Abstract
Objective
High body mass index (BMI) greater than 25 kg/m2 has a complex relationship with cancers. The aim of this systematic review and meta-analysis is to explore controversy over whether BMI is correlated with outcomes including survival and immunotherapy-related adverse events (irAEs) in cancer patients treated with immunotherapy.
Methods
We searched PubMed, Embase, Web of Science, and The Cochrane Library for relevant studies published up to June 2020. Title/abstract screening, full-text review, data extraction, and quality assessment were performed independently. Subgroup analysis was based on sex, treatment lines, the status of programmed death-ligand 1 (PD-L1), and tumor types. Sensitivity analysis was performed by synthesizing studies that adjusted for certain covariates or studies with good quality. Statistical heterogeneity was evaluated by the I2 value. Meta-analysis was performed with hazard ratio (HR) / odds ratio (OR) and 95% confidence intervals (CIs) as the effect measures.
Results
Twenty studies were included for survival and irAEs analyses. Patients with high BMI who underwent immunotherapy had longer overall survival (OS) (pooled hazard ratio, pHR = 0.71 [95% CI: 0.59–0.85]) and progression-free survival (PFS) (pHR = 0.76 [95% CI: 0.65–0.88]) than those with low BMI; at the same time, high-BMI patients had increased irAEs (OR = 2.54 [95% CI: 1.12–5.79]).
Conclusion
In general, high BMI was correlated with improved OS and PFS in patients treated with immunotherapy along with a high risk of irAEs. However, discrepant findings from subgroup analyses urgently call for further analysis.
Supplementary Information
The online version of this article (10.1007/s00262-021-02858-y) contains supplementary material, which is available to authorized users.
Keywords: BMI, Immunotherapy, Cancers, Meta-analysis, Adverse effects
Introduction
Numerous population-based studies have demonstrated that occurrence and progression of tumors are related to BMI, especially in breast cancer and colorectal cancer [1–3]. The correlation of BMI and clinical outcomes in advanced cancer patients has been investigated as well, however, without conclusive results [4–6]. Recent clinical studies have demonstrated that high BMI is associated with improved response and survival in cancer patients treated with targeted therapy and immunotherapy, but not with chemotherapy [4, 7]. Though immune checkpoint inhibitors (ICIs) such as anti-programmed death-1 (PD-1) and PD-L1 antibodies have dramatically improved survival in various cancers [8, 9], how to identify the small proportion of patients who will benefit from immunotherapy is the key challenge because many attempts have failed. Several multicenter studies have reported that patients with high BMI benefit more from ICIs treatment in solid malignant tumors, including non-small cell lung cancer (NSCLC), melanoma, and renal cell carcinoma (RCC) [10, 11]. Conversely, a retrospective multicohort analysis has reported that BMI is not associated with improved OS and PFS in immunotherapy in metastatic melanoma [12]. Moreover, a pooled analysis of 16 articles including 4090 cancer patients has shown that BMI ≥ 30 is associated with better outcomes in cancer patients treated with ICIs [13]. Since immunotherapy was first introduced, only two individual pooled analyses and a meta-analysis have focused on BMI. Based on the limited data available so far, it appears that the correlation between BMI and immunotherapeutic benefit may differ by tumor types. Besides the benefit, the correlation of BMI and irAEs has been reported in few studies recently, however, with different conclusion. The proliferation of immunotherapeutic studies involving more cancer patients and a wider spectrum of cancers provides an opportunity to confirm the correlation of BMI with survival benefits and irAEs in general and also possibly to investigate the precise relationship in subgroups of patients.
In this systematic review and meta-analysis, we explore the prognostic value of BMI in cancers treated with immunotherapy grouped by sex, treatment lines, the status of PD-L1, tumor types. Similarly, we examine the association between BMI and irAEs.
Methods
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines to report our meta-analysis[14].
Literature search
We systematically conducted an independent review of the PubMed, Embase, Web of Science, and The Cochrane Library databases on clinical trials in English. The search strategy is outlined in Supplemental Table 1. A supplementary search of the Web of Science, Embase, and The Cochrane Library databases was also performed to ensure that no additional studies were overlooked.
Eligibility criteria
The inclusion criteria were: (1) BMI and immunotherapy data; (2) study outcomes were OS, PFS, and irAEs; (3) clinical trials; (4) the effect estimates and corresponding 95% confidence intervals (CIs) were reported directly or could be calculated indirectly from published data. The references of relevant reports were also reviewed manually. If more than one publication was found for the same trial, the most recent, complete, and updated version was included in the final analysis. Subgroup analyses for survival were conducted according to tumor types, sex, treatment lines, and the status of PD-L1. The principal exclusion criteria were overlapping publications, lack of relevant outcome data; similarly, preliminary data not yet reported were not included. The flow diagram of eligible studies is shown in Fig. 1.
Fig. 1.
Flow diagram of identifying eligible studies
Data extraction and quality assessment
Data were extracted from the eligible studies included according to the PRISMA statement: (1) study characteristics (first author, year of publication, total sample), BMI cutoff value, OS, PFS and irAEs, HRs for PFS, OS and OR for irAEs with the relative 95% CI; (2) tumor types, sex, treatment lines, and the status of PD-L1. The quality of the included studies was assessed according to Newcastle–Ottawa Scale criteria [15].
Statistical analysis
Statistical analyses were carried out using the statistical package STATA (v.14.0). We used HRs to summarize the association between BMI and immunotherapy benefit, simultaneously, OR was applied to summarize the association between BMI and irAEs. If a study did not report the HR and its 95% CI directly, they were calculated from the available data. Statistical heterogeneity in the results between studies included in the meta-analysis was examined using Cochrane’s Q statistic, and inconsistency was quantified with the I2 statistic [100% × (Q − df)/Q], which estimates the percentage of total variation across studies due to heterogeneity rather than chance. P < 0.10 for the Q statistic and/or I2 > 50% were considered to show statistically significant heterogeneity. Summary HRs were calculated using random-effects (RE) or fixed-effects (FE) models depending on the heterogeneity of the included studies (RE model when I2 > 50% and FE model when I2 ≤ 50%). An overall analysis was conducted by evaluating all relevant studies. Simultaneously, funnel plots were constructed to highlight outlying studies and to examine publication bias. Forest plots were used to summarize and visualize the HR or OR with 95% CIs for each study and for the aggregated estimates from the RE or FE models.
Results
Search results and patient characteristics
There were 771 potentially relevant publications identified in this study. In the end, twenty studies were included for survival [7, 10–12, 16–29] and irAEs [7, 10, 12, 19, 30, 31] analysis. Descriptive characteristics were shown in Table 1. The primary cancers were melanoma, lung cancer, and renal cell carcinoma. Most of the patients were from the USA. The common ICIs were nivolumab, pembrolizumab, and atezolizumab. BMI cutoff value of most articles was 25 kg/m2.
Table 1.
Baseline characteristics of included retrospective studies
| Author | Year | Total sample | Male % |
Median age | Cancer types | Treatment | Region | BMI cutoff value | Primary outcomes |
|---|---|---|---|---|---|---|---|---|---|
| Cortellini [10] | 2019 | 976 | 663(67.93) | 68 | Multiple cancers | Pembrolizumab, nivolumab, or atezolizumab | Europe | 25 | OS, PFS, irAEs |
| Gomes [16] | 2017 | 187 | 108(57.75) | 58 | Metastatic melanoma | Ipilimumab | South America | 25 | PFS |
| Kichenadasse [11] | 2019 | 1434 | 890(62.06) | 64 | Advanced NSCLC | Atezolizumab | Multiple region | 25,30 | OS, PFS |
| Zhi [17] | 2018 | 703 | NA | NA | Advanced NSCLC | Nivolumab or pembrolizumab | North America | 25,30 | OS |
| McQuade 1 [7] | 2018 | 207 | 138(66.67) | NA | Metastatic melanoma | Ipilimumab plus dacarbazine | North America | 25,30 | OS, PFS, irAEs |
| McQuade 2 [7] | 2018 | 329 | 213(64.74) | NA | Metastatic melanoma | Pembrolizumab, nivolumab or atezolizumab | North America | 25,30 | OS, PFS, irAEs |
| Richtig [12] | 2018 | 76 | 46(60.53) | NA | Metastatic melanoma | Ipilimumab | Australia | 25 | OS, PFS, irAEs |
| Labomascus [18] | 2018 | 162 | 65(40.12) | 68 | Advanced NSCLC | Nivolumab or pembrolizumab | North America | 24.69 | OS |
| Dumenil [19] | 2018 | 67 | 46(68.66) | 68.5 | Advanced NSCLC | Nivolumab | Europe | 18.5 | OS, PFS, irAEs |
| Dizman [20] | 2018 | 235 | 172(73.19) | 65 | Advanced RCC | Immunotherapy | North America | 25 | OS |
| Ibrahimi [21] | 2018 | 198 | NA | 62 | Multiple cancers | Immunotherapy | North America | 30 | OS, PFS |
| Lalani [22] | 2019 | 147 | 104(70.75) | NA | Advanced RCC | Immunotherapy | North America | 25 | OS |
| Wang [23] | 2019 | 250 | 114(45.60) | 61.7 | Multiple cancers | Immunotherapy | North America | 30 | OS, PFS |
| Kondo [24] | 2018 | 39 | 24(61.54) | 65 | Metastatic melanoma | Nivolumab | Asia | 20 | PFS |
| Taniguchi [25] | 2017 | 201 | 135(67.16) | 68 | Advanced NSCLC | Nivolumab | Asia | 20 | PFS |
| Shiroyama [26] | 2018 | 201 | 135(67.16) | 68 | Advanced NSCLC | Nivolumab | Asia | 18.5 | PFS |
| Bergerot [27] | 2019 | 42 | 28(66.67) | NA | Advanced RCC | Nivolumab, atezolizumab, or avelumab | North America | 25 | OS |
| Ichihara 1 [28] | 2020 | 84 | 68(80.95) | 71 | Advanced NSCLC | pembrolizumab | Asia | 22 | OS, PFS |
| Ichihara 2 [28] | 2020 | 429 | 338(78.79) | 69 | Advanced NSCLC | Pembrolizumab, nivolumab or atezolizumab | Asia | 22 | OS, PFS |
| Sanchez [29] | 2019 | 203 | 151 (74.38) | 62 | Advanced RCC | Immunotherapy | North America | 30 | OS |
| Cortellini [30] | 2020 | 1070 | 724(67.66) | 68 | Multiple cancers | Immunotherapy | Europe | 25 | irAEs |
| Valentine [31] | 2017 | 32 | NA | NA | Metastatic melanoma | Pembrolizumab or nivolumab | Europe | 25 | irAEs |
Multiple cancers refer to NSCLC, melanoma, RCC, and others. The included articles of McQuade and Ichihara contain two cohorts, which labeled Author 1 and Author 2 in different rows of Table. NSCLC non-small cell lung cancer, RCC renal cell carcinoma, irAEs immunotherapy-related adverse events, NA not applicable
Primary outcome
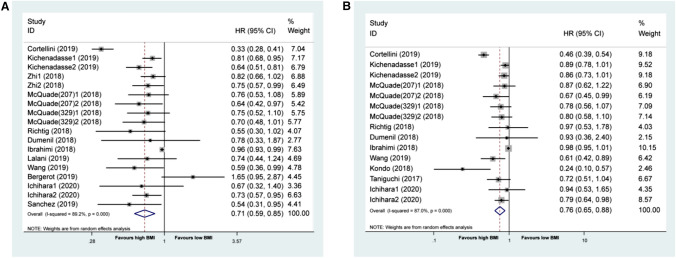
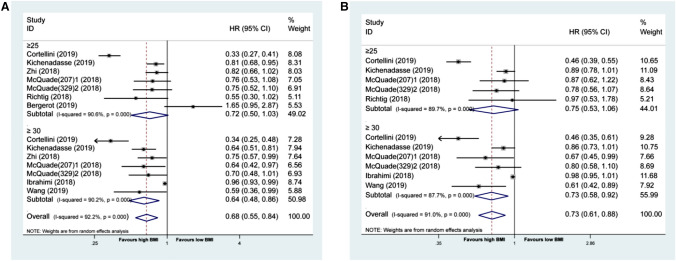
When these outcomes were analyzed according to BMI (the high or low BMI cutoff value was referenced to the article showed in Table 1), patients with high BMI who underwent immunotherapy had longer OS (pHR = 0.71 [95% CI: 0.59–0.85]) and longer PFS (pHR = 0.76 [95% CI: 0.65–0.88]) than those with low BMI (Fig. 2a, b). The χ2 test for study heterogeneity was significant (P < 0.001), suggesting that the reported results of the individual trials differ substantially. When we divided the population in the high BMI group into BMI ≥ 25 and BMI ≥ 30, respectively, we found the pHRs were 0.64 (95% CI: 0.48–0.86, P = 0.003) for OS and 0.73 (95% CI: 0.58–0.92, P = 0.007) for PFS in BMI ≥ 30 group. The pHRs were 0.72 (95% CI: 0.50–1.03, P = 0.069) for OS and 0.75 (95% CI: 0.53–1.06, P = 0.101) for PFS in BMI ≥ 25 group (Fig. 3a, b). Thus, its apparent BMI ≥ 30 benefited more from ICIs.
Fig. 2.
Association between BMI and prognosis in cancer patients treated with ICIs. a. Forest plot for association between BMI and OS in cancer patients treated with ICIs. b. Forest plot for association between BMI and PFS in cancer patients treated with ICIs. BMI body mass index, OS overall survival, PFS progression free survival, ICIs immune checkpoint inhibitors. The included articles of Kichenadasse, Zhi, McQuade, and Ichihara contain different cohorts and/or different BMI cutoff value, which labeled Author 1, Author 2 and even Author(sample) 1, Author(sample) 2. Below is the same as above
Fig. 3.
Association between BMI and prognosis in high BMI cancer patients treated with ICIs. a. Forest plot for association between BMI and OS in cancer patients treated with ICIs, stratified by BMI ≥ 25 and BMI ≥ 30. b. Forest plot for association between BMI and PFS in cancer patients treated with ICIs, stratified by BMI ≥ 25 and BMI ≥ 30
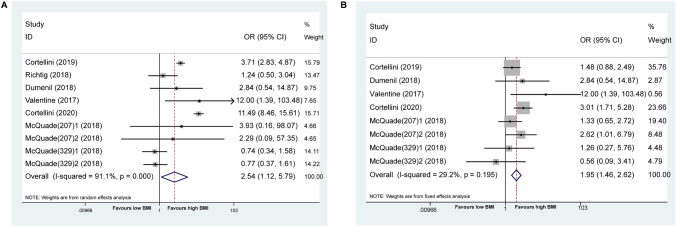
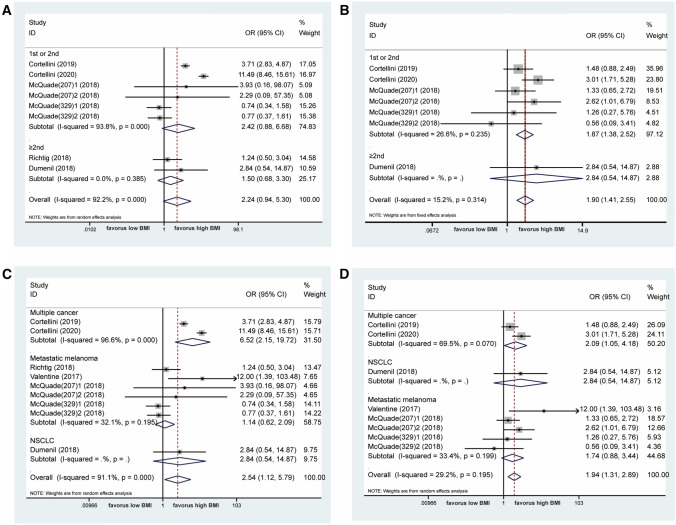
At the same time, as shown in Fig. 4a, the patients with BMI ≥ 25 experienced a higher risk of any grade of irAEs compared to those with BMI < 25 (OR = 2.54 [95% CI: 1.12–5.79], I2 = 91.1%, P = 0.026). The comparable results were seen in G3/G4 irAEs (OR = 1.95 [95% CI: 1.46–2.62], I2 = 29.2%, P < 0.001) (Fig. 4b). Of note, cancer patients with high BMI were inclined to have better OS and PFS from immunotherapy, while simultaneously exhibiting a higher risk of adverse events.
Fig. 4.
Association between BMI and irAEs in cancer patients treated with ICIs. a. Forest plot for association between BMI and any grade of irAEs treated with ICIs. b. Forest plot for association between BMI and G3/G4 irAEs treated with ICIs. irAEs immunotherapy-related adverse events
Subgroup analysis
Sex, treatment lines, the status of PD-L1, and tumor types were chosen for subgroup analysis with the aim of finding who could obtain a survival benefit in the high BMI group and analyzing the source of heterogeneity. As shown in this study, men with high BMI were more likely to get an OS benefit from immunotherapy (pHR = 0.60 [95% CI: 0.45–0.81], p = 0.001) than were women (pHR = 0.69 [95% CI: 0.46–1.06], p = 0.09), as well as for PFS (pHR = 0.62 [95% CI: 0.49–0.78, p < 0.001] vs pHR = 0.86 [95% CI: 0.51–1.44], p = 0.566, respectively), as shown in Fig. 5a and b. The overall compared result was p < 0.001 for OS and p = 0.004 for PFS. ICIs in second or subsequent line could produce longer OS (pHR = 0.71 [95% CI: 0.62–0.82], p < 0.001) than first or second line (pHR = 0.68 [95% CI: 0.46–1.00], p = 0.05 for OS), as shown in Fig. 5c. In terms of PFS, both of ≥ 2nd (pHR = 0.79 [95% CI: 0.70–0.89], p < 0.001) and first or second (pHR = 0.65 [95% CI: 0.48–0.90], p = 0.008) could benefit from immunotherapy regardless of BMI, as shown in Fig. 5d. We found an improvement in survival of patients with high BMI in advanced NSCLC (OS: pHR = 0.76 [95% CI: 0.69–0.83], PFS: pHR = 0.85 [95% CI: 0.78–0.93]) and metastatic melanoma (OS: pHR = 0.70 [95% CI: 0.58–0.84], PFS: pHR = 0.75 [95% CI: 0.60–0.93]), but not RCC (OS: pHR = 0.87 [95% CI: 0.46–1.46]), as shown in Fig. 5e, f. When we examined BMI and PD-L1 status together, we found that patients with both high BMI and positive PD-L1 had longer OS (pHR = 0.62 [95% CI: 0.45–0.84]) and longer PFS (pHR = 0.83 [95% CI: 0.73–0.95]), as shown in Fig. 5g, h.
Fig. 5.
Subgroup analyses of the relationship between BMI and prognosis in ICIs treated cancer patients. a. Forest plot for association between BMI and OS in cancer patients treated with ICIs, stratified by sex. b. Forest plot for association between BMI and PFS in cancer patients treated with ICIs, stratified by sex. c. Forest plot for association between BMI and OS in cancer patients treated with ICIs, stratified by treatment lines. d. Forest plot for association between BMI and PFS in cancer patients treated with ICIs, stratified by treatment lines. e. Forest plot for association between BMI and OS in cancer patients treated with ICIs stratified by tumor types. f. Forest plot for association between BMI and PFS in cancer patients treated with ICIs stratified by tumor types. g. Forest plot for association between BMI and OS in cancer patients treated with ICIs stratified by PD-L1 status. h. Forest plot for association between BMI and PFS in cancer patients treated with ICIs stratified by PD-L1 status
Because few studies reported data relevant to the relationship between BMI and irAEs, only treatment lines and tumor types were chosen for subgroup analysis. In general, the incidence of any grade of irAEs was independent of BMI for subgroups defined by the first or second treatment line (OR = 2.42 [95% CI: 0.88–6.68]) and by ≥ 2nd line (OR = 1.50 [95% CI: 0.68–3.30]). However, first or second line immunotherapy had a high risk of G3/G4 irAEs in the high BMI group (OR = 1.87 [95% CI: 1.38–2.52]) but not for ≥ 2nd line (OR = 2.84 [95% CI:0.0.54–14.87]), as shown in Fig. 6a, b. For the tumor types, there was no difference in metastatic melanoma for any grade of irAEs (OR = 1.14 [95% CI: 0.62–2.09]) and for G3/G4 irAEs (OR = 1.74 [95% CI: 0.88–3.44]), as shown in Fig. 6c, d.
Fig. 6.
Subgroup analyses of the relationship between BMI and irAEs in ICIs treated cancer patients. a. Forest plot for association between BMI and any grade of irAEs treated with ICIs stratified by treatment lines. b. Forest plot for association between BMI and G3/G4 irAEs treated with ICIs stratified by treatment lines. c. Forest plot for association between BMI and any grade of irAEs treated with ICIs stratified by tumor types. d. Forest plot for association between BMI and G3/G4 irAEs treated with ICIs stratified by tumor types
Heterogeneity analysis, publication bias, and sensitivity analysis
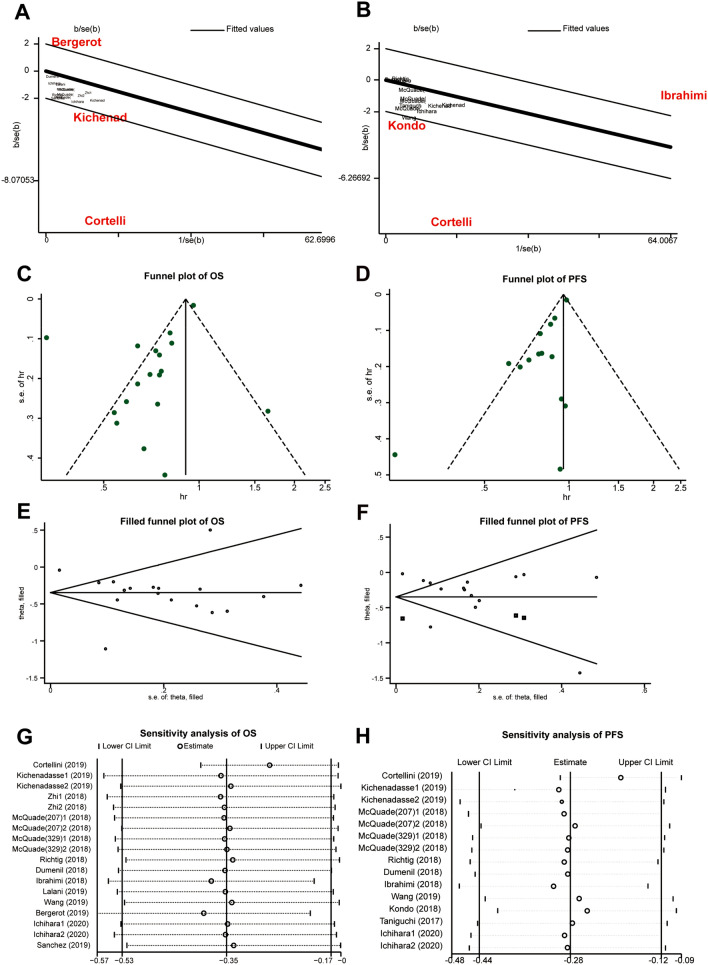
As shown in Fig. 2, there was great heterogeneity of this meta-analysis. According to subgroup analysis and Galbraith plot, the dominating sources of heterogeneity were from the studies of Cortellini [10], Bergerot [27], Kondo [24], and Ibrahim [21] (Fig. 7a, b). Indiscriminate tumor types might be the reason, which brought in considerable confounders. What's more, Kondo and Bergerot’s studies contained a very small sample size. The funnel plots, assessment of publication bias, are shown in Fig. 7c and d. Meanwhile, the Egger's regression test had significant publication biases for OS (p = 0.015) and PFS (p = 0.018). At last, filled funnel plot of OS (p < 0.001) and PFS (p = 0.001) reflected the same results (Fig. 7e and f), which indicated the result of the publication bias was robust. The sensitivity analysis for OS and PFS was performed to test the reliability of this finding. As shown in Fig. 7g and h, the result attested all the studies was located within the confidential interval and the study of Cortellini mainly resulted in the heterogeneity.
Fig. 7.
Heterogeneity analysis, publication bias, and sensitivity analysis. a. heterogeneity analysis of OS by Galbraith plot, b. heterogeneity analysis of PFS by Galbraith plot, c. funnel plot of OS, d. funnel plot of PFS, e. filled funnel plot of OS, f. filled funnel plot of PFS, g. sensitivity analysis of OS, and h. sensitivity analysis of PFS
Discussion
By pooling the individual studies, we found a significant association between high BMI and improved clinical outcomes in cancer patients receiving ICIs relative to outcomes in patients with low BMI. Moreover, we confirmed that overweight/obese patients were related to a greater incidence of irAEs (irAEs of any grade or G3/G4 irAEs). All in all, there might be an epiphenomenon: the better the outcomes among patients with higher BMI, the higher the incidence of irAEs within the same BMI categories.
BMI could potentially be used as a proxy for poor performance status (PS) in real-world data studies; for example, higher BMI is associated with better PS [17]. Some retrospective studies have also shown that PS status is closely related to the efficacy of immunotherapy [32, 33]. Both BMI and PS are partly associated with obesity; the clinical characteristics of obesity may provide some explanations of why high BMI is correlated with good outcomes and irAEs of ICIs treatments. In fact, obesity has a highly complicated association with cancers. Although obesity increases the occurrence of certain types of cancers, such as breast cancers and colorectal cancer, obesity protects against worse outcomes in patients with advanced cancers, such as lung cancers that are associated with wasting [34]. Moreover, previous studies have suggested that high BMI is associated with better outcomes from surgery, radiotherapy, and some types of chemotherapy [35–37] in patients with lung cancer [35, 36]. The biological basis of the association between obesity and the immune system is just beginning to be understood. It is possible that obesity may induce a low-grade systemic meta-inflammation and impaired immune response. Most individuals who are obese harbor inflamed adipose tissue, which resembles chronically injured tissue, with immune cell infiltration and remodeling, which have been found to possibly promote breast and other cancers [38]. Elevated plasma levels of inflammatory markers are correlated with the degree of obesity [39]. Obesity might induce macrophage activation via toll-like receptor 4 (TLR4), thereby stimulating NF-κB signaling. This, in turn, activates transcription of proinflammatory genes including COX-2, IL-6, IL-1β, and TNFα [40]. Moreover, obesity induces T-cell dysfunction and increases the exhausted PD-1–positive T-cell phenotype in fat and tumor microenvironment through leptin production, which may be the link between obesity and immune response [23, 41]. Leptin is characteristically present at high levels in obesity and can affect T-cell function [42, 43]. The increased PD-1 expression correlates with upregulation of phospho-STAT3, a major downstream mediator of leptin signaling, which is also known to induce PD-1 expression on T cells through distal regulatory elements that interact with the PD-1 gene promoter. The identified association between high BMI and OS with atezolizumab appears to be particularly strong in the PD-L1–positive population, lending further support to the presence of a T-cell dysfunction state in patients with obesity. Atezolizumab, through its mechanism of action of PD-1/PD-L1 axis inhibition on T cells, might induce a favorable response in patients with obesity with an established T-cell exhausted state. A novel idea explains that overweight/obese patients might have a different composition of gut microbiota, which would cause the different benefit from immunotherapy [44–46].
As for irAEs, the predictor is not established either. Mirsoian et al. have already revealed that obesity might play a critical role in the induction of immunotherapy toxicities [47], also confirmed in our study. Obesity is hallmarked by a self-sustaining inflammatory response termed “meta-inflammation” [48]. A recent study has attested that immunotherapy that is effective against tumors in young, lean mice can cause lethal inflammation in obese mice. Another reason might be that ICI dosages are based on weight, so we could speculate that overweight/obese patients inevitably have been exposed to higher risks of developing irAEs because of having received higher doses. However, the mechanisms by which BMI affects irAEs remain unknown.
The positive correlation of higher BMI with better survival and severe irAEs did not exist in all patient groups as found in our study. In fact, male patients reportedly tend to have better survival from ICI treatment compared to females [49]. This capacity of tumors in women to evade immune surveillance could make advanced tumors in women less immunogenic and enriched with stronger mechanisms of immune escape than similar tumors in men, and thus, they might become more resistant to immunotherapies [50]. More importantly, the increased susceptibility of women to autoimmune disorders could also make them more likely to develop immune checkpoint inhibitor-related adverse events, potentially leading to a higher rate of treatment discontinuation [51]. With regard to BMI, the correlation was only seen in male patients as well. A potential hormonal mediator of the BMI effects is related to the difference between the sexes [52]; however, the real reasons have not been clarified. Early ICI studies mainly focused on melanoma and NSCLC apparently because of their distinctive immunological characteristics, but now increasing tumor types have been found in which ICI yields an advantage, for example urothelial cancer (UC) and RCC. However, the correlation of BMI and survival has not been seen in RCC, and the different correlation may be due to small patient numbers in RCC studies or higher immunity of melanoma and NSCLC. Meanwhile, based on 204 existing meta-analyses and system reviews, Kyrgiou et al. eventually verified that the risk of eleven types of cancer (containing RCC) was strongly associated with obesity, while the association between other types of cancer (containing NSCLC and melanoma) and obesity was uncertain [53]. The same result came from the International Agency for Research on Cancer (IARC) working group [54]. Nonetheless, what surprised us was that the relationship of higher BMI and severe irAEs was not confirmed in melanoma, for which this analysis included relatively large numbers of patients and studies. The absence of correlation may be due to an included study that assessed adverse events as not more frequent in patients with normal BMI than in patients who were overweight and obese. It indicates that the correlation of BMI and irAEs needs further investigation. It is easy to understand the combination of high BMI with positive PD-L1 to find patients with better OS and PFS, since obesity induces T-cell dysfunction and increases the exhausted PD-1 positive T-cell phenotype [41]. With regard to treatment lines, our results indicated ≥ 2nd line immunotherapy with high BMI tended to have larger survival benefit than first or second line with high BMI. However, first or second line immunotherapy had a high risk of G3/G4 irAEs in high BMI group but not for ≥ 2nd line. This discrepancy may be caused by having more data available now for the second line and above.
Limitations
There are several limitations in our study: 1. Our study has the risk of publication bias. One of the bias and cause of heterogeneity is the analysis of "multiple cancers" and that the main conclusions could be clearly drawn only for melanoma and NSCLC.
Another heterogeneity roots in the disunity of the treatment regimen and sample population. 2. The cutoff value for BMI differs in the included studies. 3. Our study just evaluates the baseline BMI but not the longitudinal BMI, which is underpowered to explain the dynamic effect of BMI on immunotherapy efficacy. 4. BMI may be not a good indicator of fat accumulation. visceral fat, subcutaneous fat, and muscular tissue will be alternative.
Conclusion
Our meta-analysis provides strong evidence that cancer patients with high BMI are more likely to benefit from immunotherapy than those with normal BMI; the association is especially strong for patients who are male or PD-L1 positive or receiving second line or above treatment. BMI might be an effective prognostic marker for immunotherapy. However, high BMI is also related to higher incident of irAEs. Baseline BMI should therefore be considered as a stratification factor in future immune checkpoint inhibitor therapy trials.
Supplementary Information
Below is the link to the electronic supplementary material.
Authors Contribution
X.L.P and Y.Y.F designed the study. Y.Y.F, J.C, and P.K.W designed the statistical plan. Y.Y.F performed the key analyses. Y.Y.F, J.C, and P.K.W generated and collected the data. H.W.Z, W.L, and J.Y.N assisted in data interpretation. Y.Y.F wrote the manuscript. X.L.P revised the manuscript.
Funding
None.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
As this study was based on published data, no ethics approval was sought for the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yafei You and Chang Jiang contributed equally to this work.
Change history
3/20/2021
A Correction to this paper has been published: 10.1007/s00262-021-02907-6
References
- 1.Pearson-Stuttard J, Zhou B, Kontis V, Bentham J, Gunter MJ, Ezzati M. Worldwide burden of cancer attributable to diabetes and high body-mass index: a comparative risk assessment. Lancet Diabetes Endocrinol. 2018;6(6):e6–e15. doi: 10.1016/s2213-8587(18)30150-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schoemaker MJ, Nichols HB, Wright LB, Brook MN, Jones ME, O'Brien KM, Adami HO, Baglietto L, Bernstein L, Bertrand KA, Boutron-Ruault MC, Braaten T, Chen Y, Connor AE, Dorronsoro M, Dossus L, Eliassen AH, Giles GG, Hankinson SE, Kaaks R, Key TJ, Kirsh VA, Kitahara CM, Koh WP, Larsson SC, Linet MS, Ma H, Masala G, Merritt MA, Milne RL, Overvad K, Ozasa K, Palmer JR, Peeters PH, Riboli E, Rohan TE, Sadakane A, Sund M, Tamimi RM, Trichopoulou A, Ursin G, Vatten L, Visvanathan K, Weiderpass E, Willett WC, Wolk A, Yuan JM, Zeleniuch-Jacquotte A, Sandler DP, Swerdlow AJ. Association of body mass index and age with subsequent breast cancer risk in premenopausal women. JAMA Oncol. 2018;4(11):e181771. doi: 10.1001/jamaoncol.2018.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnold M, Pandeya N, Byrnes G, Renehan PAG, Stevens GA, Ezzati PM, Ferlay J, Miranda JJ, Romieu I, Dikshit R, Forman D, Soerjomataram I. Global burden of cancer attributable to high body-mass index in 2012: a population-based study. Lancet Oncol. 2015;16(1):36–46. doi: 10.1016/s1470-2045(14)71123-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Albiges L, Hakimi AA, Xie W, McKay RR, Simantov R, Lin X, Lee JL, Rini BI, Srinivas S, Bjarnason GA, Ernst S, Wood LA, Vaishamayan UN, Rha SY, Agarwal N, Yuasa T, Pal SK, Bamias A, Zabor EC, Skanderup AJ, Furberg H, Fay AP, de Velasco G, Preston MA, Wilson KM, Cho E, McDermott DF, Signoretti S, Heng DYC, Choueiri TK. Body mass index and metastatic renal cell carcinoma: clinical and biological correlations. J Clin Oncol: Off J Am Soc Clin Oncol. 2016;34(30):3655–3663. doi: 10.1200/jco.2016.66.7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lennon H, Sperrin M, Badrick E, Renehan AG. The obesity paradox in cancer: a review. Curr Oncol Rep. 2016;18(9):56. doi: 10.1007/s11912-016-0539-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taghizadeh N, Boezen HM, Schouten JP, Schröder CP, Elisabeth de Vries EG, Vonk JM. BMI and lifetime changes in BMI and cancer mortality risk. PLoS ONE. 2015;10(4):e0125261. doi: 10.1371/journal.pone.0125261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McQuade JL, Daniel CR, Hess KR, Mak C, Wang DY, Rai RR, Park JJ, Haydu LE, Spencer C, Wongchenko M, Lane S, Lee DY, Kaper M, McKean M, Beckermann KE, Rubinstein SM, Rooney I, Musib L, Budha N, Hsu J, Nowicki TS, Avila A, Haas T, Puligandla M, Lee S, Fang S, Wargo JA, Gershenwald JE, Lee JE, Hwu P, Chapman PB, Sosman JA, Schadendorf D, Grob JJ, Flaherty KT, Walker D, Yan Y, McKenna E, Legos JJ, Carlino MS, Ribas A, Kirkwood JM, Long GV, Johnson DB, Menzies AM, Davies MA. Association of body-mass index and outcomes in patients with metastatic melanoma treated with targeted therapy, immunotherapy, or chemotherapy: a retrospective, multicohort analysis. Lancet Oncol. 2018;19(3):310–322. doi: 10.1016/s1470-2045(18)30078-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, Barlesi F, Kohlhaufl M, Arrieta O, Burgio MA, Fayette J, Lena H, Poddubskaya E, Gerber DE, Gettinger SN, Rudin CM, Rizvi N, Crino L, Blumenschein GR, Jr, Antonia SJ, Dorange C, Harbison CT, Graf Finckenstein F, Brahmer JR. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. New Engl J Med. 2015;373(17):1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, Waterhouse D, Ready N, Gainor J, Aren Frontera O, Havel L, Steins M, Garassino MC, Aerts JG, Domine M, Paz-Ares L, Reck M, Baudelet C, Harbison CT, Lestini B, Spigel DR. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. New Engl J Med. 2015;373(2):123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cortellini A, Bersanelli M, Buti S, Cannita K, Santini D, Perrone F, Giusti R, Tiseo M, Michiara M, Di Marino P, Tinari N, De Tursi M, Zoratto F, Veltri E, Marconcini R, Malorgio F, Russano M, Anesi C, Zeppola T, Filetti M, Marchetti P, Botticelli A, Antonini Cappellini GC, De Galitiis F, Vitale MG, Rastelli F, Pergolesi F, Berardi R, Rinaldi S, Tudini M, Silva RR, Pireddu A, Atzori F, Chiari R, Ricciuti B, De Giglio A, Iacono D, Gelibter A, Occhipinti MA, Parisi A, Porzio G, Fargnoli MC, Ascierto PA, Ficorella C, Natoli C. A multicenter study of body mass index in cancer patients treated with anti-PD-1/PD-L1 immune checkpoint inhibitors: when overweight becomes favorable. J Immunother Cancer. 2019;7(1):57. doi: 10.1186/s40425-019-0527-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kichenadasse G, Miners JO, Mangoni AA, Rowland A, Hopkins AM, Sorich MJ. Association between body mass index and overall survival with immune checkpoint inhibitor therapy for advanced non-small cell lung cancer. JAMA Oncol. 2019 doi: 10.1001/jamaoncol.2019.5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Richtig G, Hoeller C, Wolf M, Wolf I, Rainer BM, Schulter G, Richtig M, Grubler MR, Gappmayer A, Haidn T, Kofler J, Huegel R, Lange-Asschenfeldt B, Pichler M, Pilz S, Heinemann A, Richtig E. Body mass index may predict the response to ipilimumab in metastatic melanoma: an observational multi-centre study. PLoS ONE. 2018;13(10):e0204729. doi: 10.1371/journal.pone.0204729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu H, Cao D, He A, Ge W. The prognostic role of obesity is independent of sex in cancer patients treated with immune checkpoint inhibitors: a pooled analysis of 4090 cancer patients. Int Immunopharmacol. 2019;74:105745. doi: 10.1016/j.intimp.2019.105745. [DOI] [PubMed] [Google Scholar]
- 14.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 16.Gomes JR. Analysis of the impact of body mass index in the treatment of metastatic melanoma with ipilimumab. J Clin Oncol. 2017;35(15_suppl):e21044. doi: 10.1200/JCO.2017.35.15_suppl.e21044. [DOI] [Google Scholar]
- 17.Zhi J, Khozin S, Kuk D, Torres A, Sorg R, Lee S, Miksad R, Pazdur R, Abernethy A. Association of baseline body mass index (BMI) with overall survival (OS) in patients (pts) with metastatic non-small cell lung cancer (mNSCLC) treated with nivolumab (N) and pembrolizumab (P) J Clin Oncol. 2018;36:6553–6553. doi: 10.1200/JCO.2018.36.15_suppl.6553. [DOI] [Google Scholar]
- 18.Labomascus S, Fughhi I, Bonomi P, McDonald A, Batus M, Fidler MJ, Basu S, Borgia J. P2.01–61 Body mass index over time is associated with overall survival in advanced NSCLC patients treated with immunotherapy. J Thorac Oncol. 2018 doi: 10.1016/j.jtho.2018.08.1115. [DOI] [Google Scholar]
- 19.Dumenil C, Massiani MA, Dumoulin J, Giraud V, Labrune S, Chinet T, Giroux Leprieur E. Clinical factors associated with early progression and grade 3–4 toxicity in patients with advanced non-small-cell lung cancers treated with nivolumab. PLoS ONE. 2018;13(4):e0195945. doi: 10.1371/journal.pone.0195945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dizman N, Bergerot P, Bergerot CD, Philip EJ, Salgia MM, Hsu J, Adashek J, Pal SK. 894PComparative effect of body-mass index on outcome with targeted therapy and immunotherapy in patients with metastatic renal cell carcinoma (mRCC) Ann Oncol. 2018 doi: 10.1093/annonc/mdy283.103. [DOI] [Google Scholar]
- 21.Ibrahimi S, Mukherjee S, Roman D, King C, Machiorlatti M, Aljumaily R. Effect of body mass index and albumin level on outcomes of patients receiving anti PD-1/PD-L1 therapy. J Clin Oncol. 2018;36:213–213. doi: 10.1200/JCO.2018.36.5_suppl.213. [DOI] [Google Scholar]
- 22.Lalani A-K, Xie W, Flippot R, Steinharter J, Harshman L, McGregor B, Heng D, Choueiri T. Impact of body mass index (BMI) on treatment outcomes to immune checkpoint blockade (ICB) in metastatic renal cell carcinoma (mRCC) J Clin Oncol. 2019;37:566–566. doi: 10.1200/JCO.2019.37.7_suppl.566. [DOI] [Google Scholar]
- 23.Wang Z, Aguilar EG, Luna JI, Dunai C, Khuat LT, Le CT, Mirsoian A, Minnar CM, Stoffel KM, Sturgill IR, Grossenbacher SK, Withers SS, Rebhun RB, Hartigan-O'Connor DJ, Mendez-Lagares G, Tarantal AF, Isseroff RR, Griffith TS, Schalper KA, Merleev A, Saha A, Maverakis E, Kelly K, Aljumaily R, Ibrahimi S, Mukherjee S, Machiorlatti M, Vesely SK, Longo DL, Blazar BR, Canter RJ, Murphy WJ, Monjazeb AM. Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade. Nat Med. 2019;25(1):141–151. doi: 10.1038/s41591-018-0221-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kondo T, Nomura M, Otsuka A, Nonomura Y, Kaku Y, Matsumoto S, Muto M. Predicting marker for early progression in unresectable melanoma treated with nivolumab. Int J Clin Oncol. 2019;24(3):323–327. doi: 10.1007/s10147-018-1345-9. [DOI] [PubMed] [Google Scholar]
- 25.Taniguchi Y, Tamiya A, Isa S-I, Nakahama K, Okishio K, Shiroyama T, Suzuki H, Inoue T, Tamiya M, Hirashima T, Imamura F, Atagi S. Predictive factors for poor progression-free survival in patients with non-small cell lung cancer treated with nivolumab. Anticancer Res. 2017;37(10):5857–5862. doi: 10.21873/anticanres.12030. [DOI] [PubMed] [Google Scholar]
- 26.Shiroyama T, Suzuki H, Tamiya M, Tamiya A, Tanaka A, Okamoto N, Nakahama K, Taniguchi Y, Isa SI, Inoue T, Imamura F, Atagi S, Hirashima T. Pretreatment advanced lung cancer inflammation index (ALI) for predicting early progression in nivolumab-treated patients with advanced non-small cell lung cancer. Cancer Med. 2018;7(1):13–20. doi: 10.1002/cam4.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bergerot PG, Bergerot CD, Philip EJ, Meza L, Dizman N, Hsu J, Pal SK. Targeted therapy and immunotherapy: effect of body mass index on clinical outcomes in patients diagnosed with metastatic renal cell carcinoma. Kidney Cancer. 2019;3(1):63–70. doi: 10.3233/kca-180047. [DOI] [Google Scholar]
- 28.Ichihara E, Harada D, Inoue K, Sato K, Hosokawa S, Kishino D, Watanabe K, Ochi N, Oda N, Hara N, Hotta K, Maeda Y, Kiura K. The impact of body mass index on the efficacy of anti-PD-1/PD-L1 antibodies in patients with non-small cell lung cancer. Lung Cancer (Amsterdam, Netherlands) 2020;139:140–145. doi: 10.1016/j.lungcan.2019.11.011. [DOI] [PubMed] [Google Scholar]
- 29.Sanchez A, Furberg H, Kuo F, Vuong L, Ged Y, Patil S, Ostrovnaya I, Petruzella S, Reising A, Patel P, Mano R, Coleman J, Russo P, Liu CH, Dannenberg AJ, Chan TA, Motzer R, Voss MH, Hakimi AA. Transcriptomic signatures related to the obesity paradox in patients with clear cell renal cell carcinoma: a cohort study. Lancet Oncol. 2020;21(2):283–293. doi: 10.1016/s1470-2045(19)30797-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cortellini A, Bersanelli M, Santini D, Buti S, Tiseo M, Cannita K, Perrone F, Giusti R, De Tursi M, Zoratto F, Marconcini R, Russano M, Zeppola T, Anesi C, Filetti M, Marchetti P, Botticelli A, Gelibter A, De Galitiis F, Vitale MG, Rastelli F, Tudini M, Silva RR, Atzori F, Chiari R, Ricciuti B, De Giglio A, Migliorino MR, Mallardo D, Vanella V, Mosillo C, Bracarda S, Rinaldi S, Berardi R, Natoli C, Ficorella C, Porzio G, Ascierto PA. Another side of the association between body mass index (BMI) and clinical outcomes of cancer patients receiving programmed cell death protein-1 (PD-1)/Programmed cell death-ligand 1 (PD-L1) checkpoint inhibitors: a multicentre analysis of immune-related adverse events. Eur J Cancer (Oxford, England: 1990) 2020;128:17–26. doi: 10.1016/j.ejca.2019.12.031. [DOI] [PubMed] [Google Scholar]
- 31.Heidelberger V, Goldwasser F, Kramkimel N, Jouinot A, Huillard O, Boudou-Rouquette P, Chanal J, Arrondeau J, Franck N, Alexandre J, Blanchet B, Leroy K, Avril M-F, Dupin N, Aractingi S. Sarcopenic overweight is associated with early acute limiting toxicity of anti-PD1 checkpoint inhibitors in melanoma patients. Invest New Drugs. 2017;35(4):436–441. doi: 10.1007/s10637-017-0464-x. [DOI] [PubMed] [Google Scholar]
- 32.Escoin-Perez C, Blasco S, Juan-Vidal O. Immune checkpoint inhibitors in special populations. a focus on advanced lung cancer patients. Lung Cancer (Amsterdam, Netherlands) 2020;144:1–9. doi: 10.1016/j.lungcan.2020.03.026. [DOI] [PubMed] [Google Scholar]
- 33.Passaro A, Spitaleri G, Gyawali B, de Marinis F. Immunotherapy in non-small-cell lung cancer patients with performance status 2: clinical decision making with scant evidence. J Clin Oncol. 2019;37(22):1863–1867. doi: 10.1200/jco.18.02118. [DOI] [PubMed] [Google Scholar]
- 34.Azvolinsky A. Cancer prognosis: role of BMI and fat tissue. J Natl Cancer Inst. 2014;106(6):dju177. doi: 10.1093/jnci/dju177. [DOI] [PubMed] [Google Scholar]
- 35.Masel EK, Berghoff AS, Füreder LM, Heicappell P, Schlieter F, Widhalm G, Gatterbauer B, Dieckmann U, Birner P, Bartsch R, Schur S, Watzke HH, Zielinski CC, Preusser M. Decreased body mass index is associated with impaired survival in lung cancer patients with brain metastases: a retrospective analysis of 624 patients. Eur J Cancer Care (Engl) 2017 doi: 10.1111/ecc.12707. [DOI] [PubMed] [Google Scholar]
- 36.Yap WK, Shih MC, Kuo C, Pai PC, Chou WC, Chang KP, Tsai MH, Tsang NM. Development and validation of a nomogram for assessing survival in patients with metastatic lung cancer referred for radiotherapy for bone metastases. JAMA Netw Open. 2018;1(6):e183242. doi: 10.1001/jamanetworkopen.2018.3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sepesi B, Gold KA, Correa AM, Heymach JV, Vaporciyan AA, Roszik J, Dmitrovsky E, Liu X. The influence of body mass index on overall survival following surgical resection of non-small cell lung cancer. J Thorac Oncol. 2017;12(8):1280–1287. doi: 10.1016/j.jtho.2017.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iyengar NM, Gucalp A, Dannenberg AJ, Hudis CA. Obesity and cancer mechanisms: tumor microenvironment and inflammation. J Clin Oncol: Off J Am Soc Clin Oncol. 2016;34(35):4270–4276. doi: 10.1200/JCO.2016.67.4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Invitti C. Obesity and low-grade systemic inflammation. Minerva Endocrinol. 2002;27(3):209–214. [PubMed] [Google Scholar]
- 40.Howe LR, Subbaramaiah K, Hudis CA, Dannenberg AJ. Molecular pathways: adipose inflammation as a mediator of obesity-associated cancer. Clin Cancer Res: An Off J Am Assoc Cancer Res. 2013;19(22):6074–6083. doi: 10.1158/1078-0432.CCR-12-2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murphy KA, James BR, Sjaastad FV, Kucaba TA, Kim H, Brincks EL, Chua SC, Jr, Wilber A, Griffith TS. Cutting edge: elevated leptin during diet-induced obesity reduces the efficacy of tumor immunotherapy. J Immunol (Baltimore, Md: 1950) 2018;201(7):1837–1841. doi: 10.4049/jimmunol.1701738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Naylor C, Petri WA. Leptin regulation of immune responses. Trends Mol Med. 2016;22(2):88–98. doi: 10.1016/j.molmed.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 43.Saucillo DC, Gerriets VA, Sheng J, Rathmell JC, Maciver NJ. Leptin metabolically licenses T cells for activation to link nutrition and immunity. J Immunol (Baltimore, Md: 1950) 2014;192(1):136–144. doi: 10.4049/jimmunol.1301158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vétizou M, Pitt JM, Daillère R, Lepage P, Waldschmitt N, Flament C, Rusakiewicz S, Routy B, Roberti MP, Duong CP, Poirier-Colame V, Roux A, Becharef S, Formenti S, Golden E, Cording S, Eberl G, Schlitzer A, Ginhoux F, Mani S, Yamazaki T, Jacquelot N, Enot DP, Bérard M, Nigou J, Opolon P, Eggermont A, Woerther PL, Chachaty E, Chaput N, Robert C, Mateus C, Kroemer G, Raoult D, Boneca IG, Carbonnel F, Chamaillard M, Zitvogel L. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science (New York, NY) 2015;350(6264):1079–1084. doi: 10.1126/science.aad1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillère R, Fluckiger A, Messaoudene M, Rauber C, Roberti MP, Fidelle M, Flament C, Poirier-Colame V, Opolon P, Klein C, Iribarren K, Mondragón L, Jacquelot N, Qu B, Ferrere G, Clémenson C, Mezquita L, Masip JR, Naltet C, Brosseau S, Kaderbhai C, Richard C, Rizvi H, Levenez F, Galleron N, Quinquis B, Pons N, Ryffel B, Minard-Colin V, Gonin P, Soria JC, Deutsch E, Loriot Y, Ghiringhelli F, Zalcman G, Goldwasser F, Escudier B, Hellmann MD, Eggermont A, Raoult D, Albiges L, Kroemer G, Zitvogel L. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science (New York, NY) 2018;359(6371):91–97. doi: 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- 46.Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, Prieto PA, Vicente D, Hoffman K, Wei SC, Cogdill AP, Zhao L, Hudgens CW, Hutchinson DS, Manzo T, Petaccia de Macedo M, Cotechini T, Kumar T, Chen WS, Reddy SM, Szczepaniak Sloane R, Galloway-Pena J, Jiang H, Chen PL, Shpall EJ, Rezvani K, Alousi AM, Chemaly RF, Shelburne S, Vence LM, Okhuysen PC, Jensen VB, Swennes AG, McAllister F, Marcelo Riquelme Sanchez E, Zhang Y, Le Chatelier E, Zitvogel L, Pons N, Austin-Breneman JL, Haydu LE, Burton EM, Gardner JM, Sirmans E, Hu J, Lazar AJ, Tsujikawa T, Diab A, Tawbi H, Glitza IC, Hwu WJ, Patel SP, Woodman SE, Amaria RN, Davies MA, Gershenwald JE, Hwu P, Lee JE, Zhang J, Coussens LM, Cooper ZA, Futreal PA, Daniel CR, Ajami NJ, Petrosino JF, Tetzlaff MT, Sharma P, Allison JP, Jenq RR, Wargo JA. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science (New York, NY) 2018;359(6371):97–103. doi: 10.1126/science.aan4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mirsoian A, Murphy WJ. Obesity and cancer immunotherapy toxicity. Immunotherapy. 2015;7(4):319–322. doi: 10.2217/imt.15.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res. 2005;96(9):939–949. doi: 10.1161/01.Res.0000163635.62927.34. [DOI] [PubMed] [Google Scholar]
- 49.Conforti F, Pala L, Bagnardi V, De Pas T, Martinetti M, Viale G, Gelber RD, Goldhirsch A. Cancer immunotherapy efficacy and patients' sex: a systematic review and meta-analysis. Lancet Oncol. 2018;19(6):737–746. doi: 10.1016/s1470-2045(18)30261-4. [DOI] [PubMed] [Google Scholar]
- 50.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science (New York, NY) 2011;331(6024):1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 51.Menzies AM, Johnson DB, Ramanujam S, Atkinson VG, Wong ANM, Park JJ, McQuade JL, Shoushtari AN, Tsai KK, Eroglu Z, Klein O, Hassel JC, Sosman JA, Guminski A, Sullivan RJ, Ribas A, Carlino MS, Davies MA, Sandhu SK, Long GV. Anti-PD-1 therapy in patients with advanced melanoma and preexisting autoimmune disorders or major toxicity with ipilimumab. Ann Oncol: Off J Eur Soc Med Oncol. 2017;28(2):368–376. doi: 10.1093/annonc/mdw443. [DOI] [PubMed] [Google Scholar]
- 52.Schneider G, Kirschner MA, Berkowitz R, Ertel NH. Increased estrogen production in obese men. J Clin Endocrinol Metab. 1979;48(4):633–638. doi: 10.1210/jcem-48-4-633. [DOI] [PubMed] [Google Scholar]
- 53.Kyrgiou M, Kalliala I, Markozannes G, Gunter MJ, Paraskevaidis E, Gabra H, Martin-Hirsch P, Tsilidis KK. Adiposity and cancer at major anatomical sites: umbrella review of the literature. BMJ. 2017;356:j477. doi: 10.1136/bmj.j477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Park Y, Colditz GA. Fresh evidence links adiposity with multiple cancers. BMJ. 2017;356:j908. doi: 10.1136/bmj.j908. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.