Abstract
V-domain immunoglobulin suppressor of T cell activation (VISTA) is an immune checkpoint molecule expressed in hematopoietic cells, granulocytes, macrophages, and monocytes. However, few studies to date have investigated VISTA expression, especially its clinical utility, in bladder cancer. The present retrospective study aimed to examine VISTA, programmed death ligand-1 (PD-L1), and CD45 expression by immunohistochemical and immunofluorescence staining of archived pathological tissue samples from 159 patients with primary bladder cancer. The correlation between VISTA expression in immune cells (ICs) and clinicopathologic variables including PD-L1 expression in ICs was examined. Briefly, the rates of VISTA-positive ICs and VISTA-positive tumor cells were 67.9% (108/159) and 30.8% (49/159), respectively. The VISTA expression in ICs of patients with bladder cancer, including those with non-muscle-invasive bladder cancer (NMIBC), was positively correlated with tumor stage, grade, size, and multiplicity. The VISTA expression in ICs was stronger in bladder cancer cases with PD-L1-positive ICs than in those with PD-L1-negative ICs (p < 0.001). The mean intravesical recurrence-free survival was shorter in NMIBC cases with VISTA-positive ICs than in those with VISTA-negative ICs (34.0 vs 39.9 months, p = 0.03, log-rank test). In this first study to investigate VISTA expression in bladder cancer, these results implicate VISTA as a potential immunotherapeutic target and immunologic biomarker in bladder cancer.
Keywords: VISTA, Bladder cancer, Non-muscle invasive, Immune checkpoint inhibitor, Immune cells
Introduction
Bladder cancer is the tenth most common cancer worldwide, with 550,000 newly diagnosed cases and 200,000 deaths in 2018 [1, 2]. About 70% of all bladder cancer cases are early-stage non-muscle-invasive bladder cancer (NMIBC) at the time of diagnosis [2]. Transurethral tumor resection is the first-line surgical approach for the diagnosis, staging, and treatment of visible NMIBC. However, in the first 12 months after initial transurethral tumor resection, 40–80% of patients with NMIBC will experience intravesical recurrence (IVR) and 25% of patients will progress to muscle-invasive bladder cancer (MIBC) or metastatic bladder cancer [3]. For the prevention of IVR and progression to MIBC, guidelines on NMIBC recommend adjuvant intravesical instillation of bacillus Calmette-Guérin (BCG) after transurethral tumor resection as an immunotherapeutic approach for NMIBC [4].
Following the discovery of immune checkpoints such as programmed death ligand-1 (PD-L1), programmed cell death protein-1 (PD-1), and cytotoxic T-lymphocyte-associated protein 4, new immunotherapeutic strategies utilizing immune checkpoint inhibitors have been recently demonstrated to achieve successful clinical outcomes in locally advanced or metastatic bladder cancer [5–8]. The enthusiasm for new immunotherapies using immune checkpoint inhibitors has extended to the treatment of localized bladder cancer including NMIBC. In particular, clinical trials for immunotherapy by intravesical instillation as well as systemic injection are ongoing in BCG-unresponsive patients [9]. The discovery of novel immune checkpoints in bladder cancer should motivate the development of new immune-oncologic drugs and immune-related tumor markers.
V-domain Ig suppressor of T cell activation (VISTA), also known as PD-1H, c10orf54, Dies1, DD1α, Gi24, and SISP1, is a B7 family member that is primarily expressed in hematopoietic cells such as myeloid cells, granulocytes, and T cells [10, 11]. Several studies have demonstrated that VISTA functions both as a ligand for antigen-presenting cells and as a receptor for T cells; VISTA also suppresses T cell activation, proliferation, and cytokine production [11, 14]. An analysis of the Cancer Genome Atlas has revealed the landscape of VISTA expression across multiple cancer types including bladder cancer, and the correlation between VISTA and cancers has also been investigated [11, 12]. However, to our knowledge, no study to date has reported the clinical role of VISTA in bladder cancer. Therefore, the present study aimed to identify the relationship between VISTA expression in tumor-infiltrating immune cells (ICs) and previously known clinicopathologic features of bladder cancer and to evaluate the potential role of VISTA as a therapeutic target and biomarker for the prevention of IVR in surgically treated NMIBC.
Materials and methods
Study patients
This retrospective study included 159 patients with available pathological tissue specimens at Inje Biobank who underwent transurethral resection of bladder cancer between 2015 and 2017 in Inje University Busan Paik Hospital. NMIBC was defined as stage Ta, Tis, or T1 tumor confining to the mucosa or invading the lamina propria according to the 8th edition of American Joint Committee on Cancer (AJCC) TNM staging system [13]. A total of 94 patients with NMIBC who were followed for > 3 months were included in analyses on IVR. All patients with NMIBC underwent cystoscopy every 3 months for the first two years, every 6 months for the third year, and annually thereafter to evaluate for intravesical recurrence. Elective abdominal and chest computed tomography and bone scans were performed annually. Intravesical recurrence was based on pathological confirmation. Clinical data on demographic characteristics and follow-up data were extracted from the electronic medical records.
The study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. The study was approved by the Busan Paik Hospital Institutional Review Board (IRB No. 17-0195).
Tissue microarray and immunohistochemistry
Nine tissue microarray (TMA) blocks comprising 477 cores were constructed by selecting areas containing viable and representative tumor cells (TCs) and stroma with tumor-infiltrating ICs, including macrophages and lymphocytes, from paraffin-embedded tissue blocks of the 159 patients. Three cores with 2-mm diameters were selected from each case.
Immunohistochemical analysis was performed on 4-µm-thick serial sections of the nine TMA blocks using the following primary antibodies: VISTA (clone D1L2G, 1:200, Cell Signaling Technology, MA, USA), PD-L1 (clone SP142, 1:200, Ventana Medical Systems, AZ, USA), and CD45 (clone 2B11, 1:500, Dako, CA, USA). Human tonsil and placental tissues treated with and without primary antibodies were used as positive and negative controls, respectively. The BenchMark XT automated immunohistochemistry system (Ventana) was used with ultraView Universal DAB detection kit (Ventana Medical Systems), according to the manufacturers’ instructions.
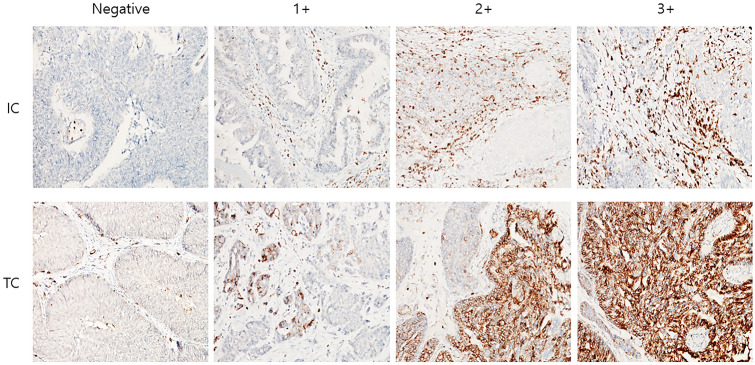
The extents of the positive staining for VISTA and PD-L1 on TCs and ICs were graded on the basis of the percentage of the positive cells. Specifically, the grading for VISTA was as follows: negative, no positive cells; 1 + , < 50% positivity, 2 + , 50–80% positivity; 3 + , > 80% positivity. The grading for PD-L1 was as follows: 0, no positive cells; 1 + , < 5% positivity; 2 + , 5–10% positivity; 3 + , > 10% positivity (Fig. 1). The three tumor TMA cores of each case were scored independently, and the average score was used for all analyses. Statistically, a score of 1 or more was defined positive. Quantitative evaluation was not performed for CD45.
Fig. 1.
Representative immunohistochemical expression by scoring of V-domain Ig-containing suppressor of T cell activation (VISTA) in immune cells (ICs) and tumor cells (TCs) in bladder cancer. Original magnification × 200
Double-staining immunofluorescence
For immunofluorescence, 3-μm-thick serial sections of TMA blocks were incubated with the following primary antibodies: VISTA (clone D1L2G, 1:100, Cell Signaling Technology, MA, USA), PD-L1 (clone E1L3N, 1:100, Cell Signaling Technology), and CD45 (clone MAB1430, 1:100, R&D Systems, MA, USA) for 1 h at room temperature and washed with phosphate-buffered saline. Next, the sections were incubated with Alexa Fluor® 555 anti-mouse and Alexa Fluor® 488 anti-rabbit (Nos. 4409 and 4412, 1:1000, Cell Signaling Technology, MA, USA) secondary antibodies for 1 h at room temperature and washed with phosphate-buffered saline. Finally, the sections were incubated with 1 μg/mL DAPI (4′,6-diamidino-2-phenylindole) to visualize the nuclei. Immunofluorescence double staining was assessed for VISTA and PD-L1 positive cells (green signals) and coexpressed CD45 (red signal) using a fluorescence microscope (BX-51; Olympus, Tokyo, Japan). Quantitative evaluation of the immunofluorescence staining was not performed.
Statistical analysis
Data were expressed as means ± standard deviation. Comparisons of the clinicopathologic variables and immunohistochemistry results according to the IVR status were performed using the chi-squared and Fisher’s exact tests for categorical data and Student’s t test for continuous data. The correlation between VISTA expression in ICs and clinicopathologic variables including PD-L1 expression in ICs was analyzed by Spearman’s rank correlation test. Probability of IVR-free survival was estimated using the Kaplan–Meier method, and log-rank test was used to assess statistical differences. All statistical analyses were performed with SPSS ver. 20.0 (IBM, Armonk, NY, USA), and statistical significance was defined as a p value of < 0.05.
Results
Patient characteristics
The clinicopathologic characteristics of 159 patients with bladder cancer are summarized in Table 1. Briefly, the mean patient age at diagnosis was 66.8 years and 127 (79.9%) patients were male. The cohort comprised 118 (74.2%) patients with NMIBC in pathological stage pTa or pT1, including 94 patients with available follow-up data, and 41 (25.8%) patients with MIBC, including 6 (3.8%) patients with metastases. Histologically high-grade tumors were present in 99 (62.3%) patients, and 36 (22.6%) patients had concomitant carcinoma in situ. The tumor size was smaller than 3 cm by preoperative computer tomography in 99 (57.2%) patients. The percentages of patients with more than three tumor foci and those with single tumors were 50.3% and 33.3%, respectively.
Table 1.
Clinicopathologic characteristics for bladder cancer patients
| Characteristics | N = 159 (%) |
|---|---|
| Age (yr) at diagnosis, mean ± SD | 66.8 ± 0.89 |
| Gender | |
| Male | 127 (79.9) |
| Female | 32 (20.1) |
| Pathologic T stage | |
| pTa | 54 (33.9) |
| pT1 | 64 (40.3) |
| ≥ pT2 | 41 (25.8) |
| Clinical N stage | |
| cN0 | 147 (92.5) |
| cN1-3 | 12 (7.5) |
| Clinical M stage | |
| cM0 | 153 (96.2) |
| cM1 | 6 (3.8) |
| Tumor gradea | |
| Low | 60 (37.7) |
| High | 99 (62.3) |
| Concomitant carcinoma in situ | |
| Absent | 123 (77.4) |
| Present | 36 (22.6) |
| Tumor size | |
| < 3 cm | 91 (57.2) |
| ≥ 3 cm | 68 (42.8) |
| Multiplicity | |
| Solitary | 53 (33.3) |
| Two | 26 (16.4) |
| ≥ Three | 80 (50.3) |
aWorld Health Organization (WHO) grading system (2016)
VISTA and PD-L1 expression in bladder cancer
The immunohistochemical VISTA protein expression, which was observed in the cytoplasm and membrane of TCs, exhibited nuclear and cytoplasmic localization in the ICs (Fig. 1). Among a total of 159 cases included in the study, 49 (30.8%) cases were VISTA-positive in TCs (negative: 110, 1 + : 7, 2 + : 29, and 3 + : 13), whereas only 13 (8.0%) cases expressed PD-L1 in TCs (negative: 146, 1 + : 6, 2 + : 2, and 3 + : 5). Tumor-infiltrating ICs were positive for VISTA in 108 (67.9%) of the cases (negative: 51, 1 + : 39, 2 + : 42, and 3 + : 27), whereas the PD-L1 positivity on ICs (negative: 93, 1 + : 36, 2 + : 25. and 3 + : 5) was observed in 66 (41.5%) of the cases (Table 2).
Table 2.
Expression of VISTA and PD-L1 in TCs and tumor-infiltrating ICs in bladder cancer
| Total n (%) |
VISTA in ICs | VISTA in TCs | |||
|---|---|---|---|---|---|
| Positive n (%) |
Negative n (%) |
Positive n (%) |
Negative n (%) |
||
| 108 (67.9) | 51 (32.1) | 49 (30.8) | 110 (69.2) | ||
| PD-L1 in ICs | p < 0.0001 | p = 0.2034 | |||
| Positive | 66 (41.5) | 59 (89.4) | 7 (10.6) | 24 (36.4) | 42 (63.6) |
| Negative | 93 (58.5) | 49 (52.7) | 44 (47.3) | 25 (26.9) | 68 (73.1) |
| PD-L1 in TCs | p = 0.05 | p = 0.2128 | |||
| Positive | 13 (8.0) | 12 (92.3) | 1 (7.7) | 6 (46.2) | 7 (53.8) |
| Negative | 146 (91.8) | 96 (65.7) | 50 (34.2) | 43 (29.5) | 103 (70.5) |
Clinicopathologic significance of VISTA expression in ICs
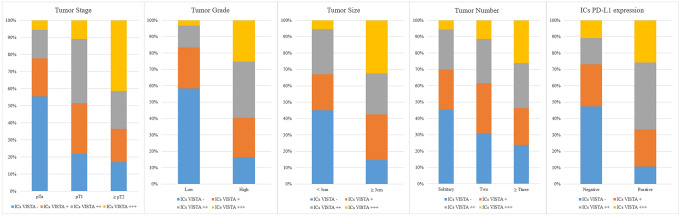
VISTA expression in the ICs showed a significant relationship with clinicopathologic features (Fig. 2). Specifically, higher VISTA expression in ICs was correlated with higher tumor stage (Spearman’s rank correlation, 0.325; p < 0.001), higher pathologic grade (Spearman’s rank correlation, 0.438; p < 0.001), tumor size larger than 3 cm (Spearman’s rank correlation, 0.322; p < 0.001), and multiple bladder cancer lesions (Spearman’s rank correlation, 0.203; p = 0.01).
Fig. 2.
Relationship between expression of V-domain Ig suppressor of T cell activation (VISTA) in ICs and clinicopathologic features. The higher VISTA expression in ICs was correlated with higher tumor stage (Spearman’s rank correlation = 0.325, p < 0.001), higher pathologic grade (Spearman’s rank correlation = 0.438, p < 0.001), tumor size larger than 3 cm (Spearman’s rank correlation = 0.322, p < 0.001), and multiple cancer lesions (Spearman’s rank correlation = 0.203, p = 0.01)
Of the 94 NMIBC cases, 62 (65.9%) exhibited VISTA positivity in ICs. Compared to the cases with VISTA-negative ICs, those with VISTA-positive ICs had a higher tumor stage (67.7% in pT1 vs. 32.3% in pTa and pTis, p < 0.001) and higher tumor grade (71.0% in pT1 vs. 29.0% in pTa and pTis, p < 0.001). Furthermore, VISTA in ICs was more frequently expressed in cases with concomitant carcinoma in situ (27.4% vs. 3.1%, p = 0.005) and in those with multiple tumors (69.4% vs. 43.7%, p = 0.028). But VISTA expression in TCs was not statistically significant association with clinicopathologic features (Table 3).
Table 3.
Relationship of VISTA expression and clinicopathologic features in NMIBC
| Characteristics | VISTA in ICs | p value | VISTA in TCs | p value | ||
|---|---|---|---|---|---|---|
| Negative | Positive | Negative | Positive | |||
| N (%) | N (%) | N (%) | N (%) | |||
| 32 (34.0%) | 62 (65.9%) | 64 (68.1%) | 30 (31.9%) | |||
| Pathologic T stage | ||||||
| pTa, pTis | 23 (71.9) | 20 (32.3) | < 0.001 | 34 (53.1) | 9 (30.0) | 0.036 |
| pT1 | 9 (28.1) | 42 (67.7) | 30 (46.9) | 21 (70.0) | ||
| Tumor gradea | ||||||
| Low | 27 (84.4) | 18 (29.0) | < 0.001 | 34 (53.1) | 11 (36.7) | 0.138 |
| High | 5 (15.6) | 44 (71.0) | 30 (46.9) | 19 (63.3) | ||
| Concomitant carcinoma in situ | ||||||
| Absent | 31 (96.9) | 45 (72.6) | 0.005 | 54 (84.4) | 22 (73.3) | 0.207 |
| Present | 1 (3.1) | 17 (27.4) | 10 (15.6) | 8 (26.7) | ||
| Tumor size | ||||||
| < 3 cm | 28 (87.5) | 41 (66.1) | 0.029 | 48 (75.0) | 21 (70.0) | 0.611 |
| ≥ 3 cm | 4 (12.5) | 21 (33.9) | 16 (25.0) | 9 (30.0) | ||
| Multiplicity | ||||||
| Solitary | 18 (56.3) | 19 (30.6) | 0.028 | 24 (37.5) | 13 (43.3) | 0.803 |
| Two | 5 (15.6) | 15 (24.2) | 15 (23.4) | 5 (16.7) | ||
| ≥ Three | 9 (28.1) | 28 (45.2) | 25 (39.1) | 12 (40.0) | ||
aWorld Health organization (WHO) grading system (2016)
VISTA and PD-L1 expression in tumor-infiltrating ICs
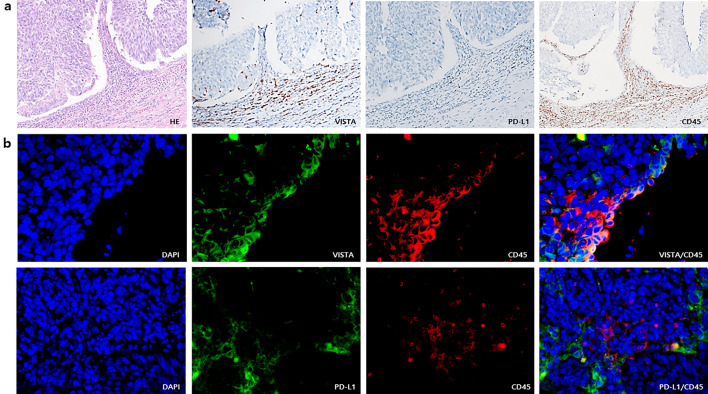
VISTA expression in ICs significantly correlated with the presence of PD-L1 expression in ICs. Briefly, 54.6% (59/108) of the cases with VISTA-positive ICs had PD-L1-positive ICs whereas 44 of the 51 cases with VISTA-negative ICs also had PD-L1-negative ICs (p < 0.001) (Table 2). The rate of cases with VISTA-positive ICs was higher in cases with PD-L1-positive ICs than in those with PD-L1-negative ICs (correlation coefficient = 0.387, p < 0.001) (Fig. 1). VISTA expression was observed in CD45-positive ICs, which, however, did not express PD-L1 by immunohistochemistry and double staining immunofluorescence (Fig. 3).
Fig. 3.
Expression of V-domain Ig suppressor of T cell activation (VISTA), programmed cell death ligand-1 (PD-L1) and CD45 by immunohistochemistry (a) and double staining immunofluorescence (b). a VISTA, PD-L1 and CD45 expressions in tumor-infiltrating ICs on serial section of same tissue. Original magnifications × 200. b Representative co-expression of VISTA and CD45 and separated expression of PD-L1 and CD45. Original magnification, × 400
Relationship of IVR-free survival with VISTA and PD-L1 expression in ICs of patients with NMIBC
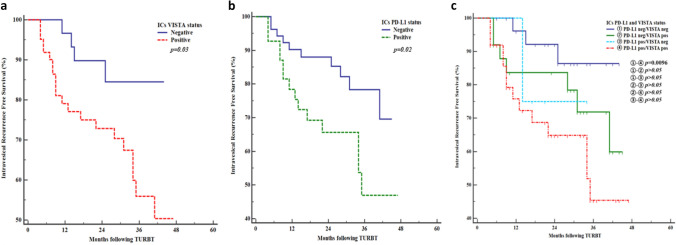
During a mean follow-up period of 36.6 months (95% confidence interval [CI] 33.3–40.0 months; median survival, not reached), 25 (26.6%) patients with NMIBC experienced IVR. The mean IVR-free survival rate was 34.0 months (95% CI 29.6–38.5 months; median survival not reached) in patients with VISTA-positive ICs and 39.9 months (95% CI 36.1–43.6; median survival, not reached) in those with VISTA-negative ICs (p = 0.03, log-rank test) (Fig. 4a). Conversely, the mean IVR-free survival rate was 32.2 months (95% CI 26.5–37.9; median survival, yet reached) in patients with PD-L1-positive ICs and 38.7 months (95% CI 35.1–42.3; median survival, not reached) in those with PD-L1-negative ICs (p = 0.02, log-rank test) (Fig. 4b). Four subgroups according to PD-L1 and VISTA expressions in ICs (PD-L1 negative/VISTA negative (n = 28), PD-L1 negative/VISTA positive (n = 25), PD-L1 positive/VISTA negative (n = 4), PD-L1 positive/VISTA positive (n = 37)) were not shown statistically significance between 4 groups but PD-L1 negative/VISTA negative group showed higher IVR-free survival rate (mean 40.5 months, 95% CI 36.7–44.2) than PD-L1 positive/VISTA positive group (mean 31.7 months, 95% CI 25.7–37.8)(p = 0.0096, log-rank test) (Fig. 4c).
Fig. 4.
Kaplan–Meier curves of intravesical recurrence (IVR)-free survival according to expression of V-domain Ig suppressor of T cell activation (VISTA) and programmed cell death ligand-1 (PD-L1) on immune cells (ICs) in patients with non-muscle-invasive bladder cancer. IVR-free survival rates in patients with VISTA-positive (a) and PD-L1 positive in ICs (b) were significantly lower than with VISTA-negative and PD-L1-negative in ICs (p = 0.03, p = 0.02). VISTA positive/PD-L1 positive in ICs group was showed significantly lower IVR-free survival rate than VISTA negative/PD-L1 negative in ICs group (p = 0.0096) (c)
Discussion
Recent advances in cancer immunotherapy utilizing inhibitors of immune checkpoint molecules, including cytotoxic T-lymphocyte-associated protein 4, PD-1, and PD-L1, have led to improved prognosis in a broad variety of solid malignancies. The current pioneering study is the first to report the clinical significance of the novel immune checkpoint VISTA in bladder cancer.
In humans, VISTA is highly expressed in myeloid cells whereas its expression is lower in CD4+ and CD8+ T cells; VISTA signaling plays an inhibitory role in T cell activation [15, 16]. Recent studies have noted the function of VISTA as an immune checkpoint molecule, demonstrating its potential role as a target for cancer immunotherapy in various solid tumors [15, 17–20]. VISTA expression has been reported to be positively associated with poor survival outcomes in melanoma, whereas contrasting results were observed in esophageal adenocarcinoma and hepatocellular carcinoma [18–20]. Several studies have recently demonstrated that VISTA is expressed in both ICs and TCs. Liao et al. reported that ICs were positive for VISTA in 90.8% of ovarian cancer tissues and associated with advanced stage in ovarian cancer [17]. In contrast, a recent study on high-grade serous ovarian cancer revealed that VISTA expression in TCs was associated with favorable prognosis [21]. In the present study, VISTA expression in TCs was observed in 30.8% of the cases although it was not significantly associated with clinicopathologic factors or IVR. VISTA might play different roles in TCs and ICs according to the cancer type; however, the underlying mechanism has not yet been elucidated. In the present study, VISTA-positive ICs were detected in 67.9% of all bladder cancer cases and in 65.9% of all NMIBC cases. Additionally, our analyses indicated that VISTA expression in ICs was associated with poor clinicopathologic features of bladder cancer. Furthermore, higher tumor stage and grade as well as large and multiple tumors were positively correlated with VISTA expression in ICs. Although the expression of VISTA alone does not account for the development and progression of bladder cancer, these results implicate that VISTA might play a role in the progression of bladder cancer and should be considered as a target for cancer immunotherapy.
The most interesting finding of the present work is the correlation between VISTA expression in ICs and IVR after tumor resection in NMIBC. The rate of IVR was higher in patients with VISTA-positive ICs than in those with VISTA-negative ICs. Clinicopathologic features such as tumor grade, size, and multiplicity are well-known predictors for IVR after primary tumor resection for NMIBC [22]. Therefore, the results of the present study could not conclusively show that VISTA alone was associated with increased risk of IVR in patients with VISTA-positive ICs. However, VISTA expression in ICs was higher in NMIBC cases with poor clinicopathologic features, suggesting that VISTA expression might directly or indirectly impact IVR in NMIBC.
Immunotherapy is utilized for the treatment of bladder cancer, especially in NMIBC. Conventional guidelines in NMIBC recommend scheduled intravesical BCG instillation as cancer immunotherapy for the prevention of IVR and bladder cancer progression to MIBC [23, 24]. The immunologic reaction to BCG involves the induction of an adaptive immune response via T cells and killer cells that are activated via mycobacterial antigen presentation, which eventually leads to the destruction of cancer cells [25, 26]. However, despite the demonstrated utility of BCG immunotherapy in the prevention of IVR, up to 40% of treated patients with NMIBC experience IVR or cancer progression [27, 28]. Patients who are nonresponsive to BCG immunotherapy or progress to MIBC eventually undergo cystectomy or cytotoxic chemotherapy, which increases medical costs while reducing patients’ quality of life [29, 30]. Intravesical immune checkpoint inhibitor treatment of NMIBC comprises one of the several approaches that are considered to overcome these limitations. Accumulating experience based on BCG treatment has become the starting point for intravesical immunotherapy in bladder cancer, whereas the results of immune checkpoint inhibitor therapy in advanced and metastatic bladder cancer have led to increased interest in their utility for the treatment of NMIBC [31]. Currently undergoing phase I and II clinical trials are evaluating the feasibility and safety of PD-L1 and PD-1 inhibitors in NMIBC [32]. Additionally, a clinical trial comparing intravesical versus intravenous immune checkpoint inhibitors in NMIBC, such as the NCT03167151 trial, is currently recruiting patients [32]. Our results showing that VISTA expression in ICs was correlated with clinicopathologic features associated with intravesical BCG treatment failure as well as with IVR suggest a role for VISTA as an alternative to BCG as a target for intravesical immunotherapy to prevent IVR in NMIBC.
The role of PD-1 and PD-L1 has been widely reported in bladder cancer, whereas the prognostic significance of PD-L1 expression remains controversial. However, the antitumor effect of atezolizumab, an anti-PD-L1 antibody, was reported to depend on PD-L1 expression level in ICs [33]. In the present study, the rate of IVR was higher in patients with PD-L1-expressing ICs than in those with ICs not expressing PD-L1, similar to that observed with VISTA expression in ICs. Although the theoretical basis has not been established, these results implicate the role of VISTA as a target for not only cancer immunotherapy in NMIBC but also combination immunotherapy with PD-1 and PD-L1 in overall bladder cancer. Indeed, in an experimental study using a murine tumor model, the antitumor efficacy was improved with simultaneous blockade of VISTA and PD-L1 [34]. Furthermore, recent studies in prostate cancer and melanoma reported that VISTA expression was increased after treatment with other immune checkpoint inhibitors [35, 36]. These results also implicate VISTA as a potential target for cancer immunotherapy of tumors that acquire resistance after treatment with immune checkpoint inhibitors. Till date, no direct relationship has been established between PD-1/PD-L1 and VISTA expressions, and no associations with other checkpoint molecules such as TIM-3 and LAG3, have been revealed, which are known play roles in the resistance anti-PD-1 and anti-CTLA-4 treatment [37]. Villarroel-Espindola et al. reported that the VISTA protein level was significantly correlated with both the PD-1/PD-L1 levels in human non-small cell lung cancer and they suggested that locally secreted factors such as interleukins or interferons could have mediated VISTA upregulation [38]. Several clinical trials evaluated the utility of PD-L1 expression as a biomarker with the goal of advancing patient options and improve response rates; however, the results regarding the correlation of PD-L1 expression with response to immune checkpoint inhibitor treatment were inconsistent [39, 40]. Although other predictors such as mutation load and molecular subtypes have been identified, treatment in real-life practice still relies on PD-L1 expression [32, 40]. The present study has found that VISTA expression in ICs was significantly and positively correlated with PD-L1 expression in ICs. Additionally, their expressions in different ICs show that they may have immunologic activities in different sites and steps. These findings support previous studies suggesting VISTA as a potential new therapeutic target for combination therapy as well as a biomarker for predicting response in immunotherapy. The underlying mechanisms of these potential roles of VISTA in bladder cancer require further experimental studies.
The present study has several limitations that should be acknowledged. First, due to the small number of patients and the short follow-up period, no complete oncological findings were identified. As a preliminary study including a small cohort of patients with bladder cancer, we were able to identify the association of VISTA expression with known risk factors for IVR and progression but could not assess its association with survival outcomes. Therefore, it is necessary to clarify the clinical significance of VISTA expression in MIBC as well as NMIBC through a large-scale prospective study. Second, the use of TMAs may not allow complete tumor assessment using immunohistochemical markers. Additional research is necessary for the establishment of VISTA scoring system in bladder tumor using the whole slide. While the present study results showed that the expression of VISTA and PD-L1 in ICs differed, we could not evaluate the IC type-specific expression either checkpoint molecule. Future investigation using multiplexed immunofluorescence is warranted. Finally, almost all patients underwent intravesical BCG instillation after transurethral resection in current study due to retrospective data. VISTA expression according to changes in the tumor immunologic environment after BCG treatment and its prognostic significances in animal model will follow in our further study.
In conclusion, this is the first study providing evidence that VISTA expression in bladder cancer is positively correlated with poor clinicopathologic features, demonstrating its clinical utility as a new immune checkpoint molecule in bladder cancer. Its association with IVR suggests that VISTA might be considered as a biomarker and target of adjuvant treatment in NMIBC. Finally, VISTA might be utilized as a target for combined immunotherapy and as an additional biomarker supplementing PD-L1 based on our results showing a relationship between the two immune checkpoint inhibitors in bladder cancer.
Acknowledgements
The biospecimens used in this study were provided by the Inje Biobank of Inje University Busan Paik Hospital, a member of Korea Biobank Network. And this work was supported by 2018 Inje University Busan Paik Hospital research grant.
Abbreviations
- AJCC
American Joint Committee on Cancer
- BCG
Bacillus Calmette-Guérin
- CI
Confidence interval
- ICs
Immune cells
- IVR
Intravesical recurrence
- MIBC
Muscle-invasive bladder cancer
- NMIBC
Non-muscle-invasive bladder cancer
- PD-1
Programmed cell death protein-1
- PD-L1
Programmed death ligand-1
- TCs
Tumor cells
- TMA
Tissue microarray
- VISTA
V-domain Ig suppressor of T cell activation
Author’s contributions
WIS: conception, writing and original manuscript preparation, CHL, WK, DSL: data collection and analysis, HYP: funding acquisition, SJJ: acquisition and interpretation of data, re-writing and editing the manuscript, JIJ, DHJ, IC, HYP: conception and design. All authors have commented on previous versions of manuscript and read and approved the final manuscript.
Funding
This work was supported by 2018 Inje University Busan Paik Hospital research grant.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Declarations
Ethical approval
This study was approved by the Busan Paik Hospital Institutional Review Board (IRB No.17-0195) and was in compliance with ethical guidelines according to the Declaration of Helsinki.
Consent to participate
Informed consent was waived by the Busan Paik Hospital Institutional Review Board on the grounds of being a retrospective study using tumor tissue already archived by the Inje Biobank.
Consent for publication
The authors transfer to Springer the non-exclusive publication rights.
Conflict of interest
The authors declare that they have no potential conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Saginala K, Barsouk A, Aluru JS, Rawla P, Padala SA, Barsouk A. Epidemiology of bladder cancer. Med Sci (Basel) 2020 doi: 10.3390/medsci8010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soukup V, Capoun O, Cohen D, Hernandez V, Babjuk M, Burger M, Comperat E, Gontero P, Lam T, MacLennan S, Mostafid AH, Palou J, van Rhijn BWG, Roupret M, Shariat SF, Sylvester R, Yuan Y, Zigeuner R. Prognostic performance and reproducibility of the 1973 and 2004/2016 World Health Organization grading classification systems in non-muscle-invasive bladder cancer: a European Association of urology non-muscle invasive bladder cancer guidelines panel systematic review. Eur Urol. 2017;72(5):801–813. doi: 10.1016/j.eururo.2017.04.015. [DOI] [PubMed] [Google Scholar]
- 4.Babjuk M, Burger M, Comperat EM, Gontero P, Mostafid AH, Palou J, van Rhijn BWG, Roupret M, Shariat SF, Sylvester R, Zigeuner R, Capoun O, Cohen D, Escrig JLD, Hernandez V, Peyronnet B, Seisen T, Soukup V. European association of urology guidelines on non-muscle-invasive bladder cancer (TaT1 and carcinoma in situ) - 2019 Update. Eur Urol. 2019;76(5):639–657. doi: 10.1016/j.eururo.2019.08.016. [DOI] [PubMed] [Google Scholar]
- 5.Chism DD. Urothelial carcinoma of the bladder and the rise of immunotherapy. J Natl Compr Canc Netw. 2017;15(10):1277–1284. doi: 10.6004/jnccn.2017.7036. [DOI] [PubMed] [Google Scholar]
- 6.Kim HS, Seo HK. Immune checkpoint inhibitors for urothelial carcinoma. Investig Clin Urol. 2018;59(5):285–296. doi: 10.4111/icu.2018.59.5.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lattanzi M, Balar AV. Current status and future direction of immunotherapy in urothelial carcinoma. Curr Oncol Rep. 2019;21(3):24. doi: 10.1007/s11912-019-0775-5. [DOI] [PubMed] [Google Scholar]
- 8.Siefker-Radtke AO, Apolo AB, Bivalacqua TJ, Spiess PE, Black PC. Immunotherapy with checkpoint blockade in the treatment of urothelial carcinoma. J Urol. 2018;199(5):1129–1142. doi: 10.1016/j.juro.2017.10.041. [DOI] [PubMed] [Google Scholar]
- 9.Hahn NM, Necchi A, Loriot Y, Powles T, Plimack ER, Sonpavde G, Roupret M, Kamat AM. Role of checkpoint inhibition in localized bladder cancer. Eur Urol Oncol. 2018;1(3):190–198. doi: 10.1016/j.euo.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 10.Wang L, Rubinstein R, Lines JL, Wasiuk A, Ahonen C, Guo Y, Lu LF, Gondek D, Wang Y, Fava RA, Fiser A, Almo S, Noelle RJ. VISTA, a novel mouse Ig superfamily ligand that negatively regulates T cell responses. J Exp Med. 2011;208(3):577–592. doi: 10.1084/jem.20100619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nowak EC, Lines JL, Varn FS, Deng J, Sarde A, Mabaera R, Kuta A, Le Mercier I, Cheng C, Noelle RJ. Immunoregulatory functions of VISTA. Immunol Rev. 2017;276(1):66–79. doi: 10.1111/imr.12525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Le Mercier I, Chen W, Lines JL, Day M, Li J, Sergent P, Noelle RJ, Wang L. VISTA regulates the development of protective antitumor immunity. Cancer Res. 2014;74(7):1933–1944. doi: 10.1158/0008-5472.CAN-13-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paner GP, Stadler WM, Hansel DE, Montironi R, Lin DW, Amin MB. Updates in the eighth edition of the tumor-node-metastasis staging classification for urologic cancers. Eur Urol. 2018;73(4):560–569. doi: 10.1016/j.eururo.2017.12.018. [DOI] [PubMed] [Google Scholar]
- 14.Mulati K, Hamanishi J, Matsumura N, Chamoto K, Mise N, Abiko K, Baba T, Yamaguchi K, Horikawa N, Murakami R, Taki M, Budiman K, Zeng X, Hosoe Y, Azuma M, Konishi I, Mandai M. VISTA expressed in tumour cells regulates T cell function. Br J Cancer. 2019;120(1):115–127. doi: 10.1038/s41416-018-0313-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xie S, Huang J, Qiao Q, Zang W, Hong S, Tan H, Dong C, Yang Z, Ni L. Expression of the inhibitory B7 family molecule VISTA in human colorectal carcinoma tumors. Cancer Immunol Immunother. 2018;67(11):1685–1694. doi: 10.1007/s00262-018-2227-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lines JL, Pantazi E, Mak J, Sempere LF, Wang L, O'Connell S, Ceeraz S, Suriawinata AA, Yan S, Ernstoff MS, Noelle R. VISTA is an immune checkpoint molecule for human T cells. Cancer Res. 2014;74(7):1924–1932. doi: 10.1158/0008-5472.CAN-13-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liao H, Zhu H, Liu S, Wang H. Expression of V-domain immunoglobulin suppressor of T cell activation is associated with the advanced stage and presence of lymph node metastasis in ovarian cancer. Oncol Lett. 2018;16(3):3465–3472. doi: 10.3892/ol.2018.9059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuklinski LF, Yan S, Li Z, Fisher JL, Cheng C, Noelle RJ, Angeles CV, Turk MJ, Ernstoff MS. VISTA expression on tumor-infiltrating inflammatory cells in primary cutaneous melanoma correlates with poor disease-specific survival. Cancer Immunol Immunother. 2018;67(7):1113–1121. doi: 10.1007/s00262-018-2169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loeser H, Kraemer M, Gebauer F, Bruns C, Schroder W, Zander T, Persa OD, Alakus H, Hoelscher A, Buettner R, Lohneis P, Quaas A. The expression of the immune checkpoint regulator VISTA correlates with improved overall survival in pT1/2 tumor stages in esophageal adenocarcinoma. Oncoimmunology. 2019;8(5):e1581546. doi: 10.1080/2162402X.2019.1581546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang M, Pang HJ, Zhao W, Li YF, Yan LX, Dong ZY, He XF. VISTA expression associated with CD8 confers a favorable immune microenvironment and better overall survival in hepatocellular carcinoma. BMC Cancer. 2018;18(1):511. doi: 10.1186/s12885-018-4435-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zong L, Zhou Y, Zhang M, Chen J, Xiang Y. VISTA expression is associated with a favorable prognosis in patients with high-grade serous ovarian cancer. Cancer Immunol Immunother. 2020;69(1):33–42. doi: 10.1007/s00262-019-02434-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamat AM, Bellmunt J, Galsky MD, Konety BR, Lamm DL, Langham D, Lee CT, Milowsky MI, O'Donnell MA, O'Donnell PH, Petrylak DP, Sharma P, Skinner EC, Sonpavde G, Taylor JA, 3rd, Abraham P, Rosenberg JE. Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of bladder carcinoma. J Immunother Cancer. 2017;5(1):68. doi: 10.1186/s40425-017-0271-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Power NE, Izawa J. Comparison of guidelines on non-muscle invasive bladder cancer (EAU, CUA, AUA, NCCN, NICE) Bladder Cancer. 2016;2(1):27–36. doi: 10.3233/BLC-150034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woldu SL, Bagrodia A, Lotan Y. Guideline of guidelines: non-muscle-invasive bladder cancer. BJU Int. 2017;119(3):371–380. doi: 10.1111/bju.13760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ratliff TL, Palmer JO, McGarr JA, Brown EJ (1987) Intravesical Bacillus Calmette-Guerin therapy for murine bladder tumors: initiation of the response by fibronectin-mediated attachment of Bacillus Calmette-Guerin. Cancer Res 47(7):1762–1766 https://cancerres.aacrjournals.org/content/47/7/1762.long [PubMed]
- 26.Lockyer CR, Gillatt DA. BCG immunotherapy for superficial bladder cancer. J R Soc Med. 2001;94(3):119–123. doi: 10.1177/014107680109400305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Witjes JA. Management of BCG failures in superficial bladder cancer: a review. Eur Urol. 2006;49(5):790–797. doi: 10.1016/j.eururo.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 28.Bohle A, Jocham D, Bock PR. Intravesical bacillus Calmette-Guerin versus mitomycin C for superficial bladder cancer: a formal meta-analysis of comparative studies on recurrence and toxicity. J Urol. 2003;169(1):90–95. doi: 10.1097/01.ju.0000039680.90768.b3. [DOI] [PubMed] [Google Scholar]
- 29.Chang SS, Boorjian SA, Chou R, Clark PE, Daneshmand S, Konety BR, Pruthi R, Quale DZ, Ritch CR, Seigne JD, Skinner EC, Smith ND, McKiernan JM. Diagnosis and treatment of non-muscle invasive bladder cancer: AUA/SUO guideline. J Urol. 2016;196(4):1021–1029. doi: 10.1016/j.juro.2016.06.049. [DOI] [PubMed] [Google Scholar]
- 30.Spiess PE, Agarwal N, Bangs R, Boorjian SA, Buyyounouski MK, Clark PE, Downs TM, Efstathiou JA, Flaig TW, Friedlander T, Greenberg RE, Guru KA, Hahn N, Herr HW, Hoimes C, Inman BA, Jimbo M, Kader AK, Lele SM, Meeks JJ, Michalski J, Montgomery JS, Pagliaro LC, Pal SK, Patterson A, Plimack ER, Pohar KS, Porter MP, Preston MA, Sexton WJ, Siefker-Radtke AO, Sonpavde G, Tward J, Wile G, Dwyer MA, Gurski LA. Bladder cancer, version 5.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2017;15(10):1240–1267. doi: 10.6004/jnccn.2017.0156. [DOI] [PubMed] [Google Scholar]
- 31.Mukherjee N, Svatek RS, Mansour AM. Role of immunotherapy in bacillus Calmette-Guerin-unresponsive non-muscle invasive bladder cancer. Urol Oncol. 2018;36(3):103–108. doi: 10.1016/j.urolonc.2017.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doyle E, Crew J, Mostafid H, Tuthill M, Cerundolo V, Gerristen W, Protheroe A. Urothelial cancer: a narrative review of the role of novel immunotherapeutic agents with particular reference to the management of non-muscle-invasive disease. BJU Int. 2019;123(6):947–958. doi: 10.1111/bju.14643. [DOI] [PubMed] [Google Scholar]
- 33.Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, Dawson N, O’Donnell PH, Balmanoukian A, Loriot Y, Srinivas S, Retz MM, Grivas P, Joseph RW, Galsky MD, Fleming MT, Petrylak DP, Perez-Gracia JL, Burris HA, Castellano D, Canil C, Bellmunt J, Bajorin D, Nickles D, Bourgon R, Frampton GM, Cui N, Mariathasan S, Abidoye O, Fine GD, Drecier R. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicenter, phase 2 trial. Lancet. 2016;387:1909–1920. doi: 10.1016/S0140-6736(16)00561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu J, Yuan Y, Chen W, Putra J, Suriawinata AA, Schenk AD, Miller HE, Guleria I, Barth RJ, Huang YH, Wang L. Immune-checkpoint proteins VISTA and PD-1 nonredundantly regulate murine T-cell responses. Proc Natl Acad Sci U S A. 2015;112(21):6682–6687. doi: 10.1073/pnas.1420370112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kakavand H, Jackett LA, Menzies AM, Gide TN, Carlino MS, Saw RPM, Thompson JF, Wilmott JS, Long GV, Scolyer RA. Negative immune checkpoint regulation by VISTA: a mechanism of acquired resistance to anti-PD-1 therapy in metastatic melanoma patients. Mod Pathol. 2017;30(12):1666–1676. doi: 10.1038/modpathol.2017.89. [DOI] [PubMed] [Google Scholar]
- 36.Gao J, Ward JF, Pettaway CA, Shi LZ, Subudhi SK, Vence LM, Zhao H, Chen J, Chen H, Efstathiou E, Troncoso P, Allison JP, Logothetis CJ, Wistuba II, Sepulveda MA, Sun J, Wargo J, Blando J, Sharma P. VISTA is an inhibitory immune checkpoint that is increased after ipilimumab therapy in patients with prostate cancer. Nat Med. 2017;23(5):551–555. doi: 10.1038/nm.4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luisa B, Francheska C, Lindsay C, Courtney M, Purushottam L, Rahul RM. Resistance to checkpoint inhibition in cancer immunotherapy. Transl Oncol. 2020;13(3):e100738. doi: 10.1016/j.tranon.2019.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Villarroel-Espindola F, Yu X, Datar I, Mani N, Sanmamed M, Velcheti V, Syrigos K, TokiM ZH, Chen L, Herbst RS, Schalper KA. Spatially resolved and quantitative analysis of VISTA/PD-1H as a novel immunotherapy target in human non-small cell lung cancer. Clin Cancer Res. 2018;24(7):1562–1573. doi: 10.1158/1078-0432.CCR-17-2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giridhar KV, Kohli M. Management of Muscle-Invasive Urothelial Cancer and the Emerging Role of Immunotherapy in Advanced Urothelial Cancer. Mayo Clin Proc. 2017;92(10):1564–1582. doi: 10.1016/j.mayocp.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 40.Fusi A, Festino L, Botti G, Masucci G, Melero I, Lorigan P, Ascierto PA. PD-L1 expression as a potential predictive biomarker. Lancet Oncol. 2015;16(13):1285–1287. doi: 10.1016/S1470-2045(15)00307-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.