Abstract
Background
Radiotherapy (RT) may function synergistically with immunotherapy and targeted agents (TA). This study aimed to assess the effectiveness and safety of RT combined with programmed death-1 (PD-1) inhibitors and lenvatinib in patients with relapsed or refractory advanced biliary tract carcinoma (BTC).
Methods
This retrospective study included patients with relapsed or refractory advanced BTC who received RT combined with PD-1 inhibitors and lenvatinib at the Peking Union Medical College Hospital (PUMCH). Overall survival (OS), progression-free survival (PFS), objective response rate (ORR), disease control rate (DCR), and safety were evaluated.
Results
Thirty-one patients who received RT combined with PD-1 inhibitors and lenvatinib as a second- or later-line therapy were analyzed. RT sites were mainly distributed in the liver lesions (64.5%) and lymph nodes (58.1%). The ORR and DCR were 32.3% (10/31; 95% CI: 14.8–49.7) and 87.1% (27/31; 95% CI: 74.6–99.6), respectively. The median PFS (mPFS) and median OS (mOS) were 7.9 (95% CI: 7.1–8.7) and 11.7 (95% CI: 8.3–15.0) months, respectively. Subgroup analyses of this cohort included 12 and 19 patients who received concurrent and salvage (> 6 weeks after commencing PD-1 inhibitor therapy) RT, respectively. The salvage RT group had higher mOS (11.7 vs. 10.5; p = 0.75) and mPFS (7.9 vs. 6.9; p = 0.85) than the concurrent RT group; however, statistical significance was not reached. All patients experienced any-grade adverse events (AEs), and excessive PD-1 inhibitors or RT toxicity were not observed.
Conclusions
RT, PD-1 inhibitors, and lenvatinib may be safely combined and have antitumor effectiveness in patients with advanced BTC.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-023-03399-2.
Keywords: Advanced biliary tract cancer, PD-1 inhibitors, Lenvatinib, Concurrent radiotherapy, Salvage radiotherapy
Introduction
Biliary tract carcinomas (BTC), including intrahepatic cholangiocarcinoma (ICC), extrahepatic cholangiocarcinoma (ECC), and gallbladder cancer (GBC), are aggressive malignancies [1]. The incidence and mortality of BTC, mainly ICC, have increased in recent years [2, 3]. BTC has a poor prognosis, and the overall 5-year overall survival rate of patients with BTC is < 20% [4]. Patients are usually diagnosed with advanced-stage disease, and a small proportion of them is eligible to undergo surgery [5].
Gemcitabine plus cisplatin (GC) is approved as first-line therapy for advanced BTC [6], and folinic acid with fluorouracil and oxaliplatin (FOLFOX) is recommended as a second-line treatment [7]. Both first- and second-line treatments have modest survival benefits. However, the subsequent available antitumor solutions are limited for patients with advanced BTC who experience disease progression after chemotherapy failure [8].
Immunotherapy and lenvatinib have shown promising effects in treating multiple tumor types [6]. However, the treatment effectiveness of either PD-1 inhibitors alone or PD-1 inhibitors combined with lenvatinib is far from satisfactory in BTC [9, 10]. A systematic review demonstrated that RT has survival benefits for patients with advanced BTC [11]. In addition, studies have shown that RT improves the effectiveness of PD-1 inhibitors [12].
Considering the different anti-malignancy mechanisms of lenvatinib, PD-1 inhibitors, and RT, combining these three modalities may have a potential synergistic effect and promising preliminary effectiveness in advanced BTC. Therefore, this study investigated the effectiveness and safety of a combination of RT with PD-1 inhibitors and lenvatinib in patients with relapsed or refractory advanced BTC.
Materials and methods
Participants and treatment
We retrospectively collected data from patients with relapsed or refractory advanced BTC who received RT combined with PD-1 inhibitors and lenvatinib at the Peking Union Medical College Hospital (PUMCH) between January 31, 2020 and November 29, 2021. The primary eligibility criteria included histologically confirmed ECC, ICC, or GBC; at least one measurable or evaluable tumor lesion according to the Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST 1.1); and the need for further systemic treatment. The disease progressed in all patients despite previously receiving at least first-line systemic therapy. In addition, data on patients’ demographic, surgical, pathological, regional, and systemic treatment information were recorded. The study protocol complied with the Declaration of Helsinki and was approved by the Institutional Review Board and Ethics Committee at Peking Union Medical College Hospital.
The patients either received RT concomitantly with PD-1 inhibitors or PD-1 inhibitors before RT. Lenvatinib was administered orally at a dose of 12 mg (for patients with body weight ≥ 60 kg) or 8 mg (for patients with body weight < 60 kg) once daily. The PD-1 inhibitor dose included a fixed dose of 200 mg (or 240 mg of toripalimab) every 3 weeks or 3 mg/kg every 3 weeks.
We assessed the effectiveness and safety of RT combined with PD-1 inhibitors and lenvatinib. In addition, we performed a subgroup analysis in the study cohort based on the time interval between RT and PD-1 inhibitor administration. The patients were grouped as follows: (1) concurrent RT group with RT administered simultaneously with PD-1 inhibitors, or no later than 6 weeks after commencing PD-1 therapy; (2) “salvage” RT group with RT administered due to clinical or radiological progression of the disease or stable disease (SD) noted > 6 weeks after commencing PD-1 inhibitor administration [13].
Patients underwent intensity-modulated radiation therapy (IMRT). The radiation dose was prescribed to the isocenter or 95% planning target volume as 24.0–60.0 Gy in 6–27 fractions or a single dose between 1.8 and 6.0 Gy for tumor sites not more than five times a week, at the physician's discretion.
Outcome assessments
The overall response was assessed using enhanced computed tomography (CT) or magnetic resonance imaging (MRI) according to RECIST 1.1 by professional radiologists at the center of PUMCH. Therapeutic effectiveness included the objective response rate (ORR) (the proportion of patients with confirmed complete response (CR) and partial response (PR)), progression-free survival (PFS), overall survival (OS), disease control rate (DCR) (the proportion of patients who achieved an objective response or SD), and safety. PFS was defined as the time from the start of PD-1 inhibitors administration to disease progression at any site or death. Additionally, OS was defined as the time from commencing PD-1 inhibitors administration to the date of death. In addition, adverse events (AEs) were categorized and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0 (CTCAE 4.0).
Statistical analysis
All statistical analyses were performed using the data obtained by the cut-off date of June 1, 2022. Continuous variables are listed as median with interquartile range (IQR) while categorical variables are listed as a percentage of the total. The Kaplan–Meier method and bilateral log-rank test were used to generate PFS and OS curves. The χ2 test, Fisher's exact test, and Spearman’s ρ coefficient test were performed as appropriate to compare the individual variables. All statistical analyses were performed using SPSS 22 (version 22.0, SPSS Inc., Chicago, IL, USA) and R (version 4.0.3).
Results
The patient demographics and baseline characteristics
Between January 31, 2020, and November 29, 2021, 31 patients with advanced BTC were included in this study. The median follow-up was 13.5 months. The baseline characteristics of the patients are shown in Table 1 (Supplymentary table S1). The median age of the patients was 61 (IQR: 54.5–65.8) years, and 58.1% were male. The primary tumor sites were as follows: 19 ICCs (61.3%), four ECCs (12.9%), and eight GBCs (25.8%). All patients had undergone at least the first line of antitumor treatment. Furthermore, local relapse was observed postoperatively in 16.1% of the patients. In addition, 34.5% of the patients received systemic chemotherapy, and 54.8% of patients chose targeted therapy due to concerns about the side effects of chemotherapy. The liver was the most common metastatic site (83.9%), and other metastatic lesions included brain (one patient), uterine (one patient), and adrenal (one patient) metastases.
Table 1.
Baseline characteristics
| Characteristics | RT plus PD-1 inhibitors and lenvatinib (n = 31) |
|---|---|
| Age, years(median, IQR) | 61(54.5 − 65.8) |
| Gender, n (%) | |
| Male | 18(58.1) |
| Female | 13(41.9) |
| Tumor subtype, n (%) | |
| Intrahepatic cholangiocarcinoma | 19(61.3) |
| Extrahepatic cholangiocarcinoma | 4(12.9) |
| Gallbladder cancer | 8(25.8) |
| ECOG performance status, n (%) | |
| 0 | 13(41.9) |
| 1 | 15(48.4) |
| 2 | 3(9.7) |
| Differentiated histology, n (%) | |
| Well | 0 |
| Moderately | 3(9.7) |
| Poorly | 9(29) |
| Moderately-poorly | 5(16.1) |
| Well-moderately | 0 |
| Unsure | 14(45.2) |
| Previous antitumor therapy, n (%) | |
| Radical surgery resection | 5(16.1) |
| Systemic chemotherapy | 11(35.4) |
| Targeted therapy | 17(54.8) |
| Site of metastases, n (%) | |
| Intrahepatic | 26(83.9) |
| Lymph nodes | 24(77.4) |
| Lung | 5(16.1) |
| Bone | 7(22.6) |
| Other (Uterus, adrenal glands, brain) | 3(9.7) |
| Radiotherapy dose (Gray) | |
| Median(range) | 46 (40–51) |
| Radiotherapy technique | |
| Intensity-modulated radiation | 31(100) |
| Radiotherapy site | |
| Liver | 20(64.5) |
| Bone | 3(9.7) |
| Lung | 9(29.0) |
| Soft tissue or lymph nodes in the abdominal cavity | 18(58.1) |
| Type of anti-PD-1 antibodies, n (%) | |
| Toripalimab | 22(71.0) |
| Camrelizumab | 7(22.6) |
| Tislelizumab | 2(6.4) |
RT sites were mainly distributed in the liver lesions (64.5%) and lymph nodes (58.1%). The median radiation dose delivered was 46 (range: 24–60) Gy in 6–27 fractions with IMRT. Nineteen (61.3%) patients received one course, and 12 (38.7%) received two courses.
Effectiveness
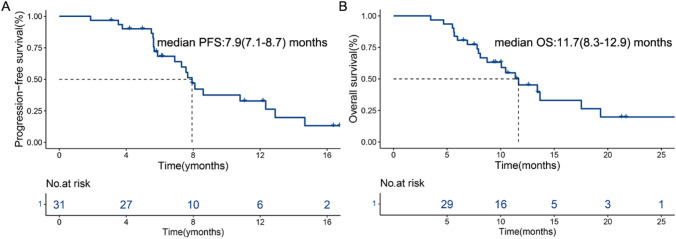
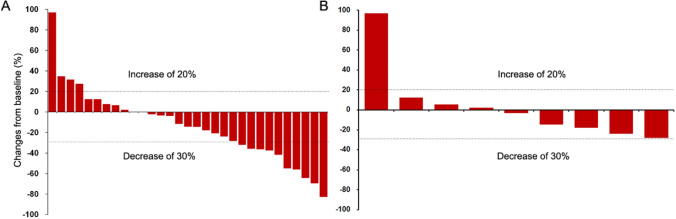
In this study, all patients underwent complete radiological evaluation. Among the 31 patients, no patient achieved CR, 10 achieved PR, 17 had SD, and 4 exhibited progressive disease (PD) (Table 2). The ORR was 32.3% (10/31; 95% CI: 14.8–49.7), and the DCR was 87.1% (27/31; 95% CI: 74.6–99.6) (Table 2). The median PFS (mPFS) was 7.9 (95% CI: 7.1–8.7) months, and the median OS (mOS) was 11.7 (95% CI: 8.3–12.9) months (Fig. 1). Figure 2A shows a waterfall plot of the target lesions from the baseline; among the 31 (64.5%) patients, 20 had decreased tumor size. Analysis of the nine measurable non-target lesions showed that five patients had a decrease in tumor size from baseline (Fig. 2B).
Table 2.
Tumour responses based on RECIST 1.1
| Evaluable patients (n = 31) | |
|---|---|
| Objective response rate (95% CI) | 32.3(14.8 − 49.7) |
| Complete response (n, %) | 0 |
| Partial response (n, %) | 10(32.3) |
| Stable disease (n, %) | 17(54.8) |
| Progressive disease (n, %) | 4(12.9) |
| DCR (n, %), 95% CI | 87.1(74.6 − 99.6) |
| Median overall survival, months (95% CI) | 11.7(8.3 − 12.9) |
| Median progression-free survival, months (95% CI) | 7.9(7.1 − 8.7) |
DCR, disease control rate; RECIST, response evaluation criteria in solid tumors
Fig. 1.
Kaplan–Meier curves for progression-free survival A and overall survival B for patients receiving RT plus PD-1 inhibitors and lenvatinib
Fig. 2.
Waterfall plots of the best percentage change. The best percentage change in targeted tumor size from baseline for 31 patients A, and non-target tumor size from baseline for nine patients B
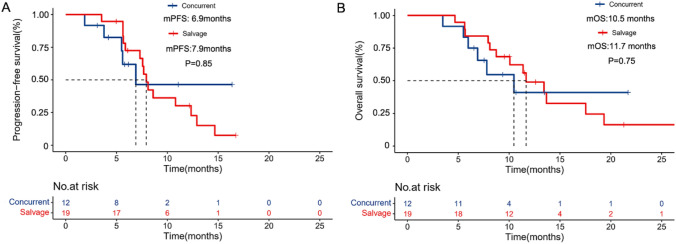
Among these patients, 12 (38.7%) received concurrent treatment with PD-1 inhibitors and RT, and 19 (61.3%) received “salvage” RT. Subgroup analyses of the two subgroups showed that the ORR and DCR were 33.3% (4/12; 95% CI: 2–64.6) and 83.3% (10/12; 95% CI: 58.6–108.1), respectively, in the concurrent RT group. However, in the salvage RT group, the ORR and DCR were 31.6% (16/19; 95% CI: 8.6–54.6) and 89.5% (17/19; 95% CI: 74.3–104.7), respectively (Table 3). Furthermore, in the salvage RT group, one patient achieved PR before commencing RT, two who achieved SD before RT achieved PR, and six achieved SD before RT. The mPFS in the salvage and concurrent RT groups was 6.9 (95% CI: 7.2–8.6) and 7.9 (95% CI: 7.2–8.6) months, respectively (p = 0.85; Fig. 3A). The mOS in the salvage and concurrent RT groups was 10.5 (95% CI: 5.8–15.2) and 11.7 (95% CI: 8.4–14.9) months, respectively (p = 0.75; Fig. 3B).
Table 3.
Subgroup analyses of effectiveness in the concurrent and salvage RT group
| Concurrent RT (n = 12) | Salvage RT (n = 19) | P | |
|---|---|---|---|
| ORR (n, 95% CI) | 33.3(26 − 4.6) | 31.6(8.6 − 54.6) | 0.61 |
| DCR (n, 95% CI) | 83.3(58.6 − 108.1) | 89.5(74.3 − 104.7) | 0.51 |
| CR (n, %) | 0 | 0 | – |
| PR (n, %) | 4 | 6 | – |
| SD (n, %) | 6 | 11 | – |
| PD (n, %) | 2 | 2 | – |
CR complete response, PR partial response SD stable disease PD progressive disease, ORR objective response rate, DCR disease control rate
Fig. 3.
Kaplan–Meier estimates the progression-free survival A and overall survival B after stratification based on the time interval between RT and PD-1 inhibitors
Safety
All patients experienced more than one AE, and no treatment-related deaths occurred in this study (Supplymentary table S2). Two patients experienced grade 4 severe AEs (SAEs) (gastrointestinal hemorrhage and diarrhea), and 22 (71%) experienced grade 3 AEs. The most common AEs (any grade) were fatigue (74.2%), hypertension (58.1%), and elevated alanine transaminase (ALT) or aspartate transaminase (AST) levels (54.8%). The most frequent grade 3 AEs were fatigue, rash, bilirubin elevation, and hypoproteinemia, with an incidence of 12.9%. All any-grade AEs recorded were reversible and controllable.
Discussion
Compared to the published literature, this is the first study to evaluate the effectiveness and safety of RT combined with PD-1 inhibitors and lenvatinib administration in patients with relapsed or refractory advanced BTC. Patients with relapsed or refractory advanced BTC who have received systemic antitumor therapy have limited treatment options, and their OS is poor. In addition, the effectiveness of PD-1 inhibitors, alone or in combination with lenvatinib, is far from satisfactory. Most of the patients enrolled in the present study were at an advanced BTC stage and had received two or more previous therapies. Nevertheless, after receiving combined PD-1 inhibitor therapy and RT, they achieved an ORR of 32.3%, DCR of 87.1%, mPFS of 7.9 (95% CI: 7.1–8.7) months, and mOS of 11.7 (95% CI: 8.3–12.9) months.Thus, RT plus PD-1 inhibitors and lenvatinib may prolong the survival and enhance the antitumor effectiveness of immunotherapy.
Due to the complexity and heterogeneity of the tumor immune microenvironment in BTC, the antitumor effectiveness of PD-1 inhibitors combined with targeted agents (TA) is ambiguous. The LEAP-005 study published an ORR of 10% (95% CI: 2‒26), DCR of 68% (95% CI: 49‒83), mPFS of 6.1 (95% CI: 2.1‒6.4) months, and mOS of 8.6 (95% CI: 5.6 to NR) months for the patients receiving lenvatinib and pembrolizumab [10]. Furthermore, a single-arm study reported that pembrolizumab combined with lenvatinib could enhance the antitumor effectiveness of immunotherapy, with an ORR of 25% and DCR of 75% in patients with refractory BTC [14]. However, the ORR of lenvatinib combined with PD-1 inhibitors varies across different centers [15, 16]. A growing body of evidence suggests that the addition of RT to PD-1 inhibitor therapy may improve the effectiveness of immune checkpoint inhibitors (ICIs) [17, 18] where RT is administered before or concurrently with ICIs[16]. Notably, ORRs, DCR, mOS, and mPFS in this study were higher than those in other studies on patients administered PD-1 inhibitors combined with TA [14, 19]. This suggests that the immune priming provided by radiation may be an integral part of enhancing the system's response to checkpoint therapy. Retrospective studies reported that patients who received ipilimumab before RT had an increased duration of irradiated tumor response compared with patients who received ipilimumab after RT [20, 21]. Nevertheless, caution must be exercised when interpreting the effectiveness of combined RT and ICI therapy.
This study analyzed concurrent and salvage RT, but no significant difference was found. Although the mOS and mPFS were higher in the salvage RT group, the ORR was higher in the concurrent RT group. In the salvage RT group, one patient who had progressive disease (PD) achieved PR after RT, two who had SD before RT achieved PR, and six who had PD before RT achieved SD. These results suggest that salvage RT may help patients survive longer, whereas concurrent RT improves treatment response and facilitates conversion surgery. Furthermore, the timing of radiation administration may produce different outcomes. In addition, we observed that both target and non-target lesions decreased in five patients. In comparison, target lesions decreased, and non-target lesions increased in four patients, indicating that RT had a synergistic effect with PD-1 inhibitors andlenvatinib.
Furthermore, RT can promote tumor cell necrosis, release tumor antigens to promote immune cell recognition, and induce the release of other cytokines to facilitate immune cell function. Therefore, we explored the existing medical literature to gather such evidence. In addition, the comparison between salvage and concurrent RT requires further investigation.
Moreover, the optimal radiation dose remains unclear. Data from mouse models bearing B78 melanoma tumors have indicated that low-dose targeted radionuclide therapy renders immunologically “cold” tumors responsive to immune checkpoint blockade [22]. Another breast and colorectal mouse model indicated that a fractionated regimen might be superior to a single dose in overcoming RT-induced adaptive resistance by upregulating PD-L1 [23].
Regarding therapeutic safety, all patients experienced no grade 5 AEs, and approximately 71% experienced grade 3 AEs. The AEs most frequently noted in this study were fatigue and hypertension. Two patients experienced grade 4 AEs, including gastrointestinal hemorrhage and diarrhea, which were controlled and improved after drug discontinuation and active management. The incorporation of RT into immunotherapy caused more AEs than traditional chemotherapy or PD-1 inhibitor therapy alone or in combination with TA. A possible reason might be that local–regional therapies such as RT may cause more side effects. However, none of the AEs in either group were unexpected; furthermore, they were generally manageable and reversible. Ultimately, the combination of RT with PD-1 inhibitors and lenvatinib showed a good safety profile.
This study had some limitations. First, retrospective research studies are prone to recall, observation, and selection bias. Hence, the effectiveness and safety of RT combined with PD-1 inhibitors and lenvatinib observed should be considered cautiously because of the study’s limited sample size and retrospective nature. Consequently, the findings of this study should be validated in prospective clinical trials. Second, selection bias from patients who probably had different tumor burdens cannot be ruled out. Third, the follow-up period was relatively short. Fourth, this study lacked a control group (FOLFOX or PD-1 inhibitors plus lenvatinib). Finally, programmed death-ligand 1 (PD-L1) expression is one of the most effectiveness-related biomarkers. However, PD-L1 expression was not evaluated in this study. Although these factors somewhat weaken the validity and reliability of this study’s conclusions, ‘real-world’ data are still helpful for a subsequent prospective study.
Conclusions
This study assessed the effectiveness and safety of RT combined with PD-1 inhibitors and lenvatinib in relapsed or refractory advanced BTC. Adding RT to PD-1 inhibitors and lenvatinib therapy may result in a good safety profile and promising antitumor effectiveness. Furthermore, we observed that concurrent and salvage RT resulted in favorable survival and control rates. Additionally, we found that salvage RT may enable patients to survive longer, whereas concurrent RT improves treatment response. However, the study was limited by a relatively small and heterogeneous population of patients; therefore, rare toxicities may not be detected. Hence, further research with larger prospective cohorts is required.
Supplementary Information
Below is the link to the electronic supplementary material.
Abbreviations
- AEs
Adverse events
- BTCs
Biliary tract cancers
- CR
Complete response
- CTCAE
Common Terminology Criteria Coastocellular Group
- DCR
Disease control rate
- ECC
Extrahepatic cholangiocarcinoma
- GBC
Gallbladder cancer
- HR
Hazard rate
- ICC
Intrahepatic cholangiocarcinoma
- Lenvatinib
Tyrosine kinase inhibitors
- ORR
Objective response rate
- OS
Overall survival
- PD
Progressive disease
- PD-1
Programmed cell death protein 1
- PD-L1
Programmed cell death ligand 1
- PFS
Progression-free survival
- PR
Partial response
- RECIST
Response evaluation criteria in solid tumors
- RT
Radiotherapy
- SD
Stable disease
- TA
Targeted agents
Author contributions
YCW and XBY collected the data and wrote the manuscript. XRH and HTZ designed and examined the study. NZ, JYL, and XY helped to collect the literature and participated in discussions. ZYX, JNX, and YYW performed the statistical analyses. All authors read and approved the final manuscript.
Funding
This work was supported by CAMS Innovation Fund for Medical Sciences (CIFMS) (2021–1-I2M-003 and 2021-I2M-1–061), CSCO-hengrui Cancer Research Fund (Y-HR2019-0239, Y-HR2020MS-0415, Y-HR2020QN-0414), CSCO-MSD Cancer Research Fund (Y-MSDZD2021-0213) and National Ten-thousand Talent Program.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Declarations
Conflict of Interest
The authors have no conflicts of interest to declare.
Ethical approval
All patients were fully informed about the objectives of this study and provided formal written consent a priori. The protocols of this study were compliant with the principles of the Declaration of Helsinki and were also approved by the institutional review board and ethics committee at Peking Union Medical College Hospital (PUMCH-JS-1391).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yunchao Wang, Xiaobo Yang and Yanyu Wang authors contributed equally to this work.
Contributor Information
Xiaorong Hou, Email: hxr_pumch@163.com.
Haitao Zhao, Email: zhaoht@pumch.cn.
References
- 1.Brindley PJ, Bachini M, Ilyas SI, Khan SA, Loukas A, Sirica AE, Teh BT, Wongkham S, Gores GJ. Cholangiocarcinoma. Nat Rev Dis Prim. 2021;7:65. doi: 10.1038/s41572-021-00300-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Valle JW, Borbath I, Khan SA, Huguet F, Gruenberger T, Arnold D. Biliary cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol Official J European Soc Med Oncol. 2016;27:v28–v37. doi: 10.1093/annonc/mdw324. [DOI] [PubMed] [Google Scholar]
- 3.Valle JW, Kelley RK, Nervi B, Oh DY, Zhu AX. Biliary tract cancer. Lancet (London, England) 2021;397:428–444. doi: 10.1016/s0140-6736(21)00153-7. [DOI] [PubMed] [Google Scholar]
- 4.Boilève A, Hilmi M, Delaye M, Tijeras-Raballand A, Neuzillet C. Biomarkers in hepatobiliary cancers: What is useful in clinical practice? Cancers. 2021 doi: 10.3390/cancers13112708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shroff RT, Kennedy EB, Bachini M, et al. Adjuvant therapy for resected biliary tract cancer: ASCO clinical practice guideline. J clin oncol offi j Am Soc Clin Oncol. 2019;37:1015–1027. doi: 10.1200/jco.18.02178. [DOI] [PubMed] [Google Scholar]
- 6.Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–1281. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 7.Lamarca A, Palmer DH, Wasan HS, et al. Second-line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer (ABC-06): a phase 3, open-label, randomised, controlled trial. Lancet Oncol. 2021;22:690–701. doi: 10.1016/s1470-2045(21)00027-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y, Yang X, Wang D, et al. lenvatinib beyond first-line therapy in patients with advanced biliary tract carcinoma. Front Oncol. 2022;12:785535. doi: 10.3389/fonc.2022.785535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piha-Paul SA, Oh DY, Ueno M, et al. Efficacy and safety of pembrolizumab for the treatment of advanced biliary cancer: results from the KEYNOTE-158 and KEYNOTE-028 studies. Int J Cancer. 2020;147:2190–2198. doi: 10.1002/ijc.33013. [DOI] [PubMed] [Google Scholar]
- 10.Villanueva L, Lwin Z, Chung HCC, Gomez-Roca CA, Graham DMJ. Lenvatinib plus pembrolizumab for patients with previously treated biliary tract cancers in the multicohort phase 2 LEAP-005 study. J Clin Oncol. 2021;39:4080. doi: 10.1200/JCO.2021.39.15_suppl.4080. [DOI] [Google Scholar]
- 11.Taggar AS, Mann P, Folkert MR, Aliakbari S, Myrehaug SD, Dawson LA. A systematic review of intraluminal high dose rate brachytherapy in the management of malignant biliary tract obstruction and cholangiocarcinoma. Radiother Oncol J European Soc Ther Radiol Oncol. 2021;165:60–74. doi: 10.1016/j.radonc.2021.10.011. [DOI] [PubMed] [Google Scholar]
- 12.Liu Y, Dong Y, Kong L, Shi F, Zhu H, Yu J. Abscopal effect of radiotherapy combined with immune checkpoint inhibitors. J Hematol Oncol. 2018;11:104. doi: 10.1186/s13045-018-0647-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liniker E, Menzies AM, Kong BY, et al. Activity and safety of radiotherapy with anti-PD-1 drug therapy in patients with metastatic melanoma. Oncoimmunology. 2016;5:e1214788. doi: 10.1080/2162402x.2016.1214788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin J, Yang X, Long J, et al. Pembrolizumab combined with lenvatinib as non-first-line therapy in patients with refractory biliary tract carcinoma. Hepatobiliary Surg Nutr. 2020;9:414–424. doi: 10.21037/hbsn-20-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jian Z, Fan J, Shi GM, et al. Lenvatinib plus toripalimab as first-line treatment for advanced intrahepatic cholangiocarcinoma: a single-arm, phase 2 trial. J Clin Oncol. 2021;39:4099. doi: 10.1200/JCO.2021.39.15_suppl.4099. [DOI] [Google Scholar]
- 16.Mody K, Starr J, Saul M, Poorman K, Shields AF. Patterns and genomic correlates of PD-L1 expression in patients with biliary tract cancers. J Gastrointest Oncol. 2019;10:1099–1109. doi: 10.21037/jgo.2019.08.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lan Y, Moustafa M, Knoll M, et al. Simultaneous targeting of TGF-β/PD-L1 synergizes with radiotherapy by reprogramming the tumor microenvironment to overcome immune evasion. Cancer Cell. 2021;39:1388–403.e10. doi: 10.1016/j.ccell.2021.08.008. [DOI] [PubMed] [Google Scholar]
- 18.Mondini M, Levy A, Meziani L, Milliat F, Deutsch E. Radiotherapy-immunotherapy combinations: perspectives and challenges. Mol Oncol. 2020;14:1529–1537. doi: 10.1002/1878-0261.12658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zuo B, Yang X, Yang X, et al. A real-world study of the efficacy and safety of anti-PD-1 antibodies plus lenvatinib in patients with advanced gallbladder cancer. Cancer immunol immunother CII. 2022 doi: 10.1007/s00262-021-03121-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chicas-Sett R, Morales-Orue I, Rodriguez-Abreu D, Lara-Jimenez P. Combining radiotherapy and ipilimumab induces clinically relevant radiation-induced abscopal effects in metastatic melanoma patients: a systematic review. Clin transl radiat oncol. 2018;9:5–11. doi: 10.1016/j.ctro.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salama AK, Postow MA, Salama JK. Irradiation and immunotherapy: from concept to the clinic. Cancer. 2016;122:1659–1671. doi: 10.1002/cncr.29889. [DOI] [PubMed] [Google Scholar]
- 22.Patel RB, Hernandez R, Carlson P, et al. Low-dose targeted radionuclide therapy renders immunologically cold tumors responsive to immune checkpoint blockade. Sci transl med. 2021 doi: 10.1126/scitranslmed.abb3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dovedi SJ, Adlard AL, Lipowska-Bhalla G, et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Can Res. 2014;74:5458–5468. doi: 10.1158/0008-5472.Can-14-1258. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its supplementary information files.