Abstract
Immunotherapy based on γδT cells has limited efficiency in solid tumors, including colon cancer (CC). The immune evasion of tumor cells may be the main cause of the difficulties of γδT cell-based treatment. In the present study, we explored whether and how B7-H3 regulates the resistance of CC cells to the cytotoxicity of Vγ9Vδ2 (Vδ2) T cells. We observed that B7-H3 overexpression promoted, while B7-H3 knockdown inhibited, CC cell resistance to the killing effect of Vδ2 T cells in vitro and in vivo. Mechanistically, we showed that B7-H3-mediated CC cell resistance to the cytotoxicity of Vδ2 T cells involved a molecular pathway comprising STAT3 activation and decreased ULBP2 expression. ULBP2 blockade or knockdown abolished the B7-H3 silencing-induced increase in the cytotoxicity of Vδ2 T cells to CC cells. Furthermore, cryptotanshinone, a STAT3 phosphorylation inhibitor, reversed the B7-H3 overexpression-induced decrease in ULBP2 expression and attenuated the killing effect of Vδ2 T cells on CC cells. Moreover, there was a negative correlation between the expression of B7-H3 and ULBP2 in the tumor tissues of CC patients. Our results suggest that the B7-H3-mediated STAT3/ULBP2 axis may be a potential candidate target for improving the efficiency of γδT cell-based immunotherapy in CC.
Electronic supplementary material
The online version of this article (10.1007/s00262-020-02771-w) contains supplementary material, which is available to authorized users.
Keywords: Colon cancer, B7-H3, Vγ9Vδ2 T cells, ULBP2, STAT3
Introduction
Colon cancer (CC) is the third most common tumor, ranks second in mortality rate among cancer-related diseases and poses a serious threat to human health [1]. Over the last few years, surgery and neoadjuvant chemotherapy have been regarded as the best clinical treatment regimen for CC patients [2]. However, most patients are diagnosed at advanced stages for the first time, which results in disappointing survival rates [3]. Immunotherapy strategies are being actively investigated that may improve the outcomes of patients with advanced-stage and distant metastatic CC.
γδ T cells account for approximately 5% of CD3 + T cells in human peripheral blood and manifest the features of both innate and adaptive immunity [4]. Vγ9Vδ2 (Vδ2) T cells, the predominant subset of peripheral blood γδ T cells (> 70%) [5], have important roles in tumor immunosurveillance against multiple malignancies such as leukemia, hepatocellular cancer and CC [6]. Vδ2 T cells are activated by nonpeptidic-phosphorylated phosphoantigens (pAgs), such as isopentenyl diphosphate (IPP) and (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate (HMBPP), which are commonly upregulated in infected, stressed cells or tumor cells in a T cell receptor (TCR)-dependent manner [7]. Recently, mounting evidence has shown that immune receptor NK gene complex group 2 (NKG2) member D (NKG2D)/NKG2D ligand (NKG2DL) interaction is another important mechanism for regulating the antitumoral activity of Vδ2 T cells [8]. Baker et al. noted that acute systemic β-adrenergic receptor activation markedly augmented the mobilization, expansion, and antitumor activity of Vδ2 T cells via NKG2D [9]. Moreover, treatment with decitabine, a DNA demethylation drug, enhanced the cytotoxic effect of Vδ2 T cells on osteosarcoma cells through the NKG2D-NKG2DL axis [10]. Given that immunotherapy based on Vδ2 T cells had a limited efficiency in solid tumors [11], exploring the regulation of NKG2D/NKG2DL interaction may improve the antitumoral activity of Vδ2 T cells.
As one member of the B7 superfamily, B7-H3 is overexpressed in a wide range of cancers, including CC and is associated with poor prognosis [12]. B7-H3 is constitutively expressed on antigen-presenting cells (APCs) [13] and induced on dendritic cells (DCs), NK cells and T cells. The reported data showed that APC-expressed B7-H3 exerted an inhibitory effect on the functions of CD8 + T cells and NK cells and promoted tumor progression [13]. Additionally, B7-H3 is also widely expressed on tumor cells and contributes to the migration and invasion, chemoresistance, glycolysis, and angiogenesis of tumor cells [14, 15]. Moreover, tumor cell-expressed B7-H3 plays a vital role in the regulation of CD8 + T cells and NK cells in a wide spectrum of tumors, such as ovarian cancer, neuroblastoma, and glioma [16, 17]. Our previous study also investigated whether B7-H3 inhibited the cytotoxicity of Vδ2 T cells against colon cancer cells via the downregulation of IFN-γ and perforin/granzyme B expression [18]. However, studies have not evaluated the roles and mechanisms of B7-H3-mediated CC cell resistance to Vδ2 T cell cytotoxicity. In this article, we explored whether and how B7-H3 protects CC cells from the cytotoxicity of Vδ2 T cells by regulating STAT3/ULBP2 axis.
Materials and methods
Colorectal cancer cell culture, cell lentivirus infection, and cell transfection
The human colon cancer cell lines HCT116 (ATCC, Manassas, Virginia, USA, #CRL-247) and RKO cells (ATCC, #CRL-2577) were incubated in complete DMEM (Biological Industries, Kibbutz Beit Haemek, Israel, #01-052-1ACS) containing 10% fetal bovine serum (FBS, Biological Industries, #04-001-1ACS) and 1% penicillin–streptomycin (Beyotime, Shanghai, China, #C0222) and were cultured at 37 °C and 5% CO2.
Stable B7-H3-overexpressing HCT116 or RKO cells (LV-B7-H3 HCT116 or LV-B7-H3 RKO) and knockdown HCT116 or RKO cells (Sh-B7-H3 HCT116 or Sh-B7-H3 RKO) were described previously [19].
Human ULBP2 siRNA (5′-CTCAATGGGAGACTGTATA-3′) and corresponding negative control siRNA (NC siRNA) were purchased from RiboBio (Guangzhou, China, #stB0013750A). According to the instructions of the manufacturer, CC cells were transfected with ULBP2 siRNA or NC siRNA using Lipofectamine 2000 (Invitrogen, Carlsbad, California, USA, #11668019). After 48 h of transfection, RT-qPCR, western blotting and flow cytometry were used to confirm the transfection efficiency.
RNA extraction and RT-qPCR
According to the manufacturer’s instructions, total RNA was extracted from cells by using the TransZol Up Plus RNA Kit (TransGen Biotech, Beijing, China, #ER501–01) reagent. cDNA was reverse-transcribed from 1 μg RNA using PrimeScript™ RT Master Mix (Takara, Shiga, Japan, #RR036A) according to the manufacturer’s instructions. RT-qPCR was performed using an AceQ qPCR SYBR Green Master Mix (without ROX) kit (Vazyme Biotech Co., Ltd, Nanjing, China, #Q121-02). The PCR protocols were as follows: 95 °C for 5 min, followed by 40 cycles of amplification for 30 s at 95 °C, 45 s at 60 °C and 45 s at 72 °C. β-Actin was used to normalize for individual gene expression. All primers used in this study are listed in Supplementary Table 1.
Flow cytometry
Digested CC cells were collected and washed with PBS (HyClone, Logan, Utah, USA, #SH30256.01B). Then, the cells were stained with PE-Cy7-conjugated anti-B7-H3 (BioLegend, San Diego, USA, Clone MIH42, #351008) or PE-conjugated anti-ULBP2 (R&D Systems, Minneapolis, Minnesota, USA, Clone 165903, #MAB-1298) and analyzed by a Millipore Guava® easyCyte 8 flow cytometer (Merck Millipore, Darmstadt, Germany).
Western blotting
Total protein was extracted from CC cells using RIPA lysis buffer (Beyotime, #P0013D) with protease inhibitor cocktail (Beyotime, #P1045). The Enhanced BCA Protein Assay Kit (Beyotime, #P0009) was used to detect the protein concentrations. Equal amounts of protein (30 μg) were separated by 10% SDS-PAGE and transferred to PVDF membranes (Merck Millipore, #FFP39). The membranes were then incubated with primary antibodies as follows: goat anti-human 4IgB7-H3 antibody (R&D Systems, #AF1027), goat anti-human ULBP2 antibody (R&D Systems, #AF1298), mouse anti-human STAT3 (CST, Boston, Massachusetts, USA, #9139), rabbit anti-human/mouse phospho-STAT3 (CST, #9145), and mouse anti-human/mouse β-actin (CST, #3700). After incubation with HRP-conjugated secondary antibodies, the membranes were detected using the Clarity Western ECL substrate kit (Bio-Rad, CA, USA, #1705060).
Vδ2 T cell line establishment
Vδ2 T cell line establishment was performed as we reported previously [20]. Briefly, peripheral blood mononuclear cells (PBMCs) from healthy donors were stimulated with zoledronic acid (5 μM, Abcam, Cambridge, UK, #ab141980), recombinant human interleukin-2 (rhIL-2, 150 IU/mL, PeproTech, Rocky Hill, Connecticut, USA, #200-02), β-mercaptoethanol (50 μM, Sigma-Aldrich, Darmstadt, Germany, #M3148), MEM with nonessential amino acids (1:100, Gibco, New York, USA, #11140050), and L-glutamine (1:100, Gibco, #25030081) for 14 days. In some experiments, we used the anti-TCR γδ MicroBead Kit (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany) to purify expanded Vδ2 T cells according to the manufacturer's instructions.
Cytotoxicity assay
The LDH Cytotoxicity Assay Kit (Beyotime, #C0017) was used to measure the cytotoxicity of Vδ2 T cells against CC cells pretreated with or without ULBP2 siRNA, NC siRNA, ULBP2 monoclonal blocking antibody (ULBP2 mAb, 1 μg/mL, R&D Systems, #MAB1298, isotype: IgG2a) or CTN (50 μM, Selleck, Houston, Texas, USA, #S2285) according to the manufacturer's instructions.
The cytotoxicity assay based on the CCK-8 assay was performed as we reported previously [20]. Briefly, CC cells (target cells, T), pretreated with or without ULBP2 siRNA, NC siRNA, ULBP2 mAb, or CTN, were incubated with mitomycin (10 μg/mL, Sigma-Aldrich, #10107409001) for 2 h. Then, Vδ2 T cells (effector cells, E) and CC cells (target cells, T) were coincubated in Vδ2 T cell growth medium in 96-well plates at different E/T ratios for 1 day. CCK-8 solution (100 μl/mL, Dojindo Laboratories, Kumamoto, Japan, #CK04) was added to each well for 4 h, and the optical density (OD) at 450 nm was measured. The cytotoxicity was calculated by the following equation: cytotoxicity (%) = 100 –100 × {(OD value of CC cells and Vδ2 T cells combination − OD value of Vδ2 T cells)/OD value of CC cells}.
Alternatively, we used the number and area of Vδ2 T cell clusters after Vδ2 T cell and CC cell coculture to evaluate the cytotoxicity as previously described [21]. Briefly, Vδ2 T cells and CC cells, pretreated with or without ULBP2 siRNA, NC siRNA, ULBP2 mAb or CTN, were coincubated in Vδ2 T cell growth medium in 96-well plates for 2 days. Then, the number and area of Vδ2 T cell clusters (at least 300 μm2) were determined using ImageJ 1.41 (National Institutes of Health, Bethesda, USA) analysis software. Three different locations per well were imaged and used to count the number and area of the clusters.
In vivo mouse experiments
All animal experiments were performed under the institutional guidelines of the Institutional Animal Care and Use Committee of Soochow University (Suzhou, China). Six-week-old female CB-17 SCID mice (Shanghai Lab. Animal Research Center, Shanghai, China) were randomly divided into four groups: LV-NC + PBS, LV-NC + γδ T, LV-B7-H3 + PBS, and LV-B7-H3 + γδ T. A total of 5 × 106 LV-NC or LV-B7-H3 HCT116 cells were separately subcutaneously injected into the right flank. On days 7 and 14, the mice in the LV-NC + γδ T and LV-B7-H3 + γδ T groups were administered Vδ2 T cells by intravenous injection via the tail vein (1 × 107). The mice in the LV-NC + PBS and LV-B7-H3 + PBS groups received PBS injection. The tumor sizes were measured by Vernier calipers every 2–3 days. The following formula was used to calculate the volume of tumors: volume (mm3) = 0.5 × L (mm) × S2 (mm2), where S and L are the smallest and largest perpendicular tumor diameters, respectively. On day 30, all the mice were sacrificed. Then, the tumor tissues were excised from tumor-bearing mice and weighed.
CC clinical tissue specimens
A total of 106 pairs of CC tissue samples and the corresponding normal adjacent tissue samples were collected from the First Affiliated Hospital of Soochow University (Suzhou, China) with the consent of all patients. The study protocol was approved by the Institutional Review Board of the First Affiliated Hospital of Soochow University. Detailed clinicopathological information is shown in Supplementary Table 2.
IHC
CC samples or xenograft tumor samples were fixed with formalin, embedded in paraffin and cut into 5 μm sections. All sections were incubated with goat anti-human 4IgB7-H3 antibody (1:200, R&D Systems) or goat anti-human ULBP2 antibody (1:200, R&D Systems) overnight at 4 °C. Then, the sections were incubated with the HRP-labeled rabbit anti-goat secondary antibody (Invitrogen, #31402) and visualized by staining with 3,3′-diaminobenzidine (Biocare Medical, California, USA, #DB801). The scoring criteria for B7-H3 and ULBP2 immunostaining were based on clinical data and adopted the semiquantitative immunoreactive score (IRS) system as we reported previously [22]. Briefly, category A, which represents the intensity of immunostaining, was scored using the following criteria: 0, negative; 1, weak; 2, moderate; 3, strong. Category B, which represents the percentage of immunoreactive cells, was scored using the following criteria: 1, (0–25%); 2, (26–50%); 3, (51–75%); and 4, (76–100%). Final scores were calculated by multiplying the scores of categories A and B in the same section; the scores ranged from 0 to 12.
Immunofluorescence (IF)
All CC sample sections and xenograft tumor sample sections were incubated with FITC-conjugated anti-TCR gamma + TCR delta antibody (1:50, Abcam, #ab171110) overnight at 4 °C. Then, the sections were counterstained with DAPI (Beyotime, #C1005) for 5 min at room temperature. All sections were visualized by immunofluorescence microscope. The number of γδT cells was counted and normalized with DAPI cells.
Expression data sets
UALCAN (https://ualcan.path.uab.edu) was used to analyze the expression of B7-H3 and ULBP2 mRNA in The Cancer Genome Atlas (TCGA) CC datasets, which contain 41 normal samples and 286 CC samples [23]. In addition, the data of B7-H3 and ULBP2 mRNA expression levels were also obtained from TCGA GDC data portal (https://portal.gdc.cancer.gov). Apart from patients with other tumors, recurrent tumors or receiving treatments, 17 normal samples, and 173 CC samples remained for subsequent experiments.
Statistical analysis
Student’s t test was used for comparisons between two groups, and one-way ANOVA was used for multiple comparisons. Pearson correlation and linear regression were used to analyze the degree of association between groups. The mean ± SD or SEM was used to present all data. Each experiment was repeated at least three times. Significant differences are displayed as follows: *p < 0.05; **p < 0.01; ***p < 0.001.
Results
B7-H3 on CC cells suppresses Vδ2 T cell-mediated lysis of CC cells in vitro and in vivo
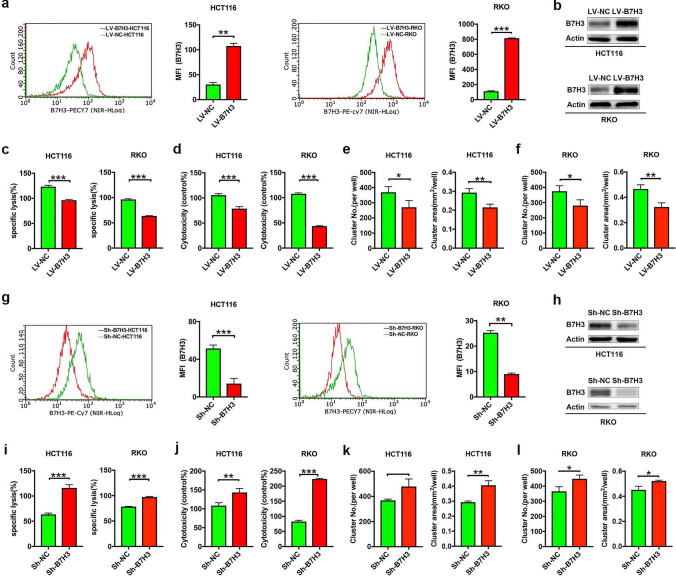
To assess whether CC cell-expressed B7-H3 affected the cytotoxicity of Vδ2 T cells, we used stable B7-H3-overexpressing HCT116 or RKO cells that we described previously [19]. Using real-time quantitative PCR (RT-qPCR), flow cytometry and western blotting assays, we showed that both the mRNA and protein levels of B7-H3 markedly increased in B7-H3-overexpressing HCT116 or RKO cells (Supplementary Fig. 1a; Fig. 1a, b). As shown in Supplementary Fig. 1b, the highest cytotoxic effect of Vδ2 T cells on HCT116 or RKO cells was observed at an effector cells:target cells (E:T) ratio of 40:1. Therefore, we examined the effects of CC cell-expressed B7-H3 on Vδ2 T cell cytotoxicity at an E:T ratio of 40:1. The lactate dehydrogenase (LDH) and Cell Counting Kit 8 (CCK-8) assay data showed that B7-H3-overexpressing HCT116 or RKO cells were resistant to the killing effect of Vδ2 T cells (Fig. 1c, d). Moreover, Vδ2 T cells formed fewer and smaller clusters around B7-H3-overexpressing HCT116 or RKO cells than around control cells (Fig. 1e, f; Supplementary Fig. 1c, d).
Fig. 1.
The expression of B7-H3 in colon cancer cells is associated with resistance to Vδ2 T cell-mediated lysis in vitro. a, b The protein expression of B7-H3 in B7-H3-overexpressing HCT116 and RKO cells was analyzed by flow cytometry (a) and western blot (b) assays. β-Actin served as a loading control. c, d The cytotoxicity of Vδ2 T cells (effector cells, E) against B7-H3-overexpressing HCT116 or RKO cells (target cells, T) at E/T ratios of 40:1 was analyzed by LDH (c) and CCK-8 (d) assays. e, f The number (NO.) and size (area) of the clusters of Vδ2 T cells formed surrounding B7-H3-overexpressing HCT116 (e) or RKO (f) cells. g, h The protein expression of B7-H3 in B7-H3 knockdown HCT116 and RKO cells was analyzed by flow cytometry (g) and western blot (h) assays. β-Actin served as a loading control. i, j The cytotoxicity of Vδ2 T cells (effector cells, E) against B7-H3 knockdown HCT116 or RKO cells (target cells, T) at E/T ratios of 40:1 was analyzed by LDH (i) and CCK-8 (j) assays. k, l The number (NO.) and size (area) of the clusters of Vδ2 T cells formed surrounding B7-H3 knockdown HCT116 (k) or RKO (l) cells. Values are expressed as the means ± SD. Data are representative of results from three independent experiments. *p < 0.05, **p < 0.01, and ***p < 0.001
In complementary loss-of-function studies, stable knockdown B7-H3 HCT116 or RKO cells were used (Supplementary Fig. 2a; Fig. 1g, h). The results of LDH, CCK-8 and Vδ2 T cell cluster assays revealed that the depletion of B7-H3 in HCT116 or RKO cells led to a substantial increase in the killing ability of Vδ2 T cells (Fig. 1i–j; Supplementary Fig. 2b, c).
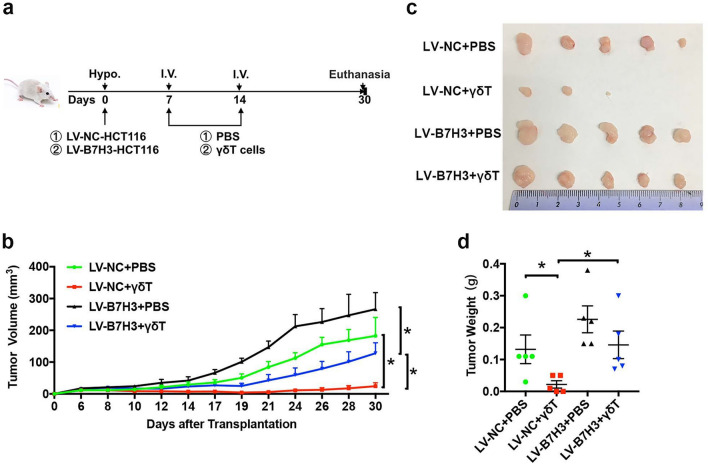
Then, we used severe combined immunodeficient (SCID) mice to establish xenograft models of B7-H3-overexpressing HCT116 tumors or control tumors, and γδ T cells were administered at different time points (Fig. 2a). As shown in Fig. 2b, B7-H3 overexpression promoted the growth of HCT116 cells in vivo. Furthermore, tumors derived from B7-H3-overexpressing HCT116 cells were significantly more resistant to γδ T cells than tumors derived from control vector HCT116 cells (Fig. 2b). Consistent with the tumor growth curve data, the tumor images and weight data also revealed that B7-H3-overexpressing HCT116 tumors exhibited increased resistance to γδ T cells (Fig. 2c, d). Together, these results illustrated that B7-H3 expression on CC cells inhibited the cytotoxicity of Vδ2 T cells to CC cells in vivo.
Fig. 2.
B7-H3-overexpressing CC cells resist Vδ2 T cell-mediated cytotoxicity in vivo. a Schematic overview of tumor models and γδ T cell treatment. b The volumes of B7-H3-overexpressing HCT116 tumors in SCID mice following Vδ2 T cell treatment. c A representative image of B7-H3-overexpressing HCT116 tumors in SCID mice following Vδ2 T cell treatment. d The weight of B7-H3-overexpressing HCT116 tumors in SCID mice following Vδ2 T cell treatment. Values are expressed as the means ± SEM. n = 5 mice per group. *p < 0.05
B7-H3 downregulates ULBP2 expression in CC cells
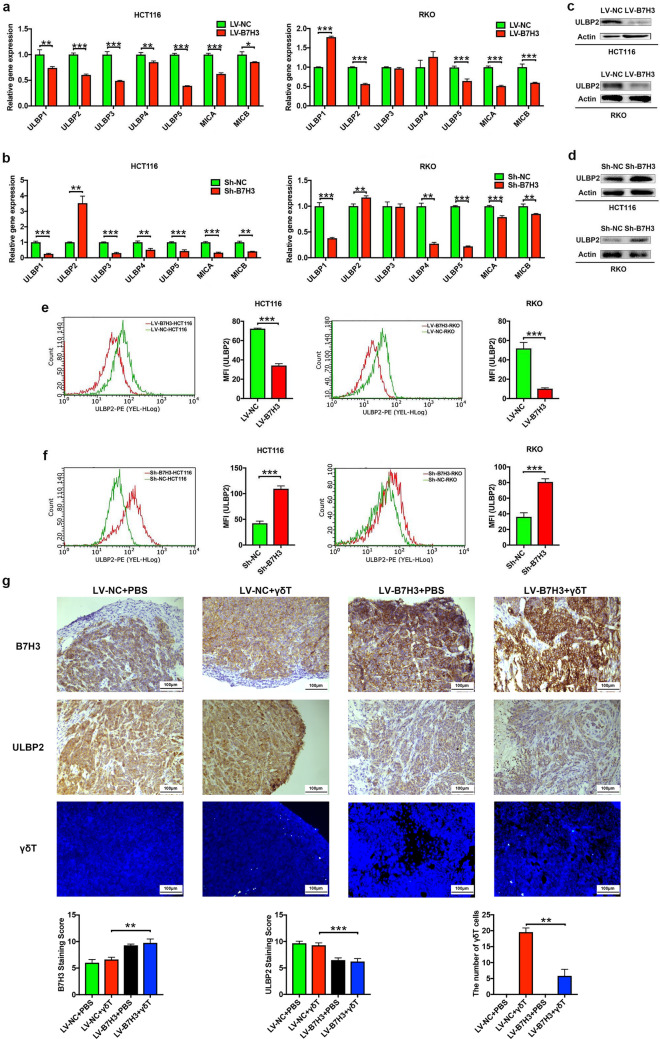
Given that the NKG2D/NKG2DL axis is an important way to regulate the antitumoral activity of Vδ2 T cells [8–10], we hypothesized that B7-H3 is involved in the regulation of NKG2DL expression in CC cells. Hence, we assessed the expression of a spectrum of key NKG2DLs, including UL16-binding protein 1 (ULBP1), ULBP2, ULBP3, ULBP4, ULBP5, MHC class I polypeptide-related sequence A (MICA), and MICB, in both B7-H3-overexpressing and B7-H3-knockdown CC cells with RT-qPCR (Fig. 3a, b). The results showed that the expression levels of ULBP2 were inversely correlated with the expression levels of B7-H3 in HCT116 and RKO cells (Fig. 3a, b). Furthermore, our western blotting assay indicated that overexpression of B7-H3 significantly reduced ULBP2 protein expression (Fig. 3c), while silencing of B7-H3 resulted in a significant increase in ULBP2 protein expression in HCT116 and RKO cells (Fig. 3d). Moreover, flow cytometry analysis showed consistent results (Fig. 3e, f). More importantly, we observed that compared with the control tumors, the B7-H3-overexpressing HCT116 tumors displayed decreased protein expression of ULBP2 (Fig. 3g). In addition, the number of γδT cells in B7-H3-overexpressing HCT116 tumors treated with Vδ2 T cells were fewer than that in tumors derived from control vector HCT116 cells after Vδ2 T cells injection (Fig. 3g; Supplementary Fig. 3). Thus, these results suggest that B7-H3 negatively regulated ULBP2 expression in CC cells.
Fig. 3.
B7-H3 suppresses the expression of ULBP2 in colon cancer cells in vitro and in vivo. a, b The mRNA expression of NKG2DLs, including ULBP1, ULBP2, ULBP3, ULBP4, ULBP5, MICA, and MICB, in B7-H3-overexpressing (a) or knockdown (b) HCT116 and RKO cells was analyzed by RT-qPCR. n = 3 (c, d) The protein expression of ULBP2 in B7-H3-overexpressing (c) or knockdown (d) HCT116 and RKO cells was analyzed by western blot assay. β-Actin served as a loading control. e, f The protein expression of ULBP2 in B7-H3-overexpressing (e) or knockdown (f) HCT116 and RKO cells was analyzed by flow cytometry assay. n = 3 (g) The expression of B7-H3, ULBP2 and infiltrating γδT cells in B7-H3-overexpressing HCT116 tumors in SCID mice following Vδ2 T cell treatment was analyzed by immunohistochemistry or immunofluorescence assay. n = 5 mice per group. Values are expressed as the means ± SEM. **p < 0.01, and ***p < 0.001
CC cell-expressed B7-H3 inhibits the cytotoxicity of Vδ2 T cells via ULBP2
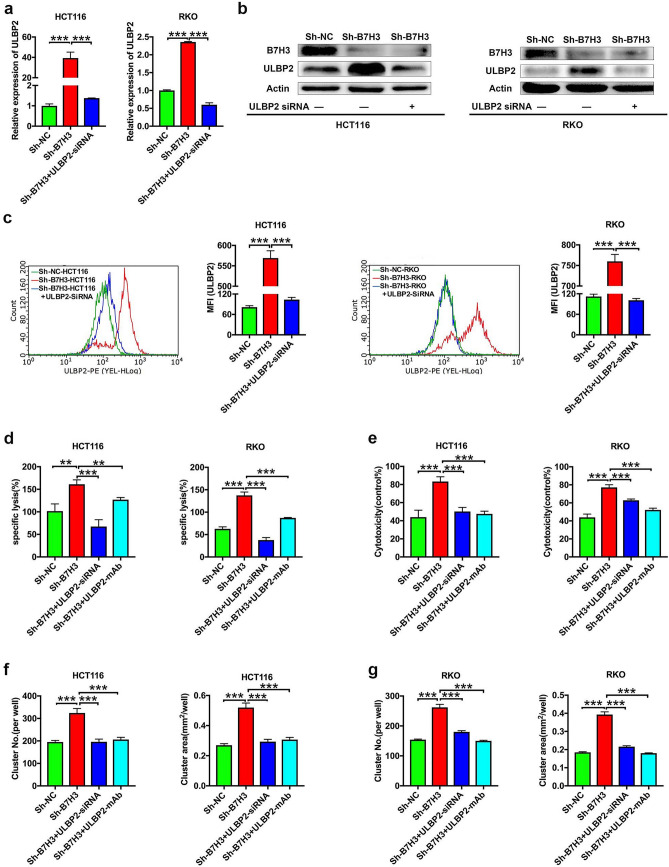
As shown in Fig. 4a, b, ULBP2 silencing by a commercial siRNA against ULBP2 (ULBP2 siRNA) reduced the mRNA and protein expression of ULBP2 induced by B7-H3 knockdown in HCT116 and RKO cells. Moreover, flow cytometry analysis also confirmed that ULBP2 silencing reversed ULBP2 expression on the B7-H3 knockdown HCT116 and RKO cell surfaces (Fig. 4c). We then investigated whether B7-H3 inhibited the killing activity of Vδ2 T cells by regulating ULBP2 in CC cells. Treatment with ULBP2 siRNA or ULBP2 mAb abolished the B7-H3 silencing-induced increase in the cytotoxicity of Vδ2 T cells to CC cells (Fig. 4d–g; Supplementary Fig. 4). Taken together, the above results suggest that B7-H3 on CC cells inhibited the cytotoxicity of Vδ2 T cells in a ULBP2-dependent manner.
Fig. 4.
ULBP2 knockdown or blockade abolishes B7-H3-mediated colon cancer cell resistance to the cytotoxicity of Vδ2 T cells. a The mRNA expression of ULBP2 in B7-H3 knockdown HCT116 and RKO cells transfected with ULBP2-siRNA was analyzed by RT-qPCR. b The protein expression of B7-H3 and ULBP2 in B7-H3 knockdown HCT116 and RKO cells transfected with ULBP2-siRNA was analyzed by western blot assay. β-Actin served as a loading control. c The protein expression of ULBP2 in B7-H3-knockdown HCT116 and RKO cells transfected with ULBP2-siRNA was analyzed by flow cytometry assay. d, e The cytotoxicity of Vδ2 T cells against B7-H3 knockdown HCT116 or RKO cells treated with ULBP2-siRNA or ULBP2 monoclonal blocking antibody (ULBP2 mAb) was analyzed by LDH (d) and CCK-8 (e) assays. f, g The number (NO.) and size (area) of the clusters of Vδ2 T cells formed surrounding B7-H3 knockdown HCT116 (f) and RKO (g) cells treated with ULBP2-siRNA or ULBP2 mAb. Values are expressed as the means ± SD. Data are representative of results from 3 independent experiments. **p < 0.01, and ***p < 0.001
B7-H3 decreases ULBP2 expression and the cytotoxicity of Vδ2 T cells through STAT3
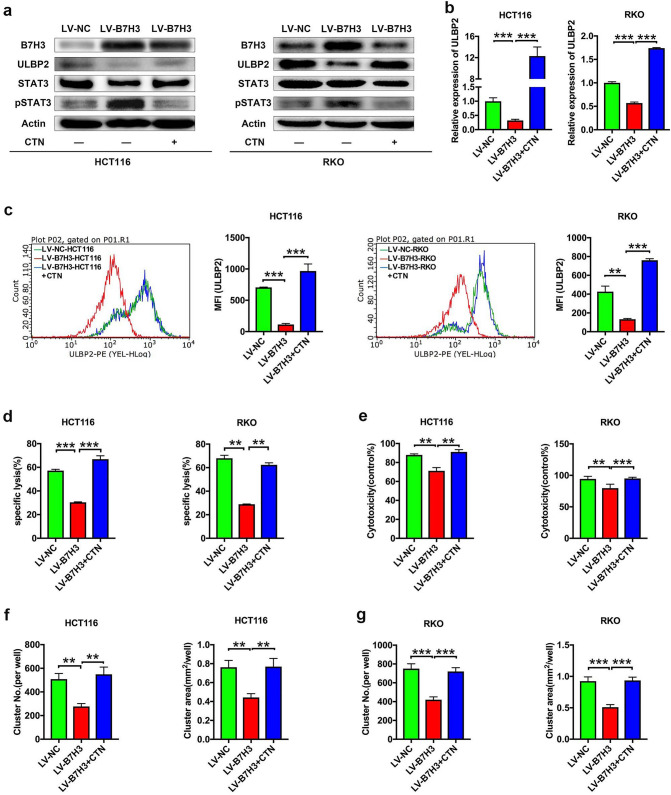
We further investigated how B7-H3 regulates ULBP2 expression in CC cells. It has been reported that inhibition of STAT3 activity significantly upregulates ULBP2 expression in tumor cells [24, 25]. Hence, we hypothesized that overexpression of B7-H3 inhibits ULBP2 expression by activating STAT3 activity. To test this hypothesis, we examined STAT3 activity in B7-H3-overexpressing CC cells. The western blotting analysis data showed that increased STAT3 activity was observed in both B7-H3-overexpressing HCT116 and RKO cells (Fig. 5a). Furthermore, the STAT3 phosphorylation inhibitor cryptotanshinone (CTN) blocked the phosphorylation of STAT3 and elevated ULBP2 protein in B7-H3-overexpressing HCT116 and RKO cells (Fig. 5a). In addition, using RT-qPCR and flow cytometry assays, we observed that CTN treatment markedly promoted the expression levels of ULBP2 in B7-H3-overexpressing HCT116 and RKO cells (Fig. 5b, c). Since B7-H3 suppressed ULBP2 expression via STAT3 activation, we further examined the role of STAT3 activation in the inhibitory effect of B7-H3 on the cytotoxicity of Vδ2 T cells. CTN treatment reversed the B7-H3 overexpression-induced decrease in the cytotoxicity of Vδ2 T cells to CC cells (Fig. 5d-g; Supplementary Fig. 5).
Fig. 5.
B7-H3 suppresses ULBP2 expression and the cytotoxicity of Vδ2 T cells through STAT3. a The protein expression of B7-H3, ULBP2, STAT3, and pSTAT3 in B7-H3-overexpressing HCT116 and RKO cells treated with or without cryptotanshinone (CTN) was analyzed by western blot assay. β-Actin served as a loading control. b The mRNA expression of ULBP2 in B7-H3-overexpressing HCT116 and RKO cells treated with or without CTN was analyzed by RT-qPCR. c The protein expression of ULBP2 in B7-H3-overexpressing HCT116 and RKO cells treated with or without CTN was analyzed by flow cytometry assay. d, e The cytotoxicity of Vδ2 T cells against B7-H3-overexpressing HCT116 or RKO cells treated with or without CTN was analyzed by LDH (d) and CCK-8 (e) assays. f, g The number (NO.) and size (area) of the clusters of Vδ2 T cells formed surrounding B7-H3-overexpressing HCT116 (f) and RKO (g) cells treated with or without CTN. Values are expressed as the means ± SD. Data are representative of results from 3 independent experiments. **p < 0.01, and ***p < 0.001
Aberrant B7-H3 expression is negatively correlated with ULBP2 in CC tissue specimens
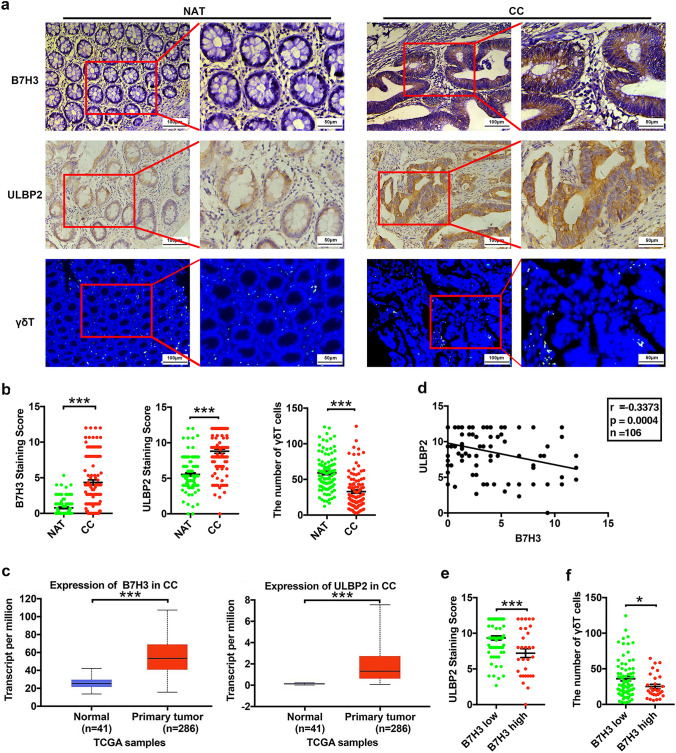
We first investigated the relationship between B7-H3 and ULBP2 expressions in the tissue specimens of patients with CC. As shown in Fig. 6a, b, and Supplementary Fig. 6, compared with the normal adjacent tissues (NAT), the tumor tissues of CC patients had increased expression of both B7-H3 and ULBP2 and declined the number of γδT cells. The mRNA expression of B7-H3 and ULBP2 was upregulated in CC patients according to the UALCAN databases (Fig. 6c). Additionally, we observed that both B7-H3 and ULBP2 mRNA levels were increased and that there was a positive correlation between B7-H3 and ULBP2 mRNA levels in these CC patients in the data downloaded from GDC portal (Supplementary Fig. 7a, b). Although both B7-H3 and ULBP2 expressions were increased in CC tissue specimens, we observed an inverse correlation between B7-H3 and ULBP2 protein levels in our cohort (Fig. 6d). Furthermore, we selected 76 cases (71.7%) with low expression of B7-H3 (immunohistochemistry (IHC) score ≤ 4) and 30 cases (28.3%) with high expression of B7-H3 (IHC score > 4). The ULBP2 expression levels and the proportions of infiltrating γδT cells in patients with high B7-H3 expression were both significantly lower than those in patients with low B7-H3 expression (Fig. 6e,f; Supplementary Fig. 6). Taken together, these clinical results revealed that B7-H3 was negatively correlated with the expression of ULBP2 and the proportions of infiltrating γδT cell in vivo.
Fig. 6.
Aberrant B7-H3 expression is negatively correlated with ULBP2 expression in colon cancer patient specimens. a A representative image of the IHC or IF assay of B7-H3, ULBP2 and infiltrating γδT cell expression in neighboring noncancerous tissue (NAT) samples and colon cancer tumor tissue (CC) samples. n = 106. b B7-H3 and ULBP2 protein expression based on their staining index in NAT and CC specimens. The expression of infiltrating γδT cells was counted in NAT and CC specimens by normalizing with DAPI cells. n = 106. c The expression of B7-H3 and ULBP2 in normal solid tissue specimens (n = 41) and primary tumor specimens (n = 286) of colon cancer patients in the TCGA cohort. d The correlation between B7-H3 and ULBP2 expression in CC patient samples. n = 106. e ULBP2 protein expression is shown in patients stratified into B7-H3 low (IHC score ≤ 4, n = 76) and B7-H3 high (IHC score > 4, n = 30) groups. f γδT cell expression is shown in patients stratified into B7-H3 low (IHC score ≤ 4, n = 76) and B7-H3 high (IHC score > 4, n = 30) groups. Values are expressed as the means ± SEM. ***p < 0.001
Discussion
The acquisition of resistance to the cytotoxicity of NK cells and various T cell subsets in cancer cells is one of the difficulties of cancer immune treatment. Hence, an increasing number of studies have been reported on the underlying molecular mechanisms by which tumor cells acquire this resistance [26, 27]. Park et al. noted that liver cancer stem cells, which highly express epithelial cell adhesion molecule (EpCAM), resisted NK cell-mediated cytotoxicity by upregulating CEACAM1 expression [26]. Targeting AXL, a member of the TAM (Tyro3, Axl, and Mer) receptor tyrosine kinase family, reversed lung cancer cell resistance to NK cell- and CTL cell-mediated cytotoxicity by upregulating ICAM1 and ULBP1 expression [27]. However, few studies have examined how CC cells escape the killing effect of γδ T cells. In the present study, we observed that B7-H3 overexpression promoted, while B7-H3 knockdown inhibited, CC cell resistance to the killing effect of Vδ2 T cells in vitro and in vivo. Therefore, we conclude that B7-H3 overexpression confers resistance to Vδ2 T cell-mediated cytotoxicity in CC cells.
B7-H3 is overexpressed in multiple malignant tumors, including CC and is positively associated with poor prognosis [28, 29]. Although the immunologic function of B7-H3 remains controversial, with conflicting costimulatory and coinhibitory functions [30], the biological functions of B7-H3 in tumor immunity have received widespread attention. Chimeric antigen receptor (CAR) T cells targeting B7-H3 (B7-H3. CAR-Ts) has been reported to control the growth of solid tumors, such as pancreatic ductal adenocarcinoma, ovarian cancer, and neuroblastoma, in vitro and in orthotopic and metastatic xenograft mouse models [31]. Cai et al. showed that tumor cells expressing B7-H3, but not host cells, inhibited antitumor T-cell functions in ovarian cancer insensitive to PD-1 blockade therapy [17]. Our previous study indicated that the expression of B7-H3 on γδT cells was increased in the peripheral blood and tumor tissues of CC patients [18]. Moreover, B7-H3 mediated the inhibition of Vδ2 T cell cytotoxicity via the downregulation of IFN-γ and perforin/granzyme B expression [18]. In this study, we explored how B7-H3 modulates CC cell-intrinsic resistance to Vδ2 T cell-mediated killing. We first surmised that B7-H3 increased CC cell resistance to Vδ2 T cell-mediated cytotoxicity through the negative regulation of ULBP2.
γδT cells exert broad innate antitumor and anti-infective activities via both indirect and direct pathways. Previous studies showed that γδT cells kill tumor cells by recognizing unprocessed PAgs via cell-surface TCRs [32], producing proinflammatory cytokines (e.g., IFN-γ, TNF-α, and IL-4) [32, 33], granzyme B and perforin [33, 34]. In addition, interactions such as NKG2D/NKG2DL, Trail/Trail-R, and Fas/FasL are also involved in the activation of the effector function of γδ T cell-mediated cytotoxicity against tumors [10]. Herein, we focused our research on B7-H3, which promotes CC cell resistance to Vδ2 T cell-mediated cytotoxicity in an NKG2D/NKG2DL interaction-dependent manner. For this reason, we analyzed the mRNA expression level of a spectrum of key NKG2DLs, including ULBP1, ULBP2, ULBP3, ULBP4, ULBP5, MICA, and MICB, in both B7-H3-overexpressing and B7-H3 knockdown CC cells. Among these NKG2DLs, the mRNA expression level of ULBP2 was negatively associated with the B7-H3 expression level in both HCT116 and RKO cells. Moreover, the western blotting and flow cytometry data indicated that B7-H3 suppressed the protein expression of ULBP2 in CC cells. Importantly, aberrant B7-H3 expression was negatively correlated with ULBP2 both in B7-H3-overexpressing HCT116 xenograft tumors and tissue specimens of patients with CC. Tumor cell-expressed immune-related proteins, such as MHC I, BTN3A1, and CD155, contributed to modifying the function of γδ T cells, NK cells and CD8 + T cells in the tumor microenvironment [35–37]. We detected and observed that the mRNA expression of MHC I, BTN3A1, and CD155 was not associated with the B7-H3 level in either HCT116 or RKO cells (Supplementary Fig. 8). These results suggest that B7-H3 negatively regulates ULBP2 expression in CC cells. ULBP2 has been associated with immune surveillance and immune escape in a wide range of cancers, such as hepatocellular carcinoma, melanoma, and CC [38–40]. In line with these studies, we showed that treatment with ULBP2 siRNA or ULBP2 mAb abolished the B7-H3 silencing-induced increase in the cytotoxicity of Vδ2 T cells to CC cells. These data indicate that B7-H3 negatively regulates ULBP2 expression and, more importantly, that B7-H3 contributes to the promotion of CC cell resistance to the killing effect of Vδ2 T cells by ULBP2. Given that NKG2D is also expressed on NK cells and various T cell subsets [41], we inferred that B7-H3 may also protect CC cells from the cytotoxic effects of NK and T cell subsets by regulating ULBP2.
Previous studies indicated that the expression of ULBP2 in cancer cells can be regulated at the transcriptional and posttranscriptional levels [42]. ULBP2 has been reported to be regulated by miR-34a/c in melanoma [43], by miR-873 in cervical cancer [44], and by miR-519a-3p in breast cancer [45]. Textor et al. revealed that wild-type p53, but not mutant p53, strongly increases the mRNA and protein expression of ULBP1 and ULBP2 [46]. Furthermore, TGF-β leads to a decrease in MICA, ULBP2, and ULBP4 transcripts in malignant glioma [47]. RAZTI1, a proteolytic enzyme, can degrade ULBP2 [42]. In addition, marine exposure induces ULBP2 expression in chronic myelogenous leukemia K562 cells by suppressing the activation of the IL-6 receptor-mediated JAK/STAT3 pathway [25]. Our previous studies showed that STAT3 signaling is a downstream target of B7-H3 in CC [48]. In this study, we observed that B7-H3 overexpression promoted STAT3 phosphorylation and inhibited ULBP2 expression in HCT116 and RKO cells. More importantly, CTN, a STAT3 phosphorylation inhibitor, blocked the phosphorylation of STAT3 and elevated ULBP2 protein in B7-H3-overexpressing HCT116 and RKO cells. Additionally, CTN treatment reversed the B7-H3 overexpression-induced decrease in the cytotoxicity of Vδ2 T cells to CC cells. These results suggest that B7-H3 inhibits ULBP2 expression and promotes CC cell resistance to Vδ2 T cell-mediated cytotoxicity dependent of the STAT3 pathway in CC. However, the current study does not clarify the precise mechanism by how B7-H3 activates the STAT3 pathway and posttranslationally modifies ULBP2 in CC cells. Further investigations on this question are needed in our future study. Besides, we observed that there was a positive correlation between B7-H3 and ULBP2 mRNA levels in CC patient tissues in the data downloaded from TCGA, but the protein expression of B7-H3 was negatively associated with ULBP2 protein levels in our cohort. Given that the regulation of ULBP2 expression in cancers is complicated [42], we inferred that different regulatory mechanisms, such as transcriptional regulation, post-transcriptional regulation, translational regulation, post-translational regulation, or mRNA degradation regulation, results in the inconsistency between mRNA and protein expression of ULBP2 in the tumor microenvironment. Therefore, it may be valuable to explore the regulation of ULBP2 expression in cancers in our future study.
Our results demonstrated that B7-H3 confers resistance to Vδ2 T cell-mediated cytotoxicity in CC cells by modulating ULBP2 expression in vitro and in vivo. Furthermore, B7-H3 mediates the inhibition of ULBP2 expression in CC cells in a STAT3 pathway-dependent manner (Fig. 7). The present study expands our understanding of B7-H3 in γδ T cell-based immunotherapy and suggests that the B7-H3/STAT3/ULBP2 axis represents a potential target for CC therapeutic intervention.
Fig. 7.
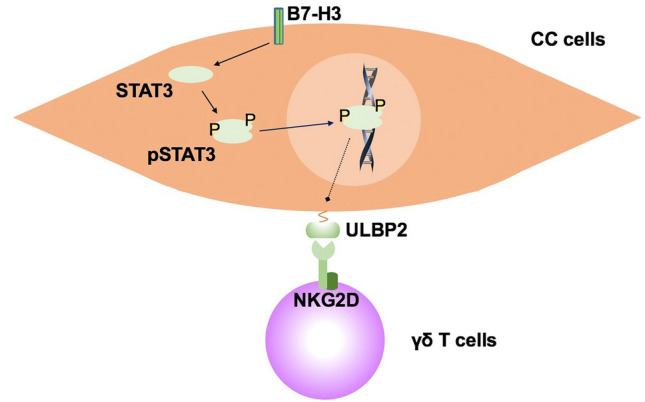
Outline of the mechanism underlying B7-H3-mediated colon cancer cell resistance to γδ T cell-mediated cytotoxicity
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank all the members of the department of gastroenterology, general surgery, and pathology of the First Affiliated Hospital of Soochow University for their help in collecting clinical samples.
Author contributions
HL, TS and WC designed the experiments; HL, YM, HW, and JL performed most of the experiments; MW and YG contributed to provide clinical samples; YM, NG, and YG assisted with experiments and analysis of the data; XZ and GZ provided administrative, technical, or material support. HL, TS, and WC wrote the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (81802843, 81672372, 81372276); Colleges and Universities Natural Science Research Project of Jiangsu Province (18KJB320023, 17KJA310004); Suzhou Science & Technology plan project (SYS2019035, SYS201747, SS2019077).
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval and consent to participate
All animal experiments were performed under the institutional guidelines of the Institutional Animal Care and Use Committee of Soochow University. The experiments involving in tissue samples of patients with CC were approved by the Institutional Review Board of the First Affiliated Hospital of Soochow University.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Tongguo Shi, Email: shitg@suda.edu.cn.
Weichang Chen, Email: weichangchen@126.com.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Tamas K, Walenkamp AM, de Vries EG, van Vugt MA, Beets-Tan RG, van Etten B, de Groot DJ, Hospers GA. Rectal and colon cancer: Not just a different anatomic site. Cancer Treat Rev. 2015;41:671–679. doi: 10.1016/j.ctrv.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Goding Sauer A, Fedewa SA, Butterly LF, Anderson JC, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020 doi: 10.3322/caac.21601. [DOI] [PubMed] [Google Scholar]
- 4.Bhat SAVD. Checkpoint blockade rescues the repressive effect of histone deacetylases inhibitors on γδ T cell function. Front Immunol. 2018;9:1615. doi: 10.3389/fimmu.2018.01615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bottino CTG, Ferrini S, et al. Two subsets of human T lymphocytes expressing gamma/delta antigen receptor are identifiable by monoclonal antibodies directed to two distinct molecular forms of the receptor. J Exp Med. 1988;168:491–505. doi: 10.1084/jem.168.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pui CH, Pei D, Cheng C, et al. Treatment response and outcome of children with T-cell acute lymphoblastic leukemia expressing the gamma-delta T-cell receptor. Oncoimmunology. 2019;8:1599637. doi: 10.1080/2162402X.2019.1599637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gober HJ, Kistowska M, Angman L, Jeno P, Mori L, De Libero G. Human T cell receptor gammadelta cells recognize endogenous mevalonate metabolites in tumor cells. J Exp Med. 2003;197:163–168. doi: 10.1084/jem.20021500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Z. X, Dual face of Vγ9Vδ2-T cells in tumor immunology: anti- versus pro-tumoral activities. Front Immunol. 2017;8:1041. doi: 10.3389/fimmu.2017.01041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baker FL, Bigley AB, Agha NH, Pedlar CR, O'Connor DP, Bond RA, Bollard CM, Katsanis E, Simpson RJ. Systemic beta-adrenergic receptor activation augments the ex vivo expansion and anti-tumor activity of Vgamma9Vdelta2 T-cells. Front Immunol. 2019;10:3082. doi: 10.3389/fimmu.2019.03082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang ZWZ, Li S, et al. Decitabine enhances Vγ9Vδ2 T cell-mediated cytotoxic effects on osteosarcoma cells the NKG2DL-NKG2D Axis. Front Immunol. 2018;9:1239. doi: 10.3389/fimmu.2018.01239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Willcox BE, Willcox CR. gammadelta TCR ligands: the quest to solve a 500-million-year-old mystery. Nat Immunol. 2019;20:121–128. doi: 10.1038/s41590-018-0304-y. [DOI] [PubMed] [Google Scholar]
- 12.Li Y, Guo G, Song J, Cai Z, Yang J, Chen Z, Wang Y, Huang Y, Gao Q. B7–H3 promotes the migration and invasion of human bladder cancer cells via the PI3K/Akt/STAT3 signaling pathway. J Cancer. 2017;8:816–824. doi: 10.7150/jca.17759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee YH, Martin-Orozco N, Zheng P, et al. Inhibition of the B7–H3 immune checkpoint limits tumor growth by enhancing cytotoxic lymphocyte function. Cell Res. 2017;27:1034–1045. doi: 10.1038/cr.2017.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu H, Tekle C, Chen YW, et al. B7–H3 silencing increases paclitaxel sensitivity by abrogating Jak2/Stat3 phosphorylation. Mol Cancer Ther. 2011;10:960–971. doi: 10.1158/1535-7163.MCT-11-0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Son Y, Kwon S-M, Cho J-Y. CD276 (B7–H3) maintains proliferation and regulates differentiation in angiogenic function in late endothelial progenitor cells. Stem Cells. 2019;37:382–394. doi: 10.1002/stem.2944. [DOI] [PubMed] [Google Scholar]
- 16.Lemke D, Pfenning PN, Sahm F, et al. Costimulatory protein 4IgB7H3 drives the malignant phenotype of glioblastoma by mediating immune escape and invasiveness. Clin Cancer Res. 2012;18:105–117. doi: 10.1158/1078-0432.CCR-11-0880. [DOI] [PubMed] [Google Scholar]
- 17.Cai D, Li J, Liu D, et al. Tumor-expressed B7–H3 mediates the inhibition of antitumor T-cell functions in ovarian cancer insensitive to PD-1 blockade therapy. Cell Mol Immunol. 2020;17:227–236. doi: 10.1038/s41423-019-0305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu H, Shi T, Wang M, Li X, Gu Y, Zhang X, Zhang G, Chen W. B7–H3 inhibits the IFN-γ-dependent cytotoxicity of Vγ9Vδ2 T cells against colon cancer cells. OncoImmunology. 2020;9:1748991. doi: 10.1080/2162402x.2020.1748991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang R, Ma Y, Zhan S, Zhang G, Cao L, Zhang X, Shi T, Chen W. B7–H3 promotes colorectal cancer angiogenesis through activating the NF-kappaB pathway to induce VEGFA expression. Cell Death Dis. 2020;11:55. doi: 10.1038/s41419-020-2252-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li X, Lu H, Gu Y, Zhang X, Zhang G, Shi T, Chen W. Tim-3 suppresses the killing effect of Vgamma9Vdelta2T cells on colon cancer cells by reducing perforin and granzyme B expression. Exp Cell Res. 2020;386:111719. doi: 10.1016/j.yexcr.2019.111719. [DOI] [PubMed] [Google Scholar]
- 21.Zhou XGY, Xiao H, et al. Combining Vγ9Vδ2 T cells with a lipophilic bisphosphonate efficiently kills activated hepatic stellate cells. Front Immunol. 2017;8:1381. doi: 10.3389/fimmu.2017.01381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma Y, Wang R, Lu H, et al. B7–H3 promotes the cell cycle-mediated chemoresistance of colorectal cancer cells by regulating CDC25A. J Cancer. 2020;11:2158–2170. doi: 10.7150/jca.37255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chandrashekar DS, Bashel B, Balasubramanya SAH, Creighton CJ, Ponce-Rodriguez I, Chakravarthi BVSK, Varambally S. UALCAN: a portal for facilitating tumor subgroup gene expression and survival analyses. Neoplasia (New York, NY) 2017;19:649–658. doi: 10.1016/j.neo.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu L, Chen X, Shen M, et al. Inhibition of IL-6-JAK/Stat3 signaling in castration-resistant prostate cancer cells enhances the NK cell-mediated cytotoxicity via alteration of PD-L1/NKG2D ligand levels. Mol Oncol. 2018;12:269–286. doi: 10.1002/1878-0261.12135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu XZZ, Jiang L, et al. Matrine increases NKG2D ligand ULBP2 in K562 cells via inhibiting JAK/STAT3 pathway: a potential mechanism underlying the immunotherapy of matrine in leukemia. Am J Transl Res. 2015;7:1838–1849. [PMC free article] [PubMed] [Google Scholar]
- 26.Park DJ, Sung PS, Kim J-H, Lee GW, Jang JW, Jung ES, Bae SH, Choi JY, Yoon SK. EpCAM-high liver cancer stem cells resist natural killer cell–mediated cytotoxicity by upregulating CEACAM1. J ImmunoTher Cancer. 2020;8:e000301. doi: 10.1136/jitc-2019-000301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Terry S, Abdou A, Engelsen AST, et al. AXL targeting overcomes human lung cancer cell resistance to NK- and CTL-mediated cytotoxicity. Cancer Immunol Res. 2019;7:1789–1802. doi: 10.1158/2326-6066.CIR-18-0903. [DOI] [PubMed] [Google Scholar]
- 28.Wu J, Wang F, Liu X, Zhang T, Liu F, Ge X, Mao Y, Hua D. Correlation of IDH1 and B7 H3 expression with prognosis of CRC patients. Eur J Surg Oncol. 2018;44:1254–1260. doi: 10.1016/j.ejso.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 29.Dong P, Xiong Y, Yue J, Hanley SJB, Watari H. B7H3 as a promoter of metastasis and promising therapeutic target. Front Oncol. 2018 doi: 10.3389/fonc.2018.00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ni L, Dong C. New B7 family checkpoints in human cancers. Mol Cancer Ther. 2017;16:1203–1211. doi: 10.1158/1535-7163.mct-16-0761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Du H, Hirabayashi K, Ahn S, et al. Antitumor responses in the absence of toxicity in solid tumors by targeting B7–H3 via chimeric antigen receptor T cells. Cancer Cell. 2019;35:221–37.e8. doi: 10.1016/j.ccell.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wohler JE, Smith SS, Barnum SR. Gammadelta T cells: the overlooked T-cell subset in demyelinating disease. J Neurosci Res. 2010;88:1–6. doi: 10.1002/jnr.22176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu YL, Ding YP, Tanaka Y, Shen LW, Wei CH, Minato N, Zhang W. Gammadelta T cells and their potential for immunotherapy. Int J Biol Sci. 2014;10:119–135. doi: 10.7150/ijbs.7823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Silva-Santos B, Serre K, Norell H. γδ T cells in cancer. Nat Rev Immunol. 2015;15:683–691. doi: 10.1038/nri3904. [DOI] [PubMed] [Google Scholar]
- 35.Riond JRS, Nicolau ML, et al. In vivo major histocompatibility complex class I (MHCI) expression on MHCIlow tumor cells is regulated by gammadelta T and NK cells during the early steps of tumor growth. Cancer Immunity. 2009;9:10. [PMC free article] [PubMed] [Google Scholar]
- 36.Zocchi MRCD, Venè R, et al. Zoledronate can induce colorectal cancer microenvironment expressing BTN3A1 to stimulate effector γδ T cells with antitumor activity. Oncoimmunology. 2017;6:e1278099. doi: 10.1080/2162402X.2016.1278099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li XY, Das I, Lepletier A, et al. CD155 loss enhances tumor suppression via combined host and tumor-intrinsic mechanisms. J Clin Invest. 2018;128:2613–2625. doi: 10.1172/JCI98769. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38.Shi L, Lin H, Li G, et al. Cisplatin enhances NK cells immunotherapy efficacy to suppress HCC progression via altering the androgen receptor (AR)-ULBP2 signals. Cancer Lett. 2016;373:45–56. doi: 10.1016/j.canlet.2016.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frazao A, Colombo M, Fourmentraux-Neves E, et al. Shifting the balance of activating and inhibitory natural killer receptor ligands on BRAFV600E melanoma lines with vemurafenib. Cancer Immunol Res. 2017;5:582–593. doi: 10.1158/2326-6066.cir-16-0380. [DOI] [PubMed] [Google Scholar]
- 40.Rothe A, Jachimowicz RD, Borchmann S, et al. The bispecific immunoligand ULBP2-aCEA redirects natural killer cells to tumor cells and reveals potent anti-tumor activity against colon carcinoma. Int J Cancer. 2014;134:2829–2840. doi: 10.1002/ijc.28609. [DOI] [PubMed] [Google Scholar]
- 41.Sun B, Yang D, Dai H, et al. Eradication of hepatocellular carcinoma by NKG2D-based CAR-T cells. Cancer Immunol Res. 2019;7:1813–1823. doi: 10.1158/2326-6066.cir-19-0026. [DOI] [PubMed] [Google Scholar]
- 42.Duan S, Guo W, Xu Z, et al. Natural killer group 2D receptor and its ligands in cancer immune escape. Mol Cancer. 2019;18:29. doi: 10.1186/s12943-019-0956-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heinemann A, Zhao F, Pechlivanis S, Eberle J, Steinle A, Diederichs S, Schadendorf D, Paschen A. Tumor suppressive microRNAs miR-34a/c control cancer cell expression of ULBP2, a stress-induced ligand of the natural killer cell receptor NKG2D. Cancer Res. 2012;72:460–471. doi: 10.1158/0008-5472.CAN-11-1977. [DOI] [PubMed] [Google Scholar]
- 44.Liang HX, Li YH. MiR-873, as a suppressor in cervical cancer, inhibits cells proliferation, invasion and migration via negatively regulating ULBP2. Genes Genom. 2020;42:371–382. doi: 10.1007/s13258-019-00905-8. [DOI] [PubMed] [Google Scholar]
- 45.Breunig C, Pahl J, Küblbeck M, et al. MicroRNA-519a-3p mediates apoptosis resistance in breast cancer cells and their escape from recognition by natural killer cells. Cell Death Dis. 2017;8:e2973-e. doi: 10.1038/cddis.2017.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Textor S, Fiegler N, Arnold A, Porgador A, Hofmann TG, Cerwenka A. Human NK cells are alerted to induction of p53 in cancer cells by upregulation of the NKG2D ligands ULBP1 and ULBP2. Cancer Res. 2011;71:5998–6009. doi: 10.1158/0008-5472.CAN-10-3211. [DOI] [PubMed] [Google Scholar]
- 47.Eisele G, Wischhusen J, Mittelbronn M, Meyermann R, Waldhauer I, Steinle A, Weller M, Friese MA. TGF-beta and metalloproteinases differentially suppress NKG2D ligand surface expression on malignant glioma cells. Brain. 2006;129:2416–2425. doi: 10.1093/brain/awl205. [DOI] [PubMed] [Google Scholar]
- 48.Shi T, Ma Y, Cao L, et al. B7–H3 promotes aerobic glycolysis and chemoresistance in colorectal cancer cells by regulating HK2. Cell Death Dis. 2019 doi: 10.1038/s41419-019-1549-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its supplementary information files.