Abstract
To obtain immunomodulatory peptides from isolated soy protein (ISP), pepsin was selected to prepare hydrolysates and 4-h treatment (Pepsin-ISPH4h) showed the highest yield and immunomodulatory activities. The Pepsin-ISPH4h was sequentially fractionated by 30, 10 and 1-kDa molecular weight cut-off (MWCO) membranes, in which 1-kDa MWCO permeate (1P) exhibited the most significant enhancement of phagocytosis activity without causing excessive inflammation as compared with Pepsin-ISPH4h. To further purify and enhance the immunomodulatory activity, 1P was distinct by high-performance liquid chromatography equipped with a reverse-phase column and in vivo immunomodulatory activity of fractions was examined in mice. Fraction 1 (F1) significantly elevated phagocytosis activity of mice spleen macrophages and neutrophils. However, increase of phagocytosis activity did not result from the induction of macrophages M1 or M2 polarization. The immunomodulatory peptide sequence, EKPQQQSSRRGS, from F1 was identified by LC–MS/MS. Phagocytosis activity and macrophage M1 polarization were elevated by synthetic peptide treatment. Hence, our results indicated that isolated soy protein hydrolysates prepared by pepsin could provide a source of peptides with immunomodulatory effects.
Graphical Abstract
Keywords: Immunomodulatory peptide, Isolated soy protein (ISP), Lipopolysaccharide (LPS), Molecular weight cut-off (MWCO), Pepsin, Soybean
Introduction
Human immunity is influenced by many factors such as age, dietary habit, exercise, stress, and so on (Hamer et al. 2012; Marques et al. 2015). Busy life style causes imbalanced immune modulations in people of modern world; therefore, maintaining normal daily life style as well as supplementing immune modulators can rectify immune imbalance (Santiago-López et al. 2016; Yu et al. 2018; Polak et al. 2021). The studies of protein enzymatic hydrolysates are initially focused on improving dietary and nutritional functions (Adler-Nissen 1986), for example human gut intestinal systems absorb small peptides (dipeptide or tripeptide) better than free amino acids (Ziegler et al. 1990; Siemensma et al. 1993; Bueno-Gavilá et al. 2021; Zaky et al. 2022). Recently, immunomodulatory peptides showed beneficial effects on human health are prepared from food-based proteins such as chum salmon (Yang et al. 2009), Alasaka pollock (Hou et al. 2012), rohu egg (Chalamaiah et al. 2014, 2018), wheat germ globulin (Wu et al. 2017), rice (Fang et al. 2019), false starwort (Pseudostellaria heterophylla; Yang et al. 2020), duck egg ovalbumin (He et al. 2021), and Stevia rebaudiana (Li et al. 2021), implying that an increasing number of scientists are attracted and devoted into this research field.
The primary function of mammalian immune system is to prevent contagious illnesses by building up a complicate firewall by cells and proteins (Bayne 2013), and can be divided into innate and adaptive immune systems (Iwasaki and Medzhitov 2015). Macrophages and neutrophils are endocytic defense cells of innate immune system that can nonspecifically engulf external pathogens and trigger inflammation responses by releasing NO and cytokines (Gordon 2016). Proper inflammation response can help human body to defense invasions of pathogens; however, hyperinflammation may cause tissues damages (Ginderachter et al. 2006). Cytokines, such as interleukin-6 (IL-6), interleukin-10 (IL-10), and tumor necrosis factor-α (TNF-α), are tiny proteins, modulating the growth and functions of immune cells (Kim et al. 2008; Ahn et al. 2015). Macrophage cells can further polarize into M1 and M2 types, in which M1 cells can produce high amount of pro-inflammatory cytokines and reactive oxygen species to promote inflammation, whereas M2 cells secrete anti-inflammatory cytokine, IL-10, to repair damaged tissues (Murray et al. 2014).
Soybean (Glycine max L.) protein is an important source with properties of high yield, low price, high nutritive value, and broad applications (Ricker et al. 2004; Coscueta et al. 2019; Akbarian et al. 2022). Peptides identified from soy protein hydrolysates have shown functions of anti-oxidation (Ranamukhaarachchi et al. 2013; Ashaolu 2020), stimulating lipolysis (Tsou et al. 2012), anti-angiotensin I-converting enzyme activity (Rho et al. 2009), and immunomodulatory effects (Egusa and Otani 2009; Dia et al. 2014; Zhang et al. 2021). The goal of this study was to isolate and purify immunomodulatory peptides from pepsin-isolated soy protein hydrolysates (Pepsin-ISPH) using a combination of molecular weight cut-off module and reverse-phase high-performance liquid chromatography. Peptides were further identified by mass spectrometry and synthetic peptides were used for investigating its mechanisms of immunomodulation functions.
Materials and methods
Materials and chemical reagents
Isolated soy protein (ISP) and NEW Soy 88 were purchased from Gemfont Co., Taipei, Taiwan. Pepsin from porcine gastric mucosa, sodium dodecyl sulfate (SDS), o-phthalaldehyde (OPA), Leu-Gly dipeptide, lipopolysaccharide (LPS), dimethyl sulfoxide (DMSO), sodium carbonate (NaHCO3), and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were obtained from MilliporeSigma, Darmstadt, Germany. Dulbecco’s modified Eagle medium (DMEM) was purchased from Gibco, TX, USA. l-Glutamate and charcoal/dextran-treated fetal bovine serum (FBS) were obtained from Biological Industries, CT, USA. Molecular weight cut-off (MWCO) membranes, ER 30 kDa, PW 10 kDa, and GE 1 kDa, were obtained from Osmonics Inc., MN, USA. All chemical reagents used were American Chemistry Society (ACS) grade or better.
Preparation of enzymatic hydrolysate, measurement of hydrolysis ratio, and determination of soluble nitrogen by Kjeldahl method
The 2.5% (w/v) ISP was dissolved in 0.2 M phosphate buffer (pH 2.0) and digested by pepsin (S:E ratio = 100:1) at 37 °C. Hydrolysates of isolated soy protein (ISPH) were collected at 0, 0.5, 1, 2, 4, and 6 h, then pepsin was inactivated by boiling for 15 min followed by storing at − 20 °C until use.
The degree of hydrolysis (DH) was measured by OPA method using the dipeptide, Leu-Gly, as standard (Nielson et al. 2001). DH (%) was indicated as:
Hsample represents α-amino group concentration (mmol/mL); Htotal represents total peptide number of ISPH (7.8 mEq α-amino group/g).
The soluble nitrogen of hydrolysate was prepared by adding 20% (w/v) trichloroacetic acid, and its nitrogen content was estimated by Kjeldahl method according to Tsou et al. (2012). Yield (N mg/mL) was indicated as:
V1: titration volume of sample (mL); V2: titration volume of blank (mL); C: concentration of HCl (0.1 N × titer); 14: atomic mass of nitrogen.
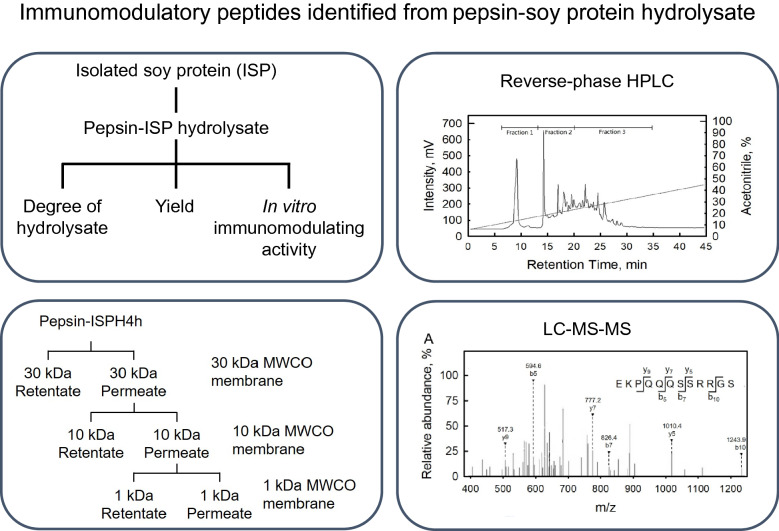
Fractionation of hydrolysate by molecular weight cut-off
Hydrolysate of 4-h pepsin-treated isolated soy protein (Pepsin-ISPH4h) was sequentially fractionated by a membrane MWCO module with 30 kDa, 10 kDa, and 1 kDa to obtain retentates and permeates. One volume of Pepsin-ISPH4h was initially filtered by a 30-kDa MWCO membrane to acquire 1:9 ratio of retentates (30R) and permeates. The 30-kDa permeate was then filtered by a 10-kDa MWCO membrane to acquire 1:9 ratio of retentates (10R) and permeates. The 10-kDa permeate was then filtered by a 1-kDa MWCO membrane to acquire 1:9 ratio of retentates (1R) and permeates (1P).
Fractionation of 1P fraction by reverse-phase HPLC
Fraction 1P was further fractionated using a reverse-phase high-performance liquid chromatography applied on an InertSustain® C18 column (10 × 250 mm, 5 μm, GL Sciences, Japan) with a linear gradient of acetonitrile from 0 to 45% in 45 min at a flow rate of 2 mL/min. The elution signals were monitored at 214 nm.
Culture of mouse macrophage cells
Mouse macrophage RAW264.7 cell line was obtained from Bioresource Collection and Research Center (BCRC 60001), Hsinchu, Taiwan. Cells were grown in DMEM medium supplemented with 10% FBS, 1.6 g/L NaHCO3, and 2 mM l-glutamine, maintaining in a 5% CO2 incubator at 37 °C. Cells were subcultured every 48–72 h, and discarded after 50 generations.
Cell viability—MTT test
The cytotoxicity activity against RAW264.7 macrophage was investigated by MTT assay (Mosmann et al. 1983; Tsou et al. 2012). Cells were cultured in a 96-well microtiter plate (1 × 105 cells/well) for 24 h followed by incubating with various ISPH for 24 h. Cells were washed with phosphate buffer saline (PBS) and then incubated with MTT solution (0.5 mg/mL) at 37 °C for 4 h. Methanol treatment was used as negative control. DMSO solution was applied to resuspend the MTT formazan for 20 min. The absorbance was determined at 595 nm using a microtiter plate reader (BioTek, VT, USA). Cell viability (%) was indicated as:
ODsample: absorbance at 595 nm of sample; ODcontrol: absorbance at 595 nm of untreated sample as control; ODmethanol: absorbance at 595 nm of sample treated with methanol as negative control.
Determination of nitrogen oxide production
The NO production was measured by Griess assay (Ahn et al. 2015). RAW264.7 macrophage cells were cultured in a 96-well microtiter plate (1 × 105 cells/well) for 24 h followed by incubated with 1 ppm LPS and/or various ISPH for 24 h. After treatment, 50 μL culture medium was mixed with 50 μL Griess reagent and then incubated in dark place for 10 min. The absorbance of the mixture was determined at 550 nm using a Microtiter plate reader (BioTek, VT, USA). The concentration of NO was calculated using a standard curve generated from sodium nitrite dissolved in DMEM medium.
Phagocytosis assay
RAW264.7 macrophage cells were cultured in a 96-well microtiter plate (1 × 105 cells/well) for 24 h followed by incubated with various ISPH for 24 h. One ppm LPS treatment was used as positive control. E. coli BL21 cells transformed with pEGFP plasmid was added in a 96-well microtiter plate (5 × 106 cells/well) and centrifuged at 120g at 4 °C for 5 min to precipitate E. coli cells to be phagocytosed by macrophage for 2 h. Trypan blue regent (2 ×) was added in a 96-well microtiter plate and incubated for 2 min. Trypan blue was removed and fluorescence signal was measured with excitation wavelength at 485 nm and emission wavelength at 538 nm. Relative phagocytosis was indicated as:
Determination of pro-inflammatory cytokines in RAW246.7 macrophage cells stimulated by LPS
The levels of interleukin-6 (IL-6) and interleukin-10 (IL-10) were measured by mouse IL-6 and IL-10 Quantikine ELISA kits (R&D systems, MN, USA) following to the manufacturer’s instruction. RAW264.7 macrophage cells were cultured in a 96-well microtiter plate (1 × 105 cells/well) for 24 h and culture media were stored at − 20 °C until use. Capture antibodies were coated onto 96-well microtiter plate at 4 °C overnight. After blocking, microtiter plate was washed three times and 100 μL/well standards or culture media were added and incubated at room temperature for 2 h. Microtiter plate was washed three times and then 100 μL/well detection antibodies were added and incubated at room temperature for 2 h. Microtiter plate was washed three times and then 100 μL/well streptavidin–HRP was added and incubated in dark for 20 min. Plate was washed three times and then 100 μL/well substrate solution was added and incubated in dark condition for 20 min. After incubation, 50 μL stop solution was added and the absorbance of the mixture was determined at 450 nm using a microtiter plate reader (BioTek, VT, USA). The concentrations of cytokinins were calculated using a standard curve generated from various concentrations of standards.
Mouse spleen cell endocytosis assay
Male C57BL/6J mouse (19 weeks) was injected intraperitoneally with 5 mg/kg samples, and killed three days after injection. Spleen was collected and placed in 5 mL YAC medium (RPMI 1640, 2.2 g/L NaHCO3, 2 mM l-glutamine, 10% FBS) and then ground with 200 mesh sieve. Supernatant was removed by centrifugation at 300g, 4 °C, for 10 min. Pellet/cell was kept and resuspended in 5 mL 0.1 × Hank’s balanced salt solution (HBSS) to disrupt red blood cells and then added 10 mL HBSS. Supernatant was removed by centrifugation at 300g, 4 °C, for 10 min, and pellet/cell was resuspended in YAC medium and adjusted to 2 × 107 cells/mL by a FACSCalibur™ Flow Cytometer (BD Bioscience, NJ, USA). Spleen lymphocyte cells were cultured in a 96-well microtiter plate (1 × 105 cells/well) and BioParticles® FITC–Escherichia coli (2.5 × 106 cells/well) was added and incubated at 37 °C for 2 h. After removing supernatant, lymphocyte cells were mixed with 100 μL trypan blue and fluorescence signal was measured by FACSCalibur™ Flow Cytometer (BD Bioscience, NJ, USA). Positive fluorescence level was indicated as:
M1: percentage cell within 101 fluorescence signal; M2: percentage cell within 102 fluorescence signal; M3: percentage cell within 103 fluorescence signal; M4: percentage cell within 104 fluorescence signal.
Differentiation of M1 and M2 macrophages
Mouse spleen lymphocyte cells were harvested as mentioned previously. Surface biomarkers, PE-anti-mouse CD68, PE/Cy5-anti-mouse CD197, and FITC-anti-mouse CD206 antibodies, were obtained from Biolegend, CA, USA. Nine μL fluorescence conjugated antibodies were mixed with 50 μL cell suspensions and incubated at 4 °C in dark for 30 min. Cells were washed and resuspended in 200 μL analysis buffer (RPMI 1640, 5% FBS, 2 mM l-glutamate, and 2 × Dulbecco’s phosphate buffer saline). Two differentiated cells, M1 (CD68+/CD197+) and M2 (CD68+/CD206+) were analyzed using a FACSCalibur™ Flow Cytometer (BD Bioscience, NJ, USA).
Identification of anti-inflammatory peptide by LC–MS/MS and peptide synthesis
Amino acid sequence identification of anti-inflammation peptide was analyzed by UPLC (UltiMate 3000, ThermoFisher, MA, USA) followed by quadruple tandem ion trap mass spectrometer (Q-TOF/MS/MS; Bruker micrOTOF-Q III, Bruker Daltonic, Germany) equipped with an electrospray ionization source in Center of Precision Instrument, Tunghai University, Taichung, Taiwan. Immunomodulatory peptides isolated from pepsin–soybean hydrolysate were chemically synthesized by Yao Hong Biotechnology Inc., New Taipei City, Taiwan. Peptides, 5 mg/kg and 25 mg/kg, were used in endocytosis assay and macrophage phenotype assay, respectively.
Statistical analysis
Results were expressed as mean ± standard deviation (SD) and analyzed using Statistical Analysis System (SAS/STAT® software, NC, USA). Mean with different letters were labeled as significantly different (p < 0.05) by Duncan’s multiple range test.
Results and discussion
Effect of pepsin hydrolysis of ISP on phagocytosis, NO formation, and cell viability in RAW264.7 macrophage cells
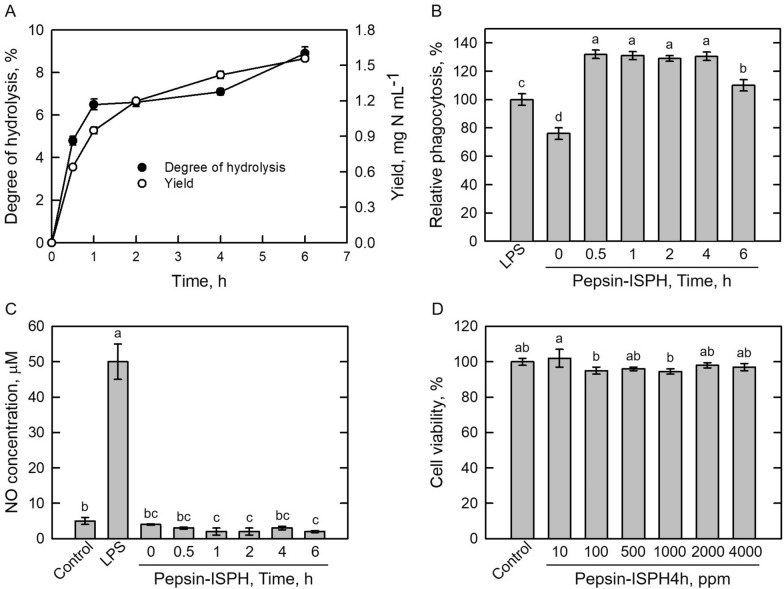
Degree of enzymatic hydrolysis has been shown to influence functions of peptides (Jamdar et al. 2010; Liu et al. 2010; Tsou et al. 2012). To investigate immunomodulatory effects of soy protein hydrolysate, hydrolysates (Pepsin-ISPH) were obtained with the degree of hydrolysis (DH, %) of 4.8%, 6.5%, 6.6%, 7.1%, and 8.9% together with yield of 0.64, 0.95, 1.2, 1.42, and 1.56 mg nitrogen per mL, respectively (Fig. 1A). The DH of Pepsin-ISPH was positively correlated to yield in peptic hydrolysis time; the result was similar to previous studies (Jamdar et al. 2010; Liu et al. 2010; Tsou et al. 2012; Toopcham et al. 2017). In terms of phagocytosis activity, Pepsin-ISPH from 0.5 to 4 h, were 1.3-fold higher than that of LPS-treated RAW264.7 macrophage cells, whereas Pepsin-ISPH6h only showed slightly 1.1-fold increase (Fig. 1B). NO formation (Fig. 1C) and cell viability (Fig. 1D) were monitored in LPS and Pepsin-ISPH-treated RAW264.7 macrophage cells. NO was significantly produced in LPS-treated RAW264.7 macrophage cells (Dia et al. 2014; Ahn et al. 2015; Li et al. 2021); however, significant effects were not observed in pepsin hydrolysates-treated RAW264.7 macrophage cells (Fig. 1C). According to MTT assay on cell viability (Fig. 1D), Pepsin-ISPH in the ranges from 10 to 4000 ppm was not toxic to RAW264.7 macrophage cells (Fang et al. 2019; Lee et al. 2021). Next, hydrolysate of isolated soy protein digested with pepsin for 4 h (Pepsin-ISPH4h) was used to further fractionation to enrich the ability of immunomodulation.
Fig. 1.
Preparation of pepsin-isolated soy protein hydrolysate (Pepsin-ISPH). A effect of different hydrolysis time of isolated soy protein hydrolysate by pepsin on degree of hydrolysis (●) and yield (○) in terms of TCA soluble nitrogen. B effect of 4000 ppm Pepsin-ISPH with different hydrolysis time on relative phagocytosis (%) in mouse RAW246.7 macrophages. C effect of 4000 ppm Pepsin-ISPH with different hydrolysis time on relative phagocytosis (%) in mouse RAW246.7 macrophages. D dosage effect of Pepsin-ISPH4h on cell viability (%) in mouse RAW246.7 macrophages. LPS (1 ppm) was used as positive control. Bars represent mean ± standard deviation (SD; n = 3). Mean with different letters are significantly different (p < 0.05) by Duncan’s multiple range test
Effect of pepsin-ISPH separated by different MWCO membranes on phagocytosis, NO formation, and cell viability in RAW264.7 macrophage cells
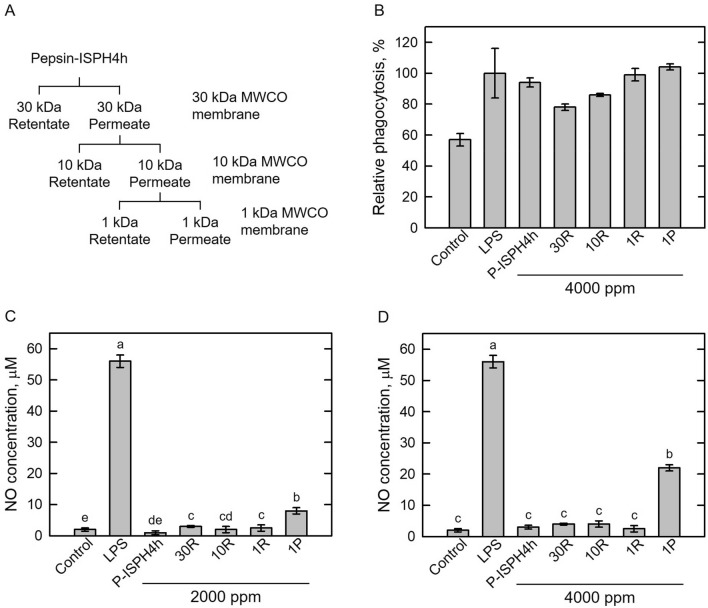
Pepsin-ISPH4h was sequentially fractionated using a membrane MWCO module with three different molecular weights, 30 kDa, 10 kDa, and 1 kDa, to acquire three retentates and one permeate fractions, namely 30R, 10R, 1R, and 1P (Fig. 2A), for further enhancing its immunomodulatory activity (Tsou et al. 2012). Next, the effects of phagocytosis of mouse macrophage cells were examined (Fig. 2B). The phagocytosis ability of the 4000 ppm 1P-treated RAW264.7 macrophage cells was slightly higher than that of LPS-treated control cells, suggesting that peptides in the 1P fraction can boost the function of RAW264.7 macrophage cells. To examine the dosage effects on NO formation, RAW264.7 macrophage cells were treated with 2000 ppm (Fig. 2C) or 4000 ppm (Fig. 2D) hydrolysate, and LPS treatment was used as positive control. Likewise, NO was significantly produced in LPS-treated RAW264.7 macrophage cells (Fig. 2C, D). NO formation by 1P treatment was increased in a dosage-dependent manner (Fig. 2C, D), indicating that peptide(s) in 1P fraction can induce NO formation in RAW264.7 macrophage cells. Previous studies had shown that proper NO formation can help immune system and macrophages to destroy tumor cells as well as invasive pathogens (Zheng et al. 2014; Fang et al. 2019; Li et al. 2021); moreover, NO formation in over 2000 ppm dosage of the 1P fraction-treated macrophages were not correlated to negative inflammation reaction (Fig. 2C). As a result, the 1P fraction was used for further fractionation to enrich the ability of immunomodulation effects.
Fig. 2.
Fractionation of Pepsin-ISPH by molecular weight cut-off (MWCO) membrane. A fraction chart. B effect of 4000 ppm Pepsin-ISPH and its retentates/permeate on relative phagocytosis (%) in mouse macrophage RAW246.7 cells. Dosage effect of Pepsin-ISPH and its retentates/permeate, 2000 ppm (C) and 4000 ppm (D) on NO concentration in mouse macrophage RAW246.7 cells. LPS (1 ppm) was used as positive control. Bars represent mean ± standard deviation (SD; n = 3). Mean with different letters are significantly different (p < 0.05) by Duncan’s multiple range test
Effect of 1-kDa permeate (1P) on phagocytosis, NO formation, cell viability, and pro-inflammatory cytokines production in RAW264.7 macrophage cells
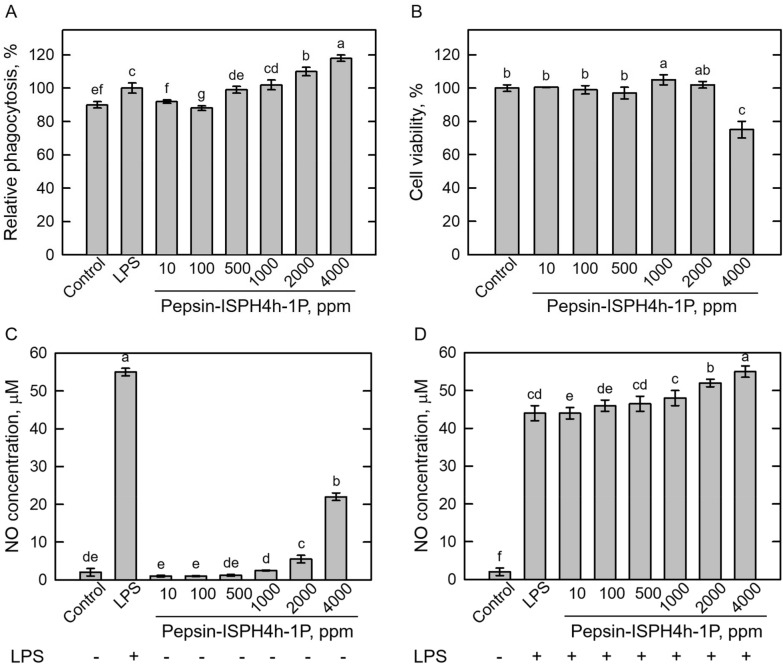
To investigate the immunomodulation function of the 1-kDa permeate (1P), phagocytosis activity (Fig. 3A), cell viability (Fig. 3B) and NO formation in the absence (Fig. 3C) or presence (Fig. 3D) of LPS were used to estimate the optimum working concentration of the 1P fraction. In Fig. 3A, the maximum phagocytosis activity was monitored in the 4000 ppm 1P-treated RAW264.7 macrophage cells. Except 4000 ppm 1P treatment, cell viabilities were not influenced by 1P treatments from 10 to 2000 ppm (Fig. 3B). Furthermore, 4000 ppm 1P treatment led to remarkable NO formations with or without LPS (Fig. 3C, D).
Fig. 3.
Determination of anti-inflammatory activity of Pepsin-ISPH4h-1P fraction. Dosage effect of Pepsin-ISPH4h-1P on relative phagocytosis (%, A), cell viability (%, B), and NO formation in the absence (C) or presence (D) of 1 ppm LPS in mouse macrophage RAW246.7 cells. Bars represent mean ± standard deviation (SD; n = 3). Mean with different letters are significantly different (p < 0.05) by Duncan’s multiple range test
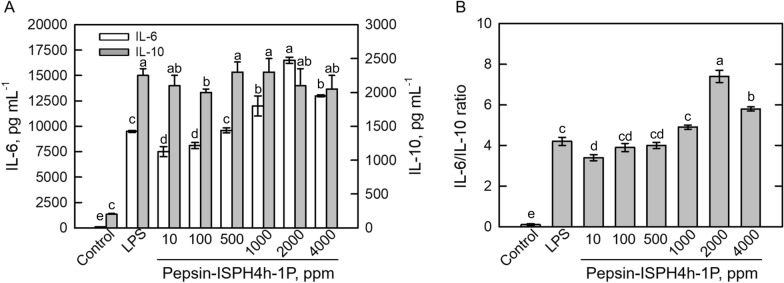
Interleukin-6 (IL-6) and interleukin-10 (IL-10) are major pro-inflammatory cytokines production in macrophage cells (Ahn et al. 2015; Toopcham et al. 2017; Lee et al. 2021; Li et al. 2021). During inflammation response, cells can release a large amount of IL-6 to promote the formation of NO as well as to activate phagocytosis of macrophage cells (Minato and Abe 2013; Li et al. 2021), whereas IL-10 exhibits anti-inflammatory ability to reduce NO formation as well as to decrease inflammatory cytokinins secretions (Asadullah et al. 2003; Lee et al. 2021). To study the effect of the 1P fraction on cytokine production, RAW264.7 macrophage cells were treated with various dosages of the 1P fraction, and LPS treatment was used as positive control. The formation of IL-6 was intercorrelated to the increased concentrations of the 1P fraction (Fig. 4A); however, the amount of IL-10 was constantly induced by 1P treatments which was independent on its dosages (Fig. 4A). In addition, IL-6/IL-10 ratio was compared in LPS-treated cells and the maximum ratio was monitored in the 2000 ppm 1P-treated macrophage cells (Fig. 4B). The higher IL-6/IL-10 ratio indicated that cells were prone to inflammatory response (Song et al. 2016; Sapan et al. 2017; Koyama et al. 2021). As a result, 1P treatment cannot trigger severe inflammation response and was had no inhibitory effect on LPS-induced inflammatory effects. Also, similar results were reported in shark derived protein hydrolysate (Mallet et al. 2014) and rice proteins (Wen et al. 2021).
Fig. 4.
Determination of pro-inflammatory cytokines in LPS–Pepsin-ISP4h-1P-costimulated RAW264.7 macrophage cells. A dosage effect of Pepsin-ISPH4h-1P with 1 ppm LPS co-stimulation on IL-6 (gray column) and IL-10 (white column) productions. B dosage effect of Pepsin-ISPH4h-1P with 1 ppm LPS co-stimulation on IL-6/IL-10 ratio. Bars represent mean ± standard deviation (SD; n = 3). Mean with different letters are significantly different (p < 0.05) by Duncan’s multiple range test
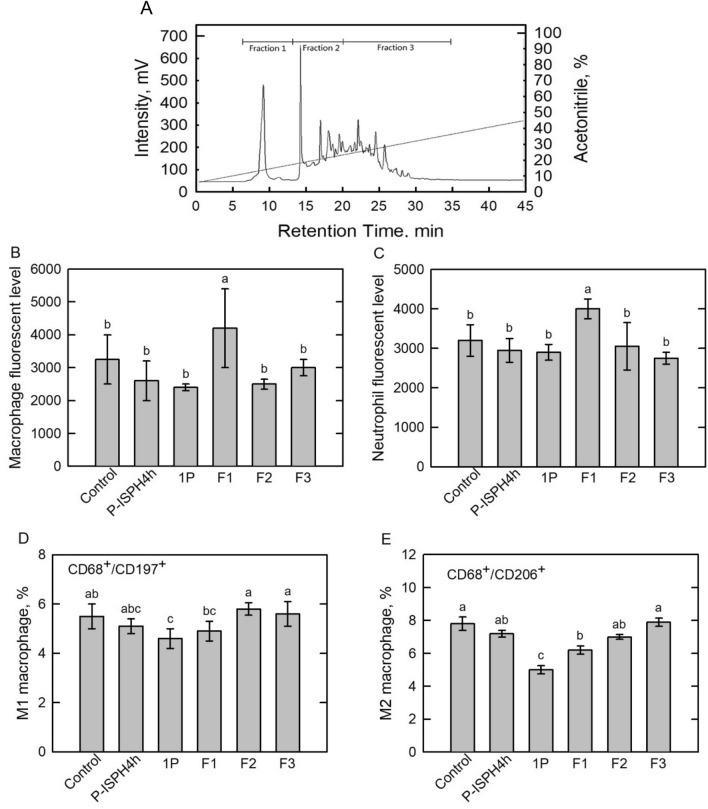
Effect of 1P fractions, F1–F3, on phagocytosis and polarization in RAW264.7 macrophage cells
Fraction 1P exhibited the immunomodulatory ability to promote cell phagocytosis was further separated by reverse-phase HPLC into three fractions (Fig. 5A). Based on its retention time, Fraction 1 (F1), Fraction 2 (F2), and Fraction 3 (F3) were in between 6 and 14 min, 14–21 min, and 21–35 min, respectively (Fig. 5A). For endocytosis activity assay, macrophage (Fig. 5B) and neutrophil cells (Fig. 5C) were harvested from mice spleens and then incubated with transgenic recombinant green fluorescence protein (GFP) produced E. coli cells (Gille et al. 2006). The fluorescent level was represented as the endocytosis activity (Jiang et al. 2007). As shown in Fig. 5C, F1 fraction significantly increased endocytosis activity in macrophage cells, whereas F2 and F3 fractions exhibited no influences as control. In addition, F1 treatment also enhanced endocytosis activity of neutrophil cells (Fig. 5D). Next, Pepsin-ISPH4h, 1P, and F1–F3 were injected intraperitoneally into mice and then spleen lymphocyte cells were harvested and differentiated cells, M1 (Fig. 5D) and M2 (Fig. 5E), were analyzed by a flow cytometer (Kim et al. 2019). Macrophage M1 cells produces NO or reactive oxygen intermediates to defense bacteria or viruses infection; M2 cells secrete certain cytokines, IL-4 or IL-10, mediating damaged tissues repair (Murray et al. 2014; Rőszer 2015; Kim et al. 2019). Macrophages M1 and M2 polarization were not affected by all pepsin hydrolysates treatments (Fig. 5D, E). Taken together, F1 fraction can increase phagocytosis activities in both mice spleen macrophage and neutrophil cells, but it cannot induce macrophages M1 or M2 polarization.
Fig. 5.
Determation of anti-inflammatory activity of Pepsin-ISPH4h-1P fractions. A Pepsin-ISPH-4 h-1P was fractionated into F1–F3 using a reverse-phase high-performance liquid chromatography. Effects of Pepsin-ISPH4h, Pepsin-ISP4h-1P, and F1–F3 fractions on positive fluorescent level in macrophages (B), neutrophil (C), M1 (D) or M2 (E) phenotype polarization in mouse spleen in vivo. Bars represent mean ± standard deviation (SD; n = 3). Mean with different letters are significantly different (p < 0.05) by Duncan’s multiple range test
Identification of anti-inflammatory peptides by LC–MS/MS from F1 fraction and investigation of synthetic peptide on phagocytosis and polarization of macrophage
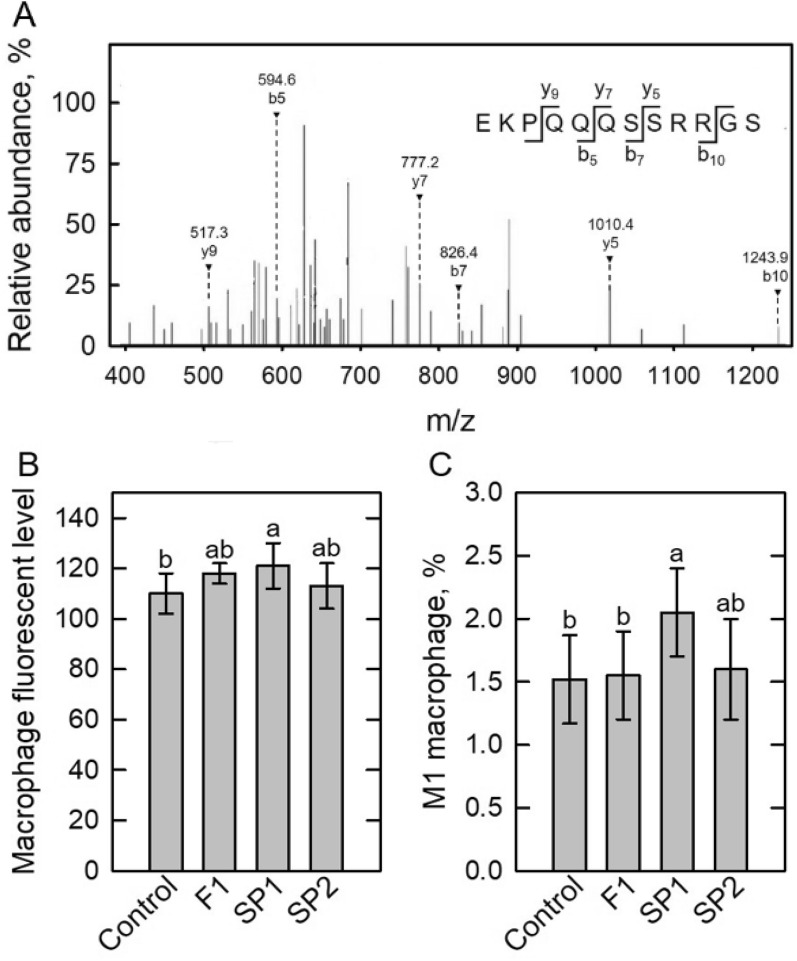
Previous studies had shown that peptides with positive charged amino acids are intercorrelated with immunomodulatory functions (Mercier et al. 2004; Kong et al. 2008; Jacquot et al. 2010; Hou et al. 2012; Hemshekhar et al. 2019). In this study, liquid chromatography with tandem mass spectrometry (LC–MS–MS) was used for peptide identification (Dia et al. 2014; Fang et al. 2019; Li et al. 2021; Wen et al. 2021). Two peptides with positively charged amino acids, EKPQQQSSRRGS (Fig. 6A) and VVQGKGAIGFAFP, were identified from F1 fraction by LC–MS–MS analysis. Accordingly, synthetic peptide 1 (SP1, EKPQQQSSRRGS) and synthetic peptide 2 (SP2, VVQGKGAIGFAFP) were used to examine its in vivo immunomodulatory effects in mice (Wen et al. 2021). Both synthetic peptides treatments showed no effect on M1 macrophage polarization (data not shown). In macrophage endocytosis analysis, the stimulating effect of the SP1 peptide was better than that of the F1 and SP2 (Fig. 6B). In macrophage polarization analysis, SP1 showed induction effect on M1 macrophage polarization, whereas F1 and SP2 had no effect on that (Fig. 6C). As a result, positive immunomodulatory activities were confirmed in synthetic peptide.
Fig. 6.
Peptide identified from Pepsin-ISPH4h-1P F1 fraction. A the mass spectrum of a peptide, EKPQQQSSRRGS, identified by LC–MS–MS. Effects of the synthetic peptides, EKPQQQSSRRGS (SP1) and VVQGKGAIGFAFP (SP2), on positive fluorescent level in macrophages (B), and M1 (C) phenotype polarization in mouse spleen in vivo. Bars represent mean ± standard deviation (SD; n = 3). Mean with different letters are significantly different (p < 0.05) by Duncan’s multiple range test
Conclusions
In this study, pepsin-treated isolated soy protein hydrolysates exhibited immunomodulatory effects such as enhancing phagocytosis activity and not causing excessive inflammatory response. Putative peptides from isolated soy protein hydrolysate by peptic hydrolysis were purified using MWCO and reverse-phase chromatography technique. Two peptides were identified by mass spectrometry. Further studies revealed that the synthetic peptide, EKPQQQSSRRGS, can increase phagocytosis activity in mice spleen macrophage cells as well as can induce macrophages M1 polarization. Taken together, this study can serve as a fundamental basis for the preparation of immunomodulatory peptides from isolated soy proteins.
Acknowledgements
This study was supported by research Grants (NSC100-2221-E029-002 and NSC101-2221-E029-026 for W.D.C.) from Ministry of Science and Technology, Taiwan.
Abbreviations
- ISPH
Isolated soy protein hydrolysate
- MWCO
Molecular weight cut-off
- P
Permeate
- F
Fraction
- LPS
Lipopolysaccharide
- IL
Interleukin
- TNF-α
Tumor necrosis factor-α
- SDS
Sodium dodecyl sulfate
- OPA
O-Phthalaldehyde
- DMSO
Dimethyl sulfoxide
- MTT
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- DMEM
Dulbecco’s modified Eagle medium
- FBS
Fetal bovine serum
- DH
Degree of hydrolysis
- BCRC
Bioresource Collection and Research Center
- PBS
Phosphate buffer saline
- SD
Standard deviation
- HBSS
Hank’s balanced salt solution
- GFP
Green fluorescence protein
- SP
Synthetic peptide
Authors’ contributions
L-SH: conceptualization, methodology, writing—original draft, writing—review and editing. M-SL: conceptualization, methodology, investigation. W-DC: conceptualization, funding acquisition, resources, supervision, writing—review and editing. All authors read and approved the final manuscript.
Funding
Ministry of Science and Technology, Taiwan, research Grants (NSC100-2221-E029-002 and NSC101-2221-E029-026 for W.D.C.)
Availability of data and materials
Data are contained in the main material.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Lu-Sheng Hsieh and Ming-Shing Lu contributed equally to this work
References
- Adler-Nissen J. Enzymic hydrolysis of food proteins. London: Elsevier Applied Science Publishers; 1986. Some fundamental aspects of food protein hydrolysis; pp. 9–24. [Google Scholar]
- Ahn C-B, Cho Y-S, Je J-Y. Purification and anti-inflammatory action of tripeptide from salmon pectoral fin byproduct protein hydrolysate. Food Chem. 2015;168:151–156. doi: 10.1016/j.foodchem.2014.05.112. [DOI] [PubMed] [Google Scholar]
- Akbarian M, Khani A, Eghbalpour S, Uversky VN. Bioactive peptides: synthesis, sources, applications, and proposed mechanisms. Int J Mol Sci. 2022;23:1445. doi: 10.3390/ijms23031445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asadullah K, Sterry W, Volk HD. Interleukin-10 therapy—review of a new approach. Pharmacol Rev. 2003;55:241–269. doi: 10.1124/pr.55.2.4. [DOI] [PubMed] [Google Scholar]
- Ashaolu TJ. Health applications of soy protein hydrolysis. Int J Pept Res Ther. 2020;26:2333–2343. doi: 10.1007/s10989-020-10018-6. [DOI] [Google Scholar]
- Bayne CJ. Origins and evolutionary relationships between the innate and adaptive arms of immune systems. Integr Comp Biol. 2013;43:293–299. doi: 10.1093/icb/43.2.293. [DOI] [PubMed] [Google Scholar]
- Bueno-Gavilá E, Abellán A, Girón-Rodríguez F, María Cayuela J, Tejada L. Bioactivity of hydrolysates obtained from chicken egg ovalbumin using artichoke (Cynara scolymus L.) proteases. Foods. 2021;10:246. doi: 10.3390/foods10020246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalamaiah M, Hemalatha R, Jyothirmayi T, Diwan PV, Uday Kumar P, Nimgulkar C, Dinesh Kumar B. Immunomodulatory effects of protein hydrolysates from rohu (Labeo rohita) egg (roe) in BALB/c mice. Food Res Int. 2014;62:1051–1061. doi: 10.1016/j.foodres.2014.05.050. [DOI] [Google Scholar]
- Chalamaiah M, Yu W, Wu J. Immunomodulatory and anticancer protein hydrolysates (peptides) from food proteins: a review. Food Chem. 2018;245:205–222. doi: 10.1016/j.foodchem.2017.10.087. [DOI] [PubMed] [Google Scholar]
- Coscueta ER, Campos DA, Osório H, Nerli BB, Pintado M. Enzymatic soy protein hydrolysis: a tool for biofunctional food ingredient production. Food Chem X. 2019;1:100006. doi: 10.1016/j.fochx.2019.100006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dia VP, Bringe NA, de Mejia EG. Peptides in pepsin-pancreatin hydrolysates from commercially available soy products that inhibit lipopolysaccharide-induced inflammation in macrophages. Food Chem. 2014;152:423–431. doi: 10.1016/j.foodchem.2013.11.155. [DOI] [PubMed] [Google Scholar]
- Egusa S, Otani H. Soybean protein fraction digested with neutral protease preparation, “Peptidase R”, produced by Rhizopus oryzae, stimulates innate cellular immune system in mouse. Int Immunopharmacol. 2009;9:931–936. doi: 10.1016/j.intimp.2009.03.020. [DOI] [PubMed] [Google Scholar]
- Fang Y, Pan X, Zhao E, Shi Y, Shen X, Wu J, Pei F, Hu Q, Qiu W. Isolation and identification of immunomodulatory selenium-containing peptides from selenium-enriched rice protein hydrolysates. Food Chem. 2019;275:696–702. doi: 10.1016/j.foodchem.2018.09.115. [DOI] [PubMed] [Google Scholar]
- Gille C, Spring B, Tewes L, Poets CF, Orlikowsky T. A new method to quantify phagocytosis and intracellular degradation using green fluorescent protein-labeled Escherichia coli: comparison of cord blood macrophages and peripheral blood macrophages of healthy adults. Cytometry A. 2006;69:152–154. doi: 10.1002/cyto.a.20222. [DOI] [PubMed] [Google Scholar]
- Gordon S. Phagocytosis: an immunological process. Immunity. 2016;44:463–475. doi: 10.1016/j.immuni.2016.02.026. [DOI] [PubMed] [Google Scholar]
- Hamer M, Endrighi R, Poole L. Physical activity, stress reduction, and mood: insight into immunological mechanisms. Methods Mol Biol. 2012;934:89–102. doi: 10.1007/978-1-62703-071-7_5. [DOI] [PubMed] [Google Scholar]
- He P, Wang Q, Zhan Q, Pan L, Xin X, Wu H, Zhang M. Purification and characterization of immunomodulatory peptides from enzymatic hydrolysates of duck egg ovalbumin. Food Funct. 2021;12:668–681. doi: 10.1039/D0FO02674C. [DOI] [PubMed] [Google Scholar]
- Hemshekhar M, Faiyae S, Faiyaz S, Choi K-YG, Krokhin OV, Mookherjee N. Immunomodulatory functions of the human cathelicidin LL-37 (aa 13–31)-derived peptides are associated with predicted α-helical propensity and hydrophobic index. Biomolecules. 2019;9:501. doi: 10.3390/biom9090501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou H, Fan Y, Li B, Xue C, Yu G, Zhang Z, Zhao X. Purification and identification of immunomodulatory peptides from enzymatic hydrolysates of Alaska pollock frame. Food Chem. 2012;134:821–828. doi: 10.1016/j.foodchem.2012.02.186. [DOI] [PubMed] [Google Scholar]
- Iwasaki A, Medzhitov R. Control of adaptive immunity by the innate immune system. Nat Immunol. 2015;16:343–353. doi: 10.1038/ni.3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquot A, Gauthier SF, Drouin R, Boutin Y. Proliferative effects of synthetic peptides from β-lactoglobulin and α-lactalbumin on murine splenocytes. Int Dairy J. 2010;20:514–521. doi: 10.1016/j.idairyj.2010.02.013. [DOI] [Google Scholar]
- Jamdar SN, Rajalakshmi V, Pednekar MD, Juan F, Yardi V, Sharma A. Influence of degree of hydrolysis on functional properties, antioxidant activity and ACE inhibitory activity of peanut protein hydrolysate. Food Chem. 2010;121:178–184. doi: 10.1016/j.foodchem.2009.12.027. [DOI] [Google Scholar]
- Jiang H, Zhang J, Shi B-Z, Xu Y-H, Li Z-H, Gu J-R. Application of EGFP-EGF fusions to explore mechanism of endocytosis of epidermal growth factor. Acta Pharmacol Sin. 2007;28:111–117. doi: 10.1111/j.1745-7254.2007.00481.x. [DOI] [PubMed] [Google Scholar]
- Kim JY, Kim TH, Kim SS. Anti-inflammatory effect of a human prothrombin fragment-2-derived peptide, NSA9, in EOC2 microglia. Biochem Biophys Res Commun. 2008;368:779–785. doi: 10.1016/j.bbrc.2008.01.142. [DOI] [PubMed] [Google Scholar]
- Kim H, Wang SY, Kwak G, Yang Y, Kwon IC, Kim SH. Exosome-guided phenotypic switch of M1 to M2 macrophages for cutaneous wound healing. Adv Sci. 2019;6:1900513. doi: 10.1002/advs.201900513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong X, Guo M, Hua Y, Cao D, Zhang C. Enzymatic preparation of immunomodulating hydrolysates from soy proteins. Bioresour Technol. 2008;99:8873–8879. doi: 10.1016/j.biotech.2008.04.056. [DOI] [PubMed] [Google Scholar]
- Koyama T, Uchida K, Fukushima K, Ohashi Y, Uchiyama K, Inoue G, Takahira N, Takaso M. Elevated levels of TNF-α, IL-1β and IL-6 in the synovial tissue of patients with labral tear: a comparative study with hip osteoarthritis. BMC Musculoskelet Disord. 2021;22:33. doi: 10.1186/s12891-020-03888-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SM, Son K-N, Shah D, Ali M, Balasubramaniam A, Shukla D. Histatin-1 attenuates LPS-induced inflammatory signaling in RAW264.7 macrophages. Int J Mol Sci. 2021;22:7856. doi: 10.3390/ijms22157856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, An L, Zhang S, Shi Z, Bao J, Tuerhong M, Abudukeremu M, Xu J, Guo Y. Structural elucidation and immunomodulatory evaluation of a polysaccharide from Stevia rebaudiana leaves. Food Chem. 2021;364:130310. doi: 10.1016/j.foodchem.2021.130310. [DOI] [PubMed] [Google Scholar]
- Liu Q, Kong B, Xiong YL, Xia X. Antioxidant activity and functional properties of porcine plasma protein hydrolysate as influenced by the degree of hydrolysis. Food Chem. 2010;118:403–410. doi: 10.1016/j.foodchem.2009.05.013. [DOI] [Google Scholar]
- Mallet J-F, Duarte J, Vinderola G, Anguenot R, Beaulieu M, Matar C. The immunopotentiating effects of shark-derived protein hydrolysate. Nutrition. 2014;30:706–712. doi: 10.1016/j.nut.2013.10.025. [DOI] [PubMed] [Google Scholar]
- Marques AH, Bjørke-Monsen A-L, Teixeira AL, Silverman MN. Maternal stress, nutrition and physical activity: impact on immune function, CNS development and psychopathology. Brain Res. 2015;1617:28–46. doi: 10.1016/j.brainres.2014.10.051. [DOI] [PubMed] [Google Scholar]
- Mercier A, Gauthier SF, Fliss I. Immunomodulating effects of whey proteins and their enzymatic digests. Int Dairy J. 2004;14:175–183. doi: 10.1016/j.idairyj.2003.08.003. [DOI] [Google Scholar]
- Minato KI, Abe C. Chapter 17—immunomodulating effect of immodulating effect of polysaccharide. In: Preedy V, editor. Bioactive food as dietary interventions for arthritis and related inflammatory diseases. Amsterdam: Elsevier; 2013. pp. 241–250. [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, Locati M, Mantovani A, Martinez FO, Mege J-L, Mosser DM, Natoli G, Saeij JP, Schultze JL, Shirey KA, Sica A, Suttles J, Udalova I, van Ginderachter JA, Vogel SN, Wynn TA. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielson P, Petersen D, Dambmann C. Improved method for determining food protein degree of hydrolysis. J Food Sci. 2001;66:642–646. doi: 10.1111/j.1365-2621.2001.tb04614.x. [DOI] [Google Scholar]
- Polak E, Stępień AE, Gol O, Tabarkiewicz J. Potential immunomodulatory effects from consumption of nutrients in whole foods and supplements on the frequency and course of infection: preliminary results. Nutrients. 2021;13:1157. doi: 10.3390/nu13041157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranamukhaarachchi S, Meissner L, Moresoli C. Production of antioxidant soy protein hydrolysates by sequential ultrafiltration and nanofiltration. J Membr Sci. 2013;429:81–87. doi: 10.1016/j.memsci.2012.10.040. [DOI] [Google Scholar]
- Rho SJ, Lee JS, Chung YI, Kim YW, Lee HG. Purification and identification of an angiotensin I-converting enzyme inhibitory peptide from fermented soybean extract. Process Biochem. 2009;44:490–493. doi: 10.1016/j.procbio.2008.12.017. [DOI] [Google Scholar]
- Ricker D, Johnson L, Murphy P. Functional properties of improved glycinin and β-nglycinin fractions. J Food Sci. 2004;69:303–311. doi: 10.1111/j.1365-2621.2004.tb06332.x. [DOI] [Google Scholar]
- Rőszer T. Understanding the mysterious M2 macrophage through activation markers and effector mechanisms. Mediat Inflamm. 2015;2015:816460. doi: 10.1155/2015/816460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago-López L, Hernández-Mendoza A, Vallejo-Cordoba B, Mata-Haro V, González-Córdova AF. Food-derived immunomodulatory peptides. J Sci Food Agric. 2016;96:3631–3641. doi: 10.1002/jsfa.7697. [DOI] [PubMed] [Google Scholar]
- Sapan HB, Paturusi I, Islam AA, Yusuf I, Patellongi I, Massi MN, Pusponegoro AD, Arief SK, Labeda I, Rendy L, Hatta M. Interleukin-6 and interleukin-10 plasma levels and mRNA expression in polytrauma patients. Chin J Traumatol. 2017;20:318–322. doi: 10.1016/j.cjtee.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemensma AD, Weijer WJ, Bak HJ. The importance of peptide lengths in hypoallergenic infant formulae. Trends Food Sci Technol. 1993;4:16–21. doi: 10.1016/S0924-2244(05)80006-8. [DOI] [Google Scholar]
- Song Y, Zhang W, Zhang L, Wu W, Zhang Y, Han X, Yang C, Zhang L, Zhou D. Cerebrospinal fluid IL-10 and IL-10/IL-6 as accurate diagnostic biomarkers for primary central nervous system large B-cell lymphoma. Sci Rep. 2016;6:38671. doi: 10.1038/srep38671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toopcham T, Mes JJ, Wicheers HJ, Yongsawatdigul J. Immunomodulatory activity of protein hydrolysates derived from Virgibacillus halodenitrificans SK1-3-7 proteinase. Food Chem. 2017;224:320–328. doi: 10.1016/j.foodchem.2016.12.041. [DOI] [PubMed] [Google Scholar]
- Tsou M-J, Lin S-B, Chao C-H, Chiang W-D. Enhancing the lipolysis-stimulating activity of soy protein using limited hydrolysis with Flavourzyme and ultrafiltration. Food Chem. 2012;134:1564–1570. doi: 10.1016/j.foodchem.2012.03.093. [DOI] [PubMed] [Google Scholar]
- Van Ginderachter JA, Movahedi K, Hassanzadeh Ghassabeh G, Meerschaut S, Beschin A, Raes G, De Baetselier P. Classical and alternative activation of mononuclear phagocytes: picking the best of both worlds for tumor promotion. Immunobiology. 2006;211:487–501. doi: 10.1016/j.imbio.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Wen L, Jiang Y, Zhou X, Bi H, Yang B. Structure identification of soybean peptides and their immunomodulatory activity. Food Chem. 2021;359:129970. doi: 10.1016/j.foodchem.2021.129970. [DOI] [PubMed] [Google Scholar]
- Wu W, Zhang M, Ren Y, Cai X, Yin Z, Zhang X, Min T, Wu H. Characterization and immunomodulatory activity of a novel peptide, ECFSTA, from wheat germ globulin. J Agri Food Chem. 2017;65:5561–5569. doi: 10.1021/acs.jafc.7b01360. [DOI] [PubMed] [Google Scholar]
- Yang R, Zhang Z, Pei X, Han X, Wang J, Wang L, Long Z, Shen X, Li Y. Immunomodulatory effects of marine oligopeptide preparation from Chum Salmon (Oncorhynchus keta) in mice. Food Chem. 2009;113:464–470. doi: 10.1016/j.foodchem.2008.07.086. [DOI] [Google Scholar]
- Yang Q, Cai X, Huang M, Wang S. A specific peptide with immunomodulatory activity from Pseudostellaria heterophylla and the action mechanism. J Funct Foods. 2020;68:103887. doi: 10.1016/j.jff.2020.103887. [DOI] [Google Scholar]
- Yu W, Field CJ, Wu J. Purification and identification of anti-inflammatory peptides from spent hen muscle proteins hydrolysate. Food Chem. 2018;253:101–107. doi: 10.1016/j.foodchem.2018.01.093. [DOI] [PubMed] [Google Scholar]
- Zaky AA, Simal-Gandara J, Eun J-B, Shim J-H, El-Aty AMA. Bioactivities, applications, safety, and health benefits of bioactive peptides from food and by-products: a review. Front Nutr. 2022;8:815640. doi: 10.3389/fnut.2021.815640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Gao S, Li H, Cao M, Li W, Liu X. Immunomodulatory effects of selenium-enriched peptides from soybean in cyclophosphamide-induced immunosuppressed mice. Food Sci Nutr. 2021;9:6322–6334. doi: 10.1002/fsn3.2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Zhao T, Feng W, Wang W, Zou Y, Zheng D, Takase M, Li Q, Wu H, Yang L, Wu X. Purification, characterization and immunomodulating activity of a polysaccharide from flowers of Abelmoschus esculentus. Carbohydr Polym. 2014;106:335–342. doi: 10.1016/j.carbpol.2014.02.079. [DOI] [PubMed] [Google Scholar]
- Ziegler F, Ollivier J, Cynober L, Masini J, Coudray-Lucas C, Levy E, Giboudeau J. Efficiency of enteral nitrogen support in surgical patients: small peptides v non-degraded proteins. Gut. 1990;31:1277–1283. doi: 10.1136/.31.11.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are contained in the main material.