Abstract
Echinococcus granulosus is a cestode parasite which causes cystic echinococcosis disease. Previously we observed that vaccination with E. granulosus antigens from human hydatid cyst fluid (HCF) significantly inhibits colon cancer growth. In the present work, we evaluate the anti-tumor immune response induced by human HCF against LL/2 lung cancer in mice. HCF vaccination protected from tumor growth, both in prophylactic and therapeutic settings, and significantly increased mouse survival compared to control mice. Considering that tumor-associated carbohydrate antigens are expressed in E. granulosus, we oxidized terminal carbohydrates in HCF with sodium periodate. This treatment abrogates the anti-tumor activity induced by HCF vaccination. We found that HCF vaccination-induced IgG antibodies that recognize LL/2 tumor cells by flow cytometry. An antigen-specific immune response is induced with HCF vaccination in the tumor-draining lymph nodes and spleen characterized by the production of IL-5 and, in less extent, IFNɣ. In the tumor microenvironment, we found that NK1.1 positive cells from HCF-treated mice showed higher expression of CD69 than control mice ones, indicating a higher level of activation. When we depleted these cells by administrating the NK-specific antibody NK1.1, a significantly decreased survival was observed in HCF-induced mice, suggesting that NK1.1+ cells mediate the anti-tumor protection induced by HCF. These results suggest that HCF can evoke an integrated anti-tumor immune response involving both, the innate and adaptive components, and provide novel insights into the understanding of the intricate relationship between HCF vaccination and tumor growth.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-021-02948-x.
Keywords: Cancer, Parasite, Echinococcus granulosus, Vaccination, Immunity
Introduction
Non-small cell lung cancer (NSCLC) remains one of the most challenging malignancies to treat. In spite of the introduction of innovative therapies over the last decade, NSCLC is still the leading cause of cancer-related mortality [1]. Although targeted therapy has been associated with a significant survival benefit in patients harboring aberrations in epidermal growth factor receptor (EGFR), anaplastic lymphoma kinase (ALK), or ROS1 [2], these gene abnormalities are present in a small percentage of patients. Emerging evidences demonstrating the anti-tumor activity of the immune system have generated great interest in immunotherapies, even for tumors that were historically considered as non-immunogenic, such as lung cancer [3]. Antibodies blocking immune checkpoint inhibitors such as CTLA-4 and PD-L1/PD-1 have had remarkable clinical success in several cancers and are less toxic than traditional chemotherapy. These drugs are currently approved therapies for NSCLC patients, showing improved and durable responses [4], but unfortunately, the majority of patients are resistant to checkpoint inhibition therapy.
Immunotherapy of cancer has emerged as an attractive approach for their capacity of breaking the immune tolerance and invoking long-term immune response, targeting cancer cells without autoimmunity [5]. However, several large phase III trials of vaccines in NSCLC reported within the last years have shown disappointing results [6]. The lack of clinically significant outcomes could, at least partly, be attributed to the inability of induced T-cell responses to overcome the tumoral mechanisms of immune escape, one of the major challenges in cancer vaccination [7]. The ability of cancers to evade immunosurveillance is consequence of multiple mechanisms [8], such as production of immunosuppressive molecules (IDO, TGF-β, and IL-10), loss of MHC antigen expression, as well as higher numbers of T-regulatory (Treg) and M2 macrophages cells in the tumor microenvironment.
We have previously identified and characterized a group of human cancer-associated simple O-glycan structures, such as Tn, TF, sialyl-Tn and Tk antigens, in several parasites [9]. Vaccination with tumor-associated antigens (TAA), coming from evolutionary distant organisms (such as parasites), should be useful to override tolerance problems encountered with human TAA-based cancer therapeutic approaches [10]. The idea of using the patient’s immune system activated by a microorganism to attack their own cancer began with Coley´s observations [11] followed by the use of Bacillus Calmette-Guerin (BCG) to treat early stage bladder cancer [12], approved by the Food and Drug Administration (FDA) in 1990. We have recently found that immunization with a Trypanosoma cruzi homogenate significantly suppresses colon and mammary tumor development in rat models reproducing human carcinogenesis [13]. In line with these observations, it has been demonstrated that Toxoplasma gondii induces anti-tumor immunity, promoting regression of established primary melanoma B16-F10 tumors [14], and that malaria infection suppresses Lewis lung cancer growth via induction of innate and specific adaptive anti-tumor responses characterized by production of Th1-type cytokines [15].
Echinococcus granulosus is a cestode parasite causing cystic echinococcosis disease. Correlation between E. granulosus infection and lower cancer incidence in humans was suggested in an epidemiological study [16]. We observed that vaccination with E. granulosus antigens from human HCF significantly inhibits colon cancer growth (both in prophylactic and therapeutic settings) via induction of anti-tumor immunity in a mice model [17]. In addition, we have found that immunization with mucin peptides derived from this parasite can potently induce anti-tumor activity by increasing the frequency of activated NK cells and providing splenocytes with the ability to mediate killing of tumor cells [18]. Based on these observations and considering that T-cell tolerance remains a particularly relevant problem for lung cancer vaccines production, we hypothesize that certain E. granulosus antigens could be involved in an effective immune response induction against this tumor. In this paper, we demonstrate that immunization with human HCF induces strong immune responses against lung cancer in mice both, in preventive and therapeutic settings, via NK1.1+ cell activation.
Materials and methods
Animals and tumor cell line
C57BL/6 J male mice were bred and maintained at the animal facility of Institut Pasteur de Montevideo (Uruguay) under specific pathogen-free conditions. Mice were housed in groups of 5 in individually ventilated cages (IVC, Tecniplast, Italy) with ad libitum access to autoclaved food (5K67, Labdiet, PMI Nutrition, MO, USA) and filtered water. Environmental enrichment was provided during the whole experiment. Rabbits were purchased from Instituto de Higiene (Facultad de Medicina, Montevideo, Uruguay). All experimental protocols were opportunely approved by the Institutional Animal Care Committee (number 010/12) and were performed following National Law of Animal Experimentation (# 18.611). The murine Lewis lung carcinoma cell line LL/2 (LLC1) was obtained from ATCC (Manassas, VA) and was cultured in DMEM (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS) (Invitrogen) at 37 °C and 5% CO2 atmosphere.
Hydatid cyst fluid
The starting material consisted of three non-complicated E. granulosus hydatid cysts (two localized in the liver and one in the spleen), obtained from the Pathological Laboratory at Hospital Pasteur, Montevideo, Uruguay. The HCF was aseptically aspirated from fertile cysts, then centrifuged at 10,000×g at 4 °C for 30 min and the supernatant was kept at − 20 °C until use. The present work was carried out using a batch of pooled three individual cysts. Periodate oxidation of HCF was carried out as described before [19]. Briefly, HCF hydatid fluid was freeze dried, resuspended in buffer sodium acetate (50 mM) pH 4.5 and incubated with sodium periodate (10 mM) for 30 min at room temperature in the dark, followed by the reduction with sodium borohydride (50 mM) in PBS. The resulting oxidized-HCF was referred as to mPox-HCF. As a control, HCF was subjected to the same treatment, without the presence of sodium periodate. Lysates were then dialyzed against PBS and their protein concentration was measured using the bicinchoninic acid assay (Sigma-Aldrich, St. Louis, MO).
Evaluation of sera reactivity by flow cytometry
Mice or rabbits were immunized three times with human HCF (100 μg/animal) in aluminum hydroxide (alum) at two-week intervals. After the last immunization, animals were bled and sera were evaluated by flow cytometry on the LL/2 cell line by flow cytometry. For that, cells were first incubated for 15 min with sera (diluted 1:100) at 4 °C in PBS containing 2% FBS and 0.1% sodium azide. Then, they were incubated for 15 min with an anti-mouse or ant-rabbit IgG goat antibody FITC labeled (Sigma, St Louis, MO). Alternatively, in order to evaluate sera recognition of intracellular antigens, cells were first permeabilized by incubating them in PBS containing 0.1% Triton-X100, 2% FBS and 0.1% sodium azide. Paraformaldehyde-fixed cells were analyzed on a CyAn ADP Analyzer (Beckman Coulter), and analyses were performed with Summit V4.3 (Beckman Coulter).
Mice tumors and HCF immunization
Six-eight week old C57BL/6 J mice were injected s.c. into the right flank with 1 × 105 LL/2 cells diluted in PBS (200 μl final volume). In prophylactic experiments, mice were vaccinated three times (days − 35, − 21 and − 7 before tumor cell challenge) via s.c. with HCF (300 μg protein/mouse) in alum. In the therapeutic setting, mice were challenged on day 0 with 1 × 105 LL/2 cells and 4, 7 and 10 days later they were injected s.c. with HCF in alum. Control mice were treated with PBS in alum. The size of the tumor was calculated by the formula V (mm3) = (4/3) × pi × R1 × R2 × R3, where R1, R2, R3 are the largest radius of the tumor in three dimensions. Survival of mice was followed for 90–120 days. Mice were humanely euthanized when the tumor diameter reached 20 mm or if they showed signs of distress or discomfort. For NK depletion, mice were injected i.p. with anti-NK1.1 antibody (300 μg) at days − 1, 1, 3, 5, 7, 9, 12, while control mice received saline buffer.
Leukocyte activation assays
Proliferation assays were performed with cells from spleens or draining lymph nodes (DLN) from naive or tumor-bearing mice. Cells (0.5–1 × 106 cells/ml) were cultured in complete medium consisting of RPMI-1640 with glutamine (PAA Laboratories, Austria) supplemented with 10% FBS, 50 μM 2-mercaptoethanol, 100 U/ml penicillin and 10 μg/ml streptomycin (Sigma-Aldrich), in the presence or absence of a LL/2 lysate (20 μg/ml), HCF (20 μg/ml) at 37 °C and 5% CO2 for 3 days. IFNγ and IL-5 levels were evaluated on culture supernatants by specific sandwich ELISA assays (BD Biosciences, NJ, USA).
Analyses of immune cells in the tumor microenvironment by flow cytometry
Cells from tumors were washed twice with PBS containing 2% FBS and 0.1% sodium azide and stained with anti-NK1.1 (PK136), -CD69 (H12F3), -CD8 (53–6.7), -CD11c (N418), -I-A/I-E (2G9), -CD11b (M1/70), -Ly6G (RB6-8C5), -Ly6C (HK1.4), then washed twice with PBS containing 2% FBS and 0.1% sodium azide, and fixed with 0.1% formaldehyde. Cell populations were analyzed by first excluding tumor cells by size and complexity. All analyses were performed using a CyAn ADP Analyzer (Beckman Coulter). Antibodies were obtained from Affymetrix or Biolegend (CA, USA).
Statistical analysis
The Student’s t test was used to compare data from various experimental groups. A p value < 0.05 was considered statistically significant. Mean and SEM are shown, unless indicated otherwise. Survival was evaluated from the day of tumor injection until euthanasia, and the Kaplan–Meier test was used to compare mouse survival between the groups. Data were processed using the graphpad Prism 6.0 software.
Results
Immunization with human HCF protects from tumor growth
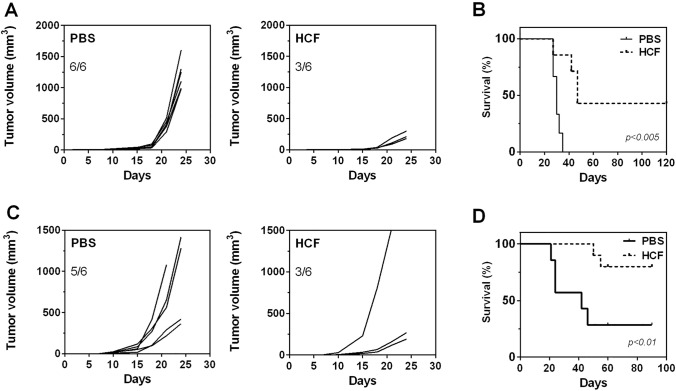
To investigate the capacity of HCF vaccination to protect from tumor growth, we used a subcutaneous lung tumor model in mice both, in prophylactic and therapeutic settings. First, we injected mice with HCF in alum three times, while control mice received PBS instead of HCF. One week after the last boost, mice were s.c. challenged with 1 × 105 LL/2 cells and both, tumor growth and mice survival, were measured (Fig. 1a, b). The average of tumor size was significantly lower in HCF-vaccinated mice comparing to control group (< 500 mm3 for protected mice and 1000–2000 mm3 for control mice). Interestingly, 6 out of 6 mice (100%) from the control group developed tumors, while only 3 out of 6 (50%) mice had tumors in the HCF-vaccinated group and they were significantly smaller than those developed in the PBS group, confirming the protection induced by HCF (Fig. 1a). Furthermore, all control mice were humanely sacrificed within 38 days following tumor challenge. In contrast, HCF-vaccinated mice that developed tumors, showed a prolonged survival, being sacrificed within 50 days after tumor challenge. In addition, 50% of HCF-vaccinated mice survived without tumor burden by the end of the experiment period (120 days) (Fig. 1b).
Fig. 1.
HCF immunization protects against LL/2 tumor growth both in prophylactic and therapeutic settings. a C57BL/6 J male mice (n = 6) were s.c. vaccinated three times in two week-intervals with human HCF (300 μg) or PBS in alum before LL/2 cell challenge (100,000 cells/mouse). Tumor growth was measured regularly using a caliper. Tumor volume (mm3) was calculated according to the following formula: 4/3 × pi × r1 × r2 × r3. b Survival of vaccinated and control mice was followed for 120 days after tumor challenge. Mice were euthanized when subcutaneous tumors reached 20 mm or when mice shows signs of distress or discomfort. c After s.c. administration of LL/2 cells (day 0), C57BL/6 J male? mice (n = 6) received a 300 μg dose of human HCF or PBS in alum on days 4, 7, and 11. d Survival of treated and control mice was followed for 90 days after tumor challenge. The data are representative of at least three independent experiments
We next evaluated the efficacy of HCF to protect against tumor growth in a therapeutic setting. In this case, mice received three vaccinations with HCF in alum at days 4, 7 and 11 after tumor cell challenge. In these conditions, 5 out of 6 mice (83%) of the control group developed tumors, while only three animals (50%) presented tumors in the vaccinated group (Fig. 1c). In the same way as prophylactic protocol, most animals from the control group developed larger tumors than those from the treated group (Fig. 1c). Interestingly, immunotherapeutic vaccination with HCF significantly increased mouse survival compared to control mice (Fig. 1d). Of note, one HCF-vaccinated mouse did not respond to the treatment since it developed a big tumor as control mice (Fig. 1c).
Glycoconjugates in HCF mediate tumor protection
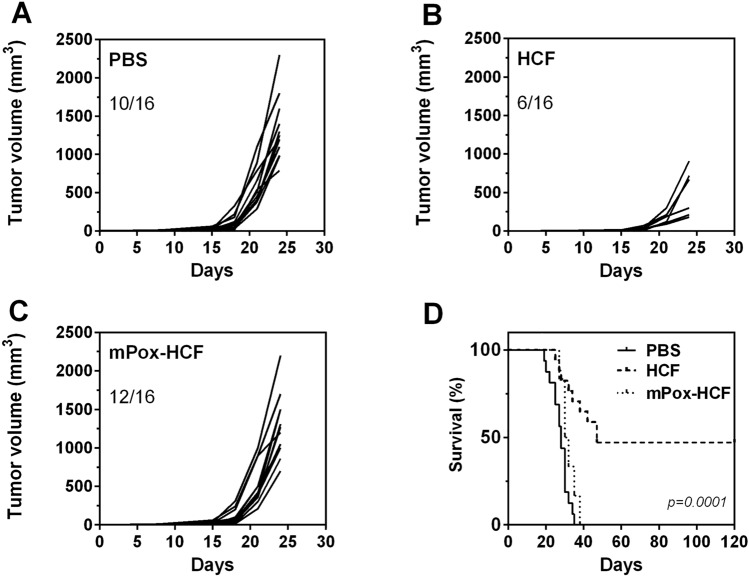
Considering that we previously found the expression of tumor-associated carbohydrate antigens (such as Tn and sialyl-Tn) in E. granulosus, and in order to establish whether or not carbohydrate structures on HCF mediate tumor protection, we oxidized terminal carbohydrates in HCF with sodium periodate. This strategy is usually used to evaluate the functional roles of glycoconjugates [20, 21]. During this process, the glycol groups in carbohydrates are oxidized to reactive aldehyde groups, which are in turn reduced with sodium borohydride. Thus, the structure of carbohydrates is lost, as well as the possible biological activity that they can mediate. Mice vaccinated with oxidized HCF (mPox-HCF), developed similar tumors to those from the control group (Fig. 2a, c). As control of the oxidation treatment, we used HCF subjected to the whole oxidation treatment excepting for the incubation with sodium periodate. Oxidation control HCF-vaccinated mice were protected from tumor growth or developed small tumors (Fig. 2b).
Fig. 2.
Glycoconjugates in HCF mediate tumor protection. a C57BL/6 J mice (n = 16) were vaccinated three times at 2 week-intervals with human HCF, m-periodate oxidized HCF (mPox-HCF) or PBS in alum before LL/2 cell challenge (100,000 cells/mouse). Tumor growth was measured regularly using a caliper. Tumor volume (mm3) was calculated according to the following formula: 4/3 × pi × r1 × r2 × r3. b Survival of HCF-vaccinated and control mice was followed for 120 days after tumor challenge
The role of glycoconjugates in anti-tumor protection was also confirmed when analyzing mouse survival, since all tumor-bearing mice from the mPox-HCF vaccinated group were humanely sacrificed before day 40, as control mice (Fig. 2d), while 50% of HCF-vaccinated mice were free of tumors until day 120. Thus, carbohydrates on HCF glycoconjugates seem to play a role in the induction of tumor protection.
HCF vaccination-Induced IgG antibodies that recognize LL/2 tumor cells
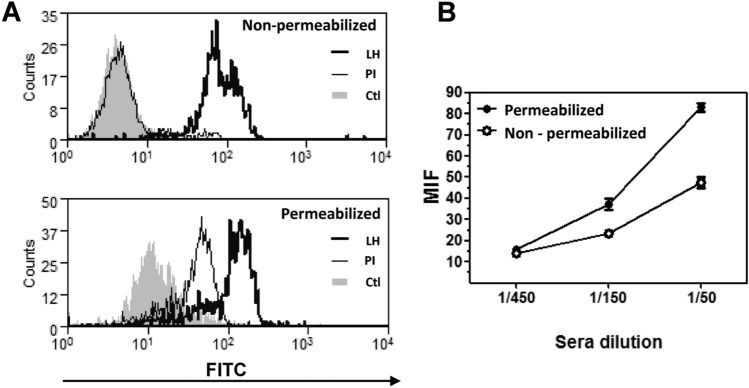
To analyze the induced anti-tumor humoral immune response, we evaluated if HCF-specific antibodies were capable of recognizing LL/2 tumor cells by flow cytometry. As shown in Fig. 3a, IgG antibodies recognized both surface and intracellular molecules from tumor cells. Furthermore, the recognition was antibody dose-dependent (Fig. 3b), indicating that HCF vaccination-induced antibodies capable of recognizing tumor cells.
Fig. 3.
Antibodies induced by HCF immunization recognize tumor cells. a Histogram plots showing antibody recognition for membrane and cytosolic antigens on LL/2 cells. Flow cytometry analyses were carried out on permeabilized or non-permeabilized LL/2 tumor cells incubated with sera (diluted 1:100) collected from animals immunized with human HCF. Controls consisted on pre-immune sera (PI) or cells incubated with secondary antibody only (Ctl). Five thousand events were collected and gated on FSC vs SSC dot plot. b Mean fluorescence intensity (MFI) representing antibody recognition of membrane and cytosolic antigens on non-permeabilized and permeabilized LL/2 cells, respectively. In this case, different sera dilutions (1:50, 1:150, 1:450) were used, and MFI values were subtracted to the corresponding pre-immune serum at same dilution
HCF-specific cells from immunized mice produce high IL-5 and IFNɣ levels
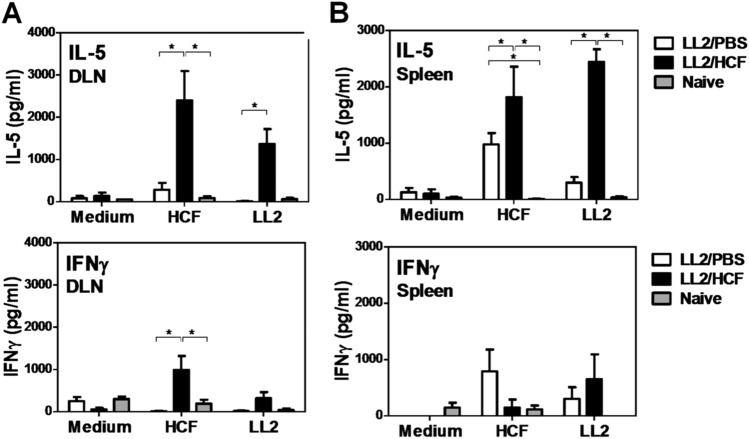
In order to study whether an antigen-specific T cell immune response was induced in HCF-treated mice, we re-stimulated cells from tumor-draining lymph nodes (DLN) or spleens of HCF-treated and control mice either with HCF or a LL/2 protein lysate, and evaluated the production of IL-5 and IFNɣ. When re-stimulated with HCF, DLN or spleen cells from treated animals produced higher levels of IL-5 than control mice. Interestingly, when these cells were re-stimulated with a LL/2 protein lysate, they produced IL-5 while cells from the control group did not (Fig. 4). Of note, tumor-DLN cells from control mice, like naive mice, did not produce significant levels of IFNɣ in any condition (Fig. 4a). Furthermore, the levels of IL-5 were higher than IFNɣ, which was only significantly detected when DLN cells from HCF-treated mice were stimulated with HCF. On the other hand, IL-4 was not detected (data not shown). These results suggest that an antigen-specific immune response is induced with HCF vaccination in the tumor DLN and spleen characterized by the production of IL-5 and in less extent IFNɣ.
Fig. 4.
HCF-specific cells from immunized mice produce high IL-5 and IFNɣ levels. After s.c. administration of LL/2 cells (100,000 cells/mouse) (day 0), C57BL/6 mice (n = 6) received a 300 μg dose of human HCF or PBS in alum on days 4, 7, and 11. At day 17 animals were sacrificed and spleens and DLN were collected. DLN cells (a) or splenocytes (b) (0.5–1 × 106/well) were cultured in presence or absence of a LL/2 lysate or HCF for three days at 37ºC. Levels of IL-5 and IFNγ were measured in cell supernatants by specific ELISA
NK1.1+ cells present a higher degree of activation in the tumor microenvironment of HCF‑treated mice
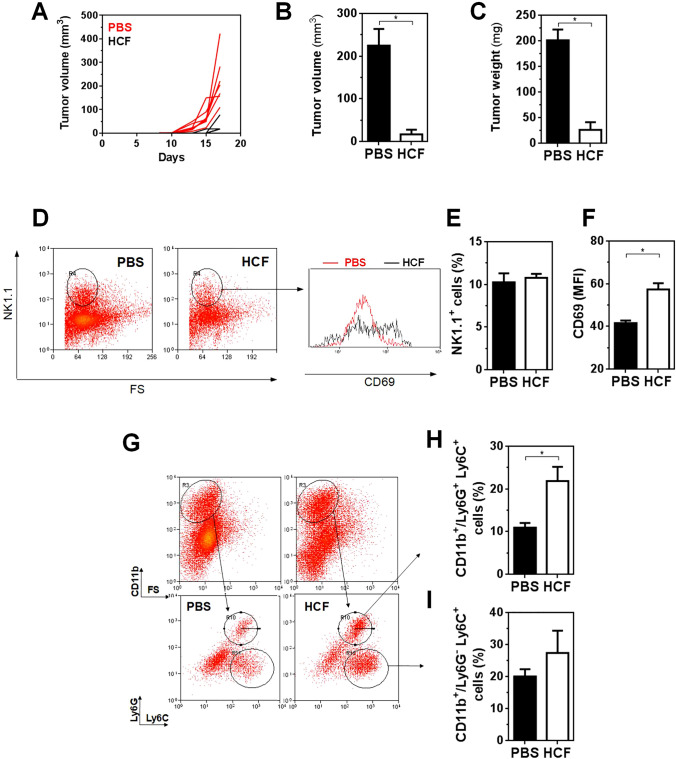
To get more insights in the cellular mechanisms that mediate tumor protection in HCF-treated mice, we evaluated a series of different immune cells in the tumor microenvironment (Fig. 5 and Supplementary Fig. 1) of HCF-treated and control mice that were sacrificed at day 17 after cell challenge (Fig. 5a). Only 3 out of 7 (42%) of HCF-treated mice developed tumors, that were significantly lower both in size and weight than control mice at the time of sacrifice (Fig. 5b, c). Flow cytometry analyses showed no changes in the percentage NK1.1 positive cells in tumors of HCF-treated mice in relation to control mice (Fig. 5d, 5e). However, NK1.1+ cells from HCF-treated mice showed higher expression of CD69 than control mice (Fig. 5d, f), indicating a higher level of activation.
Fig. 5.
Analyses of the tumor microenvironment in HCF-treated animals. a After s.c. administration of LL/2 cells (100,000 cells/mouse) (day 0), C57BL/6 J male mice (n = 7) received a 300 μg dose of human HCF or PBS in alum on days 4, 7, and 11. Tumor growth was measured regularly using a caliper. Tumor volume (mm3) was calculated according to the following formula: 4/3 × pi × r1 × r2 × r3. At day 17 animals were sacrificed and tumors were removed. Tumor volume (b) and weight (c) from HCF-treated and control animals was recorded. d Tumor cells were stained with anti-NK1.1, and -CD69 antibodies and analyzed by flow cytometry. e Frequency of NK1.1+ cells. f CD69 expression (MFI) on NK1.1+ cells. g Tumor cells were stained with anti-CD11b, Ly6G and Ly6C antibodies and analyzed by flow cytometry. h Frequency of Ly6G+ Ly6C+ cells gated in CD11b+ cells. i Frequency of Ly6G− Ly6C+ cells gated in CD11b+ cells
Tumors from HCF-treated mice also showed higher levels of granulocytes, defined as CD11b+/Ly6G+Ly6Clo cells (Fig. 5g, h). On the contrary, no significant changes were detected in the CD11b+/Ly6G−Ly6C+ cells (Fig. 5g, i), that account for monocytes.
Depletion of NK1.1+ cells abrogates HCF‑induced mice survival
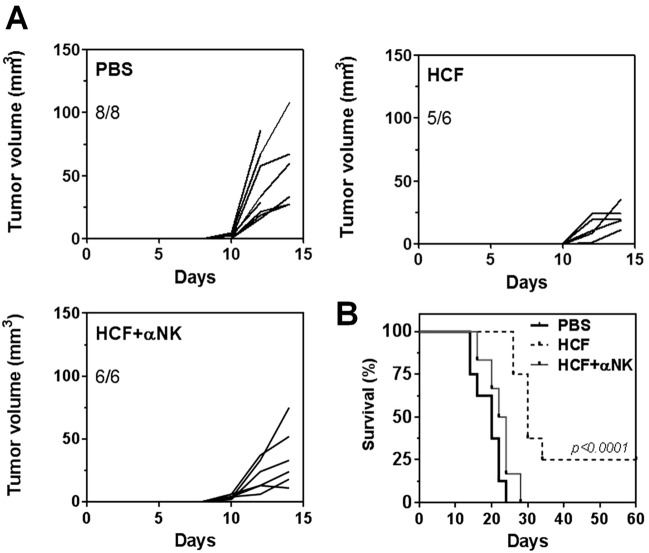
In order to determine whether NK cells were involved in the anti-tumor protection mediated by HCF, we depleted them by administrating the NK-specific antibody NK1.1. As shown in Fig. 6a, HCF-treated mice that received the anti-NK1.1 antibody developed tumors of variable size, with no significant difference between the HCF-vaccinated and control group. However, NK1.1+ cell depletion significantly decreased HCF-induced mice survival (Fig. 6b), suggesting that NK1.1+ cells mediate the anti-tumor protection induced by HCF.
Fig. 6.
NK1.1+ cells mediate HCF-induced protection. a After s.c. administration of LL/2 cells (day 0), C57BL/6 J mice received a 300 μg dose of human HCF or PBS in alum on days 4, 7, and 11. For NK1.1+ depletion, mice were injected i.p. with anti-NK1.1 antibody (300 μg/mouse) at days − 1,1, 3, 5, 7, 9, 12. Tumor growth was measured regularly using a caliper. Tumor volume (mm3) was calculated according to the following formula: 4/3 × pi x r1 x r2 x r3. b Survival of treated and control mice was followed for 60 days after tumor challenge. Mice were euthanized when subcutaneous tumors reached 20 mm or when mice shows signs of distress or discomfort. Statistically difference between PBS and HCF groups and PBS/α NK1.1+ and HCF groups
Discussion
Formulation of an effective vaccine should require a non-toxic adjuvant, which stimulates not only innate and adaptive immunity, but also reverses tumor-induced tolerance. With the identification of tumor-associated antigens (TAA) in parasites [9], the hypothesis of generating effective responses against cancer using molecules from evolutionary distant organisms, such as E. granulosus [10], is strengthened. The results of the work presented here show that human HCF induces anti-lung tumor responses, both in prophylactic as well as in therapeutic settings, with immunizations at short intervals. These results are in line with our previous report with a colon tumor model [17], demonstrating the effective memory induction for tumor rejection of a rechallenge 90 days after the first one, which reflects the activation of helper T lymphocytes.
The existence of shared antigens between tumor cells and E. granulosus, main players of cancer-parasite cross-immune reaction, also arises from our results. By flow cytometry, we observed that HCF-specific antibodies recognize both membrane and intracellular molecules in lung cancer cells. These antibodies could mediate anti-tumor activity. Recently, we demonstrated that the T. cruzi parasite also induces immune responses able to antagonize the development of cancer, using chemically induced colon and breast cancer models [13]. We also found that anti-T. cruzi antibodies were able to induce cancer cell death by antibody-dependent cellular cytotoxicity (ADCC) [13]. The potential for anti-tumor effects to be generated by anti-HCF antibodies recognizing LL/2 cancer cells is supported by the observation that the serum of patients with hydatid cyst has lethal effects on lung cancer in vitro [22].
Cancer-parasite cross-immune reactions may be due to carbohydrate antigens, for example, through the Tn and sialyl-Tn structures which are expressed in E. granulosus [23]. However, here these carbohydrate structures do not seem to play any role in cross-reactive immunity since LL/2 cells do not have Tn and sialyl-Tn determinants (results not shown). Although the identity of the parasite molecules that induce the anti-tumor response remain to be elucidated, we can state that the carbohydrates are essential for the induction of the immune response since the periodate-oxidation of HCF carbohydrates eliminated its anti-tumor activity. Possible candidates include mucins (highly glycosylated cell surface proteins), of high importance in tumor biology and in the generation of anti-cancer vaccines [24]. E. granulosus also possesses mucins. Interestingly, we have previously observed that immunization with peptides derived from an E. granulosus mucin (named Egmuc) induces potent anti-tumor activity, increasing NK cell activity and enhancing the ability of splenocytes to kill tumor cells [18]. We also demonstrated that Egmuc peptides induce, together with LPS, maturation of DCs by increasing the production of IL-12 and IL-6 and that Egmuc-treated DCs may activate NK cells. In agreement with these observations, in the present work, we found that NK1.1+ cells depletion significantly decreased HCF-induced mice survival, suggesting that these cells mediate the anti-tumor protection induced by HCF. NK cells have been shown to have an important role in anti-tumor immune response [25]. For instance, the density of NK cells in lung tumor tissue has been found to correlate positively with prognosis [26]. Natural killer cell-associated direct cytotoxicity and cytokine production are crucial mechanisms for early innate host resistance against viruses, bacteria, or protozoa. Although little is known about the role of NK cells in the defense against helminth infections [27–29], reports demonstrating that hydatid patients with active cysts have proportionally more NK cells in blood than the control group have been published [30]. Furthermore, the in vivo depletion of NK cells strongly enhances the worm load and influences IL-4 and IL-5 plasma levels [31]. Finally, early E. granulosus-infected mice present high numbers of activated NK cells in the peritoneal cavity [32]. Altogether, these data demonstrated a new role for NK cells in the host defense against filariae.
Splenocytes and tumor DLN cells from HCF-treated mice that were challenged with LL/2 cells produced high levels of IL-5 when stimulated with an LL/2 lysate. IL-5 is a key player in the coordination and orchestration of eosinophil-based inflammatory processes [33]. IL-5 is produced by activated Th2 cells and innate lymphoid cells 2 (ILC2), and in smaller amounts, by NK cells, NKT cells and eosinophils themselves [34]. IL-5 is best known for its activity on B cells and eosinophils, where it upregulates a number of surface receptors, induces granule release and promotes the growth and differentiation of eosinophils [33]. Interestingly, the high levels of IL-5 detected in HCF-treated mice were correlated with an increase of granulocytes in tumors. The increase of granulocytes in tumors from HCF-treated mice could account for neutrophils or eosinophils. Recent data have demonstrated the active role of eosinophils in tumor regression [35]. For instance, activated eosinophils were essential for tumor rejection since they produce chemoattractants that contribute to the recruitment of tumor-specific CD8+ T cells into the tumor [36]. On the other hand, neutrophils can also have anti-tumor properties. Indeed, tumor-associated neutrophils (TAN) are capable of mediate tumor-rejection [37]. Furthermore, TAN can also prevent tumor growth by the formation of neutrophil extracellular traps (NETs) [38]. The fact that HCF-treated mice presented more CD11b+/Ly6G+Ly6C+ cells in the tumor microenvironment would suggest a role of these cells in anti-tumor rejection, although more experiments are needed to confirm it.
In conclusion, we provide evidence of the anti-tumor effects of HCF and its ability to induce both humoral (antibody) and cellular immunity, with the participation of innate immunity, especially NK1.1+ cells. In agreement with our results, the ability of HCF to induce Th1 immune responses [39] or to favor the maturation of dendritic cells, with an increase in the production of pro-inflammatory cytokines IL-6 and IL-12 [40] together with our results, highlight the potential of HCF in tumor immunotherapy.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the Flow Cytometry Facility of Institut Pasteur de Montevideo for their assistance.
Author contributions
EO, TF, and EB designed the study. EB, TF, CC, ER, GFG, and NB performed experiments and analyzing the data. MC, GM and EO contributed to analysis and interpretation. TF, EO, and EB analyzed the data and wrote the manuscript. GM, NB and MC critically revised the manuscript. All authors approved the manuscript.
Funding
This work was supported by Fondo María Viñas, Agencia Nacional de Investigación e Innovación, Uruguay [Number FMV_1_2017_1_136442] (to EO), Fondo ANII-GSK, Agencia Nacional de Investigación e Innovación, Uruguay [Number FSGSK_1_2017_1_145466] (to EO), Programa Grupos de Investigación, CSIC, Universidad de la República, Uruguay [number 908] (to EO), Fondo para la Convergencia Estructural del MERCOSUR [COF 03/11] (to EB, GFG, MC, NB and EO).
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All mice were maintained under specific pathogen-free conditions and used in accordance with protocols established by the Animal Care Committee of the Institut Pasteur de Montevideo, Uruguay. All work with mice was performed according to protocol # 010/12 approved by the Institutional Animal Care Committee and following the National Law of Animal Experimentation (# 18.611).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edgardo Berriel and Teresa Freire have contributed equally to this work.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Hirsch FR, Suda K, Wiens J, Bunn PA., Jr New and emerging targeted treatments in advanced non-small-cell lung cancer. Lancet. 2016;388:1012–1024. doi: 10.1016/S0140-6736(16)31473-8. [DOI] [PubMed] [Google Scholar]
- 3.Du L, Herbst RS, Morgensztern D. Immunotherapy in lung cancer. Hematol Oncol Clin North Am. 2017;31:131–141. doi: 10.1016/j.hoc.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Doroshow DB, Sanmamed MF, Hastings K, Politi K, Rimm DL, Chen L, Melero I, Schalper KA, Herbst RS. Immunotherapy in non-small cell lung cancer: facts and hopes. Clin Cancer Res. 2019 doi: 10.1158/1078-0432.CCR-18-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schlom J, Hodge JW, Palena C, Tsang KY, Jochems C, Greiner JW, Farsaci B, Madan RA, Heery CR, Gulley JL. Therapeutic cancer vaccines. Adv Cancer Res. 2014;121:67–124. doi: 10.1016/B978-0-12-800249-0.00002-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomas A, Giaccone G. Why has active immunotherapy not worked in lung cancer? Ann Oncol. 2015;26:2213–2220. doi: 10.1093/annonc/mdv323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vinay DS, Ryan EP, Pawelec G, Talib WH, Stagg J, Elkord E, Lichtor T, Decker WK, Whelan RL, Kumara HMCS, Signori E, Honoki K, Georgakilas AG, Amin A, Helferich WG, Boosani CS, Guha G, Ciriolo MR, Chen S, Mohammed SI, Azmi AS, Keith WN, Bilsland A, Bhakta D, Halicka D, Fujii H, Aquilano K, Ashraf SS, Nowsheen S, Yang X, Choi BK, Kwon BS. Immune evasion in cancer: mechanistic basis and therapeutic strategies. Semin Cancer Biol. 2015;35:S185–S198. doi: 10.1016/j.semcancer.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 8.Tuccitto A, Shahaj E, Vergani E, Ferro S, Huber V, Rodolfo M, Castelli C, Rivoltini L, Vallacchi V. Immunosuppressive circuits in tumor microenvironment and their influence on cancer treatment efficacy. Virchows Arch. 2019;474:407–420. doi: 10.1007/s00428-018-2477-z. [DOI] [PubMed] [Google Scholar]
- 9.Osinaga E. Expression of cancer-associated simple mucin-type O-glycosylated antigens in parasites. Crit Rev Iubmb Life. 2007;59:269–273. doi: 10.1080/15216540601188553. [DOI] [PubMed] [Google Scholar]
- 10.Darani HY, Yousefi M. Parasites and cancers: parasite antigens as possible targets for cancer immunotherapy. Future Oncol. 2012;8:1529–1535. doi: 10.2217/fon.12.155. [DOI] [PubMed] [Google Scholar]
- 11.Coley WB. End results in Hodgkin's disease and lymphosarcoma treated by the mixed toxins of Erysipelas and Bacillus prodigiosus, alone or combined with radiation. Ann Surg. 1928;88:641–667. doi: 10.1097/00000658-192810000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chou R, Selph S, Buckley DI, Fu R, Griffin JC, Grusing S, Gore JL. Intravesical therapy for the treatment of nonmuscle invasive bladder cancer: a systematic review and meta-analysis. J Urol. 2017;197:1189–1199. doi: 10.1016/j.juro.2016.12.090. [DOI] [PubMed] [Google Scholar]
- 13.Ubillos L, Freire T, Berriel E, Chiribao ML, Chiale C, Festari MF, Medeiros A, Mazal D, Rondán M, Bollati-Fogolín M, Rabinovich GA, Robello C, Osinaga E. Trypanosoma cruzi extracts elicit protective immune response against chemically induced colon and mammary cancers. Int J Cancer. 2016;138:1719–1731. doi: 10.1002/ijc.29910. [DOI] [PubMed] [Google Scholar]
- 14.Baird JR, Byrne KT, Lizotte PH, Toraya-Brown S, Scarlett UK, Alexander MP, Sheen MR, Fox BA, Bzik DJ, Bosenberg M, Mullins DW, Turk MJ, Fiering S. Immune-mediated regression of established B16F10 melanoma by intratumoral injection of attenuated Toxoplasma gondii protects against rechallenge. J Immunol. 2013;190:469–478. doi: 10.4049/jimmunol.1201209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen L, He Z, Qin L, Li Q, Shi X, Zhao S, Chen L, Zhong N, Chen X. Antitumor effect of malaria parasite infection in a murine Lewis lung cancer model through induction of innate and adaptive immunity. PLoS ONE. 2011;6:e24407. doi: 10.1371/journal.pone.0024407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akgül H, Tez M, Unal AE, Keşkek M, Sayek I, Ozçelik T. Echinococcus against cancer: why not? Cancer. 2003;98(9):1999–2000. doi: 10.1002/cncr.11752. [DOI] [PubMed] [Google Scholar]
- 17.Berriel E, Russo S, Monin L, Festari MF, Berois N, Fernández G, Freire T, Osinaga E. Anti-tumor activity of human hydatid cyst fluid in a murine model of colon cancer. Sci World J. 2013 doi: 10.1155/2013/230176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noya V, Bay S, Festari MF, García E, Rodriguez E, Chiale C, Ganneau C, Baleux F, Astrada S, Bollati-Fogolín M, Osinaga E, Freire T. Mucin-like peptides from Echinococcus granulosus induce antitumor activity. Int J Oncol. 2013;43:775–784. doi: 10.3892/ijo.2013.2000. [DOI] [PubMed] [Google Scholar]
- 19.Rodríguez E, Noya V, Cervi L, Chiribao ML, Brossard N, Chiale C, Carmona C, Giacomini C, Freire T. Glycans from Fasciola hepatica modulate the host immune response and TLR-induced maturation of dendritic cells. PLoS Negl Trop Dis. 2015;9:e0004234. doi: 10.1371/journal.pntd.0004234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tawill S, le Goff L, Ali F, Blaxter M, Allen J. Both free-living and parasitic nematodes induce a characteristic Th2 response that is dependent on the presence of intact glycans. Infect Immun. 2004;72:398–407. doi: 10.1128/IAI.72.1.398-407.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klaver EJ, Kuijk LM, Laan LC, Kringel H, van Vliet SJ, Bouma G, Cummings RD, Kraal G, van Die I. Trichuris suis-induced modulation of human dendritic cell function is glycan-mediated. Int J Parasitol. 2013;43:191–200. doi: 10.1016/j.ijpara.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 22.Karadayi S, Arslan S, Sumer Z, Turan M, Sumer H, Karadayi K. Does hydatid disease have protective effects against lung cancer? Mol Biol Rep. 2013;40:4701–4704. doi: 10.1007/s11033-013-2565-8. [DOI] [PubMed] [Google Scholar]
- 23.Alvarez Errico D, Medeiros A, Míguez M, Casaravilla C, Malgor R, Carmona C, Nieto A, Osinaga E. O-glycosylation in Echinococcus granulosus: identification and characterization of the carcinoma associated Tn antigen. Exp Parasitol. 2001;98:100–109. doi: 10.1006/expr.2001.4620. [DOI] [PubMed] [Google Scholar]
- 24.Apostolopoulos V, McKenzie IFC. Cellular mucins: targets for Immunotherapy. Crit Rev Immunol. 2017;37:421–437. doi: 10.1615/CritRevImmunol.v37.i2-6.110. [DOI] [PubMed] [Google Scholar]
- 25.Kim N, Lee HH, Lee HJ, Choi WS, Lee J, Kim HS. Natural killer cells as a promising therapeutic target for cancer immunotherapy. Arch Pharm Res. 2019 doi: 10.1007/s12272-019-01143-y. [DOI] [PubMed] [Google Scholar]
- 26.Soo RA, Chen Z, Yan Teng RS, Tan HL, Iacopetta B, Tai BC, Soong R. Prognostic significance of immune cells in non-small cell lung cancer: meta-analysis. Oncotarget. 2018;9:24801–24820. doi: 10.18632/oncotarget.24835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Niederkorn JY, Stewart GL, Ghazizadeh S, Mayhew E, Ross J, Fischer B. Trichinella pseudospiralis larvae express natural killer (NK) cell-associated asialo-GM1 antigen and stimulate pulmonary NK activity. Infect Immun. 1988;56:1011–1016. doi: 10.1128/iai.56.5.1011-1016.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Babu S, Blauvelt CP, Nutman TB. Filarial parasites induce NK cell activation, type 1 and type 2 cytokine secretion, and subsequent apoptotic cell death. J Immunol. 2007;179:2445–2456. doi: 10.4049/jimmunol.179.4.2445. [DOI] [PubMed] [Google Scholar]
- 29.Babu S, Porte P, Klei TR, Shultz LD, Rajan TV. Host NK cells are required for the growth of the human filarial parasite Brugia malayi in mice. J Immunol. 1998;161:1428–1432. [PubMed] [Google Scholar]
- 30.Hernández A, O'Connor JE, Mir A. Phenotypic analysis of peripheral lymphocyte subpopulations in hydatid patients. Parasitol Res. 1999;85:948–950. doi: 10.1007/s004360050664. [DOI] [PubMed] [Google Scholar]
- 31.Korten S, Volkmann L, Saeftel M, Fischer K, Taniguchi M, Fleischer B, Hoerauf A. Expansion of NK cells with reduction of their inhibitory Ly-49A, Ly-49C, and Ly-49G2 receptor-expressing subsets in a murine helminth infection: contribution to parasite control. J Immunol. 2002;168:5199–5206. doi: 10.4049/jimmunol.168.10.5199. [DOI] [PubMed] [Google Scholar]
- 32.Mourglia-Ettlin G, Marqués JM, Chabalgoity JA, Dematteis S. Early peritoneal immune response during Echinococcus granulosus establishment displays a biphasic behavior. PLoS Negl Trop Dis. 2011;5:e1293. doi: 10.1371/journal.pntd.0001293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takatsu K. Interleukin-5 and IL-5 receptor in health and diseases. Proc Jpn Acad Ser B Phys Biol Sci. 2011;87:463–485. doi: 10.2183/pjab.87.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosenberg HF, Dyer KD, Foster PS. Eosinophils: changing perspectives in health and disease. Nat Rev Immunol. 2013;13:9–22. doi: 10.1038/nri3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Capobianco A, Manfredi AA, Monno A, Rovere-Querini P, Rugarli C. Melanoma and lymphoma rejection associated with eosinophil infiltration upon intratumoral injection of dendritic and NK/LAK cells. J Immunother. 2008;31:458–465. doi: 10.1097/CJI.0b013e318174a512. [DOI] [PubMed] [Google Scholar]
- 36.Carretero R, Sektioglu IM, Garbi N, Salgado OC, Beckhove P, Hämmerling GJ. Eosinophils orchestrate cancer rejection by normalizing tumor vessels and enhancing infiltration of CD8(+) T cells. Nat Immunol. 2015;16:609–617. doi: 10.1038/ni.3159. [DOI] [PubMed] [Google Scholar]
- 37.Buonocore S, Haddou NO, Moore F, Florquin S, Paulart F, Heirman C, Thielemans K, Goldman M, Flamand V. Neutrophil-dependent tumor rejection and priming of tumoricidal CD8+ T cell response induced by dendritic cells overexpressing CD95L. J Leukoc Biol. 2008;84:713–720. doi: 10.1189/jlb.0108075. [DOI] [PubMed] [Google Scholar]
- 38.Garley M, Jabłońska E, Dąbrowska D. NETs in cancer. Tumour Biol. 2016;37:14355–14361. doi: 10.1007/s13277-016-5328-z. [DOI] [PubMed] [Google Scholar]
- 39.Riganò R, Profumo E, di Felice G, Ortona E, Teggi A, Siracusano A. In vitro production of cytokines by peripheral blood mononuclear cells from hydatid patients. Clin Exp Immunol. 1995;99:433–439. doi: 10.1111/j.1365-2249.1995.tb05569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kanan JH, Chain BM. Modulation of dendritic cell differentiation and cytokine secretion by the hydatid cyst fluid of Echinococcus granulosus. Immunology. 2006;118:271–278. doi: 10.1111/j.1365-2567.2006.02375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.