Abstract
Existing immune checkpoint inhibitors focus on activating T cells and show limited effectiveness in gastric cancer (GC). SIGLEC10 is identified as a novel tumor-associated macrophage-related immune checkpoint in other cancer types. However, its immunosuppressive role and clinical significance in GC remain unclear. In this study, we find a dominant expression of SIGLEC10 on CD68+ macrophages in GC. SIGLEC10 can suppress the proliferation and function of tumor-infiltrating CD8+ T cells in vitro via the Akt/P38/Erk signaling pathway. Furthermore, in ex vivo and in vivo models, SIGLEC10 blockade promotes CD8+ T cell effector function. Finally, SIGLEC10+ macrophages are positively correlated with the adverse prognosis of GC. Our study highlights that SIGLEC10 directly suppresses T cell function and serves as a promising target for immunotherapy and suggests SIGLEC10+ macrophages as a novel potential predictor of the clinical prognosis of GC.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-023-03488-2.
Keywords: SIGLEC10, Macrophage, Gastric cancer, CD8+ T cell, CD68
Introduction
Over the past decade, several immunotherapies have revolutionized tumor treatment. The most prominent of these are immune checkpoint inhibitors (ICI), which are antibodies that block the inhibitory effects of checkpoint receptors, such as cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4), programmed death 1 (PD-1), and programmed death ligand-1 (PD-L1), on the immune system [1]. ICIs have achieved excellent therapeutic efficacy in a variety of tumors, including melanoma, Hodgkin lymphoma, non-small cell lung carcinoma, and gastric cancer (GC) [2–5]. Besides, the findings of the ATTRACTION-2 and CheckMate-649 studies were extraordinary milestones in the battle against GC [6, 7]. However, the response rate to monoclonal anti-PD-1 antibodies has been disappointing because of the high heterogeneity of immune evasion mechanisms, highlighting the importance of elucidating the interaction between distinct cell populations.
Tumor-associated macrophages (TAMs) are key components of the cancer microenvironment that influence tumor growth and progression. Recent studies have highlighted that TAMs secreted an array of cytokines, chemokines, and enzymes, such as IL-10, TGF-β, ARG-1, and CXCL8, which could suppress CD4+ and CD8+ T cell effector functions [8–10]. Other studies have also revealed that macrophages mediated lymphocyte trapping or dysfunction by strongly expressing PD-L1, PDL2, CTLA-4, and B7-H4 [11–13]. Therefore, the modulation of TAMs as therapeutic targets is receiving increasing attention.
SIGLEC10, known as sialic-acid-binding immunoglobulin-like lectins 10, is composed of five extracellular Ig-like domains and a cytoplasmic tail containing three putative tyrosine-based signaling motifs. It is mainly expressed by innate immune cells and has immunomodulatory functions [14–16]. SIGLEC10 plays an important role in tumor immune escape by binding to various ligands. In ovarian and breast cancers, SIGLEC10, an inhibitory receptor expressed by TAMs, interacts with CD24 expressed by tumors [17]. Previous reports showed that SIGLEC-15 and B7-H4 play a role as a “ligand” for an unknown inhibitory receptor on cytotoxic T cells, in much the same way as PD-L1 on cancer cells or tumor stroma engages with the immune checkpoint molecule PD-1 on T cells [13, 18]. SIGLEC10 has an extracellular structure similar to that of SIGLEC-15 [19]. However, the role of SIGLEC10 in the regulation of tumor-infiltrating T cells as a “ligand” in GC remains elusive.
Herein, we identify infiltration of SIGLEC10+ macrophages in GC tissue by Fluorescence Activating Cell Sorter (FACS). By analyzing single-cell RNA sequencing data of GC, we conclude the characteristic of SIGLEC10+ macrophages. Using in vitro and ex vivo models, we explore the relevant mechanism and function of the SIGLEC10+ macrophage. Finally, we analyze the correlation between the number of SIGLEC10+ macrophages and the GC progression. Collectively, we reveal the role of SIGLEC10 expressed by TAMs as a “ligand” in inhibiting the proliferation and cytotoxic function of tumor-infiltrating CD8+ T cells, as well as to establish its potential as a novel therapeutic agent for GC therapy.
Methods
Patients
The Renji Hospital (RJ) Cohort originally contained 146 patients with GC from Renji Hospital, Shanghai Jiaotong University (Shanghai, China). Twelve patients were excluded due to missing clinical data, metastatic diseases, and dot loss. The remaining 134 patients were included in this analysis. The clinicopathological characteristics of the enrolled patients are shown in Table 1. Clinical tumor stages were determined according to the 8th Edition of the American Joint Committee on Cancer (AJCC) Cancer Staging System. All follow-up data were collected from the date of surgery until December 2021. Overall survival (OS) was defined as the period between surgery and death or the last follow-up. Patient characteristics of the TCGA [20] cohorts were retrieved from http://www.cbioportal.org/study/summary?id=stad_tcga on June 4, 2018, and https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE62254 on January 15, 2019, respectively.
Table1.
Characteristics of GC patients for IF analysis
| Gastric cancer | ||
|---|---|---|
| Number | Percentage | |
| Total | 134 | 100% |
| Gender | ||
| Male | 81 | 60.45% |
| Female | 53 | 39.55% |
| Age | ||
| < 60 | 61 | 45.52% |
| > = 60 | 73 | 54.48% |
| Tumor stage | ||
| T1 | 21 | 15.67% |
| T2 | 21 | 15.67% |
| T3 | 47 | 35.07% |
| T4 | 45 | 33.58% |
| Lymph node metastasis | ||
| N0 | 44 | 32.84% |
| N1 | 90 | 67.16% |
| Distant metastasis | ||
| M0 | 110 | 82.09% |
| M1 | 24 | 17.91% |
| AJCC stage | ||
| I | 12 | 8.96% |
| II | 55 | 41.04% |
| III | 44 | 32.84% |
| IV | 23 | 17.16% |
| Histological grading | ||
| Differentiated | 77 | 57.46% |
| Undifferentiated | 57 | 42.54% |
| Tumor size (cm) | ||
| < 5 cm | 73 | 54.48% |
| > = 5 cm | 61 | 45.52% |
| Neural invasion | ||
| Absent | 102 | 76.12% |
| Present | 32 | 23.88% |
| Vascular invasion | ||
| Absent | 101 | 75.37% |
| Present | 33 | 24.63% |
| Microsatellite state | ||
| MSI | 35 | 26.12% |
| MSS | 99 | 73.88% |
| Siglec10+macrophages | ||
| High | 67 | 50.00% |
| Low | 67 | 50.00% |
Fresh tumor and peritumor tissues for flow cytometry were collected from patients with surgically resectable tumors who underwent gastrectomy between October 2018 and December 2019. All patients were pathologically diagnosed with GC, and none received any antitumor treatment prior to surgery. Tissue resection procedures were performed by radical gastrectomies and were approved by the Ethics Committee of the clinical center (no. 2017–114-CR-02).
Sample preparation and cell isolation
Fresh GC tissues were washed with phosphate-buffered saline (PBS) containing 1% fetal bovine serum (FBS, Cat# 900–18, Gemini, USA) and were subsequently minced. Then, tissue pieces were digested in 200 U/mL type IV collagenase (Cat# C5138, Sigma, St. Louis, USA) and 0.1 mg/ml DNase I (Cat# D5025, Sigma) for 30 min at 37 °C. After digestion, the samples were filtered through 70 μm and 40 μm cell strainers (Cat# 352,340 and Cat# 352,360, Falcon, New York, USA), and the cells were washed twice with PBS. Subsequently, the supernatants were discarded, and the cell pellets were suspended in 1 mL of PBS.
Flow cytometry
Fresh tumor tissues were collected during surgery. Single-cell suspensions were prepared as above described. The cell suspensions were then stained with Fixable Viability Dye 780 to distinguish live cells. Cells were stained with surface markers for 40 min at 4 °C. For intracellular staining, the Fixation/Permeabilization Solution Kit (Cat# 00–5123-43, eBioscience, San Diego, USA) was used according to the manufacturer’s instructions. Fixable viability dye and antibodies for membrane surface protein staining and intramembrane protein staining were used. For cytokine staining, cells were re-stimulated with 0.25 μg/ml PMA (Cat# P-8139, Sigma), 0.25 μg/ml ionomycin (Cat# I-0634, Sigma), and 1:1000 Golgistop (Cat# 554,724, BD Biosciences, New Jersey, USA) at 37 °C for 4 h. After live/dead and surface staining, cells were fixed and permeabilized and stained for cytokines. The antibodies used for FACS staining are listed in Supplementary Table 1. BD LSRFortessa X-20 cell analyzer and BD FACSAria III (BD Biosciences) were used for FACS analysis and sorting, respectively. Data analysis was performed using FlowJo v10 (FlowJo LLC, USA).
T cell isolation
Fresh tumor tissues and peripheral blood were collected during surgery. Single-cell suspensions were prepared as previously described. Peripheral blood mononuclear cells were isolated using Ficoll-Hypaque density gradient centrifugation. CD8+ T cells were obtained by positive selection using CD8 magnetic bead (Cat# 130–045-201, Miltenyi, Bergisch Gladbach, Germany), according to the manufacturer’s instructions, and 5 × 105 cells/mL were cultured in X-VIVO (Cat# 04-418Q, Lonza, Switzerland) medium. The purity of the separated T cell fractions was greater than 80% in all cases. In addition, sorted T cells can be further incubated in vitro and proliferate.
Western blot
The total protein extract was obtained using a lysis buffer, and protein concentrations were measured using the BCA Protein Assay Kit. Samples were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to nitrocellulose (NC) membranes (Cat# HATF00010, Millipore, Massachusetts, USA). The Antibodies of AKT (1:100), p-AKT (1:1000), P38 (1:1000), p-P38 (1:1000), Erk (1:1000), P-Erk (1:2000), GAPDH (1:5000) were used to detected corresponding proteins, respectively. The membranes were incubated with antibodies and visualized using an immobilization Western HRP chemiluminescence (Cat# WBKLS0100, Millipore).
Immunostaining
Formalin-fixed, paraffin-embedded GC surgical specimens were collected and sectioned into slides. Slides were dewaxed in an oven, washed in xylene, hydrated in ethanol, and heated in 0.01 M sodium citrate buffer (pH = 6.0) for antigen retrieval. Sections then were incubated in normal goat serum for 20 min at 37 °C. The slides were incubated with corresponding primary antibodies (Dilution, SIGLEC10, 1:200; CD68, 1:200) in a wet box at 4° C overnight. The nuclei were stained with DAPI. Then, they were incubated with species-appropriate rabbit/mouse secondary antibodies. The sections were analyzed using Leica DMi8 microsystems. For SIGLEC10+ macrophage density, we scanned immunohistochemistry sections at high magnification (× 200) and captured five independent microscopic fields.
Generation of overexpressing cell lines
SIGLEC10 was cloned into pHR lentiviral vector (pHR-SFFV-IRES-EGFP). Then, PHR-SIGLEC10-SFFV-IRES-EGFP or pHR-SFFV-IRES-EGFP, delta8.9, and pMD2.G were co-transfected with PEI reagent (Cat# 23,966, Polyscience) into HEK293T cells, when cells were grown in 10 cm cell culture dishes to 70% confluence.
The supernatant containing viruses was harvested at post-transfection 48 and 72 h and was concentrated via centrifugal filters (Cat# UFC9100, MERCK). Next, THP-1 cells were diluted into 0.2 million/well added with lentiviruses and 8 μg/mL polybrene (Cat# TR-1003, Sigma-Aldrich). Then, the 96-well plate was centrifuged at 900 rcf for 60 min at 24℃, followed by incubation at 37℃ with 5% CO2.
In vitro and Ex vivo inhibition assay
For the in vitro CD8+ T cell activation assay, CD8+ T cells were obtained from the blood and tumor tissue samples of patients, as described above. First, the CD8+ T cells were incubated with anti-CD3 and anti-CD28 antibodies. Then, CD8+ T cells were cultured with anti-SIGLEC10 (5 μg/mL; R&D Systems, Minneapolis, Minnesota, USA) or IgG1 isotype control (5 μg/mL, ab206198; Abcam, Cambridge, UK) in RPMI 1640 medium containing 10% FBS for 72 h at 37 °C and 5% CO2. For the macrophage/CD8 + T cell co-culture system, the SIGLEC10-OE macrophage and Ctrl-OE macrophage were induced from the THP-1 cell line. Briefly, 2.5 × 105 THP-1 cells were seeded in a 6-well plate and treated with PMA (100 ng/ml) for 48 h. Following that, 5 × 105 CD8+ T cells incubated with anti-CD3 and anti-CD28 antibodies were added to the same 6-well plate. After 72 h of co-culture, the CD8+ T cells were analyzed using FACS. To assess T cell proliferation, CD8+ T cells were labeled with CellTrace Violet following the protocol of the CellTrace Violet Cell Proliferation Kit before stimulation with anti-CD3/CD28 beads (Cat# MBS-C001, Gibco, Waltham, Massachusetts, USA). Cytokine production was determined by intracellular cytokine staining after 72 h of stimulation to evaluate the effector function of CD8+ T cells.
Ex vivo intervention studies were performed as described previously [27]. Fresh tumor tissues were collected during surgery. Single-cell suspensions were prepared as previously described. The cells were then cultured with anti-SIGLEC10 (5 μg/mL) or IgG1 isotype control (5 μg/mL) in RPMI 1640 medium containing 10% FBS for 24 h at 37 °C and 5% CO2. Subsequently, the cells were cultured for phenotypic analysis using FACS, as described above.
Dimension reduction, unsupervised clustering, annotation, and visualization
The Seurat package was used for cell clustering, dimensionality reduction, and annotation of cell types. In the integrated data set, 2000 highly variable genes were detected and then used for PCA analysis. PC1 to PC30 were selected for tSNE dimensionality reduction and cell clustering. After that, the cells were embedded in a two-dimensional tSNE pattern. Canonical markers of known cell types were used as a reference to annotate the main cell types, such as MYH11 and MYL9 (myofibroblasts), DCN and LUM (fibroblasts), and TRAC and CD3D (T cells). Subclustering of T cells, endothelial cells, NK cells, and fibroblasts were further performed with the same approach.
Gene set enrichment analysis
The gene set enrichment analysis for DEGs of each cluster was conducted by clusterProfiler, GSVA, and GSEABase packages, with which the enriched GO terms and Kyoto Encyclopedia of Genes and Genomes pathways were calculated.
TCGA data analysis
The TCGA data were used to test the correlation of expression in each subtype and patient survival. The preprocessed gene expression data (fragments per kilobase per million fragments, upper quartile normalized), as well as clinical data for primary tumors and normal tissue, were downloaded from UCSC Xena (http://xena.ucsc.edu/). Then, gene expression signatures were explored to refer to the expression of subpopulations. The gene signature scores were derived as the averages of the expression values (TPM) of DEGs (FDR < 0.05, log2[FC] > 0.4) of each subpopulation. The statistical analysis was performed using the survival and survminer packages. Spearman correlations between signatures were calculated using the cor(x) function in R. Differences between MSS and MSI patients were estimated using the limma package.
Cell–cell interaction analysis
The cell–cell interaction analysis was performed by CellPhoneDB, a publicly available repository of curated receptors, ligands, and their interactions. The parameter threshold was set to 10, which means that only receptors and ligands expressed in more than 10% of the cells in each cluster were considered. For a given ligand–receptor pair, cell types with an average expression level of either the ligand or the receptor of less than 1 and a P value of more than 0.01 were filtered.
PBMC-PDX mouse model
Tissue resection procedures were performed by radical gastrectomies and were approved by the Ethics Committee of the clinical center. The tumor tissue was cut into small pieces about 10 mm3. Each tumor piece is coated with a mixture of matrigel and culture medium. Typically, the right flank of NSG mice (NOD-Prkdcscid Il2rgem1/Smoc, Shanghai Model Organisms Center, China) is selected for implantation. The growing tumor tissue is referred to as F1 PDX after implantation. Once the F1 PDX reaches a size of 800mm3, the mouse is euthanized, and the tumor tissue is collected for further in vivo treatment experiments.
Patients matched PBMCs (5 × 106) were collected and injected into NSG mice to generate the humanized PBMC model. The tumor tissue from F1 PDX was then subcutaneously transplanted into the right flank of NSG mice, while the animals were under anesthesia. Anti-SIGLEC10/IgG was administered at 10 mg/kg/mouse when tumor volumes reached 100 mm3 and were then administered every 4 days for a total of four times.
Statistical analysis
The same software was used for all statistical analyses, including SPSS 23.0 and GraphPad Prism 8.0. The Student’s t test or one-way analysis of variance was used for continuous variables. Spearman’s correlation coefficient was used to evaluate the correlation between the different variables. The Kaplan–Meier method was used to determine OS. Grayscale analysis of the western blot images was performed using ImageJ (V1.8.0). All data are presented as mean ± standard deviation. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, as determined by two-tailed test, NS, not significant.
Results
SIGLEC10 is dominantly expressed in macrophages in GC
To dissect the expression of SIGLEC10 across various cell types in GC, we analyzed the single-cell RNA sequencing (scRNA-seq) dataset of GC [21]. We performed dimensionality reduction and unsupervised cell clustering using the Seurat software suite [22, 23]. The main known cell types were identified based on previously reported canonical cell markers, and the results were presented using t-distribution (Fig. 1A). SIGLEC10 was abundantly expressed by macrophages (Fig. 1B). FACS was used to examine the expression of SIGLEC10 on different cells in tumor tissues from patients with GC, and results validated that SIGLEC10 was dominantly expressed in CD68+ macrophages in GC (Fig. 1C). Further analysis proved that SIGLEC10+ macrophages were mainly present in tumor tissue rather than in paired peritumoral normal tissue (Fig. 1D&E). The above results showed that SIGLEC10 played an important role, mainly through the interaction between macrophages and other cells.
Fig. 1.
SIGLEC10+ macrophages are highly infiltrative in GC. A UMAP plot and clustering analysis of combined single-cell sequencing data from human gastric cancer. B SIGLEC10 expression in various cell types from GC single-cell sequencing data. C Tumor tissue samples werecollected from GC patients (n = 20) undergoing surgery in Renji Hospital. Representative flow cytometry showing subpopulations gated SIGLEC10+ CD45+ leucocytes from fresh human GC samples. D Normal tissue and tumor tissue samples were collected from GC patients (n = 30) undergoing surgery in Renji Hospital. The percentage of SIGLEC10+ CD68+ cells in normal tissues and tumor tissues was measured by FACS. E Immunofluorescence staining of SIGLEC10+ CD68+ cells Tregs in GC tumor tissues or normal tissue. Data are representative of three independent experiments. All data are represented as mean ± sd. ***, p < 0.001. UMAP, Uniform Manifold approximation and projection. GC, gastric cancer
Characterization of SIGLEC10+ macrophages by scRNA-seq in GC
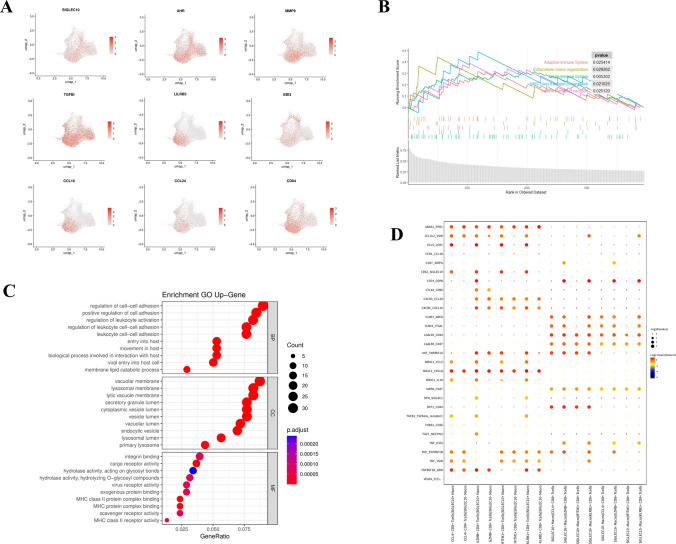
Then, we analyzed the SIGLEC10+ macrophage subpopulation in the scRNA-seq data of GC to identify the characteristics of this subpopulation. Compared with SIGLEC10− macrophage, several known chemokines related to tumor immune escape, such as CCL18 and CCL24, were highly expressed on SIGLEC10+ macrophage, and other immune microenvironment inhibitors, such as AHR, EBI3, TGFB, and LIBR5, were also highly expressed on SIGLEC10+ macrophage, indicating that this subpopulation of cells may mediate tumor immune escape (Fig. 2A). Besides, gene signatures of SIGLEC10+ macrophage were used for GO (Gene Ontology) and gene set enrichment analysis (GSEA). GSEA showed enrichment of the immune response and extracellular matrix organization pathway in SIGLEC10+ macrophages. Go analysis showed SIGLEC10+ macrophage was able to the regulation of cell–cell adhesion (Fig. 2B&C). Subsequently, using CellPhoneDB, ligand–receptor pairs pertaining to SIGLEC10+ and CD8+ T cells, such as TIGIT-NECTIN2, TNF-ICOS, and SPP1-CD44, which were identified as checkpoints in previous studies, were found in the tumor microenvironment (TME) from GC scRNA-seq data (Fig. 2D). Additionally, FACS results demonstrated that at the protein level, CD8+ T cells in tumor tissues displayed elevated expression of TIGIT, TNFRSF1B, CD44, and ICOS, and SIGLEC10+ macrophages in tumor tissues exhibited higher expression of TNF, SPP1, and NECTIN2 (Supplementary Fig. 2A&B). Furthermore, to further investigate the polarization of SIGLEC10+ macrophages toward M1 or M2 subtype, we assessed the expression of CD163 and CD206. Compared to SIGLEC10− macrophage, CD163 and CD206 were both highly expressed in SIGLEC10+ macrophages, indicating an immunosuppressive M2 phenotype (Supplementary Fig. 2C). Therefore, the SIGLEC10+ macrophages subgroup might show immune regulatory function and have a strong causative link with CD8+ T cells’ exhaustion.
Fig. 2.
Characterization of SIGLEC10+ macrophages by scRNA-seq in GC. A UMAP plot showing expression levels of differentiated genes on SIGLEC10+ macrophages. B GSEA (Gene Set Enrichment Analysis) of differentiated genes between SIGLEC10+ macrophages and SIGLEC10− macrophages. C GO pathway analysis of differentiated genes between SIGLEC10+ macrophages and SIGLEC10− macrophage. D Bubble heatmap showing the interaction strength for selected ligand–receptor pairs on SIGLEC10+ macrophages and CD8+ T cell subtypes with CellPhoneDB analysis. Dot size indicates p value, colored by interaction strength levels. UMAP, Uniform manifold approximation and projection
SIGLEC10 inhibits tumor-infiltrating CD8+ T cell function
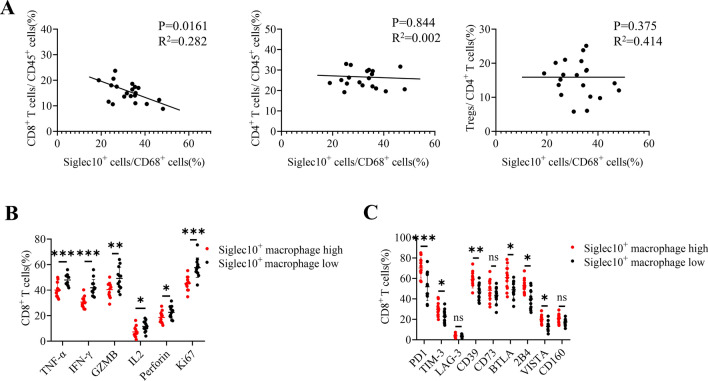
CD8+ T cells play a crucial role in controlling tumor growth. The above results indicated that SIGLEC10+ macrophages were associated with CD8+ T cells. To dissect the relationship between SIGLEC10+ macrophages and CD8+ T cells, first, we analyzed the infiltration of SIGLEC10+ macrophages and different T cell subtypes in GC microenvironment (TME) and found that the increased SIGLEC10+ macrophages infiltration was negatively correlated with the infiltration of CD8+ T cells, but not Treg cell or CD4 + T cells (Fig. 3A). Then, the relationship between the SIGLEC10+ macrophages and different CD8+ T cell activation and exhaustion markers was explored. Based on the number of SIGLEC10+ macrophages in the tumor tissue, the samples were divided into SIGLEC10+ macrophages high infiltration and low infiltration groups. Compared with the low infiltration groups, activation markers of CD8+ T cells, such as GZMB, TNF-α, IFN-γ, PRF-1, and Ki67, were significantly elevated in the high infiltration group (Fig. 3B). Additionally, increases in the levels of PD-1, TIM-3, CD39, BTLA, 2B4, VISTA were displayed in the high SIGLEC10+ macrophages infiltration group (Fig. 3C). Consequently, SIGLEC10+ macrophages might affect CD8+ T cell function in TME.
Fig. 3.
SIGLEC10+ macrophages impair GC infiltrating CD8+ T cell function. A Tumor tissue samples were collected from GC patients (n = 20) undergoing surgery in Renji Hospital. The percentage of SIGLEC10+ CD68+ cells, CD8+ T cells, CD4+ T cells, and FOXP3+ Tregs in tumor tissues were measured by FACS. The correlation between SIGLEC10+ macrophages and different T cells was assayed. B Subjects were divided into two groups. The median cutoff for SIGLEC10+ macrophages was 40 percent in GC. If the proportion of SIGLEC10+ macrophages in the subject was higher than 40%, the subject was considered as a high infiltration group. If it is lower than 40%, it is considered as a low infiltration group. Expression of CD8+ T functional markers (TNF-α, TNF-γ, GZMB, IL2, Perforin, Ki67) between the high SIGLEC10+ macrophages group and low SIGLEC10+ macrophages group was measured by FACS. C The expression of CD8+ T exhausted marker (PD-1, TIM-3, LAG-3, CD73, BTLA, 2B4, VISTA, CD160) between high SIGLEC10+ macrophages group and low SIGLEC10+ macrophages group was measured by FACS. Data are representative of three independent experiments. All data are represented as mean ± sd. *, p < 0.05, **p < 0.01; ***, p < 0.001, ns, not significant
To further elucidate the role of SIGLEC10 in CD8+ T cells, we isolated CD8+ T cells from tumor tissues and the peripheral blood of patients. SIGLEC10 recombinant human protein (rh-SIGLEC10), but not control hIg, suppressed proliferation and inhibited the IFN-γ and TNF-α release of/from CD8+ T cells from tumor tissue but not from peripheral blood in response to anti-CD3/CD28 stimulation (Fig. 4A&B). Next, we overexpressed SIGLEC10 in THP-1 cells and used FACS sorting to obtain SIGLEC10+ THP-1 cells. These cells were subsequently differentiated into macrophages (Supplementary Fig. 3A). Then, SIGLEC10-OE macrophages and Ctrl-OE macrophages were co-cultured with CD8+ T cells isolated from tumor tissue. The results indicated that compared to Ctrl-OE macrophages, SIGLEC10-OE macrophages were able to suppress the proliferation and cytotoxic cytokines release of CD8+ T cells (Supplementary Fig. 3B&C). Together, our results provided evidence for SIGLEC10 expressed on macrophages in directly suppressing tumor-infiltrating CD8+ T cell activity.
Fig. 4.
SIGLEC10+ macrophages inhibit GC-infiltrating CD8+ T cell function in vitro. A CD8+ T cells were isolated from fresh tumor tissues and PBMCs. The CD8+ T cells were labeled with CellTrace Violet (CTV) following the protocol from the CTV Proliferation Kit. CTV-labeled CD8+ T cells were after stimulation with 0.1 μg/ml of anti-CD3 in the presence of human SIGLEC10 fusion protein (hS10-hIg, 5 μg/ml or 15 μg/ml) or control (hIg, 5 μg/ml) for 3 days. B TNFα/IFN-γ expression of CD8+ T cells was measured by flow cytometry. Data are representative of three independent experiments. All data are represented as mean ± sd. ***, p < 0.001, ns, not significant
SIGLEC10 limits the activation of tumor-infiltrating CD8+ T cells by inhibiting the Akt, P38, and Erk signaling pathways
Previous studies have shown that Akt/P38/Erk signaling pathways were involved in T cell survival, proliferation, and differentiation [24–26]. Therefore, we explored whether SIGLEC10 inhibits T cells through different signaling pathways. Western blotting was performed to evaluate the inhibitory effects of SIGLEC10 on the P38/AKT/ERK pathway. CD8+ T cells from the tumor tissue samples were treated with rh-SIGLEC10/hIg and stimulated with anti-CD3/CD28 beads. The levels of phosphorylated Akt, phosphorylated P38, and phosphorylated Erk were decreased in the rh-SIGLEC10-treated groups in contrast to those in the hIg-treated group (Fig. 5). These results suggested that SIGLEC10 might inhibit the activation of CD8+ T cells through the Akt, P38, and Erk signaling pathways.
Fig. 5.

SIGLEC10 inhibits activation of CD8+ T cells through the Akt/Erk/p38 signaling pathway. CD8+ T cells were isolated from fresh tumor tissues and after stimulation with 0.1 μg/ml of anti-CD3 in the presence of 5 μg/ml human SIGLEC10 fusion protein (hS10-hIg, 5 μg/ml or 15 μg/ml) or control (hIg, 5 μg/ml) for 6 h. Representative western blotting result for relative total AKT, ERK, P38, and phosphorylation (phos) of AKT, ERK, P38 in T cells. Quantitative analysis of the band intensities for phosphorylation levels normalized by total protein levels. Data are representative of three independent experiments. All data are represented as mean ± sd. ***, p < 0.001
Anti-SIGLEC10 recovers CD8+ T cell function in TME of GC
We then used a SIGLEC10-neutralizing antibody to evaluate the effects of blocking SIGLEC10 on the TME in GC. The tumor culture system in vitro was established to simulate the in vivo tumor immune system of patients, which included tumor cells, immune cells, and other cells, according to the methods described in recent literature [27].
After incubation with SIGLEC10-neutralizing antibody for 24 h, single-cell suspensions were subjected to FACS analysis (Fig. 6A). The characteristics of CD8+ T cells were also explored. Compared to the control group, blockade of SIGLEC10 led to significant upregulation of TNF-α, IFN-γ, GZMB, PRF-1, and Ki67 in CD8+ T cells (Fig. 6B), whereas the expression of PD-1, TIM-3, BTLA4, 2B4, and VISTA of CD8+ T cells decreased markedly (Fig. 6C). Taken together, these findings implied that SIGLEC10 blocking might elicit a tumoricidal action.
Fig. 6.
SIGLEC10 blockade reactivates CD8+ T cell function. A GC tissues were digested, incubated with 50 μg/ml SIGLEC10-neutralizing antibody or isotype control mAb (Control). B and C, and subjected to flow cytometry analysis to determine the different molecules (TNF-α, IFN-γ, GZMB, IL2, PRF-1, Ki67, PD-1, TIM-3, CD39, BTLA4, 2B4, and VISTA) expression in CD8+ T cells. Data are representative of three independent experiments. All data are represented as mean ± sd. *, p < 0.05, **p < 0.01; ***, p < 0.001, ns, not significant
Then, we have constructed a humanized mouse model (Supplementary Fig. 4A). Compared to the IgG group, anti-SIGLEC10 exhibited better inhibition of tumor growth (Supplementary Fig. 4B&C). FACS analysis of the mouse tumor samples revealed a significant decrease in the percentage of SIGLEC10+ macrophages in the anti-SIGLEC10 group compared to the control groups. Additionally, the CD8+ T cells in the anti-SIGLEC10 group exhibited significantly higher levels of expression for IFN-γ and TNF-α compared to the control group. (Supplementary Fig. 4D-F).
SIGLEC10+ macrophages are related to GC progression
Lastly, we sought to explore whether the number of SIGLEC10+ macrophages correlated with the tumor histologic grade. We performed immunofluorescence staining on 134 samples (Table 1). According to the number of SIGLEC10+ macrophages, subjects were divided into high infiltration group and low infiltration group (cutoff: 46/mm2). We found that SIGLEC10+ macrophage numbers were higher in advanced disease (TNM stages III and IV) than in early disease stages (TNM stages I and II). In addition, high SIGLEC10+ macrophages infiltration was positively correlated with tumor invasion, lymph node stage, and vascular invasion, while no significant difference was observed between the two groups, regarding neural invasion, distant metastasis, and pathological classification. Interestingly, the number of SIGLEC10+ macrophages was lower in microsatellite stable (MSS) tumors than in microsatellite instable (MSI) tumors, indicating that SIGLEC10+ macrophages might be an important target for MSI tumors (Fig. 7A). To compare the prognostic value of intratumoral SIGLEC10+ macrophages in patients with GC, we conducted Kaplan–Meier curve analysis in the Renji Hospital cohort (n = 134)), TCGA cohort (n = 318). Using the medium value of all tumor-infiltrating SIGLEC10+ macrophages numbers as a comparison point, we found that a high proportion of SIGLEC10+ macrophages residing in tumors was associated with unfavorable prognosis (Fig. 7B&C). Notably, the finding that the number of intratumoral SIGLEC10+ macrophages independently predicted survival was verified by multivariate analyses using a Cox proportional hazard model (Table 2). These results suggested that increased tumor-infiltrating SIGLEC10+ macrophages were associated with the progression of GC and adverse patient survival.
Fig. 7.
The number of tumor-infiltrating SIGLEC10+ macrophages correlates with multiple clinical parameters of GC patients. A and B The number of tumor-infiltrating SIGLEC10+ macrophages was analyzed for putative correlations with multiple clinical parameters, such as tumor stage and patient overall survival time. For the cumulative survival curves, patients were separated into two groups by the median value (46/mm2) of tumor-infiltrating SIGLEC10+ macrophage numbers, and Kaplan–Meier plots were used to show cumulative survival differences. Each dot represents one patient. C Kaplan–Meier plots of TCGA database for GC patients. Diff, differentiated; Undiff, undifferentiated; ns, not significant. *P < 0.05; ***P < 0.001, ns, not significant. GC, gastric cancer
Table 2.
Univariate and multivariate analyses of factors associated with survival
| Variables | Overall survival | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | |||||||
| HR | 95.0% CI | p | HR | 95.0% CI | p | |||
| Sex (male vs. female) | 1.007 | 0.629 | 1.614 | 0.975 | ||||
| Age, years (≥ 60 vs. < 60) | 1.003 | .982 | 1.025 | .767 | ||||
| Tumor size, cm (≥ 5 vs. < 5) | 0.140 | 1.420 | 0.892 | 2.262 | ||||
| Histologic type (undiff vs. diff) | 2.816 | 1.752 | 4.526 | .000 | 2.474 | 1.193 | 0.575 | 0.636 |
| Neural invasion (present vs. absent) | 1.472 | 0.878 | 2.468 | 0.143 | ||||
| Vascular invasion (present vs. absent) | 2.86 | 1.772 | 4.617 | 0.000 | 2.054 | 0.965 | 0.453 | 0.926 |
| Tumor (T) invasion (T1 + T2 vs. T3 + T4) | 1.836 | 0.992 | 3.396 | 0.053 | 1.073 | 0.465 | 0.201 | 0.073 |
| Lymphoid nodal (N) status (N0 vs. N1 + N2 + N3) | 2.253 | 1.251 | 4.055 | 0.007 | 1.493 | 0.501 | 0.168 | 0.215 |
| Distant metastasis (M) status (M0 vs. M1) | 4.753 | 2.809 | 8.042 | 0.000 | 3.330 | 1.236 | 0.458 | 0.676 |
| TNM stage (I + II vs. III + IV) | 4.102 | 2.39 | 7.041 | 0.000 | 20.909 | 6.406 | 1.963 | 0.002 |
| Microsatellite state (MSI vs MSS) | 0.65 | 0.396 | 1.067 | 0.089 | 1.248 | 0.634 | 0.322 | 0.187 |
| SIGLEC10+ cell number (high vs. low) | 3.873 | 2.321 | 6.461 | 0.000 | 6.953 | 3.794 | 2.070 | 0.000 |
Cox proportional hazards regression model. Variables used in multivariate analysis were adopted by univariate analysis. TNM, tumor node metastasis; diff, differentiation; undiff, undifferentiation; CI, confidence interval; and HR, hazard ratio
Discussion
A limited number of patients with GC have achieved clinical benefits from PD-1/PD-L1 blockade therapy, highlighting the importance of a better selection of patients or additional treatment to overcome resistance [5]. Previous studies have shown that SIGLEC-family members were involved in the regulation of immune cell activation, proliferation, and apoptosis in autoimmune diseases, inflammatory responses, and tumorigenesis [28]. However, the immunological function of SIGLEC10 in the GC TME remains largely unknown. Here, SIGLEC10 was found to be upregulated in tumor-infiltrating macrophages, in contrast to its minimal expression level on macrophages in normal tissues. SIGLEC10 efficiently impaired the function of CD8+ T cells via Akt, p38, and Erk signaling pathways in vitro. Anti-SIGLEC10 could recover CD8+ T cell function in the TME of GC. The density of SIGLEC10+ macrophages in tumors was positively correlated with poor patient survival. This study highlighted the specific roles of SIGLEC10+ macrophages subsets and demonstrated SIGLEC10 as a promising target to enhance effector function in T cell therapies for cancer treatment.
It is challenging to identify different subgroups of macrophages due to functional and phenotypic heterogeneity, which warrants a more precise classification to distinguish different subtypes of macrophages [29]. SIGLEC10 is an inhibitory receptor widely expressed in immune cells [14]. In this study, SIGLEC10 was found mainly expressed on CD68+ macrophages in the GC TME. Moreover, the high infiltration of SIGLEC10+ TAM subgroup showed a more advanced pathological stage. Multivariate analyses revealed that stromal SIGLEC10+ TAM infiltration was an independent prognostic factor for adverse OS in GC. Furthermore, SIGLEC10+ TAM was highly present in MSI GC. It has been reported that patients with MSI-high GC drastically responded to anti-PD-1 therapy [5]; therefore, SIGLEC10 might be a new therapeutic target for patients with MSI GC. In summary, SIGLEC10 can be used as a specific pro-tumoral TAMs marker and SIGLEC10+ macrophages may represent a group of immunosuppressive macrophage subsets as well as predict poor prognosis in patients.
Recent studies have highlighted the protumorigenic roles of macrophage-mediated CD8+ T cell dysfunction. Macrophages could inhibit cytotoxic T cell responses in various ways [30]. An intravital imaging study also demonstrated long-lasting physical interactions between TAMs and CD8+ T cells which promote T cell exhaustion [31]. Therefore, exploring the molecules and signaling pathways involved in the interaction between TAMs and CD8+ T cells can help us better understand the immunosuppressive TME. By analyzing GC single-cell sequencing data and tumor patient samples, we found that the number of SIGLEC10+ macrophages was positively correlated with CD8+ T cells. Based on the infiltration of SIGLEC10+ macrophages, we divided the samples into SIGLEC10+ macrophage high/low infiltration groups. Compared with the low infiltration group, the effector markers of CD8+ T cells were higher and the exhaustion markers were lower in the high infiltration group. Previous reports showed that B7-H4 and SIGLEC-15 expressed on TAMs played a role as a “ligand” for an unknown inhibitory receptor on cytotoxic T cells, in much the same way as PD-L1 (aka B7-H1, CD274) on cancer cells or tumor stroma engaged the immune checkpoint molecule PD-1 on T cells [32]. Since SIGLEC10 had a similar ectodomain to SIGLEC-15, we sought to explore whether SIGLEC10 has a similar role as a “ligand”. Through in vitro stimulation, we found that rh-SIGLEC10 could inhibit the proliferation and secretion of cytotoxic cytokines in tumor-infiltrating T cells derived from patients with GC, mainly through the Akt/P38/Erk pathway. However, rh-SIGLEC10 was not able to affect the function and proliferation of CD8+ T cells in peripheral blood, indicating the expression of its receptors in tumor-infiltrating CD8+ T cells. Taken together, the expression of SIGLEC10 on macrophages and its receptors on CD8+ T cells can be regarded as a new immunotherapy checkpoint.
Ample therapeutic drugs targeting SIGLECs, including antibodies or glycosylated ligands, have been developed and used in the treatment of many SIGLECs-related diseases, such as lymphoma, leukemia, and autoimmune diseases. Gemtuzumab ozogamicin (GO) (Pfizer, Brooklyn, NY, USA), an anti-CD33 immunotoxin conjugated to a derivative of DNA-damaging calicheamicin, was the first immunotoxin approved by the FDA [33]. Chimeric antigen receptor T cell (CART) therapy approaches targeting CD22 have shown some promise [34]. Here, we built an ex vivo model to evaluate the effect of targeted SIGLEC10 on CD8+ T cell function in the TME of GC. Interestingly, SIGLEC10 blockade partially restored the killing function of CD8+ T cells, as shown by the reduction in the exhausted markers and the enhancement of the killing function and proliferation markers. This was also consistent with the results of a previous study in hepatocellular carcinoma [35], indicating that SIGLEC10 could be used as a better therapeutic target in various tumors.
This study has several limitations. First, the design was retrospective, and the number of patients was relatively small. In addition, we did not thoroughly investigate the paired receptor to SIGLEC10 in T cells, which could be another key immune checkpoint.
In conclusion, TAMs are phenotypically and functionally heterogeneous, and specific subsets of TAMs may play distinct roles in cancer progression and antitumor immunity. In this study, we delineate a functionally distinct subset of SIGLEC10 expressing TAMs in GC, linked SIGLEC10+ TAM abundance with CD8+ T cell exhaustion and adverse clinical outcomes, and further prove SIGLEC10 as a promising target to enhance effector function in T cell therapies for GC treatment.
Supplementary Information
Below is the link to the electronic supplementary material.
A CD163 and CD206 expression in SIGLEC10+ macrophage and SIGLEC10- macrophage were measured by FACS. B TIGIT, TNFRSF1B, CD44 and ICOS expression of CD8+ T cell in tumor tissue and normal tissue from gastric cancer subjects were measured by FACS. C TNF, SPP1 and NECTIN2 expression of SIGLEC10+ macrophage in tumor tissue and normal tissue from gastric cancer subjects were measured by FACS (TIF 2005 KB)
A SIGLEC10 expression in SIGLEC10/Ctrl-overexpression (SIGLEC10/Ctrl-OE) macrophage were measured by FACS. B CD8+ T cells were isolated by magnetic beads from GC patients’ samples. Celltrace Violet (CTV) labeled CD8+ T cell macrophage were co-culture with SIGLEC10/Ctrl-OE macrophage for 72 hrs at ratios (1:2), then the proliferate ability of CD8+ T cells was measured by FACS. C CD8+ T cells were isolated by magnetic beads from GC patients’ samples. CD8+ T cells were co-culture with SIGLEC10/Ctrl-OE macrophage for 72 hrs at ratio (macrophages:CD8+ T cells, 1:2), then the TNF-α and IFN-γ expression of CD8+ T cells was measured by FACS (TIF 1582 KB)
A NSG mice were entailed injection with tumor-matched PBMC (5×106). Then patients-derived tumor was implanted 2 days after PBMC engraftment. Mice received anti-SIGLEC10 mAb or IgG isotype control treatment 6 days after tumor engraftment. B Tumor volumes were measured every 2 days. The plot showed the tumor growth curve. C Tumor volumes on day 24. D The SIGLEC10 expression of tumor-infiltrating macrophage in different groups was measured by FACS. E&F The TNF-α and IFN-γ expression of tumor-infiltrating CD8+ T cells in different groups were measured by FACS (TIF 1919 KB)
Acknowledgements
The authors would like to thank National Natural Science Fund for Distinguished Young Scholars, National Natural Science Foundation of China, and National Key Research and Development Program of China for their financial support.
Author contributions
All authors contributed to the study conception and design. Experiment was designed and performed by YG and SK. Data collection and analysis were performed by FX and YG. Manuscript was written by YG, SK, and JC. Pathological analysis was performed by YS and XL. Bioinformatics analysis was performed by FX and DX. The manuscript was revised by JC. The study was designed and supervised by GZ, HL, and WZ. All authors read and approved the final manuscript.
Funding
Funding was provided by National Natural Science Foundation of China, (Grant Nos. 32070878, 81970497).
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Conflict of interest
The authors declare no potential conflicts of interest.
Ethical approval
All experiments were approved by the Ethical Committee of the Shanghai Jiao Tong University School of Medicine, Renji Hospital (no. 2017–114-CR-02). All procedures followed the ethical guidelines of the Declaration of Helsinki and the guidelines of the China Ethics Review Committee before starting the experiment. Informed consent or a substitute for it was obtained from all patients included in this study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yixian Guo, Shouyu Ke and Feng Xie are co-first authors.
Contributor Information
Wenyi Zhao, Email: zhaowy2win@yeah.net.
Hong Lu, Email: hlu@sjtu.edu.cn.
References
- 1.Robert C, Robert C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat Commun. 2020;11(1):3801. doi: 10.1038/s41467-020-17670-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364(26):2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 3.Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372(4):311–319. doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim ST, Cristescu R, Bass AJ, et al. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat Med. 2018;24(9):1449–1458. doi: 10.1038/s41591-018-0101-z. [DOI] [PubMed] [Google Scholar]
- 6.Kang YK, Boku N, Satoh T, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390(10111):2461–2471. doi: 10.1016/S0140-6736(17)31827-5. [DOI] [PubMed] [Google Scholar]
- 7.Janjigian YY, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021;398:27–40. doi: 10.1016/S0140-6736(21)00797-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4(1):71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 9.Doedens AL, Stockmann C, Rubinstein MP, et al. Macrophage expression of hypoxia-inducible factor-1 alpha suppresses T-cell function and promotes tumor progression. Cancer Res. 2010;70(19):7465–7475. doi: 10.1158/0008-5472.CAN-10-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin C, He H, Liu H, et al. Tumour-associated macrophages-derived CXCL8 determines immune evasion through autonomous PD-L1 expression in gastric cancer. Gut. 2019;68(10):1764–1773. doi: 10.1136/gutjnl-2018-316324. [DOI] [PubMed] [Google Scholar]
- 11.Menguy S, Prochazkova-Carlotti M, Beylot-Barry M, et al. PD-L1 and PD-L2 are differentially expressed by macrophages or tumor cells in primary cutaneous diffuse large B-cell lymphoma. Leg Type Am J Surg Pathol. 2018;42(3):326–334. doi: 10.1097/PAS.0000000000000983. [DOI] [PubMed] [Google Scholar]
- 12.Wang XB, Giscombe R, Yan Z, Heiden T, Xu D, Lefvert AK. Expression of CTLA-4 by human monocytes. Scand J Immunol. 2002;55(1):53–60. doi: 10.1046/j.0300-9475.2001.01019.x. [DOI] [PubMed] [Google Scholar]
- 13.Kryczek I, Zou L, Rodriguez P, et al. B7–H4 expression identifies a novel suppressive macrophage population in human ovarian carcinoma. J Exp Med. 2006;203(4):871–881. doi: 10.1084/jem.20050930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Munday J, Kerr S, Ni J, et al. Identification, characterization and leucocyte expression of SIGLEC-10, a novel human sialic acid-binding receptor. Biochem J. 2001;355(Pt 2):489–497. doi: 10.1042/bj3550489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yin SS, Gao FH. Molecular Mechanism of Tumor Cell Immune Escape Mediated by CD24/SIGLEC-10. Front Immunol. 2020;11:1324. doi: 10.3389/fimmu.2020.01324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bandala-Sanchez E, G Bediaga N, Goddard-Borger ED, et al. CD52 glycan binds the proinflammatory B box of HMGB1 to engage the SIGLEC-10 receptor and suppress human T cell function. Proc Natl Acad Sci U S A. 2018;115(30):7783–7788. doi: 10.1073/pnas.1722056115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barkal AA, Brewer RE, Markovic M, et al. CD24 signalling through macrophage SIGLEC-10 is a target for cancer immunotherapy. Nature. 2019;572(7769):392–396. doi: 10.1038/s41586-019-1456-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang J, Sun J, Liu LN, et al. SIGLEC-15 as an immune suppressor and potential target for normalization cancer immunotherapy. Nat Med. 2019;25(4):656–666. doi: 10.1038/s41591-019-0374-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang KY, Qi LL, Kang FB, Wang L. The intriguing roles of SIGLEC family members in the tumor microenvironment. Biomark Res. 2022;10(1):22. doi: 10.1186/s40364-022-00369-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cancer Genome Atlas Research Network Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202–209. doi: 10.1038/nature13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar V, Ramnarayanan K, Sundar R, et al. Single-cell atlas of lineage states, tumor microenvironment, and subtype-specific expression programs in gastric cancer. Cancer Discov. 2022;12(3):670–691. doi: 10.1158/2159-8290.CD-21-0683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Satija R, Farrell JA, Gennert D, Schier AF, Regev A. Spatial reconstruction of single-cell gene expression data. Nat Biotechnol. 2015;33(5):495–502. doi: 10.1038/nbt.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stuart T, Butler A, Hoffman P, et al. Comprehensive integration of single-cell data. Cell. 2019;177(7):1888–1902.e21. doi: 10.1016/j.cell.2019.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim EH, Sullivan JA, Plisch EH, et al. Signal integration by Akt regulates CD8 T cell effector and memory differentiation. J Immunol. 2012;188(9):4305–4314. doi: 10.4049/jimmunol.1103568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.D’Souza WN, Chang CF, Fischer AM, Li M, Hedrick SM. The Erk2 MAPK regulates CD8 T cell proliferation and survival. J Immunol. 2008;181(11):7617–7629. doi: 10.4049/jimmunol.181.11.7617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rincón M, Pedraza-Alva G. JNK and p38 MAP kinases in CD4+ and CD8+ T cells[J] Immunol Rev. 2003;192(1):131–142. doi: 10.1034/j.1600-065X.2003.00019.x. [DOI] [PubMed] [Google Scholar]
- 27.Adeegbe DO, Liu Y, Lizotte PH, et al. Synergistic immunostimulatory effects and therapeutic benefit of combined histone deacetylase and bromodomain inhibition in non-small cell lung cancer. Cancer Discov. 2017;7(8):852–867. doi: 10.1158/2159-8290.CD-16-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fraschilla I, Pillai S. Viewing SIGLECs through the lens of tumor immunology. Immunol Rev. 2017;276(1):178–191. doi: 10.1111/imr.12526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stoger JL, Goossens P, de Winther MPJ. Macrophage heterogeneity: relevance and functional implications in atherosclerosis. Curr Vasc Pharmacol. 2010;8(2):233–248. doi: 10.2174/157016110790886983. [DOI] [PubMed] [Google Scholar]
- 30.Petty AJ, Yang Y. Tumor-associated macrophages: implications in cancer immunotherapy. Immunotherapy. 2017;9(3):289–302. doi: 10.2217/imt-2016-0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kersten K, Hu KH, Combes AJ, et al. Spatiotemporal co-dependency between macrophages and exhausted CD8+ T cells in cancer. Cancer Cell. 2022;40(6):624–638.e9. doi: 10.1016/j.ccell.2022.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li B, Zhang B, Wang X, et al. Expression signature, prognosis value, and immune characteristics of SIGLEC-15 identified by pan-cancer analysis[J] Oncoimmunology. 2020;9(1):1807291. doi: 10.1080/2162402X.2020.1807291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stasi R. Gemtuzumab ozogamicin: an anti-CD33 immunoconjugate for the treatment of acute myeloid leukaemia. Expert Opin Biol Ther. 2008;8(4):527–540. doi: 10.1517/14712598.8.4.527. [DOI] [PubMed] [Google Scholar]
- 34.Adeel K, Fergusson NJ, Shorr R, Atkins H, Hay KA. Efficacy and safety of CD22 chimeric antigen receptor (CAR) T cell therapy in patients with B cell malignancies: a protocol for a systematic review and meta-analysis. Syst Rev. 2021;10(1):35. doi: 10.1186/s13643-021-01588-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xiao N, Zhu X, Li K, et al. Blocking SIGLEC-10hi tumor-associated macrophages improves anti-tumor immunity and enhances immunotherapy for hepatocellular carcinoma. Exp Hematol Oncol. 2021;10(1):36. doi: 10.1186/s40164-021-00230-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A CD163 and CD206 expression in SIGLEC10+ macrophage and SIGLEC10- macrophage were measured by FACS. B TIGIT, TNFRSF1B, CD44 and ICOS expression of CD8+ T cell in tumor tissue and normal tissue from gastric cancer subjects were measured by FACS. C TNF, SPP1 and NECTIN2 expression of SIGLEC10+ macrophage in tumor tissue and normal tissue from gastric cancer subjects were measured by FACS (TIF 2005 KB)
A SIGLEC10 expression in SIGLEC10/Ctrl-overexpression (SIGLEC10/Ctrl-OE) macrophage were measured by FACS. B CD8+ T cells were isolated by magnetic beads from GC patients’ samples. Celltrace Violet (CTV) labeled CD8+ T cell macrophage were co-culture with SIGLEC10/Ctrl-OE macrophage for 72 hrs at ratios (1:2), then the proliferate ability of CD8+ T cells was measured by FACS. C CD8+ T cells were isolated by magnetic beads from GC patients’ samples. CD8+ T cells were co-culture with SIGLEC10/Ctrl-OE macrophage for 72 hrs at ratio (macrophages:CD8+ T cells, 1:2), then the TNF-α and IFN-γ expression of CD8+ T cells was measured by FACS (TIF 1582 KB)
A NSG mice were entailed injection with tumor-matched PBMC (5×106). Then patients-derived tumor was implanted 2 days after PBMC engraftment. Mice received anti-SIGLEC10 mAb or IgG isotype control treatment 6 days after tumor engraftment. B Tumor volumes were measured every 2 days. The plot showed the tumor growth curve. C Tumor volumes on day 24. D The SIGLEC10 expression of tumor-infiltrating macrophage in different groups was measured by FACS. E&F The TNF-α and IFN-γ expression of tumor-infiltrating CD8+ T cells in different groups were measured by FACS (TIF 1919 KB)
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.