surface expression CD107a in a degranulation assay. Non-CAR-T cells and MC10029 CAR-T cells were generated from the same donor and incubated with Z-138 cells at an E:T ratio of 2:1 to characterize the cytotoxicity of MC10029 CAR-T cells. (Statistical analysis, Supplementary Fig. 7d). b Granzyme B ELISA shows functional potency of MC10029 CAR-T cells against Z138 cells. Non-CAR-T cells or MC10029 CAR-T cells were co-incubated Z-138 cells at an E:T ratio of 4:1 for 72 h, when the supernatants were harvested for subsequent ELISA. Graphed data are means of quadruplicate sampling. The data are representative of three independent experiments. c Bioluminescent imaging catalogs the changes of luciferase-expressing Z-138 model (Z-138-Luc, 0.5 × 106 cells/mouse) in NSG mice challenged with the tumor cells followed by treatment with one of three treatments. Six days after tumor cell injection, tumor-bearing mice were randomized into three groups (N = 5 per group). On day seven, mice received single infusion treatment with either PBS (vehicle), Non-CAR-T cells (10 × 106 total T cells), or MC10029 CAR T-cells (2 × 106 CAR-T cells out of 10 × 10.6 total T cells). Tumor burden was quantified by bioluminescence intensity. The results shown are the representative of two independent experiments using different donor T cells. d The Kaplan–Meier plot shows overall survival versus days after Z-138 tumor challenge; log rank analysis identified statistical differences between the treatment groups. (**p < 0.01; ***p < 0.001)
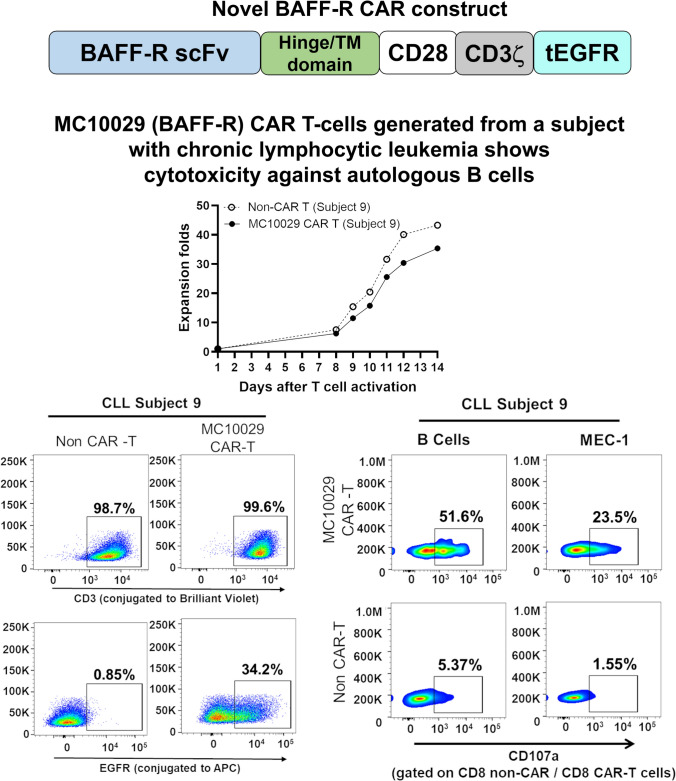
MC10029 CAR-T cells offer a promising option for chronic lymphocytic leukemia (CLL)
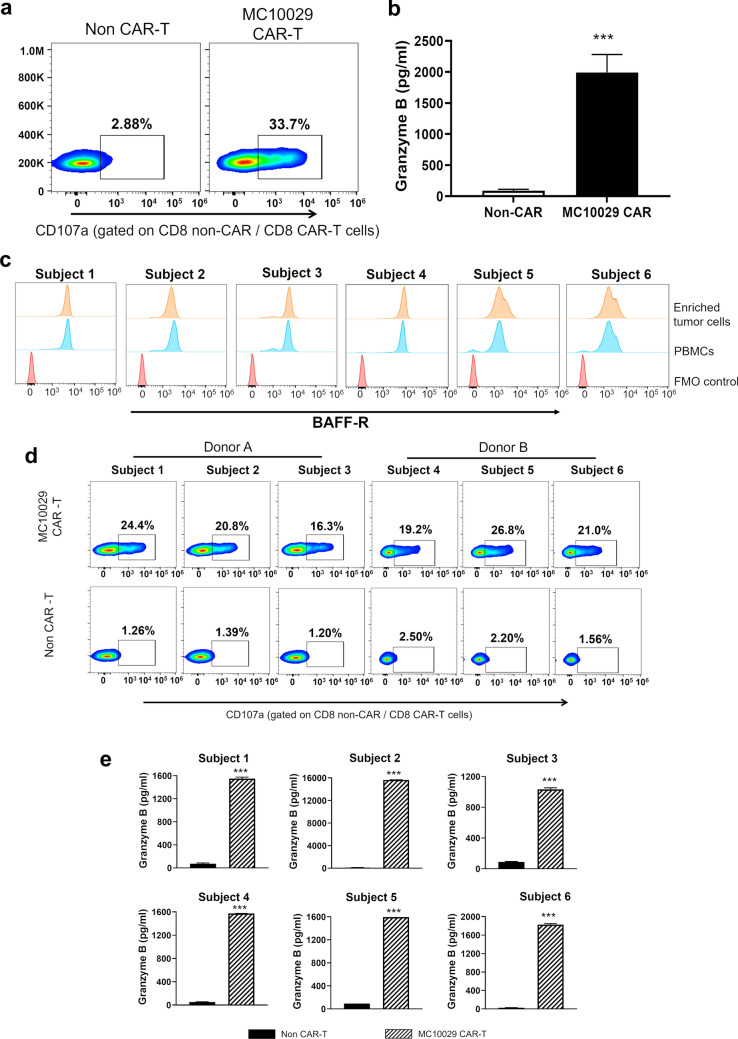
CLL stands out as a significant unmet need in the realm of CAR-T cell therapy for B-cell hematological malignancies. Although CD19 CAR-T cell therapies have been pursued for CLL patients, complete remissions have not matched those reported for ALL or diseases like FL and MCL [7–9, 22]. Considering the high expression of BAFF-R in CLL, we explored the potential of BAFF-R-targeting MC10029 CAR-T therapy as an alternative option for CLL patients. MEC-1, a CLL cell line, was first utilized to investigate the cytotoxicity of MC10029 CAR-T cells against CLL. The effectiveness of MC10029 CAR-T cells against MEC-1 cells was confirmed through granule degranulation, as shown in Fig. 4a and supplementary Fig.7e. Furthermore, the release of granzyme B in response to the tumor cells provides additional evidence supporting the efficacy of MC10029 CAR-T cells in targeting and inducing cytotoxic effects on MEC-1 cells (Fig. 4b).
Fig. 4.
Targeting chronic lymphocytic leukemia (CLL) with MC10029 CAR-T cells. a Flow cytometry contour plots show the functional potency of CAR-T cells against target cells by the surface expression CD107a in a degranulation assay. Non-CAR-T cells and MC10029 CAR-T cells were generated from the same donor and incubated with MEC-1 cells at an E:T ratio of 2:1 to characterize the cytotoxicity of MC10029 CAR-T cells. (Statistical analysis, Supplementary Fig. 7e). b A granzyme B ELISA shows functional potency of MC10029 CAR-T cells against MEC-1 cells. Non-CAR-T cells or MC10029 CAR-T cells were co-incubated MEC-1 cells at an E:T ratio of 4:1 for 72 h; the supernatants were then harvested for subsequent ELISA. Graphed data are means of quadruplicate sampling. The data are representative of three independent experiments. c Flow cytometry shows the cell surface expression of BAFF-R on enriched tumor cells and PBMCs that were collected from six subjects with CLL. d MC10029 CAR-T cells derived from two different healthy donors were incubated with the enriched primary B-cell tumor cells at an E:T ratio of 2:1. Using a CD107a degranulation assay, the cytotoxic activity of MC10029 CAR-T cells was visualized using flow cytometry. Non-CAR T cells from the same donor were used as a negative control. e The release of granzyme B confirms the functional potency of MC10029 CAR-T cells against primary CLL tumor cells. MC10029 CAR-T cells or non-CAR-T cells were co-incubated with enriched primary CLL tumor cells (E:T ratio of 4:1) for 72 h, after which the supernatants were harvested for subsequent ELISA. Graphed data are means of quadruplicate sampling. (***p < 0.001)
We next assessed our MC10029 CAR-T cells against primary B-cells isolated from subjects with CLL. The selected subjects were three males and three females with an age range of 56 to 83 years. (Supplementary Fig. 5a, Identifiers 1–6) B-cells were enriched from PBMCs of each subject, and BAFF-R expression was confirmed (Fig. 4c); B-cell enrichment effectively removed endogenous T cells (Supplementary Fig. 5b). In our initial studies, two batches of MC10029 CAR-T cells and non-CAR-T cells were engineered from T cells isolated from two healthy donors. These batches were shown to be identical in viability, identity, and potency. (Supplementary Fig. 6a–c).
We observed the activation of CD8 + MC10029 CAR-T cells was comparable across the six primary CLL tumor cells following incubation (Fig. 4d, top panels and Supplementary Fig. 5c). Furthermore, incubation of MC10029 CAR-T cells with CLL tumor cells resulted in the release of granzyme B (Fig. 4e). Four of the CLL subjects provided sufficient B-cells that could be evaluated for the release of additional granule proteins. Granzyme A, perforin, and IFN-γ were released in statistically significant amounts when the MC10029 CAR-T cells were incubated with primary CLL tumor cells. (Supplementary Fig. 5d).
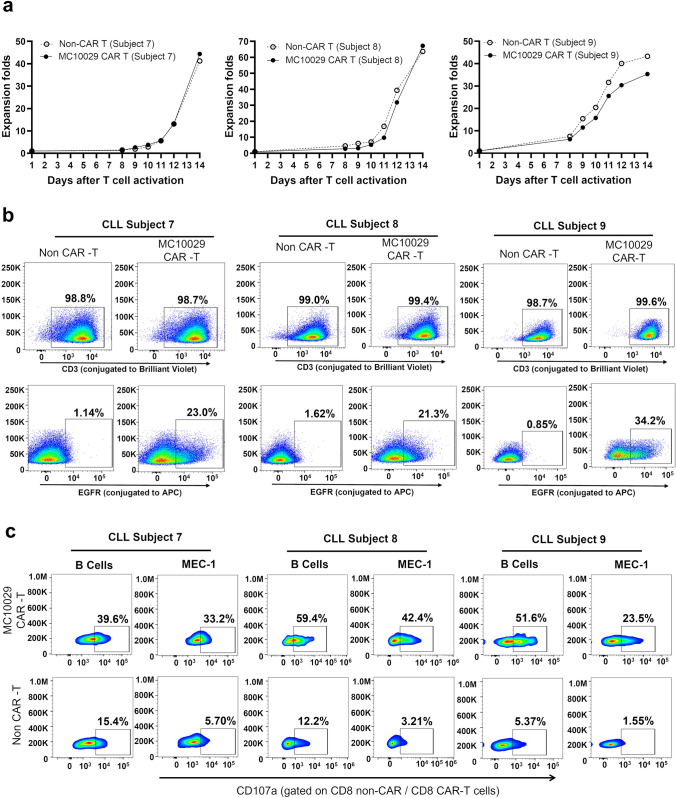
To model the efficacy of MC10029 CAR-T cells more realistically as a clinical therapeutic, we identified three additional CLL subjects (Supplementary Fig. 5a, Identifier 7– 9) to generate subject-derived MC10029 CAR-T cells for incubation with autologous B-cells. The MC10029 CAR-T cells that were engineered from three CLL subjects were characterized by monitoring fold expansion (> 25; Fig. 5a), identity (> 80% CD3 positive cells; Fig. 5b, top panels), and potency (> 10% EGFR positive cells; Fig. 5b, bottom panels). These MC10029 CAR-T cells and their corresponding non-CAR-T cells (negative control) were then incubated with either the matching autologous B-cells or MEC-1 cells (positive control). The cytotoxicity of the subject-derived MC10029 CAR-T cells against autologous tumor cells was confirmed with a CD107a degranulation assay on CD8 (Fig. 5c). Non-specific activity is consistently observed when subject-derived Non-CAR-T cells are incubated with target cells, especially autologous B-cells.
Fig. 5.
Subject-derived MC10029 CAR-T cells elicited ex vivo cytotoxicity against autologous CLL tumors. a The growth curves of MC10029 CAR-T cells and their corresponding Non-CAR-T cells, both derived from T cells isolated from the peripheral blood of three CLL subjects, were plotted side by side to compare their growth patterns. b The subject-derived MC10029 CAR-T cells were assessed for their identity (> 80% CD3 positive cells) and potency (> 10% EGFR positive cells). c. The cytotoxicity of MC10029 CAR-T cells derived from the three subjects with CLL was evaluated against autologous tumor cells using a CD107a degranulation assay. MEC-1 cells were employed as a positive control, while the corresponding Non-CAR-T cells served as a negative control. The analysis of cytotoxicity was gated on the CD8 + CAR-T cell populations
The readiness of advancing the MC10029 CAR-T cell therapy to the clinic
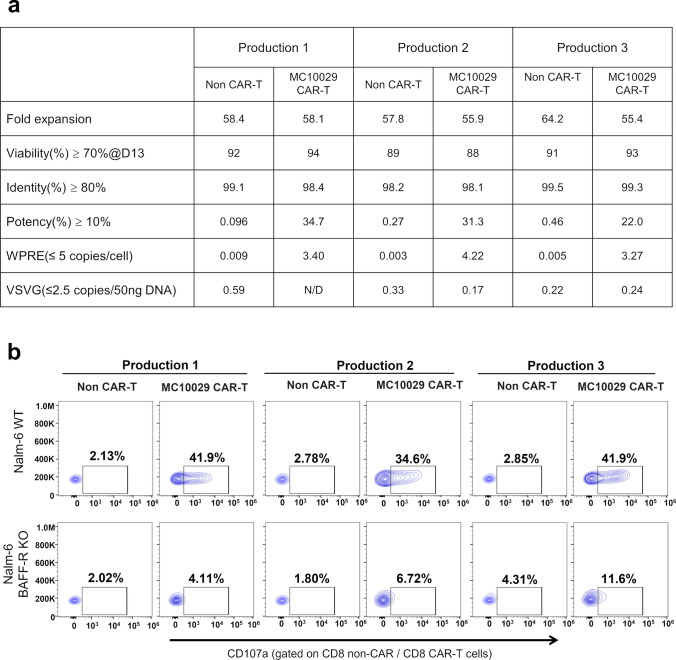
As part of our IND filing and in preparation for our Phase 1a/1b clinical trial, we have performed engineering runs to yield three production batches of CAR-T cells using a MC10029-expressing lentiviral vector that was produced under GMP conditions. We have established and validated a collection of assays that will be used as the process quality control (QC) and final product QC assays; these assays were performed on the laboratory generated production batches (Fig. 6a and b). The production batches of MC10029 CAR-T cells were evaluated for cell quality with Fold Expansion and Viability (> 70%, as determined by Muse Cell Analyzer); CAR-T cell specific characterization with Identity (> 70%, as determined by flow cytometry for CD3 positive cells) and Potency (> 10%, as determined by flow cytometry for EGFR positive T cells); and Safety from adventitious viral agents by determining lentiviral copy number by two methods (< 5 copies of Woodchuck Hepatitis Virus Posttranscriptional Regulatory Element (WPRE) per cell and < 5 copies/50 ng DNA of vesicular stomatitis virus G glycoprotein (VSVG; encodes envelope gene sequences) as detected via a real-time qPCR assay). All three production batches of MC10029 CAR-T cells met the required criteria to be qualified as a product (Fig. 6a). Furthermore, antigen-specific cytotoxicity of these MC10029 CAR-T cells was confirmed using our standard degranulation assay (Fig. 6b, Supplementary Fig. 7f). The successful qualification of these batches confirms their suitability for further clinical development.
Fig. 6.
Characterization of three qualification productions of clinical-grade MC10029 CAR-T cells. a Product release criteria for CAR-T cells. Three batches of MC10029 CAR-T cells were evaluated for cell quality with fold expansion (> 25) and viability (> 70%, as determined by Trypan Blue staining) as well as CAR-T cell specific characterization with identity (> 80%, as determined by flow cytometry for CD3 positive cells) and potency (> 10%, as determined by flow cytometry for EGFR (a transgene) positive T cells). The corresponding non-CAR-T cells were used as controls. To show that the lentiviral vector remains non-infectious and safe from adventitious viral agents, the lentiviral copy number (< 5 copies/per cell of WPRE) and VSVG (< 5 copies/50 ng DNA) were determined using a qPCR assay. b Flow cytometry contour plots of CAR-T cell functional potency as measured by a CD107a degranulation assay. MC10029 CAR-T cells (characterized in panel A) from three different healthy donors were incubated with WT Nalm-6 or BAFF-R KO Nalm-6 at an E:T ratio of 2:1. Analysis was gated on CD8 + CAR-T cell populations and showed no difference between the batches (Supplementary Fig. 7f). Non-transduced T cells (Non-CAR-T) from the same donor were used as negative controls
Discussion
Since their approval in 2017, CD19 CAR-T cell therapies have since widened their indications and numbers [2, 3]. CD19 CAR-T cell therapy has revolutionized the treatment of B-cell malignancies and has been a trailblazer for adoptive cell therapies as well as for developing strategies for treating the CAR-T cell toxicities, namely cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS). It is irrefutable that the collection of CD19 CAR-T cell therapies has been curative for many patients, has extended the lives of many other patients, and continues to change the treatment paradigm for B-cell malignancies.
The long-term surveillance of CD19 CAR-T cell-treated patients shows a reduction in overall survival (OS) after four years[23] and that not all B-cell malignancies respond to CD19 CAR-T cells with equal success. CD19 CAR-T cell therapy shows considerably lower complete remissions for patients with CLL[24], possibly related to the 72 year median age of CLL patients and the characteristic immunosuppression of this malignancy [25]. This highlights the need for alternative treatment strategies that include new target antigens as well as strategies to combat R/R disease.
The BAFF cytokine and its receptor, BAFF-R, form a critical pathway for the development, maturation, and survival of mature B-cell that results in the activation of the PI3K and NF-ĸB anti-apoptotic pathways [14, 26]. The BCR and BAFF-R signaling pathways share PI3K and NF-ĸB[26, 27], which aid in the selection and maturation of B-cell clones[13, 14]; when particular clones are expanded that has unrestricted growth, B-cell malignancies are the result [12]. In fact, BCR activation regulates the expression of BAFF-R; BAFF-R works in concert with BCR for the positive selection of B-cells during development, best exemplified in the production of auto-reactive B-cells in autoimmune disease [28]. BAFF-R targeting CAR-T cells have advanced into a Phase 1 clinical trial for patients with R/R B-cell ALL (NCT04690595 with the primary outcome of incidence of adverse events) and a Phase 1 clinical trial for patients with R/R B-cell NHL (NCT05370430 with the primary outcomes of incidence of adverse events and maximum tolerated dose). Interestingly, the literature reports only two cases of late-onset common variable immunodeficiency due to a loss of BAFF-R expression [26]. With the two clinical trials for BAFF-R CAR-T cell therapy either in early clinical trial phases or our trial that is scheduled to begin, the loss of BAFF-R antigen has yet to be observed within the context of antigen escape; however, the loss of antigen might be observed once BAFF-R CAR-T cell therapies have become more widely applied to an increased number of patients.
Like the CD19 CAR-T cell therapy, a second novel BAFF-R CAR-T cell therapy likely holds great potential for the benefit of patients. In this regard, we have successfully developed a new monoclonal antibody that targets BAFF-R and used its scFv to engineer a novel anti-BAFF-R CAR, MC10029 CAR. What further sets MC10029 CAR apart is its utilization of the CD28 costimulatory domain, distinguishing it from the first reported anti-BAFF-R CAR that is currently in clinical trials with a 4–1 BB costimulatory domain. We have validated the antigen-specific cytotoxicity of MC10029 CAR-T cells against multiple BAFF-R expressing cell lines and their corresponding BAFF-R KO cell lines in both in vitro and in vivo models, suggesting the broad utility of MC10029 CAR-T against B-cell malignancies.
The observation that the therapeutic efficacy of CD19 CAR-T cells in treating CLL was significantly lower compared to ALL (reported at 26% for CLL versus over 90% for ALL)[22] prompted us to focus our efforts on MC10029 CAR-T cells as a specific immunotherapy for CLL. We were further encouraged with the strong expression of BAFF-R in CLL [29]. Our initial evaluation of MC10029 CAR-T cells was conducted against CLL cell lines, followed by more challenging assessment using clinically derived samples. The evaluation of healthy donor MC10029 CAR-T cells against CLL subject-derived primary tumor cells demonstrated antigen-specific cytotoxicity and established our methodologies. To strengthen our hypothesis that MC10029 CAR-T cells are suitable to treat CLL, we designed an autologous assay of MC10029 CAR-T cells and primary tumor cells, both derived from the same CLL subject using a 50 ml peripheral blood collection. We show that T cells isolated from CLL patients can be engineered into MC10029 CAR-T cells having the comparable fold expansion, identity, and potency as those generated from healthy donors. Most importantly, these subject-derived MC10029 CAR-T cells show cytotoxic potency against autologous primary CLL cells, thereby providing convincing evidence for the translational significance and clinical applicability of MC10029 CAR-T cell therapy for future CLL patients.
We also noted that subject-derived non-CAR-T cells exhibited higher non-specific cytotoxicity against autologous target cells compared to non-specific cytotoxicity that we typically see with healthy donor-derived non-CAR-T cells. This may suggest that these subjects have specific tumor reactive T cell populations that have been expanded in the manufacturing process; these tumor reactive T cell populations also appear subject specific in quantity and reactivity to target cells.
With these convincing data, we filed an IND for MC10029 CAR-T cells, which has received approval. We are advancing to our next goal of a Phase 1a/1b clinical trial for MC10029 CAR-T cells with patients with R/R CLL and B-cell NHL. We believe MC10029 CAR-T cells are a therapeutic that can have a role in the current onco-hematological treatment regimens for B-cell lymphoid malignancies, specifically CLL. Our future work includes designing dual or multiantigen CAR-T cells as well as armored CAR-T cells with BAFF-R as the foundation. These next generation CAR designs remain active avenues of therapeutic development to combat lymphoid malignancies.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank the Animal Resource Center at Mayo Clinic Florida for daily care of mice used in this study and the flow cytometry facilities at Mayo Clinic Florida. The authors thank the MD Anderson Antibody Core Facility for their efforts to generate BAFF-R monoclonal antibodies.
Abbreviations
- ALL
Acute lymphocytic leukemia
- BAFF-R
B-cell activating factor receptor
- BCR
B-cell receptor
- CAR
Chimeric antigen receptor
- CLL
Chronic lymphocytic leukemia
- CR
Complete remission
- DLBCL
Diffuse large B-cell lymphoma
- EGFR
Epidermal growth factor receptor
- E:T
Effector to target cell
- FL
Follicular lymphoma
- GFP
Green fluorescent protein
- GMP
Good manufacturing practices
- IV
Intravenous
- KO
Knock out
- MCL
Mantle cell lymphoma
- MOI
Multiplicity of infection
- NHL
Non-Hodgkin lymphoma
- NSG
NOD scid gamma
- PBMCs
Peripheral blood mononuclear cells
- PBS
Phosphate-buffered saline
- scFv
Single-chain variable fragment
- R/R
Relapsed and/or refractory
- VSVG
Vesicular stomatitis virus G
- WPRE
Woodchuck hepatitis virus post-transcriptional regulatory element
- WT
Wild type
Author contributions
HQ and YL designed the project and studies. YL, YQ, AM, RR-V, SL and TT performed experiments and analyzed data. FY, HSM, and MAK-D organized subject selection and sample collection. MEG, HQ and YL contributed to manuscript writing. MEG generated final figures and participated in the final review with RD, HSM, MAK-D, and HQ. MAK-D and HQ supervised the entire project.
Funding
We would like to acknowledge the generous funding support to HQ, which includes the Florida Health Grant (#MOG07, SB2500) and the Mayo Clinic Florida CAR-T Manufacturing Program Fund. HQ and MA K-D were also supported by the Fred C. and Katherine B. Andersen Foundation, Mayo Clinic Cancer Center, and the Mayo Clinic President's Discovery Translation Program Award.
Declarations
Conflict of interest
HQ has equity with Pepromene Bio, Inc. and Innolifes Inc. The other authors have no competing interests.
Consent to participate
Informed consent was implemented for sample collection.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yan Luo and Yaqing Qie have equally contributed to the project.
Contributor Information
Mohamed A. Kharfan-Dabaja, Email: KharfanDabaja.Mohamed@mayo.edu
Hong Qin, Email: Qin.Hong@mayo.edu.
References
- 1.Wang K, Wei G, Liu D. CD19: a biomarker for B cell development, lymphoma diagnosis and therapy. Exp Hematol Oncol. 2012;1:36. doi: 10.1186/2162-3619-1-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377:2531–2544. doi: 10.1056/NEJMoa1707447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380:45–56. doi: 10.1056/NEJMoa1804980. [DOI] [PubMed] [Google Scholar]
- 4.Abramson JS, Palomba ML, Gordon LI, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396:839–852. doi: 10.1016/s0140-6736(20)31366-0. [DOI] [PubMed] [Google Scholar]
- 5.Locke FL, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel as second-line therapy for large B-cell lymphoma. N Engl J Med. 2022;386:640–654. doi: 10.1056/NEJMoa2116133. [DOI] [PubMed] [Google Scholar]
- 6.Kamdar M, Solomon SR, Arnason J, et al. Lisocabtagene maraleucel versus standard of care with salvage chemotherapy followed by autologous stem cell transplantation as second-line treatment in patients with relapsed or refractory large B-cell lymphoma (TRANSFORM): results from an interim analysis of an open-label, randomised, phase 3 trial. Lancet. 2022;399:2294–2308. doi: 10.1016/s0140-6736(22)00662-6. [DOI] [PubMed] [Google Scholar]
- 7.Wang M, Munoz J, Goy A, et al. KTE-X19 CAR T-Cell Therapy in Relapsed or Refractory Mantle-Cell Lymphoma. N Engl J Med. 2020;382:1331–1342. doi: 10.1056/NEJMoa1914347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacobson CA, Chavez JC, Sehgal AR, et al. Axicabtagene ciloleucel in relapsed or refractory indolent non-Hodgkin lymphoma (ZUMA-5): a single-arm, multicentre, phase 2 trial. Lancet Oncol. 2022;23:91–103. doi: 10.1016/s1470-2045(21)00591-x. [DOI] [PubMed] [Google Scholar]
- 9.Fowler NH, Dickinson M, Dreyling M, et al. Tisagenlecleucel in adult relapsed or refractory follicular lymphoma: the phase 2 ELARA trial. Nat Med. 2022;28:325–332. doi: 10.1038/s41591-021-01622-0. [DOI] [PubMed] [Google Scholar]
- 10.Todorovic Z, Todorovic D, Markovic V, et al. CAR T cell therapy for chronic lymphocytic leukemia: successes and shortcomings. Curr Oncol. 2022;29:3647–3657. doi: 10.3390/curroncol29050293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thompson JS, Bixler SA, Qian F, et al. BAFF-R, a newly identified TNF receptor that specifically interacts with BAFF. Science. 2001;293:2108–2111. doi: 10.1126/science.1061965. [DOI] [PubMed] [Google Scholar]
- 12.Parameswaran R, Lim M, Fei F, et al. Effector-mediated eradication of precursor B acute lymphoblastic leukemia with a novel Fc-engineered monoclonal antibody targeting the BAFF-R. Mol Cancer Ther. 2014;13:1567–1577. doi: 10.1158/1535-7163.MCT-13-1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khan WN. B cell receptor and BAFF receptor signaling regulation of B cell homeostasis. J Immunol. 2009;183:3561–3567. doi: 10.4049/jimmunol.0800933. [DOI] [PubMed] [Google Scholar]
- 14.Rowland SL, Leahy KF, Halverson R, Torres RM, Pelanda R. BAFF receptor signaling aids the differentiation of immature B cells into transitional B cells following tonic BCR signaling. J Immunol. 2010;185:4570–4581. doi: 10.4049/jimmunol.1001708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodig SJ, Shahsafaei A, Li B, Mackay CR, Dorfman DM. BAFF-R, the major B cell-activating factor receptor, is expressed on most mature B cells and B-cell lymphoproliferative disorders. Hum Pathol. 2005;36:1113–1119. doi: 10.1016/j.humpath.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 16.Qin H, Dong Z, Wang X, et al. CAR T cells targeting BAFF-R can overcome CD19 antigen loss in B cell malignancies. Sci Transl Med. 2019;11:eaaw9414. doi: 10.1126/scitranslmed.aaw9414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dietz AB, Bulur PA, Emery RL, Winters JL, Epps DE, Zubair AC, Vuk-Pavlović S. A novel source of viable peripheral blood mononuclear cells from leukoreduction system chambers. Transfusion. 2006;46:2083–2089. doi: 10.1111/j.1537-2995.2006.01033.x. [DOI] [PubMed] [Google Scholar]
- 18.Dong Z, Cheng WA, Smith DL, et al. Antitumor efficacy of BAFF-R targeting CAR T cells manufactured under clinic-ready conditions. Cancer Immunol Immunother. 2020;69:2139–2145. doi: 10.1007/s00262-020-02614-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hildebrand JM, Luo Z, Manske MK, et al. A BAFF-R mutation associated with non-Hodgkin lymphoma alters TRAF recruitment and reveals new insights into BAFF-R signaling. J Exp Med. 2010;207:2569–2579. doi: 10.1084/jem.20100857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Novak AJ, Grote DM, Stenson M, et al. Expression of BLyS and its receptors in B-cell non-Hodgkin lymphoma: correlation with disease activity and patient outcome. Blood. 2004;104:2247–2253. doi: 10.1182/blood-2004-02-0762. [DOI] [PubMed] [Google Scholar]
- 21.Takahata H, Ohara N, Ichimura K, et al. BAFF-R is expressed on B-cell lymphomas depending on their origin, and is related to proliferation index of nodal diffuse large B-cell lymphomas. J Clin Exp Hematop. 2010;50:121–127. doi: 10.3960/jslrt.50.121. [DOI] [PubMed] [Google Scholar]
- 22.Fraietta JA, Lacey SF, Orlando EJ, et al. Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat Med. 2018;24:563–571. doi: 10.1038/s41591-018-0010-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jacobson CA. Highlights in CAR T-cell therapy from the 62nd American Society of Hematology Annual Meeting and Exposition. Clin Adv Hematol Oncol. 2021;19:1–24. [PubMed] [Google Scholar]
- 24.Roessner PM, Seiffert M. T-cells in chronic lymphocytic leukemia: Guardians or drivers of disease? Leukemia. 2020;34:2012–2024. doi: 10.1038/s41375-020-0873-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lemal R, Tournilhac O. State-of-the-art for CAR T-cell therapy for chronic lymphocytic leukemia in 2019. J Immunother Cancer. 2019;7:202. doi: 10.1186/s40425-019-0686-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smulski CR, Eibel H. BAFF and BAFF-receptor in B cell selection and survival. Front Immunol. 2018;9:2285. doi: 10.3389/fimmu.2018.02285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boothby MR, Hodges E, Thomas JW. Molecular regulation of peripheral B cells and their progeny in immunity. Genes Dev. 2019;33:26–48. doi: 10.1101/gad.320192.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tussiwand R, Rauch M, Flück LA, Rolink AG. BAFF-R expression correlates with positive selection of immature B cells. Eur J Immunol. 2012;42:206–216. doi: 10.1002/eji.201141957. [DOI] [PubMed] [Google Scholar]
- 29.Darwiche W, Gubler B, Marolleau JP, Ghamlouch H. Chronic lymphocytic leukemia B-cell normal cellular counterpart: clues from a functional perspective. Front Immunol. 2018;9:683. doi: 10.3389/fimmu.2018.00683. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.